Abstract
The COVID-19 pandemic has resulted in many therapies, of which many are repurposed and used for other diseases in the last decade such in Influenza and Ebola. We intend to provide a robust foundation for cardiovascular outcomes of the therapies to better understand the rationale for the clinical trials that were conducted during the COVID-19 pandemic, and to gain more clarity on the steps moving forward should the repurposing provide clinical benefit in pandemic situations. With this state-of-the-art review, we aim to improve the understanding of the cardiovascular involvement of the therapies prior to, during, and after the COVID-19 pandemic to provide meaningful findings to the cardiovascular specialists and clinical trials for therapies, moving on from the period of pandemic urgency.
Keywords: COVID-19, Investigational therapies, Cardiovascular, Clinical trials, Long covid
1. Introduction
At the end of 2019, an outbreak of pneumonia with unknown etiology occurred in Wuhan, a city in the Hubei province in China.1 The patients were later on found to have developed pneumonia due to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The outbreak spread rapidly and was announced to be a Public Health Emergency of International Concern by the WHO at the end of January and known as coronavirus disease 2019 (COVID-19).2 Approximately 80 % of COVID-19 patients suffer from mild respiratory infections and do not require hospitalization. However, about 15 % of the patients suffer from moderate to severe pneumonia requiring supportive therapy and close monitoring. The remaining 5 % suffer from serious conditions and require intensive care treatment.3 Acute respiratory distress syndrome (ARDS) and multiple organ dysfunction syndrome (MODS) are serious complications of COVID-19 which are associated with high morbidity and mortality with no successful therapy for COVID-19 till date.4
Various clinical trials were conducted around the world for the discovery of an effective treatment for COVID-19 as part of emergency use authorization (EUA) by the Food and Drug Administration (FDA). These therapies are being assessed for their risk–benefit profile and may be associated with severe adverse effects that may overlap with the clinical features of COVID-19.6 Drugs such as hydroxychloroquine (HCQ) and chloroquine (CQ), earlier assessed as an emerging therapy in COVID-19, has increased the likelihood of cardiac arrhythmias. 5 Therapies should be used carefully in patients with underlying cardiovascular conditions and echocardiography must be done in severely ill patients before their use.6,7 Careful use of these drugs with other drugs that are associated with prolongation of QT intervals (e.g. azithromycin) and cardiac arrhythmias should be considered.8–10 Similarly, lopinavir-ritonavir can also prolong the QT interval and increase the possibility of bradycardia in the elderly and severely-ill COVID-19 patients, having been eliminated as an emerging therapy since evidence that the risks outweighed the benefits.11 Various interactions among drugs and diseases should be taken into account before their use in COVID-19 patients. In this review, we appraised emerging drugs in the management of COVID-19 and their potential cardiovascular implications.
2. Methods
A scoping review was performed using Medline and Scopus to identify relevant articles. The following search terms were used using Boolean logic: cardiovascular, in combination with COVID-19, corticosteroids, remdesivir, favipiravir, ivermectin, oleandrin, convalescent plasma, monoclonal antibodies, recombinant ACE-2, cytokine inhibitors, interferon, and stem cells. The search was conducted independently for each therapy or drug and the final results were 322 articles. Studies were incorporated which reported the cardiovascular outcomes. The authors independently screened the titles and abstracts to determine eligibility. Lateral entries were selected by conducting an umbrella review of the reference list of the selected studies.
3. Cardiovascular effects of COVID-19 therapies
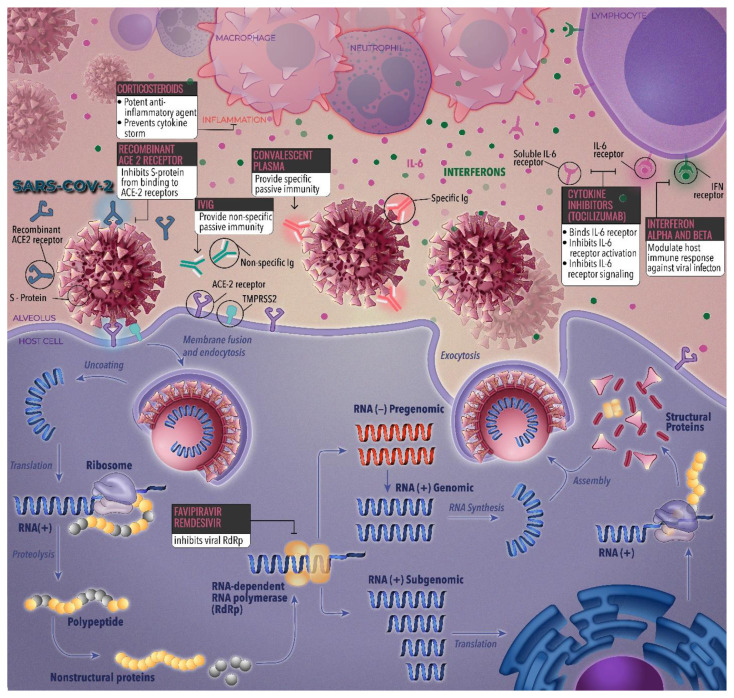
Therapies for the treatment of COVID-19 have been approved by the Food and Drug Administration (FDA) in the United States. Of these therapies, remdesivir was promising whereas other therapies were tentatively effective. Fig. 1 identifies select emerging therapies and their targets in the viral life cycle of severe acute coronavirus 2 syndrome (SARS-CoV-2).
Fig. 1.
Drug targets of select emerging therapies across the viral life cycle of SARS-CoV-2.
3.1. Corticosteroids
Corticosteroids are well-tolerated in treating COVID-19 and are being examined for their therapeutic efficacy at different stages of the disease. 12 Their downside includes an increased risk of secondary infections.13 Corticosteroids work by reducing the expression of ACE2 and TMPRSS2, which facilitates the entry of the virus into host cells.14 They may also be effective in treating complications like secondary pericarditis, myocarditis, and acute respiratory distress syndrome (ARDS).12,15 An Argentine trial evaluated high-dose dexamethasone for ARDS.16 However, the treatment comes with risks, including hypertension and insulin resistance.17
3.2. Favipiravir
Favipiravir acts as an RNA-dependent RNA polymerase inhibitor and shows broad antiviral activity against various RNA viruses.18,19,20 It is approved in Japan primarily for influenza.21,22 Early studies indicate its efficacy against SARS-CoV-2.19 A trial conducted by Peking University involved multiple centers and looked into its effectiveness in COVID-19.24,25 The drug has been associated with possible mild QT prolongation, although the evidence is limited.23
3.3. Remdesivir
Remdesivir is an FDA-approved drug used for hospitalized COVID-19 patients but has been associated with hepatotoxicity.26–30 One study reported that some patients fared better without the drug.32 It can also cause hypokalemia, which may lead to cardiac arrhythmias.31
3.4. Ivermectin
Ivermectin is known for its anti-parasitic properties and has demonstrated antiviral activity in vitro.33–35 It is approved by the FDA for treating parasitic infections but has not been endorsed for COVID-19 treatment.36,42 Generally, side effects are mild and often associated with parasite load.37–40 A study observed no significant new ECG abnormalities in a cohort with pre-existing cardiac issues.41
3.5. Oleandrin
Oleandrin, a cardiac glycoside derived from the Nerium oleander L. plant, is known for its cytotoxic properties and used in treating cardiac issues.43 It operates by inhibiting the Na–K ATPase enzyme and other cellular pathways.44 In Vero cell experiments against COVID-19, it substantially reduced viral production.45 The compound also has anti-inflammatory effects potentially beneficial in COVID-19 management.46,47 However, it can cause side effects like cardiac arrhythmias.48,49
3.6. Immunoglobulin therapy
Convalescent plasma and immunoglobulins have shown limited effectiveness in treating COVID-19 despite Emergency Use Authorization (EUA) by the FDA.50 Previous uses in other disease outbreaks had minor side effects but were generally safe.51–54 Transfusion-associated cardiac overload (TACO) is an identified risk, especially in vulnerable populations.55,56 Human monoclonal antibodies targeting IL-6 pathways are under study but may lead to dyslipidemia.57
3.7. Recombinant ACE-2
Angiotensin converting enzyme-2 (ACE-2) is a form of transmembrane metallo carboxypeptidase which is closely related to ACE, the main enzyme in RAS. This is also a primary treatment method for hypertension and other similar cardiac conditions. Recombinant ACE-2 is created through genetic modification of human ACE-2 (genetic sequencing: Gln 18–247 Ser 740) bound with polyhistidine at the C-terminal.58 ACE-2 is a precursor to angiotensin, a protein that is crucial in regulation of blood pressure, vascular health and vasomotor tone. ACE2 is present in various areas in the human body, including the vascular endothelial cells in lung and cardiac cells, kidney tubules, small and large intestines. A depletion of ACE-2 is strongly associated with hypertension, heart failure, coronary artery disease, diabetes. A recombinant vaccine combining elements of avian viruses with SARS-CoV-2 has been developed for intranasal administration.59 Promising results have been shown in animal studies.60,61
3.8. Cytokine inhibitors
COVID-19 often triggers a cytokine storm, leading to severe complications.62–65 Various IL-1 receptor antagonists, such as Anakinra and Canakinumab, are being trialed for their ability to mitigate this response.66–68 The drugs have shown promising results in reducing inflammation and improving clinical outcomes.68,69 The CANTOS trial further validates the cardiovascular safety profile of canakinumab. 70 Tocilizumab, an IL-6 receptor antagonist, has also shown efficacy but requires monitoring due to potential cardiovascular effects and drug interactions.71–75 Clinical trials with TNF-alpha blockers like adalimumab are also underway.67
3.9. Interferon alpha
Pegylated Interferon -Alpha, which was previously used in the management of SARS & MERS, was studied for the treatment of COVID-19.76,77 However, in a study involving 295 patients with chronic HCV, adverse cardiovascular effects were reported. There were six patients who had cardiac effects during the interferon therapy and four others who reported adverse effects within one year of the therapy.78 Increase in TNF-α during INF-α therapy may be the cause and underlying mechanism for the adverse cardiac effects.79 Another study of 194 patients with chronic hepatitis C reported cardiovascular complications (18 %) with pegylated interferon-alpha therapy.80 The arrhythmogenic property may be attributed to local inflammation in the conduction system induced by interferon.81,82 Hence, prudent use of interferon alpha was recommended in high-risk patients of COVID-19.
3.10. Interferon-beta
Interferon beta has been used in the treatment of multiple sclerosis.83 Studies on the efficacy of treatments for SARS and MERS provide insight into options for potential repurposing of these drugs for SARS-CoV-2 treatment.84,85 However, it was reported that interferon beta, especially type 1 interferons, accelerates atherosclerotic changes.86 A randomized clinical trial of 81 patients of COVID-19 reported 28.4 % of adverse cardiovascular events.87 This called for further detailed assessment of cardiovascular risk in patients treated with Type-1 IFN.
3.11. Stem cells
When severe, COVID-19 is a systemic illness characterized by hyper-inflammation, cytokine storm, and elevations of cardiac injury biomarkers.4 Structurally, both viruses use the ACE2 receptor to enter cells and bind with similar affinities to ACE2, therefore given these similarities to SARS-CoV, it is plausible that SARS-CoV-2 could also use ACE2 to enter adult cardiomyocytes.88 One study utilized human induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CMs) as a model to examine the mechanisms of cardiomyocyte-specific infection by SARS-CoV-2.88 Another study aimed to test whether the viral RNA of SARS-CoV-2 can be transmitted via extracellular vesicles (EVs) into cardiomyocytes.89 A case series by Singh et al. explored the safety and effectiveness of intravenous allogeneic cardiosphere-derived cells (CDCs).90
4. Discussion
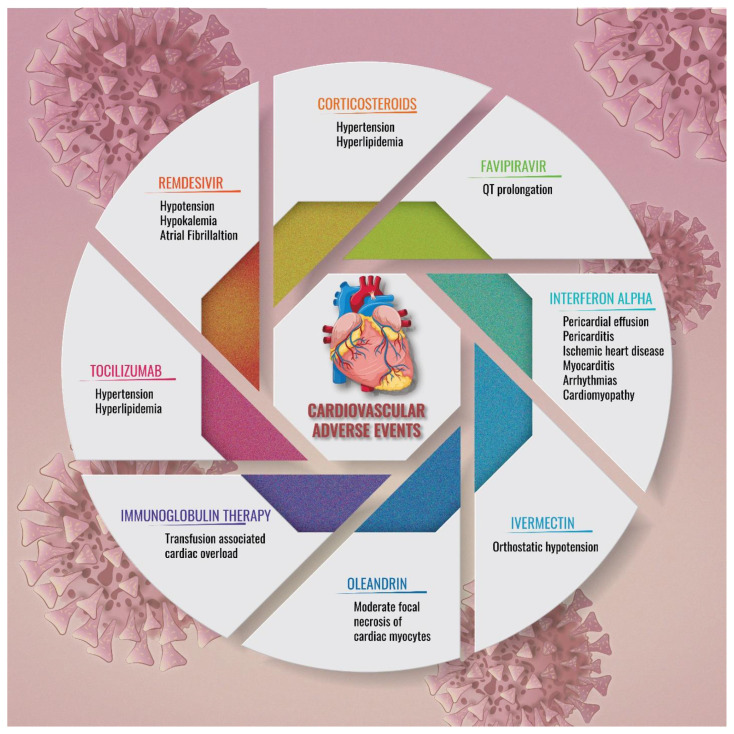
While pulmonary involvement is predominant in COVID-19, and as we move on from the COVID-19 pandemic, there are accumulating evidence of severe manifestations of the cardiovascular system. This may be of essence as we cater to cases of long COVID. The role of underlying cardiovascular conditions in precipitating the cardiovascular involvement during and after COVID-19 infection is of concern but remains unclear. Associations with acute kidney injury has been observed in nearly 60 % of COVID-19 who are hospitalized.91 Suspected contributors include i) acute hypoxemia, tachycardia and hypoxemia due to the pulmonary involvement in COVID-19 leading to type 2 myocardial involvement, ii) induction of hypercoagulability leading to acute atherothrombosis and acute coronary syndrome, iii) stress-induced cardiomyopathy known as Takotsubo syndrome, and iv) direct or indirect myocardial injury due to viral invasion.91 Fig. 2 summarizes the potential cardiovascular adverse outcomes due to COVID-19 therapies.
Fig. 2.
Adverse cardiovascular outcomes of therapies in COVID-19.
Using clinicaltrials.gov as a reference, various studies have utilized the following primary outcomes: time to 2-point reduction of symptoms, time to return of olfactory sensation, and whether or not the patient will require ICU treatment of invasive mechanical ventilation during the hospital course. Aside from corticosteroids, numerous studies established the efficacy of antiviral drugs such as favipiravir and remdesivir. For these two therapeutic treatments over hundreds of trials worldwide were conducted; the common primary outcomes comprised time to repeat negative testing on RT-PCR of a nasopharyngeal swab for SARS-CoV-2. Other treatment approaches were considered including ivermectin, oleandrin, recombinant ACE-2, and cytokine inhibitors such as tocilizumab.
The World Health Organization defines Long COVID as “the continuation or development of new symptoms 3 months after the initial SARS-CoV-2 infection, with these symptoms lasting for at least 2 months with no other explanation”.92 The use of the mentioned therapies in patients with long COVID, who are experiencing cardiovascular manifestations, has both potential benefits and risks. Careful consideration must be taken when planning the course of treatment for such patients. The following are implications of the discussed treatments:
Interferon Alpha: This treatment has been linked to adverse cardiovascular events such as ischemic heart disease, arrhythmias, and cardiomyopathy. Although it could potentially inhibit viral replication, its use should be approached with caution in patients who have a history of cardiovascular disease or are at high risk for such conditions. Regular monitoring for cardiac effects during and after therapy is necessary.
Interferon Beta: Despite its potential antiviral efficacy, this treatment may accelerate atherosclerotic changes, which could lead to increased cardiovascular risks. Therefore, the potential benefits need to be weighed against these risks, particularly in patients with pre-existing cardiovascular conditions or who are at high risk of such conditions.
Stem cells: Preliminary evidence indicates potential benefits of stem cell therapies in severe cases of COVID-19, with associated reductions in pro-inflammatory biomarkers. However, more robust, large-scale trials are needed to establish the safety and efficacy of these therapies in the management of long COVID with cardiovascular manifestations.
In general, patients with long COVID experiencing cardiovascular manifestations are recommended the following:
Prioritize comprehensive care: Because long COVID can affect multiple organ systems, patients, and their primary care providers should prioritize comprehensive, multidisciplinary care. This care team might include a primary care doctor, cardiologist, neurologist, pulmonologist, and possibly a mental health professional.
Engage in cardiovascular protective activities: This includes following a heart-healthy diet, engaging in regular physical activity, avoiding tobacco and excessive alcohol, managing stress, and keeping other health conditions like diabetes and hypertension under control.
Active monitoring: Regular follow-ups with healthcare providers for the monitoring of cardiovascular health and the effectiveness of treatment interventions are essential. This can help in early identification and management of potential complications.
Participate in rehabilitation programs: Some patients with long COVID may benefit from participating in rehabilitation programs, which can provide specialized physical and occupational therapy to help manage persistent symptoms and improve daily functioning.
Stay informed about emerging treatments: The knowledge about long COVID is continuously evolving, and new treatments are being tested. It is pertinent for patients to stay informed about these developments and discuss potential benefits and risks with their healthcare provider.
The ongoing research on long COVID is critical in understanding the disease and its effects better, which would pave the way for the development of more targeted and effective therapies. Until then, personalized care, close monitoring, and maintaining a healthy lifestyle are key to managing the condition.
5. Conclusion
The review provides a global perspective for the potential cardiovascular involvement and outcomes of COVID-19 therapies, while presenting implications for long COVID. With many treatments being repurposed for COVID-19 and used for different diseases earlier, there is ample data during, prior to, and after the COVID-19 pandemic that is indicative of the potential cardiovascular outcomes. While clinical trials are underway addressing the growing medical needs of those afflicted with long-term outcomes of the disease and therapies, this review provides a comprehensive view into the cardiovascular interactions of the therapies in context with COVID-19.
Footnotes
Author contributions: The authors contributed and are assigned authorship as per ICMJE guidelines.
Conflicts of interest: The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding: No funding was received for this study in any form.
References
- 1. Wu YC, Chen CS, Chan YJ. The outbreak of COVID-19: an overview. J Chinese Med Assoc. 2020 doi: 10.1097/JCMA.0000000000000270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO. Events as they happen. Rolling updates on coronavirus disease (COVID-19) Who; [Google Scholar]
- 3. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese center for disease control and prevention. JAMA, J Am Med Assoc. 2020;323(13):1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 4. Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020 doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wu CI, Postema PG, Arbelo E, et al. SARS-CoV-2, COVID-19, and inherited arrhythmia syndromes. Heart Rhythm. 2020;17(9):1456–1462. doi: 10.1016/j.hrthm.2020.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Roden DM, Harrington RA, Poppas A, Russo AM, Russo AM. Considerations for drug interactions on QTc in exploratory COVID-19 treatment. Circulation. 2020:E906–E907. doi: 10.1161/CIRCULATIONAHA.120.047521. [DOI] [PubMed] [Google Scholar]
- 7. Kamp TJ, Hamdan MH, January CT. Chloroquine or hydroxychloroquine for COVID-19: is cardiotoxicity a concern? J Am Heart Assoc. 2020;9(12):e016887–e016887. doi: 10.1161/JAHA.120.016887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bessière F, Roccia H, Delinière A, et al. Assessment of QT intervals in a case series of patients with coronavirus disease 2019 (COVID-19) infection treated with hydroxychloroquine alone or in combination with azithromycin in an intensive care unit. JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mercuro NJ, Yen CF, Shim DJ, et al. Risk of QT interval prolongation associated with use of hydroxychloroquine with or without concomitant azithromycin among hospitalized patients testing positive for coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020. [DOI] [PMC free article] [PubMed]
- 10. Nguyen LS, Dolladille C, Drici MD, et al. Cardiovascular toxicities associated with hydroxychloroquine and azithromycin: an analysis of the world health organization pharmacovigilance database. Circulation. 2020;142(3):303–305. doi: 10.1161/CIRCULATIONAHA.120.048238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Christophe B, Nicolas M, Alexis H, Osama AA, Yazine M. Lopinavir-ritonavir treatment for COVID-19 infection in intensive care unit. Circ Arrhythmia Electrophysiol. 2020;13(8):e008798. doi: 10.1161/CIRCEP.120.008798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Imazio M, Brucato A, Lazaros G, et al. Anti-inflammatory therapies for pericardial diseases in the COVID-19 pandemic: safety and potentiality. J Cardiovasc Med (Hagerstown) 2020;21(9):625–629. doi: 10.2459/JCM.0000000000001059. [DOI] [PubMed] [Google Scholar]
- 13. Ragni E, Mangiavini L, Viganò M, et al. Management of osteoarthritis during the COVID-19 pandemic. Clin Pharmacol Ther. 2020;108(4):719–729. doi: 10.1002/cpt.1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Peters MC, Sajuthi S, Deford P, et al. COVID-19-related genes in sputum cells in asthma: relationship to demographic features and corticosteroids. Am J Respir Crit Care Med. 2020;202(1):83–90. doi: 10.1164/rccm.202003-0821OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Coyle J, Igbinomwanhia E, Sanchez-Nadales A, Danciu S, Chu C, Shah N. A recovered case of COVID-19 myocarditis and ARDS treated with corticosteroids, tocilizumab, and experimental AT-001. Case Reports. 2020;2(9):1331–1336. doi: 10.1016/j.jaccas.2020.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Maskin LP, Olarte GL, Palizas F, et al. High dose dexamethasone treatment for Acute Respiratory Distress Syndrome secondary to COVID-19: a structured summary of a study protocol for a randomised controlled trial. Trials. 2020;21(1):743. doi: 10.1186/s13063-020-04646-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Maxwell SR, Moots RJ, Kendall MJ. Corticosteroids: do they damage the cardiovascular system? Postgrad Med. 1994;70(830):863–870. doi: 10.1136/pgmj.70.830.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bilbul M, Paparone P, Kim AM, Mutalik S, Ernst CL. Psychopharmacology of COVID-19. Psychosomatics. 2020;61(5):411–427. doi: 10.1016/j.psym.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li H, Liu Z, Ge J. Scientific research progress of COVID-19/SARS-CoV-2 in the first five months. J Cell Mol Med. 2020;24(12):6558–6570. doi: 10.1111/jcmm.15364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Akyõl FT, Karadoğan D, Gürkan CG, et al. What we learned about COVID-19 so far? Notes from underground. Turkish Thorac J. 2020;21(3):185–192. doi: 10.5152/Turk-ThoracJ.2020.20052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Takahashi N, Abe R, Hattori N, et al. Clinical course of a critically ill patient with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) J Artif Organs. 2020 June;:1–4. doi: 10.1007/s10047-020-01183-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Reiner Ž, Hatamipour M, Banach M, et al. Statins and the Covid-19 main protease: in silico evidence on direct interaction. Arch Med Sci. 2020;16(2):490–496. doi: 10.5114/aoms.2020.94655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Malvy D, Taburet AM, de Lamballerie X, Mentre F, Extramiana F. The safety profile of favipiravir should not be the first argument to suspend its evaluation in viral hemorrhagic fevers. PLoS Neglected Trop Dis. 2020;14(6):1–4. doi: 10.1371/journal.pntd.0008259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Li J, Zhang C, Wu Z, Wang G, Zhao H. The Mechanism and Clinical Outcome of patients with Corona Virus Disease 2019 Whose Nucleic Acid Test has changed from negative to positive, and the therapeutic efficacy of Favipiravir: a structured summary of a study protocol for a randomised controlled. Trials. 2020;21(1) doi: 10.1186/s13063-020-04430-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yenerçağ M, Arslan U, Doğduş M, et al. Evaluation of electrocardiographic ventricular repolarization variables in patients with newly diagnosed COVID-19. J Electrocardiol. 2020;62:5–9. doi: 10.1016/j.jelectrocard.2020.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. U.S Food & Drug Administration. Coronavirus (COVID-19) update: FDA issues emergency use authorization for potential COVID-19 treatment. [Google Scholar]
- 27. Leegwater E, Strik A, Wilms EB, et al. Drug-induced liver injury in a patient with coronavirus disease 2019: potential interaction of remdesivir with P-glycoprotein inhibitors. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mulangu S, Dodd LE, Davey RT, et al. A randomized, controlled trial of Ebola virus disease therapeutics. N Engl J Med. 2019 doi: 10.1056/NEJMoa1910993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. EM Agency. Summary on compassionate use. EMA/178637/2020 – Rev.2. [Google Scholar]
- 30. Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of covid-19 – preliminary report. N Engl J Med. 2020 doi: 10.1056/nejmoa2007764. [DOI] [PubMed] [Google Scholar]
- 31. Wang Y, Zhang D, Du G, et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebocontrolled, multicentre trial. Lancet. 2020;395(10236):1569–1578. doi: 10.1016/S0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Spinner CD, Gottlieb RL, Criner GJ, et al. Effect of remdesivir vs standard care on clinical status at 11 Days in patients with moderate COVID-19: a randomized clinical trial. JAMA. 2020 August; doi: 10.1001/jama.2020.16349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Crump A, Omura S. Ivermectin, “Wonder drug” from Japan: the human use perspective. Proc Japan Acad Ser B Phys Biol Sci. 2011;87(2):13–28. doi: 10.2183/pjab.87.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wagstaff KM, Sivakumaran H, Heaton SM, Harrich D, Jans DA. Ivermectin is a specific inhibitor of importin α/β-mediated nuclear import able to inhibit replication of HIV-1 and dengue virus. Biochem J. 2012;443(3):851–856. doi: 10.1042/BJ20120150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Caly L, Wagstaff KM, Jans DA. Nuclear trafficking of proteins from RNA viruses: potential target for antivirals? Antivir Res. 2012;95(3):202–206. doi: 10.1016/j.antiviral.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 36. Caly L, Druce JD, Catton MG, Jans DA, Wagstaff KM. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antivir Res. 2020;178:104787. doi: 10.1016/J.ANTIVIRAL.2020.104787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Saber-Ayad M, Saleh MA, Abu-Gharbieh E. The rationale for potential pharmacotherapy of covid-19. Pharmaceuticals. 2020;13(5) doi: 10.3390/ph13050096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Awadzi K, Dadzie KY, Shulz-Key H, Haddock DR, Gilles HM, Aziz MA. The chemotherapy of onchocerciasis X. An assessment of four-single dose treatment regimes of MK-933 (Ivermectin) in human onchocerciasis. Ann Trop Med Parasitol. 1985;79(1):63–78. doi: 10.1080/00034983.1985.11811889. [DOI] [PubMed] [Google Scholar]
- 39. Paasch U, Haustein UF. Management of endemic outbreaks of scabies with allethrin, permethrin, and ivermectin. Int J Dermatol. 2000;39(6):463–470. doi: 10.1046/j.1365-4362.2000.00990.x. [DOI] [PubMed] [Google Scholar]
- 40. De Sole G, Remme J, Awadzi K, et al. Adverse reactions after large-scale treatment of onchocerciasis with ivermectin: combined results from eight community trials. Bull World Health Organ. 1989;67(6):707–719. [PMC free article] [PubMed] [Google Scholar]
- 41. Dukuly ZD, Pacque M, Nara A, Taylor HR, Williams PN, Greene BM. A prospective study in high risk subjects of electrocardiographic changes with ivermectin. Trop Med Parasitol. 1990;41(1):73–74. [PubMed] [Google Scholar]
- 42. Solomon S. FDA letter to stakeholders: do not use ivermectin intended for animals as treatment for COVID-19 in humans. [Google Scholar]
- 43. Ayogu JI, Odoh AS. Prospects and therapeutic applications of cardiac glycosides in cancer remediation. ACS Comb Sci Published online. 2020 doi: 10.1021/acscombsci.0c00082. [DOI] [PubMed] [Google Scholar]
- 44. Sreenivasan Y, Sarkar A, Manna SK. Oleandrin suppresses activation of nuclear transcription factor-κB and activator protein-1 and potentiates apoptosis induced by ceramide. Biochem Pharmacol. 2003;66(11):2223–2239. doi: 10.1016/j.bcp.2003.07.010. [DOI] [PubMed] [Google Scholar]
- 45. Plante KS, Plante JA, Fernandez D, et al. Prophylactic and therapeutic inhibition of in vitro SARS-CoV-2 replication by oleandrin. bioRxiv. 2020 January;15:203489. doi: 10.1101/2020.07.15.203489. [DOI] [Google Scholar]
- 46. Dey P, Chaudhuri TK. Immunomodulatory activity of Nerium indicum through inhibition of nitric oxide and cyclooxygenase activity and modulation of TH1/TH2 cytokine balance in murine splenic lymphocytes. Cytotechnology. 2016;68(4):749–761. doi: 10.1007/s10616-014-9826-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Botelho AFM, Miranda ALS, Freitas TG, et al. Comparative cardiotoxicity of low doses of digoxin, ouabain, and oleandrin Cardiovasc toxicol. 2020 doi: 10.1007/s12012-020-09579-1. [DOI] [PubMed] [Google Scholar]
- 48. Küçükdurmaz Z, Karapinar H, Gül I, Yilmaz A. Complete atrioventricular block after self-ingestion of Nerium oleander for relief of hemorrhoidal complaints. Turk Kardiyol Dernegi Arsivi. 2012;40(2):168–170. doi: 10.5543/tkda.2012.01703. [DOI] [PubMed] [Google Scholar]
- 49. Senthilkumaran S, Meenakshisundaram R, Michaels AD, Thirumalaikolundusubramanian P. Electrocardiographic changes during inhalational oleander toxicity. Journal of Electrocardiology. 2011;44:470–472. doi: 10.1016/j.jelectrocard.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 50. FDA. Convalescent Plasma COVID-19 letter of authorization. [Google Scholar]
- 51. Cheng Y, Wong R, Soo YOY, et al. Use of convalescent plasma therapy in SARS patients in Hong Kong. Eur J Clin Microbiol Infect Dis. 2005;24(1):44–46. doi: 10.1007/s10096-004-1271-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Arabi YM, Hajeer AH, Luke T, et al. Feasibility of using convalescent plasma immunotherapy for MERS-CoV infection, Saudi Arabia. Emerg Infect Dis. 2016;22(9):1554–1561. doi: 10.3201/eid2209.151164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kraft CS, Hewlett AL, Koepsell S, et al. The use of TKM-100802 and convalescent plasma in 2 patients with ebola virus disease in the United States. Clin Infect Dis. 2015;61(4):496–502. doi: 10.1093/cid/civ334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Luke TC, Kilbane EM, Jackson JL, Hoffman SL. Meta-analysis: convalescent blood products for Spanish influenza pneumonia: a future H5N1 treatment? Ann Intern Med. 2006;145(8):599–609. doi: 10.7326/0003-4819-145-8-200610170-00139. [DOI] [PubMed] [Google Scholar]
- 55. Gosmann F, Nørgaard A, Rasmussen MB, Rahbek C, Seeberg J, Møller T. Transfusion-associated circulatory overload in adult, medical emergency patients with perspectives on early warning practice: a single-centre, clinical study. Blood Transfus. 2018;16(2):137–144. doi: 10.2450/2017.0228-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Clifford L, Jia Q, Subramanian A, Yadav H, Schroeder DR, Kor DJ. Risk factors and clinical outcomes associated with perioperative transfusion-associated circulatory overload. Anesthesiology. 2017;126(3):409–418. doi: 10.1097/ALN.0000000000001506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Joyner MJ, Bruno KA, Klassen SA, et al. Safety update: COVID-19 convalescent plasma in 20,000 hospitalized patients. Mayo Clin Proc. 2020;95(9):1888–1897. doi: 10.1016/j.mayocp.2020.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Rossi GP, Sanga V, Barton M. Potential harmful effects of discontinuing ace-inhibitors and arbs in covid-19 patients. Elife. 2020:9. doi: 10.7554/eLife.57278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Rohaim MA, Munir M. A scalable topical vectored vaccine candidate against SARS-CoV-2. Vaccines. 2020;8(3):1–16. doi: 10.3390/vaccines8030472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Yang J, Wang W, Chen Z, et al. A vaccine targeting the RBD of the S protein of SARS-CoV-2 induces protective immunity. Nature. 2020 doi: 10.1038/s41586-020-2599-8. [DOI] [PubMed] [Google Scholar]
- 61. Kalita P, Padhi AK, Zhang KYJ, Tripathi T. Design of a peptide-based subunit vaccine against novel coronavirus SARS-CoV-2. Microb Pathog. 2020:145. doi: 10.1016/j.micpath.2020.104236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Zaim S, Chong JH, Sankaranarayanan V, Harky A. COVID-19 and multiorgan response. Curr Probl Cardiol. 2020;45(8) doi: 10.1016/j.cpcardiol.2020.100618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Rizzo P, Vieceli Dalla Sega F, Fortini F, Marracino L, Rapezzi C, Ferrari R. COVID-19 in the heart and the lungs: could we “Notch” the inflammatory storm? Basic Res Cardiol. 2020;115(3):31. doi: 10.1007/s00395-020-0791-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Gurwitz D. Angiotensin receptor blockers as tentative SARS-CoV-2 therapeutics. Drug Dev Res. 2020;81(5):537–540. doi: 10.1002/ddr.21656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Wu L, O’Kane AM, Peng H, Bi Y, Motriuk-Smith D, Ren J. SARS-CoV-2 and cardiovascular complications: from molecular mechanisms to pharmaceutical management. Biochem Pharmacol. 2020;178:114114. doi: 10.1016/j.bcp.2020.114114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. McGonagle D, Sharif K, O’Regan A, Bridgewood C. The role of cytokines including interleukin-6 in COVID-19 induced pneumonia and macrophage activation syndrome-like disease. Autoimmun Rev. 2020;19(6) doi: 10.1016/j.autrev.2020.102537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Iannaccone G, Scacciavillani R, Del Buono MG, et al. Weathering the cytokine storm in COVID-19: therapeutic implications. Cardiorenal Med. 2020;10(5):277–287. doi: 10.1159/000509483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Buckley LF, Wohlford GF, Ting C, et al. Role for anti-cytokine therapies in severe coronavirus disease 2019. Crit Care Explor. 2020;2(8):e0178. doi: 10.1097/cce.0000000000000178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Szekely Y, Arbel Y. A review of interleukin-1 in heart disease: where do we stand today? Cardiol Ther. 2018;7(1):25–44. doi: 10.1007/s40119-018-0104-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Shah SR, Abbasi Z, Fatima M, et al. Canakinumab and cardiovascular outcomes: results of the CANTOS trial. J Community Hosp Intern Med Perspect. 2018;8(1):21–22. doi: 10.1080/20009666.2018.1428023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Pandey A, Nikam AN, Shreya AB, et al. Potential therapeutic targets for combating SARS-CoV-2: drug repurposing, clinical trials and recent advancements. Life Sci. 2020:256. doi: 10.1016/j.lfs.2020.117883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Kim SC, Solomon DH, Rogers JR, et al. Cardiovascular safety of tocilizumab versus tumor necrosis factor inhibitors in patients with rheumatoid arthritis: a multi-database cohort study. Arthritis Rheumatol. 2017;69(6):1154–1164. doi: 10.1002/art.40084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Driggin E, Madhavan MV, Bikdeli B, et al. Cardiovascular considerations for patients, health care workers, and health systems during the COVID-19 pandemic. J Am Coll Cardiol. 2020;75(18):2352–2371. doi: 10.1016/j.jacc.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Kawashiri SY, Kawakami A, Yamasaki S, et al. Effects of the anti-interleukin-6 receptor antibody, tocilizumab, on serum lipid levels in patients with rheumatoid arthritis. Rheumatol Int. 2011;31(4):451–456. doi: 10.1007/s00296-009-1303-y. [DOI] [PubMed] [Google Scholar]
- 75. Castagné B, Viprey M, Martin J, Schott AM, Cucherat M, Soubrier M. Cardiovascular safety of tocilizumab: a systematic review and network meta-analysis. PLoS One. 2019;14(8) doi: 10.1371/journal.pone.0220178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Haagmans BL, Kuiken T, Martina BE, et al. Pegylated interferon-α protects type 1 pneumocytes against SARS coronavirus infection in macaques. Nat Med. 2004;10(3):290–293. doi: 10.1038/nm1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Omrani AS, Saad MM, Baig K, et al. Ribavirin and interferon alfa-2a for severe Middle East respiratory syndrome coronavirus infection: a retrospective cohort study. Lancet Infect Dis. 2014;14(11):1090–1095. doi: 10.1016/S1473-3099(14)70920-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Teragawa H, Hondo T, Amano H, Hino F, Ohbayashi M. Adverse effects of interferon on the cardiovascular system in patients with chronic hepatitis C. Jpn Heart J. 1996;37(6):905–915. doi: 10.1536/ihj.37.905. [DOI] [PubMed] [Google Scholar]
- 79. Guo YR, Cao QD, Hong ZS, et al. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak-A n update on the status. Mil Med Res. 2020 doi: 10.1186/s40779-020-00240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. El-Dosouky II, El Hawari SAM, Emara MH, Hamed EF. Types and predictors of interferon/ribavirin induced cardiac complications in the Egyptian patients with chronic hepatitis C virus. J Indian Coll Cardiol. 2016;6(1):16–21. doi: 10.1016/j.jicc.2016.01.001. [DOI] [Google Scholar]
- 81. Salman H, Bergman M, Bessler H, Alexandrova S, Djaldetti M. The effect of interferon on mouse myocardial capillaries: an ultrastructural study. Cancer. 1999;85(6):1375–1379. doi: 10.1002/(SICI)1097-0142(19990315)85:6<1375::AID-CNCR22>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 82. Parlato S, Santini SM, Lapenta C, et al. Expression of CCR-7, MIP-3β, and Th-1 chemokines in type I IFN-induced monocyte-derived dendritic cells: importance for the rapid acquisition of potent migratory and functional activities. Blood. 2001;98(10):3022–3029. doi: 10.1182/blood.V98.10.3022. [DOI] [PubMed] [Google Scholar]
- 83. Lu CC, Chen MY, Lee WS, Chang YL. Potential therapeutic agents against COVID-19: what we know so far. J Chinese Med Assoc. 2020;83(6):534–536. doi: 10.1097/JCMA.0000000000000318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Shalhoub S. Interferon beta-1b for COVID-19. Lancet. 2020;395(10238):1670–1671. doi: 10.1016/S0140-6736(20)31101-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Ylldlrlm C, Nieuwenhuis S, Teunissen PF, Horrevoets AJG, Van Royen N, Van Der Pouw Kraan TCTM. Interferon-beta, a decisive factor in angiogenesis and arteriogenesis. J Interferon Cytokine Res. 2015;35(6):411–420. doi: 10.1089/jir.2014.0184. [DOI] [PubMed] [Google Scholar]
- 86. Goossens P, Gijbels MJJ, Zernecke A, et al. Myeloid type I interferon signaling promotes atherosclerosis by stimulating macrophage recruitment to lesions. Cell Metabol. 2010;12(2):142–153. doi: 10.1016/j.cmet.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 87. Davoudi-Monfared E, Rahmani H, Khalili H, et al. Arandomized clinical trial of the efficacy and safety of interferon β-1a in treatment of severe COVID-19. Antimicrob Agents Chemother. 2020;64(9) doi: 10.1128/AAC.01061-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Sharma A, Garcia G, Arumugaswami V, Svendsen C. Human iPSC-derived cardiomyocytes are susceptible to SARS-CoV-2 infection. bioRxiv Prepr Serv Biol. 2020 doi: 10.1101/2020.04.21.051912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Kwon Y, Nukala SB, Srivastava S, et al. Detection of viral RNA fragments in human iPSC-cardiomyocytes following treatment with extracellular vesicles from SARS-CoV-2 coding-sequence-overexpressing lung epithelial cells. bioRxiv Prepr Serv Biol. 2020 doi: 10.1101/2020.05.14.093583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Singh S, Chakravarty T, Chen P, et al. Allogeneic cardiosphere-derived cells (CAP-1002) in critically ill COVID-19 patients: compassionate-use case series. Basic Res Cardiol. 2020;115(4) doi: 10.1007/s00395-020-0795-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Lang JP, Wang X, Moura FA, Siddiqi HK, Morrow DA, Bohula EA. A current review of COVID-19 for the cardiovascular specialist. Am Heart J. 2020;226:29–44. doi: 10.1016/j.ahj.2020.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Sarfraz Z, Sarfraz A, Barrios A, et al. Cardio-pulmonary sequelae in recovered COVID-19 patients: considerations for primary care. J Prim Care Community Health. 2021:12. doi: 10.1177/21501327211023726. [DOI] [PMC free article] [PubMed] [Google Scholar]