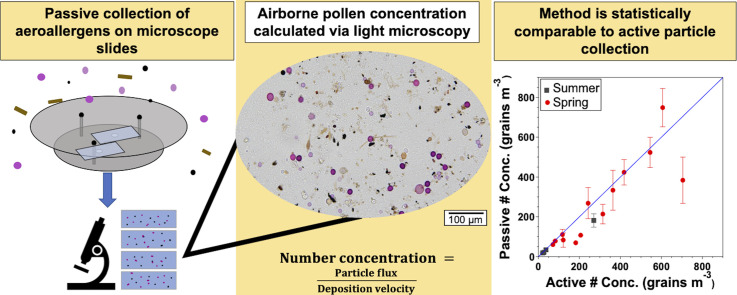
Abstract
Traditionally, airborne concentrations of aeroallergens are sampled in a single location by an active sampler, which requires electricity and regular maintenance. However, aeroallergen concentrations may vary widely over urban and rural environments, requiring a method that is cost-effective and scalable so that many measurements can be made across an air shed. We developed such a method that uses passive sampling and light microscopy for analysis. Inexpensive and easy to operate, passive samplers rely on the gravitational settling of particles onto microscope slides. This determines airborne pollen concentration through: 1) sample collection using a modified Durham sampler, 2) preparation of samples for microscopy and strategic sample imaging, and 3) simplified particle measurements and calculation of pollen concentration following deposition velocity models proposed by Scheppegrell [1] and Wagner and Leith [2]. This method was verified with two sampling campaigns during the ragweed season of 2020 and the tree pollen season of 2021. The concentrations determined with the passive and Burkard sampling methods were found to be well-correlated (r > 0.99, r = 0.87) and precise (%CV = 20 %, 21 %). The validation of passive samplers will enable measurements of aeroallergens over wider spatial scales and help determine where aeroallergen exposure risks are greatest.
-
•
An inexpensive and low-cost method was developed to determine airborne pollen counts.
-
•
The method was evaluated for its accuracy and reproducibility.
-
•
The method can be applied to examine the concentrations and spatial variability of airborne pollen.
Keywords: Aeroallergens, Microscopy, Pollen, Air quality, Image analysis
Method name: Determination of pollen concentration from passively collected samples
Graphical abstract
Specifications Table
| Subject area: | Environmental Science |
| More specific subject area: | Aeroallergens, Air Quality Monitoring |
| Name of your method: | Determination of pollen concentration from passively collected samples |
| Name and reference of original method: | Not Applicable |
| Resource availability: | Image analysis software: https://imagej.net/software/fiji/ Microscopy equipment: BX-61 Olympus Light Microscope (Waltham, MA): https://www.olympus-ims.com/en/microscope/bx61–2/ ProScan stage navigator (H101A, Prior Scientific, Rockland Park, MA): https://www.prior.com/product/h101a |
Background
A scalable sampling method, herein defined as a method simple and inexpensive enough to be duplicated simultaneously over diverse geographical regions, is required to assess the spatial variability in aeroallergen concentrations. Bioaerosol collection methods that use an active air flow, like the Burkard volumetric spore sampler [3] and the Rotorod pollen sampler [4] are expensive, require AC power, and must be maintained daily or weekly, limiting the number of samplers that can be deployed simultaneously.
Various researchers have developed methods for the passive collection of bioaerosols to cost-effectively assess spatial variability. The Tauber trap [5] and Durham shelter have been used in spatial studies of airborne pollen, including those of Crispen et al. [6]. The Tauber trap is suitable only for measurements of pollen deposition, as its exposure to rain results in the collection of pollen by both wet and dry deposition. The Durham sampler [7] is one of the most widely used passive samplers due its simplicity of relying only on the gravitational deposition of particles on a microscope slide housed between two aluminum plates. While it is generally used for measurements of deposited particle concentration, the Durham shelter has been used to measure airborne pollen concentration [8] and a modified model has been used to measure airborne particle concentration [9]. Bricchi et al. [10] and Watanabe et al. [11] demonstrated the agreement of the passive samplers with the Burkard trap in detecting relative pollen taxa frequency and deposition. In contrast, Cornell [12] observed poor agreement in observed pollen counts (r = 0.24) and Miki et al. [8] propose that correction factors to account for wind speed and turbulence are required in order for the two samplers to be comparable. Ott and Peters [9] adopted a low-cost approach similar to Durham's to assess ambient particle mass concentration across large airsheds. Measurements made with this sampler were cross validated with a Federal Reference Method monitor for the quantification of the mass concentration of coarse particulate matter, PM10–2.5 [9]. Ott and Peters [9] and Ault et al. [13] demonstrated these samplers to be effective in the study of spatial variability in atmospheric particulate matter.
The airborne pollen number concentration can be estimated from measurements of particle deposition on a surface. The method proposed by Scheppegrell [1] and improved by Cocke [14] measures pollen grains deposited on a surface per unit area over time. Assuming deposition by gravitational settling enables determination of airborne number concentrations by dividing the surface deposition by the terminal settling velocity of individual particles.
This project expands the use of passive samplers to quantify airborne pollen concentrations as a function of their size. The determination of airborne pollen concentration followed Wagner and Leith [2], as well as Scheppegrell [1] and Cocke [14], to account for particle size. The method was evaluated by comparing its results to a co-located Burkard volumetric spore trap as a reference method.
Method details
1. Sample Collection
Two sampling campaigns were conducted at the University of Iowa Air Monitoring Site in Iowa City, IA (41.6647, −91.5845). The site is peri‑urban and bordered by a wooded area, a gravel parking lot, and a partially reconstructed tallgrass prairie. The first campaign occurred over four two-week periods in 2021: August 25 to September 9, September 9 to September 23, September 23 to October 7, and October 7 to October 21. A second campaign in spring 2021 consisted of 13 overlapping 5-day sampling periods ranging April 16 to May 16.
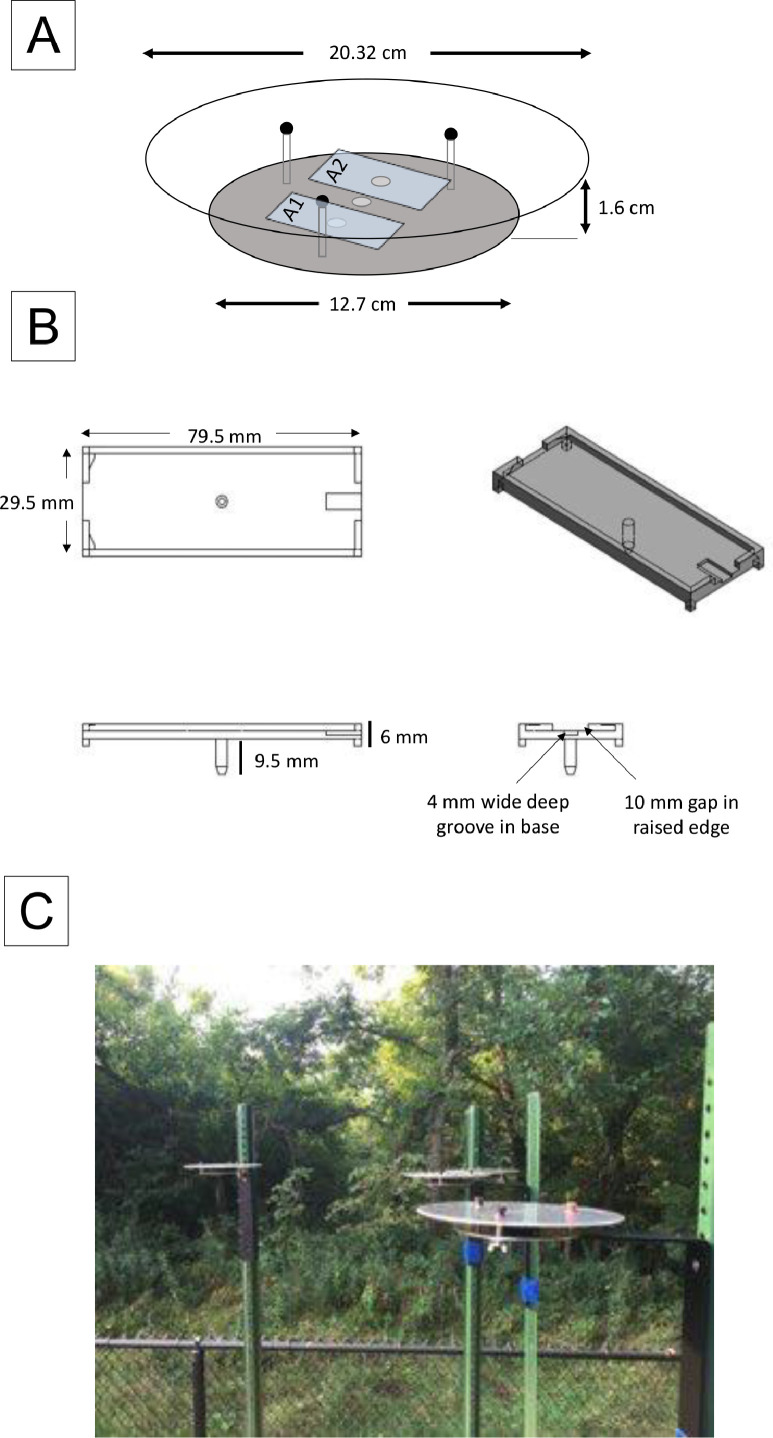
To prepare for sampling, microscope slides (75 × 25 × 1.0 mm, Micro Slides, VWR) were coated with a thin layer of stopcock grease (Lubriseal, Thomas Scientific), which acted as an adhesive. The microscope slides were placed under a Staticmaster ionizer (Thomas Scientific) to eliminate static charge and then in a passive pollen shelter, consisting of two parallel stainless-steel plates separated by three 1.6-cm metal struts (Fig. 1A, [9]). The slides were secured in plastic holders, which held the microscope slide flat on the lower plate facing up (Fig. 1B). These slide holders were designed specifically for this study and made using 3D printing. Four co-located shelters, identified as A, B, C, and D, each containing two slides identified as A1, A2, B1, B2, etc. were deployed approximately 1 meter apart. Each sampler was raised on metal poles affixed on a platform to a total of 3 m above the ground (Fig. 1C).
Fig. 1.
A: Passive pollen sampler, as described by Ott and Peters, with additional pin holes to hold multiple slide holders. Two microscope slides, labeled A1 and A2, are placed in plastic slide holders inserted into pin holes. B: Plastic microscope slide holders, made to hold standard size microscope slides. Includes groove in base for easy slide removal. Slide is raised 6 mm above the bottom plate of the shelter. C: Assembled passive pollen sampler, mounted on l-bracket and fence post 1.8 m above the ground.
Airborne pollen was actively collected with a volumetric spore trap (Burkard Manufacturing Company), which impacts pollen grains on a microscope slide coated with stopcock grease. The microscope slide was mechanically advanced in the sampler at a rate of 2 mm per hour, giving 24-hour samples with 1-hour resolution. The Burkard trap was installed on a wooden platform about 2 m above the ground. The Burkard measurements were averaged to obtain the airborne concentration for the two-week (for the summer study) or 5-day (for the spring study) periods corresponding to passive measurements. All samples were placed in slide holders and stored in a desiccator before being mounted for microscopy.
Air temperature data was collected by a Vantage Vue wireless weather station (SKU 6250, Davis Instruments) mounted approximately 4 m above the ground at the University of Iowa Air Sampling site. No meteorological data was collected from April 16 to April 20, 2021, so temperature data for these days was provided by the University of Iowa WeatherSTEM station, located approximately 4.2 km southeast from the study site (41.6587, −91.5522) [15].
2. Microscopy
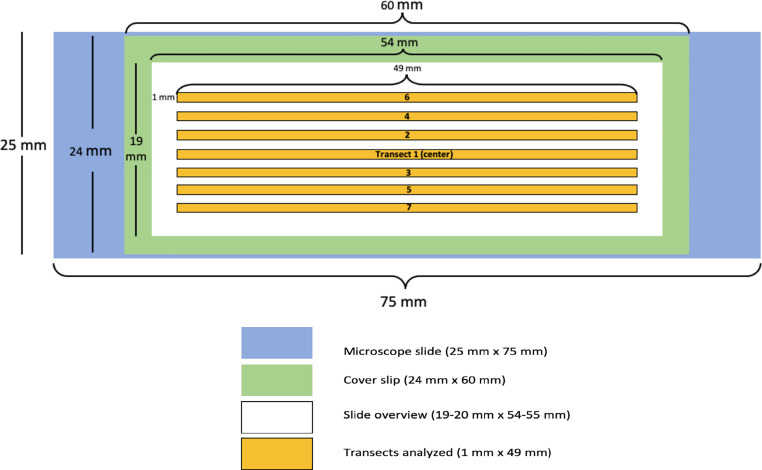
After sample collection, the passive and Burkard slides were mounted in a medium prepared from 8 g of gelatin, 70 mL of glycerin, 30 mL of distilled water, and 1 mL of phenol [16]. Basic fuchsin stain (5619–61–9, Sigma Aldrich, St. Louis, MO) was added until the desired pink color needed for pollen identification was achieved. Cover slips were applied to the slides and sealed with CoverGrip Coverslip Sealant (23,005, Biotium, Fremont, CA). The slides were viewed via bright-field microscopy using a light microscope (BX-61, Olympus, Waltham, MA) equipped with a ProScan stage navigator (H101A, Prior Scientific, Rockland Park, MA) at the Central Microscopy Research Facility at the University of Iowa. Images of the slides were captured at the 20x objective and 1960 × 1600 resolution. Images of the samplers collected via the passive sampler were taken by selecting a 49 mm x 1 mm area on the slide. Selection of a microscope field for analysis began by imaging the center transect of the slide, counting the number of particles in this area, and adding images of the sample areas adjacent to the preliminary image until at least 200 particles were analyzed. This field selection strategy is illustrated in Fig. 2. A single 48 mm x 2 mm image of each Burkard sample was captured for longitudinal analysis. High-quality images of these transects were taken efficiently by using the “focus map” function in the Olympus cellSens software associated with the microscope.
Fig. 2.
Strategy for selecting sample areas for analysis. The green area indicates where deposited particles are observable after the application of mounting medium and the coverslip. Transects are selected from the center of the slide outwards, leaving space to prevent transect overlap or analysis of areas where particulate matter may not be evenly distributed.
For method development, transects of varying positions on the microscope slide were selected and imaged. The number of particles in each transect was counted and compared. The inner transects of the slide contained more pollen and produced more consistent data than the outer transects, assessed by calculating coefficients of variation. Thus, the procedure was optimized to use images captured near the center of the cover slip, about 11 mm from the top of the slide. Additional images were taken adjacent to this center transect with a 1 mm gap between them to prevent overlap.
3. Data Analysis
Data was collected for both sample types using the image analysis software, Fiji ImageJ [17]. For the passively collected samples, the diameters of individual particles were measured and used to calculate their deposition velocities. Particles that were part of clumps greater than 20 touching pollen grains were not included in the calculation of pollen concentration. Particles in clumps of less than 20 touching pollen grains were measured individually. The particle to surface flux was divided by the deposition velocity to determine the contribution of a single particle to the number concentration, summed for all particles observed, and scaled up to calculate the particle number concentration per cubic meter. All deposition mechanisms other than gravitational settling are neglected. The pollen number concentration was calculated following Eq. (1):
| (1) |
in which is the pollen number concentration (m-3), is the particle flux to surface deposition (count s-1 m-2), is the deposition velocity (m s-1), is the number of pollen grains observed, is the total area of imaged and analyzed in meters, and t is the sampling time in seconds [1,2,14]. This value can then be summed for all particles observed and scaled up to particle number concentrations per cubic meter. The deposition velocity (Vdep) was defined following Stokes’ Law and Eq. (2):
| (2) |
in which is the particle density (1000 kg m-3), da is the particle's aerodynamic diameter (m), Cc is the Cunningham correction factor, g is the gravitational constant (9.8 m s-2), and is the viscosity of air at 19.85 °C (1.81e-5 N s m-2) [18]. The calculations conducted for this study assume a temperature gradient of zero, negligible voltage accumulation, and spherical, standard density pollen grains (1000 kg m-3). Pollen density can vary widely by pollen type and the relative humidity in which it is observed; reported values range from 550 to 1300 kg m-3 [19]. Standard density was chosen for convenience and because it falls between this range and is close to the most commonly reported density for several types of pollen [20]. The pollen types present in the sample (weed, forb, grass, or tree) were also noted [21].The daily mean pollen concentration was achieved by counting the pollen grains on the Burkard samples and scaling up following Eq. (3):
| (3) |
in which ni is the number of pollen grains observed, A is the total sample area (m2), is the image area analyzed (m2), and S is the volume of air sampled (m3).
4. Method Validation
a. Campaign 1
The first sampling campaign of the study was conducted during the ragweed pollen season from August 25 to October 21, 2020. Two-week averaged pollen concentrations measured using the passive samplers ranged from 15 to 217 grains m-3 (Table S1). The pollen concentrations in the first sampling period (8/25–9/09, ranging 112–217 grains m-3) were considered high according to the National Allergy Bureau's ragweed scale (NAB high: 50–499 grains m-3). The later three sampling periods (ranging 15–50 grains m-3) were considered moderate (NAB moderate: 10–49 grains m-3), with the exception of one sampler in the second period (B1, 50 grains m-3) which fell in the high NAB range [22]. The pollen concentration decreased between August and October as temperatures declined, which is consistent with the observation that ragweed pollen production decreases in lower temperatures (Table S1) [23]. No data was collected from sampler C2 for the fourth sampling period due to pollen disturbance during slide mounting. The precision of the co-located passive samplers was assessed by coefficients of variation (CV), which ranged 14 to 24 % with an average CV of 20 %. A minimum of 200 particles per passive sample were counted for the calculation of pollen concentration.
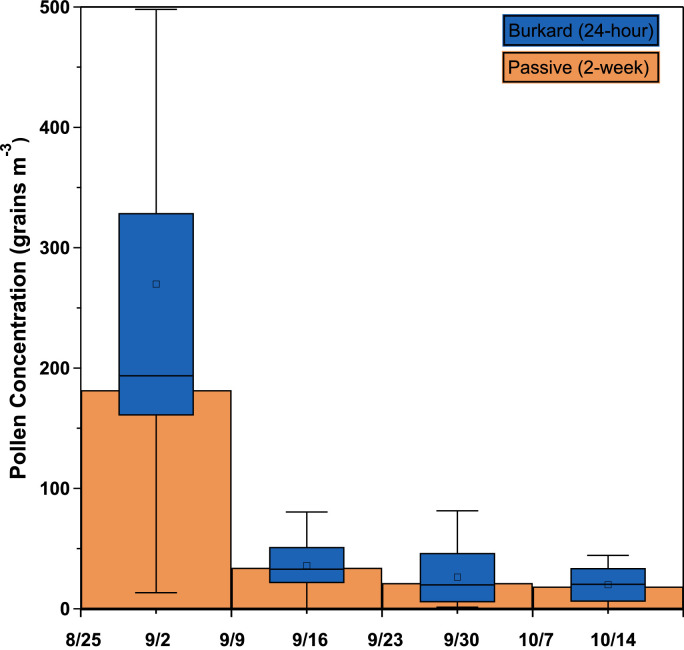
A visual comparison of the results from the active and passive sampling methods (Fig. 3) suggests general agreement, as the median two-week averaged pollen concentration measured by the Burkard spore trap is roughly equivalent to the mean concentration measured by the passive samplers. The coefficient of correlation (r) between the two data sets was >0.99, which supports similarity between the data sets. The percent difference was also calculated in this manner and ranged from 0.1 % to 83 % with an average of 26 %, further suggesting general agreement between the sampling methods. However, it should be noted that the power of these tests is limited by a small sample size.
Fig. 3.
Comparison of pollen concentrations measured with the passive sampler to those with the Burkard sampler. Data includes 14 24-hour averaged Burkard measurements and 8 two-week averaged passive measurements (A1, A2-D1, D2) per sampling period. Box indicates the interquartile range, whiskers indicate the upper and lower adjacent values, square represents mean, and central line represents median. Alignment of the Burkard median and passive mean indicates general agreement between the methods.
Assessment of Fig. 3 and Table S1 show the least agreement occurred during the first sampling period. This is most likely due to the elevated and more variable pollen concentrations detected during this time, suggesting that the accuracy of the passive samplers is less satisfactory at higher pollen concentrations. Turbulence and changes in wind speed have also been shown to decrease the accuracy of Durham-type samplers by increasing the flux of particles to the surface of the sampler, resulting in an overestimation of the particle concentration [2,8]. This may be corrected by incorporating additional deposition mechanisms in the calculation of terminal settling velocity, including convective diffusion and inertia [2,8].
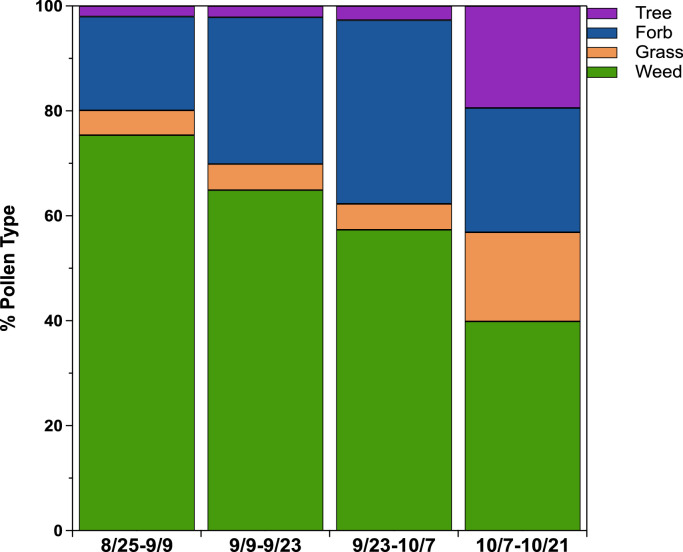
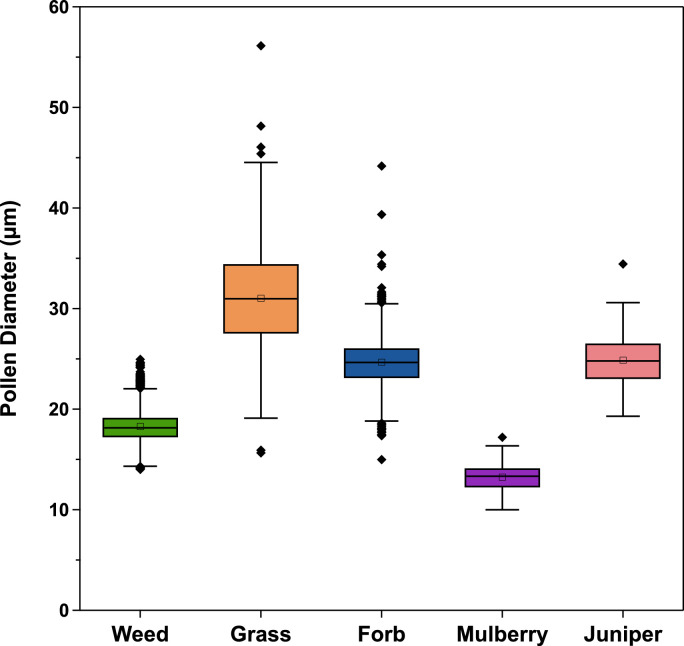
Pollen types sampled during the study included grass, weed, tree, and forb. Weed (mostly giant and common ragweed) was the predominant pollen type (Fig. 4). Mulberry and juniper were the only significant types of tree pollen. The weed pollen season is during the hotter summer months in Iowa City, so this pollen was the most prevalent during the earlier sampling periods. Juniper is a cool weather pollinator, which is reflected in the relative increase in tree pollen throughout the study [21]. Although grass and forb pollination generally peaks during warm weather [21] we saw consistent grass and forb pollen levels in these categories, even as the overall pollen concentration decreased. The size distributions for each pollen type are shown in Fig. 5. Every pollen type except grass followed a narrow size distribution. The results of the study were re-calculated using an assumed median diameter for each pollen type. Using the assumed diameter introduced a negative error of only 6 %, suggesting that identification alone may give an adequate estimate of airborne pollen concentration.
Fig. 4.
Predominant pollen types by count, including all sampling periods of the summer study. Miscellaneous pollen types with counts less than 50 observed grains were not included.
Fig. 5.
Size distribution of pollen by type. Data was collected from four sampling periods of the summer 2020 field study. Box indicates interquartile range, central line is the population median, central square is population mean, whiskers show the upper and lower adjacent values, and diamonds are outliers.
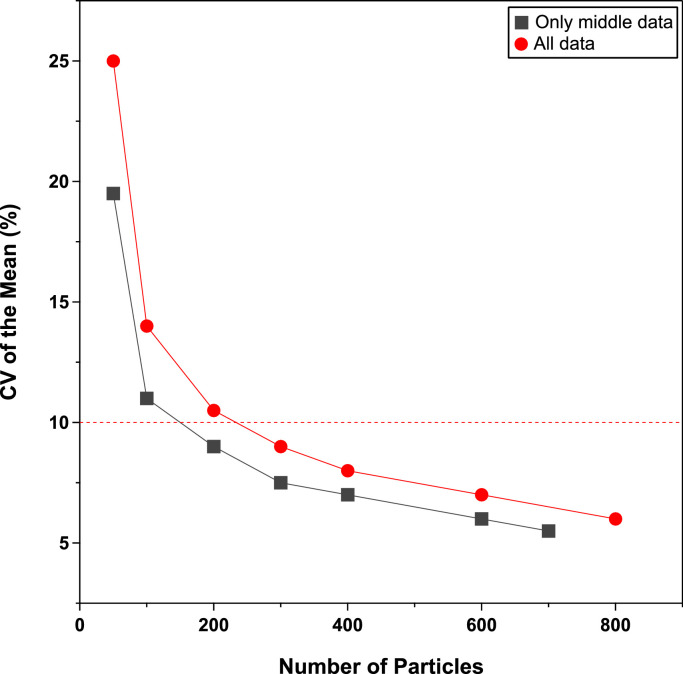
Microscopic imaging and visual analysis of each passive sample requires between 15 min to 3 h depending on pollen load and sample quality. Further statistical testing was done to optimize the analysis (Fig. 6). It was found that a sample size of 120 particles was needed to achieve a coefficient of variation of 12 %, or 10 % by analysis of 200 particles. It can take up to 3 h to analyze a fully loaded slide, but about 30 min to analyze the required 200 particles. It was also found that the center of the slide had a smaller coefficient of variation for a more limited sample size. This observation suggests that areas selected for imaging should be centered on the microscope slide for maximum efficiency, rather than by random field selection as demonstrated by previous passive sampling studies [24].
b. Campaign 2:
Fig. 6.
Sample size optimization and sample area collection; the target CV of 10 % (dashed red line) was reached at about 200 particles.
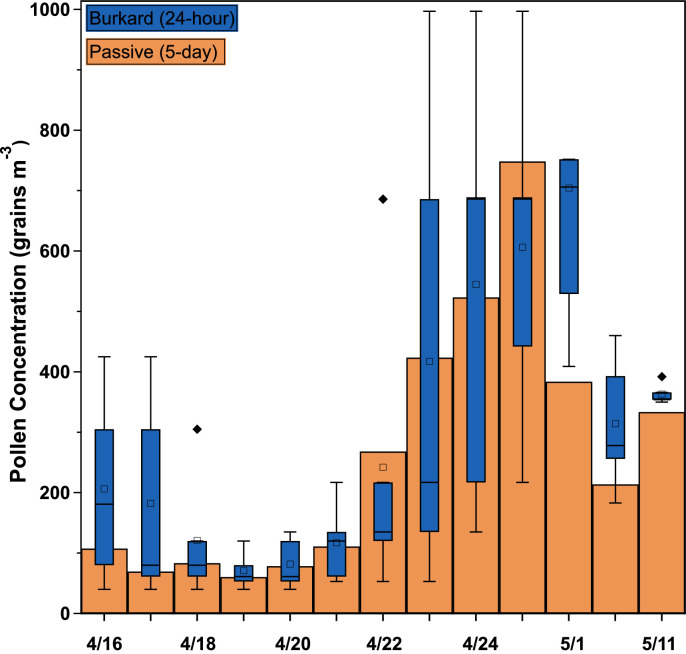
To test the efficacy of the passive sampling method in the tree pollen season, active and passive measurements were collected during the spring of 2021. Five-day averaged pollen concentrations during this study ranged from 58 to 816 grains m-3, which is considered moderate (15–89 grains m-3) to high (90–1499 grains m-3) according to the NAB scale (Table S2) and increased until mid-May. No data was collected from sampler 2 for the second and fourth sampling periods due to pollen disturbance during slide mounting and insufficient slide imaging to obtain 200 particle measurements, respectively. The coefficients of variation between the passive samplers ranged from 0.2 % to 43 %, with an average of 21 %. This is slightly higher than seen in the summer study (20 %). A minimum of 200 pollen grains per sample were counted in the calculation of pollen concentration. Almost all pollen counted were identified as tree pollen, and individual pollen grains were not classified further due to the difficulty of assigning tree pollen genera.
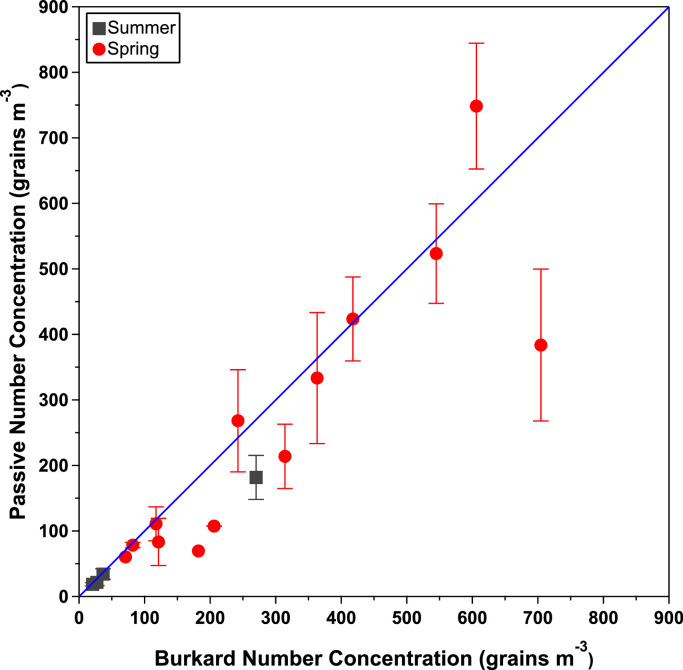
This data is visualized in Fig. 7, which shows similar results between the passive and Burkard methods. This set of passive sampler data was compared with the Burkard measurements by calculating percent differences (Table S2) and by linear regression analysis (Table S3). Regression analysis yielded a correlation coefficient of 0.87 and a slope of 0.89, suggesting that there is a strong, but not perfect linear relationship between the two populations. The intercept of the linear model was not found to be statistically significant at the 95 % confidence level. The percent differences between the passive and Burkard data ranged from 1.4 to 90 %, with an average percent difference of 28 %. This is similar to that calculated for the summer study (26 %). The agreement between sampling methods for both the spring and the summer are compared in Fig. 8.
Fig. 7.
Comparison of number concentrations measured with the passive method to that measured with the Burkard method in the spring study. Box indicates the interquartile range, whiskers are the upper and lower adjacent values, square is the mean, central line is the median, and diamonds are outliers. The mean passive concentration aligns generally with the median Burkard concentration for most sampling periods as with the summer study but has a much wider distribution within the Burkard data.
Fig. 8.
Pollen number concentration determined with passive sampler compared to that measured with the Burkard sampler. Alignment with the identity line (slope = 1) indicates similar population means. Greater agreement was observed for the summer study than the spring. Whiskers represent the standard deviations between paired passive samplers.
The wide range of pollen concentrations observed during the spring study are most likely due to the larger concentrations, greater transport capabilities, and higher point of release of tree pollen [21]. This makes tree pollen more likely to undergo significant fluctuations in concentration during gusts of wind or other local disturbances. Tree pollen has a wide range of diameters in comparison to weed pollen, with mulberry averaging at ∼13 µm and pine pollen, which can be as large as 100 µm. Because the deposition model used to calculate pollen concentration for the passive method is dependent on particle size, large amounts of very large or very small particles can have a considerable effect on the pollen concentration. This may be another source of discrepancy between the two methods as the calculation associated with the Burkard measurements is not dependent on size. Additionally, because there were trees located very close to the sampling site, differences in the volume of pollen collected by the individual samplers may be increased in relation to the summer study. This would decrease agreement between the pollen concentrations observed between individual samplers.
The effect of particle size can also influence agreement between passive samplers. For example, the two samples collected during the 4/25–4/30 period contained 281 and 279 observed particles, respectively, over an identical sample area, but yielded pollen concentrations of 681 and 816 grains m-3. It was found that only 8.9 % of sample one's particles were under 20 µm, compared to 22 % in sample two, resulting in its much larger concentration. Tree pollen varies widely by diameter and the prominence of smaller pollen grains like mulberry may increase the effect of particle size on the data collected during the spring study, which in turn would decrease the precision between samplers.
Previously published studies show varying agreement between pollen data collected by the Durham sampler and the Burkard spore trap. The results of this study agree with the observation of Bricchi et al. [10] that the passive sampler (consisting of a microscope slide housed in a Durham shelter) reports lower pollen concentrations than the Burkard spore trap, but that both methods show closely related seasonal trends in pollen concentration and that their passive sampling method was appropriate for spatial pollen analysis. Watanabe and Ohizumi [11] collected Japanese cedar and Japanese cypress pollen using the Durham shelter, calculated pollen deposition, and converted this data to airborne pollen concentration. The comparison of the Durham pollen concentrations to that collected with co-located Burkard spore traps yielded Spearman correlation coefficients comparable to the Pearson's correlation coefficients produced by this study. Cornell et al. [12] found only a slight correlation between these two samplers, but observed that the influence of pollen clumping varied between them, further suggesting that pollen clumping has a significant effect on the determination of pollen concentration using passive samplers.
Future work will include the determination of the effect pollen agglomeration has on the developed sampling procedure, and the creation of appropriate measurement protocols for pollen clumps. This methodology will be applied in an evaluation of the spatial variability in aeroallergen concentrations across the Iowa City area.
Limitations
The passive sampling method and deposition model used in this study produced statistically similar results to the conventional sampling method (Burkard spore trap). This method is appropriate for application in the quantification of airborne allergens during both the tree and ragweed pollen seasons. However, this method has not yet been validated for other seasons or geographic locations. The study conducted by Miki et al. [8] suggests that the developed sampling method may be vulnerable to influences by turbulent inertia and wind, as they observed relationships between passive sampling efficiency and wind speed and turbulence. The deposition model used in this study does not calculate pollen deposition as a function of these parameters, which may introduce error in the calculation of pollen number concentration and decrease the precision of the samplers. Error may also be attributed to the settling of pollen as clumps. Only measurements of individual pollen grains were taken, but the deposition of pollen clumps may result in true settling velocities inconsistent with those calculated following Stokes Law for a single pollen grain. Future work will include the determination of the effect pollen agglomeration has on the developed sampling procedure, and the creation of appropriate measurement protocols for pollen clumps.
CRediT authorship contribution statement
Lillian M. Jones: Methodology, Validation, Formal analysis, Investigation, Data curation, Writing – original draft, Writing – review & editing, Visualization. Chamari B.A. Mampage: Methodology, Validation, Formal analysis, Investigation, Data curation, Writing – original draft, Writing – review & editing. Thomas M. Peters: Conceptualization, Methodology, Validation, Formal analysis, Resources, Writing – original draft, Writing – review & editing, Visualization, Supervision, Project administration, Funding acquisition. Elizabeth A. Stone: Conceptualization, Methodology, Validation, Formal analysis, Resources, Writing – original draft, Writing – review & editing, Visualization, Supervision, Project administration, Funding acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Ethics statements
Not applicable
Acknowledgments
This research was funded by the University of Iowa Office of the Provost through the Interdisciplinary, Scalable Solutions for a Sustainable Futures project. We also acknowledge the Environmental Health Sciences Research Center (NIH P30 E5005605) for the loan of the Burkard spore trap; Randy Nessler, Jian Shao, and Chantal Allamargot of the University of Iowa Central Microscopy Research Facility for their assistance with microscopic imaging; and Maunika Gandhameneni for her assistance with sample analysis.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.mex.2024.102787.
Appendix. Supplementary materials
Data availability
Data will be made available on request.
References
- 1.Scheppegrell W. Hay-fever and hay-fever pollens. Arch. Intern. Med. (Chic). 1917;XIX:959–980. doi: 10.1001/archinte.1917.00080260002001. [DOI] [Google Scholar]
- 2.Wagner J., Leith D. Passive aerosol sampler. Part I: principle of operation. Aerosol. Sci. Technol. 2001;34:186–192. doi: 10.1080/027868201300034808. [DOI] [Google Scholar]
- 3.Hirst J.M. An automatic volumetric spore trap. Ann. Appl. Biol. 1952;39:257–265. doi: 10.1111/j.1744-7348.1952.tb00904.x. [DOI] [Google Scholar]
- 4.Magill P.L., Lumpkins E.D., Arveson J.S. A system for appraising airborne populations of pollens and spores. J. Occup. Environ. Hyg. 1968;29:293–298. doi: 10.1080/00028896809343003. [DOI] [PubMed] [Google Scholar]
- 5.Tauber H. A static non-overload pollen collector. New Phytol. 1974;73:359–369. doi: 10.1111/j.1469-8137.1974.tb04770.x. [DOI] [Google Scholar]
- 6.Crispen K.L., Gillespie D.N., Weiler E.C., Noonan C.W., Hamilton R.F., Ward T.J. A comparison of 1978 and 2006 peak pollen seasons and sampling methods in Missoula, Montana. Grana. 2010;49:128–133. doi: 10.1080/00173131003587049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Durham O.C. The volumetric incidence of atmospheric allergens: IV. A proposed standard method of gravity sampling, counting, and volumetric interpolation of results. J. Allerg. 1946;17:79–86. doi: 10.1016/0021-8707(46)90025-1. [DOI] [PubMed] [Google Scholar]
- 8.Miki K., Kawashima S., Kobayashi S., Takeuchi S., Tseng Y.T., Nakamura K. Evaluating inaccurate pollen concentrations caused by turbulence using passive sampler. Aerobiolog. (Bologn.) 2022;38 doi: 10.1007/s10453-021-09728-1. [DOI] [Google Scholar]
- 9.Ott D.K., Peters T.M. A shelter to protect a passive sampler for coarse particulate matter, pm10 − 2.5. Aerosol. Sci. Technol. 2008;42:299–309. doi: 10.1080/02786820802054236. [DOI] [Google Scholar]
- 10.Bricchi E., Frenguelli G., Mincigrucci G. Experimental results about Platanus pollen deposition. Aerobiolog. (Bologn.) 2000;16:347–352. doi: 10.1023/A:1026701028901. [DOI] [Google Scholar]
- 11.Watanabe K., Ohizumi T. Comparability between Durham method and real-time monitoring for long-term observation of Japanese cedar (Cryptomeria japonica) and Japanese cypress (Cryptomeria obtusa) pollen counts in Niigata prefecture, Japan. Aerobiologia. 2018;34:257–267. doi: 10.1007/s10453-018-9511-0. [DOI] [Google Scholar]
- 12.Cornell R.G., Welch S.F., Hall L.B. A comparison of gravimetric and volumetric pollen samplers. J. Allerg. 1961;32:128–134. doi: 10.1016/0021-8707(61)90065-X. [DOI] [PubMed] [Google Scholar]
- 13.Ault A.P., Peters T.M., Sawvel E.J., Casuccio G.S., Willis R.D., Norris G.A., Grassian V.H. Single-particle SEM-EDX analysis of iron-containing coarse particulate matter in an urban environment: sources and distribution of iron within Cleveland, Ohio. Environ. Sci. Technol. 2012;46:4331–4339. doi: 10.1021/es204006k. [DOI] [PubMed] [Google Scholar]
- 14.Cocke E.C. Calculating pollen concentration of the air. J. Allerg. 1937;8:601–606. doi: 10.1016/S0021-8707(37)90315-0. [DOI] [Google Scholar]
- 15.Johnson County WeatherSTEM Portal: kinnick Stadium Site. https://www.johnson.weatherstem.com/uiowa. (Accessed 05/19 2024).
- 16.Zander R.H. On mounting delicate bryophytes in glycerol. Bryologist. 1997;100:380–382. doi: 10.2307/3244509. [DOI] [Google Scholar]
- 17.Schindelin J., Arganda-Carreras I., Frise E., Kaynig V., Longair M., Pietzsch T., Preibisch S., Rueden C., Saalfeld S., Schmid B., Tinevez J.-Y., White D.J., Hartenstein V., Eliceiri K., Tomancak P., Cardona A. Fiji: an open-source platform for biological-image analysis. Nat. Method. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hinds W.C. 2nd ed. John Wiley and Sons; New York: 1999. Aerosol Technology. [Google Scholar]
- 19.Harrington J.B., Metzger K. Ragweed Pollen Density. Am. J. Bot. 1963;50:532–539. doi: 10.2307/2440028. [DOI] [Google Scholar]
- 20.Hirose Y., Osada K. Terminal settling velocity and physical properties of pollen grains in still air. Aerobiolog. (Bologn.) 2016;32:385–394. doi: 10.1007/s10453-015-9408-0. [DOI] [Google Scholar]
- 21.E.G. Smith, Sampling and Identifying Allergenic Pollens and Molds, 1st ed., Blewstone Press 1984.
- 22.Portnoy J., Barnes C., Barnes C.S. The National Allergy Bureau: pollen and spore reporting today. J. Allergy Clin. Immunol. 2004;114:1235–1238. doi: 10.1016/j.jaci.2004.07.062. [DOI] [PubMed] [Google Scholar]
- 23.Bianchi D.E., Schwemmin D.J., Wagner J.W.H. Pollen Release in the Common Ragweed (Ambrosia artemisiifolia) Botan. Gazett. 1959;120:235–243. doi: 10.1086/336030. [DOI] [Google Scholar]
- 24.Ranta H., Sokol C., Hicks S., Heino S., Kubin E. How do airborne and deposition pollen samplers reflect the atmospheric dispersal of different pollen types? An example from northern Finland. Grana. 2008;47:285–296. doi: 10.1080/00173130802457230. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.