Abstract
Plants adapt to fluctuating environmental conditions by adjusting their metabolism and gene expression to maintain fitness1. In legumes, nitrogen homeostasis is maintained by balancing nitrogen acquired from soil resources with nitrogen fixation by symbiotic bacteria in root nodules2–8. Here we show that zinc, an essential plant micronutrient, acts as an intracellular second messenger that connects environmental changes to transcription factor control of metabolic activity in root nodules. We identify a transcriptional regulator, FIXATION UNDER NITRATE (FUN), which acts as a sensor, with zinc controlling the transition between an inactive filamentous megastructure and an active transcriptional regulator. Lower zinc concentrations in the nodule, which we show occur in response to higher levels of soil nitrate, dissociates the filament and activates FUN. FUN then directly targets multiple pathways to initiate breakdown of the nodule. The zinc-dependent filamentation mechanism thus establishes a concentration readout to adapt nodule function to the environmental nitrogen conditions. In a wider perspective, these results have implications for understanding the roles of metal ions in integration of environmental signals with plant development and optimizing delivery of fixed nitrogen in legume crops.
Subject terms: Rhizobial symbiosis, Plant signalling
Zinc acts as a second messenger in root nodules and regulates nitrogen homeostasis by controlling the transition between the active state and the inactive filamentous state of the novel transcriptional regulator FIXATION UNDER NITRATE (FUN).
Main
Modulation of gene expression enables organisms to adapt their growth and metabolism to the constantly changing environment. Plants devote significant resources to acquiring growth limiting nutrients such as nitrate and phosphate, with transcriptional regulators such as NLPs9,10 and PHR111 altering plant metabolism and development in line with nutrient availability1. In addition to accessing soil nitrogen for growth, legumes can acquire fixed nitrogen through symbiosis with bacteria hosted in root organs known as nodules. To balance the costs of provision of carbon to the nitrogen-fixing bacteria with the benefit of fixed nitrogen, legume hosts modulate nodule function in response to the environment. In particular, available soil nitrate reduces nodule formation, growth and function and induces senescence of existing nodules12. A number of pathways have been shown to have a role in this regulation, including NLP and NRT2.1, which drive core nitrate signalling and acquisition6,8,13. Environmental signals are also integrated into nodulation via systemic signalling through pathways that affect root development2,3,5,7,14,15. Recently, more specific regulators of nodule function have been identified16,17, although it remains unclear how environmental signals are linked to these regulators. We designed a genetic screen to identify factors in nitrogen fixation that provide insights into the link between the environment and nodule metabolism. We describe an unexpected role for zinc as a second messenger that links the environment to nitrogen homeostasis by directly regulating a transcriptional regulator of multiple processes associated with nodule senescence.
FUN controls nitrogen fixation
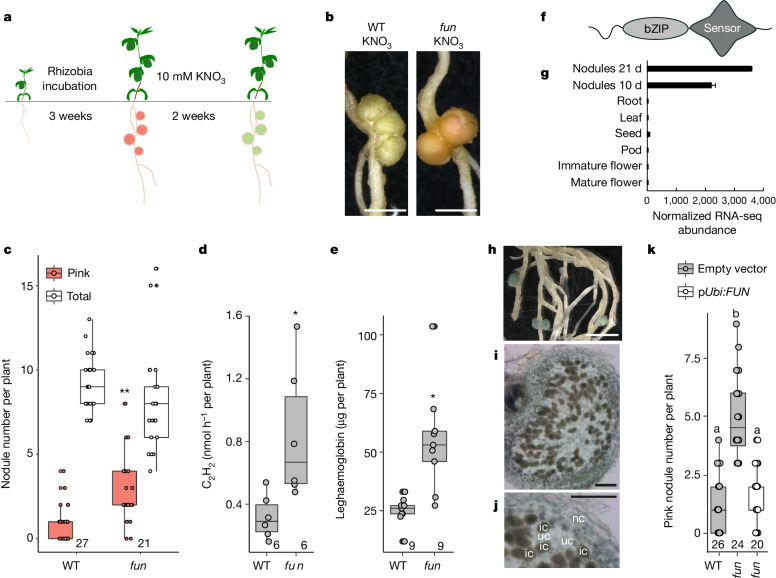
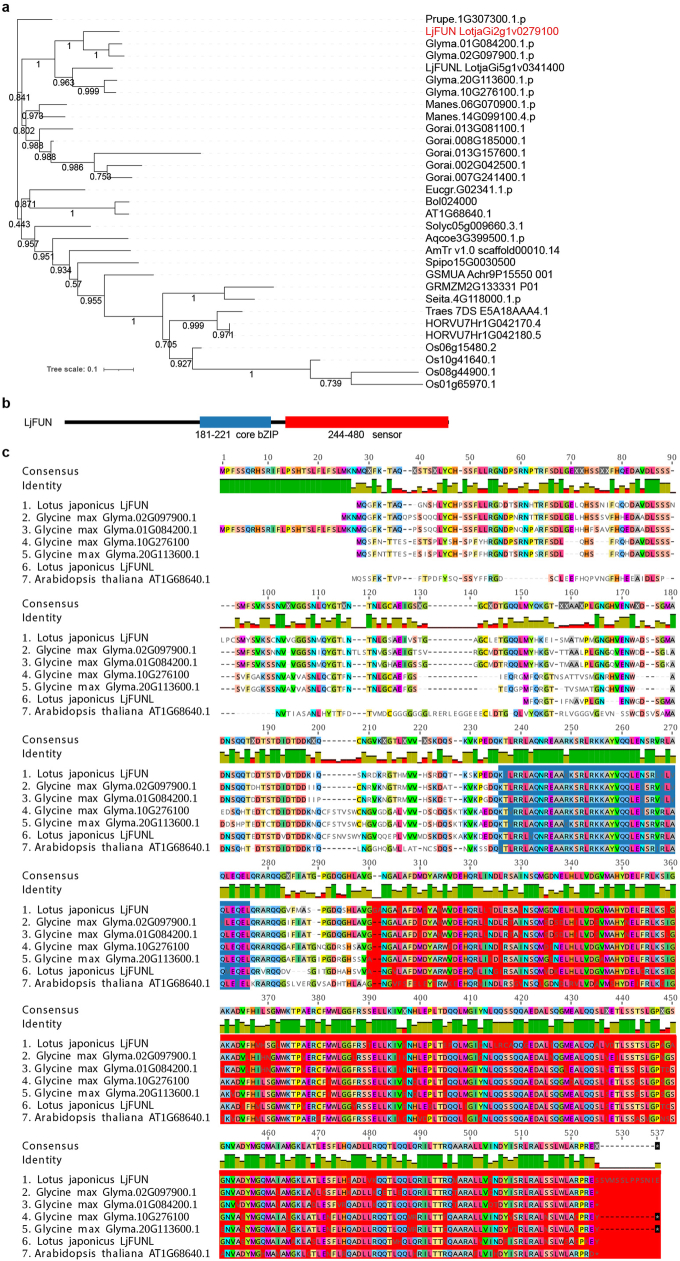
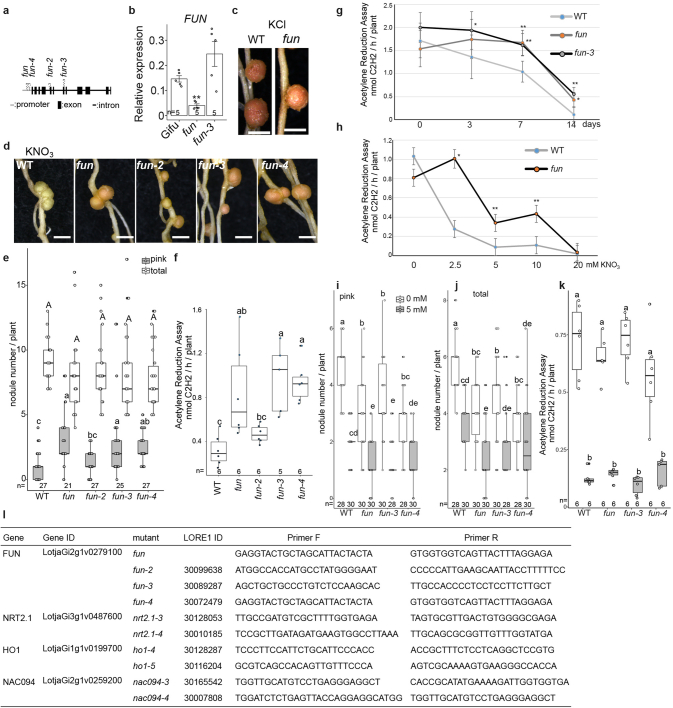
To identify environmental regulators of nodulation, we reasoned that by applying restrictive conditions after functional root nodules were formed, we could screen for mutants with specific impairments in regulating nodule function. Using the distinctive pink colour (produced by leghaemoglobin) of nitrogen-fixing nodules as opposed to the green colour of senescent nodules, we screened a population of LORE118–20 insertion mutants in the model legume Lotus japonicus (Lotus) to identify genotypes retaining nodule function despite suppressive nitrate conditions (Fig. 1a). We observed a mutant, which we named fixation under nitrate (fun), that retains a higher number of pink nodules relative to the wild type (Fig. 1b,c). The function of these pink nodules was confirmed by increased nitrogen fixation rates when assayed by acetylene reduction (Fig. 1d) and increased leghaemoglobin content (Fig. 1e). We identified a LORE1 retrotransposon insertion20 in the promoter region of a bZIP-type transcription factor, which is causative of the fun phenotype. The FUN gene encodes a protein of the TGA family of transcription factors, with greatest similarity to the Arabidopsis transcription factor PERIANTHIA (PAN)21,22. The TGA family belong to group D bZIP transcription factors23 and is characterized by the presence of a basic leucine zipper (bZIP) DNA-binding domain in the N terminus and a DOG1 domain of unknown function at the C terminus24, which we refer to as the sensor domain for reasons outlined below (Fig. 1f). Phylogenetic analysis indicates that FUN is highly conserved in legumes, with legumes carrying both a FUN and FUN-like paralogue in the PAN orthogroup (Extended Data Fig. 1a). In Lotus, FUN transcripts are detected at high levels in nodules (Fig. 1g), and promoter activity is evident in the nodules (Fig. 1h–j). We validated FUN as the causative gene by complementing the fun mutation with a constitutively expressed FUN (Fig. 1k) and by confirming that the nodulation phenotype is consistent in three independent LORE1-mutant alleles that reduce gene expression via promoter insertion (fun and fun-4) or by interrupting function via exonic insertion (fun-3) (Extended Data Fig. 2). An intronic insertion allele (fun-2) is not impaired relative to wild type (Extended Data Fig. 2). FUN regulation is restricted to mature functional nodules, since application of nitrate prior to inoculation inhibits nodulation in fun mutants to the same degree as wild type (Extended Data Fig. 2i–k).
Fig. 1. FUN is essential for the suppression of nitrogen fixation by environmental nitrate.
a, Schematic diagram of the screen that resulted in identification of FUN. Mutants producing functionally pink nodules were watered with 10 mM KNO3. Most nodules on wild-type plants became green and senescent, whereas fun mutant plants maintained pink nodules even under high concentrations of nitrate. b–e, Nodulation phenotypes of fun mutants in high-nitrate conditions. The nodule appearance (b), nodule number (c), nitrogen fixation measured by acetylene reduction assay (ARA) (d) and leghaemoglobin content (e) of fun mutant plants after 2 weeks of exposure to 10 mM KNO3. b, Scale bars, 1 cm. f, Schematic of the FUN protein, showing bZIP DNA-binding and sensor domains. g, The expression pattern of FUN in different tissues obtained from the Lotus expression atlas42. h–j, The expression pattern of the FUN promoter is revealed by beta-glucuronidase (GUS) reporter gene expression. FUN is expressed exclusively in nodules (h,i), and cross-sections (i,j) indicate that FUN is expressed predominantly in uninfected cells (uc) and nodule cortex (nc), and to a lesser extent in infected cells (ic) (j). Scale bars: 2 cm (h), 200 µm (i,j). k, Complementation of fun mutants using expression of FUN–GFP under the control of the Lotus ubiquitin promoter restores the sensitivity of fun nodules to nitrate. Letters indicate groups that are significantly different from each other (P < 0.05). c–e,k, In box plots, the centre line represents the median, box edges delineate first and third quartiles, whiskers extend to maximum and minimum values and dots show individual values. Numbers below data in box plots represent the number of biologically independent samples. P values determined by ANOVA and Tukey post hoc testing; *P < 0.05, **P < 0.01.
Extended Data Fig. 1. Phylogenetic tree and alignment of FUN.
a FUN orthologous proteins were identified using shoot.bio and a phylogenetic tree constructed with the inclusion of FUN and LjFUN-like. b The schematic diagram of the LjFUN protein. The DNA binding bZIP domain is shown in blue, while the zinc sensor domain is shown in red. c The protein alignment of selected orthologues of FUN and FUN-like. Prupe: Prunus persica, Lotus japonicus: Lj, Glycine max: Glyma, Manihot esculenta: Manes, Gossypium raimondii: Gorai, Eucalyptus grandis: Eucgr, Brassica oleracea: Bol, Arabidopsis thaliana: AT, Solanum lycopersicum: Solyc, Aquilegia coerulea: Aqcoe, Amborella trichopoda: AmTr, Spirodela polyrhiza: Spipo, Musa acuminata: GSMUA, Zea mays: GRMZM, Setaria italica: Seita, Triticum aestivum: Traes, Hordeum vulgare: HORVU, Oryza sativa: Os.
Extended Data Fig. 2. Nitrate suppression of nitrogen fixation of fun alleles.
a The diagram of FUN gene and LORE1 insertions of each allele. There are 12 exons. In fun and fun-4 (30072479), LORE1 is inserted in the promoter regions. In fun-2 (30099638), LORE1 is inserted at the end of the fourth intron. In fun-3 (30089287), LORE1 is inserted at the end of the seventh exon. b The gene expression of FUN in fun and fun-3 mutants. RNA were extracted from WT, fun, and fun-3 nodules. c-f, The nitrogen fixation of fun mutants after high concentrations of nitrate treatments. 3 week post inoculation WT and fun mutants (with mature nodules) were watered with 10 mM KNO3 for another two weeks. The nodule under KCl (c), under KNO3 (d), nodule number (e), and ARA activity (f) were counted or measured after 2 weeks of nitrate exposure. g-h, Time and dose series of nitrate treatments. The ARA activity of fun mutants (with mature nodules) exposed to 10 mM KNO3 for 0, 3, 7, and 14 days (g). ARA activity (d) of fun mutants (with mature nodules) under 2-week 0, 2.5, 5, 10 and 20 mM KNO3 exposure (h). i-k, Phenotypes of fun mutants with nitrate application prior to inoculation. Plants were grown on plates with 0 or 5 mM KNO3 and inoculated with rhizobia, pink (i) and total (j) nodule number, and ARA (k) of wild type and fun mutants were measured 3 weeks post inoculation. l The LORE1 IDs, primers, and their sequences of individual mutants used in the manuscript. Scale bars in c and d are 1 cm. Bars show mean ± SE and individual values (dots) in b. Box plots show Min, Q1, Median, Q3, Max and individual values (dots) in e-f and i-k. Significant differences among different genotypes are indicated by letters (p < 0.05) as determined by ANOVA and Tukey post-hoc testing with pairwise P-values indicated (**: p value < 0.01; *: p value < 0.05). Images and data for WT and fun are reproduced from Fig. 1b, c and d alongside the additional alleles shown here. Biological independent samples n value shown on each box plots and bar plots.
FUN regulates nodule senescence
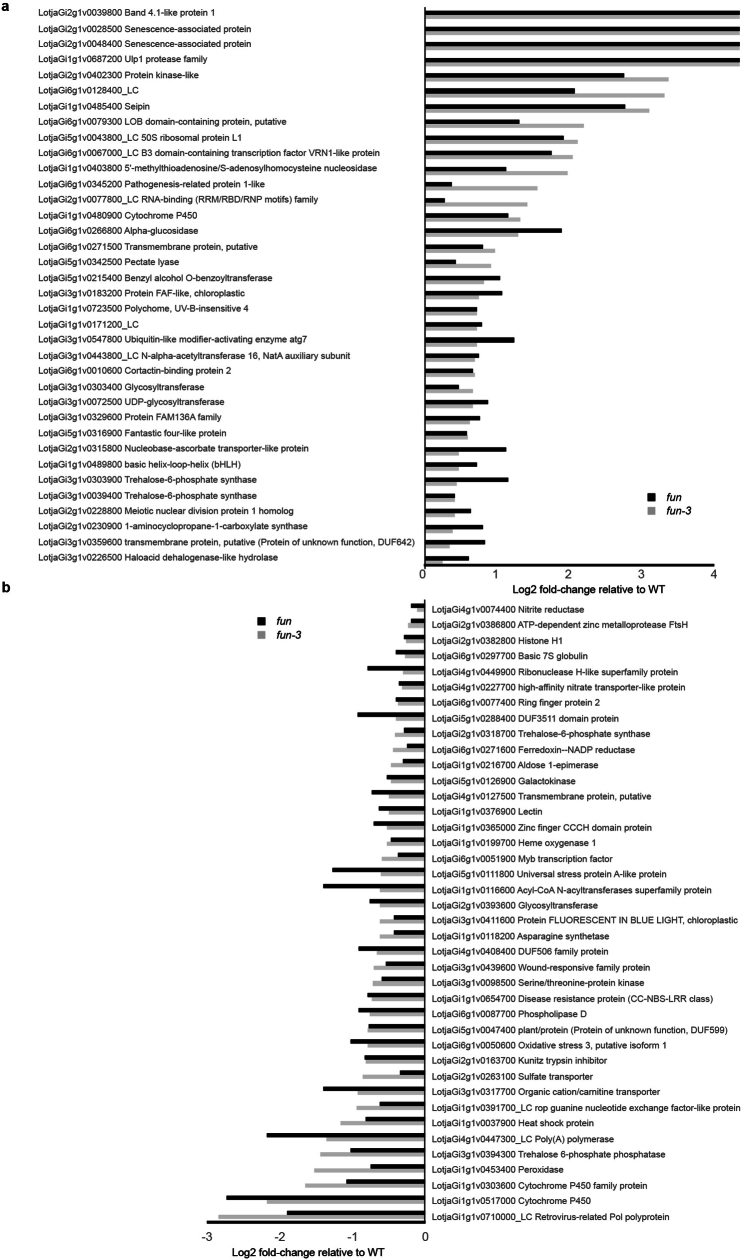
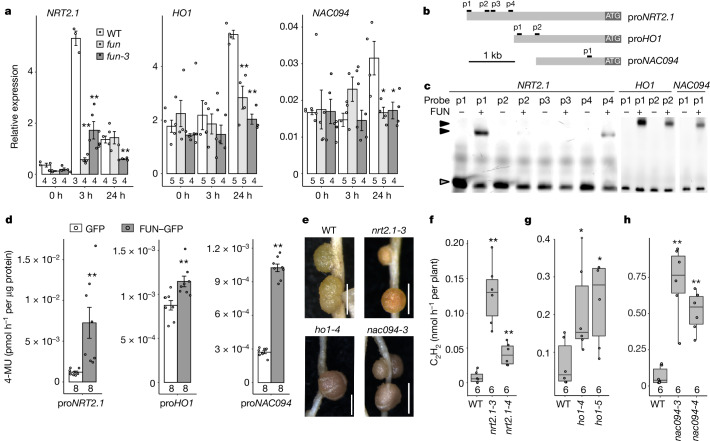
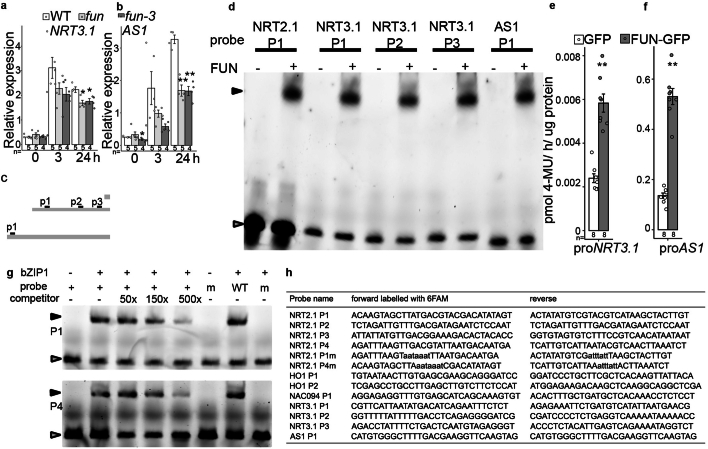
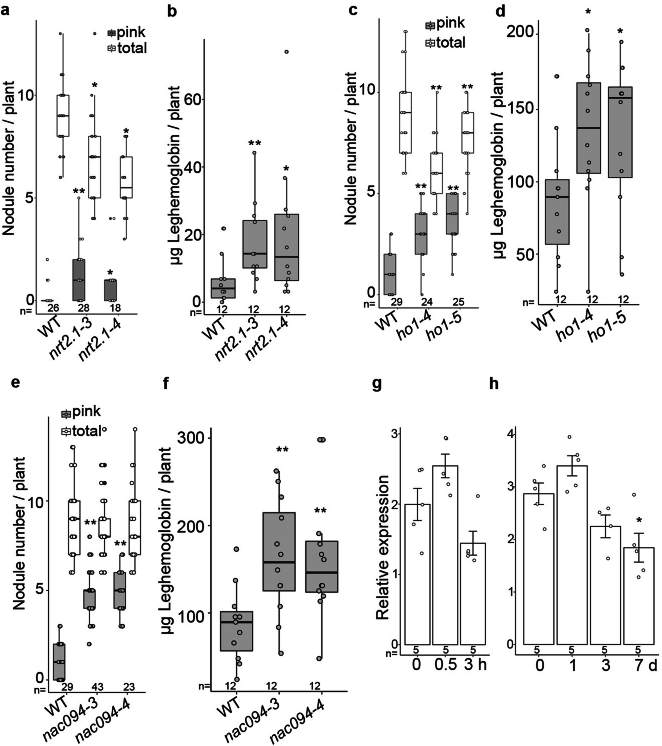
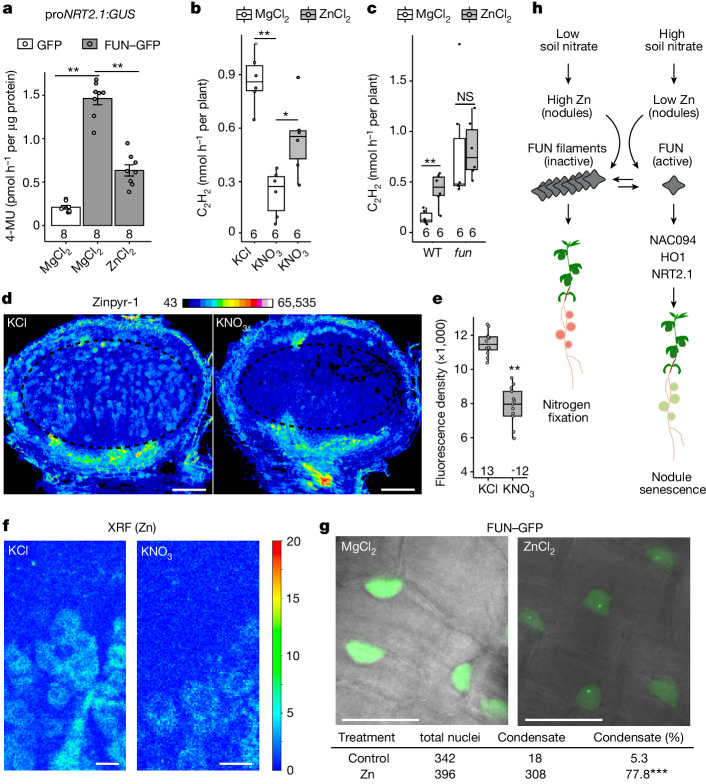
Since FUN is a transcriptional regulator, we searched for gene targets associated with nitrate signalling or nodule function that may be directly regulated. RNA-sequencing (RNA-seq) analysis identified 587 genes with greater than twofold expression change in wild-type nodules exposed to nitrate. Comparison with fun mutants showed that 106 of these genes were regulated differently in fun nodules (Extended Data Fig. 3), with several gene ontology groups detected in both up- and down-regulated gene groups by Gene Ontology with Mann–Whitney U test25 (GO-MWU) (Extended Data Fig. 4a). Notable amongst these regulated genes were HO1, whose haem oxygenase product degrades leghaemoglobin during nodule senescence26,27, the nitrate transporter gene NRT3.1 and ASPARAGINE SYNTHETASE 1 (AS1), which is important for nitrogen assimilation (Extended Data Fig. 4c,d). We were also able to identify a number of putative TGA-type binding motifs (TGACG28) in the promoter regions of two genes with similar phenotypes to fun when mutated: the nitrate transporter gene NRT2.113 and the NAC transcription factor gene NAC094, whose product triggers nodule senescence17. Induction of these genes by nitrate was attenuated in fun mutants analysed by quantitative PCR with reverse transcription and RNA-seq (Fig. 2a and Extended Data Figs. 4d and 5a,b). FUN was co-expressed in uninfected cells with NAC094 and HO1 (Fig. 1i,j), whereas nitrate regulation of NAC094—which also occurs in infected cells17—may require additional regulators. DNA probes representing the binding regions within the promoters were bound by the purified FUN DNA-binding domain in electrophoretic mobility shift assays (EMSAs) (Fig. 2b,c and Extended Data Fig. 5c,d). Mutation and competition assays with excess unlabelled DNA probes demonstrated the specificity of this interaction for the NRT2.1 promoter (Extended Data Fig. 5g). To validate the relevance of this binding in vivo, we conducted transient activation experiments in Nicotiana benthamiana for the NRT2.1, HO1, NAC094, NRT3.1 and AS1 promoters and showed that all the promoters coupled to the GUS reporter were significantly induced by FUN in this system (Fig. 2d and Extended Data Fig. 5e,f). Further supporting the view that FUN controls these pathways, nrt2.1, ho1 and nac094 mutants showed similar nodule phenotypes to the original fun mutant, including enhanced nitrogen fixation and leghaemoglobin content (Fig. 2e–h and Extended Data Fig. 6). Together, these results indicate that FUN targets nodule senescence and nitrate signalling pathways to modulate nodule function to the environment. Regulation of the nitrate signalling pathway by FUN in this way may serve to alter the sensitivity of the nodule to nitrate relative to other root tissues.
Extended Data Fig. 3. Differentially regulated genes in nodules 24 h after nitrate exposure.
Three-week post inoculation wild-type, fun and fun-3 mutants were treated with 0 or 10 mM KNO3 for 24 h. Nodules were harvested for RNA-seq. Genes with more than 2 fold changes and p value < 0.05 were selected as DE in WT and compared with genes detected as DE (without fold-change filtering) in fun.
Extended Data Fig. 4. Gene expression analysis of FUN targets.
a GO-MWU (rank-based Mann-Whitney U test) identified several ontology groups enriched among up and downregulated genes. b The primers and their sequences used in the qRT-PCR. c-d Relative expressions of genes differentially expressed in fun relative to WT are displayed. Downstream targets of FUN with identified TGA motifs within the promoters are shown in a RNAseq timeseries (n = 3 at at each timepoint) conducted by17 (c) and in our dataset (n = 4 in each condition) (d).
Fig. 2. FUN is a transcriptional regulator controlling expression of NRT2.1, HO1 and NAC094, which regulate nitrate signalling and nitrogen fixation in nodules.
a, Expression of NRT2.1, HO1 and NAC094 in nodules of fun mutants treated with nitrate for indicated times is lower than in wild type. Data are mean ± s.e.m. and dots show individual values. b, Schematic diagram of the NRT2.1, HO1 and NAC094 promoters (indicated by the prefix ‘pro’), indicating the four (p1–p4 in NRT2.1), two (p1–p2 in HO1) and one (p1–p2 in NAC094) putative FUN binding sites. c, Binding of FUN to DNA probes derived from the respective regions of NRT2.1, HO1 and NAC094 show binding to p1 and p4 in the NRT2.1 promoter, p1 and p2 in the HO1 promoter, and p1 in the NAC094 promoter. Grey arrowheads indicate free probes, while black arrowheads are probes bound by FUN. d, FUN activates the NRT2.1, HO1 and NAC094 promoters in trans-activation assays in N. benthamiana leaves. FUN was expressed as the effector, and GUS driven by NRT2.1, HO1 and NAC094 promoters was expressed as the reporter. e–h Nodule appearance (e) and ARA activity (f,h) of nrt2.1 (e,f), ho1 (e,g) and nac094 (e,h) mutants following 2 weeks of exposure to 10 mM KNO3. e, Scale bars, 1 cm. d,f–h, In box plots, the centre line represents the median, box edges delineate first and third quartiles, whiskers extend to maximum and minimum values and dots show individual values. Numbers below data in bar charts and box plots represent the number of biologically independent samples. P values determined by ANOVA and Tukey post hoc testing. *P < 0.05, **P < 0.01.
Extended Data Fig. 5. FUN regulates signalling in nodules.
a-b The expression of Nrt3.1 and AS1 in nodules of fun mutants. The induction of Nrt3.1 (a) and As1 (b) by nitrate is lower in nodules of fun mutants. c-d The binding of FUN to FBSs in promoters of Nrt3.1 and As1. c Schematic diagram of the promoter of Nrt3.1 and As1. There are three (P1-3) and one (P1) putative FUN Binding Sites (FBSs) in the promoter of Nrt3.1 and As1, respectively. d FUN binds to the P1, P2, and P3 in Nrt3.1’s promoter and P1 in Ho1’s promoter in EMSA. e-f FUN can activate the promoter of Nrt3.1 (e) and As1 (f). The transactivation assay of the promoter of Nrt3.1 and As1 by FUN in N. benthamiana leaves. FUN-GFP was expressed as the effector, and GUS driven by the promoter of Nrt3.1 and As1 as reporters. g FUN specifically binds to the P1 and P4 regions of the Nrt2.1 promoter. FUN binds to the P1 and P4 in Nrt2.1’s promoter in EMSA. DNA probes containing predicted binding sites are FAM-tagged. Competition DNA is 50, 150 and 500 times concentration of WT DNA without FAM. m: DNA probes with the mutations of TGACG, the core binding site. h The probes and their sequences used in EMSA. The mutations in the core region of FBS are shown. Probe locations are illustrated in Fig. 2b,c. Grey arrowheads indicate free probes, while black arrowheads are probes bound by FUN in d and g. Significant differences are determined by ANOVA and Tukey post-hoc testing (**: p value < 0.01; *: p value < 0.05). Bars show mean ± SE and individual values (dots) in a-b and g. Biological independent samples n value shown on each bar plots.
Extended Data Fig. 6. The nodulation and leghemoglobin content of nrt2.1, ho1 and nac094 mutants after nitrate treatments and the expression of Fun upon nitrate treatments.
a-f The nodule number (a,c,e), and leghemoglobin content (b,d,f) of nrt2.1 (a-b), ho1 (c-d) and nac094 (e-f) mutants under 2-week 10 mM KNO3 exposure. g-h FUN does not respond transcriptionally to nitrate. The expression of Fun in 3-week old nodules exposed to 10 mM KNO3 for 0, 0.5, and 3 h (g), and 0, 1, 3, and 7 days (h). There is no significant difference before and after nitrate treatments until 7 days once nodule function has ceased. Bars show mean ± SE and individual values (dots) in g and h. Box plots show Min, Q1, Median, Q3, Max and individual values (dots) in a-f. Significant differences are determined by ANOVA and Tukey post-hoc testing (**: p value < 0.01; *: p value < 0.05). Biological independent samples n value shown on each box plots and bar plots.
Zn alters the oligomeric state of FUN
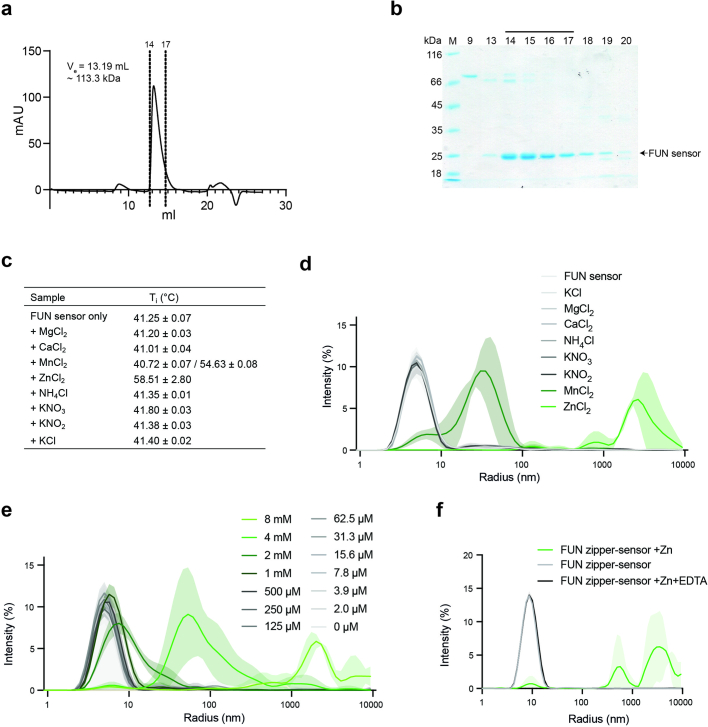
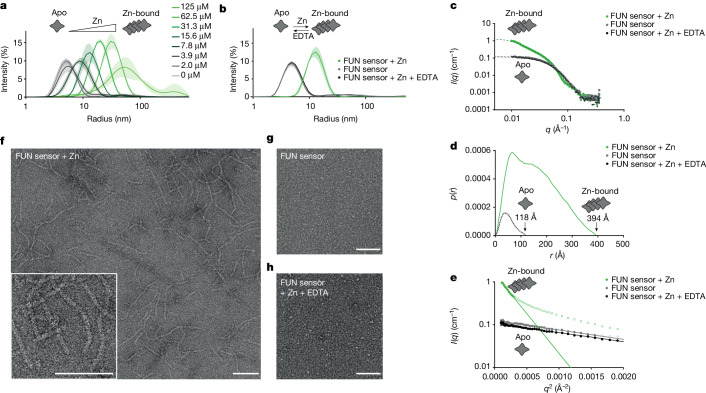
The FUN sensor domain has distant homology to metal-binding proteins29 and since we observed no transcriptional regulation of FUN in nodules (Extended Data Fig. 6g,h), the activity could be regulated at the protein level. To understand the mechanism, we expressed and purified the FUN sensor domain (Extended Data Fig. 7a,b) and screened common cellular metal ions and nitrogen compounds to determine whether these influence the FUN sensor. We found that both thermostability (assayed by nano differential scanning fluorimetry (nanoDSF)) (Extended Data Fig. 7c) and molecular size (assayed by dynamic light scattering (DLS)) (Fig. 3a and Extended Data Fig. 7d,e) of FUN increased in the presence of zinc and manganese, whereas there were no changes in response to the other compounds tested. Dose–response experiments revealed that zinc increased the molecular size of the FUN sensor at low, physiologically relevant concentrations (3.9–7.8 µM), whereas only unnaturally high levels of manganese (2–4 mM) increased its size, showing that zinc is the relevant ligand (Fig. 3a and Extended Data Fig. 7e). The changes induced by zinc were reversible when zinc was chelated using EDTA (Fig. 3b). We also confirmed similar zinc sensitivity and reversibility with a protein containing both the DNA-binding domain and sensor domain (Extended Data Fig. 7f). Further investigation by small angle X-ray scattering experiments (SAXS) provided scattering data and pair distance-distribution functions (histograms of distances between pairs of points within the structure) confirming that the FUN sensor shifts from a smaller molecular size to a larger oligomer form when zinc is present, and that this effect is reversible by removing zinc with EDTA (Fig. 3c–e). We investigated the structure of the oligomeric form of the FUN sensor using electron microscopy. Negatively stained samples reveal that large filament structures form when the FUN sensor is zinc-bound and that these filaments disassemble when zinc is removed using EDTA (Fig. 3f–h). Together, our results show that FUN binds low physiological concentrations of zinc, which changes its oligomeric form to large filaments, and that this process is dynamic and reversible, which could be a mechanism of regulating activity.
Extended Data Fig. 7. FUN specifically binds to zinc.
a-b Purification of the FUN sensor domain. Chromatogram from size exclusion chromatography (Superdex 200 increase 10/300) of the FUN sensor domain (a). SDS-PAGE analysis of SEC fractions (b). Fractions 14-17 were pooled and saved as indicated by the dashed lines on the chromatogram and horizontal line above the SDS-PAGE. c-d FUN sensor ligand screen. Inflection temperatures (Ti) from nanoDSF experiments on the FUN sensor domain with different ions at a concentration of 4 mM (c). Dynamic light scattering (DLS) experiment of the FUN sensor domain with different ions at a concentration of 4 mM (d). Increase in the hydrodynamic radius of the FUN sensor is only observed in the presence of manganese or zinc. e High concentrations of manganese increase the size of the FUN sensor. DLS experiments of the FUN sensor domain in a MnCl2 concentration series. Manganese concentrations in the millimolar range are needed to induce changes of the hydrodynamic radius of the FUN sensor. f DLS experiments of the FUN protein containing zipper and sensor domain in the presence of 100 µM ZnCl2. The zinc-induced change in hydrodynamic radius is reversed with 5 mM EDTA.
Fig. 3. The FUN sensor forms protein filaments in the presence of physiological concentrations of zinc.
a, DLS experiments with the FUN sensor in a zinc concentration series show that the particle size of the FUN sensor increases with zinc concentrations above 3.9–7.8 µM. b, The increase in particle size is reversible when zinc is removed using EDTA. c–e, SAXS analysis of the FUN sensor, showing c, scattering data, I(q), versus modulus of the scattering vector, q, of the FUN sensor in the apo and zinc-bound forms, and following zinc removal using EDTA. d, Pair distance-distribution (p(r)) plot with maximum distance (Dmax) indicated. e, Guinier plots of ln(I(q)) versus q2. Closed circles show data used in the fit and open circles are omitted data points. The p(r) function shows radii of gyration of 39 ± 1 Å for the pure FUN sensor sample and the EDTA plus zinc-containing sample, and 125 ± 1 Å for the zinc-bound sample. The values calculated from p(r) were slightly lower for all samples for the Guinier analysis. f–h, Negative-staining electron microscopy images of the FUN sensor showing filament structures in the presence of Zn (f), and no visible filaments in the absence of Zn (g) or when Zn is removed using EDTA (h). Scale bars, 100 nm.
Zn is a second messenger regulating FUN
The identification of zinc-induced FUN filaments raises the possibility that this may have a role in modulating the activity of the protein. Using the NRT2.1 promoter as a readout for FUN activity, co-infiltration with zinc significantly reduced FUN activity relative to mock (MgCl2) in N. benthamiana leaves (Fig. 4a). This indicates that the zinc-bound filamentous state of FUN is the inactive form of the protein. Further confirming the negative regulatory effect of zinc on FUN activity, addition of 500 µM zinc to nitrate-exposed wild-type plants significantly increased nodule function after 10 days, reproducing the phenotypes of fun knockout mutants as determined by acetylene reduction (Fig. 4b) and leghaemoglobin content (Extended Data Fig. 8a). This increase was dependent on the presence of FUN, as no further increase in nodule function was observed in the fun mutant (Fig. 4c and Extended Data Fig. 8b). Given the phenotypes of the fun mutant, we hypothesized that zinc may act as a messenger linking nitrate with FUN activity and nodule regulation. To test whether nitrate influences cellular zinc levels, we used the zinc-sensitive dye zinpyr-130 to evaluate Lotus nodule sections from plants grown in nitrate-free conditions as well as nodules exposed to 10 mM KNO3 for 24 h (Fig. 4d,e and Extended Data Fig. 8c). This revealed a marked reduction in zinc levels, particularly within the nitrogen fixation zone and the cortical cells of nitrate-treated nodules. Independent confirmation of this concentration reduction was obtained via micro-X-ray fluorescence (XRF) microscopy conducted on sections of nodules treated with 10 mM KNO3 for 24 h, which showed a ring-like distribution of zinc in infected cells associated with the symbiosome radial distribution and dense packaging (Fig. 4f and Extended Data Fig. 8e). Density measurements of ten infected cells from each condition confirmed that zinc reduced by half relative to untreated nodules (0.54 ± 0.06; Extended Data Fig. 8e). To confirm the in vivo relevance of zinc-dependent filamentation of FUN, we expressed a FUN–GFP construct in Lotus roots. FUN–GFP exhibited a disperse localization in 95% of nuclei in the control condition (500 µM MgCl2), whereas addition of zinc (500 µM ZnCl2) triggered relocalization to distinct sub-nuclear condensates in 77% of nuclei (Fig. 4g). This supports the view that FUN mediates a graded response, with filamentation being a dynamic response to physiological changes in zinc concentration in the cell. Consistent with the effect of zinc on protein activity in N. benthamiana, we also observed a zinc-dependent increase in condensate frequency in leaves infiltrated with zinc alongside the FUN–GFP construct (Extended Data Fig. 8d). Nuclear condensation can have roles in both sequestering inactive transcriptional regulators31 and in activation of transcription32. Together, our results show that alterations in zinc concentrations in response to soil nitrate are sufficient to alter FUN activity and thus the nitrogen fixation phenotype of the nodule.
Fig. 4. Zinc alters FUN activity and nitrogen fixation.
a, Application of zinc (500 µM ZnCl2) interferes with the activation of the NRT2.1 promoter by FUN in trans-activation assays in N. benthamiana leaves relative to mock (500 µM MgCl2). Data are mean ± s.e.m. and dots show individual values. b, Zinc application (500 µM ZnCl2) relieves the suppression of nitrogen fixation (measured by ARA) by 10 mM KNO3 in wild-type plants. c, Improved nitrogen fixation (measured by ARA) by zinc in restrictive (10 mM) nitrate conditions is dependent on FUN. d,e, Nitrate exposure triggers a reduction in cellular zinc levels within nodules, as indicated by the Zinpyr-1 fluorescent dye at 24 h post-treatment. Scale bars, 200 μm. e, The average intensity of the fixation zone indicated with the dashed circle in d. f, Lower cellular zinc levels were also evident with XRF microscopy at 24 h after nitrate treatment. Scale bars, 20 µm; colour bar represents normalized Zn–K X-ray fluorescence intensity. g, Zinc-dependent nuclear condensation of FUN–GFP was observed in Lotus roots. Scale bars, 20 μm. h, Mechanistic model of how FUN regulates nodule function. Under low soil nitrate, zinc accumulates in nodules, retaining FUN in inactive filaments and allowing continued nitrogen fixation. With high soil nitrate, cellular zinc levels decrease, liberating active FUN from filaments and increasing expression of target genes, including NAC094, HO1 and NRT2.1, that induce nodule senescence. b,c,e, In box plots, the centre line represents the median, box edges delineate first and third quartiles, whiskers extend to maximum and minimum values and dots show individual values. Numbers below data in box plots represent the number of biologically independent samples. P values determined by ANOVA and Tukey post hoc testing in a–c,e and by chi-squared test in g. *P < 0.05, **P < 0.01, ***P < 0.001; NS, not significant.
Extended Data Fig. 8. Nitrate reduces zinc levels in nodules and zinc promotes FUN condensates in the nucleus.
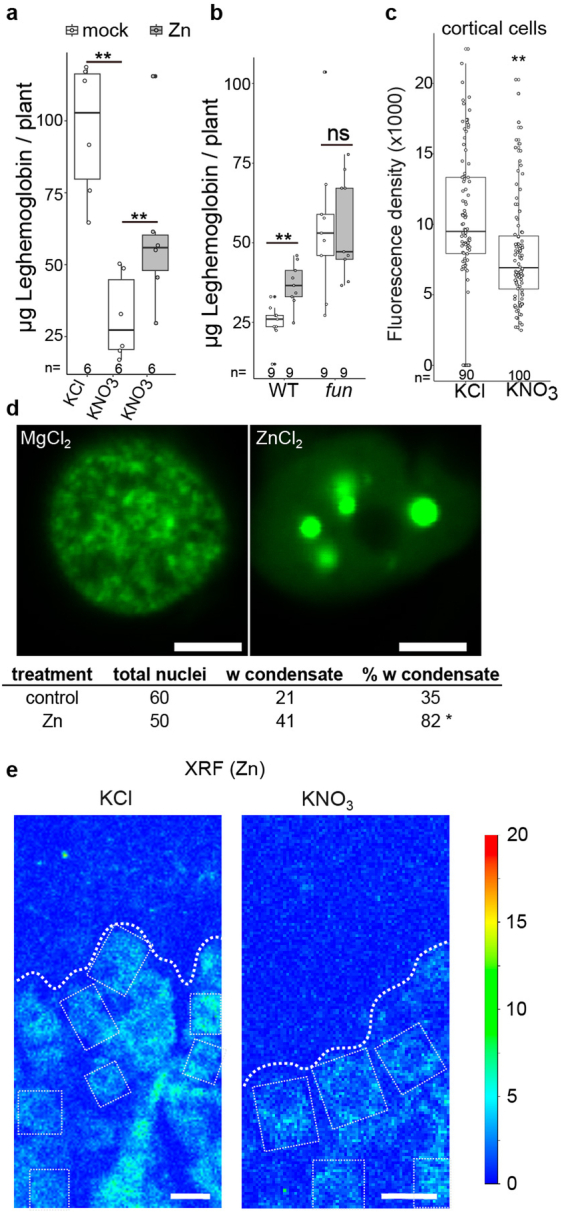
a-b The leghemoglobin corresponding to ARA in Fig. 4f,g. ns: not significant. c Nitrate exposure triggers a reduction in cellular zinc levels within nodules as indicated by the Zinpyr-1 fluorescent dye at 24 h post treatment. The average intensity of the cortical cell zone of nodules. The Box plots show Min, Q1, Median, Q3, Max and individual values (dots) in a-c. Significant differences are determined by ANOVA and Tukey post-hoc testing (**: p value < 0.01; *: p value < 0.05). Biological independent samples n value shown on each box plots and bar plots. d Co-infiltration with ZnCl2 increases the frequency of FUN nuclear condensates in N. benthamiana leaves. Leaves were infiltrated with Agrobacterium carrying a binary vector to express pro35S:FUN-GFP and subsequently infiltrated with 500 µM MgCl2 or ZnCl2 two days before confocal observation. Chi-squared testing (*: p value < 0.05). Scale bar 5 µm. e XRF regions used for quantification. Regions analysed for quantification of zinc from Fig. 4f are boxed. The dash lines indicate the boundaries between nodule cortex (above line) and infected region (below line). Scale bar 20 µm.
Discussion
Our genetic screen identified a basic leucine zipper transcription factor, FUN, as a novel regulator of nitrogen fixation in legumes. We identified a sensor domain within FUN as being crucial for its activity and demonstrated that intracellular zinc levels determine protein activity via ligand-dependent protein filamentation. We showed that FUN forms inactive filaments under high zinc concentrations that act as a molecular reservoir from which active proteins can be released when zinc levels are lowered (Fig. 4h). Cellular zinc levels have an inverse relationship with nitrate, and we show that zinc acts as a second messenger to signal nitrate availability and control the transition between inactive filamentous and active states of the FUN protein. Previous work has demonstrated that filamentation can be part of the process in condensate formation33–35, however it remains to be established how the condensation we observe in planta relates to the FUN filament structure and whether additional components are recruited to regulate condensate formation in the nuclei.
In plants, we demonstrated that altered zinc concentrations affect the activity of the FUN protein and nodule function, acting to link soil nitrate supply to transcriptional modulation of nodule metabolism. This post-translational regulation of FUN activity enables the plant to respond to a nitrate concentration gradient via a gradual decrease in zinc levels, liberating greater quantities of active FUN to tune nodule function to the environment. This stands in contrast to previously described zinc-sensitive transcription factors such as bZIP19/23, where zinc binding to a zinc-sensitive motif unrelated to the FUN sensor is likely to cause conformational changes that prevent their activity36. The precise mechanism by which intracellular zinc concentrations are affected by nitrate—for example, via transporter regulation, organellar sequestration or cellular export, remains unknown. FUN is a transcription factor in the TGA family, whose members regulate a diverse array of important plant traits including nitrate uptake37,38, pathogen response39 and flower development22. Given the presence of the identified sensor domain within homologues of the TGA family, it is plausible that zinc or other metal ions and metabolites could provide similar graded responses to environmental stimuli, enabling a connection between the environment and plant development through metal ion signalling. Manipulation of metal ion accumulation or the responsiveness of protein filamentation to these metal ions may provide novel methods for optimizing these important plant traits.
Nitrogen fixation is an energy-demanding process that requires provision of fixed carbon to symbiotic rhizobia. A regulated senescence programme enables restriction of the carbon supply to nodules and reprovisioning of nutrients to support plant growth and reproduction40. Several NAC transcription factors were recently shown to regulate pathways required for nodule senescence16,17. Our identification of FUN as a regulator of senescence-related processes through multiple pathways—including via NAC094—opens new avenues for fine-tuning these pathways to enhance tolerance of legumes to soil nitrate, and provides an opportunity to increase delivery of fixed nitrogen to agriculturally important crops. Notably, the specificity of the identified pathway to nodule functional regulation ensures that mutants do not show adverse effects associated with other genetic pathways such as nodule number regulation2,3,14 or nitrate acquisition and signalling6,13,41.
Methods
Plant lines and growth conditions
The Lotus japonicus Gifu ecotype was used as the wild type. All plants were grown at 21 °C under 16 h light/8 h dark conditions. For germination, Lotus seeds were scarified with sandpaper and surface sterilized with 1% sodium hypochlorite for 10 min. Seedlings were washed with sterile water for 5 times and germinated on wet filter paper (AGF 651; Frisenette ApS) in sterile square Petri dishes at 21 °C for 2 days. Then, seedlings were transferred into the substrate mixture (leca:vermiculite=3:1). Three weeks post-inoculation, plants were treated with 10 mM KNO3 or KCl for 14 days (or as indicated). Subsequently, nodule number, nitrogenase activity (ARA), or leghaemoglobin content were recorded. For Zn treatment, 3 weeks post-inoculation, plants were watered with 500 µM MgCl2 (mock) or 500 µM ZnCl2 for 3 days followed by 10 days 10 mM KNO3 treatments. ARA and leghaemoglobin content were recorded. LORE1 insertion mutants were ordered through LotusBase (https://lotus.au.dk) and homozygotes were isolated for phenotyping and generation of higher order mutants as described43. Line numbers and genotyping primers are provided in Extended Data Fig. 2a. Mesorhizobium loti NZP2235 was used for nodulation assays.
Mutant screening and sequence analysis
A LORE1-mutant pool, in which there are random LORE1 insertions in the genome of each individual, were germinated in substrate mixture (leca:vermiculite 3:1) and inoculated with M.loti NZP2235. Four weeks post-inoculation, plants were watered with 10 mM KNO3 for three weeks. Most nodules became green or black, and we isolated plants with pink nodules for rescreening in subsequent generations. DNA from mutant plants was isolated and LORE1-flanking sequences sequenced to identify LORE1 insertion positions as previously described19. FUN protein sequences were identified by BLAST and SHOOT44 and aligned with MAFFT 7.490 and a tree constructed using FastTree 2.1.11. The tree was visualized using iTOL 6.7.345.
Hairy root transformation
For complementation assays, the Lotus ubiquitin promoter, FUN coding sequence, and 35S terminator were cloned into the pIV10 expression vector46. To study the expression pattern of FUN, native FUN promoter, glucuronidase (GUS) and the native FUN terminator sequence (tFUN) were cloned into the pIV10 expression vector. Constructs mentioned above were transformed into Agrobacterium rhizogenes AR1193. These agrobacteria were used to transform the hypocotyl of 6-day old seedlings. After three weeks, non-transformed roots were removed, and seedlings were transferred into the substrate mixture mentioned above or onto 0.25× Broughton and Dilworth 1971 medium plates. Subsequently, plants were inoculated with rhizobia and watered with nitrate as described above.
Acetylene reduction assay
ARAs were conducted essentially as described47. The nodulated root from single plants was placed in a 5 ml glass gas chromatography vial. A syringe was used to replace 500 µl air in the vial with 2% acetylene. Samples were incubated at room temperature for 30 min before ethylene quantification using a SensorSense (Nijmegen, NL) ETD-300 ethylene detector operating in sample mode with 2.5 l h−1 flow rate and 6 min detection time. The curve was integrated using the SensorSense valve controller software to calculate the total ethylene production per sample.
Leghaemoglobin content measurement
Leghaemoglobin content measurements were conducted using a spectrophotometric method as described previously41. Fresh nodules from each individual plant were first ground and homogenized in 16-fold volumes of 0.1 M precooled PBS (Na2HPO4:NaH2PO4 buffer at 5 °C, pH 6.8). The resulting slurry was then centrifuged at 12,000g for 15 min prior to assaying the supernatant by spectrophotometry at a wavelength of 540, 520 and 560 nm. The Leghaemoglobin content was calculated from a standard curve using bovine haemoglobin as a protein standard.
GUS staining
Three weeks post-inoculation, hairy roots were put into GUS staining buffer, which contains 0.5 mg ml−1 5-bromo-4-chloro-3-indolyl-β-d-glucuronic acid, 100 mM potassium phosphate buffer (pH 7.0), 10 mM EDTA (pH 8.0), 1 mM potassium ferricyanide, 1 mM potassium ferrocyanide and 0.1% Triton X-100. The roots were incubated at 37 °C overnight. Roots were washed with 70% ethanol twice before image acquisition. Quantitative GUS assays are described below for the trans-activation assays.
Gene expression
For RNA-seq, 3 weeks post-inoculation, plants were acclimatized prior to treatment by submerging in 0.25× Long Ashton liquid medium overnight, then treated with 0 or 10 mM KNO3 for 24 h. Mature nodules were collected. mRNA was isolated using the NucleoSpin RNA Plant kit (Macherey-Nagel) and RNA-seq (PE-150 bp Illumina sequencing) was conducted by Novogene. RNA-seq analysis was performed by mapping reads to the reference transcriptome using Salmon48 and quantification performed using DEseq249. A publicly available timeseries of nitrate-treated nodules17 was obtained from GEO using accession number GSE197362. GO enrichment was performed using GO_MWU with GO terms obtained from https://lotus.au.dk.
For the expression of target genes, RevertAid Reverse Transcriptase (Thermo) was used for the synthesis of first strand cDNA. LightCycler480 instrument and LightCycler480 SYBR Green I master (Roche Diagnostics) were used for quantitative PCR with reverse transcription. Ubiquitin-conjugating enzyme was used as a reference. The cDNA concentration of target genes was calculated using amplicon PCR efficiency calculations using LinRegPCR50. Target genes were compared to the reference for each of 5 biological repetitions (each consisting of 8 to 10 nodules). At least two technical repetitions were performed in each analysis. Primers used are listed in Extended Data Fig. 4b.
Electrophoretic mobility shift assay
The DNA probes with 6-FAM-label at the 5′ end were synthesized by Eurofins and are listed in Extended Data Fig. 5h. We incubated the purified FUN DNA-binding domain (residues 178–237) with the probes at 37 °C for 60 min in EMSA buffer (25 mM Tris-HCl pH 8.0, 80 mM NaCl, 35 mM KCl, 5 mM MgCl2). After incubation, the reaction mixture was electrophoresed in 6% native polyacrylamide gel and then labelled DNA was detected with the Typhoon scanner (Fujifilm). Probes without 6-FAM-label served as competitors, while probes with mutation in the core binding sites (TGACG) served as mutants.
Transient activation assay
Promoters of FUN candidate target genes (NRT2.1, HO1, NRT3.1 and AS1), the glucuronidase (GUS) coding sequence and 35S terminator were cloned into compatible Golden Gate vectors as reporters; while the 35S promoter, FUN coding sequence, eGFP and 35S terminator were cloned as the effector. The reporters and effector were cloned into the p50507 Golden Gate binary vector. These constructs were then transformed into Agrobacterium tumefaciens strain AGL1. These A. tumefaciens were diluted to OD600 = 0.2 and were infiltrated into N. benthamiana leaves. Three days after infiltration, samples of about 20 mg were collected for protein extraction. GUS activities were measured with 4-methylumbelliferyl-β-d-glucuronide as substrate (Sigma-Aldrich) using a Thermo Scientific Varioskan flash. For Zn treatment, 2 days after A. tumefaciens infiltration, N. benthamiana leaves were infiltrated with 500 µM MgCl2 (mock), 500 µM ZnCl2, or 2.5 mM EDTA. GUS activities were measured 1 day after treatments.
Protein production and purification
The FUN sensor domain (residues 244–480) with a 3C-cleavable N-terminal tag consisting of 10 histidines, 7 arginines and a SUMO tag was obtained from GenScript together with a construct of the FUN sensor with the zipper domain (residues 178–480) N-terminally tagged with 7 histidines and a GB1 tag. The plasmids were transformed into Escherichia coli LOBSTR cells51. The expression culture was grown to OD600 = 0.6 in LB medium with 0.1 mg ml−1 ampicillin and 0.034 mg ml−1 chloramphenicol at 37oC and 110 rpm. Cells were cold shocked on ice for 30 min before expression was induced with 0.4 mM IPTG at 18oC overnight. The cells were pelleted (4,400g, 4 °C, 10 min), resuspended in lysis buffer (50 mM Tris-HCl pH 8.0, 500 mM NaCl, 10% glycerol, 10 mM imidazole, 5 mM β-mercaptoethanol and 1 mM benzamidine) and lysed by sonication. The lysate was cleared by centrifugation (30,600g, 4 °C, 30 min), and the proteins were purified from the cleared lysate using a Protino Ni-NTA 5 ml column (Machery-Nagel). The protein was eluted with a high-imidazole buffer (50 mM Tris-Hcl pH 8.0, 250 mM NaCl, 5% glycerol, 500 mM imidazole, 5 mM β-mercaptoethanol). The FUN sensor with zipper was not purified further, while the FUN sensor was dialysed overnight against 50 mM Tris-HCl pH 8.0, 250 mM NaCl, 5% glycerol, 5 mM β-mercaptoethanol with 3C protease in a 1:50 molar ratio. The cleaved tag and the protease were subsequently removed by a second Ni-IMAC step. The FUN sensor was further purified by size-exclusion chromatography on a Superdex 200 Increase 10/300 GL (GE Healthcare) in minimal buffer (10 mM Tris-HCl pH 8.0, 150 mM NaCl, 5 mM β-mercaptoethanol). For SAXS analysis, the FUN sensor was further purified on a ResourceQ 1 ml (GE Healthcare) and eluted with a linear gradient of 10–500 mM NaCl and 10 mM Tris-HCl pH 8.0 and 5 mM β-mercaptoethanol. Eluted fractions were pooled and dialysed against minimal buffer.
DLS and nanoDSF
The FUN protein was analysed on a Prometheus Panta instrument (NanoTemper Technologies) for alterations in thermal unfolding (nanoDSF) and size (DLS) upon addition of ligands. 0.8 mg ml−1 of the purified protein was incubated with 4 mM of different potential ligands or a 0–4 mM ZnCl2 series for 20 min whereupon 5 mM EDTA was added to samples analysed for reversible filamentation. Before addition, ZnCl2 was filtered using VivaSpin MWCO 5 kDa and immediately added to the protein samples. 10 consecutive DLS measurements were performed for each sample at 25 °C with 100% laser power and followed by a nanoDSF experiment measured at a temperature slope of 1 °C/min from 25–90 °C with 100% excitation power. All measurements were performed in triplicates.
SAXS
SAXS measurements were performed at the in-house NanoSTAR instrument at Aarhus University52,53 (Bruker AXS). The instrument uses a Cu rotating anode, has a scatterless pinhole in front of the sample47 and employs a two-dimensional position-sensitive gas detector (Vantec 500, Bruker AXS). The samples and buffer were measured in a homebuilt flow-through capillary. The intensity I(q) is displayed as a function of the modulus of the scattering vector. The buffer scattering was subtracted from the scattering from the samples and the intensities were converted to an absolute scale and corrected for variations in detector efficiency by normalizing to the scattering of pure water46. The data were plotted in Guinier of ln(I(q)) versus q2 to determine the radius of gyration Rg, and an indirect Fourier transformation54,55 was performed to obtain the pair distance-distribution function p(r), which is a histogram of distances between pair of points within the particles weighted by the excess scattering length density at the points. Note that the resolution of the SAXS data is about 400 Å and therefore the overall length of the fibrils induced by zinc is not resolved. The p(r) function is in this case related to the cross-section structure of the filaments.
Negative-stain electron microscopy
For electron microscopy, 0.1 mg ml−1 of the purified FUN sensor domain was incubated 20 min at room temperature with or without 100 µM ZnCl2 and with or without 5 mM EDTA. Samples for negative staining were prepared on 400 copper mesh grids that were manually covered with a collodion support film coated with carbon using a Leica EM SCD 500 High Vacuum Sputter Coater. Before staining, the grids were glow discharged with negative polarity, 25 mA for 45 s, using a PELCO easiGlow glow discharge system. 3 µl of the FUN sensor was deposited on the grid, incubated 30 s, and excess sample was removed from the grid using Whatman paper. After the blotting, the grid was floated 3 times on 2% uranyl formate solution for 15 s and then dried. Negative-staining micrographs were recorded using a Tecnai G2 Spirit microscope operating at 120 kV, equipped with a TemCam-F416 (4kx4k) TVIPS CMOS camera and a Veleta (2kx2k) CCD camera, at EMBION the Danish national cryo-EM facility in Aarhus, Denmark. Micrographs were recorded at a magnification of 42,000× and 52,000×.
Microscopy and confocal imaging
For the FUN expression pattern, the roots after GUS staining were observed by Leica M165FC Fluorescence stereomicroscope. Nodules were embedded in 3% agarose and sectioned in 100-µm slices using a vibratome. Nodule slices were observed by Zeiss Axioplan 2 light microscope. For FUN subcellular locations, Lotus hairy roots and N. benthamiana leaves expressing FUN–GFP were treated with 500 µM ZnCl2 (Zn) or MgCl2 (mock) for 3 days, and fluorescence were observed using a 491–535 nm filter on a Zeiss LSM 710 confocal microscope.
Zinpyr-1 imaging and quantification
Plants with pink nodules (3 weeks post-inoculation) were acclimatized prior to treatment by submerging in 0.25× Long Ashton liquid medium overnight, then treated with 0 or 10 mM KNO3 for 24 h. Mature nodules were embedded in 3% agarose and sectioned in 80-µm slices using a vibratome. Slides were stained with 5 µM Zinpyr-1 for 3 h and rinsed 3 times with water. Fluorescence was observed by Zeiss LSM 710 confocal microscope, using excitation at 488 nm and emission from 505 to 550 nm. Fluorescence densities were quantified by ImageJ.
Micro-XRF
XRF images were acquired at the ID21 beamline of the European Synchrotron Radiation Facility56. The scanning X-ray microscope at ID21 is equipped with a liquid nitrogen passively cooled cryogenic stage. Samples were prepared as described57. In brief, nodules were embedded in OCT medium and cryo-fixed by plunging them into liquid nitrogen-chilled isopentane. 20 mm sections of frozen samples were obtained using a Leica LN22 cryo-microtome and mounted in a liquid nitrogen-cooled sample holder between two Ultralene (Spex SamplePrep) foils. The beam was focused to 0.9 × 0.6 mm2 using Kirkpatrick–Baez mirror optics. The emitted fluorescence signal was detected with an energy-dispersive, large area (80 mm2) SDD detector equipped with a beryllium window (XFlash SGX, RaySpec). Images were acquired at a fixed energy of 9.8 keV by raster-scanning the sample with a step of 2 × 2 mm2 and a 220 ms dwell time. Elemental distribution was calculated with the PyMca software package58.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Online content
Any methods, additional references, Nature Portfolio reporting summaries, source data, extended data, supplementary information, acknowledgements, peer review information; details of author contributions and competing interests; and statements of data and code availability are available at 10.1038/s41586-024-07607-6.
Supplementary information
RNA-seq statistics
Source data
Source Data Figs. 1–4 and Source Data Extended Data Figs. 2–8
Acknowledgements
This work was supported by the project Enabling Nutrient Symbioses in Agriculture (ENSA), which is funded by Bill & Melinda Gates Agricultural Innovations (INV- 57461), the Bill & Melinda Gates Foundation and the Foreign, Commonwealth and Development Office (INV-55767), a Carlsberg Foundation grant (CF21-0139) and the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (grant agreement 834221). The authors thank F. Petersen and M. K. Sørensen for assistance in screening design and plant maintenance and P. Smith for comments on the manuscript. We acknowledge the European Synchrotron Radiation Facility (ESRF) for provision of synchrotron radiation facilities under proposal number EV246 to use beamline ID21.
Extended data figures and tables
Author contributions
J.L., J.S. and D.R. conceived the genetic screen. J.L. conducted the genetic screen, isolated mutants, and conducted plant phenotyping, molecular cloning and gene expression analysis. H.L. performed hairy root experiments. P.K.B., M.V.K., E.P. and E.D. purified proteins and performed biochemical analyses. M.V.K., E.D., T.D. and T.B. performed negative-stain electron microscopy. M.V.K. and J.S.P. performed SAXS analyses. J.L. and S.U.A. identified LORE1 insertions. M.N. performed confocal microscopy. J.L. and D.R. analysed RNA-seq data. J.L., H.C.-M., V.E. and M.G.-G. conducted synchrotron experiments. D.R. and K.R.A. coordinated and supervised the project. J.L., M.V.K., K.R.A. and D.R. wrote the manuscript with inputs from all authors.
Peer review
Peer review information
Nature thanks Rodrigo Gutierrez, Takuya Suzaki and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer review reports are available.
Funding
Open access funding provided by La Trobe University.
Data availability
The main data supporting the findings of this study are available within the article, its Extended Data Figures and supplementary information files. Raw RNA-seq data have been submitted to NCBI under accession PRJNA985805 and processed data with differential expression statistics is available as Supplementary Data File 1. Source data are provided with this paper.
Competing interests
Aarhus University has filed US provisional patent application 63/483,248 authored by J.L., P.K.B., J.S., K.R.A. and D.R. on use of the FUN gene and downstream targets to improve nitrogen fixation in legumes. The other authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Jieshun Lin, Peter K. Bjørk
Contributor Information
Jieshun Lin, Email: jslin@mbg.au.dk.
Kasper R. Andersen, Email: kra@mbg.au.dk
Dugald Reid, Email: dugald.reid@latrobe.edu.au.
Extended data
is available for this paper at 10.1038/s41586-024-07607-6.
Supplementary information
The online version contains supplementary material available at 10.1038/s41586-024-07607-6.
References
- 1.Scheible, W.-R. et al. Genome-wide reprogramming of primary and secondary metabolism, protein synthesis, cellular growth processes, and the regulatory infrastructure of Arabidopsis in response to nitrogen. Plant Physiol.136, 2483–2499 (2004). 10.1104/pp.104.047019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krusell, L., Madsen, L. H., Sato, S. & Aubert, G. Shoot control of root development and nodulation is mediated by a receptor-like kinase. Nature420, 422–426 (2002). 10.1038/nature01207 [DOI] [PubMed] [Google Scholar]
- 3.Nishimura, R. et al. HAR1 mediates systemic regulation of symbiotic organ development. Nature420, 426–429 (2002). 10.1038/nature01231 [DOI] [PubMed] [Google Scholar]
- 4.Searle, I. R. et al. Long-distance signaling in nodulation directed by a CLAVATA1-like receptor kinase. Science299, 109–112 (2003). 10.1126/science.1077937 [DOI] [PubMed] [Google Scholar]
- 5.Okamoto, S. et al. Nod factor/nitrate-induced CLE genes that drive HAR1-mediated systemic regulation of nodulation. Plant Cell Physiol.50, 67–77 (2009). 10.1093/pcp/pcn194 [DOI] [PubMed] [Google Scholar]
- 6.Lin, J.-S. et al. NIN interacts with NLPs to mediate nitrate inhibition of nodulation in Medicago truncatula. Nat. Plants4, 942–952 (2018). 10.1038/s41477-018-0261-3 [DOI] [PubMed] [Google Scholar]
- 7.Tsikou, D. et al. Systemic control of legume susceptibility to rhizobial infection by a mobile microRNA. Science362, 233–236 (2018). 10.1126/science.aat6907 [DOI] [PubMed] [Google Scholar]
- 8.Nishida, H. et al. A NIN-LIKE PROTEIN mediates nitrate-induced control of root nodule symbiosis in Lotus japonicus. Nat. Commun.9, 499 (2018). 10.1038/s41467-018-02831-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu, K.-H. et al. NIN-like protein 7 transcription factor is a plant nitrate sensor. Science377, 1419–1425 (2022). 10.1126/science.add1104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Castaings, L. et al. The nodule inception-like protein 7 modulates nitrate sensing and metabolism in Arabidopsis. Plant J.57, 426–435 (2009). 10.1111/j.1365-313X.2008.03695.x [DOI] [PubMed] [Google Scholar]
- 11.Bustos, R. et al. A central regulatory system largely controls transcriptional activation and repression responses to phosphate starvation in Arabidopsis. PLoS Genet.6, e1001102 (2010). 10.1371/journal.pgen.1001102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nishida, H. & Suzaki, T. Nitrate-mediated control of root nodule symbiosis. Curr. Opin. Plant Biol.44, 129–136 (2018). 10.1016/j.pbi.2018.04.006 [DOI] [PubMed] [Google Scholar]
- 13.Misawa, F. et al. Nitrate transport via NRT2.1 mediates NIN-LIKE PROTEIN-dependent suppression of root nodulation in Lotus japonicus. Plant Cell34, 1844–1862 (2022). 10.1093/plcell/koac046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huault, E. et al. Local and systemic regulation of plant root system architecture and symbiotic nodulation by a receptor-like kinase. PLoS Genet.10, e1004891 (2014). 10.1371/journal.pgen.1004891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Imin, N., Mohd-Radzman, N. A., Ogilvie, H. A. & Djordjevic, M. A. The peptide-encoding CEP1 gene modulates lateral root and nodule numbers in Medicago truncatula. J. Exp. Bot.64, 5395–5409 (2013). 10.1093/jxb/ert369 [DOI] [PubMed] [Google Scholar]
- 16.Yu, H. et al. GmNAC039 and GmNAC018 activate the expression of cysteine protease genes to promote soybean nodule senescence. Plant Cell35, 2929–2951 (2023). 10.1093/plcell/koad129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang, L. et al. A transcription factor of the NAC family regulates nitrate-induced legume nodule senescence. New Phytol.238, 2113–2129 (2023). 10.1111/nph.18896 [DOI] [PubMed] [Google Scholar]
- 18.Fukai, E. et al. Establishment of a Lotus japonicus gene tagging population using the exon-targeting endogenous retrotransposon LORE1. Plant J.69, 720–730 (2012). 10.1111/j.1365-313X.2011.04826.x [DOI] [PubMed] [Google Scholar]
- 19.Urbański, D. F., Małolepszy, A., Stougaard, J. & Andersen, S. U. Genome-wide LORE1 retrotransposon mutagenesis and high-throughput insertion detection in Lotus japonicus. Plant J.69, 731–741 (2012). 10.1111/j.1365-313X.2011.04827.x [DOI] [PubMed] [Google Scholar]
- 20.Małolepszy, A. et al. The LORE1 insertion mutant resource. Plant J.88, 306–317 (2016). 10.1111/tpj.13243 [DOI] [PubMed] [Google Scholar]
- 21.Running, M. P. & Meyerowitz, E. M. Mutations in the PERIANTHIA gene of Arabidopsis specifically alter floral organ number and initiation pattern. Development122, 1261–1269 (1996). 10.1242/dev.122.4.1261 [DOI] [PubMed] [Google Scholar]
- 22.Maier, A. T., Stehling-Sun, S., Offenburger, S.-L. & Lohmann, J. U. The bZIP transcription factor PERIANTHIA: a multifunctional hub for meristem control. Front. Plant Sci.2, 79 (2011). 10.3389/fpls.2011.00079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dröge-Laser, W., Snoek, B. L., Snel, B. & Weiste, C. The Arabidopsis bZIP transcription factor family—an update. Curr. Opin. Plant Biol.45, 36–49 (2018). 10.1016/j.pbi.2018.05.001 [DOI] [PubMed] [Google Scholar]
- 24.Tomaž, Š., Gruden, K. & Coll, A. TGA transcription factors—structural characteristics as basis for functional variability. Front. Plant Sci.13, 935819 (2022). 10.3389/fpls.2022.935819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nielsen, R. et al. A scan for positively selected genes in the genomes of humans and chimpanzees. PLoS Biol.3, e170 (2005). 10.1371/journal.pbio.0030170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang, L. et al. CRISPR/Cas9 knockout of leghemoglobin genes in Lotus japonicus uncovers their synergistic roles in symbiotic nitrogen fixation. New Phytol.224, 818–832 (2019). 10.1111/nph.16077 [DOI] [PubMed] [Google Scholar]
- 27.Zhou, Y. et al. Heme catabolism mediated by heme oxygenase in uninfected interstitial cells enables efficient symbiotic nitrogen fixation in Lotus japonicus nodules. New Phytol.239, 1989–2006 (2023). 10.1111/nph.19074 [DOI] [PubMed] [Google Scholar]
- 28.Bartlett, A. et al. Mapping genome-wide transcription-factor binding sites using DAP-seq. Nat. Protoc.12, 1659–1672 (2017). 10.1038/nprot.2017.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Trepreau, J. et al. Structural basis for metal sensing by CnrX. J. Mol. Biol.408, 766–779 (2011). 10.1016/j.jmb.2011.03.014 [DOI] [PubMed] [Google Scholar]
- 30.Sinclair, S. A., Sherson, S. M., Jarvis, R., Camakaris, J. & Cobbett, C. S. The use of the zinc-fluorophore, Zinpyr-1, in the study of zinc homeostasis in Arabidopsis roots. New Phytol.174, 39–45 (2007). 10.1111/j.1469-8137.2007.02030.x [DOI] [PubMed] [Google Scholar]
- 31.Ivanov, P., Kedersha, N. & Anderson, P. Stress granules and processing bodies in translational control. Cold Spring Harb. Perspect. Biol.11, a032813 (2019). 10.1101/cshperspect.a032813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boija, A. et al. Transcription factors activate genes through the phase-separation capacity of their activation domains. Cell175, 1842–1855.e16 (2018). 10.1016/j.cell.2018.10.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morelli, C. et al. RNA modulates hnRNPA1A amyloid formation mediated by biomolecular condensates. Nat. Chem.10.1038/s41557-024-01467-3 (2024). [DOI] [PMC free article] [PubMed]
- 34.Peran, I. & Mittag, T. Molecular structure in biomolecular condensates. Curr. Opin. Struct. Biol.60, 17–26 (2020). 10.1016/j.sbi.2019.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goetz, S. K. & Mahamid, J. Visualizing molecular architectures of cellular condensates: hints of complex coacervation scenarios. Dev. Cell55, 97–107 (2020). 10.1016/j.devcel.2020.09.003 [DOI] [PubMed] [Google Scholar]
- 36.Lilay, G. H. et al. Arabidopsis bZIP19 and bZIP23 act as zinc sensors to control plant zinc status. Nat. Plants7, 137–143 (2021). 10.1038/s41477-021-00856-7 [DOI] [PubMed] [Google Scholar]
- 37.Alvarez, J. M. et al. Systems approach identifies TGA1 and TGA4 transcription factors as important regulatory components of the nitrate response of Arabidopsis thaliana roots. Plant J.80, 1–13 (2014). 10.1111/tpj.12618 [DOI] [PubMed] [Google Scholar]
- 38.Ruffel, S. et al. Genome-wide analysis in response to nitrogen and carbon identifies regulators for root AtNRT2 transporters. Plant Physiol.186, 696–714 (2021). 10.1093/plphys/kiab047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kumar, S. et al. Structural basis of NPR1 in activating plant immunity. Nature605, 561–566 (2022). 10.1038/s41586-022-04699-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Puppo, A. et al. Legume nodule senescence: roles for redox and hormone signalling in the orchestration of the natural aging process. New Phytol.165, 683–701 (2005). 10.1111/j.1469-8137.2004.01285.x [DOI] [PubMed] [Google Scholar]
- 41.Jiang, S. et al. NIN-like protein transcription factors regulate leghemoglobin genes in legume nodules. Science374, 625–628 (2021). 10.1126/science.abg5945 [DOI] [PubMed] [Google Scholar]
- 42.Kamal, N. et al. Insights into the evolution of symbiosis gene copy number and distribution from a chromosome-scale Lotus japonicus Gifu genome sequence. DNA Res.27, dsaa015 (2020). 10.1093/dnares/dsaa015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mun, T., Bachmann, A., Gupta, V., Stougaard, J. & Andersen, S. U. Lotus Base: an integrated information portal for the model legume Lotus japonicus. Sci. Rep.6, 39447 (2016). 10.1038/srep39447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Emms, D. M. & Kelly, S. SHOOT: phylogenetic gene search and ortholog inference. Genome Biol.23, 85 (2022). 10.1186/s13059-022-02652-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Letunic, I. & Bork, P. Interactive Tree Of Life (iTOL) v5: an online tool for phylogenetic tree display and annotation. Nucleic Acids Res.49, W293–W296 (2021). 10.1093/nar/gkab301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stougaard, J., Abildsten, D. & Marcker, K. A. The Agrobacterium rhizogenes pRi TL-DNA segment as a gene vector system for transformation of plants. Mol. Gen. Genet.207, 251–255 (1987). 10.1007/BF00331586 [DOI] [Google Scholar]
- 47.Lin, J., Roswanjaya, Y. P., Kohlen, W., Stougaard, J. & Reid, D. Nitrate restricts nodule organogenesis through inhibition of cytokinin biosynthesis in Lotus japonicus. Nat. Commun.12, 6544 (2021). 10.1038/s41467-021-26820-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Patro, R., Duggal, G., Love, M. I., Irizarry, R. A. & Kingsford, C. Salmon provides fast and bias-aware quantification of transcript expression. Nat. Methods14, 417–419 (2017). 10.1038/nmeth.4197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol.15, 550 (2014). 10.1186/s13059-014-0550-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ramakers, C., Ruijter, J. M., Deprez, R. H. L. & Moorman, A. F. M. Assumption-free analysis of quantitative real-time polymerase chain reaction (PCR) data. Neurosci. Lett.339, 62–66 (2003). 10.1016/S0304-3940(02)01423-4 [DOI] [PubMed] [Google Scholar]
- 51.Andersen, K. R., Leksa, N. C. & Schwartz, T. U. Optimized E. coli expression strain LOBSTR eliminates common contaminants from His-tag purification. Proteins81, 1857–1861 (2013). 10.1002/prot.24364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pedersen, J. S. A flux- and background-optimized version of the NanoSTAR small-angle X-ray scattering camera for solution scattering. J. Appl. Crystallogr.37, 369–380 (2004). 10.1107/S0021889804004170 [DOI] [Google Scholar]
- 53.Lyngsø, J. & Pedersen, J. S. A high-flux automated laboratory small-angle X-ray scattering instrument optimized for solution scattering. J. Appl. Crystallogr.54, 295–305 (2021). 10.1107/S1600576720016209 [DOI] [Google Scholar]
- 54.Glatter, O. A new method for the evaluation of small-angle scattering data. J. Appl. Crystallogr.10, 415–421 (1977). 10.1107/S0021889877013879 [DOI] [Google Scholar]
- 55.Pedersen, J. S., Hansen, S. & Bauer, R. The aggregation behavior of zinc-free insulin studied by small-angle neutron scattering. Eur. Biophys. J.22, 379–389 (1994). 10.1007/BF00180159 [DOI] [PubMed] [Google Scholar]
- 56.Cotte, M. et al. The ID21 X-ray and infrared microscopy beamline at the ESRF: status and recent applications to artistic materials. J. Anal. At. Spectrom.32, 477–493 (2017). 10.1039/C6JA00356G [DOI] [Google Scholar]
- 57.Escudero, V. et al. Medicago truncatula Ferroportin2 mediates iron import into nodule symbiosomes. New Phytol.228, 194–209 (2020). 10.1111/nph.16642 [DOI] [PubMed] [Google Scholar]
- 58.Solé, V. A., Papillon, E., Cotte, M., Walter, P. & Susini, J. A multiplatform code for the analysis of energy-dispersive X-ray fluorescence spectra. Spectrochim. Acta B62, 63–68 (2007). 10.1016/j.sab.2006.12.002 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
RNA-seq statistics
Source Data Figs. 1–4 and Source Data Extended Data Figs. 2–8
Data Availability Statement
The main data supporting the findings of this study are available within the article, its Extended Data Figures and supplementary information files. Raw RNA-seq data have been submitted to NCBI under accession PRJNA985805 and processed data with differential expression statistics is available as Supplementary Data File 1. Source data are provided with this paper.