Abstract
Context
Sever's disease (calcaneal apophysitis) is a common condition in youth athletes, including those who participate in barefoot sports. Health care professionals often recommend that young athletes with Sever's disease wear heel cups in their shoes while active, but barefoot athletes are unable to use heel cups.
Objective
To compare the efficacy of 2 braces used by barefoot athletes with Sever's disease.
Design
Randomized controlled clinical trial.
Setting
Pediatric sports medicine clinic.
Patients or Other Participants
A total of 43 barefoot athletes aged 7 to 14 years were enrolled, and 32 completed the study (age = 10.3 ± 1.6 years; 29 girls, 3 boys).
Intervention(s)
Participants were randomized to the Tuli's Cheetah heel cup (n = 16) or Tuli's The X Brace (n = 16) group for use during barefoot sports over the 3-month study period.
Main Outcome Measure(s)
Participants completed self-reported assessments after diagnosis (baseline) and 1, 2, and 3 months later. The primary outcome was the Oxford Ankle Foot Questionnaire for Children (OxAFQ-C) physical score (3 months postenrollment). The secondary outcomes were OxAFQ-C school or play and emotional scores and the visual analog scale pain score.
Results
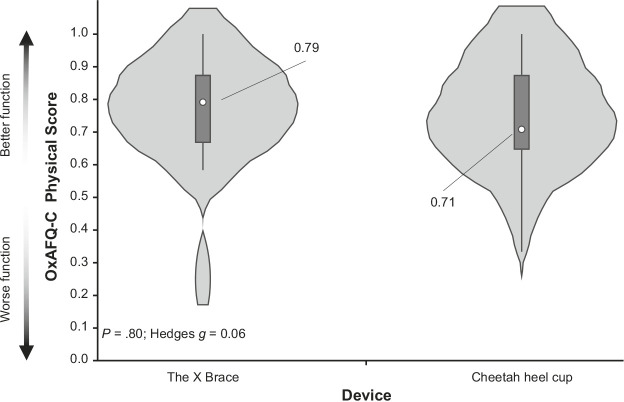
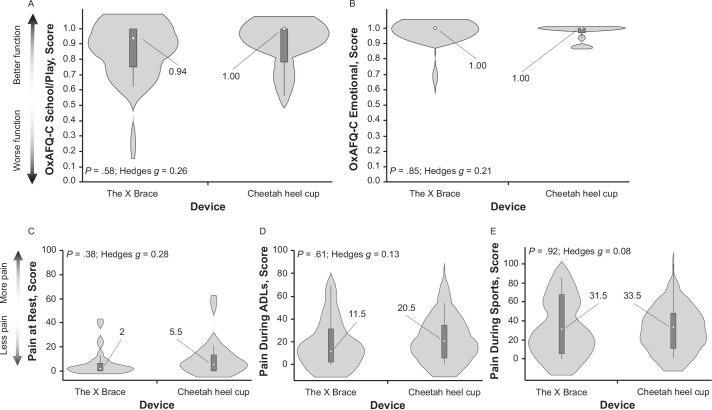
The percentage of time wearing the brace during barefoot sports was not different between the Cheetah heel cup and The X Brace groups (82% versus 64% of the time in sports; P = .08). At 3 months, we observed no differences for the OxAFQ-C physical (0.79 versus 0.71; P = .80; Hedges g = 0.06), school or play (0.94 versus 1.00; P = .58; Hedges g = 0.26), or emotional (1.00 versus 1.00; P = .85; Hedges g = 0.21) score. Visual analog scale pain scores during activities of daily living and sports were lower (better) at the 2- and 3-month time points than at baseline (P < .001).
Conclusions
Both groups demonstrated improvements in ankle and foot function across time, but no between-groups differences were seen at 3 months. Given these results, barefoot athletes with Sever's disease may consider using either brace with barefoot activity to help improve pain and functional status.
Keywords: heel pain, gymnasts, dancers, Cheetah heel cup, The X Brace
Key Points
Providers who diagnose gymnasts, dancers, acrobats, or martial arts athletes with Sever's disease can offer either the Cheetah heel cup or The X Brace to these patients.
Both braces led to improved function and pain in barefoot athletes with Sever's disease.
Sever's disease is an osteochondrosis of the calcaneal apophysis that causes heel pain.1 This overuse injury is due to repetitive foot impacts and repeated traction on the calcaneal apophysis.2 It typically occurs in physically active and skeletally immature children3 aged 8 to 14 years.1 Sever's disease accounts for 2% to 16% of all musculoskeletal concerns in children.4 Established risk factors for this condition include high levels of athletic activity,5 a high body mass index,6,7 limited ankle range of motion, and a lower foot posture index.7
The disease can negatively affect quality of life.3 Affected children often limp5 and report worse overall pain, which can lead to reduced physical activity and decreased happiness relative to their peers without Sever's disease.3 It is widely accepted that physical activity improves child health outcomes, including positive effects on adiposity, musculoskeletal health and fitness, and cardiovascular health.8 Beyond the health benefits of physical activity alone, participation in sports during childhood and adolescence enhances psychological and social health outcomes.9 Sever's disease limits physical activity and athletic participation because of heel pain with activity.10 Therefore, proper management of this condition aims to reduce pain and improve function and quality of life without losing substantial time from sport (ie, avoiding complete rest).3
Typical treatments involve wearing heel cups in shoes, activity modification, and rehabilitation. Patients who use these interventions have decreased heel pain.11–15 Previous researchers have demonstrated that treatment with a heel cup or other foot orthoses resulted in symptom improvement.15,16 Randomized, blinded, and longitudinal studies in which different treatment options for Sever's disease have been evaluated are lacking.17 Additionally, the standard treatment (ie, using a heel cup in a shoe) is not possible for young athletes who participate in barefoot sports, such as gymnastics, dance, acrobatics, or martial arts, because of the unique demands of their sports. Thus, alternative treatments are needed for the barefoot athlete population. Currently, 2 braces are available for barefoot athletes with Sever's disease: Tuli's Cheetah heel cup and Tuli's The X Brace (Medi-Dyne Healthcare Products), yet no investigators have assessed their relative efficacy in reducing pain and improving function across time. Barefoot athletes can use other standard management strategies, such as home exercises, formal physical therapy, nonsteroidal anti-inflammatory drugs (NSAIDs), and activity modification. Physical therapy is often difficult to standardize because of variability in and patient compliance with the exercises. Therefore, the purpose of our study was to compare the efficacy of the Cheetah heel cup and The X Brace to improve pain and functional outcomes over a 3-month period among barefoot youth athletes with Sever's disease. We hypothesized that both braces would provide benefits but that the Cheetah heel cup, which includes a heel cup in the brace, would reduce pain and improve function more than The X Brace. Determining the efficacy of these braces will enable barefoot athletes to make an informed decision about which brace is better for them and ultimately allow them to participate in their sports with less pain and improved function.
METHODS
Design
We conducted a single-blinded, prospective randomized clinical trial in which we recruited and enrolled adolescent barefoot athletes who were diagnosed with Sever's disease (Clinicaltrials.gov ID: NCT03494647). Participants were recruited from a single sports medicine program associated with a tertiary care regional hospital between April 18, 2018, and August 1, 2021. The final participant completed the final follow-up study questionnaire on November 1, 2021.
Participants
Volunteers aged 7 to 14 years who participated in a barefoot sport such as gymnastics, dance, martial arts, or acrobatics were eligible to enroll. We approached 51 patients who met the criteria for enrollment, and 43 patients elected to enroll in the study. The 8 participants who did not enroll either gave no reason (n = 2) or were interested but did not respond to attempts to contact them (n = 6). At the enrollment visit, we collected participant characteristics, including sex, race, age, height, and mass (Table 1). We also obtained information regarding primary barefoot sport, hours per week of sport activity, and history of brace use before enrollment. Exclusion criteria were a history of foot or ankle surgery or rheumatologic disease. No athlete was excluded due to meeting the exclusion criteria. The diagnosis of Sever's disease was made by the treating physician based on pain localized to the calcaneal apophysis that worsened with activity in a skeletally immature patient. Skeletal maturity was determined by evaluating the athlete for an open calcaneal apophysis by using ankle, foot, or calcaneal radiographs obtained in the clinic at the time of diagnosis. Six Board-certified sports medicine physicians diagnosed participants based on these criteria and referred potential participants to our study team for enrollment. The physicians were instructed to provide their standard recommendations and management strategies for all participants. These included informational handouts on Sever's disease; home exercise programs supplied by the physician or clinical athletic trainer who worked with the physician; referrals for physical therapy; and instructions on activity modification, ice, and NSAIDs. We did not require participants to go to physical therapy because differences are often present in physical therapy exercises and participant compliance with these exercises.
Table 1.
Participant Characteristics
|
Variable |
Cheetah Heel Cupa (n = 16) |
The X Bracea (n = 16) |
P Value |
|
No. (%)b |
|||
| Affected side | .26 | ||
| Left | 1 (6) | 3 (19) | |
| Right | 3 (19) | 6 (38) | |
| Bilateral | 12 (75) | 7 (44) | |
| Sex: female | 14 (88) | 15 (94) | >.99 |
| Race | |||
| American Indian or Alaska Native | 0 (0) | 1 (6) | >.99 |
| Asian | 1 (6) | 1 (6) | >.99 |
| Black or African American | 2 (13) | 1 (6) | >.99 |
| White | 11 (69) | 11 (69) | >.99 |
| >1 | 2 (13) | 2 (13) | >.99 |
| Ethnicity: Hispanic or Latino | 1 (6) | 1 (6) | >.99 |
| Previous Sever's disease diagnosis | 3 (19) | 5 (31) | .69 |
|
No. |
|||
| Sport | >.99 | ||
| Gymnastics | 13 | 14 | |
| Dance | 2 | 2 | |
| Martial arts | 1 | 0 | |
|
Mean ± SD |
|||
| Age, y | 10.6 ± 1.6 | 10.1 ± 1.6 | .44 |
| Height, cm | 138.2 ± 10.7 | 138.6 ± 9.0 | .92 |
| Mass, kg | 32.7 ± 7.6 | 31.4 ± 5.2 | .57 |
| Questionnaire completion timing | |||
| Time from consent to initiation of brace wearing, d | 6.8 ± 4.2 | 5.1 ± 3.9 | .24 |
| Follow-up, mo after enrollment | |||
| 1 | 37.3 ± 6.1 | 36.7 ± 7.3 | .79 |
| 2 | 67.8 ± 8.5 | 67.1 ± 9.7 | .84 |
| 3 | 99.6 ± 13.9 | 94.1 ± 9.1 | .21 |
Model Tuli's (Medi-Dyne).
Percentages were rounded, so sums may be >100%.
All participants and their parent or guardian provided written informed assent and consent, respectively, and the Colorado Multiple Institutional Review Board at the University of Colorado approved the study.
Interventions
Participants were randomized to receive either the Cheetah heel cup or The X Brace (Figure 1). The Cheetah heel cup is a slip-on neoprene ankle brace with a built-in multicell, multilayer waffle heel cup. The X Brace is an elastic foot brace with an integrated silicone strip on the heel strap. Both braces are marketed for use by barefoot athletes with Sever's disease. The unblinded members of the study team (C.C.L., M.N.P., C.N.S.) gave the participants and their families instructions for brace use over the subsequent 3 months. The assigned brace (2 braces if both feet were affected) was given to each participant in the clinic during the research visit or mailed after the visit. Participants were instructed to wear the brace during all barefoot activities.
Figure 1.
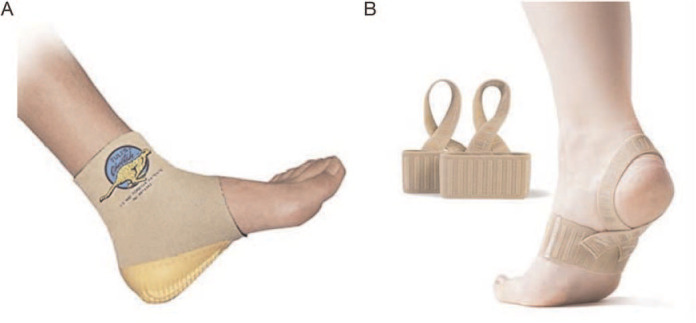
A, Cheetah heel cup (model Tuli's; Medi-Dyne Healthcare Products) and, B, The X Brace (model Tuli's; Medi-Dyne Healthcare Products).
After the initial enrollment period, we assessed outcomes at 1, 2, and 3 months. These times were chosen based on previous literature11,12 in which the use of prefabricated orthoses and shoe inserts resulted in beneficial changes in patients with Sever's disease during these time frames. Each month, participants completed an online survey using REDCap (Research Electronic Data Capture; Vanderbilt University)18 that included questions on brace acceptability and wear, the amount of barefoot activity, and the use of pain medicine (see Supplemental Figure 1, available online at https://doi.org/10.4085/1062-6050-0266.22.S1). Participants also rated the brace's appearance, comfort, and ability to improve their athletic performance. These responses were rated on a 5-point Likert scale, with 1 indicating not at all true and 5 indicating completely true. They also reported the number of hours per week they wore the brace and the number of hours per week they were active in their sports, which we used to calculate the percentage of time that they wore their assigned brace during their barefoot sports. They received an activity log calendar with their brace to assist in tracking weekly barefoot sport activity and brace use. Participants who were >5 days late in completing their surveys received a reminder call from the research team. After 3 unsuccessful attempts to contact a participant or family during the follow-up period, we considered the individual lost to follow up.
Because earlier authors11,15 found that the lack of a heel cup or other foot orthosis led to worse outcomes among patients with Sever's disease, we did not include a true control group that did not receive a brace. Instead, all participants received 1 type of brace to eliminate the concern that patients who were not provided with a brace would purchase one. In addition to instructions regarding brace wear, all treating physicians provided standard treatment recommendations that included stretching, heel cups for daily activity (to be worn in shoes during the day), activity modification, ice, NSAIDs, or a combination.
Primary and Secondary Endpoints
Participants completed a series of questionnaires at enrollment and monthly for 3 months. The primary endpoint was the outcome at 3 months after enrollment. Secondary endpoints were the change in outcome from enrollment to 1, 2, and 3 months after enrollment.
Primary and Secondary Outcome Variables
To assess ankle and foot function, we instructed participants to complete the Oxford Ankle Foot Questionnaire for Children (OxAFQ-C). The OxAFQ-C is a validated questionnaire used to measure well-being in patients aged 5 to 16 years who are affected by foot and ankle conditions.19,20 It has been used as an outcome metric for Sever's disease7,17 and consists of 15 items assessing how often the individual experiences the condition, with a higher score representing more frequent problems. The 3 domains are physical (6 items), school or play (4 items), and emotional (4 items), with responses rated on a scale of 0 (never) to 4 (always). In addition, 1 final question (not included in the 3 domains) asks about concerns regarding footwear. Within each domain, we summed the responses and divided the value by the maximum total points to create a score that was transformed to a fraction from 0 to 1. Our primary outcome was the physical domain score because of the relevance in sports.20 We also examined school or play and emotional domains as secondary outcomes.
Participants used a visual analog scale (VAS)21 to rate pain severity at rest, during activities of daily living (ADLs), and during sport participation. To rate pain severity, they indicated the part of the line on a sliding scale that corresponded to their experience (ie, at rest, during ADLs, and during sport participation). From that information, we transformed the response into a numeric score, ranging from 0 (far left; no pain) to 100 (far right; most severe pain).
Sample Size and Randomization
A power analysis was performed to determine adequate sample size based on previous studies of the OxAFQ-C.12 Means for treatment groups were estimated based on a related randomized controlled trial12 in which the minimal clinically important difference (MCID) was 0.07 and the correlation between consecutive time points was conservatively assumed to be 0.6. We estimated our sample size at 3 months after enrollment based on the average of the 2- and 6-month scores in earlier work.12 A sample size of 34 participants (17 in each group) would provide a power of 0.80 to reject the null hypothesis of no group difference at 3 months with an α level of .05. We randomized participants into each group at enrollment using a 1:1 block randomization scheme (block size = 4) to ensure equal allocation of patients to each treatment group. The statistician (D.R.H.) generated the block stratification protocol and remained blinded to group assignments until all analyses had been performed.
Statistical Analysis
Data are presented as means ± SDs for continuous variables and numbers with corresponding percentages in groups for categorical variables. Mean differences and 95% CIs for the mean differences were calculated between groups. To ensure that our randomization was successful, we compared baseline participant characteristics, brace wear, behavioral characteristics, and perceptions of the brace between groups using independent-samples t tests and the χ2 or Fisher exact test.
Given the nonnormal distribution of the data (Shapiro-Wilk test P < .05), we compared OxAFQ-C domain and VAS scores at baseline and 3 months after enrollment between brace groups using the Mann-Whitney U test. We also calculated effect sizes (Hedges g) to determine the magnitude of the effect between groups and interpreted the strength of the effect as small (0.00–0.49), medium (0.50–0.79), or high (≥0.80). As a sensitivity analysis and to address individual changes throughout the 3-month study period, we also computed pre-post changes for each participant (3-month outcomes − initial outcome) on each outcome variable of interest. We compared these change values using independent-samples t tests, as the data were normally distributed. Finally, we assessed changes across time and between groups using a 2-level (group and time) analysis of variance. If an omnibus effect was identified, we conducted between-groups or across-time Tukey post hoc tests, using adjusted P values. The P values and associated clinically meaningful values (Hedges g, mean difference, and 95% CI) are reported. The α level was set at .05, and all tests were 2 sided. Statistical analyses were performed using Stata (version 15; StataCorp LLC).
RESULTS
Of the 43 patients who enrolled, 22 were randomized to The X Brace group, and 21 were randomized to the Cheetah heel cup group (Figure 2). A total of 6 participants were lost to follow up in The X Brace group, and 5 participants were lost to follow up in the Cheetah heel cup group (74% overall participant retention rate). Thus, our final analysis reflected 16 participants in each group. No adverse events related to brace wear or unintended effects caused by study involvement were observed.
Figure 2.
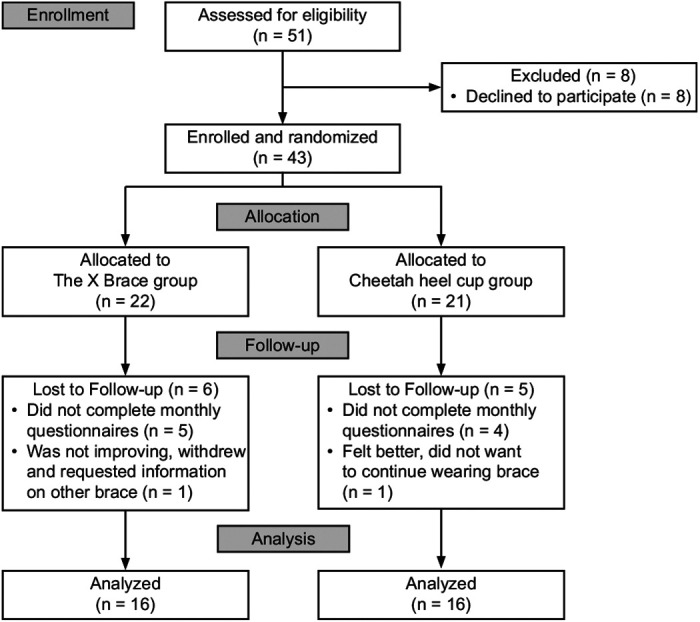
Consolidated Standards of Reporting Trials (CONSORT) diagram for the study.
No differences were found in participant characteristics, medical history, or timing of questionnaire completion (Table 1). Most participants were gymnasts, and no between-groups differences were noted regarding the type of barefoot sport (P > .99; Table 1). We saw no between-groups differences for perceptions about brace wear or behavioral characteristics (ie, stretching, NSAID use, amount of time in sport participation before enrollment in or during the study; Table 2). The Cheetah heel cup group spent a larger percentage of time wearing the brace during their barefoot sports than The X Brace group, although this finding was not different (P = .08; Hedges g = 0.62; Table 2).
Table 2.
Participant Perceptions and Behavioral Characteristics
|
Variable |
Cheetah Heel Cupa |
The X Bracea |
P Value |
| Perceptions, mean ± SDb | |||
| Brace improved athletic performancec | 3.9 ± 0.9 | 3.7 ± 1.0 | .50 |
| Brace appearance was acceptablec | 4.4 ± 0.7 | 4.4 ± 0.7 | .93 |
| Brace was comfortablec | 4.3 ± 0.7 | 3.8 ± 1.3 | .25 |
| Behavioral characteristics | |||
| Stretching frequency, d/wkd | 3.1 ± 1.5 | 3.1 ± 1.6 | .99 |
| Time in barefoot sports participation before enrollment, h/wk | 10.9 ± 7.3 | 13.3 ± 8.2 | .40 |
| Time in barefoot sports during study, h/wk | 10.0 ± 8.4 | 12.6 ± 7.7 | .36 |
| Time in barefoot sports wearing brace during study, h/wk | 7.4 ± 6.5 | 8.1 ± 6.6 | .76 |
| Time in barefoot sports wearing brace during study, %c | 81.8 ± 26.7 | 64.1 ± 29.5 | .08 |
| Total time missed because of heel pain during study, h | 6.9 ± 21.5 | 6.2 ± 13.2 | .91 |
| Nonsteroidal anti-inflammatory drug use in the month before enrollment, No. (%) | 7 (44) | 9 (56) | .48 |
| Missed sports because of heel pain during study, No. (%) | 8 (50) | 9 (56) | .72 |
Model Tuli's (Medi-Dyne Healthcare Products).
Rated on a 1–5 scale, with a higher score indicating stronger agreement.
Expressed as the mean ± SD percentage of the time athletes reported wearing their brace, calculated as the amount of time wearing the brace divided by the total time reported playing sports.
A higher score indicates more frequent stretching.
We did not demonstrate any between-groups differences for the OxAFQ-C physical score at the baseline (P = .26) or 3-month assessments (P = .80; Figure 3). In addition, we did not identify any between-groups differences for our secondary outcomes, with corresponding small effect sizes (Figure 4). No baseline differences were observed between groups for the OxAFQ-C school or play (P = .60) or emotional (P = .08) domain or VAS scores at rest (P = .24), during ADLs (P = .72), or during sports (P = .66).
Figure 3.
Comparison of the primary outcome variable between the Cheetah heel cup (model Tuli's; Medi-Dyne Healthcare Products) and The X Brace (model Tuli's; Medi-Dyne Healthcare Products) groups at the 3-month assessment: the Oxford Ankle Foot Questionnaire for Children (OxAFQ-C) physical score (mean difference = 0.01; 95% CI = −0.13, 0.15). Data are presented as median (center dot with label, with corresponding number included) and interquartile range (box around the median). The shaded area represents the probability density of data at each level of the scale, smoothed using a kernel density estimator.
Figure 4.
Comparison of the secondary outcome variables between the Cheetah heel cup (model Tuli's; Medi-Dyne Healthcare Products) and The X Brace (model Tuli's; Medi-Dyne Healthcare Products) groups at the 3-month assessment: Oxford Ankle Foot Questionnaire for Children (OxAFQ-C), A, school or play (mean difference [MD] = 0.05; 95% CI = −0.09, 0.19) and, B, emotional scores (MD = 0.02; 95% CI = −0.04, 0.08); and visual analog scale pain scores, C, at rest (MD = 3.7; 95% CI = −5.7, 13.1), D, during activities of daily living (ADLs; MD = 3.1; 95% CI = −13.5, 19.7), and E, during sports (MD = 2.5; 95% CI = −24.2, 19.2). Data are presented as median (center dot with label, with corresponding number included) and interquartile range (box around the median). The shaded area represents the probability density of data at each level of the scale, smoothed using a kernel density estimator.
When examining the pre-post changes across the study period, we found that the Cheetah heel cup group displayed greater mean functional improvement for the OxAFQ-C emotional domain than The X Brace group, with a medium effect size (P = .03; Hedges g = 0.79; Table 3). The mean change for the Cheetah heel cup group was 0.13 and for The X Brace was 0.05 (MCID = 0.07). No other pre-post change outcomes were different between groups, with small effect sizes (Table 3).
Table 3.
Between-Groups Changes Across the Measurement Outcomes Mean ± SDa
|
Variable |
Cheetah Heel Cupb |
The X Braceb |
P Value |
Hedges g |
| Oxford Ankle Foot Questionnaire for Childrenc | ||||
| Physical | 0.27 ± 0.22 | 0.22 ± 0.18 | .50 | 0.24 |
| School or play | 0.19 ± 0.19 | 0.18 ± 0.25 | .92 | 0.04 |
| Emotional | 0.13 ± 0.08 | 0.05 ± 0.11 | .03e | 0.79 |
| Visual analog scale for paind | ||||
| At rest | −9.5 ± 23.1 | −7.4 ± 14.5 | .76 | 0.10 |
| During activities of daily living | −25.4 ± 30.3 | −23.6 ± 23.2 | .85 | 0.07 |
| During sports | −42.4 ± 30.5 | −36.1 ± 25.8 | .53 | 0.22 |
Change calculated as 3-month outcome − baseline outcome.
Model Tuli's (Medi-Dyne Healthcare Products).
A positive score indicates functional improvement.
A negative score indicates less pain.
Between-groups difference (P < .05).
No between-groups differences were present at any time on any outcome measure (see Supplemental Figure 2, available online at https://doi.org/10.4085/1062-6050-0266.22.S2); however, effects of time were evident. For all 3 OxAFQ-C domains, the initial assessment scores were lower (worse) than the 2- and 3-month scores (Supplemental Figure 2A through C; P < .01). There were no effects across time for VAS scores at rest. The VAS scores during ADLs and during sports were higher (worse) at the initial visit (enrollment) scores than at 2 and 3 months (Supplemental Figure 2D through F; P < .001).
DISCUSSION
We compared the efficacy of 2 braces, Cheetah heel cup and The X Brace, in improving pain and function in barefoot athletes diagnosed with Sever's disease. The brace groups were similar in terms of demographics, brace wear, sport participation, and behavioral characteristics. Previous researchers have shown that a heel cup worn inside the athlete's shoe reduces the pressure on the heel of a child with Sever's disease13 and decreases pain.14 Because gymnasts, dancers, and martial arts athletes train and compete barefoot, they are unable to use a traditional heel cup for activities. We found that using the Cheetah heel cup or The X Brace resulted in similar patient-reported pain and function trajectories across a 3-month period after diagnosis, confirming the results observed for heel cups or other foot orthoses used within shoes.22
The primary outcome of our study was the OxAFQ-C physical score. Participants in both groups showed improvement in this domain, although no between-groups differences were present. Thus, the results of our primary aim to understand the relative efficacy between braces suggested that improvement may occur with a variety of bracing strategies among barefoot athletes. Without a control group, we could not infer whether the improvement across measures in both groups was due to the brace or spontaneous recovery. However, brace use for barefoot athletes with Sever's disease was generally well tolerated and did not result in any detrimental effect on pain and function recovery. As such, providers may consider recommending both braces as an intervention for barefoot athletes with Sever's disease.
Regarding our secondary outcomes, we did not demonstrate a difference across any of the measurements between groups except for the change in the OxAFQ-C emotional score between baseline and 3 months. Participants in the Cheetah heel cup group had greater improvement in this domain over time. Questions in the emotional domain assess aspects of the way in which participants walk, how their foot or ankle looks, if anyone is unkind because of their foot or ankle, or if they feel embarrassed. It seems that this brace may have provided some benefit to barefoot athletes compared with The X Brace. However, although the Cheetah heel cup may have led to positive emotional effects, this result may have also been spurious, given the many secondary and sensitivity analyses we performed.
Although Sever's disease is often self-limiting and resolves over 6 to 12 months,22 this condition can lead to decreased physical activity and quality of life when young athletes have symptoms.3 Compared with the time spent in sport before study participation, our athletes reported participating in >90% of the potential time in their sport, regardless of which brace they wore. This indicates that both braces provided benefits to young athletes with Sever's disease, as evident in the high rate at which they were able to continue to participate in their sport with decreased pain.
Our study had limitations, and our results should be interpreted considering them. We did not have a true control group and could not definitively determine that either brace was the single source of improvement over the 3-month study period. Our study population consisted mostly of White girls, so our results may not be generalizable to other populations. Our data, including the amount of time spent participating in sport, were susceptible to subjective reporting errors, and we did not have established MCIDs for the OxAFQ-C or VAS. Based on our power calculations, we needed 17 participants per group for acceptable power (>0.80), but our research was slightly underpowered at 16 participants per group in the final analysis. Given the restriction on in-person clinical research during the study period resulting from the COVID-19 pandemic, the timing and small sample size may have inhibited our ability to analyze an adequately powered sample. Furthermore, the COVID-19 pandemic may have altered sport participation and affected our results in ways that we did not measure.
CONCLUSIONS
Both the Cheetah heel cup and The X Brace were beneficial options for treating barefoot athletes who had Sever's disease and could not wear conventional in-shoe heel cups. Both braces, in addition to standard treatment recommendations such as stretching, ice, and NSAIDs, had similar positive effects on recovery. Providers who diagnose barefoot athletes with Sever's disease may consider recommending either brace to facilitate improvements in pain and function along with other components of management.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported through a grant from the American Medical Society for Sports Medicine (AMSSM) Young Investigator's Award (Drs Sweeney, Wilson, Potter, and Howell). Funding was provided by the AMSSM Research Committee and AMSSM Foundation.
Work by Dr Potter was made possible by the National Institutes of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award No. T32HD007490-2.
SUPPLEMENTAL MATERIAL
Supplemental Figure 1.
Initial research survey and monthly survey. a Haleon. b Johnson & Johnson. c Bayer. Abbreviations: zip, zip code; BMI, body mass index; PE, physical education.
Found at DOI: https://doi.org/10.4085/1062-6050-0266.22.S1
Supplemental Figure 2.
Longitudinal outcomes for the Cheetah heel cup (model Tuli's; Medi-Dyne Healthcare Products) and The X Brace (model Tuli's; Medi-Dyne Healthcare Products) groups for the Oxford Ankle Foot Questionnaire for Children (OxAFQ-C), A, physical, B, school or play, and C, emotional domain and the visual analog scale pain, D, at rest, E, during activities of daily living (ADLs), and F, during sport. a Indicates difference (P < .01). b Indicates difference (P < .001).
Found at DOI: https://doi.org/10.4085/1062-6050-0266.22.S2
REFERENCES
- 1. Wiegerinck JI, Yntema C, Brouwer HJ, Struijs PA. Incidence of calcaneal apophysitis in the general population. Eur J Pediatr. 2014; 173 (5): 677–679. 10.1007/s00431-013-2219-9 [DOI] [PubMed] [Google Scholar]
- 2. Launay F. Sports-related overuse injuries in children. Orthop Traumatol Surg Res. 2015; 101 (suppl 1): S139–S147. 10.1016/j.otsr.2014.06.030 [DOI] [PubMed] [Google Scholar]
- 3. Scharfbillig RW, Jones S, Scutter S. Sever's disease—does it effect quality of life? Foot (Edinb). 2009; 19 (1): 36–43. 10.1016/j.foot.2008.07.004 [DOI] [PubMed] [Google Scholar]
- 4. Scharfbillig RW, Jones S, Scutter SD. Sever's disease: what does the literature really tell us? J Am Podiatr Med Assoc. 2008; 98 (3): 212–223. 10.7547/0980212 [DOI] [PubMed] [Google Scholar]
- 5. McKenzie DC, Taunton JE, Clement DB, Smart GW, McNicol KL. Calcaneal epiphysitis in adolescent athletes. Can J Appl Sport Sci. 1981; 6 (3): 123–125. [PubMed] [Google Scholar]
- 6. Scharfbillig RW, Jones S, Scutter S. Sever's disease: a prospective study of risk factors. J Am Podiatr Med Assoc. 2011; 101 (2): 133–145. 10.7547/1010133 [DOI] [PubMed] [Google Scholar]
- 7. James AM, Williams CM, Luscombe M, Hunter R, Haines TP. Factors associated with pain severity in children with calcaneal apophysitis (Sever disease). J Pediatr. 2015; 167 (2): 455–459. 10.1016/j.jpeds.2015.04.053 [DOI] [PubMed] [Google Scholar]
- 8. Janssen I, Leblanc AG. Systematic review of the health benefits of physical activity and fitness in school-aged children and youth. Int J Behav Nutr Phys Act. 2010; 7:40. 10.1186/1479-5868-7-40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Eime RM, Young JA, Harvey JT, Charity MJ, Payne WR. A systematic review of the psychological and social benefits of participation in sport for children and adolescents: informing development of a conceptual model of health through sport. Int J Behav Nutr Phys Act. 2013; 10:98. 10.1186/1479-5868-10-98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Madden CC, Mellion MB. Sever's disease and other causes of heel pain in adolescents. Am Fam Physician. 1996; 54 (6): 1995–2000. [PubMed] [Google Scholar]
- 11. James AM, Williams CM, Haines TP. Effectiveness of interventions in reducing pain and maintaining physical activity in children and adolescents with calcaneal apophysitis (Sever's disease): a systematic review. J Foot Ankle Res. 2013; 6 (1): 16. 10.1186/1757-1146-6-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. James AM, Williams CM, Haines TP. Effectiveness of footwear and foot orthoses for calcaneal apophysitis: a 12-month factorial randomised trial. Br J Sports Med. 2016; 50 (20): 1268–1275. 10.1136/bjsports-2015-094986 [DOI] [PubMed] [Google Scholar]
- 13. Perhamre S, Lundin F, Klässbo M, Norlin R. A heel cup improves the function of the heel pad in Sever's injury: effects on heel pad thickness, peak pressure and pain. Scand J Med Sci Sports. 2012; 22 (4): 516–522. 10.1111/j.1600-0838.2010.01266.x [DOI] [PubMed] [Google Scholar]
- 14. Perhamre S, Lundin F, Norlin R, Klässbo M. Sever's injury: treat it with a heel cup. A randomized, crossover study with two insole alternatives. Scand J Med Sci Sports. 2011; 21 (6): e42–e47. 10.1111/j.1600-0838.2010.01140.x [DOI] [PubMed] [Google Scholar]
- 15. Wiegerinck JI, Zwiers R, Sierevelt IN, van Weert HC, van Dijk CN, Struijs PA. Treatment of calcaneal apophysitis: wait and see versus orthotic device versus physical therapy. A pragmatic therapeutic randomized clinical trial. J Pediatr Orthop. 2016; 36 (2): 152–157. 10.1097/BPO.0000000000000417 [DOI] [PubMed] [Google Scholar]
- 16. Micheli LJ, Ireland ML. Prevention and management of calcaneal apophysitis in children: an overuse syndrome. J Pediatr Orthop. 1987; 7 (1): 34–38. 10.1097/01241398-198701000-00007 [DOI] [PubMed] [Google Scholar]
- 17. James AM, Williams CM, Haines TP. Heel raises versus prefabricated orthoses in the treatment of posterior heel pain associated with calcaneal apophysitis (Sever's disease): a randomised control trial. JFoot Ankle Res. 2010; 3:3. 10.1186/1757-1146-3-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009; 42 (2): 377–381. 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Morris C Doll H Davies N et al. . The Oxford Ankle Foot Questionnaire for children: responsiveness and longitudinal validity. Qual Life Res. 2009; 18 (10): 1367–1376. 10.1007/s11136-009-9550-7 [DOI] [PubMed] [Google Scholar]
- 20. Morris C, Doll HA, Wainwright A, Theologis T, Fitzpatrick R. The Oxford Ankle Foot Questionnaire for children: scaling, reliability and validity. J Bone Joint Surg Br. 2008; 90 (11): 1451–1456. 10.1302/0301-620X.90B11.21000 [DOI] [PubMed] [Google Scholar]
- 21. Bijur PE, Silver W, Gallagher EJ. Reliability of the visual analog scale for measurement of acute pain. Acad Emerg Med. 2001; 8 (12): 1153–1157. 10.1111/j.1553-2712.2001.tb01132.x [DOI] [PubMed] [Google Scholar]
- 22. Perhamre S, Janson S, Norlin R, Klässbo M. Sever's injury: treatment with insoles provides effective pain relief. Scand J Med Sci Sports. 2011; 21 (6): 819–823. 10.1111/j.1600-0838.2010.01051.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.