Abstract
Extracranial vascular pathology uncommonly causes intracranial subarachnoid hemorrhage (SAH). Among possible lesions are aneurysms at the craniocervical junction arising from a posterior inferior cerebellar artery (PICA) with an extradural origin. We describe a case of a 55-year-old female presenting with a sudden and severe headache. A computed tomography scan revealed a SAH within the fourth ventricle and cervical spinal canal, and a ruptured saccular aneurysm on a PICA with extradural C2-origin. Despite difficult access anatomy, endovascular treatment was feasible and resulted in subtotal initial occlusion and preservation of distal PICA flow. Upon 3-month follow-up, the aneurysm was completely occluded with a patent PICA. The patient’s clinical status remained stable at the 1.5-year follow-up. In conclusion, we present a rare case of an aneurysm originating from a PICA with extradural C2-origin that was treated endovascularly with preservation of the PICA.
Keywords: Aneurysm, Endovascular treatment, Extracranial, Vascular variant, Coil embolization
INTRODUCTION
Rupture of aneurysms of the posterior inferior cerebellar artery (PICA) with an extradural or low intradural origin from the vertebral artery (VA) are rare causes of subarachnoid hemorrhage (SAH) [1]. These aneurysms are often located extracranially at the craniocervical junction and may be overlooked on brain computed tomography angiography (CTA) and digital subtraction angiography (DSA) [2-4].
In patients with an extradural PICA origin, the artery bifurcates from the V3-segment of the VA at the C1- or C2-levels [5,6]. Aneurysms arising on a PICA with an extradural C2-origin offer unique problems for endovascular and surgical management due to difficult access anatomy. Previously reported cases have been managed either by surgery [4,7-10] or deconstructive endovascular techniques [3]. We report a patient with a ruptured aneurysm on a PICA with an extradural C2-origin that was successfully treated by coil embolization with preservation of the parent artery. A review and discussion of the literature is provided.
CASE REPORT
Clinical Presentation and Diagnostic Work-up
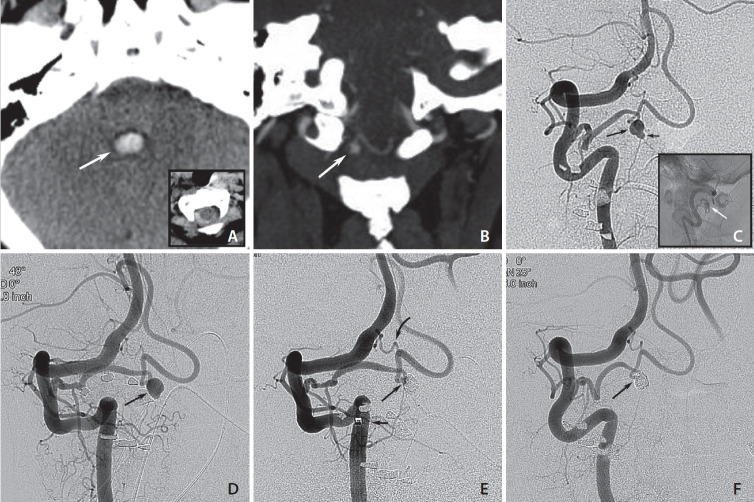
A 55-year-old female presented with a sudden onset of severe headache, photophobia, nausea, and vomiting. The patient had a Glasgow Coma Scale score of 11 without focal neurological deficits. Initial brain CT demonstrated a SAH within the fourth ventricle, resulting in acute hydrocephalus, and at the craniocervical junction surrounding the medulla oblongata and upper cervical spinal cord (Fig. 1A). A CTA was performed which suggested an extracranial intradural saccular aneurysm at the C1-level arising from a PICA with extradural C2-origin (Fig. 1B). After emergent placement of an external ventricular drain, DSA confirmed the presence of a 3.8×2.8 mm saccular aneurysm with a small bleb (Fig. 1C).
Fig. 1.
(A) Axial computed tomography (CT) scans on admission show blood in the fourth ventricle (white arrow) and subarachnoid hemorrhage at the craniocervical junction (inset). (B) Coronal CT angiography shows a small saccular structure at the C1-level (white arrow) that appears to arise from a posterior inferior cerebellar artery (PICA) with an extradural origin. (C) Right vertebral artery injections show a PICA arising from the V3-segment with an intradural aneurysm (long arrow) carrying a small bleb (short arrow). Lateral non-subtracted image shows the location of the aneurysm in the spinal canal (white arrow; inset). (D) Follow-up 9 months after aneurysm rupture shows a subtle change in shape with the bleb less clearly visible (arrow). (E) Control run after aneurysm coiling (3 coils) with a small neck remnant (long arrow), intentionally left to preserve PICA flow. Position of distal access catheter that facilitated distal microcatheter navigation into the PICA and the aneurysm (short arrow). Small anastomotic branch arising from the usual PICA origin of the V4 segment (curved arrow). (F) Follow-up angiogram 3 months after treatment shows progression of aneurysm occlusion that is now complete (arrow), while flow in the distal PICA is preserved.
Endovascular Management
Treatment options were discussed by an interdisciplinary team of neurosurgeons and interventional neuroradiologists, and it was decided to attempt endovascular therapy (EVT). The procedure remained unsuccessful due to access difficulties caused by a tortuous right subclavian artery. One month later, when the patient was readmitted for EVT, DSA showed the aneurysm unchanged in size and shape but also a small proximal, likely iatrogenic PICA dissection. At that time, the patient had made a full neurological recovery and continued to prefer EVT. While early control imaging was planned, the patient was temporarily lost to follow-up. A DSA 9 months later showed a largely healed dissection with minimal change in aneurysm shape but no decrease in size (Fig. 1D). Therefore, a second treatment attempt was undertaken using a triaxial approach (6F Chaperon™; MicroVention), 0.038″ DAC, Excelsior® SL 10 (Stryker), which allowed reaching the aneurysm. Slight under-packing of the aneurysm sac was performed using 3 detachable coils leading to subtotal occlusion while flow in the PICA was preserved (Fig. 1E). The patient woke up without neurologic deficits.
Follow-up
A follow–up DSA 3 months later showed progressive complete occlusion of the aneurysm and patency of the PICA (Fig. 1F). A clinical follow-up 1.5 years post-procedure was unremarkable.
DISCUSSION
In general, PICA aneurysms make up approximately 0.5–3% of all cerebral aneurysms, while aneurysms distal to the VA-PICA bifurcation make up 0.2% [11]. Extracranial PICA aneurysms represent an even rarer type of lesion related to anatomical variations in the origin and course of the artery. The PICA usually originates from the intradural, intracranial V4-segment of the VA, but variations of PICA anatomy are common. Intradural PICA origin below the foramen magnum occur in 17–18% [12] of cases and caudal PICA loops below the foramen magnum occur in 35% [13] of cases. Therefore, extracranial PICA aneurysms can develop proximally at a low-lying intradural VA-PICA bifurcation or distally on a caudal PICA loop [1].
The extradural PICA origin at the C1- or C2-levels represents an anatomical variant observed in 1.3% of normal subjects on CTA [14]. The anomaly is related to the embryonal lateral spinal artery (LSA) [5]. The LSA corresponds to the posterolateral arterial axis of the upper cervical spinal cord and runs between the posterior rootlets C1–C4 and the dentate ligaments. It anastomoses with small PICA branches at the restiform body and with segmental branches from the vertebral and occipital arteries. Caudally, the LSA terminates in the posterolateral arterial axis of the spinal cord at the C4- or C5-levels. An extradural PICA origin from the VA is thought to develop when a segmental transdural branch to the LSA becomes prominent and establishes a connection to the PICA [5]. Furthermore, a PICA with extradural C2-origin may also develop from the posterior spinal artery according to Siclari et al. [6].
Microsurgical studies of cadaveric specimens with the PICA origin at the C1-level have previously been performed [2,15], while the anatomy of the C2-origin has been demonstrated on imaging [5]. A PICA with an extradural C1-origin originates approximately 10 mm proximal to the dura and parallels the VA until both arteries perforate the dura together [2]. No muscular branches arise from this artery [2,15].
The PICA with extradural C2-origin originates from the vertical part of the V3-segment located between the C1 and C2 transverse processes (Fig. 2). The extradural part of the artery initially courses posteriorly around the lateral mass of the C1 where a muscular branch often arises [7,8]. It then enters the spinal canal at the C1–2 intervertebral space coursing medially below the posterior arch of C1. A slight tapering or caliber change of the artery may be visible on DSA images where the artery perforates the dura. The initial intradural segment is usually tortuous, after which the artery ascends as a vertical segment. The artery has a cranial loop after passing the foramen magnum, but often there is no caudal loop. A small intradural anastomotic vessel between the VA and PICA with extradural C2-origin may be found, which was seen in our patient.
Fig. 2.
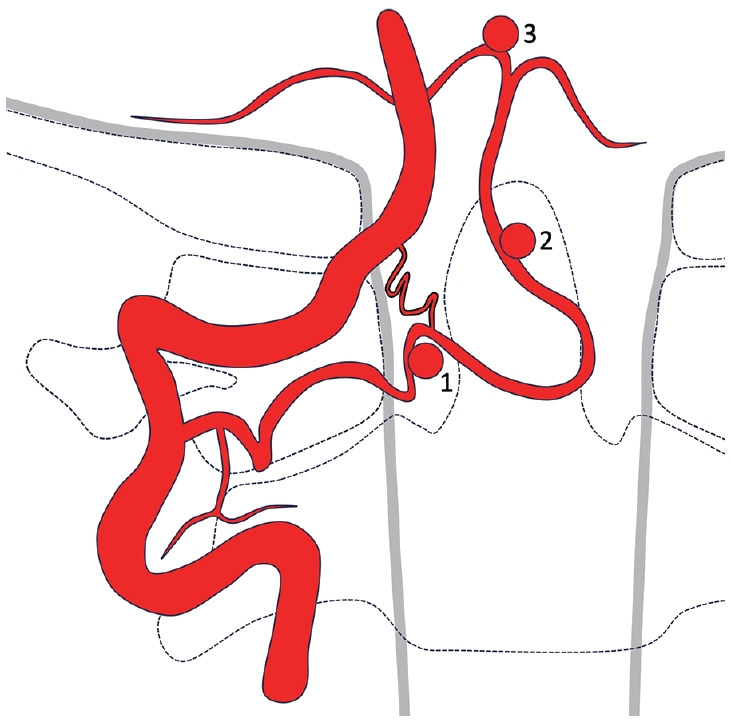
Illustration of a posterior inferior cerebellar artery (PICA) with extradural C2-origin. A muscular branch may arise from the extradural segment of the artery. An intradural small anastomotic branch between the vertebral artery and PICA is sometimes found. Extracranial aneurysms may arise proximally at the level of dural penetration (1) or distally on the ascending vertical segment (2). Aneurysms may also develop on the intracranial PICA (3).
Six patients with an aneurysm associated with an extradural PICA origin at the C2-level have previously been reported in the literature (Table 1) [3,4,7-10]. All patients presented with aneurysm rupture associated with SAH, intraventricular hemorrhage, and hydrocephalus. Five patients had extracranial aneurysms; 4 of them were located at the level of dural penetration [3,4,7,9]. One patient with extradural C2-origin of both PICAs had bilateral aneurysms located at the ascending vertical PICA-segment—1 just below and the other at the level of the foramen magnum [8]. PICA aneurysms are usually amendable to surgical treatment due to their location dorsal to the medulla. In 4 patients with extracranial aneurysms, clipping was performed after variable combinations of suboccipital craniectomies and laminectomies at the C1- and C2-levels [4,7-9]. One patient harbored an intracranial aneurysm, which was treated by a transcondylar approach providing adequate visualization of the aneurysm located anterolateral to the medulla [10]. Four out of 5 patients undergoing surgery had follow-up DSAs showing complete aneurysm occlusion [4,8-10] and documentation of PICA patency in 3 cases [4,8,10]. There were no technical complications, and surgical treatment resulted in good or excellent outcomes in all patients. The patient reported by Tanaka et al. [9] developed aphasia and right hemiparesis before undergoing surgery due to a left parietal infarct that was thought to be caused by vasospasms.
Table 1.
Aneurysms arising on posterior inferior cerebellar arteries with extradural C2-origin
| Source | Age/sex | Presentation | Aneurysm location | Side | Treatment | Complications | Aneurysm at FU | Outcome |
|---|---|---|---|---|---|---|---|---|
| Tanaka et al. [9] (1993) | 39/F | SAH, hydrocephalus | Proximal EC | Left | SOC, lam, clipping | Left parietal infarct | CO | Aphasia, |
| Abe et al. [3] (1998) | 22/M | SAH, hydrocephalus | Proximal EC | Right | PAO, coiling | Vasospasms | CO | No deficits |
| Kim et al. [10] (2001) | 53/F | SAH, IVH, hydrocephalus | IC | Left | TCA, C1-lam, clipping | None | CO | No deficits |
| Tabatabai et al. [4] (2007) | 19/M | IVH | Proximal EC | Right | SOC, C1-lam, clipping | None | CO | No deficits |
| Bhat et al. [8] (2009) | 35/F | IVH | Distal EC | Bilateral* | SOC, C1-lam, clipping | None | CO | No deficits |
| Dikshit et al. [7] (2021) | 51/M | SAH, IVH | Proximal EC | Right | C1-2-lam, clipping | None | No data | No deficits |
| Present case | 55/F | SAH, IVH, hydrocephalus | Proximal EC | Right | SAO, coiling | Proximal PICA dissection | CO | No deficits |
FU, follow-up; F, female; SAH, subarachnoid hemorrhage; EC, extracranial; SOC, suboccipital craniectomy; lam, laminectomy; CO, complete occlusion; M, male; PAO, parent artery occlusion; IVH, intraventricular hemorrhage; IC, intracranial; TCA, transcondylar approach; SAO, selective aneurysm occlusion; PICA, posterior inferior cerebellar artery.
Rupture of the left aneurysm.
Endovascular coil occlusion of an aneurysm arising from a PICA with extradural C2-origin was first reported by Abe et al. [3]. Parent artery occlusion with microcoils was performed following an asymptomatic balloon test occlusion with normal auditory brain stem monitoring. The patient showed no new symptoms after the procedure and the distal PICA territory was supplied by collaterals.
While in our case, the distal PICA territory appeared to have sufficient collateral flow, selective aneurysm occlusion (SAO) was nevertheless preferred to minimize the risk of a brain stem or cerebellar ischemia. Slight under-packing of the aneurysm with coils was considered reasonable. This strategy proved effective as complete occlusion with preserved PICA flow was confirmed after 3 months.
It has been proposed that a PICA with an extradural origin supplies a ‘less eloquent’ vascular territory because the perforators to the anterolateral medulla normally supplied by the PICA are taken over by the VA instead [16]. PICAs with extradural origins still supply the olive and the lateral and posterior medulla [2,15]. Occlusion of a PICA with extradural origin may not necessarily cause a lateral medullary infarct and the distal PICA territory may even be supplied by anterior inferior cerebellar artery anastomoses, which potentially makes its sacrifice feasible [3]. However, Won et al. [17] 2019 recently reported a patient presenting with sixth nerve palsy and diplopia after C1-2 fusion who developed a PICA infarct due to vascular injury of a PICA with an extradural C2-origin. Therefore, in our opinion, SAO, if technically feasible, should be preferred over vessel sacrifice. In cases with difficult access anatomy, triaxial techniques can help overcome tortuous vessel segments. If parent vessel occlusion is performed, a too proximal PICA occlusion should be avoided due to the risk of retrograde filling of the aneurysm through collaterals.
In conclusion, endovascular treatment of aneurysms arising from a PICA with an extradural C2-origin is feasible and can be accomplished either by SAO or sacrifice of the parent vessel. The former should be performed whenever possible to minimize the risk of ischemia, especially in patients with insufficient anastomoses to the distal PICA territory. Endovascular techniques may be particularly useful in patients with favorable anatomy and should be considered in patients who either have contraindications or are reluctant to undergo surgical treatment. Triaxial catheter techniques are recommended. Finally, in patients with SAH and intraventricular hemorrhage in the fourth ventricle, the search for a bleeding source using CTA and DSA should include the upper cervical spine at least down to the C2-level.
Footnotes
Fund
None.
Ethics Statement
Institutional Review Board approval was waived for this study. Informed consent for publication of this report was obtained from the patient.
Conflicts of Interest
The authors have no conflicts to disclose.
Author Contributions
Concept and design: RHD, GLHJ, and GB. Analysis and interpretation: RHD, GLHJ, and GB. Data collection: RHD, GLHJ, and GB. Writing the article: RHD, GLHJ, and GB. Critical revision of the article: RHD, GLHJ, ZM, SPG, and GB. Final approval of the article: RHD and GB. Overall responsibility: GB.
REFERENCES
- 1.Dammers R, Krisht AF, Partington S. Diagnosis and surgical management of extracranial PICA aneurysms presenting through subarachnoid haemorrhage: case report and review of the literature. Clin Neurol Neurosurg. 2009;111:758–761. doi: 10.1016/j.clineuro.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 2.Fine AD, Cardoso A, Rhoton AL., Jr Microsurgical anatomy of the extracranial-extradural origin of the posterior inferior cerebellar artery. J Neurosurg. 1999;91:645–652. doi: 10.3171/jns.1999.91.4.0645. [DOI] [PubMed] [Google Scholar]
- 3.Abe T, Kojima K, Singer RJ, Marks MP, Watanabe M, Ohtsuru K, et al. Endovascular management of an aneurysm arising from posterior inferior cerebellar artery originated at the level of C2. Radiat Med. 1998;16:141–143. [PubMed] [Google Scholar]
- 4.Tabatabai SA, Zadeh MZ, Meybodi AT, Hashemi M. Extracranial aneurysm of the posterior inferior cerebellar artery with an aberrant origination: case report. Neurosurgery. 2007;61:E1097–1098. doi: 10.1227/01.neu.0000303206.92617.07. discussion E1098. [DOI] [PubMed] [Google Scholar]
- 5.Lasjaunias P, Vallee B, Person H, Ter Brugge K, Chiu M. The lateral spinal artery of the upper cervical spinal cord. Anatomy, normal variations, and angiographic aspects. J Neurosurg. 1985;63:235–241. doi: 10.3171/jns.1985.63.2.0235. [DOI] [PubMed] [Google Scholar]
- 6.Siclari F, Burger IM, Fasel JH, Gailloud P. Developmental anatomy of the distal vertebral artery in relationship to variants of the posterior and lateral spinal arterial systems. AJNR Am J Neuroradiol. 2007;28:1185–1190. doi: 10.3174/ajnr.A0498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dikshit P, Sharma A, Mehrotra A, Verma PK, Das KK, Jaiswal AK. Extra-cranial proximal pica aneurysm - a rare and surreptious cause of posterior fossa sah: case report and review of literature. Br J Neurosurg. 2021 doi: 10.1080/02688697.2021.1970112. [published online ahead of print Sep 15, 2021] [DOI] [PubMed] [Google Scholar]
- 8.Bhat DI, Somanna S, Kovoor J, Chandramoul BA. Aneurysms from extracranial, extradurally originating posterior inferior cerebellar arteries: a rare case report. Surg Neurol. 2009;72:406–408. doi: 10.1016/j.surneu.2008.09.021. [DOI] [PubMed] [Google Scholar]
- 9.Tanaka A, Kimura M, Yoshinaga S, Tomonaga M. Extracranial aneurysm of the posterior inferior cerebellar artery: case report. Neurosurgery. 1993;33:742–744. doi: 10.1227/00006123-199310000-00028. discussion 744-745. [DOI] [PubMed] [Google Scholar]
- 10.Kim K, Kobayashi S, Mizunari T, Teramoto A. Aneurysm of the distal posteroinferior cerebellar artery of extracranial origin: case report. Neurosurgery. 2001;49:996–998. doi: 10.1097/00006123-200110000-00040. discussion 998-999. [DOI] [PubMed] [Google Scholar]
- 11.Kleinpeter G. Why are aneurysms of the posterior inferior cerebellar artery so unique? Clinical experience and review of the literature. Minim Invasive Neurosurg. 2004;47:93–101. doi: 10.1055/s-2004-818437. [DOI] [PubMed] [Google Scholar]
- 12.Lister JR, Rhoton AL, Jr, Matsushima T, Peace DA. Microsurgical anatomy of the posterior inferior cerebellar artery. Neurosurgery. 1982;10:170–199. [PubMed] [Google Scholar]
- 13.Margolis MT, Newton TH. Borderlands of the normal and abnormal posterior inferior cerebellar artery. Acta Radiol Diagn (Stockh) 1972;13:163–176. doi: 10.1177/02841851720130p122. [DOI] [PubMed] [Google Scholar]
- 14.Wakao N, Takeuchi M, Nishimura M, Riew KD, Kamiya M, Hirasawa A, et al. Vertebral artery variations and osseous anomaly at the C1-2 level diagnosed by 3D CT angiography in normal subjects. Neuroradiology. 2014;56:843–849. doi: 10.1007/s00234-014-1399-y. [DOI] [PubMed] [Google Scholar]
- 15.Salas E, Ziyal IM, Bank WO, Santi MR, Sekhar LN. Extradural origin of the posteroinferior cerebellar artery: an anatomic study with histological and radiographic correlation. Neurosurgery. 1998;42:1326–1331. doi: 10.1097/00006123-199806000-00079. [DOI] [PubMed] [Google Scholar]
- 16. Lasjaunias P, Berenstein A, ter Brugge KG. Craniocervical junction. In: Lasjaunias P, Berenstein A, Brugge KG. Surgical neuroangiography, 2nd ed. Vol. 1, Clinical vascular anatomy and variations. Springer, 2001;165-259. [Google Scholar]
- 17.Won D, Lee JM, Park IS, Lee CH, Lee K, Kim JY, et al. Posterior inferior cerebellar artery infarction originating at C1-2 after C1-2 fusion. Korean J Neurotrauma. 2019;15:192–198. doi: 10.13004/kjnt.2019.15.e27. [DOI] [PMC free article] [PubMed] [Google Scholar]