Abstract
Objective
This meta-analysis aimed to evaluate the impact of wilted and unwilted silage on various parameters, such as nutrient content, fermentation quality, bacterial populations, and digestibility.
Methods
Thirty-six studies from Scopus were included in the database and analyzed using a random effects model in OpenMEE software. The studies were grouped into two categories: wilting silage (experiment group) and non-wilting silage (control group). Publication bias was assessed using a fail-safe number.
Results
The results showed that wilting before ensiling significantly increased the levels of dry matter, water-soluble carbohydrates, neutral detergent fiber, and acid detergent fiber, compared to non-wilting silage (p<0.05). However, wilting significantly decreased dry matter losses, lactic acid, acetic acid, butyric acid, and ammonia levels (p<0.05). The pH, crude protein, and ash contents remained unaffected by the wilting process. Additionally, the meta-analysis revealed no significant differences in bacterial populations, including lactic acid bacteria, yeast, and aerobic bacteria, or in vitro dry matter digestibility between the two groups (p>0.05).
Conclusion
Wilting before ensiling significantly improved silage quality by increasing dry matter and water-soluble carbohydrates, as well as reducing dry matter losses, butyric acid, and ammonia. Importantly, wilting did not have a significant impact on pH, crude protein, or in vitro dry matter digestibility.
Keywords: Forage, Meta-analysis, Silage, Wilting
INTRODUCTION
Silage is the fermented and preserved feed made from grass, legumes, or whole crops. Silage quality is influenced by the resident microbial communities, which in turn affect the fermentation process. The type of forage (crop) used, growing conditions, and environmental factors during the wilting period influence the populations of different microbial communities in silos [1]. Wilting significantly impacts silage quality, and its primary objectives, as outlined by Ribas et al [2], include enhancing fermentation quality, mitigating environmental pollution, and minimizing nutrient losses in the form of gases and effluents.
The wilting process affects the moisture content in the silage, thereby influencing the quality of fermentation. Wilting before ensiling is widely practiced in many parts of the world, as it can reduce silo runoff and improve silage fermentation quality. The wilting process can influence both the physical and chemical attributes of the silage [3].
The effect of wilting on silage quality has been examined in various research studies. However, several studies cannot be considered a standard for understanding the impact of wilting on silage quality, as many of them present inconclusive data. For instance, the pH results in the research conducted by Tao et al [4] indicate a decrease after wilting, while the pH results in the study by Kim et al [5] suggest an increase after wilting. Additionally, [6] observed an increase in lactic acid, in contrast to Zheng et al [7], who reported a decrease in lactic acid due to wilting. Furthermore, conflicting results on silage digestibility were reported, with an increase according to Wan et al [8],
Determining whether wilting has a positive or negative effect on silage quality is challenging based on individual research reports. To gauge the overall impact of wilting on silage quality, a generalization process needs to be conducted on existing research using suitable statistical methods. This study aims to assess the impact of wilting on the quality of silage through a meta-analysis method. It is important to note that, while meta-analysis can provide valuable insights, it cannot replace individual research results. The accuracy of individual research results remains robust, aligned with the specific conditions of each study when silage is made, such as the type of forage species utilized, the timing of harvesting, the incorporation of additives, and the adjustment of dry matter (DM) at initial silage making [1,9,10]. These variables play a crucial role in shaping the outcomes of each study and must be considered when evaluating the overall impact of wilting on silage quality.
Meta-analysis is widely recognized as the preferred method for synthesizing research results across disciplines. Its extensive application in numerous fields has illustrated its versatility in consolidating research findings. In a meta-analysis, the data are analyzed, emphasizing the strength or size of an effect rather than the statistical significance of individual studies. The robust results of the meta-analysis can be obtained after incorporating various factors, including sample size, research methodologies, and publication [11,12]. Careful consideration of these factors enhances the reliability and generalizability of the meta-analytical findings, providing a more comprehensive and nuanced understanding of the overall impact being assessed [13,14].
MATERIALS AND METHODS
Database development
A database was developed from several types of literature that reported the effect of wilting on silage. The search of the literature was conducted using Scopus with the keywords used being ‘wilting’ and ‘silage/ensiling’. The database was made in August 2023 from the Scopus research database. The selection criteria were: i) English-language articles; ii) direct comparison between wilting and un-wilting silage; iii) comparison of chemical content, bacteria silage population, and DM digestibility and; iv) replication and variance were reported (standard deviation [SD] or standard error or means). These criteria followed the preferred reporting item for systematic reviews and meta-analysis protocol.
All relevant literature titles are collected along with other information. All literature is collected and a database is formed using a data aggregation process. Data aggregation is the arrangement of data from the literature to facilitate the analysis calculations used.
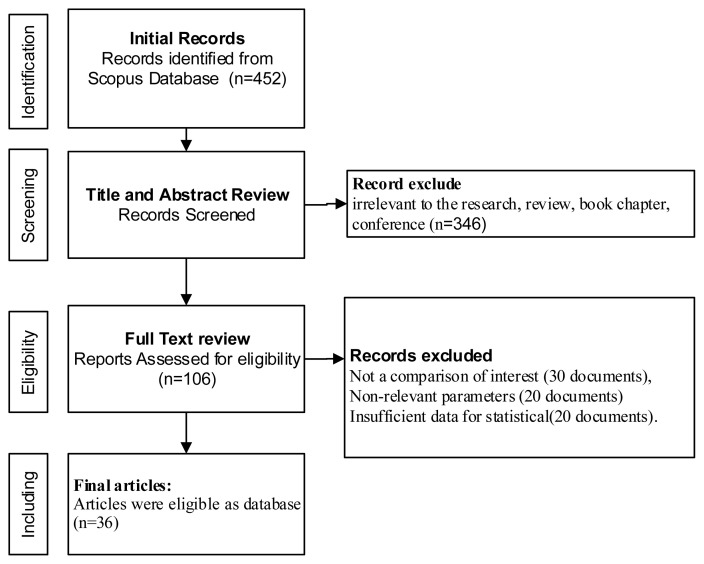
The selection process is shown in Figure 1. The initial search resulted in 452 articles. A total of 346 articles were excluded for several reasons (non-related titles, review articles, or conference proceedings). Hence, the full-text evaluation resulted in 106 articles while 70 articles were excluded due to lack of comparison (n = 30), irrelevant contents or variables (n = 20), and insufficient data (n = 20). The final articles (n = 36) after assessment were considered a database in the meta-analysis shown in Table 1.
Figure 1.
Flow chart of articles selection process based on preferred reporting item for systematic reviews and meta-analysis protocol.
Table 1.
Articles included in the meta-analysis
| No. | Reference | Forage type | Additional treatment | Storage time (d) | Wilting time (h) |
|---|---|---|---|---|---|
| 1 | [21] | Bermuda Grass | - | 30, 90 | 4 |
| 2 | [34] | Oat | With and without inoculant bacteria | 112 | 5 |
| 3 | [44] | Elephant Grass | With and without Citrus pulp | 60 | 6 |
| 4 | [4] | Alfalfa | - | 45 | 5.2, 8.5 |
| 5 | [45] | Grass | - | 110 | 6 |
| 6 | [35] | Grass Herbage | - | 210, 540 | 24, 48 |
| 7 | [24] | Comfrey (Symphytum officinale) | - | 202 | 24 |
| 8 | [46] | Perennial ryegrass | Shade and not shade, nonadditive, formic acid, formalin | 75 | 2, 68 |
| 9 | [47] | Ryegrass, Alfalfa | - | 16 | 3, 4, 8 |
| 10 | [48] | Grass | Nonadditive, formic acid, and formalin, other additive | 6 | 24 |
| 11 | [49] | Perennial ryegrass | Low nitrogen, High nitrogen | 120 | 96 |
| 12 | [50] | Catch crop: a mixture of sunflower, sorghum, peas, Vicia sp. and Trifolium alexandrinum | - | 7, 14, 28, 98 | 72, 96 |
| 13 | [51] | A mixture of Lolium perenne L. (81%), Poa pratensis L. (9%), and Annual weeds (5%) | - | 28 | 24 |
| 14 | [52] | Maize | - | 40 | 24, 72 |
| 15 | [53] | Oat | - | 100 | 14 |
| 16 | [54] | Wheat cultivar Bet Hashita flowering stage maturity | - | 210, 60 | 8 |
| 17 | [55] | Chrysanthemum coronarium L early bud maturity and late flower maturity | - | 120 | 4, 26, 47, 77, 95 |
| 18 | [56] | Wheat Forage | - | 322 | 6, 20 |
| 19 | [57] | Pure sudangrass | Nonadditive, Molasses, L. plantarum, Molasses+L. Plantarum | 60 | 8 |
| 20 | [22] | Perennial ryegrass | Inoculant, formic acid | 70 | 28, 52 |
| 21 | [58] | Elephant grass | Nonadditive, cassava meal 7.5% NM, 15% NM, 22.5% NM | 60 | 8 |
| 22 | [28] | King grass | - | 14, 30 | 12 |
| 23 | [59] | Perennial ryegrass | Nonadditive, formic acid-formalin | 122 | 48 |
| 24 | [3] | Stylosanthes guianensis Swartz | Temperature 10°C, 20ºC, 30°C, 40ºC | 125 | 6, 12 |
| 25 | [6] | Guinea grass | Nonadditive, molasess | 14, 28, 56 | 6, 7, 8 |
| 26 | [5] | Rye grass | - | 30 | 24, 48, 12, 24 |
| 27 | [33] | Moringa oleifera leaf | Nonadditive, L. plantarum | 60, 120 | 12 |
| 28 | [27] | Broussonetia papyrifera | Nonadditive, Enterococcus durans, cellulase, formic acid | 60 | 3.5 |
| 29 | [7] | Alfalfa Sanditi, Alfalfa caribou, Alfalfa WL319HQ, Alfalfa 4030 | - | 14, 28, 56 | 2, 4 |
| 30 | [60] | Medicago sativa L. 250, Medicago sativa L. 350 | - | 120 | 4.5, 2.5 |
| 31 | [23] | Mulberry | Nonadditive, L. plantarum, Commercial L. plantarum, Cellulase | 30 | 2, 4 |
| 32 | [61] | Italian ryegrass, festulolium | Nonadditive, L. Casei, L. Bucheri | 120 | 4 |
| 33 | [62] | Shorgum | - | 30 | 12 |
| 34 | [63] | Ryegrass | Additive L. plantarum, formic acid | ||
| 35 | [64] | Whole crop pea | Acid treatment | 103 | - |
| 36 | [65] | Sainfoin | - | 120 | 5–25 |
Data extraction
Data were analyzed using the random-effects meta-analysis method as described by Risyahadi et al [15]. The mathematical modeling of one-way random effects follows:
In this equation, yi represents the effect size (Hedge’s d) for the i-th observation, θ is the general parameter for the combined effect size, vi represents the actual variation in the effect size, and ɛi is the error for the i-th observation.
In brief, the effect size (d) was calculated based on Hedges’ standardized mean difference, with the formula [16]:
Where the mean of the experimental or wilting process is (E), the control group or without wilting process is (C), and the pooled standard deviation is (S) S defined as:
and J is the correction factor for the small sample size, i.e.:
where: NE, sample size of the experimental group; Nc, sample size of the control group; SE, standard deviation of the experimental group; SC, standard deviation of the control group. The variance of Hedges’d (Vd) is described as follows:
The cumulative effect size (d++) was formulated as follows:
where: Wi, the inverse of the sampling variance: Wi = 1/vd. The precision of the effect size was described using a 95% confidence interval (CI), i.e. d±(1.96×SD). All the above equations were derived from the study of Sánchez-Meca and Marín-Martínez [17]. Statistical significance was established by verifying that the CI did not encompass a null effect size. Significance was set at a p-value of 0.05. A fail-safe number (Nfs) was calculated to identify publication bias caused by non-significant studies, which were not included in the analysis. Nfs > 5N+10 was considered to provide evidence of a robust meta-analysis model. Nfs was calculated using the method of Rosenthal [18]. The smallest sample size from individual studies was applied as N. Cohen’s benchmarks were used as standard judgment borders for effect size assessment. These benchmarks were: 0.2 for small, 0.5 for medium, and 0.8 for large effect size.
Between-study variance (τ2) was estimated using DerSimonian and Laird’s method [19]
Where Q is the weighted sum square, df is the degrees of freedom, and C is the value. The meta-analysis was conducted using OpenMEE for performance variables. To address potential publication bias from omitted studies, a fail-safe number (Nfs) was computed [18], with Nfs > 5N+10 indicating robustness, applying the smallest sample size from individual studies as N.
RESULTS
All literature data were incorporated into the data table, and the summarized data were subsequently entered into the data tabulation. Once all the data had been entered, descriptive statistics and various parameters from the database were used to generate Table 2.
Table 2.
Descriptive statistics of database
| Variables | NC | Mean | Min | Max | SD | ||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
||||||
| Un-wilting | Wilting | Un-wilting | Wilting | Un-wilting | Wilting | Un-wilting | Wilting | ||
| Chemical content | |||||||||
| Dry matter (% as fed) | 71 | 19.33 | 30.03 | 9.90 | 13.4 | 39.80 | 56.10 | 1.37 | 1.79 |
| Crude protein (% DM) | 55 | 14.65 | 14.46 | 7.25 | 6.75 | 23.02 | 22.03 | 1.55 | 1.51 |
| Water-soluble carbohydrate (% DM) | 51 | 3.38 | 5.04 | 0 | 0.02 | 12.20 | 21.80 | 0.74 | 0.75 |
| Neutral detergent fiber (% DM) | 34 | 49.42 | 50.41 | 19.20 | 25.20 | 70.1 | 70.29 | 2.50 | 2.55 |
| Acid detergent fiber (% DM) | 27 | 30.66 | 31.67 | 17.20 | 19.41 | 41.50 | 42.50 | 1.56 | 1.59 |
| Ammonia (% N) | 71 | 8.24 | 6.60 | 0.25 | 0.10 | 35.25 | 17.90 | 1.05 | 0.97 |
| Ash (% DM) | 15 | 9.44 | 9.37 | 2.26 | 2.12 | 16.55 | 15.90 | 0.55 | 0.39 |
| Dry matter losses (% DM) | 30 | 3.45 | 2.80 | 0.15 | 0 | 13.00 | 10.10 | 0.65 | 0.57 |
| pH and organic acid | |||||||||
| pH | 137 | 4.59 | 4.61 | 2.13 | 2.40 | 6.77 | 7.00 | 0.87 | 0.64 |
| Lactic acid (% DM) | 127 | 4.91 | 4.28 | 0 | 0 | 27.64 | 24.59 | 0.69 | 0.53 |
| Acetic acid (% DM) | 122 | 2.16 | 1.31 | 0 | 0 | 9.34 | 7.22 | 0.22 | 0.22 |
| Propionic acid (% DM) | 45 | 0.38 | 0.70 | 0 | 0 | 2.12 | 5.89 | 0.07 | 0.11 |
| Butyric acid (% DM) | 90 | 0.62 | 0.27 | 0 | 0 | 4.41 | 2.94 | 0.11 | 0.10 |
| Microbial population (Log cfu/g) | |||||||||
| Lactic acid bacteria | 15 | 6.86 | 7.10 | 4.58 | 5.00 | 8.32 | 8.56 | 0.49 | 0.49 |
| Yeast | 14 | 3.98 | 3.97 | 0 | 0 | 5.40 | 6.13 | 0.25 | 0.24 |
| Aerobic bacteria | 14 | 4.84 | 5.09 | 2.19 | 2.59 | 7.90 | 7.90 | 0.39 | 0.42 |
| Silage digestibility | |||||||||
| In vitro dry matter digestibility (% DM) | 18 | 52.56 | 50.56 | 6.4 | 6.6 | 79.50 | 77.00 | 4.12 | 3.88 |
SD, standard deviation; DM, dry matter.
Due to conflicting research findings and a small sample size, not all results could be considered reliable owing to publication bias. Briefly, the fail-safe number (Nfs) indicated which studies were suitable to be included in the final robust conclusions. This number expressed how many sample study sizes should be added to change the initial effect size into a negligible variable. If Nfs > 5N+10, where N was the study effect size used to calculate the initial effect size, then the result could be considered as the final robust conclusion [19].
The fail-safe number rules dictate that the robust parameters for assessing silage quality include DM, lactic acid, acetic acid, butyric acid, ammonia, water-soluble carbohydrates (WSC), and acid detergent fiber (ADF). On the other hand, propionic acid, crude protein (CP), pH, and neutral detergent fiber (NDF) are deemed unrobust. Additionally, bacterial populations in silage, such as lactic acid bacteria (LAB), yeast, and aerobic bacteria, as well as in vitro dry matter digestibility (IDMD), do not meet the criteria for robust parameters.
This meta-analysis study employed the Q statistics test, τ2, and I2 to examine heterogeneity. The Q statistic was the weighted sum of the squared values of each study’s effect size deviation from the mean effect size of all studies. The estimate of the population variable tau (τ) was the standard deviation of the overall effect size, and τ2 represents the variance of the overall effect size. The I2 index was a measure of the proportion of unexplained heterogeneity.
Based on the Heterogeneity Q statistics test, τ2, and I2, it was observed that some variables exhibited high heterogeneity, while others demonstrated low heterogeneity. Concerning the chemical content of the silage, all parameters displayed excess heterogeneity when Q was higher than the degree of freedom (Nc-1). IDMD and bacterial population of silage also showed high heterogeneity.
Heterogeneity was influenced by several factors, including the number of studies in the meta-analysis, the extent of variation in study effect sizes (between-studies variance), and the amount of variance in the observed effect size for each study (within-study variance). The heterogeneity of this study was high due to different types of forage, additional treatment, and storage time. Furthermore, the differences in wilting time processes influenced the quality of silage, thereby affecting heterogeneity.
The results of the meta-analysis are presented in Table 3, providing a comprehensive evaluation of nutrient content, fermentation quality, bacterial population, and digestibility of silage using Cohen’s methodology. Compared to non-wilted silage, wilting before ensiling significantly increased DM, WSC, NDF, and ADF (p<0.05). Wilting significantly reduced DM losses (p<0.05), as well as lactic acid, acetic acid, butyric acid, and ammonia content. Notably, the pH, CP, and ash content remained unchanged during the wilting process. Furthermore, a meta-analysis showed that the bacterial population of LAB, yeast, and aerobic bacteria, as well as IDMD of silage, were not significantly affected (p>0.05).
Table 3.
Meta-analysis on wilting effects on silage quality
| Variables | NC | Estimate | Lower bound | Upper bound | Std. error | p-value | τ2 | Q | Het. p-value | I2 |
|---|---|---|---|---|---|---|---|---|---|---|
| Chemical content | ||||||||||
| Dry matter | 71 | 4.48 | 3.63 | 5.33 | 0.43 | <0.001 | 8.76 | 523.42 | <0.001 | 86.63 |
| Crude protein | 55 | −0.19 | −0.53 | 0.14 | 0.17 | 0.27 | 0.79 | 113.58 | <0.001 | 52.46 |
| Water-soluble carbohydrate | 51 | 1.34 | 0.44 | 2.23 | 0.46 | 0.003 | 8.46 | 480.63 | <0.001 | 89.59 |
| Neutral detergent fiber | 34 | 0.43 | 0.01 | 0.85 | 0.21 | 0.04 | 0.68 | 60.79 | 0.002 | 45.72 |
| Acid detergent fiber | 27 | 0.93 | 0.38 | 1.48 | 0.28 | <0.001 | 1.17 | 61.20 | <0.001 | 57.52 |
| Ammonia | 71 | −1.16 | −1.69 | −0.63 | 0.27 | <0.001 | 3.87 | 418.48 | <0.001 | 83.27 |
| Ash | 15 | 0.87 | −0.52 | 2.27 | 0.71 | 0.22 | 5.34 | 127.62 | <0.001 | 89.03 |
| Dry matter losses | 30 | −1.59 | −2.61 | −0.58 | 0.52 | 0.002 | 5.82 | 164.96 | <0.001 | 82.42 |
| pH and organic acid | ||||||||||
| pH | 138 | 0.08 | −0.36 | 0.53 | 0.23 | 0.71 | 4.78 | 805.02 | <0.001 | 83.23 |
| Lactic acid | 127 | −0.83 | −1.29 | −0.37 | 0.24 | <0.001 | 4.79 | 746.46 | <0.001 | 83.25 |
| Acetic acid | 122 | −2.37 | −2.90 | −1.85 | 0.27 | <0.001 | 6.19 | 687.13 | <0.001 | 82.39 |
| Propionic acid | 45 | −0.38 | −1.06 | 0.29 | 0.34 | 0.26 | 3.68 | 206.27 | <0.001 | 78.67 |
| Butyric acid | 90 | −2.46 | −3.14 | −1.79 | 0.34 | <0.001 | 7.10 | 653.99 | <0.001 | 87.00 |
| Microbial population | ||||||||||
| Lactic acid bacteria | 15 | 0.40 | −0.26 | 1.06 | 0.34 | 0.23 | 0.89 | 29.75 | 0.008 | 52.95 |
| Yeast | 14 | 0.12 | −1.14 | 1.38 | 0.64 | 0.85 | 3.97 | 56.60 | <0.001 | 78.80 |
| Aerobic bacteria | 14 | 0.78 | −0.02 | 1.58 | 0.41 | 0.06 | 1.25 | 29.18 | 0.004 | 58.88 |
| Silage digestibility | ||||||||||
| In vitro dry matter digestibility | 18 | −0.06 | −0.59 | 0.48 | 0.28 | 0.84 | 0.52 | 27.85 | 0.047 | 38.95 |
DISCUSSION
Effects of wilting on dry matter and water-soluble carbohydrates content
Moisture content stands out as a crucial factor influencing silage quality. Excessively wet silage may result in poor fermentation and spoilage, while overly dry silage can lead to inadequate packing and diminished nutritional value. The optimal moisture content for silage falls within the range of 60% to 65% [20].
Dry matter content serves as a more precise indicator of forage moisture levels and is the recommended metric to ensure the correct moisture level for effective silage fermentation and preservation. Digestible energy intake is estimated from DM intake by ruminants, and energy digestibility is obtained from ruminants fed at maintenance levels. Wilting brings about proportional increases in silage DM. Meta-analysis findings indicate that wilting significantly (p<0.05) influences forage silage by elevating DM content and reducing DM loss. This outcome is consistent with numerous experimental studies [21,22]. The increase in DM value results from a lesser decrease in DM losses within the silage. According to Borreani et al [1], the wilting process significantly reduces DM loss, especially in leaves, and is directly associated with the initial DM content of the forage during treatment and the severity of its condition.
The ensiling process is initiated by LAB during fermentation, utilizing water-soluble WSC as energy and carbon sources. Therefore, WSC is crucial for achieving well-preserved silages, with a recommended concentration level of 60 to 70 g/kg of DM [23]. Increasing WSC concentration can enhance fermentation efficiency, promoting faster forage preservation with minimal acid or inoculant use, which can lead to cost savings in silage production.
The meta-analysis reveals that the wilting process significantly increases (p<0.05) WSC content in silage. This finding aligns with the experimental study by Zhang et al [23]. However, it is essential to note that certain researchers have reported no significant impact of wilting on WSC levels [24]. The rise in WSC content can be attributed to the higher concentration of DM. During wilting, the concentration of carbohydrates, including WSC, increases as the forage loses moisture. This elevated concentration results in a higher quantity of WSC relative to the overall DM content of the silage. This finding corresponds with the research by Yahaya et al [25], which indicates that silage with higher DM content contains higher WSC compared to silage with medium and lower DM. Additionally, wilting slows down the respiration of plant cells, leading to reduced carbohydrate consumption. By decreasing respiration, more carbohydrates, including WSC, are preserved in the forage, contributing to higher WSC levels in the silage [1].
Effects of wilting on pH value and organic acid of silage
The pH value serves as a crucial indicator of silage quality [26]. During the ensiling process, the pH of forage is reduced to a level that inhibits the proliferation of undesirable bacteria, including clostridia, enterobacteria, yeasts, and molds. Conversely, wilting involves elevating the initial DM content of silage to a level that effectively hinders the growth of harmful bacteria, such as clostridia [20].
The meta-analysis results indicated that wilting and non-wilting treatments had no significant impact on pH reduction. This finding aligns with the report by Hao et al [27], who observed that neither wilting nor additive addition to silage affected pH value. The pH value in silage is influenced by microbial heterogeneity, which can alter the lactic acid to acetic acid ratio [28]. Other organic acids, such as propionic acid and butyric acid, also influence pH value. Microbial heterogeneity in silage is further influenced by the type and maturity of the forage, as well as the temperature at ensiling. This variation in silage material and environmental conditions affects microbial populations, which in turn impacts the fermentation process and the resulting pH value [29].
Wilting contributes to the enhancement of silage fermentation quality, particularly by impacting the lactic acid content, which can undergo degradation into other components [30]. This aligns with the findings of the meta-analysis, revealing a decrease in lactic acid levels, accompanied by reductions in acetic-propionic and butyric acids. The wilting process diminishes the overall activity of microorganisms in the silage [31], with a notable decline in clostridium bacteria, as evidenced by a significant reduction in butyric acid levels. This observation is in line with the report by Cole Diepersloot et al [21], which emphasizes that non-wilted forage fermentation yields low butyric acid levels, further emphasizing how wilting can effectively decrease butyric acid content. Low butyric acid levels indicate that silage can be considered high-quality even if lactic acid levels are relatively low and the pH is relatively high, as this suggests effective nutrient preservation [32].
Effects of wilting on crude protein and ammonia content
While this meta-analysis revealed a slight decrease in CP content in wilted silage compared to non-wilted silage, the difference was not statistically significant (p>0.05). This inconsistency in findings across studies could be attributed to varying conclusions in the research. For example, Kim et al [5] reported lower CP content in wilted silage, while Yahaya et al [25] found higher CP content. Additionally, studies by Hao et al [27] and Wang et al [33] observed both increased and decreased CP levels in treated silages. The potential reduction in CP content due to wilting could be linked to the continued activity of respiration enzymes in surviving plant cells after harvesting, albeit at a reduced level, as evidenced by the nonsignificant decrease in CP observed with wilting.
These findings support the meta-analysis result showing lower ammonia content (p<0.05) in wilted silage compared to unwilted silage, indicating reduced proteolysis activity. The main purpose of wilting before ensiling is to increase DM content, thereby reducing respiration activity and preserving nutrients like CP, which can then be used by animals during feeding. The positive effect of wilting on reducing proteolysis activity has been well-documented by many researchers including [6,23,34,35].
Effects of wilting on NDF and ADF content
Based on the results of the meta-analysis, wilting in silage leads to a significant increase (p<0.05) in NDF and ADF contents. This finding aligns with the observations made by Reppeto et al [36], who noted a substantial rise in NDF and ADF contents after 8 hours and 10 days of ensiling forage. The significance of this meta-analysis study lies in the variations reported by different researchers. Contrary to these results, Cole Diepersloot et al [21] found no effects on NDF after 30 days of storage, but observed lower levels of NDF for wilting silage after 90 days of storage. Moreover, some studies, such as Zhang et al [23] and Herrmann et al [37], reported decreases in NDF during fermentation, possibly due to solubilization by acid components affecting certain NDF fractions, and this may be linked to the degradation occurring in the wilting process [23]. Despite these differences, it is generally accepted that storage has minimal effects on NDF [38].
Effects of wilting on silage digestibility
Silage digestibility stands as a critical parameter in determining overall silage quality. The meta-analysis results suggest that wilting treatment does not have a significant impact on IVDMD of silage. This observation aligns with the primary objective of ensiling, which aims to minimize nutrient loss and preserve forage digestibility [39]. The wilting process involves a gradual reduction of moisture, with the goal being not to alter the nutritional content but rather to preserve it as effectively as possible, maintaining the digestible material. This approach differs from rapid drying methods which can lead to diminished nutrient levels and reduced digestibility across various components [40].
Effects of wilting on silage bacteria
The present meta-analysis indicated no significant influence on microbial populations in silage, including LAB, yeast, and aerobic bacteria (p>0.05). Nonetheless, the expectation for wilting silage was to have a positive effect on bacterial populations, especially in reducing unwanted clostridial populations. This anticipation is due to the diminished oxygen availability resulting from reduced water content in wilted silage, which should inhibit their growth [41]. Different studies have reported varied impacts on the LAB population in wilted silages. For instance, Wan et al [8] observed an increase, whereas Tao et al [4] noted a decrease, and Liu et al [3] recorded both increases and decreases in their respective studies. The unaltered LAB population compared to non-wilted silage might be attributed to the absence of added inoculants. Augmenting the LAB population in silage can be accomplished by including LAB inoculants [33]. According to Kung et al [32], reducing the risk of undesirable bacteria growth can be achieved by ensiling forages at a DM content above 30% to 35%. This is due to the higher DM content, which diminishes the available moisture for bacterial growth and fosters an acidic environment less favorable for harmful bacteria. Additionally, wilting before ensiling further reduces forage moisture, making the environment less conducive to bacterial proliferation [5]. The suppression of bacterial growth due to the wilting process might be indicated by the decrease in lactic acid levels, accompanied by reductions in acetic-propionic and butyric acids, as shown in the current meta-analysis results.
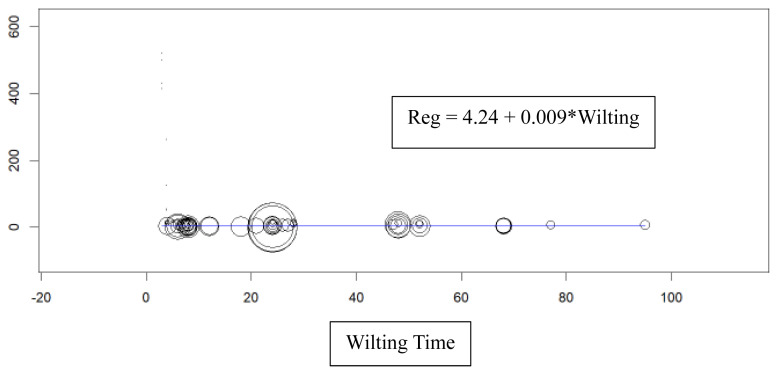
Regression of dry matter and wilting time
The wilting treatment process positively influences the DM content of forage intended for silage, as demonstrated by the regression equation: DM = 4.24+0.009×Wilting (Figure 2). A more extended wilting duration correlates with a higher DM value. It is crucial to achieve fast wilting with a shorter duration in the field to minimize DM loss [1]. Nevertheless, extended exposure of harvested forage to sunlight may negatively impact the quality of silage as it promotes the growth of undesirable microorganisms. Prolonged wilting durations could also undermine the aerobic stability and nutritional value of silages [42,43].
Figure 2.
Regression of dry matter and wilting time.
CONCLUSION
The present study found that pre-ensiling wilting of forage had a dual impact on chemical composition and silage quality. The process significantly increased DM, WSC, NDF, and ADF, while reducing DM losses, lactic acid, acetic acid, butyric acid, and ammonia. Notably, pH, CP, and ash content remained unchanged during wilting. Additionally, a meta-analysis showed that LAB, yeast, and aerobic bacteria populations, as well as IDMD, were not significantly affected.
Footnotes
CONFLICT OF INTEREST
We certify that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript.
FUNDING
The authors received no financial support for this article.
REFERENCES
- 1.Borreani G, Tabacco E, Schmidt RJ, Holmes BJ, Muck RE. Silage review: factors affecting dry matter and quality losses in silages. J Dairy Sci. 2018;101:3952–79. doi: 10.3168/jds.2017-13837. [DOI] [PubMed] [Google Scholar]
- 2.Ribas WFG, Monção FP, Júnior VRR, et al. Effect of wilting time and enzymatic-bacterial inoculant on the fermentative profile, aerobic stability and nutritional value of BRS capiaçu grass silage. R Bras Zootec. 2021;50:e20200207. doi: 10.37496/rbz5020200207. [DOI] [Google Scholar]
- 3.Liu Q, Zhang J, Shi S, Sun Q. The effects of wilting and storage temperatures on the fermentation quality and aerobic stability of stylo silage. Anim Sci J. 2011;82:549–53. doi: 10.1111/j.1740-0929.2011.00873.x. [DOI] [PubMed] [Google Scholar]
- 4.Tao L, Zhou H, Zhang N, et al. Effects of different source additives and wilt conditions on the pH value, aerobic stability, and carbohydrate and protein fractions of alfalfa silage. Anim Sci J. 2017;88:99–106. doi: 10.1111/asj.12599. [DOI] [PubMed] [Google Scholar]
- 5.Kim JG, Chung ES, Seo S, Ham JS, Kang WS, Kim DA. Effects of maturity at harvest and wilting days on quality of round baled rye silage. Asian-Australas J Anim Sci. 2001;14:1233–7. doi: 10.5713/ajas.2001.1233. [DOI] [Google Scholar]
- 6.Nishino N, Li Y, Wang C, Parvin S. Effects of wilting and molasses addition on fermentation and bacterial community in guinea grass silage. Lett Appl Microbiol. 2012;54:175–81. doi: 10.1111/j.1472-765X.2011.03191.x. [DOI] [PubMed] [Google Scholar]
- 7.Zheng M, Niu D, Zuo S, Mao P, Meng L, Xu C. The effect of cultivar, wilting and storage period on fermentation and the clostridial community of alfalfa silage. Ital J Anim Sci. 2018;17:336–46. doi: 10.1080/1828051X.2017.1364984. [DOI] [Google Scholar]
- 8.Wan JC, Xie KY, Wang YX, Liu L, Yu Z, Wang B. Effects of wilting and additives on the ensiling quality and in vitro rumen fermentation characteristics of sudangrass silage. Anim Biosci. 2021;34:56–65. doi: 10.5713/ajas.20.0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ertekin I, Atis I, Aygun YZ, Yilmaz S, Kizilsimsek M. Effects of different nitrogen doses and cultivars on fermentation quality and nutritive value of Italian ryegrass (Lolium multiflorum Lam.) silages. Anim Biosci. 2022;35:39–46. doi: 10.5713/ab.21.0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ridla M, Uchida S. Comparative study on the effects of combined treatments of lactic acid bacteria and cellulases on the fermentation characteristic and chemical composition of rhodesgrass (Chloris gayana Kunth.) and Italian ryegrass (Lolium multiflorum Lam.) silages. Asian-Australas J Anim Sci. 1999;12:525–30. doi: 10.5713/ajas.1999.525. [DOI] [Google Scholar]
- 11.Cheung MWL, Vijayakumar R. A guide to conducting a meta-analysis. Neuropsychol Rev. 2016;26:121–8. doi: 10.1007/s11065-016-9319-z. [DOI] [PubMed] [Google Scholar]
- 12.Papakostidis C, Giannoudis PV. Meta-analysis: what have we learned? Injury. 2023;54(Suppl 3):S30–4. doi: 10.1016/j.injury.2022.06.012. [DOI] [PubMed] [Google Scholar]
- 13.Haidich AB. Meta-analysis in medical research. Hippokratia. 2010;14(Suppl 1):29–37. [PMC free article] [PubMed] [Google Scholar]
- 14.Leung L. Validity, reliability, and generalizability in qualitative research. J Family Med Prim Care. 2015;4:324–7. doi: 10.4103/2249-4863.161306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Risyahadi ST, Martin RSH, Qomariyah N, Suryahadi S, Sukria HA, Jayanegara A. Effects of dietary extrusion on rumen fermentation, nutrient digestibility, performance and milk composition of dairy cattle: a meta-analysis. Anim Biosci. 2023;36:1546–57. doi: 10.5713/ab.23.0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheung MWL, Vijayakumar R. A guide to conducting a meta-analysis. Neuropsychol Rev. 2016;26:121–8. doi: 10.1007/s11065-016-9319-z. [DOI] [PubMed] [Google Scholar]
- 17.Sánchez-Meca J, Marín-Martínez F. Meta-analysis in psychological research. Int J Psychol Res. 2010;3:150–62. [Google Scholar]
- 18.Rosenthal R. The file drawer problem and tolerance for null results. Psychol Bull. 1979;86:638–41. doi: 10.1037/0033-2909.86.3.638. [DOI] [Google Scholar]
- 19.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 20.McDonald P, Henderson AR, Heron SJE. The biochemistry of silage. 2nd ed. Marlow, Bucks, UK: Chalcombe Publications; 1991. [DOI] [Google Scholar]
- 21.Cole Diepersloot E, Pupo MR, Ghizzi LG, Heinzen C, Ferraretto LF. Effect of wilting and microbial inoculation on the fermentation profile, nutrient composition, and aerobic stability of Bermuda grass silage. Anim Feed Sci Technol. 2022;290:115376. doi: 10.1016/j.anifeedsci.2022.115376. [DOI] [Google Scholar]
- 22.Dawson LER, Ferris CP, Steen RWJ, Gordon FJ, Kilpatrick DJ. The effects of wilting grass before ensiling on silage intake. Grass Forage Sci. 1999;54:237–47. doi: 10.1046/j.1365-2494.1999.00176.x. [DOI] [Google Scholar]
- 23.Zhang YC, Wang XK, Li DX, Lin YL, Yang FY, Ni KK. Impact of wilting and additives on fermentation quality and carbohydrate composition of mulberry silage. Asian-Australas J Anim Sci. 2020;33:254–63. doi: 10.5713/ajas.18.0925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wilkinson JM. A laboratory evaluation of comfrey (Symphytum officinale L.) as a forage crop for ensilage. Anim Feed Sci Technol. 2003;104:227–33. doi: 10.1016/S0377-8401(02)00293-6. [DOI] [Google Scholar]
- 25.Yahaya MS, Kawai M, Takahashi J, Matsuoka S. The effect of different moisture contents at ensiling on silo degradation and digestibility of structural carbohydrates of orchardgrass. Anim Feed Sci Technol. 2002;101:127–33. doi: 10.1016/S0377-8401(02)00080-9. [DOI] [Google Scholar]
- 26.Tavares VB, Pinto JC, Evangelista AR, Figueiredo HCP, Ávila CLS, de Lima RF. Effects of different compaction degrees, inclusion of absorbent additive and wilting on the chemical composition of tanzania grass silages. R Bras Zootec. 2009;38:40–9. doi: 10.1590/S1516-35982009000100006. [DOI] [Google Scholar]
- 27.Hao J, Sun WT, Wu CR, et al. Fermentation quality, bacterial community, and aerobic stability of perennial recut Broussonetia papyrifera silage with different additives and wilting time. Fermentation. 2022;8:262. doi: 10.3390/fermentation8060262. [DOI] [Google Scholar]
- 28.Chen R, Li M, Yang J, et al. Exploring the effect of wilting on fermentation profiles and microbial community structure during ensiling and air exposure of king grass silage. Front Microbiol. 2022;13:971426. doi: 10.3389/fmicb.2022.971426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li D, Ni K, Zhang Y, Lin Y, Yang F. Fermentation characteristics, chemical composition and microbial community of tropical forage silage under different temperatures. Asian-Australas J Anim Sci. 2019;32:665–74. doi: 10.5713/ajas.18.0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen L, Guo G, Yuan X, Zhang J, Li J, Shao T. Effects of applying molasses, lactic acid bacteria and propionic acid on fermentation quality, aerobic stability and in vitro gas production of total mixed ration silage prepared with oat-common vetch intercrop on the Tibetan Plateau. J Sci Food Agric. 2016;96:1678–85. doi: 10.1002/jsfa.7271. [DOI] [PubMed] [Google Scholar]
- 31.Santos MC, Kung L. Short communication: the effects of dry matter and length of storage on the composition and nutritive value of alfalfa silage. J Dairy Sci. 2016;99:5466–9. doi: 10.3168/jds.2016-10866. [DOI] [PubMed] [Google Scholar]
- 32.Kung L, Shaver RD, Grant RJ, Schmidt RJ. Silage review: interpretation of chemical, microbial, and organoleptic components of silages. J Dairy Sci. 2018;101:4020–33. doi: 10.3168/jds.2017-13909. [DOI] [PubMed] [Google Scholar]
- 33.Wang Y, He L, Xing Y, et al. Dynamics of bacterial community and fermentation quality during ensiling of wilted and unwilted Moringa oleifera leaf silage with or without lactic acid bacterial inoculants. mSphere. 2019;4:e00341–19. doi: 10.1128/msphere.00341-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gomes ALM, Jacovaci FA, Bolson DC, Nussio LG, Jobim CC, Daniel JLP. Effects of light wilting and heterolactic inoculant on the formation of volatile organic compounds, fermentative losses and aerobic stability of oat silage. Anim Feed Sci Technol. 2019;247:194–8. doi: 10.1016/j.anifeedsci.2018.11.016. [DOI] [Google Scholar]
- 35.McEniry J, Forristal PD, O’Kiely P. Factors influencing the conservation characteristics of baled and precision-chop grass silages. Irish J Agric Food Res. 2011;50:175–88. [Google Scholar]
- 36.Reppeto JL, Cajarville C, D’Alessandro J, Curbelo A, Soto C, Garin D. Effect of wilting and ensiling on ruminal degradability of temperate grass and legume mixtures. Anim Res. 2005;54:73–80. doi: 10.1051/animres:2005007. [DOI] [Google Scholar]
- 37.Herrmann C, Heiermann M, Idler C. Effects of ensiling, silage additives and storage period on methane formation of biogas crops. Bioresour Technol. 2011;102:5153–61. doi: 10.1016/j.biortech.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 38.Der Bedrosian MC, Nestor KE, Kung L. The effects of hybrid, maturity, and length of storage on the composition and nutritive value of corn silage. J Dairy Sci. 2012;95:5115–26. doi: 10.3168/jds.2011-4833. [DOI] [PubMed] [Google Scholar]
- 39.Grant RJ, Ferraretto LF. Silage review: silage feeding management: silage characteristics and dairy cow feeding behavior. J Dairy Sci. 2018;101:4111–21. doi: 10.3168/jds.2017-13729. [DOI] [PubMed] [Google Scholar]
- 40.Lyimo BJ, Mtengeti EJ, Urio NA, Demanisho EN. Effect of wilting, chopping length and different levels of maize bran on grass silage quality. Livest Res Rural Dev. 2018;30:112. [Google Scholar]
- 41.Yang F, Wang Y, Zhao S, Wang Y. Lactobacillus plantarum inoculants delay spoilage of high moisture alfalfa silages by regulating bacterial community composition. Front Microbiol. 2020;11:1989. doi: 10.3389/fmicb.2020.01989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brüning D, Gerlach K, Weiß K, Südekum KH. Effect of compaction, delayed sealing, and aerobic exposure on maize silage quality and on the formation of volatile organic compounds. Grass Forage Sci. 2018;73:53–66. doi: 10.1111/gfs.12288. [DOI] [Google Scholar]
- 43.Wilkinson JM, Davies DR. The aerobic stability of silage: key findings and recent developments. Grass Forage Sci. 2013;68:1–19. doi: 10.1111/j.1365-2494.2012.00891.x. [DOI] [Google Scholar]
- 44.Gomes RS, Almeida JCC, Carneiro JC, et al. Impacts of citrus pulp addition and wilting on elephant grass silage quality. Biosci J. 2017;33:675–84. doi: 10.14393/BJ-v33n3-33790. [DOI] [Google Scholar]
- 45.McEniry J, Allen E, Murphy JD, O’Kiely P. Grass for biogas production: the impact of silage fermentation characteristics on methane yield in two contrasting biomethane potential test systems. Renew Energy. 2014;63:524–30. doi: 10.1016/j.renene.2013.09.052. [DOI] [Google Scholar]
- 46.Dewhurst RJ, King PJ. Effects of extended wilting, shading and chemical additives on the fatty acids in laboratory grass silages. Grass Forage Sci. 1998;53:219–24. doi: 10.1046/j.1365-2494.1998.00130.x. [DOI] [Google Scholar]
- 47.Mangan JL, Harrison FA, Vetter RL. Immunoreactive fraction 1 leaf protein and dry matter content during wilting and ensiling of ryegrass and alfalfa. J Dairy Sci. 1991;74:2186–99. doi: 10.3168/jds.S0022-0302(91)78392-6. [DOI] [PubMed] [Google Scholar]
- 48.Haigh PM. The effect of wilting and silage additives on the fermentation of autumn made grass silage ensiled in bunkers on commercial farms in South Wales 1983–85. Grass Forage Sci. 1988;43:337–45. doi: 10.1111/j.1365-2494.1988.tb02159.x. [DOI] [Google Scholar]
- 49.Anderson R. The effect of extended moist wilting and formic acid additive on the conservation as silage of two grasses differing in total nitrogen content. J Sci Food Agric. 1983;34:808–18. doi: 10.1002/jsfa.2740340808. [DOI] [Google Scholar]
- 50.Franco RT, Buffière P, Bayard R. Optimizing storage of a catch crop before biogas production: impact of ensiling and wilting under unsuitable weather conditions. Biomass Bioenergy. 2017;100:84–91. doi: 10.1016/j.biombioe.2017.03.017. [DOI] [Google Scholar]
- 51.Narasimhalu P, Teller E, Vanbelle M, Foulon M, Dasnoy F. Apparent digestibility of nitrogen in rumen and whole tract of friesian cattle fed direct-cut and wilted grass silages. J Dairy Sci. 1989;72:2055–61. doi: 10.3168/jds.S0022-0302(89)79329-2. [DOI] [PubMed] [Google Scholar]
- 52.Kamra DN, Singh R, Jakhmola RC, Srivatsa RVN. Effect of wilting and the additive straw, molasses and urea on the fermentation pattern of maize silage. Anim Feed Sci Technol. 1983;9:185–96. doi: 10.1016/0377-8401(83)90033-0. [DOI] [Google Scholar]
- 53.Liu QH, Wu JX, Dong ZH, Wang SR, Shao T. Effects of overnight wilting and additives on the fatty acid profile, α-tocopherol and β-carotene of whole plant oat silages. Anim Feed Sci Technol. 2020;260:114370. doi: 10.1016/j.anifeedsci.2019.114370. [DOI] [Google Scholar]
- 54.Weinberg ZG, Khanal P, Yildiz C, Chen Y, Arieli A. Effects of stage of maturity at harvest, wilting and LAB inoculant on aerobic stability of wheat silages. Anim Feed Sci Technol. 2010;158:29–35. doi: 10.1016/j.anifeedsci.2010.03.006. [DOI] [Google Scholar]
- 55.Valente ME, Borreani G, Caredda S, Cavallarin L, Sulas L. Ensiling forage garland (Chrysanthemum coronarium L.) at two stages of maturity and at different wilting levels. Anim Feed Sci Technol. 2003;108:181–90. doi: 10.1016/S0377-8401(03)00123-8. [DOI] [Google Scholar]
- 56.Williams CC, Froetschel MA, Ely LO, Amos HE. Effects of inoculation and wilting on the preservation and utilization of wheat forage. J Dairy Sci. 1995;78:1755–65. doi: 10.3168/jds.S0022-0302(95)76801-1. [DOI] [PubMed] [Google Scholar]
- 57.Zou SY, Chen SK, Tang QY, et al. Effects of silage additives on quality and in vitro rumen fermentation characteristics of first season ratoon rice whole silage. Cao Ye Xue Bao. 2021;30:122–32. [Google Scholar]
- 58.Oliveira AC, Garcia R, Pires AJV, et al. Elephant grass silages with or without wilting, with cassava meal in silage production. Rev Bras Saúde Prod Anim. 2017;18:417–29. doi: 10.1590/S1519-99402017000300002. [DOI] [Google Scholar]
- 59.Haigh PM, Parker JWG. Effect of silage additives and wilting on silage fermentation, digestibility and intake, and on live weight change of young cattle. Grass Forage Sci. 1985;40:429–36. doi: 10.1111/j.1365-2494.1985.tb01774.x. [DOI] [Google Scholar]
- 60.Hartinger T, Gresner N, Südekum KH. Effect of wilting intensity, dry matter content and sugar addition on nitrogen fractions in lucerne silages. Agriculture. 2019;9:11. doi: 10.3390/agriculture9010011. [DOI] [Google Scholar]
- 61.Nishino N, Touno E. Ensiling characteristics and aerobic stability of direct-cut and wilted grass silages inoculated with Lactobacillus casei or Lactobacillus buchneri. J Sci Food Agric. 2005;85:1882–8. doi: 10.1002/jsfa.2189. [DOI] [Google Scholar]
- 62.Wahyono T, Sasongko WT, Indriatama WM, et al. Influence of different variety and wilting treatment on the nutritive value of whole plant sorghum silage. Adv Anim Vet Sci. 2022;10:1649–58. doi: 10.17582/journal.aavs/2022/10.7.1649.1658. [DOI] [Google Scholar]
- 63.Gordon FJ, Dawson LER, Ferris CP, Steen RWJ, Kilpatrick DJ. The influence of wilting and forage additive type on the energy utilisation of grass silage by growing cattle. Anim Feed Sci Technol. 1999;79:15–27. doi: 10.1016/S0377-8401(99)00013-9. [DOI] [Google Scholar]
- 64.Rondahl T, Bertilsson J, Martinsson K. Effects of maturity stage, wilting and acid treatment on crude protein fractions and chemical composition of whole crop pea silages (Pisum sativum L.) Anim Feed Sci Technol. 2011;163:11–9. doi: 10.1016/j.anifeedsci.2010.09.017. [DOI] [Google Scholar]
- 65.Cavallarin L, Antoniazzi S, Borreani G, Tabacco E. Effects of wilting and mechanical conditioning on proteolysis in sainfoin (Onobrychis viciifolia Scop) wilted herbage and silage. J Sci Food Agric. 2005;85:831–8. doi: 10.1002/jsfa.2022. [DOI] [Google Scholar]