Abstract
Bronchiectasis is marked by bronchial dilatation, recurrent infections and significant morbidity, underpinned by a complex interplay between microbial dysbiosis and immune dysregulation. The identification of distinct endophenotypes have refined our understanding of its pathogenesis, including its heterogeneous disease mechanisms that influence treatment and prognosis responses. Next-generation sequencing (NGS) has revolutionised the way we view airway microbiology, allowing insights into the “unculturable”. Understanding the bronchiectasis microbiome through targeted amplicon sequencing and/or shotgun metagenomics has provided key information on the interplay of the microbiome and host immunity, a central feature of disease progression. The rapid increase in translational and clinical studies in bronchiectasis now provides scope for the application of precision medicine and a better understanding of the efficacy of interventions aimed at restoring microbial balance and/or modulating immune responses. Holistic integration of these insights is driving an evolving paradigm shift in our understanding of bronchiectasis, which includes the critical role of the microbiome and its unique interplay with clinical, inflammatory, immunological and metabolic factors. Here, we review the current state of infection and the microbiome in bronchiectasis and provide views on the future directions in this field.
Shareable abstract
Exploring the nexus of infection and microbiome dynamics offers new avenues in understanding and treating bronchiectasis. https://bit.ly/3UtqoUl
Introduction
Bronchiectasis, characterised by irreversible and progressive bronchial dilatation, presents complex challenges due to chronic and recurrent infection, inflammation and structural damage [1]. This poses significant challenges in its management, primarily due to its complex aetiology involving microbial infection, immune dysfunction and inflammation [2]. Acknowledging disease heterogeneity as a key feature in the lack of available evidence-based therapeutics is now being increasingly addressed by advanced multiomics technologies that provide a deeper understanding of disease pathogenesis, including the role of infection and the microbiome. Here, we review the current state of the literature in relation to infection and the microbiome in bronchiectasis. We based this review on relevant PubMed/Medline articles identified using the primary search terms “infection” and “microbiome” together with “bronchiectasis” (search query: “bronchiectasis AND (infection OR microbiome)”). This synthesis underscores the urgent need for more effective, timely and targeted interventions in this field.
Infections in bronchiectasis
Infection and its related inflammation remain key pathophysiological and clinical aspects in bronchiectasis and play a crucial role in the primary development and secondary progressive process in this disease state (figure 1) [3]. The ability to distinguish between acute events, pulmonary exacerbations and chronic conditions, associated with persistent microbial colonisation, is crucial in understanding the role infections play in bronchiectasis. An acute deterioration in respiratory symptoms, termed an “exacerbation”, often indicates an infectious episode, although it is important to note that changes in the lung microbiome are not always evident during such episodes [4, 5]. While the term “colonisation” traditionally suggests a benign presence of micro-organisms, in the context of bronchiectasis, it can be associated with negative outcomes [6, 7]. Exacerbations are characterised by an acute worsening of respiratory symptoms, which may not always coincide with the detection of pathogens in cultures, underscoring the complex interactions between host immunity and microbial presence in the lungs [8–14]. Thus, defining exacerbations remains challenging because of their inherent heterogeneous nature characterised by a fluctuating course of symptoms, coupled with varied patient perceptions of severity. Consensus definitions have been established for use in clinical practice and trials [4, 15, 16]. The underlying pathophysiological mechanisms leading to an exacerbation are not fully understood; however, in recent years, increasing evidence suggests that inflammatory processes (neutrophilic and/or eosinophilic inflammation) and environmental factors play key roles [17–20]. Long-held assumptions presume that all exacerbations in bronchiectasis are infective (i.e. new pathogen, increased bacterial load from an existing pathogen or altered bacterial virulence) and hence are primarily treated with antibiotics [15, 21]. However, such views are evolving and have been questioned in the light of more recent studies, as described below.
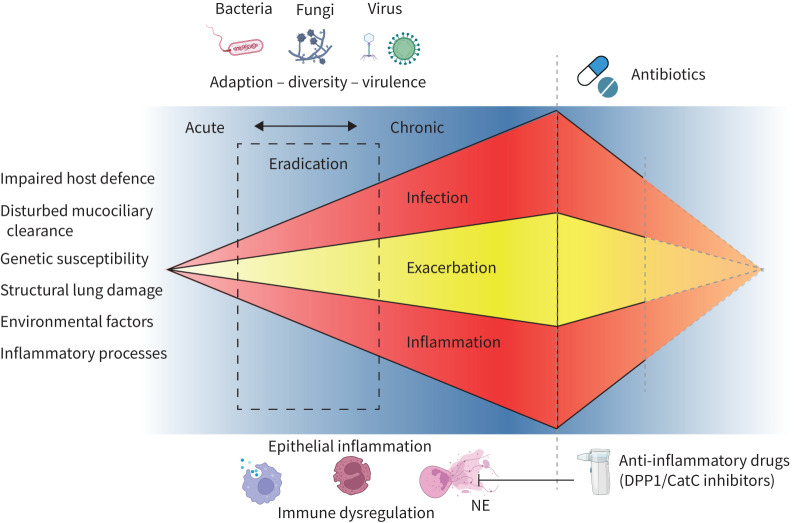
FIGURE 1.
Overview of bronchiectasis pathogenesis. A complex interaction between infection and inflammation results in a self-perpetuating cycle initially triggered by various conditions (indicated on the left). This cycle is primarily driven by bacterial infections, with growing evidence of significant contributions from viruses and fungi. The progression from acute to chronic infection hinges on factors such as pathogen virulence, adaptability and the selective pressures within the host environment. Identifying the exact stage of infection (acute or chronic) is crucial, as it influences the effectiveness of eradication efforts (broken rectangle). Central to this process is an excessive (usually neutrophilic) inflammatory response that leads to further tissue damage and impairs mucociliary clearance. This infection-driven inflammation can increase the likelihood of acute exacerbations. Therapeutic strategies aim to mitigate both infection and inflammation. This is achieved through conventional antibiotics and newer pharmacological interventions targeting neutrophilic inflammation, such as dipeptidyl peptidase 1 (DPP1)/cathepsin C (CatC) inhibitors. Despite these efforts, the structural lung damage and the conditions present at the onset of infection and inflammation preclude a reversal to the pre-disease state. Figure created with BioRender.com.
Bacterial infection in bronchiectasis
Bacterial infections remain key drivers of exacerbations, with relevant but less understood contributions from viral or fungal infection [7, 22–25]. Several studies have demonstrated a strong association between bacterial infection, increased bacterial sputum load and the heightened risk of exacerbation, while antibiotic therapy has been shown to effectively reduce inflammation, thereby mitigating the severity of exacerbations and improving patient outcomes [6, 7, 17, 18, 26, 27]. Bacteria commonly associated with exacerbations in bronchiectasis include Pseudomonas (P.) aeruginosa, Haemophilus (H.) influenzae, Staphylococcus (Sta.) aureus and, less commonly, Stenotrophomonas maltophilia (figure 2a) [28–32]. Additionally, the role of nontuberculous mycobacteria (NTM) in bronchiectasis is increasingly recognised, indicating a potential sub-phenotype of the disease that warrants further study and understanding [1, 33].
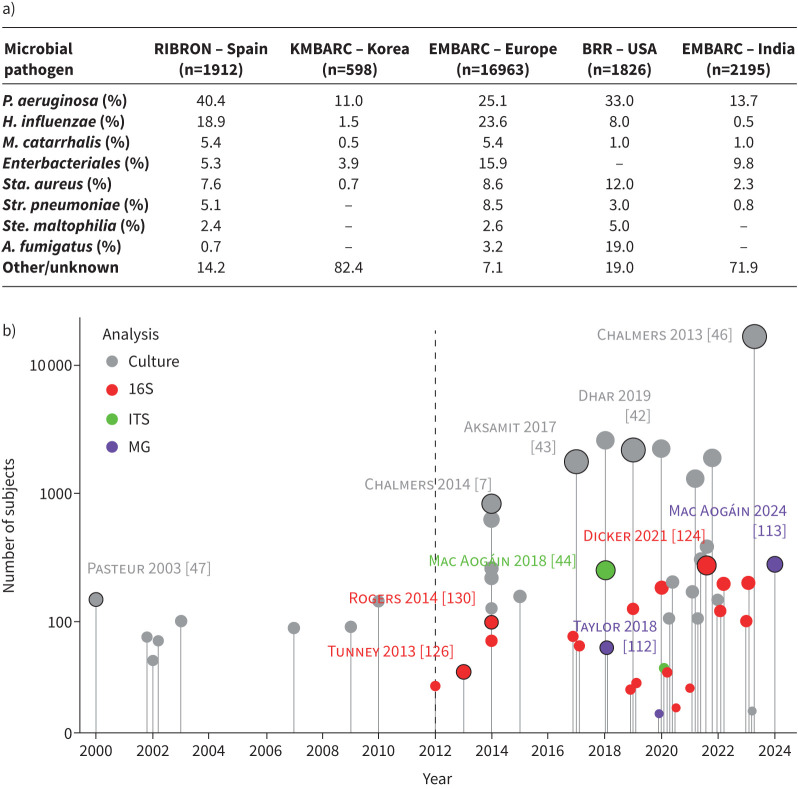
FIGURE 2.
Increasing scale of culture-based and airway microbiome studies in bronchiectasis. a) Table illustrating prevalence of key bacterial taxa from several major bronchiectasis registries. b) Visual timeline of microbiological and microbiome research outputs in bronchiectasis (2000–2024) comparing culture-based and sequencing-based (microbiome) studies. Each point on the chart indicates an individual study with the size of the circle and the length of the associated vertical lines reflecting study size. Points on the chart are colour-coded by the type of analysis conducted (grey: culture based; red: bacterial 16S rRNA analysis; green: fungal internal transcribed spacer (ITS) analysis; purple: metagenomics (MG)). Selected studies specifically discussed in the review are indicated by black borders surrounding dots and have accompanying citations. A broken line demarcates the beginning of the “microbiome era” in bronchiectasis. The complete list of studies illustrated in the figure is detailed in supplementary appendix 1. A.: Aspergillus; H.: Haemophilus; P.: Pseudomonas; Sta.: Staphylococcus; Ste.: Stenotrophomonas; Str: Streptococcus.
Viral infection in bronchiectasis
Several studies now contribute to a growing evidence base supporting viral infection as an important trigger of bronchiectasis exacerbations. Two studies have demonstrated that viruses are more frequently detected during exacerbations (20.9–49.0%) than in the stable state (11.4–18.9%) [23, 24], with the following viruses detected at highest frequency: coronavirus, rhinovirus, influenza A/B, respiratory syncytial virus B and human metapneumovirus [23, 24, 34]. Two studies from Australia and Europe investigated viruses in stable bronchiectasis and while the Australian study (n=27) showed a higher frequency of viruses in sputum during winter versus summer months (92% and 33%, respectively), the European study (n=219) did not detect any seasonal differences (13.5% in winter versus 12.3% in summer) [35, 36]. Furthermore, there appears to be links between viral upper respiratory tract infections (URTIs) and bronchiectasis exacerbations, as viral detection rates in sputum in patients experiencing exacerbations following URTIs remain higher compared to individuals either without URTI or subsequent exacerbation [37]. Some studies have proposed that detection of the Epstein–Barr virus in bronchiectasis confers a shorter time to exacerbation; however, this relationship requires validation [38]. During the coronavirus disease 2019 pandemic, studies reported a decrease in bronchiectasis exacerbations, most likely attributed to enhanced social hygiene and shielding through social distancing, resulting in reduced viral transmission [25, 39, 40]. Taken together, it appears that viruses play a relevant role in bronchiectasis exacerbations; however, their role in stable disease remains to be clarified.
Fungal infection in bronchiectasis
The role of fungi in the development of acute infection in bronchiectasis remains unclear. Other than Candida albicans, Aspergillus (A.) fumigatus represents the most common isolated fungi with significant pathogenic potential [41]. The prevalence rates of A. fumigatus in bronchiectasis are uncertain, with a European registry study illustrating it in 3% (279 out of 9226) of stable samples and 3.2% (393 out of 12152) of all stable and exacerbation samples [42]. Among patients within the US Bronchiectasis Registry, 60% (1087/1826) had at least one fungal culture, with A. fumigatus detected in 16% of those without NTM [43]. Current molecular and/or serological approaches illustrate that fungal detection and/or sensitisation may be seen in stable bronchiectasis. A. fumigatus and A. terreus are associated with more severe disease, with the latter associated with exacerbations [44]. The CAMEB (Cohort of Asian and Matched European Bronchiectasis) study, including patients from Asia and Europe, and excluding clinical allergic bronchopulmonary aspergillosis (ABPA) demonstrated serological ABPA in 18% (n=224) [45]. The most common fungal-associated aetiology in bronchiectasis is ABPA [42, 46, 47], which itself may lead to acute deterioration in individual patients. While emerging evidence suggests that fungi influence bronchiectasis, our understanding of their specific role in acute infection and on disease course remains insufficient.
Chronic infection in bronchiectasis
Chronic (bacterial) infection adversely impacts the progression of bronchiectasis, leading to a higher risk of exacerbations, hospitalisations, increased mortality, poorer lung function and reduced quality of life [6, 7, 22, 29, 45, 48]. Between research studies and clinical guidelines, the term “chronic infection” has not been uniformly defined [5, 49, 50]. An international expert group recently proposed that it be defined as “two positive cultures of the same microorganism, at least three months apart, in the context of clinically relevant bronchiectasis” [7]. Additionally, the same authors recommend avoiding the term “chronic colonisation” to allow clear emphasis on the negative impact of chronic bacterial infection on disease course [7]. Culture-based methods have commonly identified P. aeruginosa, H. influenzae, Enterobacteriales, Sta. aureus, Moraxella catarrhalis and Streptococcus pneumoniae as most abundant in bronchiectasis [43, 46, 48]. Chronic infections occur in 45–72% of cases, with P. aeruginosa and H. influenzae being most frequent (figure 2a) [6, 7, 22, 48].
Chronic infection in bronchiectasis involves complex immune dysregulation (figure 1), primarily characterised by neutrophil dominance, but an increasing recognition of a role for Th2-dominant (eosinophilic) inflammation [20]. It is important to consider the bi-directionality of this association; while inflammation may result from persistent microbial presence, it could equally contribute to an inability to effectively clear infections. This cyclical relationship complicates the determination of initial causality in chronic airway conditions, where both microbial colonisation and immune dysregulation represent parallel pathways of disease progression. A recent study demonstrated that different patient clusters may be defined based on airway and systemic inflammation with those exhibiting more pronounced neutrophilic inflammation demonstrating an increased risk for exacerbations and altered microbiomes [51]. Neutrophilic inflammation further correlates with airway bacterial load [17, 52], which triggers further recruitment of neutrophils to the airway, primarily driven by C-X-C motif ligand 8, interleukin (IL)-1β, IL-8, tumour necrosis factor-α and leukotriene B4 [53–55]. These processes activate host defence mechanisms, including phagocytosis, formation of neutrophil extracellular traps (NETs), neutrophil degranulation, production of reactive oxygen species and release of pro-inflammatory cytokines [18, 55, 56]. The serine protease neutrophil elastase (NE) has been associated with increased exacerbations, decreased lung function and disease severity in bronchiectasis [52, 57, 58]. NETs, web-like structures composed of DNA, histones and serine proteases (primarily NE), serve to contain and neutralise pathogens. Their overabundance is central to the development and progression of bronchiectasis and chronicity of infection in relation to NETs and are linked to increased disease severity, exacerbations and mortality in bronchiectasis [18]. Interestingly, P. aeruginosa, H. influenzae and Sta. aureus may induce and degrade NETs, providing a survival advantage for microbes and thus contributing to chronic infection [59–61]. Other host factors which contribute to chronic infection in bronchiectasis include impaired neutrophil phagocytosis and disrupted mucociliary clearance, both associated with high NE activity [54, 62–65]. Given the close association between neutrophilic inflammation and bacterial infection, targeting neutrophils has emerged as a promising therapy for frequent exacerbators with bronchiectasis; an approach being currently explored in clinical trials (figure 1) [17, 19, 52, 66, 67].
P. aeruginosa in bronchiectasis
The Gram-negative bacterium P. aeruginosa has major clinical relevance in bronchiectasis owing to its frequent detection and related pathogenicity. Registry data revealed its presence in 11–40% of patients, with notable geographical variation, even within continents, including an increased incidence in Southern Europe [7, 46, 48, 68]. The true prevalence of chronic infection with P. aeruginosa remains uncertain, with reported rates ranging widely between 8 and 32% across jurisdictions [7, 22, 48, 49]. Prior analysis of >10 000 individuals recorded in the European Bronchiectasis Registry revealed several independent risk factors for a new P. aeruginosa infection, including poorer lung function, history of frequent exacerbations, specific radiology, use of inhaled corticosteroids (ICS), macrolide therapy, low body mass index and increased sputum production [69]. Further studies on smaller populations have also confirmed elevated risks of new infection in patients with aetiologies such as COPD or primary ciliary dyskinesia, as well as prior infection with H. influenzae and in those in older age groups [70–72]. Numerous studies demonstrate that patients with chronic P. aeruginosa infection experience significant adverse outcomes, including poor and accelerated decline in lung function, increased disease severity, more pronounced radiological findings, poorer quality of life, and higher frequencies of exacerbation and hospitalisation with greater risk of mortality. Such findings underscore the impact that P. aeruginosa infection has on individuals with bronchiectasis, highlighting the clinical need for effective identification, eradication and therapy [6, 29, 48, 49, 73–76]. Here, eradication is defined as an absence of P. aeruginosa in respiratory cultures after targeted antibiotic treatment, coupled with clinical improvement and sustained nonrecurrence over a specified follow-up period. National and international guidelines recommend eradication in early infection, despite the uncertainty of natural progression between individual patients and the varied efficacy of early antipseudomonal antimicrobial therapy, especially in asymptomatic patients [15, 21, 50]. Retrospective studies on P. aeruginosa eradication in bronchiectasis, employing varying treatment regimens including oral, inhaled and intravenous antibiotics, have reported initial eradication rates ranging from 52 to 80%, which decrease to 36–60% after 1 year, highlighting the relevance of prompt identification and clinical action [71, 77, 78]. Prospective studies using tobramycin and colistin for eradication report initial success rates of 90.9 and 61.2% at 1 and 3 months, respectively, with rates dropping to 76.5 and 40.3% after 12 months [79, 80]. It remains unclear whether such longitudinal change in eradication success indicate treatment-related suppression of infection or if re-infection occurs following successful eradication. There remains an important research gap related to the long-term effects of P. aeruginosa eradication in bronchiectasis and how, if at all, this affects future disease course.
The microbiome in bronchiectasis
NGS approaches to assess microbiomes
Targeted amplicon sequencing
In the last 25 years, there has been an explosion of NGS technologies that offer fresh possibilities for investigating the role of bacterial, viral and fungal communities in the setting of acute and/or chronic infection in bronchiectasis. The technological advantages and limitations of the different methodologies have been extensively reviewed elsewhere [81, 82], with recommendations and guidelines for best practice now accessible [83, 84]. This review will therefore focus on how such NGS approaches have been applied to evaluate the microbiome in bronchiectasis.
The most frequently used methods to investigate bacterial microbiota in bronchiectasis have been amplicon-based approaches, targeting and selectively amplifying specific regions of the 16S rRNA gene. Typically, one to two hypervariable regions of the 16S rRNA gene (present in all prokaryotes including nonculturable organisms [85]) are amplified by PCR and sequenced. Sequencing two (rather than one) hypervariable regions allows for improved taxonomic classification but remains restricted to classifying bacteria to the genus level, with biases in identification of certain taxa possible depending on the primers and 16S rRNA region selected [86, 87]. It has been proposed that using an intergenic spacer region between the 16S and 23S rRNA gene subunits as the amplicon of choice may resolve taxonomy to subspecies level with greater confidence [88]; however, more recently, synthetic long-read sequencing methodologies have been developed including PacBio Single Molecule Real Time circular consensus sequences, Oxford Nanopore or Loop synthetic long-read sequencing [89–92]. These latter approaches enable classification to species or strain level and may provide insight into functional differences within bacterial populations [93]. Such methods exhibit higher throughput than second-generation sequencing but come with increased costs and bioinformatics requirements [94].
In terms of defining fungal taxonomy, several genetic markers are traditionally used, including the fungal 18S and 28S rRNA genes, the associated long spacer region, translation elongation factor 1-alpha, RNA polymerase II (RPB1 and RPB2) and beta-tubulin [95, 96]. For comprehensive profiling of fungi in complex ecosystems, the ITS (internal transcribed spacer) regions have emerged as markers of choice. The preference for ITS regions is attributed to species-specific variation, extensive databases and relative standardisation, although careful interpretation of sequences obtained are required [96–98]. Standardisation of fungal mycobiome sequencing has yet to reach the same level as bacterial 16S rRNA gene sequencing [97]. Challenges persist in terms of optimising detection sensitivity, achieving broad taxonomic coverage and developing efficient DNA extraction techniques [99]. Overcoming such challenges is hampered by the limited availability of fungal genomes in the public domain, leading to identification biases towards specific fungal taxa [98–101]. While the technical feasibility of dual ITS1 and ITS2 sequencing via targeted ITS shotgun metagenomics has been demonstrated, selective sequencing of either ITS1 or ITS2 has gained prominence due its relative simplicity, lower cost and less complex wet and dry lab protocols [44, 99]. The choice of specific primer sets targeting alternate ITS1 and ITS2 regions is also a key consideration, impacting the levels of detection and range of coverage in mycobiome studies. Moreover, a preference for the ITS2 region for analysis of commensal microbes has been advanced given the apparent biases associated with ITS1 [101]. Fungal ITS analysis has significantly benefited from years of bioinformatic refinement of bacterial 16S rRNA gene sequencing analysis. Specialised pipelines and tools tailored for fungal ITS data processing now include QIIME2 q2-ITSxpress plugin, DADA2 adapted for ITS sequences, the UNITE Pipeline and FUNGuild for taxonomic and functional annotation [102–105], all of which offer robust solutions for quality filtering, taxonomic classification, diversity analysis and functional annotation, enabling a comprehensive exploration of fungal communities within microbiome studies.
Metagenomics
The application of metagenomic whole genome sequencing (WGS) to airway microbiome samples now represents an attractive alternative to targeted amplicon sequencing. This approach offers assessment of the entire genomic content (bacteria, fungi and viruses) within a sample, allowing for comprehensive analysis of microbial diversity and functional potential, including antibiotic resistance genes, while minimising primer bias [106]. Metagenomics enables identification of novel micro-organisms and functions and allows a layered approach to analysis [107, 108]. Taxonomic identification is facilitated by rapid classification methods and mirrors the insights attainable with 16S rRNA, with added robust species-level resolution [109]. Functional microbiome analysis is possible and has yielded insight into the functional composition of the bronchiectasis microbiome; revealing its distinct profile and composition as correlated with exacerbation [107, 110]. A particularly noteworthy aspect of the metagenomic approach is the rich array of antimicrobial resistance genes identifiable in the bronchiectasis airway termed the “resistome” [110–112]. The presence of bacteriophages within the bronchiectasis microbiome has been investigated by this approach but has yet to reveal clear clinical association [107, 113, 114]. While WGS metagenomics offers kingdom-agnostic analysis, investigation of low-abundance taxa such as fungi may necessitate deeper sequencing rendering greater cost and time required compared to alternative targeted approaches. Limitations exist even with metagenomics and the field is still evolving toward methodological consensus, complicated by emerging novel technologies for sequencing and advancing bioinformatic approaches [115, 116]. Meta-transcriptomics offers even deeper analysis into the metabolic states associated with the bronchiectasis microbiome and has been employed in several reported studies, highlighting its ability to simultaneously capture bacterial, viral and fungal profiles in tandem with functional characterisation and host response, affording additional view of the airway microbiome [117, 118].
The bacteriome in bronchiectasis
Almost a decade since Rogers et al. [119] set out key research questions related to respiratory microbiota, knowledge of the bacteriome in bronchiectasis has significantly advanced, both during disease stability and exacerbations. This has been extensively reviewed elsewhere [120–122]. The stable sputum bacteriome in bronchiectasis is dominated by the phyla Pseudomonadota (was Proteobacteria) and Bacillota (formerly Firmicutes) whilst, at the genus level, Pseudomonas, Haemophilus, Streptococcus, Veillonella, Prevotella, Stenotrophomonas and Neisseria are typically seen alongside less abundant taxa including Rothia, Staphylococcus, Enterobacteriaceae and Leptotrichia [123–127]. Patients with more severe disease appear to have a more unbalanced or “dysbiotic” bacteriome, often dominated by Pseudomonas, Streptococcus or Haemophilus [124, 128, 129]. Early studies have often been small (<100 patients) (figure 2b), cross-sectional and focused on clinical subsets of bronchiectasis largely dominated by Caucasian subjects [58, 130, 131]. More recent studies have included cohorts of patients from different geographic regions including Asia and the Middle East [20, 44, 107, 128, 131–133]. Longitudinal studies with follow-up to 16 years have shown that individual bacterial profiles from sputum or bronchoalveolar lavage (BAL), during stable disease and exacerbations, generally remain unchanged over time [124–126, 134, 135].
Our understanding of the bronchiectasis bacteriome in upper and lower airway niches is limited. One study, with 35 paired sputum and nasopharyngeal (NP) swabs found statistically significant differences between these niches; where Bacillota and Streptococcus were more prevalent in sputum and Bacillota, Actinobacteriota, Corynebacterium and Dolosigranulum predominant in NP swabs [131]. Choo et al. [136] investigated the oropharyngeal (OP) microbiota in 84 bronchiectasis patients and showed that core OP microbiota were largely comparable to sputum but with additional genera including as Gemella, Oribacterium and Campylobacter. Three studies have utilised BAL, revealing little difference between the bacteriomes obtained from sputum and BAL [123, 134, 137]. Only a single study to date has investigated multiple body sites such as the stomach or oral cavity alongside the respiratory bacteriome in bronchiectasis [138]. Comparisons of bacteria with clinical characteristics reveal that the bacteriome is altered in patients with more severe disease (determined by FACED score (forced expiratory volume in 1 s, age, chronic P. aeruginosa colonisation, radiological extension and dyspnoea) or the bronchiectasis severity index) with lower alpha diversity and increased abundance of taxa such as Pseudomonas being associated with more severe scores and the abundance of taxa such as Rothia or Haemophilus associating with milder disease [124, 128, 139]. Importantly, associations between alpha diversity and lung function are less clear, with contradictory results currently reported [123–125, 135]. A large (n=281) observational cohort, including both cross-sectional and longitudinal sampling in bronchiectasis demonstrated that patients with a stable bacteriome dominated by Pseudomonas or Stenotrophomonas were at increased risk of mortality or severe exacerbations, indicating the potential for microbiome related disease stratification [124]. Furthermore, emerging work links bacteriome profiles to inflammatory patterns and disease endophenotypes (figure 3) [18, 20, 51, 58, 140, 141]. The chronicity of bacterial infection or airway colonisation in bronchiectasis leads to the development of aberrant neutrophilic immune responses such as those described in previous sections of this review [17, 52]. Comparable patterns are observed when comparing bacteriomes with inflammatory markers including increased levels of neutrophil-associated proteins such as NE, NETs, MPO (myeloperoxidase) or ELANE (elastase, neutrophil expressed) detected in individuals with Pseudomonadota- or Pseudomonas-dominated bacteriomes that predispose to increased sputum production and persistence of bacteria-related biofilms [18, 51, 58, 140, 141]. NTM are increasingly recognised in bronchiectasis, inducing cavitary, infiltrative and nodular lung diseases, particularly in patients with underlying pulmonary abnormalities [1, 33, 142]. The role of NTM, including species such as Mycobacterium avium complex, the most prevalent NTM, followed by Mycobacterium abscessus and Mycobacterium chelonae, is crucial, particularly given the significant challenges in their detection [43, 143]. Bronchiectasis exhibits distinct microbiome features associated with NTM infection, including decreased alpha diversity, enrichment with oral commensals and a distinct pro-inflammatory immune profile [143]. Furthermore, the interaction between NTM and the host immune system, particularly impaired interferon-γ and granulocyte–macrophage colony-stimulating factor production coupled with associations with upper airway taxa and T-helper (Th) 17 cytokines, highlights the complex microbiological landscape in the airways and the associated treatment challenges [143]. The correlation between NTM infection and increased risk of fungal colonisation by A. fumigatus represents an important example of inter-kingdom interaction in this setting [144, 145].
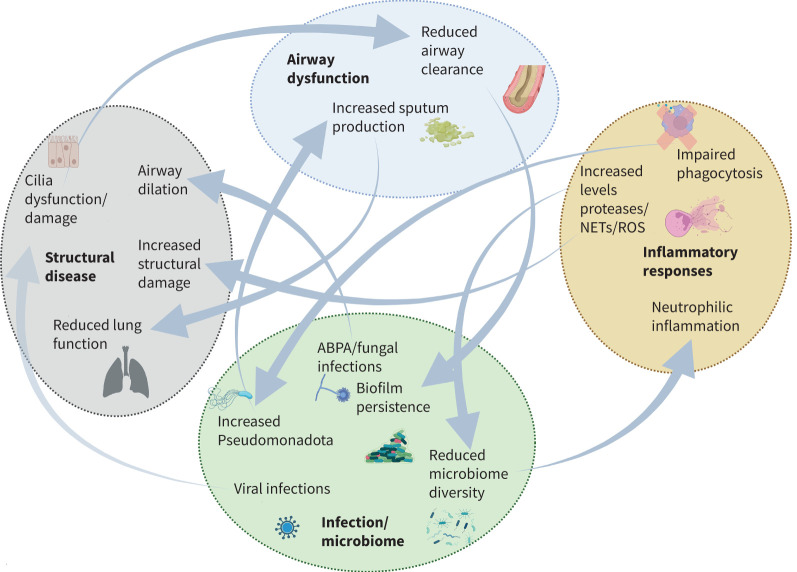
FIGURE 3.
Microbiome and microbial interactions in the vicious vortex of bronchiectasis pathogenesis, adapted from the vicious vortex hypothesis of Flume et al. [3]. NET: neutrophil extracellular trap; ROS: reactive oxygen species. Figure created with BioRender.com.
The mycobiome in bronchiectasis
Fungi are ubiquitous constituents of the air microbiome and have been detected in healthy and diseased airways [146, 147]. Though often challenging to identify and clinically characterise, they contribute significantly to airway immunopathology and remain central in allergic respiratory disease [148, 149]. Host pattern recognition receptors such as Toll-like receptors and C-type lectin receptors are central to host detection of fungal pathogen-associated molecular patterns, such as β-glucans, chitin and galactomannan [150, 151]. In this context, the mycobiome has been identified as a priority area for bronchiectasis research [21, 44, 45, 107, 138, 152, 153]. This adds to existing literature describing the mycobiome in cystic fibrosis (CF), where fungal colonisation, sensitisation and ABPA are closely correlated with clinical outcome [153–160]. Recent work in bronchiectasis highlights the increased sensitisation response, corroborating early studies describing atopy associated with non-CF disease [45]. Sensitisation in bronchiectasis and the presence of poly-sensitisation to multiple fungal and nonfungal allergens are closely correlated with poorer lung function and disease severity, while multiplex analysis of host airway immunology identified “immunoallertypes” including a fungal-driven and proinflammatory profile, seen in a subgroup of patients with bronchiectasis, where it was associated with worse clinical outcome [45]. Intriguingly, high sensitisation levels in COPD have also been linked to high-risk airway mycobiome profiles, while environmental metagenomics now highlights several airborne fungal taxa (and allergens) that trigger sensitisation responses and poorer clinical outcomes [161, 162]. While such environmental metagenomic analysis and its disease correlates are yet to be characterised in bronchiectasis, the characterisation of distinct mycobiome profiles associating with serological ABPA or sensitisation including that in bronchiectasis–COPD overlap clearly suggests a role for environmental allergens in bronchiectasis including those of fungal origin [152]. In light of such findings, a continued exploration of the airway mycobiome and associated environmental sources of fungal allergens is warranted in bronchiectasis and may offer fresh avenues for disease stratification and targeted intervention.
The virome in bronchiectasis
DNA-based microbiome analytics do not allow evaluation of RNA viruses, key organisms within the bronchiectasis microbiome. Consequently, viruses including influenza, respiratory syncytial virus, coronaviruses and rhinovirus have been overlooked by many existing studies. In the clinical setting, targeted detection of specific viruses by reverse transcriptase PCR is common and roles in other respiratory disease states recognised. Nevertheless, holistic RNA-based assessment of the virome is less commonly performed and lacks thorough application and study in bronchiectasis. While several methods related to metatranscriptomics have been proposed, these analyses face challenges including RNA degradation, reverse transcription biases, inherent complexity related to respiratory viruses, host RNA contamination and bioinformatic challenges [163, 164]. Addressing such factors necessitates a tailored experimental and analytical approach to accurately study the respiratory virome. Furthermore, metatranscriptomics will only capture RNA virus profiles, while DNA viruses (and phages) may play important roles and, therefore, to ensure a holistic assessment of the virome, analysis of both RNA and DNA viral species are necessary [165]. While it remains a challenge to apply in clinical settings, virome analysis is likely to reveal important components related to bronchiectasis pathogenesis, although type and timing of sample collection is important given the dynamics related to viral burden and host response [164, 166]. Notably, viral infection commonly precedes bacterial infection which, in turn, can influence the microbiome, highlighting the relevance of considering viral–bacterial interaction. In an important demonstration of this phenomenon, Molyneaux et al. [167] highlighted the role of rhinovirus infection in enhancing bacterial burden, noting an increase in H. influenzae within the existing microbiota. This finding points to complex interactions between viral infections and bacterial dynamics, suggesting that viruses may influence the exacerbation of underlying respiratory conditions associated with alterations in the composition of respiratory microbiota. These insights underscore the need for integrated viral and bacterial management in respiratory diseases [167]. Indeed, integration of targeted viral identification within microbiome and mycobiome data has already highlighted the relevance of such interaction, particularly in association with exacerbation [107]. The occurrence of specific bacteriophage profiles associated with clinical outcomes in bronchiectasis is yet to be elucidated and a key unmet research need in relation to identifying phage patterns and clinical outcomes continues to exist [107, 113]. Bacteriophages may reshape microbiomes and lead to altered community structures with relevant diagnostic and therapeutic implications for bronchiectasis [168, 169].
Inter-kingdom analysis and multiomic approaches in bronchiectasis
Inter-kingdom analyses related to the airway microbiome has informed mechanisms related to exacerbations in CF and bronchiectasis [107, 170]. Networks highlighting microbial interactions may even more accurately reflect clinical variation, offering a novel framework for understanding bronchiectasis and exacerbations [171, 172]. Microbial networks are significantly altered in bronchiectasis; observed interactions between bacteria, viruses and fungi offer gains in predicting exacerbation as compared to analysis of microbial relative abundance profiles in isolation [107]. The holistic capture of the inter-kingdom “interactome” provides a framework to assess the intricate and often unpredictable behaviour of pathogens in clinical settings. This approach recognises that interactions are not static but dynamic, embedded in a multidimensional space where simplistic, reductionist clustering of microbiome data can mislead or obscure clinically significant patterns. Exploring interaction profiles of specific pathogens can uncover synergistic or antagonistic relationships that may critically influence disease progression or therapeutic responses [107, 108]. This strategy is particularly relevant in chronic diseases, where understanding shifts in microbial communities could inform targeted therapies, potentially leading to more effective and personalised treatment strategies [107, 108, 122]. Furthermore, analysis of gut–lung microbiota (including bacteria and fungi) further highlights the importance of network interaction dynamics and their association with bronchiectasis phenotypes [138]. Increasing application of such “multi-biome” approaches, including longitudinal studies, will likely provide additional insight into potential interaction dynamics that correlate with health, disease and predictions of treatment response. Interestingly, it has been recently observed that particular bronchiectasis “resistotypes” are identifiable by metagenomics and correlate with clinical phenotypes [113]. The relationship between mobile genetic elements, plasmids and the airway resistome remains to be studied in bronchiectasis and may uncover further insight into bacteriome–resistome correlates of clinical relevance [173].
Microbiomes for diagnosis, stratification and prognostication in bronchiectasis
Large bronchiectasis patient registries, with associated biobanks, are now established globally (figure 2a). These have enabled, for the first time, microbiome studies to be combined with clinical data in large numbers of patients [43, 174, 175]. This undoubtedly will lead to more comprehensive, multiomic studies, replete with longitudinal follow-up and representation of more diverse patient populations and disease phenotypes. Differences in the bronchiectasis microbiome can be leveraged to stratify patients and compare clinical outcomes and disease characteristics [18, 42, 124, 129]. Importantly, however, existing studies are often comparative, with patients grouped by microbiome population medians or tertiles. Such approaches preclude the use of microbiome data as clinical, diagnostic and/or prognostication tools. Individual NGS microbiome data, however, may be utilised alongside traditional microbiology as part of enhanced clinical monitoring of “at-risk” patients, particularly for cases where standard culture has failed to identify potential causative agents related to exacerbations and/or to monitor the effectiveness of eradication therapies or novel treatments [113, 176, 177]. One of the biggest challenges to developing novel therapeutics for bronchiectasis has often been the failure of reproducible randomised controlled trials, such as the RESPIRE or ORBIT trials of dry powder or inhaled ciprofloxacin [9, 10, 178–180]. In nonalcoholic fatty liver disease, microbiome network analysis has been employed to identify responders or nonresponders to novel interventions [181]. The same approach should be considered in bronchiectasis.
Can we target the microbiome for therapeutic benefit?
Focus has traditionally been on symptom and infection control, with minimal attention on how treatments affect the microbiome in bronchiectasis. Use of macrolides and other antibiotics are central for managing infections and reducing exacerbations, yet these treatments alter resident microbial composition and affect resistance patterns, highlighting the complex interaction between microbial communities and therapeutic intervention [113, 130, 182, 183]. This emphasises the need to consider the therapeutic-related effects on the microbiome, as they may influence disease progression and future response to treatments. More recent emphasis has shifted towards targeted microbial therapies, such as narrow-spectrum antibiotics and bacteriophage therapy, aiming to address pathogens while preserving beneficial “commensal” bacteria. Such strategies represent a move towards precision medicine, by attempting to minimise the drawbacks of broad-spectrum antibiotics and target therapy at the individual level [184, 185]. Additionally, interconnections between gut and lung microbiomes, alongside environmental factors, suggest that a comprehensive treatment approach incorporating lifestyle, diet, comorbidities and the patient's living environment should be considered [186, 187]. This broader view appears essential for developing fresh therapeutic options for bronchiectasis that considers the complex interplay between microbiomes, immunity and the surrounding environment [122]. ICS have limited and poorly understood impacts on the respiratory microbiome in bronchiectasis [188]. Recent work has indicated the potential benefits of ICS in patients with a predominantly eosinophilic bronchiectasis phenotype, with microbiome composition related to this inflammatory endotype, suggesting the potential for microbiome-targeted approaches [20]. Biologics represent an additional potential treatment approach in bronchiectasis, especially with agents that target eosinophilic inflammation and Th2-mediated responses. These therapies offer a route to modulate the immune–microbiome axis and individualise therapy [56, 189, 190]. This is an exciting era for bronchiectasis, one where targeting the microbiome represents a fresh opportunity to realise precision medicine and develop more effective, targeted treatments that consider infection, inflammation and immunity (table 1) [122].
TABLE 1.
Current and emerging stratification and therapeutic targeting of the microbiome in bronchiectasis
| Current methods | Emerging/future methods |
|---|---|
| Stratification based on clinical phenotypes, e.g. frequent exacerbators | Microbiome as an integrated biomarker and treatable trait |
| Stratification based on disease severity (BSI, FACED) | Ecological metrics (α-/β-diversity), graph-theoretic measures (degree, betweenness centrality and modularity) |
| Broad antimicrobial therapy based on microbial culture results | Evaluations of pathogens and associated commensal–pathobiont interaction networks to inform antimicrobial therapies |
| Use of azithromycin, doxycycline, fluoroquinolones (pathogen-centric) for pathogen eradication | Targeted antimicrobial eradication programmes, bacteriophage therapy, antibody–drug conjugates, narrow-spectrum microbiome-targeting |
| Broad anti-inflammatory therapy, e.g. inhaled corticosteroids | Precision therapeutics, e.g. anti-IL5 and DPP1 inhibitors |
| Focus on the lung as an independent biome | Holistic integration and appreciation of multi-site “axes”, e.g. gut–lung and oral–lung |
| One size fits all treatments based on the “vicious vortex” model | Multimodal precision interventions integrating ongoing assessments of the microbiome into the vicious vortex |
| Stratification of patients into microbiome types defined in relation to other patients’ microbiomes | Personalised individual prognostic/diagnostic microbiome results |
BSI: bronchiectasis severity index; DDP1: dipeptidyl peptidase 1; FACED: forced expiratory volume in 1 s, age, chronic Pseudomonas aeruginosa colonisation, radiological extension and dyspnoea; IL: interleukin.
Conclusions and future directions
The intricate interplay between microbiome, immune response and host factors in bronchiectasis emphasises the need for targeted therapeutic strategies that address such complexity. To advance the management of bronchiectasis, future research must prioritise a better understanding of the airway microbiome and the precise role of commensals and/or pathobionts in maintaining respiratory health [132, 191, 192]. Such work entails exploring the symbiotic relationship between host and microbial communities. Crosstalk between the microbiome and other host features such as genetics, inflammation and immunity represents another crucial avenue for investigation [51, 193]. Understanding these interactions will uncover mechanisms of disease, exacerbation and risks associated with progression, which in turn will guide the development of novel therapeutics aimed at disrupting these pathological processes. The application of multiomics in bronchiectasis research holds great promise. By integrating microbiome data with that from genomics, transcriptomics, proteomics and metabolomics, a more holistic view of disease will be gained and novel biomarkers to detect, monitor and tailor treatment can be developed. Such approaches must be applied at scale in large and carefully curated global biobanks which must be maintained and supported. Identifying early or “at-risk” microbial features to arrest disease progression should be prioritised [192, 194] and the future successes for discovering novel interventions for bronchiectasis are grounded in the integration of multiomics approaches with microbiology and NGS, allowing an improved understanding of host–microbe dynamics. Employing such a strategy promises to transform the bronchiectasis landscape and move beyond the traditional symptomatic care to one addressing fundamental mechanisms that drive disease [122].
Points for clinical practice
Integrating advanced multiomics technologies to understand the complex interplay of infection and the microbiome in bronchiectasis will bring new perspectives to disease management.
The diversity of microbial dynamics should be considered when diagnosing and managing bronchiectasis, emphasising personalised treatment plans that address specific microbial imbalances and immune responses.
The application of available genomic technologies, such as targeted amplicon sequencing and shotgun metagenomics, could substantially improve therapeutic outcomes by enabling tailored microbial management strategies.
Questions for future research
To advance bronchiectasis management, future research must harness the complex interactions between microbes, immune responses, host genetics and the exposome. Increased understanding of the microbiome and its role in health, including both symbiotic and pathogenic relationships, is crucial. Future research must reveal mechanisms driving disease exacerbation and progression, guiding the development of novel, targeted and preventative therapies. Employing multiomics approaches, integrating genomic, transcriptomic, proteomic and metabolomic data, will enrich our perspective on disease. While this brings analytical, interpretive and translational challenges, it holds the potential to uncover novel biomarkers and refine treatment strategies. Implementing these methods in large, well-maintained global biobanks is vital for validating biomarkers and therapeutic targets across diverse populations. These efforts aim to shift bronchiectasis management from symptomatic treatment to a focus on fundamental disease mechanisms and diverse clinical endophenotypes.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERR-0038-2024.SUPPLEMENT (212.6KB, pdf)
Acknowledgements
The authors would like to acknowledge the Academic Respiratory Initiative for Pulmonary Health (TARIPH) and the Lee Kong Chian School of Medicine Centre for Microbiome Medicine for support.
This article has an editorial commentary: https://doi.org/10.1183/16000617.0124-2024
Provenance: Commissioned article, peer reviewed.
Previous articles in this series: No. 1: Perea L, Faner R, Chalmers JD, et al. Pathophysiology and genomics of bronchiectasis. Eur Respir Rev 2024; 33: 240055.
Number 2 in the Series “World Bronchiectasis Conference 2024” Edited by James D. Chalmers, Felix C. Ringshausen and Pieter C. Goeminne
Conflict of interest: S.H. Chotirmall serves on advisory boards for CSL Behring, Pneumagen Ltd and Boehringer Ingelheim, has received lecture fees from AstraZeneca and Chiesi Farmaceutici and has served on data safety and monitoring boards (DSMBs) for Inovio Pharmaceuticals Ltd and Imam Abdulrahman Bin Faisal University. All other authors have no conflicts to disclose.
Support statement: This work was supported by the Singapore Ministry of Health's National Medical Research Council under its Clinician-Scientist Individual Research Grant (MOH-001356) (S.H. Chotirmall) and Clinician Scientist Award (MOH-000710) (S.H. Chotirmall), and the Singapore Ministry of Education under its AcRF Tier 1 Grant (RT1/22) (S.H. Chotirmall). Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Chalmers JD, Chang AB, Chotirmall SH, et al. Bronchiectasis. Nat Rev Dis Primers 2018; 4: 45. doi: 10.1038/s41572-018-0042-3 [DOI] [PubMed] [Google Scholar]
- 2.Chalmers JD, Chotirmall SH. Bronchiectasis: new therapies and new perspectives. Lancet Respir Med 2018; 6: 715–726. doi: 10.1016/S2213-2600(18)30053-5 [DOI] [PubMed] [Google Scholar]
- 3.Flume PA, Chalmers JD, Olivier KN. Advances in bronchiectasis: endotyping, genetics, microbiome, and disease heterogeneity. Lancet 2018; 392: 880–890. doi: 10.1016/S0140-6736(18)31767-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hill AT, Haworth CS, Aliberti S, et al. Pulmonary exacerbation in adults with bronchiectasis: a consensus definition for clinical research. Eur Respir J 2017; 49: 1700051. doi: 10.1183/13993003.00051-2017 [DOI] [PubMed] [Google Scholar]
- 5.Aliberti S, Goeminne PC, O'Donnell AE, et al. Criteria and definitions for the radiological and clinical diagnosis of bronchiectasis in adults for use in clinical trials: international consensus recommendations. Lancet Respir Med 2022; 10: 298–306. doi: 10.1016/S2213-2600(21)00277-0 [DOI] [PubMed] [Google Scholar]
- 6.Chalmers JD, Aliberti S, Filonenko A, et al. Characterization of the “frequent exacerbator phenotype” in bronchiectasis. Am J Respir Crit Care Med 2018; 197: 1410–1420. doi: 10.1164/rccm.201711-2202OC [DOI] [PubMed] [Google Scholar]
- 7.Chalmers JD, Goeminne P, Aliberti S, et al. The bronchiectasis severity index. An international derivation and validation study. Am J Respir Crit Care Med 2014; 189: 576–585. doi: 10.1164/rccm.201309-1575OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Serisier DJ, Martin ML, McGuckin MA, et al. Effect of long-term, low-dose erythromycin on pulmonary exacerbations among patients with non-cystic fibrosis bronchiectasis: the BLESS randomized controlled trial. JAMA 2013; 309: 1260–1267. doi: 10.1001/jama.2013.2290 [DOI] [PubMed] [Google Scholar]
- 9.Aksamit T, De Soyza A, Bandel TJ, et al. RESPIRE 2: a phase III placebo-controlled randomised trial of ciprofloxacin dry powder for inhalation in non-cystic fibrosis bronchiectasis. Eur Respir J 2018; 51: 1702053. doi: 10.1183/13993003.02053-2017 [DOI] [PubMed] [Google Scholar]
- 10.De Soyza A, Aksamit T, Bandel TJ, et al. RESPIRE 1: a phase III placebo-controlled randomised trial of ciprofloxacin dry powder for inhalation in non-cystic fibrosis bronchiectasis. Eur Respir J 2018; 51: 1702052. doi: 10.1183/13993003.02052-2017 [DOI] [PubMed] [Google Scholar]
- 11.Chang AB, Boyd J, Bush A, et al. A core outcome set for bronchiectasis in children and adolescents for use in clinical research: an international consensus study. Lancet Respir Med 2024; 12: 78–88. doi: 10.1016/S2213-2600(23)00233-3 [DOI] [PubMed] [Google Scholar]
- 12.Spargo M, Ryan C, Downey D, et al. Development of a core outcome set for trials investigating the long-term management of bronchiectasis. Chron Respir Dis 2019; 16: 1479972318804167. doi: 10.1177/1479972318804167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sibila O, Laserna E, Shoemark A, et al. Heterogeneity of treatment response in bronchiectasis clinical trials. Eur Respir J 2022; 59: 2100777. doi: 10.1183/13993003.00777-2021 [DOI] [PubMed] [Google Scholar]
- 14.Crichton ML, Aliberti S, Chalmers JD. A systematic review of pharmacotherapeutic clinical trial end-points for bronchiectasis in adults. Eur Respir Rev 2019; 28: 180108. doi: 10.1183/16000617.0108-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hill AT, Sullivan AL, Chalmers JD, et al. British Thoracic Society guideline for bronchiectasis in adults. Thorax 2019; 74: Suppl. 1, 1–69. doi: 10.1136/thoraxjnl-2018-212463 [DOI] [PubMed] [Google Scholar]
- 16.Martínez-García M, Máiz L, Olveira C, et al. Spanish guidelines on treatment of bronchiectasis in adults. Arch Bronconeumol 2018; 54: 88–98. doi: 10.1016/j.arbr.2017.07.014 [DOI] [PubMed] [Google Scholar]
- 17.Chalmers JD, Smith MP, McHugh BJ, et al. Short- and long-term antibiotic treatment reduces airway and systemic inflammation in non-cystic fibrosis bronchiectasis. Am J Respir Crit Care Med 2012; 186: 657–665. doi: 10.1164/rccm.201203-0487OC [DOI] [PubMed] [Google Scholar]
- 18.Keir HR, Shoemark A, Dicker AJ, et al. Neutrophil extracellular traps, disease severity, and antibiotic response in bronchiectasis: an international, observational, multicohort study. Lancet Respir Med 2021; 9: 873–884. doi: 10.1016/S2213-2600(20)30504-X [DOI] [PubMed] [Google Scholar]
- 19.Chalmers JD, Usansky H, Rubino CM, et al. Pharmacokinetic/pharmacodynamic evaluation of the dipeptidyl peptidase 1 inhibitor brensocatib for non-cystic fibrosis bronchiectasis. Clin Pharmacokinet 2022; 61: 1457–1469. doi: 10.1007/s40262-022-01147-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shoemark A, Shteinberg M, De Soyza A, et al. Characterization of eosinophilic bronchiectasis: a European multicohort study. Am J Respir Crit Care Med 2022; 205: 894–902. doi: 10.1164/rccm.202108-1889OC [DOI] [PubMed] [Google Scholar]
- 21.Polverino E, Goeminne PC, McDonnell MJ, et al. European Respiratory Society guidelines for the management of adult bronchiectasis. Eur Respir J 2017; 50: 1700629. doi: 10.1183/13993003.00629-2017 [DOI] [PubMed] [Google Scholar]
- 22.Martínez-García M, de Gracia J, Vendrell Relat M, et al. Multidimensional approach to non-cystic fibrosis bronchiectasis: the FACED score. Eur Respir J 2014; 43: 1357–1367. doi: 10.1183/09031936.00026313 [DOI] [PubMed] [Google Scholar]
- 23.Gao Y-H, Guan W-J, Xu G, et al. The role of viral infection in pulmonary exacerbations of bronchiectasis in adults a prospective study. Chest 2015; 147: 1635–1643. doi: 10.1378/chest.14-1961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen C-L, Huang Y, Yuan J-J, et al. The roles of bacteria and viruses in bronchiectasis exacerbation: a prospective study. Arch Bronconeumol 2020; 56: 621–629. doi: 10.1016/j.arbr.2019.12.014 [DOI] [PubMed] [Google Scholar]
- 25.Crichton ML, Shoemark A, Chalmers JD. The impact of the COVID-19 pandemic on exacerbations and symptoms in bronchiectasis: a prospective study. Am J Respir Crit Care Med 2021; 204: 857–859. doi: 10.1164/rccm.202105-1137LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murray MP, Govan JRW, Doherty CJ, et al. A randomized controlled trial of nebulized gentamicin in non-cystic fibrosis bronchiectasis. Am J Respir Crit Care Med 2011; 183: 491–499. doi: 10.1164/rccm.201005-0756OC [DOI] [PubMed] [Google Scholar]
- 27.Laska IF, Crichton ML, Shoemark A, et al. The efficacy and safety of inhaled antibiotics for the treatment of bronchiectasis in adults: a systematic review and meta-analysis. Lancet Respir Med 2019; 7: 855–869. doi: 10.1016/S2213-2600(19)30185-7 [DOI] [PubMed] [Google Scholar]
- 28.Clemente MG, Olveira C, Girón R, et al. Impact of chronic bronchial infection by Staphylococcus aureus on bronchiectasis. J Clin Med 2022; 11: 3960. doi: 10.3390/jcm11143960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Araujo D, Shteinberg M, Aliberti S, et al. The independent contribution of Pseudomonas aeruginosa infection to long-term clinical outcomes in bronchiectasis. Eur Respir J 2018; 51: 1701953. doi: 10.1183/13993003.01953-2017 [DOI] [PubMed] [Google Scholar]
- 30.Yang S-H, Song MJ, Kim YW, et al. Understanding the effects of Haemophilus influenzae colonization on bronchiectasis: a retrospective cohort study. BMC Pulm Med 2024; 24: 7. doi: 10.1186/s12890-023-02823-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Metersky ML, Aksamit TR, Barker A, et al. The prevalence and significance of Staphylococcus aureus in patients with non-cystic fibrosis bronchiectasis. Ann Am Thorac Soc 2018; 15: 365–370. doi: 10.1513/AnnalsATS.201706-426OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Metersky ML, Choate R, Aksamit TR, et al. Stenotrophomonas maltophilia in patients with bronchiectasis: an analysis of the US Bronchiectasis and NTM Research Registry. Respir Med 2022; 193: 106746. doi: 10.1016/j.rmed.2022.106746 [DOI] [PubMed] [Google Scholar]
- 33.Mac Aogáin M, Chalmers JD, Chotirmall SH. Bronchiectasis. In: Huang YJ, Garantziotis S, eds. The Microbiome in Respiratory Disease: Principles, Tools and Applications. Cham, Springer International Publishing, 2022; pp. 179–198. [Google Scholar]
- 34.Park YE, Sung H, Oh Y-M. Respiratory viruses in acute exacerbations of bronchiectasis. J Korean Med Sci 2021; 36: e217. doi: 10.3346/jkms.2021.36.e217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mitchell AB, Mourad B, Buddle L, et al. Viruses in bronchiectasis: a pilot study to explore the presence of community acquired respiratory viruses in stable patients and during acute exacerbations. BMC Pulm Med 2018; 18: 84. doi: 10.1186/s12890-018-0636-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aliberti S, Gramegna A, Zucchetti S, et al. Respiratory viruses in stable bronchiectasis: a multicenter evaluation in Northern Italy. Respir Med 2022; 205: 107056. doi: 10.1016/j.rmed.2022.107056 [DOI] [PubMed] [Google Scholar]
- 37.Huang Y, Chen C-L, Cen L-J, et al. Sputum pathogen spectrum and clinical outcomes of upper respiratory tract infection in bronchiectasis exacerbation: a prospective cohort study. Emerg Microbes Infect 2023; 12: 2202277. doi: 10.1080/22221751.2023.2202277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen CL, Huang Y, Martinez-Garcia MA, et al. The role of Epstein–Barr virus in adults with bronchiectasis: a prospective cohort study. Open Forum Infect Dis 2020; 7: ofaa235. doi: 10.1093/ofid/ofaa235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Åstrand A, Kiddle SJ, Mudedla RSG, et al. Effect of COVID-19 on bronchiectasis exacerbation rates: a retrospective US insurance claims study. Ann Am Thorac Soc 2023; 21: 261–270. doi: 10.1513/AnnalsATS.202211-944OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martínez-Vergara A, Moreno RMG, Olveira C, et al. Impact of the SARS-CoV-2 virus pandemic on patients with bronchiectasis: a multicenter study. Antibiotics 2022; 11: 1096. doi: 10.3390/antibiotics11081096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Máiz L, Nieto R, Cantón R, et al. Fungi in bronchiectasis: a concise review. Int J Mol Sci 2018; 19: 142. doi: 10.3390/ijms19010142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dhar R, Singh S, Talwar D, et al. Bronchiectasis in India: results from the European Multicentre Bronchiectasis Audit and Research Collaboration (EMBARC) and Respiratory Research Network of India Registry. Lancet Glob Health 2019; 7: e1269–e1279. doi: 10.1016/S2214-109X(19)30327-4 [DOI] [PubMed] [Google Scholar]
- 43.Aksamit TR, O'Donnell AE, Barker A, et al. Adult patients with bronchiectasis: a first look at the US Bronchiectasis Research Registry. Chest 2017; 151: 982–992. doi: 10.1016/j.chest.2016.10.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mac Aogáin M, Chandrasekaran R, Lim AYH, et al. Immunological corollary of the pulmonary mycobiome in bronchiectasis: the CAMEB study. Eur Respir J 2018; 52: 1800766. doi: 10.1183/13993003.00766-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mac Aogáin M, Tiew PY, Lim AYH, et al. Distinct “immunoallertypes” of disease and high frequencies of sensitization in non-cystic fibrosis bronchiectasis. Am J Respir Crit Care Med 2019; 199: 842–853. doi: 10.1164/rccm.201807-1355OC [DOI] [PubMed] [Google Scholar]
- 46.Chalmers JD, Polverino E, Crichton ML, et al. Bronchiectasis in Europe: data on disease characteristics from the European Bronchiectasis registry (EMBARC). Lancet Respir Med 2023; 11: 637–649. doi: 10.1016/S2213-2600(23)00093-0 [DOI] [PubMed] [Google Scholar]
- 47.Pasteur MC, Helliwell SM, Houghton SJ, et al. An investigation into causative factors in patients with bronchiectasis. Am J Respir Crit Care Med 2000; 162: 1277–1284. doi: 10.1164/ajrccm.162.4.9906120 [DOI] [PubMed] [Google Scholar]
- 48.Martinez-García MA, Villa C, Dobarganes Y, et al. RIBRON: the Spanish online bronchiectasis registry. Characterization of the first 1912 patients. Arch Bronconeumol 2021; 57: 28–35. doi: 10.1016/j.arbr.2020.11.010 [DOI] [PubMed] [Google Scholar]
- 49.Finch S, McDonnell MJ, Abo-Leyah H, et al. A comprehensive analysis of the impact of Pseudomonas aeruginosa colonisation on prognosis in adult bronchiectasis. Ann Am Thorac Soc 2015; 12: 1602–1611. doi: 10.1513/AnnalsATS.201506-333OC [DOI] [PubMed] [Google Scholar]
- 50.Martínez-García MÁ, Máiz L, Olveira C, et al. Spanish guidelines on the evaluation and diagnosis of bronchiectasis in adults. Arch Bronconeumol 2018; 54: 79–87. doi: 10.1016/j.arbr.2017.07.013 [DOI] [PubMed] [Google Scholar]
- 51.Choi H, Ryu S, Keir HR, et al. Inflammatory molecular endotypes in bronchiectasis: a European multicenter cohort study. Am J Respir Crit Care Med 2023; 208: 1166–1176. doi: 10.1164/rccm.202303-0499OC [DOI] [PubMed] [Google Scholar]
- 52.Chalmers JD, Moffitt KL, Suarez-Cuartin G, et al. Neutrophil elastase activity is associated with exacerbations and lung function decline in bronchiectasis. Am J Respir Crit Care Med 2017; 195: 1384–1393. doi: 10.1164/rccm.201605-1027OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Angrill J, Agusti C, De Celis R, et al. Bronchial inflammation and colonization in patients with clinically stable bronchiectasis. Am J Respir Crit Care Med 2001; 164: 1628–1632. doi: 10.1164/ajrccm.164.9.2105083 [DOI] [PubMed] [Google Scholar]
- 54.Chalmers JD, Hill AT. Mechanisms of immune dysfunction and bacterial persistence in non-cystic fibrosis bronchiectasis. Mol Immunol 2013; 55: 27–34. doi: 10.1016/j.molimm.2012.09.011 [DOI] [PubMed] [Google Scholar]
- 55.Keir H, Chalmers J. Pathophysiology of bronchiectasis. Semin Respir Crit Care Med 2021; 42: 499–512. doi: 10.1055/s-0041-1730891 [DOI] [PubMed] [Google Scholar]
- 56.Giam YH, Shoemark A, Chalmers JD. Neutrophil dysfunction in bronchiectasis: an emerging role for immunometabolism. Eur Respir J 2021; 58: 2003157. doi: 10.1183/13993003.03157-2020 [DOI] [PubMed] [Google Scholar]
- 57.Shoemark A, Cant E, Carreto L, et al. A point-of-care neutrophil elastase activity assay identifies bronchiectasis severity, airway infection and risk of exacerbation. Eur Respir J 2019; 53: 1900303. doi: 10.1183/13993003.00303-2019 [DOI] [PubMed] [Google Scholar]
- 58.Oriano M, Gramegna A, Terranova L, et al. Sputum neutrophil elastase associates with microbiota and Pseudomonas aeruginosa in bronchiectasis. Eur Respir J 2020; 56: 2000769. doi: 10.1183/13993003.00769-2020 [DOI] [PubMed] [Google Scholar]
- 59.Young RL, Malcolm KC, Kret JE, et al. Neutrophil extracellular trap (NET)-mediated killing of Pseudomonas aeruginosa: evidence of acquired resistance within the CF airway, independent of CFTR. PLoS One 2011; 6: e23637. doi: 10.1371/journal.pone.0023637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Juneau RA, Pang B, Weimer KED, et al. Nontypeable Haemophilus influenzae initiates formation of neutrophil extracellular traps. Infect Immun 2011; 79: 431–438. doi: 10.1128/IAI.00660-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pilsczek FH, Salina D, Poon KKH, et al. A novel mechanism of rapid nuclear neutrophil extracellular trap formation in response to Staphylococcus aureus. J Immunol 2010; 185: 7413–7425. doi: 10.4049/jimmunol.1000675 [DOI] [PubMed] [Google Scholar]
- 62.Bedi P, Davidson DJ, McHugh BJ, et al. Blood neutrophils are reprogrammed in bronchiectasis. Am J Respir Crit Care Med 2018; 198: 880–890. doi: 10.1164/rccm.201712-2423OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Smallman LA, Hill SL, Stockley RA. Reduction of ciliary beat frequency in vitro by sputum from patients with bronchiectasis: a serine proteinase effect. Thorax 1984; 39: 663–667. doi: 10.1136/thx.39.9.663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Amitani R, Wilson R, Rutman A, et al. Effects of human neutrophil elastase and Pseudomonas aeruginosa proteinases on human respiratory epithelium. Am J Respir Cell Mol Biol 1991; 4: 26–32. doi: 10.1165/ajrcmb/4.1.26 [DOI] [PubMed] [Google Scholar]
- 65.Nair C, Shoemark A, Chan M, et al. Cyanide levels found in infected cystic fibrosis sputum inhibit airway ciliary function. Eur Respir J 2014; 44: 1253–1261. doi: 10.1183/09031936.00097014 [DOI] [PubMed] [Google Scholar]
- 66.Chalmers JD, Gupta A, Chotirmall SH, et al. A phase 2 randomised study to establish efficacy, safety and dosing of a novel oral cathepsin C inhibitor, BI 1291583, in adults with bronchiectasis: Airleaf. ERJ Open Res 2023; 9: 00633–2022. doi: 10.1183/23120541.00633-2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chalmers JD, Haworth CS, Metersky ML, et al. Phase 2 trial of the DPP-1 inhibitor brensocatib in bronchiectasis. N Engl J Med 2020; 383: 2127–2137. doi: 10.1056/NEJMoa2021713 [DOI] [PubMed] [Google Scholar]
- 68.Lee H, Choi H, Sim YS, et al. KMBARC registry: protocol for a multicentre observational cohort study on non-cystic fibrosis bronchiectasis in Korea. BMJ Open 2020; 10: e034090. doi: 10.1136/bmjopen-2019-034090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Burgel P-R, Polverino E, Harworth C, et al. Risk factors for new P. aeruginosa isolation in bronchiectasis – data from the European Bronchiectasis Registry (EMBARC). Eur Respir J 2019; 54: Suppl. 63, PA2163. doi: 10.1183/13993003.congress-2019.PA2163 [DOI] [Google Scholar]
- 70.Miszkiel KA, Wells AU, Rubens MB, et al. Effects of airway infection by Pseudomonas aeruginosa: a computed tomographic study. Thorax 1997; 52: 260–264. doi: 10.1136/thx.52.3.260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pieters A, Bakker M, Hoek RAS, et al. Predicting factors for chronic colonization of Pseudomonas aeruginosa in bronchiectasis. Eur J Clin Microbiol Infect Dis 2019; 38: 2299–2304. doi: 10.1007/s10096-019-03675-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shkeiri R, Saliba W, Stein N, et al. Exploring factors associated with acquisition and chronicity of infection in bronchiectasis: a population-based study. Respir Med 2021; 185: 106487. doi: 10.1016/j.rmed.2021.106487 [DOI] [PubMed] [Google Scholar]
- 73.Davies G, Wells AU, Doffman S, et al. The effect of Pseudomonas aeruginosa on pulmonary function in patients with bronchiectasis. Eur Respir J 2006; 28: 974–979. doi: 10.1183/09031936.06.00074605 [DOI] [PubMed] [Google Scholar]
- 74.Evans SA, Turner SM, Bosch BJ, et al. Lung function in bronchiectasis: the influence of Pseudomonas aeruginosa. Eur Respir J 1996; 9: 1601–1604. doi: 10.1183/09031936.96.09081601 [DOI] [PubMed] [Google Scholar]
- 75.Wang R, Ding S, Lei C, et al. The contribution of Pseudomonas aeruginosa infection to clinical outcomes in bronchiectasis: a prospective cohort study. Ann Med 2021; 53: 459–469. doi: 10.1080/07853890.2021.1900594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Martinez-García MA, Oscullo G, Posadas T, et al. Pseudomonas aeruginosa and lung function decline in patients with bronchiectasis. Clin Microbiol Infect 2021; 27: 428–434. doi: 10.1016/j.cmi.2020.04.007 [DOI] [PubMed] [Google Scholar]
- 77.White L, Mirrani G, Grover M, et al. Outcomes of Pseudomonas eradication therapy in patients with non-cystic fibrosis bronchiectasis. Respir Med 2012; 106: 356–360. doi: 10.1016/j.rmed.2011.11.018 [DOI] [PubMed] [Google Scholar]
- 78.Vallières E, Tumelty K, Tunney MM, et al. Efficacy of Pseudomonas aeruginosa eradication regimens in bronchiectasis. Eur Respir J 2017; 49: 1600851. doi: 10.1183/13993003.00851-2016 [DOI] [PubMed] [Google Scholar]
- 79.Blanco-Aparicio M, Canosa JLS, López PV, et al. Eradication of Pseudomonas aeruginosa with inhaled colistin in adults with non-cystic fibrosis bronchiectasis. Chronic Respir Dis 2019; 16: 147997311987251. doi: 10.1177/1479973119872513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Orriols R, Hernando R, Ferrer A, et al. Eradication therapy against Pseudomonas aeruginosa in non-cystic fibrosis bronchiectasis. Respiration 2015; 90: 299–305. doi: 10.1159/000438490 [DOI] [PubMed] [Google Scholar]
- 81.Alsayed AR, Abed A, Khader HA, et al. Molecular accounting and profiling of human respiratory microbial communities: toward precision medicine by targeting the respiratory microbiome for disease diagnosis and treatment. Int J Mol Sci 2023; 24: 4086. doi: 10.3390/ijms24044086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liu L, Li Y, Li S, et al. Comparison of next-generation sequencing systems. J Biomed Biotechnol 2012; 2012: 251364. doi: 10.1155/2012/251364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Carney SM, Clemente JC, Cox MJ, et al. Methods in lung microbiome research. Am J Respir Cell Mol Biol 2020; 62: 283–299. doi: 10.1165/rcmb.2019-0273TR [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mirzayi C, Renson A, Furlanello C, et al. Reporting guidelines for human microbiome research: the STORMS checklist. Nat Med 2021; 27: 1885–1892. doi: 10.1038/s41591-021-01552-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.López-Aladid R, Fernández-Barat L, Alcaraz-Serrano V, et al. Determining the most accurate 16S rRNA hypervariable region for taxonomic identification from respiratory samples. Sci Rep 2023; 13: 3974. doi: 10.1038/s41598-023-30764-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Klindworth A, Pruesse E, Schweer T, et al. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res 2013; 41: e1. doi: 10.1093/nar/gks808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kim M, Morrison M, Yu Z. Evaluation of different partial 16S rRNA gene sequence regions for phylogenetic analysis of microbiomes. J Microbiol Methods 2011; 84: 81–87. doi: 10.1016/j.mimet.2010.10.020 [DOI] [PubMed] [Google Scholar]
- 88.Mukherjee C, Beall CJ, Griffen AL, et al. High-resolution ISR amplicon sequencing reveals personalized oral microbiome. Microbiome 2018; 6: 153. doi: 10.1186/s40168-018-0535-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Eid J, Fehr A, Gray J, et al. Real-time DNA sequencing from single polymerase molecules. Science 2009; 323: 133–138. doi: 10.1126/science.1162986 [DOI] [PubMed] [Google Scholar]
- 90.Callahan BJ, Wong J, Heiner C, et al. High-throughput amplicon sequencing of the full-length 16S rRNA gene with single-nucleotide resolution. Nucleic Acids Res 2019; 47: e103. doi: 10.1093/nar/gkz569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jain M, Fiddes IT, Miga KH, et al. Improved data analysis for the MinION nanopore sequencer. Nat Methods 2015; 12: 351–356. doi: 10.1038/nmeth.3290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Callahan BJ, Grinevich D, Thakur S, et al. Ultra-accurate microbial amplicon sequencing with synthetic long reads. Microbiome 2021; 9: 130. doi: 10.1186/s40168-021-01072-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhang T, Li H, Ma S, et al. The newest Oxford Nanopore R10.4.1 full-length 16S rRNA sequencing enables the accurate resolution of species-level microbial community profiling. Appl Environ Microbiol 2023; 89: e0060523. doi: 10.1128/aem.00605-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Athanasopoulou K, Boti MA, Adamopoulos PG, et al. Third-generation sequencing: the spearhead towards the radical transformation of modern genomics. Life 2021; 12: 30. doi: 10.3390/life12010030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Guarro J, Gené J, Stchigel AM. Developments in fungal taxonomy. Clin Microbiol Rev 1999; 12: 454–500. doi: 10.1128/CMR.12.3.454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Schoch CL, Seifert KA, Huhndorf S, et al. Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for fungi. Proc Natl Acad Sci USA 2012; 109: 6241–6246. doi: 10.1073/pnas.1117018109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nilsson RH, Anslan S, Bahram M, et al. Mycobiome diversity: high-throughput sequencing and identification of fungi. Nat Rev Microbiol 2019; 17: 95–109. doi: 10.1038/s41579-018-0116-y [DOI] [PubMed] [Google Scholar]
- 98.Nilsson RH, Kristiansson E, Ryberg M, et al. Intraspecific ITS variability in the kingdom Fungi as expressed in the international sequence databases and its implications for molecular species identification. Evol Bioinform Online 2008; 4: 193–201. doi: 10.4137/EBO.S653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ali N, Mac Aogáin M, Morales RF, et al. Optimisation and benchmarking of targeted amplicon sequencing for mycobiome analysis of respiratory specimens. Int J Mol Sci 2019; 20: 4991. doi: 10.3390/ijms20204991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mac Aogáin M, Chaturvedi V, Chotirmall SH. MycopathologiaGENOMES: the new “Home” for the Publication of fungal genomes. Mycopathologia 2019; 184: 551–554. doi: 10.1007/s11046-019-00366-3 [DOI] [PubMed] [Google Scholar]
- 101.Nash AK, Auchtung TA, Wong MC, et al. The gut mycobiome of the Human Microbiome Project healthy cohort. Microbiome 2017; 5: 153. doi: 10.1186/s40168-017-0373-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Abarenkov K, Nilsson RH, Larsson KH, et al. The UNITE database for molecular identification and taxonomic communication of fungi and other eukaryotes: sequences, taxa and classifications reconsidered. Nucleic Acids Res 2024; 52: D791–D797. doi: 10.1093/nar/gkad1039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rivers AR, Weber KC, Gardner TG, et al. ITSxpress: software to rapidly trim internally transcribed spacer sequences with quality scores for marker gene analysis. F1000Res 2018; 7: 1418. doi: 10.12688/f1000research.15704.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Callahan BJ, McMurdie PJ, Rosen MJ, et al. DADA2: high-resolution sample inference from Illumina amplicon data. Nat Methods 2016; 13: 581–583. doi: 10.1038/nmeth.3869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Nguyen NH, Song Z, Bates ST, et al. FUNGuild: an open annotation tool for parsing fungal community datasets by ecological guild. Fungal Ecology 2016; 20: 241–248. doi: 10.1016/j.funeco.2015.06.006 [DOI] [Google Scholar]
- 106.Liu YX, Qin Y, Chen T, et al. A practical guide to amplicon and metagenomic analysis of microbiome data. Protein Cell 2021; 12: 315–330. doi: 10.1007/s13238-020-00724-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mac Aogáin M, Narayana JK, Tiew PY, et al. Integrative microbiomics in bronchiectasis exacerbations. Nat Med 2021; 27: 688–699. doi: 10.1038/s41591-021-01289-7 [DOI] [PubMed] [Google Scholar]
- 108.Narayana JK, Mac Aogáin M, Goh WWB, et al. Mathematical-based microbiome analytics for clinical translation. Comput Struct Biotechnol J 2021; 19: 6272–6281. doi: 10.1016/j.csbj.2021.11.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mostacci N, Wüthrich TM, Siegwald L, et al. Informed interpretation of metagenomic data by StrainPhlAn enables strain retention analyses of the upper airway microbiome. mSystems 2023; 8: e0072423. doi: 10.1128/msystems.00724-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mac Aogáin M, Lau KJX, Cai Z, et al. Metagenomics reveals a core macrolide resistome related to microbiota in chronic respiratory disease. Am J Respir Crit Care Med 2020; 202: 433–447. doi: 10.1164/rccm.201911-2202OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Pailhoriès H, Herrmann JL, Velo-Suarez L, et al. Antibiotic resistance in chronic respiratory diseases: from susceptibility testing to the resistome. Eur Respir Rev 2022; 31: 210259. doi: 10.1183/16000617.0259-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Taylor SL, Leong LEX, Mobegi FM, et al. Understanding the impact of antibiotic therapies on the respiratory tract resistome: a novel pooled-template metagenomic sequencing strategy. Multidiscip Respir Med 2018; 13: Suppl 1, 30. doi: 10.1186/s40248-018-0140-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mac Aogáin M, Xaverius Ivan F, Jaggi TK, et al. Airway “resistotypes” and clinical outcomes in bronchiectasis. Am J Respir Crit Care Med 2024; 210: 47–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Dominic C, Pye HV, Mishra EK, et al. Bacteriophages for bronchiectasis: treatment of the future? Curr Opin Pulm Med 2024; 30: 235–242. doi: 10.1097/MCP.0000000000001050 [DOI] [PubMed] [Google Scholar]
- 115.Xie Z, Canalda-Baltrons A, d'Enfert C, et al. Shotgun metagenomics reveals interkingdom association between intestinal bacteria and fungi involving competition for nutrients. Microbiome 2023; 11: 275. doi: 10.1186/s40168-023-01693-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Usyk M, Peters BA, Karthikeyan S, et al. Comprehensive evaluation of shotgun metagenomics, amplicon sequencing, and harmonization of these platforms for epidemiological studies. Cell Rep Methods 2023; 3: 100391. doi: 10.1016/j.crmeth.2022.100391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sulaiman I, Wu BG, Li Y, et al. Functional lower airways genomic profiling of the microbiome to capture active microbial metabolism. Eur Respir J 2021; 58: 2003434. doi: 10.1183/13993003.03434-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sulaiman I, Chung M, Angel L, et al. Microbial signatures in the lower airways of mechanically ventilated COVID-19 patients associated with poor clinical outcome. Nat Microbiol 2021; 6: 1245–1258. doi: 10.1038/s41564-021-00961-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Rogers GB, Shaw D, Marsh RL, et al. Respiratory microbiota: addressing clinical questions, informing clinical practice. Thorax 2015; 70: 74–81. doi: 10.1136/thoraxjnl-2014-205826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Richardson H, Dicker AJ, Barclay H, et al. The microbiome in bronchiectasis. Eur Respir Rev 2019; 28: 190048. doi: 10.1183/16000617.0048-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chotirmall SH, Gellatly SL, Budden KF, et al. Microbiomes in respiratory health and disease: an Asia-Pacific perspective. Respirology 2017; 22: 240–250. doi: 10.1111/resp.12971 [DOI] [PubMed] [Google Scholar]
- 122.Chotirmall SH, Bogaert D, Chalmers JD, et al. Therapeutic targeting of the respiratory microbiome. Am J Respir Crit Care Med 2022; 206: 535–544. doi: 10.1164/rccm.202112-2704PP [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Rogers GB, van der Gast CJ, Cuthbertson L, et al. Clinical measures of disease in adult non-CF bronchiectasis correlate with airway microbiota composition. Thorax 2013; 68: 731–737. doi: 10.1136/thoraxjnl-2012-203105 [DOI] [PubMed] [Google Scholar]
- 124.Dicker AJ, Lonergan M, Keir HR, et al. The sputum microbiome and clinical outcomes in patients with bronchiectasis: a prospective observational study. Lancet Respir Med 2021; 9: 885–896. doi: 10.1016/S2213-2600(20)30557-9 [DOI] [PubMed] [Google Scholar]
- 125.Woo TE, Lim R, Heirali AA, et al. A longitudinal characterization of the non-cystic fibrosis bronchiectasis airway microbiome. Sci Rep 2019; 9: 6871. doi: 10.1038/s41598-019-42862-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Tunney MM, Einarsson GG, Wei L, et al. Lung microbiota and bacterial abundance in patients with bronchiectasis when clinically stable and during exacerbation. Am J Respir Crit Care Med 2013; 187: 1118–1126. doi: 10.1164/rccm.201210-1937OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Rofael SAD, Brown J, Lipman MCI, et al. Impact of prophylactic and “rescue pack” antibiotics on the airway microbiome in chronic lung disease. BMJ Open Respir Res 2023; 10: e001335. doi: 10.1136/bmjresp-2022-001335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lee SH, Lee Y, Park JS, et al. Characterization of microbiota in bronchiectasis patients with different disease severities. J Clin Med 2018; 7: 429. doi: 10.3390/jcm7110429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Rogers GB, Zain NM, Bruce KD, et al. A novel microbiota stratification system predicts future exacerbations in bronchiectasis. Ann Am Thorac Soc 2014; 11: 496–503. doi: 10.1513/AnnalsATS.201310-335OC [DOI] [PubMed] [Google Scholar]
- 130.Rogers GB, Bruce KD, Martin ML, et al. The effect of long-term macrolide treatment on respiratory microbiota composition in non-cystic fibrosis bronchiectasis: an analysis from the randomised, double-blind, placebo-controlled BLESS trial. Lancet Respir Med 2014; 2: 988–996. doi: 10.1016/S2213-2600(14)70213-9 [DOI] [PubMed] [Google Scholar]
- 131.Shteinberg M, Chalmers JD, Narayana JK, et al. Bronchiectasis with chronic rhinosinusitis is associated with eosinophilic airway inflammation and is distinct from asthma. Ann Am Thorac Soc 2024; 21: 748–758. doi: 10.1513/AnnalsATS.202306-551OC [DOI] [PubMed] [Google Scholar]
- 132.Li L, Mac Aogáin M, Xu T, et al. Neisseria species as pathobionts in bronchiectasis. Cell Host Microbe 2022; 30: 1311–1327.e8. doi: 10.1016/j.chom.2022.08.005 [DOI] [PubMed] [Google Scholar]
- 133.Guan WJ, Yuan JJ, Li HM, et al. Altered community compositions of Proteobacteria in adults with bronchiectasis. Int J Chron Obstruct Pulmon Dis 2018; 13: 2173–2182. doi: 10.2147/COPD.S159335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Byun MK, Chang J, Kim HJ, et al. Differences of lung microbiome in patients with clinically stable and exacerbated bronchiectasis. PLoS One 2017; 12: e0183553. doi: 10.1371/journal.pone.0183553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Cox MJ, Turek EM, Hennessy C, et al. Longitudinal assessment of sputum microbiome by sequencing of the 16S rRNA gene in non-cystic fibrosis bronchiectasis patients. PLoS One 2017; 12: e0170622. doi: 10.1371/journal.pone.0170622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Choo JM, Abell GCJ, Thomson R, et al. Impact of long-term erythromycin therapy on the oropharyngeal microbiome and resistance gene reservoir in non-cystic fibrosis bronchiectasis. mSphere 2018; 3: e00103-18. doi: 10.1128/mSphere.00103-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.van der Gast CJ, Cuthbertson L, Rogers GB, et al. Three clinically distinct chronic pediatric airway infections share a common core microbiota. Ann Am Thorac Soc 2014; 11: 1039–1048. doi: 10.1513/AnnalsATS.201312-456OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Narayana JK, Aliberti S, Mac Aogáin M, et al. Microbial dysregulation of the gut–lung axis in bronchiectasis. Am J Respir Crit Care Med 2023; 207: 908–920. doi: 10.1164/rccm.202205-0893OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Carvalho FGDS, Comas TZ, Pons LR, et al. Microbiome characterization in patients with bronchiectasis and its association with severity and bronchial inflammation. Bronchi-Omics Project. Eur Respir J 2022; 60: Suppl. 66, 4594. doi: 10.1183/13993003.congress-2022.4594 [DOI] [Google Scholar]
- 140.Huang JT, Cant E, Keir HR, et al. Endotyping COPD, bronchiectasis and the “COPD–bronchiectasis association”. Am J Respir Crit Care Med 2022; 206: 417–426. doi: 10.1164/rccm.202108-1943OC [DOI] [PubMed] [Google Scholar]
- 141.Taylor SL, Rogers GB, Chen AC, et al. Matrix metalloproteinases vary with airway microbiota composition and lung function in non-cystic fibrosis bronchiectasis. Ann Am Thorac Soc 2015; 12: 701–707. doi: 10.1513/AnnalsATS.201411-513OC [DOI] [PubMed] [Google Scholar]
- 142.Cassidy PM, Hedberg K, Saulson A, et al. Nontuberculous mycobacterial disease prevalence and risk factors: a changing epidemiology. Clin Infect Dis 2009; 49: e124–e129. doi: 10.1086/648443 [DOI] [PubMed] [Google Scholar]
- 143.Sulaiman I, Wu BG, Li Y, et al. Evaluation of the airway microbiome in nontuberculous mycobacteria disease. Eur Respir J 2018; 52: 1800810. doi: 10.1183/13993003.00810-2018 [DOI] [PubMed] [Google Scholar]
- 144.Mac Aogáin M, Jaggi TK, Chotirmall SH. The airway microbiome: present and future applications. Arch Bronconeumol 2022; 58: 8–10. doi: 10.1016/j.arbres.2021.08.003 [DOI] [PubMed] [Google Scholar]
- 145.Chotirmall SH, Martin-Gomez MT. Aspergillus species in bronchiectasis: challenges in the cystic fibrosis and non-cystic fibrosis airways. Mycopathologia 2018; 183: 45–59. doi: 10.1007/s11046-017-0143-7 [DOI] [PubMed] [Google Scholar]
- 146.Tiew PY, Mac Aogáin M, Ali NABM, et al. The mycobiome in health and disease: emerging concepts, methodologies and challenges. Mycopathologia 2020; 185: 207–231. doi: 10.1007/s11046-019-00413-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Gusareva ES, Acerbi E, Lau KJX, et al. Microbial communities in the tropical air ecosystem follow a precise diel cycle. Proc Natl Acad Sci USA 2019; 116: 23299–23308. doi: 10.1073/pnas.1908493116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Knutsen AP, Bush RK, Demain JG, et al. Fungi and allergic lower respiratory tract diseases. J Allergy Clin Immunol 2012; 129: 280–291. doi: 10.1016/j.jaci.2011.12.970 [DOI] [PubMed] [Google Scholar]
- 149.Chen SC, Meyer W, Pashley CH. Challenges in laboratory detection of fungal pathogens in the airways of cystic fibrosis patients. Mycopathologia 2018; 183: 89–100. doi: 10.1007/s11046-017-0150-8 [DOI] [PubMed] [Google Scholar]
- 150.van de Veerdonk FL, Gresnigt MS, Romani L, et al. Aspergillus fumigatus morphology and dynamic host interactions. Nat Rev Microbiol 2017; 15: 661–674. doi: 10.1038/nrmicro.2017.90 [DOI] [PubMed] [Google Scholar]
- 151.Romani L. Immunity to fungal infections. Nat Rev Immunol 2011; 11: 275–288. doi: 10.1038/nri2939 [DOI] [PubMed] [Google Scholar]
- 152.Tiew PY, Lim AYH, Keir HR, et al. High frequency of allergic bronchopulmonary aspergillosis in bronchiectasis-COPD overlap. Chest 2022; 161: 40–53. doi: 10.1016/j.chest.2021.07.2165 [DOI] [PubMed] [Google Scholar]
- 153.Cuthbertson L, Felton I, James P, et al. The fungal airway microbiome in cystic fibrosis and non-cystic fibrosis bronchiectasis. J Cyst Fibros 2021; 20: 295–302. doi: 10.1016/j.jcf.2020.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Soret P, Vandenborght LE, Francis F, et al. Respiratory mycobiome and suggestion of inter-kingdom network during acute pulmonary exacerbation in cystic fibrosis. Sci Rep 2020; 10: 3589. doi: 10.1038/s41598-020-60015-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Hong G, Daniel SG, Lee JJ, et al. Distinct community structures of the fungal microbiome and respiratory health in adults with cystic fibrosis. J Cyst Fibros 2023; 22: 636–643. doi: 10.1016/j.jcf.2023.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Willger SD, Grim SL, Dolben EL, et al. Characterization and quantification of the fungal microbiome in serial samples from individuals with cystic fibrosis. Microbiome 2014; 2: 40. doi: 10.1186/2049-2618-2-40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kramer R, Sauer-Heilborn A, Welte T, et al. Cohort study of airway mycobiome in adult cystic fibrosis patients: differences in community structure between fungi and bacteria reveal predominance of transient fungal elements. J Clin Microbiol 2015; 53: 2900–2907. doi: 10.1128/JCM.01094-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Mac Aogáin M, Vidaillac C, Chotirmall SH. Fungal Infections and ABPA. In: Davis SD, Rosenfeld M, Chmiel J, eds. Cystic Fibrosis: A Multi-Organ System Approach. Cham, Springer International Publishing, 2020; pp. 93–126. [Google Scholar]
- 159.Breuer O, Schultz A, Garratt LW, et al. Infections and progression of structural lung disease in children with cystic fibrosis. Am J Respir Crit Care Med 2020; 201: 688–696. doi: 10.1164/rccm.201908-1585OC [DOI] [PubMed] [Google Scholar]
- 160.Coughlan CA, Chotirmall SH, Renwick J, et al. The effect of Aspergillus fumigatus infection on vitamin D receptor expression in cystic fibrosis. Am J Respir Crit Care Med 2012; 186: 999–1007. doi: 10.1164/rccm.201203-0478OC [DOI] [PubMed] [Google Scholar]
- 161.Tiew PY, Dicker AJ, Keir HR, et al. A high-risk airway mycobiome is associated with frequent exacerbation and mortality in COPD. Eur Respir J 2021; 57: 2002050. doi: 10.1183/13993003.02050-2020 [DOI] [PubMed] [Google Scholar]
- 162.Tiew PY, Ko FWS, Pang SL, et al. Environmental fungal sensitisation associates with poorer clinical outcomes in COPD. Eur Respir J 2020; 56: 2000418. doi: 10.1183/13993003.00418-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Rajagopala SV, Bakhoum NG, Pakala SB, et al. Metatranscriptomics to characterize respiratory virome, microbiome, and host response directly from clinical samples. Cell Rep Methods 2021; 1: 100091. doi: 10.1016/j.crmeth.2021.100091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Chiu CY, Miller SA. Clinical metagenomics. Nat Rev Genet 2019; 20: 341–355. doi: 10.1038/s41576-019-0113-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Liang G, Bushman FD. The human virome: assembly, composition and host interactions. Nat Rev Microbiol 2021; 19: 514–527. doi: 10.1038/s41579-021-00536-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Footitt J, Mallia P, Durham AL, et al. Oxidative and nitrosative stress and histone deacetylase-2 activity in exacerbations of COPD. Chest 2016; 149: 62–73. doi: 10.1378/chest.14-2637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Molyneaux PL, Mallia P, Cox MJ, et al. Outgrowth of the bacterial airway microbiome after rhinovirus exacerbation of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2013; 188: 1224–1231. doi: 10.1164/rccm.201302-0341OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Mitchell AB, Oliver BG, Glanville AR. Translational aspects of the human respiratory virome. Am J Respir Crit Care Med 2016; 194: 1458–1464. doi: 10.1164/rccm.201606-1278CI [DOI] [PubMed] [Google Scholar]
- 169.Santiago-Rodriguez TM, Hollister EB. Human virome and disease: high-throughput sequencing for virus discovery, identification of phage-bacteria dysbiosis and development of therapeutic approaches with emphasis on the human gut. Viruses 2019; 11: 656. doi: 10.3390/v11070656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Layeghifard M, Li H, Wang PW, et al. Microbiome networks and change-point analysis reveal key community changes associated with cystic fibrosis pulmonary exacerbations. NPJ Biofilms Microbiomes 2019; 5: 4. doi: 10.1038/s41522-018-0077-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Layeghifard M, Hwang DM, Guttman DS. Disentangling interactions in the microbiome: a network perspective. Trends Microbiol 2017; 25: 217–228. doi: 10.1016/j.tim.2016.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Narayana JK, Mac Aogáin M, Ali NABM, et al. Similarity network fusion (SNF) for the integration of multi-omics and microbiomes in respiratory disease. Eur Respir J 2021; 58: 2101016. doi: 10.1183/13993003.01016-2021 [DOI] [PubMed] [Google Scholar]
- 173.Carr VR, Shkoporov A, Hill C, et al. Probing the mobilome: discoveries in the dynamic microbiome. Trends Microbiol 2021; 29: 158–170. doi: 10.1016/j.tim.2020.05.003 [DOI] [PubMed] [Google Scholar]
- 174.Aliberti S, Polverino E, Chalmers JD, et al. The European Multicentre Bronchiectasis Audit and Research Collaboration (EMBARC) ERS clinical research collaboration. Eur Respir J 2018; 52: 1802074. doi: 10.1183/13993003.02074-2018 [DOI] [PubMed] [Google Scholar]
- 175.Martinez-García MA, Villa C, Dobarganes Y, et al. RIBRON: the Spanish online bronchiectasis registry. Characterization of the first 1912 patients. Arch Bronconeumol 2021; 57: 28–35. doi: 10.1016/j.arbr.2020.11.010 [DOI] [PubMed] [Google Scholar]
- 176.Yi X-Z, Yang J-H, Huang Y, et al. Differential airway resistome and its correlations with clinical characteristics in Haemophilus- or Pseudomonas-predominant microbial subtypes of bronchiectasis. Respir Res 2023; 24: 264. doi: 10.1186/s12931-023-02562-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Grimwood K, Laupland KB. Can microbiologists and infectious diseases physicians contribute to the management of bronchiectasis? A view from Down Under. J Assoc Med Microbiol Infect Dis Can 2023; 8: 161–164. doi: 10.3138/jammi-2023-07-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Serisier DJ, Bilton D, De Soyza A, et al. Inhaled, dual release liposomal ciprofloxacin in non-cystic fibrosis bronchiectasis (ORBIT-2): a randomised, double-blind, placebo-controlled trial. Thorax 2013; 68: 812–817. doi: 10.1136/thoraxjnl-2013-203207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Haworth CS, Bilton D, Chalmers JD, et al. Inhaled liposomal ciprofloxacin in patients with non-cystic fibrosis bronchiectasis and chronic lung infection with Pseudomonas aeruginosa (ORBIT-3 and ORBIT-4): two phase 3, randomised controlled trials. Lancet Respir Med 2019; 7: 213–226. doi: 10.1016/S2213-2600(18)30427-2 [DOI] [PubMed] [Google Scholar]
- 180.Chotirmall SH, Chalmers JD. RESPIRE: breathing new life into bronchiectasis. Eur Respir J 2018; 51: 1702444. doi: 10.1183/13993003.02444-2017 [DOI] [PubMed] [Google Scholar]
- 181.Cheng R, Wang L, Le S, et al. A randomized controlled trial for response of microbiome network to exercise and diet intervention in patients with nonalcoholic fatty liver disease. Nat Commun 2022; 13: 2555. doi: 10.1038/s41467-022-29968-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Ubeda C, Pamer EG. Antibiotics, microbiota, and immune defense. Trends Immunol 2012; 33: 459–466. doi: 10.1016/j.it.2012.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Taylor SL, Leong LEX, Mobegi FM, et al. Long-term azithromycin reduces Haemophilus influenzae and increases antibiotic resistance in severe asthma. Am J Respir Crit Care Med 2019; 200: 309–317. doi: 10.1164/rccm.201809-1739OC [DOI] [PubMed] [Google Scholar]
- 184.Avis T, Wilson FX, Khan N, et al. Targeted microbiome-sparing antibiotics. Drug Discov Today 2021; 26: 2198–2203. doi: 10.1016/j.drudis.2021.07.016 [DOI] [PubMed] [Google Scholar]
- 185.Chou S, Zhang S, Guo H, et al. Targeted antimicrobial agents as potential tools for modulating the gut microbiome. Front Microbiol 2022; 13: 879207. doi: 10.3389/fmicb.2022.879207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Garcia-Olivé I, Radua J, Sánchez-Berenguer D, et al. Association between environmental factors and hospitalisations for bronchiectasis in Badalona, Barcelona, Spain (2007–2015). Med Clin 2018; 150: 257–261. doi: 10.1016/j.medcli.2017.06.022 [DOI] [PubMed] [Google Scholar]
- 187.Goeminne PC, Cox B, Finch S, et al. The impact of acute air pollution fluctuations on bronchiectasis pulmonary exacerbation: a case-crossover analysis. Eur Respir J 2018; 52: 1702557. doi: 10.1183/13993003.02557-2017 [DOI] [PubMed] [Google Scholar]
- 188.Kapur N, Petsky HL, Bell S, et al. Inhaled corticosteroids for bronchiectasis. Cochrane Database Syst Rev 2018; 5: CD000996. doi: 10.1002/14651858.CD000996.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Pavord ID, Chanez P, Criner GJ, et al. Mepolizumab for eosinophilic chronic obstructive pulmonary disease. N Engl J Med 2017; 377: 1613–1629. doi: 10.1056/NEJMoa1708208 [DOI] [PubMed] [Google Scholar]
- 190.Kayongo A, Robertson NM, Siddharthan T, et al. Airway microbiome–immune crosstalk in chronic obstructive pulmonary disease. Front Immunol 2022; 13: 1085551. doi: 10.3389/fimmu.2022.1085551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Wu BG, Sulaiman I, Tsay JJ, et al. Episodic aspiration with oral commensals induces a MyD88-dependent, pulmonary T-helper cell type 17 response that mitigates susceptibility to Streptococcus pneumoniae. Am J Respir Crit Care Med 2021; 203: 1099–1111. doi: 10.1164/rccm.202005-1596OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Opron K, Begley LA, Erb-Downward JR, et al. Loss of airway phylogenetic diversity is associated with clinical and pathobiological markers of disease development in COPD. Am J Respir Crit Care Med 2024: in press [ 10.1164/rccm.202303-0489OC] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Meldrum OW, Donaldson GC, Narayana JK, et al. Accelerated lung function decline and mucus-microbe evolution in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2024: in press [ 10.1164/rccm.202306-1060OC] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Xu K, Diaz AA, Duan F, et al. Bronchial gene expression alterations associated with radiological bronchiectasis. Eur Respir J 2023; 61: 2200120. doi: 10.1183/13993003.00120-2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERR-0038-2024.SUPPLEMENT (212.6KB, pdf)