Abstract
There remains no one standard induction for nodal-based peripheral T-cell lymphoma (PTCL). We conducted a phase II study of lenalidomide plus CHOEP as a novel induction strategy. Patients received CHOEP at standard doses in combination with 10 mg of lenalidomide on days one through ten of a 21-day cycle for six cycles of therapy followed by observation, high dose therapy with autologous stem cell rescue, or maintenance lenalidomide per provider preference. Among 39 patients evaluable for efficacy, the objective response rate after six cycles was 69%, with complete response in 49%, partial response in 21%, stable disease in 0% and progressive disease in 13%. Thirty-two patients (82%) completed full induction and seven patients (18%) discontinued for toxicity, primarily hematologic. Any grade hematologic toxicity occurred in over 50% of patients, with grade 3 or 4 febrile neutropenia occurring in 35% of patients despite mandated growth factors. With a median followup of surviving patients of 21.3 months, the estimated two-year progression-free and overall survival was 55% (95% CI 37%–70%) and 78% (95% CI 59%–89%), respectively. In sum, six cycles of lenalidomide plus CHOEP resulted in a modest response rate primarily due to hematologic toxicity which prevented all patients from completing planned induction.
INTRODUCTION:
Improvements in the initial treatment of peripheral T-cell lymphomas (PTCL) are needed. While initial responses to standard therapies, such as CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) or CHOP plus etoposide (CHOEP) can be high, durable remissions are less frequent and long-term survival is unsatisfactory.1 As such, exploration of investigational induction regimens is warranted. Lenalidomide is an immunomodulatory agent with activity in PTCL.2,3 Accordingly, we performed a phase I/II evaluation of lenalidomide plus CHOEP in untreated, stage II-IV PTCL. The phase II results are presented here.
METHODS:
Design
In this multicenter phase I/II trial, adult patients with stage II-IV PTCL-NOS, angioimmunoblastic T-cell lymphoma (AITL), anaplastic lymphoma kinase-negative (ALK−) ALCL, ALK-positive ALCL (if International Prognostic Index [IPI] of 3–5), enteropathy-associated T-cell lymphoma, or hepatosplenic T-cell lymphoma were eligible. Patients were required to have absolute neutrophil count > 1000 cells/mm3 (unless cytopenias secondary to lymphoma), platelet count > 100,000/μL (or > 75,000/μL if bone marrow involvement by lymphoma or splenomegaly), serum creatinine < 2.0 mg/dL or calculated creatinine clearance > 45 mL/min, and aminotransferase levels ≤ 2.5 times the upper limit of normal (or ≤ 5 if hepatic involvement of lymphoma). No prior therapy was allowed except for prednisone. The study was registered as NCT02561273.
Treatment
The phase I portion was a 3 + 3 protocol to determine the maximum tolerated dose of lenalidomide in combination with CHOEP. Based on the phase I results, 10 mg of lenalidomide was chosen for the phase II portion. Lenalidomide was administered on days one through ten in combination with standard dose CHOEP. Therapy was administered for six planned cycles, with each cycle being 21 days. In responding patients after six cycles (complete or partial response), either high dose therapy with autologous stem cell rescue (HDT/ASCR) or maintenance lenalidomide (12 cycles of 10 mg for 21 days of a 28-day cycle) were allowed based on physician preference. Mandatory prophylaxis with anticoagulation and growth factor was required.
Assessments
Full staging with a bone marrow biopsy and positron emission tomography/computed tomography (PET/CT) was required. Response assessments occurred after two and six cycles of therapy; response category was defined as CR, partial response (PR), stable disease (SD), or progressive disease (PD) as per the Revised Response Criteria for Malignant Lymphoma. If baseline bone marrow was involved with lymphoma, documentation of clearance was required for a CR. Adverse event (AE) monitoring was performed using the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.02.
Analyses
The primary objective was to evaluate the CR rate after six cycles. We hypothesized that six cycles would achieve a CR rate of 60% versus the null hypothesis of 40% in the intent-to-treat population. According to a two-stage Simon optimum design and using a Type I error of 9% and power of 85%, 37 patients would be needed to evaluate our hypothesis (with 22 patients needing to achieve CR). An interim analysis was planned after the first 19 patients treated at the MTD were evaluable for response, at which point at least 9 patients must have achieved CR to continue accrual. Key secondary endpoints included ORR and median progression-free (PFS) and overall survival (OS).
RESULTS:
Patients
Baseline characteristics are shown in Supplemental Table 1. Upon pathology review, one patient was re-classified as having Hodgkin lymphoma; therefore, while 40 patients were evaluable for toxicity, 39 patients were evaluable for efficacy (Supplemental Figure 1).
Efficacy
Thirty-two patients (82%) completed six cycles of lenalidomide-CHOEP induction, with seven patients (18%) discontinuing for toxicity. Response rates after six cycles are shown in Table 1. The ORR was 69% with CR in 49%—as such, the hypothesized CR rate of 60% was not achieved. There was no difference in ORR by histology (p=0.97). After induction, 16 patients (41%) received HDT/ASCR, 10 patients (26%) received maintenance lenalidomide, and 1 patient (3%) was observed. Among the 32 patients who completed the full six cycles of induction, the ORR was 84% with a CR rate of 60%. There was no difference in ORR in those ≤ 60 or > 60 years (p=0.61) (Supplemental Table 2).
Table 1.
Response after Six Cycles.
| Response1 | Total (n = 39)1 |
PTCL-NOS (n = 19) |
AITL (n = 16) |
ALK− ALCL (n = 4) |
|---|---|---|---|---|
|
| ||||
| Overall response, n (%) | ||||
| CR | 19 (49) | 9 (47) | 7 (44) | 3 (75) |
| PR | 8 (21) | 3 (16) | 5 (31) | 0 (0) |
| SD | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| PD | 5 (13) | 3 (16) | 3 (19) | 0 (0) |
| Discontinued for toxicity (prior to completion of six cycles) | 7 (18) | 4 (21) | 2 (13) | 1 (25) |
|
| ||||
| Objective response rate, n (%) | 27 (69) | 12 (63) | 12 (75) | 3 (75) |
Responses to induction are shown for patients evaluable for efficacy who completed six cycles of induction therapy.
Safety
Safety data is shown in Supplemental Table 3. Over 50% of patients experienced any grade cytopenias. Grade 3/4 febrile neutropenia occurred in 38% of patients (n=15) despite mandated growth factor support. In those who discontinued due to toxicity, two discontinued after cycle one, one discontinued after cycle two, three discontinued after cycle three, and one discontinued after cycle four.
Survival
With a median followup of surviving patients of 21.3 months, the estimated two-year PFS and OS was 55% (95% CI 37%–70%) and 78% (95% CI, 59%–89%), respectively (Figure 1). By histology, two-year PFS for PTCL-NOS, AITL, and ALK− ALCL was 55% (95% CI, 29%–74%), 67% (95% CI, 38%–84%), and 25% (95% CI, 1%–67%) (p=0.38). By histology, two-year OS for PTCL-NOS, AITL, and ALK− ALCL was 75% (95% CI, 47%–90%), 93% (95% CI, 59%–99%), and 37% (95% CI, 1%–81%) (p=0.16) (Supplemental Figure 2). Among those who completed induction and received maintenance lenalidomide (n=10), completed induction and underwent HDT/ASCR (n=16), completed induction and received neither maintenance nor HDT/ASCR (n=1), discontinued due to toxicity (n=7), or experienced progression of disease with induction (n=5), two-year PFS was 56% (95% CI, 20%−81%), 81% (95% CI, 52%–94%), 100% (95% CI, 100%–100%), 27% (95% CI, 1%–67%), and 0%, respectively (p<0.001) (Supplemental Figure 3). Similarly, OS varied significantly among these five groups (p=0.0093) (Supplemental Figure 4). There was no significant difference in survival in those who completed induction and received maintenance lenalidomide (n=10) versus HDT/ASCR (n=16) (Supplemental Figures 5 and 6). Finally, there was no difference in PFS or OS in those ≤ 60 versus > 60 years (Supplemental Figures 7 and 8).
Figure 1.
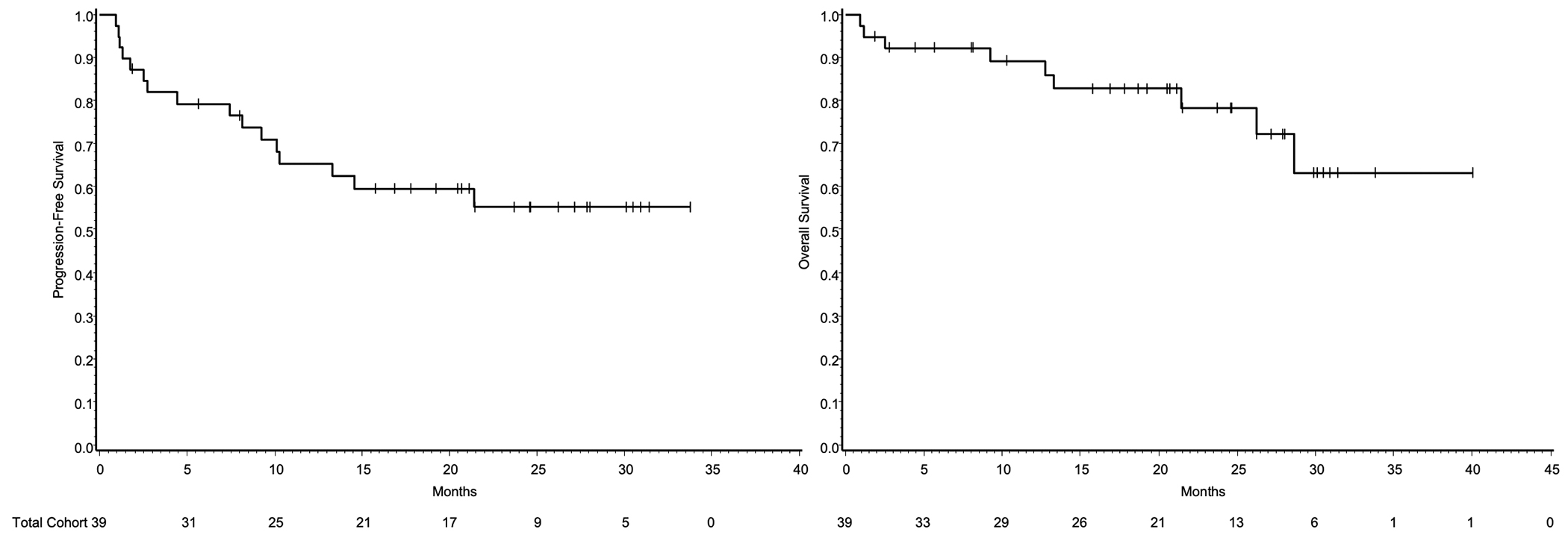
Kaplan Meier estimates for PFS and OS.
DISCUSSION:
There remains no one standard of care in the upfront management of nodal-based PTCLs. While responses to CHOP can be high, durability does not mirror respons.4 Accordingly, intensification with etoposide has been attempted. In non-randomized prospective and retrospective reports, adding etoposide to CHOP appears to improve response rates and event-free survival predominantly in younger patients, although OS benefits have not been demonstrated.5,6 In a recent analysis of over 1400 patients from the Netherlands Cancer Registry, CR rate was higher in those receiving CHOEP versus CHOP (60% versus 40%, p=0.02). However, there was no difference in OS when adjusting for age, subtype, IPI, and receipt of HDT/ASCR.6
Nevertheless, as CHOEP is one of the most active induction regimens, we used the CHOEP backbone to test lenalidomide addition in newly-diagnosed disease. This combination resulted in a modest ORR of 69% after six cycles. There was a high discontinuation rate due to adverse events, primarily hematologic. This combination failed to meet the prespecified expected CR rate of 60% after six cycles in the intent-to-treat population. In a previously reported phase II trial of lenalidomide plus CHOP in untreated patients with AITL, a complete metabolic response was observed in 41% of patients, below the prespecified expected rate of 55%.7 In our trial, response rates were predominantly affected by toxicity, and indeed, in those who actually completed induction (n=32), the ORR was 84% with a CR rate of 60%. Etoposide adds considerable hematologic toxicity to CHOP, especially in older patients, as demonstrated in the NHL-B1 and NHL-B2 trials comparing CHOP versus CHOEP in patients with aggressive lymphoma.8 While lenalidomide has been safely combined with R-CHOP in diffuse large B-cell lymphoma,9 clearly the addition of lenalidomide to CHOEP in this trial resulted in additive toxicity and a high discontinuation rate despite growth factors. Notably, in the aforementioned trial of lenalidomide plus CHOP in untreated AITL, excess hematologic toxicity was also observed.7 Potentially shorter courses of intensive therapy could be explored.
Attempts to incorporate active agents in upfront combinations for T-cell lymphoma have been challenging. The only regimen that shows a survival benefit over standard therapy in a randomized trial is brentuximab vedotin.10 However, two randomized phase III trials of alemtuzumab plus CHOP versus CHOP showed no increase in survival owing to increased toxicity.11,12 Similarly, a phase III trial comparing romidepsin plus CHOP versus CHOP failed to meet its primary endpoint of PFS,13 and a related phase II trial of romidepsin plus CHOEP and HDT/ASCR failed to meet a prespecified PFS.14 These trials demonstrate that the addition of an active agent to chemotherapy may not result in increased survival, though a critical distinction in the above examples is that alemtuzumab and romidepsin significantly increased toxicity compared to standard chemotherapy (which was not observed with BV-CHP). In our trial, lenalidomide added excess hematologic toxicity which prevented patients from actually completing induction. An ongoing randomized study (NCT04803201) is testing the addition of azacytidine15 or duvelisib to CHOP or CHOEP in untreated PTCL.
In conclusion, we report the phase II results of lenalidomide in combination with CHOEP in untreated, stage II-IV PTCL. This combination failed to reach the prespecified expected CR rate of 60% after six cycles. These findings were largely secondary to excess hematologic toxicity. Efforts to move active therapies forward in lines of therapy in T-cell lymphomas should prioritize agents with non-overlapping toxicity to chemotherapy.
Supplementary Material
Funding Sources:
This research was funded through Celgene.
Conflict of Interest Disclosure:
R.S., R.H.A., B.H., L.Q.P., E.L., and S.M.A. have no COI disclosures. S.M.H. receives research support from ADC Therapeutics, Affimed, Aileron, Celgene, Crispr Therapeutics, Daiichi Sankyo, Forty Seven, Inc., Kyowa Hakko Kirin, Millennium /Takeda, Seattle Genetics, Trillium Therapeutics, and Verastem/SecuraBio, and has served as a consultant for Acrotech Biopharma, ADC Therapeutics, Astex, Auxilus Pharma, Merck, C4 Therapeutics, Celgene, Cimieo Therapeutics, Daiichi Sankyo, Janssen, Kura Oncology, Kyowa Hakko Kirin, Myeloid Therapeutics, ONO Pharmaceuticals, Seattle Genetics, SecuraBio, Shoreline Biosciences, Inc, Takeda, Trillium Therapeutics, Tubulis, Verastem/SecuraBio, Vividion Therapeutics and Yingli Pharma Limited. J.M.V. received research support from Epizye, Kite, Loxo, and Novartis, and has served as a consultant for Abbvie, Janssen, AstraZeneca, MEI Pharma, Pharmacyclics, Genentech, Seagen, GenMab, Lilly, and Novartis. H.J.L. is an advisory board member of Century Therapeutics, has received research support from Bristol Myers-Squibb, Celgene, Octernal Therapeutics, Seagen, and Takeda, and has served as a consultant for Bristol-Myers Squibb and Guidepoint Global. N.M.S. has received research support from AstraZeneca, Bristol Myers-Squibb, Celgene, C4 Therapeutics, Corvus Pharmaceuticals, Daiichi Sankyo, Genentech/Roche, Innate Pharmaceuticals, SecuraBio/Verastem, as well as honorarium for service as a consultant for SecuraBio/Verastem, Daiichi Sankyo, C4 Therapeutics, Genentech, Karyopharm Therapeutics, Kyowa Hakko Kirin, and Ono pharmaceuticals. J.M.Z. has received research support from Seagen, Kiyowa Kirin, Myeloid, CRSPR, and AstraZeneca, and has served as a consultant for Seagen, SecuraBio/Verastam, and Kiyowa Kirin. M.J.L. has received research support from EUSA Pharma and SecuraBio/Verastem. A.J.M. has received research support form ADC Therapeutics, Beigene, Miragen, Seattle Genetics, Merck, Bristol-Myers Squibb, Incyte, and SecuraBio, and receives honorarium from Affimed, Imbrium Therapeutics L.P./Purdue, Janpix Ltd., Merck, Seattle Genetics, and Takeda. M.A.L. has served as a consultant for AbbVie, Acrotech, ADC Therapeutics, Astellas, AstraZeneca, Bristol Myers Squibb, Daiichi Sankyo, EUSA Pharma, Fate Therapeutics, Genentech, Kite, Instil Bio, MorphoSys, Nurix, Sana Pharma, and TG Therapeutics.
Footnotes
Ethics Approval Statement: The study was approved by the institutional review boards and ethics committees at all participating sites and registered at http://clinicaltrials.gov as NCT02561273.
Patient Consent Statement: All patients were required to provide written informed consent before registration.
Permission to Reproduce Material From Other Sources: N/A
Clinical Trial Registration: The trial was registered at http://clinicaltrials.gov as NCT02561273.
Data Availability Statement:
The original data presented in this study are available from the corresponding author upon reasonable request at stuverr@mskcc.org.
REFERENCES:
- 1.Vose J, Armitage J, Weisenburger D, International T-Cell Lymphoma Project. International Peripheral T-Cell and Natural Killer/T-Cell Lymphoma Study: Pathology Findings and Clinical Outcomes. Journal of Clinical Oncology. 2008;26(25):4124–4130. [DOI] [PubMed] [Google Scholar]
- 2.Morschhauser F, Fitoussi O, Haioun C, et al. A phase 2, multicentre, single-arm, open-label study to evaluate the safety and efficacy of single-agent lenalidomide (Revlimid) in subjects with relapsed or refractory peripheral T-cell non-Hodgkin lymphoma: the EXPECT trial. Eur J Cancer. 2013;49(13):2869–2876. [DOI] [PubMed] [Google Scholar]
- 3.Toumishey E, Prasad A, Dueck G, et al. Final report of a phase 2 clinical trial of lenalidomide monotherapy for patients with T-cell lymphoma. Cancer. 2015;121(5):716–723. [DOI] [PubMed] [Google Scholar]
- 4.Savage KJ, Chhanabhai M, Gascoyne RD, Connors JM. Characterization of peripheral T-cell lymphomas in a single North American institution by the WHO classification. Annals of Oncology. 2004;15(10):1467–1475. [DOI] [PubMed] [Google Scholar]
- 5.Schmitz N, Trümper L, Ziepert M, et al. Treatment and prognosis of mature T-cell and NK-cell lymphoma: an analysis of patients with T-cell lymphoma treated in studies of the German High-Grade Non-Hodgkin Lymphoma Study Group. Blood. 2010;116(18):3418–25. [DOI] [PubMed] [Google Scholar]
- 6.Brink M, Meeuwes FO, Van Der Poel MWM, et al. Impact of etoposide and ASCT on survival among patients aged <65 years with stage II to IV PTCL: a population-based cohort study. Blood. 2022;140(9):1009–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lemonnier F, Safar V, Beldi-Ferchiou A, et al. Integrative analysis of a phase 2 trial combining lenalidomide with CHOP in angioimmunoblastic T-cell lymphoma. Blood Adv. 2021;5(2):539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pfreundschuh M, Trümper L, Kloess M, et al. Two-weekly or 3-weekly CHOP chemotherapy with or without etoposide for the treatment of elderly patients with aggressive lymphomas: results of the NHL-B2 trial of the DSHNHL. Blood. 2004;104(3):634–641. [DOI] [PubMed] [Google Scholar]
- 9.Castellino A, Chiappella A, Laplant BR, et al. Lenalidomide plus R-CHOP21 in newly diagnosed diffuse large B-cell lymphoma (DLBCL): long-term follow-up results from a combined analysis from two phase 2 trials. Blood Cancer J. 2018;8:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Horwitz S, O’Connor OA, Pro B, et al. Brentuximab vedotin with chemotherapy for CD30-positive peripheral T-cell lymphoma (ECHELON-2): a global, double-blind, randomized, phase 3 trial. Lancet. 2019;393(10168):229–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.d’Amore F, Leppä S, Silva MG da, et al. Final Analysis of the Front-Line Phase III Randomized ACT-1 Trial in Younger Patients with Systemic Peripheral T-Cell Lymphoma Treated with CHOP Chemotherapy with or without Alemtuzumab and Consolidated By Autologous Hematopoietic Stem Cell Transplant. Blood. 2018;132(Supplement 1):998–998. [Google Scholar]
- 12.Wulf GG, Altmann B, Ziepert M, et al. Alemtuzumab plus CHOP versus CHOP in elderly patients with peripheral T-cell lymphoma: the DSHNHL2006–1B/ACT-2 trial. Leukemia. 2021;35(1):143–155. [DOI] [PubMed] [Google Scholar]
- 13.Bachy E, Camus V, Thieblemont C, et al. Romidepsin Plus CHOP Versus CHOP in Patients With Previously Untreated Peripheral T-Cell Lymphoma: Results of the Ro-CHOP Phase III Study (Conducted by LYSA). Journal of Clinical Oncology. 2022;40(3):242–251. [DOI] [PubMed] [Google Scholar]
- 14.Chiappella A, Dodero A, Evangelista A, et al. Romidepsin-CHOEP followed by high-dose chemotherapy and stem-cell transplantation in untreated Peripheral T-Cell Lymphoma: results of the PTCL13 phase Ib/II study. Leukemia. 2023;37(2):433–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ruan J, Moskowitz AJ, Mehta-Shah N, et al. Multicenter Phase 2 Study of Oral azacytidine (CC-486) plus CHOP as initial treatment for peripheral T-cell lymphoma. Blood. 2023;141(18):2194–2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original data presented in this study are available from the corresponding author upon reasonable request at stuverr@mskcc.org.


