Abstract
Background
Intravenous lipid emulsion is recognised as a therapy for rescue in cases of local anaesthetic toxicity, but its use in reversing overdose or toxicity related to other drugs remains the subject of debate. This in vitro study sought to expand our understanding of the importance of partitioning in determining the impact of intravenous lipid emulsion on aqueous free drug concentrations.
Methods
Twenty-seven drugs and associated metabolites were screened for the ability of intravenous lipid emulsion to reduce the amount of free drug in the aqueous phase, using specialised cassettes designed for this purpose. The relative amount of drug equilibrating across the membrane from plasma to phosphate-buffered saline was measured, using liquid chromatography–mass spectrometry, at a 6 h timepoint in plasma samples treated with intravenous lipid emulsion and paired, untreated controls.
Results
The data obtained were plotted against measures of partition (LogP and cLogD7.4) and with log-transformed non-protein bound drug. There were significant inverse correlations between the capacity for intravenous lipid emulsion to reduce drug detected in the phosphate-buffered saline compartment and LogP and cLogD7.4, and a direct association with log [non-protein-bound drug]. However, a number of drugs showed substantial variance between different plasma samples.
Conclusions
Modulation of free drug in the aqueous compartment is broadly predictable by the partition coefficient, although ramipril was identified to be an outlier in this regard. Further mechanistic and clinical exploration is merited to establish a standardised protocol for lipid emulsion therapy.
Keywords: drug overdose, drug partition, intralipid therapy, protein binding, protocol development
Drug toxicity poses a significant challenge for doctors, especially because few drugs have specific treatments, and establishing the causative agent is often difficult. Although drugs such as opioids and benzodiazepines have specific antagonists, (naloxone and flumazenil, respectively), the majority of drug toxicity treatment is simply supportive, often necessitating organ support in high dependency or intensive care units. These methods of organ support are often invasive, costly,1 and not without their own complications.
Drug overdose is an issue worldwide, but particularly prevalent in the USA2 and Europe,3 with Scotland holding the unenviable record of having the highest rate of drug-related death amongst European countries, roughly three times that of the UK as a whole.4, 5, 6 From 1996 to 2019, drug-related deaths rose from ∼250/million to ∼1200/million,2 with multiple drugs being present in >93% of cases.3 The need for a broad-spectrum treatment to reverse the effects of multiple drug overdoses is particularly urgent in Scotland, but would have benefits worldwide.
Intravenous lipid emulsion (ILE) was first recognised as a treatment for local anaesthetic toxicity in animal studies by Weinberg and colleagues in 1998,7,8 leading to clinical studies that show benefit9 and adoption as protocolised management of local anaesthetic toxicity in humans in many places.10 Subsequent trials and case reports11 have indicated that ILE may have a role in overcoming toxicity in some, but not all drug and pesticide12 overdoses, many of which can be difficult to manage. The mode of action is thought to be a combination of the ‘lipid sink’ theory,13 a concept in which lipid-soluble drugs establish a new pharmacokinetic equilibrium in an expanded lipid volume, leading to a reduction in free plasma concentration, and ‘lipid shuttle’,14, 15, 16 whereby the ILE facilitates redistribution of the toxic drug away from critical organs. Although local anaesthetic overdose reversal by ILE is partially explained by partition,17 modelling suggests that the benefits in local anaesthetic overdose extend beyond a simple lipid sink concept.13 In addition, beyond impacts of ILE on drug distribution, there is substantial evidence for ‘cardiotonic’ effects of ILE on cardiac muscle and vasculature to offer cardiovascular support.18, 19, 20, 21 Over the past decade, a number of studies showing benefit in specific drug22 and pesticide23 overdoses (or failing to do so24), systematic reviews,18 recommendations,19,25 and guidelines26,27 have been published, but the concept of use of ILE in overdose remains controversial and a globally accepted, standardised protocol has not yet been adopted.
The human and cost impact of drug toxicity calls for further treatment options in the early management of this group of patients. Given that partitioning is still considered to be an important contributory element to the potential of ILE in mitigating drug toxicity in overdose, a critical step in understanding the extent of potential of ILE is to establish the extent of partitioning of a wide range of drugs by ILE, notwithstanding that other benefits might be realised in vivo through additional benefits, such as cardiotonic effects.
The aim of this study was to measure drug partitioning into ILE of 27 commonly prescribed drugs known to have toxic effects, and to evaluate the predictive potential of partition coefficient (LogP) and the calculated distribution constant (cLogD7.4) on drug sequestration by ILE in vitro.
Methods
Materials
Drugs were procured from Cambridge Biosciences (Cambridge, UK) except for quetiapine, norquetiapine, olanzapine, carbamazepine, and trazodone, which were sourced from Merck (Glasgow, Scotland). HPLC grade acetonitrile and HPLC grade methanol were from Fisher (Loughborough, UK). Formic acid, dimethyl sulfoxide (DMSO), and ammonium formate were from Merck. Human plasma was bought from Cambridge Bioscience. Phosphate-buffered saline (PBS) pH 7.4 isotonic was from Invitrogen (Renfrew, Scotland). Rapid Equilibrium Dialysis (RED) inserts and plates were from Fisher.
Sample preparation
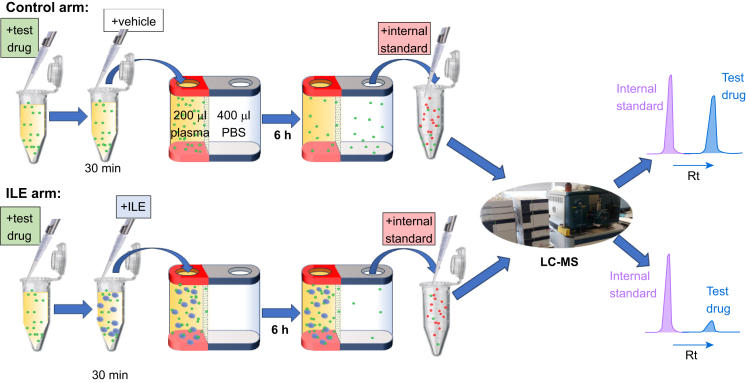
Each drug was analysed separately by taking 10 μl of 1 mg ml−1 standard prepared in methanol (apart from quetiapine and olanzapine, which were prepared in DMSO), before drying down under nitrogen and taking up into 200 μl of human plasma (Fig. 1). Intralipid (Baxter, Newbury, UK) 20%, 50 μl or PBS 50 μl (control) was then added to the plasma. Each drug was tested in triplicate with plasma from three different donors. This mixture was incubated at 37°C for 30 min with shaking (250 rpm). The mixture (200 μl) was then added to the inner chamber of the RED insert with PBS (400 μl) added to the outer chamber (Fig. 1). This was incubated at 37°C with shaking (250 rpm) for 6 h. The PBS dialysate (100 μl) from the outer chamber was then aspirated into an Eppendorf® tube containing dried down carbamazepine (5 μg) by way of an internal standard, and frozen down at –20°C overnight. The following day, dialysates were defrosted on ice and 300 μl of ice-cold acetonitrile was added. This was incubated on ice for 30 min followed by centrifugation (10 min, 4°C, 13 000 g). 200 μl of the supernatant was removed and dried down in a speedvac (miVac duo concentrator). Then, 1 ml of mobile phase was added and 10 μl was injected into the liquid chromatography-mass spectrometry (LC-MS) apparatus (Fig. 1).
Fig 1.
Experimental protocol using RED dialysis chambers before LC-MS analysis of samples taken from the PBS RED chamber. LC was used to separate test drugs from the internal standard, carbamazepine (different retention times [Rts]); data for each arm were expressed as a ratio of test drug peak area to internal standard area. This ratio was then compared between the control and ILE arms of the study. ILE, intravenous lipid emulsion; LC-MS, liquid chromatography-mass spectrometry; RED, rapid equilibrium dialysis.
LC-MS analysis
Chromatographic separation was achieved on a Shimadzu Nexera (Shimadzu, Milton Keynes, UK) using a gradient starting at water 95% + formic acid 0.1% + ammonium formate 0.1 mM (A) and acetonitrile 5% (B), isocratic for 3 min. The composition of this mobile phase was changed to 45% A and 55% B over 13 min and held for 1 min. The gradient was changed to 95% A and 5% B over 0.1 min and held for 6 min. Flow rate was 220 μl min−1 and the column used was Hypersil Gold 100 mm×2.1 mm, 1.9 μm at a temperature of 45°C. The detector used was an ABSciex Qtrap 6500 with electrospray ionisation (ESI) in multiple reaction monitoring mode. Curtain gas was 55 psi, collision energy was medium, ion source temperature was 350°C, and the ion source gases were 40 psi and 80 psi, respectively. The results were quantified by first comparing the area under the curve (AUC) of the test drug peak with the internal standard. The ratio of drug peak:internal standard was subsequently compared between the control and ILE arms of the study and the change in ratio in the ILE arm expressed as % change compared with control.
Data sources for Log P and cLogD7.4 values
A comprehensive survey was conducted of open-source values for Log P. The three most complete records for the study drugs were PubChem, ChemSpider, and DrugBank. Because values were not identical from the sources, a mean of the available values was taken for the purposes of this study. In some cases, several calculated and experimental values were quoted on a single site; in these cases, all values were included. cLog D7.4 values were taken from ChemSpider, calculated using ACD software.
Statistics
The median (95% confidence interval) values for % change in drug detected in the PBS compartment were plotted against Log P, cLogD7.4 and protein binding and analysed for correlation using Spearman's rank correlation because normal distribution could not be assumed. P<0.05 was considered to be significant.
Results
Of the 28 drugs tested in this study, viable results were achieved for 27 candidates; reproducible measurements could not be achieved for sodium valproate using the methodology available, so this drug was excluded from the study.
Variability across the three replicates for each drug was typically below 10%, with a few exceptions: only two usable replicates were obtained for propranolol and lamotrigine and variance amongst replicates for norquetiapine, lidocaine, baclofen, bisoprolol, and ramiprilat were all >20%.
Table 1 shows the ILE-induced % reduction in drug detected in the PBS compartment of the RED cassettes, along with literature values for Log P, cLogD7.4 and protein binding (%); drugs have been ranked according to the extent of reduction in drug detected in the ILE arm of the study.
Table 1.
Effect of ILE on drug detected in the PBS compartment of RED cassettes, expressed as % change from control. Literature values for relevant physicochemical characteristics of each drug are also listed. All Log P values were a mean of values quoted from three sources: PubChem, Chemspider, and Drug Bank. ILE, intravenous lipid emulsion; NF, not found; PBS, phosphate-buffered saline; RED, rapid equilibrium dialysis; sd, standard deviation. ∗Only a single value was located. ∗∗Mean of two values.
| Rank | Drug | Abbreviation (Fig 2) | Δ% Drug detected in PBS (range) | Log P (sd) | cLogD7.4 | Protein binding (%, range) |
|---|---|---|---|---|---|---|
| 1 | Sertraline | Sert | −90.4 (−92.0 to −89.8) | 5.06 (0.17) | 3.14 | 99 |
| 2 | Norfluoxetine | Nfluox | −88.4 (−89.6 to −85.9) | 3.78 (0.20) | 2.23 | 94–95 |
| 3 | Lignocaine | Ligno | −79.1 (−83.7 to −77.6) | 2.55 (0.39) | 1.26 | 60–80 |
| 4 | Verapamil | Verap | −79.6 (−82.2 to −76.6) | 4.32 (0.30) | 2.38 | 86–94 |
| 5 | Duloxetine | Dulox | −80.4 (−85.6 to −71.0) | 4.41 (0.16)∗∗ | 2.31 | >90 |
| 6 | Clomipramine | Clomip | −78.6 (−83.9 to −65.5) | 5.17 (0.10) | 3.31 | 97–98 |
| 7 | Amitryptyline | Amitr | −75.4 (−76.3 to −72.7) | 4.92 (0.03) | 2.96 | 95 |
| 8 | Amlodipine | Amlod | −71.1 (−73.8 to – 67.1) | 3.02 (0.46) | 1.91 | 98 |
| 9 | Norclomipramine | Nclomip | −65.3 (−67.2 to −64.3) | 4.71 (0.01)∗∗ | 2.26 | 97–99 |
| 10 | Nortryptyline | Ntryp | −64.8 (−64.8 to −59.7) | 4.74 (0.31) | 2.28 | 93 |
| 11 | Bupivacaine | Bupiv | −70.1 (−70.1 to −48.7) | 3.59 (0.12) | 2.68 | 95 |
| 12 | Norquetiapine | Nquetia | −47.5 (−69.8 to −46.0) | 2.90 (0.40)∗ | 2.11 | NF |
| 13 | Norverapamil | Nverap | −51.4 (−59.1 to −48.6) | 4.1∗ | 1.53 | NF |
| 14 | Fluoxetine | Fluox | −48.2 (−51.8 to −44.2) | 4.05 (0.03) | 1.75 | 94 |
| 15 | Bisoprolol | Bisop | −54.7 (−65.3 to −16.6) | 2.12 (0.08) | 0.12 | 30 |
| 16 | Propranolol | Propan | −26.8 (−28.6 to −24.9) | 2.91 (0.08) | 1.15 | 85–96 |
| 17 | Quetiapine | Quetia | −20.0 (−17.7 to −20.8) | 2.36 (0.32) | 2.29 | 83 |
| 18 | Desvenlafaxine | Dvenlafax | −5.5 (−23.8 to −2.3) | 2.51 (0.1) | 0.89 | 30 |
| 19 | Atenolol | Aten | −10.1 (−20.7 to 0.7) | 0.24 (0.09) | −0.85 | 6–16 |
| 20 | Venlafaxine | Venlafax | −6.2 (−20.3 to 13.8) | 2.93 (0.10) | 1.43 | 27–30 |
| 21 | 4-OH propranolol | 4-OH Propran | 5.0 (−6.3 to 5.7) | 2.44 (0.17)∗∗ | 0.26 | NF |
| 22 | Lamotrigine | Lamot | 8.7 (−13.2 to 30.6) | 1.4 (0.58) | 1.68 | 55 |
| 23 | Olanzapine | Olanz | 13.0 (10.0–29.5) | 2.90 (0.27) | 1.90 | 93 |
| 24 | Baclofen | Baclo | 31.9 (−65.9 to 44.2) | 0.27 (0.68) | −0.94 | 30 |
| 25 | Trazodone | Trazod | 56.8 (36.2–69.2) | 2.49 (0.28) | 2.59 | 89–95 |
| 26 | Ramiprilat | Rmprlat | 74.2 (−38.3 to 108.7) | 1.02 (0.58) | −1.58 | 56 |
| 27 | Ramipril | Rmprl | 136.7 (63.6–146.4) | 2.23 (0.55) | −0.13 | 73 |
Of the candidate drugs and active metabolites studied, ILE reduced the amount of drug detected in the PBS compartment of the cassette in 20/27 cases. There was an apparent increase in drug detected in the PBS compartment in the remaining seven candidates, but the study was not powered to establish whether the effect was significant.
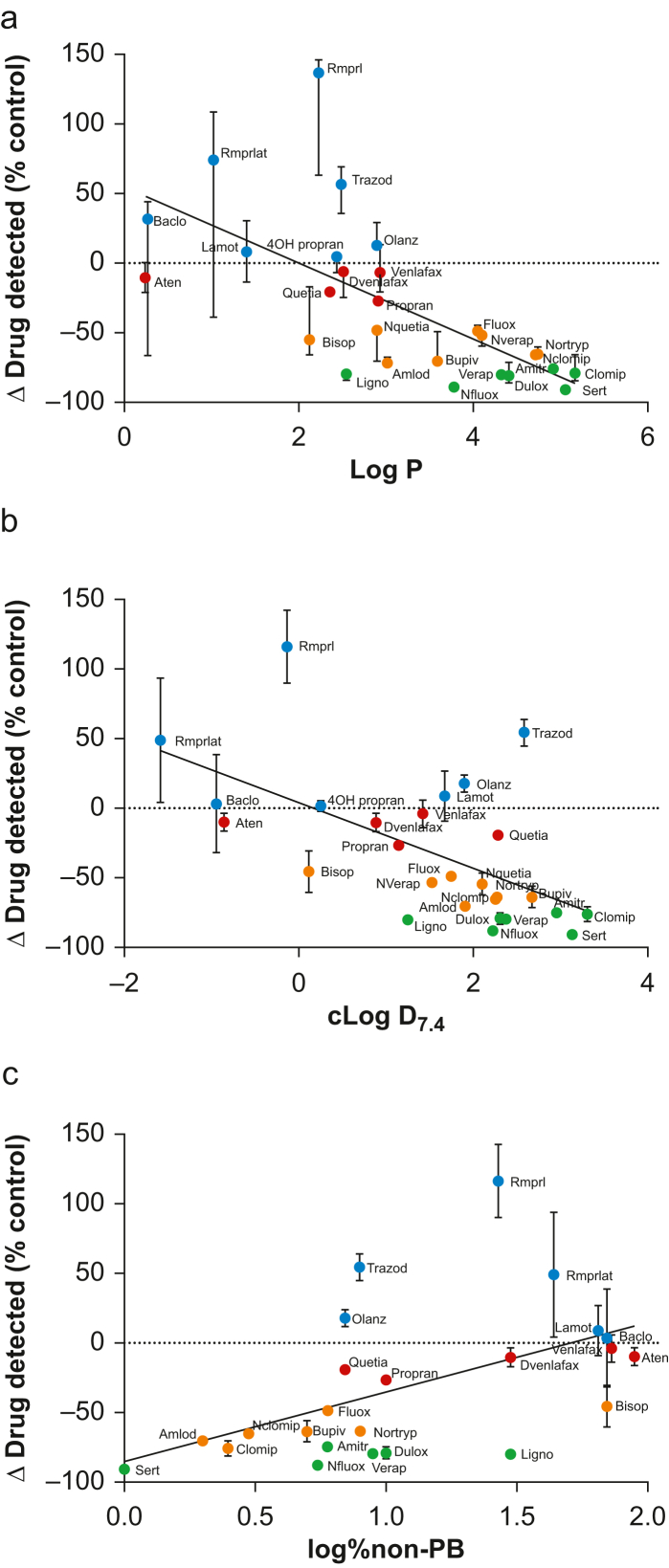
Correlation analyses of change in drug detected against each of the physicochemical characteristics listed in Table 1 revealed that there was a significant inverse association of change in drug detected with ILE with Log P (Fig. 2a; P<0.0001), cLogD7.4 (Fig. 2b; P=0.0005), and a significant association with log % non-protein binding (Fig. 2c; P=0.0060). There were examples of substantial deviation from the trend, with trazodone, ramiprilat, and ramipril in particular exhibiting unexpectedly high values in drug detected in the presence of ILE compared with the control; ramipril was identified as a statistical outlier (ROUT outlier analysis, Q=1%). The variance across three different plasma samples was particularly high for baclofen, ramipril, and ramiprilat.
Fig 2.
Correlation of median values of % change (95% confidence interval [CI]) in drug detected in the phosphate-buffered saline (PBS) chamber of the rapid equilibrium dialysis (RED) cassette with (a) Log P, (b) cLogD7.4, and (c) log % non-protein bound drug in the presence of intravenous lipid emulsion (ILE) compared with control (Spearman's rank correlation on median data (n=3 different plasma samples, each tested in triplicate): (a) r=−0.76 (−0.88 to −0.52, 95% CI), P<0.0001; (b) r=−0.60 (−0.80 to −0.27), P=0.0009; (c) r=−0.54 (0.16–0.79), P=0.0067). Abbreviations as per Table 1.
Discussion
This study set out to determine the impact of ILE added to plasma on the amount of drug able to cross the membrane into the PBS-filled compartment of RED cassettes. A total of 27 candidate drugs were assessed and found to be differentially affected in this regard, from some that were almost completely prevented from crossing the membrane to some that were apparently able to cross more readily than in parallel controls without ILE added. There was a significant inverse association of the impact of ILE with Log P and with cLogD7.4 and an unexpected association with log % non-protein bound drug. The correlations were, however, a good distance from being perfect, with some drugs presenting as notable exceptions, an effect that merits further exploration.
Partition coefficients and protein binding as determinants of ILE effect
A simplistic view in the in vitro setting would be that partition coefficients are an accurate determinant of the effectiveness of ILE to modulate drug availability in the aqueous compartment. The assumption behind this concept is that lipophilic drugs will distribute into the abundant lipid phase presented by ILE, whereas hydrophilic drugs would remain in the aqueous phase of the plasma, and would therefore be available to equilibrate across the RED membrane and be detected in the PBS compartment. To this end, Log P proved to be a relatively good predictor of ILE effect in vitro; those drugs with high Log P values (denoting high lipophilicity) showed substantially reduced accumulation across the membrane in the PBS chamber to a greater degree than those with low Log P values. However, the association was not entirely predictable, highlighting the complexity of the plasma matrix compared with the simple octanol/water model that is the basis for Log P determination. In particular, Log P makes no allowance for pH, which is a major determinant of drug ionisation, depending on the pKa of the drug in question. cLogD is a value that can be derived from Log P for a given pH (e.g. 7.4), with respect to the relevant pKa. It is somewhat surprising, therefore, that, although the association of cLogD7.4 with drug detection in the PBS compartment was retained, the association was less significant than for Log P; the allowance for pH as a factor did not improve the linearity of the fit. More puzzling still is the finding that the portion of the drug estimated to not be bound to plasma proteins was directly associated with the ability of ILE to sequester drug. One explanation for this apparent anomaly might revolve around the fact that the lipid components of ILE could block binding sites on plasma proteins, effectively displacing drugs such as ramipril and trazodone into the unbound fraction.28,29 This displacement effect is known to contribute to some drug interactions,25 but its role in the current setting would require further investigation.
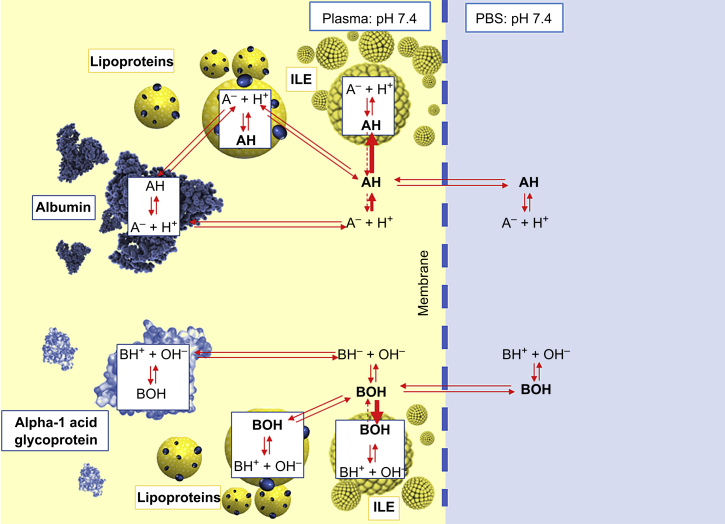
Understanding the complexities of the interactions between various drug fates is beyond the scope of this pragmatic study, but the findings serve to illustrate that predicting the potential merits of ILE in reversing drug toxicity cannot be reliably achieved through use of partition coefficients alone, and possibly not even by more comprehensive ILE capture predictors.30 It is also important to recognise that there are already additional beneficial effects recognised for ILE in the in vivo setting (e.g. cardiotonic effects)18, 19, 20 that are not considered here. Figure 3 illustrates some of the equilibria involved in determining drug distribution in plasma, and the impact of ILE on the overall equilibrium that might exist in the RED cassettes.
Fig 3.
Simplified diagram to show the complex equilibria involved in determining the amount of drug detected in the phosphate-buffered saline (PBS) (right) compartment of the rapid equilibrium dialysis (RED) cassettes for acidic (top half) and basic (bottom half) drugs.
Drugs
One of the unexpected results of this study was that several drugs showed unpredictably high concentrations in the PBS compartment; ramipril was identified as an outlier in this regard (+137% with ILE compared with PBS control). It should be emphasised that the variance across the replicates associated with ramipril and baclofen in particular was also correspondingly high compared with the other drugs, precluding any firm conclusions regarding additional availability of these drugs in the aqueous component of plasma in the presence of ILE compared with untreated plasma. The study was not powered to specifically identify significance of the impact of ILE compared with control plasma for individual drugs, but both the variance found between plasma samples from different individuals, and the clear excursion of these particular drugs from the association shared by others merits further exploration.
Study limitations
This was an entirely in vitro study designed to explore the association of influencing factors around partition on free drug that is available for equilibration in the aqueous phase across a membrane. It was designed to explore this relationship using the simplest model possible, whilst still retaining important characteristics that are relevant to the in vivo situation, not least of which is the inclusion of the highly complex matrix that is plasma. As with any model, there are a number of limitations that are important to recognise:
The in vitro nature of the study precludes identifying any in vivo impacts that ILE might have in patients (e.g. cardiotonic effects);18, 19, 20 similarly, the model adopted precludes determination of the ultimate fate of drug vis-à-vis the lipid shuttle hypothesis. The power of in vitro studies is to be able to focus on specific effects of mediators to help try to understand mechanisms.
The study was powered to establish the relationship of the various physical parameters tested on free drug available to diffuse from plasma to the PBS compartment, but not to distinguish whether the changes induced for individual drugs by ILE were significantly different from controls, particularly given the wide variance seen with a minority of the drugs tested here. Although we are confident that the variance is of biological origin (i.e. primarily associated with plasma samples from different individuals), rather than technical (i.e. between technical replicates from the same individual), further experiments are required with more replicates to establish whether some of the surprise findings are reproducible. It would also be interesting to establish the time-course of the onset of the effects of ILE in this regard, and to measure drug in all of the various compartments in plasma, and the aqueous phase to garner more information about the mechanics involved.
Both the drug concentrations and the ILE concentration should be considered to be supra-physiological in this model. The intention was to prove principle in the knowledge that some drug concentrations diffusing across the membrane might be very low, rather than necessarily to mimic in vivo concentrations. In addition, plasma pH was not measured in this study, but was assumed to be pH 7.4 on account of the high buffering capacity of plasma proteins. Future studies will not only measure pH, but actively manipulate it to establish the role of pH in determining drug distribution.
Conclusions
This study has shown that the presence of ILE in plasma has significant effects on partitioning for a number of drugs. For most cases, the studied drugs behaved as hypothesised and the presence of ILE reduced the quantity of drug which was free to diffuse across the RED dialysis membrane, although ramipril presented as an outlier in this regard. Mixed drug overdose is a highly problematic issue in clinical practice, however, the dosing and timing of ILE is yet to be settled in a standardised protocol and a more complete understanding on the spectrum of benefit across drugs has not yet been fully elucidated. Further research is merited to establish the complexity of the physicochemical impact of ILE on drug distribution between lipid, protein, and aqueous compartments of plasma, by way of a forerunner in understanding the ultimate fate of drugs influenced by ILE in overdose.
Authors’ contributions
Conceived the idea: KB, MS
Designed the study: KB, PW
Secured funding: KB, PW, IM
Interpreted data: KB, MS
Wrote the manuscript: KB, MS, IM
Edited the manuscript: KB, MS, AR, IM
Approved the manuscript: all authors
Conducted mass spectrometry experiments, analysed data: AR
Drafted the manuscript: PW
Are in agreement to be accountable for all aspects of the work: all authors
Funding
Two Endowment Awards from NHS Highland Research, Development and Innovation.
Declarations of interest
The authors declare that they have no conflicts of interest.
Handling editor: Phil Hopkins
Contributor Information
Kenneth Barker, Email: kenneth.barker2@nhs.scot.
Ian L. Megson, Email: ian.megson@uhi.ac.uk.
References
- 1.Guest J.F., Keating T., Gould D., Wigglesworth N. Modelling the annual NHS costs and outcomes attributable to healthcare-associated infections in England. BMJ Open. 2020;10 doi: 10.1136/bmjopen-2019-033367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Friedman J., Godvin M., Shover C.L., Gone J.P., Hansen H., Schriger D.L. Trends in drug overdose deaths among US adolescents, January 2010 to June 2021. JAMA. 2022;327:1398–1400. doi: 10.1001/jama.2022.2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Montane E., Castells X. Epidemiology of drug-related deaths in European hospitals: a systematic review and meta-analysis of observational studies. Br J Clin Pharmacol. 2021;87:3659–3671. doi: 10.1111/bcp.14799. [DOI] [PubMed] [Google Scholar]
- 4.Christie B. Drug deaths: record number in Scotland prompts calls for urgent UK policy reform. BMJ. 2019;366:l4731. doi: 10.1136/bmj.l4731. [DOI] [PubMed] [Google Scholar]
- 5.Iacobucci G. Drug related deaths in Scotland double in 10 years. BMJ. 2017;358:j3941. doi: 10.1136/bmj.j3941. [DOI] [PubMed] [Google Scholar]
- 6.Murray M. Drug-related deaths in Scotland: what is the solution? Br J Nurs. 2021;30:996. doi: 10.12968/bjon.2021.30.17.996. [DOI] [PubMed] [Google Scholar]
- 7.Weinberg G.L., VadeBoncouer T., Ramaraju G.A., Garcia-Amaro M.F., Cwik M.J. Pretreatment or resuscitation with a lipid infusion shifts the dose-response to bupivacaine-induced asystole in rats. Anesthesiology. 1998;88:1071–1075. doi: 10.1097/00000542-199804000-00028. [DOI] [PubMed] [Google Scholar]
- 8.Weinberg G.L. Lipid emulsion infusion: resuscitation for local anesthetic and other drug overdose. Anesthesiology. 2012;117:180–187. doi: 10.1097/ALN.0b013e31825ad8de. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taftachi F., Sanaei-Zadeh H., Sepehrian B., Zamani N. Lipid emulsion improves Glasgow coma scale and decreases blood glucose level in the setting of acute non-local anesthetic drug poisoning – a randomized controlled trial. Eur Rev Med Pharmacol Sci. 2012;16(Suppl 1):38–42. [PubMed] [Google Scholar]
- 10.Neal J.M., Barrington M.J., Fettiplace M.R., et al. The third American society of regional anesthesia and pain medicine practice advisory on local anesthetic systemic toxicity: executive summary 2017. Reg Anesth Pain Med. 2018;43:113–123. doi: 10.1097/AAP.0000000000000720. [DOI] [PubMed] [Google Scholar]
- 11.Cave G., Harvey M., Willers J., et al. LIPAEMIC report: results of clinical use of intravenous lipid emulsion in drug toxicity reported to an online lipid registry. J Med Toxicol. 2014;10:133–142. doi: 10.1007/s13181-013-0375-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pannu A.K., Garg S., Bhalla A., Dhibar D.P., Sharma N. Lipid emulsion for the treatment of acute organophosphate poisoning: an open-label randomized trial. Clin Toxicol (Phila) 2022;60:602–608. doi: 10.1080/15563650.2021.2013496. [DOI] [PubMed] [Google Scholar]
- 13.Kuo I., Akpa B.S. Validity of the lipid sink as a mechanism for the reversal of local anesthetic systemic toxicity: a physiologically based pharmacokinetic model study. Anesthesiology. 2013;118:1350–1361. doi: 10.1097/ALN.0b013e31828ce74d. [DOI] [PubMed] [Google Scholar]
- 14.Fettiplace M.R., Weinberg G. The mechanisms underlying lipid resuscitation therapy. Reg Anesth Pain Med. 2018;43:138–149. doi: 10.1097/AAP.0000000000000719. [DOI] [PubMed] [Google Scholar]
- 15.Shi K., Xia Y., Wang Q., et al. The effect of lipid emulsion on pharmacokinetics and tissue distribution of bupivacaine in rats. Anesth Analg. 2013;116:804–809. doi: 10.1213/ANE.0b013e318284123e. [DOI] [PubMed] [Google Scholar]
- 16.Fettiplace M.R., Lis K., Ripper R., et al. Multi-modal contributions to detoxification of acute pharmacotoxicity by a triglyceride micro-emulsion. J Control Release. 2015;198:62–70. doi: 10.1016/j.jconrel.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.French D., Smollin C., Ruan W., Wong A., Drasner K., Wu A.H. Partition constant and volume of distribution as predictors of clinical efficacy of lipid rescue for toxicological emergencies. Clin Toxicol (Phila) 2011;49:801–809. doi: 10.3109/15563650.2011.617308. [DOI] [PubMed] [Google Scholar]
- 18.Cao D., Heard K., Foran M., Koyfman A. Intravenous lipid emulsion in the emergency department: a systematic review of recent literature. J Emerg Med. 2015;48:387–397. doi: 10.1016/j.jemermed.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 19.Fettiplace M.R., Akpa B.S., Rubinstein I., Weinberg G. Confusion about infusion: rational volume limits for intravenous lipid emulsion during treatment of oral overdoses. Ann Emerg Med. 2015;66:185–188. doi: 10.1016/j.annemergmed.2015.01.020. [DOI] [PubMed] [Google Scholar]
- 20.Fettiplace M.R., Akpa B.S., Ripper R., et al. Resuscitation with lipid emulsion: dose-dependent recovery from cardiac pharmacotoxicity requires a cardiotonic effect. Anesthesiology. 2014;120:915–925. doi: 10.1097/ALN.0000000000000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Petersen K.M., Bogevig S., Petersen T.S., et al. Hemodynamic effects of intravenous, high-dose lipid emulsion with and without metoprolol infusion in healthy volunteers: a randomized clinical trial. Clin Pharmacol Ther. 2019;105:1009–1017. doi: 10.1002/cpt.1281. [DOI] [PubMed] [Google Scholar]
- 22.Kazemifar A.M., Yazdi Z., Bedram A., Mahmoudi J., Ziaee M. Effects of intravenous lipid emulsion on tramadol-induced seizure; a randomized clinical trial. Arch Acad Emerg Med. 2021;9:e20. doi: 10.22037/aaem.v9i1.1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chhabria B.A., Bhalla A., Shafiq N., Kumar S., Dhibar D.P., Sharma N. Lipid emulsion for acute organophosphate insecticide poisoning – a pilot observational safety study. Clin Toxicol (Phila) 2019;57:318–324. doi: 10.1080/15563650.2018.1520997. [DOI] [PubMed] [Google Scholar]
- 24.Levine M., Brent J., Wiegand T., et al. Lipid emulsion therapy during management of the critically-ill poisoned patient: a prospective cohort study. Clin Toxicol (Phila) 2023;61:584–590. doi: 10.1080/15563650.2023.2248372. [DOI] [PubMed] [Google Scholar]
- 25.Gosselin S., Hoegberg L.C., Hoffman R.S., et al. Evidence-based recommendations on the use of intravenous lipid emulsion therapy in poisoning. Clin Toxicol (Phila) 2016;54:899–923. doi: 10.1080/15563650.2016.1214275. [DOI] [PubMed] [Google Scholar]
- 26.Lavonas E.J., Akpunonu P.D., Arens A.M., et al. 2023 American Heart Association focused update on the management of patients with cardiac arrest or life-threatening toxicity due to poisoning: an update to the American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2023;148:e149–e184. doi: 10.1161/CIR.0000000000001161. [DOI] [PubMed] [Google Scholar]
- 27.American College of Medical Toxicology ACMT position statement: guidance for the use of intravenous lipid emulsion. J Med Toxicol. 2017;13:124–125. doi: 10.1007/s13181-016-0550-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schmidt S., Gonzalez D., Derendorf H. Significance of protein binding in pharmacokinetics and pharmacodynamics. J Pharm Sci. 2010;99:1107–1122. doi: 10.1002/jps.21916. [DOI] [PubMed] [Google Scholar]
- 29.Sugita O., Sawada Y., Sugiyama Y., Iga T., Hanano M. Prediction of drug-drug interaction from in vitro plasma protein binding and metabolism. A study of tolbutamide-sulfonamide interaction in rats. Biochem Pharmacol. 1981;30:3347–3354. doi: 10.1016/0006-2952(81)90611-0. [DOI] [PubMed] [Google Scholar]
- 30.Li Z., Li M., Sun H., et al. Prediction of drug capturing by lipid emulsions in vivo for the treatment of a drug overdose. J Control Release. 2022;346:148–157. doi: 10.1016/j.jconrel.2022.04.011. [DOI] [PubMed] [Google Scholar]