Abstract
This study investigates the effects of selected PGPB on lettuce growth performance under heat-stress conditions. Bacterial plant growth-promoting potentials have been characterized and identified successfully in ongoing studies. Based on in vitro plant growth-promoting potential, the top five bacteria were ranked and identified as Acinetobacter sp. GRB12, Bacillus sp. GFB04, Klebsiella sp. LFB06, Klebsiella sp. GRB10, and Klebsiella sp. GRB04. They were mixed to inoculate on lettuce (Lactuca sativa L.) in temperature-controlled greenhouses. Another in-vivo chamber experiment was conducted by using Bacillus sp. GFB04 and Klebsiella sp. GFB10. Plant physiological traits (chlorophyll fluorescence and transpiration) and nutrient contents were measured at harvest, along with growth, development, and yield component analyses. Uninoculated plants under heat-stress condition showed poor growth performance. In contrast, plants with PGPB inoculation showed improved growth under heat-stress conditions, as the uptake of nutrients was facilitated by the symbionts. Inoculation also improved lettuce photosystem II efficiency and decreased total water use under heat stress. In conclusion, the current study suggests that PGPB inoculation successfully enhances lettuce heat-tolerance. PGPB application could potentially help improve sustainable production of lettuce with less fertilization under increasing temperatures.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12298-024-01470-5.
Keywords: Heat-stress tolerance, Lettuce, Nutrient content, Photosystem II efficiency, Stomatal conductance, Transpiration
Introduction
Abnormal air temperature warming could lead to heat stress on existing plants and crops (Fahad et al. 2017). Heat stress affects plants by reducing photosynthetic capacity, membrane permeability, enzyme activity, and other metabolic functions (Bita and Gerats 2013). Heat stress causes physiological responses, such as increasing stomatal conductance and leaf temperature, thus, reducing plant growth, development, and further production for crops (Khan et al. 2020b). Therefore, the harmful effects of the increasing temperature under heat-stress conditions should be addressed for the sustainable production of crops.
Previously published climate change reports have predicted that the effects of global warming are most notable in developing countries in tropical and semi-tropical regions (IPCC 2021). The increased atmospheric temperature could affect lettuce production through water relations, transpiration, and water-use efficiency (WUE) (Sadok et al. 2021; Bita and Gerats 2013). Recently, studies indicated significant growth in the rate of plant transpiration due to heat stress. The trade-off was raised between the requirement for latent cooling driven by excessively high temperatures and the need to conserve water due to the rising demand for evaporation (Sadok et al. 2021). The production could be decreased due to plant disorders and diseases caused by high temperatures (Bisbis et al. 2018). The Ricardian method has been used for estimating the impacts of climate change in a review (Mendelsohn and Massetti 2017). The model predicted that net farm revenue would decline by 8–12% if global average temperatures rise by 2 °C and a 7% increase in precipitation. The changes would be beneficial in cold regions but disadvantageous in warm regions in agriculture. Globally, the air temperature has increased by 1.5 °C compared to the preindustrial period (IPCC 2021). Furthermore, in considerable regions of agricultural production around the world, a 2 °C increase in temperature has been recorded and threatened the productivity of crops (Cohen et al. 2021).
Plant growth-promoting bacteria (PGPB) are the bacteria that provide plant growth-promoting (PGP) traits to the host plants. PGPB colonize the rhizosphere around the root area of the plants and provide beneficial influence, such as preventing the plants from being infected by pathogens and impaired by abiotic stresses through multiple mechanisms (Porter et al. 2020; Rho et al. 2018b; Bhardwaj et al. 2017; Yang et al. 2009). In addition, the bacteria often establish a close symbiotic relationship within the plants, developing endophytic associations, which also successfully promote plant growth with PGP traits (Omomowo and Babalola 2019).
PGPB promotes plant growth by facilitating nutrient uptake by biological nitrogen fixation, production of siderophores, solubilization of phosphate, production of plant growth hormones, enhancement of nutrient mobilization, and improvement of soil structure (Bruno et al. 2020; Kumar and Verma 2018; Gouda et al. 2018; Dhawi et al. 2015). In addition, regulation of 1-aminocyclopropane-1-carboxylate (ACC) deaminase and phytohormones biosynthesized by PGPB can alleviate abiotic stress and suppress a range of pathogens without any negative impacts on plant growth (Souza et al. 2015). Under abiotic stress conditions, PGPB can survive through different mechanisms, such as heat shock proteins (HSPs) synthesis and biofilm formation (Mahapatra et al. 2022; Wang et al. 2023). PGPB can also produce antimicrobial compounds and cell wall degrading enzymes (CWDEs) when plants suffer from biotic stresses (Wang et al. 2023).
Heat stress causes adverse effects on plants, while PGPB inoculation is found to confer abiotic stress tolerance to the host plants. A study (Khan et al. 2020a) found that the inoculation of Bacillus cereus SA1 on soybean plants showed a significant increase in plant growth under heat-stress condition. SA1 was able to strengthen different reactive oxygen species (ROS) scavenging activity, such as increases in antioxidant superoxide dismutase (SOD) activity, ascorbate peroxidase (APX) activity, and glutathione (GSH) contents, eventually resulting in decreases in malondialdehyde (MDA) (Khan et al. 2020a). PGPB could help the plants to up-regulate HSPs linked to thylakoid membranes, thus protecting the electron transport chain of the photosystem II (PSII) complex under heat stress. Chlorophyll a, chlorophyll b, and carotenoid contents were observed to be decreased when the plants suffered from heat stress. PGPB inoculation increased the contents of these photosynthetic pigments (Khan et al. 2020b).
In this study, lettuce (Lactuca sativa L.) was used as a model plant of a cool-season crop. The bacteria used in this study were endophytes isolated from Welsh onion (Allium fistulosum L.) which were well-described in Wang et al. (2023). Our study aims to (1) characterize the Welsh onion endophytic PGPB, (2) evaluate the PGP traits of the selected PGPB in vitro, (3) examine the effects of the PGPB on lettuce growth performance and yield under heat-stress condition, study the effects of the PGPB on the physiological traits, nutrition contents, and growth and developmental performance of lettuce. We hypothesized that the thermotolerance of the selected PGPB strains demonstrated by the in-vitro assays is related to the symbiotic performance of the PGPB strains in providing heat-tolerance to the host, and the PGPB inoculations promote plant growth with increased biomass, increased nutrient contents, and less water loss under heat-stress condition.
Materials and methods
The experiments were separated into three parts. First, the thermo-tolerant bacteria was screened out for their PGP traits under heat-stress conditions. Second, the seeds were inoculated with the best performing select strains to determine whether they harmed the host plant in the following in-vivo experiments. Bacterial consortium inoculation and single-strain inoculations were applied in two separate experiments with different environmental settings.
In vitro characterization of thermo-tolerant plant growth promoting bacteria (PGPB)
Information about bacteria isolates
The isolation and identification of the bacteria isolates were according to and well-described in Wang et al. (2023). More than 100 bacteria strains were extracted and isolated from the plant soils, roots, stalks, and leaves of Welsh onion. The genomic DNA of bacteria was extracted from Welsh onion’s plant tissues (roots, stalks, and leaves) and soils using Genomic DNA Spin Kit for blood, bacteria, and cultured cells (Bioman Scientific Co., Ltd., New Taipei City, Taiwan). Polymerase chain reactions (PCR) amplification was processed through a bacteria-specific primer pair 27F (5’ AGAGTTTATCMTGGCTCAG-3’) and 1492R (5’-GRTACCTTGTTACGACTT-3’) (Bioman Scientific Co., Ltd.) to amplify the 16S rRNA gene of bacteria (Liu et al. 2020). A 100-bp band was observed from 1.5% agarose through electrophoresis. PCR was conducted according to the subsequent parameters: initial denaturation at 95 °C for 5 min, followed by 30 cycles comprising denaturation at 95 °C for 1 min, annealing at a temperature range of 50–58 °C for 1 min, and extension at 72 °C for 1.5 min, concluded with a final extension step at 72 °C for 7 min (Wang et al. 2023). The PCR products were sequenced by the Genomics sequencing laboratory (Genomics, New Taipei City, Taiwan). The amplified and sequenced 16S rRNA genes were identified by comparing the sequences’ similarity with the NCBI database using the BLAST search tool (https://blast.ncbi.nlm.nih.gov/Blast.cgi). Based on the preliminary data, 18 bacteria strains were identified and used as experimental materials. These 18 strains were stored at − 80 °C until examination. The freeze-preserved isolates were thawed and propagated on Petri dishes to be further examined for the PGP traits in vitro.
Characterization and screening of bacteria
In-plates, bioassays were conducted to examine the potential PGP traits under different temperature regimes. All the bacteria strains were first examined for their thermotolerance. The isolates on lysogeny broth (LB) medium (Sigma-Aldrich, St. Louis, United States) in the solid state were grown at 25 °C, 30 °C, 35 °C, and 40 °C in a growth chamber to determine their growth abilities under normal and high temperatures. All treatments were exposed to an average of 24 h / 0 h (day/night) fluorescence light, relative humidity of 80 (± 1) %, and daily light integral (DLI) of 19.0 mol m−2 d−1. The general growth behaviors were recorded and evaluated to document the thermotolerance. Then, under the same temperature settings, we conducted the bioassays to qualify and quantify PGP traits: biological nitrogen fixation (BNF), siderophore production, and phosphate solubilization assays. The BNF trait was indirectly assayed by observing morphological growth characteristics of PGPB strains propagated on the Qubit System’s nitrogen-free medium (NFM) (Doty et al. 2009). The siderophore production ability was evaluated by measuring the growth of bacteria on chrome azurol S (CAS) agar medium (Sigma-Aldrich, St. Louis, United States) (Schwyn and Neilands 1987). The CAS reagent and Fe form the blue color of the agar. The color change from blue to orange implies that Fe has been removed from the CAS reagent. The phosphate solubilization ability was examined by inoculating bacteria strains on Pikovskaya medium (PVK medium, HIMEDIA, Mumbai, India) (Franco-Correa et al. 2010). The appearance of the halo zone around the colony after seven days shows the solubilization of immobile phosphate, which is attributed to the production of acids from the strains. After the characterization, the top five isolates showing the highest degree of PGP traits were selected.
Criteria of bacteria strain selection
The top five strains were selected according to their PGP scores. The scores were based on the strains’ growth performance on different media. Qualitative assays were done on LB and NFM agar to see if the strains could grow on those media. Score 1 was given to see if they could grow; quantitative assays were done on CAS and PVK media to observe their quantitative growth performance. The scores could be given up to 3 based on the measurement method on each medium. For the CAS medium, the diameters of the orange (discolored) zones were measured; for the PVK medium, the diameters of the halo zones were measured. The strains could get up to the maximum score of 8 for each temperature test. The bioassays were tested at four growing temperatures: 25 °C, 30 °C, 35 °C, and 40 °C, in a growth chamber. Since the characterization and screening processes aimed at identifying the thermo-tolerant strains, a weighting system was built to have more complete results. The scores in 35 °C and 40 °C had different weights since those temperatures are considered heat-stress conditions for bacteria to grow. The scores at 25 °C and 30 °C remained one time, while 35 °C and 40 °C increased to 1.5 times and two times, respectively.
In vivo characterization of the lettuce host plant with the PGPB inoculation
Two in vivo inoculation experiments were conducted in different environmental conditions: a greenhouse and a growth chamber. The treatment design was also different; the greenhouse experiment used a bacterial consortium as an inoculum, while the growth chamber experiment used single-strain inoculations. Both experiments measured the same parameters to examine the physical and physiological characteristics of the host plant lettuce.
Experimental design in greenhouse
The plant material used in this experiment is a romaine-type lettuce variety (Romaine LE4712, Suntech Seed, Tainan City, Taiwan) in Taiwan, a slightly heat-tolerant variety bred by a local seed company. It was selected as the plant material to grow in a precision temperature-regulated Venlo-type glass greenhouse facility at National Taiwan University. The plants were grown in pots (width × length × height: 12 cm × 12 cm × 10 cm) with a peat moss planting medium (Professional Grow Boards, Kekkilä Professional, Vantaa, Finland). There were four treatments (2 × 2 factorial design) to examine the effects of the selected PGPB on the plant performance under heat-stress conditions and PGPB inoculation: (1) non-inoculated controls without heat stress, (2) non-inoculated controls with heat stress, (3) PGPB inoculated without heat stress, (4) PGPB inoculated with heat stress. Optimal temperature treatments were set to 20/15 °C (d/n), while heat-stress treatments were set to 30/25 °C (d/n). All treatments were exposed to 12 h/12 h (d/n) light and 80 (± 1) % relative humidity. The actual environmental data, air temperature, light intensity, and relative humidity, were recorded by HOBO data loggers (Pendant UA, Onset Computer Corporation, Bourne, MA, United States). In the 20/15 °C room, the average temperature, DLI, and relative humidity were 24.3/16.4 °C, 18.1 mol m−2 d−1, and 80 (± 1) %, respectively. While in the 30/25 °C room, the average temperature, DLI, and relative humidity were 32.3/25.3 °C, 13.1 mol m−2 d−1, and 80 (± 1) %, respectively. Each treatment consisted of 10 replicates. The seeds were grown in a seed tray for seven days and then transplanted into the pots. Pots were placed randomly according to the split-plot design of heat-stress treatment blocks as main plots within which PGPB treatment blocks were assigned as sub-plots. This greenhouse experiment was conducted over 45 days.
Experimental design in growth chamber
The same romaine-type lettuce variety was selected as the plant material to grow in a growth chamber (F-740, Hi-Point, Kaohsiung City, Taiwan) with a white light LED plate (LED SUN LIGHT Z1 White, Hi-Point, Kaohsiung City, Taiwan). The lettuce seeds were grown in pots (width × length × height: 12 cm × 12 cm × 10 cm) with a peat moss planting medium (Professional Grow Boards, Kekkilä Professional, Vantaa, Finland). There were six treatments (3 × 2 factorial design) to examine the effects of the selected PGPB on the plant performance under heat-stress conditions and PGPB inoculation: (1) non-inoculated controls without heat stress, (2) Bacillus sp. GFB04 inoculation without heat stress, (3) Klebsiella sp. GRB10 inoculation without heat stress, (4) non-inoculated controls with heat stress, (5) Bacillus sp. GFB04 inoculation with heat stress, (6) Klebsiella sp. GRB10 inoculation with heat stress. Optimal temperature treatments were set at 20/15 °C (d/n), while heat-stress treatments were set at 30/25 °C (d/n). All treatments were exposed to 12 h/12 h (d/n) light and 65 (± 1) % relative humidity. The environmental data, including temperature, light intensity, and air humidity, was recorded by HOBO data loggers (Pendant UA, Onset Computer Corporation, Bourne, MA, United States). In optimal temperature, the average temperature, DLI, and relative humidity were 22.1/16.0 °C, 46.9 mol m−2 d−1, and 65 (± 1) %, respectively. While in the 30/25 °C settings, the average temperature, DLI, and relative humidity were 32.4/24.2 °C, 46.9 mol m−2 d−1, and 65 (± 1) %, respectively. Each treatment consisted of 10 replicates. The seeds were grown in a seed tray for seven days and then transplanted into the pots. Pots were placed randomly according to the split-plot design of heat-stress treatment blocks as main plots within which PGPB treatment blocks were assigned as sub-plots. This chamber experiment was conducted over 30 days.
Biomass measurement
The fresh weight and dry weight of the shoot and root were measured. The biomass calculation compared the wet and dry weight of the whole crop before and after drying using an 80 °C oven for 48 h. The growth performance of the plants was measured at harvest.
Nutrient content analysis
A plant nutrients test was conducted to investigate the content of nutrients (N, P, K, Ca, Mg, and Fe) within the plant. After measuring the biomass, the dried samples’ nutrients were analyzed. The dried samples were ground into fine powders for acid digestion, and 0.25 g of dried samples were weighed using different treatments. The weighed samples were added with sulfuric acid (H2SO4) and hydrogen peroxide. An acid digester (Multiwave 5000, Anton Paar, Graz, Austria) then digested the mixtures until the mixtures became transparent. The transparent mixtures were filtrated and diluted. Finally, the filtrates were tested with continuous fluid analysis (CFA, Proxima, DKSH, Switzerland) and atomic absorption spectroscopy (AAS, 230ATS, Buck Scientific, Norwalk, CT, United States).
Physiological traits analysis
A porometer and pulse-amplitude modulation (PAM) fluorometer (LI-600, LI-COR Biosciences, Lincoln, Nebraska, United States) was used to measure the physiological data, which include stomatal conductance to water vapor (gs), transpiration (E), and chlorophyll fluorescence. Using the chlorophyll fluorescence parameters, the maximum quantum efficiency of PSII (Fv/Fm) of the dark-adapted leaf and the quantum efficiency of PSII (ΦPSII) of the light-adapted leaf were calculated.
Inoculation processes
The seed inoculation method was used in this experiment (Rho et al. 2018b). The selected strains were cultured in an LB (liquid) medium and placed inside an orbital shaker for 48 h. The concentration of each strain was then measured using a spectrophotometer (Biochrom, Cambridge, United Kingdom) at OD600. The OD600 of each strain was set to 0.1, approximately equal to 1 × 108 colony forming units (CFUs)/mL in the starting sample (Rutten et al. 2019). Then, the bacterial suspension was filled with LB (liquid) medium. The lettuce seeds were first imbibed to screen out good-quality seeds. Next, the surface of the seeds was sterilized with 2.5% NaClO for 10 min and rinsed three times using sterile deionized distilled water. The sterilized seeds were then placed in 1) bacteria suspension (filled with LB medium) and 2) LB (liquid) medium. The media were then put inside an orbital shaker for two hours for the inoculation. These inoculation processes were applied to the entire study.
Data analysis
All data were analyzed by the IBM SPSS Statistics 22 (IBM Corp., Armonk, N.Y., USA). Two-way analysis of variance (ANOVA) was used to examine the effects of heat stress, PGPB treatments, and their interactions on the performance of the physiology, nutrient uptake, and growth of the plants. Both heat stress and PGPB were set to fixed effects and analyzed using a fixed-effect model. Once the main effects were significant, we used Tukey’s HSD method to separate the group differences. For the growth chamber experiment, contrast matrix analysis was used for comparing means with significant differences between control vs inoculated plants, control vs BsGFB04, control vs KsGRB10, and BsGFB04 vs KsGRB10.
Results
In vitro symbiotic characterization identified five thermotolerant PGPB candidates
The results of 18-strain bioassays are shown in the tables, which are the temperature growth response (Table S1), nitrogen fixation (Table S2), phosphate solubilization (Table S3), and siderophore production (Table S4). Qualitative assays were conducted for the strains’ survival and BNF, while quantitative assays were conducted for their phosphate solubilization and siderophore production. For the phosphate solubilization assay, Klebsiella sp. GRB04 showed the best performance under 25 °C and 30 °C, and Klebsiella sp. GRB10 performed the best under 35 °C and 40 °C. Klebsiella sp. GRB10 and Klebsiella sp. LFB06 got the highest scores for the siderophore production assay. In addition, Klebsiella sp. GRB10 had the best performance in different assays under 35 °C and 40 °C. On the other hand, Microbacterium sp. GFB06 got the lowest scores since it did not perform in vitro PGP traits even if it survived under 40 °C. After summarizing and calculating the scores, the results showed that the top five bacteria were identified and ranked as Klebsiella sp. GRB10, Klebsiella sp. GRB04, Klebsiella sp. LFB06, Acinetobacter sp. GRB12, and Bacillus sp. GFB04 (Table S5). They were selected as a bacterial consortium or single strains as inoculum for inoculations on lettuce in the experiment following in vivo experiments.
In vivo characterization of the lettuce host plant with the PGPB inoculation
Assessing biomass changes due to temperature and inoculation
In the greenhouse experiment, the investigation of growth parameters (Table 1) showed that the temperature was the main factor leading to worse growth. The plants grown under heat stress conditions resulted in a significant biomass decrease compared to those under optimal temperature conditions. The heat stress caused a significant change in all measured growth parameters (P < 0.001, Table 1). Average shoot fresh weight (FW) under optimal temperature conditions was 9.70 g and 7.84 g, whereas that under high temperature conditions was 2.42 g and 4.23 g for control and bacteria-inoculated (BI) plants, respectively, a total 62% decrease by the heat stress treatment. The heat stress decreased the average total FW and dry weight (DW) by 63% and 65%, respectively. Inoculation resulted in a significant increase in root FW and DW (P < 0.05). The inoculation treatment could only increase the root FW and DW under optimal temperature conditions by 39% and 44%, respectively, while not affecting the shoot and root biomass under heat stress conditions.
Table 1.
Effects of bacterial consortium inoculation on the growth parameters (biomass of shoot and root) of lettuce under optimum temperature (20/15 °C, d/n) and heat stress (30/25 °C) conditions
| Treatments | Shoot | Root | Total | ||||
|---|---|---|---|---|---|---|---|
| TEMP | INOC | FW (g) | DW (g) | FW (g) | DW (g) | FW (g) | DW (g) |
| Optimum | Control | 9.70 ± 0.45 | 1.23 ± 0.78 | 3.91 ± 0.16 | 0.44 ± 0.01 | 13.61 ± 0.61 | 1.68 ± 0.06 |
| BI | 7.84 ± 1.07 | 1.12 ± 0.25 | 5.42 ± 0.37* | 0.63 ± 0.04* | 13.26 ± 1.43 | 1.76 ± 0.18 | |
| HT | Control | 2.42 ± 1.03 | 0.35 ± 0.18 | 1.50 ± 0.22 | 0.14 ± 0.08 | 3.92 ± 1.21 | 0.50 ± 0.15 |
| BI | 4.23 ± 1.09 | 0.56 ± 0.25 | 1.85 ± 0.44 | 0.17 ± 0.05 | 6.08 ± 1.53 | 0.72 ± 0.18 | |
| Treatment effects | |||||||
| TEMP, df = 1 | 33.107*** (0.000) | 39.066*** (0.000) | 88.44*** (0.000) | 107.139*** (0.000) | 45.629*** (0.000) | 54.306*** (0.000) | |
| INOC, df = 1 | 0.001 (0.978) | 0.099 (0.761) | 8.617* (0.019) | 9.341* (0.016) | 0.526 (0.489) | 0.973 (0.353) | |
| TEMP × INOC, df = 1 | 3.744° (0.089) | 1.887 (0.207) | 3.343 (0.105) | 5.061° (0.055) | 1.004 (0.346) | 0.272 (0.616) | |
Data are means (± SE) of three replicates, and means with the *P < 0.05 in the same temperature treatment when inoculation effects are significantly different according to one-way ANOVA. F-values from two-way ANOVA are given together with P-values in brackets. °, P < 0.1 (highlighted in italics); *P < 0.05; **P < 0.01; ***P < 0.001 (highlighted in bold)
FW fresh weight, DW dry weight, TEMP temperature treatment, INOC endophyte inoculation treatment, Control control treatment, BI bacterial consortium inoculation, HT heat stress treatment, HT + BI heat stress treatment with bacterial consortium inoculation
Furthermore, in the chamber experiment, the investigation of growth parameters (Table 4) showed that high temperature significantly decreased the shoot and root FW and DW. The heat stress caused a significant change in all measured growth parameters (P < 0.001). On the other hand, the inoculations resulted in a marginally significant change in all measured growth parameters (P < 0.001) except the root FW. In addition, significant interaction effects were observed in Temp. × Inoc. on all measured growth parameters (P < 0.001 or P < 0.05). The inoculation treatments significantly increased FW and DW of the lettuce shoot and root under heat stress conditions while showing no effects under optimal temperature conditions. In the contrast-matrix analysis, BsGFB04 did not increase the plant’s biomass under heat stress conditions compared to the control group. In contrast, KsGRB10 increased all measured growth parameters except the root FW. The biomass gained in the KsGRB10 group was more significant than the BsGFB04 group. KsGRB10 showed the highest biomass in shoot and root, which increased the total FW and DW by 43% and 63%, respectively.
Table 4.
Effects of sole bacterial inoculation with Bacillus sp. GFB04 and Klebsiella sp. GRB10 on the growth parameters (biomass of shoot and root) of lettuce under optimum temperature (20/15 °C, d/n) and heat stress (30/25 °C, d/n) conditions
| Treatments | Shoot | Root | Total | ||||
|---|---|---|---|---|---|---|---|
| TEMP | INOC | FW (g) | DW (g) | FW (g) | DW (g) | FW (g) | DW (g) |
| Optimum | Control | 23.94 ± 0.92 | 2.28 ± 0.09 | 6.30 ± 0.18 | 0.52 ± 0.02 | 30.24 ± 0.99 | 2.81 ± 0.10 |
| BsGFB04 | 22.51 ± 0.78 | 2.23 ± 0.09 | 5.64 ± 0.38 | 0.49 ± 0.04 | 28.14 ± 1.07 | 2.72 ± 0.09 | |
| KsGRB10 | 24.02 ± 1.02 | 2.42 ± 0.11 | 5.45 ± 0.30 | 0.48 ± 0.02 | 29.47 ± 1.18 | 2.90 ± 0.13 | |
| HT | Control | 18.90 ± 0.38b | 1.68 ± 0.07b | 3.56 ± 0.10b | 0.25 ± 0.01c | 22.46 ± 0.40b | 1.92 ± 0.07c |
| BsGFB04 | 20.77 ± 0.59b | 1.80 ± 0.06b | 3.88 ± 0.14b | 0.35 ± 0.01b | 24.66 ± 0.59b | 2.15 ± 0.05b | |
| KsGRB10 | 23.67 ± 0.98a | 2.30 ± 0.06a | 4.90 ± 0.18a | 0.45 ± 0.02a | 28.58 ± 1.13a | 2.74 ± 0.07a | |
| Treatment effects | |||||||
| TEMP, df = 1 | 12.848*** (.001) | 33.926*** (.000) | 77.831*** (.000) | 65.521*** (.000) | 27.892*** (.000) | 54.879*** (.000) | |
| INOC, df = 2 | 5.491** (.007) | 12.893*** (.000) | 1.614 (.208) | 6.781** (.002) | 5.305** (.005) | 15.454*** (.000) | |
| TEMP × INOC, df = 2 | 4.420* (.017) | 4.501* (0.016) | 11.022*** (.000) | 15.631*** (.000) | 6.829** (.002) | 8.618*** (.001) | |
| Contrasts | |||||||
| Control vs Inoculated | 2.482 (.121) | 4.238* (.044) | 0.017 (.898) | 3.396 (.070) | 1.58 (.214) | 4.679* (.035) | |
| Control vs BsGFB04 | 0.058 (.811) | 0.089 (.767) | 0.146 (.704) | 0.684 (.413) | 0.002 (.966) | 0.227 (.637) | |
| Control vs KsGRB10 | 5.651* (.023) | 11.816*** (.001) | 0.444 (.509) | 5.083* (.030) | 4.197* (.047) | 10.981** (.002) | |
| BsGFB04 vs KsGRB10 | 6.594* (.014) | 12.690*** (.001) | 1.550 (.221) | 2.543 (.119) | 5.938* (.020) | 12.211*** (.001) | |
Data are means (± SE) of ten replicates, and means with the same letter in the same column and temperature treatment are not significantly different at P = 0.05 according to Tukey’s HSD test. F-values from two-way ANOVA are given together with P-values in brackets. °, P < 0.1 (highlighted in italics); *P < 0.05; **P < 0.01; ***P < 0.001 (highlighted in bold). Contrast matrix was used to test the comparisons with inoculation treatments
FW fresh weight, DW dry weight, Control control treatment, BsGFB04 Bacillus sp. GFB04 inoculation, KsGRB10 Klebsiella sp. GRB10 inoculation, HT heat stress treatment, TEMP temperature treatment, INOC endophyte inoculation treatment
Nutrient content accumulations are correlated through bacterial inoculations
In the greenhouse experiment, the results of nutrient contents showed the inoculation effects on lettuce shoots (Table 2) and roots (Table 3). For the shoot, the inoculations increased lettuce K, Ca, and Mg contents under optimal temperature conditions by 32%, 57%, and 19%, respectively, while not affecting N, P, and Fe contents. The heat stress caused a significant change in P (P = 0.014) and Fe (P = 0.016) contents for the root. However, the inoculations did not improve the nutrient contents of lettuce root under both temperature conditions.
Table 2.
Effects of bacterial consortium inoculation on nutrient contents in lettuce shoot under optimum temperature (20/15 °C, d/n) and heat stress (30/25 °C, d/n) conditions
| Shoot | |||||||
|---|---|---|---|---|---|---|---|
| Treatments | Elements (μg/g) | ||||||
| TEMP | INOC | N | P | K | Ca | Mg | Fe |
| Optimum | Control | 1083.0 ± 150.0 | 2547.0 ± 62.9 | 2984.6 ± 132.9 | 1689.1 ± 182.3 | 246.0 ± 10.7 | 5.4 ± 0.6 |
| BI | 1701.5 ± 382.3 | 2394 ± 59.9 | 3952.6 ± 148.7* | 2643.7 ± 179.2* | 293.3 ± 7.7* | 5.2 ± 0.5 | |
| HT | Control | 844.6 ± 21.8 | 2744.2 ± 92.4 | 5226.2 ± 164.8 | 1831.9 ± 138.6 | 287.1 ± 6.9 | 5.3 ± 0.8 |
| BI | 918.6 ± 95.2 | 2828.1 ± 65.5 | 4093.5 ± 270.3 | 2190.7 ± 128.5 | 304.2 ± 15.7 | 5.0 ± 0.2 | |
| Treatment effects | |||||||
| TEMP, df = 1 | 4.556° (.070) | 2.667 (.141) | 6.595* (.033) | 0.972 (.353) | 3.642° (.093) | 0.063 (.808) | |
| INOC, df = 1 | 2.094 (.191) | 1.145 (.316) | 0.032 (.863) | 0.201 (.666) | 0.200 (.666) | 0.175 (.687) | |
| TEMP × INOC, df = 1 | 1.295 (.293) | 0.586 (.466) | 5.127° (.053) | 5.198° (.052) | 2.676 (.141) | 0.004 (.949) | |
Data are means (± SE) of three replicates, and means with the *P < 0.05 in the same temperature treatment when inoculation effects are significantly different according to one-way ANOVA. F-values from two-way ANOVA are given together with P-values in brackets. °, P < 0.1 (highlighted in italics); *P < 0.05; **P < 0.01; ***P < 0.001 (highlighted in bold)
TEMP temperature treatment, INOC endophyte inoculation treatment, Control control treatment, BI bacterial consortium inoculation, HT heat stress treatment, HT + BI heat stress treatment with bacterial consortium inoculation
Table 3.
Effects of bacterial consortium inoculation on nutrient contents in lettuce root under optimum temperature (20/15 °C, d/n) and heat stress (30/25 °C, d/n) conditions
| Root | |||||||
|---|---|---|---|---|---|---|---|
| Treatments | Elements (μg/g) | ||||||
| TEMP | INOC | N | P | K | Ca | Mg | Fe |
| Optimum | Control | / | 4466.7 ± 610.2 | 2320.9 ± 253.6 | 90.6 ± 4.3 | 180.9 ± 8.0 | 12.5 ± 1.9 |
| BI | / | 5656.4 ± 264.2 | 3911.6 ± 217.7 | 121.4 ± 30.3 | 167.5 ± 15.6 | 10.7 ± 2.5 | |
| HT | Control | / | 2734.5 ± 880.7 | 2653.0 ± 1084.9 | 124.5 ± 75.3 | 155.0 ± 63.5 | 5.0 ± 1.8 |
| BI | / | 3366.9 ± 662.6 | 2895.7 ± 213.1 | 83.5 ± 16.0 | 168.6 ± 15.1 | 6.9 ± 1.0 | |
| Treatment effects | |||||||
| TEMP, df = 1 | 9.761* (.014) | .351 (.570) | 0.002 (.963) | 0.134 (.723) | 9.153* (.016) | ||
| INOC, df = 1 | 2.004 (.195) | 2.520 (.151) | 0.015 (.905) | 0.000 (.996) | 0.002 (.965) | ||
| TEMP × INOC, df = 1 | 0.187 (.667) | 1.362 (.277) | 0.753 (.411) | 0.160 (.700) | 0.992 (.349) | ||
Data are means (± SE) of three replicates, and means with the *P < 0.05 in the same temperature treatment when inoculation effects are significantly different according to one-way ANOVA. F-values from two-way ANOVA are given together with P-values in brackets. °, P < 0.1 (highlighted in italics); *P < 0.05; **P < 0.01; ***P < 0.001 (highlighted in bold)
TEMP temperature treatment, INOC endophyte inoculation treatment, Control control treatment, BI bacterial consortium inoculation, HT heat stress treatment, HT + BI heat stress treatment with bacterial consortium inoculation
Additionally, in the chamber experiment, the analysis of nutrients showed no significant inoculation effect on the shoot under the optimal temperature conditions (Table 5). In contrast, the inoculations increased the N and P contents in the shoot under heat stress conditions. The heat stress caused a significant change in shoot N content (P < 0.001). On the other hand, the inoculations resulted in a marginally significant change in shoot N, P, and Mg contents (P < 0.1). In the contrast-matrix analysis, BsGFB04 inoculations significantly increased P content (P < 0.05), while KsGRB10 inoculations significantly increased N and K contents (P < 0.05) in the shoot. Both BsGFB04 and KsGRB10 inoculations improved nutrient contents. However, comparing the individual inoculation treatments, they showed a significant change in shoot Ca content, showing that the nutrient contents obtained in the KsGRB10 group were greater than the BsGFB04 group. In addition, there was no significant inoculation effect for the root under optimal temperature conditions (Table 6). The inoculations resulted in significant changes in root N, K, Ca, and Mg contents (P < 0.01 or P < 0.05), showing that root nutrient contents obtained in the inoculation treatment groups were more significant than the control group. In the contrast-matrix analysis, inoculated plants significantly increased N, P, K, Ca, and Mg contents in the root. Furthermore, the KsGRB10 inoculation showed a significant increase in N, P, K, Ca, and Mg contents in the root, compared to the control group. Therefore, comparing the individual inoculation treatments, KsGRB10 performed better than BsGFB04 regarding lettuce nutrient content. KsGRB10 increased the most of N, P, K, Ca, and Mg contents by 133%, 33%, 107%, 217%, and 219%, respectively.
Table 5.
Effects of sole bacterial inoculation with Bacillus sp. GFB04 and Klebsiella sp. GRB10 on nutrient contents in lettuce shoot under optimum temperature (20/15 °C, d/n) and heat stress (30/25 °C, d/n) conditions
| Shoot | |||||||
|---|---|---|---|---|---|---|---|
| Treatments | Elements (μg/g) | ||||||
| TEMP | INOC | N | P | K | Ca | Mg | Fe |
| Control | Control | 1093.1 ± 76.5 | 1174.4 ± 103.5 | 2633.0 ± 187.0 | 74.7 ± 28.2 | 104.4 ± 17.5 | 15.1 ± 0.4 |
| BsGFB04 | 1195.7 ± 277.2 | 1334.2 ± 151.4 | 2836.2 ± 383.7 | 69.4 ± 15.3 | 123.0 ± 21.0 | 16.5 ± 2.8 | |
| KsGRB10 | 1145.4 ± 81.5 | 1638.0 ± 295.7 | 3209.3 ± 262.4 | 151.3 ± 17.2 | 136.8 ± 5.2 | 16.7 ± 1.9 | |
| HT | Control | 735.0 ± 69.4b | 854.2 ± 69.5b | 2198.0 ± 57.6 | 88.2 ± 42.5 | 116.3 ± 22.3 | 18.5 ± 1.7 |
| BsGFB04 | 859.6 ± 35.3b | 1399.0 ± 158.0a | 2464.1 ± 289.0 | 65.0 ± 19.4 | 118.8 ± 15.4 | 12.3 ± 1.2 | |
| KsGRB10 | 1197.2 ± 38.8a | 1188.2 ± 120.0ab | 2889.4 ± 241.5 | 80.1 ± 25.5 | 121.4 ± 25.5 | 17.8 ± 2.4 | |
| Treatment effects | |||||||
| TEMP, df = 1 | 4.261° (.061) | 3.011 (.108) | 2.809 (.120) | .909 (.359) | .027 (.871) | .003 (.960) | |
| INOC, df = 2 | 2.059° (.065) | 3.456° (.065) | 2.725 (.106) | 1.815 (.205) | .489° (.0625) | 1.319 (.304) | |
| TEMP × INOC, df = 2 | 1.647 (.233) | 1.302 (.308) | .022 (.978) | 1.415 (.281) | .263 (.773) | 2.050 (.171) | |
| Contrasts | |||||||
| Control vs Inoculated | 2.180 (.159) | 6.179* (.024) | 3.146 (.095) | 0.165 (.690) | 0.992 (.334) | 0.312 (.584) | |
| Control vs BsGFB04 | 0.433 (.526) | 6.888* (.025) | 0.784° (.397) | 0.311 (.589) | 0.360 (.562) | 1.514 (.247) | |
| Control vs KsGRB10 | 6.422* (.030) | 4.110° (.070) | 6.824* (.026) | 1.232 (.293) | 1.113 (.316) | 0.064 (.805) | |
| BsGFB04 vs KsGRB10 | 0.897 (.366) | 0.054 (.821) | 1.681 (.224) | 4.239° (.067) | 0.239 (.636) | 1.784 (.211) | |
Data are means (± SE) of three replicates, and means with the same letter in the same column and temperature treatment are not significantly different at P = 0.05 according to Tukey’s HSD test. F-values from two-way ANOVA are given together with P-values in brackets. °, P < 0.1 (highlighted in italics); *P < 0.05; **P < 0.01; ***P < 0.001 (highlighted in bold). Contrast matrix was used to test the comparisons with inoculation treatments
Control control treatment, BsGFB04 Bacillus sp. GFB04 inoculation, KsGRB10 Klebsiella sp. GRB10 inoculation, HT heat stress treatment, TEMP temperature treatment, INOC endophyte inoculation treatment
Table 6.
Effects of sole bacterial inoculation with Bacillus sp. GFB04 and Klebsiella sp. GRB10 on nutrient contents in lettuce root under optimum temperature (20/15 °C, d/n) and heat stress (30/25 °C, d/n) conditions
| Root | |||||||
|---|---|---|---|---|---|---|---|
| Treatments | Elements (μg/g) | ||||||
| TEMP | INOC | N | P | K | Ca | Mg | Fe |
| Control | Control | 884.6 ± 99.4 | 1266.3 ± 49.7 | 2284.1 ± 145.6 | 1467.1 ± 236.0 | 151.6 ± 10.2 | 4.9 ± 0.3 |
| BsGFB04 | 1222.1 ± 232.5 | 1575.6 ± 239.5 | 3089.6 ± 327.4 | 1882.4 ± 249.1 | 250.8 ± 62.7 | 5.7 ± 1.9 | |
| KsGRB10 | 1100.5 ± 88.6 | 1574.6 ± 250.4 | 2857.1 ± 234.2 | 1445.7 ± 78.8 | 194.4 ± 21.9 | 3.3 ± 0.3 | |
| HT | Control | 488.4 ± 53.0b | 960.3 ± 34.4b | 1452.4 ± 264.3b | 597.0 ± 41.8b | 58.2 ± 7.7b | 4.2 ± 0.9 |
| BsGFB04 | 908.7 ± 61.3a | 1272.5 ± 72.8ab | 2635.0 ± 328.5a | 1351.1 ± 133.9a | 148.9 ± 31.3a | 4.5 ± 0.7 | |
| KsGRB10 | 1138.5 ± 166.2a | 1373.8 ± 138.5ab | 3011.7 ± 66.1a | 1893.2 ± 272.1a | 185.7 ± 19.6a | 5.6 ± 1.0 | |
| Treatment effects | |||||||
| TEMP, df = 1 | 4.256° (.061) | 4.426° (.057) | 3.505° (.086) | 4.182° (.063) | 7.004* (.021) | .031 (.862) | |
| INOC, df = 2 | 6.305* (.013) | 3.094° (.082) | 11.660** (.002) | 6.900** (.010) | 5.513* (.020) | .217 (.808) | |
| TEMP × INOC, df = 2 | 1.505 (.261) | .073 (.930) | 2.033 (.174) | 6.453* (.013) | 1.343 (.298) | 1.728 (.219) | |
| Contrasts | |||||||
| Control vs Inoculated | 8.733** (.009) | 5.288* (.035) | 13.255** (.002) | 8.097* (.012) | 7.722* (.013) | 0.193 (.667) | |
| Control vs BsGFB04 | 5.346* (.043) | 4.284° (.065) | 9.309* (.012) | 4.302° (.065) | 4.579° (.058) | 0.275 (.611) | |
| Control vs KsGRB10 | 10.685** (.008) | 5.469* (.041) | 17.627** (.002) | 5.387* (.043) | 11.230** (.007) | 0.004 (.950) | |
| BsGFB04 vs KsGRB10 | 0.124 (.732) | 0.071 (.796) | 0.078 (.785) | 0.049 (.828) | 0.057 (.816) | 0.260 (.621) | |
Data are means (± SE) of three replicates, and means with the same letter in the same column and temperature treatment are not significantly different at P = 0.05 according to Tukey’s HSD test. F-values from two-way ANOVA are given together with P-values in brackets. °, P < 0.1 (highlighted in italics); *P < 0.05; **P < 0.01; ***P < 0.001 (highlighted in bold). Contrast matrix was used to test the comparisons with inoculation treatments
Control control treatment; BsGFB04 Bacillus sp. GFB04 inoculation, KsGRB10 Klebsiella sp. GRB10 inoculation, HT heat stress treatment, TEMP temperature treatment, INOC endophyte inoculation treatment
Identification of physiological traits that associate with bacterial inoculations
In the greenhouse experiment, the results of physiological parameters showed that gs (Fig. 1) and E (Fig. 2) were significantly decreased under heat stress conditions. Inoculated plants had significant decreases in both parameters in the daytime under optimal temperature conditions by 57% and 55%, respectively, compared with controls, while no significant effect under heat stress conditions. Furthermore, the chlorophyll fluorescence parameters ΦPSII and Fv/Fm showed the same results (Figs. 3 and 4), the inoculations significantly increased both parameters under heat stress conditions in which ΦPSII was improved from 0.41 to 0.50 (increased by 20%), and Fv/Fm was improved from 0.42 to 0.58 (increased by 39%) after the inoculations under heat stress conditions.
Fig. 1.

Effects of temperature and bacterial inoculation on changes in lettuce stomatal conductance (gs) in different periods of the day in 45 days after transplanting (DAT) under two temperature conditions. A 20/15 °C and B 30/25 °C. Data are means (± SE) of 10 replicates. Symbol denote significant differences from the control: *, P < 0.05. White bars indicate control groups (Control), whereas grey bars indicate bacterial consortium inoculation groups (BI)
Fig. 2.

Effects of temperature and bacterial inoculation on changes in lettuce transpiration rate (E) in different periods of the day in 45 days after transplanting (DAT) under two temperature conditions. A 20/15 °C and B 30/25 °C. Data are means (± SE) of 10 replicates. Symbol denote significant differences from the control: *, P < 0.05. White bars indicate control groups (Control), whereas grey bars indicate bacterial consortium inoculation groups (BI)
Fig. 3.

Effects of temperature and bacterial inoculation on lettuce maximum quantum yield of photosystem II (ΦPSII) in 45 days after transplanting (DAT) under two temperature conditions: Optimal (20/15 °C, on the left set) and high temperature (HT) (30/25 °C, on the right set). Data are means (± SE) of 10 replicates. Symbol denote significant differences from the control: *, P < 0.05. White bars indicate control groups (Control), whereas grey bars indicate bacterial consortium inoculation groups (BI)
Fig. 4.
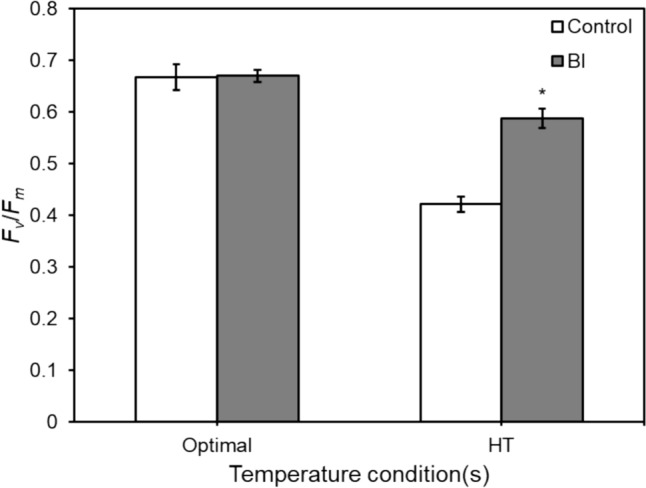
Effects of temperature and bacterial inoculation on lettuce effective quantum yield of photosystem II (Fv/Fm) in 45 days after transplanting (DAT) under two temperature conditions: Optimal (20/15 °C, on the left set) and high temperature (HT) (30/25 °C, on the right set). Data are means (± SE) of ten replicates. Symbol denote significant differences from the control: *, P < 0.05. White bars indicate control groups (Control), whereas grey bars indicate bacterial consortium inoculation groups (BI)
In addition, in the chamber experiment, the results of physiological parameters showed that gs (Fig. 5) and E (Fig. 6) were significantly decreased under heat stress conditions. All treatments had less gs but significantly increased the daytime E under heat stress conditions. Inoculation did not cause a significant change in both parameters under optimal temperature conditions. BsGFB04 and KsGRB10 treatments showed a significant decrease in gs and E in the day under heat stress conditions, respectively. Furthermore, ΦPSII and Fv/Fm showed the same results (Figs. 7 and 8). Inoculations resulted in a significant increase in both parameters under heat stress condition, not optimal temperature. Within the inoculation treatments, KsGRB10 showed the highest value on both parameters, in which ΦPSII was improved from 0.47 to 0.54 (increased by 15%), and Fv/Fm was improved from 0.64 to 0.69 (increased by 9%) after the inoculations under heat stress conditions.
Fig. 5.
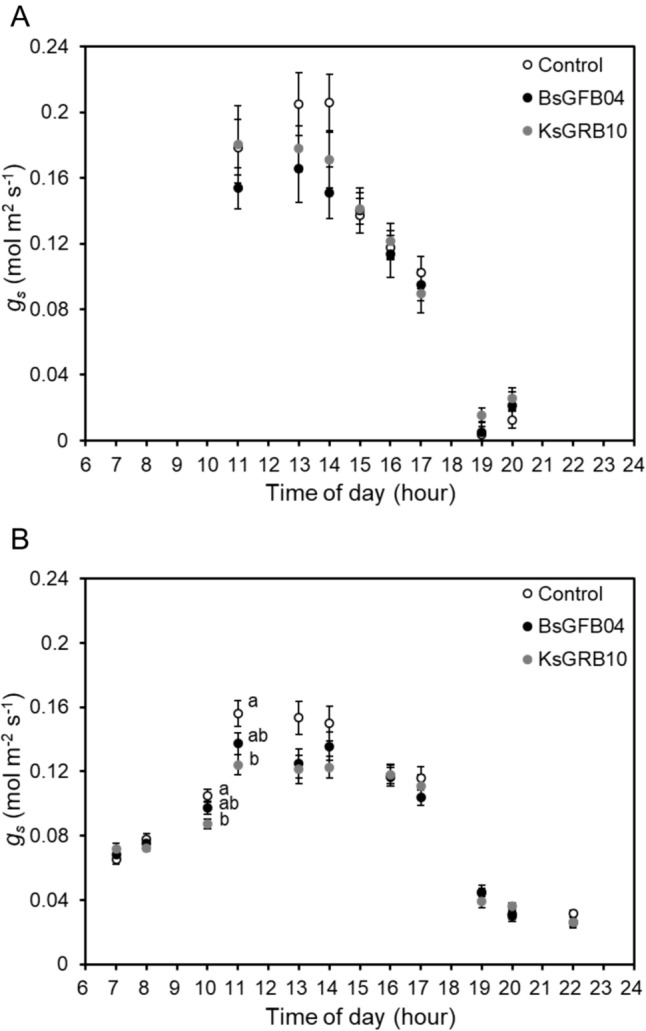
Effects of temperature and sole bacterial inoculation with Bacillus sp. GFB04 and Klebsiella sp. GRB10 on diurnal changes of lettuce stomatal conductance (gs) in 30 days after transplanting (DAT) under two temperature conditions. A 20/15 °C and B 30/25 °C. Data are means (± SE) of 10 replicates, and means with the same letter are not significantly different at P = 0.05 according to Tukey’s HSD test. Open symbols indicate control groups (Control), closed black symbols indicate Bacillus sp. GFB04 inoculation groups (BsGFB04), whereas closed grey symbols indicate Klebsiella sp. GRB10 inoculation groups (KsGRB10)
Fig. 6.
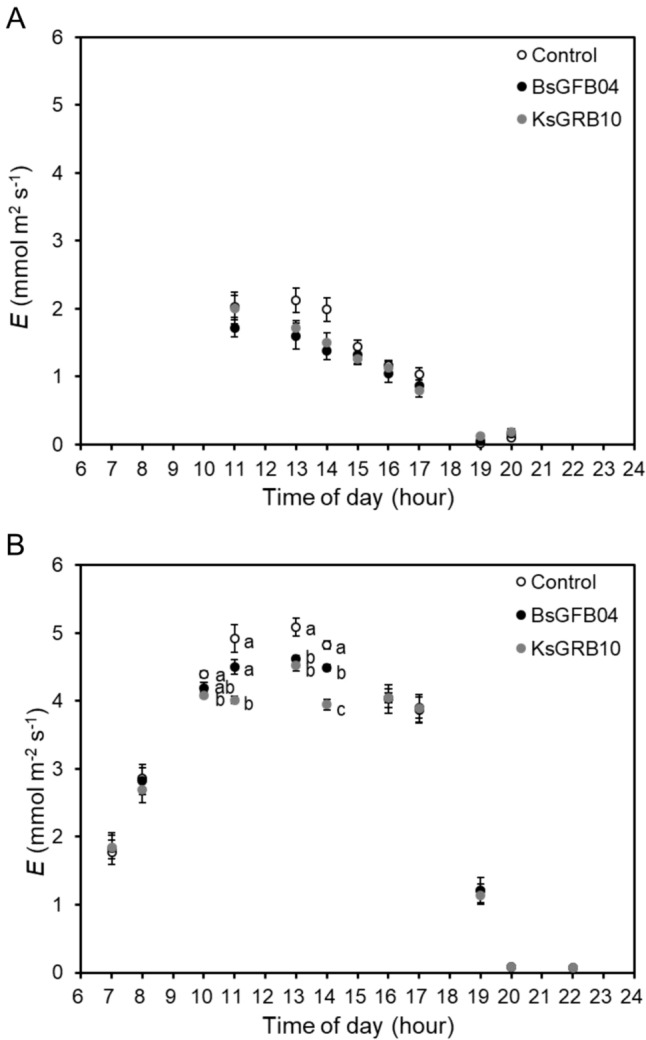
Effects of temperature and sole bacterial inoculation with Bacillus sp. GFB04 and Klebsiella sp. GRB10 on diurnal changes of lettuce transpiration rate (E) in 30 days after transplanting (DAT) under two temperature conditions. A 20/15 °C and B 30/25 °C. Data are means (± SE) of 10 replicates, and means with the same letter are not significantly different at P = 0.05 according to Tukey’s HSD test. Open symbols indicate control groups (Control), closed black symbols indicate Bacillus sp. GFB04 inoculation groups (BsGFB04), whereas closed grey symbols indicate Klebsiella sp. GRB10 inoculation groups (KsGRB10)
Fig. 7.

Effects of temperature and sole bacterial inoculation with Bacillus sp. GFB04 and Klebsiella sp. GRB10 on lettuce maximum quantum yield of photosystem II (ΦPSII) in 30 days after transplanting (DAT) under two temperature conditions. A 20/15 °C and B 30/25 °C. Data are means (± SE) of 10 replicates, and means with the same letter are not significantly different at P = 0.05 according to Tukey’s HSD test. Bars from left to right: control groups (Control), Bacillus sp. GFB04 inoculation groups (BsGFB04), and Klebsiella sp. GRB10 inoculation groups (KsGRB10)
Fig. 8.
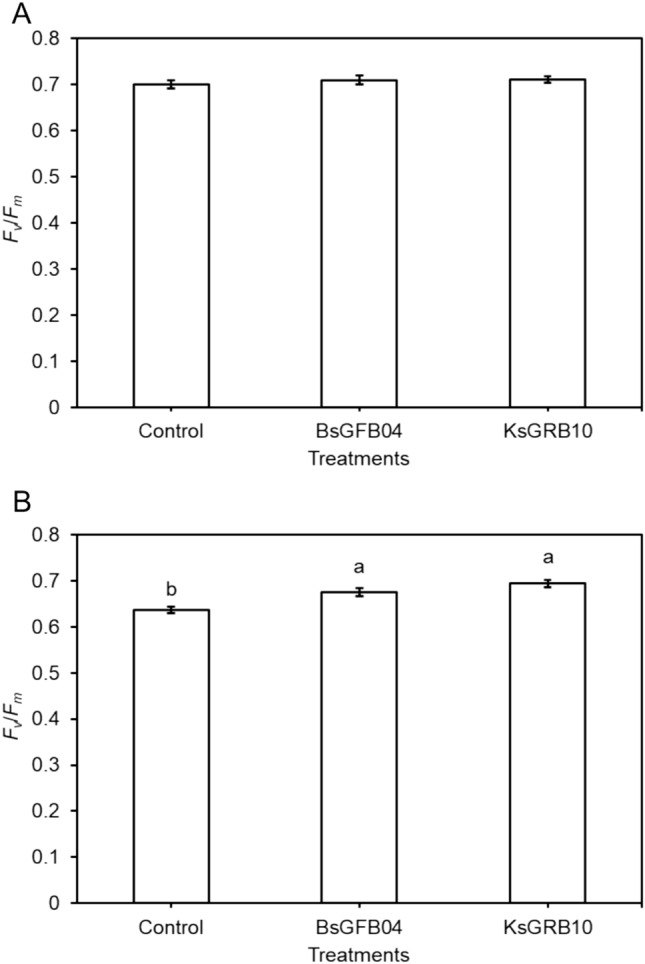
Effects of temperature and sole bacterial inoculation with Bacillus sp. GFB04 and Klebsiella sp. GRB10 on lettuce’s effective quantum yield of photosystem II (Fv/Fm) in 30 days after transplanting (DAT) under two temperature conditions. A 20/15 °C and B 30/25 °C. Data are means (± SE) of 10 replicates, and means with the same letter are not significantly different at P = 0.05 according to Tukey’s HSD test. Bars from left to right: control groups (Control), Bacillus sp. GFB04 inoculation groups (BsGFB04), and Klebsiella sp. GRB10 inoculation groups (KsGRB10)
Discussion
Heat stress reduces symbiotic performance of PGPB, enabling selection of thermo-tolerant strains
We hypothesized that the PGPB inoculations could promote plant growth due to the nutrient uptake assistance from their PGP traits. In vitro characterizations examined some of the critical PGP traits of the isolated 18 strains, which included high temperature responses, BNF, phosphate solubilization, and siderophore production. These traits were essential to consider their symbiotic potentials as an inoculum before starting the in-vivo study. The above traits involved different mechanisms by which bacteria promote plant growth, while the temperature responses showed their survival under different temperature conditions (Emami et al. 2019; Consentino et al. 2022).
There are limited studies that examine the bacterial characterizations under different temperature conditions; our research examined them to investigate their temperature responses in different bioassays and plates. The strains exhibited their characteristics under different temperature conditions (Guinebretiere et al. 2001; Saiki et al. 2014; Satti et al. 2017). In this study, most strains could perform well at 25 °C and 30 °C, but their performances started declining when the temperature exceeded their optimal growing environment. All the strains performed worst at 40 °C. Bacillus spp. developed better at 25 °C (Guinebretiere et al. 2001).
The weighted score (Table S5) reflected the importance of their performance under normal temperature (25 °C and 30 °C) and high temperature (35 °C and 40 °C) conditions. After the scores were weighted, the strains that performed better under high temperature conditions were distinguished and emphasized. They were ranked in the order of Klebsiella sp. GRB10, Klebsiella sp. GRB04, Klebsiella sp. LFB06, Acinetobacter sp. GRB12, and Bacillus sp. GFB04. The genus Klebsiella and Bacillus are well-studied PGPB under abiotic stress conditions and reported as endophytes (Bhardwaj et al. 2017; Pramanik et al. 2017; Yadav et al. 2021; Mahapatra et al. 2022; Lastochkina et al. 2017; Ferreira et al. 2018; Latif et al. 2020; Kusale et al. 2021; Noman et al. 2021). There are limited studies about Acinetobacter strains’ PGP potentials (Berza et al. 2022).
The investigation of the isolates temperature responses was essential since this research aimed to examine their inoculation effects under optimal temperature and heat stress conditions. Therefore, it was required to ensure they could survive under high temperatures. Furthermore, after the inoculations, they were assumed to promote plant growth under stress and non-stress conditions with their bacterial characteristics (Baig et al. 2011; Ahemad 2015; Emami et al. 2019).
In vivo characterization of the lettuce host plant with the PGPB inoculation
Increases in shoot and root biomass by PGPB inoculations
Lettuce is a cool-season and heat-sensitive crop. Its development and yield are greatly affected by heat stress. The lettuce plants are temperature-sensitive because the high temperatures would promote bolting while consumers are willing to purchase non-bolted lettuce in the market (Chen et al. 2022; Li et al. 2022). The biomass and morphology of lettuce reflected its yield and quality.
After harvesting the lettuces in the greenhouse experiment, the shoot and root biomass were measured (Table 1). Only root FW and DW were significantly affected in optimal temperature conditions by inoculations. The treatment effects of two factors showed that the inoculations did not affect lettuce biomass (total FW and DW); they only affected the root. The possible explanation was that the PGPB isolated from the sample plants, Welsh onion, specifically lived in lettuce roots (Wang et al. 2023). Therefore, the activities of endophytes may be emphasized in the root.
On the other hand, in the chamber experiment, inoculations only improved lettuce shoot and root biomass under heat stress conditions (Table 4). It implies that environmental factors may affect plant-PGPB interactions. Endophytes may work only if the plants need them. It involves different mechanisms regarding the characteristics of bacteria (Rho et al. 2018c).
Increases in nutrient contents by PGPB inoculations, enabling better plant growth
The analysis of nutrient contents was essential to determine the inoculation effects (Esitken et al. 2010; Consentino et al. 2022). The selected strains showed the best performances in bioassays. Therefore, they were assumed to have high PGP potentials after the inoculation (Tables 1, 2, 3, and 4). The tests were conducted to examine their effects on helping the plants obtain more nutrients from the planting medium and rhizosphere. They showed good performances in BNF, phosphate solubilization, and siderophore production; these three traits are related to nutrient acquisition.
The analysis of nutrient contents (Tables 2 and 3) in the greenhouse experiment generally showed no difference in shoot and root in four treatments. The possible reason was that the planting medium did not contain many nutrients, so the endophytes could not work in a nutrient-limited condition. It could work in a nutrient-rich condition as a bio-facilitator but not a bio-fertilizer (Consentino et al. 2022). This could explain why the inoculation did not improve biomass much in both the shoot and root. The other possible reason was the gap between in vitro and in vivo tests that remained to be discovered (Ramakrishna et al. 2019). Although the in vitro tests showed the strains’ PGP potentials, the endophytes may not work after in vivo greenhouse inoculations.
However, the chamber experiment results showed the opposite results. The results (Tables 5 and 6) showed that inoculations only increased the nutrient contents in the shoot and root under heat stress conditions. Although previous studies (Dhawi et al. 2015; Egamberdiyeva 2007) showed similar results, the inoculations successfully increased all macronutrient and micronutrient contents in maize plants. The interactions between lettuce and endophytes on nutrient absorption must be clarified in further studies.
Improvement in chlorophyll fluorescence under heat stress by PGPB inoculations, suggesting higher photosystem II photochemical efficiency
Photosynthesis is the most critical physiological trait of plants since it decides the growth performance of the plants. Active radiation was absorbed by chlorophyll during photosynthesis (Mousavi et al. 2022). The present study used chlorophyll fluorescence parameters (ΦPSII and Fv/Fm) to examine the quantum yield of PSII among the treatments. Both parameters decreased when heat stress was imposed in our study. The inoculations under optimal temperature and heat stress conditions showed significant differences (Figs. 3, 4, 7, and 8) in chlorophyll fluorescence in the greenhouse and the chamber experiment. The optimal values of Fv/Fm were consistently around 0.83 for non-stressed leaves (Baker 2008; Björkman and Demmig 1987). After the inoculations, the increased Fv/Fm values indicated that the inoculated plants had less stress and photoinhibition (Wahid et al. 2007). Inoculations could enhance the efficiency of the light-harvesting system in the plants, thus increasing the photosynthetic rate (Rho et al. 2020).
Decreases in stomatal conductance and transpiration in daytime by PGPB inoculations, suggesting less total water loss
The measurements of gs and E are critical to understanding the gas exchange in leaves. The calculation of WUE is equal to photosynthetic CO2 assimilation rate (A) divided by E (Tuzet et al. 2003). Although this study did not measure A, the chlorophyll fluorescence parameters were used as a proxy to predict A and explain the WUE of the treatments with E. In the greenhouse experiment, the treatments’ gs and E showed the same pattern (Figs. 1 and 2), in which inoculation decreased them under optimal temperature conditions while having no significant effect under the heat stress conditions in the daytime. A lower E implies less water loss through the stomatal opening under optimal temperature conditions. When comparing E and biomass (Table 1), the control and the inoculated groups did not show significant differences in biomass. In contrast, the inoculated group had a lower E. It implied that the inoculated group could harvest the same amount of active radiation as the control group but with less water loss. The growth improvement can be found in Table 1; the inoculated group and the control group under heat stress conditions had a significant marginal difference in the shoot’s FW.
Symbiotic plants could possibly avoid the need to open stomata for the uptake of CO2 from the ambient air due to the enhanced accessibility of respiratory CO2 to the process of CO2 fixation at the site of carboxylation is facilitated by its direct pathway, bypassing the diffusion of CO2 from the atmosphere to the intercellular spaces. Consequently, minimizing the distance traveled by CO2 proves advantageous for plants (Rho et al. 2018a, 2018b). Diurnal changes in gs (Fig. 5) and E (Fig. 6) were measured in the chamber experiment. Inoculations decreased both parameters to reduce total water use in the photosynthesis peak period (10 a.m. to 2 p.m.) under heat stress conditions. One of the possible reasons could be that some strains could produce abscisic acid (ABA), which is involved in regulating stomatal closure through increasing stomatal sensitivity. The upregulated ABA contents in inoculated plants could affect the stomatal apertures (Rho et al. 2018b).
Leaf temperature is affected by the evaporative cooling of stomata. Plants tend to evaporate to release heat through opening stomata in the leaves (Tuzet et al. 2003). However, the treatments showed no difference in leaf temperature under two temperature conditions in two experiments (Fig. S1 and Fig. S2), although they had lower E. The possible reason for no significant difference in leaf temperature while less E was observed in the symbiotic plants under heat stress conditions is that the plants successfully adapted to the stressful environment after inoculations. Therefore, the plants may not be required to release excessive heat.
To conclude, compared to un-inoculated plants, inoculated plants were assumed to have higher photosynthetic capacity and lower transpiration rate, thus less gas exchange under heat stress conditions. Also, the inoculated plants had higher biomass (Table 4) with less gas exchange. Therefore, the WUE was supposedly improved by inoculations.
Conclusions
The presence and function of stress-tolerant microbiomes significantly influence the mitigation of physiological abnormalities in plants induced by a range of abiotic stresses. These components play a critical role in minimizing the negative impact of abiotic stresses on plant health and functioning. Our study showed how PGPB inoculations influenced lettuce development from germination to mature stage under heat stress conditions.
Two recently characterized PGPBs were selected: Bacillus sp. GFB04 and Klebsiella sp. GRB10. The mixed strain and the single strain effects were observed in separate greenhouse and chamber experiments. The results showed that inoculations resulted in better plant growth under optimal temperature and heat stress conditions, with higher biomass, better PSII efficiency, presumable reduction in water use indicated by the reduction in stomatal conductance and transpiration during daytime, and no changes in leaf temperature.
Prospectively, the inoculation of Klebsiella sp. GRB10 in plants can be one of the directions in future studies under biotic or abiotic stresses. Besides lettuce, many crops can also be tested. More PGP traits of Klebsiella sp. GRB10, such as the production of plant growth hormones, need to be clarified for comprehensive understanding of its symbiotic mechanisms and functions. Future studies could investigate the inoculation effects under different light environments, such as light intensity and period.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The study was funded by the Ministry of Science and Technology (MOST)/National Science and Technology Council (NSTC), the Office of Research and Development (ORD) at National Taiwan University new faculty funding, MOST Project (110-2313-B-002-007). We thank Prof. Wen-Ju Yang, Prof. Shu-I Lin, and Prof, Shu-Yen Lin, the faculty members of the Department of Horticulture and Landscape Architecture and the Department of Plant Pathology and Microbiology, National Taiwan University, for providing necessary instruments and suggestions for the experimental design and the manuscript.
Author contributions
THC and HR conceived and designed research. THC conducted experiments. HAA and HR contributed analytical tools. THC analyzed data. THC wrote the draft manuscript. THC, HAA, and HR reviewed and edited the draft manuscript. All authors have read the manuscript and agreed to the manuscript submission.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Ahemad M. Enhancing phytoremediation of chromium-stressed soils through plant-growth-promoting bacteria. J Genet Eng Biotechnol. 2015;13(1):51–58. doi: 10.1016/j.jgeb.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baig KS, Arshad M, Shaharoona B, Khalid A, Ahmed I. Comparative effectiveness of Bacillus spp. possessing either dual or single growth-promoting traits for improving phosphorus uptake, growth and yield of wheat (Triticum aestivum L) Ann Microbiol. 2011;62(3):1109–1119. doi: 10.1007/s13213-011-0352-0. [DOI] [Google Scholar]
- Baker NR. Chlorophyll fluorescence: a probe of photosynthesis in vivo. Annu Rev Plant Biol. 2008;59:89–113. doi: 10.1146/annurev.arplant.59.032607.092759. [DOI] [PubMed] [Google Scholar]
- Berza B, Sekar J, Vaiyapuri P, Pagano MC, Assefa F. Evaluation of inorganic phosphate solubilizing efficiency and multiple plant growth promoting properties of endophytic bacteria isolated from root nodules Erythrina brucei. BMC Microbiol. 2022;22(1):276. doi: 10.1186/s12866-022-02688-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhardwaj G, Shah R, Joshi B, Patel P. Klebsiella pneumoniae VRE36 as a PGPR isolated from Saccharum officinarum cultivar Co99004. J Appl Biol Biotechnol. 2017 doi: 10.7324/jabb.2017.50108. [DOI] [Google Scholar]
- Bisbis MB, Gruda N, Blanke M. Potential impacts of climate change on vegetable production and product quality—a review. J Clean Prod. 2018;170:1602–1620. doi: 10.1016/j.jclepro.2017.09.224. [DOI] [Google Scholar]
- Bita CE, Gerats T. Plant tolerance to high temperature in a changing environment: scientific fundamentals and production of heat stress-tolerant crops. Front Plant Sci. 2013;4:273. doi: 10.3389/fpls.2013.00273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björkman O, Demmig B. Photon yield of O2 evolution and chlorophyll fluorescence characteristics at 77 K among vascular plants of diverse origins. Planta. 1987;170:489–504. doi: 10.1007/BF00402983. [DOI] [PubMed] [Google Scholar]
- Bruno LB, Karthik C, Ma Y, Kadirvelu K, Freitas H, Rajkumar M. Amelioration of chromium and heat stresses in Sorghum bicolor by Cr(6+) reducing-thermotolerant plant growth promoting bacteria. Chemosphere. 2020;244:125521. doi: 10.1016/j.chemosphere.2019.125521. [DOI] [PubMed] [Google Scholar]
- Chen L, Xu M, Liu C, Hao J, Fan S, Han Y. LsMYB15 regulates bolting in leaf lettuce (Lactuca sativa L.) under high-temperature stress. Front Plant Sci. 2022;13:921021. doi: 10.3389/fpls.2022.921021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen I, Zandalinas SI, Huck C, Fritschi FB, Mittler R. Meta-analysis of drought and heat stress combination impact on crop yield and yield components. Physiol Plant. 2021;171(1):66–76. doi: 10.1111/ppl.13203. [DOI] [PubMed] [Google Scholar]
- Consentino BB, Aprile S, Rouphael Y, Ntatsi G, De Pasquale C, Iapichino G, Alibrandi P, Sabatino L. Application of PGPB combined with variable N doses affects growth, yield-related traits, N-fertilizer efficiency and nutritional status of lettuce grown under controlled condition. Agronomy. 2022 doi: 10.3390/agronomy12020236. [DOI] [Google Scholar]
- Dhawi F, Datta R, Ramakrishna W. Mycorrhiza and PGPB modulate maize biomass, nutrient uptake and metabolic pathways in maize grown in mining-impacted soil. Plant Physiol Biochem. 2015;97:390–399. doi: 10.1016/j.plaphy.2015.10.028. [DOI] [PubMed] [Google Scholar]
- Doty SL, Oakley B, Xin G, Kang JW, Singleton G, Khan Z, Vajzovic A, Staley JT. Diazotrophic endophytes of native black cottonwood and willow. Symbiosis. 2009;47(1):23–33. doi: 10.1007/BF03179967. [DOI] [Google Scholar]
- Egamberdiyeva D. The effect of plant growth promoting bacteria on growth and nutrient uptake of maize in two different soils. Appl Soil Ecol. 2007;36(2–3):184–189. doi: 10.1016/j.apsoil.2007.02.005. [DOI] [Google Scholar]
- Emami S, Alikhani HA, Pourbabaei AA, Etesami H, Sarmadian F, Motessharezadeh B. Effect of rhizospheric and endophytic bacteria with multiple plant growth promoting traits on wheat growth. Environ Sci Pollut Res Int. 2019;26(19):19804–19813. doi: 10.1007/s11356-019-05284-x. [DOI] [PubMed] [Google Scholar]
- Esitken A, Yildiz HE, Ercisli S, Figen Donmez M, Turan M, Gunes A. Effects of plant growth promoting bacteria (PGPB) on yield, growth and nutrient contents of organically grown strawberry. Sci Hortic. 2010;124(1):62–66. doi: 10.1016/j.scienta.2009.12.012. [DOI] [Google Scholar]
- Fahad S, Bajwa AA, Nazir U, Anjum SA, Farooq A, Zohaib A, Sadia S, Nasim W, Adkins S, Saud S, Ihsan MZ, Alharby H, Wu C, Wang D, Huang J. Crop production under drought and heat stress: plant responses and management options. Front Plant Sci. 2017;8:1147. doi: 10.3389/fpls.2017.01147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira NC, Mazzuchelli RDCL, Pacheco AC, Araujo FFD, Antunes JEL, Araujo ASFD. Bacillus subtilis improves maize tolerance to salinity. Ciência Rural. 2018 doi: 10.1590/0103-8478cr20170910. [DOI] [Google Scholar]
- Franco-Correa M, Quintana A, Duque C, Suarez C, Rodríguez MX, Barea J-M. Evaluation of actinomycete strains for key traits related with plant growth promotion and mycorrhiza helping activities. Appl Soil Ecol. 2010;45(3):209–217. doi: 10.1016/j.apsoil.2010.04.007. [DOI] [Google Scholar]
- Gouda S, Kerry RG, Das G, Paramithiotis S, Shin HS, Patra JK. Revitalization of plant growth promoting rhizobacteria for sustainable development in agriculture. Microbiol Res. 2018;206:131–140. doi: 10.1016/j.micres.2017.08.016. [DOI] [PubMed] [Google Scholar]
- Guinebretiere MH, Berge O, Normand P, Morris C, Carlin F, Nguyen-The C. Identification of bacteria in pasteurized zucchini purees stored at different temperatures and comparison with those found in other pasteurized vegetable purees. Appl Environ Microbiol. 2001;67(10):4520–4530. doi: 10.1128/AEM.67.10.4520-4530.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IPCC (2021) Ipcc, 2021: Summary for policymakers. In: Climate change 2021: the physical science basis. Contribution of working group i to the sixth assessment report of the intergovernmental panel on climate change
- Khan MA, Asaf S, Khan AL, Jan R, Kang S-M, Kim K-M, Lee I-J. Thermotolerance effect of plant growth-promoting Bacillus cereus SA1 on soybean during heat stress. BMC Microbiol. 2020;20(1):175. doi: 10.1186/s12866-020-01822-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan MA, Asaf S, Khan AL, Jan R, Kang SM, Kim KM, Lee IJ. Extending thermotolerance to tomato seedlings by inoculation with SA1 isolate of Bacillus cereus and comparison with exogenous humic acid application. PLoS ONE. 2020;15(4):e0232228. doi: 10.1371/journal.pone.0232228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Verma JP. Does plant-microbe interaction confer stress tolerance in plants: a review? Microbiol Res. 2018;207:41–52. doi: 10.1016/j.micres.2017.11.004. [DOI] [PubMed] [Google Scholar]
- Kusale SP, Attar YC, Sayyed R, Malek RA, Ilyas N, Suriani NL, Khan N, El Enshasy HA. Production of plant beneficial and antioxidants metabolites by Klebsiella variicola under salinity stress. Molecules. 2021;26(7):1894. doi: 10.3390/molecules26071894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lastochkina O, Pusenkova L, Yuldashev R, Babaev M, Garipova S, Blagova D, Khairullin R, Aliniaeifard S. Effects of Bacillus subtilis on some physiological and biochemical parameters of Triticum aestivum L. (wheat) under salinity. Plant Physiol Biochem. 2017;121:80–88. doi: 10.1016/j.plaphy.2017.10.020. [DOI] [PubMed] [Google Scholar]
- Latif S, Mohamed AG, Sueyoshi K. Effect of Bacillus subtilis on some physiological and biochemical processes in barley (Hordeum vulgare L.) plant grown under salt stress. Egypt J Botany. 2020 doi: 10.21608/ejbo.2020.41931.1555. [DOI] [Google Scholar]
- Li Y, Zhu J, Feng Y, Li Z, Ren Z, Liu N, Liu C, Hao J, Han Y. LsARF3 mediates thermally induced bolting through promoting the expression of LsCO in lettuce (Lactuca sativa L) Front Plant Sci. 2022;13:958833. doi: 10.3389/fpls.2022.958833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y-T, Yang IC, Lin N-C. Evaluation of biocontrol potential for Fusarium yellows of celery by antagonistic and gallic acid-degrading bacteria. Biol Control. 2020 doi: 10.1016/j.biocontrol.2020.104268. [DOI] [Google Scholar]
- Mahapatra S, Yadav R, Ramakrishna W. Bacillus subtilis impact on plant growth, soil health and environment: Dr. Jekyll and Mr. Hyde. J Appl Microbiol. 2022;132(5):3543–3562. doi: 10.1111/jam.15480. [DOI] [PubMed] [Google Scholar]
- Mendelsohn RO, Massetti E. The use of cross-sectional analysis to measure climate impacts on agriculture: theory and evidence. Rev Environ Econ Policy. 2017;11:280–298. doi: 10.1093/reep/rex017. [DOI] [Google Scholar]
- Mousavi SS, Karami A, Maggi F. Photosynthesis and chlorophyll fluorescence of Iranian licorice (Glycyrrhiza glabra L.) accessions under salinity stress. Front Plant Sci. 2022;13:984944. doi: 10.3389/fpls.2022.984944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noman M, Ahmed T, Shahid M, Niazi MBK, Qasim M, Kouadri F, Abdulmajeed AM, Alghanem SM, Ahmad N, Zafar M. Biogenic copper nanoparticles produced by using the Klebsiella pneumoniae strain NST2 curtailed salt stress effects in maize by modulating the cellular oxidative repair mechanisms. Ecotoxicol Environ Saf. 2021;217:112264. doi: 10.1016/j.ecoenv.2021.112264. [DOI] [PubMed] [Google Scholar]
- Omomowo OI, Babalola OO. Bacterial and fungal endophytes: Tiny giants with immense beneficial potential for plant growth and sustainable agricultural productivity. Microorganisms. 2019 doi: 10.3390/microorganisms7110481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter SS, Bantay R, Friel CA, Garoutte A, Gdanetz K, Ibarreta K, Moore BM, Shetty P, Siler E, Friesen ML, Bennett A. Beneficial microbes ameliorate abiotic and biotic sources of stress on plants. Funct Ecol. 2020;34(10):2075–2086. doi: 10.1111/1365-2435.13499. [DOI] [Google Scholar]
- Pramanik K, Mitra S, Sarkar A, Soren T, Maiti TK. Characterization of cadmium-resistant Klebsiella pneumoniae MCC 3091 promoted rice seedling growth by alleviating phytotoxicity of cadmium. Environ Sci Pollut Res Int. 2017;24(31):24419–24437. doi: 10.1007/s11356-017-0033-z. [DOI] [PubMed] [Google Scholar]
- Ramakrishna W, Yadav R, Li K. Plant growth promoting bacteria in agriculture: two sides of a coin. Appl Soil Ecol. 2019;138:10–18. doi: 10.1016/j.apsoil.2019.02.019. [DOI] [Google Scholar]
- Rho H, Doty SL, Kim SH. Estimating microbial respiratory CO(2) from endophytic bacteria in rice. Plant Signal Behav. 2018;13(8):e1500067. doi: 10.1080/15592324.2018.1500067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rho H, Epps VV, Wegley N, Doty SL, Kim S-H. Salicaceae endophytes modulate stomatal behavior and increase water use efficiency in rice. Front Plant Sci. 2018;9:188. doi: 10.3389/fpls.2018.00188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rho H, Hsieh M, Kandel SL, Cantillo J, Doty SL, Kim SH. Do endophytes promote growth of host plants under stress? A meta-analysis on plant stress mitigation by endophytes. Microb Ecol. 2018;75(2):407–418. doi: 10.1007/s00248-017-1054-3. [DOI] [PubMed] [Google Scholar]
- Rho H, Doty SL, Kim SH. Endophytes alleviate the elevated CO2-dependent decrease in photosynthesis in rice, particularly under nitrogen limitation. J Exp Bot. 2020;71(2):707–718. doi: 10.1093/jxb/erz440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutten P, Tennant R, Beal J, Workman C, Haddock-Angelli T, Farny N, Selvarajah V (2019) Calibration protocol-conversion of OD600 to colony forming units (CFUs). 10.17504/protocols.io.5gjg3un
- Sadok W, Lopez JR, Smith KP. Transpiration increases under high-temperature stress: potential mechanisms, trade-offs and prospects for crop resilience in a warming world. Plant Cell Environ. 2021;44(7):2102–2116. doi: 10.1111/pce.13970. [DOI] [PubMed] [Google Scholar]
- Saiki T, Kimura R, Arima K. Isolation and characterization of extremely thermophilic bacteria from hot springs. Agric Biol Chem. 2014;36(13):2357–2366. doi: 10.1080/00021369.1972.10860589. [DOI] [Google Scholar]
- Satti SM, Shah AA, Auras R, Marsh TL. Isolation and characterization of bacteria capable of degrading poly(lactic acid) at ambient temperature. Polym Degrad Stab. 2017;144:392–400. doi: 10.1016/j.polymdegradstab.2017.08.023. [DOI] [Google Scholar]
- Schwyn B, Neilands JB. Universal chemical assay for the detection and determination of siderophores. Anal Biochem. 1987;160(1):47–56. doi: 10.1016/0003-2697(87)90612-9. [DOI] [PubMed] [Google Scholar]
- Souza Rd, Ambrosini A, Passaglia LMP. Plant growth-promoting bacteria as inoculants in agricultural soils. Genet Mol Biol. 2015;38(4):401–419. doi: 10.1590/S1415-475738420150053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuzet A, Perrier A, Leuning R. A coupled model of stomatal conductance, photosynthesis and transpiration. Plant, Cell Environ. 2003;26(7):1097–1116. doi: 10.1046/j.1365-3040.2003.01035.x. [DOI] [Google Scholar]
- Wahid A, Gelani S, Ashrafa M, Foolad MR. Heat tolerance in plants: an overview. Environ Exp Bot. 2007;61(3):199–223. doi: 10.1016/j.envexpbot.2007.05.011. [DOI] [Google Scholar]
- Wang JY, Jayasinghe H, Cho YT, Tsai YC, Chen CY, Doan HK, Ariyawansa HA. Diversity and biocontrol potential of endophytic fungi and bacteria associated with healthy welsh onion leaves in Taiwan. Microorganisms. 2023;11(7):1801. doi: 10.3390/microorganisms11071801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav R, Ror P, Rathore P, Kumar S, Ramakrishna W. Bacillus subtilis CP4, isolated from native soil in combination with arbuscular mycorrhizal fungi promotes biofortification, yield and metabolite production in wheat under field conditions. J Appl Microbiol. 2021;131(1):339–359. doi: 10.1111/jam.14951. [DOI] [PubMed] [Google Scholar]
- Yang J, Kloepper JW, Ryu C-M. Rhizosphere bacteria help plants tolerate abiotic stress. Trends Plant Sci. 2009;14(1):1–4. doi: 10.1016/j.tplants.2008.10.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.


