Problems in setting up the first trial of an HIV-1 vaccine in Uganda are described here by Mugerwa and colleagues. The authors explain how they solved these problems and give suggestions to help researchers who are starting future trials of HIV vaccines
Trials of the HIV-1 vaccine have been conducted in Europe, North America, Brazil, China, and Thailand.1 The first trial of a candidate vaccine in Africa was recently completed in Uganda. It involved a randomised, placebo controlled trial of a vaccine in healthy volunteers at low risk of HIV infection.2,3 The vaccine, called “ALVAC-HIV,” uses a live recombinant canarypox vector to express envelope and core genes of HIV-1.
Many commentators predicted that it would be difficult to conduct trials of HIV vaccines in developing countries because of scientific, sociobehavioural, ethical, and logistical barriers.4–8 Before we started the trial in Uganda, we gathered data to help us overcome these potential barriers. We collected epidemiological9 and sociobehavioural10 data about people who had participated in studies that looked at preparing for trials of the HIV vaccine. These data showed the prevalence and incidence of HIV, behaviours placing people at risk of becoming infected with HIV, and the social acceptability of a vaccine against HIV.9–11 The people received detailed education and counselling about infection with HIV and about HIV vaccines, and we recruited some for our trial.11 We organised three open workshops at the HIV candidate vaccine trial workshop in Kampala in 1996 to gain consensus from scientists, policy makers, community representatives, and the media about how to undertake research into HIV vaccines.
Despite these initiatives to solve problems before the trial began, we still encountered many barriers. In this article, we discuss these barriers and the strategies that we developed to overcome them.
Summary points
During Africa's first trial of a candidate HIV vaccine, we encountered social, political, legal, and ethical barriers to completing the trial
Widespread public and media fear existed about the risks of participating in the trial, including the risks of becoming infected and being experimented on
The lack of adequate ethical guidelines and a regulatory control mechanism for biomedical research led to delays in approval of the study protocol
Doctors planning trials of HIV vaccines need to interact closely with representatives from the communities involved, the media, volunteers, and other stakeholders
Social, behavioural, and ethical issues
Public misconceptions and media misinformation
Despite elaborate preparations for the Ugandan trial of the HIV vaccine, misconceptions arose about the trial's purpose among the general public and potential volunteers. Between 20 and 30 people infected with HIV, who mistakenly thought that the vaccine was therapeutic, asked to be enrolled in the study. Three themes recurred during weekly meetings of focus groups held for the benefit of potential volunteers: belief that the vaccine would protect against unsafe sex, fear that volunteers were to be injected with HIV, and fear that volunteers would be exposed deliberately to people infected with HIV.
Most of the confusion was due to widespread rumours and conflicting media reports about the vaccine. Many media writers, reporters, and editors seemed not to understand concepts such as the difference between drugs and candidate vaccines. One report in a Ugandan newspaper (New Vision 22 September 1996) referred to ALVAC-HIV as a “candidate drug”; some journalists confused the vaccine with the newly introduced combination of anti-HIV drugs. The latter error led to false hopes of treatment and to demands that ALVAC-HIV be given to as many people as possible. Meanwhile, an ongoing campaign by the Ministry of Health to increase vaccination of children against polio was disrupted by allegations that the polio vaccine was contaminated with HIV.
Political issues and intrigue
Such public stigmatisation of the trial meant that the matter had to be openly debated in parliament and approved by a ministerial cabinet before being sanctioned by the president of Uganda. Many public figures tried to make political gains from the situation, by expressing concerns about Ugandans serving as guinea pigs for experiments that could not be done in Europe or the United States. On the day of the first vaccination, a controversial but influential member of the Ugandan parliament organised a rally, which was broadcast live on a local radio station. “This vaccine should be tried on animals in the National Parks. President Museveni has sanctioned its use on Ugandans in exchange for money to finance his war in the Congo,” he declared, amid wild applause.
Ethical concerns
Before the study began, the Uganda network on law, ethics, and HIV raised concerns about the informed consent process, possible coercion of volunteers, reimbursement, and volunteers' confidentiality. The network wondered how we would ensure that volunteers fully understood the risks inherent in participation during the process of obtaining informed consent. To resolve this potential problem, we translated the consent form into the participants' language and gave them two weeks to read it and ask questions. We also tested their understanding of the trial—volunteers who failed to score at least 70% in these tests were referred for further education at focus group discussions. We felt that certain scientific terminology would be difficult for the general public to understand, so we simplified some research terms by using culturally and socially familiar analogies— “air supply” was used for placebo, “wrapping” for blinding, and “lottery” for randomisation.
To avoid the possibility of coercion, all staff involved in the study were civilian rather than from a military background. The only incentives for participants were treatments for simple conditions such as malaria, free condoms, counselling, lunch, $10 (£7) for the time spent in the trial, and reimbursement of transport costs. With respect to confidentiality, we did not discuss volunteers' participation with their spouses, family members, or friends—this decision was left to the volunteers themselves.
Safety of the trial participants
The safety of participants in the trial became a lively public issue. The annual expenditure on health in Uganda is only $6 per person. Families already overburdened with sick members would have neither the motivation nor the resources to look after relatives falling ill as a result of their participation in the vaccine trial. A woman living with AIDS was reported in a local newspaper as saying, “Researchers should take full responsibility for any consequences of the trial. If volunteers fall ill, let the scientists look after them.”
The vaccine manufacturer had arranged insurance for volunteers experiencing injuries related to the vaccine. Volunteers who tested positive for antibodies to HIV because of the vaccine were to be offered special participation cards to protect from being discriminated against for immigration, insurance, and employment purposes. Some potential volunteers declined to participate because of fears about being ineligible for future vaccine trials and the prospect of inadequate compensation for their families if they died.
The high prevalence of infection with HIV in some poor countries, combined with such countries' inadequate resources for purchasing antiretroviral medications, makes them ideal testing sites for candidate vaccines. Ugandans were concerned about reports in the foreign media that manufacturers might choose to trial vaccines in poor countries to reduce product liability in case of injury or to exploit weaker legal, ethical, or regulatory mechanisms for conducting biomedical research.
A newspaper report (New Vision 1 March 1997) asked whether the trial was an example of “hit and run research” by scientists from rich countries, in which a poor country was chosen as the setting because the study would be cheaper and fewer questions would be asked about safety and ethics. A bishop of the Church of Uganda warned that the church would resist trials of HIV vaccines if safety measures for volunteers were not guaranteed. “People made in the image of God are not to be used as objects to be disposed of when experiments fail,” he commented during a church sermon.
Scientific issues
The risks to volunteers
We believe that some objections raised in the research community and among medical professionals were of importance; others were due to jealousy on the part of professionals not involved in the trial. For example, three prominent Ugandan scientists—a geneticist, a virologist, and an immunologist—raised fears that the vaccine might undergo dangerous mutations that could lead people to develop strange abnormalities. They further argued that the virus might replicate in vaccinated individuals, causing disseminated disease, and that the individuals would then infect their partners through sexual transmission, since the vaccine uses a live vector. To these doctors, the trial was comparable to the infamous Tuskegee experiment, in which American researchers investigating the course of syphilis denied treatment to African-American patients deliberately and without the patients' knowledge. The three scientists claimed that the motive of our trial was to spread HIV and other viruses among uninfected Ugandans and eventually to wipe out the African race, even though a major safety feature of canarypox is its inability to replicate in mammals.12
The disagreements between scientists created apprehension among the public. Attempts to clear up the misunderstandings and allay the anxiety delayed the start of the trial. The delay caused further speculation about the worthiness of the vaccine, as the public wondered why the trial was taking so long to start. On 11 September 1996, New Vision quoted experts as saying that the candidate vaccine was safe; this seemed to contradict an earlier report in the same paper, which stated that the main aim of the trial was to evaluate safety in local conditions. The public became distrustful, with some asking why, if the vaccine was safe, were trials being conducted in Uganda. The public could not understand that, although no adverse effects had been found when the HIV vaccine was tested elsewhere, its safety for the indigenous peoples of Africa could not be assumed.
Recommendations based on the Ugandan experience
Many countries lack regulatory mechanisms and a legal framework for conducting biomedical research. This, together with absent or inadequate ethical guidelines, poses problems that hinder reviews and approvals of research. Any country wishing to undertake research into HIV vaccines needs to have a national plan that clearly addresses these issues. The recently formulated ethical guidelines by the joint United Nations programme on HIV and AIDS (UNAIDS) can be used as a basis for developing local guidelines.13 National regulatory agencies must be set up early enough to assess the quality, safety, and potential efficacy of new vaccines against HIV.
A related issue is the need to have at least two committees with appropriate representation to review ethical and scientific aspects of study protocols. In Uganda, the protocol for the trial had to be approved by six different local committees—ethics, science, technology, biosafety, drug authority, and social services—because there was no national regulatory and control mechanism for conducting research into HIV vaccines. This meant that no single committee was considered competent enough to handle this new and sensitive subject. The time taken to form the committees delayed the date that the first participant was enrolled by one year.
The local population has confidence in trials when national scientists are seen to participate actively in the process of developing vaccines. These national scientists should be involved, therefore, in all scientific discussions, including those about the choice of vaccine construct and study design and about implementation of the study. Early negotiations with funding and collaborating institutions should include delicate issues such as patent rights, intellectual property, compensation for injuries induced by the vaccine, appropriate treatment for volunteers who become infected with HIV naturally, and, where relevant, availability of the vaccine for the participating country once it is licensed.
Lack of information leads to misinformation. Politicians and other community leaders should be given information to encourage public support for trials of vaccines and to reassure potential study volunteers. An information strategy for the public, volunteers, and the media—who should be given regular progress reports on various aspects of the trial—is essential. An information desk to issue periodic bulletins and correct misinformation appearing in the electronic or printed media can help avert crises. An advisory board including representatives of the local community may provide a critical link between researchers and the public. Focus group discussions provide an opportunity for trial volunteers to air their concerns and for researchers to understand volunteers' problems and provide information about the ongoing study as well as about trials in other countries.
Conclusion
Before researchers start any trials of HIV vaccines, they need to gain support from politicians, the media, and the general public. Respected local scientists must be involved in the planning and implementation of studies to enhance their credibility and to identify cultural or logistical issues that might hinder recruitment. All stakeholders must be involved in discussions about important scientific, social, legal, ethical, and other concerns before the study begins. The success of future trials of HIV vaccines in poor countries depends on adequate mechanisms of regulatory control as well as a clearly defined approval process for research protocols.
Supplementary Material
Figure.
GIDEON MENDEL/NETWORK
Election posters for President Museveni of Uganda, who was accused of sanctioning the use of a trial HIV vaccine on Ugandans in exchange for money to finance war in the Congo
Figure.
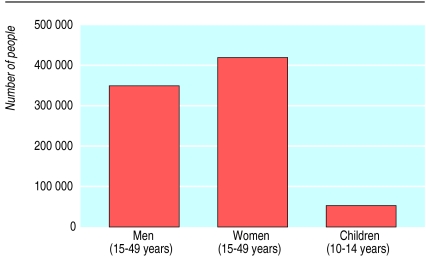
Estimated number of people living with HIV and AIDS in Uganda at the end of 1999. Data from UNAIDS/WHO epidemiological fact sheet on HIV/AIDS and sexually transmitted infections. Uganda. 2000 update
Figure.
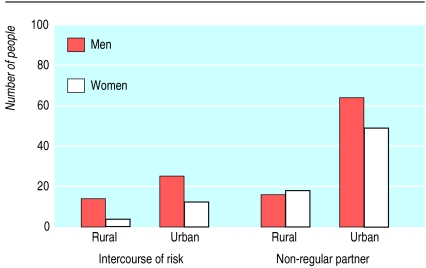
Proportion of people reporting use of condom during most recent “intercourse of risk” (left) and proportion of sexually active people having at least one sexual partner other than their regular partner in the past 12 months (right) in rural and urban areas of Uganda in 1995. Data from UNAIDS/WHO epidemiological fact sheet on HIV/AIDS and sexually transmitted infections. Uganda. 2000 update
Acknowledgments
We thank the study volunteers for their patience, understanding, and enthusiasm about being involved in this landmark trial. We thank the laboratory and clinical staff of the Joint Clinical Research Center and the Uganda Virus Research Institute. We acknowledge the support of the Ugandan Ministry of Health and UNAIDS. We thank Dr Harriet Mayanja for her advice and contribution to the drafting of this paper.
Footnotes
Funding: Funding was provided by the US National Institute of Allergy and Infectious Diseases (AI-35173 and AI-36219) and Fogarty International Center (TW-00011), Bethesda, Maryland, with support from the Government of Uganda and UNAIDS, Geneva, Switzerland. The vaccine was provided by Aventis-Pasteur (formerly Pasteur Merieux Connaught), Lyons, France.
Competing interests: None declared.
Further information about contributors appears on bmj.com
References
- 1.Esparza J, Bhamarapravati N. Accelerating the development and future availability of HIV-1 vaccines: why, when, where, and how. Lancet. 2000;355:2061–2066. doi: 10.1016/S0140-6736(00)02360-6. [DOI] [PubMed] [Google Scholar]
- 2.Kebba A, Hom DL, Mugyenyi P, Salata R, Mbidde E, Kaleebu P, et al. Phase I vaccine study of the ALVAC-HIV vCP205 in healthy Ugandan adults [abstract]. XIII International Conference on AIDS, Durban, South Africa, July 9-14, 2000.
- 3.Salmon-Ceron D, Excler JL, Finkielsztejn L, Autran B, Gluckman JC, Sicard D, et al. Safety and immunogenicity of a live recombinant canarypox virus expressing HIV type 1 gp120 MN MN tm/gag/protease LAI (ALVAC-HIV, vCP 205) followed by a p24E-V3 MN synthetic peptide (CLTB-36) administered in healthy volunteers at low risk for HIV infection. AIDS Res Hum Retrovir. 1999;15:633–645. doi: 10.1089/088922299310935. [DOI] [PubMed] [Google Scholar]
- 4.Lurie P, Bishaw M, Chesney MA, Cooke M, Fernandes ME, Hearst N, et al. Ethical, behavioural, and social aspects of HIV vaccine trials in developing countries. JAMA. 1994;271:295–301. [PubMed] [Google Scholar]
- 5.Temoshok LR. Behavioral research contributions to planning and conducting HIV vaccine efficacy studies. AIDS Res Hum Retroviruses. 1994;10 (Suppl 2):S277–S280. [PubMed] [Google Scholar]
- 6.Berkley S. HIV vaccine development for the world: an idea whose time has come? AIDS Res Hum Retroviruses. 1998;14 (Suppl 3):S191–S196. [PubMed] [Google Scholar]
- 7.Excler JL, Beyer C. Human immunodeficiency virus vaccine development in developing countries: are efficacy trials feasible. J Hum Virol. 2000;3:193–214. [PubMed] [Google Scholar]
- 8.Mbidde E. Bioethics and local circumstances. Science. 1998;279:155. doi: 10.1126/science.279.5348.155a. [DOI] [PubMed] [Google Scholar]
- 9.Mugyenyi P, Hom DL, Loughlin A, Johnson JL, McGrath JL, George K, et al. HIV-1 seroprevalence, seroincidence and risk behaviour in the Ugandan military [abstract]. XI International Conference on AIDS, Vancouver, Canada, 1996.
- 10.Hom DL, Johnson JL, Mugyenyi P, Byaruhanga R, Kityo C, Louglin A, et al. HIV-1 risk and vaccine acceptability in the Ugandan Military. J Acquir Immune Def Synd Hum Retrovir. 1997;15:375–380. doi: 10.1097/00042560-199708150-00008. [DOI] [PubMed] [Google Scholar]
- 11.McGrath JW, George K, Svilar G, Ihler E, Mafigiri D, Kabugo M, et al. Developing AIDS vaccine trials education programs in Uganda. J Acquir Immune Def Synd Hum Retrovir. 2001;26:176–181. doi: 10.1097/00042560-200102010-00012. [DOI] [PubMed] [Google Scholar]
- 12.Plotkin SA, Cadoz M, Meignier B, Meric C, Leroy O, Excler JL, et al. The safety and use of canarypox vectored vaccines. Dev Biol Stand. 1995;84:165–170. [PubMed] [Google Scholar]
- 13.Joint United Nations Programme on HIV/AIDS (UNAIDS) Ethical considerations in HIV preventive vaccine research. Geneva: Joint United Nations Programme on HIV/AIDS (UNAIDS); 2000. www.unaids.org/publications/documents/vaccines/vaccines/JC072-EthicalCons-E.pdf (accessed 29 Nov 2001). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.