Abstract
To determine whether human immunodeficiency virus type 1 (HIV-1) coreceptors besides CXCR4 and CCR5 are involved in HIV-1 infection of the thymus, we focused on CCR8, a receptor for the chemokine I-309, because of its high expression in the thymus. Similar levels of CCR8 mRNA were detected in immature and mature primary human thymocytes. Consistent with this, [125I]I-309 was shown to bind specifically and with similar affinity to the surface of immature and mature human thymocytes. Fusion of human thymocytes with cells expressing HIV-1 X4 or X4R5 envelope glycoprotein was inhibited by I-309 in a dose-dependent manner. In addition, I-309 partially inhibited productive infection of human thymocytes by X4, R5, and X4R5 HIV-1 strains. Our data provide the first evidence that CCR8 functions as an HIV-1 coreceptor on primary human cells and suggest that CCR8 may contribute to HIV-1-induced thymic pathogenesis.
As a primary site for T-cell differentiation, maturation, and selection, the thymus plays a crucial role in early childhood. Infection with human immunodeficiency virus (HIV) induces severe thymic involution in pediatric patients and results in the depletion of mature and immature thymocytes (13, 24, 29). HIV type 1 (HIV-1)-induced thymic dysfunction is associated with a fast progression to AIDS in pediatric patients (22).
CCR5-using (R5) viruses have been shown to infect mature thymocytes as well as thymic stromal cells, including macrophages, in vitro and in vivo in the SCID-hu mouse model (2, 14, 18, 35, 37). In contrast, in most cases CXCR4-using (X4) viruses have been shown to infect immature thymocytes and thymocyte precursors in vitro and to induce fast thymocyte depletion, with subsequent interruption of thymopoiesis, in vivo in SCID-hu mouse models (18, 31, 34, 35, 37, 38). Recent studies from our laboratory (42) and others (3, 17, 25, 44) have demonstrated that thymocytes express high levels of CXCR4 and low levels of CCR5 and that these receptors are involved in thymocyte infection with T-cell line-tropic and macrophage-tropic viruses, respectively (25, 42). Expression of chemokine receptor CCR8 and orphan receptors STRL33 and GPR15 has been detected in the thymus at the mRNA level (9, 20); however, whether these “minor” HIV-1 coreceptors are used in vivo for the infection of primary thymocytes or any other cell type is uncertain.
CCR8 is a human receptor for the CC chemokine I-309 (28, 36). In addition to the thymus, CCR8 mRNA is expressed in human monocytes and Th2 lymphocytes (36, 47). CCR8 has been shown to support infection by diverse HIV-1 strains, including dualtropic viruses, in CCR8-transfected cell lines (15, 30, 45). Here, we address the role of CCR8 in primary human thymocytes.
MATERIALS AND METHODS
Cell purification and culture.
Fresh thymus fragments were obtained during cardiac surgery from children (ages 1 month to 3 years) with congenital valvular malformations. The tissue was minced, large aggregates were removed by passage through a nylon mesh, and thymocytes were separated by centrifugation on a Ficoll-Paque gradient (Pharmacia Biotech, Uppsala, Sweden).
In some experiments, thymocytes were separated into CD4− CD8− double-negative (DN), CD4+ CD8+ double-positive (DP), CD8+ CD4− single-positive (CD8 SP), and CD4+ CD8− single-positive (CD4 SP) subsets using the CD4 MultiSort kit (Miltenyi Biotec Inc., Auburn, Calif.) according to the manufacturer's instructions. The separated thymocyte subsets were ≥98% pure, as verified by flow cytometry.
Chemokine binding assay.
The binding assay was performed in triplicate using 106 thymocytes per 100 μl of binding solution (1% bovine serum albumin and 0.1% sodium azide in Hanks' balanced salt solution) containing 0.2 nM [125I]I-309 and unlabeled chemokine at the concentrations indicated in the text. Following incubation at room temperature for 1 h, the cells were diluted with 1 ml of binding solution containing 0.5 M NaCl and microcentrifuged for 5 min. The supernatants were removed by aspiration, the tips of the tubes containing the pellets were excised, and radioactivity was counted in a gamma counter. The iodinated I-309 was purchased from New England Nuclear (Boston, Mass.), with a specific activity of 2,200 Ci/mmol. Unlabeled recombinant human chemokines RANTES, MIP-1α, MIP-1β, interleukin-8 (IL-8), MCP1, MCP2, MCP3, MCP4, eotaxin, lymphotactin, fractalkine, IP-10, ENA78, and TARC were purchased from Peprotech Inc. (Rocky Hill, N.J.), and I-309 was from R & D Systems (Minneapolis, Minn.). Binding data were analyzed using the program Ligand.
Flow cytometry.
The following mouse monoclonal antibodies (MAbs) against human markers were used: fluorescein isothiocyanate (FITC)-labeled anti-CD8 (Becton Dickinson, San Jose, Calif.) and Cy chrome-labeled anti-CD4 and phycoerythrin (PE)-labeled anti-CXCR4 MAbs (PharMingen, San Diego, Calif.). Cells isolated from the thymus were incubated with 5 μl of each MAb for 1 h at 4°C, washed, and analyzed on a FACScan (Becton Dickinson). Thirty thousand cells were collected per sample and analyzed with Cell Quest Software using the FL-1 (for FITC), FL-2 (for PE), and FL-3 (for Cy chrome) channels. Spectral overlap between cells stained with specific antibodies and those incubated with PE-, Cy chrome-, and FITC-conjugated isotype controls was electronically compensated for by analogue subtraction. The delta mean fluorescent channel (ΔMFC) is presented as a mean channel number derived from staining with the anti-CXCR4 MAb after subtraction of background staining with the mouse isotype control.
In some experiments, 5 × 106 thymocytes were treated with 2 μg of SDF-1α or I-309 per ml at 37°C in a CO2 incubator for 2 h before staining with anti-CXCR4 MAb.
HIV Env-dependent cell fusion assay.
12E1 T cells, which are CD4−, were infected with recombinant vaccinia viruses encoding the envelope protein (Env) from strain RF (T tropic), SF2 (T tropic), or 89.6 (dualtropic) at 10 PFU/cell. Thymocytes were mixed with either effector TF228 cells stably transfected with the IIIB/B10 envelope gene (16) or effector 12E1 cells infected with recombinant vaccinia viruses expressing T-tropic (SF2 and RF) or dualtropic (89.6) envelopes at a 1:1 ratio (105 cells each) in triplicate. Cell fusion activity was quantified after 3 to 5 h by counting syncytia. Where indicated, the chemokine SDF-1α, I-309, MIP-1β, or RANTES was added to the thymocytes for 30 to 60 min (37°C) prior to mixing with the Env-expressing effector cells.
Thymocyte infectivity assay.
CD4 SP thymocytes were inoculated with HIV-1NL4-3, HIV-1Ba-L, or the HIV-1DH125 clone of the HIV-1DH12 viral strain (32) at a multiplicity of infection (MOI) of 0.01 for 1 h, washed extensively, and cultured in the presence of IL-2 (100 U/ml) and phytohemagglutinin (1 μg/ml; Sigma) for 7 days. To determine coreceptor usage, thymocytes were incubated with MIP-1β, SDF-1α, I-309, or MCP3 (at various concentrations) for 1 to 2 h prior to infection and during the culture. Aliquots of supernatants from infected cells were collected on multiple days postinfection and analyzed for p24 by enzyme-linked immunosorbent assay (ELISA) (DuPont, Wilmington, Del.).
I-309 reverse transcriptase PCR (RT-PCR).
Total RNA was isolated from DN, DP, CD8 SP, and CD4 SP thymocyte subsets using RNAzol B solution (TelTest Inc., Friendswood, Tex.). cDNA was prepared from total RNA using oligo(dT) primers (Perkin-Elmer Cetus Inc.) and Moloney murine leukemia virus reverse transcriptase (RT) enzyme (Gibco-BRL, Gaithersburg, Md.) according to the manufacturer's instructions. Aliquots of cDNA were amplified by PCR using Taq polymerase (Perkin-Elmer Cetus, Norwalk, Conn.) and primer pairs specific for I-309 and β-actin. The CCR8 mRNA-specific primers were designed using the previously published sequence (GenBank accession no. U45983): upstream, 5′-TGG CCC TGT CTG ACC TGC TTT; downstream, 5′-GGC AGA AGT CAG CTG TTG GCT. Amplification was performed for 35 cycles with annealing temperature of 59°C for 45 s and extension at 72°C for 1 min. The amplified product had a predicted size of 599 bp. The β-actin mRNA-specific primers and PCR conditions were reported previously (43).
RESULTS
CCR8 mRNA and protein expression in thymocyte subpopulations.
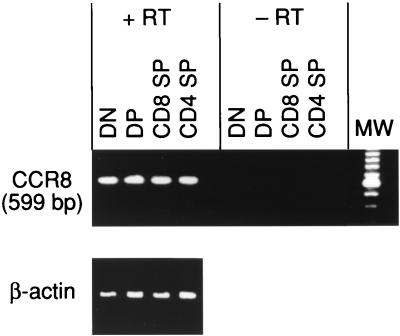
Since CCR8 mRNA was previously detected in total thymic tissue (36), it was of interest to determine the pattern of CCR8 mRNA expression in subpopulations of thymocytes. Total human thymocytes were separated into DN, DP, SP CD8, and SP CD4 thymocytes. RNA was extracted from each subset and subjected to RT-PCR using CCR8-specific primers. Similar levels of CCR8 mRNA were detected in all four thymocyte subsets (Fig. 1). In the absence of the RT during cDNA preparation, no signal was detected, confirming that the signal was not due to residual DNA.
FIG. 1.
Expression of CCR8 mRNA in thymocyte subsets. Total human thymocytes were separated into DN, DP, CD4 SP, and CD8 SP subsets. RNA was extracted from each subset and subjected to RT-PCR in the absence and presence of RT enzyme. Lane MW, size markers.
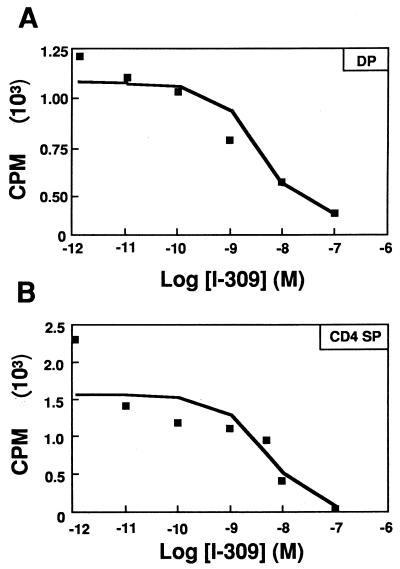
To determine whether CCR8 protein is expressed on mRNA-positive thymocytes, we carried out [125I]I-309 competition binding experiments (Fig. 2). Iodinated I-309 bound to both DP and CD4 SP thymocytes, in agreement with the pattern of mRNA expression. Binding was specific, since I-309 but not SDF-1, RANTES, MIP-1α, MIP-1β, IL-8, MCP1, MCP2, MCP3, MCP4, eotaxin, lymphotactin, fractalkine, IP-10, ENA78, and TARC could compete for labeling of the cells. Scatchard analysis of the competition curves for DP and CD4 SP cells revealed similar levels (∼10,000 sites per cell) and affinity (mean ± standard error of the mean [SEM]: Kd = 4.70 ± 1.18 nM for DP and 4.63 ± 0.35 nM for CD4 SP). The specificity and affinity agreed with CCR8 expressed in transfected 4DE4 cells, a mouse pre-B-cell lymphoma cell line (H. L. Tiffany and P. M. Murphy, unpublished data). These data are consistent with expression of CCR8 on both DP and CD4 SP thymocytes. Similar binding was also observed on DN cells (data not shown). Though CCR8 was detected on all thymocyte subsets, no Ca2+ mobilization or chemotaxis of thymocytes in response to I-309 was detected, although I-309 induced Ca2+ mobilization in CCR8-transfected cells in the same experiment. Ca2+ flux was detected in thymocytes in response to SDF-1 but not MIP-1β (42) (data not shown). Thus, the biological function of CCR8 in the thymus remains unknown.
FIG. 2.
Displacement of radiolabeled I-309 binding to immature and mature thymocytes by unlabeled I-309. Thymocytes were separated into immature DP (A) and mature CD4 SP (B) subsets and incubated with [125I]I-309 in the presence of increased concentrations of unlabeled I-309. Scatchard analysis revealed Kds (mean ± SEM, n = 3) of 4.70 ± 1.18 nM for DP and 4.63 ± 0.35 nM for CD4 SP thymocytes. The results shown are representative of three separate experiments.
I-309-mediated inhibition of thymocyte fusion with X4 and X4R5 envelope-expressing cells.
Since CCR8 has been shown to support infection of transfected cells by diverse HIV-1 strains (15, 30, 45), it was important to determine whether CCR8 could function as an HIV-1 coreceptor in primary human thymocytes. No fusion of thymocytes with R5 envelope-expressing cells was detected, as previously described (42). In contrast, thymocytes formed many syncytia with TF228 cells expressing the envelope from the prototypic X4 virus IIIB and with 12E1 expressing other X4 envelopes (Tables 1 and 2). The CXCR4 ligand SDF-1 and the CCR8 ligand I-309 inhibited fusion of primary thymocytes with X4 envelopes in a dose-dependent manner, while no inhibition by MIP-1β was observed at any dose tested (Table 1). Similar levels of fusion inhibition were observed when SDF-1 or I-309 was added to thymocyte fusion assays with other X4 envelopes (RF or SF2) and with the X4R5 envelope 89.6 (Table 2). A further increase in fusion inhibition with X4 envelopes (RF, SF2, and IIIB) was observed when both SDF-1 and I-309 were added at equal concentrations, although the additive effects never reached 100% inhibition. No inhibition was observed with the X4R5 envelope 89.6 in the presence of the β-chemokine RANTES, indicating that fusion of thymocytes with 89.6 does not involve CCR5. In control cultures, fusion between IIIB envelope-expressing cells and PM1 cells, which express CXCR4 but not CCR8, was ≥90% inhibited by SDF-1 but unaffected by I-309, confirming the specificity of the chemokine inhibition (Table 2).
TABLE 1.
I-309 and SDF-1α inhibit fusion of thymocytes with X4 envelope in a dose-dependent mannera
| Chemokine | Dose (μg/ml) | Mean no. of syncytia ± SEM | % Inhibition |
|---|---|---|---|
| None | 234 ± 26 | ||
| SDF-1 | 2.0 | 137 ± 6 | 42 |
| 1.0 | 150 ± 19 | 37 | |
| 0.1 | 177 ± 2 | 25 | |
| I-309 | 2.0 | 91 ± 7 | 61 |
| 1.0 | 130 ± 2 | 45 | |
| 0.1 | 174 ± 8 | 26 | |
| MIP-1β | 2.0 | 259 ± 7 | 0 |
| 0.1 | 246 ± 9 | 0 |
Total human thymocytes were incubated with the indicated chemokines. After 1 h of incubation, thymocytes were mixed at a 1:1 ratio with effector cells, which were TF228 cells expressing IIIB envelope. Syncytia were counted after 4 to 5 h. Percentages of inhibition were calculated using the formula (b − a)/b × 100, where a is the number of syncytia in the presence and b is the number of syncytia in the absence of chemokines. Data represent three experiments.
TABLE 2.
I-309 and SDF-1α inhibit fusion of human thymocytes with X4 and X4R5 envelope-expressing cellsa
| Cells | Envelope | Chemokine | Dose (μg/ml) | Mean no. of syncytia ± SEM | % Inhibition |
|---|---|---|---|---|---|
| Thymocytes | IIIB | None | 271 ± 12 | ||
| SDF-1 | 2 | 137 ± 25 | 50 | ||
| I-309 | 2 | 172 ± 26 | 38 | ||
| SDF-1 + 1-309 | 2 + 2 | 73 ± 2 | 74 | ||
| RANTES | 2 | 261 ± 2 | 5 | ||
| RF | None | 331 ± 15 | |||
| SDF-1 | 2 | 135 ± 15 | 59 | ||
| I-309 | 2 | 200 ± 32 | 40 | ||
| SDF-1 + I-309 | 2 + 2 | 30 ± 3 | 91 | ||
| RANTES | 2 | 338 ± 22 | 0 | ||
| SF2 | None | 110 ± 7 | |||
| SDF-1 | 2 | 59 ± 12 | 47 | ||
| I-309 | 2 | 79 ± 11 | 28 | ||
| SDF-1 + I-309 | 2 + 2 | 28 ± 7 | 75 | ||
| RANTES | 2 | 104 ± 4 | 0 | ||
| 89.6 | None | 82 ± 1 | |||
| SDF-1 | 2 | 35 ± 1 | 68 | ||
| I-309 | 2 | 60 ± 12 | 27 | ||
| SDF-1 + I-309 | 2 + 2 | 23 ± 3 | 72 | ||
| RANTES | 2 | 84 ± 16 | 0 | ||
| PM1 | IIIB | None | 387 ± 41 | ||
| SDF-1 | 1 | 36 ± 17 | 91 | ||
| I-309 | 1 | 449 ± 43 | 0 |
Thymocytes or PM1 cells were incubated with chemokines at the indicated concentrations at 37°C. After 1 h of incubation, cells were mixed (at a 1:1 ratio) with effector cells infected with recombinant vaccinia viruses expressing IIIB, RF, SF2, or 89.6 envelopes. Syncytia were scored after 4 to 5 h. Percent reduction in syncytium formation was calculated as described in Table 1, footnote a. Data represent two experiments.
Role of CCR8 in productive infection of mature thymocytes with X4, R5, and X4R5 HIV-1.
To determine whether CCR8 supported the productive infection of thymocytes, CD4 SP thymocytes were incubated with chemokines for 1 to 2 h prior to HIV-1NL4-3 adsorption, and during the course of infection. I-309 and SDF-1α, but not MCP3, inhibited p24 production by NL4-3-infected thymocytes (Table 3). Both chemokines inhibited NL4-3 infection of mature thymocytes in a dose-dependent manner; however, SDF-1α inhibited infection more efficiently than I-309. Importantly, when SDF-1α and I-309 were mixed together, an additive inhibitory effect was observed, in agreement with the results from our thymocyte fusion assay (Tables 2 and 3).
TABLE 3.
I-309 and SDF-1α inhibit infection of thymocytes with HIV-1NL4-3 in a dose-dependent mannera
| Day after infection | Chemokine | Dose (μg/ml) | Mean p24 concn (pg/ml) ± SEM | % Inhibition |
|---|---|---|---|---|
| 5 | None | 1,038.7 ± 18.7 | ||
| I-309 | 2.0 | 629.8 ± 16.7 | 40 | |
| 0.2 | 770.3 ± 2.8 | 26 | ||
| SDF-1α | 2.0 | 287.9 ± 1.1 | 72 | |
| 0.2 | 529.8 ± 27 | 49 | ||
| I-309 + SDF-1α | 1.0 + 1.0 | 76.9 ± 20.8 | 93 | |
| MCP3 | 2.0 | 1,421 ± 133.1 | 0 | |
| 7 | None | 9,847.5 ± 627.6 | ||
| I-309 | 2.0 | 4,541.2 ± 53.0 | 54 | |
| 0.2 | 8,578.7 ± 141.4 | 13 | ||
| SDF-1α | 2.0 | 1,841.2 ± 0 | 81 | |
| 0.2 | 6,185 ± 97.2 | 37 | ||
| I-309 + SDF-1α | 1.0 + 1.0 | 241.2 ± 229.7 | 98 |
All chemokines were added to CD4 SP thymocytes 1 to 2 h prior to infection and then maintained at the indicated concentrations during culture. Thymocytes were infected with HIV-1NL4-3 at an MOI of 0.01; supernatants were harvested at the indicated times and analyzed for p24 by ELISA. Chemokine-mediated inhibition of p24 production was calculated by comparison with control cultures without chemokines.
In a similar set of experiments, the CD4 SP thymocytes were infected with the HIV-1 R5 viral strain Ba-L in the absence or presence of chemokines. In agreement with data reported from our laboratory (42), the productive infection of mature thymocytes with Ba-L was inhibited by MIP-1β (99% inhibition) but not by SDF-1α (Table 4). In addition, it was found that I-309 partially inhibited p24 production by Ba-L-infected thymocytes (27 to 57%). When the inhibitory activities of MIP-1β and I-309 were compared side by side using thymocytes from the same donor, MIP-1β was found to be a more effective inhibitor than I-309 when the chemokines were used at 1.0 or 0.1 μg/ml (Table 4). The data suggested that CCR5 is the preferred coreceptor for macrophage-tropic strains during infection of SP thymocytes.
TABLE 4.
MIP-1β and I-309 but not SDF-1α inhibit infection of human thymocytes with HIV-1Ba-La
| Expt. | Chemokine | Dose (μg/ml) | Mean p24 concn (ng/ml) | % Inhibition |
|---|---|---|---|---|
| I | None | 29.4 ± 1.1 | ||
| MIP-1β | 0.1 | 1.8 ± 0.2 | 99 | |
| I-309 | 0.1 | 14.3 ± 0.6 | 40 | |
| SDF-1α | 0.1 | 27.2 ± 1.5 | 0 | |
| II | None | 187.8 ± 20.0 | ||
| MIP-1β | 1.0 | 12.4 ± 1.1 | 93 | |
| 0.1 | 22.6 ± 2.2 | 88 | ||
| I-309 | 1.0 | 136.0 ± 8.3 | 27 | |
| 0.1 | 192.0 ± 12.5 | 0 | ||
| SDF-1 | 1.0 | 223.4 ± 18.6 | 0 | |
| III | None | 612.7 ± 21.7 | ||
| MIP-1β | 0.1 | 18.2 ± 3.3 | 97 | |
| I-309 | 0.1 | 387.3 ± 22.3 | 57 | |
| MIP-1β + I-309 | 0.1 + 0.1 | 38.7 ± 2.0 | 94 | |
| MIP-1β | 0.01 | 415.2 ± 34.7 | 32 | |
| I-309 | 0.01 | 265.1 ± 18.9 | 37 | |
| MIP-1β + I-309 | 0.01 + 0.01 | 246.2 ± 18.9 | 60 |
CD4 SP thymocytes were infected with HIV-1Ba-L (MOI, 0.01). Supernatants were harvested on day 6 for experiments 1 and 2 and on day 7 for experiment 3. Chemokine treatment and p24 analyses were performed as described in Table 3, footnote a.
To determine whether CCR8 plays a role in the infection of thymocytes with X4R5 viruses, the CD4 SP thymocytes were infected with the DH125 viral strain. Production of p24 was detected in thymocytes infected with DH125, and p24 production by infected thymocytes was inhibited at low levels by I-309 and SDF-1 but not by MIP-1β (Table 5). No further increase in the inhibition of infection was observed when SDF-1 and I-309 were added together (data not shown).
TABLE 5.
Thymocyte infection with DH125 dualtropic HIV-1 is partially inhibited by I-309 and SDF-1α but not by MIP-1βa
| Chemokine | Mean p24 concn (ng/ml) ± SEM | % Inhibition |
|---|---|---|
| None | 79.0 ± 0.78 | |
| I-309 | 64.5 ± 2.3 | 18 |
| SDF-1α | 55.6 ± 0.78 | 29 |
| I-309 + SDF-1α | 61.2 ± 0.78 | 22 |
| MIP-1β | 74.0 ± 3.1 | 6 |
CD4 SP thymocytes were infected with HIV-1DH125 at an MOI of 0.01. Chemokines were used at 2 μg/ml. Supernatants were harvested on day 10. Chemokine treatment and p24 analyses were performed as described in Table 3, footnote a.
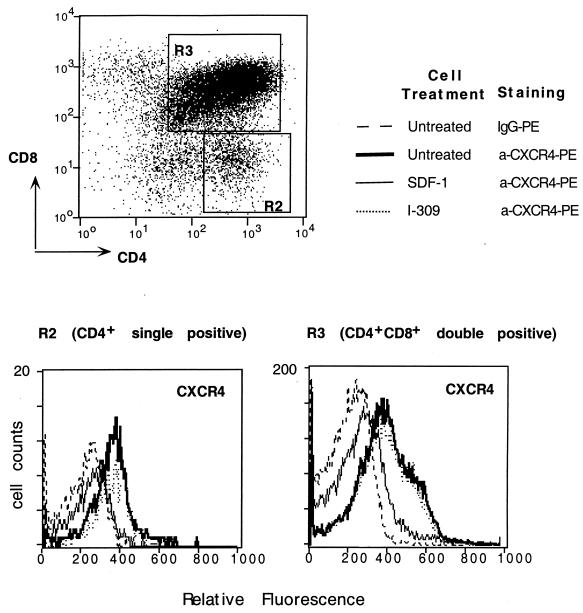
SDF-1 but not I-309 induces downregulation of CXCR4 expression on human thymocytes.
Since I-309 inhibited infection of human thymocytes with X4 viruses, it was possible that this inhibitory effect is mediated by cross-down regulation of CXCR4 induced by I-309 via CCR8. To test this hypothesis, human thymocytes were left untreated or incubated with SDF-1 or I-309 at 37°C for 2 h and subjected to three-color immunofluorescence analysis. Mature CD4 SP thymocytes and immature DP thymocytes expressed significant levels of CXCR4 (ΔMFC 133 and 198, respectively) (Fig. 3). Pretreatment of thymocytes with 2 μg of SDF-1 per ml induced a reduction in CXCR4 expression on both CD4 SP and DP thymocytes (ΔMFC 36 and 68, respectively). In contrast, I-309 (and MIP-1β; data not shown) used at the same concentration did not affect CXCR4 expression on thymocytes (ΔMFC, 148 and 193 on CD4 SP and DP thymocytes, respectively). Thus, no evidence for down modulation of surface CXCR4 following I-309 treatment of thymocytes was found.
FIG. 3.
CXCR4 expression on human thymocytes treated with SDF-1 or I-309. Three-color immunofluorescence analysis was performed for CD4/CD8 and CXCR4 markers on total human thymocytes. Cells were stained with Cy chrome-conjugated anti-CD4 and FITC-conjugated anti-CD8 MAbs. CD4 SP or DP thymocytes were gated and analyzed for CXCR4 expression using the PE-12G5 MAb in untreated thymocytes (thick lines) or thymocytes pretreated with SDF-1 (thin lines) or I-309 (dotted lines). Control cells were stained with PE-conjugated mouse isotype control immunoglobulin G (IgG) (broken lines).
DISCUSSION
Here we have demonstrated that CCR8 mRNA is expressed at similar levels in immature and mature human thymocytes, in contrast to the pattern of CCR8 distribution in the murine thymus, where CCR8 mRNA expression was shown to be associated with cells of the CD4+ lineage (47). Consistent with mRNA expression, similar levels of I-309 binding to DP and CD4 SP thymocyte subsets were detected. No signaling in response to I-309 was observed (data not shown), suggesting that CCR8 may not be involved in the migration of thymocytes. However, our data have demonstrated that CCR8 on thymocytes can be used as a coreceptor by some X4, R5, and X4R5 HIV-1 strains. This conclusion was supported by two types of experiments: I-309-mediated inhibition of thymocyte fusion with cells expressing X4 or X4R5 envelopes, and I-309-mediated inhibition of p24 production by thymocytes infected with X4, R5, and X4R5 viral strains. Our interpretation rests on the monospecificity of I-309 for CCR8, which is strongly suggested from studies of binding to primary cells and other known chemokine receptors.
More than 90% of thymocytes express CD4, and all thymocytes express CXCR4, rendering thymocytes vulnerable to infection by X4 viruses (17, 25). In our current study, we have demonstrated that productive infection of mature thymocytes by a laboratory-adapted X4 virus, NL4-3, is sensitive to both SDF-1 and I-309 inhibition. These findings suggest that both coreceptors, CXCR4 and CCR8, could be used by X4 viruses in vivo. The mechanism of I-309-mediated inhibition of X4 viral infection could not be attributed to an indirect CXCR4 down regulation, since only SDF-1 induced a reduction in the level of CXCR4 expression on thymocytes.
In the SCID-hu model, it was demonstrated that the HIV-1NL4-3 strain induces depletion of thymocytes both in the cortex and in the medulla, indicating that immature and mature thymocytes in vivo are susceptible to NL4-3 infection (2). The infection of thymocytes with R5 HIV-1 in vitro and in vivo was shown to exhibit a different pattern of pathogenesis than infection with X4 viruses (14, 18, 35, 37). In most reports, R5 infection was more restricted to mature thymocytes, macrophages, and dendritic cells (2, 6, 25). In the current study, we have confirmed the earlier findings that infection of thymocytes with R5 viruses is mediated by CCR5 (25, 42). In addition, we now demonstrate that CCR8 may also support R5 viral infection of mature thymocytes, in agreement with earlier findings that CCR8 can support HIV-1 X4- and R5-mediated infection of CCR8-transfected cells (15). When I-309 and MIP-1β were used side by side to inhibit Ba-L-mediated infection of thymocytes, MIP-1β was found to be more effective than I-309. These data suggested that R5 viruses may prefer to use CCR5 over CCR8 on SP thymocytes. However, our data do not exclude the possibility that some R5 viruses may use CCR8 to establish infection in SP or DP thymocytes in vivo.
In addition to X4 and R5 viruses, we tested the usage of CXCR4, CCR5, and CCR8 by the dualtropic viruses 89.6 and HIV-1DH125 (11, 30, 32). The ability of both dualtropic viruses to use CCR8 for infection of human thymocytes observed in our experiments is in agreement with the previously published observation that 89.6 and HIV-1DH123 can use CCR8 in transfected cell lines (30, 45). In addition, DH12 and its clones (DH123 and DH125) were shown to use CCR5 and CXCR4 (11, 19, 45). However, in our experiments, infection of thymocytes with DH125 was moderately inhibited (10 to 30%) by I-309 and SDF-1 but not by MIP-1β. These findings suggest that infection of thymocytes by DH125 is supported by CCR8 and CXCR4 but not by CCR5, although all three receptors are expressed on mature CD4 SP thymocytes. The low sensitivity of thymocyte infection with DH125 to inhibition with MIP-1β observed in our experiments is in agreement with a previously published observation on the low sensitivity of DH12-infected peripheral blood mononuclear cells to inhibition with β-chemokines (7). Similarly, the dualtropic virus 89.6 was shown to use CXCR4 and CCR5 for fusion in vitro; however, only CXCR4 was shown to support infection by 89.6 of tonsillar tissue in ex vivo culture (12). Thus, our data further support the notion that the results obtained with transfected cell lines in vitro may differ from those obtained with primary cells and that, on primary cells, dualtropic viruses may preferentially use certain coreceptors. For example, it was reported that some primary dualtropic isolates use CXCR4 on macrophages regardless of whether CCR5 is present (41). The dualtropic 89.6 strain used CCR5 for fusion with macrophages derived from normal individuals (41) and CXCR4 on macrophages derived from CCR5Δ32 homozygous individuals who lack functional CCR5 (40). These data suggest that coreceptor usage by HIV-1 strains with various tropisms may also depend on the microenvironment, i.e., the pattern of multiple coreceptors expressed by the same target cell.
It is interesting to note that in our thymocyte experiments, the sensitivity of the fusion or infection observed with the dualtropic viruses 89.6 and DH125 to chemokine-mediated inhibition was different from the sensitivity of X4 or R5 viruses. In the thymocyte fusion assay with X4 viral envelopes and in the productive infection of thymocytes with HIV-1NL4-3, an additive effect was observed in the presence of both SDF-1 and I-309. In contrast, no increase in the inhibition of thymocyte fusion with 89.6 envelope or in the thymocyte infection with DH125 was detected with both SDF-1 and I-309. The reasons for the differences in the inhibitory patterns of SDF-1 and I-309 on the fusion or infection of X4 versus X4R5 viruses with thymocytes are not understood. X4 and X4R5 viruses were shown to use different epitopes on CXCR4 (4, 21, 27), and epitopes on the chemokine receptors recognized by gp120 and by chemokines are only partially overlapping (10, 23). Therefore, it is possible that the dualtropic envelopes may interact with the epitopes within the coreceptor that are not completely masked by the chemokines. In this scenario, envelope binding to the coreceptor may proceed in spite of a previously bound chemokine molecule, albeit at a lower rate. In addition, the 2-μg/ml dose of I-309 used to inhibit HIV-1 infection of thymocytes was 1,000-fold higher than is required to saturate all CCR8 receptors expressed on the 106 thymocytes used for the infection. Thus, even at saturation, no complete inhibition was achieved, suggesting that chemokine-mediated inhibition may also depend on envelope binding affinity, cooperativity, and potentially other effects independent of the direct ligand-receptor interaction.
The potential of the immune system to regenerate in HIV-1-infected infants and young adults is largely dependent on the ability to control HIV-1 replication within the thymus. Antiretroviral therapy was shown to suppress virus replication only transiently in human thymic implants (1). However, effective inhibition of viral replication was achieved when multidrug therapy was initiated immediately after infection of the thymic implant (26). Since the thymic microenvironment preserves its ability to support endogenous progenitor cell differentiation following exposure to HIV-1 (39), it is important to identify which coreceptors within the thymus should be targeted by inhibitors in combination with multidrug therapy to ensure control of viral replication. Although multiple coreceptors have been shown to support HIV-1 infection of transfected cell lines in vitro, it was suggested that in vivo only CXCR4 and CCR5 should be targeted by inhibitors (46). In this regard, it is important to note that differential distribution of viral variants may occur among different body compartments in a single individual, resulting in the accumulation of specific and perhaps unique HIV-1 variants in some tissues, such as lungs and thymus (5, 33). Since a higher degree of V3 loop heterogeneity was reported for thymus-derived versus blood-derived HIV-1 isolates (5), it is possible that in addition to CXCR4 and CCR5, other coreceptors such as CCR8 play an important role in the infection of primary thymocytes.
Up to now, only 3 of the 15 or so HIV coreceptors have passed the important test of in vivo relevance (CCR5, CXCR4, and to a lesser degree CCR3). Our data provide direct evidence that CCR8 also can be used by HIV strains for infection of primary cells. Thus, CCR8 should be considered a target for antiretroviral drug development.
ACKNOWLEDGMENTS
M. Zaitseva was supported by a grant from the Office of Women's Health, Food and Drug Administration.
We are grateful to B. F. Akl and C. Hill of Virginia Heart Surgery Associates (Fairfax, Va.) and the cardiac operating room nurses of Fairfax Hospital (Fairfax, Va.) for assistance in obtaining the pediatric thymic tissues. We thank M. Martin and K. Peden for help with viral stocks. We thank C. Lapham and K. Peden for critical reviews of the manuscript and J. Manischewitz for technical help.
REFERENCES
- 1.Amado R G, Jamieson B D, Cortado R, Cole S W, Zack J A. Reconstitution of human thymic implants is limited by human immunodeficiency virus breakthrough during antiretroviral therapy. J Virol. 1999;73:6361–6369. doi: 10.1128/jvi.73.8.6361-6369.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berkowitz R D, Alexander S, Bare C, Linquist-Stepps V, Bogan M, Moreno M E, Gibson L, Wieder E D, Kosek J, Stoddart C A, McCune J M. CCR5- and CXCR4-utilizing strains of human immunodeficiency virus type 1 exhibit differential tropism and pathogenesis in vivo. J Virol. 1998;72:10108–10117. doi: 10.1128/jvi.72.12.10108-10117.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berkowitz R D, Beckerman K P, Schall T J, McCune J M. CXCR4 and CCR5 expression delineates targets for HIV-1 disruption of T cell differentiation. J Immunol. 1998;161:3702–3710. [PubMed] [Google Scholar]
- 4.Brelot A, Heveker N, Pleskoff O, Sol N, Alizon M. Role of the first and third extracellular domains of CXCR-4 in human immunodeficiency virus coreceptor activity. J Virol. 1997;71:4744–4751. doi: 10.1128/jvi.71.6.4744-4751.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calabrò M L, Zanotto C, Calderazzo F, Crivellaro C, Del Mistro A, De Rossi A, Chieco-Bianchi L. HIV-1 infection of the thymus: evidence for a cytopathic and thymotropic viral variant in vivo. AIDS Res Hum Retroviruses. 1995;11:11–19. doi: 10.1089/aid.1995.11.11. [DOI] [PubMed] [Google Scholar]
- 6.Cameron P U, Lowe M G, Sotzik F, Coudhlan A F, Crowe S M, Shortman K. The infection of macrophage and non-macrophage tropic isolates of HIV-1 with thymic and tonsillar dendritic cells in vitro. J Exp Med. 1996;183:1851–1856. doi: 10.1084/jem.183.4.1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cho M W, Lee M K, Carney M C, Berson J F, Doms R W, Martin M A. Identification of determinants on a dualtropic human immunodeficiency virus type 1 envelope glycoprotein that confer usage of CXCR4. J Virol. 1998;72:2509–2515. doi: 10.1128/jvi.72.3.2509-2515.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dairaghi D J, Fan R A, McMaster B E, Hanley M R, Schall T J. HHV8-encoded vMIP-I selectively engages chemokine receptor CCR8: agonist and antagonist profiles of viral chemokines. J Biol Chem. 1999;274:21569–21574. doi: 10.1074/jbc.274.31.21569. [DOI] [PubMed] [Google Scholar]
- 9.Deng H K, Unutmaz D, KewalRamani V N, Littman D R. Expression cloning of new receptors used by simian and human immunodeficiency viruses. Nature. 1997;388:296–300. doi: 10.1038/40894. [DOI] [PubMed] [Google Scholar]
- 10.Doranz B J, Orsini M J, Turner J D, Hoffman T L, Berson J F, Hoxie J A, Peiper S C, Brass L F, Doms R W. Identification of CXCR4 domains that support coreceptor and chemokine receptor functions. J Virol. 1999;73:2752–2761. doi: 10.1128/jvi.73.4.2752-2761.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dragic T, Trkola A, Lin S W, Nagashima K A, Kajumo F, Zhao L, Olson W L, Mackay C R, Allaway G P, Sakmar T P, Moore J P, Maddon P J. Amino-terminal substitutions in the CCR5 coreceptor impair gp120 binding and human immunodeficiency virus type 1 entry. J Virol. 1998;72:279–285. doi: 10.1128/jvi.72.1.279-285.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Glushakova S, Yi Y, Grivel J C, Singh A, Schols D, De Clercq E, Collman R G, Margolis L. Preferential coreceptor utilization and cytopathicity by dual-tropic HIV-1 in human lymphoid tissue ex vivo. J Clin Investig. 1999;104:R7–R11. doi: 10.1172/JCI7403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grody W W, Fligiel S, Naeim F. Thymus involution in the acquired immunodeficiency syndrome. Am J Clin Pathol. 1985;84:85–95. doi: 10.1093/ajcp/84.1.85. [DOI] [PubMed] [Google Scholar]
- 14.Hays E F, Uittenbogaart C H, Brewer J C, Vollger L W, Zack J A. In vitro studies of HIV-1 expression in thymocytes from infants and children. AIDS. 1992;6:265–272. doi: 10.1097/00002030-199203000-00003. [DOI] [PubMed] [Google Scholar]
- 15.Horuk R, Hesselgesser J, Zhou Y, Faulds D, Halks-Miller M, Harvey S, Taub D, Samson M, Parmenteir M, Rucker J, Doranz B, Doms R. The CC chemokine I-309 inhibits CCR8-dependent infection by diverse HIV-1 strains. J Biol Chem. 1998;273:386–391. doi: 10.1074/jbc.273.1.386. [DOI] [PubMed] [Google Scholar]
- 16.Jonak Z, Clark R, Matour D, Trulli S, Craig R, Henri E, Lee E, Creig R, Debouck C. A human lymphoid recombinant cell line with functional human immunodeficiency virus type 1 envelope. AIDS Res Hum Retrovir. 1993;9:23–32. doi: 10.1089/aid.1993.9.23. [DOI] [PubMed] [Google Scholar]
- 17.Kitchen S G, Zack J A. CXCR4 expression during lymphopoiesis: implications for human immunodeficiency virus type 1 infection of the thymus. J Virol. 1997;71:6928–6934. doi: 10.1128/jvi.71.9.6928-6934.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kollmann T R, Kim A, Pettoello-Mantovani M, Hachamovitch M, Rubinstein A, Goldstein M M, Goldstein H. Divergent effects of chronic HIV-1 infection on human thymocyte maturation in SCID-hu mice. J Immunol. 1995;154:907–921. [PubMed] [Google Scholar]
- 19.Lee M K, Heaton J, Cho M W. Identification of determinants of interaction between CXCR4 and gp120 of a dual-tropic HIV-1DH12 isolate. Virology. 1999;257:290–296. doi: 10.1006/viro.1999.9686. [DOI] [PubMed] [Google Scholar]
- 20.Liao F, Alkhatib G, Peden K W C, Sharma G, Berger E A, Farber J M. STRL33, a novel chemokine receptor-like protein, functions as a fusion cofactor for both macrophage-tropic and T cell line-tropic HIV-1. J Exp Med. 1997;185:2015–2023. doi: 10.1084/jem.185.11.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McKnight A, Wilkinson D, Simmons G, Talbot S, Picard L, Ahuja M, Marsh M, Hoxie J A, Clapham P R. Inhibition of human immunodeficiency virus fusion by a monoclonal antibody to a coreceptor (CXCR4) is both cell type and virus strain dependent. J Virol. 1997;71:1692–1696. doi: 10.1128/jvi.71.2.1692-1696.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nahmias A J, Clark W S, Kourtis A P, Lee F K, Cotsonis G, Ibegbu C, Thea D, Palumbo P, Vink P, Simonds R J, Nesheim S R. Thymic dysfunction and time of infection predict mortality in human immunodeficiency virus-infected infants. CDC Perinatal AIDS Collaborative Transmission Study Group. J Infect Dis. 1998;178:680–685. doi: 10.1086/515368. [DOI] [PubMed] [Google Scholar]
- 23.Olson W C, Rabut G E, Nagashima K A, Tran D N, Anselma D J, Monard S P, Segal J P, Thompson D A, Kajumo F, Guo Y, Moore J P, Maddon P J, Dragic T. Differential inhibition of human immunodeficiency virus type 1 fusion, gp120 binding, and CC-chemokine activity by monoclonal antibodies to CCR5. J Virol. 1999;73:4145–4155. doi: 10.1128/jvi.73.5.4145-4155.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Papiernik M, Brossard Y, Mulliez N, Roume C, Brechot C, Barin F, Goudeau A, Bach J F, Griscelli C, Henrion R, Vazeux R. Thymus abnormalities in fetuses aborted from human immunodeficiency virus type 1 seropositive women. Pediatrics. 1992;89:297–301. [PubMed] [Google Scholar]
- 25.Pedroza-Martins L, Gurney K B, Torbett B E, Uittenbogaart C H. Differential tropism and replication kinetics of human immunodeficiency virus type 1 isolates in thymocytes: coreceptor expression allows viral entry, but productive infection of distinct subsets is determined at the postentry level. J Virol. 1998;72:9441–9452. doi: 10.1128/jvi.72.12.9441-9452.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pettoello-Mantovani M, Kollmann T R, Katopodis N F, Raker C, Kim A, Yurasov S, Wiltshire H, Goldstein H. thy/liv-SCID-hu mice: a system for investigating the in vivo effects of multidrug therapy on plasma viremia and human immunodeficiency virus replication in lymphoid tissues. J Infect Dis. 1998;177:337–346. doi: 10.1086/514214. [DOI] [PubMed] [Google Scholar]
- 27.Picard L, Wilkinson D A, McKnight A, Gray P W, Hoxie J A, Clapham P R, Weiss R A. Role of the amino-terminal extracellular domain of CXCR-4 in human immunodeficiency virus type 1 entry. Virology. 1997;231:105–111. doi: 10.1006/viro.1997.8506. [DOI] [PubMed] [Google Scholar]
- 28.Roos R S, Loetscher M, Legler D F, Clark-Lewis I, Baggiolini M, Moser B. Identification of CCR8, the receptor for the human CC chemokine I-309. J Biol Chem. 1997;272:17251–17254. doi: 10.1074/jbc.272.28.17251. [DOI] [PubMed] [Google Scholar]
- 29.Rosenzweig M, Clark D P, Gaulton G N. Selective thymocyte depletion in neonatal HIV-1 thymic infection. AIDS. 1993;7:1601–1605. doi: 10.1097/00002030-199312000-00009. [DOI] [PubMed] [Google Scholar]
- 30.Rucker J, Edinger A L, Sharron M, Samson M, Lee B, Berson J F, Yi Y, Margulies B, Collman R G, Doranz B J, Parmentier M, Doms R W. Utilization of chemokine receptors, orphan receptors, and herpesvirus-encoded receptors by diverse human and simian immunodeficiency viruses. J Virol. 1997;71:8999–9007. doi: 10.1128/jvi.71.12.8999-9007.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schnittman S M, Denning S M, Greenhouse J J, Justement J S, Baseler M, Kurtzberg J, Haynes B F, Fauci A S. Evidence for susceptibility of intrathymic T-cell precursors and their progeny carrying T-cell antigen receptor phenotypes TCRαβ+ and TCRγδ+ to human immunodeficiency virus infection: a mechanism for CD4+ (T4) lymphocyte depletion. Proc Natl Acad Sci USA. 1990;87:7727–7731. doi: 10.1073/pnas.87.19.7727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shibata R, Hoggan M D, Broscius C, Englund G, Theodore T S, Buckler-White A, Arthur L O, Israel Z, Schultz A, Lane H C, Martin M M. Isolation and characterization of a syncytium-inducing, macrophage/T-cell line-tropic human immunodeficiency virus type 1 isolate that readily infects chimpanzee cells in vitro and in vivo. J Virol. 1995;69:4453–4462. doi: 10.1128/jvi.69.7.4453-4462.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Singh A, Besson G, Mobasher A, Collman R G. Patterns of chemokine receptor fusion cofactor utilization by human immunodeficiency virus type 1 variants from the lungs and blood. J Virol. 1999;73:6680–6690. doi: 10.1128/jvi.73.8.6680-6690.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stanley S K, McCune J M, Kaneshima H, Justement J S, Sullivan M, Boone E, Baseler M, Adelsberger J, Bonyhadi M, Orenstein J, Fox C H, Fauci A S. Human immunodeficiency virus infection of the human thymus and disruption of the thymic microenvironment in the SCID-hu mouse. J Exp Med. 1993;178:1151–1163. doi: 10.1084/jem.178.4.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Su L, Kaneshima H, Bonyhadi M, Salimi S, Kraft D, Rabin L, McCune J M. HIV-1-induced thymocyte depletion is associated with indirect cytopathicity and infection of progenitor cells in vivo. Immunity. 1995;2:25–36. doi: 10.1016/1074-7613(95)90076-4. [DOI] [PubMed] [Google Scholar]
- 36.Tiffany H, Lautens L, Gao J, Pease J, Locati M, Combadiere C, Modi W, Bonner T, Murphy P. Identification of CCR8: a human monocyte and thymus receptor for the chemokine I309. J Exp Med. 1998;186:165–170. doi: 10.1084/jem.186.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Uittenbogaart C H, Anisman D J, Jamieson B D, Kitchen S, Schmid I, Zack J A, Hays E F. Differential tropism of HIV-1 isolates for distinct thymocyte subsets in vitro. AIDS. 1996;10:F9–F16. doi: 10.1097/00002030-199606001-00001. [DOI] [PubMed] [Google Scholar]
- 38.Valentin H, Nugeyre M T, Vuillier F, Boumsell L, Schmid M, Barre-Sinoussi F, Pereira R A. Two subpopulations of human triple-negative thymic cells are susceptible to infection by human immunodeficiency virus type 1 in vitro. J Virol. 1994;68:3041–3050. doi: 10.1128/jvi.68.5.3041-3050.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Withers-Ward E S, Amado R G, Koka P S, Jamieson B D, Kaplan A H, Chen I S, Zack J A. Transient renewal of thymopoiesis in HIV-infected human thymic implants following antiviral therapy. Nat Med. 1997;3:1102–1109. doi: 10.1038/nm1097-1102. [DOI] [PubMed] [Google Scholar]
- 40.Yi Y, Rana S, Turner J D, Gaddis N, Collman R G. CXCR4 is expressed by primary macrophages and supports CCR5-independent infection by dual-tropic but not T-tropic isolates of human immunodeficiency virus type 1. J Virol. 1998;72:772–777. doi: 10.1128/jvi.72.1.772-777.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yi Y, Isaacs S N, Williams D A, Frank I, Schols D, De Clercq E, Kolson D L, Collman R G. Role of CXCR4 in cell-cell fusion and infection of monocyte-derived macrophages by primary human immunodeficiency virus type 1 (HIV-1) strains: two distinct mechanisms of HIV-1 dual tropism. J Virol. 1999;73:7117–7125. doi: 10.1128/jvi.73.9.7117-7125.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zaitseva M B, Lee S, Rabin R L, Tiffany H L, Farber J M, Peden K W, Murphy P M, Golding H. CXCR4 and CCR5 on human thymocytes: biological function and role in HIV-1 infection. J Immunol. 1998;161:3103–3113. [PubMed] [Google Scholar]
- 43.Zaitseva M B, Golding H, Betts M, Yamauchi A, Bloom E T, Butler L E, Stevan L, Golding B. Human peripheral blood CD4+ and CD8+ T cells express Th1-like cytokine mRNA and proteins following in vitro stimulation with heat-inactivated Brucella abortus. Infect Immun. 1995;63:2720–2728. doi: 10.1128/iai.63.7.2720-2728.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang L, He T, Talal A, Wang G, Frankel S S, Ho D D. In vivo distribution of the human immunodeficiency virus/simian immunodeficiency virus coreceptors CXCR4, CCR3, and CCR5. J Virol. 1998;72:5035–5045. doi: 10.1128/jvi.72.6.5035-5045.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang Y J, Dragic T, Cao Y, Kostrikis L, Kwon D S, Littman D R, Kewalramani V N, Moore J P. Use of coreceptors other than CCR5 by non-syncytium-inducing adult and pediatric isolates of human immunodeficiency virus type 1 is rare in vitro. J Virol. 1998;72:9337–9344. doi: 10.1128/jvi.72.11.9337-9344.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang Y J, Moore J P. Will multiple coreceptors need to be targeted by inhibitors of human immunodeficiency virus type 1 entry? J Virol. 1999;73:3443–3448. doi: 10.1128/jvi.73.4.3443-3448.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zingoni A, Soto H, Hedrick J A, Stoppacciaro A, Storlazzi C T, Sinigaglia F, D'Ambrosio D, O'Garra A, Robinson D, Rocchi M, Santoni A, Zlotnik A, Napolitano M. The chemokine receptor CCR8 is preferentially expressed in Th2 but not Th1 cells. J Immunol. 1998;161:547–551. [PubMed] [Google Scholar]