To The Editor:
The impact of the autologous graft (autograft) CD34+ cell dose on outcomes among patients undergoing autologous hematopoietic stem cell transplantation (HSCT) for high-risk neuroblastoma is currently unknown. Pediatric transplant physicians regularly have the option to administer CD34+ doses higher than required for hematopoietic recovery, with few published studies to guide practice when surplus CD34+ cells are available. In adult patients undergoing autologous HSCT, higher CD34+ cell doses are associated with improved survival and more rapid neutrophil engraftment.1,2 However, infusions of larger grafts may be associated with an increased incidence of endothelial injury complications (EIC), as greater numbers of immune effector cells are infused with the graft.3 Such complications are mediated by interactions between activated endothelial cells and immune effector cells (IECs), and are a major cause of non-relapse mortality (NRM).4 In the current study, we therefore sought to determine whether large CD34+ cell doses were advantageous or deleterious by examining the relationship between CD34+ dose and engraftment, relapse rates, EICs, non-relapse mortality (NRM), progression-free survival (PFS) and overall survival (OS) in children undergoing autologous HSCT for high-risk neuroblastoma.
Data were obtained from the Center for International Blood and Marrow Transplant Research (CIBMTR), and included children aged <10 years undergoing autologous peripheral blood transplant (single or tandem) for neuroblastoma in the United States or Canada between 2008 and 2018. Patients were excluded if their CD34+ cell count was not reported, or if their pre-transplant disease status was reported as stable disease (SD), progressive disease (PD), or unknown (UNK). Patients who underwent more than one autologous HSCT were analyzed based on the CD34+ cell dose of their first transplant, with the assumption that CD34+ cell dose was equivalent across all transplants. The primary study endpoint was PFS, with secondary endpoints including time-to-neutrophil-engraftment, EIC incidence, relapse, NRM, and OS. EICs were studied as a composite variable that included VOD/SOS, engraftment syndrome, idiopathic pneumonia syndrome, thrombotic microangiopathy and diffuse alveolar hemorrhage. The Kaplan-Meier estimator was utilized to calculate OS and PFS. A Cox regression model was built to examine for factors associated with relapse, NRM, PFS, and OS. Optimal cut-points for TNC and CD34+ dose were determined using the maximum likelihood method as binary variables (above/below cut-point), for PFS. P-values ≤ 0.05 were considered statistically significant. Analyses were done using SAS version 9.4 (Cary, NC).
Patient Demographics:
One hundred and eighty-three patients were included, of whom 128 (70%) received a single autologous HSCT and 55 (30%) a tandem autologous HSCT. The median age at transplant was 3 years (range <1 to 10 years), with 85% of subjects 1-5 years in age. Sixty-four (35%) patients were in complete remission (CR) at the time of transplant, with the remaining patients in either very good partial remission (VGPR)(n=56) or partial remission (PR)(n=63). Median length of follow-up was 49 months (range 3-144 months).
Autograft Characteristics:
Median infused CD34 dose was 5.2 x 106/kg (range 0.2x106 to 77.5x106/kg), with an interquartile range of 3.9-8.8x106/kg. There were no differences in patient demographics between CD34+ dose quartiles. No association was seen between patient age and CD34+ yield (p=0.74).
Optimal CD34+ dose discrimination:
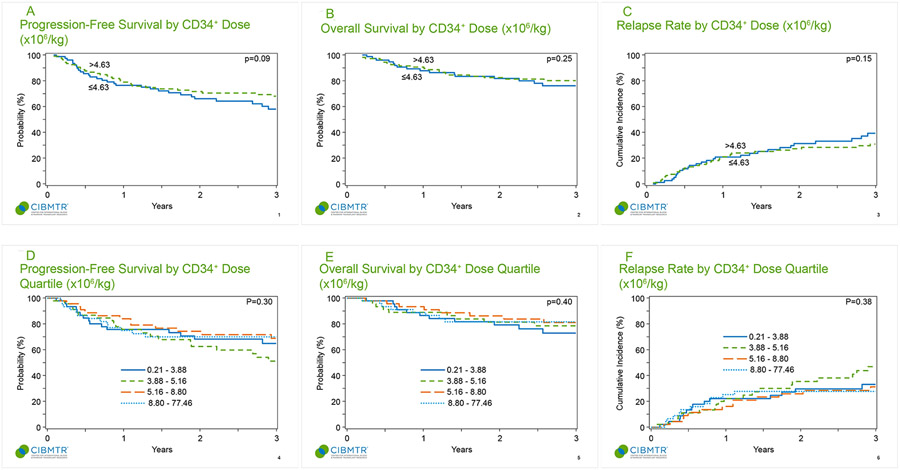
A CD34+ cell dose of 4.6x106/kg was identified as the optimal cell dose to discriminate the largest outcome differences. Autografts above this threshold approached but did not achieve statistical significance for PFS (HR = 0.67, 95% CI: 0.42-1.06, p=0.09; Figure 1A). There was no significant association between CD34+ cell dose and OS (HR = 0.72, 95% CI: 0.42-1.26, p=0.25; Figure 1B), relapse rate (HR = 0.70, 95% CI: 0.43-1.14, p=0.15; Figure 1C), or NRM (HR = 0.22, 95% CI: 0.02-2.08, p=0.18). The incidence of EICs was not significantly different between those who received cell doses ≤4.6x106/kg or >4.6x106/kg, at any of the examined time-points (100 days, 6 months, and 1-year post transplant) (p=0.82).
Figure 1.
A: Progression-free survival by CD34+ dose (x106/kg)
Figure 1B: Overall survival by CD34+ dose (x106/kg)
Figure 1C: Relapse rate by CD34+ dose (x106/kg)
Figure 1D: Progression-free survival by CD34+ dose quartile (x106/kg)
Figure 1E: Overall survival by CD34+ dose quartile (x106/kg)
Figure 1F: Relapse rate by CD34+ dose quartile (x106/kg)
Interquartile Differences in Outcome:
CD34+ cell doses (x106/kg) and clinical outcomes (PFS, OS, relapse) were analyzed by interquartile ranges. No quartile was associated with superior outcomes for PFS (p=0.3; Figure 1D), OS (p=0.40; Figure 1E), or relapse rate (p=0.38 Figure 1F) at one- or three-years post-transplant. EIC incidence did not vary according to CD34+ cell dose quartile, with similar rates of EIC seen in all 4 quartiles at the 100-day, 6 month, and 1-year post transplant timepoints (p=0.52). Among patients receiving a CD34+ dose ≤ 2x106/kg (n=16), the 3-year PFS and OS were 68.8% and 81.3%, respectively. Among patients receiving a CD34+ dose ≥ 10x106/kg (n=35), the 3-year PFS and OS were 73.5% and 80%, respectively.
Endothelial Injury Complications:
Twenty-eight of 183 patients (15%) experienced EICs post-transplant. Fourteen occurred in single transplants. The median infused CD34+ cell dose for these 14 patients was 5.0x106/kg (range 1.41-33.19), and the median infused TNC dose was 2.3x108/kg (range 0.41-24.63). Thirteen patients experienced EICs in tandem transplants, with the median infused CD34+ cell dose 4.7x106/kg (range 0 21-77.46), and median TNC dose 1.2x108/kg (range 0.42-12.08). Data was missing for the remaining 1 patient.
Neutrophil Engraftment:
No specific CD34+ dose-quartile exhibited a superior time-to-engraftment, with a median neutrophil engraftment of 10-11 days in each quartile. All patients receiving a CD34+ cell dose ≤ 2x106/kg or ≥ 10x106/kg engrafted, at a median 11 days (range 10-39 days) and 10 days (range 1-34 days), respectively.
TNC Dose and Outcomes:
For all patients, the median TNC dose was 2.4x108/kg (range 0.1-44.3x108/kg), with an interquartile range of 1.3-4.7x108/kg. The optimal TNC 'cut point' to discriminate between the largest differences in outcome was 3.7x108/kg. However, autografts containing >3.7x108 TNC/kg were not associated with improved PFS (p=0.24), OS (p=0.33), lower risk of relapse (p=0.17) or NRM (p=0.37).
We had initially hypothesized that higher CD34+ cell doses may be associated with increased relapse rates, given the potential for harvesting circulating tumor cells (CTC) with higher volume collections. Although metastatic marrow disease is present in approximately 75% of patients at the time of diagnosis of high-risk neuroblastoma,5 apheresis products are not routinely screened for CTCs. Previous studies in this population have identified detectable tumor mRNA in 50% of apheresis products, associated with inferior outcomes when present (5-year EFS 29%, 95% CI 21–38% versus 51%, 95% CI 42–60%; p=0.0003).6 Moreover, though children with less metastatic marrow involvement at the time of apheresis/stem cell collection may theoretically mobilize more CD34+ cells,1,2 we were unable to address this issue in our study, as marrow involvement at diagnosis/apheresis was not routinely collected. We had also hypothesized that larger autograft cell doses may be associated with higher rates of EICs, given the potential infusion of more immune effector cells (CD3+ or CD15+), ultimately leading to endothelial injury.4,7 8-11 This was not seen in our study though, as there was no impact of CD34 cell dose or TNC on the incidence of EIC. Ultimately, our retrospective trial failed to show a correlation between CD34+ and TNC cell dose and post-HSCT in high-risk neuroblastoma, including PFS, OS, relapse rates, NRM, EIC incidence, and time-to-neutrophil-engraftment.
ACKNOWLEDGMENTS
The authors would like to acknowledge the membership of the CIBMTR’s Pediatric Cancer Working Committee for their valuable and insightful input into this manuscript.
FUNDING STATEMENT
The CIBMTR is supported primarily by Public Health Service U24CA076518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); 75R60222C00011 from the Health Resources and Services Administration (HRSA); N00014-21-1-2954 and N00014-23-1-2057 from the Office of Naval Research; Support is also provided by Be the Match Foundation, the Medical College of Wisconsin, the National Marrow Donor Program, Gateway for Cancer Research, Pediatric Transplantation and Cellular Therapy Consortium and from the following commercial entities: AbbVie; Actinium Pharmaceuticals, Inc.; Adaptimmune; Adaptive Biotechnologies Corporation; ADC Therapeutics; Adienne SA; Allogene; Allovir, Inc.; Amgen, Inc.; Angiocrine; Anthem; Astellas Pharma US; Atara Biotherapeutics; BeiGene; bluebird bio, inc.; Bristol Myers Squibb Co.; CareDx Inc.; CRISPR; CSL Behring; CytoSen Therapeutics, Inc.; Elevance Health; Eurofins Viracor, DBA Eurofins Transplant Diagnostics; Gamida-Cell, Ltd.; GlaxoSmithKline; HistoGenetics; Incyte Corporation; Janssen Research & Development, LLC; Janssen/Johnson & Johnson; Jasper Therapeutics; Jazz Pharmaceuticals, Inc.; Karius; Kiadis Pharma; Kite, a Gilead Company; Kyowa Kirin; Legend Biotech; Magenta Therapeutics; Mallinckrodt Pharmaceuticals; Medexus Pharma; Merck & Co.; Mesoblast; Millennium, the Takeda Oncology Co.; Miltenyi Biotec, Inc.; MorphoSys; Novartis Pharmaceuticals Corporation; Omeros Corporation; Orca Biosystems, Inc.; Ossium Health, Inc.; Pfizer, Inc.; Pharmacyclics, LLC, An AbbVie Company; Pluristem; PPD Development, LP; Regimmune; Sanofi; Sanofi-Aventis U.S. Inc.; Sobi, Inc.; Stemcyte; Takeda Pharmaceuticals; Talaris Therapeutics; Vertex Pharmaceuticals; Vor Biopharma Inc.; Xenikos BV.
Footnotes
Conflicts of Interest and Financial Disclosure Statement
No funding was secured specifically for this project.
Dr. Knight reports partial salary support from Hold’Em for Life Oncology Fellowship, Garron Family Cancer Center Research Fellowship, and BMO Financial Group Oncology Fellowship.
Dr. Wall reports steering committee participation for CRISPR/Vertex Pharmaceuticals and Editas Medicine; acting as a study advisor for CRISPR/Vertex Pharmaceuticals and Editas Medicine; acting as a clinical trial site-PI for CRISPR Therapeutics, Vertex Pharmaceuticals, and Novartis; and research funding from CRISPR Therapeutics, Vertex Pharmaceuticals, and Novartis.
Dr. Rangarajan reports serving as the BMT Medical Monitor for NMDP (no financial reimbursements); and having served as honorary consultant for Medexus.
Dr. Dvorak reports consulting for Jazz Pharma, and Alexion Inc.
Dr. Auletta reports employment with National Marrow Donor Program (NMDP); and advisory council participation for Ascella Health.
Dr. Talano reports research funding from Miltenyi.
Dr. Rotz reports acting as a resource for Clinical Investigation in Blood and Marrow Transplantation (RCI BMT) (employment).
Dr. Myers reports research funding for an investigator initiated clinical trial from Incyte and sponsored research from Elixirgen Therapeutics.
Dr. Sharma reports consulting for Spotlight Therapeutics, Medexus Inc., Vertex Pharmaceuticals, and Sangamo Therapeutics; research funding from CRISPR Therapeutics. Clinical Trial site-PI: CRISPR Therapeutics, Vertex Pharmaceuticals, Novartis Pharmaceuticals, Magenta Therapeutics, Beam Therapeutics, Honoraria: Vindico Medical Education.
Dr. Qayed reports honoraria from Vertex and Novartis.
PRIOR PRESENTATIONS
Portions of the data in this manuscript were previously presented as abstracts at the Transplantation and Cellular Therapy Meetings of ASTCT and CIBMTR (“Tandem”), April 22nd-26th 2022.
DATA USE AGREEMENT
CIBMTR supports accessibility of research in accord with the National Institutes of Health (NIH) Data Sharing Policy and the National Cancer Institute (NCI) Cancer Moonshot Public Access and Data Sharing Policy. The CIBMTR only releases de-identified datasets that comply with all relevant global regulations regarding privacy and confidentiality.
REFERENCES
- 1.Pavone V, Gaudio F, Console G, et al. Poor mobilization is an independent prognostic factor in patients with malignant lymphomas treated by peripheral blood stem cell transplantation. Bone Marrow Transplant. 2006;37(8):719–724. doi: 10.1038/sj.bmt.1705298 [DOI] [PubMed] [Google Scholar]
- 2.Costa LJ, Nista EJ, Buadi FK, et al. Prediction of Poor Mobilization of Autologous CD34+ Cells with Growth Factor in Multiple Myeloma Patients: Implications for Risk-Stratification. Biol Blood Marrow Transplant. 2014;20(2):222–228. doi: 10.1016/j.bbmt.2013.11.003 [DOI] [PubMed] [Google Scholar]
- 3.Ravoet C, Feremans W, Husson B, et al. Clinical evidence for an engraftment syndrome associated with early and steep neutrophil recovery after autologous blood stem cell transplantation. Bone Marrow Transplant. 1996;18(5):943–947. [PubMed] [Google Scholar]
- 4.Palomo M, Diaz-Ricart M, Carbo C, et al. Endothelial dysfunction after hematopoietic stem cell transplantation: role of the conditioning regimen and the type of transplantation. Biol Blood Marrow Transplant J Am Soc Blood Marrow Transplant. 2010;16(7):985–993. doi: 10.1016/j.bbmt.2010.02.008 [DOI] [PubMed] [Google Scholar]
- 5.Morgenstern DA, London WB, Stephens D, et al. Prognostic significance of pattern and burden of metastatic disease in patients with stage 4 neuroblastoma: A study from the International Neuroblastoma Risk Group database. Eur J Cancer Oxf Engl 1990. 2016;65:1–10. doi: 10.1016/j.ejca.2016.06.005 [DOI] [PubMed] [Google Scholar]
- 6.Kreissman SG, Seeger RC, Matthay KK, et al. Purged versus non-purged peripheral blood stem-cell transplantation for high-risk neuroblastoma (COG A3973): a randomised phase 3 trial. Lancet Oncol. 2013;14(10):999–1008. doi: 10.1016/S1470-2045(13)70309-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dvorak CC, Higham C, Shimano KA. Transplant-Associated Thrombotic Microangiopathy in Pediatric Hematopoietic Cell Transplant Recipients: A Practical Approach to Diagnosis and Management. Front Pediatr. 2019;7:133. doi: 10.3389/fped.2019.00133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Turunen A, Valtola J, Partanen A, et al. Autograft cellular composition and outcome in NHL patients: results of the prospective multicenter GOA study. Leuk Lymphoma. 2020;61(9):2082–2092. doi: 10.1080/10428194.2020.1762879 [DOI] [PubMed] [Google Scholar]
- 9.Porrata LF, Burgstaler EA, Winters JL, et al. Immunologic Autograft Engineering and Survival in Non-Hodgkin Lymphoma. Biol Blood Marrow Transplant. 2016;22(6):1017–1023. doi: 10.1016/j.bbmt.2016.01.024 [DOI] [PubMed] [Google Scholar]
- 10.Porrata LF, Inwards DJ, Ansell SM, et al. Autograft immune content and survival in non-Hodgkin’s lymphoma: A post hoc analysis. Leuk Res. 2019;81:1–9. doi: 10.1016/j.leukres.2019.03.009 [DOI] [PubMed] [Google Scholar]
- 11.Lee SE, Lim JY, Kim TW, et al. Different role of circulating myeloid-derived suppressor cells in patients with multiple myeloma undergoing autologous stem cell transplantation. J Immunother Cancer. 2019;7(1):35. doi: 10.1186/s40425-018-0491-y [DOI] [PMC free article] [PubMed] [Google Scholar]