Abstract
Hepatitis C virus (HCV) is the leading causative agent of blood-borne chronic hepatitis and is the target of intensive vaccine research. The virus genome encodes a number of structural and nonstructural antigens which could be used in a subunit vaccine. The HCV envelope glycoprotein E2 has recently been shown to bind CD81 on human cells and therefore is a prime candidate for inclusion in any such vaccine. The experiments presented here assessed the optimal form of HCV E2 antigen from the perspective of antibody generation. The quality of recombinant E2 protein was evaluated by both the capacity to bind its putative receptor CD81 on human cells and the ability to elicit antibodies that inhibited this binding (NOB antibodies). We show that truncated E2 proteins expressed in mammalian cells bind with high efficiency to human cells and elicit NOB antibodies in guinea pigs only when purified from the core-glycosylated intracellular fraction, whereas the complex-glycosylated secreted fraction does not bind and elicits no NOB antibodies. We also show that carbohydrate moieties are not necessary for E2 binding to human cells and that only the monomeric nonaggregated fraction can bind to CD81. Moreover, comparing recombinant intracellular E2 protein to several E2-encoding DNA vaccines in mice, we found that protein immunization is superior to DNA in both the quantity and quality of the antibody response elicited. Together, our data suggest that to elicit antibodies aimed at blocking HCV binding to CD81 on human cells, the antigen of choice is a mammalian cell-expressed, monomeric E2 protein purified from the intracellular fraction.
Hepatitis C virus (HCV) is the major cause of chronic hepatitis, which can evolve into cirrhosis, liver failure, or hepatocellular carcinoma (2, 4). There is no vaccine for HCV, and the only available treatment, a combination of alpha interferon and ribavirin, is efficacious in only a minority of patients (33). Given that approximately 200 million chronic HCV infections have been estimated worldwide (52), there is a pressing need to develop new therapies and vaccination strategies. The development of such strategies will be aided greatly by a more complete picture of the structure-function features of HCV proteins.
HCV is an enveloped plus-strand RNA virus of the Flaviviridae family (24). Its genome is 9.5 kb in length with one open reading frame that is translated as a single polyprotein, which is processed by host and virus proteases into at least three structural and seven presumptive nonstructural proteins with various enzymatic activities (5, 22, 47). Two glycoproteins, E1 and E2, are probably virion envelope proteins, containing multiple N-linked glycosylation sites, and form heterodimers in vitro (23, 32, 35, 45). The coexpressed E1-E2 complex localizes to the endoplasmic reticulum (ER) and lacks complex N-linked glycans (7, 8, 13, 15, 45, 49).
Neutralizing antibodies often play a pivotal role in defeating viral infections, including prominent human pathogens such as influenza virus and hepatitis B virus (9, 28). The assessment of neutralizing antibody responses to HCV has been difficult because HCV does not grow efficiently in cell cultures. To overcome this obstacle, we developed a surrogate assay which measures the ability of antibodies to inhibit the binding of recombinant E2 to its putative cellular receptor CD81 on human cells (neutralization-of-binding [NOB] assay) (44, 46). CD81 is a membrane-associated protein belonging to the family of tetraspanins (30). Its large extracellular loop (LEL) binds E2 with a Kd of 1.8 nM (42), and this interaction appears necessary and sufficient for binding of bona fide HCV particles (44). Importantly, chimpanzee sera containing antienvelope antibodies, which are capable of preventing HCV infection in vivo, inhibit the binding of HCV to CD81 in vitro, suggesting that this interaction is relevant to infection (44).
Our research has focused primarily on comparing vaccine formulations of HCV E2, which is an obvious candidate for inclusion in a subunit vaccine because of its potential role in HCV attachment. Thus, targeting antibodies to HCV E2 could be a viable strategy for disrupting the HCV-CD81 interaction. Despite the inherent difficulties in studying HCV infection and the lack of a clear correlate of protection, there is evidence that neutralizing antibodies can be protective. Studies performed with human immunoglobulin (Ig) preparations have suggested some degree of efficacy in preventing the transmission of HCV in the transfusion setting, after liver transplants, and in sexual transmission (17, 27, 43). Relevant data on the existence of HCV-specific neutralizing antibodies also come from experimental vaccination studies in chimpanzees, the only species besides humans susceptible to infection. When vaccinated with recombinant envelope proteins (E1-E2 heterodimer), chimpanzees developed high titers of anti-E2 antibodies in serum, as determined by enzyme-linked immunosorbent assay (ELISA) and NOB assay, and were completely protected from subsequent challenge with the homologous virus (6, 46).
As with any chronic viral infection, there is a growing interest in eliciting not only antibody but also cytotoxic T-lymphocyte (CTL) responses to HCV. Therefore, we have begun to investigate DNA vaccination as an alternative or complement to a vaccine based solely on recombinant proteins. DNA vaccines have been reported to prime good antibody responses, but, unlike subunit vaccines, they offer the advantage of maximizing CTL responses (12, 41). Their efficacy has been successfully demonstrated in animal models for a wide variety of viral pathogens including hepatitis B virus, human immunodeficiency virus, and influenza virus (10, 29, 51). However, despite its advantages and success in rodent models, DNA vaccination suffers from several drawbacks in that it is still largely in the realm of basic research and nonsecreted antigens are particularly weak stimulators of antibody responses (3).
Here we compared the quantity and quality of anti-E2 antibody responses elicited by various forms of recombinant HCV E2 proteins or by DNA vaccines. Our findings indicate that an ER-retained form of recombinant HCV E2 protein is the antigen of choice for eliciting the best antibody response in terms of both quality and quantity.
MATERIALS AND METHODS
Cloning, expression, and purification of E2 protein.
E2384–661 and E2384–715 were expressed from recombinant CHO cells as described previously (46, 49). For purification of secreted E2 (S-E2), CHO cell conditioned medium was concentrated 23-fold by ultrafiltration and subjected to flowthrough chromatography on a type I ceramic hydroxyapatite (HAP) (Bio-Rad) column equilibrated in 100 mM NaCl–10 mM phosphate (pH 6.3). E2-containing flowthrough fractions, as assessed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting, were pooled and concentrated on a 30-kDa-cutoff tangential flow filter (Omega membrane; Pall Corp., Torrance, Calif.). Internal E2 (I-E2) was solubilized from CHO cells with lysis buffer (4% Triton X-100, 100 mM Tris-HCl [pH 8.0], 1 mM EDTA, protease inhibitors). The solution was loaded on a Galanthus nivalis lectin (Vector Laboratories, Burlingame, Calif.) affinity column equilibrated in 1 M NaCl–2% Triton X-100–50mM Tris-HCl (pH 8.0). The column was washed with 1 M NaCl–0.1% Triton X-100–20 mM phosphate (pH 6.0) and eluted with 1 M methyl-α-d-mannopyranoside. The eluate was diluted, loaded onto a HAP column, and subjected to a concentration step as indicated above. The HAP concentrate was processed over an S-Fractogel column (EM Separations) equilibrated and washed with 25 mM NaCl–10mM phosphate (pH 6.2). I-E2 was eluted with 150 mM NaCl–10 mM phosphate (pH 6.2). To purify the different molecular weight forms of E2, the S-E2715 concentrate and the I-E2715 eluate were adjusted to 30 mM phosphate (pH 7.0), and fractionated on a Superdex 200 (Pharmacia) gel filtration column equilibrated with phosphate-buffered saline (PBS) (pH 7.0)–1 mM EDTA. Aliquots of collected fractions were trichloracetate precipitated for analysis. Fractions containing monomers, dimers, and trimers of S-E2715 and I-E2715 were pooled and concentrated as indicated above.
Deglycosylation of internal E2715 and carbohydrate analysis.
I-E2715 was digested with α-mannosidase or endo-β-N-acetylglucosaminidase H (endo H) (Boehringer Mannheim). The exoglycosidase α-mannosidase efficiently removes free mannose (Man) residues that are α1→6 Man or α1→2 Man linked and less efficiently removes α1→3 Man-linked residues. Endo H cleaves between the two N-acetylglucosamine (GlcNAc) residues of N-linked core units, leaving one GlcNAc residue linked to asparagine. Samples were incubated with or without enzyme overnight at 37°C in 50 mM citrate (pH 5.0). α-Mannosidase digestion was monitored by SDS-PAGE, and then 100 mM borate (pH 9.5) was added to stop the reaction. The samples were then dialyzed against 10 mM phosphate (pH 8.0) to remove digested monosaccharides. Endo H-digested carbohydrates were removed by S-Fractogel chromatography as indicated above. For monosaccharide content analyses, digested E2 was treated with 3 M trifluoroacetic acid for 6 h at 100°C to release the monosaccharides. Following lyophilization, samples were derivatized at pH 8.0 with 50 mM phenylmethylpyralozone and extracted into tert-butylether. The extract was then analyzed by reverse-phase high-pressure liquid chromatography, and the monosaccharide content was determined by using an external calibration profile and an internal talose standard.
DNA constructs.
The vector pAC-FN used for DNA vaccination will be described in detail elsewhere (J. M. Heile, S. Abrignani, and G. Grandi, unpublished data). Briefly, expression of the gene of interest is driven by the human cytomegalovirus immediate-early promoter and enhancer and RNA stability is ensured by a simian virus 40 splice site and poly(A) signal. E2 DNA vaccines are derived from a previously described construct, which directs truncated E2384–715 of HCV genotype 1a into the ER by a 25-amino-acid (aa) tissue plasminogen activator (TPA)-derived signal sequence (46). The tpa-E2715 insert was transferred into the SmaI and NotI sites of the pAC-FN polylinker by standard molecular cloning techniques, resulting in pAC-E2715. For the construction of DNA vaccines encoding ER-retained E2, the following strategy has been used. pAC-E2715 was modified by replacing the C-terminal part of E2 with PCR fragments amplified from pMCMV-HC5p, which encodes HCV aa 1 to 917 (49). The fragments were cloned into the unique ApaI site at E2 aa 470 and into the NotI site. The sense primer used for all constructs (E2Apa-209; 5′-CCTCCTCGCACCAGGCGCCAAGC-3′) anneals 209 bp upstream of ApaI. All antisense primers are composed of the sequence 5′-ATCGTAGCGGCCGCTTA-3′, comprising a NotI site and the ochre stop codon, followed by the sequence 5′-CGCCTCCGCTTGGGATATG-3′ (E2746), 5′-CGCGTACGCCCGCTGGGGC-3′ (E2809), 5′-AGGCATTTTCTTTTCATCAATAAAACTGCGTCTGCTGCGCGCGTCTGCAAGCAGAAGG-3′ (E2730KK), or 5′-GAGCTCGTCCTTAATGGCCCAGGACGCG-3′ (E2715DEL). The resulting constructs are referred to as pAC-E2746, pAC-E2809, pAC-E2730KK, and pAC-E2715DEL. All PCR-derived modifications were verified using an ABI sequencer (Amersham Pharmacia Biotech).
In vitro analysis of constructs.
CHO cells were stably transfected with the E2-DNA vaccines for analysis. Selection was achieved through bicistronic expression of E2 together with a neomycin resistance gene (neo), coupled downstream of the E2 sequence via an internal ribosomal entry site. neo was amplified by PCR from pcDNAINeo (Invitrogen) and cloned into BamHI and blunt-ended NcoI sites of pCITE-2a(+) (Novagen), with NcoI providing the ATG start codon. The resulting IRES-neo fragment was excised with ApaI and XbaI and blunt ended for insertion into the NotI site of the pAC-E2 constructs. CHO DG44 cells were grown in Ham's F12 medium supplemented with 10% fetal calf serum, 2 mM L-glutamine, and 0.02% l-proline. Subconfluent monolayers grown in 100-mm-diameter dishes were transfected with 20 μg of plasmid DNA by calcium phosphate coprecipitation. Precipitates were incubated with cells for 6 h at 37°C and then given a 3-min shock with 15% glycerol in PBS. Cells were grown for 48 h without selection, divided among 15 plates, and grown for 2 weeks in medium containing 1 mg of Geneticin G418 sulfate (Gibco BRL) per ml. E2 protein was purified from pooled transfectants without further cloning. Cells were resuspended in lysis buffer (20 mM Tris-HCl [pH 7.5], 100 mM NaCl, 1 mM EDTA, 0.5% NP-40, 0.5% deoxycholate) containing protease inhibitors. E2 was immunoprecipitated with serum of chimpanzee L559, which was immunized with E1-E2 heterodimer and protected against challenge with homologous HCV (6). Using protein A-Sepharose CL-4B (Pharmacia), the lysate was precleared with L559 preimmune serum and E2 was precipitated with immune serum. The pellet was directly resuspended in 100 mM acetate (pH 5.0)–0.03% SDS and boiled for 2 min. Equal parts were incubated with or without endo H for 20 h at 37°C in the presence of protease inhibitors and then trichloroacetate precipitated. After SDS-PAGE under reducing conditions, the E2 antigen was detected by Western blotting with a monoclonal antibody (MAb) raised against E2715 expressed in insect cells (MAb 3E51), which recognizes nonconformational E2 (S. Abrignani, D. Rosa, and M. Houghton, unpublished data), followed by horseradish peroxidase-conjugated anti-mouse Ig antiserum (Amersham).
Animals and immunizations.
Hartley female guinea pigs were purchased from Elmhill, Chumford, Mass. Five animals per group were immunized intraperitoneally (i.p.) with 8 μg of S-E2715 or I-E2715 in MF59-0 adjuvant on days 0, 30, and 90, and bled on day 110. Groups of eight 6- to 8-week-old female C57BL/6 mice (Charles River, Calco, Como, Italy) were injected intramuscularly (i.m.) in both hindlegs with either DNA in PBS or protein in MF59-0 adjuvant on days 0 and 35 and bled on day 49. Plasmid DNA was prepared using endotoxin-free purification columns (Qiagen, Hilden, Germany). Per mouse and injection, a total of 100 μl of 1-μg/μl E2-DNA (100 μg) or 50-ng/μl I-E2715 protein (5 μg) was administered.
Antibody titers.
Anti-E2 antibody titers were measured by ELISA. For guinea pig sera, Nunc maxisorb microtiter plates were coated with HeLa E1-E2 antigen (0.3 μg/ml) purified as previously described (6). The sera were analyzed with horseradish peroxidase-conjugated anti-guinea pig IgG antiserum (Sigma). Mouse sera were analyzed with Nunc CovaLink microtiter plates coated with CHO I-E2715 protein at 0.5 μg/ml, using alkaline phosphatase-conjugated anti-mouse IgG (Sigma). Antibody titers were calculated as the dilution which gave an optical density (OD) that equaled the cutoff. The cutoff was established as the mean OD + 3 standard deviations for eight preimmune sera.
E2 binding assay.
Binding of E2 to target cells was measured as described previously (46). Briefly, MOLT-4 cells were incubated with different concentrations of E2 protein. Cell-bound E2 was detected with anti-E1-E2 antiserum from chimpanzee L559 followed by phycoerythrin-labeled anti-human Ig, F(ab′)2 fragment specific (Southern Biotechnology Associates, Birmingham, Ala.), or with antiserum from guinea pigs immunized with I-E2715 or S-E2715 followed by fluorescein isothiocyanate-labeled anti-guinea pig IgG, F(ab′)2 fragment specific (Jackson ImmunoResearch, West Grove, Pa.). Cell-bound fluorescence was analyzed with a FACScan flow cytometer (Beckton Dickinson). The net mean fluorescence intensity (MFI) was calculated by subtracting the value obtained in parallel with chimpanzee L559 or guinea pig preimmune serum. The specificity of E2 binding to CD81 on human cells was confirmed as described previously (44) by competing E2 binding to human cells with soluble human CD81 LEL, which was purified as described previously (42) and incubated at a concentration of 50 μg/ml with E2 prior to incubation with MOLT-4 cells.
NOB assay.
Mouse and guinea pig sera were tested for their ability to inhibit the binding of E2 protein to human target cells as previously described (25, 46). Briefly, NOB titers were determined by incubation of MOLT-4 cells with a nonsaturating concentration of biotinylated I-E2715 protein (1.5 μg/ml), which was previously incubated with a test serum at different dilutions. Following incubation of the cells with a streptavidin-phycoerythrin conjugate (Southern Biotechnology Associates) at 2.5 μg/ml, E2 binding was measured with a FACScan. NOB titers correspond to the reciprocal values of serum dilutions which inhibit 50% of binding.
RESULTS
I-E2 but not S-E2 binds human target cells and elicits NOB antibodies following immunization.
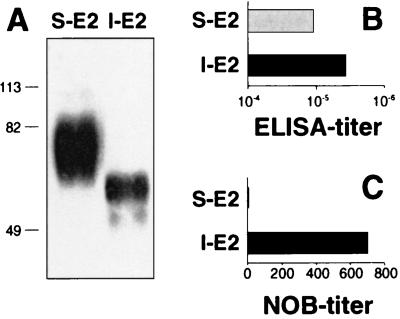
The E2 protein is processed from the HCV polyprotein, leading to a polypeptide backbone of 363 residues, spanning residues 384 to 746 (31). aa 716 to 746 serve as a membrane anchor, and their deletion can lead to secretion of the E2 protein when expressed in mammalian cells (34, 48). We previously described stably transfected CHO cell lines expressing E2, targeted to the ER via a TPA-derived leader sequence fused to its N terminus and carboxy-terminally truncated at residues 661 (E2661) or 715 (E2715). Both lines secrete a fraction of the E2 protein and retain part of it in the ER (49). Although E2661 is secreted from CHO transfectants with more efficiency than are longer truncated versions, we first aimed to purify and analyze the largest secreted E2 protein possible, and we previously reported the purification and binding to human target cells of E2715 (46). This E2 protein had been affinity purified from culture supernatants with MAb 5E5/H7 raised against the HeLa intracellular E1-E2 heterodimer, and we obtained E2 only in extremely small quantities. The low yield achieved with a conformational MAb used for purification led us to suspect that we had actually captured either a minor well-folded fraction or an intracellular fraction that had been released into the supernatant by occasional cell lysis. We then purified both the supernatant fraction (S-E2715) and the intracellular fraction (I-E2715) from the same line without the use of E2-specific antibodies. In Western blots, S-E2715 appeared as a broad band of about 74 kDa whereas I-E2715 appeared as a sharper band of about 60 kDa (Fig. 1A), indicating extensive usage of the 11 N-linked glycosylation sites within E2.
FIG. 1.
I-E2 induces higher anti-E2 NOB titers than S-E2 does. (A) E2715 protein was expressed in CHO cells and purified from the supernatant (S-E2) and from lysed cells (I-E2). The two fractions were analyzed by SDS-PAGE (10% acrylamide) under reducing conditions and Western blotting with chimpanzee anti-E1/E2 antiserum. The sizes (in kilodaltons) of protein molecular mass markers are indicated on the left. (B and C) Hartley female guinea pigs were immunized i.p. with 8 μg of S-E2 or I-E2 in MF59-0 adjuvant on days 0, 30, and 90. Sera taken on day 110 were analyzed by ELISA (B) and the NOB assay (C). Anti-E2 ELISA titers (total IgG) are expressed as the highest serum dilution resulting in an OD450 that equaled the cutoff (mean OD + 3 standard deviations for eight preimmune sera). Anti-E2 NOB titers are expressed as reciprocal values of serum dilutions which inhibit 50% of E2 binding to CD81 on MOLT-4 cells. The results are given as the mean values for five individual animals.
The E2 protein is an ideal vaccine candidate for the generation of antibodies that inhibit the binding of virus to target cells. This antibody subset can be estimated in vitro by the NOB assay. To test the quality of different E2 antigens, we used S-E2715 and I-E2715 for immunization studies in guinea pigs. Both fractions raised high antibody titers as measured by ELISA, but only the internal fraction raised NOB antibodies (Fig. 1B and C).
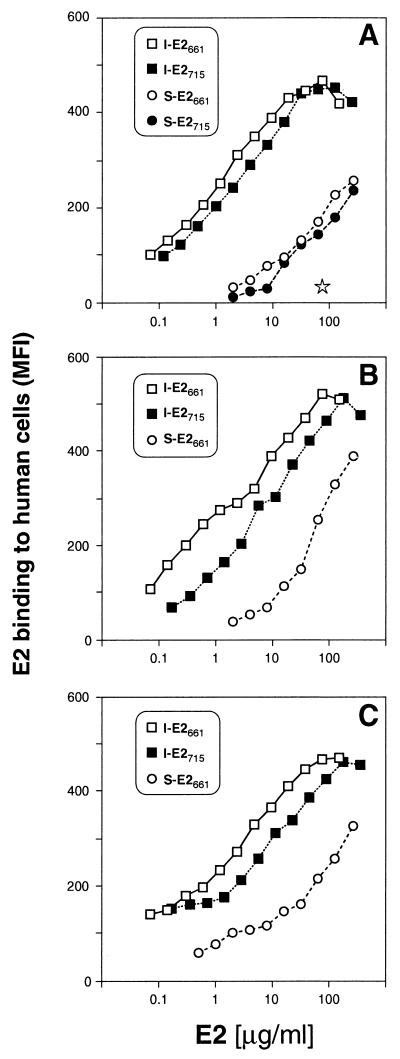
We then measured the binding of the different forms of recombinant E2715 to CD81 and also included recombinant E2661 in these studies. For both truncated forms, I-E2 binding to target cells was saturable and was detected at concentrations as low as 0.1 μg/ml. Moreover, addition of purified recombinant human CD81 LEL blocked this binding completely, demonstrating the specificity of the interaction (Fig. 2A). By contrast, S-E2 bound weakly to cells and did not reach saturation at the maximum concentration used. As previously described, cell-bound E2 was detected using anti-E2 antiserum raised in a chimpanzee immunized with intracellular E1-E2 heterodimer (46). We considered the possibility that the observed difference in binding was due to a decreased ability of this antiserum to recognize the complex glycosylated S-E2. We therefore repeated the experiments using antisera raised in guinea pigs against I-E2715 or S-E2715. Both antisera confirmed our finding that only the internal but not the secreted fractions of truncated E2 bind efficiently to CD81 (Fig. 2B and C).
FIG. 2.
Binding of various truncated E2 proteins to human cells. S-E2 (circles) and I-E2 (squares) truncated at position 661 (open symbols) or at position 715 (solid symbols) and expressed in CHO cells, were tested for their capacity to bind MOLT-4 cells. Cell-bound E2 is indicated as net MFI as detected by flow cytometry using chimpanzee anti-E1-E2 antiserum (A) or using antiserum raised in guinea pigs against I-E2715 (B) or S-E2715 (C). The star represents the effect of 50 μg soluble human CD81 LEL per ml on the binding of I-E2661.
Internal E2 monomers but not dimers or trimers bind efficiently to human cells.
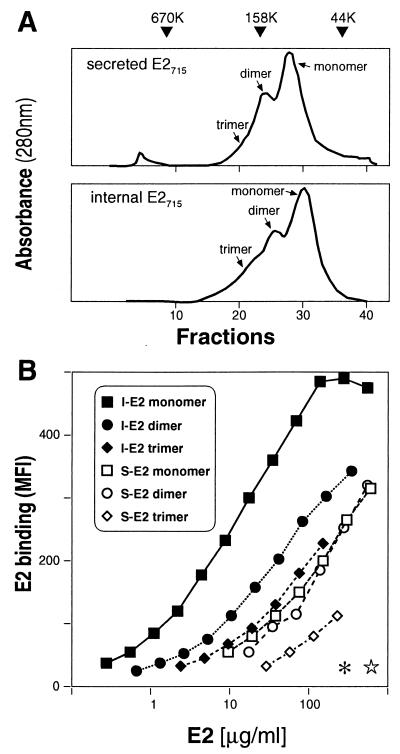
The recombinant E2 protein expressed in mammalian cells tends to aggregate, and these aggregates are stabilized by disulfide bonds. Such multimers of the HCV envelope are thought to be dead-end products (11, 34). We investigated whether the presence of oligomerized E2 protein in our preparations influences their ability to bind CD81. We separated forms of I-E2715 and S-E2715 with different molecular weights by gel filtration, through which we were able to purify monomers, dimers, and trimers of each. Both E2 forms display very similar gel filtration profiles, indicating that in both cases about 60% of the unfractionated material is monomeric (Fig. 3A). Only the monomeric fraction of I-E2715 bound efficiently to cellular targets, while the dimeric and trimeric fractions bound poorly. By contrast, neither monomers, dimers, nor trimers of S-E2715 bound CD81 efficiently. CD81 LEL blocks the binding of monomeric I-E2715 and S-E2715 completely, demonstrating the specificity of the interaction (Fig. 3B). Thus, aggregated recombinant I-E2715 fractions have an impaired capacity to present the conformational binding epitopes.
FIG. 3.
Monomers of I-E2715 bind human cells better than do dimers and trimers. Purified I-E2715 and S-E2715 were fractionated further by size exclusion chromatography into different molecular weight forms. (A) The elution profiles of I-E2715 and S-E2715 are shown. Individual fractions were monitored by SDS-PAGE under nonreducing conditions (data not shown). The monomeric, dimeric, and trimeric forms of E2 are indicated. The triangles indicate the elution time of size exclusion molecular weight standards. (B) Monomers (squares), dimers (circles), and trimers (diamonds) of I-E2715 (solid symbols) and S-E2715 (open symbols) were compared by their capacity to bind MOLT-4 cells. Cell-bound E2 was measured by flow cytometry using chimpanzee anti-E1-E2 antiserum and is indicated as net MFI. The ability of the chimpanzee serum to recognize monomers, dimers, and trimers of I-E2715 and S-E2715 was confirmed by staining Western blots of E2 SDS-PAGE gels under nonreducing conditions (data not shown). The effect of 50 μg of soluble human CD81 LEL per ml on the binding of monomeric I-E2715 (asterisk) and monomeric S-E2715 (star) is represented.
Sugar moieties are not necessary for E2 binding to human cells.
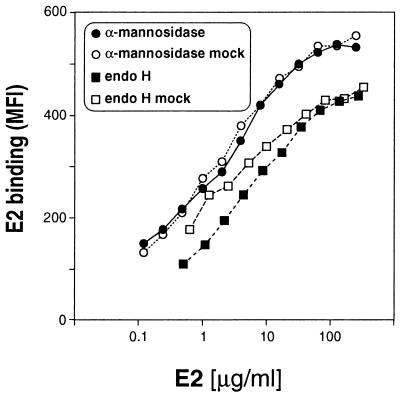
Because the E2 protein elicited NOB antibodies upon immunization and bound CD81 only when core glycosylated but not when complex glycosylated, we tested whether core glycosylation was required for these activities. To this end, we performed binding studies using deglycosylated E2. We digested I-E2715 with α-mannosidase or with endo H and analyzed the products for the presence of monosaccharides and for their binding activity. α-Mannosidase-digested E2 retains all GlcNAc residues compared to the undigested control, as well as, most probably, the β1→4 GlcNAc-linked and some α1→3 mannose-linked mannose residues, which are not efficiently cleaved by the enzyme (Table 1). Endo H digestion was complete, with approximately half of the GlcNAc residues cleaved off and only trace amounts of uncleaved mannose left. Importantly, the deglycosylated forms and the undigested controls were identical in their capacity to bind CD81 (Fig. 4). The enzymes alone have no binding activity (data not shown). Thus, core glycosylation is not necessary for E2 binding to CD81 and complex glycosylation is likely to mask the E2 binding epitope for its cellular receptor. This also may explain the inability of complex glycosylated S-E2715 to raise NOB antibodies after immunization. By treating S-E2 with peptide-N-glycosidase F (PNGase F), which removes complex sugars completely, we attempted to restore its binding activity. However, PNGase F-digested S-E2 formed high-molecular-weight aggregates, with the majority of the protein precipitating out of solution. The aggregates that remained in solution did not migrate into an SDS gel. This is presumably due to hydrophobic interactions following removal of all hydrophilic carbohydrates or due to conformational changes through the conversion of asparagine at the N-glycosylation site to aspartate. Therefore, it was not possible to compare completely deglycosylated S-E2 with untreated or endo H-treated I-E2 (data not shown).
TABLE 1.
Stoichiometry of the carbohydrate content of recombinant I-E2715a
| Expt | Treatment | Amt (mol/mol of E2) of:
|
|
|---|---|---|---|
| Mannose | Glucosamine | ||
| 1 | None | 51 | 13 |
| α-Mannosidase | 12 | 13 | |
| Mock treated | 61 | 17 | |
| 2 | None | 57 | 16 |
| Endo H | 3 | 6 | |
| Mock treated | 59 | 17 | |
Other monosaccharides, including glucose, galactose, galactosamine, xylose, and fucose, were not detected.
FIG. 4.
Deglycosylation of I-E2 does not influence its binding activity. I-E2715 was digested with α-mannosidase or with endo H. MOLT-4 binding activity of α-mannosidase-treated (solid circles) and endo H-treated (solid squares) E2 was compared to that of mock-treated E2 (open symbols). Cell-bound E2 was measured by flow cytometry using chimpanzee anti-E1-E2 antiserum and is indicated as net MFI.
Construction and in vitro analysis of ER-retained E2 DNA vaccines.
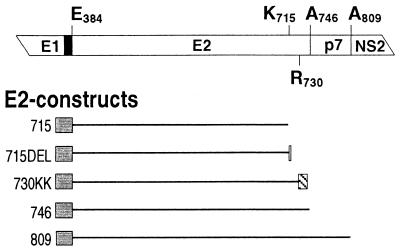
Given the potential benefits of DNA vaccines over recombinant proteins (12), we assessed the ability of different E2 forms to stimulate humoral immunity if delivered in the form of a DNA vaccine. Using the DNA vaccination vector pAC, we constructed five E2-encoding plasmids (Fig. 5). (i) pAC-E2715 corresponds to the construct used for E2715 expression in CHO cells in that it encodes the same C-terminally truncated E2, directed into the ER by a TPA-leader sequence fused to its N terminus (aa 384), and driven by the human cytomegalovirus promoter. It was the basis for the other constructs, which all have the same N terminus and were made by modifying the C terminus of E2715. (ii and iii) pAC-E2746 (ii) and pAC-E2809 (iii) encode two naturally occurring membrane-anchored E2 species differing in their C termini (aa 746 and 809, respectively). The larger product contains a small highly hydrophobic region from aa 747 to 809, known as p7. Both forms are retained in the ER (31, 36, 48). Because the cellular localization of purified E2 antigen is critical, we assumed that ER retention of E2 protein expressed by DNA vaccines would favor the induction of NOB antibodies. (iv and v) Based on the same rationale, we additionally constructed pAC-E2715DEL (iv) by adding to the C-terminal lysine (aa 715) the three amino acids DEL to obtain the canonical signal for ER retention of luminal proteins, the amino acid motif KDEL (38), and pAC-E2730KK (v) by fusing to the C terminus of the first transmembrane-spanning domain of E2 (aa 730) the cytoplasmic tail of the adenovirus E3/19K protein, which confers ER retention and retrieval to transmembrane proteins (40, 50). The latter was constructed because despite the convincing in vitro data indicating its retention in the ER, full-length E2 has been reported to be present on the plasma membrane of hepatocytes in transgenic mice (26).
FIG. 5.
Diagram of the E2 constructs used for DNA immunization. The relevant HCV proteins are shown at the top. The black box corresponds to the signal peptide at the C terminus of E1 directing E2 into the ER, and terminal amino acid positions in the constructs are indicated. HCV sequences present in each construct are indicated by black lines which are drawn to scale and oriented with respect to the HCV proteins shown at the top. Grey boxes represent an N-terminal 25-aa TPA-derived signal peptide directing E2 into the ER, the white box indicates three additional C-terminal amino acids leading to the motif KDEL, and the hatched box indicates the C-terminal cytoplasmic tail of the adenovirus E3/19K protein (SRRSFIDEKKMP).
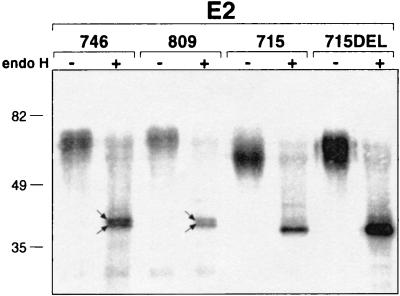
We next compared biochemical properties of E2 expressed in CHO cells stably transfected with our five DNA vaccination constructs. We were not able to detect an E2730KK protein, suggesting rapid degradation possibly due to incorrect folding. The other forms of E2 could be detected by Western blotting, and, as expected, all four are endo H sensitive, indicating an ER localization (Fig. 6). The proteins expressed from pAC-E2809 (E2-p7) and pAC-E2746 (E2) have the same apparent molecular weight, indicating that p7 is cleaved, consistent with previous reports (31, 36, 48). Endo H digestion of these two membrane-anchored E2 forms leads to the same sharp double band, suggesting inaccessibility for efficient cleavage of one core carbohydrate unit or the presence of an additional E2 form shorter than E2746. The two proteins encoded by pAC-E2715 and pAC-E2715DEL show a difference of about 5 kDa in their apparent molecular mass and one sharp band after endo H treatment. This suggests that the KDEL motif causes differences in carbohydrate trimming in the ER or when the protein is recycled between the ER and the cis Golgi.
FIG. 6.
E2 stably expressed from the different DNA immunization constructs in CHO cells is endo H sensitive. E2 was immunoprecipitated from cell lysates with chimpanzee anti-E1-E2 antiserum and treated with endo H (+) or left untreated (−). The samples were analyzed by SDS-PAGE (10% acrylamide) under reducing conditions and Western blotting. Arrows indicate the presence of two closely migrating bands. Sizes (in kilodaltons) of the molecular mass markers are indicated on the left.
Comparison of DNA and protein immunization in mice.
We analyzed the anti-E2 antibody response elicited in C57BL/6 mice after i.m. priming and boosting. As seen before (Fig. 1B), immunization with recombinant I-E2715 elicits high anti-E2 IgG titers and good NOB titers in mice (Table 2). By contrast, the antibody response generated by DNA immunization, using either of our constructs, is both quantitatively and qualitatively less efficacious. The two truncated E2 forms, E2715 and E2715DEL, elicit comparable antibody titers as measured by ELISA, but no NOB antibody titers were detected. The two membrane-anchored forms, E2746 and E2809, elicit even lower anti-E2 IgG titers. However, these two constructs did elicit low but detectable NOB titers in three out of eight mice. The anti-E2 IgG ELISA titers in individual mice in a group were all very similar (data not shown), suggesting that NOB titers do not directly correlate with total anti-E2 IgG. Intriguingly, pAC-E2730KK raises measurable anti-E2 antibody titers, although we were not able to detect the protein in transfected CHO cells.
TABLE 2.
Comparison of anti-E2 antibody responses elicited by protein or DNA immunization in mice
| Immunization | ELISA titera | NOB titerb |
|---|---|---|
| Protein | ||
| I-E2715 | 150,000 | 500 (8) |
| DNA | ||
| E2715 | 10,000 | 0 (0) |
| E2715DEL | 15,000 | 0 (0) |
| E2730KK | 3,000 | 0 (0) |
| E2746 | 6,000 | 60 (3) |
| E2809 | 7,000 | 40 (3) |
Assessed on pooled sera (eight mice per group) and expressed as the reciprocal values of serum dilutions.
Mean values of individual NOB-positive sera (the number of NOB-positive sera per group of eight mice is given in parentheses).
DISCUSSION
The experiments presented here were performed to determine the optimal form of the HCV E2 antigen to include in a vaccine from the perspective of the generation of antibodies. The quality of recombinant E2 protein was assessed by two means: (i) the capacity to bind its putative receptor CD81 on human target cells and (ii) the capacity to elicit antibodies, in particular those that inhibit receptor binding (NOB antibodies). We show that CHO cell-expressed truncated E2 protein, when derived from the core glycosylated intracellular fraction (I-E2) but not from the complex glycosylated secreted fraction (S-E2), binds with high efficiency to human cells and elicits NOB antibodies in guinea pigs. We also show that carbohydrate moieties are not necessary for I-E2 binding to cellular targets and that only the monomeric fraction binds efficiently compared to multimers. Moreover, comparing recombinant I-E2 protein to several E2-encoding DNA vaccines in mice, we found that for both quantity and quality of the antibody response, protein immunization is superior to DNA. Based on these results, we propose that, for the induction of antibody responses, the E2 antigen needed in a future HCV vaccine is a recombinant, monomeric protein purified from the intracellular fraction.
In chimpanzees vaccinated with the E1-E2 heterodimer, high NOB antibody titers correlate with protection from infection with an homologous HCV isolate (46). However, when vaccinated chimpanzees were challenged with a heterologous virus, infection occurred in all animals but 9 of the 10 vaccinees did not develop chronic infection (M. Houghton and S. Abrignani, unpublished data). Antisera from animals immunized with recombinant E1-E2 derived from the HCV-1 strain contained high titers of anti-E2 antibodies that were able to block CD81 binding of recombinant E2 derived not only from HCV subtype 1a but also from subtype 1b. By contrast, anti-E1 antibodies did not block the binding of E1-E2 to human cells. Together, immunization experiments in chimpanzees indicate that a vaccine based on HCV envelope proteins is able to prevent at least chronic HCV infection and that in the E1-E2 heterodimer, it is E2 that can generate antibodies capable of blocking virus binding to target cells. Thus, although sterilizing immunity was not achieved following challenge with heterologous virus, the prevention of chronic infection still holds promise, given that acute HCV infections are often asymptomatic and clinically inconsequential.
In an effort to produce recombinant HCV E2 in CHO cells, we originally focused on S-E2715, whose purification is easier to scale up than is that of the intracellular fraction. Although we found that a supernatant fraction of E2 obtained by affinity chromatography binds human cells (46), we realized that our culture supernatant contained only a minor fraction of biologically active (in our binding assay) E2 protein. In an attempt to define the optimal forms of E2 for binding CD81, we assessed intracellular or secreted fractions of different truncated E2 proteins expressed in CHO cells. We found that both I-E2661 and I-E2715 bind substantially better to CD81 than do S-E2661 and S-E2715. It was found previously that S-E2661 binds well to CD81 (18). However, the same authors recently reviewed this finding and reported that I-E2661 binds CD81 with higher efficiency than does S-E2661 (19). Our results not only confirm this finding but also give a functional correlate to the superior binding of I-E2 to CD81. We demonstrate, using S-E2715 or I-E2715 in immunization studies, that both fractions elicit high anti-E2 antibody titers as assessed by ELISA whereas only the I-E2 fraction raises NOB antibody titers. The finding that deglycosylated I-E2715 binds CD81 on human cells as well as untreated I-E2715 suggests that carbohydrates are not directly involved in the recognition of CD81. They could, however, play a role in the correct folding of E2 in the ER.
Given that robust virus cell entry assays and infection assays are not available, we do not know whether CD81 mediates HCV entry into cells. However, we have evidence that (i) CD81 is very inefficient in mediating internalization of bound ligands (42) and (ii) some hepatoma cell lines can bind recombinant envelope proteins in the absence of CD81 (data not shown), suggesting that human cells contain other molecules which interact with HCV and may mediate entry. Recently, the low-density lipoprotein receptor has been proposed as a possible HCV receptor (37) or, more generally, as a molecule exploited by members of the family Flaviviridae to enter human cells (1). However, until reliable infection assays are available, it will always be difficult to sort out the relative importance of candidate HCV receptors.
Little is known about the assembly and maturation of HCV. However, studies with recombinant proteins indicate that noncovalent heterodimers of E1 and E2 reside in the ER and are core glycosylated. They have been proposed as the prebudding form of the HCV envelope glycoprotein complex (11, 14). When truncated forms of E2 pass through the Golgi and are secreted, they become complex glycosylated. Our results indicate that these modifications almost completely abolish the ability of E2 to bind to CD81 on human cells and to elicit NOB antibodies. Our view is that these complex sugars mask the binding site, most probably through steric or charge interference. Based on the results reported here, I-E2 probably mimics the native envelope form present in the virus, and thus the viral envelope should not be complex glycosylated. Consistent with this view, HCV could assemble at the ER membrane, bud into the ER, and be released into the extracellular environment by host cell lysis, similar to rotaviruses (16). By this mechanism, complex carbohydrate modifications are prevented because viral particles do not pass through the Golgi. Alternatively, it may be that HCV exits through the Golgi in a form which protects at least the carbohydrates in and around the CD81 binding site from further modifications.
For the development of a vaccine against intracellular pathogens (e.g., viruses), it is advantageous to optimize antibody, T-helper cell, and CTL responses, all of which have been achieved by DNA vaccination of experimental animals (12). However, here we show that DNA vaccination elicits low ELISA titers of anti-E2 antibodies and low NOB titers compared to immunization with E2 protein. These data parallel results obtained by others with mice or small primates by using truncated E2 constructs for DNA immunization (20, 21, 39). Although NOB titers do not necessarily correlate with total anti-E2 antibody titers in our experience with human sera (46), we cannot rule out that the low efficiency of NOB antibody production following DNA immunization is a direct consequence of the inability of the same DNA constructs to induce high anti-E2 antibody titers in general. DNA vaccines encoding intracellular proteins elicit poor antibody responses in comparison to secreted proteins (3). E2-encoding DNA vaccines therefore might be limited by their inherent inaccessibility to antibodies. Another possible explanation of the lack of NOB antibody responses is that the uptake of DNA and the expression of E2 in vivo alter the glycosylation pattern, localization, and/or conformation of the E2 protein.
In conclusion, to elicit antibodies aimed at blocking HCV binding to CD81 on human cells, protein immunization is superior to DNA immunization. Cellular responses could potentially be achieved by administering DNA in combination with recombinant protein, and investigating the influence of one to another will be an important step in defining optimal vaccination strategies for HCV.
ACKNOWLEDGMENTS
Jens M. Heile and Yiu-Lian Fong contributed equally to this work.
We thank Nicholas Valiante for critical reading of the manuscript and insightful discussions, and we thank Giorgio Corsi for artwork.
J.M.H. was supported by EU grant PL 960505.
REFERENCES
- 1.Agnello V, Ábel G, Elfahal M, Knight G B, Zhang Q-X. Hepatitis C virus and other Flaviviridae viruses enter cells via low density lipoprotein receptor. Proc Natl Acad Sci USA. 1999;96:12766–12771. doi: 10.1073/pnas.96.22.12766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alberti A, Chemello L, Benvegnù L. Natural history of hepatitis C. J Hepatol. 1999;31(Suppl. 1):17–24. doi: 10.1016/s0168-8278(99)80369-9. [DOI] [PubMed] [Google Scholar]
- 3.Boyle J S, Koniaras C, Lew A M. Influence of cellular location of expressed antigen on the efficacy of DNA vaccination: cytotoxic T lymphocyte and antibody responses are suboptimal when antigen is cytoplasmic after intramuscular DNA immunization. Int Immunol. 1997;9:1897–1906. doi: 10.1093/intimm/9.12.1897. [DOI] [PubMed] [Google Scholar]
- 4.Choo Q-L, Kuo G, Weiner A J, Overby L R, Bradley D W, Houghton M. Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science. 1989;244:359–362. doi: 10.1126/science.2523562. [DOI] [PubMed] [Google Scholar]
- 5.Choo Q-L, Richman K H, Han J H, Berger K, Lee C, Dong C, Gallegos C, Coit D, Medina-Selby A, Barr P J, Weiner A J, Bradley D W, Kuo G, Houghton M. Genetic organisation and diversity of the hepatitis C virus. Proc Natl Acad Sci USA. 1991;88:2451–2455. doi: 10.1073/pnas.88.6.2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choo Q-L, Kuo G, Ralston R, Weiner A, Chien D, Van Nest G, Han J, Berger K, Thudium K, Kuo C, Kansopon J, McFarland J, Tabrizi A, Ching K, Moss B, Cummins L B, Houghton M, Muchmore E. Vaccination of chimpanzees against infection by the hepatitis C virus. Proc Natl Acad Sci USA. 1994;91:1294–1298. doi: 10.1073/pnas.91.4.1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cocquerel L, Meunier J-C, Pillez A, Wychowski C, Dubuisson J. A retention signal necessary and sufficient for endoplasmic reticulum localization maps to the transmembrane domain of hepatitis C virus glycoprotein E2. J Virol. 1998;72:2183–2191. doi: 10.1128/jvi.72.3.2183-2191.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cocquerel L, Duvet S, Meunier J-C, Pillez A, Cacan R, Wychowski C, Dubuisson J. The transmembrane domain of hepatitis C virus glycoprotein E1 is a signal for static retention in the endoplasmic reticulum. J Virol. 1999;73:2641–2649. doi: 10.1128/jvi.73.4.2641-2649.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Couch R B, Kasel J A. Immunity to influenza in man. Annu Rev Microbiol. 1983;37:529–549. doi: 10.1146/annurev.mi.37.100183.002525. [DOI] [PubMed] [Google Scholar]
- 10.Davis H L, Michel M-L, Whalen R G. DNA-based immunization induces continuous secretion of hepatitis B surface antigen and high levels of circulating antibody. Hum Mol Genet. 1993;2:1847–1851. doi: 10.1093/hmg/2.11.1847. [DOI] [PubMed] [Google Scholar]
- 11.Deleersnyder V, Pillez A, Wychowski C, Blight K, Xu J, Hahn Y S, Rice C M, Dubuisson J. Formation of native hepatitis C virus glycoprotein complexes. J Virol. 1997;71:697–704. doi: 10.1128/jvi.71.1.697-704.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Donnelly J J, Ulmer J B, Shiver J W, Liu M A. DNA vaccines. Annu Rev Immunol. 1997;15:617–648. doi: 10.1146/annurev.immunol.15.1.617. [DOI] [PubMed] [Google Scholar]
- 13.Dubuisson J, Hsu H H, Cheung R C, Greenberg H B, Russell D G, Rice C M. Formation and intracellular localization of hepatitis C virus envelope glycoprotein complexes expressed by recombinant vaccinia and Sindbis viruses. J Virol. 1994;68:6147–6160. doi: 10.1128/jvi.68.10.6147-6160.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dubuisson J, Rice C M. Hepatitis C virus glycoprotein folding: disulfide bond formation and association with calnexin. J Virol. 1996;70:778–786. doi: 10.1128/jvi.70.2.778-786.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duvet S, Cocquerel L, Pillez A, Cacan R, Verbert A, Moradpour D, Wychowski C, Dubuisson J. Hepatitis C virus glycoprotein complex localization in the endoplasmic reticulum involves a determinant for retention and not retrieval. J Biol Chem. 1998;273:32088–32095. doi: 10.1074/jbc.273.48.32088. [DOI] [PubMed] [Google Scholar]
- 16.Estes M K. Rotaviruses and their replication. In: Fields B N, Knipe D M, Howley P M, Chanock R M, Melnick J L, Monath T P, Roizman B, Strauss S E, editors. Fields virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 1625–1655. [Google Scholar]
- 17.Feray C, Gigou M, Samuel D, Ducot B, Maisonneuve P, Reynes M, Bismuth A, Bismuth H. Incidence of hepatitis C in patients receiving different preparations of hepatitis B immunoglobulins after liver transplantation. Ann Intern Med. 1998;128:810–816. doi: 10.7326/0003-4819-128-10-199805150-00003. [DOI] [PubMed] [Google Scholar]
- 18.Flint M, Maidens C, Loomis-Price L D, Shotton C, Dubuisson J, Monk P, Higginbottom A, Levy S, McKeating J A. Characterization of hepatitis C virus E2 glycoprotein interaction with a putative cellular receptor, CD81. J Virol. 1999;73:6235–6244. doi: 10.1128/jvi.73.8.6235-6244.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flint M, Dubuisson J, Maidens C, Harrop R, Guile G R, Borrow P, McKeating J A. Functional characterization of intracellular and secreted forms of a truncated hepatitis C virus E2 glycoprotein. J Virol. 2000;74:702–709. doi: 10.1128/jvi.74.2.702-709.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Forns X, Emerson S U, Tobin G J, Mushahwar I K, Purcell R H, Bukh J. DNA immunization of mice and macaques with plasmids encoding hepatitis C virus envelope E2 protein expressed intracellularly and on the cell surface. Vaccine. 1999;17:1992–2002. doi: 10.1016/s0264-410x(98)00448-4. [DOI] [PubMed] [Google Scholar]
- 21.Fournillier A, Depla E, Karayiannis P, Vidalin O, Maertens G, Trépo C, Inchauspé G. Expression of noncovalent hepatitis C virus envelope E1-E2 complexes is not required for the induction of antibodies with neutralizing properties following DNA immunization. J Virol. 1999;73:7497–7504. doi: 10.1128/jvi.73.9.7497-7504.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grakoui A, Wychowski C, Lin C, Feinstone S M, Rice C M. Expression and identification of hepatitis C virus polyprotein cleavage products. J Virol. 1993;67:1385–1395. doi: 10.1128/jvi.67.3.1385-1395.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hijikata M, Kato N, Ootsuyama Y, Nakagawa M, Shimotohno K. Gene mapping of the putative structural region of the hepatitis C virus genome by in vitro processing analysis. Proc Natl Acad Sci USA. 1991;88:5547–5551. doi: 10.1073/pnas.88.13.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Houghton M. Hepatitis C viruses. In: Fields B N, Knipe D M, Howley P M, Chanock R M, Melnick J L, Monath T P, Roizman B, Strauss S E, editors. Fields virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 1035–1058. [Google Scholar]
- 25.Ishii K, Rosa D, Watanabe Y, Katayama T, Harada H, Wyatt C, Kiyosawa K, Aizaki H, Matsuura Y, Houghton M, Abrignani S, Miyamura T. High titers of antibodies inhibiting the binding of envelope to human cells correlate with natural resolution of chronic hepatitis C. Hepatology. 1998;28:1117–1120. doi: 10.1002/hep.510280429. [DOI] [PubMed] [Google Scholar]
- 26.Kawamura T, Furusaka A, Koziel M J, Chung R T, Wang T C, Schmidt E V, Liang T J. Transgenic expression of hepatitis C virus structural proteins in the mouse. Hepatology. 1997;25:1014–1021. doi: 10.1002/hep.510250437. [DOI] [PubMed] [Google Scholar]
- 27.Knodell R G, Conrad M E, Ishak K G. Development of chronic liver disease after acute non-A, non-B post-transfusion hepatitis. Role of gamma-globulinprophylaxis in its prevention. Gastroenterology. 1977;72:902–909. [PubMed] [Google Scholar]
- 28.Krugman S, Overby L R, Mushahwar I K, Ling C-M, Frösner G G, Deinhardt F. Viral hepatitis, type B. Studies on natural history and prevention re-examined. N Engl J Med. 1979;300:101–106. doi: 10.1056/NEJM197901183000301. [DOI] [PubMed] [Google Scholar]
- 29.Letvin N L, Montefiori D C, Yasutomi Y, Perry H C, Davies M-E, Lekutis C, Alroy M, Freed D C, Lord C I, Handt L K, Liu M A, Shiver J W. Potent, protective anti-HIV immune responses generated by bimodal HIV envelope DNA plus protein vaccination. Proc Natl Acad Sci USA. 1997;94:9378–9383. doi: 10.1073/pnas.94.17.9378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Levy S, Todd S C, Maecker H T. CD81 (TAPA-1): a molecule involved in signal transduction and cell adhesion in the immune system. Annu Rev Immunol. 1998;16:89–109. doi: 10.1146/annurev.immunol.16.1.89. [DOI] [PubMed] [Google Scholar]
- 31.Lin C, Lindenbach B D, Prágai B M, McCourt D W, Rice C M. Processing in the hepatitis C virus E2-NS2 region: identification of p7 and two distinct E2-specific products with different C termini. J Virol. 1994;68:5063–5073. doi: 10.1128/jvi.68.8.5063-5073.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matsuura Y, Suzuki T, Suzuki R, Sato M, Aizaki H, Saito I, Miyamura T. Processing of E1 and E2 glycoproteins of hepatitis C virus expressed in mammalian and insect cells. Virology. 1994;205:141–150. doi: 10.1006/viro.1994.1629. [DOI] [PubMed] [Google Scholar]
- 33.McHutchison J G, Gordon S C, Schiff E R, Shiffman M L, Lee W M, Rustgi V K, Goodman Z D, Ling M H, Cort S, Albrecht J K. Interferon alfa-2b alone or in combination with ribavirin as initial treatment for chronic hepatitis C. N Engl J Med. 1998;339:1485–1492. doi: 10.1056/NEJM199811193392101. [DOI] [PubMed] [Google Scholar]
- 34.Michalak J-P, Wychowski C, Choukhi A, Meunier J-C, Ung S, Rice C M, Dubuisson J. Characterization of truncated forms of hepatitis C virus glycoproteins. J Gen Virol. 1997;78:2299–2306. doi: 10.1099/0022-1317-78-9-2299. [DOI] [PubMed] [Google Scholar]
- 35.Miyamura T, Matsuura Y. Structural proteins of hepatitis C virus. Trends Microbiol. 1993;1:229–231. doi: 10.1016/0966-842x(93)90137-g. [DOI] [PubMed] [Google Scholar]
- 36.Mizushima H, Hijikata M, Asabe S-I, Hirota M, Kimura K, Shimotohno K. Two hepatitis C virus glycoprotein E2 products with different C termini. J Virol. 1994;68:6215–6222. doi: 10.1128/jvi.68.10.6215-6222.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Monazahian M, Böhme I, Bonk S, Koch A, Scholz C, Grethe S, Thomssen R. Low density lipoprotein receptor as a candidate receptor for hepatitis C virus. J Med Virol. 1999;57:223–229. doi: 10.1002/(sici)1096-9071(199903)57:3<223::aid-jmv2>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 38.Munro S, Pelham H R B. A C-terminal signal prevents secretion of luminal ER proteins. Cell. 1987;48:899–907. doi: 10.1016/0092-8674(87)90086-9. [DOI] [PubMed] [Google Scholar]
- 39.Nakano I, Maertens G, Major M E, Vitvitski L, Dubuisson J, Fournillier A, De Martynoff G, Trépo C, Inchauspé G. Immunization with plasmid DNA encoding hepatitis C virus envelope E2 antigenic domains induces antibodies whose immune reactivity is linked to the injection mode. J Virol. 1997;71:7101–7109. doi: 10.1128/jvi.71.9.7101-7109.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nilsson T, Jackson M, Peterson P A. Short cytoplasmic sequences serve as retention signals for transmembrane proteins in the endoplasmic reticulum. Cell. 1989;58:707–718. doi: 10.1016/0092-8674(89)90105-0. [DOI] [PubMed] [Google Scholar]
- 41.Pardoll D M, Beckerleg A M. Exposing the immunology of naked DNA vaccines. Immunity. 1995;3:165–169. doi: 10.1016/1074-7613(95)90085-3. [DOI] [PubMed] [Google Scholar]
- 42.Petracca R, Falugi F, Galli G, Norais N, Rosa D, Campagnoli S, Burgio V, Di Stasio E, Giardina B, Houghton M, Abrignani S, Grandi G. Structure-function analysis of hepatitis C virus envelope-CD81 binding. J Virol. 2000;74:4824–4830. doi: 10.1128/jvi.74.10.4824-4830.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Piazza M, Sagliocca L, Tosone G, Guadagnino V, Stazi M A, Orlando R, Borgia G, Rosa D, Abrignani S, Palumbo F, Manzin A, Clementi M. Sexual transmission of the hepatitis C virus and efficacy of prophylaxis with intramuscular immune serum globulin. A randomized controlled trial. Arch Intern Med. 1997;157:1537–1544. [PubMed] [Google Scholar]
- 44.Pileri P, Uematsu Y, Campagnoli S, Galli G, Falugi F, Petracca R, Weiner A J, Houghton M, Rosa D, Grandi G, Abrignani S. Binding of hepatitis C virus to CD81. Science. 1998;282:938–941. doi: 10.1126/science.282.5390.938. [DOI] [PubMed] [Google Scholar]
- 45.Ralston R, Thudium K, Berger K, Kuo C, Gervase B, Hall J, Selby M, Kuo G, Houghton M, Choo Q-L. Characterization of hepatitis C virus envelope glycoprotein complexes expressed by recombinant vaccinia viruses. J Virol. 1993;67:6753–6761. doi: 10.1128/jvi.67.11.6753-6761.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rosa D, Campagnoli S, Moretto C, Guenzi E H, Cousens L, Chin M, Dong C, Weiner A J, Lau J Y N, Choo Q-L, Chien D, Pileri P, Houghton M, Abrignani S. A quantitative test to estimate neutralizing antibodies to the hepatitis C virus: cytofluorimetric assessment of envelope glycoprotein 2 binding to target cells. Proc Natl Acad Sci USA. 1996;93:1759–1763. doi: 10.1073/pnas.93.5.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Selby M J, Choo Q-L, Berger K, Kuo G, Glazer E, Eckart M, Lee C, Chien D, Kuo C, Houghton M. Expression, identification and subcellular localization of the proteins encoded by the hepatitis C viral genome. J Gen Virol. 1993;74:1103–1113. doi: 10.1099/0022-1317-74-6-1103. [DOI] [PubMed] [Google Scholar]
- 48.Selby M J, Glazer E, Masiarz F, Houghton M. Complex processing and protein:protein interactions in the E2:NS2 region of HCV. Virology. 1994;204:114–122. doi: 10.1006/viro.1994.1515. [DOI] [PubMed] [Google Scholar]
- 49.Spaete R R, D'Anna A, Rugroden M E, Choo Q-L, Berger K, Crawford K, Kuo C, Leng S, Lee C, Ralston R, Thudium K, Tung J W, Kuo G, Houghton M. Characterization of the hepatitis C virus E2/NS1 gene product expressed in mammalian cells. Virology. 1992;188:819–830. doi: 10.1016/0042-6822(92)90537-y. [DOI] [PubMed] [Google Scholar]
- 50.Teasdale R D, Jackson M R. Signal-mediated sorting of membrane proteins between the endoplasmic reticulum and the Golgi apparatus. Annu Rev Cell Dev Biol. 1996;12:27–54. doi: 10.1146/annurev.cellbio.12.1.27. [DOI] [PubMed] [Google Scholar]
- 51.Ulmer J B, Donnelly J J, Parker S E, Rhodes G H, Felgner P L, Dwarki V J, Gromkowski S H, Deck R R, DeWitt C M, Friedman A, Hawe L A, Leander K R, Martinez D, Perry H C, Shiver J W, Montgomery D L, Liu M A. Heterologous protection against influenza by injection of DNA encoding a viral protein. Science. 1993;259:1745–1749. doi: 10.1126/science.8456302. [DOI] [PubMed] [Google Scholar]
- 52.World Health Organization. Hepatitis C. Weekly Epidemiol Rec. 1997;72:65–69. [Google Scholar]