Abstract
Background
The rise of extended-spectrum β-lactamase-producing Enterobacterales (ESBL-E) in low- and middle-income countries limits treatment options, leading to the frequent use of broad-spectrum antibiotics. Reducing time-to-result for a urinary infection can facilitate correct antibiotic treatment and support antimicrobial and diagnostic stewardship measures. This study compared two simplified enrichment methods for detecting CTX-M directly from urine specimens.
Methods
Two enrichment methods, namely centrifugation of 2 mL urine and filtration of 1 mL urine using the DirecTool adaptor, were compared using 20 culture-positive urine samples (20 suspected ESBL-E and 20 non-ESBL-E). CTX-M production was detected using a lateral flow assay (LFA), NG-Test® CTX-MMULTI. The presence of blaCTX-M genes was confirmed by whole-genome sequencing (WGS).
Results
The results of both enrichment methods were identical, with a sensitivity of 87.5% and a specificity of 100%. In 19/20 (95%) of the urine samples, the results of the CTX-M LFA were identical with the phenotypic confirmation and WGS. Both methods could detect ESBL-E bacteriuria with ≥104 cfu/mL. All ESBL-E-negative samples were identified accurately. Both enrichment methods yielded negative results in one ESBL-E-positive (CTX-M-15) sample despite phenotypic and genotypic confirmation of ESBL production. High leukocyte count (>500 cells/µL), the presence of boric acid or polymicrobial samples did not appear to impact the performance of both enrichment methods.
Conclusions
Our study underscores the feasibility of directly detecting CTX-M in urine. Simplified enrichment methods, particularly with a filtration kit, enhance the assay’s practicality, rendering it suitable for use in primary care, emergency departments or remote laboratories without sophisticated equipment.
Background
Antimicrobial resistance (AMR) is an ongoing global challenge, with the greatest impact in low- and middle-income countries (LMICs).1,2 Overuse of broad-spectrum antibiotics has been identified as a major catalyst for the emergence and spread of AMR in LMICs. Although efforts are being made to implement antimicrobial stewardship (AMS) measures to curb overuse and discourage unwarranted antibiotic prescribing, the effectiveness of AMS programmes is hampered by the lack of rapid and reliable microbiological diagnostics.3,4
Urinary tract infections (UTIs) are one of the most common causes of infection and account for a significant proportion of antibiotic use worldwide.5,6 With the emergence and spread of extended-spectrum β-lactamase-producing Enterobacterales (ESBL-E) in LMICs, the treatment options are limited, and physicians often resort to broad-spectrum antibiotics to anticipate the high local or regional prevalence of drug-resistant bacteria.6,7 The prompt initiation of correct antibiotic therapy is critical in febrile UTIs.8 In LMICs, as ESBL-producing Escherichia coli or Klebsiella pneumoniae are commonly encountered as uropathogens, physicians often rely on their experience or local epidemiology to select a treatment choice that is likely to be effective, leading to a tendency to overprescribe broad-spectrum antibiotics as a precaution.9
Expediting the time-to-result for urine diagnostics can significantly assist physicians in making informed decisions regarding antibiotic prescriptions.3 Point-of-care diagnostic tests (POCTs), specifically using a lateral flow assay (LFA) to identify CTX-M beta-lactamases, a predominant mechanism underlying the ESBL phenotype in E. coli, directly from urine specimens, have been proposed for this purpose.10–12 However, this approach may involve enriching or pre-incubating 10 mL urine samples, demanding sophisticated instrumentation and workflow, including more hands-on time to process the samples, thus hampering the POCT approach.11 In this study, our objective was to explore whether the procedure for detecting ESBL-E directly from urine specimens can be simplified for use in resource-limited settings. As a proof-of-principle, we conducted a head-to-head comparison of two enrichment methods, employing centrifugation and the DirecTool adapter filtration system.
Methods
Collection of clinical urine specimens
Rest clinical urine specimens were collected from the routine microbiology laboratory. Twenty of the urine samples with suspected ESBL-E, defined as resistance to penicillins, second- and third-generation cephalosporins, and 20 non-ESBL-E urine samples were chosen at random. The specimens were stored at 4°C–8°C after inoculation and collected for this study after 24–48 h. The detailed procedures of the routine microbiological diagnostic (culture, species identification, AST, determination of leukocyte count) as well as the sample size calculation for this proof-of-principle study are provided in the Supplementary Appendix (available as Supplementary data at JAC-AMR Online).
Direct testing for CTX-M from urine specimens
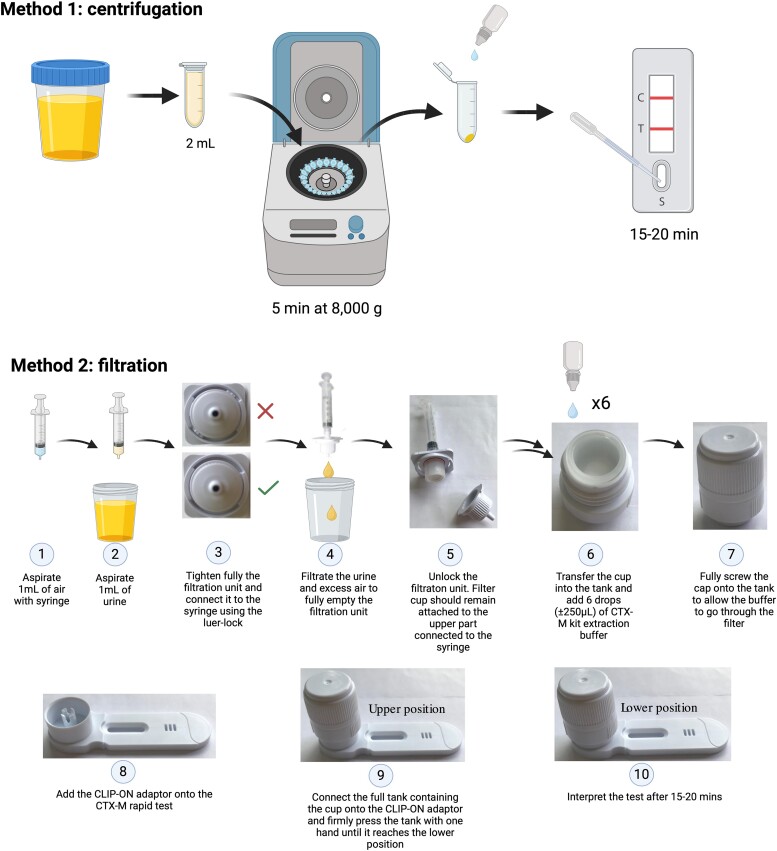
The aim of this study was to compare two different methods of preparation, by centrifugation and filtration, for the detection of CTX-M in urine samples (Figure 1). In the centrifugation approach, 2 mL of urine was centrifuged at 12 000 rpm (±8000 g, Eppendorf® MiniSpin) for 5 min, and the resulting supernatant was discarded. Approximately 150 µL of NG-Test® extraction buffer was added to the pellet, followed by vortexing to mix thoroughly. A 100 µL suspension of this mixture was immediately added to the sample well of the LFA cassette, and the results were interpreted after the control band became visible (±15–20 min). The filtration using NG-Test ® DirecTool adapter was performed according to the manufacturer’s protocol with 1 mL urine (Figure 1).
Figure 1.
Schematic diagram of the urine processing methods to detect ESBL directly from urine specimen using NG-Test® CTX-M MULTI LFA. For the centrifugation method, the urine sample in a 2 mL reaction tube was centrifuged using Eppendorf® MiniSpin at 12 000 rpm (±8000 g). The filtration method was performed using the DirecTool CLIP-ON adaptor. Illustrations for method 2 were adapted from the protocol provided by NG Biotech. Created on Biorender®.
Whole-genome sequencing
DNA was extracted using DNeasy Blood and Tissue Kit (Qiagen GmbH, Germany), and library preparation (Illumina DNA prep kit) was performed following the manufacturer’s instructions. Sequencing was done on a MiSeq Illumina platform (short-read sequencing, 2 × 300 bp). Post-sequencing quality checks and assembly were performed as described previously.13 Draft genomes were checked for AMR genes using Abricate with NCBI, CARD, ARG-ANNOT, ResFinder and MEGARES databases.
Statistical analysis
Descriptive statistics and performance of diagnostic tests (specificity and sensitivity) were analysed using Stata18 (StataCorp).
Ethical clearance
The use of leftover samples for routine microbiological diagnostics did not require additional individual consent. The ethical committee of the University of Lübeck was consulted and waived individual consent (2022-620).
Results
We randomly selected 40 culture-positive urine samples with suspected ESBL (n = 20) and non-ESBL Enterobacterales (n = 20) for this study. The molecular detection of blaCTX-M by WGS was considered the true diagnosis for assessing the performance of both enrichment methods. One E. coli isolate (UR2) could not be recultured on the ESBL selective medium for WGS. Overall, the CTX-M MULTI LFA showed concordance with the microbiological culture in 38 out of 40 samples (95%). Of the 20 suspected ESBL producers, 17 were identified as E. coli, two as K. pneumoniae and one as Citrobacter freundii. WGS analysis showed that ST131 and ST88 were the predominant sequence types; six E. coli strains belonged to sequence type 131 (ST131) and two to ST88 (Table 1). Sixteen isolates harboured blaCTX-M genes. The predominant ESBL gene detected was blaCTX-M-15 (11/16, 68.8%), followed by blaCTX-M-1 (3/16, 18.8%). Other CTX-M genes detected were blaCTX-M-27 and blaCTX-M-65 (1/16, 6.3% each). Other antibiotic resistance genes are summarized in Supplementary Table S1.
Table 1.
Overview of urine specimen and rapid test performance to detect CTX-M
| NR | Sample type | Leukocyte count (per µL) | Polymicrobial | Enterobacteralesa | Other species | NG-Test® CTX-M MULTI | ESBL confirmation (genotypic and phenotypic) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| species | quantification | ESBL phenotypeb | centrifugation | filtration | direct from colony | growth on ESBL plate | WGS/CTX-M | MLST | ||||||
| suspected ESBL | UR1 | urine | <10 | yes | E. coli | 10^6 cfu/ml | yes | Enterococcus faecalis, Acinetobacter species | pos | pos | pos | yes | CTX-M 15 | ST131 |
| UR2 | catheter urine | 10–25 | no | E. coli | 10^4 cfu/ml | yes | — | pos | pos | n/a | n/a | n/a | n/a | |
| UR3 | urine (bladder puncture) | <10 | no | E. coli | 10^4 cfu/ml | yes | — | pos | pos | pos | yes | CTX-M 27 | ST131 | |
| UR4 | midstream urine | 10–25 | yes | E. coli | 10^4 cfu/ml | yes | E. faecalis | pos | pos | pos | yes | CTX-M 15 | ST131 | |
| UR5 | catheter urine | >500 | yes | E. coli | 10^6 cfu/ml | yes | E. faecalis, Streptococcus anginosus | pos | pos | pos | yes | CTX-M 15 | ST131 | |
| UR6 | catheter urine | >500 | no | Citrobacter braakii | 10^4 cfu/ml | yes | — | neg | neg | neg | yes | no CTX-M detected, CMY 101 | ST561 | |
| UR7 | midstream urine | >500 | no | E. coli | 10^6 cfu/ml | yes | — | pos | pos | pos | yes | CTX-M 1 | ST88* | |
| UR8 | midstream urine | 10–25 | no | E. coli | 10^5 cfu/ml | yes | — | negc | neg | pos | yes | CTX-M 15 | ST131 | |
| UR9 | catheter urine | 75 | yes | E. coli | 10^5 cfu/ml | yes | Aerococcus urinae | pos | pos | pos | yes | CTX-M 15 | ST349 | |
| UR10 | midstream urine | >500 | yes | E. coli | 10^6 cfu/ml | yes | urogenital flora | pos | pos | pos | yes | CTX-M 1 | ST88 | |
| UR11 | catheter urine | 10–25 | yes | E. coli | 10^6 cfu/ml | yes | urogenital flora | pos | pos | pos | yes | CTX-M 15 | ST131 | |
| UR12 | catheter urine | 75 | yes | E. coli | 10^6 cfu/ml | yes | Klebsiella pneumoniae, Candida krusei | neg | neg | neg | yes | no CTX-M detected | ST131-1LV | |
| UR13 | midstream urine | >500 | no | E. coli | 10^6 cfu/ml | yes | pos | pos | pos | yes | CTX-M 1 | ST2015 | ||
| UR14 | midstream urine | 75 | yes | E. coli | 10^6 cfu/ml | yes | urogenital flora | neg | neg | neg | yes | no CTX-M detected | ST167 | |
| UR15 | catheter urine | >500 | yes | K. pneumoniae | 10^6 cfu/ml | yes | Morganella morganii | negc | neg | weak pos | yes | CTX-M 15 | ST432 | |
| UR16 | midstream urine | >500 | yes | E. coli | 10^6 cfu/ml | yes | urogenital flora | pos | pos | pos | yes | CTX-M 65 | ST69 | |
| UR17 | midstream urine | <10 | yes | E. coli | 10^6 cfu/ml | yes | Aerococcus urinae | pos | pos | pos | yes | CTX-M 15 | ST4891 | |
| UR18 | midstream urine | >500 | yes | K. pneumoniae | 10^5 cfu/ml | yes | Staphylococcus epidermidis | pos | pos | pos | yes | CTX-M 15 | ST1662 | |
| UR19 | catheter urine | <10 | no | E. coli | 10^6 cfu/ml | yes | pos | pos | pos | yes | CTX-M 15 | ST361 | ||
| UR20 | midstream urine | 75 | no | E. coli | 10^6 cfu/ml | yes | pos | pos | pos | yes | CTX-M 15 | ST1193 | ||
| non-ESBL | UR21 | midstream urine | 75 | no | E. coli | 10^4 cfu/ml | no | — | neg | neg | n/a | no | n/a | n/a |
| UR22 | midstream urine | 10–25 | no | E. coli | 10^6 cfu/ml | no | — | neg | neg | n/a | no | n/a | n/a | |
| UR23 | midstream urine | 75 | no | E. coli | 10^6 cfu/ml | no | — | neg | neg | n/a | no | n/a | n/a | |
| UR24 | midstream urine | 10–25 | no | E. coli | 10^6 cfu/ml | no | — | neg | neg | n/a | no | n/a | n/a | |
| UR25 | midstream urine | 75 | no | E. coli | 10^6 cfu/ml | no | — | neg | neg | n/a | no | n/a | n/a | |
| UR26 | midstream urine | 75 | no | E. coli | 10^6 cfu/ml | no | — | neg | neg | n/a | no | n/a | n/a | |
| UR27 | midstream urine | 75 | no | E. coli | 10^6 cfu/ml | no | — | neg | neg | n/a | no | n/a | n/a | |
| UR28 | midstream urine | >500 | no | E. coli | 10^6 cfu/ml | no | — | neg | neg | n/a | no | n/a | n/a | |
| UR29 | midstream urine | >500 | no | E. coli | 10^5 cfu/ml | no | — | neg | neg | n/a | no | n/a | n/a | |
| UR30 | catheter urine | >500 | yes | E. coli | 10^6 cfu/ml | no | Enterococcus faecalis | neg | neg | n/a | no | n/a | n/a | |
| UR31 | catheter urine | 75 | no | E. coli | 10^6 cfu/ml | no | — | neg | neg | n/a | no | n/a | n/a | |
| UR32 | midstream urine | >500 | no | E. coli | 10^5 cfu/ml | no | — | neg | neg | n/a | no | n/a | n/a | |
| UR33 | midstream urine | <10 | yes | urogenital flora | 10^2 cfu/ml | no | — | neg | neg | n/a | no | n/a | n/a | |
| UR34 | catheter urine | >500 | yes | P. mirabilis | 10^5 cfu/ml | no | Enterococcus faecalis | neg | neg | n/a | no | n/a | n/a | |
| UR35 | midstream urine | 75 | no | E. coli | 10^5 cfu/ml | no | — | neg | neg | n/a | no | n/a | n/a | |
| UR36 | midstream urine | <10 | yes | E. coli | 10^6 cfu/ml | no | Enterobacter cloacae | neg | neg | n/a | no | n/a | n/a | |
| UR37 | catheter urine | 10–25 | yes | K. pneumoniae | 10^6 cfu/ml | no | Staphylococcus aureus | neg | neg | n/a | no | n/a | n/a | |
| UR38 | midstream urine | <10 | no | E. coli | 10^3 cfu/ml | no | urogenital flora | neg | neg | n/a | no | n/a | n/a | |
| UR39 | catheter urine | 75 | no | E. coli | 10^4 cfu/ml | no | none | neg | neg | n/a | no | n/a | n/a | |
| UR40 | catheter urine | 75 | no | E. coli | 10^6 cfu/ml | no | none | neg | neg | n/a | no | n/a | n/a | |
ESBL, extended-spectrum beta-lactamase; WGS, whole-genome sequencing; MLST, multi-locus sequence type; pos, positive; neg, negative; n/a, not applicable.
aData acquired from the routine microbiological diagnostics.
bIsolates with suspected ESBL phenotype were defined as Enterobacterales with phenotypic resistance to penicillins and second- and third-generation cephalosporins, as determined by VITEK®2.
cDiscrepant results between direct detection of CTX-M from urine samples and from bacterial colony are indicated in bold.
The NG-Test® CTX-M MULTI results showed concordance between centrifugation and filtration pre-processing. Both pre-processing methods could detect 104 cfu/mL CTX-M-producing E. coli compared to the lower detection limit of 105 cfu/mL without pre-processing using spiked urine samples (Supplementary Methods and Figure S1). Three samples suspected of harbouring ESBL-producing C. freundii (sample UR6), E. coli (sample UR8) and K. pneumoniae (UR15) gave negative results in the LFA directly from urine samples. Further analysis of the resistome from the WGS data revealed that UR6 did not harbour any blaCTX-M genes, justifying the negative LFA result. However, in the case of samples UR8 and UR15, the direct urine LFA yielded negative results, despite the presence of CTX-M-producing E. coli and positive LFA from culture (Table 1, Supplementary Figure S2). For UR15, however, the LFA from culture only produced a very weak positive band for CTX-M (Supplementary Figure S2).
Compared with microbial culture plus detection of blaCTX-M by WGS as the gold standard, the direct urine testing, irrespective of pre-treatment methods, demonstrated a sensitivity of 87.5% (95% CI: 61.7%–98.4%) and a specificity of 100% (95% CI 83.9%–100%), a positive predictive value of 100% and a negative predictive value of 91.3%. Our proof-of-principle study suggests that high leukocyte count (>500 cells/µL), the presence of boric acid or polymicrobial samples did not have an impact on the performance of both enrichment methods (Table 1).
Discussion
CTX-M detection directly from urine without enrichment has a limit of ≥105 cfu/mL but exhibits weak band intensity, posing interpretation challenges for untrained staff (Supplementary Figure S1). Tang et al.11 recommend a prior enrichment step involving centrifugation of 10 mL urine at 3000 × g for 15 min, discarding the supernatant and additional incubation at 37°C for 20 min to enhance the sensitivity. However, this contradicts the POCT principle, as it requires equipment like a centrifuge and an incubator. This approach may be suitable for healthcare settings but proves challenging in primary care, outpatient clinics and decentralized settings in LMICs, where simple and affordable diagnostics are crucial. The versatility of this assay has been demonstrated by several studies that have evaluated the performance of this assay to detect CTX-M and carbapenemases from positive blood cultures, from urine specimens and even from rectal swabs with minimal modification of a short incubation period.10,12,14 In our study, we focused on direct testing from urine specimens as we believe this use case has the greatest potential to improve antibiotic prescribing in resource-limited settings.
In a study by Volland et al., the enrichment step consisted of filtering 3 mL of urine samples with a bacterial concentration of at least 105 cfu/mL, using similar post-filtration processing as in our study. This approach demonstrated a sensitivity of 100% (95% CI: 91.19%–100%) and a specificity of 95% (95% CI: 95.6%–100%) for beta-lactamase detection.11 In our study, we observed a sensitivity of 87.5% with a specificity of 100%. A key difference in our research is that we used 1 mL of urine instead of 3 mL and included samples with a bacterial load ≥104 cfu/mL. Furthermore, our study consisted of using a filtration device (DirecTool) adapted to an already available CE-marked product (NG-Test® CTX-M MULTI), while the existing published data were obtained from a research-use-only device (BL DetecTool). Notably, our study demonstrated the ability to detect CTX-M in samples with a bacterial load of 104 cfu/mL after enrichment. This finding is particularly relevant given that bacteriuria ≥104 cfu/mL is generally considered a reliable threshold for UTI.15
Our study has certain limitations. The sample size for this comparative analysis was small (n = 20 per group), and the study was retrospective, with samples stored in the refrigerator at least overnight, which may affect the bacterial load and sensitivity of detection. This limitation is attributed to the low prevalence of ESBL-E causing UTI in our study setting. Despite these challenges, our results demonstrate that using a smaller volume (1 mL) provides reliable results. Further, our approach proved effective in detecting ESBL-producing bacteria at concentrations as low as 104 cfu/mL. The strength of our study lies in the comprehensive comparison of different methods and incorporating CTX-M gene detection by WGS. Nevertheless, our data suggest that an easy-to-use microbiological POCT may be an alternative to the cumbersome conventional culture-based diagnostics. In this study, we only evaluated the feasibility of using the CTX-M MULTI panel, but there are other panels that could detect carbapenemase and mcr genes. Due to the low prevalence (<0.5%, based on unpublished routine data) of these genes in our study population, we were unable to validate the performance of these other panels.
Conclusions
In conclusion, our study demonstrates the feasibility of direct detection of CTX-M in urine for rapid detection of ESBL-E, which is essential for the timely initiation of appropriate treatment, reducing precautionary use of broad-spectrum antibiotics and reducing selection pressure for antibiotic-resistant strains, particularly in resource-poor regions with a high burden of AMR. Simplified enrichment methods using a filtration kit increase the practicality of the assay, which can be used as a microbiological POCT on the bedside and is suitable for remote laboratories without electricity. Given the highly prevalent carbapenem resistance, it may be necessary to complement the CTX-M MULTI LFA with a rapid test for carbapenemase-producing Enterobacterales for implementation in LMICs. Larger clinical intervention studies are essential for further validation.
Supplementary Material
Acknowledgements
We thank Melanie Albrecht and Tran Thanh Tung for their assistance in performing WGS and NG Biotech for providing the NG-Test® CTX-M MULTI test kits.
Contributor Information
Dennis Nurjadi, Department of Infectious Diseases and Microbiology, University of Lübeck and University Hospital Schleswig-Holstein Campus Lübeck, Ratzeburger Allee 160, 23562 Lübeck, Germany; German Center for Infection Research (DZIF), Partner Site Hamburg-Lübeck-Borstel-Riems, Lübeck, Germany; Vietnamese German Center for Medical Research (VG-CARE), Hanoi, Vietnam.
Arnaud Chalin, NG Biotech, R&D Department, Guipry, France.
Susanne Hauswaldt, Department of Infectious Diseases and Microbiology, University of Lübeck and University Hospital Schleswig-Holstein Campus Lübeck, Ratzeburger Allee 160, 23562 Lübeck, Germany.
Linus Olson, Department of Women’s and Children’s Health, Karolinska Institutet, Stockholm, Sweden; Department of Global Public Health, Karolinska Institutet, Stockholm, Sweden; Training and Research Academic Collaboration (TRAC), Sweden, Vietnam.
Mattias Larsson, Department of Global Public Health, Karolinska Institutet, Stockholm, Sweden; Training and Research Academic Collaboration (TRAC), Sweden, Vietnam.
Åse Östholm, Department of Infectious Diseases in Region Östergötland, Linköping, Sweden; Department of Biomedical and Clinical Sciences, Linköping University, Linköping, Sweden.
Thirumalaisamy P Velavan, Vietnamese German Center for Medical Research (VG-CARE), Hanoi, Vietnam; Institute of Tropical Medicine, Universitätsklinikum Tübingen, Tübingen, Germany; Faculty of Medicine, Duy Tan University, Da Nang, Vietnam.
Sébastien Boutin, Department of Infectious Diseases and Microbiology, University of Lübeck and University Hospital Schleswig-Holstein Campus Lübeck, Ratzeburger Allee 160, 23562 Lübeck, Germany; German Center for Infection Research (DZIF), Partner Site Hamburg-Lübeck-Borstel-Riems, Lübeck, Germany; Airway Research Center North (ARCN), German Center for Lung Research (DZL), Lübeck, Germany.
Jan Rupp, Department of Infectious Diseases and Microbiology, University of Lübeck and University Hospital Schleswig-Holstein Campus Lübeck, Ratzeburger Allee 160, 23562 Lübeck, Germany; German Center for Infection Research (DZIF), Partner Site Hamburg-Lübeck-Borstel-Riems, Lübeck, Germany.
Lennart E Nilsson, Department of Biomedical and Clinical Sciences, Linköping University, Linköping, Sweden.
Håkan Hanberger, Training and Research Academic Collaboration (TRAC), Sweden, Vietnam; Department of Infectious Diseases in Region Östergötland, Linköping, Sweden; Department of Biomedical and Clinical Sciences, Linköping University, Linköping, Sweden.
Funding
NG-Test® CTX-M MULTI LFA and the DirecTool® filtration system were kindly provided by NG Biotech. This work was supported by the Federal Ministry of Education and Research (BMBF)/German Center for Infection Research (DZIF; J.R., TTU 08.824).
Transparency declarations
Test kits were provided by NG Biotech. A.C. is an employee of NG Biotech. The company was not involved in data acquisition and interpretation of the results. D.N. and T.P.V. are members of the PAN-ASEAN Coalition for Epidemic and Outbreak Preparedness (DAAD-PACE-UP project ID: 57592343). D.N. has received speaker’s honoraria from Shionogi and Cepheid outside the scope of this work. All other authors declared no conflicts of interest.
Data availability
The sequencing data were uploaded to the NCBI GenBank under the bioproject number PRJNA1101205. Accession numbers are listed in the Supplementary Table.
Supplementary data
Figures S1 and S2, Table S1 and Appendix are available as Supplementary data at JAC-AMR Online.
References
- 1. Ikhimiukor OO, Odih EE, Donado-Godoy P et al. A bottom-up view of antimicrobial resistance transmission in developing countries. Nat Microbiol 2022; 7: 757–65. 10.1038/s41564-022-01124-w [DOI] [PubMed] [Google Scholar]
- 2. Laxminarayan R, Matsoso P, Pant S et al. Access to effective antimicrobials: a worldwide challenge. Lancet 2016; 387: 168–75. 10.1016/S0140-6736(15)00474-2 [DOI] [PubMed] [Google Scholar]
- 3. Zakhour J, Haddad SF, Kerbage A et al. Diagnostic stewardship in infectious diseases: a continuum of antimicrobial stewardship in the fight against antimicrobial resistance. Int J Antimicrob Agents 2023; 62: 106816. 10.1016/j.ijantimicag.2023.106816 [DOI] [PubMed] [Google Scholar]
- 4. Holmes AH, Moore LSP, Sundsfjord A et al. Understanding the mechanisms and drivers of antimicrobial resistance. Lancet 2016; 387: 176–87. 10.1016/S0140-6736(15)00473-0 [DOI] [PubMed] [Google Scholar]
- 5. Schmiemann G, Kniehl E, Gebhardt K et al. The diagnosis of urinary tract infection: a systematic review. Dtsch Arztebl Int 2010; 107: 361–7. 10.3238/arztebl.2010.0361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Goebel MC, Trautner BW, Grigoryan L. The five ds of outpatient antibiotic stewardship for urinary tract infections. Clin Microbiol Rev 2021; 34: e0000320. 10.1128/CMR.00003-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pujades-Rodriguez M, West RM, Wilcox MH et al. Lower urinary tract infections: management, outcomes and risk factors for antibiotic re-prescription in primary care. EClinicalMedicine 2019; 14: 23–31. 10.1016/j.eclinm.2019.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Davenport M, Mach KE, Shortliffe LMD et al. New and developing diagnostic technologies for urinary tract infections. Nat Rev Urol 2017; 14: 296–310. 10.1038/nrurol.2017.20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang D, Liu C, Zhang X et al. Does diagnostic uncertainty increase antibiotic prescribing in primary care? NPJ Prim Care Respir Med 2021; 31: 17. 10.1038/s41533-021-00229-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Volland H, Balleste-Delpierre C, Szabo D et al. Rapid detection of CTX-M-type ESBLs and carbapenemases directly from biological samples using the BL-DetecTool. J Antimicrob Chemother 2022; 77: 2867–75. 10.1093/jac/dkac264 [DOI] [PubMed] [Google Scholar]
- 11. Tang F, Lee CH, Li X et al. Evaluation of two tests for the rapid detection of CTX-M producers directly in urine samples. Antibiotics (Basel) 2023; 12: 1585. 10.3390/antibiotics12111585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fernandez-Pittol M, Bosch J, Balleste-Delpierre C et al. Multicenter study to assess the use of BL-DetecTool for the detection of CTX-M-type ESBLs and carbapenemases directly from clinical specimens. J Clin Microbiol 2024; 62: e0113623. 10.1128/jcm.01136-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Boutin S, Scherrer M, Spath I et al. Cross-contamination of carbapenem-resistant Gram-negative bacteria between patients and the hospital environment in the first year of a newly built surgical ward. J Hosp Infect 2024; 144: 118–27. 10.1016/j.jhin.2023.11.016 [DOI] [PubMed] [Google Scholar]
- 14. Vasilakopoulou A, Naas T, Gonzalez C et al. A multicentre evaluation of the NG-test DetecTool OXA-23 for the rapid detection of OXA-23 carbapenemase directly from blood cultures. JAC Antimicrob Resist 2024; 6: dlae029. 10.1093/jacamr/dlae029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kranz J, Schmidt S, Lebert C et al. Uncomplicated bacterial community-acquired urinary tract infection in adults. Dtsch Arztebl Int 2017; 114: 866–73. 10.3238/arztebl.2017.0866 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The sequencing data were uploaded to the NCBI GenBank under the bioproject number PRJNA1101205. Accession numbers are listed in the Supplementary Table.