Abstract
Results of a prospective study of stage‐adapted treatment of human immunodeficiency virus (HIV)‐associated Hodgkin lymphoma (HIV‐HL) showed a 2‐year overall survival (OS) of 90.7% with no significant difference between early favorable (EF), early unfavorable (EU), and advanced HL. Patients with EF HIV‐HL received two to four cycles of doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) + 30 Gy involved field (IF) radiation, those with EU HIV‐HL received four cycles of ABVD or BEACOPP (bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone) baseline + 30 Gy IF, and six to eight cycles of BEACOPP baseline were administered in advanced disease. The objective of the present analysis is to determine long‐term outcomes of HIV‐HL. Of 108 patients, 23 (21%) had EF HL, 14 (13%) had EU HL, and 71 (66%) had advanced‐stage HL. After a median follow‐up of 9.14 (range, 0–12.9) years, there were five primary refractory HL patients (5%) and 11 relapses (10%), of which seven were late relapses (>2 years). A second primary malignancy (SPM) occurred in 10 patients after a median of 7.3 years (range, 1.5–10.7) from HL diagnosis. The 10‐year OS for patients with EF, EU, and advanced HL was 95.7%, 84.6%, and 76.1%, respectively. By multivariate analysis, Center for Disease Control and Prevention category C (hazard ratio [HR] 3.00, 95% confidence interval [CI]: 1.16–7.74, p = 0.023) and achievement of complete remission were significant for OS (HR 0.03, 95% CI: 0.01–0.08, p = 2.45 × 10−9). In conclusion, a stage‐adapted treatment approach for HIV‐HL is highly effective with long‐term survival rates similar to those reported in HIV‐uninfected HL. However, the risk for late relapse and SPM is significant.

INTRODUCTION
Hodgkin lymphoma (HL) is one of the most common non‐AIDS‐defining malignancies seen in people living with human immunodeficiency virus (HIV) (PWH), in whom the risk of HL is increased by approximately 10–14‐fold compared to the general population. 1 The risk of HL has remained relatively stable in recent decades, with higher incidences observed in males compared to females. 2 , 3 The main risk factor for HIV‐associated HL (HIV‐HL) is a moderately lowered CD4+ T‐cell count. Despite virologic suppression, there is a marked T‐cell decline in the final months before HL diagnosis. 3 , 4 , 5 In comparative retrospective studies, no significant differences in disease‐free survival (DFS) or progression‐free survival (PFS) and overall survival (OS) were observed between people with and without HIV, with doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) being the most common regimen for HIV‐HL. 6 , 7 , 8 However, few prospective studies were performed in the era of combination antiretroviral therapy (cART). 9 , 10 , 11 , 12 The only prospective trial that investigated a stage‐ and risk‐adapted treatment approach in HIV‐HL showed 2‐year OS of 90.7% with no significant difference between early favorable (95.7%), early unfavorable (100%), and advanced HL (86.8%). 10 However, the median follow‐up was only 26.2 months, and survival rates may be less favorable with longer follow‐up. To date, no data on late relapse are available for HIV‐HL. Here, we report long‐term survival and safety data after approximately 9 years of follow‐up.
MATERIALS AND METHODS
We conducted a prospective, multicenter trial at 42 institutions in Germany and Austria (ClinicalTrials.gov: NCT01468740). The trial design has been published previously. 10 Briefly, adult PWH (18–75 years of age) with histologically confirmed early favorable HL defined as Ann Arbor stage IA/B or IIA/B without risk factors (large mediastinal tumor, extranodal involvement, ≥3 lymph node areas involved) received two courses of ABVD plus 30 Gy involved field (IF) radiation. Two further cycles of CT were recommended if partial remission (PR) was noted after two cycles. Patients with early unfavorable HL (stage IA/B or IIA/B and at least one risk factor) received four courses of BEACOPP (bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone) baseline + 30 Gy IF radiotherapy to sites of initial bulky disease (those at least 5 cm in diameter) or residual tumor of ≥2 cm in diameter. Of note, four courses of ABVD were recommended rather than BEACOPP according to a protocol amendment considering the results of the German Hodgkin Study Group (GHSG) HD11 trial. 13 The treatment for advanced HL consisted of eight courses of BEACOPP baseline. After the completion of chemotherapy for advanced HL, sites of initial bulky disease (those at least 5 cm in diameter) and residual tumor larger than 2.5 cm in diameter received 30 Gy of irradiation. In people with advanced HIV infection, with two or three of the following risk factors: CD4 lymphocyte counts <50/µL; prior AIDS‐defining opportunistic infection; and ECOG performance status >2, replacement of BEACOPP with ABVD was recommended. cART was given concomitantly with chemotherapy.
The ABVD regimen consisted of doxorubicin 25 mg/m2, bleomycin 10 U/m2, vinblastine 6 mg/m2, and dacarbazine 375 mg/m2. Drugs were administered intravenously on Days 1 and 15 of each 28‐day cycle. BEACOPP baseline was administered every 21 days, as reported previously. 10
The status of HIV infection was classified according to the 1993 Center for Disease Control and Prevention (CDC) criteria, with CDC category C indicating an AIDS‐defining illness. 13
The primary endpoint was tolerability and treatment‐related mortality. Secondary endpoints included PFS and OS.
Statistics
Associations between categorical characteristics were assessed using Fisher's exact test. PFS was calculated from the beginning of chemotherapy to the time of progression, relapse, or death. OS was measured from the beginning of treatment to the last follow‐up or death from any cause. Censoring was done at the date of last contact. Late relapse and very late relapse were defined as relapse occurring greater than 2 years and greater than 5 years following the diagnosis of HL, respectively.
The probability of PFS and OS was determined using the Kaplan–Meier method. Univariable and multivariable Cox proportional hazards models were used to explore the predictive value of each covariable. Statistical analyses were performed using the statistical software R (version 3.5.2). All p values were two‐sided. p Values of 0.05 or less were considered statistically significant.
RESULTS
Patient population
A total of 108 PWH and histologically proven HL were enrolled in the study. The baseline characteristics have been described previously (Supporting Information S1: Table S1).
Long‐term outcomes
After a median follow‐up of 9.14 (range, 0–12.9) years, there were five primary refractory HL (5%) and 11 relapses (10%), of which seven were late (n = 4) or very late (n = 3) relapses (Table 1). Thirteen of 16 cases with relapsed/refractory (r/r) disease occurred in advanced HL, and three patients with early unfavorable HL (n = 2) and early favorable HL (n = 1) experienced a relapse after complete remission (CR). At HL diagnosis, the CD4 cell count was below 200/μL in 11 of 16 r/r patients (68.8%) but only in five of 91 patients (5.5%) who sustained a CR (n = 107).
Table 1.
Patients with relapsed and refractory HL.
| Patient no. | HL stage | CDC category | First‐line chemotherapy | Result | TTR (years) | Salvage therapy | Outcomea |
|---|---|---|---|---|---|---|---|
| 1. 12‐01 | Advanced IIIB | A3 | 1× BEACOPP | PR | 0.9 | 3× DHAP, 1× R‐IMV, HDCT/ASCT | Died of interstitial lung disease/lung fibrosis 10.6 years |
| First relapse (stage IA): IF‐RT | |||||||
| 6× ABVD | Second relapse: BV (PNP), Nivo | ||||||
| 2. 15‐01 | Advanced IIIB | C3 | 6× BEACOPP | CR | 1.6 | 2× DHAP + HDCT/ASCT | Alive in CR 9.7+ years since relapse |
| 3. 28‐01 | Advanced IVB | A2 | 3× BEACOPP | NC | 0.1 | 1× DHAP | Dead with HL (neutropenic sepsis) 25 months |
| 1× DexaBEAM | |||||||
| First relapse: 2× | |||||||
| DexaBEAM, alloTx | |||||||
| Second relapse: 1× R‐Gem | |||||||
| 4. 19‐03 | Advanced IVB | B3 | 6× ABVD | PR | 0.6 | None | Died of HL 0.6 years |
| 5. 06‐08 | Advanced IVB | C3 | 3× ABVD | PD | 0.2 | None | Died of HL 0.3 years |
| 6. 38‐01 | Advanced IIIB | C3 | 8× BEACOPP | PR | 0.9 | 1× DHAP | Died of HL 1 year |
| 7. 06−10 | Advanced IVB | C3 | 3× ABVD | CR | 3.4 | 2× R‐DHAP + HDCT/ASCT | Alive in CR 4.3+ years since relapse |
| 3× BEACOPP | |||||||
| 8. 06‐15 | Early unfavorable IIB | B3 | 4× ABVD | CR | 1.6 | 3× IGEV, HDCT/ASCT | Died of CMV‐pneumonia 4 months after second CR, 11 months since relapse |
| 9. 07‐02 | Advanced IVB | C3 | 8× BEACOPP | CR | 5.9 | 2× DHAP (aplasia), 2× IGEV | Died of HL 5 months since relapse |
| 10. 25‐06 | Advanced IIIB | C3 | 6× BEACOPP | CR | 1.4 | 3× DexaBeam, HDCT/ASCT | Died of HL, 5 years since relapse |
| Second relapse: 1× DHAP, 3× BV | |||||||
| 11. 03‐17 | Advanced IVB | C3 | 6× BEACOPP | CR | 6.7 | 2× DHAP, HDCT/ASCT | Alive in CR 3.9+ months since relapse |
| 12. 03‐25 | Early unfavorable IIB | C3 | 4× ABVD | CR | 3.7 | 6× BV | Died of HL 6 months since relapse |
| 13. 19‐02 | Advanced IVB | C3 | 6× BEACOPP | CR | 1.3 | Salvage Tx abroad | Lost after relapse |
| 14. 06‐16 | Advanced IIIB | A2 | 6× BEACOPP | CR | 4.5 | 2× R‐DHAP, HDCT/ASCT | Alive in CR 2.7+ years since relapse |
| 15. 14‐01 | Early favorable IIA | B3 | 4× ABVD | CR | 9.1 | 2× DHACarbo, 2× BV, 6× Nivo | Died of HL 1.8 years since relapse |
| 16. 25‐05 | Advanced IVB | A3 | 6× BEACOPP | CR | 4.1 | 2× DHAP, HDCT/ASCT | Alive in CR 4.9+ years since relapse |
Abbreviations: ABVD, doxorubicin, bleomycin, vinblastine, and dacarbazine; BEACOPP, bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, prednisolone; BV, brentuximab vedotine; CDC, Centers for Disease Control; CMV, cytomegalovirus; CR, complete remission; DHAP, dexamethasone, high‐dose cytarabine, cisplatin; HDCT/ASCT, high‐dose chemotherapy and autologous stem cell transplantation; HL, Hodgkin lymphoma; IGEV, ifosfamide, gemcitabine, epirubicine, etoposide; nivo, nivolumab; PR, partial remission; R‐IMV, rituximab, ifosfamide, methotrexate, etoposide; TTR, time to relapse.
From HL diagnosis.
High‐dose chemotherapy (HDCT) followed by autologous stem cell transplantation (ASCT) was administered in eight of 16 patients with r/r HL. Of these, five patients were in ongoing CR at the last follow‐up, two died in CR from cytomegalovirus pneumonia (n = 1) and interstitial pneumonia (n = 1), and one patient died from refractory HL 5 years from the first relapse (Table 1).
Of eight patients not undergoing HDCT/ASCT, four died of early progression, one after having received an allogeneic SCT from a matched unrelated donor, three died of refractory HL after having relapsed from CR, and one patient underwent salvage chemotherapy abroad and was lost to follow‐up (Table 1).
Data on CD4 T‐cell counts and HIV plasma viremia at the last follow‐up were available for 67 patients (62%). The median CD4‐cell count was 620/µL (range, 168–1629), and the HIV viral load was below the detection limit of 50 HIV‐RNA copies/mL in 62 of 67 patients (93%).
An SPM was diagnosed in 10 patients (9%) after a median of 7.3 years (range, 1.5–10.7) from HL diagnosis (Table 2). Five patients had undergone primary chemotherapy with either ABVD or BEACOPP, and the CD4 cell count was below 200/µl at diagnosis of HL in seven of nine patients. Data on the CD4 cell count at the time of diagnosis of the SPM were not available. Four of the 10 SPM patients were alive and in ongoing CR. One patient died of septic pneumonia 9.7 years after diagnosis of anal cancer, and one patient each died of refractory small‐cell lung cancer, diffuse large B‐cell lymphoma, and peripheral T‐cell lymphoma. Another patient with anal cancer died of a late relapse of HL, and one patient was lost to follow‐up after being diagnosed with bladder cancer (Table 2).
Table 2.
Second primary malignancies.
| Patient no. | Ann Arbor | CDC categorya | Primary therapy | Result | SPM | Years after HL | Outcome |
|---|---|---|---|---|---|---|---|
| 1. 03‐01 | Early favorable IIA | B | 2× ABVD | CR | Squamous cell CIS vocal fold | 5.2 | Alive in CR 7.4 years after SPM, 12.9+ years after HL |
| 2. 03‐02 | Advanced IIIB | A3 | 6× BEACOPP | CR | SCLC | 8.2 | Died of SCLC at 11 months, 9.2 years after HL |
| 3. 03‐12 | Early unfavorable IIB | C3 | 4.5× ABVD | CR | DLBCL | 10.5 | Alive in CR at 11.8+ years |
| 4. 03‐19 | Advanced IVA | C3 | 7× BEACOPP | CR | DLBCL | 7.5 | Died of DLBCL 7.5 years after HL |
| 5. 07‐02 | Advanced IVB | C3 | 8× BEACOPP | CR | Anal cancer | 4.9 | Relapse of HL at 5 years −>2× DHAP + 2× IGEV |
| Died of HL 5.9 years after HL diagnosis, 1 year after anal cancer diagnosis | |||||||
| 6. 08‐01 | Advanced IIIA | B3 | 6× BEACOPP | CR | Anal cancer | 10.7 | Died of septic pneumonia 11 years after HL, 9.7 years after anal cancer |
| 7. 18‐01 | Advanced IVB | C3 | 6× ABVD | CR | PTCL | 1.5 | Died of PTCL 3.7 years since SPM, 5.2 years after HL |
| 8. 24‐03 | Advanced IIIB | B2 | 7.5× ABVD | CR | Bladder cancer | 2.4 | Lost to follow‐up |
| 9. 27‐01 | Advanced IIIB | B3 | 8× BEACOPP | CR | Vulvar cancer | 9.5 | Alive in CR 10 months after vulvar cancer, 10.2 years after HL |
| 10. 35‐03 | Early favorable IIA | A1 | 2× ABVD | CR | Malignant melanoma | 6.4 | Alive in CR 2.5 years after melanoma, 8.8 years after HL |
Abbreviations: ABVD, doxorubicin, bleomycin, vinblastine, and dacarbazine; BEACOPP, bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone; CIS, carcinoma in situ; CR, complete remission; DLBCL, diffuse large B‐cell lymphoma; HL, Hodgkin lymphoma; PTCL, peripheral T‐cell lymphoma; SCLC, small‐cell lung cancer; SPM, second primary malignancies.
At the time of HL diagnosis.
Compared with those who did not, patients who developed an SPM more frequently had a CD4 cell count <200/µL (56% vs. 41%), HIV‐RNA ≥ 50 copies/mL (60% vs. 43%), and a prior AIDS‐defining illness (40% vs. 30%) at the time of HL diagnosis. However, the differences were not statistically significant.
Of the entire cohort, 22 patients (20%) have died. Apart from neutropenic sepsis (n = 5), causes of death were r/r HL (n = 8), sepsis unrelated to HIV or HL (n = 1), interstitial lung disease/pulmonary fibrosis (n = 1), SPM (n = 3), and an AIDS‐defining illness (n = 2, one case each of progressive multifocal leukoencephalopathy and cytomegalovirus pneumonia). The causes of death could not be clarified in two patients.
The 10‐year PFS of the entire study population was 73.5% (95% confidence interval [CI]: 64.7–83.4) (Figure 1A), and the 10‐year OS rate was 81.4% (95% CI: 73.7–89.8) (Figure 1B). The PFS and OS rates in early favorable, early unfavorable, and advanced HL at 5, 9, and 10 years are listed in Table 3. Notably, the PFS in patients with advanced HL was significantly inferior to that in early favorable disease (hazard ratio [HR] 4.30, 95% CI: 1.01–18.26, p = 0.048; Figure 2A), but in terms of OS, the difference between early favorable and advanced HL did not reach statistical significance (HR 3.43, 95% CI: 0.79–14.83, p = 0.099; Figure 2B). The International Prognostic Score (IPS) did not significantly impact PFS (Figure 3A) and OS (Figure 3B).
Figure 1.
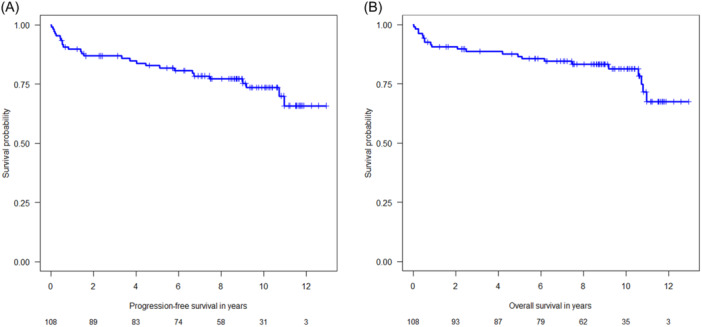
(A) Kaplan–Meier plot on progression‐free‐survival (entire study population). (B) Kaplan–Meier plot on overall survival (entire study population).
Table 3.
Progression‐free survival and overall survival.
| Early favorable (n = 23) | Early unfavorable (n = 14) | Advanced (n = 71) | ||||
|---|---|---|---|---|---|---|
| PFS/OS | % | 95% CI | % | 95% CI | % | 95% CI |
| PFS at 5 years | 95.7 | 87.7–100 | 85.1 | 68.0–100 | 78.3 | 69.1–88.7 |
| OS at 5 years | 95.7 | 87.7–100 | 84.6 | 67.1–100 | 84.2 | 76.1–93.2 |
| PFS at 9 years | 95.7 | 87.7–100 | 85.1 | 68.0–100 | 69.8 | 59.5–82.0 |
| OS at 9 years | 95.7 | 87.7–100 | 84.6 | 67.1–100 | 79.1 | 69.8–89.6 |
| PFS at 10 years | 87.0 | 70.8–100 | 85.1 | 68.0–100 | 66.9 | 55.8–80.2 |
| OS at 10 years | 95.7 | 87.7–100 | 84.6 | 67.1–100 | 76.1 | 65.9–88.0 |
Abbreviations: CI, confidence interval; OS, overall survival; PFS, progression‐free survival.
Figure 2.
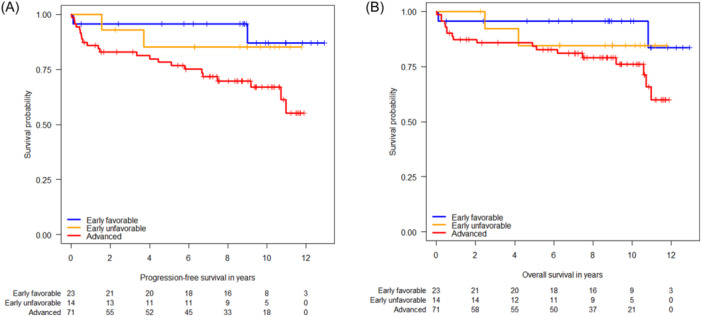
(A) Kaplan–Meier plots on progression‐free survival with respect to Hodgkin stage. (B) Kaplan–Meier plots on overall survival with respect to Hodgkin stage.
Figure 3.
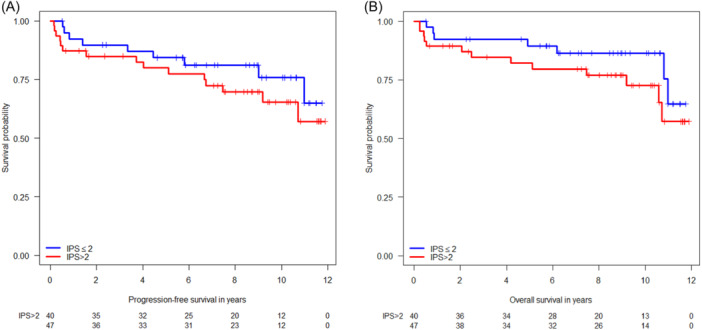
(A) Kaplan–Meier plots on progression‐free survival with respect to the International Prognostic Score (IPS). (B) Kaplan–Meier plots on overall survival with respect to the IPS.
By univariate analysis, patients with CDC category C had a significantly higher risk for progression compared to those with category A/B (HR 2.45, 95% CI: 1.15–5.23, p = 0.020). The presence of B‐symptoms was significantly associated with inferior PFS (HR 3.55, 95% CI: 1.23–10.26, p = 0.020) (Supporting Information S1: Table S2). By contrast, patients achieving a CR with upfront CT had a significantly better PFS (HR 0.036, 95% CI: 0.013 to 0.096, p = 3.93 × 10−10). A CD4 cell count <200/µL was not associated with a significantly higher risk for progression (HR 1.72, 95% CI: 0.81–3.69, p = 0.161) (Supporting Information S1: Table S2).
While age ≥45 years (HR 2.97, 95% CI: 1.21–7.29, p = 0.017) and extranodal involvement (HR 2.56, 95% CI: 1.04–6.29, p = 0.040) were associated with inferior OS, achievement of CR after CT (HR 0.035, 95% CI: 0.012–0.098, p = 2.35 × 10−10) was significantly associated with better OS (Supporting Information S1: Table S3).
By multivariable analysis, CDC category C was significant for both PFS (HR 2.90, 95% CI: 1.25–6.71, p = 0.013) and OS (HR 3.00, 95% CI: 1.16–7.74, p = 0.023), as was achievement of CR for PFS (HR 0.04, 95% CI: 0.01–0.11, p = 2.89 × 10−9) and OS (HR 0.03, 95% CI 0.01–0.08, p = 2.45 × 10−9).
DISCUSSION
There are some remarkable findings from this long‐term follow‐up study on HIV‐related HL. First, the outcome of patients with early favorable HL is excellent, with a 95.7% OS rate at 5 and 10 years. Thus, two cycles of ABVD followed by IF radiotherapy can be confirmed as a standard treatment for early favorable HIV‐HL. However, a radiation dose of 20 rather than 30 Gy and the use of smaller involved site radiation fields may now be preferred as both approaches resulted in excellent disease control in HIV‐negative patients with early favorable HL. 14
The 85.1% (95% CI: 68.0–100) 5‐year PFS rate of patients with early unfavorable HIV‐HL appears similar to the 87.7% (95% CI: 84.8–90.6) 5‐year freedom from treatment failure rate as reported in the GHSG HD14 study for HIV‐negative early unfavorable HL receiving four cycles of ABVD. 14 In the present study, no further PFS events occurred in subsequent years, so the 10‐year PFS rate remained stable at 85.1%, which is also in the range of the 10‐year PFS of 85.6% (95% CI: 82.6–88.1) reported in the GHSG HD14 study. 15 By contrast, the OS of 84.6% (95% CI: 67.1–100) at 5 and 10 years is markedly lower than the 96.8% (95% CI: 92.0–95.7) at 5‐ and 10‐year OS reported in HIV‐negative patients receiving four cycles of ABVD. 15 , 16 However, the significance of this finding is limited by the small number of 14 patients with early unfavorable HL included in the present study. Further, one of both relapsed patients with early unfavorable HIV‐HL died of cytomegalovirus pneumonia in second CR. Of note, this is one of only two deaths from an AIDS‐defining illness observed in the entire study cohort.
As compared to the previously reported 2‐year PFS rate of 87.5% (95% CI: 79.1–96.8), 10 there is a 10% decline in the PFS at 5 years (78.3% [69.1–88.7]), which, however, does not translate into a significant decline in the 5‐year OS (84.2% [76.1–93.2]), indicating successful salvage treatment approaches. Our data compare favorably with the 5‐year OS of 76% (95% CI: 65–87) reported in a retrospective study on patients with advanced‐stage HIV‐HL treated with six to eight cycles of ABVD. 17 A pooled analysis of four randomized trials on ABVD versus BEACOPP in HIV‐negative advanced HL reported a 7‐year PFS of 71.1% (95% CI: 67.1–74.6) for ABVD and 81.1% (95% CI: 77.5–84.2) for BEACOPP, 18 which is in the range of the 78.3% PFS at 5 years reported here. The 10‐year PFS rate of 66.9% (95% CI: 55.8–80.2) is mainly due to late relapses and fatal SPMs. However, it again appears in the range of what is expected with ABVD for advanced HL in the HIV‐negative setting. For HD9 trial participants with HIV‐negative advanced HL, the 15‐year PFS was reported to be 57.0% (95% CI: 50.0–64.0) for COPP/ABVD and 66.8% (95% CI: 61.9–71.8) for BEACOPP baseline. 19 The 10‐year OS rate of 76.1% (65.9–88.0) compares favorably with the 7‐year OS of 84.3% (95% CI: 80.8–87.2) for ABVD and 87.7% (95% CI: 84.5–90.2) for BEACOPP 17 and with the 15‐year OS of 72.3% (95% CI: 66.5–78.1) with COPP/ABVD, and 74.5% (95% CI: 70.1–78.9) with BEACOPP baseline as reported in HIV‐negative advanced HL. 19
It should be noted that there were four toxic deaths (5.6%) among patients who received BEACOPP, with three experiencing their fatal event after the seventh and eighth cycles. 10 Thus, no more than six cycles of BEACOPP should be applied, and caution is advised, especially in patients with low CD4 counts. Six cycles of ABVD also proved effective in a retrospective study showing no significant differences in the 5‐year event‐free survival and 5‐year OS observed between patients with and without HIV infection. 6 Further, in another retrospective study on ABVD for HIV‐HL, 42 of 51 patients (82.4%) received six rather than eight cycles of ABVD. 17 It should be noted, however, that the reduction from eight to six cycles of BEACOPP baseline has never been tested in HIV‐negative HL.
PET‐adapted therapy proved feasible in a small study on 12 PWH and advanced HL treated within the SWOG S0816 phase II trial. 11 After two cycles of ABVD, patients with a negative PET2 received four additional cycles of ABVD, while PET2‐positive patients received six cycles of BEACOPP baseline. Overall, three patients developed progressive disease (all PET2‐negative), with an estimated PFS at 2 years of 83% (95%: 46.1–95.3). More data on PET‐adapted treatment are needed before this approach can be generally recommended for advanced HIV‐HL. However, as established in the HIV‐negative setting, radiotherapy after chemotherapy for advanced HL should be restricted to patients with PET‐positive residual disease. 14 , 20 , 21 This is also because HIV infection confers an increased cardiovascular disease risk that may only become evident after more than 10 years following treatment for HL.
Recent results from the phase I/II study AMC085 of the use of brentuximab vedotin (BV) combined with AVD in patients with early unfavorable and advanced HIV‐HL are encouraging with 2‐year PFS and OS rates of 86% (95% CI: 0.71–0.94) and 92% (95% CI: 0.78–0.97), respectively. 12 However, patients who require ART that includes potent CYP3A4 inhibitors were excluded from the study; there was a 10% rate of ≥grade 3 peripheral sensory neuropathy, and PFS and OS data are very close to the 2‐year PFS and OS reported in the present study. 10 Especially in PWH, the 4.5% improvement in OS with BV‐AVD over ABVD shown in a randomized trial in HIV‐negative advanced HL must be carefully weighed against the increased neurotoxicity and myelosuppression with BV‐AVD compared to ABVD. 22 , 23 Of note, pharmacokinetic and pharmacodynamic interactions between cytotoxics and antiretroviral drugs must always be carefully considered regardless of whether BV is used or not.
Although Nivolumab‐AVD may become a new standard of care for advanced HL, no data on this regimen are available in PWH.
To date, no data have been available on late and very late relapses in HIV‐HL. In HIV‐negative HL, the rates of late or very late relapse were reported to be 4% and 5.7%, respectively. 24 , 25 According to the GHSG experience of people without HIV and complete response after first‐line treatment, the cumulative incidence of very late relapse appears to rise linearly, with rates of 2.5%, 4.3%, and 6.9% at 10, 15, and 20 years, respectively. 26 Similar data were reported from Denmark 27 and Greece. 25 Within the entire study cohort presented here, these events were seen in 4.7% and 2.8% of patients, respectively. Notably, four of seven patients were in sustained second CR after salvage therapy. Thus, our findings indicate that the risk of late and very late relapse is not increased in HIV‐HL compared to HL in people without HIV.
To our knowledge, the present study is the first to provide data on SPM in HIV‐HL survivors. Although five patients each had undergone primary chemotherapy with ABVD and BEACOPP, respectively, the impact of the chemotherapy regimen on developing an SPM cannot be conclusively assessed given the low number of patients affected. Notably, most patients experienced solid tumors, and there was no myelodysplasia or therapy‐related acute myeloid leukemia. Four of the 10 SPM were AIDS‐defining malignancies, and this may have contributed to the risk of SPM. In a recent analysis of data from the California Cancer Registry on patients diagnosed with HL from 1990 to 2015, the risk of SPM increased 2.66‐fold in HIV‐infected and 1.92‐fold in HIV‐uninfected HL survivors. 28 Kaposi sarcomas were assigned to the hematologic malignancies group, but even though and in line with our data, only 35% of HIV‐infected patients developed hematologic malignancies. Also, in HIV‐uninfected patients, SPM rates were reported to be 4% in patients treated with ABVD and 6.5% in those treated with BEACOPP baseline or BEACOPP escalated. 18 The median time to development of SPM was approximately 2 years longer than in the present study (7.3 vs. 5.4 years), but this may be due to small patient numbers. It should be noted that non‐AIDS‐defining cancers are a growing source of morbidity and mortality for PWH irrespective of previous antineoplastic therapy. 29
The IPS did not predict PFS or OS in the present trial. Further, although patients with early favorable HL had a significantly better PFS than those with advanced HL, the difference in OS was not statistically significant. However, these findings may be due to the limited number of patients included in the trial. Our observation that a CD4 cell count <200/µL did not predict poorer outcomes is in contrast to findings from a previous retrospective study of advanced‐stage HL from nine countries and three continents, where a CD4 cell count <200 cells/µL was associated with inferior PFS (HR 2.60, p = 0.002) and OS (HR, 2.04, p = 0.04). 30 However, the meaningfulness of these findings may be limited by the study's retrospective nature and differences between institutions and countries in the standard of care of PLWH. Further, given the HR for PFS of 1.72 in patients with CD4 cell count <200 cells/µL as shown in the present study, lack of statistical significance may be due to low patient numbers. Nevertheless, our finding that a prior AIDS‐defining illness at HL diagnosis (CDC category C) was associated with both inferior PFS and OS indicates that HIV‐related factors may still be able to predict the outcome of patients with HIV‐HL.
It is very encouraging that in our study, only two patients died of an opportunistic infection, underlining the beneficial effects of concomitant cART and antimicrobial prophylaxis.
Five of eight patients who underwent HDCT followed by ASCT were in sustained CR at the last follow‐up, underlining the role of intensive salvage therapies. 31
A limitation of the study is that it was not designed to show differences in mortality between different groups of patients. However, given the limited number of patients with HIV‐HL, larger trials with sufficient statistical power are hardly feasible. Further, data on risk factors that might have contributed to the development of SPM, such as behavioral factors, family history of cancer, or CD4 cell counts at the time of SPM, were unavailable. However, considering the small number of patients, it appears unlikely that any of these factors would be significantly associated with an SPM. Nevertheless, we cannot exclude the possibility that the use of consolidation radiation therapy, which was based on the size of residual lesions at the end of treatment, may have contributed to some extent to the occurrence of SPM.
In summary, a stage‐ and risk‐adapted treatment approach for HIV‐HL is highly effective with long‐term outcome rates similar to those reported in HIV‐uninfected HL. However, there is a significant risk of late relapse and SPM underlining a need for lifelong follow‐up care.
AUTHOR CONTRIBUTIONS
Marcus Hentrich and Christian Hoffmann designed the study, analyzed and interpreted data, and wrote the paper. Marcus Hentrich, Markus Müller, Christoph Wyen, Anna Pferschy, Jan Siehl, Jürgen K. Rockstroh, Dirk Schürmann, and Christian Hoffmann recruited patients for the study and analyzed and interpreted data. Marcus Hentrich and Anna Pferschy collected data. Vindi Jurinovic analyzed and interpreted data and performed statistical analysis. Marcus Hentrich had the final responsibility for submission and was the PI of the study. All authors had full access to the data in the study, approved the final version of the manuscript, and accept responsibility to submit for publication.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
FUNDING
This work was supported, in part, by grants from Deutsche AIDS Gesellschaft e.V., German Federal Ministry of Education and Research (BMBF, Grant No. 01 KI 0771; to C. W.), and Harlachinger Krebshilfe e.V.
Supporting information
Supporting information.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request. Deidentified patient data and data dictionary may be provided upon reasonable request via email to the corresponding author.
REFERENCES
- 1. Navarro J‐T, Moltó J, Tapia G, Ribera J‐M. Hodgkin lymphoma in people living with HIV. Cancers. 2021;13(17):4366. 10.3390/cancers13174366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Poizot‐Martin I, Lions C, Allavena C, et al. Spectrum and incidence trends of AIDS‐ and non‐AIDS‐defining cancers between 2010 and 2015 in the French Dat'AIDS Cohort. Cancer Epidemiol Biomarkers Prev. 2021;30(3):554‐563. 10.1158/1055-9965.EPI-20-1045 [DOI] [PubMed] [Google Scholar]
- 3. Kimani SM, Painschab MS, Horner MJ, et al. Epidemiology of haematological malignancies in people living with HIV. Lancet HIV. 2020;7(9):e641‐e651. 10.1016/S2352-3018(20)30118-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shepherd L, Ryom L, Law M, et al. Differences in virological and immunological risk factors for non‐Hodgkin and Hodgkin lymphoma. J Natl Cancer Inst. 2018;110(6):598‐607. 10.1093/jnci/djx249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hoffmann C, Schommers P, Wolf E, et al. CD4+ and CD8+ T‐cell kinetics in aviremic HIV‐infected patients developing Hodgkin or non‐Hodgkin lymphoma. AIDS. 2016;30(5):753‐760. 10.1097/QAD.0000000000000980 [DOI] [PubMed] [Google Scholar]
- 6. Montoto S, Shaw K, Okosun J, et al. HIV status does not influence outcome in patients with classical Hodgkin lymphoma treated with chemotherapy using doxorubicin, bleomycin, vinblastine, and dacarbazine in the highly active antiretroviral therapy era. J Clin Oncol. 2012;30(33):4111‐4116. 10.1200/JCO.2011.41.4193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Besson C, Lancar R, Prevot S, et al. High risk features contrast with favorable outcomes in HIV‐associated Hodgkin lymphoma in the modern cART era, ANRS CO16 LYMPHOVIR Cohort. Clin Infect Dis. 2015;61(9):1469‐1475. 10.1093/cid/civ627 [DOI] [PubMed] [Google Scholar]
- 8. Sorigué M, García O, Tapia G, et al. HIV‐infection has no prognostic impact on advanced stage Hodgkin lymphoma. AIDS. 2017;31(10):1445‐1449. 10.1097/QAD.0000000000001487 [DOI] [PubMed] [Google Scholar]
- 9. Spina M, Gabarre J, Rossi G, et al. Stanford V regimen and concomitant HAART in 59 patients with Hodgkin disease and HIV infection. Blood. 2002;100(6):1984‐1988. 10.1182/blood-2002-03-0989 [DOI] [PubMed] [Google Scholar]
- 10. Hentrich M, Berger M, Wyen C, et al. Stage‐adapted treatment of HIV‐associated Hodgkin lymphoma: results of a prospective multicenter study. J Clin Oncol. 2012;30(33):4117‐4123. 10.1200/JCO.2012.41.8137 [DOI] [PubMed] [Google Scholar]
- 11. Danilov AV, Li H, Press OW, et al. Feasibility of interim positron emission tomography (PET)‐adapted therapy in HIV‐positive patients with advanced Hodgkin lymphoma (HL): a sub‐analysis of SWOG S0816 phase 2 trial. Leuk Lymphoma. 2017;58(2):461‐465. 10.1080/10428194.2016.1201573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rubinstein PG, Moore PC, Bimali M, et al. Brentuximab vedotin with AVD for stage II‐IV HIV‐related Hodgkin lymphoma (AMC 085): phase 2 results from an open‐label, single arm, multicentre phase 1/2 trial. Lancet Haematol. 2023;10(8):e624‐e632. 10.1016/S2352-3026(23)00157-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. US Centers for Disease Control and Prevention . 1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Recomm Rep. 1993;5:1‐19. [PubMed] [Google Scholar]
- 14. Eichenauer DA, Aleman BMP, André M, et al. Hodgkin lymphoma: ESMO clinical practice guidelines for diagnosis, treatment and follow‐up. Ann Oncol. 2018;29(Suppl. 4):iv19‐iv29. 10.1093/annonc/mdy080 [DOI] [PubMed] [Google Scholar]
- 15. von Tresckow B, Plütschow A, Fuchs M, et al. Dose‐intensification in early unfavorable Hodgkin's lymphoma: final analysis of the German Hodgkin Study Group HD14 Trial. J Clin Oncol. 2012;30(9):907‐913. 10.1200/JCO.2011.38.5807 [DOI] [PubMed] [Google Scholar]
- 16. Gillessen S, Plütschow A, Fuchs M, et al. Intensified treatment of patients with early stage, unfavourable Hodgkin lymphoma: long‐term follow‐up of a randomised, international phase 3 trial of the German Hodgkin Study Group (GHSG HD14). Lancet Haematol. 2021;8(4):e278‐e288. 10.1016/S2352-3026(21)00029-6 [DOI] [PubMed] [Google Scholar]
- 17. Xicoy B, Ribera JM, Miralles P, et al. Results of treatment with doxorubicin, bleomycin, vinblastine and dacarbazine and highly active antiretroviral therapy in advanced stage, human immunodeficiency virus‐related Hodgkin's lymphoma. Haematologica. 2007;92(2):191‐198. 10.3324/haematol.10479 [DOI] [PubMed] [Google Scholar]
- 18. André MPE, Carde P, Viviani S, et al. Long‐term overall survival and toxicities of ABVD vs BEACOPP in advanced Hodgkin lymphoma: a pooled analysis of four randomized trials. Cancer Med. 2020;9(18):6565‐6575. 10.1002/cam4.3298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. von Tresckow B, Kreissl S, Goergen H, et al. Intensive treatment strategies in advanced‐stage Hodgkin's lymphoma (HD9 and HD12): analysis of long‐term survival in two randomised trials. Lancet Haematol. 2018;5(10):e462‐e473. 10.1016/S2352-3026(18)30140-6 [DOI] [PubMed] [Google Scholar]
- 20. Engert A, Haverkamp H, Kobe C, et al. Reduced‐intensity chemotherapy and PET‐guided radiotherapy in patients with advanced stage Hodgkin's lymphoma (HD15 trial): a randomised, open‐label, phase 3 non‐inferiority trial. Lancet. 2012;379(9828):1791‐1799. 10.1016/S0140-6736(11)61940-5 [DOI] [PubMed] [Google Scholar]
- 21. Gallamini A, Rossi A, Patti C, et al. Consolidation radiotherapy could be safely omitted in advanced Hodgkin lymphoma with large nodal mass in complete metabolic response after ABVD: final analysis of the randomized GITIL/FIL HD0607 trial. J Clin Oncol. 2020;38(33):3905‐3913. 10.1200/JCO.20.00935 [DOI] [PubMed] [Google Scholar]
- 22. Ansell SM, Radford J, Connors JM, et al. Overall survival with brentuximab vedotin in stage III or IV Hodgkin's lymphoma. N Engl J Med. 2022;387(4):310‐320. 10.1056/NEJMoa2206125 [DOI] [PubMed] [Google Scholar]
- 23. Hoffmann C, Hentrich M. Optimising treatment of HIV‐associated Hodgkin lymphoma. Lancet Haematol. 2023;10(8):e563‐e564. 10.1016/S2352-3026(23)00177-1 [DOI] [PubMed] [Google Scholar]
- 24. Pinczés L, Miltényi Z, Illés Á. Young adults diagnosed with Hodgkin lymphoma are at risk of relapsing late: a comprehensive analysis of late relapse in Hodgkin lymphoma. J Cancer Res Clin Oncol. 2018;144(5):935‐943. 10.1007/s00432-018-2613-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Vassilakopoulos TP, Kravvariti E, Panitsas F, et al. Very late relapses in Hodgkin lymphoma treated with chemotherapy with or without radiotherapy: linear pattern and distinct prognostic factors. Blood Cancer J. 2022;12(7):102. 10.1038/s41408-022-00674-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bröckelmann PJ, Goergen H, Kohnhorst C, et al. Late relapse of classical Hodgkin lymphoma: an analysis of the German Hodgkin study group HD7 to HD12 trials. J Clin Oncol. 2017;35(13):1444‐1450. 10.1200/JCO.2016.71.3289 [DOI] [PubMed] [Google Scholar]
- 27. Andersen MD, Hamilton‐Dutoit S, Modvig L, et al. Late recurrence of lymphoid malignancies after initial treatment for Hodgkin lymphoma—a study from the Danish Lymphoma Registry. Br J Haematol. 2022;198(1):50‐61. 10.1111/bjh.18180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Abrahão R, Brunson AM, Kahn JM, Li QW, Wun T, Keegan THM. Second primary malignancy risk after Hodgkin lymphoma treatment among HIV‐uninfected and HIV‐infected survivors. Leuk Lymphoma. 2022;63(5):1091‐1101. 10.1080/10428194.2021.2020775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chiao EY, Coghill A, Kizub D, et al. The effect of non‐AIDS‐defining cancers on people living with HIV. Lancet Oncol. 2021;22(6):e240‐e253. 10.1016/S1470-2045(21)00137-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Castillo JJ, Bower M, Brühlmann J, et al. Prognostic factors for advanced‐stage human immunodeficiency virus‐associated classical Hodgkin lymphoma treated with doxorubicin, bleomycin, vinblastine, and dacarbazine plus combined antiretroviral therapy. Cancer. 2015;121(3):423‐431. 10.1002/cncr.29066 [DOI] [PubMed] [Google Scholar]
- 31. Hübel K, Re A, Boumendil A, et al. Autologous stem cell transplantation for HIV‐associated lymphoma in the antiretroviral and rituximab era: a retrospective study by the EBMT Lymphoma Working Party. Bone Marrow Transplant. 2019;54(10):1625‐1631. 10.1038/s41409-019-0480-x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request. Deidentified patient data and data dictionary may be provided upon reasonable request via email to the corresponding author.


