Abstract
Background
Health consumers increasingly want access to accurate, evidence‐based information about their medications. Currently, education about medications (that is, information that is designed to achieve health or illness related learning) is provided predominantly via spoken communication between the health provider and consumer, sometimes supplemented with written materials. There is evidence, however, that current educational methods are not meeting consumer needs. Multimedia educational programs offer many potential advantages over traditional forms of education delivery.
Objectives
To assess the effects of multimedia patient education interventions about prescribed and over‐the‐counter medications in people of all ages, including children and carers.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library 2011, Issue 6), MEDLINE (1950 to June 2011), EMBASE (1974 to June 2011), CINAHL (1982 to June 2011), PsycINFO (1967 to June 2011), ERIC (1966 to June 2011), ProQuest Dissertation & Theses Database (to June 2011) and reference lists of articles.
Selection criteria
Randomised controlled trials (RCTs) and quasi‐RCTs of multimedia‐based patient education about prescribed or over‐the‐counter medications in people of all ages, including children and carers, if the intervention had been targeted for their use.
Data collection and analysis
Two review authors independently extracted data and assessed the risk of bias of included studies. Where possible, we contacted study authors to obtain missing information.
Main results
We identified 24 studies that enrolled a total of 8112 participants. However, there was significant heterogeneity in the comparators used and the outcomes measured, which limited the ability to pool data. Many of the studies did not report sufficient information in their methods to allow judgment of their risk of bias. From the information that was reported, three of the studies had a high risk of selection bias and one was at high risk of bias due to lack of blinding of the outcome assessors. None of the included studies reported the minimum clinically important difference for the outcomes that were measured. We have therefore reported results from the studies but have been unable to interpret whether differences were of clinical importance.
The main findings of the review are as follows.
Knowledge: There is low quality evidence that multimedia education was more effective than usual care (non‐standardised education provided as part of usual clinical care) or no education (standardised mean difference (SMD) 1.04, 95% confidence interval (CI) 0.49 to1.58, six studies with 817 participants). There was considerable statistical heterogeneity (I2 = 89%), however, all but one of the studies favoured the multimedia group. There is moderate quality evidence that multimedia education was not more effective at improving knowledge than control multimedia interventions (i.e. multimedia programs that do not provide information about the medication) (mean difference (MD) of knowledge scores 2.78%, 95% CI ‐1.48 to 7.0, two studies with 568 participants). There is moderate quality evidence that multimedia education was more effective when added to a co‐intervention (written information or brief standardised instructions provided by a health professional) compared with the co‐intervention alone (MD of knowledge scores 24.59%, 95% CI 22.34 to 26.83, two studies with 381 participants).
Skill acquisition: There is moderate quality evidence that multimedia education was more effective than usual care or no education (MD of inhaler technique score 18.32%, 95% CI 11.92 to 24.73, two studies with 94 participants) and written education (risk ratio (RR) of improved inhaler technique 2.14, 95% CI 1.33 to 3.44, two studies with 164 participants). There is very low quality evidence that multimedia education was equally effective as education by a health professional (MD of inhaler technique score ‐1.01%, 95% CI ‐15.75 to 13.72, three studies with 130 participants).
Compliance with medications: There is moderate quality evidence that there was no difference between multimedia education and usual care or no education (RR of complying 1.02, 95% CI 0.96 to 1.08, two studies with 4552 participants).
We could not determine the effect of multimedia education on other outcomes, including patient satisfaction, self‐efficacy and health outcomes, due to an inadequate number of studies from which to draw conclusions.
Authors' conclusions
This review provides evidence that multimedia education about medications is more effective than usual care (non‐standardised education provided by health professionals as part of usual clinical care) or no education, in improving both knowledge and skill acquisition. It also suggests that multimedia education is at least equivalent to other forms of education, including written education and education provided by a health professional. However, this finding is based on often low quality evidence from a small number of trials. Multimedia education about medications could therefore be considered as an adjunct to usual care but there is inadequate evidence to recommend it as a replacement for written education or education by a health professional. Multimedia education may be considered as an alternative to education provided by a health professional, particularly in settings where provision of detailed education by a health professional is not feasible. More studies evaluating multimedia educational interventions are required in order to increase confidence in the estimate of effect of the intervention.
Conclusions regarding the effect of multimedia education were limited by the lack of information provided by study authors about the educational interventions, and variability in their content and quality. Studies testing educational interventions should provide detailed information about the interventions and comparators. Research is required to establish a framework that is specific for the evaluation of the quality of multimedia educational programs. Conclusions were also limited by the heterogeneity in the outcomes reported and the instruments used to measure them. Research is required to identify a core set of outcomes which should be measured when evaluating patient educational interventions. Future research should use consistent, reliable and validated outcome measures so that comparisons can be made between studies.
Keywords: Humans; Health Knowledge, Attitudes, Practice; Multimedia; Medication Adherence; Nonprescription Drugs; Nonprescription Drugs/therapeutic use; Patient Education as Topic; Patient Education as Topic/methods; Patient Education as Topic/standards; Prescription Drugs; Prescription Drugs/therapeutic use; Randomized Controlled Trials as Topic
Plain language summary
Multimedia programs for educating patients about medications
Consumers need detailed information about their medications to enable them to use their medications safely and effectively. For information to be useful it needs to be presented in a format that can be easily understood by consumers. There is evidence that methods such as spoken communication between the health provider and consumer and written materials are not meeting consumers’ needs. Multimedia education programs use more than one format to provide information. This could include using written words, diagrams and pictures with the use of audio, animation or video. They can be provided using different technologies, such as DVD and CD‐ROM, or can be accessed over the Internet.
This review presents the evidence from 24 studies, involving 8112 participants, of multimedia education programs about medications.
We found that multimedia education programs about medications are superior to no education or education provided as part of usual clinical care in improving patient knowledge. There was wide variability in the results from the six studies that compared multimedia education to usual care or no education. However, all but one of the six studies favoured multimedia education. We also found that multimedia education is superior to usual care or no education in improving skill levels. The review also suggested that multimedia was at least as effective as other forms of education, including written education or brief education from a health provider. However, these findings were based on a small number of studies, many of which were of low quality. Multimedia education did not improve compliance with medications (i.e. the degree to which a patient correctly follows advice about his or her medication) compared with usual care or no education. We could not determine the effect of multimedia education on other outcomes, such as patient satisfaction, self‐efficacy (confidence in their ability to perform health‐related tasks) and health outcomes.
The review findings therefore suggests that multimedia education programs about medications could be used alongside usual care provided by health providers. There is not enough evidence to recommend it as a replacement for written education or education by a health professional. Multimedia education could be used instead of detailed education given by a health provider when it is not possible or practical for health professionals to provide this service.
This review found that there were differences between the types of education provided to the control groups and what results were measured. This limited the ability to summarise results across studies, so most of the conclusions of this review were based on results from a small number of studies. More studies of multimedia educational programs are needed to make the results of this review more reliable.
Summary of findings
Summary of findings for the main comparison. Summary of findings: multimedia education compared with no education or usual care.
| Multimedia education compared with no education or usual care for prescribed and over‐the‐counter medications | ||||||
|
Patient or population: patients taking prescribed and over‐the‐counter medications or their carers Intervention: multimedia education Comparison: no education or usual care | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| no education or usual care | multimedia education | |||||
|
Knowledge (< 4 weeks)
Mean number of correct responses (higher score indicates higher knowledge) Follow‐up: 0 to 3 weeks |
The mean knowledge (< 4 weeks) in the intervention groups was 1.04 higher (0.49 to 1.58 higher) | 817 (6 studies) | ++OO lowa,b | Standardised score used as one study (Deitz 2011) did not provide a denominator and therefore could not be converted to the same scale as the other studies. A standard deviation of 1.04 represents a large difference between groups. 1 further study of 167 patients measured the number whose knowledge score improved and found that this was 458 versus 202 per 1000 in the intervention and control groups respectively (RR 2.26, 95% CI 1.39 to 3.67). The quality of the evidence was lowa,c. 1 study with 8 patients measured mean knowledge (%) at 4 weeks or more and found it was 22.5 higher (0.42 lower to 45.42 higher) in the intervention group. The quality of the evidence was very lowa,c,d. |
||
|
Skill acquisition
Mean inhaler technique score (%), higher score indicates better technique. Scale from: 0 to 100. Follow‐up: 0 to 9 months |
The mean skill acquisition in the control groups was 71.22%e | The mean skill acquisition in the intervention groups was 18.32 higher (11.92 to 24.73 higher) | 94 (2 studies) | +++O moderatea | ||
|
Compliance with medication
(different measures used by the studies: patient self‐report of compliance and prescription refill data). Follow‐up: 3 to 39 weeks |
Low risk populationf | RR 1.02 (0.96 to 1.08) | 4552 (2 studies) | +++O moderatea | ||
| 443 per 1000 | 452 per 1000 (425 to 478) | |||||
| Medium risk populationf | ||||||
| 704 per 1000 | 718 per 1000 (676 to 760) | |||||
| High risk populationf | ||||||
| 965 per 1000 | 984 per 1000 (926 to 1000) | |||||
|
Health outcomes: Drug misuse and dependence CAGE screening tool. Scale from 0 to 4. Higher score indicates greater probability of drug misuse or dependence |
The mean CAGE score in the control group was 0.86 | The mean CAGE score in the intervention group was 0.38 lower (0.60 lower to 0.15 lower) | 329 (1 study) |
++OO lowa,c | 1 further study with 43 participants measure pain on a scale of 0 (no pain) to 10 (worst possible pain) and found that pain in the intervention group was 0.5 lower (1.44 lower to 0.44 higher). However, the quality of evidence was very lowc,g. | |
| Medication side effects ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | One study measured medication side effects but did not report results in a form that could be extracted for meta‐analysis. The authors reported no statistically‐significant difference in the incidence of side effects between the two groups. |
| Quality of life ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No studies measured quality of life. |
|
Self‐efficacy Self‐efficacy for medication adherence (higher score indicates greater self‐efficacy, scale not reported) |
The mean self‐efficacy in the control group was 56.88 | The mean self‐efficacy in the intervention group was 1.94 higher (0.56 lower to 4.44 higher) | 343 (1 study) |
++OO lowa,c | 1 further study with 99 patients measured self‐efficacy for avoiding drug interactions (scale of 1 to 5) and found that mean self‐efficacy in the intervention groups was 1.38 higher (0.9 to 1.86 higher). The quality of the evidence was lowa,c. | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk Ratio; SMD: Standardised Mean Difference | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
a. All of the studies had unclear risk of bias for allocation concealment, blinding of outcome assessors or both.
b. Considerable statistical heterogeneity (I2 = 89%). However, all but one of the studies (Deitz 2011) favoured the multimedia group. Heterogeneity may be due to variability in the education provided to the control group as part of usual care.
c. Results are from only one study.
d. Wide 95% confidence interval including both no effect and substantial effect in the direction of the intervention group.
e. Control groups results measured at the same time point as was used in the meta‐analysis were used to calculate mean scores across the included studies.
f. Three assumed baseline risks were provided based on the control group risks in the included studies (low and high risk) and the median risk across the two studies (medium).
g. The study was at high risk of bias due to lack of allocation concealment.
Summary of findings 2. Summary of findings: multimedia education compared with written education.
| Multimedia education compared with written education for prescribed and over‐the‐counter medications | ||||||
|
Patient or population: patients taking prescribed and over‐the‐counter medications or their carers Intervention: multimedia education Comparison: written education | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| written education | multimedia education | |||||
|
Knowledge (number whose knowledge score improved) Follow‐up: one day |
570 per 1000a | 458 per 1000 (336 to 621) |
RR 0.80 (0.59 to 1.09) | 162 (1 study) |
+OOO very lowb,c | More patients had perfect scores at baseline in the multimedia group (241 versus 165 per 1000). Improvement could not be detected in these patients which may have biased results in favour of written education. |
|
Number who improved on skill
(global inhaler technique rating) Follow‐up: mean one hour |
Low risk populationd | RR 2.14 (1.33 to 3.44) | 164 (2 studies) | +++O moderatee | 1 further study with 20 participants reported that mean inhaler technique score (%) was 31 higher (10.15 to 51.85 higher) in the intervention group. The quality of the evidence was lowb,c. | |
| 180 per 1000 | 385 per 1000 (239 to 619) | |||||
| Medium risk populationd | ||||||
| 222 per 1000 | 475 per 1000 (295 to 764) | |||||
| High risk populationd | ||||||
| 260 per 1000 | 556 per 1000 (346 to 894) | |||||
| Compliance with medication | See comment | See comment | Not estimable | ‐ | See comment | No studies measured compliance with medication. |
| Health outcomes ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No studies measured health outcomes. |
| Medication side effects ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No studies measured medication side effects. |
| Quality of life ‐ not measured | See comment | See comment | ‐ | See comment | No studies measured quality of life. | |
| Self‐efficacy ‐ not measured | See comment | See comment | ‐ | See comment | No studies measured self‐efficacy. | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk Ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
a. The assumed risk was calculated from the mean baseline risk in the single study included in the meta‐analysis.
b. The study had unclear risk of bias for allocation concealment and blinding of outcome assessors.
c. Results are from only one study.
d. Three assumed baseline risks were provided based on the control group risks in the included studies (low and high risk) and the median risk across the two studies (medium).
e. Studies had unclear risk of bias for allocation concealment.
Summary of findings 3. Summary of findings: multimedia education compared with education by a health professional.
| Multimedia education compared with education by a health professional for prescribed and over‐the‐counter medications | ||||||
|
Patient or population: patients taking prescribed and over‐the‐counter medications or their carers Intervention: multimedia education Comparison: education by a health professional | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| education by a health professional | multimedia education | |||||
| Knowledge Mean number of correct responses (%). Scale from: 0 to 100. Follow‐up: mean 1 day | The mean knowledge in the control groups was 88.9 %a | The mean knowledge in the intervention groups was 2.2 lower (8.83 lower to 4.43 higher) | 22 (1 study) | +OOO very lowb,c | ||
| Skill acquisition (< 4 weeks) Mean inhaler technique score (%), higher score indicates better technique. Scale from: 0 to 100. Follow‐up: 0 to 2 weeks | The mean skill acquisition (< 4 weeks) in the control groups was 76.7%a | The mean skill acquisition (< 4 weeks) in the intervention groups was 1.01 lower (15.75 lower to 13.72 higher) | 130 (3 studies) | +OOO very lowd,e,f | Two studies with 130 patients reported skill acquisition at 4 weeks of more and found that mean inhaler technique score (%) was 4.29 lower (17.23 lower to 8.65 higher) in the intervention group. The quality of the evidence was lowd,e. | |
| Compliance with medication ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No studies measured compliance with medication. |
|
Health outcomes Bronchial obstruction (FEV), higher score indicates less bronchial obstruction. Theoretical maximum scores were 2.72 for the multimedia and 3.14 for the control group. Follow‐up: 15 days |
The mean FEV (at 15 days) in the control group was 2.09a | The mean FEV (at 15 days) in the intervention group was 0.29 higher (0.26 lower to 0.84 higher) | 28 (1 study) |
+OOO very lowc,e,g | ||
| Medication side effects ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No studies measured medication side effects. |
| Quality of life ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No studies measured quality of life. |
| Self‐efficacy ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No studies measured self‐efficacy. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk Ratio; MD: Mean Difference; FEV: Forced Expiratory Volume | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
a. Control groups results measured at the same time point as was used in the meta‐analysis were used to calculate mean scores across the included studies.
b. The study was at high risk of bias due to lack of allocation concealment.
c. Results were based on one study with a small sample size and wide 95% confidence interval.
d. All of the studies had unclear risk of bias for blinding of outcome assessors. All of the studies except Lirsac 1991 also had unclear risk of bias for allocation concealment.
e. Wide 95% confidence interval including both no effect and substantial effect.
f. Substantial statistical heterogeneity (I² = 68%) which may be due to differences in the education provided to the control group.
g. The study had unclear risk of bias for blinding of outcome assessors.
Summary of findings 4. Summary of findings: multimedia education compared with written education and education by a health professional.
| Multimedia education compared with written education and education by a health professional for prescribed and over‐the‐counter medications | ||||||
|
Patient or population: patients taking prescribed and over‐the‐counter medications or their carers Intervention: multimedia education Comparison: written education and education by a health professional | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| written education and education by a health professional | multimedia education | |||||
| Knowledge ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No studies measured knowledge. |
| Compliance with medication ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No studies measured compliance with medication. |
| Number who improved on skill (rating of inhaler technique) Follow‐up: mean 1 day | 429 per 1000a | 502 per 1000 (300 to 832) | RR 1.17 (0.7 to 1.94) | 69 (1 study) | +OOO very lowb,c | |
| Health outcomes ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No studies measured health outcomes. |
| Medication side effects ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No studies measured medication side effects. |
| Quality of life ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No studies measured quality of life. |
| Self‐efficacy ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No studies measured self‐efficacy. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk Ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
a. The assumed risk was calculated from the mean baseline risk in the single study included in the meta‐analysis.
b. The study had unclear risk of bias for allocation concealment.
c. Results were based on a single study with a relatively small sample size and wide confidence interval.
Summary of findings 5. Summary of findings: multimedia education compared with control multimedia.
| Multimedia education compared with control multimedia for prescribed and over‐the‐counter medications | ||||||
|
Patient or population: patients taking prescribed and over‐the‐counter medications or their carers Intervention: multimedia education Comparison: control multimedia | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| control multimedia | multimedia education | |||||
| Knowledge Mean number of correct responses (%). Scale from: 0 to 100. Follow‐up: 3 to 6 months | The mean knowledge in the control groups was 61%a |
The mean knowledge in the intervention groups was 2.78 higher (1.48 lower to 7.05 higher) | 568 (2 studies) | +++O moderateb | ||
|
Skill acquisition Inhaler technique (scored 0 to 100 and reported as change in score from baseline) Follow‐up: 1 month |
The mean change in inhaler technique score in the control group was 1.62 out of 100a | The mean change in inhaler technique score in the intervention group was 13.3 higher (8.98 to 17.62 higher) |
116 (1 study) |
++OO lowb,c | Data from the last time point at which the outcome was measured included in the 'Summary of findings' table. | |
| Compliance with medication. Mean score of the Chronic Disease Compliance Instrument (CDCI), higher score indicates better compliance. Scale from: 18 to 90. Follow‐up: 3 months | The mean compliance with medication in the control groups was 78.4 out of 90a | The mean compliance with medication in the intervention groups was 2.6 higher (0.78 to 4.42 higher) | 303 (1 study) | ++OO lowc,e | ||
|
Health outcomes Peak expiratory flow readings (higher readings indicate better peak flow) Follow‐up: 1 day |
The mean peak flow in the control group was 382d | The mean peak flow in the intervention group was 21 higher (5.28 to 37.20 higher) |
116 (1 study) |
++OO lowb,c | ||
| Medication side effects ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No studies measured medication side effects |
| Quality of life (QOL). Outcome was measured on different scales in different studies (higher score indicates better QOL) Follow‐up: mean 3 months | The mean quality of life in the intervention groups was 0.2 higher (0.04 lower to 0.44 higher) | 269 (1 study) | +++O moderatec | A standard deviation of 0.2 represents a small difference between groups. SMD 0.2 (‐0.04 to 0.44) | ||
| Self‐efficacy. Self efficacy score (higher score indicates greater self‐efficacy). Scale from: 27 to 189. Follow‐up: mean 3 months | The mean self‐efficacy in the control groups was 158.8 out of 189a | The mean self‐efficacy in the intervention groups was 5.3 higher (0 to 10.6 higher) | 303 (1 study) | +++O moderatec | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk Ratio; SMD: Standardised Mean Difference | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
a. Control groups results measured at the same time point as was used in the meta‐analysis were used to calculate mean scores across the included studies.
b. One or more of the studies had unclear risk of bias for allocation concealment.
c. Results are based on only one study.
d. The assumed risk was calculated from the mean baseline risk in the single study included in the meta‐analysis.
e. Baseline compliance levels were higher in the intervention group.
Summary of findings 6. Summary of findings: multimedia education and a co‐intervention compared with co‐intervention alone.
| Multimedia education and a co‐intervention compared with the co‐intervention alone for prescribed and over‐the‐counter medications | ||||||
|
Patient or population: patients taking prescribed and over‐the‐counter medications or their carers Intervention: multimedia education and a co‐intervention Comparison: co‐intervention alone | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| co‐intervention alone | multimedia education and a co‐intervention | |||||
| Knowledge (< 4 weeks) Mean number of correct responses (%). Scale from: 0 to 100. Follow‐up: 2 to 14 days | The mean knowledge (< 4 weeks) in the control groups was 68.76%a | The mean knowledge (< 4 weeks) in the intervention groups was 24.59 higher (22.34 to 26.83 higher) | 381 (2 studies) | +++O moderateb | 1 study with 60 patients measured mean knowledge (%) at 4 weeks or more and found it was 23.3 higher (12.82 to 33.58 higher) in the intervention group. The quality of the evidence was lowb,c. | |
| Skill acquisition Mean inhaler technique score (%), higher score indicates better technique. Scale from: 0 to 100. Follow‐up: mean 2 weeks | The mean skill acquisition in the control groups was 59.1%a | The mean skill acquisition in the intervention groups was 3.4 higher (8.3 lower to 15.1 higher) | 87 (1 study) | +OOO very lowb,c,d | ||
| Non‐compliance with medications score Mean non‐compliance score (lower scores indicate better compliance). Scale from: 0 to 160. Follow‐up: 2 to 6 days | The mean non‐compliance score in the control groups was 40.45 out of 160a | The mean non‐compliance score in the intervention groups was 32.15 lower (51.13 to 13.17 lower) | 320 (1 study) | ++OO lowb,c | 1 further study with 77 patients measured the number who completed their course of medications and found that this was 659 versus 639 per 1000 in the intervention and control groups respectively (RR 0.97, 95% CI 0.7 to 1.35). The quality of the evidence was lowc,d. | |
|
Health outcome (Number who had a medical complication of pelvic inflammatory disease) Follow‐up: two weeks |
317 per 1000e | 361 per 1000 (193 to 675) |
RR 1.14 (0.61 to 2.13) | 77 (1 study) |
++OO lowc,d | |
| Medication side effects Mean adverse self‐medication behaviour score (lower scores indicate safer self‐medication behaviour). Scale from: 0 to 40. Follow‐up: mean 4 weeks | The mean medication side effects in the control groups was 21 out of 40a | The mean medication side effects in the intervention groups was 5 lower (10.06 lower to 0.06 higher) | 60 (1 study) | +OOO very lowb,c,f | Data from the last time point at which the outcome was measured included in the 'Summary of findings' table. | |
| Quality of life ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No studies measured quality of life |
| Self‐efficacy Self‐efficacy rating scale (higher score indicates greater self‐efficacy). Scale from: 1 to 5. Follow‐up: mean 4 weeks | The mean self‐efficacy in the control groups was 3 on 1‐5 rating scalea | The mean self‐efficacy in the intervention groups was 0.9 higher (0.52 to 1.28 higher) | 60 (1 study) | ++OO lowb,c | Data from the last time point at which the outcome was measured included in the 'Summary of findings' table. | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk Ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
a. Control groups results measured at the same time point as was used in the meta‐analysis were used to calculate mean scores across the included studies.
b. The studies had unclear risk of bias for allocation concealment.
c. Results were based on only one study.
d. Wide 95% confidence interval including both no effect and substantial effect in the direction of the multimedia group.
e. The assumed risk was calculated from the mean baseline risk in the single study included in the meta‐analysis.
f. Outcome measures patient behaviours that are likely to cause medication adverse effects rather than measuring medication adverse effects directly.
Background
Patient education about medications
Health consumers increasingly want access to accurate, evidence‐based information about their health condition and its treatment. There is evidence, however, that current methods for delivering this information are not meeting their needs. Studies have shown a mismatch between health professionals' and health consumers' views about the amount and type of information patients should receive about their medications (Berry 1997; Nair 2002). These groups also appear to have different priorities relating to the purpose of medication information (Grime 2007). Health consumers generally want more information than is provided to facilitate informed decision making, presented in a way that is easy to understand; whereas health professionals focus upon the need to improve medication compliance, save consultation time and establish medico‐legal evidence of informed consent.
Formats for delivering educational materials
For information to be useful for health consumers it needs to be presented in a format that they can understand. Currently, patient information about medications is presented predominantly via spoken communication between the health provider and consumer, sometimes supplemented with written materials. Studies have shown that patients find it difficult to retain information told to them by their doctors, with 40 to 80% of information forgotten immediately (Ley 1982). The amount of information retained is inversely proportional to the amount of information presented, and almost half of the information that is retained is remembered incorrectly (Kessels 2003). Written information that is provided to reinforce verbal communication has been shown to produce variable results. A Cochrane review of written information for consumers about medications found that while some studies showed that patients given written information had improved knowledge about their medications, overall the results were mixed (Nicolson 2009). Moreover the quality of written materials about medications may vary, particularly with respect to their content, structure and usability (Buchbinder 2001; Clerehan 2005; Clerehan 2009).
Potential value of multimedia education programs
Multimedia education programs which provide information using more than one format offer many potential advantages over traditional forms of information delivery. The combination of audio with graphic presentation of information, or the use of animation or video may overcome barriers that poor literacy creates for some consumers (Liao 1996; Wofford 2001). Multiple studies have shown that learning is improved when students are presented with information in an audiovisual, rather than visual only, format (Kalyuga 2000; Mayer 1998; Moreno 1999; Tindall‐Ford 1997). As opposed to spoken communication from a health professional, multimedia education programs can be viewed at a pace that suits the consumer, with information repeated as required. They can be provided using different technologies, many of which are portable or can be accessed over the Internet. This allows consumers and their families to access the information at a time and place that is convenient for them, and the information to be widely disseminated at little cost. The anonymity provided by the programs may allow patients to access information that they may be uncomfortable requesting from their doctor. One of the advantages of multimedia programs over written materials is that they often allow information to be tailored, or personalised, to individual patients and their needs. Tailored information is more likely to be used and viewed as relevant compared with generic information. There is also some evidence that it may be more effective at modifying health‐related behaviours (Lustria 2009).
Multimedia programs vary in their interactivity, which is defined as the degree of user control and program responsiveness. User control is the ability of the user to alter the form and content of a program, select topics or services and the order in which they are presented, and respond to information presented. Responsiveness is the degree to which a program is able to take into account and respond to the actions of the user. Interactivity increases user engagement, active information processing and satisfaction, and increases the effectiveness of educational materials (Street 1997).
Like any other information format, multimedia education programs may also vary in content accuracy. Additional quality concerns include the ease with which the written, spoken or graphical information is understood, and the usability of the program. The way the program is delivered may also result in the exclusion of certain sections of the population who do not have access to the technology or the technical knowledge required to use it. This may be a particular problem for people from low socioeconomic backgrounds and/or the elderly (Bozionelos 2004; Wright 2009).
The impact of patient education about medications
Tones 1994 defined health education as any intentional activity which is designed to achieve health‐ or illness‐related learning. Information about medications which aims to produce changes in areas including knowledge, beliefs, skills and behaviours would meet this definition for health education. The aims and therefore the relevant outcomes of health education vary. The most direct effect of education would be to increase knowledge and skill levels. Health consumers who are prescribed medications are expected to perform certain self‐management tasks. These include making an informed decision about taking the medication, self‐administering the medication at the right dose and time, monitoring their response to treatment, recognising side effects of the medication and alerting health professionals if they are not responding or develop side effects. These self‐management tasks are particularly important for those suffering chronic conditions. Many health consumers may take multiple medications which makes self‐management more complex and increases the risk of adverse events (Gray 2009). To perform these tasks successfully, health consumers require detailed information about their medications.
Increased knowledge about medications may lead to changes in health‐related behaviour and improved health outcomes. However, a recent overview of systematic reviews evaluating the effects of consumer‐oriented interventions on medicines use suggests that patient education limited to information provision alone is insufficient to effect change (Ryan 2011). Guidelines for the management of asthma recommend the use of additional strategies including patient self‐monitoring, regular medical review and the used of a written action plan, which, in combination with patient education, have been shown to improve health outcomes (Gibson 2002). Ryan's overview supports this approach, finding that interventions that included self‐monitoring and self‐management, and possibly the combination of education with self‐management, show promise in improving adherence and medicine use (Ryan 2011). There may also be more direct effects of education on patients’ emotions, beliefs and attitudes as well as health‐related behaviour, which have not been evaluated in these reviews.
Much of the literature related to patient education about medication and behaviour change has focused upon medication compliance or adherence, which is defined as the degree to which a person’s behaviour coincides with medical advice. This assumes that the patient has a passive role in accepting medical direction (Bajramovic 2004). Studies of educational interventions about medications have shown mixed effects on compliance, with some interventions successfully increasing knowledge without a resulting increase in compliance rate (Brus 1998; Cote 1997). A Cochrane review found that interventions that were effective for improving patient compliance with long‐term treatments were typically complex and multi‐faceted (Haynes 2008). The failure of educational interventions to improve compliance rates may be due to the frequency of intentional non‐compliance. This is where patients deliberately choose, for reasons such as side effects or perceived lack of effectiveness, not to take their medication as prescribed (Lowe 2000). These decisions are often not reported back to the treating doctor.
More recently, the emphasis in medication prescription has shifted from 'compliance' to 'concordance'. Concordance entails a partnership, with two‐way communication between the patient and the health professional. The patient is encouraged to share their preferences and beliefs, is fully informed about the benefits and risks of treatment, and participates as a partner with the health professional in reaching agreement about treatment (Stevenson 2004). This collaborative approach to prescribing medications, whereby the patient is involved in the discussion and decision‐making process, has been shown to improve patients’ knowledge and initial beliefs about the medication, medication use, and satisfaction with both medication and care (Bultman 2000). Patient education about treatment is a vital component of the concordance model. However, concordance is difficult to measure, and it has not been widely adopted as an outcome measure for evaluating the effectiveness of educational interventions. Patient‐reported outcomes measuring health consumers' perception of components which make up the concordance model are more readily measurable. These may include their attitudes and beliefs about medications, satisfaction with the education and care they received, participation in decision making, and confidence in their ability to perform health‐related tasks (self‐efficacy).
As well as examining the benefits of patient education, it is also important to ensure that it is not causing any harm. There is evidence that medication compliance is not decreased by informing patients of a medication's potential adverse events (Haynes 2008). However, particular studies have raised concern that informing patients of all potential risks of a medication may increase anxiety and the incidence of medication side effects by suggestion, overload them with too much information from which they will be unable to weigh potential risks against benefits, and lead to their missing out on beneficial treatments (Fraenkel 2002; Pullar 1990).
Relationship to other reviews
This review covers multimedia education about medications for any condition, and therefore is likely to overlap with other existing Cochrane and non‐Cochrane reviews. Two reviews, performed in 2005 and 2008, examined randomised controlled trials of multimedia patient educational interventions (Jeste 2008; Wofford 2005). The reviews used different definitions for multimedia: Wofford 2005 defined it as using graphics (animation or video) and/or audio with or without the use of supporting text, while Jeste 2008 defined it as utilising both an auditory‐verbal channel and a visual‐pictorial channel. Wofford 2005 limited interventions to those that utilised desktop computers for delivery, therefore excluding interventions delivered as videotapes or using portable devices. Jeste 2008 excluded interventions aimed at carers and studies that did not report objective measures of knowledge. Both reviews excluded studies that were not published in English and had study participants less than 18 years of age. Our review is more comprehensive in that we did not restrict inclusion to studies published in English, nor by age or category of health consumer, and we considered a wide range of outcomes, including interventions targeted to carers and outcomes for carers. Our definition of multimedia is also broader. Although both reviews focused on educational interventions they also included decision aids and interventions providing counselling, cognitive behavioural therapy, emotional support and case management. They also included interventions that contained multiple components, where the effect of multimedia education on outcomes could not be separated from the effect of other components of the intervention.
The Cochrane review by Murray et al (Murray 2005) deals with Interactive Health Communication Applications (IHCAs) for people with chronic diseases. These are defined as computer‐based information packages for patients that combine health information with at least one other intervention such as decision support, behaviour change support or peer support. Complex programs involving multiple interventions in combination with health information were excluded from our review unless the effect of the multimedia educational intervention on measured outcomes was clearly separated from the impact of the other interventions. Our review will not be limited to people with chronic conditions nor by the type of technology used to deliver the program. IHCAs are, by their definition, interactive, but do not necessarily have a multimedia component. Non‐interactive interventions such as video were therefore not considered in Murray's review.
A number of reviews examine the effectiveness of education, and other types of interventions, at improving medication adherence (Haynes 2008; M'Imunya 2012; Rueda 2006). The M'Imunya and Rueda reviews are disease specific, in that they deal with any type of intervention that aims to improve adherence to treatments for tuberculosis and HIV respectively. The Haynes review is much broader, in that it deals with any type of intervention intended to improve adherence to prescription medications used to treat any condition. All of these reviews focus on adherence and clinical outcomes. However, it is likely that they will contain some studies which overlap with our review. An overview of systematic reviews examines consumer‐oriented interventions for evidence‐based prescribing and medicines use (Ryan 2011). This overview includes a broad range of interventions and outcomes and may contain studies which overlap with our review.
A number of Cochrane reviews examine self‐management education, particularly for the management of chronic diseases (Effing 2007; Powell 2002; Shaw 2010). Self‐management education teaches skills that allow patients to identify their problems, and provides them with techniques to help them make decisions, take appropriate actions and alter those actions as they encounter changes in circumstance or disease. The development of an action plan is a central feature of this process (Bodenheimer 2002). Education is a key component of self‐management programs. Our review will focus on purely educational interventions where medication‐specific information and technical skills are transferred to a patient. Our review will include studies in which the purpose of this information transfer is to improve self‐management, but will exclude more complex self‐management education programs unless the effect of the multimedia educational component can be separated from the effect of the other components of the program.
Why it is important to do this review
Despite the potential benefits of multimedia compared with traditional methods of patient education delivery, there is concern that the application of these programs may precede evidence of their effectiveness (Robinson 1998). The development and production of multimedia programs is expensive, especially when this is compared to the limited cost involved in producing information leaflets. Although there have been a number of Cochrane reviews in the area of patient education, this will be the only review that looks specifically at the effects of multimedia presentation of information related to medication on knowledge, skills, and health outcomes.
Objectives
To assess the effects of multimedia patient education interventions about prescribed and over‐the‐counter medications in people of all ages, including children and carers.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) and quasi‐RCTs (in which methods of allocating participants to treatment are not strictly random, e.g. using alternation, date of birth, or some similar method of allocation). Some studies professing to be RCTs are actually quasi‐RCTs. However, this can only be determined during a risk of bias assessment and only in studies that adequately report their methods for randomisation. We therefore decided to include all RCTs and quasi‐RCTs and then deal with randomisation issues in the Risk of bias in included studies and in sensitivity analysis. Studies that randomised individuals or cluster‐randomised trials that randomised by groups, for example by medical practice, were eligible for inclusion.
Types of participants
Participants included people of all ages who had been prescribed (or directly administered by a health professional) a particular medication or medication regimen, or who had obtained an over‐the‐counter medication. We included children and carers if the intervention had been targeted for their use. Participants also included people who were provided with education about a particular medication, medication regimen or over‐the‐counter medication but who had not been prescribed or obtained the medication.
We excluded studies relating to unlicensed medications and complementary medicines, including vitamins and nutritional supplements.
Types of interventions
We included studies of multimedia‐based patient education about prescribed or over‐the‐counter medications.
Intervention format
Multimedia was defined as the delivery of information using a combination of formats. Formats were divided into:
text, still graphics or photographs
animation and video
audio
We included interventions that delivered information using a combination of at least two different formats. However, we excluded interventions if they contained only text with still graphics or photographs, e.g. printed pamphlets with pictures or Internet pages with text and still pictures.
We included multimedia interventions which were interactive or tailored. All mechanisms for delivering multimedia programs (such as web‐based, DVD and CD‐ROM) were included in the review. We included studies that evaluated multimedia interventions directed at participants both before they had started their medication and once they were already taking it.
Intervention content
For the study to be included in the review, the educational intervention must have included information or education about a particular medication or group of medications as its primary focus. We excluded interventions that did not contain an education component (e.g. electronic history taking).
Complex programs that included a multimedia education program as part of a number of interventions were only included if the impact of the multimedia intervention on measured outcomes could be clearly separated from the effect of the other interventions. Decision aids, which are primarily designed to assist consumers in choosing between treatment options, were not included. The intervention must have been designed to inform or educate health consumers or their carers. We excluded interventions that were aimed at educating health professionals.
Comparison interventions
The main comparison groups included:
no education;
standard or usual care i.e. where no standardised educational intervention is provided as part of the trial but participants received non‐standardised education from health professionals involved in their care;
other forms of education (not using the multimedia format)
a control multimedia education program that provides generic information, or information not pertaining to the medication or treatment being considered in the study.
The content of the comparison interventions was assessed in the same way as for the multimedia educational interventions.
Types of outcome measures
The type of outcomes reported was not used to determine study eligibility for inclusion. Both binary and continuous outcomes were selected as appropriate. We extracted data, when available, for all of the following outcomes for each of the trials, extracting data at all time points measured.
Primary outcomes
The primary outcomes were:
Patient or carer knowledge about the medication. Increased knowledge about a medication was considered the most direct effect of an educational intervention.
Any measure of skill acquisition related to the medication (e.g. ability to administer injectable medications appropriately).
Secondary outcomes
Secondary outcomes included:
Health‐related behaviour, including medication compliance/adherence or concordance.
Health outcomes, including disease‐related outcomes and safety data including adverse events related to the medication.
Patient‐ or carer‐reported outcomes: these were varied and included quality of life, self‐efficacy, emotions (including anxiety caused by the educational intervention), beliefs and attitudes about the medication, satisfaction with the care and education received, and the user's perception of the quality and usability of the educational intervention. Outcomes relating to self‐management itself (such as perception of ability to self‐manage) were included here, while measures of ability to perform specific self‐management tasks (such as safely self‐administer medication) were included under skill acquisition in the primary outcomes.
Participant usage of the intervention.
Cost, including the cost of health care, the cost‐effectiveness of the educational intervention, and resource utilisation such as the number of hospital admissions and medical reviews.
Search methods for identification of studies
Electronic searches
We searched the following electronic databases and sources to identify studies:
Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library) to June 2011;
MEDLINE (Ovid) 1950 to June 2011;
EMBASE (Ovid) 1974 to June 2011;
CINAHL (EBSCOhost) 1982 to June 2011;
PsycINFO (Ovid) 1967 to June 2011;
ERIC (Educational Resources Information Centre) 1966 to June 2011;
ProQuest Dissertation & Theses Database to June 2011.
We present the search strategies in Appendix 1 to Appendix 7. There were no language restrictions.
Searching other resources
We systematically searched the reference lists of reviews that potentially overlapped with our review, reviews identified by our search strategy and the studies retrieved in full text, to identify potentially relevant studies. We also checked the personal records of the review authors to identify any further studies for inclusion.
Data collection and analysis
Selection of studies
Two review authors (SC, RJ) independently screened the titles and abstracts of identified studies for possible inclusion, and removed duplicate records of the same report. The full text of all potentially‐relevant studies were retrieved and assessed for inclusion. If studies had insufficient information on which to base a decision, we contacted the study authors to obtain further information. Disagreements regarding inclusion were resolved by discussion and consensus, or by consulting a third author. Relevant studies that we excluded are listed in the Characteristics of excluded studies table with the primary reason for exclusion given.
Data extraction and management
Two review authors (SC, RJ) independently extracted data from all included studies using a form derived from the data extraction template of the Cochrane Consumers and Communication Review Group. Disagreements were resolved by discussion or through a third party. Extracted data included details about the participants, the intervention, the outcomes assessed and the study results. Extracted data were summarised in the Characteristics of included studies tables.
Data extracted about participants included general demographics information and where available, information about: their literacy (as defined or reported by the study authors); educational level; the medical condition being treated; their co‐morbidities; and other medications they were receiving.
Information extracted about the intervention included the format or media used, when and how it was delivered, whether it reached its intended audience, and details regarding the type and degree of interactivity or tailoring.
Based upon systemic functional linguistics, two of the authors (RC and RB) have previously developed a linguistic framework, the Evaluative Linguistic Framework (ELF), for evaluating the quality of medication leaflets intended for patients (Clerehan 2005; Clerehan 2009). From this work, which included consumer input about what information needs to be included (Hirsh 2009), we found that medication leaflets have an identifiable generic structure that may include up to ten 'moves' or identifiable sections.
These include:
Background of the drug;
Summary of use of drug;
Dosage instructions;
Outline of benefits of drug;
Account of side‐effects;
Information regarding monitoring required e.g. regular blood tests
Constraints on patient behavior (including information about drug interactions);
Contraindications to use;
Storage instructions; and
Clinical contact availability
We contacted study authors to request access to the multimedia intervention. Where this was not made available, our evaluations were based on descriptions of the intervention from the studies. The specific content of the multimedia educational interventions was extracted and assessed according to the ten ELF content areas.
In addition, we systematically evaluated and report the quality of the multimedia intervention according to the ELF (Clerehan 2009) (Table 7). The ELF was specifically developed for written patient information and was adapted for assessment of the quality of multimedia patient education interventions. It considers the generic or overall structure of the intervention, its rhetorical elements (function of each section, such as to define, instruct or inform the reader), nature of the relationship between the developer and reader (such as medical expert to layperson, etc), metadiscourse (description of the purpose or structure of the text), headings, the technicality of the medical terminology, density of the content words (or 'lexical density') and its factual content. We documented whether literacy, format, readability and usability issues were considered. In addition we determined whether health consumers were involved in its development.
1. Framework for evaluating healthcare text based upon systemic functional linguistics.
| Item | Description | Assessment |
| Overall organisational or generic structure of the text | Series of stages or moves in a text (e.g. background on drug, dosage instructions, account of side effects) | What identifiable sections of text (moves) are present? Are all essential moves included? What is the sequence of moves and is this appropriate? |
| Rhetorical elements | The function of each move in relation to the reader (e.g. to define, instruct, inform) | What are the rhetorical functions of each move in relation to the reader? Are these clearly defined and appropriate? Is there clear guidance about what to do with the presented information? Are instructions clear about what action needs to be taken? |
| Relationship between writer and reader | Nature of the relationship between the writer and reader (e.g. medical expert to layperson; doctor to his/her patient) | Is it clear who the writer and intended audience is? Is the relationship between writer and reader clear and consistent? Is the person who is expected to take responsibility for any actions clear? Is the importance and/or urgency of the action made clear? How positive (encouraging, reassuring) is the tone? |
| Meta discourse | Description of the purpose/structure of the text | Is there a clear description of the purpose of the text? |
| Headings | Signposts in the text for the reader | Are headings present? If present, are they appropriate? |
| Technicality of vocabulary | The technicality of the medical terminology/other vocabulary that is used | How technical is the vocabulary that is used in the text? Is this appropriate? |
| Lexical density | Density of the content words in the text | What is the average content density of the text (percentage of content‐bearing words)? Is this appropriate? |
| Factual content of text | Facts included in the text | Is the factual information correct and up‐to‐date? Is the source of information provided? Is the quality and strength of the evidence discussed? |
| Format | Visual aspects such as layout, font size, style, use of visual material, etc. | What is the length, layout, font size and visual aspect of the document? |
If a trial included multiple measures of a particular outcome (e.g. knowledge), we extracted only one of these measures to prevent double counting. If the study identified one of the measures as the primary outcome, or specified it in its sample size calculation, then we extracted these data. Otherwise the decision as to which measure to extract was made by consensus opinion of the authors, based on the clinical importance of the outcome, whether the measure had been validated and found to be reliable, and its similarity to those reported in other studies. Authors involved in this decision were blinded to the results for each of the outcomes.
Where studies reported outcomes at more than one time point, we examined and reported data at all time points to determine if the effects of the intervention, particularly on acquisition of knowledge and skills, persisted or changed over time. For the meta‐analysis we decided to divide time points into those that were short‐term (< 4 weeks) and long‐term (≥ 4 weeks) and if there were multiple time points within these categories, we extracted data from the last time point measured. This division of time points was a post‐hoc decision based on the range of time points reported by the included studies.
Assessment of risk of bias in included studies
We assessed and reported the risk of bias of included studies in accordance with the Cochrane Handbook (Higgins 2011a), which recommends the explicit reporting of the following individual elements for RCTs: sequence generation; allocation concealment; blinding, incomplete outcome data; selective outcome reporting; and other sources of bias. For each of these domains, we described the methods used in each study, and made a judgment about the risk of bias using the guidelines in Higgins 2011a, with each domain judged as having low, high or unclear risk of bias. Blinding was assessed for both participants and outcome assessors. Studies were judged to be at low risk of bias if both the participants and assessors were adequately blinded; if the outcomes measured were objective and unable to be influenced by lack of blinding or if the assessment occurred immediately following the intervention, minimising the potential for the lack of blinding of participants to influence outcomes. We also examined the method of intervention delivery to determine if it had the potential to cause selection bias; for example, inadvertently selecting participants from higher socioeconomic backgrounds or with higher levels of educational attainment by only including participants who have access to certain technologies. We contacted study authors for additional information about the included studies, or for clarification of the study methods as required. We incorporated the results of the 'Risk of bias' assessment into the review through systematic narrative description and commentary about each of the elements, leading to an overall assessment of the risk of bias of included studies, a judgement about the internal validity of the review’s results and a GRADE assessment of the quality of evidence for each outcome (Schünemann 2008b).
Measures of treatment effect
We analysed separately studies that compared the multimedia intervention to no intervention (including usual care), to other forms of education (written information, education by a health professional or both) and to other multimedia interventions, as well as those that compared multimedia in combination with a co‐intervention to the co‐intervention alone. Effects were expressed as mean differences (MD) and 95% confidence intervals (CI) for continuous outcomes and risk ratios (RRs) and 95% CI for dichotomous outcomes. For continuous outcomes, if individual studies used different scales to measure the same outcome (and results could not be converted into the same scale) then we used standardised mean differences (SMD).
Unit of analysis issues
We intended that potential unit of analysis errors, caused by cluster randomised trials failing to appropriately account for correlation of observations within clusters, would be corrected by incorporating an approximate analysis of the trial using methods recommended by the Cochrane Collaboration (Higgins 2011b).
Dealing with missing data
We contacted study authors to obtain missing statistical data. Outcomes were analysed using intention‐to‐treat results where possible. Where this was not possible, the data were analysed as reported by the study authors. We reported the number of participants lost to follow‐up and reasons given for attrition in each study as part of the 'Risk of bias' assessment. We calculated missing standard deviations (SDs), where possible, from other reported statistics (Higgins 2011c). Where this was not possible, the SD was imputed from the most representative study; i.e. the study with the greatest weight on the meta‐analysis. The possible impact on missing data on the findings of the review was investigated in sensitivity analyses, and discussed in the review (Higgins 2011b).
Assessment of heterogeneity
Before meta‐analysis, studies were assessed for clinical homogeneity with respect to type of multimedia program, control group, and the outcomes measured. Clinically heterogeneous studies were not combined in the analysis, but separately described. For studies judged as clinically homogeneous, we tested for statistical heterogeneity using the Chi2 test. We interpreted the Chi2 test as indicating significant statistical heterogeneity if the Chi2 significance (P value) was less that 0.10. This value was chosen instead of the conventional significance level of 0.05 to counteract the low power of the Chi2 test when meta‐analyses contain studies with small sample sizes or that are few in number. In order to assess and quantify the possible magnitude of inconsistency across studies, we examined the I2 statistic. The I2 statistic describes the percentage of the variability in effect estimates that is due to heterogeneity rather than sampling error. It was interpreted as: 0% to 40% representing heterogeneity that may not be important; 30% to 60% representing moderate heterogeneity; 50% to 90% representing substantial heterogeneity; 75% to 100% representing considerable heterogeneity (Deeks 2011).
Assessment of reporting biases
We planned to assess publication bias graphically using a funnel plot if there were at least 10 studies included in the meta‐analyses of the primary outcomes (knowledge and skill acquisition) (Sterne 2011). However, we identified fewer than 10 studies, precluding the analysis.
We assessed the potential for 'small sample' reporting bias by investigating whether the random‐effects model produced similar effect sizes as the fixed‐effect model. In the presence of small‐study effects, the intervention effect is more beneficial in the smaller studies, and the random‐effects estimate of the intervention effect will be more beneficial than the fixed‐effect estimate (Sterne 2011).
Data synthesis
All trials, regardless of their assessed risk of bias, were pooled in the primary analysis. We pooled studies using the random‐effects model due to the likely heterogeneity of the multimedia interventions being evaluated. We presented results for each of the outcomes, organised by the comparison intervention:
Multimedia compared to no education or usual care
-
Multimedia compared with other forms of education
written education
education by a health professional
written education and education by a health professional
Multimedia compared with 'control' multimedia program
Multimedia plus a co‐intervention compared with the co‐intervention alone
We used forest plots to illustrate the results of meta‐analyses where there was more than one study for that outcome. Where there was only one study for a particular outcome, we included the results in the text. We also presented results for all studies in Additional tables (Table 8).
2. Study outcome data.
| Study | Study arms | Timing of follow‐up | N at follow‐up | Outcome | Results |
| Acosta 2009 | Multimedia (I) vs control multimedia (C) | Immediately before (baseline) and after (post) the intervention and after one month. | I: 62 C: 54 |
Skill acquisition (inhaler technique) | Change in score (% correct) from baseline: Post intervention: I: 15.92 (SD 15.04), C: 1.16 (6.55), P < 0.001, mean difference 14.77, 95% CI 10.39 to 19.15. After 1 month: I: 14.92 (SD 15.49), C: 1.62 (SD 7.30) P < 0.001, mean difference 13.30 95% CI 8.74 to 17.86. |
| Peak flow | Post intervention I: 403.45 (95% CI 392.40 ‐ 414.94), C: 382.21 (95% CI 370.42 ‐ 394.01). Results are "controlled for pre‐intervention peak flows". There was no significant difference in pre intervention peak flows readings (C: 409.81, I: 386.94, P = 0.18) | ||||
| Deitz 2011 | Multimedia (I) vs control (C) | After the intervention period | Total post‐test N = 346 I: 181 C: 165 |
||
| N (who completed the knowledge questionnaire) = 329 | Knowledge of prescription drug use and dependency | Mean score (SD): Drug facts I: 13.23 (2.40), C: 12.84 (2.33), P = 0.025; Smart use I: 13.1 (1.63), C:12.9 (1.43), P = 0.457; Manage health I: 6.48 (0.96), C: 6.40 (0.89), P = 0.127. Maximum score for the scales was not reported. | |||
| 1. N = 345 2. N = 343 3. N = 342 |
Self efficacy: 1. Self‐efficacy in obtaining medical information and attention to their concerns from their doctor (PEPPI) 2. Medication adherence self‐efficacy (Medication Adherence Survey) 3. Self efficacy in managing medication problems (Ability to Manage Problems Survey) |
1. Mean score (SD): I: 17.85 (4.33), C: 17.70 (4.30), P = 0.687. Maximum score for the scale was not reported. 2. Mean score (SD): I: 58.82 (11.88), C: 56.88 (11.72), P = 0.013. Maximum score for the scale was not reported. 3. Mean score (SD): I: 28.05 (3.99), C: 27.34 (3.83), P = 0.026. Maximum score for the scale was not reported. |
|||
| N = 345 | Patients' perception of the therapeutic alliance with care givers and degree of satisfaction with treatment (Patient feedback survey) | Mean score (SD): I: 30.20 (5.81), C: 30.19 (5.05), P = 0.744. Maximum score for the scale was not reported. | |||
| N = 344 | Patients' perception of the degree of directive guidance (instructions on taking their medication properly) given by doctors and pharmacists (Purdue) | Mean score (SD): I: 17.27 (4.40), C: 17.01 (4.07), P = 0.749. Maximum score for the scale was not reported. | |||
| N = 329 | Patients' perception about their current drug use and whether it is problematic (CAGE) | Mean score (SD): I: 0.485 (0.83), C: 0.861 (1.19), P = 0.038. Maximum score for the scale was not reported. | |||
| N = 344 | Self reported drug taking for non‐medical purposes (sections of the NSDUH) | Odds ratio between intervention and control group (from logistic regression with pre‐intervention non‐medical use entered as a control variable): Non‐medical use of analgesics: OR 0.656, 95% CI 0.147 to 2.92, P = 0.579. Non‐medical use of sedatives: OR 1.41, 95% CI 0.50 to 3.97, P = 0.517. Non‐medical use of tranquillisers: OR 2.09, 95% CI 0.92 to 33.03, P = 0.132. Non‐medical use of stimulants: OR 6.47, 95% CI 0.426 to 98.13, P = 0.179. | |||
| Goodyer 2006 | Multimedia (I) vs written and verbal (V) vs written (C) | Immediately before and after the intervention | I: 34 V: 35 C: 35 |
Skill acquisition (inhaler technique) |
Change in global technique rating. Number who were: worse than baseline I: 5/34, V: 4/35, C: 5/35; same as baseline I: 12/34, V: 16/35, C: 18/35; better than baseline I: 17/34, V: 15/35, C: 9/35. Number who: check mouthpiece I: 16/34, V:11/35, C: 1/35; shake inhaler: I: 24/34, V: 35/35, C: 16/35; co‐ordination I: 11/34, V: 10/35, C: 3/35. |
| Kato 2008 | Multimedia education (I) vs control multimedia (C) | Before the intervention as well as 1 and 3 months later | Number varies for each outcome for the 3 time points. Knowledge: I: 191, 172, 164 C: 168, 146, 139 |
Knowledge about cancer | Mean score (SD). Before I: 59% (20) C: 60% (20); 1 month I: 65% (20) C: 63% (20); 3 months I: 66% (20) C: 63% (20). Significantly greater increase in knowledge over time in intervention group (P = 0.035) |
| CDCI: I: 191, 172, 163 C: 167, 147, 140 MAS: I: 190, 167, 160 C: 166, 146, 138 |
Self‐reported adherence to medications: 1. CDCI (Chronic Disease Compliance Instrument) 2. MAS (Medication Adherence Scale) |
1. CDCI mean score (SD), range of 18‐90 with higher scores representing greater adherence: Before I: 79.2 (7.9), C: 77.4 (7.5); 1 month I: 79 (8.3), C: 78.4 (7.7); 3 months I: 81 (8.7), C: 78.4 (7.5). 2. MAS mean score (SD), range from 0‐4 with higher scores indicating greater adherence: Before I: 2.9 (1.1), C: 2.9 (1.1); 1 month I: 3.0 (1.1), C: 3.1 (1.0); 3 months I: 2.9 (1.1), C: 3.0 (1.1). |
|||
| 6‐MP metabolite assays: I: 28, 24, 23 C: 26, 22, 23 MEMS (only measured at 3 months): I: 107 C: 93 |
Objective measures of adherence to medications: 1. 6 ‐MP adherence using metabolite assays: 6‐TG and 6‐MMP 2. TMP/SMX adherence using Medication Event Monitoring System (MEMS) |
1. 6‐MP mean metabolite level (SD). 6‐TG: Before I: 250.7 (245.3) C: 284.3 (206.4); 1 month I: 283 (230.1), C: 302.1 (214); 3 months I: 286.5 (307.4), C: 236.8 (148.2). 6‐MMP: Before I: 10484.6 (9920.6) C: 9218 (11004.2); 1 month I: 11168.9 (12107.5), C: 10349.9 (11667.1); 3 months I: 8499.1 (7600.3), C: 8087.0 (9123.6). 2. MEMS mean (SD) % prescribed doses taken: At 3 months. I: 62.3% (62.9). C: 52.5% (37.6) Medication adherence did not differ significantly between groups when measured using self‐report: CDCI (P = 0.78), MAS (P = 0.503) but did differ significantly on objective measures: 6‐MP metabolites (P = 0.041), MEMS (P = 0.012). |
|||
| Clinic visit attendance | Mean: 98+/‐ 1% of scheduled visits were attended across both groups with no significant difference between the groups P = 0.65 | ||||
| I: 191, 172, 164 C: 168, 148, 139 |
Self‐efficacy | Mean score (SD). Range 27‐189 with higher scores representing greater self‐efficacy. Before I: 155.9 (22.3), C: 156.6 (21.3); 1 month I: 158 (24.3), C: 157.9 ( 22.3); 3 months I: 164.1 (23.4), C: 158.8 (23.5). Significantly greater self‐efficacy in intervention group (P = 0.011). | |||
| PQL: I: 154, 143, 119 C: 134, 119, 102 FACT‐G: I: 32, 25, 23 C: 31, 29, 25 |
Quality of life (QOL): 1. PQL (Paediatric Quality of Life) 2. FACT‐G (Functional Assessment of Cancer Therapy) |
1. PQL: Mean score (SD). Range 0‐100 with higher score representing better QOL. Before I: 64.2 (15.4) C: 62.5 (17,4); 1 month 65.5 (15.1) C: 63.5 (17.6); 3 months I: 69.1 (15.1) C: 66.3 (17.3). 2. FACT‐G: Mean score (SD). Range 0‐108 with higher score representing better QOL. Before I: 11.3 (2.6), C: 10.7 (2.7); 1 month I: 11 (3.2) C: 11.1 (2.1); 3 months I: 12.2 (2.9), C: 11.3 (2.8). No significant difference between groups for QOL: PQL (P = 0.112), FACT‐G (P = 0.15). |
|||
| I: 191, 170, 162 C: 168, 146, 139 |
Perceived stress | Mean score (SD). Range 10‐50 with higher score representing more stress. Before I: 34.4 (7.4), C: 33.1 (6.6); 1 month I: 36.5 (6.6), C: 35.2 (6.8); 3 months I: 38.1 (6.9), C: 35.7 (6.2). No significant difference between groups (P = 0.931) | |||
| I: 190, 171, 164 C: 168, 147, 139 |
Health Locus of Control Subscales: 1. Self/Internal 2. Chance 3. Powerful others 4. Doctors 5. Other people |
Mean score (SD). Range 3‐36 for each sub‐scale with higher scores representing higher locus of control. Before I: 18.9 (6.1), C: 18.6 (5.3); 1 month I: 18 (5.9), C: 18.2 (5.8); 3 months I: 17.6 (6.6), C: 17.7 (6.2). Before I: 20.3 (6.6), C: 20.7 (7.3); 1 month I: 19.1 (6.1), C: 20 (6.6); 3 months I: 18.7 (6.8), C: 19.4 (6.9). Before I: 26.4 (4.7), C: 26.5 (4.6); 1 month I: 26.1 (5.1), C: 26.4 (4.6); 3 months I: 25.7 (5.3). C 26.2 (4.8). Before I: 15.2 (2.5) C:15.4 (2.6); 1 month I: 15 (2.8), C: 15.1 (2.8); 3 months I: 14.7 (2.9), C: 15 (2.6) Before I: 11.1 (3.4), C: 11.1 (3.5); 1 month I: 11.2 (3.4), C: 11.4 (3.2); 3 months I: 11 (3.7), C: 11.2 (3.3). No significant difference between groups on overall health locus of control score (P = 0.608). |
|||
| Kinnane 2008 | Multimedia and co‐intervention (I) versus co‐intervention alone (C) | At time of 2nd chemotherapy cycle (˜3 to 4 weeks post‐intervention) | I: 31 C: 29 |
Knowledge ‐ recall of content in the pre‐chemotherapy education. (results reported for each question). | No statistically‐significant difference in I and C group results for any question. The authors reported a trend towards higher recall in the video group for 3 questions: what mouth problems should be reported straight away (87% vs 78%, P = 0.45); symptoms of low red cell count (80.6% vs 66.7%, P = 0.29); prevention of constipation (74.2% vs 51.7%, P = 0.07). |
| Use of health services | Number of patient phone calls to the chemotherapy centre I: 25, C: 27; Number of phone calls relating to education content I: 20/25, C: 15/27. | ||||
| Knoerl 1999 | Multimedia (I) vs usual care (C) | Preoperatively and 4, 8 and 24 hours postoperatively | I: 38 C: 38 |
Knowledge about PCA (measured pre and 4 hours post‐op) | Mean score (SD). Pre I: 65.4% (22.4), C: 56.6% (25.9), P = 0.13; Post I: 95.6% (8.4), C: 75.8% (17.6), P = 0.00. |
| Attitude to pain medications (measured pre and 4 hours post‐op) | Mean score (SD) with higher score indicating more positive attitude. Pre I: 72% (28.5), C: 72% (28.2), P = 1.00; Post I: 95.6% (9.2), C: 72.4% (23.3), P = 0.00. | ||||
| Satisfaction with pain management | Mean (SD), range 1‐6 with high score representing low satisfaction: 4 hours post I: 1.4 (0.68), C: 1.8 (0.93), P = 0.03; 8 hours post I: 1.2 (0.37) C: 1.5 (0.60), P = 0.01; 24 hours post I: 1.2 (0.41), C: 1.5 (0.60), P = 0.10 | ||||
| Pain | Mean pain rating (SD) from 0‐10 with 0 = no pain and 10 = worst possible pain. 4 hours post I: 4.0 (2.5), C: 5.3(3), P = 0.06; 8 hours post I: 3.2 (2.3), C: 3.9 (2.4), P = 0.27; 24 hours post I: 3.0 (2.1), C: 3.5 (2.1), P = 0.42. | ||||
| Incidence of medication side effects | Percent who experienced side effect: Nausea I: 42.1%, C: 34.2%, P = 0.48; vomiting I: 18.4%, C: 10.5%, P = 0.33; pruritis I: 28.9%, C: 34.2%, P = 0.63; hypotension I and C: 5.3%, P =1.0; sedation I: 21.1%, C: 28.9%, P = 0.43; urinary retention I: 10.5%, C: 13.2%, P = 0.73. | ||||
| Lirsac 1991 | Multimedia (I) vs verbal (C) | Before the intervention and after 15 days. | I: 14, C: 14 | Inhaler technique | Mean change in inhaler technique score: I: ‐2.65, C: ‐1.65 (P < 0.05). Errors in inhaler technique following education (number of patients): I: 0 errors = 8, 1 error = 6. C: 0 errors = 6, 1 error = 4, 2 errors = 4. Mean inhaler technique score following education (SD ‐ estimated from figure) I:0.45 (0.5), C:0.85 (0.9). Baseline mean inhalation score was significantly better in the control group I: 3.1 (0.7), C: 2.5 (0.5), P < 0.05. |
| Bronchial obstruction | FEV: Baseline I: Pre MDI 1.89 (0.61), Post MDI 2.35 (0.74), C: Pre MDI 2.01 (0.7), 2.53 (0.84). 15 days after education I: Pre MDI 2.38 (0.84), Post MDI 2.66 (0.74), C: Pre MDI 2.09 (0.63), Post MDI 2.66 (0.74). Theoretical maximum FEV I: 2.73 (0.72), C: 3.14 (0.6) |
||||
| Mazor 2007 | Multimedia (I) vs usual care (C) | Before the intervention and 3 weeks later | Number varies for each outcome: I: 220, C: 87 |
Knowledge about warfarin | Mean score (SD) Before I: 57.5% (16), C: 54% (18) After I: 68.95% (16.07), C: 57% (17). |
| I: 220, C: 86 | Self‐reported non‐adherence to warfarin dosing | Percent reporting non‐adherence. Before I: 6.8%, C: 5.8%, After I: 3.6%, C: 3.5% | |||
| I: 213, C: 85 | Self‐reported intention to adhere to laboratory testing | Mean score (SD) range 1‐5 with higher score indicating stronger intent to adhere. Before I: 3.7 (1.1), C: 3.7 (1.0); After I: 3.93 (0.99), C: 3.81 (0.96). | |||
| I: 175, C: 57 | Non‐attendance at laboratory appointments | Number (%) not attending. Before I: 35/175 (20%), C: 10/57 (17.5%); After I: 38/175 (22%), C: 19/57 (33%). | |||
| Subscale 1: I: 221, C: 89 Subscale 2: I: 219, C: 89 Subscale 3: I: 215, C: 85 Subscale 4: I: 221, C: 89 |
Beliefs about warfarin sub‐scales: 1. Belief warfarin is beneficial 2. Belief warfarin is worrisome 3. Belief warfarin regimen is confusing or difficult 4. Belief that laboratory testing is important |
Mean scores (SD) with range 1‐5 with higher scores representing greater belief in benefit, less worry, confusion or difficulty and stronger belief in importance of testing. 1. Belief warfarin is beneficial: Before I: 3.45 (0.78), C: 3.47 (0.75); After I: 3.57 (0.8), C: 3.44 (0.74). 2. Belief warfarin is worrisome: Before I: 3.11 (0.94), C: 3.21 (0.89); After I: I:2.97 (0.98), C: 3.31 (0.85). 3. Belief warfarin regimen is confusing or difficult: Before I: 4.28 (0.55), C: 4.21 (0.63); After I: 4.27 (0.58), C: 4.19 (0.65) 4. Belief that laboratory testing is important: Before I: 4.16 (0.74), C: 4.06 (0.71); After I: 4.26 (0.68) C: 4.03 (0.7). |
|||
| McElnay 1989 | Multimedia plus written education (I) vs instruction by a health professional plus written education (V) vs written education (C) | Immediately after the intervention and 2 weeks later | Immediately after the intervention N = 50 in all three groups Two weeks later: I: 43 V: 40 C: 44 |
Skill acquisition (inhaler technique) | Immediately after the intervention (mean score out of 80 (SD)) I: 60 (14.85), V: 64.2 (6.36), C: 49.5 (13.44); P values: I vs V: P > 0.05, I vs C: P < 0.001; V vs C: P < 0.001 2 weeks later I: 50 (26.2), V: 59.5 (12.65), C: 47.3 (17.25); P values: I vs V: P > 0.05, I vs C: P < 0.05; V vs C: P < 0.001 |
| Navarre 2007 | Multimedia (I) vs usual care (C) | Immediately after the intervention | I: 18 C: 16 |
Knowledge of inhaler technique | Mean score (SD) I: 80.9% (17), C 67.4% (11.8), P = 0.01 |
| Skill acquisition (inhaler technique) | Mean score out of 100 (SD) I: 88.3 (12.3), C: 67.4 (19.2), P = 0.001 | ||||
| Neafsey 2001 | Multimedia (I) vs usual care (C) | Immediately after the intervention | I: 30 C: 30 |
Knowledge about interaction of prescribed medications with OTC antacids and alcohol. | Mean score (SD) I: 71.7% (19.1), C: 36.2% (16.5), (P ≤ 0.001). |
| Self‐efficacy | Mean score (SD) scored from 1‐5 with higher scores representing greater self‐efficacy I: 3.14 (0.9), C: 1.76 (0.99), (P ≤ 0.001). | ||||
| Neafsey 2002 | Multimedia plus written information (I) vs written information (C) | Immediately after the intervention, 2 and 4 weeks later (except for risk of adverse medication behaviour which was assessed pre‐intervention, 2 and 4 weeks later) | I: 30 C: 30 |
Knowledge about interaction of prescription medications with OTC preparations and alcohol. | Mean score (SD) Post intervention I: 72.8% (16.8), C: 53.7% (18.7); At 2 weeks I: 77.6% (20.5), C: 55.2% (20); At 4 weeks I: 79.9% (21), C: 56.7% (20), P < 0.05 at all time points. |
| Self‐efficacy | Mean score and SD (range 1‐5) with higher scores representing higher self‐efficacy. Post intervention I: 3.7 (0.8), C: 3.1 (0.95); At 2 weeks I:3.7 (0.8), C:2.8 (0.9), At 4 weeks I:3.9 (0.7), C:3.0 (0.8), P < 0.05 at all time points. | ||||
| Risk of adverse medication behaviour | Mean score and SD (range 0‐40) with higher score representing riskier behaviour Pre‐intervention I: 21 (11), C: 22 (14); At 2 weeks I: 18 (10.5), C: 22 (13); At 4 weeks I: 16 (10), C: 21 (10) | ||||
| Olver 2009 | Multimedia (I) vs written information (C) | 3‐4 week after the intervention | I: 47 C: 54 |
Recall of information about chemotherapy | Number who answered each question correctly. Number of drugs I: 26/47 C: 25/54, P = 0.43. Treatment length I: 30/47, C:27/54, P = 0.23. Treatment goal I: 25/47, C: 31/54, P = 0.69. |
| Satisfaction with information | Across both groups 66.4% found the information very or somewhat helpful and 53.5% felt that is was the right amount. Authors stated that there was no significant difference between groups on these variables. | ||||
| Usability of the information | Across both groups 45.5% read all of the information and 27.7% felt they understood all of the information. Authors stated that there was no significant difference between the groups in these variables. | ||||
| Anxiety level | Across both groups 30% felt the information dispelled their anxiety, 33% felt no different and 11% became more anxious. Authors stated that there was no significant difference between the groups. | ||||
| Osguthorpe 1983 | Multimedia plus written information (I) vs written information alone (W) Multimedia alone (I) vs usual care (C) |
One week before and after the intervention | N = 202. The number in each arm was not reported | Knowledge about psychiatric medications. | Mean change scores from baseline for individual questions in each cluster were reported. The authors stated that there were no differences between groups based on total change scores but these were not reported. |
| Powell 1995 | Multimedia (I) vs usual care (C) | Time of enrolment to completion of the study or termination from the HMO plan (maximum 9 months) | I: 1993 C: 2253 |
Compliance using the Medication Possession Ratio (MPR): the number of days' supply of medication obtained by patient divided by the number of days the patient was observed. | Mean MPR (SD) I: 0.70 (0.23), C: 0.70 (0.28). Number with MPR ≥ 0.8 I: 917 (46%), C: 998 (44%) |
| Savage 2003 | Multimedia (I) vs written education (C) | Immediately before and after the intervention | I: 52 C: 43 |
Skill acquisition (inhaler technique) |
Change in global inhaler technique rating: number worse than baseline I: 2/52, C: 4/43; same as baseline I: 27/52, C: 31/43; better than baseline I: 23/52, C: 8/43. Number who checks mouthpiece I: 10/52, C: 3/43; shakes inhaler I: 41/52, C: 32/43; coordination I: 28/52, C: 12/43; breathes in I: 27/52, C: 18/43. |
| Schnellinger 2010 | Multimedia (I) vs written (P) vs control (C) | Immediately before (pre) and after (post) the intervention and after 4 weeks | Post: I: 83 P: 79 C: 84 At 4 weeks: I: 65 P: 63 C: 61 |
Knowledge about appropriate antibiotic use | Median score out of 10 (range): Pre I: 9 (2‐10), P: 8 (1‐10), C: 8 (1‐10), Post I: 10 (2‐10), P: 10 (1‐10), C: 8 (0‐10), 4 weeks I: 10 (2‐10), P: 9 (2‐10), C: 8 (1‐10). Numbers who had perfect scores at baseline I: 20 (31%), P: 13 (21%), C: 11 (18%). Number who improved following intervention (based on pre and post scores): I: 38 (58%), P: 45 (71%), C: 17 (28%). With percentages calculated after excluding participants with perfect scores at baseline: I: 85%, P: 90%, C: 34%. |
| Evaluation survey | Percent answering yes: Q1. Found the study useful/interesting I: 93, P: 95, C: 80. Q2. Learnt something new about antibiotics I: 72, P: 84, C: 30. Q3. Paediatrician talked about proper use of antibiotics I: 59, P: 33, C: 70. Q4. Paediatrician talked about improper antibiotic use I: 61, P: 58, C: 66. Q5. Paediatrician offered antibiotics more often than needed I: 11, P: 11, C: 16. Q6. Would you ask Paediatrician for antibiotics for conditions mentioned in study (where antibiotics are not appropriate) I: 15, P: 35, C: 45. Q7. Do you think there is an antibiotic for every infection I: 12, P: 10, C: 12. Q8. Did you know about antibiotic resistance prior to the study I: 76, P: 63, C: 63. Q9. If you knew that doctor would not give an antibiotic would you still go to the doctor I: 58, P: 56, C: 51. | ||||
| Schwarz 2008a | Multimedia education (I) vs control multimedia (C) | Before and 6 months after the intervention | I: 127 C: 138 |
Knowledge about emergency contraception (EC) | Number who learned: one or more things about EC I: 97/127 (76%), C: 86/138 (62%), P = 0.04. Mean scores baseline I:59%, C:62%, P = 0.12. Mean scores 6 months I:59%, C:58%, P = 0.88. EC is available in California I: 24%, C: 18%, P = 0.36; EC is safe I: 26%, C: 12%, P < 0.001; EC is effective I: 22%, C: 14%, P = 0.29; EC will not adversely affect future fertility I: 37%, 20%, P = 0.005; EC will not cause birth defects or miscarriage I: 34%, C: 14%, P < 0.001; EC can be used 3‐5 days after condom breaks I: 28%, C: 19%, P = 0.005; EC does not protect from STD I: 11%, C: 6%, P = 0.31; A period is expected within 3 weeks of taking EC I: 20%, C: 14%, P = 0.33. |
| Attitude to EC | Number (%) who had personal objection to EC pre‐intervention: I: 19/219 (9%) C: 13/227 (6%). Number (%) who developed a more positive attitude toward EC after the intervention I:10/219 (8%), C: 6/227 (4%), P = 0.06. | ||||
| Self‐reported use of EC during study period |
Number who used EC I: 6%, C: 3%, P = 0.09 Number who had supply of EC in the home I: 34%, C: 7%, P < 0.001. |
||||
| Conception during study period | Number who conceived I: 2.7%, C: 5.7%, P = 0.12. | ||||
| Self 1983 | Multimedia (I) vs education by a health professional (V) vs written education (C) | All groups assessed immediately after the intervention. I and V groups also assessed between 1‐16 weeks later. | Immediately after: I: 10 V: 9 C: 10 1‐16 weeks later: I: 8 V: 7 |
Skill acquisition (inhaler technique) |
Mean inhaler technique score out of 20. Immediately after the intervention (SD) I: 16.9 (5.0), V: 16.8 (4.5), C: 10.7 (4.5). 1‐16 weeks later I: 14.3 (5.8), V: 17.4 (2). |
| Solomon 1988 | Multimedia plus verbal instruction from doctor +/‐ special pill packaging (I) vs verbal instruction from doctor +/‐ special pill packaging (C) | 2 or 6 days after starting the medication | N = 321, 38 to 42 participants in each of the 8 groups and approximately half exposed to the multimedia intervention. | Knowledge about tetracycline for treatment of STDs | Mean score out of 15. I: 14.4, C: 10.7. ANOVA F‐ratio = 442.7, P < 0.001. |
| Non‐compliance with medication | Mean score (range 0‐160 with higher scores indicating worse compliance) I: 8.3, C: 40.45. Authors reported that the multimedia group had better compliance based on ANOVA result (F‐ratio = 114.2, P < 0.001). | ||||
| Premature resumption of sexual activity | Median percentage who resumed sexual activity prematurely I: 3%, C: 22%, ANOVA F‐ratio = 30.2, P < 0.001. | ||||
| Satisfaction with care | Authors reported that results for these variables were significantly improved in patients who had received multimedia education and/or special pill packaging compared to patients who had received neither: F (1312) = 5.1 P < 0.025. |
||||
| Perception of disease severity | F(1312) = 5.1 P < 0.026 | ||||
| Confidence in medication | F(1312) = 6.1 P < 0.015 | ||||
| Stone 1989 | Multimedia (I) vs education by a health professional (C) | Immediately before and after the intervention | I: 9 C: 13 |
Knowledge about warfarin | Mean score out of 18 (SD) Before I:12.6 (2.6), C:12.8 (1.4); After I:15.6 (1.4), C:16 (1.4), P = 0.60. |
| Satisfaction with education | Mean score out of 25 I: 23.2, C: 24.8 Authors state that there was no significant difference. | ||||
| Use of health services | Time required for teaching (mean time in minutes) I: 17.6 (duration of video), C:26 (5.7) (mean (SD) duration of nurse lecture). Time required for questions after education (mean time in minutes) I: 7.5 (7.2) C: 6.3 (5.3) | ||||
| Trent 2010 | Multimedia plus usual care (I) vs usual care alone (C) | After two‐week treatment period | I: 36 C: 41 |
Adherence with medications | Number that completed the medication course: I: 23 (64%), C: 27 (66%). |
| Attendance at 72 hour clinical visit | Number who attended: I: 11 (31%), C: 6 (15%). | ||||
| Abstinence from intercourse | Number who abstained: I: 25 (69%), C: 31 (76%) | ||||
| Partner notification | Number who notified partner: I: 30 (83%). C: 35 (85%) | ||||
| Partner treatment | Number who reported that partner had been treated: I: 24 (67%), C: 20 (49%) | ||||
| Complications | Number who reported complications: I: 13 (36%), C: 13 (32%). | ||||
| Van Der Palen 1997 | Multimedia (I) vs education by a health professional (V) vs usual care (C) | At first scheduled clinic visit up to 9 months later | I: 38 V: 77 C: 33 |
Skill acquisition (inhaler technique) | Mean inhaler checklist scores I: 91%, V: 91%, C: 74%; Essential checklist items (mean score) I: 92%; V: 95% C: 76%; Percent of patients with a 100% score I: 76%, V: 86%, C: 49%. |
| Voris 1982 | Multimedia (I) vs usual care (C) | Before the intervention, and both 1‐5 days and 4 weeks after the intervention | After 1‐5 days: I: 6 C: 5 After 4 weeks: I: 4 C: 4 |
Knowledge about tricyclic antidepressants. | After 1‐5 days (mean score out of 20 (and range)) I: 19 (18‐20), C: 13.6 (8‐17); After 4 weeks I: 17.3 (16‐19), C: 12.8 (7‐18). |
We used narrative synthesis where studies were not suitable for meta‐analysis, presented by comparison. For example, if the mean and SD could not be extracted for continuous outcomes, we presented the summary statistic and measure of variance, where available. These data were also presented in Additional tables (Table 8). If no data could be extracted from a study, we reported this in the notes section of the Characteristics of included studies table.
We used 'Summary of findings' tables to present the main results for each of the comparison groups, as recommended by the Cochrane Collaboration (Schünemann 2008a). We selected the following outcomes as the most important, and included them in 'Summary of findings' tables:
patient or carer knowledge about the medication;
skill acquisition;
compliance;
disease‐related outcomes;
adverse effects related to the medication,
quality of life, and
self‐efficacy.
Each table contains information about the number of studies and participants included, the outcome being examined, the magnitude of effect, and a GRADE assessment of the quality of evidence for each outcome (Schünemann 2008b).
Within each of these comparison groups, we explored possible reasons for variability of findings. These possible effect modifiers included the type or quality of the intervention, or variations in the characteristics of the population studied, including those outlined in the secondary objectives (age, literacy or disease chronicity).
Subgroup analysis and investigation of heterogeneity
Subgroup analyses were planned according to:
age group (examining the effect on the elderly (over 65 years) compared to those in other age groups);
literacy (groups with low literacy compared to others);
medications treating acute or chronic conditions;
-
specific characteristics of the multimedia intervention according to:
-
degree of interactivity:
not interactive; e.g. video or DVD that can only be watched from beginning to end
limited interactivity; e.g. DVD or computer program where the individual can choose the information components that they want to access and the order in which they access them
fully interactive; in which the responses of the program are constantly changing in response to user actions e.g. a computer game or simulation
tailoring (where the information provided is personalised, based on an individual's characteristics) versus generic information
-
Sensitivity analysis
We performed sensitivity analyses to examine the effect of adequate allocation concealment and blinding of outcome assessor (trials with low risk of bias) on effect size for the primary outcomes. We also performed sensitivity analyses to investigate the effect of imputation of missing data (e.g. SD). Where the random‐effects model was used, we repeated the analysis using a fixed‐effect model and compared the results to examine the influence of small‐study effects on the intervention effect estimate.
Consumer participation
We collected information about consumer involvement in the development of the educational interventions. As part of the standard editorial process of the Cochrane Consumers and Communication Review Group, the protocol (Ciciriello 2010) and this review were reviewed by a consumer referee. A consumer representative was a co‐author for this review (TdK). She reviewed the protocol and provided input on how it could be improved so that it was more relevant to consumers. She also contributed to the synthesis of results and was part of the team that wrote the review.
Results
Description of studies
Results of the search
The search strategies identified 6113 studies (to June 2011), with 4562 remaining once duplicates were removed. We identified a further 977 studies when the search was updated to the end of June 2011, bringing the total to 5539 and leaving 5529 once duplicates were removed. Of these, 5346 were excluded as they were judged as not relevant to the review based on information in the study title and abstract, leaving 193 references for which the full text was retrieved. On examination of the full text, we identified 71 studies as clearly not fulfilling the inclusion criteria for this review. We excluded these studies because they were not randomised or quasi‐randomised controlled trials (N = 57) or did not contain any multimedia intervention (N = 14). We examined the remaining 122 studies in greater detail, from which 24 studies were found that met all inclusion criteria for this review. We outline the reasons for exclusion of the other studies below (Excluded studies). No further studies meeting criteria for inclusion were found following examination of the references of included studies or relevant reviews (those identified as potentially overlapping with the current review and those identified from current database searches). We illustrate the results of the search strategy and screening process in Figure 1.
1.
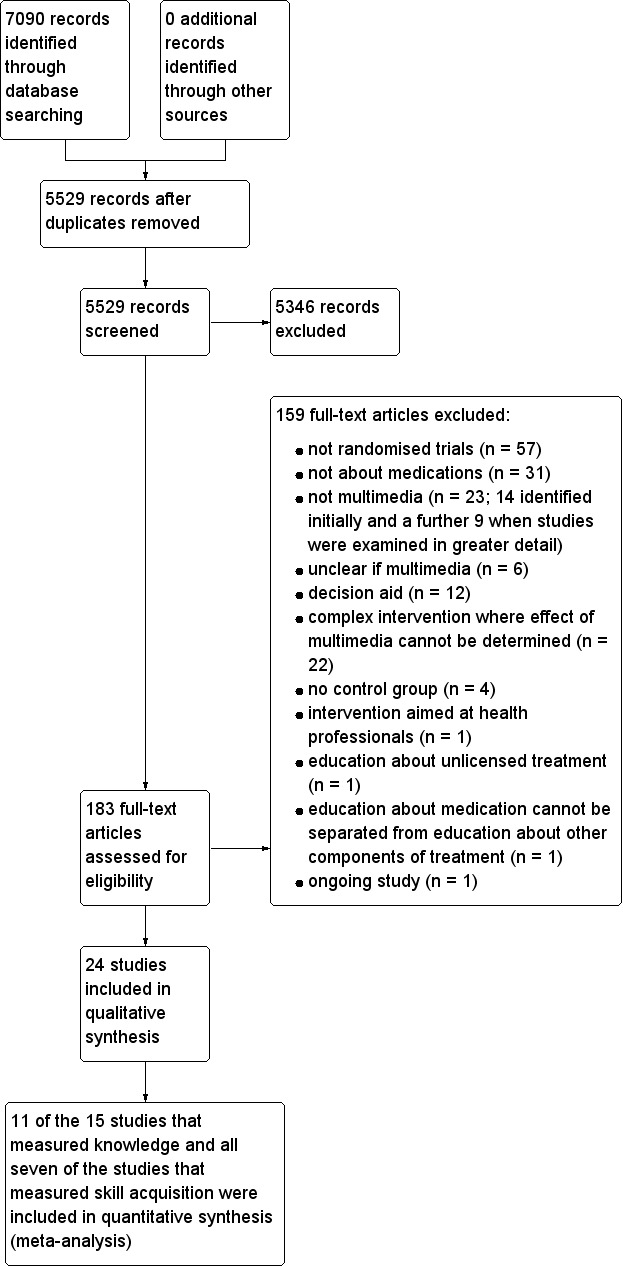
Study flow diagram.
We were able to contact 10 of the study authors to request further information. All 10 authors were contacted to request access to the multimedia educational program. This was provided by six authors (Deitz 2011; Kinnane 2008; Mazor 2007; Navarre 2007; Olver 2009; Trent 2010), while three authors provided copies of the voice‐over script (Goodyer 2006; Savage 2003; Schwarz 2008a) and one author informed us that the multimedia program was no longer available (Van Der Palen 1997). Further outcome data were requested from 4 of the 10 authors. One author was able to provide this information (Schwarz 2008a), while three authors informed us that this information was not available (Deitz 2011; Kinnane 2008; Olver 2009).
Included studies
We included 24 studies. Study characteristics are summarised below. Key participant and intervention characteristics are summarised across the studies in Table 9 and outlined in more detail for each of the individual studies in the Characteristics of included studies tables. Study duration varied from one day to nine months and the studies were published from 1982 to 2011. English was the first language of participants, and therefore the only language in which education was provided, in all but five studies (Acosta 2009; Goodyer 2006; Kato 2008; Lirsac 1991; Van Der Palen 1997). .
3. Summary of key participant and intervention characteristics across included studies.
| Characteristics of study participants | |||
| Characteristic | Number of studies | Study titles | |
| Country in which study is set | USA | 16 | Acosta 2009; Deitz 2011; Knoerl 1999; Mazor 2007; Navarre 2007; Neafsey 2001; Neafsey 2002; Osguthorpe 1983; Powell 1995; Schnellinger 2010; Schwarz 2008a; Self 1983; Solomon 1988; Stone 1989; Trent 2010; Voris 1982 |
| United Kingdom | 3 | Goodyer 2006; McElnay 1989; Savage 2003 | |
| Australia | 2 | Kinnane 2008; Olver 2009 | |
| Netherlands | 1 | Van Der Palen 1997 | |
| France | 1 | Lirsac 199 | |
| Multi‐centre study in the USA, Canada and Australia | 1 | Kato 2008 | |
| Setting from which participants were recruited | Community or hospital‐based outpatient clinics, treatment centres and inpatient wards | 15 | Kato 2008; Kinnane 2008; Knoerl 1999Lirsac 1991; Mazor 2007; Navarre 2007; Olver 2009; Osguthorpe 1983; Savage 2003; Self 1983; Solomon 1988; Stone 1989; Trent 2010; Van Der Palen 1997; Voris 1982 |
| Emergency departments or urgent care clinics | 3 | Acosta 2009; Schnellinger 2010; Schwarz 2008a | |
| Local seniors centres | 2 | Neafsey 2001; Neafsey 2002 | |
| Turkish social clubs | 1 | Goodyer 2006 | |
| HMO members who had pharmacy claims for particular medications | 1 | Powell 1995 | |
| Volunteers from staff at participating hospitals | 2 | Deitz 2011; McElnay 1989 | |
| Gender | Only women | 3 | Deitz 2011; Schwarz 2008a; Trent 2010 |
| Only men | 1 | Osguthorpe 1983 | |
| Age | Adults only (17 years or older). Three studies dealt with specific age groups; Neafsey 2001 and Neafsey 2002 recruiting seniors aged 60 years or older and Schwarz 2008a recruiting women of childbearing age (aged 18 to 45 years) | 19 | Acosta 2009; Deitz 2011; Goodyer 2006; Kinnane 2008; Knoerl 1999; Mazor 2007; McElnay 1989; Navarre 2007; Neafsey 2001; Neafsey 2002; Olver 2009; Osguthorpe 1983; Powell 1995; Schnellinger 2010; Schwarz 2008a; Self 1983; Stone 1989; Van Der Palen 1997; Voris 1982 |
| Also recruited children. Kato 2008 recruited children, adolescents and young adults aged 13 to 29 years, Trent 2010 recruited adolescents aged 15 to 21 years, Savage 2003 included patients aged 12 to 87 years old, Lirsac 1991 recruited participants aged 10 to 71 years old and Solomon 1988 included participants aged 14 to 59 years old (mean age 23). | 5 | Kato 2008; Lirsac 1991; Savage 2003; Solomon 1988; Trent 2010 | |
| Language in which education was provided | English | 19 | Deitz 2011; Kinnane 2008; Knoerl 1999; Mazor 2007; McElnay 1989; Navarre 2007; Neafsey 2001; Neafsey 2002; Olver 2009; Osguthorpe 1983; Powell 1995; Savage 2003; Schnellinger 2010; Schwarz 2008a; Self 1983; Solomon 1988; Stone 1989; Trent 2010; Voris 1982 |
| Dutch | 1 | Van Der Palen 1997 | |
| Turkish | 1 | Goodyer 2006 | |
| French | 1 | Lirsac 1991 | |
| More than one language (Kato 2008 using English, French or Spanish and Acosta 2009 using both English and Spanish) | 2 | Acosta 2009; Kato 2008 | |
| Educational level | Mean number of years of education completed. Combined results from the 4 studies showed that the 243 participants completed a mean of 13.5 years of education. | 4 | Knoerl 1999; Neafsey 2001; Neafsey 2002; Stone 1989 |
| Highest level of education completed. Of the combined 2616 participants from the 11 studies, 49% had completed some primary or secondary school while 48% had attended tertiary level education. | 11 | Acosta 2009; Deitz 2011; Kato 2008; Kinnane 2008; Mazor 2007; Navarre 2007; Olver 2009; Osguthorpe 1983; Schnellinger 2010; Schwarz 2008a; Solomon 1988 | |
| Low versus high level. One study reported that 82% of its 148 participants had a low educational level and 18% had a high level but did not provide definitions for these levels. | 1 | Van Der Palen 1997 | |
| Participants' level of educational attainment was not reported. | 8 | Goodyer 2006; Lirsac 1991; McElnay 1989; Powell 1995; Savage 2003; Self 1983; Trent 2010; Voris 1982 | |
| Literacy | Comprehension score of at least grade six on the Rapid Estimate of Adult Literacy Measure (REALM) | 2 | Neafsey 2001; Neafsey 2002 |
| One study reported that over 80% of the participants were literate in Turkish when assessed using a language proficiency test devised by their language department. | 1 | Goodyer 2006 | |
| No information reported about literacy | 21 | All other studies | |
| Primary diagnosis and treatment; topic of the educational program | Respiratory illnesses treated with metered dose inhalers (MDIs); MDI technique | 7 | Acosta 2009; Goodyer 2006; Lirsac 1991; Navarre 2007; Savage 2003; Self 1983; Van Der Palen 1997 |
| Malignancies treated with chemotherapy; chemotherapy and management of its side effects | 3 | Kato 2008; Kinnane 2008; Olver 2009 | |
| Conditions requiring anticoagulation with warfarin; warfarin | 2 | Mazor 2007; Stone 1989 | |
| Psychiatric illnesses treated with specific medications; specific psychiatric medications | 2 | Osguthorpe 1983; Voris 1982 | |
| Elderly patients taking particular over‐the‐counter medications e.g. antacids and pain relievers; interaction between prescription and over‐the counter medications | 2 | Neafsey 2001; Neafsey 2002 | |
| Sexually transmitted diseases (STD) or pelvic inflammatory disease (PID) treated with antibiotics; treatment of STD or PID with antibiotics | 2 | Solomon 1988; Trent 2010 | |
| Hypertension, hypercholesterolaemia or menopause treated with specific medications; specific medications for these conditions | 1 | Powell 1995 | |
| Undergoing a surgical procedure requiring post‐operative patient‐controlled analgesia (PCA); PCA | 1 | Knoerl 1999 | |
| Did not require participants to have a particular medical condition or to have taken a particular medication: female hospital employees ‐ education about medications with abuse potential including strategies for avoiding drug use and dependence; volunteers with no experience of MDI use ‐ education about MDI technique; parents of children presenting for acute care ‐ education about proper antibiotic use; women of child‐bearing age attending emergency department ‐ education about emergency contraception. | 4 | Deitz 2011; McElnay 1989; Schnellinger 2010; Schwarz 2008a | |
| Acuity of the primary diagnosis | Acute | 3 | Knoerl 1999; Solomon 1988; Trent 2010 |
| Chronic | 16 | Acosta 2009; Goodyer 2006; Kato 2008; Kinnane 2008; Lirsac 1991; Mazor 2007; Navarre 2007; Neafsey 2002; Olver 2009; Osguthorpe 1983; Powell 1995; Savage 2003; Self 1983; Stone 1989; Van Der Palen 1997; Voris 1982 | |
| Unclear | 1 | Neafsey 2001 | |
| Characteristics of interventions | |||
| Format | Video: 10 studies used videotapes, two studies DVDs and two studies did not report the technology used to deliver the video. | 14 | Acosta 2009; Kinnane 2008; Knoerl 1999; Lirsac 1991; Mazor 2007; McElnay 1989; Osguthorpe 1983; Powell 1995; Schnellinger 2010; Self 1983; Solomon 1988; Stone 1989; Trent 2010; Van Der Palen 1997 |
| Computer‐based program: four studies used programs with touch‐screen technology and the program used in one study was a computer game. | 9 | Deitz 2011; Goodyer 2006; Kato 2008; Navarre 2007; Neafsey 2001; Neafsey 2002; Olver 2009; Savage 2003; Schwarz 2008a | |
| Slide audiovisual presentation | 1 | Voris 1982 | |
| Source of the multimedia program | Developed by the study authors | 19 | Deitz 2011; Goodyer 2006; Kato 2008; Kinnane 2008; Mazor 2007; McElnay 1989; Navarre 2007; Neafsey 2001; Neafsey 2002; Olver 2009; Osguthorpe 1983; Savage 2003; Schnellinger 2010; Schwarz 2008a; Self 1983; Solomon 1988; Stone 1989; Trent 2010; Voris 1982 |
| Developed by other organisations ‐ pharmaceutical companies and a national medical media company | 2 | Powell 1995; Van Der Palen 1997 | |
| Not reported | 3 | Acosta 2009; Knoerl 1999; Lirsac 1991 | |
| Consumer involvement in development of the program | Studies that reported consumer involvement in the development of the multimedia program | 6 | Kato 2008; Mazor 2007; McElnay 1989; Neafsey 2001; Neafsey 2002; Trent 2010 |
| Number of times the multimedia program was viewed and success at reaching target audience | All participants viewed the program on at least one occasion | 20 |
Acosta 2009; Goodyer 2006; Kinnane 2008; Knoerl 1999; Lirsac 1991; McElnay 1989; Navarre 2007; Neafsey 2001; Neafsey 2002; Olver 2009; Osguthorpe 1983; Savage 2003; Schnellinger 2010; Schwarz 2008a; Self 1983; Solomon 1988; Stone 1989; Trent 2010; Van Der Palen 1997; Voris 1982 |
| Known number of participants who did not view the program on at least one occasion ‐ 4% (Deitz 2011), 13% (Kato 2008) | 2 | Deitz 2011; Kato 2008 | |
| Number who did not view the program not known ‐ Powell 1995 sent a questionnaire to a subset of 205 of the 1993 participants of which 97 (47%) were returned. Of these, 13% reported that they had not viewed the video. | 2 | Mazor 2007; Powell 1995 | |
| Rates of repeat viewing of the program reported ‐ 36.2% and 88% viewed program more than once in Olver 2009, Deitz 2011 respectively, 33% viewed program for the requested duration of one hour/week in Kato 2008 and the program was viewed a median of three times (range 1 to 50) in Van Der Palen 1997 | 4 | Deitz 2011; Kato 2008; Olver 2009; Van Der Palen 1997 | |
| Rates of repeat viewing not measured or reported | 2 | Mazor 2007; Powell 1995 | |
| Assistance from staff | Studies reporting that research staff or health professionals provided assistance in viewing or using the multimedia program | 4 | Goodyer 2006; Navarre 2007; Olver 2009; Savage 2003 |
| Degree of interactivity of the computer based programs (none of the programs using other formats contained any interactive features) |
Limited | 6 | Goodyer 2006; Neafsey 2001; Neafsey 2002; Olver 2009; Savage 2003; Schwarz 2008a |
| Sections with both limited and full interactivity | 1 | Deitz 2011 | |
| Full | 1 | Kato 2008 | |
| Not reported | 1 | Navarre 2007 | |
| Tailoring | Program tailored to an individual users' characteristics | 2 | Deitz 2011; Olver 2009 |
Participants
The 24 included studies enrolled a total of 8112 participants. Sample sizes for the trials varied from 11 (Voris 1982) to 4246 participants (Powell 1995). Nineteen of the studies involved only adults, while five studies also recruited children (Kato 2008; Lirsac 1991; Savage 2003; Solomon 1988; Trent 2010). Four of the studies selected participants of a particular gender: Deitz 2011; Schwarz 2008a and Trent 2010 only included women while Osguthorpe 1983 only included men admitted to a Veterans' psychiatric hospital.
Only three studies reported patients' literacy levels. Neafsey 2001 and Neafsey 2002 recruited only participants who had a reading comprehension score of at least grade six on the Rapid Estimate of Adult Literacy Measure (REALM). Goodyer 2006 reported that over 80% of the participants were literate in Turkish when assessed using a language proficiency test devised by the language department at King's College London.
Twenty of the studies recruited patients who were taking, or were about to start taking, particular medications for a variety of medical conditions. Only 3 of these 20 studies were performed in patients with acute conditions (Knoerl 1999; Solomon 1988; Trent 2010), the chronicity of the underlying conditions was unclear in Neafsey 2001, and the other 16 studies dealt with chronic conditions. The remaining four studies recruited participants who were not necessarily taking the medication under consideration at the time. Of these, three studies recruited demographic groups that were likely to have taken the relevant medication or to require them in the future. This included female hospital employees (Deitz 2011), parents of children presenting to an emergency department for acute care (Schnellinger 2010), and women of childbearing age who were attending two urgent care clinics for any reason (Schwarz 2008a). One study recruited healthy volunteers who were not taking the relevant medication and were unlikely to require it in the future (McElnay 1989).
Thirteen studies reported that they had obtained institutional ethics approval and informed consent from the study participants (Acosta 2009; Kato 2008; Kinnane 2008; Knoerl 1999; Goodyer 2006; Olver 2009; Navarre 2007; Savage 2003; Schnellinger 2010; Schwarz 2008a; Solomon 1988; Trent 2010; Van Der Palen 1997). Five studies reported informed consent but did not provide information about ethics approval (Deitz 2011; Neafsey 2001; Neafsey 2002; Osguthorpe 1983; Voris 1982). One study reported ethics approval but not informed consent (Mazor 2007) and five studies reported neither ethics approval nor informed consent (Lirsac 1991; McElnay 1989; Powell 1995; Self 1983; Stone 1989).
Interventions
The multimedia interventions consisted of videos in 14 studies (Acosta 2009; Kinnane 2008; Knoerl 1999; Lirsac 1991; Mazor 2007; McElnay 1989; Osguthorpe 1983; Powell 1995; Schnellinger 2010; Self 1983; Solomon 1988; Stone 1989; Trent 2010; Van Der Palen 1997), computer‐based programs in 9 studies (Deitz 2011; Goodyer 2006; Kato 2008; Navarre 2007; Neafsey 2001; Neafsey 2002; Olver 2009; Savage 2003; Schwarz 2008a), including a computer game (Kato 2008), and in 1 study a slide‐tape audiovisual presentation (Voris 1982).
The length of the videos ranged from 3 minutes (Schnellinger 2010) to 30 minutes (Powell 1995). The complexity of the computer‐based programs also varied from short videos accompanied by text and voice‐over, to programs that provided detailed information utilising text, still graphics, animations and video and broken into multiple sections through which the user was able to navigate freely.
The usage of the programs also varied. In 20 studies all of the participants viewed the multimedia program on at least one occasion (Acosta 2009; Goodyer 2006; Kinnane 2008; Knoerl 1999; Lirsac 1991; McElnay 1989; Navarre 2007; Neafsey 2001; Neafsey 2002; Olver 2009; Osguthorpe 1983; Savage 2003; Schnellinger 2010; Schwarz 2008a; Self 1983; Solomon 1988; Stone 1989; Trent 2010; Van Der Palen 1997; Voris 1982). In six studies the participants had the opportunity to view the program on more than one occasion if they wished (Deitz 2011; Kato 2008; Mazor 2007; Olver 2009; Powell 1995; Van Der Palen 1997). However, rates of repeat usage varied. For example, Olver 2009 and Van Der Palen 1997 reported that the multimedia program was viewed more than once by 36.2% and 88% of participants respectively.
Information about the program's degree of interactivity or tailoring was able to be determined for eight of the studies using computer‐based multimedia interventions. Six of the computer programs allowed limited interactivity, with users having some control over the order in which they accessed content and the pace at which they moved through the program (Goodyer 2006; Neafsey 2001; Neafsey 2002; Olver 2009; Savage 2003; Schwarz 2008a). The program used in Deitz 2011 also contained fully interactive components where users could determine their fitness level and determine if they needed help with their use of prescription medication. The program used in Kato 2008 was a fully interactive computer game, with the actions of users determining the direction that the game takes. Two programs were tailored to an individual users' characteristics (Deitz 2011; Olver 2009).
The educational content of the multimedia programs focused on metered dose inhaler (MDI) technique in eight studies (Acosta 2009; Goodyer 2006; Lirsac 1991; McElnay 1989; Navarre 2007; Savage 2003; Self 1983; Van Der Palen 1997), chemotherapy and the management of its side effects in three studies (Kato 2008; Kinnane 2008; Olver 2009), anticoagulation therapy with warfarin in two studies (Mazor 2007; Stone 1989) and the interaction between alcohol, over‐the‐counter medications and prescription medications in two studies (Neafsey 2001; Neafsey 2002). The remaining studies each dealt with a different topic.
Six of the studies reported consumer involvement in the development of the multimedia program (Kato 2008; Mazor 2007; McElnay 1989; Neafsey 2001; Neafsey 2002; Trent 2010).
Evaluation of the educational interventions using the Evaluative Linguistic Framework (ELF)
The 24 studies used 22 different multimedia educational interventions. Six of the multimedia programs were available or were provided by the study authors (Deitz 2011; Kinnane 2008; Mazor 2007; Navarre 2007; Olver 2009; Trent 2010). Program evaluation was based on the voice‐over script for three programs that were used in four studies (Goodyer 2006; Savage 2003; Schwarz 2008a; Voris 1982) and from information provided on a website (including the user manual, screen shots and short video excerpts) for one study (Kato 2008). Evaluations of the remaining 12 multimedia programs were based on descriptions provided by the authors in the published studies. The detail in which the characteristics of the multimedia program were reported varied. The information provided in six studies was insufficient to allow any evaluation of the multimedia program (Acosta 2009; Knoerl 1999; Lirsac 1991; Schnellinger 2010; Self 1983; Van Der Palen 1997). Five studies provided information which allowed evaluation which was mostly limited to the program's generic structure and format (McElnay 1989; Osguthorpe 1983; Powell 1995; Solomon 1988; Stone 1989). The multimedia program used in two studies (Neafsey 2001; Neafsey 2002), was evaluated based on information from a publication describing the program's development and testing (Strickler 2002). We present detailed information regarding the quality of the multimedia programs in Additional tables (Table 10).
4. Description and evaluation of interventions.
| Study | Intervention | Comparators |
| Acosta 2009 |
Source of information about intervention: Information provided by the author in the published thesis. Description of intervention: Video demonstrating steps for correct MDI use. Name of program: Not reported Country of origin: USA Language: English and Spanish Year program used in the study was produced: Not reported Stated expertise of content developers: Not reported Stated expertise of program developers: Not reported Process of development and testing: Not reported. Availability: Not reported Purpose: To demonstrate correct inhaler technique to patients with asthma Content: Video containing pictures describing the different steps in properly using an MDI. Relationship between developer and user: Health professionals to patients. Target audience: Asthma patients Format: Video Hardware required: Television and video player Media: Video and audio Delivery: Viewed on site in the emergency department Length of program: 10 minutes Number of times program was used: Patients only viewed the video on one occasion. Time point of delivery: Participants had existing asthma therefore it is assumed that they were using MDIs prior to the study. 58% had previously received training about correct use of an MDI. Success at reaching target audience: The video was administered on site therefore all participants in the intervention arm viewed the program. Assistance: Not reported Interactivity: No Tailoring: No |
Source of information about comparators: Information provided by the author in the published thesis Description of comparator: Video about asthma. Name of program: Not reported Country of origin: USA Language: English and Spanish Year program used in the study was produced: Not reported Stated expertise of content developers: Not reported Stated expertise of program developers: Not reported Process of development and testing: Not reported. Availability: Not reported Purpose: To provide general information about asthma and its triggers. Content: Video explains issues faced by patients with asthma and asthma triggers but does not contain any MDI related instructions. Relationship between developer and user: Health professionals to patients. Target audience: Asthma patients Format: Video Hardware required: Television and video player Media: Video and audio Delivery: Viewed on site in the emergency department Length of program: 10 minutes Number of times program was used: Patients only viewed the video on one occasion. Time point of delivery: Success at reaching target audience: The video was administered on site therefore all participants in the control arm viewed the program. Assistance: Not reported Interactivity: No Tailoring: No |
|
Evaluation of intervention: Inadequate information was provided on which to base an assessment |
Evaluation of comparator: Inadequate information was provided on which to base an assessment |
|
| Deitz 2011 |
Source of information about intervention: Copy of the program as well as information provided by the author in the published study. Description of intervention: Multimedia program designed to prevent the misuse of psychoactive prescription drugs. Name of program: SmartRx: Your Prescription for Good Health Country of origin: USA Language: English Year program used in the study was produced: Not reported Stated expertise of content developers: Although this was not explicitly stated, the study implied that the multimedia program was developed by the study authors. The study authors included psychologists specialising in substance abuse prevention. Stated expertise of program developers: The authors have experience in the development of Internet and DVD‐based health programs. Process of development and testing: The SmartRx program is based on the Cook and Youngblood conceptual model guiding the development of workplace substance use and misuse prevention interventions. The multimedia approach "was designed to make the program more appealing and engaging". No further information was reported regarding development or testing. Availability: Access to the program was provided on request by the study authors. Purpose: To prevent the misuse of psychoactive prescription medications. Content: Information about five classes of medications: analgesics, sedative‐hypnotics, stimulants, antidepressants and tranquillisers. The program consists of three sections. 1. Medication facts ‐ providing information about the pharmaceutical properties of medications including therapeutic action, potential side effects and recommended guidelines for self‐administration. 2. Smart treatment use ‐ instructs users on the safe administration and responsible use of medications. It contains information on drug tolerance, abuse and dependence and information, how to determine if they need help and what to do if they think they have a problem. 3. Managing your health ‐ focuses on alternatives to medications and suggestions on ways to improve health including exercise, relaxation and yoga. Relationship between developer and user: Health professionals to patients. Target audience: Women who are currently using or anticipate the use of medications with abuse potential. Format: Web‐based multimedia program Hardware required: Computer with Internet access. Media: Text, graphics, animations, video and audio Delivery: Participants accessed the program over the Internet. Length of program: The program consisted of 110 pages. The length of the program varies depending on what sections the user accesses. The length of time that participants used the program was recorded with results showing 8% used it for less than 20 minutes, 32% for 20 minutes to one hour, 28.5% one to two hours and 31.5% over tow hours. Average utilisation was one hour and 53 minutes. Number of times program was used: The number of times participants logged into the program was recorded with results showing that seven participants did not use the program. Of those that did 12% accessed the program on one occasion, 24% on two occasions, 32.5% on three to four occasions, 18.5% on five to six occasions and 13% on seven or more occasions. Time point of delivery: Participants were considered to be at risk of pharmaceutical misuse due to their profession. However, they were not necessarily taking any of the medications targeted by the program. Success at reaching target audience: Seven participants in the multimedia group did not use the program. Assistance: None Interactivity: Limited ‐ Fully. Participants are able to navigate through the program and choose the information components that they want to access and the order in which they access them. However there are fully interactive segments within the program where users are able to complete self‐assessments to determine if they need help with their use of prescription medication and evaluate their fitness level. Tailoring: Yes. The program contained self‐assessments on current or anticipated prescription drug use and self‐reported learning interests. Assessment results were used to recommend sections of the program that were most applicable to the user. |
Source of information about comparators: Information provided by the author in the published study Description of comparator: Waiting list controls who did not receive any education. |
|
Generic structure: The content included seven moves: Background on drug; Summary of use; Dosage instructions; Account of side effects; Constraints on patient behaviour; Outline of benefits and Clinical contact availability. Rhetorical elements: There rhetorical elements in the program included are to inform, instruct and define. Terminology used to describe medications and their actions was defined for each of the medication classes. Instructions are clear and consistently identify whose role it is to perform the task. Tenor: The tenor in the program is medical expert/health professional to patient. However, this is implied as the presenter in the program does not introduce themselves and provides no information regarding their background or qualifications. The presenter refers to themselves as "we" e.g. "we recommend that you..." but does not identify who this refers to. In the "Smart Use" section there is video of women discussing their medication with their health professional but the tenor remains the same. Metadiscourse: The introduction to the program clearly states the purpose of the program before outlining the different sections and how they can be used. It also instructs users on how to navigate through the program and access further information. Headings: Each section has a heading and a series of subheadings. Section headings are also listed in a panel to the left of the main screen and can be used to navigate through the program. Technicality of language: Medical terminology was used but it was accompanied by a definition or explanation. Lexical density: Overall lexical density was not calculated due to the variation in content. Lexical density was calculated from three segments of the "Medication facts" section. The introduction which provided background information about the medications had lexical density of 51%. A segment providing specific information about one of the classes of medication had lexical density of 65% and a video of a conversation between a patient and a doctor had lexical density of 31%. Factual content: The program did not state the source of the information or the date it was produced. Format: The program is divided into three main sections. 1. The Medication Facts section provides a description of how medications work and common concerns presented with graphics and animation. The"concerns" section contains a list of commonly asked questions with video segments of pharmacists answering each question. 2. The Smart Use section "contains information about drug tolerance, abuse and dependence and the warning signs of each. Real‐life video testimonials are provided of women who have experienced problems with prescription medications and there is a self‐assessment on how to determine if one needs help with one's use of prescription medication". 3. The Managing Your Health section presents alternatives to medications with interactive segments on evaluating one's fitness level and tips for starting an exercise regime as well as printable pages listing particular exercises. |
Evaluation of comparator: Not applicable |
|
| Goodyer 2006 |
Source of information about intervention: Information provided by the authors in the published study and a copy of the voice‐over script for the English version of the program. Description of intervention: Multimedia program demonstrating metered dose inhaler technique. This study used the Turkish version of the program used in Savage 2003. Name of program: Multimedia Touch Screen System (MTS) Country of origin: England Language: Turkish (however the program is available in other languages including English) Year program used in the study was produced: Not reported Stated expertise of content developers: Pharmacy Practice Group King's College London. Stated expertise of program developers: Not reported Process of development and testing: Not reported other than to state that the content was based on that of the "how to use your inhaler" section of the Ventolin PIL. Availability: No longer available. Purpose: To demonstrate correct inhaler technique to Turkish patients with asthma Content: The program covered eight information points relating to correct inhaler technique and additional information about posture. It consisted of video clip demonstrations of correct inhaler technique with a voice‐over explanation. Relationship between developer and user: Health professionals to patients. Target audience: Turkish asthma patients with poor English skills Format: Computer‐based multimedia touch‐screen system Hardware required: Computer with touch‐screen Media: Video and audio Delivery: Viewed on a computer in the patient's home Length of program: Not reported Number of times program was used: Patients only used the program on one occasion but were allowed to use it for as long as they wished Time point of delivery: Patients were longstanding inhaler users (mean years (SD) = 8.8 (8.9) Success at reaching target audience: Assessors went to the patients' homes, administered the program and conducted assessments on one visit therefore all participants in the intervention arm viewed the program. Assistance: The authors state that patients required "little instruction on use of the system" but do not describe this further Interactivity: Limited. Users could interact with the program using a touch‐screen that allowed them to navigate through the program. Tailoring: No |
Source of information about comparators: Information provided by the authors in the published study. The evaluation of the PIL used the current version of the Ventolin PIL produced by Allen and Hanbury (dated June 2009). Description of comparators: 1. Patient information leaflet (PIL) A translated (into Turkish) patient information leaflet. The leaflet was produced by a study author and was an exact translation of the current PIL produced by the drug manufacturer (Allen and Hanbury) for Ventolin MDI. 2. Verbal support Written information was followed by verbal support from a translator who identified areas where the patient's technique could be improved and then spent up to 15 minutes discussing this with the patient. |
|
Evaluation of intervention: Generic structure: The program focused on the information contained in the "How to use your inhaler" section of the PIL. It therefore only contained the "Dosage instructions" move. Rhetorical elements: The program consisted almost entirely of instructions. The instructions used the imperative form and responsibility for the actions was clear. The importance and urgency of actions was clearly stated. Tenor: Unable to assess Metadiscourse: The purpose of the program was clearly stated at the beginning. Headings: Unable to assess Technicality of language: Lay language was used with minimal medical terminology. Lexical density: 42%. Calculated from the script for the program voice‐over. Factual content: Unable to assess Format: A touch screen multimedia program in which a white male model demonstrates the steps required for correct MDI use accompanied by a voiceover. Key points from the voiceover were reinforced as on‐screen text. Users could choose to replay steps. |
Evaluation of comparator: Ventolin PIL Generic structure: The leaflet was divided into six sections: 1. What ventolin is and what it is used for; 2. Before you use ventolin; 3. How to use you inhaler (ventolin); 4. Possible side effects; 5. How to store ventolin and 6. Further information. All of the possible moves other than "Information regarding monitoring" were identifiable. The sequence of moves was typical for PILs. Rhetorical elements: The predominant rhetorical elements in the leaflet were to inform and instruct. Instructions used the imperative form and responsibility for the actions was clear. The importance and urgency of actions was clearly stated. Tenor: The leaflet was written by the drug manufacturer for patients using ventolin. The intended reader was clearly identified at the beginning of the leaflet ("Information for the user") and the relationship was clear and consistent throughout. The identity of the writer was not explicitly stated but implied by the drug company logo and copyright at the end of the leaflet. Metadiscourse: The purpose of the leaflet was stated as providing information about ventolin for the user. The sections contained in the leaflet were also listed at the beginning. Headings: Headings were present, clearly formatted and clearly signposted the information contained in each section. Technicality of language: There was minimal use of medical terminology and where it was used its meaning was explained. Risk was explained using words (e.g. common, uncommon) and numeric (e.g. less than one in 10) descriptors. Lexical density: 46%. The lexical density was calculated from the "how to use your inhaler" section of the leaflet. Factual content: The source and evidence base for the information contained in the leaflet was not stated. However, the leaflet included the date it was created/updated. Format: The leaflet was a double sided A4 page that contained a mix of prose and bullet point and numbered lists. It used a font size smaller than 12‐point but provided contact information for accessing a large print version of the leaflet. It contained a shaded box providing instructions on the use of an MDI broken into nine steps and accompanied by photographs of a person performing six of the steps. Inadequate information was provided on which to base an assessment of the verbal support provided. |
|
| Kato 2008 |
Source of information about intervention: Information provided by the authors in the published study and information from the re‐mission website including the game manual, screen shots and short video excerpts from the game. Description of intervention: Name of program: Re‐Mission Country of origin: USA Language: English, Spanish and French Year program used in the study was produced: 2006 Stated expertise of content developers: Scientific and medical consultants contributed to the content of the game. Stated expertise of program developers: Video game developers and animators were involved in the construction of the video game. Process of development and testing: Developed by the study authors. Behavioural objectives identified from literature reviews and pre‐production studies were translated into game structure on the basis of principles from the self‐regulation model of health and illness. "Teens and young adults with cancer participated actively throughout the game development process to ensure that the game was fun, and that it really spoke to the issues that they confront every day in their fight against cancer." Availability: A trailer of the game can be viewed on the website www.re‐mission.net and the game can be ordered in CD or DVD format from the website. It is available free of charge. Purpose: To improve health‐related behaviours such as treatment adherence, cancer‐related self‐efficacy and knowledge in adolescents and young adults with malignancies. Content: The game does not provide any structured information, but the game play includes destroying cancer cells and managing common treatment related effects such as bacterial infections, nausea and constipation by taking appropriate medications and engaging in positive self‐care behaviours. Tenor: Developed by health professionals for patients. Target audience: Adolescents and young adults with a diagnosis of a malignancy. Format: Video game for personal computer Hardware required: Computer Media: Text, audio and animation. Delivery: Played on computers supplied to the patients in the patient's own home. Length of program: The game's length is variable depending on how the patient plays it. There are 20 levels and the patient must complete the mission successfully before moving on to the next level. Number of times program was used: Patients were asked to play the video game for at least one hour per week for a three month period. However, only 33% of the intervention group (and 22% of the control group) used the game for the requested period of time. Time point of delivery: Patients had been diagnosed with cancer and commenced their treatment regimes. Success at reaching target audience: 13% of the intervention group did not use their computer or attempt to play the game at all (compared with 9% of the control group). Assistance: None Interactivity: Fully interactive Tailoring: No |
Source of information about comparator: Information provided by the authors in the published study. Description of comparator: A commercial PC game (Indiana Jones and the Emperor's Tomb) was used as it had similarities with the "play structure and controller interface" of Re‐Mission. |
|
Evaluation of intervention: Generic structure: From the information available, the game contains at least four moves: Summary of use; Account of side effects; Constraints on patient behaviour and Outline of benefits. Rhetorical elements: Users are given audio instructions and suggestions throughout the mission. The main rhetorical elements in the game are to inform and instruct. Tenor: Health professional to patient. The mission briefing is given by a doctor who outlines the assignment. Metadiscourse: At the beginning of each mission users are provided with a briefing of their assignment and a list of objectives that they need to complete in order to be successful in the mission. Headings: The game does not contain headings but uses a "heads up display (HUD)" as a signpost throughout the game. The HUD includes radar that identifies the position of the user and navigational arrows to guide the user through the mission. Technicality of language: Medical terminology is used but defined. Lexical density: Unable to assess Factual content: The source of the content is identified in the program information. This contains disclaimers stating that the content is for informational purposes only and does not take the place of medical advice and that the publisher "makes reasonable efforts to include accurate and current information whenever possible, but makes no warranties as to its accuracy or completeness". The date the program was produced is shown in the copyright. Format: A fully interactive game which is available on CD, DVD and as a download (Windows only) from the Internet. The game contains 20 missions each presenting a clinical scenario of a patient with a particular type of cancer who is facing particular treatment related issues. At the beginning of each mission, the user is provided with a brief medical history of the patient as well as a list of objectives for their mission. The user then navigates through the mission in order to complete these objectives e.g. use antibiotics to blast bacteria which are causing infection following a course of chemotherapy. |
Evaluation of comparator: Not applicable |
|
| Kinnane 2008 |
Source of information about intervention: Information provided by the authors in the published study as well as a copy of the DVD. Description of intervention: Video about chemotherapy, its side effects and how to prevent and manage these side effects given as well as standard pre‐chemotherapy education. Name of program: Staying well during chemotherapy Country of origin: Australia Language: The video is available in 12 languages. Only the English version was used for this study Year program used in the study was produced: 2005 Stated expertise of content developers: Developed by oncology nursing staff from the Southern Health chemotherapy unit with input from The Cancer Council Victoria, Cancer Nurses Society of Australia and Southern Health Oncology and Medical Haematology Focus Group. Stated expertise of program developers: Video was filmed and produced by LOTE Marketing Process of development and testing: No information was provided other than that the video was developed by oncology nurses with input from the organisations listed above. Availability: Not reported Purpose: To improve patient safety when at home while they are undergoing chemotherapy treatment and to help them better understand the effects of treatments and assist in the management of side effects Content: Generic information about chemotherapy and how to manage side effects.The video outlines the most important take home information on dealing with nausea and vomiting, taking anti‐emetics, monitoring for and reporting signs of infection, low platelet count and anaemia, mouth care, advice concerning eating well and drinking fluids, prevention and control of constipation and diarrhoea. Target audience: Patients about to start chemotherapy Format: DVD Hardware required: Television and DVD player Media: Video with audio voice‐over Delivery: Patients watched the video, with family members who were present on the day, in a separate room of the treatment centre Length of program: 10 minutes and 30 seconds Number of times program was used: Once Time point of delivery: Prior to starting chemotherapy Success at reaching target audience: All participants viewed the computer program on site at the treatment centre Assistance: No medical or nursing staff were present during the video. Interactivity: No Tailoring: No |
Source of information about comparator: Information provided by the authors in the published study. Description of comparator: Standard care Standard pre‐chemotherapy education consisting of a one hour education session with a member of the nursing staff and written information. The nurse explained how the chemotherapy drugs work, side effects, self‐help concepts including dietary and fluid intake advice, special instructions regarding mouth care, how and when to take antiemetics, monitoring for problems associated with low blood counts and advice regarding the prevention and control of diarrhoea and constipation. Written information included a Neutropenic Alert card detailing contact numbers and information for healthcare professionals to use if the patient presented unwell, details of the chemotherapy regime, side effects and their management, self‐help concepts and circumstances in which the patient should contact the treatment centre. |
|
Evaluation of intervention: Generic structure: There are five moves contained within the video: Summary of use; Account of side effects; Information regarding monitoring; Constraints on patient behaviour and Clinical contact availability. Rhetorical elements: The main rhetorical elements in the video are to inform and instruct. Instructions are clear and consistently identify whose role it is to perform the task. Tenor: The health professional to patient relationship is not stated explicitly but is implied by the language e.g. "during our care for you". Metadiscourse: The purpose of the video is stated in the introduction which also outlines the main topics of information that will be presented. Headings: None Technicality of language: Medical terminology was largely avoided. However, where it was used, it was accompanied by an explanation. Lexical density: 51%. Calculated from the first three minutes of the video. Factual content: The source of the content and the date it was last updated is implied by the date and acknowledgement of the Southern Health department of medical oncology and clinical haematology on the DVD. Format: Although the program is produced using DVD format, this only became evident when a copy of the program was obtained. The program is described by the study authors as a video and does not use any of the functionality of the DVD format. It is designed to be viewed as a video, from beginning to end, with no section breaks or menu to allow navigation. It consists of video images, and one short animation, illustrating information provided by a voiceover with background music. |
Evaluation of comparators: Generic structure: Based on the information provided by the authors the content included at least five moves: Summary of use; Account of side effects; Information regarding monitoring; Constraints on patient behaviour and Clinical contact availability. Rhetorical elements: Unable to assess Tenor: Nurse to patient Metadiscourse: Unable to assess Headings: Unable to assess Technicality of language: Unable to assess Lexical density: Unable to assess Factual content: Unable to assess Format: One‐hour standardised education session given by a nurse and written information including a Neutropenic Alert card |
|
| Knoerl 1999 |
Source of information about intervention: Information provided by the authors in the published study. Description of intervention: Instructional video about the use of PCA Name of program: Not reported Country of origin: USA Language: English Year program used in the study was produced: Not reported Stated expertise of content developers: Not reported Stated expertise of program developers: Not reported Process of development and testing: Not reported Relationship between developers and users: Not reported Availability: Not reported Purpose: To provide preoperative teaching regarding the use of PCA therapy that increases patient knowledge about PCA and corrects mistaken beliefs about pain medicine. Content: Not reported Target audience: Patients undergoing a surgical procedure who are expected to use PCA postoperatively. Format: Videotape Hardware required: Television and VCR Media: Video with audio Delivery: The video was viewed in the Ambulatory Surgical Unit prior to the patients going to theatre. Length of program: 11 minutes Number of times program was used: Once Time point of delivery: Patients viewed the video preoperatively and started PCA in the immediate postoperative period. Success at reaching target audience: All participants viewed the presentation on site. Assistance: Not reported Interactivity: No Tailoring: No |
Source of information about comparator: Information provided by the authors in the published study. Description of comparator: Usual care: informal teaching provided by hospital medical staff about PCA as part of usual perioperative care. |
|
Evaluation of intervention: Inadequate information was provided on which to base an assessment |
Evaluation of comparator: Not applicable |
|
| Lirsac 1991 |
Source of information about intervention: Information provided by the authors in the published study. Description of intervention: Video about the use of MDIs Name of program: Not reported Country of origin: France Language: French Year program used in the study was produced: Not reported Stated expertise of content developers: Not reported Stated expertise of program developers: Not reported Process of development and testing: Not reported Relationship between developers and users: Not reported Availability: Not reported Purpose: To demonstrate correct use of MDIs. Content: Described the use of MDIs Target audience: Patients prescribed MDIs. Format: Videotape Hardware required: Television and VCR Media: Video with audio Delivery: Not reported. However, the article implied that the video was viewed on site. Length of program: Five minutes Number of times program was used: Once Time point of delivery: Patients were regular users of MDIs prior to viewing the video. Success at reaching target audience: All participants viewed the presentation on site. Assistance: Not reported Interactivity: No Tailoring: No |
Source of information about comparators: Information provided by the authors in the published study. Description of comparator: Instruction sheet read by a doctor while participants looked at pictorial representations. Education lasted three to four minutes. |
|
Evaluation of intervention: Inadequate information was provided on which to base an assessment |
Evaluation of comparators: Inadequate information was provided on which to base an assessment |
|
| Mazor 2007 |
Source of information about intervention: Copy of the videotape as well as information provided by the authors in the published study. Description of intervention: Video showing a physician‐patient encounter about oral anticoagulant medication management. Name of program: Patients' views about anti‐coagulation therapy: A research study Country of origin: USA Language: English Year program used in the study was produced: 2005 Stated expertise of content developers: Health professionals Stated expertise of program developers: Not reported Process of development and testing: The videos were produced by the authors for this study. Draft scripts were created and reviewed by the authors before being filmed. Draft versions of the videos were pilot tested with four patients attending the anticoagulant clinic. Final versions of the scripts were reviewed by the two physician authors for clinical accuracy. Relationship between developers and users: Health professional to patient Availability: Not reported Purpose: To educate patients on anticoagulant medication using different methods (narrative evidence, statistical evidence or both) to communicate evidence to patients. Content: The videos depicted a physician talking to a patient about anticoagulant medication management. The physician covered issues such as dosing, target INR's and symptoms indicating side effects of the medication. There were three versions of the video presenting evidence using narrative (patient anecdotes), statistical or a combination of both methods. Target audience: Patients taking anticoagulant medications. Format: Videotape Hardware required: Television and VCR Media: Video with audio Delivery: Video was watched in the participant's home. Length of program: Not reported Number of times program was used: Patients were asked to view the video once, but were free to watch it as many times as they liked. Time point of delivery: Patients had been prescribed warfarin and receiving care at an anticoagulation clinic for at least three months. Success at reaching target audience: It is unknown how many participants viewed the videos, but questionnaires were returned by fewer patients in the intervention than the control groups and 44/350 patients from the intervention groups reported that they were unable to watch the video as they did not have access to a VCR. Assistance: None Interactivity: No Tailoring: No |
Source of information about comparator: Information provided by the authors in the published study. Description of comparator: Usual care: participants did not receive a video or other form of education as part of this study. |
|
Evaluation of intervention: Generic structure: The content of the video contained seven moves. The three moves not included were contraindications, outline of benefits and storage instructions. Rhetorical elements: The majority of the video's content informed users about warfarin and its use. Tenor: The video consists of a conversation between a doctor and patient. The person viewing the video is instructed to imagine that the doctor in the video is talking to them. Metadiscourse: Patients are told that they are about to see a video of a doctor and patient talking about warfarin. However, the purpose of the video is not presented. Headings: None Technicality of language: Medical terminology was avoided or used with accompanying explanations Lexical density: 43%. Calculated from the script for the video. Factual content: The year that the video was produced was shown as part of the copyright on a label on the videocassette. The statistical evidence version of the video stated that certain information was based on research findings. However, the source of the evidence is not given. The narrative version illustrated the same information using patient anecdotes. Format: Videotaped conversation between a doctor and patient |
Evaluation of comparators: Not applicable. |
|
| McElnay 1989 |
Source of information about intervention: Information provided by the authors in the published study. Description of intervention: Video about the use of MDIs Name of program: Not reported Country of origin: UK Language: English Year program used in the study was produced: Not reported Stated expertise of content developers: The video was developed by the study authors who were health professionals. Stated expertise of program developers: The Audio‐Visual Unit at The Queen's University of Belfast. Process of development and testing: A short video was prepared on the correct use of the metered dose inhaler. The adequacy of the film was assessed in a pilot study involving 30 students studying non‐medical subjects. Based on the results obtained, a second improved video film was prepared for use in the study. Relationship between developers and users: Developed by health professional for patients Availability: Not reported Purpose: To demonstrate the correct use of the metered dose bronchodilator inhaler. Content: The correct use of a metered dose inhaler illustrating six steps 1. Shake the inhaler and remove the cap. 2. Hold the inhaler upright. 3. Breathe out fully through your mouth, emptying as much air from your lungs as possible. 4. Close lips tightly around the mouthpiece. 5. Press the canister as you start to take a long deep breath‐in through your mouth. 6. Close mouth and hold breath for five to ten seconds before slowly breathing out. Target audience: Adults who have been prescribed a metered dose inhaler. Format: Videotape Hardware required: Television and VCR Media: Video with audio Delivery: The video was viewed on site. Length of program: Five minutes Number of times program was used: Once Time point of delivery: The participants in this study were healthy volunteers who had no previous experience in the use of MDIs Success at reaching target audience: All participants viewed the video on site. Assistance: Not reported Interactivity: No Tailoring: No |
Source of information about comparators: Information provided by the authors in the published study. The evaluation of the PIL used the current version of the Ventolin PIL produced by Allen and Hanbury (dated June 2009). Description of comparators: 1. Ventolin PIL Written instruction consisting of the drug manufacturer's (Allen & Hanbury) PIL for Ventolin. 2. Personal instruction Personal instruction consisted of a five minute demonstration of correct inhaler technique by a pharmacist, review of a custom‐designed instruction sheet outlining the six steps required for using a MDI correctly and the Ventolin PIL. |
|
Evaluation of intervention: Generic structure: The program illustrated six steps involved in correct MDI use. It therefore only contained the "Dosage instructions" move. Rhetorical elements: Unable to assess Tenor: Unable to assess Metadiscourse: Unable to assess Headings: Unable to assess Technicality of language: Unable to assess Lexical density: Unable to assess Factual content: Unable to assess Format: A short five minute video demonstrating correct MDI use. |
Evaluation of comparators: Evaluation of the PIL presented in Goodyer 2006. Inadequate information was provided on which to base an assessment of the personal instruction provided. |
|
| Navarre 2007 |
Source of information about intervention: The current version of the tutorial available online as well as information provided by the authors in the published study. Description of intervention: Name of program: Metered Dose Inhalers Country of origin: USA Language: English Year program used in the study was produced: 2000 Stated expertise of content developers: Faculty at the University of Michigan College of Pharmacy. Stated expertise of program developers: Developed with the assistance of the University of Michigan Center for Information Technology. Website design and art direction by Steve Burdick, University of Michigan ITCS Web and Database Services. Process of development and testing: Not reported Relationship between developers and users: Health professional to patient Availability: The tutorial was also created for access through a website and information regarding the website is available from Dr Erickson at the University of Michigan Purpose: To demonstrate the correct use of MDIs Content: The tutorial covers the steps of inhaler technique Target audience: Adults using MDIs Format: Computer program available over the Internet or on CD‐ROM. Hardware required: Computer Media: Text, video of an actor‐patient and animation Delivery: Viewed on computers on site at either the clinic or pharmacy. Length of program: Not reported Number of times program was used: Once Time point of delivery: At least six months after commencing inhaler. Success at reaching target audience: All participants viewed program on site. Assistance: The research assistant oriented the patient on the use of the computer and was available to answer any question regarding navigation through the program. Interactivity: Not reported Tailoring: Not reported |
Source of information about comparator: Information provided by the authors in the published study. Description of comparator: Usual care: the participants did not receive any education as part of this study. |
|
Evaluation of intervention: Generic structure: 15 steps outlining the correct use of an MDI. The content is limited to the "Dosage instructions" move. Rhetorical elements: The program provides instructions but also informs users of why the action is important and the consequences of performing the action incorrectly. Tenor: The organisation and individuals who created the program were identified in a heading at the top of the page and in a separate "credits" page. The user was clearly responsible for each of the actions presented. Metadiscourse: There is no information provided regarding the purpose of the program other than the title stating that it is part of the inhaler instructional series. Headings: Each step is shown with a heading. Selecting the heading then takes you to another page where information about that step is presented Technicality of language: The acronym MDI was used without accompanying explanation. Some technical and medical language was also used without explanation e.g. propellant, aerosol impaction, tachycardia. Lexical density: 45%. Calculated from the first two sections, containing nine of the 15 steps. Factual content: The authors of the program were identified and the year in which the program was developed was presented as part of the copyright. Format: The tutorial is divided into six sections containing a total of 15 steps which are presented in a summary page. When the link for each step is selected a page presenting written instructions is shown with an accompanying window in which a short audiovisual presentation can be played. This consists of a video of an "actor‐patient" performing that step, some supporting animations and a voiceover reading the written instructions. Users can navigate through the steps using "next" and "back" arrows or can select individual steps from links on the summary page. |
Evaluation of comparators: Not applicable. |
|
| Neafsey 2002 |
Source of information about intervention: Information provided by the authors in the published study and in a previous publication describing the development and testing of the program (Strickler 2002). Description of intervention: Computer program about the interaction between prescription medications and over‐the‐counter medications and alcohol. Name of program: Preventing Medicine Conflicts Country of origin: USA Language: English Year program used in the study was produced: Not reported Stated expertise of content developers: Pharmacologist, visual communications designer and design students Stated expertise of program developers: Animations were produced by a professional visual communications designer using Adobe Illustrator and Aftereffects. Process of development and testing: The program was designed based on evidence on the learning styles of older adults. Patients in focus groups evaluated aspects of the program including visual features, language used in the text and the interactive questions on multiple occasions as the program was being developed leading to changes in the program. It was revised again following pilot testing with 60 older adults (Neafsey 2001). Relationship between developers and users: Developed by health professionals for patients Availability: The program can be purchased from the University of Connecticut School of Nursing Purpose: To teach older adults about potential drug interactions that can results from self‐medication with over‐the‐counter agents and alcohol Content: Information about potential interactions between prescribed medications with over‐the‐counter medications and alcohol. Target audience: Older adults (aged 60 or over) living independently in the community Format: CD‐ROM using Macromedia Authorware software Hardware required: Notebook computers with infra‐red touch‐screen Media: Text, still graphics and animations (no audio). Delivery: Viewed on computers provided at the participating senior centre clinics Length of program: 80 minutes Number of times program was used: Once Time point of delivery: Participants were already taking prescription medications in conjunction with OTC preparations. Success at reaching target audience: All participants viewed the computer program on site Assistance: Not reported Interactivity: Limited: There are questions with multiple choice answers throughout the program. Users are able to use buttons on the touch‐screen to answer questions and control the animations. Users can also skip questions. Feedback is given based on the response to each question. Tailoring: None |
Source of information about comparator: Information provided by the authors in the published study. Description of comparator: 1. Written information The written information group received an information booklet containing the same information as the computer program but presented using only text. The text is set at 14 point Arial typeface and is written at a 6th grade reading level. 2. No information The control group did not receive any education as part of this study. |
|
Evaluation of intervention: Generic structure: The focus of the program is the interaction between prescription medications and over‐the‐counter medications and alcohols. This is contained within the "constraints on patient behaviour" move. Unable to assess whether information from other moves was also included in the program. Rhetorical elements: Unable to assess Tenor: Unable to assess Metadiscourse: Unable to assess Headings: Headings are used in the program. The information hierarchy and placement of elements on the page were tested with participants during development of the program. Technicality of language: The authors stated that the health information within the program was at Fleish‐Kincaid grade 5 reading level. Particular vocabulary and descriptions within the program were tested in focus groups to identify and change vocabulary that the target population found difficult to understand. Lexical density: Unable to assess. Factual content: Unable to assess Format: Computer with touch screen allowing on screen buttons for navigation ("menu", "back" and "next") and for control of animations ("replay", "pause", "play"). Program content is divided into four main sections: blood pressure medicines, blood thinners, antacids and acid reducers and pain relievers. The font used for the text was 18 to 20 point Stone Sans Bold with 28 and 32 point font size used for headings. Text line lengths were typically between two to four words in length and the longest block of continuous text was five lines. |
Evaluation of comparators: Inadequate information was provided on which to base an assessment |
|
| Neafsey 2001 | Name of program: This is an earlier version of the "Preventing Medicine Conflicts" interactive computer program (Neafsey 2002). Description and evaluation of the intervention are the same as those in Neafsey 2002. |
Source of information about comparators: Information provided by the authors in the published study. Description of comparators: The control group did not receive any education as part of this study. |
| Olver 2009 |
Source of information about intervention: Copy of the CD‐ROM and information provided by the authors in the published study. Description of intervention: CD‐ROM Name of program: Understanding cancer Country of origin: Australia Language: English Year program used in the study was produced: Not reported Stated expertise of content developers: Health professionals including oncologists and psychologists Stated expertise of program developers: The CD‐ROM was developed with media producers Steve Whitham Media and M‐Plex Multimedia Process of development and testing: The CD‐ROM was designed using the "medical expertise of the researchers in association with local media expertise". Relationship between developers and users: Developed by health professionals for patients. Availability: Not reported Purpose: To provide chemotherapy treatment information as part of the informed consent process Content: The authors state that the CD‐ROM contains the same information about chemotherapy as their standard written information and consent sheet but do not outline what information this contains. The CD‐ROM also has additional information about cancer and its treatment under the headings of type of cancer, nutrition, patient's perspective, further information, prevention, help, frequently asked questions, glossary of terms and consent form. Target audience: Adults who are about to start chemotherapy Format: CD‐ROM using Macromedia software Hardware required: Computers Media: Text, still graphics, video and audio Delivery: Viewed on computers provided on site at the Chemotherapy Day Centre. Participants were also able to take the CD‐ROM home for further viewing. Length of program: Depends on which sections of program the user chooses to view Number of times program was used: Viewed on one occasion on site and then at the participant's discretion off site Time point of delivery: Prior to starting chemotherapy Success at reaching target audience: All participants viewed CD‐ROM on site, 36.2% viewed the CD‐ROM again at home Assistance: A nurse was available on site to help with navigation if participants were unfamiliar with the technology Interactivity: Limited. patients are able to view components of the program in their entirety or choose particular areas that they are interested in viewing. Tailoring: Yes ‐ patients can select from a menu the type of cancer they have been diagnosed with. They are then presented with information specific to that cancer and its treatment. |
Source of information about comparators: Information provided by the authors in the published study. Description of comparators: Written information consisting of the hospital's standard information sheet and consent form. All patients (intervention and control) were also provided with usual care consisting of verbal information about their chemotherapy by the medical or nursing staff. They were free to ask questions and access other information. |
|
Evaluation of intervention: Generic structure: All of the moves except for "Dosage instructions", "Contraindications" and "Storage instructions" were included in the program. Rhetorical elements: Most of the information is presented to inform and instruct the user. However, there is lack of clarity at times as to whether the information is being presented to instruct or inform and whose responsibility it is to perform the action. Tenor: For most of the components of the program the tenor is health professional to patient. This is stated in the introduction where the doctor and the cancer centre are identified. The user is referred to using both impersonal (the patient) and personal (you) language in the program. The program also contains videotaped interviews with patients providing their perspective on their diagnosis and treatment. For this component, the tenor is patient to patient. Metadiscourse: The introduction to the program clearly states the purpose of the program before outlining the different sections and how they can be used. It also instructs users on how to navigate through the program and access further information. Headings: Headings are used consistently throughout the program and are used to aid navigation. Technicality of language: The information contains frequent use of medical terminology. The definition for some but not all of this terminology can be obtained by selecting the relevant word if it is highlighted or via the "Glossary of terms" section. Lexical density: Overall lexical density could not be calculated due to the variation in content. Information about the cancer and chemotherapy, presented with text and voice‐over, in the "Type of cancer" section had lexical density of 56%. A video of a health professional providing information about nutrition had lexical density of 49% and a video of a patient describing their experience of cancer and its treatment had lexical density of 29%. Factual content: The source of the information was identified as the Royal Adelaide Hospital Cancer Centre. Although there was no date showing when the program was last updated, patients were advised that they could use the program to link to up to date information on the Internet. Format: CD‐ROM with the main content headings presented in a menu on the left. These include: Type of cancer; Nutrition; FAQs, Patient's perspective; Prevention; Glossary of terms; Further information; Help and Consent form. Selecting some headings leads to a menu of subheadings and information presented as text, voice‐over which reads the text and accompanying animations. Users can navigate through this information using "previous" and "next" arrows at the bottom of the screen. Words highlighted in blue can be linked to in order to access further information. Selection of other headings leads to videos of health professionals or patients. Users can pause and rewind these videos. |
Evaluation of comparators: Inadequate information was provided on which to base an assessment |
|
| Osguthorpe 1983 |
Source of information about intervention: Information provided by the authors in the published study. Description of intervention: Video about medications used to treat psychiatric illnesses Name of program: Videotaped Nurse Explanation Country of origin: USA Language: English Year program used in the study was produced: Not reported Stated expertise of content developers: Script was developed by the study investigators (nurses) Stated expertise of program developers: The hospital's audiovisual department Process of development and testing: The script was developed by the investigators and the video produced in the hospital's audiovisual department. No testing was reported Relationship between developer and user: Health professional to patient. Availability: Not stated Purpose: To provide patients with general information about study medications Content: A videotaped nurse explanation providing general information (not specific to each medication) about 9 medications used for treatment of psychiatric illnesses. This included general information common to all of the medications such as their purpose, side effects, precautions and helpful hints. Target audience: Psychiatric inpatients Format: Videotape Hardware required: Television and VCR Media: Video, Audio, Text and Cartoons Delivery: Viewed in a conference room on the hospital ward Length of program: Eight minutes Number of times program was used: Once Time point of delivery: Patients had started the medication within 3 weeks before watching the video Success at reaching target audience: All participants viewed the presentation on site. Assistance: Not reported Interactivity: No Tailoring: No |
Source of information about comparator: Information provided by the authors in the published study. Description of comparator: Written information consisting of a drug information sheet containing the same information as the videotaped nurse explanation except that it identified the particular medication by name, picture and dosage. |
|
Evaluation of intervention: Generic structure: The authors stated that the video "was a general discussion and was not specific to each drug. The content followed the same outline as the drug information sheet. No new information was included". Although not specifically stated this implied that the video contained the same information as the "general information" section of the leaflet which covers four moves: Summary of use, Account of side effects, Constraints on patient behaviour and Clinical contact availability. Rhetorical elements: Unable to assess Tenor: Nurse to patient. Metadiscourse: Unable to assess Headings: Unable to assess Technicality of language: Unable to assess Lexical density: Unable to assess Factual content: Unable to assess Format: An eight minute video consisting of a nurse explanation interspersed with cartoons and printed materials to emphasise important points. Important points were also summarised at the end of the video. |
Evaluation of comparators: Drug information sheet Generic structure: The authors described the content of the information sheet as "Information was specific in identifying the particular medication by name, by picture and by dosage. General information described the purpose, side effects, precautions and helpful hints common to all of the medications included in the study. The drug information sheet ended with a place for the telephone number of the ward to be written in so the patient could telephone the ward if questions emerged". This suggests that the information contains 6 moves: Background on the drug, Summary of use, Dosage instructions, Account of side effects, Constraints on patient behaviour and Clinical contact availability. Rhetorical elements: Unable to assess Tenor: Unable to assess Metadiscourse: Unable to assess Headings: Unable to assess Technicality of language: Materials were aimed at fifth grade reading level with important medical symptoms translated into layman's terms. Lexical density: Unable to assess Factual content: Unable to assess Format: The information sheet was constructed so that it could be folded into billfold size and carried easily |
|
| Powell 1995 |
Source of information about intervention: Information provided by the authors in the published study. Description of intervention: Name of programs: Six videotapes: 1. "The Health Challenge: Managing Hypertension", 2. "Taking Lotensin", 3. "Taking Lopressor" 4. "Discussing menopause". 5. "Using Estraderm" and 6. "The Health Challenge: Managing High Cholesterol, Taking Zocor." Country of origin: USA Language: English Year program used in the study was produced: Not reported Stated expertise of content developers: Not reported Stated expertise of program developers: "The videotapes were produced by a national medical media company." Process of development and testing: Not reported Availability: Not reported Relationship between developers and users: Not reported Purpose: To enhance compliance with prescribed drug therapy. Content: Each video included "1) an explanation of the condition or disease process, 2) suggested changes in behaviour that can help ameliorate the problem and contribute to general wellness, 3) an explanation of how the prescribed drug works to control disease processes and symptoms, 4) a discussion of potential adverse effects and contraindications, 5) advice on the importance of compliance, including the need to refill the prescription as prescribed." Target audience: Adults who have been prescribed benazepril, metoprolol, simvastatin or transdermal oestrogen. Format: Videotape Hardware required: Television and VCR Media: Video with audio Delivery: In the participant's home. Length of program: Approximately 30 minutes. Number of times program was used: Not recorded Time point of delivery: Participants had already been prescribed the medications. Success at reaching target audience: The numbers of participants that watched the videos is unknown. However a subset of participants were sent a questionnaire "to determine general reactions to the videotapes, the percentage of tapes actually received and the level of interest in this type of educational program." 205 questionnaires were sent out, of which 97 (47%) were returned. Of these 87% reported that they viewed the videotape. Assistance: None Interactivity: No Tailoring: No |
Source of information about comparators: Information provided by the authors in the published study. Description of comparators: The control group did not receive any education as part of this study. |
|
Evaluation of intervention: Generic structure: Based on the information provided by the authors the content included at least four moves: Summary of use; Account of side effects; Contraindications and Outline of benefits. Rhetorical elements: Unable to assess Tenor: Unable to assess Metadiscourse: Unable to assess Headings: Unable to assess Technicality of language: Unable to assess Lexical density: Unable to assess Factual content: Unable to assess Format: Videotapes of approximately 30 minute duration. |
Evaluation of comparators: No applicable. |
|
| Savage 2003 |
Source of information about intervention: Information provided by the authors in the published study and a copy of the voice‐over script for the English version of the program, Description of intervention: Multimedia program about metered dose inhaler technique. This study used the English version of the program used in Goodyer 2006. Name of program: Multimedia Touch Screen System (MTS) Country of origin: England Language: English (the program is also available in other languages). Year program used in the study was produced: Not reported Stated expertise of content developers: Pharmacy Practice Group King's College London. Stated expertise of program developers: Not reported Process of development and testing: Not reported other than to state that content was based on the "how to use your inhaler" section of the Ventolin PIL. Relationship between developers and users: Health professionals to patients Availability: No longer available Purpose: To demonstrate correct inhaler technique Content: The program covered the eight "key information points" on correct inhaler technique that are included in the Ventolin PIL provided by the drug manufacturer Allen & Hanburys. Target audience: Adults using bronchodilator MDIs. Format: Computer program Hardware required: Multimedia touch screen computer Media: Video, audio and text. Delivery: Viewed on computers on‐site. Length of program: Not reported Number of times program was used: Once Time point of delivery: Patients were longstanding users of bronchodilators Success at reaching target audience: All participants viewed the program on site. Assistance: Patients were supervised when viewing the program. The authors state that "using the touch screen was easy, with few people of any age required even minimal prompting" Interactivity: Limited. Users interacted with the computer through a touch screen which allowed some control of navigation through the program Tailoring: None |
Source of information about comparator: Information provided by the authors in the published study. The evaluation of the PIL used the current version of the Ventolin PIL produced by Allen and Hanbury (dated June 2009). Description of comparator: Written instruction consisting of the drug manufacturer's (Allen & Hanbury) PIL for Ventolin. |
|
Evaluation of intervention: Evaluation of the multimedia program is presented in Goodyer 2006. |
Evaluation of comparators: Evaluation of the PIL is presented in Goodyer 2006. |
|
| Schnellinger 2010 |
Source of information about intervention: Information provided by the authors in the published study. Description of intervention: Animated video about appropriate use of antibiotics. Name of program: Not reported Country of origin: USA Language: English. Year program used in the study was produced: Not reported Stated expertise of content developers: Study researchers including paediatricians. Stated expertise of program developers: Eight Point Productions was hired to produce and edit the video. Process of development and testing: The script and characters were developed by the study authors. The video was reviewed at multiple points during its four month development by the authors and a group consisting of hospital administrators, health educators and the director of the Children's Office of Health Care Equity. The video was approved by the institutional review board prior to implementation. Relationship between developers and users: Health professionals to patients Availability: Not reported Purpose: To educate parents about appropriate use of antibiotics. Content: Information about the appropriate use of antibiotics. Target audience: Parents Format: Video Hardware required: DVD player Media: Video animations with audio Delivery: Viewed on a portable DVD player in the emergency department. Length of program: Three minutes Number of times program was used: The video was viewed once with no opportunity to review it. Time point of delivery: During presentation to the emergency department for acute care. Assistance: Not reported Interactivity: None Tailoring: None |
Source of information about comparator: Information provided by the authors in the published study. Description of comparator: Written instruction consisting of the American Academy of Pediatrics pamphlet about antibiotic use and antibiotic resistance. Participants were able to read this pamphlet on one occasion for 15 minutes before returning it to the research assistant. |
|
Evaluation of intervention: Inadequate information was provided on which to base an assessment. |
Evaluation of comparator: Inadequate information was provided on which to base an assessment. |
|
| Schwarz 2008a |
Source of information about intervention: Information provided by the authors in the published study and in response to request for specific information as well as a copy of the script. Description of intervention: Computer program about emergency contraception (EC) Name of program: EC Video Doctor Country of origin: USA Language: English Year program used in the study was produced: Not reported Stated expertise of content developers: Study author who is a specialist in women's reproductive health. Stated expertise of program developers: Study authors oversaw the programming. Process of development and testing: The program was pilot tested with women seeking urgent care until it was "bug free". Relationship between developers and users: Health professionals to patients Availability: Not reported Purpose: To educate women of child‐bearing age about EC Content: Covered nine key areas about EC: 1. What is EC, 2. When to use EC, 3. What to expect when you use EC, 4. Why to use EC, 5. How to use, 6. Where to get EC, 7. The cost of EC, 8. How does EC work 9. Why is EC important. Target audience: Women of child‐bearing age. Format: Computer program Hardware required: Computer Media: Short video segments, graphics and audio. Delivery: In two separate Urgent Care Clinics where the women were attending appointments. Length of program: 15 minutes Number of times program was used: Once, with the participant having to use all parts of the program. Time point of delivery: The program was shown to the participants after which they were given a sample pack of EC for them to use in the future if required. Success at reaching target audience: All participants used the computer program on site Assistance: Short introductory video instructed users on how to use the mouse to click on each of the nine questions. Interactivity: Limited. Participants could select any of the nine section in any order but had to watch all of them before they could complete the program. Tailoring: No |
Source of information about comparator: Information provided by the authors in the published study. Description of comparator: The control group viewed a computer program about preconception folate supplementation presented using the same format as the EC program. |
|
Evaluation of intervention: Generic structure: The content included six moves: Background,on drug; Summary of use; Dosage instructions; Account of side effects; Contraindications and Outline of benefits. The program also included information on how to access EC and its cost. Rhetorical elements: The program primarily informs women about EC. The program included some instructions but presented them as statements using the passive voice which made their function unclear e.g. "The instructions included in the package suggest taking one pill as soon as possible, and a second pill 12 hours after the first one." Tenor: A video doctor answered questions about EC that the user had selected. The doctor introduced themselves at the beginning of the program. In many instances, the language used was impersonal referring to "a woman" or "some women" rather than directly addressing the user. Metadiscourse: The purpose of the program is stated in the introduction Headings: Unable to assess Technicality of language: The authors aimed for a seventh grade reading level and provided an audio voice‐over so that the program could be used in non‐literate populations. Lexical density: 51%. Calculated from the script. Factual content: Unable to assess Format: Users select from nine questions each leading to short video segments during which the "video doctor" answers the question. The videos are accompanied by additional graphics and text where appropriate. |
Evaluation of comparators: The computer program was the same as that in the intervention arm except that it provided information about preconception folate instead of emergency contraception. |
|
| Self 1983 |
Source of information about intervention: Information provided by the authors in the published study. Description of intervention: Video about MDI use Name of program: Not reported Country of origin: USA Language: English Year program used in the study was produced: Not reported Stated expertise of content developers: Pharmacists and Medical Practitioners Stated expertise of program developers: Technical assistance in videotape production was provided by two individuals from Memphis University School. Process of development and testing: Not reported other than to state that the video was produced by the investigators. Relationship between developers and users: Health professional to patient. Availability: Not reported Purpose: To demonstrate the correct use of inhalers. Content: The video provided instructions based on ten steps for the correct use of an inhaler. Target audience: Adults with an asthma diagnosis who use inhalers. Format: Videotape Hardware required: Television and VCR Media: Video Delivery: The video was viewed in an allergy clinic. Length of program: Not reported Number of times program was used: Once Time point of delivery: 12/29 had previously used inhalers but had not seen a demonstration of proper technique. The others were starting an inhaler for the first time. Success at reaching target audience: All participants viewed the presentation on site. Assistance: Not reported Interactivity: No Tailoring: No |
Source of information about comparators: Information provided by the authors in the published study. Description of comparators: 1. Written information An information sheet "reflecting the manufacturer's directions and the current literature". 2. Personal instruction Personal instruction in the use of an inhaler by a pharmacist. |
|
Evaluation of intervention: Inadequate information was provided on which to base an assessment. |
Evaluation of comparators: Inadequate information was provided on which to base an assessment. |
|
| Solomon 1988 |
Source of information about intervention: Information provided by the authors in the published study. Description of intervention: Video about tetracycline for treatment of STDs Name of program: So they gave me these pills... Country of origin: USA Language: English Year program used in the study was produced: Not reported Stated expertise of content developers: Developed by the authors. Stated expertise of program developers: Not reported Process of development and testing: Video was developed by the authors. Relationship between developer and user: Health and educational professionals to patients. Availability: Copies of the video are available from the project officer at the Centre for Disease Control. Purpose: To provide instructions on the correct administration of tetracycline for STD. Content: The video provides basic information about tetracycline and the importance of correct administration. Step by step instructions are given for patients to build "an individualized schedule" for taking their medication. Target audience: Patients diagnosed with a sexually transmitted disease requiring treatment with Tetracycline. The videotape was designed for patients of inner‐city clinics who are predominantly young, black and poorly educated. Format: Videotape Hardware required: Television and VCR Media: Video and audio Delivery: The video was viewed in the STD clinic. Length of program: 10 minutes Number of times program was used: Once Time point of delivery: Patients viewed the video on the day they commenced their medication. Success at reaching target audience: All participants viewed the video on site. Assistance: The doctor observed the patient watching the video noting any difficulties the patient may have in devising their schedule. The patient was then seen by the doctor immediately after viewing the videotape. The doctor and patient review the schedule card together and answered any questions the patient had. Interactivity: No Tailoring: No |
Source of information about comparators: Information provided by the authors in the published study. Description of comparators: Patients in both the intervention and control group were randomised to receive either special pill packaging or usual care: 1. Special pill packaging Consisting of seven foil packed strips (one for each day of treatment) each with four individually wrapped pills. Each pill was labelled with the day, the number of the pill and instructions relating to when to take the pill and how long to wait until eating. 2. Usual care (verbal instructions) Received a standard set of instructions provided verbally by the treating doctor to all study participants. The instructions consisted of the doctor reading out a paragraph which included the name of the medication, instructions on when and how to take the medication and how long to wait before resuming sexual activity. |
|
Evaluation of intervention: Generic structure: Based on the information provided by the authors the content of the video focuses primarily on the move "Dosage instructions" but also contains the moves "Summary of use" and "Constraints on patient behaviour". Rhetorical elements: The video primarily instructs patients on the correct use of tetracycline as well as informing them of the consequences of improper use. Tenor: Doctor to patient "a physician/narrator speaks directly to patients" and also patient to patient "testimonials from a young couple" who have taken the medication Metadiscourse: Unable to assess Headings: Unable to assess Technicality of language: The physician/narrator "uses easy to understand language and concrete illustrations" Lexical density: Unable to assess Factual content: Unable to assess Format: A 10 minute video of a physician narrator speaking directly to patients juxtaposed with testimonials from a young couple who explain what happened when they inadvertently took the medication incorrectly. |
Evaluation of comparator: Verbal instructions given in the usual care group. Generic structure: Consists of nine short sentences which contained only two of the possible 10 moves ‐ Dosage instructions and Constraints on patient behaviour. The instructions did not contain one of the two "obligatory moves" ‐ Account of side effects. Rhetorical elements: Seven of the nine sentences instruct the patient while the other two inform. Instructions used the imperative form and responsibility for the actions was clear. Tenor: Doctor to patient. Metadiscourse: None. Headings: Not applicable. Technicality of language: Language does not contain any technical or medical terminology. Lexical density: 53% Factual content: The source and evidence base for the content was not given but the content consisted primarily of dosing instructions. Format: Short paragraph read by a doctor to the patient. |
|
| Stone 1989 |
Source of information about intervention: Information provided by the authors in the published study. Description of intervention: Video about anticoagulant therapy Name of program: Not reported Country of origin: USA Language: English Year program used in the study was produced: Not reported Stated expertise of content developers: Produced by the study authors Stated expertise of program developers: Not reported Process of development and testing: The video was produced by the study authors but no information was provided about its development or testing Relationship between developers and users: Health professional to patient Availability: Not reported Purpose: To teach patients starting anticoagulant medications about anticoagulant therapy Content: The videotape reviewed the reasons for anticoagulant therapy, the complications of warfarin, how to monitor therapy and other general points about anticoagulant therapy Target audience: Patients starting treatment with anticoagulants Format: Videotape Hardware required: Television and VCR Media: Video and audio Delivery: The video was viewed at the anticoagulation clinic Length of program: 15 minutes Number of times program was used: Once Time point of delivery: Prior to starting anticoagulant medication Success at reaching target audience: All participants viewed the video on site Assistance: Not reported Interactivity: No Tailoring: No |
Source of information about comparator: Information provided by the authors in the published study. Description of comparator: Personal instruction Lecture from one of two nurses specially trained in anticoagulation therapy. The nurse lecture was standardised and contained the same information as the videotape. A question and answer session with a health professional was held following both the video and personal instruction. |
|
Evaluation of intervention: Generic structure: Based on the information provided by the authors the content was identical to that in the control intervention and included at least three moves: Summary of use; Account of side effects and Information regarding monitoring. Rhetorical elements: Unable to assess Tenor: Unable to assess Metadiscourse: Unable to assess Headings: Unable to assess Technicality of language: Unable to assess Lexical density: Unable to assess Factual content: Unable to assess Format: 15 minute videotape |
Evaluation of comparators: Generic structure: Based on the information provided by the authors the content was identical to that in the multimedia intervention and included at least three moves: Summary of use; Account of side effects and Information regarding monitoring. Rhetorical elements: Unable to assess Tenor: Nurse to patient Metadiscourse: Unable to assess Headings: Unable to assess Technicality of language: Unable to assess Lexical density: Unable to assess Factual content: Unable to assess Format: Standardised lecture given by one of two nurses |
|
| Trent 2010 |
Source of information about intervention: Copy of the video and information provided by the authors in the published study. Description of intervention: Video about self‐care of PID in addition to standardised care. Name of program: Pelvic inflammatory disease ‐ patient education video Country of origin: USA Language: English Year program used in the study was produced: Not reported but copyright at the end of the video was from 2005. Stated expertise of content developers: Developed by the authors who are adolescent health specialists. Stated expertise of program developers: The program was "created by a professional production team" Process of development and testing: Video was developed by the authors "using the health belief model as a conceptual framework. It was refined through qualitative research with adolescents who had a history of STI". Relationship between developer and user: Health professionals to patients. Availability: Publicly available on "YouTube" Purpose: To educate patients about PID self‐care in order to improve adherence Content: "The video tells the story of PID as related by a universal patient created by the voices and images of seven different female adolescents. The video portrays the patient's interface with the health provider as well as the male partner's interface and allows the universal girl to acknowledge the barriers and benefits of PID self‐care while providing cues for action by the individual patient". Target audience: Adolescent girls diagnosed with PID. Format: Not reported Hardware required: Not reported Media: Video and audio with some simple diagrams and animations. Delivery: The video was viewed at the clinical sites. Length of program: Six minutes Number of times program was used: Once Time point of delivery: Patients viewed the video on the day they were diagnosed and prior to being discharged with a course of the medication. Success at reaching target audience: All participants viewed the video on site. Assistance: Not reported Interactivity: No Tailoring: No |
Source of information about comparator: Information provided by the authors in the published study. Description of comparator: Standardised care consisting of detailed discharge instructions based on the 2006 Centre for Disease Control STD treatment guidelines, a 14‐day course of medication and a written hand‐out to facilitate self‐care. |
|
Evaluation of intervention: Generic structure: The content included five moves: Summary of use; Dosage instructions; Constraints on patient behaviour; Outline of benefits and Clinical contact available. Rhetorical elements: The program predominantly informed patients but specific instructions were also provided in the final minute of the video. Tenor: Patient to patient. However, actors were used rather than actual patients. A doctor was also shown but directed their conversation to the patient/actor. Metadiscourse: The purpose of the program was not provided within the video. However, accompanying text stated that the video was intended to improve adherence to treatment, follow‐up care and partner notification. It is unclear whether this text was shown to patients who participated in the study. Headings: None Technicality of language: Medical terminology was used infrequently and when used, was accompanied by an explanation. Lexical density: 36%. Calculated from the first minute of the video. Factual content: Program producers, their institution and sources of funding were identified in the end credits. The year that the program was developed was identified from the copyright shown at the end of the program. Format: Six minute video showing actors speaking directly to camera accompanied by a few simple diagrams and animations. |
Evaluation of comparators: Inadequate information was provided on which to base an assessment |
|
| Van Der Palen 1997 |
Source of information about intervention: Information provided by the authors in the published study. Description of intervention: Video about the use of MDIs Name of program: Not reported Country of origin: Netherlands Language: Dutch Year program used in the study was produced: Not reported Stated expertise of content developers: The videos were not designed for this study but were "readily available from various pharmaceutical companies" Stated expertise of program developers: Not reported Process of development and testing: Not reported Relationship between developer and user: Not reported Availability: The videos were available from the pharmaceutical companies at the time of the study Purpose: To instruct patients on correct inhaler technique Content: Not reported Target audience: Patients using MDIs Format: Videotape Hardware required: Television and VCR Media: Video and audio Delivery: In the participant's home Length of program: Not reported Number of times program was used: Patients watched it a median of three times (range one to 50) Time point of delivery: Patients had been using inhaled medications for at least one month Success at reaching target audience: Patients were given the video to take home and it was reported that all of them watched it at least once during the study period. Assistance: None Interactivity: No Tailoring: No |
Source of information about comparators: Information provided by the authors in the published study. Description of comparators: 1. Personal instruction Patients demonstrated their inhaler technique to a pulmonary function technician. Errors in technique were then corrected using verbal instructions and visual demonstrations. Patients continued to demonstrate their technique until no errors were made. 2. Group instruction A group of five to seven patients received instruction from a specialised registered nurse. Each patient demonstrated their inhaler technique in front of the group. The average session lasted 45 minutes. 3. Control group The control group did not receive any education as part of this study. |
|
Evaluation of intervention: Inadequate information was provided on which to base an assessment |
Evaluation of comparators: Inadequate information was provided on which to base an assessment |
|
| Voris 1982 |
Source of information about intervention: Information provided by the authors in the published study which included the script for the educational program. Description of intervention: Slide‐tape presentation about tricyclic antidepressants Name of program: The Tricyclic Antidepressant Education Program Country of origin: USA Language: English Year program used in the study was produced: Not reported Stated expertise of content developers: Developed by the first author (psychiatric pharmacy fellow) Stated expertise of program developers: Assistance from the Biomedical Communications Department at the University of Nebraska Medical Centre. Process of development and testing: "The initial script and slide descriptions were written and sent to selected staff pharmacists and psychiatrists for review and to assure pharmaceutical and medical exactness." An education reading specialist tested the program for suitability for readers at the seventh grade level. Relationship between developers and users: Health professionals to patients. Availability: Not reported Purpose: To increase both short‐term and long‐term knowledge about tricyclic antidepressants and depression. Content: Program consisted of four sections: an introduction citing the objectives of the program; a section describing depression including possible causes, common signs and symptoms; the largest section dealing with facts on tricyclic antidepressants (basic mechanism of action, dosing, importance of maintaining adequate blood levels, common side effects and what to do if they occur, consequences of under and over‐dosing); and a brief conclusion. Target audience: Adults who are commencing a tricyclic antidepressants Format: Slide‐tape audiovisual presentation (either a carousel projector, screen and tape player or a carousel and a cassette playback synchronizer). Hardware required: Carousel projector, screen and a cassette player. Media: Text or still graphics and audio Delivery: Viewed on a projector screen at the hospital. Length of program: Seven minutes Number of times program was used: Once Time point of delivery: Patients had recently commenced a tricyclic antidepressant. Success at reaching target audience: All participants viewed the presentation on site. Assistance: The investigator was not present during the program but was available to answer questions at the completion of the presentation. Interactivity: No Tailoring: No |
Source of information about comparator: Information provided by the authors in the published study. Description of comparator: Usual care: "Information which nurses and physicians decided to give them as part of the usual hospital routine." |
|
Evaluation of intervention: Generic structure: The education program contained seven moves: Background on drug, Summary of use, Dosage instructions, Side effects, Constraints on patient behaviour, Outline of benefits and Clinical contact available. Users were advised of situations in which they should contact their doctor but were not provided with information on how to do so. Rhetorical elements: The education primarily informed and instructed patients. The function was unclear in some areas e.g. "The medicine works best when taken every day. Most people take their medicine at the same time every day" ‐ it is unclear whether this is informing patients of common practice or instructing them on when to take their medication. Tenor: Health professional to patient. The identity of the authors and the intended user was clearly stated at the beginning of the program. The person responsible for actions is stated throughout. Metadiscourse: There is a clear description of the purpose of the education in the introduction. This outlines what information the education will provide and what it is designed to teach patients. Headings: Unable to assess Technicality of language: The authors state that "the terminology used in the program was designed to be understood by the majority of patients' and that the program was suitable at the seventh grade reading level. Medical terminology was used but an explanation of its meaning was also provided. Lexical density: 52%. Calculated from the script for the audiovisual presentation. Factual content: The institutions involved in developing the content were identified. The script also alluded to some of the information being based on scientific studies. Format: A seven minute slide‐tape audiovisual presentation |
Evaluation of comparator: Not applicable. |
MDI ‐ Metered Dose Inhaler, PIL ‐ Patient Information Leaflet, PCA ‐ Patient Controlled Analgesia, INR ‐ International Normalised Ratio, OTC ‐ over‐the‐counter, EC ‐ Emergency Contraception, STD ‐ Sexually Transmitted Disease, STI ‐ Sexually Transmitted Infection, PID ‐ Pelvic Inflammatory Disease.
Generic structure
Sixteen multimedia programs, used in 18 studies, provided adequate information on which to base an assessment of their generic structure (Deitz 2011; Goodyer 2006; Kato 2008; Kinnane 2008; Mazor 2007; McElnay 1989; Navarre 2007; Neafsey 2001; Neafsey 2002; Olver 2009; Osguthorpe 1983; Powell 1995; Savage 2003; Schwarz 2008a; Solomon 1988; Stone 1989; Trent 2010; Voris 1982). The programs contained one to seven of the ten possible 'moves' or identifiable sections of medication information. The move 'Summary of use', which outlines what the medication does, was the most frequently used (in 11 programs). This was followed by 'Dosage instructions', 'Account of side effects' and 'Constraints on patient behaviour' which were each present in nine programs. None of the programs contained the 'Storage instructions' move. Three programs, used in four studies, which focused on instructing patients on the correct use of MDIs, included only one move ('Dosage instructions') (Goodyer 2006; McElnay 1989; Navarre 2007; Savage 2003).
Rhetorical elements
Eleven programs (used in 12 studies) provided adequate information on which to base an assessment of their rhetorical elements (Deitz 2011; Goodyer 2006; Kato 2008; Kinnane 2008; Mazor 2007; Navarre 2007; Olver 2009; Savage 2003; Schwarz 2008a; Solomon 1988; Trent 2010; Voris 1982). The two main rhetorical elements employed by the programs were 'to inform' and 'to instruct'. Most of the programs contained both elements. However, the program used in Goodyer 2006 and Solomon 1988 included only instructions regarding correct MDI use, while Mazor 2007 informed users about the management of oral anticoagulant medications without providing any direct instructions. In 10 of the 11 programs where the tenor was able to be assessed, the relationship with the user was health professional or medical expert to patient (Deitz 2011; Kato 2008; Kinnane 2008; Lirsac 1991; Navarre 2007; Olver 2009; Osguthorpe 1983; Schwarz 2008a; Solomon 1988; Voris 1982). Two of the programs also contained components where the relationship was patient to patient (Olver 2009; Solomon 1988) and one program consisted entirely of a patient to patient interaction (Trent 2010). In some of the programs, the relationship was explicit, e.g. the health professional introduced themselves or was clearly identified as a health professional based on their clothing and setting (Kato 2008; Mazor 2007; Navarre 2007; Olver 2009; Schwarz 2008a; Trent 2010; Voris 1982). However, in others the relationship was not explicitly stated but implied by the language used e.g. "during our care for you" (Deitz 2011; Kinnane 2008).
Metadiscourse
Ten programs used in 11 studies provided adequate information on which to base an assessment of their metadiscourse (the description of the purpose or structure of the program) (Deitz 2011; Goodyer 2006; Kato 2008; Kinnane 2008; Lirsac 1991; Navarre 2007; Olver 2009; Savage 2003; Schwarz 2008a; Trent 2010; Voris 1982). Of these, seven programs (used in eight studies) clearly stated their purpose, generally as part of their introduction (Deitz 2011; Goodyer 2006; Kato 2008; Kinnane 2008; Olver 2009; Savage 2003; Schwarz 2008a; Voris 1982). One program did not contain any metadiscourse but had separate text accompanying the video that explained the purpose of the program (Trent 2010). It was unclear whether this text was shown to patients who participated in the study.
Factual content
Eight programs provided adequate information on which to base assessment of their factual content (Deitz 2011; Kato 2008; Kinnane 2008; Mazor 2007; Navarre 2007; Olver 2009; Trent 2010; Voris 1982). The source of the information, i.e. the organisation who had developed the program, was identified in six of the programs (Kato 2008; Kinnane 2008; Navarre 2007; Olver 2009; Trent 2010; Voris 1982). This was mostly presented as an acknowledgement at the end of the program. None of the programs explicitly stated when the information was last updated. This information was implied from the copyright date presented in five programs (Kato 2008; Kinnane 2008; Mazor 2007; Navarre 2007; Trent 2010).
Lexical density
Lexical density was calculated as the percentage of content words within a program or a section from the program. The higher the lexical density, the more densely packed the information, which may make it more difficult to understand. Lexical density was able to be calculated for 9 of the programs used in 10 studies (Deitz 2011; Goodyer 2006; Kinnane 2008; Mazor 2007; Navarre 2007; Olver 2009; Savage 2003; Schwarz 2008a; Trent 2010; Voris 1982). Lexical density in the programs varied from 29% to 65%. The lowest lexical density was found in programs or sections of programs that depicted patients presenting information or having a conversation (Olver 2009 29%; Deitz 2011 31% and Trent 2010 36%).
Comparator
We were able to obtain a copy of the written information leaflet used as the comparator in four studies (Goodyer 2006; McElnay 1989; Savage 2003; Solomon 1988). Four other studies provided a description of the comparator on which to base a limited evaluation of its quality (Kinnane 2008; Osguthorpe 1983; Schwarz 2008a; Stone 1989). Information regarding the quality of the comparators is presented in Table 10. Insufficient information was available on which to evaluate the quality of the comparator or co‐interventions for the remaining eight studies (Acosta 2009; Lirsac 1991; Neafsey 2002; Olver 2009; Schnellinger 2010; Self 1983; Trent 2010; Van Der Palen 1997).
Comparators and co‐interventions
Multimedia versus usual care or no education
Ten studies compared multimedia education to usual care or no education (Deitz 2011; Knoerl 1999; Mazor 2007; Navarre 2007; Neafsey 2001; Osguthorpe 1983; Powell 1995; Schnellinger 2010; Van Der Palen 1997; Voris 1982). Usual care was defined as non‐standardised education that was provided by health professionals as part of standard clinical practice. Participants in the control groups did not received any education as part of the study. Of these ten studies, five recruited patients who were taking particular medications and then presented those randomised to the intervention group with multimedia education about that medication (Mazor 2007; Navarre 2007; Neafsey 2001; Powell 1995; Van Der Palen 1997). In three of these five studies, the inclusion criteria required that the patients had been taking the medication for a minimum period of time, ranging from one to six months. Two of the studies reported patients' mean duration of treatment, showing that it was five years or longer (Navarre 2007; Van Der Palen 1997). As education about medications is commonly provided at the time a medication is first prescribed, this suggests that many of the control group participants would not have received any recent education about their medications.
Three studies recruited patients who were recently started on a medication, and randomised them to receive multimedia education or usual care (Knoerl 1999; Osguthorpe 1983; Voris 1982). In Knoerl 1999 this consisted of informal and non‐standardised teaching about patient‐controlled analgesia (PCA) provided by hospital staff. In Osguthorpe 1983 and Voris 1982 psychiatric hospital inpatients and outpatients who were recently started on a medication received information provided by nurses and physicians as part of usual hospital care.
Two studies recruited participants from particular demographic groups which were the appropriate target for the educational intervention but were not necessarily receiving the medications of interest at that time, and compared the intervention to a control group which did not receive any education about the medication (Deitz 2011; Schnellinger 2010).
Multimedia versus other forms of education
Five studies compared multimedia education to written information (Goodyer 2006; Olver 2009; Savage 2003; Schnellinger 2010; Self 1983). Goodyer 2006, Savage 2003 and Self 1983 used patient information leaflets about MDIs, while Olver 2009 compared multimedia education to the hospital's standard information sheet and consent form for chemotherapy, and Schnellinger 2010 to an information pamphlet about antibiotic use and resistance. Both the multimedia education and written information groups from Olver 2009 were also provided with verbal information about their chemotherapy and the opportunity to ask questions of medical or nursing staff.
Six studies compared multimedia education to education provided verbally by a health professional (Goodyer 2006; Lirsac 1991; McElnay 1989; Self 1983; Stone 1989; Van Der Palen 1997). In McElnay 1989 and Self 1983, patients in the control arm were individually instructed in the use of MDIs by a pharmacist. McElnay 1989 also provided a custom designed printed instruction sheet outlining the steps required to use an MDI and the manufacturer's package insert for the MDI to both the control and multimedia arms. In Van Der Palen 1997, the comparators included personal instruction, in which patients received individual instruction in the use of MDIs by a pulmonary function technician, and group instruction, in which groups of five to seven patients were educated by a specialised registered nurse. In Stone 1989, individual patients received a standardised presentation, with content identical to the multimedia program, delivered by a nurse specially trained in anticoagulant therapy. In Goodyer 2006, the control arm was initially assessed after receiving written information. They then received personal instruction from a translator before being assessed again, providing results for the combination of written information and instruction from a health professional. In Lirsac 1991, patients received education from a doctor, who read from an instruction sheet outlining the correct use of MDIs, while patients viewed pictorial representations.
Three studies compared a multimedia education program to a control multimedia program (Acosta 2009; Kato 2008; Schwarz 2008a). In Schwarz 2008a the control program consisted of a multimedia program about another medication while Kato 2008 used a commercial computer game that contained no information about medications and Acosta 2009 used a video which contained general information about asthma. In Acosta 2009 and Kato 2008, the participants were all undergoing treatment with the medication of interest; therefore the control group would have received education or instruction about the medication from treating health professionals as part of usual care. In Schwarz 2008a, only 27% of participants in the control group had ever used emergency contraception. Therefore, it is likely that many in the control group had not received any education about this medication.
Multimedia and a co‐intervention versus the co‐intervention alone
Six studies compared multimedia education in combination with a co‐intervention to the co‐intervention alone, e.g. video plus written information versus written information alone (Kinnane 2008; McElnay 1989; Neafsey 2002; Osguthorpe 1983; Solomon 1988; Trent 2010). Two of these studies compared the multimedia intervention in combination with written patient education materials to the same written patient education materials alone (Neafsey 2002; Osguthorpe 1983). The written materials consisted of a drug information sheet about the medication (Osguthorpe 1983) and a patient information booklet (Neafsey 2002). One study compared multimedia and written education to written education alone, but used different written materials in the two arms (McElnay 1989). The multimedia education group received a custom designed instruction sheet outlining the steps required for correct MDI use in combination with the manufacturer's package insert for the MDI, while the written information group received only the manufacturer's package insert. Both the multimedia and control arms in Kinnane 2008 received a one‐hour standardised education session from a registered nurse, as well as written information about chemotherapy and the management of side effects. In Solomon 1988, patients from both the multimedia and control arms were provided with standardised verbal instructions from their doctor and randomised to receive special pill packaging labelled with instructions for when to take the medication, or no further intervention. The verbal instructions consisted of a single paragraph stating the name of the medication and instructions on how to take the medication correctly. In Trent 2010, all participants received standardised education including detailed discharge instructions, a 14‐day course of the medication and a written handout.
Outcome measures
Knowledge and skill acquisition
All but 2 of the 24 studies (Powell 1995; Trent 2010) measured either knowledge or skill acquisition and one study measured both (Navarre 2007).
Fifteen studies measured patient knowledge, each using different scales testing knowledge derived from the content of the educational interventions used in that study (Deitz 2011; Kato 2008; Kinnane 2008; Knoerl 1999; Mazor 2007; Navarre 2007; Neafsey 2001; Neafsey 2002; Olver 2009; Osguthorpe 1983; Schnellinger 2010; Schwarz 2008a; Solomon 1988; Stone 1989; Voris 1982). Fourteen of these studies used scales that were developed by the authors for the purpose of the study, while Schwarz 2008a used a pre‐existing questionnaire developed for a nationwide telephone survey about emergency contraception.
Skill acquisition was measured in eight studies that examined MDI technique in participants with asthma (Acosta 2009; Goodyer 2006, Lirsac 1991; Savage 2003; Self 1983), chronic obstructive pulmonary disease (COPD) (Van Der Palen 1997), asthma or COPD (Navarre 2007) and healthy volunteers (McElnay 1989). In all studies, MDI technique was assessed using inhaler checklists that outline the steps required for correct inhaler use. The inhaler checklists, as well as the methods used to observe and score inhaler technique, varied in each of the trials. However, the content of the different checklists was similar across the studies. Four of the seven studies reported that inhaler technique assessors were blinded (Acosta 2009; Goodyer 2006; McElnay 1989; Savage 2003).
Compliance or adherence
Five studies measured compliance or adherence to the prescribed medication (Kato 2008; Mazor 2007; Powell 1995; Solomon 1988; Trent 2010) while Mazor 2007 also measured patient compliance with laboratory monitoring. Trent 2010 measured compliance with medical instructions, including attendance at a 72‐hour follow‐up visit, abstinence from sexual activity while undergoing treatment for pelvic inflammatory disease, and the rates of notification and treatment of sexual partners. Solomon 1988 also measured rates of abstinence from sexual activity during the course of treatment.
The methods used to measure compliance varied. Mazor 2007 used a patient‐reported measure of non‐adherence that was developed by the authors for the purposes of this study. Powell 1995 monitored prescription refill data over a six month period. Kato 2008 assessed patient compliance with medications using five different measures: attendance at clinics; drug assays measuring levels of medication metabolites in blood tests; two existing measures of patient‐reported adherence (the Chronic Disease Compliance Instrument (CDCI) and the Medication Adherence Scale (MAS)); and the Medication Event Monitoring System (MEMS), which measures adherence to antibiotics using a medication container containing a microprocessor that records the dates and times the container is opened. Trent 2010 assessed medication adherence and adherence with other medical instructions using a telephone interview. Solomon 1988 also ascertained patient compliance using a telephone interview, but designed a scoring system where errors in medication compliance were given a numerical value according to the degree to which they might compromise treatment effectiveness.
Mazor 2007 measured compliance with the recommended laboratory testing required to monitor treatment with warfarin using two methods. Compliance was measured as the percent of patients who were documented to have missed at least one monitoring appointment, as well as the patient's reported intention to adhere to the laboratory testing using a two‐item scale, where higher scores represented a stronger intention to adhere.
Use of the study medication
Schwarz 2008a measured the number of patients who used emergency contraception or had a supply of emergency contraception in the home during the study period. Knoerl 1999 recorded PCA parameters, as well as the type and amount of analgesics used by patients in the post‐operative period. Deitz 2011 measured participants' self‐reported use of prescription medications for non‐medical purposes.
Health outcomes and medication side effects
Seven studies measured health outcomes. Knoerl 1999 measured patients' ratings of post‐operative pain on a scale of zero (no pain) to ten (worst possible pain). Lirsac 1991 measured bronchial obstruction using the forced expiratory volume (FEV) on spirometry and Acosta 2009 measured peak expiratory flow on a peak flow meter. Schwarz 2008a measured the number of patients who conceived during the study period, and Trent 2010 measured the rate of complications from pelvic inflammatory disease or its treatment.
Deitz 2011 used the CAGE questionnaire (Ewing 1984) to assess the risk of prescription drug misuse and addiction. Knoerl 1999 also recorded the incidence of medication‐related side effects. Although Neafsey 2002 did not directly measure the incidence of medication side effects, the authors developed a rating of patient self‐medication behaviours that were likely to lead to adverse events. This involved patients completing a questionnaire about their medication use behaviour, and the responses then being scored based on expert panel ratings of how likely each behaviour was to cause an adverse event, giving an overall adverse self‐medication behaviour score.
Other patient‐reported outcomes
Self‐efficacy was measured in four studies, each using different self‐efficacy questionnaires. All four of the studies used scales that were developed by the authors for the purpose of that study. Two of the studies measured self‐efficacy for avoiding drug and alcohol interactions with prescribed medications (Neafsey 2001; Neafsey 2002), Kato 2008 measured self‐efficacy to manage cancer and its treatment and Deitz 2011 measured self‐efficacy to take medications as prescribed and manage medication problems. Deitz 2011 also used an established scale (Perceived Efficacy in Patient‐Physician Interactions (PEPPI) Maly 1999) to measure patient self‐efficacy for obtaining medical information and attention for their medical concerns from physicians.
Five studies measured patients' satisfaction ‐ three measuring satisfaction with medical care (Deitz 2011; Knoerl 1999; Solomon 1988) and two measuring satisfaction with the education they received (Olver 2009; Stone 1989). Both Knoerl 1999 and Solomon 1988 measured patients' satisfaction with medical care using a single question with either a five‐ or six‐point Likert response scale. Olver 2009 and Stone 1989 measured patients' satisfaction with the education they had received using scales developed by the authors for the purpose of the study. Deitz 2011 used components of an established instrument (the Patient Feedback System (Forman 2003)) to measure patients' perceptions of their therapeutic alliance with caregivers, and satisfaction with treatment.
Five studies measured patients' attitudes or beliefs about medicines, each using different methods. Knoerl 1999 and Mazor 2007 used questionnaires that the authors adapted from existing measures. Knoerl 1999 modified the Ward's Barriers Questionnaire (Ward 1993) to measure patient attitudes to using narcotic pain medicine to relieve pain. Mazor 2007 modified the Beliefs about Medicines Questionnaire (Horne 1999) to measure patients' beliefs about warfarin and laboratory monitoring. Schnellinger 2010 used three questions relating to parents' attitudes to antibiotic prescribing using a yes/no response format. The remaining two studies used a single question measuring patients' attitudes to emergency contraception using a yes/no response format (Schwarz 2008a) or their confidence in the treatment using a five‐point rating scale (Solomon 1988).
Two studies measured patients' perceptions about their health. Solomon 1988 assessed patients' perception of the severity of their illness using a single question with a five‐point rating scale, while Kato 2008 measured patients' perception of their control over their health using an existing measure (the Multidimensional Health Locus of Control Scale). Deitz 2011 measured patients' perception of the care (directive guidance) they received from doctors and pharmacists using an established measure (the Purdue Pharmacist Directive Guidance Scale (Gupchup 1996)). Schnellinger 2010 measured patients' perception of the education they received using a single question asking if they had learnt something about antibiotics that they had not known previously.
Quality of life was measured in only one study (Kato 2008) using two existing questionnaires designed for adult (Functional Assessment of Cancer Therapy ‐ General (FACT‐G)) and paediatric (Paediatric Quality of Life Inventory (PQL)) populations.
Patient usage of the educational intervention
Only one study (Olver 2009) measured patient usage of the educational intervention, reporting patients' perception of how much of the information contained in the written or multimedia education they accessed and understood.
Use of health services
Two studies measured the use of health services. Stone 1989 reported the amount of time that was required for delivery of education via video or nurse lecture and for health professionals to answer patient questions after they had received the education. Kinnane 2008 measured the number and content of telephone calls from patients to the outpatient clinic during the study period. No studies measured economic outcomes.
Adverse effects of the educational intervention
Adverse effects of the educational intervention were measured in two studies using existing tools (Kato 2008; Olver 2009). Adverse effects were measured indirectly by determining patients' self‐perceived stress (Kato 2008) and self‐perceived anxiety (Olver 2009) before and after the educational interventions.
Excluded studies
Of the 122 studies that we examined in further detail, 10 were found to be duplicate publications of the same study and we excluded 87 from the review. The reasons for excluding these studies are listed in Characteristics of excluded studies. The most common reasons for exclusion were:
the primary focus of the educational intervention was not medications (N = 31),
the effect of the multimedia intervention on measured outcomes could not be separated from the effect of co‐interventions (N = 22), and
the intervention met the definition for a decision aid (N = 12).
We excluded a further nine studies because they did not contain any multimedia intervention. We excluded seven studies as there was inadequate information on which to determine their suitability for inclusion in the review. One of these studies was excluded after the authors were contacted and reported that the intervention consisted of text and still pictures, therefore not meeting the definition for multimedia (Jank 2009). We contacted the authors of two studies for further information but no response was received (Edworthy 1999; Peet 1996). Contact details for the other study authors were not available (Bakker 1999; Charles 1999; Petro 2005; Yakirevitch 2010). The published protocol of one study met criteria for inclusion in the review but the study is currently ongoing and results were not available at the time of this review (Smith 2010). We report details regarding this study in the Characteristics of ongoing studies table.
Risk of bias in included studies
Many of the studies provided inadequate information to allow a judgement to be made about the adequacy of their methods, resulting in an unclear risk of bias. Risk of bias is summarised below and in Figure 2, and further detail is provided in the Characteristics of included studies tables.
2.
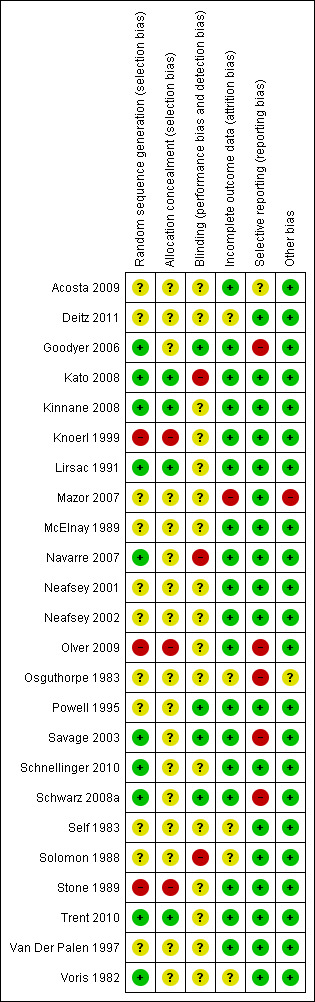
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Ten of the 24 studies reported adequate methods for sequence generation (Goodyer 2006; Kato 2008; Kinnane 2008; Lirsac 1991; Navarre 2007; Savage 2003; Schnellinger 2010; Schwarz 2008a; Trent 2010; Voris 1982). However, only four of these studies also reported adequate allocation concealment (Kato 2008; Kinnane 2008; Lirsac 1991; Trent 2010). The other six studies provided insufficient details on which to judge the adequacy of methods used. The methods used for both sequence generation and allocation concealment were inadequate in three studies (Knoerl 1999; Olver 2009; Stone 1989). The remaining 11 studies did not provide enough information to allow a judgement to be made about the adequacy of randomisation or allocation concealment methods.
Blinding
Blinding of participants is difficult in studies of educational interventions. Four studies reported the methods used to blind participants (Goodyer 2006; Kato 2008; Powell 1995; Schwarz 2008a). Participants in Goodyer 2006 and Kato 2008 did not have advance knowledge of the type of education that they would receive. This was considered adequate blinding in Goodyer 2006, where outcomes were measured immediately following the educational intervention, but not in Kato 2008, where outcomes were measured one and three months after the intervention. Powell 1995 and Schwarz 2008a attempted to blind participants by withholding information about the purpose of the study. In Powell 1995 the participants in the multimedia group were informed that the videotapes were part of a patient education program but they were not informed that their medication compliance was being assessed through prescription refill records. Participants in the control group received no education and presumably were not aware that they were participating in a study. Schwarz 2008a attempted to blind participants by not mentioning emergency contraception in the consent documents. Participants therefore did not know that there were intervention and control multimedia programs that were being compared. Two studies reported that the participants were not blinded (Savage 2003; Solomon 1988). In Savage 2003 outcomes were measured immediately following the intervention, minimising the potential for the lack of blinding influencing outcomes. The remaining 18 studies did not provide any information about participant blinding.
Eight studies reported that assessors were adequately blinded (Acosta 2009; Goodyer 2006; Kato 2008; McElnay 1989; Olver 2009; Savage 2003; Schwarz 2008a; Solomon 1988), while one study reported that they were not (Navarre 2007). The remaining 15 studies did not provide any information about blinding of assessors. Of these, four studies used subjective measures or patient interview where the lack of blinding of assessors could influence outcomes (Lirsac 1991; Self 1983; Trent 2010; Van Der Palen 1997). Powell 1995 used a completely objective outcome and the other 10 studies used patient‐reported questionnaires which minimised the potential for the lack of blinding of assessors influencing outcomes.
Incomplete outcome data
The missing outcome data were greater in the intervention (33%) than control groups (21%) in Mazor 2007. The reasons given for the missing data were also unbalanced. At least 13% of participants in the intervention group did not provide outcome data as they lacked access to a television or video recorder and could therefore not view the intervention. Lack of access to this technology did not preclude participation in the control arm. This imbalance is likely to impact on outcomes including knowledge through selection bias. Five studies did not report sufficient information about attrition or the reasons for missing data to allow a judgement about risk of bias (Deitz 2011; Osguthorpe 1983; Self 1983; Solomon 1988; Voris 1982). The remaining 18 studies adequately reported the reasons for missing outcome data, which were balanced across intervention groups, or reported no missing data.
Selective reporting
Five studies did not report results from all of the outcomes specified in their methods. Goodyer 2006 and Savage 2003 assessed inhaler technique using a checklist consisting of eight steps, but then only reported the results for a subset of 'key' steps that had not been pre‐specified. Both Olver 2009 and Osguthorpe 1983 did not report the results for two of the questions used in their knowledge or information recall instrument. The methods from Schwarz 2008a outlined that knowledge would be reported both limited to participants who completed follow‐up and using an intention‐to‐treat analysis, where it was assumed that participants lost to follow‐up had learnt nothing. However, only a P value was reported for the intention‐to‐treat analysis. The remaining 19 studies reported all outcomes that were specified in their methods. However, in Acosta 2009, although only inhaler technique scores were specified as outcomes in the study methods, peak flow was also measured at three time points. Results were only reported for measurements made at two of the time points.
Other potential sources of bias
The methods used by Mazor 2007 led to selection bias, as only participants who had access to a television or video recorder would have been able to watch the videotape and therefore participate in the study in the intervention arm, while lack of access to a television or video recorder did not exclude participants from the control arm. Non‐respondent numbers were higher in the intervention arm than in the control group. Lack of access to technology is more prevalent in groups with lower levels of educational attainment and from lower socioeconomic backgrounds. The exclusion of participants who did not have access to a television or video recorder from the intervention arm may therefore have led to selection of participants who were better educated and from a higher socioeconomic group in the intervention arm, potentially biasing the results for knowledge and skill acquisition in favour of the multimedia intervention.
There may have been a risk of recruitment bias in Osguthorpe 1983, a cluster randomised trial in which individuals were recruited to the study after the hospital wards had been randomised. The authors did not report whether staff involved in the recruitment and admission of patients were blinded to the ward allocation. Foreknowledge of the intervention assigned to each ward could have influenced the types of participants recruited. Osguthorpe 1983 did not account for cluster randomisation in statistical analyses and did not report outcomes in sufficient detail for them to be statistically adjusted for inclusion in the meta‐analysis.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6
The results of studies with sufficient data to be included in the meta‐analyses are displayed in the Data and analyses tables and are described below. A summary of the results from all of the studies, including those with insufficient data to be included in meta‐analyses, are displayed in Additional tables (Table 8) and are also described below. None of the included studies reported the minimum clinically important difference for the outcomes that were measured. We have therefore reported results from the studies, but have been unable to interpret whether differences were of clinical importance.
Multimedia education versus usual care or no education
Ten studies compared multimedia education to usual care or no education (Deitz 2011; Knoerl 1999; Mazor 2007; Navarre 2007; Neafsey 2001; Osguthorpe 1983; Voris 1982; Powell 1995; Schnellinger 2010; Van Der Palen 1997). We present the main findings for this comparator in Table 1.
Knowledge
Eight of the 10 studies comparing multimedia education to usual care or no education measured knowledge. Six of these studies, with a total of 817 participants, provided sufficient data for meta‐analysis (Deitz 2011; Knoerl 1999, Neafsey 2001, Mazor 2007, Navarre 2007; Voris 1982). Voris 1982 reported mean scores with a score range but no standard deviation (SD) or other measure of variance from which the SD could be calculated. We therefore imputed the SD using the SD from the study with the greatest weight in the meta‐analysis (Mazor 2007). Pooled results from the six studies showed that participant knowledge, measured within four weeks of the intervention, was greater in the multimedia education group (SMD 1.04, 95% CI 0.49 to1.58, Analysis 1.1, Figure 3). A sensitivity analysis to examine the effect of the imputed SD shows that removal of Voris 1982 from the pooled analysis does not affect the results of the meta‐analysis (SMD 0.99, 95% CI 0.42 to 1.57).
1.1. Analysis.
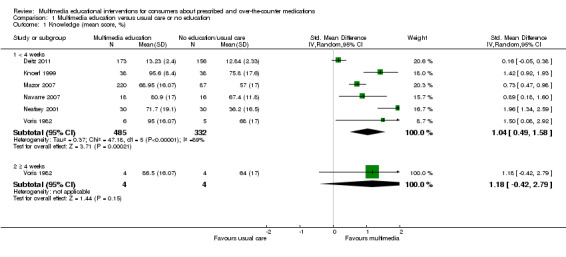
Comparison 1 Multimedia education versus usual care or no education, Outcome 1 Knowledge (mean score, %).
3.
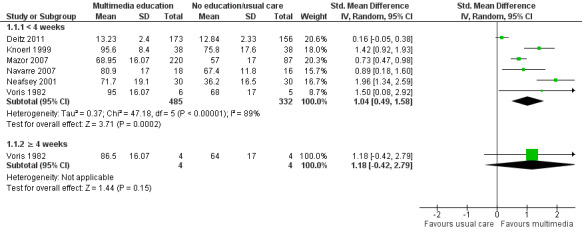
Forest plot of comparison: 1 Multimedia education versus usual care or no education, outcome: 1.1 Knowledge (mean score, %).
Tests for heterogeneity revealed considerable heterogeneity (I2 = 89%). Examination of the forest plot revealed that although there was heterogeneity with non‐overlapping confidence intervals, all but one of the studies (Deitz 2011) favoured the multimedia education group. Results of the meta‐analysis were unchanged when the fixed‐effect model was used.
Schnellinger 2010 reported that multimedia education performed better than the control group when knowledge was measured immediately after the intervention and four weeks later (P < 0.001) in 167 patients. However, data could not be extracted to verify this result as the authors reported the median and range of scores. From the scores, it was evident that results were skewed, with high knowledge scores at baseline. This suggested that the scale used was poorly targeted to knowledge levels in this population and therefore insensitive to change due to a ceiling effect. Schnellinger 2010 also reported that knowledge scores increased following the intervention in 71% of the multimedia education group compared with 28% of the control group (RR 2.26, 95% CI 1.39 to 3.67, Analysis 1.2). At baseline, 31% and 18% of participants had perfect scores in the multimedia education and control groups respectively; therefore improvement in knowledge following the intervention could not be detected in these participants. As the number with perfect scores was higher in the multimedia education group, this would be expected to underestimate the effect of the multimedia intervention.
1.2. Analysis.
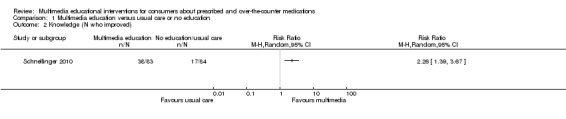
Comparison 1 Multimedia education versus usual care or no education, Outcome 2 Knowledge (N who improved).
The remaining study did not provide data in a format suitable for meta‐analysis. Osguthorpe 1983 reported no statistically‐significant difference one week after the educational intervention. The author attributed the lack of effect on the content of the intervention being overly simplified to the point that it did not add to baseline knowledge, and the outcome measure being insensitive to change.
Only Voris 1982 measured long‐term knowledge, although this was measured only four weeks after the intervention. Voris 1982 was a small study with only four patients remaining in each arm at this time point. While the authors reported that the results favoured the multimedia education group, when we analysed the results using the imputed SDs the results were not statistically significant (MD 22.50, 95% CI ‐0.42 to 45.40, Analysis 1.1).
Skill acquisition
Two of the studies comparing multimedia education to usual care or no education measured skill acquisition and provided sufficient data for meta‐analysis (Navarre 2007; Van Der Palen 1997). The studies examined MDI technique in a total of 94 participants and showed that inhaler technique was superior in the multimedia education group compared with usual care or no education (MD of inhaler technique score (percent) 18.32, 95% CI 11.92 to 24.73, Analysis 1.3). Van Der Palen 1997 reported an SD for the overall sample rather than separate SDs for the intervention and control groups. The individual group SDs for the meta‐analysis were imputed from the overall sample SD. The results of the meta‐analysis also favoured the multimedia education group if the Van Der Palen 1997 data were removed (MD 20.90, 95% CI 9.91 to 31.89).
1.3. Analysis.
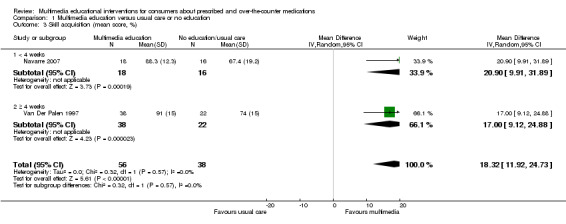
Comparison 1 Multimedia education versus usual care or no education, Outcome 3 Skill acquisition (mean score, %).
Navarre 2007 measured inhaler technique directly after the educational intervention, while Van Der Palen 1997 measured it at the first scheduled clinic visit up to nine months later (mean of 19 weeks for the multimedia education group and 25 weeks for the usual care or no education group). The results from both studies were comparable, suggesting that the effect of multimedia education on inhaler technique may persist over time. There was no evidence of significant heterogeneity (I2 = 0%).
Compliance
Compliance with medications
Two studies comparing multimedia education to usual care or no education measured patient compliance with prescribed medications (Mazor 2007; Powell 1995) in a total of 4552 patients. The methods used to measure compliance and the timing of assessments varied. Mazor 2007 used a patient‐reported measure of non‐adherence that was developed by the investigators for the purposes of this study and administered three weeks after the intervention. Powell 1995 monitored prescription refill data over a six month period. Both studies reported no significant difference in patient medication compliance between the intervention and control groups: 96% versus 97% compliance in Mazor 2007 and 46% versus 44% compliance in Powell 1995 (RR 1.02, 95% CI 0.96 to 1.08, Analysis 1.4).
1.4. Analysis.
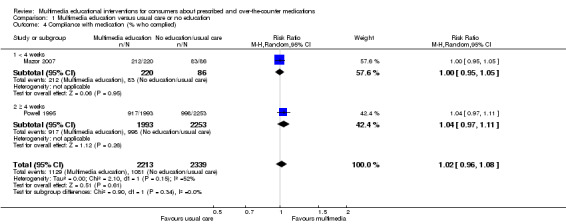
Comparison 1 Multimedia education versus usual care or no education, Outcome 4 Compliance with medication (% who complied).
Compliance with monitoring
Mazor 2007 also reported compliance with recommended laboratory testing to monitor treatment with warfarin in 232 patients. The study measured the percent of patients who were documented to have missed at least one monitoring appointment. This result was reversed to obtain the percent of patients who attended all monitoring appointments, and showed a trend favouring the multimedia education group (78% in the multimedia education group versus 66% in the control group, RR 1.17, 95% CI 0.96 to 1.43, Analysis 1.5). This study also reported the patients' intention to adhere to the laboratory testing using a two‐item scale where higher scores represented a stronger intention to adhere (score range one to five). The study reported no significant difference between the intervention and control groups (mean score for intervention 3.93 (SD 0.99) and control 3.81 (SD 0.96)).
1.5. Analysis.
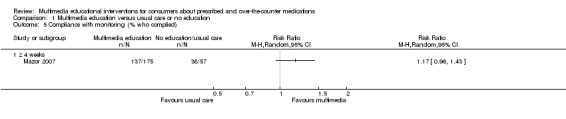
Comparison 1 Multimedia education versus usual care or no education, Outcome 5 Compliance with monitoring (% who complied).
Use of study medication
Knoerl 1999 recorded pain medication that was administered in the post‐operative period. The study found that there were no statistically‐significant differences between the multimedia education and control groups in the amount or type of narcotic administered by PCA, in other PCA parameters including loading dose, lockout interval and control button dose, the amount of narcotic administered by nurses, or the amount of supplemental analgesics used in conjunction with PCA. These findings could not be verified as the authors reported no data.
Deitz 2011 recorded patients' self‐reported use of prescription medications for non‐medical purposes, and found that there was no statistically‐significant difference between the multimedia education and usual care or no education groups. No data could be extracted for meta‐analysis as the results were reported separately for each group of medications with no overall incidence of non‐medical use reported.
Medication safety
Knoerl 1999 recorded the incidence of narcotic medication‐related side effects including nausea, vomiting, pruritis, respiratory depression, hypotension, sedation and urinary retention. There was no statistically‐significant difference in the incidence of each side effect between the intervention and control groups. These results were not included in the meta‐analysis as the authors did not report the overall incidence of side effects, instead providing the incidence of each individual side effect.
Health outcomes
Knoerl 1999 measured 72 patients' ratings of post‐operative pain on a scale of 0 (no pain) to 10 (worst possible pain) at 4, 8 and 24 hours after surgery, and found that there was no statistically‐significant difference between the intervention and control groups at all time points. At the 24 hour time point, patients reported mean pain scores of 3.00 (SD 2.10) and 3.50 (SD 2.10) in the intervention and control groups respectively (MD ‐0.50, 95% CI ‐1.44 to 0.44, Analysis 1.6).
1.6. Analysis.
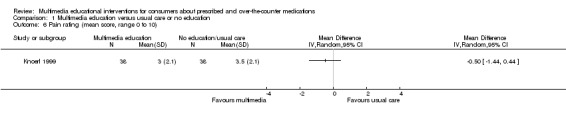
Comparison 1 Multimedia education versus usual care or no education, Outcome 6 Pain rating (mean score, range 0 to 10).
Deitz 2011 measured the risk of prescription drug misuse and dependence in 329 participants using the CAGE screening tool. They reported lower scores (where lower scores indicate a lower risk of drug misuse or dependence) in the multimedia education group (mean score 0.49 (0.83) versus 0.86 (1.19), P = 0.038, MD ‐0.38, 95% CI ‐0.60 to ‐0.15, Analysis 1.7). Results from Deitz 2011 and Knoerl 1999 were not pooled as the health outcomes measured were considered clinically heterogenous.
1.7. Analysis.
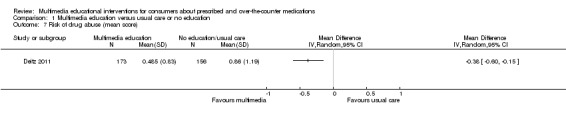
Comparison 1 Multimedia education versus usual care or no education, Outcome 7 Risk of drug abuse (mean score).
Satisfaction with care
Two studies measured different aspects of patient satisfaction with care (Deitz 2011; Knoerl 1999). Results from the two studies were not pooled as the outcomes measured were considered clinically heterogeneous. Knoerl 1999 measured 72 patients' satisfaction with post‐operative pain management using a six‐point Likert scale, where one indicated patients were very satisfied and six indicated they were very dissatisfied. The study reported that, although patients in the multimedia education group were more satisfied at four hours (mean score 1.80 (SD 0.93) versus 1.40 (SD 0.68), P = 0.03) and eight hours (1.50 (SD 0.60) versus 1.20 (SD 0.37), P = 0.01) after their operation, the difference between the groups was no longer statistically significant the day after surgery (1.50 (SD 0.60) versus 1.20 (SD 0.41), P = 0.10). However, when we used the mean score to calculate the MD (reversing the scale so that a higher score represented greater satisfaction), the results at the day after surgery time point continued to favour the multimedia education group (MD 0.30, 95% CI 0.07 to 0.53, Analysis 1.8).
1.8. Analysis.
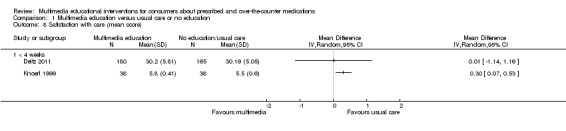
Comparison 1 Multimedia education versus usual care or no education, Outcome 8 Satisfaction with care (mean score).
Deitz 2011 measured participants' satisfaction with treatment and their therapeutic alliance with their physician. The 345 participants were women working at two hospitals, 61% of whom were medical professionals or assistants. The participants were not required to have a medical condition or to have received any therapy. The authors reported no difference between the multimedia education and usual care or no education groups (mean score 30.20 (SD 5.81) versus 30.19 (SD 5.05), MD 0.01, 95% CI ‐1.14 to 1.16, Analysis 1.8).
Perception of care
Deitz 2011 measured patients' perception of the directive guidance (instructions on taking their medications properly) they received from doctors and pharmacists and found that there was no difference between the multimedia education and usual care or no education group (mean score 17.27 (4.40) versus 17.01 (4.07), MD 0.26, 95% CI ‐0.64 to 1.16, Analysis 1.9).
1.9. Analysis.
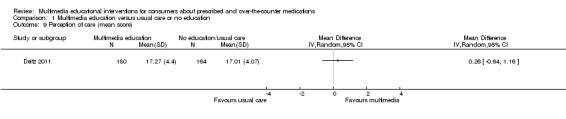
Comparison 1 Multimedia education versus usual care or no education, Outcome 9 Perception of care (mean score).
Self‐efficacy
Two studies measured self‐efficacy for different health‐related tasks (Deitz 2011; Neafsey 2001). The results from the studies were not pooled as the outcomes were judged to be clinically heterogeneous. Neafsey 2001 tested self‐efficacy for avoiding drug and alcohol interactions with prescribed medications in 60 patients. The study used a questionnaire developed by the authors that provided a mean self‐efficacy score of one to five, with higher scores indicating greater self‐efficacy. The authors reported that the multimedia education group had significantly greater self‐efficacy than the control group (multimedia education 3.14 (SD 0.90), control 1.76 (SD 0.99), MD 1.38, 95% CI 0.90 to 1.86, Analysis 1.10).
1.10. Analysis.
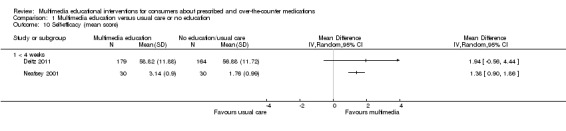
Comparison 1 Multimedia education versus usual care or no education, Outcome 10 Self‐efficacy (mean score).
Deitz 2011 used separate measures to examine self‐efficacy in performing 3 different health‐related tasks in 343 participants. The measures tested self‐efficacy for taking medications as prescribed (medication adherence); managing medication problems; and obtaining medical information and attention for medical concerns from physicians (interacting with doctors). Data from the medication adherence self‐efficacy scale were extracted by consensus opinion of the review authors as its content was thought to be the most clinically relevant. The review authors were not aware of the results when making this decision. Deitz 2011 reported greater self‐efficacy for medication adherence in the multimedia education group, based on an analysis of covariance (ANCOVA) (mean score 58.82 (11.88) versus 56.88 (11.72), P = 0.013). However, when the mean scores were used to calculate the MD there was no statistically‐significant difference between the two groups (MD 1.94, 95% CI ‐0.56 to 4.44). Results reported by the study authors for the other measures of self‐efficacy were mixed. Multimedia education performed better than usual care or no education for "managing medication problems" self‐efficacy (P = 0.03) but the difference in mean scores between the two groups was very small (28.05 multimedia education versus 27.34 usual care or no education). There was no difference reported between the two groups for self‐efficacy in "interacting with doctors" (P = 0.69).
Beliefs about medication
Three studies comparing multimedia education to usual care or no education measured patients' beliefs about their medications using different questionnaires in each study (Knoerl 1999; Mazor 2007; Schnellinger 2010). The results from these three studies were not pooled due to the clinical heterogeneity in the types of beliefs measured and the methods used to measure them.
Knoerl 1999 assessed 72 patients' attitudes to using narcotic pain medicines to relieve pain using a six‐item questionnaire with agree, disagree and don't know response options. Each item was scored as correct or incorrect and the results reported as the mean percentage of correct responses. Higher scores indicated a more favourable attitude towards the medication. Knoerl 1999 reported that patients' mean belief scores were significantly higher in the multimedia education group (mean 96% (SD 9) versus 72% (SD 23), a result verified by our analysis (MD 23.20, 95% CI 15.24 to 31.16, Analysis 1.11).
1.11. Analysis.
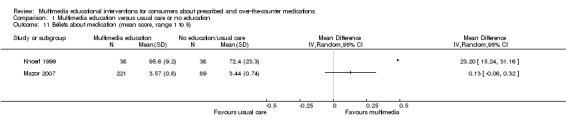
Comparison 1 Multimedia education versus usual care or no education, Outcome 11 Beliefs about medication (mean score, range 1 to 5).
Mazor 2007 evaluated 310 patients' beliefs about warfarin using a questionnaire with four sub‐scales with five‐point Likert response options (strongly agree to strongly disagree). Each of the scales had scores ranging from one to five, with higher scores representing more positive beliefs about warfarin. Three of the sub‐scales tested beliefs about warfarin while the other tested beliefs about laboratory monitoring. Results from the sub‐scale "warfarin is beneficial" were extracted by consensus opinion of the authors as its content was thought to be the most representative of the beliefs about warfarin scale. The review authors were not aware of the results when making this decision. The study authors reported a positive effect of the multimedia intervention on patients' belief that warfarin is beneficial compared with usual care or no education (P = 0.01) based on a regression analysis with the change score from baseline as the dependant variable. However, when we used the mean scores post‐intervention to calculate the MD, there was no statistically‐significant difference between the intervention and control groups (MD 0.13, 95% CI ‐0.06 to 0.32, Analysis 1.11). The reported baseline scores were similar in both groups. The results reported by the study authors for the other sub‐scales were mixed. Multimedia education performed better than usual care or no education for the "lab testing is important" sub‐scale (P = 0.01), but there was no significant difference for the "taking warfarin is worrisome" and "taking warfarin is confusing or difficult" sub‐scales.
Schnellinger 2010 evaluated 167 parents' attitudes towards antibiotics using three items with yes/no response options. Data from the item "Based on what you learned in the study, would you ever ask your paediatrician for an antibiotic if your child had one of the illnesses we talked about today?" were extracted by consensus opinion of the authors as the item's content was thought to be the most representative of parent attitude to antibiotics that the study aimed to change. The study authors reported a positive effect of multimedia education, with more parents in the multimedia education group stating that they would not inappropriately request antibiotics (71/83 versus 46/84, RR 1.56, 95% CI 1.26 to 1.93, Analysis 1.12). There was no significant difference between the multimedia education and control groups for the other two items "Do you think that doctors have an antibiotic for every infection?" and "If your child had one of the illnesses we talked about and you knew that the doctor would not give you an antibiotic, would you go see your doctor anyway?".
1.12. Analysis.
Comparison 1 Multimedia education versus usual care or no education, Outcome 12 Beliefs about medication (N who answered correctly).
Perception of the education
Schnellinger 2010 evaluated 167 participants' perception of the education they received, asking if they "learnt something about antibiotics that they did not know before". Results favoured the multimedia education group (60/83 versus 25/84, RR 2.43, 95% CI 1.70 to 3.46, Analysis 1.13).
1.13. Analysis.
Comparison 1 Multimedia education versus usual care or no education, Outcome 13 Perception of the education (N who learnt something new).
Outcomes not evaluated
None of the studies comparing multimedia education to usual care or no education evaluated the effect on quality of life.
Multimedia education versus other form of education
Nine studies compared multimedia education to another form of education. Five of the studies compared multimedia education to written education (Goodyer 2006; Olver 2009; Savage 2003; Schnellinger 2010; Self 1983), five compared it to education by a health professional (Lirsac 1991; McElnay 1989; Self 1983; Stone 1989; Van Der Palen 1997) and one study compared multimedia education to written education in combination with education from a health professional (Goodyer 2006). The main findings for these comparators are presented in Table 2; Table 3 and Table 4 respectively.
Knowledge
Only three of the seven studies comparing multimedia to other forms of education measured knowledge. The results could not be pooled for meta‐analysis. Schnellinger 2010 compared multimedia education to a written intervention in 162 participants, and reported that multimedia education performed somewhat better at the four week time point (P < 0.04), but not immediately following the intervention. However, data could not be extracted to verify this result as the authors reported the median and range of knowledge scores. Knowledge was also reported as the number of participants whose score improved following the intervention. Improvement was seen in 58% of the multimedia education group and 71% of the written education group (RR 0.80, 95% CI 0.59 to 1.09, Analysis 2.1). However, 31% and 21% of participants had perfect scores at baseline in the video and pamphlet groups respectively, therefore improvement in knowledge following the intervention could not be detected in these participants. As the number with perfect scores at baseline was higher in the multimedia education group, this would be expected to underestimate the effect of the multimedia intervention.
2.1. Analysis.
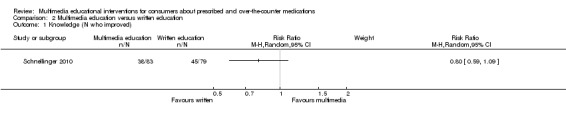
Comparison 2 Multimedia education versus written education, Outcome 1 Knowledge (N who improved).
Olver 2009 reported no significant difference in knowledge between the multimedia and written education groups in a study of 101 patients. Data could not be extracted to verify this result as the authors reported the number of correct responses for the individual knowledge questions without an overall summated knowledge score. Stone 1989 compared multimedia education to education by a health professional in a total of 22 patients. Patient knowledge was measured using true/false questions derived from the content of the educational intervention and developed by the authors for the purpose of the study. There was no significant difference in knowledge scores between the intervention and control groups (mean score intervention 87% (SD 8), control 89% (SD 8), MD ‐2.20 95% CI ‐8.83 to 4.43 Analysis 3.1).
3.1. Analysis.
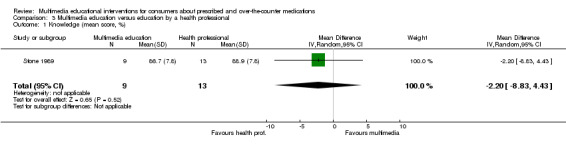
Comparison 3 Multimedia education versus education by a health professional, Outcome 1 Knowledge (mean score, %).
Skill acquisition
Three studies comparing multimedia education to written education measured skill acquisition in a total of 184 participants and all provided sufficient data for meta‐analysis. Self 1983 reported inhaler technique using a continuous scale with results favouring multimedia education (mean score multimedia education group 85% (SD 25), control group 54% (SD 23), MD 31.00, 95% CI 10.15 to 51.85, Analysis 2.2). Goodyer 2006 and Savage 2003 reported the number of participants in whom inhaler technique improved following the intervention, with results favouring multimedia education (RR 2.14, 95% CI 1.33 to 3.44, Analysis 2.3, Figure 4). In Goodyer 2006 the control group participants, who had previously received written instruction about inhaler technique, then received personal instruction by a translator before their inhaler technique was re‐assessed. When global inhaler technique was evaluated following this additional instruction, there was no statistically‐significant difference in the number of participants in whom technique improved between the multimedia education and control groups (17/34 versus 15/35, RR 1.17, 95% CI 0.70 to 1.94, Analysis 4.1). Results of the meta‐analyses were unchanged when the fixed‐effect model was used.
2.2. Analysis.
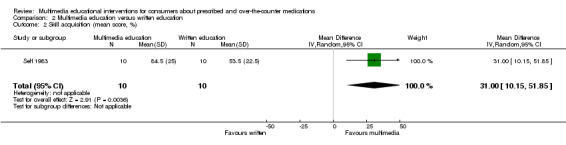
Comparison 2 Multimedia education versus written education, Outcome 2 Skill acquisition (mean score, %).
2.3. Analysis.
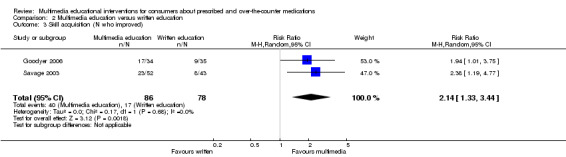
Comparison 2 Multimedia education versus written education, Outcome 3 Skill acquisition (N who improved).
4.
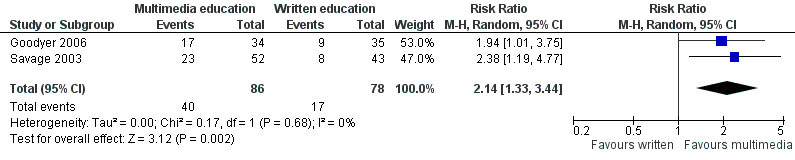
Forest plot of comparison: 2 Multimedia education versus written education, outcome: 2.3 Skill acquisition (N who improved).
4.1. Analysis.
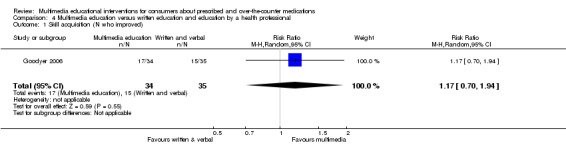
Comparison 4 Multimedia education versus written education and education by a health professional, Outcome 1 Skill acquisition (N who improved).
Four studies comparing multimedia education to education by a health professional measured skill acquisition in a total of 245 participants and provided sufficient data for meta‐analysis (Self 1983; Lirsac 1991; McElnay 1989; Van Der Palen 1997). The studies measured inhaler technique at different time points. McElnay 1989 and Self 1983 assessed inhaler technique immediately after the intervention and then repeated assessment at either 2 weeks (McElnay 1989) or between 1 to 16 weeks (mean 6 weeks) (Self 1983) after the intervention. Lirsac 1991 measured inhaler technique before and 15 days after the intervention and Van Der Palen 1997 assessed inhaler technique at the first scheduled clinic visit up to 9 months later (mean of 19 weeks for the multimedia education group and 23 weeks for those educated by a health professional). Meta‐analysis was performed separately for studies reporting short‐term skill acquisition (less than four weeks) (Lirsac 1991; McElnay 1989; Self 1983) and those reporting more long‐term skill acquisition (greater than or equal to four weeks) (Self 1983; Van Der Palen 1997).
Skill acquisition assessed within four weeks showed no difference between multimedia education and education by a health professional (MD inhaler technique score (percent) ‐1.01, 95% CI ‐15.75 to 13.72, Analysis 3.2). However, there was substantial statistical heterogeneity (I2 = 68%). Examination of the forest plot revealed that Lirsac 1991 favoured the intervention while McElnay 1989 favoured the control and Self 1983 suggested no difference. Although the multimedia interventions used in the studies were similar, the control interventions differed. In McElnay 1989 and Self 1983, participants received personal instruction from a health professional consisting of a demonstration of correct inhaler technique. In Lirsac 1991 the health professional read out instructions for correct technique while the participant looked at illustrations. The participants were not shown a demonstration. This difference may account for the heterogeneity in the meta‐analysis. Removal of Lirsac 1991 showed a non‐significant trend favouring education by a health professional (MD ‐8.84, 95% CI ‐19.32 to 1.63). Skill acquisition measured after four weeks or more also showed a non‐significant trend favouring education by a health professional (MD ‐4.29, 95 %CI ‐17.23 to 8.65, Analysis 3.2, Figure 5). Results of the meta‐analyses were unchanged when the fixed‐effect model was used.
3.2. Analysis.
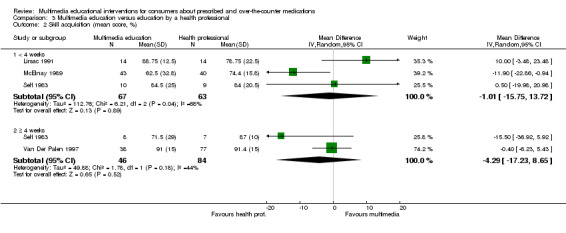
Comparison 3 Multimedia education versus education by a health professional, Outcome 2 Skill acquisition (mean score, %).
5.
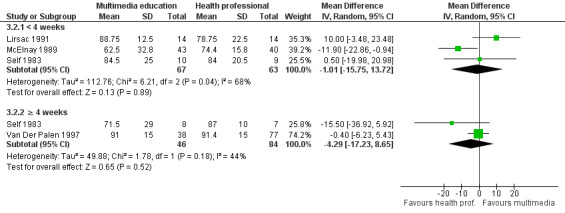
Forest plot of comparison: 3 Multimedia education versus education by a health professional, outcome: 3.2 Skill acquisition (mean score, %).
Van Der Palen 1997 reported an SD for the overall sample rather than separate SDs for the intervention and control groups. The individual group SDs for the meta‐analysis were imputed from the overall sample SD. Removal of the Van Der Palen 1997 data from the meta‐analysis for long‐term skill acquisition did not change the results, which continue to show a trend favouring education by a health professional (MD ‐15.50, 95% CI ‐36.92 to 5.92).
Health outcomes
Lirsac 1991 measured bronchial obstruction before and after using an MDI in 28 patients with poor MDI technique. The study authors reported that both groups had significant improvement in bronchial obstruction following MDI use at baseline despite poor technique. Fifteen days after receiving education, the multimedia education group had significant improvement in their FEV measured before using the MDI while the group who received education from a health professional did not (multimedia education group 1.89 to 2.38 litres (P < 0.001), control group 2.01 to 2.09 litres (not significant)). However, there was no difference in the mean FEV between the two groups (MD 0.29, 95% CI ‐0.26 to 0.84, Analysis 3.3). Both groups had improved FEV measurements following MDI use, but only the multimedia education group achieved FEV scores equivalent to their theoretical maximum.
3.3. Analysis.
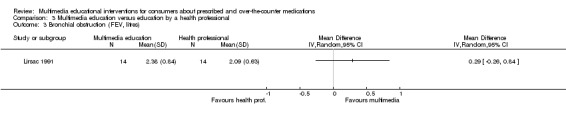
Comparison 3 Multimedia education versus education by a health professional, Outcome 3 Bronchial obstruction (FEV, litres).
Satisfaction
Satisfaction with education
Olver 2009 reported patients' satisfaction with the education they had received, stating that 66% found the information very or somewhat helpful, and that 54% felt that they had received the right amount of information. The study authors reported that there were no significant differences between the written education and multimedia education groups on these variables. No data could be extracted to verify these results. Stone 1989 measured patient satisfaction with the education they received using a 12‐item satisfaction questionnaire with a maximum satisfaction score of 25. The authors reported no significant differences between multimedia education and education by a health professional, with mean satisfaction scores of 23.20 and 24.80 respectively. Data could not be extracted for meta‐analysis from this study as the SD was not reported and could not be calculated or imputed from other studies.
Use of health services
Stone 1989 reported the amount of time that was required for delivery of the education via video or nurse lecture and for health professionals to answer patient questions following the education. The duration of the video was 17.6 minutes while the nurse lecture presentation had a mean duration of 26 minutes (SD 5.7). The authors reported that there was no statistically‐significant difference in the time required for questions between the groups (mean time in minutes: multimedia education 7.5 (SD 7.2), nurse lecture 6.3 (SD 5.3)).
Beliefs about medication
Schnellinger 2010 evaluated parents' attitudes towards antibiotics. The study authors reported a positive effect of multimedia education compared with written information, with more parents in the multimedia education group stating that they would not inappropriately request antibiotics from health professionals (71/83 versus 51/79, RR 1.33, 95% CI 1.10 to 1.60, Analysis 2.4). There was no significant difference between the multimedia and written education groups for the other two items.
2.4. Analysis.
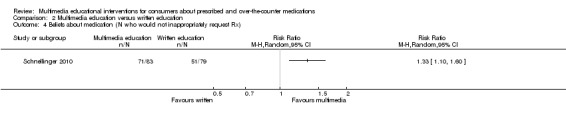
Comparison 2 Multimedia education versus written education, Outcome 4 Beliefs about medication (N who would not inappropriately request Rx).
Patient usage of the education
Olver 2009 recorded patients' perception of how much of the information contained in the written or multimedia education they accessed and understood. They reported that overall, 28% of patients understood all of the information presented and that there was no significant difference in understanding between the multimedia and written education groups. The information provided was read in its entirety by 46% of patients with no significant difference between the groups. No data could be extracted in order to verify these findings.
Perception of the education
Schnellinger 2010 evaluated participants' perception of the education, asking if they "learnt something about antibiotics that they did not know before". There was no significant difference between the multimedia (60/83) and the written education (66/79) groups (RR 0.87, 95% CI 0.73 to 1.02, Analysis 2.5).
2.5. Analysis.
Comparison 2 Multimedia education versus written education, Outcome 5 Perception of the education (N who learnt something new).
Adverse effects of the educational intervention
Olver 2009 measured patients' perception of their anxiety level after the educational intervention. The authors reported combined scores from both groups, with 11% feeling more anxious, 30% feeling less anxious and 33% feeling the same level of anxiety after receiving written or multimedia education. The authors stated that there was no significant difference between the groups but no data could be extracted to verify this.
Outcomes not evaluated
None of the studies comparing multimedia education to written education evaluated the effect on compliance with medications, health outcomes, medication side effects, quality of life or self‐efficacy. None of the studies comparing multimedia education to education by a health professional evaluated the effect on compliance with medications, medication side effects, quality of life or self‐efficacy. The single study that compared multimedia education to the combination of written education and education by a health professional only examined the effect on skill acquisition.
Multimedia education versus control multimedia
Three studies compared multimedia education to a control multimedia program (Acosta 2009; Kato 2008; Schwarz 2008a). We present the main findings for this comparator in Table 5.
Knowledge
Two studies comparing multimedia education to a control multimedia program measured knowledge in a total of 568 patients. Kato 2008 measured knowledge before the intervention, and one and three months after the intervention. Baseline knowledge levels were comparable, but the study authors reported a significantly greater increase in knowledge over time in the intervention group (P = 0.035). Although there was a slightly greater change in mean scores from baseline to the three month time point in the intervention group (59% to 66%) than the control group (60% to 63%) there was no statistically‐significant difference in the mean scores at the three month time point (66% versus 63%, MD 3.00, 95% CI ‐1.52 to 7.52, Analysis 5.1, Figure 6). Schwarz 2008a measured knowledge about emergency contraception at baseline and six months later, and reported that significantly more patients in the multimedia education group than the control group learned one or more things about emergency contraception (76% and 62% respectively). Mean scores for the knowledge test were not reported in the study but were made available by the authors on request. Baseline scores in both groups were comparable (59% intervention and 62% control group, P = 0.12) and there was no difference in mean scores between the two groups at six months (mean score 59% intervention versus 58% control group, P = 0.88). Pooled data from both studies revealed no difference in mean scores between the two groups (MD 2.78, 95% CI ‐1.48 to 7.05).
5.1. Analysis.
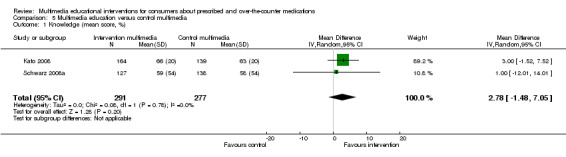
Comparison 5 Multimedia education versus control multimedia, Outcome 1 Knowledge (mean score, %).
6.
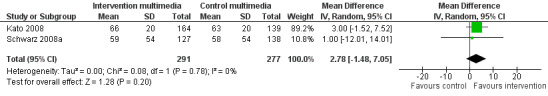
Forest plot of comparison: 5 Multimedia education versus control multimedia, outcome: 5.1 Knowledge (mean score, %).
Skill acquisition
Acosta 2009 measured inhaler technique in 116 patients immediately before and after the intervention and at one month follow‐up. The change from baseline score (percent of steps performed correctly) in the post‐intervention time point was significantly higher in the multimedia education group: 15.92 (SD 15.04) compared with the control group: 1.16 (SD 6.55) (MD 14.76, 95% CI 10.63 to 18.89, Analysis 5.2). This difference was maintained after one month with a change of score from baseline of 14.92 (SD 15.49) in the multimedia education group and 1.62 (SD 7.30) in the control group (MD 13.30, 95% CI 8.98 to 17.62, Analysis 5.2).
5.2. Analysis.
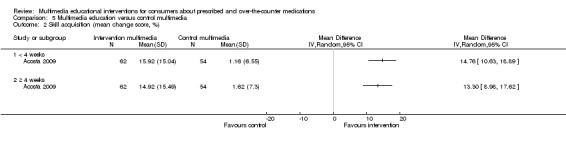
Comparison 5 Multimedia education versus control multimedia, Outcome 2 Skill acquisition (mean change score, %).
Compliance with medications
Kato 2008 assessed patient compliance with medications in 303 patients using 5 different measures: attendance at clinics, 6‐MP blood metabolite assays (indicate adherence to 6‐MP), Chronic Disease Compliance Instrument or CDCI (a patient‐reported measure of adherence to medical treatment), Medication Adherence Scale or MAS (a patient‐reported measure of general adherence to treatment) and the Medication Event Monitoring System or MEMS (which measures adherence to antibiotics using a medication container containing a microprocessor that records the dates and times the container is opened). Data from the CDCI were chosen for the meta‐analysis as the CDCI was measured in all study participants and it had better measurement properties than the MAS. CDCI scores ranged from 18 to 90 with higher scores representing better compliance. Outcomes were measured before the intervention, then one and three months following the intervention. The three‐month time point was used for the analysis.
For the CDCI results, the authors stated that there was no statistically‐significant difference in compliance between the intervention and control groups when analysed using a repeated‐measures mixed‐effect linear model that tested differences between treatment groups at the three time points (P = 0.78). However, when we used the unadjusted means from the three month time point, the results favoured multimedia education (MD 2.60, 95% CI 0.78 to 4.42, Analysis 5.3). The unadjusted means show that the level of compliance was higher in the intervention than the control groups at baseline (79.20 (SD 7.90) versus 77.40 (SD 7.50)). It is therefore possible that our result could be confounded by the higher levels of baseline compliance in the intervention group.
5.3. Analysis.
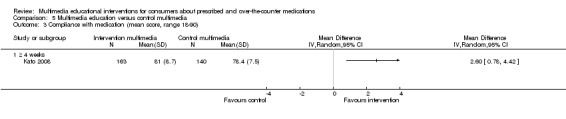
Comparison 5 Multimedia education versus control multimedia, Outcome 3 Compliance with medication (mean score, range 18‐90).
Results for the other compliance measures were mixed. There was no significant difference between compliance in the intervention and control groups when measured using the patient‐reported MAS (P = 0.50). However, objective measures of compliance demonstrated higher medication compliance in the multimedia education group (MEMS P = 0.01, 6‐MP assay P = 0.04). The objective measures were only available for the subset of patients who were prescribed that particular medication (MEMS in 200/304 and 6‐MP assay in 46/304 patients). Compliance with clinic attendance (percentage of scheduled visits that patients attended) was equally high in both the intervention and control groups (mean 98% +/‐ 1% in both groups, P = 0.65).
Use of study medication
Schwarz 2008a measured the number of patients who used emergency contraception during the seven‐month study period. The study found that there was no statistically‐significant difference in the percentage of enrolled patients who accessed emergency contraception in the intervention and control groups (6% versus 3%, RR 2.25, 95% CI 0.87 to 5.80, Analysis 5.4). However, patients in the multimedia education group were more likely to have a supply of emergency contraception at home (34% vs 7%, P < 0.001).
5.4. Analysis.
Comparison 5 Multimedia education versus control multimedia, Outcome 4 Use of medication (N who used medication).
Health outcomes
Schwarz 2008a measured the number of patients who conceived during the study period and found that there was no statistically‐significant difference between the intervention and control groups (3% vs 6%, P = 0.12). The study authors did not report whether these were planned pregnancies or resulted from lack of patient knowledge on the use of emergency contraception. Acosta 2009 reported that peak flow readings following the intervention (controlled for pre‐intervention peak flows) were statistically better in the intervention group (P = 0.001). This was confirmed when the MD was calculated using SDs obtained from the reported 95% CIs (MD of peak flow readings (litres per second) 21.24, 95% CI 5.28 to 37.20, Analysis 5.5).
5.5. Analysis.
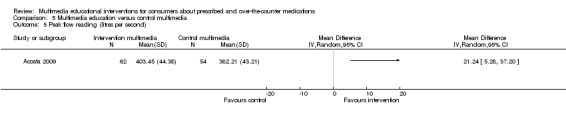
Comparison 5 Multimedia education versus control multimedia, Outcome 5 Peak flow reading (litres per second).
Self‐efficacy
Kato 2008 measured perceived self‐efficacy to manage cancer and its treatment in 303 patients. Self‐efficacy was measured using a scale designed for the purpose of this study, with possible scores ranging from 7 to 189 where higher scores reflect greater self‐efficacy. The study analysed results using a mixed‐effect linear model and found that patients in the intervention and control groups had similar levels of self‐efficacy at baseline. There was a significant increase in self‐efficacy over time in the intervention group (P = 0.01). Calculation of the MD using the mean self‐efficacy scores post‐intervention also favoured the multimedia education group (intervention 164.00 (SD 23.40), control 158.80 (SD 23.50), MD 5.30, 95% CI 0.00 to 10.60, Analysis 5.6).
5.6. Analysis.
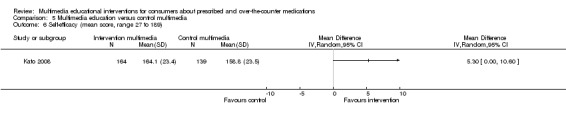
Comparison 5 Multimedia education versus control multimedia, Outcome 6 Self‐efficacy (mean score, range 27 to 189).
Quality of life
Kato 2008 measured quality of life (QOL) using two established questionnaires designed for adult (Functional Assessment of Cancer Therapy ‐ General (FACT‐G)) and paediatric (Paediatric Quality of Life Inventory (PQL)) populations. QOL was measured one and three months following the intervention. The authors stated that there was no significant difference in QOL between the intervention and control groups over the different time points (PQL P = 0.11, FACT‐G P = 0.15). The results from the paediatric and adult sub‐groups, at the three month time point, were pooled (N = 269), showing a trend favouring multimedia education, although this did not reach statistical significance (SMR 0.20, 95% CI ‐0.04 to 0.44, Analysis 5.7).
5.7. Analysis.
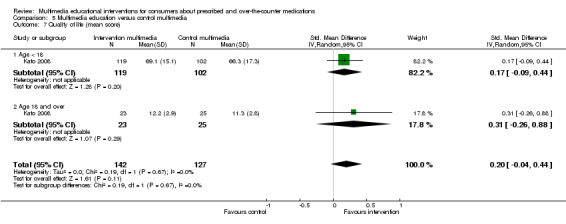
Comparison 5 Multimedia education versus control multimedia, Outcome 7 Quality of life (mean score).
Beliefs about medication
Schwarz 2008a measured patient attitudes towards emergency contraception in 446 participants and found that there was a trend favouring the intervention, with a greater number of participants developing a more positive attitude towards emergency contraception after the multimedia intervention (8% versus 4%, RR 1.73, 95% CI 0.64 to 4.67, Analysis 5.8).
5.8. Analysis.

Comparison 5 Multimedia education versus control multimedia, Outcome 8 Beliefs about medication (N with more positive beliefs).
Health locus of control
Kato 2008 measured 303 patients' perception of their control over their health using the Multidimensional Health Locus of Control Scale, and found that there was no significant difference between the intervention and control groups (P = 0.61). We were unable to extract data to verify this result, as the authors reported mean scores for each of the questionnaire's individual dimensions but not an overall locus of control score.
Adverse effects of the educational intervention
Kato 2008 measured 301 patients' self‐perceived stress using the Perceived Stress Scale. Possible scores for this scale range from 10 to 50, with higher scores representing greater stress. The study reported that there was no significant difference between the intervention and control groups using a repeated‐measures mixed‐effect linear model that tested differences between treatment groups at the three time points. When the unadjusted mean scores from the three month time point were used to calculate the MD, the results suggested that self‐perceived stress levels were higher in the intervention group (intervention 38.10 (SD 6.90), control 35.70 (6.20), MD 2.40, 95% CI 0.92 to 3.88, Analysis 5.9). Baseline levels of self‐perceived stress were higher in the intervention than control group (34.40 (SD 7.40) versus 33.10 (SD 6.60)). It is therefore possible that our result could be confounded by the higher levels of baseline self‐perceived stress in the intervention group.
5.9. Analysis.
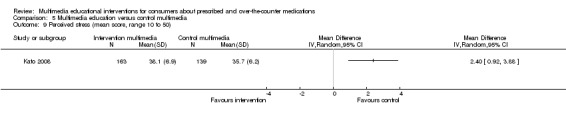
Comparison 5 Multimedia education versus control multimedia, Outcome 9 Perceived stress (mean score, range 10 to 50).
Outcomes not evaluated
None of the studies comparing multimedia education to a control multimedia program evaluated the effect on medication side effects.
Multimedia education and a co‐intervention versus the co‐intervention alone
Six studies compared multimedia education in combination with a co‐intervention to the co‐intervention alone (Kinnane 2008; McElnay 1989; Neafsey 2002; Osguthorpe 1983; Solomon 1988; Trent 2010). We present the main findings for this comparator in Table 6.
Knowledge
Four studies comparing multimedia education with a co‐intervention to the co‐intervention alone measured knowledge (Kinnane 2008; Neafsey 2002; Osguthorpe 1983; Solomon 1988). Two of the studies, with a total of 381 patients, provided sufficient data for meta‐analysis. Neafsey 2002 measured knowledge immediately after patients had received education, as well as two and four weeks later. The authors reported that knowledge scores were significantly higher in the multimedia education group at all of the time points. Calculation of the MD using mean scores from the two week time point (intervention 78% (SD 21) versus control 55% (SD 20), MD 22.40, 95% CI 12.15 to 32.65) and four week time point (intervention 80% (SD 21), control 57% (SD 20), MD 23.20, 95% CI 12.82 to 33.58) confirmed higher knowledge levels in the multimedia education group. Solomon 1988 evaluated patient knowledge on either the second or sixth day after the intervention, with the results strongly favouring the multimedia education group (mean score intervention 96% (SD 11), control 71% (SD 11). Pooled results for short term (less than four weeks) knowledge from both Solomon and Neafsey strongly favoured the multimedia education group (MD 24.59, 95% CI 22.34 to 26.83, Analysis 6.1, Figure 7). Results of the meta‐analysis were unchanged when we used the fixed‐effect model.
6.1. Analysis.
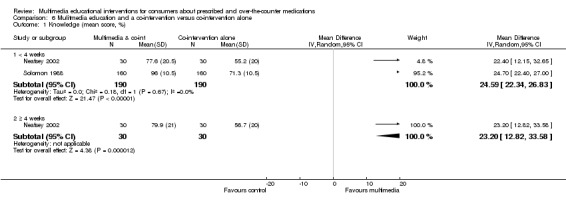
Comparison 6 Mulitmedia education and a co‐intervention versus co‐intervention alone, Outcome 1 Knowledge (mean score, %).
7.
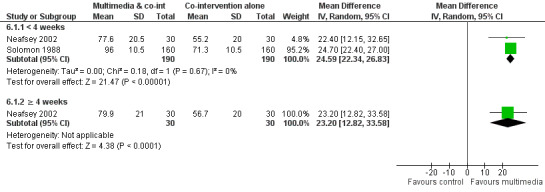
Forest plot of comparison: 6 Mulitmedia education and a co‐intervention versus co‐intervention alone, outcome: 6.1 Knowledge (mean score, %).
Kinnane 2008 and Osguthorpe 1983 measured patient knowledge but did not provide sufficient data for meta‐analysis. Both studies reported results for each of the knowledge questions individually, with no overall summated knowledge score. The authors of both studies reported no statistically‐significant difference in knowledge between the intervention and control groups.
Skill acquisition
McElnay 1989 compared inhaler technique in healthy volunteers receiving written information in combination with a multimedia education program to those receiving written information alone. Inhaler technique was measured in 87 volunteers immediately following the education and two weeks later. Results from the first time point indicated greater inhaler technique scores in the multimedia education group, with mean scores of 75% versus 62% (MD 13.10, 95% CI 6.16 to 20.04). However, testing of inhaler technique two weeks later showed that this difference between the two groups was not maintained, with scores of 63% versus 59% (MD 3.40, 95% CI ‐8.30 to 15.10, Analysis 6.2). The authors postulated that the deterioration in inhaler technique scores over the two weeks may have been due to the healthy volunteers not using the inhalers between the two time points, and hypothesised that scores at the second time point would be higher in an asthmatic population who were using inhalers daily.
6.2. Analysis.
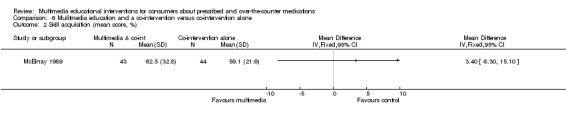
Comparison 6 Mulitmedia education and a co‐intervention versus co‐intervention alone, Outcome 2 Skill acquisition (mean score, %).
Compliance
Compliance with medications
Two studies measured compliance with medications. We did not pool the results, as data from both studies could not be converted to dichotomous or continuous measures. Solomon 1988 measured patient compliance in 321 patients. Compliance was assessed via interviews with patients and scored using a system, devised by the authors for the purposes of this study, that assigned a numerical value according to the degree to which that error might compromise treatment effectiveness. Possible scores ranged from 0 to 160, with higher scores indicating worse compliance. The study reported that patients in the multimedia education group had significantly better compliance scores than those who did not view the multimedia intervention, and this result was confirmed when the MD was calculated from the mean scores (multimedia education 8.3 (SD 86.6) versus control 40.45 (SD 86.6), MD ‐32.15, 95% CI ‐51.13 to ‐13.17, Analysis 6.3).
6.3. Analysis.
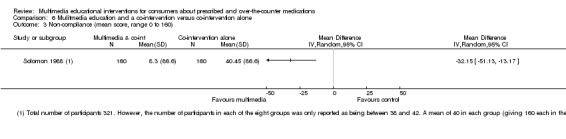
Comparison 6 Mulitmedia education and a co‐intervention versus co‐intervention alone, Outcome 3 Non‐compliance (mean score, range 0 to 160).
Trent 2010 measured compliance with medications in 77 patients. Compliance was assessed via telephone interviews where participants were asked if they had completed a two week course of antibiotics. There was no difference in compliance rates between the two groups (intervention 23/36, control 27/41, RR 0.97, 95% CI 0.70 to 1.35, Analysis 6.4).
6.4. Analysis.

Comparison 6 Mulitmedia education and a co‐intervention versus co‐intervention alone, Outcome 4 Compliance with medication (N).
Compliance with treatment instructions
Solomon 1988 examined the percentage of patients who prematurely resumed sexual activity while being treated with tetracycline for a sexually transmitted disease. The authors reported that a smaller percentage of patients prematurely resumed sexual activity in the multimedia education group (median percentage 3% versus 22%, F‐ratio = 30.2, P < 0.001). These results could not be verified as the authors only reported the median number of patients.
Trent 2010 measured patient compliance with treatment instructions, including the number of participants who attended a medical follow‐up appointment, abstained from sexual intercourse during treatment, and notified their partners of the sexually transmitted disease diagnosis and the number of partners who were then treated. We extracted patient attendance at the follow‐up appointment, as this was identified as a primary outcome measure by the study authors. The authors reported that there was a higher rate of compliance with the follow‐up appointment in the multimedia education group (11/36 (31%) versus 6/41 (15%), RR 2.09, 95% CI 0.86 to 5.08, Analysis 6.5). The multimedia education group also had higher rates of partner treatment (67% versus 49%) but there was no significant difference in rates of sexual abstinence (69% versus 76%) or partner notification (83% versus 85%).
6.5. Analysis.
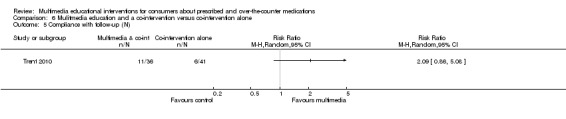
Comparison 6 Mulitmedia education and a co‐intervention versus co‐intervention alone, Outcome 5 Compliance with follow‐up (N).
Health outcomes
Trent 2010 measured complications from pelvic inflammatory disease or its treatment, and found that there was no difference between the multimedia education and control groups (13/36 (36%) versus 13/41 (32%), RR 1.14, 95% CI 0.61 to 2.13, Analysis 6.6)
6.6. Analysis.

Comparison 6 Mulitmedia education and a co‐intervention versus co‐intervention alone, Outcome 6 Medical complication (N).
Adverse medication behaviour
Neafsey 2002 measured adverse self‐medication behaviour (patient medication behaviours that were likely to lead to adverse events) in a total of 60 patients. Possible scores ranged from 0 to 40, with higher scores representing greater risk for adverse events. The authors reported a significant decrease in the adverse behaviour score compared with baseline measurements for patients receiving multimedia and written education, but not those receiving written information alone. There were no significant differences in group scores at baseline. The statistical significance of the difference in behaviour scores between the multimedia education and control group following the intervention was not reported. When the MD was calculated from the mean scores, it revealed a trend favouring the multimedia education arm which became more evident with time from the intervention (at two weeks MD ‐4.00, 95% CI ‐9.98 to 1.98; at four weeks MD ‐5.00, 95% CI ‐10.06 to 0.06, Analysis 6.7).
6.7. Analysis.
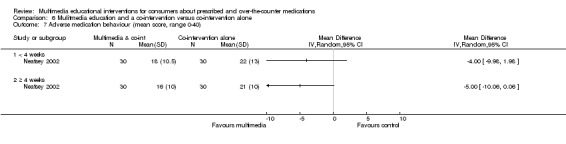
Comparison 6 Mulitmedia education and a co‐intervention versus co‐intervention alone, Outcome 7 Adverse medication behaviour (mean score, range 0‐40).
Self‐efficacy
Neafsey 2002 measured self‐efficacy for avoiding drug and alcohol interactions with prescribed medications in a total of 60 patients, using a self‐efficacy questionnaire that was developed by the authors for the purpose of this study. Possible scores ranged from zero to five, with higher scores representing greater self‐efficacy. The authors reported higher self‐efficacy in the multimedia and written education group compared with the group that received the written information alone. This effect persisted from immediately following the intervention (mean score intervention 3.70 (SD 0.80), control 2.80 (SD 0.90), MD 0.90, 95% CI 0.47 to 1.33, Analysis 6.8), to the last time point at which outcomes were measured, four weeks after the intervention (intervention 3.90 (SD 0.70), control 3.00 (SD 0.80), MD 0.90, 95%CI 0.52 to 1.28 Analysis 6.8).
6.8. Analysis.
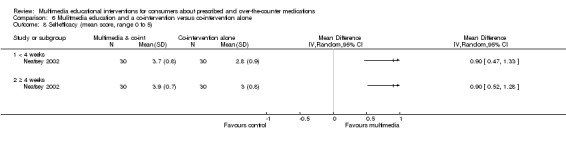
Comparison 6 Mulitmedia education and a co‐intervention versus co‐intervention alone, Outcome 8 Self‐efficacy (mean score, range 0 to 5).
Satisfaction with care
Solomon 1988 assessed patients' satisfaction with care using a five‐point rating scale. We could not extract data for this outcome as the effect of the multimedia intervention could not be separated from that of the special pill packaging. However, the authors reported that patients who had received the multimedia intervention, the pill packaging or both had significantly higher satisfaction than those who had received neither intervention.
Beliefs about medication
Solomon 1988 assessed patients' confidence in the treatment regimen using a five‐point rating scale. We could not extract data for this outcome as the effect of the multimedia intervention could not be separated from that of the special pill packaging. However, the authors reported that patients who had received the multimedia intervention, the pill packaging or both had significantly greater confidence than those who had received neither intervention.
Perception of illness
Solomon 1988 assessed patients' perception of the severity of their illness using a five‐point rating scale. We could not extract data for this outcome as the effect of the multimedia intervention could not be separated from that of the co‐intervention. However, the authors reported that patients who had received the multimedia intervention, the pill packaging or both had significantly higher ratings of perceived disease severity than those who had received neither intervention.
Use of health services
Kinnane 2008 measured the number and content of telephone calls from patients to the outpatient clinic during the study period. The education provided to patients included information about the circumstances in which patients were to contact the clinic. The study reported no significant difference in the number of calls made by the multimedia and control groups (25 and 27 telephone calls respectively). However, the study authors noted that calls made by the multimedia education group were more likely to be about medical problems covered in the education (80% and 56% of the telephone calls respectively).
Outcomes not evaluated
None of the studies comparing multimedia education in combination with a co‐intervention to the co‐intervention alone evaluated the effect on quality of life.
Sensitivity analysis
We performed sensitivity analyses examining the effect of adequate allocation concealment and blinding of outcome assessors on the effect size of knowledge and skill acquisition. There were three studies appropriate for sensitivity analysis of the effect of allocation concealment on the effect size of skill acquisition in the multimedia education versus education by a health professional comparison group (Lirsac 1991; McElnay 1989; Self 1983). Results of the analysis with the inclusion of the three studies showed no difference between the multimedia education and comparison groups (MD ‐1.01, 95% CI ‐15.75 to13.72). The results continued to show no statistically‐significant difference when only data from the study at low risk of bias were examined (Lirsac 1991) (MD 10.00, 95% CI ‐3.48 to 23.48).
There were two studies appropriate for sensitivity analysis of the effect of blinding of outcome assessors on the effect size of knowledge in the multimedia education and co‐intervention versus co‐intervention alone comparison group (Neafsey 2002; Solomon 1988). Results of the analysis did not change with the inclusion of both studies (MD 24.59, 95% CI 22.34 to 26.83) or only data from the study at low risk of bias for the blinding of assessors (Solomon 1988) (MD 24.70, 95% CI 22.40 to 27.00). Three studies measuring skill acquisition in the multimedia education versus education by health professionals comparison group were also appropriate for sensitivity analysis of the effect of blinding of assessors (Lirsac 1991; McElnay 1989; Self 1983). The results when the three studies were included showed no difference between the multimedia education and control group (MD ‐1.01, 95% CI ‐15.75 to 13.72). When data from the study at low risk of bias for the blinding of assessors were included (McElnay 1989), the results showed a trend favouring education by a health professional (MD ‐11.90, 95% CI ‐22.86 to ‐0.94). As was discussed earlier, the variability of results from these studies may reflect differences in the education received in the control groups.
Discussion
Summary of main results
This systematic review summarises the evidence from randomised controlled trials of multimedia educational interventions about prescribed and over‐the‐counter medications. We identified 24 studies that enrolled a total of 8112 participants. However, due to variability in the comparators and outcomes measured, results from the review were based on subsets of these studies and participants. Although the studies varied in the outcomes that they measured, all but two measured our primary outcomes of interest ‐ knowledge and/or skill acquisition. The heterogeneity in the outcomes reported and the instruments used to measure these outcomes limited meta‐analysis for all comparisons. We were unable to comment on the clinical importance of results due to lack of information regarding the minimum clinically important difference of the outcomes measured. Also, variability of the aims and content of the multimedia and control educational interventions, and lack of information on which to base evaluation of their quality, limited conclusions from this review.
Summary of results
Knowledge
This review provides evidence that multimedia education is superior to no education or non‐standardised education provided as part of usual clinical care in improving patient knowledge (SMD 1.04, 95% CI 0.49 to1.58, six studies with 817 participants: RR 2.26, 95% CI 1.39 to 3.67, one study with 817 participants). There was considerable statistical heterogeneity (I2 = 89%), however, all but one of the studies favoured the multimedia group. The review found that multimedia education was not superior to control multimedia interventions (i.e. multimedia programs that do not provide information about the medication) in improving knowledge (two studies with 568 participants: MD 2.78, 95% CI ‐1.48 to 7.05). The amount of information provided to the control group participants about the relevant medications varied and, in some instances, appeared to inversely correlate with the size of the effect of the multimedia intervention.
The review provides evidence that multimedia education has a superior effect on knowledge when added to co‐interventions consisting of written information or brief standardised information provided by a health professional compared with the co‐interventions alone (two studies with 381 participants: MD 24.59, 95% CI 22.34 to 26.83). Multimedia education was only compared to written education in a single study with methodological limitations that would be expected to underestimate the effect of the multimedia education. In this study, multimedia education was equally effective as written information (one study with 162 participants: RR 0.80, 95% CI 0.59 to 1.09). A single study also showed that multimedia education was equally effective as a standardised 25‐minute lecture provided by a health professional which had identical content to the multimedia intervention (one study with 22 participants: MD 2.2, 95% CI ‐8.83 to 4.43). The inconsistency of results in comparing multimedia education to that provided by a health professional may be explained by differing content and quality of the education provided in the control group. Overall, the results suggest that multimedia education is superior to brief standardised education provided by a health professional but not to more intensive interventions where the health professional provides the same or similar information to what is included in the multimedia education program.
Skill acquisition
All of the studies measuring skill acquisition were evaluating education about metered dose inhalers. The review found that multimedia education was more effective at improving skill acquisition than usual care or no education (MD 18.32, 95% CI 11.92 to 24.73, two studies with 94 participants), written education (MD 31.00, 95% CI 10.14 to 51.85 and RR 2.14, 95% CI 1.33 to 3.44, three studies with 184 participants) and a control multimedia intervention (MD 13.30, 95% CI 8.98 to 17.62, one study with 116 participants). Multimedia education was equally effective as education by a health professional (three studies with 130 participants: MD ‐1.01, 95% CI ‐15.75 to 13.72). However, there was substantial statistical heterogeneity (I2 = 68%) in this meta‐analysis which may have been due to differences in the content and quality of the education used as a comparator.
Multimedia education was equally effective as the combination of written education and education by a health professional (one study with 69 participants: RR 1.17, 95% CI 0.7 to 1.94). Multimedia education was also equally effective when added to a co‐intervention consisting of written education compared with written education alone (one study with 87 participants: MD 3.40, 95% CI ‐8.30 to 15.10). This is inconsistent with the results from other studies that used written education as a comparator. The results from this study did show that multimedia education was more effective when the outcome was measured immediately following the intervention. but the effect was not maintained at two weeks which is the time point extracted for meta‐analysis. The study used healthy volunteers, and the study authors postulated that the deterioration in inhaler technique scores over the two weeks may have been because the volunteers, unlike asthma patients, were not using the inhalers between the two time points.
Compliance
The review found that multimedia education did not improve compliance with medications compared with usual care or no education (RR 1.02, 95% CI 0.96 to 1.08, two studies with 4552 participants).
Other outcomes
We were not able to pool data for any other outcome measures. Only two studies measured potential adverse effects from the educational intervention and both did so indirectly by determining patients' self‐perceived stress or anxiety before and after the intervention (Kato 2008; Olver 2009). Data could only be extracted from one study, comparing multimedia education to a control multimedia program. These data suggest that self‐perceived stress levels were higher in the multimedia education group (MD 2.40, 95% CI 0.92‐ 3.88). However, baseline levels of self‐perceived stress were higher in the intervention group, which may have confounded the result.
Overall completeness and applicability of evidence
Multimedia patient education about medications would most likely be used as an adjunct to, rather than a replacement of, patient education provided by a health professional as part of usual care. Multimedia education was shown to be more effective at improving patient knowledge and skill acquisition when compared with no education or non‐standardised education provided by health professionals as part of usual care. However, only two studies examined the addition of multimedia education to standardised education by a health professional and compared it to education by a health professional alone. Results from the studies were inconclusive, with Solomon 1988 reporting a large increase in knowledge as well as increased compliance in the group which received the multimedia education program, while Kinnane 2008 reported no difference in knowledge between the two groups. The variability in results may be due to differences in the quality of the education provided by the health professionals. In Solomon 1988 both groups received standardised verbal instructions from their doctor consisting of a short paragraph outlining proper use of the medication. In Kinnane 2008 the patients received much more intensive education from a health professional, consisting of a detailed, one‐hour standardised education session from a registered nurse as well as written information about the medication and management of side effects.
This review found that multimedia education had no greater effect on medication compliance than usual care or no education. This is in keeping with previous studies and reviews that have found that improving patient education does not necessarily lead to improved rates of compliance (Brus 1998; Cote 1997). As previously discussed, the concept of compliance assumes that patients play a passive role in accepting medical direction. The degree of intentional and rational non‐compliance with medications suggests that this is not the case (Lowe 2000). The patient’s role in decision‐making is acknowledged in the concept of concordance, where patients and health professionals work together to reach consensus decisions about the patient’s care (Stevenson 2004). None of the studies included in the review measured concordance.
The development and production of multimedia programs is expensive, especially when this is compared to the limited cost involved in producing written information leaflets. None of the studies in this review evaluated the cost‐effectiveness of multimedia education. Only two studies measured resource utilisation. Stone 1989 reported the amount of time required to deliver the educational intervention (multimedia education 17.6 minutes compared with nurse lecture 26 minutes (SD 5.7)) and reported no difference in the time needed for questions following the intervention between the two groups. Kinnane 2008 measured the number and content of telephone calls from patients to the outpatient clinic during the study period and reported no difference in the number of calls made by the multimedia education and control groups, but noted that calls made by the multimedia education group were more likely to be about medical problems covered in the education program. Of the 22 multimedia programs used by the studies included in this review, only 6 were still available or could be obtained from the authors. It is not known how many of the programs evaluated in the studies are still in clinical use.
We could not extract data from several studies for meta‐analysis. The primary outcome of knowledge was not measured in two of the ten studies comparing multimedia education to usual care or no education and data could not be extracted from a further study. Three of the seven studies comparing multimedia education to written information or education by a health professional measured knowledge. Data could only be extracted from two of these studies and the results could not be pooled. Two of the three studies comparing multimedia education to a control multimedia program reported results for patient knowledge in a form that could be extracted for meta‐analysis. One of the five studies comparing multimedia education with a co‐intervention to the co‐intervention alone did not measure knowledge, and in a further two studies, data could not be extracted for this outcome. The other primary outcome of skill acquisition was measured in 8 of the 24 studies. All of these studies measured inhaler technique and reported data suitable for meta‐analysis.
Limited conclusions based on single studies could be reached regarding the effect of multimedia education on other outcomes including patient satisfaction, self‐efficacy, quality of life, medication side effects and health outcomes. Our capacity to pool information from studies was limited due to heterogeneity in the comparisons used, the outcomes measured and the instruments used to measure them. A number of studies did not report data in a format suitable for meta‐analysis. One of the secondary objectives of this review was to examine whether the effects of multimedia education persisted over time. Five of the included studies measured outcomes at one month after the intervention and five studies measured outcomes beyond one month (six weeks to nine months). We discussed the results of these studies at different time points in Effects of interventions but, due to the heterogeneity of study outcomes and comparators, we could not provide an overall assessment of the persistence of multimedia education effects over time.
Quality of the evidence
Many of the studies did not report sufficient information in their methods to allow judgment of their risk of bias. From the information that was reported, three of the studies had a high risk of selection bias, and one was at high risk of assessments being biased due to lack of blinding of the outcome assessors. The quality of the evidence is outlined below for each of the comparison groups.
Multimedia education compared with usual care or no education
Conclusions regarding the superior effect of multimedia education on knowledge were based on the results from six studies containing 817 participants. The evidence was downgraded to low quality due to the studies having an unclear risk of bias for allocation concealment, blinding of outcome assessors or both, and due to considerable statistical heterogeneity (I2 = 89%). Results from all of the studies included in the meta‐analysis favoured the multimedia education group, although the confidence interval for one of the studies crossed zero. We extracted dichotomous data from a further study of 167 participants, but these were not combined with continuous data from the other studies. The evidence from this study was also downgraded to low quality due to the results being based on a single study with unclear risk of bias for allocation concealment and blinding of outcome assessors. However, the results from this study showed a consistent effect favouring multimedia education, despite methodological limitations which may have biased the results in favour of the control group.
Conclusions regarding the superior effect of multimedia education on skill acquisition were based on data from two studies with 94 participants. Two studies containing a total of 4552 participants provided evidence that multimedia education was not effective in improving patient compliance with medications. The quality of the evidence for both of these outcomes was downgraded to moderate due to unclear risk of bias.
Conclusions for post‐operative pain, risk of drug misuse and dependence, self‐efficacy for medication adherence and for avoiding interactions with prescribed medications were based on single studies of varying size (43 to 343 participants). All of these outcomes, apart from post‐operative pain, were downgraded to low quality of evidence, due to conclusions being drawn from a single study with unclear risk of bias. The quality of the post‐operative pain outcome was downgraded to very low, as the study was at high risk of bias due to lack of allocation concealment.
Multimedia compared with other forms of education
The equivalent effect of multimedia education compared with written education on knowledge was based on very low quality evidence. The conclusion was based on a single study of 162 participants. The study had unclear risk of bias for both allocation concealment and blinding of outcome assessors and had methodological limitations which may have biased the results in favour of written education. A further study with 101 participants also reported no difference in effect, but data could not be extracted to verify this result. The superior effect of multimedia education compared with written education on skill acquisition was based on 3 studies with 184 participants. Dichotomous data were pooled from two of the studies while continuous data from the third study were reported separately. The three studies provided low to moderate quality evidence and all had unclear risk of bias for allocation concealment and or blinding of outcome assessors.
The equivalent effect of multimedia education compared with education by a health professional on knowledge was based on very low quality evidence. The conclusion was based on a single study of 22 participants which was at high risk of bias due to lack of allocation concealment. The effect of multimedia education compared with education by a health professional on skill acquisition was based on 4 studies with 245 participants. Data were pooled for three studies measuring skill acquisition within four weeks of the intervention (N = 130) and two studies measuring this outcome four weeks or more after the intervention (N = 130). Within four weeks, multimedia education was equivalent to education by a health professional, but there was significant heterogeneity in the results of the studies. This may be explained by differences in the amount of education received by the control group. Two of the studies had unclear risk of bias for allocation concealment and blinding of outcome assessors. When skill acquisition was measured at four weeks or more, the results were also equivalent. However, the 95% CI was wide and included both no effect and a substantial effect in the direction of the control group.
The equivalent effect of multimedia education on skill acquisition when compared with the combination of written education and education by a health professional was based on a single study of 69 participants. The quality of the evidence was downgraded to very low due to the single study having unclear risk of bias for allocation concealment, and the wide 95% CI crossing zero and including both no effect and a moderate sized effect favouring the multimedia education.
Multimedia education compared with a control multimedia program
The equivalent effect of multimedia education on knowledge when compared with a control multimedia program was based on 2 studies with 568 participants. The evidence was of moderate quality and was downgraded due to unclear risk of bias for allocation concealment. Conclusions regarding the superior effect of multimedia education on skill acquisition, medication compliance, self‐efficacy and peak expiratory flow readings were all based on single studies. The evidence for the effect on self‐efficacy was of moderate quality, and was only downgraded because it was based on a single study. The quality of the evidence for the other outcomes was further downgraded to low. The results for compliance may have been confounded by higher levels of baseline compliance in the intervention group. Conclusions for other outcomes were based on studies with unclear risk of bias for allocation concealment. Conclusions regarding the effect on quality of life were based on a single study of 269 participants. The 95% CI crossed zero and included both no effect and a small to moderate sized effect favouring the multimedia education.
Multimedia education with a co‐intervention compared with the co‐intervention alone
Conclusions regarding the superior effect of the addition of multimedia education to a co‐intervention on knowledge was based on the results of 2 studies with 381 participants. The evidence was of moderate quality and was downgraded due to the studies having unclear risk of bias for allocation concealment. The lack of effect on skill acquisition of the addition of multimedia education to written education was based on a single study of 87 participants. The evidence was of very low quality due to the single study having unclear risk of bias for allocation concealment and due to a wide 95% CI that included both no effect and substantial effect in the direction of the multimedia group. The results were inconsistent with findings from other studies that used written education as a comparator. The study showed that multimedia education was more effective than written education when the outcome was measured immediately following the intervention (MD 13.10, 95% CI 6.16 to 20.04). However, this effect was not maintained at two weeks which is the time point extracted for meta‐analysis. The study used healthy volunteers rather than patients and the study authors postulated that the deterioration in inhaler technique scores over the two weeks may have been because the volunteers, unlike asthma patients, were not using the inhalers between the two time points.
Conclusions for the superior effect of multimedia education on self‐efficacy was based on a single study with 60 participants. Conclusions for the lack of effect of the addition of multimedia education on medical complications from the underlying illness and medication side effects were based on single studies. The evidence was downgraded to low or very low due to study limitations including unclear risk of bias, wide 95% CI or indirect measurement of the outcome. Medication compliance was measured in 2 studies with 397 participants. However, results were not pooled as dichotomous data were reported for one study and continuous data for the other. There was an inconsistent effect of multimedia education on medication compliance, with one study showing better compliance in the multimedia education group while the other study reported no difference. The quality of the evidence for both studies was low due to unclear risk of bias for allocation concealment or a wide 95% CI that included both no effect and a substantial effect favouring multimedia education .
Limitations of the review
Heterogeneity in the content and quality of the comparators
In order to evaluate whether multimedia delivery of education is effective compared with existing forms of patient education, it is important that the educational content provided to participants is similar in both the intervention and control arms. However, in a number of studies, this was not the case.
This review provides evidence that multimedia education about medications is superior to usual care or no education in improving patient knowledge and skill acquisition. However, the effect of multimedia education may have been overestimated by the design of some of the studies. Three of the ten studies comparing multimedia education to usual care or no education required patients to have taken the medication for a certain period of time in order to be eligible to participate in the study (Mazor 2007; Navarre 2007; Van Der Palen 1997). This resulted in a patient group which had started the medication, and probably received education about the medication, months to years before being enrolled in the study. The control groups did not receive any information about the medication as part of the study and were compared to the multimedia group who received education about the medication a short time before outcomes were measured. A fourth study (Neafsey 2001) reported a large effect size in the multimedia education group due to extremely low levels of knowledge in the control group who were unlikely to have received any information about the area of knowledge being tested.
Eight studies used written educational materials in the control arm. In five studies, the written materials were directly compared to multimedia education (Goodyer 2006; Olver 2009; Savage 2003; Schnellinger 2010; Self 1983), while in three studies, they were used as a co‐intervention (McElnay 1989; Neafsey 2002; Osguthorpe 1983). The information provided in the written educational materials was similar to that in the multimedia intervention in all of the studies. Nine studies used standardised education by a health professional in the control arm. In six studies, education by a health professional was compared directly to multimedia education (Goodyer 2006; Lirsac 1991; McElnay 1989; Self 1983; Stone 1989; Van Der Palen 1997), while in three studies it was used as a co‐intervention (Kinnane 2008; Solomon 1988; Trent 2010). There was marked variability in the content of the standardised education provided by health professionals and its similarity to the information provided in the multimedia intervention. Five of the six studies comparing education by a health professional to multimedia education provided information about MDIs. In four of the studies, the control arm received direct instruction from a health professional, either individually or in a group setting. This instruction included the health professional demonstrating correct inhaler technique and, in at least two of the studies, viewing patients' inhaler technique and correcting errors. However, in Lirsac 1991 the health professional only read out instructions for correct technique while the participant looked at illustrations. The participants were not shown a demonstration. In the remaining study (Stone 1989), the control arm received a 25‐minute lecture about anticoagulant therapy with identical content to the multimedia program. The education provided by health professionals as a co‐intervention also varied. In Kinnane 2008, the patients received a one‐hour standardised education session from a registered nurse while in Trent 2010, all participants received detailed discharge instructions. However, in Solomon 1988, the verbal instructions consisted only of a single paragraph stating the name of the medication and instructions on how to take the medication correctly. This variability in the comparators may account for the heterogeneity of results seen when comparing multimedia education to standardised education by a health professional. Overall, the results suggest that multimedia education is superior to brief standardised education provided by a health professional but not to more intensive interventions where the health professional provides the same or similar information to what is included in the multimedia program. Multimedia education may still be preferable and more practical in these situations due to the time required to provide the more intensive interventions.
The quality of the comparator could only be evaluated in 4 of the 16 studies which compared multimedia to another form of education, We obtained a copy of the printed information leaflet used as the comparator in three studies (Goodyer 2006; McElnay 1989; Savage 2003), allowing complete evaluation of its quality. Solomon 1988 published the transcript of the standardised verbal instructions provided by health professionals, also allowing for evaluation of its quality. For a further four studies, we performed a limited evaluation based on a description of the education provided in the study publication.
Heterogeneity in the content and quality of the multimedia interventions
Heterogeneity in the content and quality of the multimedia educational interventions may also explain some of the variability of results in the review. However, our ability to evaluate the educational interventions was limited by the paucity of information reported by the study authors. We intended to determine an overall grading of the quality of the educational interventions and comparators based upon our application of the Evaluative Linguistic Framework (ELF). This was not performed as quality could only be fully evaluated for the six studies for which the multimedia intervention was available and provided by the study authors (Deitz 2011; Kinnane 2008; Mazor 2007; Navarre 2007; Olver 2009; Trent 2010). For the another seven studies, we performed a limited evaluation based on the voice‐over script (Goodyer 2006; Savage 2003; Schwarz 2008a; Voris 1982) or descriptions of the multimedia program (Kato 2008; Neafsey 2001; Neafsey 2002). Only 2 of 16 studies that compared multimedia to a different form of education provided enough information to allow assessment of both the multimedia and comparison educational interventions (Goodyer 2006; Savage 2003). The remaining 11 studies provided insufficient information on which to base an assessment of the quality of the intervention.
Although the ELF was a useful starting point for evaluating the quality of the educational interventions, it was designed to evaluate written materials, and does not capture many aspects of multimedia interventions. We could assess the generic structure for the multimedia programs used in 18 of the studies. None of the programs contained all ten of the possible moves (sections of information) identified by the ELF. However, the number of moves included varied based on the aims of the program, and therefore did not necessarily reflect program quality. For example, three programs included only one move ('dosage instructions'). However, this was appropriate as the programs focused on instructing patients on the correct use of MDIs. We calculated lexical density for nine of the programs and this varied from 29% to 65%. The lowest lexical density was found in programs or sections of programs that depicted patients presenting information or having a conversation (29% to 36%). This degree of lexical density is in keeping with that seen in natural speech and informal text (Halliday 1985). The lexical density in the other programs, where the information was presented by a health professional or narrator, varied from 42% to 65%. This is in keeping with the lexical density seen in formal or technical texts (Halliday 1985) raising the possibility that the information, when presented as text, would be difficult for patients to understand. However, presentation of this information as a voice‐over may overcome this difficulty.
Outcome measures
The heterogeneity in the outcomes reported and the instruments used to measure these outcomes, and the lack of data regarding the minimum clinically important difference for the outcomes, limited the conclusions that could be drawn from this review. Instruments used to measure outcomes were often developed by the study authors with limited reporting of their reliability and validity. Fourteen of the 15 studies that measured patient knowledge used scales developed by the study authors for the purpose of the study or similar preceding studies. The other scale had previously been used in a telephone survey about emergency contraception. Of these 15 studies, 2 reported measures of scale reliability such as internal consistency or test‐retest reliability. Six reported that the scale was pilot tested with the target population and three reported content validity based on expert review. Only one study reported testing discriminant validity of the scale. The use of scales which have undergone limited testing of their measurement properties raises the possibility that a lack of effect may be due to the scale being insensitive to change, rather than inefficacy of the intervention. This was particularly evident in Schnellinger 2010 where the results from the knowledge scale were skewed to the left, and 23% of participants had perfect scores at baseline. This suggests that the scale was insensitive to change due to a ceiling effect, and poorly targeted to knowledge in the sample population.
Potential biases in the review process
The broad search strategy used in this review minimised the risk of missing relevant studies. The success of the search strategy in capturing relevant literature was supported by the lack of further studies being identified for inclusion when reference lists from included studies and relevant reviews were examined. Screening of titles, abstracts and the full text of studies to identify those meeting the inclusion criteria. as well as data extraction from the included studies were performed independently by two review authors, minimising the risk of bias and errors. Sensitivity analyses showed that imputation of missing SDs did not change the results of the meta‐analyses, and results did not differ with the use of random‐effects or fixed‐effect models, suggesting that results of meta‐analyses were robust and not being biased by the chosen methodology.
We extracted post‐intervention outcome data and used them for the meta‐analyses without any statistical adjustment for pre‐intervention scores. For most of the studies this would not have led to bias in the results, due to randomisation resulting in similar baseline scores. However, in some cases the results of the meta‐analysis conflicted with results reported by study authors that were calculated using statistical methods that incorporated change over time or adjusted for baseline scores. For example, Kato 2008 reported no statistically‐significant difference in compliance between the intervention and control groups when using a repeated‐measures mixed‐effect linear model that tested differences between treatment groups at the three measured time points. The use of unadjusted means from the three‐month time point for the meta‐analysis favoured multimedia, but may have been confounded by higher levels of baseline compliance in the multimedia group. All cases where the meta‐analysis findings conflicted with those of the study authors were reported either in the text of the review or in the accompanying tables.
Agreements and disagreements with other studies or reviews
This review provides more conclusive evidence for the effectiveness of multimedia education about medications in improving patient knowledge and skill acquisition than previous reviews in this area (Jeste 2008; Wofford 2005). The results from Jeste 2008 were inconclusive, with four of the eight studies finding that multimedia education about medications was superior to the comparator in improving patient knowledge. Wofford 2005 did not provide a synthesis of the effectiveness of multimedia interventions due to variability in the topics of the educational interventions and the reported outcome measures in the included studies. We also found that there was variability in the outcomes measured and reported by the included studies, and that this limited conclusions about the effectiveness of multimedia education on outcomes other than knowledge and skill acquisition.
The inclusion criteria for our review differed from previous reviews of multimedia education, in that it focused only on purely educational interventions about prescription and over‐the‐counter medications, where the effect of the multimedia educational component on outcomes could be separated from the effect of other components of the intervention. Unlike previous reviews, this review did not place any restrictions on the language in which the study was published, the age of participants, the method used to deliver the multimedia program or the outcomes measured. Twenty of the 24 studies included in this review had not been included in either of the previous reviews.
The results from this review differed from the finding in Ryan 2011 that there was insufficient evidence that education alone improves knowledge about medications. However, the results from this review are consistent with the finding from Haynes 2008 and Ryan 2011 that education alone is ineffective at improving medication compliance.
Authors' conclusions
Implications for practice.
This review provides evidence that multimedia education about medications was more effective than no education or non‐standardised education provided by health professionals as part of usual clinical care in improving both knowledge and skill acquisition. It also suggests that multimedia education is at least equivalent to other forms of education including written education and education provided by a health professional. However, this is based on often low quality evidence from a small number of trials. Multimedia education about medications could therefore be considered as an adjunct to usual care but there is currently inadequate evidence to recommend it as a replacement for written education or education by a health professional. Multimedia education may be considered as an alternative to education provided by a health professional, in settings where provision of detailed education by a health professional is not feasible. This review was not able to examine potential harms of multimedia education, the feasibility of producing multimedia education programs or their cost‐effectiveness, as several important outcomes were not assessed.
Implications for research.
Many of the conclusions of this review were based on results from a small number of studies. More studies evaluating multimedia educational interventions are required in order to increase confidence in the estimate of effect of the intervention. Conclusions regarding the effect of multimedia education were limited by the variability in content and quality of both the multimedia and comparison educational interventions, and by the limited information provided about the educational interventions in the published studies. Studies testing educational interventions should provide detailed information about the educational interventions and comparators. Research is needed to establish a framework that is specific to the evaluation of the quality of multimedia educational programs. The Evaluative Linguistic Framework (ELF) may provide a useful starting point in developing this framework. Conclusions were also limited by the heterogeneity in the outcomes reported and the instruments used to measure them. The variability in outcomes suggests a lack of consensus regarding which outcomes are important to measure when evaluating patient educational interventions. Research is required to build consensus in this area and to identify a core set of outcomes which should be measured when evaluating educational interventions. This research should include patients to ensure that the outcomes selected are relevant to both patients and health professionals. Future research should use consistent, reliable and validated outcome measures so that comparisons can be made between studies.
Acknowledgements
We acknowledge the contribution of Cochrane Consumers and Communication Review Group editors and staff, particularly Sophie Hill, Rebecca Ryan, Anneliese Synnot and Megan Prictor, as well as John Kis‐Rigo who designed and implemented the search strategies.
Appendices
Appendix 1. MEDLINE search strategy
1. health education/
2. consumer health information/
3. patient education as topic/
4. health promotion/
5. counseling/
6. information dissemination/
7. information services/
8. (inform* adj5 (patient* or client* or consumer* or user* or carer* or caregiver* or care giver*)).tw.
9. drug information services/
10. (drug information or medical information or (health adj (education or information or communication or promotion))).tw.
11. ((educat* or instruct* or advis* or advice* or counsel* or teach* or train* or coach* or learn*) and (patient* or client* or consumer* or user* or carer* or caregiver* or care giver*)).tw.
12. or/1‐11
13. exp educational technology/
14. communications media/
15. medical informatics/
16. information systems/
17. exp computers/
18. software/
19. online systems/
20. user computer interface/
21. computer graphics/
22. Internet/
23. (Internet or web or website*).tw.
24. video recording/
25. hypermedia/
26. computer assisted instruction/
27. (computer adj (assisted or based or mediated or generated)).tw.
28. computeri#ed.tw.
29. (interactive adj3 (program* or software or online or on‐line or media or technolog* or communication or health*)).tw.
30. video games/
31. videoconferencing/
32. (multimedia or multi media or video* or dvd* or film? or television or cd‐rom* or animation or audiovisual* or audio visual*).tw.
33. ((audio* or cassette* or tape? or compact dis* or cd or cds or radio or phone? or telephone? or iphone? or helpline*) and (visual or print* or written or text or pamphlet* or leaflet* or flyer* or brochure* or booklet* or publication* or card? or graphic* or picture* or image* or pictorial* or pictogram* or illustrat* or computer* or software or program* or online or on‐line or email* or mail* or letter*)).tw.
34. telemedicine/
35. (telehealth or telemedicine or telepharmac* or e‐health or ehealth or e‐pharmac*).tw.
36. or/13‐35
37. exp drug therapy/
38. drug therapy.fs.
39. exp pharmaceutical preparations/
40. exp pharmaceutical services/
41. exp therapeutic uses/
42. (medication* or medicines or medicament* or drug? or pharmac* or prescription? or over the counter).tw.
43. or/37‐42
44. 12 and 36 and 43
45. randomized controlled trial.pt.
46. controlled clinical trial.pt.
47. randomized.ab.
48. placebo.ab.
49. drug therapy.fs.
50. randomly.ab.
51. trial.ab.
52. groups.ab.
53. or/45‐52
54. exp animals/ not humans.sh.
55. 53 not 54
56. 44 and 55
Appendix 2. CENTRAL search strategy
| ID | Search | Hits |
| #1 | "health education":kw | 2979 |
| #2 | ((patient or client) next education):kw | 5159 |
| #3 | "health promotion":kw | 2608 |
| #4 | counseling:kw | 2856 |
| #5 | ((patient or medical or health or drug) next information):kw | 216 |
| #6 | (information next (service or dissemination)):kw | 301 |
| #7 | (inform* near (patient or client or consumer or user or carer or caregiver or care‐giver)):ti,ab | 3679 |
| #8 | ("drug information" or "medical information" or (health next (education or information or communication or promotion))):ti,ab | 2325 |
| #9 | ((educat* or instruct* or advis* or advice* or counsel* or teach* or train* or coach* or learn*) and (patient or client or consumer or user or carer or caregiver or care‐giver)):ti,ab | 20956 |
| #10 | teaching:kw | 2177 |
| #11 | learning:kw | 3346 |
| #12 | "education program":kw | 335 |
| #13 | (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12) | 36691 |
| #14 | MeSH descriptor Educational Technology explode all trees | 1749 |
| #15 | "educational technology" | 49 |
| #16 | ((communication or telecommunication or mass or instructional) next media):kw | 200 |
| #17 | "mass communication":kw | 4 |
| #18 | ((information or online or on‐line) next system):ti,ab,kw | 534 |
| #19 | "medical informatics":kw | 58 |
| #20 | MeSH descriptor Computers explode all trees | 816 |
| #21 | "computers":kw | 594 |
| #22 | microcomputers:kw | 230 |
| #23 | (computer next (program or interaction or interface or graphics)):kw | 782 |
| #24 | (computer next (system or network or terminal)):kw | 215 |
| #25 | MeSH descriptor Software, this term only | 558 |
| #26 | hypermedia:ti,ab,kw | 7 |
| #27 | (internet or web or website):ti,ab,kw | 2357 |
| #28 | "computer assisted instruction":kw | 593 |
| #29 | (computer next (assisted or based or mediated or generated)):ti,ab | 2048 |
| #30 | computeri?ed:ti,ab,kw | 3031 |
| #31 | (interactive near/3 (program or software or online or on‐line or media or technolog* or communication or health*)):ti,ab | 203 |
| #32 | ((video or computer) next games):ti,ab,kw | 143 |
| #33 | teleconferencing | 42 |
| #34 | (multimedia or multi‐media or video* or dvd* or film* or television or cd‐rom* or animation or audiovisual* or audio‐visual*):ti,ab,kw | 6760 |
| #35 | ((audio* or cassette or tape or compact dis* or cd or cds or radio or phone* or telephone* or iphone or helpline) and (visual or print* or written or text or pamphlet or leaflet or flyer or brochure or booklet or publication or card or graphic* or picture* or image* or pictorial* or pictogram or illustrat* or computer or software or program* or online or on‐line or email* or mail* or letter)):ti,ab,kw | 3611 |
| #36 | (telehealth or telemedicine or telepharmac* or e‐health or ehealth or e‐pharmac*):ti,ab,kw | 926 |
| #37 | (#14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36) | 18970 |
| #38 | (#13 AND #37) | 5126 |
| #39 | MeSH descriptor Drug Therapy explode all trees | 96760 |
| #40 | dt:kw | 19082 |
| #41 | MeSH descriptor Pharmaceutical Preparations explode all trees | 47140 |
| #42 | MeSH descriptor Pharmaceutical Services explode all trees | 1237 |
| #43 | MeSH descriptor Therapeutic Uses explode all trees | 199748 |
| #44 | (medication or "medicines" or medicament or drug or pharmac* or prescription or "over the counter"):ti,ab,kw | 289749 |
| #45 | (#39 OR #40 OR #41 OR #42 OR #43 OR #44) | 323806 |
| #46 | (#38 AND #45) | 1038 |
| #47 | (#46) ……….[in Clinical Trials] | 932 |
Appendix 3. EMBASE search strategy
1. health education/
2. patient education/
3. health promotion/
4. consumer health information/
5. counseling/
6. directive counseling/
7. patient counseling/
8. patient guidance/
9. patient information/
10. information dissemination/
11. information service/
12. (inform* adj5 (patient* or client* or consumer* or user* or carer* or caregiver* or care giver*)).tw.
13. drug information/
14. medical information/
15. (drug information or medical information or (health adj (education or information or communication or promotion))).tw.
16. teaching/
17. ((educat* or instruct* or advis* or advice* or counsel* or teach* or train* or coach* or learn*) and (patient* or client* or consumer* or user* or carer* or caregiver* or care giver*)).tw.
18. or/1‐17
19. educational technology/
20. exp audiovisual equipment/
21. videorecording/
22. hypermedia/
23. mass communication/
24. mass medium/
25. exp telecommunication/
26. medical informatics/
27. information system/
28. medical information system/
29. exp computer/
30. computer program/
31. computer graphics/
32. human computer interaction/
33. computer interface/
34. online system/
35. internet/
36. (internet or web or website*).tw.
37. (computer adj (assisted or based or mediated or generated)).tw.
38. computeri#ed.tw.
39. (interactive adj3 (program* or software or online or on‐line or media or technolog* or communication or health*)).tw.
40. videoconferencing/
41. (multimedia or multi media or video* or dvd* or film? or television or cd‐rom* or animation or audiovisual* or audio visual*).tw.
42. ((audio* or cassette* or tape? or compact dis* or cd or cds or radio or phone? or telephone? or iphone? or helpline*) and (visual or print* or written or text or pamphlet* or leaflet* or flyer* or brochure* or booklet* or publication* or card? or graphic* or picture* or image* or pictorial* or pictogram* or illustrat* or computer* or software or program* or online or on‐line or email* or e‐mail* or letter*)).tw.
43. (telehealth or telemedicine or telepharmac* or e‐health or ehealth or e‐pharmac*).tw.
44. or/19‐43
45. exp drug therapy/
46. dt.fs.
47. exp drug/
48. exp pharmaceutics/
49. exp pharmacology/
50. exp "drug use"/
51. (medication* or medicines or medicament* or drug? or pharmac* or prescription? or over the counter).tw.
52. or/45‐51
53. 18 and 44 and 52
54. exp controlled clinical trial/
55. single blind procedure/ or double blind procedure/
56. crossover procedure/
57. random*.tw.
58. placebo*.tw.
59. ((singl* or doubl*) adj (blind* or mask*)).tw.
60. (crossover or cross over or factorial* or latin square).tw.
61. (assign* or allocat* or volunteer*).tw.
62. or/54‐61
63. nonhuman/
64. 62 not 63
65. 53 and 64
Appendix 4. CINAHL search strategy
| Query | Limiters/Expanders | |
| S50 | s49 | Limiters ‐ Exclude MEDLINE records Search modes ‐ Boolean/Phrase |
| S49 | s38 and s48 | Search modes ‐ Boolean/Phrase |
| S48 | S39 or S40 or S41 or S42 or S43 or S44 or S45 or S46 or S47 | Search modes ‐ Boolean/Phrase |
| S47 | TI (singl* or doubl* or tripl* or trebl*) and TI (blind* or mask*) | Search modes ‐ Boolean/Phrase |
| S46 | AB (singl* or doubl* or tripl* or trebl*) and AB (blind* or mask*) | Search modes ‐ Boolean/Phrase |
| S45 | AB (random* or trial or groups or placebo*) or TI (random* or trial or groups or placebo*) | Search modes ‐ Boolean/Phrase |
| S44 | MH Quantitative Studies | Search modes ‐ Boolean/Phrase |
| S43 | MH Placebos | Search modes ‐ Boolean/Phrase |
| S42 | MH Random Assignment | Search modes ‐ Boolean/Phrase |
| S41 | MH Clinical Trials+ | Search modes ‐ Boolean/Phrase |
| S40 | PT Clinical Trial | Search modes ‐ Boolean/Phrase |
| S39 | randomi?ed controlled trial* | Search modes ‐ Boolean/Phrase |
| S38 | s9 and s32 and s37 | Search modes ‐ Boolean/Phrase |
| S37 | s33 or s34 or s35 or s36 | Search modes ‐ Boolean/Phrase |
| S36 | medication* or drug* or pharmac* or medicines or prescription* | Search modes ‐ Boolean/Phrase |
| S35 | MH drugs+ | Search modes ‐ Boolean/Phrase |
| S34 | MW drug therapy | Search modes ‐ Boolean/Phrase |
| S33 | MH drug therapy+ | Search modes ‐ Boolean/Phrase |
| S32 | s10 or s11 or s12 or s13 or s14 or s15 or s16 or s17 or s18 or s19 or s20 or s21 or s22 or s23 or s24 or s25 or s26 or s27 or s28 or s29 or s30 or s31 | Search modes ‐ Boolean/Phrase |
| S31 | telehealth or telemedicine or telepharmac* or e‐health or ehealth or e‐pharmac* | Search modes ‐ Boolean/Phrase |
| S30 | (audio* or cassette* or tape* or compact dis* or cd or cds or radio or phone* or telephone* or iphone* or helpline*) and (visual or print* or written or text or pamphlet* or leaflet* or flyer* or brochure* or booklet* or publication* or card* or graphic* or picture* or image* or pictorial* or pictogram* or illustrat* or computer* or software or program* or online or on‐line or email* or e‐mail* or letter*) | Search modes ‐ Boolean/Phrase |
| S29 | multimedia or multi media or video* or dvd* or film* or television or cd‐rom* or animation or audiovisual* or audio visual* | Search modes ‐ Boolean/Phrase |
| S28 | interactive | Search modes ‐ Boolean/Phrase |
| S27 | computer assisted or computer based or computer generated or computer mediated or computeri?ed | Search modes ‐ Boolean/Phrase |
| S26 | hypertext or hypermedia | Search modes ‐ Boolean/Phrase |
| S25 | internet or web or website* | Search modes ‐ Boolean/Phrase |
| S24 | user computer interface | Search modes ‐ Boolean/Phrase |
| S23 | computer graphics | Search modes ‐ Boolean/Phrase |
| S22 | computer terminals | Search modes ‐ Boolean/Phrase |
| S21 | MH software | Search modes ‐ Boolean/Phrase |
| S20 | computer program* | Search modes ‐ Boolean/Phrase |
| S19 | MH computer systems | Search modes ‐ Boolean/Phrase |
| S18 | MH health information networks | Search modes ‐ Boolean/Phrase |
| S17 | online system* | Search modes ‐ Boolean/Phrase |
| S16 | MH health information systems | Search modes ‐ Boolean/Phrase |
| S15 | MH information systems | Search modes ‐ Boolean/Phrase |
| S14 | MH health informatics+ | Search modes ‐ Boolean/Phrase |
| S13 | communications media | Search modes ‐ Boolean/Phrase |
| S12 | educational technology | Search modes ‐ Boolean/Phrase |
| S11 | MH "computers and computerization" | Search modes ‐ Boolean/Phrase |
| S10 | MH informatics | Search modes ‐ Boolean/Phrase |
| S9 | s1 or s2 or s3 or s4 or s5 or s6 or s7 or s8 | Search modes ‐ Boolean/Phrase |
| S8 | TI (educat* or instruct* or advis* or advice* or counsel* or teach* or train* or coach* or learn*) and TI (patient* or client* or consumer* or user* or carer* or caregiver* or care giver*) | Search modes ‐ Boolean/Phrase |
| S7 | AB (educat* or instruct* or advis* or advice* or counsel* or teach* or train* or coach* or learn*) and AB (patient* or client* or consumer* or user* or carer* or caregiver* or care giver*) | Search modes ‐ Boolean/Phrase |
| S6 | MH drug information services | Search modes ‐ Boolean/Phrase |
| S5 | MH information services | Search modes ‐ Boolean/Phrase |
| S4 | MH counseling | Search modes ‐ Boolean/Phrase |
| S3 | MH health information+ | Search modes ‐ Boolean/Phrase |
| S2 | MH health promotion | Search modes ‐ Boolean/Phrase |
| S1 | MH health education+ | Search modes ‐ Boolean/Phrase |
Appendix 5. PsycINFO search strategy
1. health education/
2. client education/
3. drug education/
4. health promotion/
5. counseling/
6. information dissemination/
7. information services/
8. (inform* adj5 (patient* or client* or consumer* or user* or carer* or caregiver* or care giver*)).ti,ab,id.
9. (drug information or medical information or (health adj (education or information or communication or promotion))).ti,ab,id.
10. ((educat* or instruct* or advis* or advice* or counsel* or teach* or train* or coach* or learn*) and (patient* or client* or consumer* or user* or carer* or caregiver* or care giver*)).ti,ab,id.
11. or/1‐10
12. exp audiovisual instruction/
13. instructional media/
14. exp audiovisual communications media/
15. communications media/
16. mass media/
17. telecommunications media/
18. exp electronic communication/
19. exp human computer interaction/
20. exp information systems/
21. exp computers/
22. computer software/
23. exp computer assisted instruction/
24. (internet or web or website* or portal*).ti,ab,id.
25. websites/
26. hypermedia/
27. (computer adj (assisted or based or mediated or generated)).ti,ab,id.
28. (interactive adj3 (program* or software or online or on‐line or media or technolog* or communication or health*)).ti,ab,id.
29. teleconferencing/
30. (multimedia or multi media or video* or dvd* or film? or television or cd‐rom* or animation or audiovisual* or audio visual*).ti,ab,id.
31. ((audio* or cassette* or tape? or compact dis* or cd or cds or radio or phone? or telephone? or iphone? or helpline*) and (visual or print* or written or text or pamphlet* or leaflet* or flyer* or brochure* or booklet* or publication* or card? or graphic* or picture* or image* or pictorial* or pictogram* or illustrat* or computer* or software or program* or online or on‐line or email* or mail* or letter*)).ti,ab,id.
32. telemedicine/
33. (telehealth or telemedicine or telepharmac* or e‐health or ehealth or e‐pharmac*).ti,ab,id.
34. or/12‐33
35. exp drug therapy/
36. exp drugs/
37. prescribing drugs/
38. pharmacology/
39. self medication/
40. (medication* or medicines or medicament* or drug? or pharmac* or prescription? or over the counter).ti,ab,id.
41. or/35‐40
42. 11 and 34 and 41
43. random*.ti,ab,hw,id.
44. trial*.ti,ab,hw,id.
45. placebo*.ti,ab,hw,id.
46. ((singl* or doubl* or trebl* or tripl*) and (blind* or mask*)).ti,ab,hw,id.
47. (cross over or crossover or factorial* or latin square).ti,ab,hw,id.
48. (assign* or allocat* or volunteer*).ti,ab,hw,id.
49. treatment effectiveness evaluation/
50. mental health program evaluation/
51. exp experimental design/
52. "2000".md.
53. or/43‐52
54. animal.po.
55. 53 not 54
56. 42 and 55
Appendix 6. ERIC search strategy
KW=(educat* or instruct* or advis* or advice* or counsel* or teach* or train* or coach* or learn* or inform* or promot* or communicat*) AND KW=(multimedia or multi media or video* or dvd* or film* or television or cd‐rom* or animation or audiovisual* or audio visual* or telehealth or telemedicine or telepharmac* or e‐health or ehealth or e‐pharmac* or interactive or computer* or software or media or hypermedia or internet or web or website* or portal* or online or on‐line or informatics or information system* or ((audio* or cassette* or tape* or compact dis* or cd or cds or radio or phone* or telephone* or iphone* or helpline*) and (visual or print* or written or text or pamphlet* or leaflet* or flyer* or brochure* or booklet* or publication* or card* or graphic* or picture* or image* or pictorial* or pictogram* or illustrat* or program* or email* or mail* or letter*))) AND KW=(medication* or medicines or medicament* or drug* or pharmac* or prescription*) AND KW=(random* or trial* or placebo* or assign* or allocat* or volunteer* or crossover or cross over or factorial* or singl* blind* or doubl* blind* or control group* or experimental group* or intervention group* or controlled stud* or clinical stud*)
Appendix 7. ProQuest search strategy
In Advanced Search:
educat* or advis* or teach* or train* or learn* or inform* Citation and abstract
AND
educational technology or multimedia or multi media or video* or dvd* or film* or television or cd‐rom* or audiovisual* or audio visual* or interactive or computer* or software or internet or web or website* or online or on‐line Citation and abstract
AND
medication* or medicines or drug* or pharmac* Abstract
AND
random* or trial* or assign* or allocat* or doubl* blind* Citation and abstract
Data and analyses
Comparison 1. Multimedia education versus usual care or no education.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Knowledge (mean score, %) | 6 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 < 4 weeks | 6 | 817 | Std. Mean Difference (IV, Random, 95% CI) | 1.04 [0.49, 1.58] |
| 1.2 ≥ 4 weeks | 1 | 8 | Std. Mean Difference (IV, Random, 95% CI) | 1.18 [‐0.42, 2.79] |
| 2 Knowledge (N who improved) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3 Skill acquisition (mean score, %) | 2 | 94 | Mean Difference (IV, Random, 95% CI) | 18.32 [11.92, 24.73] |
| 3.1 < 4 weeks | 1 | 34 | Mean Difference (IV, Random, 95% CI) | 20.90 [9.91, 31.89] |
| 3.2 ≥ 4 weeks | 1 | 60 | Mean Difference (IV, Random, 95% CI) | 17.0 [9.12, 24.88] |
| 4 Compliance with medication (% who complied) | 2 | 4552 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.96, 1.08] |
| 4.1 < 4 weeks | 1 | 306 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.95, 1.05] |
| 4.2 ≥ 4 weeks | 1 | 4246 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.97, 1.11] |
| 5 Compliance with monitoring (% who complied) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5.1 ≥ 4 weeks | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Pain rating (mean score, range 0 to 10) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 7 Risk of drug abuse (mean score) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 8 Satisfaction with care (mean score) | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 8.1 < 4 weeks | 2 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 Perception of care (mean score) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 10 Self‐efficacy (mean score) | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 10.1 < 4 weeks | 2 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 11 Beliefs about medication (mean score, range 1 to 5) | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 12 Beliefs about medication (N who answered correctly) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 13 Perception of the education (N who learnt something new) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 2. Multimedia education versus written education.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Knowledge (N who improved) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2 Skill acquisition (mean score, %) | 1 | 20 | Mean Difference (IV, Random, 95% CI) | 31.0 [10.15, 51.85] |
| 3 Skill acquisition (N who improved) | 2 | 164 | Risk Ratio (M‐H, Random, 95% CI) | 2.14 [1.33, 3.44] |
| 4 Beliefs about medication (N who would not inappropriately request Rx) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5 Perception of the education (N who learnt something new) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 3. Multimedia education versus education by a health professional.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Knowledge (mean score, %) | 1 | 22 | Mean Difference (IV, Random, 95% CI) | ‐2.20 [‐8.83, 4.43] |
| 2 Skill acquisition (mean score, %) | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 < 4 weeks | 3 | 130 | Mean Difference (IV, Random, 95% CI) | ‐1.01 [‐15.75, 13.72] |
| 2.2 ≥ 4 weeks | 2 | 130 | Mean Difference (IV, Random, 95% CI) | ‐4.29 [‐17.23, 8.65] |
| 3 Bronchial obstruction (FEV, litres) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 4. Multimedia education versus written education and education by a health professional.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Skill acquisition (N who improved) | 1 | 69 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.70, 1.94] |
Comparison 5. Multimedia education versus control multimedia.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Knowledge (mean score, %) | 2 | 568 | Mean Difference (IV, Random, 95% CI) | 2.78 [‐1.48, 7.05] |
| 2 Skill acquisition (mean change score, %) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.1 < 4 weeks | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 ≥ 4 weeks | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Compliance with medication (mean score, range 18‐90) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.1 ≥ 4 weeks | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Use of medication (N who used medication) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5 Peak flow reading (litres per second) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6 Self‐efficacy (mean score, range 27 to 189) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 7 Quality of life (mean score) | 1 | 269 | Std. Mean Difference (IV, Random, 95% CI) | 0.20 [‐0.04, 0.44] |
| 7.1 Age < 18 | 1 | 221 | Std. Mean Difference (IV, Random, 95% CI) | 0.17 [‐0.09, 0.44] |
| 7.2 Age 18 and over | 1 | 48 | Std. Mean Difference (IV, Random, 95% CI) | 0.31 [‐0.26, 0.88] |
| 8 Beliefs about medication (N with more positive beliefs) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 9 Perceived stress (mean score, range 10 to 50) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 6. Mulitmedia education and a co‐intervention versus co‐intervention alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Knowledge (mean score, %) | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 < 4 weeks | 2 | 380 | Mean Difference (IV, Random, 95% CI) | 24.59 [22.34, 26.83] |
| 1.2 ≥ 4 weeks | 1 | 60 | Mean Difference (IV, Random, 95% CI) | 23.20 [12.82, 33.58] |
| 2 Skill acquisition (mean score, %) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Non‐compliance (mean score, range 0 to 160) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4 Compliance with medication (N) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5 Compliance with follow‐up (N) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 6 Medical complication (N) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 7 Adverse medication behaviour (mean score, range 0‐40) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 7.1 < 4 weeks | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 ≥ 4 weeks | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Self‐efficacy (mean score, range 0 to 5) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 8.1 < 4 weeks | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.2 ≥ 4 weeks | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Acosta 2009.
| Methods |
Aim of study: To determine if a video educational intervention will result in the correct use of a Metered Dose Inhaler (MDI). Aim of the intervention:To demonstrate the steps required for correct inhaler technique to patients with asthma. Study design: Randomised controlled trial with two arms: video demonstrating correct MDI use and video providing information about asthma and its triggers. Study duration: One month. Recruitment process: Patients presenting to St Barnabas Hospital Emergency Department with symptoms related to asthma were invited to participate in the study and enrolled prior to being discharged. Inclusion criteria: Patients over 18 years of age who have had two asthma attacks in the past three months and are able to understand English or Spanish. Exclusion criteria: Pregnant females, Patients with allergies to MDIs, previous participation in the study, co‐morbid conditions such as cardiac, hypertension, diabetes, COPD or end stage renal disease, psychiatric patients unable to sign the consent form and any institutionalised patients (nursing home, group home or incarcerated). Informed consent: Yes (implied in the exclusion criteria). Ethics approval: Yes "The study was approved by the hospital Institutional Review Board (IRB)". |
|
| Participants |
Description: Adults with asthma. Location: St. Barnabas Hospital, Bronx, New York, USA. Number: 133 patients enrolled, 116 completed the study: 62 in the intervention and 54 in the control group. Age: I: 37% 18‐25, 15% 26‐35, 18% 36‐45, 14% 46‐55, 3% > 56. C: 32% 18‐25, 32% 26‐35, 20% 36‐45, 11% 46‐55, 4% > 56 (some groups do not add to 100% due to missing data). Gender: I: 46% male, C: 50% male. Ethnicity: I: 20% Caucasian, 51% Hispanic, 17% African American, 7% other. C: 32% Caucasian, 44% Hispanic, 11% African American, 7% other. Educational level: I: 13% junior high, 45% high school, 40% some college, 11% bachelor's degree or higher. C: 13% junior high, 26% high school, 44% some college, 13% bachelor's degree or higher. Literacy: Not reported. Primary diagnosis being treated: Asthma. Acute or chronic: Chronic. Co‐morbidities: Excluded from study if had co‐morbidities. Treatment: Inhaled medications for asthma. |
|
| Interventions |
Topic of education: Information about correct MDI technique for asthma. Intervention: A 10‐minute video demonstrating steps for correct MDI use. Co‐intervention: None. Comparison intervention: A 10‐minute video explaining how asthma is triggered and different issues faced by patients with asthma but not containing any MDI‐related instruction. |
|
| Outcomes |
Primary outcomes: Assessment of inhaler technique. Secondary outcomes: Peak flow, patient questionnaire regarding the video and their asthma control. Methods of assessing outcomes: Examiner assessed MDI use based on a checklist of 8 individual steps which were scored as correct or incorrect. The authors appear to have converted this to a percentage of correct steps and from this calculated and reported the change score between the pre and post intervention samples. Peak flow was measured using a peak flow meter. The patient questionnaire consisted of five multiple choice questions asking patient to rate how helpful they found the video and whether they would recommend it to family and friends, recall the number of asthma attacks and days they missed from work in the last month and identify where they had learnt to use and MDI for the first time. Reliability and validity of outcome measures: The source of the checklist used to assess MDI technique was not reported. The patient questionnaire appears to have been developed by the author specifically for this study but no information was provided regarding its development or testing. Timing of outcome assessments: Peak flow and MDI use were measured immediately pre and post intervention and one month after the intervention. The patient questionnaire was completed only at the one month follow‐up. Follow‐up of non‐respondents: Not reported. Adverse effects of the intervention: Authors note that the education may lead to the patient becoming "overconfident of knowing how to use the MDI and possibly get hurt if not used appropriately when the patient experiences an asthma attack". |
|
| Sources of funding | Grant from the New York College of Osteopathic Medicine of New York. | |
| Consumer involvement | None specified. | |
| Notes | Results from the questionnaire assessing patients' perception of the intervention and their asthma control were not included in the review as a single combined result of all participants was reported which did not allow comparison between the study arms. Results of peak flow were reported with 95% confidence intervals. These were converted to SD for the meta‐analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided other than a statement that patients were randomised into an experimental or control group. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | The assessor was blinded to participant group assignment "The examiner was blinded to the intervention". No information was provided about patient blinding. Assessments made immediately before and after the intervention had minimal potential for the lack of blinding influencing outcomes. However, assessments were also performed a month later which raises the possibility that the lack of blinding may influence the outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 133 patients were enrolled in the study and 116 completed it: 54/63 in the intervention arm and 62/70 in the control arm. The authors did not report the reasons for patients not completing the study. However, as the proportion of patients excluded from analyses was similar in both groups, we do not expect the analyses to favour one group. |
| Selective reporting (reporting bias) | Unclear risk | Although, mean inhaler technique scores were not reported for any time point, change scores (from baseline) were reported. Only inhaler technique scores were specified as outcomes in the study methods. Peak flow was measured at all three time points but only the results for measurements made immediately pre and post intervention were reported. The post‐intervention peak flow score was "controlled for pre‐peak flows" which were higher in the control group than the intervention group although this difference was reported as not statistically significant (P = 0.18). The results from the patient questionnaire were only reported as the number and percent of all participants (in both the intervention and control groups) selecting each response therefore no comparison could be made between the groups. |
| Other bias | Low risk | There was no selection bias as the video was delivered on site using the available facilities. |
Deitz 2011.
| Methods |
Aim of study: To test the effectiveness of a web‐based program designed to prevent the misuse of psychoactive prescription drugs in a population of working women. Aim of the intervention: To prevent the misuse of psychoactive prescription drugs. Study design: Randomised controlled trial with two arms: multimedia program and wait‐list control group. Study duration: The intervention and assessments occurred over six to eight weeks. Recruitment process: All female employees of the participating hospitals were invited to participate via announcements placed on the hospital intranet, in the wellness departments of the hospitals and in the staff cafeteria. Inclusion criteria: Female employees of the participating hospitals who had access to a computer with Internet at work and/or at home. Exclusion criteria: None reported. Informed consent: Yes: "Women interested in participating in the study were told about the nature of the study and were asked to read and sign a consent document describing requirements of participation." Ethics approval: Not reported. |
|
| Participants |
Description: Female employees of participating hospitals. Location: Two hospitals in West Virginia and Ohio, USA. Number: 362 enrolled in the study and completed the pretest survey (185 in the intervention group and 177 in the control group) while 346 completed the posttest (181 in the intervention group and 165 in the control group). Age: Range 21 to 75 years, mean 44 years. Gender: Female. Ethnicity: Not reported. Educational level: 83% had at least some college experience. Literacy: Not reported. Primary diagnosis being treated: Not applicable. Acute or chronic: Not applicable. Co‐morbidities: Not applicable. Treatment: At baseline 28% were using antidepressants, 22% analgesics, 16% tranquillizer medications, 9% sedatives and 3% stimulants. |
|
| Interventions |
Topic of education: Information about medications with abuse potential, proper administration of these medications, avoidance of drug use and dependence and non‐drug options for managing health issues. Intervention: Web‐based multimedia program. Co‐intervention: None. Comparison intervention: No education. |
|
| Outcomes |
Primary outcomes: Knowledge, physician‐patient interaction and self‐efficacy for pharmaceutical adherence and managing medication‐related problems. Secondary outcomes: Self‐reported use of pharmaceutical medications for non‐medical purposes, patients' perception of their drug use and patients' perception of the multimedia program. Methods of assessing outcomes: Online SmartRx questionnaire consisting of multiple measures. These included the following established instruments: the Purdue Pharmacist Directive Guidance Scale (measuring patient perception of the directive guidance given by doctors and pharmacists); Patient Feedback Survey (measuring the therapeutic alliance between patient and caregiver and patient satisfaction with treatment); sections of the National Survey on Drug Use and Health (NSDUH) (patient self‐reported use of pharmaceutical medications for non‐medical purposes); the five‐item version of the Perceived Efficacy in Patient‐Physician Interactions (PEPPI) (measures patient confidence in their ability to communicate with their physicians and get them to address their medical concerns); and the CAGE for Prescription Medications (measures patient perceptions about their current drug use). The authors also developed four instruments specifically for this study: the Knowledge of Prescription Drug Abuse and Dependency scale consisting of true/false and multiple choice items constructed to measure knowledge from the multimedia program and organised into three sub‐scales according to the relevant section from the program; the Treatment Seeking Self‐Efficacy and the Confidence in Ability to Address Drug Problems scales were "designed to assess whether individuals feel that they have the information, personal knowledge and skills necessary to adequately address problems from the administration of prescriptions when they arise". The scales consist of items with four to five‐point response scales; Reactions to the Program contained items assessing the degree to which the program was clear, informative, interesting, useful, accurate, comprehensive etc. Reliability and validity of outcome measures: The knowledge scale developed by the authors was based on actual content provided in the multimedia program and was reported to have "high face validity" but no information was provided as to how this was determined. No further information about its validity or reliability was reported. The Cronbach's alpha for two scales developed by the authors was reported: Treatment Seeking Self‐Efficacy alpha = 0.94, Confidence in Ability to Address Drug Problems alpha = 0.78. The authors also reported the Cronbach's alpha for some of the established instruments that they used: Purdue Pharmacist Directive Guidance Scale alpha = 0.89; Patient Feedback Survey alpha = 0.95; PEPPI alpha = 0.94; CAGE alpha = 0.63. Timing of outcome assessments: Participants completed the SmartRx questionnaire after a 4‐week period in which patients in the intervention group had access to the multimedia program. Follow‐up of non‐respondents: Participants were given two weeks to complete the pretest survey and were sent reminders every few days with information about the deadline. Four email reminders were sent out during the four week period encouraging participants to view the multimedia program. No follow‐up of post‐test survey non‐respondents was reported (although the authors may have used the same reminder system as for the pretest survey). Adverse effects of the intervention: Not reported. |
|
| Sources of funding | Funded from a NIDA grant. | |
| Consumer involvement | None reported. | |
| Notes | Results from the Reactions to Program measure were not included in the review as they were only measured in the multimedia group therefore there was no control group. The study reported results from each of the three sub‐scales of the knowledge questionnaire separately. Results from the Drug Facts sub‐scale was used for the meta‐analysis based on consensus opinion of the review authors that this was the most representative of medication knowledge. The Drug Facts sub‐scale measures knowledge about the pharmaceutical properties of the medications (e.g. therapeutic action, side effects, guidelines for self‐administration) while the other two sub‐scales measure knowledge about drug abuse and dependence (Smart Use) or non‐pharmaceutical alternatives to medications (Managing one's Health). Three separate measures were used to examine self‐efficacy in performing three different health‐related tasks: taking medications as prescribed (medication adherence); managing medication problems; obtaining medical information and attention for medical concerns from physicians. Data from the medication adherence self‐efficacy scale were extracted for the meta‐analysis by consensus opinion of the review authors as its content was thought to be the most clinically relevant. The authors reported different number of participants who completed each of the questionnaires but did not specify the numbers in the intervention and control group separately. For the purposes of the meta‐analysis, the same proportion of participants was assumed to have been missing from both groups. Non medical use of prescription medications is reported in the text but not included in the meta‐analysis as results are presented for each medication group separately with no overall incidence rate. The authors were contacted to request the denominators for the outcome measures but were unable to provide this information. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were "randomly assigned" to an experimental or control group. The methods used for sequence generation were not reported. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No information was provided regarding blinding of participants or investigators. Participants were free to access further information which raises the possibility that any lack of patient blinding may influence the outcomes. Assessments consisted of questionnaires testing both relatively objective (knowledge) and subjective (self‐efficacy) outcomes. However, the use of questionnaires minimised the potential for the lack of blinding of assessors influencing outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 362 participants were enrolled in the study and completed the pre‐test survey (185 in the intervention group and 177 in the control group) while 346 (94%) completed at least some of the post‐test survey (181 in the intervention group and 165 in the control group). Although there were more non‐respondents in the control group (7% versus 2%) the overall number of non‐respondents was small therefore we do not expect the analyses to favour one group. Greater numbers did not complete certain sections of the post‐test survey. For example, 329/362 (91%) completed the knowledge questionnaire. The authors did not report the number of respondents for the control or intervention group therefore it is not known whether the number of non‐respondents was equivalent across both groups. |
| Selective reporting (reporting bias) | Low risk | All of the outcomes outlined in the methods section were reported. |
| Other bias | Low risk | There was no selection bias as participants in both the intervention and control arm were required to have access to the Internet either at home and/or work. |
Goodyer 2006.
| Methods |
Aim of study: To evaluate two interventions providing the same information to patients of Turkish origin: a translated patient information leaflet plus verbal support from a translator and a multimedia touch screen system (MTS) to determine if the MTS system reduces the need for translator support. Aim of the intervention: To demonstrate correct inhaler technique to Turkish patients with asthma. Study design: Randomised controlled trial with two arms: multimedia and leaflet followed by verbal support. Study duration: Duration of the recruitment period was not stated. The intervention and assessments occurred on one visit. Recruitment process: Turkish‐speaking patients were initially recruited from general practice surgeries in Camden but only 10 from 100 eligible patients agreed to participate. Recruitment was therefore expanded with potentially eligible participants identified through contacts in social clubs and informal networks. Inclusion criteria: Turkish as a first language, not fluent in English, who had been using an MDI for more than three months. Exclusion criteria: None specified. Informed consent: Yes, "All recruits gave their informed consent to the study". Ethics approval: Yes, "approved by the relevant local ethics committees". |
|
| Participants |
Description: Turkish speaking patients with asthma. Location: Camden and Tower Hamlets, London, England. Number: 69 patients were recruited with 34 randomised to the intervention and 35 to the comparison group. Age: Mean 42 years (SD 17.5). Gender: 74% female, 26% male. Ethnicity: Turkish. Educational level: Not reported. Literacy: Over 80% were literate in Turkish. Primary diagnosis being treated: Asthma. Acute or chronic: Chronic. Co‐morbidities: None reported. Treatment: Inhaled medications for asthma. |
|
| Interventions |
Topic of education: Information about correct inhaler technique for asthma. Intervention: A multimedia touch screen system showing video clips demonstrating inhaler technique with Turkish voice‐over instruction. Co‐intervention: None. Comparison intervention: A translated (into Turkish) patient information leaflet followed by verbal support from a translator who identified areas where the patient's technique could be improved and then spent up to 15 minutes discussing this with the patient. |
|
| Outcomes |
Primary outcomes: Assessment of inhaler technique. Secondary outcomes: None. Methods of assessing outcomes: Video recordings of patients demonstrating their inhaler technique were assessed by two pharmacists with experience in respiratory medicine. The assessors gave an overall rating (poor, adequate and good) and a more detailed assessment based on a checklist of eight individual steps which were scored as correct or incorrect. Inhaler shaking counts and length of inspiration were also recorded. The authors reported the number of participants whose global technique improved, deteriorated or stayed the same compared with baseline as well as the number who performed individual steps correctly. Reliability and validity of outcome measures: Intra‐rater and inter‐rater reliability was measured for the two assessors using a sample of 12 patients chosen to illustrate the range of good and poor techniques that were each rated twice by both of the assessors. Intra‐rater reliability kappa score = 0.795 P < 0.0001, inter‐rater reliability, median for comparison of all observations, kappa = 0.5 (quartile range 0.32 to 0.75). Timing of outcome assessments: The multimedia group was assessed immediately pre and post intervention. The comparison arm was assessed immediately before and after receiving the written information leaflet and then again after receiving verbal support from a translator. Follow‐up of non‐respondents: Not applicable as all assessments were performed during a single visit. Adverse effects of the intervention: None specified. |
|
| Sources of funding | Funded by the Department of Health and the North London Primary Care Research Network (Nocten). | |
| Consumer involvement | None specified. | |
| Notes | Two studies were reported: one comparing inhaler technique in patients with fluent and poor English and the other testing two educational interventions in the poor English group. Only the results of the second study are relevant to this review. Outcomes were tested immediately before and after the intervention. The control group was evaluated at baseline, after receiving written information and then after receiving additional personal verbal instruction from a translator. The intervention group was only evaluated on two occasions before and after the multimedia education. Results comparing multimedia to written education and multimedia to the combination of written information and education by a health professional were used in separate meta‐analyses. The global inhaler technique rating was chosen as the measure of skill acquisition for the meta‐analysis as it was an overall assessment of the participants' skill and evidence had been provided for its reliability. The rating was converted to a dichotomous outcome: improved (those whose technique rating improved following the intervention) and did not improve (those whose technique rating worsened or stayed the same). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patient allocation was done using random number tables" The investigators used a random component for sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. It is unclear whether the random number table was open or concealed from investigators. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Assessors were blinded to participant group assignment "The assessors did not attend experimental sessions and had no contact with patient volunteers. They were therefore blind to the information format". Patients did not know in advance which information method they would receive. Assessments occurred immediately following the intervention minimising the potential for the lack of blinding influencing outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Although no information was provided regarding missing outcomes it is assumed that they were available from all participants as the study was completed in one visit. |
| Selective reporting (reporting bias) | High risk | The inhaler technique was assessed based on a checklist of 8 steps but only 3 "key steps" were reported. This was not prespecified in the methods. |
| Other bias | Low risk | There was no selection bias as the program was delivered on site using the available facilities. |
Kato 2008.
| Methods |
Aim of study: To determine the effectiveness of a video‐game intervention for improving adherence and other behavioural outcomes for adolescents and young adults with malignancies including acute leukaemia, lymphoma, and soft tissue sarcoma. Aim of the intervention: To improve health‐related behaviours such as treatment adherence, cancer‐related self‐efficacy and knowledge in adolescents and young adults with malignancies. Study design: Multi‐centre randomised trial with two arms: intervention video game and control video game. Study duration: Three months. Recruitment process: Patients were recruited by fliers and staff contact at 34 academic medical centres and community practices in the USA, Canada and Australia. Inclusion criteria: Patients aged between 13 and 29 years, who had a malignancy diagnosis (new or relapsed), undergoing treatment (chemotherapy, radiotherapy or stem cell transplantation) that was expected to last at least four months after enrolment. Exclusion criteria: A history of seizures as a result of photosensitivity (e.g. flashing lights); inability to communicate with study personnel in English, French or Spanish; or inability to follow the study schedule or directions. Informed consent: Yes. "Written informed consent was obtained from adult participants or from a minor's parent or legal guardian". Ethics approval: Yes. "All procedures were approved by local institutional review boards". |
|
| Participants |
Description: Adolescents and young adults with a diagnosis of a malignancy. Location: Unites States, Canada and Australia. Number: 375 were enrolled, 197 were randomised to the intervention arm and 178 to the control group. At the three month follow ups 164/197 were analysed in the intervention group and 140/178 were analysed in the control group. Age N (%): I: 13‐14 years : 71 (36.4), 15‐16 years: 56 (28.7), 17‐18 years 47 (24.1), 19‐29 years: 21 (10.8). C: 13‐14 years: 60 (34.1), 15‐16years 58 (32.9), 17‐18 years: 32 (18.2), 19‐29 years: 26 (14.8). Gender N (%): Male I: 132 (67.7) C: 119 (67.6). Ethnicity N (%): I: White 109 (55.9), Hispanic 42 (21.5), Black/African American 19 (9.7), Mixed 9 (4.6), Asian 4 (2.1), Native American 2 (1), Pacific Islander 0 (0), Decline to answer 6 (3.1) Missing 4 (2.1). C: White 101 (57.4), Hispanic 34 (19.3), Black/African American 15 (18.5), Mixed 6 (3.4) Asian 6 (3.4), Native American 1 (0.6), Pacific Islander 3 (1.7), Decline to answer 2 (1.1), Missing 8 (4.5). Educational level N (%): I: Less than high school 76 (39), High school 81 (14.5), Some college or more 33 (16.9). C: Less than high school 57 (32.4), High school 77 (43.8), Some college or more 33 (18.8). Literacy: Not reported. Primary diagnosis being treated N (%): I: Acute Lymphoblastic Leukaemia 76 (38.9), Acute myelogenous leukaemia 15 (7.7), Hodgkins Lymphoma 19 (9.7), Non‐Hodgkin's Lymphoma 17 (8.7), Brain tumour 14 (7.2), Osteosarcoma 24 (12.3), Ewing Sarcoma 9(4.6), Other 21 (10.8). C: Acute Lymphoblastic Leukaemia 74 (42.1), Acute myelogenous leukaemia 15 (8.5), Hodgkin's Lymphoma 16 (9.1), Non‐Hodgkin's Lymphoma 17 (8.7), Brain tumour 14 (7.9), Osteosarcoma 18 (10.2), Ewing Sarcoma 9 (4.6), Other 20 (11.4). Acute or chronic: Chronic. Co‐morbidities: None reported. Treatment: Cancer regimes. |
|
| Interventions |
Topic of education: Cancer regimes focusing on chemotherapy, antibiotics, antiemetics and stool softeners as well as "managing common treatment‐related adverse effects." Intervention: An interactive video game played on PC which promotes treatment adherence and positive self care behaviours. Co‐intervention: A commercial PC game (Indiana Jones and the Emperor's Tomb). Comparison intervention: The commercial PC game (see above) alone. |
|
| Outcomes |
Primary outcomes: Adherence to prescribed cancer treatment regime (specifically oral chemotherapy and adjunct antibiotic therapy). Secondary outcomes: Patient's self‐efficacy to manage cancer, their knowledge about cancer, their health locus of control, stress and quality of life. Methods of assessing outcomes: Adherence to general cancer treatment was measured in five ways: attendance at clinics, 6‐MP blood assays (indicate adherence to 6‐MP), CDCI (self‐reported measure of adherence to medical treatment‐18 items with responses rated on scale of one to five and total scores ranging from 18 to 90; with higher scores representing greater adherence), MAS (self‐reported measure of general adherence to treatment; zero to four scale, higher scores representing greater adherence) and the Medication Event Monitoring System (measures adherence to antibiotics by using a medication container which contains a microprocessor that records the dates and times the container is opened). Secondary outcomes were measured using six different questionnaires: self efficacy scale (27 items with seven‐point Likert response scale, score range 27 to 189 with higher scores representing greater self‐efficacy), cancer knowledge scale (18 MCQ items, reported as mean percent of correct responses), Multidimensional Health Locus of Control Scale Form C (items rated on scale of one to six, total sub‐scale scores range from 3 to 36, higher scores represent higher locus of control) and the Perceived Stress Scale (items rated on scale of 1‐5, total scores range from 10 to 50 with higher scores representing more stress). Functional status was measured using the Functional Assessment of Cancer Therapy‐General scale (FACT‐G, 0 to 104‐point scale) in those 18 or over and the Paediatric Quality of Life scale (PQL) on those < 18 years. Reliability and validity of outcome measures: Two of the questionnaires used for secondary outcomes were developed specifically for this study. The Self‐Efficacy scale was developed "in accordance with the standard methods for designing self‐efficacy scales." Cronbach's alpha was 0.93. The Cancer Knowledge Scale was also developed specifically for this study "as a measure of patient's knowledge about cancer". No information is given regarding its reliability or validity. The other questionnaires used were established instruments. The authors report the internal consistency for each instrument (Cronbach's alpha) CDCI = 0.83, MSA = 0.57 respectively, PQL = 0.91, FACT‐G = 0.92, the Multidimensional Health Locus of Control Scale Form C = 0.56‐0.77 and the Perceived Stress Scale = 0.85. Timing of outcome assessments: Outcomes were measured at baseline (pre‐intervention), one and three months after the patients were given the video games. Follow‐up of non‐respondents: Not described. Adverse effects of the intervention: There was one adverse event in the control group with a patient reporting dizziness only while playing the control game. This was investigated with no physiological cause found. The patient was withdrawn from the study. |
|
| Sources of funding | The "research was supported by HopeLab Foundation, a non profit organization." One of the investigators was also received grants from the National Cancer Institute, National Institute of Health. | |
| Consumer involvement | Teens and young adults with cancer participated actively throughout the game development process to ensure that the game was fun, and that it really spoke to the issues that they confront every day in their fight against cancer | |
| Notes | Outcomes were measured pre‐intervention and then one and three months following the intervention. Data from the last time point assessed (at three months) were used for the meta‐analysis. Five measures were used in the study to assess the primary outcome of adherence: two objective measures of adherence to medications measured only in the subgroup taking that medication, one objective measure of adherence to clinic visits and two subjective measures of adherence to treatment (CDCI and MAS). CDCI and MAS were measured in all patients and reported in a method that could be analysed. CDCI was chosen as the measure of adherence used in the meta‐analysis as it has better measurement properties (Cronbach's alpha 0.83 compared with 0.57 for the MAS). The quality of life instruments for both age groups were used, with the age groups divided into sub‐groups. Health locus of control was not included in the meta‐analysis as it was a multidimensional instrument with scores given for each dimension but no overall score. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Computer allocation was randomised within sites on the basis of a computerized random‐number generator" The investigators used a random component for sequence generation. |
| Allocation concealment (selection bias) | Low risk | "After baseline assessments, a site associate contacted a study coordinator at a central office, who gave the associate a number indicating a specific computer to be distributed to the participant (i.e. a computer implementing the control or experimental condition)." |
| Blinding (performance bias and detection bias) All outcomes | High risk | Both investigators and participants were blinded, although participants became aware of which video game they had received once they logged onto their computer. "Condition assignment of each participant was concealed from study personnel, but participants became aware of their treatment assignment once they logged onto their assigned computers". Assessments were not made directly after the intervention and participants were free to access further information which raises the possibility that the lack of blinding may influence the outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Of the 375 participants that were randomly assigned at the commencement of the study, four dropped out due to inadequate consent, withdrawal of consent or ineligibility prior to receiving the intervention or control video game (one drop out from intervention group and three from control group). In addition, at the commencement of the study 3/197 patients crossed over from the intervention group to the control group prior to receiving the video game, and 2/178 patient crossed over to the intervention group and received the intervention prior to study commencement. At one month follow up I: 14/197 withdrawals (1 disease progression, 2 other, 1 parent/guardian request to withdraw, 10 participant request to withdraw), C: 23/178 withdrawals (1 adverse event, 1 death. 1 disease progression, 2 other, 3 parent/guardian request to withdraw, 15 participant request to withdraw). At 3 month follow up I: 16/197 withdrawals (1 death, 2 disease progression, 8 other, 5 participant request to withdraw) and one lost to follow up, C: 12/178 withdrawals (2 death, 1 disease progression, 3 other, 6 participant request to withdraw). At the completion of the study, analysis was performed on up to 164/197 of the intervention group and 140/178 of the control group. As the proportion of patients excluded from analyses was similar in both groups (17% intervention versus 21% control), we do not expect the analyses to favour one group. |
| Selective reporting (reporting bias) | Low risk | All outcomes outlined in the methods sectioned were reported. |
| Other bias | Low risk | There was no selection bias as all participants were given a computer to use for playing the video game. |
Kinnane 2008.
| Methods |
Aim of study: 1. To compare recall of information regarding self‐care and side effects in patients who watched a video as part of pre‐chemotherapy education with those who received standard care. 2. To survey patients and evaluate the usefulness of the video in assisting them in managing side effects of chemotherapy in the home setting. Aim of the intervention: To help patients better understand the anticipated side effects of treatment they are undergoing, assist in management of common side effects with a range of self‐help concepts and improve patient safety. Study design: Randomised controlled trial with two arms: videotape and usual care. Study duration: Recruitment occurred between September 2004 and October 2005. Study follow‐up continued until the patient completed chemotherapy or, if chemotherapy was still ongoing, 20 weeks. Recruitment process: Patients referred to the chemotherapy day unit for adjuvant breast or colorectal chemotherapy treatment between September 2004 and October 2005 were identified from referral documentation and medical history. Those meeting inclusion criteria were approached by designated nursing staff and invited to participate in the study. Inclusion criteria: Patients newly diagnosed with cancer, 18 to 75 years old, English as primary language, capable of hearing a normal conversation and viewing videotape, oriented to time and place, living in community setting, had European Co‐operative Oncology Group score of zero to two and either diagnosed with colorectal cancer and scheduled to receive adjuvant weekly 5‐FU and Folinic acid or diagnosed with breast cancer and receiving 5‐FU, Epirubicin and Cyclophosphamide. Exclusion criteria: If they had other serious illnesses or medical conditions that would prohibit the understanding and giving of informed consent/education such as brain metastasis, history of significant neurological or psychiatric disorders including psychotic disorder, a diagnosis of clinical depression, were visually or hearing impaired or unable to complete the questionnaire or if they or any immediate family members had prior chemotherapy for cancer. Informed consent: Yes "all patients who agreed to participate gave informed consent". Ethics approval: Yes "The study received approval by the Research and Ethics Committee associated with our institution". |
|
| Participants |
Description: Patients about to start chemotherapy for breast or colorectal cancer. Location: Monash Medical Centre, Melbourne, Australia. Number: Following dropouts 60 patients remained in the study; 29 in the control and 31 in the intervention group. Age (mean): C: 54.8 years, I: 49 years. Gender: C: 7 male, 22 female, I: 5 male, 26 female. Ethnicity: Not reported. Educational level: C: up to high school 82.8%, Tertiary 17.2% I: Up to high school 80.7%, Tertiary 19.3%. Literacy: Not reported. Primary diagnosis being treated: breast or colorectal cancer. Acute or chronic: Chronic. Co‐morbidities: Not reported. Treatment: Chemotherapy. |
|
| Interventions |
Topic of education: Generic information about chemotherapy and how to manage side effects.The video outlines the most important take home information on dealing with nausea and vomiting, taking anti‐emetics, monitoring for and reporting signs of infection, low platelet count and anaemia, mouth care, advice concerning eating well and drinking fluids, prevention and control of constipation and diarrhoea. Intervention: An educational video about managing chemotherapy related side effects Co‐intervention: Standard education (see below). Comparison intervention: Standard education ‐ a one hour standardised education session by nursing staff covering how the drugs work, side effects, self‐help concepts such as dietary and fluid intake advice, instructions about mouth care, how and when to take anti‐emetics, monitoring for problems associated with low blood counts and advice on prevention and control of constipation and diarrhoea. Written information with details of chemo regime, side effects, self‐help concepts and guidelines for managing side effects, circumstances in which to contact health professionals and contact details. |
|
| Outcomes |
Primary outcomes: Recall of information contained in the standard and video education (knowledge). Secondary outcomes: Number and content of phone calls from patients to the treatment centre over the study period, patient evaluation of video including rating of satisfaction. Methods of assessing outcomes: Knowledge was tested using a 15‐item MCQ test of information recall and reported as the number of correct responses for each question; nurses recorded the number and content of phone calls. The video was evaluated by the video arm only using a satisfaction questionnaire. This outcome was not included in the review as there was no comparison arm. Reliability and validity of outcome measures: Information recall questions were developed by the study investigators and styled on information provided in the written patient information that was also covered in the video. No testing of the reliability or validity of these questions was reported. Timing of outcome assessments: Patients completed the information recall questionnaire when they attended for their second cycle of chemotherapy (no information was reported regarding the timing of this second visit. It was assumed to be within three to four weeks based on typical cycle frequency for the chemotherapy combinations used). The number and content of telephone calls to the treatment centre were from noted from the first cycle of chemo until 20 weeks or completion of chemo, a questionnaire evaluating the video was administered to patients who had viewed the video at 20 weeks or on completion of chemotherapy. Follow‐up of non‐respondents: Patients completed recall questionnaire when attending for their second cycle of chemo therefore there were no non‐respondents. Adverse effects of the intervention: Not reported |
|
| Sources of funding | None reported. However the video acknowledged funding and support for the production of the program from the Australian Council for Quality and Safety in Health care. | |
| Consumer involvement | None reported | |
| Notes | No data were able to be extracted for the meta‐analysis as knowledge was reported as the number of correct responses for each question in the knowledge test with no overall score. The study authors were contacted to request mean knowledge results but responded that this information was not available. Results regarding the number of phone calls made by patients to the chemotherapy centre during the study period were reported in the text but not included in the meta‐analysis. Satisfaction with the education was only measured in the multimedia group and was not included in the review as there was no control group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A randomised schedule of allocation sequence was generated by computer." The investigators used a random component for sequence generation. |
| Allocation concealment (selection bias) | Low risk | "Each patient allocation was marked, folded and then placed in a sealed envelope and allocation was provided by an independent person working in the department without knowledge of the patient or their medical history. Allocations were consecutive and the date and allocation time were recorded on the Patient Case Form" |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No information was provided regarding blinding of participants or investigators. Assessments were not made directly after the intervention and participants were free to access further information which raises the possibility that patients not being blinded may influence the outcome despite the outcome measure being a multiple choice information recall questionnaire which is relatively objective. The use of questionnaires minimised the potential for the lack of blinding of assessors influencing outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3/67 patients approached declined to participate in study (before randomisation) as felt overwhelmed by their situation. Following randomisation 3/32 patients withdrew from the control arm; two due to toxicity leading to treatment discontinuation and one mistakenly recruited outside the age range. 1/32 withdrew from the intervention arm due to progressive disease leading to change in treatment. One patient in the intervention arm did not complete the video evaluation because they were inadvertently not given the evaluation form. Results were calculated based on the participants remaining in the study at that time point. Some recall questions and questions in the video evaluation form were not answered by all respondents. No reason was given for the missing data. Percentage correct scores were calculated using the denominator of the number of respondents who had answered each question. As the proportion of patients excluded from analyses was similar in both groups we do not expect the analyses to favour one group. |
| Selective reporting (reporting bias) | Low risk | All of the outcomes outlined in the methods section were reported. |
| Other bias | Low risk | There was no selection bias as the program was delivered on site using the available facilities. |
Knoerl 1999.
| Methods |
Aim of study: To compare the effectiveness of a structured preoperative teaching program to that of the usual informal teaching provided by the hospital staff in alleviating postoperative pain through more effective use of Patient Controlled Analgesia (PCA) therapy. Aim of the intervention: To educate patients about PCA therapy in order to alleviate post‐operative pain through more effective use of PCA therapy. Study design: Randomised control trial with two arms: video and usual care. Study duration: 24 hours. Recruitment process: Patients attending an Ambulatory Surgical Unit who were scheduled for a surgical procedure that day were asked to participate. Inclusion criteria: Patient prescribed PCA therapy using meperidine or morphine for post operative pain for greater than 12 hours, 18 to 67 years, ability to read and speak English, American Society of Anaesthesiology (ASA) physical status class I and II, general anaesthesia for the surgical procedure. Exclusion criteria: Patients who were health care providers that had used the PCA device in patient care, Patients who had a prior history of using PCA therapy. Informed consent: Yes "informed consent was obtained prior to patient's inclusion in the trial." Ethics approval: Yes "approval for this study was granted by the institutional review board". |
|
| Participants |
Description: Patients being admitted to the Ambulatory Surgical Unit for a hospital based surgical procedure. Location: "Mid‐Western State" in USA. Number: 76 patients; 38 in the control group and 38 in the intervention group. Age mean (SD): I: 46.6 (8.6), C: 43.8 (8.7). Gender N (%): I: female 33 (78.9), male 5 (13.2), C: female 30 (78.9), male 8 (21.1). Ethnicity N (%): I: African‐American 15 (39.5), Caucasian 20 (52.6), Hispanic 3 (7.9), Other 0 (0), C: African‐American 14 (36.8), Caucasian 20 (52.6), Hispanic 3 (7.9), Other 1 (1.3). Educational level (mean years (SD)): I: 15.2 (2.7), C: 14.5 (2.7). Literacy: Not reported. Primary diagnosis being treated N (%): I:General Surgery 7 (18.4), Orthopaedic 8 (21.1), Neurosurgery 2 (5.3), Gynaecology 14 (36.8), Urogynecology 5 (13.2), Plastics 1 (2.6), ENT 1 (2.6), C: General Surgery 5 (13.2), Orthopaedic 5 (13.2), Neurosurgery 4 (10.5), Gynaecology 21 (55,3), Urogynecology 3 (7.9), Plastics 0 (0), ENT 0 (0). Acute or chronic: Acute. Co‐morbidities: Not reported. Treatment: Patient controlled analgesia. |
|
| Interventions |
Topic of education: Patient Controlled Analgesia (PCA) Intervention: An instructional video on the use of PCA . Co‐intervention: At the completion of the video participants were able to practice pressing a control button on a PCA device. Comparison intervention: Usual care. |
|
| Outcomes |
Primary outcomes: Knowledge, attitudes towards pain medication, pain intensity, satisfaction with pain management. Secondary outcomes: Amount of analgesic consumed, incidence of narcotic related side effects. Methods of assessing outcomes: A six‐item questionnaire with agree/disagree/don't know response options measured overall knowledge (reported as mean score (% correct)) and another six‐item scale measured attitudes to using narcotic medication to relieve pain (reported as mean score (% who chose the desired option)). An 11‐point numeric rating scale was used to assess pain intensity (0 = no pain and 10 = worst possible pain) and an analgesic data form to record total amount of analgesic used. Satisfaction was measured using a six‐point Likert scale 1 = very satisfied and 6 = very dissatisfied. Reliability and validity of outcome measures: The questionnaire assessing patient knowledge about how to use PCA therapy, was developed from a literature review on the subject and evidence for content validity was provided by two experts in the field of pain management evaluating the appropriateness of each test item. The questionnaire assessing patient attitude towards pain medication was "derived from Ward's Barrier's Questionnaire" by selecting only items that were relevant to acute pain management. It was also evaluated for content validity by two experts. Both scales were pilot tested over a two week period in the target population for evidence of face validity. The authors state that the 11‐point numerical rating scale used to measure pain intensity "is both valid and reliable compared with other types of pain measurement instruments." The patient outcome questionnaire looking at patient satisfaction was "recommended by the Quality Assistance Committee of the American Pain Society". Timing of outcome assessments: All patients performed a baseline pretest looking at their knowledge of, and attitude towards PCA's. Knowledge and attitudes were tested again four hours after PCA therapy was initiated. Satisfaction and pain intensity scores were also considered at 4, 8 and 24 hours (or whenever PCA therapy was discontinued) after PCA initiation. Follow‐up of non‐respondents: Not applicable as all assessments were performed during the one admission. Adverse effects of the intervention: Not reported |
|
| Sources of funding | None stated | |
| Consumer involvement | None stated | |
| Notes | The last time point reported for each outcome was used for the meta‐analysis. This was four hours post PCA initiation for PCA knowledge and attitudes and 24 hours post PCA initiation or when PCA was discontinued for the other outcomes. For the meta‐analysis, the scale for patient satisfaction was reversed so that a higher score represented greater satisfaction. The incidence of narcotic related side effects is reported in the text but not included in the meta‐analysis as results are presented as the number in each arm who developed each side effect with no overall incidence rate. Parameters relating to analgesics used in the postoperative period are also reported in the text but not included in the meta‐analysis as no data were provided by the authors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Participants were "randomly assigned into treatment groups according to their order of admission into the Ambulatory Surgery Unit." Sequence generation used a non‐random approach using order of hospital admission. |
| Allocation concealment (selection bias) | High risk | Randomisation was based on the order in which participants were admitted to hospital. This method would allow allocation to be foreseen. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No information was provided regarding blinding of participants or assessors. All outcomes were measured via questionnaires. Although knowledge is a relatively objective outcome, the others including patients satisfaction, beliefs about pain medications and perception of pain are subjective. The use of questionnaires minimised the potential for the lack of blinding of assessors influencing outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | It appears that patients were randomised preoperatively but the decision to treat patients with post‐operative PCA was made in the perioperative period. Of the 157 patients who were initially considered for this study "78/157 were not eligible because they were not prescribed PCA therapy for their post‐operative pain management", 2/157 refused consent preoperatively and 1/157 was unable to participate due to severe postoperatively nausea and vomiting. Thus a total of 76 patients were retained in the study, 38 in each arm. As the proportion of patients excluded from analyses was similar in both groups we do not expect the analyses to favour one group. |
| Selective reporting (reporting bias) | Low risk | All outcomes identified in the methods section were reported. |
| Other bias | Low risk | There was no selection bias as the program was delivered on site using the available facilities. |
Lirsac 1991.
| Methods |
Aim of study: To test the efficacy of two learning materials, comparing the quality of inhalation and the level of lung obstruction, before and after education by a standardised instruction card or by watching a film, in asthmatic patients using metered dose inhalers daily, but not knowing how to inhale correctly. Aim of the intervention: To improve metered dose inhaler technique. Study design: Randomised controlled trial with 3 arms: videotape, education by a doctor and a positive control group who received an extended videotape providing instructions on the use of a spacer. The positive control arm was not included in this review. Study duration: 15 days. Recruitment process: Not explicitly stated but the authors thank doctors from the Perpignan respiratory centre for enrolling patients in the study. Inclusion criteria: Patients with asthma (characterised by paroxysmal dyspnoea with wheeze and by reversible lung obstruction demonstrated by Forced Expiratory Volume (FEV) in the previous year) who spoke and understood French, were cooperative, were using MDIs daily but did not know how to use them correctly (defined as an inhalation score greater than one) and were not familiar with the use of spacers. Exclusion criteria: None reported. Informed consent: Not reported. Ethics approval: Not reported. |
|
| Participants |
Description: Patients with asthma who were using MDIs daily but not using them correctly. Location: Perpignan, France. Number: 45 patients participated in the study: 14 in the video arm, 14 in the education from a doctor arm and 17 in the positive control group. The positive control arm was not included in this review. Age (mean years (SD)): video 35 (19), doctor 48 (17). Gender: video: 7/14 male, doctor: 11/14 male. Ethnicity: Not reported. Educational level: Not reported. Literacy: Not reported. Primary diagnosis being treated: Asthma. Acute or chronic: Chronic. Co‐morbidities: Not reported. Treatment: MDIs containing terbutaline. |
|
| Interventions |
Topic of education: Information about the correct use of MDIs for asthma. Intervention: A video demonstrating the correct use of a MDI. Co‐intervention: None. Comparison intervention: Verbal instruction from a doctor reading from an instruction sheet while the patient looks at pictures. |
|
| Outcomes |
Primary outcomes: Assessment of inhaler technique. Secondary outcomes: Assessment of bronchial obstruction. Methods of assessing outcomes: Inhaler technique was assessed using a predetermined scoring system with a maximum score of four, where a lower score represented better inhaler technique. One point was given if each of the following steps was completed incorrectly: 1. expiration; 2. positioning of the inhaler; 3. coordination of release and inspiration; and 4. holding breath for at least 10 seconds after inspiration. Bronchial obstruction was measured before and after using the MDI by measuring the FEV on spirometry. Reliability and validity of outcome measures: Not reported Timing of outcome assessments: Inhaler technique and FEV were measured before and 15 days following the intervention. FEV was measured twice at both time points ‐ before and after using the MDI. Follow‐up of non‐respondents: All participants attended both assessments. Adverse effects of the intervention: Not reported. |
|
| Sources of funding | Not reported. | |
| Consumer involvement | Not reported. | |
| Notes | The positive control arm which received a video about MDIs and spacers was not included in this review. The mean inhaler technique score post education was shown as a graph with no numeric value reported. The score was calculated from the reported baseline and mean change data and the SD was estimated from the graph. To allow comparison with scales used in other studies in the meta‐analysis the score was converted to a score out of 100 with higher scores indicating better inhaler technique. Bronchial obstruction was reported as the FEV measured before and after MDI at baseline and 15 days following the intervention. The FEV measured before MDI was selected for the meta‐analysis as it was judged to be the most clinically meaningful. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were "randomly allocated into one of the three following groups, according to a previously established randomisation code." |
| Allocation concealment (selection bias) | Low risk | The randomisation code was "revealed, for a given patient, just before the education". |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No information was provided regarding blinding of participants or assessors. Assessments of inhaler technique were based on a pre‐determined scoring system but remained subjective. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No participants were reported as having been lost to follow up. |
| Selective reporting (reporting bias) | Low risk | All of the outcomes outlined in the methods section were reported. |
| Other bias | Low risk | Although the site where the video intervention was delivered was not explicitly reported the article implies that it was delivered onsite. There was therefore no selection bias. |
Mazor 2007.
| Methods |
Aim of study: "To determine the relative impact of incorporating narrative evidence, statistical evidence or both into patient education about Warfarin." Aim of the intervention: To provide education for patients taking anticoagulant medication. Study design: Randomised control trial with four arms: narrative evidence video, statistical evidence video, narrative and statistical evidence video and usual care Study duration: Between March and July 2005. Recruitment process: 680 patient medical records were randomly selected from an anticoagulant clinic's patient list. These records were reviewed to find eligible participants. From this group 600 eligible participants were posted out a letter stating they would be mailed a questionnaire unless the patient requested otherwise. Inclusion criteria: Adults receiving treatment at an anticoagulation clinic for at least 3 months prior to recruitment date and who had current active prescriptions for warfarin. Exclusion criteria: Non English speaking, cognitive impairment or medical problems that would preclude participation, or not residing in the area at the time of the study. Informed consent: States that participants were asked to sign a form to permit investigators to access protected patient information. However does not state that written informed consent was obtained for the study. Ethics approval: Yes. "The Institutional Review Board of the University of Massachusetts Medical School reviewed and approved the study protocol". |
|
| Participants |
Description: Adult patients receiving care from an anticoagulation clinic. Location: University of Massachusetts Medical Center, Massachusetts, USA. Number: 317/600 completed the study with 77 in the narrative video group, 82 in the statistical video group, 77 in the combined video group and 90 in the control group. Age N (%): Narrative: < 44 years 0 (0.0), 45‐64 years 14 (17.1), 55‐64 years 19 (23.2), 65‐74 years 13 (15.9), 75 years or older 36 (43.9) missing 0 (0.0). Statistical: < 44 years 8 (11.8), 45‐64 years 9 (13.2), 55‐64 years 14 (20.6), 65‐74 years 11 (16.2), 75 years or older 25 (36.8) missing 1 (1.5). Combined: < 44 years 3 (3.9), 45‐64 years 5 (6.5), 55‐64 years 21 (27.3), 65‐74 years 18 (23.4), 75 years or older 30 (39) missing 0 (0.00). Control: < 44 years 6 (6.7), 45‐64 years 11 (12.2), 55‐64 years 21 (23.3), 65‐74 years 23 (25.6), 75 years or older 27 (30) missing 2 (2.2). Gender N (%): Narrative: Male 55 (65.1), Female 27 (32.9), Missing 0 (0.0). Statistical: Male 42 (61.8), Female 25 (36.8), Missing 1 (1.5). Combined: Male 44 (57.1), Female 33 (42.9), Missing 0 (0.0). Control: Male 55 (61.1) Female 34 (37.8), Control 1 (1.1) Ethnicity N (%): Narrative: American Indian or Alaska Native 0 (0), Asian 0 (0), Black 2 (2.4), Latino 0 (0), White 78 (95.1), Other 1 (1.2), Multiple 1 (1.2), Missing 0 (0.0). Statistical: American Indian or Alaska Native 0 (0), Asian 0 (0), Black 1 (1.5), Latino 0 (0), White 65 (95.6), Other 0 (0.0), Multiple 1 (1.5), Missing 1 (1.5). Combined: American Indian or Alaska Native 0 (0), Asian 1 (1.3), Black 0 (0.0), Latino 2 (2.6), White 71 (92.2), Other 2 (2.6), Multiple 1 (1.3), Missing 0 (0.0). Control: American Indian or Alaska Native 0 (0), Asian 0 (0), Black 1 (1.1), Latino 2 (2.2), White 80 (88.9), Other 3 (3.3), Multiple 2 (2.2), Missing 2 (2.2) Educational level N (%): Narrative: 8th grade or less 4 (4.0), Some high school 8 (9.8), High school graduate 18 (22.0), Some college or a two year degree 22 (26.8), four year college graduate 9 (11.0), More than four year college degree 20 (24.4), Missing 1 (1.2). Statistical: eight grade or less 4 (5.9), Some high school 5 (7.4), High school graduate 19.1 (20), Some college or a two year degree 16 (20.8), four year college graduate 12 (15.6), More than four year college degree 19 (24.7), Missing 1 (1.3). Combined: eight grade or less 4 (5.2), Some high school 5 (6.5), High school graduate 20 (26.0), Some college or a two year degree 16 (20.8), four year college graduate 12 (15.6), More than four year college degree 19 (24.7), Missing 1 (1.3). Control: eight grade or less 1 (1.1), Some high school 11 (12.2), High school graduate 24 (26.7), Some college or a two year degree 28 (31.1), four year college graduate 8 (8.9), More than four year college degree 17 (18.9), Missing 1 (1.1). Literacy: Not reported. Primary diagnosis being treated: Not reported. Acute or chronic: Chronic. Co‐morbidities: Not reported. Treatment: Warfarin. |
|
| Interventions |
Topic of education: Information about Warfarin. Intervention: Three separate videos looking at anticoagulation education. One video used narrative evidence, one video used statistical evidence and one video used both narrative and statistical evidence. Co‐intervention: None. Comparison intervention: Standard care. |
|
| Outcomes |
Primary outcomes: Knowledge and beliefs about warfarin, self‐reported adherence to warfarin, belief that lab testing is important, intent to adhere to lab testing and adherence to lab appointments. Secondary outcomes: None. Methods of assessing outcomes: Warfarin related knowledge was tested by a 22‐item questionnaires with "True", "False" or "Don't Know" response options. Items were scored correct or incorrect and a total score calculated as the percent of correct responses. Self‐reported non‐adherence was based on responses to the five‐point Likert strongly agree‐strongly disagree items "I sometimes take a little extra warfarin when I feel it's was too low" and "I sometimes skip a does of warfarin because I feel better without it. The items were scored dichotomously: patient was judged to be non‐adherent if they agreed or chose the neutral option. Patients' intention to adhere with recommended lab testing was measured using two items with five‐point Likert response options (one to five). Actual attendance for lab monitoring was documented in the 270 (85%) patients who completed the study and consented for their laboratory values to be checked by study staff. This was measured for the 3/12 period following completion of the post‐intervention questionnaire. Beliefs about warfarin and lab testing were measured using four sub‐scales: 1. lab testing is important; 2. warfarin is beneficial; 3. taking warfarin is worrisome; 4. taking warfarin is confusing or difficult. Items used five‐point Likert scale scored one to five with higher scores representing greater belief, less worry and confusion. Reliability and validity of outcome measures: The questionnaire at baseline and post‐intervention was developed by the authors. The questionnaire covers three main areas; warfarin related knowledge, beliefs and adherence. The items were reviewed by two physicians and pre‐tested with four coagulation clinic patients using cognitive interviews. It appears that the psychometric analyses for this questionnaire were carried out on the study sample. The knowledge section consists of 22 items, Cronbach's alpha was 0.79. The beliefs section initially contained 33 items, 8 of which were drawn from the Beliefs About Medication Scale and 25 that were developed by the authors. The authors state that 22 of the 33 items were retained based on their factor loadings and reliability and were found to form five subsets. However only 17 items in 4 subsets are presented and discussed: Cronbach's alpha for each was: beliefs about medication as beneficial (four items) 0.83, beliefs that Warfarin is worrisome (four items) 0.75, belief that taking warfarin is confusing/difficult (five items) 0.70 and for the belief that lab monitoring was important alpha was (four items) 0.72. Adherence was measured using four items. Timing of outcome assessments: All participants received a baseline questionnaire and a follow up questionnaire +/‐ a video three weeks later. Follow‐up of non‐respondents: None reported. Adverse effects of the intervention: None reported |
|
| Sources of funding | The study was supported by the "Meyers Primary Care Institute, a joint endeavour of the University of Massachusetts Medical School, Fallon Clinic Foundation and Fallon Community Health Plan, and the Rosalie Wolf Interdisciplinary geriatric Health Care Research Center at the University of Massachusetts, which is funded through a joint program of RAND Health and the John Hartford Foundation. | |
| Consumer involvement | "Preliminary versions of the full questionnaire were pre‐tested using cognitive interviewing with a convenience sample of four anticoagulation clinic patients in 1:1 sessions." The three videos were also pilot tested with four clinic patients. | |
| Notes | The study compares three videos that use different methods for conveying evidence to patients to usual care. For the purpose of this review the three video arms were merged together and compared to usual care. There were two measures for adherence to lab monitoring. Documentation of patients' actual attendance at monitoring appointments was chosen as a more accurate measure than patients' intention to adhere to monitoring measured via a questionnaire. Adherence data were changed from the number who were non‐adherent to the number that were adherent. Three sub‐scales were used to measure beliefs about medicines. The "warfarin is beneficial" sub‐scale was used for the meta‐analysis based on consensus opinion of the authors that it was the most representative sub‐scale for the beliefs about medicines concept. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were "randomly assigned." The methods used for sequence generation were not reported. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No information was provided regarding blinding of participants or investigators. Assessments were not made directly after the intervention and participants were free to access further information which raises the possibility that any lack of patient blinding may influence the outcomes. Assessments consisted of questionnaires testing both relatively objective (knowledge) and subjective (self‐reported adherence and beliefs about medications) outcomes. However, the use of questionnaires minimised the potential for the lack of blinding of assessors influencing outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 600 patients met inclusion criteria, were randomised and were sent an initial mail out describing the study at which point 8/600 patients declined to participate (opted out). The baseline questionnaire was mailed out 16/600 were undeliverable, 12/600 opted out, 79/600 did not respond to the questionnaire and 16/600 were excluded (died, discontinued warfarin, currently hospitalised, residing out of area, cognitive ability, non English speaking). A further 5/600 returned this questionnaire but opted out of further participation. At this point 464/600 were remaining in the study: 121 in the narrative video group, 110 in the statistical video group, 119 in the combined video group and 114 in the usual care group. For the third mail out of the video and follow‐up questionnaire: the narrative video had 0/121 undeliverable, 2/121 opt out, 22/121 had no response and 13/121 no VCR. The Statistical video had 1/110 undeliverable, 1/110 opt out, 25/110 non response and 15/110 no VCR. The combined narrative and statistical video had 4/119 undeliverable, 2/119 opt out, 19/119 no response, 1/119 patient excluded and 16/119 no VCR. The questionnaire alone had 1/114 undeliverable, 1/114 opt out, 20/114 no response and 2/114 patients excluded. Therefore from the 464/600 who received the third mailing 83/121 (69%) remained in the narrative video group, 68/100 (68%) in the statistical video group, 77/119 (65%) in the combined video group and 90/114 (79%) in the control group. Results were calculated only for available data therefore denominators for each outcome measure varied depending on the number of non‐responders. The proportion of patients excluded from analyses was greater in the multimedia groups compared with the control group. Patients were excluded from the multimedia but not the control groups if they did not have access to a VCR. Lack of access to technology is more likely in lower socioeconomic groups and those with lower levels of educational attainment. This may therefore have biased the results towards overestimating the effect of the intervention on knowledge and skill acquisition. |
| Selective reporting (reporting bias) | Low risk | All outcomes described in the methods section are reported. |
| Other bias | High risk | Only participants who had access to a TV/VCR would have been able to watch the video and presumably complete the questionnaire. In the 3 video groups (narrative, statistical and both) 13, 15 and 16 participants respectively, reported not being able to view the video as they did not have access to a VCR. It is not known how many other non‐respondents also had this barrier. Non‐respondent numbers were higher in the video groups than in the control group. |
McElnay 1989.
| Methods |
Aim of study: To examine whether a short instruction video, suitable for use in the community or hospital environment, could be used effectively instead of personal instruction about inhaler technique. Aim of the intervention: To demonstrate correct usage of a bronchodilator inhaler. Study design: Randomised controlled trial with three arms: video, personal instruction and written information. Study duration: Two weeks. Recruitment process: Non medical staff at The Royal Victoria Hospital Belfast volunteered to participate in this study. Inclusion criteria: Non medical staff who had "no previous experience of the use of metered dose inhalers." Exclusion criteria: None stated. Informed consent: Not reported. Ethics approval: Not reported. |
|
| Participants |
Description: Non medical staff of the participating hospital. Location: Belfast, Northern Ireland, UK. Number: 150 participants were recruited with 50 participants enrolled in each group. Age (mean(range)): Written 29 (17‐61), Personal 29 (18‐64), Video 29 (17‐61). Gender: Males: written 15/50, personal 19/50, video 11/50. Ethnicity: Not reported. Educational level: Not reported. Literacy: Not reported. Primary diagnosis being treated: Healthy volunteers. Acute or chronic: Not applicable. Co‐morbidities: Not applicable. Treatment: Bronchodilators. |
|
| Interventions |
Topic of education: Correct technique for the use of the bronchodilator inhaler. Intervention: A video demonstrating the correct use of a metered dose inhaler. Co‐intervention: None. Comparison intervention: One group received written instruction on the correct use of the inhaler and another received personal instruction of the correct use of the inhaler by a pharmacist. |
|
| Outcomes |
Primary outcomes: Assessment of inhaler technique. Secondary outcomes: None. Methods of assessing outcomes: Inhaler technique was assessed using a pre‐determined scoring system where 6 steps were each scored and added together giving a total maximum of 80 points, with a higher score indicating better technique. Reliability and validity of outcome measures: Although the development of the scoring system took into findings from a pilot study that used the system "together with consultation with pulmonary physicians" no testing of its reliability or validity was reported. Timing of outcome assessments: All groups were tested after the intervention. They were then asked to demonstrate their technique two weeks later (not having any exposure to inhalers or information in the two weeks period). Follow‐up of non‐respondents: Not reported. Adverse effects of the intervention: Not reported. |
|
| Sources of funding | None stated. | |
| Consumer involvement | A pilot study of the video involved 30 students studying non‐medical subjects. The "adequacy of the film was assessed in the students and, based on results obtained, a second improved video film was prepared." | |
| Notes | Although this study has three arms (video, personal instruction and written information) the video and personal instruction groups both received written information as a co‐intervention. The review divided the study into two comparisons: video plus written vs written alone and video plus written vs personal instruction plus written. The last time point reported for the outcome (two weeks post‐intervention) was used for the meta‐analysis. Inhaler technique scores were converted to a percentage for the meta‐analysis. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were "randomly assigned to one of three groups". The methods used for sequence generation were not reported. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No information was provided regarding blinding of participants. Assessors who evaluated inhaler technique were blinded "the members of the scoring panel were unaware of the group to which each subject belonged." No other outcomes were measured. Assessments were made immediately following the intervention but also 2 weeks later raising the possibility that any lack of patient blinding might have influenced the outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 23/150 of the volunteer participants did not attend the second assessment 2 weeks later: six from Group A (written instruction), 10 from Group B (personal instruction) and seven from Group C (video instruction). Results appear to have been calculated based on results for participants remaining in the study at that time point. As the proportion of patients excluded from analyses was similar in all groups we do not expect the analyses to favour one group. |
| Selective reporting (reporting bias) | Low risk | The outcome identified in the methods section was reported. |
| Other bias | Low risk | There was no selection bias as the program was delivered on site using the available facilities. |
Navarre 2007.
| Methods |
Aim of study: To determine whether a computer‐based tutorial on inhaler technique could improve patients' knowledge and ability to correctly demonstrate inhaler technique. Aim of the intervention: To enhance the education of patients as to appropriate use of inhaler delivered pulmonary medication. Study design: Randomised controlled trial with two arms: computer based tutorial versus usual care Study duration: Patients were recruited over a three month period. Intervention and assessment occurred during one visit. Recruitment process: Participants were recruited from a university‐affiliated pulmonary clinic and a community pharmacy located in the same city. Inclusion criteria: Adults > 18 years, prescribed and using at least one inhaled pulmonary medication daily for a minimum of six months. Either receiving care at a university‐affiliated pulmonary clinic or having a prescription filled at a community pharmacy located in the same city over a 3 month period. Clinic participants had a documented diagnosis of asthma or COPD, while pharmacy participants reported this diagnosis when asked. Exclusion criteria: Participants who "relied exclusively on spacer devices to aid in inhaled drug delivery." Informed consent: Yes, "written informed consent was obtained from all subjects". Ethics approval: Yes, "the study protocol was approved by the University of Michigan Medical School." |
|
| Participants |
Description: Adults with pulmonary disease and experience using inhalers. Location: Michigan, USA. Number: 34 patients were recruited and randomised with 18 randomised in the intervention and 16 in the control group. Age (mean (SD)): I: 57 years (13.7). C: 51.8 (12.4). Gender: I: 33% male. C: 25% male. Ethnicity: I: 88.9% white, 11.1% other. C: 64.3% white, 35.7% other. Educational level: I: High school or less 38.9%, college 61.1%. C: High school or less 33.3%, college 66.7%. Literacy: Not reported. Primary diagnosis being treated: Asthma or COPD. Acute or chronic: Chronic. Co‐morbidities: Not reported. Treatment: Using at least one inhaled pulmonary medication daily for at least 6 months. |
|
| Interventions |
Topic of education: Information about the correct use of inhalers for asthma and COPD. Intervention: A computer‐assisted inhaler technique tutorial. Co‐intervention: None. Comparison intervention: No education. |
|
| Outcomes |
Primary outcomes: Observed inhaler technique and inhaler technique knowledge. Secondary outcomes: Patient assessment of the usability of the computer tutorial. Methods of assessing outcomes: Inhaler technique was observed by a research assistant who documented it on the observed inhaler technique checklist. This was then scored using a range of 0 to 100. The value was derived by dividing the number of steps performed correctly by the total number of possible correct steps and then multiplying by 100. The higher the score the better the inhaler technique. Inhaler technique knowledge was assessed using a questionnaire, the inhaler technique knowledge test, which lists the inhaler technique steps, with two or three possible responses for each step, one of which was correct. Scores were derived by dividing the number of correctly answered steps by the total number of steps and multiplying by 100 (range from 0 to 100; higher score indicates better knowledge). Reliability and validity of outcome measures: The observed inhaler technique evaluation checklist and the inhaler technique knowledge test were developed based on the albuterol inhaler package insert and the NIH clinical practice guidelines expert panel report on guidelines for the diagnosis and management of asthma. Timing of outcome assessments: Immediately after the intervention. Follow‐up of non‐respondents: Study performed during one visit therefore there were no non‐respondents. Adverse effects of the intervention: None reported. |
|
| Sources of funding | Provided by the University of Michigan Office of Information Technology Fund for Innovations that Enhance the Quality of Student Learning via Computer‐Based Learning Grant. | |
| Consumer involvement | None reported. | |
| Notes | Patient assessment of the usability of the computer tutorial was measured both in the intervention group and also in members of the control group who chose to use the tutorial after completing the study. As there was no comparison group this outcome was not included in the review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomisation was conducted using a predetermined randomisation table." |
| Allocation concealment (selection bias) | Unclear risk | No information provided. It is unclear whether the randomisation table was open or concealed from investigators. |
| Blinding (performance bias and detection bias) All outcomes | High risk | No information was provided regarding blinding of participants. Assessors evaluating inhaler technique were not blinded "research assistants evaluating inhaler technique were not blinded to patient study group assignment. The assistants performed the randomisation, facilitated use of the computer program and performed evaluation and data collection." Attempts were made to standardise the interaction of investigators with participants as well as inhaler technique assessments but these remain subjective. The research assistants "underwent standardized training and passed a test in an attempt to ensure similar assessments." "The research assistant was available to answer any question regarding navigation through the program; however, specific questions regarding inhaler technique....were deferred to discussion after the study was completed." Knowledge was tested using a questionnaire immediately following the intervention minimising the potential for the lack of participant blinding influencing this outcome. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Although no information was provided regarding missing outcomes it is assumed that they were available from all participants as the study was completed in one visit. |
| Selective reporting (reporting bias) | Low risk | All of the outcomes outlined in the methods section were reported. |
| Other bias | Low risk | There was no selection bias as the program was delivered on site using the available facilities. |
Neafsey 2001.
| Methods |
Aim of study: To determine effectiveness of the Personal Education Program (PEP) in improving the knowledge of older adults about interactions between prescribed medications and over‐the‐counter (OTC) medications and alcohol, and their self‐efficacy in avoiding interactions. Aim of the intervention: "To increase older adults' knowledge of potential interactions of prescription medications with OTC drugs and alcohol and to increase their confidence (self efficacy) about how to avoid such interactions." Study design: Randomised controlled trial with two arms: computer program and no education Study duration: Interventions and assessments occurred over one visit. Recruitment process: Subjects were recruited from four local senior centres after responding to a flier. Inclusion criteria: Age ≥ 60, independent physical & cognitive function (defined as: perform activities of daily living on Katz tool, 7/8 combined Nagi and Breslau functional items, 6/10 items on short portable mental status questionnaire, reading comprehension score of at least grade six on rapid estimate of adult literacy, be living independently and have visual acuity of at least 20/100 with corrective lenses.), and be taking calcium supplements, antacids or H2 blockers. Exclusion criteria: None. Informed consent: Yes; "All study participants were asked to sign an informed consent form that was read to them and explained if needed". Ethics approval: Not reported. |
|
| Participants |
Description: Seniors who attend four local senior centres. Location: Connecticut, USA. Number: 60 participants were recruited for the study, 30 in each arm. Age (mean (SD)): I: 73 (7.0), C: 68.8 (11.9). Gender: Both groups contained 88% women. Ethnicity: Not stated. Educational level (mean years (SD)): I:13.5 (2.9), C: 14.5 (3.1). Literacy: Reading comprehension of at least Grade six on the Rapid Estimate of Adult Literacy (REALM). Primary diagnosis being treated: Not reported. Acute or chronic: Not reported. Co‐morbidities: Not reported. Treatment: Calcium supplements, antacids or H2 blockers. |
|
| Interventions |
Topic of education: Information about potential interactions between prescription and over‐the‐counter drugs and alcohol. Intervention: An interactive computer program combining text, animation and graphics. Co‐intervention: None. Comparison intervention: No education. |
|
| Outcomes |
Primary outcomes: Knowledge, self efficacy and satisfaction with the computer program. Secondary outcomes: None. Methods of assessing outcomes: Knowledge was measured using an eight‐item MCQ based on the content of the intervention, and results reported as mean knowledge score (%). Self‐efficacy was measured using an eight‐item questionnaire with five‐point Likert response scale (1 = not sure to 5 = totally sure); mean scores were reported and ranged from one to five points, with a higher score indicating greater self‐efficacy. Participant satisfaction with the intervention was measured across all participants once they had watched the intervention (the control group watched the PEP at the end of the visit). Satisfaction was not included in the review as there was no comparison group. Reliability and validity of outcome measures: Instruments to measure self‐efficacy, knowledge and satisfaction were developed and validated by the authors. Information from focus groups guided choice of phrasing. Instruments were reviewed for content validity by a nurse researcher, pharmacologist and instrument development specialist. The instruments were pilot tested and the results analysed to determine instrument validity. Data for the self‐efficacy measure were analysed with exploratory factor analysis (principal component) showing it was unidimensional with factor loads > 0.78 and Cronbach's alpha of 0.94. One month test‐retest reliability = 0.82 (P < 0.001). For the knowledge test difficulty and discrimination indices were used to select most valid items which were then retested. Cronbach's alpha 0.55, test‐retest reliability = 0.65. No testing of the satisfaction scale was reported. Timing of outcome assessments: Immediately after the computer program in the intervention group and prior to the control group seeing the computer program Follow‐up of non‐respondents: Not reported. Adverse effects of the intervention: Not reported. |
|
| Sources of funding | Patrick and Catherine Weldon Donaghue Medical Research Foundation. | |
| Consumer involvement | Consumers were involved in the development of the PEP and the self medication questionnaire. They were also involved in pilot testing these instruments. | |
| Notes | Patient satisfaction with the multimedia intervention was not included in the review as there was no control group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "The sample was randomly divided into experimental and wait‐list control groups". The methods used for sequence generation were not reported. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No information was provided regarding blinding of participants or investigators. Outcomes were measured directly after the intervention therefore limiting the chance that participants accessed information from other sources. Assessment consisted of questionnaires testing both relatively objective (knowledge) and subjective (self‐efficacy and satisfaction) outcomes. However, the use of questionnaires minimised the potential for the lack of blinding of assessors influencing outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Although no information was provided regarding missing outcomes it is assumed that they were available from all participants as the study was completed in one visit. |
| Selective reporting (reporting bias) | Low risk | All of the outcomes outlined in the methods section were reported. |
| Other bias | Low risk | There was no selection bias as the program was delivered on site using the available facilities. |
Neafsey 2002.
| Methods |
Aim of study: To determine effectiveness of the Personal Education Program (PEP) in improving the knowledge of older adults about interactions between prescribed and over‐the‐counter (OTC) medications, their self‐efficacy in avoiding interactions and self‐medication practices. Aim of the intervention: To increase older adults' knowledge, self efficacy and safety relating to potential drug interactions arising from self‐medication Study design: Randomised controlled trial with three arms: PEP (multimedia intervention plus booklet), conventional care (booklet only), control (usual care). Study duration: Four weeks Recruitment process: Subjects were recruited from local seniors centres surrounding a research university in rural New England by nursing students Inclusion criteria: Age ≥ 60, independent physical & cognitive function (defined as: perform activities of daily living on Katz tool, 7/8 combined Nagi and Breslau functional items, 6/10 items on short portable mental status questionnaire, reading comprehension score of at least grade six on rapid estimate of adult literacy, be living independently and have visual acuity of at least 20/100 with corrective lenses.), taking antihypertensives or anticoagulants with OTC calcium supplement, antacids, H2 blockers or pain relievers. Exclusion criteria: None Informed consent: Yes; "All study participants were asked to sign an informed consent form that was read to them and explained if needed". Ethics approval: Not reported. |
|
| Participants |
Description: Seniors who attend local senior centres. Location: New England, USA. Number: 98 participants were recruited, 13 were omitted from the study leaving 85: 30 in the PEP arm, 30 in the conventional group and 25 in the control group. Age: Mean age of participants was 73.8 (SD = 6.5). No information was given about participant age in the different arms. Gender: Female 73%. Ethnicity: Caucasian 98%. Educational level: Mean years of education of participants was 12.6 (SD = 3.2). No information was given about education level in the different arms. Literacy: Reading comprehension score of at least grade six on rapid estimate of adult literacy (REALM). Primary diagnosis being treated: Hypertension or condition requiring anticoagulation. Acute or chronic: Chronic. Co‐morbidities: Not reported. Treatment: Taking antihypertensives or anticoagulants with OTC calcium supplement, antacids, H2 blockers or pain relievers. |
|
| Interventions |
Topic of education: Potential interactions between prescribed and over‐the‐counter medications focusing on interactions of antihypertensives and anticoagulants with antacids and analgesics. Intervention: An 80 minute interactive computer program combining text, graphics and animations. Co‐intervention: An information booklet that contains the same information as the computer program but is presented using only text (no graphics). Comparison intervention: Information booklet only (conventional) or no education (control). |
|
| Outcomes |
Primary outcomes: Knowledge, self‐efficacy and adverse self‐medication behaviours. Secondary outcomes: Participant satisfaction with the intervention (measured across all groups once they have watched the intervention i.e. control group watched PEP at end of visit three). Methods of assessing outcomes: Knowledge was measured using a 17‐item MCQ knowledge questionnaire based on the content of PEP, and was reported as mean correct responses (%). Self‐efficacy was measured using a 13‐item questionnaire with five‐point Likert response options (1 = not sure, 5 = totally sure); the mean score was reported with possible scores of one to five with higher scores representing greater self‐efficacy. Adverse self‐medication risk scores were calculated from adverse self‐medication behaviours reported on a medication use questionnaire. The behaviours were weighted based on a mean expert panel rating of the likelihood of each behaviour leading to a drug interaction (1 = very unlikely to 5 = likely). Satisfaction with the intervention was tested using a 14‐item questionnaire with a 5‐point Likert scale. Satisfaction was not included in the review as there was no comparison group. Reliability and validity of outcome measures: Instruments to measure self‐efficacy, knowledge, self‐reported medication use, estimate of adverse self‐medication behaviour and satisfaction were developed and validated by the authors. Information from focus groups guided choice of phrasing. Instruments were reviewed for content validity by a nurse researcher, pharmacologist and instrument development specialist. They were written at fifth grade reading level. They were each pilot tested with the target population and the results analysed to determine instrument validity. Data for self‐efficacy measure analysed with EFA (principal component) showing it was unidimensional with factor loads > 0.63 and Cronbach's alpha of 0.95. One month test‐retest reliability = 0.81 (P < 0.001). For the knowledge test, difficulty and discrimination indices were used to select the most valid items which were then retested. Cronbach's alpha 0.68, test‐retest reliability 0.50. For the medication use instrument, concurrent validity of self‐report was tested by having married couples complete the questionnaire based on their own and their spouses pattern of behaviour. All sections of the instrument were found to have a match rate >85% and Kappa values >0.75. No testing of the satisfaction scale was reported. Timing of outcome assessments: Immediately after intervention, two weeks and four weeks Follow‐up of non‐respondents: Not reported. Adverse effects of the intervention: Not reported. |
|
| Sources of funding | Patrick and Catherine Weldon Donaghue Medical Research Foundation. | |
| Consumer involvement | Consumers were involved in focus groups that were used in the development of the multimedia program and the self‐medication questionnaire. They were also involved in pilot testing of these instruments. | |
| Notes | Only the PEP (multimedia intervention plus booklet) and conventional care (booklet only) arms were included in this review. Knowledge, self‐efficacy and adverse medication behaviour scores were reported in graphical format only. Thus for the purposes of meta‐analysis in this review, we estimated the means and SD from the graphs. Data from the two and four week time points were used for the meta‐analysis. Patient satisfaction with the multimedia intervention was not included in the review as there was no control group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants "were randomly assigned to one of three groups". The methods used for sequence generation were not reported. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No information was provided regarding blinding of participants or investigators. Assessments were not made directly after the intervention and participants were free to access further information which raises the possibility that any lack of patient blinding may influence the outcomes. Assessments consisted of questionnaires testing both relatively objective (knowledge) and subjective (self‐efficacy, self‐medication behaviours and satisfaction) outcomes. However, the use of questionnaires minimised the potential for the lack of blinding of assessors influencing outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 13/98 subjects were omitted from the study: 3/33 from the PEP arm, 3/33 from the conventional arm and 4/32 from the control group due to illness of the participant or their spouse. A further 3/32 from control group were omitted because they declined to use the intervention at the end of their third visit. 85/98 participants in total were retained in study. Results appear to have been calculated based on the participants remaining in the study at that time point. As the proportion of patients excluded from analyses was similar in both groups we do not expect the analyses to favour one group. |
| Selective reporting (reporting bias) | Low risk | All of the outcomes outlined in the methods section were reported. |
| Other bias | Low risk | There was no selection bias as the program was delivered on site using the available facilities. |
Olver 2009.
| Methods |
Aim of study: To determine whether an interactive CD‐ROM improved cancer patients' recall of chemotherapy treatment information compared with standard written information, and whether demographic, cognitive, and psychological factors better predicted recall than the format of delivery. Aim of the intervention: To provide informed consent information about chemotherapy. Study design: Pseudo‐randomised controlled trial with two arms: CD‐ROM and written information. Study duration: Four weeks. Recruitment process: Consecutive chemotherapy naive cancer patients who were not involved in clinical trials were approached during inpatient and outpatient attendance at the Royal Adelaide Hospital Medical Oncology Unit by consulting clinicians. Inclusion criteria: Aged 18 years or over, have a life expectancy of at least 12 weeks, English speaking, and with the ability to provide consent. Exclusion criteria: Co‐morbility involving a significant psychiatric or cognitive disability, or if cognitive function was affected e.g. cerebral metastases. Informed consent: Yes, "patients provided their clinician with written consent to enter the trial". Ethics approval: Yes, "approval by the institutional ethics committee" |
|
| Participants |
Description: Patients starting treatment with chemotherapy. Location: Royal Adelaide Hospital Medical Oncology Unit, Australia. Number: 101 patients were recruited and randomised; 47 into the intervention and 54 into the control group. The demographics reported are for the 77 who completed follow‐up assessments. Age (mean (SD)): I: 54.3 (15.1), C: 59.9 (12.3). Gender: I: 62.2% male. C: 57.5% male. Ethnicity: 82% Australian/New Zealand, 17% United Kingdom, 1% Japanese. Educational level: I: Primary 13.9%, Secondary 61.1%, Tertiary 25%. C: Primary 12.8%, Secondary 59%, Tertiary 28.2%. Literacy: Not reported. Primary diagnosis being treated: Cancer. Acute or chronic: Chronic. Co‐morbidities: Not reported. Treatment: Chemotherapy. |
|
| Interventions |
Topic of education: Information about chemotherapy presented with a consent form. Intervention: Multimedia CD‐ROM and consent form providing at least the same information as in the standard written information sheet and consent form as well as additional information about the type of cancer, nutrition, patient's perspective, prevention, further information and frequently asked questions (FAQs). Co‐intervention: Usual care: both arms received verbal information about chemotherapy, the opportunity to ask questions of medical and nursing staff and were free to access further information at their own discretion. Comparison intervention: Standard written information sheet and consent form. |
|
| Outcomes |
Primary outcomes: Patient recall of treatment information. Secondary outcomes: Patient viewpoint of where they obtained most of their information, their satisfaction with the information and how much of the information they accessed and understood. Methods of assessing outcomes: Patient recall of treatment information was assessed during an interview with a nurse who asked patients five questions about their treatment. Satisfaction with the information was based on the response to three questions asking patients whether they read all the information provided, found the information useful and of the right amount. Questions also assessed how much of the information that accessed and understood and patients' perception of their anxiety level. Reliability and validity of outcome measures: The recall questions had been used in two previous studies evaluating a written consent form. Reliability and validity were not reported. Timing of outcome assessments: Baseline psychological and cognitive assessment occurred immediately prior to the intervention. Recall and other measures such as patient satisfaction as well as patient demographics were measured when patients attended for their second cycle of chemo (three to four weeks after the intervention). Follow‐up of non‐respondents: Patients completed recall questions and questionnaire when attending for their second cycle of chemo therefore there were no non‐responders. Adverse effects of the intervention: Patient anxiety. |
|
| Sources of funding | Partial support by grant from the Cancer Council South Australia in 2003. | |
| Consumer involvement | None stated. | |
| Notes | No data were able to be extracted for the meta‐analysis as patient recall was reported as the mean number of correct responses for three of the five individual items in the scale with no summated score. The authors were contacted to request mean scores but responded that this information was not available. Other results were reported as a single percentage combining results from both the control and intervention groups, therefore no comparison between the groups could be made. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "Patients were randomly allocated by data managers to one of two arms of the study using odd/even hospital identification numbers." Sequence generation used a non‐random approach using hospital record number. |
| Allocation concealment (selection bias) | High risk | "Allocation was performed by data managers therefore clinicians remained blinded to group allocation." However the sequence generation was based on the hospital identification numbers and would allow clinicians to foresee allocation. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No information was provided regarding blinding of participants. Clinicians, who were involved in outcome assessment were blinded to group allocation. Patients had the opportunity to interact with medical staff and seek further information prior to evaluation which raises the possibility that any lack of patient blinding may influence the outcomes including relatively objective outcomes such as patient recall of information. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 101 patients were recruited and randomised. 6/47 patients in the intervention arm and 7/54 in the control arm did not complete time one data as they were too ill at the time. A further 4/47 patients in the intervention arm and 6/54 in the control arm did not complete time two data as they were too ill or refused to participate. A further 1/54 in the control arm completed time two but did not complete recall data. This left 37/47 in the intervention group and 40/54 in the control group. Data were analysed using intention‐to‐treat analyses; t‐test analyses, performed to determine if patients who did not complete time two assessments were different from those that did, showed that those who completed assessments had greater immediate thematic data recall. This suggests that patients with better performance were more likely to complete the study, however, numbers dropping out were relatively equal in both groups. |
| Selective reporting (reporting bias) | High risk | The results for only three of the five patient recall questions were reported. These are identified as the main recall questions in the results but this was not pre‐specified in the methods. Authors suggest in the limitations section that analysis of the other recall questions has not yet been performed: "Analyses of additional treatment information, like the number of correctly recalled side effects of chemotherapy, are further needed". The data for other outcomes including patient satisfaction, perception of understanding and anxiety were only presented as an overall number and percentage across all participants with a statement that there were no significant differences between the control and intervention groups on these variables. |
| Other bias | Low risk | There was no selection bias as the program was delivered on site using the available facilities. |
Osguthorpe 1983.
| Methods |
Aim of study: To determine the most effective method of teaching psychiatric inpatients about their medication. Aim of the intervention: To provide medication information to patients. Study design: Cluster randomised trial with a four group design: video, written information, both video and written information, no education. Study duration: March 1978 to July 1979. Each patient was evaluated over a three‐week period. Recruitment process: Investigators visited participating general psychiatry wards at a Veterans Affairs psychiatric hospital to identify eligible patients and invite them to participate. Inclusion criteria: Male, able to read English, who were taking any of the following drugs orally and regularly: Serentil, Haldol, Trilafon, Mellaril, Prolixin, Loxitane, Navane, Thorazine or Stelazine. Exclusion criteria: Physically unable to tolerate participation. Informed consent: Yes "The investigators schedule appointments for patients to learn about the project and consider participation, standard information about the study, explained its purpose, procedures and potential gains and risks". Ethics approval: Not reported. |
|
| Participants |
Description: Men admitted to a general psychiatry ward at Veterans Administration Psychiatric hospital. Location: Large, urban Veterans Administration Psychiatric hospital in California, USA. Number: 202 completed the study (did not report number of patients in each of the study arms). Age: Ranged from 20 to 64 with mean of 39. No data reported for separate arms except for the statements "age was not distributed evenly across the four groups". Gender: Male 100%. Ethnicity: White 53.5%, black 40.1%, Hispanic 5.9%, other 0.5%. Educational level: Eight grade or below 5%, eighth to twelfth grade 61%, some college 29%, not stated 5%. Educational level was significantly higher in the Video only and no education arms than in the information sheet only and the information sheet and video combined arms. Literacy: Not reported. Primary diagnosis being treated: Psychiatric illness: psychotic 51%, mixed 20%, other 16%, uncodable 13%. Acute or chronic: Chronic. Co‐morbidities: Not reported. Treatment: Serentil, Haldol, Trilafon, Mellaril, Prolixin, Loxitane, Navane, Thorazine or Stelazine. |
|
| Interventions |
Topic of education: Information about psychiatric medications. Intervention: A videotaped nurse explanation providing general information (not specific to each medication) about the medications included in the study. This included general information about the purpose, side effects, precautions and helpful hints common to all the medications. Co‐intervention: Drug information sheet given as a co‐intervention with the video in one arm. Comparison intervention: Drug information sheet containing the same general information as the video as well information specific to the particular medication (identifying the medication by name, picture and dosage) or no education. |
|
| Outcomes |
Primary outcomes: Patient knowledge and medication recognition. Secondary outcomes: None. Methods of assessing outcomes: An 11‐item knowledge questionnaire containing nine multiple choice items testing knowledge and two testing medication recognition. Reliability and validity of outcome measures: Questions were written aimed at a grade five readability level. Content validity was judged by a panel of nurses who examined questions for accuracy of drug data and for consistency with information provided in the information sheet and video. Both patients and nurses read the questions for clarity. Test‐retest reliability of the instrument was established by the control group (but not reported). Timing of outcome assessments: Pre and post test questionnaire were administered a week before and a week after the intervention. Follow‐up of non‐respondents: Not reported. Adverse effects of the intervention: Not reported. |
|
| Sources of funding | None stated. | |
| Consumer involvement | Patients were involved in pilot testing of the outcome measure questions. | |
| Notes | No data could be extracted for the meta‐analysis as knowledge was reported as the mean change score from baseline for 9 of the 11 individual questions in each of the 4 study arms but with no overall change score reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Wards rather than patients were randomised but the methods used for sequence generation were not reported: "the wards were randomly assigned to one of four groups". |
| Allocation concealment (selection bias) | Unclear risk | No information provided. Patients were admitted to the wards after the wards had been randomised. It is unclear if the assignment of the wards was known or concealed from study personnel and admissions staff. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No information was provided regarding blinding of participants or investigators. Knowledge, measured using a questionnaire, is a relatively objective outcome. Although patients were able to ask questions of the investigators, protocols were in place to standardise the interaction between investigators and participants. "Investigators followed an outline of standard information. Questions about specific drugs and about information beyond that covered in the Drug Information Sheet or the Videotaped Nurse Explanation were deferred until the posttest was administered. Questions were answered when they arose if they clarified the presented materials." However, outcomes were assessed one week following the intervention so patients had the opportunity to interact with medical staff and seek further information prior to evaluation raising the possibility that any lack of blinding may have influenced outcomes including knowledge. The use of questionnaires minimised the potential for the lack of blinding of assessors influencing outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Patients who were taken off the medications during the three week study period were excluded from the analysis. However, no information was provided about the total number of participants who were randomised, the number of participants missing from follow‐up assessments or the number excluded from the analysis. |
| Selective reporting (reporting bias) | High risk | Only the change scores between pre‐ and post‐test knowledge questions with the results of ANOVA on the change scores and some significance levels were reported. No results from the two medication recognition questions were reported. |
| Other bias | Unclear risk | There was no selection bias as the program was delivered on site using the available facilities. There may have been a risk of recruitment bias as individuals were recruited to the trial after the wards had been randomised, and foreknowledge of the intervention each cluster was receiving could have influenced the types of participants recruited or admitted to each ward. |
Powell 1995.
| Methods |
Aim of study: "To assess the value of mailed educational videotapes as a means of enhancing medication compliance." Aim of the intervention: To enhance compliance with prescribed drug therapy. Study design: Randomised controlled trial with two arms: video and no education. Study duration: Subjects were enrolled over six months (July 1 1993 to January 2 1994) and refill data were collected over a nine month period (July 1 1993 to April 1 1994). Recruitment process: HMO members with a pharmacy claims for one of the four drugs being considered in the study. Inclusion criteria: If a participant had been prescribed either benazepril, metoprolol, simvastatin or transdermal oestrogen and were receiving medical and prescription coverage through the participating HMO plan. Exclusion criteria: None. Informed consent: Not reported. Ethics approval: Not reported. |
|
| Participants |
Description: Members of a HMO with a pharmacy claim for benazepril, metoprolol, simvastatin or transdermal oestrogen. Location: USA. Number: 4246 participants with 2253 in the control group and 1993 in the study group. Age (mean (range)): I: 55 (20‐97), C:54 (20‐94). Gender (N (%)): male I: 704(35), C: 731 (32), Female I: 1289 (65), C:1522 (68). Ethnicity: Not reported. Educational level: Not reported. Literacy: Not reported. Primary diagnosis being treated: Hypertension, hyperlipidaemia or menopause (inferred from the medications prescribed). Acute or chronic: Chronic. Co‐morbidities: Not reported. Treatment: Metoprolol, simvastatin, benazepril or transdermal oestrogen. |
|
| Interventions |
Topic of education: There were four programs comprising of six videotapes 1. "The Health Challenge: Managing Hypertension", 2. "Taking Lotensin", 3. "Taking Lopressor" 4. "Discussing menopause". 5. "Using Estraderm" and 6. "The Health Challenge: Managing High Cholesterol, Taking Zocor." Intervention: Videotapes presenting information on the drug prescribed and inferred disease state. Co‐intervention: None. Comparison intervention: No education. |
|
| Outcomes |
Primary outcomes: Medication compliance. Secondary outcomes: None. Methods of assessing outcomes: Compliance was monitored through prescription refill data available to the HMO. The medication possession ratio (MPR) was calculated as the number of days' supply of the medication the participant obtained during the study period divided by the number of days the participant was in the study. Reliability and validity of outcome measures: Not applicable. Timing of outcome assessments: MPR was measured from the time a participant was enrolled in the study until the study period finished or the participant was terminated from the HMO plan. Follow‐up of non‐respondents: Not applicable. Adverse effects of the intervention: Not reported. |
|
| Sources of funding | The study was funded by educational grants from Civa‐Geigy Coorporation and Merck and Company. | |
| Consumer involvement | None reported. | |
| Notes | The number of patients who were compliant in each group (defined by the study authors as those with an MPR of 0.8 or greater) was used for the meta‐analysis as this was thought to more meaningful than a mean MPR score. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were "randomly assigned to a study group or a control group." The methods used for sequence generation were not reported. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants in the videotape group "were informed that the videotapes were part of a patient education program but not that medication compliance was being assessed." The control group received no education and presumably were not aware that they were participating in a study. Although no information was provided regarding blinding of investigators the methods used to measure compliance were objective. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All suitable participants were mailed the relevant videotape, their compliance was monitored via prescription refill data available through their HMO and all participants were included in the data analysis within the group to which they had been assigned. |
| Selective reporting (reporting bias) | Low risk | The outcomes identified in the methods section was reported. |
| Other bias | Low risk | Participants were randomised to the intervention and control groups without knowledge of who had facilities to watch video. All patients in the intervention group were analysed in that group regardless of whether or not they had watched the video. |
Savage 2003.
| Methods |
Aim of study: To compare the effects of standard information on correct inhaler use presented via a patient information leaflet (PIL) or a multimedia touch screen computer (MTS). Aim of the intervention: To improve metered dose inhaler technique. Study design: Single‐blind randomised controlled trial with two arms: patient information leaflet and a multimedia touch screen computer. Study duration: Intervention and assessments occurred over one visit. Recruitment process: Participants were recruited from four general practices. Patients who had used a metered dose inhaler in the past six months were identified through their medical records and mailed an invitation letter and asked to complete and return a consent form if they wanted to participate in the study. As there was a lower than predicted response rate in the 17‐34 age group, recruitment was extended to students from King's College London using the college's email system. Inclusion criteria: Patients > 12 years and used a bronchodilator MDI in past six months. Exclusion criteria: "Problem patients, and those known not to speak English well". Informed consent: Yes, "those wishing to participate returned a consent from to the practice" Participants also gave written consent for a video to be made of them demonstrating of their inhaler technique. Ethics approval: Yes. "The study was approval by the relevant Local Research Ethics Committees and the Ethics Committee at King's College London". |
|
| Participants |
Description: Patients over 12 years using a bronchodilator MDI in the past six months. Location: London, UK. Number: 110 patients were recruited and randomised, having excluded the five drop outs, 57 were allocated to receive the multimedia intervention and 48 allocated to receive the leaflet. Age (N (%)): I: < 25 10 (17.5%), 25‐50 14 (24.6%), 50+ 33 (57.9%). C: < 25 7 (14.6%), 25‐50 14 (29.2%), 50+ 27 (56.3%). Gender: I: 53% female. C: 54% female. Ethnicity: Not reported. Educational level: Not reported. Literacy: Not reported. Primary diagnosis being treated: Not reported. Acute or chronic: Chronic. Co‐morbidities: Not reported. Treatment: Bronchodilator. |
|
| Interventions |
Topic of education: Information about the correct use of inhalers. Intervention: Demonstration of correct inhaler technique via a multimedia touch screen computer. Co‐intervention: None. Comparison intervention: 1996 version of the ventolin package insert PIL. |
|
| Outcomes |
Primary outcomes: Assessment of inhaler technique. Secondary outcomes: Information acceptability and usefulness. Methods of assessing outcomes: Video recordings of patients demonstrating their inhaler technique were evaluated by a blinded assessor. The assessor gave a rating of poor, adequate or good for global technique and a more detailed assessment based on a checklist of eight inhaler steps that were scored as performed correctly or incorrectly. Inhaler shaking counts and length of inspiration were also recorded. Information about the acceptability and usefulness of the education was based on the response to 18 statements about information and content as part of an interview with the investigator. Reliability and validity of outcome measures: Intra‐rater reliability for the global technique rating was assessed using repeated ratings of 12 patients, pre‐ and post‐information, chosen to illustrate the range of good and poor technique. There was good agreement between the two data sets (kappa 0.795 P < 0.0001). Timing of outcome assessments: Immediately before and after the intervention. Follow‐up of non‐respondents: Study performed during one visit therefore there were no non‐responders. Adverse effects of the intervention: Not reported. |
|
| Sources of funding | Provided by the Department of health and the North London Primary Research Network. | |
| Consumer involvement | None reported. | |
| Notes | The global inhaler technique rating was chosen as the measure of skill acquisition for the meta‐analysis as it was an overall assessment of the participants' skill and evidence had been provided for its reliability. The rating was converted to a dichotomous outcome: improved (those whose technique rating improved following the intervention) and did not improve (those whose technique rating worsened or stayed the same). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients allocation was done using random number tables". The investigators used a random component for sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | This was a single blind study with blinding of assessor who evaluated inhaler technique "assessed blind by an assessor who did not attend experimental sessions and had no contact with patient volunteers." The participants were not blinded however, assessments occurred immediately following the intervention minimising the potential for the lack of blinding influencing outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Of the 110 participants that were randomised 5/110 did not continue with the study as 4/110 could not use an MDI without a spacer and 1/110 no longer used this medication. No information was provided regarding which of the groups these participants were randomised into. 105 patients completed the study, 57 in the MTS group and 48 in the PIL group. However, 5/105 (2/57 MTS group and 3/48 PIL group) participants were not included in the results as the video of their inhaler technique was insufficiently clear to be rated (3/105) or the "the assessor was unable to decide on a global rating" (2/105). Results appear to have been calculated based on the participants remaining in the study at that time point. As the proportion of patients excluded from analyses was similar in both groups we do not expect the analyses to favour one group. |
| Selective reporting (reporting bias) | High risk | Inhaler technique assessed using global rating and scoring correct or incorrect performance of eight steps. However, only results for four "key steps", that were not pre‐specified, were reported. Authors state that full results of the questionnaire evaluating acceptability and usefulness of the education provided will be reported elsewhere. |
| Other bias | Low risk | There was no selection bias as the program was delivered on site using the available facilities. |
Schnellinger 2010.
| Methods |
Aim of study: To create an animated video about the appropriate use of antibiotics and to compare its effect on parent knowledge to that of the equivalent American Academy of Pediatrics pamphlet. Aim of the intervention: To teach parents about the appropriate use of antibiotics. Study design: Prospective randomised controlled trial with three arms: control group (C), pamphlet group (P) and animated video group (I). Study duration: Enrolment occurred during a four month period between October 2008 and January 2009. Participants were followed up for four weeks. Recruitment process: Parents/guardians of children presenting to the emergency department for acute care during the recruitment period were approached by a research assistant and asked to participate in the study. Inclusion criteria: "English speaking parents/guardians who presented to the paediatric emergency department with their child aged 0‐18 years for an acute care visit" Exclusion criteria: Parents/guardians whose child required urgent medical care or who enrolled but did not complete the postintervention knowledge survey. Informed consent: Yes "the same research assistant obtained consent from and enrolled all participants" Ethics approval: Yes "The institutional review board at Children's Hopsitals and Clinics of Minnesota approved the study" |
|
| Participants |
Description: Parents/guardians of children presenting to the emergency department for acute care. Location: Children's Hospitals and Clinics of Minnesota, USA Number: 246 were included in the study: 84 in the control group, 79 pamphlet group and 83 video group. 189 completed the four week follow up: 61 control, 63 pamphlet and 65 video. Age of parent (median (range)): C: 32 (16‐53), P: 31.5 (17‐51), I: 33 (17‐72). Age of child: C: 23% < 1 year, 42% 1‐5, 24% 6‐11, 12% >11, P: 29% < 1 year, 43% 1‐5, 19% 6‐11, 9% > 11, I: 15% < 1 year, 48% 1‐5, 15% 6‐11, 23% > 11. Gender of parent (% female): C: 82, P: 76, I: 80. Gender of child (% female): C: 49, P: 49, I: 55. Ethnicity: C: 44% Caucasian, 33% black, 23% other, P: 56% Caucasian, 23% black, 22% other, I: 57% Caucasian, 22% black, 22% other. Educational level: C: 67% college, 20% high school, 13% less than high school, P: 70% college, 19% high school, 11% less than high school, I: 68% college, 19% high school, 13% less than high school. Literacy: Not reported. Primary diagnosis being treated: Not reported. Acute or chronic: Acute. Co‐morbidities: Not reported. Treatment: Not reported. |
|
| Interventions |
Topic of education: Appropriate antibiotic use. Intervention: Video about appropriate use of antibiotics. Co‐intervention: None. Comparison intervention: Pamphlet about antibiotic use and antibiotic resistance, or no education. |
|
| Outcomes |
Primary outcomes: Knowledge. Secondary outcomes: Evaluation survey testing participant attitudes to antibiotics, their perception of the study, their health provider's prescribing behaviours and whether the provider provided relevant information about antibiotics. Methods of assessing outcomes: Knowledge was measured using a 10‐item questionnaire created specifically for this study with response options of yes/no/not sure which were scored as correct or incorrect. Participant attitudes and perceptions were measured using a nine‐item "evaluation survey" also created for this study. As part of this survey participants were asked if they found the intervention interesting or useful, whether they learnt something, if their paediatrician had talked about proper and improper use of antibiotics, if they felt that paediatricians offered antibiotics more often than necessary, if they thought there was an antibiotic for every infection, knew about antibiotic resistance prior to the study and if they would have presented to a doctor or the emergency department if they knew that there was no antibiotic for their child's infection. Reliability and validity of outcome measures: The knowledge tested in the knowledge survey was based on published studies and provider input. The language in the survey met a sixth grade level of understanding. The items were pilot tested with 15 parents/guardians and revised based on their feedback prior to use in this study. No information was reported regarding the development of the evaluation survey and no testing of the reliability or validity of the surveys was reported. Timing of outcome assessments: Knowledge was measured at three time points: before the intervention, 1.5 to 2 hours after the intervention and four weeks after the intervention. The evaluation survey was administered after the knowledge survey at 1.5 to 2 hours after the intervention. Follow‐up of non‐respondents: Participants were given a $10 gift card after they completed the four week follow‐up survey. Further follow‐up of non‐respondents was not reported. Adverse effects of the intervention: Not reported. |
|
| Sources of funding | The study was funded by the Children's Hospitals and Clinics of Minnesota Internal Research Grant Program. | |
| Consumer involvement | Parents/guardians pilot tested the knowledge survey and their feedback was used to refine the survey prior to its use as an outcome measure in this study. | |
| Notes | Although this study has three arms (video, pamphlet and no education) the review compared video vs pamphlet and video vs no education. Knowledge was reported both as a median score and as the number of participants who had improved scores following the intervention. Data using the second method of reporting were extracted for meta‐analysis. Results were skewed and knowledge scores were high at baseline, suggesting that the scale used was poorly targeted to knowledge levels in this population and therefore insensitive to change due to a ceiling effect. 31%, 21% and 18% of participants had perfect scores at baseline in the video, pamphIet and no education groups respectively, therefore improvement in knowledge following the intervention could not be detected in these participants. Results for five of the nine items in the evaluation survey are listed in Table 8 but not included in the review as they are not relevant to the evaluation of educational interventions (items measure participants' perception of the study, health providers' prescribing behaviour and whether they had provided participants with information). Three of the items assess participants' attitude to antibiotics. As the trial authors reported the percentage of patients who answered "yes" to each item separately, with no overall score, we extracted data from one item, "Based on what you learnt in this study, would you ever ask your paediatrician for an antibiotic if your child had one of the illnesses we talked about today" as the item most relevant to the aims of the study. Scores for this item were converted to the number of respondents who had chosen the correct response (No). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomized assignment was accomplished by using a random‐number‐generated list". |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No information was provided regarding blinding of participants or assessors. Assessments used scored questionnaires testing both objective (knowledge) and subjective (attitudes) outcomes. The assessment that occurred immediately following the intervention minimised the potential for the lack of blinding of participants influencing outcomes. However, this is not the case with the assessment four weeks following the intervention. The use of questionnaires minimised the potential for the lack of blinding of assessors influencing outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 41 participants were excluded from the study as they did not complete the knowledge survey following the intervention. 246 completed this survey and were included in the study and 189 completed the four week follow‐up. The number of patients completing the four week follow‐up was similar in all groups. |
| Selective reporting (reporting bias) | Low risk | All outcomes described in the methods section are reported. |
| Other bias | Low risk | There was no selection bias as the program was delivered on site using the available facilities. |
Schwarz 2008a.
| Methods |
Aim of study: To evaluate whether computerised counselling about Emergency Contraception (EC) with the provision of a free sample of EC can increase knowledge and use of EC among women seen in an urgent care clinic. Aim of the intervention: To provide counselling about emergency contraception. Study design: Randomised controlled trial with two arms: computerised counselling about emergency contraception and control computerised counselling about preconception folate. Study duration: Six months. Recruitment process: Participants were recruited from the waiting areas of two urgent care clinics. Inclusion criteria: English speaking adults (aged 18 to 45 years old). Exclusion criteria: Women were excluded if they were unlikely to become pregnant in the next year (because they were currently pregnant, had undergone a hysterectomy or tubal ligation, had an intrauterine device in place, had a partner who had undergone a vasectomy, were a lesbian, or were over 45 years of age). Also women who did not have a telephone or were planning on relocating were excluded. Informed consent: Yes, "all participants provided written informed consent". Ethics approval: Yes. "Approval obtained by the Committee on Human Research at the University of California, San Francisco and the Institutional Review Board of the University of Pittsburgh". |
|
| Participants |
Description: Females of child‐bearing age attending an urgent care clinic. Location: Two urgent care clinics in San Francisco, USA. Number: 446 women were enrolled and randomised with 219 participants in the intervention group and 227 in the control group. 265 completed the follow‐up survey; 127 intervention and 138 control. Age N (%): I: 18‐24 years 56 (26%), 25‐30 years 87 (40), 31‐35 years 49 (22), 36‐45 years 43 (20). C: 18‐24 years 60 (27), 25‐30 years 70 (31), 31‐35 years 57 (25), 36‐45 years 38 (17). Gender: 100% female. Ethnicity N (%): I: White 95 (43), Black 24 (11), Latina 30 (14), Asian 37 (17), Other 30 (14). C: White 100(44), Black 29 (13), Latina 34 (15), Asian 40 (18), Other 23 (10). Educational level N (%): I: Less than high school 14 (6), High school graduate 28 (13), Vocational school or some college 74 (34), College degree 61 (28), Graduate or professional degree 41 (19). C: Less than high school 9 (4), High school graduate 25 (11), Vocational school or some college 80 (35), College degree 78 (34), Graduate or professional degree 34 (15). Literacy: Not reported. Primary diagnosis being treated: Not applicable. Acute or chronic: Acute. Co‐morbidities: None. Treatment: Option to use emergency contraception . |
|
| Interventions |
Topic of education: Emergency contraception. Intervention: Computerised educational program providing information about emergency contraception (EC) including 1. What is EC, 2. When to use EC, 3. What to expect when you use EC, 4. Why to use EC, 5. How to use, 6. Where to get EC, 7. The cost of EC, 8. How does EC work 9. Why is EC important. Co‐intervention: None. Comparison intervention: Alternative computer educational program on taking folate (covering the same questions as in the EC program 1. What is folate, 2. When to use folate etc). |
|
| Outcomes |
Primary outcomes: Self reported use of emergency contraception. Secondary outcomes: Knowledge of and attitudes towards EC, self‐reported pregnancy. Methods of assessing outcomes: A computerised survey measured baseline EC knowledge, attitudes and ability to access EC and collected demographic information. The follow‐up survey was conducted over the phone by a research assistant using a standardised script that contained the same questions as the baseline survey. Knowledge about EC was tested using nine items; seven with yes/no response options and two with six‐point Likert responses (extremely safe (0) to extremely dangerous (5) or extremely effective (0) to very ineffective (5)). Beliefs about EC was measured using an item asking if participants had personal objections to EC. Reliability and validity of outcome measures: The questionnaire testing participants knowledge of and attitude towards EC contained questions developed by the Kaiser Family Foundations. These questions had previously been used in a nationwide telephone survey about EC. The other questions were "previously used to evaluate contraceptive and pregnancy experiences." The entire questionnaire was pre‐tested in the target population "and ambiguous questions were reworded until consistently interpreted." Timing of outcome assessments: At baseline (before the intervention) and six months later. Follow‐up of non‐respondents: Multiple attempts were made to contact non‐responders by phone. Adverse effects of the intervention: None reported. |
|
| Sources of funding | "Funds for this study were provided by UCSF/Mt Zion Health Fund. Duramed Pharmaceuticals donated samples of emergency contraception but had no involvement in the study design or drafting of the manuscript." | |
| Consumer involvement | Questionnaire used to test patient's knowledge of, attitude towards and ability to access emergency contraception was pre‐tested in the target population prior to use in the current study. | |
| Notes | The number of participants who conceived during the study period were reported in the text but not included in the meta‐analysis. The authors were contacted and provided mean EC knowledge scores with P values. SD were calculated from the reported P values. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomisation was performed by a computer‐generated sequence". The investigators used a random component for sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | "Allocation was concealed from research assistants." No further information was provided on how this was achieved. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Assessors were blinded at all stages of the study. At baseline "allocation was concealed from research assistants until after the participant had completed the educational module." At the six month follow‐up "research assistants, who were blinded to whether subjects were in the intervention or control groups, called all subjects and administered the follow‐up survey". Attempts were also made to blind patients by not mentioning EC in the consent documents. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | It was anticipated that approximately 10% of the women would be lost to follow up so extra numbers were recruited to deal with this. Of the 446 participants enrolled and randomised 181 (92 from intervention and 89 from control group) were not able to be contacted for the follow‐up telephone call (41%). Therefore numbers that completed the follow up survey were 127/219 in the intervention group and 138/227 in the control group. The investigators performed "both an intention‐to‐treat analysis (in which we assumed women who were lost to follow‐up never used EC) and an analysis limited to participants that completed follow‐up" for the use of EC and knowledge outcomes. For intention to treat analyses patients who were lost to follow‐up were assumed not to have used EC or to have no increase in knowledge from the first assessment. |
| Selective reporting (reporting bias) | High risk | All outcomes described in the methods section are reported. However knowledge outcomes were only reported as the number who have improved compared with their baseline knowledge and the intention to treat analysis for knowledge was reported as only as a P value for the comparison of the accuracy of answers in the two groups. |
| Other bias | Low risk | There was no selection bias as the program was delivered on site using the available facilities. |
Self 1983.
| Methods |
Aim of study: To compare the effectiveness of having a pharmacist instruct patients, in person or on videotape, with the usefulness of the standard package insert. Aim of the intervention: To instruct patients in the use of an inhaler. Study design: Randomised controlled trial with three arms: personal instruction by a pharmacist, videotape and standard written information. Study duration: Intervention and assessments occurred over two visits (with the second visit occurring somewhere between one and 16 weeks after the first instructional session). Recruitment process: Mild asthmatics were recruited from an allergy clinic. Inclusion criteria: Mild asthmatics attending an allergy clinic. Exclusion criteria: None stated. Informed consent: Not reported. Ethics approval: Not reported. |
|
| Participants |
Description: Patient attending an allergy clinic. Location: USA. Number: 29 patients were assigned to one of three groups: 10 in the control group, nine in the personal demonstration of inhaler technique group and 10 in the video intervention group. Age: Mean age for both groups was 39 years. Gender: 20 women and 9 men. Ethnicity: Not reported. Educational level: Not reported. Literacy: Not reported. Primary diagnosis being treated: Asthma. Acute or chronic: Chronic. Co‐morbidities: Not reported. Treatment: Bronchodilators. |
|
| Interventions |
Topic of education: Correct use of inhalers in asthma. Intervention: A video demonstrating correct inhaler technique. Co‐intervention: None. Comparison intervention: Leaflet group received an information sheet based on the manufacturer's directions and current literature, personal instruction were shown the use of the inhaler by one of the pharmacists. |
|
| Outcomes |
Primary outcomes: Observed inhaler technique. Secondary outcomes: None. Methods of assessing outcomes: Inhaler technique was assessed by a "specially trained technician" using a 10‐point checklist based on the instructions that had been given to the patient. Each patient was scored twice based on two inhaler technique demonstrations and their scores added (maximum score of 20) with higher scores indicating better technique. Reliability and validity of outcome measures: Not reported. Timing of outcome assessments: Patients were assessed immediately after the intervention, those in the personal demonstration and videotape groups were re‐tested between one to 16 weeks later (mean six weeks). Follow‐up of non‐respondents: Not reported. Adverse effects of the intervention: Not reported. |
|
| Sources of funding | Grants from Shering Cooperation and Boehringer Ingelheim. | |
| Consumer involvement | None reported. | |
| Notes | Although this study has three arms (leaflet, video and pharmacist demonstration) the review compared video vs written leaflet and video vs pharmacist demonstration. Inhaler technique scores were converted to a percentage for the meta‐analysis. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were "randomly assigned.... to three roughly equal groups". The methods used for sequence generation were not reported. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No information was provided regarding blinding of participants or assessors. Assessments of inhaler technique were based on a checklist but remained subjective. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | All patients completed the first assessment immediately following the intervention. 2/9 patients assigned to the group receiving personal instruction on inhaler technique and 2/10 patients assigned to the videotape intervention did not return for their follow up visit and assessment. No reasons were provided for participant attrition. Mean scores appear to have been calculated based on results for participants remaining in the study at that time point. |
| Selective reporting (reporting bias) | Low risk | The outcome stated in the method section was reported. |
| Other bias | Low risk | There was no selection bias as the program was delivered on site using the available facilities. |
Solomon 1988.
| Methods |
Aim of study: "To determine whether patient education could facilitate compliance with a prescribed medical regimen for tetracycline among inner‐city patients with a positive diagnosis for a sexually transmitted disease." Aim of the intervention: "To provided step‐by‐step instructions for building an individualised schedule for taking the four pills (tetracycline)." Study design: Randomised controlled trial with eight arms: four intervention groups: video plus special pill packaging, video alone, special packaging alone and neither video nor special packaging, with each of these groups divided into two arms: telephone interview on the second or the sixth day of the treatment regimen. Study duration: Intervention and assessments occurred over a period of up to seven days Recruitment process: Patients attending a STD clinic who required a course of tetracycline were asked to participate in the study. Inclusion criteria: All patients with a positive diagnosis of a sexually transmitted disease who were to be treated with a seven day course of tetracycline and no other medication were eligible for the study. Exclusion criteria: None reported. Informed consent: Yes "the doctor explained the purpose of the study and solicited their informed consent to participate" Ethics approval: Yes "the study procedure was reviewed and approved by Education Development Center's Institutional Review Board" |
|
| Participants |
Description: Patients attending a sexual health clinic for treatment of a sexually transmitted disease (STD). Location: Washington DC General Hospital STD clinic, USA. Number: The final sample consisted of 321 participants with each group having between 38 and 42 participants. Age: Patients ranged in age from 14 to 59 years with a median age of 23 years. Gender: Male 95.3%. Ethnicity: Black 97.2%. Educational level: 35.5% had only completed junior high school and 56.4 had completed high school. Literacy: Not reported. Primary diagnosis being treated: STDs with 87.9% being non‐gonococcal urethritis. Acute or chronic: Acute. Co‐morbidities: Not reported. Treatment: Tetracycline. |
|
| Interventions |
Topic of education: Information about and instructions on the correct method for taking tetracycline for an STD. Intervention: An instructional video given in conjunction with a schedule card for patients to complete while watching the video. The schedule card is designed to be an integral part of the videotape with the video providing step by step instructions for patients to fill in an individualised schedule for taking the Tetracycline tablets. The schedule was reviewed by the doctor who suggested changes as necessary. Co‐intervention: A standardised set of instructions was given by the doctor to all patient groups. Patients were also given the opportunity to ask the doctor questions. Comparison intervention: The patients in the special packaging arms received seven pack strips (one for each day) with each pill labelled with the day, the number of the pill, the time of day to take and instructions to wait one hour after taking the tablet before eating. The doctor introduced the patient to the special packaging, showed them how they pills were organised and read through the labels. |
|
| Outcomes |
Primary outcomes: Compliance with taking tetracycline. Secondary outcomes: Knowledge of the medication prescribed, satisfaction with care received, perceptions of illness and confidence in treatment regime. Methods of assessing outcomes: A scoring system looking at patient compliance was devised for the current study by assigning a numerical value to each recorded error in compliance according to the degree to which that error might compromise treatment effectiveness. Compliance errors were recorded based on patient interviews. Questionnaires were used to assess knowledge (15 true/false items derived from the content of the video) as well as satisfaction with care received, perception of illness, and confidence in the treatment regime. Reliability and validity of outcome measures: Not reported. Timing of outcome assessments: On either the second or sixth day the doctor contacted the patients and asked them a series of questions regarding their medication‐taking behaviour on the previous day. Follow‐up of non‐respondents: The doctor attempted to contact the patient via telephone on either day two or day six post commencement of treatment. If the patient was unable to be contacted on that day no further phone calls were made. Adverse effects of the intervention: Not reported. |
|
| Sources of funding | This study was supported by a grant from the Education Development Centre to the Centre for Disease Control, Centre for Prevention Services, Division of Sexually Transmitted Diseases. | |
| Consumer involvement | None reported. | |
| Notes | For the knowledge and compliance outcomes the SD was calculated from the P value of the AVOVA F‐test. The number in each group was not reported other than stating that there were between 38 and 42 in each arm, therefore the SD was estimated using the average number of patients in each group (40). The trial authors reported knowledge as the mean number of correct responses out of a possible total of 15. From this the mean percentage of correct responses was calculated for use in the meta‐analysis. Data from the premature resumption of sexual activity outcome could not be extracted for meta‐analysis as the authors reported medians only. No data could be extracted for the satisfaction with care, perceptions of illness and confidence in treatment regime outcomes as the effect of the multimedia intervention was not reported separately from that of the special pill packaging. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were "assigned at random." No further information was given. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding (performance bias and detection bias) All outcomes | High risk | The patients and the doctor who gave the patients instructions were not blinded. However, the doctor who assessed the patients on day two or six via telephone was blinded "When calling the doctor had only the patient's name and telephone number in front of him and no information about that person's experimental condition." The participants had the opportunity to seek further information prior to evaluation raising the possibility that the lack of blinding may influence outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 51/372 participants were unable to be contacted via telephone follow up. The number of participants missing for each of the groups was not reported apart from the statement that they were "evenly distributed throughout the eight experimental conditions." Each group retained between 38 and 52 participants. The results appear to have been calculated based on results for participants remaining in the study at that time point. |
| Selective reporting (reporting bias) | Low risk | The outcomes stated in the method section have been reported. |
| Other bias | Low risk | There was no selection bias as the program was delivered on site using the available facilities. |
Stone 1989.
| Methods |
Aim of study: To examine videotape instruction as a teaching method and to assess specifically its effectiveness and acceptability by patients when compared with individual instruction for patients beginning oral anticoagulant medication. Aim of the intervention: To educate patients about warfarin. Study design: Randomised controlled trial with two arms: videotape and nurse lecture. Study duration: Intervention and assessments occurred over one visit. Recruitment process: Patients referred to hospital‐based anticoagulant clinic who had not received anticoagulation therapy in the previous five years were enrolled. Further details regarding recruitment were not reported. Inclusion criteria: Patient referred to hospital based anticoagulant clinic. Exclusion criteria: Excluded if received anticoagulant therapy in previous five years. Informed consent: Not reported. Ethics approval: Not reported. |
|
| Participants |
Description: Patients referred to a hospital based anticoagulation clinic. Location: Hospital‐based coagulation clinic in Massachusetts, USA. Number: 22 patients were enrolled and randomised: 13 into the personalised education and 9 into the video group. Age (mean years): I: 60, C: 58.6. Gender: Not reported. Ethnicity: Not reported. Educational level (mean years): I: 10.9, C: 12.1. Literacy: Not reported. Primary diagnosis being treated (N): Deep venous thrombosis I: 2, C: 3, atrial fibrillation I: 3, C: 3, other I: 4, C: 7. Acute or chronic: Chronic. Co‐morbidities: Not reported. Treatment: Warfarin. |
|
| Interventions |
Topic of education: Information about warfarin. Intervention: Video outlining reasons for anticoagulation therapy, complications of warfarin, how to monitor therapy and other general points about anticoagulation. Co‐intervention: Question and answer session with nurse after watching the video. Comparison intervention: Individual 25‐minute lecture given by a nurse followed by a question and answer session. |
|
| Outcomes |
Primary outcomes: Knowledge about warfarin. Secondary outcomes: Time required for teaching and questions, patients' impression of learning, patient satisfaction with the education and level of comfort with the medication. Methods of assessing outcomes: Knowledge about warfarin was measured using an 18 item true/false questionnaire, and the mean score out of a possible 18 was reported. A 12‐item satisfaction questionnaire tested satisfaction with the teaching method, whether patients felt that they learned from the presentation and their level of comfort with the medication after instruction. Items were scored one to five with highest being the most positive response to the instruction giving a maximum total score of 25. The time required for teaching and the question/answer sessions was also recorded. Reliability and validity of outcome measures: 18‐item knowledge questionnaire was developed by authors. The questionnaire was pilot tested on a control group of individuals outside the medical field prior to the study with questions removed if they did not discriminate between warfarin knowledge or lack of knowledge. No information was reported of the reliability or validity of this measure. Scores were summated but no analysis of dimensionality of the scale was reported. A 12‐item satisfaction questionnaire developed by the authors with no information reported of its development or testing. Timing of outcome assessments: Immediately before and after the intervention. Follow‐up of non‐respondents: Study all performed during one visit therefore there were no non‐respondents. Adverse effects of the intervention: Not recorded. |
|
| Sources of funding | None stated. | |
| Consumer involvement | None reported. | |
| Notes | Patient knowledge was reported as a mean number of correct answers out of a possible highest score of 18, and SE; from this we calculated the mean percentage of correct responses for the purposes of meta‐analysis, and converted the SE to a SD. Patient satisfaction with the education was not included in the meta‐analysis as no SD was reported or could be calculated or imputed. The authors reported the mean time required for the question and answer session following the education in each arm. This was described in the text and not included in the meta‐analysis. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "Patients were randomised based on odd or even medical record numbers". Sequence generation used a non‐random approach using hospital record number. |
| Allocation concealment (selection bias) | High risk | Allocation was based on hospital record number and would allow investigators to foresee allocation. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No information was provided regarding blinding of participants or investigators. Assessment consisted of a questionnaire testing knowledge administered immediately before and after the control or intervention education. Education provided in the control and intervention groups was standardised. Although these factors minimise the potential for the lack of blinding influencing outcomes a judgement could not be made based on the information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Although no information was provided regarding missing outcomes it is assumed that they were available from all participants as the study was completed in one visit. |
| Selective reporting (reporting bias) | Low risk | All outcomes outlined in the methods section are reported. |
| Other bias | Low risk | There was no selection bias as the program was delivered on site using the available facilities. |
Trent 2010.
| Methods |
Aim of study: "To examine the effectiveness of a brief behavioral intervention at the time of pelvic inflammatory disease (PID) diagnosis on subsequent patient adherence behaviours". Aim of the intervention: To educate patients regarding PID self‐care in order to improve patient adherence behaviours. Study design: Randomised controlled trial with two arms: Video plus standardised care versus standardised care alone. Study duration: The intervention and assessment period for each study participant was two weeks. Recruitment process: Trained research assistants screened patients presenting to the participating centres to determine eligibility for participation. Inclusion criteria: Patients diagnosed with mild to moderate PID who were to be treated as outpatients, were permanent residents of the metropolitan area under study, had access to a telephone for follow‐up and were willing to participate in the study. Exclusion criteria: Patients 14 years and younger and those with severe PID defined as those with potential surgical emergencies, significant nausea, vomiting or high fever, evidence of tuboovarian abscess or other extenuating medical circumstances. Patients who were pregnant, were concurrently diagnosed with sexual assault, were unable to communicate with staff due to cognitive, mental or language difficulties or had been previously enrolled and re‐diagnosed with PID. Informed consent: Yes, "The study was explained in detail using an interactive informed‐consent procedure". Ethics approval: Yes, "The study was approved by the Johns Hopkins School of Medicine Institutional Review Board and the Saint Agnes Hospital Institutional Review Board. Additional approval was obtained from the Maryland State Attorney General for the recruitment of children who were wards of the state". |
|
| Participants |
Description: Urban adolescents from a sexually transmitted infection prevalent community diagnosed with mild to moderate PID. Location: Patients were recruited from five clinical sites in two institutions (a large academic medical centre and a community hospital with close ties to the academic centre) in a large urban centre on the east coast of the USA that has been disproportionately affected by sexually transmitted infections. These clinical sites included the paediatric and adult emergency departments and general paediatric and adolescent medicine clinics. Number: 126 patients were enrolled. Of these 61 were in the intervention group, 65 in the control group. A further 5 were enrolled but not included in the study due to "data loss". Age: Range 15‐21 years. Mean age 17.3 years (SD 1.7). Gender: Female. Ethnicity: 90% African‐American, 6% European‐American, 4% other, no Latino/Hispanics. Educational level: Not reported. Literacy: Not reported. Primary diagnosis being treated: Pelvic inflammatory disease. Acute or chronic: Acute. Co‐morbidities: Not reported. Treatment: Antibiotics. |
|
| Interventions |
Topic of education: Self‐care of PID. Intervention: Video about the barriers and benefits of PID self‐care. Co‐intervention: Standardised care consisting of detailed discharge instructions based on the 2006 Centre for Disease Control STD treatment guidelines, a 14‐day course of medication and a written hand‐out to facilitate self‐care. Comparison intervention: Standardised care as described above. |
|
| Outcomes |
Primary outcomes: Medication adherence and attendance at the 72‐hour follow‐up visit. Secondary outcomes: Partner notification and treatment, temporary abstinence from sexual intercourse and development of complications such as worsening abdominal pain, inability to tolerate medications and requirement of additional clinical care beyond the expected 72‐hour follow‐up visit. Methods of assessing outcomes: Participants completed an audio computerised self‐interview prior to the intervention which collected detailed demographic measures as well as sexual and STD history, sexual abstinence, self‐care self efficacy and perceived benefits of treatment and follow‐up. They were then contacted after completing the two week treatment period to organise a face to face interview. At the interview a disease intervention specialist ("experienced individuals who worked with the city health department to locate community members with reportable or communicable infectious diseases") asked patients about the course of their illness using a standardised interview structure and completion of a form. Reliability and validity of outcome measures: Not applicable as outcomes were based on patient report obtained in an interview. Timing of outcome assessments: Baseline data were obtained prior to the intervention and outcomes were measured following the completion of the two week treatment period. Follow‐up of non‐respondents: Not reported. Participants were reimbursed $10 for the baseline interview and $25 for the follow‐up interview. Adverse effects of the intervention: Not reported. |
|
| Sources of funding | From the Robert Wood Johnson Generalist Faculty Scholars Program, the Centre for Disease Control and Prevention, the Thomas Wilson Foundation for the Children of Baltimore City and the John and Mary McCarthy Foundation. | |
| Consumer involvement | Adolescents who had a history of STD were involved in qualitative research to refine the video intervention. | |
| Notes | Patient compliance with specific treatment instructions was measured. These included the number of participants who attended a medical follow‐up appointment, abstained from sexual intercourse during treatment, notified their partners of the sexually transmitted disease diagnosis and the number of partners who were treated. Patient attendance at the follow‐up appointment was selected for the meta‐analysis as this was identified as a primary outcome measure by the study authors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised "by a computer generated randomisation system". |
| Allocation concealment (selection bias) | Low risk | "Envelopes containing the group assignment were opened by participants after informed consent to participate had been obtained from each of them". |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No information was provided regarding blinding of participants or investigators. Adherence with medication and 72‐hour follow up were subjective measures drawn from a telephone interview. Assessments were not made directly after the intervention and participants were free to access further information which raises the possibility that any lack of patient blinding may influence the outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 131 participants were enrolled but 126 were included in the study as baseline data were not available for the other five participants. Of the 126 participants who were randomised, 77 completed the two week outcome assessment and were included in the analysis: 36/61 in the intervention arm and 41/65 in the control arm. As the proportion of patients excluded from analyses was similar in both groups we do not expect the analyses to favour one group. |
| Selective reporting (reporting bias) | Low risk | All of the outcomes outlined in the methods section were reported. |
| Other bias | Low risk | There was no selection bias as the program was delivered on site using the available facilities. |
Van Der Palen 1997.
| Methods |
Aim of study: To compare the long‐term efficacy of three modes of inhaler technique instruction. Aim of the intervention: To provide instruction on correct inhaler technique. Study design: Randomised controlled trial with four arms: videotape, personal instruction, group instruction and usual care. Study duration: Up to nine months. Recruitment process: All COPD patients who attended the pulmonary outpatient department between February and June 1994. Inclusion criteria: Patients aged 18 to 65 with COPD. Exclusion criteria: Those who had used inhaled medication for less than one month or with limited ability to understand and speak Dutch. Informed consent: Yes "informed consent was obtained from all COPD patients". Ethics approval: Yes "Approval for the study was acquired from the hospital's ethical committee". |
|
| Participants |
Description: Patients with COPD attending a pulmonary outpatient department. Location: The Netherlands. Number: 152 patients: 33 control (C), 37 group instruction (GI), 40 personal instruction (PI), 38 video instruction (VI), 4 excluded. Age (mean (SD)): C: 56 (8), GI: 54 (9), PI: 55 (9), VI: 56 (9). Gender N (%) : Male C: 20(61), GI: 24(65), PI: 25 (62), VI: 26 (68) Female C: 13 (39), GI: 13 (35), PI: 15 (38), VI: 12 (32). Ethnicity: Not reported. Educational level N (%): Low C: 26 (79), GI: 31 (86), PI: 35 (87), VI: 28 (74). High C: 7 921, GI: 5 (14), PI: 5 (13), VI: 10 (26). Literacy: Not reported. Primary diagnosis being treated: COPD. Acute or chronic: Chronic. Co‐morbidities: Not reported. Treatment: Inhaled medication. |
|
| Interventions |
Topic of education: Inhaler technique instruction. Intervention: Instructional video. Co‐intervention: Inhaler checklist with the patients errors marked were given to participants in all intervention groups to take home. Comparison interventions: Personal instruction (PI) from pulmonary function technician. Errors were corrected using verbal instructions and visual demonstrations and patients demonstrated their inhalation technique until no errors were made. Group instruction (GI) with five to seven patients educated by specialised registered nurse. All patients had to demonstrate their technique in front of the group. Average session lasted 45 minutes. Control group received no education. |
|
| Outcomes |
Primary outcomes: Inhaler technique. Secondary outcomes: None. Methods of assessing outcomes: Inhaler technique was assessed by well‐trained pulmonary function technicians using purpose designed inhaler specific checklists. Three inhaler technique scores were calculated: all checklist items, essential items only (based on previous work to determine essential steps for different inhalers) and percentage of patients completing all essential items correctly. Reliability and validity of outcome measures: The checklists were adapted from those of the Dutch Asthma Foundation and were tested in a pilot study. Timing of outcome assessments: Baseline (pre‐intervention) and at the next scheduled visit to the chest physician up to 9 months later (mean time 25 weeks for control, 26 weeks for group and 19 weeks for both video and personal arms). Follow‐up of non‐respondents: Not reported. Adverse effects of the intervention: Not reported. |
|
| Sources of funding | Netherlands Asthma Foundation, the OostNederland Health Care Insurance Fund, GlaxoWellcome and Astra Pharmaceuticals provided financial support, videotapes and placebo inhalers. | |
| Consumer involvement | None reported. | |
| Notes | The four arms from this study (videotape, personal instruction, group instruction and usual care) were included in the review as the comparisons of videotape vs instruction by a health professional (combining results from the personal and group instruction arms) and videotape vs usual care. The inhaler technique score based on all checklist items was selected for the meta‐analysis as it was an overall measure of patients' skill and presented as the first outcome in the study report. As no SD was reported for the individual arms the SD reported for the mean overall score across all arms was used for all of the groups. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants "were randomised into one of four groups". The methods used for sequence generation were not reported. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No information was provided regarding blinding of participants or assessors. Assessments of inhaler technique were based on a checklist but remained subjective. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4/152 patients did not attend their follow‐up visit with the chest physician and were excluded from the analysis. No information was provided regarding what group these patients had been randomised into. As the proportion of patients excluded from analyses was small we do not expect the analyses to favour one group. |
| Selective reporting (reporting bias) | Low risk | All outcomes identified in the methods section were reported. |
| Other bias | Low risk | Randomisation was stratified by possession of a video recorder. |
Voris 1982.
| Methods |
Aim of study: 1. To develop an individualised tricyclic audio‐visual education program for patients with depression. 2. To compare the program's effectiveness with that of traditional education in increasing a patient's short‐term knowledge about tricyclic antidepressants and depression. 3. to compare the program's effectiveness with that of traditional education in increasing a patient's long‐term knowledge about tricyclic antidepressants and depression. Aim of the intervention: To increase both short‐term and long‐term knowledge about tricyclic antidepressants and depression. Study design: Randomised controlled trial with two arms: audiovisual presentation and usual care. Study duration: Four weeks. Recruitment process: Possible study participants were identified "through discussions with team members, chart reviews and medication order sheet reviews" Inclusion criteria: Age ≥ 19, taking tricyclic antidepressants, able to read and speak English. Exclusion criteria: Must not have documented organic brain syndrome or schizo‐affective disorder. Informed consent: Yes, "physician and patient consent were obtained". Ethics approval: Not reported. |
|
| Participants |
Description: Patients who have recently commenced a tricyclic antidepressant. Location: Nebraska Psychiatric Institute and the University of Nebraska Hospital and Clinic in Omaha, Nebraska, USA. Number: 11 patients were recruited for the study: five control, six intervention. Age: Not reported other than inclusion criteria that participants be aged ≥ 19. Gender: Not reported. Ethnicity: Not reported. Educational level: Not reported. Literacy: Not reported. Primary diagnosis being treated: Depression. Acute or chronic: Chronic. Co‐morbidities: Not reported. Treatment: Tricyclic antidepressants. |
|
| Interventions |
Topic of education: Information about tricyclic antidepressants. Intervention: A slide‐tape audiovisual presentation of information about tricyclic antidepressants. Co‐intervention: None. Comparison intervention: Standard education. |
|
| Outcomes |
Primary outcomes: Patient knowledge. Secondary outcomes: None. Methods of assessing outcomes: Knowledge was tested using a questionnaire with 20 MCQ or true/false items. A mean knowledge score (maximum score of 20) was reported. Reliability and validity of outcome measures: Questions were developed by the authors (pharmacists) based on the content of the intervention. The wording of the question was examined by a specialist in educational reading to ensure that they were readable at a sixth to seventh grade level. No other testing of the questionnaire was reported. Timing of outcome assessments: Both groups received the pretest questionnaire once they were started on the medication and their dose was stable. The intervention group received the program within 48 hours of completing the pretest. The post test was then administered between 24 hours and three days after the program. The control group received the post test between four and five days after receiving the pretest. Both groups received a follow up test approximately four weeks after the post test. Follow‐up of non‐respondents: Not reported. Adverse effects of the intervention: Not reported. |
|
| Sources of funding | Supported in part by NIMH grant. | |
| Consumer involvement | None reported. | |
| Notes | The SD was not reported, could not be calculated from other reported statistics and was therefore imputed from Mazor 2007. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A table of random numbers was used to allocate the patients into groups. The investigators used a random component for sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. It is unclear whether the random number table was open or concealed from investigators. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No information was provided regarding blinding of participants, health professionals who provided patients with information as part of usual care or the investigator who answered participants' questions in both groups. Knowledge, measured using a questionnaire, is a relatively objective outcome. However, participants were instructed by health professionals approximately four days before knowledge was tested raising the potential that any lack of blinding might affect outcomes. The use of a questionnaire minimised the potential for the lack of blinding of assessors influencing outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | All participants who completed the baseline assessment also completed the follow‐up assessment three to five days later. 1/5 participants in the control group and 2/6 from the intervention group did not complete assessments performed four weeks later. No reasons were provided for the participant attrition. Mean scores appear to have been calculated based on results for participants remaining in the study at that time point. |
| Selective reporting (reporting bias) | Low risk | All of the outcomes outlined in the methods section were reported. |
| Other bias | Low risk | There was no selection bias as the program was delivered on site using the available facilities. |
MDI ‐ Metered Dose Inhaler, COPD ‐ Chronic Obstructive Pulmonary Disease, MCQ ‐ Multiple Choice Question, PCA ‐ Patient Controlled Analgesia, FEV ‐ Forced Expiratory Volume, OTC ‐ Over the Counter, EC ‐ Emergency Contraception, STD ‐ Sexually Transmitted Disease, VCR ‐ Videocassette Recorder.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Agertoft 1998 | All participants received the multimedia education |
| Albert 2007 | Primary focus of education was not medications |
| Allen LaPointe 2006 | Complex intervention, cannot examine effect of multimedia education alone |
| Anderson 2004 | Primary focus of education was not medications |
| Bakker 1999 | Unable to determine if the intervention met the definition of multimedia used for this review |
| Barry 1997 | Decision aid |
| Bartholomew 2006 | Complex intervention, cannot examine effect of multimedia education alone |
| Bauchner 2001 | Cannot examine effect of multimedia education alone |
| Bieber 2004 | Primary focus of education was not medications |
| Bourgeois 2008 | Primary focus of education was not medications |
| Brock 2007 | Cannot examine effect of multimedia education alone |
| Brook 2003 | Complex intervention, cannot examine effect of multimedia education alone |
| Brown 1997 | Primary focus of education was not medications |
| Chan 2003 | Complex intervention, cannot examine effect of multimedia education alone |
| Chan 2007 | Complex intervention, cannot examine effect of multimedia education alone |
| Charles 1999 | Unable to determine if the intervention met the definition of multimedia used for this review |
| Chewning 1999 | Decision aid |
| Cordina 2001 | Complex intervention, cannot examine effect of multimedia education alone |
| Cromheecke 2000 | All participants received the multimedia education |
| Currier 2004 | Primary focus of education was not medications |
| D'Alessandro 2004 | Complex intervention, cannot examine effect of multimedia education alone or determine which participants, if any, accessed the multimedia component of the recommended websites |
| Done 1998 | Primary focus of education was not medications |
| Dunn 1998 | Decision aid |
| Edworthy 1999 | Unable to determine if the intervention met the definition of multimedia used for this review |
| Elkjaer 2010 | Complex intervention, cannot examine effect of multimedia education alone |
| Fureman 1997 | Education about an experimental (unlicensed) medication |
| Gilliam 2004 | Complex intervention, cannot examine effect of multimedia education alone |
| Goldsmith 1999 | Primary focus of education was not medications |
| Hawkins 1993 | Primary focus of education was not medications |
| Holzheimer 1998 | Primary focus of education was not medications |
| Homer 2000 | Primary focus of education was not medications |
| Homer 2005 | Education aimed at health professionals |
| Howell 2006 | Primary focus of education was not medications |
| Huang 2009 | Primary focus of education was not medications |
| Huss 2003 | Primary focus of education was not medications |
| Ingersoll 2011 | Complex intervention, cannot examine effect of multimedia education alone |
| Jank 2009 | Intervention was not multimedia |
| Kerner 1999 | Decision aid |
| Kobulnicky 2002 | Complex intervention, cannot examine effect of multimedia education alone |
| Krishna 2003 | Primary focus of education was not medications |
| Kulp 2004 | Primary focus of education was not medications |
| Liang 2006 | Intervention was not multimedia |
| Lin 2008 | Primary focus of education was not medications |
| Linne 1999 | Program contains text with still graphics/illustrations but no audio or video and therefore does not meet definition for multimedia |
| Linne 2000 | Program contains text with still graphics/illustrations but no audio or video and therefore does not meet definition for multimedia |
| Linne 2001 | Program contains text with still graphics/illustrations but no audio or video and therefore does not meet definition for multimedia |
| Linne 2006 | Program contains text with still graphics/illustrations but no audio or video and therefore does not meet definition for multimedia |
| Lovell 2010 | Primary focus of education was not medications |
| Maasland 2007 | Primary focus of education was not medications |
| Madoff 1996 | Not multimedia |
| May 2003 | All participants received the multimedia education |
| McGinley 2002 | Program contains text with still graphics/illustrations but no audio or video and therefore does not meet definition for multimedia |
| McPherson 2006 | Primary focus of education was not medications |
| Moldofsky 1979 | Primary focus of education was not medications |
| Montgomery 2003 | Cannot examine effect of multimedia education alone |
| Mullan 2009 | Decision aid |
| Murray 2001 | Decision aid |
| Nannenga 2009 | Decision aid |
| Nolte 2006 | Not education about medication |
| Nordfeldt 2005 | Cannot examine effect of multimedia education alone |
| Oliveira 2003 | Primary focus of education was not medications |
| Peet 1996 | Unable to determine if the intervention met the definition of multimedia used for this review |
| Petro 2005 | Unable to determine if the intervention met the definition of multimedia used for this review |
| Powell 1979 | Cannot examine effect of multimedia education alone |
| Radcliffe‐Branch 1998 | Primary focus of education was not medications |
| Riesen 2008 | Complex intervention, cannot examine effect of multimedia education alone |
| Rostom 2002 | Decision aid |
| Sampaio‐Sa 2008 | Primary focus of education was not medications |
| Saver 2007 | Decision aid |
| Schneider 2008 | All participants received the multimedia education |
| Schwarz 2008b | Primary focus of education was not medications |
| Sheridan 2006 | Decision aid |
| Siminoff 2006 | Decision aid |
| Soflin 1977 | Cannot examine effect of multimedia education alone |
| Sorrell 1983 | Program contains text only and therefore does not meet definition for multimedia |
| Steinberg 1996 | Primary focus of education was not medications |
| Steiner 2009 | Complex intervention with no multimedia component reported |
| Sundberg 2005 | Primary focus of education was not medications |
| Syrjala 2008 | Cannot examine effect of multimedia education alone |
| Taylor 2005 | Cannot examine effect of multimedia education alone |
| Thomas 2000 | Education about medication and other therapy. Only 37% taking medication and cannot separate effect of education about medication on this group. |
| Thomas 2009 | Primary focus of education was not medications |
| Thomson 2007 | Decision aid |
| Van Der Meer 2009 | Complex intervention, cannot examine effect of multimedia education alone |
| Williams 2005 | Primary focus of education was not medications |
| Yakirevitch 2010 | Unable to determine if the intervention met the definition of multimedia used for this review |
| Yuan 2005 | Primary focus of education was not medications |
Characteristics of ongoing studies [ordered by study ID]
Smith 2010.
| Trial name or title | Trial of an educational intervention on patients' knowledge of atrial fibrillation (AF) and anticoagulant therapy, International Normalised Ratio (INR) control, and outcome of treatment with warfarin |
| Methods |
Aim of study: To examine the effects of an intensive educational intervention on patients' INR control. Aim of the intervention: To provide information about oral anticoagulant therapy for atrial fibrillation. Study design: Randomised controlled trial with two arms: intensive education and usual care. Study duration: 12 months. Recruitment process: Patients recruited from the outpatient AF clinics at participating hospitals. Patients eligible for oral anticoagulant therapy received a standard explanation of the need for this therapy and its risk/benefits. Those who accepted this treatment were approached to participate in the study. Inclusion criteria: AF patients newly referred for warfarin therapy with ECG‐documented AF. Exclusion criteria: Age <18 years, contraindications to warfarin, prior use of warfarin, valvular heart disease, cognitive impairment, unable to speak or read English and have any disease likely to cause death within 12 months. Informed consent: Yes "All participants will provide written informed consent. Ethics approval: Yes "granted by the Black Country Research Ethics Committee". |
| Participants |
Description: Patients with AF newly referred for warfarin therapy. Location: The outpatient AF clinics at City Hospital, Sanwell District General Hospital and Good Hope Hospital in the United Kingdom. Primary diagnosis being treated: Atrial fibrillation. Acute or chronic: Chronic. Co‐morbidities: Not reported. Treatment: Warfarin. |
| Interventions |
Topic of education: Oral anticoagulant therapy for AF. Intervention: Group session of two to eight patients in which they are shown the DVD, encouraged to ask questions and complete a worksheet‐based exercise to reinforce the information presented. Co‐intervention: Educational booklet. Comparison intervention: Usual care consisting of information provided by dosing officers and anticoagulation nurses and an educational booklet. |
| Outcomes |
Primary outcomes: Proportion of time spent within the therapeutic INR range Secondary outcomes: Patient knowledge, beliefs about medication, anxiety and depression, illness representations, cost effectiveness of the intervention and the incidence of strokes, minor and major bleeding and thromboembolic events. Methods of assessing outcomes: All INR results between 1 and 12 months after starting warfarin were recorded and will be used to calculate the proportion of time each patient spent in the therapeutic INR range (2‐3). Incidence of strokes, bleeding and thromboembolic events was determined from the computerised clinical information system at the hospital. Other secondary outcomes were measured using questionnaires: A 14‐item questionnaire tested knowledge about AF and anticoagulant therapy. The other questionnaires consisted of the Beliefs about Medication Questionnaire (BMQ) Horne 1999, the hospital anxiety and depression scale Zigmond 1983 and the Illness Perception Questionnaire (IPQ‐B) Weinman 1996. Reliability and validity of outcome measures: The knowledge questionnaire was devised by the authors for use in a previously published before and after study of written information about AF and warfarin Lane 2006. No testing of its reliability or validity was reported. The other questionnaires were established instruments. Timing of outcome assessments: 1, 2, 6 and 12 months after starting anticoagulant therapy. Follow‐up of non‐respondents: "If returned questionnaires are not fully completed, an independent researcher will contact the patient by telephone to facilitate 100% completion of the questionnaires". Adverse effects of the intervention: Not reported |
| Starting date | 1st January 2009 |
| Contact information | Deirdre A Lane: deirdre.lane@swbh.nhs.uk |
| Notes | We contacted the study authors. Data collection phase of the study has recently been completed but the data are yet to be analysed. |
INR ‐ international normalised ratio (therapeutic range 2.0 to 3.0), AF ‐ atrial fibrillation, ECG ‐ Electrocardiogram.
Differences between protocol and review
The protocol (Ciciriello 2010) for this review specified that participants would include those prescribed (or directly administered by a health professional) a particular medication or medication regimen, or who had obtained an over‐the‐counter medication. We had intended to include participants who were yet to start taking the medication. When we examined the studies identified, we found that a number provided education about medications to participants who had not necessarily been prescribed or taken the medication of interest. As we felt that this population was equivalent to those who had been prescribed the medication but not yet taken it, we broadened our selection criteria to include participants who were provided with education about a particular medication, medication regimen or over‐the‐counter medication but who had not been prescribed or obtained the medication.
The protocol specified that the results of the meta‐analysis would be organised by the comparison intervention with the groups including: no education or usual care; another form of education; and control multimedia programs. When we examined the studies identified, we determined that combining studies that used different types of comparison education was not appropriate as the control arms were not clinically homogeneous. We therefore only combined studies that used the same form of education in the comparison arm. We also identified studies that compared multimedia in combination with a co‐intervention to the co‐intervention alone. These studies were also examined separately.
In order to avoid a large number of meta‐analyses each containing a small number of studies, we intended to divide studies into those dealing with acute or chronic conditions and calculate the overall pooled effect for health outcomes across all studies within these two groups. We also intended to calculate the pooled effect on health outcomes of individual clinical conditions when they had been studied in three trials or more. The threshold of three trials was chosen based on the precedent set in other Cochrane reviews (Hróbjartsson 2010). This was not required, as only four of the seven studies that measured health outcomes were suitable for inclusion in the meta‐analysis. The studies each reported different health outcomes for different clinical conditions.
The protocol specified that we would extract data from all time points. In this review, data from all time points are reported in Table 8 and discussed in the review. However, for the meta‐analysis we decided to divide time points into those that were short‐term (< 4 weeks) and long‐term (≥ 4 weeks) and if there were multiple time points within these categories we extracted the last time point measured. This was a post‐hoc decision based on the range of time points that the included studies measured and reported.
We had intended to correct the potential unit of analysis error in cluster randomised trials that failed to appropriately account for correlation of observations within clusters, by incorporating an approximate analysis of the trial using methods outlined in Higgins 2011b. This was not possible as the only cluster randomised trial included did not report outcomes in sufficient detail for them to be statistically adjusted for inclusion in the meta‐analysis.
We intended to assess publication bias graphically using a funnel plot if there were 10 or more studies included in the meta‐analysis of the primary outcomes (knowledge and skill acquisition). This was not performed as none of the meta‐analyses contained 10 or more studies.
We intended to determine an overall grading of the quality of the educational interventions and comparators (High, Moderate, Low) based upon our application of the ELF. This was not performed as adequate information on which to base the quality assessment was only available for the multimedia program used in six of the studies and the comparators from four of the studies. Information regarding the quality of the interventions and comparators is presented in Table 10 and summarised in the Description of studies section.
We intended to conduct subgroup analyses where data were available according to:
age group (examining the effect on the elderly (over 65 years) compared to those in other age groups);
literacy (groups with low literacy, as defined by the study authors, compared to others);
medications treating acute or chronic conditions;
quality of the intervention (high versus moderate and low quality according to the ELF); and
-
specific characteristics of the multimedia intervention according to:
-
degree of interactivity:
not interactive; e.g. video or DVD that can only be watched from beginning to end,
limited interactivity; e.g. DVD or computer program in which the individual can choose the information components that they want to access and the order in which they access them,
fully interactive; in which the responses of the program are constantly changing in response to user actions e.g. a computer game or simulation;
tailored (where the information provided is personalised, based on an individual's characteristics) versus generic information.
-
Subgroup analysis was not possible as there were an inadequate number of studies identified to allow comparison for these characteristics.
Since publication of the protocol (Ciciriello 2010), Claire O'Neill was added as a review co‐author.
Contributions of authors
SC and RB conceived and designed the review. SC and RJ screened search results and papers and extracted and analysed data from the papers. CO assisted SC with extraction of data for the Characteristics of studies and Description and evaluation of intervention tables. RC provided advice on the evaluation of the educational interventions. The review was written by SC, RB and RJ. TdK wrote the plain language summary. IW and RO revised all drafts of the review. TdK, CO and RC provided general advice on the review.
Sources of support
Internal sources
-
Department of Epidemiology & Preventive Medicine, School of Public Health & Preventive Medicine, Monash University, Australia.
In‐kind support
-
Cabrini Institute, Cabrini Medical Centre, Australia.
In‐kind support
-
Centre for Rheumatic Diseases, Department of Medicine (RMH/WH), The University of Melbourne, Australia.
In‐kind support
External sources
No sources of support supplied
Declarations of interest
The authors SC, RO, IW and RB have been involved in the development of a multimedia patient education program about methotrexate treatment for rheumatoid arthritis and two outcome measures for use in the evaluation of patient education interventions: the Medication Education Impact Questionnaire (MeiQ) and the Methotrexate in Rheumatoid Arthritis Knowledge test (MiRAK). The authors have, or have had in the past, affiliations with The University of Melbourne, Monash University and Deakin University, and these institutions have taken steps to protect intellectual property rights associated with the above.
New
References
References to studies included in this review
Acosta 2009 {published data only}
- Acosta J. Educational intervention in adult asthma: a randomized clinical trial to determine if adult patients with asthma can learn how to use a metered dose inhaler (MDI). Weill Medical College of Cornell University 2009.
Deitz 2011 {published data only (unpublished sought but not used)}
- Deitz DK, Cook RF, Hendrickson A. Preventing prescription drug misuse: field test of the SmartRx web program. Substance Use & Misuse 2011;46:678‐86. [DOI] [PubMed] [Google Scholar]
Goodyer 2006 {published data only}
- Goodyer L, Savage I, Dikmen Z. Inhaler technique in Turkish people with poor English: a case of information discrimination?. Pharmacy World and Science 2006;28(2):107‐14. [DOI] [PubMed] [Google Scholar]
Kato 2008 {published data only}
- Kato P, Cole S, Bradlyn A, Pollock B. A video game improves behavioral outcomes in adolescents and young adults with cancer: a randomized trial. Pediatrics 2008;122(2):e305‐17. [DOI] [PubMed] [Google Scholar]
Kinnane 2008 {published data only (unpublished sought but not used)}
- Kinnane N, Thompson L. Evaluation of the addition of video‐based education for patients receiving standard pre‐chemotherapy education. European Journal of Cancer Care 2008;17(4):328‐39. [DOI] [PubMed] [Google Scholar]
Knoerl 1999 {published data only}
- Knoerl DV, Faut‐Callahan M, Paice J, Shott S. Preoperative PCA teaching program to manage postoperative pain. MEDSURG Nursing: Official Journal of the Academy of Medical‐Surgical Nurses 1999;8(1):25‐33, 36. [PubMed] [Google Scholar]
Lirsac 1991 {published data only}
- Lirsac B, Braunstein G. A randomised assessment of two methods of using aerosols [Evaluation randomisee de deux methodes d'apprentissage de l'utilisation des aerosols‐doseurs]. Revue des Maladies Respiratoires 1991;8:559‐65. [PubMed] [Google Scholar]
Mazor 2007 {published data only}
- Mazor K, Baril J, Dugan E, Spencer F, Burgwinkle P, Gurwitz J. Patient education about anticoagulant medication: is narrative evidence or statistical evidence more effective?. Patient Education and Counseling 2007;69(1‐3):145‐57. [DOI] [PubMed] [Google Scholar]
McElnay 1989 {published data only}
- McElnay JC, Scott MG, Armstrong AP, Stanford CF. Audiovisual demonstration for patient counselling in the use of pressurised aerosol bronchodilator inhalers. Journal of Clinical Pharmacy and Therapeutics 1989;14:135‐44. [DOI] [PubMed] [Google Scholar]
Navarre 2007 {published data only}
- Navarre M, Patel H, Johnson C, Durance A, McMorris M, Bria W, et al. Influence of an interactive computer‐based inhaler technique tutorial on patient knowledge and inhaler technique. Annals of Pharmacotherapy 2007;41(2):216‐21. [DOI] [PubMed] [Google Scholar]
Neafsey 2001 {published data only}
- Neafsey PJ, Strickler Z, Shellman J, Padula AT. Delivering health information about self‐medication to older adults: use of touchscreen‐equipped notebook computers. Journal of Gerontological Nursing 2001;27(11):19‐27. [DOI] [PubMed] [Google Scholar]
Neafsey 2002 {published data only}
- Neafsey PJ, Strickler Z, Shellman J, Chartier V, Neafsey PJ, Strickler Z, et al. An interactive technology approach to educate older adults about drug interactions arising from over‐the‐counter self‐medication practices. Public Health Nursing 2002;19(4):255‐62. [DOI] [PubMed] [Google Scholar]
Olver 2009 {published data only (unpublished sought but not used)}
- Olver IN, Whitford HS, Denson LA, Peterson MJ, Olver SI. Improving informed consent to chemotherapy: a randomized controlled trial of written information versus an interactive multimedia CD‐ROM. Patient Education & Counseling 2009;74(2):197‐204. [DOI] [PubMed] [Google Scholar]
Osguthorpe 1983 {published data only}
- Osguthorpe N, Roper J, Saunders J. The effect of teaching on medication knowledge. Western Journal of Nursing Research 1983;5(3):205‐16. [DOI] [PubMed] [Google Scholar]
Powell 1995 {published data only}
- Powell K, Edgren B. Failure of educational videotapes to improve medication compliance in a health maintenance organization. American Journal of Health‐System Pharmacy 1995;52(20):2196‐9. [DOI] [PubMed] [Google Scholar]
Savage 2003 {published data only}
- Savage I, Goodyear L. Providing information on metered dose inhaler technique: is multimedia as effective as print?. Family Practice 2003;20(5):552‐7. [DOI] [PubMed] [Google Scholar]
Schnellinger 2010 {published data only}
- Schnellinger M, Finkelstein M, Thygeson M, Vander Velden H, Karpas A, Madhok M. Animated video vs pamphlet: comparing the success of educating patients about proper antibiotic use. Pediatrics 2010;125(5):990‐6. [DOI] [PubMed] [Google Scholar]
Schwarz 2008a {published and unpublished data}
- Schwarz E, Gerbert B, Gonzales R. Computer‐assisted provision of emergency contraception a randomized controlled trial. Journal of General Internal Medicine 2008;23(6):794‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Self 1983 {published data only}
- Self TH, Brooks JB, Lieberman P, Ryan MR. The value of demonstration and role of the pharmacist in teaching the correct use of pressurized bronchodilators. Canadian Medical Association Journal 1983;128(2):129‐31. [PMC free article] [PubMed] [Google Scholar]
Solomon 1988 {published data only}
- Solomon MZ, DeJong W, Jodrie TA. Improving drug regimen adherence among patients with sexually transmitted disease. The Journal of Compliance in Health Care 1988;3(1):41‐56. [Google Scholar]
Stone 1989 {published data only}
- Stone S, Holden A, Knapic N, Ansell J. Comparison between videotape and personalized patient education for anticoagulant therapy. Journal of Family Practice 1989;29(1):55‐7. [PubMed] [Google Scholar]
Trent 2010 {published data only}
- Trent M, Chung S, Burke M, Walker A, Ellen JM. Results of a randomized controlled trial of a brief behavioral intervention for pelvic inflammatory disease in adolescents. Journal of Pediatric and Adolescent Gynaecology 2010;23:96‐101. [DOI] [PubMed] [Google Scholar]
Van Der Palen 1997 {published data only}
- Palen J, Klein J, Kerkhoff A, Herwaarden C, Seydel E. Evaluation of the long‐term effectiveness of three instruction modes for inhaling medicines. Patient Education and Counseling 1997;32(suppl 1):s87‐95. [DOI] [PubMed] [Google Scholar]
Voris 1982 {published data only}
- Voris JC, Wilson JE, DuBe JE. Tricyclic antidepressant education program and its evaluation. Hospital Pharmacy 1982;17(4):184‐6. [PubMed] [Google Scholar]
References to studies excluded from this review
Agertoft 1998 {published data only}
- Agertoft L, Pedersen S. Importance of training for correct Turbuhaler use in preschool children. Acta Paediatrica 1998;87(8):842‐7. [DOI] [PubMed] [Google Scholar]
Albert 2007 {published data only}
- Albert N, Buchsbaum R, Li J. Randomized study of the effect of video education on heart failure healthcare utilization, symptoms, and self‐care behaviors. Patient Education and Counseling 2007;69(1‐3):129‐39. [DOI: ] [DOI] [PubMed] [Google Scholar]
Allen LaPointe 2006 {published data only}
- Allen LaPointe N, DeLong E, Chen A, Hammill B, Muhlbaier L, Califf R, et al. Multifaceted intervention to promote beta‐blocker use in heart failure. American Heart Journal 2006;151(5):999‐1005. [DOI: ] [DOI] [PubMed] [Google Scholar]
Anderson 2004 {published data only}
Bakker 1999 {published data only}
- Bakker DA, Blais D, Reed E, Vaillancourt C, Gervais S, Beaulieu P. Descriptive study to compare patient recall of information: nurse taught versus video supplement. Canadian Oncology Nursing Journal 1999;9(3):115‐20. [DOI] [PubMed] [Google Scholar]
Barry 1997 {published data only}
- Barry M, Cherkin D, Chang Y, Fowler F, Skates S. A randomised trial of a multimedia shared decision‐making program for men facing a treatment decision for benign prostate hyperplasia. Disease Management and Clinical Outcomes 1997;1(1):5‐14. [Google Scholar]
Bartholomew 2006 {published data only}
Bauchner 2001 {published data only}
Bieber 2004 {published data only}
- Bieber C, Muller KG, Blumenstiel K, Schuller‐Roma B, Richter A, Hochlehnert A, et al. Shared decision making (SDM) with chronic pain patients. The patient as a partner in the medical decision making process. Bundesgesundheitsblatt, Gesundheitsforschung, Gesundheitsschutz 2004; Vol. 47, issue 10:985‐91. [DOI] [PubMed]
Bourgeois 2008 {published data only}
- Bourgeois F, Simons W, Olson K, Brownstein J, Mandl K. Evaluation of influenza prevention in the workplace using a personally controlled health record: randomized controlled trial. Journal of Medical Internet Research 2008;10(1):e5. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Brock 2007 {published data only}
- Brock G, Carrier S, Casey R, Tarride J, Elliot S, Dugre H, et al. Can an educational program optimise PDE5i therapy? A study of Canadian primary care practices. Journal of Sexual Medicine 2007;4(5):1404‐13. [DOI] [PubMed] [Google Scholar]
Brook 2003 {published data only}
- Brook O, Hout H, Nieuwenhuyse H, Heerdink E. Impact of coaching by community pharmacists on drug attitude of depressive primary care patients and acceptability to patients; a randomized controlled trial. European Neuropsychopharmacology 2003;13(1):1‐9. [DOI: ] [DOI] [PubMed] [Google Scholar]
Brown 1997 {published data only}
- Brown S, Lieberman D, Gemeny B, Fan Y, Wilson D, Pasta D. Educational video game for juvenile diabetes: results of a controlled trial. Medical Informatics 1997;22(1):77‐89. [DOI] [PubMed] [Google Scholar]
Chan 2003 {published data only}
Chan 2007 {published data only}
- Chan D, Callahan C, Hatch‐Pigott V, Lawless A, Proffitt H, Manning N, et al. Internet‐based home monitoring and education of children with asthma is comparable to ideal office‐based care: results of a 1‐year asthma in‐home monitoring trial. Pediatrics 2007;119(3):569‐78. [DOI: ] [DOI] [PubMed] [Google Scholar]
Charles 1999 {published data only}
- Charles E. Education of hypertensive patients assisted by computers [Education assistee par ordinateur pour patients hypertendus]. Revue de L'Infirmiere 1999;52:44‐5. [PubMed] [Google Scholar]
Chewning 1999 {published data only}
Cordina 2001 {published data only}
Cromheecke 2000 {published data only}
- Cromheecke M, Levi M, Colly L. Self management of long term oral anticoagulation was as effective as specialist anticoagulation clinic management. Evidence‐Based Medicine 2001;6(2):41. [DOI: ] [Google Scholar]
Currier 2004 {published data only}
- Currier RO. The relation between knowledge of ADHD and treatment acceptability in a multi‐disciplinary pediatric clinic [PhD thesis]. Louisiana, United States: Louisiana State University and Agricultural & Mechanical College, 2004. [Google Scholar]
D'Alessandro 2004 {published data only}
- D'Alessandro D, Kreiter C, Kinzer S, Peterson M. A randomized controlled trial of an information prescription for pediatric patient education on the Internet. Archives of Pediatrics and Adolescent Medicine 2004;158(9):857‐62. [DOI: ] [DOI] [PubMed] [Google Scholar]
Done 1998 {published data only}
- Done ML, Lee A. The use of a video to convey preanesthetic information to patients undergoing ambulatory surgery. Anesthesia and Analgesia 1998;87(3):531‐6. [DOI] [PubMed] [Google Scholar]
Dunn 1998 {published data only}
- Dunn RA, Shenouda PE, Martin DR, Schultz AJ. Videotape increases parent knowledge about poliovirus vaccines and choices of polio vaccination schedules. Pediatrics 1998;102(2):e26‐31. [DOI] [PubMed] [Google Scholar]
Edworthy 1999 {published data only}
- Edworthy SM, Devins GM. Improving medication adherence through patient education distinguishing between appropriate and inappropriate utilization. Patient education study group. Journal of Rheumatology 1999;26(8):1793‐801. [PubMed] [Google Scholar]
Elkjaer 2010 {published data only}
- Elkjaer M, Shuhaibar M, Burisch J, Bailey Y, Scherfig H, Laugesen B, et al. E‐health empowers patients with ulcerative colitis: a randomised controlled trial of the web‐guided "constant care" approach. Gut 2010;59:1652‐61. [DOI] [PubMed] [Google Scholar]
Fureman 1997 {published data only}
- Fureman I, Meyers K, McLellan AT, Metzger D, Woody G. Evaluation of a video‐supplement to informed consent: injection drug users and preventive HIV vaccine efficacy trials. AIDS Education & Prevention 1997;9(4):330‐41. [PubMed] [Google Scholar]
Gilliam 2004 {published data only}
- Gilliam M, Knight S, McCarthy M, Jr. Success with oral contraceptives: a pilot study. Contraception 2004;69(5):413‐8. [DOI] [PubMed] [Google Scholar]
Goldsmith 1999 {published data only}
- Goldsmith DM, Safran C. Using the Web to reduce postoperative pain following ambulatory surgery. Proceedings / AMIA 1999;Annual Symposium:780‐4. [PMC free article] [PubMed] [Google Scholar]
Hawkins 1993 {published data only}
- Hawkins R, Price K. The effects of an education video on patients' requests for postoperative pain relief. Australian Journal of Advanced Nursing 1993;10(4):32‐40. [PubMed] [Google Scholar]
Holzheimer 1998 {published data only}
- Holzheimer L, Mohay H, Masters IB. Educating young children about asthma: comparing the effectiveness of a developmentally appropriate asthma education video tape and picture book. Child: Care, Health & Development 1998;24(1):85‐99. [DOI] [PubMed] [Google Scholar]
Homer 2000 {published data only}
- Homer C, Susskind O, Alpert H, Owusu C, Schneider L, Rappaport L, et al. An evaluation of an innovative multimedia educational software program for asthma management: report of a randomized, controlled trial. Pediatrics 2000; Vol. 106, issue 1:210‐5. [PubMed]
Homer 2005 {published data only}
- Homer C, Forbes P, Horvitz L, Peterson L, Wypij D, Heinrich P. Impact of a quality improvement program on care and outcomes for children with asthma. Archives of Pediatrics and Adolescent Medicine 2005;159(5):464‐9. [DOI: ] [DOI] [PubMed] [Google Scholar]
Howell 2006 {published data only}
- Howell KJ. "Quest for the Code": A study of a computer based education program for children with asthma [PhD thesis]. New York: Syracuse University, 2006. [Google Scholar]
Huang 2009 {published data only}
- Huang TT, Li YT, Wang CH. Individualized programme to promote self‐care among older adults with asthma: randomized controlled trial. Journal of Advanced Nursing 2009;65(2):348‐58. [DOI] [PubMed] [Google Scholar]
Huss 2003 {published data only}
Ingersoll 2011 {published data only}
- Ingersoll KS, Farrell‐Carnahan L, Cohen‐Filipic J, Heckman CJ, Ceperich SD, Hettema J, et al. A pilot randomized clinical trial of two medication adherence and drug use interventions for HICV+ crack cocaine users. Drug and Alcohol Dependence 2011;116:177‐87. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jank 2009 {published data only}
- Jank S, Kappler M, Markmann A, Bertsche T, Klimm H, Haefeli W. Impact of an individualized computer‐assisted educational intervention on management of anticoagulant therapy. Basic and Clinical Pharmacology and Toxicology Conference: 10th Annual Congress of the German Association of Clinical Pharmacology. Conference Publication 2009;104(6):526. [Google Scholar]
Kerner 1999 {published data only}
- Kerner DN. Impact of a decision aid videotape on young women's attitudes and knowledge about hormone replacement therapy [PhD thesis]. San Diego: University of California, San Diego and San Diego State University, 1999. [Google Scholar]
Kobulnicky 2002 {published data only}
- Kobulnicky CJH. The behavioral impact of teaching patients to monitor side effects of chemotherapy: an intervention study [PhD thesis]. Madison, US: The University of Wisconsin ‐ Madison, 2002. [Google Scholar]
Krishna 2003 {published data only}
- Krishna S, Francisco B, Andrew Balas E, Konig P, Graff G, Madsen R. Internet‐enabled interactive multimedia asthma education program: a randomized trial. Pediatrics 2003;111(3):503‐19. [DOI: ] [DOI] [PubMed] [Google Scholar]
Kulp 2004 {published data only}
- Kulp JL, Rane S, Bachman G. Impact of preventive osteoporosis education on patients behavior: immediate and 3‐month follow‐up. Menopause: The Journal of The North American Menopause Society 2004;11(1):116‐9. [DOI] [PubMed] [Google Scholar]
Liang 2006 {published data only}
- Liang H, Xue Y, Berger BA. Web‐based intervention support system for health promotion. Decision Support Systems 2006;42(1):435‐49. [DOI: ] [Google Scholar]
Lin 2008 {published data only}
Linne 1999 {published data only}
- Linne A, Liedholm H, Israelsson B. Effects of systematic education on heart failure patients' knowledge after 6 months. A randomised, controlled trial. European Journal of Heart Failure 1999;1(3):219‐27. [DOI: ] [DOI] [PubMed] [Google Scholar]
Linne 2000 {published data only}
- Linne AB. Randomised, controlled trials of patient education with special reference to heart failure and joint diseases [PhD thesis]. Lund, Sweden: Lund University, 2000. [Google Scholar]
Linne 2001 {published data only}
- Linne AB, Liedholm H, Jacobsson L. The effects on knowledge of the systematic education of patients with joint diseases treated with NSAIDs and diuretics. Patient Education and Counseling 2001;42(2):165‐74. [DOI: ] [DOI] [PubMed] [Google Scholar]
Linne 2006 {published data only}
- Linne AB, Liedholm H. Effects of an interactive CD‐program on 6 months readmission rate in patients with heart failure ‐ a randomised, controlled trial [NCT00311194]. BMC Cardiovascular Disorders 2006;6:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lovell 2010 {published data only}
- Lovell MR, Forder PM, Stockler MR, Butow P, Briganti EM, Chye R, et al. A randomized controlled trial of a standardized educational intervention for patients with cancer. Journal of Pain and Symptom Management 2010;40(1):49‐59. [DOI] [PubMed] [Google Scholar]
Maasland 2007 {published data only}
- Maasland E, Koudstaal P, Habbema J, Dippel D. Effects of an individualized multimedia computer program for health education in patients with a recent minor stroke or transient ischemic attack ‐ a randomized controlled trial. Acta Neurologica Scandinavica 2007;115(1):41‐8. [DOI: ] [DOI] [PubMed] [Google Scholar]
Madoff 1996 {published data only}
- Madoff SA, Pristach CA, Smith CM, Pristach EA. Computerized medication instruction for psychiatric inpatients admitted for acute care. MD Computing 1996;13(5):427‐41. [PubMed] [Google Scholar]
May 2003 {published data only}
- May S, West R, Hajek P, Nilsson F, Foulds J, Meadow A. The use of videos to inform smokers about different nicotine replacement products. Patient Education and Counseling 2003;51(2):143‐7. [DOI: ] [DOI] [PubMed] [Google Scholar]
McGinley 2002 {published data only}
- McGinley AM. Effect of web‐based computer‐tailoring on women's intention to continue or begin to use hormone replacement therapy to lower their risk for osteoporosis [PhD thesis]. University of Pennsylvania, 2002. [978‐0‐493‐57810‐1] [Google Scholar]
McPherson 2006 {published data only}
- McPherson A, Glazebrook C, Forster D, James C, Smyth A. A randomized, controlled trial of an interactive educational computer package for children with asthma. Pediatrics 2006;117(4):1046‐54. [DOI: ] [DOI] [PubMed] [Google Scholar]
Moldofsky 1979 {published data only}
- Moldofsky H, Broder I, Davies G, Leznoff A. Videotape educational program for people with asthma. Canadian Medical Association Journal 1979;120(6):669‐72. [PMC free article] [PubMed] [Google Scholar]
Montgomery 2003 {published data only}
- Montgomery A, Fahey T, Peters T. A factorial randomised controlled trial of decision analysis and an information video plus leaflet for newly diagnosed hypertensive patients. British Journal of General Practice 2003;53(491):446‐53. [PMC free article] [PubMed] [Google Scholar]
Mullan 2009 {published data only}
- Mullan R, Montori V, Shah N, Christianson T, Bryant S, Guyatt G, et al. The diabetes mellitus medication choice decision aid: a randomized trial. Archives of Internal Medicine 2009;169(17):1560‐8. [DOI: ] [DOI] [PubMed] [Google Scholar]
Murray 2001 {published data only}
- Murray E, Davis H, Tai SS, Coulter A, Gray A, Haines A, et al. Randomised controlled trial of an interactive multimedia decision aid on hormone replacement therapy in primary care. BMJ 2001; Vol. 323, issue 7311:490‐3. [0959‐8138] [DOI] [PMC free article] [PubMed]
Nannenga 2009 {published data only}
- Nannenga MR, Montori VM, Weymiller AJ, Smith SA, Christianson TJ, Bryant SC, et al. A treatment decision aid may increase patient trust in the diabetes specialist. The statin choice randomized trial. Health Expectations 2009;12(1):38‐44. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nolte 2006 {published data only}
- Nolte S, Donnelly J, Kelly S, Conley P, Cobb R. A randomized clinical trial of a videotape intervention for women with chemotherapy‐induced alopecia: a gynecologic oncology group study. Oncology Nursing Forum 2006;33(2):305‐11. [DOI] [PubMed] [Google Scholar]
Nordfeldt 2005 {published data only}
- Nordfeldt S, Johansson C, Carlsson E, Hammersjo JA. Persistent effects of a pedagogical device targeted at prevention of severe hypoglycaemia: a randomized, controlled study. Acta Paediatrica 2005;94(10):1395‐401. [DOI] [PubMed] [Google Scholar]
Oliveira 2003 {published data only}
- Oliveira AC. The effects of a brief psychoeducational video on cervical strain symptoms six months post‐injury [PhD thesis]. San Diego, USA: California School of Professional Psychology ‐ San Diego, 2003. [Google Scholar]
Peet 1996 {published data only}
- Peet M, Turpin G, Nielson T. Teaching schizophrenic patients about their depot neuroleptic medication (conference abstract). XXth Collegium Internationale Neuro‐psychopharmacologicum. Melbourne, Australia, 23 ‐ 27 June, 1996.
Petro 2005 {published data only}
- Petro W, Schuppenies A. Inhalation therapy by dose‐inhalers: analysis of patients performance and possibilities for improvement. Pneumologie 2005;59(5):316‐20. [DOI] [PubMed] [Google Scholar]
Powell 1979 {published data only}
- Powell MF, Burkhart VD, Lamy PP. Diabetic patient compliance as a function of patient counseling. Drug Intelligence & Clinical Pharmacy 1979;13(9):506‐11. [DOI] [PubMed] [Google Scholar]
Radcliffe‐Branch 1998 {published data only}
- Radcliffe‐Branch D, Overbury O, Kasner OP, Renaud M. Effects of educational interventions on medication compliance, health beliefs, and knowledge in glaucoma patients (conference abstract). Investigative Opthalmology & Visual Science 1998.
Riesen 2008 {published data only}
- Riesen W, Noll G, Dariolu R. Impact of enhanced compliance initiatives on the efficacy of rosuvastatin in reducing low density lipoprotein cholesterol levels in patients with primary hypercholesterolaemia. Swiss Medical Weekly 2008;138(29‐30):420‐6. [DOI] [PubMed] [Google Scholar]
Rostom 2002 {published data only}
- Rostom A, O'Connor A, Tugwell P, Wells G. A randomized trial of a computerized versus an audio‐booklet decision aid for women considering post‐menopausal hormone replacement therapy. Patient Education and Counseling 2002;46(1):67‐74. [DOI: ] [DOI] [PubMed] [Google Scholar]
Sampaio‐Sa 2008 {published data only}
- Sampaio‐Sa M, Page‐Shafer K, Bangsberg D, Evans J, Dourado M, Teixeira C, et al. 100% adherence study: educational workshops vs. video sessions to improve adherence among ART‐naive patients in Salvador, Brazil. AIDS and Behavior 2008;12(suppl 1):s54‐62. [DOI: ] [DOI] [PubMed] [Google Scholar]
Saver 2007 {published data only}
- Saver BG, Gustafson D, Taylor TR, Hawkins RP, Woods NF, Dinauer S, et al. A tale of two studies: the importance of setting, subjects and context in two randomized, controlled trials of a web‐based decision support for perimenopausal and postmenopausal health decisions. Patient Education & Counseling 2007; Vol. 66, issue 2:211‐22. [DOI] [PubMed]
Schneider 2008 {published data only}
- Schneider N, Cortner C, Justice M, Gould JL, Amor C, Hartman N, et al. Preferences among five nicotine treatments based on information versus sampling. Nicotine & Tobacco Research 2008;10(1):179‐86. [DOI] [PubMed] [Google Scholar]
Schwarz 2008b {published data only}
- Schwarz K, Garrett B, Lee J, Thompson D, Thiel T, Alter M, et al. Positive impact of a shelter‐based hepatitis B vaccine program in homeless Baltimore children and adolescents. Journal of Urban Health 2008;85(2):228‐38. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sheridan 2006 {published data only}
- Sheridan S, Shadle J, Simpson, Jr, Pignone M. The impact of a decision aid about heart disease prevention on patients' discussions with their doctor and their plans for prevention: a pilot randomized trial. BMC Health Services Research 2006;6:121. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Siminoff 2006 {published data only}
- Siminoff L, Gordon N, Silverman P, Budd T, Ravdin P. A decision aid to assist in adjuvant therapy choices for breast cancer. Psycho‐Oncology 2006;15(11):1001‐13. [DOI: ] [DOI] [PubMed] [Google Scholar]
Soflin 1977 {published data only}
- Soflin D, Young WW, Clayton BD. Development and evaluation of an individualized patient education program about digoxin. American Journal of Hospital Pharmacy 1977;34(4):367‐71. [PubMed] [Google Scholar]
Sorrell 1983 {published data only}
- Sorrell SP. Enhancement of adherence to tricyclic antidepressants by computerized supervision [PhD thesis]. Illinois Institute of Technology, 1983. [Google Scholar]
Steinberg 1996 {published data only}
- Steinberg T, Diercks M, Millspaugh J. An evaluation of the effectiveness of a videotape for discharge of organ transplant recipients. Journal of Transplant Coordination 1996;6(2):59‐63. [DOI] [PubMed] [Google Scholar]
Steiner 2009 {published data only}
Sundberg 2005 {published data only}
- Sundberg R, Tunsater A, Palmqvist M, Ellbjar S, Lowhagen O, Toren K. A randomized controlled study of a computerized limited education program among young adults with asthma. Respiratory Medicine 2005;99(3):321‐8. [DOI: ] [DOI] [PubMed] [Google Scholar]
Syrjala 2008 {published data only}
- Syrjala K, Abrams J, Polissar N, Hansberry J, Robison J, DuPen S, et al. Patient training in cancer pain management using integrated print and video materials: a multisite randomized controlled trial. Pain 2008;135(1‐2):175‐86. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Taylor 2005 {published data only}
- Taylor J, Kwan‐Gett T, McMahon, Jr. Effectiveness of a parental educational intervention in reducing antibiotic use in children: a randomized controlled trial. Pediatric Infectious Disease Journal 2005;24(6):489‐93. [DOI: ] [DOI] [PubMed] [Google Scholar]
Thomas 2000 {published data only}
- Thomas R, Daly M, Perryman B, Stockton D. Forewarned is forearmed ‐ benefits of preparatory information on video cassette for patients receiving chemotherapy or radiotherapy ‐ a randomised controlled trial. European Journal of Cancer 2000;36(12):1536‐43. [DOI] [PubMed] [Google Scholar]
Thomas 2009 {published data only}
- Thomas C. Psychoeducational DVD intervention for acute low back pain [PhD thesis]. San Diego, CA: Alliant International University, 2009:161. [Google Scholar]
Thomson 2007 {published data only}
- Thomson R, Eccles M, Steen I, Greenaway J, Stobbart L, Murtagh M, et al. A patient decision aid to support shared decision‐making on anti‐thrombotic treatment of patients with atrial fibrillation: randomised controlled trial. Quality and Safety in Health Care 2007;16(3):216‐23. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Van Der Meer 2009 {published data only}
- Meer V. Summaries for patients. Internet‐based program to assist patients in asthma care. Annals of Internal Medicine 2009;151(2):110‐20. [DOI] [PubMed] [Google Scholar]
Williams 2005 {published data only}
- Williams G, McGregor H, Zeldman A, Freedman Z, Deci E, Elder D. Promoting glycemic control through diabetes self‐management: evaluating a patient activation intervention. Patient Education and Counseling 2005;56(1):28‐34. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Yakirevitch 2010 {published data only}
- Yakirevitch J, Marchevsky S, Abramovitch Y, Kotler M. Special informative Internet site for Schizophrenic patients: Its feasibility and contribution to pharmacotherapy. European Psychiatry Conference: 18th European Congress of Psychiatry Munich Germany Conference Publication. 2010; Vol. 25:1134.
Yuan 2005 {published data only}
- Yuan L, Manderson L, Ren M, Li G, Yu D, Fang J. School‐based interventions to enhance knowledge and improve case management of schistosomiasis: a case study from Hunan, China. Acta Tropica 2005;96(2‐3):248‐54. [DOI: ] [DOI] [PubMed] [Google Scholar]
References to ongoing studies
Smith 2010 {published data only}
- Smith DE, Xuereb CB, Pattison HM, Lip GYH, Lane DA. Trial of an educational intervention on patients' knowledge of atrial fibrillation and anticoagulant therapy, INR control, and outcome of treatment with warfarin. BMC Cardiovascular Disorders 2010;10(12):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Bajramovic 2004
- Bajramovic J, Emmerton L, Tett SE. Perceptions around concordance ‐ focus groups and semi‐structured interviews conducted with consumers, pharmacists and general practitioners. Health Expectations 2004;7(3):221‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Berry 1997
- Berry DC, Michas IC, Gillie A, Forster M. What do patients want to know about their medicines and what do doctors want to tell them? A comparative study. Psychology & Health 1997;12:467‐80. [Google Scholar]
Bodenheimer 2002
- Bodenheimer T, Lorig K, Homan H, Grumbach K. Patient self‐management of chronic disease in primary care. JAMA 2002;288:2469‐75. [DOI] [PubMed] [Google Scholar]
Bozionelos 2004
- Bozionelos N. Socio‐economic background and computer use: the role of computer anxiety and computer experience in their relationship. International Journal of Human‐Computer Studies 2004;61(5):725‐46. [Google Scholar]
Brus 1998
- Brus H, Laar M, Taal E, Rasker J, Wiegman O. Effects of patient education on compliance with basic treatment regimens and health in recent onset active rheumatoid arthritis. Annals of the Rheumatic Diseases 1998;57:146‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Buchbinder 2001
- Buchbinder R, Hall S, Grant G, Mylvaganam A, Patrick MR. Readability and content of supplementary written drug information for patients used by Australian rheumatologists. Medical Journal of Australia 2001;174(11):575‐8. [DOI] [PubMed] [Google Scholar]
Bultman 2000
- Bultman DC, Svarstad BL. Effects of physician communication style on client medication beliefs and adherence with antidepressant treatment. Patient Education and Counseling 2000;40(2):173‐85. [DOI] [PubMed] [Google Scholar]
Clerehan 2005
- Clerehan R, Buchbinder R, Moodie J. A linguistic framework for assessing the quality of written patient information: its use in assessing methotrexate information for rheumatoid arthritis. Health Education and Research 2005;20(3):334‐44. [DOI] [PubMed] [Google Scholar]
Clerehan 2009
- Clerehan R, Hirsh D, Buchbinder R. Medication information leaflets for patients: the further validation of an analytic linguistic framework. Communication and Medicine 2009;6(2):117‐27. [PubMed] [Google Scholar]
Cote 1997
- Cote J, Cartier A, Robichaud P, Boutin H, Malo JL, Rouleau M, et al. Influence on asthma morbidity of asthma education programs based on self management plans following treatment optimisation. American Journal of Respiratory and Critical Care Medicine 1997;155:1509‐14. [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. The Cochrane Collaboration.
Effing 2007
- Effing T, Monninkhof EEM, Valk PP, Zielhuis GGA, Walters EH, Palen JJ, et al. Self‐management education for patients with chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858.CD002990.pub2] [DOI] [PubMed] [Google Scholar]
Ewing 1984
- Ewing JA. Detecting alcoholism: the CAGE questionnaire. Journal of the American Medical Association 1984;252(14):1905‐7. [DOI] [PubMed] [Google Scholar]
Forman 2003
- Forman RF, Krejci J, Selzer J, Hantula DA, Potter JS, Grissom G, et al. Patient feedback manual, version 1.0. Philadelphia PA, University of Pennsylvania, Treatment Research Institute 2003.
Fraenkel 2002
- Fraenkel L, Bogardus S, Concato J, Felson D. Unwillingness of rheumatoid arthritis patients to risk adverse events. Rheumatology 2002;41:253‐61. [DOI] [PubMed] [Google Scholar]
Gibson 2002
- Gibson PG, Powell H, Wilson A, Hensley MJ, Abramson MJ, Bauman A, et al. Limited (information only) patient education programs for adults with asthma. Cochrane Database of Systematic Reviews 2002, Issue 1. [DOI: 10.1002/14651858.CD001005] [DOI] [PubMed] [Google Scholar]
Gray 2009
- Gray CL, Gardner C. Adverse drug events in the elderly: an ongoing problem. Journal of Managed Care Pharmacy 2009;15(7):568‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Grime 2007
- Grime J, Blenkinsopp A, Raynor DK, Pollock K, Knapp P. The role and value of written information for patients about individual medicines: a systematic review. Health Expectations 2007;10:286‐98. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gupchup 1996
- Gupchup GV, Wolfgang AP, Thomas J. Development of a scale to measure directive guidance by pharmacists. Annals of Pharmacotherapy 1996;30(12):1369‐75. [DOI] [PubMed] [Google Scholar]
Halliday 1985
- Halliday MAK. Spoken and Written Language. Victoria: Deakin University, 1985. [Google Scholar]
Haynes 2008
- Haynes RB, Ackloo E, Sahota N, McDonald HP, Yao X. Interventions for enhancing medication adherence. Cochrane Database of Systematic Reviews 2008, Issue 2. [DOI: 10.1002/14651858.CD000011.pub3] [DOI] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011b
- Higgins JPT, Deeks JJ, Altman DG (editors). Chapter 16: Special topics in statistics. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011c
- Higgins JPT, Deeks JJ (editors). Chapter 7: Selecting studies and collecting data. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hirsh 2009
- Hirsh D, Clerehan R, Staples M, Osborne RH, Buchbinder R. Patient assessment of medication information leaflets and validation of the Evaluative Linguistic Framework (ELF). Patient Education and Counseling 2009;77:248‐54. [DOI] [PubMed] [Google Scholar]
Horne 1999
- Horne R, Weinman J, Hankins M. The development and evaluation of a new method for assessing the cognitive representation of medication. Psychology Health 1999;14:1‐24. [Google Scholar]
Hróbjartsson 2010
- Hróbjartsson A, Gøtzsche PC. Placebo interventions for all clinical conditions. Cochrane Database of Systematic Reviews 2010, Issue 1. [DOI: 10.1002/14651858.CD003974.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jeste 2008
- Jeste DV, Dunn LB, Folsom DP, Zisook D. Multimedia educational aids for improving consumer knowledge about illness management and treatment decisions: a review of randomized controlled trials. Journal of Psychiatric Research 2008;42:1‐21. [DOI] [PubMed] [Google Scholar]
Kalyuga 2000
- Kalyuga S, Chandler P, Sweller J. Incorporating learner experience into the design of multimedia instruction. Journal of Educational Psychology 2000;92(1):126‐36. [Google Scholar]
Kessels 2003
- Kessels RPC. Patients' memory for medical information. Journal of the Royal Society of Medicine 2003;96(5):219‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lane 2006
- Lane DA, Ponsford J, Shelley A. Patient knowledge and perceptions of atrial fibrillation and anticoagulant therapy: effects of an educational intervention programme. The West Birmingham atrial fibrillation project. International Journal of Cardiology 2006;110:354‐8. [DOI] [PubMed] [Google Scholar]
Ley 1982
- Ley P. Satisfaction, compliance and communication. British Journal of Clinical Psychology 1982;21(Pt 4):241‐54. [DOI] [PubMed] [Google Scholar]
Liao 1996
- Liao L, Jollis JG, DeLong ER, Peterson ED, Morris KG, Mark DB. Impact of an interactive video on decision making of patients with ischemic heart disease. Journal of General Internal Medicine 1996;11(6):373‐6. [DOI] [PubMed] [Google Scholar]
Lowe 2000
- Lowe CJ, Raynor DK. Intentional non‐adherence in elderly patients: fact or fiction. The Pharmaceutical Journal 2000;265(7114):R19‐20. [Google Scholar]
Lustria 2009
- Lustria MLA, Cortese J, Noar SM, Glueckauf RL. Computer tailored health interventions delivered over the web: review and analysis of key components. Patient Education and Counselling 2009;74:156‐73. [DOI] [PubMed] [Google Scholar]
M'Imunya 2012
- M'Imunya JM, Kredo T, Volmink J. Patient education and counselling for promoting adherence to treatment for tuberculosis. Cochrane Database of Systematic Reviews 2012, Issue 5. [DOI: 10.1002/14651858.CD006591.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Maly 1999
- Maly RC, Bourque LB, Engelhardt RF. A randomized controlled trial of facilitating information giving to patients with chronic medical conditions: effects on outcomes of care. The Journal of Family Practice 1999;49(5):356‐63. [PubMed] [Google Scholar]
Mayer 1998
- Mayer RE, Moreno R. A split‐attention effect in multimedia learning: evidence for dual processing systems in working memory. Journal of Educational Psychology 1998;90(2):312‐20. [Google Scholar]
Moreno 1999
- Moreno R, Mayer RE. Cognitive principals of multimedia learning: the role of modality and contiguity. Journal of Educational Psychology 1999;91(2):358‐68. [Google Scholar]
Murray 2005
- Murray E, Burns J, See Tai S, Lai R, Nazareth I. Interactive Health Communication Applications for people with chronic disease. Cochrane Database of Systematic Reviews 2005, Issue 4. [DOI: 10.1002/14651858.CD004274.pub4] [DOI] [PubMed] [Google Scholar]
Nair 2002
- Nair K, Dolovich L, Cassels A, McCormack J, Levine M, Gray J, et al. What patients want to know about their medications. Canadian Family Physician 2002;48:104‐10. [PMC free article] [PubMed] [Google Scholar]
Nicolson 2009
- Nicolson D, Knapp P, Raynor DK, Spoor P. Written information about individual medicines for consumers. Cochrane Database of Systematic Reviews 2009, Issue 2. [DOI: 10.1002/14651858.CD002104.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Powell 2002
- Powell H, Gibson PG. Options for self‐management education for adults with asthma. Cochrane Database of Systematic Reviews 2002, Issue 3. [DOI: 10.1002/14651858.CD004107] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pullar 1990
- Pullar T, Wright V, Feely M. What do patients and rheumatologists regard as an 'acceptable' risk in the treatment of rheumatic disease?. British Journal of Rheumatology 1990;29:215‐8. [DOI] [PubMed] [Google Scholar]
Robinson 1998
- Robinson TN, Patrick K, Eng TR, Gustafson D. An evidence‐based approach to interactive health communication: a challenge to medicine in the information age. JAMA 1998;280(14):1264‐9. [DOI] [PubMed] [Google Scholar]
Rueda 2006
- Rueda S, Park‐Wyllie LY, Bayoumi A, Tynan AM, Antoniou T, Rourke S, et al. Patient support and education for promoting adherence to highly active antiretroviral therapy for HIV/AIDS. Cochrane Database of Systematic Reviews 2006, Issue 3. [DOI: 10.1002/14651858.CD001442.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ryan 2011
- Ryan R, Santesso N, Hill S, Lowe D, Kaufman C, Grimshaw J. Consumer‐oriented interventions for evidence‐based prescribing and medicines use: an overview of systematic reviews. Cochrane Database of Systematic Reviews 2011, Issue 5. [DOI: 10.1002/14651858.CD007768.pub2] [DOI] [PubMed] [Google Scholar]
Schünemann 2008a
- Schünemann HJ, Oxman AD, Higgins JPT, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and ‘Summary of findings' tables. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1 (updated September 2008). The Cochrane Collaboration, 2008. Available from www.cochrane‐handbook.org.
Schünemann 2008b
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1 (updated September 2008). The Cochrane Collaboration, 2008. Available from www.cochrane‐handbook.org.
Shaw 2010
- Shaw EJ, Stokes T, Camosso‐Stefinovic J, Baker R, Baker GA, Jacoby A. Self‐management education for adults with epilepsy. Cochrane Database of Systematic Reviews 2010, Issue 10. [DOI: 10.1002/14651858.CD004723.pub3] [DOI] [PubMed] [Google Scholar]
Sterne 2011
- Sterne JAC, Egger M, Moher D (editors). Chapter 10: Addressing reporting biases. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Intervention. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Stevenson 2004
- Stevenson FA, Cox K, Britten N, Dundar Y. A systematic review of the research on communication between patients and health care professionals about medicines: the consequences of concordance. Health Expectations 2004;7(3):235‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Street 1997
- Street RL, Gold WR, Manning T. Health Promotion and Interactive Technology: Theoretical Applications and Future Directions. New Jersey: Lawrence Erlbaum Associates, 1997. [Google Scholar]
Strickler 2002
- Strickler Z, Neafsey P. Visual design of interactive software for older adults: preventing drug interactions in older adults. Visible Language 2002;36(1):4. [Google Scholar]
Tindall‐Ford 1997
- Tindall‐Ford S, Chandler P, Sweller J. When two sensory modes are better than one. Journal of Experimental Psychology: Applied 1997;3(4):257‐87. [Google Scholar]
Tones 1994
- Tones K, Tilford S. Health Education. Effectiveness, Efficiency and Equity. London: Chapman & Hall, 1994. [Google Scholar]
Ward 1993
- Ward SE, Goldberg N, Miller‐McCauley V, Mueller C, Nolan A, Pawlik‐Plank D. Patient‐related barriers to management of cancer pain. Pain 1993;52:319‐24. [DOI] [PubMed] [Google Scholar]
Weinman 1996
- Weinman J, Petrie KJ, Moss‐Morris R. The illness perception questionnaire: a new method for assessing the cognitive representation of illness. Psychology and Health 1996;11:431‐46. [Google Scholar]
Wofford 2001
- Wofford JL, Currin D, Michielutte R, Wofford MM. The multimedia computer for low‐literacy patient education: a pilot project of cancer risk perceptions. Medscape General Medicine 2001;3(2):23. [PubMed] [Google Scholar]
Wofford 2005
- Wofford JL, Smith ED, Miller DP. The multimedia computer for office‐based patient education: a systematic review. Patient Education and Counseling 2005;59:148‐57. [DOI] [PubMed] [Google Scholar]
Wright 2009
- Wright DW, Hill TJ. Prescription for trouble: Medicare part D and patterns of computer and internet access among the elderly. Journal of Aging & Social Policy 2009;21(2):172‐86. [DOI] [PubMed] [Google Scholar]
Zigmond 1983
- Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatrica Scandinavia 1983;67:361‐70. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Ciciriello 2010
- Ciciriello S, Johnston RV, Osborne RH, Wicks I, deKroo T, Clerehan R, et al. Multimedia educational interventions for consumers about prescribed and over the counter medications. Cochrane Database of Systematic Reviews 2010, Issue 3. [DOI: 10.1002/14651858.CD008416] [DOI] [PMC free article] [PubMed] [Google Scholar]


