Abstract
G protein-coupled receptors (GPCRs) exhibit remarkable structural plasticity, which underlies their capacity to recognize a wide range of extracellular molecules and interact with intracellular partner proteins. Nuclear magnetic resonance (NMR) spectroscopy is uniquely well-suited to investigate GPCR structural plasticity, enabled by stable-isotope “probes” incorporated into receptors that inform on structure and dynamics. Progress with stable-isotope labeling methods in Eukaryotic expression systems has enabled production of native or nearly-native human receptors with varied and complementary distributions of NMR probes. These advances have opened up new avenues for investigating the roles of conformational dynamics in signaling processes, including by mapping allosteric communication networks, understanding the specificity of GPCR interactions with partner proteins and exploring the impact of membrane environments on GPCR function.
Introduction
G protein-coupled receptors (GPCRs) are sensory integral membrane proteins that recognize an enormous range of extracellular stimuli and interact with numerous intracellular partner proteins to initiate cellular signaling events. It is widely appreciated that the functions of GPCRs are enabled by their inherent structural plasticity, i.e., conformational dynamics, and a complete view of GPCR function must also include knowledge of their dynamic behavior [1,2]. While crystallography and cryo-EM have made tremendous progress determining GPCR structures, concurrently, great advances investigating conformational dynamics of GPCRs have been made by spectroscopic methods, especially nuclear magnetic resonance (NMR) spectroscopy [3]. Indeed, current understanding of GPCR molecular recognition mechanisms are highly informed from NMR studies [3].
NMR spectroscopy provides several significant advantages for studying GPCR conformational dynamics, including that experiments can be carried out at physiological temperatures, do not require bulky tags, and frequently utilize proteins with native or nearly-native amino acid sequences. Importantly, NMR data provide information on GPCR structures and dynamics at the level of individual nuclei. This unique capability is enabled by stable-isotopes, which act as “probes” that sense changes in local structure, dynamics, and environments. By distributing NMR probes throughout the receptor, one can obtain a global view of GPCR conformational dynamics at atomic resolution. With advances in stable-isotope labeling approaches, inroads have been made into NMR studies with more challenging proteins, including GPCRs.
This review surveys stable-isotope labeling approaches for NMR studies of GPCRs, emphasizing methods that utilize NMR-observable nuclei other than 19F, i.e., 13C, 15N, 2H, and 1H. 19F-NMR complements experiments with these nuclei, as reviewed elsewhere [4,5]. Examples from the literature are presented that illustrate a range of expression systems for producing GPCRs and various methods for incorporating NMR probes, including stable-isotope labeling via chemical modification and via biosynthetic approaches. We discuss how advances in stable-isotope labeling and production of GPCRs have led to a more complete view of their functions by providing insights from NMR into GPCR-drug interactions, interactions with partner proteins, and impacts of the cellular environment on GPCR structure and conformational dynamics.
Overview of stable-isotope labeling approaches for NMR
Table 1 presents a survey from the literature of GPCRs expressed for NMR studies, employed expression systems, stable-isotope labeling schemes and employed membrane mimetics. GPCR NMR studies have used two general approaches for incorporating stable-isotope labels: post-translational chemical modification, especially reductive methylation of lysines, or incorporation via biosynthesis during protein expression. The majority of GPCR NMR studies have employed Eukaryotic expression systems, with insect cells (Sf9) being the most widely used organism. Most studies incorporated stable-isotopes via biosynthesis rather than chemical modification. To date, studies in solution have used mostly detergent micelles as membrane mimetics and have exclusively focused on class A GPCRs.
Table 1.
| GPCR | Expression system | Isotope labeling | Membrane Mimetic | Ref. |
|---|---|---|---|---|
| A2AAR | Yeast (P. pastoris) | ε−13CH3-Ile, 2H | Detergent micelles | [6] |
| u-15N, ~70%2H | Detergent micelles | [7], [8], [9] | ||
| ε−13CH3-Met | Lipid nanodiscs | [10] | ||
| α1AAR | E.coli | ε−13CH3-Met | Detergent micelles | [11] |
| β1AR | Insect cells (Sf9) | 15N-Valine | Detergent micelles | [12] , [13], [14] |
| ε−13CH3-Met | Detergent micelles | [15] | ||
| Insect cells (Sf9) | u-15N, >60%2H | Detergent micelles | [16] | |
| Mammalian cells | ε−13CH3-Met | Detergent micelles | [17] | |
| β2AR | Insect cells (Sf9) | (13CH3)-Lys reductive dimethylation | Detergent micelles | [18], [19] |
| ε−13CH3-Met | Detergent micelles | [20,21] | ||
| ε−13CH3-Met, 2H | Lipid nanodiscs | [22] | ||
| β−13CH3-Ala, 2H | Detergents micelles Lipid nanodiscs |
[23] | ||
| Insect cells (Sf9) and E.coli | ε−13CH3-Met, 2H, c-term-2H, 13C, 15N | Lipid nanodiscs | [24] | |
| [2,3,3-2H, 15N]-leucine | Detergent micelles | [25] | ||
| BLT2 | E.coli | ε−13CH3-Met and ε−13CH3-Ile | Lipid nanodiscs | [26] |
| CB2 | Yeast (P. pastoris) | ε−13CH3-Ile | Detergent micelles | [27] |
| ACKR3 | Insect cells | ε−13CH3-Met | Detergent micelles | [28] |
| H1R | Yeast (P. pastoris) | u-15N, ~70%2H | Detergent micelles | [29] |
| M2R | Insect cells (Sf9) | ε−13CH3-Met | Detergent micelles | [30] |
| NTR1 | E.coli | 13C-MMTS | Detergent micelles | [31] |
| ε−13CH3-Met | Detergent micelles | [32] | ||
| μOR | Insect cells | ε−13CH3-Met, 2H | Detergent micelles | [33] |
| (13CH3)-Lys reductive dimethylation | Detergent micelles | [34], [35] | ||
| OX2R | Yeast (P. pastoris) | ε−13CH3-Ile | Detergent micelles | [36] |
| CB1 | Yeast (P. pastoris) | ε−13CH3-Ile | Detergent micelles | [36] |
| Rhodopsin | Mammalian cells | α,ε−15N-Trp | Detergent micelles | [37],[38] |
| 13Cβ-Ser, 13Cβ-Cys, 13Cα-Gly | [39] |
Abbreviations: A2AAR, adenosine A2A receptor; α1AAR, α1A-adrenergic receptor; β1AR, β1-Adrenergic receptor; β2AR, β2-Adrenergic receptor; BLT2, leukotriene B4 receptor 2; CB2, Cannabinoid receptor type 2; ACKR3, atypical chemokine receptor 3; H1R, histamine H1 receptor; M2R, muscarinic acetylcholine receptor M2; NTR1, neurotensin receptor type 1; μOR, μ-opioid receptor; OX2R, orexin receptor type 2; CB1, cannabinoid receptor type 1.
Studies of GPCR complexes with small molecules
A central question in GPCR signaling is how information from ligand binding at the orthosteric pocket is transmitted ~30 Å to the intracellular surface of the receptor. NMR studies covering a growing number of class A GPCRs have provided insight into allosteric transmission processes.
The adenosine A2A receptor, A2AAR, a class A GPCR that regulates dopamine release and myocardial blood flow, has been the focus of multiple NMR studies. Expression of A2AAR in Pichia pastoris enabled uniform incorporation of stable-isotopes and extensive deuteration. This allowed highly resolved NMR spectra to be recorded that provided a global view of A2AAR structural plasticity. 2D [15N,1H]-transverse relaxation-optimized spectroscopy (TROSY) [40] spectra of A2AAR revealed the impact of drugs and mutations to receptor hot spots on signal transduction (Figure 1, a and b) [7]. The same methodology was employed to study A2AAR complexes with partial agonists [9], leading to the observation of conformations for highly conserved residues Trp6.48 and Phe6.44 unique from those observed in full agonist complexes (superscripts denote Ballesteros-Weinstein nomenclature). Extrinsic tryptophans were introduced using the same expression methodology to provide novel, well-dispersed 15N–1H indole signals, which showed different responses at helices V, VI, and VII correlating with changes in the efficacy of bound drugs and a ternary complex with bound agonist and polypeptide derived from the carboxy terminus of GαS [8]. 2D [13C,1H]-HMQC spectra of uniformly deuterated A2AAR with 1H/13C-labels at isoleucine δ1 methyl groups enabled experiments correlating fast side chain motions with the efficacy of bound drugs and sodium concentration [6] (Figure 1, c and d).
Figure 1.
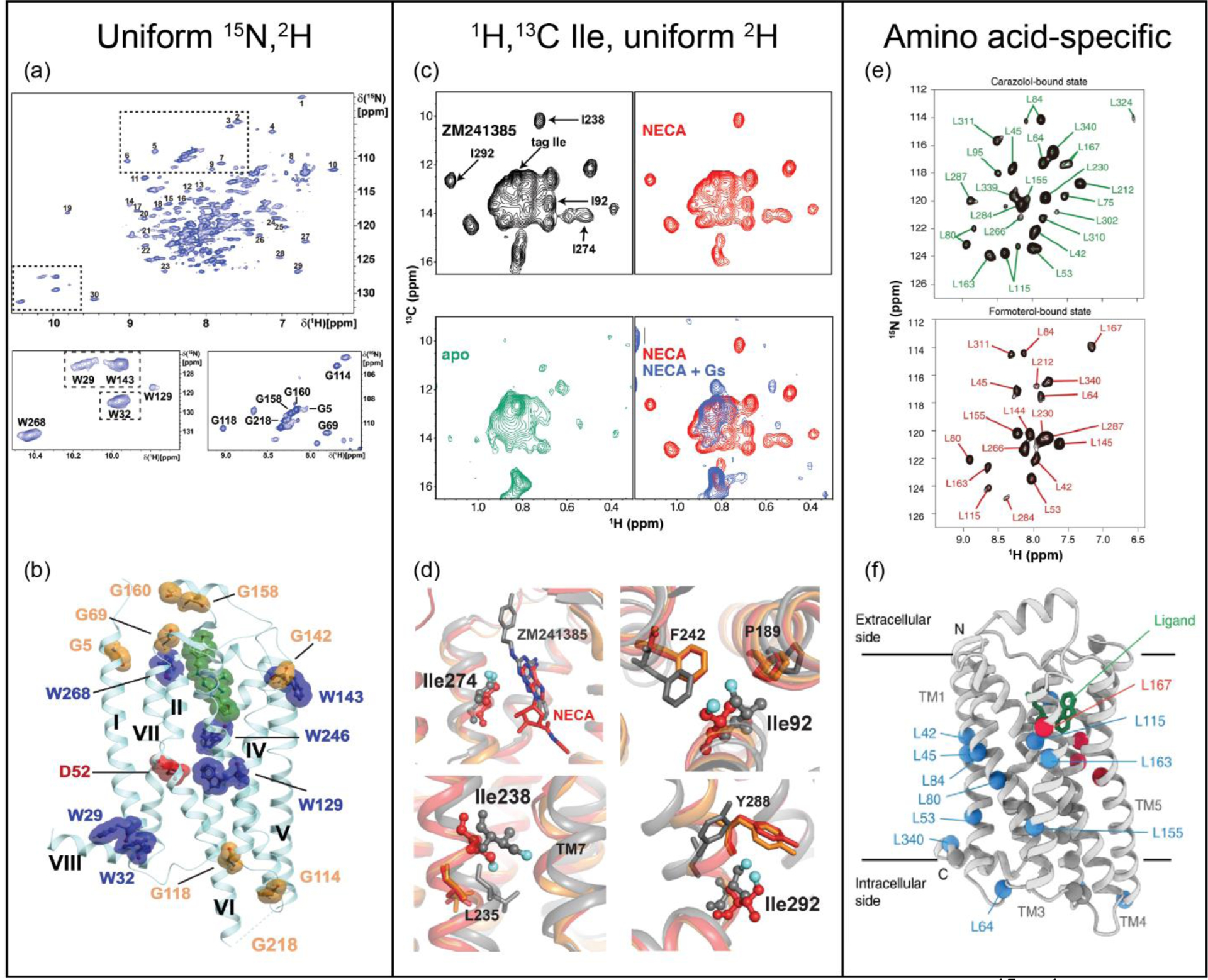
Insights from NMR into ligand-stimulated GPCR activation. (a) 2D [15N,1H]-TROSY spectrum of [u-15N, ~70% 2H]-A2AAR in complex with the antagonist ZM241385. Regions containing Trp indole 15N−1H and Gly backbone signals are expanded. (b) Assigned signals mapped onto an A2AAR crystal structure (PDB 6AQF) with the antagonist ZM241385 shown in green and conserved residue Asp52 in red. (c) [13C,1H]-HMQC spectra of several complexes of [1H,13C-Ile δ1, u-2H]-A2AAR with assigned signals annotated. (d) Superimposed crystal structures of A2AAR in complex with the antagonist ZM241385 (gray, PDB 4EIY), agonist NECA (red, PDB 2YDV), and agonist UK432097 (orange, PDB 3QAK), highlighting regions where significant chemical shift changes were observed. (e) 2D [15N,1H]-TROSY correlation spectra of [2,3,3-2H,15N Leu]-β2AR in complex with the antagonist carazalol and agonist formoterol. (f) Chemical shift differences observed between antagonist- and agonist-bound β2AR mapped onto the crystal structure of β2AR in complex with carazolol (PDB 2RH1); red spheres and blue spheres denote amide signals of leucine residues with chemical shift differences >0.4 ppm and <0.4ppm, respectively. Panels a and b adapted from reference 7, panels c and d adapted from reference 6, and panels e and f adapted from reference 24, with permission.
Adrenergic receptors, targets of catecholamine neurotransmitters, are one of the most studied class A GPCR subfamilies. NMR studies of β2AR have so far exclusively produced the receptor in insect cells and have utilized both chemical modification and biosynthesis stable-isotope labeling approaches. Early studies of the β2AR labeled with ε−13CH3-methionine observed functionally important conformational states not represented among available crystal or cryo-EM structures [20] and demonstrated how drug efficacy influenced the equilibrium of different conformational states [21]. Improvements in signal-to-noise of NMR experiments with β2AR in lipid nanodiscs were obtained by substituting a selected set of amino acids with 2H-labeled amino acids in protein expression media also containing ε−13CH3-methionine [22] or β−13CH3-Alanine [23]. Paramagnetic relaxation enhancement (PRE) experiments with 15N,2H-leucine labeled β2AR yielded a structural model of the agonist-bound receptor that significantly differed from available crystal structures [25] (Figure 1, e and f). NMR studies of the related β1AR incorporated 15N,2H-valines throughout the protein, which enabled visualization of how drug binding altered allosteric networks [12] and characterization of distinct conformers and quantitative measurement of their rates of exchange [13].
NMR studies have identified significant differences among the energy landscapes of class A receptors and propensities for activating specific signaling pathways. Utilizing ε−13CH3-methionine labeling, NMR studies of an α1AA receptor engineered for E. coli expression correlated chemical shifts with ligand efficacies and conformations of receptor microswitches [11]. Microswitches are conserved clusters of amino acids though to play important roles in allosteric transmission of drug binding, as reviewed elsewhere [41]. In contrast, NMR observations of M2R containing ε−13CH3-methionine observed no clear correlations between chemical shifts and the efficacy of bound drugs, suggesting a more complex energy landscape comprising multiple distinct receptor conformations [30]. Stable isotope labeling with ε−13CH3-methionine in combination with reductive methylation of lysine residues [35] and ε−13CH3- methionine in a deuterated background [34] were employed to investigate the effects of ligand pharmacology on μ-OR signaling bias. The intrinsically biased receptor ACKR3 was studied using ε−13CH3-methionine labeling, correlating conformational changes in the extracellular ligand-binding pocket with changes in the intracellular β-arrestin–coupling region [28]. NMR studies of a growing number of class A receptors have provided additional insights (see Table 1).
Investigations of GPCR ternary complexes
NMR experiments have expanded on work with GPCR binary complexes with ligands to studies of ternary complexes with partner signaling proteins. Observations from NMR experiments have provided insights into mechanisms of partner protein recognition and allosteric modulation of partner protein complex formation on orthosteric ligand binding
Single domain antibodies, termed nanobodies, have been used as mimetics of G proteins to investigate GPCR complex formation with partner proteins by structural and biophysical techniques, including NMR spectroscopy [12,15,17,19,20,28,30,33,35,42]. [1H,15N]-TROSY spectra of 15N-valine labeled β1AR in complex with nanobody Nb80 revealed allosteric communication pathways from the receptor’s intracellular surface to the orthosteric binding pocket [12]. β1AR labeled with ε−13CH3-methionine showed rigid receptor dynamics in a ternary complex with agonist and nanobdy Nb6b9 compared to intermediate timescale motion for complexes with agonists alone [15]. A comparison of ε−13CH3-methionine labeled β1AR in complex with Nb80 and the engineered GS protein, ‘mini-Gs”, showed highly similar responses of the receptor in both complexes (Figure 2, a and b) [17].
Figure 2.
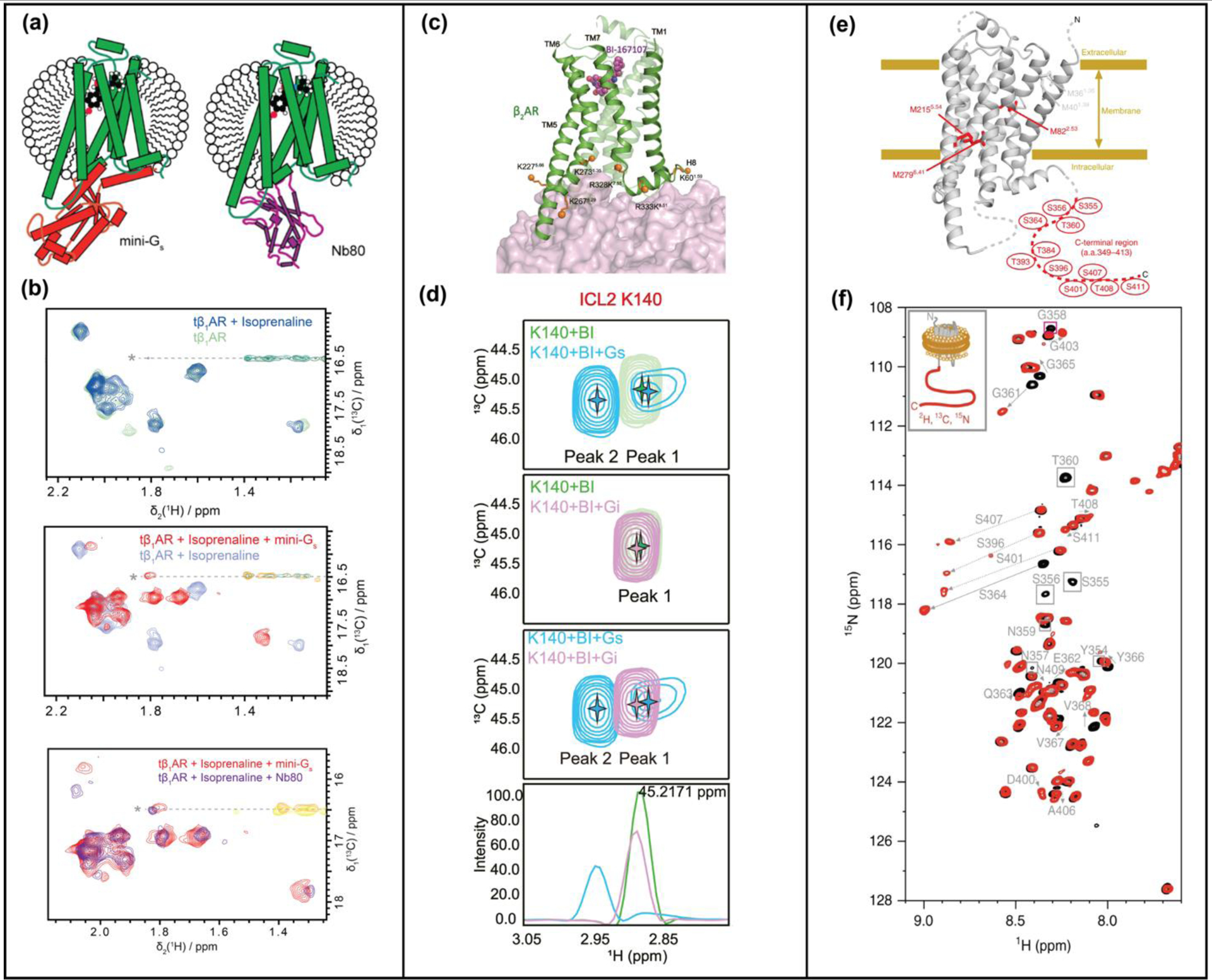
NMR studies of GPCR ternary complexes. (a) Complexes of tβ1AR with an engineered Gs protein (mini-Gs) and G protein-mimicking nanobody, Nb80, compared by NMR. Schematics show β1AR ternary complexes with an agonist and mini-Gs or nanobody NB80. (b) Superimposed [13C,1H]-HMQC spectra of β1AR in complex with an agonist and in ternary complexes with either mini-GS or Nb80. (c) β2AR-G protein interactions studied by reductive methylation of lysines. Lysines used as NMR probes are shown as orange solid spheres on the structure of the β2AR-GS complex (PDB 3SN6). (d) Expanded panels from [13C,1H]-HSQC spectra of β2AR highlighting chemical shift differences of K140between complexes with GS and Gi, indicating involvement of ICL2 in differentiating GS and Gi. (e) Phosphorylated β2AR and β2AR in complex with arrestin studied with ε−13CH3-methionine labeling and segmental labeling. (f) Superimposed [15N,1H]-HSQC spectra of β2AR (black) and phosphorylated β2AR (red) with segmentally-labeled C-terminus. Significant chemical shift changes are indicated with arrows. Panels a and b adapted from reference 17, c and d adapted from reference 19, and e and f adapted from reference 23, with permission.
The structural basis for GPCR-G protein selectivity is not well understood. This problem was explored by NMR with reductively 13C-methylated β2AR to investigate the structural determinants as to why β2AR preferentially forms complexes with GS over GI [19]. Significant chemical shift differences between complexes with GS and Gi were observed for methylated lysine residues located on the intracellular loop 2 (ICL2) of β2AR (Figure 2, c and d) [19]. Interactions between β2AR ICL2 and G proteins were found to be important determinants for selectivity of GS over Gi in signaling complexes [19].
Mechanisms of arrestin-receptor complex formation have also been investigated by NMR spectroscopy. Early studies of [u-15N,2H]-arrestin-1 interaction with rhodopsin observed global structural changes of arrestin-1 upon complex formation and indicated arrestin adopted a dynamic conformational ensemble [43]. A critical step preceding arrestin recruitment is phosphorylation of the disordered receptor C-terminus. The impact of phosphorylation on the conformation of the β2AR C-terminus was studied using a segmentally [13C,15N]-labelled C-terminus covalently attached to the unlabeled receptor TM region using intein chemistry (Figure 2, e and f) [24]. Phosphorylation of the β2AR C-terminus was found to bring the C-terminus proximate to the membrane surface in samples reconstituted in lipid nanodiscs, placing residues in the C-terminus closer to the TM core to facilitate arrestin binding simultaneously to both receptor regions [24].
GPCR-lipid interactions explored by NMR
Increasing evidence from experimental and computational studies highlight the critical impact of lipids on GPCR function both through specific receptor-lipid interactions and by changing the bulk physical properties of the membrane bilayer [44]. NMR studies are investigating these dual roles, utilizing membrane mimetics including lipid nanodiscs and vesicles.
Cholesterol has been thought to modulate GPCR activity both directly as an orthosteric ligand, as in the case with the class F receptor Smoothened [45], and as a potential allosteric modulator [46]. Earlier saturation-transfer NMR experiments showed β2AR associated preferentially with cholesterol over ergosterol [47]. The role of cholesteryl hemisuccinate (CHS), a more soluble analog of cholesterol, has been investigated as a potential negative allosteric modulator of the β1AR (Figure 3, a–c) [14]. Pressure-dependent 1H–15N TROSY spectra of the G protein binding-competent 15N-valine-labelled β1AR complex in the presence and absence of CHS were collected. Combining high-pressure NMR with crystallography, the location of a cavity in the receptor structure was found to correlate with a cholesterol-binding pocket. The presence of CHS was thus shown to prevent this pocket from collapsing and to block conformational changes of activation microswitches [14].
Figure 3.
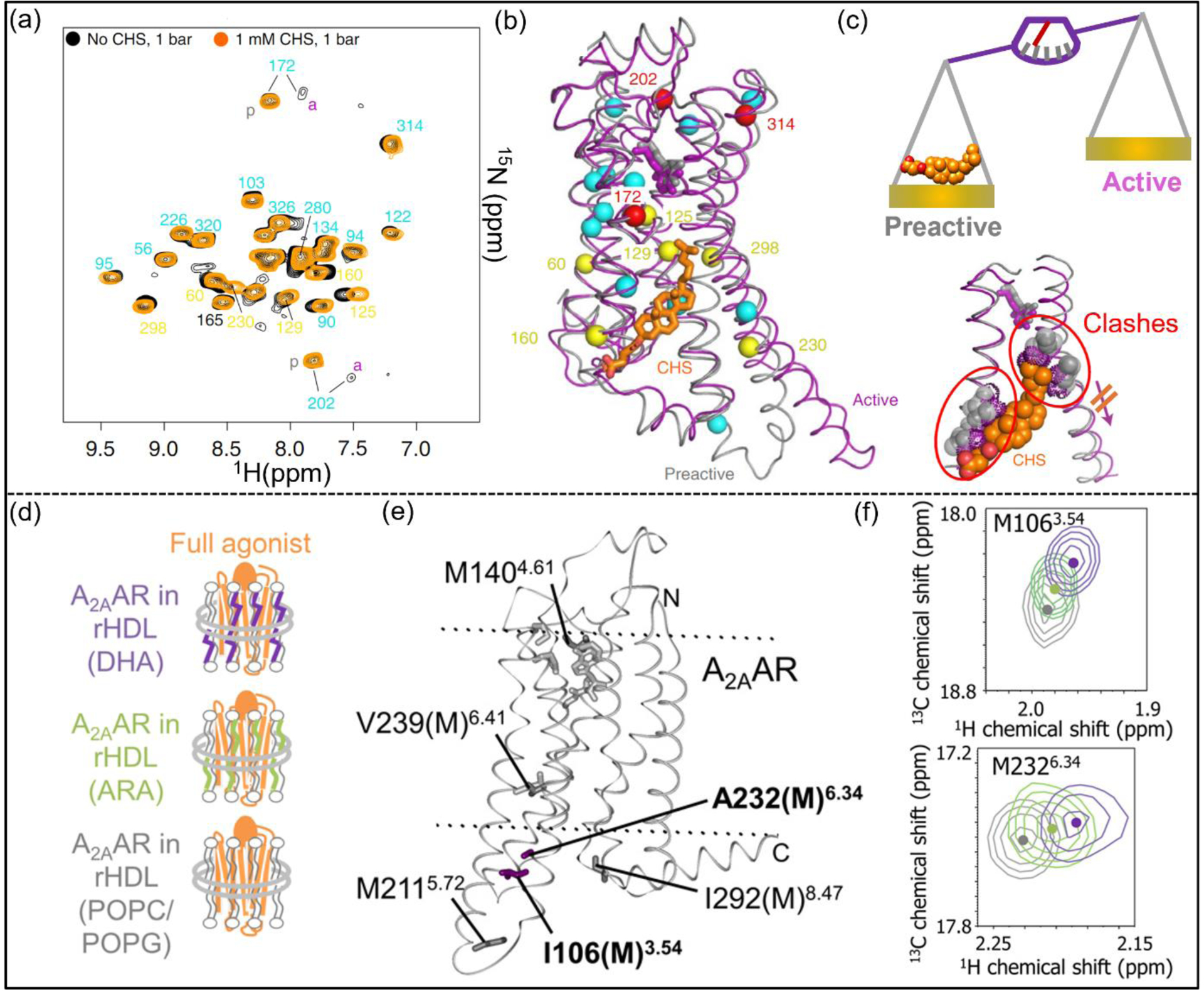
NMR investigations of membrane composition on GPCR function-related dynamics. (a) Superposition of [15N,1H]-TROSY spectra of 15N-valine β1AR in the absence (black) or presence (orange) of CHS. (b) Responses to CHS (orange sticks) mapped onto the crystal structures of pre-active (PDB 2Y03, grey) and active (PDB 6H7J, magenta) β1AR. Valine 15N−1H amides in (a) are shown as spheres colored by their response to CHS (red, increasing pre-active conformation population; yellow, moderate chemical shift change; cyan, small chemical shift change). (c) NMR data and crystal structures provided a view of CHS as a negative allosteric modulator of activation. (d) Schematics of A2AAR reconstituted in nanodiscs containing lipids and DHA or ARA. (e) ε−13CH3-methionine used to monitor the receptor’s response to DHA and ARA. I106(M) and A232(M) showed larger chemical shift differences and are shown as purple sticks. NECA and the less affected methionines are shown as grey sticks. (f) Signals for M106 and M232 in superimposed 1H-13C HMQC spectra. Peak colors correspond to the colored text in (d). Panels a-c adapted from reference 14, and panels d-f adapted from reference 10, with permission.
Observations correlating higher abundance of specific lipids with higher expression of certain GPCRs in some cell types led to the hypothesis that organ-specific GPCR functions may be driven by lipid-receptor interactions. For example, docosahexaenoic acid (DHA) and arachidonic acid (ARA) make up ~14% of the total lipid content in the mammalian brain striatum where A2AAR is also extensively expressed [48]. 2D HMQC spectra of [[α,β,β−2H,methyl-13C] Met,u-2H] A2AAR showed distinct changes for A2AAR in nanodiscs with and without DHA, especially near the intracellular surface in TM3 and TM6 (Figure 3 d–f) [10]. These changes correlated with a significant increase in GTP uptake by G proteins in complex with A2AAR in the same lipid compositions [10].
Conclusions and Outlook
NMR has provided insights into GPCR structural plasticity so far predominantly for class A receptors (Table 1). Future experiments will expand on these initial studies to include more class A subfamilies and additional classes, facilitating comparison of function-related dynamics among more receptors. Exploration of GPCR complex formation with partner proteins by NMR is at the early stages, but initial literature data hint at the promise of NMR to provide improved understanding of the roles of post-translational modifications and membranes in signaling complex formation. Potentially transient complexes difficult to capture by structural techniques, such as GPCR interactions with kinases, may be more amenable to investigation by NMR. Flexible regions involved in the formation of signaling complexes, including the receptor C-terminus, are accessible to NMR and can be independently expressed, stable-isotope labeled and covalently attached to receptor cores via chemical ligation methods [24,49].
An emerging area of research where NMR will likely play a key role are investigations of the impact of the cellular environment, especially lipid membranes, on GPCR structure-function relationships. A seemingly limitless range of membrane and membrane-mimicking environments is accessible to NMR, including micelles, bicelles, nanodiscs for experiments in aqueous solutions, and vesicles for experiments in solids. Integrating data from NMR with cryo-EM structures of receptors in membrane mimetics will likely be a powerful combination to address questions on receptor-lipid interactions, including the affinities of lipids for different structural regions. Ultimately, membrane mimetics may not even be needed. The advent of technologies for enhancing the sensitivity of NMR, including dynamic nuclear polarization [50,51], promises to provide opportunities to study GPCRs directly in situ in their native cellular environments.
Acknowledgements
This work was supported by the National Institutes of Health grant R35GM138291.
Footnotes
Conflict of interest statement
None declared
References
- 1.Gusach A, et al. : Beyond structure: Emerging approaches to study GPCR dynamics. Curr Opin Struct Biol 2020, 63:18–25. [DOI] [PubMed] [Google Scholar]
- 2.Latorraca NR, Venkatakrishnan AJ, Dror RO: GPCR dynamics: Structures in motion. Chem Rev 2017, 117:139–155. [DOI] [PubMed] [Google Scholar]
- 3.Shimada I, et al. : GPCR drug discovery: Integrating solution NMR data with crystal and cryo-em structures. Nat Rev Drug Discov 2019, 18:59–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Picard L-P, Prosser RS: Advances in the study of GPCRs by 19F NMR. Curr Opin Struct Biol 2021, 69:169–176. [DOI] [PubMed] [Google Scholar]
- 5.Didenko T, et al. : Fluorine-19 NMR of integral membrane proteins illustrated with studies of GPCRs. Curr Opin Struct Biol 2013, 23:740–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clark LD, et al. : Ligand modulation of sidechain dynamics in a wild-type human GPCR. Elife 2017, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** First report of ILV-labeled human GPCR produced in a deuterated background revealing fast dynamics induced by different bound ligands
- 7.Eddy MT, et al. : Allosteric coupling of drug binding and intracellular signaling in the A2A adenosine receptor. Cell 2018, 172:68–80. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** NMR data of uniform 2H,15N-labeled GPCR provided global view of the impact of drugs and mutations on activation hotspots and signal transduction
- 8.Eddy MT, et al. : Extrinsic tryptophans as NMR probes of allosteric coupling in membrane proteins: Application to the A2A adenosine receptor. J Am Chem Soc 2018, 140:8228–8235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eddy MT, Martin BT, Wuthrich K: A2A adenosine receptor partial agonism related to structural rearrangements in an activation microswitch. Structure 2021, 29:170–176 e173. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** Structural basis for partial agonism relating distinct conformation of activation hot spot to structural changes at receptor intracellular surface
- 10.Mizumura T, et al. : Activation of adenosine A2A receptor by lipids from docosahexaenoic acid revealed by NMR. Sci Adv 2020, 6:eaay8544. [DOI] [PMC free article] [PubMed] [Google Scholar]; * NMR study relating presence of specific lipids to changes in G protein activity for GPCR in lipid nanodiscs
- 11.Wu FJ, et al. : Probing the correlation between ligand efficacy and conformational diversity at the α1A-adrenoreceptor reveals allosteric coupling of its microswitches. J Biol Chem 2020, 295:7404–7417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Isogai S, et al. : Backbone NMR reveals allosteric signal transduction networks in the β1-adrenergic receptor. Nature 2016, 530:237–241. [DOI] [PubMed] [Google Scholar]; ** NMR data of 15N-valine labeled β1AR show allosteric impact of partner protein binding on the GPCR orthosteric ligand pocket
- 13.Grahl A, et al. : A high-resolution description of β1-adrenergic receptor functional dynamics and allosteric coupling from backbone NMR. Nat Commun 2020, 11:2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abiko LA, et al. : Filling of a water-free void explains the allosteric regulation of the β1-adrenergic receptor by cholesterol. Nat Chem 2022, 14:1133–1141. [DOI] [PubMed] [Google Scholar]; ** Integrating NMR data and crystallography to provide a structural basis for allosteric modulation of a receptor by cholesteryl hemisuccinate
- 15.Solt AS, et al. : Insight into partial agonism by observing multiple equilibria for ligand-bound and Gs-mimetic nanobody-bound β1-adrenergic receptor. Nat Commun 2017, 8:1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Opitz C, Isogai S, Grzesiek S: An economic approach to efficient isotope labeling in insect cells using homemade 15N-, 13C- and 2H-labeled yeast extracts. J Biomol NMR 2015, 62:373–385. [DOI] [PubMed] [Google Scholar]
- 17.Rossler P, et al. : GPCR activation states induced by nanobodies and mini-G proteins compared by NMR spectroscopy. Molecules 2020, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bokoch MP, et al. : Ligand-specific regulation of the extracellular surface of a G-protein-coupled receptor. Nature 2010, 463:108–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ma X, et al. : Analysis of β2AR-Gs and β2AR-Gi complex formation by NMR spectroscopy. Proc Natl Acad Sci U S A 2020, 117:23096–23105. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** Structural basis for β2AR G protein selectivity determined to involve key residues in intracellular loop 2.
- 20.Nygaard R, et al. : The dynamic process of β2-adrenergic receptor activation. Cell 2013, 152:532–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kofuku Y, et al. : Efficacy of the β2-adrenergic receptor is determined by conformational equilibrium in the transmembrane region. Nat Comm 2012, 3:1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kofuku Y, et al. : Functional dynamics of deuterated β2-adrenergic receptor in lipid bilayers revealed by NMR spectroscopy. Angew Chem 2014, 53:13376–13379. [DOI] [PubMed] [Google Scholar]
- 23.Kofuku Y, et al. : Deuteration and selective labeling of alanine methyl groups of β2-adrenergic receptor expressed in a baculovirus-insect cell expression system. J Biomol NMR 2018, 71:185–192. [DOI] [PubMed] [Google Scholar]
- 24.Shiraishi Y, et al. : Phosphorylation-induced conformation of β2-adrenoceptor related to arrestin recruitment revealed by NMR. Nat Commun 2018, 9:194. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Structural basis for arrestin recruitment involved interactions of a phosphorylated receptor C-terminus with the surface of a membrane mimetic.
- 25.Imai S, et al. : Structural equilibrium underlying ligand-dependent activation of β2-adrenoreceptor. Nat Chem Biol 2020, 16:430–439. [DOI] [PubMed] [Google Scholar]; ** NMR PRE experiments provided structural model of a GPCR bound to an agonist with significant differences from available crystal structures.
- 26.Casiraghi M, et al. : Functional modulation of a G protein-coupled receptor conformational landscape in a lipid bilayer. J Am Chem Soc 2016, 138:11170–11175. [DOI] [PubMed] [Google Scholar]
- 27.Yeliseev A: Expression and preparation of a G-protein-coupled cannabinoid receptor CB2 for NMR structural studies. Curr Protoc Protein Sci 2019, 96:e83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kleist AB, et al. : Conformational selection guides β-arrestin recruitment at a biased G protein-coupled receptor. Science 2022, 377:222–228. [DOI] [PMC free article] [PubMed] [Google Scholar]; * NMR observations of β-arrestin recruitment to an atypical chemokine receptor by tuning receptor dynamics through bound ligands.
- 29.Mulry E, Ray AP, Eddy MT: Production of a human histamine receptor for NMR spectroscopy in aqueous solutions. Biomolecules 2021, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu J, et al. : Conformational complexity and dynamics in a muscarinic receptor revealed by NMR spectroscopy. Mol Cell 2019, 75:53–65 e57. [DOI] [PubMed] [Google Scholar]
- 31.Goba I, et al. : Probing the conformation states of neurotensin receptor 1 variants by NMR site-directed methyl labeling. ChemBioChem 2021, 22:139–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bumbak F, et al. : Optimization and 13CH3 methionine labeling of a signaling competent neurotensin receptor 1 variant for NMR studies. Biochim Biophys Acta Biomembr 2018, 1860:1372–1383. [DOI] [PubMed] [Google Scholar]
- 33.Sounier R, et al. : Propagation of conformational changes during mu-opioid receptor activation. Nature 2015, 524:375–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Okude J, et al. : Identification of a conformational equilibrium that determines the efficacy and functional selectivity of the mu-opioid receptor. Angew Chem 2015, 54:15771–15776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cong X, et al. : Molecular insights into the biased signaling mechanism of the mu-opioid receptor. Mol Cell 2021, 81:4165–4175 e4166. [DOI] [PMC free article] [PubMed] [Google Scholar]; *NMR data support structural basis for biased agonism of a GPCR through involvment of intracellular loops and helix 8.
- 36.Clark L, et al. : On the use of pichia pastoris for isotopic labeling of human GPCRs for NMR studies. J Biomol NMR 2018, 71:203–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stehle J, et al. : Characterization of the simultaneous decay kinetics of metarhodopsin states ii and iii in rhodopsin by solution-state NMR spectroscopy. Angew Chem 2014, 53:2078–2084. [DOI] [PubMed] [Google Scholar]
- 38.Pope AL, et al. : A conserved proline hinge mediates helix dynamics and activation of rhodopsin. Structure 2020, 28:1004–1013 e1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ahuja S, et al. : Helix movement is coupled to displacement of the second extracellular loop in rhodopsin activation. Nat Struct Mol Biol 2009, 16:168–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pervushin K, et al. : Attenuated t2 relaxation by mutual cancellation of dipole–dipole coupling and chemical shift anisotropy indicates an avenue to NMR structures of very large biological macromolecules in solution. Proc. Natl. Acad. Sci. USA 1997:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Katritch V, Cherezov V, Stevens RC: Structure-function of the G protein–coupled receptor superfamily. Annu Rev Pharmacol Toxicol 2013, 53:531–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Manglik A, et al. : Structural insights into the dynamic process of β2-adrenergic receptor signaling. Cell 2015, 161:1101–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhuang T, et al. : Involvement of distinct arrestin-1 elements in binding to different functional forms of rhodopsin. Proc Natl Acad Sci 2013, 110:942–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jones AJY, et al. : Structure and dynamics of GPCRs in lipid membranes: Physical principles and experimental approaches. Molecules 2020, 25:4729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Luchetti G, et al. : Cholesterol activates the G-protein coupled receptor smoothened to promote hedgehog signaling. eLife 2016, 5:e20304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van der Westhuizen ET, et al. : Endogenous allosteric modulators of G protein–coupled receptors. J Pharmacol Exp Ther 2015, 353:246–260. [DOI] [PubMed] [Google Scholar]
- 47.Gater Deborah L, et al. : Two classes of cholesterol binding sites for the β2AR revealed by thermostability and NMR. Biophys J 2014, 107:2305–2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xiao Y, Huang Y, Chen Z-Y: Distribution, depletion and recovery of docosahexaenoic acid are region-specific in rat brain. Brit J Nutr 2005, 94:544–550. [DOI] [PubMed] [Google Scholar]
- 49.Li Y, et al. : Chemical synthesis of a full-length G-protein-coupled receptor β2-adrenergic receptor with defined modification patterns at the c-terminus. J Am Chem Soc 2021, 143:17566–17576. [DOI] [PubMed] [Google Scholar]
- 50.Su Y, Andreas L, Griffin RG: Magic angle spinning NMR of proteins: High-frequency dynamic nuclear polarization and 1H detection. Annu Rev Biochem 2015, 84:465–497. [DOI] [PubMed] [Google Scholar]
- 51.Barnes AB, et al. : High-field dynamic nuclear polarization for solid and solution biological NMR. Appl Mag Res 2008, 34:237–263. [DOI] [PMC free article] [PubMed] [Google Scholar]


