Summary
Background
The Northern Territory (NT) has the highest prevalence of chronic hepatitis B (CHB) in Australia. The Hep B PAST program aims to improve health outcomes for people living with CHB.
Methods
This mixed methods study involves First Nations peoples living in the NT. We used participatory action research principles across three steps: 1. Foundation step: establishing hepatitis B virus (HBV) status and linkage to care; 2. Capacity building: training the health workforce; 3. Supported transition to primary healthcare: implementation of the “Hub and Spoke” model and in-language resources. Analysis occurred at three time points: 1. Pre-Hep B PAST (2018); 2. Foundation step (2020); and 3. Completion of Hep B PAST (2023). Evaluation focuses on four key indicators, the number of people: 1) with documented HBV status; 2) diagnosed with CHB; 3) receiving care; and 4) receiving treatment.
Findings
Hep B PAST (2018–23) reached 40,555 people. HBV status was documented in 11% (1192/10,853), 79.2% (26,075/32,915) and 90.8% (28,675/31,588) of people at pre-Hep B PAST, foundation step, and completion respectively. An estimated 99.9% (821/822) of people were diagnosed, 86.3% (709/822) engaged in care, and 24.1% (198/822) on antiviral treatment at completion. CHB prevalence in the study population is 2.6%, decreasing from 6.1% to 0.4% in the pre- and post-vaccination cohorts.
Interpretation
Hep B PAST is an effective model of care. Partner health services are exceeding elimination targets. This model could enable other countries to enhance the cascade of care and work towards eliminating HBV.
Funding
National Health and Medical Research Council.
Keywords: Hepatitis B virus, Chronic hepatitis B, Global public health, First Nations peoples, Primary healthcare
Research in context.
Evidence before this study
There is a global commitment to eliminate viral hepatitis as a public health threat by 2030. No countries are currently on track to meet this target. Testing and treatment coverage remains unacceptably low globally, with most care delivered through hospital outpatient settings. The Global Health Sector Strategy 2022–30 promotes simplified, decentralised service delivery models, supporting primary healthcare clinicians to provide care and treatment, and mobilising and strengthening community engagement. There is currently limited evidence detailing successful examples of these alternative models of care for people living with chronic hepatitis B (CHB). To meet global targets to eliminate CHB as a public health threat by 2030, a substantial increase in access to testing, care, and treatment is required. Although there has been some recent progress towards elimination, including decreased incident cases due to vaccination, it is estimated that 89.7% of people living with CHB globally remain undiagnosed. Scale-up of screening and treatment needs to be urgently implemented. Current models of care are failing to meet elimination targets.
Added value of this study
Australia is a high-income, low-CHB-prevalence country. CHB disproportionately affects First Nations and migrant populations in Australia, with evidence that culturally and linguistically diverse communities have healthcare access disparities. Our study setting in Australia's Northern Territory (NT) is geographically, socially, and culturally complex. The NT has the highest overall prevalence of CHB in Australia, with endemic levels of CHB in the First Nations population. This study used participatory action research methodology to implement a decentralised model of care that significantly improved the cascade of care for First Nations peoples living with CHB. These improvements were sustained across the five years of the study, including through the COVID-19 pandemic, resulting in an estimated 99.9% of people living with CHB being diagnosed, 86.3% engaged in care, and 24.1% receiving antiviral treatment, exceeding national and global CHB elimination targets.
Implications of all the available evidence
CHB care and treatment are still often restricted to tertiary care or provided by specialists. A paradigm shift to primary healthcare is needed to increase access and equity and to achieve elimination. Our findings provide new evidence that a decentralised, comprehensive primary healthcare model can significantly improve the hepatitis B virus cascade of care. The results have direct implications for Australia, and we call for increased investment to expand and roll out this model nationally. Globally, our model has the potential to be translated, including to low-to-middle-income countries.
Introduction
Global epidemiology and World Health Organization elimination commitment
Chronic hepatitis B (CHB) is a major public health issue, with an estimated 296 million people (3.8% of the world's population) living with this chronic infection.1 CHB disproportionately affects First Nations populations worldwide.2 Without appropriate care, CHB can lead to hepatocellular carcinoma (HCC) and cirrhosis in up to 25% of people,3 with over 800,000 people dying annually.1 CHB has considerable social and personal impacts,4 affecting health, well-being, and quality of life. HCC and cirrhosis in people living with CHB can be substantially reduced with engagement in care, monitoring, and treatment.3
The World Health Organization's (WHO) Global Health Sector Strategies 2022–30 (GHSS) prioritise the triple elimination of HIV, hepatitis C virus (HCV) and hepatitis B virus (HBV) as public health threats by 2030.5 Highly effective prevention and treatment interventions, such as vaccination, antenatal care and antiviral medication, have made CHB elimination possible.6 Recent efforts have shown progress in reducing incident cases due to vaccination.5 However, funding and strategies remain inadequate to meet elimination goals, leaving 89.7% undiagnosed globally.5 In many places worldwide, CHB remains in specialist care; however, most care can be successfully delivered in primary healthcare (PHC).5 The GHSS highlights the importance of removing structural and systemic barriers to healthcare access and addressing inequities by promoting a PHC framework.5 This includes simplified, decentralised service delivery models, capacity-building of the PHC workforce to deliver care and treatment, and strengthening community engagement.5
CHB in Australia
In Australia in 2021, an estimated 200,385 people were living with CHB.7 The Australian government supports global elimination goals, with the Third National Hepatitis B Strategy8 setting clear targets to improve access to care and reduce CHB-related morbidity and mortality.8 Targets to be achieved by 2022 were 80% of people living with CHB to be diagnosed, 50% engaged in care, and 20% on antiviral treatment.8 Australia supports access to antiviral medication, PHC, antenatal care, and HBV vaccine with public funding. Yet even with these public health interventions, to address HBV-related mortality and achieve elimination in Australia, substantial improvements in access to care, monitoring, and treatment are required.9
The Northern Territory (NT) has the highest prevalence of CHB in Australia (1.73% compared to 0.78%).7 NT First Nations peoples are disproportionately affected, with a 6% overall prevalence, declining in younger age groups since universal vaccination.10
HBV vaccine has been on the NT childhood immunisation schedule since 1990, with 93% of children fully immunised,11 exceeding the GHSS target of >90%.5 First Nations peoples in the NT have a unique HBV sub-genotype C4, which causes more aggressive disease.12 The HBV vaccine has a serotype mismatch with C4.13 Evidence suggests the vaccine is effective in preventing chronic infection but sub-optimal at protecting against hepatitis B core antibody positivity.13 First Nations peoples in the NT have an age-adjusted HCC incidence of 23 per 100,000 compared to 4 per 100,000 for non-Indigenous people, giving an incidence rate ratio of 5.9.14
Objectives
The Hep B PAST program aims to eliminate CHB as a public health threat in the NT, with elimination defined as no new incident cases and minimal morbidity and mortality associated with CHB. We aimed to understand the population's HBV status and epidemiology of CHB, improve the cascade of care, and build health workforce capacity to provide culturally safe care for CHB in PHC.
Methods
Study design
Hep B PAST is a co-designed population-based partnership that uses participatory action research (PAR) methodology. PAR situates power towards those most affected by a program's outcomes, recognised for strengthening social and emotional well-being and promoting decolonisation in First Nations peoples research.15
Study setting
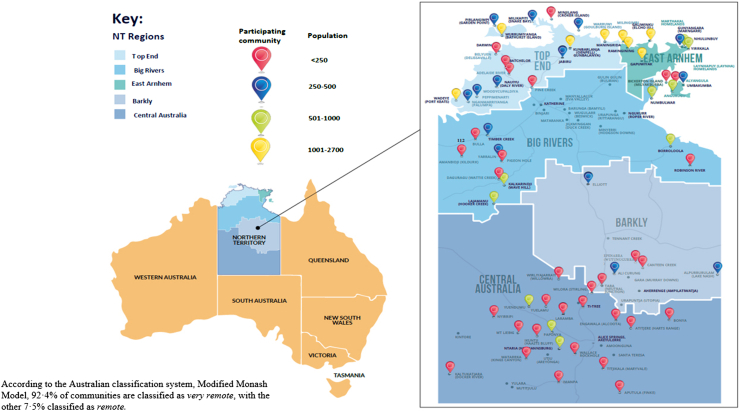
The NT covers a large geographical area, 1.3 million km2, and is sparsely populated, with 233,000 people.16 First Nations peoples comprise 26.3% of the population in the NT, with 77% living remotely in small communities.16
Participants
A total of 66 out of 87 (75.9%) remote community clinics in the NT are included in this study. All clinical primary healthcare services providing care to Aboriginal and Torres Strait Islander people in the NT were eligible to join the study. The study was promoted through direct contact with health services, Aboriginal community health boards, NT-wide high-level advisory committees, and the First Nations Reference Group. Services that had the capacity to join the Hep B PAST program in 2018 were recruited into the study and are included in this analysis. Reasons given by non-participating services for not joining the study included limited human resources to commit staff time, moratoriums on research and competing priorities. Subsequently, further services are now part of Hep B PAST and their data will be analysed separately once they complete all steps. The intention is to enable this program for all NT services. This study was approved by the Human Research Ethics Committee of NT Health and Menzies School of Health Research–HREC 2018–3242. Following extensive community consultation and with Indigenous Reference Group input into the consent processes, a hybrid consent model was used for different aspects of the Hep B PAST program, as detailed below. Consent for Step 1—Foundation Step–Hepatitis B status allocation and linkage to care was provided through health service level consent. Every individual enrolled in the health service was included in this part of the foundation step (Appendix pp. 19–41).
For inclusion into Step 1—Creation of the “Hep B Hub” the two consent process options were:
-
1.
Service level consent—every individual who is known to be living with CHB will be part of the “Hep B Hub” as a part of their usual care. with the option to opt-out on an individual basis.
-
2.
Individual consent–if the patient wishes to be included in the “Hep B Hub” but does not attend a service which has given service level consent, then a verbal discussion with their health care professional will occur. Individual written consent will be completed.
Recruitment into the evaluation of Step 2—Capacity building of the health workforce was by individual participants' written consent. Entry into Step 3—Supported transition to PHC required service level consent with the option to opt-out on an individual basis.
Procedures
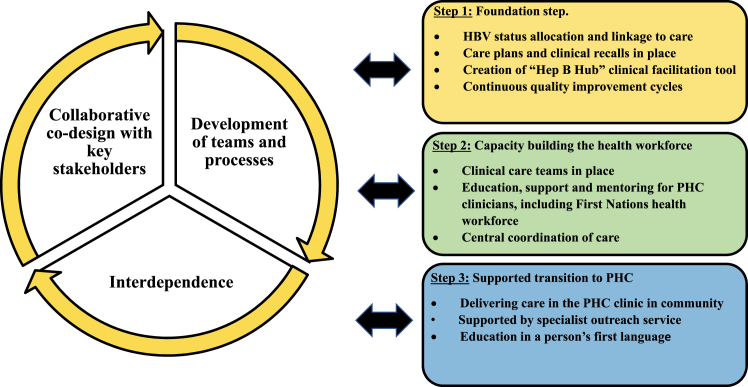
The Hep B PAST program model uses a systematic approach driven by a collaborative, reciprocal, iterative research process between the Hep B PAST team, First Nations Reference Group and communities, and those with lived experience of CHB (Fig. 1).
Fig. 1.
Hep B PAST research methodology model.
Step 1: foundation step
Hepatitis B status allocation and linkage to care
Eligible participants are defined as all First Nations people (0–100 years old) registered with a Hep B PAST partner health service clinic. We undertook the foundation step to establish participants’ HBV status, with a clinician systematically reviewing all existing pathology and vaccination data from multiple electronic health record systems (EHR), as described elsewhere.17 Each person was allocated a clearly defined HBV status, using consistent terminology: fully vaccinated, immune by exposure, infected, non-immune or unknown. The HBV status was added to the front screen of the EHRs, thus clearly visible to PHC clinicians. All people were linked to appropriate care, and clinicians were alerted to the follow-up actions required. Referral processes, and algorithms were defined in local guidelines and communicated regularly with clinicians (Appendix pp. 36 and 37). All people living with CHB were added to the “Hep B Hub”. This step took two years and was completed by trained study nurses.17
Creation of “Hep B Hub”
Embedded within an existing EHR system, we created and implemented an electronic register and care facilitation tool for people living with CHB–the “Hep B Hub”–to streamline clinical care through recalls, reminders and pre-populated care plans18 that included required investigations. Inclusion criteria for the “Hep B Hub” was all people living with CHB in the NT. Cascade of care analysis focused on First Nations people living with CHB in Hep B PAST partner clinics.
Continuous quality improvement (CQI) cycles
CQI cycles occurred every six months and included extraction and interpretation of HBV test and vaccination data from the “Hep B Hub”, to establish an HBV status and link people to care. Coordinators provided tailored reports for clinics, communicated with stakeholders, building trust and ownership and undertook in-person visits and teleconferences. Reports measured cascade of care outcomes and identified: priority people overdue for CHB care; people whose HBV status remained unknown (requiring testing); and non-immune people (requiring vaccination).
Step 2: clinical care teams and capacity building of the health workforce
Remote PHC clinics are staffed by a mix of First Nations health workforce and resident nurses, supported by visits and telehealth from doctors. In the hub and spoke model, the “Hep B Hub” clinical facilitation tool is the hub with clusters of communities as the spokes, allocated to clinical care teams consisting of a trained and supported: i) First Nations health worker, ii) General Practitioner (GP) or Nurse Practitioner (NP) who can prescribe antiviral treatment; and iii) access to ultrasound and transient elastography to measure liver stiffness (FibroScan®). Accredited GP and NP training is provided twice a year through the program. As there was no pre-existing CHB-specific education for the First Nations health workforce, we co-designed a “Managing hepatitis B” course. We describe this course elsewhere.19,20
Step 3: supported transition to primary healthcare
Delivery of CHB care in PHC clinical care teams was implemented through systematic care planning, PHC workforce education,19,20 CHB identified positions embedded in PHC, availability of ultrasound and FibroScan®, support by the specialist outreach teams21 and culturally appropriate in-language consumer resources.22 We have previously reported that having an educational tool in a person's first language is crucial to developing treatment partnerships for First Nations peoples living with CHB.23 The “Hep B Story” app is available in nine Aboriginal languages, covering 70% of NT First Nations peoples in their first languages.
Outcomes
We evaluated the effect of the Hep B PAST model of care on the cascade of care components critical to achieving HBV elimination. The key indicators are the number of people 1) with a known and documented HBV status, 2) diagnosed, 3) engaged in care, and 4) receiving antiviral treatment. Success is if the outcome improved over time (1–4) and elimination targets for each indicator were met (2–4). Analysis was at the health service level at three pre-specified time points: pre-Hep B PAST (2018), after the completion of the foundation step (2020) and at completion of the Hep B PAST program (2023). Differences in individuals included at each time point are due to population movement, births, and deaths. Whilst the main Hep B PAST study commenced in 2018, pilot studies commenced in some communities before this.17 Clinics that had not received pilot interventions before 2018 were analysed as a subset (Pre-Hep B PAST) to estimate the true baseline status of HBV care. Those people in the pre-Hep B PAST subset are included in the denominator of the foundation step.
For calculating the proportion of people diagnosed in the cascade of care, the number of people living with chronic hepatitis B in the group with unknown serostatus (i.e. not yet diagnosed) was assumed to be equivalent to the CHB prevalence in the population with known serostatus. To estimate the number of people infected, the total number of people living with CHB at completion of Hep B PAST was calculated as the sum of people diagnosed plus the estimated number with undiagnosed infection. The percentage prevalence of HBV infection (diagnosed and undiagnosed) at the commencement of Hep B PAST (2018) was assumed to be the same as that estimated at the completion of Hep B PAST (2023). This process assumes all HBV infections found during Hep B PAST were among people with previously unknown serostatus and that the true prevalence of HBV infection in our study cohort did not change substantially over time.
The definition of “engaged in care” is based on the number of people living with CHB who had HBV viral load monitoring within the past 15 months. The definition of “receiving antiviral treatment” is the number of people living with CHB prescribed antiviral medication. Current guidelines set eligibility criteria for treatment and are based on an assessment of the phase of the disease and whether liver damage is occurring. Current targets aim for 20% of people living with CHB to receive antiviral treatment.8
Statistical analysis and data validation
All data were extracted from the “Hep B Hub” and participating partner clinics EHR systems, compiled in Microsoft 365 Apps for enterprise, Access, and analysed in STATA v16 (StataCorp, College Station, Texas, USA). The data management team performed detailed quality checks on all extracted data, including triangulation using multiple EHR systems. Binomial confidence intervals were calculated for proportions. Differences in proportions between different stages of Hep B PAST were assessed by chi-square test. Statistical significance was defined as a p < 0.0001.
Role of the funding source
The study's funders had no role in the study design, data collection, analysis, interpretation, or writing of the report.
Results
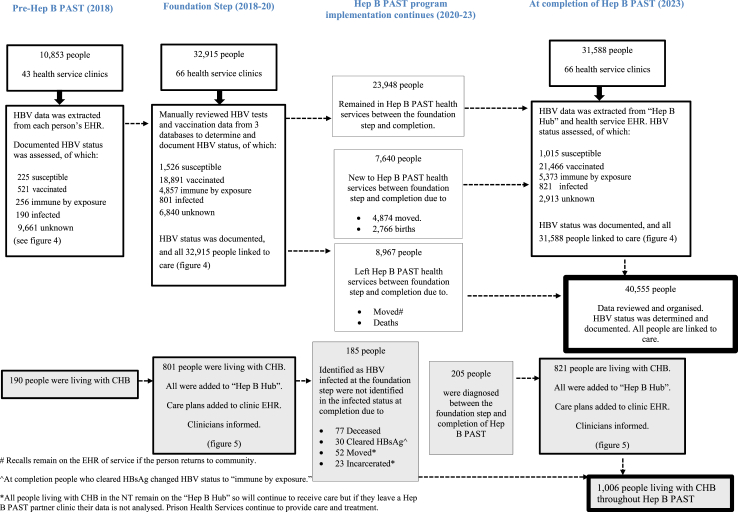
Participants A total of 40,555 First Nations people were part of the Hep B PAST program. At completion (2023), 31,588 people remained residents in participating health services (Fig. 2), representing 51.6% of the First Nations population in the NT.16
Fig. 2.
Flowchart detailing the movement of participants through Hep B PAST health services throughout the program (2018–23)Descriptive data.
The demographics of the study population remained broadly consistent throughout the study period (Table 1) and were representative of NT's First Nations population.16 The population of participating communities ranged from 20 to 2671 people (Fig. 3).
Table 1.
Demographics of the study population Pre-Hep B PAST (2018), foundation step (2020) and Completion of Hep B PAST (2023).
| Pre-Hep B PAST, 2018 N = 10,853 (%)a | Foundation step, 2020 N = 32,915 (%)a | Completion, 2023 N = 31,588 (%)a | |
|---|---|---|---|
| Sex | |||
| Male | 5113 (47.1) | 16,206 (49.2) | 15,428 (48.8) |
| Female | 5626 (51.8) | 16,535 (50.2) | 16,042 (50.8) |
| Unknown | 114 (1.1) | 174 (0.5) | 118 (0.4) |
| Age (years) | |||
| <10 | 2092 (19.3) | 5947 (18.1) | 5506 (17.4) |
| 10–19 | 2033 (18.7) | 6565 (20.0) | 6235 (19.7) |
| 20–29 | 1642 (15.1) | 5497 (16.7) | 5948 (18.8) |
| 30–39 | 1391 (12.8) | 4609 (14.0) | 5059 (16.0) |
| 40–49 | 1067 (9.8) | 3695 (11.2) | 3878 (12.3) |
| 50–59 | 733 (6.8) | 2563 (7.8) | 2930 (9.3) |
| 60–69 | 337 (3.1) | 1413 (4.3) | 1422 (4.5) |
| 70–79 | 126 (1.2) | 562 (1.7) | 446 (1.4) |
| 80–89 | 34 (0.3) | 197 (0.6) | 112 (0.4) |
| 90+ | 2 (0.0) | 89 (0.3) | 19 (0.1) |
| Unknown | 1396 (12.9) | 1778 (5.4) | 33 (0.1) |
| Median (IQR) | 23.5 (11.2, 39.5) | 25.5 (12.7, 41.9) | 26.6 (13.8, 42.1) |
| Residenceb | |||
| Remote | (0.0) | 2504 (7.6) | 1075 (3.4) |
| Very remote | 10,853 (100.0) | 30,411 (92.4) | 30,513 (96.6) |
All values n (%N) unless otherwise stated.
According to the Australian classification system, Modified Monash Model.
Fig. 3.
Map of Australia and NT, indicating health services participating in Hep B PAST.
HBV status allocation
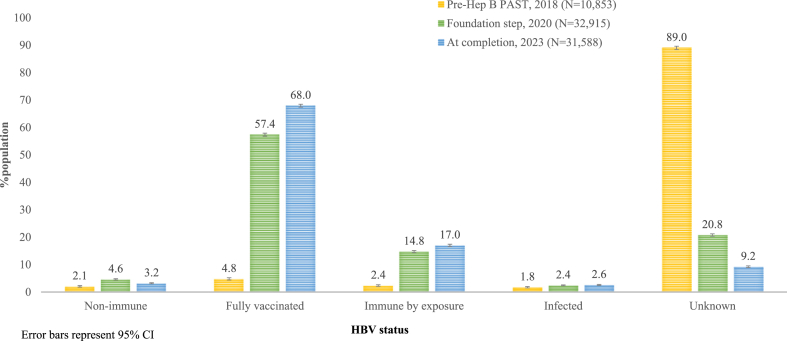
The foundation step of determining and allocating HBV status and subsequent CQI cycles found statistically significant improvements in all HBV status allocations and at all timepoints (p < 0.0001) (Fig. 4, Appendix pp. 3–9). Pre-Hep B PAST, 1192/10,853, 11.0% of people had a known and documented HBV status, meaning (9661/10,853), 89.0% [95% CI 88.4–89.6] had an unknown and undocumented status. On completion of the foundation step, this increased to (26,075/32,915), 79.2% of people who had enough existing clinical data to determine an HBV status, meaning that (6840/32,915), 20.8% had an unknown status. At completion of Hep B PAST (28,675/31,588), 90.8% of the study population had a known and documented HBV status, with (2913/31,588), 9.2% [95% CI 9.2–9.5] remaining unknown. At the foundation step, the non-immune, susceptible to HBV status was 4.6% [95% CI 4.4–4.8]. All people non-immune at the foundation step and at each CQI cycle had a vaccination care plan added to their EHR. As a result, the proportion who remained susceptible to HBV at completion of Hep B PAST declined to 3.2% [95% CI 3.2–3.4]. Improvements were also reflected in the vaccination status code.
Fig. 4.
Documented HBV status of the study population, comparing time points 1. Pre–Hep B PAST (2018), 2. Foundation step (2020), and 3. Completion of Hep B PAST (2023).
There were 23,948 people who were included in both the foundation step and the completion of Hep B PAST (Fig. 2). Of which 19,539 (81.6%) people's HBV status remained the same 2995 (12.5%) went from unknown to a known status, and 626 (2.6%) changed from non-immune to vaccinated between the two time points.
CHB cascade of care
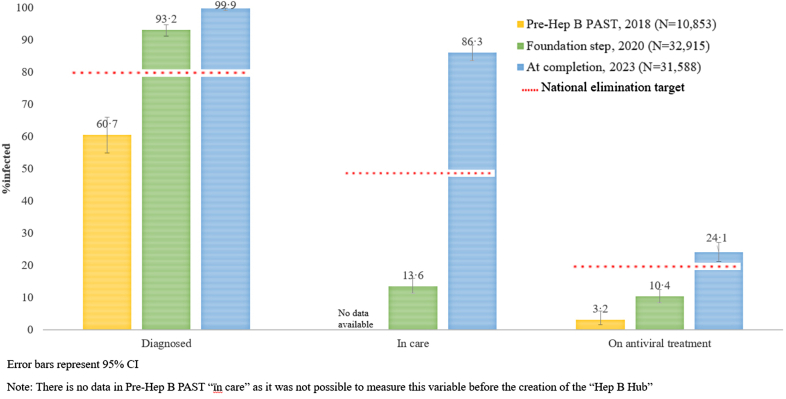
As part of the foundation step and ongoing CQI, all people living with CHB were added to the “Hep B Hub” to facilitate care. We found statistically significant improvements in all domains of the cascade of care (p < 0.0001). At completion of Hep B PAST, the estimated cascade of care was (821/822), 99.9% [95% CI 99.3–99.9] diagnosed, (709/822), 86.3% [95% CI 83.7–88.5] engaged in care and (198/822), 24.1% [95% CI 21.2–27.2] on antiviral treatment (Fig. 5).
Fig. 5.
Cascade of care of the study population comparing time points 1. Pre-Hep B PAST (2018), 2. Foundation step (2020), and 3. Completion of Hep B PAST (2023).
CHB prevalence
In the Pre-Hep B PAST cohort (N = 10,853), there were 190 people diagnosed with CHB. The foundation step (N = 32,915) identified 801 people living with CHB; a care plan was initiated, and each person was included in the “Hep B Hub”, thus entering them into a cascade of care. There were 185 people who were HBV positive at foundation step who were no longer counted in the HBV infected status at completion of Hep B PAST (2023), of which 76 people had died, 30 people had cleared their hepatitis B surface antigen (no longer infected), and 78 people had moved to a non-participating health service (Fig. 2, Appendix p. 14).
Between the foundation step and completion of the study, 205 additional people were diagnosed with CHB, accounting for 24.9% of the total people living with CHB at completion. At the completion of Hep B PAST (N = 31,588), 821 people were living with CHB, giving an overall prevalence of 2.6% [95% CI 2.4–2.8] in participating health services.
The CHB prevalence among people born before 1990, when universal vaccination was introduced, was 6.1% [95% CI 5.6–6.5], decreasing to 0.4% [95% CI 0.3–0.5] in the post-vaccination cohort (Appendix p. 12). The overall prevalence in males was 3.7% and in females 2.2% (Appendix p. 11). Further analyses of subgroups can be found in the Appendix.
Discussion
We demonstrated marked improvements in the CHB cascade of care, achieved through partnerships and a co-designed and implemented model of care. Hep B PAST partner services are exceeding national HBV elimination targets. Success factors include: systematic data organisation,17 regular CQI cycles, a hub and spoke model of care, a trained First Nations and PHC health workforce,19,20 dedicated staffing, outreach access to diagnostic technology and in-language consumer health resources.22 The Hep B PAST program consists of a diverse, multidisciplinary team that includes researchers, epidemiologists, public health experts, lab scientists, clinical doctors, nurses, Aboriginal health workers, and people living with CHB. This collaborative approach, coupled with a culture of true partnerships and flattened hierarchies, has been key to its success. Australia also benefits from universal access to publicly funded healthcare. Implementing this model could enable other regions and countries to improve CHB care in PHC settings, enhance the cascade of care and work towards eliminating HBV as a public health threat.
Over time, Australia has seen improvements in the CHB cascade of care. In 2021, an estimated 73% of people living with CHB were aware of their diagnosis, 26% were engaged in care and 13% receiving antiviral treatment.7 Despite significant progress, Australia is still well below elimination targets. Hep B PAST cascade of care metrics are now 99.9% diagnosed, 86.3% in care and 24.1% on treatment. The NT is predicted to be the only jurisdiction in Australia where CHB-related mortality is continuing to decline.9
Models of care
The GHSS aims to guide strategically focused responses to the elimination of HIV, HCV, and HBV by 2030.5 The HIV response has benefited from decades of global action, supported by strong community engagement, a public health approach, financial investment and resourcing, and expansion of services with a focus on addressing inequities.5 Similarly, integration within PHC, with safe and well-tolerated treatments, has improved the HCV cascade of care24,25 and reduced HCV prevalence.25 Despite the GHSS vision for simplified, decentralised CHB care,5 many people living with CHB globally still rely on care in hospitals.5
Although less is described about CHB models of care, a recent systematic review assessed the effectiveness of interventions targeting social determinants of health in the context of HBV.26 Interventions targeting race, culture, language, and socioeconomic status successfully enhanced HBV prevention, care, and treatment,26 with key themes including knowledge, education, diagnosis, screening, clinical engagement, treatment, upstream prevention, and vaccination found across the studies.26 The health and life expectancy of First Nations peoples in Australia have been adversely affected by the social, cultural, and commercial determinants of health.27 In our study context, the social determinants of health, including remoteness, systemic racism, colonisation, overcrowded housing, comorbidities, limited healthcare access, and poverty, all heighten vulnerability to HBV transmission and increase the potential for adverse CHB outcomes. The Hep B PAST program includes equitable and social determinants-focused interventions involving the community at all stages. From the beginning, PAR principles prioritised community input which shaped the program's direction. Concerns about additional blood tests prompted a focus on data linkage. The community also expressed a desire for on-country care and more information about CHB, leading to training for the primary healthcare workforce, enabling local CHB management and improving access to care. The one-stop liver shop and the development of an app directly responded to community requests, improving access to culturally safe care and information in preferred languages. The Hep B PAST program provides new evidence that a comprehensive PHC model can significantly improve the CHB cascade of care.
Effective data utilisation and linkage to care are crucial. The foundation step exposed substantial amounts of existing vaccine and pathology data. We demonstrated that systematic data organisation and interpretation by a clinician, using simplified, visible HBV codes, and the introduction of the “Hep B Hub” substantially improved understanding of HBV status and management among PHC clinicians. The process facilitated the identification of people living with CHB, enabled a better understanding of CHB epidemiology and improved the cascade of care, highlighting linkage to care as a priority for focused improvement strategies. Globally, limited accessibility to screening often serves as the primary factor contributing to a high proportion of people with unknown HBV status.
Continuous quality improvement (CQI) processes are essential for ongoing clinical and data management and program sustainability. CQI is a systematic, cyclical process using objective measurements and clear indicators to enhance the implementation of best-practice management in PHC.28 Hep B PAST used CQI processes to link people to care and provide each clinic with regular, tailored reports, resulting in testing and vaccination completion. Collaboration between the Hep B PAST team and the trained Aboriginal health workforce was instrumental in identifying priority people and addressing barriers to engagement. This partnership fostered ownership and sustainability and tailored local solutions.
Globally, a decrease in CHB prevalence since 2016 is likely due to increasing universal infant vaccination coverage, mortality and better-quality prevalence data collection.29 All of these factors were observed in our study, with a prevalence of 0.4% in the post-vaccination cohort, improved data organisation providing greater visibility into CHB prevalence, and sadly, we also noted a reduction in CHB prevalence due to mortality.
Hub and spoke support model and trained health workforce
A CHB register can result in improved treatment uptake.30 We found the “Hep B Hub” clinical facilitation tool to be a robust system to support guideline-based CHB care delivery and analysis of antiviral uptake. However, the other components of the Hep B PAST program are also required to achieve elimination of CHB. In the NT, the “One Stop Liver Shop” implemented in one remote community showed improvements in the CHB cascade of care.21 Hep B PAST used the hub and spoke model to expand this approach. Successful transition of CHB care to PHC teams requires a trained and supported health workforce. Limited adherence to CHB clinical guidelines in PHC may be due to gaps in health provider knowledge and high staff turnover.21 We delivered prescriber training twice a year throughout Hep B PAST. We co-designed and delivered training for the First Nations health workforce, which led to increased and sustained CHB-related knowledge and engagement.19,20 Ongoing education, mentoring and support were provided to all members of the clinical care teams. Sustained funding has been committed from the NT government to continue to deliver these trainings twice-yearly.
Limitations and next steps
Despite our CHB management success in participating health services, there remain health services in the NT that are yet to benefit from the Hep B PAST model of care. We have begun working with many of these services and seek to secure funding to expand the program. An economic evaluation is underway of the Hep B PAST program to assess the feasibility and scalability to other contexts and to quantify the cost-benefit analysis of the model of care versus the status quo approach.
In-line with NT guidelines, people born since the implementation of universal infant HBV vaccination in 1990 were classified as fully vaccinated if they received three documented vaccinations as infants, regardless of serological evidence confirming immunity or the absence of infection. Ideally, all people should have an HBV test, especially in high CHB-prevalence settings, particularly considering sub-genotype C4 has a serotype mismatch with the HBV vaccine.12 Hep B PAST has directly influenced policy and guidelines locally and nationally, including a recommendation for one-off HBV testing for everyone in the NT. Subsequent research generated from this study will include analysis of serological data within this post-vaccine cohort. One further limitation was the lack of a control group for analysis making it possible that other factors outside of the Hep B PAST program contributed to the observed improvements.
Conclusion
The Hep B PAST program facilitated testing, treatment, and the provision of culturally safe care in PHC. It is an effective model of care that strengthens partnerships, communication and connections between people living with CHB, community, health organisations and healthcare professionals. It promotes patient-centred care and a decentralised service model. Partner services are exceeding national HBV elimination targets. We encourage broad translation and implementation of this transferrable model, including to low-to-middle-income countries, other complex environments, and other conditions. Using PAR approaches, we successfully transferred the Hep B PAST model to NT's remote community COVID-19 response. Success requires time, ethical space, and ensuring the voice of lived experience is included in adaptation. Achieving HBV elimination requires investment, leadership, political commitment, and strong public policy.
Contributors
KH, SYCT, JSD, and JD conceived of the concept, design, and methodology of the study. KH, TAF, PN, PM, KF, BGS, JTB, CG, and PB contributed to data acquisition and curation. KH, JD, PB, JSD and DB conducted formal analysis, and KH and JD accessed and verified the data. JD, CC, KH, SYCT and JSD obtained funding for the study. All authors contributed to the investigation. MMc, EVC, TAF, PM, CG, SS, and PN provided project administration, material, or technical support. SB, GG, TDS, PMW, KH, PB developed resources, JD, CC, and JSD provided supervision, KH writing—original draft, all authors contributed to writing–review & editing of manuscript. KH had final responsibility for the decision to submit for publication.
Data sharing statement
Ethical and privacy considerations restrict public access to the data collected and analysed in this study. Data will not be shared prior to the final analysis is published by the study's authors. Following this time, data access proposals will require consideration and approval by the study management committee (the data custodians). Data requests can be made through the Hep B PAST steering committee, email: Hepbpast@menzies.edu.au. The study protocol and analysis plan are available in (Appendix pp. 13–48).
Editor note
The Lancet Group takes a neutral position with respect to territorial claims in published maps and institutional affiliations.
Declaration of interests
JD, JSD, and SYCT received a National Health and Medical Research Council partnership grant to undertake this work. This grant and the in-kind contributions of the partner organisations have supported many of the authors in undertaking fieldwork (GS) and travel for meetings (JSD, KF, NA, SB, BGS) and conferences (PB, TDS, TF, PW, EVC, GG). KH, BGS, JTB and JD report payment/honoraria for ASHM presentations donated to ASHM partners. BGS received donation of fibroscan from NSW health to NT health.
Acknowledgements
The authors acknowledge and pay respect to the past and present Traditional Custodians and Elders and the continuation of all Aboriginal and Torres Strait Islander peoples' cultural, spiritual, educational, health and research practices. The Aboriginal and Torres Strait Islander authors of this paper have given permission to use the terminology First Nations peoples for an international audience. Hep B PAST was supported by an Australian Government National Health and Medical Research Council (NHMRC) partnership grant–GNT1151837. KH is undertaking a PhD and has an NHRMC scholarship, GNT1190918. Funders played no role in the study design, analysis, or the decision to publish. We are grateful to all partners in Hep B PAST (Appendix pp. 49–53), and we would like to thank and acknowledge all people living with CHB and all our dedicated clinicians and health workforce.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lanwpc.2024.101116.
Appendix A. Supplementary data
References
- 1.World Health Organization . WHO; 2023. Hepatitis B fact sheet.https://www.who.int/news-room/fact-sheets/detail/hepatitis-b [Google Scholar]
- 2.World Health Organization . 2017. Global hepatitis report.https://apps.who.int/iris/bitstream/handle/10665/255016/9789241565455-eng.pdf;jsessionid=226A79C9E3F0795555FE78576DBA98DB?sequence=1 Geneva. [Google Scholar]
- 3.Suk-Fong Lok A. Hepatitis B treatment: what we know now and what remains to be researched. Hepatol Commun. 2019;3(1):8–19. doi: 10.1002/hep4.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tu T., Block J.M., Wang S., Cohen C., Douglas M.W. The lived experience of chronic hepatitis B: a broader view of its impacts and why we need a cure. Viruses. 2020;12(5):515. doi: 10.3390/v12050515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization Global health sector strategies on, respectively, HIV, viral hepatitis and sexually transmitted infections for the period 2022-2030. 2022. https://www.who.int.publications/i/item/9789240053779
- 6.Cooke G.S., Andrieux-Meyer I., Applegate T.L., et al. Accelerating the elimination of viral hepatitis: Lancet gastroenterology & hepatology commission. Lancet Gastroenterol Hepatol. 2019;4(2):135–184. doi: 10.1016/S2468-1253(18)30270-X. [DOI] [PubMed] [Google Scholar]
- 7.MacLachlan J.H., Romero N., Purcell I., Cowie B.C. Viral hepatitis mapping project: hepatitis B. National Report 2021. Darlinghurst, NSW: Australasian Society for HIV, Viral Hepatitis, and Sexual Health Medicine (ASHM) 2023. ashm.org.au/vh-mapping-project
- 8.Australian Government Department of Health . Commonwealth of Australia; Canberra: 2018. Third national hepatitis B strategy 2018-2022.https://www1.health.gov.au/internet/main/publishing.nsf/Content/ohp-bbvs-1/$File/Hep-B-Third-Nat-Strategy-2018-22.pdf [Google Scholar]
- 9.McCulloch K., Romero N., Allard N., MacLachlan J.H., Cowie B.C. Modelling jurisdictional disparities in the cascade of care for chronic hepatitis B in Australia: impact of treatment uptake on mortality. Aust N Z J Public Health. 2023;47(1) doi: 10.1016/j.anzjph.2022.100011. [DOI] [PubMed] [Google Scholar]
- 10.Davies J., Li S.Q., Tong S.Y., et al. Establishing contemporary trends in hepatitis B sero-epidemiology in an Indigenous population. PLoS One. 2017;12(9) doi: 10.1371/journal.pone.0184082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Australian Government Department of Health and Ageing . Commonwealth of Australia; Canberra: 2023. Current coverage data tables for all children. Current coverage data tables for all children | Australian Government Department of Health and Aged Care. [Google Scholar]
- 12.Littlejohn M., Davies J., Yuen L., et al. Molecular virology of hepatitis B virus, subgenotype C4 in northern Australian Indigenous populations. J Med Virol. 2014;86:695–706. doi: 10.1002/jmv.23888. [DOI] [PubMed] [Google Scholar]
- 13.Cheah B.C., Davies J., Singh G.R., et al. Sub-optimal protection against past hepatitis B virus infection where subtype mismatch exists between vaccine and circulating viral genotype in northern Australia. Vaccine. 2018;36(24):3533–3540. doi: 10.1016/j.vaccine.2018.01.062. [DOI] [PubMed] [Google Scholar]
- 14.Parker C., Tong S.Y., Dempsey K., et al. Hepatocellular carcinoma in Australia's northern territory: high incidence and poor outcome. Med J Aust. 2014;201(8):470–474. doi: 10.5694/mja13.11117. [DOI] [PubMed] [Google Scholar]
- 15.Dudgeon P., Bray A., Darlaston-Jones D., Walker R. Lowitja Institute; Australia: 2020. Aboriginal participatory action research: an Indigenous research methodology strengthening decolonisation and social and emotional wellbeing.https://policycommons.net/artifacts/10779440/aboriginal-participatory-action-research/11657560/ [Google Scholar]
- 16.Australian Bureau of Statistics. Snapshot of Northern Territory . Commonwealth of Australia; Canberra, Australia: 2022. High level summary for northern territory in 2021. Snapshot of Northern Territory | Australian Bureau of Statistics (abs.gov.au) [Google Scholar]
- 17.Hosking K., Stewart G., Mobsby M., et al. Data linkage and computerised algorithmic coding to enhance individual clinical care for Aboriginal people living with chronic hepatitis B in the Northern Territory of Australia - is it feasible? PLoS One. 2020;15(4) doi: 10.1371/journal.pone.0232207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xiao Y., van Gemert C., Howell J., et al. A survey of knowledge, attitudes, barriers and support needs in providing hepatitis B care among GPs practising in Australia. BMC Primary Care. 2022;23(1):137. doi: 10.1186/s12889-021-11916-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hosking K., De Santis T., Vintour-Cesar E., et al. “The most culturally safe training I’ve ever had”: the co-design of a culturally safe managing hepatitis B training course with and for the Aboriginal health workforce of the Northern Territory of Australia. BMC Health Serv Res. 2023;23(1):935. doi: 10.1186/s12913-023-09902-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hosking K., De Santis T., Vintour-Cesar E., et al. "Putting the power back into community": a mixed methods evaluation a chronic hepatitis B training course for the Aboriginal health workforce of Australia's Northern Territory. PLoS One. 2024;19(1):e0288577. doi: 10.1371/journal.pone.0288577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hla T.K., Bukulatjpi S.M., Binks P., Gurruwiwi G.G., Dhurrkay R.G., Davies J. A "one stop liver shop" approach improves the cascade-of-care for aboriginal and torres strait islander Australians living with chronic hepatitis B in the northern territory of Australia: results of a novel care delivery model. Int J Equity Health. 2020;19(1):64. doi: 10.1186/s12939-020-01180-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Binks P., Ross C., Gurruwiwi G.G., et al. Adapting and translating the “Hep B Story” App the right way: a transferable toolkit to develop health resources with, and for, Aboriginal people. Health Promot J Austr. 2024 doi: 10.1002/hpja.858. [DOI] [PubMed] [Google Scholar]
- 23.Davies J., Bukulatjpi S., Sharma S., Davis J., Johnston V. "Only your blood can tell the story" - a qualitative research study using semi- structured interviews to explore the hepatitis B related knowledge, perceptions and experiences of remote dwelling Indigenous Australians and their health care providers in northern Australia. BMC Public Health. 2014;14(1):1233. doi: 10.1186/1471-2458-14-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oru E., Trickey A., Shirali R., Kanters S., Easterbrook P. Decentralisation, integration, and task-shifting in hepatitis C virus infection testing and treatment: a global systematic review and meta-analysis. Lancet Global Health. 2021;9(4):e431–e445. doi: 10.1016/S2214-109X(20)30505-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hassanin A., Kamel S., Waked I., Fort M. Egypt's ambitious strategy to eliminate hepatitis C virus: a case study. Glob Health Sci Pract. 2021;9(1):187–200. doi: 10.9745/GHSP-D-20-00234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anyiwe K., Erman A., Hassan M., et al. Characterising the effectiveness of social determinants of health-focused hepatitis B interventions: a systematic review. Lancet Infect Dis. 2024;24(6):e366–e385. doi: 10.1016/S1473-3099(23)00590-X. [DOI] [PubMed] [Google Scholar]
- 27.Commonwealth of Australia . 2022. Commonwealth closing the gap annual report. Canberra, Australia. Commonwealth Closing the Gap Annual Report 2022 (niaa.gov.au) (accessed Sep 2023) [Google Scholar]
- 28.Ivers N., Jamtvedt G., Flottorp S., et al. Audit and feedback: effects on professional practice and healthcare outcomes. Cochrane Database Syst Rev. 2012;6 doi: 10.1002/14651858.CD000259.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Razavi-Shearer D., Gamkrelidze I., Pan C., et al. Global prevalence, cascade of care, and prophylaxis coverage of hepatitis B in 2022: a modelling study. Lancet Gastroenterol Hepatol. 2023;10 doi: 10.1016/S2468-1253(23)00197-8. [DOI] [PubMed] [Google Scholar]
- 30.Robotin M.C., Masgoret X., Porwal M., Goldsbury D., Khoo C., George J. Using a chronic hepatitis B registry to support population-level liver cancer prevention in Sydney, Australia. Clin Epidemiol. 2018;10:41–49. doi: 10.2147/CLEP.S146275. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.