Abstract
Background
In the mammalian retina, intrinsically-photosensitive retinal ganglion cells (ipRGC) detect light and integrate signals from rods and cones to drive multiple non-visual functions including circadian entrainment and the pupillary light response (PLR). Non-visual photoreception and consequently non-visual sensitivity to light may change across child development. The PLR represents a quick and reliable method for examining non-visual responses to light in children. The purpose of this study was to assess differences in the PLRs to blue and red stimuli, measured one hour prior to bedtime, between children and adolescents.
Methods
Forty healthy participants (8–9 years, n = 21; 15–16 years, n = 19) completed a PLR assessment 1 h before their habitual bedtime. After a 1 h dim-light adaptation period (< 1 lx), baseline pupil diameter was measured in darkness for 30 s, followed by a 10 s exposure to 3.0 × 1013 photons/cm2/s of either red (627 nm) or blue (459 nm) light, and a 40 s recovery in darkness to assess pupillary re-dilation. Subsequently, participants underwent 7 min of dim-light re-adaptation followed by an exposure to the other light condition. Lights were counterbalanced across participants.
Results
Across both age groups, maximum pupil constriction was significantly greater (p < 0.001, ηp2 = 0.48) and more sustained (p < 0.001, ηp2 = 0.41) during exposure to blue compared to red light. For adolescents, the post-illumination pupillary response (PIPR), a hallmark of melanopsin function, was larger after blue compared with red light (p = 0.02, d = 0.60). This difference was not observed in children. Across light exposures, children had larger phasic (p < 0.01, ηp2 = 0.20) and maximal (p < 0.01, ηp2 = 0.22) pupil constrictions compared to adolescents.
Conclusions
Blue light elicited a greater and more sustained pupillary response than red light in children and adolescents. However, the overall amplitude of the rod/cone-driven phasic response was greater in children than in adolescents. Our findings using the PLR highlight a higher sensitivity to evening light in children compared to adolescents, and continued maturation of the human non-visual photoreception/system throughout development.
Supplementary Information
The online version contains supplementary material available at 10.1186/s40101-024-00363-6.
Keywords: Children, Adolescents, Pupillary light reflex, Light exposure, Intrinsically photosensitive retinal ganglion cells
Background
Intrinsically photosensitive retinal ganglion cells (ipRGCs) are ocular photoreceptors that express the photopigment melanopsin and are maximally sensitive to short-wavelength blue light, with a peak sensitivity of ~ 480 nm [1, 2]. ipRGCs integrate their intrinsic response to inputs from rods and cones, before transmitting the signal to non-visual centers in the brain such as the suprachiasmatic nucleus (SCN), the central timekeeper of bodily circadian rhythms [3, 4], the olivary pretectal nucleus (OPN) which controls the pupillary light response (PLR) [5, 6], and centers involved in sleep and alertness [7]. Stimulation of ipRGCs by light in the evening can suppress the production and release of the sleep-promoting hormone melatonin from the pineal gland, delay the timing of the circadian clock, and decrease nighttime sleepiness [8, 9]. These effects may be particularly strong in children, compared with adolescents or adults [10–12].
The PLR offers a fast and reliable biomarker for testing the physiology and integrity of visual and non-visual photoreception. It generally consists of a rapid constriction of the pupil to an abrupt light stimulus. This transient (or phasic) constriction response is largely attributed to outer-retinal (rod/cone) activity [6]. If the light stimulation is maintained, the transient constriction is followed by a sustained constriction of the pupil (steady-state). The steady-state response consists of input from all photoreceptors; however, the ipRGC’s intrinsic contribution to this state is three-folds higher than long-wavelength (L-cones) and medium-wavelength (M-cones) cones [5]. After cessation of the light stimulus, signals from both the rods and ipRGCs contribute to the post-illumination pupil response (PIPR) in the first 1.7 s, after which the PIPR is entirely driven by ipRGCs [13]. Chromatic pupillometry, using light stimuli of various colors/wavelengths, is increasingly used to measure retinal health and development [14, 15]. By employing light exposure patterns of different wavelengths, this technique has been suggested to differentiate pupillometric signals originating from the outer (rods and cones) and inner (intrinsic ipRGC response) retina according to the irradiance and spectral sensitivities of photoreceptors. In adults, exposure to short-wavelength blue light results in faster, greater, and more sustained pupillary constriction than long-wavelength red light [16–18]. This more sustained response to blue light is consistent with greater stimulation of the ipRGCs. Therefore, components of the chromatic PLR can be used as an indicator of the ipRGC response and can be utilized as a marker in health and disease [15, 19, 20], even in clinical settings [21].
Baseline pupil diameter increases throughout childhood, peaking in adolescence [22–24], then slowly decreases throughout adulthood [10, 25, 26]. In adults age 21–70 years, a larger baseline pupil diameter was associated with a larger PIPR 6 s after exposure to blue light [25]. However, when controlling for baseline pupil diameter, age was not associated with PIPR, indicating that the inputs of the ipRGC to the pupillary pathway do not diminish with advancing age. Data on the maturation of the PLR throughout child development, however, remain limited. In premature infants (gestational age 38 weeks), the PLR is observed in response to blue but not red light [24]. However, the magnitude of the pupillary constriction, as well as baseline pupil size, is significantly smaller in premature infants than at 2 years of age [24]. In a longitudinal study of the PLR elicited by green light (530 nm) across children aged 6–24 months, baseline size, minimal pupil size, and maximum pupil constriction (% constriction with respect to baseline) increased with age, whereas latency (the time from light onset to the start of pupil constriction) decreased with age [27]. In a sample of children aged 6–17 years, the amplitude of pupillary constriction in response to green light increased until age 8, then plateaued [23]. PLR latency decreased across ages 6–9, then stabilized. Constriction and re-dilation time were not associated with age. Across children ages 5–15 years, the ipRGC-driven pupil response to light was observed to be robust and similar to those measured in adults [28]. Lastly, in another study examining the PLR between children aged 3–10 and 10–18 years, the younger group required a brighter red light stimulus to evoke a 5% pupil constriction than the older group [14].
The circadian timing of a light exposure can influence the PLR [29]. In adults, circadian fluctuations in post-stimulus pupil size were observed in response to blue (463 nm) but not red (635 nm) light [30]. Additionally, the ipRGC-driven pupil response in children is influenced by individual differences in 24-h light history [28]. Furthermore, across the lifespan, humans spend the vast majority of their time in indoor environments [31, 32], resulting in less exposure to bright light during the day and greater exposure to artificial light at night [33]. Both the circadian timing of assessments and prior light history are largely unreported and uncontrolled for in previous studies of the PLR in children. The purpose of the present research was to (1) study the chromatic pupillary light reflex in response to red and blue evening light in children and adolescents while controlling for the confounding factors mentioned above; and (2) examine age-related differences across 2 distinct stages of child development. The measurement was conducted in the hour before participants’ habitual bedtimes in order to simulate conditions under which children are often exposed to artificial light and a time when the non-visual circadian system is highly responsive to light exposure.
Methods
Participants
Children aged 8.0–9.9 years (n = 21, 12 female, 81.0% white, 4.8% Asian, 14.3% more than one race reported, 90.5% non-Hispanic, M = 8.99 years, SD = 0.51 years) and adolescents aged 15.0–16.9 years (n = 19, 5 female, 94.7% white, 5.3% more than one race reported, 100% non-Hispanic, M = 15.78 years, SD = 0.55 years) completed a 6-day study protocol. Participants were recruited from the greater Boulder, CO, USA area. Prospective interested parents were screened through an online questionnaire and subsequent phone interview. Participants were excluded from the study for parent report of any of the following: clinical sleep disorders; behavioral problems; personal or family history of diagnosed narcolepsy, depression, psychosis, or bipolar disorder; pre-term delivery (< 35 weeks); low birth weight (< 5.5 lbs.); current use of caffeine, nicotine, alcohol, or medications affecting sleep, the circadian system, or light sensitivity; active allergies or asthma; developmental disabilities; neurological disorders; metabolic disorders; chronic medical conditions; head injury involving loss of consciousness; travel > 2 time zones in the 2 months before participation; sleep schedule varying > 2 h between weekdays and weekends; migraine or frequent headaches; eye disorders (excluding corrected refractive error) or color blindness (confirmed using the Ishihara Color Vision Test). All study procedures were approved by the University of Colorado Boulder Institutional Review Board. Parents provided written, informed consent and children/adolescents provided written assent to participate. Families were compensated for their participation.
Protocol
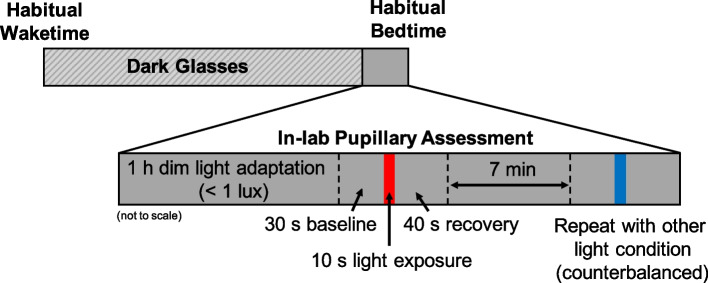
Data collection occurred between June and October in 2021 and September through October in 2022. For 5 days, children followed a strict parent-selected sleep schedule (bedtime and wake time) with ≥ 9 h time in bed, whereas adolescents followed a self-selected sleep schedule of ≥ 8 h time in bed [34]. Adherence to the sleep schedule was confirmed through a wrist-worn actigraph (Spectrum Plus, Philips Respironics, Pittsburgh, PA, USA) which recorded sleep–wake states via motor activity in 1 min epochs, as well as a daily sleep diary (parent-reported for the school-aged children and self-reported for the adolescents). Participants were instructed to avoid light exposure between bedtime and wake. Starting at wake time on Day 6, participants wore a pair of dark glasses (UVEX by Honeywell, Espresso lens) with an advertised visible light transmission of 15% [35] in order to limit exposure to bright light and reduce individual variability in light history (Fig. S1). The transmittance of the glasses at 480 nm was measured as 8.9%. Participants were instructed to remain indoors wearing the glasses throughout the day until beginning the dim-light adaptation at the laboratory that evening (Fig. 1). Participants arrived at the lab 2.5 h before their scheduled bedtime for the pupillary assessment. They then entered a dimly-lit room (< 1 lx at angle of gaze) beginning 2 h before their scheduled bedtime and adapted to the dim-light for 1 h.
Fig. 1.
Pupillary assessment protocol. On the day of the pupillary assessment, participants remained indoors wearing a pair of dark-tinted glasses from their habitual wake time until the start of the dim-light adaptation at the laboratory (2 h before scheduled bedtime). Following a 1 h dim-light adaptation, pupil diameter was measured during a 30 s baseline, 10 s light exposure, and 40 s recovery. After a 7 min dim-light re-adaptation, the measurement was repeated for the remaining light condition, counterbalanced across participants.
The pupillary assessment then began 1 h before each participant’s scheduled bedtime. Researchers fitted the participant with a head-mounted eye tracking unit (ETL-100F, ISCAN, Inc., Woburn, MA, USA), which measures pupil diameter at a rate of 60 Hz. Participants were seated at a table and instructed to keep their chin on a chinrest ~ 42 cm from the light source and look straight ahead during all measurements. Pupil diameter was recorded during a 30 s baseline in darkness, a 10 s light stimulus, and a 40 s recovery period in darkness to measure pupillary re-dilation. Participants were exposed to either a blue or red narrow-band LED light source matched in photon flux at 3.0 × 1013 photons/cm2/s (Table 1; Fig. S2). The light source consisted of LED strips (YUNBO, Changzhou, China) arranged on a backplane of a 61 cm × 61 cm × 12 cm deep white box with an acrylic diffusing panel at the front. A research spectrometer (MSC15, Gigahertz-Optik, Amesbury, MA, USA; calibrated 04/01/2021) was used to verify light intensity on the vertical plane from the chinrest before each participant. Following a 7 min re-adaptation to the dim-light, the measurement was repeated for the remaining lighting condition, the order of which was counterbalanced across participants. For four participants (three children, one adolescent), a visual scan of the data revealed an error in the recording. These children were re-adapted to the dim light for an additional 7 min before the measurement was repeated. Light metrics are reported in accordance with the ENLIGHT Checklist and Guidelines [36].
Table 1.
Spectral characteristics for each experimental light source
| Light parameter | Blue | Red |
|---|---|---|
| Peak wavelength (nm) | 459.08 (0.36) | 627.12 (0.29) |
| Full-width half maximum (nm) | 24.56 (0.15) | 20.67 (0.13) |
| Photopic illuminance (lux) | 7.17 (0.24) | 22.15 (0.67) |
| Melanopic equivalent daylight illuminance (lux) | 70.09 (2.32) | 0.10 (0.01) |
Measurements were obtained with a research spectrometer before each participant’s assessment. Means (standard deviations) across all participants are presented in the table above
Analysis
Actigraphy data were scored using our standard, published methods [37]. Light exposure at the wrist was recorded by the actigraph in 1 min epochs. Sleep parameters (sleep duration, sleep efficiency, wake after sleep onset (WASO)) were calculated by averaging across the five nights prior to the pupillary measurement. Light history across the first five days of the protocol was calculated as the average across each epoch between 00:00 on Day 1 and 23:59 on Day 5 of the protocol. Light history on Day 6 was calculated as the average across epochs between 00:00 and each participant’s arrival at the lab for their pupillary assessment (2.5 h before their scheduled bedtime), at which time the actigraph was removed and each participant was exposed to the same lighting environment in the lab prior to the PLR measurement.
Pupil diameter data were analyzed only from the right eye of each participant and recorded in raw pixels. All data processing was performed using Matlab R2021b (The MathWorks, Inc., Natick, MA, USA). Prior to extracting variables, the data were filtered and smoothed to remove blinks. This was achieved by first manually setting upper and lower thresholds to exclude outliers due to blinks. Then, the data were smoothed using the Matlab ‘lowess’ smooth function with a span of 0.01. The PLR variables (adapted from Najjar et al. 2023 [15]) were then extracted from this smoothed data (Table 2).
Table 2.
Description of each pupillary variable calculated
| Variable Name (units) | Definition |
|---|---|
| Baseline Pupil Diameter (pixels) | Median pupil diameter in the 30 s preceding light onset |
| Phasic Constriction (% from baseline) | Median % constriction between 300–700 ms after light onset |
| Constriction Latency (s) | Time required after light onset for the pupil to reach 10% of constriction |
| Maximum Pupil Constriction (% from baseline) | |
| Sustained Slope (%/s) | Slope of best fit linear regression from 3–10 s after light onset |
| Post-Illumination Pupil Response (PIPR, % from baseline) | Median percent constriction between 5 to 7 s after light offset |
| Area Under the Curve (AUC, %.s) | Absolute value of a trapezoidal numerical integral of the data between light offset and 12 s after light offset |
First, a baseline pupil size, in pixels, was calculated as the median diameter across the 30 s prior to light onset. Each data set was then normalized to this baseline to translate the pupil size in pixels to percent constriction away from baseline, with 0% representing baseline pupil size. Phasic constriction and constriction latency could not be determined for two children due to a technical error in recording the exact moment of light onset during one of their measurements.
All statistical analyses were conducted with SPSS Statistics 28.0 (IBM Corp. Armonk, NY, USA). Due to non-normal distributions, Mann–Whitney U tests were used to examine group differences in light history. A Wilcoxon signed rank test was used to examine individual changes in light history between Days 1 through 5 compared with Day 6. Independent samples t-test were utilized to examine group differences in sleep parameters and baseline pupil diameter. PLR data were analyzed with mixed-model ANOVAs to examine differences in response to the two light conditions (Red, Blue) and across the age groups (Children, Adolescents). Effect sizes for significant results are provided as partial-eta squared. Post-hoc comparisons were performed with repeated measures t-tests, and effect sizes were determined using Cohen’s d. An alpha-level of 0.05 was used to determine statistical significance.
Results
Two participants were myopic, but did not wear corrective lenses during the pupillary assessments. Analyses were performed both including and excluding their data. As expected from the previous literature [38], we observed no differences in the results of the analyses after excluding these participants.
Sleep parameters
Average sleep duration across the 5 nights preceding the in-lab assessment was significantly longer for the children than the adolescents, commensurate with reductions in sleep duration observed across typical development (Table 3) [39]. However, no differences in sleep efficiency or wake after sleep onset (WASO) were observed, indicating comparable sleep quality across the two age groups in the days leading up to the pupillary measurement.
Table 3.
Actigraphically-derived average sleep duration and quality
| Variable | Children | Adolescents | Statistics |
|---|---|---|---|
| Sleep Duration (min) | 578.71 (25.13) | 504.21 (31.25) | t(38) = 8.35, p < 0.001 |
| Sleep Efficiency (%) | 85.28 (3.32) | 84.66 (5.62) | t(38) = 0.43, p = 0.67 |
| Wake After Sleep Onset (min) | 63.34 (14.87) | 62.34 (23.28) | t(38) = 0.16, p = 0.87 |
Prior light exposure
Across the first five days of the protocol, average light exposure as measured from the wrist was significantly greater for children (M = 248.93 lx, SD = 80.88) than adolescents (M = 162.08 lx, SD = 147.94; z = -2.99, p < 0.01, r = 0.47). On the day of the pupillary assessment (Day 6), however, when participants were instructed to remain indoors throughout the day, no difference in light exposure between the two age groups was observed (children: M = 91.23 lx, SD = 70.28, adolescents: M = 64.82 lx, SD = 52.20; z = -1.26, p = 0.22). Additionally, across individual participants, average light exposure on Day 6 was significantly less than on Days 1 through 5 (z = -4.80, p < 0.001, r = 0.76), suggesting participants adhered to researcher instructions to remain indoors until coming to the laboratory in the evening.
Baseline pupil size
Baseline pupil diameter before each participant’s first light exposure was not significantly different between children (M = 231.62 pixels, SD = 35.31) and adolescents (M = 242.88 pixels, SD = 29.23; t(38) = -1.09, p = 0.28). Neither was any group difference observed in baseline pupil diameter before each participant’s second light exposure (t(38) = -1.69, p = 0.10; M = 225.49 pixels, SD = 34.42 for children; M = 242.18 pixels, SD = 27.31 for adolescents). A paired-samples t-test indicated a non-significant difference in baseline pupil diameter between the first and second measurements (t(39) = 1.85, p = 0.07). Descriptive statistics for each PLR variable across light color and age group are presented in Table S1.
Features of pupillary light constriction
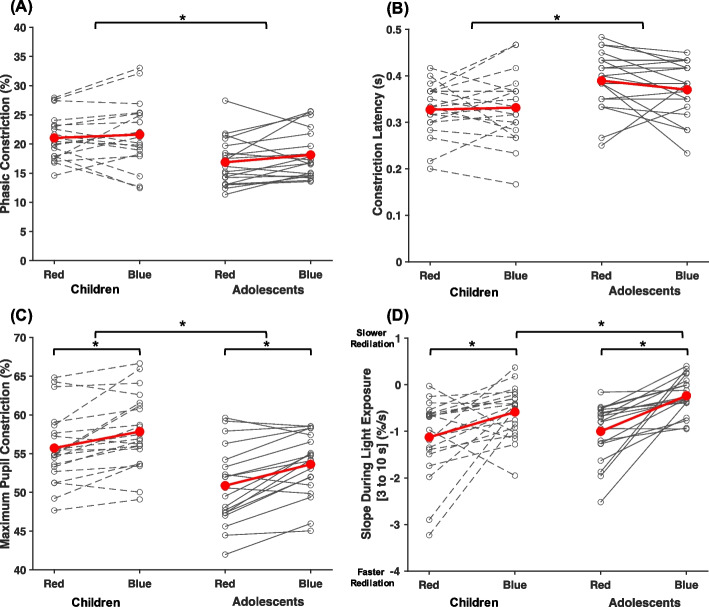
Detailed descriptions of how each variable was calculated are provided in Table 2. Average pupil constriction (as a % away from baseline) was plotted for each age group and light condition (Fig. 2). Across both age groups, phasic constriction was marginally greater in response to blue than red light (p = 0.054, ηP2 = 0.10; Fig. 3A). Regardless of the wavelength of the stimulation, children had both a significantly greater phasic constriction (p < 0.01, ηP2 = 0.18) and smaller constriction latency (p = 0.01, ηP2 = 0.17; Fig. 3B) than adolescents, indicating a faster and more robust initial pupillary response to light in general. Maximum pupil constriction was greater in response to blue light compared to red light for both age groups (p < 0.01, ηP2 = 0.48; Fig. 3C). Children also exhibited a greater maximum constriction than adolescents (p < 0.01, ηP2 = 0.22). Between 3 to 10 s after light onset, pupil constriction was more sustained (i.e., a smaller magnitude slope was observed) during blue compared to red light across both age groups (p < 0.01, ηP2 = 0.41; Fig. 3D). Conversely, an independent samples t-test revealed a larger magnitude slope during blue light in children than in adolescents (t(38) = 2.32, p = 0.03, d = 0.74), indicating a less sustained response.
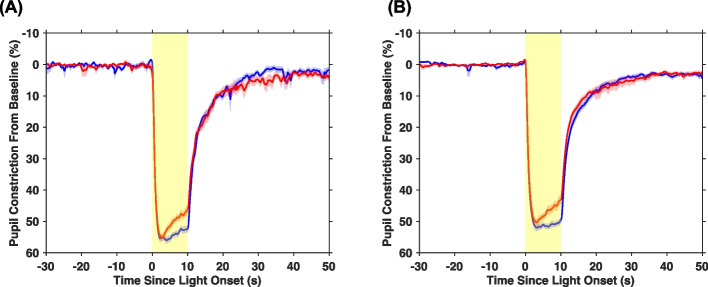
Fig. 2.
Average pupil constriction adjusted to baseline pupil diameter across the 80 s measurement. Average pupil constriction is displayed at each time point for (A) children and (B) adolescents for red and blue light separately. The shaded red and blue areas around each average line represent the standard error of the mean. The shaded yellow area denotes the timing of the 10 s light exposure
Fig. 3.
PLR metrics during light onset. Results depicting (A) phasic constriction; (B) constriction latency; (C) maximum pupil constriction; and (D) slope during light exposure broken down by age group and experimental light condition. Asterisks denote statistical significance (p < 0.05)
Features of pupillary re-dilation
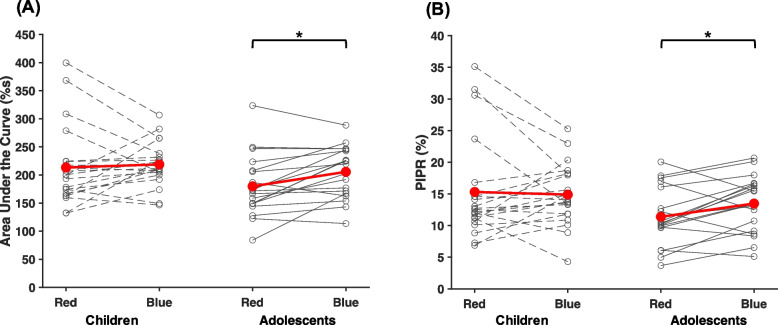
The area under the curve (AUC) 0–12 s after light offset was significantly larger for blue compared to red light (p = 0.045, ηP2 = 0.10; Fig. 4A). However, post-hoc t-tests revealed that this was observed for adolescents (p < 0.01, d = 0.72), but not children (p = 0.66). Similarly, the PIPR was larger following blue compared to red light in adolescents (p = 0.02, d = 0.60; Figure 4B), but not in children (p = 0.76). The PIPR following the red light was 3.92% larger in children than adolescents, though this difference did not reach statistical significance (t(38) = 1.88, p = 0.07). The PIPR following the blue light was 1.41% larger for children than adolescents, indicating the lack of difference between light stimuli for the children was the result of a more sustained response to red light rather than a less sustained response to blue.
Fig. 4.
PLR metrics following light offset. Results depicting (A) area under the curve and (B) post-illumination pupil response (PIPR) broken down by age group and experimental light condition. Asterisks denote statistical significance (p < 0.05)
Discussion
In this well-controlled, repeated-measures study examining age-related changes in the pupillary light reflex, we observed a greater and more sustained pupillary response to blue compared to red light presented in the hour before habitual bedtime for both school-aged children and adolescents. Additionally, although children exhibited a faster and greater amplitude of transient and maximal pupillary constriction across both light colors compared to adolescents, they yielded a reduced sustained response to blue light.
Previously, Crippa and colleagues [14] reported that children aged 3–10 years required a brighter red light stimulus to reach 5% pupil constriction than children 10–18 years. Additionally, in children aged 6–17 years, pupillary constriction in response to a green light stimulus has been shown to increase until age 8 before plateauing [23]. Similarly, latency was observed to decrease across ages 6–9, then stabilized. In contrast, our findings suggest that maximum constriction to both red and blue light decreases and latency increases again between school-age and adolescence. By examining more narrow age groups (8–9 and 15–16 years), our data offer a more fine-grained examination of age-related differences in the PLR than prior published findings, with particular focus around the onset of puberty where changes in the non-visual response to light have been reported [12]. It is difficult to compare findings across the few studies examining age-related changes in the PLR due to differences in protocols and light stimuli, highlighting the need for greater research in these populations and the development of well-controlled standardized procedures.
Our findings contribute to a growing literature demonstrating maturation of the non-visual system throughout development. Previous work by Crowley, et al. [12] demonstrated a reduced circadian response to light in later adolescence. Pre-pubertal adolescents exhibited significantly more melatonin suppression to evening light exposure than post-pubertal adolescents. Additionally, school-aged children display nearly twice as much melatonin suppression to evening bright light as adults [10]. Our data are also in agreement with experimental findings in mice showing that the pupillary light reflex continues to develop until adulthood. In that study, the PLR in response to blue and red light was compared in 1-month-old mice to 2-month-old mice and adult 4-month-old mice. The 1-month-old mice exhibited greater maximal constriction to both light stimuli than 2-month-old mice, as well as a smaller sustained constriction response to blue light [40], strongly mirroring our present findings in humans.
The post-illumination pupil response, especially 1.7 s after light offset, is reported to be entirely driven by melanopsin [13]. Consistent with previous findings in adults [30, 41], post-illumination metrics from our study (i.e., AUC and PIPR) were greater in response to blue compared to red light in adolescents. This difference, however, was not observed in our sample of school-aged children, although the amplitude of the post-illumination response was at least as large as that of the adolescents. Coupled with the children’s smaller sustained response to blue light compared with adolescents, these findings could suggest continued development of the melanopsin-driven pupillary response to evening light during youth. In addition, our findings suggest that the extrinsic input (from rods and cones) to ipRGCs develop differently from the ipRGCs’ intrinsic, melanopsin-driven, response. In fact, with development the extrinsic input to ipRGCs originating from rods and cones appears to decline (lower phasic constriction, slower latency of constriction, and reduced maximum constriction), while the intrinsic melanopsin-driven input appears to increase or at least remain stable with development (increased PIPR and AUC to blue only in adolescents). Whether this reduction in outer retinal input to the PLR would be reflected in circadian entrainment is unclear and an important topic for future research. Moreover, data in adults indicate a reduction in the sustained post-illumination pupil response in the evening compared to daytime [29, 30]. Assuming a similar circadian-dependent pattern in the response is present in children, examining these outcomes near the peak of the ipRGC-driven response during the daytime could potentially result in different outcomes and is another area for future examination.
Although the circadian response to evening light has been shown to decrease between pre-pubertal and post-pubertal children [12], there is a substantial lack of research on typical ipRGC development and changes in connectivity across childhood. However, research on outer retinal photoreceptors indicates age-related changes across early and late adulthood. For example, in human adolescents and adults, cone density decreases with axial length and age [42, 43]. Additionally, outer-retinal cells undergo extensive reorganization during normal human aging [44]. The longitudinal assessment of these and other mechanisms in early childhood-adolescence is crucial to confirm our findings but also to determine whether our findings of age-related differences in the PLR reflect structural changes in the retina, connectivity between outer- and inner-retinal photoreceptors, or maturational changes farther downstream.
Our data were collected utilizing a rigorous protocol designed to control for several confounding variables that could influence the pupillary light reflex. By remaining indoors and wearing a pair of dark glasses throughout Day 6 of the protocol, participants were exposed to significantly less light on the day of the assessment, with less individual and group variability in light history. Additionally, participants restricted light exposure between bedtime and wake time throughout the protocol to provide consistent light/dark timing. Finally, the pupillary assessment began 1 h before each participant’s habitual bedtime in order to anchor the assessment within a consistent circadian window across individuals. By focusing only on the hour before bedtime, our findings offer a more translatable examination of how the light exposure children and adolescents receive before bedtime stimulates the non-visual system, potentially leading to circadian disruption.
Our study has a few limitations. The phase angle between melatonin onset (a marker of the timing of the circadian clock) and bedtime grows wider across adolescence [45]. Because we anchored the protocol to habitual bedtime and did not assess melatonin onset, the exact circadian timing of the pupillary assessment could have differed between individuals and age groups. Furthermore, adherence to wearing the dark glasses on the final day of the protocol was confirmed by self- or parental-report and could not be verified objectively. Wearing the dark glasses throughout the day and the 1 h dim-light adaptation prior to the PLR measurements could also have influenced the response of the ipRGC, as prior dim-light adaptation can increase the sensitivity of the circadian system to light exposure [46, 47]. Finally, participants in our sample were predominantly reported as Caucasian by their parents (87.5%). Previous studies with children and adults reported racial differences in the pupillary light reflex and circadian sensitivity to light [48, 49]. Therefore, given the lack of diversity in our sample, our findings may not be representative of the PLR in children and adolescents across different racial and ethnic identities.
Conclusions
In conclusion, in this well-controlled, repeated-measures study, we highlight differences in the pupillary response to blue and red light between children (8–9 years) and adolescents (15–16 years). While our findings demonstrate an overall heightened sensitivity to evening light in children compared to adolescents, they also suggest continued maturation of the human non-visual photoreception/system throughout development.
Supplementary Information
Acknowledgements
We thank the participants and families who took part in this research. We also thank the students and staff of the Sleep and Development Lab at the University of Colorado Boulder who assisted in data collection.
Abbreviations
- ANOVA
Analysis of variance
- AUC
Area under the curve
- ipRGC
Intrinsically photosensitive retinal ganglion cells
- LED
Light emitting diode
- OPN
Olivary pretectal nucleus
- PIPR
Post-illumination pupillary response
- PLR
Pupillary light response
- SCN
Suprachiasmatic nucleus
- SD
Standard deviation
Authors’ contributions
LEH, MKL, and RPN conceptualized and designed the study. MTD designed and built the light stimulus apparatuses. LEH collected the data. LEH, MTD, and RPN analyzed and interpreted the data. LEH and MTD created the figures. LEH drafted the initial manuscript. All authors revised the drafted manuscript and read and approved the final manuscript.
Funding
This work was funded by the Eunice Kennedy Shriver National Institute of Child Health & Human Development (F32-HD103390; R01-HD087707) and the National Heart, Lung, And Blood Institute of the National Institutes of Health (T32-HL149646). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
All study procedures were approved by the University of Colorado Boulder Institutional Review Board. Parents provided written, informed consent and children/adolescents provided written assent to participate.
Consent for publication
Not applicable.
Competing interests
LEH and MTD have no financial or personal conflicts to declare. MKL reports receiving travel funds from the Australian Research Council and research support from the National Institutes of Health, beyond the submitted work. RPN has a patent application for a handheld pupillometer (PCT/SG2018/050204): Handheld ophthalmic and neurological screening device. The device was not used in this study.
Footnotes
Dr. LeBourgeois passed away in November 2023.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295:1070–1073. doi: 10.1126/science.1067262. [DOI] [PubMed] [Google Scholar]
- 2.Enezi Ja, Revell V, Brown T, Wynne J, Schlangen L, Lucas R. A “melanopic” spectral efficiency function predicts the sensitivity of melanopsin photoreceptors to polychromatic lights. J Biol Rhythms. 2011;26(4):314–23. doi: 10.1177/0748730411409719. [DOI] [PubMed] [Google Scholar]
- 3.Brainard GC, Hanifin JP. Photons, clocks, and consciousness. J Biol Rhythm. 2005;20(4):314–325. doi: 10.1177/0748730405278951. [DOI] [PubMed] [Google Scholar]
- 4.Gooley JJ, Lu J, Chou TC, Scammell TE, Saper CB. Melanopsin in cells of origin of the retinohypothalamic tract. Nat Neurosci. 2001;4(12):1165. doi: 10.1038/nn768. [DOI] [PubMed] [Google Scholar]
- 5.Tsujimura Si, Ukai K, Ohama D, Nuruki A, Yunokuchi K. Contribution of human melanopsin retinal ganglion cells to steady-state pupil responses. Proceedings of the Royal Society B: Biological Sciences. 2010;277(1693):2485–92. doi: 10.1098/rspb.2010.0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McDougal DH, Gamlin PD. The influence of intrinsically-photosensitive retinal ganglion cells on the spectral sensitivity and response dynamics of the human pupillary light reflex. Vision Res. 2010;50(1):72–87. doi: 10.1016/j.visres.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Z, Beier C, Weil T, Hattar S. The retinal ipRGC-preoptic circuit mediates the acute effect of light on sleep. Nat Commun. 2021;12(1):5115. doi: 10.1038/s41467-021-25378-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duffy JF, Wright KP., Jr Entrainment of the human circadian system by light. J Biol Rhythm. 2005;20(4):326–338. doi: 10.1177/0748730405277983. [DOI] [PubMed] [Google Scholar]
- 9.Zeitzer JM, Dijk DJ, Kronauer RE, Brown EN, Czeisler CA. Sensitivity of the human circadian pacemaker to nocturnal light: melatonin phase resetting and suppression. J Physiol. 2000;526(3):695–702. doi: 10.1111/j.1469-7793.2000.00695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Higuchi S, Nagafuchi Y, Lee SI, Harada T. Influence of light at night on melatonin suppression in children. J Clin Endocrinol Metab. 2014;99(9):3298–3303. doi: 10.1210/jc.2014-1629. [DOI] [PubMed] [Google Scholar]
- 11.Hartstein LE, Diniz Behn C, Akacem LD, Stack N, Wright Jr KP, LeBourgeois MK. High sensitivity of melatonin suppression response to evening light in preschool‐aged children. J Pineal Res. 2022:e12780. 10.1111/jpi.12780. [DOI] [PMC free article] [PubMed]
- 12.Crowley SJ, Cain SW, Burns AC, Acebo C, Carskadon MA. Increased sensitivity of the circadian system to light in early/mid-puberty. J Clin Endocr Metab. 2015;100(11):4067–4073. doi: 10.1210/jc.2015-2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adhikari P, Feigl B, Zele AJ. Rhodopsin and melanopsin contributions to the early redilation phase of the post-illumination pupil response (PIPR) PLoS ONE. 2016;11(8):e0161175. doi: 10.1371/journal.pone.0161175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crippa SV, Pedrosa Domellöf F, Kawasaki A. Chromatic pupillometry in children. Front Neurol. 2018;9:669. doi: 10.3389/fneur.2018.00669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Najjar RP, Rukmini A, Finkelstein MT, Nusinovici S, Mani B, Nongpiur ME, et al. Handheld chromatic pupillometry can accurately and rapidly reveal functional loss in glaucoma. Br J Ophthalmol. 2023;107(5):663–670. doi: 10.1136/bjophthalmol-2021-319938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Young RS, Kimura E. Pupillary correlates of light-evoked melanopsin activity in humans. Vision Res. 2008;48(7):862–871. doi: 10.1016/j.visres.2007.12.016. [DOI] [PubMed] [Google Scholar]
- 17.Ishikawa H, Onodera A, Asakawa K, Nakadomari S, Shimizu K. Effects of selective-wavelength block filters on pupillary light reflex under red and blue light stimuli. Jpn J Ophthalmol. 2012;56(2):181–186. doi: 10.1007/s10384-011-0116-1. [DOI] [PubMed] [Google Scholar]
- 18.Kardon R, Anderson SC, Damarjian TG, Grace EM, Stone E, Kawasaki A. Chromatic pupil responses: preferential activation of the melanopsin-mediated versus outer photoreceptor-mediated pupil light reflex. Ophthalmology. 2009;116(8):1564–1573. doi: 10.1016/j.ophtha.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 19.Najjar RP, Sharma S, Atalay E, Rukmini AV, Sun C, Lock JZ, et al. Pupillary responses to full-field chromatic stimuli are reduced in patients with early-stage primary open-angle glaucoma. Ophthalmology. 2018;125(9):1362–1371. doi: 10.1016/j.ophtha.2018.02.024. [DOI] [PubMed] [Google Scholar]
- 20.Tan TE, Finkelstein MT, Tan GSW, Tan ACS, Chan CM, Mathur R, et al. Retinal neural dysfunction in diabetes revealed with handheld chromatic pupillometry. Clin Experiment Ophthalmol. 2022;50(7):745–756. doi: 10.1111/ceo.14116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Finkelstein MT, Najjar RP, Chougule P, Mathur R, Milea D. Chromatic pupillometry in multiple evanescent white dot syndrome masquerading as atypical optic neuritis. Acta Ophthalmol. 2022;100(6):713–715. doi: 10.1111/aos.14998. [DOI] [PubMed] [Google Scholar]
- 22.MacLachlan C, Howland HC. Normal values and standard deviations for pupil diameter and interpupillary distance in subjects aged 1 month to 19 years. Ophthalmic Physiol Opt. 2002;22(3):175–182. doi: 10.1046/j.1475-1313.2002.00023.x. [DOI] [PubMed] [Google Scholar]
- 23.Daluwatte C, Miles JH, Christ SE, Beversdorf DQ, Lofgreen A, Berliner N, et al. Age-dependent pupillary light reflex parameters in children. 2012 Annual International Conference of the IEEE Engineering in Medicine and Biology Society. San Diego: IEEE; 2012. p. 3776–9. 10.1109/EMBC.2012.6346789. [DOI] [PubMed]
- 24.Ikeda T, Ishikawa H, Shimizu K, Asakawa K, Goseki T. Pupillary size and light reflex in premature infants. Neuro-Ophthalmology. 2015;39(4):175–178. doi: 10.3109/01658107.2015.1055363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Adhikari P, Pearson CA, Anderson AM, Zele AJ, Feigl B. Effect of age and refractive error on the melanopsin mediated post-illumination pupil response (PIPR) Sci Rep. 2015;5:17610. doi: 10.1038/srep17610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Biçer GY, Zor KR, Küçük E. Do static and dynamic pupillary parameters differ according to childhood, adulthood, and old age? A quantitative study in healthy volunteers. Indian J Ophthalmol. 2022;70(10):3575–3578. doi: 10.4103/ijo.IJO_1254_22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kercher C, Azinfar L, Dinalankara DM, Takahashi TN, Miles JH, Yao G. A longitudinal study of pupillary light reflex in 6-to 24-month children. Sci Rep. 2020;10(1):1–9. doi: 10.1038/s41598-020-58254-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ostrin LA. The ip RGC-driven pupil response with light exposure and refractive error in children. Ophthalmic Physiol Opt. 2018;38(5):503–515. doi: 10.1111/opo.12583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zele AJ, Feigl B, Smith SS, Markwell EL. The circadian response of intrinsically photosensitive retinal ganglion cells. PLoS ONE. 2011;6(3):e17860. doi: 10.1371/journal.pone.0017860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Münch M, Léon L, Crippa SV, Kawasaki A. Circadian and wake-dependent effects on the pupil light reflex in response to narrow-bandwidth light pulses. Invest Ophthalmol Vis Sci. 2012;53(8):4546–4555. doi: 10.1167/iovs.12-9494. [DOI] [PubMed] [Google Scholar]
- 31.Klepeis NE, Nelson WC, Ott WR, Robinson JP, Tsang AM, Switzer P, et al. The National Human Activity Pattern Survey (NHAPS): a resource for assessing exposure to environmental pollutants. J Eposure Sci Environ Epidemiol. 2001;11(3):231–252. doi: 10.1038/sj.jea.7500165. [DOI] [PubMed] [Google Scholar]
- 32.Matz CJ, Stieb DM, Davis K, Egyed M, Rose A, Chou B, et al. Effects of age, season, gender and urban-rural status on time-activity: Canadian Human Activity Pattern Survey 2 (CHAPS 2) Int J Environ Res Public Health. 2014;11(2):2108–2124. doi: 10.3390/ijerph110202108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wright KP, McHill AW, Birks BR, Griffin BR, Rusterholz T, Chinoy ED. Entrainment of the human circadian clock to the natural light-dark cycle. Curr Biol. 2013;23(16):1554–1558. doi: 10.1016/j.cub.2013.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hirshkowitz M, Whiton K, Albert SM, Alessi C, Bruni O, DonCarlos L, et al. National Sleep Foundation’s updated sleep duration recommendations. Sleep Health. 2015;1(4):233–243. doi: 10.1016/j.sleh.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 35.UVEX H. Uvex - Ultra Violet Excluded SCT Filters and Tints Brochure. https://prodedam.honeywell.com/content/dam/honeywell-edam/sps/his/en-us/products/head-eye-faceprotection/documents/eye/sps-his-ultra-violet-excluded-sct-brochure-na-eng.pdf.
- 36.Spitschan M, Kervezee L, Lok R, McGlashan E, Najjar RP, Allen AE, et al. ENLIGHT: A consensus checklist for reporting laboratory-based studies on the non-visual effects of light in humans. EBioMedicine. 2023;98:104889. [DOI] [PMC free article] [PubMed]
- 37.LeBourgeois MK, Carskadon MA, Akacem LD, Simpkin CT, Wright KP, Jr, Achermann P, et al. Circadian phase and its relationship to nighttime sleep in toddlers. J Biol Rhythm. 2013;28(5):322–331. doi: 10.1177/0748730413506543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rukmini A, Chew MC, Finkelstein MT, Atalay E, Baskaran M, Nongpiur ME, et al. Effects of low and moderate refractive errors on chromatic pupillometry. Sci Rep. 2019;9(1):1–10. doi: 10.1038/s41598-019-41296-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Iglowstein I, Jenni OG, Molinari L, Largo RH. Sleep duration from infancy to adolescence: reference values and generational trends. Pediatrics. 2003;111(2):302–307. doi: 10.1542/peds.111.2.302. [DOI] [PubMed] [Google Scholar]
- 40.Kircher N, Crippa SV, Martin C, Kawasaki A, Kostic C. Maturation of the pupil light reflex occurs until adulthood in mice. Front Neurol. 2019;10:56. doi: 10.3389/fneur.2019.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Adhikari P, Zele AJ, Feigl B. The post-illumination pupil response (PIPR) Invest Ophthalmol Vis Sci. 2015;56(6):3838–3849. doi: 10.1167/iovs.14-16233. [DOI] [PubMed] [Google Scholar]
- 42.Song H, Chui TYP, Zhong Z, Elsner AE, Burns SA. Variation of cone photoreceptor packing density with retinal eccentricity and age. Invest Ophthalmol Vis Sci. 2011;52(10):7376–7384. doi: 10.1167/iovs.11-7199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eckmann-Hansen C, Hansen MH, Laigaard PP, Sander BA, Munch IC, Olsen EM, et al. Cone photoreceptor density in the copenhagen child cohort at age 16–17 years. Ophthalmic Physiol Opt. 2021;41(6):1292–1299. doi: 10.1111/opo.12889. [DOI] [PubMed] [Google Scholar]
- 44.Eliasieh K, Liets LC, Chalupa LM. Cellular reorganization in the human retina during normal aging. Invest Ophthalmol Vis Sci. 2007;48(6):2824–2830. doi: 10.1167/iovs.06-1228. [DOI] [PubMed] [Google Scholar]
- 45.Crowley SJ, Acebo C, Fallone G, Carskadon MA. Estimating dim light melatonin onset (DLMO) phase in adolescents using summer or school-year sleep/wake schedules. Sleep. 2006;29(12):1632–1641. doi: 10.1093/sleep/29.12.1632. [DOI] [PubMed] [Google Scholar]
- 46.Chang AM, Scheer FA, Czeisler CA. The human circadian system adapts to prior photic history. J Physiol. 2011;589(Pt 5):1095–1102. doi: 10.1113/jphysiol.2010.201194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Smith KA, Schoen MW, Czeisler CA. Adaptation of human pineal melatonin suppression by recent photic history. J Clin Endocrinol Metab. 2004;89(7):3610–3614. doi: 10.1210/jc.2003-032100. [DOI] [PubMed] [Google Scholar]
- 48.Brown JT, Connelly M, Nickols C, Neville KA. Developmental changes of normal pupil size and reactivity in children. J Pediatr Ophthalmol Strabismus. 2015;52(3):147–151. doi: 10.3928/01913913-20150317-11. [DOI] [PubMed] [Google Scholar]
- 49.Higuchi S, Motohashi Y, Ishibashi K, Maeda T. Influence of eye colors of Caucasians and Asians on suppression of melatonin secretion by light. Am J Physiol Regul Integr Comp Physiol. 2007;292(6):R2352–R2356. doi: 10.1152/ajpregu.00355.2006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.