Abstract
Background
Cerebral cavernous malformations (CCMs) frequently manifest with haemorrhages. Stereotactic radiosurgery (SRS) has been employed for CCM not suitable for resection. Its effect on reducing haemorrhage risk is still controversial. The aim of this study was to expand on the safety and efficacy of SRS for haemorrhagic CCM.
Methods
This retrospective multicentric study included CCM with at least one haemorrhage treated with single-session SRS. The annual haemorrhagic rate (AHR) was calculated before and after SRS. Recurrent event analysis and Cox regression were used to evaluate factors associated with haemorrhage. Adverse radiation effects (AREs) and occurrence of new neurological deficits were recorded.
Results
The study included 381 patients (median age: 37.5 years (Q1–Q3: 25.8–51.9) with 414 CCMs. The AHR from diagnosis to SRS excluding the first haemorrhage was 11.08 per 100 CCM-years and was reduced to 2.7 per 100 CCM-years after treatment. In recurrent event analysis, SRS, HR 0.27 (95% CI 0.17 to 0.44), p<0.0001 was associated with a decreased risk of haemorrhage, and the presence of developmental venous anomaly (DVA) with an increased risk, HR 1.60 (95% CI 1.07 to 2.40), p=0.022. The cumulative risk of first haemorrhage after SRS was 9.4% (95% CI 6% to 12.6%) at 5 years and 15.6% (95% CI% 9 to 21.8%) at 10 years. Margin doses> 13 Gy, HR 2.27 (95% CI 1.20 to 4.32), p=0.012 and the presence of DVA, HR 2.08 (95% CI 1.00 to 4.31), p=0.049 were factors associated with higher probability of post-SRS haemorrhage. Post-SRS haemorrhage was symptomatic in 22 out of 381 (5.8%) patients, presenting with transient (15/381) or permanent (7/381) neurological deficit. ARE occurred in 11.1% (46/414) CCM and was responsible for transient neurological deficit in 3.9% (15/381) of the patients and permanent deficit in 1.1% (4/381) of the patients. Margin doses >13 Gy and CCM volume >0.7 cc were associated with increased risk of ARE.
Conclusion
Single-session SRS for haemorrhagic CCM is associated with a decrease in haemorrhage rate. Margin doses ≤13 Gy seem advisable.
Keywords: Haemorrhage, Stroke, Vascular Malformations, Brain, Intervention
WHAT IS ALREADY KNOWN ON THIS TOPIC.
The radiosurgical treatment of cerebral cavernous malformation (CCM) is controversial.
WHAT THIS STUDY ADDS
This large, multicentric series shows that stereotactic radiosurgery is associated with haemorrhage risk reduction using recurrent analysis.
A dose >13 Gy is associated with rebleeding and adverse radiation effects.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
A margin dose of less than 13 Gy seems advisable to treat patient for haemorrhagic CCM.
Further analysis on haemorrhagic risk reduction should use recurrent analysis.
Introduction
The prevalence of cerebral cavernous malformations (CCMs) is estimated between 0.2% and 0.5%.1 Twenty-five per cent present with symptomatic, intracerebral haemorrhage (ICH).1 2 The 5-year risk of repeat haemorrhage is estimated to be as high as 30.8% in patients with brainstem CCM presenting with haemorrhage or focal neurological deficit (FND).2 Resection is the primary treatment for haemorrhagic CCM, with an estimated permanent morbidity rate of approximately 3%.3 However, this rate is highly dependent on the location; brainstem CCM resection carries significantly higher morbidity and mortality rate of 16% and 1.5%, respectively.4
Stereotactic radiosurgery (SRS) can be an alternative management option for patients with CCMs not amenable for resection.1 5 CCM radiosurgery remains a subject of controversy,6 despite several studies reporting a reduction in post-SRS haemorrhage rates.7–11 The major points of the contention include the lack of a distinct radiographic endpoint to evaluate the efficacy of SRS,7 8 10–12 and the high risk of SRS-related complications in the earlier reports.12–14 Since the efficacy of SRS seems to appear after a latency period of 2 years, one additional concern is that the observed effect might actually reflect the natural history of CCMs, as the haemorrhages occur in cluster.6 15 16
The purpose of this study was to evaluate the safety and efficacy of single-session SRS for haemorrhagic CCM and to determine predictors of outcomes.
Methods
Patient population and inclusion criteria
This retrospective, multicentre study follows the guidelines set forth by the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE). All patients with haemorrhagic CCM (sporadic or familial) treated with single-session SRS were included in the study. Patients lacking follow-up after SRS or presenting with progressive FND and seizures without evidence of clinical and radiological prior ICH were excluded (online supplemental figure 1).
svn-2023-002380supp001.pdf (116.7KB, pdf)
This study included 381 patients treated between 1995 and 2021 at 11 centres of the International Radiosurgery Research Foundation. Each centre obtained approval for sharing deidentified data (IRB number: 17972). Data from each cohort were checked for internal consistency and any missing data or discrepancies were resolved by request to the collaborators.
SRS technique
SRS was delivered using the Leksell Gamma-Knife available at each participating centre. Stereotactic, high-resolution brain MRI and/or CT scanning were used for planning.
Follow-up and study endpoints
Clinical and imaging follow-up was performed by the participating centres according to local protocols, usually every 6 months after SRS for 2 years and annually thereafter. The Zabramski stage was defined before and after treatment.17 Outcome measures included pre-SRS and post-SRS symptomatic ICH rate as the primary endpoint, occurrence and evolution of neurological deficit, occurrence of adverse radiation effect (ARE) and epilepsy evolution.
The study adheres to the standards set by the Angioma Alliance Scientific Advisory Board that define haemorrhage as ‘a clinical event involving acute or subacute onset symptoms (any headache, epileptic seizure, impaired consciousness or new/worsened focal neurological deficit referable to the anatomic location of the CCM) accompanied by radiological, pathological, surgical, or rarely only cerebrospinal fluid evidence of recent extra- or intralesional hemorrhage. The definition includes neither an increase in CCM diameter without other evidence of recent hemorrhage, nor the existence of a hemosiderin halo’.18 Neurological symptoms were classified as improved, stable or worsening. A worsening condition was defined as the occurrence of a new permanent symptom and/or worsening of at least one neurological symptom. Neither epilepsy (new-onset or pre-existing) nor headache was included in this evaluation of neurological symptom evolution.
AREs were defined as perilesional T2 hyperintensity or cyst development. They were classified as symptomatic or asymptomatic. The CCM volume was contoured in the GammaPlan software for treatment purposes on T2 or T1 with gadolinium in function of where the CCM was more clearly visualised. The lesion was contoured in each slide, excluding the haemosiderin rim. The same sequences were used to compare the CCM at last follow-up. The lesion volume evolution was defined as enlarged if the lesion was more than 20% at last follow-up compared with SRS target volume, decrease if the volume decreased of more than 20% from baseline and stable otherwise.
Patients presenting with at least one epileptic seizure prior to SRS were classified in four categories: no additional seizures and no antiepileptic medication, no seizure with medication, improvement of at least 50% of the frequency and/or intensity of seizures with medication and seizure refractory to medication. Post-SRS seizure outcomes were defined using the Engel classification at last follow-up.19
Statistical analysis
The statistical analysis was performed using R (R Foundation for Statistical Computing). Normality of continuous variables was assessed graphically and with the Shapiro-Wilk test; with normality not verified, continuous variables are presented as median, first and third quartile (Q1–Q3). A p value <0.05 was considered significant.
No data were imputed. Analysis was performed per patient for relevant characteristics and per cavernoma for efficacy and adverse event. Patients treated for multiple CCM at different timepoints were handled as different patients (n=4 patients for 5 CCM).
The post-SRS annual haemorrhage rate (AHR) was calculated by dividing the cumulative number of haemorrhages by the cumulative number of contributed years of follow-up by each lesion. Each lesion contributed risk time from the date of SRS to the date of last follow-up, death or new procedure for CCM. Due to the discrepancy on the best method to calculate the pre-SRS AHR, all three methods previously reported in the literature were used: (1) CCMs are congenital lesions; the observation period is calculated from birth until SRS, all haemorrhages are included, (2) CCMs are acquired lesions; the observation period is calculated from diagnosis to SRS, all haemorrhages are included, (3) the period is calculated from first haemorrhage to SRS but the first haemorrhage is excluded if this is the reason for diagnosis.10–12 The pre-SRS AHRs were compared with the overall and first 2-year post-SRS AHR using the methodology described by Sahai et al.20
As haemorrhages occurred multiple times pre-SRS and post-SRS, univariate and multivariate recurrent event analysis was performed using the Prentice, William and Peterson Gap-Time (PWP-GT) model,21 using the third method of haemorrhagic rate calculation. This method was chosen since CCMs are not congenital lesions, can form during lifetime, with the risk of bleeding being non-constant since birth and increasing following a haemorrhage.2 17 Optimal cut-off points for continuous variable were calculated with the Youden index. Statistically significant factors and clinically relevant ones with a p value <0.20 were included in the multivariate analysis.
We investigated the risk factors associated with new haemorrhage after SRS and ARE. As the number of recurrent events (second and third haemorrhage) after SRS was low, a Cox regression instead of a PWP-GT analysis was performed.22 Kaplan-Meier curves for first haemorrhage after SRS were plotted. A logistic regression model was employed to evaluate the risk factors for AREs after SRS.
Results
Demographics
A total of 381 patients (211 (55.4%) female, median age of 37.5 years (Q1–Q3: 25.8–51.9) at SRS) were included. The presentation leading to the discovery of the CCM was haemorrhage in 94.5% of patients (360/381), seizure without evidence of haemorrhage in 2.4% (9/381) and progressive FND in 1.6% (6/381), and an incidental discovery in 1.6% (6/381). The 13 patients not presenting with haemorrhage on diagnosis experienced haemorrhagic events in the time interval between diagnosis and radiosurgery. Two patients had a genetic mutation identified (0.6%): one a CCM1/KRIT1 mutation, and the other a CCM2 mutation (table 1).
Table 1.
Demographic characteristics of the 381 included patients
| Demographics | n (%) |
| Age at diagnosis (years), median (Q1–Q3) | 34.7 (24.3–48.6) |
| Age at SRS (years), median (Q1–Q3) | 37.5 (25.8–51.9) |
| Sex | |
| Male | 170 (44.6%) |
| Female | 211 (55.4%) |
| Genetic mutation identified | 2 (0.6%) |
| Initial presentation | |
| Incidental* | 6 (1.6%) |
| Seizure* | 9 (2.4%) |
| Haemorrhage | 360 (94.5%) |
| Focal neurological deficit* | 6 (1.6%) |
| Clinical symptoms pre-SRS† | |
| None | 29 (7.6%) |
| Motor deficit | 86 (22.6%) |
| Sensory deficit | 62 (16.3%) |
| Cerebellar symptom | 26 (6.8%) |
| Cranial nerve deficit | 98 (25.7%) |
| Seizure | 77 (20.2%) |
| Headaches | 72 (18.9%) |
| Others‡ | 56 (14.7%) |
| Pre-SRS seizure control (n=77) | |
| No seizure without medication | 2 (2.6%) |
| No seizure with medication | 34 (44.2%) |
| Improvement of at least 50% in frequency or intensity under medication | 14 (18.2%) |
| Improvement of less than 50% under medication | 27 (35.1%) |
*Not associated with acute or subacute haemorrhage; patients were included due to haemorrhagic events occurring in the time interval between diagnosis and radiosurgery.
†Patients may exhibit several symptoms pre-SRS. The percentages are calculated for each symptom per patient.
‡Speech disorder, memory loss, unspecified gait trouble.
Q1–Q3, first to third quartiles; SRS, stereotactic radiosurgery.
Twenty-four (6.3%) patients had more than one CCM treated in the same SRS session: 18 patients with two lesions, 4 patients with three CCMs, 1 patient with four CCMs and 1 patient had five CCMs treated; in total, 414 CCMs were treated and included. Nineteen patients (5%) had been previously managed surgically for 21 CCMs (5.1%); the median time from resection to SRS was 3 years (Q1–Q3: 1–6). Seven patients had bleeding events after surgery; the remaining 14 were treated for a recurrent/residual CCM. Most treated CCMs (171/414 (41.3%)) were located in either supratentorial lobar areas or the brainstem (155/414 (37.4%)); basal ganglia and thalamic CCM (60/414 (14.5%)) or cerebellar CCM (28/414 (6.8%)]) were less common. A median margin dose of 12 Gy (Q1–Q3: 12.0–14.0) was employed and the median target volume was 0.6 cm3 (Q1–Q3: 0.2–1.5) (table 2).
Table 2.
Clinical, radiological and treatment characteristics of 414 CCMs
| Clinical and radiological data per CCM | n (%) |
| Previous surgery | 21 (5.1%) |
| Location characteristics | |
| Adjacent to the cortex | 253 (61.1%) |
| Adjacent to ependymal plane | 100 (24.2%) |
| Location (anatomic) | |
| Brainstem | 155 (37.4%) |
| Basal ganglia and thalamus | 60 (14.5%) |
| Supratentorial lobar area | 171 (41.3%) |
| Cerebellum | 28 (6.8%) |
| Associated developmental venous anomaly | 50 (12.1%) |
| Number of pre-SRS haemorrhages | |
| 1 | 324 (78.3%) |
| 2 | 71 (17.1%) |
| 3 | 16 (3.9%) |
| 4 | 1 (0.2%) |
| 5 | 2 (0.5%) |
| Median (Q1–Q3) time from diagnosis to SRS, years | 0.9 (0.3–3.1) |
| Zabramski classification | |
| 1 | 78 (19.0%) |
| 2 | 307 (74.7%) |
| 3 | 25 (6.1%) |
| 4 | 1 (0.2%) |
| Unknown | 3 |
| Dosimetric parameter per CCM, median (Q1–Q3) | |
| Target volume, cm3, median (Q1–Q3) | 0.6 (0.2–1.5) |
| Margin dose, Gy, median (Q1–Q3) | 12 (12c14) |
| Isocentres, median (Q1–Q3) | 4 (2–8) |
| Isodose line %, median (Q1–Q3) | 50 (50–51.5) |
CCM, cerebral cavernous malformation; Q1-Q3, first to third quartiles; SRS, stereotactic radiosurgery.
Haemorrhagic risk
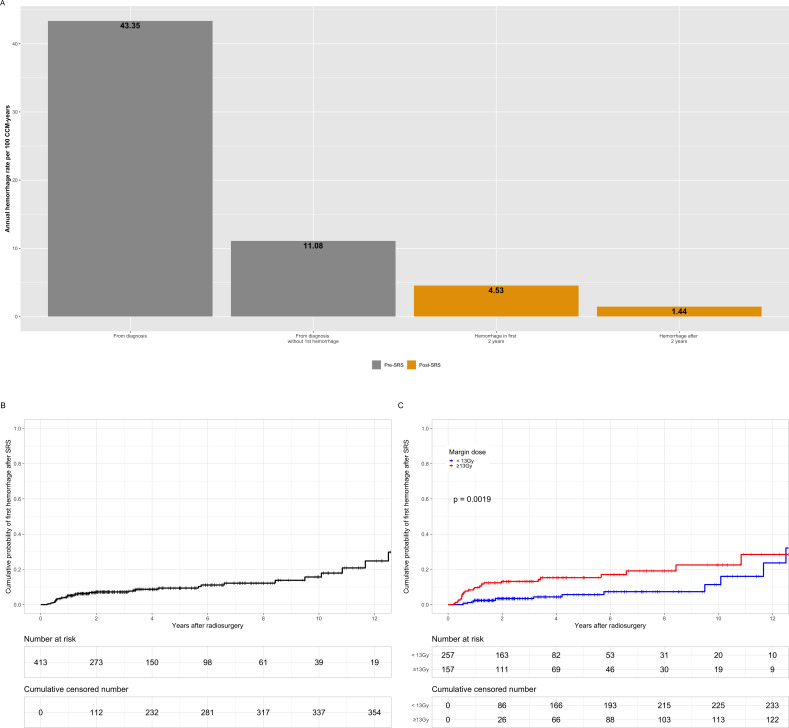
The cumulative number of pre-SRS haemorrhages was 528; 324 out of 414 (78.3%) CCM had a single haemorrhagic event, 71 out of 414 (17.1%) CCM bled twice, 16 out of 414 (3.9%) had three haemorrhages, 1 out of 414 (0.2%) bled four times and finally 2 out of 414 CCM (0.5%) bled five times. The calculated pre-SRS AHR varied based on the methodology used; 3.31 per 100 CCM-years (follow-up from birth: 15 931.2 years; 528 haemorrhages), 43.35 per 100 CCM-years (follow-up from diagnosis: 1218 years; 528 haemorrhages) and 11.08 per 100 CCM-years (follow-up from diagnosis: 1218 years; 135 haemorrhages after the exclusion of haemorrhages leading to CCM diagnosis).
The post-SRS AHR was 2.7 per 100 CCM-years, with a total of 50 haemorrhages occurring over 1850.9 years of follow-up. Specifically, 34 out of 414 (8.2%) CCM had a single post-SRS haemorrhagic event, 5 out of 414 (1.2%) bled twice and 2 out of 414 (0.5%) bled three times. Of these, 34 occurred in the first 2 years after SRS. With a cumulative follow-up of 750.2 years, this led to an AHR of 4.53 per 100-CCM years in the first 2 years after SRS. Sixteen haemorrhages occurred after 2 years over 1108.3 years of follow-up, which led to an AHR after 2 years of 1.44 per 100 CCM-years. There was a statistically significant reduction in haemorrhage rate post-SRS (−8.33 per 100 CCM-years, 95% CI 6.67 to 10, p<0.0001), when comparing pre-SRS (from diagnosis after the exclusion of first haemorrhages) and post-SRS. The different calculated AHR can be found (table 3, figure 1A).
Table 3.
Annual haemorrhage rate per 100 CCM-years
| Annual haemorrhage rate per 100 CCM-years | ||||
| Pre-SRS | Post-SRS | |||
| <2-year post-SRS | ≥2-year post-SRS | Overall | ||
| Overall cohort | ||||
| From birth | 3.31 | 4.53 | 1.44 | 2.7 |
| From diagnosis with first haemorrhage included | 43.35 | |||
| From diagnosis with first haemorrhage excluded* | 11.08 | |||
| Single haemorrhage before SRS (n=324) | ||||
| From birth | 2.66 | 3.6 | 1.48 | 2.38 |
| From diagnosis with first haemorrhage included | 47.86 | |||
| From diagnosis with first haemorrhage excluded* | 3.1 | |||
| Multiple haemorrhages before SRS (n=90) | ||||
| From birth | 5.45 | 7.78 | 1.36 | 3.68 |
| From diagnosis with first haemorrhage included | 37.71 | |||
| From diagnosis with first haemorrhage excluded* | 21.07 | |||
*Except when the diagnosis was before the first haemorrhage (n=21 cases for overall series and single bleed patients).
CCM, cerebral cavernous malformations; SRS, stereotactic radiosurgery.
Figure 1.
Bar plot of annual haemorrhage rate per 100 CCM-years in the overall cohort (A). Kaplan-Meier curve for first new haemorrhage after SRS (B) in function of margin dose (C). CCM, cerebral cavernous malformation; SRS, stereotactic radiosurgery.
In the multivariate recurrent event analysis, SRS (HR 0.27, 95% CI 0.17 to 0.44, p<0.0001) was associated with a significant reduction in haemorrhage rate. The presence of a DVA was associated with an increased risk of haemorrhage (HR 1.60, 95% CI 1.07 to 2.40, p=0.022) (table 4).
Table 4.
Recurrent event analysis using the Prentice, Williams and Peterson Gap-Time model for risk factors associated with haemorrhage in 411 CCMs
| Factor | Univariate analysis | Multivariate analysis | ||
| P value | HR (95% CI) | P value | HR (95% CI) | |
| Gender female | 0.243 | 1.21 (0.88 to 1.67) | ||
| Familial form | 0.821 | 0.77 (0.06 to 9.43) | ||
| Developmental venous anomaly | 0.014 | 1.69 (1.11 to 2.57) | 0.022 | 1.60 (1.07 to 2.40) |
| Location brainstem vs others | 0.022 | 1.44 (1.05 to 1.96) | 0.072 | 1.31 (0.98 to 1.75) |
| Prior surgery | 0.559 | 1.18 (0.68 to 2.04) | ||
| Stereotactic radiosurgery | <0.0001 | 0.25 (0.15 to 0.41) | <0.0001 | 0.27 (0.17 to 0.44) |
CCM, cerebral cavernous malformation.
The 2-year, 5-year and 10-year cumulative probability of a new, post-SRS, first haemorrhage was 7.2% (95% CI 4.4% to 9.7%), 9.4% (95% CI 6% to 12.6%) and 15.6% (95% CI 9% to 21.8%), respectively (figure 1B). In the multivariate Cox regression analysis, a margin dose >13 Gy (HR 2.27, 95% CI 1.20 to 4.32, p=0.012) and the presence of a DVA (HR 2.08, 95% CI 1.00 to 4.31, p=0.049) were associated with a higher probability of bleeding after SRS (table 5, figure 1C).
Table 5.
Cox analysis for factor associated with new haemorrhage after SRS in 413 CCMs*
| Factor | Univariate analysis | Multivariate analysis | ||
| P value | HR (95% CI) | P value | HR (95% CI) | |
| Gender female | 0.68 | 1.14 (0.61 to 2.13) | ||
| Developmental venous anomaly | 0.037 | 2.15 (1.05 to 4.40) | 0.049 | 2.08 (1.00 to 4.31) |
| Age at SRS >65 | 0.037 | 3.02 (1.07 to 8.52) | 0.052 | 2.81 (0.99 to 7.95) |
| Volume >0.7 cc | 0.042 | 1.94 (1.02 to 3.66) | 0.064 | 1.85 (0.96 to 3.53) |
| Margin >13 Gy | 0.003 | 2.63 (1.39 to 4.98) | 0.012 | 2.27 (1.20 to 4.32) |
| Location brainstem vs others | 0.65 | 1.16 (0.62 to 2.15) | ||
| Multiple haemorrhages pre-SRS | 0.048 | 1.90 (1.00 to 3.60) | 0.071 | 1.80 (0.95 to 3.42) |
*The analysis was performed on 413 CCM, as volumetric measurements on one CCM were missing
CCM, cerebral cavernous malformation; SRS, stereotactic radiosurgery.
Imaging outcomes
With a median imaging follow-up of 3.1 years from SRS (Q1–Q3: 1.8–6.1), the CCM volume was stable in 232 out of 412 (56.3%), decreased in 171 out of 412 (41.5%) and increased in 9 out of 412 (2.2%). Among the nine patients with an increased CCM volume, eight had a rebleed of their CCM. The pre-SRS and post-SRS Zabramski scale was available in 378 CCMs. At last follow-up, in 288 of them (76.2%), it was unchanged (table 2).
Adverse radiation effects
ARE occurred in 42 patients and 46 CCM (11.1%), with 95.6% (44/46) presenting as T2 perilesional hyperintensity. Of these 42, 25 patients were managed with observation, 13 required a corticosteroids regimen, 1 was treated with corticosteroid and bevacizumab, and in 1 case, the treatment was unknown. Two CCMs developed delayed cysts. One cyst was managed conservatively, and one required stereotactic aspiration.
On multivariate logistic regression, CCM volume >0.7 cc (OR 5.19, 95% CI 2.41 to 12.5, p<0.001) and margin dose >13 Gy (OR 5.17, 95% CI 2.55 to 11.2, p<0.001) were associated with the occurrence of ARE (online supplemental table 2).
Clinical outcomes
At a median clinical follow-up of 3.78 from SRS (Q1–Q3: 1.71–6.54) years, 60 (15.7%) patients developed new or worsening neurological deficits. Post-SRS haemorrhages were responsible for 15 (3.9%) cases of transient neurological deficits, 7 (1.9%) cases of permanent neurological deficits, 16 (4.2%) cases of headache and 1 (0.3%) case of seizures. AREs were responsible for 15 cases (3.9%) of transient deficits, 2 (0.5%) cases of new seizures and 4 cases (1.1%) of permanent deficits. Twenty-one (5.5%) patients were asymptomatic.
In four (1.1%) patients, the neurological deterioration (two transient and two permanent) was linked to the CCM without evidence of new haemorrhage or ARE on MRI. Two (0.5%) patients developed Todd’s paresis. Eight (2.1%) more patients developed neurological deficits (4 (1.1%) of them transient and 4 (1.1%) permanent) with no clear aetiology. In three (0.9%) patients, new neurological symptoms (one transient, two permanent) developed following a first-ever haemorrhage of coexisting, non-haemorrhagic CCM. Furthermore, one (0.3%) patient developed normal-pressure hydrocephalus, and one (0.3%) patient presented with ischaemic stroke. Overall, accounting for all post-SRS events, 119 out of 371 (32.1%) patients had improved neurological function at a last follow-up, 83 out of 371 (22.4%) patients remained stable and 20 out of 371 (5.4%) deteriorated, 149 out of 371 (40.1%) had no symptoms prior SRS and did not develop new symptoms. Six patients (1.6%) died during the follow-up, with repeat CCM haemorrhage being the cause in one patient. The cause of death was either unrelated to the CCM (three cases) or unknown (two cases) in the other five patients.
Seizure
Seventy-seven patients (20.2%) presented with at least one seizure prior to SRS. Of these 77 patients, 2 (2.6%) had no further seizure and required no medication, 34 (44.2%) had no more seizure under medication, 14 (18.2%) had an improvement of at least 50% in the frequency/intensity of seizures under medication and 27 (35.1%) had an improvement of less than 50% in the frequency/intensity of epileptic activity under medication or had medically refractory seizures (table 1).
New-onset, post-SRS seizure occurred in three patients; in two of them due to ARE. Overall, at a last clinical follow-up, 46 patients (57.5%) were Engel class I, 11 (13.75%) were Engel class 2, 8 (10%) were Engel class 3, 12 (15%) showed no improvement (Engel class 4A and B) and 3 (3.75%) had worse symptoms (Engel class 4C). In 14 (17.5%) patients, seizure medications were completely withdrawn (online supplemental table 1).
Additional management
Further treatment was required in eight patients, with three undergoing CCM resection and four repeat SRS. One patient underwent a stereotactic aspiration of a cyst and a thalamotomy for tremor. The median time from SRS to new treatment was 3.1 years (Q1–Q3: 1.3–6.5).
Discussion
Haemorrhage risk reduction
This multicentric study included 381 patients harbouring 414 haemorrhagic CCMs. Only CCMs with at least one haemorrhagic episode were included to ensure a more homogeneous population. To the best of our knowledge, this is the largest report on SRS-managed haemorrhagic CCM to date.
Using recurrent event analysis, SRS appears to significantly reduce the risk of haemorrhage (HR 0.29, p<0.001). Several publications have underlined the importance of using specific statistical methodology for recurrent events.21 23 With the number of haemorrhages being the main outcome used to evaluate SRS efficacy for CCM and also a proven risk factor of subsequent haemorrhages, we believe that the PWP-GT model was more suitable.21 22 While a reduction of the AHR after SRS treatment has been previously reported in the literature,10 11 15 it is the first time that a recurrent event analysis model is used to evaluate the efficacy of SRS. The advantage of this method is that it takes into consideration the natural history of CCM with multiple haemorrhages and their effect on the risk of future bleeding events. These results are in accordance with a recent meta-analysis showing that SRS showed high efficacy in preventing future haemorrhage (86%, 95% CI 81% to 90%) with a low risk of long-term morbidity (10%, 95% CI 7 to 13%), while the rates were 77% (95% CI 75% to 83%) and 21% (95% CI 16% to 28%) for observation, respectively.24
Some uncertainty concerning the efficacy of SRS is that the observed risk reduction stems from the tendency of CCM to present with closely spaced clusters of haemorrhage interspersed with long haemorrhage-free intervals.16 It is currently unknown if and when the bleeding risk returns to baseline levels.2 25 Unfortunately, only prospective clinical trials with a control group could completely elucidate the effect of SRS on haemorrhage risk. The difficulty of performing such a clinical trial was previously demonstrated by the inability to recruit enough patients.6 However, in a retrospective study by Lee et al, no significant difference in the haemorrhage rate during the first 2 years after SRS was observed when comparing patients that were treated after multiple haemorrhages to patients treated after a single haemorrhage.26 It should be noted though, that the risk factors linked to an aggressive behaviour with repeat haemorrhages are not well understood,27 and that comparisons with natural history studies would be biased, as most of them only include the first haemorrhage.2 A better understanding of the natural history and the development of imaging protocols or plasma biomarkers could help to better define the efficacy of SRS.28 29
Interestingly, we found margin dose >13 Gy (HR 2.27, p=0.012) to be associated with an increased risk of new post-SRS haemorrhage. This association might initially seem counterintuitive. Shin et al demonstrated the presence of vascular endothelial growth factor (VEGF)-staining small capillaries and venules in irradiated CCM that were resected after a new bleeding. They formulated the hypotheses that these vessels are foci of neovascularisation, which may progress to the characteristic thin-walled large lumen vessel responsible for subsequent haemorrhage.30 Kim et al showed in vivo that endothelial cell irradiation with more than 20 or 30 Gy in single fraction was responsible for increasing vascular endothelial growth factor A (VEGF-A) production.31 Given that usually a 50% isodose line is used in Gamma Knife radiosurgery, maximum doses >26 Gy would have frequently been delivered with prescription doses> 13 Gy. While this radiobiological mechanism could support our results, there is paucity of data regarding the optimal radiation dose that would adequately reduce the risk of haemorrhage, while at the same time not inducing overexpression of VEGF. In the same way, the reason for rebleeding in the first 2 years after SRS or later could be attributed to different pathophysiological phenomenon. This association has never been described before, with most studies either reporting no differences in haemorrhage rate after SRS in function of the dose7 11 32 or not investigating for associated risk factors.8 12
The presence of a DVA (HR 1.60, p=0.022 with PWP-GT) as a risk factor for new haemorrhage after SRS is in accordance with the literature.33 34 The implications for the radiosurgical management of CCM are unknown, other than that targeting the DVA is not recommended due to an increased risk of complications.35 CCMs located in the brainstem reportedly have a worse natural history2 and are associated with higher rebleeding risk after SRS,8 a finding that was not validated in our model. Similarly, no increased risk of post-SRS haemorrhage in patients with multiple pre-SRS haemorrhages as compared with patients with a single haemorrhage was observed.7 8
Adverse radiation effects
AREs occurred in 46 CCMs (11.1%) and were associated with transient neurological symptoms in 15 patients, permanent in 4 and seizures in 2 cases. A recent meta-analysis reported a 4% risk of permanent deficit due to ARE.36 A volume >0.7 cc (OR 5.19, p<0.001) and a margin dose >13 Gy (OR 5.17, p<0.001) were associated with increased risk of ARE. This is concordant with other literature reports.37 The haemosiderin ring has been hypothesised to act as a radiosensitiser due to its elevated iron content.12 38 This characteristic could explain the higher risk of ARE with similar doses, volume and location compared with arteriovenous malformation.13 It is currently recommended to avoid the inclusion of haemosiderin in the treatment plan.38
Limitations
Even though the multicentric design can partially mitigate the effect of individual centre biases, its retrospective nature makes it subject to selection bias, institutional treatment practices and reporting bias. The reason for treating patient with SRS over surgery could not be reliably captured.
As seizure control was not the primary goal of treatment, the complete Engel classification was not employed to report outcomes.
The imaging was not centrally reviewed. New haemorrhage was defined in this study as evidence of acute or subacute bleeding and new symptoms as recommended.18 Asymptomatic haemorrhage was not captured and the haemorrhage rate could be underestimated. Due to the long-time interval in which patients were treated, various MRI sequences and/or CT scan were used. This could have introduced bias in the evaluation of haemorrhage, DVA and ARE; however, the direction of this bias could not be ascertained, and careful description of new symptom onset was performed. Moreover, despite the fact that the median follow-up time in this study is comparable to a recent meta-analysis study evaluating the natural history of CCM, it is conceivable that delayed complications, such as adverse radiation effects or de novo CCM, could be missed.2
Of the included CCM in the study, 5.1% were surgically managed before SRS. This rate is in accordance with rate of remnant or recurrence (4.3% to 6.6%) found in the open surgery reports.39 40 The differences between residual or recurrent CCM after surgery could not be reliably captured, as imaging availability and techniques evolved with time.
Genetic mutations were confirmed in 0.6% of the patients. Patients were not uniformly tested for genetic mutations in all participating centres due to the differences in genetic testing availability and the inclusion of patients treated over a long period. As such, the number of patients harbouring gene mutations associated with CCM formation is probably underestimated.
We made the choice to have broad inclusion criteria, rather than exclude specific patient subgroups (with a familial form, a previous surgery) to be closer to clinical practice. This choice can increase heterogeneity in the cohort.
Generalisability
Due to the multicentric nature of the study, the results can apply to haemorrhagic CCM treated with radiosurgery but not to the group with progressive FND without clear evidence of new haemorrhage.
Conclusion
Single-session SRS decreases the risk of repeat haemorrhage in haemorrhagic CCM. Further evidence is needed to confirm the efficacy of SRS and improve case selection. Prescription doses ≤13 Gy could reduce SRS-related complications and the risk of repeat haemorrhage.
Acknowledgments
CD gratefully acknowledges receipt of a grant for mobility from the “Hospices civils de Lyon”, France, from the “Institut Servier”, France, from the “Societe française of Neurochirurgie (SFNC)”, France, from the “Fondation Planiol”, France, and from the “Phillip foundation”.
Footnotes
@wrhernandez4
Contributors: Conception and design: JS, CD, SP, GM. Acquisition of data: CD, SP, YS, GDA, AMN, WAR, SRT, KA, AME-S, RMEE, AHE, NMM, RMÁ, RL, JM, DM, J-NT, MT, AR, NK, RK, PP, AF, HS, WH, REB, REW, JA, DK, GNB, SP. Analysis and interpretation of data: CD, GM. Drafting the article: CD, GM. Critically revising the article: JS, GM, SD, ZX, YS, NMM, RL, DM, MT, DK. Reviewed submitted version of manuscript: All authors. Statistical analysis: CD, GM. Study supervision: JS.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. Data are available on reasonable request to the corresponding author.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1. Akers A, Al-Shahi Salman R, A. Awad I, et al. Synopsis of guidelines for the clinical management of cerebral cavernous malformations: consensus recommendations based on systematic literature review by the Angioma alliance scientific advisory board clinical experts panel. NEUROSURGERY 2017;80:665–80. 10.1093/neuros/nyx091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Horne MA, Flemming KD, Su I-C, et al. Clinical course of untreated cerebral cavernous malformations: a meta-analysis of individual patient data. Lancet Neurol 2016;15:166–73. 10.1016/S1474-4422(15)00303-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Harris L, Poorthuis MHF, Grover P, et al. Surgery for cerebral cavernous malformations: a systematic review and meta-analysis. Neurosurg Rev 2022;45:231–41. 10.1007/s10143-021-01591-5 [DOI] [PubMed] [Google Scholar]
- 4. Gross BA, Batjer HH, Awad IA, et al. Brainstem cavernous malformations: 1390 surgical cases from the literature. World Neurosurg 2013;80:89–93. 10.1016/j.wneu.2012.04.002 [DOI] [PubMed] [Google Scholar]
- 5. Awad IA, Polster SP. Cavernous Angiomas: Deconstructing a neurosurgical disease. J Neurosurg 2019;131:1–13. 10.3171/2019.3.JNS181724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Flemming KD, Lanzino G. Stereotactic Radiosurgery for cavernous malformations: natural history or treatment effect Neurology 2019;93:921–2. 10.1212/WNL.0000000000008516 [DOI] [PubMed] [Google Scholar]
- 7. Jacobs R, Kano H, Gross BA, et al. Defining long-term clinical outcomes and risks of stereotactic Radiosurgery for brainstem cavernous malformations. World Neurosurg 2018. 10.1016/j.wneu.2018.11.226 [DOI] [PubMed] [Google Scholar]
- 8. Nagy G, Stokes SS, Erőss LG, et al. Contemporary Radiosurgery of cerebral cavernous malformations: part 2. treatment outcome for Hemispheric lesions. J Neurosurg 2018:1–9. 10.3171/2018.2.JNS171267 [DOI] [PubMed] [Google Scholar]
- 9. Kida Y, Hasegawa T, Iwai Y, et al. Radiosurgery for symptomatic cavernous malformations: A multi-institutional retrospective study in Japan. Surg Neurol Int 2015;6(Suppl 5):S249–57. 10.4103/2152-7806.157071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lee C-C, Wang W-H, Yang H-C, et al. Gamma knife Radiosurgery for cerebral cavernous malformation. Sci Rep 2019;9:19743. 10.1038/s41598-019-56119-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Karaaslan B, Gülsuna B, Erol G, et al. Stereotactic Radiosurgery for cerebral cavernous malformation: comparison of hemorrhage rates before and after stereotactic Radiosurgery. J Neurosurg 2022;136:655–61. 10.3171/2021.2.JNS21138 [DOI] [PubMed] [Google Scholar]
- 12. Lunsford LD, Khan AA, Niranjan A, et al. Stereotactic Radiosurgery for symptomatic solitary cerebral cavernous malformations considered high risk for resection. J Neurosurg 2010;113:23–9. 10.3171/2010.1.JNS081626 [DOI] [PubMed] [Google Scholar]
- 13. Pollock BE, Garces YI, Stafford SL, et al. Stereotactic Radiosurgery for cavernous malformations. J Neurosurg 2000;93:987–91. 10.3171/jns.2000.93.6.0987 [DOI] [PubMed] [Google Scholar]
- 14. Kondziolka D, Lunsford LD, Flickinger JC, et al. Reduction of hemorrhage risk after stereotactic Radiosurgery for cavernous malformations. J Neurosurg 1995;83:825–31. 10.3171/jns.1995.83.5.0825 [DOI] [PubMed] [Google Scholar]
- 15. Poorthuis MHF, Rinkel LA, Lammy S, et al. Stereotactic Radiosurgery for cerebral cavernous malformations: A systematic review. Neurology 2019;93:e1971–9. 10.1212/WNL.0000000000008521 [DOI] [PubMed] [Google Scholar]
- 16. Barker FG, Amin-Hanjani S, Butler WE, et al. Temporal clustering of hemorrhages from untreated cavernous malformations of the central nervous system. Neurosurgery 2001;49:15–24; 10.1097/00006123-200107000-00002 [DOI] [PubMed] [Google Scholar]
- 17. Zabramski JM, Wascher TM, Spetzler RF, et al. The natural history of familial cavernous malformations: results of an ongoing study. J Neurosurg 1994;80:422–32. 10.3171/jns.1994.80.3.0422 [DOI] [PubMed] [Google Scholar]
- 18. Al-Shahi Salman R, Berg MJ, Morrison L, et al. Hemorrhage from cavernous malformations of the brain: definition and reporting standards. Angioma Alliance Scientific Advisory Board Stroke 2008;39:3222–30. 10.1161/STROKEAHA.108.515544 [DOI] [PubMed] [Google Scholar]
- 19. Engel J, Wiebe S, French J, et al. Practice parameter: temporal lobe and localized neocortical Resections for epilepsy: report of the quality standards subcommittee of the American Academy of neurology. Neurology 2003;60:538–47. 10.1212/01.WNL.0000055086.35806.2D [DOI] [PubMed] [Google Scholar]
- 20. Sahai H, Khurshid A. Statistics in epidemiology: methods, techniques, and applications. Boca Raton: CRC Press, 1996. [Google Scholar]
- 21. Thenmozhi M, Jeyaseelan V, Jeyaseelan L, et al. Survival analysis in longitudinal studies for recurrent events: applications and challenges. Clinical Epidemiology and Global Health 2019;7:253–60. 10.1016/j.cegh.2019.01.013 [DOI] [Google Scholar]
- 22. Amorim L, Cai J. Modelling recurrent events: a Tutorial for analysis in epidemiology. Int J Epidemiol 2015;44:324–33. 10.1093/ije/dyu222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rauch G, Kieser M, Binder H, et al. Time-to-first-event versus recurrent-event analysis: points to consider for selecting a meaningful analysis strategy in clinical trials with composite endpoints. Clin Res Cardiol 2018;107:437–43. 10.1007/s00392-018-1205-7 [DOI] [PubMed] [Google Scholar]
- 24. Bubenikova A, Skalicky P, Benes V, et al. Overview of cerebral cavernous malformations: comparison of treatment approaches. J Neurol Neurosurg Psychiatry 2022;93:475–80. 10.1136/jnnp-2021-328658 [DOI] [PubMed] [Google Scholar]
- 25. Steiner L, Karlsson B, Yen C-P, et al. Radiosurgery in cavernous malformations: anatomy of a controversy. J Neurosurg 2010;113:16–21; 10.3171/2009.11.JNS091733 [DOI] [PubMed] [Google Scholar]
- 26. Lee SH, Choi HJ, Shin HS, et al. Gamma knife Radiosurgery for brainstem cavernous malformations: should a patient wait for the Rebleed Acta Neurochir (Wien) 2014;156:1937–46. 10.1007/s00701-014-2155-0 [DOI] [PubMed] [Google Scholar]
- 27. Santos AN, Rauschenbach L, Gull HH, et al. Central nervous system cavernous malformations: cross-sectional study assessing Rebleeding risk after a second haemorrhage. Eur J Neurol 2023;30:144–9. 10.1111/ene.15574 [DOI] [PubMed] [Google Scholar]
- 28. Girard R, Zeineddine HA, Fam MD, et al. Plasma biomarkers of inflammation reflect seizures and hemorrhagic activity of cerebral cavernous malformations. Transl Stroke Res 2018;9:34–43. 10.1007/s12975-017-0561-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sone JY, Hobson N, Srinath A, et al. Perfusion and permeability MRI predicts future cavernous Angioma hemorrhage and growth. J Magn Reson Imaging 2022;55:1440–9. 10.1002/jmri.27935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shin SS, Murdoch G, Hamilton RL, et al. Pathological response of cavernous malformations following Radiosurgery. J Neurosurg 2015;123:938–44. 10.3171/2014.10.JNS14499 [DOI] [PubMed] [Google Scholar]
- 31. Kim EJ, Lee H, Lee Y-J, et al. Ionizing radiation regulates vascular endothelial growth factor-A transcription in cultured human vascular endothelial cells via the PERK/Eif2Α/Atf4 pathway. Int J Radiat Oncol Biol Phys 2020;107:563–70. 10.1016/j.ijrobp.2020.03.003 [DOI] [PubMed] [Google Scholar]
- 32. López-Serrano R, Martínez NE, Kusak ME, et al. Significant hemorrhage rate reduction after gamma knife Radiosurgery in symptomatic cavernous malformations: long-term outcome in 95. Stereotact Funct Neurosurg 2017;95:369–78. 10.1159/000480664 [DOI] [PubMed] [Google Scholar]
- 33. Idiculla PS, Gurala D, Philipose J, et al. Cerebral cavernous malformations, developmental venous anomaly, and its coexistence: A review. Eur Neurol 2020;83:360–8. 10.1159/000508748 [DOI] [PubMed] [Google Scholar]
- 34. Zhang S, Ma L, Wu C, et al. A rupture risk analysis of cerebral cavernous malformation associated with developmental venous anomaly using susceptibility-weighted imaging. Neuroradiology 2020;62:39–47. 10.1007/s00234-019-02274-1 [DOI] [PubMed] [Google Scholar]
- 35. Lindquist C, Guo WY, Karlsson B, et al. Radiosurgery for venous Angiomas. J Neurosurg 1993;78:531–6. 10.3171/jns.1993.78.4.0531 [DOI] [PubMed] [Google Scholar]
- 36. Shanker MD, Webber R, Pinkham MB, et al. Gamma knife® stereotactic Radiosurgery for intracranial cavernous malformations. Journal of Clinical Neuroscience 2022;106:96–102. 10.1016/j.jocn.2022.10.015 [DOI] [PubMed] [Google Scholar]
- 37. Liscák R, Vladyka V, Simonová G, et al. Gamma knife surgery of brain cavernous Hemangiomas. J Neurosurg 2005;102 Suppl:207–13. 10.3171/jns.2005.102.s_supplement.0207 [DOI] [PubMed] [Google Scholar]
- 38. George EJS, Perks J, Plowman PN. “Stereotactic Radiosurgery XIV: the role of the Haemosiderin “ring” in the development of adverse reactions following Radiosurgery for intracranial cavernous malformations: a sustainable hypothesis”. British Journal of Neurosurgery 2002;16:385–91. 10.1080/026886902100007632 [DOI] [PubMed] [Google Scholar]
- 39. Singh H, Elarjani T, da Silva HB, et al. Brain stem cavernous malformations: operative nuances of a less-invasive resection technique. Operative Surg 2018;15:153–73. 10.1093/ons/opx231 [DOI] [PubMed] [Google Scholar]
- 40. Garcia RM, Oh T, Cole TS, et al. Recurrent brainstem cavernous malformations following primary resection: blind spots, fine lines, and the right-angle method. J Neurosurg 2020;135:671–82. 10.3171/2020.6.JNS201555 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
svn-2023-002380supp001.pdf (116.7KB, pdf)
Data Availability Statement
Data are available upon reasonable request. Data are available on reasonable request to the corresponding author.