Key Points
Question
Is there an association between common environmental toxicants and depressive symptoms among US adults?
Findings
This cross-sectional study of 3427 adults from the National Health and Nutrition Examination and Survey found that 27 environmental toxicant levels measured in blood or urine samples were associated with depressive symptoms, as assessed by the 9-item Patient Health Questionnaire. Systemic inflammation assessed by peripheral white blood cell count mediated these associations.
Meaning
These findings suggest that many common environmental toxicants are associated with depressive symptoms, providing potential targets for intervention measures and mechanistic research.
This cross-sectional study examines the association between environmental toxicant exposure and depressive symptoms in a population of US adults using an exposome approach based on data from the National Health and Nutrition Examination Survey.
Abstract
Importance
Recognizing associations between exposure to common environmental toxicants and mental disorders such as depression is crucial for guiding targeted mechanism research and the initiation of disease prevention efforts.
Objectives
To comprehensively screen and assess the associations between potential environmental toxicants and depressive symptoms and to assess whether systemic inflammation serves as a mediator.
Design, Setting, and Participants
A total of 3427 participants from the 2013-2014 and 2015-2016 waves of the National Health and Nutrition Examination and Survey who had information on blood or urine concentrations of environmental toxicants and depression scores assessed by the 9-item Patient Health Questionnaire (PHQ-9) were included. Statistical analysis was performed from July 1, 2023, to January 31, 2024.
Exposures
Sixty-two toxicants in 10 categories included acrylamide, arsenic, ethylene oxide, formaldehyde, iodine, metals, nicotine metabolites, polycyclic aromatic hydrocarbons, volatile organic compound (VOC) metabolites; and perchlorate, nitrate, and thiocyanate.
Main Outcomes and Measures
An exposome-wide association study and the deletion-substitution-addition algorithm were used to assess associations with depression scores (PHQ-9 ≥5) adjusted for other important covariates. A mediation analysis framework was used to evaluate the mediating role of systemic inflammation assessed by the peripheral white blood cell count.
Results
Among the 3427 adults included, 1735 (50.6%) were women, 2683 (78.3%) were younger than 65 years, and 744 (21.7%) were 65 years or older, with 839 (24.5%) having depressive symptoms. In terms of race and ethnicity, 570 participants (16.6%) were Mexican American, 679 (19.8%) were non-Hispanic Black, and 1314 (38.3%) were non-Hispanic White. We identified associations between 27 chemical compounds or metals in 6 of 10 categories of environmental toxicants and the prevalence of depressive symptoms, including the VOC metabolites N-acetyl-S-(2-hydroxy-3-butenyl)-l-cysteine (odds ratio [OR], 1.74 [95% CI, 1.38, 2.18]) and total nicotine equivalent-2 (OR, 1.42 [95% CI, 1.26-1.59]). Men and younger individuals appear more vulnerable to environmental toxicants than women and older individuals. Peripheral white blood cell count mediated 5% to 19% of the associations.
Conclusions and Relevance
In this representative cross-sectional study of adults with environmental toxicant exposures, 6 categories of environmental toxicants were associated with depressive symptoms with mediation by systemic inflammation. This research provides insight into selecting environmental targets for mechanistic research into the causes of depression and facilitating efforts to reduce environmental exposures.
Introduction
Depression is one of the key diseases covered by the World Health Organization’s Mental Health Gap Action Programme. An estimated 3.8% of the population is affected by depression globally.1,2 Mental illnesses have led to the loss of several billion dollars annually in the US. The international community has invested in a concerted effort to improve mental health, but satisfactory outcomes have yet to be achieved as of 2020.3 Therefore, the identification of modifiable risk factors for depression is critical.4
Psychiatric disorders have complex, multifaceted, and interrelated environmental origins.5 With increasing environmental pollution, many pollutants may impact human disease.6 Previous studies focused on the use of a priori knowledge to assess associations between individual or single categories of candidate exposures and depression7 and have obtained sufficient evidence that hundreds of natural or synthetic toxicants, such as volatile organic compounds (VOCs), metals, and polycyclic aromatic hydrocarbons (PAHs), are associated with depression in humans.8,9,10,11,12 However, there are inherent limitations to this approach. For example, some toxicants that show an association with depression when studied in isolation may not prove robust or as clinically relevant when considered in combination with other toxicants.13
Exposome studies provide a scientific framework to uncover the biological consequences of exposure to a wide range of risk factors, allowing for the avoidance of problems related to selective reporting and confounding by coexposures.14,15 To date, exposome studies have uncovered the associations of multiple behavioral risk factors and social factors with depression,16,17 while exposome studies exploring the associations of environmental toxicants with depression remain limited.8 In addition, exploration of the associations of environmental toxicants with depressive disorders has been limited by sample size and the lack of comprehensive measurements of environmental toxicants. Therefore, a comprehensive exposome analysis of environmental toxicants is essential for investigating the association between these toxicants and depression.
Previous studies have identified the activation of inflammatory pathways as one of the key mechanisms involved in the pathogenesis of depression and have revealed several inflammatory biomarkers associated with depression, including total white blood cells (WBCs) and C-reactive protein.18,19 Previous studies indicate that inflammation could serve as a crucial link between environmental toxicants and depression.20,21,22,23 However, there are significant knowledge gaps in addressing multivariate mediation involving not only high-dimensional mediators but also multiple toxicants with inherent covariance and grouping structures. Therefore, a framework for the analysis of high-dimensional mediators is required to explore a mixed mediation setting involving multiple exposure categories and groups of endogenous biomarker mediators.24,25
Based on a no-hypothesis approach, the purpose of this study was to comprehensively examine the association between environmental toxicant exposure and depressive symptoms in a population of US adults using an exposome approach based on data from the National Health and Nutrition Examination Survey (NHANES) to provide a theoretical basis for policy-driven exposure reduction and depression prevention interventions. In addition, a mediation analysis framework was implemented to examine the mediating function of systemic inflammation.
Methods
Study Design and Dataset Generation
NHANES is a nationally representative, cross-sectional study that collects health survey data from the US resident and ambulatory civilian population, with a questionnaire administered in the home followed immediately by a standardized health survey administered in a specially equipped mobile examination center.26 In this study, we included survey results from 3427 participants who had environmental toxicants characterized and who completed the 9-item Patient Health Questionnaire (PHQ-9) in the 2013-2014 and 2015-2016 NHANES waves, representing approximately 240 million US adults 18 years and older (eMethods and eFigure 1 in Supplement 1).
Race and ethnicity were categorized as Mexican American, non-Hispanic Black, non-Hispanic White, other Hispanic, and other race (which comprised participants who identified as non-Hispanic multiracial). These data were collected as a confounding factor owing to the differences among individuals of different races and ethnicities regarding susceptibility to depression and ability to metabolize environmental toxins.
NHANES was approved by the Research Ethics Review Board of the US Centers for Disease Control and Prevention National Center for Health Statistics, and written informed consent was obtained from all adult participants. We followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline for cross-sectional studies.
Outcome Definition
The PHQ-9, a depression screening instrument, was administered to determine the frequency of depressive symptoms over the past 2 weeks. The instrument incorporates Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition) depression diagnostic criteria.27 Responses were categorized as “not at all,” “several days,” “more than half the days,” or “nearly every day,” with scores ranging from 0 to 3. The total score is based on the sum of the points for each item and ranges from 0 to 27. Participants can be classified into 4 categories of depression based on the questionnaire scores: asymptomatic (0-4), mild (5-9), moderate (10-14), moderately severe (15-19), and severe (20-27). We considered a score of 5 or greater to be positive for depression, but additionally grouped the participants based on PHQ-9 scores of at least 10 and at least 15 for sensitivity analyses.
Exposure Definition
In this study, a total of 89 environmental toxicants in 13 categories were measured in the participants who were selected by NHANES investigators based on the subsample A weights. After the questionnaire survey, blood and urine samples were collected from the participants for the evaluation of the exposure level of environmental toxicants. Toxicants were assessed by either the measurement of hemoglobin adducts for reactive organic compounds such as acrylamide, glycidamide, ethylene oxide, and formaldehyde or by assaying inorganic elemental toxicants and metals in the urine. Detailed information and measurement methods for the 89 environmental toxicants and the contaminants included in each toxicant category are available in the eMethods and eTable 1 in Supplement 1. Following consideration of missing data and values below the lower limit of detection (eMethods in Supplement 1), a total of 62 types in 10 categories of environmental toxicants were included in the primary analysis. The concentrations of hemoglobin adduct were expressed as the number per milligram of hemoglobin, with analyses adjusted for hemoglobin concentration. The urine toxicants were adjusted per milligram of urine creatine to adjust for urinary dilution.
Statistical Analysis
Data were analyzed from July 1, 2023, to January 31, 2024. We performed statistical analyses of the exposome using 2 methods, including the exposome-wide association study (ExWAS), which considers the effects of each environmental toxicant independently, and the deletion-substitution-addition (DSA) algorithm, which considers all environmental toxicants simultaneously.28 More details are described in the eMethods in Supplement 1. We conducted sensitivity ExWAS analyses to enhance the robustness of our findings. First, we performed sensitivity analyses based on different cutoff points (≥10 and ≥15) of the PHQ-9. We also conducted ExWAS analysis based on the data that did not impute missing values and did not exclude participants with missing values for more than one-third of all environmental toxicants.
We developed a mediation analysis framework consisting of 2 analyses, with the objective of examining whether the associations between environmental toxicants and depressive symptoms were mediated by natural log-transformed total WBC count as a surrogate for systemic inflammation. The initial analysis performed was a 1-way pairwise mediation, while the second method involved exposure dimension reduction followed by pairwise mediation analysis. Additionally, a reverse mediation analysis was conducted to confirm the absence of reverse mediation. Exposure dimension reduction followed by pairwise mediation analysis is another approach to mediation analysis that aims to reduce the dimensionality of exposures to reduce the number of mediating role models. The approach of the second option is to use exposure-class risk scores (ERS) for mediation analysis. More details about the mediation analysis framework are available in the eMethods in Supplement 1.
We included covariates in the ExWAS, DSA algorithm, and mediation analysis framework based on previous research, consisting of sex, age, race and Hispanic ethnicity, educational level, the ratio of family income to poverty, body mass index, sleep duration, alcohol consumption, estimated glomerular filtration rate, sample collection time, 6-month survey period, and survey cycles. We used the rexposome package to conduct ExWAS analyses, the dsa package for the DSA algorithm, the mediation package to analyze the mediation effects, and the gcdnet package for adaptive elastic net regularization. The DSA package was executed in R, version 2.15.3 (R Project for Statistical Computing), while the remaining analyses were performed using R, version 4.3.1. Two-sided P < .05 indicated statistical significance.
Results
Among the 3427 participants, 1692 (49.4%) were men, 1735 (50.6%) were women, 2683 (78.3%) were younger than 65 years, and 744 (21.7%) were 65 years or older. In terms of race and ethnicity, 570 participants (16.6%) were Mexican American, 679 (19.8%) were non-Hispanic Black, 1314 (38.3%) were non-Hispanic White, 382 (11.1%) were other Hispanic, and 482 (14.1%) were other race. During the 2013-2014 survey period, the crude prevalence of depression was 427 of 1754 participants (24.3%), and during the 2015-2016 survey period, 412 of 1673 (24.6%), for an overall prevalence of 839 of 3427 (24.5%) (Table 1). The baseline data before the imputation of missing values are shown in eTable 2 in Supplement 1 and have a distribution similar to the results before the interpolation process.
Table 1. Participant Characteristics.
| Characteristic | Participant group, No. (%) | ||
|---|---|---|---|
| All (N = 3427) | No depression (n = 2588) | Depression (n = 839) | |
| Sex | |||
| Men | 1692 (49.4) | 1343 (51.9) | 349 (41.6) |
| Women | 1735 (50.6) | 1245 (48.1) | 490 (58.4) |
| Age, y | |||
| <65 | 2683 (78.3) | 2037 (78.7) | 646 (77.0) |
| ≥65 | 744 (21.7) | 551 (21.3) | 193 (23.0) |
| Race and ethnic origin | |||
| Mexican American | 570 (16.6) | 440 (17.0) | 130 (15.5) |
| Non-Hispanic Black | 679 (19.8) | 493 (19.0) | 186 (22.2) |
| Non-Hispanic White | 1314 (38.3) | 988 (38.2) | 326 (38.9) |
| Other Hispanic | 382 (11.1) | 284 (11.0) | 98 (11.7) |
| Other racea | 482 (14.1) | 383 (14.8) | 99 (11.8) |
| Educational level | |||
| Less than 9th grade | 371 (10.8) | 263 (10.2) | 108 (12.9) |
| 9th-11th grade | 405 (11.8) | 266 (10.3) | 139 (16.6) |
| High school graduate, GED, or equivalent | 799 (23.3) | 592 (22.9) | 207 (24.7) |
| Some college or AA degree | 1852 (54.0) | 1467 (56.7) | 385 (45.9) |
| Ratio of family income to poverty | |||
| <1.07 | 899 (26.2) | 600 (23.2) | 299 (35.6) |
| 1.07 to <2.06 | 834 (24.3) | 622 (24.0) | 212 (25.3) |
| 2.06 to <3.93 | 845 (24.7) | 651 (25.2) | 194 (23.1) |
| ≥3.93 | 849 (24.8) | 715 (27.6) | 134 (16.0) |
| BMI | |||
| <25 | 1020 (29.8) | 783 (30.3) | 237 (28.2) |
| 25 to <30 | 1094 (31.9) | 857 (33.1) | 237 (28.2) |
| ≥30 | 1313 (38.3) | 948 (36.6) | 365 (43.5) |
| Sleep duration, h | |||
| 6-8 | 2372 (69.2) | 1871 (72.3) | 501 (59.7) |
| <6 | 381 (11.1) | 230 (8.9) | 151 (18.0) |
| >8 | 674 (19.7) | 487 (18.8) | 187 (22.3) |
| Alcohol consumption | |||
| Yes | 2402 (70.1) | 1802 (69.6) | 600 (71.5) |
| No | 1025 (29.9) | 786 (30.4) | 239 (28.5) |
| eGFR, mL/min/1.73 m2 | |||
| <66.56 | 854 (24.9) | 643 (24.8) | 211 (25.1) |
| 66.56 to <84.02 | 855 (24.9) | 666 (25.7) | 189 (22.5) |
| 84.02 to <100.18 | 850 (24.8) | 631 (24.4) | 219 (26.1) |
| ≥100.18 | 868 (25.3) | 648 (25.0) | 220 (26.2) |
| Sample collection time | |||
| Morning | 1612 (47.0) | 1243 (48.0) | 369 (44.0) |
| Afternoon | 1254 (36.6) | 928 (35.9) | 326 (38.9) |
| Evening | 561 (16.4) | 417 (16.1) | 144 (17.2) |
| 6-mo Survey period | |||
| November 1 through April 30 | 1716 (50.1) | 1288 (49.8) | 428 (51.0) |
| May 1 through October 31 | 1711 (49.9) | 1300 (50.2) | 411 (49.0) |
| Survey cycles | |||
| 2013-2014 | 1754 (51.2) | 1327 (51.3) | 427 (50.9) |
| 2015-2016 | 1673 (48.8) | 1261 (48.7) | 412 (49.1) |
Abbreviations: AA, Associate in Arts; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); eGFR, estimated glomerular filtration rate; GED, General Educational Development.
Includes participants who identified as non-Hispanic multiracial.
Among 89 toxicants, a total of 62 with sufficient data were included. Of these, 10 were analyzed dichotomously based on detection limits, and the remaining 50 were log(ln) transformed to approximate a normal distribution (eTable 1 in Supplement 1). As shown in eFigure 2 in Supplement 1, there were different degrees of correlation among the 62 environmental toxicants investigated in this study. The correlation coefficients ranged from −0.227 to 0.988, with most toxicants having a correlation coefficient of less than 0.800.
The ExWAS analysis accounted for potential confounding factors and revealed 27 environmental toxicants in 6 categories with a positive association with depressive symptoms. These categories include acrylamide and glycidamide, ethylene oxide, metals (2 types), nicotine metabolites (3 types), PAH (6 types), and VOC metabolites (14 types). In particular, individuals with detectable levels of MHBMA2 had a risk of depressive symptoms 1.74 (95% CI, 1.38-2.18) times higher than those with undetectable levels. Furthermore, total nicotine equivalent-2 (TNE-2) and total hydroxycotinine, which are nicotine metabolites, were also associated with depressive symptoms. For each 1-U increase in IQR, the likelihood of depressive symptoms increased by 42% (95% CI, 26%-59%) for TNE-2 and 41% (95% CI, 26%-59%) for hydroxycotinine (Table 2 and Figure 1). According to the DSA algorithm, TNE-2 and N-acetyl-S-(2-carboxyethyl)-l-cysteine (CEMA; a VOC metabolite) were identified as key factors associated with the prevalence of depressive symptoms.
Table 2. Associations Between Environmental Toxicants (62 Exposures) and Depressive Symptoms in 3427 Adults (ExWAS Analysis).
| Exposure family by toxicant exposure | Processing | Median (IQR) | Model 1a | Model 2b | ||
|---|---|---|---|---|---|---|
| OR (95% CI) | P valuec | OR (95% CI) | P valuec | |||
| Acrylamide and glycidamide | ||||||
| Acrylamide | log(ln) | 42.9 (32.8-64.2) | 1.18 (1.09-1.28) | <.001 | 1.14 (1.05-1.24) | .007 |
| Glycidamide | log(ln) | 38.4 (28.1-54.5) | 1.15 (1.06-1.25) | .002 | 1.10 (1.01-1.20) | .07 |
| Speciated arsenics | ||||||
| Arsenobetaine | 2 Categories | NA | 0.97 (0.83-1.14) | .79 | 1.02 (0.86-1.20) | .87 |
| Arsenocholine | 2 Categories | NA | 0.87 (0.70-1.07) | .22 | 0.90 (0.72-1.12) | .43 |
| Arsenous acid | 2 Categories | NA | 0.83 (0.71-0.98) | .04 | 0.90 (0.76-1.07) | .34 |
| Dimethylarsinic acid | log(ln) | 3.5 (2.2-5.7) | 0.95 (0.86-1.05) | .38 | 0.95 (0.86-1.06) | .48 |
| Monomethylarsonic acid | 2 Categories | NA | 0.88 (0.75-1.04) | .17 | 0.95 (0.81-1.13) | .65 |
| Ethylene oxide | ||||||
| Ethylene oxide | log(ln) | 21.1 (15.1-42.2) | 1.23 (1.15-1.31) | <.001 | 1.17 (1.09-1.25) | <.001 |
| Formaldehyde | ||||||
| Formaldehyde | log(ln) | 132.0 (121.0-144.0) | 1.01 (0.94-1.09) | .79 | 1.02 (0.94-1.11) | .65 |
| Iodine | ||||||
| Iodine | log(ln) | 124.3 (76.9-222.9) | 0.98 (0.89-1.09) | .79 | 0.94 (0.84-1.05) | .37 |
| Metals | ||||||
| Copper | log(ln) | 115.1 (99.0-133.6) | 1.24 (1.12-1.37) | <.001 | 1.04 (0.93-1.17) | .55 |
| Selenium | log(ln) | 128.8 (118.9-139.8) | 0.90 (0.82-0.99) | .05 | 0.95 (0.86-1.05) | .45 |
| Zinc | log(ln) | 80.4 (70.9-90.4) | 0.88 (0.79-0.97) | .02 | 0.93 (0.83-1.04) | .3 |
| Barium | log(ln) | 1.1 (0.6-2.0) | 0.93 (0.84-1.02) | .16 | 0.94 (0.85-1.04) | .34 |
| Cadmium | log(ln) | 0.2 (0.1-0.4) | 1.23 (1.10-1.37) | <.001 | 1.13 (0.99-1.28) | .12 |
| Cobalt | log(ln) | 0.4 (0.3-0.6) | 1.10 (1.00-1.21) | .08 | 1.02 (0.91-1.13) | .83 |
| Cesium | log(ln) | 4.2 (3.1-5.9) | 0.91 (0.82-1.01) | .09 | 0.91 (0.81-1.01) | .14 |
| Mercury | 2 categories | NA | 0.93 (0.79-1.09) | .46 | 1.01 (0.85-1.20) | .92 |
| Manganese | 2 categories | NA | 1.32 (1.11-1.56) | .002 | 1.21 (1.02-1.45) | .07 |
| Molybdenum | log(ln) | 37.2 (24.8-54.4) | 0.94 (0.85-1.03) | .23 | 0.93 (0.84-1.03) | .22 |
| Lead | log(ln) | 0.3 (0.2-0.5) | 1.12 (1.01-1.24) | .06 | 1.05 (0.94-1.18) | .48 |
| Antimony | log(ln) | 0 (0-0.1) | 1.17 (1.07-1.28) | .002 | 1.09 (0.99-1.21) | .13 |
| Tin | log(ln) | 0.4 (0.2-0.9) | 1.29 (1.17-1.42) | <.001 | 1.16 (1.04-1.30) | .02 |
| Strontium | log(ln) | 96.2 (59.4-148.8) | 0.99 (0.90-1.08) | .79 | 0.99 (0.90-1.09) | .92 |
| Thallium | log(ln) | 0.2 (0.1-0.2) | 0.87 (0.79-0.96) | .008 | 0.89 (0.80-0.99) | .07 |
| Tungsten | log(ln) | 0.1 (0-0.1) | 1.11 (1.00-1.22) | .06 | 1.08 (0.97-1.20) | .22 |
| Uranium | log(ln) | 0 (0-0) | 1.22 (1.11-1.34) | <.001 | 1.17 (1.06-1.30) | .01 |
| Nicotine metabolites | ||||||
| TNE-2 | log(ln) | 0 (0-1.3) | 1.51 (1.37-1.67) | <.001 | 1.42 (1.26-1.59) | <.001 |
| Total cotinine | log(ln) | 0.5 (0.2-86.1) | 1.44 (1.31-1.58) | <.001 | 1.33 (1.20-1.49) | <.001 |
| Total hydroxycotinine | log(ln) | 0.9 (0.3-157.2) | 1.51 (1.37-1.67) | <.001 | 1.41 (1.26-1.59) | <.001 |
| PAH | ||||||
| 1-Napthol | log(ln) | 1392.5 (708.1-4718.5) | 1.39 (1.26-1.54) | <.001 | 1.28 (1.15-1.42) | <.001 |
| 2-Napthol | log(ln) | 5664.4 (2898.7-10 933.4) | 1.42 (1.26-1.59) | <.001 | 1.21 (1.07-1.37) | .008 |
| 3-Hydroxyfluorene | log(ln) | 71.7 (42.5-189.7) | 1.30 (1.19-1.43) | <.001 | 1.21 (1.09-1.34) | .001 |
| 2-Hydroxyfluorene | log(ln) | 174.8 (109.8-382.4) | 1.39 (1.27-1.53) | <.001 | 1.27 (1.14-1.40) | <.001 |
| 1-Hydroxyphenanthrene | log(ln) | 104.6 (69.1-169.7) | 1.22 (1.10-1.35) | <.001 | 1.13 (1.01-1.26) | .06 |
| 1-Hydroxypyrene | log(ln) | 131.6 (80.5-225.0) | 1.27 (1.14-1.41) | <.001 | 1.15 (1.03-1.29) | .04 |
| 2- and 3-Hydroxyphenanthrene | log(ln) | 127.5 (82.8-209.0) | 1.26 (1.14-1.40) | <.001 | 1.16 (1.03-1.29) | .03 |
| Perchlorate, nitrate, and thiocyanate | ||||||
| Nitrate | log(ln) | 42 911.3 (30 973.3-63 739.6) | 1.01 (0.92-1.10) | .92 | 1.04 (0.95-1.14) | .52 |
| Thiocyanate | log(ln) | 1049.0 (532.2-2212.7) | 1.18 (1.07-1.29) | .002 | 1.10 (1.00-1.22) | .10 |
| Perchlorate | log(ln) | 2.6 (1.6-4.3) | 0.96 (0.87-1.07) | .54 | 0.96 (0.86-1.07) | .54 |
| VOC metabolites | ||||||
| 2,2DCVMA | 2 categories | NA | 1.04 (0.89-1.22) | .66 | 1.35 (0.80-2.27) | .34 |
| 2MHA | log(ln) | 31.8 (14.8-73.1) | 1.16 (1.04-1.29) | .01 | 1.14 (1.02-1.28) | .05 |
| 3 and 4 MHA | log(ln) | 189.7 (94.1-508.0) | 1.29 (1.14-1.45) | <.001 | 1.25 (1.09-1.42) | .005 |
| AAMA | log(ln) | 50.9 (31.8-90.0) | 1.20 (1.09-1.32) | <.001 | 1.14 (1.02-1.26) | .04 |
| AMCC | log(ln) | 145.1 (78.3-262.9) | 1.29 (1.16-1.43) | <.001 | 1.17 (1.04-1.31) | .02 |
| ATCA | log(ln) | 121.9 (60.8-227.2) | 1.09 (0.98-1.21) | .13 | 0.96 (0.85-1.08) | .55 |
| BMA | log(ln) | 6.5 (4.0-11.4) | 0.98 (0.89-1.07) | .67 | 0.92 (0.84-1.02) | .19 |
| BPMA | log(ln) | 3.9 (1.7-9.8) | 0.89 (0.79-0.99) | .05 | 0.92 (0.82-1.03) | .24 |
| CEMA | log(ln) | 97.2 (61.9-161.1) | 1.40 (1.27-1.54) | <.001 | 1.29 (1.17-1.43) | <.001 |
| CYMA | log(ln) | 1.7 (1.0-8.7) | 1.28 (1.20-1.37) | <.001 | 1.22 (1.14-1.31) | <.001 |
| DHBMA | log(ln) | 303.3 (232.3-400.9) | 1.20 (1.10-1.30) | <.001 | 1.12 (1.02-1.23) | .04 |
| GAMA | 2 Categories | NA | 1.37 (1.17-1.60) | <.001 | 1.31 (1.11-1.55) | .005 |
| HEMA | 2 Categories | NA | 1.35 (1.16-1.58) | <.001 | 1.27 (1.08-1.50) | .01 |
| 2HPMA | log(ln) | 27.8 (17.7-51.3) | 1.21 (1.10-1.32) | <.001 | 1.19 (1.08-1.30) | .002 |
| 3HPMA | log(ln) | 238.0 (146.2-460.5) | 1.28 (1.17-1.40) | <.001 | 1.22 (1.11-1.35) | <.001 |
| MA | log(ln) | 133.0 (93.0-196.2) | 1.25 (1.14-1.36) | <.001 | 1.16 (1.06-1.28) | .007 |
| MHBMA2 | 2 Categories | NA | 2.02 (1.63-2.50) | <.001 | 1.74 (1.38-2.18) | <.001 |
| MHBMA3 | log(ln) | 4.7 (2.9-9.6) | 1.31 (1.20-1.43) | <.001 | 1.22 (1.11-1.34) | <.001 |
| PHEMA | 2 Categories | NA | 1.35 (1.16-1.58) | <.001 | 1.21 (1.03-1.43) | .05 |
| PGA | log(ln) | 201.7 (144.6-289.9) | 1.15 (1.06-1.26) | .002 | 1.08 (0.99-1.18) | .16 |
| PMA | 2 Categories | NA | 1.00 (0.85-1.17) | .97 | 0.98 (0.83-1.16) | .83 |
| HPMMA | log(ln) | 214.3 (148.7-427.0) | 1.31 (1.21-1.43) | <.001 | 1.22 (1.11-1.33) | <.001 |
Abbreviations: 2,2DCVMA, N-acetyl-S-(2,2-dichlorovinyl)-l-cysteine; AAMA, 2-carbamoylethylmercapturic acid; AMCC, methylcarbamoylmercapturate; ATCA, 2-aminothiazoline-4-carboxylic acid; BMA, benzylmercapturic acid; BPMA, N-acetyl-S-(n-propyl)-l-cysteine; CEMA, N-acetyl-S-(2-carboxyethyl)-l-cysteine; CYMA, N-acetyl-S-(2-cyanoethyl)-l-cysteine; DHBMA, dihydroxy-butyl-mercapturic acid; ExWAS, exposome-wide association study; GAMA, carbamoyl-2-hydroxyethylmercapturate; HEMA, 2-hydroxyethylmercapturic acid; HPMA, hydroxypropylmercapturic acid; HPMMA, 3-hydroxy-1-methyl-propylmercapturic acid; MA, mandelic acid; MHA, methylhippuric acid; MHBMA, N-acetyl-S-(2-hydroxy-3-butenyl)-l-cysteine; NA, not applicable; OR, odds ratio; PAH, polycyclic aromatic hydrocarbon; PGA, polyglutamic acid; PHEMA, poly(2-hydroxyethyl methacrylate); PMA, phenylmercapturic acid; TNE-2, total nicotine equivalent-2; VOC, volatile organic compound.
Unadjusted model.
Constructed by adjusting for sex, age, race and Hispanic ethnicity, educational level, the ratio of family income to poverty, body mass index, sleep duration, alcohol consumption, estimated glomerular filtration rate, sample collection time, 6-month survey period, and survey cycles.
Adjusted to control the false discovery rate at 5%.
Figure 1. Adjusted Association Between Environmental Toxicants (62 Exposures) and Depressive Symptoms in 3427 Adults (Exposome-Wide Association Study Analysis).
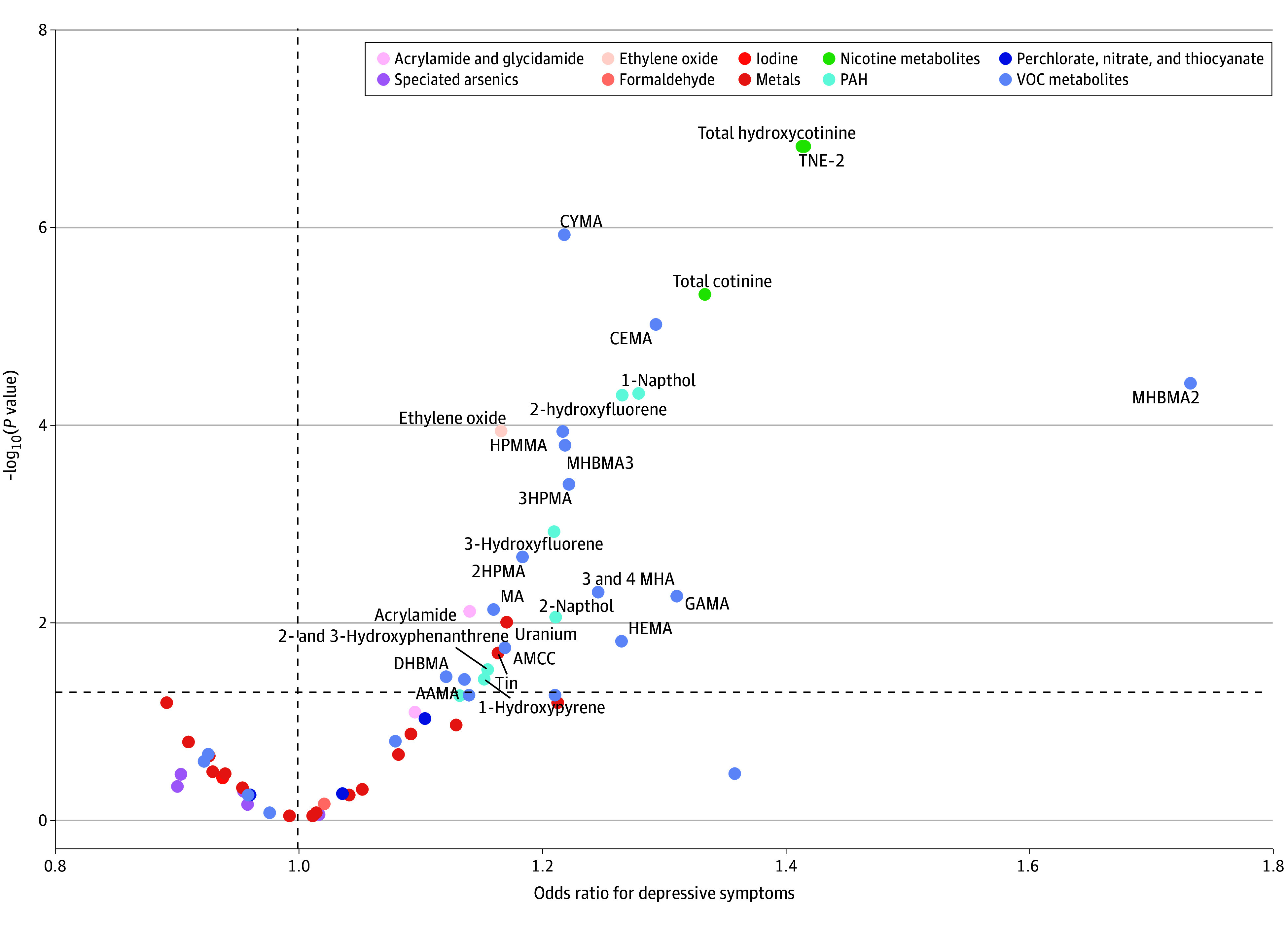
AAMA indicates 2-carbamoylethylmercapturic acid; AMCC, methylcarbamoylmercapturate; CEMA, cyanoethyl mercapturic acid; CYMA, N-acetyl-S-(2-cyanoethyl)-l-cysteine; DHBMA, dihydroxy-butyl-mercapturic acid; GAMA, carbamoyl-2-hydroxyethylmercapturate; HEMA, 2-hydroxyethylmercapturic acid; HPMA, hydroxypropylmercapturic acid; HPMMA, 3-hydroxy-1-methyl-propylmercapturic acid; MA, mandelic acid; MHA, methylhippuric acid; MHBMA, monohydroxybutenyl-mercapturic acid; PAH, polycyclic aromatic hydrocarbon; TNE-2, total nicotine equivalent-2; and VOC, volatile organic compound.
The analysis was stratified by age and sex. The findings suggest that more types of environmental toxicants were associated with depressive symptoms among men (20 toxicants) and individuals younger than 65 years (23 toxicants) compared with women and individuals 65 years or older, particularly for nicotine and VOC metabolites (Figure 2 and eTable 3 in Supplement 1).
Figure 2. Adjusted Association Between Environmental Toxicants (62 Exposures) and Depressive Symptoms in 3427 Adults (Exposome-Wide Association Study Analysis) Stratified by Age and Sex.
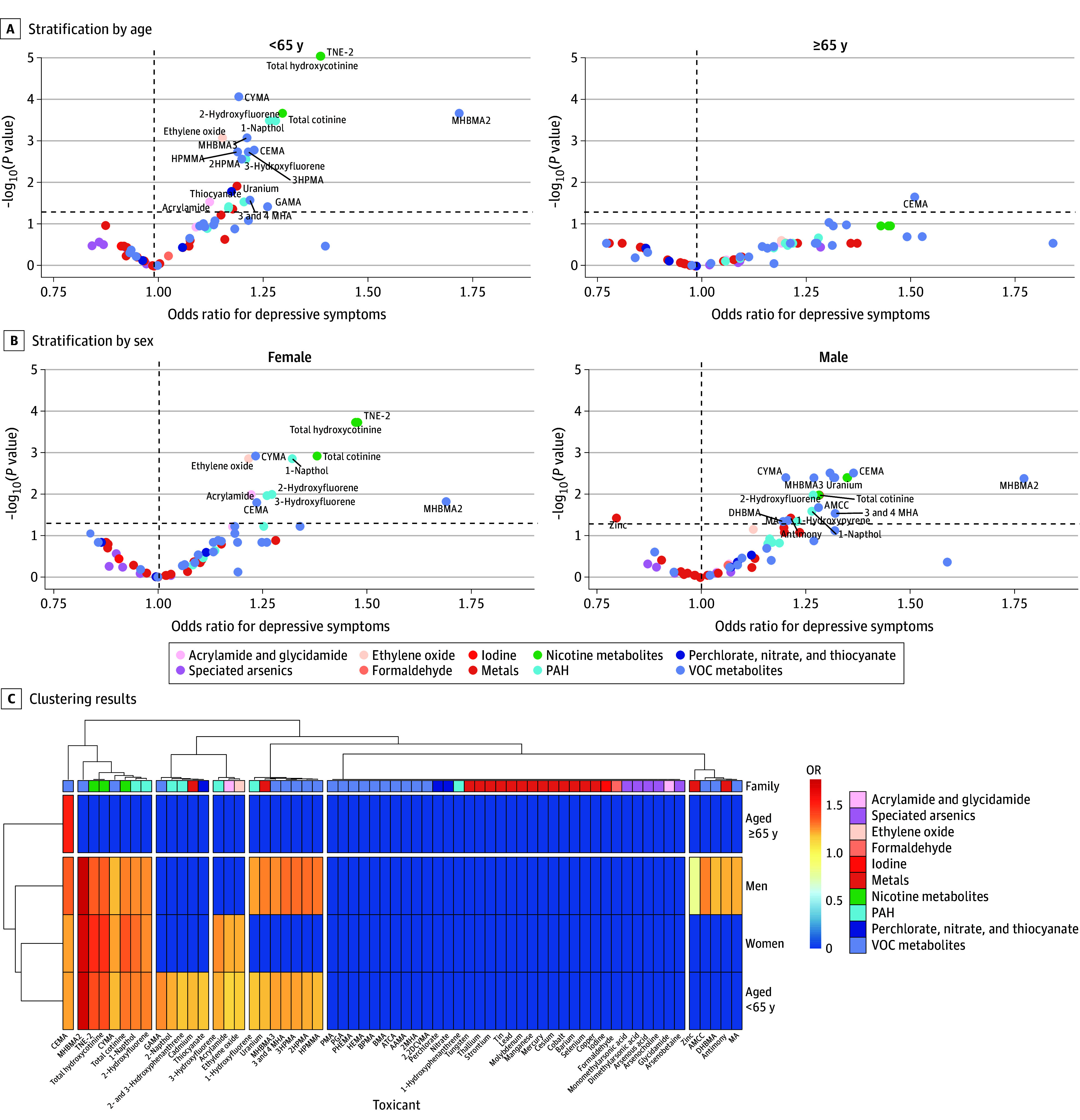
2,2DCVMA indicates N-acetyl-S-(2,2-dichlorovinyl)-l-cysteine; AAMA, 2-carbamoylethylmercapturic acid; AMCC, methylcarbamoylmercapturate; ATCA, 2-aminothiazoline-4-carboxylic acid; BMA, benzylmercapturic acid; BPMA, N-acetyl-S-(n-propyl)-l-cysteine; CEMA, cyanoethyl mercapturic acid; CYMA, N-acetyl-S-(2-cyanoethyl)-l-cysteine; DHBMA, dihydroxy-butyl-mercapturic acid; GAMA, carbamoyl-2-hydroxyethylmercapturate; HEMA, 2-hydroxyethylmercapturic acid; HPMA, hydroxypropylmercapturic acid; HPMMA, 3-hydroxy-1-methyl-propylmercapturic acid; MA, mandelic acid; MHA, methylhippuric acid; MHBMA, monohydroxybutenyl-mercapturic acid; PAH, polycyclic aromatic hydrocarbon; PGA, polyglutamic acid; PHEMA, poly(2-hydroxyethyl methacrylate); PMA, phenylmercapturic acid, TNE-2, total nicotine equivalent-2; and VOC, volatile organic compound.
Sensitivity analyses were conducted to confirm the robustness of our observed connections. Using PHQ-9 scores of 10 or more and 15 or more as cutoff values for grouping, our findings revealed that when using a PHQ-9 score of 10 or more as the cutoff, a total of 23 environmental toxicants across 7 categories were associated with depressive symptoms. When the cutoff was increased to a PHQ-9 score of 15 or more, 8 environmental toxicants across 3 categories remained associated with depressive symptoms. Notably, 4 environmental toxicants across 2 categories were consistently associated with depressive symptoms, regardless of the cutoff value, including nicotine metabolites and thallium. It is noteworthy that some environmental toxicants, such as thallium and arsenocholine, were negatively correlated with depressive symptoms at increasing PHQ-9 cutoff values. Furthermore, MHBMA2 continued to be associated with depressive symptoms (eFigure 3 and eTable 4 in Supplement 1). In addition, we also conducted an ExWAS analysis based on the data that did not impute missing values and did not exclude participants with missing values for more than one-third of all environmental toxicants. The results suggest that a total of 29 environmental toxicants across 6 categories were associated with depressive symptoms based on the data that did not impute missing values, and a total of 28 environmental toxicants across 6 categories were associated with depressive symptoms based on the data that did not exclude participants with missing values for more than one-third of all environmental toxicants (eFigure 4 and eTable 5 in Supplement 1).
A mediation analysis framework was used to further explore the mechanisms associated with the prevalence of depressive symptoms due to environmental toxicants. We found that total WBC count had a mediating role in associations of 33 environmental toxicants with depressive symptoms, as shown in Figure 3 and eTable 6 in Supplement 1. There were 17 direct associations between environmental toxicants and depressive symptoms in 33 mediated models, and inflammatory biomarkers mediated 5% to 19% of these associations in 17 mediated models. Reverse mediation analysis revealed that inflammatory biomarkers played a mediating role in 31 inverse associations of environmental toxicants and depressive symptoms (eFigure 5 and eTable 7 in Supplement 1) . However, for the associations of environmental toxicants and depressive symptoms, 9 intermediate models met the criterion of P < .05/50, while in reverse associations of environmental toxicants and depressive symptoms, none met the criteria. This suggests that the total WBC count, possibly acting as a surrogate for systemic inflammation, may mediate the association between environmental toxicants and depressive symptoms. In addition, we assessed the mediating role of total WBC count in ERS and depressive symptoms associations and found that total WBC count mediated the association of ERS (nicotine metabolites, VOC metabolites, PAHs, and metals) with depressive symptoms, as shown in Figure 3 and eTable 8 in Supplement 1.
Figure 3. Mediation of Association Between Environmental Toxicants and Depressive Symptoms by Inflammation Biomarkers.
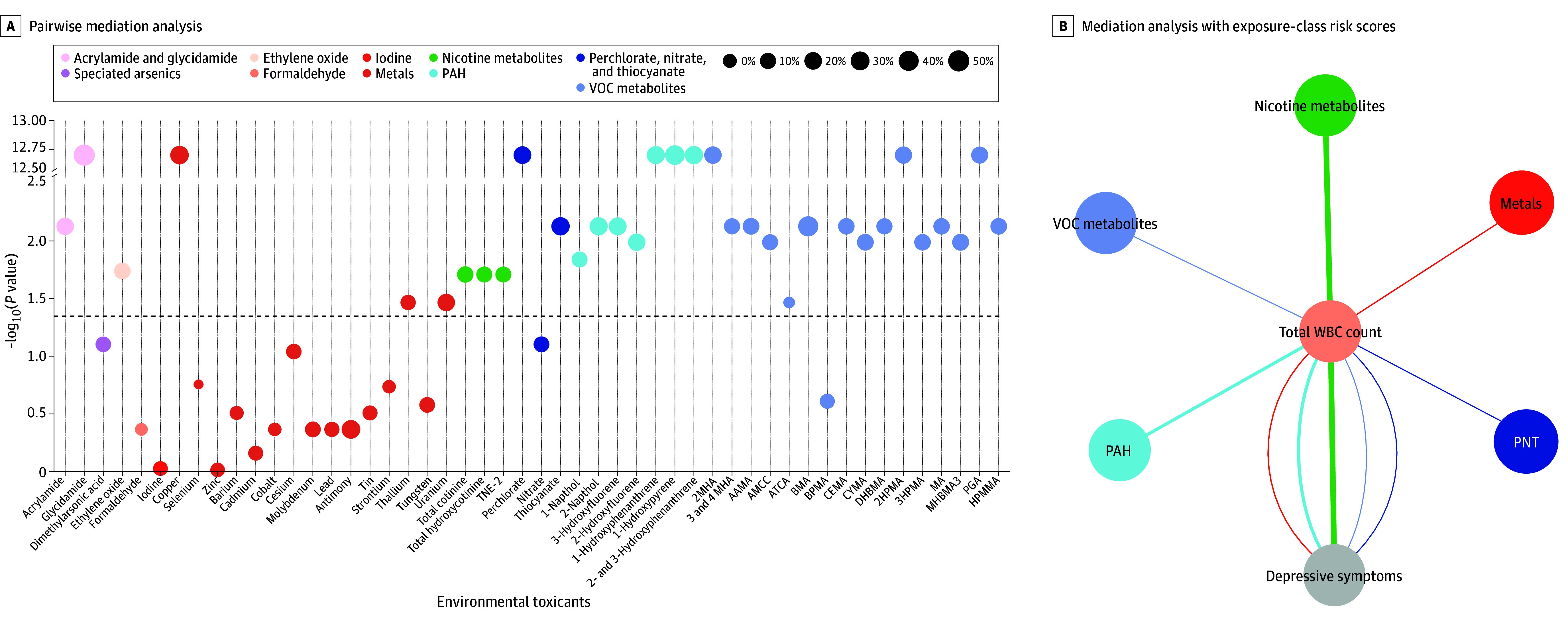
AAMA indicates 2-carbamoylethylmercapturic acid; AMCC, methylcarbamoylmercapturate; ATCA, 2-aminothiazoline-4-carboxylic acid; BMA, benzylmercapturic acid; BPMA, N-acetyl-S-(n-propyl)-l-cysteine; CEMA, cyanoethyl mercapturic acid; CYMA, N-acetyl-S-(2-cyanoethyl)-l-cysteine; DHBMA, dihydroxy-butyl-mercapturic acid; HPMA, hydroxypropylmercapturic acid; HPMMA, 3-hydroxy-1-methyl-propylmercapturic acid; MA, mandelic acid; MHBMA, monohydroxybutenyl-mercapturic acid; PAH, polycyclic aromatic hydrocarbon; PGA, polyglutamic acid; PNT, perchlorate, nitrate, and thiocyanate; TNE-2, total nicotine equivalent-2; VOC, volatile organic compound; and WBC, white blood cell.
Discussion
In this cross-sectional profiling of environmental toxicants to uncover risk factors for depressive symptoms, we found that 6 of 10 categories of toxicants were associated with the risk of depressive symptoms. Furthermore, men and individuals younger than 65 years appear more vulnerable to environmental toxicants than women and older individuals. Systemic inflammation may mediate the association between environmental toxicants (or ERS) and depressive symptoms. This suggests that more attention should be paid to exposure to environmental toxicants in the population to reduce the risk of depression.
Our findings indicate that of the 10 categories of toxicants we surveyed, 6 were associated with the risk of depressive symptoms: nicotine metabolites, VOC metabolites, PAH, acrylamide and glycidamide, ethylene oxide, and metals. Our finding that nicotine exposure was associated with depressive symptoms is supported by previous studies.11,29 Although many smokers report that smoking can alleviate the negative effects of depression, a longitudinal birth cohort study found an association when it was assessed prospectively.30 A meta-analysis suggested that nicotine has a negative impact on childhood brain development during adolescence and may have long-term negative effects on the brain and behavior.31,32 In addition, our study is the first, to our knowledge, to systematically examine the association between VOC metabolites and depressive symptoms, which may reflect the metabolism of VOCs in the body and specific toxic byproducts.12 Our findings suggest that most of the VOC metabolites, especially MHBMA2 and CEMA, are associated with depressive symptoms, which is also consistent with previous studies.12,33
A previous meta-analysis34 revealed that PAH compounds were associated with an increase in depressive symptoms among adults, with 1-napthol showing the strongest overall association, consistent with our findings. Experimental studies have also indicated that PAH exposure can lead to brain development defects through oxidative stress and can result in specific anxiety-related behavioral disorders.35 In addition, previous studies have shown associations between depressive symptoms and 4 other categories of toxicants, including acrylamide,21,36 ethylene oxide,37 and heavy metals.38,39,40,41 Overall, ample evidence supports our exposome research on toxicants. Implementing targeted measures based on these findings could play a pivotal role in preventing and treating depression.
Sensitivity analyses showed that our results remained relatively robust. However, we found a positive association of arsenocholine and thallium with depressive symptoms, and there are several possible reasons for this. First, the use of antidepressants can lead to the development of obesity, which can cause some lipophilic environmental toxins to accumulate in adipose tissue, reducing their concentration in the blood or urine.42 Second, magnesium therapy can lead to rapid recovery from major depression.43 However, magnesium supplementation may affect the metabolism of some metals in the body, which may explain the finding in this study that exposure to thallium and cesium is protective against major depression. In addition, arsenocholine is found in seafood and is less harmful, and levels in the body can be altered by dietary changes. More evidence is needed with further studies.44
Furthermore, we observed differential susceptibility to toxicants across various demographic characteristics, with a greater association among men and young individuals than among women and older individuals. Previous research has shown that men may be more vulnerable to the effects of certain environmental toxicants than women due to the impact of testosterone on neurotoxicants. Women also have increased levels of glutathione and estrogen, which provide protection against environmental toxicants.45,46,47 In addition, young individuals may have more routes of exposure to environmental toxicants, such as occupational exposures, cosmetics, and home renovations, than older individuals.48 Furthermore, the brain’s development can continue until early adulthood, and during this developmental process, the brain may be more susceptible to environmental pollution.49 Therefore, it is crucial to pay more attention to the exposure of young people to environmental toxicants.
Environmental exposure–induced oxidative stress leading to inflammation is a critical factor contributing to a range of human diseases.20 Depression is associated with chronic systemic inflammation, cell-mediated immune activation, and chronic inflammation. Therefore, inflammation could act as an intermediary factor in the link between environmental chemical exposure and the onset of depression. In our study, we used a mediation analysis framework with distinct penalization and estimation algorithms for each type of analysis. However, the consistent findings throughout these methods highlight the importance of the associations, increasing our confidence in the actual mediating pathways. Using both methods, we found that inflammation plays a mediating role in the associations between depressive symptoms and VOCs, nicotine, metal, and PAH. Previous evidence also supports our findings.22,23 For example, imbalances in specific metal levels can weaken the structure, regulation, and catalytic functions of enzymes, proteins, receptors, and transport proteins, which, induced by oxidative stress, can lead to inflammation and decreased metal proteins, contributing to the development of various neurological disorders.22 Additionally, inflammation has been identified as a crucial factor linking nicotine and depression. Omega-3 fatty acids prevent nicotine withdrawal–induced exacerbation of anxiety and depression in rats by modulating oxidative stress and the inflammatory response.23 Further research is necessary to explore the role of inflammation in the association between other toxicants and depression.
Strengths and Limitations
To the best of our knowledge, the present study is the largest and most recent exposome study of environmental toxicant exposure that simultaneously considers the associations between a comprehensive set (10 classes and 62 types) of toxicants and depressive symptoms. We used a no-hypothesis approach, which is characterized by the systematic testing of many variables in relation to a single outcome, to identify all toxicants associated with the development of depressive symptoms.
Our study has several limitations that warrant consideration. First, the emergence of a plethora of newly synthesized chemicals has resulted in the existence of thousands of chemical compounds.15 The environmental toxicants encompassed in this study represent only a fraction of this diversity, highlighting the need for further identification of environmental toxicants that may have stronger associations with depressive symptoms. Second, several environmental toxicants were excluded due to detection limits, underscoring the necessity of enhancing the sensitivity of detection instruments. Third, our cross-sectional study did not directly establish a causal relationship between environmental toxins and depressive symptoms; furthermore, assessing depressive symptoms solely through scales does not constitute a diagnosis of depression. Fourth, there may be some unadjusted confounders, such as genetic risks of depression and social factors. Fifth, despite demonstrating the unidirectionality of mediation using bidirectional mediation, which is an effective method for analyzing mediation in cross-sectional studies,50 prospective research is also required for additional validation. Finally, instead of using inflammatory biomarkers directly, the total WBC count was used as a surrogate for systemic inflammation in this study.
Conclusions
In this cross-sectional study, a total of 27 environmental toxicants in 6 categories were found to be associated with the prevalence of depressive symptoms. The susceptibility to environmental toxicants varied across populations, especially among men and individuals younger than 65 years, compared with women and older individuals. Systemic inflammation, as assessed by the total WBC, may mediate multiple associations of environmental toxicants and depressive symptoms. This research highlights the significance of preventing and regulating important environmental toxicants to gain fresh insights into preventing and potentially treating depression.
eMethods. Data Collection, Toxicant Detection, and Analysis
eTable 1. Characteristics of 89 Environmental Contaminants or Metabolites of Environmental Contaminants
eTable 2. Characterization of Participants in This Study Before Data Interpolation
eTable 3. Adjusted Association Between Environmental Toxicants (62 Exposures) and Depressive Symptoms in 3427 Adults (ExWAS Analysis) Stratified by Age and Sex
eTable 4. Adjusted Association Between Environmental Toxicants (62 Exposures) and Depressive Symptoms in 3427 Adults (ExWAS Analysis), Compared With Threshold-Based Results
eTable 5. Sensitivity Analyses of the Adjusted Associations Between the Environmental Toxicants (62 Exposures) and Depressive Symptoms (ExWAS Analysis)
eTable 6. Mediation of Association Between Environmental Toxicants and Depressive Symptoms by Inflammation Biomarkers
eTable 7. Mediation of the Inverse Association Between Environmental Toxicants and Depressive Symptoms by Inflammation Biomarkers
eTable 8. Mediation of the Association Between Exposure-Class Risk Score (ERS) and Depressive Symptoms by Inflammation Biomarkers
eFigure 1. Flowchart of Sample Selection
eFigure 2. Correlation of the Environmental Toxicants (62 Exposures)
eFigure 3. Adjusted Associations Between the Environmental Exposome (62 Exposures) and Depressive Symptoms in 3427 Adults (ExWAS Analysis), Compared With Threshold-Based Results
eFigure 4. Sensitivity Analyses of Adjusted Associations Between the Environmental Exposome (62 Exposures) and Depressive Symptoms (ExWAS Analysis), Compared With Threshold-Based Results
eFigure 5. Mediation of the Inverse Association Between Environmental Toxicants and Depressive Symptoms by Total WBC by Pairwise Mediation Analysis
eReferences
Data Sharing Statement
References
- 1.GBD 2019 Mental Disorders Collaborators . Global, regional, and national burden of 12 mental disorders in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Psychiatry. 2022;9(2):137-150. doi: 10.1016/S2215-0366(21)00395-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Evans-Lacko S, Aguilar-Gaxiola S, Al-Hamzawi A, et al. Socio-economic variations in the mental health treatment gap for people with anxiety, mood, and substance use disorders: results from the WHO World Mental Health (WMH) surveys. Psychol Med. 2018;48(9):1560-1571. doi: 10.1017/S0033291717003336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization . World misses most 2020 mental health targets; extension of WHO Mental Health Action Plan to 2030 provides new opportunity for progress. October 8, 2021. Accessed December 29, 2023. https://www.who.int/news/item/08-10-2021-who-report-highlights-global-shortfall-in-investment-in-mental-health
- 4.Kioumourtzoglou MA. Identifying modifiable risk factors of mental health disorders—the importance of urban environmental exposures. JAMA Psychiatry. 2019;76(6):569-570. doi: 10.1001/jamapsychiatry.2019.0010 [DOI] [PubMed] [Google Scholar]
- 5.Lund C, Brooke-Sumner C, Baingana F, et al. Social determinants of mental disorders and the Sustainable Development Goals: a systematic review of reviews. Lancet Psychiatry. 2018;5(4):357-369. doi: 10.1016/S2215-0366(18)30060-9 [DOI] [PubMed] [Google Scholar]
- 6.Pandics T, Major D, Fazekas-Pongor V, et al. Exposome and unhealthy aging: environmental drivers from air pollution to occupational exposures. Geroscience. 2023;45(6):3381-3408. doi: 10.1007/s11357-023-00913-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Siroux V, Agier L, Slama R. The exposome concept: a challenge and a potential driver for environmental health research. Eur Respir Rev. 2016;25(140):124-129. doi: 10.1183/16000617.0034-2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reuben A, Manczak EM, Cabrera LY, et al. The interplay of environmental exposures and mental health: setting an agenda. Environ Health Perspect. 2022;130(2):25001. doi: 10.1289/EHP9889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bratman GN, Anderson CB, Berman MG, et al. Nature and mental health: an ecosystem service perspective. Sci Adv. 2019;5(7):eaax0903. doi: 10.1126/sciadv.aax0903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rahman HH, Niemann D, Munson-McGee SH. Association among urinary polycyclic aromatic hydrocarbons and depression: a cross-sectional study from NHANES 2015-2016. Environ Sci Pollut Res Int. 2022;29(9):13089-13097. doi: 10.1007/s11356-021-16692-3 [DOI] [PubMed] [Google Scholar]
- 11.Dickerson AS, Wu AC, Liew Z, Weisskopf M. A scoping review of non-occupational exposures to environmental pollutants and adult depression, anxiety, and suicide. Curr Environ Health Rep. 2020;7(3):256-271. doi: 10.1007/s40572-020-00280-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang M, Kim SH, Kim JC, Shin T, Moon C. Toluene induces depression-like behaviors in adult mice. Toxicol Res. 2010;26(4):315-320. doi: 10.5487/TR.2010.26.4.315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ioannidis JPA. Neglecting major health problems and broadcasting minor, uncertain issues in lifestyle science. JAMA. 2019;322(21):2069-2070. doi: 10.1001/jama.2019.17576 [DOI] [PubMed] [Google Scholar]
- 14.Burkett JP, Miller GW. Using the exposome to understand environmental contributors to psychiatric disorders. Neuropsychopharmacology. 2021;46(1):263-264. doi: 10.1038/s41386-020-00851-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vermeulen R, Schymanski EL, Barabási AL, Miller GW. The exposome and health: where chemistry meets biology. Science. 2020;367(6476):392-396. doi: 10.1126/science.aay3164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choi KW, Stein MB, Nishimi KM, et al. ; 23andMe Research Team; Major Depressive Disorder Working Group of the Psychiatric Genomics Consortium . An exposure-wide and mendelian randomization approach to identifying modifiable factors for the prevention of depression. Am J Psychiatry. 2020;177(10):944-954. doi: 10.1176/appi.ajp.2020.19111158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pries LK, Moore TM, Visoki E, Sotelo I, Barzilay R, Guloksuz S. Estimating the association between exposome and psychosis as well as general psychopathology: results from the ABCD Study. Biol Psychiatry Glob Open Sci. 2022;2(3):283-291. doi: 10.1016/j.bpsgos.2022.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mazza MG, De Lorenzo R, Conte C, et al. ; COVID-19 BioB Outpatient Clinic Study group . Anxiety and depression in COVID-19 survivors: role of inflammatory and clinical predictors. Brain Behav Immun. 2020;89:594-600. doi: 10.1016/j.bbi.2020.07.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eswarappa M, Neylan TC, Whooley MA, Metzler TJ, Cohen BE. Inflammation as a predictor of disease course in posttraumatic stress disorder and depression: a prospective analysis from the Mind Your Heart Study. Brain Behav Immun. 2019;75:220-227. doi: 10.1016/j.bbi.2018.10.012 [DOI] [PubMed] [Google Scholar]
- 20.Peters A, Nawrot TS, Baccarelli AA. Hallmarks of environmental insults. Cell. 2021;184(6):1455-1468. doi: 10.1016/j.cell.2021.01.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang A, Wan X, Zhuang P, et al. High fried food consumption impacts anxiety and depression due to lipid metabolism disturbance and neuroinflammation. Proc Natl Acad Sci U S A. 2023;120(18):e2221097120. doi: 10.1073/pnas.2221097120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tarnacka B, Jopowicz A, Maślińska M. Copper, iron, and manganese toxicity in neuropsychiatric conditions. Int J Mol Sci. 2021;22(15):7820. doi: 10.3390/ijms22157820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Amiry GY, Haidary M, Azhdari-Zarmehri H, Beheshti F, Ahmadi-Soleimani SM. Omega-3 fatty acids prevent nicotine withdrawal-induced exacerbation of anxiety and depression by affecting oxidative stress balance, inflammatory response, BDNF and serotonin metabolism in rats. Eur J Pharmacol. 2023;947:175634. doi: 10.1016/j.ejphar.2023.175634 [DOI] [PubMed] [Google Scholar]
- 24.Aung MT, Song Y, Ferguson KK, et al. Application of an analytical framework for multivariate mediation analysis of environmental data. Nat Commun. 2020;11(1):5624. doi: 10.1038/s41467-020-19335-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park SK, Zhao Z, Mukherjee B. Construction of environmental risk score beyond standard linear models using machine learning methods: application to metal mixtures, oxidative stress and cardiovascular disease in NHANES. Environ Health. 2017;16(1):102. doi: 10.1186/s12940-017-0310-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.National Center for Health Statistics . National Health and Nutrition Examination Survey: Analytic Guidelines, 2011–2014 and 2015–2016. Centers for Disease Control and Prevention; 2018. [Google Scholar]
- 27.Spitzer RL, Kroenke K, Williams JB; Primary Care Evaluation of Mental Disorders . Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study: primary care evaluation of mental disorders: patient health questionnaire. JAMA. 1999;282(18):1737-1744. doi: 10.1001/jama.282.18.1737 [DOI] [PubMed] [Google Scholar]
- 28.Agier L, Portengen L, Chadeau-Hyam M, et al. A systematic comparison of linear regression-based statistical methods to assess exposome-health associations. Environ Health Perspect. 2016;124(12):1848-1856. doi: 10.1289/EHP172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schmitt HJ, Calloway EE, Sullivan D, et al. Chronic environmental contamination: a systematic review of psychological health consequences. Sci Total Environ. 2021;772:145025. doi: 10.1016/j.scitotenv.2021.145025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boden JM, Fergusson DM, Horwood LJ. Cigarette smoking and depression: tests of causal linkages using a longitudinal birth cohort. Br J Psychiatry. 2010;196(6):440-446. doi: 10.1192/bjp.bp.109.065912 [DOI] [PubMed] [Google Scholar]
- 31.Leslie FM. Unique, long-term effects of nicotine on adolescent brain. Pharmacol Biochem Behav. 2020;197:173010. doi: 10.1016/j.pbb.2020.173010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iñiguez SD, Warren BL, Parise EM, et al. Nicotine exposure during adolescence induces a depression-like state in adulthood. Neuropsychopharmacology. 2009;34(6):1609-1624. doi: 10.1038/npp.2008.220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stingone JA, McVeigh KH, Claudio L. Early-life exposure to air pollution and greater use of academic support services in childhood: a population-based cohort study of urban children. Environ Health. 2017;16(1):2. doi: 10.1186/s12940-017-0210-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhen H, Zhang F, Cheng H, et al. Association of polycyclic aromatic hydrocarbons exposure with child neurodevelopment and adult emotional disorders: a meta-analysis study. Ecotoxicol Environ Saf. 2023;255:114770. doi: 10.1016/j.ecoenv.2023.114770 [DOI] [PubMed] [Google Scholar]
- 35.Lin YC, Wu CY, Hu CH, Pai TW, Chen YR, Wang WD. Integrated hypoxia signaling and oxidative stress in developmental neurotoxicity of benzo[a]pyrene in zebrafish embryos. Antioxidants (Basel). 2020;9(8):731. doi: 10.3390/antiox9080731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bian HT, Xiao L, Liang L, Xie YP, Wang HL, Wang GH. RGFP966 is protective against lipopolysaccharide-induced depressive-like behaviors in mice by inhibiting neuroinflammation and microglial activation. Int Immunopharmacol. 2021;101(Pt B):108259. doi: 10.1016/j.intimp.2021.108259 [DOI] [PubMed] [Google Scholar]
- 37.Estrin WJ, Bowler RM, Lash A, Becker CE. Neurotoxicological evaluation of hospital sterilizer workers exposed to ethylene oxide. J Toxicol Clin Toxicol. 1990;28(1):1-20. doi: 10.3109/15563659008993472 [DOI] [PubMed] [Google Scholar]
- 38.Baj J, Forma A, Sitarz E, et al. Beyond the mind-serum trace element levels in schizophrenic patients: a systematic review. Int J Mol Sci. 2020;21(24):9566. doi: 10.3390/ijms21249566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shi W, Li T, Zhang Y, et al. Depression and anxiety associated with exposure to fine particulate matter constituents: a cross-sectional study in North China. Environ Sci Technol. 2020;54(24):16006-16016. doi: 10.1021/acs.est.0c05331 [DOI] [PubMed] [Google Scholar]
- 40.Dinocourt C, Culeux C, Legrand M, Elie C, Lestaevel P. Chronic exposure to uranium from gestation: effects on behavior and neurogenesis in adulthood. Int J Environ Res Public Health. 2017;14(5):536. doi: 10.3390/ijerph14050536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tian X, Xue B, Wang B, et al. Physical activity reduces the role of blood cadmium on depression: a cross-sectional analysis with NHANES data. Environ Pollut. 2022;304:119211. doi: 10.1016/j.envpol.2022.119211 [DOI] [PubMed] [Google Scholar]
- 42.Patten SB, Williams JV, Lavorato DH, Brown L, McLaren L, Eliasziw M. Major depression, antidepressant medication and the risk of obesity. Psychother Psychosom. 2009;78(3):182-186. doi: 10.1159/000209349 [DOI] [PubMed] [Google Scholar]
- 43.Eby GA, Eby KL. Rapid recovery from major depression using magnesium treatment. Med Hypotheses. 2006;67(2):362-370. doi: 10.1016/j.mehy.2006.01.047 [DOI] [PubMed] [Google Scholar]
- 44.Marafante E, Vahter M, Dencker L. Metabolism of arsenocholine in mice, rats and rabbits. Sci Total Environ. 1984;34(3):223-240. doi: 10.1016/0048-9697(84)90065-2 [DOI] [PubMed] [Google Scholar]
- 45.Kern JK, Geier DA, Homme KG, et al. Developmental neurotoxicants and the vulnerable male brain: a systematic review of suspected neurotoxicants that disproportionally affect males. Acta Neurobiol Exp (Wars). 2017;77(4):269-296. doi: 10.21307/ane-2017-061 [DOI] [PubMed] [Google Scholar]
- 46.Richter Schmitz CR, Eichwald T, Branco Flores MV, et al. Sex differences in subacute manganese intoxication: oxidative parameters and metal deposition in peripheral organs of adult Wistar rats. Regul Toxicol Pharmacol. 2019;104:98-107. doi: 10.1016/j.yrtph.2019.03.005 [DOI] [PubMed] [Google Scholar]
- 47.Yamagata AT, Guimarães NC, Santana DF, et al. Gender influence on manganese induced depression-like behavior and Mn and Fe deposition in different regions of CNS and excretory organs in intraperitoneally exposed rats. Toxicology. 2017;376:137-145. doi: 10.1016/j.tox.2016.05.012 [DOI] [PubMed] [Google Scholar]
- 48.Papadopoli R, Nobile CGA, Trovato A, Pileggi C, Pavia M. Chemical risk and safety awareness, perception, and practices among research laboratories workers in Italy. J Occup Med Toxicol. 2020;15:17. doi: 10.1186/s12995-020-00268-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bethlehem RAI, Seidlitz J, White SR, et al. ; 3R-BRAIN; AIBL; Alzheimer’s Disease Neuroimaging Initiative; Alzheimer’s Disease Repository Without Borders Investigators; CALM Team; Cam-CAN; CCNP; COBRE; cVEDA; ENIGMA Developmental Brain Age Working Group; Developing Human Connectome Project; FinnBrain; Harvard Aging Brain Study; IMAGEN; KNE96; Mayo Clinic Study of Aging; NSPN; POND; PREVENT-AD Research Group; VETSA . Brain charts for the human lifespan. Nature. 2022;604(7906):525-533. doi: 10.1038/s41586-022-04554-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lin L, Yi X, Liu H, et al. The airway microbiome mediates the interaction between environmental exposure and respiratory health in humans. Nat Med. 2023;29(7):1750-1759. doi: 10.1038/s41591-023-02424-2 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Data Collection, Toxicant Detection, and Analysis
eTable 1. Characteristics of 89 Environmental Contaminants or Metabolites of Environmental Contaminants
eTable 2. Characterization of Participants in This Study Before Data Interpolation
eTable 3. Adjusted Association Between Environmental Toxicants (62 Exposures) and Depressive Symptoms in 3427 Adults (ExWAS Analysis) Stratified by Age and Sex
eTable 4. Adjusted Association Between Environmental Toxicants (62 Exposures) and Depressive Symptoms in 3427 Adults (ExWAS Analysis), Compared With Threshold-Based Results
eTable 5. Sensitivity Analyses of the Adjusted Associations Between the Environmental Toxicants (62 Exposures) and Depressive Symptoms (ExWAS Analysis)
eTable 6. Mediation of Association Between Environmental Toxicants and Depressive Symptoms by Inflammation Biomarkers
eTable 7. Mediation of the Inverse Association Between Environmental Toxicants and Depressive Symptoms by Inflammation Biomarkers
eTable 8. Mediation of the Association Between Exposure-Class Risk Score (ERS) and Depressive Symptoms by Inflammation Biomarkers
eFigure 1. Flowchart of Sample Selection
eFigure 2. Correlation of the Environmental Toxicants (62 Exposures)
eFigure 3. Adjusted Associations Between the Environmental Exposome (62 Exposures) and Depressive Symptoms in 3427 Adults (ExWAS Analysis), Compared With Threshold-Based Results
eFigure 4. Sensitivity Analyses of Adjusted Associations Between the Environmental Exposome (62 Exposures) and Depressive Symptoms (ExWAS Analysis), Compared With Threshold-Based Results
eFigure 5. Mediation of the Inverse Association Between Environmental Toxicants and Depressive Symptoms by Total WBC by Pairwise Mediation Analysis
eReferences
Data Sharing Statement


