Abstract
Objectives
The cognitive outcome of CPR is poor. This study aims to evaluate if enhancing blood flow to the brain and oxygen dissociation from the hemoglobin improve cerebral O2 transport during CPR in cardiac arrest swine.
Methods
Standard swine-CPR model of induced VF and recovery was treated with an auto-transfusion tourniquet (A-TT®; HemaShock® (HS) Oneg HaKarmel Ltd. Israel) and ventilation with a novel mixture of 30% Oxygen, 5% CO2, and 65% Argon (COXAR™). Five swine received the study treatment and 5 controls standard therapy. Animals were anesthetized, ventilated, and instrumented for blood draws and pressure measurements. Five minutes of no-CPR arrest were followed by 10 min of mechanical CPR with and without COXAR-HS™ enhancement followed by defibrillation and 45 min post ROSC follow-up.
Results
All 5 COXAR-HS™ animals were resuscitated successfully as opposed to 3 of the control animals. Systolic (p < 0.05), and diastolic (p < 0.01) blood pressures, and coronary (p < 0.001) and cerebral (p < 0.05) perfusion pressures were higher in the COXAR-HS™ group after ROSC, as well as cerebral flow and O2 provided to the brain (p < 0.05). Blood pressure maintenance after ROSC required much higher doses of norepinephrine in the 3 resuscitated control animals vs. the 5 COXAR-HS™ animals (p < 0.05). jugular vein PO2 and SO2 exceeded 50 mmHg and 50%, respectively with COXAR-HS™.
Conclusions
In this pilot experimental study, COXAR-HS™ was associated with higher diastolic blood pressure and coronary perfusion pressure with lower need of vasopressors after ROSC without significant differences prior to ROSC. The higher PjvO2 and SjvO2 suggest enhanced O2 provision to the brain mitochondria, while limb compression by the HS counteracts the vasodilatory effect of the CO2. Further studies are needed to explore and validate the COXAR-HS™ effects on actual post-ROSC brain functionality.
Keywords: COXAR, HemaShock®, Cardiac Arrest, Cardio-pulmonary resuscitation, Cerebral oxygen delivery
Introduction
The physiological analysis of the cascade of brain survival outlined in Part A led us to develop a new modality aimed at addressing some of the suboptimal elements of the current AHA protocol of CPR. This modality consists of ventilation with a novel gas mixture of 30% O2, 5% CO2 and 65% Argon together with prompt application of the Auto-transfusion Tourniquets (A-TT®; HemaShock®) on the swine legs soon after the onset of VF. We use the phrase COXAR-HS™ for this combined modality. The rationale for using COXAR-HS™ to treat cardiac arrest is as follows:
-
a.
Ventilation with elevated FICO2 induces cerebral and global vasodilation AND shifts to the right the O2-Hgb dissociation curve with higher O2 delivery to the tissue per ml of blood flowing through the tissue (Bohr Effect).1, 2, 3
-
b.
Use of A-TT™ counteracts the dilation effect of CO2 on the peripheral blood vessels of the legs so that the vasodilation is focused to the circulation of the brain and the core organs. The use of A-TT™ also increases venous return to the heart, prevents “wasting” of the CPR cardiac output on perfusion of the legs, increases cerebral blood flow and increases peripheral resistance thereby elevating diastolic blood pressure and coronary perfusion pressure.4, 5, 6, 7
-
c.
Ventilation with FIO2 that is only mildly elevated (30%) to maintain SaO2 > 90% without elevating PaO2 to extreme and potentially toxic levels.8, 9, 10, 11
-
d.
Ventilation with 65% Argon counteracts the effects of the oxygen free radicals on the brain tissues starting upon the onset of care. In another study, 70% Argon was shown to improve neurological outcome in pigs.12 In that study, Argon was started only after ROSC was achieved, while during CPR 100% O2 was used.
-
e.
Minimize the use of vasoactive drugs – epinephrine − during CPR and norepinephrine after ROSC. Both drugs constrict blood vessels in the whole body, including the cerebral circulation.13, 14
In this context, the goal of the present study is to compare the potential beneficial effects of the bundled therapy combining limb constriction and ventilation with a gas mixture containing Argon, CO2 and O2 vs standard cardiopulmonary resuscitation in a swine model of cardiac arrest. We evaluated ROSC occurrence, hemodynamics, gas exchanges and cerebral oxygen delivery and consumption.
Methods
General
This investigation was approved by the Ecole Nationale Vétérinaire d’Alfort (France) ethical review board, in compliance with national and European regulations regarding animal use for research.
Animal preparation and surgical instrumentation
Domestic swine (35 kg) from a single source who underwent similar pre-study husbandry and health evaluation were anesthetized with zolazepam (10 mg/kg IM) and tiletamine (10 mg/kg IM; Zoletil©). Methadone (0.75 mg/kg IM; Confortan©) was also administered for analgesia. A catheter was then inserted in an ear vein in order to induce anesthesia with propofol (5 mg/kg IV; Propovet©). After endotracheal intubation, anesthesia was further maintained with propofol (10 mg/kg/h IV). Artificial mechanical ventilation was undertaken (tidal volume = 6 ml/kg; positive end-expiratory pressure = 5 cmH2O; respiratory rate = 20 ± 5 breaths/minute with aim to keep PaCO2 at 40 ± 4 mmHg; Monnal T60® respirator, Air Liquide, France).
A pressure gauge (Millar®, SPR-524, Houston, TX, USA) was inserted into the cerebral cortex through a small-hole craniotomy to monitor intracranial pressure (ICP). Carotid blood flow (CBF) was monitored by a 3 mm blood flow probe (PS-Series Probes®, Transonic, NY, USA), placed around the internal carotid artery. At the same time, several venous and arterial catheters were inserted using the Seldinger technique under ultrasound guidance as follows: a femoral artery catheter for blood pressure monitoring and arterial blood sampling, a jugular vein catheter for blood gas sampling, a 9Fr femoral vein sheath (Arrow Medical Limited, Kington, United Kingdom) for insertion of several catheters dedicated for drug and fluid administration, right atrial pressure assessment and intra-cardiac electric stimulation for cardiac arrest induction.
Experimental protocol
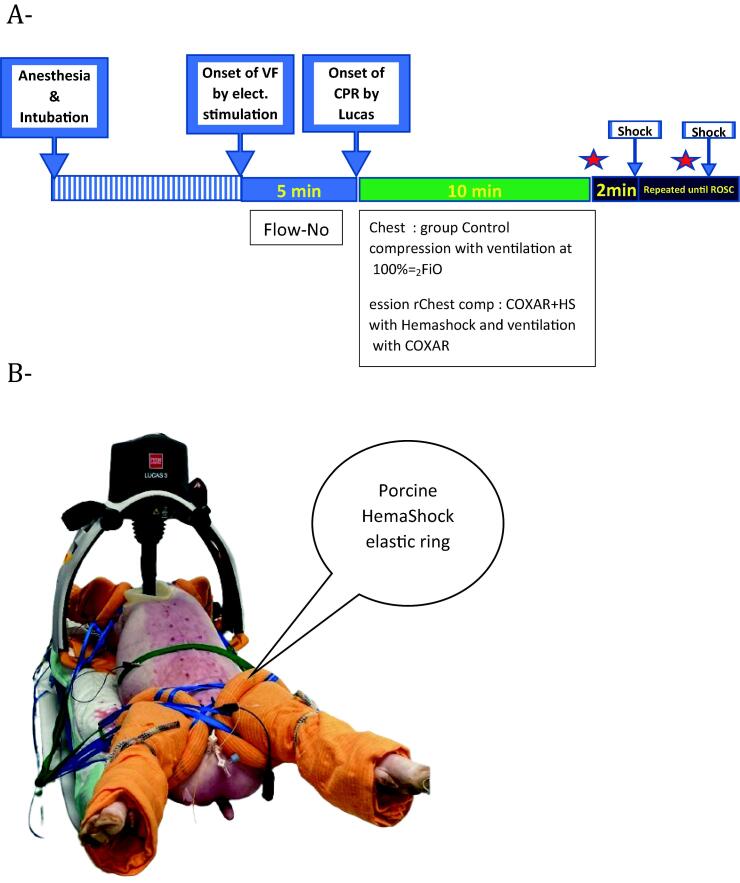
After verification of the depth of anesthesia, cardiac arrest (i.e. ventricular fibrillation) was induced by the administration of alternating current (A/C 10 V) for 1 s through the stimulation electrode. Cardiac arrest was left untreated for a period of 5 min (“No Flow” period). After this period, cardiopulmonary resuscitation (CPR) was started by an automated cardiac massage (Lucas 3.0, Stryker®) and resumption of artificial ventilation (tidal volume = 8 ml/kg; respiratory rate = 10; I/E ratio = 1/5; end-expiratory pressure = 0). Respiratory parameters were not modified throughout CPR and were the same in both groups. Animals were divided into two experimental groups starting at the onset of CPR, as illustrated in Fig. 1A. The animal allocation was performed blindly, using a block randomization. However, the study was not blinded due to the presence of the HemaShock® system on the legs in the corresponding group.
Fig. 1.
Panel A, Experimental timeline (star = epinephrine administration if diastolic blood pressure was inferior to 30 mmHg). Panel B, Porcine HemaShock applied to all 4 limbs of a swine. The device is rolled up the leg by pulling the straps which are then secured to the table. Note the white (bloodless) feet compared to the pink abdomen. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Control group, with mechanical ventilation with FIO2 = 100% from the onset of CPR.
COXAR™ + HemaShock® (“COXAR-HS™”) group, with mechanical ventilation with a gas mixture containing 30% of O2, 5% of CO2 and 65% of Argon. In this group, limb constriction was applied with the HemaShock® system (Fig. 1B). The HemaShock® was applied to the 4 legs during the no-flow period and limb constriction was completed before the onset of CPR. Exposure to COXAR™ was started at the onset of CPR by connecting a tube from the calibrated gas cylinder to the mechanical ventilator inlet. Two teams applied the HS, one on the forelegs and one on the hindlegs. The HS application and securing took 2–2.5 min.
After 10 min of CPR, electric shocks (200–270 J) were administered every 2 min in both groups until recovery of spontaneous cardiac activity (ROSC). ROSC was defined as the onset of sinus rhythm and systolic blood pressure sustainably above 60 mmHg without mechanical chest compression. Epinephrine was administered (10 µg/kg IV) every 4 min until ROSC. In the absence of ROSC after 30 min of CPR or in case of lung hemorrhage, resuscitation failure was considered, and cardiac massage was discontinued. In the presence of ROSC, resuscitated animals were monitored to assess hemodynamic stability during the post-ROSC period until 60 min after the end of cardiac arrest. After ROSC, mechanical ventilation parameters were set as follows: tidal volume = 6 ml/kg; positive end-expiratory pressure = 5 cmH2O; respiratory rate = 20 ± 5 breaths per minute. In the Control group, FIO2 was adjusted to target normoxia. In the COXAR™ + HemaShock® group, limb constriction and ventilation with COXAR™ gas continued until the end of the experiment. Norepinephrine was given IV to maintain mean arterial pressure above 75 mmHg after ROSC and the total amount of norepinephrine was recorded.
Investigational parameters
Hemodynamic parameters were recorded using a dedicated software for signal analysis (Notocord Systems, Croissy-sur-Seine, France). For each investigated parameter, average values were analyzed; in all animals at baseline and 5, 9 and 12 min after the onset of CPR. The “CPR 12 min” time point demonstrates the effect of epinephrine (first administration at CPR10’). Since CPR 15 min, parameters were only analyzed and represented for animals achieving ROSC.
Blood gases and blood lactate were analyzed using a dedicated analyzer (Cobas, Roche Diagnostics, Switzerland). Samples were withdrawn from arterial, right atrial and jugular vein at baseline and 5, 10, 15, 30 and 60 min after cardiac arrest. Again, those values were analyzed in all animals at baseline and 5 and 10 min of CPR, but only in animals achieving ROSC at 15, 30 and 60 min.
Statistical analysis
Data were expressed as mean ± SD. A two-way analysis of variance (ANOVA) was performed for the parameters measured at baseline and during CPR (Graphpad Prism®). Another ANOVA was performed for the parameters measured after ROSC in resuscitated animals. In both cases, a PLSD Fisher test was used when a significant time and group × time interaction was observed. All exact p values are shown in Supplemental material. Statistical significance was considered as p < 0.05 but p values below 0.01 or 0.001 are indicated when applicable. As this was designed as a preliminary and exploratory study, we only included 5 animals in each group (total n = 10). All p values are shown in Table 1.
Table 1.
P values of the group, time and group × time effects of the two-way analyses of variance (ANOVA) for the different parameters measured before or after ROSC. When a group or group × time interaction effect was observed, post-hoc p values were calculated using a Fisher's PLSD test. Values of p less than 0.05 are marked in red.
 |
Results
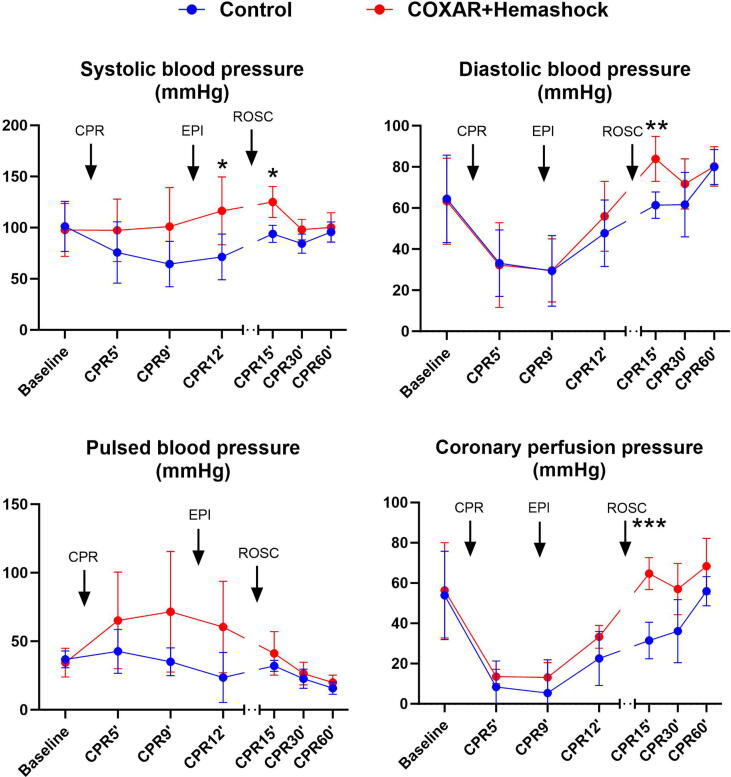
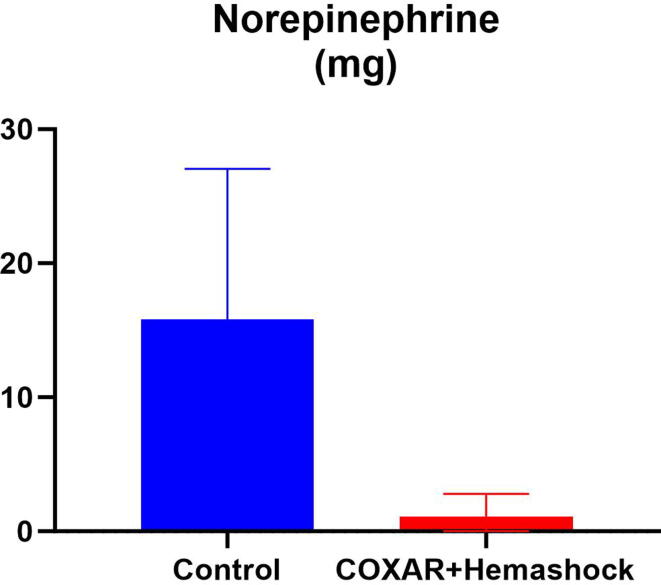
Five animals were included in each experimental group. Fig. 2 illustrates the hemodynamic parameters of the pigs at baseline, during CPR and after ROSC for the COXAR-HS™ group and for the Control group. Systolic, diastolic, pulse pressure and coronary perfusion pressure (CoPP) were not significantly different between the groups during CPR, but after ROSC the pressures were significantly higher in the COXAR-HS™ vs Control groups, respectively. After the defibrillation attempts started at CPR12′, sustained ROSC was obtained in 5/5 and 3/5 animals in COXAR-HS™ vs Control groups, respectively. The 5 COXAR-HS™ resuscitated swine required very small doses of norepinephrine (NE) to maintain their mean blood pressure above 70 mmHg after ROSC. The three converted control animals required much higher doses of NE (Fig. 3).
Fig. 2.
Hemodynamic parameters (Systolic, diastolic, pulsed blood, and coronary perfusion pressure). EPI, epinephrine, CPR, resumption, ROSC, resumption of spontaneous circulation; *p < 0.05; **p < 0.01; ***p < 0.001; N = 5 in both groups from Baseline to CPR12′; N = 3 and 5 in Control and COXAR + HemaShock group from CPR15′ to CPR60′ (only animals achieving ROSC). All values are represented as mean + SD.
Fig. 3.
Total dose of norepinephrine, expressed in mg, administered in animals achieving resumption of spontaneous circulation after resuscitation (n = 5 and 3 in Control and COXAR + HemaShock groups, respectively).
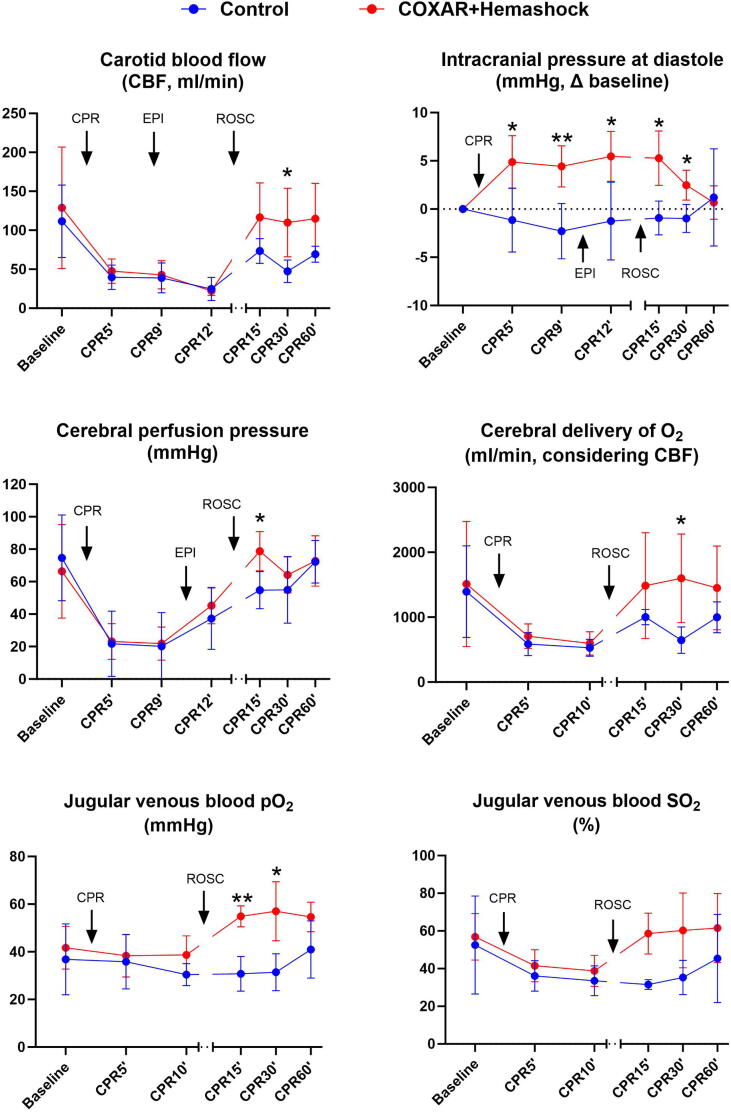
Fig. 4 illustrates the changes in the cerebral oxygen transport variables. Baseline parameters were similar for both groups. During CPR, intracranial pressure was significantly higher in the COXAR-HS™ group. This is rather expected by the known CO2-induced cranial vasodilation.2 Importantly, this difference disappeared after ROSC. During CPR, carotid blood flow and cerebral perfusion pressure were similar in the COXAR-HS™ group as in Control. However, post ROSC, carotid blood flow, cerebral perfusion pressure and O2 delivery were significantly higher in the COXAR-HS™ group, resulting in a much higher jugular vein PO2 and SO2.
Fig. 4.
Parameters demonstrating cerebral O2 transport. CPR, resumption, ROSC, resumption of spontaneous circulation; *p < 0.05; **p < 0.01; N = 5 in both groups from Baseline to CPR12′; N = 3 and 5 in Control and COXAR + HemaShock group from CPR15′ to CPR60′ (only animals achieving ROSC). All values are represented as mean + SD.
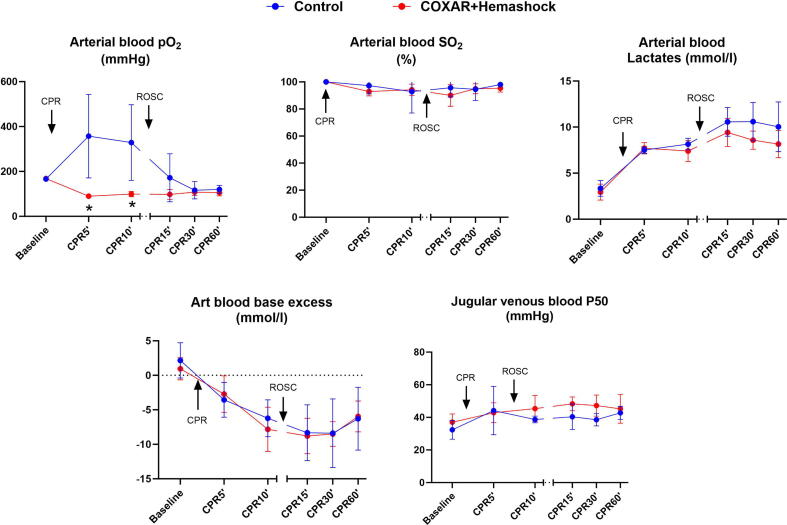
Fig. 5 illustrates the changes in the systemic oxygenation during the study. In Control conditions, PaO2 during CPR was high, as expected with FIO2 = 1.0. In the COXAR-HS™ group, PaO2 group was lower and around 100 mmHg. This PO2 provides SO2 of at least 90% which is sufficient for metabolic use without causing O2 toxicity.8 Arterial base excess was similar in both COXAR-HS™ and Control groups, but arterial lactate levels tended to be lower by about 1.5 mmol/l in the COXAR-HS™ group (NS) after ROSC. Jugular vein P50 values were higher after ROSC in the COXAR-HS™ group. Venous PO2/FIO2 values were higher in the COXAR-HS™ group.
Fig. 5.
Parameters demonstrating global gas exchange. CPR, resumption, ROSC, resumption of spontaneous circulation; *p < 0.05; N = 5 in both groups from Baseline to CPR12′; N = 3 and 5 in Control and COXAR + HemaShock group from CPR15′ to CPR60′ (only animals achieving ROSC). All values are represented as mean + SD.
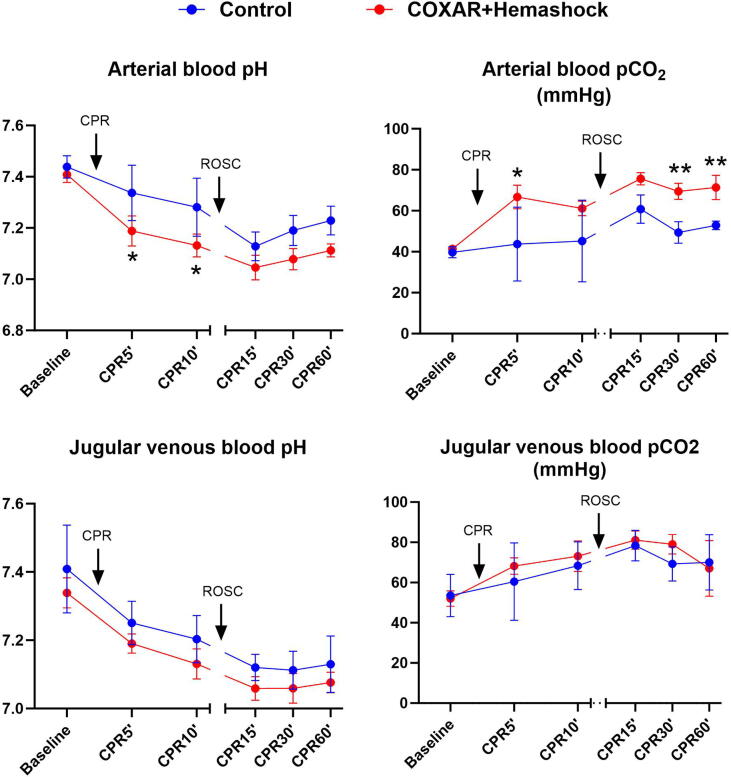
Fig. 6 illustrates the changes in blood gases during the study. Arterial pH was lower in the COXAR-HS™ group than in the Control group. The difference is explained by the respiratory acidosis caused by the higher FICO2 which clearly elevates PaCO2. Jugular venous pH is also lower in the COXAR-HS™ group, but the difference in the jugular PCO2 is very small.
Fig. 6.
Parameters demonstrating blood acid-base balance. CPR, resumption, ROSC, resumption of spontaneous circulation; *p < 0.05; **p < 0.01; N = 5 in both groups from Baseline to CPR12′; N = 3 and 5 in Control and COXAR + HemaShock group from CPR15′ to CPR60′ (only animals achieving ROSC). All values are represented as mean + SD.
Table 1 shows the p values of the group, time and group × time effects of the two-way analyses of variance (ANOVA) for the different parameters measured before or after ROSC. When a group or group × time interaction effect was observed, post-hoc p values were calculated using a Fisher's PLSD test.
Discussion
The combined data from this study clearly show beneficial effects of COXAR-HS™ during CPR and particularly after ROSC when compared to control. Oxygen transport to the brain was most significant, particularly after ROSC. The higher cerebral perfusion pressure and carotid blood flow resulted in higher delivery and utilization of O2 by the brain. The higher ROSC rate (100% vs. 60%) in the COXAR-HS™ group and the substantially lower need for norepinephrine after ROSC are global indicators of the better outcome in the COXAR-HS™ group. The fact that this was achieved with a much lower FIO2 of 0.3 vs. 1.0 is also important, given the known toxicity of hyperoxia. The PaO2 was around 100 mmHg and arterial O2 saturation was above 90% in the COXAR-HS™. In addition, we did not observe any negative effect of high Argon levels which is in agreement with observations in another study by Fumagalli et al.12
The fact that PjvO2 was much higher in the COXAR-HS™ group is most significant in our view. Following the physiological analysis of Part A, we can safely conclude that the PO2 at the end of the cerebral capillaries was also high, which means that the diffusion gradient from the capillary to the most distant and distal mitochondria is sufficient to deliver the O2 needed for the mitochondria to generate ATP at a normal rate. We think that the high PjvO2 results from the combined effects of the high cerebral perfusion pressure and blood flow together with the fact that each ml of blood transfers more oxygen to the tissue at normal or high PO2. This is a direct result of the shift to the right of the O2-Hgb dissociation curve as seen from the higher P50. The shift of the dissociation curve is caused by the lower pH and the higher PCO2. The similar BE in the two groups and the tendency for lower lactate levels in the COXAR-HS™ group indicate that the greater acidosis is essentially respiratory in nature, caused by the high FiCO2.
We identified two parameters that may have negative effects on the brain health in the COXAR-HS™ group. One is the higher intracranial pressure, and the other is the increased acidemia of the blood. However, the increased ICP by 5 mmHg was small relative to the substantially higher systolic and mean arterial pressures. As such, the higher ICP, which was expected from the known effects of hypercarbia on the brain blood vessels, did neither interfere with the carotid blood flow nor cerebral perfusion pressure. The effects of low pH at about 7.1 on the brain are not known. Respiratory acidosis (e.g. during exacerbation of COPD) or metabolic acidosis (e.g. in diabetes keto-acidosis) do alter mentation acutely but are reversible when the acidemia is corrected.
The other potentially important observation in this study is the higher coronary perfusion pressure with COXAR-HS™ (Fig. B3). This tendency was apparent but not significant during CPR (±10 mmHg after epinephrine administration at CPR 12 min) and more so after ROSC. One would expect a higher effect on CoPP in humans since the blood volume of the 4 limbs of a domestic pig is only about 16% of the total blood volume which is significantly less than the 24% legs blood volume of humans.
Study limitations
This study population of 5 + 5 animals is small since it was intended as a pilot exploratory evaluation of this new method. This limits the statistical analysis to illumination of only very substantial difference. Also, the fact that 2 of the control animals failed ROSC further reduced the group size and statistical analysis. Clearly more animals should be studied in the future. Another limitation is the duration of post-ROSC follow up. As such, we were not able to evaluate the physical and cognitive recovery of the pigs, nor the procedure of getting the animals off COXAR-HS™. This should also be addressed in subsequent study(ies). An inevitable limitation is the use of swine for this study. The pig’s 4 legs only contain approximately 16% of the body mass and blood volume, whereas human’s 2 legs contain 24%. A related issue is the time it takes to apply and secure HemaShock® on the swine – 2–2.5 min by two teams as opposed to 20 s or less on each leg of an adult person. As such, this study should only be considered as a pilot study, according to the low number of animals. In addition, it will be important to evaluate the effect of HemaShock® and COXAR™ on further neurological recovery after cardiac arrest.
Conclusions
In this pilot experimental study, COXAR-HS™ was associated with higher diastolic blood pressures and coronary perfusion pressures with lower need of vasopressors after ROSC without significant differences prior to ROSC. COXAR-HS™ also increased elevated O2 transport to the brain as indicated by the higher PjvO2. Further studies are needed to explore and validate the COXAR-HS™ effects on actual post-ROSC brain functionality.
Credit authorship contribution statement
Noam Gavriely: Writing – original draft, Supervision, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization. Jukka O Rasanen: Writing – review & editing, Methodology, Investigation, Formal analysis. Sharon Abadi Saar: Writing – review & editing, Project administration. Lionel Lamhaut: Writing – review & editing, Investigation. Alice Hutin: Writing – review & editing, Investigation. Fanny Lidouren: Writing – review & editing, Resources, Project administration, Methodology, Investigation. Yara Abi Zeid Daou: Writing – review & editing, Resources, Methodology, Investigation. Renaud Tissier: Writing – review & editing, Supervision, Resources, Project administration, Methodology, Investigation, Formal analysis, Data curation, Conceptualization.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: ‘NG is an executive and stakeholder in Oneg HaKarmel Ltd. and a retiree of the Technion, Israel Institute of Technology. No other sources of support. JOR is a professor emeritus of Mayo Clinic and a consultant to Oneg HaKarmel Ltd. SAS is an executive and stakeholder in Oneg HaKarmel Ltd. LL does not have anything to disclose. FL does not have anything to disclose. YAZD does not have anything to disclose. RT is a stakeholder of a company dedicated to liquid ventilation in critical care conditions (not related to the present work). He is also co-inventor of a patent in the field of hypothermia and liquid ventilation. This study was funded by Oneg HaKarmel Ltd. Tirat Carmel, Israel’.
Footnotes
Supplementary material to this article can be found online at https://doi.org/10.1016/j.resplu.2024.100681.
Appendix A. Supplementary material
The following are the Supplementary material to this article:
References
- 1.Crystal, George J. PhD. Carbon Dioxide and the Heart: Physiology and Clinical Implications. Anesthesia & Analgesia 121(3): p 610-623, September 2015. [DOI] [PubMed]
- 2.J.M. Pollock, A.R. Deibler, C.T. Whitlow, H. Tan, R.A. Kraft, J.H. Burdette and J.A. Maldjian Hypercapnia-Induced Cerebral Hyper perfusion: An Underrecognized Clinical Entity | American Journal of Neuroradiology American Journal of Neuroradiology February 2009, 30 (2) 378-385. [DOI] [PMC free article] [PubMed]
- 3.Carbogen Inhalation Therapy - Medical Clinical Policy Bulletins | Aetna [Accessed January 25, 2024].
- 4.Zhengfei Yang, David Tang, Xiaobo Wu, Xianwen Hu, Jiefeng Xu, Jie Qian, Min Yang, Wanchun Tang, A tourniquet assisted cardiopulmonary resuscitation augments myocardial perfusion in a porcine model of cardiac arrest, 2014, Resuscitation, (86), pgs. 49-53. [DOI] [PubMed]
- 5.Gavriely O, Nave T, Sivan S, Shabtai-Musih Y, Gavriely N. Auto Transfusion and Blood Pressure Elevation by Elastic Leg Compression in Normal Subjects. Rappaport, Technion; Haifa: Israel Institute of Technology; 2000. [Accessed January 25, 2024]. Available at: http://www.emergencyeed.com/uploads/1/9/1/4/19141635/study_-_auto_transfusion_mk0036200_compressed_2.pdf.
- 6.Martin Perez, David Tang, Noam Gavriely, Auto-Transfusion Tourniquet (A-TT) reanimation of cardiac arrest patients – retrospective charts review (Manuscript in review).
- 7.Acute Ischemic Stroke Trial in Seoul (carbogen, phenylephrine) | Clincosm [Accessed January 25, 2024].
- 8.Singer M., Young P.J., Laffey J.G., Asfar P., Taccone F.S., Skrifvars M.B., Meyhoff C.S., Radermacher P. Dangers of hyperoxia. Crit Care. 2021 Dec 19;25(1):440. doi: 10.1186/s13054-021-03815-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rhodes CE, Denault D, Varacallo M. Physiology, Oxygen Transport. [Updated 2022 Nov 14]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan. [PubMed]
- 10.Dell’Anna, A.M., Lamanna, I., Vincent, JL. et al. How much oxygen in adult cardiac arrest? 2014, Critical Care vol 18, article number 555, Pgs 2-7. [DOI] [PMC free article] [PubMed]
- 11.Ashkanian M., Gjedde A., Mouridsen K., Vafaee M., Hansen K.V., Østergaard L., Andersen G. Carbogen inhalation increases oxygen transport to hypoperfused brain tissue in patients with occlusive carotid artery disease. Increased oxygen transport to hypoperfused brain. Brain Research. 2009;1304:90–95. doi: 10.1016/j.brainres.2009.09.076. [DOI] [PubMed] [Google Scholar]
- 12.Francesca Fumagalli, PhD; Davide Olivari, MBiol; Antonio Boccardo et Al. Ventilation With Argon Improves Survival with Good Neurological Recovery After Prolonged Untreated Cardiac Arrest in Pigs. 2020, Journal of the American Heart Association, (9) 1-15. [DOI] [PMC free article] [PubMed]
- 13.Yannopoulos D., Matsuura T., Schultz J., Rudser K., Halperin H.R., Lurie K.G. Sodium nitroprusside enhanced cardiopulmonary resuscitation improves survival with good neurological function in a porcine model of prolonged cardiac arrest. Crit Care Med. 2011 Jun;39(6):1269–1274. doi: 10.1097/CCM.0b013e31820ed8a6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ripeckyj A., Kosmopoulos M., Shekar K., Carlson C., Kalra R., Rees J., Aufderheide T.P., Bartos J.A., Yannopoulos D. Sodium Nitroprusside-Enhanced Cardiopulmonary Resuscitation Improves Blood Flow by Pulmonary Vasodilation Leading to Higher Oxygen Requirements. JACC Basic Transl Sci. 2020 Feb 5;5(2):183–192. doi: 10.1016/j.jacbts.2019.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.