Summary
Background
Survival among people with HIV (PWH) has vastly improved globally over the last few decades but remains lower than among the general population. We aimed to estimate time trends of survival among PWH and their families from 1995 to 2021.
Methods
We conducted a registry-based, nationwide, population-based, matched cohort study. We included all Danish-born PWH from 1995 to 2021 who had been on antiretroviral therapy for 90 days, did not report intravenous drug use, and were not co-infected with hepatitis C (n = 4168). We matched population controls from the general population 10:1 to PWH by date of birth and sex (n = 41,680). For family cohorts, we identified siblings, mothers, and fathers of PWH and population controls. From Kaplan–Meier tables with age as time scale, we estimated survival from age 25. We compared PWH with population controls and families of PWH with families of population controls to calculate mortality rate ratios adjusted for sex, age, comorbidities, and education (aMRR).
Findings
The median age of death among PWH increased from 27.5 years in 1995–1997 to 73.9 years (2010–2014), but thereafter survival increased only marginally. From 2015 to 2021, mortality was increased among PWH (aMRR 1.87 (95% CI: 1.65–2.11)) and siblings (aMRR: 1.25 (95% CI: 1.07–1.47)), mothers (aMRR: 1.30 (95% CI: 1.17–1.43)), and fathers (aMRR: 1.15 (95% CI: 1.03–1.29)) of PWH compared to their respective control cohorts. Mortality among siblings of PWH who reported heterosexual route of HIV transmission (aMRR: 1.51 (95% CI: 1.16–1.96)) was higher than for siblings of PWH who reported men who have sex with men as route of HIV transmission (aMRR 1.19 (95% CI: 0.98–1.46)).
Interpretation
Survival among PWH improved substantially until 2010, after which it increased only marginally. This may partly be due to social and behavioural factors as PWH families also had higher mortality.
Funding
Preben and Anna Simonsen’s Foundation and Independent Research Fund Denmark.
Keywords: HIV, Survival analysis, Cohort studies, Denmark
Research in context.
Evidence before this study
We searched PubMed until February 1, 2024 for studies with the following MeSH-terms ((“HIV Infections” [Mesh]) AND “Denmark” [Mesh]) AND ((((((“Survival Analysis” [Mesh]) OR “Survival Rate” [Mesh]) OR “Mortality” [Mesh]) OR “Life Expectancy” [Mesh]) OR “Cohort Studies” [Majr:NoExp]) OR “Epidemiologic Studies” [Mesh]).
Studies have shown that survival among persons living with HIV in Denmark has improved over time from 1995 to 2015, from the initial introduction of highly active antiretroviral therapy through to changes in treatment initiation and regimens over the past several decades. Despite this improvement, survival among people with HIV has remained lower than that of the general population.
Added value of this study
In this study, we conducted a nationwide, population-based cohort study using the Danish HIV Cohort Study as well as administrative and health registers, which enabled us to include people with HIV (PWH) and population controls as well as family members to both PWH and population controls. Our results demonstrated that the increase in survival among PWH has plateaued in recent years with minimal improvement since 2010. Additionally, survival among family members of PWH was also lower than that of family members of population controls. The observed increase in mortality among PWH and their families cannot be explained by differences in education and comorbidity.
Implications of all the available evidence
Recent findings have shown that survival among PWH remains lower than that of the general population. Our findings in this study indicate that factors other than HIV impact survival among PWH and their families, which should be further investigated and addressed.
Introduction
Over the past several decades, there have been vast improvements in life expectancy among people living with HIV (PWH) worldwide.1 Though mortality among PWH is still higher than that of the general population,2 recent improvements in survival can largely be attributed to effective and widely available antiretroviral therapy (ART). However, the discrepancy between the life expectancies of PWH and the general population persists.3
Similar trends have been observed in Denmark. The Danish HIV Cohort Study (DHCS) is a nationwide, prospective cohort of all PWH connected to care in Denmark since 19954 and has previously been used to investigate survival among PWH compared to population controls from 1995 through 2015.5,6 With each passing period during this timeframe, survival among PWH continued to improve and move closer to that of population controls. Additionally, the median expected age of death for a 25-year-old PWH across the same time period increased from 35 to 74 years, though this improvement was still below the median age of death among population controls at 80 years.6
The numerous administrative and health-related registers in Denmark allow for individual-level linkage of data regarding education and comorbidities.7 In addition to surveilling the health outcomes among PWH, the DHCS has also previously been used to investigate various health outcomes among siblings of PWH, including excess mortality8 and mental illness.9 This study includes siblings, mothers, and fathers of PWH in the DHCS and population controls to further investigate trends in survival among family members of PWH compared to family members of the general population. We hypothesise that familial factors influence the increased mortality among PWH, which would be reflected in increased mortality among family of PWH as well.
Survival of PWH remains a topic of significant public health interest, as does the investigation into factors that can predict and influence life expectancy as PWH live longer. This study aims to update the survival estimates among PWH in Denmark through 2021 and compare them with the general population as well as siblings, mothers, and fathers of both PWH and population controls.
Methods
We performed a nationwide, population-based, matched cohort study using family cohorts as described previously.8
Setting
Denmark had a population of 5.2–5.8 million during the years of this study with an estimated prevalence of HIV-infection of 0.11%.10 In Denmark, medical care is provided free of charge to all residents. All residents of Denmark are assigned an individual and unique ten digit personal identification number at birth or upon immigration.7 This is used to track individuals through the Danish health and administrative registers.
Data sources
The DHCS is a complete record of all PWH 16 years of age and older receiving care at one of the eight HIV centres in Denmark since January 1, 19954 and includes data regarding demographics as well as other information related to HIV infection and care. Enrolment is ongoing and participants are included in the cohort upon their first visit to an HIV treatment centre.
The Danish Civil Registration System is a national register containing information on all residents of Denmark, including date of birth, vital status, date of migration, date of death, place of residence, and familial relationships, among others.11
The National Patient Registry was established in 1977 and collects information on all hospital admissions in Denmark.12 Data from outpatient and emergency department visits were added in 1995. For each hospital contact, the National Patient Registry registers dates of admission and discharge and up to 20 discharge diagnoses according to the International Classification of Diseases, 8th revision (ICD-8) through 1993 and the tenth version (ICD-10) from 1994 onwards assigned by the attending physician.
The Danish educational registers hold information on the highest attained education.13
Study cohorts
We included all Danish-born PWH from the DHCS who did not report intravenous drug use as the route of HIV transmission, who were not positive for hepatitis C virus, and who initiated ART before November 1, 2021. To determine whether additional factors unrelated to injection drug use may contribute to excess mortality among PWH and their families, we excluded individuals who reported intravenous drug use as the route of HIV transmission and PWH with hepatitis C infection, as hepatitis C infection is closely related to injection drug use in PWH of Danish origin.14 Date of study inclusion was January 1, 1995, the 25th birthdate, the date of immigration (to account for Danish-born PWH living abroad) or 90 days after ART initiation, whichever came last (the first 90 days were excluded to account for mortality known to occur shortly after diagnosis and initiation of ART). Using the linkage between the DHCS and the Civil Registration System, we constructed a control cohort, which included ten Danish-born population controls per PWH, who were matched on date of birth and sex and who were alive and residing in Denmark at the date of study inclusion of the PWH they were matched to. Furthermore, we identified siblings, mothers, and fathers of both PWH and population controls, who were alive and residing in Denmark at the date of study inclusion of the PWH or population control to whom they had a familial link. Population controls as well as siblings, mothers, and fathers of both controls and PWH were assigned the same date of study inclusion as their corresponding PWH.
Confounding factors
We identified comorbidity status from the National Patient Registry according to Charlson Comorbidity Index (CCI) using diagnosis codes, excluding AIDS.15,16 We used CCI as an ordinal variable grouped as CCI 0, 1, 2, 3, 4, 5, and ≥6. We obtained the highest attained education of all individuals from the Danish educational registers, which we used as an ordinal variable stratified as low education level (up to and including secondary school), middle education level (bachelor's and vocational degrees) or higher education level (master's degree or higher). We determined confounding factors at the date of HIV diagnosis of the PWH and the population controls and at date of study inclusion for the family members. However, when we compared survival among siblings with population controls, we determined confounding factors for siblings at date of HIV diagnosis of the PWH to whom the controls and siblings were attached.
Statistical methods
We calculated time from the date of study inclusion until either the date of emigration, November 1, 2021 or the date of death, whichever came first. The outcome of interest was time to all-cause mortality.
We used Kaplan Meier analyses to compute cumulative survival and median age of death after age 25 years with age as the time scale as previously described.5,6 For PWH, analyses were stratified by the following seven calendar periods: 1995–1996 (pre-HAART), 1997–1999 (early HAART), 2000–2004, 2005–2009, 2010–2014, 2015–2018, and 2019–2021. These periods correspond to time periods used in previous studies5,6 as well as the development and initiation of PWH on antiretroviral therapies. For the sibling and family cohorts, the analyses were stratified into three calendar periods: 1995–2004, 2005–2014, and 2015–2021 to ensure proper statistical power in each time period. For the sibling cohorts, analyses continued to ages 55, 65, and 75 years for the three time periods, respectively, and to age 85 years for the overall parent and family cohorts.
We used a Cox regression model to compute mortality rate ratios (MRR) and 95% confidence intervals with age as time scale. PWH were included in the analyses at the age at which they entered the study and exited at the age of death or censoring. We calculated MRRs and adjusted MRRs (aMRR) in the 2015–2021 period between PWH and population controls, as well as between siblings, mothers, and fathers of PWH and population cohorts. We also compared mortality between families of the PWH and population controls, in which families were defined as the combination of siblings, mothers, and fathers. We stratified the Cox regression analyses on route of transmission (men who have sex with men (MSM), heterosexual) for PWH and siblings of PWH. Finally, MRRs for the sibling-, father-, mother-, and family cohorts were adjusted for sex, age, comorbidity, and education to compute aMRRs and 95% confidence intervals). As the PWH and control cohorts were matched for date of birth and sex, we only adjusted for comorbidity and education in the analyses comparing PWH with controls.
IRB approval
According to Danish law, registry-based studies do not require approval from an ethics board. The study was approved by the Danish Data Protection Agency (registration number: P-2021-446).
Role of the funding source
The funding sources played no role in the study design, data collection, analysis, interpretation, writing of this manuscript, or decision to submit for publication.
Results
Study population
We included 4168 PWH, of whom 3650 (88%) were men with a median age of 42 years at study inclusion and a total of 49,156 person-years of observation (PYR) (Table 1). We also included 41,680 population controls with a total of 613,307 PYR. Additionally, 5590 siblings, 3217 mothers, and 3091 fathers of PWH were included as well as 52,491 siblings, 32,077 mothers, and 31,431 fathers of population controls with a total of 1,419,336 PYR. Regarding route of HIV transmission, 2539 (61%) of PWH reported MSM, 1345 (32%) reported heterosexual sexual contact, and 284 (7%) were categorised as other/unknown. More PWH had a CCI above 0 than the population controls. PWH had attained lower levels of education than population controls, with 1659 (40%) having achieved a low education level, 1616 (39%) with a middle education level, and 893 (21%) were highly educated compared to 12,928 (31%), 19,469 (47%), and 9283 (22%), respectively. Siblings of PWH were less educated compared to siblings of population controls, despite the median age being the same across the two groups.
Table 1.
Characteristics of study cohorts.
| PWH | Population controls | Siblings of PWH | Siblings of population controls | Mothers of PWH | Mothers of population controls | Fathers of PWH | Fathers of population controls | |
|---|---|---|---|---|---|---|---|---|
| Number | 4168 | 41,680 | 5590 | 52,491 | 3217 | 32,077 | 3091 | 31,431 |
| Male, n (%) | 3650 (88) | 36,500 (88) | 2937 (53) | 27,224 (52) | 0 | 0 | 3091 (100) | 31,431 (100) |
| Age, years (IQR) | 42 (34–51) | 42 (34–51) | 38 (31–46) | 38 (31–45) | 66 (59–74) | 65 (58–73) | 69 (61–78) | 68 (61–77) |
| Route of transmissiona, n (%) | ||||||||
| MSM | 2539 (61) | 25,390 (61) | 3604 (64) | 33,592 (64) | 2057 (64) | 20,395 (64) | 1984 (64) | 20,016 (64) |
| Heterosexual sex | 1345 (32) | 13,439 (32) | 1687 (30) | 15,495 (30) | 960 (30) | 9505 (30) | 914 (30) | 9268 (29) |
| Other/unknown | 284 (7) | 2851 (7) | 299 (5) | 3404 (6) | 200 (6) | 2177 (7) | 193 (6) | 2147 (7) |
| Charlson comorbidity index score, n (%) | ||||||||
| 0 | 3428 (82) | 36,792 (88) | 4996 (89) | 47,257 (90) | 1980 (62) | 20,442 (64) | 1687 (55) | 17,573 (56) |
| 1 | 339 (8) | 2816 (7) | 369 (7) | 3340 (6) | 427 (13) | 3923 (12) | 479 (15) | 4692 (15) |
| 2 | 255 (6) | 1281 (3) | 143 (3) | 1297 (2) | 399 (12) | 3921 (12) | 447 (14) | 4065 (13) |
| 3 | 73 (2) | 400 (1) | 46 (1) | 367 (1) | 147 (5) | 1444 (5) | 185 (6) | 1952 (6) |
| 4 | 35 (1) | 148 (0) | 16 (0) | 90 (0) | 62 (2) | 555 (2) | 99 (3) | 965 (3) |
| 5 | 10 (0) | 60 (0) | 6 (0) | 40 (0) | 26 (1) | 262 (1) | 47 (2) | 419 (1) |
| ≥6 | 28 (1) | 183 (0) | 14 (0) | 100 (0) | 176 (5) | 1530 (5) | 147 (5) | 1765 (6) |
| Education level, n (%) | ||||||||
| Low | 1659 (40) | 12,928 (31) | 2393 (43) | 19,106 (36) | 1891 (59) | 18,029 (56) | 1548 (50) | 15,180 (48) |
| Middle | 1616 (39) | 19,469 (47) | 2039 (36) | 21,408 (41) | 923 (29) | 9583 (30) | 1142 (37) | 11,950 (38) |
| High | 893 (21) | 9283 (22) | 1158 (21) | 11,977 (23) | 403 (13) | 4465 (14) | 401 (13) | 4301 (14) |
| Attrition, n (%) | ||||||||
| Emigrated | 101 (2) | 407 (1) | 53 (9) | 552 (1) | 19 (1) | 107 (0) | 33 (1) | 206 (1) |
| Lost to follow-up | ≤3 (0) | 10 (0) | ≤3 (0) | 13 (0) | ≤3 (0) | ≤3 (0) | ≤3 (0) | ≤3 (0) |
| Observation time, PYR | 49,156 | 613,307 | 77,563 | 745,212 | 29,025 | 313,592 | 20,423 | 233,521 |
| Death, n (%) | 1069 (26) | 4684 (11) | 315 (6) | 2240 (4) | 1599 (50) | 13,931 (43) | 1945 (63) | 18,455 (59) |
PYR: person-years of observation, PWH: people with HIV, MSM: men who have sex with men.
Route of Transmission for controls refers to the route of transmission for their matched PWH.
Survival of PWH compared to population controls
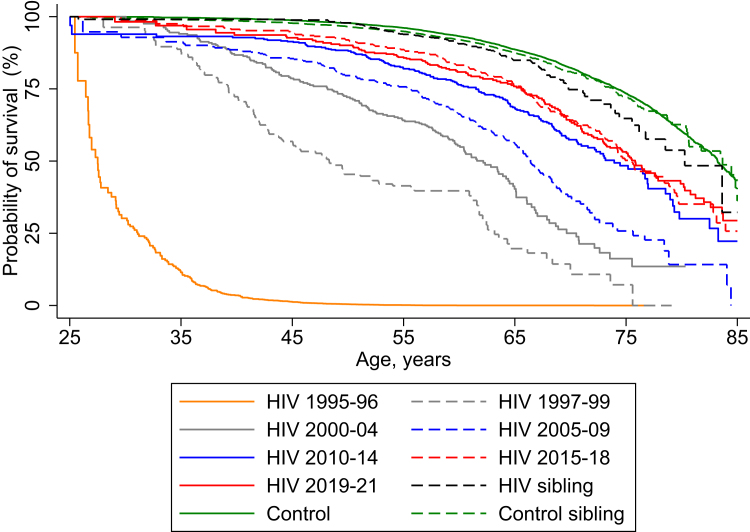
Among PWH, median survival from age 25 years increased from the 1995 to 1996 period through to the later calendar periods (Table 2). In contrast to earlier time-periods, however, the three calendar periods from 2010 to 2021 only provided a marginal increase in survival among PWH (Table 2, Fig. 1). Age of death among PWH in the 1995–1996 period was 27.5 (25.5–29.4) (MRR: 79.31 (68.48–91.84)) and increased from 73.9 (69.8–77.0) (MRR: 2.31 (1.96–2.73)) in 2010–2014 to 75.8 (72.9–79.9) (MRR: 1.86 (1.55–2.21)) in 2019–2021. In the stratified analyses, between 2015 and 2021, PWH with heterosexual transmission had an aMRR of 2.19 (95% CI: 1.80–2.67), whereas PWH with MSM transmission had an aMRR of 1.66 (95% CI: 1.40–1.98), compared to population controls.
Table 2.
Mortality rate ratios (MRR), adjusted MRRs (aMRR), and median age of death from age 25 years among people with HIV (PWH) for seven time periods and their same-sex siblings and population controls and their same-sex siblings from 2015 to 2021.
| Number of deaths | MRRa (95% Confidence Interval) | aMRRa,b (95% Confidence Interval) | Median age of death (Years, Interquartile range) | |
|---|---|---|---|---|
| Population group | ||||
| Population controls (2015–2021) | 2191 | 1 (ref.) | 1 (ref.) | 83.2 (82.9–83.6) |
| Persons with HIV | ||||
| 1995–1996 | 226 | 79.39 (68.55–91.94) | 79.09 (68.28–91.61) | 27.5 (25.5–29.4) |
| 1997–1999 | 91 | 12.50 (10.08–15.51) | 12.25 (9.87–15.2) | 48.3 (42.9–55.7) |
| 2000–2004 | 151 | 6.61 (5.58–7.83) | 6.41 (5.41–7.6) | 62.2 (59.6–64.4) |
| 2005–2009 | 163 | 4.09 (3.48–4.81) | 3.93 (3.34–4.62) | 66.4 (63.2–68.5) |
| 2010–2014 | 152 | 2.31 (1.96–2.73) | 2.22 (1.88–2.63) | 73.9 (69.8–77.0) |
| 2015–2018 | 151 | 1.95 (1.65–2.3) | 1.87 (1.58–2.21) | 75.5 (73.9–78.4) |
| 2019–2021 | 132 | 1.86 (1.56–2.21) | 1.80 (1.51–2.14) | 75.8 (72.9–79.9) |
| Combined 2015–2021 | 283 | 1.90 (1.68–2.16) | 1.84 (1.62–2.08) | 75.7 (74.3–77.7) |
| Siblings of population controls (2015–2021) | 1220 | 1.01 (0.92–1.1) | 0.99 (0.90–1.08) | 84.5 (83.5–85.2) |
| Siblings of PWH (2015–2021) | 175 | 1.31 (1.07–1.59) | 1.25 (1.03–1.52) | 83.6 (78.4–84.5) |
Mortality rate ratios were compared to population controls.
Adjusted for Charlson Comorbidity Index and education.
Fig. 1.
Cumulative survival from age 25 years stratified by calendar period of observation for PWH and their same-sex siblings (HIV sibling) and population controls (Control) and their same-sex siblings (Control sibling) (seeTable S1for numbers at risk and censored individuals).
Survival of families of PWH and population controls
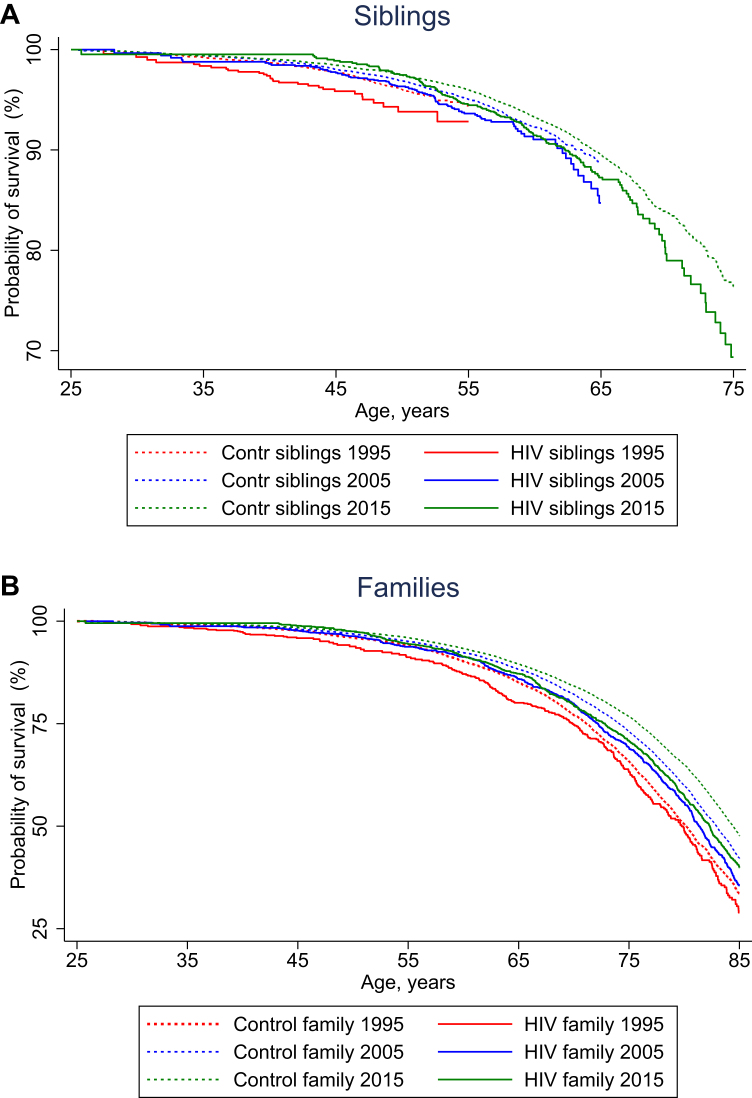
Siblings of PWH had slightly decreased survival compared to population controls, while survival among siblings of population controls did not differ from that of population controls (Fig. 1, Table 2). Median survival from age 25 years was decreased for siblings and families of PWH compared to siblings and families of population controls in all time periods (Fig. 2). Additionally, while the survival of siblings and families of population controls continued to increase over the three time periods, survival among siblings and families of PWH in the 2005–2015 periods more or less overlapped with that of the 2015–2021 period (Fig. 2). Mortality in the 2015–2021 periods was increased among siblings of PWH compared to siblings of population controls (aMRR: 1.25 (95% CI: 1.07–1.47)) (Table 3). Siblings of PWH with heterosexual transmission of HIV had higher MRR than siblings of PWH with MSM transmission (aMRR: 1.51 (95% CI: 1.16–1.96) and aMRR: 1.19 (95% CI: 0.98–1.46)). Families of PWH also had increased mortality compared to families of population controls (aMRR: 1.24 (95% CI: 1.16–1.32)). More specifically, mothers of PWH had an aMRR of 1.30 (95% CI: 1.17–1.43) compared to mothers of population controls, whereas fathers of PWH had an aMRR of 1.15 (95% CI: 1.03–1.29) compared to fathers of population controls. Additionally, mothers to PWH with heterosexual route of transmission had a greater increase in mortality (1.49 (1.26–1.77)) than was observed among mothers of MSM PWH (1.22 (1.07–1.38)).
Fig. 2.
Cumulative survival from age 25 years for A) siblings of PWH (solid lines) and siblings of population controls (dotted lines) and B) families of PWH (solid lines) and families of population controls (dotted lines), stratified by calendar period of observation (seeTable S2for numbers at risk and censored individuals).
Table 3.
Mortality rate ratios (MRR) for PWH, siblings, mothers, and fathers of PWH and families of PWH versus families of population controls stratified by route of HIV transmission and adjusted for sex, Charlson Comorbidity Index (CCI), and education level, 2015–2021.
| Populations | Adjustment |
|
|---|---|---|
| Unadjusted MRR (95% Confidence Interval) | Adjusted MRR (95% Confidence Interval) | |
| PWH versus population controls | 1.92 (1.70–2.17) | 1.87 (1.65–2.11) |
| PWH with heterosexual transmission versus population controls | 2.34 (1.92–2.84) | 2.19 (1.80–2.67) |
| PWH with MSM transmission versus population controls | 1.66 (1.39–1.97) | 1.66 (1.40–1.98) |
| Siblings of PWH versus siblings of population controls | 1.30 (1.11–1.52) | 1.25 (1.07–1.47) |
| Siblings of PWH with heterosexual transmission versus siblings of population controls | 1.53 (1.18–1.99) | 1.51 (1.16–1.96) |
| Siblings of PWH with MSM transmission versus siblings of population controls | 1.23 (1.01–1.51) | 1.19 (0.98–1.46) |
| Mothers of PWH versus mothers of population controls | 1.32 (1.20–1.46) | 1.30 (1.17–1.43) |
| Mothers of PWH with heterosexual transmission versus mothers of population controls | 1.49 (1.26–1.77) | 1.41 (1.19–1.67) |
| Mothers of PWH with MSM transmission versus mothers of population controls | 1.22 (1.07–1.38) | 1.22 (1.07–1.38) |
| Fathers of PWH versus fathers of population controls | 1.13 (1.01–1.26) | 1.15 (1.03–1.29) |
| Fathers of PWH with heterosexual transmission versus fathers of population controls | 1.04 (0.83–1.29) | 1.05 (0.84–1.31) |
| Fathers of PWH with MSM transmission versus fathers of population controls | 1.15 (1.01–1.31) | 1.18 (1.04–1.34) |
| Familiesa of PWH versus familiesa of population controls | 1.23 (1.15–1.32) | 1.24 (1.16–1.32) |
| Familiesa of PWH with heterosexual transmission versus familiesa of population controls | 1.32 (1.18–1.49) | 1.30 (1.15–1.47) |
| Familiesa of PWH with MSM transmission versus familiesa of population controls | 1.19 (1.09–1.29) | 1.20 (1.11–1.30) |
All analyses were adjusted for CCI and education. Analyses of siblings, mothers, and fathers were also adjusted for age and sex.
Defined as siblings, mothers, and fathers.
Discussion
In this registry-based, nationwide, population-based matched cohort study, we found that while survival among PWH improved significantly over the past several decades and approached that of the general population, this progress plateaued from 2010 and onward. Additionally, survival rates among siblings, mothers, and fathers of PWH were lower than that of the siblings, mothers, and fathers of controls. Finally, siblings of PWH who had a heterosexual route of HIV-transmission had a higher relative mortality compared to siblings of population controls than siblings of PWH with MSM route of HIV transmission.
It is unclear why the increase in survival with calendar time seemed to plateau in 2010 for PWH and their families, after which the overall increased survival in the general population no longer translated to PWH. By excluding PWH with hepatitis C co-infection and intravenous drug use as route of HIV transmission, we have already excluded PWH with the highest mortality, as it has been previously reported that siblings of hepatitis C co-infected PWH have increased mortality compared to siblings of PWH without hepatis C co-infection.8 Previous studies that used the DHCS to investigate survival of PWH included individuals with intravenous drug use as route of HIV transmission, a significant point of departure from our study design, which may explain differences in survival compared to our actual study.5,6 Furthermore, adjusting for comorbidities and education only marginally changed our risk estimates. This suggests that neither differences in comorbidity nor socioeconomic status can fully explain the differences in survival among PWH and the general population. By including family cohorts, we were able to demonstrate an increased mortality among families of PWH compared to families of population controls, which might represent certain social and behavioural factors that are not captured by adjusting the survival analyses for comorbidities and education. This is supported by the fact that unhealthy behaviours such as smoking, have been reported to be more common among PWH than the general population.17
There could be several reasons for the higher mortality observed among PWH than among siblings of PWH. We hypothesise that certain social and behavioural factors are adopted across the family and might contribute to the increase in mortality among HIV families. These factors and the subsequent increase in risk observed across the family may however be especially heightened among PWH and contribute to both the risk of HIV infection and higher mortality observed among PWH than among HIV families. Additionally, we hypothesise that the aforementioned social inequality as well as behavioural and social factors may be more widely adopted among PWH reporting heterosexual HIV transmission and their families than for PWH reporting MSM transmission and their families. A reason for this could be that MSM, a known risk group for HIV, is a characteristic attributed on the level of the individual rather than a shared familial lifestyle behaviour or indicator of social inequality. This is therefore reflected in the difference in mortality risk when we stratified by mode of HIV transmission among PWH and their siblings. Finally, the increase in mortality among mothers and fathers of PWH compared to mothers and fathers of population controls, respectively, could also be attributed to behavioural and health-related risk factors observed within the family. For example, previous studies have found an increase in risk of certain smoking-related comorbidities, namely lung and head and neck cancers as well as myocardial infarction among families to PWH,18, 19, 20 which is in line with our findings of increased mortality among families of PWH. Additionally, mothers had larger increase in mortality related to route of infection, a trend also observed among both PWH and siblings to PWH. The same trend was not observed among fathers, though we hypothesize that this was a chance finding due to less statistical power among fathers.
In addition to social and behavioural factors, the biological effects of HIV itself also impact survival. HIV infection has been linked to various inflammatory diseases, including cardiovascular disease, cancer, and liver and kidney disease.21, 22, 23 Inflammation-related conditions have also been linked to premature aging among PWH, who have similar comorbidities and overall health status to those from the general population who are ten years older.21 Behaviour-related comorbidities, such as smoking, human papilloma virus, and certain cancers, are also more prevalent among PWH.24 Fewer PWH are dying from HIV-related factors and more from non-communicable comorbidities, though it is not always clear to what degree HIV and lifestyle play an underlying role in these deaths.25 This shift in disease burden of HIV from a disease with high morbidity and mortality to a treatable, chronic disease will likely continue as PWH live longer.24 Chronic health effects related to HIV infection add an additional burden to PWH and their survival, a burden that we would not expect to see among their HIV-negative siblings.
Trickey et al. used the Antiretroviral Therapy Cohort Collaboration and found that life expectancy for PWH on ART after 2015 is slightly below that of the general population.26 This study utilised data from 20 different North American and European HIV cohorts from countries with varying healthcare systems and access to ART and could not consider factors such as education in their analyses due to lack of access to data regarding socioeconomic status. Gueler et al. used the Swiss HIV Cohort Study and found that while PWH were still found to have shorter life expectancies compared to the general population across all education levels, life expectancy among highly educated PWH was comparable to that of those in the general population with low education levels.27 Guaraldi et al. used the Italian Collaborative HIV Aging Cohort and found that there was no significant difference in life expectancy among PWH aged 40 who had complete immune recovery and the general population of the same age, while PWH aged 25 were found to have decreased life expectancy by five years, despite immune recovery.28 The authors of this study acknowledge, however, that differences in risk-taking behaviours likely exist between Italian and northern European PWH, which could limit the generalisability of this study to the Danish context. Lastly, de Coninick et al. used the Swedish National HIV Register and found that despite adherence to ART, PWH were three times as likely to die as the general population.29 However, this study included PWH with injection drug use as mode of HIV transmission as well as foreign-born PWH, two factors that differ substantially from our study design and could lead to differences in study population and subsequent findings.
This study does not address potential discrepancies in survival rates among foreign-born PWH in Denmark compared to Danish-born PWH and their family members due to the exclusion of foreign-born PWH, which may limit the generalisability of our findings. However, the inclusion of only Danish-born PWH allowed for the enrichment of the DHCS with data from various nationwide registers as well as the linkage to siblings, mothers, and fathers, which is not available for foreign-born PWH. Further, it mitigated potential confounding as differences in survival among foreign-born and Danish-born residents likely would have influenced the results if we had included foreign-born PWH. Another limitation may be the use of education as the only measure of socioeconomic status, which may leave some residual confounding. Additionally, we cannot exclude the possibility that some PWH with intravenous drug use as route of HIV transmission reported sexual transmission to avoid stigmatisation that exists regarding drug abuse in Denmark. Denmark efficiently implemented universal access to ART for all PWH,30 which is why our results may not be generalisable to countries with delayed implementation of or limited access to ART. Finally, it is a limitation that other potential confounding factors, such as smoking, alcohol intake and physical activity, were not included in this study. These factors are not included in any health registries and were therefore not available to us at the time of the study.
The high quality and completeness of data within the DHCS is a strength of this study and makes the cohort a powerful tool with which to further investigate continued health outcomes and mortality among PWH in Denmark. However, the most important and unique aspect of this study is the inclusion of the family cohorts, which allows for the investigation of social and behavioural factors that run through generations of a family. Additionally, the inclusion of family cohorts is not available in most other research settings, which makes their inclusion a unique opportunity in the Danish context. Lastly, the highly registered nature of data within the Danish healthcare system allowed us to capture potential differences in socioeconomic status (education) and overall health (CCI) between PWH, their families and the general population.
We conclude that there has been an impressive increase in survival of PWH from 1995 to 2010, but there remains a gap in survival between PWH and the general population, PWH and their family members, as well as family members of PWH and family members of population controls. These discrepancies may in part be attributed to shared socioeconomic factors as well as social and behavioural factors within families. Future research should continue to follow the trends in survival among PWH and their families as well as further investigate the potential factors contributing to decreased relative survival compared to the general population.
Contributors
CE, LHO, MMT, and NO were involved in the conceptualisation, methodology, and formal analysis of the study. LHO, JC, GK, ISJ, CL, AP, MDP, S. Lunding, S. Leth, LN, MMT, and NO were involved in data curation. CE, LHO, MMT, and NO were responsible for the writing of the original draft as well as review and editing. CE and LHO share first authorship.
Data sharing statement
The ethical approval of this study from the Danish Data Protection Agency states the data that has been used in this article cannot be shared publicly.
Declaration of interests
Malte Mose Tetens has received travel grants outside this work from GlaxoSmithKline Pharma A/S, and funding of salary from the Research Fund of Copenhagen University Hospital–Rigshospitalet. The remaining authors declare no conflicts of interest.
Acknowledgements
Funding: Niels Obel and the Danish HIV Cohort study have received grants from Preben and Anna Simonsen’s Foundation. Lars Haukali Omland received funding from Independent Research Fund Denmark (grant ID: 10.46540/4262-00020B).
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lanepe.2024.100956.
Appendix A. Supplementary data
References
- 1.Teeraananchai S., Kerr S.J., Amin J., Ruxrungtham K., Law M.G. Life expectancy of HIV-positive people after starting combination antiretroviral therapy: a meta-analysis. HIV Med. 2017;18(4):256–266. doi: 10.1111/hiv.12421. [DOI] [PubMed] [Google Scholar]
- 2.Patterson S., Cescon A., Samji H., et al. Life expectancy of HIV-positive individuals on combination antiretroviral therapy in Canada. BMC Infect Dis. 2015;15:274. doi: 10.1186/s12879-015-0969-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wandeler G., Johnson L.F., Egger M. Trends in life expectancy of HIV-positive adults on antiretroviral therapy across the globe: comparisons with general population. Curr Opin HIV AIDS. 2016;11(5):492–500. doi: 10.1097/COH.0000000000000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Omland L.H., Ahlström M.G., Obel N. Cohort profile update: the Danish HIV cohort study (DHCS) Int J Epidemiol. 2014;43(6):1769-9e. doi: 10.1093/ije/dyu153. [DOI] [PubMed] [Google Scholar]
- 5.Lohse N., Hansen A.B., Pedersen G., et al. Survival of persons with and without HIV infection in Denmark, 1995-2005. Ann Intern Med. 2007;146(2):87–95. doi: 10.7326/0003-4819-146-2-200701160-00003. [DOI] [PubMed] [Google Scholar]
- 6.Lohse N., Obel N. Update of survival for persons with HIV infection in Denmark. Ann Intern Med. 2016;165(10):749–750. doi: 10.7326/L16-0091. [DOI] [PubMed] [Google Scholar]
- 7.Schmidt M., Schmidt S.A.J., Adelborg K., et al. The Danish health care system and epidemiological research: from health care contacts to database records. Clin Epidemiol. 2019;11:563–591. doi: 10.2147/CLEP.S179083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hansen A.B., Gerstoft J., Kronborg G., Pedersen C., Sorensen H.T., Obel N. Mortality in siblings of patients coinfected with HIV and hepatitis C virus. J Infect Dis. 2007;195(2):230–235. doi: 10.1086/510246. [DOI] [PubMed] [Google Scholar]
- 9.Vollmond C.V., Tetens M.M., Paulsen F.W., et al. Risk of depression in people with human immunodeficiency virus: a nationwide population-based matched cohort study. Clin Infect Dis. 2023;77(11):1569–1577. doi: 10.1093/cid/ciad415. [DOI] [PubMed] [Google Scholar]
- 10.Statens Serum Institut HIV 2022 Denmark 2023. https://en.ssi.dk/surveillance-and-preparedness/surveillance-in-denmark/annual-reports-on-disease-incidence/hiv-2022 Available from:
- 11.Pedersen C.B. The Danish Civil registration system. Scand J Public Health. 2011;39(7 Suppl):22–25. doi: 10.1177/1403494810387965. [DOI] [PubMed] [Google Scholar]
- 12.Schmidt M., Schmidt S.A., Sandegaard J.L., Ehrenstein V., Pedersen L., Sørensen H.T. The Danish National Patient Registry: a review of content, data quality, and research potential. Clin Epidemiol. 2015;7:449–490. doi: 10.2147/CLEP.S91125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jensen V.M., Rasmussen A.W. Danish education registers. Scand J Public Health. 2011;39(7 Suppl):91–94. doi: 10.1177/1403494810394715. [DOI] [PubMed] [Google Scholar]
- 14.Larsen M.V., Omland L.H., Gerstoft J., et al. Impact of injecting drug use on response to highly active antiretroviral treatment in HIV-1-infected patients: a nationwide population-based cohort study. Scand J Infect Dis. 2010;42(11–12):917–923. doi: 10.3109/00365548.2010.511258. [DOI] [PubMed] [Google Scholar]
- 15.Obel N., Omland L.H., Kronborg G., et al. Impact of non-HIV and HIV risk factors on survival in HIV-infected patients on HAART: a population-based nationwide cohort study. PLoS One. 2011;6(7) doi: 10.1371/journal.pone.0022698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zavascki A.P., Fuchs S.C. The need for reappraisal of AIDS score weight of Charlson comorbidity index. J Clin Epidemiol. 2007;60(9):867–868. doi: 10.1016/j.jclinepi.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 17.Rahmanian S., Wewers M.E., Koletar S., Reynolds N., Ferketich A., Diaz P. Cigarette smoking in the HIV-infected population. Proc Am Thorac Soc. 2011;8(3):313–319. doi: 10.1513/pats.201009-058WR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Engsig F.N., Gerstoft J., Kronborg G., et al. Head and neck cancer in HIV patients and their parents: a Danish cohort study. Clin Epidemiol. 2011;3:217–227. doi: 10.2147/CLEP.S19875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Engsig F.N., Kronborg G., Larsen C.S., et al. Lung cancer in HIV patients and their parents: a Danish cohort study. BMC Cancer. 2011;11:272. doi: 10.1186/1471-2407-11-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rasmussen L.D., Omland L.H., Pedersen C., et al. Risk of myocardial infarction in parents of HIV-infected Individuals: a population-based Cohort Study. BMC Infect Dis. 2010;10:169. doi: 10.1186/1471-2334-10-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Serrano-Villar S., Moltó-Marhuenda J., Montero-Alonso M., Diaz-Torné C., López-Cavanillas M., Pérez de Isla L. 2023. Knowledge, attitudes and practices in HIV-related chronic inflammation and cardiovascular risk in Spain. Enfermedades infecciosas y microbiologia clinica (English ed) [DOI] [PubMed] [Google Scholar]
- 22.Lerner A.M., Eisinger R.W., Fauci A.S. Comorbidities in persons with HIV: the lingering challenge. JAMA. 2020;323(1):19–20. doi: 10.1001/jama.2019.19775. [DOI] [PubMed] [Google Scholar]
- 23.Omland L.H., Gerstoft J., Kronborg G., et al. Cancer risk and temporal trends in people with HIV during a quarter of a century–a nationwide population-based matched cohort study. Infect Dis (Lond) 2023:1–8. doi: 10.1080/23744235.2023.2260864. [DOI] [PubMed] [Google Scholar]
- 24.Deeks S.G., Lewin S.R., Havlir D.V. The end of AIDS: HIV infection as a chronic disease. Lancet. 2013;382(9903):1525–1533. doi: 10.1016/S0140-6736(13)61809-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hentzien M., Drame M., Delpierre C., et al. HIV-related excess mortality and age-related comorbidities in patients with HIV aged >/=60: a relative survival analysis in the French Dat'AIDS cohort. BMJ Open. 2019;9(1) doi: 10.1136/bmjopen-2018-024841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Trickey A., Sabin C.A., Burkholder G., et al. Life expectancy after 2015 of adults with HIV on long-term antiretroviral therapy in Europe and North America: a collaborative analysis of cohort studies. Lancet HIV. 2023;10(5):e295–e307. doi: 10.1016/S2352-3018(23)00028-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gueler A., Moser A., Calmy A., et al. Life expectancy in HIV-positive persons in Switzerland: matched comparison with general population. AIDS. 2017;31(3):427–436. doi: 10.1097/QAD.0000000000001335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guaraldi G., Cossarizza A., Franceschi C., et al. Life expectancy in the immune recovery era: the evolving scenario of the HIV epidemic in northern Italy. J Acquir Immune Defic Syndr. 2014;65(2):175–181. doi: 10.1097/QAI.0000000000000018. [DOI] [PubMed] [Google Scholar]
- 29.de Coninck Z., Hussain-Alkhateeb L., Bratt G., et al. Non-AIDS mortality is higher among successfully treated people living with HIV compared with matched HIV-negative control persons: a 15-year follow-up cohort study in Sweden. AIDS Patient Care STDS. 2018;32(8):297–305. doi: 10.1089/apc.2018.0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vourli G., Noori T., Pharris A., et al. Human immunodeficiency virus continuum of care in 11 European union countries at the end of 2016 overall and by key population: have we made progress? Clin Infect Dis. 2020;71(11):2905–2916. doi: 10.1093/cid/ciaa696. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.