Abstract
We report here the first demonstration of dengue virus infection and vasoactive cytokine response of a cell of the mast cell/basophil lineage. Infection of KU812 cells was dependent on dengue-specific antibody and gave rise to infectious virions. This antibody-enhanced dengue virus infection triggered a four- to fivefold increase in the release of interleukin-1β (IL-1β) and a modest increase for IL-6 but not for an alternate cytokine, granulocyte-macrophage colony-stimulating factor. The results suggest a potential role for mast cells/basophils in the pathogenesis of dengue virus-induced disease.
Dengue virus infection is associated with disease, ranging from dengue fever to dengue hemorrhagic fever (DHF) and/or dengue shock syndrome (DSS). Host immunological factors play a role in DHF and/or DSS, since severe disease occurs most often in individuals experiencing secondary dengue virus infections (42). Subneutralizing levels of dengue-specific antibodies have been shown to potentiate dengue virus infection via antibody-dependent enhancement (16). Antibody-enhanced dengue infection of monocytes (17, 18) stimulates the production of cytokines, such as tumor necrosis factor alpha (TNF-α), which act on endothelium (2). Specific cytokines are also found to be elevated in sera from patients with DHF and/or DSS (12, 13, 23, 24, 41, 49, 52).
The role of mast cells/basophils has not yet been explored with regard to dengue pathogenesis. Mast cells play an important role in inflammation (reviewed in reference 30) and in the host defense against foreign pathogens (9, 10, 28). These cells mediate immune responses by selective production and secretion of a variety of soluble mediators including chemokines, vasoactive cytokines, such as interleukin-1β (IL-1β), IL-6, and TNF-α (5, 7, 11, 32–34), lipid mediators, and granule-associated products (44). Mast cells reside mainly in the tissues and associate closely with blood vessels (1, 3, 36, 40, 45, 46) and nerves (35, 48, 51), while basophils normally circulate in the blood. Mast cell activation is closely linked with local increases in vascular permeability in allergic disease.
Mast cells/basophils express both FcɛRI (the high-affinity human immunoglobulin E [IgE] receptor) and some Fcγ (14, 47, 50) receptors. As such, they are potential targets for antibody-enhanced virus infection as well as for the consequent induction of powerful vasoactive cytokines. We therefore sought to investigate the human mast cell/basophil KU812 cell line with respect to dengue virus susceptibility and concomitant vasoactive cytokine responses.
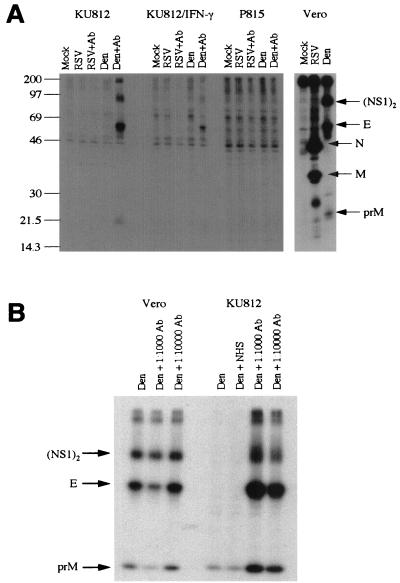
Human mast cell/basophil KU812 cells, maintained in RPMI 1640 (Life Technologies, Grand Island, N.Y.) supplemented with 10% fetal calf serum, 10 mM HEPES, 100 U of penicillin/ml, and 100 μg of streptomycin (Life Technologies)/ml and, where noted, treated for 8 days with 0.3 mM sodium butyrate and 40 ng of gamma interferon (IFN-γ) (R&D Systems, Minneapolis, Minn.) /ml, and P815 mouse mastocytoma cells were mock inoculated or inoculated with either dengue virus or respiratory syncytial virus (RSV) (an unrelated virus) in the presence or absence of corresponding human immune serum (1:1,000 final dilution). Cultures were incubated at 37°C and radiolabeled with [35S]methionine-cysteine (NEN, Mississauga, Ontario, Canada) from 24 h postinfection for 3 to 4 h followed by 12 to 14 h chase. Cell supernatants were harvested and immunoprecipitated with dengue virus immune sera and protein A-bearing, formalin-fixed Staphylococcus aureus as previously described (20). Immunoprecipitates were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (25) using 10% polyacrylamide gels. Gels were impregnated with 1 M sodium salicylate and fluorographed by exposure to Kodak X-ray film at −70°C. As shown in Fig. 1A, both undifferentiated and sodium butyrate- and IFN-γ-differentiated KU812 cells were permissive to dengue virus infection in the presence of human dengue virus immune sera (even though treatment with sodium butyrate and IFN-γ reduced the level of infection, possibly due to the antiviral properties of residual IFN-γ). In contrast, the mouse mastocytoma P815 cells were much less permissive to dengue virus infection, with or without human dengue virus immune serum (Fig. 1A). P815 cells have been previously shown to be susceptible to antibody-enhanced dengue virus infection (27). However, our data show clearly that KU812 cells are superior to P815 cells in their permissiveness to antibody-enhanced dengue virus infection. No antibody-enhanced infection of RSV was observed. Dengue virus-infected KU812 cells produced infectious virions by 24 h postinfection which began to decline at 72 h postinfection (Fig. 2). A requirement for human dengue virus immune serum (rather than normal human serum) in enhanced dengue virus infection of KU812 cells is shown in Fig. 1B. As expected, Vero cells showed infection with dengue virus alone which was not subject to antibody enhancement (Fig. 1B).
FIG. 1.
Antibody-enhanced dengue virus infection of KU812 cells. (A) Cultures of Vero, P815, or KU812 cells were inoculated with dengue type 2 virus strain 16681 (19) (multiplicity of infection [MOI], 0.1), RSV (MOI, 0.1), or combinations of either virus with the respective human immune serum (final dilution, 1:1000). Virus infection was monitored by radiolabeling with [35S]methionine-cysteine, followed by immunoprecipitation and fluorographic sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The positions of dengue virus proteins (NS1)2, E, and prM and of RSV proteins N and M are indicated. (B) Vero cells (positive control) and KU812 cells were inoculated with dengue virus alone (Den) (MOI, 0.2), and normal human serum (NHS) (final dilution, 1:1,000) or dengue virus and human dengue virus immune sera (Ab; final dilutions of 1:1,000 or 1:10,000). The positions of the radiolabeled (NS1)2 and E proteins are indicated. Data are representative of nine separate experiments. Human sera, described in reference 20, were obtained from patients convalescing from dengue virus type 2 infection; normal human AB sera and RSV-positive sera were obtained from volunteer donors.
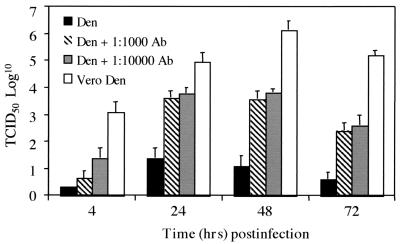
FIG. 2.
Infectious virion production in dengue virus-infected KU812 and Vero cells. Infection conditions were as described in the legend to Fig. 1 (MOI, 0.1 to 0.3). Virion production was assessed by a 50% tissue culture infective dose assay (43) on Vero cells at 4, 24, 48, and 72 h postinfection. Data are representative of three separate experiments and are expressed as the mean ± the standard error of the mean.
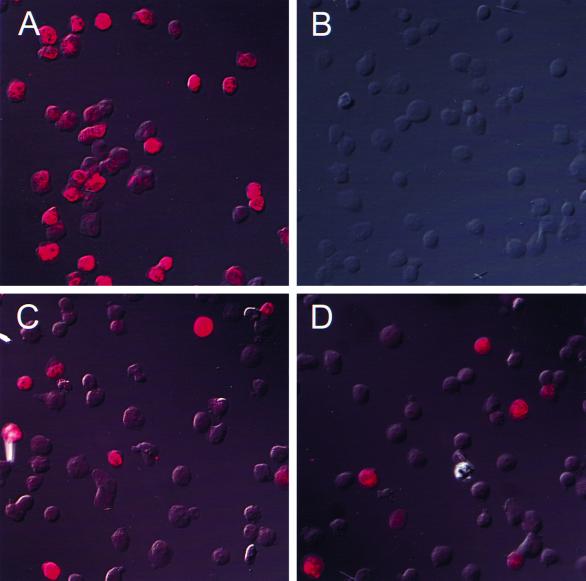
Fluorescence microscopy was used to investigate the number of dengue virus-infected cells. Vero cells inoculated with dengue virus and KU812 cells inoculated with dengue virus, dengue virus and normal human serum, or dengue virus and human dengue virus immune serum combinations were harvested 24 h postinfection and examined for virus antigen expression by fluorescence microscopy (Fig. 3). Cells were fixed with 4% paraformaldehyde, washed, resuspended in 10% dimethyl sulfoxide (DMSO) in phosphate-buffered saline and frozen at −80°C. Following permeabilization with 0.1% saponin for 1 h at room temperature, samples were washed and resuspended in 1% bovine serum albumin (BSA)-0.2% sodium azide in phosphate-buffered saline. Mouse anti-dengue monoclonal antibody 1B7 (22) or isotype-matched mouse anti-IgG2a antibody (negative control) was employed as a primary antibody and incubated on ice for 1 h. Subsequently, samples were washed and incubated with goat anti-mouse fluorescein isothiocyanate (FITC)- or Texas Red-labeled antibody for 1 h on ice. Cytospin preparations were made of each sample and viewed via fluorescence microscopy to assess the number of infected cells. A total of 1,000 cells was counted under UV illumination with the number of positive green (fluorescein isothiocyanate) or red (Texas Red) fluorescent cells recorded to give the percentage of cells infected with dengue virus. Vero cells inoculated with dengue virus alone were 30% fluorescence positive at 24 h (Fig. 3A). KU812 cells showed no virus-positive cells when inoculated with dengue virus either alone or in combination with normal human sera (Fig. 3B). However, KU812 cells inoculated with dengue virus in combination with human dengue virus immune serum at a dilution of 1:1,000 showed an infection rate of 0.6 to 11% (0.6, 11.0, and 5.5% in three experiments) (Fig. 3C). KU812 cells inoculated with dengue virus and human dengue virus immune serum at a 1:10,000 dilution showed an infection rate of 2 to 14% (2.4, 14.4, and 10.5%) (Fig. 3D). All isotype staining controls were negative.
FIG. 3.
Immunofluorescence of dengue virus-infected KU812 cells. Vero and KU812 cells inoculated with dengue virus (MOI, 0.1 to 0.3) or dengue virus-antiserum combinations were harvested 24 h postinfection. Dengue virus-infected Vero cells (A), KU812 cells inoculated with dengue virus and normal human serum (1:1,000 final dilution) (B), antibody-enhanced dengue virus-infected KU812 cells with a 1:1,000 final dilution of human dengue virus immune serum (C), and antibody-enhanced dengue virus-infected KU812 cells with a 1:10,000 final dilution of human dengue virus immune serum (D) are shown. The data are representative of three separate experiments.
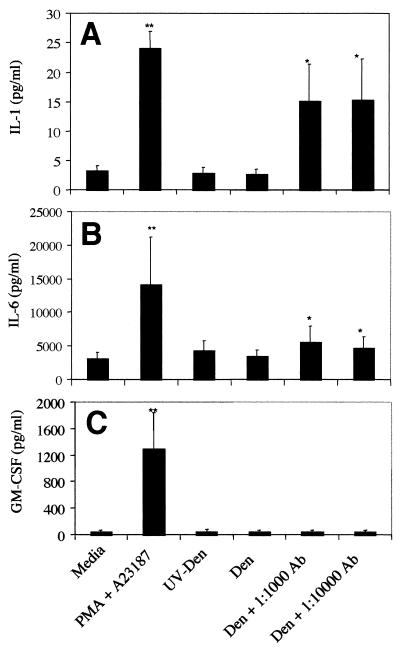
To analyze cytokine production in response to antibody-enhanced dengue virus infection of KU812 cells, supernatants were harvested from KU812 cultures at 72 h. IL-1β production was significantly enhanced in supernatants obtained from dengue virus-infected cultures, as analyzed by enzyme-linked immunosorbent assay (ELISA) using a matched antibody pair (IL-1β M-412B-E, M-420B-B; Endogen, Woburn, Mass.) (Fig. 4A). IL-6 was found to follow a similar, though less dramatic, pattern of enhanced production (Fig. 4B) with the appropriate ELISA antibody pair (IL-6 M-620-E, M-621-B; Endogen). In contrast to that for both IL-1β and IL-6, granulocyte-macrophage colony-stimulating factor (GM-CSF) production, analyzed by ELISA as previously described (53), was not elevated in antibody-enhanced dengue virus-infected KU812 cell supernatants compared to levels for controls (Fig. 4C). No increased cytokine production (for IL-1β, IL-6, or GM-CSF) was detected in KU812 cells inoculated with UV-inactivated virus–human dengue virus immune serum combinations or dengue virus-normal human serum combinations (data not shown). These data thus indicate that KU812 cells are stimulated by antibody-enhanced dengue virus infection to produce selective vasoactive cytokines.
FIG. 4.
Cytokine responses in dengue virus-infected KU812 cells. (A) IL-1β production assayed at 72 h postinfection by antibody-enhanced dengue virus-infected KU812 cells. Infection of KU812 cells was carried out as described in the legend to Fig. 1 (MOI, 0.1 to 0.3). KU812 cells incubated with media alone (Media) indicates the level of constitutive production; 25 ng of phorbol myristate acetate (PMA)/ml- and 5 × 10−7 M A23187 (calcium ionophore)-treated KU812 cells were used as positive controls. For some experiments, virus was inactivated with UV light (UV-Den) as previously described (2). The data are from six separate experiments with triplicate samples and using one serum sample from a patient convalescing from dengue virus infection, sample 7873. (B) IL-6 production assayed at 72 h postinfection by antibody-enhanced dengue virus-infected KU812 cells. The data are from five separate experiments with triplicate samples. (C) GM-CSF production assayed at 72 h postinfection by antibody-enhanced dengue virus-infected KU812 cells. The data are from three separate experiments with triplicate samples. Significant differences from the dengue-alone samples are indicated by ∗ (P < 0.05) and ∗∗ (P < 0.01). Data are represented as the mean ± the standard error of the mean. Statistical significance was assessed using a nonparametric approach. GM-CSF and IL-1β were initially analyzed using Friedman's test for all data obtained, followed by examination of specific groups using Dunn's multiple comparison test. In view of the large differences between baseline responses for individual experiments, IL-6 data was analyzed using Freidman's test followed by the Wilcoxon signed rank test to compare responses between specific groups. Sensitivity of the IL-6 and IL-1β assays was 1.95 pg/ml; that of the GM-CSF assay was 3 pg/ml.
Two potentially important vasoactive factors, histamine and TNF-α, could not be measured in the dengue virus-KU812 cell system. KU812 cells are relatively poorly granulated and therefore contain little histamine (53). They also release little TNF-α upon appropriate stimulation (unpublished observations). Nevertheless, our findings demonstrate that human KU812 mast cells/basophils undergo antibody-enhanced dengue virus infection and that antibody-enhanced dengue virus infection results in selective vasoactive IL-1β and IL-6 cytokine release. Selective production of the vasoactive cytokines IL-1β and IL-6, by antibody-enhanced dengue virus infection of KU812 cells, may provide additional insights into the pathogenic mechanisms of severe dengue disease. IL-6 is an endogenous pyrogen (21) known to mediate increased endothelial cell permeability (31). In rodent systems, mast cells have been shown to be a much more potent source of IL-6 than other cell types, such as the macrophage (26). Elevated serum levels of IL-6, but not of IL-1β, have been reported in patients with DHF and/or DSS (23). Our finding of increased IL-1β production in antibody-enhanced dengue virus-infected KU812 cells raises the possibility that mast cells/basophils may represent a local source of IL-1β in dengue virus infection. Locally produced IL-1β from dengue virus-infected tissue mast cells that reside in close proximity to blood vessels may then act directly on endothelial cells. IL-1β is recognized to induce fever (4), inflammation, and shock (reviewed in reference 8). IL-1β induces production of IL-6 and TNF-α and activates endothelial cells (reviewed in reference 37), modulating the expression of the adhesion molecules (6, 38, 39) as well as altering endothelial cell morphology (38). Such enhanced adhesion molecule expression may induce inflammatory cell activation and migration with consequent potential vasculitic damage.
Our findings that mast cell/basophil KU812 cells are permissive to dengue virus infection which leads to infectious virion production and vasoactive cytokine production support the hypothesis that mast cells/basophils may contribute to the vascular pathology seen in severe dengue disease. Mast cells are resident tissue cells and are present in large numbers in the skin (29), while basophils comprise approximately 1% of total circulating cells. Mast cells are therefore present at the site of dengue virus infection in the skin, whereas basophils would be accessible to dengue virus in the circulation.
The observation that virus-antibody complexes are much more potent than virus alone in inducing mast cells to produce vasoactive cytokines is consistent with the known epidemiological evidence that preexisting immunity is a risk factor for DHF and/or DSS in human dengue virus infections (15). Our present study employed homotypic (dengue virus type 2) convalescent-phase sera, although antibody-dependent enhancement of dengue virus infection can be readily achieved with either homo- or heterotypic antibodies (15). In attempting to extrapolate our in vitro results to clinical disease, it will be important to determine the relative contributions of different cell types involved in virus amplification and in modulating hemostasis. So far, this has only been achieved for circulating monocytes (42). The contribution to pathogenesis of other cell types, including cells of the basophil/mast cell lineage, is unknown. Nevertheless, the results of the present study support further investigation to eventually identify the full spectrum of cell types which are infected by dengue virus in vivo and which contribute to perturbation of vascular function.
Acknowledgments
This work was supported by the Natural Sciences and Engineering Research Council of Canada and the Medical Research Council of Canada.
We are grateful to B. Innis and A. King of the Walter Reed Army Institute of Research for providing the human dengue virus immune sera used in this study.
REFERENCES
- 1.Alving K. Airways vasodilation in the immediate allergic reaction. Involvement of inflammatory mediators and sensory nerves. Acta Physiol Scand Suppl. 1991;597:1–64. [PubMed] [Google Scholar]
- 2.Anderson R, Wang S, Osiowy C, Issekutz A C. Activation of endothelial cells via antibody-enhanced dengue virus infection of peripheral blood monocytes. J Virol. 1997;71:4226–4232. doi: 10.1128/jvi.71.6.4226-4232.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anton F, Morales C, Aguilar R, Bellido C, Aguilar E, Gaytan F. A comparative study of mast cells and eosinophil leukocytes in the mammalian testis. Zentbl Vetmed A. 1998;45:209–218. doi: 10.1111/j.1439-0442.1998.tb00819.x. [DOI] [PubMed] [Google Scholar]
- 4.Atkins E. Pathogenesis of fever. Physiol Rev. 1960;40:580–646. doi: 10.1152/physrev.1960.40.3.580. [DOI] [PubMed] [Google Scholar]
- 5.Benyon R C, Bissonnette E Y, Befus A D. Tumor necrosis factor-alpha dependent cytotoxicity of human skin mast cells is enhanced by anti-IgE antibodies. J Immunol. 1991;147:2253–2258. [PubMed] [Google Scholar]
- 6.Bevilacqua M P, Pober J S, Mendrick D L, Cotran R S, Gimbrone M A., Jr Identification of an inducible endothelial leukocyte adhesion molecule, ELAM-1. Proc Natl Acad Sci USA. 1987;84:9238–9242. doi: 10.1073/pnas.84.24.9238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bradding P, Feather I H, Wilson S, Bardin P G, Heusser C H, Holgate S T, Howarth P H. Immunolocalization of cytokines in the nasal mucosa of normal and perennial rhinitic subjects. The mast cell as a source of IL-4, IL-5, and IL-6 in human allergic mucosal inflammation. J Immunol. 1993;151:3853–3865. [PubMed] [Google Scholar]
- 8.Dinarello C A. Biologic basis for interleukin-1 in disease. Blood. 1996;87:2095–2147. [PubMed] [Google Scholar]
- 9.Echtenacher B, Mannel D N, Hultner L. Critical protective role of mast cells in a model of acute septic peritonitis. Nature. 1996;381:75–77. doi: 10.1038/381075a0. [DOI] [PubMed] [Google Scholar]
- 10.Galli S J, Maurer M, Lantz C S. Mast cells as sentinels of innate immunity. Curr Opin Immunol. 1999;11:53–59. doi: 10.1016/s0952-7915(99)80010-7. [DOI] [PubMed] [Google Scholar]
- 11.Grabbe J, Welker P, Moller A, Dippel E, Ashman L K, Czarnetzki B M. Comparative cytokine release from human monocytes, monocyte-derived immature mast cells, and a human mast cell line (HMC-1) J Investig Dermatol. 1994;103:504–508. doi: 10.1111/1523-1747.ep12395649. [DOI] [PubMed] [Google Scholar]
- 12.Green S, Vaughn D W, Kalayanarooj S, Nimmannitya S, Suntayakorn S, Nisalak A, Rothman A L, Ennis F A. Elevated plasma interleukin-10 levels in acute dengue correlate with disease severity. J Med Virol. 1999;59:329–334. [PubMed] [Google Scholar]
- 13.Green S, Vaughn D W, Kalayanarooj S, Nimmannitya S, Suntayakorn S, Nisalak A, Lew R, Innis B L, Kurane I, Rothman A L, Ennis F A. Early immune activation in acute dengue illness is related to development of plasma leakage and disease severity. J Infect Dis. 1999;179:755–762. doi: 10.1086/314680. [DOI] [PubMed] [Google Scholar]
- 14.Guo C B, Kagey-Sobotka A, Lichtenstein L M, Bochner B S. Immunophenotyping and functional analysis of purified human uterine mast cells. Blood. 1992;79:708–712. [PubMed] [Google Scholar]
- 15.Halstead S B. Pathogenesis of dengue: challenges to molecular biology. Science. 1988;239:476–481. doi: 10.1126/science.3277268. [DOI] [PubMed] [Google Scholar]
- 16.Halstead S B. Antibody, macrophages, dengue virus infection, shock, and hemorrhage: a pathogenetic cascade. Rev Infect Dis. 1989;11(Suppl. 4):S830–S839. doi: 10.1093/clinids/11.supplement_4.s830. [DOI] [PubMed] [Google Scholar]
- 17.Halstead S B, O'Rourke E J. Dengue viruses and mononuclear phagocytes. I. Infection enhancement by non-neutralizing antibody. J Exp Med. 1977;146:201–217. doi: 10.1084/jem.146.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Halstead S B, Porterfield J S, O'Rourke E J. Enhancement of dengue virus infection in monocytes by flavivirus antisera. Am J Trop Med Hyg. 1980;29:638–642. doi: 10.4269/ajtmh.1980.29.638. [DOI] [PubMed] [Google Scholar]
- 19.Halstead S B, Simasthien P. Observations related to pathogenesis of dengue hemorrhagic fever. II. Antigenic and biologic properties of dengue viruses and their association with disease responses in the host. Yale J Biol Med. 1970;42:276–292. [PMC free article] [PubMed] [Google Scholar]
- 20.He R T, Innis B L, Nisalak A, Usawattanakul W, Wang S, Kalayanarooj S, Anderson R. Antibodies that block virus attachment to Vero cells are a major component of the human neutralizing antibody response against dengue virus type 2. J Med Virol. 1995;45:451–461. doi: 10.1002/jmv.1890450417. [DOI] [PubMed] [Google Scholar]
- 21.Helle M, Brakenhoff J P, De-Groot E R, Aarden L A. Interleukin 6 is involved in interleukin 1-induced activities. Eur J Immunol. 1988;18:957–959. doi: 10.1002/eji.1830180619. [DOI] [PubMed] [Google Scholar]
- 22.Henchal E A, McCown J M, Burke D S, Seguin M C, Brandt W E. Epitopic analysis of antigenic determinants on the surface of dengue-2 virions using monoclonal antibodies. Am J Trop Med Hyg. 1985;34:162–169. doi: 10.4269/ajtmh.1985.34.162. [DOI] [PubMed] [Google Scholar]
- 23.Hober D, Poli L, Roblin B, Gestas P, Chungue E, Granic G, Imbert P, Pecarere J L, Vergez-Pascal R, Wattre P, Maniez-Montreuil M. Serum levels of tumor necrosis factor-alpha (TNF-alpha), interleukin-6 (IL-6) and interleukin-1 beta (IL-1 beta) in dengue-infected patients. Am J Trop Med Hyg. 1993;48:324–331. doi: 10.4269/ajtmh.1993.48.324. [DOI] [PubMed] [Google Scholar]
- 24.Hober D, Nguyen T L, Shen L, Ha D Q, Huong V, Benyoucef S, Nguyen T, Bui T, Loan H, Le B, Bouzidi A, De Groote D, Drouet M, Deubel V, Wattre P. Tumor necrosis factor alpha levels in plasma and whole-blood culture in dengue-infected patients: relationship between virus detection and pre-existing specific antibodies. J Med Virol. 1998;54:210–218. doi: 10.1002/(sici)1096-9071(199803)54:3<210::aid-jmv12>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 25.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 26.Leal-Berumen I, Conlon P, Marshall J S. IL-6 production by rat peritoneal mast cells is not necessarily preceded by histamine release and can be induced by bacterial lipopolysaccharide. J Immunol. 1994;152:5468–5476. [PubMed] [Google Scholar]
- 27.Legrand L F, Hotta H, Hotta S, Homma M. Antibody-mediated enhancement of infection by dengue virus of the P815 murine mastocytoma cell line. Biken J. 1986;29:51–55. [PubMed] [Google Scholar]
- 28.Malaviya R, Ikeda T, Ross E, Abraham S N. Mast cell modulation of neutrophil influx and bacterial clearance at sites of infection through TNF-alpha. Nature. 1996;381:77–80. doi: 10.1038/381077a0. [DOI] [PubMed] [Google Scholar]
- 29.Marshall J S, Ford G P, Bell E B. Formalin sensitivity and differential staining of mast cells in human dermis. Br J Dermatol. 1987;117:29–36. doi: 10.1111/j.1365-2133.1987.tb04087.x. [DOI] [PubMed] [Google Scholar]
- 30.Marshall J S, Bienenstock J. The role of mast cells in inflammatory reactions of the airways, skin, and intestine. Curr Opin Immunol. 1994;6:853–859. doi: 10.1016/0952-7915(94)90004-3. [DOI] [PubMed] [Google Scholar]
- 31.Maruo N, Morita I, Shirao M, Murota S. IL-6 increases endothelial permeability in vitro. Endocrinology. 1992;131:710–714. doi: 10.1210/endo.131.2.1639018. [DOI] [PubMed] [Google Scholar]
- 32.Moller A, Henz B M, Grutzkau A, Lippert U, Aragane Y, Schwarz T, Kruger-Krasagakes S. Comparative cytokine gene expression: regulation and release by human mast cells. Immunology. 1998;93:289–295. doi: 10.1046/j.1365-2567.1998.00425.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moller A, Lippert U, Lessmann D, Kolde G, Hamann K, Welker P, Schadendorf D, Rosenbach T, Luger T, Czarnetzki B M. Human mast cells produce IL-8. J Immunol. 1993;151:3261–3266. [PubMed] [Google Scholar]
- 34.Nilsson G, Svensson V, Nilsson K. Constitutive and inducible cytokine mRNA expression in the human mast cell line HMC-1. Scand J Immunol. 1995;42:76–81. doi: 10.1111/j.1365-3083.1995.tb03628.x. [DOI] [PubMed] [Google Scholar]
- 35.Olsson Y. Mast cells in human peripheral nerve. Acta Neurol Scand. 1971;47:357–368. doi: 10.1111/j.1600-0404.1971.tb07490.x. [DOI] [PubMed] [Google Scholar]
- 36.Pesci A, Majori M, Piccoli M L, Casalini A, Curti A, Franchini D, Gabrielli M. Mast cells in bronchiolitis obliterans organizing pneumonia. Mast cell hyperplasia and evidence for extracellular release of tryptase. Chest. 1996;110:383–391. doi: 10.1378/chest.110.2.383. [DOI] [PubMed] [Google Scholar]
- 37.Pober J S. Cytokine-mediated activation of vascular endothelium: physiology and pathology. Am J Pathol. 1988;133:426–433. [PMC free article] [PubMed] [Google Scholar]
- 38.Pober J S, Lapierre L A, Stolpen A H, Brock T A, Springer T A, Friers W, Bevilacqua M P, Mendrick D L, Gimbrone M A., Jr Activation of cultured human endothelial cells by recombinant lymphotoxin: comparison with tumor necrosis factor and interleukin 1 species. J Immunol. 1987;138:3319–3324. [PubMed] [Google Scholar]
- 39.Pober J S, Bevilacqua M P, Mendrick D L, Lapierre L A, Friers W, Gimbrone M A., Jr Two distinct monokines, interleukin 1 and tumor necrosis factor, each independently induce biosynthesis and transient expression of the same antigen on the surface of cultured human vascular endothelial cells. J Immunol. 1986;136:1680–1687. [PubMed] [Google Scholar]
- 40.Pulimood A B, Mathan M M, Mathan V I. Quantitative and ultrastructural analysis of rectal mucosal mast cells in acute infectious diarrhea. Dig Dis Sci. 1998;43:2111–2116. doi: 10.1023/a:1018875718392. [DOI] [PubMed] [Google Scholar]
- 41.Raghupathy R, Chaturvedi U C, Al-Sayer H, Elbishbishi E A, Agarwal R, Nagar R, Kapoor S, Misra A, Mathur A, Nusrat H, Azizieh F, Khan M A Y, Mustafa A S. Elevated levels of IL-8 in dengue hemorrhagic fever. J Med Virol. 1998;56:280–285. doi: 10.1002/(sici)1096-9071(199811)56:3<280::aid-jmv18>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 42.Rothman A L, Ennis F A. Immunopathogenesis of dengue hemorrhagic fever. Virology. 1999;257:1–6. doi: 10.1006/viro.1999.9656. [DOI] [PubMed] [Google Scholar]
- 43.Russell P K, Buescher E L, McCown J M, Ordonez J. Recovery of dengue viruses from patients during epidemics in Puerto Rico and East Pakistan. Am J Trop Med. 1966;15:573–579. doi: 10.4269/ajtmh.1966.15.573. [DOI] [PubMed] [Google Scholar]
- 44.Schwartz L B, Austen K F. Structure and function of the chemical mediators of mast cells. Prog Allergy. 1984;34:271–321. [PubMed] [Google Scholar]
- 45.Selye H. Mast cells and necrosis. Science. 1966;152:1371–1372. doi: 10.1126/science.152.3727.1371. [DOI] [PubMed] [Google Scholar]
- 46.Selye H, Somogyi A, Mecs I. Influence of mast cells and vasoconstrictors upon vasious acute connective-tissue reactions. Angiologica. 1968;5:172–185. [PubMed] [Google Scholar]
- 47.Sperr W R, Bankl H C, Mundigler G, Klappacher G, Grosschmidt K, Agis H, Simon P, Laufer P, Imhof M, Radaszkiewicz T, Glogar D, Lechner K, Valent P. The human cardiac mast cell: localization, isolation, phenotype, and functional characterization. Blood. 1994;84:3876–3884. [PubMed] [Google Scholar]
- 48.Stead R H, Dixon M F, Branwell N H, Riddell R H, Bienenstock J. Mast cells are closely apposed to nerves in the human gastrointestinal mucosa. Gastroenterology. 1989;97:575–585. doi: 10.1016/0016-5085(89)90627-6. [DOI] [PubMed] [Google Scholar]
- 49.Vitarana T, de Silva H, Withana N, Gunasekera C. Elevated tumour necrosis factor in dengue fever and dengue hemorrhagic fever. Ceylon Med J. 1991;36:63–65. [PubMed] [Google Scholar]
- 50.Wedi B, Lewrick H, Butterfield J H, Kapp A. Human HMC-1 mast cells exclusively express the Fc gamma RII subtype of IgG receptor. Arch Dermatol Res. 1996;289:21–27. doi: 10.1007/s004030050147. [DOI] [PubMed] [Google Scholar]
- 51.Weisner-Menzel L, Schulz B, Vakilzadeh F, Czarnetzki B M. Electron microscope evidence for a direct contact between nerve fibers and mast cells. Acta Dermato-Venereol. 1981;61:465–469. doi: 10.2340/0001555561465469. [DOI] [PubMed] [Google Scholar]
- 52.Yadav M, Kamath K R, Iyngkaran N, Sinniah M. Dengue hemorrhagic fever and dengue shock syndrome: are they tumour necrosis factor-mediated disorders? FEMS Microbiol Immunol. 1991;89:45–50. doi: 10.1111/j.1574-6968.1991.tb04969.x. [DOI] [PubMed] [Google Scholar]
- 53.Zhu F, Gomi K, Marshall J S. Short-term and long-term cytokine release by mouse bone marrow mast cells and the differentiated KU812 cell line are inhibited by Brefeldin A. J Immunol. 1998;161:2541–2551. [PubMed] [Google Scholar]