Abstract
Males and females in sexually dimorphic species show differences in their physiology and behaviour due to differences in energetic investment into reproduction and soma. This means that the two sexes may show different patterns of parasitism at different times of the year. In this study, we evaluate the abundance of fecal eggs and larvae of 5 parasite types (Strongyles, Nematodirus spp., Marshallagia marshalli., Protostrongylus spp. lungworms, and Eimeria spp.) in relation to season and sex in Rocky Mountain bighorn sheep (Ovis canadensis). We use fecal egg counts (FEC) as a proxy for infection intensity. Parasite FECs differed between male and female bighorn sheep and varied with season. We found pronounced fluctuations in fecal egg counts of various parasite species in males and females across different seasons and reproductive stages. Strongyle counts were significantly higher during late gestation and lactation/summer, and particularly pronounced in males. Nematodirus counts were highest during late gestation in females and during the rut in males. Marshallagia counts peaked during late gestation in females and during the rut in males. Protostrongylus spp. lungworm counts were highest during late gestation in females and in males during lactation/summer and the rut. Eimeria oocyst counts varied across seasons, with higher counts in males during the rut and in females during winter and late gestation. Additionally, significant differences in Strongyle counts were observed between coursing and tending rams, with tending rams exhibiting higher counts. We discuss why the sexes might differ in FECs and suggest that differences between FECs of the parasites across seasons may be due to different life cycles and cold tolerance of the parasites themselves.
Keywords: Parasite egg counts, Seasonality, Sex differences, Reproductive schedules, Bighorn sheep
Graphical abstract
Highlights
-
•
Strong seasonal and sex differences in fecal parasite egg counts of bighorn sheep.
-
•
Nematodirus and Marshallagia counts peaked during late gestation in females and rut in males.
-
•
Protostrongylus spp. lungworm counts highest during late gestation in females and summer/rut in males.
-
•
Eimeria oocyst counts varied, with higher counts in males during rut and females in winter/late gestation.
-
•
Significant Strongyle count differences between coursing and tending rams, with higher counts in tending rams.
1. Introduction
Traditional disease models assume random host contacts, yet parasite intensity often adheres to the 80/20 rule, with a small portion of the population driving most infections (Woolhouse et al., 1997; Poulin 1996a, 1996b), suggesting non-random disease spread (Ferrari et al., 2010’ Salih et al., 1979; McKenna 1981; Altizer and Oberhauser 1999). Seasonality is thought to be one of the factors that contribute to individual differences in parasite infection intensity (Altizer et al., 2006), via host-specific life history trade-offs (Stearns 1992). Seasonal variations in parasite infection are common and can result from 1) environmental factors, like temperature and precipitation (Nelson and Demas 1996; Barber et al., 2016), 2) parasite biology and their adaptation to different conditions (Merilä et al., 1995), 3) host biology, including events like birthing and lactation in females (Turner et al., 2012), and reproductive effort in males (Ezenwa et al., 2012) or 4) host immunity and sex (Poulin 1996b).
For instance, in red deer (Cervus elaphus), the intensity of gastrointestinal helminths (Fasciola hepatica, Elaphostrongylus cervi, and Strongyles) peaked in spring and summer but decreased in winter (Albery et al., 2018). Similarly, springbok (Antidorcas marsupialis) in Namibia exhibited higher infection intensity of Eimeria spp., helminth strongyles, and Strongyloides during wetter seasons compared to drier seasons (Cizauskas et al., 2015). Furthermore, previous exposure, age, sociality, and sex, influence parasite infection intensity and seasonality (Ferrari et al., 2010; Patterson and Ruckstuhl 2013; Wesołowska, 2022). Male hosts typically exhibit higher parasite prevalence than females of the same species (Poulin 1996b; Schmid-Hempel, 2021).
For instance, arctic foxes (Vulpes lagopus) show sex-biased dietary preferences, with females favouring birds and males consuming rodents (Friesen et al., 2015). Male arctic foxes consequently harbour more cestodes (Taenia spp. and Echinococcus multilocularis), which are reliant on rodents as intermediate hosts. Similarly, behavioural differences can drive parasite prevalence. For example, bachelor Grant’s gazelles (Nanger granti) exhibit lower helminth parasite infection and reduced territorial behaviour compared to reproductively active males (Ezenwa and Snider 2016). Certain rodent species (Apodemus agrarius, Apodemus flavicollis, and Myodes glareolus) display male-biased flea infestations, possibly due to larger male territories (Kowalski et al., 2015). European bat species (Spinturnix spp.) exhibit female-biased mite parasitism, attributed to maternity roost crowding (Christe et al., 2007), illustrating behaviour-driven exposure in a segment of the population.
Physiological disparities, like sex hormones, also affect parasite infection (Klein 2004; Zuk and McKean 1996). Testosterone, for instance, may have immunosuppressive effects, as evidenced by higher ectoparasite abundance in testosterone-injected male barn swallows (Hirundo rustica) and increased nematode infection in testosterone-treated male red grouse (Lagopus lagopus scotica) (Saino et al., 1995; Mougeot et al., 2006). Iteroparous females investing in reproduction would, on the other hand, trade off their energy between immune function and body condition versus reproductive costs during gestation, birthing, and lactation; i.e., shown for Trichostrongylus retortaeformis in rabbits Oryctolagus cuniculus (Cattadori et al., 2005), or Dall’s sheep, Ovis dalli dalli, for whom infection intensity with Mashallagia marshalli was significantly higher in pregnant than yield ewes and those in poorer body condition (Aleuy et al., 2018). Sex hormones, often linked to reproductive states, can lead to differences in sex-specific immunity and susceptibility to parasites (i.e., summary in Schmid-Hempel, 2021). However, the interactions between testosterone, corticosterone, behaviour and parasite prevalence are complex. For example, in Grant’s gazelles (Nanger granti)), the relationship between parasites and host testosterone levels varied depending on the parasite but were also mediated by changes in behaviour (Ezenwa et al., 2012).
Few studies have explored the relationship between host sex, reproductive status and seasonal parasite infection variation in marked individuals over multiple seasons and years, despite established roles of host sex in parasite prevalence and transmission (i.e., Ferrari et al., 2010; Hayward et al., 2022; Sweeny et al., 2022). Here, we investigate the pattern of parasite abundance of five parasites in male and female bighorn sheep; Strongyles, Nematodirus spp., Marshallagia marshalli, Protostrongylus spp., and Eimeria. We compare the parasite abundance (via fecal egg counts; FECs) of individual bighorn sheep throughout different seasons over a total of 29 months. We hypothesize that parasite FECs will differ between seasons, due to differences in parasite and host biology. We also hypothesize that the pattern of parasite abundance will be different between males and females, due to differences in their reproductive schedules. Because winter is the coldest period of the year and includes the pre-rut, rut, and early gestation, we expect to see higher levels of fecal egg counts in males due to energetic demands of mating and hypophagia (Pelletier and Festa-Bianchet 2006). On the other hand, late spring and summer include energetically demanding late gestation and early lactation for females. Therefore, we expect fecal egg outputs to be higher for females, compared to males in the late gestation and lactation period, but higher for males than females during the rutting season and winter. Lastly, we predict that fecal egg counts will be affected by age, with younger animals and very old animals having the highest counts, because of naivety or senescence in their respective immune responses. A study on wild Soay sheep (Ovis aries), for example, has shown a clear decline in circulating anti-bodies against a highly prevalent nematode (Teladorsagia circumcincta, a strongyle nematode), linked to low survival (Froy et al., 2019).
2. Methods
2.1. Study area and animals
The study was conducted in Sheep River Provincial Park, in southwestern Alberta, Canada (50.63° N, 114.38° W) on a population of Rocky Mountain bighorn sheep. At the time of the study, the sheep population included approximately 35 resident rams (>2 years of age) and 80+ ewes. Lambs are caught a few months after birth (September to November) and are individually marked with coloured and numbered ear tags (Festa-Bianchet 1988). Thus, over 90% of sheep were individually marked. Temperatures in the study area range from −30 to +30 °C with average temperatures in July around 16°. Elevations in the park vary between 1400 and 1600 m above sea level.
2.2. Fecal collection
Fecal samples were collected from focal male and female bighorn sheep, aged 1–18 (oldest male 14), within the park from May 2016 to November 2018 for a total of 29 months; except for males in December, due to rut-induced hypophagia (Pelletier and Festa-Bianchet 2006) that almost halts fecal production in males. We were also unable to collect any fecal samples from females between June and September as they spent these months in the alpine areas, outside their winter range. Individual male and female bighorn sheep were followed within Sheep River Provincial Park and fecal samples were collected at least twice a month from each individual if possible. We were able to collect fecal samples from 90 individually marked sheep. For some individuals we only had one fecal sample while others were sampled up to 13 times over the period of the study (samples per ID: mean ± SD = 4.45 ± 2.70).
We collected samples from the same individuals repeatedly so that we could follow the seasonal changes in parasite infection intensity within an individual. Individual hosts differ in the number of eggs/larvae/oocysts shed in the feces, i.e., the fecal egg count (FEC). For the purpose of this exercise, we assume that FEC correlates with parasite burden (directly or indirectly), as the true intensity can otherwise only be measured by terminal sampling to recover adult parasites. This assumption is based on several studies that have found strong correlations between infection intensities and parasite burden, for example, both McKenna (1981) and Cabaret et al. (1998) showed that strongyle counts in feces were strongly correlated with adult worm burdens in domestic sheep (Ovis aries), as did Rehbein and Hamel (2022) with Protostrongylus rufescens lungworm burdens in domestic sheep. Similarly, Seivwright et al. (2004) found that fecal egg counts provide a reliable measure of Trichostrongylus tenuis (strongyle) infection intensities in red grouse (Lagopus lagopus scoticus).
When encountering sheep, observers remained at least 50 m away from them to minimize disturbance. The most common way to collect fecal samples was to wait for all the sheep to bed down. Once they had settled down, a laying association map was drawn, with each ear-tagged individual’s spot and key landmarks noted to help identify the exact location of each sheep. Once the herd moved, an assistant was directed to the location where each sheep had been bedded. Fecal samples were only collected if found in the spots identified in the laying associations and that could unequivocally be attributed to the individual. The second most common fecal sample collection method was observing sheep defecate while they were standing or moving and then collecting the samples once the animals had moved on. The sample was only collected if there was no fecal contamination of other individuals nearby. The total number of known-age individuals sampled was 88 animals (M = 47; F = 41), with most individuals having been sampled at least twice, resulting in a total of 390 fecal samples.
Each fecal sample was collected in a separate, individually tagged, zip-lock bag. Once samples were in the bag, the air was pushed out to ensure that the eggs did not continue to develop, since hatching, development, or death of parasite eggs can negatively affect fecal analysis (Nielsen et al., 2010). Fecal samples were stored in a cooler box with an icepack to ensure that the temperature remained relatively cool and consistent. The fecal samples were removed from the cooler as soon as possible (15 min–2 h) and stored in a refrigerator at 4 °C (Nielsen et al., 2010; Sengupta et al., 2016).
2.3. Parasite extraction and counts
The Modified Wisconsin Double Centrifugation method was used to concentrate parasite eggs and oocysts from the fecal pellets. We followed the Standard Operating Procedure – 3, from the Alberta CWHC Parasitology Lab (WCVM Parasitology diagnostic techniques handbook, 2002) with the modification of using 4 g of fecal sample and two layers of cheesecloth to strain the fecal solution. The egg/oocyst count data were divided by the weight of the fecal sample in grams, to calculate the number of eggs/oocysts per gram of feces (EPG). After processing the samples, egg counts were done using a microscope (10 × 10 for ocular objective = 100 power) in the lab, or the nearby R. B. Miller Research Station. All samples were processed within 5 days of collection, well within the 8 days recommended (Crawley et al., 2016). In total, sheep feces had the following parasite eggs/oocysts and larvae: proportion of samples (prop) with strongyles = 0.81, Nematodirus spp. (prop = 0.79), Marshallagia marshalli (prop = 0.86), Protostrongylus spp. Lungworms (prop = 0.70), Eimeria spp. (prop = 0.74), Moniezia (prop = 0.20) and Trichiurus (prop. = 0.08). As few individuals were detected with Moniezia or Trichiurus, we did not analyse the counts of either parasite.
2.4. Seasons and reproductive status
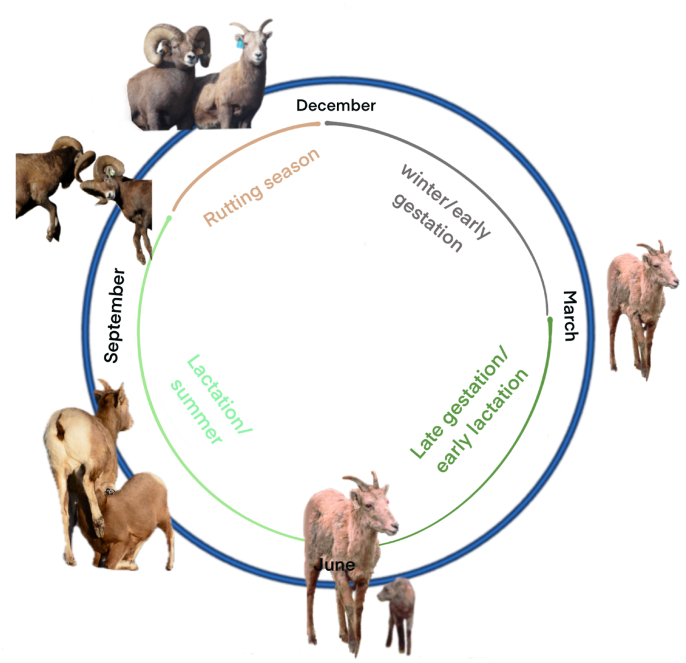
Since it was not always possible to resample individuals every two weeks, the year was divided into four distinct, biologically meaningful seasons: Jan–March = Winter/early gestation, April–June = late gestation/early lactation, July–October = lactation/summer, November and December = rut (Fig. 1). The pre-rut is from October to mid-November when males mostly fight for dominance (image of two rams fighting) (Fig. 1). The rut takes place in late November and December (image of ram with ewe) and is the only time of the year when adult males and females are in the same groups (Fig. 1). Gestation lasts from December to late May or early June (image of lone ewe) (Fig. 1). Lactation is from June till late November (image of ewe with lamb) (Fig. 1). Females mostly stay in the alpine area from June to September/October.
Fig. 1.
Schematic of the reproductive biology and seasons of bighorn sheep. The blue circle represents the entire year, where the top is December, 3 o’clock March, 6 o’clock June, 10 o’clock October etc. The grey quarter circle represents the season Jan–March = Winter/early gestation; the dark green quarter circles represent the season from April–June = late gestation/early lactation; the light green line represents the season between July and October, which is also representing lactation/summer; and the brown line is representing November and December, or the rutting season. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Data on the reproductive status of each individual was collected for each year of the study. Male reproductive (mating) tactics were assigned based on rut observations, while female reproductive state was assessed based on the presence of swollen udders and suckling lambs. All females older than 1 year were assumed to be pregnant, while those that did not have lambs with them when they came back from the alpine area in September/October were labeled as not lactating. Female reproductive status is strongly temporally correlated with season: females are pregnant in winter and give birth in late May early June, followed by a period of lactation into October/November. To distinguish between reproductive types, female reproductive status was divided into either pregnant, lactating, or non-lactating and male reproductive status was divided into those males who employed the tending or coursing tactic during the rut, as these tactics have different costs associated with them, with tending being more costly than coursing (i.e., in Himalayan tahr (Hemitragus jemlahicus) (Forsyth et al., 2005)). We checked if non-lactating and lactating females differed in their parasite FECs. However, we only had parasite counts for lactating females between September and December. At this time of the year, there were no significant differences in parasite counts between non-lactating and lactating females (all parasite comparisons; p > 0.05). We thus only report on effects of mating tactic in males on parasite shedding.
2.5. Statistical analyses
The fecal egg count data followed a negative binomial distribution, was heavily overdispersed, and zero-inflated (Denwood et al., 2008; Chipeta et al., 2014). Furthermore, the variance was not equal to the mean for any of the parasite species, and we thus used a negative binomial distribution family for all further analyses. All statistical models were run in the programming language, R, (R Core Team, 2023), using the statistical packages, ‘glmmTMB’ (Brooks et al., 2017) and ‘DHARMa’ (Hartig 2019), emmeans (Lenth et al., 2018), and ‘ggplot2’ (Wickham 2016).
For each parasite, a generalised linear mixed effects model (GLMM) was fit as follows:
| glmmTMB(Parasite_name ∼ Sex + Age + Season + Sex:Season +(1|ID), ziformula = ∼1, family = nbinom2, data = AllData, REML = FALSE). |
We evaluated the significance of the fixed effects by sequentially dropping the fixed terms from the full model and using likelihood ratio tests to compare the nested models. We first tested the sex:season interaction, before then testing the significance of the main effects. If the interaction was significant then we did not test the constituent main effects directly - as the interaction is conditional on them being present. For strongyles, the sex:season interaction was non-significant (α = 0.05) and was dropped from the final model, but for all other parasites, the interaction term was significant and was maintained. When the interaction was significant, we carried out post-hoc contrasts of the marginal means to compare the sex and status groupings, using the emmeans package (Lenth et al., 2018), reporting the significance of the overall interaction effect as well as that of specific contrasts. Because of the large number of potential contrasts, we focussed on the within-sex contrasts across seasons and the between-sex contrasts within seasons (and not the between-sex contrasts across seasons). All contrasts are in Appendix A1, Appendix A2, Appendix A3, Appendix A4, Appendix A5. While age was not significant in any of the models, we left it in the models as we had predicted a priori that it would affect parasite counts and we wanted to provide an estimate for its effect in each case, conditional on season and sex.
For males, we also fitted an additional model for each parasite that compared the FEC of individuals that adopted the tending or coursing tactic during the rut. The model structure was the same as above, but with on a single fixed categorical factor defining the mating tactic. These models were fitted to 223 counts from male faecal samples (n = 47 males).
3. Results
3.1. Strongyles
Season (χ2 = 129.19, df = 3, p < 0.001) and sex (χ2 = 32.34, df = 1, p < 0.001) each significantly affected strongyle fecal egg counts as main effects, while age did not (χ2 = 0.03, df = 1, p = 0.86; Table 1). The interaction between season and sex was not significant (χ2 = 2.06, df = 3, p = 0.56).
Table 1.
GLMM estimates, standard errors (SE), Z, and p-values for the effects of age, sex, season on strongyle fecal egg counts. Sex (M) indicates estimates for males compared to females. Late gestation/early lactation is the reference category for seasonal comparisons. Significant p-values are bolded, while near-significant p-values are in Italics. Zero-inflation intercept ±SE = −3.09 ± 0.56, z = −5.51, p < 0.001. Estimates are provided on the [log]-link scale.
| Strongyles | Estimate | Std Error | Z | p-value | |
|---|---|---|---|---|---|
| Intercept | 3.03 | 0.20 | 14.90 | <0.0001 | |
| Age | −0.00 | 0.02 | −0.18 | 0.86 | |
| Sex (M) | 1.11 | 0.18 | 6.33 | <0.0001 | |
| Season |
|
0.31 | 0.16 | −1.93 | 0.054 |
| −2.04 | 0.23 | −8.96 | <0.0001 | ||
| −2.79 | 0.25 | −11.51 | <0.0001 | ||
| Random Effect |
Variance |
SD |
|||
| Individual ID (n = 88) | 0.21 | 0.46 | |||
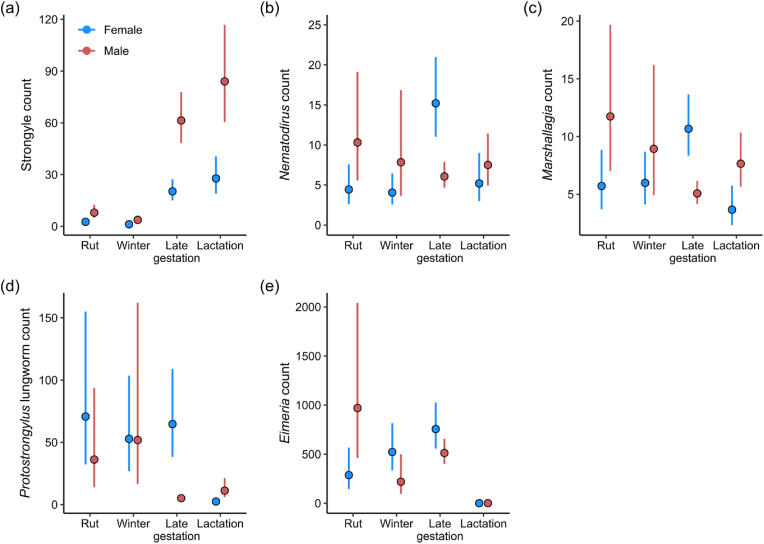
During the rut and the following early gestation/winter months fecal egg counts were very low for both sexes (November–March) while both males and females had significantly higher strongyle counts during the late gestation and lactation/summer seasons (Fig. 2a, Appendix A1). Males had significantly higher strongyle counts than females in all seasons except during winter/early gestation. (Fig. 2a–Appendix A1). The mating tactic males (reproductive status in figure) employed during the rut significantly affected their fecal strongyle egg counts with tending rams having higher egg counts than coursing rams (Estimate = 0.61, SE = 0.20, χ2 = 8.50, df = 1, p = 0.004; Fig. 3).
Fig. 2.
Seasonal differences in fecal egg counts in female (blue) and male (red) bighorn sheep. Point intervals display the mean count ±95% confidence intervals as predicted by generalised linear mixed effects models. Seasons are: Late gestation (Late gestation/early lactation between April to June); Lactation/summer (between July and October); Rut (November and December); Winter (Winter/early gestation from January to March). Parasites are a) Strongyle; b) Nematodirus; c) Marshallagia; d) Protostrongylus lungworm; e) Eimeria. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Fig. 3.
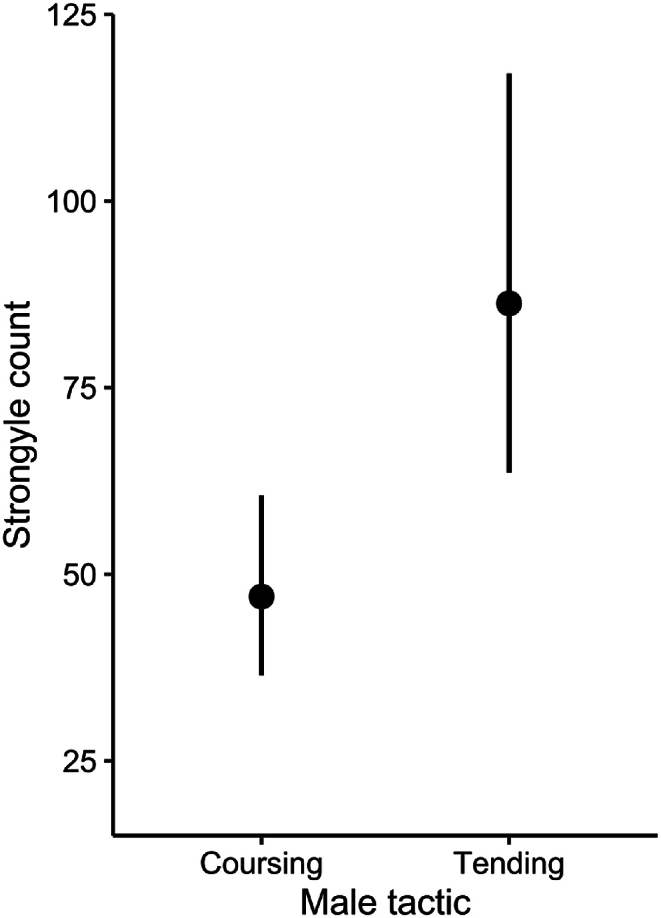
Differences in mean and standard error in strongyle counts between males that use the coursing or tending mating tactic. Point intervals display the mean count ±95% confidence intervals as predicted by the generalised linear mixed effects model.
3.2. Nematodirus
Season and sex significantly affected Nematodirus fecal egg counts in an interaction (χ2 = 28.44, df = 3, p < 0.001), while age had no effect (χ2 = 0.11, df = 1, p = 0.74; Table 2).
Table 2.
GLMM estimates, standard errors (SE), Z, and p-values for the effects of age, sex, season, and the interaction sex:season on Nematodirus fecal egg counts. Sex (M) indicates estimates for males compared to females. Late gestation/early lactation was the reference category for seasonal comparisons. Significant p-values are bolded. Zero-inflation intercept ±SE = −3.04 ± 1.02, z = −3.00, p = 0.03. Estimates are provided on the [log]-link scale.
| Nematodirus | Estimate | SE | Z-value | p-value | |
|---|---|---|---|---|---|
| (Intercept) | 2.76 | 0.21 | 12.96 | <0.001 | |
| Age | −0.01 | 0.21 | −0.34 | 0.74 | |
| Sex (M) | −0.92 | 0.21 | −4.50 | <0.001 | |
| Season |
|
−1.07 | 0.30 | −3.59 | <0.001 |
| −1.23 | 0.30 | −4.10 | <0.001 | ||
| −1.32 | 0.26 | −5.07 | <0.001 | ||
| Sex (M): Lactation/summer | 1.29 | 0.37 | 3.51 | <0.001 | |
| Sex (M): Rutting season | 1.76 | 0.44 | 4.01 | <0.001 | |
| Sex (M): Winter/early gestation | 1.58 | 0.47 | 3.32 | <0.001 | |
| Random Effect |
Variance |
SD |
|||
| Individual ID (n = 88) | 0.20 | 0.44 | |||
In females the Nematodirus fecal egg counts were highest during the late gestation/spring season and significantly lower during the rutting and winter (Fig. 2b–Appendix A2). Males seemed to shed the highest number of eggs during the rut and the lowest during the late gestation season, but none of these means were signficantly different (Fig. 2b–Appendix A2). There were signficant differences in Nematodirus counts between the sexes in the late gestation and rut season: while females had significantly higher counts in late gestation, males had significantly higher counts of Nematodirus during the rutting season (Fig. 2b–Appendix A2).
The mating tactic males employed during the rut did not affect their fecal Nematodirus egg count (Estimate = −0.13, SE = 0.26, χ2 = 0.24, df = 1, p = 0.63).
3.3. Marshallagia
Season and sex significantly affected fecal Marshallagia fecal counts in an interaction (χ2 = 36.50, df = 3, p < 0.001), while age had no effect (χ2 = 2.34, df = 1, p = 0.13; Table 3).
Table 3.
GLMM estimates, standard errors (SE), Z, and p-values for the effects of age, sex, and season on Marshallagia fecal egg counts. Sex (M) indicates estimates for males that are compared to females. Late gestation/early lactation was the reference category for seasonal comparisons. Significant p-values are bolded. Zero-inflation intercept ±SE = −3.10 ± 0.68, z = −4.57, p < 0.001. Estimates are provided on the [log]-link scale.
| Marshallagia | Estimate | SE | Z-value | p-value | |
|---|---|---|---|---|---|
| (Intercept) | 2.23 | 0.16 | 13.76 | <0.001 | |
| Age | 0.03 | 0.02 | 1.54 | 0.12 | |
| Sex (M) | −0.74 | 0.16 | −4.70 | <0.001 | |
| Season |
|
−1.07 | 0.25 | −4.30 | <0.001 |
| −0.62 | 0.24 | −2.60 | 0.009 | ||
| −0.58 | 0.21 | −2.77 | 0.006 | ||
| Sex (M): Lactation/summer | 1.48 | 0.30 | 4.92 | <0.001 | |
| Sex (M): Rutting season | 1.46 | 0.36 | 4.07 | <0.001 | |
| Sex (M): Winter/early gestation | 1.15 | 0.37 | 3.06 | 0.002 | |
| Random Effect |
Variance |
SD |
|||
| Individual ID (n = 88) | 0.09 | 0.31 | |||
Female fecal egg counts of Marshallagia were significantly higher during late gestation/spring (April–June) compared to lactation (lowest after returning from the alpine areas September/October), the rut or winter (Fig. 2c–Appendix A3). Males had significantly higher Marshallagia egg counts during the rut than during the late gestation season (Fig. 2c–Appendix A3). Females shed significantly higher numbers of Marshallagia eggs in their feces compared to males during the late gestation period, while males had higher egg counts during lactation and the rut. There was no difference between the sexes in winter (Fig. 2c–Appendix A3). The mating tactic males employed during the rut did not affect their fecal Marshallagia egg count (Estimate = −0.15, SE = 0.20, χ2 = 0.57, df = 1, p = 0.45).
3.4. Protostrongylus spp. lungworms
Season and sex significantly affected fecal Protostrongylus spp. lungworm egg counts in an interaction (χ2 = 49.89, df = 3, p < 0.001), while age had no effect (χ2 = 0.46, df = 1, p = 0.50; Table 4).
Table 4.
GLMM estimates, standard errors (SE), Z, and p-values for the effects of age, sex and season on Protostrongylus spp lungworm fecal egg counts. Sex (M) indicates estimates for males compared to females. Significant p-values are bolded. Late gestation/early lactation was the reference category for seasonal comparisons. Applying a zero-inflation parameter did all data points did not improve model fit so it was not included. Estimates are provided on the [log]-link scale.
| Protostrongylus lungworms | Estimate | SE | Z-value | p-value | |
|---|---|---|---|---|---|
| (Intercept) | 4.04 | 0.35 | 11.69 | <0.001 | |
| Age | 0.03 | 0.04 | 0.68 | 0.50 | |
| Sex (M) | −2.53 | 0.35 | −7.30 | <0.001 | |
| Season |
|
−3.25 | 0.45 | −7.15 | <0.001 |
| 0.09 | 0.44 | 0.21 | 0.84 | ||
| −0.20 | 0.39 | −0.52 | 0.60 | ||
| Sex (M): Lactation/summer | 4.04 | 0.57 | 7.14 | <0.001 | |
| Sex (M): Rutting season | 1.86 | 0.66 | 2.81 | 0.005 | |
| Sex (M): Winter/early gestation | 2.51 | 0.72 | 3.51 | <0.001 | |
| Random Effect |
Variance |
SD |
|||
| Individual ID (n = 88) | 0.89 | 0.94 | |||
Female fecal egg counts of Protostrongylus spp. lungworms were significantly lower during lactation/summer than during late gestation, the rut or winter (Fig. 2d–Appendix A4). The Protostrongylus spp. lungworm fecal egg count of males was significantly lower during late gestation/early lactation compared to the rut and winter (Fig. 2d–Appendix A4). The sexes only differed in Protostrongylus spp. lungworm egg counts during late gestation and lactation. While females had significantly higher counts during late gestation, males had higher counts during lactation/summer (Fig. 2d–Appendix A4). The mating tactic males employed during the rut did not affect their fecal Protostrongylus lungworm egg/larvae count (Estimate = −0.69, SE = 0.54 χ2 = 1.67, df = 1, p = 0.20).
3.5. Eimeria
Season and sex significantly affected fecal Eimeria spp. oocyst counts in an interaction (χ2 = 16.95, df = 3, p < 0.001), while age had no effect (χ2 = 0.48, df = 1, p = 0.49; Table 5).
Table 5.
GLMM estimates, standard errors (SE), Z, and p-values for the effects of sex, age, season and reproductive status on Eimeria spp. lungworm fecal egg counts. Sex (M) indicates estimates for males compared to females. Late gestation/early lactation was the reference category for seasonal comparisons. Zero-inflation intercept ±SE = −2.57 ± 0.35, z = −7.28, p < 0.001. Near-significant p-values are in Italics and significant ones are bolded. Estimates are provided on the [log]-link scale.
| Eimeria | Estimate | SE | Z-value | p-value | |
|---|---|---|---|---|---|
| (Intercept) | 6.71 | 0.20 | 33.54 | <0.001 | |
| Age | −0.02 | 0.02 | −0.70 | 0.49 | |
| Sex (M) | −0.39 | 0.20 | −1.95 | 0.051 | |
| Season |
|
−9.94 | 1.04 | −9.53 | <0.001 |
| −0.97 | 0.37 | −2.64 | 0.008 | ||
| −0.37 | 0.25 | −1.46 | 0.14 | ||
| Sex (M): Lactation/summer | 2.29 | 1.11 | 2.06 | 0.039 | |
| Sex (M): Rutting season | 1.61 | 0.53 | 3.00 | 0.003 | |
| Sex (M): Winter/early gestation | −0.48 | 0.50 | −0.95 | 0.34 | |
| Random Effect |
Variance |
SD |
|||
| Individual ID (n = 88) | 0.19 | 0.43 | |||
Females had significantly higher fecal Eimeria oocyst counts in late gestation compared to lactation/summer or the rut (Fig. 2d–Appendix A5), but higher counts during the rut and winter than during lactation/summer, when there were no Eimeria oocysts found in feces. Males had the highest Eimeria counts during the rut compared to winter and the lactation/summer, with hardly any Eimeria oocysts found during the lactation season (Fig. 2e–Appendix A5). Males had significantly higher fecal Eimeria occyst counts than female during the rut, whereas there were no significant sex differences in other seasons. (Fig. 2e). The mating tactic males employed during the rut did not affect their fecal Eimeria oocyst count (Estimate = −0.26, SE = 0.23, χ2 = 1.29, df = 1, p = 0.25).
4. Discussion
Our study showed that host sex and season explained differences in parasite infection intensity for all five parasite groups. Seasonal trends varied between the sexes and with the parasite type, with all models, except for strongyles, showing a significant interaction term of season and sex, indicating that the relative abundance of egg counts between males and females differed at different times of the year for most parasites. We will thus discuss the differences in fecal parasite prevalence from the model-predicted marginal means and their associated contrasts. Strongyle infection intensity was higher for both sexes in the seasons of late gestation and lactation, than during the rut or winter/early gestation season when counts were very low. In general, the strongyle fecal egg count was higher in males than in females, except for winter when there was a trend for males to have higher counts. Within males, strongyle counts were higher in tending rams than in coursing rams. None of the other parasite types were impacted by the reproductive status of the rams. Age was not a significant predictor of fecal egg counts in any of the five parasite types evaluated.
Female bighorn sheep had the highest fecal egg counts during the late gestation/early lactation season, except in Protostrongylus spp lungworms which remained high during the rut and winter. Males on the other hand had the highest fecal egg counts and higher than females for Nematodirus, Marshallagia and Eimeria during the rut season. The peak in Protostrongylus lungworms was during winter and in strongyles during lactation which coincides with the pre-rut. These result hint towards a possible trade-off between immunocompetence and reproductive costs assuming that for females, late gestation, and for males, the rut, are the costliest seasons in terms of reproductive effort. Our results are very similar to those found by Pelletier et al. (2005), for Protostrongylus larvae counts in bighorn sheep. That we found similar correlations with sex and season for several other parasite types, notably Nematodirus. Marshallagia, and to a certain extend also Eimeria, reinforces the idea of parasites taking advantage of the host’s energetic costs being channeled towards reproductive effort rather than immunity (Albery et al., 2021; Nordling et al., 1998).
The one parasite where we observed a totally different pattern is strongyles which are almost absent in the fecal counts in both sexes during the rut and winter seasons. Strongyle eggs have been shown to need relatively high temperatures to hatch (Rossanigo and Gruner 1995; Hoar 2012) and they are relatively easily damaged by freezing (Foreyt 1986; Wharton and Allan, 1989). The few eggs found in feces in the winter season may thus reflect selection against egg shedding by the parasite under unfavourable conditions for larval development. In reindeer (Rangifer tarandus) a similar difference between Marshallagia being more prominent in winter and strongyles being more prominent in summer has been described by Irvine et al. (2000).
The differences in fecal egg counts/infection intensity of parasites between the sexes, reproductive states and over the seasons could be explained by the differences in host biology and parasite abundance, as well as parasite biology (Sweeny et al., 2022). Sweeny et al. (2022), for example, also found that nematode prevalence (particularly strongyles) in Soay sheep changed according to season, with reproductive status, and age. Strongyles egg counts were highest in spring and summer and primarily in adults (Sweeny et al., 2022).
In Eimeria, temperature sensitivity is very variable. They sporulate relatively rapidly in the environment and tend to be important livestock pathogens, especially for caves/lambs in the early summer (Foreyt 1990; Rind and Brohi, 2001; Yan et al., 2021). However, the ideal temperature ranges from 24 to 32 °C. At these temperatures, most species sporulate within 5 days (Engidaw et al., 2015). Given the large range of temperature tolerance of oocysts, there may be differences in oocyst production based on the species (Kutz et al., 2012). It’s likely a reflection of the parasite’s behaviour, biology, or environmental conditions, that leads to the very low numbers of oocysts found in the feces of both sexes during the lactation season, something that needs to be researched in more depth, under experimental conditions.
As mentioned above, Marshallagia and Nematodirus spp. fecal egg counts were significantly higher in late gestation for females, compared to the other seasons, and higher in males than females during the rut and winter. Both Nematodirus spp. and Marshallagia marshalli may have escaped temperature limitation due to tolerance of very low temperatures, (van Dijk and Morgan, 2008; Hoar 2012), despite some heat requirements during development from the egg to infective larval stages (Aleuy et al., 2020, Aleuy et al., 2023). The same may be true for Protostrongylus spp. lungworms whose larvae (L1 stage) can tolerate very low temperatures (Cabaret et al., 1991). This could give the parasite the freedom to be most active at times when hosts are most restricted in terms of energy expenditure (i.e.; trading off immunocompetence and reproductive effort). For example, energy demands can be 300% that of normal maintenance during lactation (Randolph et al., 1977 or Prentice and Prentice 1988). In bighorn sheep, birthing and lactation take place during the non-winter months, with the majority of birthing between late May to early June and lambs being mostly weaned by November (Festa-Bianchet 1988).
A peri-parturient rise in parasite egg counts has also been reported in domestic sheep (for example in West African Dwarf sheep (Agyei et al., 1991) and in Bulkhi sheep (Chaudhry et al., 2009) where infection intensity in females increases during the first few weeks before and after birthing. We did not sample females during late pregnancy and early lactation as they migrated into the alpine and remained in inaccessible areas during this time. Therefore, given the patterns present in other ungulates, it is possible that maximum fecal egg counts in females were missed in our study. During the first couple of years of their life, all lambs and yearlings stay in nursery groups with their mothers (Ruckstuhl 1998). During this time, the lambs and yearlings are naïve potential hosts for parasites. Thus, Armour (1980) posited that infection intensity during non-winter months in females could increase as some of these eggs and larvae could establish within the newly born lambs and yearlings. However, in this study, age was not a significant factor explaining variation in parasite infection intensity, and we did not sample lambs.
Conversely, the winter season is expected to be more costly for males since this includes the pre-rut, rut, and post-rut periods when males spend most of their energy establishing dominance hierarchies and mating. The pre-rut is costly for males because of an increase in social interactions to establish their hierarchy (Geist 1971; Pelletier and Festa-Bianchet 2006), while the rut is costly since the males are either engaged in mate guarding or trying to secure mating opportunities with a guarded estrous female (Hogg 1984). Courting and mate guarding are energy-expensive activities. Males often forego food consumption to maximize mating success by searching for estrus females or guarding females (e.g., bighorn sheep,– Pelletier et al., 2009; Alpine chamois, Rupicapra rupicapra, – Willisch and Ingold, 2007; long-tailed macaques, Macaca fascicularis, – Girard-Buttoz et al., 2014; isopods, Lirceus fontinalis, – Sparkes et al., 1996). To meet the demands of mating costs, males may reallocate resources from immune function to mating and thus have higher fecal egg counts and parasite loads in winter, compared to non-winter. Parasites of animals living in temperate locations such as mountain ungulates may have evolved to tolerate a high range of temperatures in an attempt to infect females and males at different times of the year when the host’s defences are weakened (Irvine et al., 2000), or their energy demand is highest.
In a study on FECs in red deer, Albery et al. (2018) found that some nematodes may decrease egg production during winter, due to the eggs’ low over-winter survival, rather than being indicative of a reduced parasite burden in the hosts. This seems consistent with the results found in our study. The female bighorn sheep of our study population migrate to the mountains to give birth towards the end of May, while the adult males remain in Sheep River Provincial Park (Ruckstuhl 1998). We speculate that higher elevations and lower ambient temperatures during these birth migrations could mean that strongyle eggs would struggle to develop.
It would be interesting to explore host behaviours and how they could affect parasite prevalence and burden. It is not unreasonable to speculate that females might migrate to the alpine to avoid predation, as was suggested for Dall’s sheep (Rachlow and Bowyer 1998) and parasites, but further research is needed to test the idea that females might migrate to colder areas to avoid parasites. A strong indication for a parasite avoidance mechanism is that while females had very high parasite egg counts in late gestation, these counts dropped to relatively low numbers at the time they were back at lower elevations in September to October (lactation season) while male fecal egg counts did increase from late gestation to lactation with the already mentioned exception in Eimeria counts.
Finally, this study showed several significant correlations between the FECs of different parasite groups with interactions between sex and season. Interestingly, when calculating correlation coefficients for the different parasite pairs, we found a negative correlation between strongyles and Protostrongylus spp. lungworm larvae (r = −0.14, p < 0.001), which could potentially indicate parasite competition, but again it is unclear whether this is a causal relationship or a mere correlation. More research is needed to look at interactions of different parasite groups within a single host and its sex biases.
In conclusion our results show strong indications that parasites are able to target host reproductive effort by increasing reproduction during the time of maximal energy need of their host, targeting male and female hosts accordingly. Further, different parasite life-history dictates and limits season of egg/larvae shedding. Finally, we argue that it is possible that female seasonal migration may have a positive effect on parasite reduction.
CRediT authorship contribution statement
Samridhi Rijal: Writing – original draft, Visualization, Validation, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization. Peter Neuhaus: Writing – review & editing, Writing – original draft, Supervision, Methodology, Conceptualization. Jack Thorley: Formal analysis, Visualization, Writing – review & editing. Nigel Caulkett: Writing – review & editing, Resources. Susan Kutz: Writing – review & editing, Resources, Methodology, Conceptualization. Kathreen E. Ruckstuhl: Writing – review & editing, Writing – original draft, Visualization, Supervision, Resources, Project administration, Methodology, Funding acquisition, Formal analysis, Data curation, Conceptualization.
Declaration of competing interest
We hereby declare now conflict of interest.
Acknowledgements and funding
We would like to thank the University of Calgary for the use of the R. B. Miller Research Station. K.E.R. received support from the Natural Sciences and Engineering Research Council of Canada (NSERC; funding reference number 316189-2012-RGPIN). Further funding was also provided by an Alberta Conservation Association Grants in Biodiversity (RES0035325-S001) awarded to SR.
Appendix A1. Seasonal contrast for fecal prevalence of strongyle parasite eggs in bighorn sheep. The interaction between sex and season was not significant, so all contrasts apply to both males and females. Given are the contrast estimates, standard errors (SE), t ratios and p-values. Significant terms are bolded and near significant ones in italics. All contrasts are provided on the [log]-link scale
| Seasonal Contrasts for Strongyles | Estimate | SE | t ratio | p-value |
|---|---|---|---|---|
| Gestation-Lactation | −0.31 | 0.16 | −1.93 | 0.22 |
| Gestation-Rut | 2.05 | 0.23 | 8.96 | <0.0001 |
| Gestation-Winter | 2.80 | 0.25 | 11.15 | <0.0001 |
| Lactation-Rut | 2.36 | 0.25 | 9.29 | <0.0001 |
| Lactation-Winter | 3.11 | 0.28 | 11.32 | <0.0001 |
| Rut-Winter | 0.75 | 0.31 | 2.42 | 0.075 |
Appendix A2. Seasonal and sex contrast for fecal prevalence of Nematodirus parasite eggs in bighorn sheep. Given are the contrast estimates, standard errors (SE), t ratios and p-values. Significant terms are bolded and near significant ones in italics. All contrasts are provided on the [log]-link scale
| Seasonal Contrasts for Nematodirus | Sex | Estimate | SE | t ratio | p-value |
|---|---|---|---|---|---|
| Gestation-Lactation | F | 1.07 | 0.30 | 3.59 | 0.002 |
| Gestation-Rut | F | 1.23 | 0.30 | 4.11 | 0.0002 |
| Gestation-Winter | F | 1.32 | 0.26 | 5.07 | <0.0001 |
| Lactation-Rut | F | 0.16 | 0.37 | 0.43 | 0.97 |
| Lactation-Winter | F | 0.25 | 0.34 | 0.73 | 0.89 |
| Rut-Winter | F | 0.09 | 0.34 | 0.26 | 1.00 |
| Gestation-Lactation | M | −0.21 | 0.22 | −0.97 | 0.77 |
| Gestation-Rut | M | −0.53 | 0.32 | −1.65 | 0.35 |
| Gestation-Winter | M | −0.26 | 0.40 | −0.64 | 0.92 |
| Lactation-Rut | M | −0.32 | 0.36 | −0.88 | 0.81 |
| Lactation-Winter | M | −0.04 | 0.44 | −0.10 | 1.00 |
| Rut-Winter | M | 0.28 | 0.48 | 0.58 | 0.94 |
| Season | Sex Contrast | Estimate | SE | t ratio | p-value |
|---|---|---|---|---|---|
| Gestation | F-M | 0.92 | 0.21 | 4.50 | <0.0001 |
| Lactation | F-M | −0.37 | 0.33 | −1.10 | 0.27 |
| Rut | F-M | −0.84 | 0.42 | −2.03 | 0.043 |
| Winter | F-M | −0.66 | 0.45 | −1.47 | 0.14 |
Appendix A3. Seasonal and sex contrast for fecal prevalence of Marshallagiamarshalli parasite eggs in bighorn sheep. Given are the contrast estimates, standard errors (SE), t ratios and p-values. Significant terms are bolded and near significant ones in italics. All contrasts are provided on the [log]-link scale
| Seasonal Contrasts for Marshallagia | Sex | Estimate | SE | t ratio | p-value |
|---|---|---|---|---|---|
| Gestation-Lactation | F | 1.07 | 0.25 | 4.30 | <0.0001 |
| Gestation-Rut | F | 0.62 | 0.24 | 2.60 | 0.047 |
| Gestation-Winter | F | 0.58 | 0.21 | 2.77 | 0.030 |
| Lactation-Rut | F | −0.45 | 0.30 | −1.48 | 0.45 |
| Lactation-Winter | F | −0.49 | 0.28 | −1.75 | 0.30 |
| Rut-Winter | F | −0.04 | 0.27 | −0.16 | 1.00 |
| Gestation-Lactation | M | −0.41 | 0.17 | −2.45 | 0.071 |
| Gestation-Rut | M | −0.84 | 0.27 | −3.12 | 0.010 |
| Gestation-Winter | M | −0.57 | 0.31 | −1.82 | 0.27 |
| Lactation-Rut | M | −0.43 | 0.29 | −1.48 | 0.45 |
| Lactation-Winter | M | −0.16 | 0.33 | −0.47 | 0.97 |
| Rut-Winter | M | 0.27 | 0.38 | 0.71 | 0.89 |
| Season | Sex Contrast | Estimate | SE | t ratio | p-value |
|---|---|---|---|---|---|
| Gestation | F-M | 0.75 | 0.16 | 4.70 | <0.0001 |
| Lactation | F-M | −0.74 | 0.27 | −2.71 | 0.007 |
| Rut | F-M | −0.72 | 0.34 | −2.13 | 0.034 |
| Winter | F-M | −0.40 | 0.35 | −1.14 | 0.26 |
Appendix A4. Seasonal and sex contrast for fecal prevalence of Protostrongylus lungworm parasite eggs/larvae in bighorn sheep. Given are the contrast estimates, standard errors (SE), t ratios and p-values. Significant terms are bolded and near significant ones in italics. All contrasts are provided on the [log]-link scale
| Seasonal Contrasts for Protostrongylus | Sex | Estimate | SE | t ratio | p-value |
|---|---|---|---|---|---|
| Gestation-Lactation | F | 3.25 | 0.46 | 7.15 | <0.0001 |
| Gestation-Rut | F | −0.09 | 0.44 | −0.21 | 1.00 |
| Gestation-Winter | F | 0.20 | 0.39 | 0.52 | 0.95 |
| Lactation-Rut | F | −3.34 | 0.53 | −6.26 | <0.0001 |
| Lactation-Winter | F | −3.05 | 0.51 | −5.93 | <0.0001 |
| Rut-Winter | F | 0.29 | 0.48 | 0.61 | 0.93 |
| Gestation-Lactation | M | −0.78 | 0.34 | −2.34 | 0.091 |
| Gestation-Rut | M | −1.95 | 0.50 | −3.90 | 0.0007 |
| Gestation-Winter | M | −2.31 | 0.60 | −3.84 | 0.0008 |
| Lactation-Rut | M | −1.52 | 0.56 | −2.10 | 0.16 |
| Lactation-Winter | M | −1.5229 | 0.65 | −2.33 | 0.092 |
| Rut-Winter | M | −0.35 | 0.70 | −0.52 | 0.96 |
| Season | Sex Contrast | Estimate | SE | t ratio | p-value |
|---|---|---|---|---|---|
| Gestation | F-M | 2.53 | 0.35 | 7.30 | <0.0001 |
| Lactation | F-M | −1.51 | 0.53 | −2.86 | 0.005 |
| Rut | F-M | 0.67 | 0.63 | 1.06 | 0.29 |
| Winter | F-M | 0.02 | 0.68 | 0.03 | 0.98 |
Appendix A5. Seasonal and sex contrast for fecal prevalence of Eimeria parasite oocysts in bighorn sheep. Given are the contrast estimates, standard errors (SE), t ratios and p-values. Significant terms are bolded and near significant ones in italics. All contrasts are provided on the [log]-link scale
| Seasonal Contrasts for Eimeria | Sex | Estimate | SE | t ratio | p-value |
|---|---|---|---|---|---|
| Gestation-Lactation | F | 9.94 | 1.04 | 9.53 | <0.0001 |
| Gestation-Rut | F | 0.97 | 0.37 | 2.64 | 0.043 |
| Gestation-Winter | F | 0.37 | 0.25 | 1.46 | 0.46 |
| Lactation-Rut | F | −8.97 | 1.08 | −8.29 | <0.0001 |
| Lactation-Winter | F | −9.57 | 1.06 | −9.06 | <0.0001 |
| Rut-Winter | F | −0.60 | 0.40 | −1.51 | 0.43 |
| Gestation-Lactation | M | 7.64 | 0.39 | 19.65 | <0.0001 |
| Gestation-Rut | M | −0.64 | 0.39 | −1.64 | 0.36 |
| Gestation-Winter | M | 0.85 | 0.43 | 1.97 | 0.20 |
| Lactation-Rut | M | −8.28 | 0.54 | −15.43 | <0.0001 |
| Lactation-Winter | M | −6.80 | 0.56 | −12.00 | <0.0001 |
| Rut-Winter | M | 1.49 | 0.55 | 2.70 | 0.036 |
| Season | Sex Contrast | Estimate | SE | t ratio | p-value |
|---|---|---|---|---|---|
| Gestation | F-M | 0.39 | 0.20 | 1.95 | 0.052 |
| Lactation | F-M | −1.90 | 1.10 | −1.73 | 0.085 |
| Rut | F-M | −1.22 | 0.51 | −2.38 | 0.018 |
| Winter | F-M | 0.87 | 0.48 | 1.81 | 0.071 |
References
- Agyei A.D., Sapong D., Probert A.J. Periparturient rise in faecal nematode egg counts in West African Dwarf sheep in Southern Ghana in the absence of arrested strongyle larvae. Vet. Parasitol. 1991;39:79–88. doi: 10.1016/0304-4017(91)90064-3. [DOI] [PubMed] [Google Scholar]
- Albery G.F., Kenyon F., Morris A., Morris S., Nussey D.H., Pemberton J.M. Seasonality of helminth infection in wild red deer varies between individuals and between parasite taxa. Parasitology. 2018;145:1–11. doi: 10.1017/S0031182018000185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albery G.F., Morris A., Morris S., Kenyon F., Nussey D.H., Pemberton J.M. Fitness costs of parasites explain multiple life-history trade-offs in a wild mammal. Am. Nat. 2021;197 doi: 10.1086/712633. [DOI] [PubMed] [Google Scholar]
- Aleuy O.A., Ruckstuhl K., Hoberg E.P., Veitch A., Simmons N., Kutz S.J. Diversity of gastrointestinal helminths in Dall’s sheep and the negative association of the abomasal nematode, Marshallagia marshalli, with fitness indicators. PLoS One. 2018;13(3) doi: 10.1371/journal.pone.0192825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aleuy O.A., Peacock S., Hoberg E.P., Ruckstuhl K.E., Brooks T., Aranas M., Kutz S. Phenotypic plasticity and local adaptation in freeze tolerance: implications for parasite dynamics in a changing world. Int. J. Parasitol. 2020;50(2):161–169. doi: 10.1016/j.ijpara.2019.12.004. [DOI] [PubMed] [Google Scholar]
- Aleuy O.A., Peacock S.J., Molnár P.K., Ruckstuhl K.E., Kutz S.J. Local thermal adaptation and local temperature regimes drive the performance of a parasitic helminth under climate change: the case of Marshallagia marshalli from wild ungulates. Global Change Biol. 2023;29(22):6217–6233. doi: 10.1111/gcb.16918. [DOI] [PubMed] [Google Scholar]
- Altizer S.M., Oberhauser K. Effects of the protozoan parasite Ophryocystis elektroscirrhaon the fitness of monarch butterflies (Danaus plexippus) J. Invertebr. Pathol. 1999;74:76–88. doi: 10.1006/jipa.1999.4853. [DOI] [PubMed] [Google Scholar]
- Altizer S., Dobson A., Hosseini P., Hudson P., Pascual M., Rohani P. Seasonality and the dynamics of infectious diseases. Ecol. Lett. 2006;9:467–484. doi: 10.1111/j.1461-0248.2005.00879.x. [DOI] [PubMed] [Google Scholar]
- Armour J. The epidemiology of helminth disease in farm animals. Vet. Parasitol. 1980;6:7–46. [Google Scholar]
- Barber I., Berkhout B.W., Ismail Z. Thermal change and the dynamics of multi-host parasite life cycles in aquatic ecosystems. Integr. Comp. Biol. 2016;56(4):561–572. doi: 10.1093/icb/icw025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks M.E., Kristensen K., van Benthem K.J., Magnusson A., Berg C.W., Nielsen A., Skaug H.J., Maechler M., Bolker B.M. glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. The R Journal. 2017;9(2):378–400. [Google Scholar]
- Cabaret J., Gasnier N., Jacquiet P. Faecal egg counts are representative of digestive-tract strongyle worm burdens in sheep and goats. Parasite. 1998;5:137–142. doi: 10.1051/parasite/1998052137. [DOI] [PubMed] [Google Scholar]
- Cattadori I.M., Boag B., Bjørnstad O.N., Cornell S.J., Hudson P.J. Peak shift and epidemiology in a seasonal host–nematode system. P. Roy. Soc. B-Biol. Sci. 2005;272(1568):1163–1169. doi: 10.1098/rspb.2004.3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabaret J., Riseani S.R., Baéza E. Survival of sheep and goat first stage protostrongylid larvae in experimental conditions: influence of humidity and temperature. J. Helminthol. 1991;65(3):201–207. doi: 10.1017/s0022149x00010713. [DOI] [PubMed] [Google Scholar]
- Chaudhry F.R., Qayyum M., Khan M.F., Ahmad T., Khanum A., Shakir M.R., James D.H., Miller E. Peri-parturient rise in faecal nematode egg counts with reference to Haemonchus contortus in bulkhi ewes in Northern Punjab, Pakistan. Pakistan J. Zool. 2009;41:437–443. [Google Scholar]
- Chipeta M.G., Ngwira B.M., Simoonga C., Kazembe L.N. Zero adjusted models with applications to analyzing helminths count data. BMC Res. Notes. 2014;7:856. doi: 10.1186/1756-0500-7-856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christe P., Glaizot O., Evanno G., Bruyndonckx N., Devevey G., Yannic G., Patthey P., Maeder A., Vogel P., Arlettaz R. Host sex and ectoparasites choice: preference for, and higher survival on female hosts. J. Anim. Ecol. 2007;76:703–710. doi: 10.1111/j.1365-2656.2007.01255.x. [DOI] [PubMed] [Google Scholar]
- Cizauskas C.A., Turner W.C., Pitts N., Getz W.M. Seasonal patterns of hormones, macroparasites, and microparasites in wild African ungulates: the interplay among stress, reproduction, and disease. PLoS One. 2015;10:1–29. doi: 10.1371/journal.pone.0120800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawley J.A.H., Chapman S.N., Lummaa V., Lynsdale C.L. Testing storage methods of faecal samples for subsequent measurement of helminth egg numbers in the domestic horse. Vet. Parasitol. 2016;221:130–133. doi: 10.1016/j.vetpar.2016.03.012. [DOI] [PubMed] [Google Scholar]
- Denwood M., Stear M., Matthews L., Reid S., Toft N., Innocent G. The distribution of the pathogenic nematode Nematodirus battus in lambs is zero-inflated. Parasitology. 2008;135:1225–1235. doi: 10.1017/S0031182008004708. [DOI] [PubMed] [Google Scholar]
- Engidaw S., Anteneh M., Demis C. Coccidiosis in small ruminants. Afr. J. Basic Appl. Sci. 2015;7(6):311–319. [Google Scholar]
- Ezenwa V.O., Stefan Ekernas L., Creel S. Unravelling complex associations between testosterone and parasite infection in the wild. Funct. Ecol. 2012;26:123–133. doi: 10.1111/j.1365-2435.2011.01919.x. [DOI] [Google Scholar]
- Ezenwa V.O., Snider M.H. Reciprocal relationships between behaviour and parasites suggest that negative feedback may drive flexibility in male reproductive behaviour. P. Roy. Soc. B-Biol. Sci. 2016;283(1831) doi: 10.1098/rspb.2016.0423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari N., Rosà R., Lanfranchi P., Ruckstuhl K.E. Effect of sexual segregation on host–parasite interaction: model simulation for abomasal parasite dynamics in alpine ibex (Capra ibex) Int. J. Parasitol. 2010;40(11):1285–1293. doi: 10.1016/j.ijpara.2010.03.015. [DOI] [PubMed] [Google Scholar]
- Festa-Bianchet M. Nursing behaviour of bighorn sheep: correlates of Ewe age, parasitism, lamb age, birthdate and sex. Anim. Behav. 1988;36(5):1445–1454. [Google Scholar]
- Foreyt W.J. Recovery of nematode eggs and larvae in deer: evaluation of fecal preservation methods. J. Am. Vet. Med. Assoc. 1986;189:1065–1067. [PubMed] [Google Scholar]
- Foreyt W.J. Coccidiosis and cryptosporidiosis in sheep and goats. Vet. Clin. N. Am. 1990;6:655–670. doi: 10.1016/s0749-0720(15)30838-0. [DOI] [PubMed] [Google Scholar]
- Forsyth D.M., Duncan R.P., Tustin K.G., Gaillard J.-M. A substantial energetic cost to male reproduction in a sexually dimorphic ungulate. Ecology. 2005;86:2154–2163. [Google Scholar]
- Friesen O.C., Roth J.D., Graham L.C. Sex-biased parasitism in monogamous arctic foxes is driven by diet. J. Mammal. 2015;96(2):417–424. doi: 10.1093/jmammal/gyv043. [DOI] [Google Scholar]
- Froy H., Sparks A.M., Watt K., Sinclair R., Bach F., Pilkington J.G., Pemberton J.M., McNeilly T.N., Nussey D.H. Senescence in immunity against helminth parasites predicts adult mortality in a wild mammal. Science. 2019;365:1296–1298. doi: 10.1126/science.aaw5822. [DOI] [PubMed] [Google Scholar]
- Geist V. University of Chicago Press; Chicago: 1971. Mountain Sheep. [Google Scholar]
- Girard-Buttoz C., Heistermann M., Rahmi E., Agil M., Fauzan P.A. Costs of mate-guarding in wild male long-tailed macaques (Macaca fascicularis): physiological stress and aggression. Horm. Behav. 2014;66:637–648. doi: 10.1016/j.yhbeh.2014.09.003. [DOI] [PubMed] [Google Scholar]
- Hartig F. DHARMa: residual diagnostics for hierarchical (multi-level/mixed) regression models. R package version 0.2.4. 2019 https://CRAN.R-project.org/package=DHARMa [Google Scholar]
- Hayward A.D., Behnke J.M., Childs D.Z., Corripio-Miyar Y., Fenton A., Fraser M.D., Kenyon F., McNeilly T.N., Pakeman R.J., Pedersen A.B., Pemberton J.M., Sweeny A.R., Wilson K., Pilkington J.G. Long-term temporal trends in gastrointestinal parasite infection in wild Soay sheep. Parasitology. 2022;149:1749–1759. doi: 10.1017/S0031182022001263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoar B.M. University of Calgary; Calgary, Alberta: 2012. Ecology and Transmission Dynamics of Ostertagia Gruehneri in Barrenground Caribou [PhD Thesis] [Google Scholar]
- Hogg J.T. Mating in bighorn sheep: multiple creative male strategies. Science. 1984;225:526–529. doi: 10.1126/science.6539948. [DOI] [PubMed] [Google Scholar]
- Irvine R.J., Stien A., Halvorsen O., Langvatn R., Albon S.D. Life-history strategies and population dynamics of abomasal nematodes in Svalbard reindeer ( Rangifer tarandus platyrhynchus ) Parasitology. 2000;120:297–311. doi: 10.1017/S0031182099005430. [DOI] [PubMed] [Google Scholar]
- Klein S.L. Hormonal and immunological mechanisms mediating sex differences in parasite infection. Parasite Immunol. 2004;26(6‐7):247–264. doi: 10.1111/j.0141-9838.2004.00710.x. [DOI] [PubMed] [Google Scholar]
- Kowalski K., Bogdziewicz M., Eichert U., Rychlik L. Sex differences in flea infections among rodent hosts: is there a male bias? Parasitol. Res. 2015;114(1):337–341. doi: 10.1007/s00436-014-4231-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutz S.J., Ducrocq J., Verocai G.G., Hoar B.M., Colwell D.D., Beckmen K.B., Polley L., Elkin B.T., Hoberg E.P. Advances in Parasitology. Elsevier; 2012. Parasites in ungulates of arctic north America and Greenland; pp. 99–252. [DOI] [PubMed] [Google Scholar]
- Lenth R., Singmann H., Love J., Buerkner P., Herve M. R Package Version 4.0-3; 2018. Package “emmeans”.http://cran.r-project.org/package=emmeans [Google Scholar]
- McKenna P.B. The diagnosis value and interpretation of faecal egg counts in sheep. N. Z. Vet. J. 1981;29(8):129–132. doi: 10.1080/00480169.1981.34821. [DOI] [PubMed] [Google Scholar]
- Merilä J., Björklund M., Bennett G.F. Geographic and individual variation in haematozoan infections in the greenfinch, Carduelis chloris. Can. J. Zool. 1995;73(10):1798–1804. [Google Scholar]
- Mougeot F., Redpath S.M., Piertney S.B. Elevated spring testosterone increases parasite intensity in male red grouse. Behav. Ecol. 2006;17(1):117–125. doi: 10.1093/beheco/arj005. [DOI] [Google Scholar]
- Nelson R.J., Demas G.E. Seasonal changes in immune function. Q. Rev. Biol. 1996;71:511–548. doi: 10.1086/419555. [DOI] [PubMed] [Google Scholar]
- Nielsen M.K., Vidyashankar A.N., Andersen U.V., DeLisi K., Pilegaard K., Kaplan R.M. Effects of fecal collection and storage factors on strongylid egg counts in horses. Vet. Parasitol. 2010;167:55–61. doi: 10.1016/j.vetpar.2009.09.043. [DOI] [PubMed] [Google Scholar]
- Nordling D., Andersson M., Zohari S., Lars G. Reproductive effort reduces specific immune response and parasite resistance. Proc. Roy. Soc. Lond. B. 1998;265:1291–1298. doi: 10.1098/rspb.1998.0432. [DOI] [Google Scholar]
- Patterson J.E.H., Ruckstuhl K.E. Parasite infection and host group size: a meta-analytical review. Parasitology. 2013;140:803–814. doi: 10.1017/S0031182012002259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier F., Page K.A., Ostiguy T., Festa‐Bianchet M., Lundberg P. Fecal counts of lungworm larvae and reproductive effort in bighorn sheep, Ovis canadensis. Oikos. 2005;110:473–480. [Google Scholar]
- Pelletier F., Festa‐Bianchet M. Sexual selection and social rank in bighorn rams. Anim. Behav. 2006;71:649–655. [Google Scholar]
- Pelletier F., Mainguy J., Cote S.D. Rut-induced hypophagia in male bighorn sheep and mountain goats: foraging under time budget constraints. Ethology. 2009;115:141–151. [Google Scholar]
- Poulin R. Helminth growth in vertebrate hosts: does host sex matter? Int. J. Parasitol. 1996;26(11):1311–1315. doi: 10.1016/S0020-7519(96)00108-7. [DOI] [PubMed] [Google Scholar]
- Poulin R. Sexual inequalities in helminth infections: a cost of being a male? Am. Nat. 1996;147:287–295. [Google Scholar]
- Prentice A.M., Prentice A. Energy costs of lactation. Annu. Rev. Nutr. 1988;8:63–79. doi: 10.1146/annurev.nu.08.070188.000431. [DOI] [PubMed] [Google Scholar]
- R Core Team . R: A language and environment for statistical computing. R. Foundation for Statistical Computing; Vienna, Austria: 2023. https://www.R-project.org/ [Google Scholar]
- Rachlow J.L., Bowyer R.T. Habitat selection by Dall’s sheep (Ovis dalli): maternal trade‐offs. J. Zool. 1998;245(4):457–465. doi: 10.1111/j.1469-7998.1998.tb00120.x. [DOI] [Google Scholar]
- Randolph P.A., Randolph J.C., Mattingly K., Foster M.M. Energy costs of reproduction in the cotton rat. Sigmodon Hispidus. Ecol. 1977;58:31–45. [Google Scholar]
- Rehbein S., Hamel D. A note on the relationship between fecal larval excretion and Protostrongylus rufescens lungworm burden in sheep. Parasitol. Res. 2022;121:1539–1543. doi: 10.1007/s00436-022-07485-9. [DOI] [PubMed] [Google Scholar]
- Rind R., Brohi M.R. Factors affecting the survival and sporulation of Eimeria oocysts of cattle. Pakistan J. Biol. Sci. 2001;4:487–491. [Google Scholar]
- Rossanigo C.E., Gruner L. Moisture and temperature requirements in faeces for the development of free-living stages of gastrointestinal nematodes of sheep, cattle and deer. J. Helminthol. 1995;69:357–362. doi: 10.1017/s0022149x00014954. [DOI] [PubMed] [Google Scholar]
- Ruckstuhl K. Foraging behaviour and sexual segregation in bighorn sheep. Anim. Behav. 1998;56:99–106. doi: 10.1006/anbe.1998.0745. [DOI] [PubMed] [Google Scholar]
- Saino N., Møller A.P., Bolzerna A.M. Testosterone effects on the immune system and parasite infestations in the barn swallow (Hirundo rustica): an experimental test of the immunocompetence hypothesis. Behav. Ecol. 1995;6(4):397–404. doi: 10.1093/beheco/6.4.397. [DOI] [Google Scholar]
- Salih S.Y., Marshall T.F., Radalowicz A. Morbidity in relation to the clinical forms and to intensity of infection in Schistosoma mansoni infections in the Sudan. Ann. Trop. Med. Parasitol. 1979;73:439–449. doi: 10.1080/00034983.1979.11687283. [DOI] [PubMed] [Google Scholar]
- Schmid-Hempel P. Evolutionary Parasitology: The Integrated Study of Infections, Immunology, Ecology, and Genetics. 2nd ed. Oxford University Press; Oxford: 2021. [DOI] [Google Scholar]
- Seivwright L.J., Redpath S., Mougeot F., Watt L., Hudson P.J. Faecal egg counts provide a reliable measure of Trichostrongylus tenuis intensities in free-living red grouse Lagopus lagopus scoticus. J. Helminthol. 2004;78:69–76. doi: 10.1079/joh2003220. [DOI] [PubMed] [Google Scholar]
- Sengupta M.E., Thapa S., Thamsborg S.M., Mejer H. Effect of vacuum packing and temperature on survival and hatching of strongyle eggs in faecal samples. Vet. Parasitol. 2016;217:21–24. doi: 10.1016/j.vetpar.2015.12.014. [DOI] [PubMed] [Google Scholar]
- Sparkes T.C., Keogh D.P., Pary R.A. Energetic costs of mate guarding behavior in male stream dwelling isopods. Oecologia. 1996;106:166–171. doi: 10.1007/BF00328595. [DOI] [PubMed] [Google Scholar]
- Stearns S.C. Oxford University Press; Oxford: 1992. The Evolution of Life Histories. [Google Scholar]
- Sweeny A.R., Corripio-Miyar Y., Bal X., Hayward A.D., Pilkington J.G., McNeilly T.N., Nussey D.H., Kenyon F. Longitudinal dynamics of co-infecting gastrointestinal parasites in a wild sheep population. Parasitology. 2022:1–12. doi: 10.1017/S0031182021001980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner W.C., Versfeld W.D., Kilian J.W., Getz W.M. Synergistic effects of seasonal rainfall, parasites and demography on fluctuations in springbok body condition. J. Anim. Ecol. 2012;81:58–69. doi: 10.1111/j.1365-2656.2011.01892.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dijk J., Morgan E.R. The effect of temperature on the development, hatching and survival of Nematodirus battus larvae. Parasitology. 2008;135:269–283. doi: 10.1017/S0031182007003812. [DOI] [PubMed] [Google Scholar]
- Wesołowska A. Sex-the most underappreciated variable in research: insights from helminth-infected hosts. Vet. Res. 2022;53(1):94. doi: 10.1186/s13567-022-01103-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wharton D.A., Allan G.S. Cold tolerance mechanisms of the free-living stages of Trichostrongylus colubriformis (Nematoda) J. Exp. Biol. 1989;145:353–369. doi: 10.1242/jeb.145.1.353. [DOI] [PubMed] [Google Scholar]
- Wickham H. Springer-Verlag; New York: 2016. ggplot2: Elegant Graphics for Data Analysis. [Google Scholar]
- Willisch C.S., Ingold P. Feeding or resting? The strategy of rutting male Alpine chamois. Ethology. 2007;113(1):97–104. doi: 10.1111/j.1439-0310.2006.01301.x. [DOI] [Google Scholar]
- Woolhouse M.E., Dye C., Etard J.-F., Smith T., Charlwood J.D., Garnett G.P., Hagan P., Hii J.L.K., Ndhlovu P.D., Quinnell R.J., et al. Heterogeneities in the transmission of infectious agents: implications for the design of control programs. P. Natl. A Sci. USA. 1997;94(1):338–342. doi: 10.1073/pnas.94.1.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan X., Liu M., He S., Tong T., Liu Y., Ding K., Deng H., Wang P. An epidemiological study of gastrointestinal nematode and Eimeria coccidia infections in different populations of Kazakh sheep. PLoS One. 2021 doi: 10.1371/journal.pone.0251307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuk M., McKean K.A. Sex differences in parasite infections: patterns and processes. Int. J. Parasitol. 1996;26(10):1009–1024. doi: 10.1016/s0020-7519(96)80001-4. [DOI] [PubMed] [Google Scholar]