Abstract
Attributing to their broad pharmacological effects encompassing anti-inflammation, antitoxin, and immunosuppression, glucocorticoids (GCs) are extensively utilized in the clinic for the treatment of diverse diseases such as lupus erythematosus, nephritis, arthritis, ulcerative colitis, asthma, keratitis, macular edema, and leukemia. However, long-term use often causes undesirable side effects, including metabolic disorders-induced Cushing's syndrome (buffalo back, full moon face, hyperglycemia, etc.), osteoporosis, aggravated infection, psychosis, glaucoma, and cataract. These notorious side effects seriously compromise patients' quality of life, especially in patients with chronic diseases. Therefore, glucocorticoid-based advanced drug delivery systems for reducing adverse effects have received extensive attention. Among them, prodrugs have the advantages of low investment, low risk, and high success rate, making them a promising strategy. In this review, we propose the strategies for the design and summarize current research progress of glucocorticoid-based prodrugs in recent decades, including polymer-based prodrugs, dendrimer-based prodrugs, antibody-drug conjugates, peptide-drug conjugates, carbohydrate-based prodrugs, aliphatic acid-based prodrugs and so on. Besides, we also raise issues that need to be focused on during the development of glucocorticoid-based prodrugs. This review is expected to be helpful for the research and development of novel GCs and prodrugs.
Keywords: Glucocorticoids, Prodrug design, Targeted drug delivery, Research progress
Graphical abstract

1. Introduction
Glucocorticoids (GCs) exhibit a wide range of pharmacological effects and extensive clinical applications, primarily encompassing the following aspects [1], [2], [3], [4]. (1) Anti-inflammatory effects: GCs effectively inhibit the synthesis and release of inflammatory mediators, thereby reducing tissue inflammation and swelling. As a result, they are extensively utilized in the treatment of various inflammatory conditions, including rheumatoid arthritis, inflammatory bowel disease, skin inflammation and coronavirus pneumonia (COVID-19) [5], [6], [7]. (2) Immunosuppressive effect: GCs suppress the function of the immune system, leading to a decreased immune response. This makes them highly effective in treating autoimmune diseases such as systemic lupus erythematosus and rheumatoid arthritis, and they are also employed for immunosuppression in organ transplant patients. (3) Anti-allergic effect: GCs are beneficial in reducing allergic reactions, and are commonly employed in the treatment of allergic rhinitis, urticaria, and other allergic diseases. (4) Anti-tumor effect: GCs are often combined with cytotoxic drugs such as cisplatin, paclitaxel and docetaxel in cancer treatment to mitigate the side effects of chemotherapy [8], [9], [10]. In addition, GCs can be employed in the treatment of specific malignant diseases, such as leukemia and lymphoma. Moreover, they have the potential to enhance the anti-solid tumor efficacy of cytotoxic drugs by regulating the tumor microenvironment [8], [9], [10], [11], [12], [13], [14]. These diverse pharmacological effects make GCs indispensable in the management of a broad spectrum of medical conditions.
However, it is essential to employ them judiciously due to the potential for adverse effects, especially with prolonged or excessive use. High-dose or long-term systemic use of GCs may result in adverse effects due to nonspecific distribution, such as Cushing's syndrome from endocrine system disorders, osteoporosis from osteoblast destruction, hyperglycemia from glucose metabolism disorders, and increased risk of infection due to immunosuppression [15], [16], [17]. Besides, topical ocular administration may also result in certain side effects, such as the development of glaucoma and cataracts [1,2,[18], [19], [20], [21], [22]]. Overall, GCs are a double-edged sword. Therefore, healthcare practitioners must carefully consider the benefits and risks, tailor the dosage and treatment duration to individual patients, and continually monitor their response to ensure optimal therapeutic outcomes while minimizing side effects.
Representative dosage forms and products of GCs approved by U.S. Food and Drug Administration (FDA) are summarized in Table 1. Among them, orally administered tablets and capsules are the most frequently utilized in clinical practice, and drugs are absorbed through the gastrointestinal tract into the systemic circulation. Injectables have a more rapid onset of action and are often used for first aid. Although tablets and injectables are convenient for patients, they are also the most likely to cause side effects due to their systemic absorption. Additionally, to minimize the systemic adverse effects of drugs, various locally administered formulations have been developed successively, such as suspensions, emulsions, ointments, creams, oils, sprays, patches, foams, shampoo and implants. Despite some success with the aforementioned formulations, topically administered treatments are mainly limited to diseases affecting easily accessible organs such as the eyes, lungs and skin. Unfortunately, there are still no suitable products available to treat conditions like rheumatoid arthritis, lupus nephritis (LN) and ulcerative colitis. Meanwhile, the market of targeted formulations for systemic administration also remains blank.
Table 1.
Representative dosage forms and products of GCs approved by FDA.
| Dosage forms | Active ingredient | Route of administration | Specifications | Proprietary Name |
|---|---|---|---|---|
| Tablet | Dex | Oral | 1.5 mg;4 mg;6 mg | DEXAMETHASONE |
| Fludrocortisone acetate | Oral | 0.1 mg | FLUDROCORTISONE ACETATE | |
| Prednisone | Oral | 2.5 mg;5 mg;10 mg;20 mg;50 mg | PREDNISONE | |
| Prednisolone | Oral | 5 mg | PREDNISOLONE | |
| Capsule | Budesonide | Oral | 4 mg | TARPEYO |
| Injectable | Dex sodium phosphate | Injection | 4 mg/ml | DEXAMETHASONE SODIUM PHOSPHATE |
| Prednisolone sodium phosphate | Injection | 15 mg/ml | PREDNISOLONE SODIUM PHOSPHATE | |
| Methylprednisolone acetate | Injection | 80 mg/ml | DEPO-MEDROL | |
| Triamcinolone acetonide | Injection | 40 mg/ml | KENALOG | |
| Methylprednisolone sodium succinate | Injection | 40 mg/ml; 125 mg/ml |
SOLU-MEDROL | |
| Emulsion | Difluprednate | Ophthalmic | 0.05% | DUREZOL |
| Suspension | Dex; tobramycin | Ophthalmic | 0.1%;0.3% | TOBRAMYCIN AND DEXAMETHASONE |
| Triamcinolone acetonide | Injection | 40 mg/ml | XIPERE | |
| Budesonide | Inhalation | 0.25 mg/2ml;0.5 mg/2 ml;1 mg/2 ml | BUDESONIDE | |
| Loteprednol etabonate | Ophthalmic | 0.2% | ALREX | |
| Oil | Fluocinolone acetonide | Topical | 0.01% | DERMA-SMOOTHE/FS |
| Ointment | Triamcinolone acetonide | Topical | 0.1% | MYKACET |
| Betamethasone dipropionate | Topical | 0.05% | DIPROLENE | |
| Halobetasol propionate | Topical | 0.05% | HALOBETASOL PROPIONATE | |
| Hydrocortisone valerate | Topical | 0.2% | HYDROCORTISONE VALERATE | |
| Cream | Desonide | Topical | 0.05% | DESOWEN |
| Hydrocortisone valerate | Topical | 0.2% | HYDROCORTISONE VALERATE | |
| Clobetasol propionate | Topical | 0.05% | CORMAX | |
| Spray | Triamcinolone acetonide | Nasal | 0.055 mg/spray | NASACORT ALLERGY 24 HOUR |
| Flunisolide | Nasal | 0.025 mg/spray | FLUNISOLIDE | |
| Mometasone furoate | Nasal | 0.025 mg/spray | RYALTRIS | |
| Paste | Triamcinolone acetonide | Dental | 0.1% | TRIAMCINOLONE ACETONIDE |
| Foam | Halobetasol propionate | Topical | 0.05% | LEXETTE |
| Clobetasol propionate | Topical | 0.05% | CLOBETASOL PROPIONATE | |
| Shampoo | Clobetasol propionate | Topical | 0.05% | CLOBEX |
| Fluocinolone acetonide | Topical | 0.01% | CAPEX | |
| Implant | Fluocinolone acetonide | Intravitreal | 0.18 mg | YUTIQ |
| Dex | Intravitreal | 0.7 mg | QZURDEX | |
Therefore, novel drug delivery systems such as liposomes [23], [24], [25], [26], micelles [27], [28], [29], [30], inorganic carriers [31], [32], [33] and prodrugs [34], [35], [36], [37] have attracted the attention of researchers for enhancing drug targeting and reducing side effects in recent decades. However, there are limited marketed products based on liposomes and polymeric micelles, and only 13 products such as amphotericin B, doxorubicin hydrochloride, irinotecan hydrochloride, vincristine sulfate, dibucaine, cytarabine and daunorubicin compound liposomes are currently approved by FDA, while micelle-based products are even less available. Compared with liposomes or micelles, prodrugs have gained favor in new drug research due to their low investment, small risk profile and high success rate. Currently, prodrugs account for approximately 10 % of marketed drugs, and this proportion is increasing as more drug developers adopt prodrug strategies to improve drug formulations [34,38].
Prodrugs are compounds that are inactive or less active in vitro and release active drugs in vivo through enzymatic or non-enzymatic transformation [35]. They are typically designed to address the issue of poor solubility, poor permeability, instability, toxicity, and non-targeting of the parent drug during the administration process [39]. Some glucocorticoid prodrugs are already available, such as dexamethasone (Dex) sodium phosphate and methylprednisolone sodium succinate, but they mainly improve the hydrophilicity of the parent drug for a rapid onset of action. In recent years, a large number of smart responsive (pH, enzymes, etc.) prodrugs have been widely investigated as a potential strategy to reduce side reactions. In this review, we will summarize the current strategies and research progress of various GCs-based prodrugs (Scheme 1), such as polymer-based prodrugs (HPMA, PEG, PEI, etc.), dendrimer-based prodrugs, antibody-based prodrugs, peptide-based prodrugs, carbohydrate-based prodrug, aliphatic acids-based prodrugs and other types of prodrugs. Furthermore, the pertinent issues to be addressed during the development of these prodrugs have been deliberated upon.
Scheme 1.
Schematic illustration of advantages and classification of GCs-based prodrugs.
2. Strategies in prodrug design of GCs
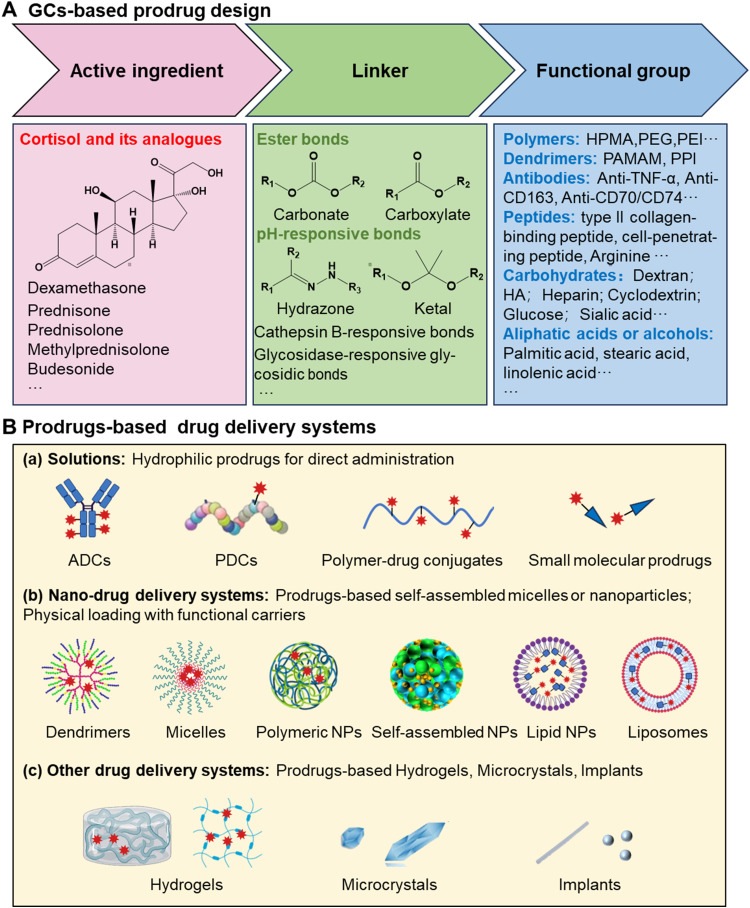
The design of GCs prodrugs follows general principles, focusing on three essential components: the active ingredient, the linker, and the functional group (Fig. 1A).
Fig. 1.
(A) Strategies in prodrug design of GCs and (B) prodrug-based drug delivery systems.
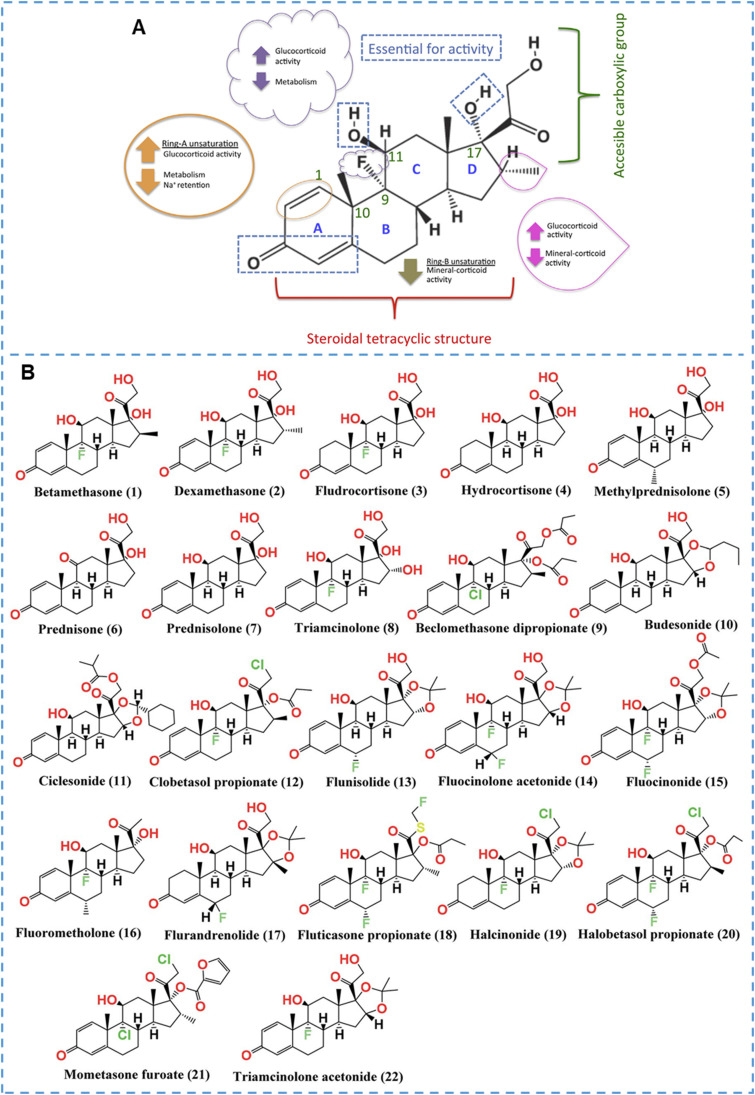
The active ingredient refers to the GCs, and the basic structure of GCs contains the stem nucleus of pregnane, Δ4–3,20-Dione, 17α‑hydroxy, and 11‑hydroxy or keto group (Fig. 2A) [2]. Due to its broad-spectrum activity in inhibiting immune responses, anti-inflammatory processes, antitoxin effects, and anti-shock capabilities, the GCs structure underwent further modifications to derive more potent compounds. Taking the potent GCs, Dex, as an example (Fig. 2A), the structures of C3, C4∼5, C11 and C17 are the basic structures for maintaining GCs activity; the introduction of an unsaturated double bond at the C1∼2 position and a methyl group at the C16 position can increase GCs activity while decreasing Na+ retention; the introduction of a fluorine atom at the C9 position can increase its activity and decrease its metabolic rate; besides, the hydroxyl group at position 21 can be used to design ester prodrugs such as acetate, phosphate esters. Overall, several structures with high specificity and low adverse reactions have been discovered after in-depth studies of the structure-activity relationship, of which more than 20 structures have been approved by FDA (Fig. 2B) [40]. In the design of prodrugs, these GCs can be used as active ingredients, considering factors like potency, therapeutic efficacy, and side effect profile. However, not all drugs can be used to design prodrug, and the following issues need to be in mind. (1) Active drugs need to contain functional groups that can be modified effectively, such as hydroxyl, carboxyl, amino or carbonyl. For GCs, the hydroxyl group at the C21 and the carbonyl group at the C3 are often used for modification. (2) Active drugs need to have an excellent stability. It is not only expected to remain stable in chemical synthesis but also required to remain stable at the onset site. For example, despite the fact that prodrug can selectively accumulate at the colonic site and release prednisolone in designing sodium sulfate-based prodrugs for oral targeted treatment of colitis, prednisolone itself rapidly decreases under the action of intestinal reductases, thus limiting its therapeutic efficacy [41], [42], [43], [44]. (3) The properties of the active drug should be mastered, such as solubility, action target, and toxicity. GCs exert their effects mainly intracellularly, so their modification cannot affect their cellular internalization.
Fig. 2.
Structure-activity relationship of GCs and structures of the screened FDA-approved GCs. (A) Structure-activity relationship of GCs, Dex as an example. Reprinted with permission [1], copyright 2016, Elsevier. (B) Chemical structures of the GCs (1∼22) approved by FDA. Reprinted with permission [40], copyright 2021, The Royal Society of Chemistry.
Besides, the linker is a pivotal element in prodrug design as it connects the active ingredient to the functional group. The choice of linker directly influences the stability of the prodrug. GCs exert pharmacological effects mainly through binding to the glucocorticoid receptors (GRs) inside the cell and then entering the nucleus to regulate related genes [1,2,45]. GCs with good lipophilicity can easily enter cells through passive diffusion and bind to GRα inside the cells [45,46]. After binding to GCs, GRs transform from inactive complexes to activated transcription factors, dissociating from multimeric complex proteins (especially Hsp90), thereby exposing the nuclear localization signal and rapidly translocating to the nucleus. Ligand-receptor complexes can modulate transcriptional responses by binding to GCs response elements and protein-protein interactions. Considering that GCs need to be free in the cytoplasm to exert their pharmacological effects, linkers must be cleavable at the disease site or within the cell. The cleavage of prodrugs is directly tied to their pharmacological effects. If the linker cleaves easily in the body, the active drug will be released quickly, which helps the drug work quickly, such as phosphate prodrugs. However, such linkers are not suitable for sustained-release drug delivery. In contrast, if linkers are difficult to cleave in the body and release the active drug, no pharmacological activity will occur. Therefore, linkers with suitable cleavage speed or selective cleavage properties under pathological conditions are more popular in recent prodrug studies. Based on the pathological characteristics of the disease site, the commonly used linkers include phosphate bonds, carbonate bonds, pH-sensitive hydrazone bonds and ketal bonds, reactive oxygen species (ROS)-responsive thioether bonds and disulfide bonds, cathepsin B and matrix metalloproteinase-responsive peptide chains, etc.
In addition, it should be noted that the same chemical bond may also lead to inconsistent drug release behavior. This is mainly because the adjacent electron-withdrawing or electron-donating group will impact the cleavable behavior of chemical bond. For example, Jia et al. introduced double bonds and aromatic structures next to the hydrazone bond to alter local electron conjugation, thereby adjusting the cleavage rate of the original hydrazone bond [47]. Furthermore, the ester bond that readily breaks in plasma becomes stable after being changed to carbamate [48]. Lastly, the safety of the linker itself, as well as that of its possible metabolites, needs to be considered, especially for some linkers with relatively large molecular weights.
The functional group is a distinctive feature of the prodrug, conferring additional properties or targeting capabilities. This group can modulate the prodrug's physicochemical characteristics and interaction with biological systems. Moreover, functional groups can be tailored for specific purposes, such as achieving site-specific drug release or enhancing cellular uptake. For example, grafting polar groups such as sodium phosphate or hydrogen succinate can improve the water solubility of GCs, and these prodrugs are hydrolyzed in vivo to generate active GCs. They facilitate the production of injectable solutions, which are more rapid-acting than oral solid dosage forms. Some water-soluble polymers, such as (N-(2-hydroxypropyl)-methacrylamide) (HPMA), polyethylene glycol (PEG), polyethyleneimine (PEI), etc., can be used to prepare conjugate prodrugs to improve the water solubility and injectability of GCs. In addition, some specific antibodies and targeting peptides can also be used to construct functional GCs prodrugs to improve the targeting of GCs. Hydrophobic fatty acid chains can also be used to construct hydrophobic prodrugs to enhance their sustained release effects. Besides, some active small molecules such as sialic acid, carnitine, and fumaric acid can respectively improve the inflammatory targeting, lung targeting, and anti-inflammatory effects of GCs. It is worth noting that these prodrugs can also be prepared into different drug delivery systems. For example, some amphipathic prodrugs can be prepared into micelles, some prodrugs can be prepared into nanoparticles, liposomes, etc., and some can be prepared into hydrogels and implants, the application of these drug delivery systems will further improve the therapeutic effects and side effects of GCs (Fig. 1B).
In summary, the design of GCs prodrugs involves a meticulous selection of the active GCs, a strategic choice of linker for controlled release, and the incorporation of functional groups to impart specific attributes. These considerations collectively aim to enhance the therapeutic profile of GCs, addressing limitations associated with their free forms.
3. Polymer-based prodrugs
Revolutionary changes have taken place in the application of polymers in industry and medicine fields, and polymer-drug conjugates have also received extensive attention in drug delivery systems. Researchers grafted hydrophobic GCs onto water-soluble polymers, such as HPMA, PEG, PEI, etc., to form polymer prodrugs to improve the hydrophilicity, targeting ability and side effects of GCs [49]. Representative polymer-based GCs prodrugs are summarized in Table 2.
Table 2.
Representative polymer-based GCs prodrugs.
| Prodrug types | Prodrug name | Carrier | Linker | Route of administration | Applications and Advantages | Ref. |
|---|---|---|---|---|---|---|
| HPMA-based prodrugs | P-Dex | PHPMA (73.0 kDa) |
Acid-activated hydrazone | Intravenous injection | To treat AIA in rats; Strong, long-lasting anti-inflammatory effect. | [51] |
| P-Dex | PHPMA (34.0 kDa) |
Acid-activated Hydrazone | Intravenous injection | To treat AIA in rats; Controllable molecular weight and drug content; Strong, long-lasting anti-inflammatory and joint protection effects. | [52] | |
| P-Dex | PHPMA (35.0 kDa) |
Acid-activated Hydrazone | Intravenous injection | To treat AIA in rats; Explored the effects of hydrazone bonds with different activation rates on efficacy and side effects; | [47] | |
| P-Dex | PHPMA (36 kDa) |
Acid-activated Hydrazone | Intravenous injection | To treat CIA in mice; Single intravenous administration can last for 30 d; Reduced skeletal toxicity. | [56] | |
| ProGel-Dex | PHPMA (6.8 kDa) |
Acid-activated Hydrazone | Intra-articular injection | To treat arthritis and osteoarthritis in rats; Local administration and last for one month; Sustained resolution of arthritis pain and inflammation without apparent GCs-associated toxicity. | [55] | |
| P-Dex | PHPMA (41.8 kDa) |
Acid-activated Hydrazone | Intravenous injection | To treat Lupus Nephritis; Monthly administration reduces proteinuria and mortality in mice with LN without osteoporosis. | [58] | |
| P-Dex | PHPMA (37.1 kDa) |
Acid-activated Hydrazone | Intravenous injection | To treat murine calvaria osteolysis; Sustained retention for 6 d in disease sites; Excellent osteoprotective effects. | [60] | |
| P-Dex | PHPMA (36.8 kDa) |
Acid-activated Hydrazone | Intravenous injection | To treat murine osteolysis; Comparable therapeutic effect; Reduced systemic toxicity. | [61] | |
| P-Dex | PHPMA (15.0–45.0 kDa) |
Acid-activated Hydrazone | Intravenous injection | Evaluated the impact of molecular weight and drug content on the pharmacokinetic and biodistribution profiles of P-Dex in an osteolysis mouse model. | [62] | |
| P-Dex | PHPMA (36.8 kDa) |
Acid-activated Hydrazone | Intravenous injection | To treat SDS-induced ulcerative colitis; A single injection of P-Dex with 1/4 equivalent Dex dose had a better therapeutic effect than daily free Dex. | [63] | |
| Polymer-DEX/DOX | PHPMA (27.0-43.0 kDa) | Esterase/ Hydrolysis-activated Ester | Intravenous injection | To treat murine lymphoma; Significant long-term survival of Polymer-DEX/DOX compared free drug solution. | [64] | |
| PEG-based prodrugs | PEG-Dex | PEG (10 kDa) |
Esterase/ Hydrolysis-activated Ester | Oral and Intravenous injection | Increased the hydrophilicity of Dex; Improved oral bioavailability. | [65] |
| mPEG-Dex | PEG (2.0 kDa) |
Acid-activated Hydrazone | Intravenous injection | To treat AIA in rats; Provided sustained (>15 d) amelioration of ankle joint inflammation. | [66] | |
| DHAc-PEG | PEG (22 kDa) |
Acid-activated Hydrazone | —— | Lysosomal escape and drug release at the cellular level were studied. | [68] | |
| ZSJ-0228 | PEG (1.9 kDa) |
Acid-activated Hydrazone | Intravenous injection | To treat Lupus Nephritis; Sustained therapeutic effect for one month; Better biosafety. | [69] | |
| PEG-Dex | PEG (5.0 kDa) |
Acid-activated Hydrazone | Intravenous injection | To treat AIA in rats; Long-acting for one week; Explored the effect of Dex content on the fate of PEG-Dex micelles in vivo. | [70] | |
| SA-PEG-Dex | PEG (2.0 kDa) |
Esterase/ Hydrolysis-activated Ester | Intravenous injection | To treat Acute Kidney Injury; Active targeted drug delivery; Enhanced therapeutic effects. | [71] | |
| PEI-based prodrugs | PEI-Dexa | PEI (branched, 2 kDa) | Thioether | —— | Used as a gene delivery vehicle; Increased the gene transfection efficiency. | [79], [80], [81] |
| PEG-Glu-PEI-Dex | PEI (branched, 2 kDa) | Thioether | —— | Used as a gene delivery vehicle; Reduced toxicity and improved the transfection efficiency of PEI. | [82] | |
| Au-PEI/DNA/PEI-Dex | PEI (branched, 1.2 kDa) | Thioether | Intravenous injection | For in vivo gene delivery; Improved gene transfection efficiency; Enhanced tumor suppression effect. |
[83] | |
| DXM-PEI-mannose | PEI (branched, 2 kDa) | Acid-activated Imine bond | Intravenous injection | To treat Acute lung injury; Long-term lung targeting effect; Good therapeutic effect. | [85] | |
| PVP-base prodrugs | PVP-prednisolone | PVP (16.9 kDa) |
Acid-activated Hydrazone | —— | To treat LPS-induced cell inflammation; Desired anti-inflammatory effect; Achieved a pathological microenvironment-responsive drug release. | [87] |
| Polydexame thasone prodrugs | Poly-Dex | Adipic acid dihydrazide | Acid-activated Hydrazone | Intravenous injection | To treat LPS-induced cell inflammation; Ultra-high drug loading of 74 wt%; Enhanced anti-inflammatory capacity. | [88] |
| Dendrimer-based prodrugs | PAMAM-Dex | PAMAM (NH2) G4 | Thioether | —— | Used for in vitro cellular gene delivery; Enhanced the transfection efficiency and reduced cytotoxicity of dendrimers. | [89,90] |
| PPI-Dex | PPI (NH2) G4 and G5 | Thioether | —— | Used for in vitro gene delivery; Improved PPI cytotoxicity and transfection; | [91] | |
| PAMAM-Dex | PAMAM (NH2) G4 | Esterase/ Hydrolysis-activated Succinate | —— | Used for anti-inflammatory at cellular level; More efficient therapeutic effect compared with Dex-loaded liposome. | [92] | |
| D-Dex | PAMAM (OH) G4 | Esterase/ Hydrolysis-activated Succinate | Subconjunctival injection; | To treat corneal inflammation of rat mild alkali burn; Long-acting for two weeks; Enhanced therapeutic efficiency and reduced side effects. | [93] | |
| D-Dex | PAMAM (OH) G4 | Esterase/ Hydrolysis-activated Glutarate | Subconjunctival injection; | To treat autoimmune dacryoadenitis in rabbits; Long-acting for two weeks; Improved efficacy and patient compliance. | [94] | |
CIA: collagen-induced arthritis; LPS: lipopolysaccharide; SDS: sodium dextran sulfate.
3.1. HPMA-based prodrugs
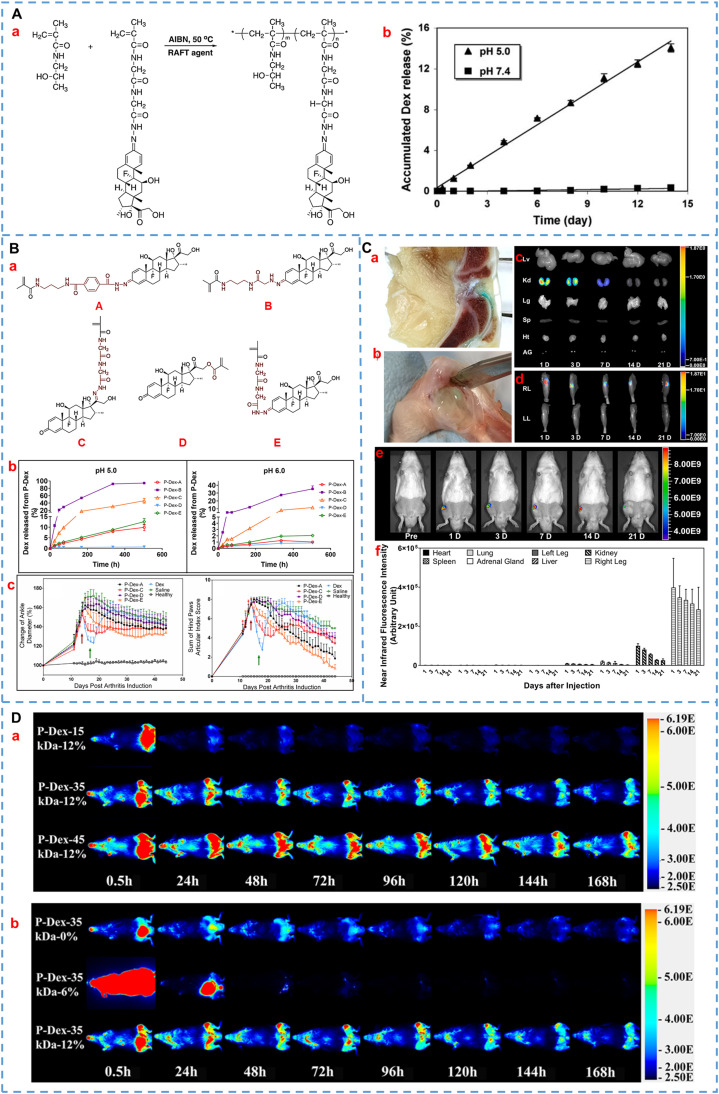
Since Kopeček et al. first synthesized HPMA in 1973, HPMA has gained widespread popularity in biomedicine fields (such as drug copolymer carriers and hydrogel matrix) for more than 40 years because of convenient modifiability (containing active groups of hydroxyl and amino groups) and good biocompatibility [36,49,50]. For GCs, Wang and Liu et al. innovatively designed and synthesized HPMA-Dex polymer prodrugs bridged by hydrazone bonds for the treatment of arthritis (P-Dex, as shown in Fig. 3A) [51,52]. This pH-sensitive hydrazone linkage can selectively release drugs in an acidic environment of disease, facilitating the targeted treatment of arthritis. The results showed that systemic administration of P-Dex exhibited superior and more sustained anti-inflammatory effects in comparison to free Dex in adjuvant-induced arthritis (AIA) rats. Moreover, P-Dex showed a greater preservation effect on bone and cartilage than Dex. In the following study, the authors utilized fluorescence-activated cell sorting (FACS) and immunohistochemical staining techniques to investigate the prolonged mechanism of fluorescent-labeled P-Dex [53]. On one hand, inflamed joints exhibit enhanced vascular permeability, which facilitates joint targeting of macromolecular prodrugs. On the other hand, inflammation-activated synovial fibroblasts and myeloid cells promote dynamic uptake of prodrugs followed by gradual release of active drugs in an acidic lysosomal environment, resulting in sustained anti-inflammatory signaling via blockade of pro-inflammatory cytokine production. Therefore, extravasation of nanomedicine through leaky vasculature at sites of inflammation and subsequent inflammatory cell-mediated sequestration are two crucial factors contributing to its long-acting mechanism. In addition, Quan and co-workers studied the influence of molecular weight and drug content on the biodistribution of polymer prodrugs in vivo by labeling polymers with 125I [54]. The results suggested that the increase in molecular weight and drug loading of Dex would promote the joint targeting effect of P-Dex by increasing the circulating half-life and enhancing the uptake of inflammatory cells in the disease site.
Fig. 3.
Representative HPMA-based GCs prodrugs. (A)(a) Synthesis process of HPMA–Dex conjugates. (b) In vitro release profiles of P-Dex in pH 5.0 and 7.4 buffers. Reprinted with permission [52], copyright 2008, Springer Nature. (B)(a) Design of Dex-containing monomers (A, B, C, D, E) of HPMA-Dex prodrugs with different releasing rates. (b) Dex release behavior of various P-Dex prodrugs in the different mediums. (c) Pharmacodynamic results of different P-Dex prodrugs on joint inflammation in AIA rats. Reprinted with permission [47], copyright 2020, Elsevier. (C) Retention of the ProGel-Dex in arthritic joints. (a, b) Retention of ProGel-Dex in the knee joints and dissected ankle joints of rats was observed at 7 d and 28 d after intra-articular injection, respectively. NIR optical images of various organs (c) and hind limbs (d, e) at different time points. (f) The semi-quantitative results of (c) and (d). Reprinted with permission [55], copyright 2021, Elsevier. (D) The images were acquired following a single intravenous administration of P-Dex-IRDye conjugate with different MW (a) and different Dex content (b). The left and contralateral femurs of mice were challenged with poly (methyl methacrylate) particles and PBS, respectively. Reprinted with permission [62], copyright 2017, American Chemical Society.
In a recent study, Jia and colleagues explored the correlation of HPMA-Dex prodrugs linked by five different chemical bonds (ester bonds and hydrazone bonds at different positions) in vitro and in vivo. As shown in Fig. 3B [47], the findings of Dex release of the five prodrugs showed a broad spectrum of activation kinetics. The therapeutic effects and side effects after a single administration were also evaluated and compared in the AIA rats. The results served as a reminder that the faster in vitro drug release led to quicker therapeutic efficacy in vivo, albeit with an increased risk of relapse. On the contrary, slow drug release might lead to poor effects on the body. Therefore, appropriate drug release kinetics was essential to design an ideal prodrug. In addition to intravenous administration, Gao et al. reported an HPMA-Dex prodrug hydrogel for the treatment of joint pain by intra-articular administration [55]. They observed that as the drug loading of Dex reached 20%, the prodrug solution underwent a gradual transformation into a hydrogel upon increasing the temperature from 4 °C to 30 °C. After intra-articular injection, the prodrug solution underwent gelation and exhibited sustained retention within the joint for over one month (Fig. 3C), prolonging the alleviation of joint inflammation and pain in arthritis rats.
In addition to being used to treat rheumatoid arthritis [47,[51], [52], [53], [54],56], HPMA-Dex has also been widely extended to other diseases, such as LN [57], [58], [59], inflammation caused by implants [60], [61], [62], inflammatory bowel disease [63], and tumors [64]. Among them, Wang's team not only studied the therapeutic effect of HMPA-Dex conjugates in the murine prosthesis failure model but also explored the influence of the molecular weight of prodrugs on both the therapeutic effect and the biodistribution in the body. The biodistribution of P-Dex-IRDye conjugates with varying molecular weights or drug content in poly (methyl methacrylate) particles-induced peri‑prosthetic inflammation mice were investigated using near-infrared ray (NIR) optical images, as shown in Fig. 3D [61,62]. The study revealed that molecular weight was the primary factor influencing the pharmacokinetics and biodistribution of P-Dex, while Dex content played a secondary role. Increasing the molecular weight of P-Dex reduced clearance, prolonged half-life, and facilitated targeted accumulation at inflammatory sites. Higher Dex content also resulted in greater systemic exposure and longer retention of the conjugate at sites of inflammation. However, P-Dex with a Dex content of 6 wt% exhibited abnormally rapid elimination due to aggregation, which made it more susceptible to internalization and clearance by the mononuclear phagocytic system. Considering that this study only investigated the in vivo fate of three drug-loaded P-Dex, the optimal Dex content should be determined in follow-up studies. Overall, this study provided a reference for the subsequent study of copolymer prodrugs. In another study, Ren et al. injected HPMA-Dex prodrugs intravenously to treat sodium dextran sulfate-induced ulcerative colitis mice [63]. Even though the prodrug was equivalent to a quarter of the free drug dose, it still exhibited superior therapeutic efficacy compared to the unbound drug (histologic colitis score, P < 0.05), which was exciting for the treatment of colitis. Besides, Kostková et al. grafted Dex and doxorubicin together on HPMA polymers of different molecular weights to synergistically treat lymphoma, which effectively prolonged the survival period of diseased mice [64]. This provided a new option for the treatment of tumors, but different linkers may cause different drug release rates, and the synergistic mechanism of the two drugs has not been explored.
As mentioned above, extensive research has been conducted on HPMA-based prodrugs of GCs, and they have demonstrated remarkable advantages, including long-lasting therapeutic effects and reduced adverse reactions. However, certain considerations warrant attention during the development of this type of prodrug. These include exploring the metabolic pathway of HPMA, ensuring reproducibility in the synthesis process, investigating the impact of various linkers, and assessing the influence of diverse administration routes.
3.2. PEG-based prodrugs
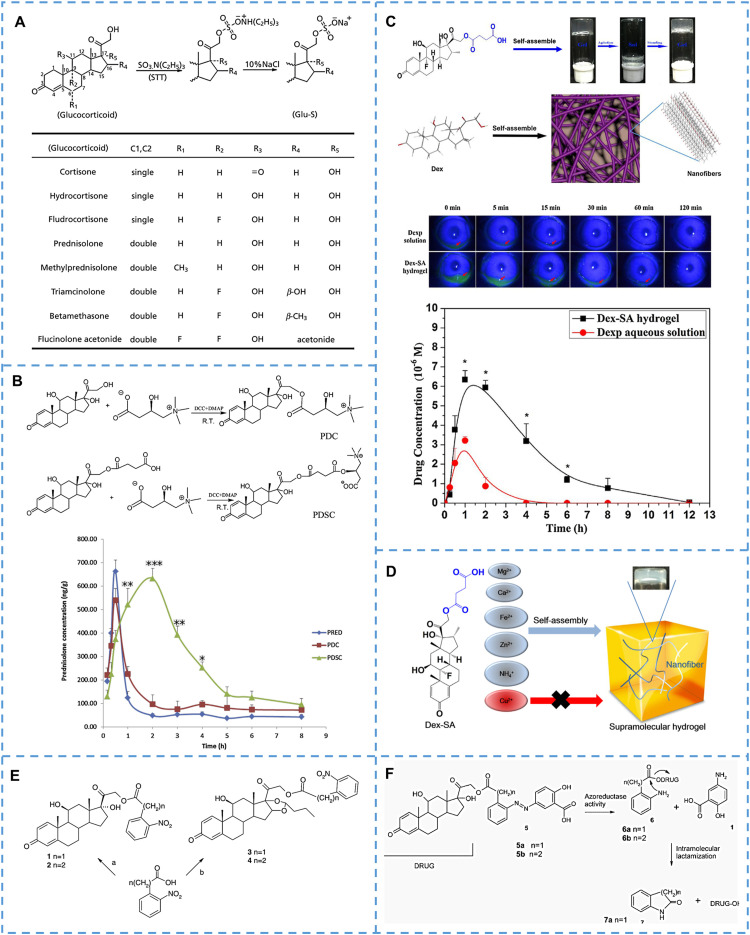
PEG with good biocompatibility has been widely employed in drug delivery [65], [66], [67], [68], [69], [70], [71]. Originally, Zacchigna et al. synthesized a drug-polymer conjugate (mPEG-Dex, as shown in Fig. 4A) [65] by covalently linking Dex to mPEG-NH2 (Mw:10 kDa) through a succinate linker. Although the mPEG-Dex increased the hydrophily of Dex, the area under the concentration-time curve (AUC) and mean residence time of the drug in blood did not show significant improvement following intravenous administration. This might be attributed to the premature release of Dex from mPEG-Dex triggered by esterase. In later studies, the scientists introduced acid-sensitive hydrazone bonds into the prodrugs to avoid premature release of the drug [66,[68], [69], [70]]. Among them, Liu and his colleagues synthesized a linear PEG-Dex prodrug via a hydrazone bond using a click reaction for arthritis therapy. As illustrated in Fig. 4B [66], the prodrug was capable of selectively releasing Dex under low pH conditions (about pH 5.0). And it could continue to improve ankle joint inflammation in AIA rats (> 15 d), while the same dose of free Dex could only temporarily relieve arthritis and relapse after stopping the drug. Furthermore, PEG-Dex had no obvious side effects on bone density in comparison to the free drug. It seems to be an effective strategy for treating arthritis.
Fig. 4.
Representative PEG-based GCs prodrugs. (A) Chemical structure of mPEG-Dex coniugate. Reprinted with permission [65], copyright 2008, Elsevier. (B) Chemical structure of Click PEG-Dex; The release behaviors of click PEG-Dex at the medium of different pH; Ankle joint diameter changes of the various treatment groups during the entire experiment. Reprinted with permission [66], copyright 2010, American Chemical Society. (C) Design of ZSJ-0228 that self-assembles into micelles in water, with good renal targeting and the effect of reducing proteinuria caused by nephritis. Reprinted with permission [69], copyright 2010, American Chemical Society. (D) Chemical structure of PEG-Dex conjugate; pharmacokinetics behaviors and pharmacokinetic parameters after intravenous injection of various Dex-based formulations. Reprinted with permission [70], copyright 2018, Springer Nature. (E) Chemical structure of SA-PEG-Dex conjugates; the renal fluorescence signal and the semi-quantitative values in AKI mice treated with fluorescent probe-labeled micelles at different time points. Reprinted with permission [71], copyright 2017, Ivyspring International Publisher.
In later research, Jia et al. developed a macromolecular PEG-Dex prodrug (ZSJ-0228) that can self-assemble into micelles for the treatment of LN (Fig. 4C) [59,69]. In this amphiphilic prodrug, the Dex dimer acts as the hydrophobic group and the PEG chain acts as the hydrophilic group. Dex was also linked to the entire material through an acid-sensitive hydrazone bond, which was conducive to the specific release of the drugs in the pathological environment. The therapeutic efficacy was significantly improved by monthly intravenous injection of ZSJ-0228 for a duration of 2 months without obvious side effects (adrenal gland atrophy and osteopenia) compared with the equivalent dose of daily Dex sodium phosphate (P < 0.01). Besides, the researchers further studied the tissue distribution and cellular localization of the fluorescent dye-labeled prodrug, which proved that the prodrugs could passively accumulate to the inflamed kidney by the ‘ELVIS’ mechanism (the Extravasation of the nanomedicine through Leaky Vasculature at sites of inflammation and its subsequent Inflammatory cell-mediated Sequestration). Similarly, Wang et al. linked Dex to the wheat-like PEG derivatives through the hydrazone bond and designed an acid-responsive polymer micelle for the treatment of RA [70]. As shown in Fig. 4D, they studied the differences in drug release, biodistribution and therapeutic effects of polymer micelles with different drug loadings. It provided a structural basis for the design and optimization of nanocarriers.
In addition to passive targeted therapy, active targeted delivery of nanoparticles has also received extensive attention. As shownin Fig. 4E [71], Hu and his colleagues designed an E-selectin-targeted Sialic acid-PEG-Dex micelle (SA-PEG-Dex) for enhanced therapeutic efficacy of acute kidney injury (AKI). The conjugate could undergo spontaneous self-assembly to form micelles in an aqueous system and could actively deliver the drug to inflammation sites through the interaction of SA and overexpressed E-selectin receptors on the inflammatory vascular endothelial cells. Biodistribution experiments verified that SA-PEG-Dex micelles accumulated more in inflammatory cells and inflammatory kidneys than PEG-Dex. As a result, SA-PEG-Dex micelles had better therapeutic efficacy in treating AKI than free Dex and PEG-Dex (P < 0.05).
Currently, PEG-based prodrugs are primarily utilized for treating inflammatory diseases through intravenous injection, mainly owing to PEG's capability to prolong the circulation time of nanocarriers in the bloodstream. Moreover, these prodrugs can help reduce side effects by altering the biodistribution of drugs within the body. However, it is essential to note that the molecular weight of PEG in these prodrugs varies significantly, ranging from 2 to 22 kDa, and there is still relatively limited research on how molecular weight influences the fate of PEG-based prodrugs in vivo. Besides, some studies have shown that the repeated use of PEG may also cause some problems, such as the production of anti-PEG antibodies, allergic reactions, and accelerated blood clearance (ABC), which may produce toxic substances and greatly affect its therapeutic effect [72], [73], [74]. Therefore, these factors also need to be considered comprehensively during the development of PEG-based prodrugs.
3.3. PEI-based prodrugs
PEI is a hydrophilic linear or branched cationic polymer containing amino groups. With its ability to form nanocomplexes with drugs, RNA or DNA through electrostatic interactions or covalent bonds, it was widely used in drug delivery, molecular imaging, etc. [75], [76], [77], [78]. GCs have been reported to expand the nuclear pore and translocate into the nucleus upon binding with GRs, thereby facilitating DNA transport into the nucleus. Therefore, many studies have modified GCs on the carrier surface to facilitate the nuclear transport of cargo [79], [80], [81], [82], [83], [84]. For example, researchers synthesized Dex-modified PEI (PEI-Dex) by one-step reaction and then used them for various DNA delivery, as shown in Fig. 5A [79], [80], [81], [82]. The results proved that the gene transfection efficiency was not only improved but also the cytotoxicity was reduced compared to PEI alone, which makes PEI-Dex an excellent non-viral gene carrier. Furthermore, Kim et al. modified PEG on the surface of PEI-Dex to improve solubility, stability and cytotoxicity, which were expected to prolong the residence time of nanocomposites in vivo [82]. Based on in vitro cell-level gene delivery, Chen et al. designed the Dex-modified PEI-coated gold nanocomposites (Au-PEI/DNA/PEI-Dex) for in vivo gene delivery (Fig. 5B) [83]. The outer layer of PEI-Dex not only protected DNA from DNase degradation but also enhanced DNA transfection efficiency as a nuclear targeting agent, which was also validated in tumor-bearing mice. In addition to use as the nuclear-targeting agents, PEI-Dex can also be used to treat inflammatory diseases. Dex and mannose co-modified PEI prodrug (Dex-PEI-mannose) nanoparticles were constructed for the targeted treatment of acute lung injury (ALI) by specifically binding to mannose receptors on macrophages in Su's research [85]. Dex-PEI linked by imine bonds has pH-responsive drug release behavior, which can avoid premature drug leakage to a certain extent. Modification of mannitol improved the disease targeting and therapeutic effect of ALI.
Fig. 5.
Representative polymer-based GCs prodrugs. (A) The synthesis scheme of PEI-Dex. Reprinted with permission [79], copyright 2007, American Chemical Society. (B) Schematic diagram of preparation and gene delivery of Au-PEI/DNA/PEI-Dex. Reprinted with permission [83], copyright (2014), American Chemical Society. (C) Synthetic route of Dex-PEI-mannose. Reprinted with permission [85], copyright 2021, Elsevier. (D) Schematic diagram of prednisolone-PVP prodrug modified nerve electrode. Reprinted with permission [87], copyright 2010, American Chemical Society. (E) Schematic diagram of preparation and anti-inflammatory treatment of poly-Dex-prodrug nanocapsules. Reprinted with permission [88], copyright 2021, The Royal Society of Chemistry.
In this part, PEI-based GCs prodrugs are mainly used for gene delivery due to the nuclear targeting of Dex and the proton sponge effect of PEI. It is worth mentioning that although PEI-Dex prodrugs have been extensively studied, the average molecular weights of PEI were all less than 2 kDa, which may be related to the higher toxicity of PEI with larger molecular weights. In addition, the development of PEI-related materials with better biocompatibility, specific targeting effect, and buffering capacity will facilitate the further development of this class of prodrugs [76].
3.4. PVP-based prodrugs
Polyvinylpyrrolidone (PVP) is a hydrophilic polymer that has been extensively used as a carrier in the fields of medicine, biomedicine, and nutrition. The exceptional versatility of PVP renders it one promising polymer for developing innovative pharmaceuticals [86]. A study of PVP-based GCs prodrugs was investigated by Cao and his colleagues. As shown in Fig. 5D [87], they constructed the prodrug by linking prednisolone to functionalized PVP via an acid-sensitive hydrazone bond and then deposited it on a nerve electrode to alleviate inflammation at the nerve interface. The pH-sensitive drug release behavior mediated by hydrazone bond contributed to the rapid drug release under conditions of inflammation-induced tissue acidosis. In vitro anti-inflammatory ability and biocompatibility further confirmed the application value of PVP-based GCs prodrugs on implantable nerve electrodes. Of course, its application still needs to be verified in a large number of animal experiments before entering clinical studies.
3.5. Poly-Dex phosphate prodrug
Unlike other polymer-based prodrugs, poly-Dex phosphate prodrugs (Poly-Dex) were constructed by copolymerization of drugs with a dihydrazine adipate monomer via a hydrazone linkage [88]. The total concentration of Dex in the Poly-Dex polymer was 74 wt%, which was significantly higher than that of conventional polymer prodrugs. The authors then encapsulated Poly-Dex into mesoporous silica nanocapsules to avoid premature leakage of drugs. Upon uptake by target cells, the pH-sensitive linker can be cleaved in weakly acidic conditions of lysosomes, leading to the release of active drugs. Importantly, in vitro anti-inflammatory viability studies showed significant advantages of this nano-drug delivery system compared to either unencapsulated poly-prodrugs or Dex sodium phosphate (the relative IL-6 expression, P < 0.01). This poly-prodrug delivery system provides an idea for the targeted delivery of water-soluble small-molecule drugs.
4. Dendrimer-based prodrugs
Dendrimers are three-dimensional spherical chemical structures with high monodispersity, dimensional adjustability and surface functionalization [95], [96], [97], [98]. These unique features enable dendrimers to link, coordinate, or encapsulate therapeutic drugs (as shown in Fig. 6A) to achieve desired pharmacokinetics and biodistribution, which has been developed as a potential nanotherapeutic platform. This section mainly summarizes the preclinical development of dendrimer-based GCs prodrugs and representative prodrugs are summarized in Table 2.
Fig. 6.
Representative dendrimer-based GCs prodrugs. (A) Diagrammatic representation of dendrimer-based drug delivery systems. Reprinted with permission [96], copyright 2021, Elsevier. (B) Synthesis routes of PAMAM G4 (NH2)-Dex conjugate. Reprinted with permission [92], copyright 2013, Elsevier. (C) Synthetic route of G4(Phe) and G4(Phe)-Dex. Reprinted with permission [90], copyright 2020, The authors. (D) Preparation of d-Dex and d-Dex-loaded injectable hydrogel. (a, b) Synthesis of d-Dex and gel precursors. (c) Schematic diagram of the formation of a final injectable hydrogel. Reprinted with permission [93], copyright 2017, Elsevier.
The GCs receptor is a nuclear receptor that is primarily located in the cytoplasm. When the GR binds to a ligand, the receptor-ligand complex is transported from the cytoplasm to the nucleus. It is reported that the receptor enlarges the nuclear pore to 60 nm during the translocation process, which facilitates the translocation of the polymer/DNA complex into the nucleus [99,100]. Based on the reports that GCs can promote nuclear pore expansion, researchers constructed a Dex-modified fourth-generation poly(amidoamine) (PAMAM, as shown in Fig. 6C) to improve the transfection efficiency of gene [89,90]. In these studies, Dex acted as an auxiliary rather than as the main active ingredient. The transfection efficiency of DNA-loaded PAMAM-Dex was significantly enhanced compared with PAMAM (P < 0.05). In a similar study, the researchers grafted Dex onto another cationic dendrimer material (polypropylenimine (PPI)) for DNA delivery. They developed efficient gene carriers by the conjugation of Dex at various percentages to the 4th and 5th-generation PPIs (PPIG4s and PPIG5s) [91]. The results indicated that PPIG5s exhibit superior transfection efficiency compared to PPIG4s (P < 0.05), with a Dex grafting ratio of 10 % being the optimal condition. It was suggested that the structure of the dendrimer and the ratio of Dex on its surface were very critical factors. In another study, Choksi and his colleagues compared the anti-inflammatory ability of PAMAM dendrimer–Dex (Fig. 6B) with Dex-loaded liposomes [92]. It was found that dendrimer–Dex had a better ability to inhibit pro-inflammatory factors in vitro than Dex-loaded liposomes, which may be because dendrimers with cationic charges on the surface are more easily internalized by cells. However, the authors did not conduct experiments to verify this perspective. Besides, they also did not compare the in vivo anti-inflammatory ability, which was not comprehensive enough.
In addition to systemic administration, dendrimers have also played an important role in topical ocular therapy. For instance, Soiberman et al. designed a subconjunctival injectable dendrimer-Dex (D-Dex) hydrogel to treat mild alkali burn-induced corneal inflammation [93]. As shown in Fig. 6D [93], Dex was bound to fourth-generation PAMAM (OH) via a succinate bond, which was then embedded in a hyaluronic acid hybrid gel to construct a sustained-release drug delivery system. After subconjunctival injection, d-Dex was able to distribute into inflamed corneal macrophages and persistently reduced inflammation for more than 2 weeks. Besides, d-Dex hydrogel also mitigated the adverse effects caused by frequent administration of free Dex, such as elevation of intraocular pressure. Thereafter, the team also investigated the efficacy of subconjunctival injections of d-Dex alone in the treatment of autoimmune dacryoadenitis [94]. Encouragingly, obvious restoration of lacrimal gland function and attenuation of inflammation were also achieved after two weeks of treatment with a single injection of d-Dex compared with free Dex solution. By comparing the above two studies, we believe that d-Dex may be eliminated faster than d-Dex-loaded hyaluronic acid hybrid gel due to the lack of a hydrogel barrier, but all of them were able to exert their effects for 2 weeks. Therefore, the in vivo clearance mechanism of d-Dex remains to be elucidated. The elucidation of the clearance mechanism of d-Dex will not only help understand the fate of drugs in the body, thereby better predicting and optimizing their pharmacodynamic performance, but also help to optimize drug dosing regimens, including dosage and dosing frequency. Furthermore, it is critical to assess its accumulation and potential toxic effects in the body.
Dendrimer-based prodrugs of GCs can be used both intravenously and locally for drug delivery. Despite great potential in the field of drug delivery, synthetic complexity, unclear metabolic mechanisms, and potential toxicity need to be addressed in the following study. Dendrimers often involve complex synthetic processes, and simplifying synthetic routes and optimizing reaction conditions are crucial to ensure reproducibility and scalability and accessibility to applications. Besides, understanding how dendrimer-based prodrugs are metabolized in vivo is fundamental for predicting their pharmacokinetics and pharmacodynamics. Identifying metabolic pathways can inform potential side effects and guide dosage and dosing frequency. Moreover, in-depth investigation of the safety of dendrimers and their prodrugs, including acute and chronic toxicity, immunogenicity, and potential long-term effects, can guide the design of safer dendrimers-based prodrugs. In conclusion, acknowledging and proactively addressing synthetic complexity, metabolic mechanisms, and potential toxicity will undoubtedly contribute to the successful development and clinical translation of dendrimer-based prodrugs of GCs.
5. Antibody-drug conjugates
Antibody-drug conjugates (ADCs), consisting of a monoclonal antibody covalently connected to a cytotoxic payload via a chemical linker, were initially developed as an innovative strategy for targeted cancer therapy [101], [102], [103], [104]. Currently, more than ten ADCs have been approved by the FDA for treating various tumors, and at least 80 ADCs are currently in clinical development [105,106]. In addition to their application in the field of oncology, researchers have also been exploring the potential for targeted delivery of GCs to immune cells as a means of treating immune diseases [105,[107], [108], [109], [110], [111], [112], [113], [114]]. GCs-based ADCs also have the potential to be an effective means of enhancing efficacy and reducing or eliminating the adverse indications of GCs. This section summarizes recent developments in GCs-based ADCs (Table 3 and Fig. 7 [105,[107], [108], [109], [110], [111], [112], [113]]).
Table 3.
Representative antibody, peptide, carbohydrate-drug conjugates of GCs.
| Prodrug name | Carrier | Linker | Route of administration | Applications and Advantages | Ref. |
|---|---|---|---|---|---|
| Antibody-drug conjugates | |||||
| anti-CD163-Dex conjugate | Anti-CD163 | Esterase/ Hydrolysis-activated Succinate | Intravenous injection | To treat endotoxemia and nonalcoholic steatohepatitis; Precise targeting to macrophages; The in vivo potency of conjugated Dex was bout 50-fold that of nonconjugated Dex; Reduced systematic side effects. | [107,108] |
| α-hCD70 Antibody- Dex conjugate | Human CD70 antibody | Esterase/ Hydrolysis-activated Phosphate ester | At cellular level | Reduced the GCs-induced leucine zipper mRNA levels; Precise targeting to immune cells; The linker has strong plasma stability and lysosomal-responsive cleavage. | [109] |
| α-hCD70 Antibody- budesonide conjugate | Human CD70 antibody | Cathepsin B/Esterase-activated Phosphate based linkers | At cellular level | The linkers allow for payload attachment at an aliphatic alcohol; Precise targeting to immune cells. | [110] |
| anti-CD74- fluticasone propionate conjugates | Human CD74 antibody | Esterase-activated phosphate ester | At cellular level | Precise targeting of immune cells; Allows lower drug doses while achieving superior results. | [111] |
| anti-PRLR- budesonide conjugates | PRLR antibody | Cathepsin B- sensitive linkers | Intraperitoneal injection | To treat LPS-induced endotoxemia; Exhibited greater potency than free drug without safety liability. | [105] |
| Anti-α-TNF- GR modulators conjugates | Mouse α-TNF antibody | Protease-activated Maleimide-Gly-Ala-Ala | Intraperitoneal injection | To treat mouse contact hypersensitivity and chronic mouse arthritis; Enhanced therapeutic efficiency; Sufficient therapeutic window between efficacy and unwanted effects. | [112] |
| Peptide-drug conjugates | |||||
| Valine–valine-Dex (VVD) | Valine–Valine | Esterase/ Hydrolysis-activated Valine ester | —— | The prodrug could improve the encapsulation efficiency of hydrophilic drugs in nanoparticles by forming hydrophobic ion pairs with dextran sulphate. | [119] |
| 1-Dex-P | NapPhe-Phe-Lys-Tyr-OH | Esterase/ Hydrolysis-activated Succinate | Intraperitoneal injection | To treat hepatic fibrosis in mice; Exhibited a much stronger anti-hepatic fibrosis effect than Dex. | [120] |
| L-SS-Dex | Lys(Z)-NCA | Redox and pH-activated Disulfide bond | Intravenous injection | To treat colorectal cancer in mice; Increased the tumoral accumulation and superior antitumor activity than Dex. | [11] |
| Dex-GGD | Gly-Gly-Asp | Esterase/ Hydrolysis-activated Succinate | Intravitreal injection | To treat experimental autoimmune uveitis in rats; Superior therapeutic efficacy; Reduced the apparent ocular side-effects. | [121] |
| Dex-SA-FFFE | Phe-Phe-Phe-Glu | Esterase/ Hydrolysis-activated Succinate | Topical instillation | To treat endotoxin-induced uveitis in rabbits; Comparable in vivo therapeutic efficacy with Dex; Showed good ocular tolerance. | [122] |
| WYRGRLGE-Dex | WYRGRL | Esterase/ Hydrolysis-activated ester | At cellular level | Increased the binding and therapeutic efficacy inside cartilage compared to the free drug. | [124] |
| Dex-poly(L-aspartic acid) conjugate | Poly(L-aspartic acid) (30 kDa) |
Esterase/ Hydrolysis-activated ester | Intragastrically (Oral) | Enhanced colon targeting (Increased the drug in colon and reduced the drug in blood). | [126] |
| BUD-l-Arg | Arg | Esterase/ Hydrolysis-activated ester | Intravenous injection | To treat kidney injury and atherosclerosis in mice; Actively target the site of inflammation; Enhanced the therapeutic efficiency. | [127] |
| Carbohydrate-based prodrugs | |||||
| Dex-β-d-glucoside | β-d-glucoside | ß-glucosidase -activated Glycosidic bond | Intragastrically (Oral) | To treat colitis in guinea pigs and rats; Improved colon targeting of oral administration; Enhanced colitis treatment without adrenal atrophy. | [131], [132], [133] |
| Prednisolone-β-d-glucoside | β-d-glucoside | ß-glucosidase -activated Glycosidic bond | Intragastrically (Oral) | Improved colon targeting of oral administration of free drug. | [134] |
| Budesonide -β-d-glucoside | β-d-glucoside | ß-glucosidase -activated Glycosidic bond | Intragastrically (Oral) | To treat acetic acid-induced pancolitis; Accelerated colitis healing with limited adrenal suppression. | [135] |
| Prednisolone succinate/α-cyclodextrin | α-cyclodextrin | Esterase/ Hydrolysis-activated Succinate | Intragastrically (Oral) | To treat TNBS-induced colitis in rats; Delayed-release drug; Comparable the therapeutic effect; Reduced the systemic side effect. | [140] |
| Dex /Methylprednisolone- dextran | Dextran (72.6 kDa) |
Esterase/ Hydrolysis-activated Succinate and Glutarate | Intragastrically (Oral) | To treat acetic acid-induced colitis; Facilitated mucosal repair in rat colitis without adrenal suppression. | [136,143] |
| Dex-succinate-dextran (DSD) | Dextran (70 kDa) |
Esterase/ Hydrolysis-activated Succinate | Intragastrically (Oral) | The dextran prodrug was stable in rat stomach and small intestine and negligibly absorbed from these tracts; Can selectively deliver GCs to the colon. | [142] |
| Dextran–budesonide conjugates | Dextran (10, 70 and 500 kDa) |
Esterase/ Hydrolysis-activated Succinate | Intragastrically (Oral) | The influence of the molecular weight of orally administered macromolecular prodrugs on colon targeting was discussed. | [138] |
| Dex-sialic acid | Sialic acid | Acid-activated Hydrazone | Intravenous injection | To treat IR-induced AKI in mice; Actively targets the site of inflammation; Reduced the side effects. | [137] |
| Prednisolone–glucosamine conjugate | Glucosamine | Carbamate | Intravenous injection | To treat IR-induced AKI in mice; Actively targets the site of inflammation; Enhanced therapeutic effect and reduced side effects. | [48] |
| Heparin-Dex prodrug | Heparin | Acid-activated Hydrazone | At cellular level | Self-assemble into spherical polymeric micelles; Acid-sensitive release characteristics; Exhibited obvious synergistic anti-tumor effect. | [139] |
| Hyaluronic acid-Dex | Hyaluronic acid (12 kDa) | Esterase/ Acid-activated Ester | Intravenous injection | To treat glomerulonephritis; “Homing” to inflammatory renal tissue with 4.33-fold improvement; Superior therapeutic effect via collaborative two-pronged anti-inflammatory therapy. | [141] |
TNBS: Trinitrobenzenesulfonic acid.
Fig. 7.
Representative antibody-drug conjugates of GCs. (A) The chemical structure and mechanism of the anti-CD163- Dex conjugates. Reprinted with permission [107], copyright 2012, Elsevier. Reprinted with permission [108], copyright 2016, The authors. (B) Phosphate diester linked α-hCD70 antibody-GCs conjugates. Reprinted with permission [109], copyright 2016, American Chemical Society. (C) Cathepsin B-cleavable linker and self-immolation spacer linked antibody-budesonide conjugate. Reprinted with permission [110], copyright 2016, American Chemical Society. (D) Conjugation process for GCs-ADCs; representative structures of budesonide analogues. Reprinted with permission [105], copyright 2021, American Chemical Society. (E) The structure of anti-α-TNF-Ala-Ala-GCs and therapeutical effects on collagen-induced arthritis mice. Representative pharmacodynamic data of ADCs. Reprinted with permission [112], copyright 2022, American Chemical Society.
In 2012, Jonas H Gravesen and colleagues were the first to develop a biodegradable CD163 antibody-drug conjugate that delivered precisely the Dex to macrophages by specifically binding to the CD163 on rat macrophages [107]. As shown in Fig. 7A [107], each antibody could not only load four Dex molecules but also maintain a high affinity for CD163. The findings indicated that the therapeutic efficacy of ADCs was approximately 50-fold greater than that of unconjugated Dex. More importantly, ADCs did not produce significant side effects such as thymic and splenic lymphocyte apoptosis and weight loss in rats caused by the free Dex. Thereafter, these investigators also showed that the low-dose anti-CD163-Dex conjugates significantly suppressed severe nonalcoholic steatohepatitis [108]. It not only significantly attenuated inflammation, glycogen deposition and fibrosis but also had no significant systemic adverse effects. Macrophage-directed CD163 antibody-drug conjugates showed great potential as a future anti-inflammatory therapy. However, the ester-based linker used in this ADC disappeared 50% from the plasma within 20 min, possibly due to its rapid hydrolysis in the blood and, thus, its limited circulation stability. Therefore, it was imperative to develop innovative linkers for the targeted delivery of GCs that enable their stable adhesion to antibodies in circulation and efficient release of payloads after antibodies were internalized into antigen-positive cells. Kern et al. designed a phosphate ester-linked anti-human CD70 antibody-Dex conjugate (Fig. 7B) for targeted delivery of cargo to immune cells that specifically express CD70 [109]. In this study, the authors developed a variety of phosphate ester bond-related linkers and characterized them by in vitro lysosomal lysate stability as well as at the cellular mRNA level. These soluble phosphate ester linkers have been confirmed to possess desirable plasma stability and tunable drug release behavior in a lysosomal environment. In addition, the authors combined a phosphate linkage with a cathepsin B-responsive dipeptide to construct a novel linker (CatPhos linkers)-linked anti-human CD70 antibody-budesonide conjugate for immune cell targeting [110]. As shown in Fig. 7C [110], cathepsins first cleave the drug linkage to produce budesonide phosphate, which is then degraded by phosphatases to release free budesonide. Besides, these CatPhos linkers not only could solve the defect that carbamates cannot release drugs but also improve the poor stability of carbonate linkages in blood. Furthermore, this linker can connect various classes of drugs containing fatty alcohols, expanding the range of ADCs loading options. Similarly, Brandish et al. reported an anti-human CD74-fluticasone propionate conjugate that could achieve precise delivery of GCs to immune cells with the phosphate ester linkage adopted in this ADC [111]. Although phosphate ester-based linkers were associated with numerous advantages, the utility of such linkers and the potential to achieve targeted delivery of GCs need to be validated under in vivo conditions. Besides, the cathepsin B-responsive ADCs were developed by Han et al. (Fig. 7D) [105]. The authors first optimized the structure of budesonide and obtained the more potent lead compounds P3 and P12, which showed 100-fold selectivity for GR compared to other nuclear receptors and no safety concerns. The lead compounds were conjugated to the human PRLR antibody through a cathepsin B-responsive dipeptide bond, ensuring high stability in plasma and specific release of GCs in antigen-positive cells to potentially reduce systemic side effects. In a recent study, Hobson and team designed an innovative protease cleavable dipeptide (Ala-Ala) as a linker and then firmly linked the potent GCs together with the mouse α-TNF antibody to constitute a new type of ADCs [112]. As shown in Fig. 7E [112], the ADCs exhibited greater potency in murine models of contact hypersensitivity and chronic arthritis than their parent α-TNF antibodies (P < 0.05). Notably, analysis of biomarkers for procollagen type 1N-terminal propeptide and corticosterone indicated that the ADCs had a suitable therapeutic window.
In general, different linkers were used to construct GCs-based ADCs in this section, including carbonate bonds, phosphate bonds, carbamates, cathepsin-responsive linkers, and protease-responsive linkers. Among them, the carbonate linkage is less stable in the blood, which will cause premature release of the active drug, while the carbamate linkage is too stable, resulting in limited release of the active drug. In contrast, phosphate bonds have been reported to have better plasma stability and tunable drug release behavior in a lysosomal environment. Moreover, linkers with specific enzyme-responsive cleavage, such as cathepsin-responsive linkers and protease-responsive bonds, can selectively release active drugs and, therefore, have better targeting properties, helping to reduce the side effects of drugs in non-target organs. Additionally, although the therapeutic efficacy of some ADCs of GCs has been verified in animal models [105,107,108,112], there are still some ADCs that only stay at the cellular level [109], [110], [111], which needs to be further explored. In addition, some ADCs were administered intraperitoneally [105,112] rather than intravenously in vivo studies, and the effect of the administration route on drug efficacy also needs to be considered based on the following factors. (1) Drugs administered by intraperitoneal injection are usually gradually absorbed into the systemic circulation through the abdominal mesenteric vein, and the drug absorption rate is relatively slow. (2) The bioavailability of drugs injected intraperitoneally is usually affected by the molecular weight and logP of the ADCs, and is lower than that injected intravenously. (3) ADCs drugs currently approved by the FDA are administered through intravenous injection, while intraperitoneal injection is generally suitable for those drugs that treat peritoneal cancer and peritoneal infection. Hence, the selection of the route of administration in preclinical studies should be carefully considered in alignment with clinical applications. The route of administration significantly influences drug absorption, distribution, metabolism, and excretion (ADME). A route mimicking the clinical scenario will provide more accurate insights into these pharmacokinetic parameters. Besides, ensuring alignment between the route of administration in preclinical studies and the intended clinical applications is pivotal for the successful translation of research outcomes from bench to bedside. This alignment will enhance the predictive value of preclinical findings and facilitate a smoother transition to clinical trials.
6. Peptide-drug conjugates
Compared to ADCs, peptide-drug conjugates (PDCs) represent a burgeoning category of prodrugs, which are mainly composed of peptide chains, linkers and active drugs. PDCs possess the characteristics of low molecular weight, high tumor penetration, reduced immunogenicity and facile solid-phase synthesis, rendering them a subject of widespread interest in recent decades. PDCs are poised to emerge as the next generation of targeted anti-tumor therapeutics following small molecule targeted agents, monoclonal antibodies, and ADCs [115], [116], [117], [118]. Although antitumor drugs are used as the main object of research, some other drugs that are widely used in clinics have also received wide attention, such as GCs [119], [120], [121], [122], [123], [124], [125], [126]. This section mainly reviews the preclinical development of PDCs of GCs and representative PDCs, which are summarized in Table 3 and Fig. 8 [119], [120], [121], [122], [123], [124], [125], [126].
Fig. 8.
Representative peptide-based GCs prodrugs. (A) Chemical structure of valine-valine-Dex dipeptide prodrug. Reprinted with permission [119], copyright 2011, Taylor & Francis. (B) Molecular structure of the Dex-SA-FFFE. Reprinted with permission [122], copyright 2018, Dove Medical Press Limited. (C) Chemical structure of the cell-penetrating peptide-Dex conjugates. Reprinted with permission [125], copyright 2020, Elsevier. (D) Schematic representation diagram of l-SS-DEX for modulating the tumor microenvironment. Reprinted with permission [11], copyright 2020, Elsevier. (E) Diagram of 1-Dex-P self-assembled nanofibers for the treatment of liver fibrosis and therapeutical mechanism. Reprinted with permission [120], copyright 2018, American Chemical Society. (F) Synthesis of WYRGLRGE-Dex and the interaction between collagen type II. Reprinted with permission [124], copyright 2019, Elsevier. (G) Diagram of the preparation and targeted treatment for inflammatory vascular diseases of BUD-l-Arg@PSA. Reprinted with permission [127], copyright 2023, American Chemical Society.
Gaudana et al. designed a valine-valine-Dex dipeptide prodrug by reacting Dex with Boc-valine–valine-OH (Fig. 8A) and complexed it with dextran sulfate using hydrophobic ion-pairing technology [119]. Then the complex and PLGA (poly(lactic-co-glycolic acid)) were prepared into a sustained-release nanoparticle with a diameter of 130∼150 nm. This design significantly improved the encapsulation efficiency of Dex and achieved the expected sustained release effect in vitro [119]. In another study, Tang and his colleagues developed a hydrogelator precursor polypeptide-Dex prodrug (Nap-Phe-Phe-Lys(Dex)-Tyr(H2PO3)-OH, 1-Dex-P) through solid-phase peptide synthesis for liver fibrosis therapy [120]. As illustrated in Fig. 8E [120], the prodrug was initially dephosphorylated by alkaline phosphatase to form 1-Dex that can self-assemble into nanofibers after entering the body. At the same time, Dex was slowly released under the action of esterase. This tandem enzyme strategy can achieve stronger therapeutic effects than free drugs and has the potential to be widely used in the future. Besides, Ma et al. synthesized a redox- and pH-responsive poly-lysine-Dex conjugates (L-SS-Dex) for the treatment of mice colon cancer [11]. The conjugates can self-assemble into nanoparticles and have tumor microenvironment-responsive drug release behavior as illustrated in Fig. 8D [11]. The findings indicated that l-SS-Dex significantly enhanced the tumor accumulation of Dex compared with free Dex (P < 0.01), improving the therapeutic effect. The study also found that l-SS-Dex could inhibit tumors by modulating the immunosuppressive microenvironment, which provided a therapeutic option for cancer therapy by utilizing anti-inflammatory drugs.
In addition, Li's group synthesized several Dex-polypeptide amphiphilic prodrugs (Dex-D, Dex-GD, Dex-GGD, and Dex-GGGD) by a classical solid-phase synthesis method. Among them, Dex-GGD, capable of rapidly forming supramolecular hydrogels in PBS, was used to treat uveitis [121]. Dex-GGD effectively reduced inflammatory cell influx and inhibited retinal macroglia and microglia activation after intravitreal injection, thus alleviating uveitis. Additionally, it significantly mitigated adverse reactions (such as elevated intraocular pressure) in comparison to the free drug solution (P < 0.05). However, the authors did not investigate the in vitro drug release behavior and the existence form in the body. In addition to self-assembly hydrogels, Li's group also designed a Dex-polypeptide conjugate (Dex-SA-FFFE, Fig. 8B) by a classic solid-phase peptide synthesis method using 2-chlorotrityl chloride resin and N-Fmoc-protected amino acids [122]. It consists of Dex, phenylalanine, glutamic acid and succinic acid. Dex-SA-FFFE could self-assemble into nanoparticles with an average hydration radius of about 150 nm, and it had an esterase-responsive drug release behavior. After topical ocular instillation, the therapeutic effect for rabbit uveitis of Dex-SA-FFFE is equivalent to that of free Dex. Regarding the ability of some peptide-drug conjugates to self-assemble into supramolecular materials, Sis et al. [123] designed two Dex-peptide conjugates (Dex-VVVAAKK) linked by different linkers (ester and hydrazone) and studied the effect of linkers on supramolecular assembly. It has been observed that linkers exerted an influence on the assembly mechanism and energy barriers, the microscopic and macroscopic properties of the resulting supramolecular materials, which provides a reference for the study of supramolecular self-assembled drug-peptide conjugates.
Except for some non-functionalized peptide-modified GCs conjugates, peptides with biological functions have also been widely studied. For example, Dex was linked to the type II collagen-binding peptide (WYRGLRGE) via an ester bond to enhance drug retention in the joints of rheumatoid arthritis patients (Fig. 8F) [124]. The findings demonstrated that the conjugate exhibited a remarkable ability to transport Dex into the deep regions of cartilage by selectively interacting with cartilage-specific collagen type II, thereby augmenting its therapeutic efficacy. In another study, Bhattacharya et al. designed three cathepsin d-responsive cell-penetrating peptide-Dex conjugates (GRKKRRQRRPPQ, FNLPLPSRPLLR and AAVLLPVLLAAP, Fig. 8C) to treat retinal diseases by intravitreal injection [125]. The conjugates with good chemical stability in the vitreous could increase the internalization of retinal pigment epithelial cells for enhancing pharmacological effects. Compared with free drugs, the Dex conjugates modified with cell-penetrating peptides have demonstrated an increased drug residence in both the vitreous and aqueous humor, presenting a promising therapeutic strategy for posterior ocular diseases. It is worth mentioning that Dex was not released as a pure drug from the conjugate but rather as a drug-related fragment (Dex-arginine) in this study. The authors found that the binding site of this fragment is the same as that of Dex on the GR, so it can exert pharmacological effects similar to Dex. In a recent study, the minimalist prodrug of l-arginine and budesonide was synthesized through ester bonds (BUD-l-Arg@PSA) to regulate vascular endothelial dysfunction and inflammation (Fig. 8G) [127]. In this prodrug, l-arginine can not only serve as a substrate for nitric oxide synthase to promote the release of nitric oxide but also can modify the properties of budesonide to readily complex with polysialic acid to form nanoparticles. The therapeutic efficacy of this prodrug-based nanoplatform had been verified in the renal injury model and atherosclerosis model in mice, and the excellent results showed the promising potential of the prodrug.
In this section, a diverse array of amino acids or peptides are utilized to construct GCs-based PDCs, which include active amino acid (L-arginine [127]), targeting peptides (WYRGLRGE [124]), membrane-penetrating peptides (GRKKRRQRRPPQ, FNLPLPSRPLLR and AAVLLPVLLAAP [125]), and cathepsin-responsive peptides (KGKPILFFRLK [125]). These various peptides confer unique advantages to PDCs; some PDCs can be directly administered, while others serve as intermediates for preparations such as hydrogels and nanoparticles, broadening their applicability in treating a wide range of diseases. However, there are still some factors that need to be taken into consideration during the development of PDCs. Firstly, the size and structure of peptide molecules may restrict the loading capacity of PDCs. The larger peptides may provide more sites for drug reaction, potentially increasing drug loading capacity. Specific structural amino acids or sequences within peptides may provide more favorable sites for drug conjugation, influencing loading efficiency. Secondly, some specific amino acid sequence and structural characteristics can affect susceptibility to proteolytic degradation and clearance. As an illustration, natural l-amino acids are readily identified and cleaved by proteases in vivo. To mitigate protease degradation, a strategy involves substituting natural l-amino acids with d-amino acids, which are less susceptible to protease recognition. Alternatively, the altering sequence of amino acids within a polypeptide can also contribute to reducing vulnerability to protease-mediated degradation. Besides, lage-scale production of PDCs can be challenging due to the complexity of synthesis and potential issues related to reproducibility. Ongoing research focuses on streamlining synthetic procedures, optimizing reaction conditions, and employing efficient purification techniques to enhance scalability. Overall, despite these challenges, continuous research and optimization efforts hold the promise of overcoming limitations. This would enable the development of more stable, efficient, and cost-effective PDCs, thereby enhancing their therapeutic benefit.
7. Carbohydrate-based prodrugs
Carbohydrates, classified as monosaccharides, oligosaccharides and polysaccharides, are essential substances for natural life activities. Natural carbohydrates and their derivatives have been widely investigated for treating various diseases. In the last 20 years, as many as 54 carbohydrate-based drugs have been approved either as therapeutic or diagnostic agents [128], [129], [130]. This section reviews the application of carbohydrate-based GCs prodrugs over the past few decades (Table 3 and Fig. 9) [131], [132], [133], [134], [135], [136], [137], [138], [139], [140], [141], [142].
Fig. 9.
Representative carbohydrates-based GCs prodrugs. (A) The chemical structure of Dex/prednisolone/Budesonide-β-D-glucoside prodrug. (B) The prodrug structure of budesonide-dextran and the budesonide release in various conditions. Reprinted with permission [138], copyright 2009, Elsevier. (C) The structure of α-cyclodextrin-succinate-prednisolone prodrug and targeting mechanism after oral administration. Reprinted with permission [140], copyright 2002, Elsevier. (D) The synthesis and targeting mechanism of Dex-SA. Reprinted with permission [137], copyright 2022, Elsevier. (E) The preparation of amphiphilic heparin-Dex prodrug and micelles. Reprinted with permission [139], copyright 2013, Elsevier. (F) The synthesis of prodrug and diagram of the anti-inflammatory effect of MM/HA-Dex. Reprinted with permission [141], copyright 2022, Elsevier.
As early as the 1980s, some GCs-monosaccharide prodrugs have been synthesized specifically for treating inflammatory bowel disease in the colon (Fig. 9A) [131], [132], [133], [134], [135]. On the one hand, glycoside prodrugs are generally more hydrophilic than the parent drug, which can reduce drug absorption in the stomach and small intestine and increase the distribution of the prodrug in the colon [133]. On the other hand, the unique glycosidase activity of the colon microbiota helps to precisely cleave glycosidic bonds and release drugs at the target site [134]. Therefore, such glycosidic-linked carbohydrate-based prodrugs are often used for colon-targeted drug delivery. For example, Friend and colleagues showed that nearly 60% of prodrugs could reach the cecum, while only less than 1% of Dex reached the cecum after intragastric administration of the prodrug and Dex at an equivalent dose in healthy rats [134]. In another study, about 20%-30% of the prodrugs can reach the colon in guinea pigs with inflammatory bowel disease after oral administration [133]. These inconsistent results with previous research may be related to animal species and disease status. Based on this, Haeberlin and team have specifically evaluated the levels of enzymes in the gastrointestinal tract of rats in different states (such as conventional and colitis rats) and the hydrolysis of Dex-β-d-glucoside [132]. The results showed that the β-d-glucosidase activity in the intestinal tract of colitis rats was significantly lower than that of conventional rats (P < 0.05), which directly led to the reduced Dex release in the colon. However, there are significant activity gradients of β-d-glucosidase in the small intestine and large intestine of different types of rats, which provides favorable prerequisites for the targeted release of glycoside prodrugs in the colon. In addition, the efficacy of Dex-β-d-glucoside in vivo has also been studied in colitis rats [131]. The findings showed that the prodrugs exhibited a significant impact on promoting mucosal healing and stopping diarrhea compared to free Dex (P < 0.05), and could also enhance the absorption of sodium chloride. This was mainly because the prodrug can deliver higher concentrations of Dex to the colon. In subsequent studies, Yano et al. [140] designed an α-cyclodextrin (cyclic hexose)-succinate-prednisolone prodrug for the colon-targeted treatment of colitis based on a specific colon microenvironment. As shown in Fig. 9C [140], hydrophobic prednisolone was encapsulated in the cavity of cyclodextrin, which could reduce absorption in the upper gastrointestinal tract after intragastric administration. With the help of glucosidase produced by microorganisms, the structure of cyclodextrins was destroyed, and drugs were quickly released into the colon to exert their effects. The pharmacological research in the colitis mice also confirms that the prodrug could reduce toxicity by reducing systemic absorption of the drug. In addition to monosaccharides and oligosaccharides, polysaccharides were also used to synthesize GCs prodrugs for colon targeting [136,138,142,143]. Unlike Dex-β-d-glucoside prodrug, GCs-dextran prodrugs were mainly linked via succinate or glutarate rather than glycosidic bonds. The mechanism of colon-targeted drug release was mainly that macromolecular dextran (usually 10-500 kDa) could prevent drugs from the influence of enzymes in the upper gastrointestinal tract, while the drug was released under the action of dextranases and esterase in the colon rich in microorganisms [138,143]. Typically, the Dex released from Dex-succinate-dextran prodrugs in the colon and cecum was about 6 and 12-fold that of free Dex after oral administration [136,142]. Moreover, the release of drug in the cecum and colon was notably greater compared to that in the small intestine. More importantly, these prodrugs could better treat colitis without adrenal suppression [143]. Another valuable study examined the effects of different dextran molecular weights on the release of budesonide-succinate-dextran prodrug [138]. As shown in Fig. 9B [138], low molecular weight dextran prodrugs (10 kDa) could rapidly release drugs in both the small intestine and colon contents, indicating that low molecular dextran cannot effectively protect ester bonds from hydrolysis. While the high molecular weight dextran prodrugs (500 kDa) rarely release drugs, which was related to their very low solubility. However, prodrugs of medium molecular weight (70 kDa) were able to achieve both colon targeting and selective drug release. This study suggested that appropriate dextran molecular weight is critical for colon-targeted drug delivery of prodrugs.
In addition to oral administration for colon targeting as mentioned above, carbohydrate-based prodrugs of GCs have also been applied for intravenous drug delivery. For example, our group linked sialic acid, an acidic amino sugar-containing nine carbon atoms, with Dex via hydrazone linkage to obtain Dex-sialic acid prodrug (Dex-SA) [137]. This prodrug enables precise delivery of Dex to inflamed sites by specifically binding sialic acid to E-selectin receptors that are overexpressed in inflammatory vascular endothelial cells as shown in Fig. 9D. Pharmacodynamic studies in AKI mice demonstrated that this prodrug achieved an equivalent efficacy to that of free drug while significantly reducing side effects after intravenous administration. Notably, this prodrug did not show a more robust effect on the anti-inflammatory effect, mainly because the modification of sialic acid changed the hydrophilicity of Dex and thus was readily cleared from the body. Therefore, drugs that can prolong retention in the body and target inflammation are the goal of the next effort. In recent years, polysaccharides such as heparin sodium and hyaluronic acid have also been used to construct prodrugs for GCs. For example, to co-load two hydrophobic drugs, Dex and doxorubicin, into a nanoplatform for treating multiple myeloma, Li et al. combined water-soluble heparin polysaccharides with Dex through acid-sensitive hydrazone bonds to obtain an amphiphilic prodrug (Fig. 9E) [139]. The prodrugs could form into polymer micelles in an aqueous system, while they can load doxorubicin into the hydrophobic core. This strategy could efficiently deliver Dex and doxorubicin together, potentially treating multiple myeloma. In a similar study, Zhang and his colleagues prepared the self-assembled micelles of Dex prodrugs by connecting Dex to hyaluronic acid through ester bonds as shown in Fig. 9F [141]. Then, natural macrophage membranes were used to wrap them into biomimetic nanoparticles for the precise treatment of glomerulonephritis. After reaching the target organ, drugs could be released in weakly acidic environments through ester bond cleavage, and then hyaluronic acid and Dex could synergistically treat diseases by polarizing macrophages and inhibiting mesangial cell proliferation, respectively.
In this section, extensive research has been conducted on carbohydrate-based GC prodrugs, particularly focusing on glycosidic bond-linked prodrugs for oral drug delivery to achieve colon targeting. This approach leverages the responsiveness of the glycosidic bond to intestinal microbial enzymes. Additionally, some drugs are utilized for intravenous injection, taking advantage of the targeting ability of cell membrane receptors specific to certain sugar molecules. Water-soluble carbohydrates can enhance the hydrophilicity of the prodrug and thereby reduce the transmembrane ability of the drug (usually lipophilic small molecules are more likely to cross the membrane through passive diffusion), thus reducing the absorption of the drug in the small intestine and oral bioavailability. Therefore, these prodrugs are usually administered orally for the local treatment of intestinal diseases rather than systemic diseases. Besides, for intravenous injection, these hydrophilic prodrugs could accelerate the drug's elimination from the body. This occurs primarily because compounds in phase II metabolism undergo glucuronic acid coupling reactions, increasing their polarity and accelerating excretion. Carbohydrate compounds, especially small molecule monosaccharides, have similar structures to glucuronic acid, so prodrugs with higher hydrophilicity and polarity are easily excreted directly from the body [137,144]. Consequently, the development of such prodrugs should carefully consider these factors to optimize their pharmacokinetics and therapeutic efficacy.
8. Long-chain aliphatic acids or aliphatic alcohols-based prodrugs
Prodrugs based on long-chain fatty acids or fatty alcohols can improve the lipophilicity of the drug and help improve the drug loading capacity and sustained release effect in lipid-based drug delivery systems. On the one hand, lipophilic prodrugs have an improved solubility in lipid phases, facilitating their incorporation into lipid-based drug delivery systems. This increased solubility allows for higher drug loading in lipid carriers such as nanoparticles or liposomes. On the other hand, lipophilic prodrugs tend to partition preferentially into the lipid phase of the carrier, and this high-affinity property with lipid materials will exhibit slower release kinetics and, therefore, contribute to sustained release and prolonged therapeutic effects [145]. These prodrugs are usually made from aliphatic acids or aliphatic alcohols linked to the drug through different linkers, with aliphatic compounds comprising 16 carbons or 18 carbons being the most common. The representative long-chain aliphatic acid or aliphatic alcohol-based GCs prodrugs are summarized in Table 4 and Fig. 10.
Table 4.
Representative long-chain aliphatic acids or aliphatic alcohols-based GCs prodrugs.
| Prodrug name | Carrier | Linker | Route of administration | Applications and Advantages | Ref. |
|---|---|---|---|---|---|
| Aliphatic acids-based prodrugs | |||||
| Dex palmitate | Palmitic acid /Emulsion | Esterase/ Hydrolysis-activated Ester |
Intravenous injection | To treat VEGF-induced rabbit vascular leakage and laser-induced rat choroidal neovascularization; Last-longing effect for 9 months following a single injection; Good biosafety. | [149] |
| Dex palmitate | Palmitic acid /Nanocrystal | Esterase/ Hydrolysis-activated Ester |
Intravitreous injection | To treat age-related macular degeneration of rabbits; Last-longing effect for 1 month following a single injection. | [159] |
| Dex palmitate | Palmitic acid /Liposome | Esterase/ Hydrolysis-activated Ester |
Inhalation | To treat endotoxin-induced lung inflammation; Increased drug accumulation in lung; Enhanced therapeutic effects. | [151] |
| Dex palmitate | Palmitic acid /Liposome | Esterase/ Hydrolysis-activated Ester |
Intravenous injection | To treat AIA and S180-bearing in mice; Improved encapsulation efficiencies of Dex; Actively targets the disease sites; Enhanced therapeutic effects. | [12,145,152] |
| Dex palmitate | Palmitic acid /Nanoparticle | Esterase/ Hydrolysis-activated Ester |
Intravenous injection | To treat CIA in mice; Increased drug accumulation in arthritic joints; Enhanced therapeutic effects; Reduced the side effects. | [147] |
| Budesonide palmitate | Palmitic acid /Nanoparticle | Esterase/ Hydrolysis-activated Ester |
At cellular level; | To treat LPS-induced cell inflammation; Modification of mannitol increases cellular internalization of liposomes; Enhanced anti-inflammatory ability. | [156] |
| Aliphatic alcohols-based prodrugs | |||||
| Dex-ALA | α-linolenic acid/nanoparticles | Esterase/ Hydrolysis-activated Ester |
Intravenous injection | To treat 4T1 breast cancer in mice; High drug loading; Enhanced anti-tumor ability through combined therapy. | [161] |
| Dex-Linoleyl alcohol | Linoleyl alcohol/Lipid nanoparticle | Esterase/ Hydrolysis-activated Succinate |
Intravenous injection | As potent suppressors of the immunostimulatory effects of lipid nanoparticle formulations of nucleic acids; Enhanced the immunostimulatory effects compared with free drug. | [162] |
| AKP-Dex | Fatty alcohols/ Nanoparticles | Acid-activated Acetone-Based Ketal | Intravenous injection | To treat CIA in rats; Studied the relationships between AKP-dex structure and properties; Showed higher accumulation in inflamed joints and better therapeutic efficacy than free Dex with less-severe side effects. |
[163] |
| Stearoxyl-ketal-Dex | Stearyl alcohol/ Microcrystal | Acid-activated Acetone-Based Ketal | Intra-articular injection | To treat CIA in rats; Evaluated the influence of particle size and injection dose on anti-inflammatory effect after intra-articular injection; Exhibited long-acting anti-arthritis effects with good safety. | [166] |
VEGF: vascular endothelial growth factor;.
Fig. 10.
Representative long-chain aliphatic acids or aliphatic alcohols-based GCs prodrugs. (A) DXP-loaded nanoparticles improve pharmacokinetic properties. Reprinted with permission [154], copyright 2019, American Chemical Society. (B) Preparation and antitumor study of Dex-ALA/DTX nanoparticles. Reprinted with permission [161], copyright 2022, Elsevier. (C) Structures of lipophilic Dex prodrugs [162]. Reprinted with permission, copyright 2018, Elsevier. (D) Preparation and structural screening of AKP-Dex prodrug nanoparticles. a) AKP-Dexs were synthesized from Dex and isopropenyl ethers and were then co-assembled with DSPE−mPEG2000 by a nanoprecipitation method to form AKP-Dex-loaded NPs. (b) Promoiety structures and designations for the corresponding AKP-Dexs. Screening of promoieties for AKP-Dex-loaded NPs on the basis of size, colloidal stability, and acid sensitivity of the linker. Reprinted with permission [163], copyright 2020, American Chemical Society. (E) SKD nanocrystals were used to treat arthritis: (a) Preparation of SKD MCs: the larger MCs (3.1 µm) were formulated by anti-solvent crystallization method, and the smaller ones (1.1 µm) were further fabricated by wet grinding of larger MCs. (b) In a CIA rat model, MCs exhibited sustained release of native Dex, biocompatible stearyl alcohol and the metabolite acetone in the acidic inflammatory joints post IA injection. Attributing to the stronger sustained-release effect, the therapeutic effect of MCs with an average particle size of 3.1 µm was better than that of 1.1 µm. Reprinted with permission [166], copyright 2021, Elsevier.
Dex palmitate (DXP) stands out as one of the extensively researched GCs prodrugs. Limethason®, a lipid emulsion injection loaded with DXP, has been available in Japan since 1988 for treating arthritis [146], [147], [148]. Encouraged by this, DXP-related drug delivery systems have gained much attention from researchers, such as emulsions [149,150], liposomes [12,145,151,152], lipid nanoparticles [147,[153], [154], [155], [156]], and microparticles [157]. Representatively, Daull et al. prepared DXP emulsion and preliminarily evaluated its sustained-release effect after intravitreous injection [149]. The results showed that Dex maintained a higher level for prolonged periods in the rabbit retina and choroid after a single administration, which could inhibit effectively vascular leakage for 9 months, indicating that DXP emulsion has the potential to treat chronic eye diseases. Similarly, Mizushima et al. studied the anti-inflammatory activity of DXP emulsion using the preformed carrageenan granuloma pouch method [150]. The study showed that the anti-inflammatory activity of DXP lipid emulsion was 5.6 times that of the same amount of free Dex. DXP liposomes have also been studied for the treatment of pneumonia [151], arthritis [152] and tumors [12]. For example, the DXP liposomes were demonstrated by Hu et al. to accumulate to a higher extent in joints and have a stronger anti-inflammatory effect in inhibiting arthritis [152]. In addition, some DXP nanoparticles, such as lipid nanoparticles [153,158], polymer nanoparticles [159], and low-density lipoprotein nanoparticles [160], have also been used for anti-inflammatory, age-related macular degeneration and anti-tumor applications. For example, DXP nanoparticles were employed to treat age-related macular degeneration [159]. These nanoparticles exhibited sustained drug efficacy for up to 1 month following a single vitreous injection. Overall, in comparison to Dex-loaded nano-formulations, DXP exhibited not only significantly improved drug loading and encapsulation efficiency but also achieved a sustained-release effect, which proves beneficial for disease treatment.
In addition to palmitic acid, other aliphatic acids, such as linoleic and linolenic acid, have also been used to construct prodrugs for GCs. As illustrated in Fig. 10B [161], Zhang and his colleagues designed a Dex-α-linolenic acid prodrug (Dex-ALA) by direct reaction of the carboxyl group of ALA with the hydroxyl group at position C21 of Dex. The authors found that this prodrug could form into nanoparticles and had the behavior of esterase-responsive drug release. The prodrug could also be used as a carrier to load the cytotoxic drug to prepare an excipient-free nanodrug delivery system for synergistic tumor therapy. In the prepared Dex-ALA/DTX nanoparticles, Dex significantly augmented the tumor suppressive effect of Docetaxel (DTX). This enhancement could be attributed to two key factors. Firstly, Dex effectively increased the accumulation of nanoparticles at the tumor site by reducing interstitial pressure (data indicated a reduction from 20 mmHg to 10 mmHg). Secondly, Dex exerted inhibitory effects on inflammation-related tumor progression by suppressing the expression of NF-κB and TNF-α. This study not only provided a new carrier but also explored the way for the combined use of Dex with chemotherapeutic drugs.
There are still some studies linking fatty alcohols with GCs drugs through different linkers. For example, Chen et al. designed five Dex prodrugs containing the C18 hydrocarbon group (LD001∼LD005, Fig. 10C) through succinate bond and loaded them into lipid nanoparticles (LNPs) of nucleic acids for inhibition of immunostimulatory effects [162]. The authors finally selected LD003, with higher encapsulation efficiency and esterase-responsive Dex release, as the candidate drug for subsequent studies. In vivo studies demonstrated that cytokine production was significantly inhibited after intravenous administration of LNPs containing LD003, and LD003 at an equivalent dose of 0.5 mg/kg Dex was more potent than 20 mg/kg free Dex. Such prodrugs were also promised for other immune-based therapies, and their use at low doses would help avoid GCs-related side effects. In another study, Xu and his co-workers synthesized various Dex-fatty alcohol prodrug nanoparticles (AKP-Dex) to address the deficiencies of both premature drug release and low drug loading capacity of traditional physical entrapped nanoparticles [163]. Based on the fact that the pH value of arthritic synovial fluid is lower than the range of normal synovial fluid (6.6–7.2 vs 7.4–7.8) [164,165], the authors chose a pH-sensitive ketal bond as the linker, which would help the prodrug selectively release the active drug at the pathological site while reducing side effects in non-target organs. As shown in Fig. 10D [163], the authors finally selected two prodrug nanoparticles to treat arthritis by comparing the logP, particle size, as well as the release drug behavior of eight fatty alcohol prodrugs. The in vivo results demonstrated better joint targeting and therapeutic efficacy, and lower side effects of these pH-activated prodrug nanoparticles compared with free Dex solution (P < 0.05). This team also developed a pH-responsive stearoxyl ketal-Dex prodrug (SKD). Different from previous studies, the author prepared the prodrug into microcrystals and injected it into the joint cavity to treat rheumatoid arthritis (Fig. 10E) [166]. In this study, researchers focused on investigating microcrystalline size and dose for the efficacy of a single intra-articular injection. The therapeutic effect of microcrystals with an average particle size of 3.1 µm was better than that of 1.1 µm, mainly because smaller particles that release drugs faster were prone to be cleared from the body. The pH-activated prodrug microcrystals provided a new option for the development of long-acting injections.
In this section, the prodrugs of long-chain fatty acids and fatty alcohols were found to increase the hydrophobicity of the drug, resulting in a slow drug release effect. Unlike the above-mentioned hydrophilic polymer prodrugs, ADCs and PDCs, hydrophobic prodrugs typically possess low water solubility and are characterized by their tendency to form aggregates or precipitates in aqueous solutions. This results in rapid clearance by the reticuloendothelial system, limiting their circulation time and therapeutic efficacy. Additionally, aggregated hydrophobic prodrugs may cause local irritation or toxicity at the injection site. Hence, hydrophobic prodrugs typically necessitate the aid of various nanocarriers, including liposomes, nanoparticles, and micelles, to ameliorate the aforementioned limitations. By encapsulating hydrophobic prodrugs within nanocarriers, these delivery systems not only mitigate the challenges associated with direct injection but also offer opportunities for targeted and controlled drug delivery. Alternatively, such hydrophobic prodrugs can also be directly prepared into nanocrystals [159] or microcrystals [166] for local long-acting drug delivery, such as intravitreal injection and intra-articular injection. This type of prodrug shows significant potential for clinical application, as evidenced by the presence of lipid emulsion injection of DXP (Limethason®) already in the market.
In addition to the major classes of GCs prodrugs mentioned above, we summarize some other types of prodrugs in this section (Table 5 and Fig. 11, Fig. 11).
Table 5.
Representative other types of GCs prodrugs.
| Prodrug types | Prodrug name | Carrier | Linker | Route of administration | Applications and Advantages | Ref. |
|---|---|---|---|---|---|---|
| Sulfate sodium-based prodrugs | Prednisolone/ Dex/ methylprednisolone 21-sulfate sodium | Sulfate sodium | Sulfatase-activated Sulfate ester | Intragastric (Oral) | To treat TNBS-induced colitis in rats; Improve colon targeting; Enhanced the therapeutic effect of colitis. | [[41], [42], [43], [44],167] |
| L-Carnitine based prodrugs | Prednisolone- l-carnitine | L-Carnitine | Esterase/ Hydrolysis-activated Ester |
Intra-tracheal instillation | To treat chicken OVA-induced asthma in guinea pig; Actively targets the lung; Enhanced thera-peutic effects. | [168,169] |
| Succinic acid-based prodrugs | Methylprednisolone- succinate | Succinic acid /Liposome | Esterase/ Hydrolysis-activated Ester |
Intravenous injection | To treat AIA in rats; Improved encapsulation efficiency of drug in liposome; Sustained release effect; Potent therapeutic effects. | [170] |
| Dex-succinate | Succinic acid /Hydrogel | Esterase/ Hydrolysis-activated Ester |
Topical ocular instillation | Improved the precorneal retention time and the intraocular bioavailability; Comparable anti-inflammatory effects and good biosafety. | [171,172] | |
| Nitrophenyl-based prodrugs | Prednisolone/ budesonide- O-nitrophenyl acetic acid/ propionate | Aromatic nitro compounds | Nitro reductase-activated linker | Intragastric (Oral) | Used for the colonic delivery of oral drugs; Nitroreductase-responsive drug release in the colonic microbiota. | [174] |
| Dex- 5-aminosalicylic acid | 5-aminosalicylic acid | Azoreductase-activated Azo group | Intragastric (Oral) | To treat DSS-induced colitis; Comparable therapeutic efficacy and reduced adverse reactions. | [175] | |
| GCs dimer prodrugs | GCs dimer prodrugs | Dex, prednisolone, triamcinolone acetonide and hydrocortisone | Esterase/ Hydrolysis-activated Triethylene gycol (TEG) |
Intravitreal injection | To treat LPS-induced uveitis and VEGF-induced blood-retinal barrier breakdown; Carrier-free dimeric prodrug implant; Long-lasting effect; Without safety concerns. | [20] |
| Dex- MMF | Dex- MMF | MMF | Esterase/ Hydrolysis-activated Ester |
At cellular level | To treat LPS-induced cell inflammation; Exert synergistic therapeutic effects; Improved the cell membrane penetration of the parent drugs. | [176] |
| Carbon quantum dot-Dex | CD-Dex | Carbon quantum dots | Esterase/ Hydrolysis-activated Ester |
Intravenous injection | To treat CCl4-induced liver fibrosis; Small-sized CD-Dex can traverse the hepatic sinusoidal barrier; Enhanced the synergistic therapeutic effects. | [177] |
OVA: ovalbumin; DSS: Dextran sodium sulfate.
Fig. 11.
Representative Sulfate sodium/L-Carnitine/Succinic acid/Nitrophenyl-based GCs prodrugs. (A) Reaction scheme of sulfate sodium-based GCs prodrugs. Reprinted with permission [41], copyright 2011, Oxford University Press. (B) The synthesis of PDC and PDSC and mean lung prednisolone concentration-time curves of PRED, PDC, and PDSC solutions. Reprinted with permission [168], copyright 2011, American Chemical Society. Reprinted with permission [169], copyright 2014, Elsevier. (C) Preparation, precorneal retention and in vitro drug release of Dex-SA hydrogel. Reprinted with permission [171], copyright 2018, Elsevier. (D) Dex-SA can form supermolecule hydrogels with various cations. Reprinted with permission [172], copyright 2018, Elsevier. (E) The chemical structure of the nitro-substituted 21-ester for the GCs. Reprinted with permission [174], copyright 2013, Elsevier. (F) Cyclization-activated steroid prodrugs for the colon. Reprinted with permission [175], copyright 2009, American Chemical Society.
9. Sulfate sodium-based prodrugs
Kim's team synthesized oral prednisolone-sodium sulfate prodrugs to treat inflammatory bowel disease [44]. Herein, the authors expected that the water-soluble prodrugs can inhibit the drug's absorption in the upper gastrointestinal tract and specifically release drugs in the colon by sulfatase hydrolysis. The findings indicated that although the prodrug could selectively release prednisolone in the cecal content, the prednisolone in the content decreases rapidly, which may affect the final efficacy. The authors believed that the instability of prednisolone was mainly related to the nature of the drug itself. Therefore, the stability of commonly used GCs in buffer containing cecal content was investigated in the subsequent study (the structures were shown in Fig. 11A) [41], [42], [43]. Studies have found that Dex had a stable inhibitory effect on the biological inactivation of cecal content compared to prednisolone, hydrocortisone, and cortisone, which was an ideal candidate for developing colon-specific prodrugs. Then Ho Kim et al. synthesized Dex-sodium sulfate prodrugs and verified their stability in different intestinal segments of healthy rats. The results suggested that this prodrug could achieve colon-specific drug delivery [43]. In addition to screening for GCs that are stable in the cecal content in developing promising GCs-sulfate prodrug candidates, the addition of reduction inhibitors can also significantly inhibit the loss of GCs that are susceptible to metabolism in the cecal content. Taking methylprednisolone-sodium sulfate prodrug as an example, glycyrrhizin, a reduction inhibitor, not only increased the accumulation of methylprednisolone released from the prodrugs in the cecal content but also improved the therapeutic effect on colitis mice [41,167]. In summary, GCs-sodium sulfate prodrugs can be a promising colon-targeted delivery strategy after the metabolic susceptibility of GCs is overcome.
10. L-carnitine-based prodrugs
L-carnitine (β-Hydroxyl-γ-Trimethylaminobutyric acid) is a zwitterionic molecule that enters cells mainly via the organic cation/carnitine transporter (OCTN). In this section, l-carnitine was modified on the prednisolone molecule to improve the targeting of the drug via OCTN [168]. As shown in Fig. 11B [168], the authors synthesized two prednisolone (PRED)-l-carnitine prodrugs (PDC and PDSC) and compared their differential uptake in bronchial epithelial cells. The results indicated that PDSCs were more readily taken up by cells that highly express OCTN through a carrier-mediated pathway. Further cellular experiments showed that PDSCs exhibited superior ability in suppressing the production of pro-inflammatory cytokines. Subsequently, the potential of PDSCs for treating asthma was validated in another study [169]. They examined the pharmacokinetic behaviors of the three drugs (PRED, PDC and PDSC), and it can be seen from Fig. 11B that PDSCs significantly increased their retention time and accumulation amount in the lungs compared to PDC and PRED. It further exhibited the optimal therapeutic effect with a 3.8-fold reduction in infiltrating inflammatory cells, alleviating the lesion extent in the lungs and bronchi of asthmatic guinea pigs. Taken together, this OCTN-mediated prednisolone-l-carnitine prodrug is a promising way for pulmonary drug delivery. Notably, the stability of this class of prodrugs before entering targeted cells after administration is a matter of concern.
11. Succinic acid-based prodrugs
Unlike succinic acid as a linker in the previously mentioned some prodrugs, it was directly linked to the drug to form the GCs-succinate prodrugs in this section. To address the shortcomings of traditional GCs liposomes, such as inadequate drug loading, low encapsulation efficiency, and uncontrolled release, Avnir et al. synthesized an amphiphilic weak acid GCs prodrug (methylprednisolone-succinate prodrug) and actively loaded it into liposomes through the calcium acetate gradient method [170]. The prepared liposomes not only exhibited an improved encapsulation efficiency of 94% and drug lipid ratio of 0.41, but also had an excellent sustained release effect. In addition, pharmacodynamic trials in arthritis rats have also shown potent therapeutic effects in the early stages and even at the peak of the disease. Similarly, the Dex-succinate prodrugs (Dex-SA) were synthesized by Li et al. [171,172]. Unlike loading it into liposomes, the authors found that it can self-assemble into the supramolecular hydrogel to achieve self-delivery of ophthalmic drugs as shown in Fig. 11C [171]. This pH-dependent prodrug hydrogel could provide sustained drug release for 5 d Moreover, it significantly improved the precorneal retention time and the intraocular bioavailability after topical instillation compared with Dex solution. It is noteworthy that the normal physiological environmental pH of the human body is around 7.0, which may be lower in disease states [27,173]. However, the Dex-SA hydrogel released less Dex at pH 7.0 than that at pH 8.0, only about 10 % was released within 96 h. This limited release of active drugs may directly affect the final therapeutic effect on inflammation, thereby hindering its clinical application. Therefore, this characteristic of the hydrogel might be contradictory to practical application. In addition, researchers have proposed a new supramolecular hydrogel of Dex-SA prodrugs via metal ions (e.g., Mg2+, Ca2+, Zn2+ and Fe2+) coordination strategy (Fig. 11D) [172]. Among them, the Ca2+ coordinated Dex-SA hydrogel exhibited excellent mechanical properties. It was not only capable of slowly releasing drugs but also could finely regulate the release behavior of drugs by changing the Ca2+ concentration. Importantly, this prodrug hydrogel not only had good biosafety but also showed comparable anti-inflammatory effects with Dex solution in vitro, suggesting that it was a potential anti-inflammatory drug delivery platform. Overall, the synthesis process of Dex-SA is straightforward, and its structure is well-defined. Whether chelated with metal cations to form a hydrogel or loaded into liposomes, it demonstrates a potent anti-inflammatory effect. As a result, Dex-SA exhibits significant potential for clinical applications.
12. Nitrophenyl/Nitroazo-based prodrugs
Nitrophenyl-based prodrugs were mainly used for the colonic delivery of oral drugs. Its design principle was mainly that the prodrugs could be able to reduce drug's absorption in the intestine, while the intermediate-containing amino with cyclization activity was released under the action of nitroreductase of the colonic microbiota, which then underwent intramolecular amidation to release the active drugs. Gilmer et al. synthesized prodrugs of prednisolone and budesonide with o-nitrophenylacetic acid or o-nitrophenylpropionic acid as donors of nitrobenzene (as shown in Fig. 11E) [174]. The authors evaluated the release profile and intestinal permeation properties of the prodrugs through Clostridium perfringens and Caco-2 monolayers, respectively. Besides, the stability of each prodrug in artificial gastric and intestinal fluids was also examined. The findings demonstrated that the utilization of nitroreductase-induced cyclization and drug release was a highly effective strategy for the targeted delivery of drugs to colorectal tissues. In another study, different from the reduction triggered drug release of nitrobenzene, the authors extended the use of reduction of azo groups as carriers for drug release [175]. In this prodrug, prednisolone was attached via an ester linkage to an arylazo carrier (Fig. 11F). The azo groups can be selectively reduced to amino groups by azoreductases in the colon and subsequently undergo lactamization to release the drug. Besides, 5-aminosalicylic acid coupled with the other side of the azo group was also able to be used for the treatment of colitis, contributing to enhanced therapeutic efficacy. In vivo pharmacodynamic results of murine colitis showed that prodrug 5b exhibited comparable therapeutic efficacy to prednisolone but didn't induce thymic atrophy. This illustrated that the prodrug could decrease drug's absorption in the small intestine, reducing the drug's adverse reactions. However, where the study is still underpowered is the fact that this drug was a dual prodrug that lacked control for 5-aminosalicylic acid in the pharmacodynamic study. Overall, nitrophenyl or nitroazo-based GCs prodrugs have the potential to treat inflammatory bowel diseases while reducing side effects. However, there are still some aspects that deserve attention in future design, such as the safety of the byproducts in the metabolism process of these prodrugs and the stability of the active drugs themselves in the gut.
13. GCs dimer prodrugs
To address the issue of restricted drug loading in polymer-based delivery systems, Battiston et al. developed a carrier-free GCs dimeric prodrug and prepared it into the implants without any excipients [20]. In this study, the Dex dimer prodrug (Dex-TEG-Dex) was synthesized using two Dex molecules linked by a triethylene glycol (TEG) linker. Due to the smart design, the degradation products (Dex, TEG, CO2) of prodrugs would not cause any safety concerns. As shown in Fig. 12A, these dimer-based implants achieved the sustained release of the drug through surface erosion and linker hydrolysis. Based on this, the desired release profiles can be obtained by adjusting the surface area. Additionally, this study investigated the feasibility of various GCs (prednisolone, triamcinolone acetonide, and hydrocortisone) dimer implants and the effect of hydrophobic linkers (hexanediol) on drug release. Importantly, Dex-TEG-Dex dimeric prodrug implants were effective in suppressing lipopolysaccharide-induced inflammatory responses. Furthermore, it exhibited predictable pharmacokinetics and significantly prolonged efficacy (greater than 6 months) in a rabbit model of blood-retinal barrier disruption. Overall, this study has significant implications for drug delivery, providing a strategy for the field to be able to precisely regulate drug release for the treatment of multiple diseases.
Fig. 12.
Representative other types of GCs prodrugs. (A) Schematic structure of the dimer prodrug and release mechanism of drug from implants. Reprinted with permission [20], copyright (2021), Springer Nature. (B) Representative pharmacodynamic results and diagram of the synergistic mechanism of Dex-MMF prodrug. Reprinted with permission [176], copyright (2021), American Chemical Society. (C) Diagram of the esterase-responsive CD-Dex prodrugs for the treatment of liver fibrosis. Reprinted with permission [177], copyright (2021), Wiley-VCH GmbH.
14. Dex-monomethyl fumarate prodrug
Dual prodrugs that can exert synergistic therapeutic effects are a novel strategy to reduce the adverse reactions caused by high-dose use of GCs through lowering the dosage. In a recent study, the authors evaluated the synergistic inhibition of macrophage inflammation by a combination of multiple anti-inflammatory agents (including Dex, curcumin, monomethyl fumarate (MMF) etc.). Two drugs with the optimal synergistic effect (Dex with MMF) were linked together by an esterification reaction to form the Dex-MMF dual prodrugs [176]. Surprisingly, the prodrugs exhibited potent anti-inflammatory effects in both mouse and human macrophages that outperformed the unconjugated drug mixture (Fig. 12B). This was mainly because the prodrugs improved the cell membrane penetration of the parent drugs, especially MMF. Despite satisfactory results at the cellular level, whether these ester-linked prodrugs can pass the test of esterase in plasma and then enter target cells is the key to success.
15. Carbon quantum dot-Dex prodrug
Recently, Xu et al. constructed a novel GCs prodrug (CD-Dex) by grafting Dex on carbon quantum dots (CDs) via ester bonds [177]. In this study, it was anticipated that small-sized CDs (23 nm) possessing reactive oxygen species (ROS) scavenging properties would be capable of traversing the hepatic sinusoidal barrier, homing in on hepatocytes and scavenging excessive ROS within the pathological microenvironment. As shown in Fig. 12C [177], this esterase-responsive CD-Dex could treat liver fibrosis by scavenging ROS and inhibiting inflammation after targeting pathological microstructures. In vivo anti-liver fibrosis experiments showed that CD-Dex could significantly attenuate liver injury and collagen deposition, thereby preventing the progression of liver fibrosis. Despite the reasonable therapeutic results, whether the ester bond-linked Dex in prodrugs would release prematurely in the blood circulation was ignored by the authors.
16. Clinical applications of GCs-based prodrugs
This section summarizes marketed products and ongoing clinical trials involving GCs-based prodrugs. The overview of these products, including prodrug categories, dosage forms, manufacturers, target diseases and other information, is shown in Table 6, Table 7. We found that many prodrugs in clinic improve the water solubility of GCs by grafting polar groups such as sodium phosphate, hydrogen succinate or sodium succinate, including hydrocortisone hydrogen succinate, prednisolone sodium succinate, prednisolone sodium phosphate, Dex sodium phosphate and betamethasone sodium phosphate. All these esters are hydrolyzed in vivo to yield the active GCs, and they can facilitate oral absorption or preparation of liquid formulations, such as oral solutions, eye drops, or injectable solutions. Moreover, these prodrugs usually take effect quickly and are suitable for first aid use. In contrast, GC prodrugs enhance the long-acting effects of GCs by adding long carbon side chains (such as dipropionate, pyruvate, or valerate moieties) to the GCs structure and can be administered through the skin, inhalation, or nasal cavity. This simple modification has also resulted in a number of commercial products, such as beclomethasone dipropionate and mometasone furoate. The increased lipophilicity ensures high local retention in mucosa, tissue or skin, thereby reducing systemic exposure and potential side effects. Besides, Dex palmitate emulsion injection is also a marketed product for the treatment of rheumatoid arthritis. It has a good sustained-release effect and is usually administered once every 2 weeks in clinical practice. Additionally, it is exciting that there are also ADCs drugs based on GCs in clinical trials, and their success will bring hope to patients with rheumatoid arthritis and B-cell malignant tumors. It is worth mentioning that these prodrugs in clinic are mainly small molecule prodrugs, while some polymer prodrugs and prodrug-based nanomedicine delivery are very limited, which may be related to their complex production processes and safety, etc. In the following section, we will discuss its opportunities and challenges in more detail.
Table 6.
Representative marketed products of GCs-based prodrugs.
| Proprietary Name | Active ingredients | Administration route | Manufacturer | Country |
|---|---|---|---|---|
| Solu-Cortef | Hydrocortisone sodium succinate | Intravenous | PHARMACIA AND UPJOHN | America |
| Oralsone Adultos | Hydrocortisone hydrogen succinate | Buccal | Laboratorios Viñas, S.A. | Spain |
| Predasol | Prednisolone sodium succinate | Intraarticular | Sun-Farm Sp. Z.O.O. | Netherlands |
| Prednisolon Unimedic | Prednisolone sodium phosphate | Ocular | Unimedic Pharma Ab | Denmark |
| Aacidexam | Dex sodium phosphate | Intraarticular | Aspen Pharma Trading Limited | Belgium |
| Dexmathasone sodium phosphate | Dex sodium phosphate | Intravenous | Gland Pharma Ltd | China |
| Dexa-Ophtal | Dex sodium phosphate | Ocular | Dr. Gerhard Mann Chem.-Pharm. Fabrik Gmbh | Germany |
| Decadron | Dex sodium phosphate | Oral | I.B.N. Savio S.R.L. | Italy |
| Albaflo | Betamethasone sodium phosphate | Intramuscular | Esseti Farmaceutici S.R.L | Italy |
| Betnesol | Betamethasone sodium phosphate | Oral | Alfasigma S.P.A | France |
| Qvar Redihaler | Beclomethasone dipropionate | Inhalation | Norton Waterford | America |
| Ryaltris | Mometasone furoate | Nasal | GLENMARK SPECIALTY | America |
| Limethason | Dex palmitate | Intravenous | Mitsubishi Tanabe Pharma Korea Co., Ltd. | Korea |
Table 7.
Representative products of GCs-based prodrugs in clinical trials.
| Registered number | Drug name | Adaptation disease | Stage | Country |
|---|---|---|---|---|
| EUCTR2017–003,590–33-DK | Prednisolone sodium phosphate | Asthma | Phase Ⅳ | Denmark |
| EUCTR2012–005,123–32-GB | Prednisolone sodium phosphate | Otitis media with effusion | Phase Ⅲ | United Kingdom |
| EUCTR2010–020,448–37-BE | Prednisolone sodium phosphate | Colitis | Phase Ⅱ | Belgium; Netherlands |
| CTR20131261 | Dex palmitate | Rheumatoid arthritis | PhaseⅡ | China |
| NCT05571046 | Betamethasone sodium phosphate | Radiculopathy lumbar | Phase Ⅳ | Turkey |
| NCT04734210 | Betamethasone sodium phosphate | Dry eye disease | Phase Ⅱ | United States |
| NCT04544683 | Dex sodium phosphate | Cervical radiculopathy; Cervical spondylosis; Disk, Herniated | Phase Ⅳ | United States |
| NCT04432012 | Dex sodium phosphate | Total knee replacement | Phase Ⅳ | Switzerland |
| CTRI/2020/04/024,470 | Dex sodium phosphate | Oral submucous fibrosis | PhaseⅡ /Phase Ⅲ |
India |
| NCT03823391 | ABBV-3373 (ADCs) | Rheumatoid arthritis | Phase Ⅱ | United States |
| NCT05512390 | ABBV-319 (ADCs) | B cell malignant tumor | Phase Ⅰ | United States |
17. Opportunities and challenges
In this review, we summarized the advances of various GCs-based prodrugs in recent years, including polymers, carbohydrates, dendrimers, antibodies, polypeptides as well as some other small molecule-based prodrugs. Although these prodrugs are widely studied, the current drugs that successfully enter the clinical stage are still limited. To further promote the clinical transformation of GCs-based prodrugs, several issues and challenges still need to be addressed.
17.1. Pharmacokinetic challenges
Firstly, achieving consistent and predictable bioavailability is often challenging due to factors such as variable absorption rates, metabolism, and systemic clearance. Secondly, prodrugs relying on enzymatic activation or the microenvironment of disease may face variability in enzyme levels among individuals. Thirdly, achieving targeted drug delivery to specific tissues while minimizing systemic exposure is also a huge challenge. In response to the above challenges, we can establish in vivo and in vitro drug release correlation models to predict their distribution and metabolism in the body. We can also design prodrugs that can adapt to individual differences based on the patient's genotype and phenotype, improving the level of individualization of treatment. In addition, various nano- or micro-drug delivery strategies can also be used to improve the druggability of prodrugs.
17.2. Safety and toxicity considerations
Prodrugs or metabolites may be considered foreign bodies, causing immune responses, including allergic reactions or immune system activation. It may also affect immune system function in the long term. Therefore, more biocompatible materials should be selected to reduce activation of the immune system through surface modification. Besides, linkers and functional groups in prodrugs may cause safety concerns. Whether it is a polymer, antibody, amino acid, carbohydrate or other carrier, its safety is paramount. The functional groups and linkers should be able to be metabolized out of the body, and their metabolites and metabolic pathways should also be clear.
17.3. Regulatory landscape
The design concept of prodrugs is usually to improve the physicochemical and pharmacokinetic properties of active pharmaceutical ingredients to address the problems currently encountered in clinical practice, such as poor patient compliance and severe side effects. Therefore, the development of prodrugs should be driven by clinical needs, which is in line with the guidelines of regulatory agencies such as the FDA and the European Medicines Agency (EMA). Generally speaking, prodrug design makes it easier to discover new drugs with suitable ADME (absorption, distribution, metabolism, and excretion) properties than to search for completely new chemical entities. In some cases, prodrugs can not only be more effective than parent drugs, but their entire development cycle is also much shorter than traditional new chemical entities because the main ingredients have been proven to be safe and effective. From the FDA's point of view, some prodrug applications can be reviewed via the 505(b)(2) pathway. In this case, adequate pharmacokinetic studies are necessary in early development stage, and then what kind of pharmacodynamic and safety studies will be determined based on pharmacokinetic results. In clinical research, pharmacokinetic study is more important because it can effectively bridge with the parent drug and reduce unnecessary clinical research. In addition, we'd better to pay close attention to the regulatory policies, actively communicate with regulatory agencies to understand the approval process and reasonably plan time and expenses, which will facilitate the successful development of GCs prodrugs.
18. Conclusions
In summary, GCs-based prodrugs are a promising strategy to address the clinical problems of GCs by improving the absorption, distribution, metabolism or excretion of GCs. In this review, we proposed the strategies for the design and summarized the current research progress of GCs-based prodrugs in recent decades. Besides, we raise opportunities and challenges that need to be focused on during the development of GCs-based prodrugs. Although the development of prodrugs faces many challenges, we strongly believe that there will be more prodrugs from bench to bedside under the constant efforts of scientists.
Conflicts of interest
There are no conflicts to declare.
Acknowledgments
This work was supported by the National Natural Science Foundation of China [82172086], National Key R&D Program of China [2020YFE0201700], Shenyang Science and Technology Talent Support Program [RC210447], Career Development Program for Young and Middle-aged Teachers of Shenyang Pharmaceutical University [ZQN2019004] and “Dual Service” Program of University in Shenyang.
References
- 1.Rodríguez Villanueva J., Rodríguez Villanueva L., Guzmán Navarro M. Pharmaceutical technology can turn a traditional drug, dexamethasone into a first-line ocular medicine. A global perspective and future trends. Int J Pharm. 2017;516(1–2):342–351. doi: 10.1016/j.ijpharm.2016.11.053. [DOI] [PubMed] [Google Scholar]
- 2.Fung A.T., Tran T., Lim L.L., Samarawickrama C., Arnold J., Gillies M., et al. Local delivery of corticosteroids in clinical ophthalmology: a review. Clin Experiment Ophthalmol. 2020;48(3):366–401. doi: 10.1111/ceo.13702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meduri G.U., Annane D., Confalonieri M., Chrousos G.P., Rochwerg B., Busby A., et al. Pharmacological principles guiding prolonged glucocorticoid treatment in ARDS. Intensive Care Med. 2020;46(12):2284–2296. doi: 10.1007/s00134-020-06289-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hardy R.S., Raza K., Cooper M.S. Therapeutic glucocorticoids: mechanisms of actions in rheumatic diseases. Nat Rev Rheumatol. 2020;16(3):133–144. doi: 10.1038/s41584-020-0371-y. [DOI] [PubMed] [Google Scholar]
- 5.Talebian S., Wallace G.G., Schroeder A., Stellacci F., Conde J. Nanotechnology-based disinfectants and sensors for SARS-CoV-2. Nat Nanotechnol. 2020;15(8):618–621. doi: 10.1038/s41565-020-0751-0. [DOI] [PubMed] [Google Scholar]
- 6.Águas R., Mahdi A., Shretta R., Horby P., Landray M., White L., et al. Potential health and economic impacts of dexamethasone treatment for patients with COVID-19. Nat Commun. 2021;12(1):915. doi: 10.1038/s41467-021-21134-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ledford H. Steroid is first drug shown to prevent deaths from COVID-19. Nature. 2020;582(7813):469. doi: 10.1038/d41586-020-01824-5. [DOI] [PubMed] [Google Scholar]
- 8.Martin J.D., Panagi M., Wang C., Khan T.T., Martin M.R., Voutouri C., et al. Dexamethasone increases cisplatin-loaded nanocarrier delivery and efficacy in metastatic breast cancer by normalizing the tumor microenvironment. ACS Nano. 2019;13(6):6396–6408. doi: 10.1021/acsnano.8b07865. [DOI] [PubMed] [Google Scholar]
- 9.Zhang L., Su H., Liu Y., Pang N., Li J., Qi X.-R. Enhancing solid tumor therapy with sequential delivery of dexamethasone and docetaxel engineered in a single carrier to overcome stromal resistance to drug delivery. J Controll Release. 2019;294:1–16. doi: 10.1016/j.jconrel.2018.12.004. [DOI] [PubMed] [Google Scholar]
- 10.Popilski H., Abtew E., Schwendeman S., Domb A., Stepensky D. Efficacy of paclitaxel/dexamethasone intra-tumoral delivery in treating orthotopic mouse breast cancer. J Controll Release. 2018;279:1–7. doi: 10.1016/j.jconrel.2018.04.010. [DOI] [PubMed] [Google Scholar]
- 11.Ma S., Song W.T., Xu Y.D., Si X.H., Zhang D.W., Lv S.X., et al. Neutralizing tumor-promoting inflammation with polypeptide-dexamethasone conjugate for microenvironment modulation and colorectal cancer therapy. Biomaterials. 2020;232 doi: 10.1016/j.biomaterials.2019.119676. [DOI] [PubMed] [Google Scholar]
- 12.Sun J., Song Y.Z., Lu M., Lin X.Y., Liu Y., Zhou S.L., et al. Evaluation of the antitumor effect of dexamethasone palmitate and doxorubicin co-loaded liposomes modified with a sialic acid-octadecylamine conjugate. Eur J Pharmaceut Sci. 2016;93:177–183. doi: 10.1016/j.ejps.2016.08.029. [DOI] [PubMed] [Google Scholar]
- 13.Chen M.C., Tan Y.J., Dong Z.L., Lu J.Q., Han X., Jin Q.T., et al. Injectable anti-inflammatory nanofiber hydrogel to achieve systemic immunotherapy post local administration. Nano Lett. 2020;20(9):6763–6773. doi: 10.1021/acs.nanolett.0c02684. [DOI] [PubMed] [Google Scholar]
- 14.Mi D., Li J., Wang R., Li Y., Zou L., Sun C., et al. Postsurgical wound management and prevention of triple-negative breast cancer recurrence with a pryoptosis-inducing, photopolymerizable hydrogel. J Controll Release. 2023;356:205–218. doi: 10.1016/j.jconrel.2023.02.042. [DOI] [PubMed] [Google Scholar]
- 15.Pofi R., Caratti G., Ray D.W., Tomlinson J.W. Treating the side effects of exogenous glucocorticoids: can we separate the good from the bad? Endocr Rev. 2023;44(6):975–1011. doi: 10.1210/endrev/bnad016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nieman L.K. Cushing's syndrome: update on signs, symptoms and biochemical screening. Eur J Endocrinol. 2015;173(4) doi: 10.1530/EJE-15-0464. M33-M8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schäcke H., Döcke W.D., Asadullah K. Mechanisms involved in the side effects of glucocorticoids. Pharmacol Ther. 2002;96(1):23–43. doi: 10.1016/s0163-7258(02)00297-8. [DOI] [PubMed] [Google Scholar]
- 18.Da Silva J.A., Jacobs J.W., Kirwan J.R., Boers M., Saag K.G., Ines L.B., et al. Safety of low dose glucocorticoid treatment in rheumatoid arthritis: published evidence and prospective trial data. Ann Rheum Dis. 2006;65(3):285–293. doi: 10.1136/ard.2005.038638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Malkawi A.K., Masood A., Shinwari Z., Jacob M., Benabdelkamel H., Matic G., et al. Proteomic analysis of morphologically changed tissues after prolonged dexamethasone treatment. Int J Mol Sci. 2019;20(13):3122. doi: 10.3390/ijms20133122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Battiston K., Parrag I., Statham M., Louka D., Fischer H., Mackey G., et al. Polymer-free corticosteroid dimer implants for controlled and sustained drug delivery. Nat Commun. 2021;12(1):2875. doi: 10.1038/s41467-021-23232-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aref A.A., Scott I.U., Oden N.L., Ip M.S., Blodi B.A., VanVeldhuisen P.C., et al. Incidence, risk Factors, and timing of elevated intraocular pressure after intravitreal triamcinolone acetonide injection for macular edema secondary to retinal vein occlusion SCORE study report 15. JAMA Ophthalmol. 2015;133(9):1022–1029. doi: 10.1001/jamaophthalmol.2015.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tripathi R.C., Parapuram S.K., Tripathi B.J., Zhong Y., Chalam K.V. Corticosteroids and glaucoma risk. Drugs Aging. 1999;15(6):439–450. doi: 10.2165/00002512-199915060-00004. [DOI] [PubMed] [Google Scholar]
- 23.Ren H.W., He Y.W., Liang J.M., Cheng Z.K., Zhang M., Zhu Y., et al. Role of liposome size, surface charge, and PEGylation on rheumatoid arthritis targeting therapy. ACS Appl Mater Interfaces. 2019;11(22):20304–20315. doi: 10.1021/acsami.8b22693. [DOI] [PubMed] [Google Scholar]
- 24.Deshantri A.K., Fens M.H., Ruiter R.W.J., Metselaar J.M., Storm G., van Bloois L., et al. Liposomal dexamethasone inhibits tumor growth in an advanced human-mouse hybrid model of multiple myeloma. J Controll Release. 2019;296:232–240. doi: 10.1016/j.jconrel.2019.01.028. [DOI] [PubMed] [Google Scholar]
- 25.Jia M.D., Deng C.F., Luo J.W., Zhang P., Sun X., Zhang Z.R., et al. A novel dexamethasone-loaded liposome alleviates rheumatoid arthritis in rats. Int J Pharm. 2018;540(1–2):57–64. doi: 10.1016/j.ijpharm.2018.02.001. [DOI] [PubMed] [Google Scholar]
- 26.Zhao M.N., Wang R.J., Yang K.M., Jiang Y.H., Peng Y.C., Li Y.K., et al. Nucleic acid nanoassembly-enhanced RNA therapeutics and diagnosis. Acta Pharm Sin B. 2023;13(3):916–941. doi: 10.1016/j.apsb.2022.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.He L.M., Qin X.Y., Fan D.H., Feng C.L., Wang Q., Fang J.Y. Dual-stimuli responsive polymeric micelles for the effective treatment of rheumatoid arthritis. ACS Appl Mater Interfaces. 2021;13(18):21076–21086. doi: 10.1021/acsami.1c04953. [DOI] [PubMed] [Google Scholar]
- 28.Wang Q., Jiang J.Y., Chen W.F., Jiang H., Zhang Z.R., Sun X. Targeted delivery of low-dose dexamethasone using PCL-PEG micelles for effective treatment of rheumatoid arthritis. J Controll Release. 2016;230:64–72. doi: 10.1016/j.jconrel.2016.03.035. [DOI] [PubMed] [Google Scholar]
- 29.Wang Q., Jiang H., Li Y., Chen W., Li H., Peng K., et al. Targeting NF-kB signaling with polymeric hybrid micelles that co-deliver siRNA and dexamethasone for arthritis therapy. Biomaterials. 2017;122:10–22. doi: 10.1016/j.biomaterials.2017.01.008. [DOI] [PubMed] [Google Scholar]
- 30.Xu X.Y., Sun L.P., Zhou L., Cheng Y.J., Cao F. Functional chitosan oligosaccharide nanomicelles for topical ocular drug delivery of dexamethasone. Carbohydr Polym. 2020;227 doi: 10.1016/j.carbpol.2019.115356. [DOI] [PubMed] [Google Scholar]
- 31.Komane P.P., Kumar P., Choonara Y.E. Atrial natriuretic peptide antibody-functionalised, PEGylated multiwalled carbon nanotubes for targeted ischemic stroke intervention. Pharmaceutics. 2021;13(9):1357. doi: 10.3390/pharmaceutics13091357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Motiei Pour M., Moghbeli M.R., Larijani B. Akbari Javar H. pH-Sensitive mesoporous bisphosphonate-based TiO2 nanoparticles utilized for controlled drug delivery of dexamethasone. Chemical Papers. 2021;76(1):439–451. [Google Scholar]
- 33.García-Fernández A., Sancho M., Bisbal V., Amorós P., Marcos M.D., Orzáez M., et al. Targeted-lung delivery of dexamethasone using gated mesoporous silica nanoparticles. A new therapeutic approach for acute lung injury treatment. J Controll Release. 2021;337:14–26. doi: 10.1016/j.jconrel.2021.07.010. [DOI] [PubMed] [Google Scholar]
- 34.Nguyen A., Bttger R., Li S.D. Recent trends in bioresponsive linker technologies of prodrug-based self-assembling nanomaterials. Biomaterials. 2019;2021 doi: 10.1016/j.biomaterials.2021.120955. [DOI] [PubMed] [Google Scholar]
- 35.Li G.T., Sun B.J., Li Y.Q., Luo C., He Z.G., Sun J. Small-molecule prodrug nanoassemblies: an emerging nanoplatform for anticancer drug delivery. Small. 2021;17(52) doi: 10.1002/smll.202101460. [DOI] [PubMed] [Google Scholar]
- 36.Yuan F., Quan L.D., Cui L., Goldring S.R., Wang D. Development of macromolecular prodrug for rheumatoid arthritis. Adv Drug Deliv Rev. 2012;64(12):1205–1219. doi: 10.1016/j.addr.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tessier B., Tsapis N., Fattal E., Moine L. Emerging nanoparticle platforms to improve the administration of glucocorticoids. J Controll Release. 2023;358:273–292. doi: 10.1016/j.jconrel.2023.04.039. [DOI] [PubMed] [Google Scholar]
- 38.Saravanakumar G., Kim J., Kim W.J. Reactive-oxygen-species-responsive drug delivery systems: promises and challenges. Adv Sci. 2017;4(1) doi: 10.1002/advs.201600124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang F.H., Porter M., Konstantopoulos A., Zhang P.C., Cui H.G. Preclinical development of drug delivery systems for paclitaxel-based cancer chemotherapy. J Controll Release. 2017;267:100–118. doi: 10.1016/j.jconrel.2017.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Elmaaty A.A., Alnajjar R., Hamed M.I.A., Khattab M., Khalifa M.M., Al-Karmalawy A.A. Revisiting activity of some glucocorticoids as a potential inhibitor of SARS-CoV-2 main protease: theoretical study. RSC Adv. 2021;11(17):10027–10042. doi: 10.1039/d0ra10674g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kong H., Lee Y., Kim H., Hong S., Kim D.D., Yoon J.H., et al. Susceptibility of glucocorticoids to colonic metabolism and pharmacologic intervention in the metabolism: implication for therapeutic activity of colon-specific glucocorticoid 21-sulfate sodium at the target site. J Pharmacy Pharmacol. 2012;64(1):128–138. doi: 10.1111/j.2042-7158.2011.01386.x. [DOI] [PubMed] [Google Scholar]
- 42.Kong H., Kim Y., Lee Y., Choi B., Jung S., Jung Y., et al. Sulfate-conjugated methylprednisolone: evaluation as a colon-specific methylprednisolone prodrug and comparison with sulfate-conjugated prednisolone and dexamethasone. J Drug Target. 2009;17(2):159–167. doi: 10.1080/10611860802546637. [DOI] [PubMed] [Google Scholar]
- 43.Ho Kim I., Sik Kong H., Im Choi B., Soo Kim Y., Jung Kim H., Wook Yang Y., et al. Synthesis and in vitro properties of dexamethasone 21-sulfate sodium as a colon-specific prodrug of dexamethasone. Drug Dev Ind Pharm. 2008;32(3):389–397. doi: 10.1080/03639040500519441. [DOI] [PubMed] [Google Scholar]
- 44.Doh M.J., Jung Y.J., Kim I.H., Kong H.S., Kim Y.M. Synthesis and properties of prednisolone 21-sulfate sodium as a colon-specific prodrug of prednisolone. Arch Pharm Res. 2003;26(4):258–263. doi: 10.1007/BF02976952. [DOI] [PubMed] [Google Scholar]
- 45.Smoak K.A., Cidlowski J.A. Mechanisms of glucocorticoid receptor signaling during inflammation. Mech Ageing Dev. 2004;125(10):697–706. doi: 10.1016/j.mad.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 46.Ozbakir B., Crielaard B.J., Metselaar J.M., Storm G., Lammers T. Liposomal corticosteroids for the treatment of inflammatory disorders and cancer. J Controll Release. 2014;190:624–636. doi: 10.1016/j.jconrel.2014.05.039. [DOI] [PubMed] [Google Scholar]
- 47.Jia Z.S., Zhao G., Wei X., Kong D.X., Sun Y.Y., Zhou Y., et al. Structural optimization of HPMA copolymer-based dexamethasone prodrug for improved treatment of inflammatory arthritis. J Controll Release. 2020;324:560–573. doi: 10.1016/j.jconrel.2020.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lin Y., Li Y.P., Wang X.H., Gong T., Zhang L., Sun X. Targeted drug delivery to renal proximal tubule epithelial cells mediated by 2-glucosamine. J Controll Release. 2013;167(2):148–156. doi: 10.1016/j.jconrel.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 49.Fang G., Zhang Q.H., Pang Y.Z., Thu H.E., Hussain Z. Nanomedicines for improved targetability to inflamed synovium for treatment of rheumatoid arthritis: multi-functionalization as an emerging strategy to optimize therapeutic efficacy. J Controll Release. 2019;303:181–208. doi: 10.1016/j.jconrel.2019.04.027. [DOI] [PubMed] [Google Scholar]
- 50.Kopeček J., Kopečková P. HPMA copolymers: origins, early developments, present, and future. Adv Drug Deliv Rev. 2010;62(2):122–149. doi: 10.1016/j.addr.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang D., Miller S.C., Liu X.-M., Anderson B., Wang X.S., Goldring S.R. Novel dexamethasone-HPMA copolymer conjugate and its potential application in treatment of rheumatoid arthritis. Arthritis Res Ther. 2007;9(1):R2. doi: 10.1186/ar2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu X.M., Quan L.D., Tian J., Alnouti Y., Fu K., Thiele G.M., et al. Synthesis and evaluation of a well-defined HPMA copolymer-dexamethasone conjugate for effective treatment of rheumatoid arthritis. Pharm Res. 2008;25(12):2910–2919. doi: 10.1007/s11095-008-9683-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Quan L.D., Purdue P.E., Liu X.M., Boska M.D., Lele S.M., Thiele G.M., et al. Development of a macromolecular prodrug for the treatment of inflammatory arthritis: mechanisms involved in arthrotropism and sustained therapeutic efficacy. Arthritis Res Ther. 2010;12(5):R170. doi: 10.1186/ar3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Quan L.D., Yuan F., Liu X.M., Huang J.G., Alnouti Y., Wang D. Pharmacokinetic and biodistribution studies of N-(2-Hydroxypropyl)methacrylamide copolymer-dexamethasone conjugates in adjuvant-induced arthritis rat model. Mol Pharm. 2010;7(4):1041–1049. doi: 10.1021/mp100132h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhao G., Ren R.G., Wei X., Jia Z.S., Chen N.R., Sun Y.Y., et al. Thermoresponsive polymeric dexamethasone prodrug for arthritis pain. J Controll Release. 2021;339:484–497. doi: 10.1016/j.jconrel.2021.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Quan L., Zhang Y., Dusad A., Ren K., Purdue P.E., Goldring S.R., et al. The evaluation of the therapeutic efficacy and side effects of a macromolecular dexamethasone prodrug in the collagen-induced arthritis mouse model. Pharm Res. 2015;33(1):186–193. doi: 10.1007/s11095-015-1776-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yuan F., Nelson R.K., Tabor D.E., Zhang Y., Akhter M.P., Gould K.A., et al. Dexamethasone prodrug treatment prevents nephritis in lupus-prone (NZB x NZW)F1 mice without causing systemic side effects. Arthritis Rheum. 2012;64(12):4029–4039. doi: 10.1002/art.34667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wei X., Zhao G., Wang X., Gautam N., Jia Z., Zhao Z., et al. Head-to-head comparative pharmacokinetic and biodistribution (PK/BD) study of two dexamethasone prodrug nanomedicines on lupus-prone NZB/WF1 mice. Nanomedicine. 2020;29 doi: 10.1016/j.nano.2020.102266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhao Z., Jiang H., Xu X., Jia Z., Ren R., Foster K.W., et al. Polymeric dexamethasone prodrugs attenuate lupus nephritis in MRL/lpr mice with reduced glucocorticoid toxicity. Nanomedicine. 2022;44 doi: 10.1016/j.nano.2022.102579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ren K., Purdue P.E., Burton L., Quan L-D, Fehringer E.V., Thiele G.M., et al. Early detection and treatment of wear particle-induced inflammation and bone loss in a mouse calvarial osteolysis model using HPMA copolymer conjugates. Mol Pharm. 2011;8(4):1043–1051. doi: 10.1021/mp2000555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ren K., Dusad A., Yuan F., Yuan H., Purdue P.E., Fehringer E.V., et al. Macromolecular prodrug of dexamethasone prevents particle-induced peri-implant osteolysis with reduced systemic side effects. J Controll Release. 2014;175:1–9. doi: 10.1016/j.jconrel.2013.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wei X., Li F., Zhao G., Chhonker Y.S., Averill C., Galdamez J., et al. Pharmacokinetic and biodistribution studies of HPMA copolymer conjugates in an aseptic implant loosening mouse model. Mol Pharm. 2017;14(5):1418–1428. doi: 10.1021/acs.molpharmaceut.7b00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ren K., Yuan H., Zhang Y., Wei X., Wang D. Macromolecular glucocorticoid prodrug improves the treatment of dextran sulfate sodium-induced mice ulcerative colitis. Clin Immunol. 2015;160(1):71–81. doi: 10.1016/j.clim.2015.03.027. [DOI] [PubMed] [Google Scholar]
- 64.Kostková H., Etrych T., Říhová B., Ulbrich K. Synergistic effect of HPMA copolymer-bound doxorubicin and dexamethasone in vivo on mouse lymphomas. J Bioact Compat Polym. 2011;26(3):270–286. [Google Scholar]
- 65.Zacchigna M., Cateni F., Di Luca G., Voinovich D., Perissutti B., Drioli S., et al. Synthesis of a new mPEG-dexamethasone conjugate and preliminary bioavailability studies in rabbits. J Drug Deliv Sci Technol. 2008;18(3):155–159. [Google Scholar]
- 66.Liu X.M., Quan L.D., Tian J., Laquer F.C., Dong W. Syntheses of click PEG-dexamethasone conjugates for the treatment of rheumatoid arthritis. Biomacromolecules. 2010;11(10):2621–2628. doi: 10.1021/bm100578c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hu J.B., Song G.L., Liu D., Li S.J., Wu J.H., Kang X.Q., et al. Sialic acid-modified solid lipid nanoparticles as vascular endothelium-targeting carriers for ischemia-reperfusion-induced acute renal injury. Drug Deliv. 2017;24(1):1856–1867. doi: 10.1080/10717544.2017.1410258. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 68.Funk D., Schrenk H.-H., Frei E. Development of a novel polyethylene glycol-corticosteroid-conjugate with an acid-cleavable linker. J Drug Target. 2010;19(6):434–445. doi: 10.3109/1061186X.2010.504271. [DOI] [PubMed] [Google Scholar]
- 69.Jia Z., Wang X., Wei X., Zhao G., Foster K.W., Qiu F., et al. Micelle-forming dexamethasone prodrug attenuates nephritis in lupus-prone mice without apparent glucocorticoid side effects. ACS Nano. 2018;12(8):7663–7681. doi: 10.1021/acsnano.8b01249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang Q., Li Y., Chen X., Jiang H., Zhang Z., Sun X. Optimized in vivo performance of acid-liable micelles for the treatment of rheumatoid arthritis by one single injection. Nano Res. 2018;12(2):421–428. [Google Scholar]
- 71.Hu J.B., Kang X.Q., Liang J., Wang X.J., Xu X.L., Yang P., et al. E-selectin-targeted sialic acid-PEG-dexamethasone micelles for enhanced anti-inflammatory efficacy for acute kidney injury. Theranostics. 2017;7(8):2204–2219. doi: 10.7150/thno.19571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yao X., Qi C., Sun C., Huo F., Jiang X. Poly(ethylene glycol) alternatives in biomedical applications. Nano Today. 2023;48 [Google Scholar]
- 73.Tenchov R., Sasso J.M., Zhou Q.A. PEGylated lipid nanoparticle formulations: immunological safety and efficiency perspective. Bioconjug Chem. 2023;34(6):941–960. doi: 10.1021/acs.bioconjchem.3c00174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Guo C., Yuan H.Y., Wang Y.X., Feng Y.P., Zhang Y., Yin T., et al. The interplay between PEGylated nanoparticles and blood immune system. Adv Drug Deliv Rev. 2023;200 doi: 10.1016/j.addr.2023.115044. [DOI] [PubMed] [Google Scholar]
- 75.Zakeri A., Kouhbanani M.A.J., Beheshtkhoo N., Beigi V., Mousavi S.M., Hashemi S.A.R., et al. Polyethylenimine-based nanocarriers in co-delivery of drug and gene: a developing horizon. Nano Rev Exp. 2018;9(1) doi: 10.1080/20022727.2018.1488497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jiang C.P., Chen J.T., Li Z.T., Wang Z.T., Zhang W.L., Liu J.P. Recent advances in the development of polyethylenimine-based gene vectors for safe and efficient gene delivery. Expert Opin Drug Deliv. 2019;16(4):363–376. doi: 10.1080/17425247.2019.1604681. [DOI] [PubMed] [Google Scholar]
- 77.Fahira A.I., Amalia R., Barliana M.I., Gatera V.A., Abdulah R. Polyethyleneimine (PEI) as a polymer-based co-delivery system for breast cancer therapy. Breast Cancer-Targets Therapy. 2022;14:71–83. doi: 10.2147/BCTT.S350403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Li J., Yu X., Shi X., Shen M. Cancer nanomedicine based on polyethylenimine-mediated multifunctional nanosystems. Prog Mater Sci. 2022;124 [Google Scholar]
- 79.Bae Y.M., Choi H., Lee S., Kang S.H., Kim Y.T., Nam K., et al. Dexamethasone-conjugated low molecular weight polyethylenimine as a nucleus-targeting lipopolymer gene carrier. Bioconjug Chem. 2007;18(6):2029–2036. doi: 10.1021/bc070012a. [DOI] [PubMed] [Google Scholar]
- 80.Kim H., Kim H.A., Bae Y.M., Choi J.S., Lee M. Dexamethasone-conjugated polyethylenimine as an efficient gene carrier with an anti-apoptotic effect to cardiomyocytes. J Gene Med. 2009;11(6):515–522. doi: 10.1002/jgm.1320. [DOI] [PubMed] [Google Scholar]
- 81.Kim H.A., Park J.H., Yi N., Lee M. Delivery of hypoxia and glioma dual-specific suicide gene using dexamethasone conjugated polyethylenimine for glioblastoma-specific gene therapy. Mol Pharm. 2014;11(3):938–950. doi: 10.1021/mp4006003. [DOI] [PubMed] [Google Scholar]
- 82.Kim T.H., Choi H., Yu G.S., Choi J.S. Synthesis of polyethylene glycol-oligo (glutamic acid) conjugated with polyethylenimine-dexamethasone for gene delivery applications. J Nanosci Nanotechnol. 2013;13(11):7325–7330. doi: 10.1166/jnn.2013.8084. [DOI] [PubMed] [Google Scholar]
- 83.Chen Z.Z., Zhang L.F., He Y.L., Li Y.F. Sandwich-type Au-PEI/DNA/PEI-Dexa nanocomplex for nucleus-targeted gene delivery in vitro and in vivo. ACS Appl Mater Interfaces. 2014;6(16):14196–14206. doi: 10.1021/am503483w. [DOI] [PubMed] [Google Scholar]
- 84.Choi M., Gu J., Lee M., Rhim T. A new combination therapy for asthma using dual-function dexamethasone-conjugated polyethylenimine and vitamin D binding protein siRNA. Gene Ther. 2017;24(11):727–734. doi: 10.1038/gt.2017.83. [DOI] [PubMed] [Google Scholar]
- 85.Su M., Yang B., Xi M., Qiang C., Yin Z. Therapeutic effect of pH-Responsive dexamethasone prodrug nanoparticles on acute lung injury. J Drug Deliv Sci Technol. 2021;66 doi: 10.1016/j.jddst.2021.102738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Franco P., De Marco I. The use of Poly(-vinyl pyrrolidone) in the delivery of drugs: a review. Polymers (Basel) 2020;12(5):1114. doi: 10.3390/polym12051114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cao Y., He W. Synthesis and characterization of glucocorticoid functionalized poly(N-vinyl pyrrolidone): a versatile prodrug for neural interface. Biomacromolecules. 2010;11(5):1298–1307. doi: 10.1021/bm100095t. [DOI] [PubMed] [Google Scholar]
- 88.Li M.Y., Jiang S., Haller A., Wirsching S., Fichter M., Simon J., et al. Encapsulation of polyprodrugs enables an efficient and controlled release of dexamethasone. Nanoscale Horiz. 2021;6(10):791–800. doi: 10.1039/d1nh00266j. [DOI] [PubMed] [Google Scholar]
- 89.Choi J.S., Ko K.S., Park J.S., Kim Y.H., Kim S.W., Lee M. Dexamethasone conjugated poly(amidoamine) dendrimer as a gene carrier for efficient nuclear translocation. Int J Pharm. 2006;320:171–178. doi: 10.1016/j.ijpharm.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 90.Zuo M., Li X., Liu S., Chen B., Cheng D. Nuclear-targeting delivery of CRISPRa system for upregulation of β-defensin against virus infection by dexamethasone and phenylalanine dual-modified dendrimer. Adv Polymer Technol. 2020;2020:1–13. [Google Scholar]
- 91.Malaekeh-Nikouei B., Rezaee M., Gholami L., Sanjar Mousavi N., Kazemi Oskuee R. Synthesis, characterization and evaluation of transfection efficiency of dexamethasone conjugated poly(propyleneimine) nanocarriers for gene delivery. Pharm Biol. 2018;56(1):519–527. doi: 10.1080/13880209.2018.1517183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Choksi A., Sarojini K.V.L., Vadnal P., Dias C., Suresh P.K., Khandare J. Comparative anti-inflammatory activity of poly(amidoamine) (PAMAM) dendrimer–dexamethasone conjugates with dexamethasone-liposomes. Int J Pharm. 2013;449(1–2):28–36. doi: 10.1016/j.ijpharm.2013.03.056. [DOI] [PubMed] [Google Scholar]
- 93.Soiberman U., Kambhampati S.P., Wu T., Mishra M.K., Oh Y., Sharma R., et al. Subconjunctival injectable dendrimer-dexamethasone gel for the treatment of corneal inflammation. Biomaterials. 2017;125:38–53. doi: 10.1016/j.biomaterials.2017.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lin H., Liu Y., Kambhampati S.P., Hsu C.C., Kannan R.M., Yiu S.C. Subconjunctival dendrimer-drug therapy for the treatment of dry eye in a rabbit model of induced autoimmune dacryoadenitis. Ocular Surface. 2018;16(4):415–423. doi: 10.1016/j.jtos.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Surekha B., Kommana N.S., Dubey S.K., Kumar A.V.P., Shukla R., Kesharwani P. PAMAM dendrimer as a talented multifunctional biomimetic nanocarrier for cancer diagnosis and therapy. Colloids Surfaces B: Biointerfaces. 2021;204 doi: 10.1016/j.colsurfb.2021.111837. [DOI] [PubMed] [Google Scholar]
- 96.Saluja V., Mishra Y., Mishra V., Giri N., Nayak P. Dendrimers based cancer nanotheranostics: an overview. Int J Pharm. 2021;600 doi: 10.1016/j.ijpharm.2021.120485. [DOI] [PubMed] [Google Scholar]
- 97.Kojima C. Preclinical studies of dendrimer prodrugs. Expert Opin Drug Metab Toxicol. 2015;11(8):1303–1315. doi: 10.1517/17425255.2015.1052404. [DOI] [PubMed] [Google Scholar]
- 98.Hsu H.J., Bugno J., Sr Lee, Hong S. Dendrimer-based nanocarriers: a versatile platform for drug delivery. WIREs Nanomedi Nanobiotechnol. 2016;9:e1409. doi: 10.1002/wnan.1409. [DOI] [PubMed] [Google Scholar]
- 99.Shahin V., Albermann L., Schillers H., Kastrup L., Schäfer C., Ludwig Y., et al. Steroids dilate nuclear pores imaged with atomic force microscopy. J Cell Physiol. 2005;202(2):591–601. doi: 10.1002/jcp.20152. [DOI] [PubMed] [Google Scholar]
- 100.Mi Bae Y., Choi H., Lee S., Ho Kang S., Tae Kim Y., Nam K., et al. Dexamethasone-conjugated low molecular weight polyethylenimine as a nucleus-targeting lipopolymer gene carrier. Bioconjug Chem. 2007;18(6):2029–2036. doi: 10.1021/bc070012a. [DOI] [PubMed] [Google Scholar]
- 101.Leong S., Lam H.P.J., Kirkham Z., Popat R. Antibody drug conjugates for the treatment of multiple myeloma. Am J Hematol. 2023;98(S2):S22–S34. doi: 10.1002/ajh.26750. [DOI] [PubMed] [Google Scholar]
- 102.Tolcher A.W. Antibody drug conjugates: the dos and don'ts in clinical development. Pharmacol Ther. 2022;240 doi: 10.1016/j.pharmthera.2022.108235. [DOI] [PubMed] [Google Scholar]
- 103.Hurvitz S.A. Recent progress in antibody–drug conjugate therapy for cancer. Nat Cancer. 2022;3(12):1412–1413. doi: 10.1038/s43018-022-00495-7. [DOI] [PubMed] [Google Scholar]
- 104.Fatima S.W., Khare S.K. Benefits and challenges of antibody drug conjugates as novel form of chemotherapy. J Controll Release. 2022;341:555–565. doi: 10.1016/j.jconrel.2021.12.013. [DOI] [PubMed] [Google Scholar]
- 105.Han A., Olsen O., D'Souza C., Shan J., Zhao F., Yanolatos J., et al. Development of novel glucocorticoids for use in antibody-drug conjugates for the treatment of inflammatory diseases. J Med Chem. 2021;64(16):11958–11971. doi: 10.1021/acs.jmedchem.1c00541. [DOI] [PubMed] [Google Scholar]
- 106.Fu Z., Li S., Han S., Shi C., Zhang Y. Antibody drug conjugate: the “biological missile” for targeted cancer therapy. Signal Transduct Target Ther. 2022;7(1):93. doi: 10.1038/s41392-022-00947-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Graversen J.H., Svendsen P., Dagnæs-Hansen F., Dal J., Anton G., Etzerodt A., et al. Targeting the hemoglobin scavenger receptor CD163 in macrophages highly increases the anti-inflammatory potency of dexamethasone. Molecular Therapy. 2012;20(8):1550–1558. doi: 10.1038/mt.2012.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Svendsen P., Graversen J.H., Etzerodt A., Hager H., Roge R., Gronbæk H., et al. Antibody-directed glucocorticoid targeting to CD163 in M2-type macrophages attenuates fructose-induced liver inflammatory changes. Molecular Therapy-Methods Clin Develop. 2017;4:50–61. doi: 10.1016/j.omtm.2016.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kern J.C., Cancilla M., Dooney D., Kwasnjuk K., Zhang R., Beaumont M., et al. Discovery of pyrophosphate diesters as tunable, soluble, and bioorthogonal linkers for site-specific antibody-drug conjugates. J Am Chem Soc. 2016;138(4):1430–1445. doi: 10.1021/jacs.5b12547. [DOI] [PubMed] [Google Scholar]
- 110.Kern J.C., Dooney D., Zhang R., Liang L.D., Brandish P.E., Cheng M.G., et al. Novel phosphate modified cathepsin B linkers: improving aqueous solubility and enhancing payload scope of ADCs. Bioconjug Chem. 2016;27(9):2081–2088. doi: 10.1021/acs.bioconjchem.6b00337. [DOI] [PubMed] [Google Scholar]
- 111.Brandish P.E., Palmieri A., Antonenko S., Beaumont M., Benso L., Cancilla M., et al. Development of anti-CD74 antibody-drug conjugates to target glucocorticoids to immune cells. Bioconjug Chem. 2018;29(7):2357–2369. doi: 10.1021/acs.bioconjchem.8b00312. [DOI] [PubMed] [Google Scholar]
- 112.Hobson A.D., McPherson M.J., Waegell W., Goess C.A., Stoffel R.H., Li X., et al. Design and development of glucocorticoid receptor modulators as immunology antibody-drug conjugate payloads. J Med Chem. 2022;65(6):4500–4533. doi: 10.1021/acs.jmedchem.1c02099. [DOI] [PubMed] [Google Scholar]
- 113.Dragovich P.S. Antibody-drug conjugates for immunology. J Med Chem. 2022;65(6):4496–4499. doi: 10.1021/acs.jmedchem.2c00339. [DOI] [PubMed] [Google Scholar]
- 114.Buttgereit F., Aelion J., Rojkovich B., Zubrzycka-Sienkiewicz A., Chen S., Yang Y., et al. Efficacy and safety of ABBV-3373, a novel anti-tumor necrosis factor glucocorticoid receptor modulator antibod-drug conjugate, in adults with moderate-to-severe rheumatoid arthritis despite methotrexate therapy: a randomized, double-blind, active-controlled proof-of-concept phase IIa trial. Arthritis Rheumatol. 2023;75(6):879–889. doi: 10.1002/art.42415. [DOI] [PubMed] [Google Scholar]
- 115.Dissanayake S., WA Denny, Gamage S., Sarojini V. Recent developments in anticancer drug delivery using cell penetrating and tumor targeting peptides. J Controll Release. 2017;250:62–76. doi: 10.1016/j.jconrel.2017.02.006. [DOI] [PubMed] [Google Scholar]
- 116.Cooper B.M., Iegre J., O' Donovan D.H., Halvarsson M.Ö., Spring D.R. Peptides as a platform for targeted therapeutics for cancer: peptide-drug conjugates (PDCs) Chem Soc Rev. 2021;50(3):1480–1494. doi: 10.1039/d0cs00556h. [DOI] [PubMed] [Google Scholar]
- 117.Wang Y., Cheetham A.G., Angacian G., Su H., Xie L.S., Cui H.G. Peptide-drug conjugates as effective prodrug strategies for targeted delivery. Adv Drug Deliv Rev. 2017;110:112–126. doi: 10.1016/j.addr.2016.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhu Y.-S., Tang K., Lv J. Peptide–drug conjugate-based novel molecular drug delivery system in cancer. Trends Pharmacol Sci. 2021;42(10):857–869. doi: 10.1016/j.tips.2021.07.001. [DOI] [PubMed] [Google Scholar]
- 119.Gaudana R., Parenky A., Vaishya R., Samanta S.K., Mitra A.K. Development and characterization of nanoparticulate formulation of a water soluble prodrug of dexamethasone by HIP complexation. J Microencapsul. 2010;28(1):10–20. doi: 10.3109/02652048.2010.520093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Tang W., Zhao Z.B., Chong Y.Y., Wu C.F., Liu Q.Z., Yang J.B., et al. Tandem enzymatic self-assembly and slow release of dexamethasone enhances its antihepatic fibrosis effect. ACS Nano. 2018;12(10):9966–9973. doi: 10.1021/acsnano.8b04143. [DOI] [PubMed] [Google Scholar]
- 121.Zhang R., Zhou J., Lin D., Hu Y., Jin B., Wang Y., et al. Dexamethasone-peptide prodrug supramolecular hydrogel effectively alleviates experimental autoimmune uveitis (EAU) Chem Eng J. 2021;421 [Google Scholar]
- 122.Yu X.X., Zhang R.S., Lei L., Song Q.Q., Li X.Y. High drug payload nanoparticles formed from dexamethasone-peptide conjugates for the treatment of endotoxin-induced uveitis in rabbit. J Microencapsul. 2019;14:591–603. doi: 10.2147/IJN.S179118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sis M.J., Ye Z., La Costa K., Webber M.J. Energy landscapes of supramolecular peptide-drug conjugates directed by linker selection and drug topology. ACS Nano. 2022;16(6):9546–9558. doi: 10.1021/acsnano.2c02804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Formica F.A., Barreto G., Zenobi-Wong M. Cartilage-targeting dexamethasone prodrugs increase the efficacy of dexamethasone. J Controll Release. 2019;295:118–129. doi: 10.1016/j.jconrel.2018.12.025. [DOI] [PubMed] [Google Scholar]
- 125.Bhattacharya M., Sadeghi A., Sarkhel S., Hagström M., Bahrpeyma S., Toropainen E., et al. Release of functional dexamethasone by intracellular enzymes: a modular peptide-based strategy for ocular drug delivery. J Controll Release. 2020;327:584–594. doi: 10.1016/j.jconrel.2020.09.005. [DOI] [PubMed] [Google Scholar]
- 126.Leopold C.S., Friend D.R. In vivo pharmacokinetic study for the assessment of poly(L-aspartic acid) as a drug carrier for colon-specific drug delivery. J Pharmacokinetics Biopharmaceut. 1995;23(4):397–406. doi: 10.1007/BF02353640. [DOI] [PubMed] [Google Scholar]
- 127.Wang S., Wang Y.Q., Lai X.X., Sun J.W., Hu M., Chen M., et al. Minimalist nanocomplex with dual regulation of endothelial function and inflammation for targeted therapy of inflammatory vascular diseases. ACS Nano. 2023;17(3):2761–2781. doi: 10.1021/acsnano.2c11058. [DOI] [PubMed] [Google Scholar]
- 128.Sedighi M., Mahmoudi Z., Ghasempour A., Shakibaie M., Ghasemi F., Akbari M., et al. Nanostructured multifunctional stimuli-responsive glycopolypeptide-based copolymers for biomedical applications. J Controll Release. 2023;354:128–145. doi: 10.1016/j.jconrel.2022.12.058. [DOI] [PubMed] [Google Scholar]
- 129.Cao X., Du X.J., Jiao H., An Q.L., Chen R.X., Fang P.F., et al. Carbohydrate-based drugs launched during 2000-2021. Acta Pharm Sin B. 2022;12(10):3783–3821. doi: 10.1016/j.apsb.2022.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Su L., Feng Y.L., Wei K.C., Xu X.Y., Liu R.Y., Chen G.S. Carbohydrate-based macromolecular biomaterials. Chem Rev. 2021;121(18):10950–11029. doi: 10.1021/acs.chemrev.0c01338. [DOI] [PubMed] [Google Scholar]
- 131.Fedorak R.N., Cui N., Friend D.R., Madsen K.L., Empey L.R. A novel colon-specific steroid prodrug enhances sodium chloride absorption in rat colitis. Am J Physiol. 1995;269(2):G210. doi: 10.1152/ajpgi.1995.269.2.G210. [DOI] [PubMed] [Google Scholar]
- 132.Haeberlin B., Rubas W., Iii H., Friend D.R. In vitro evaluation of dexamethasone-β-D-Glucuronide for colon-specific drug delivery. Pharm Res. 1993;10(11):1553–1562. doi: 10.1023/a:1018956232628. [DOI] [PubMed] [Google Scholar]
- 133.Colon-specific delivery of dexamethasone from a glucoside prodrug in the guinea pig. Pharm Res. 1991;8(4):445. doi: 10.1023/a:1015838825437. [DOI] [PubMed] [Google Scholar]
- 134.Friend D.R., Chang G.W. A colon-specific drug-delivery system based on drug glycosides and the glycosidases of colonic bacteria. J Med Chem. 2002;27(3):261–266. doi: 10.1021/jm00369a005. [DOI] [PubMed] [Google Scholar]
- 135.Cui N., Friend D.R., Fedorak R.N. A budesonide prodrug accelerates treatment of colitis in rats. Gut. 1994;35(10):1439–1446. doi: 10.1136/gut.35.10.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Mcleod A.D., Tolentino L., Tozer T.N. Glucocorticoid-dextran conjugates as potential prodrugs for colon-specific delivery: steady-state pharmacokinetics in the rat. Biopharm Drug Dispos. 2010;15(2):151–161. doi: 10.1002/bdd.2510150207. [DOI] [PubMed] [Google Scholar]
- 137.Liu H.B., Liu D.D., Ji M.S., Xiao P.F., Qin Y., Zhao J.S., et al. Inflammation-targeted sialic acid-dexamethasone conjugates for reducing the side effects of glucocorticoids. Int J Pharm. 2022;622 doi: 10.1016/j.ijpharm.2022.121900. [DOI] [PubMed] [Google Scholar]
- 138.Varshosaz J., Emami J., Tavakoli N., Fassihi A., Minaiyan M., Ahmadi F., et al. Synthesis and evaluation of dextran-budesonide conjugates as colon specific prodrugs for treatment of ulcerative colitis. Int J Pharm. 2009;365(1–2):69–76. doi: 10.1016/j.ijpharm.2008.08.034. [DOI] [PubMed] [Google Scholar]
- 139.Li N.-N., Lin J., Gao D., Zhang L-M. A macromolecular prodrug strategy for combinatorial drug delivery. J Colloid Interface Sci. 2014;417:301–309. doi: 10.1016/j.jcis.2013.11.061. [DOI] [PubMed] [Google Scholar]
- 140.Yano H., Hirayama F., Kamada M., Arima H., Uekama K. Colon-specific delivery of prednisolone-appended α-cyclodextrin conjugate: alleviation of systemic side effect after oral administration. J Controll Release. 2002;79(1):103–112. doi: 10.1016/s0168-3659(01)00532-6. [DOI] [PubMed] [Google Scholar]
- 141.Zhang H.J., He Q.Q., Wang J.J., Wang Y.P., Xuan X.Y., Sui M.L., et al. Biomimetic micelles to accurately regulate the inflammatory microenvironment for glomerulonephritis treatment. Pharmacol Res. 2022;181 doi: 10.1016/j.phrs.2022.106263. [DOI] [PubMed] [Google Scholar]
- 142.Pang Y.N., Yan Z., Zhang Z.R. Synthesis of an enzyme-dependent prodrug and evaluation of its potential for colon targeting. World J Gastroenterol. 2002;8(5):5. doi: 10.3748/wjg.v8.i5.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.McLeod A.D., Fedorak R.N., Friend D.R., Tozer T.N., Cui N. A glucocorticoid prodrug facilitates normal mucosal function in rat colitis without adrenal suppression. Gastroenterology. 1994;106(2):405–413. doi: 10.1016/0016-5085(94)90599-1. [DOI] [PubMed] [Google Scholar]
- 144.Zhang Z., Tang W. Drug metabolism in drug discovery and development. Acta Pharm Sin B. 2018;8(5):721–732. doi: 10.1016/j.apsb.2018.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Li J., Yang J., Wang W.X., Yu J.C., Fu J.G., Wang X.L. A novel liposomal dexamethasone palmitate formulation and anti-inflammatory effects on mice. Chin J Chem. 2009;27(7):1411–1414. [Google Scholar]
- 146.Simón-Vázquez R., Tsapis N., Lorscheider M., Rodríguez A., Calleja P., Mousnier L., et al. Improving dexamethasone drug loading and efficacy in treating arthritis through a lipophilic prodrug entrapped into PLGA-PEG nanoparticles. Drug Deliv Transl Res. 2022;12(5):1270–1284. doi: 10.1007/s13346-021-01112-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lorscheider M., Tsapis N., ur-Rehman M., Gaudin F., Stolfa I., Abreu S., et al. Dexamethasone palmitate nanoparticles: an efficient treatment for rheumatoid arthritis. J Controll Release. 2019;296:179–189. doi: 10.1016/j.jconrel.2019.01.015. [DOI] [PubMed] [Google Scholar]
- 148.Yokoyama K., Watanabe M. Limethason as a lipid microsphere preparation: an overview. Adv Drug Deliv Rev. 1996;20(2–3):195–201. [Google Scholar]
- 149.Daull P., Paterson C.A., Kuppermann B.D., Garrigue J.S. A preliminary evaluation of dexamethasone palmitate emulsion: a novel intravitreal sustained delivery of corticosteroid for treatment of macular edema. J Ocular Pharmacol Therapeut. 2013;29(2):258–269. doi: 10.1089/jop.2012.0044. [DOI] [PubMed] [Google Scholar]
- 150.Mizushima Y., Hamano T., Yokoyama K. Tissue distribution and anti-inflammatory activity of corticosteroids incorporated in lipid emulsion. Ann Rheum Dis. 1982;41(3):263–267. doi: 10.1136/ard.41.3.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Wijagkanalan W., Higuchi Y., Kawakami S., Teshima M., Sasaki H., Hashida M. Enhanced anti-inflammation of inhaled dexamethasone palmitate using mannosylated liposomes in an endotoxin-induced lung inflammation model. Mol Pharmacol. 2008;74(5):1183–1192. doi: 10.1124/mol.108.050153. [DOI] [PubMed] [Google Scholar]
- 152.Hu L., Luo X., Zhou S.L., Zhu J.Y., Xiao M.Y., Li C., et al. Neutrophil-mediated delivery of dexamethasone palmitate-loaded liposomes decorated with a sialic acid conjugate for rheumatoid arthritis treatment. Pharm Res. 2019;36(7):97. doi: 10.1007/s11095-019-2609-4. [DOI] [PubMed] [Google Scholar]
- 153.Gürcan S., Tsapis N., Reynaud F., Denis S., Vergnaud J., Özer Ö., et al. Combining dexamethasone and TNF-α siRNA within the same nanoparticles to enhance anti-inflammatory effect. Int J Pharm. 2021;598 doi: 10.1016/j.ijpharm.2021.120381. [DOI] [PubMed] [Google Scholar]
- 154.Lorscheider M., Tsapis N., Simón-Vázquez R., Guiblin N., Ghermani N., Reynaud F., et al. Nanoscale lipophilic prodrugs of dexamethasone with enhanced pharmacokinetics. Mol Pharm. 2019;16(7):2999–3010. doi: 10.1021/acs.molpharmaceut.9b00237. [DOI] [PubMed] [Google Scholar]
- 155.Kim J.K., Howard M.D., Dziubla T.D., Rinehart J.J., Jay M., Lu X.L. Uniformity of drug payload and its effect on stability of solid lipid nanoparticles containing an ester prodrug. ACS Nano. 2011;5(1):209–216. doi: 10.1021/nn102357y. [DOI] [PubMed] [Google Scholar]
- 156.Pinheiro do Nascimento L., Tsapis N., Reynaud F., Desmaële D., Moine L., Vergnaud J., et al. Mannosylation of budesonide palmitate nanoprodrugs for improved macrophage targeting. Eur J Pharmaceut Biopharmaceut. 2022;170:112–120. doi: 10.1016/j.ejpb.2021.12.001. [DOI] [PubMed] [Google Scholar]
- 157.N'Guessan A., Fattal E., Chapron D., Gueutin C., Koffi A., Tsapis N. Dexamethasone palmitate large porous particles: a controlled release formulation for lung delivery of corticosteroids. Eur J Pharmaceut Sci. 2018;113:185–192. doi: 10.1016/j.ejps.2017.09.013. [DOI] [PubMed] [Google Scholar]
- 158.Kim J.K., Yuan H., Nie J., Yang Y.T., Leggas M., Potter P.M., et al. High payload dual therapeutic-imaging nanocarriers for triggered tumor delivery. Small. 2012;8(18):2895–2903. doi: 10.1002/smll.201200437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Canioni R., Reynaud F., Leite-Nascimento T., Gueutin C., Guiblin N., Ghermani N.-E., et al. Tiny dexamethasone palmitate nanoparticles for intravitreal injection: optimization and in vivo evaluation. Int J Pharm. 2021;600 doi: 10.1016/j.ijpharm.2021.120509. [DOI] [PubMed] [Google Scholar]
- 160.Tauchi Y., Chono S., Morimoto K. Cellular uptake of a dexamethasone palmitate-low density lipoprotein complex by macrophages and foam cells. J Drug Target. 2003;11(3):163–168. doi: 10.1080/10611860310001595247. [DOI] [PubMed] [Google Scholar]
- 161.Zhang L., Zhou Y., Chai X., Yang Z., Pang N., Du Y., et al. Excipient-free prodrug-based three-in-one nanoparticles co-deliver diversified agents to amplify tumor therapy. Chem Eng J. 2022;435 [Google Scholar]
- 162.Chen S., Zaifman J., Kulkarni J.A., Zhigaltsev I.V., Tam Y.K., Ciufolini M.A., et al. Dexamethasone prodrugs as potent suppressors of the immunostimulatory effects of lipid nanoparticle formulations of nucleic acids. J Controll Release. 2018;286:46–54. doi: 10.1016/j.jconrel.2018.07.026. [DOI] [PubMed] [Google Scholar]
- 163.Xu Y., Mu J.Q., Xu Z.K., Zhong H.P., Chen Z.Q., Ni A.K., et al. Modular acid-activatable acetone-based ketal-linked nanomedicine by dexamethasone prodrugs for enhanced anti-rheumatoid arthritis with low side effects. Nano Lett. 2020;20(4):2558–2568. doi: 10.1021/acs.nanolett.9b05340. [DOI] [PubMed] [Google Scholar]
- 164.Goldie I., Nachemson A. Synovial pH in rheumatoid knee joints:II. the effect of local corticosteroid treatment. Acta Orthop Scand. 2009;41(3):354–362. doi: 10.3109/17453677008991521. [DOI] [PubMed] [Google Scholar]
- 165.Yan Y., Lu A., Dou Y., Zhang Z., Wang X.Y., Zhai L., et al. Nanomedicines reprogram synovial macrophages by scavenging nitric oxide and silencing CA9 in progressive osteoarthritis. Adv Sci. 2023;10(11) doi: 10.1002/advs.202207490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Xu Y., Chen Z., Xu Z., Du Y., Han J., Yuan X., et al. Intra-Articular injection of acid-sensitive stearoxyl-ketal-dexamethasone microcrystals for long-acting arthritis therapy. Asian J Pharm Sci. 2021;16(2):213–221. doi: 10.1016/j.ajps.2020.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Lee Y., Jeong S., Kim W., Kim H., Yoon J.H., Jeong S.H., et al. Glycyrrhizin enhances therapeutic activity of a colon-specific methylprednisolone prodrug against experimental colitis. Dig Dis Sci. 2013;58(5):1226–1234. doi: 10.1007/s10620-012-2495-7. [DOI] [PubMed] [Google Scholar]
- 168.Mo J.X., Shi S.J., Zhang Q., Gong T., Sun X., Zhang Z.R. Synthesis, transport and mechanism of a type I prodrug: l-carnitine ester of prednisolone. Mol Pharm. 2011;8(5):1629–1640. doi: 10.1021/mp100412z. [DOI] [PubMed] [Google Scholar]
- 169.Mo J., Lim L.Y., Zhang Z.R. l-Carnitine ester of prednisolone: pharmacokinetic and pharmacodynamic evaluation of a type I prodrug. Int J Pharm. 2014;475(1–2):123–129. doi: 10.1016/j.ijpharm.2014.08.049. [DOI] [PubMed] [Google Scholar]
- 170.Avnir Y., Ulmansky R., Wasserman V., Even-Chen S., Broyer M., Barenholz Y., et al. Amphipathic weak acid glucocorticoid prodrugs remote-loaded into sterically stabilized nanoliposomes evaluated in arthritic rats and in a Beagle dog: a novel approach to treating autoimmune arthritis. Arthritis Rheumatism. 2007;58(1):119–129. doi: 10.1002/art.23230. [DOI] [PubMed] [Google Scholar]
- 171.Zhang Z.L., Yu J., Zhou Y.F., Zhang R.S., Song Q.Q., Lei L., et al. Supramolecular nanofibers of dexamethasone derivatives to form hydrogel for topical ocular drug delivery. Colloids Surfaces B-Biointerfaces. 2018;164:436–443. doi: 10.1016/j.colsurfb.2018.01.051. [DOI] [PubMed] [Google Scholar]
- 172.Zhou Y.F., Lei L., Zhang Z.L., Zhang R.S., Song Q.Q., Li X.Y. Cation instructed steroidal prodrug supramolecular hydrogel. J Colloid Interface Sci. 2018;528:10–17. doi: 10.1016/j.jcis.2018.05.059. [DOI] [PubMed] [Google Scholar]
- 173.Chao Y., Liu Z. Biomaterials tools to modulate the tumour microenvironment in immunotherapy. Nature Rev Bioeng. 2023;1(2):125–138. [Google Scholar]
- 174.Marquez Ruiz J.F., Kedziora K., Pigott M., Keogh B., Windle H., Gavin J., et al. A nitrophenyl-based prodrug type for colorectal targeting of prednisolone, budesonide and celecoxib. Bioorg Med Chem Lett. 2013;23(6):1693–1698. doi: 10.1016/j.bmcl.2013.01.060. [DOI] [PubMed] [Google Scholar]
- 175.Ruiz J.F.M., Radics G., Windle H., Serra H.O., Simplício A.L., Kedziora K., et al. Design, synthesis, and pharmacological effects of a cyclization-activated steroid prodrug for colon targeting in inflammatory bowel disease. J Med Chem. 2009;52(10):3205–3211. doi: 10.1021/jm8016317. [DOI] [PubMed] [Google Scholar]
- 176.Genito C.J., Eckshtain-Levi M., Piedra-Quintero Z.L., Krovi S.A., Kroboth A., Stiepel R.T., et al. Dexamethasone and fumaric acid ester conjugate synergistically inhibits inflammation and NF-κB in macrophages. Bioconjug Chem. 2021;32(8):1629–1640. doi: 10.1021/acs.bioconjchem.1c00200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Xu Y., Chen J., Jiang W., Zhao Y., Yang C., Wu Y., et al. Multiplexing nanodrug ameliorates liver fibrosis via ROS elimination and inflammation suppression. Small. 2021;18(3) doi: 10.1002/smll.202102848. [DOI] [PubMed] [Google Scholar]