Abstract
Insulin receptor substrate (IRS) proteins are key mediators in insulin signaling pathway. In social insect lives, IRS proteins played important roles in caste differentiation and foraging, but there function in disease defenses such as active immunization has not been reported yet. To investigate the issue, we successfully suppressed the IRS gene 3 days after dsRNA injection. Suppressing IRS gene increased the contents of glucose, trehalose, glycogen, and triglyceride and decreased the content of pyruvate in termites, and led to the metabolic disorder of glucose and lipids. IRS suppressing significantly enhanced grooming behaviors of nestmates of fungus-contaminated termites and hence increased the conidial load in the guts of the nestmates. Additionally, IRS suppressing led to significant downregulation of the immune genes Gram-negative bacteria-binding protein2 (GNBP2) and termicin and upregulation of the apoptotic gene caspase8, and hence diminished antifungal activity of nestmates of fungus-contaminated termites. The above abnormal behavioral and physiological responses significantly decreased the survival rate of dsIRS-injected nestmates of the fungus-contaminated termites. These findings suggest that IRS is involved in regulation of active immunization in termites, providing a better understanding of the link between insulin signaling and the social immunity of termites.
Keywords: insulin signaling, Reticulitermes chinensis, Metarhizium anisopliae, social immunity, survival
Introduction
In social insect colonies, pathogenic infections have the potential to spread rapidly and cause disease outbreaks (Pull et al. 2018). However, social insects have the ability to protect themselves by utilizing individual defenses and colony-wide systematic responses against pathogens, providing the colony with protection known as “social immunity” (Cremer 2019, Liu et al. 2019). Social immunity includes a series of immune strategies such as chemical communication, hygienic behavior, and physiological immune response to resist infections by pathogens at individual and colony levels (Liu et al. 2015, Xiong et al. 2019, Hassan et al. 2021). Grooming behavior is one of the most important behavioral immunities, by which nestmates can remove infectious particles from the body surfaces of exposed individuals (Tragust et al. 2013, Theis et al. 2015, Zhou et al. 2021). Pathogens are transferred from pathogen-exposed insects to the nestmates and cause low-level infections during grooming (Shimizu and Yamaji 2003, Yanagawa and Shimizu 2007). Low-level infections rarely result in death but instead upregulate immune genes and hence promote an enhanced ability to inhibit growth of pathogens (Konrad et al. 2012). Individuals in the colony get immunization against a specific pathogen during infection process, which helps to fight against the same pathogens in the future, this process is termed as active immunization (Masri and Cremer 2014, Liu et al. 2015). Distinguished from passive immunity, which relies on acquiring immune substances to enhance disease resistance, active immunity activates the host’s own immune system (Konrad et al. 2012, Masri and Cremer 2014).
As a key regulator of glucose homeostasis, insulin plays important anabolic functions throughout the body and is involved in regulating the growth, reproduction, and caste differentiation of social insects (Corona et al. 2016). The insulin signaling pathway also regulates the aging of termite Reticulitermes chinensis (Snyder) (Haroon et al. 2021). Insulin signaling can affect the metabolism of the organism and promote the storage of carbohydrates and lipids in Drosophila, which is reflected in its ability to control the adipocyte cell number and triglyceride storage (DiAngelo et al. 2009). Defects in the insulin/insulin-like growth factor signaling pathway manifest as a collection of metabolic conditions in Drosophila (Das and Dobens 2015). Modulation of insulin receptor substrate (IRS) was sufficient to alter the developmental fate of a queen-destined larva into a worker phenotype in honeybees Apis mellifera (Mutti et al. 2011, Wolschin et al. 2011). A study of the ant Diacamma sp. found that the asymmetry in reproductive potential between ants was correlated with insulin receptor expression in the ovaries (Okada et al. 2010). The interplay between vitellogenin, juvenile hormone, and insulin signaling regulates queen lifespan of honeybees A. mellifera without sacrificing fecundity (Corona et al., 2007). Expression of peripheral IRS can also affect food choice in foraging behavior of the honeybee A. mellifera (Wang et al. 2010). Insulin signaling is involved in the regulation of worker division of labor in honeybee colonies and soldier-specific morphological changes in termite Hodotermopsis sjostedti (Hattori et al. 2013). The insulin receptor holds potential as an important target for the development of new drugs and pesticides, given its physiological effects and molecular mechanisms (Zhang et al. 2022). These studies show the close relationship between insulin signaling and social insect lives. However, as social immunity is also an important aspect of social insect lives, whether insulin signaling participates in such colony-level disease defenses is still unclear.
The subterranean termite R. chinensis Snyder is an important pest of forest trees and urban buildings in China, including Beijing, Tianjin, Shanxi, and the Yangtze River drainage basin (Huang et al. 2013, Gao et al. 2018). The entomopathogenic fungus (EPF) Metarhizium anisopliae (Metchnikoff) Sorokin is an ideal experimental fungus for studying insect social immunity and is being developed in biological pest control (Masri and Cremer 2014, Liu et al. 2019). Here, we used R. chinensis and M. anisopliae as the tested materials to determine the effect of insulin signaling on active immunization in termites. To address it, we suppressed the IRS gene and then detected the changes of metabolites (glucose, trehalose, glycogen, pyruvate, and triglyceride) in termites, and tested the behavioral immunity (grooming behavior and conidial load) and physiological immunity (antifungal activity and immune-related gene expressions) in nestmates of the fungus-contaminated termites. We further tested the survival rate of dsIRS-injected nestmates of fungus-contaminated termites so as to verify the effect of IRS-mediated disruption of active immunization on the resistance of termite groups to fungal infections. This study will offer a new avenue to link insulin signaling pathway to active immunization in termites, and will help to build a theoretical basis for deeply revealing the social immunity of termites and other social insects.
Materials and Methods
Experimental Termites
Worker termites were collected from the 9 R. chinensis colonies in Shizi Hill, Wuhan City, Hubei Province, China. The termites from each colony were reared in a plastic container (25 cm × 15 cm × 10 cm) under laboratory conditions of 25 ± 1 °C, 80 ± 5% RH, and 24 h of darkness. We used pine as the food source for termites. Healthy adult workers were chosen for the subsequent experiments.
Fungal Pathogens
The R. chinensis workers were contaminated with the EPF M. anisopliae (IBCCM321.93). M. anisopliae was cultivated on potato dextrose agar (PDA) (Rishui, China), and plates were placed upside down in an artificial climate box that maintained a constant temperature of 25 °C for approximately 10 days. The spores of M. anisopliae were collected with 0.1% Tween 80 and stored in a refrigerator at 4 °C. The spore germination rate used in all the experiments was greater than 90% (Liu et al. 2019). In the fungal treatment group of subsequent experiments, we followed the previously published protocol (Cremer 2019) by pipetting 0.35 μl of the spore suspension (108 conidia/ml) onto the tergites of the termite abdomen. The termites were then placed into a 4 °C refrigerator for 30 min to reduce movement, allowing the conidia to attach to their body surface (Traniello et al. 2002). In the control group, 0.35 μl of sterile 0.1% Tween 80 solution was placed on the tergites of the termite abdomen and placed in 4 °C refrigerator for 30 min.
Cloning and Phylogenetic Analysis of the IRS Gene
Total RNA was extracted from 3 R. chinensis workers using TRIzol (Takara, Japan). The quality and concentration of the extracted RNA were measured with a Nanodrop 2000 spectrophotometer (Thermo Fisher Scientific, USA). We synthesized cDNA from 500 ng of total RNA by using the PrimeScript RT Reagent Kit with gDNA Eraser (Perfect Real Time) (Takara, Japan) according to the manufacturer’s instructions. The primers for gene amplification (Supplementary Table S1) were designed based on the transcriptome database of R. chinensis (unpublished). The polymerase chain reaction (PCR) products were purified by using the AxyPrep DNA Gel Extraction Kit (Axygen Scientific, USA), cloned into the pMD18-T (Takara, Japan), transformed by using Trans1-T1 Phage Resistant Chemically Competent Cells (Transgen Biotech, China), and sequenced by Tsingke Biological Technology (China). The results were analyzed by NCBI BLAST (National Center for Biotechnology Information) (http://www.ncbi.nlm.nih.gov/). Primers based on the sequencing results were designed to verify the size of the target gene (Supplementary Table S1). The protein alignment and phylogenetic analysis were performed using MEGA 7 software. The multiple sequence alignment was conducted with the ClustalW algorithm, and the phylogenetic tree was constructed using the Neighbor-Joining method. The reliability of the tree structure was assessed using a bootstrap procedure based on 1,000 replicates.
IRS Gene Expression in Active Immunization
One fungus-contaminated termite and 5 naïve nestmates were placed together in a petri dish (D = 3.5 cm) with moist filter paper at the bottom. The head of the fungus-contaminated termite was marked with a black marker. After 1 day, 3 nestmates of the fungus-contaminated termites were randomly selected from each petri dish and placed in a 1.5-ml centrifuge tube to extract the total RNA. The nestmates of the Tween 80-treated termites were used as the control samples. There were 9 replications from 3 termite colonies, each colony with 3 replications.
Approximately 500 ng of RNA was converted to cDNA. RT-qPCR primers were designed using NCBI (https://www.ncbi.nlm.nih.gov/tools/primer-blast) (Supplementary Table S1) and synthesized by Tsingke Biological Technology (China). RT-qPCR was performed in a Bio-Rad CFX Connect Real-Time PCR System (Bio-Rad, USA). The PCR conditions consisted of 3 min at 95 °C followed by 40 cycles of 10 s at 95 °C and 30 s at 58 °C. The reaction mixtures were prepared according to the manufacturer’s protocol (Yeasen, China). The relative expression level of the IRS gene was normalized to the expression level of Heat Shock Protein 70 (HSP70) and β-actin, which were used as reference genes, with the 2−ΔΔCT method (Livak&Schmittgen 2001).
RNAi Efficiency after Injecting dsIRS
To verify the function of IRS gene in active immunization of termites, we synthesized dsIRS and estimated the RNAi efficiency of dsIRS. Primers (Supplementary Table S1) containing the T7 RNA polymerase promoter were used to amplify dsGFP and dsIRS by PCR. The dsRNA was synthesized and purified according to the manufacturer’s instructions (Thermo Fisher Scientific, USA). Purity of the extracted dsRNA was checked through Nanodrop 2000 spectrophotometer (Thermo Fisher Scientific, USA).
We injected 100 nl (2 μg) dsRNA between the second and third thoracic segment of the termites with a microinjector (PV820, WPI Inc, Germany) (Zhou et al. 2006). After injection, the termites were placed in a plastic petri dish (D = 9 cm) with wet filter paper at the bottom and reared for 3 days. The efficiency of RNAi was detected by RT-qPCR. The termites injected with double-stranded IRS were called as the dsIRS-treated group, and the termites injected with double-stranded green fluorescent protein (GFP) were called as the dsGFP-treated group (the control). There were 9 replications from 3 termite colonies, each colony with 3 replications.
Determination of Metabolic Substances
The content of all metabolites was determined by using available assay kits (Nanjing Jiancheng Bioengineering Institute, China) based on the manufacturer’s protocols of these kits. We determined the levels of glucose, trehalose, glycogen, pyruvate, and triglyceride in termites injected with dsIRS and dsGFP using the GPO-PAP method at respective wavelengths of 505, 620, 620, 505, and 510 nm. There were 9 replications from 3 termite colonies, each colony with 3 replications.
Behavioral Immunophenotypes
We investigated the grooming behavior changes of dsIRS-injected nestmates toward infected individuals. Three days after dsRNA (dsIRS or dsGFP) injection, 5 dsRNA-injected nestmates were placed in a petri dish (D = 3.5 cm) with 1 fungus-contaminated termite. The head of the fungus-contaminated termite was marked with a black marker. To examine whether suppressing IRS affected the grooming behavior of the termites, a camera (acA1920-40gc, Basler) was used to continuously record the grooming behavior of termites for 40 min. EthoVision 14.0 tracking software (Noldus Information Technology, Netherlands) was used to analyze the frequency and cumulative time of grooming behavior during which the nestmates groomed the infected individuals. There were 9 replications from 3 termite colonies, each colony with 3 replications.
After 40 min of grooming behavior, the termites injected with dsIRS or dsGFP were dissected, and their guts were obtained and placed in 50 μl of 0.1% Tween 80, respectively. The guts were disrupted by a glass grinding rod, transferred to PDA selection medium (containing 100 μg/ml streptomycin, 100 μg/ml chloramphenicol, and 100 μg/ml kanamycin), and then placed in 25 ± 1 °C incubator for 1 wk so as to count the number of colony-forming units (CFUs) of M. anisopliae in the guts. There were 5 replications from 2 termite colonies.
Physiological Immunophenotypes
Socially transferred conidia may activate the immune response of the hosts to enhance their antifungal activity, so we analyzed the alterations of the nestmates’ antifungal activity after injection of dsIRS. Two days after dsRNA (dsIRS or dsGFP) injection, 5 dsRNA-injected nestmates were reared in a petri dish (D = 3.5 cm) with 1 fungus-contaminated termite for 1 day. The 5 dsRNA-injected nestmates of the fungus-contaminated termites were ground into powder, dissolved in 0.9% NaCl, and subsequently centrifuged to obtain the supernatant. We used 96-well microplates to measure the antifungal activity. We added 50 μl of Sabouraud Dextrose Broth (SDB) and 4 μl of 0.9% NaCl for the standards and 50 μl of SDB, 2 μl of 0.9% NaCl, and 2 μl of M. anisopliae spore suspension for the growth control. We added 50 μl of SDB, 2 μl of sample supernatant, and 2 μl of M. anisopliae spore suspension for the samples. The microplates were incubated in a constant temperature oscillator for 24 h (25 °C, 200 rpm). The absorbance of each well was measured with a microplate reader (SPAPK, TECAN, Switzerland) at a wavelength of 600 nm every hour to calculate the fungal growth inhibition rate in order to compare the changes in the antifungal activity. Fungal growth inhibition rate = (ODgrowth control − ODsample)/(ODgrowth control − ODstandard) (Zhao et al. 2020). There were 9 replications from 3 termite colonies, each colony with 3 replications.
Socially transferred conidia may also improve immune gene expression in hosts, so we examined how the expression of the immune gene Gram-negative bacteria-binding protrin2 (GNBP2), termicin, and apoptosis gene caspase8, changed in the nestmates after injection of dsIRS. Two days after dsIRS or dsGFP injection, 5 dsRNA-injected nestmates were reared in a petri dish (D = 3.5 cm) with 1 fugus-contaminated termite for 1 day. RT-qPCR primers were designed using NCBI (https://www.ncbi.nlm.nih.gov/tools/primer-blast) (Supplementary Table S1). We used RT-qPCR and the 2−ΔΔCT method (Livak and Schmittgen 2001) to determine the change in the expression of the genes GNBP2, termicin, and caspase8. There were 9 replications from 3 termite colonies, each colony with 3 replications.
Survival
To explore whether suppressing IRS can improve the lethal effect of M. anisopliae on termites, we observed the changes in the survival of the nestmate termites reared with 1 fungus-contaminated termite after injection of dsIRS. Two days after dsIRS or dsGFP injection, 5 dsRNA-injected nestmates were reared in a petri dish (D = 3.5 cm) with 1 fugus-contaminated termite for 20 days. The death of the nestmate termites in the petri dish was recorded every day, and dead termites were removed in a timely manner. The control groups included dsIRS- or dsGFP-injected termites reared with 1 Tween 80-treated termite. There were 6 replications from 3 termite colonies, each replication had 5 workers used to count the survival rate, and a total of 120 termites were used in this experiment.
Statistical Analysis
First, we used the Shapiro–Wilk test to detect whether the data conformed to a normal distribution. Data that did not conform to a normal distribution were analyzed for the significance of differences using the Wilcoxon test. The data that obeyed the normal distribution were analyzed for significant differences using independent t-tests. Cox proportional regression was used to analyze the effect of termite colonies and experimental treatments on the survival of the termites, and then we used Kaplan–Meier methods to analyze the significant differences in the survival of the termites between the different experimental treatments.
Results
Cloning, Expression, and RNAi Efficiency of IRS in R. chinensis
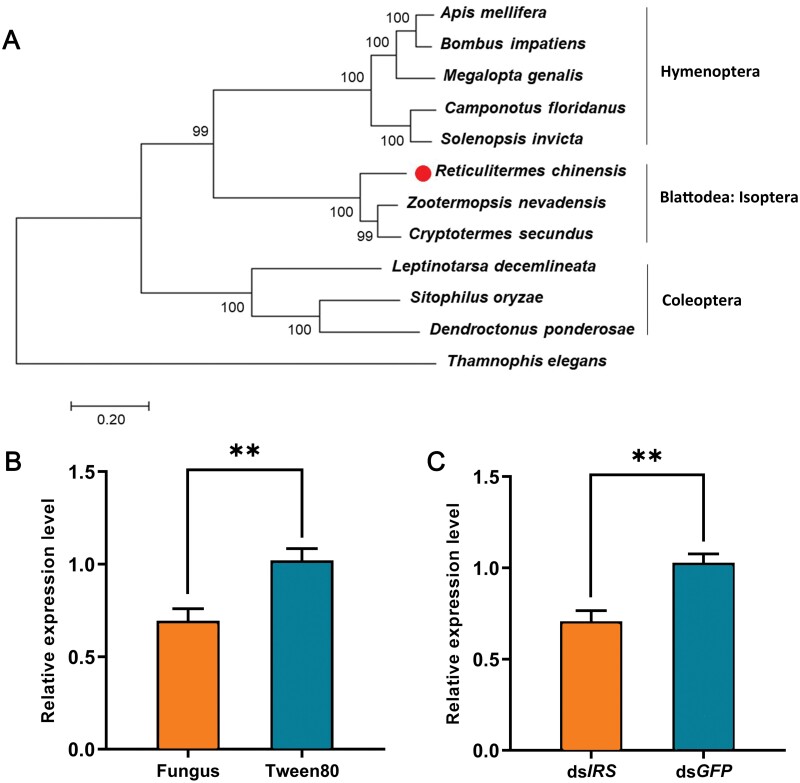
The phylogenetic trees showed that the amino acid sequences of IRS from different insect species are highly conserved (Fig. 1A). Moreover, the IRS proteins of R. chinensis, Zootermopsis nevadensis, and Cryptotermes secundus are in the same clade with A. mellifera, Bombus impatiens, Megalopta genalis, Camponotus floridanus, and Solenopsis invicta, which shows that they are more closely related. However, the IRS proteins of Leptinotarsa decemlineata, Sitophilus oryzae, and Dendroctonus ponderosae are not in the same branch with the 3 termite species, indicating that Coleoptera and termites are farther from each other.
Fig. 1.
Cloning, expression, RNAi efficiency, and metabolic effect of IRS gene in R. chinensis. A) The phylogenetic tree of IRS from several insects. R. chinensis was marked with ‘●’. The scale bar indicates an average of 0.05 substitutions per site. B) IRS expression in the nestmates of the fungus-contaminated termites. C) IRS expression 3 days after dsIRS injection. The data are shown as mean + SEM (**, P < 0.01).
One day after rearing together, the nestmates of the fungus-contaminated termites exhibited significantly lower IRS expression than the nestmates of the Tween 80-treated termites (Fig. 1B: t = 3.608, df = 16, P = 0.002), indicating that IRS may be involved in regulating active immunization against M. anisopliae in R. chinensis.
Three days after injections, IRS expression in the dsIRS-injected termites was significantly decreased compared to that in the control termites (Fig. 1C: t = 4.211, df = 16, P = 0.001).
Effects of Suppressing IRS Gene on Metabolic Substances Levels of R. chinensis
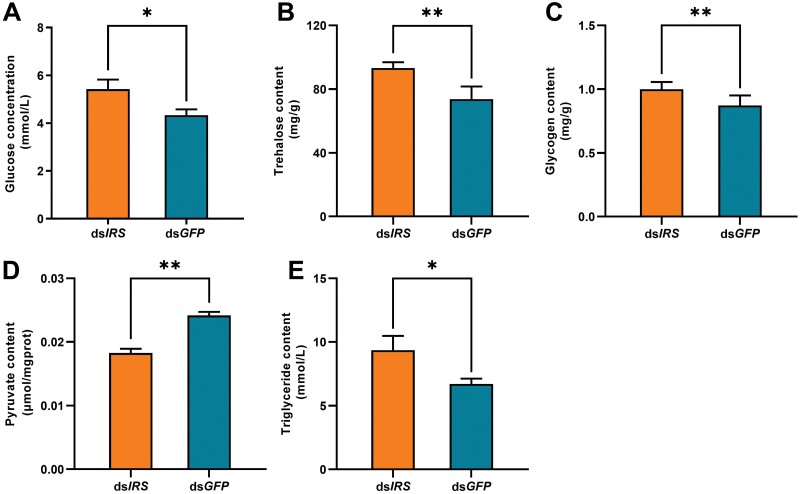
After suppressing with IRS gene, glucose (Fig. 2A: Z = 2.252, N = 9, P = 0.024), trehalose (Fig. 2B: Z = 3.488, N = 9, P < 0.001), glycogen (Fig. 2C: Z = 2.608, N = 9, P = 0.009), and triglyceride (Fig. 2D: Z = 2.428, N = 9, P = 0.015) increased significantly in R. chinensis. In addition, suppressing IRS gene led to the decrease of pyruvate content in R. chinensis (Fig. 2E: t = 6.916, df = 16, P < 0.001).
Fig. 2.
Effect of silencing IRS on the level of glucose, trehalose, and triglyceride in R. chinensis. A) Glucose level 3 days after dsIRS injection. B) Trehalose level 3 days after dsIRS injection. C) Glycogen level 3 days after dsIRS injection. D) Pyruvate level 3 days after dsIRS injection. E) Triglyceride level 3 days after dsIRS injection. The data are shown as mean + SEM (*, P < 0.01; **, P < 0.01).
Effect of Suppressing IRS on the Grooming Behavior of R. chinensis
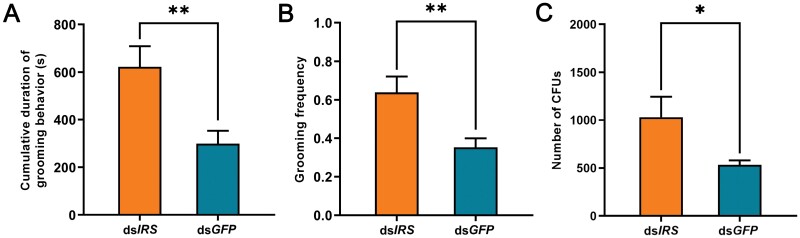
The cumulative duration of grooming behavior was significantly higher in dsIRS-injected nestmates of the fungus-contaminated termites than in the controls (Fig. 3A: t = 3.173, df = 16, P = 0.006). The grooming frequency was significantly higher in dsIRS-injected nestmates of the fungus-contaminated termites than in the controls (Fig. 3B: t = 3.033, df = 16, P = 0.008). The number of CFUs was significantly higher in dsIRS-injected nestmates of the fungus-contaminated termites than in the controls (Fig. 3C: Z = 1.984, N = 5, P = 0.047).
Fig. 3.
Effect of silencing IRS on grooming behavior of the nestmates of the fungus-contaminated termites. A) The cumulative duration of the grooming behavior. B) The grooming frequency. C) The number of colony-forming units of M. anisopliae in the gut. The data are shown as mean + SEM (*, P < 0.05; **, P < 0.01).
Impact of IRS Knockdown on Antifungal Activity and Expressions of Apoptotic and Immune Genes
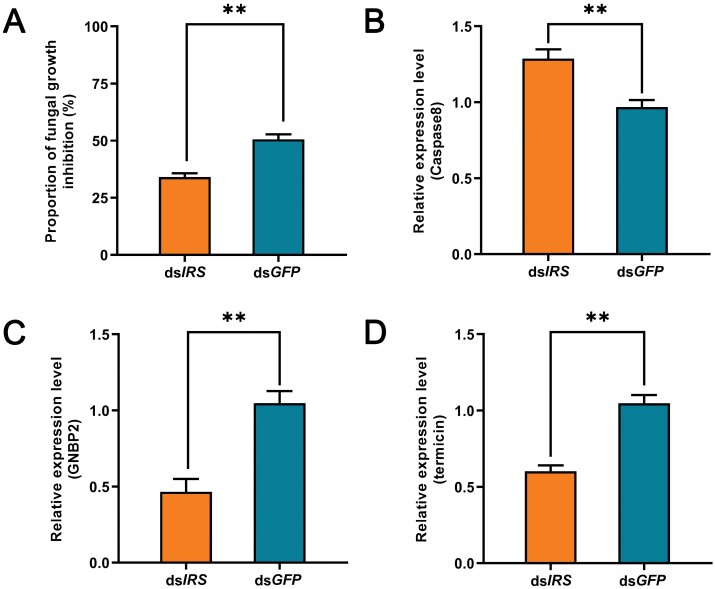
The antifungal activity of the dsIRS-injected nestmates of the fungus-contaminated termites was significantly lower than that of the control (Fig. 4A: t = 5.980, df = 16, P < 0.001). Additionally, Caspase8 expression in the dsIRS-infected nestmates of the fungus-contaminated termites was significantly higher than that in the control (Fig. 4B: t = 4.175, df = 16, P = 0.001), but the expressions of GNBP2 and Termicin in the dsIRS-infected nestmates of the fungus-contaminated termites was significantly lower than those in the controls (Fig. 4C: t = 5.024, df = 16, P < 0.001; Fig. 4D: t = 6.773, df = 16, P < 0.001, respectively).
Fig. 4.
Impact of silencing IRS on antifungal activity and expressions of apoptotic and immune genes in the nestmates of the fungus-contaminated termites. A) The antifungal activity. B) The expression of the apoptotic gene Caspase8. C) The expression of the immune gene GNBP2. D) The expression of the immune gene termicin. The data are shown as mean + SEM (**, P < 0.01).
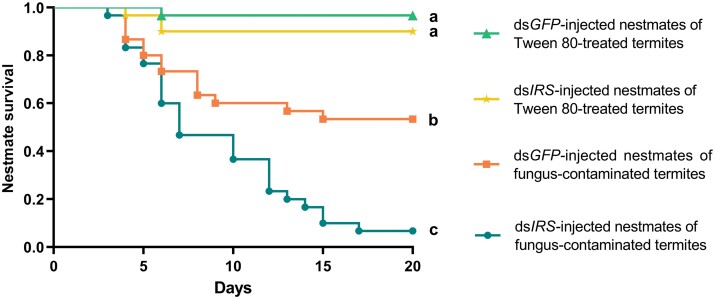
Influence of IRS downregulation on the survival of termites. Different treatments have a significant effect on nestmate survival (Wald test = 42.924, df = 1, P < 0.001), while different colonies have no significant effect on nestmate survival (Wald test = 3.450, df = 1, P = 0.063). The survival of the dsIRS-infected nestmates of the fungus-contaminated termites was significantly lower than that of the nestmates in the other groups (dsIRS-Fungus vs. dsGFP-Fungus: χ2 = 14.490, P < 0.001; dsIRS-Fungus vs. dsIRS-Tween 80: χ2 = 40.257, P < 0.001; dsIRS-Fungus vs. dsGFP-Tween 80: χ2 = 49.387, P < 0.001) (Fig. 5). The survival of the dsGFP-injected nestmates of the fungus-contaminated termites was significantly lower than that of the dsGFP-injected nestmates of the Tween 80-treated termites (dsGFP-Fungus vs. dsGFP-Tween 80: χ2 = 14.659, P < 0.001) (Fig. 5). The survival of the dsGFP-injected nestmates of the fungus-contaminated termites was significantly lower than that of the dsIRS-injected nestmates of the Tween 80-treated termites (dsGFP-Fungus vs. dsIRS-Tween 80: χ2 = 9.510, P = 0.002) (Fig. 5). There was no significant difference in survival between the dsIRS- and dsGFP-injected nestmates of the Tween 80-treated termites (dsGFP-Tween 80 vs. dsIRS-Tween 80: χ2 = 1.086, P = 0.297) (Fig. 5).
Fig. 5.
Influence of silencing IRS on the survival of the nestmates of the fungus-contaminated termites. Different letters indicate significant differences among the 4 groups (P < 0.01).
Discussion
Insulin signaling plays an important role in maintaining metabolic homeostasis in organisms. Insect insulin regulates insects’ metabolism and immunity through signal transduction between cells (Xu et al. 2012, Spellberg and Marr 2015). The downregulated IRS gene increased the contents of glucose, trehalose, glycogen and triglyceride in termites, and decreased the content of pyruvate. The previous study showed that via ablation of the NSCs that produce insulin-like peptides, Drosophila had elevated levels of trehalose, glycogen, and lipids (Broughton et al. 2005). As another example, flies lacking the IRS Chico also exhibit elevated whole-body lipid levels (Böhni et al. 1999). Our results also prove this point, elevated sugars and lipids level in termites after suppressed IRS indicates that insulin signal is an important regulator of energy metabolism. Suppressing IRS may affect the homeostatic control of blood sugar in termites and causes termites to develop into a pathologically hyperglycemic and high triglyceride status. There is a tight coupling between immune function and metabolic function in the human (Konstantinos et al. 2015, Pearce 2021). The physiological and pathological processes of glucose and lipid metabolism disorders in humans involve the activation of the innate immune system, including the release of pro-inflammatory lipoproteins, disorders of antiinflammatory lipotroponin, insulin resistance and changes in monocyte chemokines (Reddy et al. 2019, Olona et al. 2021). The metabolic disorder of termites caused by injection of dsIRS may cause the imbalance of blood sugar of termites, then destroy the physiological immunity of termites, affect the immunity of termites to pathogenic fungi, and suppress with the active immunity.
Insulin signaling plays important role in driving behavioral immunity during active immunization in termite colonies. The interplay between immune response and insulin signaling has evolved as a means of the energy distribution during infection. The immune response reduces growth and nutrient storage in Drosophila by attenuating insulin signaling (DiAngelo et al. 2009). Inhibiting the insulin signaling pathway prolongs the survival of Drosophila against certain bacteria (McCormack et al. 2016). In our experiments, active immunization resulted in downregulation of the IRS gene (Fig. 1B), which shows that there is also an interaction between insulin signaling and active immunization in termites. Previous studies have found that insulin signaling can regulate caste-specific behaviors in social insects (Stern 2003, Erion and Sehgal 2013, Smykal and Raikhel 2015). In our study, we found that insulin signaling affected the hygiene behavior: The downregulated IRS enhanced grooming behavior of nestmates toward fungus-contaminated termites (Fig. 3A and B). When grooming, nestmates lick the pathogenic spores on the surface of infected termites (Liu et al. 2022). Enhanced grooming behaviors meant that more pathogenic spores were licked by the caregivers, and hence the downregulated IRS led to a larger number of CFUs in the gut of the nestmates of fungus-contaminated termite (Fig. 3C). These results indicate that IRS knockdown induces the nestmates of the fungus-contaminated termites to invest more in behavioral immunity during active immunization. However, once the conidial load exceeds the level that the caregivers can tolerate, they will face a great survival challenge.
IRS suppressing led to diminished physiological immunity and made nestmates more vulnerable to the social transferred fungal conidia. Pseudomonas aeruginosa can suppress host immunity by activating the DAF-2 insulin-like signaling pathway in Caenorhabditis elegans (Evans et al. 2008). Chico gene of Drosophila is a homologue of vertebrate IRS1-4, the functional loss of which reduced insulin signaling in D. melanogaster but increased the resistance to gram-positive and gram-negative bacterial infections (Libert et al. 2008). These suggest that insulin signaling and IRS play important roles in regulating immune responses. Normally fungus-exposed individuals led to low-level infections and enhanced the antifungal activity of nestmates due to social contact (grooming behavior) (Liu et al. 2015, Zhao et al. 2020). The previous studies showed that insulin signaling can modulate the innate immune response to bacterial pathogens, and also affect antimicrobial peptide activity (Spellberg and Marr 2015). In our study, suppressing IRS reduced the antifungal activity (Fig. 4A) and survival (Fig. 5) of nestmates grooming toward fungus-contaminated termites, indicating that their physiological immunity was weakened. Physiological immunity is an important component of active immunization, and weakening physiological immunity will eventually result in disruption of active immunization (Cremer 2019). Our suppressing IRS led to high-level of conidia load but diminished physiological immunity, which resulted in high risk of infection and mortality of the nestmates, and was more conducive to the epidemic outbreak in termite colonies.
IRS mediated active immunization by influencing the expression of immune and apoptotic genes in termites. Termicin is a defensin-like antimicrobial peptide from termites and shows strong antifungal activity. GNBP2 can exhibit direct antifungal activity by breaking down β-1,3-glucan in the fungal cell wall, playing a direct role in termite antifungal defense (Kim et al. 2000, Lamberty et al. 2001, Xu et al. 2009, Hamilton and Bulmer 2012). Gene GNBP2 and termicin are significantly upregulated in termites infected with pathogenic bacteria and fungi (Gao and Thompson 2015). The dsIRS-injected nestmates of the fungus-contaminated termite reduced their antifungal activity, possibly by inhibiting the upregulation of immune genes GNBP2 and termicin (Fig. 4C and D). In addition, after suppressing IRS, the expression of apoptosis gene caspase8 in the recipient termites was upregulated (Fig. 4B). The previous study showed that overexpressed IRS inhibited infection-induced apoptosis (Kaburagi et al. 2003, Shirakawa et al. 2013), while a small reduction in IRS also increased apoptosis (Lingohr et al. 2003, Ramocki et al. 2008). And hyperglycemia will induce apoptosis (Habib 2013), which is consistent with our previous results. We propose that suppressing IRS may lead to excessive apoptosis and synergistically disrupt active immunization in termites.
In summary, the knockdown of IRS gene resulted in more frequent and greater uptake of M. anisopliae spores by nestmate termites, which increased the chance of spore dispersal. In this process, suppressing IRS decreased the expressions of immune genes of the recipient termites, together with the upregulation of the cell apoptosis gene, weakened antifungal ability and therefore destroyed the physiological base of active immunization. Active immunity can reduce spore infection and protect termite colonies. However, the destruction of physiological immunity makes the high-frequency grooming behavior increase the infection amount, resulting in a burden on the nestmate termites. It was demonstrated that suppressing IRS gene impaired the active immunization of R. chinensis against M. anisopliae. Our study provides a novel technique to connect the insulin signaling pathway to immune response in termites. It suggests a potential strategy of using biological agents that combine EPF with dsIRS to efficiently manage termite pests in the field. Future research should focus on more efficient and precise techniques for delivering dsRNA into termite bodies to improve the effectiveness of IRS gene suppression. Exploring the potential synergies of combining IRS gene suppression with other biological or chemical control methods could lead to the development of integrated pest management strategies.
Supplementary Material
Contributor Information
Wei Zhou, Hubei Insect Resources Utilization and Sustainable Pest Management Key Laboratory, Huazhong Agricultural University, Wuhan 430070, China.
Xingying Zhao, Hubei Insect Resources Utilization and Sustainable Pest Management Key Laboratory, Huazhong Agricultural University, Wuhan 430070, China.
Ali Hassan, Hubei Insect Resources Utilization and Sustainable Pest Management Key Laboratory, Huazhong Agricultural University, Wuhan 430070, China.
Bao Jia, Hubei Insect Resources Utilization and Sustainable Pest Management Key Laboratory, Huazhong Agricultural University, Wuhan 430070, China; Nanning Institute of Termite Control, Nanning 530023, China.
Long Liu, Henan International Laboratory for Green Pest Control, Henan Engineering Laboratory of Pest Biological Control, College of Plant Protection, Henan Agricultural University, Zhengzhou 450002, China.
Qiuying Huang, Hubei Insect Resources Utilization and Sustainable Pest Management Key Laboratory, Huazhong Agricultural University, Wuhan 430070, China.
Funding
This work was supported by grants from National Natural Science Foundation of China (32170500 and 31572322).
Author Contributions
Wei Zhou (Data curation [equal], Formal analysis [equal], Investigation [equal], Validation [equal], Validation [equal], Visualization [equal], Writing—original draft [equal], Writing—original draft [equal]), Xingying Zhao (Conceptualization [equal], Methodology [equal]), Ali Hassan (Visualization [equal], Writing—review & editing [equal]), Bao Jia (Investigation [equal], Writing—review & editing [equal]), Long Liu (Methodology [equal], Writing—review & editing [equal]), and Qiuying Huang (Conceptualization [equal], Funding acquisition [equal], Project administration [equal], Resources [equal], Supervision [equal], Writing—review & editing [equal])
References
- Böhni R, Riesgo EJ, Oldham S, Brogiolo W, Hafen E.. Autonomous control of cell and organ size by CHICO, a Drosophila homolog of vertebrate IRS1-4. Cell. 1999:97(7):865–875. [DOI] [PubMed] [Google Scholar]
- Broughton SJ, Piper MDW, Ikeya T, Bass TM, Jacobson J, Driege Y, Martinez P, Hafen E, Withers DJ, Leevers SJ, et al. Longer lifespan, altered metabolism, and stress resistance in Drosophila from ablation of cells making insulin-like ligands. Proc Natl Acad Sci USA. 2005:102(8):3105–3110. 10.1073/pnas.0405775102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corona M, Libbrecht R, Wheeler DE.. Molecular mechanisms of phenotypic plasticity in social insects. Curr Opin Insect Sci. 2016:13:55–60. 10.1016/j.cois.2015.12.003 [DOI] [PubMed] [Google Scholar]
- Corona M, Velarde RA, Remolina S, Moran-Lauter A, Wang Y, Hughes KA, Robinson GE.. Vitellogenin, juvenile hormone, insulin signaling, and queen honey bee longevity. Proc Natl Acad Sci USA. 2007:104(17):7128–7133. 10.1073/pnas.0701909104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremer S. Social immunity in insects. Curr Biol. 2019:29(11):R458–R463. 10.1016/j.cub.2019.03.035 [DOI] [PubMed] [Google Scholar]
- Das R, Dobens LL.. Conservation of gene and tissue networks regulating insulin signalling in flies and vertebrates. Biochem Soc Trans. 2015:43(5):1057–1062. 10.1042/BST20150078 [DOI] [PubMed] [Google Scholar]
- DiAngelo JR, Bland ML, Bambina S, Cherry S, Birnbaum MJ.. The immune response attenuates growth and nutrient storage in Drosophila by reducing insulin signaling. Proc Natl Acad Sci USA. 2009:106(49):20853–20858. 10.1073/pnas.0906749106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erion R, Sehgal A.. Regulation of insect behavior via the insulin-signaling pathway. Front Physiol. 2013:4:00353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans EA, Kawli T, Tan MW.. Pseudomonas aeruginosa suppresses host immunity by activating the DAF-2 insulin-like signaling pathway in Caenorhabditis elegans. PLoS Pathog. 2008:4(10):e1000175. 10.1371/journal.ppat.1000175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Q, Thompson GJ.. Social context affects immune gene expression in a subterranean termite. Insectes Soc. 2015:62(2):167–170. 10.1007/s00040-015-0389-3 [DOI] [Google Scholar]
- Gao YY, Yu SX, Li JJ, Sun P, Xiong M, Lei C, Zhang Z, Huang Q.. Bioactivity of diatomaceous earth against the subterranean termite Reticulitermes chinensis Snyder (Isoptera: Rhinotermitidae). Environ Sci Pollut Res. 2018:25(28):28102–28108. 10.1007/s11356-018-2718-3 [DOI] [PubMed] [Google Scholar]
- Habib SL. Diabetes and renal tubular cell apoptosis. World J Diabetes 2013:4(2):27–30. 10.4239/wjd.v4.i2.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton C, Bulmer MS.. Molecular antifungal defenses in subterranean termites: RNA interference reveals in vivo roles of termicins and GNBPs against a naturally encountered pathogen. Dev Comp Immunol. 2012:36(2):372–377. 10.1016/j.dci.2011.07.008 [DOI] [PubMed] [Google Scholar]
- et al. , Ma XM, Li YX, et al. Transcriptomic evidence that insulin signalling pathway regulates the ageing of subterranean termite castes. Sci Rep. 2021:10(1):6145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan A, Huang QY, Mehmood N, Xu H, Zhou W, Gao YY.. Alteration of termite locomotion and allogrooming in response to infection by pathogenic fungi. J Econ Entomol. 2021:114(3):1256–1263. 10.1093/jee/toab071 [DOI] [PubMed] [Google Scholar]
- Hattori A, Sugime Y, Sasa C, Miyakawa H, Ishikawa Y, Miyazaki S, Okada Y, Cornette R, Lavine LC, Emlen DJ, et al. Soldier morphogenesis in the Damp-Wood termite is regulated by the insulin signaling pathway. J Exp Zoolog B Mol Dev Evol. 2013:320(5):295–306. 10.1002/jez.b.22501 [DOI] [PubMed] [Google Scholar]
- Huang QY, Li GH, Husseneder C, Lei CL.. Genetic analysis of population structure and reproductive mode of the termite Reticulitermes chinensis Snyder. PLoS One. 2013:8(7):e69070. 10.1371/journal.pone.0069070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaburagi Y, Satoh S, Yamamoto HR, Ito Y, Akanuma Y, Sekihara H, Yasuda K, Sasazuki T, Kadowaki T, Yazaki Y. Protection of insulin receptor substrate-3 from staurosporine-induced apoptosis. Biochem Biophys Res Commun. 2003:300(2):371–377. [DOI] [PubMed] [Google Scholar]
- Kim YS, Ryu JH, Han SJ, et al. Gram-negative bacteria-binding protein, a pattern recognition receptor for lipopolysaccharide and beta-1,3-glucan that mediates the signaling for the induction of innate immune genes in Drosophila melanogaster cells. J Biol Chem. 2000:275(42):32721–32727. [DOI] [PubMed] [Google Scholar]
- Konrad M, Vyleta ML, Theis FJ, Stock M, Tragust S, Klatt M, Drescher V, Marr C, Ugelvig LV, Cremer S.. Social transfer of pathogenic fungus promotes active immunisation in ant colonies. PLoS Biol. 2012:10(4):e1001300. 10.1371/journal.pbio.1001300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konstantinos K, Ana A, Peter G.. Infection homeostasis: implications for therapeutic and immune programming of metabolism in controlling infection. Med Microbiol Immunol. 2015:204(4):395–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamberty M, Zachary D, Lanot R, Bordereau C, Robert A, Hoffmann JA, Bulet P.. Insect immunity-constitutive expression of a cysteine-rich antifungal and a linear antibacterial peptide in a termite insect. J Biol Chem. 2001:276(6):4085–4092. 10.1074/jbc.m002998200 [DOI] [PubMed] [Google Scholar]
- Libert S, Chao YF, Zwiener J, Pletcher SD.. Realized immune response is enhanced in long-lived puc and chico mutants but is unaffected by dietary restriction. Mol Immunol. 2008:45(3):810–817. 10.1016/j.molimm.2007.06.353 [DOI] [PubMed] [Google Scholar]
- Lingohr MK, Dickson LM, Wrede CE, Briaud I, McCuaig JF, Myers MG, Rhodes CJ.. Decreasing IRS-2 expression in pancreatic beta-cells (INS-1) promotes apoptosis, which can be compensated for by introduction of IRS-4 expression. Mol Cell Endocrinol. 2003:209(1–2):17–31. 10.1016/j.mce.2003.08.003 [DOI] [PubMed] [Google Scholar]
- Liu L, Li GH, Sun PD, Lei CL, Huang QY.. Experimental verification and molecular basis of active immunization against fungal pathogens in termites. Sci Rep. 2015:5(1):15106. 10.1038/srep15106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Wang W, Liu YL, Sun PD, Lei CL, Huang QY.. The influence of allogrooming behavior on individual innate immunity in the subterranean termite Reticulitermes chinensis (Isoptera: Rhinotermitidae). J Insect Sci. 2019:19(1):6. 10.1093/jisesa/iey119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Yan FM, Zhao CC, Su LJ, Huang QY, Tang QB.. MicroRNAs shape social immunity: a potential target for biological control of the termite Reticulitermes chinensis. J Pest Sci. 2022:96(1):265–279. 10.1007/s10340-022-01495-3 [DOI] [Google Scholar]
- Livak KJ, Schmittgen TD.. Analysis of relative gene expression data using real-time quantitative PCR and the 2(T)(-Delta Delta C) method. Methods. 2001:25(4):402–408. 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- Masri L, Cremer S.. Individual and social immunisation in insects. Trends Immunol. 2014:35(10):471–482. 10.1016/j.it.2014.08.005 [DOI] [PubMed] [Google Scholar]
- McCormack S, Yadav S, Shokal U, Kenney E, Cooper D, Eleftherianos I.. The insulin receptor substrate Chico regulates antibacterial immune function in Drosophila. Immun Ageing: I & A 2016:13:15. 10.1186/s12979-016-0072-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutti NS, Dolezal AG, Wolschin F, Mutti JS, Gill KS, Amdam GV.. IRS and TOR nutrient-signaling pathways act via juvenile hormone to influence honey bee caste fate. J Exp Biol. 2011:214(Pt 23):3977–3984. 10.1242/jeb.061499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada Y, Miyazaki S, Miyakawa H, Ishikawa A, Tsuji K, Miura T.. Ovarian development and insulin-signaling pathways during reproductive differentiation in the queenless ponerine ant Diacamma sp. J Insect Physiol. 2010:56(3):288–295. 10.1016/j.jinsphys.2009.10.013 [DOI] [PubMed] [Google Scholar]
- Olona A, Hateley C, Muralidharan S, Wenk MR, Torta F, Behmoaras J.. Sphingolipid metabolism during Toll-like receptor 4 (TLR4)-mediated macrophage activation. Br J Pharmacol. 2021:178(23):4575–4587. 10.1111/bph.15642 [DOI] [PubMed] [Google Scholar]
- Pearce EL. Metabolism as a driver of immunity. Nat Rev Immunol. 2021:21(10):618–619. 10.1038/s41577-021-00601-3 [DOI] [PubMed] [Google Scholar]
- Pull CD, Ugelvig LV, Wiesenhofer F, Grasse AV, Tragust S, Schmitt T, Brown MJ, Cremer S.. Destructive disinfection of infected brood prevents systemic disease spread in ant colonies. Elife 2018:7:e32073. 10.7554/eLife.32073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramocki NM, Wilkins HR, Magness ST, Simmons JG, Scull BP, Lee GH, McNaughton KK, Lund PK.. Insulin receptor substrate-1 deficiency promotes apoptosis in the putative intestinal crypt stem cell region, limits Apc(min/+) tumors, and regulates Sox9. Endocrinology. 2008:149(1):261–267. 10.1210/en.2007-0869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy P, Lent SD, Ramakrishnan N, McLaughlin M, Jialal I.. Metabolic syndrome is an inflammatory disorder: a conspiracy between adipose tissue and phagocytes. Clin Chim Acta. 2019:496:35–44. 10.1016/j.cca.2019.06.019 [DOI] [PubMed] [Google Scholar]
- Shimizu S, Yamaji M.. Effect of density of the termite, Reticulitermes speratus Kolbe (Isoptera: Rhinotermitidae), on the susceptibilities to Metarhizium anisopliae. Appl Entomol Zool. 2003:38(1):125–130. 10.1303/aez.2003.125 [DOI] [Google Scholar]
- Shirakawa J, Togashi Y, Sakamoto E, Kaji M, Tajima K, Orime K, Inoue H, Kubota N, Kadowaki T, Terauchi Y.. Glucokinase activation ameliorates ER stress-induced apoptosis in pancreatic beta-Cells. Diabetes. 2013:62(10):3448–3458. 10.2337/db13-0052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smykal V, Raikhel AS.. Nutritional control of insect reproduction. Curr Opin Insect Sci. 2015:11:31–38. 10.1016/j.cois.2015.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spellberg MJ, Marr MT.. FOXO regulates RNA interference in Drosophila and protects from RNA virus infection. Proc Natl Acad Sci USA. 2015:112(47):14587–14592. 10.1073/pnas.1517124112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern D. Body-size control: How an insect knows it has grown enough. Curr Biol. 2003:13(7):R267–R269. 10.1016/s0960-9822(03)00197-0 [DOI] [PubMed] [Google Scholar]
- Theis FJ, Ugelvig LV, Marr C, Cremer S.. Opposing effects of allogrooming on disease transmission in ant societies. Philos Trans R Soc Lond Ser B. 2015:370(1669):20140108. 10.1098/rstb.2014.0108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tragust S, Mitteregger B, Barone V, Konrad M, Ugelvig LV, Cremer S.. Ants disinfect fungus-exposed brood by oral uptake and spread of their poison. Curr Biol. 2013:23(1):76–82. 10.1016/j.cub.2012.11.034 [DOI] [PubMed] [Google Scholar]
- Traniello JF, Rosengaus RB, Savoie K.. The development of immunity in a social insect: evidence for the group facilitation of disease resistance. Proc Natl Acad Sci USA. 2002:99(10):6838–6842. 10.1073/pnas.102176599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Mutti NS, Ihle KE, Siegel A, Dolezal AG, Kaftanoglu O, Amdam GV.. Down-regulation of honey bee IRS gene biases behavior toward food rich in protein. PLoS Genet. 2010:6(4):e1000896. 10.1371/journal.pgen.1000896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolschin F, Mutti NS, Amdam GV.. Insulin receptor substrate influences female caste development in honeybees. Biol Lett. 2011:7(1):112–115. 10.1098/rsbl.2010.0463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong H, Cai J, Chen X, Liang S, Wen X, Wang C.. The effects of trichoderma fungi on the tunneling, aggregation, and colony-initiation preferences of black-winged subterranean termites, Odontotermes formosanus (Blattodea: Termitidae). Forests 2019:10(11):1020. 10.3390/f10111020 [DOI] [Google Scholar]
- Xu P, Shi M, Chen XX.. Positive selection on termicins in one termite species, Macrotermes barneyi (Isoptera: Termitidae). Sociobiology 2009:53(3):739–753. [Google Scholar]
- Xu XJ, Gopalacharyulu P, Seppnen LT, et al. Insulin signaling regulates fatty acid catabolism at the level of CoA activation. PLoS Genet. 2012:8(1):e1002478. 10.1371/journal.pgen.1002478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagawa A, Shimizu S.. Resistance of the termite, Coptotermes formosanus Shiraki to Metarhizium anisopliae due to grooming. Biocontrol 2007:52(1):75–85. 10.1007/s10526-006-9020-x [DOI] [Google Scholar]
- Zhang XH, Zhu XZ, Bi XY, Huang JG, Zhou LJ.. The insulin receptor: an important target for the development of novel medicines and pesticides. Int J Mol Sci . 2022:23(14):7793. 10.3390/ijms23147793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Liu L, Zhou W, Cai Q, Huang QY.. Roles of selenoprotein T and transglutaminase in active immunization against entomopathogenic fungi in the termite Reticulitermes chinensis. J Insect Physiol. 2020:125:104085–104085. 10.1016/j.jinsphys.2020.104085 [DOI] [PubMed] [Google Scholar]
- Zhou W, Huang QY, Zhao XY, Liu L, Mehmood N.. Silencing of selenium-binding protein disrupted the active immunization of the termite Reticulitermes chinensis and improved the lethal effect of the entomopathogenic fungus Metarhizium anisopliae. Biol Control. 2021:157:104588. 10.1016/j.biocontrol.2021.104588 [DOI] [Google Scholar]
- Zhou XG, Oi FM, Scharf ME.. Social exploitation of hexamerin: RNAi reveals a major caste-regulatory factor in termites. Proc Natl Acad Sci USA. 2006:103(12):4499–4504. 10.1073/pnas.0508866103 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.