Highlights
-
•
This study describes the genetic characteristics, clustering patterns, and phylogeographic dynamics of the JC polyomavirus (JCV) from 1993 to 2023.
-
•
All the available complete genomes (n = 656), VP1 (n = 1547), and large t antigen (n = 724) isolated globally were investigated.
-
•
All the JCV strains are classified into four major phylogenetic clades, i.e., GI-GIV, where GI is further grouped into two types (GI.1 and GI.2), each with five sub-clades (GI.1/2 a-e), GII into three (GII a-c), GIII as a separate clade, and GIV into seven sub-clades (GIV a-g).
-
•
The phylogeographic network analysis indicated four major clusters corresponding to GI-GIV clades, each with multiple subclusters and mutational sub-branches corresponding to the subclades.
-
•
Genetic recombination analysis identified an inter-genotype recombinant within the NCCR region, while the amino acid variability analysis revealed high entropy across all proteins.
-
•
The study provides a comprehensive classification, distribution, and genetic variability patterns of JCV strains during 1993 to 2023.
Keywords: JC polyomavirus, Classification, Clustering, Recombination, Variation
Abstract
The human JC polyomavirus (JCV) is a widespread, neurotropic, opportunistic pathogen responsible for progressive multifocal leukoencephalopathy (PML) as well as other diseases in immunosuppressed individuals, including granule cell neuronopathy, JCV-associated nephropathy, encephalitis, and meningitis in rare cases. JCV classification is still unclear, where the ICTV (International Committee on Taxonomy of Viruses) has grouped all the strains into human polyomavirus 2, with no classification on clade and subclade levels. Therefore, JCV strains were previously classified using different genomic regions, e.g., full-length, VP1, and the V-T intergenic region etc., and the strains were grouped into several types related to various geographic locations and human ethnicities. However, neither of these classifications and nomenclature contemplates all the groups described so far. Herein, we evaluated all the available full-length coding genomes, VP1, and large T antigen nucleotide sequences of JCV reported during 1993–2023 and classified them into four major phylogenetic clades, i.e., GI-GIV, where GI is further grouped into two types GI.1 and GI.2 with five sub-clades each (GI.1/GI.2 a-e), GII into three (GII a-c), GIII as a separate clade, and GIV into seven sub-clades (GIV a-g). Similarly, the phylogeographic network analysis indicated four major clusters corresponding to GI-GIV clades, each with multiple subclusters and mutational sub-branches corresponding to the subclades. GI and GIV clusters are connected via GI.1-e reported from Europe and America, GII, GIII and GIV clusters are connected by GII-b and GII-c strains reported from Africa, while GIV cluster strains are connected to the Russia-Italy JCV haplotype. Furthermore, we identified JCV-variant-GS/B-Germany-1997 (GenBank ID: AF004350.1) as an inter-genotype recombinant having major and minor parents in the GI.1-e and GII-a clades, respectively. Additionally, the amino acid variability analysis revealed high entropy across all proteins. The large T antigen exhibited the highest variability, while the small t antigen showed the lowest variability. Our phylogenetic and phylogeographic analyses provide a new approach to genotyping and sub-genotyping and present a comprehensive classification system of JCV strains based on their genetic characteristics and geographic distribution, while the genetic recombination and amino acid variability can help identify pathogenicity and develop effective preventive and control measures against JCV infections.
1. Introduction
The JC polyomavirus (Human polyomavirus 2, JCPyV or JCV) was discovered in 1964 in a deceased patient's degenerated brain tissue who was suffering from progressive multifocal leukoencephalopathy (PML) (Zu Rhein and Chou, 1965). A couple of years later, the virus was also detected in primary cultures of human fetal glial cells (PHFG) via inoculating a similar brain lesion extract acquired from a Hodgkin's disease patient (Padgett BL et al., 1971). Recent studies suggest that JCV is widespread in different parts of the world, causing primarily PML as well as other diseases in immunosuppressed individuals, like individuals having treatment with immunosuppressive drugs or HIV AIDS patients (Levican et al., 2019; Ferenczy et al., 2012). JCV may infect individuals in a very early stage of life, possibly before six months old, while a significant number of infants become seropositive within two years of age (Elia et al., 2017). With age, the seroprevalence increases, where 50 to 80 percent of prevalence among healthy adults has been reported globally during multiple seroepidemiological studies (Elia et al., 2017; Padgett and Walker, 1973; Taguchi et al., 1982; DeCaprio and Garcea, 2013; Šroller et al., 2014; Gossai et al., 2016).
The virus persists and replicates asymptomatically in the infected individual's urinary tract and other organs (Grinnell et al., 1983). The virus can be rarely detected outside the immunocompetent individual's urinary tract (Koralnik et al., 1999), however, during treatment with certain immunomodulating drugs or immunosuppression, the virus may develop into lytic infection in oligodendrocytes, resulting in PML (Berger and Concha, 1995; Koralnik, 2004). Oligodendrocytes infection causes extensive demyelination leading to neuronal dysfunction and death. The symptoms of PML include motor dysfunction, multiple sclerosis, visual defects, stroke, and speech impairment (Ferenczy et al., 2012; Astrom et al., 1958; Richardson, 1961), while some patients may develop seizures (Miskin et al., 2016). Besides PML, JCV can also cause granule cell neuronopathy (GCN), JCV-associated nephropathy, encephalitis, and meningitis in rare cases (Tan and Koralnik, 2010). GCN, like PML, is also a brain infection but it attacks the cerebellum's granule cells instead of astrocytes and oligodendrocytes in the case of PML (Du Pasquier et al., 2003). Since both the PML and GCN infections occur within the CNS, they likely have comorbidity (Wüthrich et al., 2009). The meningitis and encephalitis by JCV also occur rarely and are related to individuals with immunocompromised conditions, while the JCV-associated nephropathy also leads to mild symptoms of nephropathy as compared to nephropathy with other viruses (Blake et al., 1992; Viallard et al., 2005; Kantarci et al., 2011).
JCV persists in at least two forms within an infected individual, e.g., the non-pathogenic form and the latent form (Yogo et al., 1990; Bellizzi et al., 2012; Snopková et al., 2019). The NCCR (non-coding control region; see below) is found in a rearranged form during the neurotropic phase and is typically present in a PML patient's brain, CSF (cerebrospinal fluid), or blood. In the non-pathogenic form on the other hand, the NCCR is not rearranged and is present frequently in patient's urine (Yogo et al., 1990). Rearrangements of the NCCR result in duplications and deletions of specific sequence elements (Yogo and Sugimoto, 2001) that are believed to have a role in virus pathogenesis by altering its cellular tropism. Moreover, point mutations observed in VP1 capsid protein have also been reported to be involved in PML (Reid et al., 2011; Sunyaev et al., 2009; H.-Y. Zheng et al., 2005; H.-Y. Zheng et al., 2005). However, there is no consensus on whether specific genotypes of JCV are preferentially associated with PML and it is unclear from the existing literature that whether the pathogenic form arises from the latent virus alterations or is acquired as a new infection (Agostini et al., 2000; Agostini et al., 1996; Ciappi et al., 1999).
JCV belongs to the betapolyomavirus in the family Polyomaviridae, characterized by a small (∼45 nm), icosahedral, non-enveloped virus having a supercoiled, closed circular dsDNA (double-stranded DNA) genome of approximately 5 kbp in length (Frisque et al., 1984; Pinto and Dobson, 2014; Torres, 2020). The genome is organized into two protein coding regions, e.g., early and late, which are transcribed in opposite directions starting from a common NCCR (noncoding control region) portion. The early region encodes the small t (tumor) antigen (STA), large T antigen (LTA) and several splice variants of T antigen, while the late region encodes the agnoprotein and VP1-VP3 proteins (DeCaprio and Garcea, 2013; Frisque et al., 1984; White et al., 2009). The NCCR contains the origin of replication (ORI) of the virus and the promoter for the early and late genes. The NCCR in the archetype consists of six blocks, e.g., a-f, where the block f and the ORI are conserved among different species while the middle blocks are variable (Ault and Stoner, 1993; Assetta and Atwood, 2017). Duplications and/or rearrangements in these blocks are believed to generate more binding sites that confer advantages to the JCV (Frisque et al., 1984). For example, the NCCR in archetype has fewer binding sites for Spi-B, Oct-6/SCIP, and DDX-1 transcription factor proteins, which leads to lower efficacy of the viral gene transcription compared to the NCCR in the rearranged form (Assetta and Atwood, 2017; Sunden et al., 2007; Vaz et al., 2000). Spi-B is reported to enhance the viral gene transcription in primary astrocytes and B-cells, while DDX-1 can act as a coactivator to enhance NF-κB-mediated early and late transcription (Assetta and Atwood, 2017; Marshall et al., 2012). Furthermore, c block repeats increase the NFI binding sites, which are essential while activating viral gene transcription in lymphoid and brain tissues (Assetta and Atwood, 2017; Monaco et al., 2001). Like other viruses, the proteins encoded by the early region have regulatory functions or participate in the replication of the virus, while the proteins encoded by the late region are structural proteins. In addition, the genome also contains other ORFs (open reading frames), but their functions and expression, in many cases, remain unknown (Moens et al., 2017; White et al., 2013).
Previously, JCV strains were classified using different nomenclatures and methods, grouping the strains into several types related to different geographic locations and human ethnicities (Stoner et al., 2000; Sugimoto et al., 1997; Yogo et al., 2004). In addition, the phylogenetic relationships were mapped using different genomic regions, including the full-length genomes, VP1, and the V-T intergenic region, e.g., the 610 bp region corresponding to the 5′ end of T genes and 3′ end of VP1 (Torres, 2020). Herein, we re-analyzed all the available full-length genomes, in addition to VP1 and LTA nucleotide sequences of JCV, available on NCBI GenBank and isolated globally during 1993–2023 to map the latest genetic clustering, phylogeographic dynamics, and genetic characteristics of JCV isolates. We have also revised the genotyping and sub-genotyping of JCV with a new approach that will provide a robust and simple classification system, placing all the existing and new strains in their respective groups.
2. Materials and methods
2.1. Dataset
In this study, we retrieved the available complete (coding regions, without NCCR) genomes (n = 656), VP1 (n = 1547) and large T antigen (n = 724) nucleotide sequences of JCV from NCBI GenBank database isolated during 1993–2023, isolated from different sources of human body (Supplementary Table 1). The strains were identified and formatted using the GenBank accession number, virus name, isolate/strain, region/country, and year of report in the NCBI GenBank database. Based on full-length coding genomes, they were isolated from 49 different countries/regions of the world (Table 1).
Table 1.
Geographic distribution of JCV full-length genomes-based genotypes, 1993–2023.
| Genotype | Asia | Africa | America | Europe | Oceania | Eurasia | Total | |
|---|---|---|---|---|---|---|---|---|
| GI.1 | GI.1-a | China (15), Malaysia (3), Vietnam (3), Myanmar (22), Philippines (12), Indonesia (2), Thailand (3), Mongolia (1) | Zambia (1), South Africa (1), Mauritius (2), | USA (4) | – | Kiribati (5) | – | 74 |
| GI.1-b | China (5), Philippine (2), Thailand (1) | – | – | – | – | – | 8 | |
| GI.1-c | Japan (29), China (20), Mongolia (2), South Korea (7) | – | USA (6) | – | – | – | 64 | |
| GI.1-d | Philippines (7), China (9), Malaysia (1), India (1) | Mauritius (2) | – | – | – | – | 20 | |
| GI.1-e | – | – | USA (17) | Belgium (3), Germany (2), Greece (1), Netherlands (1), Finland (1), Macedonia (1) | – | – | 26 | |
| GI.2 | GI.2-a | Japan (25), South Korea (7), | – | USA (9), Canada (5), Guatemala (5), Mexico (6), Peru (5) | – | – | – | 62 |
| GI.2-b | Japan (3) | – | – | – | – | – | 3 | |
| GI.2-c | China (4), Mongolia (4), Japan (2), Myanmar (4), Uzbekistan (2), Nepal (1), Sri Lanka (5) | South Africa (1) | USA (2) | – | – | Russia (4), Turkey (1) | 30 | |
| GI.2-d | Saudi Arabia (2), | – | USA (1) | Greece (1) | – | – | 4 | |
| GI.2-e | Philippines (5), Indonesia (2) | – | – | – | Kiribati (11) | – | 18 | |
| GII | GII-a | Iran (6), Uzbekistan (4), India (5), Saudi Arabia (4) | Sudan (2), Ethiopia (27), Kenya (5), Zambia (2), Tanzania (3), South Africa (2), | – | Greece (1), Macedonia (1) | Australia (1) | Turkey (2) | 65 |
| GII-b | – | Niger (4), Morocco (3), Central African Republic (2), Mauritania (2), Ethiopia (4) | USA (3) | – | – | – | 18 | |
| GII-c | – | South Africa (9) | USA (1) | – | – | – | 10 | |
| GIII | – | Ghana (4) | – | – | – | – | 4 | |
| GIV | GIV-a | Japan (1) | – | USA (51) | Finland (1), Germany (1), Italy (1) | – | Russia (2) | 57 |
| GIV-b | – | – | USA (37) | Finland (4) | – | – | 41 | |
| GIV-c | – | – | USA (38) | – | – | – | 38 | |
| GIV-d | Japan (6), South Korea (1) | South Africa (1) | USA (1), Canada (4) | Netherlands (1), Finland (10), Sweden (1) | – | Russia (3) | 28 | |
| GIV-e | USA (13) | Finland (19), Italy (3), Germany (2), Netherlands (1), Norway (4), UK (1), Switzerland (1), Belgium (1), Spain (1) | Russia (3) | 49 | ||||
| GIV-f | – | Morocco (1) | USA (3) | Belgium (8), Finland (9), Spain (1), UK (1), Netherlands (1), Greece (1) | – | Russia (2) | 27 | |
| GIV-g | Japan (4) | – | – | – | – | Russia (4) | 8 | |
| Total | 242 | 79 | 211 | 84 | 17 | 21 | 654 | |
2.2. Phylogenetic tree construction and genetic similarity analysis
All the retrieved sequences were aligned using the ClustalW multiple alignment and edited with BioEdit v7.2.5. Following the alignment, the maximum likelihood (ML) phylogenetic trees were constructed with the help of IQ-TREE multicore version 1.6.12, using the best-fitting substitution model TIM3+F + I + G4 for full-length genomes, TIM3+F + I + G4 for VP1 and TN+F + I + G4 for LTA. The trees were constructed with 1000 bootstrap replicates, while the tree branches were tested by SH-like aLRT (Shimodaira-Hasegawa-like approximate likelihood ratio tests) with 1000 replicates (Nguyen et al., 2015; Hoang et al., 2018). The trees were visualized and modified with the help of FigTree v1.4. Moreover, the genetic similarity of the full-length JCV genomes was mapped using SimPlot v3.5.1.
2.3. Phylogeographic clustering analysis
Using phylogeographic network analysis on population-specific genetic data is a more effective method of mapping the genetic connections between intra-specific genetic sequences and illustrating individual relationships (Leigh and Bryant, 2015). Hence, we implemented the Minimum Spanning Network (MSN) offered by the PopArt v1.7 (Leigh and Bryant, 2015) to analyze all the complete genome sequences of JCV. In addition, the nucleotide diversity, number of segregating sites, and Tajima's D statistic (D) data were also recorded using the PopArt v1.7 (Leigh and Bryant, 2015).
2.4. Genetic recombination analysis of JCV
All the complete genome sequences of JCV were analyzed to detect the potential recombination events using the RDP4 (Recombination Detection Program 4) v4.101 (Martin et al., 2015). The recombination events were identified using each of the seven algorithms within the RDP4 software package, e.g., Chimaera, RDP, MaxChi, BootScan, 3seq, SiScan, and GENECONV. A recombination event confirmed by at least five of these approaches was considered as a real recombination event.
2.5. Shannon entropy analysis for amino acids variability landscape
Entropy is a statistical measure of site variability that gives low scores to less variable sites and a high score to more variable sites. Thus, we separately retrieved the amino acids sequences of the ORFs of JCV encoding the agnoprotein, VP1, VP2, VP3, large T antigen (LTA) and small t antigen (STA) from the NCBI GenBank database and were separately aligned with ClustalW multiple alignments and edited using the BioEdit v7.2.5. Following the alignment, the amino acids sequences were submitted to LANL (Los Alamos National Laboratory) website (https://www.hiv.lanl.gov/content/sequence/ENTROPY/entropy_one.html) in order to determine the Shannon entropy for each amino acid position in the consensus sequence of each protein of JCV.
3. Results
3.1. Genotyping of JCV based on full-length genomes or coding sequences of VP1 and LTA
We first evaluated the full-length coding sequences of JCV (a total of 656) available in the NCBI GenBank database to date (isolated during 1993–2023) to analyze their latest phylogenetic characteristics and genetic clustering. We inferred the full-length genomes-based ML phylogenetic tree with best-fit model TIM3+F + I + G4 and 1000 bootstraps, while the tree branches were tested by SH-like aLRT (Shimodaira-Hasegawa-like approximate likelihood ratio tests) with 1000 replicates (Nguyen et al., 2015; Hoang et al., 2018). The final dataset of ML (maximum likelihood) method consisted of 4756 positions, 1121 distinct patterns, 411 singleton sites, and 3723 constant sites.
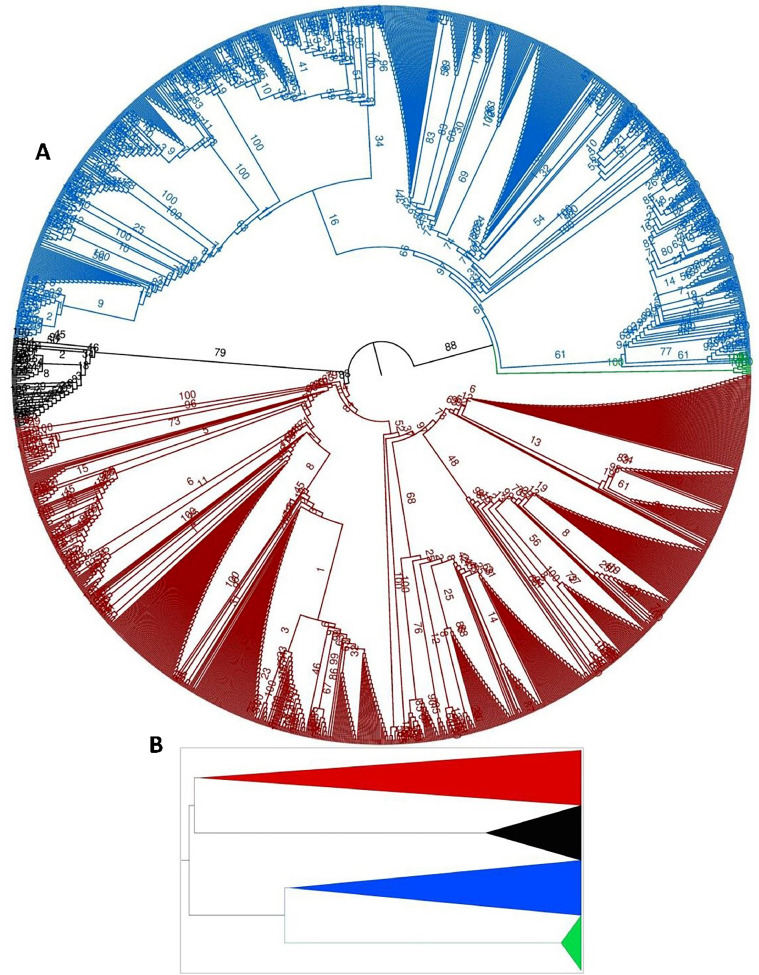
Our phylogenetic tree indicated that all the JCV strains are classified into four major groups, e.g., GI-GIV (Fig. 1A and B), where the GI is further grouped into two subtypes, e.g., GI.1 and GI.2, each with five sub-clades (GI.1/GI.2 a-e). Similarly, GII is grouped into three (GII a, b, and c) and GIV into seven sub-clades (GIV a-g) (Fig. 1C, Supplementary Fig. 1). Interestingly, four of the JCV strains isolated in Ghana during 2000–2001, e.g., strains GH-1, GH-2, GH-3 and GH-4 (GenBank IDs: AB038252.1, AB038253.1, AB048545.1 and AB048546.1 respectively) clustered as a separate clade from all the remaining JCV strains (Fig. 1A, B and C, Supplementary Fig. 1). All the sub-groups were highly diverse, clustering strains from different regions of the world, except the GI.2-b that was limited to strains from Japan, and GIV-c limited to strains from USA only. Similarly, the GI.1-e clustered strains from the USA and Europe, GII-c from South Africa and the USA, GIV-b from Finland and the USA, GIV-g from Japan and Russia, and GI.2-d from Saudi Arabia, the USA, and Greece (Table 1). The highest number of strains were clustered in GI.1-a (n = 74) and GII-a (n = 65), majorly from Asia and Africa, followed by GI.1-c (n = 64) and GI.2-a (n = 62) clustering strains from Asia and America. GII-a and GIV-d were the most diver sub-clades, clustering strains from all the six major regions of the world, except America for the first and Oceania for the latter. Most of the strains reported from Europe clustered in GI.1-e, GIV-d, GIV-e, and GIV-f, while those reported from America in GI.2-a. Similarly, the remaining sub-clades clustered strains reported in different major regions of the world, e.g., Asia, Africa, America, Europe, Oceania, and Eurasia (Table 1, Fig. 1, Supplementary Fig. 1). These results suggest that the JCV strains should be grouped and classified based on phylogenetic clustering, rather than based on geographic regions and distribution.
Fig. 1.
The phylogenetic tree based on the full-length coding genomic sequences of JCV strains isolated during 1993–2023. (A) The phylogenetic tree representing each group (GI-GIV) collapsed, and (B) the radial representation of the tree with four groups (GI-GIV) and two distinct types within the first clade (GI.1 and GI.2). (C) The ML phylogenetic tree of 656 complete coding genome sequences of JCV, classifying all the strains into four major groups (GI-GIV). GI is further classified into two types GI.1 and GI.2, each with five sub-clades (GI a-e), GII into three (GII a-c), GIII as a separate clade, and GIV into seven (GIV a-g) sub-clades. The trees are rooted on the midpoint. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1000 replicates) are indicated at each node. The tree branches were tested by Shimodaira-Hasegawa-like approximate likelihood ratio tests (SH-like aLRT) with 1000 replicates. The evolutionary distances were computed using the best-fit substitution model TIM3+F + I + G4. The tree was visualized and modified using FigTree v1.4. Each clade and sub-clade is indicated in different colors.
In addition, we analyzed the genetic similarities of twenty-two JCV strains as representative from each clade and sub-clade, taking JCV-1993 (GenBank ID: NC_001699.1) as query strain (Fig. 2) to further evaluate the genetic diversity of JCVs. The genetic similarity map indicated high diversity (<95 %) in the VP1 coding region of JCV genomes, followed by the large T-antigen regions and VP2-VP3. The highest variability was shown by JCV-AT-8-Japan-2001, JCV-GH-4-Ghana-2001 and JCV-UC167V3-C026-South_Africa-2022 (GenBank ID: AB048577.1, AB048546.1, and OP549863.1 respectively). The highest conservation was shown by the agnoprotein, followed by the small T-antigen, respectively (Fig. 2).
Fig. 2.
Genetic similarity map of the complete coding genome sequences of representative JCV strains. (A) Schematic diagram of JCV complete genome structure. From 5′end to 3′ end are the ORFs encoding the agnoprotein, VP2, VP3, VP1, Large T-antigen, and the Small T-antigen. (B) SimPlot similarity analysis results, using the JCV-1993 (GenBank ID: NC_001699.1) as the query sequence to compare with twenty-two other representative strains of JCV from each clade and sub-clade.
Since the JCVs are also classified using the VP1 and other partial genomic sequences, we constructed additional phylogenetic trees based on VP1 and large T-antigen nucleotide sequences. Firstly, we inferred the phylogenetic tree based on all the available VP1 nucleotide sequences (n = 1547) at the NCBI GenBank database (Fig. 3, Supplementary Fig. 2). In consistency with our complete genome sequences-based phylogenetic analysis, the VP1-based phylogenetic tree also classified all the JCV strains into four potential major groups, e.g., GI-GIV. All the strains isolated in Ghana that appeared as a distinct clade within our full-length coding genomes-based tree (GIII, Fig. 1, Supplementary Fig. 1) clustered as a separate clade within the VP1-based tree (indicated in green color), while the strains clustered in GI-c in the full-length genomes-based tree also appeared as a separate clade in VP1-based phylogenetic tree also (indicated in black color) (Fig. 3, Supplementary Fig. 2). These results validate our full-length genomes-based phylogeny that the JCV strains are classified into four major clades, however, the complete genomes-based phylogenetic classification provides a more robust grouping than the VP1-based phylogeny, as the VP1 coding region indicated high variability, as well as the sub-clade level grouping within the VP1-based phylogenetic classification, is not well allocated.
Fig. 3.
The phylogenetic tree based on VP1 nucleotide sequences of JCV strains. (A) The ML phylogenetic tree of 1547 VP1 nucleotide sequences of JCV, classifying all the strains into four major groups, e.g., GI (red), GII (black), GIII (blue) and GIV (green). (B) The phylogenetic tree representing each group collapsed. The tree is rooted on the midpoint; major clades and sub-clades of JCV are indicated. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1000 replicates) are indicated at each node. The tree branches were tested by Shimodaira-Hasegawa-like approximate likelihood ratio tests (SH-like aLRT) with 1000 replicates. The evolutionary distances were computed using the best-fit substitution model TIM3+F + I + G4. The tree was visualized and modified using FigTree v1.4.
For further validation, we inferred an additional tree based on the available (n = 724) nucleotide sequences of the large T-antigen (LTA) of the JCV (Fig. 4, Supplementary Fig. 3). The LTA-based grouping of JCV strains showed high diversity, where the strains can be classified into four groups like full-length and VP1-based trees, e.g., GI (indicated in red), GII (blue), GIII (black) and GIV (green). However, the JCV-strain-317A-Ethiopia-2004 (GenBank ID: AY356539.1) clustered separately from all the other strains. Interestingly, this strain clustered together with the GII-a strains within the full-length (Fig. 1, Supplementary Fig. 1) while within the GII clade in the VP1-based phylogenetic tree (Fig. 3, Supplementary Fig. 2). In conclusion, the VP1 phylogenetic tree is closely related to the full-length coding sequences-based phylogenetic tree, while the LTA-based phylogeny is dissimilar to both phylogenetic classifications. Thus, it may be concluded from these results that the full-length genomes-based phylogenetic classification provides a more reliable method for grouping all the available and future strains.
Fig. 4.
The phylogenetic tree based on large T-antigen nucleotide sequences of JCV strains. (A) The ML phylogenetic tree of 724 LTA nucleotide sequences of JCV, classifying all the strains into four potential major groups, e.g., GI (red), GII (blue), GIII (black) and GIV (green), while the JCV-strain-317A-Ethiopia-2004 (GenBank ID: AY356539.1) appeared separately from all the other JCV strains. (B) The phylogenetic tree representing each group collapsed. The tree is rooted on the midpoint, major clades, and sub-clades of JCV are indicated. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1000 replicates) are indicated at each node. The tree branches were tested by Shimodaira-Hasegawa-like approximate likelihood ratio tests (SH-like aLRT) with 1000 replicates. The evolutionary distances were computed using the best-fit substitution model TN+F + I + G4. The tree was visualized and modified using FigTree v1.4.
3.2. The phylogeographic dynamics of JCV based on full-length genomes
To further investigate the regional level spread and phylogeographic dynamics of JCV strains, we inferred the MSN phylogeographic network of all the full-length genomes. In consistence with our full-length phylogenetic tree (Fig. 1), the network analysis indicated great diversity among the JCV strains, showing four major clusters (corresponding to GI-GIV) with multiple subclusters and mutational sub-branches (corresponding to the sub-clades) (Fig. 5). In consistence to our phylogenetic analysis, the phylogeographic network indicated clustering of strains based on genetic relationships, rather than geographic locations, where each cluster grouped strains from diverse populations and regions. Like the complete coding sequences based phylogenetic tree, the phylogeographic network indicated the GI as the largest cluster, with sub-clusters corresponding to GI.1 and GI.2 (Fig. 5). However, their sub-clades diverged with each other, especially the GI.1c and GI.2-e, suggesting a common ancestor for both types. In addition, the GII and GIV strains appeared as distinct major clusters, each with their corresponding subclade. The GIII strains in the phylogenetic tree also clustered as a distinct branch that is connected to the GII-b strain JCV-318C-Ethiopia (GenBank ID: AY364314.1) following 56 mutational steps, while 4, 17, 17 and 21 mutational steps within (Fig. 5).
Fig. 5.
The Phylogeographic network of the full-length coding genomes of JCV, 1993–2023. The phylogeographic network of 654 full-length genomes of JCV was constructed using the MSN network offered by PopArt v1.7. The strains are grouped in four major clusters, e.g., GI-GIV, with multiple sub-mutational branches. The GI and GIV clusters are connected via GI.1-e reported from Europe and America, GII, GIII, and GIV clusters are connected by GII-b and GII-c strains reported from Africa, while the GIV strains are connected to Russia-Italy haplotype of JCV, e.g., JCV-isolate-1017-Russia-2022 and isolate-IT-8-Italy-2002 (GenBank IDs: MT448664.1 and AB074584.1 respectively). The distance of branches is proportional to the number of mutations. Numbers represent the mutational steps. Each country is represented with a different color.
The GIV strains are connected to the Russia-Italy haplotype of JCV, e.g., JCV-isolate-1017-Russia-2022 and isolate-IT-8-Italy-2002 (GenBank IDs: MT448664.1 and AB074584.1 respectively). Similarly, the JCV-strain-317A-Ethiopia-2004 (GenBank ID: AY356539.1), which was appeared separately from all the JCV strains based on LTA phylogeny (Fig. 4), clustered within the GII cluster in the phylogenetic network like the full-length genomes based phylogenetic tree. The JCV-variant-GS/B-Germany-1997 (GenBank ID: AF004350.1) that appeared as a distanced strain within the GI.1-e in the full-length genomes-base tree (Fig. 1, Supplementary Fig. 1) also clustered within the GI.1-e in the phylogeographic network and was connected to JCV-variant-GS/K-Germany-1997 (GenBank ID: AF004349.1) and JCV-isolate-B129jc-Belgium-2023 (GenBank ID: OQ230881.1) following one and two mutational steps respectively. In addition, the GII, GIII, and GIV clusters are connected by GII-b and GII-c strains that are reported from Africa, while the GI and GIV clusters are connected via GI.1-e, which are reported from Europe and America. GI clade clusters strains majorly from Asia, except GI.2-a, which is limited to America and Japan; GII and GIII majorly cluster the JCVs reported from Africa, while GIV clusters JCVs from Europe and America (Fig. 5).
The population genetics statistics calculated using the PopArt v1.7 indicated an overall nucleotide diversity (pi) of pi=0.0540843, with a total of 671 segregating sites, indicating the presence of genetic variation within the JCV population. Similarly, Tajima's D statistic was calculated to be D = 1.78276. This positive value suggests a departure from neutral expectations and indicates the influence of non-neutral evolutionary forces. The Tajima's D p-value [p (D >= 1.78276)] was calculated to be statistically significant, e.g., p = 0.0486, further supporting the departure from neutral expectations in the JCV population. In conclusion, these results suggest the possibility of genetic exchange and potential genetic drift among the JCVs.
3.3. Genetic recombination patterns of JCV full-length genomes
Since phylogenetic and phylogeographic analyses indicated high diversity, potential genetic exchange, and drift among the JCVs, we assessed the full-length genomes (including the NCCR region) to determine the genetic recombination patterns of JCVs. We performed the recombination analysis of all the full-length genomes using the seven algorithms embedded in the RDP4 software package to identify the recombination patterns and genomic breakpoints. The genetic recombination analysis did not identify any recombination in the coding regions of JCV genomes; however, an intergenotype recombination event was identified among the GI and GII clades. Interestingly, this recombinant strain was the JCV-variant-GS/B-Germany-1997 (GenBank ID: AF004350.1) that appeared as a distanced strain within the GI.1-e in the full-length coding sequences-base tree (Fig. 1, Supplementary Fig. 1) and clustered within the GI.1-e in the phylogeographic network (Fig. 5). The major parent was the GS/K-Germany-1997 (GenBank ID: AF004349.1) within the GI.1-e sub-clade, while the minor parent was the JCV strain-315A-Ethiopia-2204 (GenBank ID: AY342299.1) from the GII-a sub-clade. The genomic breakpoints of the recombinant were nucleotide no 4905 to 5208, corresponding to the non-coding control region (NCCR) of the recombinant strain (Fig. 6). The results indicate the potential genetic exchange among the JCV strains within the NCCR regions, which is in consistence with the fact that the NCCR is the most diverse region of JCV.
Fig. 6.
Genetic recombination analysis of 656 full-length genomes of JCV, 1993–2023. (A) Diagram of the full-length genome of JCV, recombinant strain, their major and minor parents, and the genomic breakpoints. (B) A detailed illustration of the recombination event detected by the RDP4 software. The recombination event was detected (+) by six of the seven algorithms, e.g., Chimaera, MaxChi, BootScan, 3seq, SiScan, and GENECONV, while not detected (-) by only one algorithm, e.g., RDP within the RDP4 software package.
3.4. Amino acids variability landscape of the JCV proteins
The amino acids variability across the JCV proteins, e.g., agnoprotein, VP1, VP2, VP3, LTA (Large T antigen) and STA (small t antigen) were analyzed using the Shannon entropy offered by the LANL database (https://www.hiv.lanl.gov/content/sequence/ENTROPY/entropy_one.html). The consensus sequence of the agnoprotein consisted of 73 amino acids, VP1 of 354, VP2 of 344, VP3 of 225, large T antigen (LTA) of 689 and small t antigen (STA) of 172 amino acids. The Shannon entropy indicated significant variability across all the proteins, where multiple regions within each protein exhibited high entropy scores (Fig. 7). The highest variability was shown by the LTA protein (Fig. 7E), especially aa position 625 to 686, followed by the agnoprotein aa position 43 to 71 (Fig. 7A), VP2 aa position 249 to 286 (Fig. 7C), VP3 aa position 124 to 167 (Fig. 7D), and the VP1 aa position 113–117, 158, 321 and 332 (Fig. 7B). Among them, the agnoprotein exhibited entropy nearly across the full-length sequence, while the STA on the other hand exhibited the lowest variability (Fig. 7F), making it the most conserved protein of the JCV. These results indicate significant amino acid variability across all the proteins, which further validates the genetic diversity and speculates the possibility of genetic drift and exchange among the JCVs as indicated by our phylogenetic and phylogeographic analyses.
Fig. 7.
Amino acids variability patterns of the full-length proteins of JCV, 1993–2023. The plot represents the amino acid variations in the ORFs-encoded agnoprotein, VP1, VP2, VP2, Large T antigen, and small t antigen. All the available amino acid sequences of each protein were used to acquire the consensus amino acid sequences using the Shannon entropy one offered by the Los Alamos National Laboratory (LANL). The X-axis represents the amino acids position, while the Y-axis represents the Shannon Entropy, where low scores represent less variable sites and a high score represents more variable sites.
4. Discussion
Although the International Committee on Taxonomy of Viruses (ICTV) has placed all the JCV strains within the family Polyomaviridae, genus Betapolyomavirus, species human polyomavirus 2, there is no classification system on clade and sub-clade levels by the ICTV (ICTV 2022). Therefore, JCV strains are classified using different nomenclatures and methods, grouping the strains into several types related to different geographic locations and human ethnicities (Stoner et al., 2000; Sugimoto et al., 1997; Yogo et al., 2004). Previously, JCVs are classified based on the phylogenetic analysis of the full-length genomes (Jobes et al., 1998; Sugimoto et al., 2002), V-T intergenic region, e.g., the 610 bp region corresponding to the 5′ end of T genes and 3′ end of VP1 (Sugimoto et al., 1997; Guo et al., 1996), or partial VP1 region's signature mutations analysis, e.g., the 215 bp region corresponding to the 5′ end of VP1 (Agostini et al., 1997; Ault and Stoner, 1992). The two most widely used nomenclature systems are based on the phylogenetic analysis of the full-length genomes, classifying the JCV strains into genotypes 1–8 with their respective sub-genotypes and lineages Af, B, Cy, Eu, My, and SC associated with different geographic locations (Torres, 2020; Jobes et al., 1998; Sugimoto et al., 2002). For example, the genotype 1, sub-genotype 1A, lineage Eu-a were reported to include strains from Northern, Western and Southern Europe, Eastern Asia, North-eastern, Siberia, Arctic areas of North America, and the 1B-Eu-a from Northern, Western, Southern and Eastern Europe, North America, Eastern Asia, North and South America. Similar classes were suggested for the remaining seven genotypes (2–8), while an extra two unassigned classes, grouping strains from Central Africa, and North-eastern Siberia and Japan respectively (Torres, 2020; Jobes et al., 1998; Sugimoto et al., 2002). However, neither of these classifications and nomenclature contemplates all the groups described so far, making the JCV classification complex and leaving gaps in the JCV classification system. Thus, to fulfill these gaps, we analyzed all the available full-length coding nucleotide sequences, in addition to the VP1 and LTA nucleotide sequences of JCV available on NCBI GenBank and isolated globally during 1993–2023 to map the latest genetic clustering, phylogeographic dynamics and genetic characteristics of JCV strains.
In a previous study, Hatwell and Sharp conducted a phylogenetic classification using 20 whole genome sequences to categorize the strains into three different genotypes, type 1–3, where type 2 was further classified into type 2A and 2B (Hatwell and Sharp, 2000). Moreover, various investigations conducted previously have shown that the ancestral JCV strains are grouped into three separate superclusters (Yogo et al., 2004; Sugimoto et al., 2002). Similarly, Jobes and colleagues examined 22 whole genome sequences and identified four genotypes (type 1–4) by creating a phylogenetic tree using different methods, including neighbor-joining, UPGMA, and maximum parsimony (Jobes et al., 1998). According to Jobs et al., Type 1 was of European origin, Type 2 was Asian, Type 3 was found in individuals of African descent, and Type 4 was reported as a potential recombinant of Types 1 and 3 and was widely distributed throughout the population of the United States (Jobes et al., 1998). Our study also reports four different genotypes similar to the previous study (Jobes et al., 1998); however, our findings offer a more robust and latest phylogenetic analysis, including all the available (n = 656) whole genome sequences isolated and reported from 1993 to 2023, which can help categorize both the known and recently discovered strains. Our study classified the viral strains into four major groups (GI-GIV), with additional sub-classifications within each group. The groups are divided into sub-groups, where the GI clade is the largest cluster, thus sub-divided into two types, e.g., GI.1 and GI.2 with their respective sub-clades (GI.1/GI.2 a-e). Similarly, the GII is divided into two (GII a, b) and GIV into seven sub-genotypes (GIV a-g) based on complete coding sequences available to date.
The sub-groups displayed a high level of diversity, with strains from various regions worldwide. For example, the GII-a and GIV-d were the most diverse subgroups, which included strains from Asia, Africa, America, Europe, Oceania, and Eurasia, except in America for the first and Oceania for the latter. The GI.2-b included strains from Japan, while the GIV-c was from the USA only, making them among the least diverse sub-groups. Furthermore, the GIII clade exclusively included strains from Ghana (GH-1 to GH-4) and appeared as a distanced separate clade. The study by Shackelton et al. was similar to our study in which the analysis of whole genome sequences revealed that JCV has not been strictly codiverged within the human population (Shackelton et al., 2006). However, certain genotypes are more common in specific populations, and some parts of JCV suggest evolutionary history. Wooding proposed a similar geographical distribution of humans and JCV based on differences in demographic history. In his study, both JCV and human subpopulations show unique genetic characteristics, possibly due to population isolation and genetic drift (Wooding, 2001). However, human population structure does not account for all the observed phylogenetic patterns in JCV, like the similarities between viruses from African and Asian subpopulations and the genetic diversity of European strains.
Genetic recombination plays a vital role in virus evolution by maintaining or creating diversity. The present study also reported the events of genetic recombination at nucleotide number 4905–5208 in NCCR of the recombinant, resulting in the emergence of recombinant strain. The recombinant strain [JCV-variant-GS/B-Germany-1997 (GenBank ID: AF004350.1)] is the recombination of the GS/K-Germany-1997 within the GIII-c sub-clade (GenBank ID: AF004349.1) and the JCV strain-315A-Ethiopia-2204 from the GII-a sub-clade (GenBank ID: AY342299.1). The results are in agreement with a previous study that has also reported similar kinds of recombinant strains emerging from Germany and African American JCV strains (Hatwell and Sharp, 2000).
JCV genotypes are determined solely by the DNA sequence of the viral genome. The DNA sequence variability across JCV genotypes varies in distinct genomic elements outside the viral control region (NCCR) which may lead to amino acids variability across the JCV proteins. The amino acids variability in JCV proteins including VP1, VP2, VP3, agnoprotein, LTA, and STA were analyzed using Shannon entropy. The results of our study revealed the highest variability across the LTA protein followed by agnoprotein, VP2, VP3, and VP1 while the least variability was shown by STA protein. Our results contrast the findings of the previous studies (ICTV 2022; Jobes et al., 1998), which identified highly conserved areas in agnoprotein genes, suggesting genetic variability in strains of JCV. Agnoprotein, expressed in the late phase of the JCV life cycle, is a small accessory protein that can be found in the cytoplasmic perinuclear regions and the nucleus sometimes (Assetta and Atwood, 2017). Studies suggest that the agnoprotein plays its role in the propagation and release of the virus and acts as a viroporin (Assetta and Atwood, 2017; Okada et al., 2005; Suzuki et al., 2010). Moreover, the variability of amino acids in the genome, especially in proteins related to viral replication, host-cell binding, and evasion from the immune system, can impact its ability to cause disease, transmissibility, and pathogenicity. Specific mutations have been reported in the VP1 protein that caused the JC virus to evolve into the form linked to PML by altering the virus's affinity for its cellular receptors (Sunyaev et al., 2009). The present study shows significant variation in amino acids among all proteins from 1993 to 2023, including agnoprotein, supporting genetic diversity, genetic drift, and interchange among the JCVs, as demonstrated by our phylogenetic and phylogeographic analyses.
In summary, this study offers the most recent findings on the phylogenetic traits, geographical spread, and genetic diversity patterns of the JC polyomavirus using complete genomic sequences collected between 1993 and 2023. Organizing JCV into four main clades with additional sub-clades could provide a reliable method for categorizing both current and forthcoming strains. Furthermore, genetic recombination and amino acid variability can help identify pathogenicity and develop effective vaccines for preventing and controlling JCV.
Ethical approval
Formal consent is not required for this comparative genomic and phylogenetic type of study.
Funding information
We acknowledge the grants from the National Natural Science Foundation of China (No. 82072287 and No. 32371283), the Taishan Scholars Program (E31108R0Y5), and the Special Supporting Funds for Leading Talents at or above the Provincial level in Yantai City (E32012R0Y5).
CRediT authorship contribution statement
Pir Tariq Shah: Writing – original draft, Visualization, Methodology, Investigation, Formal analysis, Conceptualization. Mohammad Ejaz: Writing – original draft. Kosar Tamanna: Writing – original draft, Data curation. Muhammad Nasir Riaz: Writing – review & editing, Validation, Data curation. Zhenyong Wu: Writing – review & editing, Supervision. Chengjun Wu: Writing – review & editing, Writing – original draft, Validation, Supervision, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
We thank everyone who contributed to the collection and production of JCV genomic sequences in the NCBI GenBank database.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.virusres.2024.199414.
Contributor Information
Zhenyong Wu, Email: wuzhenyong@simm.ac.cn.
Chengjun Wu, Email: wcj5532@dlut.edu.cn.
Appendix. Supplementary materials
Data availability
No data was used for the research described in the article.
References
- Agostini H.T., Ryschkewitsch C.F., Stoner G.L. Genotype profile of human polyomavirus JC excreted in urine of immunocompetent individuals. J. Clin. Microbiol. 1996;34(1):159–164. doi: 10.1128/jcm.34.1.159-164.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agostini H.T., et al. Asian genotypes of JC virus in Native Americans and in a Pacific Island population: markers of viral evolution and human migration. Proc. Natl. Acad. Sci. 1997;94(26):14542–14546. doi: 10.1073/pnas.94.26.14542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agostini H., et al. Influence of JC virus coding region genotype on risk of leukoencephalopathy. J. Neurovirol. 2000;6(2):S101–S108. [PubMed] [Google Scholar]
- Assetta B., Atwood W.J. The biology of JC polyomavirus. Biol. Chem. 2017;398(8):839–855. doi: 10.1515/hsz-2016-0345. [DOI] [PubMed] [Google Scholar]
- Astrom K., et al. Progressive multifocal leukoencephalopathy (PML): a 50-years old disease. Brain. 1958;81:93–111. doi: 10.1093/brain/81.1.93. [DOI] [PubMed] [Google Scholar]
- Ault G.S., Stoner G.L. Two major types of JC virus defined in progressive multifocal leukoencephalopathy brain by early and late coding region DNA sequences. J. Gen. Virol. 1992;73(10):2669–2678. doi: 10.1099/0022-1317-73-10-2669. [DOI] [PubMed] [Google Scholar]
- Ault G.S., Stoner G.L. Human polyomavirus JC promoter/enhancer rearrangement patterns from progressive multifocal leukoencephalopathy brain are unique derivatives of a single archetypal structure. J. Gen. Virol. 1993;74(8):1499–1507. doi: 10.1099/0022-1317-74-8-1499. [DOI] [PubMed] [Google Scholar]
- Bellizzi A., et al. Human polyomavirus JC reactivation and pathogenetic mechanisms of progressive multifocal leukoencephalopathy and cancer in the era of monoclonal antibody therapies. J. Neurovirol. 2012;18:1–11. doi: 10.1007/s13365-012-0101-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger J.R., Concha M. Progressive multifocal leukoencephalopathy: the evolution of a disease once considered rare. J. Neurovirol. 1995;1(1):5–18. doi: 10.3109/13550289509111006. [DOI] [PubMed] [Google Scholar]
- Blake K., et al. JC virus associated meningoencephalitis in an immunocompetent girl. Arch. Dis. Child. 1992;67(7):956. doi: 10.1136/adc.67.7.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciappi S., et al. Archetypal and rearranged sequences of human polyomavirus JC transcription control region in peripheral blood leukocytes and in cerebrospinal fluid. J. Gen. Virol. 1999;80(4):1017–1023. doi: 10.1099/0022-1317-80-4-1017. [DOI] [PubMed] [Google Scholar]
- DeCaprio J.A., Garcea R.L. A cornucopia of human polyomaviruses. Nat. Rev. Microbiol. 2013;11(4):264–276. doi: 10.1038/nrmicro2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Pasquier R., et al. Productive infection of cerebellar granule cell neurons by JC virus in an HIV+ individual. Neurology. 2003;61(6):775–782. doi: 10.1212/01.wnl.0000081306.86961.33. [DOI] [PubMed] [Google Scholar]
- Elia F., et al. JC virus infection is acquired very early in life: evidence from a longitudinal serological study. J. Neurovirol. 2017;23:99–105. doi: 10.1007/s13365-016-0477-9. [DOI] [PubMed] [Google Scholar]
- Ferenczy M.W., et al. Molecular biology, epidemiology, and pathogenesis of progressive multifocal leukoencephalopathy, the JC virus-induced demyelinating disease of the human brain. Clin. Microbiol. Rev. 2012;25(3):471–506. doi: 10.1128/CMR.05031-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisque R.J., Bream G.L., Cannella M.T. Human polyomavirus JC virus genome. J. Virol. 1984;51(2):458–469. doi: 10.1128/jvi.51.2.458-469.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossai A., et al. Seroepidemiology of human polyomaviruses in a US population. Am. J. Epidemiol. 2016;183(1):61–69. doi: 10.1093/aje/kwv155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinnell B.W., Padgett B.L., Walker D.L. Distribution of nonintegrated DNA from JC papovavirus in organs of patients with progressive multifocal leukoencephalopathy. J. Infect. Dis. 1983;147(4):669–675. doi: 10.1093/infdis/147.4.669. [DOI] [PubMed] [Google Scholar]
- Guo J., et al. Geographical distribution of the human polyomavirus JC virus types A and B and isolation of a new type from Ghana. J. Gen. Virol. 1996;77(5):919–927. doi: 10.1099/0022-1317-77-5-919. [DOI] [PubMed] [Google Scholar]
- Hatwell J.N., Sharp P.M. Evolution of human polyomavirus JC. J. Gen. Virol. 2000;81(5):1191–1200. doi: 10.1099/0022-1317-81-5-1191. [DOI] [PubMed] [Google Scholar]
- Hoang D., Chernomor O., von Haeseler A., Minh B.Q., Vinh L.S. UFBoot2: improving the ultrafast bootstrap approximation. Mol. Biol. Evol. 2018;35:518–522. doi: 10.1093/molbev/msx281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ICTV . International Committee on Taxonomy of Viruses; 2022. Taxon Details: Betapolyomavirus secuhominis. [Google Scholar]
- Jobes D.V., et al. Phylogenetic analysis of 22 complete genomes of the human polyomavirus JC virus. J. Gen. Virol. 1998;79(10):2491–2498. doi: 10.1099/0022-1317-79-10-2491. [DOI] [PubMed] [Google Scholar]
- Kantarci G., et al. JC virus-associated nephropathy in a renal transplant recipient and comparative analysis of previous cases. Transplant Infect. Dis. 2011;13(1):89–92. doi: 10.1111/j.1399-3062.2010.00567.x. [DOI] [PubMed] [Google Scholar]
- Koralnik I., et al. JC virus DNA load in patients with and without progressive multifocal leukoencephalopathy. Neurology. 1999;52(2):253. doi: 10.1212/wnl.52.2.253. [DOI] [PubMed] [Google Scholar]
- Koralnik I.J. New insights into progressive multifocal leukoencephalopathy. Curr. Opin. Neurol. 2004;17(3):365–370. doi: 10.1097/00019052-200406000-00019. [DOI] [PubMed] [Google Scholar]
- Leigh J.W., Bryant D. POPART: full-feature software for haplotype network construction. Methods Ecol. Evol. 2015;6(9):1110–1116. [Google Scholar]
- Levican J., et al. JC polyomavirus circulation in one-year surveillance in wastewater in Santiago. Chile. Infect. Genet. Evol. 2019;71:151–158. doi: 10.1016/j.meegid.2019.03.017. [DOI] [PubMed] [Google Scholar]
- Marshall L.J., et al. JC virus promoter/enhancers contain TATA box-associated Spi-B-binding sites that support early viral gene expression in primary astrocytes. J. Gen. Virol. 2012;93(3):651–661. doi: 10.1099/vir.0.035832-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin D.P., et al. RDP4: detection and analysis of recombination patterns in virus genomes. Virus Evol. 2015;1(1):vev003. doi: 10.1093/ve/vev003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miskin D.P., et al. Predictors and characteristics of seizures in survivors of progressive multifocal leukoencephalopathy. J. Neurovirol. 2016;22(4):464–471. doi: 10.1007/s13365-015-0414-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moens U., et al. Biology, evolution, and medical importance of polyomaviruses: an update. Infect. Genet. Evol. 2017;54:18–38. doi: 10.1016/j.meegid.2017.06.011. [DOI] [PubMed] [Google Scholar]
- Monaco M.C.G., et al. JC virus multiplication in human hematopoietic progenitor cells requires the NF-1 class D transcription factor. J. Virol. 2001;75(20):9687–9695. doi: 10.1128/JVI.75.20.9687-9695.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen L.-T., et al. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2015;32(1):268–274. doi: 10.1093/molbev/msu300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada Y., et al. Dissociation of heterochromatin protein 1 from lamin B receptor induced by human polyomavirus agnoprotein: role in nuclear egress of viral particles. EMBO Rep. 2005;6(5):452–457. doi: 10.1038/sj.embor.7400406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padgett B.L., Walker D.L. Prevalence of antibodies in human sera against JC virus, an isolate from a case of progressive multifocal leukoencephalopathy. J. Infect. Dis. 1973;127(4):467–470. doi: 10.1093/infdis/127.4.467. [DOI] [PubMed] [Google Scholar]
- Padgett BL W.D., ZuRhein G.M., Eckroade R.J., Dessel B.H. Cultivation of papova-like virus from human brain with progressive multifocal leucoencephalopathy. Lancet. 1971;1:1257–1260. doi: 10.1016/s0140-6736(71)91777-6. [DOI] [PubMed] [Google Scholar]
- Pinto M., Dobson S. BK and JC virus: a review. J. Infect. 2014;68:S2–S8. doi: 10.1016/j.jinf.2013.09.009. [DOI] [PubMed] [Google Scholar]
- Reid C.E., et al. Sequencing and analysis of JC virus DNA from natalizumab-treated PML patients. J. Infect. Dis. 2011;204(2):237–244. doi: 10.1093/infdis/jir256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson E.P., Jr Progressive multifocal leukoencephalopathy. N. Engl. J. Med. 1961;265(17):815–823. doi: 10.1056/NEJM196110262651701. [DOI] [PubMed] [Google Scholar]
- Shackelton L.A., et al. JC virus evolution and its association with human populations. J. Virol. 2006;80(20):9928–9933. doi: 10.1128/JVI.00441-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snopková S., et al. Progressive multifocal leukoencephalopathy-epidemiology, immune response, clinical differences, treatment. Kidney. 2019;23(24):28. [PubMed] [Google Scholar]
- Šroller V., et al. Seroprevalence rates of BKV, JCV, and MCPyV polyomaviruses in the general Czech Republic population. J. Med. Virol. 2014;86(9):1560–1568. doi: 10.1002/jmv.23841. [DOI] [PubMed] [Google Scholar]
- Stoner G.L., et al. JC virus as a marker of human migrationto the Americas. Microbes Infect. 2000;2(15):1905–1911. doi: 10.1016/s1286-4579(00)01339-3. [DOI] [PubMed] [Google Scholar]
- Sugimoto C., et al. Typing of urinary JC virus DNA offers a novel means of tracing human migrations. Proc. Natl. Acad. Sci. 1997;94(17):9191–9196. doi: 10.1073/pnas.94.17.9191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto C., et al. Evolution of human polyomavirus JC: implications for the population history of humans. J. Mol. Evol. 2002;54:285–297. doi: 10.1007/s00239-001-0009-x. [DOI] [PubMed] [Google Scholar]
- Sunden Y., et al. DDX1 promotes proliferation of the JC virus through transactivation of its promoter. Microbiol. Immunol. 2007;51(3):339–347. doi: 10.1111/j.1348-0421.2007.tb03907.x. [DOI] [PubMed] [Google Scholar]
- Sunyaev S.R., et al. Adaptive mutations in the JC virus protein capsid are associated with progressive multifocal leukoencephalopathy (PML) PLoS Genet. 2009;5(2) doi: 10.1371/journal.pgen.1000368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T., et al. The human polyoma JC virus agnoprotein acts as a viroporin. PLoS Pathog. 2010;6(3) doi: 10.1371/journal.ppat.1000801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taguchi F., Kajioka J., Miyamura T. Prevalence rate and age of acquisition of antibodies against JC virus and BK virus in human sera. Microbiol. Immunol. 1982;26(11):1057–1064. doi: 10.1111/j.1348-0421.1982.tb00254.x. [DOI] [PubMed] [Google Scholar]
- Tan C.S., Koralnik I.J. Progressive multifocal leukoencephalopathy and other disorders caused by JC virus: clinical features and pathogenesis. Lancet Neurol. 2010;9(4):425–437. doi: 10.1016/S1474-4422(10)70040-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres C. Evolution and molecular epidemiology of polyomaviruses. Infect. Genet. Evol. 2020;79 doi: 10.1016/j.meegid.2019.104150. [DOI] [PubMed] [Google Scholar]
- Vaz B., et al. Analysis of the transcriptional control region in progressive multifocal leukoencephalopathy. J. Neurovirol. 2000;6(5):398–409. doi: 10.3109/13550280009018304. [DOI] [PubMed] [Google Scholar]
- Viallard J.-F., et al. JC virus meningitis in a patient with systemic lupus erythematosus. Lupus. 2005;14(12):964–966. doi: 10.1191/0961203305lu2229cr. [DOI] [PubMed] [Google Scholar]
- Wüthrich C., et al. Frequent infection of cerebellar granule cell neurons by polyomavirus JC in progressive multifocal leukoencephalopathy. J. Neuropathol. Experim. Neurol. 2009;68(1):15–25. doi: 10.1097/NEN.0b013e3181912570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White M.K., Safak M., Khalili K. Regulation of gene expression in primate polyomaviruses. J. Virol. 2009;83(21):10846–10856. doi: 10.1128/JVI.00542-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White M.K., Gordon J., Khalili K. The rapidly expanding family of human polyomaviruses: recent developments in understanding their life cycle and role in human pathology. PLoS Pathog. 2013;9(3) doi: 10.1371/journal.ppat.1003206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wooding S. Do human and JC virus genes show evidence of host–parasite codemography? Infect. Genet. Evol. 2001;1(1):3–12. doi: 10.1016/s1567-1348(01)00002-8. [DOI] [PubMed] [Google Scholar]
- Yogo Y., Sugimoto C. The archetype concept and regulatory region rearrangement. Human Polyomavirusess. 2001:127–148. [Google Scholar]
- Yogo Y., et al. Isolation of a possible archetypal JC virus DNA sequence from nonimmunocompromised individuals. J. Virol. 1990;64(6):3139–3143. doi: 10.1128/jvi.64.6.3139-3143.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yogo Y., et al. JC virus genotyping offers a new paradigm in the study of human populations. Rev. Med. Virol. 2004;14(3):179–191. doi: 10.1002/rmv.428. [DOI] [PubMed] [Google Scholar]
- Zheng H.-Y., et al. Characterization of the VP1 loop mutations widespread among JC polyomavirus isolates associated with progressive multifocal leukoencephalopathy. Biochem. Biophys. Res. Commun. 2005;333(3):996–1002. doi: 10.1016/j.bbrc.2005.06.012. [DOI] [PubMed] [Google Scholar]
- Zheng H.-Y., et al. New sequence polymorphisms in the outer loops of the JC polyomavirus major capsid protein (VP1) possibly associated with progressive multifocal leukoencephalopathy. J. Gen. Virol. 2005;86(7):2035–2045. doi: 10.1099/vir.0.80863-0. [DOI] [PubMed] [Google Scholar]
- Zu Rhein G.M., Chou S.-M. Particles resembling papova viruses in human cerebral demyelinating disease. Science. 1965;148(3676):1477–1479. doi: 10.1126/science.148.3676.1477. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No data was used for the research described in the article.