Abstract
Background:
Brain imaging studies may provide etiologic insight into observed links between lung function and dementia and stroke.
Objective:
We evaluated associations of lung function measures with brain MRI markers of vascular and neurodegenerative disease in the ARIC Neurocognitive Study, as few studies have examined the associations.
Methods:
Lung function was measured at participants’ midlife in 1990–1992 (mean age=56±5 years) and later-life in 2011–2013 (mean age=76±5 years), and brain MRI was performed in 2011–2013. Linear regression models were used to examine the associations of lung function with brain and white matter hyperintensity (WMH) volumes, and logistic regression models were used for cerebral infarcts and microbleeds, adjusting for potential confounders.
Results:
In cross-sectional analysis (i.e., examining later-life lung function and MRI markers, n=1223), higher forced-expiratory volume in one second (FEV1) and forced vital capacity (FVC) were associated with larger brain and lower WMH volumes [e.g., 8.62 (95%CI:2.54–14.71) cm3 greater total brain volume per one-liter higher FEV1]. No association was seen with microbleeds in the overall sample, but higher FVC was associated with lower odds of microbleeds in never-smokers and higher odds in ever-smokers. In the cross-temporal analysis (i.e., associations with midlife lung function, n=1787), higher FVC levels were significantly associated with lower later-life brain volumes.
Conclusions:
Our results support modest associations of better lung function with less neurodegenerative and cerebrovascular pathology, although findings for microbleeds were unexpected in ever-smokers.
Keywords: Lung Function, Brain Volumes, Cerebral Infarct, White Matter Hyperintensity Volume
INTRODUCTION
A growing body of literature suggests potential links between lung function measures and adverse neurological outcomes, including cognitive decline, dementia, and stroke [1–7]. Potential suggested mechanisms for the associations, although not clearly understood, include systemic inflammation, chronic hypoxia, and shared underlying pathophysiology between pulmonary and cardiovascular systems [7–10]. Brain imaging studies can improve the understanding of underlying neurodegenerative and cerebrovascular disease processes associated with poor lung function and provide more cogent evidence for lung function associations with dementia and stroke. A few studies have evaluated lung function measures in relation to brain neuropathological changes, including reduced brain volumes [11, 12], white matter abnormalities [12–17], microbleeds [18], and infarcts [13–15], although findings have not been consistent [11, 13, 19, 20]; most of these studies examined white matter abnormalities, and other brain pathologies such as infarcts and microbleeds remain minimally explored. Further, the majority of these were conducted in non-US populations of European descent, and very few studies have included Black participants. Therefore, we examined midlife and later-life lung function measures in relation to comprehensive later-life brain magnetic resonance imaging (MRI) markers, including brain volumes, white matter hyperintensity (WMH) volume, cerebral infarcts, and microbleeds, in the more diverse Atherosclerosis Risk in Communities (ARIC) Study, a community-based cohort of Black and White adults.
METHODS
Study population
Between 1987 and 1989 (visit 1), the ARIC study recruited 15,972 (primarily) Black and White adults aged 45–64 years from four United States communities (Forsyth County, North Carolina; Jackson, Mississippi; Washington County, Maryland; and suburbs of Minneapolis, Minnesota) [21, 22]. ARIC participants have undergone numerous comprehensive clinical examinations and are continually surveilled for vital status. For the current investigation, we used data from the visit 2 (1990–1992) and visit 5 (2011–2013) exams which were completed by 14,348 and 6,538 participants, respectively. All eligible participants underwent lung function testing at the visit 2 and 5 exams. At the visit 5 exam, a subset of participants (n=1979) underwent brain MRI as a component of the ARIC Neurocognitive Study. Participants were selected for brain MRI if they exhibited evidence of cognitive impairment at their visit 5 cognitive evaluation or previously participated in the ARIC Brain MRI study in 2004–2006. Further, a random (age- and center-stratified) sample of the remaining cognitively normal participants was included. The study was approved by institutional review boards at participating study centers.
For the visit 2 lung function analysis, of the 1979 participants who underwent MRI at visit 5, we excluded 47 with missing lung function data, six who reported race other than Black or White (due to small numbers), nine Black participants from the Minnesota and Maryland field centers (due to race and centers aliasing and small numbers in these centers), 13 with prevalent stroke, and 117 with missing data on covariates of interest, which resulted in an analytic sample of 1787 (Supplementary Figure 1). Likewise, for the visit 5 lung function analysis, of the 1979, we excluded 482 with missing lung function data, three who reported race other than Black or White, six Black participants from the Minnesota and Maryland field centers, 179 with missing data on covariates, 56 with prevalent dementia, and 30 with stroke at visit 5, resulting in 1223 participants in the final analytic sample. We also performed the visit 5 analysis including prevalent dementia in a sensitivity analysis.
Lung function
Lung function was measured at the visit 2 exam using the Collins Survey II water-seal spirometer and at the visit 5 exam using the SensorMedics model 1022 dry-rolling seal volume spirometer by trained and certified technicians, in accordance with the American Thoracic Society/European Respiratory Society guidelines [23, 24]. Participants were asked to perform up to eight forced expirations to attain two reproducible spirograms (out of three acceptable maneuvers); lung function measures for the analysis were obtained from the best spirogram. Spirometry procedures, acceptability and reproducibility criteria, and quality control can be found elsewhere [23, 24]. We used three spirometry measures: forced vital capacity (FVC, the total amount of air that can be forcefully exhaled after maximal inspiration), forced expiratory volume in one second (FEV1, the amount of air exhaled in the first second of the FVC maneuver), and FEV1/FVC ratio (expressed as a percentage). We also examined associations with clinical lung conditions (i) obstructive lung condition (defined as FEV1/FVC < lower limits of normal (LLN)); (ii) restrictive condition (defined as FEV1/FVC≥ LLN and FVC <LLN); and (iii) normal individuals (those who did not belong to either obstructive or restrictive categories) in our secondary analyses [25–28]. More details on this are provided in the Supplemental Document.
Neuroimaging
Brain MRI scans were performed at 3 Tesla and analyzed at the ARIC MRI Reading Center (Mayo Clinic, Rochester, MN). Details on MRI analysis are described elsewhere [29, 30]. Briefly, cortical brain and total intracranial volumes were measured on magnetization-prepared rapid acquisition gradient echo using Freesurfer version 5.1. For the brain volumetric analysis, we considered total and regional cortical (i.e., frontal, temporal, occipital, parietal, deep gray matter, and temporal-parietal lobe meta-region of interest (ROI)) brain volumes. The temporal-parietal lobe meta-ROI volume was measured as the combined volume of the regions shown to be susceptible to Alzheimer’s disease, which included the parahippocampal gyrus, entorhinal cortex, inferior parietal lobules, hippocampus, precuneus, and cuneus [31]. WMH volume and presence of any infarcts were assessed on axial T2 fluid-attenuated inversion recovery sequences. The presence of any microbleeds was characterized on axial T2*gradient recalled echo sequences.
Covariates
We obtained information on sex, race, study field center, education (< high school, high school or equivalent, > high school), and APOE ε4 carrier status (none or ≥ one alleles, determined using TaqMan assays and the ABI 7700 Sequence Detection System, Applied Biosystems, Foster City, CA) from visit 1. Field center and race were combined to create five categories: Mississippi Black, Maryland White, Minnesota White, North Carolina Black, and North Carolina White. Information on age, height, cigarette smoking status (current, former, never), weight, physical activity (the sport index score of the Baecke Physical Activity Questionnaire),[32] diabetes (fasting glucose ≥ 126 mg/dL or non-fasting glucose ≥ 200 mg/dL, self-reported physician’s diagnosis, or use of anti-diabetic medications), total cholesterol level, hypertension status [hypertension (defined as systolic blood pressure ≥ 140 mm Hg, or diastolic blood pressure ≥ 90 mm Hg or use of antihypertensive medications); prehypertension (defined as systolic blood pressure ≥ 120 and <140 mmHg); and normal blood pressure], and prevalent heart diseases from both visit 2 and visit 5 exams.
Statistical analysis
Primary analysis
We used multivariable linear regression models to examine associations of lung function measures with brain and WMH volumes. WMH volume was positively skewed and thus natural log-transformed. We used logistic regression models to estimate odds ratios (ORs) for presence of cerebral infarcts and microbleeds. All models were adjusted for age, sex, field center-race, height, smoking status, weight (used as a measure of obesity in place of body mass index to avoid potential collinearity with height), education, physical activity, hypertension, diabetes, total cholesterol, coronary heart diseases, APOE ε4 carrier status, and total estimated intracranial volume. We used visit 5 covariates for cross-sectional analyses (i.e. visit 5 lung function on visit 5 MRI outcomes) and visit 2 covariates for cross-temporal analyses (i.e. visit 2 lung function on visit 5 MRI outcomes).
For the visit 2 lung function analysis, we applied inverse probability of attrition weights (IPW) to regression models to account for selective attrition from visit 2 to visit 5 (such that weighted estimates would represent associations in the eligible visit 2 population). Specifically, we estimated predicted probabilities of (i) being alive at visit 5; (ii) remaining in the study at visit 5 among those who were alive; and (iii) being selected for MRI among those who made to visit 5, using three separate logistic regression models with visit 2 lung function measures and other covariates (age, sex, field center-race, height, smoking status, weight, education, physical activity, diabetes, hypertension, total cholesterol, coronary heart diseases, and APOE ε4 status) as predictors. Overall inverse probability of attrition weights were then constructed by multiplying the three inverse probabilities together and truncating at the 99th percentile.
Secondary/sensitivity analysis
We repeated visit 5 lung function analyses by incorporating sampling weights to account for the sampling strategy used to select participants for MRI. While all analyses were based on complete cases, to assess the impact of missing covariates, we repeated the visit 5 lung function analyses employing multiple imputation by chained equations; briefly, we created 30 datasets imputing values for missing covariates, performed regression analysis in each dataset, and obtained pooled parameter estimates. We also repeated visit 2 lung function analysis without applying any IPW (i.e., unweighted analyses).
As smoking is a strong risk factor of lung function impairment, we conducted two additional analyses to explore the influence of smoking on the associations. First, we estimated all associations separately for never and ever smokers and tested for statistical interactions between lung function measures and smoking. Second, for the visit 2 analysis, we additionally adjusted for cigarette pack years (amount of smoking and time since quitting information needed to update pack-years were not collected at the visit 5 exam).
RESULTS
Table 1 presents characteristics of visit 2 (mean age±SD = 56±5 years) and visit 5 (mean age±SD = 76±5 years) analytic samples (39% male; 28% Black race, brain MRI characteristics are presented in Supplementary Table 1). As expected, participants had worse lung function and cardiovascular risk factors at visit 5 than at visit 2 (Table 1). Further, compared to the total visit 2 population (n=14,348), participants included in the visit 2 analytic sample (n=1787) were younger, and had higher education, better lung function, and cardiovascular profiles (Supplementary Table 2).
Table 1:
Characteristics of participants at visit 2 and visit 5 in the Atherosclerosis Risk in Communities Neurocognitive Study
| Characteristics | Midlife (Visit 2) characteristics in the analytic sample for cross-temporal analysis, n=1787 | Later-life (Visit 5) characteristics in the analytic sample for cross-sectional analysis, n=1223 |
|---|---|---|
| FEV1 (L), mean (SD) | 2.83 (0.69) | 2.09 (0.62) |
| FVC (L), mean (SD) | 3.75 (0.91) | 2.91 (0.86) |
| FEV1/FVC%, mean (SD) | 75.75 (6.41) | 72.34 (9.16) |
| Lung function category, n (%) | ||
| Normal | 1480 (83%) | 965 (79%) |
| Restrictive lung condition | 56 (3%) | 78 (6%) |
| Obstructive lung condition | 251 (14%) | 180 (15%) |
| Age (years), mean (SD) | 55.64 (5.22) | 75.93 (5.25) |
| Male sex, n (%) | 722 (40%) | 481 (39%) |
| Smoking status, n (%) | ||
| Never | 845 (47%) | 537 (44%) |
| Former | 670 (37%) | 630 (52%) |
| Current | 272 (15%) | 56 (5%) |
| Black race, n (%) | 495 (28%) | 347 (28%) |
| Center, n (%) | ||
| Forsyth, NC | 453 (25%) | 273 (22%) |
| Jackson, MS | 462 (26%) | 329 (27%) |
| Minneapolis, MN | 418 (23%) | 281 (23%) |
| Washington, MD | 454 (25%) | 340 (28%) |
| Education, n (%) | ||
| < High school | 242 (14%) | 152 (12%) |
| High school | 732 (41%) | 493 (40%) |
| > High school | 813 (45%) | 578 (47%) |
| BMI (kg/m2), mean (SD) | 27.42 (5.02) | 28.38 (5.54) |
| Weight (kg), mean (SD) | 80.37 (17.57) | 77.67 (16.08) |
| Height (m), mean (SD) | 1.68 (0.09) | 1.68 (0.09) |
| Physical activity index, mean (SD) | 2.41 (0.56) | 2.27 (0.64) |
| Coronary heart disease, n (%) | 26 (1%) | 103 (8%) |
| Diabetes, n (%) | 105 (6%) | 392 (32%) |
| Blood Pressure, n (%) | ||
| Normal | 980 (55%) | 152 (12%) |
| Prehypertension | 362 (20%) | 171 (14%) |
| Hypertension | 445 (25%) | 900 (74%) |
| Total cholesterol (mmol/L) | 5.38 (0.95) | 4.76 (1.09) |
| APOE ε4 status (≥1 ε4 allele)(n (%) | 526 (29%) | 351 (29%) |
| Cognition status, n (%) | ||
| Normal | 1787 (100%) | 816 (67%) |
| Mild cognitive impairment | - | 406 (33%) |
Note: BMI, Body Mass Index; FEV1, Forced Expiratory Volume in one second; FVC, Forced Vital Capacity; SD, Standard Deviation.
Information on sex, race, education, center, and APOE ε4 carrier status was obtained from ARIC visit 1. Other than for these characteristics, we present descriptive statistics for visit 2 and visit 5 characteristics. All participants were cognitively normal at visit 2 exam.
Table 1 presents the unweighted characteristics.
Cross-sectional associations: later-life (visit 5) lung function and brain MRI measures
Higher later-life FEV1 and FVC were associated with larger concurrent total and regional brain volumes (Table 2, Figures 1 and 2). All the associations were statistically significant except those of FEV1 and FVC with occipital lobe volume and that of FVC with deep gray matter volume. For instance, one liter higher FEV1 was associated with 8.62 (95% CI: 2.54, 14.71) cm3 and 1.53 (95% CI: 0.33, 2.73) cm3 greater total and frontal lobe volumes, respectively. Higher FEV1 and FVC were significantly associated with lower WMH volume [FEV1, β= −0.12 (95% CI: −0.22, −0.02) and FVC, β= −0.14 (95% CI: −0.23, −0.05)]. None of these measures were significantly associated with cerebral microbleeds or infarcts. FEV1/FVC was not associated with any brain MRI measures. Findings were similar when prevalent dementia cases were included in the analysis (Supplementary Table 3) or when multiple imputation was employed for missing covariates (Supplementary Table 4). The analysis that accounted for sampling weights used to select participants for MRI yielded qualitatively similar findings, although a few associations were no longer statistically significant (Supplementary Table 5).
Table 2:
Cross-sectional associations between later-life (visit 5) lung function measures and later-life (visit 5) brain MRI outcomes in the Atherosclerosis Risk in Communities Neurocognitive Study (n=1223)
| FEV1 (per 1L) | FVC (per 1L) | FEV1/FVC (per 1%) | |
|---|---|---|---|
| MRI outcomes | Beta (95% CI) | Beta (95% CI) | Beta (95% CI) |
| Brain volume (cm3)a | |||
| Total braina | 8.62 (2.54, 14.71) | 10.00 (4.80, 15.20) | −0.11 (−0.42, 0.19) |
| Frontal lobea | 1.53 (0.33, 2.73) | 1.27 (0.24, 2.30) | 0.03 (−0.03, 0.09) |
| Temporal lobea | 1.47 (0.56, 2.39) | 1.15 (0.37, 1.94) | 0.02 (−0.02, 0.07) |
| Occipital lobea | 0.38 (−0.11, 0.87) | 0.34 (−0.08, 0.76) | −0.00 (−0.03, 0.02) |
| Parietal lobea | 1.03 (0.13, 1.94) | 0.98 (0.20, 1.76) | 0.01 (−0.04, 0.05) |
| Deep gray mattera | 0.42 (0.05, 0.79) | 0.30 (−0.02, 0.62) | 0.01 (−0.01, 0.03) |
| Temporal-parietal lobe meta ROIa | 0.80 (0.26, 1.35) | 0.65 (0.18, 1.12) | 0.01 (−0.02, 0.04) |
| WMH volume (natural log-cm3)a | −0.12 (−0.22, −0.02) | −0.14 (−0.23, −0.05) | 0.00 (−0.00, 0.01) |
| OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| Microbleedsb | 1.06 (0.77, 1.47) | 1.02 (0.77, 1.34) | 1.01 (0.99, 1.02) |
| Infarctsb | 0.81 (0.59, 1.12) | 0.84 (0.64, 1.11) | 1.00 (0.98, 1.02) |
Note: CI, Confidence Intervals; FEV1, Forced Expiratory Volume in one second; FVC, Forced Vital Capacity; MRI, Magnetic Resonance Imaging; OR, Odds Ratio; ROI, Regions of Interest; WMH, White Matter Hyperintensity
Linear regression models were used for the continuous outcomes (i.e., brain and WMH volumes).
Logistic regression models were used for the dichotomous outcomes (i.e., microbleeds and infarcts).
Models were adjusted for age, sex, education, center-race, smoking status, height, weight, physical activity, diabetes, prevalent coronary heart disease, hypertension status, total cholesterol, APOE ε4 carrier status, and total estimated intracranial volume.
FEV1, FVC, and FEV1/FVC were modeled separately.
Figure 1:
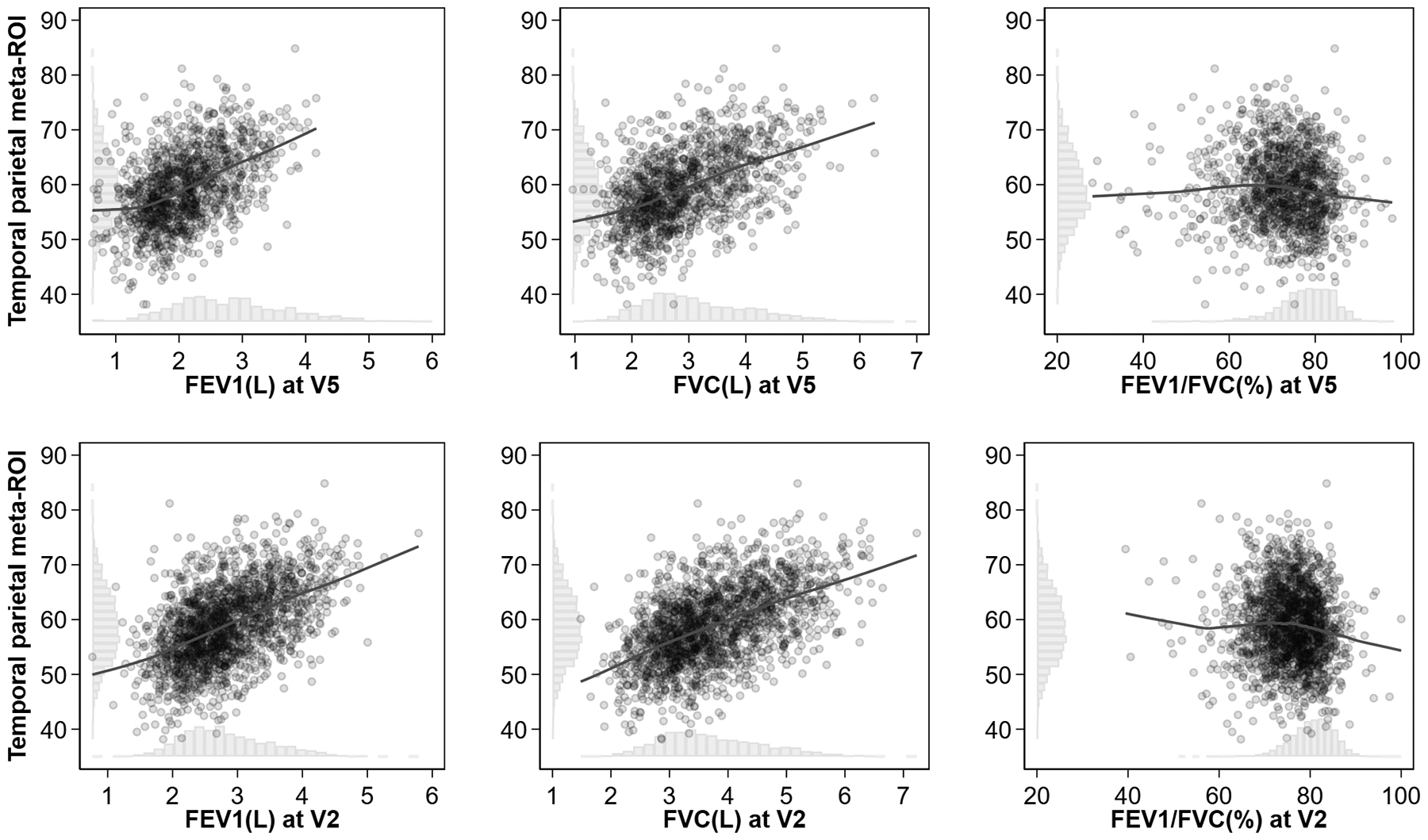
Scatter plots and locally weighted smoothing plots of temporal-parietal meta-ROI volume and lung function measures and their histograms in the Atherosclerosis Risk in Communities Neurocognitive Study
Histograms on the X- and Y-axes show the distribution of lung function measures and temporal-parietal meta-ROI volume and lung function measures, respectively. Temporal-parietal meta-ROI volume was expressed in cm3, FEV1 and FVC in liters, and FEV1/FVC in %.
Abbreviations: FEV1, Forced Expiratory Volume in one second; FVC, Forced Vital Capacity; ROI, region of interest; V2, Visit 2; V5, Visit 5
Figure 2:
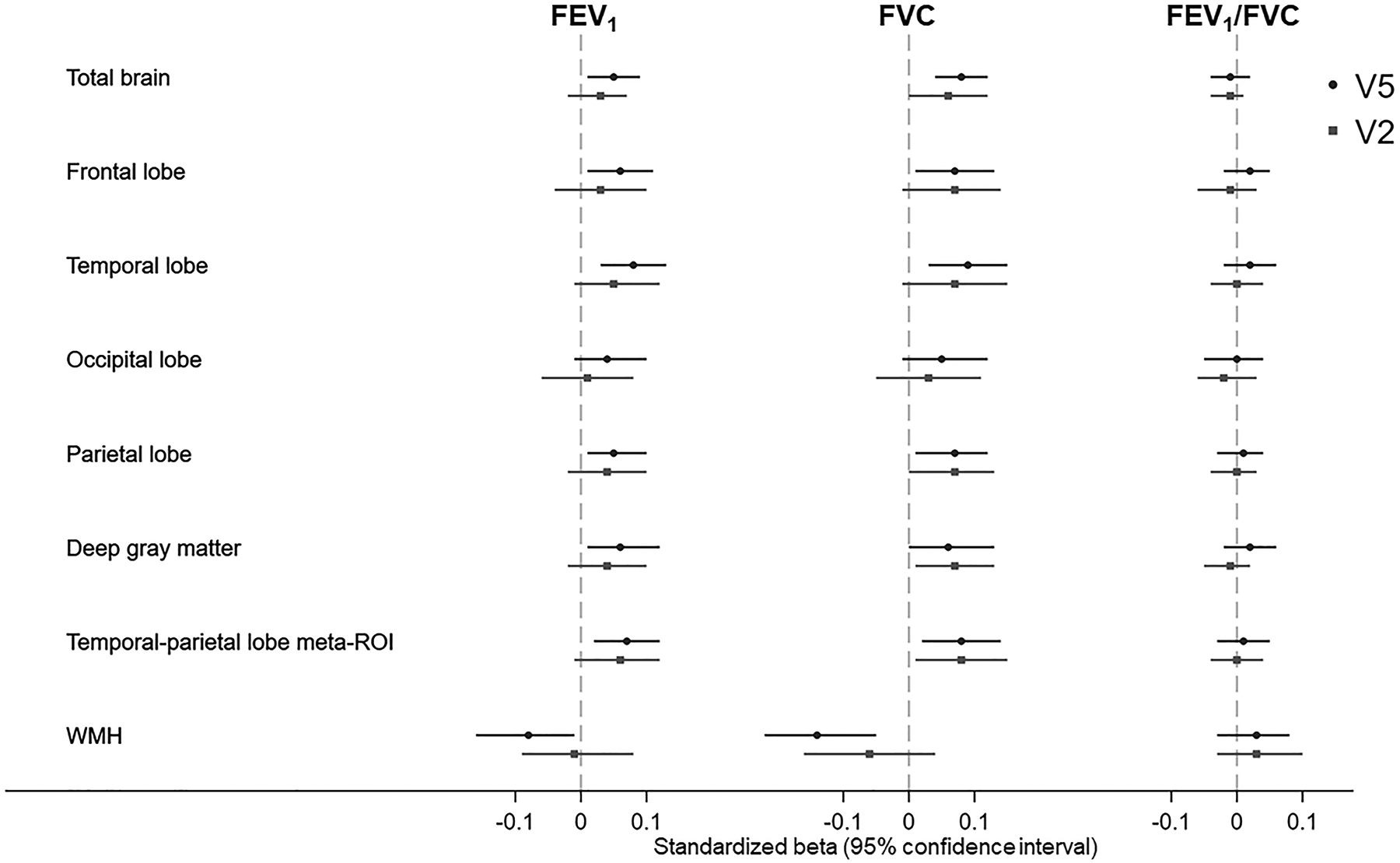
Associations of lung function measures with brain region and white matter hyperintensity volumes in the Atherosclerosis Risk in Communities Neurocognitive Study
All lung function measures and brain measures were standardized (See Supplementary Table 8 for details on standardization procedures and standardized beta estimates and their 95% confidence intervals). Brain volumes were expressed in cm3, WMH in natural log-cm3, FEV1 and FVC in liters, and FEV1/FVC in %. Round and square data points indicate associations for visit 5 and visit 2 lung function measures, respectively.
Models were adjusted for age, sex, education, center-race, education, smoking status, height, weight, physical activity, diabetes, prevalent coronary heart disease, hypertension status, total cholesterol, APOE ε4 carrier status, and estimated total estimated intracranial volume.
Abbreviations: CI, Confidence Intervals; FEV1, Forced Expiratory Volume in one second; FVC, Forced Vital Capacity; SD, Standard Deviation; WMH, White Matter Hyperintensity Volume; V2, Visit 2; V5, Visit 5
In the analysis examining clinical lung conditions, compared to individuals with normal lung function, those with restrictive (n=78) and obstructive lung (n=180) conditions generally had smaller brain volumes (Supplementary Table 6). However, associations with restrictive conditions were statistically significant for only total brain, temporal, and parietal lobe regions, and those with obstructive conditions were significant for only temporal lobe and deep gray matter volumes. Restrictive conditions were also associated with higher WMH volume (β=0.37 (95% CI: 0.18, 0.55) and higher odds of infarcts (OR=1.96 (95% CI: 1.14, 3.39, 26 of the 78 had infarcts)).
FEV1 and FVC were generally positively associated with brain volume measures in both never- (n=537) and ever-smokers (n=686) (Supplementary Table 7). However, there was also no clear pattern of associations across the never- and ever-smoker groups. For example, FEV1 and FVC were significantly associated with total brain volume only among ever-smokers, but frontal lobe volume showed significant association with FEV1 only in never-smokers and with FVC only in ever-smokers. The interaction terms between smoking status and lung function measures were not significant for any volumetric measures (i.e., p ≥ 0.05). Associations with WMH volume were generally similar between never- and ever-smokers. FVC was associated with lower odds of microbleeds among never-smokers and higher odds among ever smokers (p-interaction ≤0.05).
Cross-temporal associations: midlife lung function and brain MRI measures
In the primary analysis incorporating IPW (Table 3), only FVC was significantly associated with total brain, parietal, deep gray matter, and temporal-parietal lobe volumes. Although statistically not significant, higher midlife FEV1 and FVC in general were associated with larger brain volumes, with magnitudes generally similar to those of later-life lung function measures (Figures 1 and 2, Standardized results in Supplementary Table 8). Higher FEV1 and FEV1/FVC had supported associations with higher odds of microbleeds, which appeared to be driven by the ever-smokers (see below). In the unweighted regression analyses (Supplementary Table 9), FEV1 associations were similar, but FVC associations were attenuated compared to the weighted analyses.
Table 3:
Cross-temporal associations between midlife (visit 2) lung function measures and later-life (visit 5) brain MRI outcomes in the Atherosclerosis Risk in Communities Neurocognitive Study (n=1787)
| FEV1 (per 1L) | FVC (per 1L) | FEV1/FVC (per 1%) | |
|---|---|---|---|
| MRI outcomes | Beta (95% CI) | Beta (95% CI) | Beta (95% CI) |
| Brain volume (cm3)a | |||
| Total braina | 3.93 (−3.67, 11.52) | 7.36 (0.41, 14.30) | −0.24 (−0.72, 0.23) |
| Frontal lobea | 0.69 (−0.88, 2.25) | 1.16 (−0.14, 2.47) | −0.03 (−0.14, 0.07) |
| Temporal lobea | 0.88 (−0.22, 1.98) | 0.88 (−0.10, 1.86) | −0.00 (−0.07, 0.07) |
| Occipital lobea | 0.08 (−0.48, 0.63) | 0.18 (−0.29, 0.65) | −0.02 (−0.05, 0.02) |
| Parietal lobea | 0.75 (−0.31, 1.80) | 0.90 (0.03, 1.77) | −0.01 (−0.08, 0.06) |
| Deep gray mattera | 0.25 (−0.10, 0.60) | 0.33 (0.05, 0.62) | −0.01 (−0.03, 0.02) |
| Temporal-parietal lobe meta ROIa | 0.57 (−0.09, 1.22) | 0.61 (0.07, 1.15) | −0.00 (−0.05, 0.05) |
| WMH volume (natural log-cm3)a | −0.01 (−0.12, 0.10) | −0.06 (−0.16, 0.04) | 0.00 (−0.00, 0.01) |
| OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| Microbleedsb | 1.35 (1.01, 1.82) | 1.10 (0.84, 1.45) | 1.03 (1.01, 1.05) |
| Infarctsb | 0.80 (0.58, 1.12) | 0.87 (0.65, 1.16) | 0.99 (0.97, 1.01) |
Note: CI, Confidence Intervals; FEV1, Forced Expiratory Volume in one second; FVC, Forced Vital Capacity; MRI, Magnetic Resonance Imaging; OR, Odds Ratio; ROI, Regions of Interest; WMH, White Matter Hyperintensity
Linear regression models were used for the continuous outcomes (i.e., brain and WMH volumes).
Logistic regression models were used for the dichotomous outcomes (i.e., microbleeds and infarcts).
Models were adjusted for age, sex, education, center-race, smoking status, height, weight, physical activity, diabetes, prevalent coronary heart disease, hypertension status, total cholesterol, APOE ε4 carrier status, and total estimated intracranial volume.
FEV1, FVC, and FEV1/FVC were modeled separately. We applied inverse probability of attrition weights to regression models to account for selective attrition from visit 2 to visit 5 and selection for MRI.
Restrictive (n=56) and obstructive (n=251) clinical conditions were also associated with smaller brain volumes, although none were statistically significant. Higher odds of cerebral infarcts were seen in restrictive conditions (OR=1.94 (95% CI: 0.99, 3.77), restrictive cases with infarcts=21) and obstructive conditions (OR=1.44 (95% CI: 1.00, 2.07), obstructive cases with infarcts=68). Only restrictive conditions were associated with higher WMH volume (β=0.30 (95% CI: 0.07, 0.54), Supplementary Table 10).
When adjusted for cigarette pack-years, the findings were comparable to those of primary analyses (Supplementary Table 11). Further, when associations were estimated by smoking status, we did not see any significant associations in never-smokers (n=845), but higher FEV1 and FVC were significantly associated with higher brain volumes of most of the regions in ever-smokers (n=942) (Supplementary Table 12). The interaction terms of smoking status with FEV1 and FVC were significant (p≤0.05) for the total brain, parietal, and temporal-parietal lobe meta ROI volumes, and additionally with FVC for frontal lobe volume. Higher FEV1 and FEV1/FVC were associated with elevated microbleeds prevalence only in ever-smokers. Statistical associations between visit 2 FEV1 levels and visit 5 microbleeds appeared to be driven by smokers, and potentially by a small number of participants with extremely low visit 2 FEV1 levels and rare microbleed occurrence (Supplementary Figure 3).
DISCUSSION
In this study of community-dwelling United States adults, better midlife FVC and later-life FEV1 and FVC were associated with larger (better) later-life brain volumes, while clinical lung conditions were often associated with smaller brain volumes. Better later-life lung function measures in general were also associated with lower WMH volume, but only midlife restrictive lung condition showed an association with WMH volume. In addition, restrictive lung condition in both midlife and later-life and obstructive condition in later-life were associated with elevated cerebral infarct prevalence (although these analyses were based on a small number of exposed infarct cases). Findings for cerebral microbleeds, however, were somewhat unexpected, with higher lung function measures associated with elevated prevalence specifically in ever-smokers at both mid- and later-life.
Previously, in a subsample of the ARIC study (n=1917), lower FEV1 and FVC assessed at the visit 1 and 2 exams (1987–1992) were associated with higher prevalence odds of subclinical cerebral infarction and white matter lesions at visit 3 in 1993–1995 [15]. In the 10-year follow-up (n=1112), higher FEV1 was associated with slower progression of ventricular size worsening but not with WMH or infarct worsening [20]. Our current investigation extends these prior ARIC studies by evaluating comprehensive brain MRI markers in relation to both midlife and later-life lung function measures. However, as the earlier visits used 1.5T scans (compared to 3T scans in the visit 5 exam) and different MRI protocols for neuropathology grading and as there was a limited overlapping of participants, we were not able to examine longitudinal changes in brain neuropathology in our current analysis.
Only a few other studies have evaluated associations between lung function measures and brain volumes [11, 12, 33, 34]; while their findings are consistent with ours in the overall sample, they did not examine potential heterogeneity in the associations with brain volumes by smoking status. For example, in the US-based Rush Memory and Aging Project (MAP) (mean age=79±8 years, n=351), a better composite lung function score was associated with larger concurrent total brain and white and gray matter volumes [12]. In a cross-sectional study of 60–64 years olds from Australia (n=469), higher FEV1 and FVC but not FEV1/FVC were associated with reduced mid-ventricular ventricle-to-brain ratio [11]. A recent 2022 study that performed a coordinated analysis using data from six community-based cohorts from the US and Europe (n=11,901) reported cross-sectional associations of higher FEV1, FVC, and FEV1/FVC ratio with larger brain volumes; this analysis also included cross-sectional associations between visit 5 lung function and brain volumes from ARIC participants [33]. Further, in a recently published (2022) study using the UK Biobank data (n=30,159), both obstructive and restrictive lung impairments were associated with smaller brain volumes of some brain regions [34].
Likewise, studies have linked lung function with subclinical cerebrovascular disease markers, including white matter abnormalities and lacunar infarcts, although inconsistently [11–14, 16, 17, 33, 35]. Especially, with microbleeds for which we found contrasting results, in the Rotterdam study (median age=78 years, n=810), COPD was associated with higher microbleeds prevalence overall and higher incidence in deep or infra-tentorial regions (median follow-up: 3.42 years); associations with prevalent microbleeds in deep infra-tentorial regions were also observed for restrictive lung disease and other lung function measures [18]; however, a recent 2022 Rotterdam Study-based investigation (n=3941) did not find association with cerebral microbleeds [19].
To evaluate the influence of smoking on the associations of lung function with brain MRI markers, we estimated associations separately for ever-smokers and never-smokers. However, the results were not straightforward. For example, the associations of later-life lung function measures with concurrent brain and WMH volumes were somewhat comparable among never-smokers and ever-smokers, suggesting these later-life associations are not explained by smoking in entirety. However, the associations of midlife measures were seen only in ever-smokers suggesting that smoking may have driven the associations with midlife lung function. Contrary to this, in the recent cohort-wide investigation in the ARIC study, midlife lung function measures were associated with reduced dementia risk and cognitive decline even among never-smokers [27, 36]. Most of the prior studies with brain MRI markers only considered adjusting for smoking in their analysis; a few prior studies that evaluated associations by smoking status did not find any difference between the groups [13, 35]. Further, for microbleeds, better lung function was associated with higher prevalence in ever-smokers, which is unexpected. Prior studies have linked smoking with higher microbleeds, although inconsistently; in the ARIC study, smoking was not associated with cerebral microbleeds [37–39]. Further, cerebral microbleeds have been shown to correlate with other subclinical markers of cerebrovascular disease and linked with dementia, stroke, and other cerebrovascular outcomes, although not consistently [38, 40, 41]. We do not have plausible explanations for why we would see such contrasting findings only for cerebral microbleeds. One possibility for such unexpected findings is that the dropout rate of individuals with cerebral microbleeds and poor lung function (due to smoking) from the study might have been so extreme that it led to the spurious reverse associations. Alternatively, these could be chance findings. Further, although we adjusted for smoking in the primary analyses, we were unable to account for more in-depth information such as depth of inhaling, cigarette types, and other smoking patterns. So, the primary analyses and the analysis restricted to ever-smokers could be subject to bias due to residual confounding.
Mechanistically, how poor lung function may lead to adverse brain neuropathology is not clearly understood. Poor lung function is associated with systemic inflammation, oxidative stress, and chronic hypoxia which may alter brain pathophysiology through multiple pathways promoting neurodegenerative processes and cerebrovascular dysfunctions [8–10, 42–44]. A shared underlying pathology between pulmonary and cardiovascular conditions and/or correlated exposures associated with both conditions is another potential explanation. A recently published (2022) Mendelian Randomization (MR) study suggested associations of lung function measures with a range of cardiovascular risk factors and risk of stroke, implicating the role of lung function-mediated vascular contribution to brain neuropathological changes [45]. However, another MR study did not find evidence for the association between lung function and Alzheimer’s dementia, suggesting that we cannot rule out that the associations with some markers could be due to unexplained and residual confounding [46].
Using data from the well-characterized ARIC study, we were able to examine a broad range of MRI markers; however, our study has several limitations. The cross-temporal and cross-sectional nature of our analyses limit us from making any causal inferences. Although we had information on both mid- and later-life lung function measures, we did not have repeated brain MRI assessments that would have allowed examination of longitudinal changes in both lung function and brain MRI and thus have provided additional understanding of the associations. Further, brain MRI outcomes were measured among those who survived or agreed to participate in visit 5. Poor lung function is associated with mortality and other adverse health outcomes. While we applied inverse probability of weights to account for potential bias due to selective attrition to make inferences about all visit 2 participants for midlife analysis, we cannot completely rule out bias due to selective participation. Further, the restrictive lung function analysis (due to a small number of participants with restrictive lung conditions) and smoking-stratified analysis (especially, for current smokers) are more likely to be affected by the issues arising from small sample sizes, including low statistical power and potential sparse data bias. Lastly, our study findings may not be generalized to individuals who are not of Black or White race.
In conclusion, our findings suggest very modest associations of lung function measures with brain volumes and, to some extent, with some markers of subclinical cerebrovascular diseases. These associations persisted even after accounting for baseline cardiovascular risk factors which have been generally attributed to the link between lung function and adverse brain neuropathology. Specially, associations of midlife lung function and future brain volumes measured 23 years later hint at the etiological relevance of lung function in early neurodegenerative pathology, although future investigations on longitudinal changes of these MRI markers are needed for more definite evidence.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank the staff and participants of the ARIC study for their important contributions.
FUNDING
The Atherosclerosis Risk in Communities study has been funded in whole or in part with Federal funds from the National Heart, Lung, and Blood Institute (NHLBI), National Institutes of Health (NIH), Department of Health and Human Services, under Contract nos. (75N92022D00001, 75N92022D00002, 75N92022D00003, 75N92022D00004, 75N92022D00005). The ARIC Neurocognitive Study is supported by U01HL096812, U01HL096814, U01HL096899, U01HL096902, and U01HL096917 from the NIH (NHLBI, National Institute of Neurological Disorders and Stroke (NINDS), National Institute on Aging, and National Institute on Deafness and Other Communication Disorders). Dr. Gottesman is supported by the NINDS Intramural Research Program. Dr. Lutsey is supported by NHLBI K24-HL159246. Dr. London is supported by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences (ZO1 ES043012).
Footnotes
CONFLICT OF INTERESTS
The authors have no conflict of interest to report.
DATA AVAILABILITY
The ARIC study data used for this analysis are available to qualified investigators on request. Details on data availability and study protocols can be accessed at the ARIC website https://sites.cscc.unc.edu/aric/
REFERENCES
- [1].Gilsanz P, Mayeda ER, Flatt J, Glymour MM, Quesenberry CP Jr., Whitmer RA (2018) Early Midlife Pulmonary Function and Dementia Risk. Alzheimer Dis Assoc Disord 32, 270–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Guo X, Waern M, Sjogren K, Lissner L, Bengtsson C, Bjorkelund C, Ostling S, Gustafson D, Skoog I (2007) Midlife respiratory function and Incidence of Alzheimer’s disease: a 29-year longitudinal study in women. Neurobiol Aging 28, 343–350. [DOI] [PubMed] [Google Scholar]
- [3].Portegies ML, Lahousse L, Joos GF, Hofman A, Koudstaal PJ, Stricker BH, Brusselle GG, Ikram MA (2016) Chronic Obstructive Pulmonary Disease and the Risk of Stroke. The Rotterdam Study. Am J Respir Crit Care Med 193, 251–258. [DOI] [PubMed] [Google Scholar]
- [4].Qiao H, Chen M, Li S, Li Y, Sun Y, Wu Y (2020) Poor lung function accelerates cognitive decline in middle-aged and older adults: Evidence from the English Longitudinal Study of Ageing. Arch Gerontol Geriatr 90, 104129. [DOI] [PubMed] [Google Scholar]
- [5].Russ TC, Kivimaki M, Batty GD (2020) Respiratory Disease and Lower Pulmonary Function as Risk Factors for Dementia: A Systematic Review With Meta-analysis. Chest 157, 1538–1558. [DOI] [PubMed] [Google Scholar]
- [6].Silvestre OM, Nadruz W Jr., Querejeta Roca G, Claggett B, Solomon SD, Mirabelli MC, London SJ, Loehr LR, Shah AM (2018) Declining Lung Function and Cardiovascular Risk: The ARIC Study. J Am Coll Cardiol 72, 1109–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Lahousse L, Tiemeier H, Ikram MA, Brusselle GG (2015) Chronic obstructive pulmonary disease and cerebrovascular disease: A comprehensive review. Respir Med 109, 1371–1380. [DOI] [PubMed] [Google Scholar]
- [8].Dodd JW (2015) Lung disease as a determinant of cognitive decline and dementia. Alzheimers Res Ther 7, 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Gibson GE, Pulsinelli W, Blass JP, Duffy TE (1981) Brain dysfunction in mild to moderate hypoxia. Am J Med 70, 1247–1254. [DOI] [PubMed] [Google Scholar]
- [10].Row BW (2007) Intermittent hypoxia and cognitive function: implications from chronic animal models. Adv Exp Med Biol 618, 51–67. [DOI] [PubMed] [Google Scholar]
- [11].Sachdev PS, Anstey KJ, Parslow RA, Wen W, Maller J, Kumar R, Christensen H, Jorm AF (2006) Pulmonary function, cognitive impairment and brain atrophy in a middle-aged community sample. Dement Geriatr Cogn Disord 21, 300–308. [DOI] [PubMed] [Google Scholar]
- [12].Wang J, Song R, Dove A, Qi X, Ma J, Laukka EJ, Bennett DA, Xu W (2021) Pulmonary function is associated with cognitive decline and structural brain differences. Alzheimers Dement. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Guo X, Pantoni L, Simoni M, Gustafson D, Bengtsson C, Palmertz B, Skoog I (2006) Midlife respiratory function related to white matter lesions and lacunar infarcts in late life: the Prospective Population Study of Women in Gothenburg, Sweden. Stroke 37, 1658–1662. [DOI] [PubMed] [Google Scholar]
- [14].Kim Y, Lee H, Son TO, Jang H, Cho SH, Kim SE, Kim SJ, Lee JS, Kim JP, Jung YH, Lockhart SN, Kim HJ, Na DL, Park HY, Seo SW (2020) Reduced forced vital capacity is associated with cerebral small vessel disease burden in cognitively normal individuals. Neuroimage Clin 25, 102140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Liao D, Higgins M, Bryan NR, Eigenbrodt ML, Chambless LE, Lamar V, Burke GL, Heiss G (1999) Lower pulmonary function and cerebral subclinical abnormalities detected by MRI: the Atherosclerosis Risk in Communities study. Chest 116, 150–156. [DOI] [PubMed] [Google Scholar]
- [16].Longstreth WT Jr., Manolio TA, Arnold A, Burke GL, Bryan N, Jungreis CA, Enright PL, O’Leary D, Fried L (1996) Clinical correlates of white matter findings on cranial magnetic resonance imaging of 3301 elderly people. The Cardiovascular Health Study. Stroke 27, 1274–1282. [DOI] [PubMed] [Google Scholar]
- [17].Takamatsu K, Park K, Yokoyama A (2021) Association between airflow limitation and leukoaraiosis of the brain. Respir Investig 59, 320–326. [DOI] [PubMed] [Google Scholar]
- [18].Lahousse L, Vernooij MW, Darweesh SK, Akoudad S, Loth DW, Joos GF, Hofman A, Stricker BH, Ikram MA, Brusselle GG (2013) Chronic obstructive pulmonary disease and cerebral microbleeds. The Rotterdam Study. Am J Respir Crit Care Med 188, 783–788. [DOI] [PubMed] [Google Scholar]
- [19].Xiao T, Wijnant SRA, van der Velpen I, Terzikhan N, Lahousse L, Ikram MK, Vernooij MW, Brusselle GG, Ikram MA (2022) Lung function impairment in relation to cognition and vascular brain lesions: the Rotterdam Study. J Neurol 269, 4141–4153. [DOI] [PubMed] [Google Scholar]
- [20].Knopman DS, Penman AD, Catellier DJ, Coker LH, Shibata DK, Sharrett AR, Mosley TH Jr. (2011) Vascular risk factors and longitudinal changes on brain MRI: the ARIC study. Neurology 76, 1879–1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Wright JD, Folsom AR, Coresh J, Sharrett AR, Couper D, Wagenknecht LE, Mosley TH Jr., Ballantyne CM, Boerwinkle EA, Rosamond WD, Heiss G (2021) The ARIC (Atherosclerosis Risk In Communities) Study: JACC Focus Seminar 3/8. J Am Coll Cardiol 77, 2939–2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].ARIC Investigators (1989) The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. Am J Epidemiol 129, 687–702. [PubMed] [Google Scholar]
- [23].The ARIC Investigators (1987) Atherosclerosis Risk in Communities Study Manual 4: Pulmonary Function. National Heart, Lung, and Blood Institute of the National Institutes of Health, Collaborative Studies Coordinating Center, Chapel Hill, NC. https://sites.cscc.unc.edu/aric/sites/default/files/public/manuals/Pulmonary_Function_Assessment.1_4.pdf Accessed on February 20, 2021 [Google Scholar]
- [24].The ARIC Pulmonary Function Expert Team (2011) Atherosclerosis Risk in Communities Study Manual 4a: Pulmonary Function. https://sites.cscc.unc.edu/aric/sites/default/files/public/manuals/Manual%204a%20Pulmonary%20Function.pdf Accessed on February 20, 2021
- [25].Baugh AD, Shiboski S, Hansel NN, Ortega V, Barjakteravic I, Barr RG, Bowler R, Comellas AP, Cooper CB, Couper D, Criner G, Curtis JL, Dransfield M, Ejike C, Han MK, Hoffman E, Krishnan J, Krishnan JA, Mannino D, Paine R 3rd, Parekh T, Peters S, Putcha N, Rennard S, Thakur N, Woodruff PG (2022) Reconsidering the Utility of Race-Specific Lung Function Prediction Equations. Am J Respir Crit Care Med 205, 819–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Elmaleh-Sachs A, Balte P, Oelsner EC, Allen NB, Baugh A, Bertoni AG, Hankinson JL, Pankow J, Post WS, Schwartz JE, Smith BM, Watson K, Barr RG (2022) Race/Ethnicity, Spirometry Reference Equations, and Prediction of Incident Clinical Events: The Multi-Ethnic Study of Atherosclerosis (MESA) Lung Study. Am J Respir Crit Care Med 205, 700–710. [DOI] [PubMed] [Google Scholar]
- [27].Lutsey PL, Chen N, Mirabelli MC, Lakshminarayan K, Knopman DS, Vossel KA, Gottesman RF, Mosley TH, Alonso A (2019) Impaired Lung Function, Lung Disease, and Risk of Incident Dementia. Am J Respir Crit Care Med 199, 1385–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Quanjer PH, Stanojevic S, Cole TJ, Baur X, Hall GL, Culver BH, Enright PL, Hankinson JL, Ip MS, Zheng J, Stocks J, Initiative ERSGLF (2012) Multi-ethnic reference values for spirometry for the 3–95-yr age range: the global lung function 2012 equations. Eur Respir J 40, 1324–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Knopman DS, Griswold ME, Lirette ST, Gottesman RF, Kantarci K, Sharrett AR, Jack CR Jr., Graff-Radford J, Schneider AL, Windham BG, Coker LH, Albert MS, Mosley TH Jr., ARIC Neurocognitive Investigators (2015) Vascular imaging abnormalities and cognition: mediation by cortical volume in nondemented individuals: atherosclerosis risk in communities-neurocognitive study. Stroke 46, 433–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Schneider ALC, Selvin E, Sharrett AR, Griswold M, Coresh J, Jack CR Jr., Knopman D, Mosley T, Gottesman RF (2017) Diabetes, Prediabetes, and Brain Volumes and Subclinical Cerebrovascular Disease on MRI: The Atherosclerosis Risk in Communities Neurocognitive Study (ARIC-NCS). Diabetes Care 40, 1514–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Sullivan KJ, Ranadive R, Su D, Neyland BR, Hughes TM, Hugenschmidt CE, Lockhart SN, Wong DF, Jack CR Jr., Gottesman RF, Mosley TH Jr., Griswold ME, Windham BG (2021) Imaging-based indices of Neuropathology and gait speed decline in older adults: the atherosclerosis risk in communities study. Brain Imaging Behav 15, 2387–2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Baecke JA, Burema J, Frijters JE (1982) A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr 36, 936–942. [DOI] [PubMed] [Google Scholar]
- [33].Frenzel S, Bis JC, Gudmundsson EF, O’Donnell A, Simino J, Yaqub A, Bartz TM, Brusselle GGO, Bulow R, DeCarli CS, Ewert R, Gharib SA, Ghosh S, Gireud-Goss M, Gottesman RF, Ikram MA, Knopman DS, Launer LJ, London SJ, Longstreth WT, Lopez OL, Melo van Lent D, O’Connor G, Satizabal CL, Shrestha S, Sigurdsson S, Stubbe B, Talluri R, Vasan RS, Vernooij MW, Volzke H, Wiggins KL, Yu B, Beiser AS, Gudnason V, Mosley T, Psaty BM, Wolters FJ, Grabe HJ, Seshadri S (2022) Associations of Pulmonary Function with MRI Brain Volumes: A Coordinated Multi-Study Analysis. J Alzheimers Dis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Zhou L, Yang H, Zhang Y, Li H, Zhang S, Li D, Ma Y, Hou Y, Lu W, Wang Y (2022) Association of impaired lung function with dementia, and brain magnetic resonance imaging indices: a large population-based longitudinal study. Age Ageing 51. [DOI] [PubMed] [Google Scholar]
- [35].van Dijk EJ, Vermeer SE, de Groot JC, van de Minkelis J, Prins ND, Oudkerk M, Hofman A, Koudstaal PJ, Breteler MM (2004) Arterial oxygen saturation, COPD, and cerebral small vessel disease. J Neurol Neurosurg Psychiatry 75, 733–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Shrestha S, Zhu X, London SJ, Sullivan KJ, Lutsey PL, Windham BG, Griswold ME, Mosley TH Jr. (2023) Association of Lung Function With Cognitive Decline and Incident Dementia in the Atherosclerosis Risk in Communities Study. Am J Epidemiol 192, 1637–1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Ding J, Sigurdsson S, Garcia M, Phillips CL, Eiriksdottir G, Gudnason V, van Buchem MA, Launer LJ (2015) Risk Factors Associated With Incident Cerebral Microbleeds According to Location in Older People: The Age, Gene/Environment Susceptibility (AGES)-Reykjavik Study. JAMA Neurol 72, 682–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Graff-Radford J, Simino J, Kantarci K, Mosley TH Jr., Griswold ME, Windham BG, Sharrett AR, Albert MS, Gottesman RF, Jack CR Jr., Vemuri P, Knopman DS (2017) Neuroimaging Correlates of Cerebral Microbleeds: The ARIC Study (Atherosclerosis Risk in Communities). Stroke 48, 2964–2972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Lu D, Liu J, MacKinnon AD, Tozer DJ, Markus HS (2021) Prevalence and Risk Factors of Cerebral Microbleeds: An Analysis From the UK Biobank. Neurology. [DOI] [PubMed] [Google Scholar]
- [40].Charidimou A, Shams S, Romero JR, Ding J, Veltkamp R, Horstmann S, Eiriksdottir G, van Buchem MA, Gudnason V, Himali JJ, Gurol ME, Viswanathan A, Imaizumi T, Vernooij MW, Seshadri S, Greenberg SM, Benavente OR, Launer LJ, Shoamanesh A, International M-MI (2018) Clinical significance of cerebral microbleeds on MRI: A comprehensive meta-analysis of risk of intracerebral hemorrhage, ischemic stroke, mortality, and dementia in cohort studies (v1). Int J Stroke 13, 454–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Wiegman AF, Meier IB, Schupf N, Manly JJ, Guzman VA, Narkhede A, Stern Y, Martinez-Ramirez S, Viswanathan A, Luchsinger JA, Greenberg SM, Mayeux R, Brickman AM (2014) Cerebral microbleeds in a multiethnic elderly community: demographic and clinical correlates. J Neurol Sci 345, 125–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Daulatzai MA (2013) Death by a thousand cuts in Alzheimer’s disease: hypoxia--the prodrome. Neurotox Res 24, 216–243. [DOI] [PubMed] [Google Scholar]
- [43].Lall R, Mohammed R, Ojha U (2019) What are the links between hypoxia and Alzheimer’s disease? Neuropsychiatr Dis Treat 15, 1343–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Austin V, Crack PJ, Bozinovski S, Miller AA, Vlahos R (2016) COPD and stroke: are systemic inflammation and oxidative stress the missing links? Clin Sci (Lond) 130, 1039–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Au Yeung SL, Borges MC, Lawlor DA, Schooling CM (2022) Impact of lung function on cardiovascular diseases and cardiovascular risk factors: a two sample bidirectional Mendelian randomisation study. Thorax 77, 164–171. [DOI] [PubMed] [Google Scholar]
- [46].Higbee D, Granell R, Walton E, Korologou-Linden R, Davey Smith G, Dodd J (2021) Examining the possible causal relationship between lung function, COPD and Alzheimer’s disease: a Mendelian randomisation study. BMJ Open Respir Res 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The ARIC study data used for this analysis are available to qualified investigators on request. Details on data availability and study protocols can be accessed at the ARIC website https://sites.cscc.unc.edu/aric/


