Abstract
Objective:
Detecting oral lesions at high risk of becoming cancer may enable early interventions to prevent oral cancer. The diagnosis of dysplasia in an oral lesion is used to predict this risk but is subject to interobserver and intraobserver variability. Studying biomarkers or molecular markers that reflect underlying molecular alterations can serve as an additional and objective method of risk assessment. E-cadherin and beta-catenin, molecular markers of epithelial–mesenchymal transition (EMT), potentially contribute to early malignant progression in oral tissue. This narrative review provides an overview of EMT, its relation to oral cancer, and the interaction among E-cadherin, beta-catenin, and the Wnt pathway in malignant progression of oral tissue.
Methods:
Full-text literature on EMT, E-cadherin, beta-catenin, oral epithelial dysplasia, and oral cancer was retrieved from PubMed and Google Scholar.
Results:
Sixty original research articles, reviews, and consensus statements were selected for review.
Discussion:
EMT, a biological mechanism characterized by epithelial and mesenchymal changes, can contribute to cancer development. Molecular markers of EMT including TWIST, vimentin, and N-cadherin may serve as prognostic markers of oral cancer. Dependent on Wnt pathway activity and the loss of membranous E-cadherin, E-cadherin and beta-catenin can play various roles along the spectrum of malignant progression, including tumour inhibition, early tumour progression, and late-stage tumour progression. Cross-sectional immunohistochemical research has found changes in expression patterns of E-cadherin and beta-catenin from normal oral tissue, oral epithelial dysplasia, to oral squamous cell carcinoma.
Conclusion:
Future research should explore the longitudinal role of EMT markers in predicting malignant progression in oral tissue.
Keywords: beta catenin, cadherins, epithelial–mesenchymal transition, malignant transformation, mouth, neoplasms, oral epithelial dysplasia, risk
Abstract
Objectif :
La détection de lésions buccales présentant un risque élevé d’évoluer en cancer peut permettre des interventions précoces pour prévenir le cancer de la bouche. Le diagnostic de dysplasie dans le cas de lésions buccales sert à prédire ce risque, mais il est soumis à une variabilité d’un observateur à l’autre et avec le même observateur. L’étude de marqueurs biologiques ou de marqueurs moléculaires correspondant à des altérations moléculaires sous-jacentes peut constituer une méthode objective supplémentaire d’évaluation des risques. L’E-cadhérine et la bêta-caténine, des marqueurs moléculaires de la transition épithélio-mésenchymateuse (TEM), pourraient contribuer aux premières étapes de l’évolution maligne du tissu buccal. Cette revue narrative donne un aperçu de la TEM, de ses liens avec le cancer de la bouche et de l’interaction entre l’E-cadhérine, la bêta-caténine et la voie de signalisation Wnt dans l’évolution maligne du tissu buccal.
Méthodes :
On a obtenu le texte intégral d’études portant sur la TEM, l’E-cadhérine, la bêta-caténine, la dysplasie épithéliale buccale et le cancer de la bouche sur PubMed et Google Scholar.
Résultats :
Soixante articles sur des études originales, des revues et des déclarations de consensus ont été sélectionnés aux fins d’examen.
Discussion :
La TEM, un mécanisme biologique caractérisé par des changements épithéliaux et mésenchymateux, peut contribuer à l’apparition d’un cancer. Les marqueurs moléculaires de la TEM, notamment TWIST, la vimentine et la N-cadhérine, peuvent servir de marqueurs pronostiques du cancer de la bouche. En fonction de l’activité de la voie de signalisation Wnt et de la perte de l’E-cadhérine membraneuse, l’E-cadhérine et la bêta-caténine peuvent jouer divers rôles dans le spectre de l’évolution maligne, notamment l’inhibition tumorale, la progression tumorale précoce et l’évolution tumorale avancée. Des études transversales d’immunohistochimie ont révélé des changements dans les modèles d’expression de l’E-cadhérine et de la bêta-caténine avec le passage du tissu buccal normal, de la dysplasie épithéliale buccale au carcinome squameux de la bouche.
Conclusion :
À l’avenir, des études devraient explorer le rôle longitudinal des marqueurs de la TEM dans la prévision de l’évolution maligne dans les tissus buccaux.
PRACTICAL IMPLICATIONS OF THIS RESEARCH.
Understanding the complex biological mechanism, epithelial–mesenchymal transition (EMT), and its relationship to oral cancer and potentially oral epithelial dysplasia is important for oral health professionals.
Understanding the role of the molecular markers of EMT, specifically E-cadherin and beta-catenin, in oral malignant progression may improve the accuracy of lesion risk assessment.
Future research on EMT and oral dysplasia should be undertaken to guide lesion risk assessment and clinical management.
INTRODUCTION
In 2020, there were almost 400,000 new cases of cancers of the lip and oral cavity worldwide.1 In the United States alone, in 2021, there was an estimate of over 50,000 new cases and 10,000 deaths related to oral and pharyngeal cancer.2 In Canada, there is a 64% five-year survival rate for oral cancer,3 with an estimated 7500 new cases of head and neck cancer and 2100 deaths in 2022.4 Despite the continuous advances in research and technology, new cases and deaths from oral cancer still occur, largely because late-stage diagnosis of this disease results in high treatment-related morbidity.5 Research efforts must focus on early detection and intervention for improved patient survival rates from oral cancer.5
An oral lesion’s risk of becoming cancer is assessed based on patient demographics and risk habits, and clinical and histological findings.6 Currently, the gold standard for determining a lesion’s risk is diagnosing the presence and severity of dysplasia.7 Dysplasia is diagnosed based on architectural and cytological changes in the epithelium that show an increased risk for malignant progression. Architectural changes include abnormal epithelial stratification, drop-shaped rete ridges, mitosis high in the epithelium, generalized premature keratinization, keratin pearls within rete ridges, and the loss of epithelial cell cohesion and polarity in basal cells. Additional architectural changes include a sharply defined margin to changes, an expanded proliferative compartment, altered keratin pattern for oral subsite, verrucous or papillary architecture, changes extending to minor gland ducts, multiple different patterns of dysplasia, multifocal or skip lesions, and basal cell clustering or nesting. Cytological changes include abnormal mitotic figures, apoptotic mitoses, single cell keratinization, hyperchromasia, increased nucleus to cytoplasm ratio, increased number and size of nucleoli, and irregular cell and nucleus size and/or shape.7
Although histological diagnosis of dysplasia is important in determining a lesion’s risk, it comes with limitations. There is often interobserver and intraobserver variability in the diagnosis of dysplasia due to the lack of uniform and specific diagnostic criteria.8 For that reason, studying biomarkers or molecular markers may provide an additional and objective avenue for assessing a lesion’s risk for malignant progression.9 As clinical and histological changes reflect underlying molecular events, molecular markers can indicate alterations during carcinogenesis.9 A biological process that is indicated by multiple molecular markers is epithelial–mesenchymal transition (EMT). This process is marked by a progressive decrease in epithelial cell features and an increase in mesenchymal cell features.10 It has been shown to play a role in cancer development,11 but may or may not contribute to early malignant progression, such as in dysplasia. One event of EMT that may play a part in both early and late malignant progression is the interaction of its molecular markers E-cadherin and beta-catenin in the Wingless/Integrated (Wnt) pathway.
This article will review the basics of EMT, EMT’s relation to oral cancer, and the interaction of E-cadherin, beta-catenin, and the Wnt pathway in malignant progression and the oral tissue. A greater understanding of this topic may contribute to lesion risk assess,ment and future guidance for appropriate clinical management.
METHODS
This narrative review selected full-text literature from PubMed and Google Scholar. Keywords used in the search were oral OR mouth* OR lip* OR tongue* OR palate* OR head and neck, cancer* OR carcinoma* OR malignan* OR neoplasm* OR tumo?r* OR mass*, E-cadherin* OR uvomorulin*, beta-catenin*, and dysplasia*. There were no restrictions on publication dates to ensure necessary information was included. Publications not in English were excluded.
RESULTS
Thirty-eight (38) original research articles, 20 literature and narrative reviews, 1 systematic review, and 1 consensus statement were selected. EMT markers TWIST, vimentin, and N-cadherin may be prognostic for oral cancer based on their correlation with clinical features of oral cancer. Expression patterns of E-cadherin and beta-catenin change from normal oral tissue to oral squamous cell carcinoma and may be related to their interaction with the Wnt pathway, affecting different steps of malignant progression.
DISCUSSION
Epithelial–mesenchymal transition
Epithelial–mesenchymal transition (EMT) was originally referred to as “epithelial–mesenchymal transformation” by Elizabeth Hay in 1995.12 Modification of the term has occurred over the years, and EMT is now known as “epithelial–mesenchymal transition” to represent the biological plasticity of this mechanism.11 EMT involves a loss of epithelial features and an increase in mesenchymal traits.10 Specifically, there is a loss of epithelial cell polarity, disruption to epithelial cell junctions, degradation of the basement membrane, and reorganization of the extracellular matrix.13 As the cells gain mesenchymal features and increased motility, there is a loss of the cobblestone arrangement in epithelial cells.13 In EMT, cells exhibit both epithelial and mesenchymal features, and are rarely in a complete mesenchymal state.13 Interestingly, EMT can be reversed in the mesenchymal–epithelial transition (MET) process where mesenchymal cells regain epithelial cell traits.11
EMT occurs in different biological contexts, which consequently categorizes it into 3 types. Type I EMT is involved in embryogenesis, implantation, and organ development.11 Type II EMT plays roles in wound healing, tissue regeneration, and organ fibrosis. It typically occurs as a repair process following trauma or inflammation and stops once the repair process is complete. Type III EMT has been suggested as an important mechanism in cancer development. The mesenchymal characteristics gained increase motility of the cells and promote invasiveness and metastasis. When cancer cells have reached distant organ sites, MET may facilitate secondary tumour formation and initiate colonization.11 However, it is still unknown whether the occurrence of EMT is necessary for tumour cells to complete metastasis.14
EMT starts with the activation of transcription factors (e.g., zinc finger E-box binding homeobox [ZEB], TWIST, and SNAIL), which promote and repress genes that facilitate epithelial and mesenchymal changes.13 In addition to the activation of transcription factors, there is the expression of cell-surface proteins, reorganization and expression of cytoskeletal proteins, production of enzymes that degrade the extracellular matrix, and contribution to changes in expression of microRNAs.11 The molecular markers of EMT that indicate these processes include transcription factors, cell surface markers, cytoskeletal markers, extracellular proteins, epigenetic markers, and microRNAs.11 Common epithelial markers that are progressively lost include E-cadherin, cytokeratin, and laminin-1; common mesenchymal markers that are increasingly gained include N-cadherin, vimentin, and beta-catenin.11
EMT markers in oral cancer and dysplasia
EMT-inducing transcription factors are upregulated in EMT. TWIST is a transcription factor that regulates organ development but is highly expressed in tumour cells in the oral tissue,15–17 and has been suggested as a prognostic factor for oral cancer.17 Zhou et al.17 show that TWIST expression may be correlated with clinical features of oral cancer including low differentiation, advanced clinical stage, lymph node metastasis, and local recurrence. Other EMT-inducing transcription factors such as SNAIL, ZEB1, and ZEB2 also show increased expression in oral cancer but the expression is smaller when compared to TWIST.16 Göppel et al.16 suggest that, in head and neck cancer, these transcription factors may play a less important role than TWIST. Vimentin, a type III intermediate filament normally found in mesenchymal cells, is categorized as a cytoskeletal marker of EMT.10 Vimentin is a potential prognostic factor for patients with oral squamous cell carcinoma (OSCC).18 Liu et al.18 found that, in squamous cell carcinoma of the tongue, high vimentin expression is associated with poor cell differentiation and lymph node metastasis. Combined results of vimentin and E-cadherin expression show better prognostic significance than the prognostic value of these 2 proteins individually.19 Monitoring expression levels of cadherins in EMT has been termed “cadherin switch.” The cadherin switch from E-cadherin to N-cadherin expression has been used to observe EMT in embryonic development and cancer.10 In adult tissue, N-cadherin contributes to synapse function, vascular stability, and bone homeostasis.20 It is expressed in neural cells, endothelial cells, stromal cells, and osteoblasts, but is absent or rarely expressed in normal epithelial cells.20 In OSCC, high expression of N-cadherin and low expression of E-cadherin are closely related,21, 22 and are correlated with histological differentiation, pattern of invasion, and lymph node metastasis.21 Thus, the cadherin switch from E-cadherin to N-cadherin has been suggested to play a role in cancer development and serve as a prognostic factor.18, 21, 22
Freitas de Morais et al.23 recently published a systematic review on the immunohistochemical expression of EMT markers in oral epithelial dysplasia (OED). With increasing grades of OED, they found a gain in nuclear transcription factors and mesenchymal markers along with a decrease in epithelial and cell adhesion markers. Specifically, they noted a loss of the cell-adhesion marker claudin-1. Similar to studies on TWIST in oral cancer, there was an increase in TWIST expression in OED, while other EMT-inducing transcription factors need further research. Currently, there is an absence of research on epigenetic EMT markers in OED. Longitudinal research is needed to associate EMT with the risk of malignant transformation of potentially malignant oral lesions.23
E-cadherin and beta-catenin
One of the main events of EMT is the loss of E-cadherin.10, 11 E-cadherin is a classical cadherin encoded by the CDH1 gene.24 Cadherins are molecules characterized by extracellular cadherin repeat domains and are dependent on homophilic Ca2+ for maintaining cell-to-cell adhesion.25,26 Classical cadherins feature 5 extracellular cadherin repeats and are a major component of the adherens junctions, which are involved in epithelial cell-to-cell connections.27, 28 Adherens junctions are composed of a classical cadherin (e.g., E-cadherin), beta-catenin, alpha-catenin, and p120-catenin.27 Because the loss of E-cadherin reduces epithelial cell adhesion, its loss is often thought to contribute to the latter stages of cancer development, such as invasion and metastasis.29 E-cadherin loss may result from mutation, epigenetic changes, proteolytic cleavage, degradation or transcriptional repression (e.g., by EMT-inducing transcription factors).24, 30 Some authors have proposed that the loss of E-cadherin may also contribute to early malignant progression via beta-catenin signaling through the canonical Wnt pathway, which will be referred to as the Wnt pathway in the remainder of this text.31, 32 Like E-cadherin, beta-catenin is responsible for cell adhesion.33 It is an Armadillo repeat protein that binds E-cadherin to alpha-catenin, which is bound to the actin cytoskeleton to maintain cell adhesion.33
The Wnt pathway
The Wnt pathway is active in adult tissue homeostasis and stem cell renewal, cell proliferation, and cell differentiation during embryonic development.34, 35 When this pathway is deregulated, it can lead to diseases such as cancer.34, 35
When the Wnt pathway is inactive, a destruction complex consisting of Axin, adenomatous polyposis coli (APC), glycogen synthase kinase 3 (GSK-3), casein kinase 1 (CK1), and protein phosphatase 2A (PP2A) initiates the degradation of beta-catenin.32, 36 As a result, beta-catenin accumulation in the cytoplasm and translocation to the nucleus to affect Wnt target genes does not occur.32 There are high amounts of membranous beta-catenin compared to cytoplasmic and nuclear beta-catenin.32
During Wnt pathway activation, Wnt proteins bind to the Frizzled (FZD)/low density lipoprotein receptor-related protein (LRP) complex on the cell surface, which destroys the destruction complex.32 There is no degradation of beta-catenin, which leads to an accumulation of beta-catenin in the cytoplasm and eventual translocation to the nucleus. In the nucleus, beta-catenin interacts with the T cell-factor/lymphoid enhancer-binding factor (TCF/LEF) transcription factors to activate Wnt target genes involved in cancer formation (i.e., c-myc, cyclin D1, and survivin).32, 37–39 During embryogenesis and tissue homeostasis, the Wnt pathway is normally activated through binding of Wnt proteins.32 In disease, dysregulation of the Wnt pathway can be caused by mutations, but mutations of beta-catenin, Axin 1, and APC are not common in oral malignancy.40,41 Other processes that may dysregulate the Wnt pathway in OED and OSCC include an increase in Wnt ligands, interactions with other pathways, epigenetic alterations, endosomal sequestration of the destruction complex, and etiological factors.42–49
E-cadherin and beta-catenin in the Wnt pathway
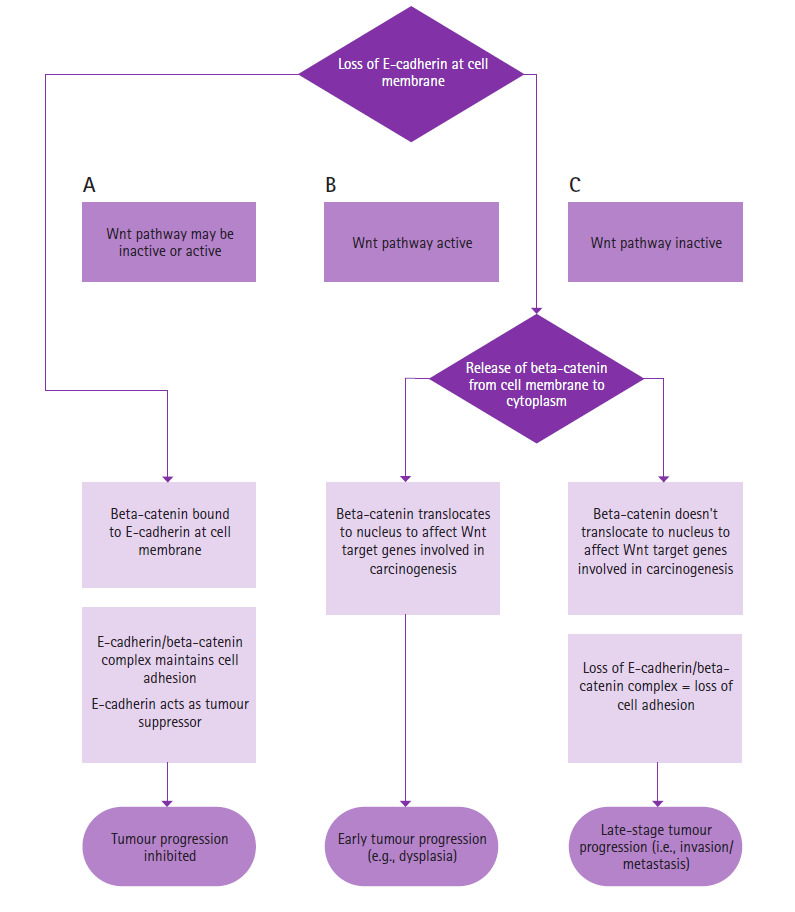
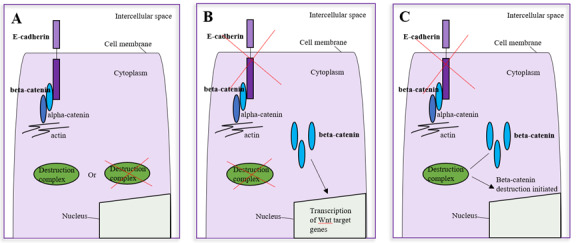
E-cadherin and beta-catenin in the Wnt pathway can contribute to different aspects of malignant progression depending on the loss of E-cadherin at the cell membrane and whether the Wnt pathway is active. They play a role in 1) inhibition of tumour progression; 2) early tumour progression; and 3) late-stage tumour progression.
Inhibition of tumour progression
In this context, E-cadherin is present at the cell membrane. Regardless of the Wnt pathway being inactive or active, beta-catenin is bound to E-cadherin at the cell membrane.31 Since E-cadherin and beta-catenin are bound together, cell-to-cell adhesion is maintained and E-cadherin acts as a tumour suppressor (A in Figure 1 and Figure 2).31
Early tumour progression
In this scenario, there is a loss of E-cadherin at the cell membrane (e.g., during EMT) and thus a release of beta-catenin from the cell membrane to the cytoplasm.31, 50, 51 The Wnt pathway is also active. Beta-catenin accumulated in the cytoplasm will translocate to the nucleus to affect Wnt target genes involved in carcinogenesis.31 Jeanes et al.31 propose that the loss of E-cadherin can amplify Wnt signaling when the Wnt pathway is active. Thus this scenario may play a role in early tumour progression, such as in dysplasia (B in Figure 1 and Figure 2).50
Late-stage tumour progression
This scenario also involves the loss of E-cadherin at the cell membrane and consequently an increase in cytoplasmic beta-catenin, but with an inactive Wnt pathway. Since the Wnt pathway is inactive, the destruction complex is intact, and the influx of beta-catenin in the cytoplasm is destroyed. Beta-catenin does not translocate to the nucleus to affect Wnt target genes involved in malignant progression.31,50 However, because there is a loss of E-cadherin and beta-catenin at the cell membrane, the adherens junction is incomplete and there is a loss of cell-to-cell adhesion, which may contribute to the latter half of tumour progression (i.e., invasion and metastasis) (C in Figure 1 and Figure 2).50
E-cadherin and beta-catenin expression in normal oral mucosa, OED, and OSCC
Williams et al.52 in 1998 were the first to report the expression patterns of E-cadherin and beta-catenin in OED. Their early results were based on staining intensity and expression patterns, but are similar to the findings of current studies.52 The details of E-cadherin and beta-catenin expression in normal oral mucosa (NOM), OED, and OSCC in the current literature are discussed in-depth in the lead author’s (IY) master’s thesis, but will be discussed briefly in this review.53
E-cadherin expression in oral tissue
In studies performing immunohistochemical analysis, a progressive decrease in membranous E-cadherin expression is evident from NOM, OED, to OSCC (Table 1).52,54–61 There is also reduced membranous E-cadherin expression in progressing severities of OED, but not all studies showed significant results.57, 58 Chaw et al.58 proposed that, in their study, the insignificant decrease in E-cadherin expression from NOM, mild, moderate, and severe OED, to OSCC may indicate that tumour cells are in a “semi-mesenchymal” state and thus cells may not exhibit complete mesenchymal characteristics, including an increased loss of E-cadherin. In addition to a loss of membranous E-cadherin, an increase in cytoplasmic E-cadherin may be observed, although mainly in OSCC.52,55,59–61 It has been suggested that cytoplasmic expression of E-cadherin in tumour cells represents a state where E-cadherin is no longer functional or useful to the cell.59, 62
Figure 1.
E-cadherin, beta-catenin, and the Wnt pathway in malignant progression
Adapted from Yim53
Figure 2.
E-cadherin, beta-catenin, and the Wnt pathway in malignant progression in the cell
Adapted from Yim53
Beta-catenin expression in oral tissue
Immunohistochemical research on beta-catenin expression has sh own a decrease in membranous beta-catenin and an increase in cytoplasmic and nuclear beta-catenin from NOM to OED (Table 2).42, 52, 54, 58, 61, 63–66 In NOM, beta-catenin is expressed mainly in the cell membrane.52, 58, 61, 63, 64, 66 In OED, however, there is a decrease in beta-catenin expression in the cell membrane.54, 58, 65 Studies have also found decreasing membranous beta-catenin expression with increasing severities of OED.58, 65 In addition to a decrease in membranous beta-catenin in OED, there is an increase in cytoplasmic and nuclear beta-catenin.58, 61, 63–65 Of interest, Chaw et al.58 and Reyes et al.64 found an increase in cytoplasmic and nuclear beta-catenin expression in moderate and severe OED. In OSCC, there is also a decrease in membranous beta-catenin and an increase in cytoplasmic and nuclear beta-catenin when compared to NOM.42, 52, 54, 58, 61, 64–67 However, when comparing cytoplasmic and nuclear beta-catenin expression between OED and OSCC, some studies found a decrease in expression from OED to OSCC, whereas other studies found an increase from OSCC to OED.42, 54, 61, 64 Reyes et al.68 suggest that greater amounts of nuclear beta-catenin in OED rather than OSCC play an important role in cell proliferation in OED, contributing to early malignant progression.
E-cadherin and beta-catenin expression in oral tissue
Studies show mixed results when analysing the expression of E-cadherin and beta-catenin together. Kaur et al.54 found that a loss of membranous E-cadherin was significantly associated with a loss of membranous beta-catenin in oral leukoplakia and OSCC. Expression of E-cadherin and beta-catenin analysed separately and in combination showed significant correlation with enhanced tumour invasiveness, late clinical stage, nodal metastasis, and poor prognosis.54 However, in the results from Chaw et al.58 membranous E-cadherin and membranous or cytoplasmic/nuclear expression of beta-catenin were not significantly correlated. They suggest that the translocation of beta-catenin to the nucleus may not be related to the loss of E-cadherin.58
Gaps in the research
Due to the lack of uniform scoring criteria in immunohistochemical expression, it is difficult to compare the literature on expression of EMT markers that may be involved in oral malignant progression. There are oft,en differences in quantification of the amount of staining expression and intensity. Some studies have multiple percentage categories (i.e., absence of staining, less than 10% of cells stained, 10% to 50% cells stained, and more than 50% cells stained), whereas other studies have only 2 categories: positive or negative (i.e., positive meaning more than a certain percentage of cells are stained).55, 63 As for staining intensity, some studies have categories to show increasing intensity, where others do not assess for intensity.42, 55, 63, 65 There are also methods that combine scoring intensity and percentage stained.57, 58, 60 However, in addition to creating challenges for the comparison of results, the semiquantitative methods used may not best represent biological data. Quantitative digital analysis is needed for robust comparisons.
Table 1.
Expression patterns of E-cadherin in normal oral mucosa, oral epithelial dysplasia, and oral squamous cell carcinoma
|
NOM |
OED |
OSCC |
|
|
Cell membrane |
High |
Decreaseda (except Lopes et al.59) |
Decreasedab (except Lopes et al.59) |
|
Cytoplasm |
None (Kaur et al.54 indicate occasional expression) |
None (except Kyrodimou et al.61) |
Increasedab |
|
Nucleus |
None |
None |
None |
NOM: normal oral mucosa; OED: oral epithelial dysplasia; OSCC: oral squamous cell carcinoma
acompared to NOM
bcompared to OED
Adapted from Yim53
Table 2.
Expression patterns of E-cadherin in normal oral mucosa, oral epithelial dysplasia, and oral squamous cell carcinoma
|
NOM |
OED |
OSCC |
|
|
Cell membrane |
High |
Decreaseda |
Decreaseda |
|
Cytoplasm |
None (Kaur et al.54 and Ishida et al.42 indicate minimal expression) |
Increaseda |
Increaseda (Mixed results when comparing expression in OSCC to OED) |
|
Nucleus |
None (Kaur et al.54 indicate minimal expression) |
Increaseda (Especially in severe OED – Chaw et al.,58 Reyes et al.,64 Lo Muzio et al.65) |
Increaseda (Mixed results when comparing expression between OSCC and OED) |
NOM: normal oral mucosa; OED: oral epithelial dysplasia; OSCC: oral squamous cell carcinoma
acompared to NOM
Adapted from Yim53
As EMT is a complex biological process, studies must eval uate multiple EMT markers simultaneously to better understand how EMT plays a role in malignant progression. In addition, to understand the complexity of this process, future research should study EMT through single cells using single-cell imaging, lineage tracing, and analysis of gene expression, genetics, and epigenetics.14 The development of mathematical models can also help outline this complex mechanism.14
There are many studies showing the altered expression of E-cadherin and beta-catenin from NOM, OED, to OSCC, but there is a lack of longitudinal research on the role of these proteins as predictors in malignant progression, especially in early malignant progression.52, 54-61, 63-65, 69, 70 Being able to identify lesions at high risk of progression can facilitate early management. The lead author’s (IY) master’s thesis was a pilot study that explored the expression of E-cadherin and beta-catenin in OED to determine whether the expression pattern of these proteins predict malignant progression.53
CONCLUSION
Oral cancer continues to be highly prevalent, with head and neck cancer being one of the most common cancers in the world. Identifying oral lesions at high risk of transforming into cancer may help with early intervention and reduce the rates of oral cancer or at least improve patient survival rates. Histological diagnosis of dysplasia is the main approach in assessing a lesion’s risk of becoming cancer, but there may be interobserver and intraobserver variability resulting in inaccurate diagnosis and risk assessment. Molecular markers of EMT that are involved in embryogenesis, tissue healing, and cancer development may aid in lesion risk assessment. EMT markers that serve as potential prognostic factors of oral cancer include TWIST, vimentin, and N-cadherin. The EMT markers E-cadherin and beta-catenin may play a role in early malignant progression through the Wnt pathway—a regulatory pathway often involved in disease. Dependent on the loss of E-cadherin and the activation of the Wnt pathway, there may be inhibition of tumour progression, early tumour progression or late-stage tumour progression. Altered expression levels of E-cadherin and beta-catenin from NOM, OED, to OSCC have been shown in immunohistochemical analysis. However, future research should focus on the longitudinal role of these proteins and use a combination of additional EMT markers in predicting early malignant progression.
CONFLICTS OF INTEREST
The authors have declared no conflicts of interest.
Acknowledgments
This work was supported by a grant from the Canadian Foundation for Dental Hygiene Research and Education (CFDHRE). IY received a Canadian Institutes of Health Research (CIHR) Canada Graduate Scholarship Master’s Award.
Footnotes
CDHA Research Agenda category: risk assessment and management
References
- Sung H , Ferlay J , Siegel RL , Laversanne M , Soerjomataram I , Jemal A , et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries CA Cancer J Clin 2021 ; 71 ( 3 ): 209 – 249 [DOI] [PubMed] [Google Scholar]
- National Cancer Institute Surveillance, Epidemiology, and End Results Program. Cancer Stat Facts: Oral Cavity and Pharynx Cancer [Internet]. [cited 2021 Dec 31]. Available from: https://seer.cancer.gov/statfacts/html/oralcav.html
- Canadian Cancer Statistics Advisory Committee. Canadian cancer statistics. Toronto (ON): CCSAC; 2019. [Google Scholar]
- Brenner DR , Poirier A , Woods RR , Ellison LF , Billette JM , Demers AA , et al. Projected estimates of cancer in Canada in 2022 CMAJ 2022 ; 194 ( 17 ): E601 – E607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein JB , Zhang L , Rosin M Advances in the diagnosis of oral premalignant and malignant lesions J Can Dent Assoc 2002 ; 68 ( 10 ): 617 – 621 [PubMed] [Google Scholar]
- Napier SS , Speight PM Natural history of potentially malignant oral lesions and conditions: an overview of the literature J Oral Pathol Med 2007 ; 37 ( 1 ): 1 – 10 [DOI] [PubMed] [Google Scholar]
- WHO Classification of Tumours Editorial Board. Head and neck tumours. 5th ed. Lyon (France): International Agency for Research on Cancer; 2022. [Google Scholar]
- Abbey LM , Kaugars GE , Gunsolley JC , Burns JC , Page DG , Svirsky JA , et al. Intraexaminer and interexaminer reliability in the diagnosis of oral epithelial dysplasia Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1995 ; 80 ( 2 ): 188 – 191 [DOI] [PubMed] [Google Scholar]
- Nikitakis NG , Pentenero M , Georgaki M , Poh CF , Peterson DE , Edwards P , et al. Molecular markers associated with development and progression of potentially premalignant oral epithelial lesions: current knowledge and future implications Oral Surg Oral Med Oral Pathol Oral Radiol 2018 ; 125 ( 6 ): 650 – 669 [DOI] [PubMed] [Google Scholar]
- Zeisberg M , Neilson EG Biomarkers for epithelial–mesenchymal transitions J Clin Invest 2009 ; 119 ( 6 ): 1429 – 1437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalluri R , Weinberg RA The basics of epithelial–mesenchymal transition J Clin Invest 2009 ; 119 ( 6 ): 1420 – 1428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay ED An overview of epithelio–mesenchymal transformation Acta Anat (Basel) 1995 ; 154 ( 1 ): 8 – 20 [DOI] [PubMed] [Google Scholar]
- Dongre A , Weinberg RA New insights into the mechanisms of epithelial–mesenchymal transition and implications for cancer Nat Rev Mol Cell Biol 2019 ; 20 : 69 – 84 [DOI] [PubMed] [Google Scholar]
- Yang J , Antin P , Berx G , Blanpain C , Brabletz T , Bronner M , et al. Guidelines and definitions for research on epithelial–mesenchymal transition Nat Rev Mol Cell Biol 2020 ; 21 ( 6 ): 341 – 352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gou Y , Zhang T , Xu J Transcription factors in craniofacial development: from receptor signaling to transcriptional and epigenetic regulation Curr Top Dev Biol 2015 ; 115 : 377 – 410 [DOI] [PubMed] [Google Scholar]
- Göppel J , Möckelmann N , Münscher A , Sauter G , Schumacher U Expression of epithelial–mesenchymal transition regulating transcription factors in head and neck squamous cell carcinomas Anticancer Res 2017 ; 37 ( 10 ): 5435 – 5440 [DOI] [PubMed] [Google Scholar]
- Zhou Y , Zhang H , Zhuo X , Liu Y , Zhang G , Tan Y Over-expression of TWIST, an epithelial–mesenchymal transition inducer, predicts poor survival in patients with oral carcinoma Int J Clin Exp Med 2015 ; 8 ( 6 ): 9239 – 9247 [PMC free article] [PubMed] [Google Scholar]
- Liu PF , Kang BH , Wu YM , Sun JH , Yen LM , Fu TY , et al. Vimentin is a potential prognostic factor for tongue squamous cell carcinoma among five epithelial–mesenchymal transition-related proteins PLoS One 2017 ; 12 ( 6 ): e0178581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wangmo C , Charoen N , Jantharapattana K , Dechaphunkul A , Thongsuksai P Epithelial–mesenchymal transition predicts survival in oral squamous cell carcinoma Pathol Oncol Res 2020 ; 26 ( 3 ): 1511 – 1518 [DOI] [PubMed] [Google Scholar]
- Mrozik KM , Blaschuk OW , Cheong CM , Zannettino ACW , Vandyke K N-cadherin in cancer metastasis, its emerging role in haematological malignancies and potential as a therapeutic target in cancer BMC Cancer 2018 ; 18 ( 1 ): 1 – 16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen PT , Kudo Y , Yoshida M , Kamata N , Ogawa I , Takata T N-cadherin expression is involved in malignant behavior of head and neck cancer in relation to epithelial–mesenchymal transition Histol Histopathol 2011 ; 26 ( 2 ): 147 – 156 [DOI] [PubMed] [Google Scholar]
- Pyo SW , Hashimoto M , Kim YS , Kim CH , Lee SH , Johnson KR , et al. Expression of E-cadherin, P-cadherin and N-cadherin in oral squamous cell carcinoma: correlation with the clinicopathologic features and patient outcome J Cranio-Maxillofacial Surg 2007 ; 35 ( 1 ): 1 – 9 [DOI] [PubMed] [Google Scholar]
- de Morais EF , Pinheiro JC , Lira JAS , Mafra RP , Barboza CAG , de Souza LB , et al. Prognostic value of the immunohistochemical detection of epithelial–mesenchymal transition biomarkers in oral epithelial dysplasia: a systematic review Med Oral Patol Oral Cir Bucal 2020 ; 25 ( 2 ): e205 – e216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruner HC , Derksen PWB Loss of E-cadherin-dependent cell-cell adhesion and the development and progression of cancer Cold Spring Harb Perspect Biol 2018 ; 10 ( 3 ): a029330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulpiau P , van Roy F Molecular evolution of the cadherin superfamily Int J Biochem Cell Biol 2009 ; 41 ( 2 ): 349 – 369 [DOI] [PubMed] [Google Scholar]
- Takeichi M Cadherin cell adhesion receptors as a morphogenetic regulator Science 1991 ; 251 ( 5000 ): 1451 – 1455 [DOI] [PubMed] [Google Scholar]
- Hartsock A , Nelson WJ Adherens and tight junctions: structure, function and connections to the actin cytoskeleton Biochim Biophys Acta 2008 ; 1778 ( 3 ): 660 – 669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Roy F Beyond E-cadherin: roles of other cadherin superfamily members in cancer Nat Rev Cancer 2014 ; 14 ( 2 ): 121 – 134 [DOI] [PubMed] [Google Scholar]
- Behrens J , Mareel MM , Van Roy FM , Birchmeier W Dissecting tumor cell invasion: epithelial cells acquire invasive properties after the loss of uvomorulin-mediated cell-cell adhesion J Cell Biol 1989 ; 108 ( 6 ): 2435 – 2447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krisanaprakornkit S , Iamaroon A Epithelial–mesenchymal transition in oral squamous cell carcinoma International Scholarly Research Notices 2012 ; 2012 : 1 – 10 [Google Scholar]
- Jeanes A, Gottardi CJ, Yap AS Cadherins and cancer: how does cadherin dysfunction promote tumor progression? Oncogene 2008;27(55):6920–6929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan CY , Nusse R The Wnt signaling pathway in development and disease Annu Rev Cell Dev Biol 2004 ; 20 : 781 – 810 [DOI] [PubMed] [Google Scholar]
- Ozawa M , Ringwald M , Kemler R Uvomorulin-catenin complex formation is regulated by a specific domain in the cytoplasmic region of the cell adhesion molecule Proc Natl Acad Sci USA 1990 ; 87 ( 11 ): 4246 – 4250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhart Z , Angers S Wnt signaling in development and tissue homeostasis Development 2018 ; 145 ( 11 ): dev146589 [DOI] [PubMed] [Google Scholar]
- Komiya Y , Habas R Wnt signal transduction pathways Organogenesis 2008 ; 4 ( 2 ): 68 – 75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamos JL , Weis WI The β-catenin destruction complex Cold Spring Harb Perspect Biol 2013 ; 5 ( 1 ): a007898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He TC , Sparks AB , Rago C , Hermeking H , Zawel L , Da Costa LT , et al. Identification of c-myc as a target of the apc pathway Science 1998 ; 281 ( 5382 ): 1509 – 1512 [DOI] [PubMed] [Google Scholar]
- Shtutman M , Zhurinsky J , Simcha I , Albanese C , D’Amico M , Pestell R , et al. The cyclin d1 gene is a target of the β-catenin/lef-1 pathway Proc Natl Acad Sci USA 1999 ; 96 ( 10 ): 5522 – 5527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T , Otevrel T , Gao Z , Gao Z , Ehrlich SM , Fields JZ , et al. Evidence that APC regulates survivin expression Cancer Res 2001 ; 61 ( 24 ): 8664 – 8667 [PubMed] [Google Scholar]
- Yeh KT , Chang JG , Lin TH , Wang YF , Chang JY , Shih MC , et al. Correlation between protein expression and epigenetic and mutation changes of Wnt pathway-related genes in oral cancer Int J Oncol 2003 ; 23 ( 4 ): 1001 – 1007 [PubMed] [Google Scholar]
- Iwai S , Katagiri W , Kong C , Amekawa S , Nakazawa M , Yura Y Mutations of the APC, beta-catenin, and axin 1 genes and cytoplasmic accumulation of beta-catenin in oral squamous cell carcinoma J Cancer Res Clin Oncol 2005 ; 131 ( 12 ): 773 – 782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida K , Ito S , Wada N , Deguchi H , Hata T , Hosoda M , et al. Nuclear localization of beta-catenin involved in precancerous change in oral leukoplakia Mol Cancer 2007 ; 6 ( 1 ): 62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes M , Peña-Oyarzun D , Maturana A , Torres VA Nuclear localization of β-catenin and expression of target genes are associated with increased Wnt secretion in oral dysplasia Oral Oncol 2019 ; 94 : 58 – 67 [DOI] [PubMed] [Google Scholar]
- Odajima T , Sasaki Y , Tanaka N , Kato-Mori Y , Asanuma H , Ikeda T , et al. Abnormal β-catenin expression in oral cancer with no gene mutation: correlation with expression of cyclin D1 and epidermal growth factor receptor, Ki-67 labeling index, and clinicopathological features Hum Pathol 2005 ; 36 ( 3 ): 234 – 241 [DOI] [PubMed] [Google Scholar]
- Lee CH , Hung HW , Hung PH , Shieh YS Epidermal growth factor receptor regulates β-catenin location, stability, and transcriptional activity in oral cancer Mol Cancer 2010 ; 9 ( 1 ): 64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S , Chen L , Mashrah M , Zhu Y , Liu J , Yang X , et al. Deregulation of secreted frizzled-related proteins is associated with aberrant β-catenin activation in the carcinogenesis of oral submucous fibrosis Onco Targets Ther 2015 ; 8 : 2923 – 2931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes M , Peña-Oyarzún D , Silva P , Venegas S , Criollo A , Torres VA Nuclear accumulation of β-catenin is associated with endosomal sequestration of the destruction complex and increased activation of Rab5 in oral dysplasia FASEB J 2020 ; 34 ( 3 ): 4009 – 4025 [DOI] [PubMed] [Google Scholar]
- Hu Z , Müller S , Qian G , Xu J , Kim S , Chen Z , et al. Human papillomavirus 16 oncoprotein regulates the translocation of β-catenin via the activation of epidermal growth factor receptor Cancer 2015 ; 121 ( 2 ): 214 – 225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenner M, Yosef B, Huebbers CU, Preuss SF, Dienes HP, Speel EJM, et al. Nuclear translocation of β-catenin and decreased expression of epithelial cadherin in human papillomavirus-positive tonsillar cancer: an early event in human papillomavirus-related tumour progression? Histopathology 2011;58(7):1117–1126 [DOI] [PubMed] [Google Scholar]
- González-Moles MA , Ruiz-Ávila I , Gil-Montoya JA , Plaza-Campillo J , Scully C β-catenin in oral cancer: an update on current knowledge Oral Oncol 2014 ; 50 ( 9 ): 818 – 824 [DOI] [PubMed] [Google Scholar]
- Paluszczak J The significance of the dysregulation of canonical Wnt signaling in head and neck squamous cell carcinomas Cells 2020 ; 9 ( 3 ): 723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams HK , Sanders DSA , Jankowski JAZ , Landini G , Brown AMS Expression of cadherins and catenins in oral epithelial dysplasia and squamous cell carcinoma J Oral Pathol Med 1998 ; 27 ( 7 ): 308 – 317 [DOI] [PubMed] [Google Scholar]
- Yim ISY. E-cadherin and beta-catenin expression in oral epithelial dysplasia: predicting progression to cancer [master’s dissertation]. Vancouver (BC): University of British Columbia; 2022. Available from: https://open.library.ubc.ca/collections/ubctheses/24/items/1.0422755 [Google Scholar]
- Kaur J , Sawhney M , Dattagupta S , Shukla NK , Srivastava A , Walfish PG , et al. Clinical significance of altered expression of β-catenin and E-cadherin in oral dysplasia and cancer: potential link with ALCAM expression PLoS One 2013 ; 8 ( 6 ): e67361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A , Sharma S , Batra M , Abidullah M , Bhuvinder S , Katragadda P Role of E-cadherin in progression of oral squamous cell carcinoma: a retrospective immunohistochemical study J Contemp Dent Pract 2018 ; 19 ( 9 ): 1105 – 1110 [PubMed] [Google Scholar]
- Guo M , Mu Y , Yu D , Li J , Chen F , Wei B , et al. Comparison of the expression of TGF-β1, E-cadherin, N-cadherin, TP53, RB1CC1 and HIF-1α in oral squamous cell carcinoma and lymph node metastases of humans and mice Oncol Lett 2018 ; 15 ( 2 ): 1639 – 1645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharada P , Swaminathan U , Nagamalini BR , Kumar KV , Ashwini BK , Lavanya VLN Coalition of E-cadherin and vascular endothelial growth factor expression in predicting malignant transformation in common oral potentially malignant disorders J Oral Maxillofac Pathol 2018 ; 22 ( 1 ): 40 – 47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaw SY , Majeed AA , Dalley AJ , Chan A , Stein S , Farah CS Epithelial to mesenchymal transition (EMT) biomarkers—E-cadherin, beta-catenin, APC and Vimentin—in oral squamous cell carcinogenesis and transformation Oral Oncol 2012 ; 48 : 997 – 1006 [DOI] [PubMed] [Google Scholar]
- Lopes NM , Xavier FCA , Ortiz RC , Amôr NG , Garlet GP , Lara VS , et al. Subcellular localization and expression of E-cadherin and SNAIL are relevant since early stages of oral carcinogenesis Pathol Res Pract 2018 ; 214 ( 8 ): 1185 – 1191 [DOI] [PubMed] [Google Scholar]
- Von Zeidler SV , de Souza Botelho T , Mendonça EF , Batista AC E-cadherin as a potential biomarker of malignant transformation in oral leukoplakia: a retrospective cohort study BMC Cancer 2014 ; 14 ( 1 ): 972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyrodimou M , Andreadis D , Drougou A , Amanatiadou EP , Angelis L , Barbatis C , et al. Desmoglein-3/γ-catenin and E-cadherin/ß-catenin differential expression in oral leukoplakia and squamous cell carcinoma Clin Oral Investig 2014 ; 18 ( 1 ): 199 – 210 [DOI] [PubMed] [Google Scholar]
- Kaur G , Carnelio S , Rao N , Rao L Expression of E-cadherin in primary oral squamous cell carcinoma and metastatic lymph nodes: an immunohistochemical study Indian J Dent Res 2009 ; 20 ( 1 ): 71 – 76 [DOI] [PubMed] [Google Scholar]
- de Freitas Silva BS , de Castro CA , Von Zeidler SLV , de Sousa SCOM , Batista AC , Yamamoto-Silva FP Altered β-catenin expression in oral mucosal dysplasia: a comparative study J Appl Oral Sci 2015 ; 23 ( 5 ): 472 – 478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes M , Rojas-Alcayaga G , Maturana A , Aitken JP , Rojas C , Ortega AV Increased nuclear β-catenin expression in oral potentially malignant lesions: a marker of epithelial dysplasia Med Oral Patol Oral Cir Bucal 2015 ; 20 ( 5 ): e540 – e546 e546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo Muzio L , Lo Russo L , Falaschini S , Ciavarella D , Pentenero M , Arduino P , et al. Β- and γ-catenin expression in oral dysplasia Oral Oncol 2009 ; 45 ( 6 ): 501 – 504 [DOI] [PubMed] [Google Scholar]
- Chowdhury P , Nagamalini B , Singh J , Ashwini B , Sharada , Swaminathan U Expression of β-catenin in oral leukoplakia and oral submucous fibrosis: an immunohistochemical study J Oral Maxillofac Pathol 2021 ; 25 ( 1 ): 124 – 130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii M , Katase N , Lefeuvre M , Gunduz M , Buery RR , Tamamura R , et al. Dickkopf (dkk)-3 and β-catenin expressions increased in the transition from normal oral mucosal to oral squamous cell carcinoma J Mol Histol 2011 ; 42 ( 6 ): 499 – 504 [DOI] [PubMed] [Google Scholar]
- Reyes M , Flores T , Betancur D , Peña-Oyarzún D , Torres VA Wnt/β-catenin signaling in oral carcinogenesis Int J Mol Sci 2020 ; 21 ( 13 ): 1 – 22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puneeta N , Santosh T , Mishra I , Gaikwad P , Sahu A Evaluation of E-cadherin and Vimentin expression for different grades of oral epithelial dysplasia and oral squamous cell carcinoma—an immunohistochemical study J Oral Maxillofac Pathol 2022 ; 26 ( 2 ): 285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahire MS , D'souza ZI , Chettiankandy TJ , Nagar SR , Sinha A , Tupkari JV Demographic study of 366 cases of oral leukoplakia and immunohistochemical analysis—an institutional study J Oral Maxillofac Pathol 2021 ; 25 ( 3 ): 478 – 484 [DOI] [PMC free article] [PubMed] [Google Scholar]


