Abstract
Objectives
To identify the occurrence rate and predictors of futile recanalisation after endovascular therapy (EVT) for acute vertebrobasilar artery occlusion (VBAO).
Methods
Participants of the Endovascular Treatment Key Technique and Emergency Workflow Improvement of Acute Ischaemic Stroke (ANGEL-ACT) registry were selected for the analysis. Futile recanalisation was defined as patients did not achieve a 90-day good outcome (modified Rankin Scale ≤3) despite successful recanalisation (modified Treatment in Cerebral Ischaemia Scale ≥2b) after the procedure. Multivariable logistic regression analysis was conducted to find independent predictors of futile recanalisation in VBAO patients undergoing EVT.
Results
Three hundred and fifteen patients with VBAO who achieved successful recanalisation after EVT were included in current analysis, of whom, 155 (49.2%) suffered futile recanalisation, and 160 achieved effective recanalisation. After the multivariable analysis, we found admission National Institutes of Health Stroke Scale (NIHSS) ≥19 (OR 4.81, 95% CI 2.76 to 8.39, p<0.001), platelet-lymphocyte ratio (PLR) ≥162.2 (OR 1.93, 95% CI 1.14 to 3.27, p=0.001), onset-to-puncture time (OTP) ≥334 min (OR 2.15, 95% CI 1.25 to 3.68, p=0.005) and use of general anesthesia (GA) (OR 1.87, 95% CI 1.09 to 3.22, p=0.024) were associated with futile recanalisation.
Conclusions
Futile recanalisation after EVT occurred 49.2% of VBAO patients in the ANGEL-ACT registry. NIHSS≥19, PLR≥162.2, OTP≥334 min and use of GA were independent predictors of futile recanalisation.
Keywords: Stroke, Thrombectomy, Cerebral Infarction
WHAT IS ALREADY KNOWN ON THIS TOPIC
There is little literature about futile recanalisation after endovascular therapy (EVT) for vertebrobasilar artery occlusion (VBAO), especially in the Asian population. Age, initial National Institutes of Health Stroke Scale, Posterior Circulation Alberta Stroke Programme Early CT Score, intravenous thrombolysis, number of passes ≤3 and first pass modified Treatment in Cerebral Ischaemia Scale 2b-3 reperfusion were reported as associated independently with futile recanalisation after EVT for VBAO previously.
WHAT THIS STUDY ADDS
We observed that futile recanalisation occurred in 49.2% of patients with VBAO, and added platelet-lymphocyte ratio ≥162.2, onset-to-puncture time ≥334 min and use of general anesthesia as the new predictors of futile recanalisation.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
The rate and several predictors of futile recanalisation after EVT for VBAO have been identified in this analysis, which could add them to the global data.
Introduction
Whether endovascular therapy (EVT) is superior to medical therapy for acute ischaemic stroke (AIS) caused by acute vertebrobasilar artery occlusion (VBAO) is still unclear. At present, four randomised controlled trials (RCTs) have been completed. BEST (Basilar Artery Occlusion Endovascular Intervention vs Standard Medical Treatment) and BASICS (Basilar Artery International Cooperation Study) RCTs did not find the superiority of EVT for VBAO.1 2 However, the other RCTs in China, BAOCHE (Basilar Artery Occlusion Chinese Endovascular Trial) and ATTENTION (Endovascular Treatment for Acute Basilar Artery Occlusion), found that EVT could lead to better outcomes than medical therapy in VBAO patients.3 4 Among the four RCTs, the successful recanalisation rate in the EVT group varied from 71% to 93% and 90-day good outcome (modified Rankin Scale(mRS) 0–3) rate after EVT varied from 42% to 46%.1–4 Therefore, there were still some VBAO patients who did not achieve a good outcome at 90 days despite successful recanalisation after EVT, which we call futile recanalisation. Futile recanalisation of EVT in AIS caused by large-vessel occlusion in the anterior circulation has been explored by many studies.5 However, regarding the VBAO, especially in the Asian population, the studies are still scarce.6–11
Therefore, we conducted an analysis using the data from a Chinese, nationwide, prospective, multicentre, registry database, aiming to explore the rate and predictors of futile recanalisation in VBAO patients undergoing EVT.
Methods
Study population
ANGEL-ACT registry (Endovascular Treatment Key Technique and Emergency Workflow Improvement of Acute Ischaemic Stroke) is a multicentre, prospective registry study, carried out from November 2017 to March 2019 at 111 sites in 26 Chinese provinces. Data were used from the above registry. The exclusion criteria and inclusion criteria, imaging interpretation method, and data collection method were reported previously.12 The inclusion and exclusion criteria of the current study were that: inclusion criteria: (1) age ≥18 years old; (2) AIS due to LVO; (3) the patient or the patient’s legal representative signed the informed consent; (4) undergoing EVT; exclusion criteria: (1) EVT records unavailable; (2) unsuccessful recanalisation; (3) mRS missing at 90 days; (4) posterior cerebral artery occlusion and (5) anterior circulation stroke.
Data collection
ANGEL-ACT registry collected all information prospectively, such as medical history, demographic characteristics, laboratory results, baseline National Institutes of Health Stroke Scale (NIHSS), vital signs, periprocedural management, procedural variables, key time points and clinical outcomes at 90 days assessed by mRS. The investigators received training and got the qualification certificates to record NIHSS and mRS. By the phone interview, only investigators blinded to all clinical information evaluated mRS at 90 days on the protocol of standardised interview.
ANGEL-ACT registry had an independent neuroimaging core lab to assess all imaging that included digital subtraction angiography, baseline MRI/MR angiography, CT/ CT angiography and CT after the procedure. The imaging interpretation included underlying intracranial atherosclerotic disease, tandem lesion, intraprocedural complications, modified Treatment in Cerebral Ischaemia Scale (mTICI)13 after the procedure, baseline Posterior Circulation Alberta Stroke Programme Early CT Score (PC-ASPECTS) and haemorrhage transformation after the procedure.
Futile recanalisation and effective recanalisation
We defined futile recanalisation as VBAO patients experiencing a 90-day poor outcome (mRS 4–6) despite successful recanalisation (mTICI≥2b) after EVT and effective recanalisation as VBAO patients achieving a 90-day good outcome (mRS≤3) with successful recanalisation after EVT.
Statistical analysis
We used numbers (percentages) to express categorical variables and median (IQR) to express continuous variables. The Mann-Whitney test and Pearson χ2 test/Fisher’s exact test were performed as univariable analyses to perform the comparison of baseline and procedure variables between the futile recanalisation and effective recanalisation groups. Before multivariable analysis, the variance inflation factors were calculated to assess the multicollinearity among the variables with p<0.10 in the univariable analysis. Next, in order to identify the best cut-off values of onset-to-puncture time (OTP), NIHSS, baseline neutrophil-lymphocyte ratio (NLR), procedure duration and baseline platelet-lymphocyte ratio (PLR), to predict futile recanalisation, we performed receiver operating characteristic analyses. Then we converted the NIHSS, PLR, NLR, OTP and procedure duration into two categorical variables according to the best cut-off values. Finally, in order to identify the independent predictors of futile recanalisation, we conducted binary logistic regression analysis with back-stepwise including NLR, PLR, NIHSS, OTP, procedure duration, use of general anaesthesia (GA) and heparin. All statistical analyses were conducted by the SAS software (V.9.4, SAS Institute). A p<0.05 (two tailed) is considered statistical significance.
Results
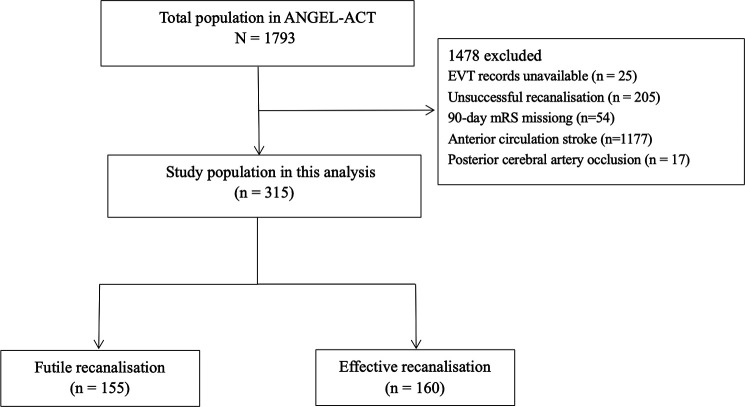
As shown in figure 1, 315 of 1793 patients with VBAO in the ANGEL-ACT registry who achieved successful recanalisation were included in this analysis, of whom, 155 experienced futile recanalisation and 160 achieved effective recanalisation.
Figure 1.
Flow chart of patient selection. ANGEL-ACT; Endovascular Treatment Key Technique and Emergency Workflow Improvement of Acute Ischaemic Stroke; EVT, endovascular treatment; mRS, modified Rankin scale.
As shown in table 1, patients with futile recanalisation had higher admission NIHSS (29 (19–35) vs 15 (8–26), p<0.001), higher admission PLR (179.4 (125.8–230.7) vs 149.0 (103.1–215.7), p=0.026), higher admission NLR (7.8 (3.8–11.7) vs 5.8 (3.1–9.3), p=0.018), a higher rate of use of GA (64.5% vs 51.3%, p=0.017) and OTP (341 (230–439) min vs 275 (185–399) min, p=0.007) than those with effective recanalisation.
Table 1.
Baseline and procedure characteristics of patients with futile recanalisation and effective recanalisation
| Baseline and procedure variables | Futile recanalisation (n=155) |
Effective recanalisation (n=160) |
P value |
| Age, years, median (IQR) | 64 (55–72) | 64 (54–72) | 0.499 |
| Male sex, n (%) | 120 (77.4) | 130 (81.3) | 0.401 |
| Admission mode, n (%) | 0.490 | ||
| Mothership | 90 (58.1) | 99 (61.9) | |
| Drip and ship | 65 (41.9) | 61 (38.1) | |
| Hypertension, n (%) | 105 (67.7) | 113 (70.6) | 0.580 |
| DM, n (%) | 37 (23.9) | 33 (20.6) | 0.488 |
| Hyperlipidaemia, n (%) | 18 (11.6) | 26 (16.3) | 0.235 |
| Coronary heart disease, n (%) | 26 (16.8) | 25 (15.6) | 0.782 |
| Atrial fibrillation, n (%) | 21 (13.6) | 23 (14.4) | 0.832 |
| Prior stroke, n (%) | 44 (28.4) | 44 (27.5) | 0.861 |
| Smoking history, n (%) | 0.233 | ||
| Never smoking | 83 (53.6) | 76 (47.5) | |
| Previous smoking | 11 (7.1) | 20 (12.5) | |
| Current smoking | 61 (39.4) | 64 (40.0) | |
| SBP, mmHg | 150 (135–167) | 153 (136–164) | 0.644 |
| Admission NIHSS* | 29(19-35) | 15(8-26) | <0.001 |
| Admission PC-ASPECTS† | 9 (6–10) | 9 (7–10) | 0.149 |
| Serum glucose, mmol/L, median (IQR) | 7.9 (6.6–10.5) | 7.5 (6.4–10.1) | 0.355 |
| Blood WCC, ×109 /L, median (IQR) | 10.3 (8.2–13.4) | 10.0 (8.0–12.5) | 0.113 |
| NLR, median (IQR) | 7.8 (3.8–11.7) | 5.8 (3.1–9.3) | 0.018 |
| PLR, median (IQR) | 179.4 (125.8–230.7) | 149.0 (103.1–215.7) | 0.026 |
| PLT, ×109/L, median (IQR) | 221.5 (184.0–251.0) | 216.0 (182.0–262.0) | 0.967 |
| Prior use of antiplatelet agents, n (%) | 24 (15.5) | 33 (20.6) | 0.236 |
| Prior use of anticoagulants, n (%) | 3 (1.9) | 5 (3.1) | 0.723 |
| Prior IVT, n (%) | 38(24.5) | 45(28.1) | 0.467 |
| Tandem occlusion, n (%) | 33 (21.3) | 23 (14.4) | 0.109 |
| Underlying ICAD, n (%) | 0.760 | ||
| Yes | 76 (49.0) | 73 (45.6) | |
| No | 63 (40.7) | 67 (41.9) | |
| Undetermined | 16 (10.3) | 20 (12.5) | |
| TOAST subtypes, n (%) | 0.106 | ||
| Large artery atherosclerosis | 96 (61.9) | 108 (67.5) | |
| Cardioembolism | 29 (18.7) | 30 (18.8) | |
| Other or unknown | 22 (14.2) | 21 (13.1) | |
| Undetermined | 8 (5.2) | 1 (0.6) | |
| General anaesthesia, n (%) | 100 (64.5) | 82 (51.3) | 0.017 |
| Heparin, n (%) | 89 (57.4) | 75 (46.9) | 0.061 |
| GP IIb/IIIa receptor inhibitor, n (%) | 109 (70.3) | 99 (61.9) | 0.114 |
| Stent retriever as first line, n (%) | 107 (69.0) | 105 (65.6) | 0.519 |
| Direct aspiration as first line, n (%) | 10 (6.5) | 6 (3.8) | 0.275 |
| Direct aspiration+stent retriever as first-line | 13 (8.4) | 18 (11.3) | 0.394 |
| IAT, n (%) | 13 (8.4) | 12 (7.5) | 0.771 |
| Rescue balloon/ stenting angioplasty, n (%) | 51 (32.9) | 48 (30.0) | 0.579 |
| Complete recanalisation, n (%) | 104 (67.1) | 111 (69.4) | 0.664 |
| No. of MT passes, median (IQR) | 1 (1–2) | 1 (1–2) | 0.151 |
| OTP, min, median(IQR) | 341 (230–439) | 275 (185–399) | 0.007 |
| Procedure duration, min, median(IQR) | 96(60-145) | 84(53-120) | 0.058 |
Bold values indicate statistical significance.
*Two missing data.
†Six missing data.
‡
DM, diabetes mellitus; IAT, intra-arterial thrombolysis; ICAD, intracranial atherosclerotic disease; IVT, intravenous thrombolysis; MT, mechanical thrombectomy; NIHSS, National Institute of Health Stroke Scale; NLR, neutrophil to lymphocyte ratio; OTP, Onset-to-puncture time; PC-ASPECTS, Posterior Circulation Alberta Stroke Programme Early CT Score; PLR, platelet to lymphocyte ratio; PLT, platelet; SBP, systolic blood pressure; TOAST, Trial of ORG 10172 in Acute Stroke Treatment; WCC, white cell count.
As shown in table 2, online supplemental file 1, we identified that admission NIHSS≥19 (OR 4.81, 95% CI 2.76 to 8.39, p<0.001), PLR≥162.2 (OR 1.93, 95% CI 1.14 to 3.27, p=0.001), OTP≥334 min (OR 2.15, 95% CI 1.25 to 3.68, p=0.005) and use of GA (OR 1.87, 95% CI 1.09 to 3.22, p=0.024) were related to the futile reperfusion after EVT for VBAO patients after the multivariable logistic analysis .
Table 2.
Independent predictors of futile recanalisation
| Predictors | Unadjusted OR (95% CI) | P value | Adjusted OR (95% CI) | P value |
| Admission NIHSS | ||||
| ≥19 vs <19 | 4.01 (2.47 to 6.51) | <0.001 | 4.81 (2.76 to 8.39) | <0.001 |
| PLR | ||||
| ≥162.2 vs <162.2 | 2.18 (1.35 to 3.52) | 0.002 | 1.93 (1.14 to 3.27) | 0.001 |
| OTP | ||||
| ≥334 min vs <334 min | 2.17 (1.38 to 3.43) | 0.001 | 2.15 (1.25 to 3.68) | 0.005 |
| General anaesthesia | ||||
| Yes vs no | 1.73 (1.10 to 2.72) | 0.018 | 1.87 (1.09 to 3.22) | 0.024 |
NIHSS, National Institute of Health Stroke Scale; OTP, onset-to-puncture time; PLR, platelet to lymphocyte ratio.
svn-2022-002185supp001.pdf (175.2KB, pdf)
Discussion
We found that futile recanalisation of EVT occurred in 49.2% of patients with VBAO, which was similar to the ETIS (Endovascular Treatment in Ischaemic Stroke) registry reported (49.5%) and lower than the BASILAR (Endovascular Treatment for Acute Basilar Artery Occlusion Study) registry reported (62.8%).10 11 Furthermore, the use of GA, admission NIHSS≥19, PLR≥162.2 and OTP≥334 min could predict independently futile recanalisation after EVT for VBAO.
Several previous studies have investigated the rate and independent predictors of futile recanalisation in VBAO patients undergoing EVT.6–11 However, the definition of futile recanalisation only in the ETIS registry and BASILAR registry was the same as our study (successful recanalisation after EVT with good outcome (90-day mRS 0–3)).7 10 11 ETIS registry and BASILAR registry reported that age, initial NIHSS, PC-ASPECTS, intravenous thrombolysis, number of passes≤3, first pass mTICI 2b-3 reperfusion, diabetes mellitus, NLR, procedure duration, incomplete recanalisation and collateral circulation were associated with futile recanalisation.7 10 11 Our study added that PLR≥162.2, OTP≥334 min and the use of GA as the new predictors of futile recanalisation of EVT for VBAO.
PLR is an easily available parameter before the procedure. Previous studies demonstrated that high admission PLR could predict poor outcomes in patients with AIS.14–16 Our study first demonstrated that PLR≥162.2 was an independent predictor of futile recanalisation. PLR has been reported as a biomarker that could reflect the inflammation intensity and the thrombotic pathways synthetically.17 Immune system is activated once AIS occurs. Then the lymphocytes are suppressed, and lymphocyte counts decrease.18 Low lymphocyte count is a general feature of an inflammatory process, which has been proven by both clinical and animal studies, can also accelerate atherosclerotic progression.19 Platelets play a critical role in atherosclerotic plaque development, atherosclerotic plaque destabilisation and atherosclerotic plaque rupture, and also in circulating arterial platelet–fibrin thrombus formation.20 The proinflammatory chemokines released by platelets participate in thrombosis and inflammation, which is also important in the formation of thrombus in reaction to endothelial cell erosion or atherosclerosis plaque rupture.14 Moreover, when the platelets are activated, they can release inflammatory mediators, which could also aggravate inflammation reaction at the vascular lesion site.21 Furthermore, platelets also can be in conjunction with neutrophils and fibrinogen, which can damage the blood-brain barrier.15 Additionally, Li et al demonstrated that PLR was associated with a high risk of stroke-related pneumonia, which may also be a reason for poor outcomes of AIS.22 In summary, it is reasonable and understandable that PLR was associated with the futile recanalisation after EVT for VBAO.
Several previous studies have found that a longer OTP could predict poor outcomes after EVT for VBAO.23–27 Regarding futile recanalisation, OTP≥334 min was found to be one independent predictor of it in our cohort, which was similar to what Ouyang et al reported (futile recanalisation was defined as 90-day mRS 0–2 with successful recanalisation), although different definitions of futile recanalisation in the two studies.9 Therefore, shortening the time delay before the procedure should be highlighted during the treatment, including early identification of stroke, fast arrival at the stroke centre and rapid intrahospital transport.
The best anaesthesia strategy during the procedure in patients with VBAO is still unknown. Recently, a two-centre RCT demonstrated that VBAO patients undergoing EVT with GA had a similar 90-day mRS 0–2 rate with those with conscious sedation (CS).28 Similarly, both the BASILR registry and ETIS registry also suggested GA could lead to similar clinical outcomes with local anaesthesia (LA)/CS in VBAO patients after EVT.29 30 Unlike them, several other studies found GA was associated with poor outcomes in such patients.25 31 Our study further found that GA was related to the futile recanalisation of EVT for VBAO. Long-time delay to recanalisation, blood pressure reduction and other undesirable physiologic effects such as changes in immune reaction, loss of airway reflexes and respiratory depression more frequently occur in GA, which may explain the association.28 32 33 However, the anaesthesia protocol of the ANGEL-ACT registry recommended that LA was the first-line anaesthesia strategy, using CS when necessary. GA should be used if the patient is expected to cooperate poorly during the procedure even with the use of CS, or with high-risk airway conditions, or with high-risk CS, which may bias our finding. Therefore, future large, multicentre RCTs are required to validate this finding.
Limitations
Our study had several limitations. First, our study is an observational study, which might introduce a selection bias. Second, some potential predictors such as blood pressure during the procedure, pons-midbrain index,34 baseline infarct volum and collateral circulation (posterior circulation CT angiography score or Basilar Artery on CT Angiography Score) were not collected, which may bias our conclusions. Third, all participants in our study are limited to Chinese. Hence, the conclusions may not be generalised to other races easily.
Conclusions
Futile recanalisation in the ANGEL-ACT registry was observed in 49.2% of the VBAO patients who achieved successful recanalisation after EVT. Moreover, we found that the predictors of futile recanalisation were admission NIHSS≥19, PLR≥162.2, OTP≥334 min and the use of GA.
Acknowledgments
We thank all clinicians, statisticians, imaging and laboratory technologists who were involved in the ANGEL-ACT registry.
Footnotes
DS and XY contributed equally.
Contributors: ZM, XH, and DS were involved in the study conception. Statistical analysis was conducted by AW. The article was drafted by DS. All authors reviewed and approved the final article. Guarantor: ZM
Funding: The ANGEL-ACT registry was supported by the National Key Research and Development Program of China, grant number 2016YFC1301500.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available on reasonable request.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants and the study was approved by the ethics committees of Beijing Tiantan Hospital, Capital Medical University, and all other participating centres. The ID of the approval is KY2017-048-01. Participants gave informed consent to participate in the study before taking part.
References
- 1. Liu X, Dai Q, Ye R, et al. Endovascular treatment versus standard medical treatment for Vertebrobasilar artery occlusion (BEST): an open-label, randomised controlled trial. Lancet Neurol 2020;19:115–22. 10.1016/S1474-4422(19)30395-3 [DOI] [PubMed] [Google Scholar]
- 2. Langezaal LCM, van der Hoeven EJRJ, Mont’Alverne FJA, et al. Endovascular therapy for stroke due to basilar-artery occlusion. N Engl J Med 2021;384:1910–20. 10.1056/NEJMoa2030297 [DOI] [PubMed] [Google Scholar]
- 3. Tao C, Nogueira RG, Zhu Y, et al. Trial of Endovascular treatment of acute basilar-artery occlusion. N Engl J Med 2022;387:1361–72. 10.1056/NEJMoa2206317 [DOI] [PubMed] [Google Scholar]
- 4. Jovin TG, Li C, Wu L, et al. Trial of Thrombectomy 6 to 24 hours after stroke due to basilar-artery occlusion. N Engl J Med 2022;387:1373–84. 10.1056/NEJMoa2207576 [DOI] [PubMed] [Google Scholar]
- 5. Deng G, Xiao J, Yu H, et al. Predictors of futile Recanalization after Endovascular treatment in acute ischemic stroke: a meta-analysis. J Neurointerv Surg 2022;14:881–5. 10.1136/neurintsurg-2021-017963 [DOI] [PubMed] [Google Scholar]
- 6. Alonso de Leciñana M, Kawiorski MM, Ximénez-Carrillo Á, et al. Mechanical Thrombectomy for basilar artery thrombosis: a comparison of outcomes with anterior circulation Occlusions. J Neurointerv Surg 2017;9:1173–8. 10.1136/neurintsurg-2016-012797 [DOI] [PubMed] [Google Scholar]
- 7. de Havenon A, Elhorany M, Boulouis G, et al. Thrombectomy in basilar artery Occlusions: impact of number of passes and futile reperfusion. J Neurointerv Surg 2023;15:422–7. 10.1136/neurintsurg-2022-018715 [DOI] [PubMed] [Google Scholar]
- 8. Meinel TR, Kaesmacher J, Chaloulos-Iakovidis P, et al. Mechanical Thrombectomy for basilar artery occlusion: efficacy, outcomes, and futile Recanalization in comparison with the anterior circulation. J Neurointerv Surg 2019;11:1174–80. 10.1136/neurintsurg-2018-014516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ouyang K, Kang Z, Liu Z, et al. Posterior circulation ASPECTS on CT angiography predicts futile Recanalization of Endovascular Thrombectomy for acute basilar artery occlusion. Front Neurol 2022;13:831386. 10.3389/fneur.2022.831386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pop R, Finitsis SN, Arquizan C, et al. Poor clinical outcome despite successful basilar occlusion Recanalization in the early time window: incidence and predictors. J Neurointerv Surg 2023;15:415–21. 10.1136/neurintsurg-2022-018769 [DOI] [PubMed] [Google Scholar]
- 11. Yang J, Jin Z, Song J, et al. Futile Recanalization after Endovascular treatment in patients with acute basilar artery occlusion. Neurosurgery 2023;92:1006–12. 10.1227/neu.0000000000002313 [DOI] [PubMed] [Google Scholar]
- 12. Jia B, Ren Z, Mokin M, et al. Current status of Endovascular treatment for acute large vessel occlusion in China: A real-world nationwide Registry. Stroke 2021;52:1203–12. 10.1161/STROKEAHA.120.031869 [DOI] [PubMed] [Google Scholar]
- 13. Zaidat OO, Yoo AJ, Khatri P, et al. Recommendations on angiographic Revascularization grading standards for acute ischemic stroke: a consensus statement. Stroke 2013;44:2650–63. 10.1161/STROKEAHA.113.001972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kim S-Y, Yi HJ, Shin D-S, et al. Prognostic significance of platelet-to-lymphocyte and platelet-to-neutrophil ratios in patients with mechanical Thrombectomy for acute ischemic stroke. J Cerebrovasc Endovasc Neurosurg 2022;24:221–31. 10.7461/jcen.2022.E2021.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sharma D, Bhaskar SMM. Prognostic role of the platelet-lymphocyte ratio in acute ischemic stroke patients undergoing reperfusion therapy: A meta-analysis. J Cent Nerv Syst Dis 2022;14:11795735221110373. 10.1177/11795735221110373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Y-K Y, H H, D-P L, et al. Prognostic value of the platelet-to-lymphocyte ratio for outcomes of stroke: a systematic review and meta-analysis. Eur Rev Med Pharmacol Sci 2021;25:6529–38. [DOI] [PubMed] [Google Scholar]
- 17. Kurtul A, Ornek E. Platelet to lymphocyte ratio in cardiovascular diseases: A. Angiology 2019;70:802–18. 10.1177/0003319719845186 [DOI] [PubMed] [Google Scholar]
- 18. Sun D, Jia B, Tong X, et al. Predictors of Parenchymal hemorrhage after Endovascular treatment in acute ischemic stroke: data from ANGEL-ACT Registry. J Neurointerv Surg 2023;15:20–6. 10.1136/neurintsurg-2021-018292 [DOI] [PubMed] [Google Scholar]
- 19. Núñez J, Miñana G, Bodí V, et al. Low lymphocyte count and cardiovascular diseases. Curr Med Chem 2011;18:3226–33. 10.2174/092986711796391633 [DOI] [PubMed] [Google Scholar]
- 20. Altintas O, Altintas MO, Tasal A, et al. The relationship of platelet-to-lymphocyte ratio with clinical outcome and final infarct core in acute ischemic stroke patients who have undergone Endovascular therapy. Neurol Res 2016;38:759–65. 10.1080/01616412.2016.1215030 [DOI] [PubMed] [Google Scholar]
- 21. Bakogiannis C, Sachse M, Stamatelopoulos K, et al. Platelet-derived Chemokines in inflammation and Atherosclerosis. Cytokine 2019;122. 10.1016/j.cyto.2017.09.013 [DOI] [PubMed] [Google Scholar]
- 22. Li W, He C, Abdulhay E. Association of platelet-to-lymphocyte ratio with stroke-associated pneumonia in acute ischemic stroke. J Healthc Eng 2022;2022:1033332. 10.1155/2022/1033332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sommer P, Scharer S, Posekany A, et al. Thrombectomy in basilar artery occlusion. Int J Stroke 2022;17:1006–12. 10.1177/17474930211069859 [DOI] [PubMed] [Google Scholar]
- 24. Gwak D-S, Choi W, Kim Y-W, et al. Predictors and outcomes of salvaging the Corticospinal tract after Thrombectomy in basilar artery occlusion stroke. Front Neurol 2022;13:878638. 10.3389/fneur.2022.878638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sun D, Huo XRaynald et al. Predictors of poor outcome after Endovascular treatment for acute Vertebrobasilar occlusion: data from ANGEL-ACT Registry. Neuroradiology 2023;65:177–84. 10.1007/s00234-022-03065-x [DOI] [PubMed] [Google Scholar]
- 26. Sang HF, Yuan JJ, Qiu ZM, et al. Association between time to Endovascular therapy and outcomes in patients with acute basilar artery occlusion. Neurology 2021;97:e2152–63. 10.1212/WNL.0000000000012858 [DOI] [PubMed] [Google Scholar]
- 27. Lee S-J, Hong JM, Choi JW, et al. Predicting Endovascular treatment outcomes in acute Vertebrobasilar artery occlusion: A model to aid patient selection from the ASIAN KR Registry. Radiology 2020;294:628–37. 10.1148/radiol.2020191227 [DOI] [PubMed] [Google Scholar]
- 28. Liang F, Wu Y, Wang X, et al. General anesthesia vs conscious sedation for Endovascular treatment in patients with posterior circulation acute ischemic stroke. JAMA Neurol 2023;80:64–72. 10.1001/jamaneurol.2022.3018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Li F, Wan J, Song J, et al. Impact of anesthetic strategy on outcomes for patients with acute basilar artery occlusion undergoing mechanical Thrombectomy. J Neurointerv Surg 2022;14:1073–6. 10.1136/neurintsurg-2021-018000 [DOI] [PubMed] [Google Scholar]
- 30. Skutecki J, Audibert G, Finitsis S, et al. General anesthesia or conscious sedation for Endovascular therapy of basilar artery Occlusions: ETIS Registry results. Rev Neurol (Paris) 2022;178:771–9. 10.1016/j.neurol.2022.03.020 [DOI] [PubMed] [Google Scholar]
- 31. Du H, Tong X, Sun X, et al. Effect of anesthesia strategy during Endovascular therapy on 90-day outcomes in acute basilar artery occlusion: a retrospective observational study. BMC Neurol 2020;20:398. 10.1186/s12883-020-01979-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Crosby G, Muir KW. Anesthesia and neurologic outcome of Endovascular therapy in acute ischemic stroke: MR (not so). Neurology 2016;87:648–9. 10.1212/WNL.0000000000002989 [DOI] [PubMed] [Google Scholar]
- 33. Petersen NH, Silverman A, Kimmel AC, et al. Decreases in blood pressure during Thrombectomy are associated with larger infarct volumes and worse functional outcome. Stroke 2019;50:e321–2. 10.1161/STROKEAHA.119.027098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sun D, Huo XRaynald et al. Outcome prediction value of critical area perfusion score for acute basilar artery occlusion. Interv Neuroradiol 2022:15910199221125853. 10.1177/15910199221125853 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
svn-2022-002185supp001.pdf (175.2KB, pdf)
Data Availability Statement
Data are available on reasonable request.