Abstract
Background
Acupuncture involving the limb region may be effective for stroke rehabilitation clinically, but the visualised and explanatory evidence is limited. Our objectives were to assess the specific effects of acupuncture for ischaemic stroke (IS) patients with hemiparesis and investigate its therapy-driven modification in functional connectivity.
Methods
IS patients were randomly assigned (2:1) to receive 10 sessions of hand-foot 12 needles acupuncture (HA, n=30) or non-acupoint (NA) acupuncture (n=16), enrolling gender-matched and age-matched healthy controls (HCs, n=34). The clinical outcomes were the improved Fugl-Meyer Assessment scores including upper and lower extremity (ΔFM, ΔFM-UE, ΔFM-LE). The neuroimaging outcome was voxel-mirrored homotopic connectivity (VMHC). Static and dynamic functional connectivity (sFC, DFC) analyses were used to study the neuroplasticity reorganisation.
Results
46 ISs (mean(SD) age, 59.37 (11.36) years) and 34 HCs (mean(SD) age, 52.88 (9.69) years) were included in the per-protocol analysis of clinical and neuroimaging. In clinical, ΔFM scores were 5.00 in HA group and 2.50 in NA group, with a dual correlation between ΔFM and ΔVMHC (angular: r=0.696, p=0.000; cerebellum: r=−0.716, p=0.000) fitting the linear regression model (R2=0.828). In neuroimaging, ISs demonstrated decreased VMHC in bilateral postcentral gyrus and cerebellum (Gaussian random field, GRF corrected, voxel p<0.001, cluster p<0.05), which fitted the logistic regression model (AUC=0.8413, accuracy=0.7500). Following acupuncture, VMHC in bilateral superior frontal gyrus orbital part was increased with cerebro-cerebellar changes, involving higher sFC between ipsilesional superior frontal gyrus orbital part and the contralesional orbitofrontal cortex as well as cerebellum (GRF corrected, voxel p<0.001, cluster p<0.05). The coefficient of variation of VMHC was decreased in bilateral posterior cingulate gyrus (PPC) locally (GRF corrected, voxel p<0.001, cluster p<0.05), with integration states transforming into segregation states overall (p<0.05). There was no acupuncture-related adverse event.
Conclusions
The randomised clinical and neuroimaging trial demonstrated acupuncture could promote the motor recovery and modified cerebro-cerebellar VMHC via bilateral static and dynamic reorganisations for IS patients with hemiparesis.
Keywords: Stroke, Magnetic Resonance Imaging, Stroke Rehabilitation, Clinical Trial, Cerebral Infarction
WHAT IS ALREADY KNOWN ON THIS TOPIC
Hemiparesis is an inevitable complication impeding the function recovery and neuroplasticity restoration for ischaemic stroke patients.
Acupuncture is a promising adjunct to western medicine for poststroke rehabilitation with the macrobilateral and microbilateral regulation.
WHAT THIS STUDY ADDS
Standardised acupuncture protocol with non-acupoint control could affirm the specific effects of acupuncture on brain and behaviour.
Per-protocol analysis of clinical and neuroimaging can provide visualised and micromodification evidence for acupuncture efficacy.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
High-quality acupuncture trials and further mechanistic investigations are required to enrich pragmatic outcomes and explanatory modification for poststroke rehabilitation, so as to enhance the importance and applicability of acupuncture in healthcare decision-making.
Introduction
Age-standard stroke has ranked as the leading cause of death and disability-adjusted life-years in China’s periodic governmental reports, with rising prevalence and incidence leading to 83.3% in-hospital burden.1 2 Stroke lesions frequently result in 50%–80% hemiparesis across the recovery stages, which is an inevitable complication of motor impairment contralateral to the affected brain hemisphere and creates a greater demand for poststroke rehabilitation.3 Notably, poststroke outcomes have improved with the implementation of integrative medicine protocol, especially routine rehabilitation in conjunction with acupuncture.4
Despite multiple acupuncture regimens that have been recommended to promote the recovery of poststroke hemiparesis,4–6 few explanatory or visualised efficacy studies of these treatments demonstrated clear benefits.7 Conflicting results on acupoint specificity and underestimated efficacy of acupuncture were posing challenges for clinical application.8 Randomised controlled trials of acupuncture combined with mechanistic investigation are essential to improve the quality of efficacy evidence.9 Recent studies have investigated acupuncture improving limbs dysfunction from neuroplasticity reorganisation, particularly the excitability of intracortical neuronal circuits involved interhemispheric inhibition (IHI) and cerebellar brain inhibition (CBI).10 11 Although our previous studies reported the bilateral modulations of acupuncture contributed to motor recovery from a single acupoint of Yanglingquan (GB34) to acupoints formula,12 13 neuroimaging outcomes supporting the maximum applicability of acupuncture have been insufficient so far.
Functional MRI (fMRI) provides a promising insight for visualisation and micromodification of acupuncture efficacy. Based on the spontaneous synchrony of functional homotopy, voxel-mirrored homotopic connectivity (VMHC) is widely used to evaluate interhemispheric integration in neurodegenerative and psychiatric disorders.14 Yet it has been inadequately used to reveal the neuroplasticity reorganisation during the stages of stroke recovery. Static and dynamic function connectivities (sFC, DFC) reflect the spatiotemporal imhomeostasis and configuration of stroke.15 Importantly, DFC clustering model has determined the unstable process wherein both time courses and regional interactions, involving high levels of intranetwork connectivity as well as anticorrelated internetwork connectivity and decreased interhemispheric connectivity within and across the domains of cortical, subcortical and cerebellar areas.15 16 However, the bilateral visualisation and modification are focusing on physiology or pathology rather than therapeutic efficacy. Clinical prediction based on machine learning could make up for the deficiency by capturing the pathological characteristics and identifying the prognostic outcomes across clinical and imaging data accurately,17 18 so as to optimise novel rehabilitation strategies based on acupuncture.
The main objectives of the codesigned study were to assess the specific effects of acupuncture involving the bilateral limbs compared with non-acupoint (NA) acupuncture for unilateral-lesion ischaemic stroke (IS) patients and increase the relevance of clinical and neuroimaging outcomes. We hypothesised there would be pathological characteristics for unilateral-lesion IS, on which acupuncture could take effect to improve motor function and modify the static and dynamic functional connectivities bilaterally.
Methods
Trail design
This single-centre, randomised, non-acupoint-controlled and participant-and-assessor-blinded trial was conducted at Dongzhimen Hospital, Beijing University of Chinese Medicine (Beijing, China), with per-protocol analysis of clinical and neuroimaging outcomes. It was registered on Chinese Clinical Trial Registry (ChiCTR1800016263). The study followed the Consolidated Standards of Reporting Trials and Standards for Reporting Interventions in Clinical Trials of Acupuncture.19 20
Participants
We consecutively enrolled IS inpatients with symptom onset at early subacute stage, matched with healthy controls (HCs) according to age and gender via poster and newspaper advertisements. Patients were eligible if they were 40–75 years old; dextromanual; diagnosed definitely with IS by two neurologists referred to Chinese guidelines for diagnosis and treatment of acute ischaemic stroke 2018 (https://rs.yiigle.com/cmaid/1062684); and reported motor impairment resulted from a first-episode duty lesion located on the basal ganglia or corona radiata on fMRI, with the causative cerebrovascular event involved the deep perforator of the unilateral middle cerebral artery in the anterior circulation. Patients were excluded if they received thrombolysis or ongoing acupuncture therapy; had central nervous system diseases (eg, epilepsy, Parkinson’s disease, Alzheimer’s disease and vascular dementia), psychiatric disorders (eg, depression and schizophrenia) or other serious primary diseases (eg, tumours, organ failure, atrial fibrillation and thrombotic diseases); were reported suspicious heterocrania or intracranial space-occupying lesions on fMRI; had pacemaker or metallic implants device; had substandard head motion (mean framewise displacements (FD) >0.5 mm or maximum FD>1 mm); and withdrew the trial.
Randomisation and masking
Eligible ISs were randomly assigned (2:1) to receive either hand-foot 12 needles acupuncture (HA) or the NA acupuncture using random envelopes. Randomisation was achieved by enrolment order via Rand function, and the randomisation sequence was created by a chief principal who did not participate the trial. Except acupuncturists, patients, outcome assessors and statisticians were blinded to treatment allocation.
Interventions
Acupuncture treatments were administered by licensed acupuncturists with a unified training before study. ISs received HA or NA acupuncture per day for 10 consecutive days, with the secondary prevention medication for cerebrovascular diseases as conventional regimens.
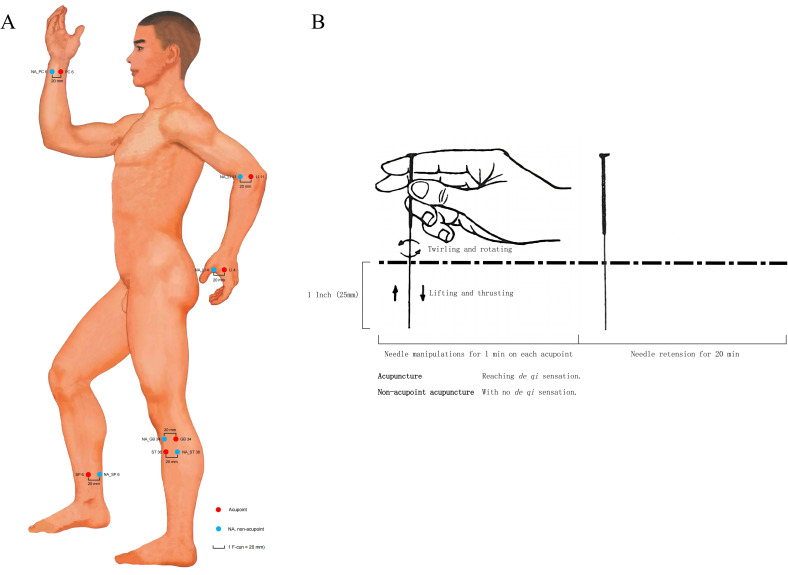
HA group received acupuncture at the 12 standard acupoints (figure 1A), including bilateral Hegu (LI4), Neiguan (PC6), Quchi (LI11), Zusanli (ST36), Yanglingquan (GB34) and Sanyinjiao (SP6),21 which are symmetrically located on the muscles of the forearm or calf as well as their distal or proximal joints. After skin disinfection, disposable sterile needles (Beijing Zhongyan Taihe Medical Instrument Co., LTD., specifications: Φ 0.25×40 mm) were inserted 1 inch (1 inch=25 mm) into the skin with an angle of 90°. Then, the even reinforcing-reducing manipulation (equally twirling and rotating, lifting and thrusting) was conducted on each acupoint for 1 min by turns to elicit de qi sensation (soreness, numbness, distention, heaviness or aching) (figure 1B).22 NA group received same stimulation dosage without de qi at NAs, which located 1 F-cun (1 F-cun=20 mm) lateral to the acupoints.23 Totally, 12 times 1 min manipulation followed by a 20 min needle retention in a session of acupuncture. ISs were treated separately to prevent communication.
Figure 1.
Acupuncture regimens. (A) Location of acupoints and non-acupoints. Standard measuring unit for acupoints was B-cun, which is one equal portion of the length between two points of particular joints. Measuring unit for non-acupoints was F-cun (1 F-cun=20 mm), which is defined as the width of the interphalangeal joint of patient’s thumb. (B) Acupuncture manual manipulation. Measurements involved the needle size and dosage were described with inch (1 inch=25 mm). NA, non-acupoint.
Acupoints location with standard measuring units followed GB/T 12346-2021 Nomenclature and location of meridian points (https://std.samr.gov.cn//gb/search/gbDetailed?id=D1E86BE73ADD430EE05397BE0A0A206B), illustrated as online supplemental tables S1 and S2.
svn-2023-002785supp001.pdf (307.2KB, pdf)
Outcomes
The primary outcome was the variation of Fugl-Meyer Assessment scores (ΔFM). Secondary outcomes included the improved FM Assessment scores of upper and lower extremity (ΔFM-UE, ΔFM-LE). Neuroimaging outcome was VMHC calculated from fMRI using 3.0 Tesla scanner (Siemens, Verio, Germany). Preacupuncture fMRI assessment was conducted in both of the IS and HC group to explore the pathological characteristics. Postacupuncture assessment was underway exclusively in the IS group to examine the therapeutic effects of acupuncture. Adverse events were documented by investigators and were categorised by acupuncturists and neurologists as acupuncture-related or non-acupuncture-related within 24 hours of occurrence.
Statistical analysis
Narrowing inclusion criteria of patients within a short time-window early after stroke combined with biomarkers could reduce intersubject variability and enrich the sample.9 Previous clinical and neuroimaging studies had a median sample size ranging from 14.5 to 50.24 We designed a sample size of 45 patients and 30 healthy individuals considering a 10% loss because of the clear aetiology and the homogeneity of motor impairment among subjects. Given the standardised clinical intervention as well as neuroimaging quality control, normalised data processing and regularised effect modification, the sample size could provide quite reliable power to detect the between-group difference and clinical prediction for brain and behaviour.25 26
The clinical and neuroimaging outcomes were conducted per-protocol analysis.27 Participants with missing data or substandard head motion were excluded. We defined the left-lesion hemisphere as the affected side and right hemispheric lesions were flipped along the midsagittal plane.28 Acupuncture efficacy analyses were implemented by motor-impairment subgroup analysis and Spearman correlation analysis to examine the macrovisualisation to microvisualisation quantitatively (the mild to moderate subgroup: initial FM scores ≥50; the severe subgroup: initial FM scores <50).29 Acupuncture mechanistic analyses were investigated via sFC and DFC clustering models based on sliding windows (size=30 TR, step=1 TR).15 Logistic regression and linear regression models based on machine learning were to modify the reliability and validity of pathological and therapeutic results within a small sample range, with an 8:2 ratio of training set and test set and 5-fold cross-validation.
Categorical variables were described with frequencies and percentages and used χ2 test. Continuous variables were reported as mean (SD) or median (IQR), with two-sample t-test or Mann Whitney U test for between-group differences, and paired t-test or paired Wilcoxon test for therapeutic differences (p<0.05). The Gaussian random field (GRF) multiple comparisons were conducted in imaging statistics (voxel p<0.001, cluster p<0.05).
Clinical statistical analysis was performed using SPSS V.29.0 (IBM). Imaging statistical analyses were finished by SPM12 (http://www.fil.ion.ucl.ac.uk/spm) and DynamicBC 2.2 (http://restfmri.net/forum/DynamicBC) via MATLAB 2023a, with gender, age and mean FD as covariates.
Results
Clinical and neuroimaging characteristics
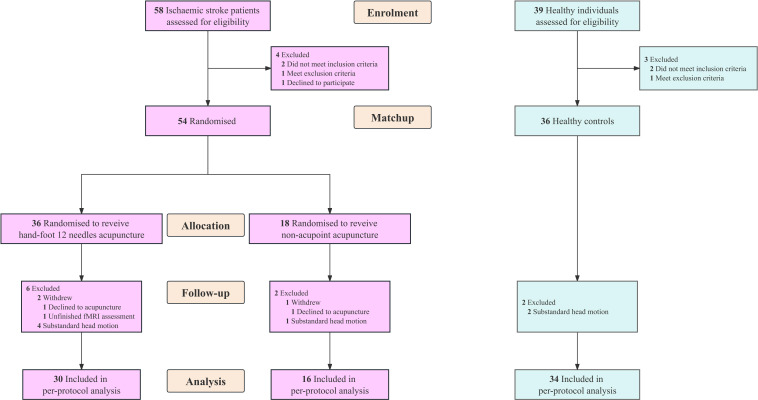
During 1 March 2018 to 30 April 2023, we screened 97 participants for eligibility. Totally, 54 patients were randomly assigned to receive either HA (n=36) or NA acupuncture (n=18), and 36 healthy individuals were matched. Finally, 46 ISs (mean (SD) age, 59.37 (11.36) years) matched with 34 HCs (mean (SD) age, 52.88 (9.69) years) completed the trial and neuroimaging study, of whom 30 received HA and 16 received NA acupuncture (figure 2). The ISs and HCs presented no significant difference in demographic and initial clinical characteristics within gender-impairment or motor-impairment severity subgroups, and baseline characteristics were similar between HA and NA group (table 1).
Figure 2.
Study flow chart. fMRI, functional MRI.
Table 1.
Demographics and baseline characteristics
| Characteristics | IS group (n=46) | HC group (n=34) | P value | |
| HA group (n=30) | NA group (n=16) | |||
| Gender, n (%) (male/female)* |
31 (67.39)/15 (32.61) | 21 (61.76)/13 (38.24) | 0.602 | |
| Age, mean (SD), years (male/female)† |
57.57 (13.68)/62.89 (9.79) | 58.00 (9.89)/62.67 (5.50) | 50.76 (10.89)/56.31 (6.33) | 0.082/0.069 0.086/0.050 |
| Location of lesion, n (%) (left/right)* |
15 (50.00)/15 (50.00) | 7 (43.75)/9 (56.25) | 0.686 | |
| Severity of motor impairment, n (%) (mild to moderated/severed)* |
20 (66.67)/10 (33.33) | 11 (68.75)/5 (31.25) | 0.886 | |
| Duration, median (IQR), days ‡ | 11.50 (7.00–20.50) | 12.50 (4.30–34.80) | 0.936 | |
| HAMD, median (IQR), scores‡ | 6.00 (2.00–13.00) | 7.50 (4.00–11.80) | 0.835 | |
| MMSE, median (IQR), scores‡ | 28.00 (24.80–29.00) | 27.00 (24.30–29.80) | 0.953 | |
| NIHSS, median (IQR), scores‡ | 3.00 (1.00–5.00) | 2.50 (1.00–5.80) | 0.861 | |
| FM, median (IQR), scores‡ | 74.00 (39.30–96.30) | 85.00 (39.00–97.80) | 0.579 | |
| Antihypertensives, n (%) (yes/no)* |
14 (46.67)/16 (53.33) | 7 (43.75)/9 (56.25) | 0.850 | |
| Lipid-lowering agents, n (%) (yes/no)* |
5 (16.67)/25 (83.33) | 6 (37.50)/10 (62.50) | 0.115 | |
| Hypoglycaemic agents, n (%) (yes/no)* |
9 (30.00)/21 (70.00) | 3 (18.75)/13 (81.25) | 0.408 | |
*χ² test. In order to avoid the information bias resulted from matching numbers, we adopted χ² test in HC group and IS group received acupuncture or non-acupoint acupuncture in sexual subgroups, respectively.
†Two-sample t-test.
‡Mann-Whitney U test.
FM, Fugl-Meyer; HA, hand-foot 12 needles acupuncture; HAMD, Hamilton Depression Scale; HC, healthy control; IS, ischaemic stroke; MMSE, Mini-mental State Examination; NA, non-acupoint; NIHSS, National Institute of Health Stroke Scale.
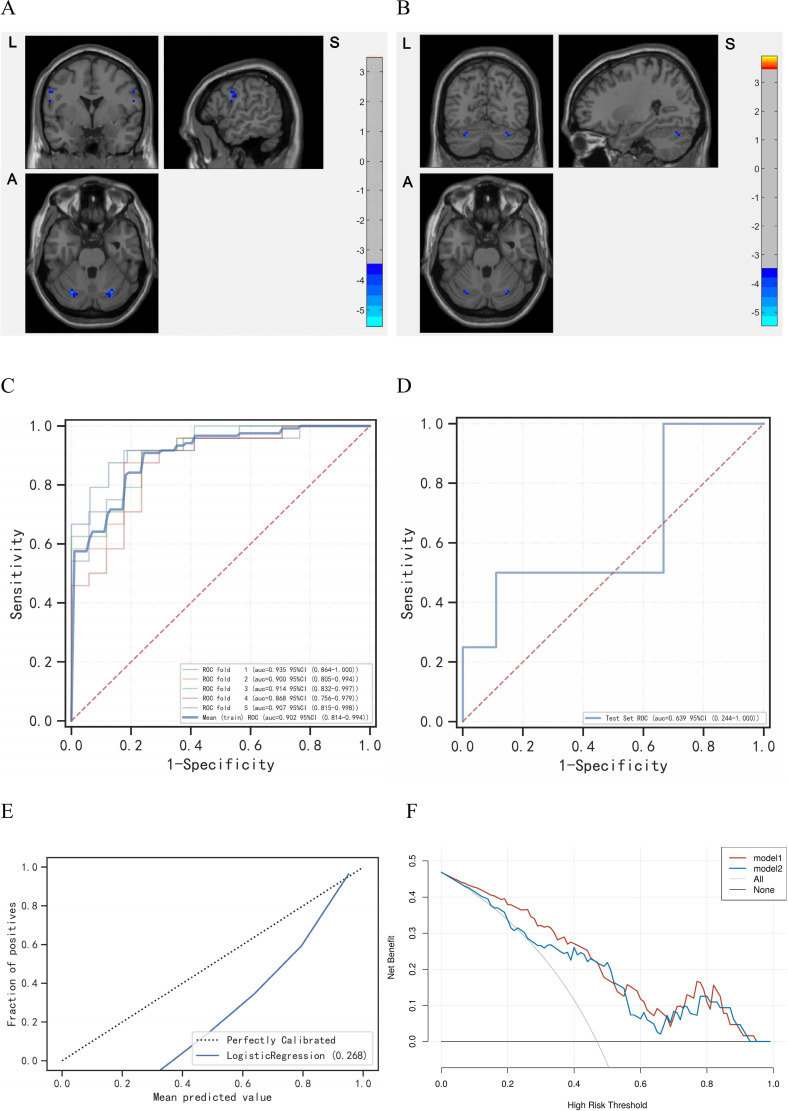
ISs demonstrated decreased VMHC in bilateral postcentral gyrus and cerebellum (GRF corrected, voxel p<0.001, cluster p<0.05) compared with HCs (figure 3A,B). To be specific, the mild to moderate ISs were reported the bilateral postcentral gyrus and cerebellum crus1, while the severe ISs were the bilateral cerebellum crus1 and cerebellum_6 (online supplemental table S3). Based on the logistic-regression machine learning, the test set (area under curve (AUC)=0.8413, accuracy=0.7500) could distinguish unilateral-lesion IS patients with hemiparesis from healthy individuals within aforesaid featured regions (figure 3C,D), with the calibration curve reflecting the predicted probability verged on empirical probability (figure 3E), and the decision curve analysis supporting both the models of decreased VMHC in cerebrum and cerebellum contributed to the identification of IS patients with hemiparesis in the threshold interval ranged from 0% to 95% (figure 3F).30
Figure 3.
The pathologically cerebro-cerebellar VMHC captured by logistic regression based on machine learning. (A) The decreased VMHC in postcentral gyrus and cerebellum crus1 among the mild to moderate ISs. Red is increasing and blue is decreasing. (B) The decreased VMHC in cerebellum crus1 and cerebellum_6 among the severe ISs. Red is increasing and blue is decreasing. (C) The ROC of train set. (D) The ROC of test set. (E) The calibration curve of the logistic regression. (F) The test decision curve of the logistic regression. The model 1 is VMHC_Postcentral, and the model 2 is VMHC_Cerebellum. AUC, area under curve; IS, ischaemic stroke; ROC, receiver operating characteristic curve; VMHC, voxel-mirrored homotopic connectivity.
Primary and secondary outcomes
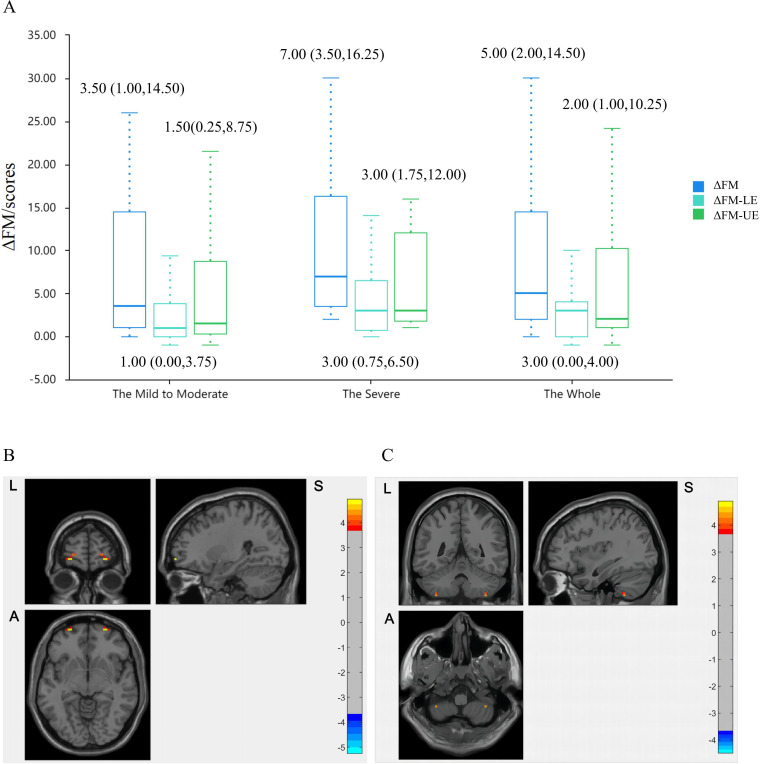
In terms of clinical outcomes, the median (IQR) ΔFM scores were 5.00 (2.00–14.50) in HA group and 2.50 (0.25–15.75) in NA group (mean difference −1.00 (95% CI −7.00 to 1.00, p<0.001)). Compared with the NA group, FM-UE scores of HA group had a slight increase from baseline with between-group differences of −1.00 points (95% CI −4.00 to 1.00 points, p<0.05), and FM-LE scores with 0.00 points (95% CI −2.00 to 1.00 points, p<0.001) (table 2). Similar changed results were observed across motor-impairment-severity subgroups (figure 4A).
Table 2.
Primary and secondary outcomes
| Variable | HA group (n=30) | NA group (n=16) | Effect size (95% CI)* | P value |
| Primary outcomes† | ||||
| ΔFM, median (IQR), scores | 5.00 (2.00–14.50) | 2.50 (0.25–15.75) | −1.00 (−7.00 to 1.00) | 0.000 |
| Secondary outcomes† | ||||
| ΔFM-UE, median (IQR), scores | 2.00 (1.00–10.25) | 2.00 (0.00–7.75) | −1.00 (−4.00 to 1.00) | 0.012 |
| ΔFM-LE, median (IQR), scores | 3.00 (0.00–4.00) | 1.50 (0.00–3.75) | 0.00 (−2.00 to 1.00) | 0.000 |
*Estimated mean differences for clinical outcomes.
†Scheirer-Ray-Hare test.
FM, Fugl-Meyer; HA, hand-foot 12 needles acupuncture; IS, ischaemic stroke; LE, lower extremity; NA, non-acupoint; UE, upper extremity.
Figure 4.
The clinical and neuroimaging outcomes. (A) The improvement of FM scores in HA group across motor-impairment severity subgroups. (B) The increased VMHC_Frontal_Sup_Orb in HA group following acupuncture. Red is increasing and blue is decreasing. (C) The higher VMHC_Cerebellum_8 in HA group compared with NA group in postacupuncture stage. Red is high and blue is low. FM, Fugl-Meyer; HA, hand-foot 12 needles acupuncture; LE, lower extremity; NA, non-acupoint; UE, upper extremity; VMHC, voxel-mirrored homotopic connectivity.
As to neuroimaging outcomes, VMHC in bilateral superior frontal gyrus orbital part was increased than preacupuncture level within HA group (GRF corrected, voxel p<0.001, cluster p<0.05) (figure 4B). Among the mild to moderate individuals, HA group demonstrated higher VMHC in bilateral cerebellum_8 than that in NA group in postacupuncture stage (figure 4C). No significant outcomes were observed in NA group. There were no acupuncture-related adverse events in either group.
Brain and behaviour effects of acupuncture
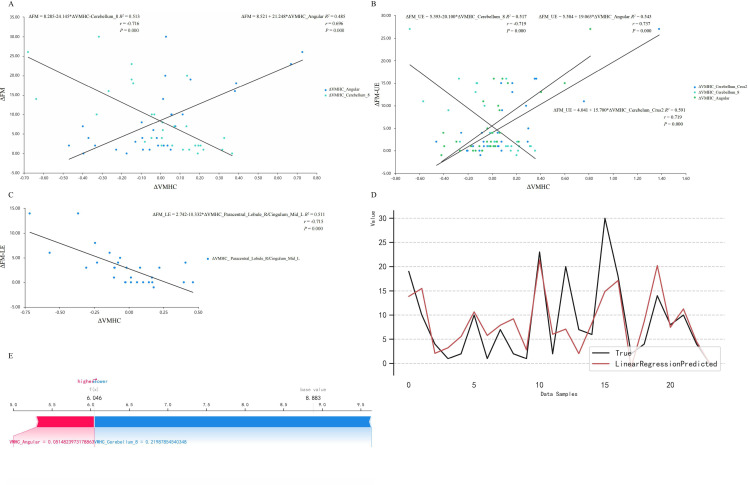
Spearman correlation analysis revealed a dual correlation between the ΔFM and the altered VMHC (ΔVMHC) (angular: r=0.696, p=0.000; cerebellum_8: r=−0.716, p=0.000) (figure 5A). The linear regression equation was ΔFM=8.389+13.199×ΔVMHC_Angular−16.089×ΔVMHC_Cerebellum_8 (R2=0.643, p=0.000). Additionally, ΔFM-UE was mainly correlated with ΔVMHC of cerebellar areas (figure 5B), and ΔFM-LE was negatively correlated with ΔVMHC in right paracentral lobule and light median cingulate and paracingulate gyri (figure 5C). There were no significant motor-related correlations within NA group.
Figure 5.
The brain and behaviour effects of acupuncture. (A) The ΔFM was positively correlated with ΔVMHC_Angular and negatively with ΔVMHC_Cerebellum_8. (B) The ΔFM-UE was positively correlated with ΔVMHC_Cerebellum_Crus2 and ΔVMHC_Angular, and negatively with ΔVMHC_Cerebellum_8. (C) The ΔFM-LE was negatively correlated with ΔVMHC_Paracentral_Lobule_R/Cingulum_Mid_L. (D) The fitting curve of true value and predicted value based on the linear regression model. (E) The SHAP values of the ΔVMHC with its predictive values meaning the contribution to the ΔFM. The predicted positive contribution of angular gyrus was smaller than the negative contribution of cerebellum (base value=8.883, predicted value=6.046). FM, Fugl-Meyer; LE, lower extremity; SHAP, shapley additive explanation; UE, upper extremity; VMHC, voxel-mirrored homotopic connectivity.
The linear regression model based on machine learning captured the ΔVMHC_Angular and ΔVMHC_Cerebellum_8 as predictors for ΔFM (validation set R2=0.31±0.57, test set R2=0.828) (figure 5D). Notably, ΔVMHC_Cerebellum_8 plays a more pronounced role in FM reduction than ΔVMHC_Angular impacting on FM increase (base value=8.883, predicted value=6.046) (figure 5E).
Static and dynamic modification of acupuncture
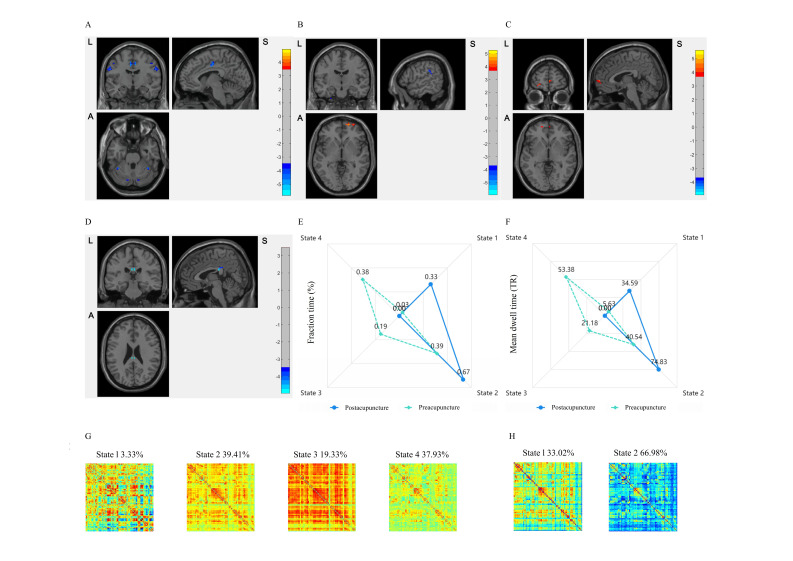
In postacupuncture stage, we observed decreased VMHC in bilateral precentral gyrus and cingulate gyrus within HA group besides postcentral gyrus and cerebellum (figure 6A). Lower VMHC occurred in the bilateral superior temporal gyrus, median cingulate and paracingulate gyrus, and the contralesional supplementary motor area (SMA) within the mild to moderate ISs, and disappeared in cerebellum within severe subgroup (online supplemental table S5). Taking the bilateral superior frontal gyrus orbital part as region of interest (ROI), sFC was higher between ipsilesional superior frontal gyrus orbital part with the contralesional orbitofrontal cortex and cerebellum, accompanied with bidirectional changed sFC in limbic system, such as supramarginal, superior temporal gyrus and superior occipital gyrus (figure 6B,C).
Figure 6.
The static and dynamic modification of acupuncture. (A) Regions reported decreased VMHC in HA group compared with HCs. (B) Changes on sFC with Frontal_Sup_Orb_R as ROI following acupuncture. (C) Changes on sFC with Frontal_Sup_Orb_L as ROI following acupuncture. (D) The decreased CV_VMHC of PCC following acupuncture. Red is increasing and blue is decreasing. (E) FT changes among DFC states following acupuncture. (F) MDT changes among DFC states following acupuncture. (G) The pattern of DFC states clustering in preacupuncture stage. (H) The pattern of DFC states clustering in postacupuncture stage. Red is positive weights and blue is negative weights. CV_VMHC, coefficient of variability of VMHC; DFC, dynamic functional connectivity; HA, hand-foot 12 needles acupuncture; HC, healthy control; PCC, posterior cingulate gyrus; ROI, region of interest; sFC, static function connectivity; VMHC, voxel-mirrored homotopic connectivity.
Following acupuncture, we found decreased coefficient of variation of VMHC (CV_VMHC) in bilateral posterior cingulate gyrus (PCC) locally (GRF corrected, voxel p<0.001, cluster p<0.05) (figure 6D). On global level, integration states (state 3, state 4) were disappeared and segregation states (state 1, state 2) were increased. State 2 was transformed from integration (overall frequency: 39.41%) to segregation (overall frequency: 66.98%) (figure 6G,H). Relevantly, there were shorter fraction time (FT) and mean dwell time (MDT) of integration states and longer periods of segregation states (p<0.05) (figure 6E,F).
Discussion
Over a 10-day treatment period, acupuncture involving limb region demonstrated greater FM improvement related to cerebro-cerebellar bilaterality than NA acupuncture, with therapy-driven modification on IHI and CBI. Since the low incidence of adverse events, acupuncture may provide a reasonable rehabilitation option for poststroke patients with hemiparesis to facilitate motor recovery.
Standardised acupuncture: central and peripheral closed-loop for motor recovery
Based on the bilateral meridian theory, symmetrical location of limbs and balanced manipulation on muscles, acupuncture at these standardised acupoints could promote poststroke recovery extensively, including improving the balance function, reducing spasticity, increasing muscle strength and general well-being.31 Clinical trial and meta-analysis yielded significant efficacy of acupuncture on motor function (SD −0.78, 95% CI −1.22 to −0.34; I2=86%),21 with heterogeneity on sham controls (OR 0.35, 95% CI 0.30 to 0.41).32 Similar to these results, the median (IQR) scores of ΔFM were 5.00 (2.00–14.50) in HA group and 2.50 (0.25–15.75) in NA group, with a more obvious and motor-region-correlated improvement in HA group. Notably, the median (IQR) scores of ΔFM (7.00 (3.50–16.25)) and ΔFM-UE (3.00 (1.75–12.00)) within the severe patients were higher than whole level, highlighting that acupuncture might be particularly beneficial to severe motor impairment. Thus, results of this study demonstrated the proximal specific effects of acupuncture on motor behaviour macroscopically.
Our previous studies have revealed the acupuncture taking effect on neuroplasticity from the motor region level to motor network level.10 13 In this study, VMHC in superior frontal gyrus orbital part was increased, accompanied with expansive lower VMHC regions within cerebro-cerebellar networks following acupuncture, while we did not found similar results in NA group. We inferred the imbalanced IHI and CBI may be potential target effectors to adjust the movement isolation and action integration,33 34 that is, the distal specific effects of acupuncture on brain plasticity microscopically. Altogether, the proximal and distal effects perform a central and peripheral closed-loop for motor rehabilitation at early subacute stage. Highly standardised control of NA could eliminate the non-specific effects from acupuncture.8
Decreased homotopic connectivity: visualised evidence for acupuncture efficacy
Objective and quantitative evaluation of VMHC in cerebrum and cerebellum could provide visualised evidence for the therapeutic assessment of acupuncture. In our prospectively pathological study, it demonstrated that decreased VMHC in bilateral postcentral gyrus and cerebellum could serve as underlying indicators for unilateral-lesion IS. Although the reported clusters of voxels were bilateral postcentral gyrus in the anatomical automatic labelling template, they were located on the transitional area of sensorimotor cortex and accounted for 75% postcentral gyrus on the primary motor cortex (M1) and 25% precentral gyrus on the primary somatosensory cortex (S1), indicating pathway-specific structure-function covariations involved IHI and CBI.14 26 On the corpus callosum and corticospinal tract (CST) pathway, our findings implied that lower homotopic connectivity in postcentral gyrus and cerebellum may hinder motor commands which were afferent from M1, efferent to S1 as well as cerebellum and projected to hand, thus impeding the inhibition of competing and feedback decoupling.35 36 Under the balance-recovery model, M1 injury and interrupted cerebro-cerebellar interactions reinforced the negative effect of CST injury on upper limbs recovery, especially in the severe subgroup.33 36–38 Within the sensorimotor network and cerebellar network, these results reflected decreased M1 intereffectors linked to adjacent S1, SMA and cerebellum, which delayed functional compensations for both the affected hemisphere and distal effectors after stroke involved unilateral middle cerebral artery.35 It confirms the potential significance of our prior hypotheses.
In our therapeutic study, we observed that the ΔVMHC in bilateral angular and cerebellum_8 had a strong dual correlation with ΔFM, as reliable predictors for motor function. This finding not only provides neuroimaging evidence in support of acupuncture efficacy, but also underscores the robustness of the interaction between macro and micro therapeutic effects. Notably, the ΔFM-UE displayed bidirectional correlations with the ΔVMHC in cerebellar areas, meaning upper capacity could be an important aspect for the motor rehabilitation.37 38 The shapley additive explanations plots showcased a consistent trend on individual level that aligned with the dual correlations on group level, illustrating that for the motor recovery, the predicted positive contribution of angular gyrus is smaller than the negative contribution of cerebellum. Closed to the supramarginal gyrus and cingulated gyrus anatomically, the angular gyrus may provide the reliable functional proof of overall motor function, while the cerebellum is more likely related to upper limb function. Collectively, our pathological and therapeutic studies support the decreased cerebro-cerebellar VMHC serving as visualised evidence for acupuncture efficacy.
Therapy-driven modification: bilateral static and dynamic reorganisation
Along with the acupuncture therapeutic efficacy, our study revealed a bilateral modification on IHI and CBI via static and dynamic functional connectivity. To be specific, there was increased VMHC in bilateral superior frontal gyrus orbital part and decreased CV_VMHC in bilateral PCC, indicating static reorganisation and dynamic integration and segregation, respectively. On the static function level, we observed bilateral alterations featured with increased sFC between the affected superior frontal gyrus orbital part and the unaffected frontal gyrus orbital part as well as cerebellum following acupuncture. As the effectors connected with M1, we noticed enhanced links within somato-cognitive action network, including higher-order sensorimotor control and cerebellar-mediated feedback.26 28 35 36 By increasing the coupling of the cerebro-cerebellar interactions, patients gained clinical benefit of acupuncture in compensating the lateralised IHI and CBI resulted from affected hemisphere. On the dynamic function level, locally decreased CV_VMHC in bilateral PCC promoted a stable cortical-subcortical coupling associated with motor inputs incorporating somatosensory information, basal ganglia feedback and prefrontal cortex, in a longer ‘temporal receptive window’ and larger posterior ‘midline’ regions.39 Overall, the DFC clustering model showed acupuncture modifying on the abnormal imbalance among dynamical states with high integration but weak correlations. Specifically, shorter periods and less shifts towards states of integration, and more frequent states of segregation indicated the functional reorganisation tending to regain a healthy profile, especially the transforming of state 1 and state 2 as pathological states.15 16 Meanwhile, dynamic functional connectivity anomalies would disappear to reach the balanced static functional connectivity, thus to restore the lesion-induced coactivation.15 40 In brief, the comodulation of sFC and DFC modifies the lateralised brain state in a bilateral therapy-driven process. Nonetheless, the exact lateralised modification of acupuncture for hemiparesis warrants further investigation.
Strengths and limitations
The strengths of this trial lie in its standardised interventions with NA control and the per-protocol analysis of clinical and neuroimaging. With the rigorous methodological designs, we believe that the results of this trial added evidence of visualisation and micromodification supporting acupuncture efficacy on poststroke rehabilitation.
The study has some limitations. First, this trial included patients with unilateral focal lesions in the subcortical ganglia, and the results may not be generalisable to the patients with multifocal or massive cerebral infraction. Second, physiological effects of NA acupuncture may underestimate the specific effects of acupuncture. Third, no adjustment was made for the mixed effects from the indirect laterality, such as right handedness or asymmetrical non-motor networks. Fourth, per-protocol analysis limited the randomised matching and modelling selection within samples based on machine learning. Fifth, applying standard statical and dynamical approaches may decrease the modification on the specific ‘stroke state’,15 including image flip, head control and sliding-window analysis with the clustering algorithm.
Conclusion
In conclusion, this randomised clinical and neuroimaging trial demonstrated that acupuncture improved the motor recovery and modified cerebro-cerebellar VMHC via bilateral static and dynamic reorganisations for IS patients with hemiparesis. These outcomes affirm the clinical efficacy of acupuncture from imaging visualisation and neuroplasticity. Acupuncture may be a compelling adjunct to the routine rehabilitation promoting poststroke motor recovery at the early subacute stage.
Acknowledgments
We appreciate the support from the personnel working at the Department of Neurology, Rehabilitation and Acupuncture of Dongzhimen Hospital, Beijing University of Chinese Medicine.
Footnotes
Contributors: TC and YZ conceptualised and designed the study. TC conducted the trial and wrote the manuscript. TC contributed to the statistical analysis and the proofreading. YZ contributed to the consultation of clinical and imaging diagnosis. KW contributed to the data collection. YZ organised the project and supervised the revision. YZ accepts full responsibility for the work and the conduct of the study, had access to the data, and controlled the decision to publish.
Funding: The study was funded by the National Natural Science Foundation of China (Grant No. 82174331, Grant No. 81873257) and the Beijing Municipal Natural Science Foundation (Grant No. 7182104).
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. Not applicable.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
All procedures were in accordance with the Declaration of Helsinki and approved by DZMEC-KY-2018-04 obtained at the Ethical Committee of Dongzhimen Hospital, Beijing University of Chinese Medicine. Participants gave informed consent to participate in the study before taking part.
References
- 1. Zhou M, Wang H, Zeng X, et al. Mortality, morbidity, and risk factors in China and its provinces, 1990-2017: a systematic analysis for the global burden of disease study 2017. Lancet 2019;394:1145–58. 10.1016/S0140-6736(19)30427-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wu S, Wu B, Liu M, et al. Stroke in China: advances and challenges in epidemiology, prevention, and management. Lancet Neurol 2019;18:394–405. 10.1016/S1474-4422(18)30500-3 [DOI] [PubMed] [Google Scholar]
- 3. Micera S, Caleo M, Chisari C, et al. Advanced neurotechnologies for the restoration of motor function. Neuron 2020;105:604–20. 10.1016/j.neuron.2020.01.039 [DOI] [PubMed] [Google Scholar]
- 4. Zhong LL, Zheng Y, Lau AY, et al. Would integrated Western and traditional Chinese medicine have more benefits for stroke rehabilitation? A systematic review and meta-analysis. Stroke Vasc Neurol 2022;7:77–85. 10.1136/svn-2020-000781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. NIH consensus conference. Acupuncture. JAMA 1998;280:1518–24. [PubMed] [Google Scholar]
- 6. Zhang S, Wu B, Liu M, et al. Acupuncture efficacy on ischemic stroke recovery: multicenter randomized controlled trial in China. Stroke 2015;46:1301–6. 10.1161/STROKEAHA.114.007659 [DOI] [PubMed] [Google Scholar]
- 7. Fei Y-T, Cao H-J, Xia R-Y, et al. Methodological challenges in design and conduct of randomised controlled trials in acupuncture. BMJ 2022;376:e064345. 10.1136/bmj-2021-064345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhang Y-Q, Jiao R-M, Witt CM, et al. How to design high quality acupuncture trials-a consensus informed by evidence. BMJ 2022:e067476. 10.1136/bmj-2021-067476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stinear CM, Lang CE, Zeiler S, et al. Advances and challenges in stroke rehabilitation. Lancet Neurol 2020;19:348–60. 10.1016/S1474-4422(19)30415-6 [DOI] [PubMed] [Google Scholar]
- 10. Chen TZ, Zou YH, Du ZM, et al. Effect of “hand and foot acupuncture with twelve needles” on the functional connection between primary motor areas of the brain and cerebellum in patients with hemiplegia after ischemic stroke. J Tradit Chin Med 2021;62:1514–21. 10.13288/j.11-2166/r.2021.17.010 [DOI] [Google Scholar]
- 11. Yang Y, Eisner I, Chen S, et al. Neuroplasticity changes on human motor cortex induced by acupuncture therapy: a preliminary study. Neural Plast 2017;2017:4716792. 10.1155/2017/4716792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lu M, Du Z, Zhao J, et al. Neuroimaging mechanisms of acupuncture on functional reorganization for post-stroke motor improvement: a machine learning-based functional magnetic resonance imaging study. Front Neurosci 2023;17:1143239. 10.3389/fnins.2023.1143239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang Y, Lu M, Liu R, et al. Acupuncture alters brain’s dynamic functional network connectivity in stroke patients with motor dysfunction: a randomised controlled neuroimaging trial. Neural Plasticity 2023;2023:1–14. 10.1155/2023/8510213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zuo X-N, Kelly C, Di Martino A, et al. Growing together and growing apart: regional and sex differences in the lifespan developmental trajectories of functional homotopy. J Neurosci 2010;30:15034–43. 10.1523/JNEUROSCI.2612-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Favaretto C, Allegra M, Deco G, et al. Subcortical-cortical dynamical states of the human brain and their breakdown in stroke. Nat Commun 2022;13:5069. 10.1038/s41467-022-32304-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bonkhoff AK, Espinoza FA, Gazula H, et al. Acute ischaemic stroke alters the brain’s preference for distinct dynamic connectivity states. Brain 2020;143:1525–40. 10.1093/brain/awaa101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Scott IA. Machine learning and evidence-based medicine. Ann Intern Med 2018;169:44–6. 10.7326/M18-0115 [DOI] [PubMed] [Google Scholar]
- 18. Rajkomar A, Dean J, Kohane I. Machine learning in medicine. N Engl J Med 2019;380:1347–58. 10.1056/NEJMra1814259 [DOI] [PubMed] [Google Scholar]
- 19. Schulz KF, Altman DG, Moher D, et al. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340:c332. 10.1136/bmj.c332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. MacPherson H, Altman DG, Hammerschlag R, et al. Revised standards for reporting interventions in clinical trials of acupuncture (STRICTA): extending the CONSORT statement. PLoS Med 2010;7:e1000261. 10.1371/journal.pmed.1000261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Beijing Hospital of Traditional Chinese Medicine . Gold needle wang leting. Beijing: Beijing Publishing House, 1984: 78. [Google Scholar]
- 22. Zhou K, Fang J, Wang X, et al. Characterization of de Qi with electroacupuncture at acupoints with different properties. J Altern Complement Med 2011;17:1007–13. 10.1089/acm.2010.0652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liu Z, Liu Y, Xu H, et al. Effect of electroacupuncture on urinary leakage among women with stress urinary Incontinence: a randomized clinical trial. JAMA 2017;317:2493–501. 10.1001/jama.2017.7220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Szucs D, Ioannidis JP. Sample size evolution in neuroimaging research: an evaluation of highly-cited studies (1990-2012) and of latest practices (2017-2018) in high-impact journals. Neuroimage 2020;221. 10.1016/j.neuroimage.2020.117164 [DOI] [PubMed] [Google Scholar]
- 25. Poldrack RA, Huckins G, Varoquaux G. Establishment of best practices for evidence for prediction: a review. JAMA Psychiatry 2020;77:534–40. 10.1001/jamapsychiatry.2019.3671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yu Q, Yin D, Kaiser M, et al. Pathway-specific mediation effect between structure, function, and motor impairment after subcortical stroke. Neurology 2023;100:e616–26. 10.1212/WNL.0000000000201495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hernán MA, Robins JM. Per-protocol analyses of pragmatic trials. N Engl J Med 2017;377:1391–8. 10.1056/NEJMsm1605385 [DOI] [PubMed] [Google Scholar]
- 28. Grefkes C, Nowak DA, Eickhoff SB, et al. Cortical connectivity after subcortical stroke assessed with functional magnetic resonance imaging. Ann Neurol 2008;63:236–46. 10.1002/ana.21228 [DOI] [PubMed] [Google Scholar]
- 29. Dawson J, Liu CY, Francisco GE, et al. Vagus nerve stimulation paired with rehabilitation for upper limb motor function after ischaemic stroke (VNS-REHAB): a randomised, blinded, pivotal, device trial. Lancet 2021;397:1545–53. 10.1016/S0140-6736(21)00475-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Vickers AJ, Elkin EB. Decision curve analysis: a novel method for evaluating prediction models. Med Decis Making 2006;26:565–74. 10.1177/0272989X06295361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chavez LM, Huang S-S, MacDonald I, et al. Mechanisms of acupuncture therapy in ischemic stroke rehabilitation: a literature review of basic studies. Int J Mol Sci 2017;18:2270. 10.3390/ijms18112270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wu P, Mills E, Moher D, et al. Acupuncture in poststroke rehabilitation: a systematic review and meta-analysis of randomized trials. Stroke 2010;41:e171–9. 10.1161/STROKEAHA.109.573576 [DOI] [PubMed] [Google Scholar]
- 33. Di Pino G, Pellegrino G, Assenza G, et al. Modulation of brain plasticity in stroke: a novel model for neurorehabilitation. Nat Rev Neurol 2014;10:597–608. 10.1038/nrneurol.2014.162 [DOI] [PubMed] [Google Scholar]
- 34. Stoodley CJ, Schmahmann JD. Functional topography in the human cerebellum: a meta-analysis of neuroimaging studies. Neuroimage 2009;44:489–501. 10.1016/j.neuroimage.2008.08.039 [DOI] [PubMed] [Google Scholar]
- 35. Gordon EM, Chauvin RJ, Van AN, et al. A somato-cognitive action network alternates with effector regions in motor cortex. Nature 2023;617:351–9. 10.1038/s41586-023-05964-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Boven E, Pemberton J, Chadderton P, et al. Cerebro-cerebellar networks facilitate learning through feedback decoupling. Nat Commun 2023;14:51. 10.1038/s41467-022-35658-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hayward KS, Ferris JK, Lohse KR, et al. Observational study of neuroimaging biomarkers of severe upper limb impairment after stroke. Neurology 2022;99:e402–13. 10.1212/WNL.0000000000200517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lin DJ, Cloutier AM, Erler KS, et al. Corticospinal tract injury estimated from acute stroke imaging predicts upper extremity motor recovery after stroke. Stroke 2019;50:3569–77. 10.1161/STROKEAHA.119.025898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Foster BL, Koslov SR, Aponik-Gremillion L, et al. A tripartite view of the posterior cingulate cortex. Nat Rev Neurosci 2023;24:173–89. 10.1038/s41583-022-00661-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Peng X, Liu Q, Hubbard CS, et al. Robust dynamic brain coactivation states estimated in individuals. Sci Adv 2023;9:eabq8566. 10.1126/sciadv.abq8566 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
svn-2023-002785supp001.pdf (307.2KB, pdf)
Data Availability Statement
Data are available upon reasonable request. Not applicable.