Abstract
Chronic graft-versus-host-disease (cGVHD) is divided into two subtypes: classic (absence of acute GVHD features) and overlap cGVHD (‘ocGVHD’), in which both chronic and acute GVHD clinical features are present simultaneously. While worse outcomes with ocGVHD have been reported, there are few recent analyses. We performed a secondary analysis of data from the ABA2 trial (N=185), in which detailed GVHD data were collected prospectively and systematically adjudicated. Analyses included cumulative incidence of classic versus ocGVHD, their specific organ manifestations, global disease severity scores, non-relapse mortality (NRM), disease-free survival (DFS) and overall survival (OS) in these two cGVHD subtypes. Of 92 patients who developed cGVHD, 35 were classified as ocGVHD. The 1-year cumulative incidence, organ involvement, and global severity of classic and ocGVHD were similar between ABA2 patients receiving CNI/MTX+placebo and CNI/MTX+abatacept; thus, cohorts were combined for ocGVHD evaluation. This analysis identified ocGVHD as having significantly higher severity at presentation and at maximum global severity compared to classic cGVHD. OS and DFS were significantly lower for ocGVHD versus classic cGVHD. OcGVHD is associated with increased cGVHD severity scores, and is associated with decreased OS and DFS compared to classic cGVHD, underscoring the high risks with this cGVHD subtype.
Introduction:
Chronic Graft-versus-host disease (cGVHD) is a major complication of allogeneic hematopoietic stem cell transplantation (HCT), and depending on graft source, GVHD prophylaxis, and HLA matching, can affect as many as 70% of transplant recipients (1–4). The proposed pathophysiology of cGVHD is complex and involves multiple phases; early post-transplant inflammation secondary to tissue injury is followed by ongoing chronic inflammation, thymic injury, and dysregulation of both innate and adaptive immunity (5). Chronic GVHD can affect nearly any organ system, but most commonly involves barrier sites such as skin, mouth and liver. Corticosteroids, newly FDA-approved cGVHD therapies, and other agents, as well as organ-specific supportive care, are the mainstays of therapy for cGVHD (6–8). Steroid-dependent and -refractory disease remains a clinical challenge, with approximately half of patients with cGVHD requiring secondary treatments within 2 years (9). Furthermore, despite the availability of newer agents for the treatment of cGVHD, there is still a large portion of patients that do not respond or do not have sustained responses (10).
Chronic GVHD is divided into classic (absence of acute GVHD features) and overlap cGVHD (‘ocGVHD’) subtypes. The 2005 National Institutes of Health (NIH) cGVHD working group and the subsequent 2014 update defined the term ‘ocGVHD’ to describe the situation when clinical findings of both acute GVHD (aGVHD) and cGVHD are present simultaneously (11, 12). The reported proportion of ocGVHD within patients who develop cGVHD ranges between 16–89%; this broad range can be attributed to differences in study design, patient population, differences in graft source and conditioning regimens, and application of NIH diagnostic criteria in the contributing reports (13–16). The incidence of ocGVHD in the pediatric population is generally lower, with reports documenting an incidence of 3–39% (17, 18).
One of the unmet needs in the field is to understand the impact of ocGVHD on post-transplant outcomes with previous reports varying substantially (19–25) and with few recent analyses (26–28). Furthermore, many other reports combine classic cGVHD and ocGVHD for post-transplant analyses, which may mask the unique role of ocGVHD in patient outcomes (29–32). Here we take advantage of detailed aGVHD, cGVHD and ocGVHD data from a recently published phase II trial (33) (‘ABA2’) to determine the incidence and severity of ocGVHD and classic cGVHD, compare their outcomes, and assess the impact of T cell costimulation blockade with abatacept (ABA) on this disease entity.
Methods:
Patients evaluated:
The ABA2 trial (NCT01743131) enrolled children (≥6 years) and adults undergoing HCT for hematologic malignancies under two strata: a randomized, double-blind, placebo-controlled stratum (8/8 HLA-matched unrelated donor (URD), comparing calcineurin inhibitor (CNI) and methotrexate (MTX) plus 4 doses of ABA versus CNI/MTX plus placebo (8/8 ABA n=73, 8/8 placebo n=69, respectively), and a single arm stratum (7/8-HLA mismatched URD receiving CNI/MTX plus ABA (7/8 ABA, n=43). For the current analysis, the 2014 NIH consensus conference criteria were used to score cGVHD (11). The severity of cGVHD was assessed at the time of cGVHD diagnosis (presentation) and at maximum cGVHD severity in the first-year post-transplant (11). A blinded analysis was performed by an endpoint review committee, who reviewed and adjudicated severity scores of acute and chronic GVHD for each patient for the first-year post-transplant. The “overlap” designation was adjudicated when one or more aGVHD manifestations were present in a patient with a simultaneous diagnostic and/or distinctive feature of chronic GVHD. Examples of acute manifestations that led to an “ocGVHD” designation included inflammatory changes in skin without sclerotic changes, acute manifestations of the GI tract such as diarrhea, anorexia or vomiting, and acute manifestations of the liver such as cholestasis. The presence of isolated hepatitis was considered a cGHVD feature. The presence or absence and the preceding type of aGVHD was also described for this patient population. Specifically, the onset of aGVHD was further classified into “classic aGVHD”, when the initial diagnosis of aGVHD occurred within the first 100 days post-transplant; “late onset aGVHD”, when the initial diagnosis of aGVHD occurred beyond day 100 with no prior history of classic aGVHD; “recurrent onset aGVHD”, when a recurrence of aGVHD occurred after day 100 with a prior history of classic aGVHD that was controlled, inactive or resolved; and “persistent aGVHD” if active aGVHD started before, and persisted beyond day 100 (34).
Statistical analysis:
Baseline characteristics were described separately for patients developing classic and overlap cGVHD. Global cGVHD severity score and cGVHD organ involvement were described at the time of diagnosis (presentation) of cGVHD and at maximum organ involvement/score (maximum severity) during the first-year post HCT. Comparisons between groups of any categorical baseline variables, global cGVHD severity scores, or frequencies of cGVHD organ involvement were performed using Fisher’s exact test. Comparisons between groups of the distribution of the grade of cGVHD organ involvement were performed using the Kruskal-Wallis test. Comparisons between groups of any numerical baseline variables were performed using the Wilcoxon rank sum test. For all analyses, separate comparisons were performed of the 8/8 ABA group to 8/8 placebo, and 8/8 ABA to 7/8 ABA. When differences were not detected, the groups were combined for comparison of overlap and classic cGVHD outcomes. All outcomes that are described and compared for ocGVHD vs classic cGVHD include only patients that developed chronic GVHD within one-year post-transplant.
As noted above, chronic GVHD endpoints included classic cGVHD and ocGVHD. The timing of these outcomes was defined as the number of days after transplant until the onset of cGVHD. In the analysis of the ABA2 dataset, there were no patients who developed new onset acute GVHD manifestations after a diagnosis of classic cGVHD; therefore, there were no patients for whom the sub-category of cGVHD changed from an initial diagnosis of classic cGVHD to ocGVHD. It should also be noted that once a diagnosis of ocGVHD was made, this diagnosis was not altered even if the acute component of the ocGVHD resolved with treatment. As such, in the present analysis, the designation of classic cGVHD and ocGVHD were fixed outcomes. Competing risks for cGVHD endpoints were relapse or death in the absence of cGVHD. In the case of classic cGVHD and ocGVHD, the opposite type of cGVHD was also a competing risk. Cumulative incidence estimates were provided for cGVHD outcomes, and Gray’s test was used for comparisons between groups.
Non-relapse mortality (NRM), time to relapse (TTR), disease-free survival (DFS), and overall survival (OS) were described and compared for the patients developing classic cGVHD and overlap cGVHD. NRM was defined as time from transplant until death in the absence of relapse, with relapse as a competing risk. TTR was defined as time from transplant until relapse, with death in the absence of relapse as a competing risk. Cumulative incidence estimates were provided for NRM and TTR, with Gray’s test for comparisons between classic and overlap. DFS was defined as time from transplant until relapse or death. OS was defined as time from transplant until death from any cause. Kaplan-Meier survival estimates were provided for DFS and OS, with the log-rank test used for comparisons. Point-estimates of NRM, TTR, DFS, and OS are provided at one- and two-years from transplant, along with 95% confidence intervals using Greenwood’s formula for DFS and OS and using the Aalen formula for NRM and TTR. Analysis was conducted using SAS v. 9.4 (SAS Institute, Cary, NC, USA) and R (r-project.org, Vienna, Austria).
Results:
Cumulative incidence and organ involvement for classic cGVHD and ocGVHD:
The cumulative incidence of all grades of cGVHD through one-year post-transplant on ABA2 was 43.1% for 8/8 placebo patients, 49.7% for 8/8 ABA patients, and 63.7% for 7/8 ABA patients (Figure 1A, p = 0.54 for the 8/8 placebo versus 8/8 ABA comparison, and p = 0.19 for the 8/8 ABA versus 7/8 ABA comparison). Of the 92 patients diagnosed with cGVHD within one-year of HCT, 35 developed ocGVHD (Table 1). As shown in Table 1, the ocGVHD and classic cGVHD groups were balanced for baseline characteristics, except for older age at transplant for patients with ocGVHD compared to classic cGVHD (mean 54.5 vs 37.1 years respectively, p=0.02). The most common organs involved in both classic cGVHD and ocGVHD are shown in Table 2A–B. The most common organs in classic cGVHD, when analyzed at the time of presentation, were the mouth (82%), eyes (39%), liver (39%) and skin (37%). The most common organs involved in ocGVHD when analyzed at the time of presentation were the skin (86%), mouth (80%) and GI tract (54%) (Table 2A). The most common organs in classic cGVHD, when analyzed at the time of maximum disease severity, were the mouth (84%), eyes (49%), skin (39%) and liver (39%). The three most common organs involved in ocGVHD when analyzed at the time of maximum disease severity were the skin (91%), mouth (83%) and GI tract (60%) (Table 2B). At the time of ocGVHD diagnosis, 97% (34/35) of patients had diagnostic features of cGVHD (oral 88.6%, eyes 3%, skin 6%, GU 3%, GI 3%). Three percent (1/35) had myositis, a distinctive feature of cGVHD (Supplemental Table 1)8. Within the 8/8 cohort (8/8 ABA and 8/8 placebo), there was no difference in the cumulative incidence of classic cGVHD (29.7% 8/8 placebo vs 30.4% 8/8 ABA, p=0.98, Figure 1B) or ocGVHD (13.4% 8/8 placebo vs 19.3% 8/8 ABA, p=0.34, Figure 1C). Within the ABA cohort (8/8 ABA and 7/8 ABA), there was no difference in the cumulative incidence of classic cGVHD (30.4% 8/8 ABA vs. 35.5% 7/8 ABA, p=0.61, Figure 1B) or ocGVHD (19.3% 8/8 ABA vs. 28.2% 7/8 ABA, p=0.30, Figure 1C). Additionally, there was no significant difference in the cGVHD global severity score at cGVHD diagnosis or at maximum cGVHD presentation within the 8/8 placebo versus 8/8 ABA cohorts or within the 7/8 versus 8/8 ABA cohorts (Table 3). Because of the similarities between the incidence and severity of classic cGVHD and ocGVHD in the 8/8 placebo, 8/8 ABA and 7/8 ABA cohorts, these cohorts were combined for most of the downstream analyses.
gure 1. One-year cumulative incidence of cGVHD by ABA2 treatment cohort.
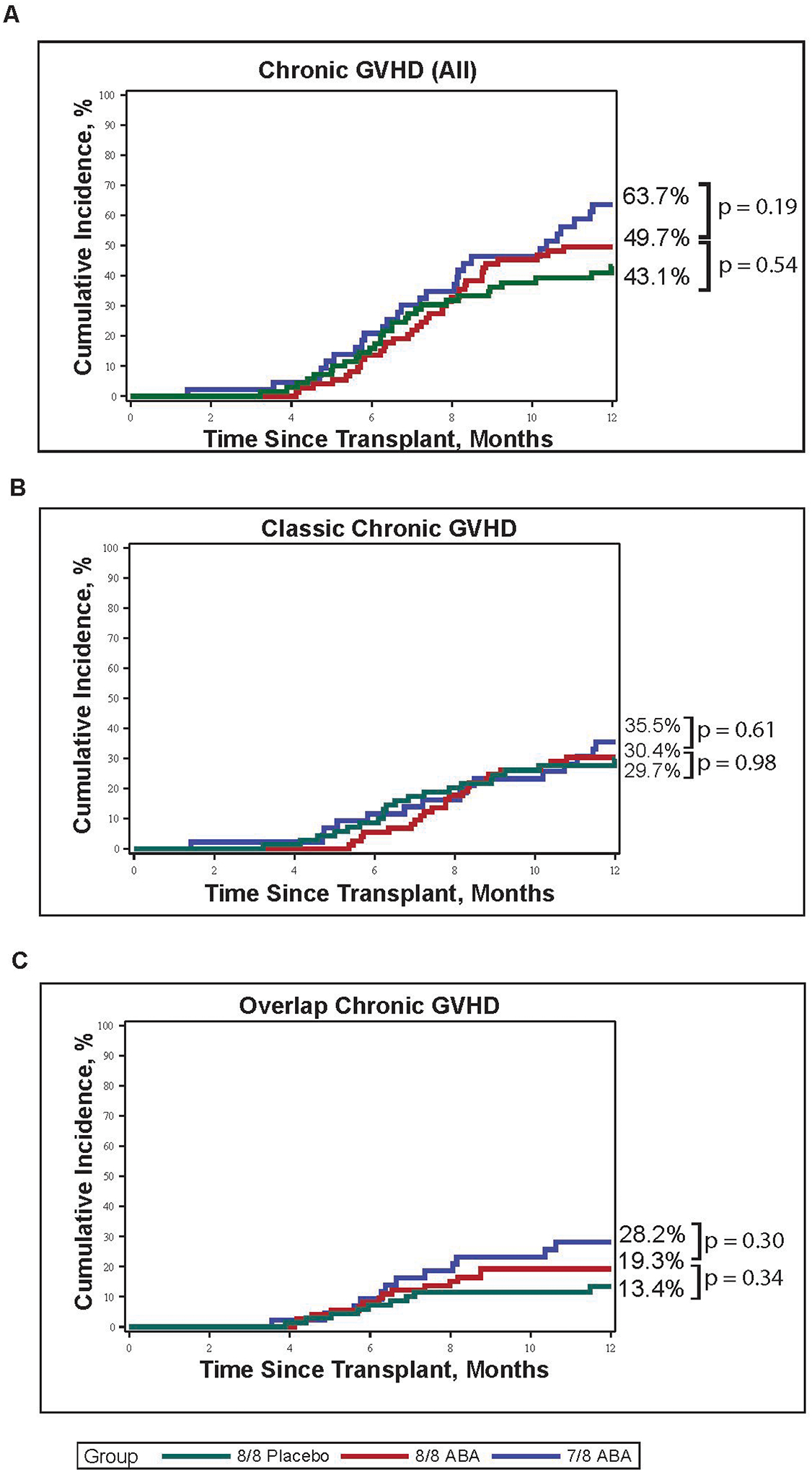
(A) Cumulative incidence of all cGVHD in 8/8 placebo (green), 8/8 ABA (red) and 7/8 ABA (blue) patients. Within the 8/8 cohort, the cumulative 1-year incidence cGVHD was 43.1% (8/8 placebo) vs 49.7% (8/8 ABA), p=0.54. Within the ABA cohort, the cumulative 1-year incidence of cGVHD was 49.7% (8/8 ABA) vs 63.7% (7/8 ABA), p=0.19. (B) Cumulative incidence of classic cGVHD in 8/8 placebo (green), 8/8 ABA (red) and 7/8 ABA (blue) patients. Within the 8/8 cohort, the cumulative incidence of classic cGVHD was 29.7% (8/8 placebo) vs 30.4% (8/8 ABA), p=0.98. Within the ABA cohort, the cumulative incidence of classic cGVHD was 30.4% (8/8 ABA) vs. 35.5% (7/8 ABA), p=0.61. (C) Cumulative incidence of ocGVHD in 8/8 placebo (green), 8/8 ABA (red) and 7/8 ABA (blue) patients. Within the 8/8 cohort, the cumulative incidence of ocGVHD was 13.4% (8/8 placebo) vs 19.3% (8/8 ABA), p=0.34. Within the ABA cohort, the cumulative incidence of ocGVHD was 19.3% (8/8 ABA) vs. 28.2% (7/8 ABA), p = 0.30.
Table 1.
Baseline characteristics of ABA2 participants with chronic GVHD.
| Characteristic Total N =92 | Classic cGVHD N=57 (62% of patients with cGVHD) | ocGVHD N=35 (38% of patients with cGVHD) | P value |
|---|---|---|---|
| Age, median years (range) | 37.1 (7.9 – 76.2) | 54.5 (7.0 – 76.5) | 0.02 |
| Age group, no. (%) | 0.602 | ||
| Pediatric (age < 21) | 13 (22.8) | 6 (17.1) | |
| Adult (age ≥21) | 44 (77.2) | 29 (82.9) | |
| Mean platelet count (103/mcL) at cGVHD diagnosis (range) | 168 (82–301) | 152 (41–335) | 0.28 |
| Time to cGVHD diagnosis, median days from transplant (range) | 220 (43 – 364) | 191 (108 – 349) | 0.0516 |
| Treatment group, no. (%) | 0.59 | ||
| 8/8 Aba | 22 (38.6) | 14 (40) | |
| 8/8 Placebo | 20 (35.1) | 9 (25.7) | |
| 7/8 Aba | 15 (26.3) | 12 (34.3) | |
| Performance Score > 90%, no. (%) | 42 (73.7) | 27 (77.1) | 0.81 |
| Disease, no. (%) | 0.85 | ||
| AML | 23 (40.4) | 11 (31.4) | |
| ALL | 17 (29.8) | 10 (28.6) | |
| CML | 3 (5.3) | 2 (5.7) | |
| MDS | 12 (21.1) | 10 (28.6) | |
| HL | 0 (0) | 0 (0) | |
| Other | 2 (3.5) | 2 (5.7) | |
| Conditioning regimen, no. (%) | 0.11 | ||
| Busulfan/Fludarabine | 6 (10.5) | 0 (0) | |
| Busulfan/cyclophosphamide | 24 (42.1) | 14 (40) | |
| TBI/ cyclophosphamide | 18 (31.6) | 10 (28.6) | |
| Fludarabine/Melphalan | 9 (15.8) | 11 (31.4) | |
| Conditioning intensity, no. (%) | 0.12 | ||
| Myeloablative | 48 (84.2) | 24 (68.6) | |
| Reduced intensity | 9 (15.8) | 11 (31.4) | |
| Graft type, no. (%) | 0.82 | ||
| Peripheral blood | 37 (64.9) | 24 (68.6) | |
| Bone marrow | 20 (35.1) | 11 (31.4) | |
Table 2.
Comparison of the involved cGVHD organ systems between classic cGVHD and ocGVHD at the time of initial presentation (Table 2A) and at maximum severity of cGVHD (Table 2B).
| Number of patients with cGVHD subtype (% of patients with cGVHD subtype compared to total number with cGVHD) | 57 (62%) | 35 (38%) | |
| Skin | 21 (37) | 30 (86) | <0.001a |
| Grade 1 | 10 (18) | 10 (29) | |
| Grade 2 | 6 (11) | 10 (29) | |
| Grade 3 | 5 (9) | 10 (29) | |
| Mouth | 47 (82) | 28 (80) | 0.79b |
| Eyes | 22 (39) | 16 (46) | 0.52b |
| GI | 14 (25) | 19 (54) | 0.002a |
| Grade 1 | 7 (12) | 9 (26) | |
| Grade 2 | 7 (12) | 3 (9) | |
| Grade 3 | 0 (0) | 7 (20) | |
| Liver | 22 (39) | 12 (34) | 0.94a |
| Grade 1 | 9 (16) | 5 (14) | |
| Grade 2 | 12 (21) | 1 (3) | |
| Grade 3 | 1 (2) | 6 (17) | |
| Lungs | 5 (9) | 0 (0) | 0.15b |
| Fascia | 5 (9) | 1 (3) | 0.40b |
| GU | 2 (4) | 1 (3) | >0.99b |
| Other | 23 (40) | 12 (34) | 0.66b |
| Skin | 22 (39) | 32 (91) | <0.001a |
| Grade 1 | 11 (19) | 10 (29) | |
| Grade 2 | 6 (11) | 11 (31) | |
| Grade 3 | 5 (9) | 11 (31) | |
| Mouth | 48 (84) | 29 (83) | >0.99b |
| Eyes | 28 (49) | 18 (51) | >0.99b |
| GI | 19 (33) | 21 (60) | 0.005a |
| Grade 1 | 10 (18) | 10 (29) | |
| Grade 2 | 9 (16) | 2 (6) | |
| Grade 3 | 0 (0) | 9 (26) | |
| Liver | 22 (39) | 14 (40) | 0.73a |
| Grade 1 | 8 (14) | 6 (17) | |
| Grade 2 | 12 (21) | 2 (6) | |
| Grade 3 | 2 (4) | 6 (17) | |
| Lungs | 9 (16) | 1 (3) | 0.08b |
| Fascia | 8 (14) | 1 (3) | 0.15b |
| GU | 4 (7) | 2 (6) | >0.99b |
| Other | 24 (42) | 14 (40) | >0.99b |
All p-values reported are nominal p-values with no adjustment for multiplicity of testing.
Kruskal-Wallis test by ranks on the distributions of the ordered grades of cGVHD organ involvement.
Fisher’s exact test on whether or not there was cGVHD involvement for specified organ system.
Table 3.
Comparison of global cGVHD severity scores between the treatment arms (8/8 placebo vs. 8/8 ABA vs 7/8 ABA) at presentation and at maximum severity of cGVHD.
| Mild, no. (%) | Moderate, no. (%) | Severe, no. (%) | P value | |
|---|---|---|---|---|
| Distribution at presentation: | ||||
| 8/8 placebo | 9 (31) | 12 (41) | 8 (28) | 0.53 (vs 8/8 ABA) |
| 8/8 ABA | 7 (19) | 19 (53) | 10 (28) | |
| 7/8 ABA | 4 (14) | 15 (54) | 9 (32) | 0.69 (vs 8/8 ABA) |
| Distribution at maximum: | ||||
| 8/8 placebo | 4 (14) | 17 (59) | 8 (28) | 0.94 (vs 8/8 ABA) |
| 8/8 ABA | 5 (14) | 19 (53) | 12 (33) | |
| 7/8 ABA | 3 (11) | 13 (46) | 12 (43) | 0.58 (vs 8/8 ABA) |
Distinguishing disease characteristics between classic cGVHD and ocGVHD:
As shown in Figure 2, patients with ocGVHD had significantly more severe cGVHD at initial diagnosis and at maximum severity within the first year post-HCT compared to those who developed classic cGVHD. The distribution of affected organs was also different for the two cGVHD subtypes (Table 2). Notably, the aGVHD components of disease made a substantial impact on the severity of cGVHD in many ocGVHD patients; for example, for patients with moderate-severe ocGVHD, more than a third of these patients would have been reclassified as having mild cGVHD if only their chronic organ involvement was scored. The severity of disease at presentation for these patients was driven by the aGVHD aspects of their disease (Figure 3). This increase in severity was also evident in the sub-category of aGVHD (described in Methods) that was present in ocGVHD patients. Thus, at presentation of ocGVHD, 60% of patients had “recurrent onset” of their aGVHD, while “late onset” and “persistent onset” each represented 20% of patients, according to the EBMT-NIH-CIBMTR task force classification system(34) (Supplemental Table 2). In contrast, at presentation of classic cGVHD, only 10.5% of patients had previously experienced “recurrent onset” aGVHD (p<0.0001 vs the rate of recurrent onset aGVHD in ocGVHD), with “late” and “persistent onset” aGVHD representing 5.3% (p=0.004 vs ocGVHD) and 1.8% of cases (p=0.04 vs ocGVHD), respectively. Consistent with the diagnosis of classic cGVHD, all aGVHD subtypes had resolved prior to the cGVHD diagnosis in these patients (Supplemental Table 2).
Figure 2. The distribution of global cGVHD severity scores comparing classic cGVHD to ocGVHD.
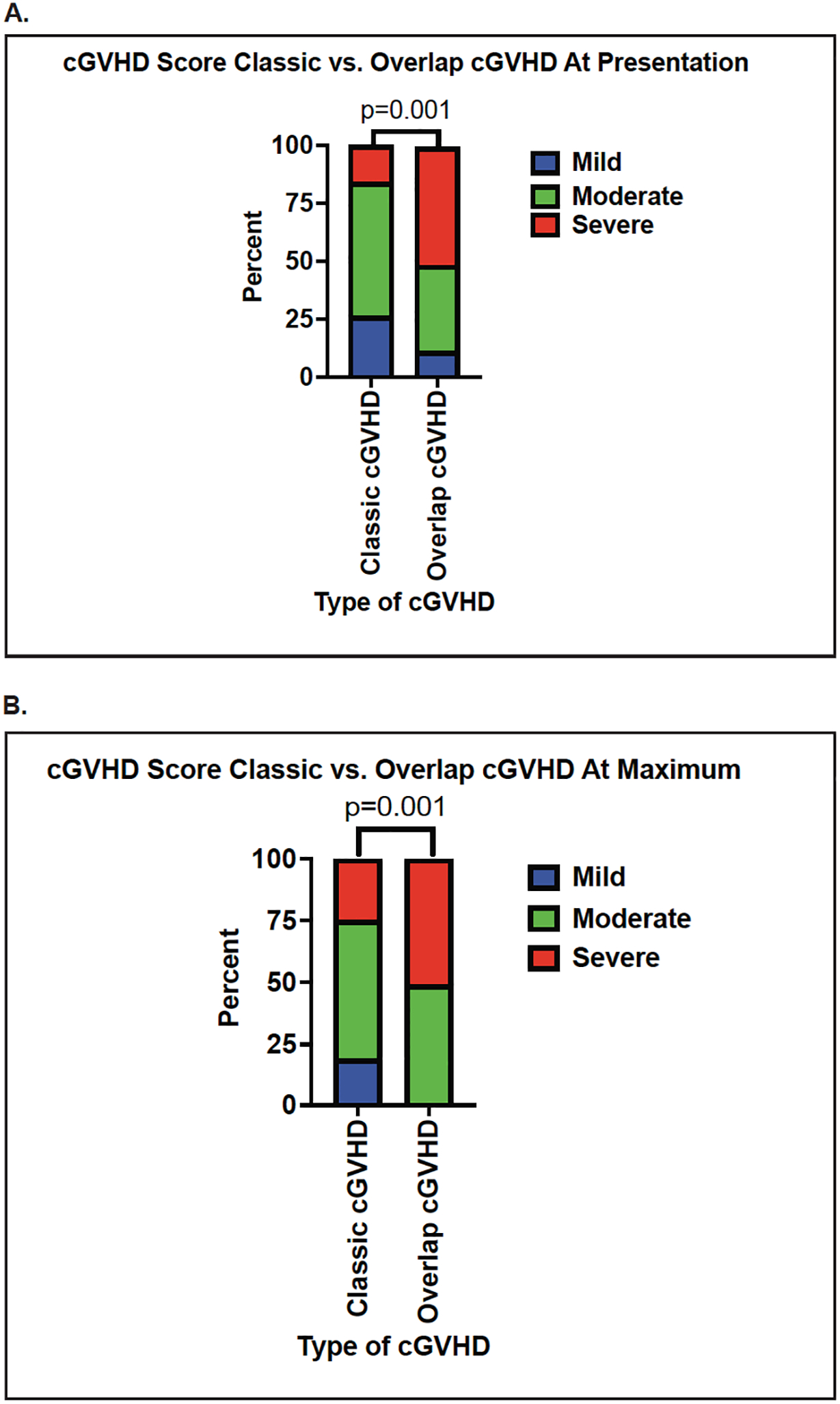
(A) Comparison at initial diagnosis of cGVHD. (B) Comparison at maximum severity of cGVHD. Blue bar: mild cGVHD global severity score; green bar: moderate cGVHD global severity score; red bar: severe cGVHD global severity score.
Figure 3. Contribution of the aGVHD component to the global severity score of ocGVHD.
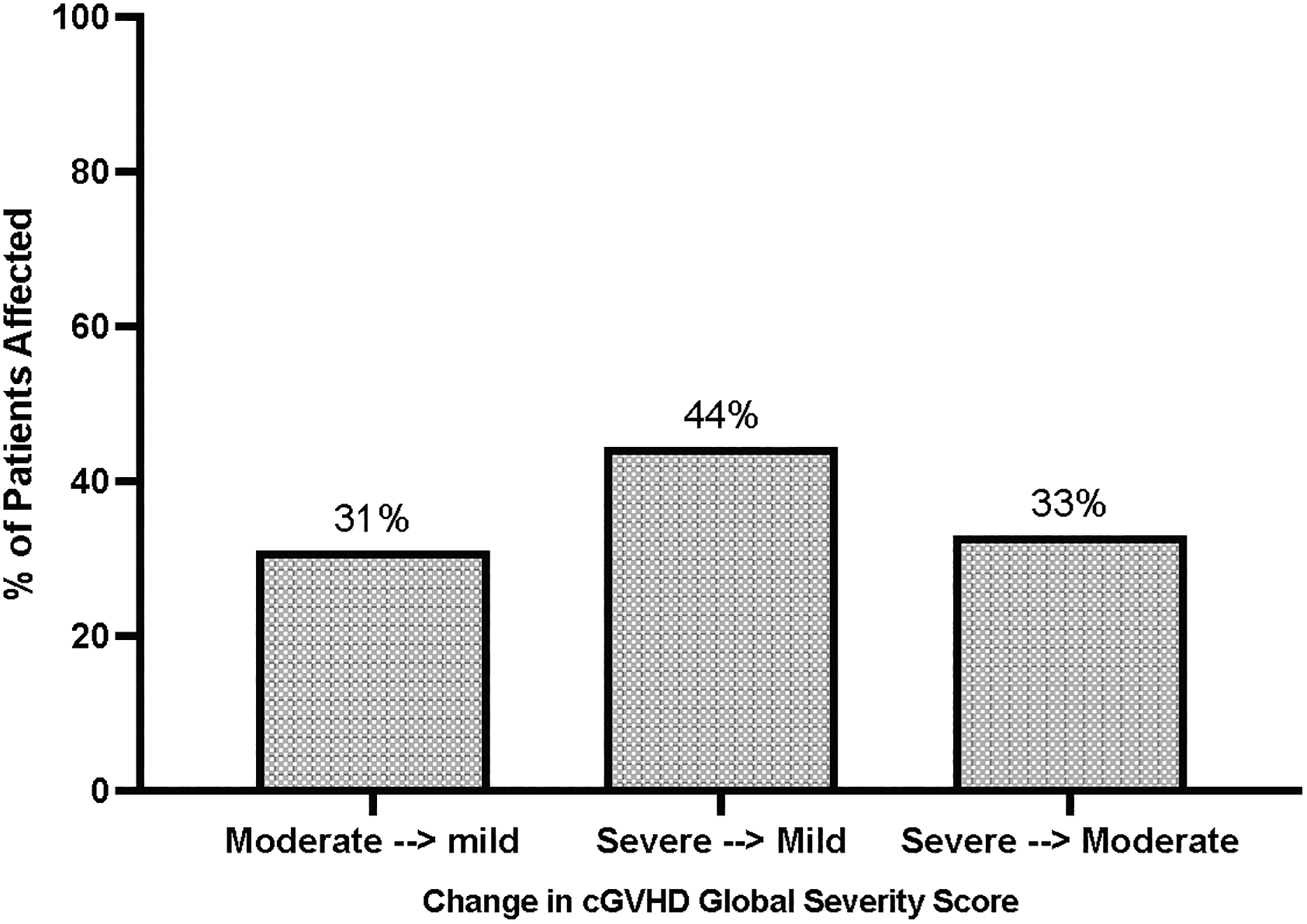
Left bar: Proportion of patients with moderate ocGVHD at presentation who would have been down-graded to mild cGVHD if the aGVHD contributions were omitted (31%). Middle bar: Proportion of patients with severe ocGVHD at presentation who would have been down-graded to mild cGVHD if the aGVHD contributions were omitted (44%). Right bar: Proportion of patients with severe ocGVHD at presentation who would have been down-graded to moderate cGVHD if the aGVHD contributions were omitted (33%).
OcGVHD is associated with decreased survival versus classic cGVHD:
As shown in Figure 4 and Table 4, compared to patients with classic cGVHD, patients with ocGVHD had significantly decreased survival. Thus, OS was significantly lower for patients with ocGVHD versus classic GVHD (p=0.003, Figure 4A). This was reflected in the estimated probability of OS in ocGVHD compared with classic cGVHD at 1 year (83% vs 100%, respectively) and 2 years (64% vs 92% respectively) (Table 4). DFS was also decreased in patients with ocGVHD versus classic GVHD (Figure 4B, p= 0.014), with a similar trend compared to OS. Thus, the 1-year estimated DFS was 83% for ocGVHD versus 100% for classic cGVHD, while 2-year DFS was 65% for ocGVHD and 84% for classic cGVHD. For NRM, there was a trend for increased NRM in patients with ocGVHD versus classic cGVHD (Figure 4C, p = 0.068), with ocGVHD patients experiencing earlier NRM than those with classic cGVHD. Indeed, for the 15 patients who experienced NRM (8 with ocGVHD, 7 with classic cGVHD), those with ocGVHD had shorter survival after cGVHD diagnosis compared to those with classic cGVHD (86 days versus 465 days, p=0.007). NRM at 1-year was 17% for those with ocGVHD versus 0% for patients with classic cGVHD, while 2-year NRM was 26% for ocGVHD and 8% for classic cGVHD. Of note, for patients with ocGVHD, many of the deaths (58%) were GVHD-related (Supplemental Table 3), further highlighting the impact that ocGVHD made to patient outcomes. Indeed, the majority of NRM recorded in cGVHD patients on ABA2 occurred in those who had severe cGVHD at diagnosis, and 87% of deaths in ocGVHD patients occurred in those who had severe aGVHD manifestations in the GI tract and liver (Supplemental Table 4). Time-to-relapse was similar for patients with classic cGVHD and ocGVHD (Figure 4D) but with a trend towards increased later relapses apparent in ocGVHD patients. Together, the pattern of mortality risk for patients with ocGVHD versus classic cGVHD suggests a more significant difference in early versus late mortality with ocGVHD, consistent with the acute attributes of this GVHD subtype.
Figure 4. Key outcomes of classic cGVHD versus ocGVHD.
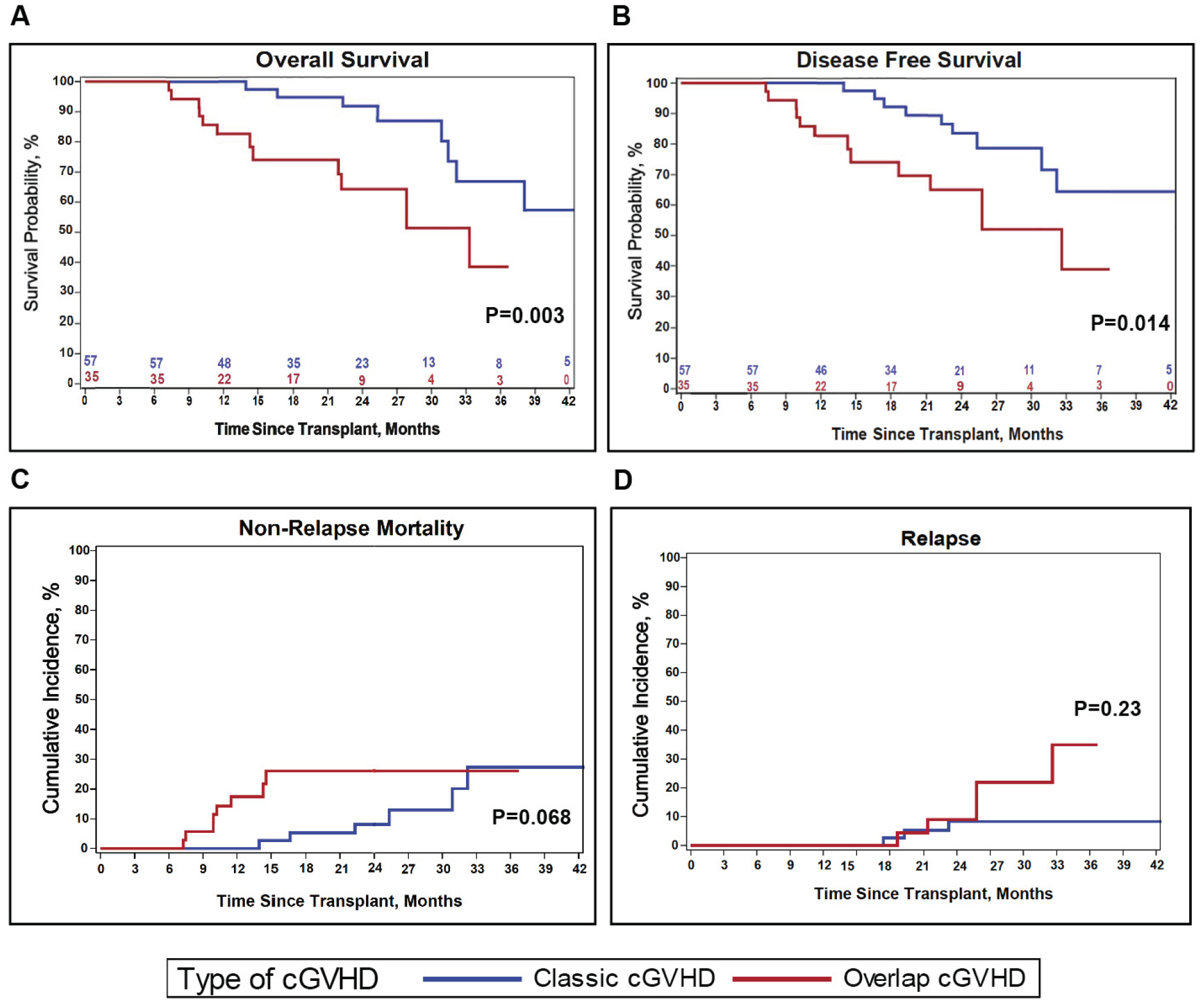
(A) Probability of OS. (B) Probability of DFS. (C) Cumulative incidence of NRM. (D) Cumulative incidence of TTR. For each graph, patient outcomes extend to 42 months. Blue: classic cGVHD. Red: ocGVHD. Note that the ocGVHD curves end at 37 months, which corresponds to the timepoint at which the patient in this group with the longest follow-up is censored. Beyond 42 months, 4 additional patients in the classic cGVHD group were censored, and there was 1 additional non-relapse death.
Table 4.
Comparison of key survival outcomes between classic cGVHD and ocGVHD.
| Classic cGVHD N=57 | ocGVHD N=35 | p-value | |
|---|---|---|---|
| OS survival probability, % (95% CI) | |||
| 1-year | 100 | 83 (65–92) | |
| 2-year | 92 (77–97) | 64 (43–80) | |
| DFS survival probability, % (95% CI) | |||
| 1-year | 100 | 83 (65–92) | |
| 2-year | 84 (67–92) | 65 (44–80) | |
| NRM cumulative incidence, % (95% CI) | |||
| 1-year | 0 | 17 (7–32) | |
| 2-year | 8 (2–20) | 26 (12–43) | |
| Relapse cumulative incidence, % (95% CI) | |||
| 1-year | 0 | 0 | |
| 2-year | 8 (2–20) | 9 (1–25) | |
Log-rank test for equality of survival functions including all follow-up.
Gray’s test for equality of cumulative incidence functions
To determine whether there were any notable differences between the placebo and ABA patients with regard to ocGVHD, we performed a sub-analysis of the 8/8 cohort for OS and DFS, comparing outcomes between classic cGVHD and ocGVHD amongst 8/8 Placebo versus 8/8 ABA patients. Although, as shown in Supplemental Table 5, the global severity of both classic cGVHD and ocGVHD was similar for 8/8 Placebo versus 8/8 ABA, there were survival differences between these two cohorts. Thus, in the 8/8 Placebo group, patients who developed ocGVHD demonstrated significantly lower OS and DFS compared to those with classic cGVHD (p = 0.004 and 0.003, respectively, Supplemental Table 6). In contrast, in the 8/8 ABA group, OS and DFS were similar (p = 0.74 and 0.698 respectively) suggesting that ABA’s protective effect on aGVHD had a positive impact on ocGVHD outcomes.
Discussion:
Using rigorously adjudicated GVHD data from the ABA2 clinical trial, here we demonstrate that ocGVHD was associated with significantly decreased OS and DFS compared to classic cGVHD, and with NRM occurring earlier in ocGVHD. These data underscore the substantial risks associated with this sub-type of cGVHD, as demonstrated in previous clinical trials(19). This analysis also highlighted the impact that the acute elements of ocGVHD made to disease severity, with these elements driving disease severity and early mortality in a substantial proportion of patients.
The present analysis also underscored the general similarity in both the placebo and ABA cohorts of the ABA2 trial with respect to the incidence of both overlap and classic cGVHD, but highlighted the potential of ABA to decrease the risk of death in patients who develop the ocGVHD subtype of disease. While this was a post-hoc analysis, and ABA2 was not powered to specifically address questions focused on ocGVHD, this observation underscores the potential importance of targeting both the inflammatory as well as fibrotic components of ocGVHD when considering prevention and treatment choices.
This analysis also highlights the challenges inherent in rigorously analyzing the impact of ocGVHD on transplant outcomes. One of the key challenges is in accurately differentiating ocGVHD from late aGVHD or other causes of organ impairment (35).The real-time adjudication process and the detailed information obtained for the diagnosis of cGVHD in ABA2 increased the clarity in classifying the diagnostic and/or distinctive features of cGVHD. As expected, more patients with ocGVHD had recurrent, late, or persistent aGVHD compared to patients with classic cGVHD. Interestingly, in most cases of ocGVHD, there was a recurrence of aGVHD prior to developing the diagnostic or distinctive signs of cGVHD. As such, it is possible that the late onset of aGVHD, irrespective of the chronic cGVHD component, was the main contributor to the worse survival outcomes in these patients. While there are conflicting reports in the literature concerning whether a late onset of aGVHD is associated with worse survival outcomes compared to classic onset aGVHD (36–40); a recent report links the increased clinical severity of late aGVHD with higher-risk aGVHD biomarker features (41). Given that the time to onset, history of prior immunosuppression, and the presence of antecedent aGVHD differ between ocGVHD, classic cGVHD, and late aGVHD, future studies will be necessary to definitively identify the biological differences between these disease entities.
Our study has several strengths. These include the prospective nature of the ABA2 trial, and the real-time, blinded data review and GVHD adjudication that occurred in this trial. This enabled the accurate assignment of cGVHD subtype and severity, given the prospective collection of granular data (including source document verification) on both acute and chronic GVHD signs and symptoms through one-year post-HCT. It should be noted that for this analysis, due to differences in the timing of key survival events in chronic versus ocGVHD, performing a landmark analysis (for instance considering events only after 1-year post-transplant) was not appropriate, as it would have disproportionally affected one of the two comparator arms. Alternatively, performing analyses from onset of cGVHD was considered, but ultimately deemed not appropriate as most survival analyses in the HCT literature use the date of stem cell infusion to anchor key survival events. Therefore, events were evaluated from the time of transplant onward. This study also has several limitations, including the fact that this was a post-hoc analysis, and therefore sample size for each cGVHD cohort was relatively small, and the fact that cGVHD adjudication was limited to cases diagnosed within one year of transplant. The small sample size may have limited the strength of some statistical analyses, and it precluded determining the impact of ocGVHD specifically in the pediatric sub-population. With respect to timing of cGVHD diagnosis, some outcome measures may be underestimated in this analysis, given that only patients diagnosed with cGVHD within the first year were included in subsequent comparisons. Because the median time to diagnosis of classic cGVHD was slightly later than ocGVHD (220 versus 191 days post-HCT), some classic GVHD cases may have been missed with this cut-off. The impact of this limitation is expected to be small, however, given that the median onset of classic cGVHD was still well within one-year post-transplant.
This study underscores the high risk that HCT patients face when they develop ocGVHD. It demonstrates that their onset of symptoms is worse, that they are harder to treat, and that they face major mortality risks associated with this disease. As we continue to refine our prevention and treatment of cGVHD, patients with overlap disease remain an under-studied and at-risk cohort. They would benefit from prevention and treatment trials that target the complex clinical and pathophysiologic origins of disease.
Supplementary Material
Key Points:
Overlap cGVHD is associated with significantly higher severity at presentation and at maximum global severity compared to classic cGVHD.
Overlap cGVHD is associated with significantly lower OS and DFS compared to classic cGVHD.
Author’s disclosures of potential conflicts of interest:
Muna Qayed
Consulting or Advisory Role: Novartis, Mesoblast
Travel, Accommodations, Expenses: Novartis
Leslie S. Kean
Consulting or Advisory Role: HiFiBio, Mammoth Biosciences
Research Funding: Regeneron, Gilead Sciences, Novartis, Tessera Therapeutics, Tonix Pharmaceuticals, Bristol Myers Squibb
Patents, Royalties, Other Intellectual Property: Licensing Fees for ABA2 clinical trial data
No other potential conflicts of interest were reported.
Data availability statement:
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References:
- 1.Lee SJ, Flowers ME. Recognizing and managing chronic graft-versus-host disease. Hematology Am Soc Hematol Educ Program. 2008:134–41. [DOI] [PubMed] [Google Scholar]
- 2.Arai S, Arora M, Wang T, Spellman SR, He W, Couriel DR, et al. Increasing incidence of chronic graft-versus-host disease in allogeneic transplantation: a report from the Center for International Blood and Marrow Transplant Research. Biol Blood Marrow Transplant. 2015;21(2):266–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flowers ME, Inamoto Y, Carpenter PA, Lee SJ, Kiem HP, Petersdorf EW, et al. Comparative analysis of risk factors for acute graft-versus-host disease and for chronic graft-versus-host disease according to National Institutes of Health consensus criteria. Blood. 2011;117(11):3214–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saliba RM, Alousi AM, Pidala J, Arora M, Spellman SR, Hemmer MT, et al. Characteristics of Graft-Versus-Host Disease (GvHD) After Post-Transplantation Cyclophosphamide Versus Conventional GvHD Prophylaxis. Transplant Cell Ther. 2022;28(10):681–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zeiser R, Blazar BR. Pathophysiology of Chronic Graft-versus-Host Disease and Therapeutic Targets. N Engl J Med. 2017;377(26):2565–79. [DOI] [PubMed] [Google Scholar]
- 6.Ryan CE, Sahaf B, Logan AC, O’Brien S, Byrd JC, Hillmen P, et al. Ibrutinib efficacy and tolerability in patients with relapsed chronic lymphocytic leukemia following allogeneic HCT. Blood. 2016;128(25):2899–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cutler C, Lee SJ, Arai S, Rotta M, Zoghi B, Lazaryan A, et al. Belumosudil for chronic graft-versus-host disease after 2 or more prior lines of therapy: the ROCKstar Study. Blood. 2021;138(22):2278–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zeiser R, Polverelli N, Ram R, Hashmi SK, Chakraverty R, Middeke JM, et al. Ruxolitinib for Glucocorticoid-Refractory Chronic Graft-versus-Host Disease. N Engl J Med. 2021;385(3):228–38. [DOI] [PubMed] [Google Scholar]
- 9.Inamoto Y, Flowers ME, Sandmaier BM, Aki SZ, Carpenter PA, Lee SJ, et al. Failure-free survival after initial systemic treatment of chronic graft-versus-host disease. Blood. 2014;124(8):1363–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zeiser R, Lee SJ. Three US Food and Drug Administration-approved therapies for chronic GVHD. Blood. 2022;139(11):1642–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jagasia MH, Greinix HT, Arora M, Williams KM, Wolff D, Cowen EW, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. The 2014 Diagnosis and Staging Working Group report. Biol Blood Marrow Transplant. 2015;21(3):389–401.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11(12):945–56. [DOI] [PubMed] [Google Scholar]
- 13.Sato T, Ichinohe T, Kanda J, Yamashita K, Kondo T, Ishikawa T, et al. Clinical significance of subcategory and severity of chronic graft-versus-host disease evaluated by National Institutes of Health consensus criteria. Int J Hematol. 2011;93(4):532–41. [DOI] [PubMed] [Google Scholar]
- 14.Moon JH, Sohn SK, Lambie A, Ellis L, Hamad N, Uhm J, et al. Validation of National Institutes of Health global scoring system for chronic graft-versus-host disease (GVHD) according to overall and GVHD-specific survival. Biol Blood Marrow Transplant. 2014;20(4):556–63. [DOI] [PubMed] [Google Scholar]
- 15.Arora M, Nagaraj S, Witte J, DeFor TE, MacMillan M, Burns LJ, et al. New classification of chronic GVHD: added clarity from the consensus diagnoses. Bone Marrow Transplant. 2009;43(2):149–53. [DOI] [PubMed] [Google Scholar]
- 16.Kim DY, Lee JH, Lee JH, Kim SH, Lim SN, Kim SD, et al. Reevaluation of the National Institutes of Health criteria for classification and scoring of chronic GVHD. Bone Marrow Transplant. 2010;45(7):1174–80. [DOI] [PubMed] [Google Scholar]
- 17.Cuvelier GDE, Nemecek ER, Wahlstrom JT, Kitko CL, Lewis VA, Schechter T, et al. Benefits and challenges with diagnosing chronic and late acute GVHD in children using the NIH consensus criteria. Blood. 2019;134(3):304–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takahashi T, Arora M, Okoev G, DeFor TE, Weisdorf DJ, MacMillan ML. Late-Onset Acute and Chronic Graft-versus-Host Disease in Children: Clinical Features and Response to Therapy. Transplant Cell Ther. 2021;27(8):667 e1–e5. [DOI] [PubMed] [Google Scholar]
- 19.Pidala J, Vogelsang G, Martin P, Chai X, Storer B, Pavletic S, et al. Overlap subtype of chronic graft-versus-host disease is associated with an adverse prognosis, functional impairment, and inferior patient-reported outcomes: a Chronic Graft-versus-Host Disease Consortium study. Haematologica. 2012;97(3):451–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arora M, Pidala J, Cutler CS, Chai X, Kurland B, Jacobsohn DA, et al. Impact of prior acute GVHD on chronic GVHD outcomes: a chronic graft versus host disease consortium study. Leukemia. 2013;27(5):1196–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cho BS, Min CK, Eom KS, Kim YJ, Kim HJ, Lee S, et al. Feasibility of NIH consensus criteria for chronic graft-versus-host disease. Leukemia. 2009;23(1):78–84. [DOI] [PubMed] [Google Scholar]
- 22.Perez-Simon JA, Encinas C, Silva F, Arcos MJ, Diez-Campelo M, Sanchez-Guijo FM, et al. Prognostic factors of chronic graft-versus-host disease following allogeneic peripheral blood stem cell transplantation: the national institutes health scale plus the type of onset can predict survival rates and the duration of immunosuppressive therapy. Biol Blood Marrow Transplant. 2008;14(10):1163–71. [DOI] [PubMed] [Google Scholar]
- 23.Jagasia M, Giglia J, Chinratanalab W, Dixon S, Chen H, Frangoul H, et al. Incidence and outcome of chronic graft-versus-host disease using National Institutes of Health consensus criteria. Biol Blood Marrow Transplant. 2007;13(10):1207–15. [DOI] [PubMed] [Google Scholar]
- 24.Aisa Y, Mori T, Kato J, Yamane A, Kohashi S, Kikuchi T, et al. Validation of NIH consensus criteria for diagnosis and severity-grading of chronic graft-versus-host disease. Int J Hematol. 2013;97(2):263–71. [DOI] [PubMed] [Google Scholar]
- 25.Kuzmina Z, Eder S, Bohm A, Pernicka E, Vormittag L, Kalhs P, et al. Significantly worse survival of patients with NIH-defined chronic graft-versus-host disease and thrombocytopenia or progressive onset type: results of a prospective study. Leukemia. 2012;26(4):746–56. [DOI] [PubMed] [Google Scholar]
- 26.Lueck C, Tzalavras A, Wohlfarth P, Meedt E, Kiehl M, Turki AT, et al. Impact of chronic graft-versus-host-disease on intensive care outcome in allogeneic hematopoietic stem cell recipients. Bone Marrow Transplant. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hamilton BK, Rybicki L, Arai S, Arora M, Cutler CS, Flowers MED, et al. Association of Socioeconomic Status with Chronic Graft-versus-Host Disease Outcomes. Biol Blood Marrow Transplant. 2018;24(2):393–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Csanadi M, Agh T, Tordai A, Webb T, Jeyakumaran D, Sengupta N, et al. A systematic literature review of incidence, mortality, and relapse of patients diagnosed with chronic graft versus host disease. Expert Rev Hematol. 2019;12(5):311–23. [DOI] [PubMed] [Google Scholar]
- 29.Okoev G, Weisdorf DJ, Wagner JE, Blazar BR, MacMillan ML, DeFor T, et al. Outcomes of chronic graft-versus-host disease following matched sibling donor versus umbilical cord blood transplant. Bone Marrow Transplant. 2021;56(6):1373–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schultz KR, Kariminia A, Ng B, Abdossamadi S, Lauener M, Nemecek ER, et al. Immune profile differences between chronic GVHD and late acute GVHD: results of the ABLE/PBMTC 1202 studies. Blood. 2020;135(15):1287–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tang FF, Cheng YF, Xu LP, Zhang XH, Yan CH, Han W, et al. Incidence, Risk Factors, and Outcomes of Chronic Graft-versus-Host Disease in Pediatric Patients with Hematologic Malignancies after T Cell-Replete Myeloablative Haploidentical Hematopoietic Stem Cell Transplantation with Antithymocyte Globulin/Granulocyte Colony-Stimulating Factor. Biol Blood Marrow Transplant. 2020;26(9):1655–62. [DOI] [PubMed] [Google Scholar]
- 32.Cuvelier GDE, Ng B, Abdossamadi S, Nemecek ER, Melton A, Kitko CL, et al. A diagnostic classifier for pediatric chronic graft-versus-host disease: results of the ABLE / PBMTC 1202 study. Blood Adv. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Watkins B, Qayed M, McCracken C, Bratrude B, Betz K, Suessmuth Y, et al. Phase II Trial of Costimulation Blockade With Abatacept for Prevention of Acute GVHD. J Clin Oncol. 2021;39(17):1865–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schoemans HM, Lee SJ, Ferrara JL, Wolff D, Levine JE, Schultz KR, et al. EBMT-NIH-CIBMTR Task Force position statement on standardized terminology & guidance for graft-versus-host disease assessment. Bone Marrow Transplant. 2018;53(11):1401–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pusic I, Pavletic SZ. Challenges in Conducting Studies in Chronic Graft-versus-Host Disease. Clin Hematol Int. 2019;1(1):36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Holtan SG, Khera N, Levine JE, Chai X, Storer B, Liu HD, et al. Late acute graft-versus-host disease: a prospective analysis of clinical outcomes and circulating angiogenic factors. Blood. 2016;128(19):2350–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moon JH, Kim SN, Kang BW, Chae YS, Kim JG, Ahn JS, et al. Early onset of acute GVHD indicates worse outcome in terms of severity of chronic GVHD compared with late onset. Bone Marrow Transplant. 2010;45(10):1540–5. [DOI] [PubMed] [Google Scholar]
- 38.Omer AK, Weisdorf DJ, Lazaryan A, Shanley R, Blazar BR, MacMillan ML, et al. Late Acute Graft-versus-Host Disease after Allogeneic Hematopoietic Stem Cell Transplantation. Biol Blood Marrow Transplant. 2016;22(5):879–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vigorito AC, Campregher PV, Storer BE, Carpenter PA, Moravec CK, Kiem HP, et al. Evaluation of NIH consensus criteria for classification of late acute and chronic GVHD. Blood. 2009;114(3):702–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee SE, Cho BS, Kim JH, Yoon JH, Shin SH, Yahng SA, et al. Risk and prognostic factors for acute GVHD based on NIH consensus criteria. Bone Marrow Transplant. 2013;48(4):587–92. [DOI] [PubMed] [Google Scholar]
- 41.Akahoshi Y, Spyrou N, Hogan WJ, Ayuk F, DeFilipp Z, Weber D, et al. Incidence, clinical presentation, risk factors, outcomes, and biomarkers in de novo late acute GVHD. Blood Adv. 2023;7(16):4479–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.


