Abstract
The role of negatively signaling NK cell receptors of the Ly49 family on the specificity of the acute CD8+ cytotoxic T-lymphocyte (CTL) response was investigated in lymphocytic choriomeningitis virus (LCMV)-infected C57BL/6 mice. Activated CD8+ T cells coexpressing Ly49G2 expanded during LCMV infection, and T-cell receptor analyses by flow cytometry and CDR3 spectratyping revealed a unique polyclonal T-cell population in the Ly49G2+ fraction. These cells lysed syngeneic targets infected with LCMV or coated with two of three LCMV immunodominant peptides examined. Transfection of these sensitive targets with H2Dd, a ligand for Ly49G2, inhibited lysis. This was reversed by antibody to Ly49G2, indicating effective negative signaling. LCMV characteristically induces an anti-H2d allospecific T-cell response that includes T-cell clones cross-reactive between allogeneic and LCMV-infected syngeneic targets. The CD8+ Ly49G2+ population mediated no allospecific killing, nor was any NK-like killing observed against YAC-1 cells. This study shows that CD8+ Ly49G2+ cells participate in the virus-induced CTL response but lyse a more restricted range of targets than the rest of the virus-induced CTL population.
Many viruses induce a strong cytotoxic T-lymphocyte (CTL) response, occurring as a consequence of the selective expansion of preexisting CD8+ cells whose T-cell receptors (TCR) can recognize virus-encoded peptides in association with major histocompatibility (MHC) class I molecules (30). These differentiated T-cell clones are endowed with cytolytic and gamma interferon-producing capacities, enabling them to manifest antiviral activity against virus-infected target cells displaying those peptides. Our understanding of this response is complicated by its degeneracy, as many virus-specific CD8+ T-cell clones cross-react with cells infected with other viruses or with uninfected cells displaying foreign MHC antigens. Indeed, high levels of allospecific CTL activity directed against discrete MHC class I alloantigens have been described in mice infected with lymphocytic choriomeningitis virus (LCMV), vaccinia virus, murine cytomegalovirus, herpes simplex virus, and Pichinde virus (7, 15, 31) and in humans with Epstein-Barr virus (2, 27, 28).
A further complication to understanding the nature of the virus-induced CTL response relates to the recent observation that, like all NK cells, some T cells express negatively regulating signaling molecules. Ly49 is a multigenic family which encodes homodimeric C-type lectin-like receptors expressed primarily on murine NK cells (reviewed in reference 23). Inhibitory Ly49 receptors have been identified on a small, predominantly CD8+ subset of murine T cells, many of which coexpress the NK cell marker, NK1.1 (10, 17). Interactions of most Ly49 receptors with their ligands, the α1/α2 peptide-binding domains of specific MHC class I alleles, result in the delivery of inhibitory signals to the cell (26). As such, targets expressing H2Dd and/or H2Ld inhibit cytotoxicity mediated by NK cells expressing the receptor Ly49G2, for which these class I molecules are ligands. This inhibition is negated by the addition of monoclonal antibodies (MAbs) that recognize Ly49G2 protein (4D11), H-2Dd, or H-2Ld (12). Similarly, interaction of the Ly49G2 receptor on interleukin-2 (IL-2)-activated CD3+ NK1.1+ T cells with H2Dd inhibited lysis and cytokine production, and this effect was negated by antibodies to Ly49G2 or H2Dd (10, 17).
The above studies were performed either with NK cells or with IL-2-activated T cells that manifest NK-like activity, but the role of these negative regulatory molecules on the repertoire and function of T cells during viral infection is not understood. LCMV infection of mice represents a useful model system for studying the activation and function of CD8+ T cells (15), and in this study we report on a distinct subset of CD8+ Ly49G2+ T cells which kill LCMV-infected syngeneic targets, but are impaired in their ability to lyse allogeneic P815 (H2d) targets.
MATERIALS AND METHODS
Infection of mice.
C57BL/6 (H2b) male mice (Jackson Laboratory, Bar Harbor, Maine) 6 to 8 weeks of age were infected intraperitoneally with 4 × 104 PFU of the Armstrong strain of LCMV as described previously (29).
Cytotoxicity assay.
Cell-mediated cytotoxicity was determined by using a 51Cr release assay as described previously (15). Selected wells contained 5 μg of anti-Ly49G2 MAb (4D11 [12]). Lytic units were calculated by the exponential fit method (20). One lytic unit was defined as the number of cells required to lyse 15% of a population of 5 × 103 target cells in a 6-h assay. Lytic unit values are expressed as mean ± standard error of the mean. The spontaneous release for each target used in these assays was <20%.
Target cells.
MC57G (H2b), a methylcholanthrene-induced fibroblast cell line from C57BL/6 mice, and P815 (H2d), a DBA/2-derived mastocytoma, were propagated as described previously (15). The B-cell line L5 MF22 (H2Db) was transfected with a neomycin resistance gene alone (L5neo3) or in conjunction with H2Dd (L5cDd104; provided by I. Nakamura, Buffalo, N.Y.) and was maintained in complete RPMI 1640 further supplemented with 0.2 mg of geneticin (G418; Sigma Chemicals, St. Louis, Mo.) per ml. To prepare target cells, MC57G cells were infected with LCMV at a multiplicity of infection of 0.1 and incubated for 2 days at 37°C. L5neo3 and L5cDd104 cells were pulsed overnight with 0.01, 0.1, or 1 μM H2Db-restricted, LCMV immunodominant peptide (6) GP33-41 (glycoprotein amino acids 33 to 41 [KAVYNFATC]), GP276-286 (SGVENPGGYCL), or NP396-404 (nucleoprotein amino acids 396-404 [FQPQNGQFI]) as described previously (25).
Effector cells and flow cytometry.
To isolate CD8+ Ly49G2+ and CD8+ Ly49G2− populations, spleen cells were stained with 0.5 μl of anti-CD8-phycoerythrin (PE) and 0.4 μl of anti-Ly49G2-fluorescein isothiocyanate (FITC) MAb per 107 cells/100 μl (Pharmingen, San Diego, Calif.). Nonspecific binding was blocked by preincubation with 1 μl of unlabeled MAb 2.4G2 (anti-FcγIII/II receptor; 0.5 mg/ml; Pharmingen) and 0.5 μl of normal mouse serum per 107 cells/100 μl for 10 min. After 30 min on ice, cells were washed with RPMI 1640 and then sorted (FACSTAR; Becton Dickinson & Co., San Jose, Calif.). Freshly isolated CD8+ Ly49G2+ and CD8+ Ly49G2− cells (104/well) were expanded for 4 days in culture with irradiated LCMV-infected peritoneal exudate cells (PECs; 4 × 104/well) and splenic leukocyte feeders (1.25 × 105/well [9, 25]). Cells were cultured in AIM-V medium (Gibco BRL, Grand Island, N.Y.) further supplemented with 14% fetal calf serum, 2 mM l-glutamine, 7.2 × 10−5 M 2-mercaptoethanol, and 17% culture supernatant from the IL-2-secreting gibbon lymphoma cell line MLA.144 (American Type Culture Collection, Manassas, Va.) (21). For staining, 106 freshly isolated spleen cells were pretreated with 1 μl of unlabeled MAb 2.4G2 and 10 μl of normal mouse serum for 10 min before being triple stained with combinations of MAb conjugates anti-CD8-allophycocyanin (APC), anti-Ly49G2-FITC, anti-Ly49A-FITC, anti-NK1.1-PE, anti-CD44-PE (Pharmingen), in a 100-μl volume. Cells were then fixed with 1% paraformaldehyde (J. T. Baker, Inc., Phillipsburg, N.J.), and analyzed using CELLQuest software (FacsCalibur; Becton Dickinson).
CDR3 length spectratyping.
Total RNA from sorted CD8+ Ly49G2+ and CD8+ Ly49G2− populations was purified using Trizol total RNA isolation reagent (GibcoBRL, Grand Island, N.Y.) as instructed by the manufacturer. Purified RNA was converted to cDNA by reverse transcription-PCR (RT-PCR) using Vβ8.1 and Cβ primers, and CDR3 length spectratyping was performed as described previously (9, 18).
RESULTS
A subset of CD8+ T cells coexpressing Ly49G2 is expanded following LCMV infection.
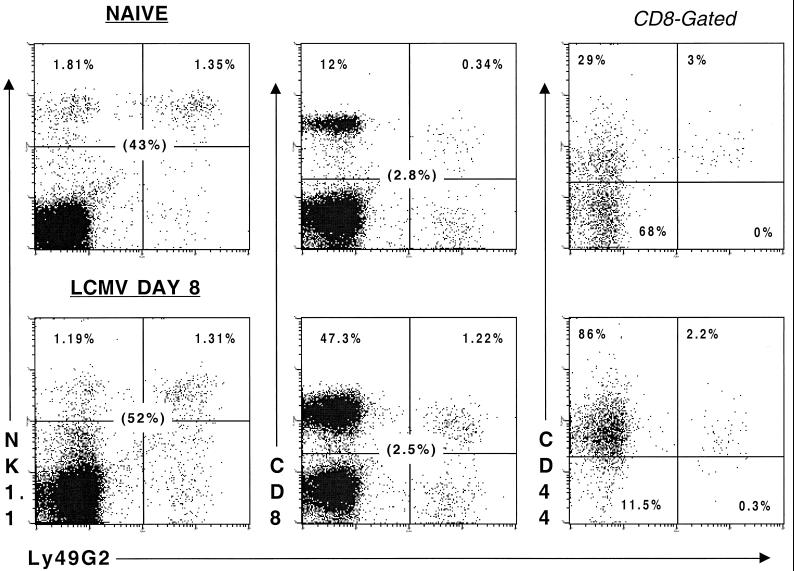
The anti-Ly49G2 MAb (4D11) identifies a substantial subset of NK cells in the spleens from naive C57BL/6J mice (Jackson Laboratory), and a smaller but significant subpopulation of CD8+ T cells (Fig. 1). Minor subpopulations of CD8+ cells bearing Ly49A and Ly49C/I, but not Ly49D, were also detected (data not shown), as described previously (17). Interestingly, the overwhelming majority of these naive CD8+ Ly49G2+ cells coexpressed the activation marker CD44, consistent with a memory phenotype and having been activated in vivo. The intensity of CD8 staining on Ly49G2+ cells was about 2.5-fold lower than on Ly49G2− cells (mean fluorescence indices of 84 and 212, respectively), further suggesting a state of enhanced activation. Ly49G2 was still coexpressed by a small but significant subset (2.5%) of a now highly expanded CD8+ T-cell population on day 8 of an intraperitoneal infection with 4 × 104 PFU of the Armstrong strain of LCMV (Fig. 1) (29). This represented an approximately fivefold increase in the number of CD8+ Ly49G2+ cells per spleen from days 0 to 8 (3.9 × 105 to 1.9 × 106 cells). Both Ly49G2+ and Ly49G2− subsets exhibited a low intensity of CD8 staining on day 8 (mean fluorescence indices of 71 and 131, respectively) and high proportions of CD44.
FIG. 1.
Ly49G2 is expressed on an activated CD8+ T-cell population, which expands in response to LCMV infection. C57BL/6 mice (6 to 8 weeks old) were inoculated intraperitoneally with 4 × 104 PFU of LCMV. Splenocytes were harvested from naive or day 8 LCMV-infected mice and examined for Ly49G2 expression on NK cells and CD8 cells (left-hand and central panels, respectively). The percentage of lymphocytes present in the upper two quadrants of each panel is indicated, and the percentage that Ly49G2+ cells represent of the NK1.1+ and CD8+ populations is shown in parentheses. CD8+ cells were gated, and the percentage of the Ly49G2− and Ly49G2+ fractions coexpressing CD44 is shown (right-hand panels).
The LCMV-induced CD8+ Ly49G2+ population displays distinct TCR usage.
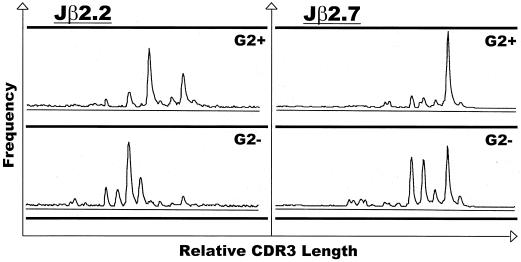
CD8+ T cells expressing Ly49G2 might have represented a small subset of the entire CD8+ population, perhaps expressing this receptor at a specific stage of development, or alternatively might have denoted a distinct subpopulation characterized by a distinctive TCR repertoire and antigen specificity. The day 8 CD8+ Ly49G2+ population was heterogeneous in that it, like the CD8+ Ly49G2− population, costained with several Vβ-specific antibodies: Vβ6 (G2+, 10%; G2−, 12%), Vβ8 (G2+, 17%; G2−, 15%), and Vβ11 (G2+, 10%; G2−, 9%). For a more detailed examination of TCR usage, we performed CDR3 length spectratyping on sorted CD8+ Ly49G2+ cells from the spleens of day 8 LCMV-infected mice. An RT-PCR with specific Vβ and Cβ primers competitively amplifies TCR with specific Vβ sequences but different CDR3 regions (18). The Vβ8.1 TCR was chosen because LCMV-specific lysis is highly represented within this population (15). Whereas the Vβ8.1 spectratype of T cells from naive mice has a characteristic Gaussian distribution, T cells from LCMV-infected mice have a skewed appearance, indicative of expanding clones of T cells (9). Extension of PCR-amplified DNA using Jβ2.2 and Jβ2.7 primers showed that the CDR3 lengths used by CD8+ clones in the Ly49G2+ fraction differed from those in the Ly49G2− subset, suggesting a distinct subset within the larger CD8+ population (Fig. 2). This conclusion was supported by our observation that Ly49G2-depleted CD8+ cells adoptively transferred into αβ TCR knockout mice failed to upregulate expression of this marker in response to LCMV infection (data not shown).
FIG. 2.
CDR3 spectratyping of LCMV-induced CD8+ Vβ8.1+ cells reveals a unique TCR usage by the Ly49G2+ subset. CD8+ Ly49G2+ (G2+) and CD8+ Ly49G2− (G2−) cells obtained from the spleens of day 8 LCMV-infected C57BL/6 mice were examined for CDR3 size patterns. RT-PCR products from these populations were subjected to runoff reactions using fluorescently labeled Jβ2.2 or Jβ2.7 primers.
The cytotoxic activity of LCMV-induced CD8+ Ly49G2+ cells ex vivo.
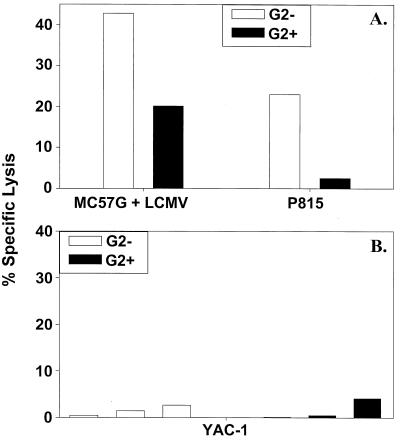
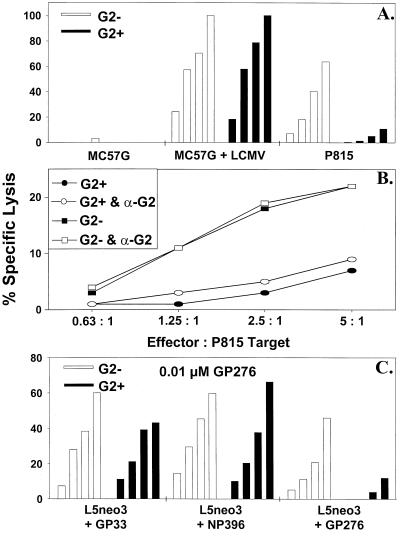
Freshly sorted CD8+ Ly49G2− spleen cells, when tested directly in a 51Cr release cytotoxicity assay, lysed both LCMV-infected syngeneic MC57G and uninfected allogeneic P815 targets, as described previously (15). The CD8+ Ly49G2+ population, however, demonstrated poor killing against P815 targets but lysed LCMV-infected syngeneic MC57G cells (Fig. 3A). Neither Ly49G2+ nor Ly49G2− CD8+ cells mediated any substantial killing against the prototypic NK cell target, YAC-1 (Fig. 3B), which we routinely use as a very sensitive target in NK cell assays (31).
FIG. 3.
CD8+ Ly49G2+ cells lyse syngeneic LCMV-infected but not H2d allogeneic P815 or YAC-1 targets. Fresh CD8+ Ly49G2− (G2−) and CD8+ Ly49G2+ (G2+) cells, sorted by flow cytometry, were used in CTL assays against LCMV-infected syngeneic (H2b) MC57G and allogeneic (H2d) P815 cells (effector-to-target ratio = 4:1) (A), and the prototypic NK cell target cell line, YAC-1 (effector-to-target ratios = 1.25:1, 2.5:1, and 5:1) (B).
Analysis of cultured LCMV-induced CD8+ Ly49G2+ cells.
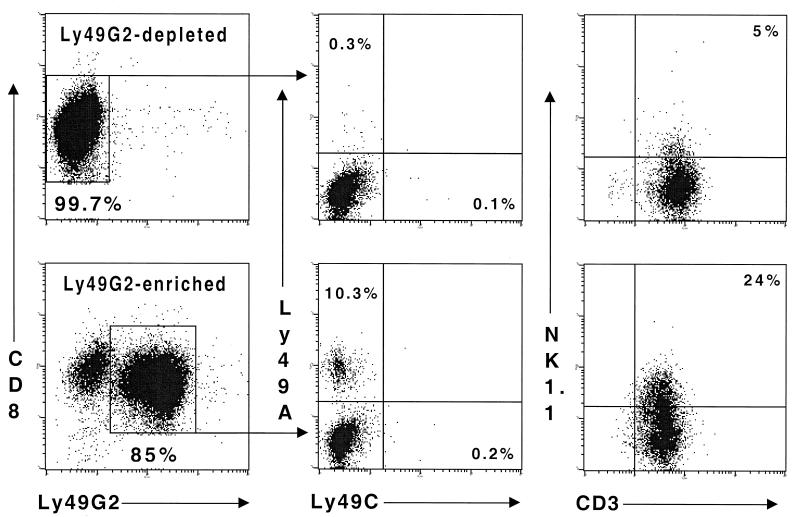
To address the limitation of low cell numbers (∼1 × 105 to 2 × 105) yielded by flow cytometric sorting of cells freshly isolated ex vivo, we propagated Ly49G2-enriched and -depleted fractions of CD8+ cells in culture for 4 days, using irradiated LCMV-infected PECs as stimulators. The two resultant populations maintained the integrity of their expression of the Ly49G2 receptor from that observed immediately postsort (Fig. 4). Hence, Ly49G2 appears to be stably expressed on a CD8+ T-cell subset and is not turned on and off on subpopulations of CD8+ cells. A significant percentage of CD8+ cells expressing Ly49G2 were positive for another Ly49 family member, Ly49A, which also inhibits NK cell activity upon interaction with H2Dd (4), but none were positive for Ly49C, which can recognize autologous MHC (1). By comparison, both Ly49A and Ly49C are coexpressed on subsets of NK1.1+ Ly49G2+ cells in naive C57BL/6 mice (24). Expression of Ly49G2 by CD8+ T cells was not restricted to the NK1.1+ T-cell subset (10, 17), but it is notable that a significant proportion of the CD8+ Ly49G2+ and not the CD8+ Ly49G2− population displayed this NK cell marker (Fig. 4).
FIG. 4.
CD8+ Ly49G2+ cells maintain expression of Ly49G2 in culture. Populations of CD8+ Ly49G2− and CD8+ Ly49G2+ cells were sorted from the spleens of day 8 LCMV-infected C57BL/6J mice by flow cytometry (final purity = 99 and 84%, respectively) and expanded in culture for 4 days by stimulation against LCMV-infected PECs. Ly49G2 and CD8 expression was reexamined at this time (left-hand panels). Ly49G2-depleted and Ly49G2-enriched CD8+ populations were also investigated for coexpression of the inhibitory Ly49 family members (central panels), Ly49A and Ly49C. The relationship between NK1.1 and Ly49G2 expression by CD8+ cells was also assessed (right-hand panels). The percentage of lymphocytes in each quadrant is indicated.
This distinctive phenotype of cultured CD8+ Ly49G2+ cells from day 8 LCMV-infected C57BL/6J mice was associated with a characteristic cytotoxic profile, reflecting that of the freshly isolated cells. Whereas both CD8+ Ly49G2+ and CD8+ Ly49G2− cells lysed syngeneic MC57G cells infected with LCMV, only the Ly49G2− cells lysed allogeneic, H2d+ P815 cells, typical of LCMV-induced CTL (Fig. 5A) (15). CD8+ Ly49G2+ cells exhibited negligible lysis against uninfected syngeneic (H2b) MC57G (Fig. 5A) or L5neo3 (data not shown) targets, in contrast to the considerable lysis mediated against the latter target by IL-2-treated, CD3+ Ly49G2+ cells examined in a previous study (17). As with freshly isolated CD8+ Ly49G2+ cells (Fig. 3B), lysis mediated by cultured CD8+ Ly49G2+ cells against YAC-1 targets was less than 3% at an effector/target ratio of 10:1, again in contrast to IL-2-activated CD3+ Ly49G2+ cells (17). Hence, the activity of our CD8+ Ly49G2+ fraction appears to be more typical of T cells than of NK cells.
FIG. 5.
Low LCMV-induced Ly49G2+ CTL-mediated lysis of allogeneic (H2d) P815 and GP276-pulsed syngeneic (H2b) targets, and failure to rescue allospecific killing with anti-Ly49G2 MAb. Cultured CD8+ Ly49G2− (G2−) and CD8+ Ly49G2+ (G2+) cells were used in CTL assays against a variety of targets: LCMV-infected syngeneic (H2b) MC57G and allogeneic (H2d) P815 cells (effector-to-target ratios = 2.5:1, 5:1, 10:1, and 20:1) (A); P815 cells in the presence and absence of anti-Ly49G2 MAb (α-G2; 5 μg/well) (B); and syngeneic (H2Db) L5neo3 cells pulsed with 1 μM LCMV immunodominant peptide GP33, NP396, or GP276 (effector-to-target ratios = 0.31:1, 0.63:1, 1.25:1, and 2.5:1) (C).
The absence of allospecific CTL activity against H2d in the CD8+ Ly49G2+ fraction could have been due to a lack of Ly49G2+ precursor cells with TCR that can recognize H2d or to the inhibited expansion of a potentially alloreactive CTL population due to the presence of Ly49G2 on the CD8+ cell surface. Alternatively, the cytotoxic activity of LCMV-induced allospecific CTL may have been inhibited by the interaction of Ly49G2 with its H2d ligand on the target cell. Killing against P815 could not be restored with a blocking MAb against Ly49G2 MAb (Fig. 5B), a result which favors the hypothesis that allospecific CTL are excluded from the Ly49G2+ fraction.
Specificity of the LCMV-specific response mediated by cultured CD8+ Ly49G2+ cells.
The bulk of the CTL response to LCMV in H2b mice is directed against three structural epitopes, GP33, GP276, and NP396, which are presented by MHC class I H2Db molecules (6, 25). Studies were done to examine the viral peptide specificity of the two CD8+ cell populations. L5neo3 cells were pulsed overnight with GP33, GP276, or NP396. The CD8+ Ly49G2+ and Ly49G2− subsets both demonstrated effective killing against L5neo3 (H2Db) targets pulsed with 1 μM GP33 or NP396. However, killing against GP276-pulsed targets by CD8+ Ly49G2+ cells was substantially reduced (Fig. 5C). Similar results were obtained with 100-fold-lower (0.01 μM) concentrations of peptide (data not shown). In replicate experiments using 0.01 μM GP276-pulsed L5neo3 targets, the average lytic activity mediated by the CD8+ Ly49G2+ subset (110 ± 17) was approximately 26% of that by the CD8+ Ly49G2− population (420 ± 95). It is not known whether there is a relationship between this low level of killing by CD8+ Ly49G2+ cells against GP276-pulsed syngeneic targets and that against allogeneic P815 (H2d) targets. This could occur if a GP276-induced conformational change in H2b resembled a ligand for Ly49G2, but that may be unlikely in light of previous studies which found that the nature of the peptide bound to H2Dd did not influence recognition by Ly49A expressed on NK cells (3, 16). Nevertheless, some killer cell immunoglobulin-like receptors (KIR) display a degree of peptide selectivity in their recognition of HLA-B or HLA-C ligands (11, 22).
Negative signaling in CD8+ cells expressing the Ly49G2 receptor.
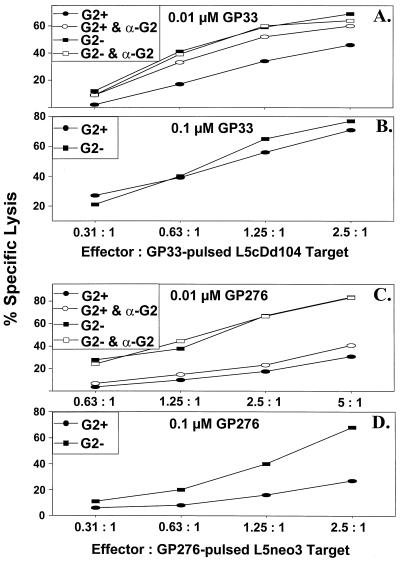
We questioned whether Ly49G2 could deliver a negative signal to LCMV-specific CTL and whether coexpression of H2Dd, a recognized Ly49G2 receptor ligand, influenced killing of H2Db targets (L5cDd104) bearing LCMV immunodominant peptides. CD8+ Ly49G2+ cells, in contrast to the CD8+ Ly49G2− subset, exhibited only modest levels of killing against Dd-expressing, GP33-pulsed (0.01 μM) L5cDd104 targets. This modest killing was substantially enhanced in the presence of MAb specific for Ly49G2 (Fig. 6A). In three replicate experiments, CD8+ Ly49G2+ cell average lytic unit values (214 ± 59) approximately doubled as a result of anti-Ly49G2 MAb treatment (429 ± 84). By contrast, the cytolytic activity of CD8+ Ly49G2− cells against L5cDd104 targets pulsed with 0.01 μM GP33 was unaffected by the addition of anti-Ly49G2 MAb (Fig. 6A). Reduced lysis of L5cDd104 targets pulsed with 0.01 μM NP396 was also enhanced by anti-Ly49G2 MAb treatment of CD8+ Ly49G2+ cells in a preliminary experiment (data not shown). Interestingly, when the H2Dd-expressing H2b targets were pulsed at the higher GP33 concentration of 0.1 μM, the level of killing mediated by CD8+ Ly49G2+ cells was similar to that of CD8+ Ly49G2− cells (Fig. 6B). This result supports the existence of a balance between negative (H2Dd) and positive (H2Db plus peptide) signals received by the CD8+ T cell, where there has been a shift toward the latter as a result of an increased concentration of GP33. In this regard, we might consider the possibility of a role for Ly49 receptors in establishing an appropriate threshold that activating signals delivered via the TCR must cross.
FIG. 6.
Anti-Ly49G2 MAb enhances CD8+ Ly49G2+ cell-mediated lysis of GP33-pulsed H2Db targets coexpressing H2Dd but does not restore killing against GP276-pulsed syngeneic targets. Cultured CD8+ Ly49G2− (G2−) and CD8+ Ly49G2+ (G2+) cells were assessed for lytic activity against H2Dd-coexpressing syngeneic (H2b) targets (L5cDd104) pulsed with 0.01 (A) and 0.1 (B) μM GP33, or syngeneic (H2b) L5neo3 targets pulsed with 0.01 μM GP276 (C), in the presence or absence of anti-Ly49G2 MAb (α-G2; 5 μg/well).
The low level of killing mediated by CD8+ Ly49G2+ cells against GP276-pulsed syngeneic targets might have been due to negative signaling through the Ly49G2 receptor. However, anti-Ly49G2 MAb did not significantly enhance killing against 0.01 μM GP276-pulsed L5neo3 (H2Db) cells (Fig. 6C). This suggests that the low levels of killing exhibited by CD8+ Ly49G2+ cells against this peptide are due to a failure to expand CTL clones capable of mediating this effect, rather than inhibition of a preexisting GP276-specific CTL population. Moreover, a 10-fold-higher concentration of GP276 peptide did not result in any significant enhancement of killing against syngeneic (H2b) L5neo3 targets presenting this peptide, further supporting this hypothesis (Fig. 6D).
DISCUSSION
We have described a distinct subset of CD8+ cells bearing an activated/memory phenotype, a skewed TCR repertoire, and the inhibitory MHC class I receptor, Ly49G2. Following LCMV infection, these cells display a characteristic pattern of target cell lysis, distinguished by poor killing of allogeneic H2d targets and of syngeneic targets presenting the GP276 immunodominant peptide. Ly49G2 negatively regulated the killing of H2Dd-expressing syngeneic (H2b) cells pulsed with GP33 or NP396 peptides, but negative signaling through this receptor could not be shown to account for the absence of allospecific or GP276-specific killing in the CD8+ Ly49G2+ fraction. In support of our observations is a recent report (32) which describes reduced numbers of GP276 peptide-loaded MHC class I tetramer+ and GP276-specific gamma interferon-producing CD8+ cells in day 8, LCMV-infected C57BL/6 mice, transgenic for the Ly49A receptor (for which H2d is also a ligand), relative to nontransgenic controls. Consistent with our Ly49G2 data for normal mice, the numbers of CD8+ cells specific for GP33 and NP396 in Ly49A-transgenic and nontransgenic mice were similar. Here we provide the first report demonstrating negative signaling through an Ly49 receptor on a population of virus-specific T cells from normal, wild-type mice. In humans, members of another family of MHC class I inhibitory receptors, KIR, have been found in a small but significant population of mainly CD8+ T cells, coexpressing surface markers consistent with a memory phenotype (13, 14). At an effector level, cytotoxicity mediated by superantigen-stimulated human T cells that express the KIR NKB1 was diminished by ligation to its HLA Bw4 ligand (19). High proportions of KIR+ T cells have been detected in human immunodeficiency virus (HIV) patients; these include HIV-specific CTLs, to which MAb against KIR restored cytolytic activity and cytokine production (5, 8). Collectively, these results indicate that NK cell receptors may regulate virus-induced T-cell responses.
Further studies are required to elucidate the significance of inhibitory MHC class I receptors on the T-cell response to viral infection, and in this regard mice transgenic for Ly49 family members will be beneficial. One appealing possibility is that the presence of Ly49 receptors on the surface of CD8+ T cells, with the ability to interact with antigen-modified self-MHC class I molecules, may modulate the virus-induced TCR repertoire. Here we have provided evidence for the exclusion of elements of the CD8+ T-cell repertoire in a subpopulation coexpressing the negative-signaling NK cell receptor molecule, Ly49G2.
ACKNOWLEDGMENTS
This research was supported by NIH grant CA34461.
We thank Carey O'Donnell and Keith Daniels for technical assistance during these studies.
REFERENCES
- 1.Brennan J, Mager D, Jeffries W, Takei F. Expression of different members of the Ly-49 gene family define distinct natural killer cell subsets and cell adhesion properties. J Exp Med. 1994;180:2287–2295. doi: 10.1084/jem.180.6.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burrows S R, Khanna R, Burrows J M, Moss D J. An alloresponse in humans is dominated by cytotoxic T lymphocytes (CTL) cross-reactive with a single Epstein-Barr Virus CTL epitope: implications for graft-versus-host disease. J Exp Med. 1994;179:1155–1161. doi: 10.1084/jem.179.4.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Correa I, Raulet D H. Binding of diverse peptides to MHC class I molecules inhibits target cell lysis by activated natural killer cells. Immunity. 1995;2:61–71. doi: 10.1016/1074-7613(95)90079-9. [DOI] [PubMed] [Google Scholar]
- 4.Daniels B F, Karlhofer F M, Seaman W E, Yokoyama W M. A natural killer cell receptor specific for a major histocompatibility complex class I molecule. J Exp Med. 1994;180:687–692. doi: 10.1084/jem.180.2.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Maria A, Ferraris A, Guastella M, Pilia S, Cantoni C, Polero L, Mingari M C, Bassetti D, Fauci A S, Moretta L. Expression of HLA class I-specific inhibitory natural killer cell receptors in HIV-specific cytolytic T lymphocytes: impairment of specific cytolytic functions. Proc Natl Acad Sci USA. 1994;94:10285–10288. doi: 10.1073/pnas.94.19.10285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hudrisier D, Mazarguil H, Laval F, Oldstone M B A, Gairin J E. Binding of viral antigens to major histocompatibility complex class I H2Db molecules is controlled by dominant negative elements at peptide non-anchor residues. Implications for peptide selection and presentation. J Biol Chem. 1996;271:17829–17836. doi: 10.1074/jbc.271.30.17829. [DOI] [PubMed] [Google Scholar]
- 7.Jennings S R. Cross-reactive recognition of mouse cells expressing the bm3 and bm11 mutations within H2Kb by H2Kb-restricted herpes simplex virus-specific cytotoxic T lymphocytes. J Immunol. 1985;135:3530–3536. [PubMed] [Google Scholar]
- 8.Kagi D, Hengartner H. Different roles for cytotoxic T cells in the control of infections with cytopathic versus noncytopathic viruses. Curr Opin Immunol. 1996;8:472–477. doi: 10.1016/s0952-7915(96)80033-1. [DOI] [PubMed] [Google Scholar]
- 9.Lin M Y, Welsh R M. Stability and diversity of T cell receptor repertoire usage during lymphocytic choriomeningitis virus infection of mice. J Exp Med. 1998;188:1993–2005. doi: 10.1084/jem.188.11.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.MacDonald H R, Lees R K, Held W. Developmentally regulated extinction of Ly49 receptor expression permits maturation an selection of NK1.1+ T cells. J Exp Med. 1998;187:2109–2114. doi: 10.1084/jem.187.12.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Malnati M S, Peruzzi M, Parker K C, Biddison W E, Ciccone E, Moretta A, Long E O. Peptide specificity in the recognition of MHC class I by natural killer cell clones. Science. 1995;267:1016–1018. doi: 10.1126/science.7863326. [DOI] [PubMed] [Google Scholar]
- 12.Mason L H, Ortaldo J R, Young H A, Kumar V, Bennett M, Anderson S K. Cloning and functional characteristics of murine large granular lymphocyte-1: a member of the Ly-49 gene family (Ly-49G2) J Exp Med. 1995;182:293–303. doi: 10.1084/jem.182.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mingari M C, Vitale C, Cambiaggi A, Schiavetti F, Melioli G, Ferrini S, Poggi A. Cytolytic T lymphocytes displaying natural killer (NK)-like activity: expression of NK-related functional receptors for HLA class I molecules (p58 and CD94) and inhibitory effect on the TCR-mediated target cell lysis or lymphokine production. Int Immunol. 1995;7:697–703. doi: 10.1093/intimm/7.4.697. [DOI] [PubMed] [Google Scholar]
- 14.Mingari M C, Vitale C, Cambiaggi A, Schiavetti F, Melioli G, Ferrini S, Poggi A. Effect of superantigens on human thymocytes: selective proliferation of V beta 2+ cells in response to toxic shock syndrome toxin-1 and their deletion upon secondary stimulation. Int Immunol. 1996;8:203–209. doi: 10.1093/intimm/8.2.203. [DOI] [PubMed] [Google Scholar]
- 15.Nahill S R, Welsh R M. High frequency of cross-reactive cytotoxic T lymphocytes elicited during the virus-induced polyclonal cytotoxic T lymphocyte response. J Exp Med. 1992;177:317–327. doi: 10.1084/jem.177.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Orihuela M, Margulies D H, Yokoyama W M. The natural killer cell receptor Ly-49A recognizes a peptide induced conformational determinant on its major histocompatibility complex class I ligand. Proc Natl Acad Sci USA. 1996;93:11792–11797. doi: 10.1073/pnas.93.21.11792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ortaldo J R, Winkler-Pickett R, Mason A T, Mason L H. The Ly49 family: regulation of cytotoxicity and cytokine production in murine CD3+ cells. J Immunol. 1998;160:1158–1165. [PubMed] [Google Scholar]
- 18.Pannetier C, Evan J, Kourilsky P. T cell receptor repertoire diversity and clonal expansions in normal and clinical samples. Immunol Today. 1995;16:176–181. doi: 10.1016/0167-5699(95)80117-0. [DOI] [PubMed] [Google Scholar]
- 19.Phillips J H, Gumperz J E, Parham P, Lanier L L. Superantigen-dependent, cell-mediated cytotoxicity inhibited by class I receptors on T lymphocytes. Science. 1995;268:403–405. doi: 10.1126/science.7716542. [DOI] [PubMed] [Google Scholar]
- 20.Pross H F, Baines M G, Rubin P, Shragge P, Paterson M S. Spontaneous human lymphocyte-mediated cytotoxicity against tumor target cells. J Clin Immunol. 1981;1:51–63. doi: 10.1007/BF00915477. [DOI] [PubMed] [Google Scholar]
- 21.Rabin H, Hopkins III R F, Ruscetti F W, Neubauer R H, Brown R L, Kawakami T G. Spontaneous release of a factor with properties of T cell growth factor from a continuous line of primate tumor T cells. J Immunol. 1981;127:1852–1856. [PubMed] [Google Scholar]
- 22.Rajagopalan S, Long E O. The direct binding of a p58 killer cell inhibitory receptor to human histocompatibility leukocyte antigen (HLA)-Cw4 exhibits peptide selectivity. J Exp Med. 1997;185:1523–1528. doi: 10.1084/jem.185.8.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rolstad B, Seaman W E. Natural killer cells and recognition of MHC class I molecules: new perspectives and challenges in immunology. Scand J Immunol. 1998;47:412–425. doi: 10.1046/j.1365-3083.1998.00358.x. [DOI] [PubMed] [Google Scholar]
- 24.Salcedo M, Diehl A D, Olsson-Alheim M Y, Sundback J, Van Kaer L, Karre K, Ljunggren H-G. Altered expression of Ly49 inhibitory receptors on natural killer cells from MHC class I-deficient mice. J Immunol. 1997;158:3174–3180. [PubMed] [Google Scholar]
- 25.Selin L K, Welsh R M. Cytolytically active memory CTL present in lymphocytic choriomeningitis virus-immune mice after clearance of virus infection. J Immunol. 1997;158:5366–5373. [PubMed] [Google Scholar]
- 26.Sentman C L, Olsson M Y, Salcedo M, Hoglund P, Lendahl U, Karre K. H2 allele-specific protection from NK cell lysis in vitro for lymphoblasts but not tumor targets. Protection mediated by alpha1/alpha2 domains. J Immunol. 1994;153:5482–5490. [PubMed] [Google Scholar]
- 27.Strang G, Rickinson B. Multiple HLA class I-dependent cytotoxicities constitute the “non-HLA-restricted” response in infectious mononucleosis. Eur J Immunol. 1987;17:1007–1013. doi: 10.1002/eji.1830170717. [DOI] [PubMed] [Google Scholar]
- 28.Tomkinson B E, Maziarz R, Sullivan J L. Characterization of the T cell-mediated cellular cytotoxicity during infectious mononucleosis. J Immunol. 1989;143:660–670. [PubMed] [Google Scholar]
- 29.Welsh R M. Cytotoxic cells induced during lymphocytic choriomeningitis virus infection of mice. I. Characterization of natural killer cell induction. J Exp Med. 1978;148:163–181. doi: 10.1084/jem.148.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Welsh R M, Lin M Y, Lohman B L, Varga S M, Zarozinski C C, Selin L K. Alpha beta and gamma delta T-cell networks and their roles in natural resistance to viral infections. Immunol Rev. 1997;159:79–93. doi: 10.1111/j.1600-065x.1997.tb01008.x. [DOI] [PubMed] [Google Scholar]
- 31.Yang H, Dundon P L, Nahill S R, Welsh R M. Virus-induced polyclonal cytotoxic T lymphocyte stimulation. J Immunol. 1989;136:1186–1193. [PubMed] [Google Scholar]
- 32.Zajac A J, Vance R E, Held W, Sourdive D J D, Altman J D, Raulet D H, Ahmed R. Impaired anti-viral T cell responses due to expression of the Ly49A inhibitory receptor. J Immunol. 1999;163:5526–5534. [PubMed] [Google Scholar]