Abstract
A potent neutralizing Fab fragment from a long-term survivor of simian immunodeficiency virus (SIVsm) infection was used to construct a recombinant macaque immunoglobulin G1κ (IgG1κ) molecule, designated IgG1-201. A Chinese hamster ovary cell line expressing IgG1-201 was derived by stable transfection and optimized for antibody secretion by methotrexate selection and dihydrofolate reductase gene amplification. IgG1-201 effectively neutralized the homologous, molecularly cloned SIVsmH4 virus but had no activity against the heterologous SIVmac251/BK28 virus. The previously characterized, neutralization-resistant SIVsmE543-3 virus was also not neutralized by IgG1-201. Binding to SIVsmH4 gp120 was enhanced in the presence of recombinant soluble CD4, suggesting that IgG1-201 bound a CD4-induced epitope. IgG1-201 immunoprecipitated the SIVsmH4 but not the SIVsmE543-3 envelope despite a close relationship between these two clones. Immunoprecipitation of a panel of SIVsmH4/SIVsmE543-3 chimeric viruses tentatively assigned the neutralization epitope to the third constant domain, immediately C terminal to the V3 loop. These findings suggest the presence of at least one CD4-induced neutralization epitope on SIV, as is the case with human immunodeficiency virus type 1.
Initial efforts to develop an AIDS vaccine focused upon eliciting neutralizing antibodies using envelope-based immunogens. More recent efforts have included additional viral gene products with the goal of generating both cytotoxic T lymphocytes and neutralizing antibodies (5, 27, 33). The ability to generate a neutralizing antibody response that is capable of broadly neutralizing primary isolates of human immunodeficiency virus (HIV) has proven difficult and remains a major goal (5, 33). Despite the difficulties in generating a broadly neutralizing antibody response through immunization, passive immunoprophylaxis experiments with a number of different animal models have provided evidence that, under certain circumstances, neutralizing antibodies are indeed capable of preventing or modulating infection. A number of human monoclonal antibodies have been generated that, alone or in combination, effectively neutralize primary HIV type 1 (HIV-1) isolates in vitro (4, 10, 12, 23, 30, 31, 34, 40, 41). Some of these HIV-1 human monoclonal antibodies can protect against infection in the hu-PBL SCID model (1, 13, 35, 36). Passive infusion of a human monoclonal antibody specific for a conserved epitope on gp41 did not prevent infection of chimpanzees, but the treated animals controlled viremia better (9). Most convincingly, recent studies have demonstrated that neutralizing antibodies provided passively by human monoclonal antibodies alone or in combination with HIV immunoglobulin (Ig) can be highly effective in blocking infection in a pathogenic simian/human immunodeficiency virus (SHIV) macaque model (32). In a similar vein, IgG purified from an HIV-1-infected chimpanzee was effective in blocking SHIV infection of macaques (39).
Fewer studies have been performed with the simian immunodeficiency virus (SIV) macaque model (8, 15, 22, 29, 37, 43) due to a paucity in macaque monoclonal antibodies (14, 38). However, many of the neutralizing epitopes for SIV have been partially mapped by peptide scanning (20, 21) and mutational analysis (3, 6, 7, 25, 44). A number of studies conducted with variants of the SIVmac lineage have shown that discrete amino acid substitutions in the envelope glycoprotein can alter the neutralization phenotype (3, 25, 44). Such changes include the acquisition of novel glycosylation sites as a viral mechanism for avoiding recognition by neutralizing antibody (6).
In a previous report, we described the isolation of Fab fragments from a long-term survivor of SIVsm infection using phage display technology (2, 14). One of these Fab fragments, Fab 201, potently neutralized homologous SIVsm isolates but was ineffective in neutralizing the heterologous SIVmac isolates (14). Fab 201 competed with the mouse monoclonal antibodies, KK5 and KK9, that react with a conformational-dependent epitope on the V3 to V4 region of the SIV envelope (20, 21). In an effort to develop a SIV-specific antibody reagent that is suitable for passive immunotherapy trials, the present report describes the conversion of Fab 201 into a recombinant macaque IgG1κ molecule and its unique biological properties. In order to generate a neutralizing macaque monoclonal IgG antibody, it was necessary to (i) generate macaque heavy chain immunoglobulin genes, (ii) insert macaque heavy and light chain genes into appropriate eukaryotic expression vectors, and (iii) develop stable transfectants that expressed the recombinant IgG.
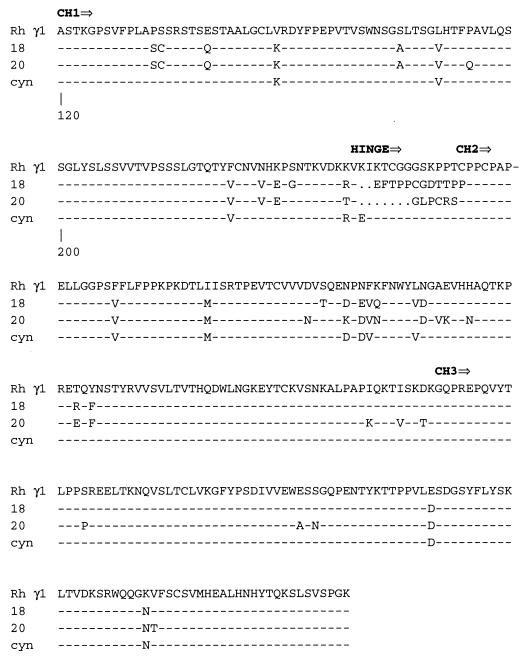
Rhesus macaque heavy chain immunoglobulin genes were amplified by PCR from bone marrow cDNA of RhE544, the macaque that was the source of Fab 201 (14). The PCR amplification used two N-terminal primers (5′-AGG TGC AGC TGC TCG AGT CTG G-3′ and 5′-CAG GTG CAG CTG CTC GAG TCG GG-3′) (13) and a single primer corresponding to the carboxy-terminal end of the fourth constant domain (5′-ATC ATA CGT AGA TAT CTA TCA TTT ACC CGG AGA CAC GGA GAG-3′). Twenty clones were obtained that included three unique sequences, as shown by the amino acid alignment of the constant regions of three representative clones in Fig. 1. Rhesus IgG1 clones similar to a cDNA clone isolated from cynomolgus monkey (Macaca fascicularis) (28) predominated (15 out of 20) and clearly were the simian equivalent of the human IgG1 isotype. The majority of variability between the unique clones was observed within the hinge region bridging the first and second constant domains. The two other clones (γ18 and γ20) were not related to any particular human gamma chain, although the hinge region of γ18 showed significant similarity to a human IgG pseudogene (19).
FIG. 1.
Alignment of rhesus monkey CH region amino acid sequences with the cynomolgus monkey CH1 sequence. The sequences are numbered according to the system described by Kabat et al. (19). A dash indicates identity to the rhesus monkey CH1 sequence, and a dot indicates a gap introduced to maximize alignment.
One of the rhesus IgG1 clones was used to generate a macaque heavy chain expression vector. The rhesus clone (a XhoI-BsaBI fragment) was substituted for the human homologue in the human heavy chain eukaryotic expression plasmid pCDHC68b (SmithKline Beecham Pharmaceuticals, King of Prussia, Pa.) (42). This created the macaque heavy chain expression vector pMmHCγ1, in which the gene was under the control of the human cytomegalovirus (CMV) promoter and included the amplifiable marker dihydrofolate reductase (dhFr) for selection purposes. The variable domain of the heavy chain portion of Fab 201 was then inserted into pMmHCγ1 using conserved XhoI and KasI sites to generate pMmHCγ1-201. To generate a light chain expression plasmid, the light chain of Fab 201 was inserted into the human light chain expression vector pCNHLC under the control of the human CMV promoter (SmithKline Beecham Pharmaceuticals) using SstI and XbaI sites to create pMmLCκ-201. This light chain vector encoded the neomycin resistance gene for selection in mammalian cells. Both clones contained the simian virus 40 origin of replication to allow expression in COS cells.
Expression and secretion of IgG were confirmed by cotransfection of COS cells with pMmHCγ1-201 and pMmLCκ-201 (data not shown). Stable IgG1-201-expressing cell clones were then established by cotransfection by electroporation of 10 μg each of the linearized heavy (pMmHCγ1-201) and light (pMmLCκ-201) chain plasmids into Chinese hamster ovary (CHO) cells lacking the gene for dihydrofolate reductase. Clones were selected with 300 μg of G418 sulfate (Gibco/BRL, Gaithersburg, Md.) per ml. Individual CHO clones were isolated and screened for antibody expression by enzyme-linked immunosorbent assay (ELISA) for IgG in the culture supernatant using goat anti-human IgG to coat plates and alkaline phosphatase-conjugated goat anti-human IgG Fc antibody (Pierce, Rockford, Ill.) for detection. Macaque polyclonal rhesus IgG was used to establish a standard curve for this assay. Those CHO clones that expressed the highest amount of antibody were selected for gene amplification with methotrexate. A stepwise increase in the concentration of methotrexate (5 μM, 10 μM, and 50 μM) resulted in 100-fold amplification of secretion (a maximum concentration of approximately 8 μg/ml). Analysis of this protein G-purified IgG1-201 by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) revealed the expected light and heavy chain proteins (data not shown).
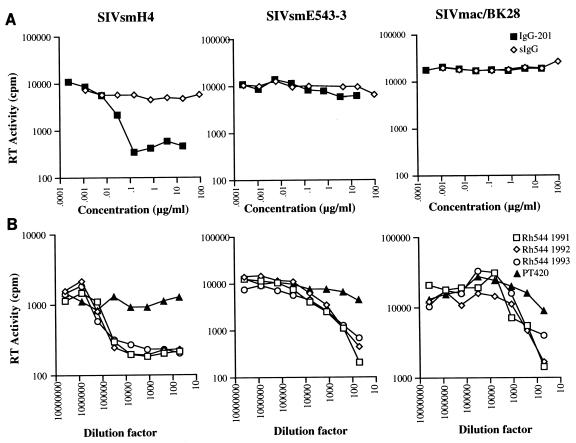
To evaluate the functional activity of the recombinant antibody, the ability of IgG1-201 to neutralize various SIV isolates was assessed using an assay based upon reduction in reverse transcriptase (RT) activity in culture supernatants. To ensure viral homogeneity we utilized molecularly cloned viruses as targets for neutralization. Cell-free virus stocks were produced by transfection of the various clones into 293 cells by the calcium phosphate method (Pharmacia, Piscataway, N.J.) and 100 50% tissue culture infectious doses (TCID50) of each virus stock were used for neutralization assays. As shown in Fig. 2, IgG1-201 efficiently neutralized SIVsmH4 (90% reduction in RT activity) but failed to neutralize the homologous neutralization-resistant strain SIVsmE543-3 (16) or the heterologous, laboratory-adapted strain SIVmac251/BK28 (26). To determine whether this strain-restricted pattern of neutralization was representative of the neutralizing activity in the serum of the donor animal, sequential plasma samples from the donor macaque (RhE544) were analyzed for neutralizing activity using plasma from an uninfected macaque (PT 420) as a negative control for neutralization. As shown in Fig. 2, plasma samples from RhE544 (from 1991 through 1993) efficiently neutralized SIVsmH4 but were much less effective in neutralizing either SIVsmE543-3 or SIVmac251/BK28. Thus, while 90% reduction endpoints were achieved with SIVsmH4, endpoints of only 50% were achieved with SIVsmE543-3 or SIVmac251/BK28.
FIG. 2.
In vitro neutralization of various molecularly cloned simian immunodeficiency viruses. (A) Neutralization profiles of IgG1-201 and polyclonal rhesus IgG (sIgG) against SIVsmH4 (17), SIVsmE543-3 (16), and SIVmac251/BK28 (23, 26); (B) neutralization profiles of sequential plasma samples from the donor monkey RhE544. Cell-free virus stocks were produced by transfection of the various clones into 293 cells by the calcium phosphate method (CellPhect kit; Pharmacia). Each stock was titrated for infectivity on CEMx174 cells. The neutralizing assay used inhibition of RT as an endpoint. Plasma samples were heat inactivated by incubation at 56°C for 30 min, diluted with phosphate-buffered saline, filtered, and stored at −20°C until use. Fivefold dilution series of purified antibody or plasma were prepared in triplicate, mixed with 100 TCID50 of virus in a 96-well plate and incubated for 1 h at 37°C. Exponentially growing CEMx174 cells (2 × 104) were added to each well. Cultures were fed at day 3 with a medium change of half of the culture supernatant, and on day 5, the supernatants were harvested and the RT transcriptase activity was determined.
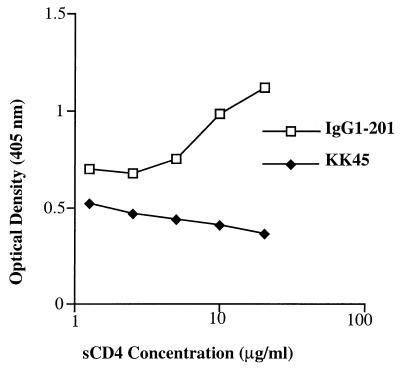
Our previous studies with Fab 201 suggested that it bound a conformational epitope spanning the V3 to V4 region of the SIV envelope, since it competed with the mouse monoclonal antibodies, KK5 and KK9, that had been mapped to bind in this region of the SIVmac envelope (20, 21). To more closely define the epitope recognized by IgG1-201, the ability of IgG1-201 to compete with soluble CD4 (sCD4) was evaluated. To evaluate interactions between IgG1-201 and recombinant sCD4, an ELISA was developed. Briefly, recombinant sCD4 and a mouse monoclonal antibody (sim.4) specific for human CD4 were obtained from the AIDS Research and Reference Reagent Program. ELISA plates (96-well) were coated overnight with recombinant SIVsm gp130 (a gift from Nancy Haigwood), blocked, and incubated with serial dilutions of sCD4 for 3 h at 37°C. Unbound sCD4 was removed by washing with Tris-borate-saline solution supplemented with 0.05% Tween 20, and fixed concentrations of the gp120-specific antibodies IgG1-201 and KK45 were added for 1 h at 37°C using triplicate samples for each concentration of antibody. As a positive control for sCD4 interaction with SIV gp120, sim.4 binding was detected with an alkaline phosphatase-conjugated mouse IgG-specific antibody (Jackson Immunolabs). Prebinding of increasing amounts of soluble human CD4 to recombinant monomeric SIVsmH4 gp120 produced in CHO cells actually enhanced the binding of IgG1-201 rather than blocking binding (Fig. 3). As a negative control, the binding of a mouse monoclonal antibody specific for a linear determinant in the V3 loop (KK45) was unaffected by sCD4. These data confirm the CD4 binding domain of gp120 is not a part of the epitope recognized by IgG1-201 but suggests that it may be in close proximity. The enhanced binding defines the IgG1-201 epitope as CD4 induced, similar to the observations for the human HIV-1-specific 17b monoclonal antibody (40, 41).
FIG. 3.
Enhanced binding of IgG1-201 in the presence of sCD4. Binding of IgG1-201 to SIV gp130 in the presence of increasing concentrations of sCD4. ELISA plates (96-well) were coated with recombinant SIVsmH4 gp120 (a gift from Nancy Haigwood) and incubated overnight. Plates were blocked and incubated with serial dilutions of sCD4 (AIDS Research and Reference Reagent Program, National Institute of Allergy and Infectious Diseases) for 3 h at 37°C. Unbound sCD4 was removed by washing with Tris-borate-saline solution supplemented with 0.05% Tween 20, and fixed concentrations of the gp120-specific antibodies IgG1-201 and KK45 were added for 1 h at 37°C.
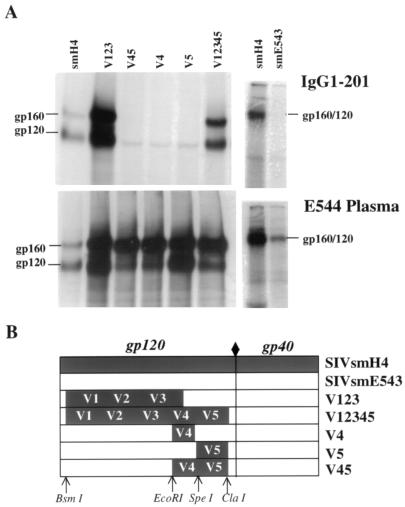
Since IgG1-201 was effective in neutralizing SIVsmH4 but had no effect on the closely related SIVsmE543-3, we used immunoprecipitation of radiolabeled cell lysates of virus-transfected 293 cells to determine whether IgG1-201 could bind the envelope glycoprotein of both of these viruses. As shown in Fig. 4, IgG1-201 immunoprecipitated envelope glycoproteins of SIVsmH4 but failed to react with the SIVsmE543-3 envelope glycoprotein. Control immunoprecipitations with plasma from an infected animal confirmed that both of these envelope glycoproteins were expressed at detectable levels. These data suggested that the epitope bound by IgG1-201 was either masked or not conserved between the SIVsmH4 and SIVsmE543-3 envelopes. This is an unanticipated finding, since SIVsmH4 is a molecular clone from the SIVsmF236 isolate used to inoculate rhesus E543, the source of SIVsmE543-3, and thus the two clones are closely related (92% identity in envelope).
FIG. 4.
Radioimmunoprecipitation of envelope proteins from cell lysates of 293 cells transfected with SIVsmH4, SIVsmE543-3, or chimeric envelopes that contained portions of the smH4 envelope (V12345, V123, V45, V4, and V5) as described in the text. 293 cells were transfected with cloned proviral DNA, pulsed with 35S-labeled cysteine and methionine for 24 h, and solubilized in 20 mM Tris buffer, pH 7.5, supplemented with 1% NP-40, 0.5% deoxycholate, and 313 mM NaCl. Radioimmunoprecipitation assays were carried out as previously described (11, 17) using protein A coupled to agarose beads. All samples were analyzed on a SDS–10% PAGE gel under reducing conditions. (A) Immunoprecipitation of the envelope glycoproteins of SIV chimeras from cell lysates of transfected 293 cells with recombinant antibody IgG1-201 (top) and plasma from an SIV-infected animal (bottom). (B) Schematic of the construction of the five envelope chimeras and parental clones used for immunoprecipitation shown in panel A. Sequences from SIVsmH4 are indicated in black and SIVsmE543-3 sequences are indicated in white, with relative locations of variable regions (V1 to V5) and restriction enzyme sites used for construction of chimeras indicated.
To more closely confirm the location of the IgG1-201 epitope, a series of chimeric viruses with exchanges of SIVsmH4 fragments into SIVsmE543-3 were generated using conserved restriction sites. Five chimeric viruses containing different segments of the smH4 envelope gene were constructed as detailed in Fig. 4B. These chimeras contained the SIVsmE543-3 genome with substitution of smH4 sequences for the entire envelope (BsmI to ClaI; V12345), the V1 to V3 region (BsmI to EcoRI; V123), the V4 to V5 region, including the CD4 binding site (EcoRI to ClaI; V45), the V4 region (EcoRI to SpeI; V4), or the V5 region (SpeI to ClaI; V5). Each chimeric envelope was cloned into the 3′-half clone of SIVsmE543-3 using the BsmI and ClaI sites. Full-length infectious clones were generated by ligation of the Csp45I to SalI fragment of the 3′-half clone with the 5′-half clone of SIVsmE543-3, as detailed previously for other chimeric SIV clones (18). The parental and chimeric virus clones were transiently transfected into 293 cells, and the ability of IgG1-201 to immunoprecipitate their envelope glycoproteins was evaluated.
As expected, IgG1-201 immunoprecipitated the envelope of the chimera which contained the entire gp120 envelope gene of smH4 (V12345). The only other envelope to be immunoprecipitated by IgG1-201 was from the chimera expressing the V1 to V3 region of SIVsmH4 (V123) (Fig. 4). The absence of binding to the V45 chimera confirmed that the CD4 binding site was not a part of the epitope recognized by IgG1-201. Based upon the proportion of precursor gp160 and gp120 visualized in immunoprecipitations, two of the chimeric envelopes exhibited an envelope-processing defect (V4 and V5). These virus supernatants displayed poor infectivity (data not shown) and were not evaluated further. However, as predicted by the binding assay, the chimeric virus that expressed the V1 to V3 domains of SIVsmH4 (the neutralization-sensitive virus clone) was efficiently neutralized by IgG1-201 (data not shown).
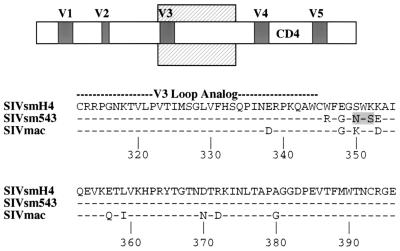
In a previous study (13), the epitope recognized by Fab 201 was mapped indirectly by competition ELISA with monoclonal antibodies of known specificity. Fab 201 competed with mouse monoclonal antibodies (KK5 and KK9) that react with a conformation-dependent epitope in the V3 to V4 region (7, 21). Previous studies with these mouse monoclonal antibodies have demonstrated that they are highly sensitive to substitutions within the V4 region (25). However, the V123 chimera but not the V4 or the V45 chimera was recognized by IgG1-201, restricting the region of the epitope to the V3 to C3 region. Examination of the amino acid sequence of this portion of the envelope revealed six substitutions in SIVsmE543-3 relative to smH4 (Fig. 5). Two of these substitutions (S-350-N and K-352-S) are responsible for introduction of an N-linked glycosylation site that is unique to SIVsmE543-3. This novel glycosylation site may be responsible for masking the neutralizing epitope in SIVsmE543-3. However, further site-directed mutagenesis will be necessary to confirm this hypothesis.
FIG. 5.
Alignment of SIVsmH4, SIVsmE543-3 (16), and SIVmac amino acid envelope (23) sequences from the V3 loop analog to the EcoRI site, the presumed area of the IgG1-201 epitope binding. Schematic representation of the envelope is shown above, with the region of the alignment designated by a cross-hatched box. The SIVsmH4 sequence is shown on the top, with dashes for SIVsmE543-3 and SIVmac below indicating amino acid identity. The SIVmac sequence is conserved between SIVmac239 and SIVmac251/BK28. The shaded box indicates a unique glycosylation site in the SIVsmE543-3 envelope.
Considering the immunoprecipitation data, CD4-induced binding, and relative substitutions in the envelope sequences of SIVsmH4 and SIVsmE543-3, we believe that residues immediately C terminal to the V3 loop are important components of the IgG1-201 epitope. The biological characteristics of IgG1-201 resemble those of the HIV-1-specific monoclonal antibodies 17b and 48d that block gp120-chemokine receptor binding and recognize an epitope that is induced by binding of gp120 to CD4 (40, 41). These two human monoclonal antibodies neutralize HIV-1 poorly in the absence of interaction with CD4, suggesting that this epitope is masked prior to conformational changes induced by binding to CD4. It will be critical to determine whether exposure of SIVsmH4 or SIVsmE543-3 to sCD4 would enhance neutralization of these isolates by IgG1-201. The present data suggest similarities in neutralizing epitopes between SIV-infected macaques and HIV-infected humans that confirm the usefulness of this model for addressing the role of neutralizing antibody in mediating vaccine protection.
Acknowledgments
We thank G. Dapolito, A. Hahn, and D. Adger-Johnson for technical assistance, D. Montefiori for critical review of the manuscript, and N. Haigwood for supplying recombinant SIVsm gp120.
REFERENCES
- 1.Andrus L, Prince A M, Bernal I, McCormack P, Lee D H, Gorny M K, Zolla-Pazner S. Passive immunization with a human immunodeficiency virus type 1-neutralizing monoclonal antibody in hu-PBL-SCID mice: isolation of a neutralization escape variant. J Infect Dis. 1998;177:889–897. doi: 10.1086/515251. [DOI] [PubMed] [Google Scholar]
- 2.Barbas C F, III, Kang A S, Lerner R A, Benkovic S J. Assembly of combinatorial antibody libraries on phage surfaces: the gene III site. Proc Natl Acad Sci USA. 1991;88:7978–7982. doi: 10.1073/pnas.88.18.7978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burns D P, Collignon C, Desrosiers R C. Simian immunodeficiency virus mutants resistant to serum neutralization arise during persistent infection of rhesus monkeys. J Virol. 1993;67:4104–4113. doi: 10.1128/jvi.67.7.4104-4113.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burton D R, Pyati J, Koduri R, Sharp S J, Thornton G B, Parren P W, Sawyer L S, Hendry R M, Dunlop N, Nara P L, et al. Efficient neutralization of primary isolates of HIV-1 by a recombinant human monoclonal antibody. Science. 1994;266:1024–1027. doi: 10.1126/science.7973652. [DOI] [PubMed] [Google Scholar]
- 5.Burton D R. A vaccine for HIV type 1: the antibody perspective. Proc Natl Acad Sci USA. 1997;94:10018–10023. doi: 10.1073/pnas.94.19.10018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chackerian B, Rudensey L M, Overbaugh J. Specific N-linked and O-linked glycosylation modifications in the envelope V1 domain of simian immunodeficiency virus variants that evolve in the host alter recognition by neutralizing antibodies. J Virol. 1997;71:7719–7727. doi: 10.1128/jvi.71.10.7719-7727.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choi W S, Collignon C, Thiriart C, Burns D P, Stott E J, Kent K A, Desrosiers R C. Effects of natural sequence variation on recognition by monoclonal antibodies neutralize simian immunodeficiency virus infectivity. J Virol. 1994;68:5395–5402. doi: 10.1128/jvi.68.9.5395-5402.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clements J E, Montelaro R C, Zink M C, Amedee A M, Miller S, Trichel A M, Jagerski B, Hauer D, Martin L N, Bohm R P, Murphey-Corb M. Cross-protective immune responses induced in rhesus macaques by immunization with attenuated macrophage-tropic simian immunodeficiency virus. J Virol. 1995;69:2737–2744. doi: 10.1128/jvi.69.5.2737-2744.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Conley A J, Kessler II J A, Boots L J, McKenna P M, Schleif W A, Emini E A, Mark III G E, Katinger H, Cobb E K, Lunceford S M, Rouse S R, Murthy K K. The consequence of passive administration of an anti-human immunodeficiency virus type 1 neutralizing monoclonal antibody before challenge of chimpanzees with a primary virus isolate. J Virol. 1996;70:6751–6758. doi: 10.1128/jvi.70.10.6751-6758.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ditzel H J, Parren P W, Binley J M, Sodroski J, Moore J P, Barbas III C F, Burton D R. Mapping the protein surface of human immunodeficiency virus type 1 gp120 using human monoclonal antibodies from phage display libraries. J Mol Biol. 1997;267:684–695. doi: 10.1006/jmbi.1997.0912. [DOI] [PubMed] [Google Scholar]
- 11.Freed E O, Risser R. The role of envelope glycoprotein processing in murine leukemia virus infection. J Virol. 1987;61:2852–2856. doi: 10.1128/jvi.61.9.2852-2856.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gauduin M C, Allaway G P, Maddon P J, Barbas III C F, Burton D R, Koup R A. Effective ex vivo neutralization of human immunodeficiency virus type 1 in plasma by recombinant immunoglobulin molecules. J Virol. 1996;70:2586–2592. doi: 10.1128/jvi.70.4.2586-2592.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gauduin M C, Parren P W, Weir R, Barbas C F, Burton D R, Koup R A. Passive immunization with a human monoclonal antibody protects hu-PBL-SCID mice against challenge by primary isolates of HIV-1. Nat Med. 1997;3:1389–1393. doi: 10.1038/nm1297-1389. [DOI] [PubMed] [Google Scholar]
- 14.Glamann J, Burton D R, Parren P W, Ditzel H J, Kent K A, Arnold C, Montefiori D, Hirsch V M. Simian immunodeficiency virus (SIV) envelope-specific Fabs with high-level homologous neutralizing activity: recovery from a long-term-nonprogressor SIV-infected macaque. J Virol. 1998;72:585–592. doi: 10.1128/jvi.72.1.585-592.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haigwood N L, Watson A, Sutton W F, McClure J, Lewis A, Ranchalis J, Travis B, Voss G, Letvin N L, Hu S L, Hirsch V M, Johnson P R. Passive immune globulin therapy in the SIV/macaque model: early intervention can alter disease profile. Immunol Lett. 1996;51:107–114. doi: 10.1016/0165-2478(96)02563-1. [DOI] [PubMed] [Google Scholar]
- 16.Hirsch V, Adger-Johnson D, Campbell B, Goldstein S, Brown C, Elkins W R, Montefiori D C. A molecularly cloned, pathogenic, neutralization-resistant simian immunodeficiency virus, SIVsmE543-3. J Virol. 1997;71:1608–1620. doi: 10.1128/jvi.71.2.1608-1620.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hirsch V M, Olmsted R A, Murphey-Corb M, Purcell R H, Johnson P R. An African primate lentivirus (SIVsm) closely related to HIV-2. Nature. 1989;339:389–392. doi: 10.1038/339389a0. [DOI] [PubMed] [Google Scholar]
- 18.Hirsch V M, Martin J E, Dapolito G, Elkins W R, London W T, Goldstein S, Johnson P R. Spontaneous substitutions in the vicinity of the V3 analog affect cell tropism and pathogenicity of simian immunodeficiency virus. J Virol. 1994;68:2649–2661. doi: 10.1128/jvi.68.4.2649-2661.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kabat E A, Wu T T, Perry H M, Gottesman K S, Foeller C. Sequences of proteins of immunological interest. 5th ed. Bethesda, Md: U.S. Department of Health and Human Services; 1991. [Google Scholar]
- 20.Kent K A, Gritz L, Stallard G, Cranage M P, Collignon C, Thiriart C, Corcoran T, Silvera P, Stott E J. Production of monoclonal antibodies to simian immunodeficiency virus envelope glycoproteins. AIDS. 1991;5:829–836. doi: 10.1097/00002030-199107000-00006. [DOI] [PubMed] [Google Scholar]
- 21.Kent K A, Rud E, Corcoran T, Powell C, Thiriart C, Collignon C, Stott E J. Identification of two neutralizing and 8 non-neutralizing epitopes on simian immunodeficiency virus envelope using monoclonal antibodies. AIDS Res Hum Retrovir. 1992;8:1147–1151. doi: 10.1089/aid.1992.8.1147. [DOI] [PubMed] [Google Scholar]
- 22.Kent K A, Kitchin P, Mills K H, Page M, Taffs F, Corcoran T, Silvera P, Flanagan B, Powell C, Rose J, et al. Passive immunization of cynomolgus macaques with immune sera or a pool of neutralizing monoclonal antibodies failed to protect against challenge with SIVmac251. AIDS Res Hum Retrovir. 1994;10:189–194. doi: 10.1089/aid.1994.10.189. [DOI] [PubMed] [Google Scholar]
- 23.Kessler J A, II, McKenna P M, Emini E A, Chan C P, Patel M D, Gupta S K, Mark III G E, Barbas III C F, Burton D R, Conley A J. Recombinant human monoclonal antibody IgG1b12 neutralizes diverse human immunodeficiency virus type 1 primary isolates. AIDS Res Hum Retrovir. 1997;13:575–582. doi: 10.1089/aid.1997.13.575. [DOI] [PubMed] [Google Scholar]
- 24.Kestler H W D, Li Y, Naidu Y M, Butler C V, Ochs M F, Jaenel G, King N W, Daniel M D, Desrosiers R C. Comparison of simian immunodeficiency virus isolates. Nature. 1988;331:619–622. doi: 10.1038/331619a0. [DOI] [PubMed] [Google Scholar]
- 25.Kinsey N E, Anderson M G, Unangst T J, Joag S V, Narayan O, Zink M C, Clements J E. Antigenic variation of SIV: mutations in V4 alter the neutralization profile. Virology. 1996;221:14–21. doi: 10.1006/viro.1996.0348. [DOI] [PubMed] [Google Scholar]
- 26.Kornfeld H, Riedel N, Viglianti G A, Hirsch V, Mullins J I. Cloning of HTLV-4 and its relation to simian and human immunodeficiency viruses. Nature. 1987;326:610–613. doi: 10.1038/326610a0. [DOI] [PubMed] [Google Scholar]
- 27.Letvin N L. Progress in the development of an HIV-1 vaccine. Science. 1998;280:1875–1880. doi: 10.1126/science.280.5371.1875. [DOI] [PubMed] [Google Scholar]
- 28.Lewis A P, Barber K A, Cooper H J, Sims M J, Worden J, Crowe J S. Cloning and sequence analysis of κ and γ cynomolgus monkey immunoglobulin cDNAs. Dev Comp Immunol. 1993;17:549–560. doi: 10.1016/s0145-305x(05)80010-2. [DOI] [PubMed] [Google Scholar]
- 29.Lewis M G, Elkins W R, McCutchan F E, Benveniste R E, Lai C Y, Montefiori D C, Burke D S, Eddy G A, Shafferman A. Passively transferred antibodies directed against conserved regions of SIV envelope protect macaques from SIV infection. Vaccine. 1993;11:1347–1355. doi: 10.1016/0264-410x(93)90106-8. [DOI] [PubMed] [Google Scholar]
- 30.Li A, Katinger H, Posner M R, Cavacini L, Zolla-Pazner S, Gorny M K, Sodroski J, Chou T C, Baba T W, Ruprecht R M. Synergistic neutralization of simian-human immunodeficiency virus SHIV-vpu+ by triple and quadruple combinations of human monoclonal antibodies and high-titer anti-human immunodeficiency virus type 1 immunoglobulins. J Virol. 1998;72:3235–3240. doi: 10.1128/jvi.72.4.3235-3240.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mascola J R, Louder M K, VanCott T C, Sapan C V, Lambert J S, Muenz L R, Bunow B, Birx D L, Robb M L. Potent and synergistic neutralization of human immunodeficiency virus (HIV) type 1 primary isolates by hyperimmune anti-HIV immunoglobulin combined with monoclonal antibodies 2F5 and 2G12. J Virol. 1997;71:7198–7206. doi: 10.1128/jvi.71.10.7198-7206.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mascola J R, Lewis M G, Stiegler G, Harris D, VanCott T C, Hayes D, Louder M K, Brown C R, Sapan C V, Frankel S S, Lu Y, Robb M L, Katinger H, Birx D L. Protection of macaques against pathogenic simian/human immunodeficiency virus 89.6PD by passive transfer of neutralizing antibodies. J Virol. 1999;73:4009–4018. doi: 10.1128/jvi.73.5.4009-4018.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Montefiori D C, Evans T G. Toward an HIV type 1 vaccine that generates potent, broadly cross-reactive neutralizing antibodies. AIDS Res Hum Retrovir. 1999;15:689–698. doi: 10.1089/088922299310773. [DOI] [PubMed] [Google Scholar]
- 34.Moore J P, Cao Y, Qing L, Sattentau Q J, Pyati J, Koduri R, Robinson J, Barbas III C F, Burton D R, Ho D D. Primary isolates of human immunodeficiency virus type 1 are relatively resistant to neutralization by monoclonal antibodies to gp120, and their neutralization is not predicted by studies with monomeric gp120. J Virol. 1995;69:101–109. doi: 10.1128/jvi.69.1.101-109.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Okamoto Y, Eda Y, Ogura A, Shibata S, Amagai T, Katsura Y, Asano T, Kimachi K, Makizumi K, Honda M. In SCID-hu mice, passive transfer of a humanized antibody prevents infection and atrophic change of medulla in human thymic implant due to intravenous inoculation of primary HIV-1 isolate. J Immunol. 1998;160:69–76. [PubMed] [Google Scholar]
- 36.Parren P W, Ditzel H J, Gulizia R J, Binley J M, Barbas III C F, Burton D R, Mosier D E. Protection against HIV-1 infection in hu-PBL-SCID mice by passive immunization with a neutralizing human monoclonal antibody against the gp120 CD4-binding site. AIDS. 1995;9:F1–F6. doi: 10.1097/00002030-199506000-00001. [DOI] [PubMed] [Google Scholar]
- 37.Putkonen P, Thorstensson R, Ghavamzadeh L, Albert J, Hild K, Biberfeld G, Norrby E. Prevention of HIV-2 and SIVsm infection by passive immunization in cynomolgus monkeys. Nature. 1991;352:436–438. doi: 10.1038/352436a0. [DOI] [PubMed] [Google Scholar]
- 38.Robinson J E, Cole K S, Elliott D L, Lam H, Amedee A M, Means R, Desrosiers R C, Clements J, Montelaro R C, Murphey-Corb M. Production and characterization of SIV envelope-specific rhesus monoclonal antibodies from a macaque asymptomatically infected with a live SIV vaccine. AIDS Res Hum Retrovir. 1998;14:1253–1262. doi: 10.1089/aid.1998.14.1253. [DOI] [PubMed] [Google Scholar]
- 39.Shibata R, Igarashi T, Haigwood N, Buckler-White A, Ogert R, Ross W, Willey R, Cho M W, Martin M A. Neutralizing antibody directed against the HIV-1 envelope glycoprotein can completely block HIV-1/SIV chimeric virus infections of macaque monkeys. Nat Med. 1999;5:204–210. doi: 10.1038/5568. [DOI] [PubMed] [Google Scholar]
- 40.Sullivan N, Sun Y, Sattentau Q, Thali M, Wu D, Denisova G, Gershoni J, Robinson J, Moore J, Sodroski J. CD4-induced conformational changes in the human immunodeficiency virus type 1 gp120 glycoprotein: consequences for virus entry and neutralization. J Virol. 1998;72:4694–4703. doi: 10.1128/jvi.72.6.4694-4703.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thali M, Moore J P, Furman C, Charles M, Ho D D, Robinson J, Sodroski J. Characterization of conserved human immunodeficiency virus type 1 gp120 neutralization epitopes exposed upon gp120-CD4 binding. J Virol. 1993;67:3978–3988. doi: 10.1128/jvi.67.7.3978-3988.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Trill J J, Shatzman A R, Ganguly S. Production of monoclonal antibodies in COS and CHO cells. Curr Opin Biotechnol. 1995;6:553–560. doi: 10.1016/0958-1669(95)80092-1. [DOI] [PubMed] [Google Scholar]
- 43.Van Rompay K K A, Otsyula M G, Tarara R P, Canfield D R, Berardi C, McChesney M B, Marthas M L. Vaccination of pregnant macaques protects newborns against mucosal simian immunodeficiency virus infection. J Infect Dis. 1996;173:1327–1335. doi: 10.1093/infdis/173.6.1327. [DOI] [PubMed] [Google Scholar]
- 44.Wu Z, Qian G, Zhen Q L, Narayan O, Stephens E B. Neutralization of SIVmac239/17E in lymphocyte cultures involves virus strain-specific linear and conformational epitopes encoded by different regions of the env gene including the “V3” domain. Virology. 1996;222:184–192. doi: 10.1006/viro.1996.0409. [DOI] [PubMed] [Google Scholar]