ABSTRACT
Introduction
Military trainees are at increased risk for infectious disease outbreaks because of the unique circumstances of the training environment (e.g., close proximity areas and physiologic/psychologic stress). Standard medical countermeasures in military training settings include routine immunization (e.g., influenza and adenovirus) as well as chemoprophylaxis [e.g., benzathine penicillin G (Bicillin) for the prevention of group A streptococcal disease] for pathogens associated with outbreaks in these settings. In a population of U.S. Army Infantry trainees, we evaluated changes in the oral microbiome during a 14-week military training cycle.
Materials and Methods
Trainees were enrolled in an observational cohort study in 2015–2016. In 2015, Bicillin was administered to trainees to ameliorate the risk of group A Streptococcus outbreaks, whereas in 2016, trainees did not receive a Bicillin inoculation. Oropharyngeal swabs were collected from participants at days 0, 7, 14, 28, 56, and 90 of training. Swabs were collected, flash frozen, and stored. DNA was extracted from swabs, and amplicon sequencing of the 16s rRNA gene was performed. Microbiome dynamics were evaluated using the QIIME 2 workflow along with DADA2, SINA with SILVA, and an additional processing in R.
Results
We observed that microbiome samples from the baseline (day 0) visit were distinct from one another, whereas samples collected on day 14 exhibited significant microbiome convergence. Day 14 convergence was coincident with an increase in DNA sequences associated with Streptococcus, though there was not a significant difference between Streptococcus abundance over time between 2015 and 2016 (P = .07), suggesting that Bicillin prophylaxis did not significantly impact overall Streptococcus abundance.
Conclusions
The temporary convergence of microbiomes is coincident with a rise in communicable infections in this population. The dynamic response of microbiomes during initial military training supports similar observations in the literature of transient convergence of the human microbiome under cohabitation in the time frame including in this experiment. This population and the associated longitudinal studies allow for controlled studies of human microbiome under diverse conditions.
INTRODUCTION
Advances in high-throughput sequencing and bioinformatics have greatly accelerated our insights into and understanding of the human microbiome, where it is recognized that a tremendous diversity of microbial communities—both within and between human hosts—exists.1 One such niche, the oral cavity, is home to a distinct microbiota, and although several studies have characterized the bacterial composition of the oral cavity in healthy individuals,1–3 fewer have described changes in the composition of these communities as a result of cohabitation and/or antibiotic treatment.4,5
Military recruits are at increased risk for pharyngitis and transmission of acute Streptococcus pyogenes [otherwise known as group A strep (GAS)],6 which could result in invasive disease. To ameliorate the risk of GAS outbreaks during training, intramuscular benzathine penicillin G (Bicillin) is routinely administered to military recruits upon entry, a practice first initiated in the 1950s that continues today.7–9 In 2016, however, a decline in the production of Bicillin resulted in a nationwide shortage of the drug (https://www.cdc.gov/std/funding/docs/STD-PCHD-TA-Notes-11c-Benzathine-Penicillin-G.pdf), which in turn prompted the suspension of GAS chemoprophylaxis among incoming trainees at Fort Benning, GA,9 a major training installation for the U.S. Army.
From 2015 to 2016, we conducted a longitudinal, observational cohort study among U.S. Army Infantry trainees at Fort Benning, GA, to examine the natural history of Staphylococcus aureus colonization and infection. A secondary objective of this study was to evaluate the longitudinal changes in host microbiome in a population of military trainees during the 14-week training cycle [One Station Unit Training (OSUT)]. Because the study period spanned the periods of use of chemoprophylaxis with Bicillin (2015) and non-use (2016) at Fort Benning, we were then afforded an opportunity to evaluate the longitudinal impact of mass antibiotic administration on the oral microbiome in a unique setting, namely a cohort of close-quartered military trainees whose daily diet, activities, and environment were shared for an extended period of time. Herein, we report the results of our study.
METHODS
Study Participants
In 2015, all incoming trainees received 1.2 million units of intramuscular Bicillin for prevention of GAS infection during training. Injections were administered during medical in-processing within 24 hours of arrival at Fort Benning. In 2016, because of a nationwide shortage of Bicillin, all GAS chemoprophylaxis strategies at Fort Benning were suspended, and none of the trainees entering OSUT that year received either Bicillin or azithromycin for GAS chemoprophylaxis.9
Sample Collection
We conducted a longitudinal, observational study among U.S. Army Infantry trainees at Fort Benning, GA, from September 2015 through December 2016.10 All trainees designated as entering previously identified training units were briefed on the study’s scientific rationale, voluntary nature of participation, and expected schedule of visits. Those who agreed to participate in the study provided written informed consent. Participants were asked to complete a total of five study visits at which specimens and data would be collected: Day 0 (i.e., the day of enrollment within 24 hours of their arrival at OSUT) and on days 14, 28, 56, and 90 of infantry training. At each of these visits, microbiome specimens were collected from oropharyngeal sites using BD BBL Culture Swabs (Becton Dickinson, Sparks, MD). Culture swabs were gently rotated three times in a circular fashion along the surface of the oropharynx while avoiding the tongue. Specimens were placed immediately onto dry ice and subsequently stored in −80 °C freezers until time of processing. This study was approved by the Uniformed Services University Institutional Review Board (IDCRP-090).
DNA Extraction, Amplification, and Sequencing
Total genomic DNA was extracted from 407 swabs at Omega Biosciences, with 196 of these samples from 43 of the 2015 participants and 211 of these samples from 48 of the 2016 participants. Samples were chosen with the intent of maximizing the number of total data points while still balancing samples between the 2 years and accounting for trainees who did not have all five time points. DNA concentration was quantified using PicoGreen, and samples with >2 ng/μL were processed for amplicon sequencing. After DNA extraction, the V1–V3 region of the 16S rRNA gene was amplified from each sample using the KAPA HiFi kit for the 16S rRNA gene (29). Libraries were evaluated using TapeStation and then sequenced on an Illumina MiSeq using paired end 2x300, targeting 50K reads per sample. A total of 22.8 m reads were generated across all samples, with a minimum read depth of 673, a median and mean of 56K, and a maximum of 116.5K reads per sample with no significant differences between the 2015 and 2016 sample depths.
Sequence Processing
Quality control and microbiome analysis were conducted via the QIIME 2 next-generation microbiome bioinformatics platform (release 2018.11).11 Further quality control and sequence clustering were conducted via the QIIME 2 DADA2 implementation.12 Before clustering, at a minimum, the leftmost 10 nucleotides of every read were trimmed to reduce the number of low-quality bases. Additionally, the median quality score was calculated for every read position, and reads were trimmed where the median quality score for the sequencing run fell below 20 for three consecutive bases. Paired-end reads were joined, and clustering was conducted using the default DADA2 parameters in QIIME 2, notably, using a maximum expected error rate of two nucleotides per read for each sequence cluster. After this processing, six samples, all from different 2016 participants (two from day 14, three from day 28, and one from day 90) were left with fewer than 1,000 reads and were removed from the analysis. Of the remaining 401 samples used for analysis (196 for the 43 individuals comprising the 2015 cohort and 205 for the 48 individuals comprising the 2016 cohort), the highest read count after quality control (QC) and read-joining was 40K, whereas the lowest was 4K. The DADA2 clustering resulted in amplicon sequence variants (ASVs), which are similar to operational taxonomic units but are characterized by their discrete nucleotide differences rather than by their taxonomic classifications. An advantage of ASVs is that they more easily enable sub-species and strain-level analysis than operational taxonomic units since they are defined by their nucleotide sequence differences. Each of the unique ASVs generated was subsequently classified using the SINA classifier with the SILVA SSU Ref NR 99 reference database (release 132).13,14 A total of 7,423 unique ASVs were identified across the samples with 6,442 of these ASVs classified to the genus level and spread across 268 unique genera.
Diversity Analysis and Statistics
Diversity analyses were performed using raw ASV counts for the 401 samples which passed QC (196 samples from 43 individuals for the 2015 cohort and 205 from 48 individuals for the 2016 cohort). β-diversity was measured using a weighted UniFrac metric across all samples and visualized using principal coordinate analysis (PCoA). Significance of clustering from PCoA was determined via a permutational multivariate analysis of variance (PERMANOVA) comparing patients within 2015 and 2016 as well as time points within each year (P-value < .05). For comparison of genera across year, raw ASV counts were normalized using percent abundance and aggregated to the genus level. Genera <0.5% abundance across all samples were aggregated as were ASVs not classified to the genus level.
RESULTS
Study Design and Sample Numbers
From 2015 to 2016, we conducted an observational, longitudinal cohort study among males undergoing U.S. Army Infantry training at Fort Benning, GA. As a part of this study, oropharyngeal swabs were collected from each participant on days 0, 7, 14, 28, 56, and 90. Further, in 2015, all recruits received intramuscular Bicillin, whereas in 2016, a nationwide shortage resulted in a lack of Bicillin for recruits. Microbiome swabs were obtained and processed from 43 individuals in 2015 and 48 in 2016.
Base Microbiome Composition Differs Between Years and Changes Over Time Throughout OSUT
Microbiome data was labeled based on cohort year (196 samples from 43 individuals for the 2015 cohort and 205 from 48 individuals for the 2016 cohort) and showed significant differences between years (P < .001, PERMANOVA). Within years, sampling day was tested as a categorical variable. In 2015, all but two pairs were significantly different (q < 0.05) (Table I), whereas in 2016, all but three were significantly different (Table II), indicating dynamic microbiomes throughout the training period.
TABLE I.
PERMANOVA Demonstrating All Time Points Within 2015 Except Day 14 Versus Day 56 Are Significantly Different
| 2015 microbiome β-diversity significance | |||||
|---|---|---|---|---|---|
| Day | Day | Sample size | Pseudo-F | P-value | q-value |
| Day 0 | Day 14 | 71 | 3.60740388 | 0.004 | 0.005 |
| Day 0 | Day 28 | 86 | 12.2634169 | 0.001 | 0.0016667 |
| Day 0 | Day 56 | 85 | 8.75342044 | 0.001 | 0.0016667 |
| Day 0 | Day 90 | 83 | 6.31929605 | 0.001 | 0.0016667 |
| Day 14 | Day 28 | 71 | 6.25814952 | 0.001 | 0.0016667 |
| Day 14 | Day 56 | 70 | 1.25901953 | 0.242 | 0.242 |
| Day 14 | Day 90 | 68 | 2.25123087 | 0.047 | 0.0522222 |
| Day 28 | Day 56 | 85 | 8.04246254 | 0.001 | 0.0016667 |
| Day 28 | Day 90 | 83 | 8.39147083 | 0.001 | 0.0016667 |
| Day 56 | Day 90 | 82 | 4.93174039 | 0.002 | 0.0028571 |
TABLE II.
PERMANOVA Demonstrating Days 14 Versus 56, 14 Versus 90, and 56 Versus 90 Are Not Significantly Different in 2016
| 2016 microbiome β-diversity significance | |||||
|---|---|---|---|---|---|
| Day | Day | Sample size | Pseudo-F | P-value | q-value |
| Day 0 | Day 14 | 89 | 7.31672375 | 0.001 | 0.0025 |
| Day 0 | Day 28 | 86 | 12.0390167 | 0.001 | 0.0025 |
| Day 0 | Day 56 | 85 | 14.019431 | 0.001 | 0.0025 |
| Day 0 | Day 90 | 83 | 10.0948933 | 0.001 | 0.0025 |
| Day 14 | Day 28 | 83 | 2.63684685 | 0.028 | 0.04 |
| Day 14 | Day 56 | 82 | 2.02877693 | 0.068 | 0.085 |
| Day 14 | Day 90 | 80 | 0.9036784 | 0.437 | 0.437 |
| Day 28 | Day 56 | 79 | 2.95981882 | 0.007 | 0.0116667 |
| Day 28 | Day 90 | 77 | 3.49839051 | 0.004 | 0.008 |
| Day 56 | Day 90 | 76 | 1.40603628 | 0.209 | 0.2322222 |
Streptococcus ASVs Are More Abundant on Day 28 in Both 2015 Cohort and 2016 Cohort
The most abundant genus in the oral cavity for both the 2015 cohort and the 2016 cohort was Streptococcus with 225 ASVs at the genus level (Fig. 1B). Though species-level classification was not able to be achieved, at the ASV level, a single ASV of Streptococcus dominated the genus (Fig. 1C and D). This ASV was most prevalent in both the 2015 cohort and the 2016 cohort. Further, in the 2015 cohort, this ASV showed a peak in abundance at day 28, which generally coincides with the incidence of acute respiratory infection in military training populations.9 Overall, a significant difference in total Streptococcus abundance (all Streptococcus ASVs) between the 2015 cohort and the 2016 cohort was not observed (Fig. 1A).
FIGURE 1.
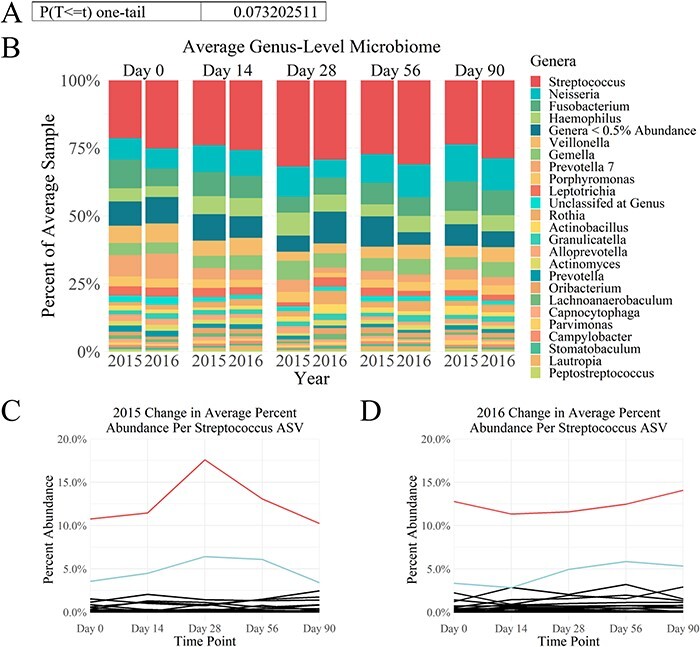
Streptococcus ASVs dominate the oral cavity microbiome. (A) There was not a significant difference between Streptococcus abundance over time between the 2015 cohort and the 2016 cohort (P = .07). (B) Comparison of the average percent abundance of ASVs agglomerated at the genus level between 2015 and 2016 illustrates that Streptococcus is the dominant genera across both years for all time points. (C) 2015 cohort Streptococcus change in abundance over time by ASV and (D) 2016 cohort Streptococcus change in abundance over time by ASV show that both years’ cohort oral microbiomes are dominated by the same two Streptococcus ASVs. The most abundant Streptococcus ASV is the top line for both years in red, whereas the second most abundant Streptococcus ASV is the next highest line for both years in light blue. All other Streptococcus ASVs are below and in black.
Recruits Have Diverse Microbiome Trajectories
To investigate the trajectory of individual microbiomes, data from the PCoA were extracted for visualization (Fig. 2). Some individuals showed little change in microbiome as indicated by a tight cluster of points, although others showed distinct changes throughout the training period. Participants who exhibit a wider, more linear spread have more changes in their microbiome over time than participants who have tightly grouped points.
FIGURE 2.
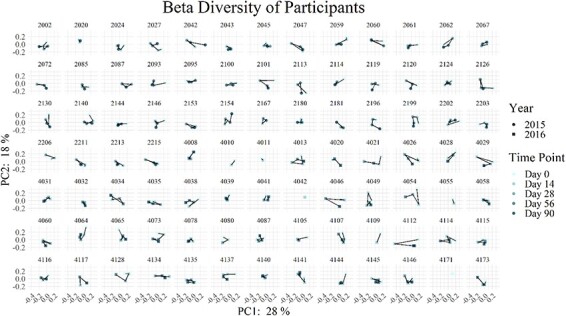
Trajectory of microbiome β-diversity changes over time and differs between participants. β-diversity was determined using weighted UniFrac to compare across time points for an individual. Each plot is of one individual trainee, with the first 43 of these being trainees from the 2015 cohort and the latter 48 of these being trainees from the 2016 cohort.
DISCUSSION
Prophylactic measures to prevent infectious disease outbreaks in congregate military populations are important public health tools; however, they also impose significant health care, financial, and operational impacts. Understanding the effect of mass antibiotic administration on microbiome dynamics and the relationship of the microbiome to disease prevalence can potentially provide insights into alternative methods to help maintain a healthy population. Cohabitation has been shown to result in convergence of individual microbiomes,15 and further, it has been demonstrated that the built environment changes with human habitation.16,17 Similarly, it is well established that close quarters can result in increased transmission of infectious disease.18 Thus, taken altogether, it is valuable to understand the effects of prolonged close-proximity on the human microbiome with regard to pathogenic organisms as well as to non-pathogenic organisms. Although it is easy to assume that close quarters will inevitably lead to pathogen exchange between cohabitants, understanding the full spectrum of bacteria exchanged between cohabitants, and the time frame and trajectory of this exchange could lead to more efficient and effective use of disease prophylactics.
The population used in this study allows for group- and individual-based data on the host microbiome, in the context of living in close proximity with others. Further, these individuals are subject to controlled living conditions, including similar diet, exercise, and activity regimes. This represents an ideal and relatively homogenous population for the study of cohabitation and transmission dynamics in the microbiome. An important limitation of note is that although study enrollment exceeded 80% of the entire platoon, it was not 100% in either year group. Additionally, some data points were missing either because of swabs not being collected or failing to pass sequencing quality control. However, the statistical analyses used were not limited by missing data since both the 2015 and 2016 cohorts still had a large, comparable number of high-quality samples left for analysis (196 and 205 from 43 and 48 individuals, respectively). That said, if samples were obtained from a greater number of individuals for both years, this might have enabled significant differentiation in some aspects of Streptococcus abundance between the 2 years.
In this study, we observed that Streptococcus was the primary genus represented in the oropharynx microbiome.2 We observed that colonization of Streptococcus ASVs persisted in spite of Bicillin prophylaxis, as shown previously.19 In fact, Streptococcus abundance over time did not significantly differ between the 2015 cohort and the 2016 cohort (P = .07). However, we were not able to obtain strain-level classification for these ASVs, a limitation which prevented us from determining any effect the Bicillin administration might have had on particular Streptococcus species or strains. However, the same two ASVs were dominant across both the 2015 cohort and the 2016 cohort, suggesting that the Bicillin did not have a significant effect on the most abundant Streptococcus strains. Thus, other interventions such as azithromycin prophylaxis may be beneficial20 for reduction of Streptococcus in OSUT populations. Between the 2015 cohort and the 2016 cohort, we observed significantly different microbiome communities; however, the study design did not allow us to determine whether any part of the difference was a direct result of Bicillin administration versus general year-to-year variation. However, these results are consistent with other studies, demonstrating that antibiotic treatments affect diverse host microbiome systems on the whole.4,21–23
A primary result of this study is the observation that the oropharynx microbiome changes throughout the 14 weeks of OSUT. In both the 2015 cohort and the 2016 cohort, the majority of pairwise comparisons of sampling days were significantly different, indicating a continuously changing microbiome throughout training. Although the cause of this change is unknown, cohabitation,15 changes to diet,24 changes in environment,25 and changes in activity levels26 may all contribute to microbiome dynamics in this population. Additional analyses of this population could help resolve cohabitation versus other factors. Although the research presented in this manuscript analyzed samples at the company level (all members of the 2015 cohort were in the same company of 200 recruits, whereas all members of the 2016 cohort were in the same company of 200 recruits), the analysis could be further conducted at the platoon level (each company is composed of four platoons of 50 recruits each). As the different platoons have different schedules, any differences between the microbiomes of platoons could help further resolve possible contributing factors.
Together, this work highlights the unique access to controlled populations as a tool to study microbiome dynamics. We observed a variation in the 2015 cohort and the 2016 cohort oropharynx microbiomes of OSUT recruits, as well as a dynamic change from entry into training through completion. Further work on regional incoming microbiomes as well as host and environmental factors that contribute to microbiome stability and convergence can be investigated with this population. This work, and future studies of this population, can inform microbiome management strategies to maintain recruit health and reduce transmitted infections.
ACKNOWLEDGMENTS
We are indebted to the U.S. Army Training and Doctrine Command, the 30th Adjutant General Reception Battalion, and the 198th Infantry Battalion at Fort Benning, GA, for their support of clinical research studies among the U.S. Army Infantry training population, and most especially, to the U.S. Army Infantrymen who agreed to participate in the study in spite of the demands of the military training. We are grateful for the Infectious Disease Clinical Research Program (IDCRP) site managers, clinical research coordinators, and laboratory personnel who contributed to the successful execution of the study. We also extend our gratitude to the investigative team members from the Uniformed Services University, Walter Reed Army Institute of Research, and the Benning Martin Army Community Hospital for their assistance in the execution of study activities and to Leigh Carson for editorial assistance with the manuscript.
CLINICAL TRAIL REGISTRATION
Not applicable.
INSTITUTIONAL REVIEW BOARD (HUMAN SUBJECTS)
This study (IDCRP-090) was approved by the Uniformed Services University Institutional Review Board.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC)
Not applicable.
INDIVIDUAL AUTHOR CONTRIBUTION STATEMENT
K.K.Z. conducted the bioinformatics analysis and drafted the original manuscript. R.P. performed informatics analysis. A.E. provided genomic guidance and drafted the original manuscript. C.M.T. secured and designed the genomic analysis and drafted the original manuscript. C.E.E. carried out data collection and analysis. M.W.E. contributed to concept development and initial study design. D.R.T. contributed to concept development and initial study design and carried out data analysis and interpretation. D.S.M. designed the original study, provided guidance on the bioinformatics analysis, and drafted the original manuscript. J.W.B. secured, designed, and conducted the original study. E.V.M. secured, designed, and conducted the original study and drafted the original manuscript. All authors reviewed and approved the final manuscript.
INSTITUTIONAL CLEARANCE
Institutional clearance approved.
Contributor Information
Kristina K Zudock, Research and Exploratory Development Department, Johns Hopkins University Applied Physics Laboratory, Laurel, MD 20723, USA.
Robert Player, Asymmetric Operations Sector, Johns Hopkins University Applied Physics Laboratory, Laurel, MD 20723, USA.
Amanda Ernlund, Research and Exploratory Development Department, Johns Hopkins University Applied Physics Laboratory, Laurel, MD 20723, USA.
Collin M Timm, Asymmetric Operations Sector, Johns Hopkins University Applied Physics Laboratory, Laurel, MD 20723, USA.
Caroline E English, Infectious Disease Clinical Research Program, Department of Preventive Medicine and Biostatistics, Uniformed Services University of the Health Sciences, Bethesda, MD 20814, USA; Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc., Bethesda, MD 20817, USA.
Michael W Ellis, Division of Infectious Diseases, University of Toledo College of Medicine and Life Sciences, Toledo, OH 43614, USA.
David R Tribble, Infectious Disease Clinical Research Program, Department of Preventive Medicine and Biostatistics, Uniformed Services University of the Health Sciences, Bethesda, MD 20814, USA.
D Scott Merrell, Department of Microbiology and Immunology, Uniformed Services University of the Health Sciences, Bethesda, MD 20814, USA.
Jason W Bennett, Multidrug-Resistant Organism Repository and Surveillance Network, Bacterial Diseases Branch, Walter Reed Army Institute of Research, Silver Spring, MD 20910, USA; Department of Medicine, Uniformed Services University of the Health Sciences, Bethesda, MD 20814, USA.
Eugene V Millar, Infectious Disease Clinical Research Program, Department of Preventive Medicine and Biostatistics, Uniformed Services University of the Health Sciences, Bethesda, MD 20814, USA; Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc., Bethesda, MD 20817, USA.
FUNDING
This work (IDCRP-90) was conducted by the Infectious Disease Clinical Research Program, a United States Department of Defense program executed by the Uniformed Services University through a cooperative agreement with the Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc. This project was supported with federal funds from the National Institute of Allergy and Infectious Diseases, National Institues of Health, under Inter‐Agency Agreement Y1-Al-5072 and from the Defense Health Program, U.S. DoD, under award HU0001190002.
CONFLICT OF INTEREST STATEMENT
None declared.
DATA AVAILABILITY
The data that support the findings of this study are available on request from the corresponding author.
REFERENCES
- 1. Costello EK, Lauber CL, Hamady M, Fierer N, Gordon JI, Knight R: Bacterial community variation in human body habitats across space and time. Science 2009; 326(5960): 1694–7.doi: 10.1126/science.1177486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Aas JA, Paster BJ, Stokes LN, Olsen I, Dewhirst FE: Defining the normal bacterial flora of the oral cavity. J Clin Microbiol 2005; 43(11): 5721–32.doi: 10.1128/JCM.43.11.5721-5732.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nasidze I, Li J, Quinque D, Tang K, Stoneking M: Global diversity in the human salivary microbiome. Genome Res 2009; 19(4): 636–43.doi: 10.1101/gr.084616.108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jakobsson HE, Jernberg C, Andersson AF, Sjolund-Karlsson M, Jansson JK, Engstrand L: Short-term antibiotic treatment has differing long-term impacts on the human throat and gut microbiome. PLoS One 2010; 5(3): e9836.doi: 10.1371/journal.pone.0009836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Adamsson I, Nord CE, Lundquist P, Sjostedt S, Edlund C: Comparative effects of omeprazole, amoxycillin plus metronidazole versus omeprazole, clarithromycin plus metronidazole on the oral, gastric and intestinal microflora in Helicobacter pylori-infected patients. J Antimicrob Chemother 1999; 44(5): 629–40.doi: 10.1093/jac/44.5.629 [DOI] [PubMed] [Google Scholar]
- 6. Gray GC, Callahan JD, Hawksworth AW, Fisher CA, Gaydos JC: Respiratory diseases among U.S. military personnel: countering emerging threats. Emerg Infect Dis 1999; 5(3): 379–85.doi: 10.3201/eid0503.990308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. McFarland RB, Colvin VG, Seal JR: Mass prophylaxis of epidemic streptococcal infections with benzathine penicillin G. II. Experience at a naval training center during the winter of 1956-57. N Engl J Med 1958; 258(26): 1277–84.doi: 10.1056/NEJM195806262582601 [DOI] [PubMed] [Google Scholar]
- 8. Schreier AJ, Hockett VE, Seal JR: Mass prophylaxis of epidemic streptococcal infections with benzathine penicillin G. I. Experience at a naval training center during the winter of 1955-56. N Engl J Med 1958; 258(25): 1231–8.doi: 10.1056/NEJM195806192582501 [DOI] [PubMed] [Google Scholar]
- 9. Webber BJ, Kieffer JW, White BK, Hawksworth AW, Graf PCF, Yun HC: Chemoprophylaxis against group A Streptococcus during military training. Prev Med 2019; 118: 142–9.doi: 10.1016/j.ypmed.2018.10.023 [DOI] [PubMed] [Google Scholar]
- 10. Blum FC, Whitmire JM, Bennett JW, et al. : Nasal microbiota evolution within the congregate setting imposed by military training. Sci Rep 2022; 12(1): 11492.doi: 10.1038/s41598-022-15059-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bolyen E, Rideout JR, Dillon MR, et al. : Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nature Biotechnol 2019; 37(8): 852–7.doi: 10.1038/s41587-019-0209-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJ, Holmes SP: DADA2: high-resolution sample inference from Illumina amplicon data. Nat Methods 2016; 13(7): 581–3.doi: 10.1038/nmeth.3869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pruesse E, Peplies J, Oliver Glöckner F: SINA: accurate high-throughput multiple sequence alignment of ribosomal RNA genes. Bioinformatics 2021; 28(14): 1823–9.doi: 10.1093/bioinformatics/bts252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Quast C, Pruesse E, Yilmaz P, et al. : The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res 2013; 41(D1): D590–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ross AA, Doxey AC, Neufeld JD: The skin microbiome of cohabitating couples. mSystems 2017; 2(4): e00043–17.doi: 10.1128/mSystems.00043-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Avila-Herrera A, Thissen J, Urbaniak C, et al. : Crewmember microbiome may influence microbial composition of ISS habitable surfaces. PLoS One 2020; 15(4): e0231838.doi: 10.1371/journal.pone.0231838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lax S, Smith DP, Hampton-Marcell J, et al. : Longitudinal analysis of microbial interaction between humans and the indoor environment. Science 2014; 345(6200): 1048–52.doi: 10.1126/science.1254529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Centers for Disease Control and Prevention : COVID-19 guidance for shared or congregate housing. Available at https://www.cdc.gov/coronavirus/2019-ncov/community/shared-congregate-house/guidance-shared-congregate-housing.html, December 31, 2020; accessed December 10, 2021.
- 19. Gray GC, Escamilla J, Hyams KC, Struewing JP, Kaplan EL, Tupponce AK: Hyperendemic Streptococcus pyogenes infection despite prophylaxis with penicillin G benzathine. N Engl J Med 1991; 325(2): 92–7.doi: 10.1056/NEJM199107113250204 [DOI] [PubMed] [Google Scholar]
- 20. Putnam SD, Gray GC, Biedenbach DJ, Jones RN: Pharyngeal colonization prevalence rates for Streptococcus pyogenes and Streptococcus pneumoniae in a respiratory chemoprophylaxis intervention study using azithromycin. Clin Microbiol Infect 2000; 6(1): 2–8.doi: 10.1046/j.1469-0691.2000.00001.x [DOI] [PubMed] [Google Scholar]
- 21. Nogueira T, David PHC, Pothier J: Antibiotics as both friends and foes of the human gut microbiome: the microbial community approach. Drug Dev Res 2019; 80(1): 86–97.doi: 10.1002/ddr.21466 [DOI] [PubMed] [Google Scholar]
- 22. Raymond F, Deraspe M, Boissinot M, Bergeron MG, Corbeil J: Partial recovery of microbiomes after antibiotic treatment. Gut Microbes 2016; 7(5): 428–34.doi: 10.1080/19490976.2016.1216747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shaw LP, Bassam H, Barnes CP, Walker AS, Klein N, Balloux F: Modelling microbiome recovery after antibiotics using a stability landscape framework. ISME J 2019; 13(7): 1845–56.doi: 10.1038/s41396-019-0392-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Singh RK, Chang HW, Yan D, et al. : Influence of diet on the gut microbiome and implications for human health. J Transl Med 2017; 15(1): 73.doi: 10.1186/s12967-017-1175-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Phillips ML: Gut reaction: environmental effects on the human microbiota. Environ Health Perspect 2009; 117(5): A198–205.doi: 10.1289/ehp.117-a198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mailing LJ, Allen JM, Buford TW, Fields CJ, Woods JA: Exercise and the gut microbiome: a review of the evidence, potential mechanisms, and implications for human health, exercise and sport sciences reviews. Am Coll Sports Med 2019; 47(2): 75–85.doi: 10.1249/JES.0000000000000183 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author.


