ABSTRACT
Objective
To evaluate the profile of patients with diabetic kidney disease (DKD) with and without concomitant diabetic retinopathy (DR) to identify clinical predictors of the development of both complications together.
Subjects and methods
Cross-sectional study including patients with type 1 and type 2 diabetes and DKD followed at the endocrinology division of a public hospital in Southern Brazil and referred for retinography assessment. The definition of DR was the occurrence of any diabetes-related damage identified in color fundus photographs under mydriasis. Urinary albumin excretion ≥ 14 mg/L and/or glomerular filtration rate < 60 mL/min/1.73 m2 (CKD-EPI equation) were used to define DKD. Factors evaluated included the clinical differences of the participants according to the presence or absence of DR. Multiple regression models were used to identify predictors of DR presence according to the clinical characteristics evaluated.
Results
The study included 517 patients with DKD, 433 (83.7%) of whom had type 2 diabetes (median age 64.7 years [interquartile range 59-73 years] years, 59.8% women, 83.4% white) and 84 (16.3%) had type 1 diabetes (median age 46.6 years [interquartile range 33.5-54.2 years], 46.4% women, 91.7% white). Patients with type 2 diabetes and DR (versus those without DR) were more often on insulin (odds ratio [OR] 3.63, 95% confidence interval [CI] 1.89-7.00), had diabetes for longer (OR 1.04, 95% CI 1.02-1.07), and had higher systolic blood pressure (OR 1.01, 95% CI 1.00-1.02). No predictors of DR presence were identified in participants with type 1 diabetes.
Conclusion
Among patients with DKD and type 2 diabetes, insulin use, longer diabetes duration, and higher systolic blood pressure level were associated with the presence of DR.
Keywords: Diabetic retinopathy, diabetic kidney disease, diabetes mellitus, diabetic complications
INTRODUCTION
Diabetes mellitus is a metabolic disease that may lead to chronic microvascular and macrovascular complications (1). Diabetic retinopathy (DR) and diabetic kidney disease (DKD) are microvascular complications that may manifest independently or concurrently, contributing to increased morbidity, mortality, and medical costs and reduced quality of life (1-3). Both complications remain highly prevalent worldwide. Indeed, DR affects about 22% of the patients with diabetes and is one of the most common causes of preventable blindness in the economically active population, while DKD affects between 21%-83% of individuals with diabetes and is the main cause of end-stage renal disease in most developed countries (4-6).
Despite being independent entities, DKD and DR are often present in association, as they may share common pathogenetic mechanisms (7). Corroborating this premise, studies have shown that DKD markers, such as increased albuminuria and reduced glomerular filtration rate (GFR), are predictors of DR (9,10), and albuminuria remission seems to have a protective effect in DR development (11). Furthermore, baseline DR severity may constitute a prognostic factor for future DKD progression, especially in patients with type 2 diabetes (10).
Therefore, the identification of clinical predictors that could discern which patients with DKD are at greater risk of developing DR may support early and aggressive interventions to prevent this outcome. Based on these considerations, the aim of this study was to compare the characteristics of patients with DKD with and without DR to identify clinical predictors of developing both complications (DKD and DR) together.
SUBJECTS AND METHODS
Design and participants
This was a cross-sectional study in patients with DKD due to type 1 or 2 diabetes who were followed at the endocrinology division of a public hospital in Southern Brazil and were referred for retinography assessment. Clinical and demographic data were collected by interviews and extracted from medical records between 2019 and 2021. The inclusion criteria included patients of both sexes, aged ≥ 18 years, nonpregnant, and diagnosed with type 1 or type 2 diabetes according to American Diabetes Association criteria (12). Participants whose results of color fundus photographs (CFPs) were impossible to classify and those without serum creatinine and urinary albumin concentration (UAC) measurements available in medical records were excluded.
The study was reported according to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guideline (13).
Ethical aspects
The study was carried out in accordance with the Declaration of Helsinki 2004 and followed all relevant guidelines and regulations. The study protocol was approved by the Research Ethics Committee of Hospital das Clínicas de Porto Alegre (2019-0113, CAAE 07744819.9.0000.5327). All authors signed a confidentiality document for data use, and all participants signed an informed consent form.
Study procedures
Patients who attended the main institution for CFP examination from June 2019 to March 2020 were invited to participate. An interview was performed to gather clinical data (age, skin color, smoking habit, age at diagnosis of diabetes) and information about other diagnoses and medication use (antidiabetics, insulin, antihypertensives, lipid-lowering drugs). Subsequently, blood and urinary samples were collected for laboratory tests (creatinine, urea, glucose, glycated hemoglobin [HbA1c], total cholesterol, HDL cholesterol, triglycerides, and UAC) if the patient had not performed these laboratory tests in the same institutional laboratory in the previous year. Data on weight and height (for body mass index calculation), as well as blood pressure, were collected from the electronic medical records of the health appointment in which the CFP was requested. Information about the presence of cardiovascular disease was obtained from medical records. Cardiovascular disease was defined as the presence of previous cardiovascular events, including coronary heart disease, stroke, or heart failure. Hypertension was defined as systolic blood pressure (SBP) ≥ 140 mmHg and/or diastolic blood pressure (DBP) ≥ 90 mmHg and/or use of antihypertensive treatment.
To achieve adequate power for the proposed analyses, a second round of participants were enrolled. At this point, patients who had a CFP carried out between January 2021 and November 2021 were included. These patients also had their clinical information collected from the electronic medical record of the medical appointment closest to the CFP.
Levels of HbA1c were measured using the high-performance liquid chromatography method, as employed by the Diabetes Control and Complications Trial (DCCT) (14). Levels of total cholesterol, HDL cholesterol, and triglycerides were determined using a standard analytical method, and LDL cholesterol was calculated using the Friedewald formula (15). All samples for laboratory tests were collected in the same institutional laboratory, and the results were recorded by study researchers in an electronic database.
A trained technician obtained retinal images to evaluate the presence of DR. The examination was performed under mydriasis, and two images of the posterior segment of each patient’s eye were obtained (one centered on the macula and the other centered on the optic nerve). A retina specialist interpreted the results. Retinal images were obtained using a Canon CR-2 retinal camera equipped with a Canon EOS 40D digital camera (Canon Inc., Tokyo, Japan). The CFP results were classified according to the International Council of Ophthalmology Diabetic Retinopathy (ICDR), and the result of the worst eye was considered (16).
The presence of DKD was assessed with measurement of UAC and calculation of the estimated GFR using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation (17). The occurrence of DKD was defined as a GFR < 60 mL/min/1.73 m2 and/or UAC from a single urine sample ≥ 14 mg/L (18-20).
Study outcome
The primary outcome was the clinical characteristics of the participants with DKD categorized by the presence or absence of DR and type of diabetes.
Statistical analysis
Demographics and clinical data are presented as mean ± standard deviation for variables exhibiting normal distribution and median (interquartile range [IQR]), frequencies, and percentages for those demonstrating asymmetric distribution. For the analysis, patients with DKD were stratified according to the presence or absence of DR. The groups were compared using the chi-square test in the case of categorical variables and the Mann-Whitney test in the case of continuous variables. A multivariable logistic regression model was designed to assess the association between the participants’ clinical characteristics and DR presence, controlling for potential confounders. The logistic regression model incorporated the variables “duration of diabetes since diagnosis,” “SBP,” “insulin use,” “HbA1c value,” and “GFR.” The first model was designed for type 1 diabetes, and the second for type 2 diabetes. The data are presented as odds ratios (ORs) and 95% confidence intervals (CIs). P levels ≤ 0.05 were considered statistically significant.
The statistical analysis was performed using the software SPSS, Version 20.0 (IBM Corp., Armonk, NY, USA).
RESULTS
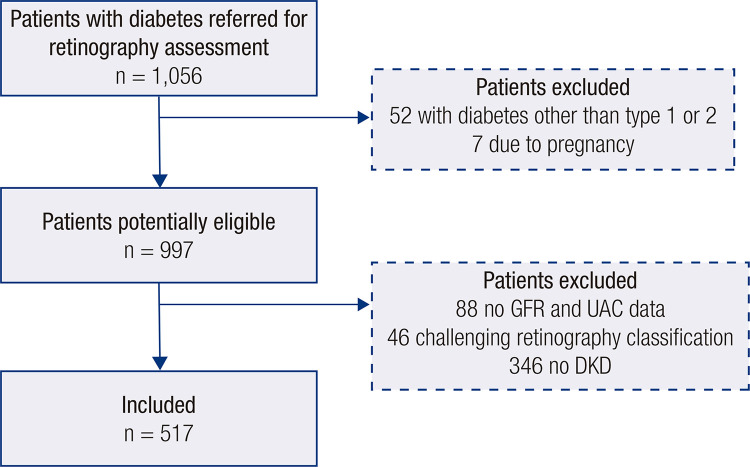
Among 997 potentially eligible participants identified (Figure 1), 517 with DKD were included. Of these, 433 (83.8%) had type 2 diabetes and 236 (45.6%) had DR. Among the patients with DR, 81 (34.3%) had mild nonproliferative DR, 84 (35.6%) had moderate nonproliferative DR, 16 (6.8%) had severe nonproliferative DR, and 55 (23.3%) had proliferative DR. Macular edema was identified in 34 (3.8%) participants. In 37 (4.1%) participants, the CFP alterations found were impossible to classify.
Figure 1. Flow diagram of the study.
Abbreviations: DKD, diabetes kidney disease; GFR, glomerular filtration rate; UAC, urinary albumin concentration (obtained from a single urine sample).
Patients with type 1 diabetes (n = 84, 16.2%) had a median age of 44.6 years (33.5-54.2 years); 46.4% were women and 91.7% were white. The median duration of diabetes since diagnosis was 29.1 years (21.0-36.5 years), and the median HbA1c level was 9.2% (8.0-10.6%) or 77.0 mmol/mol (64.0-92.0 mmol/mol). Most patients with type 1 diabetes (n = 48, 57.1%) had DR (Table 1).
Table 1. Baseline characteristics of the patients with type 1 diabetes mellitus and diabetic kidney disease.
| Without DR (n = 36) | With DR (n = 48) | P values | |
|---|---|---|---|
| Age (years) | 32.8 (21-40.7) | 43.4 (35.2-50.7) | <0.01 |
| Sex (% women) | 21 (58.3) | 18 (37.5) | 0.06 |
| Skin color (% white) | 34 (94.4) | 43 (89.6) | 0.42 |
| BMI (kg/m2) | 24.6 (22.3-26.6) | 26.5 (22.6-30.1) | 0.26 |
| Diabetes duration (years) | 16.1 (8.2-19) | 24.5 (17-33) | <0.01 |
| HbA1c (%) / (mmol/mol) | 9.8 (8.2-11) /84.0 (66.0-97.0) | 9.3 (8.2-10.2) /78.0 (66.0-88.0) | 0.28 |
| Systolic blood pressure (mmHg) | 116.5 (102-124.5) | 128.4 (110-140) | 0.03 |
| Diastolic blood pressure (mmHg) | 71 (60-80) | 86.5 (70-90) | 0.08 |
| UAC (mg/L) | 134.6 (17-51.5) | 291.9 (20-190) | 0.14 |
| GFR (mL/min/1.73 m2) | 103.8 (98.7-118.7) | 78 (47-107.7) | <0.01 |
| Total cholesterol (mg/dL) | 184.4 (150-200) | 171.4 (141.5-191.5) | 0.49 |
| HDL cholesterol (mg/dL) | 46.7 (35-54) | 48.2 (38.5-54) | 0.66 |
| Triglycerides (mg/dL) | 253.6 (95-209.5) | 136.2 (70.7-143) | 0.07 |
| Smoking | 1 (2.8) | 7 (14.6) | 0.07 |
| Cardiovascular disease | 3 (8.3) | 8 (17) | 0.24 |
| Use of ACE inhibitors | 13 (36.1) | 19 (39.6) | 0.74 |
| Use of antihypertensives | 15 (41.7) | 27 (56.2) | 0.18 |
| Use of statins | 8 (22.2) | 17 (35.4) | 0.19 |
Data are shown as median (interquartile range) or n (%). P values ≤ 0.05 indicate a significant difference. Abbreviations: ACE, angiotensin-converting enzyme; BMI, body mass index; DKD, diabetic kidney disease; DR, diabetic retinopathy; GFR, glomerular filtration rate; HbA1c, glycated hemoglobin; HDL cholesterol, high-density lipoprotein cholesterol; UAC: urinary albumin concentration (obtained from a single urine sample).
Among patients with type 2 diabetes (n = 433, 83.8%), the median age was 64.7 years (59.0-73.0 years), 59.8% were women, and 83.4% were white. The median duration of diabetes since diagnosis was 18.6 years (11-25.5 years), and the mean HbA1c level was 8.9% (6.9-10.0%) or 74.0 mmol/mol (52.0-86.0 mmol/mol). Only a minority of patients with type 2 diabetes had DR (188, 43.4%) (Table 2).
Table 2. Baseline characteristics of patients with type 2 diabetes mellitus and diabetic kidney disease.
| Without DR (n = 245) | With DR (n = 188) | P values | |
|---|---|---|---|
| Age (years) | 60.4 (53 – 69) | 61.1 (54-68) | 0.98 |
| Sex (% women) | 147 (60) | 112 (59.6) | 0.93 |
| Skin color (% white) | 203 (82.9) | 158 (84) | 0.74 |
| BMI (kg/m2) | 33.4 (28.1-37.2) | 32 (27.3-35.5) | 0.06 |
| Diabetes duration (years) | 13.8 (6-19) | 18.1 (11-23) | <0.01 |
| HbA1c (%) / (mmol/mol) | 8.6 (7.3-9.6) / 70.0 (56.0-81.0) | 9.2 (8-10.1) / 77.0 (64.0-87.0) | <0.01 |
| Systolic blood pressure (mmHg) | 132.7 (120-140) | 139.2 (121.2-150.7) | <0.01 |
| Diastolic blood pressure (mmHg) | 78.3 (70-86) | 79.8 (70-90) | 0.47 |
| Hypertension (%) | 207 (84.5) | 165 (87.8) | 0.33 |
| UAC (mg/L) | 219.3 (18-136) | 349.9 (26-280) | <0.01 |
| GFR (mL/min/1.73 m2) | 73.6 (48-97) | 67.2 (45-89) | 0.05 |
| Total cholesterol (mg/dL) | 179.9 (142-205.5) | 185.3 (152-207) | 0.29 |
| HDL cholesterol (mg/dL) | 45.4 (34-53) | 46.1 (35.7-52.2) | 0.37 |
| Triglycerides (mg/dL) | 209.9 (95.2-210.7) | 296.5 (108-216) | 0.34 |
| Smoking | 25 (10.2) | 18 (9.6) | 0.83 |
| Cardiovascular disease | 80 (32.9) | 65 (34.8) | 0.69 |
| Use of ACE inhibitors | 139 (56.7) | 118 (62.8) | 0.20 |
| Use of antihypertensives | 211 (86.1) | 165 (87.8) | 0.61 |
| Use of statins | 168 (68.6) | 133 (70.7) | 0.62 |
| Use of metformin | 198 (80.8) | 139 (73.9) | 0.09 |
| Use of sulfonylurea | 70 (28.6) | 41 (21.8) | 0.11 |
| Use of iSGLT2 | 32 (13.1) | 21 (11.2) | 0.55 |
| Use of insulin | 164 (66.9) | 173 (92.0) | <0.01 |
Data are shown as median (interquartile range) or n (%). P values ≤ 0.05 indicate a significant difference. Abbreviations: ACE, angiotensin-converting enzyme; BMI, body mass index; DKD, diabetic kidney disease; DR, diabetic retinopathy; GFR, glomerular filtration rate; HbA1c, glycated hemoglobin; HDL cholesterol, high-density lipoprotein cholesterol; iSGLT2, sodium-glucose cotransporter type 2 inhibitors; UAC: urinary albumin concentration (obtained from a single urine sample).
Table 3 shows the results of the logistic regression analysis evaluating the association between the participants’ clinical characteristics and the presence of DR in patients with type 1 diabetes. The assessed associations were not significant.
Table 3. Logistic regression analysis evaluating the association between the participants’ clinical characteristics and the presence of diabetic retinopathy in patients with type 1 diabetes.
| Variables | Presence of DR | P |
|---|---|---|
| Age | 1.00 (0.95-1.06) | 0.85 |
| Diabetes duration | 0.95 (0.89-1.02) | 0.20 |
| Systolic blood pressure | 0.99 (0.96-1.02) | 0.74 |
| GFR | 1.01 (1.00-1.03) | 0.06 |
Abbreviation: DR, diabetic retinopathy; GFR, glomerular filtration rate.
Table 4 shows the results of the logistic regression analysis evaluating the association between the participants’ clinical characteristics and the presence of DR in patients with type 2 diabetes. Patients with DR, relative to those without DR, were more frequently on insulin (OR 3.63, 95% CI 1.89-7.00), had a longer duration of diabetes since diagnosis (OR 1.04, 95% CI 1.02-1.07), and had higher SBP level (OR 1.01, 95% CI 1.00-1.02).
Table 4. Logistic regression analysis evaluating the association between the participants’ clinical characteristics and the presence of diabetic retinopathy in patients with type 2 diabetes.
| Variables | Presence of DR | P |
|---|---|---|
| Insulin use | 3.63 (1.89-7.00) | <0.01 |
| Diabetes duration | 1.04 (1.02-1.07) | <0.01 |
| Systolic blood pressure | 1.01 (1.00-1.02) | <0.01 |
| GFR | 1.00 (0.99-1.01) | 0.62 |
| HbA1c | 1.09 (0.97-1.23) | 0.14 |
| UAC | 1.00 (1.00-1.00) | 0.08 |
Abbreviations: GFR, glomerular filtration rate; HbA1c, glycated hemoglobin; UAC, urinary albumin concentration (obtained from a single urine sample).
DISCUSSION
The present study, including 517 patients with DKD, found associations between some clinical features and the presence of DR in patients with type 2 diabetes. These clinical features included insulin use, longer diabetes duration since diagnosis, and higher SBP levels. These findings are relevant as they indicate that, because both complications (DR and DKD) share common pathogenetic mechanisms (21), some patients with DKD are at increased risk of presenting DR associated with DKD. This evidence may help guide strategies to optimize DR screening in patients with DKD. In contrast, the present study found no characteristics associated with the presence of DR in patients with type 1 diabetes and DKD.
The identification of clinical features associated with a higher risk of development of DR enables the detection of patients with DKD who require closer monitoring for the potential for the development of DR. The prevalence of DR in patients with diabetes globally is around 22.3% (4), but the present study found a substantially higher prevalence (45.6%). This may have resulted from the selected patients with DKD having a possible higher risk of DR development. Corroborating this hypothesis, Pavkov et al. have demonstrated that the DKD prevalence is higher in patients with DR and reported a DKD prevalence of 23.4% in patients without DR and 36.2% in those with DR (22). Additionally, the patients included in the present study may have had diabetes of more difficult control and, consequently, more prone to the development of chronic complications. This hypothesis is in line with other characteristics of the participants included in the present study, such as the high frequency of insulin use among patients with type 2 diabetes. Moreover, the study was conducted in a tertiary care public hospital, where the most severe cases of diabetes are treated. This factor, along with the high rate of patients with type 2 diabetes using insulin and the longer duration and severity of diabetes, contribute to the high prevalence of DKD observed in the cohort.
Regarding the clinical predictors included in the analysis, the duration of diabetes is an established predictor for the development of DR and other microvascular complications in patients with type 2 diabetes (23-25). This evidence is aligned with the finding that the patients with DR had longer diabetes duration and possibly more advanced disease. Previous studies have also demonstrated that SBP levels are higher in patients with concomitant DKD and DR and contribute not only to the emergence of DR but also to its progression (26,27).
The results of the present study showed a trend towards lower GFR and higher UAC in patients with DKD and DR, which were no longer present after correction for confounders in the multivariable analysis. However, previous studies in patients with type 2 diabetes have shown that DR is an independent predictor of worsening renal function (28). In addition, studies suggest that the DR severity at baseline is associated with a faster decline in renal function and progression of albuminuria, with a risk of DKD progression 2.9 times higher in patients with nonproliferative DR and 16.6 times higher in patients with proliferative DR (29).
For type 1 diabetes, the present study found no association between clinical predictors and the presence of DR. This may have resulted from the small number of patients with this type of diabetes in the study sample. A Brazilian multicenter study including 1,760 patients with type 1 diabetes showed that a longer duration of diabetes and high levels of HbA1c are associated with the development of DR. However, the study included patients with and without DKD, which differs from the present study (30). Another analysis from this same cohort showed that female sex, longer duration of diabetes, low economic status, and high levels of HbA1c, uric acid, and SBP were associated with DKD (31).
The present study used CFPs as a diagnostic method for DR, but another, more sensitive imaging modality, optical coherence tomography (OCT), has recently been used for DR evaluation. A type of fundus examination, OCT obtains information on retinal layers using tomographic scans that can detect early changes in vascular and retinal morphology, either by measuring inner retinal thickness or choroidal thickness changes, making it an important tool for the management and monitoring of retinal diseases (32,33). The choroid may be affected by diabetes even before clinical signs of DR are present in the CFP, especially in patients with some degree of DKD, and the choriocapillaris may be preserved in the early stages (34). Microaneurysms, venous beading, vascular leakage, and nonperfused areas can be visualized by fundus fluorescein angiography, but this method is invasive, time-consuming, and not quantitative. Although OCT cannot determine blood flow, optical coherence angiography (OCTA) is a rapid, noninvasive, and high-resolution ocular fundus examination that can simultaneously perform tomographic scans and obtain blood flow information. Studies have suggested that OCTA may outperform traditional imaging techniques for the evaluation of early peripheral retinal vascular change (35).
Our study has some limitations. First, the cross-sectional design precludes the inference of causality since a temporal sequence cannot be established. Second, patients were selected from a single tertiary hospital, and the population evaluated is composed of patients with diabetes and DKD referred for retinography assessment, which limits external validity and makes it impossible to extrapolate the results to patients with diabetes in the general population. Third, GFR was estimated and not measured by the gold standard method (36), and a single urine sample was used to define DKD, which may not have ensured the exclusion of other causes of increased albuminuria, resulting in overreporting or underreporting of the true DKD prevalence in this population. In the present study, the DKD prevalence was 41.1% in patients with type 1 diabetes and 65.1% in patients with type 2 diabetes. As previous evidence suggests, differences in DKD prevalence are found all over the world and have been attributed to different methodological, social, and ethnic factors (5). In the study by Gomes et al., the prevalence of DKD in patients with type 1 diabetes was 33.7% (31). The longer diabetes duration in the patients in our cohort compared with that in the study by Gomes et al. may explain the difference in DKD prevalence between both studies. Also, the patients included in the present study were those with diabetes more difficult to control, referred for follow-up for a more complex level of care. Fourth, the definition of DKD was based on single urine and blood samples, and the definition of DKD applied, although highly accurate, was not the same as that defined by current guidelines (spot UAC > 30 mg/L or GFR < 60 mL/min/1.73 m2 for longer than 3 months) (19,18). Fifth, the present study was based on the evaluation of CFPs, while studies have shown that OCT can detect earlier changes in the vascular and retinal morphology, making it an important tool for the management and follow-up of retinal diseases, such as macular edema (32,33).
In conclusion, in summary, the present study showed that insulin use, longer diabetes duration, and higher SBP levels were predictors for the presence of DR in patients with type 2 diabetes and DKD. This finding suggests that patients with these characteristics should be monitored more closely for early identification of DR development. Studies with larger sample sizes are needed to identify DR predictors among patients with type 1 diabetes and DKD. The main limitations of the study include the fact that the definition of DKD adopted was not the same as that defined by current guidelines and the population evaluated was composed of patients with diabetes and DKD referred for CFP evaluation, thus the findings may not be applicable to patients with diabetes in the general population.
Acknowledgments
none.
Footnotes
Ethics approval and consent to participate: The study was approved by the Comissão Nacional de Ética em Pesquisa (CONEP) under the number 2019-0113.
Consent for publication: all authors have reviewed the final version of the manuscript and agree with the publication of the results presented.
Availability of data and materials: the datasets used and/or analyzed during the current study will be made available from the corresponding author upon reasonable request.
Sponsorship: The present study was conducted with support from the Fundo de Incentivo à Pesquisa e Eventos (FIPE) of Hospital de Clínicas de Porto Alegre and the Postgraduate Program in Medical Sciences: Endocrinology from the Universidade Federal do Rio Grande do Sul Medical School. The study was funded in part by the Coordination for the Improvement of Higher Education Personnel – Brazil (Capes) – Finance Code 001.
REFERENCES
- 1.ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, et al. 12. Retinopathy, Neuropathy, and Foot Care: Standards of Care in Diabetes-2023. Diabetes Care. 2023;46(Suppl 1):S203–S215. doi: 10.2337/dc23-S012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bahia LR, Araujo DV, Schaan BD, Dib SA, Negrato CA, Leão MP, et al. The costs of type 2 diabetes mellitus outpatient care in the Brazilian public health system. Value Health. 2011;14(5) Suppl 1:S137–S140. doi: 10.1016/j.jval.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 3.Ben ÂJ, de Souza CF, Locatelli F, Rosses APO, Szortika A, de Araujo AL, et al. Health-related quality of life associated with diabetic retinopathy in patients at a public primary care service in southern Brazil. Arch Endocrinol Metab. 2021;64(5):575–583. doi: 10.20945/2359-3997000000223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Teo ZL, Tham YC, Yu M, Chee ML, Rim TH, Cheung N, et al. Global Prevalence of Diabetic Retinopathy and Projection of Burden through 2045: Systematic Review and Meta-analysis. Ophthalmology. 2021;128(11):1580–1591. doi: 10.1016/j.ophtha.2021.04.027. [DOI] [PubMed] [Google Scholar]
- 5.Koye DN, Koye DN, Magliano DJ, Nelson RG, Pavkov ME. The Global Epidemiology of Diabetes and Kidney Disease. Adv Chronic Kidney Dis. 2018;25(2):121–132. doi: 10.1053/j.ackd.2017.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adler AI, Stevens RJ, Manley SE, Bilous RW, Cull CA, Holman RR, UKPDS GROUP Development and progression of nephropathy in type 2 diabetes: the United Kingdom Prospective Diabetes Study (UKPDS 64) Kidney Int. 2003;63(1):225–232. doi: 10.1046/j.1523-1755.2003.00712.x. [DOI] [PubMed] [Google Scholar]
- 7.Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res. 2010;107(9):1058–1070. doi: 10.1161/CIRCRESAHA.110.223545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hsieh YT, Tsai MJ, Tu ST, Hsieh MC. Association of Abnormal Renal Profiles and Proliferative Diabetic Retinopathy and Diabetic Macular Edema in an Asian Population with Type 2 Diabetes. JAMA Ophthalmol. 2018;136(1):68–74. doi: 10.1001/jamaophthalmol.2017.5202. published correction appears in JAMA Ophthalmol. 2019 Feb 1;137(2):233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu J, Geng J, Liu L, Teng W, Liu L, Chen L. The Relationship between Estimated Glomerular Filtration Rate and Diabetic Retinopathy. 326209J Ophthalmol. 2015;2015 doi: 10.1155/2015/326209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rodríguez-Poncelas A, Mundet-Tudurí X, Miravet-Jiménez S, Casellas A, Barrot-De la Puente JF, Franch-Nadal J, et al. Chronic Kidney Disease and Diabetic Retinopathy in Patients with Type 2 Diabetes. PLoS One. 2016;11(2):e0149448. doi: 10.1371/journal.pone.0149448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hsieh YT, Hsieh MC. Time-sequential correlations between diabetic kidney disease and diabetic retinopathy in type 2 diabetes - an 8-year prospective cohort study. Acta Ophthalmol. 2021;99(1):e1–e6. doi: 10.1111/aos.14487. [DOI] [PubMed] [Google Scholar]
- 12.ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, et al. 2. Classification and Diagnosis of Diabetes: Standards of Care in Diabetes-2023. Diabetes Care. 2023;46(Suppl 1):S19–S40. doi: 10.2337/dc23-S002. published correction appears in Diabetes Care. 2023 Feb 01. published correction appears in Diabetes Care. 2023 Sep 1;46(9):1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, STROBE Initiative The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. Int J Surg. 2014;12(12):1495–1499. doi: 10.1016/j.ijsu.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 14.Diabetes Control and Complications Trial Research Group. Nathan DM, Genuth S, et al. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 15.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502. [PubMed] [Google Scholar]
- 16.Wilkinson CP, Ferris FL, 3rd, Klein RE, et al. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology. 2003;110(9):1677–1682. doi: 10.1016/S0161-6420(03)00475-5. [DOI] [PubMed] [Google Scholar]
- 17.Stevens PE, Levin A, Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group Members Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med. 2013;158(11):825–830. doi: 10.7326/0003-4819-158-11-201306040-00007. [DOI] [PubMed] [Google Scholar]
- 18.Incerti J, Zelmanovitz T, Camargo JL, Gross JL, de Azevedo MJ. Evaluation of tests for microalbuminuria screening in patients with diabetes. Nephrol Dial Transplant. 2005;20(11):2402–2407. doi: 10.1093/ndt/gfi074. [DOI] [PubMed] [Google Scholar]
- 19.ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, et al. 11. Chronic Kidney Disease and Risk Management: Standards of Care in Diabetes-2023. Diabetes Care. 2023;46(Suppl 1):S191–S202. doi: 10.2337/dc23-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zelmanovitz T, Gross JL, Oliveira JR, Paggi A, Tatsch M, Azevedo MJ. The receiver operating characteristics curve in the evaluation of a random urine specimen as a screening test for diabetic nephropathy. Diabetes Care. 1997;20(4):516–519. doi: 10.2337/diacare.20.4.516. [DOI] [PubMed] [Google Scholar]
- 21.Romero-Aroca P, Mendez-Marin I, Baget-Bernaldiz M, Fernéndez-Ballart J, Santos-Blanco E. Review of the relationship between renal and retinal microangiopathy in diabetes mellitus patients. Curr Diabetes Rev. 2010;6(2):88–101. doi: 10.2174/157339910790909387. [DOI] [PubMed] [Google Scholar]
- 22.Pavkov ME, Harding JL, Chou CF, Saaddine JB. Prevalence of Diabetic Retinopathy and Associated Mortality Among Diabetic Adults with and without Chronic Kidney Disease. Am J Ophthalmol. 2019;198:200–208. doi: 10.1016/j.ajo.2018.10.019. [DOI] [PubMed] [Google Scholar]
- 23.Zoungas S, Woodward M, Li Q, Cooper ME, Hamet P, Harrap S, et al. Impact of age, age at diagnosis and duration of diabetes on the risk of macrovascular and microvascular complications and death in type 2 diabetes. Diabetologia. 2014;57(12):2465–2474. doi: 10.1007/s00125-014-3369-7. [DOI] [PubMed] [Google Scholar]
- 24.Liu DP, Molyneaux L, Chua E, Wang YZ, Wu CR, Jing H, et al. Retinopathy in a Chinese population with type 2 diabetes: factors affecting the presence of this complication at diagnosis of diabetes. Diabetes Res Clin Pract. 2002;56(2):125–131. doi: 10.1016/s0168-8227(01)00349-7. [DOI] [PubMed] [Google Scholar]
- 25.Voigt M, Schmidt S, Lehmann T, Köhler B, Kloos C, Voigt UA, et al. Prevalence and Progression Rate of Diabetic Retinopathy in Type 2 Diabetes Patients in Correlation with the Duration of Diabetes. Exp Clin Endocrinol Diabetes. 2018;126(9):570–576. doi: 10.1055/s-0043-120570. [DOI] [PubMed] [Google Scholar]
- 26.Stratton IM, Kohner EM, Aldington SJ, Turner RC, Holman RR, Manley SE, et al. UKPDS 50: risk factors for incidence and progression of retinopathy in Type II diabetes over 6 years from diagnosis. Diabetologia. 2001;44(2):156–163. doi: 10.1007/s001250051594. [DOI] [PubMed] [Google Scholar]
- 27.UK Prospective Diabetes Study Group Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK Prospective Diabetes Study Group [published correction appears in BMJ 1999 Jan 2;318(7175):29] BMJ. 1998;317(7160):703–713. [PMC free article] [PubMed] [Google Scholar]
- 28.Wu D, Xuan Y, Ruan Y, Feng X, Zhu Y, Jia C, et al. Prevalence of macro- and microvascular complications in patients with type 2 diabetes and kidney disease with or without albuminuria in a single Chinese Diabetes Centre. Diab Vasc Dis Res. 2016;13(1):21–30. doi: 10.1177/1479164115610247. [DOI] [PubMed] [Google Scholar]
- 29.Park HC, Lee YK, Cho A, Han CH, Noh JW, Shin YJ, et al. Diabetic retinopathy is a prognostic factor for progression of chronic kidney disease in the patients with type 2 diabetes mellitus. PLoS One. 2019;14(7):e0220506. doi: 10.1371/journal.pone.0220506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Melo LGN, Morales PH, Drummond KRG, Santos DC, Pizarro MH, Barros BSV, et al. Current epidemiology of diabetic retinopathy in patients with type 1 diabetes: a national multicenter study in Brazil. 989BMC Public Health. 2018;18(1) doi: 10.1186/s12889-018-5859-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gomes MB, Pizarro MH, Muniz LH, Barros BSV, Melo LGN, Santos DC, et al. Prevalence of chronic kidney disease in an admixed population of patients with type 1 diabetes. A multicenter study in Brazil. 108490Diabetes Res Clin Pract. 2020;170 doi: 10.1016/j.diabres.2020.108490. [DOI] [PubMed] [Google Scholar]
- 32.Vujosevic S, Muraca A, Alkabes M, Villani E, Cavarzeran F, Rossetti L, et al. Early microvascular and neural changes in patients with type 1 and type 2 diabetes mellitus without clinical signs of diabetic retinopathy. Retina. 2019;39:435–445. doi: 10.1097/IAE.0000000000001990. [DOI] [PubMed] [Google Scholar]
- 33.Lavinsky F, Lavinsky D. Novel perspectives on swept-source optical coherence tomography. 25Int J Retina Vitreous. 2016;2 doi: 10.1186/s40942-016-0050-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Da Silva MO, Chaves AECDC, Gobbato GC, Lavinsky F, Schaan BD, Lavinsky D. Early choroidal changes detected by swept-source OCT in type 2 diabetes and their association with diabetic kidney disease. BMJ Open Diabetes Res Care. 2022;10(6):e002938. doi: 10.1136/bmjdrc-2022-002938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zeng Y, Liu M, Li M, Wei D, Mao M, Liu X, et al. Early changes to retinal structure in patients with diabetic retinopathy as determined by ultrawide swept-source optical coherence tomography-angiography. 1143535Front Endocrinol (Lausanne) 2023;14 doi: 10.3389/fendo.2023.1143535. PMID: 37223042; PMCID: PMC10200911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brändström E, Grzegorczyk A, Jacobsson L, Friberg P, Lindahl A, Aurell M. GFR measurement with iohexol and 51Cr-EDTA. A comparison of the two favoured GFR markers in Europe. Nephrol Dial Transplant. 1998;13(5):1176–1182. doi: 10.1093/ndt/13.5.1176. [DOI] [PubMed] [Google Scholar]