Abstract
Developing a metallic catalyst for converting furfural (FAL) to highly valuable products such as cyclopentanone (CPO) is important for fine chemical synthesis by the efficient utilization of biomass resources. The presence of diverse unsaturated carbon atoms in FAL and the rearrangement of oxygen atoms hinder the production of CPO. We developed an optimal nickel (Ni)-to-platinum (Pt) molar ratio (1:0.007) for a bimetallic Ni–Pt/alumina (Al2O3) catalyst with a low Pt loading via an impregnation method to efficiently catalyze the selective hydrogenation of FAL in an aqueous solution to form CPO. The comprehensive characterizations by X-ray diffraction and X-ray absorption near edge structure analyses elucidated the formation of Ni0/Pt0 and Ni2+/Pt4+ after reduction by H2. The addition of a low amount of the Pt–Ni/Al2O3 catalyst resulted in an alleviation of H2 reduction behavior detected by hydrogen temperature-programmed reduction, accompanied by low H2 desorption ability observed by hydrogen temperature-programmed desorption. The catalytic activity of Ni–Pt/Al2O3 was higher than those of Ni/Al2O3 and Pt/Al2O3 catalysts. The maximum CPO yield was 66% with 93% FAL conversion under the optimized conditions (160 °C, 20 bar of H2 pressure, and 2 h). Isotopic deuterium oxide (D2O) labeling revealed the transfer of deuterium (D) atoms from D2O to the intermediates and products during hydrogenation and rearrangement, which confirmed that water was a medium for rearrangement and the source of hydrogen for the reaction. This study developed an efficient catalyst for the catalytic hydrogenation and ring rearrangement of FAL into CPO.
1. Introduction
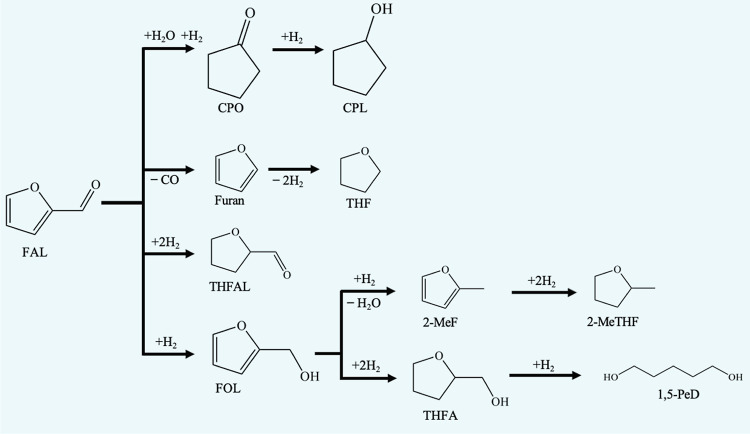
A sustainable and economic route to obtain high value-added chemicals via biomass biorefining has attracted extensive attention. The development of renewable biomass energy is crucial for resolving energy and ecology issues1,2 owing to the escalating depletion of fossil fuel sources.3 Biomass valorization has emerged as a potential process to meet the increasing demand for fuel and chemicals.4,5 Metal catalysts with scale-up strategies are crucial for the catalysis of biomass-derived molecules.6 Furfural (FAL), a biomass-derived resource rich in oxygen, is obtained via the dehydration of xylose. The hydrogenation of FAL produces furfuryl alcohol (FOL), tetrahydrofurfural (THFAL), tetrahydrofurfuryl alcohol (THFA), furan, tetrahydrofuran (THF), cyclopentanone (CPO), cyclopentanol (CPL), 2-methylfuran (2-MeF), 2-methyltetrahydrofuran (2-MeTHF), and pentanediols (PeDs) (Figure 1).7−10 The potential challenge is to attain the highest selectivity of the desired product via FAL hydrogenation with a multiproduct formation capability. The hydrogenative ring rearrangement of biomass-derived FAL to produce CPO has been extensively studied.11 CPO and CPL obtained through the selective hydrogenation and rearrangement of FAL are important precursors of various flavor-based compounds, pharmaceuticals, pesticides, electronic components, etc.12,13 CPO is a building block for fungicides, rubber-based chemicals, pharmaceuticals, and flavor- and fragrance-based compounds.14 It can also be used for the synthesis of polyamides, showcasing its potential for industrial applications. Alternately, the CPO intermediate is important for the synthesis of pharmaceuticals, insecticides, dyes, herbicides, and fragrances. The production of CPO from FAL was enhanced in the presence of water as a solvent,15 and the hydrogenative ring rearrangement was catalyzed by metals.16 Noble transition metal-based catalysts, especially platinum (Pt), were used for heterogeneous hydrogenation.17
Figure 1.
Reaction pathway of FAL hydrogenation into valuable products.
Highly efficient nickel (Ni)-based catalysts were used for a sustainable and cost-effective approach to produce CPO via FAL hydrogenation. Ni–nickel oxide (NiO)/titanium oxide (TiO2) and Ni nanoparticles encapsulated in carbon (C) layers (Ni@NP-C) were used to catalyze the selective hydrogenation of FAL to CPO in water at temperature <150 °C.18,19 Hronec and Fulajtarová successfully synthesized CPO (76.50 mol % yield) by reacting FAL with hydrogen catalyzed by 5 wt % platinum (Pt)/C. Despite conducting aqueous hydrogenation at a relatively low temperature (160 °C), the industrial applicability of this method might be hindered by the scarcity of noble metals.20 Mironenko et al. investigated the mechanism of FAL hydrogenation in water catalyzed by palladium (Pd)/C. FAL hydrogenation proceeds through four pathways involving various acid-catalyzed furan ring opening and reduction reactions.12 However, the possible reaction mechanism of the hydrogenative rearrangement of FAL in water is still not clear.
Herein, we attempted to incorporate Pt and Ni on a γ-alumina (γ-Al2O3) support to synthesize the Pt–Ni/Al2O3 catalyst via an impregnation method using various Ni-to-Pt molar ratios. The prepared catalysts were characterized via nitrogen (N2) sorption, X-ray diffraction (XRD), X-ray absorption near edge structure (XANES), chemisorption, and transmission electron microscopy (TEM). The catalytic activity of Pt–Ni/Al2O3 for FAL hydrogenation to CPO in an aqueous phase was higher than those of the Ni/Al2O3 and Pt/Al2O3 catalysts. The isotopic deuterium oxide (D2O) labeling was investigated to comprehend the possible reaction mechanism.
2. Experimental Section
2.1. Synthesis of Catalysts
The bimetallic Ni–Pt-supported Al2O3 (Ni–Pt/Al2O3) catalysts with different molar ratios of Ni to Pt were prepared via a conventional wetness impregnation method (Scheme S1). Nickel(II) nitrate hexahydrate [Ni(NO3)2·6H2O; CARLO ERBA, purity ≥98.5%] and chloroplatinic acid solution (H2PtCl6; Sigma-Aldrich, 8 wt % in H2O) were used as the metal salts. Al2O3 used as a support was obtained from the Sasol Company, Germany. A mixture of an aqueous solution of Ni(NO3)2·6H2O and H2PtCl6 was added to an Al2O3 support. The obtained catalyst was dried at 110 °C for 6 h and calcined in stagnant air at 500 °C for 5 h at a heating rate of 5 °C min–1. The calcined catalyst was reduced at 500 °C for 3 h under a flow of pure hydrogen. The synthesized bimetallic Ni–Pt/Al2O3 catalysts with different Ni/Pt molar ratios of 1:0.004, 1:0.007, and 1:0.015 were denoted as Ni1Pt0.004/Al2O3, Ni1Pt0.007/Al2O3, and Ni1Pt0.015/Al2O3, respectively. The samples were analyzed via inductively coupled plasma-optical emission spectrometry (ICP-OES). Monometallic Ni/Al2O3 and Pt/Al2O3 catalysts were prepared following the same procedure using Ni(NO3)2·6H2O and H2PtCl6 precursors, respectively.
2.2. Characterization of Catalysts
The total pore volume, specific surface area, and average pore diameter of the synthesized catalysts were characterized via nitrogen desorption–adsorption at −196 °C using a gas sorption analyzer (Autosorb iQ Station 2). The undesired volatile substances were removed before analysis by pretreating the samples under a vacuum at 200 °C for 12 h. The phase and crystallinity were analyzed via XRD using an X-ray diffractometer (D8 ADVANCE, Bruker, Ltd.) with Cu Kα X-ray source radiation in the range of 2θ from 10 to 80° within a step time of 0.5 s at 40 kV and 30 mA. XANES analysis was used to study the change in the oxidation states of the reduced catalysts. The XANES spectra at the Ni K-edge and Pt L3-edge were obtained using transmission and fluorescent modes, respectively, using a Ge(220) monochromator crystal for scanning photon energy at Beamline 8 at the Synchrotron Light Research Institute, Thailand. The calibration of the photon energy was performed using Ni and Pt foil with reference K-edge and L3-edge energies of 8979 and 10,535 eV, respectively. The fluorescent mode of the Pt L3-edge was implemented by the investigation of the self-absorption effect using a platinum oxide (PtO2) standard. The linear combination fitting (LCF) and normalization of the XANES spectra were performed using the ATHENA program. The reducibility and metal–support interaction of catalysts were observed via hydrogen temperature-programmed reduction (H2-TPR) using a chemisorption analyzer (Quantachrome TPRWin v4.10). The calcined catalysts were pretreated at 200 °C for 1 h under an argon (Ar) flow rate of 50 cm3 min–1. The H2-TPR profiles were recorded using a thermal conductivity detector (TCD) under a flow of 10 vol % H2/Ar in a temperature range of 100–900 °C with a heating rate of 10 °C min–1. The acidity of the catalyst was observed via ammonia temperature-programmed desorption (NH3-TPD) using a similar system as that used for H2-TPR. The calcined catalysts were reduced in situ at 500 °C for 2 h at a rate of 10 °C min–1 under a 10 vol % H2/Ar flow rate of 50 cm3 min–1. Five vol % ammonia/helium (He) catalysts were adsorbed at 50 °C for 2 h accompanied by unabsorbed NH3 removal using an inert gas. The signal of NH3 desorption was recorded in the range of 50–1000 °C at a ramp temperature of 10 °C min–1 via TCD under a He gas. The ability of H2 adsorption–desorption was explored via hydrogen TPD (H2-TPD). The calcined catalyst was reduced in situ at 500 °C for 2 h at a heating temperature of 10 °C min–1 under a 10 vol % H2/Ar flow rate of 50 cm3 min–1. The catalysts were adsorbed with 10 vol % H2/Ar at 50 °C for 2 h, and the desorption of H2 was recorded in the temperature range of 50–1000 °C with a ramp temperature of 10 °C min–1 via TCD under He gas. The metal composition of the calcined catalysts was analyzed via ICP-OES (PerkinElmer, NexION 2000). The calcined catalyst was digested using a mixed acid solution of hydrochloric acid (HCl, 37% AR. grade; QReC, New Zealand) and nitric acid (HNO3, 65% AR. grade; QReC, New Zealand) at a volume ratio of 3:1 under microwave irradiation before the ICP-OES measurement. The metal species distribution and morphology of reduced Ni–Pt7/Al2O3, Ni/Al2O3, and Pt/Al2O3 were observed via high-resolution TEM (HR-TEM; JEOL/JEM-218 2100Plus). Finally, the weight loss of the reduced and used catalysts in the air was analyzed via thermogravimetric analysis (TGA; TGA 5500) in the temperature range of 30–1000 °C with a heating rate of 10 °C min–1.
2.3. Procedure for the Catalytic Conversion of Furfural
The hydrogenation and rearrangement of FAL were performed in a 100 mL batch reactor (Scheme S2). FAL (1 g, C5H4O2; Sigma-Aldrich, purity 99%), 40 mL of deionized water, and 0.1–0.3 g of reduced catalyst were loaded into a reactor. The reactor was flushed with H2 gas three times to remove the inside air and was subsequently pressurized to the desired operating pressure. The reaction temperature was maintained at the desired condition using a temperature controller under stirring at 600 rpm. The reactor was placed in cool water to quench the reaction. The products were collected and separated via filtration. The obtained liquid product was quantitatively analyzed via gas chromatography (GC) equipped with a flame ionization detector (FID) (GC 2014, Shimadzu) and a CP-Wax 52 CB column (30 m × 0.25 mm × 0.25 μm). The operating conditions for GC-FID are summarized in Table S1. The FAL conversion and product yields (FOL, CPO, and CPL) were calculated using eqs 1 and 2.
| 1 |
| 2 |
2.4. Isotopic D2O Labeling
The reaction mechanism of the hydrogenation and ring rearrangement of FAL to CPO was investigated via the isotopic D2O-labeling experiment and was compared with that conducted in water (H2O) as the solvent at 140 °C, 20 bar H2, and 2 h reaction time in the presence of reduced Ni1Pt0.007/Al2O3. The liquid product was extracted from the aqueous phase by using toluene and analyzed via GC–mass spectrometry (MS) (7890A, Agilent Technologies).
3. Results and Discussion
3.1. Catalyst Characterization
3.1.1. N2 Sorption and ICP-OES Analysis
Type IV isotherms with H1-type hysteresis loops are observed in the adsorption–desorption isotherm of the calcined catalysts, confirming the appearance of mesoporous material (Figure S1; Table 1).21 The Brunauer–Emmett–Teller (BET) surface area of the pristine Al2O3 support is 194 m2 g–1 with a pore volume (Vp) of 0.50 cm3 g–1.22 The BET surface area of calcined Ni/Al2O3 is 187 m2 g–1 with a total Vp of 0.50 m2 g–1, whereas the BET surface area of calcined Pt/Al2O3 is 207 m2 g–1 with a total Vp of 0.51 m2 g–1. Thus, the porosity of Al2O3 is modified by the addition of Pt.23 The BET surface area of Pt–Ni/Al2O3 is reduced by ∼12.7 to 20.6% than that of Ni/Al2O3 either owing to the blockage or converging of pores during metal loading. The Vp of the highest Pt-loaded Ni1Pt0.015/Al2O3 is reduced. However, the average pore size diameters of Ni1Pt0.004/Al2O3, Ni1Pt0.007/Al2O3, and Ni1Pt0.015/Al2O3 are nearly similar, indicating the partial coverage of metal components on the external area of the Al2O3 support. The average pore size analyzed via the Barret–Joyner–Halenda (BJH) method is 7.4 nm for all of the calcined catalysts. The loaded Ni content is 10.2 ± 0.9 wt % for all the Ni-containing catalysts, while the loaded Pt content is low (0.126–0.584 wt %) for Ni1Pt0.004/Al2O3, Ni1Pt0.007/Al2O3, and Ni1Pt0.015/Al2O3, as analyzed via ICP-OES (Table 1).
Table 1. Porosity Obtained via N2 Sorption Experiments and Metal Composition Obtained via ICP-OES of the Calcined Catalystsa.
| catalyst | N2 sorption |
metal
contentd |
Pt/Nid mole ratio | |||
|---|---|---|---|---|---|---|
| SBET (m2 g–1)a | Vp (cm3 g–1)b | Dp (nm)c | Ni (wt %) | Pt (wt %) | ||
| Ni/Al2O3 | 187 | 0.50 | 7.4 | 9.69 | 0 | |
| Pt/Al2O3 | 207 | 0.51 | 7.4 | 0 | 10.58 | |
| Ni1Pt0.004/Al2O3 | 164 | 0.42 | 7.4 | 9.6 | 0.126 | 0.004 |
| Ni1Pt0.007/Al2O3 | 148 | 0.40 | 7.4 | 10.16 | 0.242 | 0.007 |
| Ni1Pt0.015/Al2O3 | 149 | 0.38 | 7.4 | 11.54 | 0.584 | 0.015 |
SBET obtained from the adsorption branch of the N2 isotherm.
Vp calculated from N2 adsorption at a relative pressure of ∼0.990.
Dp obtained from the desorption branch via the BJH method.
Metal composition determined via ICP-OES analysis.
3.1.2. XRD Analysis
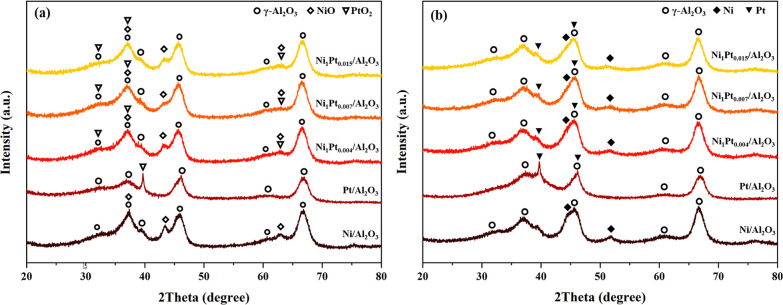
The phase purity and crystallinity of all of the reduced and calcined Ni/Al2O3, Pt/Al2O3, Ni1Pt0.004/Al2O3, Ni1Pt0.007/Al2O3, and Ni1Pt0.015/Al2O3 were analyzed via XRD (Figure 2). The XRD patterns of the catalysts show the characteristic 2θ peaks at 19, 32, 38, 39, 45, and 67° corresponding to (101), (112), (103), (202), (220), and (224) planes, respectively, which are attributed to the Al2O3 phase (PDF 01-074-4629).22,24−27Figure 2a indicates the NiO phase (PDF 01-080-5508) of calcined Ni/Al2O3 and Ni–Pt/Al2O3 with the diffraction peaks at 37, 44, and 63°, indicating the (111), (200), and (220) planes, respectively.22 The peaks of calcined Pt/Al2O3 and Ni–Pt/Al2O3 at 32, 37, and 63° correspond to the (111), (222), and (311) planes, respectively, ascribed to the PtO2 phase (PDF 01-089-3603). The peaks of reduced Ni/Al2O3 and Ni–Pt/Al2O3 (Figure 2b) at 44 and 52° correspond to metallic Ni(111) and (200) planes, respectively (PDF 00-004-0850).22 Alternately, the peaks at 39 and 46° of reduced Pt/Al2O3 and Ni–Pt/Al2O3 corresponding to (111) and (200) planes, respectively, are ascribed to the metallic Pt phase (PDF 00-004-0802).25−27 Thus, NiO and PtO2 were reduced to Ni and Pt under H2 at 500 °C before the catalytic performance.
Figure 2.
XRD patterns of (a) calcined and (b) reduced Ni/Al2O3, Pt/Al2O3, Ni1Pt0.004/Al2O3, Ni1Pt0.007/Al2O3, and Ni1Pt0.015/Al2O3.
3.1.3. XANES Analysis
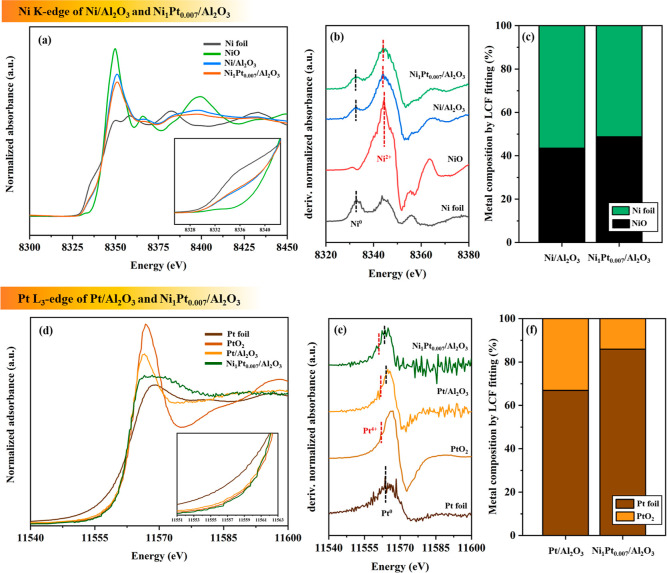
The reduced Ni1Pt0.007/Al2O3 was selected and analyzed via XANES because of its highest activity for CPO production among the three synthesized bimetallic catalysts. Figure 3 illustrates the results of XANES for the Pt L3-edge and Ni K-edge of reduced Ni1Pt0.007/Al2O3 and Ni/Al2O3 with the probable Ni and Pt standards. The valence state of the reduced sample is transformed, as indicated by the edge energy, oscillation shape, white line peak, and first derivative spectra. The Ni K-edge region (Figure 3a) shows that Ni/Al2O3 and Ni1Pt0.007/Al2O3 peaks shift to low energy with a decrease in white line intensity than that of the NiO standard, indicating a partial reduction of NiO to Ni. The normalized first derivative of the XANES spectra confirms that Ni of reduced Ni/Al2O3 and Ni1Pt0.007/Al2O3 exists as Ni2+ and metallic Ni0 owing to the similar shape and edge position of Ni foil and NiO standards (Figure 3b). LCF (Figure 3c) shows the coexistence of NiO and metallic Ni0 in the reduced Ni/Al2O3 and Ni1Pt0.007/Al2O3. The Ni0 fraction of Ni1Pt0.007/Al2O3 is higher than that of Ni/Al2O3 because of the H2 spillover effect from Pt to Ni. The Pt L3-edge results of reduced Pt/Al2O3 and Ni1Pt0.007/Al2O3 (Figure 3d) are similar to the Pt standard than that of the PtO2 standard. Thus, Pt0 is the major fraction of the reduced catalysts. The first derivative spectra (Figure 3e) of reduced Ni1Pt0.007/Al2O3 and Ni/Al2O3 show that the shift of the edge position is identical to that of the Ni foil standard. A higher Pt0 content (∼80%) of Ni1Pt0.007/Al2O3 is indicated via LCF compared to the lower Pt0 content (∼67%) of Pt/Al2O3 (Figure 3f). Thus, PtOx strongly interacts with the Al2O3 support in the Pt/Al2O3 catalyst.
Figure 3.
(a,d) Normalized XANES spectra, (b,e) first derivative, and (c,f) metal composition via LCF fitting of (a–c) Ni K-edges and (d–f) Pt L3-edges of reduced Ni/Al2O3 and Ni1Pt0.007/Al2O3 containing all combinations of Ni and Pt standards.
3.1.4. Chemisorption Analysis
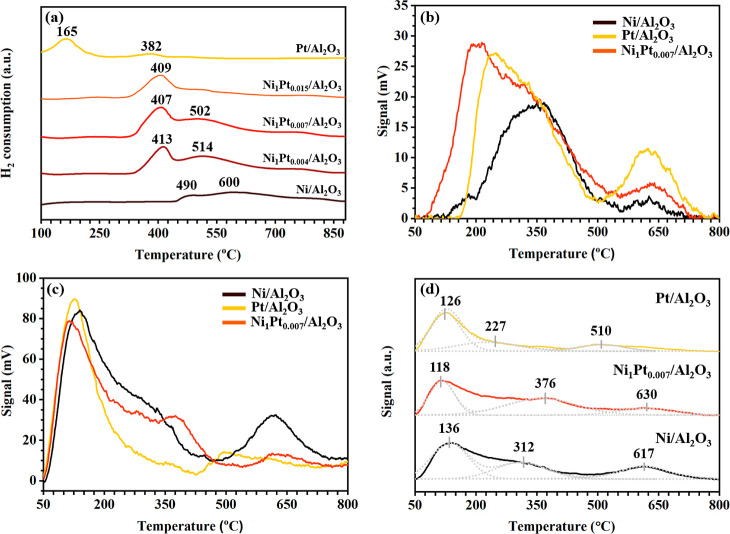
The H2-TPR of the calcined catalysts was initially measured to investigate the catalyst reducibility and metal–support interactions (Figure 4a). The signal of H2 consumption of calcined Ni/Al2O3 was detected in the temperature range of 400−800 °C owing to the weak interaction of bulk NiO and the strong interaction of surface NiO with Al2O3.28,29 The H2-TPR profile of Pt/Al2O3 comprises two reduction peaks in the ranges of 100–300 and 350–450 °C, indicating the reduction of PtOx and the strong interaction of PtOx with Al2O3, respectively. The H2-TPR profiles of Ni–Pt/Al2O3 shift to a lower reduction temperature than that of Ni/Al2O3. Thus, the H2 spillover effect from Pt to Ni is pronounced after the addition of a low amount of Pt to Ni/Al2O3. Two major reduction peaks of Ni–Pt/Al2O3 in the ranges of 350–490 and 490–700 °C indicate the reduction of NiO and stable NiO interaction with Al2O3, respectively.
Figure 4.
(a) H2-TPR of Ni/Al2O3, Pt/Al2O3, Ni1Pt0.004/Al2O3, Ni1Pt0.007/Al2O3, and Ni1Pt0.015/Al2O3. (b) H2-TPD and (c) NH3-TPD profiles with (d) deconvoluted NH3-TPD profiles of Ni/Al2O3, Pt/Al2O3, and Ni1Pt0.007/Al2O3.
The effect of Pt addition was investigated on the ability of H2 adsorption–desorption, and the H2-TPD experiments were performed for reduced Ni/Al2O3, Pt/Al2O3, and Ni1Pt0.007/Al2O3 (Figure 4b). Two H2 desorption peaks of reduced Ni/Al2O3 are present in the ranges of 155–500 and 550–750 °C, whereas Pt/Al2O3 showed two peaks in the ranges of 200–500 and 500–775 °C. Thus, the H2 desorption ability of Pt/Al2O3 is weaker than that of Ni/Al2O3. The H2-TPD profile of Ni–Pt/Al2O3 comprises two wide peaks (80–500 and 540–800 °C) similar to that of Ni/Al2O3. Thus, the H2 desorption ability of Ni-containing catalysts is associated with weak and strong chemisorbed H2 in the dissociate states. H2 desorbed at a low temperature is the H2 adsorbed by metal particles, while H2 desorbed at a high temperature is from the strongly adsorbed H2 at the metal–support interface, which conforms with the reported behavior of Ni-based catalysts.30,31 The H2 desorption peak of Ni–Pt/Al2O3 shifts to a lower temperature than those of Ni/Al2O3 and Pt/Al2O3 with a low molar ratio of Pt to Ni, indicating that the interaction of H2 with Ni–Pt/Al2O3 is weakened by the addition of Pt to Ni catalysts.
The acidity of the catalysts was analyzed via NH3-TPD (Figure 4c,d). The NH3 desorption profiles displayed differences in the desorption temperature and peak intensity, indicating that the acidity affects the strength and quantity of the catalysts. The deconvoluted peaks were indicated as weak (50–240 °C), medium (320–450 °C), and strong (550–700 °C) acid sites (Figure 4d) in accordance with literature to distinguish the strengths of acids.28 The intensity of the Ni/Al2O3 peak for weak and strong acid sites is the highest among the other catalysts, whereas that of Pt/Al2O3 shows the lowest intensity. Thus, Ni incorporated into Al2O3 exhibits more acidic sites than Pt incorporated into Al2O3 (Figure 4c). The acidity and strength of Ni–Pt/Al2O3 decrease following the addition of Pt to Ni/Al2O3 owing to the decreasing number and strength of acid sites with a relative acidity distribution (Table S2). The moderate catalyst acidity based on the strength and quantity is positive for the hydrogenation and rearrangement of FAL to CPO.
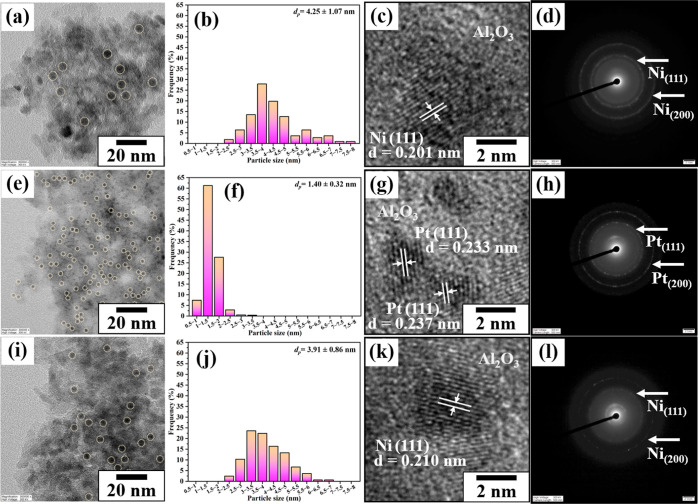
3.1.5. TEM Results
The TEM results of reduced Ni1Pt0.007/Al2O3 were compared with those of Ni/Al2O3 and Pt/Al2O3 to observe the particle size and distribution morphology (Figure 5). Metal particles were detected as dark spots on Al2O3 with different metal particle sizes. The average particle size of Ni/Al2O3 is 4.25 ± 1.07 nm (Figure 5a,b), while Pt/Al2O3 exhibits a small metal particle size of 1.40 ± 0.32 nm (Figure 5e,f). The particle size of Ni1Pt0.007/Al2O3 (3.91 ± 0.86 nm) is lower than that of Ni/Al2O3 (Figure 5i,j). Additionally, d-spacing (Figure 5c,k) with the diffraction patterns (Figure 5d,l) of Ni-containing catalysts was obtained via HR-TEM, which indicates the (111) lattice plane of metallic Ni particles with a spacing of 0.201–0.210 nm.32,33 Thus, the formation of Ni0 after reduction by H2 is confirmed. The d-spacing (Figure 5g) of Pt/Al2O3 with the diffraction pattern (Figure 5h) indicates the (111) lattice plane of metallic Pt with a spacing of 0.233–0.237 nm,34−36 indicating the formation of Pt0 after H2 reduction.
Figure 5.
(a,e,i) TEM images, (b,f,j) particle size distribution, and (c,g,k) HR-TEM images with (d,h,l) diffraction patterns of reduced (a–d) Ni/Al2O3, (e–h) Pt/Al2O3, and (i–l) Ni1Pt0.007/Al2O3.
3.2. Evaluation of Catalytic Performance
3.2.1. Effect of the Ni-to-Pt Molar Ratio
The catalytic activity of Ni–Pt/Al2O3 with different molar ratios (1:0.004–1:0.015) of Ni to Pt was investigated and compared with that of Ni/Al2O3 and Pt/Al2O3 at 140 °C, 20 bar H2, and 2 h reaction time (Figure 6). FAL conversion to CPO is <100% in the presence of the examined catalysts, and Ni–Pt/Al2O3 demonstrates superior catalytic performance. The conversion (85%) of FAL catalyzed by Ni/Al2O3 is similar to that by Ni1Pt0.004/Al2O3 and Ni1Pt0.007/Al2O3. An increase in the Ni-to-Pt molar ratio of up to 1:0.015 (Ni1Pt0.015/Al2O3) decreases FAL conversion. The lowest FAL conversion (52.5%) was obtained in the presence of Pt/Al2O3. The highest yield of CPO in the presence of Ni1Pt0.007/Al2O3 is 24.2%, while an 18.3% yield was obtained in the presence of Ni/Al2O3. Thus, a low amount of Pt improves the hydrogenation and rearrangement of FAL to CPO at a low FAL conversion level. Pt/Al2O3 catalyzed the FAL conversion to form CPO in a 5.8% yield with the generation of undesired or intermediate products up to 45%. Thus, the optimal Ni1Pt0.007/Al2O3 catalyst was selected for further investigation.
Figure 6.
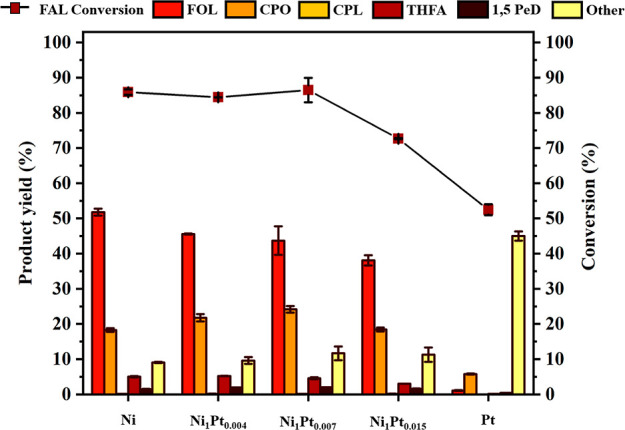
FAL conversion and product yield catalyzed by different catalysts at 140 °C, 20 bar H2, and 2 h reaction time. The catalyst loading was 20 wt % based on the initial mass of FAL. The experiments were conducted using 1 g of FAL feedstock in 40 g of water with 20% loading of the catalyst based on the initial mass of FAL.
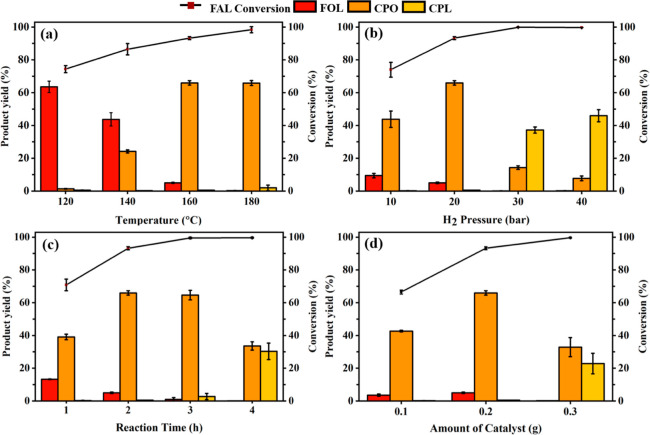
3.2.2. Effect of Operating Conditions
The effects of the reaction temperature, reaction time, H2 pressure, and catalyst loading were systematically investigated to obtain the highest yield of CPO catalyzed by Ni1Pt0.007/Al2O3 (Figure 7). The effect of the reaction temperature in the range of 120–180 °C under constant parameters (20 bar H2 pressure and 2 h reaction time) was explored. Figure 7a shows that FAL conversion increases with the reaction temperature. The CPO yield increased from 120 to 160 °C and remained stable (∼66% yield) at 160 and 180 °C. FOL was formed as an intermediate at low temperatures, which disappeared at high temperatures. Thus, the rearrangement of FAL and hydrogenation to form FOL followed by the formation of CPO are catalyzed at high temperatures. The highest (66%) CPO yield formed via 93% of FAL conversion was obtained at 160 °C. The effect of H2 pressure of 10–40 bar was examined to facilitate the solubility of H2 in an aqueous system at the optimum temperature (160 °C). Figure 7b shows an increase in FAL conversion in the H2 pressure from 10 to 30 bar, which becomes 100% at 40 bar. The maximum CPO yield was achieved at 20 bar. A further increase in the H2 pressure to 30 bar formed CPL via the direct hydrogenation of CPO. The effect of the reaction time was evaluated from 1 to 4 h under optimized 20 bar H2 pressure at 160 °C (Figure 7c). The highest FAL conversion and CPO yield were obtained by increasing the reaction time. At the initial stage (1–2 h), FA was preliminarily generated via direct FAL hydrogenation. An extended reaction time of 2–3 h induced the rearrangement of FOL to produce CPO, which further produced CPL by the hydrogenation of the C=O group of CPO by increasing the reaction time to 4 h. The catalyst amount was varied from 0.1 to 0.3 g to obtain 10, 20, and 30% catalyst loading based on the initial mass of FAL (Figure 7d). The highest FAL conversion and CPO yield were obtained with a catalyst loading of 20% of FAL feedstock. Increasing catalyst loading to 30% formed CPL via CPO hydrogenation. The maximum yield of CPO was 66% with 93% FAL conversion in the optimal conditions of 20% catalyst, 160 °C, 20 bar H2, and 2 h reaction time.
Figure 7.
Effects of the reaction temperature, reaction time, H2 pressure, and catalyst loading of FAL conversion catalyzed by Ni1Pt0.007/Al2O3. Reaction conditions are (a) 20% catalyst, 20 bar of H2 pressure, and 2 h reaction time; (b) 20% catalyst, 160 °C, and 2 h; (c) 20% catalyst, 160 °C, and 20 bar; and (d) 160 °C, 20 bar, and 2 h. The experimental measurements were conducted using 1 g of FAL feedstock in 40 g of water.
3.2.3. Catalyst Reusability
The reusability experiment of the optimal Ni1Pt0.007/Al2O3 was evaluated for three consecutive cycles under the optimal conditions (140 °C, 20 bar H2 pressure, and 2 h reaction time) (Figure S2). The catalyst used was separated from the reaction mixture and reactivated via calcination and reduction. The CPO yield and FAL conversion decreased after three consecutive experiments, indicating that the catalyst was deactivated during the reaction (Figure S2a). The XRD results of the used catalyst show no remarkable alteration of the catalyst structure before and after the reaction (Figure S2b). The used Ni1Pt0.007/Al2O3 was analyzed via TGA under a supply of air to estimate the amount of deposited carbon (Figure S2c). The catalyst lost weight at <200 °C owing to the removal of moisture. The combustion of carbon or an intermediate deposit was detected at >280 °C. The used Ni1Pt0.007/Al2O3 exhibited only 20% weight loss. Therefore, carbon formation is the primary factor for catalyst deactivation during the catalytic hydrogenation and rearrangement of FAL to CPO.
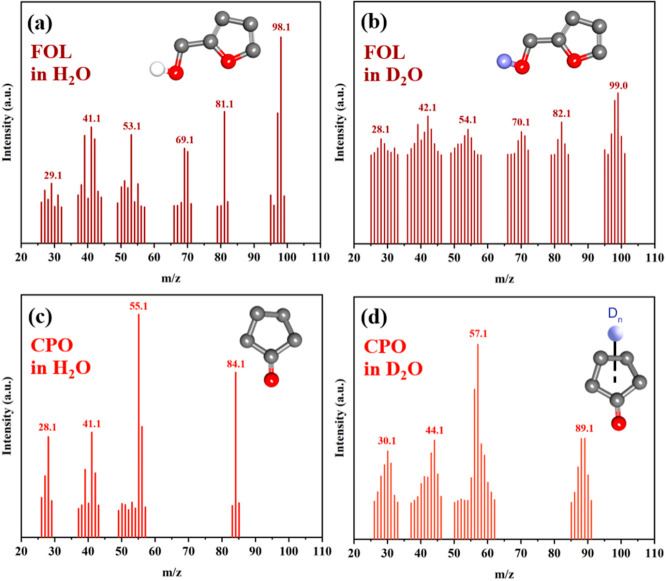
3.3. Isotopic D2O Labeling
The reaction mechanism of the hydrogenation and ring rearrangement of FAL to CPO was investigated via isotopic D2O labeling and was compared with another set of reactions performed in water as the solvent under the optimal conditions (140 °C, 20 bar H2 pressure, and 2 h reaction time). Figure 8a,c shows the GC–MS peaks of FOL (m/z = 98) and CPO (m/z = 84). FOL (m/z = 99) and CPO (m/z = 89) were generated in D2O under H2 pressure (Figure 8b,d), indicating that deuterium (D) was substituted via an isotopic exchange between D from D2O and the H atom in FAL. Additionally, the reaction in an aqueous solution was performed without pressurized H2 gas under the optimized reaction condition to form 8.8% FAL conversion and 0% CPO yield, indicating that the reaction proceeds through the H2–D2O exchange process.
Figure 8.
Mass spectra of FOL and CPO fragmentation patterns during the catalytic hydrogenation and rearrangement of FAL in (a,c) H2O and (b,d) D2O at 140 °C and 20 bar H2 pressure for 2 h. The catalyst loading was 20 wt % based on the initial mass of FAL.
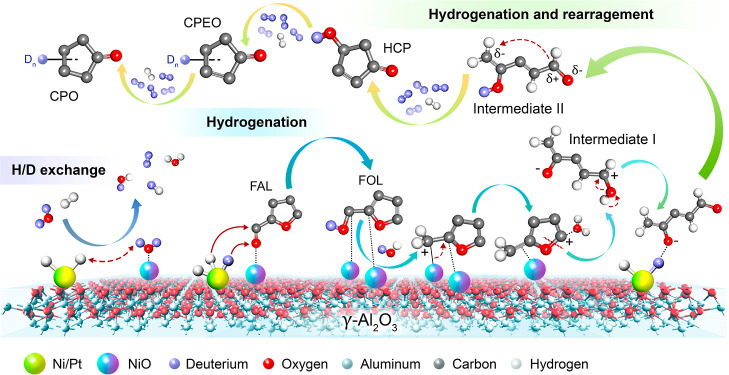
Therefore, the proposed reaction mechanism is as described in Scheme 1.37−40 H2 gas is more preferentially adsorbed and dissociated by metallic active sites than oxophilic active sites.23,28,41 The XRD and XANES results indicate the coexistence of Ni0/NiO states and metallic Pt0 as prominent valence states after the reduction of Ni–Pt/Al2O3 by hydrogen. The adsorption and dissociation of H2 molecules occur at the metallic sites of Ni0/Pt0, whereas the oxophilic NiO sites are crucial for the C=O and C–OH adsorption of FAL and FOL, respectively. The H2–D2O exchange generates the minor products of HD, D2, HOD, and H2O.37 H2 or D2 is dissociated on the metallic sites of Ni0/Pt0 during hydrogenation to generate H or D atoms. The C=O group of FAL is preferentially adsorbed by the NiO sites. D or H atoms adsorbed by the metallic sites are transferred to the carbonyl group of FAL to produce FOL via direct hydrogenation. FOL is adsorbed by oxophilic NiO sites and forms a FOL carbocation with water. The carbocation rearranges via nucleophilic attack by water to generate Intermediate I. The oxygen anion of Intermediate I is attacked by H/D atoms to form Intermediate II. The ring closure of Intermediate II via 4π-conrotatory cyclization forms 4-hydroxy-2-cyclopentenone (HCP) after hydrogenation. 2-Cyclopentenone (CPEO) is generated via dehydration. Finally, the hydrogenation of CPEO generates CPO under a H2 or D2 atmosphere.42−44 The generated CPO in D2O shows an increased mass/charge ratio (m/z) than that in water, confirming the transfer of D atoms from D2O to intermediates and products during hydrogenation and rearrangement processes. This investigation confirms that water is a reactive solvent for the rearrangement process and a hydrogen source for the reaction.
Scheme 1. Schematic Representation of the Proposed Reaction Mechanism for the Catalytic Hydrogenation and Rearrangement of FAL into CPO Catalyzed by Ni–Pt/Al2O3 under Atmospheric D2O Solvent.
4. Conclusions
A low Pt-loaded Ni–Pt/Al2O3 catalyst with different Ni-to-Pt molar ratios was successfully prepared via an impregnation method. Ni–Pt/Al2O3 catalyzed the hydrogenation of FAL to CPO in water, and the catalytic activity was compared with that of Ni/Al2O3 and Pt/Al2O3. The coexistence of Ni0/Pt0 and Ni2+/Pt4+ generated after reduction by H2 was elucidated via XRD and XANES. The H2 spillover effect from Pt to Ni was promoted after the addition of a low amount of Pt to Ni/Al2O3, as confirmed via H2-TPR. The addition of a low amount of Pt to Ni reduced the H2 desorption ability of Ni–Pt/Al2O3 than those of Ni/Al2O3 and Pt/Al2O3. The catalyst acidity decreased after the incorporation of Pt to Ni/Al2O3. Additionally, the particle size of Ni–Pt/Al2O3 was smaller than that of Ni/Al2O3. The maximum yield of CPO was 66% with 93% FAL conversion under the optimal conditions (160 °C, 20 bar H2, and 2 h) catalyzed by 20% of Ni1Pt0.007/Al2O3. Isotopic D2O labeling showed the transfer of D atoms in D2O to the intermediates and products during the hydrogenation and rearrangement processes, confirming that water was the solvent for rearrangement and H was the source for the reaction.
Acknowledgments
This research project is supported by the Mahidol University [Fundamental Fund: fiscal year 2024 by the National Science Research and Innovation Fund (NSRF)]. The Mahidol University under an International Postdoctoral Fellowship 2024 is additionally acknowledged.
Data Availability Statement
All data related to the findings of this study are accessible from the corresponding author Atthapon Srifa on reasonable request.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.4c02827.
Schematic illustration of catalyst preparation and the experimental setup; operating conditions of GC equipped with a FID; relative acidity distribution of reduced catalysts; N2 adsorption and desorption isotherms and pore size distribution; reusability experiment with XRD patterns; and TGA of reduced and spent catalysts (PDF)
Author Contributions
A.K.: investigation, validation, and writing—original draft; I.A.: validation and writing—review & editing; W.P.: validation; S.R., W.C., and W.K.: resources; W.K. and W.L.: validation and supervision; S.A.: writing—review & editing; A.S.: conceptualization, methodology, writing—review & editing, visualization, and funding acquisition.
The authors declare no competing financial interest.
Supplementary Material
References
- Li X.; Zhang L.; Zhou R.; Chen S.; Wang J.; Zeng Z.; Zou J.-J.; Deng S.; Deng Q. Bifunctional Role of Hydrogen in Aqueous Hydrogenative Ring Rearrangement of Furfurals over Co@Co-NC. ACS Sustainable Chem. Eng. 2022, 10 (22), 7321–7329. 10.1021/acssuschemeng.2c01080. [DOI] [Google Scholar]
- Tong Z.; Li X.; Dong J.; Gao R.; Deng Q.; Wang J.; Zeng Z.; Zou J.-J.; Deng S. Adsorption Configuration-Determined Selective Hydrogenative Ring Opening and Ring Rearrangement of Furfural over Metal Phosphate. ACS Catal. 2021, 11 (11), 6406–6415. 10.1021/acscatal.0c05497. [DOI] [Google Scholar]
- Feng Y.; Li Z.; Long S.; Sun Y.; Tang X.; Zeng X.; Lin L. Direct conversion of biomass derived l-rhamnose to 5-methylfurfural in water in high yield. Green Chem. 2020, 22 (18), 5984–5988. 10.1039/D0GC02105A. [DOI] [Google Scholar]
- Li H.; Zhong Y.; Wang L.; Deng Q.; Wang J.; Zeng Z.; Cao X.; Deng S. Functionalized metal-organic frameworks with strong acidity and hydrophobicity as an efficient catalyst for the production of 5-hydroxymethylfurfural. Chin. J. Chem. Eng. 2021, 33, 167–174. 10.1016/j.cjche.2020.09.018. [DOI] [Google Scholar]
- Nilaphai O.; Thepwatee S.; Kaeopookum P.; Chuaitammakit L. C.; Wongchaichon C.; Rodjang O.; Pudsong P.; Singhapon W.; Burerat T.; Kamtaw S.; et al. Synthesis of 5-(Hydroxymethyl)furfural Monoesters and Alcohols as Fuel Additives toward Their Performance and Combustion Characteristics in Compression Ignition Engines. ACS Omega 2023, 8 (19), 17327–17336. 10.1021/acsomega.3c02385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomishige K.; Nakagawa Y.; Tamura M. Selective hydrogenolysis and hydrogenation using metal catalysts directly modified with metal oxide species. Green Chem. 2017, 19 (13), 2876–2924. 10.1039/C7GC00620A. [DOI] [Google Scholar]
- Deng Q.; Gao R.; Li X.; Wang J.; Zeng Z.; Zou J.-J.; Deng S. Hydrogenative Ring-Rearrangement of Biobased Furanic Aldehydes to Cyclopentanone Compounds over Pd/Pyrochlore by Introducing Oxygen Vacancies. ACS Catal. 2020, 10 (13), 7355–7366. 10.1021/acscatal.0c01666. [DOI] [Google Scholar]
- Zhang G.-S.; Zhu M.-M.; Zhang Q.; Liu Y.-M.; He H.-Y.; Cao Y. Towards quantitative and scalable transformation of furfural to cyclopentanone with supported gold catalysts. Green Chem. 2016, 18 (7), 2155–2164. 10.1039/C5GC02528A. [DOI] [Google Scholar]
- Khemthong P.; Yimsukanan C.; Narkkun T.; Srifa A.; Witoon T.; Pongchaiphol S.; Kiatphuengporn S.; Faungnawakij K. Advances in catalytic production of value-added biochemicals and biofuels via furfural platform derived lignocellulosic biomass. Biomass Bioenergy 2021, 148, 106033. 10.1016/j.biombioe.2021.106033. [DOI] [Google Scholar]
- Weerachawanasak P.; Krawmanee P.; Inkamhaeng W.; Cadete Santos Aires F. J.; Sooknoi T.; Panpranot J. Development of bimetallic Ni-Cu/SiO2 catalysts for liquid phase selective hydrogenation of furfural to furfuryl alcohol. Catal. Commun. 2021, 149, 106221. 10.1016/j.catcom.2020.106221. [DOI] [Google Scholar]
- Niu H.; Cheng Y.; Li C.; Li S.; Luo J.; Liang C. Construction of Cu-M-Ox (M = Zn or Al) Interface in Cu Catalysts for Hydrogenation Rearrangement of Furfural. Ind. Eng. Chem. Res. 2021, 60 (47), 16939–16950. 10.1021/acs.iecr.1c03429. [DOI] [Google Scholar]
- Mironenko R. M.; Belskaya O. B.; Talsi V. P.; Likholobov V. A. Mechanism of Pd/C-catalyzed hydrogenation of furfural under hydrothermal conditions. J. Catal. 2020, 389, 721–734. 10.1016/j.jcat.2020.07.013. [DOI] [Google Scholar]
- Yang Y.; Du Z.; Huang Y.; Lu F.; Wang F.; Gao J.; Xu J. Conversion of furfural into cyclopentanone over Ni-Cu bimetallic catalysts. Green Chem. 2013, 15 (7), 1932–1940. 10.1039/c3gc37133f. [DOI] [Google Scholar]
- Dutta S.; Bhat N. S. Catalytic Transformation of Biomass-Derived Furfurals to Cyclopentanones and Their Derivatives: A Review. ACS Omega 2021, 6 (51), 35145–35172. 10.1021/acsomega.1c05861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.; Woo J.; Nguyen-Huy C.; Lee M. S.; Joo S. H.; An K. Highly dispersed Pd catalysts supported on various carbons for furfural hydrogenation. Catal. Today 2020, 350, 71–79. 10.1016/j.cattod.2019.06.032. [DOI] [Google Scholar]
- Hronec M.; Fulajtarová K.; Liptaj T. Effect of catalyst and solvent on the furan ring rearrangement to cyclopentanone. Appl. Catal., A 2012, 437–438, 104–111. 10.1016/j.apcata.2012.06.018. [DOI] [Google Scholar]
- Li J.; Wang Z.; Ma Y.; Xu C.; Zhou S. Synthesis of Mesoporous Silica-Supported NiCo Bimetallic Nanocatalysts and Their Enhanced Catalytic Hydrogenation Performance. ACS Omega 2023, 8 (13), 12339–12347. 10.1021/acsomega.3c00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Z.; Xie A.; Chen C.; Zou Z.; Shen Y.; Fu Z.; Zhang Y.; Zhang H.; Zhao H.; Wang G. Facile synthesis of N, P co-doped carbon encapsulated Ni catalyst for green production of cyclopentanone from biomass derivative furfural. Fuel 2022, 319, 123815. 10.1016/j.fuel.2022.123815. [DOI] [Google Scholar]
- Chen S.; Qian T.-T.; Ling L.-L.; Zhang W.; Gong B.-B.; Jiang H. Hydrogenation of Furfural to Cyclopentanone under Mild Conditions by a Structure-Optimized Ni-NiO/TiO2 Heterojunction Catalyst. ChemSusChem 2020, 13 (20), 5507–5515. 10.1002/cssc.202001424. [DOI] [PubMed] [Google Scholar]
- Hronec M.; Fulajtarová K. Selective transformation of furfural to cyclopentanone. Catal. Commun. 2012, 24, 100–104. 10.1016/j.catcom.2012.03.020. [DOI] [Google Scholar]
- Sun Y.; Xiong C.; Liu Q.; Zhang J.; Tang X.; Zeng X.; Liu S.; Lin L. Catalytic Transfer Hydrogenolysis/Hydrogenation of Biomass-Derived 5-Formyloxymethylfurfural to 2, 5-Dimethylfuran Over Ni-Cu Bimetallic Catalyst with Formic Acid As a Hydrogen Donor. Ind. Eng. Chem. Res. 2019, 58 (14), 5414–5422. 10.1021/acs.iecr.8b05960. [DOI] [Google Scholar]
- Kalong M.; Hongmanorom P.; Ratchahat S.; Koo-amornpattana W.; Faungnawakij K.; Assabumrungrat S.; Srifa A.; Kawi S. Hydrogen-free hydrogenation of furfural to furfuryl alcohol and 2-methylfuran over Ni and Co-promoted Cu/γ-Al2O3 catalysts. Fuel Process. Technol. 2021, 214, 106721. 10.1016/j.fuproc.2020.106721. [DOI] [Google Scholar]
- Chuseang J.; Nakwachara R.; Kalong M.; Ratchahat S.; Koo-amornpattana W.; Klysubun W.; Khemthong P.; Faungnawakij K.; Assabumrungrat S.; Itthibenchapong V.; Srifa A. Selective hydrogenolysis of furfural into fuel-additive 2-methylfuran over a rhenium-promoted copper catalyst. Sustainable Energy Fuels 2021, 5 (5), 1379–1393. 10.1039/D1SE00036E. [DOI] [Google Scholar]
- Li K.; Jiao Y.; Yang Z.; Zhang J. A comparative study of Ni/Al2O3-SiC foam catalysts and powder catalysts for the liquid-phase hydrogenation of benzaldehyde. J. Mater. Sci. Technol. 2019, 35 (1), 159–167. 10.1016/j.jmst.2018.09.018. [DOI] [Google Scholar]
- Zhang Y.; Zhou Y.; Wang Q.; Shi J.; Peng C.; He L.; Shi L. Manipulating catalytic activity and durability of Pt-modified Cu-Fe-La/γ-Al2O3 ternary catalyst for catalytic wet air oxidation: effect of calcination temperature. RSC Adv. 2018, 8 (1), 547–556. 10.1039/C7RA11899F. [DOI] [Google Scholar]
- Cen B.; Wang W.; Zhao P.; Liu C.; Chen J.; Lu J.; Luo M. Revealing the Different Roles of Sulfates on Pt/Al2O3 Catalyst for Methane and Propane Combustion. Catal. Lett. 2022, 152 (3), 863–871. 10.1007/s10562-021-03675-9. [DOI] [Google Scholar]
- Ho P. H.; Woo J.-W.; Feizie Ilmasani R.; Han J.; Olsson L. The role of Pd-Pt Interactions in the Oxidation and Sulfur Resistance of Bimetallic Pd-Pt/γ-Al2O3 Diesel Oxidation Catalysts. Ind. Eng. Chem. Res. 2021, 60 (18), 6596–6612. 10.1021/acs.iecr.0c05622. [DOI] [Google Scholar]
- Kalong M.; Srifa A.; Ratchahat S.; Koo-amornpattana W.; Poo-arporn Y.; Limphirat W.; Khemthong P.; Assabumrungrat S.; Tomishige K.; Kawi S. Continuous flow hydrogenolysis of 5-hydroxymethylfurfural into 2,5-dimethylfuran over alumina-supported nickel-iron alloy catalysts. Sustainable Energy Fuels 2023, 7 (4), 934–948. 10.1039/D2SE01683D. [DOI] [Google Scholar]
- Srifa A.; Kaewmeesri R.; Fang C.; Itthibenchapong V.; Faungnawakij K. NiAl2O4 spinel-type catalysts for deoxygenation of palm oil to green diesel. Chem. Eng. J. 2018, 345, 107–113. 10.1016/j.cej.2018.03.118. [DOI] [Google Scholar]
- Yang W.; Feng Y.; Chu W. Promotion Effect of CaO Modification on Mesoporous Al2O3-Supported Ni Catalysts for CO2 Methanation. Int. J. Chem. Eng. 2016, 2016, 2041821. 10.1155/2016/2041821. [DOI] [Google Scholar]
- Liang N.; Zhang X.; An H.; Zhao X.; Wang Y. Direct synthesis of 2-ethylhexanol via n-butanal aldol condensation-hydrogenation reaction integration over a Ni/Ce-Al2O3 bifunctional catalyst. Green Chem. 2015, 17 (5), 2959–2972. 10.1039/C5GC00223K. [DOI] [Google Scholar]
- Chen T.; Bian S.; Yang X.; Lu W.; Wang K.; Guo Y.; Zhang C.; Zhang Q. A hollow urchin-like metal-organic framework with Ni-O-cluster SBUs as a promising electrode for an alkaline battery-supercapacitor device. Inorg. Chem. Front. 2023, 10 (8), 2380–2386. 10.1039/D3QI00123G. [DOI] [Google Scholar]
- Chen T.; Deng F.; Zhu J.; Chen C.; Sun G.; Ma S.; Yang X. Hexagonal and cubic Ni nanocrystals grown on graphene: phase-controlled synthesis, characterization and their enhanced microwave absorption properties. J. Mater. Chem. 2012, 22 (30), 15190–15197. 10.1039/c2jm31171b. [DOI] [Google Scholar]
- Ayad M. M.; Torad N. L.; El-Nasr A. A.; Amer W. A. Study on catalytic efficiency of platinum and silver nanoparticles confined in nanosized channels of a 3-D mesostructured silica. J. Porous Mater. 2021, 28 (1), 65–79. 10.1007/s10934-020-00960-7. [DOI] [Google Scholar]
- Zienkiewicz-Strzałka M.; Pikus S. Microstructure characterization of noble metal-silica nanocomposites. Acta Phys. Pol., A 2016, 130 (4), 972–974. 10.12693/APhysPolA.130.972. [DOI] [Google Scholar]
- Scofield M. E.; Koenigsmann C.; Bobb-Semple D.; Tao J.; Tong X.; Wang L.; Lewis C. S.; Vukmirovic M. B.; Zhu Y.; Adzic R. R.; et al. Correlating the chemical composition and size of various metal oxide substrates with the catalytic activity and stability of as-deposited Pt nanoparticles for the methanol oxidation reaction. Catal. Sci. Technol. 2016, 6 (7), 2435–2450. 10.1039/c5cy01444a. [DOI] [Google Scholar]
- Cai W.; Li Y.; Zheng Q.; Song M.; Ma P.; Fang W.; Song W.; Lai W. Hydrogenative rearrangement of bioderived furfurals to cyclopentanones over Ni/Nb2O5 catalysts: Promotion effect of reducible NbOx and water. Fuel 2023, 338, 127345. 10.1016/j.fuel.2022.127345. [DOI] [Google Scholar]
- Kadam V. M.; Yadav G. D. Development of a Green Process for the Synthesis of Cyclopentanone Using Selective Aqueous Phase Hydrogenation of Furfural over Ni-Cu@MOF-5 Catalyst. Ind. Eng. Chem. Res. 2023, 62 (43), 17408–17427. 10.1021/acs.iecr.3c01203. [DOI] [Google Scholar]
- Date N. S.; Kondawar S. E.; Chikate R. C.; Rode C. V. Single-Pot Reductive Rearrangement of Furfural to Cyclopentanone over Silica-Supported Pd Catalysts. ACS Omega 2018, 3 (8), 9860–9871. 10.1021/acsomega.8b00980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X.; Deng Q. Review on Metal-Acid Tandem Catalysis for Hydrogenative Rearrangement of Furfurals to C5 Cyclic Compounds. Trans. Tianjin Univ. 2023, 29 (5), 347–359. 10.1007/s12209-023-00367-w. [DOI] [Google Scholar]
- Srifa A.; Kalong M.; Praikaew W.; Ratchahat S.; Chaiwat W.; Koo-Amornpattana W.; Klysubun W.; Limphirat W.; Assabumrungrat S.; Kawi S. Regulation of Pt Loading on Co/Al2O3 Catalysts for Selective Hydrogenation and Hydrogenolysis of 5-Hydroxymethylfurfural to 2,5-Bis(hydroxymethyl)furan and 2,5-Dimethylfuran. ChemCatChem 2024, 16 (5), e202301360 10.1002/cctc.202301360. [DOI] [Google Scholar]
- Pino N.; Hincapie G. M.; López D. P. Hydrodeoxygenation of furfuryl alcohol over Cu/MgAl and Cu/ZnAl catalysts derived from hydrotalcite-like precursors: A reaction for bio-oil upgrading. Ing. Invest. 2017, 37 (1), 34–42. 10.15446/ing.investig.v37n1.57991. [DOI] [Google Scholar]
- Shen T.; Hu R.; Zhu C.; Li M.; Zhuang W.; Tang C.; Ying H. J. R. a. Production of cyclopentanone from furfural over Ru/C with Al11.6 PO23.7 and application in the synthesis of diesel range alkanes. RSC Adv. 2018, 8 (66), 37993–38001. 10.1039/c8ra08757a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verrier C.; Moebs-Sanchez S.; Queneau Y.; Popowycz F. J. O.; Chemistry B. The Piancatelli reaction and its variants: recent applications to high added-value chemicals and biomass valorization. Org. Biomol. Chem. 2018, 16 (5), 676–687. 10.1039/c7ob02962d. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data related to the findings of this study are accessible from the corresponding author Atthapon Srifa on reasonable request.