Abstract
Background:
The relationships between opioid use disorder (OUD), chronic pain, and mental health distress are complex and multidirectional. The objective of this exploratory study was to examine the relationship between mental health conditions and Chronic pain severity and interference among patients stabilized on either buprenorphine or methadone.
Methods:
We report baseline data from a randomized trial of a mind-body intervention conducted at 5 outpatient clinics that provided either buprenorphine or methadone treatment. Validated scales were used to measure substance use, mental health distress, and pain severity and interference. Statistical analyses examined the relationship between mental health conditions and pain severity and interference.
Results:
Of 303 participants, 57% (n = 172) reported Chronic pain. A total of 88% (n = 268) were prescribed buprenorphine. Mental health conditions were common, with one-quarter of the sample screening positive for all 3 mental health conditions (anxiety, depression, and posttraumatic stress disorder [PTSD]). Compared to participants without Chronic pain, participants with Chronic pain were more likely to screen positive for moderate-severe anxiety (47% vs 31%); moderate-severe depression (54% vs 41%); and the combination of anxiety, depression, and PTSD (31% vs 18%). Among participants with Chronic pain, mental health conditions were associated with higher pain interference. Pain severity was higher among participants with mental health conditions, but only reached statistical significance for depression. Pain interference scores increased with a higher number of co-occurring mental health conditions.
Conclusions:
Among individuals stabilized on either buprenorphine or methadone, highly symptomatic and comorbid mental health distress is common and is associated with increased pain interference. Adequate screening for, and treatment of, mental health conditions in patients with OUD and Chronic pain is needed.
Keywords: opioid use disorder, mental health, chronic pain, pain interference, methadone, buprenorphine, posttraumatic stress disorder, depression, anxiety
Introduction
Opioid use disorder (OUD) is a chronic condition associated with significant morbidity and mortality.1 Chronic pain is a common co-occurring condition present in approximately two-thirds of patients with OUD.2–4 Chronic pain and OUD are related, and worsening of either condition may result in exacerbation and maintenance of both conditions over time.5 Complicating matters further, mental health conditions such as depressive disorders, anxiety disorders, and posttraumatic stress disorder (PTSD) commonly occur among both patients with OUD and patients with Chronic pain.6–9 Major depressive disorder, in particular, is implicated in the development and maintenance of both OUD and Chronic pain.10,11 The relationships between OUD, Chronic pain, and mental health conditions are complex and may be mediated by a variety of mechanisms including allostatic overload (eg, the cumulative impact of attempts to maintain physiologic stability in the face of stressors), negative reinforcement, and social, emotional, and cognitive processes.5
Pharmacologic treatment of OUD with methadone, a full opioid agonist, or buprenorphine, a partial opioid agonist, is associated with substantial reductions in all cause and overdose mortality.12 Buprenorphine exerts its analgesic effect through partial agonism at the mu opioid receptor. It is also an antagonist at the kappa opioid receptor and is being explored as a possible treatment option for patients with major depressive disorder, treatment resistant depression, and suicidal ideation.13,14 Despite its analgesic (buprenorphine and methadone) and antidepressant (buprenorphine) effects, a subset of patients with OUD and Chronic pain treated with medications for OUD (MOUD) continue to experience high levels of pain interference (the interference of pain on daily activities15), poor social functioning, mental health distress, nonprescribed substance use, and low utilization of coping strategies.16–21 Patients with co-occurring OUD and Chronic pain treated with MOUD are more likely to report cravings for opioids than patients without Chronic pain.22 Although presence of Chronic pain in people with OUD is not associated with a return to nonprescribed opioid use,22,23 highly volatile pain (ie, significant fluctuation or variability of pain scores over time) and severe pain are risk factors for return to use.24–26 Similarly, high levels of depression and/or high pain severity throughout treatment also appear to be risk factors for return to substance use.27
While numerous studies demonstrate a relationship between mental health distress and the presence and severity of Chronic pain among persons stabilized on either buprenorphine or methadone, there is a paucity of research specifically exploring the relationship between mental health conditions and pain interference in this population.6,21,28–30 Pain interference is important to measure as it is significantly related to Chronic pain treatment targets such as functional status, pain acceptance, and pain catastrophizing.31,32 A secondary analysis of the Prescription Opioid Addiction Treatment Study demonstrated an association between the severity of depression and pain interference.33 However, the relationship of other mental health conditions with pain interference was not assessed. In another trial, patients with co-occurring Chronic pain, OUD on buprenorphine, and PTSD had higher pain severity and pain interference than patients without PTSD.34 Other studies show a relationship between a combined measure of pain severity and interference and PTSD, depression, and anxiety.7,8,35 However, the specific relationship of pain interference to these mental health conditions is not fully explored. Furthermore, there is little understanding of the impact of co-occurring mental health conditions on Chronic pain interference in this population.
This study examines data collected at baseline for a randomized trial of Mindful Awareness in Body-Oriented Therapy (MABT) in patients stabilized on either buprenorphine or methadone. The purpose of the present exploratory study is to examine the relationship between Chronic pain and mental health conditions among patients stabilized on buprenorphine or methadone. Specific objectives include the following: (1) compare mental health conditions (anxiety, depression, PTSD) in those with versus without Chronic pain; (2) examine whether anxiety, depression, and PTSD are associated with pain severity and interference among patients with Chronic pain; (3) explore whether the number of co-occurring mental health conditions (ie, anxiety, depression, and PTSD alone or in combination) are associated with pain severity and interference among patients with Chronic pain.
Methods
Setting
We report data collected at baseline from a 2-group randomized trial (N = 303) of MABT as an adjunct to MOUD treatment. Participants were recruited from 5 Washington state outpatient clinics in urban and rural settings. One clinic was an urban opioid treatment program prescribing predominantly methadone. One clinic was a mental health clinic offering buprenorphine treatment. Three clinics were primary care clinics with embedded buprenorphine programs.
Participants
We included all participants (N = 303) enrolled in the MABT randomized trial described above. In the trial, patients with adequate treatment engagement and clinical stability to participate in the MABT intervention were recruited. For buprenorphine, this was defined as at least 4 weeks of medication treatment and appointment frequency less than once weekly. Because it typically takes a longer period of time to reach a therapeutic dose of methadone, medication dose stability was defined as at least 90 days in methadone treatment with a minimum dose of 60 mg and no more than 3 missed doses or any missed dose evaluation appointments in the past 30 days. Patients also needed to speak English and be willing to attend MABT sessions when offered. They were excluded if they were not willing or able to remain in MOUD treatment for the duration of the 1-year trial, or if they showed evidence of overt psychosis or cognitive impairment.
Measures
Demographics, socioeconomic, and health attributes were assessed by patient self-report. Substance use was assessed using the Timeline Follow-Back Interview (TLFB).36 The TLFB is a calendar method used to identify past 90-day substance use, including frequency and quantity of use.
Mental health distress was measured with 3 well-validated scales. The Patient Health Questionnaire-9 (PHQ-9) is a 9-item scale designed to assess levels and severity of depression, with a cutoff point of ≥10 for moderate-severe depression.37(p9) The General Anxiety Disorder-7 (GAD-7) is a 7-item scale designed to assess levels and severity of generalized anxiety, with a cutoff point of ≥10 for moderate-severe anxiety.38 The Posttraumatic Stress Disorder Checklist for Diagnostic and Statistical Manual of Mental Disorders-5 (PCL-5) is a validated 20-item self-report measure that assesses the DSM-5 symptoms of PTSD; a cutoff score of 33 was used to indicate probable PTSD.39
The presence of Chronic pain was determined by a survey question: “Are you currently experiencing any bodily pain that has been present for 3 months or more?” Pain severity and interference were measured using the Brief Pain Inventory (BPI).40 The BPI is a well-validated, widely used measurement tool for assessing clinical pain. The assessment includes 11 questions, which provide information on pain severity and pain interference in the past week. Pain severity is rated from 0 (“No pain”) to 10 (“Worst pain ever”), and participants are asked to rate their pain currently, at its worst and its best over the past week, and on average for the past 3 months. Pain interference is the average of 7 items asking about the impact of pain on daily activities, mood, and enjoyment of life, which participants rate on a scale from 0 (does not interfere) to 10 (completely interferes). Different cut points have been validated for different painful conditions, and per national guidelines regarding pain measures in clinical trials, a minimally important difference on the pain interference scale is a decrease of 1 point.41,42
Data Analysis
Demographic and clinical characteristics were summarized using descriptive statistics. To examine differences between participants with and without Chronic pain, independent sample t tests were used for continuous measures and chi-square tests for categorical measures. Two-sided significance tests were used for all analyses with a significance level of P < .05. Differences in clinical characteristics including time in treatment, MOUD type, percent days abstinent, pain severity, pain interference, and binary measures of mental health conditions (moderate to severe symptoms vs no or mild symptoms) and a categorical measure of the number of mental health conditions were compared by Chronic pain status.
Among the subgroup of patients who reported Chronic pain, we first examined differences in pain severity and interference by groups based on each mental health condition (anxiety, depression, and PTSD). To examine the effect of the number of mental health conditions on pain severity and interference we conducted 1-way ANOVAs to compare the means of these groups using a Bonferroni correction for multiple comparisons. To examine differences in pain severity and interference by combinations of mental health conditions, we classified patients in 8 categories: no mental health conditions, anxiety, depression, PTSD, anxiety and depression, depression and PTSD, anxiety and PTSD, and anxiety, depression, and PTSD. Box plots were used to show the range, quartiles, and medians of these differences by category since the study was not powered to detect differences between specific combinations of mental health conditions. A sensitivity analysis was performed to examine for differences in these analyses based on medication (buprenorphine or methadone). Because the sensitivity analysis yielded only one significantly different result (the ANOVA test reported in the Results section), we looked at a combined group (ie, participants on either buprenorphine or methadone) for the primary analyses, which increased the power of the study.
Data analyses were performed using Stata version 18 (StataCorp LLC, College Station, TX, USA).
Results
Participant Characteristics
As shown in Table 1, participants’ age was on average 42.3 (SD, 12.2) years and 52% (n = 157) were female. The majority of the sample (79%, n = 238) self-identified as white, and 9% (n = 27) identified as Hispanic. A total of 44% (n = 132) completed their education with high school or General Educational Development (GED), and 34% (n = 103) of patients completed 2 years of technical training, or an associate degree. A total of 66% (n = 199) were unemployed, and 97% (n = 293) reported stable housing. As presented in Table 2, 67% (n = 203) of participants had been engaged in MOUD treatment for more than 12 months, and there was no significant difference between time in treatment among participants with and without Chronic pain (P = .778). Most participants (88%, n = 268) were prescribed buprenorphine. MOUD type (ie, methadone vs buprenorphine) was not significantly different in patients with and without Chronic pain (P = .063). On average, participants reported abstinence from any nonprescribed opioid on 94.6% of the prior 90 days and reported abstinence from any nonprescribed substance (excluding cannabis) and heavy drinking days on 87.8% of the prior 90 days. Percent days abstinent was not significantly different between participants with and without Chronic pain (P = .066 for any opioid, P = .681 for nonprescribed substances and heavy drinking). Compared to participants without Chronic pain, participants with Chronic pain had higher pain severity (4.9 [SD, 1.6]) vs 2.4 [SD, 1.6], P < .001) and interference (5.0 [SD, 2.3] vs 2.2 [SD, 1.9], P < .001) as measured by the BPI.
Table 1.
Demographic Characteristics.
| Characteristic | Total | No chronic pain | Chronic pain |
|---|---|---|---|
| N | 303 | 131 | 172 |
| Gender identity | |||
| Male | 144 (48%) | 64 (49%) | 80 (47%) |
| Female | 157 (52%) | 65 (50%) | 92 (53%) |
| Nonbinary or other | 2 (1%) | 2 (2%) | 0 (0%) |
| Age | 42.3 (12.2) | 39.3 (11.3) | 44.6 (12.3) |
| Race | |||
| American Indian or Alaska Native | 13 (4%) | 5 (4%) | 8 (5%) |
| Asian | 3 (1%) | 0 (0%) | 3 (2%) |
| Black or African American | 16 (5%) | 5 (4%) | 11 (6%) |
| Native Hawaiian or Other Pacific Islander | 4 (1%) | 2 (2%) | 2 (1%) |
| White | 238 (79%) | 110 (84%) | 128 (75%) |
| More than one race | 28 (9%) | 9 (7%) | 19 (11%) |
| Hispanic | 27 (9%) | 7 (5%) | 20 (12%) |
| Education | |||
| 11th grade or less | 36 (12%) | 9 (7%) | 27 (16%) |
| High school or GED | 132 (44%) | 67 (51%) | 65 (38%) |
| 2 year college or technical school | 103 (34%) | 44 (34%) | 59 (34%) |
| College degree ± advanced degree | 32 (11%) | 11 (8%) | 21 (12%) |
| Stable housing | 293 (97%) | 126 (96%) | 167 (97%) |
| Employment | |||
| Unemployed | 199 (66%) | 81 (62%) | 118 (69%) |
| Full-time or part-time work | 104 (35%) | 50 (38%) | 54 (32%) |
GED: Graduate Equivalency Degree
Table 2.
Substance Use and Pain Characteristics by Chronic Pain Status.
| Characteristic | Total | No chronic pain | Chronic pain | P |
|---|---|---|---|---|
| N | 303 | 131 | 172 | |
| Time in treatment | .778 | |||
| <3 months | 28 (9%) | 10 (8%) | 18 (10%) | |
| 3-6 months | 26 (9%) | 10 (8%) | 16 (9%) | |
| 6-12 months | 46 (15%) | 21 (16%) | 25 (15%) | |
| >12 months | 203 (67%) | 90 (69%) | 113 (66%) | |
| Medication for opioid use disorder | .063 | |||
| Methadone | 35 (12%) | 10 (8%) | 25 (15%) | |
| Buprenorphine | 268 (88%) | 121 (92%) | 147 (85%) | |
| % Days abstinent nonprescribed opioid(s) | 96.4 (13.8) | 98.0 (9.0) | 95.1 (16.5) | .066 |
| % Days abstinent heavy drinking days and nonprescribed substances* | 87.8 (26.5) | 88.5 (26.1) | 87.2 (26.9) | .681 |
| Pain severity | 3.9 (2.0) | 2.4 (1.6) | 4.9 (1.6) | <.001 |
| Pain interference | 3.8 (2.5) | 2.2 (1.9) | 5.0 (2.3) | <.001 |
Mean (SD): P value from a pooled independent sample t test. Frequency (%): P value from chi-square test.
% Days abstinent heavy drinking days and nonprescribed substances excludes cannabis.
Mental Health Conditions in Participants With and Without Chronic Pain
As presented in Table 3, mental health conditions were highly prevalent among participants with and without Chronic pain. Compared to participants without Chronic pain, participants with Chronic pain were more likely to screen positive for moderate-severe anxiety (47% vs 31%, P = .007) and moderate-severe depression (54% vs 41%, P = .027). More participants with Chronic pain screened positive for PTSD (45% vs 36%), though the association was not statistically significant (P = .119). As shown in Table 3, while there was not a statistically significant difference in the proportion of those with 0, 1, 2, or 3 concurrent mental health conditions who reported Chronic pain (P = .062), those with all 3 mental health conditions were more likely than others to report experiencing Chronic pain (P = .013).
Table 3.
Mental Health Conditions by Chronic Pain Status.
| Total | No chronic pain | Chronic pain | P | |
|---|---|---|---|---|
| N | 303 | 131 | 172 | |
| Anxiety | 121 (40%) | 41 (31%) | 80 (47%) | .007 |
| Depression | 147 (49%) | 54 (41%) | 93 (54%) | .027 |
| PTSD | 124 (41%) | 47 (36%) | 77 (45%) | .119 |
| Number of mental health conditions | .062 | |||
| 0 | 116 (38%) | 59 (45%) | 57 (33%) | |
| 1 | 59 (19%) | 26 (20%) | 33 (19%) | |
| 2 | 51 (17%) | 22 (17%) | 29 (17%) | |
| 3 (anxiety, depression, and PTSD) | 77 (25%) | 24 (18%) | 53 (31%) |
Anxiety = moderate to severe anxiety symptoms (GAD-7 ≥ 10); depression = moderate to severe depression symptoms (PHQ-9 ≥ 10); PTSD = PCL-5 ≥ 31; P value from chi-square test.
Abbreviations: PTSD, posttraumatic stress disorder.
Pain Severity and Interference by Mental Health Conditions in Participants With Chronic Pain
As presented in Table 4, among participants with Chronic pain, a positive screen for moderate-severe anxiety, moderate-severe depression, or PTSD was associated with higher pain interference (anxiety: 5.6 vs 4.4, P < .001; depression: 5.9 vs 3.9, P < .001; PTSD 5.9 vs 4.2, P < .001). Pain severity trended toward being higher among participants with mental health conditions, but only reached statistical significance for depression (5.2 vs 4.7, P = .04).
Table 4.
Pain Severity and Interference by Mental Health Condition for Participants With Chronic Pain.
| Mental health conditions | N | Pain severity, mean (SD) | Pain interference, mean (SD) |
|---|---|---|---|
| Anxiety | |||
| No | 91 | 4.8 (1.4) | 4.4 (2.1) |
| Yes | 80 | 5.1 (1.8) | 5.6 (2.3) |
| P value | .12 | <.001 | |
| Depression | |||
| No | 78 | 4.7 (1.5) | 3.9 (2.1) |
| Yes | 93 | 5.2 (1.7) | 5.9 (2.0) |
| P value | .04 | <.001 | |
| PTSD | |||
| No | 94 | 4.7 (1.6) | 4.2 (2.2) |
| Yes | 77 | 5.2 (1.6) | 5.9 (2.1) |
| P value | .06 | <.001 |
Anxiety = moderate to severe anxiety symptoms (GAD-7 ≥ 10); depression = moderate to severe depression symptoms (PHQ-9 ≥ 10); PTSD = PCL-5 ≥ 31; P value from t test.
Abbreviations: PTSD, Post Traumatic Stress Disorder.
Pain Severity and Interference by Number of Co-occurring Mental Health Conditions in Participants With Chronic Pain
Given limited power, box plots were used to compare the range and distribution of pain severity and interference scores by categories of mental health conditions within the subgroup of patients with Chronic pain (Figure 1). The median pain severity was approximately 5 across all the mental health categories. The highest median scores (between 5 and 6) for pain interference occur in categories including depression, particularly the category of all 3 mental health conditions.
Figure 1.
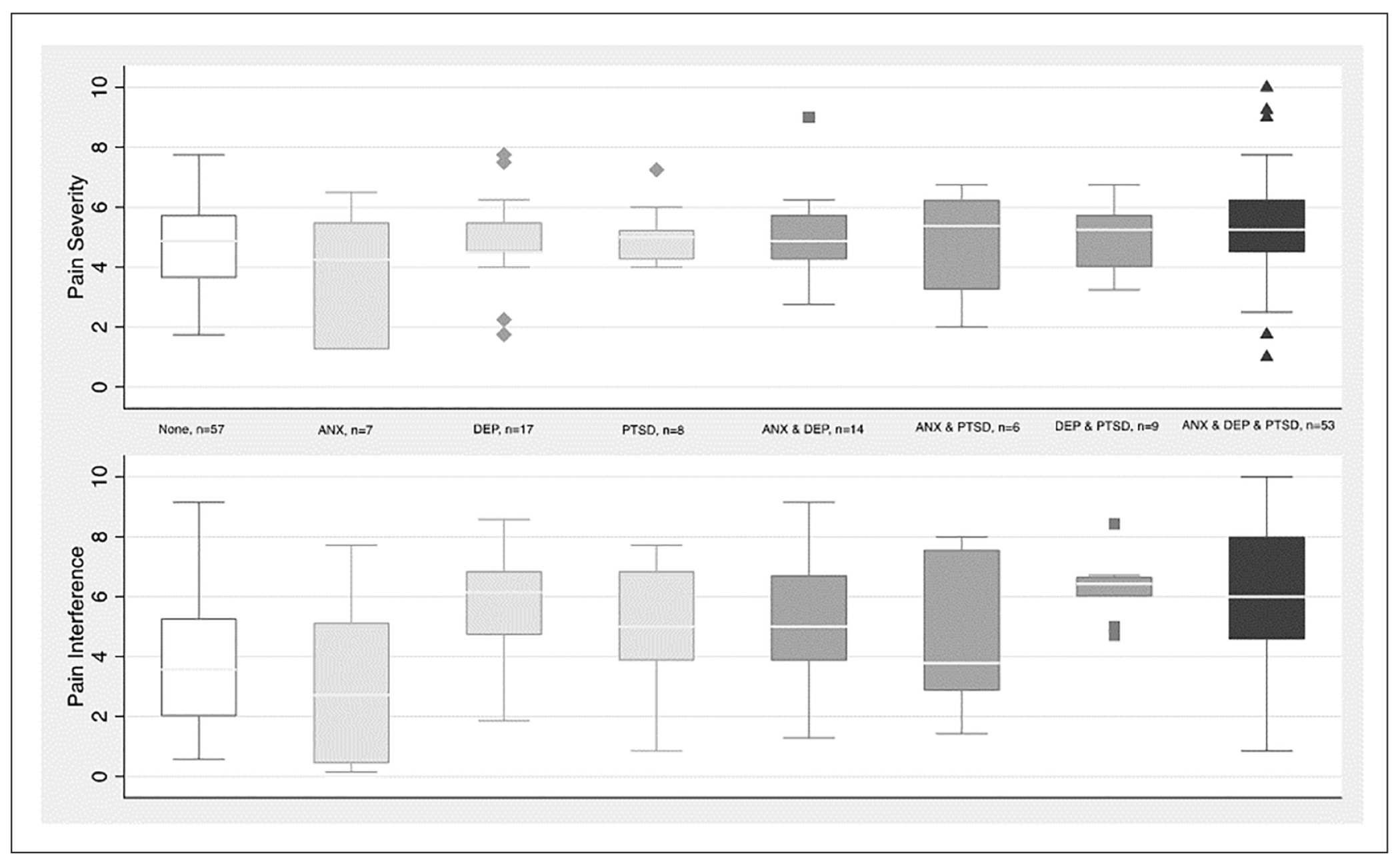
Pain severity and interference by mental health condition(s) for participants with chronic pain.
Bars represent range, boxes represent interquartile range, and lines represent medians.
Abbreviations: Anx, Anxiety; Dep, Depression; PTSD, Post-traumatic Stress Disorder
The 1-way ANOVA for pain severity shown in Table 5 revealed no significant differences among the groups based on the number of mental health conditions (F[3, 167] = 1.80, P = .148). The pain interference analysis, however, showed a significant increase in symptoms among the groups based on the number of mental health conditions (F[3, 168] = 13.5, P < .001). There were increases in pain interference symptoms for 2 mental health conditions versus 0 (mean difference = 1.7; 95% CI: 0.5-3.0); 3 versus 0 (mean difference = 2.5 95% CI: 1.4-3.5); and 3 versus 1 (mean difference = 1.3; 95% CI: 0.04-2.5). In a sensitivity analysis excluding patients receiving methadone, the 1-way ANOVA between pain severity and the number of co-occurring mental health conditions became significant (F test = 3.09, P = .029). Post hoc comparisons using a Bonferroni correction for multiple comparisons showed a significant difference (P = .025) in pain severity between those who had 0 mental health conditions and those who had 3 mental health conditions.
Table 5.
Pain Severity and Interference by Number of Mental Health Conditions for Participants With Chronic Pain.
| 0 | 1 | 2 | 3 | F(3, 167-3, 168) | P | |
|---|---|---|---|---|---|---|
| N | 57 | 33 | 29 | 53 | ||
| Pain severity | 4.7 (1.5) | 4.7 (1.5) | 5.0 (1.7) | 5.3 (1.7) | 1.80 | .148 |
| Pain interference | 3.7a (2.0) | 4.9b (2.3) | 5.4a (2.1) | 6.2a,b (2.1) | 13.53 | <.001 |
Means in a row sharing a subscript are significantly different from one another. F and P values are from 1-way ANOVA tests using a Bonferroni correction for multiple comparisons.
Discussion
In this sample of patients with OUD stabilized on either buprenorphine or methadone, highly symptomatic and co-occurring mental health conditions are prevalent, and are associated with increased pain interference. Previous research has examined an association between individual mental health conditions and Chronic pain in patients treated with MOUD.6,7,17,18,20,27,28 However, our study is the first to report that the presence of multiple, co-occurring mental health conditions are associated with higher pain interference. These findings have implications for the longitudinal care of patients treated with MOUD, especially those also experiencing Chronic pain.
Despite participant stability on MOUD (two-thirds of the sample were engaged in treatment for >1 year and rates of abstinence were high), moderate-severe anxiety, moderate-severe depression, and PTSD were highly prevalent, and frequently co-occurring, with one-quarter of the cohort screening positive for all 3 mental health conditions. Over half of participants experienced Chronic pain and had an average pain severity and interference of 4.9 and 5.0, respectively. This corresponds to moderate-severe pain, depending on the clinical cut points used.43–45 These findings comport with previously published studies demonstrating that despite the antidepressant, analgesic, and anxiolytic properties of buprenorphine, a subset of patients continue to experience high levels of pain and mental health distress.19,20,27
Compared to participants without Chronic pain, participants with Chronic pain were more likely to screen positive for moderate-severe anxiety and moderate-severe depression. This finding is congruent with previously published research that described high rates of co-occurring Chronic pain, anxiety, and depression within the general population46–49 and, specifically, among patients with OUD.2,50,51 A high prevalence of co-occurring PTSD and Chronic pain severity and interference have also been established among the general population,52 veterans,53 and patients with OUD receiving MOUD.8,9 In the present study, PTSD was more prevalent in participants with Chronic pain (45% vs 36%), but the association was not statistically significant (P = .119). Clearly, patients with Chronic pain require careful assessment of multiple mental health comorbidities.
Notably, almost one-third of participants with Chronic pain screened positive for all 3 conditions (ie, anxiety, depression, and PTSD), compared to 18% of participants without Chronic pain. Furthermore, there was an additive effect that was both statistically and clinically significant. Per Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials (IMMPACT) guidelines, a difference of 1 point on the pain interference scale indicates a minimally important difference in clinical trials.42 We found, for example, a 1.3-point higher mean pain interference score in participants with 3 mental health conditions compared to those with 1 mental health condition. Similar findings have been reported among the general population. For example, results from a large nationally representative cross-sectional survey commissioned by the New Zealand Ministry of Health revealed that symptoms of anxiety and depression interacted synergistically with Chronic pain to increase the odds of reporting Chronic pain.54 We extend these findings to include PTSD and document the additive effects of the number of mental health conditions on Chronic pain among a sample of patients with OUD stabilized on either buprenorphine or methadone.
Our results show that among participants with Chronic pain, co-occurring mental health conditions were more strongly associated with pain interference than pain severity. This finding has clinical implications as patients are most often asked to report their pain using a brief “0-to-10” assessment of pain intensity. Pain intensity is an important component of pain assessments; however, it does not allow for recognition of the multidimensional nature of pain. Assessing pain interference, in addition to pain severity, aids the measurement of the amount or frequency of “the interference of pain on daily activities” with the inclusion of physical, psychological, and social activities15 and thus is more clinically relevant, particularly in light of its demonstrated relationship to mental health distress.
Results revealing additive effects of co-occurring mental health conditions on patient-reported pain may be explained through models of allostatic load.55,56 Simplistically, allostasis encompasses the mechanisms through which individuals adapt to stressors to maintain physiological stability. Allostatic (over)load is the cumulative effect on the brain and body arising from attempts to adapt and maintain allostasis. Psychological, behavioral, and social demands relating to the management of multiple mental health conditions, or more directly through dysregulated physiological mechanisms, may limit and/or impair coping strategies. Poor coping strategies, such as avoidance, have been found to contribute to poor emotion regulation, a trans-diagnostic factor underlying mental health disorders and substance use disorder,31,32 and critical for the capacity to manage mental health distress and Chronic pain57 and highly relevant to those with OUD.57,58
Clinically, these findings support the identified need for greater mental health support for individuals receiving methadone or buprenorphine treatment, and point to the importance of complementary and integrative health (CIH) approaches that promote regulatory skills.57 CIH approaches, and specifically mind-body interventions, are considered best practice for the treatment of Chronic pain and stress-related disease.59,60 Theoretical models of mindfulness,61,62 including the mindfulness stress buffering theory, postulate that mind-body training can facilitate the capacity to observe and experience internal reactions to a stressor with acceptance and equanimity. In turn, this impartial receptiveness buffers initial threat appraisals and, subsequently, reduces emotional reactivity,63 leading to greater emotional and physical regulation and improved health.64 Therefore, mind-body interventions may promote more adaptive responses to stressors, increasing the capacity to manage mental health distress and Chronic pain within the context of OUD.
This study has multiple strengths. First, it is a multisite study including participants from urban and rural areas and multiple practice settings (opioid treatment program, mental health clinic, addiction clinic, and primary care clinic). Patients reported a high proportion of days abstinent, and most had been engaged in methadone or buprenorphine treatment for over a year, reducing the possibility that mental health symptoms were primarily substance-induced. In addition to presence or absence of Chronic pain, pain severity and interference were measured using the BPI. Pain interference is significantly related to Chronic pain treatment targets such as functional status, pain acceptance, and pain catastrophizing.31,32 There is a paucity of literature on the relationship between pain interference and mental health conditions in patients with OUD treated with MOUD. This study extends current knowledge by demonstrating an additive association between multiple mental health conditions and pain interference.
There are also several limitations worth noting. This was a convenience sample of participants already stabilized on MOUD, and results may not be generalizable to less stable populations of patients who are actively using substances. The majority (97%) indicated stable housing. Of note, this is not necessarily an indicator of permanent housing, but that housing was perceived to be stable by the participant. Though the screening forms for mental health conditions are well-validated, patients were not assessed using the gold standard DSM-5 diagnostic interview. Different BPI cut points and minimally important clinical differences have been validated for different painful conditions, but to our knowledge, there are no standardized cut points specifically for individuals with Chronic pain and OUD.34 Because this is an analysis of the baseline data only, we are unable to comment on the directionality of observed associations between mental health conditions and Chronic pain severity and interference. Most patients received buprenorphine (88%), and the present analysis was not designed to assess differences in the relationship between Chronic pain and mental health distress by MOUD type. Because most patients received buprenorphine, the results of the present study may not be generalizable to patients receiving methadone. We did not collect data on use of other medications (including medications for pain, depression, anxiety, and PTSD), so we cannot comment on whether the mental health conditions were treatment resistant. Finally, although we include an exploratory analysis, the study was not powered to detect interactions between specific combinations of mental health conditions (eg, PTSD + depression vs PTSD + anxiety), a potential question for further research.
Conclusions
Among patients with OUD stabilized on either buprenorphine or methadone, highly symptomatic and co-occurring mental health conditions are common, and are associated with increased pain interference. Treatment should not stop with MOUD; increased screening and treatment for Chronic pain and mental health conditions in this population are needed.
Highlights.
Among patients with opioid use disorder stabilized on either buprenorphine or methadone, mental health conditions (anxiety, depression, and post-traumatic stress disorder) are highly prevalent and frequently co-occurring
For patients with both chronic pain and opioid use disorder stabilized on either buprenorphine or methadone, mental health conditions are associated with increased pain interference
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This publication was made possible by Grant Numbers R33AT009932 from the National Center for Complementary and Integrative Health (NCCIH) and R01 AT010742 from NCCIH and the National Institute of Neurological Disorders and Stroke (NINDS). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NCCIH, NINDS, or the National Institutes of Health.
Footnotes
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
This study was approved by the University of Washington IRB.
Trial Registration
Registration in ClinicalTrials.gov: NCT04082637.
Data Availability Statement:
All deidentified self-report data are available at https://www.openicpsr.org/openicpsr/workspace?goToPath=/openicpsr/205381&goToLevel=project
References
- 1.Taylor JL, Samet JH. Opioid use disorder. Ann Intern Med. 2022;175(1):ITC1–ITC16. doi: 10.7326/AITC202201180 [DOI] [PubMed] [Google Scholar]
- 2.Hser YI, Mooney LJ, Saxon AJ, Miotto K, Bell DS, Huang D. Chronic pain among patients with opioid use disorder: results from electronic health records data. J Subst Abuse Treat. 2017;77:26–30. doi: 10.1016/j.jsat.2017.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Voon P, Karamouzian M, Kerr T. Chronic pain and opioid misuse: a review of reviews. Subst Abuse Treat Prev Policy. 2017;12(1):36. doi: 10.1186/s13011-017-0120-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.John WS, Wu LT. Chronic non-cancer pain among adults with substance use disorders: prevalence, characteristics, and association with opioid overdose and healthcare utilization. Drug Alcohol Depend. 2020;209:107902. doi: 10.1016/j.drugalcdep.2020.107902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ditre JW, Zale EL, LaRowe LR. A reciprocal model of pain and substance use: transdiagnostic considerations, clinical implications, and future directions. Annu Rev Clin Psychol. 2019;15:503–528. doi: 10.1146/annurev-clinpsy-050718-095440 [DOI] [PubMed] [Google Scholar]
- 6.Rosenblum A, Joseph H, Fong C, Kipnis S, Cleland C, Portenoy RK. Prevalence and characteristics of chronic pain among chemically dependent patients in methadone maintenance and residential treatment facilities. JAMA. 2003;289(18):2370–2378. doi: 10.1001/jama.289.18.2370 [DOI] [PubMed] [Google Scholar]
- 7.Barry DT, Beitel M, Garnet B, Joshi D, Rosenblum A, Schottenfeld RS. Relations among psychopathology, substance use, and physical pain experiences in methadone-maintained patients. J Clin Psychiatry. 2009;70(9):1213–1218. doi: 10.4088/JCP.08m04367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barry DT, Beitel M, Cutter CJ, et al. Exploring relations among traumatic, posttraumatic, and physical pain experiences in methadone-maintained patients. J Pain. 2011;12(1):22–28. doi: 10.1016/j.jpain.2010.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bilevicius E, Sommer JL, Asmundson GJG, El-Gabalawy R. Posttraumatic stress disorder and chronic pain are associated with opioid use disorder: results from a 2012-2013 American nationally representative survey. Drug Alcohol Depend. 2018;188:119–125. doi: 10.1016/j.drugalcdep.2018.04.005 [DOI] [PubMed] [Google Scholar]
- 10.Edwards RR, Dolman AJ, Michna E, et al. Changes in pain sensitivity and pain modulation during oral opioid treatment: the impact of negative affect. Pain Med. 2016;17(10):1882–1891. doi: 10.1093/pm/pnw010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Volkow ND, Jones EB, Einstein EB, Wargo EM. Prevention and treatment of opioid misuse and addiction: a review. JAMA Psychiatry. 2019;76(2):208–216. doi: 10.1001/jama-psychiatry.2018.3126 [DOI] [PubMed] [Google Scholar]
- 12.Sordo L, Barrio G, Bravo MJ, et al. Mortality risk during and after opioid substitution treatment: systematic review and meta-analysis of cohort studies. BMJ. 2017;357:j1550. doi: 10.1136/bmj.j1550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spreen LA, Dittmar EN, Quirk KC, Smith MA. Buprenorphine initiation strategies for opioid use disorder and pain management: a systematic review. Pharmacotherapy. 2022;42(5):411–427. doi: 10.1002/phar.2676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stefanowski B, Antosik-Wójcińska A, Święcicki Ł. The use of buprenorphine in the treatment of drug-resistant depression—an overview of the studies. Psychiatr Pol. 2020;54(2):199–207. doi: 10.12740/PP/102658 [DOI] [PubMed] [Google Scholar]
- 15.Varni JW, Stucky BD, Thissen D, et al. PROMIS Pediatric Pain Interference Scale: an item response theory analysis of the pediatric pain item bank. J Pain. 2010;11(11):1109–1119. doi: 10.1016/j.jpain.2010.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Madison CA, Eitan S. Buprenorphine: prospective novel therapy for depression and PTSD. Psychol Med. 2020;50(6):881–893. doi: 10.1017/S0033291720000525 [DOI] [PubMed] [Google Scholar]
- 17.Ilgen MA, Trafton JA, Humphreys K. Response to methadone maintenance treatment of opiate dependent patients with and without significant pain. Drug Alcohol Depend. 2006;82(3):187–193. doi: 10.1016/j.drugalcdep.2005.09.005 [DOI] [PubMed] [Google Scholar]
- 18.Dunn KE, Brooner RK, Clark MR. Severity and interference of chronic pain in methadone-maintained outpatients. Pain Med. 2014;15(9):1540–1548. doi: 10.1111/pme.12430 [DOI] [PubMed] [Google Scholar]
- 19.Dunn KE, Finan PH, Tompkins DA, Fingerhood M, Strain EC. Characterizing pain and associated coping strategies in methadone and buprenorphine-maintained patients. Drug Alcohol Depend. 2015;157:143–149. doi: 10.1016/j.drugalcdep.2015.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lazaridou A, Paschali M, Edwards RR, Gilligan C. Is buprenorphine effective for chronic pain? A systematic review and meta-analysis. Pain Med. 2020;21(12):3691–3699. doi: 10.1093/pm/pnaa089 [DOI] [PubMed] [Google Scholar]
- 21.Stein MD, Herman DS, Bailey GL, et al. Chronic pain and depression among primary care patients treated with buprenorphine. J Gen Intern Med. 2015;30(7):935–941. doi: 10.1007/s11606-015-3212-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsui JI, Lira MC, Cheng DM, et al. Chronic pain, craving, and illicit opioid use among patients receiving opioid agonist therapy. Drug Alcohol Depend. 2016;166:26–31. doi: 10.1016/j.drugalcdep.2016.06.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weiss RD, Potter JS, Fiellin DA, et al. Adjunctive counseling during brief and extended buprenorphine-naloxone treatment for prescription opioid dependence: a 2-phase randomized controlled trial. Arch Gen Psychiatry. 2011;68(12):1238–1246. doi: 10.1001/archgenpsychiatry.2011.121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fox AD, Sohler NL, Starrels JL, Ning Y, Giovanniello A, Cunningham CO. Pain is not associated with worse office-based buprenorphine treatment outcomes. Subst Abus. 2012;33(4):361–365. doi: 10.1080/29767342.2011.638734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Worley MJ, Heinzerling KG, Shoptaw S, Ling W. Pain volatility and prescription opioid addiction treatment outcomes in patients with chronic pain. Exp Clin Psychopharmacol. 2015;23(6):428–435. doi: 10.1037/pha0000039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Messina BG, Worley MJ. Effects of craving on opioid use are attenuated after pain coping counseling in adults with chronic pain and prescription opioid addiction. J Consult Clin Psychol. 2019;87(10):918–926. doi: 10.1037/ccp0000399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Griffin ML, McDermott KA, McHugh RK, Fitzmaurice GM, Jamison RN, Weiss RD. Longitudinal association between pain severity and subsequent opioid use in prescription opioid dependent patients with chronic pain. Drug Alcohol Depend. 2016;163:216–221. doi: 10.1016/j.drugalcdep.2016.04.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Delorme J, Pennel L, Brousse G, et al. Prevalence and characteristics of chronic pain in buprenorphine and methadone-maintained patients. Front Psychiatry. 2021;12:641430. doi: 10.3389/fpsyt.2021.641430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jamison RN, Kauffman J, Katz NP. Characteristics of methadone maintenance patients with chronic pain. J Pain Symptom Manage. 2000;19(1):53–62. doi: 10.1016/s0885-3924(99)00144-x [DOI] [PubMed] [Google Scholar]
- 30.Dennis BB, Bawor M, Naji L, et al. Impact of chronic pain on treatment prognosis for patients with opioid use disorder: a systematic review and meta-analysis. Subst Abuse. 2015;9:59–80. doi: 10.4137/SART.S30120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mun CJ, Beitel M, Oberleitner L, et al. Pain catastrophizing and pain acceptance are associated with pain severity and interference among methadone-maintained patients. J Clin Psychol. 2019;75(12):2233–2247. doi: 10.1002/jclp.22842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vowles KE, Witkiewitz K, Levell J, Sowden G, Ashworth J. Are reductions in pain intensity and pain-related distress necessary? An analysis of within-treatment change trajectories in relation to improved functioning following interdisciplinary acceptance and commitment therapy for adults with chronic pain. J Consult Clin Psychol. 2017;85(2):87–98. doi: 10.1037/ccp0000159 [DOI] [PubMed] [Google Scholar]
- 33.Edwards KA, Vowles KE, McHugh RK, Venner KL, Witkiewitz K. Changes in pain during buprenorphine maintenance treatment among patients with opioid use disorder and chronic pain. J Consult Clin Psychol. 2022;90(4):314–325. doi: 10.1037/ccp0000692 [DOI] [PubMed] [Google Scholar]
- 34.Peck KR, Moxley-Kelly N, Badger GJ, Sigmon SC. Posttraumatic stress disorder in individuals seeking treatment for opioid use disorder in Vermont. Prev Med. 2021;152(pt 2):106817. doi: 10.1016/j.ypmed.2021.106817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dhingra L, Masson C, Perlman DC, et al. Epidemiology of pain among outpatients in methadone maintenance treatment programs. Drug Alcohol Depend. 2013;128(1 -2):161–165. doi: 10.1016/j.drugalcdep.2012.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Robinson SM, Sobell LC, Sobell MB, Leo GI. Reliability of the timeline followback for cocaine, cannabis, and cigarette use. Psychol Addict Behav. 2014;28(1):154–162. doi: 10.1037/a0030992 [DOI] [PubMed] [Google Scholar]
- 37.Kroenke K, Spitzer RL, Williams JB. The PHQ-9:validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613. doi: 10.1046/j.1525-1497.2001.016009606.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Spitzer RL, Kroenke K, Williams JBW, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166(10):1092–1097. doi: 10.1001/archinte.166.10.1092 [DOI] [PubMed] [Google Scholar]
- 39.Blevins CA, Weathers FW, Davis MT, Witte TK, Domino JL. The Posttraumatic Stress Disorder Checklist for DSM-5 (PCL-5): development and initial psychometric evaluation. J Trauma Stress. 2015;28(6):489–498. doi: 10.1002/jts.22059 [DOI] [PubMed] [Google Scholar]
- 40.Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singap. 1994;23(2):129–138. [PubMed] [Google Scholar]
- 41.Farrar JT. Cut-points for the measurement of pain: the choice depends on what you want to study. Pain. 2010;149(2):163–164. doi: 10.1016/j.pain.2010.03.013 [DOI] [PubMed] [Google Scholar]
- 42.Dworkin RH, Turk DC, Wyrwich KW, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain. 2008;9(2):105–121. doi: 10.1016/j.jpain.2007.09.005 [DOI] [PubMed] [Google Scholar]
- 43.Atkinson TM, Mendoza TR, Sit L, et al. The Brief Pain Inventory and its “pain at its worst in the last 24 hours” item: clinical trial endpoint considerations. Pain Med. 2010; 11 (3):337–346. doi: 10.1111/j.1526-4637.2009.00774.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Serlin RC, Mendoza TR, Nakamura Y, Edwards KR, Cleeland CS. When is cancer pain mild, moderate or severe? Grading pain severity by its interference with function. Pain. 1995;61(2):277–284. doi: 10.1016/0304-3959(94)00178-H [DOI] [PubMed] [Google Scholar]
- 45.Alschuler KN, Jensen MP, Ehde DM. Defining mild, moderate, and severe pain in persons with multiple sclerosis. Pain Med. 2012;13(10):1358–1365. doi: 10.1111/j.1526-4637.2012.01471.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fishbain DA, Cutler R, Rosomoff HL, Rosomoff RS. Chronic pain-associated depression: antecedent or consequence of chronic pain? A review. Clin J Pain. 1997;13(2):116–137. doi: 10.1097/00002508-199706000-00006 [DOI] [PubMed] [Google Scholar]
- 47.McIntosh AM, Hall LS, Zeng Y, et al. Genetic and environmental risk for chronic pain and the contribution of risk variants for major depressive disorder: a family-based mixed-model analysis. PLoS Med. 2016;13(8):e1002090. doi: 10.1371/journal.pmed.1002090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McWilliams LA, Cox BJ, Enns MW. Mood and anxiety disorders associated with chronic pain: an examination in a nationally representative sample. Pain. 2003;106(1-2):127–133. doi: 10.1016/s0304-3959(03)00301-4 [DOI] [PubMed] [Google Scholar]
- 49.Pitcher MH, Von Korff M, Bushnell MC, Porter L. Prevalence and profile of high-impact chronic pain in the United States. J Pain. 2019;20(2):146–160. doi: 10.1016/j.jpain.2018.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Barry DT, Beitel M, Cutter CJ, et al. Psychiatric comorbidity and order of condition onset among patients seeking treatment for chronic pain and opioid use disorder. Drug Alcohol Depend. 2021;221:108608. doi: 10.1016/j.drugalcdep.2021.108608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Higgins C, Smith BH, Matthews K. Comparison of psychiatric comorbidity in treatment-seeking, opioid-dependent patients with versus without chronic pain. Addiction. 2020;115(2):249–258. doi: 10.1111/add.14768 [DOI] [PubMed] [Google Scholar]
- 52.Siqveland J, Hussain A, Lindstrøm JC, Ruud T, Hauff E. Prevalence of posttraumatic stress disorder in persons with chronic pain: a meta-analysis. Front Psychiatry. 2017;8:164. doi: 10.3389/fpsyt.2017.00164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.El-Gabalawy R, Blaney C, Tsai J, Sumner JA, Pietrzak RH. Physical health conditions associated with full and subthreshold PTSD in U.S. military veterans: results from the National Health and Resilience in Veterans Study. J Affect Disord. 2018;227:849–853. doi: 10.1016/j.jad.2017.11.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dominick CH, Blyth FM, Nicholas MK. Unpacking the burden: understanding the relationships between chronic pain and comorbidity in the general population. Pain. 2012;153(2):293–304. doi: 10.1016/j.pain.2011.09.018 [DOI] [PubMed] [Google Scholar]
- 55.McEwen BS, Getz L. Lifetime experiences, the brain and personalized medicine: an integrative perspective. Metabolism. 2013;62(suppl. 1):S20–S26. doi: 10.1016/j.metabol.2012.08.020 [DOI] [PubMed] [Google Scholar]
- 56.McEwen BS, Gianaros PJ. Central role of the brain in stress and adaptation: links to socioeconomic status, health, and disease. Ann N Y Acad Sci. 2010;1186:190–222. doi: 10.1111/j.1749-6632.2009.05331.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Aaron RV, McGill LS, Finan PH, Wegener ST, Campbell CM, Mun CJ. Determining profiles of pain-specific and general emotion regulation skills and their relation to 12-month outcomes among people with chronic pain. J Pain. 2023;24(4):667–678. doi: 10.1016/j.jpain.2022.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Garland EL, Bryan CJ, Nakamura Y, Froeliger B, Howard MO. Deficits in autonomic indices of emotion regulation and reward processing associated with prescription opioid use and misuse. Psychopharmacology (Berl). 2017;234(4):621–629. doi: 10.1007/s00213-016-4494-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Greeson JM, Chin GR. Mindfulness and physical disease: a concise review. Curr Opin Psychol. 2019;28:204–210. doi: 10.1016/j.copsyc.2018.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Institute of Medicine (US) Committee on Advancing Pain Research, Care, and Education. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. National Academies Press (US); 2011. Accessed August 28, 2023. http://www.ncbi.nlm.nih.gov/books/NBK91497/ [PubMed] [Google Scholar]
- 61.Baer RA. Mindfulness training as a clinical intervention: a conceptual and empirical review. Clin Psychol Sci Pract. 2003;10(2):125–143. [Google Scholar]
- 62.Brown KW, Ryan RM, Creswell JD. Mindfulness: theoretical foundations and evidence for its salutary effects. Psychol Inq. 2007;18(4):211–237. [Google Scholar]
- 63.Colgan DD, Klee D, Memmott T, Proulx J, Oken B. Perceived stress mediates the relationship between mindfulness and negative affect variability: a randomized controlled trial among middle-aged to older adults. Stress Health. 2019;35(1):89–97. doi: 10.1002/smi.2845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Creswell JD, Pacilio LE, Lindsay EK, Brown KW. Brief mindfulness meditation training alters psychological and neuroendocrine responses to social evaluative stress. Psychoneuroendocrinology. 2014;44:1–12. doi: 10.1016/j.psyneuen.2014.02.007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All deidentified self-report data are available at https://www.openicpsr.org/openicpsr/workspace?goToPath=/openicpsr/205381&goToLevel=project


