Abstract
We constructed rRSV/mIL-2, a recombinant respiratory syncytial virus (rRSV) containing the coding sequence of murine interleukin-2 (mIL-2) in a transcription cassette inserted into the G-F intergenic region. The recovered virus (rRSV/mIL-2) expressed high levels (up to 2.8 μg/ml) of mIL-2 in cell culture. Replication of rRSV/mIL-2 in vitro was reduced up to 13.6-fold from that of wild-type (wt) rRSV, an effect that was due to the presence of the foreign insert but was not specific to mIL-2. Replication of the rRSV/mIL-2 virus in the upper and lower respiratory tracts of BALB/c mice was reduced up to 6.3-fold, an effect that was specific to mIL-2. The antibody response, including the levels of RSV-specific serum immunoglobulin G1 (IgG1), IgG2a, IgA, and total IgG, and the level of protective efficacy against wt RSV challenge were not significantly different from those of wt rRSV. Analysis of total pulmonary cytokine mRNA isolated 1 and 4 days following infection with rRSV/mIL-2 revealed elevated levels of mRNA for IL-2, gamma interferon (IFN-γ), IL-4, IL-5, IL-6, IL-10, IL-13, and IL-12 p40 compared to those for wt rRSV. Flow cytometry of total pulmonary mononuclear cells isolated 10 days following infection with rRSV/mIL-2 revealed increased levels of CD4+ T lymphocytes expressing either IFN-γ or IL-4 compared to those of wt rRSV. These elevations in cytokine mRNA or cytokine-expressing CD4+ cells relative to those of wt rRSV-primed animals were not observed following challenge with wt RSV on day 28. Thus, the expression of mIL-2 by rRSV was associated with a modest attenuation of virus growth in vivo, induction of serum antibodies at levels comparable to that of wt rRSV, and transient increases in both the Th1 and Th2 CD4+ lymphocytes and cytokine mRNAs compared to those of wt rRSV.
Human respiratory syncytial virus (RSV) is an enveloped, nonsegmented negative-strand RNA virus of the paramyxovirus family. RSV is the most important viral agent of serious respiratory tract disease in infants and young children worldwide and is an important cause of disease in certain immunocompromised individuals and the elderly (4). A licensed vaccine against RSV is not yet available, although significant progress has been made towards development of a live attenuated vaccine for intranasal administration (5, 26). The single-stranded negative-sense RSV genome is 15.2 kb long and is transcribed by a sequential stop-restart mechanism to yield 10 mRNAs encoding 11 proteins. These include the two major protective and neutralization antigens, namely, the attachment G glycoprotein and the fusion F glycoprotein (4).
Severe RSV disease peaks 2 months after birth, so a pediatric RSV vaccine should be given prior to that time (4–6). However, immune responses in young infants are reduced due to (i) immunologic immaturity and (ii) the immunosuppressive effects of maternally derived, RSV-specific serum immunoglobulin G (IgG) present in infants in that age group. Furthermore, the immunity induced by natural infection with wild-type (wt) RSV typically does not confer solid resistance to reinfection even in adults. For these reasons, it would be highly desirable to develop methods to augment immune responses to an RSV vaccine.
Studies with vaccinia virus recombinants pioneered the strategy of enhancing and manipulating the immune response to the virus by coexpression of one (or more) cytokines from genes inserted into the viral genome (9, 23). This has been explored with other viruses such as simian immunodeficiency virus (13) as well as with plasmid-based vaccines, and coadministration of cytokines with subunit vaccines is also an active area of research (11, 20). We previously showed that the expression of murine interferon gamma (mIFN-γ) by recombinant RSV (rRSV) resulted in attenuation of virus replication in vivo while simultaneously augmenting the immune response (2). In this study, we explored the effects of coexpression of murine interleukin-2 (mIL-2) by RSV. IL-2 is produced by CD4+ and CD8+ T lymphocytes (for reviews, see references 10 and 25). Its pleotropic effects include stimulation of proliferation, cytolytic activity, and cytokine secretion of T lymphocytes and natural killer (NK) cells; stimulation of IL-2-regulated genes, including several chemokine receptors; stimulation of proliferation and antibody secretion by activated B cells; and stimulation of proliferation and activity of cells of the monocyte-macrophage lineage (10, 25).
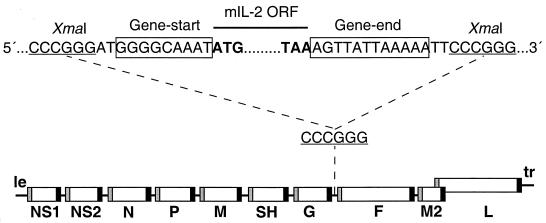
To insert the mIL-2 gene into rRSV, a transcription cassette was made by PCR in which the mIL-2 open reading frame (ORF) was flanked by the RSV gene-start and gene-end transcription signals (Fig. 1). This cassette was inserted into the G-F intergenic region of a complete RSV antigenomic cDNA, increasing its length by 549 nucleotides (nt) from 15,223 to 15,772 nt and the number of encoded mRNAs from 10 to 11. The encoded virus, designated rRSV/mIL-2, was recovered as described previously (3).
FIG. 1.
Map of the genome of rRSV/mIL-2. A cDNA of the mIL-2 ORF, whose translational stop and start codons are in bold type, was modified by PCR to be flanked by RSV-specific gene-start and gene-end transcription signals (boxed) and XmaI sites (underlined). The resulting mIL-2 transcription cassette was inserted into the intergenic region between the G and F genes using an XmaI site which had been placed there previously (1). le, leader region; tr, trailer region.
HEp-2 cells were infected with rRSV/mIL-2 or wt recombinant RSV (wt rRSV), and total intracellular RNA was harvested and analyzed by Northern blot hybridization with individual probes against the mIL-2, G, F, or L gene (results not shown). This confirmed that the IL-2 gene was expressed as a separate mRNA of the expected size. Also, the rRSV/mIL-2 virus expressed small amounts of G–mIL-2 and mIL-2–F readthrough mRNAs and did not express a G-F readthrough mRNA, changes that are consistent with the change in gene order due to the inserted gene. The overall amounts of intracellular viral mRNA that accumulated in cells infected with wt rRSV or in cells infected rRSV/mIL-2 were similar, although there appeared to be a small (two- to fourfold) decrease in the accumulation of mRNAs representing genes downstream of the IL-2 insert (data not shown). We are presently comparing a number of rRSVs containing different-sized foreign genes to determine the effect of gene insertion on transcription. However, it is evident that, with small inserts such as mIL-2, the effect on RNA synthesis is not great.
The recovered chimeric rRSV/mIL-2 virus formed plaques that were slightly smaller (10 to 15% reduction in size) than those of wt rRSV (data not shown) and were similar in size to plaques of rRSV bearing the chloramphenicol acetyltransferase (CAT) gene (rRSV/CAT virus) or the mIFN-γ gene (rRSV/mIFNγ virus) (1, 2). The kinetics of growth of the wt rRSV, rRSV/mIL-2, and rRSV/CAT viruses were compared in HEp-2 cells that were infected at a multiplicity of infection (MOI) of 2 PFU (not shown). The rRSV/mIL-2 virus, bearing the 549-nt mIL-2 insert, was moderately restricted in growth compared to wt rRSV, with a maximum difference of 18-fold at 40 h postinfection. The rRSV/CAT virus, bearing the 761-nt CAT insert, was slightly more attenuated (maximum difference of 52-fold at 40 h postinfection compared to wt rRSV). This is consistent with the general observation that insertion of an additional gene into rRSV attenuates its growth in vitro. The basis for this effect is not yet known and might involve effects on RNA synthesis, packaging, or both. Our experience from the construction of numerous recombinants bearing various foreign genes is that longer inserts are associated with greater attenuation, suggesting that the increase in genome length is a factor. In any case, the observation that the attenuation of rRSV/mIL-2 in vitro was comparable to that of rRSV/CAT indicates that the effect was not specific to the encoded mIL-2 protein.
When the rRSV/mIL-2 virus was subjected to eight serial passages in vitro and intracellular RNA was isolated and analyzed by reverse transcription-PCR with primers that flank the mIL-2 gene, a single PCR product was observed by gel electrophoresis (results not shown). There was no evidence of shorter products that might correspond to partial or complete deletion of the insert. Inserts of foreign sequences in recombinant nonsegmented negative-strand RNA viruses have been found to be surprisingly stable in general (1, 2, 24; A. Bukreyev and P. L. Collins, unpublished data). For example, when 25 viral clones were isolated biologically from a pool of rRSV/CAT that had been passaged eight times in vitro, each one efficiently expressed enzymatically active CAT (1).
To quantitate the expression of mIL-2, HEp-2 cells were infected with rRSV/mIL-2 (passage 8) at an MOI of 2 PFU per cell and aliquots of harvested medium were assayed by enzyme-linked immunosorbent assay (ELISA) using the Quantikine M Mouse IL-2 Immunoassay (R&D systems). The concentration of secreted mIL-2 was 1.7 ng/ml at 8 h postinfection and increased more than 1,000-fold to a maximum of 2.8 μg/ml at 120 h postinfection (data not shown).
To evaluate the replication of rRSV/mIL-2 in vivo, BALB/c mice were infected intranasally with 106 PFU of rRSV/mIL-2, rRSV/CAT, or wt rRSV per animal. Animals from each group were sacrificed on days 3, 4, and 5 postinfection, and the concentration of virus in the upper (nasal turbinates) and the lower (lungs) respiratory tract was determined by plaque assay. The replication of rRSV/mIL-2 was moderately attenuated at both locations (Fig. 2). The maximum levels of attenuation compared to that of wt rRSV were 5.0-fold in the upper respiratory tract on day 3 and 6.3-fold in the lower respiratory tract on day 5. The observed attenuation of rRSV/mIL-2 was statistically significant for all of the time points at each location with one exception, which was the nasal turbinates on day 5. In contrast, the replication of rRSV/CAT was not significantly different from that of wt rRSV at either location for all of the time points with one exception. The one exception was that, in the lungs on day 3, the titer of rRSV/CAT was lower than that of wt rRSV and was similar to that of rRSV/mIL-2. Thus, as we have described before (2), the presence of the 761-nt CAT insert did not significantly attenuate RSV in vivo. Hence, the attenuation of rRSV/IL-2, bearing the 549-nt mIL-2 gene at the same genome location, appeared to be specific to mIL-2.
FIG. 2.
Replication of rRSV/mIL-2, rRSV/CAT, and wt rRSV in the upper (nasal turbinates) and lower (lungs) respiratory tracts of BALB/c mice. Each animal was infected intranasally with 106 PFU of the indicated virus. Five animals per group were sacrificed on the indicated days, the nasal turbinates and lungs were harvested, and viral titers were determined by plaque assay. The data are given as average concentrations of the virus (log10 PFU/gram of tissue) and standard deviations. Statistical significance of the reduced titer of rRSV/mIL-2 compared to wt rRSV by Student's t test: for nasal turbinates, day 3, P < 0.01; day 4, P < 0.02; and day 5, P < 0.1; for lungs, day 3, P < 0.05; day 4, P < 0.05; and day 5, P < 0.001.
To evaluate the immunogenicity of rRSV/mIL-2, mice were infected with rRSV/mIL-2, rRSV/CAT, or wt rRSV as described above, and serum samples were taken on days 0 (immediately before infection), 28, and 56 (Table 1). Each of the viruses induced a high titer of RSV-neutralizing serum antibodies, and the three viruses were indistinguishable on this basis. In addition, there were no significant differences between the three viruses with regard to the induction of RSV-specific serum IgA, IgG1, IgG2a, and total IgG, as determined by ELISA with purified RSV F protein as antigen (Table 1). The mice in each group were then challenged on day 56 by the intranasal inoculation of 106 PFU of wt RSV per animal. Four days later, on day 60, the mice were sacrificed and virus titers in the upper and lower respiratory tracts were determined (data not shown). All of the previously infected animals exhibited a high level of resistance to challenge virus replication (data not shown). Replication of the challenge virus was undetectable in animals which had been previously infected with rRSV/CAT or rRSV/mIL-2 (mean titers of <2.0 log10 PFU/g in the nasal turbinates and <1.7 log10 PFU/g in the lungs), whereas a low level of RSV was detected in animals which had been immunized with wt rRSV (mean titers of 2.3 log10 PFU/g in the nasal turbinates and <1.7 log10 PFU/g in the lungs). In contrast, animals which had not been previously infected had mean titers of 4.7 log10 PFU/g in the nasal turbinates and lungs. Thus, the three viruses were indistinguishable with regard to the ability to induce a high level of resistance to reinfection.
TABLE 1.
RSV serum antibody response to infection with rRSV/mIL-2a
| Virus | Serum ELISA antibody titer (reciprocal mean log2 ± SE) to RSV F proteinb on the indicated day postinfection
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IgA
|
IgG1
|
IgG2A
|
Total IgG
|
RSV-neutralizing serum antibodiesc
|
|||||||||||
| 0 | 28 | 56 | 0 | 28 | 56 | 0 | 28 | 56 | 0 | 28 | 56 | 0 | 28 | 56 | |
| rRSV/mIL-2 | 7.3 | 10.6 ± 0.7 | 9.6 ± 1.1 | <5.3 | 9.6 ± 0.5 | 8.8 ± 1.4 | <5.3 | 10.6 ± 0.5 | 10.3 ± 0.4 | <5.3 | 10.8 ± 0.6 | 11.1 ± 0.3 | <3.3 | 9.9 ± 0.1 | 11.3 ± 0.4 |
| rRSV/CAT | 7.3 | 12.1 ± 0.7 | 7.3 ± 1.3 | <5.3 | 11.3 ± 0.5 | 7.8 ± 1.1 | <5.3 | 10.1 ± 0.4 | 10.1 ± 0.4 | <5.3 | 10.8 ± 0.3 | 10.3 ± 0.4 | <3.3 | 9.9 ± 1.2 | 11.7 ± 0.7 |
| wt rRSV | 7.3 | 11.8 ± 0.8 | 9.1 ± 1.1 | <5.3 | 10.8 ± 1.2 | 10.1 ± 0.6 | <5.3 | 11.1 ± 0.3 | 11.6 ± 0.3 | <5.3 | 11.8 ± 0.3 | 10.6 ± 0.4 | <3.3 | 9.8 ± 0.3 | 11.2 ± 0.5 |
| None (mock) | 7.3 | 7.8 ± 0.3 | 4.1 ± 0.4 | <5.3 | <5.3 | <5.3 | <5.3 | <5.3 | <5.3 | <5.3 | 7.32 ± 0.0 | <5.3 | <3.3 | <3.3 | <3.3 |
Each of the eight mice per group was infected on day 0 with 106 PFU of the indicated virus in a 0.1-ml inoculum. Antibody titers on day 56 were determined in a separate assay.
The titer of isotype-specific serum ELISA antibodies specific to the RSV F protein was determined as previously described (2).
RSV-neutralizing serum antibodies on the indicated day postinfection were measured by a complement-enhanced 60% plaque reduction assay (7).
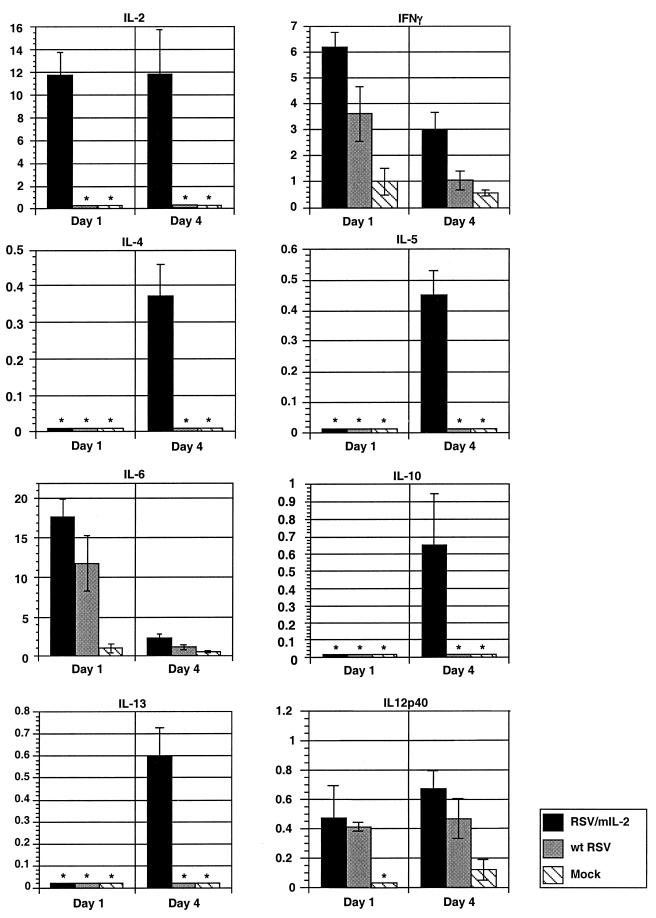
The levels of pulmonary mRNAs for selected cytokines were measured in mice following infection with 106 PFU of rRSV/mIL-2 or wt rRSV or in mock-infected mice. This assay has the advantage that it does not require in vitro stimulation or manipulation of cells and measures the aggregate response of all pulmonary cells. Four or five mice from each group were sacrificed, and lungs were harvested on days 1 and 4 postinfection. These days were chosen because they coincide with the period of active RSV replication, and abundant expression of cytokine mRNA had been demonstrated in this time period (12). Total lung RNA was isolated and analyzed by an RNase protection assay, using previously described methods (2). RNA from each individual animal was assayed separately. The cytokine-specific gel bands displayed on sequencing gels were quantitated by phosphorimagery, and the amount of each band for each mouse was expressed as a percentage of the L-32 housekeeping gene mRNA from the same gel lane for the same mouse. Then, the mean value and standard deviation for each group of mice were determined (Fig. 3).
FIG. 3.
Accumulation of pulmonary mRNA for IL-2, IFN-γ, IL-4, IL-5, IL-6, IL-10, IL-13, and IL-12 p40 in mice which were infected with 106 PFU of rRSV/mIL-2 or wt rRSV per animal or which received medium alone (mock). Four or five mice per group were sacrificed on days 1 and 4, and total pulmonary RNA was isolated and analyzed by an RNase protection assay as described previously (2) using the RiboQuant Multi-Probe RNAse Protection Assay System (PharMingen) and two different probe template sets, namely, mCK-1 and mCK-2B. Each mouse was analyzed separately, and each protected species detected by polyacrylamide gel electrophoresis was quantitated by phosphorimagery and calculated as a percentage of the amount of L-32 housekeeping gene mRNA in the same sample. Mean values of each group per day and standard deviations are shown. Note that each y axis has a different scale.
This analysis showed that infection with wt rRSV stimulated the abundant accumulation of mRNA for the Th1 cytokine IFN-γ and the Th2 cytokine IL-6 and also stimulated the accumulation mRNA for IL-12 p40, which is the inducible subunit of the IL-12 heterodimer. IL-12 is produced by monocytes and macrophages, among other cells, but not by T lymphocytes, and its production is enhanced by IFN-γ. These three abundant mRNAs were also observed in rRSV/IL-2-infected mice and accumulated to somewhat higher levels than with wt rRSV. Infection with rRSV/mIL-2 also resulted in the accumulation of IL-2 mRNA, which was not observed in wt rRSV-infected animals and likely was encoded directly by the virus. Infection with rRSV/mIL-2, but not wt rRSV, also stimulated the accumulation of several less abundant Th2 cytokine mRNAs, namely, IL-4, IL-5, IL-10, and IL-13. Thus, coexpression of IL-2 from rRSV was associated with an increase in the accumulation of mRNAs for both Th1 and Th2 marker cytokines.
Mice from each of the same groups were challenged with wt RSV on day 28, and lungs were harvested for analysis on days 29 and 32 (1 or 4 days postchallenge). As described previously (2), mice that had been infected with wt rRSV and challenged 28 days later with wt RSV exhibited elevated levels of mRNAs for IL-6, IFN-γ, and IL-12 p40, and to a lesser extent, elevated amounts of mRNAs for IL-2 and IL-10, whereas mRNAs for IL-4, IL-5, and IL-13 were not detected (data not shown). Mice that had been infected with rRSV/mIL-2 and challenged with wt RSV exhibited the same pattern with one exception: with regard to IL-12 p40 mRNA, there was no significant difference on day 29, but on day 32 the rRSV/mIL-2-primed group had a small decrease (32%, P < 0.01) from that of the wt rRSV-primed group (not shown).
We also examined the total pulmonary CD4+ T lymphocyte response to rRSV/mIL-2 versus wt rRSV. Specifically, intracellular cytokine immunostaining and flow cytometry were used to quantitate pulmonary CD4+ lymphocytes expressing the Th1 marker IFN-γ or the Th2 marker IL-4 (16, 21, 22). Mice were infected with 106 PFU of rRSV/mIL-2 or wt rRSV or were mock infected. Four animals from each group were sacrificed each on days 4 and 10, and lungs were harvested and processed as described below. The remaining mice in each group were challenged intranasally on day 28 with 106 PFU of wt RSV, four mice from each group were sacrificed 4 and 10 days later (days 32 and 38), and their lungs were harvested and processed. The lungs were minced and digested with DNase I and collagenase, and total pulmonary mononuclear cells were isolated by centrifugation and banding in Ficoll-Paque Plus medium (Amersham Pharmacia Biotech), with material from each animal processed separately. The cells were stimulated in vitro by incubation at 37°C for 4 h with nonspecific mitogen (2.5 ng of phorbol 12-myristate 13-acetate per ml and 250 ng of ionomycin per ml) in the presence of monensin, which blocks exocytosis and causes cytokines to accumulate intracellularly. Fc receptors were blocked by preincubating cells with purified rat anti-mouse CD16/CD32 (FcγIII/II receptor) for 15 min at 4°C. The cells were fixed with paraformaldehyde solution (Cytofix Buffer [PharMingen]; 20 min at 4°C), permeabilized (PermWash [PharMingen]; 20 min at 4°C), and stained for CD4+ (Tri-Color conjugated rat IgG2a clone CT-CD4 [Caltag Laboratories]), IFN-γ (fluorescein isothiocyanate [FITC]-conjugated rat IgG1 clone XMG1.2 [PharMingen]), and IL-4 (R-phycoerythrin [R-PE]-conjugated rat IgG2b clone BVD4-1D11 [PharMingen]) molecules. The immunostaining was for 30 min at 4°C in the dark using a preoptimized amount of each labeled antibody. The specificity of staining was confirmed with controls in which (i) reactivity was blocked by preincubation for 30 min at 4°C with an unconjugated preparation of the same antibody, and (ii) reactivity was lost when the primary antibody was replaced with one of the same isotype but having a heterologous specificity. Published work indicated that the in vitro stimulation step does not alter the pattern of cytokine expression (16). The lymphocyte fraction was gated as previously described (16) and analyzed by three-color flow cytometry using a FACSCalibur flow cytometer (Becton Dickinson). Approximately 60,000 gated lymphocytes were analyzed per sample. It is noteworthy that total pulmonary lymphocytes were examined, rather than a subpopulation such as isolated by lavage.
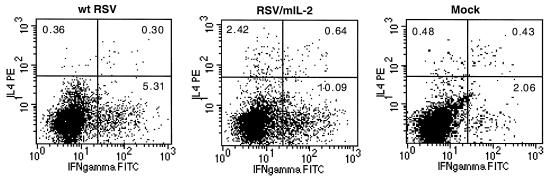
Approximately half of the total pulmonary mononuclear cells were gated as lymphocytes, and this percentage was not significantly altered in response to a primary infection or challenge with either rRSV/mIL-2 or wt rRSV compared to that in uninfected controls (not shown). The percentage of the mononuclear cells identified as CD4+ lymphocytes was essentially unchanged following initial infection with either virus (mean percentages shown in parentheses) (7.6 and 7.9 on days 4 and 10, respectively, for wt rRSV; 7.5 and 10.7 on days 4 and 10, respectively, for rRSV/mIL-2) compared to the uninfected controls (9.0 and 7.2 on days 4 and 10, respectively). However, that percentage was nearly doubled on days 32 and 38 following the challenge (mean percentages shown in parentheses) (18.2 and 15.4 on days 32 and 38, respectively, for wt rRSV; 15.7 and 14 on days 32 and 38, respectively, for rRSV/mIL-2) compared to the uninfected control (as described above). This is indicative of a strong secondary immune response despite the very restricted replication of the challenge virus. The CD4+ population was then examined for expression of IFN-γ versus IL-4. Figure 4 shows examples of data for three individual animals that were infected with rRSV/mIL-2 or with wt rRSV or mock infected and were analyzed on day 10. The complete experiment is summarized in Table 2.
FIG. 4.
Dot plots showing flow cytometric analysis of the CD4+ lymphocytes expressing IL-4 (IL4 PE) or IFN-γ (IFN gamma FITC). Mice were infected with 106 PFU of wt rRSV or rRSV/mIL-2 or were mock infected. The animals were sacrificed 10 days later, and pulmonary CD4+ cells were harvested and analyzed by flow cytometry. The percentage of CD4+ cells in three quadrants is shown for each plot. Each plot represents cells from an individual mouse.
TABLE 2.
Flow cytometric analysis of pulmonary CD4+ lymphocytes expressing IFN-γ, IL-4, or both from mice infected with wt RSV or rRSV/mIL-2a
| Virus | Day 4
|
Day 10b
|
Day 32
|
Day 38
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IFN-γ+ | IL-4+ | Dbl+ | IFN-γ+ | IL-4+ | Dbl+ | IFN-γ+ | IL-4+ | Dbl+ | IFN-γ+ | IL-4+ | Dbl+ | |
| rRSV/mIL-2 | 3.3 ± 0.32 | 0.99 ± 0.21 | 0.20 ± 0.06 | 8.8 ± 1.10* | 2.4 ± 0.09** | 0.61 ± 0.01** | 1.3 ± 0.34 | 0.78 ± 0.12 | 0.04 ± 0.02 | 20 ± 3.83 | 0.56 ± 0.07 | 0.22 ± 0.06 |
| wt rRSV | 2.7 ± 0.66 | 0.95 ± 0.28 | 0.14 ± 0.05 | 4.2 ± 1.11 | 0.65 ± 0.18 | 0.15 ± 0.06 | 4.1 ± 0.66 | 1.1 ± 0.28 | 0.09 ± 0.04 | 19.3 ± 1.35 | 0.43 ± 0.05 | 0.14 ± 0.00 |
| None (mock) | 0.48 ± 0.03 | 0.28 ± 0.00 | 0.03 ± 0.00 | 1.7 ± 0.25 | 1.1 ± 0.21 | 0.31 ± 0.10 | ND | ND | ND | 5.8 ± 0.10c | 0.70 ± 0.14c | 0.10 ± 0.02c |
Mice were infected intranasally on day 0 with 106 PFU of the indicated virus per animal or were mock infected. Animals from each group were sacrificed on days 4 and 10, as indicated. The remaining animals (including the mock-infected group) were challenged on day 28 with 106 PFU of wt RSV per animal, and the animals were sacrificed on days 32 and 38 (4 and 10 days postchallenge) as indicated. Total pulmonary mononuclear cells were analyzed by flow cytometry using immunostaining for CD4, IFN-γ, and IL-4. Values are expressed as the percentage of CD4+ lymphocytes. Each group contained four mice per day with the following exceptions: the wt RSV group on day 4 had three animals, the mock-infected group on day 4 had two animals, and the mock-infected group on days 10 and 38 had three animals each. The cells of each animal were processed separately, and each group is expressed as the mean of the individual data for the two to four mice with the standard error indicated. Dbl+, doubly positive (IFN-γ+ and IL-4+); ND, not determined.
Statistical significance calculated by Student's t-test compared to that of wt rRSV control: ∗, P < 0.005; ∗∗, P < 0.001.
Note that the animals in the Mock group were mock infected on day 0 but received the RSV challenge on day 28. Hence, the day 38 point corresponds to the day 10 point for the wt RSV group.
On day 4 following the initial infection, animals which received rRSV/mIL-2 or wt rRSV exhibited increased levels of CD4+ lymphocytes which were IFN-γ positive, IL-4 positive, or doubly positive, but the magnitude of the response was very similar for the two viruses (Table 2). On day 10, the average number of the cells which were IFN-γ positive, IL-4 positive, or doubly positive was statistically significantly increased in rRSV/mIL-2-infected mice than in wt rRSV-infected mice: 2.1-fold (P < 0.05), 3.6-fold (P < 0.001), and 4.1-fold (P < 0.001), respectively. Thus, the increase in Th1 and Th2 cytokine mRNAs noted above on day 4 (Fig. 3) was reflected by cytokine synthesis by CD4+ lymphocytes on day 10 but not day 4. This delay might reflect lower sensitivity for the latter assay, or a lag in expression, or, in the case of IFNγ, synthesis by a source other than CD4+ lymphocytes such as NK cells (15).
When animals were challenged on day 28 and pulmonary CD4+ cells were examined on day 32, the number of IFN-γ-positive cells in animals which had been primed with rRSV/mIL-2 was threefold lower than in wt rRSV-immunized mice (P < 0.001). The percentages of IL-4-positive and doubly positive cells were similar in both groups of mice. The observed reduction in IFN-γ-expressing CD4+ cells was not reflected in the amount of total pulmonary IFN-γ mRNA, indicating that cells other than CD4+ lymphocytes contribute to the overall level of this mRNA, such as NK cells. The reduction in IFN-γ-positive cells was transient, and on day 38, there were no significant differences in the number of IFN-γ or IL-4-expressing cells between mice which had originally been primed with rRSV/mIL-2 or wt rRSV. At this time point, the percentages of total pulmonary CD4+ cells expressing IFN-γ or IL-4 were ∼19 and ∼0.5, respectively.
In summary, coexpression of mIL-2 by recombinant RSV in the BALB/c mouse model (i) resulted in a modest attenuation of virus growth, (ii) increased the expression of Th1 and Th2 cytokines as detected by analysis of total pulmonary mRNA, and (iii) increased the response of total pulmonary CD4+ T lymphocytes expressing IFN-γ or IL-4. The elevated immune response to rRSV/mIL-2 likely accounts for the modest attenuation compared to that of wt rRSV. Attenuation of virus growth might be a consequence of the observed increase in the CD4+ T lymphocyte response or the observed increase in IFN-γ production or might involve other factors that were not monitored here such as activation and proliferation of CD8+ or NK cells, or stimulation of the secretion of other antiviral cytokines such as type I IFNs or tumor necrosis factor alpha (17–19). The augmentation in the accumulation of Th1 and Th2 cytokine mRNAs and CD4+ T lymphocytes was observed only during the initial infection by rRSV/mIL-2 and was not observed during subsequent challenge with wt RSV. Indeed, there was a modest reduction in IFN-γ-positive CD4+ T lymphocytes and IL-12 p40 mRNA 4 days after challenge, effects that likely are related. However, the diminution in IFN-γ-positive CD4+ T lymphocytes was transient and was not observed on day 10 following challenge. The elevated immune response during the initial infection by rRSV/mIL-2, evidenced by increased cytokine mRNAs and CD4+ T lymphocytes, was not reflected in increased RSV-specific serum antibodies or increased protective efficacy. However, the titer of RSV-specific antibodies and level of protective immunity induced by RSV infection in mice are so high that it is unclear whether they would be sensitive to further stimulation. For example, when mice that were previously infected with RSV are challenged, little or no challenge virus replication is observed, and hence a further increase in protective immunity would likely be missed. It will be important to evaluate the replication and immunogenicity of rRSV/mIL-2 in nonhuman primates, where the immune response to RSV is less robust. The available rRSV/mIL-2 virus might be used in such a study, since there appears to be considerable cross-species IL-2 activity between humans and mice (8, 14), or a recombinant RSV expressing human IL-2 could be constructed.
Acknowledgments
We thank Chris J. Cho, Myron Hill, and Cai-Yen Firestone for technical assistance. We also thank Kevin Holmes and David Stephany of the NIAID Flow Cytometry Section for assistance, advice, and the use of equipment.
REFERENCES
- 1.Bukreyev A, Camargo E, Collins P L. Recovery of infectious respiratory syncytial virus expressing an additional, foreign gene. J Virol. 1996;70:6634–6641. doi: 10.1128/jvi.70.10.6634-6641.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bukreyev A, Whitehead S S, Bukreyeva N, Murphy B R, Collins P L. Interferon gamma expressed by a recombinant respiratory syncytial virus attenuates virus replication in mice without compromising immunogenicity. Proc Natl Acad Sci USA. 1999;96:2367–2372. doi: 10.1073/pnas.96.5.2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Collins P L, Hill M G, Camargo E, Grosfeld H, Chanock R M, Murphy B R. Production of infectious human respiratory syncytial virus from cloned cDNA confirms an essential role for the transcription elongation factor from the 5′ proximal open reading frame of the M2 mRNA in gene expression and provides a capability for vaccine development. Proc Natl Acad Sci USA. 1995;92:11563–11567. doi: 10.1073/pnas.92.25.11563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Collins P L, McIntosh K, Chanock R M. Respiratory syncytial virus. In: Fields B N, Knipe D M, Howley P M, Chanock R M, Melnick J L, Monath T P, Roizman B, Straus S E, editors. Fields virology. 3rd ed. Vol. 2. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 1313–1352. [Google Scholar]
- 5.Collins P L, Whitehead S S, Bukreyev A, Fearns R, Teng M N, Juhasz K, Chanock R M, Murphy B R. Rational design of live-attenuated recombinant vaccine virus for human respiratory syncytial virus by reverse genetics. Adv Virus Res. 1999;54:423–451. doi: 10.1016/s0065-3527(08)60374-7. [DOI] [PubMed] [Google Scholar]
- 6.Crowe J E, Jr, Collins P L, Chanock R M, Murphy B R. Vaccines against respiratory syncytial virus and parainfluenza virus type 3. In: Levine M M, Woodrow G C, Kaper J B, Cobon G S, editors. New generation vaccines. 2nd ed. New York, N.Y: Marcel Dekker, Inc.; 1997. pp. 711–725. [Google Scholar]
- 7.Crowe J E, Jr, Collins P L, London W T, Chanock R M, Murphy B R. A comparison in chimpanzees of the immunogenicity and efficacy of live attenuated respiratory syncytial virus (RSV) temperature-sensitive mutant vaccines and vaccinia virus recombinants that express the surface glycoproteins of RSV. Vaccine. 1993;11:1395–1404. doi: 10.1016/0264-410x(93)90168-w. [DOI] [PubMed] [Google Scholar]
- 8.Flexner C, Hugin A, Moss B. Prevention of vaccinia virus infection in immunodeficient mice by vector-directed IL-2 expression. Nature. 1987;330:259–262. doi: 10.1038/330259a0. [DOI] [PubMed] [Google Scholar]
- 9.Flexner C, Moss B, London W T, Murphy B R. Attenuation and immunogenicity in primates of vaccinia virus recombinants expressing human interleukin-2. Vaccine. 1990;8:17–21. doi: 10.1016/0264-410x(90)90171-h. [DOI] [PubMed] [Google Scholar]
- 10.Gaffen S L, Goldsmith M A, Greene W C. Interleukin-2 and interleukin-2 receptor. San Diego, Calif: Academic Press; 1998. [Google Scholar]
- 11.Geissler M, Gesien A, Tokushige K, Wands J R. Enhancement of cellular and humoral immune responses to hepatitis C virus core protein using DNA-based vaccines augmented with cytokine-expressing plasmids. J Immunol. 1997;158:1231–1237. [PubMed] [Google Scholar]
- 12.Graham B S, Henderson G S, Tang Y W, Lu X, Neuzil K M, Colley D G. Priming immunization determines T helper cytokine mRNA expression patterns in lungs of mice challenged with respiratory syncytial virus. J Immunol. 1993;151:2032–2040. [PubMed] [Google Scholar]
- 13.Gundlach B R, Linhart H, Dittmer U, Sopper S, Reiprich S, Fuchs D, Fleckenstein B, Hunsmann G, Stahl-Hennig C, Uberla K. Construction, replication, and immunogenic properties of a simian immunodeficiency virus expressing interleukin-2. J Virol. 1997;71:2225–2232. doi: 10.1128/jvi.71.3.2225-2232.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hugin A W, Flexner C, Moss B B. Clearance of recombinant vaccinia virus expressing IL-2: role of local host immune responses. Cell Immunol. 1993;152:499–509. doi: 10.1006/cimm.1993.1307. [DOI] [PubMed] [Google Scholar]
- 15.Hussell T, Openshaw P J. Intracellular IFN-gamma expression in natural killer cells precedes lung CD8+ T cell recruitment during respiratory syncytial virus infection. J Gen Virol. 1998;79:2593–2601. doi: 10.1099/0022-1317-79-11-2593. [DOI] [PubMed] [Google Scholar]
- 16.Hussell T, Spender L C, Georgiou A, O'Garra A, Openshaw P J. Th1 and Th2 cytokine induction in pulmonary T cells during infection with respiratory syncytial virus. J Gen Virol. 1996;77:2447–2455. doi: 10.1099/0022-1317-77-10-2447. [DOI] [PubMed] [Google Scholar]
- 17.Karupiah G, Blanden R V, Ramshaw I A. Interferon gamma is involved in the recovery of athymic nude mice from recombinant vaccinia virus/interleukin 2 infection. J Exp Med. 1990;172:1495–1503. doi: 10.1084/jem.172.5.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karupiah G, Coupar B E, Andrew M E, Boyle D B, Phillips S M, Mullbacher A, Blanden R V, Ramshaw I A. Elevated natural killer cell responses in mice infected with recombinant vaccinia virus encoding murine IL-2. J Immunol. 1990;144:290–298. [PubMed] [Google Scholar]
- 19.Karupiah G, Woodhams C E, Blanden R V, Ramshaw I A. Immunobiology of infection with recombinant vaccinia virus encoding murine IL-2. Mechanisms of rapid viral clearance in immunocompetent mice. J Immunol. 1991;147:4327–4332. [PubMed] [Google Scholar]
- 20.Lin R, Tarr P E, Jones T C. Present status of the use of cytokines as adjuvants with vaccines to protect against infectious diseases. Clin Infect Dis. 1995;21:1439–1449. doi: 10.1093/clinids/21.6.1439. [DOI] [PubMed] [Google Scholar]
- 21.Openshaw P, Murphy E E, Hosken N A, Maino V, Davis K, Murphy K, O'Garra A. Heterogeneity of intracellular cytokine synthesis at the single-cell level in polarized T helper 1 and T helper 2 populations. J Exp Med. 1995;182:1357–1367. doi: 10.1084/jem.182.5.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prussin C, Metcalfe D D. Detection of intracytoplasmic cytokine using flow cytometry and directly conjugated anti-cytokine antibodies. J Immunol Methods. 1995;188:117–128. doi: 10.1016/0022-1759(95)00209-x. [DOI] [PubMed] [Google Scholar]
- 23.Ramshaw I A, Andrew M E, Phillips S M, Boyle D B, Coupar B E. Recovery of immunodeficient mice from a vaccinia virus/IL-2 recombinant infection. Nature. 1987;329:545–546. doi: 10.1038/329545a0. [DOI] [PubMed] [Google Scholar]
- 24.Schnell M J, Buonocore L, Whitt M A, Rose J K. The minimal conserved transcription stop-start signal promotes stable expression of a foreign gene in vesicular stomatitis virus. J Virol. 1996;70:2318–2323. doi: 10.1128/jvi.70.4.2318-2323.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thorpe R. Interleukin-2. San Diego, Calif: Academic Press; 1998. [Google Scholar]
- 26.Whitehead S S, Bukreyev A, Teng M N, Firestone C Y, St. Claire M, Elkins W R, Collins P L, Murphy B R. Recombinant respiratory syncytial virus bearing a deletion of either the NS2 or SH gene is attenuated in chimpanzees. J Virol. 1999;73:3438–3442. doi: 10.1128/jvi.73.4.3438-3442.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]