Abstract
Studies of potent antiretroviral combination regimens were undertaken in young infants to evaluate the potential for long-term suppression of viral replication and to evaluate the immune consequences of such therapies. Early combination antiretroviral therapy led to a loss of plasma viremia, cultivable virus, and labile extrachromosomal replication intermediates. Despite preservation of immune function, persistent human immunodeficiency type 1 (HIV-1)-specific immune responses were not detected in most infants. The absence of detectable, persisting immune responses in most HIV-1-infected infants treated early contrasts with what is typically seen in adults who are treated early. These results are consistent with the notion that early combination antiretroviral therapy of HIV-1-infected infants allows the long-term suppression of viral replication.
The integration of sensitive molecular techniques for the measurement of viral nucleic acids into clinical trials evaluating potent antiretroviral therapies has allowed significant advances in our understanding of the pathogenesis of human immunodeficiency virus type 1 (HIV-1) infection (reviewed in reference 7). Data from such clinical trials in adults and children suggest that HIV-1 replication plays a central role in the pathogenesis of HIV-1 infection. Even in early vertical HIV-1 infection (i.e., the first few months of life), plasma viral turnover is extremely rapid (21), and early plasma HIV-1 viral load appears to be predictive of subsequent disease course (1, 10). In the macaque model of simian immunodeficiency virus infection, the administration of antiretroviral therapy in early acute infection interferes with the establishment of infection (34, 35). It has therefore been suggested that controlling HIV-1 replication through the use of early, potent combination therapy regimens would provide the best opportunity to control viral replication and to preserve the immune system following vertical infection.
Data from the first trial of potent combination therapy in early vertical infection suggested that long-term suppression of HIV-1 replication was feasible (17). While the long-term suppression of viral replication was associated with preservation of the immune system, persistent HIV-1-specific immune responses were not detected following the suppression of viral replication. Further studies of potent antiretroviral combination regimens were therefore undertaken in young infants to better evaluate the potential for long-term suppression of viral replication and to evaluate the immune consequences of such therapies.
MATERIALS AND METHODS
Study population.
These studies were conducted through the Pediatric AIDS Clinical Trials Group (PACTG protocols 180 and 356). Plasma HIV-1 RNA levels fell to <50 copies/ml in 15 (62%) of 24 infants who initiated therapy at <3 months of age through PACTG 356. We have also continued long-term follow-up (50 months) of two infants from a prior early therapy study (17).
Infants were defined as infected and eligible for enrollment into one of these protocols if HIV-1 nucleic acids were detected in peripheral blood lymphocytes using PCR on at least one occasion and HIV-1 was subsequently isolated from the peripheral blood mononuclear cells (PBMC). All infants studied were either asymptomatic or only mildly symptomatic (Centers for Disease Control and Prevention clinical category N, A, or B [6]). Individual patient characteristics and antiretroviral regimens are outlined in Table 1.
TABLE 1.
Characteristics of the study population
| Patient | Antiretroviral therapya | Age (mo) | Baseline plasma RNA (log copies/ml) | Baseline % CD4 | Time (wk) to RNA <400 | Length of follow-up (mo) |
|---|---|---|---|---|---|---|
| P-1048 | ZDV/3TC/NVP | 0.5 | 4.5 | 34 | 12 | 29 |
| P-1216 | ZDV/3TC/NVP | 2.8 | 5.7 | 14 | 12 | 27 |
| P-1228 | ZDV/3TC/NVP | 1.1 | 5.3 | 47 | 16 | 32 |
| P-1232 | ZDV/3TC/NVP/ABV | 3.0 | 5.0 | 40 | 16 | 21 |
| P-1235 | ZDV/3TC/NVP/ABV | 2.2 | 5.3 | 24 | 2 | 27 |
| P-1238 | ZDV/3TC/NVP/ABV | 3.0 | 3.3 | 52 | 0.5 | 24 |
| P-1246 | ZDV/3TC/NVP/ABV | 0.8 | 5.2 | 54 | 8 | 24 |
| P-1247 | ZDV/3TC/NVP/ABV | 1.1 | 4.3 | 63 | 4 | 21 |
| P-1222 | d4T/3TC/NVP/NLF | 1.8 | 5.5 | 36 | 16 | 18 |
| P-1223 | d4T/3TC/NVP/NLF | 1.5 | 5.2 | 34 | 8 | 18 |
| P-1231 | d4T/3TC/NVP/NLF | 1.7 | 5.8 | 52 | 16 | 16 |
| P-1233 | d4T/3TC/NVP/NLF | 1.9 | 5.6 | 30 | 12 | 16 |
| P-1251 | d4T/3TC/NVP/NLF | 2.2 | 5.2 | 19 | 8 | 18 |
| P-1252 | d4T/3TC/NVP/NLF | 1.6 | 5.4 | 46 | 8 | 18 |
| P-1260 | d4T/3TC/NVP/NLF | 2.4 | 5.2 | 55 | 12 | 18 |
| P-3743 | ZDV/ddI/NVP | 2.5 | 5.5 | 44 | 8 | 56 |
| P-3742 | ZDV/ddI/NVP | 2.5 | 5.5 | 57 | 20 | 56 |
ABV, abacavir.
The human subjects committees at the participating clinical sites approved these studies. Written informed consent was obtained from the children's legal guardians. The guidelines of the U.S. Department of Health and Human Services governing experimentation in humans were followed.
Preparation of plasma and PBMC for assays.
Blood samples were collected in evacuated specimen tubes containing either acid-citrate-dextrose or EDTA (Vacutainer; Becton-Dickinson, Mountainview, Calif.). Within 6 h of phlebotomy, the specimen tubes were centrifuged at 1,500 × g for 15 min. Following repeat centrifugation of plasma at 1,500 × g for 10 min at room temperature, the supernatant was removed, divided into aliquots of 0.5 ml, and frozen promptly at −70°C. PBMC were recovered from the cell layer by Ficoll-Paque (Pharmacia, Piscataway, N.J.) density centrifugation (3).
Quantification of plasma HIV-1 RNA copy number by reverse transcriptase (RT)-mediated PCR.
HIV-1 RNA was quantified in 200 μl of EDTA-anticoagulated plasma (stored at −70°C within 6 h following phlebotomy) by PCR after reverse transcription (Amplicor; Roche). Plasma samples with values below the detection limit of the standard assay (<400 copies/ml) were subsequently tested using 450 μl of plasma and a modified assay with a detection limit of 50 HIV-1 RNA copies/ml. All assays were performed in a single laboratory that participates in an ongoing quality certification program for HIV-1 RNA quantitation sponsored by the National Institutes of Health.
Enumeration of lymphocyte subsets in the peripheral blood.
The relative percentages of CD3+ CD4+ lymphocytes in the peripheral blood were enumerated using direct immunofluorescence with fluorescein isothiocyanate (FITC)- or phycoerythrin-conjugated mouse monoclonal antibodies (Becton Dickinson). Samples were analyzed using flow cytometry (Becton Dickinson FACScan).
HIV-1 serology.
Plasma HIV-1 immunoglobulin G antibodies were detected using a commercial enzyme-linked immunosorbent assay (ELISA) (Vironostika HIV-1 Microelisa system; Organon-Teknika, Durham, N.C.). Antibody specificity was ascertained by Western blotting (Calyptebiomedical, Rockville, Md.).
Measurement of plasma immunoglobulins.
Plasma immunoglobulin levels were measured in the University of Massachusetts Medical Center clinical laboratory.
Lymphoproliferative assays.
Lymphoproliferative assays were performed by the methods of Rosenberg et al. (30), except that the recombinant HIV-1 p24 and gp160 proteins were used at a final concentration of 5 μg/ml. Pokeweed mitogen (PWM) (Sigma) was used at a concentration of 10 μg/ml. Tetanus toxoid (Connaught) was used at 1.25 and 2.5 μg/ml. For the recombinant HIV-1 proteins, the stimulation index (SI) was defined as the ratio of the mean counts per minute of the HIV-1 protein wells to the mean counts per minute of the control protein wells. For PWM and tetanus toxoid, the SI was defined as the ratio of the mean counts per minute of the stimulated wells to the mean counts per minute of the stimulated wells to the mean counts per minute of the wells containing cells and medium alone. Assays were considered valid only if the counts per minute measured in each of the control wells (i.e., the control protein wells and the wells containing cells and media alone) was less than 1,000.
Detection of HIV-1-specific CTL precursors following virus-specific in vitro stimulation of PBMC.
We (18, 28) have previously demonstrated the detection of cytotoxic T-lymphocyte (CTL) precursors in viably cryopreserved PBMC from infants who did not receive early, potent combination antiretroviral therapy. Freshly isolated PBMC were viably cryopreserved using a KRYO 10 series cell freezer and stored in liquid nitrogen until use. PBMC were used for these studies only if their viability (determined by trypan blue exclusion) was greater than 90%. Cryopreserved patient PBMC (3 × 106 to 5 × 106) were thawed and cultured with psoralen-fixed, autologous B-lymphoblastoid cell lines (B-LCL) infected with a recombinant vaccinia virus expressing the HIV-1 gag, envelope, and polymerase gene products (VT408; Therion Biologics, Cambridge, Mass.). At 14 days, the resultant cell lines were tested for HIV-1-specific cytotoxicity using autologous B-LCL infected with vaccinia virus or vaccinia virus recombinant expressing HIV-1 gag (vABT 141) or env (vABT 299) gene products (18) in a 51Cr release assay using an effector/target ratio of 50:1.
Detection and enumeration of HIV-1-specific CD8+ T cells using HLA-peptide tetramers.
HLA A*0201 heavy chain and human β2-microglobulin in the procaryotic expression system (pET R + D) were obtained from David Garboczi (National Institutes of Health). The 3′ end of the HLA A*0201 heavy chain was modified by adding a Gly/Ser linker to amino acid (aa) 276 of the HLA sequence followed by the BirA biotinylation site and a stop codon (2, 9). These expression plasmids in XA-90 cells were grown to mid-log phase and induced with 0.5 mM isopropyl-β-thiogalactosidase (IPTG). Inclusion bodies were purified as described previously (13) and solubilized in 6 M guanidine-HCl (pH 8.2). The heavy chain and β2-microglobulin were refolded with each of the following peptides: the HIV-1 Gag p17 peptide (LAI aa 77 to 85; SLYNTVATL), the HIV-1 RT peptide (LAI aa 476 to 484; ILKEPVHGV), or the Epstein-Barr virus (EBV) BMLF1 peptide (aa 280 to 288/GLCTLVAML). Purified monomers were biotinylated with BirA (Avidity, Denver, Colo.) overnight at 30°C and separated from free biotin using Centricon-30 centrifugation concentrators. Biotinylated monomers were mixed with Neutravidin–R-phycoerythrin conjugate (Sigma) at a molar ratio of 4:1 to form tetramers (2).
Cryopreserved PBMC (106) from individual study participants were thawed and stained with 1 μl of tetramer along with CD3-Cychrome and CD8-FITC (Pharmingen, La Jolla, Calif.) and analyzed by flow cytometry. All available samples from an individual study participant were stained and analyzed together. A positive control (an epitope-specific, HLA A*0201-restricted CTL line) and negative controls (PBMC from HIV-1-uninfected, HLA A*0201-positive individuals) were included in each assay. Tetramer staining of PBMC from HIV-1-uninfected, HLA A*0201-positive individuals was always <0.02%.
Detection of HIV-1 2-LTR circles in PBMC.
PBMC pellets were resuspended in buffer P1, and extrachromosomal DNA was purified by a QIAprep spin miniprep kit (Qiagen, Valencia, Calif.), using the modification for the isolation of low-copy-number plasmids as recommended by the manufacturer. Chromosomal DNA was recovered from the sodium acetate-sodium dodecyl sulfate precipitate using DNAzol reagent (Life Technologies, Gaithersburg, Md.) according to the manufacturer's protocol. Total cellular DNA was purified using an Isoquick nucleic acid extraction kit (ORCA Research, Bothell, Wash.). 2-LTR (long terminal repeat) circle junctions were amplified from 10 to 30 μl of extrachromosomal DNA in a 50-μl reaction containing 1× HotStarTaq buffer, 200 nM deoxynucleoside triphosphates, 400 nM primers, and 1.5 U of HotStarTaq (Qiagen). The reverse and forward primers were 5′-cagatctggtctaaccagaga-3′ and 5′-gtaactagagatccctcagac-3′, which annealed to nucleotides 9157 to 9137 (HIV-1 LTR R region) and nucleotides 130 to 150 (HIV-1 LTR U5 region) of HIV-1LAI, respectively (accession number K02013). After an initial denaturation step (95°C, 10 min), PCR amplification proceeded for 45 cycles (95°C, 30 s; 60°C, 30 s; 72°C, 60 s) followed by a final extension (72°C, 5 min). To control for the effect of sequence polymorphisms at primer binding sites, amplification was performed with internal primers that were reversed in orientation to those listed above. Amplification with the internal LTR primers proceeded for 35 cycles using conditions outlined above. Polymorphisms in the region of the LTR that is recognized by the fluorogenic probe can affect annealing of the probe and potentially result in false negatives. To accommodate this, Taqman reaction products were subsequently analyzed on agarose-Tris-borate-EDTA gels and stained with ethidium bromide to ensure that those reactions did not contain episome-specific PCR products. For quantitation of 2-LTR circle frequency in patient PBMC, PCRs were performed using an ABI Prism 7700 sequence detection system with the addition of 200 nM fluorogenic probe to the reaction (5′-agtggcgagccctcagatgctgc-3′) which anneals to nucleotides 9081 to 9103 of HIV-1LAI. The oligonucleotide probe was modified with 6-FAM (6-carboxyfluorescein) reporter dye on the 5′ end and 6-TAMRA (6-carboxytetramethylrhodamine) quencher dye on the 3′ end. Copy number estimates of 2-LTR circles were determined either by extrapolation from a plot of standards versus band intensity or by using the ABI Prism 7700 quantitation software.
RESULTS
Long-term suppression of plasma HIV-1 load following the initiation of early combination antiretroviral therapy.
These studies focus on 17 HIV-1-infected infants who initiated open-label, three- or four-drug antiretroviral regimens between 15 days and 3 months of age, with subsequent suppression of plasma HIV-1 RNA levels to <50 copies/ml. Individual patient characteristics and treatment regimens are provided in Table 1.
Prior to the initiation of study drug therapy, plasma HIV-1 RNA copy numbers ranged from 103.3 to 105.8 copies (median, 105.3/ml [Table 1]). As described previously (21), a biphasic clearance of HIV-1 virions from the plasma was observed following the initiation of antiretroviral therapy. An initial, rapid exponential decline (median half-life = 0.9 days) in plasma HIV-1 virions was followed by a slower second phase decline (median half-life = 15 days). Over 90% of plasma virions were cleared during the first phase of viral decay, and the plasma HIV-1 RNA copy number fell below 400 copies/ml by 20 weeks (range, 0.5 to 20 weeks; median, 12 weeks) in all infants.
An ultrasensitive assay was then used to detect and measure plasma HIV-1 RNA copy numbers in subsequent plasma samples. Plasma HIV-1 RNA fell below 50 copies/ml in 13 infants (76%) within 24 weeks of initiating therapy. By 40 weeks of therapy, plasma HIV-1 RNA was <50 copies/ml in all but one infant; plasma HIV-1 RNA fell to <50 copies/ml by 68 weeks in that infant.
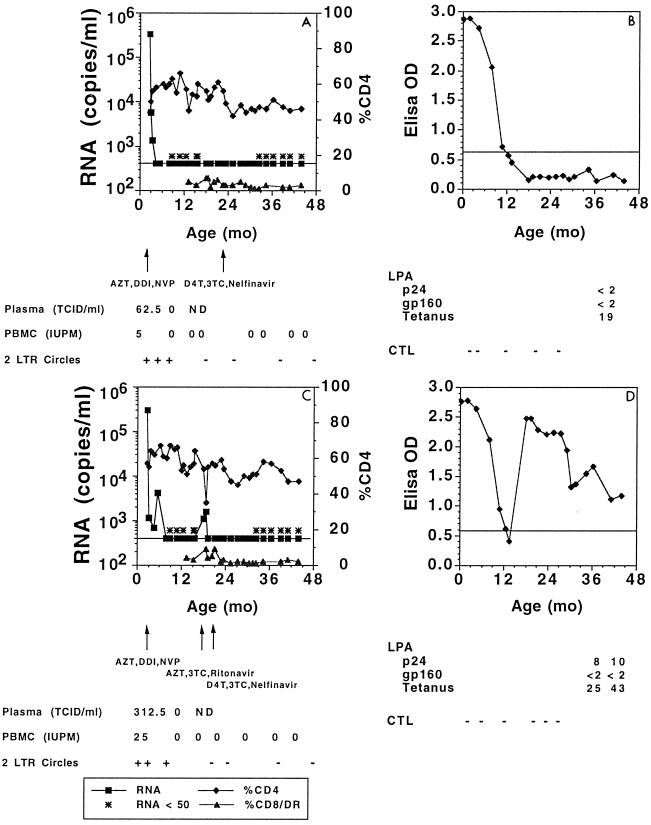
Intensive follow-up of these infants has continued through a median of 21 months (range, 16 to 56 months [Table 1]). After 15 months of therapy, HIV-1 RNA (1,000 to 1,700 copies/ml) was again detectable in the plasma of one infant (P-3742) (Fig. 1). Following a change in regimen from zidovudine (ZDV), didanosine (ddI), and nevirapine (NVP) to stavudine (D4T), lamivudine (3TC), and nelfinavir (NLF), plasma RNA copy number again dropped below 50/ml and has remained less than 50/ml for an additional 41 months. Plasma HIV-1 RNA copy number has remained <50 copies/ml in all other infants.
FIG. 1.
Peripheral blood HIV-1 load and CD4 T-lymphocyte percentages (A and C) and HIV-1-specific immune responses (B and D) in two children (P-3743 [A and B] and P-3742, [C and D]) with prolonged suppression of HIV-1 replication following early antiretroviral therapy.
Preservation of peripheral blood CD4 T cells following the suppression of viral replication.
Prior to the initiation of therapy, the percentage of CD4 T cells in the peripheral blood of the infants studied ranged from 14 to 63% (median, 44%) (Table 1). The percentage of CD4 T cells in the peripheral blood at baseline was normal for age in 14 infants and <25% in only 3 infants. At 48 weeks of therapy, the percentage of CD4 T cells in the peripheral blood ranged from 29 to 59% (median, 45%). The peripheral blood CD4 count therefore normalized or remained normal for age in all infants following the suppression of viral replication.
HIV-1 specific antibody responses following the initiation of combination antiretroviral therapy.
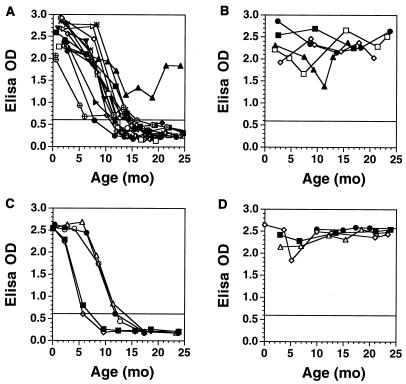
Following the initiation of the combination antiretroviral therapies, a reduction in HIV-1 antibody titers was observed in all infants, and all but one infant became seronegative by 16 months of age (Fig. 2). The decline in antibody titers was accompanied by loss of antibody binding in Western blot assays. The kinetics of plasma HIV-1-specific antibody clearance in infants who received early therapy are similar to those observed in uninfected infants born to HIV-1-seropositive women (Fig. 2), suggesting that these infants did not actively generate HIV-1-specific antibodies. This was observed despite normal plasma immunoglobulins for age (data not shown). By contrast, infants with persistent viral replication maintained high titers of HIV-1-specific antibodies in their peripheral blood.
FIG. 2.
HIV-1-specific antibodies measured by ELISA in plasma from infants with durable (>48 weeks; n = 15; A) or incomplete/transient (n = 4; B) suppression of HIV-1 replication following early antiretroviral therapy. HIV-1-specific antibodies measured by ELISA in plasma from HIV-1-uninfected infants born to HIV-1-infected women are depicted in panel C (n = 5); HIV-1 antibodies measured in the plasma of HIV-1-infected infants who did not receive therapy over the first 12 months of life are depicted in panel D (n = 4).
HIV-1-specific antibodies were promptly detected by ELISA and by Western blotting (gp160, gp120, p66, P55, p51, gp41, p24, p17) in the plasma of the infant (P-3742) who experienced viral breakthrough after 15 months of therapy (Fig. 1). Over the subsequent 41 months following the resuppression of viral replication, this child has remained seropositive by ELISA, although decreased antibody binding in the ELISAs has been observed. Antibody binding to only gp160, gp120, and p24 has persisted on Western blotting. Altogether, these data suggest that the generation of antibody responses is sensitive to the presence of replicating virus. The decrease in the titers and breadth of antibodies in infant P-3742 following the control of viral replication also suggest that the maintenance of antibody responses is sensitive to the presence of replicating virus.
HIV-1-specific lymphoproliferative responses following the initiation of combination antiretroviral therapy.
Sufficient PBMC were available from 10 infants to evaluate HIV-1-specific lymphoproliferative responses (Table 2). Responses to PWM and tetanus were detected in all infants. HIV-1 p24-specific lymphoproliferative responses were detected at multiple time points in one infant (P-3742 [Table 2]) following viral breakthrough. HIV-1 p24-specific lymphoproliferative responses (SI = 7 to 8) were also detected at one time point in each of two additional infants but were not detected on subsequent specimens. HIV-1 p24-specific lymphoproliferative responses were not detected (SI < 3) in the other seven infants.
TABLE 2.
HIV-1-specific lymphoproliferative responses in infants treated with early, potent combination therapy
| Patient | Age (mo) | Study wk | PWM | Tetanus | p24 | gp160 |
|---|---|---|---|---|---|---|
| P-1228 | 16 | 68 | 240 | 29 | 0.2 | 0.3 |
| 23 | 104 | 863 | 130 | 0 | ND | |
| 26 | 116 | 153 | 80 | 1 | ND | |
| P-1048 | 7 | 32 | 75 | 2 | 2 | 1.6 |
| 11 | 48 | 250 | 6 | 1 | 0.5 | |
| P-1235 | 8 | 24 | 154 | 1 | 1 | 2 |
| 14 | 48 | 293 | 11 | 2 | 3 | |
| P-1238 | 8 | 24 | 486 | 1 | 0.7 | 1.7 |
| 15 | 48 | 157 | 32 | 1.1 | 1.8 | |
| 18 | 68 | 305 | 150 | 1.2 | 0.7 | |
| 21 | 80 | 143 | 35 | 0.5 | 0.5 | |
| P-1246 | 8 | 32 | 889 | 62 | 8 | 1.1 |
| 12 | 68 | 988 | 123 | 0 | 0 | |
| 15 | 80 | 643 | 219 | 1.4 | 0.5 | |
| P-1247 | 7 | 24 | 64 | 32 | 7 | 1.7 |
| 18 | 68 | 188 | 24 | 1.4 | 1.1 | |
| P-1252 | 14 | 48 | 398 | 12 | 1.0 | 0.6 |
| P-1222 | 8 | 24 | 158 | 6 | 0.6 | 1 |
| P-3742 | 39 | 208 | 106 | 25 | 8 | <2 |
| 41 | 216 | 251 | 43 | 10 | <2 | |
| 58 | 284 | 98 | 12 | 14 | 1 | |
| P-3743 | 35 | 192 | 22 | 6 | <2 | <2 |
| 40 | 212 | 393 | 19 | <2 | <2 |
HIV-1 gp160-specific lymphoproliferative responses were not detected in any of the infants studied.
Longitudinal analyses of HIV-1-specific CTL responses.
Sufficient PBMC were available from eight infants to evaluate HIV-1-specific CTL responses between 24 and 48 weeks following the initiation of therapy. Efforts to generate HIV-1-specific CTL lines were made by culturing PBMC from serial blood specimens obtained from each of six infants with psoralen-fixed B-LCL infected with vaccinia vectors expressing HIV-1 gene products (16). Lytic activity of the CTL lines was evaluated using 51Cr release assays. HIV-1-specific CTL responses to HIV-1 Gag and envelope were not detected in the peripheral blood of any of the six infants studied in this manner.
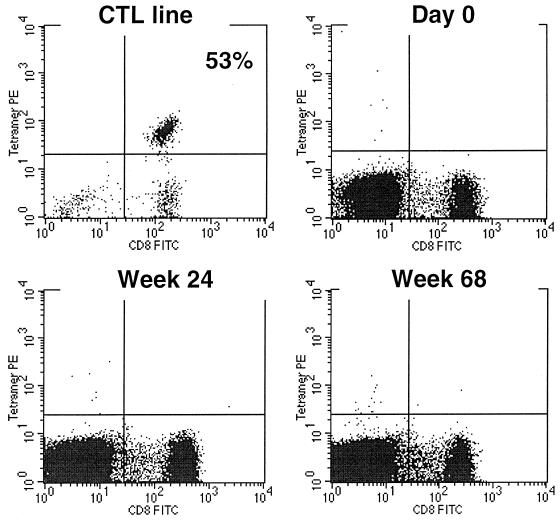
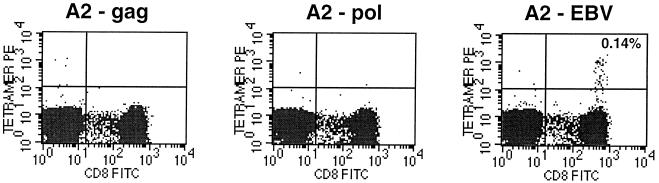
In vitro stimulation has traditionally been used to detect and quantify virus-specific CTL. It has long been recognized, however, that this method might underestimate CTL frequency since it relies on sufficient propagation of cells in culture to the point that they can be detected by the 51Cr release assay readout. A powerful and precise method that uses labeled major histocompatibility complex-peptide complexes (tetramers) to directly stain antigen-specific CD8 T cells in the peripheral blood has recently been described (2). Serial PBMC specimens from three HLA A*0201-positive infants with prolonged suppression of HIV-1 replication were stained with HLA A*0201/HIV-1 Gag p17 (LAI aa 77 to 85; SLYNTVATL) and RT peptide (LAI aa 476 to 484; ILKEPVHGV) tetramers. Responses to these epitopes are commonly detected in the peripheral blood of individuals with the HLA A*0201 allele and established HIV-1 infection (4); HLA A*0201/HIV-1 Gag p17 tetramer-staining cells have been detected in the circulation of the majority of HIV-1-infected, HLA A*0201-positive adults and children in our clinics. HIV-1-specific CD8+ T cells were not detected after direct tetramer staining (Fig. 3) of serial PBMC specimens from any of these infants or after in vitro peptide-specific stimulation of PBMC followed by tetramer staining. One of these three infants was noted to be EBV seropositive at 14 months of age. At weeks 56 and 80 of therapy, PBMC were stained also stained with a tetramer representing an HLA A*0201-restricted, EBV lytic protein epitope (BMLF1 aa 280 to 288/GLCTLVAML). Of note is that while HIV-1-specific CD8+ T lymphocytes were not detected in the peripheral blood of this infant, EBV tetramer-staining cells were detected at a frequency of 0.14% of PBMC (Fig. 4). The detection of EBV tetramer-staining cells in the peripheral blood suggests that this infant is capable of generating virus-specific CTL in response to replicating virus.
FIG. 3.
HLA A∗0201/Gag p17 tetramer staining of PBMC obtained prior to therapy (day 0) and at weeks 24 and 68 of therapy from an infant (P-1228) with durable control of HIV-1 replication (>96 weeks) after initiating ZDV/3TC/NVP therapy at 1 month of age. An HLA A∗0201-restricted, epitope-specific CTL line derived from an adult long-term nonprogressor was used as a positive control (53% of cells stained with tetramer; upper left).
FIG. 4.
HLA A∗0201/HIV Gag p17, (A2-gag), HLA A∗0201/HIV RT (A2-pol), or HLA A∗0201/EBV BMLF1 (A2-EBV) tetramer staining of PBMC obtained at 56 weeks of therapy from HIV-1 infected, EBV-seropositive infant P-1252.
Extrachromosomal HIV-1 infection intermediates.
An important question in these studies is the extent to which viral replication has been controlled in these infants. We have recently shown that extrachromosomal HIV-1 infection intermediates (2-LTR DNA circles) are labile products of HIV-1 replication and as such are indicative of recent infection events. These replication intermediates have been used to detect ongoing viral replication in individuals on potent combination antiretroviral therapy with plasma HIV-1 RNA levels below the detection levels of currently available plasma viral RNA assays (32). We therefore evaluated whether we could detect 2-LTR circle junctional sequences in PBMC infants from our study cohort. While 2-LTR circle junctional sequences were detected in the PBMC of three infants prior to therapy, 2-LTR circle sequences have not been detected in PBMC obtained from any of these infants at multiple time points after 48 weeks of therapy (e.g., Fig. 1). We then looked for the presence of 2-LTR circles in PBMC obtained from five additional infants at or after 48 weeks of therapy. In a PBMC sample obtained from one infant (P-1238) after 48 weeks of therapy, a product of appropriate molecular size was detected after 48 cycles in one of three replicate amplifications of the specimen; identity of the product as a 2-LTR circle junction was confirmed by sequencing. 2-LTR DNA circle junction sequences were not detected in the PBMC of any of the other four infants.
DISCUSSION
Recent studies have estimated the kinetics of HIV-1 replication in infected individuals and defined the central role of HIV-1 replication in pathogenesis of HIV-1 infection (15, 21). The present study was undertaken to evaluate the potential for long-term suppression of viral replication following early therapy of vertical infection and to determine the immune consequences of such therapies.
Infants constitute an ideal population for studies investigating the virologic and immunologic consequences of early, potent combination therapies. Since the vertical transmission of HIV-1 from a woman to her infant is the predominant mode of pediatric HIV-1 infection, infants at risk can be readily identified and evaluated for HIV-1 infection. Available data suggest that the majority of infants acquire HIV-1 infection at delivery (14). This, along with the ability to diagnose HIV-1 infection in the majority of infected infants within the first 4 weeks of life (20), allows the identification and therapy of infants within days to weeks of acquiring infection.
In this cohort of infants who began antiretroviral therapy within 3 months of birth, early combination therapy was associated with a loss of plasma viremia and labile extrachromosomal replication intermediates. HIV-1 provirus has been persistently detected in the PBMC of all infants. However, limited availability of PBMC from these infants has precluded large-scale virus cocultivation to evaluate the replication competence of the detected provirus. Despite apparently normal immune systems, most infants did not develop persistent HIV-1-specific immune responses. Altogether, our data suggest that early, potent combination therapy may allow extremely stringent, if not complete, control of viral replication.
The lack of persistently detectable HIV-1 specific immune responses in these infants was surprising given the high levels of plasma virus (median, 105.3 RNA copies/ml) that were detected prior to therapy; in addition, plasma HIV-1 RNA was detected in most infants by ultrasensitive assay for up to 68 weeks. Plasma RNA presumably represented replicating virus and therefore should have served as an appropriate stimulus for both humoral and cell-mediated immune responses; however, even a nonreplicating source of antigen should have evoked antibody responses.
The lack of persistent HIV-1-specific immune responses in infants contrasts with the persistent HIV-1-specific immune responses reported in adults who receive combination antiviral therapy within 3 to 4 months of acquisition of infection (22, 23, 25, 27, 30). Several investigators (22, 27, 30, 31) have reported persistently detectable HIV-1-specific CD4 T-lymphocyte responses in adults who received combination therapy during primary infection and have suggested that early, aggressive treatment of primary infection may facilitate the generation of these responses. While some groups have reported reduced antibody (25) and CTL (23) responses in adults treated during primary infection, the lack of persistently detectable responses is unusual (8).
Clonal deletion of CD8+ T cell responses in the presence of very high levels of antigen has been previously reported in the context of acute murine lymphocytic choriomeningitis virus infection (26). At least two lines of evidence argue against clonal deletion in our study population. First, the detection of HIV-1-specific antibody and lymphoproliferative responses in the peripheral blood of infant P-3742 following the breakthrough in viral replication argues against clonal deletion of HIV-1-reactive CD4 T lymphocytes. Additionally, the persistent detection of antibody and CD8 T-lymphocyte-mediated, HIV-1-specific CTL responses (4, 5, 18, 24, 28) in infants with uncontrolled viral replication argues against this.
The paucity of persistently detectable HIV-1-specific immune responses despite prolonged plasma viremia suggests that there may be a period during which the generation of immune responses is inefficient in infants. These data are compatible with prior data from our laboratory (18, 19, 28) and others (11, 12) suggesting a reduced ability to generate virus-specific immune responses in young infants. This might be due to a reduced efficiency of antigen presentation by infant dendritic cells (particularly to naïve T lymphocytes (33) or to a diminished ability of infant lymphocytes to produce factors, such as cytokines (36), important for the generation and maintenance of cell-mediated immunity. Suppression of viral replication during this period of relative immunological immaturity might critically limit the priming and expansion of virus-specific immune responses.
The development of normal antibody and cell-mediated immune responses to vaccine antigens (e.g., tetanus) or other viral (e.g., EBV) infections in these infants suggests that HIV-1-specific immune responses are particularly impaired. Factors that could contribute to this include the acquisition of infection in the presence of high titers of passively acquired, maternal HIV-1-specific antibodies and/or the selective depletion of HIV-1-specific CD4 T cells through the cytopathic effects of the virus or other mechanisms. Studies to better understand the factors that influence the generation of HIV-1-specific immune responses in these infants are under way. These studies include assays that detect intracellular cytokines in CD4 T lymphocytes by flow cytometry following in vitro stimulation, which appear to be more sensitive than lymphoproliferative assays for the detection of HIV-1-specific CD4 T-lymphocyte responses (29; A. C. McNeil, W. L. Shupert, J. A. Mican, and M. Connors, presented at the 7th Conference on Retroviruses and Opportunistic Infections, San Francisco, Calif., 30 January–2 February 2000).
In summary, early, potent combination antiretroviral therapy of HIV-1-infected infants allowed the long-term suppression of viral replication and preservation of immune function. In contrast to what is typically seen in adults treated early, persistent HIV-1-specific immune responses were not detected in most infants. The absence of detectable, persistent HIV-1-specific immune responses suggests that vaccine-induced, HIV-specific immune responses may be helpful in further controlling the virus early in life.
ACKNOWLEDGMENTS
We thank the children who participated in this study and their guardians. We also thank Sally Thomas and Bobbie Graham for support in protocol development and implementation; Kate Bak, Bruce Blais, Brittany Boisvert, Robin Brody, Kevin Byron, Richard Hudson, Erik Larson, and Linda Lambrecht for technical support; and Keilia Hodgkinson for preparation of the manuscript.
This publication was made possible by funding from the Pediatric AIDS Clinical Trial Group (AIDS Clinical Trials Group Protocols 180 and 356), National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH; AI32907 and Virology/Immunology Core Laboratories); by funding from the National Institute of Child Health and Human Development (NICHD) Perinatal/Pediatric HIV Clinical Trials Network, NICHD, NIH (contract N01-HD-3-3162); by grants AI32391 (K.L.) and AI26507 (J.L.S.) from the NIAID, NIH; by the University of Massachusetts Center for AIDS Research (AI-42845); by funding from the Elizabeth Glaser Pediatric AIDS Foundation; and by funding from Boehringer-Ingelheim Pharmaceuticals, Inc. K.L. is an Elizabeth Glaser Scientist of the Elizabeth Glaser Pediatric AIDS Foundation.
REFERENCES
- 1.Abrams E J, Weedon J, Steketee R W, Lambert G, Bamji M, Brown T, Kalish M L, Schoenbaum E E, Thomas P A, Thea D M New York City Collaborative Study Group. Association of human immunodeficiency virus (HIV) load early in life with disease progression among HIV-infected infants. J Infect Dis. 1998;178:101–108. doi: 10.1086/515596. [DOI] [PubMed] [Google Scholar]
- 2.Altman J, Moss P, Goulder P, Barouch D, McHeyzer-Williams M, Bell J I, McMichael A J, Davis M M. Direct visualization and phenotypic analysis of virus-specific T lymphocytes in HIV-infected individuals. Science. 1996;274:94–96. [Google Scholar]
- 3.Boyum A. Isolation of mononuclear cells and granulocytes from human blood. Scand J Clin Lab Investig. 1968;97:77–89. [PubMed] [Google Scholar]
- 4.Brander C, Goulder P, Luzuriaga K, Yang O, Hartman K, Jones N, Walker B, Kalams S. Persistent HIV-1 specific CTL clonal expansion despite high viral burden post in-utero HIV-1 infection. J Immunol. 1999;162:4796–4800. [PubMed] [Google Scholar]
- 5.Buseyne F, Burgard M, Teglas J P, Bui E, Rouzioux C, Mayaux M J, Blanche S, Riviere Y. Early HIV-specific cytotoxic T lymphocytes and disease progression in children born to HIV-infected mothers. AIDS Res Hum Retroviruses. 1998;14:1435–1444. doi: 10.1089/aid.1998.14.1435. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. Revised classification system for HIV-1 infection in children less than 13 years of age. Morb Mortal Wkly Rep. 1994;43(RR-12):1–10. [Google Scholar]
- 7.Coffin J M. HIV population dynamics in vivo: implications for genetic variation, pathogenesis and therapy. Science. 1995;267:483–489. doi: 10.1126/science.7824947. [DOI] [PubMed] [Google Scholar]
- 8.Daar E S. Virology and immunology of acute HIV type 1 infection. AIDS Res Hum Retroviruses. 1998;14:S-229–S-234. [PubMed] [Google Scholar]
- 9.Davis M M. Correction. Science. 1998;280:1821. [Google Scholar]
- 10.Dickover R, Dillon M, Gillette S, Deveikis A, Keller M, Plaeger-Marshall S, Chen I, Diagne A, Stiehm E R, Bryson Y. Rapid increases in load of human immunodeficiency virus correlate with early disease progression and loss of CD4 cells in vertically-infected infants. J Infect Dis. 1994;170:1279–1284. doi: 10.1093/infdis/170.5.1279. [DOI] [PubMed] [Google Scholar]
- 11.Gans H, Maldonado Y, Yasukawa L L, Beeler J, Audet S, Rinki M M, DeHovitz R, Arvin A M. IL-12, IFN-γ, and T cell proliferation to measles in immunized infants. J Immunol. 1999;162:5569–5575. [PubMed] [Google Scholar]
- 12.Gans H A, Arvin A M, Galinus J, Logan L, DeHovitz R, Maldonado Y. Deficiency of the humoral immune response to measles vaccine in infants immunized at age 6 months. JAMA. 1998;280:527–532. doi: 10.1001/jama.280.6.527. [DOI] [PubMed] [Google Scholar]
- 13.Garboczi D N, Hung D T, Wiley D C. HLA-A2-peptide complexes: refolding and crystallization of molecules expressed in Escherichia coli and complexed with single antigenic peptides. Proc Natl Acad Sci USA. 1992;89:3429–3433. doi: 10.1073/pnas.89.8.3429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garcia P M, Kalish L A, Pitt J, Minkoff H, Quinn T C, Burchett S K, Kornegay J, Jackson B, Moye J, Hanson C, Zorrilla C, Lew J F. Maternal levels of plasma human immunodeficiency virus type 1 RNA and the risk of perinatal transmission. N Engl J Med. 1999;341:394–402. doi: 10.1056/NEJM199908053410602. [DOI] [PubMed] [Google Scholar]
- 15.Ho D D. Dynamics of HIV-1 replication in vivo. J Clin Investig. 1997;99:2565–2567. doi: 10.1172/JCI119443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lubaki M N, Egan M A, Siliciano R F, Weinhold K J, Bollinger R C. A novel method for detection and ex vivo expansion of HIV type 1-specific cytolytic T lymphocytes. AIDS Res Hum Retroviruses. 1994;10:1427–1431. doi: 10.1089/aid.1994.10.1427. [DOI] [PubMed] [Google Scholar]
- 17.Luzuriaga K, Bryson Y, Krogstad P, Robinson J, Stechenberg B, Lamson M, Cort S, Sullivan J L. Combination treatment with zidovudine, didanosine, and nevirapine in infants with human immunodeficiency virus type 1 infection. N Engl J Med. 1997;336:1343–1349. doi: 10.1056/NEJM199705083361902. [DOI] [PubMed] [Google Scholar]
- 18.Luzuriaga K, Holmes D, Hereema A, Wong J, Panicali D L, Sullivan J L. HIV-1-specific cytotoxic T lymphocyte responses in the first year of life. J Immunol. 1995;154:433–443. [PubMed] [Google Scholar]
- 19.Luzuriaga K, Koup R A, Pikora C A, Brettler D B, Sullivan J L. Deficient human immunodeficiency virus type 1-specific cytotoxic T cell responses in vertically infected children. J Pediatr. 1991;119:230–236. doi: 10.1016/s0022-3476(05)80732-2. [DOI] [PubMed] [Google Scholar]
- 20.Luzuriaga K, Sullivan J L. DNA polymerase chain reaction for the diagnosis of vertical HIV infection. JAMA. 1996;275:1360–1361. [PubMed] [Google Scholar]
- 21.Luzuriaga K, Wu H L, McManus M, Britto P, Smith B, Mofenson L, Sullivan J L. Dynamics of HIV-1 replication in vertically-infected infants. J Virol. 1999;73:362–367. doi: 10.1128/jvi.73.1.362-367.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malhotra U, Berrey M M, Huang Y, Markee J, Brown D J, Ap S, Musey L, Schacker T, Corey L, McElrath M J. Effect of combination antiretroviral therapy on T-cell immunity in acute human immunodeficiency virus type 1 infection. J Infect Dis. 2000;181:121–131. doi: 10.1086/315202. [DOI] [PubMed] [Google Scholar]
- 23.Markowitz M, Vesanen M, Tenner-Racz K, Cao Y, Binley J M, Talal A, Hurley A, Ji X, Chaudhry M R, Yaman M, Frankel S, Heath-Chiozzi M, Londard J M, Moore J P, Racz P, Nixon D F, Ho D D. The effect of commencing combination antiretroviral therapy soon after human immunodeficiency virus type 1 infection on viral replication and antiviral immune responses. J Infect Dis. 1999;179:525–537. doi: 10.1086/314628. [DOI] [PubMed] [Google Scholar]
- 24.McFarland E J, Harding P A, Luckey D, Conway B, Young R K, Kuritzkes D R. High frequency of gag- and env-specific cytotoxic T lymphocyte precursors in children with vertically-acquired human immunodeficiency virus type 1 infection. J Infect Dis. 1994;170:766–774. doi: 10.1093/infdis/170.4.766. [DOI] [PubMed] [Google Scholar]
- 25.Morris L, Binley J M, Clas B A, Bonhoeffer S, Astill T P, Kost R, Hurley A, Cao Y, Markowitz M, Ho D D, Moore J P. HIV-1 antigen-specific and -nonspecific B cell responses are sensitive to combination antiretroviral therapy. J Exp Med. 1998;188:233–245. doi: 10.1084/jem.188.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moskophidis D, Lechner F, Pircher H, Zinkernagel R M. Virus persistence in acutely infected immunocompetent mice by exhaustion of antiviral cytotoxic effector T cells. Nature. 1993;362:758–761. doi: 10.1038/362758a0. [DOI] [PubMed] [Google Scholar]
- 27.Oxenius A, Price D A, Easterbrook P J, O'Callaghan C A, Kelleher A D, Whelan J A, Sontag G, Sewell A K, Phillips R E. Early highly active antiretroviral therapy for acute HIV-1 infection preserves immune function of CD8+ and CD4+ T lymphocytes. Proc Natl Acad Sci USA. 2000;97:3382–3387. doi: 10.1073/pnas.97.7.3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pikora C A, Sullivan J L, Panicali D, Luzuriaga K. Early HIV-1 envelope-specific cytotoxic T lymphocyte responses in vertically infected infants. J Exp Med. 1997;185:1153–1161. doi: 10.1084/jem.185.7.1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pitcher C J, Quittner C, Peterson D M, Connors M, Koup R A, Maino V C, Picker L J. HIV-1 specific CD4+ T cells are detectable in most individuals with active HIV-1 infection, but decline with prolonged viral suppression. Nature Med. 1999;5:518–525. doi: 10.1038/8400. [DOI] [PubMed] [Google Scholar]
- 30.Rosenberg E S, Billingsley J M, Caliendo A M, Boswell S L, Sax P E, Kalams S A, Walker B D. Vigorous HIV-1 specific CD4 T cell responses associated with control of viremia. Science. 1997;278:1447–1450. doi: 10.1126/science.278.5342.1447. [DOI] [PubMed] [Google Scholar]
- 31.Rosenberg E S, LaRosa L, Flynn T, Robbins G, Walker B D. Characterization of HIV-1 specific T-helper cells in acute and chronic infection. Immunol Lett. 1999;66:89–93. doi: 10.1016/s0165-2478(98)00165-5. [DOI] [PubMed] [Google Scholar]
- 32.Sharkey M, Teo I, Greenough T, Sharkova N, Luzuriaga K, Sullivan J L, Bucy R P, Kostrikis L G, Haase A, Davaro R, Cheeseman S H, Daly J, Bova C, Ellison R T I, Mady B, Lai K K, Moyle G, Nelson M, Gazzard B, Shaunak S, Stevenson M. Persistence of episomal HIV-1 infection intermediates in patients on highly active antiretroviral therapy. Nat Med. 2000;6:76–81. doi: 10.1038/71569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith S, Jacobs R F, Wilson C B. Immunobiology of childhood tuberculosis: a window on the ontogeny of cellular immunity. J Pediatr. 1997;131:16–26. doi: 10.1016/s0022-3476(97)70120-3. [DOI] [PubMed] [Google Scholar]
- 34.Van Rompay K K, Dailey P J, Tarara R P, Canfield D R, Aguirre N L, Cherrington J M, Lamy P D, Bischofberger N, Pedersen N C, Marthas M L. Early short-term 9-[2-(R)-(phosphonomethoxy)propyl]adenine treatment favorably alters the subsequent disease course in simian immunodeficiency virus-infected newborn rhesus macaques. J Virol. 1999;73:2947–2955. doi: 10.1128/jvi.73.4.2947-2955.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Van Rompay K K, Otsyula M G, Marthas M L, Miller C J, McChesney M B, Pedersen N C. Immediate zidovudine treatment protects simian immunodeficiency virus-infected newborn macaques against rapid onset of AIDS. Antimicrob Agents Chemother. 1995;39:125–131. doi: 10.1128/aac.39.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wilson C B, Westall J, Johnston L, Lewis D B, Dower S K, Alpert A R. Decreased production of interferon gamma by human neonatal cells: intrinsic and regulatory deficiencies. J Clin Investig. 1986;77:860–867. doi: 10.1172/JCI112383. [DOI] [PMC free article] [PubMed] [Google Scholar]