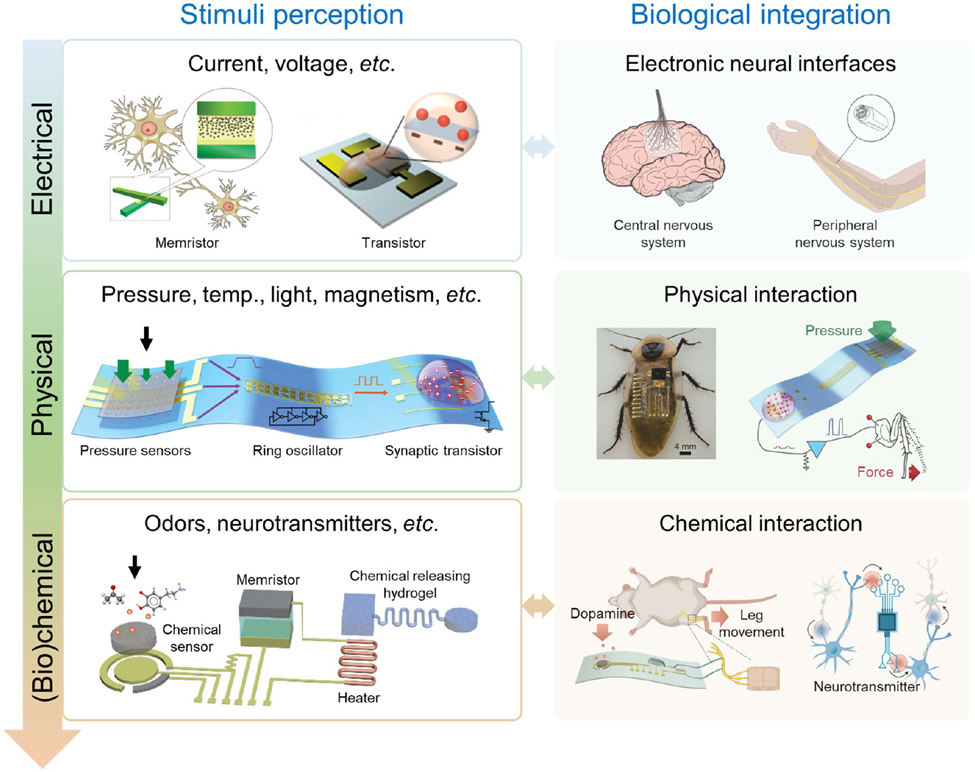
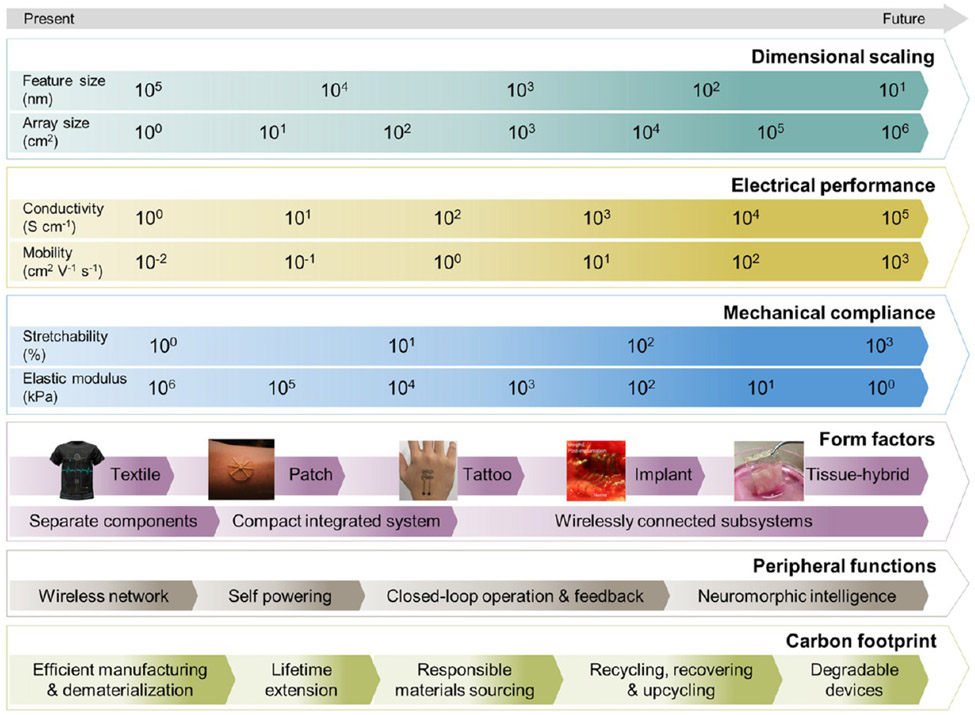
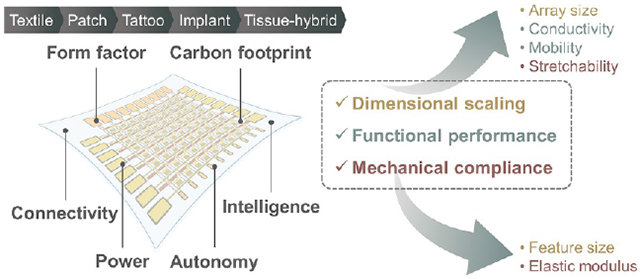
Abstract
Humans rely increasingly on sensors to address grand challenges and to improve quality of life in the era of digitalization and big data. For ubiquitous sensing, flexible sensors are developed to overcome the limitations of conventional rigid counterparts. Despite rapid advancement in bench-side research over the last decade, the market adoption of flexible sensors remains limited. To ease and to expedite their deployment, here, we identify bottlenecks hindering the maturation of flexible sensors and propose promising solutions. We first analyze challenges in achieving satisfactory sensing performance for real-world applications and then summarize issues in compatible sensor-biology interfaces, followed by brief discussions on powering and connecting sensor networks. Issues en route to commercialization and for sustainable growth of the sector are also analyzed, highlighting environmental concerns and emphasizing nontechnical issues such as business, regulatory, and ethical considerations. Additionally, we look at future intelligent flexible sensors. In proposing a comprehensive roadmap, we hope to steer research efforts towards common goals and to guide coordinated development strategies from disparate communities. Through such collaborative efforts, scientific breakthroughs can be made sooner and capitalized for the betterment of humanity.
Keywords: soft materials, mechanics engineering, flexible electronics, conformable sensors, bioelectronics, human-machine interfaces, body area sensor networks, technology translation, sustainable electronics
Graphical Abstract
Living things are equipped with biological sensory systems for light, sound, smell, etc. to monitor and to adapt to the environment. In addition to the natural senses, humans use synthetic, fabricated sensors—devices that allow users to measure the values of physical and psychological conditions of interest using the inherent physical properties of the sensors1—to augment our natural abilities of perceiving the world, enabling us to interact with the environment and to improve our living conditions.
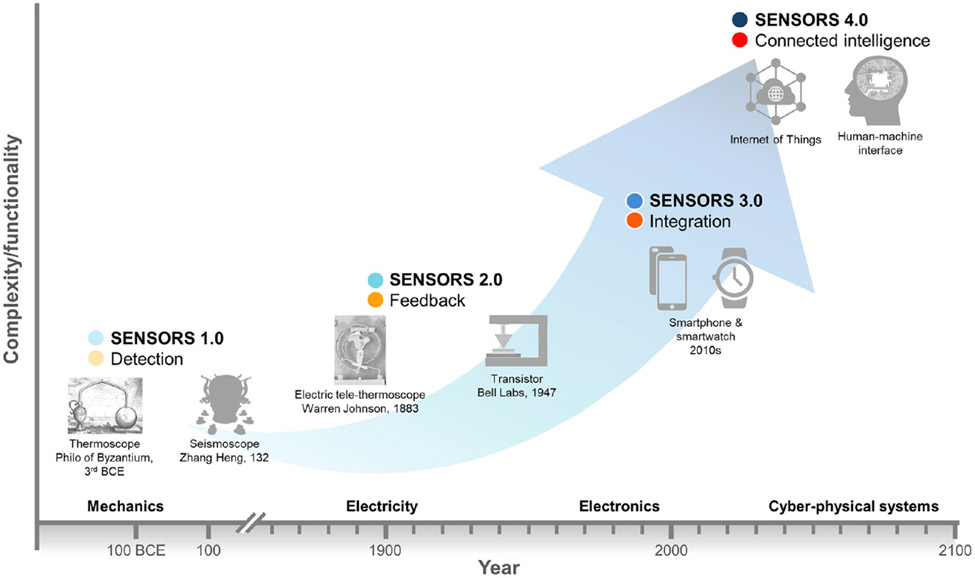
Amongst the first documented sensors in human history are the thermoscope by Philo of Byzantium in the 3rd century BCE for temperature change detection2 and the seismoscope by Zhang Heng in 132, used to detect the occurrence of earthquakes and approximate their directions.3 Sensors in this early era (what we define as Sensors 1.0, Figure 1) convert physical quantities/events to mechanical outputs that are easily observable. Later, with the discovery of electricity and the invention of electric generators, sensors were designed to convert physical parameters to electric signals, enabling control function. For instance, the electric tele-thermoscope invented by Warren Johnson in 1883 could not only monitor temperature, but could also modulate the function of an automatic temperature control system.4 This marked the era of Sensors 2.0. Moving to Sensors 3.0, the electronics industry promoted the miniaturization and integration of sensors with other electronic components, giving birth to smart devices such as smartphones and smartwatches, where dozens of sensors collectively provide an impressive user experience. In recent years, advances in the Internet of Things (IoT), Industry 4.0, big data, artificial intelligence (AI), robotics, and digital health5 have prompted sensors to become more connected and intelligent, entering Sensors 4.0. For instance, a large number and variety of sensors are embedded in autonomous vehicles with wireless connectivity for adaptive self-driving, and connected IoT sensors integrated with AI provide effective solutions to energy management of buildings and industrial facilities.6
Figure 1.
Evolution of sensor technology. Sensors 1.0 only served the function of detection. Sensors 2.0 had the hallmark of electrical feedback. The first transistors achieved sensing as well as the amplification of current.7,8 Sensors 3.0 are characterized by miniaturization and integration. A smartphone is equipped with pressure sensors, light sensors, sound sensors, temperature sensors, image sensors, motion sensors, location sensors, among many others; smartwatches are likewise equipped with an increasing cohort of diverse sensors. Budding Sensors 4.0 technologies involve sensor networks and advanced algorithms to achieve enhanced perception capabilities and intimate cooperation between humans and machines.
Sensors that translate the physical world into data serve a foundational role in the era of digital transformation. In the digital era, connectivity and decision-making rely heavily on high-quality big data—any data bias or inaccuracy may lead to distorted conclusions and/or incorrect decisions, and the consequences can be catastrophic.9,10 Therefore, it is critical to develop sensors that can acquire accurate and reliable data at large scales. This capability would potentially expedite solutions to the grand challenges facing humanity,11 such as aging populations,12 infectious diseases,13-15 food security,16-19 energy crises,6,20 climate and environment crises,21-24 and would improve our quality of life. For example, sensors could be employed to test patients for common diseases and even to predict them, to detect bacterial growth in every food package, or to monitor pollution in every lake, stream, and river.
However, conventional sensor technology is usually incapable of such massive-scale ubiquitous monitoring. Being highly integrated and miniaturized, modern sensors serve adequately as the components of smart electronics/machines, but their small and rigid form factors restrict their usage in many applications such as healthcare wearables, interactive robots, smart packaging, and building-integrated electronics, where flexible sensors would enable advances (Table 1). In this Review, we define flexible sensors broadly to include all types of sensors that can withstand mechanical deformation (>10 m−1 bending curvature or >1% strain on a device/system) without device failure or significant alteration in sensing performance. We include bendable, rollable, foldable, stretchable, twistable, and conformable sensors.25 Flexible sensors enable measurements on dynamic and/or shape-changing objects and large-area non-flat surfaces,26 due to their mechanical flexibility and stretchability, shape adaptability, and fabrication scalability, with which rigid sensors typically struggle. Flexible sensors are lightweight, thanks to the use of organic materials and/or thin-film form factors, benefiting integration, distribution, and application. Furthermore, some flexible sensors can be manufactured using low-cost materials and large-scale processes such as printing,27,28 making mass deployment economically viable. Importantly, the use of organic materials, the thin-film form factor, and the additive manufacturing of flexible sensors may provide more environmentally sustainable ways of sensor production and disposal, tackling the escalating electronic waste problem.29
Table 1.
Comparison of Conventional Rigid Sensors and Emerging Flexible Sensors
| Form factor/ appearance |

|

|
|---|---|---|
| Rigid sensors | Flexible sensors | |
| Performance | ||
| Stretchability | < 1%a,30 | Up to 1000%31,32 |
| Young’s modulusb,33 | 1–200 GPa | 10 kPa–200 GPa |
| Conformability on non-flat surfaces | No | Yes |
| Measurement during mechanical deformation | No | Yes |
| Manufacturing | ||
| Methods | MEMSc techniques | Printing, MEMS techniques, etc. |
| Scale27 | 0.01–0.1 m2 (wafer) | Up to 1–100 m2 (web)28 |
| Single-step throughput27 | 0.001−1 m2 min−1 | Up to 10–1000 m2 min−1d |
| Potential cost per unit area34 |
High |
Low |
| Carbon footprint | High | Low |
| Applications | ||
| Smartphones, autonomous vehicles, industrial robots, etc. | Conformal skin patches, smart textiles, sticker sensors for industrial IoT, supply chain, food, etc. |
Stretchability of rigid sensors is approximated as the fracture strains of conventional electronic materials. However, the real stretchability would be smaller taking performance alteration in consideration.
Ranges cover the Young’s moduli of common materials (packaging materials included) used in rigid and flexible sensors.
MEMS, microelectromechanical systems.
Assuming 1 m wide web on a roll-to-roll platform.
The above features make flexible sensors well positioned for applications that have demanding requirements in mechanical compliance, integration density and scale, manufacturability, and cost. For example, the physiological parameters that current wearable sensing technologies (e.g., continuous glucose monitors, smartwatches) can measure are limited.35 Medicalgrade measurements of electrocardiogram and sweat metabolites require conformal contact between the sensor and the skin, but this goal is hardly achievable by rigid sensors without causing discomfort due to the surface micro-texture and deformation experienced by the skin.36 In contrast, soft and stretchable sensors can address these issues, offering disruptive solutions for future healthcare.37-39 For robots to interact safely with humans,40 high densities of sensors (>10 sensors per cm2 to be comparable to human skin41) would need to cover the curved robotic surface over large areas (~m2).42 In this regard, monolithically manufacturing sensor arrays on flexible substrates is much more efficient than individually placing rigid sensor pixels.43,44 Furthermore, smart packaging with embedded sensors for product tracking and quality monitoring will be critical to efficient and sustainable supply chains,45 yet sensor stickers need to be manufactured at costs as low as a few cents to realize this large-scale (and often disposable) application. Likewise, to install sensing maps in industrial facilities such as pipes, walls, and floors, low-cost and large-area manufacturability is pivotal. To this end, printed sensors using solution-processable organic/carbon materials provide the most promising solutions. In all, the advantages offered by flexible sensors target the issues of data quantity and quality in the digital era, which will make flexible sensors one of the most valuable players in Sensors 4.0.
Flexible sensors have matured tremendously since the beginning of the 21st century. Starting with pressure sensor arrays on plastic films,43 flexible sensors now cover a wide range of physical and chemical sensing modalities, including temperature, strain, electrophysiology, ions, biomarkers, metabolites, gases, and many more.46-55 Substrates are not limited to plastic films but can also be ultra-thin plastic foils,56 porous polymer mats/meshes,57 paper,58,59 elastomers, and hydrogels.60 From single sensors to sensor arrays, from stand-alone sensors to integrated sensing systems,28 the development has been astonishing, and fascinating applications including sweat-based stress monitoring54,61,62 and remote robotic control through skin sensor-enriched virtual reality63 have been demonstrated.
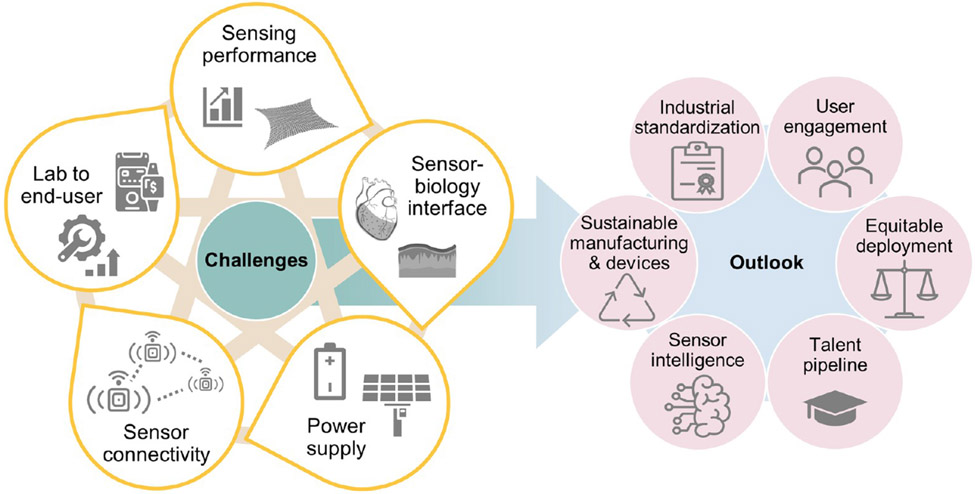
Despite significant research achievements, the adoption of and market for flexible sensors often falls short of predicted levels.27 Some flexible sensors still have a long way to go to meet the stringent demands posed by real-world problems. It is therefore timely to identify the bottlenecks hindering flexible sensor deployment, not only technical challenges, but also cultural and regulatory hurdles (Figure 2, left). Here, we summarize promising on-going efforts to address these challenges and propose further plausible solutions. In doing so, we hope to steer and to accelerate research efforts towards faster translation of laboratory innovations and prototypes into widely used products. Additionally, we anticipate long-term issues facing future sensor deployment (Figure 2, right). Early awareness of these issues will prompt crafting and development of effective solutions. We conclude by predicting what future intelligent flexible sensors will do. These challenges and prospects are summarized into a comprehensive roadmap, in the hope of guiding collective and cooperative development strategies towards common goals by the research community and beyond.
Figure 2.
An overview of issues covered in this Review, including challenges for flexible sensors in the near future (left) and issues to address in the long run (right). The five aspects of challenges are interrelated, reflected by the tan lines in the background.
SENSING PERFORMANCE
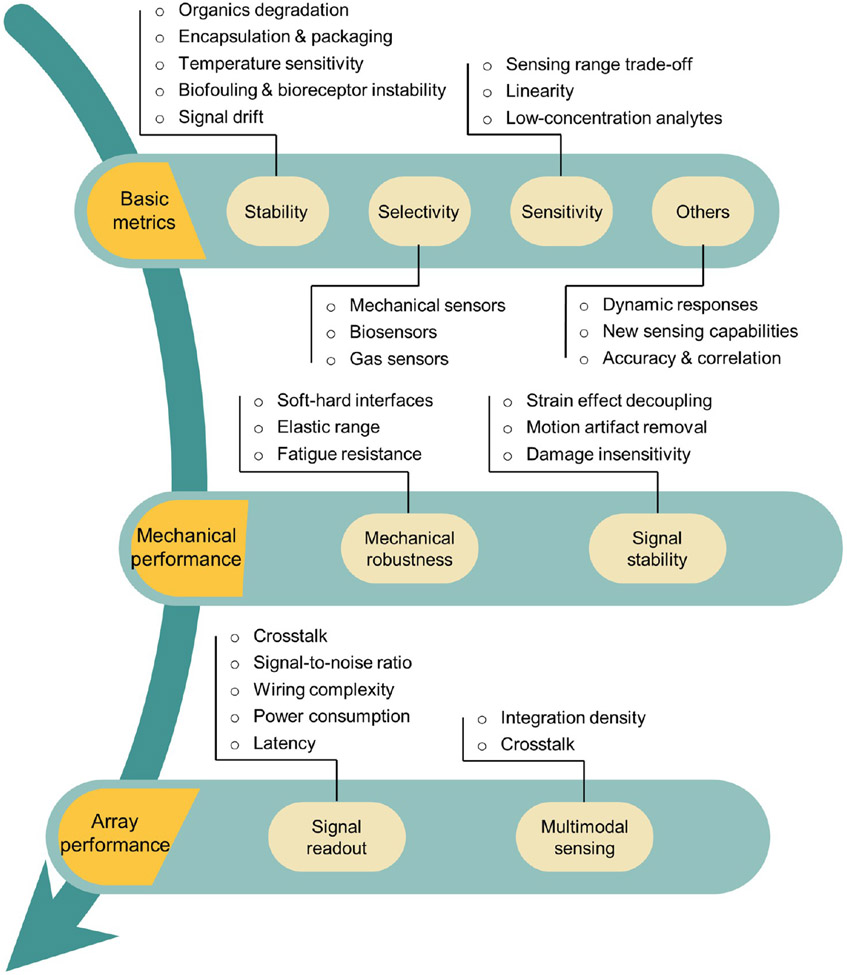
Sensing performance is of paramount importance for any sensor. Performance of flexible sensors encompasses the basic metrics that apply to both rigid and flexible sensors, including the classical 3S’s (stability, selectivity, and sensitivity), as well as aspects unique to flexible sensors, including tolerance to mechanical deformation and monolithic integration into large-area sensing arrays. We identify key issues in these three aspects: basic metrics, mechanical performance, and array performance (Figure 3), and provide detailed discussions on existing solutions and research gaps.
Figure 3.
Overview of key issues in the sensing performance of flexible sensors.
Basic Performance Metrics Comparable to Rigid Sensors.
Stability, selectivity, and sensitivity are primary metrics used to assess sensor performance. Because of the materials, manufacturing techniques, and sensing mechanisms used in flexible sensors, their performance often falls short of rigid sensors, even when no mechanical deformation is involved. Here, we discuss some most prominent issues in the 3S’s—stability being the most challenging and pressing problem for real-world applications, particularly when trying to achieve long-term sensing. We also briefly discuss considerations other than the 3S’s, including the dynamic responses of mechanical sensors and sensing capabilities enabled by wearable biosensors. We summarize sensor performance by emphasizing holistic approaches to sensor accuracy and fundamental correlation-establishing studies.
Stability in Harsh Environments Despite the Use of Unstable Materials.
Stability is essential for deployable sensors because it ensures repeatable and reliable usage in changing environments, especially for long-term monitoring. Here two dimensions are considered: time and stress (e.g., temperature, humidity).64 The challenge of stability for flexible sensors often stems from the use of organic and polymeric materials in their fabrication, which tend to degrade over time and whose properties are easily altered by environmental factors. Furthermore, wearable biosensors incorporating biological receptors face additional bio-instability. The challenge is exacerbated by the degradative environments flexile sensors are exposed to, such as in vivo tissues and biofluids, the deep sea, and high altitudes, where extreme physicochemical stresses are present.
The most straightforward approach to tackling the stability challenge is to improve the environmental stability of sensor materials. Engineering conventional rigid sensor materials into flexible and stretchable form factors is one effective approach, but the fabrication complexity can limit scalability and cost effectiveness. A special case of this strategy is the recently discovered giant magnetoelastic effect in soft systems for pressure sensing, where inorganic magnetic particles are embedded in elastomers to induce changes in magnetic fields under deformation.65 The sensing materials and mechanism employed are intrinsically waterproof and environmentally stable.65-68 While materials synthesis and modification towards environmentally stable organics69-71 and other emerging materials (e.g., perovskites)72,73 are a constant pursuit, this strategy is fundamentally challenging, governed by the physicochemical nature of these materials.
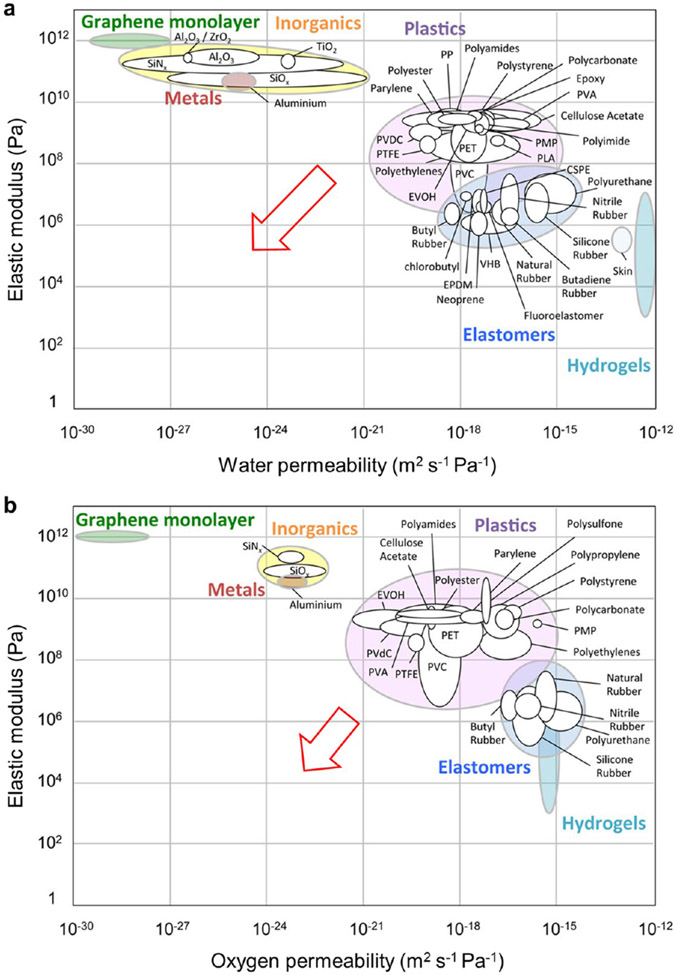
Therefore, when no direct contact between the stimulus and the sensing material is required (in mechanical, temperature, and light sensors, for example), a more viable approach is to apply protective layers to sensitive materials and the entire device.74 To this end, high-performance humidity and oxygen barrier materials are in great demand. However, flexible materials themselves usually have poor barrier properties (Figure 4) due to the intrinsic free volume in polymers and defects in inorganics,74 and barrier properties degrade with repeated mechanical deformation. Such issues are most severe for the elastic packaging of mechanical sensors and other stretchable sensors, when both elasticity and barrier properties are required.75 Adding thin-film coatings76 and filler additives to packaging materials are two frequently employed strategies,77 but there remains a lack of effective methods to improve the barrier properties of elastic substrates and packaging layers.60 In addition to specialized barrier layers, effective sealing is also critical for devices made of fluidic or liquid-containing materials (e.g., liquid metal, electrolyte, hydrogel) and devices used in liquid environments (e.g., in vivo biological tissues,76 sea-water78).
Figure 4.
Barrier properties against (a) water and (b) oxygen of common materials used in flexible sensors, plotted versus elastic modulus. Softer materials are more flexible and usually more stretchable but suffer from poorer barrier properties. Soft barrier materials with moduli comparable to elastomers and hydrogels, as well as permeabilities comparable to inorganics are highly desirable for effective encapsulation (red arrows). Adapted with permission from ref 75. Copyright 2018 American Chemical Society.
Encapsulation, and packaging in general, are of paramount importance to flexible sensors, especially wearable sensors. Proper packaging enables users to wear devices over extended periods with minimal noise or signal drift. This issue is often ignored by the academic community and is more typically considered by industry. However, packaging must be addressed in order to obtain meaningful on-body data in population studies.
Temperature is an environmental stress affecting almost all flexible sensors because most sensing materials, including rigid ones, are sensitive to temperature changes.79 This problem cannot be solved simply through material optimization or encapsulation. Introducing additional compensation elements such as temperature sensors and feedback circuits80,81 is generally more effective. Alternatively, exploring sensing mechanisms that circumvent the temperature-sensitive aspects of the sensing material82,83 is viable, although not as a general solution. Overall, to suppress temperature effects with simple device structures and high integration remains a challenge.
Stability is a significant challenge for flexible chemical sensors, especially wearable biosensors,36 where bio-fouling and bioreceptor inactivation are two major factors that affect long-term (several days) sensor performance. The fouling layer strongly influences the selectivity and binding affinity of biorecognition events and results in strong background signals as well as poor signal-to-noise ratios. One of the most commonly used strategies to combat biofouling is drop-casting protective polymeric membranes such as Nafion and chitosan.84,85 Other anti-fouling coatings such as bovine serum albumin (BSA) and poly-(ethylene glycol) are also effective.86-88 However, a drawback of this surface-coating strategy is the reduction or blockade of electronic conduction between the biorecognition moiety and the transducing electrode. To tackle this problem, three-dimensional (3D) nanocomposites composed of anti-fouling agents (e.g., BSA) and conductive materials (e.g., gold nanowires, carbon nanotubes, CNTs) have been engineered.89 Alternatively, surface roughness and wettability control can also circumvent this problem. For example, nanoporous Au electrodes minimized fouling by slowing down mass transport while allowing efficient small-molecule exchange.90 Insights from skin biology, such as materials chemistry and surface texture, may provide inspirations to tackle biofouling.
Low stability of immobilized bioreceptors in the uncontrolled conditions of wearable applications (e.g., changing temperature and pH) is another issue. Bioreceptors such as enzymes can easily detach from anchoring substrates/electrodes in fluidic environments (and even more so if mechanical deformation is involved) and lose their recognition function outside their operational windows.36 To improve the long-term stability of enzymatic sensors, a nanoporous membrane with effective enzyme immobilization was robustly anchored to nanotextured electrodes, achieving continuous glucose sensing with minimal signal drift for up to 20 h.91 Encapsulation of enzymes within electrodes through a monolithic 3D printing process is another way to improve stability.92 Alternatively, nanozymes, i.e., artificial enzymes made of nanomaterials, can be used,93-95 though often-times compromising selectivity. Molecularly imprinted polymers (MIPs), known as “artificial receptors”, can also overcome the stability issue of bioreceptors while achieving good selectivity.96
Besides biofouling and bioreceptor instability, there are other issues impairing biosensor stability. For example, many reported electrochemical biosensors utilize a mediator layer to reduce the potential required to trigger redox reactions for reduced interference from other electroactive molecules,97 yet the Faradaic signal could decay over time, limiting long-term reliability. Furthermore, charge accumulation on electrode surfaces or material interlayers can lead to signal drift, which can be mitigated by nanotextured electrodes with larger surface areas and more robust bonding with sensing layers.98 Moreover, the interactions between the active layers and biomarkers may alter the surrounding electric field, introducing microenvironmental changes as an interfering factor.
Signal drift over a relevant period of operation is an issue not only for biosensors, but also may be the number one challenge for any sensor technology. However, this issue is often ignored by the academic community outside of electrical engineering. The magnitude and predictability of signal drift often determine the lowest concentration that a sensor can accurately report over its lifetime. Flexible sensors often suffer from much larger signal drifts than their rigid counterparts, which effectively leads to high noise levels. In this regard, it is important for the community to report signal drift and its measurement carefully and precisely, and to understand the exact cause for each emerging sensor technology so that the sensor drift can be tackled effectively. For example, by designing better sensor architectures or customizing compensation algorithms and driving circuits, sensor drift can be reduced.
Stability is central to sensor practicality, yet it is often neglected in academic research. We urge the research community to place more emphasis on stability to push flexible sensors closer to commercialization. When long-term stability is not achievable, making sensors at a low cost such that they can be frequently replaced and disposed of might be another viable route to mass adoption.
Selectivity to Complex Mechanical and Chemical Stimuli.
Selectivity refers to the ability of a sensor to discriminate between the analyte of interest and possible interferences.99 It was originally defined for chemical100 and biological101 sensors but may be extended to include mechanical sensors (e.g., pressure sensors, strain sensors, torsion sensors, etc.). In real application scenarios, a wide range of chemical species and mechanical forces are usually present simultaneously, and they interact with sensing materials through similar mechanisms, thus producing ambiguous sensor responses.
There are two general approaches to sensor selectivity: specific sensors and selective sensor arrays.101 Ideally, a specific sensor only responds to one analyte, and an array of such sensors would tell the exact composition of a mixture without needing a great deal of data analysis. Such specific sensors are often hard to realize. In contrast, in a selective sensor array, each sensor responds to a collection of analytes differently, and the array response collectively produces a fingerprint for a mixture. With proper data analysis, the mixture composition can be accessed. These two principles are widely applied for mechanical sensors, biosensors, and gas sensors.
Mechanical force applied on a sensor is often a mixture of pressure, tension, shear, and torsion. Decoupling these modes is important for gesture recognition, robotic control, and prosthetics. There have been attempts to fabricate ‘specific’ mechanical sensors.83,102-106 For instance, a stiff and anisotropically resistive material was structured into micro-meanders and encapsulated in elastic films such that the sensor was responsive to only one direction of tensile strain and insensitive to bending and twisting.102 Stiff platforms were embedded underneath pyramid microstructures for pressure sensors to achieve undisturbed performance at up to 50% tensile strain.104 Although the insensitivity to other mechanical stimuli of these ‘specific’ sensors is not ideal due to materials and geometric limits, their performance is sufficient for non-critical applications or large values of a strain of interest (e.g., joint movements). ‘Specific’ sensors have been integrated to achieve multi-modal mechanical sensing, where careful mechanical design is needed to isolate and distribute different mechanical stimuli to the desired sensors so that each deformation can be sensed independently.107-109
The other ‘selective sensor array’ principle takes many forms for mechanical sensors. The simplistic implementation is to fabricate deformable sensing materials110,111 and/or design 3D sensor structures112-116 to make the response curves different for different forms of deformation. With proper signal analyses, the correct deformation can be identified. A similar method is to integrate multiple sensors on a miniaturized 3D structure117 or a two-dimensional (2D) surface.118,119 The response of individual sensors differs according to the stress applied; holistic analyses of all sensor outputs derive the deformations experienced. However, using the above methods, it may be difficult to decouple a simultaneous combination of deformations (e.g., compression plus shear) because the signals overlap. Advanced algorithms, such as machine learning, might be able to solve this problem. A third approach is to use materials or devices that are sensitive to several stimuli, but the stimuli can be distinguished by different measurement modes (e.g., resistance and capacitance).111 Readout electronics will be more complex for integrated devices using this strategy, which further increases system-level power consumption and hardware cost in parallel.
Biosensors are used to analyze complex mixtures present in biological samples, which may contain ions, small molecules (metabolites, cytokines, lipids, neurotransmitters, etc.), macromolecules (peptides, proteins, nucleic acids, etc.), and even viruses, bacteria, and cells. Selectivity becomes critically important in analyzing such complex mixtures as closely related interferents (e.g., biological precursors and metabolites) are often present.120 In this regard, nature provides many biorecognition elements that offer high specificity through interactions with metabolites and biomarkers. The utilization of bioaffinity-based receptors, including ionophores, DNAs/RNAs, aptamers, and antibodies on flexible biosensors allows selective in situ target recognition,96 although sometimes at the cost of complicated fabrication and handling, as well as relatively poor stability. In this regard, artificial bioreceptors such as MIPs offer a more stable and easily processible option without sacrificing binding specificity in some cases.96 Effective transduction mechanisms that transform the receptor-target binding to measurable electrical or optical signals are critical. Some promising examples include aptamer-functionalized field-effect transistors,120 molecular pendulum-based biomolecular sensors,121 as well as redox probe-tagged electrochemical aptasensors122 and MIP-based sensors.62 More complex biorecognition elements, including cell membranes123 and whole cells124-126 provide improved specificity towards some analytes, but this advantage comes with increased fabrication complexity and storage requirements. For biosensors that do not rely on bioaffinity for sensing, careful engineering of catalytic nanomaterials can achieve desirable selectivity in the (electro)-chemical recognition of some analytes.127-129
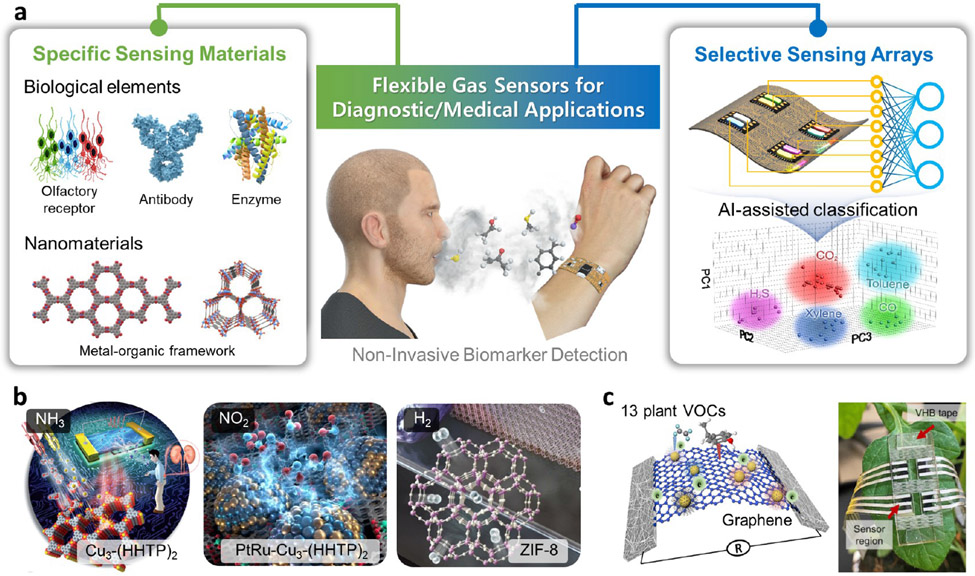
While biosensors are most often used for biofluid analyses, they can also be engineered to detect airborne pathogens130 and biologically relevant gases. Gas sensors are an emerging field for flexible sensors. They provide noninvasive means of biomarker detection to inform metabolic processes and disease progression in humans and plants,131-137 and are thus attractive for real-time health monitoring and point-of-care diagnostics (Figure 5a).138
Figure 5.
Selective gas sensors. (a) Overview of the strategies to achieve selective gas sensing, including specific sensing materials and selective sensing arrays. Flexible gas sensors target applications in health monitoring and point-of-care diagnostics. AI, artificial intelligence. (b) Examples of metal–organic framework-based nanomaterials with high sensing specificity to gas molecules. Cu3-(HHTP)2 (HHTP=2,3,6,7,10,11-hexahydroxytriphenylene) thin film for room-temperature NH3 detection. Adapted with permission from ref 139. Copyright 2017 Wiley-VCH Verlag GmbH &Co. KGaA, Weinheim. Bimetallic nanoparticles (PtRu) confined in two-dimensional Cu3-(HHTP)2 for NO2 detection. Adapted with permission from ref 140. Copyright 2021 Wiley-VCH GmbH. Zn-based zeolite imidazole framework (ZIF-8) as molecular sieving layer for selective H2 filtration on Pd nanowires. Adapted with permission from ref 141. Copyright 2017 American Chemical Society. (c) An example of nanomaterial-based selective sensing array for volatile organic compound (VOC) identification. A graphene-based stretchable chemiresistive sensor array for identification of 13 plant VOCs. Reproduced with permission from ref 142. Copyright 2021 Elsevier.
Biomarker volatile organic compounds (VOCs) present in complex mixtures (often with more than a dozen of components) and a complete profile is often required for the determination of physiological status.133,142 Some VOCs have similar molecular structures, making specific sensing challenging. Although there have been attempts to utilize biological olfactory elements, such as olfactory receptors (ORs), olfactory cells, and even olfactory organs,143 as well as other biomolecules (e.g., enzymes, antibodies, aptamers) as the recognition moieties (Figure 5a, left),144,145 insufficient understanding of biological olfactory systems poses fundamental challenges for bioaffinity-based gas sensors. For example, the pairing relationships and the binding/unbinding interactions between gas species and ORs are largely unknown.145 Other factors impeding the development of bioaffinity gas sensors include design complexity for liquid-phase reactions and the high cost and low stability associated with bioreagents, given that gas sensors are currently primarily used for industrial and environmental applications.
The growing healthcare/medical gas sensors area133 may provide an impetus to continue to develop bioaffinity-based sensing.144 In comparison, nanomaterials with tunable structures and chemistries capable of dry-phase sensing seem to be more technically and economically viable.52,146,147 Metal–organic frameworks (MOFs) are particularly attractive because their porous structures can selectively adsorb or filter gas molecules (Figure 5b).148 However, a limited understanding of gas–MOF interactions, as well as the structure–property relationships of MOFs prevents generalized design methodologies for MOF-based gas sensors to cover wide ranges of VOCs.
In contrast to specific VOC sensors, selective sensing arrays are more widely used to recognize gas mixtures (Figure 5a, right). Combined with machine learning, this strategy has seen commercial success in electronic noses.24 Nanomaterials are also a go-to option for selective sensing arrays due to their high sensitivity and ease of tuning surface interactions.149 For instance, graphene functionalized with various ligands and coupled with Au nanoparticles was used to construct an 8-sensor array that could classify 13 individual plant VOCs at >97% classification accuracy (Figure 5c).142 A recent approach achieved the fabrication and utilization of an array of 108 graphene-based sensors functionalized with 36 chemical receptors for the discrimination of 6 gas species within a minute,150 shedding light on rapid VOC detection using largescale sensor arrays. Overall, recent advances in flexible room-temperature gas sensor arrays have achieved lower power consumption, reduced fabrication cost, and greater wearability without sacrificing sensing performance.142,151-155 Although machine learning algorithms capable of higher prediction accuracy can compensate for sensor selectivity short-falls,151,156,157 improving the specificity of each sensor remains a critical challenge.
An interesting application of selective array sensing was recently reported for triboelectricity-based material identification.158 An array of sensors with differential triboelectric properties generated a fingerprint signal pattern when in contact with a particular material. Combined with machine learning, the accuracy for materials classification reached 97% when four sensors in an array were used. Such strategies may find wider application in flexible sensors to enable more sophisticated sensing capabilities.
Sensitivity with Wide-Range Linearity and to Low-Concentration Analytes.
High sensitivity allows sensors to detect minute changes in a stimulus, to reduce false-negative signals, and to improve signal-to-noise ratio and accuracy. The sensitivity of most flexible physical sensors (e.g., mechanical sensors, temperature sensors, photodetectors) is sufficient for common applications. A notable issue is the trade-off between sensitivity and sensing range in mechanical sensors. In comparison, sensitivity is more of a concern for chemical sensors, specifically biosensors that detect low concentrations of analytes present in biofluids.
The trade-off between sensitivity and sensing range, and the issue of nonlinearity exist in most mechanical sensors,111,159-163 and are especially prominent for pressure sensors.164 Ideally, high sensitivity across a wide force/pressure range is desirable, but is hardly achievable in bulk piezoresistive/piezocapacitive sensors, because of the stiffening effect of soft materials upon compression. Microstructuring is a common strategy to improve sensitivity,165,166 yet this approach mostly works at low pressures. There have been many attempts to address this problem. Structure-wise, intrafillable microstructures accommodate deformed surface structures in the underlying undercuts and grooves, thereby retarding the saturation of porous structures.167 Mechanism-wise, combined piezoresistivity and piezocapacitivity significantly increase sensitivity, even at large stress of up to 50 kPa.168 The magnetoelastic effect is useful for pressure sensing over a wide range, from 3.5 Pa to 2000 kPa,169 and its sensitivity is comparable to those of piezoresistive and piezocapacitive sensors.
The above methods do not solve the nonlinearity problem. One solution is using hierarchical microstructures, such as micropillars on hemisphere arrays.170 Adding gradient charge distribution within the active material may be able to solve the nonlinearity issue. This strategy has been demonstrated in a capacitive pressure sensor, reaching a record-high linearity range up to 1000 kPa. The mechanism is gradient compressibility and dielectric property with increasing pressure, realized by a skin-like hierarchical microstructure made of materials of different permittivities.171 This strategy may be extended to other types of pressure sensors based on gradient conductivity or gradient ionogels. Another perspective on addressing this sensitivity-range conflict is to program the sensor performance on demand based on application requirements, since extraordinarily high sensitivity is usually required for small pressure detection, whereas for large pressure, a wide sensing range is more important. A stiffness memory ionogel was developed,172 whose stiffness could be tuned by pressure plus thermal treatment. The programmable stiffness led to programmable pressure ranges, detection limits, and sensitivity. Although an interesting concept, the practical applicability of such customizable sensors should be carefully evaluated, taking account of reproducibility, calibration, etc.
Generally, for mechanical sensors and other sensors involving mechanics sensing (e.g., vibration sensors, ultrasound imagers173), sensitivity–deformability entails a balance of rigid and soft materials in rationally designed structures—rigid materials usually lead to good sensitivity, whereas soft materials enable large deformability. Integration density, system complexity, and manufacturability are key factors to consider when devising wide-range sensitive systems.
Highly sensitive wearable and implantable biosensors are strongly desired for on-body and in-body chemical sensing to aid diagnostics and therapeutics, but this technology is relatively underdeveloped. Currently reported biosensors primarily focus on biomarkers at the levels of tens of μM or higher.174-176 There are a number of clinically relevant biomarkers such as proteins, peptides, hormones, small molecules, and drugs existing in sweat or saliva at nanomolar levels and lower.177 To enable the detection of these biomarkers, the sensitivity of flexible biosensors needs improvement.
Various nanomaterials such as conducting polymer nano-fibers,178 graphene,179 nanostructured gold,180 MOFs,181 and transition metal nanoparticles (e.g., Fe3O4 and NiO)127 are often utilized on the working electrode in electrochemical sensors as they can enhance the electrochemically active surface area and electron transfer dynamics, resulting in higher detection signals.52,176 Recent reports show that laser-engraved graphene enabled the detection of sweat uric acid, tyrosine, and cortisol at sub-micromolar levels,61,182 and dendritic gold nanostructures were successfully used to monitor micromolar levels of vitamin C and glucose in sweat.91,183 Besides nanomaterials, micro- to macro-scale approaches can also increase electroactive surface areas, using printable ink formulations and 3D hybrid electrode structures.184,185
Signal transduction is important to sensitivity—effective transduction can amplify binding events to reach measurable signals. Transistors, including field-effect transistors (FETs)54,120,186-189 and organic electrochemical transistors (OECTs),190-192 are effective amplification devices.191,193,194 When the channel of a FET is reduced to the nanoscale, the high surface-to-volume ratio enables highly sensitive detection.195 By employing this mechanism with an aptamer, cortisol at a concentration down to 1 pM could be selectively detected.54 In addition, reducing the molecular size of surface-bound bioreceptors, such as using oligonucleotides in place of DNAs187 and nanobodies in place of antibodies,192 can bring the target-binding event closer to the transducer and may therefore enhance sensitivity. This consideration can also be useful in the design and selection of aptamers, to ensure that significant conformational changes in the artificial receptor occur close to the surface so as to gate the FET channel optimally.120 Successful engineering of peptides196 and DNA197 into semiconductors may allow the unification of analyte binding, transduction, and amplification in a single material, offering improvement in sensitivity and response time. Devices capable of effective amplification should be explored further for wearable biosensors. For example, subthreshold Schottky-barrier thin-film transistors demonstrate exceptional intrinsic gain of up to 1,100 V V−1.198 Schottky-contacted nanowire sensors were found to enhance the sensitivity of Ohmiccontacted sensors to light, gas, and (bio)chemicals by orders of magnitude.199
Colorimetric biosensors are attractive due to low cost, simplicity, and automated operation, but their poor sensitivities call for effective signal-amplifying mechanisms. Fluorescent biosensors could be a good alternative as fluorescence can boost sensitivity by up to 1000× that of colorimetry.200 Nanocatalysts are also promising, previously achieving 100× amplification in antibody-based lateral flow immunoassays.201 Careful design of the catalytic inorganic nanoparticles with organic recognition moieties is critical in achieving desirable sensing performance. Nevertheless, current methods for colorimetric and fluorometric signal detection by the naked eye, in-built detectors, or external cameras suffer from drawbacks such as subjectiveness, device bulkiness, and manual operation. Simple methods to quantify colorimetric and fluorometric signals digitally from wearable biosensors are needed.
Another potential way to enhance the sensitivity of biosensors is the preconcentration of target analytes through ion concentration polarization202 or dielectrophoresis.203 Target preconcentration has been used for wearable real-time monitoring of low-level heavy metals in sweat.204,205 A further strategy being explored is to amplify signals using low-noise and high-gain circuits, such as differential amplifiers and charge-coupled devices.206
Considerations beyond the 3S’s. Dynamic Responses of Mechanical Sensors.
Since mechanical deformations occur time-dependently, the dynamic responses of mechanical sensors to varying strains and stresses critically determine sensor accuracy in practical use. There are three major issues in this regard: hysteresis, response time, and strain-rate dependency, which are highly interrelated.
Hysteresis refers to differing response curves between loading and unloading, presenting a fundamental challenge for mechanical sensors. It stems from the viscoelasticity of common soft materials (e.g., elastomers, gels) used in flexible mechanical sensors,110 especially when doped with conducting fillers. Micro-/nano-structuring for enhanced sensitivity adds another source of energy dissipation from interfacial contact.207 Moreover, flexible mechanical sensors usually possess longer response times than rigid counterparts due to sluggish polymer chain movements. This difference precludes time-critical applications such as in robotic control and high-frequency applications, such as motion tracking in racing sports. Strain-rate dependency refers to the differing response curves under varying deformation rates or frequencies, leading to inaccurate readings in many applications, since most deformations encountered in daily life are not at constant speed. This phenomenon is often closely related to long response times, i.e., when the structural or molecular changes in sensors cannot catch up with the macroscale exerted stress, the sensors deviate from equilibrium states to varying extents at different strain rates. Sometimes, strain-rate dependency is an intrinsic characteristic dictated by the sensing mechanism (for instance, pressure sensing based on magnetoelastic generators depends on the rate of change in magnetic flux65,169,208). An effective strategy to overcome hysteresis and related issues is to use rigid materials with special structural designs for strain sensing, while soft materials are still required for deformability.209,210 Microstructuring is effective for pressure sensors through a reduction in contact area.164 Alternatively, careful engineering of polymeric networks can mitigate hysteresis,211-215 yet the materials fabrication can be complex and thus difficult for device integration. An emerging approach leverages machine learning to correct the errors associated with the viscoelastic properties of soft sensors for better prediction and analyses.216
The dynamic performance of mechanical sensors may appear trivial, yet it is critically important to practical measurements, deserving of greater attention. For example, although stretchable strain sensors using conductive elastomeric composites have been widely reported, their dynamic performance has rarely been investigated. Most studies only focus on quasi-static electric behavior, where the sensing performance was evaluated in a static state or in slow stretching–releasing processes (deformation speed <30 mm min−1, strain rate <10% s−1).217 Few studies have paid attention to the signal fidelity of strain sensors at higher deformation speeds,215,218 which is more relevant to dynamic motions in real life, such as limb movements and hand gestures (speed >100 mm min−1, strain rate >20% s−1).217 In monitoring these dynamic motions, strain sensors using elastomeric composites usually experience signal distortion, which is a common yet often overlooked problem.219-222 Dynamic responses at high and varying strain rates should be included as an essential performance metric when reporting mechanical sensors.
Sensing Capabilities of Wearable Biosensors.
Wearable biosensors are still in early stages of development and many sensing capabilities await exploration and development.36,223-225 The first area of improvement is to expand the portfolio of biomarkers that can be detected, to approach and to exceed current clinical assays. Complex biomarkers (e.g., proteins, hormones, nucleic acids, small molecules, and pathogens) usually require bioaffinity-based sensing, and this strategy demands the design of effective biorecognition moieties and proper immobilization and stabilization. Sensitive and selective aptamers are being developed for a wider array of targets and they could be deployed for this purpose.120 Moreover, some approaches require multi-step preparation (e.g., immunobiosensors using antibodies),88 making them challenging to integrate into wearable platforms. Microfluidics is one promising approach,15,226-229 which helps to collect, to contain, and to drive biofluids, as well as to deliver and to wash out unbound detection probes or labeling reagents. In addition, to reduce the number of preparation steps and time consumed in immunosensing, development of label-free, reagent-free, and wash-free methods is also necessary.200 Surface-enhanced Raman spectroscopy (SERS) has emerged as a powerful tool in this regard, but it requires a standalone spectrometer for signal readout.230,231 Recent work proposed an indirect electrochemical approach based on MIPs coupled with redox-active reporters, which enabled the detection of non-electroactive species in sweat, including amino acids, vitamins, metabolites, lipids, hormones, and drugs.228 This approach may be customized to detect a more diverse range of biomarkers.
A second area worth exploring is to realize continuous monitoring of these biomarkers, which enables real-time monitoring and prompt detection of abnormalities. Common bioaffinity assays (e.g., immunoassays) of disease biomarkers involve complex steps, require accurately controlled sample volumes and receptor regeneration, and are not reversible. These features make immunoassays not amenable to continuous on-body operation. Innovative strategies need to be crafted to overcome these challenges. For example, modulating intermolecular forces between the bioreceptor and the target using proper stimuli such as heat, ultrasound, electric/magnetic fields, and chemical cues might be a viable approach to sensor regeneration.51 Using this strategy, the regeneration of MIP-based electrochemical biosensors by current or voltage has been demonstrated.228 A resettable electrochemical sweat lactate sensor has been developed through reversible redox reactions in a biofuel cell.232 Regeneration of aptamers for cocaine sensing has been realized through pH-modulated conformational changes.233 Microfluidics are a promising platform for continuous-monitoring wearable biosensors. For instance, stretchable microfluidics can expel sweat from filled channels to enable multiple usage.234 Rational design in channel shape and wettability can accelerate sweat collection and realize continuous sampling.235,236 High temporal resolution can be achieved through encapsulating biofluids in water-in-oil droplets and assessing the droplets sequentially, although system compactness needs improvement.237 Meanwhile, safe, continuous, controllable, and quantitative biofluid sampling is also an important aspect of continuous biosensing. Passive micro-fluidics,238-240 porous/hydrogel absorption pads,128,180,241-244 microneedles,245,246 iontophoresis and reverse iontophoresis174,247,248 are common solutions, but none can simultaneously satisfy all requirements. Lastly, while wearable sweat sensors are the most often studied, other bodily fluids such as saliva, tears,249 and wound exudate should also be explored,224 as they may provide biomedical insights inaccessible via other means.
Equally important to technological advancement, robust knowledge of the clinical and biomedical relevance and correlation of various bodily chemicals is needed to guide the design and engineering of practically relevant biosensors. This knowledge often involves metabolites in biofluids not traditionally studied.51 In each case, the contents of the fluids will need to be compared to current gold standards (typically blood) to determine whether the fluid is representative of physiological state and what conversion factors are appropriate to analyze the data obtained. Then, the advantages of more frequent and, in some cases, continuous monitoring can be realized.
Holistic Approach to Accuracy Assurance.
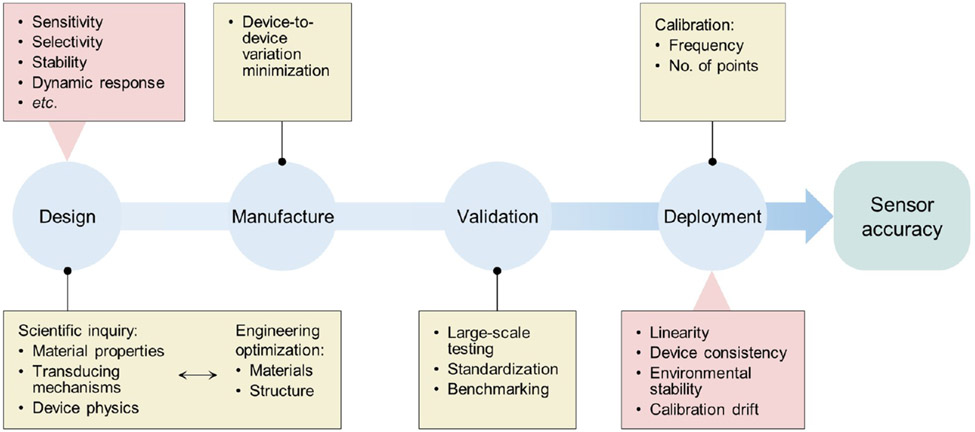
Reporting accurate values of the parameters of interest is essential to sensors. To ensure sensor accuracy, it is important to take a holistic approach spanning the entire life cycle from the development to the deployment of a sensor technology (Scheme 1). First, during the design stage, fundamental materials research is required to understand the materials properties, transduction mechanisms, and device physics. This knowledge leads to optimized materials and device structures. Going back and forth between scientific inquiry and engineering optimization would lead to improved sensor accuracy, while the 3S’s and other factors should be considered. Moving from design to deployment, well-controlled fabrication to produce consistent devices is critical. Moreover, large-scale validation with standardized procedures and benchmarking against gold-standard measurements are necessary to obtain reliable and trustworthy calibration curves. For biomedical sensors, validation experiments can be designed in accordance with the guidelines of the Clinical and Laboratory Standards Institute.
Scheme 1. A Holistic Approach to Sensor Accuracy Assurance during the Design, Manufacture, Validation, and Deployment of a Sensor Technologya.
aFactors to consider are highlighted in red boxes.
In real-world deployment, calibration can be a complex issue. There are two levels of consideration: the frequency of calibration during the entire sensor lifetime (manufacture, shipment, and usage) and the number of calibration points for each calibration. Calibration frequency usually concerns whether calibration can be performed by the manufacturer prior to shipment of the product. Most commercial sensors fall in this category. In this case, the cost of calibration is typically one third of the total cost of most commercial sensors today. However, the exact cost depends on the number of calibration points that are needed. If the sensor has a linear response in the needed dynamic range and if it has the same sensitivity in that range for all manufactured components but different offset values, then a single-point calibration is all that is needed. If the manufactured sensors do not have any offset in their base value with the same sensitivity, then no calibration is needed (which is rare but would significantly reduce costs). If a sensor has a linear response in the needed dynamic range but there is variability in the sensitivity from sensor to sensor, then two-point calibration is needed. If the sensor response is not linear, then multi-point calibration is needed (which is commercially unattractive). Furthermore, calibration against temperature, humidity and other environmental factors may be needed. For some emerging sensor technologies, the calibration can shift over time, for example, from the time of manufacturing to the time of usage. In that case, additional one-point or two-point calibrations may be needed prior to use, which complicate use. Therefore, it is critically important for the community to report linear response ranges, sensor-to-sensor variability, stability against environmental factors, calibration method, and calibration drift over time.
Reliable Correlations between Sensor Signals and Object Status.
Data without interpretation is of little use. Making sense of data collected by sensors is equally, if not more important with high-quality data acquisition. As flexible sensors enable many parameters to be acquired in unconventional situations or from previously inaccessible locations, the correlations between these parameters and the status of the monitored objects, environments, etc. should be carefully examined.250 Even for a single physical parameter, the underlying meaning can be complicated to unravel. For instance, facial strain was recently verified as an indicator of language commands through theoretical analysis and simulation,250 permitting the use of conformal strain sensors on face to deliver language commands silently.
The correlation issue is especially concerning for biomedical applications, such as biomarker measurement for disease diagnosis251 and physiological monitoring for health assessment.13 A recent report found close correlations between tear glucose levels and blood glucose levels with a lag time of 10 min,252 indicating promising noninvasive glucose monitoring by contact lenses. The study was conducted on three rabbits in the experiment group and the control group respectively, which may not be sufficient as biologically conclusive or generalizable to humans. Sweat is another biofluid in which glucose monitoring is extensively conducted.253 Nevertheless, the correlation between sweat glucose and blood glucose can be easily altered by sample collection methods as well as skin and environmental conditions.254 The large uncertainty renders wearable sweat glucose sensors255 only sufficient for range estimation but not currently qualified for guiding medical interventions. Large-scale tests with standardized protocols are needed to reach robust conclusions. In addition, equal gender representation in clinical trials is also curial for flexible sensor development and their practical usage in public. Recent results on a conformable multimodal sensory face mask performed on an equal number of male and female subjects indicate that current face masks are not suitable for women subjects in general.256 This result suggests a comprehensive mandate to be inclusive in human subject studies to have technologies be beneficial for all.
As flexible sensor technology is collecting signal types some of which are traditionally inaccessible, problems emerge in terms of the implications of sensor data. This issue calls for extensive fundamental and biomedical research, where investigations involving gold-standard tests on adequate sample sizes257,258 are needed to test the existence of correlations and to generate reliable reference databases.
Tolerance to Mechanical Deformation and Damage.
A major advantage of flexible sensors is the ability to withstand significant deformation without physical failure or performance degradation. This feature permits many use cases with which conventional rigid sensors struggle, such as conformal skin patches/tattoos and smart clothing. Nonetheless, this flexibility also poses great challenges in maintaining sensor integrity and performance under often-unpredictable mechanical interactions between the sensor and the environment.
Mechanical Robustness in Long-Term Use and at Large Deformation.
Mechanical robustness describes the sensor’s ability to withstand different forms of deformation without mechanical failure. Some extreme cases include exceptionally large strain and high impact,102 prolonged cycling strain, and constant friction. While conventional rigid sensors can be protected from mechanical damage using high-performance ceramics, metals, and thermosets, deformability of flexible sensors does not permit the use of these mechanical protective materials in conventional ways. Furthermore, due to the wide variety of materials used in flexible sensors, each having distinct mechanical properties (e.g., elastic modulus, Poisson’s ratio, viscoelasticity) and surface properties (e.g., surface energy, chemical composition), interfacial mismatch contributes a major factor to mechanical instability. The exact deformation a flexible sensor experiences varies greatly according to application, and hence exceptional mechanical robustness is not always required. Nevertheless, we highlight the most significant issues, and the principles should benefit the development of a number of flexible sensors.
Robust Soft-Hard Interfaces.
One of the most prominent mechanical challenges in flexible sensors is the interfacial instability between dissimilar materials. Stress and/or strain concentration occurs at soft-hard interfaces, leading to a major source of failure through delamination/detachment. Soft-hard interfaces exist in many forms: nanocomposites, layer-by-layer laminates, interconnects, etc. The general principles in tackling soft-hard instability are (1) improving interfacial adhesion and (2) avoiding abrupt softness/hardness difference. Specific methods vary in different scenarios, but the principles hold.
For example, using a single materials system (or at least reducing the number of materials) can eliminate many interfacial issues.118 Recently, a capacitive pressure sensor made entirely of CNT-doped polydimethylsiloxane (PDMS) was fabricated.259 Tuning the dopant concentration around the percolation threshold realized either electrode or dielectric properties with little change in mechanical softness. The interlayers were bonded together due to the similar chemistry of the layers. The resulting sensor could maintain stable performance under 100,000 cycles of rubbing and other harsh deformation conditions. Substrate mechanical engineering is effective in mitigating stress concentration in heterogeneous stretchable electronics.260-264 By synthesizing elastomers of different stiffness or embedding rigid islands, the area under rigid components is made harder than the surrounding area. Consequently, abrupt soft-hard transition between rigid functional components and soft substrates is avoided. Rational geometric engineering of the rigid islands can substantially suppress crack propagation at their interfaces with elastomers, extending failure strain and fatigue life.265 Recently, a mesh polyimide network was used in place of elastomers as substrate and superstrate for hybrid integrated systems.266 The mesh networks have reduced contact area with and more similar Young’s modulus to rigid interconnects and chips, largely alleviating the soft-hard interfacial problem. Other methods include using materials with gradients of stiffness,267 adding an interface material with medium stiffness between the soft sensor and the rigid interconnect,268 and developing ultrathin tough adhesive films for interlayer stabilization. In nanocomposites, surface chemistry engineering269 and interfacial microstructuring270,271 are common strategies to improve the bonding stability between nanomaterials and polymer matrices.272
A particularly challenging issue within the scope of soft-hard interfaces is interconnection, which is especially a concern for system integration.273 In particular, wearable and implantable sensors can be made ultrathin, soft, and stretchable, but poor mechanical strength challenges reliable connection with the rest of the system.274,275 Moreover, sensor arrays (e.g., microelectrode arrays for brain mapping276) face additional challenges in connecting high-density thin wires across long distances of soft-hard transition.
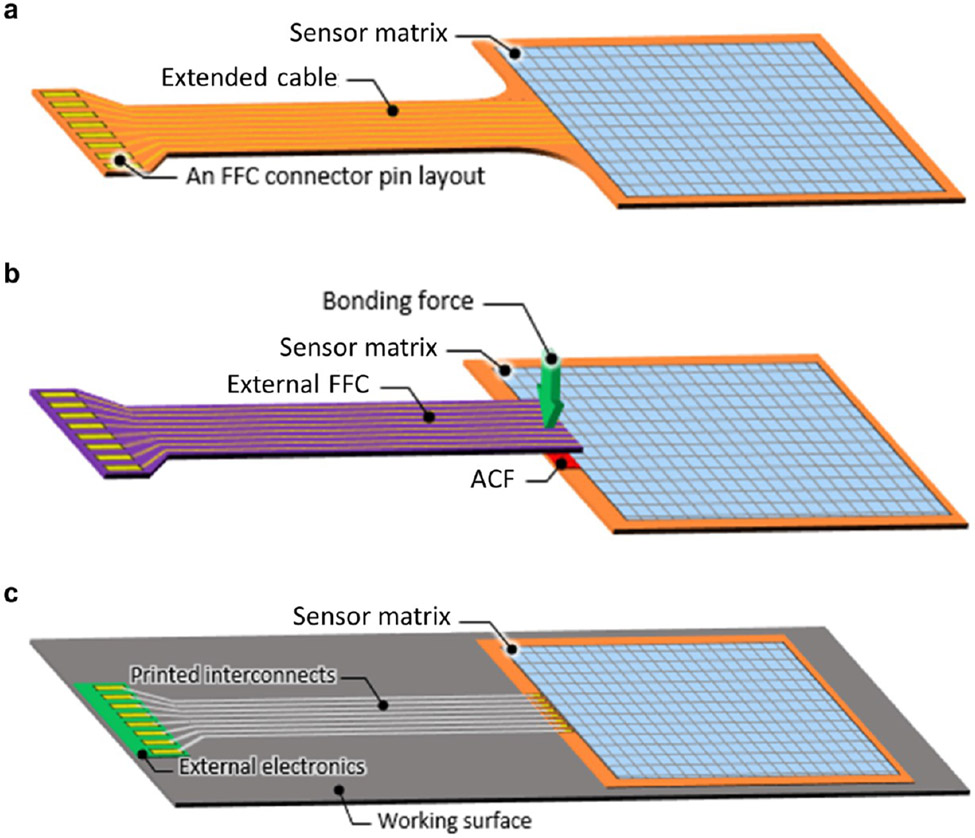
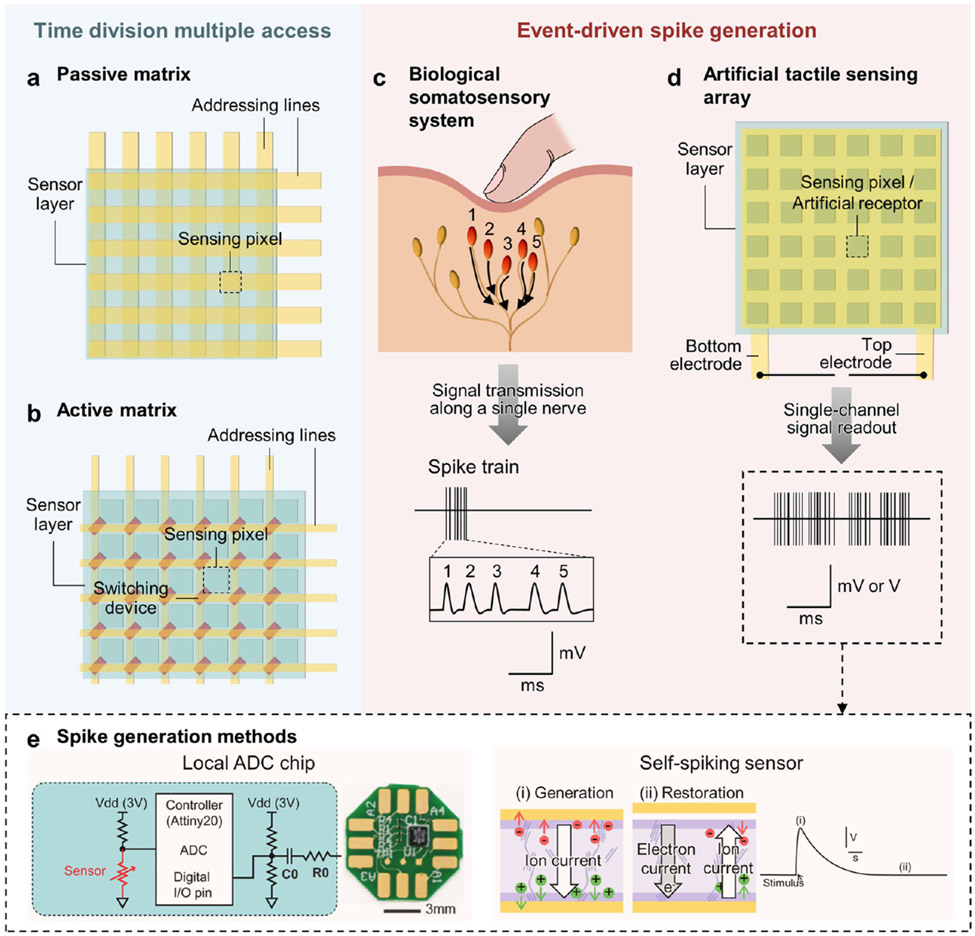
To address this problem, there are several options currently available, without using rigid wires and solders: (1) to integrate a flexible electric cable and pins into the sensor system so that it fits into a standard flat flexible cable (FFC) connector (Figure 6a);276-278 (2) to apply an anisotropic conductive film (ACF) and bond an external FFC to the sensor system (Figure 6b);279-282 and (3) to print interconnects over the surface of the sensor system down to the electric pads of the external electronics (Figure 6c).273,280 The first approach is more monolithic and can be accomplished in fewer process steps, while requiring substantially larger areas for a single device. The second approach is superior in that cables and sensor systems can be fabricated separately, thereby offering high resolution and performance, but extra steps for cable bonding are required before use. The third approach is preferred in applications that require customizability in interconnection layout or do not critically demand high resolution. Besides these options, fully soft and stretchable interconnects with robust adhesion are under investigation,283-287 which are expected to reduce interfacial mismatch with soft components significantly. Electrically conductive adhesives are commercially available as printable inks; modifying these materials to a lower rigidity will be instrumental to the large-scale manufacturing of mechanically robust systems.288 Liquid metal and conductive polymers are likely to play notable roles in these pursuits.
Figure 6.
Current interconnection approaches for flexible sensors and arrays. (a) Extended flexible sensor system layout contains flexible cable and pins suitable for a flat flexible cable (FFC) connector. (b) External FFC is bonded to the flexible sensor system via an anisotropic conductive film (ACF). (c) Printed conductors connect flexible sensor system with external electronics directly.
In parallel to engineering more robust interconnects, it is sometimes desirable to construct highly integrated systems without long distance interconnections, to minimize risk in failure and measurement instability. Such systems are like soft printed circuit boards (PCBs), where prepatterned interconnects on soft substrates are bonded to sensors and other functional components.289 Furthermore, future flexible sensing systems will be multilayer assemblies for high-density integration and sophisticated functions. Vertical interconnect access (VIA) will then be essential. There have been efforts on VIA, but rigid materials remain the most common.266,290,291 Future work may explore the principles introduced above for soft single-layer interconnections.285 Another important factor to consider is the reusability of interconnects, which allows for replacement of flexible sensors. This strategy will be useful for disposable sensors, where rigid electronics (often integrated on a flexible PCB) are designed to be reusable. Reliable and reversible bonding is needed in this case.292
When physically integrated systems cannot deliver desirable mechanical stability, wirelessly connecting soft and hard subsystems can circumvent the issues associated with interconnection and sometimes even completely eliminate soft-hard interfaces.293,294 In such cases, wireless communication will be challenged in bandwidth for large-scale sensing arrays and in reliability for sensors in deformation or motion.295 With the fastdeveloping soft devices,296-300 more components within a sensor system will be soft and flexible, and the soft-hard interface will be less and less an issue. The ultimate vision will be a fully soft system where soft-hard interfaces are mostly absent.
Large Elastic Range.
Flexible sensors possessing reversible deformability should ideally have all the materials in the sensor deformed within their elastic limits. In some circumstances, large deformations are expected, such as devices integrated directly onto the skin (up to 60–70%33) or other organs (e.g., heart, bladder). However, conventional electronic materials usually undergo brittle fracture or plasticity under a strain of ~1%.30 Hence, extending the elastic range on a system level beyond the intrinsic elasticity of constituting materials is often needed. Additionally, for practical applications, overengineering to strain values well exceeding the largest possible deformation that a sensor may experience is necessary for guaranteed mechanical safety.
There are well-established approaches to extend the elastic ranges of sensors made of brittle materials. Reducing thickness is the first approach and has been widely used to enhance the bendability of flexible electronics. Essentially, anything thinner can be flexed more.301 2D materials having atomic thickness are therefore intrinsically bendable, making them a popular choice for flexible sensors,302,303 let alone their outstanding electrical, optical, and chemical properties. The second approach is to place brittle components on the neutral axis of a multilayer stack. This strategy often takes the form of polymeric encapsulations around a brittle layer, but the situation can become complex with increasing numbers of layers and large elastic mismatches between layers.304-306 There is rich mechanics to explore for mechanically heterogeneous laminations. Building on bendability, stretchability is endowed by both structural307 and materials308 engineering. Wavy structures (both in-plane serpentine shapes309 and out-of-plane buckling310,311), fractal patterns,312 microcracks,313 honeycomb architectures,314,315 fibrous mats,155 and woven textiles32 are effective structural designs to confer minimally stretchable materials with large elastic stretchability. The mechanism is to convert macroscale stretching to localized bending and twisting, which are permitted by reduced thickness. Origami and kirigami employ similar concepts.316 Moreover, for relatively rigid substrates such as leather and polyimide, adding a strain-isolation layer with substantially lower modulus (e.g., PDMS) between inorganic active components and the substrate can effectively extend the system stretchability.317,318 Structural design utilizes conventional electronic materials and processing techniques thereby offering greater electronic performance and industrial compatibility, but it introduces soft-hard interfacial instability and often sacrifices integration density to make room for sophisticated patterns.
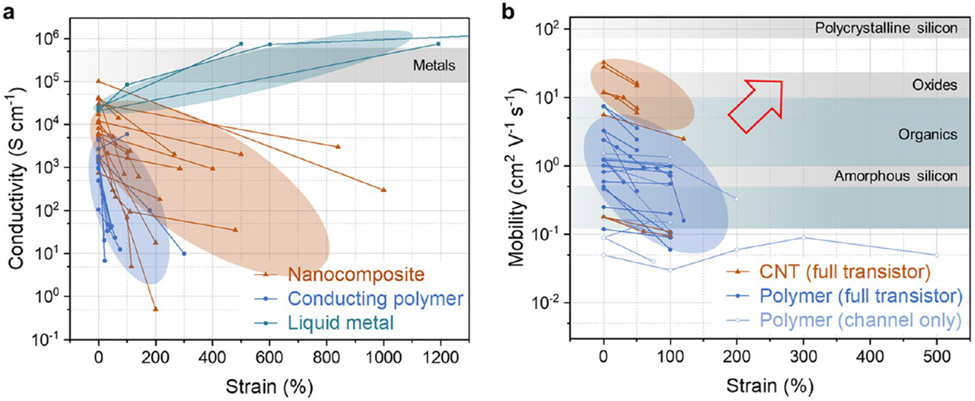
Materials engineering towards intrinsically stretchable materials provides alternatives to these problems.308,319-321 These strategies include fabricating nanocomposites with stretchable matrices, depositing liquid metal on elastic substrates or enclosing it within 2D or 3D elastic matrices, synthesizing functional stretchable polymers, and utilizing ionically conductive materials. Nanocomposites are a promising form of stretchable conductors, with the conductivities of the best performing composites approaching bulk metals (Figure 7a). Given the many available conductive fillers and matrix materials, composite materials properties can have wide ranges.322 Current technical challenges are primarily low-cost and large-scale manufacturing and high-resolution patterning. On the other hand, functional stretchable polymer syntheses have advanced rapidly. Polymeric semiconductors have achieved carrier mobilities at 100% strain exceeding non-stretchable amorphous silicon,323,324 although there remains room for improvement in mobility and stretchability (Figure 7b). In comparison, semiconducting CNTs325 can achieve higher mobility, but strain usually causes permanent morphological changes in CNT networks, and strain-insensitive electrical performance is thus challenging to achieve.326 In addition, energy dissipating (tough) interlayers with covalent bonding to interfacing materials could substantially increase the crack onset strain and thus delay the performance drop of conducting/semi-conducting thin films on elastic substrates upon stretching.327 Moving forward, to realize different types of sensing functions, stretchable material designs need to be created for other types of functional properties, such as electroluminescent and photo-responsive properties for optical sensing and electrochemical properties for biochemical sensing.127,328
Figure 7.
Performance of intrinsically stretchable (a) conductors and (b) semiconductors. (a) Intrinsically stretchable conductors are realized in three materials categories: nanocomposites, conducting polymers, and liquid metals; ionic conductors are not included. Conductivities of stretched liquid metals are calculated from resistance changes under unidirectional strain, based on the assumption of incompressible solid undergoing uniform deformation. Non-stretchable bulk metals are plotted in the gray band for comparison. Values extracted from refs 269, 285, 329-345. (b) Stretchable semiconductors are based on semiconducting CNTs or conjugated polymers. Mobility measurement was done in either stretched materials transferred to non-stretchable substrates (channel only) or fully stretchable transistors and transistor arrays (full transistor). Values for stretch directions both parallel and perpendicular to the channel length are included. Typical mobilities of common non-stretchable semiconductors are plotted in bands for comparison.346 Red arrow indicates area for improvement. Values extracted from refs 323, 324, 347-364.
The above mentioned materials target electronic sensors, where the mechanical brittleness of conventional electronic materials poses great challenges to sensor deformability. However, in biology, tissues are highly deformable yet have sophisticated sensing capabilities. Biological sensors work by the transport of ions, instead of electrons. Inspired in this direction, a strong impetus to develop ionotronics utilizing ionically conductive and highly deformable materials such as hydrogels,296 ionogels,172,365 and ionic elastomers366,367 has emerged.368 Without the requirement of incorporating electronic conduction, materials design is much simpler. To date, temperature sensors,366,369 strain sensors,365 pressure sensors,366,370 electrophysiological electrodes,371,372 and others have been realized. Major challenges in ionic sensors include adequate sensing modality and performance on par with those of electronic sensors, compatibility with electronic data processing modules, and device miniaturization.
We note that pursuing record high deformability may not always have practical significance. The purpose of high deformability should be articulated with respect to the intended application scenarios. Even for on-skin application alone, the range of tensile strain spans from 1% to 63% according to body location.33 Therefore, the true requirement for practical applications should be determined case by case. For a sensor designed for skin integration, a stretchability of 1000% might be practically unnecessary.
Fatigue Resistance at Materials Interfaces.
Improving fatigue resistance is important to sensor durability under cyclic loading and is particularly critical for mechanical sensors. The primary concern regarding fatigue resistance lies in interfacial delamination rather than the intrinsic fatigue properties of materials. This issue is closely related to soft-hard interfacial instability.265 Hence, significant efforts should focus on optimizing sensor structures with coordinated materials properties to reduce stress concentration. Due to the multitude of materials and multilayer structures employed in flexible sensors, the mechanical interactions between layers and components can be complex. Therefore, fundamental studies on the failure mechanisms and failure criteria are important. For example, Cheng et al. developed an anti-fatigue strategy to prolong the fatigue life of 3D ribbon-shaped flexible electronics by switching metal-dominated failure to desired polymer-dominated failure.373 Crack propagation in microcracked structures is another issue. Microcracks are introduced to render stretchability313 or to improve sensitivity,210 but they are usually unstable under cyclic loading. Crack engineering thus becomes important. Examples include substrate structural design374 and initial crack length control.375 On the other hand, improving the fatigue resistance of emerging soft materials such as hydrogels213,376 may broaden their application space in flexible sensors.
Signal Stability under Mechanical Interference.
Flexible sensors other than mechanical sensors should not respond to mechanical deformation, which can be regarded as a source of interference. Mechanical stability is an aspect of sensor stability. Because it is special for flexible sensors and represents a major challenge in sensing performance, we dedicate a detailed separate discussion to this issue.
Strain Effect Decoupling.
Coupling of strain into the sensing function undesirably causes signals to shift under bending or stretching. Decoupling of deformation effect is therefore important to sensing accuracy. Most of the approaches discussed above to extend elastic range contribute to alleviating this problem, yet perfectly strain-invariant performance remains challenging to attain. The key is to minimize strain experienced by active components or to synthesize strain-insensitive active materials. In particular, the island-bridge layout is effective for array-type devices.109,377 Active components with critical sensing functionality are connected by highly stretchable interconnects, and the majority of strain is borne by the interconnects, thereby minimizing alterations to the sensor output. To enhance the protection to active components, the island area is made more rigid than the surroundings by either inserting a hard platform262 or tuning the chemistry of substrates.378 Soft-hard interfacial instability is an important consideration in such systems. Another strategy is to use built-in circuits to compensate for strain-induced variation.81,379 Since these strategies are poised to increase system design complexity, the realization of strain-insensitive sensor arrays with high density will rely on innovations in integration strategies and high-resolution high-yield fabrication. A recently proposed strategy380 with potential to overcome these limitations is to bridge brittle functional thin films and stretchable conductors through ACFs, which, despite the cracking of functional thin films under tensile strain, offer alternative electronic conduction pathways that are unaffected by strain. The laminates demonstrated nearly strain-insensitive electrochemical sensing and stimulation using a library of brittle functional materials.
Motion Artifact Removal.
Motion artifacts are noise in sensor output from motion and surface deformation of the monitored object. They overshadow true signals and cause measurement inaccuracy or reduced signal-to-noise ratio. Motion artifacts are a common problem for wearable and implantable sensors, and remain one of the biggest challenges for electrophysiology and its application in wearable healthcare and human-machine interfaces. The problem comes from sensor system mechanical instability, sensor-wearer interface instability, the complexity of human body movements, electrical signals generated by muscle movement, and disturbance of ionic charge distribution and dynamics inside tissues by deformation and perspiration. The first aspect has been addressed in multiple topics including soft-hard interfaces, elastic range, and strain effect decoupling. Here, we focus on strategies addressing the rest of the problems from sensor hardware optimization and signal processing.
For hardware optimization, the mainstream approach is to improve the conformability and adhesion of sensors on tissues (often skin).381 Better conformability eliminates gaps between the sensor and the skin, and better adhesion promotes conformal contact382 and stabilizes the interface during motion. These two factors together facilitate intimate and unaltered contact between the sensor and the skin during motion, thus reducing motion artifacts. To improve conformability, making sensors soft and stretchable is effective. This goal can be achieved through reducing device thickness for lower bending stiffness383-386 and using polymeric materials with intrinsic low modulus and high stretchability.387,388 For enhancing adhesion with skin,387,389 van der Waals interactions are sufficient for ultrathin (<5 μm) devices,382,390 while adhesive polymers or functional groups are necessary for thicker devices.387,388 Electronic tattoo stickers390-393 and in situ sensor fabrication directly on skin from liquid precursors394,395 offer convenient ways to achieve conformability and adhesiveness simultaneously.
Pretreatment of skin, e.g., hair trimming, alcohol wiping, helps to reduce surface roughness and to remove contamination for better conformability and adhesion, but compromises the convenience of point-of-care sensors. The recently proposed concept of using a damping hydrogel to eliminate low-frequency mechanical noises (such as walking and breathing) for measurement of high-frequency signals (such as speech and electrophysiological signals)396 offers another materials engineering perspective to tackle motion artifacts.
On the systems level, special sensor layouts and system designs can also mitigate motion artifacts. For example, preparing arrays of sensors can enable a system to measure some physiological parameters despite small relative motion of the sensor and the body.397 Employing multiple sensors with carefully designed application positions can help cancel motion artifacts in certain sensors.398,399 Integrating other sensing modalities or mechanisms such as force, heat, magnetism, light, sound, and chemical, can buffer the influence of motion artifacts.
On the other hand, motion artifacts can also be properly handled by signal processing. Some easily distinguishable motion artifacts can be filtered by near-sensor circuits. Flexible organic electronic components, such as differential amplifiers and adaptive filters, can be seamlessly integrated with wearable sensors to perform noise reduction.400 Noise can also be attenuated by algorithms. Beyond simple low-pass, high-pass, and band-pass filters, advanced signal processing methods such as wavelets and short-time Fourier transform have shown effectiveness in removing motion artifacts. More targeted motion artifact removal utilizes motion reference signals detected by micro inertial sensors, such as accelerometers or gyroscopes.401 In such cases, motion artifact reduction in irregular and muscle strength exercises is challenging because accelerometer-based signals do not directly reflect muscular activities.402 Recently, an “interface sensor” was proposed to capture the dynamic interactions between the skin and the sensor through proximity and pressure sensing. Using these types of reference signals, the estimated heart rate was determined more accurately than by conventional accelerometer-based methods.403 Customized signal-processing strategies are needed to address human motions at different frequency, magnitude, and body position, as well as associated muscle electrical activity, deformation, and skin condition changes (e.g., perspiration-caused impedance change).
Machine learning is promising for compensating for motion artifacts with greater customizability. For example, a deep learning framework accurately determined heart rate from photoplethysmography sensors without the use of reference motion sensors.404 A neural network was trained to denoise the pulse wave signals by filtering the motion artifacts caused by respiration.405 Such a data-based approach is more versatile and capable than optimizing hardware, first, because materials and device designs are likely to differ from application to application, and second, because amplifiers and filters can only remove noise over a limited specific spectrum. In contrast, machine learning algorithms can be iterated and updated to develop personalized motion artifact filtering protocols.
Overall, most work on minimizing motion artifacts targets electrophysiological sensors and optical heart rate monitors; future research should expand to explore other types of sensors. In particular, flexible optical sensors relying on light-matter interactions are sensitive to sensor-object relative orientation and motion, and most recently developed flexile optical sensors did not demonstrate reliable function in dynamic deformation or motion.406-409 This issue presents a daunting challenge for flexible optical sensors. Signal processing through artificial neural networks has shown promise in overcoming this challenge.410
Damage Insensitivity.
To maintain or to recover sensor function autonomously despite the presence of mechanical damage is the ultimate form of mechanical stability. To achieve this goal, mechanically tough, self-healing, and stiffening materials were developed, and damage-insensitive or protective structural designs were proposed.
Increasing fracture toughness helps prevent sudden failure in the presence of minor cracks. Common elastic materials used in stretchable sensors such as silicone rubbers easily break in the presence of notches due to low fracture toughness (e.g., 310 J m−2 for PDMS259). To overcome this challenge, supramolecular elastomers with high fracture toughness (e.g., 12,000 J m−2 and 30,000 J m−2)411,412 were synthesized and further integrated with conductive materials to enable notch-insensitive anti-tearing stretchable conductors. Even at 250% of tensile strain, a notched conductor remained conductive without failure.412 Meanwhile, great progress has been made over the past decade in improving the toughness of stretchable hydrogels (e.g., 9,000 J m−2 by Sun et al.413 and 14,000 J m−2 by Yang et al.414),415 making them more stretchable and tough materials than some commercial elastomers. Furthermore, efforts in toughening 2D materials are producing promising results. The ability of graphene to resist fracture was significantly improved through topological design (i.e., controlled distribution of topological defects including disclinations, dislocations, and grain boundaries)416 and integration with CNTs.417 Hexagonal boron nitride, a dielectric 2D material, was reported to exhibit extremely high toughness,418 which could provide mechanical protection in 2D devices. These advances offer potential opportunities for graphene and other 2D materials in flexible sensors.
Self-healing materials are designed to repair mechanical damage and to restore the sensing function, and the vision is to improve sensor durability and longevity, with an additional benefit of reduction in electronic waste.419 Despite significant progress in synthesizing functional self-healing materials, including conductors211,420 and semiconductors,348,421 and fabricating self-healing sensors, such as strain sensors,420,421 proximity sensors,422 and humidity sensors,423,424 self-healable sensors face many challenges for practical applications. Usually, the performance of self-healable materials and sensors is inferior to non-self-healable counterparts because self-healing materials are predominantly polymers with dynamic bonds and low glass transition temperatures. Furthermore, it is challenging to fabricate sensors using all self-healing materials and to ensure simultaneous self-healing of multiple components. Therefore, it might be wiser to incorporate only self-healing properties in the most mechanically fragile parts of the devices, such as surfaces superficial to the system. Lastly and critically, most devices that include self-healing materials simply demonstrate functionality. More effort should be directed towards understanding whether self-healing materials can bring value to durability and longevity at device and system levels.425
Soft materials that stiffen at increasing strain, strain rate, etc. might serve as a mechanical protection mechanism for flexible sensors. Some examples include mechanochemical systems (e.g., hydrogels) that become stiffer, stronger, and tougher after repetitive stretching,426 stretchable composites and ionic elastomers with the J-shaped stress-strain curves exhibited by the skin,367,427,428 and flexible, stiffness-changeable, and impact-protective polymers for potential impact- and puncture-resistant sensors.429
Besides engineering material properties, structural design provides another route to protection against damage. Discrete dispersion of rigid components in a soft polymer matrix has shown effectiveness in preventing damage by puncture or cut to the active components.430 Stretchable armors composed of hard scales connected by soft elastomers enhance resistance to puncture, scratch, and cut, when used as an additional protective layer for flexible devices431 or functionalized as standalone flexible sensors.432 Such artificial armors with nature-inspired designs433 may find wider application in wearable sensors and electronic skin. Additionally, design principles for mechanical metamaterials with extraordinary mechanical properties such as lightweight, high strength, large elastic deformation, high toughness, and supersonic impact resilience,434-439 can be leveraged to engineer flexible sensors with high mechanical robustness.
The need to achieve mechanical robustness and stable electronic performance can be addressed, for some types of applications, with the use of liquid metals (e.g., eutectic gallium indium),440 which will likely be important components in stretchable sensors.441 Liquid metals are softer than most sensor materials,442 can sustain the largest strains of stretchable conductors,285,345 suffer no fatigue issues,285,342,443 and automatically self-heal when damaged.342,345 There has been much progress in patterning liquid metals on flexible and stretchable substrates with sub-10-μm resolution285,342,444-450 and processing liquid metals with industrially compatible techniques.451 The key remaining challenges include: eliminating the risk of leakage,452 tackling surface chemical instability,453 and verifying biocompatibility for bio-interfacing applications.454-456 Proper encapsulation will be critical to the practical application of liquid metals.457
Large-Area and High-Density Sensor Array Integration.
Improving individual sensor performance sets the foundation for any large-scale integration. Flexible sensor arrays with large area and/or high spatial resolution find application in electronic skins, medical imaging, interactive displays, building-integrated electronics, among others; in these uses, flexible sensors have significant advantages over rigid counterparts. However, during array integration, challenges concerning pixel density and quality, readout efficiency, power management, and manufacturability arise; many of these issues are interrelated and call for holistic design and manufacturing strategies. Here, we discuss the most prominent challenges in signal readout and multimodal sensing.
Efficient Matrix Signal Readout.
Efficient and high-quality signal readout is challenging in flexible sensing arrays that have high-density sensor pixels at large scales. Key targets in devising readout strategies include minimal crosstalk between sensor pixels, high signal-to-noise ratios, acceptable wiring complexity, low power consumption, small latency, small data size for transmission, manageable heat dissipation, and balanced demand on computational power for data processing.
Passive matrix and active matrix are the dominant architectures for array signal readout. A passive-matrix design, which consists of row and column lines and the sensors at the intersections (Figure 8a), is relatively easy to implement and to manufacture.281,458 However, crosstalk between sensors via undesired current paths limits signal fidelity. Although highly complex readout circuits can solve this issue,459 they introduce other problems such as reduced reading speed and accuracy, which can easily happen when the numbers of pixels become large (e.g., 1000 × 1000 matrices). An active-matrix design tackles the issues of crosstalk and wiring complexity by placing electrical switches, such as transistors and diodes, at individual sensors (Figure 8b).43,460,461 Transistors can also form amplifier circuits for local amplification at each sensing pixel.360,462,463 Flexible active matrix is a mature technology in commercial displays.
Figure 8.
Conventional time division multiple access (blue panel) and emerging event-driven spike generation (red panel) as array data collection strategies. (a,b) Passive-matrix and active-matrix designs are the most commonly used array readout strategies. (c) Biological somatosensory system uses spike trains to encode tactile information with high spatiotemporal resolution and energy efficiency. (d) Artificial sensory systems can mimic the structure and function of the biological somatosensory system. Each sensing pixel generates potential spikes and is thus called an artificial receptor. (e) Two reported methods of generating spikes in individual artificial receptors include integrating an analog-to-digital converter (ADC) at each pixel and using sensing materials that can self-generate potential spikes. Circuit and photograph of one sensing pixel incorporating ADC adapted with permission from ref 464. Copyright 2019 American Association for the Advancement of Science. Schematics of self-spiking ion-electron conductor adapted with permission from ref 465. Copyright 2022 American Association for the Advancement of Science.
Active matrices face challenges in electric interconnects and circuit solutions. For interconnection, the high impedance (>1 kΩ) of thin-film interconnects may lead to significant over-heating and voltage drop when high electric currents (10–100 mA) pass through,466 causing circuit failure and sensitivity loss, respectively. It is therefore beneficial to have high impedance ratios between sensors and connection lines, and the impedance of switching transistors in the ON state should be considered.467 Furthermore, the high impedance of long interconnects and numerous overlaps between them lead to large time constants, crosstalk, common-mode noise and pickup of external electromagnetic interference during operation. In this regard, differential signal readout approach might be a universal solution.468 For circuit solutions to drive pixels in an active matrix, the incompatible technologies for organic semiconductors (mostly p-type) and inorganic semiconductors (mostly n-type) result in difficult fabrication of complementary circuits (based on both n-type and p-type semiconductors).469 Moreover, the possible variation of parameters between transistors in the matrix using immature materials or processes may lead to offsets, dead pixels, and variations in sensitivity. Bootstrap-type circuits will be strong candidates for overcoming these challenges.470,471
Transistors play pivotal roles in matrix signal readout, conditioning, and processing—their performance critically determines the signal-to-noise ratio and power consumption of the sensor matrix.44 In this regard, current research aims at achieving comparable-to-rigid performance in stretchable transistors and arrays.326,378,472 Improvements in operating frequency and voltage, strain-independent electrical characteristics, and power consumption are needed for effective matrix addressing. Meanwhile, unconventional operational principles may bring about substantial improvements in transistor performance. For example, subthreshold Schottky-barrier thin-film transistors (SB-TFTs) offer an ultralow-power and high-gain solution (Figure 9a-d).473 Organic SB-TFTs can also be inkjet-printed while outperforming their inorganic counterparts (Figure 9e-g).198 The electrical characteristics of SB-TFTs are geometry-independent, thereby accommodating the large dimensional variations in inkjet-printed devices. This feature is especially desirable in printed arrays. Amplifiers made of such SB-TFTs have ultralow power consumption (~600 pW peak power) with high gain (260 V V−1 peak gain), thereby achieving higher resolution (down to 3.8 μV) than other TFT technologies in electrooculographical recording.198 While stretchable form factors of organic SB-TFTs are feasible via a helix structure,474 future research in exploring large-area implementations of SB-TFT arrays may substantially improve the power efficiency and signal quality in flexible sensing arrays.
Figure 9.
Subthreshold Schottky-barrier thin-film transistors (SB-TFTs). (a) Schematic of a metal oxide-based inorganic SB-TFT. IGZO, indium-gallium-zinc-oxide; LC, less compensated; MC, more compensated. (b,c) Measured input characteristics in linear scale (b), indicating VT (the threshold voltage), and in logarithmic scale (c), indicating Vref (the reference voltage), respectively. IG, gate leakage current. (d) Conceptual color bar of output power consumption (Pout) for a 1 V supply, normalized with W (channel width), clearly indicating each operational regime. Frames a–d adapted with permission from ref 473. Copyright 2016 American Association for the Advancement of Science. (e) Schematic of an organic SB-TFT. CYTOP, a commercial fluoropolymer; C8-BTBT, 2,7-dioctyl[1] benzothieno[3,2-b][1]benzothiophene; PS, polystyrene; PVC, polyvinyl cinnamate; PEN, polyethylene naphthalate. (f,g) Comparison of intrinsic gain (f) and transconductance efficiency (g) of different transistors. Organic SB-TFTs have the best performance. MOSFET, metal-oxide-semiconductor field-effect transistor. Frames e–g adapted with permission from ref 198. Copyright 2019 American Association for the Advancement of Science.
Conventional matrix solutions usually use time division multiple access for data collection, in which the changes of resistance or capacitance in all the pixels are sequentially scanned while continuously applying a bias (Figure 8, blue panel).475 This approach suffers from limitations in energy efficiency, reading speed, and latency for high-fidelity large-scale array sensing. A hint for efficient signal readout with high spatiotemporal resolution might be obtained from the biological somatosensory system (Figure 8c). The sensory receptors generate potential spikes when an external stimulus is given.476 As many as ~40 receptors are connected to one capillary nerve fiber, and the spike signals sequentially generated from the receptors form a bundle, called a spike train. Recognition is achieved by analyzing the patterns of the spike trains. Artificial sensor arrays mimicking such biological somatosensory systems (Figure 8d) can have multiple pixels sharing the same readout electrode/wire, thereby significantly reducing wiring complexity and readout latency. Moreover, pixel crosstalk is intrinsically not an issue, though more computational power is required for spike train analyses.464 Lastly, using potential signal greatly reduces power consumption because the sensor pixels can be driven at low current.
Several recent reports employed this strategy and offered substantially improved sensor array readout performance. For example, Lee et al. proposed an asynchronously coded electronic skin (ACES) to address the large amount of pressure and temperature sensor data in electronic skins.464 Analog signals from each sensor were converted to potential spikes by a microcontroller incorporating an ADC in each pixel (Figure 8e, left). Exceptional temporal precision of <60 ns was realized in a 240-pixel sensor array. However, distributing tiny rigid chips on flexible/stretchable substrates is a potential challenge for larger-area ACES. It would also limit device density (1 cm pixel pitch as reported464) and flexibility compared to active/passive matrices (<1 mm pixel pitch and full flexibility459). Kim et al. tackled this challenge by using a mixed ion-electron conductor with tunable ion relaxation time as the pressure-sensing material (Figure 8e, right).465 An array of this material with differential properties encoded contact information (contact position and area) in output spike train signatures without in-pixel conversion by circuits, thereby improving integration density (529 pixels in 2 cm × 2 cm), system flexibility (fully flexible without rigid components), and power efficiency (no additional energy required to drive microcontrollers). Spatiotemporal resolution comparable to human skin was achieved (12–132 cm−2 and ≤250 Hz). Nonetheless, due to the simplistic system design, data complexity is inferior to the prior method—no quantitative contact pressure was directly attainable. Other self-spiking sensors, such as piezoelectric and triboelectric477 tactile sensors and pyroelectric temperature sensors, are worthy of exploration and study.
Certain sensing mechanisms can also enable more efficient array signal readout. For instance, a potentiometric mechanotransduction mechanism for pressure sensing has been developed.105 Unlike traditional resistive or capacitive sensors, this mechanism transduces pressure input into potential differences between two electrodes, allowing the configuration of a single-electrode-mode array with a common reference electrode, substantially reducing the number of wires and improving pixel density. Furthermore, crosstalk between pixels is minimal due to the negligible current flow, allowing simultaneous data acquisition from all pixels. Lastly, potentiometric sensing requires significantly less power (<1 nW) than conventional electronic skins. Tactile sensing arrays based on the triboelectric mechanism also have similar desirable features, as demonstrated in a 4 × 4 array.477
Optical readout, as an alternative to electrical readout, utilizes sensing materials or structures that report stimuli through luminescence,478 color changes,479,480 marker displacements,481 or related means. It may offer better options for areal mapping because the optical output from the entire area can be accessed simultaneously without wires and circuits using well-established high-resolution cameras. However, image capture in real-world scenarios can lead to significant sensitivity loss and signal error due to suboptimal and changing lighting conditions, representing a major challenge for optical sensors. To tackle this challenge, a near-distance imaging scheme for mechanoluminescence-based pressure sensing was developed.478 The proximity of the pressure-sensitive film and the underlying image sensor significantly improved sensitivity, giving rise to a small detection threshold at the kPa level (vs. typically being on the order of MPa in previous work). The use of micro- and nanoparticles also enabled high spatial resolution with a pixel size of ~100 μm. However, the bulky and rigid complementary metal-oxide-semiconductor (CMOS) image sensor limits the wearability of the system. Nevertheless, portable optical imagers with compact design are practical to implement481,482 and have been commercialized for tactile sensing.483 In addition, magnetic sensing is an alternative wireless technique, which has been successfully applied for position and motion sensing,484,485 as well as large-area tactile sensing.486
Compared with the readout circuits of temperature, pressure, and touch sensor arrays, data acquisition systems for ultrasound487 and photoacoustic imagers are more complicated. Not only are the complexity of interconnects, but also the multichannel high-frequency sampling (usually >20 MHz for each channel) and ultrawide communication bandwidths (usually >1 Gbps for a system) are challenges. To address wiring complexity, row-column array is a simple and practical technique, although the crosstalk may be relatively high.488,489 Semiconductor technology can integrate many channels of high-frequency ADCs into a small chip, or even design a local ADC bonded onto the sensor to address the multi-channel sampling problem.490 Meanwhile, data pre-processing techniques such as transformation domain, sparse, and neural-network encoders that compress and package data at the front end in real time can help break the limitations of speed and latency in data transmission and post-processing.491,492 High circuit sensitivity usually demands high power budgets.397 Two recently reported techniques, resonant noise matching493 and coherent detection,494 were shown to increase sensitivity with modest power budgets.
While most current sensor matrices are developed for physical sensing, chemical sensing with high spatiotemporal resolution will offer vast opportunities in neuroscience and many other biomedical fields.55,120,189,495-497 To this end, ensuring consistent and stable performance of each sensing pixel and integrating biosensing materials into microfabricated devices are great challenges.498
Multimodal Sensing in Compact Systems.
In many scenarios, a large variety of sensors need to be implemented to obtain complex and comprehensive environmental and physiological information. The use of more than one type of sensors can also improve measurement accuracy and reduce the number of sensors required to generate insightful feedback.499 Multimodal sensing500 should therefore be an important feature of flexible sensors. There are generally two ways to achieve multimodal sensing: integration of multiple sensors into a single device or detection of multiple stimuli with a single sensor.501
The first strategy directly integrates various sensors by means of a matrix network or stack architecture.109,290,502-504 Sensors in these systems are usually well established and have reliable performance. However, these systems require complex structure designs, fabrication, and signal readout and conditioning, hindering high-density array integration. Tackling these issues, simplistic array structure and sensor miniaturization through microfabrication of rigid materials have produced multimodal sensing arrays with reasonably high device density (ca. 1 mm sensor pitch).109,505 Furthermore, the use of the same sensing materials118,506 and the same type of output signals (and sensing mechanisms)80,507,508 simplify manufacture and signal conditioning, respectively.
The second strategy is to realize simultaneous detection of multiple stimuli by a single sensor so as to allow high integration. This strategy will be helpful for applications with physical constraints in size, weight, and distribution. Such multimodal sensors should be able to decouple multiple stimuli without crosstalk. The most common method is to exploit differences in sensor responses to multiple stimuli for identification; some mathematics,509 and/or machine learning algorithms510,511 for data analysis are often needed. This approach might utilize the intrinsic multi-responsiveness of a single material,82,510,511 a combination of different sensing materials in different parts of functional devices,509 or arrangements of multiple sub-sensing units in 3D structures.117 Another method is to use multiple measurement modes to decouple the signals,111,512-515 which might suffer from complexity in signal readout and latency. The number of measurement modes (equations) should be equal to or greater than the number of stimuli (unknown variables). Performance of each sensing modality may not be optimal and difficult to improve simultaneously in such highly integrated sensors. Moreover, when a sensor becomes too multimodal, i.e., simultaneously responsive to many stimuli, it can become difficult to differentiate the stimuli. In this case, integrating multiple sensors of slightly different responsiveness and analyzing the signals holistically may be a solution (much like selective sensing arrays).155
With regard to multimodal sensing, special concerns go to flexible image sensors and gel-based physical sensors. At present, most flexible image sensors cannot recognize multiple colors at the same time. Although color image sensing can be achieved with the help of bandpass filters, its process complexity and cost have yet to be resolved. Array integration of filter-free narrowband flexible photodetectors is critical to the preparation of color image sensors. An intrinsically stretchable phototransistor array was developed with the capability of red-green-blue (RGB) color image recognition using quantum dot-based nanocomposites for color sensitivity and an artificial neural network for compensation of mechanical deformation-caused errors.410 Although the pixel resolution was rather low (~1 cm pitch), this phototransistor array represents a big advance in flexible color image sensors. For gel-based strain/pressure/temperature sensors, there are many reports of multifunctional sensing under ideal experimental conditions where the stimuli not of interest are kept constant.110,516,517 Future research should demonstrate decoupling these stimuli. Otherwise, the sensors can only be used in highly constrained situations, such as when there is only one stimulus without environmental interference or no quantitative information is required.517
SENSOR-BIOLOGY INTERFACE
A prominent advantage of flexible sensors is the ability to attach conformally on non-flat surfaces and to withstand dynamic deformations during use. This feature makes flexible sensors well suited for measurements on biological objects, including humans, animals,518 plants, and even tissues and cells. Some future applications include wearable/implantable sensors for health monitoring and disease management,49,50,54,519 neuroscience and biomedical studies,520 human-machine interfaces, on-plant sensors for precision agriculture,521-523 and many others. There have been tremendous efforts and progress in developing bio-interfacing flexible sensors, but most remain far from translation to practical applications. Bio-interfacing sensors should, on the one hand, acquire high-quality bio-signals across the biotic-abiotic interface and, on the other hand, not interfere with the normal function of biological organisms. These properties largely rely on compatible interfaces between biology and electronics, which, due to their distinct physiochemical properties, raise additional challenges (Tables 2 and 3 and Figure 10) on top of the sensing performance issues discussed previously. Although the issues presented here are categorized as materials orientated and form-factor orientated, many of them are interrelated (solid lines and arrows in Figure 10) and can be solved through the synergy of materials and form-factor approaches. For example, low bending stiffness, believed to render better biocompatibility to the sensing device, can be achieved through reducing the thickness of rigid electronic materials, varying film morphology and topology, synthesizing functional polymeric materials, or combinations thereof.
Table 2.
Materials Considerations in Achieving Compatible Sensor-Biology Interfacesa
| Issues | Importance | Challenges | Solutions and their limitations | Ref |
|---|---|---|---|---|
| Materials issues | 49,50,374,519,520,526,527,535-539 | |||
| Tissue-like mechanical properties (e.g. Young’s modulus, bending stiffness, stretchability) | Biocompatibility | Intrinsic limitation of electronic materials | Size reduction to the subcellular level: difficult handling and connection, simple functionality | 540-543 |
| Sensor integrity and stability | Microscopic perception by cells | Functional hydrogels: limited performance and functionality, difficult miniaturization | 370,372,544-548 | |
| Conformability | Insufficient mechanical match of plastics and elastomers | Soft network materials: complex fabrication, challenging design and fabrication of 3D tissue-like materials | 549 | |
| Wearing comfort | Unusual mechanical properties of tissues (e.g., viscoelasticity, J-shape stress-strain curves) | Hierarchical structures: limited fabrication methods and materials | 550 | |
| Stiffness-varying polymers: unexplored device integration, unverified biocompatibility | 551,552 | |||
| Tissue adhesion | Conformability | Robustness in dynamic deformation and harsh environmental conditions (e.g., UV light, heat) | Mechanical contact by pressure: discomfort, risk of sensor sliding and friction, associated signal inaccuracy and tissue irritation | 553 |
| Motion artifacts | Robustness on contaminated surfaces (e.g., water, perspiration, skin secretion, cosmetics) | Medical tape: weakened by sweat and water, painful peel-off, no stretchability | 554 | |
| Device fixation | On-demand removal without residual or pain | Physical adhesion: only applicable to ultrathin devices | 390,555 | |
| Wearing comfort | Biocompatibility | Bioinspired adhesive architectures: complex manufacture, added volume | 242,556-558 | |
| Tissue-like mechanical properties | Adhesive hydrogels: large thickness, humidity sensitivity, poor permeability | 387-389,546,559-563 | ||
| Dry adhesive polymers: limited mechanical compliance, unverified biocompatibility | 564-567 | |||
| Additional adhesion control layer: one-time use, added volume, weight and complexity | 568 | |||
| Biocompatibility | Health and safety | Intrinsic limitation of electronic materials | Approved materials and biological materials: limited materials choices, limited performance and functionality | |
| Sensor stability | Lack of knowledge of emerging materials | Surface treatment (nanostructures, hydrogel coatings, etc.): complex fabrication | 524,569-571 | |
| Nonhostile immune responses | Biohybrid implants: challenging design and fabrication | 528 | ||
| Long-term effects | Tissue-like mechanical properties: limitations therein | |||
| Large-scale, systematic tests: lengthy process, application dependence, variance in materials | ||||
| Biodegradability | Surgical device removal | Whole-device biodegradation | Dissolvable inorganics plus organic substrates: limited choice of materials, risk of high elemental dosage | 107,572-574 |
| Tissue regrowth | Biocompatible degradation products | Functional organics: limited performance and functionality | 107,575 | |
| Chronic health risk if not retrieved | Tuneable device lifetime | Components other than sensor outside body: wireless communication limitations, safety risks associated with percutaneous wires | 572 | |
| Systemic biocompatibility tests: lengthy process, species dependence | ||||
| Electrochemical compatibility | Signal-to-noise ratio | Ionic-electronic transduction | Conducting polymers: insufficient conductivity, difficult miniaturization | 343,546,547,576,577 |
| Electrode size (array resolution) | Low frequency signals | Hydrogels and their composites: insufficient conductivity, difficult miniaturization | 524,578 | |
| Biocompatibility | ||||
| Growth adaptability | Conformability | Intrinsic limitation of manmade solid materials | Viscoplastic electronic materials: initial demonstrations only | 579 |
| Biocompatibility | Minimal physical constraint on tissues | |||
| Fast-growing tissues and/or long-term use |
Their importance, specific challenges, reported solutions to these challenges, and limitations of the solutions are listed briefly.
Table 3.
Form-Factor Considerations in Achieving Compatible Sensor-Biology Interfacesa
| Issues | Importance | Challenges | Solutions and their limitations | Ref |
|---|---|---|---|---|
| Form-factor issues | 33,49,529,532,580,581 | |||
| Conformability | Signal quality | Intrinsic limitation of electronic materials | Ultrathin films including 2D materials: mechanical fragility, unstable interface to external circuits | 386,390-392,460,555,582 |
| Motion artifacts | Microscale surface morphology | Soft, stretchable, viscoelastic, and adhesive polymers: limited performance and functionality, risk of irritation, difficult miniaturization | 372,387-389,545,345-347,548,560,564,565 | |
| Heat and mass transfer | Irregular and complex 3D shapes | Substrate structural engineering: complex manufacturing, added volume | 583,584 | |
| Dynamic surfaces | 2D to 3D transformation: simple topography, tissue-incompatible processes Sol-gel materials: simple functionality | 585-588 371,589 | ||
| Draw-on, print-on, and spray-on sensors: simple functionality, poor reproducibility | 394,395,590-593 | |||
| Permeability | Wearing comfort | Not a designed function of conventional sensors | Nanomeshes, 2D materials, and porous materials: mechanical fragility, unstable interface to external circuits | 461,504,555,582,594-599 |
| Biocompatibility | Encapsulation | |||
| Reliable adhesion | Whole-device permeability | Textiles (fibers, yarns, fabrics) and multifunctional integration: degradation upon washing, rigid processors and other modules impairing wearing comfort | 32,103,600-606 | |
| Long-term use | Ultrathin hydrogels: initial demonstrations only | 607 | ||
| Imperceptibility (Light weight, miniaturization, tissue-like mechanical properties, no tether) | Invasiveness | System-level imperceptibility | Ultrathin and mesh films including 2D materials: mechanical fragility, challenging system integration | 56,57,309,390-392,582,596,608 |
| Comfort and convenience | Packaging volume | Fiber sensors: difficult handling unless integrated into textiles | 600,609,610 | |
| User compliance | ||||
| Minimal invasiveness | Health risks | Skin barrier | Noninvasive deep-tissue sensing techniques: limited penetration depth, spatial resolution, temporal resolution, and wearability | 390,566,611 |
| User acceptance | Deep-tissue signals | Microneedles: complex fabrication, low motion tolerance, ineffective passive sampling, difficult quantitative chemical sensing | 612-619 | |
| Biofluid sampling | Active biofluid induction: additional electrode and power supply, risk of irritation, concerns on drug intake | 174,616 | ||
| Large device implantation | Injectable microsensors: difficult handling and connection, simple functionality | 540,542 | ||
| Fiber sensors: small sensing area, difficult implantation, challenging system integration | 127,544,620,621 | |||
| Contact lenses: small area, difficult integration | 249,252,445,622,623 | |||
| 3D tissue coverage | Surface sensing on complex 3D structures | Minimal invasiveness | Self-expandable or multimodule microsensors: difficult handling and connection, simple functionality | 540,542,624 |
| Interior sensing of bulk tissues | Precise positioning | |||
| Volumetric mapping | Spatial resolution | Cell seeding on electronic scaffolds: inapplicable to grown tissues | 625 | |
| End-of-life withdrawal/degradation | Developmental biology-driven 3D assembly: inapplicable to grown tissues, cell-type limitation | 527,543,626 | ||
| Immune response | Mechanics-guided 3D assembly: complex inverse design, challenging micro/nano-scale fabrication | 627-630 |
Their importance, specific challenges, reported solutions to these challenges, and limitations of the solutions are listed briefly.
Figure 10.
Challenges in achieving compatible sensor-biology interfaces. Arrows indicate the influence of one property (open dots) on another (solid dots). The issues stemming from different features of biological tissues are stated at the sensor-tissue interface and indicated by thick gray arrows.
Since there is already a large and expanding collection of reviews on the topic of sensor-biology interfaces33,49,50,74,138,249,519-522,524-534 dissecting the challenges and issues in great depth and detail, we will only touch on the overall trends in the field and highlight critical challenges that deserve special attention, in the general context of flexible sensor technology. Readers can refer to references in Tables 2 and 3 for further details.
Bio-Interfacing Materials.
The most important consideration in developing bio-interfacing materials is biocompatibility. Although biocompatibility is a term frequently appearing in publications on bio-interfacing materials and sensors, more thorough understanding and investigation are needed. Biocompatibility tests are specified in a set of standards: ISO 10993-Biological evaluation of medical devices.537 Depending on the position and duration of a tissue-contacting device, the tests required to perform vary. These may include tests for cytotoxicity, sensitization, irritation or intracutaneous reactivity, systematic/acute toxicity, subacute and subchronic toxicity, genotoxicity, among many others. Cytotoxicity tests alone do not determine biocompatibility, and biocompatibility without considering position and duration of tissue contact is meaningless, because the biological effects a material/device imposes on tissues vary significantly with these two factors. Researchers should be cautious when commenting on the biocompatibility of their devices–by providing strong evidence, defining clear scopes, and making conservative conclusions–as in refs 547 and 569.
There are many discrepancies in the biocompatibility claims of emerging materials for flexible sensors, especially nanomaterials like graphene and CNTs. This issue stems partly from the non-standardized tests conducted in different studies, and partly from the large variations in material properties due to poorly controlled syntheses and large varieties of sizes, geometries, and surface states that nanomaterials can have.525,631,632 Defining the biocompatibility of nanomaterials requires standardization and large-scale efforts, which will take years and even decades to carry out. In this process, standards organizations (e.g., International Organization for Standardization, ISO; International Electrotechnical Commission, IEC),633 regulatory bodies (e.g., the U.S. Food and Drug Administration, FDA),536 as well as consortia and communities in related areas (e.g., Institute of Electrical and Electronics Engineers, IEEE; International Union of Pure and Applied Chemistry, IUPAC) should lead the effort. Before specific standards are available, researchers should refer to similar standards like ISO 10993537 for experimental design and report details in materials, equipment, procedures, and results using standard methods, without bias.
One specific biocompatibility problem is immune response, which should be carefully dealt with for user safety and acceptance and for sensor performance. Immune response varies greatly from person to person; some people are allergic to materials claimed to be biocompatible for the majority of user populations. For example, in rare cases, people with circulating anti-PEG (poly(ethylene glycol)) antibodies can experience fatal anaphylaxis to PEG-grafted drugs.634 Risks associated with hypersensitive immune systems should be evaluated and clearly communicated to potential users. On the other hand, although some immune responses are not hostile and detrimental to the human body, such as fibrous capsule formation, where no serious inflammation occurs, the insulating capsule greatly deteriorates sensor performance. In such cases, it is desirable to eliminate, not only to suppress, immune responses. Current strategies focus on controlling the mechanical properties of sensors, such as reducing bending stiffness by reducing thickness and utilizing soft polymeric materials. However, device surface chemistry and morphology also play important roles in cellmaterials interaction,635 which should be explored for biointerfacing sensors.
A major trend in materials engineering towards a more compatible sensor-biology interface is to synthesize tissue-like polymeric materials with mechanical, electrical, optical, or other functional properties (Figure 11, left). Thanks to the multi-length scale and diverse molecular design in polymeric materials, many properties can be precisely tuned and combined in a single materials system, such as softness, stretchability, adhesiveness, conductivity, biodegradability, stimuli-responsiveness, etc. Supramolecular polymeric materials636 and conjugated polymers are examples of promising polymer platforms. Hydrogel, in particular, is gaining traction because of its compositional resemblance to biological tissues—water-rich and ion-conductive.637,638 Synthetic hydrogels and hydrogels derived from biopolymers (e.g., proteins, nucleic acids) have advanced significantly in functionality and performance in the past decade, and there is a rapid expansion of hydrogel-based or hydrogel-enhanced sensors.296,524,639-641 Nonetheless, hydrogels, and polymers in general, still often fall short in functional performance relevant to sensing, particularly in conductivity and stability, compared to conventional inorganic electronic materials. Moreover, miniaturization is challenging, and fabrication is incompatible with current microfabrication facilities. These factors make the adoption of emerging polymeric materials challenging.
Figure 11.
Major innovations in materials and form factors, respectively, towards seamless sensor-biology interfaces. Materials innovations images (from left to right, top to bottom): Adapted with permission from ref 296. Copyright 2018 Springer Nature. Adapted under the terms of the Creative Commons CC BY license from ref 565; published 2022 Springer Nature. Adapted under the terms of the Creative Commons CC BY license from ref 367; published 2021 Springer Nature. Adapted with permission from ref 337. Copyright 2018 Springer Nature. Adapted with permission from ref 643. Copyright 2016 Springer Nature. Form factor images (from left to right, top to bottom): Adapted with permission from ref 56. Copyright 2013 Springer Nature. Adapted with permission from ref 540. Copyright 2015 Springer Nature. Adapted with permission from ref 644. Copyright 2017 American Chemical Society. Adapted with permission from ref 625. Copyright 2016 Springer Nature. Adapted with permission from ref 605. Copyright 2021 Springer Nature. Adapted with permission from ref 290. Copyright 2018 Springer Nature. PAA, polyacrylic acid; SBS, poly(styrene-butadiene-styrene).
Tackling the limitations of polymeric materials, a second important research direction is the discovery/endowment of bio-relevant properties in conventional electronic materials, so as to leverage their advantages in patternability, processability, and electronic performance. Biodegradation of metals (Mg, Zn, Fe, etc.), semiconductors (Si, Ge), and ceramics (SiO2, MgO, Si3N4) has been exploited for transient and bioresorbable bioelectronics.519 When made ultrathin (<1 μm), the mechanical mismatch between these intrinsically rigid materials and biological tissues can be reduced. Many bioresorbable sensors made of thin-film inorganics have been reported to demonstrate good performance and biocompatible degradation in vivo.572-574 However, large-scale, long-term, and systemic tests need to be done on more animal species including humans before conclusions can be reliably drawn on the biocompatibility of degradation products. Surface nanotexturing of inorganic electrodes has shown improved cell attachment and suppressed inflammation for neuroprobes.569,570 Compositing inorganic materials with soft polymeric matrices reduces mechanical mismatch with tissues, and often requires nanofillers such as nanoparticles and nanowires.642
Overall, to solve the sensor-biology mismatch problem from a materials perspective, it is challenging to achieve all desirable properties (e.g., mechanical compliance, adhesion, biocompatibility, electrochemical compatibility, growth adaptability) in one material110,546,548 while retaining sensing performance comparable to conventional sensor materials, and it is even more daunting to ensure all materials within a system possess these properties. Therefore, rational design in device architecture to combine materials with complementary properties is necessary to achieve device-level tissue compatibility.
Biofriendly Form Factors.
Form factors of bio-interfacing flexible sensors are evolving to thinner, lighter, more miniaturized, intricately structured and porous, highly integrated, and customized architectures (Figure 11, right). These features aim for the common goal of minimal interference with biological activities yet intimate tissue contact for better signal quality. The realization of these advanced form factors relies heavily on nano-/microfabrication, which endows sensors based on conventional electronic materials with almost all desirable bio-interfacing properties (Table 3), i.e., conformability, permeability, imperceptibility,596 minimal invasiveness, and 3D tissue coverage.542 In addition, 2D materials, such as graphene,608 MoS2,460,582 PtSe2, and PtTe2,645 provide another means to attain the desirable form factors. Nevertheless, the fundamental limitation of these form factors lies in mechanical fagility,555 because rigid materials have to be made ultrathin (<100 nm) with cell-compatible feature sizes (<10 μm) to be tissue-compatible. The lack of mechanical robustness makes manufacturing, handling, and applications challenging and impairs sensor stability. These issues often prevent real-world deployment, despite the use of well-established materials and processes. Improving mechanical robustness should be a priority for future research on imperceptible bio-interfacing flexible sensors.
Flexible hybrid electronics is one of the most promising form factors for bio-interfacing flexible sensors38,50,529 to be deployable in the near future. Yet compared with other form factors such as textiles and tattoos, flexible hybrid electronics are still relatively bulky and hardly permeable. To tackle this problem, a possible evolution pathway of flexible hybrid systems could be to move non-sensor components away from directly contacting the tissue. This strategy can eliminate many issues arising from device-tissue interfaces. Meanwhile, more effort should focus on sensor optimization to make it perfectly match the tissue, and the communication between the sensor and the rest of the system should rely on wireless technology, which is critical for Sensors 4.0 in general. This concept has been demonstrated to resolve the soft-hard interface instability issue.293 Many more issues could be solved using this strategy.
Textiles are another form factor that holds great promise.646-648 Many advanced functions have been demonstrated on textile platforms, and integrated systems can achieve energy harvesting, energy storage, sensing, display, and simple signal processing.604,649,650 Furthermore, industrial scale or industry-compatible production has been reported,553,605,606,651,652 and commercial products have started to emerge.646 Smart textiles might not be far from large-scale deployment. Nevertheless, textile sensing systems face challenges in washability, durability, wearing comfort, necessity of rigid modules, and aesthetics.
Another trend is the shift from 2D planar devices to 3D volumetric devices, in order to acquire information on 3D structured surfaces or across 3D volumes of biological tissues. While the field is in its infancy,532 progress has been made, such as injectable self-expanding neural microelectrodes,540,542 hybrid cardiac patches with multifunctional electronics,625 and cyborg organoids.626 A recent concept was proposed to build tissue-like systems from the bottom up largely or entirely using synthetic materials, mimicking the morphology, hierarchical structures, and functional properties of biological tissues.530,653 In situ fabrication of materials and devices within biological organisms and tissues530,654,655 is blurring the boundaries between manmade devices and natural organisms. More advanced functions will come at the interfaces between electronics and biology.
The form-factor challenges in compatible sensor-biology interfaces include mechanical robustness of nano-/micro-fabricated materials and devices, unaltered sensing performance, and reliable system integration between system components and with biological tissues. Innovations in device structural design, system layout and operation, and materials manufacture will bring about more effective solutions to these challenges and form factors that are presently rare (e.g., mask,130,256,656,657 suture,658 or bandage563). Importantly, to design and to engineer materials and form factors that allow seamless integration with biological tissues, it is essential to understand in detail and in depth the anatomy, physiology, material properties, biological functions, etc. of the tissues of interest.
POWER SUPPLY
Power supply is foundational to the proper functioning of sensing systems. As flexible sensors take on more advanced functions and diverse form factors in more use cases, challenges emerge in sustainably and reliably powering sensing systems and networks.659
The power consumption of integrated sensing systems, including sensors, signal processing circuits, microcontrollers, communication modules, etc. as well as the interconnections between these elements, can be substantially higher than sensors alone660 (as a reference, the power consumption of a smartwatch fluctuates within 10 mW–10 W whereas that of commercial sensors normally sits in the range of 0.1–10 mW). Large-scale (and multimodal) sensor arrays requiring simultaneous readouts of massive sensor pixels impose huge energy budgets. Systems performing continuous monitoring demand constant power supplies. All these factors contribute to high power demands of next-generation flexible sensing systems, which are not met by conventional energy storage devices.
As more sensing architectures and frameworks emerge, associated physical and resource constraints limit the use of conventional power supply strategies. For example, highly dispersed building-integrated sensor networks can have hundreds of sensor nodes. Installing power points to each sensor node or replacing batteries periodically is expensive, cumbersome, and wasteful. Body area networks employing dozens of body-worn sensors require tetherless power sources that do not need frequent battery replacement for individual sensors.
The form factors of traditional rigid and bulky batteries hinder system miniaturization and introduce soft-hard interfacial instability,61,63,661 retarding progress towards compact and compliant sensing systems.274
Battery safety is a significant issue and is in the spotlight after incidents of fires and explosions associated with battery malfunction. For human-centric sensing applications, safety of the power supply system is of paramount importance. Accidentproof designs are required, as are biocompatibility and heatgeneration considerations.
Last but not least, against the backdrop of pressing sustainability crises, the currently environment-damaging materials, manufacture, and disposal of batteries call for greener energy sources in place of fossil fuels and rare materials.
Here, we discuss potential solutions to these challenges in four areas: ambient energy harvesters, energy storage devices, wireless power transfer, and system power management (Figure 12).
Figure 12.
An energy-efficient sensing system incorporating multiple strategies for power management: reliable and high-power ambient energy harvesting (representative devices for light, mechanical, and chemical energy harvesting are shown; full list of devices is provided in Table 4), large-capacity energy storage in flexible form factors, reliable and efficient wireless power transfer, and systemic power management. TENG, triboelectric nanogenerator; PTE, power transfer efficiency.
High-Power Ambient Energy Harvesters.
The idea of having a sustainable power source near the sensor can be realized by miniaturized ambient energy harvesters integrated into the sensor powering system, which convert energy in the surroundings of the sensor into usable electricity.662 This extra energy source then provides power that can be additional to batteries for power-demanding systems and may be sufficient on its own to power devices or systems. Some energy harvesters also have sensing functions, thus working as self-powered sensors. Ambient energy harvesters make battery-free sensors possible, significantly simplifying maintenance and reducing carbon footprint.
Common types of ambient energy harvesters used in flexible sensing systems are summarized in Table 4. Mechanical,663,664 thermal,665-667 electromagnetic, and chemical energies668,669 can be harvested using portable devices, and their flexible and stretchable formats facilitate compatible integration with flexible sensors.299,670-674 In addition, the recently demonstrated thermoradiative diode675 may find use in cold environments in the future. Among these technologies, photovoltaics are the most mature with a long market history. Current research seeks to endow photovoltaic devices with greater biofunctionality such as conformability, softness, ultralightweight,676 biocompatibility, biodegradability,677 etc.,672,678 as well as to develop printable manufacturing.28,679-681 Meanwhile, exploring cheaper, safer, more stable and efficient materials is a constant pursuit.682,683 Solely photovoltaically powered systems are feasible676 due to the high energy density of solar radiation and the high power density of photovoltaic devices, and tuning the responsive wavelength to the near-infrared region allows for subcutaneous power delivery using an external light source.677,684
Table 4.
Ambient Energy Harvesters Used in Flexible Sensing Systemsa
| Energy source | Mechanism | Sensing function | Peak power density | Flexible form factor | Ref |
|---|---|---|---|---|---|
| Movement (touch, friction, bending, stretching, impact, vibration, sound & ultrasound, etc.) | Piezoelectric (also for acoustic wireless power transfer) | Strain, pressure, pulse | 3–9.3 kW m−3 | Sheets, (ultra)thin films | 685-687 |
| 1.25 W m−2 | Fibers, fabrics (stretchable) | 688,689 | |||
| 26 mW m−2 | Stretchable sponges | 690 | |||
| Triboelectric | Touch, pressure, strain, breath, vibration, sound, acceleration, gas, humidity | Up to 10 MW m−2 | Sheets, sponges | 691,692 | |
| 4.3 μW m−1, 1.77 W m−2, 230 mW m−2 | Fibers, yarns, fabrics (stretchable) | 689,693-695 | |||
| 35 mW m−2 | (Self-healable) stretchable slabs | 696,697 | |||
| Piezoelectric + triboelectric | Touch, bending, stepping | 2.34 W m−2 | Stretchable fabrics | 698 | |
| Magnetoelastic | Pulse, breath, touch, pressure, bending, stepping | 20–43.2 W m−2 | Stretchable slabs, sheets | 65,68,169,208 | |
| 6.67 W m−2 | Stretchable fibers and fabrics | 66,67 | |||
| Magnetoelectrical | Arm waving, strain | 3.2 W m−2 | Yarns, fabrics | 699 | |
| Peak power: 20 μW | Stretchable yarn and ring | 700 | |||
| Electrostatic | Stretch, twist, compression | 0.55 mW m−2 | Stretchable slabs | 701 | |
| Radiation (sunlight, light emitting diode, laser, etc.) | Photovoltaic | No | 11.5 W g−1 (PCEb 10.5%), 4.4 W g−1 (PCE 5.8%), 180 W m−2 (PCE ~19%) | (Ultra)thin films | 676,682,702,703 |
| 2.53 mW m−1, 0.61 W m−2 (PCE 11.9%) | Fibers, fabrics | 678,704 | |||
| Heat | Thermoelectric | Temperature | 111 W m−2 at 50 K ΔT, 188 W m−2 at 80 K ΔT | Sheets, films | 705,706 |
| 6.5 W m−2 at 60-K ΔT | Stretchable slabs | 707 | |||
| 110 W m−2 at 70-K ΔT, 23 W m−2 at 60-K ΔT | Fibers, fabrics | 708-711 | |||
| Thermocell | No | 0.6–0.7 mW m−2 K−2 | Stretchable slabs | 712,713 | |
| Water (moisture, humidity, sweat) | Hydrovoltaic | No | 760 μW m−2 at 25% RHc and 55.2 mW m−2 at 85% RH | Sheets | 714 |
| ~10 mW m−2 immersion in DI waterd | Thin films | 715 | |||
| ~70 W m−3 (= 21 mW m−2 at 0.3 mm thickness) at ~80% RH | Fabrics | 716 | |||
| Hydrovoltaic + thermoelectric | No | 720 mW m−2 at 70% RH and 10 K ΔT | Sheets | 717 | |
| Hydrovoltaic + photovoltaic | No | 880 mW m−2 at 50% RH and 1 sun illumination | Sheets | 718 | |
| Biofluids (sweat, blood, interstitial fluid) | Biofuel cell (on-skin) | Glucose, lactate, etc. | 35 W m−2 | Integrated skin patches | 719 |
| 83 mW m−2 | Integrated sensor chips | 720 | |||
| 130 mW m−2 at 30 mM lactate | Integrated skin patches | 232 | |||
| 12 W m−2 | Stretchable sheets | 721 | |||
| Biofuel cell (implantable) | Glucose, lactate, etc. | 44 mW m−2 on implantation and 25 mW m−2 after a month | Fibers | 722 | |
| 430 mW m−2 in 0.5 M glucose | Nanomembrane on Si substrate | 723 | |||
| 4.8 W m−2 in 5 mM glucose and 1.6 W m−2 after 7 days | Bioresorbable sheets | 724 | |||
| CNT-OH interaction (implantable) | No | 570 mW g−1 in 2.5 M HCl | Fibers | 725 |
Some state-of-the-art reports are listed.
PCE, power conversion efficiency.
RH, relative humidity.
DI water, deionized water.
Another promising device is the triboelectric nanogenerator (TENG).726 Despite its short history,727 TENG has witnessed rapid development, and is a highly promising technology for sustainable power supplies.728 It has high output performance (output energy density of 104 J m−3 and instantaneous power density of 10 MW m−2),692,729 as well as ultra-broad materials availability at relatively low cost,730 simple fabrication, and versatile operation modes, enabling cost-effective mass production and customizability to suit different applications. Its biocompatibility stems from diverse materials choices, including for implantable applications.731,732 Due to the sensitivity to deformation, TENGs can serve as self-powered sensors for various mechanical stimuli, such as pulse, breath,693 sound, touch, and body motions;733 with proper modification, gas734 and humidity735 can also be sensed.
The biggest challenge facing ambient energy harvesters is typically to produce enough power for an entire sensing system. Beyond triboelectric and photovoltaic energy conversion, the power generation efficiency and/or power density of most current technologies are insufficient to support complex sensing systems fully. While discovering energy conversion mechanisms that are intrinsically efficient (e.g., magnetoelastic effect65) or improving current technologies through materials innovation and structural engineering (e.g., using nanomaterials to increase reactive surface area) will help, integrating low-cost large-area energy harvesters in an imperceptible way might be another solution. For instance, clothing is a promising platform to integrate textile energy harvesters without significant interference to wearers’ daily activities, and the surface area across the body provides ample space to collect sufficient power.670,678,689,691,736,737 Nevertheless, comfort, convenience, aesthetics, washability, and interconnection are problems to address. Although not yet able to power integrated complex systems, ambient energy harvesters, especially self-powered sensors, can support the function of simple wireless sensors, such as active RFID tags,738 triboelectric pressure sensors,739-741 and magnetoelastic generator-based human-machine interfaces.68
Intermittency in power generation is a second problem. Ambient energy sources are usually not constantly available, including sunlight and body motion. Hybrid energy harvesters that combine two or more transducing mechanisms and scavenge energy from multiple energy sources may help alleviate this problem.742 In addition, output power usually fluctuates with the intensity of energy sources, requiring power management circuits and energy storage devices to level the curve and to provide sustained and constant power.685,743 However, power management circuits themselves usually require power to function.744 To minimize additional power requirements, a power management circuit for TENGs was designed to perform effective power regulation without any additional power input,745 which may inspire other energy harvesters. On the other hand, self-powered sensors are best positioned to address the problem of intermittency because they generate power exactly when demanding power. However, performance of selfpowered sensors needs improvement. For example, the sensitivity of piezoelectric and pyroelectric sensors is relatively low. Triboelectric sensors are prone to external noise and humidity.746 Magnetoelastic sensors need improvements in device weight and miniaturization.65,169
Large-Capacity Energy Storage Devices.
Power delivery through electrochemical energy storage devices (ESDs) is more reliable than in situ energy harvesting. Common ESDs for flexible sensors include batteries (lithium-ion batteries,747 zinc-ion batteries,605 etc.)300 and supercapacitors.604 Goals in devising ESDs for flexible sensors include high capacity (high energy density), low-profile/imperceptible form factor (flexibility, stretchability, miniaturization), and high cycling stability (electrical and mechanical cycling). These goals, however, often entail contradictory materials and device design principles, raising significant challenges in crafting effective ESD solutions for flexible sensors.
In terms of current materials, electrodes and electrolytes need better designs. Flexible form factors are usually realized by reducing the thickness of electrodes, which reduces energy capacity, as well.748,749 To improve energy capacity, stacks/arrays of flexible ESDs315,750 and large-area fabrics made from ESD yarns604 are viable approaches. This approach usually requires more substrate/encapsulation materials than conventional structures, reducing the overall energy density. Moreover, the use of polymeric binders to enhance electrode mechanical robustness further impairs energy density. Nanomaterials and polymers possessing both robust mechanical flexibility and electrochemical activity are needed.315,751 Electrolytes also face performance trade-off. Conventional liquid (predominantly organic) electrolytes are the most conductive but least stable, leading to high safety risks (due to chemical reactions and leakage).749 Electrolyte leakage and solvent evaporation also reduce energy capacity and device lifetime.752 At the other extreme, solid electrolytes are highly stable but poorly conductive or deformable. A possible trade-off would be gel electrolytes, currently dominant in flexible ESDs. Supra-molecular polymer electrolytes were recently shown to break this trade-off, achieving high stretchability, toughness, and ionic conductivity simultaneously.753
In terms of device performance, energy capacity, cycling stability, and safety are key metrics to improve. While energy density is a figure of merit for fair comparison among devices,754 during practical usage, it is the total energy capacity of an (array of) ESD(s) that determines the lifetime of a sensing system, before the next charging. For a device with ultralight weight, the energy density can be extraordinarily high, but if it cannot be scaled up and integrated with other components reliably, the device would be of little practical value. Therefore, reporting energy density and demonstrating high capacity are both essential in ESD research. Furthermore, the stabilities of current ESDs against charge cycling and mechanical cycling are not sufficient for real-world applications. Most ESDs suffer from either capacity degradation or device failure after bending and/or stretching cycles.755 Fiber batteries woven into textiles seem to be the most mechanically robust choice, and they allow largearea incorporation for higher energy capacity.651,678,756 Recent reports on industrial-scale production and integration651,756 demonstrate promise for practical use. Critically, safety evaluation is often overlooked in current flexible ESD research. For wearable and implantable applications, tests in physiological environments, potential extreme conditions, and simulated long-term use should be conducted.757 The work on sweat-activated battery758 is a good example of prioritizing biosafety in ESD design. Recent developments in sweat-activated batteries have improved capacity759 and power density759,760 as well as realized more form factors such as bandage761 and textiles.762,763 Mechanistically safer ESDs such as zinc-ion batteries757 and supercapacitors might be suitable for safety-demanding applications. Some cutting-edge design principles for safe ESDs764-766 can be applied to support flexible sensors, and fundamental understandings of ESD safety such as thermal runaway in lithium-ion or lithium batteries767-769 will be critical in guiding the design of safer lithium-based ESDs.
Materials research on ESDs and sensors are mutually beneficial and synergistic. Some materials that are initially developed for sensing applications may be repurposed for energy storage and vice versa. For example, membrane materials developed for sodium-ion batteries may double as materials for sodium sensing. Self-healing conductive hydrogels may function as both strain sensors420 and electrolytes in supercapacitors.770
Efficient Wireless Power Transfer.
With power generated by ambient energy harvesters and held in energy storage devices, the next problem is to transfer the power conveniently, efficiently, and reliably to sensors. The conventional method relies on wired power transmission and may not work effectively for emerging flexible sensor technologies, such as body area networks. In these scenarios, integrating an energy harvester and/or an energy storage device to every sensor node via wired connections causes significant installation and maintenance challenges, and limits sensor node mobility/wearability. If multiple distant sensors share the same power source, wired connections can become cumbersome and unsafe. To address these issues, wireless power transfer (WPT) is likely a more suitable strategy.
Current dominant WPT methods include near-field and farfield radio-frequency (RF) techniques. These RF technologies have both power transfer and data communication capabilities and thus can enable highly autonomous or fit-and-forget sensors that are lightweight, tether-less, and require minimal maintenance, which are particularly suitable for automation, security, safety, and productivity related applications. Near-field technologies are based on inductive coupling and magnetic resonance,622,771 and far-field technologies are based on radiative power transfer.772,773 Near-field techniques can achieve high-efficiency power transmission yet only over a limited distance (a few centimeters), and there are strict requirements for transmitter-receiver alignment. Far-field techniques can cover large areas (a few square meters), but due to omnidirectionality, their power transmission efficiency is low and subject to obstructive interference (especially from the human body). Mechanisms such as coherently enhanced WPT and exceptional point WPT, as well as metamaterials and metasurfaces for WPT774 may offer better solutions over traditional methods. Besides electromagnetic techniques, ultrasound is also a practical method, currently a mainstream technique used for implants, due to its low attenuation by biological tissues and high safety.775-777 But because it is highly directional, small-area single-node applications are most suitable. Recently demonstrated body-coupled electromagnetic power transmission also showed significantly (30–70 dB) lower path losses through the human body than far-field RF transmission.778 Without limitations in the location of transmitters and receivers, it can cover the whole body from head to toe (2 μW extracted on the head from a 1.2 mW transmitter on a foot, sufficient to operate low-power sensors), and is thus a promising technology for body area networks.
The abovementioned technologies based on electromagnetic energy transmission can also be used to harvest ambient electromagnetic energy emitted from power lines and electronic devices, as well as pervasive wireless communication networks, leading to an energy recycling effort for sustainability. However, ambient electromagnetic energy shares the same instability/intermittency issue with natural ambient energy sources yet has relatively low power density and recoverable power (10 nW–100 mW772,778). Hence, ambient electromagnetic energy might be a good add-on but is likely not a staple energy source.
Major challenges in WPT lie in device miniaturization, coupling distance increment, and transmission efficiency improvement. For human-centric sensor networks, convenient power transmitter location and usage293 should be devised to circumvent the requirement of skillful periodic charging. Biocompatibility and long-term stability of implantable wireless power modules need to be investigated. Effective WPT solutions for kilometer-range sensor networks also need to be developed for agricultural, industrial, and environmental settings where minimal human intervention is present.779
Holistic System Power Management.
Holistic power management at systems levels can be implemented from multiple perspectives (Figure 12, red panel).780 First, reducing the power consumption of individual modules in a sensor system is fundamental. Some examples of recent efforts to this end include: sensing mechanisms or materials engineering that lead to ultralow- to zero-power sensors,44,781,782 power-efficient readout architectures for sensor arrays (detailed in section Efficient Matrix Signal Readout),465 low-power wireless communications technologies (detailed in section Sensor Connectivity), flexible memory with ultralow switching current density,783 and flexible complementary circuits with ultralow driving voltages.469 In addition, low-impedance interconnections in integrated systems are also critical for improved power efficiency.
Second, a combination of multiple energy harvesting and storage strategies according to application requirements and constraints should be considered.742,784 In such cases, power management circuits that solve the impedance mismatch between high-impedance energy harvesters (such as triboelectric and piezoelectric nanogenerators) and low-impedance energy storage devices (such as supercapacitors and lithium-ion batteries) are critical.785 Such impedance mismatches can significantly reduce system efficiency during operation. However, the additional power modulation circuits and elements660,738,743,785 (e.g., transformers to regulate generators’ voltage output, charge pump circuits to increase power output of biofuel cells, maximum power point tracking circuitry to vary optimum electrical operating point of photovoltaics786) increase design and manufacturing complexity as well as implementation cost, and reduce system compactness. Thus, the integration levels of various energy harvesting and storage devices with other components in the system is critical.787 One attempt to address this issue is a battery-in-sensor developed by inserting an isolation layer into a solid-state zinc-ion battery. The device delivers power that changes with the external pressure, thereby achieving self-powered pressure sensing with high integration.788 More inspiration can be drawn from the simplistic integration of self-powered sensors and wireless communication modules towards fully power-autonomous systems.739-741 Nevertheless, there is a trade-off between sensor accuracy/controllability/reliability and system complexity, awaiting better solutions.
Biofuel cells are poised to be incorporated into system-level designs. Because their output power depends on the concentration of an analyte (e.g., glucose, lactate, alcohol), they can be used as biosensors in addition to energy harvesters.232,720,789 They have been integrated with piezoelectric nanogenerators720 for self-powered touch-based sweat sensing, and with TENGs and supercapacitors for textile sensing systems.784 Furthermore, biofuel cells have been integrated with near-field communication electronics,790 magnetic human body communication,789 and electrochromic displays232 to realize battery-free data communication and readout. Nonetheless, biofuel cell implementation is often limited by their operational lifetime, which is on the order of a few days, before enzyme degradation impairs power output. Future work utilizing either non-enzymatic sensing or engineered enzymes and biomaterials for improved operational stability as well as further integration with other energy harvesting mechanisms is expected to make a significant impact on self-powered biosensors and holistic power management approaches.
Another system-level approach is to use microcontroller units or power management integrated circuits (ICs) to manage the power flow and usage by various system components for maximum energy efficiency.691,786,791,792 For example, the system could be in a sleep mode when no alarming stimulus is present, but the energy harvesters could be functioning to store energy in batteries. Once the sensor is triggered by an alarming stimulus (most likely a self-powered sensor), the power management circuit instructs the communication module to start functioning to transfer sensor data wirelessly for prompt action. When multiple components are working simultaneously and the energy storage level is low in the battery, the system can enter a power-saving mode with reduced signal processing and data exchange. This strategy is much like how smartphones manage power usage. Power consumption of the power management system itself is an important factor to consider.
SENSOR CONNECTIVITY
Sensor connectivity refers to the information exchange among sensors, as well as between sensors and control devices (e.g., smartphones, computers). Connectivity is important because in many cases, a large group of sensors holistically reflect the status of the monitored subject/environment (e.g., health monitoring,793 posture and motion tracking,293,794 environmental monitoring21), or spatially distant subjects need to be monitored simultaneously (e.g., large-scale behavioral neuroscience in animals795). Sometimes it is the sensor-sensor interactions that produce meaningful data (e.g., COVID-19 contact tracing). A connected sensor network can be established through either wired or wireless communication. The latter is gaining traction in recent years because it can utilize the power of cloud computing and convenient data sharing and management, improve the wearability and implantability of bio-interfacing sensors, and simplify sensor installation in IoT applications, enabling sensing paradigms including Wireless Sensor Network (WSN)796-798 and Wireless Body Area Network (WBAN, IEEE 802.15.6).787
There are several information-carrying media through which wireless communication can be established, such as acoustic waves,775,799 optical signals,684,800 and RF electromagnetic waves.801 RF communication methods are most commonly used, because of their versatility through different data transfer mechanisms (magnetic inductive coupling, magnetic resonance, far-field radiation, etc.) and a wide frequency range, leading to communication protocols with distinct characteristics suitable for different applications (Table 5).
Table 5.
Radio-Frequency Wireless Communication Protocols Commonly Used in Wireless Sensor Networksa
| Technology | Range | Frequency | Data rate | Network topology and transceiver type |
Power consumption | Existing applications | Examples relevant to flexible sensors |
|---|---|---|---|---|---|---|---|
| NFC | 5–25 cm | 13.56 MHz | 106, 212, 424 kbps | Peer-to-peer | 3–15 mW | Contactless payment, ticketing, medicine and healthcare (e.g., patient charts tagging) | 445,502,802 |
| Passive, semi-passive | |||||||
| RFID | 25 cm-100 m (frequency-dependent) | LF: 120–140 kHz | 10–640 kbps | Peer-to-peer (unidirectional) | 10 nW–200 mW (frequency-dependent) | Manufacturing parts, retail items (apparel, footwear) | 293,803 |
| HF: 13.56 MHz | Passive, active | ||||||
| UHF: 865–956 MHz (far field) | |||||||
| Microwave: 2.45–5.8 GHz (far field) | |||||||
| RuBee | 15–30 m | 131 kHz | 1.2 kbps | Peer-to-peer | 40 nW804 | Real-time asset visibility in harsh environments, defense and industrial IoT805 | – |
| Active | |||||||
| UWB | 1–1000 m | 3.1–10.6 GHz | 22–600 Mbps | Peer-to-peer, star, mesh | <50 mW | Radar imaging | 492 |
| Active | |||||||
| Bluetooth | 10–300 m | 2.4 GHz | 780 kbps–3 Mbps, up to 25 Mbps | Peer-to-peer, star (seven slaves) | 10–100 mW | Earphones, smartphones, smartwatches | 63,661,806,807 |
| Active | |||||||
| Wi-Fi | 70–250 m | 2.4 and 5 GHz | 11–54 and 150 Mbps | Infrastructure-based | 835 mW | Smart home, wearables, streaming | 63,657 |
| Active | |||||||
| ZigBee | 10–100 m | 868 and 915 MHz, 2.4 GHz | 20, 40, 250 kbps | Peer-to-peer, star, mesh, cluster tree | 36.9 mW | Home automation, traffic management | 808,809 |
| Active | |||||||
| LoRa | Urban: 2–5 km | 869 and 915 MHz | 290 bps–50 kbps | Peer-to-peer, star | 1.5–100 mW | Smart cities, logistics and transportation management, smart buildings | 810,811 |
| Suburban: 15 km | Active | ||||||
| NB-IoT | Urban: 1–8 km | LTE frequencies (several bands within 0.41–5.9 GHz) | 160–250 kbps | N.A. | 106 mW | Asset tracking, sensor networks, smart cities, industrial monitoring | 812 |
| Suburban: 35 km | |||||||
| Cellular network (e.g., GSM, GPRS, LTE) | 1–10 km | 4G: 2–8 GHz | 4G: 100 Mbps | N.A. | High | Cell phones, tablets, IoT | – |
| 5G: 450 MHz–6 GHz, 24–53 GHz | 5G: 20 Gbps |
NFC, near-field communication. RFID, radio-frequency identification. LF, low frequency. HF, high frequency. UHF, ultrahigh frequency. UWB, ultra-wideband. LoRa, long range. NB-IoT, narrowband-Internet of Things. GSM, global system for mobile communications. GPRS, general packet radio service. LTE, long-term evolution.
Each RF technology has its pros and cons, and there is no one-size-fits-all solution. RF data transmission can be categorized by its range of operation into near-field and far-field technologies. Near-field technologies are characterized by short ranges and low data rates. Established protocols include radio-frequency identification (RFID)796,803,813,814 and near-field communication (NFC),815 for instance, while other near-field protocols designed for specific sensors have been reported as well,622,816,817 which require specially designed readers. These technologies have the advantage of supporting wireless power in addition to data transfer, and are therefore particularly well-suited to operate passive sensors with no battery and minimal electronics.818 However, near-field technologies are sensitive to transmitter-receiver misalignment and limited by the short range of operation (usually <5 cm). In contrast, far-field technologies support long-range, high-data rate transmission. They include standards such as Bluetooth, Wi-Fi, and 5G, which can be used by sensors to stream data continuously to a base station several meters away. However, far-field transmission requires power at the sensor. Because of radiative losses, power consumption of far-field communication modules often occupies the majority of a sensor’s energy budget. Far-field signals are also radiated far from the sensor, which raises security concerns due to the possibility of eavesdropping.
The connectivity requirements of different types of IoT networks vary widely, depending on applied purpose and resource constraints including battery life, available bandwidth, buffer size, processing capacity, form factor, transmission media, etc. To construct an efficient wireless sensor network, it is wise to select the best fit (often a trade-off) for each sensor node and it is often necessary to combine several protocols in a suitable topology to provide full network connectivity. For example, in a complex WBAN for health management, sensors carrying various forms of information are worn, attached, or implanted on or in the human body. These sensors can be connected via short-range wireless technologies, such as ZigBee, Wi-Fi, and Bluetooth for continuous data streaming to a gateway device such as a smartphone. This strategy will ensure compact form factors at sensor nodes and acceptable power consumption that can be handled by portable batteries. The gateway device then forwards the data to a remote server for access and feedback by healthcare providers. This remote data transfer can be realized by telecommunication techniques such as Worldwide Interoperability for Microwave Access (WiMax), long-term evolution (LTE), or Satellite, which also allow communications between several gateway devices.787
Improved throughput, reliability, and security are the primary goals of modern communication technologies.801 General research directions towards faster data transfer, lower latency, smaller circuit footprint, enhanced energy efficiency, etc. will benefit flexible sensor networks. For instance, high speed and low latency will be critical for real-time feedback systems as well as sensor arrays. Meanwhile, there are some issues that are specific to flexible sensor networks, regarding power consumption, body interference, and data security, dictated by emerging application requirements.
Lowering Power Consumption.
Data transmission puts a heavy energy burden on wireless sensor networks. Depending on application, power requirements vary, but in most cases, the wireless communication network consumes more power than sensors per se, and sometimes power consumption by communication can account for nearly 80–90% of total power consumption. Adding large-capacity batteries and energy harvesters around sensors to support such high energy demand is a straightforward solution, but it is not always feasible or ideal for a WSN.787 Hence, reducing the power consumption of wireless communication is of paramount importance for flexible sensors.
There has been much work on improving the energy efficiency in data transmission through antenna configuration, circuit design, modulation scheme, network topology, etc. For example, combining surface and bulk acoustic wave resonators with active CMOS circuits for RF transmitters and receivers has great potential for both ultra-low power consumption (nW to pW) and good noise performance.819 An 800 MHz on-off keying (OOK) transmitter utilized a MEMS-based RF oscillator for carrier frequency generation, leading to a 120 Mbps data rate with an energy efficiency of 5 pJ b−1.820 For receivers, the passive gain approach could be effective in achieving good sensitivity at low power consumption, as demonstrated in high-Q film bulk acoustic resonators.821 In addition, emerging techniques like long range (LoRa)822 and ultra-wideband (UWB)798,823 are amendable to low-power implementation through spreadspectrum modulation techniques. On the network level, the mesh topology is favorable in that data are passed through intermediate devices to reach their destination, allowing reduced power consumption and dynamic network connections.786 Software Defined Networking824 is expected to reduce network management complexity and power consumption at sensor nodes, although the actual benefits will need to be verified in specific application scenarios.787
Besides improving the energy efficiency of communication technologies, reducing the amount of data transferred can alleviate the issue starting at the source. This efficiency can be achieved through edge computing systems integrated near sensors to process signals for reduced data size prior to transmission. Specifically, edge systems can decide which data to transmit and which to discard, trim redundant data, and instruct the transmission of meaningful data only. For example, in realtime monitoring of building structures or environmental safety, only when abnormal events occur do data need to be transmitted immediately for swift action. Other data can be stored temporarily in local memory and deleted after a period of time. In more advanced edge systems, signals can be processed into digitally interpretable features and labeled data with significantly compressed size. All these functions will contribute to faster and more energy-efficient data communication.
Overcoming Body Interferences and Constraints.
Flexible sensors have broad applications in physiological monitoring on the human body, yet the body poses several critical challenges in wireless communication.787,801 First, biological tissues absorb electromagnetic radiation strongly within 1–10 GHz, where common RF wireless techniques reside.825,826 This absorption causes significant path loss and energy waste (significant attenuation, ca. 80 dB, when the antenna is in the vicinity of or attached to the human body826,827). Alternative signal carrying media with lower tissue absorption include ultrasound775,777 and near-field electromagnetic waves,801 but these techniques suffer from difficulty in miniaturization and sensitivity to transmitter-receiver misalignment, which severely impairs connection stability during body movements. Furthermore, body movement also leads to unreliable connections when body parts obscure the transmission pathway. For the same reason, the medium through which signals are transmitted keeps changing, making design of communication systems challenging. On the other hand, wearing comfort and implant safety raise additional materials and form-factor requirements in the design of communication modules, such as softness, stretchability, miniaturization (especially antennas), biocompatibility, among many other aspects in achieving a compatible sensor-biology interface.
Multiple directions of research are meant to address the above issues. The first is to utilize clothing as a medium for confined signal transmission, targeting wearable sensors all around the body.828 Conductive traces laid out around the body act like highways for wireless signals to travel, circumventing the issue of body absorption and enhancing transmission efficiency. For instance, far-field RF signals can be guided by metamaterial textiles with comb-like motifs that support the propagation of surface waves much like surface plasmons of optical frequency.829 When devices are placed near clothing, the emitted wireless signals can be confined to within 10 cm of the body and the transmission efficiency increased by 3 orders of magnitude. This approach can be used to extend the range of near-field transmission.794,830 Using embroidery or a heat press process, near-field relays can be attached onto clothing to establish near-field communication between nodes more than 1 m apart. Recent advances have realized washable and stretchable electronic textiles for wireless communication.802
The second major on-body wireless communication paradigm under investigation is body-coupled communication or body channel communication (BCC), where the human body is exploited as a medium for signal transmission. This can be done in three ways: capacitive coupling, galvanic coupling, and magnetic resonance coupling. Capacitive coupling is currently the most popular method because of less body attenuation at high frequencies (>10 MHz), leading to long range and high data rate.827,831,832 A transmission loss <30 dB was achievable over 1 m distance on the body,833 meaning that capacitively coupled BCC can potentially cover the whole body, within and between the torso, limbs, and the head. Moreover, the use of high frequencies and capacitive coupling ensures that the signals do not interfere with electrophysiological monitoring and are safe within specific absorption rate limits. However, capacitively coupled BCC suffers from several challenges in practical use: varying ground effect, varying skin-electrode impedance, varying body composition between body parts and individuals, multipath and interference issues. Some solutions to these problems include constant impedance monitoring and compensation, and pseudo and hybrid orthogonal frequency-division multiplexing (OFDM) transceivers.831,834 Magnetic resonance coupling, as a relatively less studied means of BCC,835 has potential advantages of insensitivity to body motion with comparable or even less pass loss than capacitive coupling.836 For all BCC methods, standards that guarantee the safety and performance on users with different body size, composition, etc. are needed.
The third branch of research efforts to improve the communication reliability of on-body sensors is devising form factors of RF devices that can adapt to body movements, geometry limitations,787 and tissue softness. The antenna is an important component in RF communication, occupying a relatively large volume in the communication system. Consequently, there have been many efforts in rendering flexibility, stretchability, and miniaturized size to antennas.293,295,445,799 Structural designs, including 2D serpentine, 3D helical, 3D spiral, and 3D buckled shapes, are effective to achieve good mechanical compliance while maintaining high conductivity. Unconventional materials such as liquid metal and nanomaterials offer another dimension of design freedom in achieving multiple antenna forms and functions. Textile-based antennas are integral to smart clothing systems. Although comparable-to-convention performance has been achieved in some of these form factors, issues and challenges remain, such as maintaining electromagnetic performance (bandwidth, gain, radiation efficiency, working frequency) during miniaturization, shielding interference from surrounding biofluids, ensuring reproducibility and environment-resistance of textile antennas, among many others. On the other hand, circuits play decisive roles in RF communication performance, being the ‘mini-brains’ of communication systems. While silicon-based CMOS chips offer superior performance in this regard, their rigidness leads to interfacial mismatch with biological tissues and soft-hard interfacial instability in an integrated system. TFT-based flexible communication chips may alleviate these issues. With the downscaling of TFTs and improved circuit design, TFT-based RFID and NFC chips will continuously bring down the power consumption,466 and may be extended to cover a larger variety of wireless techniques. Recently, intrinsically stretchable transistors378,837 and diodes298 were fabricated, and simple wirelessly accessed sensors without any rigid components were demonstrated.293,298 These efforts will contribute to fully soft wireless communication systems. In all, as wireless technologies continue to evolve, the design of unconventional form factors will face great opportunities and challenges.801
Enhancing Data Security.
Data security has always been a concern for wireless communication because wireless signals are often dispersed in free space, prone to eavesdropping. In humancentric applications, this problem becomes more critical when sensitive personal data are collected, or healthcare decisions are made on the basis of sensor data. In these scenarios, data breaches can have life-threatening consequences.
There are generally three ways to enhance data security in wireless sensor networks. The first strategy is to use transmission schemes that are insensitive to eavesdropping, targeting shortrange communication from the fundamental. In this aspect, near-field techniques805 are better than far-field ones because of small range, small electrical size, and low-power operation. Moreover, UWB is inherently secure. Its low signal energy reduces the probability of detection.798 Recently proposed textile-facilitated far-field and near-field communication technologies address this problem by confining wireless signals within 10 cm from the human body.794,829,830 BCC also guarantees highly secure data transmission by constraining signals within the human body.
The second approach is to strengthen cryptographic systems through data encryption and authentication.838 As the computational capability of supercomputers and quantum computers keeps evolving, it becomes increasingly easier for hackers to crack encrypted information.839,840 Light encryption methods based on quantum-resistant algorithms are in high demand. On the other hand, adding authentication mechanisms throughout the network for data input, access, and sharing among nodes787 allows only authorized users to access data. Biometric authentication, including basic fingerprint and facial recognition, as well as emerging ideas on biochemical and biophysical status (breath odor,841 sweat composition, heart rate, blood oxygen,842 etc.), is a reliable method based on the distinct and unique profile of each individual. However, this method requires an additional set of authentication sensors on each sensor node, complicating system design and increasing cost. Two-factor authentication (2FA), also known as two-step verification, using communication devices outside the sensor network is a simpler solution. Note that any implementation of security measures puts additional management complexity and energy budget demands on the network. Lightweight techniques are highly desirable.843
Recently proposed networking and data management frameworks can also deliver enhanced data security. For example, Software Defined Networking is a networking framework with reduced network management complexity and power consumption, and has recently been proposed to provide security and authentication services in sensor networks.787 Blockchain is another promising technology to be explored in distributed sensor networks to store personal data securely while allowing data tracking.10,787,844
Besides the above mentioned three critical challenges, there are many other issues in the connectivity of flexible sensor networks. For example, changing data volume over time (i.e., dynamic traffic) leads to load imbalances in data storing and processing at back-end servers,787 and is an emerging issue for continuous monitoring. There will be interference between sensor nodes and from other wireless devices operating at overlapping frequencies, as the number of wirelessly connected devices experiences dramatic growth. Overheating on sensor nodes787 will be an issue scaling with data volume. If higher frequency communication modules are incorporated (e.g., 5G), heat management will be a great challenge concerning user safety. Continuous chip innovation to reduce power consumption is key. Convenience, safety, and reliability of wireless communication are constant pursuits for flexible sensor networks.
Closer collaborations between researchers in the fields of flexible sensors and wireless technologies are needed to tap the full potentials of both areas. Despite ongoing research advances in wireless technologies (e.g., 13.56 Mbps data rate achieved by near-field inductive coupling845),801 a majority of reported flexible sensing systems still use commercial and conventional wireless communication modules21,61,63,661 with rigid form factors, limited ranges, and high power consumption. These choices are understandable based on the consideration of the need for system robustness and compatibility with established protocols. However, to integrate sensor and communication technologies seamlessly in terms of device structure and operation mechanisms, as well as to exploit the state-of-the-art advances in both areas,502,658 teamwork between sensor developers and wireless technology engineers will be greatly beneficial. In this way, both sensor and communication technologies can be tailored to suit each other’s need for potential synergistic effects; systems can be designed from the bottom up to achieve high levels of integration, and additional merits or functionalities of wireless communication can be discovered. Such advances would likely impact other areas of data networking.
On the basis of reliable implementation of wireless communication on a single sensor node or multiple sensor nodes,293,794,829,830 the next step is to expand to networks, where dozens of sensor nodes communicate with each other and with gateways and servers (Figure 13). More complex network and data management problems will emerge in this endeavor, requiring cutting-edge solutions developed for conventional sensors (e.g., wireless technologies in IoT and WBAN), and tailor-made solutions for flexible sensor networks.
Figure 13.
Outlook for flexible sensor networks. Black arrows denote connectivity. A group of sensor nodes can communicate within themselves and may be accessed by more than one cloud server. Due to dense connection, the two networks merge into one. Ultimately, numerous sensor nodes may be connected in a ‘meganetwork’ involving more gateways and servers (dot-outlined shapes). Cloud computing and fog computing will be instrumental in the realization of extensive sensor connectivity. Images for “imperceptible form factors”: Adapted with permission from ref 829. Copyright 2019 Springer Nature. Adapted with permission from ref 658. Copyright 2021 Springer Nature. Adapted with permission from ref 298. Copyright 2021 Springer Nature.
Data storage is essential to support connectivity. Data can be stored in local memories at sensor nodes or transferred to and stored in centralized data centers. The first strategy will benefit fast data communication but requires either heterogeneous integration of rigid memories or flexible memory devices, which are still in the early stages of research.783,846,847 The second strategy is suitable for long-term, robust storage of large datasets. However, the escalating energy demand of data centers is a pressing issue to address.848,849 Meanwhile, algorithms that compress data size should be implemented near sensors and/or in the cloud. Overall, high-density and low-power memory devices are critical to support the expanding sensor adoption.850
LAB TO END-USER
Flexible sensors will have societal impact only when they go out of laboratories. The route from lab to end-user is thorny because the market for flexible sensors is complex—they are not a single type of product nor are they being proposed for a single application; products and processes are at varying stages of technology readiness; manufacturing flexible sensing systems involves many players spanning various value chains; the software, data, and customer service associated with sensing technology require long-term sustainable management.
Here, we identify four challenges along the translation path (Figure 14). First, killer applications in which flexible sensors are likely to be the dominant technology should be identified so that research and development can be focused and efficient. Second, effective design and fabrication strategies are needed to facilitate rapid prototyping, where critical modifications to the systems can be made for greater usability and reliability in real-world settings. Third, going from lab/prototype-scale to industrial-scale manufacturing is essential for mass deployment. Production automation and fundamental process understanding are crucial. Fourth, regulatory strategies targeting issues arising from unconventional use cases deserve early attention, and companies should ensure they comply with the regulations.
Figure 14.
Key issues to address during the translation of flexible sensors from labs to end-users. Images under “Customers and competitors” designed by Freepik. Images under “Design” adapted with permission from ref 628. Copyright 2020 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim. Adapted with permission from ref 851. Copyright 2022 Wiley-VCH GmbH. Images under “Fabrication” adapted with permission from ref 852. Copyright 2020 Springer Nature. Adapted with permission from ref 853. Copyright 2021 American Association for the Advancement of Science. Images under “Modular system” adapted with permission from ref 854. Copyright 2019 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim. Adapted with permission from ref 84. Copyright 2016 Springer Nature. Images under “Printing” adapted with permission from ref 855. Copyright 2018 American Chemical Society. Adapted with permission from ref 397. Copyright 2021 American Chemical Society. Images under “Polymers & nanomaterials” adapted with permission from ref 837. Copyright 2021 American Association for the Advancement of Science. Adapted with permission from ref 856. Copyright 2021 Springer Nature. Images under “System integration” adapted with permission from ref 276. Copyright 2022 American Association for the Advancement of Science. Adapted with permission from ref 290. Copyright 2018 Springer Nature. Images under “Data security & privacy” and “Quality, safety & sustainability” designed by Freepik.
Killer Applications.
Flexible sensors are expected to revolutionize many fields because of the capability of continuously and wirelessly reporting the physicochemical status of irregularly shaped and dynamically deforming objects. A survey of research over the past decade has revealed the potential use of flexible sensors in many fields beyond healthcare, such as interactive teaching and surgical modeling, expression and creation (music, visual arts, textiles, and fashion), robotics, prosthetics, brain-computer interfaces, urban planning, buildings and infrastructure, agriculture and veterinary care, climate, renewable energy, and ocean and space exploration.857 Which of these potential applications demonstrated in labs are worth the translational effort remains a tough yet critical question. Not all flexible sensor technologies are equal and distinguishing between hype and reality is an increasing challenge for stakeholders. The following principles provide useful guidance to avoid common pitfalls.
First, the sensor technology should solve real and prevalent problems (i.e., ‘important problems’), which will determine the existence and scale of potential markets. This would require a mindset shift from ‘a solution looking for a problem’ to ‘a problem looking for a solution’. Decades of research have built up versatile toolkits for flexible sensor design and manufacture, which should be leveraged to engineer practical solutions to real-life problems. However, many researchers continue blindly improving a single or few performance metrics of flexible sensors while ignoring the specificity of signals to be measured. For example, existing flexible pulse sensors858 rarely take account of the comprehensive requirements of sensor performance. To detect a clear, undistorted pulse waveform, the sensor must have high sensitivity and good linearity within the measurement range under a specific preload (20–100 kPa)859 and in the frequency band of 0.1–20 Hz.860
To identify ‘important problems’, researchers should be guided by a combination of empathy and statistics, to seek out high-granularity problems that cause intense human suffering. Looking at the lists of the top 100 causes of hospitalization, the top 100 crop-killing parasites, the top sources of foodborne illness, etc. can help determine whether the sensor technology can be applied to a problem that is not being well addressed and if addressed would greatly improve life. For example, according to the World Health Organization, among the leading causes of global death in 2019 are cardiovascular, respiratory, and neonatal conditions; ischaemic heart disease, dementia, and diabetes are the fastest growing conditions and are among the top 10 causes—chronic health conditions are plaguing the aging population. Therefore, long-term daily health management could be an important application space for flexible sensors.10,39 Similar guides can give a sense of the magnitude of environmental or industrial problems.
Second, deeper understanding of the problems, markets, and potential customers requires close discussions between sensor developers and end users.861 Design thinking would be helpful in this process,862-864 which entails that sensor designers should put themselves in the shoes of the intended users and try to understand the genuine constraints of a particular problem. Specifically, it is critical to speak to many potential customers and understand whether they will certainly purchase the hypothetical product in large amounts once it is available. This sort of inquiry is critical to the success and direction of any new enterprise. In general, academic startups can be at risk because few scientists are trained in the techniques of customer validation. Many academic labs spend years developing viable technologies and then spend years chasing nonexistent business opportunities with those technologies. Small commercialization grants with strict research deliverables can become a distraction if they lead the scientists-turned-entrepreneurs away from finding customers and generating revenue. The tension between the academic instinct to raise technology readiness levels (TRLs) and the commercial need to find customers can lead to the undoing of startup companies.
Taking healthcare sensors as an example, identifying unmet clinical needs requires partnerships, or at least conversations, between researchers and physicians/clinicians. One recent example is that through collaboration with neurosurgeons, researchers identified that current neurostimulators cannot accommodate tissue growth in pediatric populations.579 To solve this problem, the researchers developed a morphing electronic stimulator, which can expand together with growing tissues for chronic neuromodulation. Another approach is to identify real-world medical problems by focusing on a specific disease. Some diseases might be readily monitored or treated via flexible sensors with application-specific designs. One successful example is the prevention of pressure injuries through continuous monitoring of pressure and temperature at multiple body locations for patients confined to beds.865 Beyond developing soft, skin-mountable flexible sensors, a few technical advances, such as battery-free and wireless designs and multiplesite measurements, were crafted to solve problems encountered in operating hospital settings.
It is critical to determine market sizes for new technologies. This can be done by multiplying the number of probable customers by the expected price of the product and the expected quantity of the product they will purchase. When imagining a customer for a hypothetical product, it is important to understand the spending power of that customer, and how well the product would compete with other priorities that the customer may have. Furthermore, it is important to assess whether a hypothetical product will solve an urgent need for a customer. Many potential sensor applications could be described as convenient, or nice to have, but they do not have the urgency necessary to drive sales. When market size estimates are made cautiously, rather than optimistically, they can be useful in assessing the viability of business opportunities.
The third consideration is competitiveness against conventional sensor technology. Modern electronics has advanced in both performance and cost. In many applications, miniaturized rigid sensors are strong competitors to flexible sensors. It is therefore important to develop flexible sensors that bridge the gap between conventional MEMS sensors and targeted applications and that offer distinct value propositions over incumbent technologies. One area where flexible sensors are strong players is human-centric applications, where sensors are worn on the body surface, penetrate the skin, or are implanted deep inside tissues. These human-centric applications could branch into many subcategories, such as daily health/fitness monitoring, athletic performance analysis, point-of-care diagnostics, remote patient monitoring, vital sign monitoring in hospitals, soft robotics, (neuro)prosthetics, extended reality,866 and the metaverse. Soft and stretchable skin electronics monitoring vital signs for neonatal intensive care502,867 are a promising area. Because of the small body size and sensitive skin of newborns, attaching multiple bulky electronic monitors around their bodies causes much discomfort and even damage, and prevents skin contact with parents and easy caregiving. In such cases, soft wireless sensors offer obvious advantages over conventional monitoring devices. This technology has led to a spin-off company, Sibel Health, which recently received FDA 510(k) clearance. Very low cost per unit sensors (most probably realized by printed sensors) are likely to have a strong presence in ubiquitous sensing, such as food spoilage detection in packaging,45,868 temperature mapping in industrial and electronic applications, building and equipment-integrated sensing for preventative maintenance, and hazardous gas detection in military, industrial, and public settings.
The fourth prerequisite for any translational effort is that the sensor technology developed in labs must demonstrate an adequate TRL of at least 6. Most reported flexible sensors are around TRL 4 or below, where proofs-of-concept are demonstrated in laboratory conditions.869 Reaching a TRL of 6 entails “prototype demonstration in a relevant operational environment”,869 meaning that the sensor technology should work properly under real-world conditions, including when subjected to unpredictable handling by users. However, moving from well-controlled laboratory settings to uncontrolled practical settings is the hardest step in technology translation, and so TRL 4 to TRL 6 is often referred to as the “valley of death”, where many promising technologies fail. In this regard, packaging is critically important to successful sensor deployment in real-world scenarios. We need to face the reality that most academic labs do not have the necessary resources or motivation to work on high TRL technologies. Instead, institutions with focused resources dedicated to translational work may be more productive. Imec870 and InnovationLab871 are good examples, bridging the gap between academic research and industrial applications. Existing infrastructure in manufacturing facilities, supply chains, peripheral services, trained workforces, policies, and legislation all impact the feasibility of technology translation.872
Rapid Prototyping.
After the initial demonstration of proof-of-concept sensor systems, modifications need to be made to satisfy the practical requirements in real-world settings, and a large amount of data might need to be collected to define the final product. This step requires fast prototyping, i.e., producing customized devices over a short period of time and in large quantities. During this prototyping stage, integration strategy and the coordination between components and subsystems are critical to system performance.869 Sometimes, the individual components may not be cutting-edge technologies, but proper integration can present a system with advanced functions, where stability is the key.806 Note that a complete set of solutions, from hardware to software, from device to data, should be prototyped holistically for a particular real-world scenario,63,502,661,794,873,874 because data without interpretation are of minimal use.
Effective design tools can help expedite prototyping and break the limitation of lack of access to fabrication facilities. This primarily relies on electronic computer-aided design (ECAD), also known as electronic design automation (EDA), to construct a virtual version of the designed device, to simulate, to analyze, and to verify the performance, and to generate essential process parameters for manufacturing. Mechanical simulation such as finite element analysis (FEA) is frequently used and has proven helpful for mechanics optimization in stretchable systems.875 Molecular dynamics simulations facilitate the understanding and design of functional polymers372,876,877 and nanomaterials.878 Numerical simulations also help with microfluidics optimization in wearable biosensors.228 Such electronic design processes allow for more cost-effective, systemic, and logical optimization, yet they require a solid understanding of the processes happening across materials and interfaces. Moreover, design is a holistic process involving materials science, mechanical engineering, electrical engineering, etc., demanding a collective effort from multidisciplinary teams.
Despite the convenience offered by EDA tools, current design processes still rely on human proposal and manual input, resulting in tedious iteration and slow optimization. Machine learning may be effective to expedite complex design tasks. For example, FEA-trained neural networks could propose the correct 2D design for 3D shapes,851 which are relevant to 3D conformal sensors.585-587 Although there are ongoing efforts on machine-learning-enabled flexible systems optimization focusing on mechanical879 and electrical880,881 performance,882 many other performance metrics and manufacturing parameters need to be considered. The ultimate goal is the incorporation of electrical, mechanical, thermal, power, and other features into one design package, where AI self-proposes several designs and fabrication procedures with different trade-off considerations. Nevertheless, empirical validation should never be overlooked.
Following design is fabrication. A number of fabrication strategies for rapid prototyping have been proposed, such as printing, vinyl cutting, and laser patterning. These fabrication processes generally share the features of digital control, customizability, simplicity, low-cost equipment, moderate scalability, and short production time. For example, inkjet printing of conductive inks, extrusion of insulating pastes, plus in situ plasma-activation of electrode surfaces produced implantable neuromuscular electrodes that can be customized to fit different tissue sizes and shapes.852 3D printing is also a good option for prototyping. There are already commercial 3D printers tailored for soft and stretchable electronics (e.g., NOVA by Voltera). Notable advances were recently made on 3D-printed sensors and electronics,883-888 such as fully 3D-printed photodetector arrays889 and microelectrode glucose biosensors.92 By printing different materials monolithically, device robustness is generally improved over made-and-assembly devices. Vinyl cutters are handy equipment to fabricate 2D structures in stretchable devices, offering significant savings in time and cost compared to photolithography.890 Although the minimal feature size attainable is relatively large (~250 μm), this scale is sufficient for skin electronics. Combined with multiple processing and transferring steps, “cut-solder-paste” fabrication was used to produce modular and reconfigurable electronic tattoos within 2 h.854 This cutting method applies to most metals,891 as well as graphene.391,392 Lasers can be used in many ways for prototyping. For example, laser scribing is used to pattern functional materials such as graphene on polymer substrates,182 and ongoing efforts focus on expanding the materials library that can be processed by this method.892 Laser patterning of absorbent substrates has been used to define the traces for aqueous MXene ink deposition.853 Lasers have also been used to tune the sensitivity of strain sensors through substrate micropatterning.893 A common limitation of the above methods is that these processes primarily deal with patterning of conductive materials, and the customized sensors are therefore mainly electrodes, except for 3D printing, which can fabricate more complex sensors. Expanding processing capabilities to cover wider ranges of materials and devices would facilitate prototyping of flexible sensors with greater functionality in various form factors.
At system levels, modular design854,894,895 offers more customizability, reduces design cost, and improves development efficiency, in analogy to the chiplet technology for rigid ICs.896 In this LEGO-like integration paradigm, compatibility between modules becomes critical. In particular, circuit design is of paramount importance for smooth information and power flow across modules. Simple circuits near sensors for signal preprocessing to standardize the data format enable modular sensing integration, without the need for changing the central control unit during customization (e.g., microcontrollers).897,898 Additionally, such circuits can be fabricated via low-cost solution processes together with sensors, offering greater convenience during prototyping.
A prototyping platform with industrially relevant facilities for both design and manufacture allows sensor developers to test their designs on larger scales and to collect more data for design refinement. This would be extremely helpful in crossing the “valley of death”. Nevertheless, building such an infrastructural and technological platform is a daunting task, calling for large investments as well as participation from industry and academia, across the entire value chain. Governments will need to play leading roles in this endeavor.
Scalable Manufacturing.
Successful prototyping does not guarantee market launch. The next important step is to move from prototypes to samples that are manufactured in production lines. Scalable manufacturing entails the ability to produce devices in large quantities with consistent performance at reasonable costs. Throughput of lab-scale production is minuscule compared to industrial manufacture27 and lab-produced sensors usually suffer from large device-to-device variation, impairing the reproducible and reliable sensor performance required in commercial products. Moreover, during manufacture translation, sensor and system performance should not be affected significantly although certain compromise might be needed.
A general guideline for scalable manufacture is that manufacturing processes should be largely, if not fully, automated, with detailed in-depth study of process parameters. Instead of reinventing the wheel, utilizing existing industrial manufacturing facilities with slight modification is more viable to large-scale fabrication of flexible sensors. Such facilities, based on current knowledge, include those used for microelectronics, MEMS, displays, textiles, polymer processing, and paper printing. A few examples implementing this tactic are stretchable circuits by transfer printing of microfabricated silicon nanoribbon-based circuits,377 silicon microneedle arrays on flexible substrates by dual-side lithographic microfabrication,618 and large-area pressure sensors made via a track-etch membrane template.899 Printed electronics, in particular, are at the confluence of electronic materials processing and traditional printing on paper or textiles.
Printed electronics technology enables fabrication of largescale, low-cost electronic devices on a variety of substrates (e.g., paper, plastics, textiles, leather, wood, metals, ceramics, glass), instrumental in the development and manufacture of flexible sensors. It presents major cost and scalability advantages over conventional MEMS sensors, enabling many killer applications. Moreover, printing offers a promising strategy for monolithic integration of various devices onto flexible substrates.28 A wide variety of printing techniques are available and have been extensively reviewed.28,900 Nanomaterials-based printing techniques, formulation of printable inks, post-printing treatment, and integration of functional devices are important topics and have recently progressed substantially.900-902 Fully printed sensors,874 displays,903 energy devices,680,904 memory devices,905 transistors and circuits,198,397 and integrated systems906 have been demonstrated.28,44,679,900,907 Nevertheless, printed devices are limited in feature resolution (1–100 μm) and device consistency. While targeting low-performance (and low-cost) applications can circumvent these limitations, crafting solutions to improve processing quality is essential to expand the application space of printed sensors.907 Two specific areas facing considerable challenges are: tailoring ink compositions to a specific printing technique while keeping the printing process low-cost and high-speed, and obtaining decent electrical conductivity at sufficiently low temperatures to prevent degradation and damage of plastic substrates or biological tissues.
Polymer-based functional materials possess advanced mechanical (e.g., stiffness variability)419,551 and biological (e.g., self-healing) 424 functionalities while preserving adequate sensing performance. They are the enablers of next-generation flexible sensors including intrinsically stretchable sensors and soft sensors for imperceptible skin electronics and implantable bioelectronics.297,908 Moreover, their solution processability and potential recyclability909 promise reduced environmental impact compared to inorganic-based electronics. However, scalable and high-quality manufacturing is a bottleneck for real-world deployment of polymer-based sensors. This impediment is because the processing of recently developed functional polymers (often with lower modulus and/or higher stretchability) is generally incompatible with electronics microfabrication, leading to difficult device integration.
Poor stability of polymers in harsh processing conditions is a major source of manufacturing incompatibility. For example, thermal expansion of polymers, especially stretchable ones, needs to be managed for high-resolution devices. Otherwise, different layers do not match well, leading to low device yield and consistency. Another problem is the poor chemical orthogonality between organic photoresist and organic electronic materials. Complex processing steps and additional material layers are needed to circumvent this issue,353,547,910 leading to low device uniformity and resolution (feature size >100 μm). A monolithic microlithography technique was recently reported to overcome this challenge,837 where UV light directly wrote micropatterns into electronic layers. The key to this technique lies in stretchable polymers with both electronic functionality (conductor, semiconductor, insulator, and dielectric) and UV-crosslinkability. The technique achieved record high device density for stretchable arrays (42,000 transistors per square centimeter) and small feature sizes (2-μm channel length) and enabled wafer-scale fabrication of ICs with high device yield, 98.5%. Future research should employ similar polymer design principles to expand the materials libraries that can be processed using this technique.911 Overall, developing materials with better tolerance to processing conditions is critical to high-quality manufacture of polymer-based sensors.
Nanomaterials integration in flexible sensing systems presents another manufacturing challenge.912 Large-scale production with consistent materials properties from batch to batch is a prerequisite for industrial applications but is currently unavailable for most nanomaterials. Furthermore, defect-free processing and precise patterning are critical for sensors that require high-quality nanomaterials.272,913-916 In particular, for 2D materials,917 the interfaces with substrates, metal contacts,856,918 and dielectrics should be cleanly controlled, as the properties of atomically thin 2D materials are greatly affected by the quality of their surfaces and interfaces. Moreover, integration processes that are compatible with current MEMS fabrication should be developed. Alternatively, formulating printable inks is another route to apply nanomaterials at scale.680,900
The last manufacturing challenge lies in system integration. At present, heterogeneous integration or flexible hybrid integration is probably the most feasible, scalable, and cost-effective option for building advanced sensing systems.28 Miniaturized microelectronic devices based on silicon technology are mass-produced with high performance, while organic or nanomaterial-based devices can meet requirements like large area and flexibility. Integrating these two kinds of devices takes advantages of the two approaches in both performance and manufacture. Importantly, the integration process can be achieved by adaptation of existing equipment and processes used in electronic packaging, polymer processing, and textile industries.
Soft-hard interfaces, interconnections, and packaging are major challenges in integrating devices with great variations in sizes and mechanical properties on a single thin-film or fibrous substrate. We lack effective strategies addressing these issues to ensure system reliability. A feasible route is to increase the level of integration in such heterogeneous systems towards monolithic integration. Instead of manufacturing individual components separately and assembling them on a common substrate, most system components could be manufactured monolithically on a single platform, with the addition of a few separately manufactured parts. For example, a sensor system might be entirely manufactured on a printing platform, but with pick-and-place mounting of a few silicon chips for essential needs.28 This strategy requires the printing platform to be extremely versatile to handle multiple types of materials with sometimes incompatible processing conditions, which is a major challenge in printed systems. Such monolithic integration currently costs considerably more than heterogeneous integration. Using fewer materials to realize more functions is a rational strategy to alleviate this issue.444
Scientific and engineering development being one aspect, financial and infrastructural supports are pivotal in tapping the full potential of research outcomes. Currently, a large fraction of funding for sensor research is directed towards exploratory endeavors, but the development of production equipment that could later be used to generate revenue tends to be excluded from funding. Such funding often puts startups in difficult positions because all of their time and energy is dedicated to tasks that cannot generate revenue. The only path to revenue for most companies is to make large and reliable batches of their products. Therefore, to jumpstart the field of flexible sensors, a large amount of funding should be earmarked for the development of high-risk but potentially high-reward manufacturing capabilities. Moreover, academic institutions would be wise to nurture laboratories that invest heavily in developing the next generation of automated production equipment.
New materials and manufacturing technologies require years of development prior to commercialization. It took the semiconductor industry decades to evolve to a high-speed high-performance mass manufacturing technology.79 Luckily, flexible sensors have the existing semiconductor industry and other related sectors as stepping stones. We might not be too far from their large-scale manufacturing.
Regulatory Frameworks.
It is essential for any technology to be legally approved before its market launch. This gives consumers assurance and fosters trust in the products. As flexible sensors collect massive data and are often used in close proximity to humans, explicit regulations and restrictions need to be in place to ensure user safety, data privacy, and proper handling of ethics issues. Physical safety of sensor devices should be thoroughly examined in terms of toxic substance, biocompatibility, electrical safety, mechanical safety, etc.,537 especially for devices employing unconventional materials and/or designs and deployed close to humans. Regulations for healthcare and medical sensors are more stringent due to critical health consequences associated.10,537 Therefore, it is important to receive feedback from regulatory bodies such as FDA early when designing sensors for medical use.520 Special safety concerns for bio-interfacing devices, beyond the biocompatible considerations outlined above, should be addressed. For example, accidental ingestion is a big risk for small, wireless monitoring devices used on infants.919 Catastrophic battery failure or circuit overloading could lead to injurious thermal loads on the skin.920 Repeated application/removal of skin-interfaced devices can lead to skin injuries in vulnerable populations.568 Furthermore, there should also be data privacy regulations to prevent data breach and misuse. As flexible sensors enable many unconventional use cases, regulatory frameworks will need time to refine and to suit different products, and there will likely be disputes and ambiguities in the early deployment stages. In addition, for healthcare sensors, reimbursement coverage by governments and/or insurance companies will be crucial for widespread adoption.
Since flexible sensors manufacturing involves materials and processes, regulations in manufacturing should also be considered with the aims of quality assurance, worker safety, and environmental protection. Quality management systems and standard operating procedures should be set up and documented properly. Equipment, facilities, and processes should be qualified and validated in terms of reliability. All these manufacturing compliances should be audited periodically. Safe work practices with nanomaterials and organic materials during production, storage, transportation, and processing should be determined and codified. This process entails systematic examination of potential health and environmental risks posed by newly introduced materials and processes and coming up with proper handling protocols.921
Although complying with all related regulations may restrict and retard the broader deployment of flexible sensors, it is worth the effort for a sustainable growth of the sector. Regulatory considerations should be factored in as early as possible in the design process to avoid repetitive and costly adjustments at later stages.921
In addition to challenges in market identification, prototyping, manufacturing, and regulations, there are other business considerations along the commercialization path of flexible sensors. For example, how a startup should define the scope of its product is a question. It can be attractive to try to produce a complete sensor product, but it may be better to produce components and sell them to existing sensor manufacturers. When working with large entities that are potential partners, it is critical to understand common conventions of business development. Generally, partnering conversations are cordial and slow-paced, and there is no expectation of over sharing information. It can be disheartening to see that good intentions to help people using new technologies would not make their way to end-users because of poor market entry strategies, small market size, difficult fund raising, improper business models, etc. To maximize the chances of market success, researchers should improve their business awareness.
Case Study.
When considering future commercial applications of flexible sensors, it can be helpful to study successful devices on the market as well as their failed predecessors. Although not a flexible sensor technology yet, continuous glucose monitoring (CGM), which uses wearable sensors to assess glucose levels continuously, resonates with the idea of using flexible sensors on the body for health monitoring. Since its conception in the early 2000s, CGM has emerged as a flagship technology for biosensing and has started to revolutionize diabetic care.922 With a large worldwide prevalence of 540 million people in 2021923 and a health expenditure of $760 billion in 2019,924 diabetes remains a prime target for innovative sensing technologies. The CGM offers advantages in continuous monitoring, increased comfort, and more convenience than conventional finger prick tests. Major CGM manufacturers, Abbott, Dexcom, and Medtronic, all utilize subdermally implanted sensors to monitor interstitial glucose levels. The strong correlation between glucose levels in blood and readily accessible interstitial fluid has been pivotal to the commercialization of CGM devices. This highlights the potential of detecting well-known analytes in minimally invasive biofluids (e.g., sweat, tears) and the importance of establishing strong correlations with gold-standard bio-samples (e.g., blood, cerebrospinal fluid). Patient adoption of CGM devices has been rapidly expanding, as evidenced by a 237% revenue increase of Dexcom between 2018–2021 and a forecasted global CGM market of $15 billion by 2032.925 The success of CGMs has also led to the idea of assigning glucotypes in non-diabetic individuals,926 in which people can track their glycemic responses to get more precise ideas of their predisposition to diabetes. Companies such as Levels Health and Nutrisense now sell CGMs with related software and information to those interested in monitoring their glucose responses in the context of weight loss, athletic performance, and improved general wellbeing. The commercial integration of CGM devices with autonomous insulin delivery pumps also represents a key innovation in the field of closed-loop therapeutics.
In addition to the success stories, understanding where certain technologies fail can be just as insightful. The first noninvasive (wearable and not subdermal) glucose meter to receive FDA approval was the GlucoWatch by Cygnus, Inc., in 2001. Instead of relying on an implanted sensor, the device used reverse iontophoresis, in which mild electric currents are applied on the skin to extract interstitial glucose. However, due to a combination of regulatory and technical setbacks, the device was removed from the market in 2007. Regarding regulatory hurdles, after FDA approval but without established insurance reimbursement codes, the high cost of the device limited its use. This highlights the importance of understanding not just regulatory pathways, but also insurance and healthcare reimbursement processes. On the technical side, some users reported discomfort after a few days of use, likely due to the current densities required for glucose extraction. The intellectual property was eventually purchased by Johnson & Johnson, and substantial research efforts continue to address the technical limitations of noninvasive biofluid sampling.
Within the flexible sensor sector, integrated skin patches for vital sign monitoring are an active area where many startups have emerged in recent years, such as MC10, iRhythm, VitalConnect, Chero, LifeSignals, BioIntelliSense, Sibel Health, and Sonica. These sensors are designed to generate medical grade data for clinical decision-making to improve in-patient experience or shift in-patient care to homes through remote monitoring. The market entry strategy is mainly through hospitals and healthcare providers, where insurance reimbursement can strongly affect patient adoption. Although some sensors from these companies have received FDA approval for medical use, the real clinical benefits, and thus the market, still await large-scale validation. To this end, many startups have been partnering with medical researchers and companies to conduct clinical trials, first to help simplify the trial process and second to test patient satisfaction and clinical outcomes when dealing with specific medical conditions. Likewise, MC10, a pioneer in skin-compliant multimodal vital sign monitors, was recently acquired by Medidata, who conducts clinical trials through its Sensor Cloud platform. Some companies have been experiencing rapid growth in sales since the COVID-19 pandemic, largely driven by the strong push for telemedicine in the healthcare industry. This accelerated digitalization is likely to continue providing strong momentum for remote patient monitoring technologies.
A few lessons can be gleaned from these early flexible sensor startups. First, most of the commercialized sensors use incumbent semiconductor technology to manufacture their key components, and it is the packaging that gives rise to mechanical compliance (e.g., thin-film plastic substrates, silicone encapsulation). This highlights the pivotal role of packaging strategies. Second, medical validation is a lengthy process that involves multiple parties and a large population of study subjects. Extensive collaboration is therefore critical to startups developing healthcare sensing technologies. Third, most companies offer not only sensor hardware, but also data analysis capabilities, for instance, to diagnose a certain disease from sensor signal patterns. Although well-engineered flexible sensors can generate high-quality data, their real value depends on the usefulness of these data. Therefore, demonstrating the benefits of leveraging sensor data over conventional decision-making practices is important for any sensor technology to move towards commercialization.
In all, given the large diversity of potential applications of flexible sensors, the pathway from lab to end-user is equally diverse. Customized strategies should be devised for each use case. En route to ubiquitous adoption, many issues outside the domains of science and engineering will emerge.927
OUTLOOK
We have discussed the challenges in sensor and system performance and the issues along the way to commercialization. For long-term sustainable growth of flexible sensing technologies, several issues need to be factored in to guide research and development efforts, including environmental sustainability, industrial standardization, user engagement, and deployment to larger and diverse populations.
Sustainable Manufacturing and Devices.
Flexible sensors are meant to be mass-deployable technologies applied in many aspects of life. An accompanying problem is the environmental impact such a prevalent technology may produce.29 Electronic waste and semiconductor manufacturing have put a huge burden on our planet. Flexible sensors should at a minimum not exacerbate the problem, and even better if they can ameliorate it.
Hazardous, resource-intensive, and environment-damaging materials sourcing practices such as mining should be minimized as much as possible. Carbon as an abundant element, compared to many metals (e.g., precious metals and rare earth metals), is a promising ecofriendly alternative as a functional material. Different forms of carbon (carbon black, graphite, graphene, graphene oxide, reduced graphene oxide, single-walled and multi-walled carbon nanotubes with or without functional modifications, and many more) via various production and processing techniques can cover a wide spectrum of properties, including insulating, semiconducting or conducting,928 mechanical flexibility or rigidity, electrochemical activity, etc. Toxic elements should also be avoided (e.g., developing lead-free perovskites) to minimize environmental and biological damage after product disposal.
Manufacturing of flexible sensors should adopt a low-carbon approach, such as additive manufacturing, solution processing, and low-temperature processing. Printed electronics often follow these principles and are therefore more environmentally benign than conventional microelectronics towards large-scale flexible sensor deployment. There is strong industrial interest in replacing PCBs, which are made using energy-demanding processes and harsh chemicals, with hybrid printed electronics (e.g., the ECOTRON project by TNO at the Holst Centre929). In addition, sustainable manufacturing can be facilitated by industrial IoT and data analytics. For example, using sensor technology to detect leaks and improper material usage can help minimize waste. Digital data analysis can help remove superfluous steps and illustrate where to focus efforts to eliminate excess material and energy consumption. Such digital strategies towards sustainable manufacturing are being explored by many well-known brands, such as Apple, Microsoft, and Amazon.930
End-of-life disposal of used devices should be responsibly dealt with from the initial design stage. If the device is designed to be a long-term functional product, materials and systems durability are priorities, so that the product lifetime can be extended. If the device is designed to be disposable, the materials and device structure should allow easy recycling or impose minimal environmental impact when disposed of in landfills. Electronics recycling is not an easy task due to the highly mixed nature of materials in downscaling to nanostructures, use of nanocomposites and nanoinks, etc. Therefore, there is a pressing need for effective recycling strategies of electronic waste. A few examples of recent progress in this area909,931-933 include aqueously recyclable circuits,934 recyclable and reconfigurable hybrid integrated sensor patches,935 and upcycling of compact discs for stretchable biosensors.936 Nanomaterials manufactured bottom-up show promising recycling potential when the polymer substrate/matrix and the working solution are properly paired.937,938 A more advanced concept over recycling is disassembly for remanufacturing,939,940 which may be explored in the future. Besides recycling, another strategy is to synthesize or to utilize degradable materials to minimize the impact of disposal (e.g., gelatin-based all degradable on-skin sensor patches,941 degradable pressure sensors based on MXene/tissue paper942). The use of biological or biologically derived materials (e.g., silk,372,943,944 pollen,945,946 wood,947 leather,948-950 biosynthesis by bacteria951) in flexible sensors is a budding field and might be the ultimate solution to ‘environmental imperceptibility’.952 Still in their infancy, biological materials-based devices are rather primitive with unsatisfying performance, stability, and reproducibility. Overall, life-cycle analysis is necessary to get a full picture of the environmental impact of a certain material or device.
Lastly, policies and legislation are critical to overcome the inertia in traditional manufacturing and end-of-life practices. Since many flexible sensors are manufactured using cheap plastics, they tend to be single-use and disposable without sufficient long-term stability. The economics alone will not encourage sustainable handling of such devices, as the cost of recycling will outweigh that of manufacturing new devices. In this case, governmental intervention through policies and legislation such as carbon taxes will help shift the balance and encourage responsible sensor manufacture and disposal. In the long run, research on converting waste plastics into high-value materials or products953 would drive a sustainable ‘circular sensor economy’.11
Industrial Standardization.
As sensor systems become more complex, involving a multiplicity of disciplines and technologies, standardization becomes more important than ever. Standardization is needed in terminology and nomenclature, materials specification and characterization, device/component specification, sensor/system performance evaluation, software interfaces, communication protocols, data management, etc. Among these aspects, materials specification and sensor/system performance evaluation deserve special attention.
Specifications in materials properties are crucial for downstream materials processing and device fabrication—they directly affect the quality of produced parts. Nanomaterials properties are sensitive to minute structural alterations. Hence, standardized descriptions of nanomaterials morphology is key to their ubiquitous adoption.954 For instance, a Technical Committee has been assembled by ISO to establish base values for 19 measurable characteristics (e.g., lateral size, number of layers, oxygen content) of graphene materials. Moreover, standard testing methods are of paramount importance to fair reporting nanomaterials’ intrinsic properties633 because the high surface activity of nanomaterials makes them prone to influence from the testing environment and interfacing materials.914 Strain coupling into materials’ performance adds another dimension of testing complexity. For example, when characterizing the conductivity of stretchable conductors (and mobility of stretchable semiconductors), the interfacial contact with rigid electrodes (and rigid dielectrics for semiconductors) during stretching should be well maintained to eliminate any interfacial effects that can distort the true properties of materials under test. However, standardized materials specifications are hard to achieve in newly developed materials, due to a lack of batch-to-batch consistency in production. Many of the newer elastomer products have yet to develop sufficiently to have standard and reliable synthesis protocols. In addition, since stretchable electronics are in early stages of development, many of the commercial elastomers are not produced in high volumes, so there has been little incentive to develop highly standardized synthesis methods. The same problem exists for nanomaterials, whose unreliable production hinders standardization in material specifications.
As flexible sensors expand the functionality of incumbent sensor technologies, many new performance metrics need to be well defined and tested using explicitly specified protocols for fair comparison between devices and products.648 For example, how permeability tests should be done for ‘breathable’ sensors, what parameter should be used to quantify breathability, and how much of this parameter indicates safe usage for skin? There are contradictory claims on the permeability of PDMS (positive claims in refs 60 and 955 and negative claims in ref 602), which causes much confusion when selecting the encapsulation material for on-skin sensors. This discrepancy likely originates from non-standardized testing methods (sample thickness, temperature, humidity at both sides of material, etc.),956 and the variability in intended application scenarios (skin position, skin sensitivity of subjects, etc.). Given that the insensible perspiration rate and exercise perspiration rate can differ by one or two orders of magnitude,580,602 and the water permeability of normal skin is between 240 to 1,920 g m−2 day−1,60 a ‘safe’ range and a ‘comfortable’ range of water permeability should be defined accordingly, and the same applies for gas permeability. Likewise, there is an urgent need to define the performance metrics of other descriptive terms, such as stretchable, adhesive, conformal, and to specify the best benchmarking practices.957 Moreover, for flexible and stretchable sensor systems, mechanical reliability tests need to be standardized.
Standardization spells out the foundations and best practices for sensor design, manufacturing, and operation, thus facilitating communication and collaboration between various partners in the field to expedite technology maturation. It drives economies of scale and eliminates barriers to trade; it also boosts consumer confidence and promotes sustainable development, thereby fostering sector prosperity in the long run. However, the process of standardization faces many challenges. It usually requires some degree of market penetration, so that industrial players can be involved in the process and real-world issues can be properly addressed. However, most flexible sensor technologies remain in research or in the process of lab-to-market translation; they are not sufficiently mature to put forward clear incentives for standardization, since standardization is a time-consuming and complex process.
User Acceptance and Engagement.
There can be barriers to accepting flexible sensor technologies that interface intimately with the human body. There are also risks of stigmatization, particularly for wearers with certain diseases. One way to address these issues is to conduct comprehensive user studies to identify the optimal body locations to place sensors, with a balance between data collection quality and user preferences.958 Alternatively, developing transparent, ultrathin, and invisible sensors would reduce the risk of stigmatization. Every stakeholder within the field of on-body sensors has a responsibility to address fears, preconceptions, and misconceptions. To this end, researchers can work with designers and artists to package sensors with artistic and fashionable designs. Such convergence can also lead to artwork and exhibitions to improve the public’s awareness of on-body sensors and the importance of health monitoring. Different sociocultural ecosystems must be factored into designs as well. We believe that public opinion will lean towards acceptance as knowledge increases and human-centric applications begin to improve lives.79
There will also be anxieties about privacy and data security.79 To address these concerns, regulatory frameworks and legal structures must be in place, clearly communicated, and strictly enforced. Medical and health data must remain individual property, and their use should require consent. It should be articulated who has access to which portions of the user data, and confusing and vague privacy policies should be avoided. For instance, researchers may use health data with users’ consent for the benefit of society without knowing the identities of the individuals. These principles are to ensure that data are not exploited for discriminative or malicious purposes, such as setting insurance premiums on the basis of health data or selling data for profit. On the technical front, security protocols should be developed to guard against attacks, so as to gain user trust.
Translating data into useful forms rooted in human needs79 and presenting them in a user-friendly way will be essential for long-term user engagement. This issue is often overlooked by sensor developers. For example, in spite of strong sales, around 50% of wearable fitness monitors are abandoned in the first year of use.959,960 This attrition was found to derive from decreased interest in the technology after its novelty wore off, perceived lack of benefit relative to intrusiveness, poor experience with the user interface, and frustration with technical problems.960-963 Therefore, it is critical to make technologies simple and convenient to use with tangible improvements to life. Raw data are of little use for a general user,79 especially when a handful of sensors work cooperatively. Instead, sensor data should prompt action (e.g., by alerting the wearer to the risk of heart attack and suggesting intervention measures)79 or provide insightful analyses of states (e.g., by estimating the remaining service life of a structure or facility). Reward or feedback mechanisms may be included to make sensor use more interactive and interesting. When users find sensors helpful in improving their daily lives, they will tend to appreciate the benefits and thus be more compliant with the instructions and eventually rely on the technology.
For human-centric applications beyond healthcare, such as augmented reality and virtual reality, social-science studies will be crucial to understanding the short- and long-term impacts of these forms of human-machine interactions—how they shift our mindsets and change our behaviors—and to revealing unintended consequences. We must develop flexible sensor technology responsibly, being mindful of its repercussions.79
Equitable Deployment.
Flexible sensors are expected to help solve critical challenges facing humanity, including those in healthcare, aging populations, food security, among many others. The benefits should be shared by both developing and developed countries. In addition, some flexible sensors can be manufactured using less expensive materials and processes than conventional sensors/machines, making them suited for deployment in less developed regions, for instance, to replace bulky high-tech equipment in hospitals for more accessible clinical care.964 There have been promising attempts to deploy flexible sensors in low-resource settings.502,793,867,965 However, the costeffectiveness of these technologies has yet to be examined, which is essential for sustainable deployment in the long run. In particular, medical devices are usually single-use and disposable, which makes them costly if used in large numbers. Making devices reusable is an effective way to normalize costs but is often limited by sterilization and regulatory concerns. To deploy sensor technologies in less developed regions, infrastructural and regulatory support should be taken into account. For example, is wireless infrastructure available and capable of supporting data transmission within the sensor networks? Are power sources readily available for frequent charging? Is there a regulatory body taking charge of the sensor product and are the regulatory frameworks in place for market entry? All these questions need to be answered and integrated into sensor design and translation for wide deployment around the world.
Talent Pipeline.
To sustain the growth of flexible sensing technology, a talent pipeline that continuously provides a qualified workforce is critically important. As sensing is an interdisciplinary subject, conventional curriculum structures and rigid course selection confined within specified majors do little to help prepare engineers and innovators for the future sensor sector. Educational innovation is an acute need and is underway in some institutions. For example, programming for signal processing and data analysis is such an important component of sensing that it should be a core part of graduate training programs in chemistry, biochemistry, and biomedical engineering, to train next-generation chemists, biologists, and engineers working at the interfaces of (bio)chemistry and engineering.
Sensor Intelligence.
Intelligence is the most prominent differentiator of Sensors 4.0 versus previous generations of sensors. Although intelligence is not exclusive to flexible sensors, much research in intelligent sensors and flexible sensors goes hand in hand. Here, we provide a brief overview of the ongoing efforts and future directions for intelligent sensors in general, with special focus on their demonstration and application in flexible sensors.
We envision that future intelligent sensors should possess the following characteristics (Figure 15): (1) fully autonomous (closed-loop) operation from stimuli detection and signal processing, to data analysis and feedback, while maintaining communication with operators/users,563,771,807,966-968 (2) capability to analyze complex sensor signals (multimodal and multiplexed signals, array signals, and sensor networks) to provide accurate and customized analysis of specific situations and to produce actionable feedback, (3) robust performance under non-ideal conditions including tolerance to errors and noise, and adaptability to changing environments, (4) learning capability to refine and improve performance with continued usage, (5) fast response (real-time feedback) with high energy efficiency, and (6) compact, lightweight (and flexible for biointegration) form factors. These features will empower intelligent sensors to solve complex and unstructured real-world problems efficiently and reliably, while requiring less maintenance and management.
Figure 15.
A vision for intelligent sensing systems with advantages listed on the left. Edge computing (including near-sensor and in-sensor computing) and neuromorphic computing are plausible ways of achieving sensor intelligence. They are not mutually exclusive and may be implemented in a single system. AI algorithms will be implemented in both central and edge processors. A feedback mechanism not only informs the user but also completes closed-loop operation. Integration with biological tissues permits two-way communication in various forms (details in Figure 16). Dashed black arrows indicate the direction of data flow. Images of non-neural network ML (machine learning) adapted with permission from ref 156. Copyright 2018 American Chemical Society. Neural network schematic adapted with permission from ref 969. Copyright 2020 Springer Nature. Deep learning schematic reproduced with permission from ref 970. Copyright 2015 Nature Publishing Group. Haptics image adapted with permission from ref 895. Copyright 2022 Springer Nature. Display image adapted with permission from ref 605. Copyright 2021 Springer Nature.
To achieve these ideal characteristics in future intelligent sensors, research efforts are underway in innovating advanced algorithms for data analysis, implementing edge computing to extend sensor capabilities, and inventing neuromorphic hardware for next-generation sensory computing architectures.
Advanced Algorithms.
Conventional algorithms for signal processing and data analyses perform well enough for simple data forms. However, they become inefficient or incapable when datasets become large,10,209 complex,10,661 non-linear,209 high-dimensional,971 erroneous,972,973 or with unclear correlations.390 Therefore, more powerful algorithms, predominantly machine learning (ML) methods have been proposed to overcome these challenges and shown promise in flexible sensor applications, such as sign language recognition,969,974,975 electronic skins,41,976,977 human-machine interfaces,972 and biosensing.224,973 Besides handling challenging data, ML can also be used to compensate for sensor performance deficiency, such as signal noise,410,978 drift, and limited range of detection.979 Additionally, ML allows for the fusion of multiple types of data for more accurate10,499,969 and/or more insightful10 analyses.500 ML can be used to adapt to the dynamic properties of uncertain systems and can support fast and real-time analyses of big data.
Core ML methods for flexible sensors include non-neural network methods (e.g., linear regression, principal component analysis, support vector machines) and neural network methods (e.g., multilayer perceptron, convolutional neural network, recurrent neural network).973 To deal with the increasing amounts and complexity of sensor data, deep learning, a subset of ML, is being developed, which can automatically learn features or rules from raw data with less human intervention than traditional ML algorithms.
Despite the benefits offered by ML algorithms, they suffer from major drawbacks including reliance on training quality, heavy computing burden, and data transfer issues. As ML algorithms rely on big dataset training to refine model accuracy, a reliable source of high-quality training data is important to algorithm performance. However, high-quality training data are not always accessible in sensor applications. Deviations in training datasets from data collected in real-world settings can lead to suboptimal performance in deployment. Furthermore, the implementation of ML algorithms often requires powerful computers, which are not accessible to general sensor users. Hence, cloud computing through online servers is a more viable option, which demands frequent data transfer between sensor nodes and cloud servers. The resultant heavy data traffic puts pressure on high-speed low-latency wireless communications, as well as power supplies on sensor nodes. Energy consumption for algorithm implementation should be considered, given the ballooning carbon footprint of cloud computing data centers.980
Meanwhile, we should be careful in the development and use of ML for sensor data interpretation. The lack of a physics foundation in black-box models hinders interpretation and generalization. Sound and relevant physical, chemical, and biological knowledge should be incorporated in the learning process of ML models with frequent reality checks.981 Theory-driven ML that seeks causality by integrating prior knowledge and big data has shown effectiveness in solving problems in biology,982 climate,983,984 and many other scientific and engineering fields.985-987 Likewise, appropriate sensing theories can be integrated in the loss function, initialization, architecture design, etc. for hybrid modelling of ML algorithms986 used in flexible sensors.
Edge Computing.
Expanding sensor networks will generate huge amounts of data in widely distributed sensor nodes. Conventional processing architectures, where data in the sensor nodes are transferred to centralized processing units for computing and storage, will lead to high energy consumption and large latency.41,988,989 To tackle these problems, edge computing is preferred, where data are processed locally near the sensor nodes. Edge computing distributes computation tasks throughout a sensor network, facilitates quick data analyses, and reduces computational burden on the central processing unit. It can also offer a layer of encryption to sensitive data before transmission.990 Near-sensor computing and in-sensor computing are two available paradigms to implement edge computing.991
Near-Sensor Computing.
The most straightforward way to realize edge computing is to place processing units beside sensors and integrate them on a single platform. Because conventional processing units are based on rigid chips, flexible hybrid integration is the common approach.502 Here, the main challenge lies in reliable integration and manufacture to ensure mechanical stability. In addition, these systems often present low-density and low-level sensory processing capabilities; much effort is therefore needed to improve integration and functional complexity of hybrid sensing systems.266,290,468 Moreover, long distances (from micrometer to millimeter scales) between sensing and processing units are unavoidable due to different manufacturing processes, resulting in long parasitic resistance–capacitance time delays and high-power consumption.991 Emerging integration concepts, such as 3D monolithic integration currently under development for rigid electronics,992-994 should be explored in flexible sensing systems.
To overcome the mechanical and manufacturing incompatibilities between rigid processing units and flexible sensors in flexible hybrid systems, flexible and even stretchable ICs (and memory) are emerging solutions and have seen significant recent progress.326,378,466,469,783,995,996 Processing functionality, including strain compensation,379 signal amplification,400 frequency modulation,997 and sensory adaptation998 have been demonstrated. However, the scale and complexity of flexible ICs need improvement towards elevated signal processing and computing power. In particular, high-performance n-type organic semiconductors should be developed for complementary circuit implementation.999 In addition to conventional interface circuits, emerging computing devices offer opportunities in near-sensor computing.1000 For example, recent work integrated flexible memristor arrays with pressure sensor arrays to deliver ultrafast (400 ns), energy-efficient (1000-fold power reduction) tactile sensory processing.1001
To boost the computational capabilities of near-sensor processing units, state-of-the-art technologies such as on-chip AI processors could be incorporated.996 Compared to conventional AI methods implemented on a cloud, edge AI can realize real-time data fusion and offer lower power consumption. A critical challenge for edge AI implementation is near-sensor model update. Current practice usually requires offline training before being implemented in a near-sensor system.1002,1003 When the training data fail to capture a broad spectrum of conditions in deployment, without prompt model update, classification accuracy of the model will decrease.1004 This issue is particularly problematic for low-power systems operating lightweight algorithms. Algorithm design with balanced training requirements and model performance is necessary, and effective online training approaches should be developed.1005
In-Sensor Computing.
In-sensor computing is a paradigm where computation tasks are executed within sensors at the device or material level, unifying sensing and processing in a single device. Such processing approaches reduce data transfer and data format conversion that occur in physically separate sensing and processing units, realizing ultrafast and energyefficient responsive systems in a simplified architecture.
In-sensor computing can be realized by rational device design with careful materials engineering. Environmental adaptation has been demonstrated using this strategy.1006 For example, a bioinspired organic transistor was designed with two functionally complementary bulk-heterojunctions to realize active adaptation to light intensities.1007 Likewise, a dynamically adaptive vision sensor with both photopic and scotopic adaptation was achieved using a 2D MoS2-based phototransistor array.1008 Processing functions other than environmental adaptation have also been reported. For instance, Mennel and co-workers developed an image sensor that could capture optical information and simultaneously implement an artificial neural network for image processing directly in the sensor.1009 Innovations in device physics and in-depth investigations into energy-matter interactions are needed to discover and to engineer more functionality into in-sensor computing devices.
On the other hand, intelligent matter, implemented by molecular systems and soft materials, has been explored to execute in-sensor computing.1010-1013 Intelligent matter can interact with the environment by receiving and responding to external stimuli, and simultaneously implement data processing and storage at the matter level by adapting structures or internal states. As a source of inspiration, an octopus has more than half of its neurons distributed in its arms. Although mimicking the sophistication of an octopus is incredibly challenging, there are some examples of simple logic being done in soft materials without conventional logic devices.1014-1017 While in-sensor computing using intelligent matter is still in its infancy, it is an area of opportunity for truly intelligent materials that can process information locally and respond appropriately.
Neuromorphic hardware.
Human sensory systems can perform sophisticated functions while being highly energyefficient. They rely largely on the structure and operation of the nervous system including the brain, spinal cord, and peripheral nerves. Capable of emulating aspects of nervous system sophistication and efficiency in sensory information processing, neuromorphic electronics may play important roles in intelligent sensors. However, conventional sensors, circuits, and processors are not built to implement neuromorphic sensory processing and computing efficiently. To fill this gap, neuromorphic devices with various materials and device physics have been proposed and intensively studied.1018-1020
Promising neuromorphic devices include memristors,1021,1022 spintronics,917,1023 synaptic transistors,1024-1029 and memtransistive synapses (a combination of memristors and transistors),1030,1031 with features including analog computing and parallel storage and processing, in stark contrast to conventional digital processers. Individual neuromorphic devices when integrated with sensors or embedded with sensing functions can preprocess sensory information in a delocalized manner, providing a promising route to edge computing. When interconnected in large scales, neuromorphic device arrays can be used to implement artificial neural networks more efficiently than conventional processers.1032,1033 However, much current work only simulates neural networks by extracting device parameters, instead of implementing physical demonstrations of array hardware.1034,1035 Actual physical implementations face challenges in device yield and consistency, array integration, system robustness, etc.
Sensory demonstration based on neuromorphic devices has been successful in producing artificial sensory systems mimicking biological counterparts. The building blocks of artificial sensory systems, besides sensors, are artificial synapses and artificial neurons. An artificial synapse emulates the characteristics of a biological synapse, such as long-term and short-term plasticity modulated by spike inputs, enabling memorization, learning, adaptation, and other biological computational capabilities. Currently reported artificial synapses are modulated by diverse stimuli such as electrical input,1018 light,1036,1037 chemicals (neurotransmitters),1038-1041 temperature,1042 and combinations of the above (e.g., electrical and optical inputs1027,1043). Electrical modulation offers greater convenience in integration with other electronic devices including sensors, while other modulation modes permit direct sensing by artificial synapses.
Artificial neurons transduce analog sensory stimuli into spikes (although some stimuli are naturally in a spike form such as sound and vibration1044) and subsequently relay the spike-encoded information to artificial synapses for processing. The spike form can encode rich information in its frequency, duty cycle, number of spikes, etc., and is easily handled by digital processors and synaptic devices. It offers superior power efficiency, as noted above, due to event-driven operation. However, research on artificial spiking neurons that do not rely on complex ICs513,978,1045-1051 has been hampered by a dearth of available materials and devices. Recent advances in artificial neurons and artificial synapses include reconfigurations between the two on demand in a single device,1052 which will enable more powerful and adaptive algorithm implementation.
Integrating sensors, artificial synapses, and artificial neurons produces artificial sensory systems (Figure 16, left).1019,1039,1053,1054 Artificial tactile systems513,978,1055-1058 and artificial visual systems1048,1059,1060 are the most developed; fusion of these two sensory modalities for improved recognition accuracy1061 or biomimetic learning behavior1062 has also been demonstrated. Artificial olfactory systems1049 and artificial gustatory systems1063 have been proposed, representing emerging directions for chemical sensing. In addition to the five senses, artificial reflex arcs can also be produced by integrating additional actuators,1064,1065 and artificial proprioception was recently demonstrated in animal leg movement control.1066
Figure 16.
Evolution of flexible artificial sensory devices and systems for bio-integration. Memristor schematic adapted with permission from ref 1067. Copyright 2010 American Chemical Society. Transistor schematic adapted with permission from ref 1053. Copyright 2019 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. Electronic neural interface schematics adapted with permission from ref 1068. Copyright 2020 Springer Nature. Pressure sensory artificial neuron and bio-integration images adapted with permission from ref 1055. Copyright 2018 American Association for the Advancement of Science. Chemical sensory artificial neuron and bio-integration schematics adapted with permission from ref 1039. Copyright 2022 Springer Nature.
The development of this area has shifted from pursuing biological fidelity in artificial devices to exploiting the advantages of biology-like perception to empower and to connect humans and machines. To this end, research efforts have been dedicated to making artificial sensory systems flexible and stretchable.1019,1055,1057,1060,1066 This will enable seamless integration of machines for intelligent robots1000,1058,1069,1070 and connection to biological tissues1039,1055,1066,1071 for human-machine interfaces, neuroprostheses, and cyborgs to repair, strengthen, and augment human perception (Figure 16, right).468,908,1072,1073 Challenges exist in thoroughly understanding biological sensory systems (as a source of inspiration for the conceptual design of artificial counterparts), array and system integration with densities and complexities on par with biological systems, and functional bio-integration.
CONCLUSIONS AND PROSPECTS
Sensors have become indispensable in modern society and in the era of big data and digitalization. To achieve massive sensing scales and sensing on ubiquitous objects, flexible sensors enable us to advance beyond conventional rigid sensors by enabling high-fidelity measurements on complexly shaped and deforming surfaces, as well as (potential) low-cost large-area manufacturing. These features allow flexible sensors to excel in various applications, such as wearable health monitoring, smart packaging, and building-integrated sensing. Despite the strong impetus and fast progress in research, industry and market adoption of flexible sensors awaits the future. With the objective of identifying roadblocks, we analyzed key challenges concerning the performance, fabrication, and commercialization of flexible sensors and systems, and proposed possible solutions to these problems.
The performance of flexible sensors pertaining to stability, selectivity, and sensitivity needs improvement. Stability is the greatest challenge, calling for effective packaging strategies and encapsulation materials. Nonetheless, temperature-associated instabilities are hard to solve using these strategies. Specifically, for wearable biosensors, biofouling and bioreceptor instability are the key issues. Smart surface engineering and immobilization strategies are needed, and molecularly imprinted polymers have emerged as one promising alternative to biological receptors. Signal drift over relevant periods of operation should be properly reported and handled for emerging sensor technologies.
Selectivity is a major concern for mechanical sensors, biosensors, and gas sensors. Specific sensing materials and selective sensing arrays are two general approaches to improve selectivity. Although both strategies have produced promising results for mechanical sensors, trade-offs in the complexities of sensor design and data analyses exist. Selective biosensors usually require the use of biological recognition elements, which offer exceptional specificity yet are sometimes hampered by poor stability. Nanocatalysts can deliver specificity in some cases. Closely related to biosensors, flexible gas sensors are an emerging field, where materials engineering towards specific gas sensing remains a fundamental challenge. Selective sensing arrays are more commonly used, in which algorithms for pattern recognition are decisive in detection accuracy.
Simultaneous achievement of high sensitivity, wide sensing ranges, and linearity is a major challenge for mechanical sensors. Traditional strategies of microstructuring and materials engineering can be used to achieve compromise. Recently proposed sensing mechanisms still need refinement, and the emerging idea of on-demand performance programming offers customizable sensors for different applications. Sensitivity is a critical challenge for biosensors to detect low-concentration biomarkers that are clinically relevant. Nanomaterials play central roles in this regard, being catalysts, electrodes, and/or parts of transducing devices. Although fluorometric sensing offers substantially improved sensitivity over colorimetric sensing, simple and accurate signal detection is a bottleneck. Analyte preconcentration and signal amplification by circuits are also options to improve biosensor sensitivity.
In addition to sensitivity, selectivity, and stability, the dynamic responses of mechanical sensors should be considered as an essential performance metric and properly tested and reported in future research. Hysteresis, response time, and strain-rate dependency are major concerns. Although structural and materials engineering helps to some extent, effective solutions remain elusive. Wearable biosensing awaits improvements in diversifying accessible biomarkers and consolidating continuous monitoring capabilities. To this end, label-free, reagent-free, and wash-free sensing methods, as well as regeneration strategies are needed. Importantly, sensor accuracy assurance should be factored in during the entire process of sensor development to deployment. Calibration is particularly critical yet often overlooked by the research community. Equally important to accurate sensing is establishing robust correlations between sensor data and the status of the monitored objects/environments. This is a serious issue for health monitoring sensors, where many biomedical questions await large-scale systemic investigation and validation.
Mechanical tolerance is the second important aspect of flexible sensor performance. Flexible sensors need to survive mechanical deformations during use. The most prominent challenge is at soft-hard interfaces, and interconnections are particularly concerning. Improving interfacial adhesion and adopting a gradual change in modulus are proven principles, and ongoing work proposes various implementations. Many solutions have been proposed for interconnections in flexible hybrid systems, but the results are not yet satisfactory. Soft interconnects with strong bonding in versatile application scenarios are desirable. Alternatively, wireless connections between subsystems can be adopted. A second issue is extending the elastic deformation range of flexible sensors, which has been addressed by structural engineering and materials innovations. Notably, ionically conductive materials such as hydrogels offer soft and stretchable materials platforms for sensor development. However, there is still much to do to achieve satisfactory performance and realistic manufacturing. Third, fatigue should be considered for sensors undergoing repetitive deformations. Interfacial engineering and crack stabilization are important topics.
Flexible sensors also need to retain stable function under deformation. Decoupling the strain effect on signal output is a challenge. Although efforts to extend the elastic range contribute to mitigating this problem, perfectly strain-insensitive performance is challenging to achieve. Motion artifacts are serious problems for wearable sensors. Enhancing sensor conformability and adhesion and incorporating multiple sensors in a system are effective approaches, but it is challenging to remove all motion-associated noise solely via hardware optimization. Signal processing for noise filtering requires customized solutions to address complex human motions. Machine learning is showing early promise for this purpose. Specifically, developing flexible optical sensors that can retain stable performance under deformation or motion remains a fundamental challenge. Lastly, to make flexible sensors resistant to mechanical damage, tough, self-healing, and stiffening materials are being developed. However, sensor performance with these materials is usually unsatisfactory, and system-level validation of device longevity and durability is largely lacking. Structural design provides an effective alternative. Flexible electronic armors and mechanical metamaterials are worth exploring.
Array integration is a third aspect of flexible sensor performance. Signal readout through passive and active matrices are established solutions, but issues exist in electrical interconnects and circuit solutions. Low-impedance interconnects and high-performance transistor arrays in flexible and stretchable form factors are desirable. Readout strategies mimicking biological sensory systems may fundamentally overcome limitations in spatiotemporal resolution, energy efficiency, and wiring complexity of current electrical readout strategies. Optical readout circumvents many issues encountered in electrical readout, but reliable image capture becomes a bottleneck. Ultrasound and photoacoustic imagers impose more demanding requirements in array signal readout, calling for better solutions. Chemical sensing arrays offer a plethora of exciting opportunities. Multimodal sensing is an important feature of sensor arrays. Challenges include compact and simplistic design, high-density integration, addressability, and effective stimuli decoupling. Flexible imagers responsive to multiple colors are particularly difficult to realize, but recent developments in quantum-dot-based photodetectors are shedding light on this topic.
Compatible interfaces between flexible sensing systems and biological tissues are another important area of research, primarily for human and animal body-integrated sensing. While this topic has been extensively reviewed previously, we highlighted emerging trends. Soft, stretchable, adhesive, biocompatible, biodegradable, electrochemically compatible, and growth adaptable materials are desirable. Biocompatibility is a top priority when designing materials for bio-interfacing. Correct interpretation of the term and thorough experimental validation are required. Functional polymeric materials, such as conjugated polymers and hydrogels, are promising materials, and bioresorbable inorganics have been demonstrated with respectable performance and straightforward fabrication in nonpermanent implantable devices. Achieving biofunctionality without compromising sensing performance is a challenge for materials development. Conformability, permeability, imperceptibility, minimal invasiveness, and 3D coverage are some key form-factor features that need to be achieved. Microfabrication and nanomaterials form the pillars of advanced form factors; however, mechanical fragility is a challenge for practical applications. Flexible hybrid electronics and smart textiles are promising systems. Electronics-biology integration in 3D on cellular and even subcellular levels is merging these two conventionally distinct fields, where exciting opportunities await.
In addition to sensor performance, power supply and data communication also critically affect system performance. With more demanding power usage and physical constraints, there are both needs and opportunities in high-power ambient energy harvesters, large-capacity energy storage devices, and efficient wireless power transfer in flexible, stretchable, and miniaturized form factors. Holistic power management strategies are necessary for complex systems. Connectivity, especially wireless communication, will be integral to future sensing systems. Reducing power consumption, overcoming interferences and constraints associated with the human body, and enhancing data security are primary issues concerning flexible sensing networks.
Nanomaterials and nanotechnology play pivotal roles in solving the challenges facing flexible sensors.1074 The small dimensions and large surface areas of nanomaterials render high sensitivity to external stimuli, as well as fast reactions and mass transport. Hence, nanomaterials and nanoengineering have been key to improving the sensitivity and selectivity of biosensors.200 Nanocatalysts, nanoenzymes, and nano-structured membranes and interfaces help tackle the instability of bioreceptors. Nanomaterials provide viable routes to specific gas sensing through precise structural and chemical control. Micro- and nano-structuring have been sought-after solutions to improve the sensing performance of mechanical sensors, including sensitivity, linearity, and sensing range. Quantum-dot-based photodetectors are showing promise in RGB-responsive flexible imagers. Moreover, nanoscale dimensions are beneficial to mechanical compliance and biofriendly form factors, leading to better biocompatibility and signal quality. For example, the addition of nanofillers in elastomeric matrices enables intrinsically stretchable functional materials. Electronic tattoos based on 2D materials enable conformal and imperceptible sensing, and nanomeshes permit unobstructive mass transport on biological surfaces. Nanotexturing device surfaces also helps combat biofouling and enhances cell adhesion and proliferation. Furthermore, the exceptional electronic, optoelectronic, electrochemical, and chemical properties of some nanomaterials enable high-performance devices. For instance, nanomaterials are extensively studied for energy harvesting and storage devices. Their high surface area and electrochemical activity promote energy conversion at materials interfaces.
Despite the desirable device features offered by nanomaterials and nanotechnology, there remain many challenges limiting their adoption in commercial flexible sensors. First, there is a lack of understanding of analyte-materials interactions in sensitive and/or selective nanomaterials and structure–property relationships of highly tunable nanostructures. This lack of fundamental understanding will hamper materials design and optimization for more versatile applications. Second, the biocompatibility of nanomaterials is intrinsically challenging to examine due to diverse morphologies and surface states, calling for concerted efforts from the community to standardize the related materials and processes. This standardization is not only important for human-interfacing devices, but also for workers handling nanomaterials. Moreover, the environmental impact of nanomaterials is a complex issue concerning materials and device disposal. Nanomaterials recycling should be considered in design. Regulations and legal frameworks are currently largely missing in nanomaterials safety. Third, the mechanical fragility of nanomaterials impedes their practical application. More robust materials should be fabricated, and more user-friendly protocols should be devised. Fourth, high-quality syntheses and processing, high-resolution patterning, and device integration are current issues in the manufacturing of nanomaterial-based devices. Ink formulation offers a viable route to the wide adoption of nanomaterials in printed electronics. Further improvements in functional performance, yield, resolution, and mechanical robustness are needed.
From laboratories to end-users, there are daunting challenges facing flexible sensors. Identification of killer applications entails extensive surveys of real-life problems, close discussions with potential customers, assessments of competitiveness with conventional sensors, and evaluations of technology readiness levels, among many other considerations. Subsequent sensor development should adopt application-specific designs. Tools and processes that facilitate rapid prototyping help shorten prototype-to-product times. Importantly, investment in pilotscale production and testing facilities will be instrumental for translational efforts. Further expanding manufacturing capabilities to industrial scales is essential for commercialization. Special concerns relate to printed electronics, functional polymer processing, nanomaterial production and processing, and system integration. Although scientific and engineering efforts from the research community have proposed many promising solutions to these problems, their industrial relevance may be questionable given the limited interactions between research laboratories and industrial facilities. Therefore, support for access to these facilities and the development of new manufacturing capabilities should be a priority of research governing bodies and funding agencies. Finally, regulations addressing user safety, data privacy, ethics, quality assurance, worker safety, and environmental impact need to be developed to support the sector of flexible sensor technology.
For sustainable growth of this sector, long-term issues in environmental sustainability, industrial standardization, user acceptance and engagement, equitable deployment, and talent pipelines should be addressed. Future flexible sensing systems are expected to be intelligent, built on the basis of ongoing progress in artificial intelligence, edge computing, and neuromorphic computing.
Technology Roadmap.
The above discussions break down the complex landscape of sensor types, performance metrics, form factors, system components, manufacturing, and commercialization of flexible sensor technology, focusing on challenges in different aspects of system performance and various stages of technology development. To put forward a cohesive vision of the development trajectory, here we propose a comprehensive, unified technology roadmap for flexible sensors. This roadmap is designed to connect the dots in different areas of research towards common and long-term goals and to promote disruptive, nonlinear technological advances for a ubiquitous flexible sensor future. The key to realizing these goals will be a dual strategy that elevates sensor performance, while simultaneously digging into the ‘important problems’ not well addressed by current solutions.
We sketch out a broad flexible sensor developmental narrative in six areas—dimensional scaling, functional performance (electrical performance as a representative), mechanical compliance, form factors, peripheral functions, and carbon footprint—to provide direction and extent of development for flexible sensors to head towards realistic deployment and to stay on a fast-growing trajectory (Figure 17). The narrative provides no timelines or progress guarantees but identifies critical steps, grounded in the current progress of flexible sensors combined with scenarios of state-of-the-art rigid sensors. Such a narrative is expected to provide guidance for multidisciplinary and crossvalue-chain development strategies.
Figure 17.
A technology roadmap for flexible sensors and systems. Evolution with time is not drawn to scale. Images from left to right: Adapted with permission from ref 647. Copyright 2021 American Chemical Society. Adapted with permission from ref 235. Copyright 2021 Wiley-VCH GmbH. Adapted with permission from ref 118. Copyright 2019 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. Adapted with permission from ref 579. Copyright 2020 Springer Nature. Adapted with permission from ref 625. Copyright 2016 Nature Publishing Group.
Dimensional Scaling.
Scaling in terms of feature size reduction and array size increment will enable applications requiring high spatiotemporal resolution or large-area coverage. The feature size offered by printed electronics will continue evolving while microfabrication of emerging materials such as functional polymers might eventually achieve feature sizes comparable to rigid microelectronics. Areal scaling will target robotics, vehicles, and buildings.
Electrical Performance.
Performance including conductivity, carrier mobility, and dielectric properties is the foundation of electronic (and ionic) sensors. These properties are currently compromised by the flexible form factor. Ongoing efforts in the rational design of materials and devices are heading towards performance comparable to rigid counterparts. Other functional performance, such as optoelectronic, electrochemical, (electro)-magnetic, thermal, and optical properties, will improve for various sensing modalities.
Mechanical Compliance.
This represents a major advancement of flexible sensors over rigid sensors. Beyond being bendable, soft and stretchable devices are the future for more compatible integration with biological systems, primarily targeting human-centric applications. The ultimate goal will be achieving mechanical properties similar to human tissues, including other aspects such as viscoelasticity, strain-hardening, and self-healing.
Form Factors.
Form factors of flexible sensing systems will be highly diverse. The near-term achievable form factors might be textiles or clothing-integrated systems and flexible hybrid systems. In clothing-integrated systems, interconnection and wireless communication modules are likely the first components, besides sensors, to be manufactured on fabrics or in fibers; other components will still be of a rigid form factor, and the components will be spatially separated. Flexible hybrid integration will produce compact systems that enable wearable skin patches. More challenging form factors such as tattoos, implants, and tissue-hybrids will require entirely soft or miniaturized devices, and their system integration will likely be through wireless communication.
Peripheral Functions.
They extend the functionality of flexible sensors, enabling more sophisticated and diverse uses. Autonomous wireless sensor networks that require minimal maintenance after installation will be integral to the IoT and intelligent machines. Feedback mechanisms including electrical, visual,967 auditory, haptic895 and even olfactory stimulations will provide natural and immersive experience in human-machine interactions and the metaverse.968 Intelligent sensors that emulate the human sensory systems represent an unbounded scientific pursuit to replicate and extend natural capabilities, and the potential applications are unimaginable.
Carbon Footprint.
The carbon footprint of flexible sensors and systems greatly affects the sustainability of the sector. Nearterm reductions in carbon footprint are likely through more efficient manufacturing consuming less material and energy, as well as dematerialization through lighter and thinner form factors.29 Lifetime extension should focus on mechanical and functional durability. More sustainable ways of materials sourcing will entail the use of more abundant elements and environmentally benign extraction processes. Recovering materials from waste and upcycling waste into valuable products will be essential for a circular economy. Fully degradable devices that can be disposed of without harming ecosystems will be especially beneficial to single-use applications.
There are and will be numerous challenges as we navigate this roadmap. Yet, we are optimistic that the benefits of flexible sensors will outweigh the challenges. In drafting this forwardlooking Review, we call for action from the community to address the bottlenecks impeding the maturation of flexible sensor technologies and propose groundbreaking concepts that will accelerate the development and commercialization of flexible sensors. We hope to inspire more scientists, engineers, innovators, and entrepreneurs with diverse backgrounds to participate in and contribute to this exciting field—multidisciplinary collaboration is the key to breakthroughs in a disruptive technology like flexible sensors.
ACKNOWLEDGMENTS
Y.L., Z.L., M.Z., and X.C. acknowledge the National Research Foundation, Singapore (NRF) under NRF’s Medium Sized Centre: Singapore Hybrid-Integrated Next-Generation μ-Electronics (SHINE) Centre funding programme, and AME programming funding scheme of Cyber Physiochemical Interface (CPI) project (no. A18A1b0045). Y.L. acknowledges National Natural Science Foundation of China (62201243). C.J. acknowledges funding support from the National Key R&D Program of China (no. 2019YFA0706100), the National Natural Science Foundation of China (82151305), Lingang Laboratory (LG-QS-202202-09). T.Q.T. and N.E.L. acknowledge support by the Basic Science Research Program (no. 2020R1A2C3013480) through the National Research Foundation of Korea (NRF) funded by the Ministry of Science and ICT. A.F. acknowledges the AFOSR (grant FA9550-22-1-0423). Y.L. and Y.Z. would like to acknowledge the NSF (award no. 2134664) and NIH (award no. R01HD108473) for financial support. X.F. acknowledges the support from the National Natural Science Foundation of China (grant no. U20A6001). L.Y. would like to thank the A*STAR Central Research Fund (CRF) and the AME Programmatic A18A1b0045 (Cyber Physiochemical Interfaces) for funding support. C.F.G. acknowledges the National Natural Science Foundation of China (no. T2225017). T.Q.T. acknowledges the Brain Pool Program (No. 2020H1D3A2A02111068) through the National Research Foundation (NRF) funded by the Ministry of Science. Z.L. acknowledges the support from RIE2020 AME Programmatic Grant funded by A*STAR-SERC, Singapore (Grant No. A18A1b0045). X.G. acknowledges funding support through the Shanghai Science and Technology Commission (grant no. 19JC1412400), the National Science Fund for Excellent Young Scholars (grant no. 61922057). C.D. acknowledges National Science Foundation CAREER: Conformable Piezoelectrics for Soft Tissue Imaging (grant no. 2044688) and MIT Media Lab Consortium funding. D.K. and O.G.S. acknowledge Leibniz Association and the German Research Foundation DFG (Gottfried Wilhelm Leibniz Program SCHM 1298/22-1, KA5051/1-1 and KA 5051/3-1), as well as the Leibniz association (Leibniz Transfer Program T62/2019). C.W. acknowledges the National Key Research and Development Program of China (grant no. 2021YFA1202600), National Natural Science Foundation of China (grant no. 62174082). A.V.-Y.T., E.Z., Y.Z., X.Z., and J.P. acknowledge the National Research Foundation, Singapore (NRF) under NRF’s Medium Sized Centre: Singapore Hybrid-Integrated Next-Generation μ-Electronics (SHINE) Centre funding programme, and AME programming funding scheme of Cyber Physiochemical Interface (CPI) project (no. A18A1b0045). R.Z. acknowledges National Natural Science Foundation of China (grant no. 51735007) and Beijing Natural Science Foundation (grant no. 3191001). N.M. acknowledges the support by JST PRESTO Grant Number JPMJPR20B7 and JST Adaptable and Seamless Technology transfer Program through Target-driven R&D (A-STEP) grant number JPMJTM22BK. C.P. acknowledges the Korean government (Ministry of Science and ICT, MSIT) (2022R1A4A3032923). M.W. acknowledges the National Key R&D Program of China under Grant (2021YFB3601200). X.Z. acknowledges National Natural Science Foundation of China (no. 62074029). S.X. acknowledges the 3M nontenured faculty award. T.-W.L. and D.-G.S. acknowledge the Pioneer Research Center Program through the National Research Foundation of Korea funded by the Ministry of Science, ICT & Future Planning (grant no. NRF-2022M3C1A3081211). C.T.L. would like to acknowledge support from the Institute for Health Innovation and Technology (iHealthtech), the MechanoBioEngineering Laboratory at the Department of Biomedical Engineering and the Institute for Functional Intelligent Materials (I-FIM) at the National University of Singapore (NUS). C.T.L. also acknowledges support from the National Research Foundation and A*STAR, under its RIE2020 Industry Alignment Fund – Industry Collaboration Projects (IAF-ICP) (grant no. I2001E0059) – SIA-NUS Digital Aviation Corp Lab and the NUS ARTIC Research (grant no. HFM-RP1). X.Y. acknowledges funding support by City University of Hong Kong (grant no. 9667221). T.X. and X.Z. acknowledge National Natural Science Foundation of China (22234006). B.C.K.T. acknowledges Cyber-Physiochemical Interfaces CPI, A*STAR A18A1b0045. H.G. acknowledges a research start-up grant (002479-00001) from Nanyang Technological University and the Agency for Science, Technology and Research (A*STAR) in Singapore. W.G. acknowledges National Science Foundation grant 2145802. D.J.L. acknowledges support from the US National Science Foundation grant number CBET-2223566. G.Y. acknowledges support from The Welch Foundation award F-1861, and Camille Dreyfus Teacher-Scholar Award. M.D.D. acknowledges funding support from NSF (grant no. EEC-1160483). J.-H.A acknowledges the National Research Foundation of Korea (NRF-2015R1A3A2066337). J.C. acknowledges the Henry Samueli School of Engineering & Applied Science and the Department of Bioengineering at the University of California, Los Angeles for startup support and a Brain & Behavior Research Foundation Young Investigator Grant. K.T. acknowledges JST AIP Accelerated Program (no. JPMJCR21U1) and JSPS KAKENHI (grant no. JP22H00594). P.S.W. acknowledges the National Science Foundation (CMMI1636136) for support. A.M.A., M.C.H., and P.S.W. thank the National Institute on Drug Abuse (DA045550) for support. S.M. and X.C. appreciated the support from the Smart Grippers for Soft Robotics (SGSR) Programme under the National Research Foundation, Prime Minister’s Office, Singapore under its Campus of Research Excellence and Technological Enterprise (CREATE) programme.
VOCABULARY
- Bending curvature
the inverse of bending radius
- Bioaffinity
the molecular interactions between a bioreceptor and the analyte that give rise to specific binding. Bioaffinity biosensors use such molecular binding (of molecular components such as antibodies, proteins, nucleic acids, aptamers, molecularly imprinted polymers) for sensing1075,1076
- Bioreceptor
a biologically derived material (e.g., enzymes, aptamers, DNA, antibodies, cells) or biomimetic component (e.g., molecularly imprinted polymers, nanozymes) that specifically recognizes the analyte.1077,1078 Bioreceptors are also known as biological recognition elements or biorecognition elements1077,1079
- Biorecognition
the process of signal generation (in the form of light, heat, pH, charge or mass change, etc.) upon interaction of the bioreceptor with the analyte1077
- Crosstalk
interference on sensor output from neighboring sensor pixels in an array or other sensing modes in a multimodal sensor1080
- Drift
a unidirectional change in signal that results in the average value changing monotonically (though not necessarily linearly) with time99
- Elastic range
the strain and stress ranges within which deformation can be immediately and completely recovered upon unloading1081
- Heterogeneous integration
the integration of separately manufactured components into a higher-level assembly that, in the aggregate, provides enhanced functionality and improved operating characteristics. In this definition, components should be taken to mean any units, whether individual dies, MEMS devices, passive components, and assembled packages or subsystems, that are integrated into a single package1082
- Immunosensor/immunoassay
a sensor or assay based on antibody-antigen interactions1075,1076
- Latency
in sensor signal readout, latency is the delay in time between sensor signal generation and the receival of that signal from readout electronics. In networking, latency describes the delay in time it takes a data packet to travel from one network node to another
- Linearity
the quality of having a linear correlation between sensor output signal and stimulus intensity. This is reflected in a straight line of the response-vs-sensing range curve
- Mechanical metamaterials
artificial structures with unusual mechanical properties defined by rationally designed structures of precise geometrical arrangements rather than their composition1083,1084
- Multimodal sensing
detection of more than one type of stimulus by a sensing device/system. Detection of multiple chemical species is normally not referred to as multimodal sensing51
- Multiplexed sensing
simultaneous detection of signals from multiple channels (sensing units) of an integrated sensing device. Often used to describe the simultaneous detection of multiple analytes by a biosensor51,1085
- Reliability
the degree to which an individual sensor repeatedly yields the same signal for the same stimuli under the variety of ambient conditions expected for a particular sensor application64,99
- Repeatability
the degree to which an individual sensor repeatedly yields the same signal for the same stimuli under the same operating conditions. Repeatability necessarily depends on stability99
- Response time
the time interval between the stimulus and the sensor response.1086 When the sensor signal gradually increases to a stable value corresponding to the intensity of the stimulus, usually a percentage of maximum is indicated to define the response time
- Selectivity
the ability of a sensor to discriminate between the analyte/stimulus of interest and possible interferences99
- Sensitivity
for a reversible sensor, sensitivity is defined as the change in sensor output signal obtained for an incremental change in the stimulus, i.e., the slope of the response-vs-sensing range curve. It is often confused with limit of detection (LOD), i.e., the lowest concentration of an analyte or the smallest value of a stimulus that can, with some level of statistical confidence, be differentiated from zero99
- Sensor hysteresis
the degree of difference between the response curves when going up and down the sensing range
- Somatosensory system
the network of neural structures in the brain and body that produces the perception of touch (haptic perception), temperature (thermoception), body position (proprioception), movement, and pain
- Stability
the ability of a sensor to maintain accurate measurement in the presence of possible environmental variation and in both short term and long term relative to the specified service life99
- Technology readiness levels (TRLs)
a systemic qualitative assessment system used to estimate the maturity level of a particular technology, and to compare the maturity between different types of technology869,1087
- Time division multiple access
a channel access method that divides signals into different time slots, used to facilitate channel sharing without interference. In array signal readout, this dictates that sensor pixels are read in rapid succession.
Footnotes
The authors declare the following competing financial interest(s): A.M.A. and P.S.W. have a number of patent applications related to the technologies described in this article.
Contributor Information
Yifei Luo, Institute of Materials Research and Engineering (IMRE), Agency for Science, Technology and Research (A*STAR), Singapore 138634, Republic of Singapore; Innovative Centre for Flexible Devices (iFLEX), School of Materials Science and Engineering, Nanyang Technological University, Singapore 639798, Singapore.
Mohammad Reza Abidian, Department of Biomedical Engineering, University of Houston, Houston, Texas 77024, United States.
Jong-Hyun Ahn, School of Electrical and Electronic Engineering, Yonsei University, Seoul 03722, Republic of Korea.
Deji Akinwande, Department of Electrical and Computer Engineering, The University of Texas at Austin, Austin, Texas 78712, United States; Microelectronics Research Center, The University of Texas at Austin, Austin, Texas 78758, United States.
Anne M. Andrews, Department of Chemistry and Biochemistry, California NanoSystems Institute, and Department of Psychiatry and Biobehavioral Sciences, Semel Institute for Neuroscience and Human Behavior, and Hatos Center for Neuropharmacology, University of California, Los Angeles, Los Angeles, California 90095, United States
Markus Antonietti, Colloid Chemistry Department, Max Planck Institute of Colloids and Interfaces, 14476 Potsdam, Germany.
Zhenan Bao, Department of Chemical Engineering, Stanford University, Stanford, California 94305, United States.
Magnus Berggren, Laboratory of Organic Electronics, Department of Science and Technology, Campus Norrköping Linköping University, 83 Linköping, Sweden; Wallenberg Initiative Materials Science for Sustainability (WISE) and Wallenberg Wood Science Center (WWSC), SE-100 44 Stockholm, Sweden.
Christopher A. Berkey, Department of Materials Science and Engineering, Stanford University, Stanford, California 94301, United States
Christopher John Bettinger, Department of Biomedical Engineering and Department of Materials Science and Engineering, Carnegie Mellon University, Pittsburgh, Pennsylvania 15213, United States.
Jun Chen, Department of Bioengineering, University of California, Los Angeles, Los Angeles, California 90095, United States.
Peng Chen, School of Chemistry, Chemical Engineering and Biotechnology, Nanyang Technological University, Singapore 637457, Singapore.
Wenlong Cheng, Nanobionics Group, Department of Chemical and Biological Engineering and Monash Institute of Medical Engineering, Monash University, Clayton, Australia 3800.
Xu Cheng, Applied Mechanics Laboratory, Department of Engineering Mechanics, Laboratory of Flexible Electronics Technology, Tsinghua University, Beijing 100084, PR China.
Seon-Jin Choi, Division of Materials of Science and Engineering, Hanyang University, Seongdong-gu, Seoul 04763, Republic of Korea.
Alex Chortos, School of Mechanical Engineering, Purdue University, West Lafayette, Indiana 47906, United States.
Canan Dagdeviren, Media Lab, Massachusetts Institute of Technology, Cambridge, Massachusetts 02139, United States.
Reinhold H. Dauskardt, Department of Materials Science and Engineering, Stanford University, Stanford, California 94301, United States
Chong-an Di, Beijing National Laboratory for Molecular Sciences, CAS Key Laboratory of Organic Solids, Institute of Chemistry, Chinese Academy of Sciences, Beijing 100190, China.
Michael D. Dickey, Department of Chemical and Biomolecular Engineering, North Carolina State University, Raleigh, North Carolina 27606, United States
Xiangfeng Duan, Department of Chemistry and Biochemistry, California NanoSystems Institute, University of California, Los Angeles, Los Angeles, California 90095, United States.
Antonio Facchetti, Department of Chemistry and the Materials Research Center, Northwestern University, Evanston, Illinois 60208, United States.
Zhiyong Fan, Department of Electronic and Computer Engineering and Department of Chemical and Biological Engineering, The Hong Kong University of Science and Technology, Kowloon, Hong Kong SAR, China.
Yin Fang, School of Chemistry, Chemical Engineering and Biotechnology, Nanyang Technological University, Singapore 637457, Singapore.
Jianyou Feng, State Key Laboratory of Molecular Engineering of Polymers, Department of Macromolecular Science, and Laboratory of Advanced Materials, Fudan University, Shanghai 200438, PR China.
Xue Feng, Laboratory of Flexible Electronics Technology, Department of Engineering Mechanics, Tsinghua University, Beijing 100084, China.
Huajian Gao, School of Mechanical and Aerospace Engineering, Nanyang Technological University, Singapore 639798, Singapore; Institute of High Performance Computing (IHPC), Agency for Science, Technology and Research (A*STAR), Singapore 138632, Republic of Singapore.
Wei Gao, Andrew and Peggy Cherng Department of Medical Engineering, California Institute of Technology, Pasadena, California 91125, United States.
Xiwen Gong, Department of Chemical Engineering, Department of Materials Science and Engineering, Department of Electrical Engineering and Computer Science, Applied Physics Program, and Macromolecular Science and Engineering Program, University of Michigan, Ann Arbor, Michigan 48109, United States.
Chuan Fei Guo, Department of Materials Science and Engineering, Southern University of Science and Technology, Shenzhen 518055, China.
Xiaojun Guo, National Key Laboratory of Science and Technology on Micro/Nano Fabrication, School of Electronic Information and Electrical Engineering, Shanghai Jiao Tong University, Shanghai 200240, China.
Martin C. Hartel, Department of Bioengineering, University of California, Los Angeles, Los Angeles, California 90095, United States
Zihan He, Beijing National Laboratory for Molecular Sciences, CAS Key Laboratory of Organic Solids, Institute of Chemistry, Chinese Academy of Sciences, Beijing 100190, China.
John S. Ho, Institute for Health Innovation and Technology, National University of Singapore, Singapore 117599, Singapore; Department of Electrical and Computer Engineering, National University of Singapore, Singapore 117583, Singapore; The N.1 Institute for Health, National University of Singapore, Singapore 117456, Singapore
Youfan Hu, School of Electronics and Center for Carbon-Based Electronics, Peking University, Beijing 100871, China.
Qiyao Huang, School of Fashion and Textiles, The Hong Kong Polytechnic University, Kowloon, Hong Kong SAR, China.
Yu Huang, Department of Materials Science and Engineering, California NanoSystems Institute, University of California, Los Angeles, Los Angeles, California 90095, United States.
Fengwei Huo, Key Laboratory of Flexible Electronics (KLOFE) and Institute of Advanced Materials (IAM), Nanjing Tech University (NanjingTech), Nanjing 211816, PR China.
Muhammad M. Hussain, mmh Labs, Elmore Family School of Electrical and Computer Engineering, Purdue University, West Lafayette, Indiana 47906, United States
Ali Javey, Electrical Engineering and Computer Sciences, University of California, Berkeley, California 94720, United States; Materials Sciences Division, Lawrence Berkeley National Laboratory, Berkeley, California 94720, United States.
Unyong Jeong, Department of Materials Science and Engineering, Pohang University of Science and Engineering (POSTECH), Pohang, Gyeong-buk 37673, Korea.
Chen Jiang, Department of Electronic Engineering, Tsinghua University, Beijing 100084, China.
Xingyu Jiang, Department of Biomedical Engineering, Southern University of Science and Technology, Shenzhen, Guangdong 518055, PR China.
Jiheong Kang, Department of Materials Science and Engineering, Korea Advanced Institute of Science and Technology (KAIST), Daejeon 34141, Republic of Korea.
Daniil Karnaushenko, Research Center for Materials, Architectures and Integration of Nanomembranes (MAIN), Chemnitz University of Technology, Chemnitz 09126, Germany.
Ali Khademhosseini, Terasaki Institute for Biomedical Innovation, Los Angeles, CA, USA.
Dae-Hyeong Kim, Center for Nanoparticle Research, Institute for Basic Science (IBS), School of Chemical and Biological Engineering, Seoul National University, Seoul 08826, Republic of Korea.
Il-Doo Kim, Department of Materials Science and Engineering, Korea Advanced Institute of Science and Technology (KAIST), Daejeon 34141, Republic of Korea.
Dmitry Kireev, Department of Electrical and Computer Engineering, The University of Texas at Austin, Austin, Texas 78712, United States; Microelectronics Research Center, The University of Texas at Austin, Austin, Texas 78758, United States.
Lingxuan Kong, School of Chemistry, Chemical Engineering and Biotechnology, Nanyang Technological University, Singapore 637457, Singapore.
Chengkuo Lee, Department of Electrical and Computer Engineering, National University of Singapore, Singapore 117583, Singapore; Center for Intelligent Sensors and MEMS (CISM), National University of Singapore, Singapore 117608, Singapore; National University of Singapore Suzhou Research Institute (NUSRI), Suzhou Industrial Park, Suzhou 215123, China; NUS Graduate School-Integrative Sciences and Engineering Programme (ISEP), National University of Singapore, Singapore 119077, Singapore.
Nae-Eung Lee, School of Advanced Materials Science and Engineering, Sungkyunkwan University, Suwon, Kyunggi-do 16419, Republic of Korea.
Pooi See Lee, School of Materials Science and Engineering, Nanyang Technological University, Singapore 639798, Singapore; Singapore-HUJ Alliance for Research and Enterprise (SHARE), Campus for Research Excellence and Technological Enterprise (CREATE), Singapore 138602, Singapore.
Tae-Woo Lee, Department of Materials Science and Engineering, School of Chemical and Biological Engineering, Institute of Engineering Research, Research Institute of Advanced Materials, and Interdisciplinary Program in Bioengineering, Seoul National University, Seoul 08826, Republic of Korea.
Fengyu Li, College of Chemistry and Materials Science, Jinan University, Guangzhou, Guangdong 510632, China.
Jinxing Li, Department of Biomedical Engineering, Department of Electrical and Computer Engineering, Neuroscience Program, BioMolecular Science Program, and Institute for Quantitative Health Science and Engineering, Michigan State University, East Lansing, Michigan 48823, United States.
Cuiyuan Liang, School of Chemistry and Chemical Engineering, Harbin Institute of Technology, Harbin, Heilongjiang 150001, China.
Chwee Teck Lim, Department of Biomedical Engineering, National University of Singapore, Singapore 117583, Singapore; Mechanobiology Institute, National University of Singapore, Singapore 117411, Singapore; Institute for Health Innovation and Technology, National University of Singapore, Singapore 119276, Singapore.
Yuanjing Lin, School of Microelectronics, Southern University of Science and Technology, Shenzhen 518055, China.
Darren J. Lipomi, Department of Nano and Chemical Engineering, University of California, San Diego, La Jolla, California 92093-0448, United States
Jia Liu, John A. Paulson School of Engineering and Applied Sciences, Harvard University, Boston, Massachusetts 02134, United States.
Kai Liu, School of Chemistry and Chemical Engineering, Frontiers Science Center for Transformative Molecules, Shanghai Jiao Tong University, Shanghai 200240, PR China.
Nan Liu, Beijing Key Laboratory of Energy Conversion and Storage Materials, College of Chemistry, Beijing Normal University, Beijing 100875, PR China.
Ren Liu, John A. Paulson School of Engineering and Applied Sciences, Harvard University, Boston, Massachusetts 02134, United States.
Yuxin Liu, Department of Biomedical Engineering, N.1 Institute for Health, Institute for Health Innovation and Technology (iHealthtech), National University of Singapore, Singapore 119077, Singapore; Institute of Materials Research and Engineering (IMRE), Agency for Science, Technology and Research (A*STAR), Singapore 138634, Republic of Singapore.
Yuxuan Liu, Department of Mechanical and Aerospace Engineering, North Carolina State University, Raleigh, North Carolina 27695, United States.
Zhiyuan Liu, Neural Engineering Centre, Shenzhen Institute of Advanced Technology, Chinese Academy of Sciences, Shenzhen, China 518055.
Zhuangjian Liu, Institute of High Performance Computing (IHPC), Agency for Science, Technology and Research (A*STAR), Singapore 138632, Republic of Singapore.
Xian Jun Loh, Institute of Materials Research and Engineering (IMRE), Agency for Science, Technology and Research (A*STAR), Singapore 138634, Republic of Singapore.
Nanshu Lu, Department of Aerospace Engineering and Engineering Mechanics, Department of Electrical and Computer Engineering, Department of Mechanical Engineering, Department of Biomedical Engineering, Texas Materials Institute, The University of Texas at Austin, Austin, Texas 78712, United States.
Zhisheng Lv, Institute of Materials Research and Engineering (IMRE), Agency for Science, Technology and Research (A*STAR), Singapore 138634, Republic of Singapore.
Shlomo Magdassi, Institute of Chemistry and the Center for Nanoscience and Nanotechnology, The Hebrew University of Jerusalem, Jerusalem 9190401, Israel.
George G. Malliaras, Electrical Engineering Division, Department of Engineering, University of Cambridge, CB3 0FA Cambridge, United Kingdom
Naoji Matsuhisa, Institute of Industrial Science, The University of Tokyo, Meguro-ku, Tokyo 153-8505, Japan.
Arokia Nathan, Darwin College, University of Cambridge, Cambridge CB3 9EU, United Kingdom.
Simiao Niu, Department of Biomedical Engineering, Rutgers University, Piscataway, New Jersey 08854, United States.
Jieming Pan, Department of Electrical and Computer Engineering, National University of Singapore, Singapore 117583, Singapore.
Changhyun Pang, School of Chemical Engineering and Samsung Advanced Institute for Health Science and Technology, Sungkyunkwan University, Suwon 16419, Republic of Korea.
Qibing Pei, Department of Materials Science and Engineering, Department of Mechanical and Aerospace Engineering, California NanoSystems Institute, University of California, Los Angeles, Los Angeles, California 90095, United States.
Huisheng Peng, State Key Laboratory of Molecular Engineering of Polymers, Department of Macromolecular Science, and Laboratory of Advanced Materials, Fudan University, Shanghai 200438, PR China.
Dianpeng Qi, School of Chemistry and Chemical Engineering, Harbin Institute of Technology, Harbin, Heilongjiang 150001, China.
Huaying Ren, Department of Chemistry and Biochemistry, University of California, Los Angeles, Los Angeles, California 90095, United States.
John A. Rogers, Querrey Simpson Institute for Bioelectronics, Northwestern University, Evanston, Illinois 60208, United States; Department of Materials Science and Engineering, Department of Mechanical Engineering, Department of Biomedical Engineering, Departments of Electrical and Computer Engineering and Chemistry, and Department of Neurological Surgery, Northwestern University, Evanston, Illinois 60208, United States
Aaron Rowe, Becton, Dickinson and Company, Anaheim, California 92807, United States; Ready, Set, Food!, Encino, California 91436, United States.
Oliver G. Schmidt, Research Center for Materials, Architectures and Integration of Nanomembranes (MAIN), Chemnitz University of Technology, Chemnitz 09126, Germany; Material Systems for Nanoelectronics, Chemnitz University of Technology, Chemnitz 09107, Germany; Nanophysics, Faculty of Physics, TU Dresden, Dresden 01062, Germany
Tsuyoshi Sekitani, The Institute of Scientific and Industrial Research (SANKEN), Osaka University, Osaka, Japan 5670047.
Dae-Gyo Seo, Department of Materials Science and Engineering, Seoul National University, Seoul 08826, Republic of Korea.
Guozhen Shen, School of Integrated Circuits and Electronics, Beijing Institute of Technology, Beijing 100081, China.
Xing Sheng, Department of Electronic Engineering, Beijing National Research Center for Information Science and Technology, Institute for Precision Medicine, Center for Flexible Electronics Technology, and IDG/McGovern Institute for Brain Research, Tsinghua University, Beijing 100084, China.
Qiongfeng Shi, Department of Electrical and Computer Engineering, National University of Singapore, Singapore 117583, Singapore; Center for Intelligent Sensors and MEMS (CISM), National University of Singapore, Singapore 117608, Singapore; National University of Singapore Suzhou Research Institute (NUSRI), Suzhou 215123, China.
Takao Someya, Department of Electrical Engineering and Information Systems, Graduate School of Engineering, The University of Tokyo, Tokyo 113-8656, Japan.
Yanlin Song, Key Laboratory of Green Printing, Institute of Chemistry, Chinese Academy of Sciences, Beijing, Beijing 100190, China.
Eleni Stavrinidou, Laboratory of Organic Electronics, Department of Science and Technology, Linköping University, SE-601 74 Norrkoping, Sweden.
Meng Su, Key Laboratory of Green Printing, Institute of Chemistry, Chinese Academy of Sciences, Beijing, Beijing 100190, China.
Xuemei Sun, State Key Laboratory of Molecular Engineering of Polymers, Department of Macromolecular Science, and Laboratory of Advanced Materials, Fudan University, Shanghai 200438, PR China.
Kuniharu Takei, Department of Physics and Electronics, Osaka Metropolitan University, Sakai, Osaka 599-8531, Japan.
Xiao-Ming Tao, Research Institute for Intelligent Wearable Systems, School of Fashion and Textiles, Hong Kong Polytechnic University, Hong Kong, China.
Benjamin C. K. Tee, Materials Science and Engineering, National University of Singapore, Singapore 117575, Singapore; iHealthtech, National University of Singapore, Singapore 119276, Singapore
Aaron Voon-Yew Thean, Department of Electrical and Computer Engineering, National University of Singapore, Singapore 117583, Singapore; Singapore Hybrid-Integrated Next-Generation μ-Electronics Centre (SHINE), Singapore 117583, Singapore.
Tran Quang Trung, School of Advanced Materials Science and Engineering, Sungkyunkwan University, Suwon, Kyunggi-do 16419, Republic of Korea.
Changjin Wan, School of Electronic Science and Engineering, Nanjing University, Nanjing 210023, China.
Huiliang Wang, Department of Biomedical Engineering, University of Texas at Austin, Austin, Texas 78712, United States.
Joseph Wang, Department of Nanoengineering, University of California, San Diego, California 92093, United States.
Ming Wang, Frontier Institute of Chip and System, State Key Laboratory of Integrated Chip and Systems, Zhangjiang Fudan International Innovation Center, Fudan University, Shanghai 200433, China; the Shanghai Qi Zhi Institute, Shanghai 200232, China.
Sihong Wang, Pritzker School of Molecular Engineering, The University of Chicago, Chicago, Illinois 60637, United States.
Ting Wang, State Key Laboratory of Organic Electronics and Information Displays and Jiangsu Key Laboratory for Biosensors, Institute of Advanced Materials (IAM), Nanjing University of Posts and Telecommunications, Nanjing 210023, China.
Zhong Lin Wang, Beijing Institute of Nanoenergy and Nanosystems, Chinese Academy of Sciences, Beijing 100083, China; Georgia Institute of Technology, Atlanta, Georgia 30332-0245, United States.
Paul S. Weiss, California NanoSystems Institute, Department of Chemistry and Biochemistry, Department of Bioengineering, and Department of Materials Science and Engineering, University of California, Los Angeles, Los Angeles, California 90095, United States
Hanqi Wen, School of Chemistry, Chemical Engineering and Biotechnology, Nanyang Technological University, Singapore 637457, Singapore; Institute of Flexible Electronics Technology of THU, Jiaxing Zhejiang, China 314000.
Sheng Xu, Department of Nanoengineering, Department of Electrical and Computer Engineering, Materials Science and Engineering Program, and Department of Bioengineering, University of California San Diego, La Jolla, California 92093, United States.
Tailin Xu, School of Biomedical Engineering, Health Science Center, Shenzhen University, Shenzhen, Guangdong 518060, PR China.
Hongping Yan, Department of Chemical Engineering, Stanford University, Stanford, California 94305, United States.
Xuzhou Yan, School of Chemistry and Chemical Engineering, Frontiers Science Center for Transformative Molecules, Shanghai Jiao Tong University, Shanghai 200240, PR China.
Hui Yang, Tianjin Key Laboratory of Molecular Optoelectronic Sciences, Department of Chemistry, School of Science, Tianjin University, Tianjin, China 300072.
Le Yang, Institute of Materials Research and Engineering (IMRE), Agency for Science, Technology and Research (A*STAR), Singapore 138634, Republic of Singapore; Department of Materials Science and Engineering, National University of Singapore (NUS), Singapore 117575, Singapore.
Shuaijian Yang, School of Biomedical Sciences, Faculty of Biological Sciences, University of Leeds, Leeds LS2 9JT, United Kingdom.
Lan Yin, School of Materials Science and Engineering, The Key Laboratory of Advanced Materials of Ministry of Education, State Key Laboratory of New Ceramics and Fine Processing, and Center for Flexible Electronics Technology, Tsinghua University, Beijing 100084, China.
Cunjiang Yu, Department of Engineering Science and Mechanics, Department of Biomedical Engineering, Department of Material Science and Engineering, Materials Research Institute, Pennsylvania State University, University Park, Pennsylvania 16802, United States.
Guihua Yu, Materials Science and Engineering Program and Walker Department of Mechanical Engineering, The University of Texas at Austin, Austin, Texas 78712, United States.
Jing Yu, School of Materials Science and Engineering, Nanyang Technological University, Singapore 639798, Singapore.
Shu-Hong Yu, Department of Chemistry, Institute of Biomimetic Materials and Chemistry, Hefei National Research Center for Physical Science at the Microscale, University of Science and Technology of China, Hefei 230026, China.
Xinge Yu, Department of Biomedical Engineering, City University of Hong Kong, Hong Kong, China.
Evgeny Zamburg, Department of Electrical and Computer Engineering, National University of Singapore, Singapore 117583, Singapore; Singapore Hybrid-Integrated Next-Generation μ-Electronics Centre (SHINE), Singapore 117583, Singapore.
Haixia Zhang, National Key Laboratory of Science and Technology on Micro/Nano Fabrication; Beijing Advanced Innovation Center for Integrated Circuits, School of Integrated Circuits, Peking University, Beijing 100871, China.
Xiangyu Zhang, Department of Electrical and Computer Engineering, National University of Singapore, Singapore 117583, Singapore; Singapore Hybrid-Integrated Next-Generation μ-Electronics Centre (SHINE), Singapore 117583, Singapore.
Xiaosheng Zhang, School of Electronic Science and Engineering, University of Electronic Science and Technology of China, Chengdu 611731, China.
Xueji Zhang, School of Biomedical Engineering, Health Science Center, Shenzhen University, Shenzhen, Guangdong 518060, PR China.
Yihui Zhang, Applied Mechanics Laboratory, Department of Engineering Mechanics; Laboratory of Flexible Electronics Technology, Tsinghua University, Beijing 100084, PR China.
Yu Zhang, Department of Electrical and Computer Engineering, National University of Singapore, Singapore 117583, Singapore; Singapore Hybrid-Integrated Next-Generation μ-Electronics Centre (SHINE), Singapore 117583, Singapore.
Siyuan Zhao, John A. Paulson School of Engineering and Applied Sciences, Harvard University, Boston, Massachusetts 02134, United States.
Xuanhe Zhao, Department of Mechanical Engineering, Department of Civil and Environmental Engineering, Massachusetts Institute of Technology, Cambridge, Massachusetts 02139, United States.
Yuanjin Zheng, Center for Integrated Circuits and Systems, School of Electrical and Electronic Engineering, Nanyang Technological University, Singapore 639798, Singapore.
Yu-Qing Zheng, National Key Laboratory of Science and Technology on Micro/Nano Fabrication; School of Integrated Circuits, Peking University, Beijing 100871, China.
Zijian Zheng, Department of Applied Biology and Chemical Technology, Faculty of Science, Research Institute for Intelligent Wearable Systems, Research Institute for Smart Energy, The Hong Kong Polytechnic University, Hung Hom, Kowloon, Hong Kong SAR, China.
Tao Zhou, Center for Neural Engineering, Department of Engineering Science and Mechanics, The Huck Institutes of the Life Sciences, Materials Research Institute, The Pennsylvania State University, University Park, Pennsylvania 16802, United States.
Bowen Zhu, Key Laboratory of 3D Micro/Nano Fabrication and Characterization of Zhejiang Province, School of Engineering, Westlake University, Hangzhou 310024, China.
Ming Zhu, Institute for Digital Molecular Analytics and Science (IDMxS), Nanyang Technological University, Singapore 636921, Singapore.
Rong Zhu, Department of Precision Instrument, Tsinghua University, Beijing 100084, China.
Yangzhi Zhu, Terasaki Institute for Biomedical Innovation, Los Angeles, California 90064, United States.
Yong Zhu, Department of Mechanical and Aerospace Engineering, Department of Materials Science and Engineering, and Department of Biomedical Engineering, North Carolina State University, Raleigh, North Carolina 27695, United States.
Guijin Zou, Institute of High Performance Computing (IHPC), Agency for Science, Technology and Research (A*STAR), Singapore 138632, Republic of Singapore.
Xiaodong Chen, Innovative Center for Flexible Devices (iFLEX), Max Planck-NTU Joint Laboratory for Artificial Senses, School of Materials Science and Engineering, Nanyang Technological University, Singapore 639798, Singapore; Institute of Materials Research and Engineering (IMRE), Agency for Science, Technology and Research (A*STAR), Singapore 138634, Republic of Singapore.
REFERENCES
- (1).Huddleston C. What Are Intelligent Sensors, and Why Should I Care About Them? In Intelligent Sensor Design Using the Microchip Dspic, Huddleston C. Ed.; Elsevier, 2006; pp 1–19. [Google Scholar]
- (2).McGee TD Principles and Methods of Temperature Measurement; John Wiley & Sons, 1988. [Google Scholar]
- (3).Heng Zhang Seismoscope. https://www.atlasobscura.com/places/zhang-heng-seismoscope (accessed 2021-10-15). [Google Scholar]
- (4).Johnon Warren S.. In Wikipedia. https://en.wikipedia.org/wiki/Warren_S._Johnson (accessed 2021-10-15) [Google Scholar]
- (5).Global Strategy on Digital Health 2020-2025; 978-92-4-002092-4; World Health Organization, Geneva, 2021. https://www.who.int/health-topics/digital-health#tab=tab_1 (accessed 2022-09-15). [Google Scholar]
- (6).Sassanelli C; Arriga T; Zanin S; D’Adamo I; Terzi S Industry 4.0 Driven Result-Oriented PSS: An Assessment in the Energy Management. Int. J. Energy Econ. Policy 2022, 12, 186–203. [Google Scholar]
- (7).History of the Transistor. In Wikipedia. https://en.wikipedia.org/wiki/History_of_the_transistor#First_working_transistor (accessed 2021-10-15).
- (8).Point-Contact Transistor. In Wikipedia. https://en.wikipedia.org/wiki/Point-contact_transistor (accessed 2021-10-15)
- (9).Patel R. Reboot AI with Human Values. Nature 2021, 598, 27–28. [Google Scholar]
- (10).Krittanawong C; Rogers AJ; Johnson KW; Wang Z; Turakhia MP; Halperin JL; Narayan SM Integration of Novel Monitoring Devices with Machine Learning Technology for Scalable Cardiovascular Management. Nat. Rev. Cardiol 2021, 18, 75–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Pokrajac L; Abbas A; Chrzanowski W; Dias GM; Eggleton BJ; Maguire S; Maine E; Malloy T; Nathwani J; Nazar L; et al. Nanotechnology for a Sustainable Future: Addressing Global Challenges with the International Network4sustainable Nanotechnology. ACS Nano 2021, 15, 18608–18623. [DOI] [PubMed] [Google Scholar]
- (12).Haque A; Milstein A; Fei-Fei L Illuminating the Dark Spaces of Healthcare with Ambient Intelligence. Nature 2020, 585, 193–202. [DOI] [PubMed] [Google Scholar]
- (13).Mishra T; Wang M; Metwally AA; Bogu GK; Brooks AW; Bahmani A; Alavi A; Celli A; Higgs E; Dagan-Rosenfeld O; et al. Pre-Symptomatic Detection of COVID-19 from Smartwatch Data. Nat. Biomed. Eng 2020, 4, 1208–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Lukas H; Xu C; Yu Y; Gao W Emerging Telemedicine Tools for Remote COVID-19 Diagnosis, Monitoring, and Management. ACS Nano 2020, 14, 16180–16193. [DOI] [PubMed] [Google Scholar]
- (15).Najjar D; Rainbow J; Sharma Timilsina S; Jolly P; de Puig H; Yafia M; Durr N; Sallum H; Alter G; Li JZ; et al. A Lab-on-a-Chip for the Concurrent Electrochemical Detection of SARS-CoV-2 RNA and Anti-SARS-CoV-2 Antibodies in Saliva and Plasma. Nat. Biomed. Eng 2022, 6, 968–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Ma Z; Chen P; Cheng W; Yan K; Pan L; Shi Y; Yu G Highly Sensitive, Printable Nanostructured Conductive Polymer Wireless Sensor for Food Spoilage Detection. Nano Lett. 2018, 18, 4570–4575. [DOI] [PubMed] [Google Scholar]
- (17).Guembe-Garcia M; Gonzalez-Ceballos L; Arnaiz A; Fernandez-Muino MA; Sancho MT; Oses SM; Ibeas S; Rovira J; Melero B; Represa C; et al. Easy Nitrite Analysis of Processed Meat with Colorimetric Polymer Sensors and a Smartphone App. ACS Appl. Mater. Interfaces 2022, 14, 37051–37058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Grell M; Barandun G; Asfour T; Kasimatis M; Collins ASP; Wang J; Güder F Point-of-Use Sensors and Machine Learning Enable Low-Cost Determination of Soil Nitrogen. Nat. Food 2021, 2, 981–989. [DOI] [PubMed] [Google Scholar]
- (19).Ilic IK; Lamanna L; Cortecchia D; Cataldi P; Luzio A; Caironi M Self-Powered Edible Defrosting Sensor. ACS Sens. 2022, 7, 2995–3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Elsisi M; Tran MQ; Mahmoud K; Lehtonen M; Darwish MMF Deep Learning-Based Industry 4.0 and Internet of Things Towards Effective Energy Management for Smart Buildings. Sensors 2021, 21, 1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Kim BH; Li K; Kim JT; Park Y; Jang H; Wang X; Xie Z; Won SM; Yoon HJ; Lee G; et al. Three-Dimensional Electronic Microfliers Inspired by Wind-Dispersed Seeds. Nature 2021, 597, 503–510. [DOI] [PubMed] [Google Scholar]
- (22).Iyer V; Gaensbauer H; Daniel TL; Gollakota S Wind Dispersal of Battery-Free Wireless Devices. Nature 2022, 603, 427–433. [DOI] [PubMed] [Google Scholar]
- (23).Merkoçi A. Smart Nanobiosensors in Agriculture. Nat. Food 2021, 2, 920–921. [DOI] [PubMed] [Google Scholar]
- (24).Prasad P; Raut P; Goel S; Barnwal RP; Bodhe GL Electronic Nose and Wireless Sensor Network for Environmental Monitoring Application in Pulp and Paper Industry: A Review. Environ. Monit. Assess 2022, 194, 855. [DOI] [PubMed] [Google Scholar]
- (25).Cheng I-C; Wagner S Overview of Flexible Electronics Technology. In Flexible Electronics: Materials and Applications, Wong WS, Salleo A. Eds.; Springer; US, 2009; pp 1–28. [Google Scholar]
- (26).Wang Y; Yin L; Bai Y; Liu S; Wang L; Zhou Y; Hou C; Yang Z; Wu H; Ma J; et al. Electrically Compensated, Tattoo-Like Electrodes for Epidermal Electrophysiology at Scale. Sci. Adv 2020, 6, No. eabd0996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Luo Y; Wang M; Wan C; Cai P; Loh XJ; Chen X Devising Materials Manufacturing toward Lab-to-Fab Translation of Flexible Electronics. Adv. Mater 2020, 32, 2001903. [DOI] [PubMed] [Google Scholar]
- (28).Khan Y; Thielens A; Muin S; Ting J; Baumbauer C; Arias AC A New Frontier of Printed Electronics: Flexible Hybrid Electronics. Adv. Mater 2020, 32, 1905279. [DOI] [PubMed] [Google Scholar]
- (29).Olivetti EA; Cullen JM Toward a Sustainable Materials System. Science 2018, 360, 1396. [DOI] [PubMed] [Google Scholar]
- (30).Rogers JA Materials for Semiconductor Devices That Can Bend, Fold, Twist, and Stretch. MRS Bull. 2014, 39, 549–556. [Google Scholar]
- (31).Souri H; Banerjee H; Jusufi A; Radacsi N; Stokes AA; Park I; Sitti M; Amjadi M Wearable and Stretchable Strain Sensors: Materials, Sensing Mechanisms, and Applications. Adv. Intell. Syst 2020, 2, 2000039. [Google Scholar]
- (32).Liu Z; Zhu T; Wang J; Zheng Z; Li Y; Li J; Lai Y Functionalized Fiber-Based Strain Sensors: Pathway to Next-Generation Wearable Electronics. Nano-Micro Lett. 2022, 14, 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Liu S; Rao Y; Jang H; Tan P; Lu N Strategies for Body-Conformable Electronics. Matter 2022, 5, 1104–1136. [Google Scholar]
- (34).Arias AC; MacKenzie JD; McCulloch I; Rivnay J; Salleo A Materials and Applications for Large Area Electronics: Solution-Based Approaches. Chem. Rev 2010, 110, 3–24. [DOI] [PubMed] [Google Scholar]
- (35).Ates HC; Nguyen PQ; Gonzalez-Macia L; Morales-Narvaez E; Guder F; Collins JJ; Dincer C End-to-End Design of Wearable Sensors. Nat. Rev. Mater 2022, 7, 887–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Bandodkar AJ; Jeerapan I; Wang J Wearable Chemical Sensors: Present Challenges and Future Prospects. ACS Sens. 2016, 1, 464–482. [Google Scholar]
- (37).Ling Y; An T; Yap LW; Zhu B; Gong S; Cheng W Disruptive, Soft, Wearable Sensors. Adv. Mater 2020, 32, 1904664. [DOI] [PubMed] [Google Scholar]
- (38).Gao Y; Yu L; Yeo JC; Lim CT Flexible Hybrid Sensors for Health Monitoring: Materials and Mechanisms to Render Wearability. Adv. Mater 2020, 32, 1902133. [DOI] [PubMed] [Google Scholar]
- (39).Chen S; Qi J; Fan S; Qiao Z; Yeo JC; Lim CT Flexible Wearable Sensors for Cardiovascular Health Monitoring. Adv. Healthc. Mater 2021, 10, 2100116. [DOI] [PubMed] [Google Scholar]
- (40).Heng W; Solomon S; Gao W Flexible Electronics and Devices as Human-Machine Interfaces for Medical Robotics. Adv. Mater 2022, 34, 2107902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Wang M; Luo Y; Wang T; Wan C; Pan L; Pan S; He K; Neo A; Chen X Artificial Skin Perception. Adv. Mater 2021, 33, 2003014. [DOI] [PubMed] [Google Scholar]
- (42).Massari L; Fransvea G; D’Abbraccio J; Filosa M; Terruso G; Aliperta A; D’Alesio G; Zaltieri M; Schena E; Palermo E; et al. Functional Mimicry of Ruffini Receptors with Fibre Bragg Gratings and Deep Neural Networks Enables a Bio-Inspired Large-Area Tactile-Sensitive Skin. Nat. Mach. Intell 2022, 4, 425–435. [Google Scholar]
- (43).Someya T; Sekitani T; Iba S; Kato Y; Kawaguchi H; Sakurai T A Large-Area, Flexible Pressure Sensor Matrix with Organic Field-Effect Transistors for Artificial Skin Applications. Proc. Natl. Acad. Sci. U. S. A 2004, 101, 9966–9970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Jiang C; Cheng X; Nathan A Flexible Ultralow-Power Sensor Interfaces for E-Skin. Proc. IEEE 2019, 107, 2084–2105. [Google Scholar]
- (45).Yousefi H; Su HM; Imani SM; Alkhaldi K; CD MF; Didar TF Intelligent Food Packaging: A Review of Smart Sensing Technologies for Monitoring Food Quality. ACS Sens. 2019, 4, 808–821. [DOI] [PubMed] [Google Scholar]
- (46).Rao Z; Ershad F; Almasri A; Gonzalez L; Wu X; Yu C Soft Electronics for the Skin: From Health Monitors to Human-Machine Interfaces. Adv. Mater. Technol 2020, 5, 2000233. [Google Scholar]
- (47).Wang T; Wang M; Yang L; Li Z; Loh XJ; Chen X Cyber-Physiochemical Interfaces. Adv. Mater 2020, 32, 1905522. [DOI] [PubMed] [Google Scholar]
- (48).Yu Y; Nyein HYY; Gao W; Javey A Flexible Electrochemical Bioelectronics: The Rise of in Situ Bioanalysis. Adv. Mater 2020, 32, 1902083. [DOI] [PubMed] [Google Scholar]
- (49).Xu C; Yang Y; Gao W Skin-Interfaced Sensors in Digital Medicine: From Materials to Applications. Matter 2020, 2, 1414–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Lim HR; Kim HS; Qazi R; Kwon YT; Jeong JW; Yeo WH Advanced Soft Materials, Sensor Integrations, and Applications of Wearable Flexible Hybrid Electronics in Healthcare, Energy, and Environment. Adv. Mater 2020, 32, 1901924. [DOI] [PubMed] [Google Scholar]
- (51).Sempionatto JR; Lasalde-Ramírez JA; Mahato K; Wang J; Gao W Wearable Chemical Sensors for Biomarker Discovery in the Omics Era. Nat. Rev. Chem 2022, 6, 899–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Hou HL; Anichini C; Samorì P; Criado A; Prato M 2D Van der Waals Heterostructures for Chemical Sensing. Adv. Funct. Mater 2022, 32, 2207065. [Google Scholar]
- (53).Yang B; Jiang X; Fang X; Kong J Wearable Chem-Biosensing Devices: From Basic Research to Commercial Market. Lab Chip 2021, 21, 4285–4310. [DOI] [PubMed] [Google Scholar]
- (54).Wang B; Zhao C; Wang Z; Yang K-A; Cheng X; Liu W; Yu W; Lin S; Zhao Y; Cheung KM; et al. Wearable Aptamer-Field-Effect Transistor Sensing System for Noninvasive Cortisol Monitoring. Sci. Adv 2022, 8, No. eabk0967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).Zhao C; Man T; Cao Y; Weiss PS; Monbouquette HG; Andrews AM Flexible and Implantable Polyimide Aptamer-Field-Effect Transistor Biosensors. ACS Sens. 2022, 7, 3644–3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (56).Kaltenbrunner M; Sekitani T; Reeder J; Yokota T; Kuribara K; Tokuhara T; Drack M; Schwodiauer R; Graz I; Bauer-Gogonea S; et al. An Ultra-Lightweight Design for Imperceptible Plastic Electronics. Nature 2013, 499, 458–463. [DOI] [PubMed] [Google Scholar]
- (57).Lee S; Franklin S; Hassani FA; Yokota T; Nayeem MOG; Wang Y; Leib R; Cheng G; Franklin DW; Someya T Nanomesh Pressure Sensor for Monitoring Finger Manipulation without Sensory Interference. Science 2020, 370, 966–970. [DOI] [PubMed] [Google Scholar]
- (58).Liana DD; Raguse B; Gooding JJ; Chow E Recent Advances in Paper-Based Sensors. Sensors 2012, 12, 11505–11526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (59).Singh AT; Lantigua D; Meka A; Taing S; Pandher M; Camci-Unal G Paper-Based Sensors: Emerging Themes and Applications. Sensors 2018, 18, 2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (60).Li H; Ma Y; Huang Y Material Innovation and Mechanics Design for Substrates and Encapsulation of Flexible Electronics: A Review. Mater. Horiz 2021, 8, 383–400. [DOI] [PubMed] [Google Scholar]
- (61).Torrente-Rodriguez RM; Tu J; Yang Y; Min J; Wang M; Song Y; Yu Y; Xu C; Ye C; IsHak WW; et al. Investigation of Cortisol Dynamics in Human Sweat Using a Graphene-Based Wireless Mhealth System. Matter 2020, 2, 921–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (62).Tang W; Yin L; Sempionatto JR; Moon JM; Teymourian H; Wang J Touch-Based Stressless Cortisol Sensing. Adv. Mater 2021, 33, 2008465. [DOI] [PubMed] [Google Scholar]
- (63).Liu Y; Yiu C; Song Z; Huang Y; Yao K; Wong T; Zhou J; Zhao L; Huang X; Nejad Sina K; et al. Electronic Skin as Wireless Human-Machine Interfaces for Robotic VR. Sci. Adv 2022, 8, No. eabl6700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (64).Kiran DR Reliability Engineering. In Total Quality Management: Key Concepts and Case Studies, BSP Books, 2017; pp 391–404. [Google Scholar]
- (65).Zhou Y; Zhao X; Xu J; Fang Y; Chen G; Song Y; Li S; Chen J Giant Magnetoelastic Effect in Soft Systems for Bioelectronics. Nat. Mater 2021, 20, 1670–1676. [DOI] [PubMed] [Google Scholar]
- (66).Chen G; Zhao X; Andalib S; Xu J; Zhou Y; Tat T; Lin K; Chen J Discovering Giant Magnetoelasticity in Soft Matter for Electronic Textiles. Matter 2021, 4, 3725–3740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (67).Zhao X; Zhou Y; Xu J; Chen G; Fang Y; Tat T; Xiao X; Song Y; Li S; Chen J Soft Fibers with Magnetoelasticity for Wearable Electronics. Nat. Commun 2021, 12, 6755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (68).Xu J; Tat T; Zhao X; Zhou Y; Ngo D; Xiao X; Chen J A Programmable Magnetoelastic Sensor Array for Self-Powered Human-Machine Interface. Appl. Phys. Rev 2022, 9, 031404. [Google Scholar]
- (69).Shehzad K; Xu Y; Gao C; Li H; Dang ZM; Hasan T; Luo J; Duan X Flexible Dielectric Nanocomposites with Ultrawide Zero-Temperature Coefficient Windows for Electrical Energy Storage and Conversion under Extreme Conditions. ACS Appl. Mater. Interfaces 2017, 9, 7591–7600. [DOI] [PubMed] [Google Scholar]
- (70).Lee J; Kim JW; Park SA; Son SY; Choi K; Lee W; Kim M; Kim JY; Park T Study of Burn-in Loss in Green Solvent-Processed Ternary Blended Organic Photovoltaics Derived from Uv-Crosslinkable Semiconducting Polymers and Nonfullerene Acceptors. Adv. Energy Mater 2019, 9, 1901829. [Google Scholar]
- (71).Sun W; Xie L; Guo X; Su W; Zhang Q Photocross-Linkable Hole Transport Materials for Inkjet-Printed High-Efficient Quantum Dot Light-Emitting Diodes. ACS Appl. Mater. Interfaces 2020, 12, 58369–58377. [DOI] [PubMed] [Google Scholar]
- (72).Zhang Y; Ma Y; Wang Y; Zhang X; Zuo C; Shen L; Ding L Lead-Free Perovskite Photodetectors: Progress, Challenges, and Opportunities. Adv. Mater 2021, 33, 2006691. [DOI] [PubMed] [Google Scholar]
- (73).Hao D; Zou J; Huang J Recent Developments in Flexible Photodetectors Based on Metal Halide Perovskite. InfoMat 2020, 2, 139–169. [Google Scholar]
- (74).Song E; Li J; Won SM; Bai W; Rogers JA Materials for Flexible Bioelectronic Systems as Chronic Neural Interfaces. Nat. Mater 2020, 19, 590–603. [DOI] [PubMed] [Google Scholar]
- (75).Le Floch P; Meixuanzi S; Tang J; Liu J; Suo Z Stretchable Seal. ACS Appl. Mater. Interfaces 2018, 10, 27333–27343. [DOI] [PubMed] [Google Scholar]
- (76).Mariello M; Kim K; Wu K; Lacour SP; Leterrier Y Recent Advances in Encapsulation of Flexible Bioelectronic Implants: Materials, Technologies, and Characterization Methods. Adv. Mater 2022, 34, 2201129. [DOI] [PubMed] [Google Scholar]
- (77).Vacca P. Flexible Barriers and Packaging. In Organic Flexible Electronics, Cosseddu P, Caironi M Eds.; Woodhead Publishing, 2021; pp 225–248. [Google Scholar]
- (78).Shaikh SF; Mazo-Mantilla HF; Qaiser N; Khan SM; Nassar JM; Geraldi NR; Duarte CM; Hussain MM Noninvasive Featherlight Wearable Compliant ″Marine Skin″: Stand-alone Multisensory System for Deep-Sea Environmental Monitoring. Small 2019, 15, 1804385. [DOI] [PubMed] [Google Scholar]
- (79).Chu B; Burnett W; Chung JW; Bao Z Bring on the Bodynet. Nature 2017, 549, 328–330. [DOI] [PubMed] [Google Scholar]
- (80).Zhao S; Zhu R Flexible Bimodal Sensor for Simultaneous and Independent Perceiving of Pressure and Temperature Stimuli. Adv. Mater. Technol 2017, 2, 1700183. [Google Scholar]
- (81).Wang L; Zhu R; Li G Temperature and Strain Compensation for Flexible Sensors Based on Thermosensation. ACS Appl. Mater. Interfaces 2020, 12, 1953–1961. [DOI] [PubMed] [Google Scholar]
- (82).You I; Mackanic DG; Matsuhisa N; Kang J; Kwon J; Beker L; Mun J; Suh W; Kim Tae Y; Tok Jeffrey BH; et al. Artificial Multimodal Receptors Based on Ion Relaxation Dynamics. Science 2020, 370, 961–965. [DOI] [PubMed] [Google Scholar]
- (83).Yang JC; Kim JO; Oh J; Kwon SY; Sim JY; Kim DW; Choi HB; Park S Microstructured Porous Pyramid-Based Ultrahigh Sensitive Pressure Sensor Insensitive to Strain and Temperature. ACS Appl. Mater. Interfaces 2019, 11, 19472–19480. [DOI] [PubMed] [Google Scholar]
- (84).Gao W; Emaminejad S; Nyein HYY; Challa S; Chen K; Peck A; Fahad HM; Ota H; Shiraki H; Kiriya D; et al. Fully Integrated Wearable Sensor Arrays for Multiplexed in Situ Perspiration Analysis. Nature 2016, 529, 509–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (85).Tai LC; Gao W; Chao M; Bariya M; Ngo QP; Shahpar Z; Nyein HYY; Park H; Sun J; Jung Y; et al. Methylxanthine Drug Monitoring with Wearable Sweat Sensors. Adv. Mater 2018, 30, 1707442. [DOI] [PubMed] [Google Scholar]
- (86).Lin PH; Li BR Antifouling Strategies in Advanced Electrochemical Sensors and Biosensors. Analyst 2020, 145, 1110–1120. [DOI] [PubMed] [Google Scholar]
- (87).Jiang C; Wang G; Hein R; Liu N; Luo X; Davis JJ Antifouling Strategies for Selective in Vitro and in Vivo Sensing. Chem. Rev 2020, 120, 3852–3889. [DOI] [PubMed] [Google Scholar]
- (88).Li Z; Liu Y; Chen X; Cao H; Shen H; Mou L; Deng X; Jiang X; Cong Y Surface-Modified Mesoporous Nanofibers for Microfluidic Immunosensor with an Ultra-Sensitivity and High Signal-to-Noise Ratio. Biosens. Bioelectron 2020, 166, 112444. [DOI] [PubMed] [Google Scholar]
- (89).Sabate Del Rio J; Henry OYF; Jolly P; Ingber DE An Antifouling Coating That Enables Affinity-Based Electrochemical Biosensing in Complex Biological Fluids. Nat. Nanotechnol 2019, 14, 1143–1149. [DOI] [PubMed] [Google Scholar]
- (90).Patel J; Radhakrishnan L; Zhao B; Uppalapati B; Daniels RC; Ward KR; Collinson MM Electrochemical Properties of Nanostructured Porous Gold Electrodes in Biofouling Solutions. Anal. Chem 2013, 85, 11610–11618. [DOI] [PubMed] [Google Scholar]
- (91).Lin Y; Bariya M; Nyein HYY; Kivimäki L; Uusitalo S; Jansson E; Ji W; Yuan Z; Happonen T; Liedert C; et al. Porous Enzymatic Membrane for Nanotextured Glucose Sweat Sensors with High Stability toward Reliable Noninvasive Health Monitoring. Adv. Funct. Mater 2019, 29, 1902521. [Google Scholar]
- (92).Dadras-Toussi O; Khorrami M; Louis Sam Titus ASC; Majd S; Mohan C; Abidian MR Multiphoton Lithography of Organic Semiconductor Devices for 3D Printing of Flexible Electronic Circuits, Biosensors, and Bioelectronics. Adv. Mater 2022, 34, 2200512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (93).Chong Y; Liu Q; Ge C Advances in Oxidase-Mimicking Nanozymes: Classification, Activity Regulation and Biomedical Applications. Nano Today 2021, 37, 101076. [Google Scholar]
- (94).Gooding JJ Can Nanozymes Have an Impact on Sensing? ACS Sens. 2019, 4, 2213–2214. [DOI] [PubMed] [Google Scholar]
- (95).Wu J; Wang X; Wang Q; Lou Z; Li S; Zhu Y; Qin L; Wei H Nanomaterials with Enzyme-Like Characteristics (Nanozymes): Next-Generation Artificial Enzymes (II). Chem. Soc. Rev 2019, 48, 1004–1076. [DOI] [PubMed] [Google Scholar]
- (96).Tu J; Torrente-Rodríguez RM; Wang M; Gao W The Era of Digital Health: A Review of Portable and Wearable Affinity Biosensors. Adv. Funct. Mater 2020, 30, 1906713. [Google Scholar]
- (97).Teymourian H; Barfidokht A; Wang J Electrochemical Glucose Sensors in Diabetes Management: An Updated Review (2010-2020). Chem. Soc. Rev 2020, 49, 7671–7709. [DOI] [PubMed] [Google Scholar]
- (98).Wang Y; Lin Y Enhanced Ion Sensing Stability with Nanotextured Biosensors. In IEEE 16th International Conference on Nano/Micro Engineered and Molecular Systems (NEMS), April 25–29, 2021, Xiamen, China; IEEE, 2021. [Google Scholar]
- (99).Ballantine DS; Martin SJ; Ricco AJ; Frye GC; Wohltjen H; White RM, Zellers ET Chapter 5 - Chemical and Biological Sensors. In Acoustic Wave Sensors, Ballantine DS; Martin SJ; Ricco AJ; Frye GC; Wohltjen H; White RM; Zellers ET Eds.; Academic Press, 1997; pp 222–330. [Google Scholar]
- (100).Zipser L. Selectivity of Sensor Systems. Sensor. Actuat. A: Phys 1993, 37-38, 286–289. [Google Scholar]
- (101).Peveler WJ; Yazdani M; Rotello VM Selectivity and Specificity: Pros and Cons in Sensing. ACS Sens. 2016, 1, 1282–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (102).Araromi OA; Graule MA; Dorsey KL; Castellanos S; Foster JR; Hsu WH; Passy AE; Vlassak JJ; Weaver JC; Walsh CJ; et al. Ultra-Sensitive and Resilient Compliant Strain Gauges for Soft Machines. Nature 2020, 587, 219–224. [DOI] [PubMed] [Google Scholar]
- (103).Liu Z; Zheng Y; Jin L; Chen K; Zhai H; Huang Q; Chen Z; Yi Y; Umar M; Xu L; et al. Highly Breathable and Stretchable Strain Sensors with Insensitive Response to Pressure and Bending. Adv. Funct. Mater 2021, 31, 2007622. [Google Scholar]
- (104).Su Q; Zou Q; Li Y; Chen Y; Teng S-Y; Kelleher JT; Nith R; Cheng P; Li N; Liu W; et al. A Stretchable and Strain-Unperturbed Pressure Sensor for Motion Interference-Free Tactile Monitoring on Skins. Sci. Adv 2021, 7, No. eabi4563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (105).Wu X; Ahmed M; Khan Y; Payne ME; Zhu J; Lu C; Evans JW; Arias AC A Potentiometric Mechanotransduction Mechanism for Novel Electronic Skins. Sci. Adv 2020, 6, No. eaba1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (106).Lee S; Reuveny A; Reeder J; Lee S; Jin H; Liu Q; Yokota T; Sekitani T; Isoyama T; Abe Y; et al. A Transparent Bending-Insensitive Pressure Sensor. Nat. Nanotechnol 2016, 11, 472–478. [DOI] [PubMed] [Google Scholar]
- (107).Boutry CM; Kaizawa Y; Schroeder BC; Chortos A; Legrand A; Wang Z; Chang J; Fox P; Bao Z A Stretchable and Biodegradable Strain and Pressure Sensor for Orthopaedic Application. Nat. Electron 2018, 1, 314–321. [Google Scholar]
- (108).Zeng X; Liu Y; Liu F; Wang W; Liu X; Wei X; Hu Y A Bioinspired Three-Dimensional Integrated E-Skin for Multiple Mechanical Stimuli Recognition. Nano Energy 2022, 92, 106777. [Google Scholar]
- (109).Hua Q; Sun J; Liu H; Bao R; Yu R; Zhai J; Pan C; Wang ZL Skin-Inspired Highly Stretchable and Conformable Matrix Networks for Multifunctional Sensing. Nat. Commun 2018, 9, 244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (110).Xu L; Huang Z; Deng Z; Du Z; Sun TL; Guo ZH; Yue K A Transparent, Highly Stretchable, Solvent-Resistant, Recyclable Multifunctional Ionogel with Underwater Self-Healing and Adhesion for Reliable Strain Sensors. Adv. Mater 2021, 33, 2105306. [DOI] [PubMed] [Google Scholar]
- (111).Cai Y; Shen J; Yang C-W; Wan Y; Tang H-L; Aljarb Areej A; Chen C; Fu J-H; Wei X; Huang K-W; et al. Mixed-Dimensional MXene-Hydrogel Heterostructures for Electronic Skin Sensors with Ultrabroad Working Range. Sci. Adv 2020, 6, No. eabb5367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (112).Pang C; Lee GY; Kim TI; Kim SM; Kim HN; Ahn SH; Suh KY A Flexible and Highly Sensitive Strain-Gauge Sensor Using Reversible Interlocking of Nanofibres. Nat. Mater 2012, 11, 795–801. [DOI] [PubMed] [Google Scholar]
- (113).Park J; Lee Y; Hong J; Lee Y; Ha M; Jung Y; Lim H; Kim SY; Ko H Tactile-Direction-Sensitive and Stretchable Electronic Skins Based on Human-Skin-Inspired Interlocked Micro-structures. ACS Nano 2014, 8, 12020–12029. [DOI] [PubMed] [Google Scholar]
- (114).Mu C; Song Y; Huang W; Ran A; Sun R; Xie W; Zhang H Flexible Normal-Tangential Force Sensor with Opposite Resistance Responding for Highly Sensitive Artificial Skin. Adv. Funct. Mater 2018, 28, 1707503. [Google Scholar]
- (115).Choi D; Jang S; Kim JS; Kim H-J; Kim DH; Kwon J-Y A Highly Sensitive Tactile Sensor Using a Pyramid-Plug Structure for Detecting Pressure, Shear Force, and Torsion. Adv. Mater. Technol 2019, 4, 1800284. [Google Scholar]
- (116).Bai H; Li S; Barreiros J; Tu Y; Pollock CR; Shepherd RF Stretchable Distributed Fiber-Optic Sensors. Science 2020, 370, 848. [DOI] [PubMed] [Google Scholar]
- (117).Won SM; Wang H; Kim BH; Lee K; Jang H; Kwon K; Han M; Crawford KE; Li H; Lee Y; et al. Multimodal Sensing with a Three-Dimensional Piezoresistive Structure. ACS Nano 2019, 13, 10972–10979. [DOI] [PubMed] [Google Scholar]
- (118).Gong S; Yap LW; Zhu B; Zhai Q; Liu Y; Lyu Q; Wang K; Yang M; Ling Y; Lai DTH; et al. Local Crack-Programmed Gold Nanowire Electronic Skin Tattoos for In-Plane Multisensor Integration. Adv. Mater 2019, 31, 1903789. [DOI] [PubMed] [Google Scholar]
- (119).Gao Y; Zhang B; Liu Y; Yao K; Huang X; Li J; Wong TH; Huang Y; Li J; Zhou J; et al. Mechanoreceptor Inspired Electronic Skin for Multi-Modal Tactile Information Decoding. Adv. Mater. Technol 2023, 8, 2200759. [Google Scholar]
- (120).Nakatsuka N; Yang K-A; Abendroth JM; Cheung KM; Xu X; Yang H; Zhao C; Zhu B; Rim You S; Yang Y; et al. Aptamer-Field-Effect Transistors Overcome Debye Length Limitations for Small-Molecule Sensing. Science 2018, 362, 319–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (121).Das J; Gomis S; Chen JB; Yousefi H; Ahmed S; Mahmud A; Zhou W; Sargent EH; Kelley SO Reagentless Biomolecular Analysis Using a Molecular Pendulum. Nat. Chem 2021, 13, 428–434. [DOI] [PubMed] [Google Scholar]
- (122).Arroyo-Curras N; Somerson J; Vieira PA; Ploense KL; Kippin TE; Plaxco KW Real-Time Measurement of Small Molecules Directly in Awake, Ambulatory Animals. Proc. Natl. Acad. Sci. U. S. A 2017, 114, 645–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (123).Bernards DA; Malliaras GG; Toombes GES; Gruner SM Gating of an Organic Transistor through a Bilayer Lipid Membrane with Ion Channels. Appl. Phys. Lett 2006, 89, 053505. [Google Scholar]
- (124).Tang TC; Tham E; Liu X; Yehl K; Rovner AJ; Yuk H; de la Fuente-Nunez C; Isaacs FJ; Zhao X; Lu TK Hydrogel-Based Biocontainment of Bacteria for Continuous Sensing and Computation. Nat. Chem. Biol 2021, 17, 724–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (125).Liu X; Tang TC; Tham E; Yuk H; Lin S; Lu TK; Zhao X Stretchable Living Materials and Devices with Hydrogel-Elastomer Hybrids Hosting Programmed Cells. Proc. Natl. Acad. Sci. U.S.A 2017, 114, 2200–2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (126).Atkinson JT; Su L; Zhang X; Bennett GN; Silberg JJ; Ajo-Franklin CM Real-Time Bioelectronic Sensing of Environmental Contaminants. Nature 2022, 611, 548–553. [DOI] [PubMed] [Google Scholar]
- (127).Li J; Liu Y; Yuan L; Zhang B; Bishop ES; Wang K; Tang J; Zheng Y-Q; Xu W; Niu S; et al. A Tissue-Like Neurotransmitter Sensor for the Brain and Gut. Nature 2022, 606, 94–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (128).Bae CW; Toi PT; Kim BY; Lee WI; Lee HB; Hanif A; Lee EH; Lee NE Fully Stretchable Capillary Microfluidics-Integrated Nanoporous Gold Electrochemical Sensor for Wearable Continuous Glucose Monitoring. ACS Appl. Mater. Interfaces 2019, 11, 14567–14575. [DOI] [PubMed] [Google Scholar]
- (129).Shu Y; Su T; Lu Q; Shang Z; Xu Q; Hu X Highly Stretchable Wearable Electrochemical Sensor Based on Ni-Co MOF Nanosheet-Decorated Ag/RGO/PU Fiber for Continuous Sweat Glucose Detection. Anal. Chem 2021, 93, 16222–16230. [DOI] [PubMed] [Google Scholar]
- (130).Wang B; Yang D; Chang Z; Zhang R; Dai J; Fang Y Wearable Bioelectronic Masks for Wireless Detection of Respiratory Infectious Diseases by Gaseous Media. Matter 2022, 5, 4347–4362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (131).Leemans M; Bauër P; Cuzuel V; Audureau E; Fromantin I Volatile Organic Compounds Analysis as a Potential Novel Screening Tool for Breast Cancer: A Systematic Review. Biomark. Insights 2022, 17, 117727192211007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (132).Hu W; Wu W; Jian Y; Haick H; Zhang G; Qian Y; Yuan M; Yao M Volatolomics in Healthcare and Its Advanced Detection Technology. Nano Res. 2022, 15, 8185–8213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (133).Arnold C. Diagnostics to Take Your Breath Away. Nat. Biotechnol 2022, 40, 990–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (134).Holopainen JK; Gershenzon J Multiple Stress Factors and the Emission of Plant VOCs. Trends Plant Sci. 2010, 15, 176–184. [DOI] [PubMed] [Google Scholar]
- (135).MacDougall S; Bayansal F; Ahmadi A Emerging Methods of Monitoring Volatile Organic Compounds for Detection of Plant Pests and Disease. Biosensors 2022, 12, 239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (136).Volatile Biomarkers for Human Health: From Nature to Artificial Senses, Haick H., Ed.; The Royal Society of Chemistry, 2022. [Google Scholar]
- (137).Nakhleh MK; Amal H; Jeries R; Broza YY; Aboud M; Gharra A; Ivgi H; Khatib S; Badarneh S; Har-Shai L; et al. Diagnosis and Classification of 17 Diseases from 1404 Subjects via Pattern Analysis of Exhaled Molecules. ACS Nano 2017, 11, 112–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (138).Wang Y; Haick H; Guo S; Wang C; Lee S; Yokota T; Someya T Skin Bioelectronics Towards Long-Term, Continuous Health Monitoring. Chem. Soc. Rev 2022, 51, 3759–3793. [DOI] [PubMed] [Google Scholar]
- (139).Yao MS; Lv XJ; Fu ZH; Li WH; Deng WH; Wu GD; Xu G Layer-by-Layer Assembled Conductive Metal-Organic Framework Nanofilms for Room-Temperature Chemiresistive Sensing. Angew. Chem. Int. Ed 2017, 56, 16510–16514. [DOI] [PubMed] [Google Scholar]
- (140).Park C; Koo WT; Chong S; Shin H; Kim YH; Cho HJ; Jang JS; Kim DH; Lee J; Park S; et al. Confinement of Ultrasmall Bimetallic Nanoparticles in Conductive Metal-Organic Frameworks via Site-Specific Nucleation. Adv. Mater 2021, 33, 2101216. [DOI] [PubMed] [Google Scholar]
- (141).Koo WT; Qiao S; Ogata AF; Jha G; Jang JS; Chen VT; Kim ID; Penner RM Accelerating Palladium Nanowire H2 Sensors Using Engineered Nanofiltration. ACS Nano 2017, 11, 9276–9285. [DOI] [PubMed] [Google Scholar]
- (142).Li Z; Liu Y; Hossain O; Paul R; Yao S; Wu S; Ristaino JB; Zhu Y; Wei Q Real-Time Monitoring of Plant Stresses via Chemiresistive Profiling of Leaf Volatiles by a Wearable Sensor. Matter 2021, 4, 2553–2570. [Google Scholar]
- (143).Anderson MJ; Sullivan JG; Horiuchi TK; Fuller SB; Daniel TL A Bio-Hybrid Odor-Guided Autonomous Palm-Sized Air Vehicle. Bioinspir. Biomim 2020, 16, 26002. [DOI] [PubMed] [Google Scholar]
- (144).Mitsubayashi K; Toma K; Iitani K; Arakawa T Gas-Phase Biosensors: A Review. Sensor. Actuat. B: Chem 2022, 367, 132053. [Google Scholar]
- (145).Manzini I; Schild D; Di Natale C Principles of Odor Coding in Vertebrates and Artificial Chemosensory Systems. Physiol. Rev 2022, 102, 61–154. [DOI] [PubMed] [Google Scholar]
- (146).Lu J; Xu C; Wang Y; Zhang Y; Fu Z Corrugated Cobalt Titanate/Partially Reduced Graphene Oxide Heterojunctions for a Selective Isopentanol Gas Sensor. ACS Appl. Nano Mater 2022, 5, 4721–4730. [Google Scholar]
- (147).Shin H; Kim DH; Jung W; Jang JS; Kim YH; Lee Y; Chang K; Lee J; Park J; Namkoong K; et al. Surface Activity-Tuned Metal Oxide Chemiresistor: Toward Direct and Quantitative Halitosis Diagnosis. ACS Nano 2021, 15, 14207–14217. [DOI] [PubMed] [Google Scholar]
- (148).Yuan H; Li N; Fan W; Cai H; Zhao D Metal-Organic Framework Based Gas Sensors. Adv. Sci 2022, 9, 2104374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (149).Song Z; Ye W; Chen Z; Chen Z; Li M; Tang W; Wang C; Wan Z; Poddar S; Wen X; et al. Wireless Self-Powered High-Performance Integrated Nanostructured-Gas-Sensor Network for Future Smart Homes. ACS Nano 2021, 15, 7659–7667. [DOI] [PubMed] [Google Scholar]
- (150).Capman NSS; Zhen XV; Nelson JT; Chaganti VRSK; Finc RC; Lyden MJ; Williams TL; Freking M; Sherwood GJ; Bühlmann P; et al. Machine Learning-Based Rapid Detection of Volatile Organic Compounds in a Graphene Electronic Nose. ACS Nano 2022, 16, 19567–19583. [DOI] [PubMed] [Google Scholar]
- (151).Guo L; Wang T; Wu Z; Wang J; Wang M; Cui Z; Ji S; Cai J; Xu C; Chen X Portable Food-Freshness Prediction Platform Based on Colorimetric Barcode Combinatorics and Deep Convolutional Neural Networks. Adv. Mater 2020, 32, 2004805. [DOI] [PubMed] [Google Scholar]
- (152).Ge L; Ye X; Yu Z; Chen B; Liu C; Guo H; Zhang S; Sassa F; Hayashi K A Fully Inkjet-Printed Disposable Gas Sensor Matrix with Molecularly Imprinted Gas-Selective Materials. npj Flex. Electron 2022, 6, 1. [Google Scholar]
- (153).Leong SX; Leong YX; Tan EX; Sim HYF; Koh CSL; Lee YH; Chong C; Ng LS; Chen JRT; Pang DWC; et al. Noninvasive and Point-of-Care Surface-Enhanced Raman Scattering (SERS)-Based Breathalyzer for Mass Screening of Coronavirus Disease 2019 (COVID-19) under 5 min. ACS Nano 2022, 16, 2629–2639. [DOI] [PubMed] [Google Scholar]
- (154).Vishinkin R; Busool R; Mansour E; Fish F; Esmail A; Kumar P; Gharaa A; Cancilla JC; Torrecilla JS; Skenders G; et al. Profiles of Volatile Biomarkers Detect Tuberculosis from Skin. Adv. Sci 2021, 8, 2100235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (155).Wang B; Thukral A; Xie Z; Liu L; Zhang X; Huang W; Yu X; Yu C; Marks TJ; Facchetti A Flexible and Stretchable Metal Oxide Nanofiber Networks for Multimodal and Monolithically Integrated Wearable Electronics. Nat. Commun 2020, 11, 2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (156).Jalal AH; Alam F; Roychoudhury S; Umasankar Y; Pala N; Bhansali S Prospects and Challenges of Volatile Organic Compound Sensors in Human Healthcare. ACS Sens. 2018, 3, 1246–1263. [DOI] [PubMed] [Google Scholar]
- (157).Tang W; Chen Z; Song Z; Wang C; Wan Z; Chan CLJ; Chen Z; Ye W; Fan Z Microheater Integrated Nanotube Array Gas Sensor for Parts-Per-Trillion Level Gas Detection and Single Sensor-Based Gas Discrimination. ACS Nano 2022, 16, 10968–10978. [DOI] [PubMed] [Google Scholar]
- (158).Qu X; Liu Z; Tan P; Wang C; Liu Y; Feng H; Luo D; Li Z; Wang ZL Artificial Tactile Perception Smart Finger for Material Identification Based on Triboelectric Sensing. Sci. Adv 2022, 8, No. eabq2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (159).Zhai W; Zhu J; Wang Z; Zhao Y; Zhan P; Wang S; Zheng G; Shao C; Dai K; Liu C; et al. Stretchable, Sensitive Strain Sensors with a Wide Workable Range and Low Detection Limit for Wearable Electronic Skins. ACS Appl. Mater. Interfaces 2022, 14, 4562–4570. [DOI] [PubMed] [Google Scholar]
- (160).Tan XC; Xu JD; Jian JM; Dun GH; Cui TR; Yang Y; Ren TL Programmable Sensitivity Screening of Strain Sensors by Local Electrical and Mechanical Properties Coupling. ACS Nano 2021, 15, 20590–20599. [DOI] [PubMed] [Google Scholar]
- (161).Zhang H; Liu D; Lee JH; Chen H; Kim E; Shen X; Zheng Q; Yang J; Kim JK Anisotropic, Wrinkled, and Crack-Bridging Structure for Ultrasensitive, Highly Selective Multidirectional Strain Sensors. Nano-Micro Lett. 2021, 13, 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (162).Lee J; Shin S; Lee S; Song J; Kang S; Han H; Kim S; Kim S; Seo J; Kim D; et al. Highly Sensitive Multifilament Fiber Strain Sensors with Ultrabroad Sensing Range for Textile Electronics. ACS Nano 2018, 12, 4259–4268. [DOI] [PubMed] [Google Scholar]
- (163).Cai Y; Shen J; Ge G; Zhang Y; Jin W; Huang W; Shao J; Yang J; Dong X Stretchable Ti3C2Tx MXene/Carbon Nanotube Composite Based Strain Sensor with Ultrahigh Sensitivity and Tunable Sensing Range. ACS Nano 2018, 12, 56–62. [DOI] [PubMed] [Google Scholar]
- (164).Ha KH; Huh H; Li Z; Lu N Soft Capacitive Pressure Sensors: Trends, Challenges, and Perspectives. ACS Nano 2022, 16, 3442–3448. [DOI] [PubMed] [Google Scholar]
- (165).Qin J; Yin LJ; Hao YN; Zhong SL; Zhang DL; Bi K; Zhang YX; Zhao Y; Dang ZM Flexible and Stretchable Capacitive Sensors with Different Microstructures. Adv. Mater 2021, 33, 2008267. [DOI] [PubMed] [Google Scholar]
- (166).Huang Y-C; Liu Y; Ma C; Cheng H-C; He Q; Wu H; Wang C; Lin C-Y; Huang Y; Duan X Sensitive Pressure Sensors Based on Conductive Microstructured Air-Gap Gates and Two-Dimensional Semiconductor Transistors. Nat. Electron 2020, 3, 59–69. [Google Scholar]
- (167).Bai N; Wang L; Wang Q; Deng J; Wang Y; Lu P; Huang J; Li G; Zhang Y; Yang J; et al. Graded Intrafillable Architecture-Based Iontronic Pressure Sensor with Ultra-Broad-Range High Sensitivity. Nat. Commun 2020, 11, 209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (168).Ha KH; Zhang W; Jang H; Kang S; Wang L; Tan P; Hwang H; Lu N Highly Sensitive Capacitive Pressure Sensors over a Wide Pressure Range Enabled by the Hybrid Responses of a Highly Porous Nanocomposite. Adv. Mater 2021, 33, 2103320. [DOI] [PubMed] [Google Scholar]
- (169).Zhao X; Chen G; Zhou Y; Nashalian A; Xu J; Tat T; Song Y; Libanori A; Xu S; Li S; et al. Giant Magnetoelastic Effect Enabled Stretchable Sensor for Self-Powered Biomonitoring. ACS Nano 2022, 16, 6013–6022. [DOI] [PubMed] [Google Scholar]
- (170).Bai N; Wang L; Xue Y; Wang Y; Hou X; Li G; Zhang Y; Cai M; Zhao L; Guan F; et al. Graded Interlocks for Iontronic Pressure Sensors with High Sensitivity and High Linearity over a Broad Range. ACS Nano 2022, 16, 4338–4347. [DOI] [PubMed] [Google Scholar]
- (171).Ji B; Zhou Q; Hu B; Zhong J; Zhou J; Zhou B Bio-Inspired Hybrid Dielectric for Capacitive and Triboelectric Tactile Sensors with High Sensitivity and Ultrawide Linearity Range. Adv. Mater 2021, 33, 2100859. [DOI] [PubMed] [Google Scholar]
- (172).Zhuo S; Song C; Rong Q; Zhao T; Liu M Shape and Stiffness Memory Ionogels with Programmable Pressure-Resistance Response. Nat. Commun 2022, 13, 1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (173).Hu H; Zhu X; Wang C; Zhang L; Li X; Lee S; Huang Z; Chen R; Chen Z; Wang C; et al. Stretchable Ultrasonic Transducer Arrays for Three-Dimensional Imaging on Complex Surfaces. Sci. Adv 2018, 4, No. eaar3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (174).Bariya M; Nyein HYY; Javey A Wearable Sweat Sensors. Nat. Electron 2018, 1, 160–171. [Google Scholar]
- (175).Kim J; Campbell AS; de Avila BE; Wang J Wearable Biosensors for Healthcare Monitoring. Nat. Biotechnol 2019, 37, 389–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (176).Yang Y; Gao W Wearable and Flexible Electronics for Continuous Molecular Monitoring. Chem. Soc. Rev 2019, 48, 1465–1491. [DOI] [PubMed] [Google Scholar]
- (177).Heikenfeld J; Jajack A; Feldman B; Granger SW; Gaitonde S; Begtrup G; Katchman BA Accessing Analytes in Biofluids for Peripheral Biochemical Monitoring. Nat. Biotechnol 2019, 37, 407–419. [DOI] [PubMed] [Google Scholar]
- (178).Yang G; Kampstra KL; Abidian MR High Performance Conducting Polymer Nanofiber Biosensors for Detection of Bio-molecules. Adv. Mater 2014, 26, 4954–4960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (179).Lee H; Choi TK; Lee YB; Cho HR; Ghaffari R; Wang L; Choi HJ; Chung TD; Lu N; Hyeon T; et al. A Graphene-Based Electrochemical Device with Thermoresponsive Microneedles for Diabetes Monitoring and Therapy. Nat. Nanotechnol 2016, 11, 566–572. [DOI] [PubMed] [Google Scholar]
- (180).Lee H; Song C; Hong YS; Kim M; Cho HR; Kang T; Shin K; Choi SH; Hyeon T; Kim D-H Wearable/Disposable Sweat-Based Glucose Monitoring Device with Multistage Transdermal Drug Delivery Module. Sci. Adv 2017, 3, No. e1601314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (181).Xiao J; Fan C; Xu T; Su L; Zhang X An Electrochemical Wearable Sensor for Levodopa Quantification in Sweat Based on a Metal-Organic Framework/Graphene Oxide Composite with Integrated Enzymes. Sensor. Actuat. B: Chem 2022, 359, 131586. [Google Scholar]
- (182).Yang Y; Song Y; Bo X; Min J; Pak OS; Zhu L; Wang M; Tu J; Kogan A; Zhang H; et al. A Laser-Engraved Wearable Sensor for Sensitive Detection of Uric Acid and Tyrosine in Sweat. Nat. Biotechnol 2020, 38, 217–224. [DOI] [PubMed] [Google Scholar]
- (183).Zhao J; Nyein HYY; Hou L; Lin Y; Bariya M; Ahn CH; Ji W; Fan Z; Javey A A Wearable Nutrition Tracker. Adv. Mater 2021, 33, 2006444. [DOI] [PubMed] [Google Scholar]
- (184).Goh WP; Jiang C; Yu Y; Zheng X; Liu Y; Yang L Screen-Printing Ink, Method of Manufacturing Same, Method of Producing Screen-Printed Electrode and Screen-Printed Electrode, Patent PCT/SG2022/050407; Singapore: 2021.
- (185).Yu Y; Jiang C; Zheng XT; Liu Y; Goh WP; Lim RHH; Tan SCL; Yang L Three-Dimensional Highway-Like Graphite Flakes/Carbon Fiber Hybrid Electrode for Electrochemical Biosensor. Mater. Today Adv 2022, 14, 100238. [Google Scholar]
- (186).Syu Y-C; Hsu W-E; Lin C-T Review-Field-Effect Transistor Biosensing: Devices and Clinical Applications. ECS J. Solid State Sci. Technol 2018, 7, 3196–3207. [Google Scholar]
- (187).Ye D; Wang J; Shen H; Feng X; Xiang L; Jin W; Zhao W; Ding J; He Z; Zou Y; et al. An Oligonucleotide-Distortion-Responsive Organic Transistor for Platinum-Drug-Induced DNA-Damage Detection. Adv. Mater 2021, 33, 2100489. [DOI] [PubMed] [Google Scholar]
- (188).Cheung KM; Abendroth JM; Nakatsuka N; Zhu B; Yang Y; Andrews AM; Weiss PS Detecting DNA and RNA and Differentiating Single-Nucleotide Variations via Field-Effect Transistors. Nano Lett. 2020, 20, 5982–5990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (189).Zhao C; Cheung KM; Huang IW; Yang H; Nakatsuka N; Liu W; Cao Y; Man T; Weiss PS; Monbouquette HG; et al. Implantable Aptamer-Field-Effect Transistor Neuroprobes for in Vivo Neurotransmitter Monitoring. Sci. Adv 2021, 7, No. eabj7422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (190).Rivnay J; Inal S; Salleo A; Owens RM; Berggren M; Malliaras GG Organic Electrochemical Transistors. Nat. Rev. Mater 2018, 3, 17086. [Google Scholar]
- (191).Deng Y; Qi H; Ma Y; Liu S; Zhao M; Guo Z; Jie Y; Zheng R; Jing J; Chen K; et al. A Flexible and Highly Sensitive Organic Electrochemical Transistor-Based Biosensor for Continuous and Wireless Nitric Oxide Detection. Proc. Natl. Acad. Sci. U. S. A 2022, 119, No. e2208060119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (192).Guo K; Wustoni S; Koklu A; Diaz-Galicia E; Moser M; Hama A; Alqahtani AA; Ahmad AN; Alhamlan FS; Shuaib M; et al. Rapid Single-Molecule Detection of COVID-19 and MERS Antigens via Nanobody-Functionalized Organic Electrochemical Transistors. Nat. Biomed. Eng 2021, 5, 666–677. [DOI] [PubMed] [Google Scholar]
- (193).Sun C; Wang X; Auwalu MA; Cheng S; Hu W Organic Thin Film Transistors-Based Biosensors. EcoMat 2021, 3, No. e12094. [Google Scholar]
- (194).Ren H; Xu T; Liang K; Li J; Fang Y; Li F; Chen Y; Zhang H; Li D; Tang Y; et al. Self-Assembled Peptides-Modified Flexible Field-Effect Transistors for Tyrosinase Detection. iScience 2022, 25, 103673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (195).Zhao C; Liu Q; Cheung KM; Liu W; Yang Q; Xu X; Man T; Weiss PS; Zhou C; Andrews AM Narrower Nanoribbon Biosensors Fabricated by Chemical Lift-Off Lithography Show Higher Sensitivity. ACS Nano 2021, 15, 904–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (196).Tao K; Makam P; Aizen R; Gazit E Self-Assembling Peptide Semiconductors. Science 2017, 358, 885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (197).Zhang F; Lemaur V; Choi W; Kafle P; Seki S; Cornil J; Beljonne D; Diao Y Repurposing DNA-Binding Agents as H-Bonded Organic Semiconductors. Nat. Commun 2019, 10, 4217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (198).Jiang C; Choi HW; Cheng X; Ma H; Hasko D; Nathan A Printed Subthreshold Organic Transistors Operating at High Gain and Ultralow Power. Science 2019, 363, 719–723. [DOI] [PubMed] [Google Scholar]
- (199).Yu R; Niu S; Pan C; Wang ZL Piezotronic Effect Enhanced Performance of Schottky-Contacted Optical, Gas, Chemical and Biological Nanosensors. Nano Energy 2015, 14, 312–339. [Google Scholar]
- (200).Huang X; Liu Y; Yung B; Xiong Y; Chen X Nano-technology-Enhanced No-Wash Biosensors for in Vitro Diagnostics of Cancer. ACS Nano 2017, 11, 5238–5292. [DOI] [PubMed] [Google Scholar]
- (201).Loynachan CN; Thomas MR; Gray ER; Richards DA; Kim J; Miller BS; Brookes JC; Agarwal S; Chudasama V; McKendry RA; et al. Platinum Nanocatalyst Amplification: Redefining the Gold Standard for Lateral Flow Immunoassays with Ultrabroad Dynamic Range. ACS Nano 2018, 12, 279–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (202).Yang RJ; Pu HH; Wang HL Ion Concentration Polarization on Paper-Based Microfluidic Devices and Its Application to Preconcentrate Dilute Sample Solutions. Biomicrofluidics 2015, 9, 014122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (203).Sanghavi BJ; Varhue W; Chavez JL; Chou CF; Swami NS Electrokinetic Preconcentration and Detection of Neuropeptides at Patterned Graphene-Modified Electrodes in a Nanochannel. Anal. Chem 2014, 86, 4120–4125. [DOI] [PubMed] [Google Scholar]
- (204).Kim J; de Araujo WR; Samek IA; Bandodkar AJ; Jia W; Brunetti B; Paixão TRLC; Wang J Wearable Temporary Tattoo Sensor for Real-Time Trace Metal Monitoring in Human Sweat. Electrochem. Commun 2015, 51, 41–45. [Google Scholar]
- (205).Gao W; Nyein HYY; Shahpar Z; Fahad HM; Chen K; Emaminejad S; Gao Y; Tai L-C; Ota H; Wu E; et al. Wearable Microsensor Array for Multiplexed Heavy Metal Monitoring of Body Fluids. ACS Sens. 2016, 1, 866–874. [Google Scholar]
- (206).Nakata S; Shiomi M; Fujita Y; Arie T; Akita S; Takei K A Wearable pH Sensor with High Sensitivity Based on a Flexible Charge-Coupled Device. Nat. Electron 2018, 1, 596–603. [Google Scholar]
- (207).Niu H; Chen Y; Kim E-S; Zhou W; Li Y; Kim N-Y Ultrasensitive Capacitive Tactile Sensor with Heterostructured Active Layers for Tiny Signal Perception. Chem. Eng. J 2022, 450, 138258. [Google Scholar]
- (208).Chen G; Zhou Y; Fang Y; Zhao X; Shen S; Tat T; Nashalian A; Chen J Wearable Ultrahigh Current Power Source Based on Giant Magnetoelastic Effect in Soft Elastomer System. ACS Nano 2021, 15, 20582–20589. [DOI] [PubMed] [Google Scholar]
- (209).Yao H; Yang W; Cheng W; Tan YJ; See HH; Li S; Ali HPA; Lim BZH; Liu Z; Tee BCK Near-Hysteresis-Free Soft Tactile Electronic Skins for Wearables and Reliable Machine Learning. Proc. Natl. Acad. Sci. U. S. A 2020, 117, 25352–25359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (210).Kang D; Pikhitsa PV; Choi YW; Lee C; Shin SS; Piao L; Park B; Suh KY; Kim TI; Choi M Ultrasensitive Mechanical Crack-Based Sensor Inspired by the Spider Sensory System. Nature 2014, 516, 222–226. [DOI] [PubMed] [Google Scholar]
- (211).Kong W; Yang Y; Wang Y; Cheng H; Yan P; Huang L; Ning J; Zeng F; Cai X; Wang M An Ultra-Low Hysteresis, Self-Healing and Stretchable Conductor Based on Dynamic Disulfide Covalent Adaptable Networks. J. Mater. Chem. A 2022, 10, 2012–2020. [Google Scholar]
- (212).Meng X; Qiao Y; Do C; Bras W; He C; Ke Y; Russell TP; Qiu D Hysteresis-Free Nanoparticle-Reinforced Hydrogels. Adv. Mater 2022, 34, 2108243. [DOI] [PubMed] [Google Scholar]
- (213).Lei H; Dong L; Li Y; Zhang J; Chen H; Wu J; Zhang Y; Fan Q; Xue B; Qin M; et al. Stretchable Hydrogels with Low Hysteresis and Anti-Fatigue Fracture Based on Polyprotein Cross-Linkers. Nat. Commun 2020, 11, 4032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (214).Shen Z; Zhang Z; Zhang N; Li J; Zhou P; Hu F; Rong Y; Lu B; Gu G High-Stretchability, Ultralow-Hysteresis Conducting Polymer Hydrogel Strain Sensors for Soft Machines. Adv. Mater 2022, 34, 2203650. [DOI] [PubMed] [Google Scholar]
- (215).Wang Y; Yu X; Zhang H; Fan X; Zhang Y; Li Z; Miao Y-E; Zhang X; Liu T Highly Stretchable, Soft, Low-Hysteresis, and Self-Healable Ionic Conductive Elastomers Enabled by Long, Functional Cross-Linkers. Macromolecules 2022, 55, 7845–7855. [Google Scholar]
- (216).Nguyen XA; Gong S; Cheng W; Chauhan S A Stretchable Gold Nanowire Sensor and Its Characterization Using Machine Learning for Motion Tracking. IEEE Sens. J 2021, 21, 15269–15276. [Google Scholar]
- (217).Yuan J; Zhang Y; Li G; Liu S; Zhu R Printable and Stretchable Conductive Elastomers for Monitoring Dynamic Strain with High Fidelity. Adv. Funct. Mater 2022, 32, 2204878. [Google Scholar]
- (218).Bartlett MD; Markvicka EJ; Majidi C Rapid Fabrication of Soft, Multilayered Electronics for Wearable Biomonitoring. Adv. Funct. Mater 2016, 26, 8496–8504. [Google Scholar]
- (219).Lin L; Deng H; Gao X; Zhang S; Bilotti E; Peijs T; Fu Q Modified Resistivity-Strain Behavior through the Incorporation of Metallic Particles in Conductive Polymer Composite Fibers Containing Carbon Nanotubes. Polym. Int 2013, 62, 134–140. [Google Scholar]
- (220).Lin L; Liu S; Zhang Q; Li X; Ji M; Deng H; Fu Q Towards Tunable Sensitivity of Electrical Property to Strain for Conductive Polymer Composites Based on Thermoplastic Elastomer. ACS Appl. Mater. Interfaces 2013, 5, 5815–5824. [DOI] [PubMed] [Google Scholar]
- (221).Liu H; Li Y; Dai K; Zheng G; Liu C; Shen C; Yan X; Guo J; Guo Z Electrically Conductive Thermoplastic Elastomer Nanocomposites at Ultralow Graphene Loading Levels for Strain Sensor Applications. J. Mater. Chem. C 2016, 4, 157–166. [Google Scholar]
- (222).Wang Y; Jia Y; Zhou Y; Wang Y; Zheng G; Dai K; Liu C; Shen C Ultra-Stretchable, Sensitive and Durable Strain Sensors Based on Polydopamine Encapsulated Carbon Nanotubes/Elastic Bands. J. Mater. Chem. C 2018, 6, 8160–8170. [Google Scholar]
- (223).Chung M; Fortunato G; Radacsi N Wearable Flexible Sweat Sensors for Healthcare Monitoring: A Review. J. R. Soc. Interface 2019, 16, 20190217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (224).Jin X; Liu C; Xu T; Su L; Zhang X Artificial Intelligence Biosensors: Challenges and Prospects. Biosens. Bioelectron 2020, 165, 112412. [DOI] [PubMed] [Google Scholar]
- (225).Daus A; Jaikissoon M; Khan AI; Kumar A; Grady RW; Saraswat KC; Pop E Fast-Response Flexible Temperature Sensors with Atomically Thin Molybdenum Disulfide. Nano Lett. 2022, 22, 6135–6140. [DOI] [PubMed] [Google Scholar]
- (226).Huynh VL; Trung TQ; Meeseepong M; Lee HB; Nguyen TD; Lee NE Hollow Microfibers of Elastomeric Nanocomposites for Fully Stretchable and Highly Sensitive Microfluidic Immunobiosensor Patch. Adv. Funct. Mater 2020, 30, 2004684. [Google Scholar]
- (227).Lee HB; Meeseepong M; Trung TQ; Kim BY; Lee NE A Wearable Lab-on-a-Patch Platform with Stretchable Nanostructured Biosensor for Non-Invasive Immunodetection of Biomarker in Sweat. Biosens. Bioelectron 2020, 156, 112133. [DOI] [PubMed] [Google Scholar]
- (228).Wang M; Yang Y; Min J; Song Y; Tu J; Mukasa D; Ye C; Xu C; Heflin N; McCune JS; et al. A Wearable Electrochemical Biosensor for the Monitoring of Metabolites and Nutrients. Nat. Biomed. Eng 2022, 6, 1225–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (229).Xi W; Kong F; Yeo JC; Yu L; Sonam S; Dao M; Gong X; Lim CT Soft Tubular Microfluidics for 2D and 3D Applications. Proc. Natl. Acad. Sci. U. S. A 2017, 114, 10590–10595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (230).Paria D; Kwok KS; Raj P; Zheng P; Gracias DH; Barman I Label-Free Spectroscopic SARS-CoV-2 Detection on Versatile Nanoimprinted Substrates. Nano Lett. 2022, 22, 3620–3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (231).Liu L; Martinez Pancorbo P; Xiao TH; Noguchi S; Marumi M; Segawa H; Karhadkar S; Gala de Pablo J; Hiramatsu K; Kitahama Y; et al. Highly Scalable, Wearable Surface-Enhanced Raman Spectroscopy. Adv. Opt. Mater 2022, 10, 2200054. [Google Scholar]
- (232).Hartel MC; Lee D; Weiss PS; Wang J; Kim J Resettable Sweat-Powered Wearable Electrochromic Biosensor. Biosens. Bioelectron 2022, 215, 114565. [DOI] [PubMed] [Google Scholar]
- (233).Chen S; Liu TL; Dong Y; Li J A Wireless, Regeneratable Cocaine Sensing Scheme Enabled by Allosteric Regulation of pH Sensitive Aptamers. ACS Nano 2022, 16, 20922–20936. [DOI] [PubMed] [Google Scholar]
- (234).Reeder JT; Xue Y; Franklin D; Deng Y; Choi J; Prado O; Kim R; Liu C; Hanson J; Ciraldo J; et al. Resettable Skin Interfaced Microfluidic Sweat Collection Devices with Chemesthetic Hydration Feedback. Nat. Commun 2019, 10, 5513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (235).Son J; Bae GY; Lee S; Lee G; Kim SW; Kim D; Chung S; Cho K Cactus-Spine-Inspired Sweat-Collecting Patch for Fast and Continuous Monitoring of Sweat. Adv. Mater 2021, 33, 2102740. [DOI] [PubMed] [Google Scholar]
- (236).Wang X; Liu Y; Cheng H; Ouyang X Surface Wettability for Skin-Interfaced Sensors and Devices. Adv. Funct. Mater 2022, 32, 2200260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (237).Nightingale AM; Leong CL; Burnish RA; Hassan SU; Zhang Y; Clough GF; Boutelle MG; Voegeli D; Niu X Monitoring Biomolecule Concentrations in Tissue Using a Wearable Droplet Microfluidic-Based Sensor. Nat. Commun 2019, 10, 2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (238).Koh A; Kang D; Xue Y; Lee S; Pielak RM; Kim J; Hwang T; Min S; Banks A; Bastien P; et al. A Soft, Wearable Microfluidic Device for the Capture, Storage, and Colorimetric Sensing of Sweat. Sci. Transl. Med 2016, 8, 366ra165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (239).Anastasova S; Crewther B; Bembnowicz P; Curto V; Ip HM; Rosa B; Yang GZ A Wearable Multisensing Patch for Continuous Sweat Monitoring. Biosens. Bioelectron 2017, 93, 139–145. [DOI] [PubMed] [Google Scholar]
- (240).Choi J; Kang D; Han S; Kim SB; Rogers JA Thin, Soft, Skin-Mounted Microfluidic Networks with Capillary Bursting Valves for Chrono-Sampling of Sweat. Adv. Healthc. Mater 2017, 6, 1601355. [DOI] [PubMed] [Google Scholar]
- (241).Shay T; Dickey MD; Velev OD Hydrogel-Enabled Osmotic Pumping for Microfluidics: Towards Wearable Human-Device Interfaces. Lab Chip 2017, 17, 710–716. [DOI] [PubMed] [Google Scholar]
- (242).Baik S; Lee J; Jeon EJ; Park B.-y.; Kim DW; Song JH; Lee HJ; Han SY; Cho S-W; Pang C Diving Beetle-Like Miniaturized Plungers with Reversible, Rapid Biofluid Capturing for Machine Learning-Based Care of Skin Disease. Sci. Adv 2021, 7, No. eabf5695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (243).Saha T; Fang J; Mukherjee S; Knisely CT; Dickey MD; Velev OD Osmotically Enabled Wearable Patch for Sweat Harvesting and Lactate Quantification. Micromachines 2021, 12, 1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (244).Nyein HYY; Bariya M; Tran B; Ahn CH; Brown BJ; Ji W; Davis N; Javey A A Wearable Patch for Continuous Analysis of Thermoregulatory Sweat at Rest. Nat. Commun 2021, 12, 1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (245).Li CG; Joung HA; Noh H; Song MB; Kim MG; Jung H One-Touch-Activated Blood Multidiagnostic System Using a Minimally Invasive Hollow Microneedle Integrated with a Paper-Based Sensor. Lab Chip 2015, 15, 3286–3292. [DOI] [PubMed] [Google Scholar]
- (246).Mohan AMV; Windmiller JR; Mishra RK; Wang J Continuous Minimally-Invasive Alcohol Monitoring Using Microneedle Sensor Arrays. Biosens. Bioelectron 2017, 91, 574–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (247).Lipani L; Dupont BGR; Doungmene F; Marken F; Tyrrell RM; Guy RH; Ilie A Non-Invasive, Transdermal, Path-Selective and Specific Glucose Monitoring via a Graphene-Based Platform. Nat. Nanotechnol 2018, 13, 504–511. [DOI] [PubMed] [Google Scholar]
- (248).Chen Y; Lu S; Zhang S; Li Y; Qu Z; Chen Y; Lu B; Wang X; Feng X Skin-Like Biosensor System via Electrochemical Channels for Noninvasive Blood Glucose Monitoring. Sci. Adv 2017, 3, No. e1701629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (249).Zhu Y; Li S; Li J; Falcone N; Cui Q; Shah S; Hartel MC; Yu N; Young P; de Barros NR; et al. Lab-on-a-Contact Lens: Recent Advances and Future Opportunities in Diagnostics and Therapeutics. Adv. Mater 2022, 34, 2108389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (250).Sun T; Tasnim F; McIntosh RT; Amiri N; Solav D; Anbarani MT; Sadat D; Zhang L; Gu Y; Karami MA; et al. Decoding of Facial Strains via Conformable Piezoelectric Interfaces. Nat. Biomed. Eng 2020, 4, 954–972. [DOI] [PubMed] [Google Scholar]
- (251).Madhvapathy SR; Wang H; Kong J; Zhang M; Lee JY; Park JB; Jang H; Xie Z; Cao J; Avila R; et al. Reliable, Low-Cost, Fully Integrated Hydration Sensors for Monitoring and Diagnosis of Inflammatory Skin Diseases in Any Environment. Sci. Adv 2020, 6, No. eabd7146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (252).Kim SK; Lee GH; Jeon C; Han HH; Kim SJ; Mok JW; Joo CK; Shin S; Sim JY; Myung D; et al. Bimetallic Nanocatalysts Immobilized in Nanoporous Hydrogels for Long-Term Robust Continuous Glucose Monitoring of Smart Contact Lens. Adv. Mater 2022, 34, 2110536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (253).Peng Z; Xie X; Tan Q; Kang H; Cui J; Zhang X; Li W; Feng G Blood Glucose Sensors and Recent Advances: A Review. J. Innov. Opt. Health Sci 2022, 15, 2230003. [Google Scholar]
- (254).Moyer J; Wilson D; Finkelshtein I; Wong B; Potts R Correlation between Sweat Glucose and Blood Glucose in Subjects with Diabetes. Diabetes Technol. Ther 2012, 14, 398–402. [DOI] [PubMed] [Google Scholar]
- (255).Ahmadian N; Manickavasagan A; Ali A Comparative Assessment of Blood Glucose Monitoring Techniques: A Review. J. Med. Eng. Technol 2023, 47, 121. [DOI] [PubMed] [Google Scholar]
- (256).Kim J-H; Marcus C; Ono R; Sadat D; Mirzazadeh A; Jens M; Fernandez S; Zheng S; Durak T; Dagdeviren C A Conformable Sensory Face Mask for Decoding Biological and Environmental Signals. Nat. Electron 2022, 5, 794–807. [Google Scholar]
- (257).Baker LB; Model JB; Barnes KA; Anderson ML; Lee SP; Lee KA; Brown SD; Reimel AJ; Roberts TJ; Nuccio RP; et al. Skin-Interfaced Microfluidic System with Personalized Sweating Rate and Sweat Chloride Analytics for Sports Science Applications. Sci. Adv 2020, 6, No. eabe3929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (258).Wang C; Li X; Hu H; Zhang L; Huang Z; Lin M; Zhang Z; Yin Z; Huang B; Gong H; et al. Monitoring of the Central Blood Pressure Waveform via a Conformal Ultrasonic Device. Nat. Biomed. Eng 2018, 2, 687–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (259).Zhang Y; Yang J; Hou X; Li G; Wang L; Bai N; Cai M; Zhao L; Wang Y; Zhang J; et al. Highly Stable Flexible Pressure Sensors with a Quasi-Homogeneous Composition and Interlinked Interfaces. Nat. Commun 2022, 13, 1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (260).Li E; Rao Z; Wang X; Liu Y; Yu R; Chen G; Chen H; Guo T Direct Fabrication of Stretchable Electronics on a Programmable Stiffness Substrate with 100% Strain Isolation. IEEE Electron Device Lett. 2021, 42, 1484–1487. [Google Scholar]
- (261).Lin R; Li Y; Mao X; Zhou W; Liu R Hybrid 3D Printing All-in-One Heterogenous Rigidity Assemblies for Soft Electronics. Adv. Mater. Technol 2019, 4, 1900614. [Google Scholar]
- (262).Romeo A; Liu Q; Suo Z; Lacour SP Elastomeric Substrates with Embedded Stiff Platforms for Stretchable Electronics. Appl. Phys. Lett 2013, 102, 131904. [Google Scholar]
- (263).Graz IM; Cotton DPJ; Robinson A; Lacour SP Silicone Substrate with in Situ Strain Relief for Stretchable Thin-Film Transistors. Appl. Phys. Lett 2011, 98, 124101. [Google Scholar]
- (264).Libanori R; Erb RM; Reiser A; Le Ferrand H; Suess MJ; Spolenak R; Studart AR Stretchable Heterogeneous Composites with Extreme Mechanical Gradients. Nat. Commun 2012, 3, 1265. [DOI] [PubMed] [Google Scholar]
- (265).Yang Jun C; Lee S; Ma Boo S; Kim J; Song M; Kim Su Y; Kim Da W; Kim T-S; Park S Geometrically Engineered Rigid Island Array for Stretchable Electronics Capable of Withstanding Various Deformation Modes. Sci. Adv 2022, 8, No. eabn3863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (266).Song H; Luo G; Ji Z; Bo R; Xue Z; Yan D; Zhang F; Bai K; Liu J; Cheng X; et al. Highly-Integrated, Miniaturized, Stretchable Electronic Systems Based on Stacked Multilayer Network Materials. Sci. Adv 2022, 8, No. eabm3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (267).Wang M; Wang K; Ma C; Uzabakiriho PC; Chen X; Zhao G Mechanical Gradients Enable Highly Stretchable Electronics Based on Nanofiber Substrates. ACS Appl. Mater. Interfaces 2022, 14, 35997–36006. [DOI] [PubMed] [Google Scholar]
- (268).Li M; Chen S; Fan B; Wu B; Guo X Printed Flexible Strain Sensor Array for Bendable Interactive Surface. Adv. Funct. Mater 2020, 30, 2003214. [Google Scholar]
- (269).Ma R; Kang B; Cho S; Choi M; Baik S Extraordinarily High Conductivity of Stretchable Fibers of Polyurethane and Silver Nanoflowers. ACS Nano 2015, 9, 10876–10886. [DOI] [PubMed] [Google Scholar]
- (270).Liu Z; Wang H; Huang P; Huang J; Zhang Y; Wang Y; Yu M; Chen S; Qi D; Wang T; et al. Highly Stable and Stretchable Conductive Films through Thermal-Radiation-Assisted Metal Encapsulation. Adv. Mater 2019, 31, 1901360. [DOI] [PubMed] [Google Scholar]
- (271).Wang Y; Gong S; Wang SJ; Yang X; Ling Y; Yap LW; Dong D; Simon GP; Cheng W Standing Enokitake-Like Nanowire Films for Highly Stretchable Elastronics. ACS Nano 2018, 12, 9742–9749. [DOI] [PubMed] [Google Scholar]
- (272).Huang Q; Zhu Y Patterning of Metal Nanowire Networks: Methods and Applications. ACS Appl. Mater. Interfaces 2021, 13, 60736–60762. [DOI] [PubMed] [Google Scholar]
- (273).Behfar MH; Khorramdel B; Korhonen A; Jansson E; Leinonen A; Tuomikoski M; Mäntysalo M Failure Mechanisms in Flip-Chip Bonding on Stretchable Printed Electronics. Adv. Eng. Mater 2021, 23, 2100264. [Google Scholar]
- (274).Miyamoto A; Kawasaki H; Lee S; Yokota T; Amagai M; Someya T Highly Precise, Continuous, Long-Term Monitoring of Skin Electrical Resistance by Nanomesh Electrodes. Adv. Healthc. Mater 2022, 11, 2102425. [DOI] [PubMed] [Google Scholar]
- (275).Jang H; Sel K; Kim E; Kim S; Yang X; Kang S; Ha KH; Wang R; Rao Y; Jafari R; et al. Graphene E-Tattoos for Unobstructive Ambulatory Electrodermal Activity Sensing on the Palm Enabled by Heterogeneous Serpentine Ribbons. Nat. Commun 2022, 13, 6604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (276).Tchoe Y; Bourhis Andrew M; Cleary Daniel R; Stedelin B; Lee J; Tonsfeldt Karen J; Brown Erik C; Siler Dominic A; Paulk Angelique C; Yang Jimmy C; et al. Human Brain Mapping with Multithousand-Channel PtNRGrids Resolves Spatiotemporal Dynamics. Sci. Transl. Med 2022, 14, No. eabj1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (277).Katerinopoulou D; Zalar P; Sweelssen J; Kiriakidis G; Rentrop C; Groen P; Gelinck GH; van den Brand J; Smits ECP Large-Area All-Printed Temperature Sensing Surfaces Using Novel Composite Thermistor Materials. Adv. Electron. Mater 2019, 5, 1800605. [Google Scholar]
- (278).Song E; Chiang CH; Li R; Jin X; Zhao J; Hill M; Xia Y; Li L; Huang Y; Won SM; et al. Flexible Electronic/Optoelectronic Microsystems with Scalable Designs for Chronic Biointegration. Proc. Natl. Acad. Sci. U. S. A 2019, 116, 15398–15406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (279).Norton JJ; Lee DS; Lee JW; Lee W; Kwon O; Won P; Jung SY; Cheng H; Jeong JW; Akce A; et al. Soft, Curved Electrode Systems Capable of Integration on the Auricle as a Persistent Brain-Computer Interface. Proc. Natl. Acad. Sci. U. S. A 2015, 112, 3920–3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (280).Palavesam N; Marin S; Hemmetzberger D; Landesberger C; Bock K; Kutter C Roll-to-Roll Processing of Film Substrates for Hybrid Integrated Flexible Electronics. Flex. Print. Electron 2018, 3, 014002. [Google Scholar]
- (281).Kang M; Jeong H; Park S-W; Hong J; Lee H; Chae Y; Yang S; Ahn J-H Wireless Graphene-Based Thermal Patch for Obtaining Temperature Distribution and Performing Thermography. Sci. Adv 2022, 8, No. eabm6693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (282).Dagdeviren C; Yang BD; Su Y; Tran PL; Joe P; Anderson E; Xia J; Doraiswamy V; Dehdashti B; Feng X; et al. Conformal Piezoelectric Energy Harvesting and Storage from Motions of the Heart, Lung, and Diaphragm. Proc. Natl. Acad. Sci. U. S. A 2014, 111, 1927–1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (283).Takakuwa M; Fukuda K; Yokota T; Inoue D; Hashizume D; Umezu S; Someya T Direct Gold Bonding for Flexible Integrated Electronics. Sci. Adv 2021, 7, No. eabl6228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (284).Zhu M; Ji S; Luo Y; Zhang F; Liu Z; Wang C; Lv Z; Jiang Y; Wang M; Cui Z; et al. A Mechanically Interlocking Strategy Based on Conductive Microbridges for Stretchable Electronics. Adv. Mater 2022, 34, 2101339. [DOI] [PubMed] [Google Scholar]
- (285).Liu S; Shah DS; Kramer-Bottiglio R Highly Stretchable Multilayer Electronic Circuits Using Biphasic Gallium-Indium. Nat. Mater 2021, 20, 851–858. [DOI] [PubMed] [Google Scholar]
- (286).Hwang H; Kong M; Kim K; Park D; Lee S; Park S; Song H-J; Jeong U Stretchable Anisotropic Conductive Film (S-ACF) for Electrical Interfacing in High-Resolution Stretchable Circuits. Sci. Adv 2021, 7, No. eabh0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (287).Tang L; Yang S; Zhang K; Jiang X Skin Electronics from Biocompatible in Situ Welding Enabled by Intrinsically Sticky Conductors. Adv. Sci 2022, 9, 2202043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (288).Dou J; Tang L; Mou L; Zhang R; Jiang X Stretchable Conductive Adhesives for Connection of Electronics in Wearable Devices Based on Metal-Polymer Conductors and Carbon Nanotubes. Compos. Sci. Tech 2020, 197, 108237. [Google Scholar]
- (289).Lopes PA; Santos BC; de Almeida AT; Tavakoli M Reversible Polymer-Gel Transition for Ultra-Stretchable Chip-Integrated Circuits through Self-Soldering and Self-Coating and Self-Healing. Nat. Commun 2021, 12, 4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (290).Huang Z; Hao Y; Li Y; Hu H; Wang C; Nomoto A; Pan T; Gu Y; Chen Y; Zhang T; et al. Three-Dimensional Integrated Stretchable Electronics. Nat. Electron 2018, 1, 473–480. [Google Scholar]
- (291).Biswas S; Schoeberl A; Hao Y; Reiprich J; Stauden T; Pezoldt J; Jacobs HO Integrated Multilayer Stretchable Printed Circuit Boards Paving the Way for Deformable Active Matrix. Nat. Commun 2019, 10, 4909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (292).Kim J; Sempionatto JR; Imani S; Hartel MC; Barfidokht A; Tang G; Campbell AS; Mercier PP; Wang J Simultaneous Monitoring of Sweat and Interstitial Fluid Using a Single Wearable Biosensor Platform. Adv. Sci 2018, 5, 1800880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (293).Niu S; Matsuhisa N; Beker L; Li J; Wang S; Wang J; Jiang Y; Yan X; Yun Y; Burnett W; et al. A Wireless Body Area Sensor Network Based on Stretchable Passive Tags. Nat. Electron 2019, 2, 361–368. [Google Scholar]
- (294).Kim Y; Suh JM; Shin J; Liu Y; Yeon H; Qiao K; Kum HS; Kim C; Lee HE; Choi C; et al. Chip-Less Wireless Electronic Skins by Remote Epitaxial Freestanding Compound Semiconductors. Science 2022, 377, 859–864. [DOI] [PubMed] [Google Scholar]
- (295).Xie Z; Avila R; Huang Y; Rogers JA Flexible and Stretchable Antennas for Biointegrated Electronics. Adv. Mater 2020, 32, 1902767. [DOI] [PubMed] [Google Scholar]
- (296).Yang C; Suo Z Hydrogel Ionotronics. Nat. Rev. Mater 2018, 3, 125–142. [Google Scholar]
- (297).Sim K; Rao Z; Ershad F; Yu C Rubbery Electronics Fully Made of Stretchable Elastomeric Electronic Materials. Adv. Mater 2020, 32, 1902417. [DOI] [PubMed] [Google Scholar]
- (298).Matsuhisa N; Niu S; O’Neill SJK; Kang J; Ochiai Y; Katsumata T; Wu HC; Ashizawa M; Wang GN; Zhong D; et al. High-Frequency and Intrinsically Stretchable Polymer Diodes. Nature 2021, 600, 246–252. [DOI] [PubMed] [Google Scholar]
- (299).Vallem V; Sargolzaeiaval Y; Ozturk M; Lai YC; Dickey MD Energy Harvesting and Storage with Soft and Stretchable Materials. Adv. Mater 2021, 33, 2004832. [DOI] [PubMed] [Google Scholar]
- (300).Ye T; Wang J; Jiao Y; Li L; He E; Wang L; Li Y; Yun Y; Li D; Lu J; et al. A Tissue-Like Soft All-Hydrogel Battery. Adv. Mater 2022, 34, 2105120. [DOI] [PubMed] [Google Scholar]
- (301).Rogers JA; Lagally MG; Nuzzo RG Synthesis, Assembly and Applications of Semiconductor Nanomembranes. Nature 2011, 477, 45–53. [DOI] [PubMed] [Google Scholar]
- (302).Yang T; Jiang X; Huang Y; Tian Q; Zhang L; Dai Z; Zhu H Mechanical Sensors Based on Two-Dimensional Materials: Sensing Mechanisms, Structural Designs and Wearable Applications. iScience 2022, 25, 103728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (303).Orts Mercadillo V; Chan KC; Caironi M; Athanassiou A; Kinloch IA; Bissett M; Cataldi P Electrically Conductive 2D Material Coatings for Flexible and Stretchable Electronics: A Comparative Review of Graphenes and MXenes. Adv. Funct. Mater 2022, 32, 2204772. [Google Scholar]
- (304).Shi Y; Rogers JA; Gao C; Huang Y Multiple Neutral Axes in Bending of a Multiple-Layer Beam with Extremely Different Elastic Properties. J. Appl. Mech 2014, 81, 114501. [Google Scholar]
- (305).Li S; Su Y; Li R Splitting of the Neutral Mechanical Plane Depends on the Length of the Multi-Layer Structure of Flexible Electronics. Proc. R. Soc. A 2016, 472, 20160087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (306).Li S; Liu X; Li R; Su Y Shear Deformation Dominates in the Soft Adhesive Layers of the Laminated Structure of Flexible Electronics. Int. J. Solids Struct 2017, 110–111, 305–314. [Google Scholar]
- (307).Xue Z; Song H; Rogers JA; Zhang Y; Huang Y Mechanically-Guided Structural Designs in Stretchable Inorganic Electronics. Adv. Mater 2020, 32, 1902254. [DOI] [PubMed] [Google Scholar]
- (308).Kim DC; Shim HJ; Lee W; Koo JH; Kim DH Material-Based Approaches for the Fabrication of Stretchable Electronics. Adv. Mater 2020, 32, 1902743. [DOI] [PubMed] [Google Scholar]
- (309).Kim DH; Lu N; Ma R; Kim YS; Kim RH; Wang S; Wu J; Won SM; Tao H; Islam A; et al. Epidermal Electronics. Science 2011, 333, 838–843. [DOI] [PubMed] [Google Scholar]
- (310).Sun Y; Choi WM; Jiang H; Huang YY; Rogers JA Controlled Buckling of Semiconductor Nanoribbons for Stretchable Electronics. Nat. Nanotechnol 2006, 1, 201–207. [DOI] [PubMed] [Google Scholar]
- (311).Lee G; Zarei M; Wei Q; Zhu Y; Lee SG Surface Wrinkling for Flexible and Stretchable Sensors. Small 2022, 18, 2203491. [DOI] [PubMed] [Google Scholar]
- (312).Fan JA; Yeo WH; Su Y; Hattori Y; Lee W; Jung SY; Zhang Y; Liu Z; Cheng H; Falgout L; et al. Fractal Design Concepts for Stretchable Electronics. Nat. Commun 2014, 5, 3266. [DOI] [PubMed] [Google Scholar]
- (313).Lacour SP; Chan D; Wagner S; Li T; Suo Z Mechanisms of Reversible Stretchability of Thin Metal Films on Elastomeric Substrates. Appl. Phys. Lett 2006, 88, 204103. [Google Scholar]
- (314).Lv Z; Luo Y; Tang Y; Wei J; Zhu Z; Zhou X; Li W; Zeng Y; Zhang W; Zhang Y; et al. Editable Supercapacitors with Customizable Stretchability Based on Mechanically Strengthened Ultralong MnO2 Nanowire Composite. Adv. Mater 2018, 30, 1704531. [DOI] [PubMed] [Google Scholar]
- (315).Lv Z; Tang Y; Zhu Z; Wei J; Li W; Xia H; Jiang Y; Liu Z; Luo Y; Ge X; et al. Honeycomb-Lantern-Inspired 3D Stretchable Supercapacitors with Enhanced Specific Areal Capacitance. Adv. Mater 2018, 30, 1805468. [DOI] [PubMed] [Google Scholar]
- (316).Fernandez SV; Cai F; Chen S; Suh E; Tiepelt J; McIntosh R; Marcus C; Acosta D; Mejorado D; Dagdeviren C On-Body Piezoelectric Energy Harvesters through Innovative Designs and Conformable Structures. ACS Biomater. Sci. Eng 2021, DOI: 10.1021/acsbiomaterials.1c00800. [DOI] [PubMed] [Google Scholar]
- (317).Kim D-H; Kim Y-S; Wu J; Liu Z; Song J; Kim H-S; Huang YY; Hwang K-C; Rogers JA Ultrathin Silicon Circuits with Strain-Isolation Layers and Mesh Layouts for High-Performance Electronics on Fabric, Vinyl, Leather, and Paper. Adv. Mater 2009, 21, 3703–3707. [Google Scholar]
- (318).Sun J-Y; Lu N; Yoon J; Oh K-H; Suo Z; Vlassak JJ Inorganic Islands on a Highly Stretchable Polyimide Substrate. J. Mater. Res 2009, 24, 3338–3342. [Google Scholar]
- (319).Zhao Y; Kim A; Wan G; Tee BCK Design and Applications of Stretchable and Self-Healable Conductors for Soft Electronics. Nano Converg. 2019, 6, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (320).Zhang Q; Liang J; Huang Y; Chen H; Ma R Intrinsically Stretchable Conductors and Interconnects for Electronic Applications. Mater. Chem. Front 2019, 3, 1032–1051. [Google Scholar]
- (321).Tien H-C; Huang Y-W; Chiu Y-C; Cheng Y-H; Chueh C-C; Lee W-Y Intrinsically Stretchable Polymer Semiconductors: Molecular Design, Processing and Device Applications. J. Mater. Chem. C 2021, 9, 2660–2684. [Google Scholar]
- (322).Choi S; Han SI; Kim D; Hyeon T; Kim DH High-Performance Stretchable Conductive Nanocomposites: Materials, Processes, and Device Applications. Chem. Soc. Rev 2019, 48, 1566–1595. [DOI] [PubMed] [Google Scholar]
- (323).Zhang G; McBride M; Persson N; Lee S; Dunn TJ; Toney MF; Yuan Z; Kwon Y-H; Chu P-H; Risteen B; et al. Versatile Interpenetrating Polymer Network Approach to Robust Stretchable Electronic Devices. Chem. Mater 2017, 29, 7645–7652. [Google Scholar]
- (324).Xu J; Wang S; Wang Ging-Ji N; Zhu C; Luo S; Jin L; Gu X; Chen S; Feig VR; To JWF; et al. Highly Stretchable Polymer Semiconductor Films through the Nanoconfinement Effect. Science 2017, 355, 59–64. [DOI] [PubMed] [Google Scholar]
- (325).Koo JH; Song JK; Kim DH Solution-Processed Thin Films of Semiconducting Carbon Nanotubes and Their Application to Soft Electronics. Nanotechnology 2019, 30, 132001. [DOI] [PubMed] [Google Scholar]
- (326).Dai Y; Hu H; Wang M; Xu J; Wang S Stretchable Transistors and Functional Circuits for Human-Integrated Electronics. Nat. Electron 2021, 4, 17–29. [Google Scholar]
- (327).Kang J; Mun J; Zheng Y; Koizumi M; Matsuhisa N; Wu H-C; Chen S; Tok JBH; Lee GH; Jin L; et al. Tough-Interface-Enabled Stretchable Electronics Using Non-Stretchable Polymer Semiconductors and Conductors. Nat. Nanotechnol 2022, 17, 1265–1271. [DOI] [PubMed] [Google Scholar]
- (328).Dai Y; Dai S; Li N; Li Y; Moser M; Strzalka J; Prominski A; Liu Y; Zhang Q; Li S; et al. Stretchable Redox-Active Semiconducting Polymers for High-Performance Organic Electro-chemical Transistors. Adv. Mater 2022, 34, 2201178. [DOI] [PubMed] [Google Scholar]
- (329).Park M; Im J; Shin M; Min Y; Park J; Cho H; Park S; Shim MB; Jeon S; Chung DY; et al. Highly Stretchable Electric Circuits from a Composite Material of Silver Nanoparticles and Elastomeric Fibres. Nat. Nanotechnol 2012, 7, 803–809. [DOI] [PubMed] [Google Scholar]
- (330).Kim Y; Zhu J; Yeom B; Di Prima M; Su X; Kim JG; Yoo SJ; Uher C; Kotov NA Stretchable Nanoparticle Conductors with Self-Organized Conductive Pathways. Nature 2013, 500, 59–63. [DOI] [PubMed] [Google Scholar]
- (331).Stoyanov H; Kollosche M; Risse S; Wache R; Kofod G Soft Conductive Elastomer Materials for Stretchable Electronics and Voltage Controlled Artificial Muscles. Adv. Mater 2013, 25, 578–583. [DOI] [PubMed] [Google Scholar]
- (332).Ma R; Lee J; Choi D; Moon H; Baik S Knitted Fabrics Made from Highly Conductive Stretchable Fibers. Nano Lett. 2014, 14, 1944–1951. [DOI] [PubMed] [Google Scholar]
- (333).Lee S; Shin S; Lee S; Seo J; Lee J; Son S; Cho HJ; Algadi H; Al-Sayari S; Kim DE; et al. Ag Nanowire Reinforced Highly Stretchable Conductive Fibers for Wearable Electronics. Adv. Funct. Mater 2015, 25, 3114–3121. [Google Scholar]
- (334).Matsuhisa N; Kaltenbrunner M; Yokota T; Jinno H; Kuribara K; Sekitani T; Someya T Printable Elastic Conductors with a High Conductivity for Electronic Textile Applications. Nat. Commun 2015, 6, 7461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (335).Liang J; Tong K; Pei Q A Water-Based Silver-Nanowire Screen-Print Ink for the Fabrication of Stretchable Conductors and Wearable Thin-Film Transistors. Adv. Mater 2016, 28, 5986–5996. [DOI] [PubMed] [Google Scholar]
- (336).Matsuhisa N; Inoue D; Zalar P; Jin H; Matsuba Y; Itoh A; Yokota T; Hashizume D; Someya T Printable Elastic Conductors by in Situ Formation of Silver Nanoparticles from Silver Flakes. Nat. Mater 2017, 16, 834–840. [DOI] [PubMed] [Google Scholar]
- (337).Choi S; Han SI; Jung D; Hwang HJ; Lim C; Bae S; Park OK; Tschabrunn CM; Lee M; Bae SY; et al. Highly Conductive, Stretchable and Biocompatible Ag-Au Core-Sheath Nanowire Composite for Wearable and Implantable Bioelectronics. Nat. Nanotechnol 2018, 13, 1048–1056. [DOI] [PubMed] [Google Scholar]
- (338).Lu Y; Jiang J; Yoon S; Kim KS; Kim JH; Park S; Kim SH; Piao L High-Performance Stretchable Conductive Composite Fibers from Surface-Modified Silver Nanowires and Thermoplastic Polyurethane by Wet Spinning. ACS Appl. Mater. Interfaces 2018, 10, 2093–2104. [DOI] [PubMed] [Google Scholar]
- (339).Wang P; Peng Z; Li M; Wang Y Stretchable Transparent Conductive Films from Long Carbon Nanotube Metals. Small 2018, 14, 1802625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (340).Mou L; Qi J; Tang L; Dong R; Xia Y; Gao Y; Jiang X Highly Stretchable and Biocompatible Liquid Metal-Elastomer Conductors for Self-Healing Electronics. Small 2020, 16, 2005336. [DOI] [PubMed] [Google Scholar]
- (341).Jung D; Lim C; Shim Hyung J; Kim Y; Park C; Jung J; Han Sang I; Sunwoo S-H; Cho Kyoung W; Cha Gi D; et al. Highly Conductive and Elastic Nanomembrane for Skin Electronics. Science 2021, 373, 1022–1026. [DOI] [PubMed] [Google Scholar]
- (342).Veerapandian S; Jang W; Seol JB; Wang H; Kong M; Thiyagarajan K; Kwak J; Park G; Lee G; Suh W; et al. Hydrogen-Doped Viscoplastic Liquid Metal Microparticles for Stretchable Printed Metal Lines. Nat. Mater 2021, 20, 533–540. [DOI] [PubMed] [Google Scholar]
- (343).Jiang Y; Zhang Z; Wang Y-X; Li D; Coen C-T; Hwaun E; Chen G; Wu H-C; Zhong D; Niu S; et al. Topological Supramolecular Network Enabled High-Conductivity, Stretchable Organic Bioelectronics. Science 2022, 375, 1411–1417. [DOI] [PubMed] [Google Scholar]
- (344).Xu F; Zhu Y Highly Conductive and Stretchable Silver Nanowire Conductors. Adv. Mater 2012, 24, 5117–5122. [DOI] [PubMed] [Google Scholar]
- (345).Lee W; Kim H; Kang I; Park H; Jung J; Lee H; Park H; Park JS; Yuk JM; Ryu S; et al. Universal Assembly of Liquid Metal Particles in Polymers Enables Elastic Printed Circuit Board. Science 2022, 378, 637–641. [DOI] [PubMed] [Google Scholar]
- (346).Wang D; Liu Z; Li J; Tang W; Huang Y; Yu J; Xu L; Huang Q; Song Y; Wang L; et al. Thin-Film Transistor Arrays for Biological Sensing Systems. Flex. Print. Electron 2022, 7, 023004. [Google Scholar]
- (347).Lu C; Lee WY; Gu X; Xu J; Chou HH; Yan H; Chiu YC; He M; Matthews JR; Niu W; et al. Effects of Molecular Structure and Packing Order on the Stretchability of Semicrystalline Conjugated Poly(Tetrathienoacene-Diketopyrrolopyrrole) Polymers. Adv. Electron. Mater 2017, 3, 1600311. [Google Scholar]
- (348).Oh JY; Rondeau-Gagne S; Chiu YC; Chortos A; Lissel F; Wang GN; Schroeder BC; Kurosawa T; Lopez J; Katsumata T; et al. Intrinsically Stretchable and Healable Semiconducting Polymer for Organic Transistors. Nature 2016, 539, 411–415. [DOI] [PubMed] [Google Scholar]
- (349).Sun T; Scott JI; Wang M; Kline RJ; Bazan G; O’Connor BT Reversible Plastic Deformation of Polymer Blends as a Means to Achieve Stretchable Organic Transistors. Adv. Electron. Mater 2017, 3, 1600388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (350).Kim HJ; Thukral A; Sharma S; Yu C Biaxially Stretchable Fully Elastic Transistors Based on Rubbery Semiconductor Nanocomposites. Adv. Mater. Technol 2018, 3, 1800043. [Google Scholar]
- (351).Mun J; Wang GJN; Oh JY; Katsumata T; Lee FL; Kang J; Wu HC; Lissel F; Rondeau-Gagné S; Tok JBH; et al. Effect of Nonconjugated Spacers on Mechanical Properties of Semiconducting Polymers for Stretchable Transistors. Adv. Funct. Mater 2018, 28, 1804222. [Google Scholar]
- (352).Wang G-JN; Zheng Y; Zhang S; Kang J; Wu H-C; Gasperini A; Zhang H; Gu X; Bao Z Tuning the Cross-Linker Crystallinity of a Stretchable Polymer Semiconductor. Chem. Mater 2019, 31, 6465–6475. [Google Scholar]
- (353).Wang S; Xu J; Wang W; Wang GN; Rastak R; Molina-Lopez F; Chung JW; Niu S; Feig VR; Lopez J; et al. Skin Electronics from Scalable Fabrication of an Intrinsically Stretchable Transistor Array. Nature 2018, 555, 83–88. [DOI] [PubMed] [Google Scholar]
- (354).Sim K; Rao Z; Kim H-J; Thukral A; Shim H; Yu C Fully Rubbery Integrated Electronics from High Effective Mobility Intrinsically Stretchable Semiconductors. Sci. Adv 2019, 5, No. eaav5749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (355).Chen J-Y; Hsieh H-C; Chiu Y-C; Lee W-Y; Hung C-C; Chueh C-C; Chen W-C Electrospinning-Induced Elastomeric Properties of Conjugated Polymers for Extremely Stretchable Nanofibers and Rubbery Optoelectronics. J. Mater. Chem. C 2020, 8, 873–882. [Google Scholar]
- (356).Guo X; Han L; Huang Y; Tang W Development of Organic TFT Technology for Active-Matrix Display Backplane. SID Symposium Digest of Technical Papers 2021, 52, 9–12. [Google Scholar]
- (357).Minemawari H; Yamada T; Matsui H; Tsutsumi J; Haas S; Chiba R; Kumai R; Hasegawa T Inkjet Printing of Single-Crystal Films. Nature 2011, 475, 364–367. [DOI] [PubMed] [Google Scholar]
- (358).Zhao X; Ding X; Tang Q; Tong Y; Liu Y Photolithography-Compatible Conformal Electrodes for High-Performance Bottom-Contact Organic Single-Crystal Transistors. J. Mater. Chem. C 2017, 5, 12699–12706. [Google Scholar]
- (359).Liu K; Ouyang B; Guo X; Guo Y; Liu Y Advances in Flexible Organic Field-Effect Transistors and Their Applications for Flexible Electronics. npj Flex. Electron 2022, 6, 1. [Google Scholar]
- (360).Sim K; Ershad F; Zhang Y; Yang P; Shim H; Rao Z; Lu Y; Thukral A; Elgalad A; Xi Y; et al. An Epicardial Bioelectronic Patch Made from Soft Rubbery Materials and Capable of Spatiotemporal Mapping of Electrophysiological Activity. Nat. Electron 2020, 3, 775–784. [Google Scholar]
- (361).Liang J; Li L; Chen D; Hajagos T; Ren Z; Chou SY; Hu W; Pei Q Intrinsically Stretchable and Transparent Thin-Film Transistors Based on Printable Silver Nanowires, Carbon Nanotubes and an Elastomeric Dielectric. Nat. Commun 2015, 6, 7647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (362).Zhang Z; Du C; Jiao H; Zhang M Polyvinyl Alcohol/SiO2 Hybrid Dielectric for Transparent Flexible/Stretchable All-Carbon-Nanotube Thin-Film-Transistor Integration. Adv. Electron. Mater 2020, 6, 1901133. [Google Scholar]
- (363).Liu N; Chortos A; Lei T; Jin L; Kim TR; Bae W-G; Zhu C; Wang S; Pfattner R; Chen X; et al. Ultratransparent and Stretchable Graphene Electrodes. Sci. Adv 2017, 3, No. e1700159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (364).Chortos A; Koleilat GI; Pfattner R; Kong D; Lin P; Nur R; Lei T; Wang H; Liu N; Lai YC; et al. Mechanically Durable and Highly Stretchable Transistors Employing Carbon Nanotube Semiconductor and Electrodes. Adv. Mater 2016, 28, 4441–4448. [DOI] [PubMed] [Google Scholar]
- (365).Cao Y; Tan YJ; Li S; Lee WW; Guo H; Cai Y; Wang C; Tee BCK Self-Healing Electronic Skins for Aquatic Environments. Nat. Electron 2019, 2, 75–82. [Google Scholar]
- (366).Li Q; Liu Z; Zheng S; Li W; Ren Y; Li L; Yan F Three-Dimensional Printable, Highly Conductive Ionic Elastomers for High-Sensitivity Iontronics. ACS Appl. Mater. Interfaces 2022, 14, 26068–26076. [DOI] [PubMed] [Google Scholar]
- (367).Zhang W; Wu B; Sun S; Wu P Skin-Like Mechanoresponsive Self-Healing Ionic Elastomer from Supramolecular Zwitterionic Network. Nat. Commun 2021, 12, 4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (368).Lei Z; Wu P Bioinspired Quasi-Solid Ionic Conductors: Materials, Processing, and Applications. Acc. Mater. Res 2021, 2, 1203–1214. [Google Scholar]
- (369).Wang Y; Jia K; Zhang S; Kim HJ; Bai Y; Hayward RC; Suo Z Temperature Sensing Using Junctions between Mobile Ions and Mobile Electrons. Proc. Natl. Acad. Sci. U. S. A 2022, 119, 2117962119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (370).Dobashi Y; Yao D; Petel Y; Nguyen Tan N; Sarwar Mirza S; Thabet Y; Ng Cliff LW; Scabeni Glitz E; Nguyen Giao Tran M; Plesse C; et al. Piezoionic Mechanoreceptors: Force-Induced Current Generation in Hydrogels. Science 2022, 376, 502–507. [DOI] [PubMed] [Google Scholar]
- (371).Luo Y; Li W; Lin Q; Zhang F; He K; Yang D; Loh XJ; Chen X A Morphable Ionic Electrode Based on Thermogel for Non-Invasive Hairy Plant Electrophysiology. Adv. Mater 2021, 33, 2007848. [DOI] [PubMed] [Google Scholar]
- (372).Chen G; Matsuhisa N; Liu Z; Qi D; Cai P; Jiang Y; Wan C; Cui Y; Leow WR; Liu Z; et al. Plasticizing Silk Protein for On-Skin Stretchable Electrodes. Adv. Mater 2018, 30, 1800129. [DOI] [PubMed] [Google Scholar]
- (373).Cheng X; Zhang F; Bo R; Shen Z; Pang W; Jin T; Song H; Xue Z; Zhang Y An Anti-Fatigue Design Strategy for 3D Ribbon-Shaped Flexible Electronics. Adv. Mater 2021, 33, 2102684. [DOI] [PubMed] [Google Scholar]
- (374).Zhu P; Du H; Hou X; Lu P; Wang L; Huang J; Bai N; Wu Z; Fang NX; Guo CF Skin-Electrode Iontronic Interface for Mechanosensing. Nat. Commun 2021, 12, 4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (375).Matsuhisa N; Jiang Y; Liu Z; Chen G; Wan C; Kim Y; Kang J; Tran H; Wu HC; You I; et al. High-Transconductance Stretchable Transistors Achieved by Controlled Gold Microcrack Morphology. Adv. Electron. Mater 2019, 5, 1900347. [Google Scholar]
- (376).Liang X; Chen G; Lin S; Zhang J; Wang L; Zhang P; Lan Y; Liu J Bioinspired 2D Isotropically Fatigue-Resistant Hydrogels. Adv. Mater 2022, 34, 2107106. [DOI] [PubMed] [Google Scholar]
- (377).Kim DH; Song J; Choi WM; Kim HS; Kim RH; Liu Z; Huang YY; Hwang KC; Zhang YW; Rogers JA Materials and Noncoplanar Mesh Designs for Integrated Circuits with Linear Elastic Responses to Extreme Mechanical Deformations. Proc. Natl. Acad. Sci. U.S.A 2008, 105, 18675–18680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (378).Wang W; Wang S; Rastak R; Ochiai Y; Niu S; Jiang Y; Arunachala PK; Zheng Y; Xu J; Matsuhisa N; et al. Strain-Insensitive Intrinsically Stretchable Transistors and Circuits. Nat. Electron 2021, 4, 143–150. [Google Scholar]
- (379).Zhu C; Chortos A; Wang Y; Pfattner R; Lei T; Hinckley AC; Pochorovski I; Yan X; To JWF; Oh JY; et al. Stretchable Temperature-Sensing Circuits with Strain Suppression Based on Carbon Nanotube Transistors. Nat. Electron 2018, 1, 183–190. [Google Scholar]
- (380).Zhao Y; Wang B; Tan J; Yin H; Huang R; Zhu J; Lin S; Zhou Y; Jelinek D; Sun Z; et al. Soft Strain-Insensitive Bioelectronics Featuring Brittle Materials. Science 2022, 378, 1222–1227. [DOI] [PubMed] [Google Scholar]
- (381).Gong S; Yap LW; Zhang Y; He J; Yin J; Marzbanrad F; Kaye DM; Cheng W A Gold Nanowire-Integrated Soft Wearable System for Dynamic Continuous Non-Invasive Cardiac Monitoring. Biosens. Bioelectron 2022, 205, 114072. [DOI] [PubMed] [Google Scholar]
- (382).Jeong JW; Yeo WH; Akhtar A; Norton JJ; Kwack YJ; Li S; Jung SY; Su Y; Lee W; Xia J; et al. Materials and Optimized Designs for Human-Machine Interfaces via Epidermal Electronics. Adv. Mater 2013, 25, 6839–6846. [DOI] [PubMed] [Google Scholar]
- (383).Zhao Y; Zhang S; Yu T; Zhang Y; Ye G; Cui H; He C; Jiang W; Zhai Y; Lu C; et al. Ultra-Conformal Skin Electrodes with Synergistically Enhanced Conductivity for Long-Time and Low-Motion Artifact Epidermal Electrophysiology. Nat. Commun 2021, 12, 4880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (384).Nawrocki RA; Jin H; Lee S; Yokota T; Sekino M; Someya T Self-Adhesive and Ultra-Conformable, Sub-300 nm Dry Thin-Film Electrodes for Surface Monitoring of Biopotentials. Adv. Funct. Mater 2018, 28, 1803279. [Google Scholar]
- (385).Dagdeviren C; Shi Y; Joe P; Ghaffari R; Balooch G; Usgaonkar K; Gur O; Tran PL; Crosby JR; Meyer M; et al. Conformal Piezoelectric Systems for Clinical and Experimental Characterization of Soft Tissue Biomechanics. Nat. Mater 2015, 14, 728–736. [DOI] [PubMed] [Google Scholar]
- (386).Jiang Z; Chen N; Yi Z; Zhong J; Zhang F; Ji S; Liao R; Wang Y; Li H; Liu Z; et al. A 1.3-Micrometre-Thick Elastic Conductor for Seamless On-Skin and Implantable Sensors. Nat. Electron 2022, 5, 784–793. [Google Scholar]
- (387).Yang H; Ji S; Chaturvedi I; Xia H; Wang T; Chen G; Pan L; Wan C; Qi D; Ong Y-S; et al. Adhesive Biocomposite Electrodes on Sweaty Skin for Long-Term Continuous Electrophysiological Monitoring. ACS Mater. Lett 2020, 2, 478–484. [Google Scholar]
- (388).Ji S; Wan C; Wang T; Li Q; Chen G; Wang J; Liu Z; Yang H; Liu X; Chen X Water-Resistant Conformal Hybrid Electrodes for Aquatic Endurable Electrocardiographic Monitoring. Adv. Mater 2020, 32, 2001496. [DOI] [PubMed] [Google Scholar]
- (389).Du X; Niu Z; Li R; Yang H; Hu W Highly Adhesive, Washable and Stretchable On-Skin Electrodes Based on Polydopamine and Silk Fibroin for Ambulatory Electrocardiography Sensing. J. Mater. Chem. C 2020, 8, 12257–12264. [Google Scholar]
- (390).Kireev D; Sel K; Ibrahim B; Kumar N; Akbari A; Jafari R; Akinwande D Continuous Cuffless Monitoring of Arterial Blood Pressure via Graphene Bioimpedance Tattoos. Nat. Nanotechnol 2022, 17, 864–870. [DOI] [PubMed] [Google Scholar]
- (391).Kabiri Ameri S; Ho R; Jang H; Tao L; Wang Y; Wang L; Schnyer DM; Akinwande D; Lu N Graphene Electronic Tattoo Sensors. ACS Nano 2017, 11, 7634–7641. [DOI] [PubMed] [Google Scholar]
- (392).Kireev D; Ameri SK; Nederveld A; Kampfe J; Jang H; Lu N; Akinwande D Fabrication, Characterization and Applications of Graphene Electronic Tattoos. Nat. Protoc 2021, 16, 2395–2417. [DOI] [PubMed] [Google Scholar]
- (393).Tang L; Shang J; Jiang X Multilayered Electronic Transfer Tattoo That Can Enable the Crease Amplification Effect. Sci. Adv 2021, 7, No. eabe3778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (394).Ershad F; Thukral A; Yue J; Comeaux P; Lu Y; Shim H; Sim K; Kim NI; Rao Z; Guevara R; et al. Ultra-Conformal Drawn-on-Skin Electronics for Multifunctional Motion Artifact-Free Sensing and Point-of-Care Treatment. Nat. Commun 2020, 11, 3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (395).Patel S; Ershad F; Lee J; Chacon-Alberty L; Wang Y; Morales-Garza MA; Haces-Garcia A; Jang S; Gonzalez L; Contreras L; et al. Drawn-on-Skin Sensors from Fully Biocompatible Inks toward High-Quality Electrophysiology. Small 2022, 18, 2107099. [DOI] [PubMed] [Google Scholar]
- (396).Park B; Shin Joo H; Ok J; Park S; Jung W; Jeong C; Choy S; Jo Young J; Kim T -i. Cuticular Pad-Inspired Selective Frequency Damper for Nearly Dynamic Noise-Free Bioelectronics. Science 2022, 376, 624–629. [DOI] [PubMed] [Google Scholar]
- (397).Baek S; Lee Y; Baek J; Kwon J; Kim S; Lee S; Strunk KP; Stehlin S; Melzer C; Park SM; et al. Spatiotemporal Measurement of Arterial Pulse Waves Enabled by Wearable Active-Matrix Pressure Sensor Arrays. ACS Nano 2022, 16, 368–377. [DOI] [PubMed] [Google Scholar]
- (398).Jeong H; Lee Jong Y; Lee K; Kang Youn J; Kim J-T; Avila R; Tzavelis A; Kim J; Ryu H; Kwak Sung S; et al. Differential Cardiopulmonary Monitoring System for Artifact-Canceled Physiological Tracking of Athletes, Workers, and COVID-19 Patients. Sci. Adv 2021, 7, No. eabg3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (399).Ha T; Tran J; Liu S; Jang H; Jeong H; Mitbander R; Huh H; Qiu Y; Duong J; Wang RL; et al. A Chest-Laminated Ultrathin and Stretchable E-Tattoo for the Measurement of Electrocardiogram, Seismocardiogram, and Cardiac Time Intervals. Adv. Sci 2019, 6, 1900290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (400).Sugiyama M; Uemura T; Kondo M; Akiyama M; Namba N; Yoshimoto S; Noda Y; Araki T; Sekitani T An Ultraflexible Organic Differential Amplifier for Recording Electrocardiograms. Nat. Electron 2019, 2, 351–360. [Google Scholar]
- (401).Lee H; Chung H; Lee J Motion Artifact Cancellation in Wearable Photoplethysmography Using Gyroscope. IEEE Sens. J 2019, 19, 1166–1175. [Google Scholar]
- (402).Zhang Z; Pi Z; Liu B Troika: A General Framework for Heart Rate Monitoring Using Wrist-Type Photoplethysmographic Signals During Intensive Physical Exercise. IEEE Trans. Biomed. Eng 2015, 62, 522–531. [DOI] [PubMed] [Google Scholar]
- (403).Wang L; Liu S; Li G; Zhu R Interface Sensors with Skin Piezo-Thermic Transduction Enable Motion Artifact Removal for Wearable Physiological Monitoring. Biosens. Bioelectron 2021, 188, 113325. [DOI] [PubMed] [Google Scholar]
- (404).Biswas D; Everson L; Liu M; Panwar M; Verhoef BE; Patki S; Kim CH; Acharyya A; Van Hoof C; Konijnenburg M; et al. CorNET: Deep Learning Framework for PPG-Based Heart Rate Estimation and Biometric Identification in Ambulant Environment. IEEE Trans. Biomed. Circuits Syst 2019, 13, 282–291. [DOI] [PubMed] [Google Scholar]
- (405).Fang Y; Zou Y; Xu J; Chen G; Zhou Y; Deng W; Zhao X; Roustaei M; Hsiai TK; Chen J Ambulatory Cardiovascular Monitoring via a Machine-Learning-Assisted Textile Triboelectric Sensor. Adv. Mater 2021, 33, 2104178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (406).Fuentes-Hernandez C; Chou W-F; Khan Talha M; Diniz L; Lukens J; Larrain Felipe A; Rodriguez-Toro Victor A; Kippelen B Large-Area Low-Noise Flexible Organic Photodiodes for Detecting Faint Visible Light. Science 2020, 370, 698–701. [DOI] [PubMed] [Google Scholar]
- (407).Lu W; Bai W; Zhang H; Xu C; Chiarelli Antonio M; Vázquez-Guardado A; Xie Z; Shen H; Nandoliya K; Zhao H; et al. Wireless, Implantable Catheter-Type Oximeter Designed for Cardiac Oxygen Saturation. Sci. Adv 2021, 7, No. eabe0579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (408).van Breemen AJJM; Ollearo R; Shanmugam S; Peeters B; Peters LCJM; van de Ketterij RL; Katsouras I; Akkerman HB; Frijters CH; Di Giacomo F; et al. A Thin and Flexible Scanner for Fingerprints and Documents Based on Metal Halide Perovskites. Nat. Electron 2021, 4, 818–826. [Google Scholar]
- (409).Rao Z; Lu Y; Li Z; Sim K; Ma Z; Xiao J; Yu C. Curvy, Shape-Adaptive Imagers Based on Printed Optoelectronic Pixels with a Kirigami Design. Nat. Electron 2021, 4, 513–521. [Google Scholar]
- (410).Song JK; Kim J; Yoon J; Koo JH; Jung H; Kang K; Sunwoo SH; Yoo S; Chang H; Jo J; et al. Stretchable Colour-Sensitive Quantum Dot Nanocomposites for Shape-Tunable Multi-plexed Phototransistor Arrays. Nat. Nanotechnol 2022, 17, 849–856. [DOI] [PubMed] [Google Scholar]
- (411).Kang J; Son D; Wang GN; Liu Y; Lopez J; Kim Y; Oh JY; Katsumata T; Mun J; Lee Y; et al. Tough and Water-Insensitive Self-Healing Elastomer for Robust Electronic Skin. Adv. Mater 2018, 30, 1706846. [DOI] [PubMed] [Google Scholar]
- (412).Yan X; Liu Z; Zhang Q; Lopez J; Wang H; Wu HC; Niu S; Yan H; Wang S; Lei T; et al. Quadruple H-Bonding Cross-Linked Supramolecular Polymeric Materials as Substrates for Stretchable, Antitearing, and Self-Healable Thin Film Electrodes. J. Am. Chem. Soc 2018, 140, 5280–5289. [DOI] [PubMed] [Google Scholar]
- (413).Sun JY; Zhao X; Illeperuma WR; Chaudhuri O; Oh KH; Mooney DJ; Vlassak JJ; Suo Z Highly Stretchable and Tough Hydrogels. Nature 2012, 489, 133–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (414).Yang Y; Wang X; Yang F; Wang L; Wu D Highly Elastic and Ultratough Hybrid Ionic-Covalent Hydrogels with Tunable Structures and Mechanics. Adv. Mater 2018, 30, 1707071. [DOI] [PubMed] [Google Scholar]
- (415).Maiti C; Imani KBC; Yoon J Recent Advances in Design Strategies for Tough and Stretchable Hydrogels. ChemPlusChem 2021, 86, 601–611. [DOI] [PubMed] [Google Scholar]
- (416).Zhang T; Li X; Gao H Designing Graphene Structures with Controlled Distributions of Topological Defects: A Case Study of Toughness Enhancement in Graphene Ruga. Extreme Mech. Lett 2014, 1, 3–8. [Google Scholar]
- (417).Hacopian EF; Yang Y; Ni B; Li Y; Li X; Chen Q; Guo H; Tour JM; Gao H; Lou J Toughening Graphene by Integrating Carbon Nanotubes. ACS Nano 2018, 12, 7901–7910. [DOI] [PubMed] [Google Scholar]
- (418).Yang Y; Song Z; Lu G; Zhang Q; Zhang B; Ni B; Wang C; Li X; Gu L; Xie X; et al. Intrinsic Toughening and Stable Crack Propagation in Hexagonal Boron Nitride. Nature 2021, 594, 57–61. [DOI] [PubMed] [Google Scholar]
- (419).Tan YJ; Susanto GJ; AnwarAli HP; Tee BCK Progress and Roadmap for Intelligent Self-Healing Materials in Autonomous Robotics. Adv. Mater 2021, 33, 2002800. [DOI] [PubMed] [Google Scholar]
- (420).Jiang PP; Qin H; Dai J; Yu SH; Cong HP Ultrastretchable and Self-Healing Conductors with Double Dynamic Network for Omni-Healable Capacitive Strain Sensors. Nano Lett. 2022, 22, 1433–1442. [DOI] [PubMed] [Google Scholar]
- (421).Oh JY; Son D; Katsumata T; Lee Y; Kim Y; Lopez J; Wu H-C; Kang J; Park J; Gu X; et al. Stretchable Self-Healable Semiconducting Polymer Film for Active-Matrix Strain-Sensing Array. Sci. Adv 2019, 5, No. eaav3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (422).Guo H; Tan YJ; Chen G; Wang Z; Susanto GJ; See HH; Yang Z; Lim ZW; Yang L; Tee BCK Artificially Innervated Self-Healing Foams as Synthetic Piezo-Impedance Sensor Skins. Nat. Commun 2020, 11, 5747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (423).Wu J; Wu Z; Xu H; Wu Q; Liu C; Yang B-R; Gui X; Xie X; Tao K; Shen Y; et al. An Intrinsically Stretchable Humidity Sensor Based on Anti-Drying, Self-Healing and Transparent Organohydrogels. Mater. Horiz 2019, 6, 595–603. [Google Scholar]
- (424).Khatib M; Zohar O; Haick H Self-Healing Soft Sensors: From Material Design to Implementation. Adv. Mater 2021, 33, 2004190. [DOI] [PubMed] [Google Scholar]
- (425).Markvicka EJ; Bartlett MD; Huang X; Majidi C An Autonomously Electrically Self-Healing Liquid Metal-Elastomer Composite for Robust Soft-Matter Robotics and Electronics. Nat. Mater 2018, 17, 618–624. [DOI] [PubMed] [Google Scholar]
- (426).Matsuda T; Kawakami R; Namba R; Nakajima T; Gong Jian P Mechanoresponsive Self-Growing Hydrogels Inspired by Muscle Training. Science 2019, 363, 504–508. [DOI] [PubMed] [Google Scholar]
- (427).Jang KI; Chung HU; Xu S; Lee CH; Luan H; Jeong J; Cheng H; Kim GT; Han SY; Lee JW; et al. Soft Network Composite Materials with Deterministic and Bio-Inspired Designs. Nat. Commun 2015, 6, 6566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (428).Zhalmuratova D; La TG; Yu KT; Szojka ARA; Andrews SHJ; Adesida AB; Kim CI; Nobes DS; Freed DH; Chung HJ Mimicking ″J-Shaped″ and Anisotropic Stress-Strain Behavior of Human and Porcine Aorta by Fabric-Reinforced Elastomer Composites. ACS Appl. Mater. Interfaces 2019, 11, 33323–33335. [DOI] [PubMed] [Google Scholar]
- (429).Liu K; Cheng L; Zhang N; Pan H; Fan X; Li G; Zhang Z; Zhao D; Zhao J; Yang X; et al. Biomimetic Impact Protective Supramolecular Polymeric Materials Enabled by Quadruple H-Bonding. J. Am. Chem. Soc 2021, 143, 1162–1170. [DOI] [PubMed] [Google Scholar]
- (430).Wang T; Cui Z; Liu Y; Lu D; Wang M; Wan C; Leow WR; Wang C; Pan L; Cao X; et al. Mechanically Durable Memristor Arrays Based on a Discrete Structure Design. Adv. Mater 2022, 34, 2106212. [DOI] [PubMed] [Google Scholar]
- (431).Choi J; Han S; Baliwag M; Kim BH; Jang H; Kim J-T; Hong I; Kim T; Kang SM; Lee K-T; et al. Artificial Stretchable Armor for Skin-Interfaced Wearable Devices and Soft Robotics. Extreme Mech. Lett 2022, 50, 101537. [Google Scholar]
- (432).Jiang S; Liu J; Xiong W; Yang Z; Yin L; Li K; Huang Y A Snakeskin-Inspired, Soft-Hinge Kirigami Metamaterial for Self-Adaptive Conformal Electronic Armor. Adv. Mater 2022, 34, 2204091. [DOI] [PubMed] [Google Scholar]
- (433).Yang W; Chen IH; Gludovatz B; Zimmermann EA; Ritchie RO; Meyers MA Natural Flexible Dermal Armor. Adv. Mater 2013, 25, 31–48. [DOI] [PubMed] [Google Scholar]
- (434).Meza LR; Das S; Greer JR Strong, Lightweight, and Recoverable Three-Dimensional Ceramic Nanolattices. Science 2014, 345, 1322–1326. [DOI] [PubMed] [Google Scholar]
- (435).Zheng X; Lee H; Weisgraber TH; Shusteff M; DeOtte J; Duoss EB; Kuntz JD; Biener MM; Ge Q; Jackson JA; et al. Ultralight, Ultrastiff Mechanical Metamaterials. Science 2014, 344, 1373–1377. [DOI] [PubMed] [Google Scholar]
- (436).Zhang X; Vyatskikh A; Gao H; Greer JR; Li X Lightweight, Flaw-Tolerant, and Ultrastrong Nanoarchitected Carbon. Proc. Natl. Acad. Sci. U. S. A 2019, 116, 6665–6672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (437).Feng X; Surjadi JU; Fan R; Li X; Zhou W; Zhao S; Lu Y Microalloyed Medium-Entropy Alloy (MEA)Composite Nanolattices with Ultrahigh Toughness and Cyclability. Mater. Today 2021, 42, 10–16. [Google Scholar]
- (438).Portela CM; Edwards BW; Veysset D; Sun Y; Nelson KA; Kochmann DM; Greer JR Supersonic Impact Resilience of Nanoarchitected Carbon. Nat. Mater 2021, 20, 1491–1497. [DOI] [PubMed] [Google Scholar]
- (439).Surjadi JU; Feng X; Fan R; Lin W; Li X; Lu Y Hollow Medium-Entropy Alloy Nanolattices with Ultrahigh Energy Absorption and Resilience. NPG Asia Mater. 2021, 13, 36. [Google Scholar]
- (440).Kalantar-Zadeh K; Tang J; Daeneke T; O’Mullane AP; Stewart LA; Liu J; Majidi C; Ruoff RS; Weiss PS; Dickey MD Emergence of Liquid Metals in Nanotechnology. ACS Nano 2019, 13, 7388–7395. [DOI] [PubMed] [Google Scholar]
- (441).Yang Z; Yang D; Zhao X; Zhao Q; Zhu M; Liu Y; Wang Y; Lu W; Qi D From Liquid Metal to Stretchable Electronics: Overcoming the Surface Tension. Sci. China Mater 2022, 65, 2072–2088. [Google Scholar]
- (442).Dickey MD Stretchable and Soft Electronics Using Liquid Metals. Adv. Mater 2017, 29, 1606425. [DOI] [PubMed] [Google Scholar]
- (443).Wang J; Cai G; Li S; Gao D; Xiong J; Lee PS Printable Superelastic Conductors with Extreme Stretchability and Robust Cycling Endurance Enabled by Liquid-Metal Particles. Adv. Mater 2018, 30, 1706157. [DOI] [PubMed] [Google Scholar]
- (444).Wang M; Ma C; Uzabakiriho PC; Chen X; Chen Z; Cheng Y; Wang Z; Zhao G Stencil Printing of Liquid Metal Upon Electrospun Nanofibers Enables High-Performance Flexible Electronics. ACS Nano 2021, 15, 19364–19376. [DOI] [PubMed] [Google Scholar]
- (445).Kim J; Park J; Park YG; Cha E; Ku M; An HS; Lee KP; Huh MI; Kim J; Kim TS; et al. A Soft and Transparent Contact Lens for the Wireless Quantitative Monitoring of Intraocular Pressure. Nat. Biomed. Eng 2021, 5, 772–782. [DOI] [PubMed] [Google Scholar]
- (446).Dong R; Wang L; Hang C; Chen Z; Liu X; Zhong L; Qi J; Huang Y; Liu S; Wang L; et al. Printed Stretchable Liquid Metal Electrode Arrays for in Vivo Neural Recording. Small 2021, 17, 2006612. [DOI] [PubMed] [Google Scholar]
- (447).Wang Q; Yu Y; Yang J; Liu J Fast Fabrication of Flexible Functional Circuits Based on Liquid Metal Dual-Trans Printing. Adv. Mater 2015, 27, 7109–7116. [DOI] [PubMed] [Google Scholar]
- (448).Kim D; Yoon Y; Kauh SK; Lee J Towards Sub-Microscale Liquid Metal Patterns: Cascade Phase Change Mediated Pick-N-Place Transfer of Liquid Metals Printed and Stretched over a Flexible Substrate. Adv. Funct. Mater 2018, 28, 1800380. [Google Scholar]
- (449).Li G; Lee DW An Advanced Selective Liquid-Metal Plating Technique for Stretchable Biosensor Applications. Lab Chip 2017, 17, 3415–3421. [DOI] [PubMed] [Google Scholar]
- (450).Dejace L; Chen H; Furfaro I; Schiavone G; Lacour SP Microscale Liquid Metal Conductors for Stretchable and Transparent Electronics. Adv. Mater. Technol 2021, 6, 2100690. [Google Scholar]
- (451).Ozutemiz KB; Majidi C; Ozdoganlar OB Scalable Manufacturing of Liquid Metal Circuits. Adv. Mater. Technol 2022, 7, 2200295. [Google Scholar]
- (452).Zhang L; Gao M; Wang R; Deng Z; Gui L Stretchable Pressure Sensor with Leakage-Free Liquid-Metal Electrodes. Sensors 2019, 19, 1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (453).Lee GH; Lee YR; Kim H; Kwon DA; Kim H; Yang C; Choi SQ; Park S; Jeong JW; Park S Rapid Meniscus-Guided Printing of Stable Semi-Solid-State Liquid Metal Microgranular-Particle for Soft Electronics. Nat. Commun 2022, 13, 2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (454).Wang D; Dong R; Wang X; Jiang X Flexible Electronic Catheter Based on Nanofibers for the in Vivo Elimination of Circulating Tumor Cells. ACS Nano 2022, 16, 5274–5283. [DOI] [PubMed] [Google Scholar]
- (455).Hang C; Ding L; Cheng S; Dong R; Qi J; Liu X; Liu Q; Zhang Y; Jiang X A Soft and Absorbable Temporary Epicardial Pacing Wire. Adv. Mater 2021, 33, 2101447. [DOI] [PubMed] [Google Scholar]
- (456).Cheng S; Hang C; Ding L; Jia L; Tang L; Mou L; Qi J; Dong R; Zheng W; Zhang Y; et al. Electronic Blood Vessel. Matter 2020, 3, 1664–1684. [Google Scholar]
- (457).Yeo JC; Yu J; Koh ZM; Wang Z; Lim CT Wearable Tactile Sensor Based on Flexible Microfluidics. Lab Chip 2016, 16, 3244–3250. [DOI] [PubMed] [Google Scholar]
- (458).Sundaram S; Kellnhofer P; Li Y; Zhu JY; Torralba A; Matusik W Learning the Signatures of the Human Grasp Using a Scalable Tactile Glove. Nature 2019, 569, 698–702. [DOI] [PubMed] [Google Scholar]
- (459).Lee W; Kim D; Matsuhisa N; Nagase M; Sekino M; Malliaras GG; Yokota T; Someya T Transparent, Conformable, Active Multielectrode Array Using Organic Electrochemical Transistors. Proc. Natl. Acad. Sci. U. S. A 2017, 114, 10554–10559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (460).Park YJ; Sharma BK; Shinde SM; Kim MS; Jang B; Kim JH; Ahn JH All MoS2-Based Large Area, Skin-Attachable Active-Matrix Tactile Sensor. ACS Nano 2019, 13, 3023–3030. [DOI] [PubMed] [Google Scholar]
- (461).Gwon G; Choi H; Bae J; Zulkifli NAB; Jeong W; Yoo S; Hyun DC; Lee S An All-Nanofiber-Based Substrate-Less, Extremely Conformal, and Breathable Organic Field Effect Transistor for Biomedical Applications. Adv. Funct. Mater 2022, 32, 2204645. [Google Scholar]
- (462).Kim DH; Viventi J; Amsden JJ; Xiao J; Vigeland L; Kim YS; Blanco JA; Panilaitis B; Frechette ES; Contreras D; et al. Dissolvable Films of Silk Fibroin for Ultrathin Conformal Bio-Integrated Electronics. Nat. Mater 2010, 9, 511–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (463).Yokota T; Sekitani T; Tokuhara T; Take N; Zschieschang U; Klauk H; Takimiya K; Huang T-C; Takamiya M; Sakurai T; et al. Sheet-Type Flexible Organic Active Matrix Amplifier System Using Pseudo-CMOS Circuits with Floating-Gate Structure. IEEE Trans. Electron Devices 2012, 59, 3434–3441. [Google Scholar]
- (464).Lee WW; Tan Yu J; Yao H; Li S; See Hian H; Hon M; Ng Kian A; Xiong B; Ho John S; Tee Benjamin CK A Neuro-Inspired Artificial Peripheral Nervous System for Scalable Electronic Skins. Sci. Robot 2019, 4, No. eaax2198. [DOI] [PubMed] [Google Scholar]
- (465).Kim T; Kim J; You I; Oh J; Kim S-P; Jeong U Dynamic Tactility by Position-Encoded Spike Spectrum. Sci. Robot 2022, 7, No. eabl5761. [DOI] [PubMed] [Google Scholar]
- (466).Myny K. The Development of Flexible Integrated Circuits Based on Thin-Film Transistors. Nat. Electron 2018, 1, 30–39. [Google Scholar]
- (467).Bao B; Rivkin B; Akbar F; Karnaushenko DD; Bandari VK; Teuerle L; Becker C; Baunack S; Karnaushenko D; Schmidt OG Digital Electrochemistry for On-Chip Heterogeneous Material Integration. Adv. Mater 2021, 33, 2101272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (468).Becker C; Bao B; Karnaushenko DD; Bandari VK; Rivkin B; Li Z; Faghih M; Karnaushenko D; Schmidt OG A New Dimension for Magnetosensitive E-Skins: Active Matrix Integrated Micro-Origami Sensor Arrays. Nat. Commun 2022, 13, 2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (469).Yao Y; Huang W; Chen J; Wang G; Chen H; Zhuang X; Ying Y; Ping J; Marks TJ; Facchetti A Flexible Complementary Circuits Operating at Sub-0.5 V via Hybrid Organic-Inorganic Electrolyte-Gated Transistors. Proc. Natl. Acad. Sci. U. S. A 2021, 118, No. e2111790118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (470).Kondo M; Melzer M; Karnaushenko D; Uemura T; Yoshimoto S; Akiyama M; Noda Y; Araki T; Schmidt OG; Sekitani T Imperceptible Magnetic Sensor Matrix System Integrated with Organic Driver and Amplifier Circuits. Sci. Adv 2020, 6, No. eaay6094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (471).Chen Y; Geng D; Lin T; Mativenga M; Jang J Full-Swing Clock Generating Circuits on Plastic Using a-IGZO Dual-Gate TFTs with Pseudo-CMOS and Bootstrapping. IEEE Electron Device Lett. 2016, 37, 882–885. [Google Scholar]
- (472).Wang X; Liu Y; Chen Q; Yan Y; Rao Z; Lin Z; Chen H; Guo T Recent Advances in Stretchable Field-Effect Transistors. J. Mater. Chem. C 2021, 9, 7796–7828. [Google Scholar]
- (473).Lee S; Nathan A Subthreshold Schottky-Barrier Thin-Film Transistors with Ultralow Power and High Intrinsic Gain. Science 2016, 354, 302–304. [DOI] [PubMed] [Google Scholar]
- (474).Jiang C; Tsangarides CP; Cheng X; Ding L; Ma H; Nathan A High Stretchability Ultralow-Power All-Printed Thin Film Transistor Amplifier on Strip-Helix-Fiber. Proceedings from the 2021 IEEE International Electron Devices Meeting (IEDM), December 11–16, 2021, San Francisco, CA; IEEE, 2021. [Google Scholar]
- (475).Kim TY; Suh W; Jeong U Approaches to Deformable Physical Sensors: Electronic Versus Iontronic. Mater. Sci. Eng. R 2021, 146, 100640. [Google Scholar]
- (476).Mackevicius EL; Best MD; Saal HP; Bensmaia SJ Millisecond Precision Spike Timing Shapes Tactile Perception. J. Neurosci 2012, 32, 15309–15317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (477).An T; Anaya DV; Gong S; Yap LW; Lin F; Wang R; Yuce MR; Cheng W Self-Powered Gold Nanowire Tattoo Triboelectric Sensors for Soft Wearable Human-Machine Interface. Nano Energy 2020, 77, 105295. [Google Scholar]
- (478).Zhuang Y; Li X; Lin F; Chen C; Wu Z; Luo H; Jin L; Xie RJ Visualizing Dynamic Mechanical Actions with High Sensitivity and High Resolution by Near-Distance Mechanoluminescence Imaging. Adv. Mater 2022, 34, 2202864. [DOI] [PubMed] [Google Scholar]
- (479).Quan YJ; Kim YG; Kim MS; Min SH; Ahn SH Stretchable Biaxial and Shear Strain Sensors Using Diffractive Structural Colors. ACS Nano 2020, 14, 5392–5399. [DOI] [PubMed] [Google Scholar]
- (480).Miller BH; Liu H; Kolle M Scalable Optical Manufacture of Dynamic Structural Colour in Stretchable Materials. Nat. Mater 2022, 21, 1014–1018. [DOI] [PubMed] [Google Scholar]
- (481).Yuan W; Dong S; Adelson EH Gelsight: High-Resolution Robot Tactile Sensors for Estimating Geometry and Force. Sensors (Basel) 2017, 17, 2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (482).Sun H; Kuchenbecker KJ; Martius G A Soft Thumb-Sized Vision-Based Sensor with Accurate All-Round Force Perception. Nat. Mach. Intell 2022, 4, 135–145. [Google Scholar]
- (483).Gelsight. https://www.gelsight.com/ (accessed 2022-09-12).
- (484).Melzer M; Monch JI; Makarov D; Zabila Y; Canon Bermudez GS; Karnaushenko D; Baunack S; Bahr F; Yan C; Kaltenbrunner M; et al. Wearable Magnetic Field Sensors for Flexible Electronics. Adv. Mater 2015, 27, 1274–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (485).Melzer M; Kaltenbrunner M; Makarov D; Karnaushenko D; Karnaushenko D; Sekitani T; Someya T; Schmidt OG Imperceptible Magnetoelectronics. Nat. Commun 2015, 6, 6080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (486).Bhirangi R; Hellebrekers T; Majidi C; Gupta A Reskin: Versatile, Replaceable, Lasting Tactile Skins. arXiv (Robotics), 2111.00071, ver. 2, 2021. https://arxiv.org/abs/2111.00071 (accessed 2022-09-12). [Google Scholar]
- (487).Tan P; Lu N Seeing inside a Body in Motion. Science 2022, 377, 466–467. [DOI] [PubMed] [Google Scholar]
- (488).Bouzari H; Engholm M; Nikolov SI; Stuart MB; Thomsen EV; Jensen JA Imaging Performance for Two Row-Column Arrays. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2019, 66, 1209–1221. [DOI] [PubMed] [Google Scholar]
- (489).Sauvage J; Poree J; Rabut C; Ferin G; Flesch M; Rosinski B; Nguyen-Dinh A; Tanter M; Pernot M; Deffieux T 4D Functional Imaging of the Rat Brain Using a Large Aperture Row-Column Array. IEEE Trans. Med. Imaging 2020, 39, 1884–1893. [DOI] [PubMed] [Google Scholar]
- (490).Rothberg JM; Ralston TS; Rothberg AG; Martin J; Zahorian JS; Alie SA; Sanchez NJ; Chen K; Chen C; Thiele K; et al. Ultrasound-on-Chip Platform for Medical Imaging, Analysis, and Collective Intelligence. Proc. Natl. Acad. Sci. U. S. A 2021, 118, No. e2019339118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (491).Qaisar S; Bilal RM; Iqbal W; Naureen M; Lee S Compressive Sensing: From Theory to Applications, a Survey. J. Commun. Netw 2013, 15, 443–456. [Google Scholar]
- (492).Luo Y; Li Y; Thean AV-Y; Heng C-H A 70-μm 1.35-mm2 Wireless Sensor with 32 Channels of Resistive and Capacitive Sensors and Edge-Encoded PWM UWB Transceiver. IEEE J. Solid-State Circuits 2021, 56, 2065–2076. [Google Scholar]
- (493).Yang C; Sun H; Liu S; Qiu L; Fang Z; Zheng Y A Broadband Resonant Noise Matching Technique for Piezoelectric Ultrasound Transducers. IEEE Sens. J 2020, 20, 4290–4299. [Google Scholar]
- (494).Yang C; Zheng Z; Fang Z; Tang X; Tang K; Liu S; Lou L; Zheng Y A Super-Sensitivity Photoacoustic Receiver System-on-Chip Based on Coherent Detection and Tracking. IEEE Trans. Biomed. Circuits Syst 2021, 15, 454–463. [DOI] [PubMed] [Google Scholar]
- (495).Wu G; Zhang N; Matarasso A; Heck I; Li H; Lu W; Phaup JG; Schneider MJ; Wu Y; Weng Z; et al. Implantable Aptamer-Graphene Microtransistors for Real-Time Monitoring of Neurochemical Release in Vivo. Nano Lett. 2022, 22, 3668–3677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (496).Alivisatos AP; Andrews AM; Boyden ES; Chun M; Church GM; Deisseroth K; Donoghue JP; Fraser SE; Lippincott-Schwartz J; Looger LL; et al. Nanotools for Neuroscience and Brain Activity Mapping. ACS Nano 2013, 7, 1850–1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (497).Liu Q; Zhao C; Chen M; Liu Y; Zhao Z; Wu F; Li Z; Weiss PS; Andrews AM; Zhou C Flexible Multiplexed In2O3 Nanoribbon Aptamer-Field-Effect Transistors for Biosensing. iScience 2020, 23, 101469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (498).Liao W-S; Cheunkar S; Cao HH; Bednar HR; Weiss PS; Andrews AM Subtractive Patterning via Chemical Lift-off Lithography. Science 2012, 337, 1517–1521. [DOI] [PubMed] [Google Scholar]
- (499).Liu M; Zhang Y; Wang J; Qin N; Yang H; Sun K; Hao J; Shu L; Liu J; Chen Q; et al. A Star-Nose-Like Tactile-Olfactory Bionic Sensing Array for Robust Object Recognition in Non-Visual Environments. Nat. Commun 2022, 13, 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (500).Duan S; Shi Q; Wu J Multimodal Sensors and ML-Based Data Fusion for Advanced Robots. Adv. Intell. Syst 2022, 4, 2200213. [Google Scholar]
- (501).Yang R; Zhang W; Tiwari N; Yan H; Li T; Cheng H Multimodal Sensors with Decoupled Sensing Mechanisms. Adv. Sci 2022, 9, 2202470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (502).Chung HU; Kim BH; Lee JY; Lee J; Xie Z; Ibler EM; Lee K; Banks A; Jeong JY; Kim J; et al. Binodal, Wireless Epidermal Electronic Systems with In-Sensor Analytics for Neonatal Intensive Care. Science 2019, 363, 947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (503).Sempionatto JR; Lin M; Yin L; De la paz E; Pei K; Sonsaard T; de Loyola Silva AN; Khorshed AA; Zhang F; Tostado N; et al. An Epidermal Patch for the Simultaneous Monitoring of Haemodynamic and Metabolic Biomarkers. Nat. Biomed. Eng 2021, 5, 737–748. [DOI] [PubMed] [Google Scholar]
- (504).Zhu Y; Haghniaz R; Hartel MC; Guan S; Bahari J; Li Z; Baidya A; Cao K; Gao X; Li J; et al. A Breathable, Passive-Cooling, Non-Inflammatory, and Biodegradable Aerogel Electronic Skin for Wearable Physical-Electrophysiological-Chemical Analysis. Adv. Mater 2022, No. e2209300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (505).Han M; Chen L; Aras K; Liang C; Chen X; Zhao H; Li K; Faye NR; Sun B; Kim JH; et al. Catheter-Integrated Soft Multilayer Electronic Arrays for Multiplexed Sensing and Actuation During Cardiac Surgery. Nat. Biomed. Eng 2020, 4, 997–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (506).Yao S; Myers A; Malhotra A; Lin F; Bozkurt A; Muth JF; Zhu Y A Wearable Hydration Sensor with Conformal Nanowire Electrodes. Adv. Healthc. Mater 2017, 6, 1601159. [DOI] [PubMed] [Google Scholar]
- (507).Zhao S; Zhu R Electronic Skin with Multifunction Sensors Based on Thermosensation. Adv. Mater 2017, 29, 1606151. [DOI] [PubMed] [Google Scholar]
- (508).Li G; Liu S; Wang L; Zhu R Skin-Inspired Quadruple Tactile Sensors Integrated on a Robot Hand Enable Object Recognition. Sci. Robot 2020, 5, No. eabc8134. [DOI] [PubMed] [Google Scholar]
- (509).Tien NT; Jeon S; Kim DI; Trung TQ; Jang M; Hwang BU; Byun KE; Bae J; Lee E; Tok JB; et al. A Flexible Bimodal Sensor Array for Simultaneous Sensing of Pressure and Temperature. Adv. Mater 2014, 26, 796–804. [DOI] [PubMed] [Google Scholar]
- (510).Wakabayashi S; Arie T; Akita S; Nakajima K; Takei K A Multitasking Flexible Sensor via Reservoir Computing. Adv. Mater 2022, 34, 2201663. [DOI] [PubMed] [Google Scholar]
- (511).Yang C; Wang H; Yang J; Yao H; He T; Bai J; Guang T; Cheng H; Yan J; Qu L A Machine-Learning-Enhanced Simultaneous and Multimodal Sensor Based on Moist-Electric Powered Graphene Oxide. Adv. Mater 2022, 34, 2205249. [DOI] [PubMed] [Google Scholar]
- (512).Cai P; Wan C; Pan L; Matsuhisa N; He K; Cui Z; Zhang W; Li C; Wang J; Yu J; et al. Locally Coupled Electromechanical Interfaces Based on Cytoadhesion-Inspired Hybrids to Identify Muscular Excitation-Contraction Signatures. Nat. Commun 2020, 11, 2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (513).Zhu J; Zhang X; Wang R; Wang M; Chen P; Cheng L; Wu Z; Wang Y; Liu Q; Liu M A Heterogeneously Integrated Spiking Neuron Array for Multimode-Fused Perception and Object Classification. Adv. Mater 2022, 34, 2200481. [DOI] [PubMed] [Google Scholar]
- (514).Liu W; Duo Y; Liu J; Yuan F; Li L; Li L; Wang G; Chen B; Wang S; Yang H; et al. Touchless Interactive Teaching of Soft Robots through Flexible Bimodal Sensory Interfaces. Nat. Commun 2022, 13, 5030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (515).Cui Z; Wang W; Xia H; Wang C; Tu J; Ji S; Tan JMR; Liu Z; Zhang F; Li W; et al. Freestanding and Scalable Force-Softness Bimodal Sensor Arrays for Haptic Body-Feature Identification. Adv Mater 2022, 34, 2207016. [DOI] [PubMed] [Google Scholar]
- (516).Chen L; Chang X; Wang H; Chen J; Zhu Y Stretchable and Transparent Multimodal Electronic-Skin Sensors in Detecting Strain, Temperature, and Humidity. Nano Energy 2022, 96, 107077. [Google Scholar]
- (517).Ge G; Lu Y; Qu X; Zhao W; Ren Y; Wang W; Wang Q; Huang W; Dong X Muscle-Inspired Self-Healing Hydrogels for Strain and Temperature Sensor. ACS Nano 2020, 14, 218–228. [DOI] [PubMed] [Google Scholar]
- (518).Kakei Y; Katayama S; Lee S; Takakuwa M; Furusawa K; Umezu S; Sato H; Fukuda K; Someya T Integration of Body-Mounted Ultrasoft Organic Solar Cell on Cyborg Insects with Intact Mobility. npj Flex. Electron 2022, 6, 78. [Google Scholar]
- (519).Li C; Guo C; Fitzpatrick V; Ibrahim A; Zwierstra MJ; Hanna P; Lechtig A; Nazarian A; Lin SJ; Kaplan DL Design of Biodegradable, Implantable Devices Towards Clinical Translation. Nat. Rev. Mater 2020, 5, 61–81. [Google Scholar]
- (520).Obidin N; Tasnim F; Dagdeviren C The Future of Neuroimplantable Devices: A Materials Science and Regulatory Perspective. Adv. Mater 2020, 32, 1901482. [DOI] [PubMed] [Google Scholar]
- (521).Lee G; Wei Q; Zhu Y Emerging Wearable Sensors for Plant Health Monitoring. Adv. Funct. Mater 2021, 31, 2106475. [Google Scholar]
- (522).Qu CC; Sun XY; Sun WX; Cao LX; Wang XQ; He ZZ Flexible Wearables for Plants. Small 2021, 17, 2104482. [DOI] [PubMed] [Google Scholar]
- (523).Yin H; Cao Y; Marelli B; Zeng X; Mason AJ; Cao C Soil Sensors and Plant Wearables for Smart and Precision Agriculture. Adv. Mater 2021, 33, 2007764. [DOI] [PubMed] [Google Scholar]
- (524).Yuk H; Lu B; Zhao X Hydrogel Bioelectronics. Chem. Soc. Rev 2019, 48, 1642–1667. [DOI] [PubMed] [Google Scholar]
- (525).Huang H; Su S; Wu N; Wan H; Wan S; Bi H; Sun L Graphene-Based Sensors for Human Health Monitoring. Front. Chem 2019, 7, 399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (526).Woods GA; Rommelfanger NJ; Hong G Bioinspired Materials for in Vivo Bioelectronic Neural Interfaces. Matter 2020, 3, 1087–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (527).Lin Y; Fang Y; Yue J; Tian B Soft-Hard Composites for Bioelectric Interfaces. Trends Chem. 2020, 2, 519–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (528).Rochford AE; Carnicer-Lombarte A; Curto VF; Malliaras GG; Barone DG When Bio Meets Technology: Biohybrid Neural Interfaces. Adv. Mater 2020, 32, 1903182. [DOI] [PubMed] [Google Scholar]
- (529).Fallegger F; Schiavone G; Lacour SP Conformable Hybrid Systems for Implantable Bioelectronic Interfaces. Adv. Mater 2020, 32, 1903904. [DOI] [PubMed] [Google Scholar]
- (530).Fang Y; Yang X; Lin Y; Shi J; Prominski A; Clayton C; Ostroff E; Tian B Dissecting Biological and Synthetic Soft-Hard Interfaces for Tissue-Like Systems. Chem. Rev 2022, 122, 5233–5276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (531).Yang L; Liu Q; Zhang Z; Gan L; Zhang Y; Wu J Materials for Dry Electrodes for the Electroencephalography: Advances, Challenges, Perspectives. Adv. Mater. Technol 2022, 7, 2100612. [Google Scholar]
- (532).Li H; Liu H; Sun M; Huang Y; Xu L 3D Interfacing between Soft Electronic Tools and Complex Biological Tissues. Adv. Mater 2021, 33, 2004425. [DOI] [PubMed] [Google Scholar]
- (533).Nan K; Feig VR; Ying B; Howarth JG; Kang Z; Yang Y; Traverso G Mucosa-Interfacing Electronics. Nat. Rev. Mater 2022, 7, 908–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (534).Sunwoo SH; Ha KH; Lee S; Lu N; Kim DH Wearable and Implantable Soft Bioelectronics: Device Designs and Material Strategies. Annu. Rev. Chem. Biomol. Eng 2021, 12, 359–391. [DOI] [PubMed] [Google Scholar]
- (535).Akouissi O; Lacour SP; Micera S; DeSimone A A Finite Element Model of the Mechanical Interactions between Peripheral Nerves and Intrafascicular Implants. J. Neural Eng 2022, 19, 046017. [DOI] [PubMed] [Google Scholar]
- (536).Pashuck ET; Stevens MM Designing Regenerative Biomaterial Therapies for the Clinic. Sci. Transl. Med 2012, 4, 160sr164. [DOI] [PubMed] [Google Scholar]
- (537).Ramakrishna S; Tian L; Wang C; Liao S; Teo WE Safety Testing of a New Medical Device. In Medical Devices: Regulations, Standards and Practices, Woodhead Publishing, 2015; pp 137–153. [Google Scholar]
- (538).Ashammakhi N; Hernandez AL; Unluturk BD; Quintero SA; Barros NR; Hoque Apu E; Bin Shams A; Ostrovidov S; Li J; Contag C; et al. Biodegradable Implantable Sensors: Materials Design, Fabrication, and Applications. Adv. Funct. Mater 2021, 31, 2104149. [Google Scholar]
- (539).Ferro MD; Melosh NA Electronic and Ionic Materials for Neurointerfaces. Adv. Funct. Mater 2018, 28, 1704335. [Google Scholar]
- (540).Liu J; Fu TM; Cheng Z; Hong G; Zhou T; Jin L; Duvvuri M; Jiang Z; Kruskal P; Xie C; et al. Syringe-Injectable Electronics. Nat. Nanotechnol 2015, 10, 629–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (541).Zhou T; Hong G; Fu TM; Yang X; Schuhmann TG; Viveros RD; Lieber CM Syringe-Injectable Mesh Electronics Integrate Seamlessly with Minimal Chronic Immune Response in the Brain. Proc. Natl. Acad. Sci. U. S. A 2017, 114, 5894–5899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (542).Yang X; Zhou T; Zwang TJ; Hong G; Zhao Y; Viveros RD; Fu TM; Gao T; Lieber CM Bioinspired Neuron-Like Electronics. Nat. Mater 2019, 18, 510–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (543).Le Floch P; Li Q; Lin Z; Zhao S; Liu R; Tasnim K; Jiang H; Liu J Stretchable Mesh Nanoelectronics for 3D Single-Cell Chronic Electrophysiology from Developing Brain Organoids. Adv. Mater 2022, 34, 2106829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (544).Park S; Yuk H; Zhao R; Yim YS; Woldeghebriel EW; Kang J; Canales A; Fink Y; Choi GB; Zhao X; et al. Adaptive and Multifunctional Hydrogel Hybrid Probes for Long-Term Sensing and Modulation of Neural Activity. Nat. Commun 2021, 12, 3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (545).Tringides CM; Vachicouras N; de Lazaro I; Wang H; Trouillet A; Seo BR; Elosegui-Artola A; Fallegger F; Shin Y; Casiraghi C; et al. Viscoelastic Surface Electrode Arrays to Interface with Viscoelastic Tissues. Nat. Nanotechnol 2021, 16, 1019–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (546).Li G; Huang K; Deng J; Guo M; Cai M; Zhang Y; Guo CF Highly Conducting and Stretchable Double-Network Hydrogel for Soft Bioelectronics. Adv. Mater 2022, 34, 2200261. [DOI] [PubMed] [Google Scholar]
- (547).Liu Y; Liu J; Chen S; Lei T; Kim Y; Niu S; Wang H; Wang X; Foudeh AM; Tok JB; et al. Soft and Elastic Hydrogel-Based Microelectronics for Localized Low-Voltage Neuromodulation. Nat. Biomed. Eng 2019, 3, 58–68. [DOI] [PubMed] [Google Scholar]
- (548).Han IK; Song KI; Jung SM; Jo Y; Kwon J; Chung T; Yoo S; Jang J; Kim YT; Hwang DS; et al. Electroconductive, Adhesive, Non-Swelling, and Viscoelastic Hydrogels for Bioelectronics. Adv. Mater 2023, 35, 2203431. [DOI] [PubMed] [Google Scholar]
- (549).Liu J; Yan D; Pang W; Zhang Y Design, Fabrication and Applications of Soft Network Materials. Mater. Today 2021, 49, 324–350. [Google Scholar]
- (550).Wang L; Xie S; Wang Z; Liu F; Yang Y; Tang C; Wu X; Liu P; Li Y; Saiyin H; et al. Functionalized Helical Fibre Bundles of Carbon Nanotubes as Electrochemical Sensors for Long-Term in Vivo Monitoring of Multiple Disease Biomarkers. Nat. Biomed. Eng 2020, 4, 159–171. [DOI] [PubMed] [Google Scholar]
- (551).Gao M; Meng Y; Shen C; Pei Q Stiffness Variable Polymers Comprising Phase-Changing Side-Chains: Material Syntheses and Application Explorations. Adv. Mater 2022, 34, 2109798. [DOI] [PubMed] [Google Scholar]
- (552).Chen L; Zhao C; Huang J; Zhou J; Liu M Enormous-Stiffness-Changing Polymer Networks by Glass Transition Mediated Microphase Separation. Nat. Commun 2022, 13, 6821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (553).Wicaksono I; Tucker CI; Sun T; Guerrero CA; Liu C; Woo WM; Pence EJ; Dagdeviren C A Tailored, Electronic Textile Conformable Suit for Large-Scale Spatiotemporal Physiological Sensing in Vivo. npj Flex. Electron 2020, 4, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (554).Gao M; Wu H; Plamthottam R; Xie Z; Liu Y; Hu J; Wu S; Wu L; He X; Pei Q Skin Temperature-Triggered, Debonding-on-Demand Sticker for a Self-Powered Mechanosensitive Communication System. Matter 2021, 4, 1962–1974. [Google Scholar]
- (555).Wang Y; Lee S; Wang H; Jiang Z; Jimbo Y; Wang C; Wang B; Kim JJ; Koizumi M; Yokota T; et al. Robust, Self-Adhesive, Reinforced Polymeric Nanofilms Enabling Gas-Permeable Dry Electrodes for Long-Term Application. Proc. Natl. Acad. Sci. U. S. A 2021, 118, No. e2111904118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (556).Min H; Baik S; Kim J; Lee J; Bok BG; Song JH; Kim MS; Pang C Tough Carbon Nanotube-Implanted Bioinspired Three-Dimensional Electrical Adhesive for Isotropically Stretchable Water-Repellent Bioelectronics. Adv. Funct. Mater 2022, 32, 2107285. [Google Scholar]
- (557).Liu X; Liu J; Wang J; Wang T; Jiang Y; Hu J; Liu Z; Chen X; Yu J Bioinspired, Microstructured Silk Fibroin Adhesives for Flexible Skin Sensors. ACS Appl. Mater. Interfaces 2020, 12, 5601–5609. [DOI] [PubMed] [Google Scholar]
- (558).Baik S; Lee HJ; Kim DW; Kim JW; Lee Y; Pang C Bioinspired Adhesive Architectures: From Skin Patch to Integrated Bioelectronics. Adv. Mater 2019, 31, 1803309. [DOI] [PubMed] [Google Scholar]
- (559).Xue B; Gu J; Li L; Yu W; Yin S; Qin M; Jiang Q; Wang W; Cao Y Hydrogel Tapes for Fault-Tolerant Strong Wet Adhesion. Nat. Commun 2021, 12, 7156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (560).Xue Y; Zhang J; Chen X; Zhang J; Chen G; Zhang K; Lin J; Guo C; Liu J Trigger-Detachable Hydrogel Adhesives for Bioelectronic Interfaces. Adv. Funct. Mater 2021, 31, 2106446. [Google Scholar]
- (561).Deng J; Yuk H; Wu J; Varela CE; Chen X; Roche ET; Guo CF; Zhao X Electrical Bioadhesive Interface for Bioelectronics. Nat. Mater 2021, 20, 229–236. [DOI] [PubMed] [Google Scholar]
- (562).Yang Q; Wei T; Yin RT; Wu M; Xu Y; Koo J; Choi YS; Xie Z; Chen SW; Kandela I; et al. Photocurable Bioresorbable Adhesives as Functional Interfaces between Flexible Bioelectronic Devices and Soft Biological Tissues. Nat. Mater 2021, 20, 1559–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (563).Jiang Y; Trotsyuk AA; Niu S; Henn D; Chen K; Shih CC; Larson MR; Mermin-Bunnell AM; Mittal S; Lai JC; et al. Wireless, Closed-Loop, Smart Bandage with Integrated Sensors and Stimulators for Advanced Wound Care and Accelerated Healing. Nat. Biotechnol 2022, DOI: 10.1038/s41587-022-01528-3. [DOI] [PubMed] [Google Scholar]
- (564).Yuk H; Varela CE; Nabzdyk CS; Mao X; Padera RF; Roche ET; Zhao X Dry Double-Sided Tape for Adhesion of Wet Tissues and Devices. Nature 2019, 575, 169–174. [DOI] [PubMed] [Google Scholar]
- (565).Tan P; Wang H; Xiao F; Lu X; Shang W; Deng X; Song H; Xu Z; Cao J; Gan T; et al. Solution-Processable, Soft, Self-Adhesive, and Conductive Polymer Composites for Soft Electronics. Nat. Commun 2022, 13, 358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (566).Wang C; Chen X; Wang L; Makihata M; Liu H-C; Zhou T; Zhao X Bioadhesive Ultrasound for Long-Term Continuous Imaging of Diverse Organs. Science 2022, 377, 517–523. [DOI] [PubMed] [Google Scholar]
- (567).Zhang L; Kumar KS; He H; Cai CJ; He X; Gao H; Yue S; Li C; Seet RC; Ren H; et al. Fully Organic Compliant Dry Electrodes Self-Adhesive to Skin for Long-Term Motion-Robust Epidermal Biopotential Monitoring. Nat. Commun 2020, 11, 4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (568).Jinkins KR; Li S; Arafa H; Jeong H; Lee Young J; Wu C; Campisi E; Ni X; Cho D; Huang Y; et al. Thermally Switchable, Crystallizable Oil and Silicone Composite Adhesives for Skin-Interfaced Wearable Devices. Sci. Adv 2022, 8, No. eabo0537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (569).Ereifej ES; Smith CS; Meade SM; Chen K; Feng H; Capadona JR The Neuroinflammatory Response to Nanopatterning Parallel Grooves into the Surface Structure of Intracortical Micro-electrodes. Adv. Funct. Mater 2018, 28, 1704420. [Google Scholar]
- (570).Kim E; Kim J-Y; Choi H An SU-8-Based Microprobe with a Nanostructured Surface Enhances Neuronal Cell Attachment and Growth. Micro and Nano Systems Letters 2017, 5, 28. [Google Scholar]
- (571).Abidian MR; Martin DC Multifunctional Nanobiomaterials for Neural Interfaces. Adv. Funct. Mater 2009, 19, 573–585. [Google Scholar]
- (572).Kang SK; Murphy RK; Hwang SW; Lee SM; Harburg DV; Krueger NA; Shin J; Gamble P; Cheng H; Yu S; et al. Bioresorbable Silicon Electronic Sensors for the Brain. Nature 2016, 530, 71–76. [DOI] [PubMed] [Google Scholar]
- (573).Choi YS; Yin RT; Pfenniger A; Koo J; Avila R; Benjamin Lee K; Chen SW; Lee G; Li G; Qiao Y; et al. Fully Implantable and Bioresorbable Cardiac Pacemakers without Leads or Batteries. Nat. Biotechnol 2021, 39, 1228–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (574).Yang SM; Shim JH; Cho HU; Jang TM; Ko GJ; Shim J; Kim TH; Zhu J; Park S; Kim YS; et al. Hetero-Integration of Silicon Nanomembranes with 2D Materials for Bioresorbable, Wireless Neurochemical System. Adv. Mater 2022, 34, 2108203. [DOI] [PubMed] [Google Scholar]
- (575).Curry EJ; Ke K; Chorsi MT; Wrobel KS; Miller AN III; Patel A; Kim I; Feng J; Yue L; Wu Q; et al. Biodegradable Piezoelectric Force Sensor. Proc. Natl. Acad. Sci. U. S. A 2018, 115, 909–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (576).Yao B; Wu S; Wang R; Yan Y; Cardenas A; Wu D; Alsaid Y; Wu W; Zhu X; He X Hydrogel Ionotronics with Ultra-Low Impedance and High Signal Fidelity across Broad Frequency and Temperature Ranges. Adv. Funct. Mater 2022, 32, 2109506. [Google Scholar]
- (577).Ouyang L; Wei B; Kuo C-C; Pathak S; Farrell B; Martin DC Enhanced Pedot Adhesion on Solid Substrates with Electro-grafted P(EDOT-NH2). Sci. Adv 2017, 3, 1600448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (578).Pan L; Cai P; Mei L; Cheng Y; Zeng Y; Wang M; Wang T; Jiang Y; Ji B; Li D; et al. A Compliant Ionic Adhesive Electrode with Ultralow Bioelectronic Impedance. Adv. Mater 2020, 32, 2003723. [DOI] [PubMed] [Google Scholar]
- (579).Liu Y; Li J; Song S; Kang J; Tsao Y; Chen S; Mottini V; McConnell K; Xu W; Zheng YQ; et al. Morphing Electronics Enable Neuromodulation in Growing Tissue. Nat. Biotechnol 2020, 38, 1031–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (580).Huang Q; Zheng Z Pathway to Developing Permeable Electronics. ACS Nano 2022, 16, 15537–15544. [DOI] [PubMed] [Google Scholar]
- (581).Someya T; Bauer S; Kaltenbrunner M Imperceptible Organic Electronics. MRS Bull. 2017, 42, 124–130. [Google Scholar]
- (582).Yan Z; Xu D; Lin Z; Wang P; Cao B; Ren H; Song F; Wan C; Wang L; Zhou J; et al. Highly Stretchable Van Der Waals Thin Films for Adaptable and Breathable Electronic Membranes. Science 2022, 375, 852–859. [DOI] [PubMed] [Google Scholar]
- (583).Park Y; Shim J; Jeong S; Yi GR; Chae H; Bae JW; Kim SO; Pang C Microtopography-Guided Conductive Patterns of Liquid-Driven Graphene Nanoplatelet Networks for Stretchable and Skin-Conformal Sensor Array. Adv. Mater 2017, 29, 1606453. [DOI] [PubMed] [Google Scholar]
- (584).Pang C; Koo JH; Nguyen A; Caves JM; Kim MG; Chortos A; Kim K; Wang PJ; Tok JB; Bao Z Highly Skin-Conformal Microhairy Sensor for Pulse Signal Amplification. Adv. Mater 2015, 27, 634–640. [DOI] [PubMed] [Google Scholar]
- (585).Choi J; Han C; Cho S; Kim K; Ahn J; Del Orbe D; Cho I; Zhao Z-J; Oh Yong S; Hong H; et al. Customizable, Conformal, and Stretchable 3D Electronics via Predistorted Pattern Generation and Thermoforming. Sci. Adv 2021, 7, No. eabj0694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (586).Liu J; Jiang S; Xiong W; Zhu C; Li K; Huang Y Self-Healing Kirigami Assembly Strategy for Conformal Electronics. Adv. Funct. Mater 2022, 32, 2109214. [Google Scholar]
- (587).Rich SI; Lee S; Fukuda K; Someya T Developing the Nondevelopable: Creating Curved-Surface Electronics from Non-stretchable Devices. Adv. Mater 2022, 34, 2106683. [DOI] [PubMed] [Google Scholar]
- (588).Sim K; Chen S; Li Z; Rao Z; Liu J; Lu Y; Jang S; Ershad F; Chen J; Xiao J; et al. Three-Dimensional Curvy Electronics Created Using Conformal Additive Stamp Printing. Nat. Electron 2019, 2, 471–479. [Google Scholar]
- (589).Wang C; Wang H; Wang B; Miyata H; Wang Y; Nayeem Md Osman G; Kim Jae J; Lee S; Yokota T; Onodera H; et al. On-Skin Paintable Biogel for Long-Term High-Fidelity Electroencephalogram Recording. Sci. Adv 2022, 8, No. eabo1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (590).Lee GH; Woo H; Yoon C; Yang C; Bae JY; Kim W; Lee DH; Kang H; Han S; Kang SK; et al. A Personalized Electronic Tattoo for Healthcare Realized by On-the-Spot Assembly of an Intrinsically Conductive and Durable Liquid-Metal Composite. Adv. Mater 2022, 34, 2204159. [DOI] [PubMed] [Google Scholar]
- (591).Kucukdeger E; Tong Y; Singh M; Zhang J; Harding LK; Salado A; Ellingson SW; Johnson BN Conformal 3D Printing of Non-Planar Antennas on Wrinkled and Folded Kapton Films Using Point Cloud Data. Flex. Print. Electron 2021, 6, 044002. [Google Scholar]
- (592).Zhu Z; Guo SZ; Hirdler T; Eide C; Fan X; Tolar J; McAlpine MC 3D Printed Functional and Biological Materials on Moving Freeform Surfaces. Adv. Mater 2018, 30, 1707495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (593).Ding L; Hang C; Yang S; Qi J; Dong R; Zhang Y; Sun H; Jiang X In Situ Deposition of Skin-Adhesive Liquid Metal Particles with Robust Wear Resistance for Epidermal Electronics. Nano Lett. 2022, 22, 4482–4490. [DOI] [PubMed] [Google Scholar]
- (594).Zheng S; Li W; Ren Y; Liu Z; Zou X; Hu Y; Guo J; Sun Z; Yan F Moisture-Wicking, Breathable, and Intrinsically Anti-bacterial Electronic Skin Based on Dual-Gradient Poly(Ionic Liquid) Nanofiber Membranes. Adv. Mater 2022, 34, 2106570. [DOI] [PubMed] [Google Scholar]
- (595).Yang X; Wang S; Liu M; Li L; Zhao Y; Wang Y; Bai Y; Lu Q; Xiong Z; Feng S; et al. All-Nanofiber-Based Janus Epidermal Electrode with Directional Sweat Permeability for Artifact-Free Biopotential Monitoring. Small 2022, 18, 2106477. [DOI] [PubMed] [Google Scholar]
- (596).Miyamoto A; Lee S; Cooray NF; Lee S; Mori M; Matsuhisa N; Jin H; Yoda L; Yokota T; Itoh A; et al. Inflammation-Free, Gas-Permeable, Lightweight, Stretchable On-Skin Electronics with Nanomeshes. Nat. Nanotechnol 2017, 12, 907–913. [DOI] [PubMed] [Google Scholar]
- (597).Zhou W; Yao S; Wang H; Du Q; Ma Y; Zhu Y Gas-Permeable, Ultrathin, Stretchable Epidermal Electronics with Porous Electrodes. ACS Nano 2020, 14, 5798–5805. [DOI] [PubMed] [Google Scholar]
- (598).Sun B; McCay RN; Goswami S; Xu Y; Zhang C; Ling Y; Lin J; Yan Z Gas-Permeable, Multifunctional On-Skin Electronics Based on Laser-Induced Porous Graphene and Sugar-Templated Elastomer Sponges. Adv. Mater 2018, 30, No. e1804327. [DOI] [PubMed] [Google Scholar]
- (599).Yao S; Zhou W; Hinson R; Dong P; Wu S; Ives J; Hu X; Huang H; Zhu Y Ultrasoft Porous 3D Conductive Dry Electrodes for Electrophysiological Sensing and Myoelectric Control. Adv. Mater. Technol 2022, 7, 2101637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (600).Yan W; Noel G; Loke G; Meiklejohn E; Khudiyev T; Marion J; Rui G; Lin J; Cherston J; Sahasrabudhe A; et al. Single Fibre Enables Acoustic Fabrics via Nanometre-Scale Vibrations. Nature 2022, 603, 616–623. [DOI] [PubMed] [Google Scholar]
- (601).Clevenger M; Kim H; Song HW; No K; Lee S Binder-Free Printed Pedot Wearable Sensors on Everyday Fabrics Using Oxidative Chemical Vapor Deposition. Sci. Adv 2021, 7, No. eabj8958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (602).Ma Z; Huang Q; Xu Q; Zhuang Q; Zhao X; Yang Y; Qiu H; Yang Z; Wang C; Chai Y; et al. Permeable Superelastic Liquid-Metal Fibre Mat Enables Biocompatible and Monolithic Stretchable Electronics. Nat. Mater 2021, 20, 859–868. [DOI] [PubMed] [Google Scholar]
- (603).Tian B; Fang Y; Liang J; Zheng K; Guo P; Zhang X; Wu Y; Liu Q; Huang Z; Cao C; et al. Fully Printed Stretchable and Multifunctional E-Textiles for Aesthetic Wearable Electronic Systems. Small 2022, 18, 2107298. [DOI] [PubMed] [Google Scholar]
- (604).Shi J; Liu S; Zhang L; Yang B; Shu L; Yang Y; Ren M; Wang Y; Chen J; Chen W; et al. Smart Textile-Integrated Microelectronic Systems for Wearable Applications. Adv. Mater 2020, 32, 1901958. [DOI] [PubMed] [Google Scholar]
- (605).Shi X; Zuo Y; Zhai P; Shen J; Yang Y; Gao Z; Liao M; Wu J; Wang J; Xu X; et al. Large-Area Display Textiles Integrated with Functional Systems. Nature 2021, 591, 240–245. [DOI] [PubMed] [Google Scholar]
- (606).Choi HW; Shin DW; Yang J; Lee S; Figueiredo C; Sinopoli S; Ullrich K; Jovancic P; Marrani A; Momente R; et al. Smart Textile Lighting/Display System with Multifunctional Fibre Devices for Large Scale Smart Home and IoT Applications. Nat. Commun 2022, 13, 814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (607).Cheng S; Lou Z; Zhang L; Guo H; Wang Z; Guo C; Fukuda K; Ma S; Wang G; Someya T; et al. Ultrathin Hydrogel Films toward Breathable Skin-Integrated Electronics. Adv. Mater 2022, 35, No. e2206793. [DOI] [PubMed] [Google Scholar]
- (608).Kireev D; Kampfe J; Hall A; Akinwande D Graphene Electronic Tattoos 2.0 with Enhanced Performance, Breathability and Robustness. npj 2D Mater. Appl 2022, 6, 46. [Google Scholar]
- (609).Xi W; Yeo JC; Yu L; Zhang S; Lim CT Ultrathin and Wearable Microtubular Epidermal Sensor for Real-Time Physiological Pulse Monitoring. Adv. Mater. Technol 2017, 2, 1700016. [Google Scholar]
- (610).Yu L; Yeo JC; Soon RH; Yeo T; Lee HH; Lim CT Highly Stretchable, Weavable, and Washable Piezoresistive Microfiber Sensors. ACS Appl. Mater. Interfaces 2018, 10, 12773–12780. [DOI] [PubMed] [Google Scholar]
- (611).Lin M; Hu H; Zhou S; Xu S Soft Wearable Devices for Deep-Tissue Sensing. Nat. Rev. Mater 2022, 7, 850–869. [Google Scholar]
- (612).Jin Q; Chen HJ; Li X; Huang X; Wu Q; He G; Hang T; Yang C; Jiang Z; Li E; et al. Reduced Graphene Oxide Nanohybrid-Assembled Microneedles as Mini-Invasive Electrodes for Real-Time Transdermal Biosensing. Small 2019, 15, 1804298. [DOI] [PubMed] [Google Scholar]
- (613).Paul R; Saville AC; Hansel JC; Ye Y; Ball C; Williams A; Chang X; Chen G; Gu Z; Ristaino JB; et al. Extraction of Plant DNA by Microneedle Patch for Rapid Detection of Plant Diseases. ACS Nano 2019, 13, 6540–6549. [DOI] [PubMed] [Google Scholar]
- (614).Cao Y; Lim E; Xu M; Weng JK; Marelli B Precision Delivery of Multiscale Payloads to Tissue-Specific Targets in Plants. Adv. Sci 2020, 7, 1903551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (615).He R; Liu H; Fang T; Niu Y; Zhang H; Han F; Gao B; Li F; Xu F A Colorimetric Dermal Tattoo Biosensor Fabricated by Microneedle Patch for Multiplexed Detection of Health-Related Biomarkers. Adv. Sci 2021, 8, 2103030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (616).Kusama S; Sato K; Matsui Y; Kimura N; Abe H; Yoshida S; Nishizawa M Transdermal Electroosmotic Flow Generated by a Porous Microneedle Array Patch. Nat. Commun 2021, 12, 658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (617).Lee W; Jeong S.-h.; Lim Y-W; Lee H; Kang J; Lee H; Lee I; Han H-S; Kobayashi S; Tanaka M; et al. Conformable Microneedle pH Sensors via the Integration of Two Different Siloxane Polymers for Mapping Peripheral Artery Disease. Sci. Adv 2021, 7, No. eabi6290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (618).Lee SH; Thunemann M; Lee K; Cleary DR; Tonsfeldt KJ; Oh H; Azzazy F; Tchoe Y; Bourhis AM; Hossain L; et al. Scalable Thousand Channel Penetrating Microneedle Arrays on Flex for Multimodal and Large Area Coverage Brainmachine Interfaces. Adv. Funct. Mater 2022, 32, 2112045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (619).Tehrani F; Teymourian H; Wuerstle B; Kavner J; Patel R; Furmidge A; Aghavali R; Hosseini-Toudeshki H; Brown C; Zhang F; et al. An Integrated Wearable Microneedle Array for the Continuous Monitoring of Multiple Biomarkers in Interstitial Fluid. Nat. Biomed. Eng 2022, 6, 1214–1224. [DOI] [PubMed] [Google Scholar]
- (620).Wang J; Wang L; Feng J; Tang C; Sun X; Peng H Long-Term in Vivo Monitoring of Chemicals with Fiber Sensors. Adv. Fiber Mater 2021, 3, 47–58. [Google Scholar]
- (621).Feng J; Chen C; Sun X; Peng H Implantable Fiber Biosensors Based on Carbon Nanotubes. Acc. Mater. Res 2021, 2, 138–146. [Google Scholar]
- (622).Yang C; Wu Q; Liu J; Mo J; Li X; Yang C; Liu Z; Yang J; Jiang L; Chen W; et al. Intelligent Wireless Theranostic Contact Lens for Electrical Sensing and Regulation of Intraocular Pressure. Nat. Commun 2022, 13, 2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (623).Li S; Zhu Y; Haghniaz R; Kawakita S; Guan S; Chen J; Li Z; Mandal K; Bahari J; Shah S; et al. A Microchambers Containing Contact Lens for the Noninvasive Detection of Tear Exosomes. Adv. Funct. Mater 2022, 32, 2206620. [Google Scholar]
- (624).Zhao Z; Zhu H; Li X; Sun L; He F; Chung JE; Liu DF; Frank L; Luan L; Xie C Ultraflexible Electrode Arrays for Months-Long High-Density Electrophysiological Mapping of Thousands of Neurons in Rodents. Nat. Biomed. Eng 2022, DOI: 10.1038/s41551-022-00941-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (625).Feiner R; Engel L; Fleischer S; Malki M; Gal I; Shapira A; Shacham-Diamand Y; Dvir T Engineered Hybrid Cardiac Patches with Multifunctional Electronics for Online Monitoring and Regulation of Tissue Function. Nat. Mater 2016, 15, 679–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (626).Li Q; Nan K; Le Floch P; Lin Z; Sheng H; Blum TS; Liu J Cyborg Organoids: Implantation of Nanoelectronics via Organo-genesis for Tissue-Wide Electrophysiology. Nano Lett. 2019, 19, 5781–5789. [DOI] [PubMed] [Google Scholar]
- (627).Park Y; Franz CK; Ryu H; Luan H; Cotton KY; Kim JU; Chung TS; Zhao S; Vazquez-Guardado A; Yang DS; et al. Three-Dimensional, Multifunctional Neural Interfaces for Cortical Spheroids and Engineered Assembloids. Sci. Adv 2021, 7, No. eabf9153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (628).Fan Z; Yang Y; Zhang F; Xu Z; Zhao H; Wang T; Song H; Huang Y; Rogers JA; Zhang Y Inverse Design Strategies for 3D Surfaces Formed by Mechanically Guided Assembly. Adv. Mater 2020, 32, 1908424. [DOI] [PubMed] [Google Scholar]
- (629).Cheng X; Zhang Y Micro/Nanoscale 3D Assembly by Rolling, Folding, Curving, and Buckling Approaches. Adv. Mater 2019, 31, 1901895. [DOI] [PubMed] [Google Scholar]
- (630).Bai Y; Wang H; Xue Y; Pan Y; Kim JT; Ni X; Liu TL; Yang Y; Han M; Huang Y; et al. A Dynamically Reprogrammable Surface with Self-Evolving Shape Morphing. Nature 2022, 609, 701–708. [DOI] [PubMed] [Google Scholar]
- (631).Zhu W; von dem Bussche A; Yi X; Qiu Y; Wang Z; Weston P; Hurt RH; Kane AB; Gao H Nanomechanical Mechanism for Lipid Bilayer Damage Induced by Carbon Nanotubes Confined in Intracellular Vesicles. Proc. Natl. Acad. Sci 2016, 113, 12374–12379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (632).Lucherelli MA; Qian X; Weston P; Eredia M; Zhu W; Samori P; Gao H; Bianco A; dem Bussche A Boron Nitride Nanosheets Can Induce Water Channels across Lipid Bilayers Leading to Lysosomal Permeabilization. Adv. Mater 2021, 33, No. e2103137. [DOI] [PubMed] [Google Scholar]
- (633).Clifford CA; Martins Ferreira EH; Fujimoto T; Herrmann J; Hight Walker AR; Koltsov D; Punckt C; Ren L; Smallwood GJ; Pollard AJ The Importance of International Standards for the Graphene Community. Nat. Rev. Phys 2021, 3, 233–235. [Google Scholar]
- (634).Kozma GT; Shimizu T; Ishida T; Szebeni J Anti-PEG Antibodies: Properties, Formation, Testing and Role in Adverse Immune Reactions to PEGylated Nano-Biopharmaceuticals. Adv. Drug Deliv. Rev 2020, 154–155, 163–175. [DOI] [PubMed] [Google Scholar]
- (635).Anderson JM; Rodriguez A; Chang DT Foreign Body Reaction to Biomaterials. Semin. Immunol 2008, 20, 86–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (636).Liu K; Jiang Y; Bao Z; Yan X Skin-Inspired Electronics Enabled by Supramolecular Polymeric Materials. CCS Chem. 2019, 1, 431–447. [Google Scholar]
- (637).Vo R; Hsu HH; Jiang X Hydrogel Facilitated Bioelectronic Integration. Biomater. Sci 2021, 9, 23–37. [DOI] [PubMed] [Google Scholar]
- (638).Yuk H; Wu J; Zhao X Hydrogel Interfaces for Merging Humans and Machines. Nat. Rev. Mater 2022, 7, 935–952. [Google Scholar]
- (639).Banerjee H; Suhail M; Ren H Hydrogel Actuators and Sensors for Biomedical Soft Robots: Brief Overview with Impending Challenges. Biomimetics 2018, 3, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (640).Sun X; Agate S; Salem KS; Lucia L; Pal L Hydrogel-Based Sensor Networks: Compositions, Properties, and Applications-A Review. ACS Appl. Bio Mater 2021, 4, 140–162. [DOI] [PubMed] [Google Scholar]
- (641).Wang L; Xu T; Zhang X Multifunctional Conductive Hydrogel-Based Flexible Wearable Sensors. Trends Anal. Chem 2021, 134, 116130. [Google Scholar]
- (642).Cho KW; Sunwoo SH; Hong YJ; Koo JH; Kim JH; Baik S; Hyeon T; Kim DH Soft Bioelectronics Based on Nanomaterials. Chem. Rev 2022, 122, 5068–5143. [DOI] [PubMed] [Google Scholar]
- (643).Yu KJ; Kuzum D; Hwang SW; Kim BH; Juul H; Kim NH; Won SM; Chiang K; Trumpis M; Richardson AG; et al. Bioresorbable Silicon Electronics for Transient Spatiotemporal Mapping of Electrical Activity from the Cerebral Cortex. Nat. Mater 2016, 15, 782–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (644).Vitale F; Vercosa DG; Rodriguez AV; Pamulapati SS; Seibt F; Lewis E; Yan JS; Badhiwala K; Adnan M; Royer-Carfagni G; et al. Fluidic Microactuation of Flexible Electrodes for Neural Recording. Nano Lett. 2018, 18, 326–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (645).Kireev D; Okogbue E; Jayanth RT; Ko TJ; Jung Y; Akinwande D Multipurpose and Reusable Ultrathin Electronic Tattoos Based on PtSe2 and PtTe2. ACS Nano 2021, 15, 2800–2811. [DOI] [PubMed] [Google Scholar]
- (646).Libanori A; Chen G; Zhao X; Zhou Y; Chen J Smart Textiles for Personalized Healthcare. Nat. Electron 2022, 5, 142–156. [Google Scholar]
- (647).Chen G; Xiao X; Zhao X; Tat T; Bick M; Chen J Electronic Textiles for Wearable Point-of-Care Systems. Chem. Rev 2022, 122, 3259–3291. [DOI] [PubMed] [Google Scholar]
- (648).Shuvo II; Shah A; Dagdeviren C Electronic Textile Sensors for Decoding Vital Body Signals: State-of-the-Art Review on Characterizations and Recommendations. Adv. Intell. Syst 2022, 4, 2100223. [Google Scholar]
- (649).Liu Y; Zhou X; Yan H; Zhu Z; Shi X; Peng Y; Chen L; Chen P; Peng H Robust Memristive Fiber for Woven Textile Memristor. Adv. Funct. Mater 2022, 32, 2201510. [Google Scholar]
- (650).Wang T; Meng J; Zhou X; Liu Y; He Z; Han Q; Li Q; Yu J; Li Z; Liu Y; et al. Reconfigurable Neuromorphic Memristor Network for Ultralow-Power Smart Textile Electronics. Nat. Commun 2022, 13, 7432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (651).He J; Lu C; Jiang H; Han F; Shi X; Wu J; Wang L; Chen T; Wang J; Zhang Y; et al. Scalable Production of High-Performing Woven Lithium-Ion Fibre Batteries. Nature 2021, 597, 57–63. [DOI] [PubMed] [Google Scholar]
- (652).Alshabouna F; Lee HS; Barandun G; Tan E; Cotur Y; Asfour T; Gonzalez-Macia L; Coatsworth P; Núnez-Bajo E; Kim J-S; et al. PEDOT:PSS-Modified Cotton Conductive Thread for Mass Manufacturing of Textile-Based Electrical Wearable Sensors by Computerized Embroidery. Mater. Today 2022, 59, 56–57. [Google Scholar]
- (653).Fang Y; Han E; Zhang X-X; Jiang Y; Lin Y; Shi J; Wu J; Meng L; Gao X; Griffin PJ; et al. Dynamic and Programmable Cellular-Scale Granules Enable Tissue-Like Materials. Matter 2020, 2, 948–964. [Google Scholar]
- (654).Liu J; Kim Yoon S; Richardson CE; Tom A; Ramakrishnan C; Birey F; Katsumata T; Chen S; Wang C; Wang X; et al. Genetically Targeted Chemical Assembly of Functional Materials in Living Cells, Tissues, and Animals. Science 2020, 367, 1372–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (655).Chen B; Kang W; Sun J; Zhu R; Yu Y; Xia A; Yu M; Wang M; Han J; Chen Y; et al. Programmable Living Assembly of Materials by Bacterial Adhesion. Nat. Chem. Biol 2022, 18, 289–294. [DOI] [PubMed] [Google Scholar]
- (656).Zhong J; Li Z; Takakuwa M; Inoue D; Hashizume D; Jiang Z; Shi Y; Ou L; Nayeem MOG; Umezu S; et al. Smart Face Mask Based on an Ultrathin Pressure Sensor for Wireless Monitoring of Breath Conditions. Adv. Mater 2022, 34, 2107758. [DOI] [PubMed] [Google Scholar]
- (657).Fang Y; Xu J; Xiao X; Zou Y; Zhao X; Zhou Y; Chen J A Deep-Learning-Assisted On-Mask Sensor Network for Adaptive Respiratory Monitoring. Adv. Mater 2022, 34, 2200252. [DOI] [PubMed] [Google Scholar]
- (658).Kalidasan V; Yang X; Xiong Z; Li RR; Yao H; Godaba H; Obuobi S; Singh P; Guan X; Tian X; et al. Wirelessly Operated Bioelectronic Sutures for the Monitoring of Deep Surgical Wounds. Nat. Biomed. Eng 2021, 5, 1217–1227. [DOI] [PubMed] [Google Scholar]
- (659).Song Y; Mukasa D; Zhang H; Gao W Self-Powered Wearable Biosensors. Acc. Mater. Res 2021, 2, 184–197. [Google Scholar]
- (660).Zeng X; Peng R; Fan Z; Lin Y Self-Powered and Wearable Biosensors for Healthcare. Mater. Today Energy 2022, 23, 100900. [Google Scholar]
- (661).Lee K; Ni X; Lee JY; Arafa H; Pe DJ; Xu S; Avila R; Irie M; Lee JH; Easterlin RL; et al. Mechano-Acoustic Sensing of Physiological Processes and Body Motions via a Soft Wireless Device Placed at the Suprasternal Notch. Nat. Biomed. Eng 2020, 4, 148–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (662).Gao M; Wang P; Jiang L; Wang B; Yao Y; Liu S; Chu D; Cheng W; Lu Y Power Generation for Wearable Systems. Energy Environ. Sci 2021, 14, 2114–2157. [Google Scholar]
- (663).Sezer N; Koç M A Comprehensive Review on the State-of-the-Art of Piezoelectric Energy Harvesting. Nano Energy 2021, 80, 105567. [Google Scholar]
- (664).Dagdeviren C; Joe P; Tuzman OL; Park K-I; Lee KJ; Shi Y; Huang Y; Rogers JA Recent Progress in Flexible and Stretchable Piezoelectric Devices for Mechanical Energy Harvesting, Sensing and Actuation. Extreme Mech. Lett 2016, 9, 269–281. [Google Scholar]
- (665).Wang Y; Yang L; Shi XL; Shi X; Chen L; Dargusch MS; Zou J; Chen ZG Flexible Thermoelectric Materials and Generators: Challenges and Innovations. Adv. Mater 2019, 31, 1807916. [DOI] [PubMed] [Google Scholar]
- (666).Zhang L; Shi X-L; Yang Y-L; Chen Z-G Flexible Thermoelectric Materials and Devices: From Materials to Applications. Mater. Today 2021, 46, 62–108. [Google Scholar]
- (667).Ryu H; Kim SW Emerging Pyroelectric Nanogenerators to Convert Thermal Energy into Electrical Energy. Small 2021, 17, 1903469. [DOI] [PubMed] [Google Scholar]
- (668).Shen D; Duley WW; Peng P; Xiao M; Feng J; Liu L; Zou G; Zhou YN Moisture-Enabled Electricity Generation: From Physics and Materials to Self-Powered Applications. Adv. Mater 2020, 32, 2003722. [DOI] [PubMed] [Google Scholar]
- (669).Zhang Z; Li X; Yin J; Xu Y; Fei W; Xue M; Wang Q; Zhou J; Guo W Emerging Hydrovoltaic Technology. Nat. Nanotechnol 2018, 13, 1109–1119. [DOI] [PubMed] [Google Scholar]
- (670).Zhou Y; Xiao X; Chen G; Zhao X; Chen J Self-Powered Sensing Technologies for Human Metaverse Interfacing. Joule 2022, 6, 1381–1389. [Google Scholar]
- (671).Dagdeviren C; Li Z; Wang ZL Energy Harvesting from the Animal/Human Body for Self-Powered Electronics. Annu. Rev. Biomed. Eng 2017, 19, 85–108. [DOI] [PubMed] [Google Scholar]
- (672).Gong S; Cheng W Toward Soft Skin-Like Wearable and Implantable Energy Devices. Adv. Energy Mater 2017, 7, 1700648. [Google Scholar]
- (673).Huang X; Wang L; Wang H; Zhang B; Wang X; Stening RYZ; Sheng X; Yin L Materials Strategies and Device Architectures of Emerging Power Supply Devices for Implantable Bioelectronics. Small 2020, 16, 1902827. [DOI] [PubMed] [Google Scholar]
- (674).Liu R; Wang ZL; Fukuda K; Someya T Flexible Self-Charging Power Sources. Nat. Rev. Mater 2022, 7, 870–886. [Google Scholar]
- (675).Nielsen MP; Pusch A; Sazzad MH; Pearce PM; Reece PJ; Ekins-Daukes NJ Thermoradiative Power Conversion from HgCdTe Photodiodes and Their Current-Voltage Characteristics. ACS Photonics 2022, 9, 1535–1540. [Google Scholar]
- (676).Park S; Heo SW; Lee W; Inoue D; Jiang Z; Yu K; Jinno H; Hashizume D; Sekino M; Yokota T; et al. Self-Powered Ultra-Flexible Electronics via Nano-Grating-Patterned Organic Photo-voltaics. Nature 2018, 561, 516–521. [DOI] [PubMed] [Google Scholar]
- (677).Lu L; Yang Z; Meacham K; Cvetkovic C; Corbin EA; Vázquez-Guardado A; Xue M; Yin L; Boroumand J; Pakeltis G; et al. Biodegradable Monocrystalline Silicon Photovoltaic Microcells as Power Supplies for Transient Biomedical Implants. Adv. Energy Mater 2018, 8, 1703035. [Google Scholar]
- (678).Zhang N; Huang F; Zhao S; Lv X; Zhou Y; Xiang S; Xu S; Li Y; Chen G; Tao C; et al. Photo-Rechargeable Fabrics as Sustainable and Robust Power Sources for Wearable Bioelectronics. Matter 2020, 2, 1260–1269. [Google Scholar]
- (679).Griffith MJ; Holmes NP; Elkington DC; Cottam S; Stamenkovic J; Kilcoyne ALD; Andersen TR Manipulating Nanoscale Structure to Control Functionality in Printed Organic Photovoltaic, Transistor and Bioelectronic Devices. Nanotechnology 2020, 31, 092002. [DOI] [PubMed] [Google Scholar]
- (680).Lin Y; Gao Y; Fang F; Fan Z Recent Progress on Printable Power Supply Devices and Systems with Nanomaterials. Nano Res. 2018, 11, 3065–3087. [Google Scholar]
- (681).Chen C; Chen J; Han H; Chao L; Hu J; Niu T; Dong H; Yang S; Xia Y; Chen Y; et al. Perovskite Solar Cells Based on Screen-Printed Thin Films. Nature 2022, 612, 266–271. [DOI] [PubMed] [Google Scholar]
- (682).Nassiri Nazif K; Daus A; Hong J; Lee N; Vaziri S; Kumar A; Nitta F; Chen ME; Kananian S; Islam R; et al. High-Specific-Power Flexible Transition Metal Dichalcogenide Solar Cells. Nat. Commun 2021, 12, 7034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (683).Brinkmann KO; Becker T; Zimmermann F; Kreusel C; Gahlmann T; Theisen M; Haeger T; Olthof S; Tuckmantel C; Gunster M; et al. Perovskite-Organic Tandem Solar Cells with Indium Oxide Interconnect. Nature 2022, 604, 280–286. [DOI] [PubMed] [Google Scholar]
- (684).Lee S; Cortese AJ; Gandhi AP; Agger ER; McEuen PL; Molnar ACA 250 μm × 57 μm Microscale Opto-Electronically Transduced Electrodes (MOTEs) for Neural Recording. IEEE Trans. Biomed. Circuits Syst 2018, 12, 1256–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (685).Petritz A; Karner-Petritz E; Uemura T; Schaffner P; Araki T; Stadlober B; Sekitani T Imperceptible Energy Harvesting Device and Biomedical Sensor Based on Ultraflexible Ferroelectric Transducers and Organic Diodes. Nat. Commun 2021, 12, 2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (686).Karan SK; Maiti S; Agrawal AK; Das AK; Maitra A; Paria S; Bera A; Bera R; Halder L; Mishra AK; et al. Designing High Energy Conversion Efficient Bio-Inspired Vitamin Assisted Single-Structured Based Self-Powered Piezoelectric/Wind/Acoustic Multi-Energy Harvester with Remarkable Power Density. Nano Energy 2019, 59, 169–183. [Google Scholar]
- (687).Zheng Q; Zhang H; Mi H; Cai Z; Ma Z; Gong S High-Performance Flexible Piezoelectric Nanogenerators Consisting of Porous Cellulose Nanofibril (CNF)/Poly(dimethylsiloxane) (PDMS) Aerogel Films. Nano Energy 2016, 26, 504–512. [Google Scholar]
- (688).Song S; Yun K-S Design and Characterization of Scalable Woven Piezoelectric Energy Harvester for Wearable Applications. Smart Mater. Struct 2015, 24, 045008. [Google Scholar]
- (689).Dong K; Peng X; Wang ZL Fiber/Fabric-Based Piezoelectric and Triboelectric Nanogenerators for Flexible/Stretchable and Wearable Electronics and Artificial Intelligence. Adv. Mater 2020, 32, 1902549. [DOI] [PubMed] [Google Scholar]
- (690).Zhang Y; Jeong CK; Yang T; Sun H; Chen L-Q; Zhang S; Chen W; Wang Q Bioinspired Elastic Piezoelectric Composites for High-Performance Mechanical Energy Harvesting. J. Mater. Chem. A 2018, 6, 14546–14552. [Google Scholar]
- (691).Song Y; Min J; Yu Y; Wang H; Yang Y; Zhang H; Gao W Wireless Battery-Free Wearable Sweat Sensor Powered by Human Motion. Sci. Adv 2020, 6, No. eaay9842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (692).Wu H; Wang S; Wang Z; Zi Y Achieving Ultrahigh Instantaneous Power Density of 10 MW/m2 by Leveraging the Opposite-Charge-Enhanced Transistor-Like Triboelectric Nanogenerator (OCT-TENG). Nat. Commun 2021, 12, 5470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (693).Ning C; Cheng R; Jiang Y; Sheng F; Yi J; Shen S; Zhang Y; Peng X; Dong K; Wang ZL Helical Fiber Strain Sensors Based on Triboelectric Nanogenerators for Self-Powered Human Respiratory Monitoring. ACS Nano 2022, 16, 2811–2821. [DOI] [PubMed] [Google Scholar]
- (694).Gong J; Xu B; Guan X; Chen Y; Li S; Feng J Towards Truly Wearable Energy Harvesters with Full Structural Integrity of Fiber Materials. Nano Energy 2019, 58, 365–374. [Google Scholar]
- (695).Dong K; Wu Z; Deng J; Wang AC; Zou H; Chen C; Hu D; Gu B; Sun B; Wang ZL A Stretchable Yarn Embedded Triboelectric Nanogenerator as Electronic Skin for Biomechanical Energy Harvesting and Multifunctional Pressure Sensing. Adv. Mater 2018, 30, 1804944. [DOI] [PubMed] [Google Scholar]
- (696).Pu X; Liu M; Chen X; Sun J; Du C; Zhang Y; Zhai J; Hu W; Wang ZL Ultrastretchable, Transparent Triboelectric Nanogenerator as Electronic Skin for Biomechanical Energy Harvesting and Tactile Sensing. Sci. Adv 2017, 3, No. e1700015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (697).Xiong J; Thangavel G; Wang J; Zhou X; Lee PS Self-Healable Sticky Porous Elastomer for Gas-Solid Interacted Power Generation. Sci. Adv 2020, 6, No. eabb4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (698).Jiang F; Zhou X; Lv J; Chen J; Chen J; Kongcharoen H; Zhang Y; Lee PS Stretchable, Breathable, and Stable Lead-Free Perovskite/Polymer Nanofiber Composite for Hybrid Triboelectric and Piezoelectric Energy Harvesting. Adv. Mater 2022, 34, 2200042. [DOI] [PubMed] [Google Scholar]
- (699).Wang R; Du Z; Xia Z; Liu J; Li P; Wu Z; Yue Y; Xiang Y; Meng J; Liu D; et al. Magnetoelectrical Clothing Generator for High-Performance Transduction from Biomechanical Energy to Electricity. Adv. Funct. Mater 2022, 32, 2107682. [Google Scholar]
- (700).Li S; Cao P; Li F; Asghar W; Wu Y; Xiao H; Liu Y; Zhou Y; Yang H; Zhang Y; et al. Self-Powered Stretchable Strain Sensors for Motion Monitoring and Wireless Control. Nano Energy 2022, 92, 106754. [Google Scholar]
- (701).Vallem V; Roosa E; Ledinh T; Jung W; Kim TI; Rashid-Nadimi S; Kiani A; Dickey MD A Soft Variable-Area Electrical-Double-Layer Energy Harvester. Adv. Mater 2021, 33, 2103142. [DOI] [PubMed] [Google Scholar]
- (702).Jinno H; Yokota T; Koizumi M; Yukita W; Saito M; Osaka I; Fukuda K; Someya T Self-Powered Ultraflexible Photonic Skin for Continuous Bio-Signal Detection via Air-Operation-Stable Polymer Light-Emitting Diodes. Nat. Commun 2021, 12, 2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (703).Lei Y; Chen Y; Zhang R; Li Y; Yan Q; Lee S; Yu Y; Tsai H; Choi W; Wang K; et al. A Fabrication Process for Flexible Single-Crystal Perovskite Devices. Nature 2020, 583, 790–795. [DOI] [PubMed] [Google Scholar]
- (704).Kang X; Zhu Z; Zhao T; Zhai W; Xu J; Lin Z; Zeng K; Wang B; Sun X; Chen P; et al. Hierarchically Assembled Counter Electrode for Fiber Solar Cell Showing Record Power Conversion Efficiency. Adv. Funct. Mater 2022, 32, 2207763. [Google Scholar]
- (705).Feng R; Tang F; Zhang N; Wang X Flexible, High-Power Density, Wearable Thermoelectric Nanogenerator and Self-Powered Temperature Sensor. ACS Appl. Mater. Interfaces 2019, 11, 38616–38624. [DOI] [PubMed] [Google Scholar]
- (706).Varghese T; Dun C; Kempf N; Saeidi-Javash M; Karthik C; Richardson J; Hollar C; Estrada D; Zhang Y Flexible Thermoelectric Devices of Ultrahigh Power Factor by Scalable Printing and Interface Engineering. Adv. Funct. Mater 2020, 30, 1905796. [Google Scholar]
- (707).Han Y; Simonsen LE; Malakooti MH Printing Liquid Metal Elastomer Composites for High-Performance Stretchable Thermoelectric Generators. Adv. Energy Mater 2022, 12, 2201413. [Google Scholar]
- (708).Morata A; Pacios M; Gadea G; Flox C; Cadavid D; Cabot A; Tarancon A Large-Area and Adaptable Electrospun Silicon-Based Thermoelectric Nanomaterials with High Energy Conversion Efficiencies. Nat. Commun 2018, 9, 4759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (709).Zhang T; Li K; Zhang J; Chen M; Wang Z; Ma S; Zhang N; Wei L High-Performance, Flexible, and Ultralong Crystalline Thermoelectric Fibers. Nano Energy 2017, 41, 35–42. [Google Scholar]
- (710).Shi X-L; Chen W-Y; Zhang T; Zou J; Chen Z-G Fiber-Based Thermoelectrics for Solid, Portable, and Wearable Electronics. Energy Environ. Sci 2021, 14, 729–764. [Google Scholar]
- (711).Chen W-Y; Shi X-L; Zou J; Chen Z-G Wearable Fiber-Based Thermoelectrics from Materials to Applications. Nano Energy 2021, 81, 105684. [Google Scholar]
- (712).Han C-G; Qian X; Li Q; Deng B; Zhu Y; Han Z; Zhang W; Wang W; Feng S-P; Chen G; et al. Giant Thermopower of Ionic Gelatin near Room Temperature. Science 2020, 368, 1091–1098. [DOI] [PubMed] [Google Scholar]
- (713).Lei Z; Gao W; Wu P Double-Network Thermocells with Extraordinary Toughness and Boosted Power Density for Continuous Heat Harvesting. Joule 2021, 5, 2211–2222. [Google Scholar]
- (714).Wang H; Sun Y; He T; Huang Y; Cheng H; Li C; Xie D; Yang P; Zhang Y; Qu L Bilayer of Polyelectrolyte Films for Spontaneous Power Generation in Air up to an Integrated 1,000 V Output. Nat. Nanotechnol 2021, 16, 811–819. [DOI] [PubMed] [Google Scholar]
- (715).Liu X; Ueki T; Gao H; Woodard TL; Nevin KP; Fu T; Fu S; Sun L; Lovley DR; Yao J Microbial Biofilms for Electricity Generation from Water Evaporation and Power to Wearables. Nat. Commun 2022, 13, 4369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (716).Zhang Y; Guo S; Yu ZG; Qu H; Sun W; Yang J; Suresh L; Zhang X; Koh JJ; Tan SC An Asymmetric Hygroscopic Structure for Moisture-Driven Hygro-Ionic Electricity Generation and Storage. Adv Mater 2022, 34, No. e2201228. [DOI] [PubMed] [Google Scholar]
- (717).Bai J; Hu Y; Guang T; Zhu K; Wang H; Cheng H; Liu F; Qu L Vapor and Heat Dual-Drive Sustainable Power for Portable Electronics in Ambient Environments. Energy Environ. Sci 2022, 15, 3086–3096. [Google Scholar]
- (718).Bai J; Huang Y; Wang H; Guang T; Liao Q; Cheng H; Deng S; Li Q; Shuai Z; Qu L Sunlight-Coordinated High-Performance Moisture Power in Natural Conditions. Adv. Mater 2022, 34, 2103897. [DOI] [PubMed] [Google Scholar]
- (719).Yu Y; Nassar J; Xu C; Min J; Yang Y; Dai A; Doshi R; Huang A; Song Y; Gehlhar R; et al. Biofuel-Powered Soft Electronic Skin with Multiplexed and Wireless Sensing for Human-Machine Interfaces. Sci. Robot 2020, 5, No. eaaz7946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (720).Yin L; Moon J-M; Sempionatto JR; Lin M; Cao M; Trifonov A; Zhang F; Lou Z; Jeong J-M; Lee S-J; et al. A Passive Perspiration Biofuel Cell: High Energy Return on Investment. Joule 2021, 5, 1888–1904. [Google Scholar]
- (721).Bandodkar AJ; You J-M; Kim N-H; Gu Y; Kumar R; Mohan AMV; Kurniawan J; Imani S; Nakagawa T; Parish B; et al. Soft, Stretchable, High Power Density Electronic Skin-Based Biofuel Cells for Scavenging Energy from Human Sweat. Energy Environ. Sci 2017, 10, 1581–1589. [Google Scholar]
- (722).Guo Y; Chen C; Feng J; Wang L; Wang J; Tang C; Sun X; Peng H An Anti-Biofouling Flexible Fiber Biofuel Cell Working in the Brain. Small Methods 2022, 6, 2200142. [DOI] [PubMed] [Google Scholar]
- (723).Simons P; Schenk SA; Gysel MA; Olbrich LF; Rupp JLM A Ceramic-Electrolyte Glucose Fuel Cell for Implantable Electronics. Adv. Mater 2022, 34, 2109075. [DOI] [PubMed] [Google Scholar]
- (724).Huang X; Li H; Li J; Huang L; Yao K; Yiu CK; Liu Y; Wong TH; Li D; Wu M; et al. Transient, Implantable, Ultrathin Biofuel Cells Enabled by Laser-Induced Graphene and Gold Nanoparticles Composite. Nano Lett. 2022, 22, 3447–3456. [DOI] [PubMed] [Google Scholar]
- (725).He S; Zhang A; Wang D; Song H; Chu H; Ni F; Zhang Y; Chen P; Zhang B; Qiu L; et al. An Implantable Flexible Fiber Generator without Encapsulation Made from Differentially Oxidized Carbon Nanotube Fibers. Chem. Eng. J 2022, 441, 136106. [Google Scholar]
- (726).Wang ZL; Lin L; Chen J; Niu S; Zi Y Triboelectric Nanogenerators, 1st ed.; Springer; Cham, 2016. [Google Scholar]
- (727).Fan F-R; Tian Z-Q; Lin Wang Z Flexible Triboelectric Generator. Nano Energy 2012, 1, 328–334. [Google Scholar]
- (728).Zhao X; Askari H; Chen J Nanogenerators for Smart Cities in the Era of 5G and Internet of Things. Joule 2021, 5, 1391–1431. [Google Scholar]
- (729).Fu J; Xia X; Xu G; Li X; Zi Y On the Maximal Output Energy Density of Nanogenerators. ACS Nano 2019, 13, 13257–13263. [DOI] [PubMed] [Google Scholar]
- (730).Dudem B; Dharmasena R; Riaz R; Vivekananthan V; Wijayantha KGU; Lugli P; Petti L; Silva SRP Wearable Triboelectric Nanogenerator from Waste Materials for Autonomous Information Transmission via Morse Code. ACS Appl. Mater. Interfaces 2022, 14, 5328–5337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (731).Zheng Q; Tang Q; Wang ZL; Li Z Self-Powered Cardiovascular Electronic Devices and Systems. Nat. Rev. Cardiol 2021, 18, 7–21. [DOI] [PubMed] [Google Scholar]
- (732).Hinchet R; Yoon H-J; Ryu H; Kim M-K; Choi E-K; Kim D-S; Kim S-W Transcutaneous Ultrasound Energy Harvesting Using Capacitive Triboelectric Technology. Science 2019, 365, 491–494. [DOI] [PubMed] [Google Scholar]
- (733).Gao Q; Cheng T; Wang ZL Triboelectric Mechanical Sensors-Progress and Prospects. Extreme Mech. Lett 2021, 42, 101100. [Google Scholar]
- (734).Shen S; Xiao X; Xiao X; Chen J Triboelectric Nanogenerators for Self-Powered Breath Monitoring. ACS Appl. Energy Mater 2022, 5, 3952–3965. [Google Scholar]
- (735).Guo H; Chen J; Tian L; Leng Q; Xi Y; Hu C Airflow-Induced Triboelectric Nanogenerator as a Self-Powered Sensor for Detecting Humidity and Airflow Rate. ACS Appl. Mater. Interfaces 2014, 6, 17184–17189. [DOI] [PubMed] [Google Scholar]
- (736).Wen Z; Yeh M-H; Guo H; Wang J; Zi Y; Xu W; Deng J; Zhu L; Wang X; Hu C; et al. Self-Powered Textile for Wearable Electronics by Hybridizing Fiber-Shaped Nanogenerators, Solar Cells, and Supercapacitors. Sci. Adv 2016, 2, No. e1600097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (737).Yang B; Xiong Y; Ma K; Liu S; Tao X Recent Advances in Wearable Textile-Based Triboelectric Generator Systems for Energy Harvesting from Human Motion. EcoMat 2020, 2, No. e12054. [Google Scholar]
- (738).Chen Y-L; Liu D; Wang S; Li Y-F; Zhang X-S Self-Powered Smart Active RFID Tag Integrated with Wearable Hybrid Nanogenerator. Nano Energy 2019, 64, 103911. [Google Scholar]
- (739).Tan X; Zhou Z; Zhang L; Wang X; Lin Z; Yang R; Yang J A Passive Wireless Triboelectric Sensor via a Surface Acoustic Wave Resonator (SAWR). Nano Energy 2020, 78, 105307. [Google Scholar]
- (740).Wen F; Wang H; He T; Shi Q; Sun Z; Zhu M; Zhang Z; Cao Z; Dai Y; Zhang T; et al. Battery-Free Short-Range Self-Powered Wireless Sensor Network (SS-WSN) Using TENG Based Direct Sensory Transmission (TDST) Mechanism. Nano Energy 2020, 67, 104266. [Google Scholar]
- (741).Zhang C; Chen J; Xuan W; Huang S; You B; Li W; Sun L; Jin H; Wang X; Dong S; et al. Conjunction of Triboelectric Nanogenerator with Induction Coils as Wireless Power Sources and Self-Powered Wireless Sensors. Nat. Commun. 2020, 11, 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (742).Shi Q; Sun Z; Zhang Z; Lee C Triboelectric Nanogenerators and Hybridized Systems for Enabling Next-Generation IoT Applications. Research 2021, 2021, 6849171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (743).Hu T; Wang H; Harmon W; Bamgboje D; Wang Z-L Current Progress on Power Management Systems for Triboelectric Nanogenerators. IEEE Trans. Power Electron 2022, 37, 9850–9864. [Google Scholar]
- (744).Toh WY; Tan YK; Koh WS; Siek L Autonomous Wearable Sensor Nodes with Flexible Energy Harvesting. IEEE Sens. J 2014, 14, 2299–2306. [Google Scholar]
- (745).Harmon W; Bamgboje D; Guo H; Hu T; Wang ZL Self-Driven Power Management System for Triboelectric Nanogenerators. Nano Energy 2020, 71, 104642. [Google Scholar]
- (746).Wei X; Wang B; Wu Z; Wang ZL An Open-Environment Tactile Sensing System: Toward Simple and Efficient Material Identification. Adv. Mater 2022, 34, 2203073. [DOI] [PubMed] [Google Scholar]
- (747).Li H; Wang H; Chan D; Xu Z; Wang K; Ge M; Zhang Y; Chen S; Tang Y Nature-Inspired Materials and Designs for Flexible Lithium-Ion Batteries. Carbon Energy 2022, 4, 878–900. [Google Scholar]
- (748).Chang J; Huang Q; Gao Y; Zheng Z Pathways of Developing High-Energy-Density Flexible Lithium Batteries. Adv. Mater 2021, 33, 2004419. [DOI] [PubMed] [Google Scholar]
- (749).Chen M; Zhang Y; Xing G; Chou S-L; Tang Y Electrochemical Energy Storage Devices Working in Extreme Conditions. Energy Environ. Sci 2021, 14, 3323–3351. [Google Scholar]
- (750).Lv Z; Wang C; Wan C; Wang R; Dai X; Wei J; Xia H; Li W; Zhang W; Cao S; et al. Strain-Driven Auto-Detachable Patterning of Flexible Electrodes. Adv. Mater 2022, 34, 2202877. [DOI] [PubMed] [Google Scholar]
- (751).Lv Z; Li W; Wei J; Ho F; Cao J; Chen X Autonomous Chemistry Enabling Environment-Adaptive Electrochemical Energy Storage Devices. CCS Chem. 2023, 5, 11–29. [Google Scholar]
- (752).Ma X; Jiang Z; Lin Y Flexible Energy Storage Devices for Wearable Bioelectronics. J. Semicond 2021, 42, 101602. [Google Scholar]
- (753).Mackanic DG; Yan X; Zhang Q; Matsuhisa N; Yu Z; Jiang Y; Manika T; Lopez J; Yan H; Liu K; et al. Decoupling of Mechanical Properties and Ionic Conductivity in Supramolecular Lithium Ion Conductors. Nat. Commun 2019, 10, 5384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (754).Chang J; Huang Q; Zheng Z A Figure of Merit for Flexible Batteries. Joule 2020, 4, 1346–1349. [Google Scholar]
- (755).Lin Y; Gao Y; Fan Z Printable Fabrication of Nanocoral-Structured Electrodes for High-Performance Flexible and Planar Supercapacitor with Artistic Design. Adv. Mater 2017, 29, 1701736. [DOI] [PubMed] [Google Scholar]
- (756).Liao M; Wang C; Hong Y; Zhang Y; Cheng X; Sun H; Huang X; Ye L; Wu J; Shi X; et al. Industrial Scale Production of Fibre Batteries by a Solution-Extrusion Method. Nat. Nanotechnol 2022, 17, 372–377. [DOI] [PubMed] [Google Scholar]
- (757).Li H; Han C; Huang Y; Huang Y; Zhu M; Pei Z; Xue Q; Wang Z; Liu Z; Tang Z; et al. An Extremely Safe and Wearable Solid-State Zinc Ion Battery Based on a Hierarchical Structured Polymer Electrolyte. Energy Environ. Sci 2018, 11, 941–951. [Google Scholar]
- (758).Bandodkar AJ; Lee SP; Huang I; Li W; Wang S; Su CJ; Jeang WJ; Hang T; Mehta S; Nyberg N; et al. Sweat-Activated Biocompatible Batteries for Epidermal Electronic and Microfluidic Systems. Nat. Electron 2020, 3, 554–562. [Google Scholar]
- (759).Liu Y; Huang X; Zhou J; Yiu CK; Song Z; Huang W; Nejad SK; Li H; Wong TH; Yao K; et al. Stretchable Sweat-Activated Battery in Skin-Integrated Electronics for Continuous Wireless Sweat Monitoring. Adv. Sci 2022, 9, 2104635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (760).Wu M; Shi R; Zhou J; Wong TH; Yao K; Li J; Huang X; Li D; Gao Y; Liu Y; et al. Bio-Inspired Ultra-Thin Microfluidics for Soft Sweat-Activated Batteries and Skin Electronics. J. Mater. Chem. A 2022, 10, 19662–19670. [Google Scholar]
- (761).Liu Y; Huang X; Zhou J; Li J; Nejad SK; Yiu CK; Li H; Wong TH; Park W; Yao K; et al. Bandage Based Energy Generators Activated by Sweat in Wireless Skin Electronics for Continuous Physiological Monitoring. Nano Energy 2022, 92, 106755. [Google Scholar]
- (762).Huang X; Liu Y; Zhou J; Nejad SK; Wong TH; Huang Y; Li H; Yiu CK; Park W; Li J; et al. Garment Embedded Sweat-Activated Batteries in Wearable Electronics for Continuous Sweat Monitoring. npj Flex. Electron 2022, 6, 10. [Google Scholar]
- (763).Lv J; Thangavel G; Li Y; Xiong J; Gao D; Ciou J; Tan MWM; Aziz I; Chen S; Chen J; et al. Printable Elastomeric Electrodes with Sweat-Enhanced Conductivity for Wearables. Sci. Adv 2021, 7, No. eabg8433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (764).Xia H; Lv Z; Zhang W; Wei J; Liu L; Cao S; Zhu Z; Tang Y; Chen X Hygroscopic Chemistry Enables Fire-Tolerant Supercapacitors with a Self-Healable ″Solute-in-Air″ Electrolyte. Adv. Mater 2022, 34, 2109857. [DOI] [PubMed] [Google Scholar]
- (765).He N; Song J; Liao J; Zhao F; Gao W Separator Threads in Yarn-Shaped Supercapacitors to Avoid Short-Circuiting Upon Length. npj Flex. Electron 2022, 6, 19. [Google Scholar]
- (766).Lin W; Wang F; Wang H; Li H; Fan Y; Chan D; Chen S; Tang Y; Zhang Y Thermal-Stable Separators: Design Principles and Strategies Towards Safe Lithium-Ion Battery Operations. ChemSus-Chem 2022, 15, No. e202201464. [DOI] [PubMed] [Google Scholar]
- (767).Chen R; Nolan AM; Lu J; Wang J; Yu X; Mo Y; Chen L; Huang X; Li H The Thermal Stability of Lithium Solid Electrolytes with Metallic Lithium. Joule 2020, 4, 812–821. [Google Scholar]
- (768).Hou J; Lu L; Wang L; Ohma A; Ren D; Feng X; Li Y; Li Y; Ootani I; Han X; et al. Thermal Runaway of Lithium-Ion Batteries Employing LiN(SO2F)2-Based Concentrated Electrolytes. Nat. Commun 2020, 11, 5100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (769).Zhang L; Zhao P; Xu M; Wang X Computational Identification of the Safety Regime of Li-Ion Battery Thermal Runaway. Appl. Energy 2020, 261, 114440. [Google Scholar]
- (770).Qin H; Liu P; Chen C; Cong HP; Yu SH A Multi-Responsive Healable Supercapacitor. Nat. Commun 2021, 12, 4297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (771).Mickle AD; Won SM; Noh KN; Yoon J; Meacham KW; Xue Y; McIlvried LA; Copits BA; Samineni VK; Crawford KE; et al. A Wireless Closed-Loop System for Optogenetic Peripheral Neuromodulation. Nature 2019, 565, 361–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (772).Jiang C; Li X; Lian SWM; Ying Y; Ho JS; Ping J Wireless Technologies for Energy Harvesting and Transmission for Ambient Self-Powered Systems. ACS Nano 2021, 15, 9328–9354. [DOI] [PubMed] [Google Scholar]
- (773).Ho JS; Yeh AJ; Neofytou E; Kim S; Tanabe Y; Patlolla B; Beygui RE; Poon AS Wireless Power Transfer to Deep-Tissue Microimplants. Proc. Natl. Acad. Sci. U. S. A 2014, 111, 7974–7979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (774).Song M; Jayathurathnage P; Zanganeh E; Krasikova M; Smirnov P; Belov P; Kapitanova P; Simovski C; Tretyakov S; Krasnok A Wireless Power Transfer Based on Novel Physical Concepts. Nat. Electron 2021, 4, 707–716. [Google Scholar]
- (775).Jin P; Fu J; Wang F; Zhang Y; Wang P; Liu X; Jiao Y; Li H; Chen Y; Ma Y; et al. A Flexible, Stretchable System for Simultaneous Acoustic Energy Transfer and Communication. Sci. Adv 2021, 7, No. eabg2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (776).Zhang T; Liang H; Wang Z; Qiu C; Peng Yuan B; Zhu X; Li J; Ge X; Xu J; Huang X; et al. Piezoelectric Ultrasound Energy-Harvesting Device for Deep Brain Stimulation and Analgesia Applications. Sci. Adv 2022, 8, No. eabk0159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (777).Piech DK; Johnson BC; Shen K; Ghanbari MM; Li KY; Neely RM; Kay JE; Carmena JM; Maharbiz MM; Muller R A Wireless Millimetre-Scale Implantable Neural Stimulator with Ultrasonically Powered Bidirectional Communication. Nat. Biomed. Eng 2020, 4, 207–222. [DOI] [PubMed] [Google Scholar]
- (778).Li J; Dong Y; Park JH; Yoo J Body-Coupled Power Transmission and Energy Harvesting. Nat. Electron 2021, 4, 530–538. [Google Scholar]
- (779).Choi KW; Ginting L; Aziz AA; Setiawan D; Park JH; Hwang SI; Kang DS; Chung MY; Kim DI Toward Realization of Long-Range Wireless-Powered Sensor Networks. IEEE Wirel. Commun 2019, 26, 184–192. [Google Scholar]
- (780).Dieffenderfer J; Goodell H; Mills S; McKnight M; Yao S; Lin F; Beppler E; Bent B; Lee B; Misra V; et al. Low-Power Wearable Systems for Continuous Monitoring of Environment and Health for Chronic Respiratory Disease. IEEE J. Biomed. Health Inform 2016, 20, 1251–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (781).Qian Z; Kang S; Rajaram V; Cassella C; McGruer NE; Rinaldi M Zero-Power Infrared Digitizers Based on Plasmonically Enhanced Micromechanical Photoswitches. Nat. Nanotechnol 2017, 12, 969–973. [DOI] [PubMed] [Google Scholar]
- (782).Ma C; Xu D; Huang YC; Wang P; Huang J; Zhou J; Liu W; Li ST; Huang Y; Duan X Robust Flexible Pressure Sensors Made from Conductive Micropyramids for Manipulation Tasks. ACS Nano 2020, 14, 12866–12876. [DOI] [PubMed] [Google Scholar]
- (783).Khan Asir I; Daus A; Islam R; Neilson Kathryn M; Lee Hye R; Wong HSP; Pop E Ultralow-Switching Current Density Multilevel Phase-Change Memory on a Flexible Substrate. Science 2021, 373, 1243–1247. [DOI] [PubMed] [Google Scholar]
- (784).Yin L; Kim KN; Lv J; Tehrani F; Lin M; Lin Z; Moon JM; Ma J; Yu J; Xu S; et al. A Self-Sustainable Wearable Multi-Modular E-Textile Bioenergy Microgrid System. Nat. Commun 2021, 12, 1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (785).Niu S; Wang X; Yi F; Zhou YS; Wang ZL A Universal Self-Charging System Driven by Random Biomechanical Energy for Sustainable Operation of Mobile Electronics. Nat. Commun 2015, 6, 8975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (786).Wu T; Wu F; Redoute J-M; Yuce MR An Autonomous Wireless Body Area Network Implementation Towards IoT Connected Healthcare Applications. IEEE Access 2017, 5, 11413–11422. [Google Scholar]
- (787).Hasan K; Biswas K; Ahmed K; Nafi NS; Islam MS A Comprehensive Review of Wireless Body Area Network. J. Netw. Comput. Appl 2019, 143, 178–198. [Google Scholar]
- (788).Zhang Q; Lei D; Liu N; Liu Z; Ren Z; Yin J; Jia P; Lu W; Gao Y A Zinc-Ion Battery-Type Self-Powered Pressure Sensor with Long Service Life. Adv. Mater 2022, 34, 2205369. [DOI] [PubMed] [Google Scholar]
- (789).De la Paz E; Maganti NH; Trifonov A; Jeerapan I; Mahato K; Yin L; Sonsa-Ard T; Ma N; Jung W; Burns R; et al. A Self-Powered Ingestible Wireless Biosensing System for Real-Time in Situ Monitoring of Gastrointestinal Tract Metabolites. Nat. Commun 2022, 13, 7405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (790).Bandodkar AJ; Gutruf P; Choi J; Lee K; Sekine Y; Reeder JT; Jeang WJ; Aranyosi AJ; Lee SP; Model JB; et al. Battery-Free, Skin-Interfaced Microfluidic/Electronic Systems for Simultaneous Electrochemical, Colorimetric, and Volumetric Analysis of Sweat. Sci. Adv 2019, 5, No. eaav3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (791).Zhao J; Lin Y; Wu J; Nyein HYY; Bariya M; Tai LC; Chao M; Ji W; Zhang G; Fan Z; et al. A Fully Integrated and Self-Powered Smartwatch for Continuous Sweat Glucose Monitoring. ACS Sens. 2019, 4, 1925–1933. [DOI] [PubMed] [Google Scholar]
- (792).Baltsavias S; Van Treuren W; Sawaby A; Baker SW; Sonnenburg JL; Arbabian A Gut Microbiome Redox Sensors with Ultrasonic Wake-up and Galvanic Coupling Wireless Links. IEEE Trans. Biomed. Eng 2023, 70, 76–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (793).Ryu D; Kim DH; Price JT; Lee JY; Chung HU; Allen E; Walter JR; Jeong H; Cao J; Kulikova E; et al. Comprehensive Pregnancy Monitoring with a Network of Wireless, Soft, and Flexible Sensors in High- and Low-Resource Health Settings. Proc. Natl. Acad. Sci. U. S. A 2021, 118, No. e2100466118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (794).Lin R; Kim HJ; Achavananthadith S; Kurt SA; Tan SCC; Yao H; Tee BCK; Lee JKW; Ho JS Wireless Battery-Free Body Sensor Networks Using near-Field-Enabled Clothing. Nat. Commun 2020, 11, 444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (795).Qazi R; Parker KE; Kim CY; Rill R; Norris MR; Chung J; Bilbily J; Kim JR; Walicki MC; Gereau GB; et al. Scalable and Modular Wireless-Network Infrastructure for Large-Scale Behavioural Neuroscience. Nat. Biomed. Eng 2022, 6, 771–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (796).Costa F; Genovesi S; Borgese M; Michel A; Dicandia FA; Manara G A Review of RFID Sensors, the New Frontier of Internet of Things. Sensors 2021, 21, 3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (797).Feng X; Yan F; Liu X Study of Wireless Communication Technologies on Internet of Things for Precision Agriculture. Wirel. Pers. Commun 2019, 108, 1785–1802. [Google Scholar]
- (798).Azim A; Matin MA; Asaduzzaman AN UWB Technology for WSN Applications. In Novel Applications of the UWB Technologies, Lembrikov BI, Ed.; IntechOpen, 2011. [Google Scholar]
- (799).Zhang Y; Huo Z; Wang X; Han X; Wu W; Wan B; Wang H; Zhai J; Tao J; Pan C; et al. High Precision Epidermal Radio Frequency Antenna via Nanofiber Network for Wireless Stretchable Multifunction Electronics. Nat. Commun 2020, 11, 5629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (800).Bihr U; Liu T; Ortmanns M Telemetry for Implantable Medical Devices: Part 3 - Data Telemetry. IEEE Solid-State Circuits Magazine 2014, 6, 56–62. [Google Scholar]
- (801).Kim H-J; Hirayama H; Kim S; Han KJ; Zhang R; Choi J-W Review of Near-Field Wireless Power and Communication for Biomedical Applications. IEEE Access 2017, 5, 21264–21285. [Google Scholar]
- (802).Lin R; Kim HJ; Achavananthadith S; Xiong Z; Lee JKW; Kong YL; Ho JS Digitally-Embroidered Liquid Metal Electronic Textiles for Wearable Wireless Systems. Nat. Commun 2022, 13, 2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (803).Amendola S; Lodato R; Manzari S; Occhiuzzi C; Marrocco G RFID Technology for IoT-Based Personal Healthcare in Smart Spaces. IEEE Internet Things J 2014, 1, 144–152. [Google Scholar]
- (804).Rubee: Key Factsheet. CSE Communications & Security Pte Ltd. https://cse-comsec.com/rubee/ (accessed 2022-10-04). [Google Scholar]
- (805).Stevens J; Weich C; GilChrist R Rubee (IEEE 1902.1) - the Physics Behind, Real-Time, High Security Wireless Asset Visibility Networks in Harsh Environments. Proceedings from the International Security Conference, Taiwan; 2010. [Google Scholar]
- (806).Kim YS; Kim J; Chicas R; Xiuhtecutli N; Matthews J; Zavanelli N; Kwon S; Lee SH; Hertzberg VS; Yeo WH Soft Wireless Bioelectronics Designed for Real-Time, Continuous Health Monitoring of Farmworkers. Adv. Healthc. Mater 2022, 11, 2200170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (807).Choi Yeon S; Jeong H; Yin Rose T; Avila R; Pfenniger A; Yoo J; Lee Jong Y; Tzavelis A; Lee Young J; Chen Sheena W; et al. A Transient, Closed-Loop Network of Wireless, Body-Integrated Devices for Autonomous Electrotherapy. Science 2022, 376, 1006–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (808).Huang Y; Fang D; Wu C; Wang W; Guo X; Liu P A Flexible Touch-Pressure Sensor Array with Wireless Transmission System for Robotic Skin. Rev. Sci. Instrum 2016, 87, 065007. [DOI] [PubMed] [Google Scholar]
- (809).Sun S; Liu Y; Chang X; Jiang Y; Wang D; Tang C; He S; Wang M; Guo L; Gao Y A Wearable, Waterproof, and Highly Sensitive Strain Sensor Based on Three-Dimensional Graphene/Carbon Black/Ni Sponge for Wirelessly Monitoring Human Motions. J. Mater. Chem. C 2020, 8, 2074–2085. [Google Scholar]
- (810).Shahidul Islam M; Islam MT; Almutairi AF; Beng GK; Misran N; Amin N Monitoring of the Human Body Signal through the Internet of Things (IoT) Based LoRa Wireless Network System. Appl. Sci 2019, 9, 1884. [Google Scholar]
- (811).Jiang X; Waimin JF; Jiang H; Mousoulis C; Raghunathan N; Rahimi R; Peroulis D Wireless Sensor Network Utilizing Flexible Nitrate Sensors for Smart Farming. Proceedings from the 2019 IEEE SENSORS, October 27–30, 2019, Montreal, QC, Canada, 2019; IEEE, pp 1–4. [Google Scholar]
- (812).Hu G; Yi Z; Lu L; Huang Y; Zhai Y; Liu J; Yang B Self-Powered 5G NB-IoT System for Remote Monitoring Applications. Nano Energy 2021, 87, 106140. [Google Scholar]
- (813).Alvarez Lopez Y; Franssen J; Alvarez Narciandi G; Pagnozzi J; Gonzalez-Pinto Arrillaga I; Las-Heras Andres F RFID Technology for Management and Tracking: E-Health Applications. Sensors 2018, 18, 2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (814).Duan K-K; Cao S-Y Emerging RFID Technology in Structural Engineering-A Review. Structures 2020, 28, 2404–2414. [Google Scholar]
- (815).Di Rienzo F; Virdis A; Vallati C; Carbonaro N; Tognetti A Evaluation of NFC-Enabled Devices for Heterogeneous Wearable Biomedical Application. IEEE Journal of Radio Frequency Identification 2020, 4, 373–383. [Google Scholar]
- (816).Herbert R; Lim H-R; Rigo B; Yeo W-H Fully Implantable Wireless Batteryless Vascular Electronics with Printed Soft Sensors for Multiplex Sensing of Hemodynamics. Sci. Adv 2022, 8, No. eabm1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (817).Liu T-L; Dong Y; Chen S; Zhou J; Ma Z; Li J Battery-Free, Tuning Circuit-Inspired Wireless Sensor Systems for Detection of Multiple Biomarkers in Bodily Fluids. Sci. Adv 2022, 8, No. eabo7049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (818).Stuart T; Cai L; Burton A; Gutruf P Wireless and Battery-Free Platforms for Collection of Biosignals. Biosens. Bioelectron 2021, 178, 113007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (819).Tang K; Yang C; Fang Z; Wang W; Wang N; Zhu Y; Ng EJ; Heng C-H; Zheng YA 75.3 pJ/b Ultra-Low Power MEMS-Based FSK Transmitter in ISM-915 MHz Band for Pico-IoT Applications. Proceedings from the 2021 IEEE International Symposium on Circuits and Systems (ISCAS), May 22–28, 2021, Daegu, Korea; IEEE, 2021. [Google Scholar]
- (820).Tao J; Wang N; Ng EJ; Zhu Y; Heng C-HA 5-pJ/Bit OOK Transmitter Using MEMS-Based RF Oscillator for IoT Application in 180-Nm CMOS. IEEE Microwave Wireless Compon. Lett 2021, 31, 1158–1161. [Google Scholar]
- (821).Wang PP; Mercier PP 28.2 A 220 μW -85 dBm Sensitivity BLE-Compliant Wake-up Receiver Achieving -60dB SIR via Single-Die Multi-Channel FBAR-Based Filtering and a 4-Dimentional Wake-up Signature. Proceedings from the 2019 IEEE International Solid-State Circuits Conference-(ISSCC), February 17–21, 2019, San Francisco, CA; IEEE, 2019; pp 440–442. [Google Scholar]
- (822).Sinha RS; Wei Y; Hwang S-H A Survey on LPWA Technology: LoRa and NB-IoT. ICT Express 2017, 3, 14–21. [Google Scholar]
- (823).Kshetrimayum R. An Introduction to UWB Communication Systems. IEEE Potentials 2009, 28, 9–13. [Google Scholar]
- (824).Casado M; Foster N; Guha A Abstractions for Software-Defined Networks. Commun. ACM 2014, 57, 86–95. [Google Scholar]
- (825).Welch TB; Musselman RL; Emessiene BA; Gift PD; Choudhury DK; Cassadine DN; Yano SM The Effects of the Human Body on UWB Signal Propagation in an Indoor Environment. IEEE J. Sel. Area. Comm 2002, 20, 1778–1782. [Google Scholar]
- (826).Zasowski T; Meyer G; Althaus F; Wittneben A UWB Signal Propagation at the Human Head. IEEE Trans. Microw. Theory Tech 2006, 54, 1836–1845. [Google Scholar]
- (827).Saadeh W; Altaf MAB; Alsuradi H; Yoo J A Pseudo OFDM with Miniaturized FSK Demodulation Body-Coupled Communication Transceiver for Binaural Hearing Aids in 65 Nm CMOS. IEEE J. Solid-State Circuits 2017, 52, 757–768. [Google Scholar]
- (828).Takahashi R; Yukita W; Yokota T; Someya T; Kawahara Y Meander Coil++: a Body-Scale Wireless Power Transmission Using Safe-to-Body and Energy-Efficient Transmitter Coil. Proceedings from the CHI Conference on Human Factors in Computing Systems, April 30-May 5, 2022, New Orleans, LA, 2022. [Google Scholar]
- (829).Tian X; Lee PM; Tan YJ; Wu TLY; Yao H; Zhang M; Li Z; Ng KA; Tee BCK; Ho JS Wireless Body Sensor Networks Based on Metamaterial Textiles. Nat. Electron 2019, 2, 243–251. [Google Scholar]
- (830).Hajiaghajani A; Afandizadeh Zargari AH; Dautta M; Jimenez A; Kurdahi F; Tseng P Textile-Integrated Metamaterials for near-Field Multibody Area Networks. Nat. Electron 2021, 4, 808–817. [Google Scholar]
- (831).Saadeh W; Altaf MAB; Alsuradi H; Yoo JA 1.1-mW Ground Effect-Resilient Body-Coupled Communication Transceiver with Pseudo OFDM for Head and Body Area Network. IEEE J. Solid-State Circuits 2017, 52, 2690–2702. [Google Scholar]
- (832).Tomlinson WJ; Banou S; Yu C; Stojanovic M; Chowdhury KR Comprehensive Survey of Galvanic Coupling and Alternative Intra-Body Communication Technologies. IEEE Commun. Surv. Tut 2019, 21, 1145–1164. [Google Scholar]
- (833).Bae J; Cho H; Song K; Lee H; Yoo H-J The Signal Transmission Mechanism on the Surface of Human Body for Body Channel Communication. IEEE Trans. Microw. Theory Tech 2012, 60, 582–593. [Google Scholar]
- (834).Saadeh W; Alsuradi H; Altaf MAB; Yoo JA 1.1mW Hybrid OFDM Ground Effect-Resilient Body Coupled Communication Transceiver for Head and Body Area Network. Proceedings from the 2016 IEEE Asian Solid-State Circuits Conference (A-SSCC), November 7–9, 2016; IEEE, 2016; pp 201–204. [Google Scholar]
- (835).Park J; Mercier PP Magnetic Human Body Communication. Proceedings from the 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), August 25–29, 2015, IEEE, 2015; pp 1841–1844. [DOI] [PubMed] [Google Scholar]
- (836).Park J; Mercier PP A Sub-10-pj/Bit 5-Mb/s Magnetic Human Body Communication Transceiver. IEEE J. Solid-State Circuits 2019, 54, 3031–3042. [Google Scholar]
- (837).Zheng Y-Q; Liu Y; Zhong D; Nikzad S; Liu S; Yu Z; Liu D; Wu H-C; Zhu C; Li J; et al. Monolithic Optical Micro-lithography of High-Density Elastic Circuits. Science 2021, 373, 88. [DOI] [PubMed] [Google Scholar]
- (838).He D; Chen C; Chan S; Bu J; Vasilakos AV A Distributed Trust Evaluation Model and Its Application Scenarios for Medical Sensor Networks. IEEE Trans. Inf. Technol. Biomed 2012, 16, 1164–1175. [DOI] [PubMed] [Google Scholar]
- (839).Castelvecchi D The Race to Save the Internet from Quantum Hackers. Nature 2022, 602, 198–201. [DOI] [PubMed] [Google Scholar]
- (840).Joseph D; Misoczki R; Manzano M; Tricot J; Pinuaga FD; Lacombe O; Leichenauer S; Hidary J; Venables P; Hansen R Transitioning Organizations to Post-Quantum Cryptography. Nature 2022, 605, 237–243. [DOI] [PubMed] [Google Scholar]
- (841).Jirayupat C; Nagashima K; Hosomi T; Takahashi T; Samransuksamer B; Hanai Y; Nakao A; Nakatani M; Liu J; Zhang G; et al. Breath Odor-Based Individual Authentication by an Artificial Olfactory Sensor System and Machine Learning. Chem. Commun 2022, 58, 6377–6380. [DOI] [PubMed] [Google Scholar]
- (842).Lin S; Zhu J; Yu W; Wang B; Sabet KA; Zhao Y; Cheng X; Hojaiji H; Lin H; Tan J; et al. A Touch-Based Multimodal and Cryptographic Bio-Human-Machine Interface. Proc. Natl. Acad. Sci. U. S. A 2022, 119, No. e2201937119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (843).He D; Chen C; Chan S; Bu J; Vasilakos AV Retrust: Attack-Resistant and Lightweight Trust Management for Medical Sensor Networks. IEEE Trans. Inf. Technol. Biomed. 2012, 16, 623–632. [DOI] [PubMed] [Google Scholar]
- (844).Ng WY; Tan T-E; Movva PVH; Fang AHS; Yeo K-K; Ho D; Foo FSS; Xiao Z; Sun K; Wong TY; et al. Blockchain Applications in Health Care for COVID-19 and Beyond: A Systematic Review. The Lancet Digital Health 2021, 3, e819–e829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (845).Kiani M; Ghovanloo MA 13.56-Mbps Pulse Delay Modulation Based Transceiver for Simultaneous near-Field Data and Power Transmission. IEEE T. Biomed. Circ. S 2015, 9, 1–11. [DOI] [PubMed] [Google Scholar]
- (846).Kim D; Kim I-J; Lee J-S Memory Devices for Flexible and Neuromorphic Device Applications. Adv. Intell. Syst 2021, 3, 2000206. [Google Scholar]
- (847).Sattari-Esfahlan SM; Kim C-H Flexible Graphene-Channel Memory Devices: A Review. ACS Appl. Nano Mater 2021, 4, 6542–6556. [Google Scholar]
- (848).Kuncoro IW; Pambudi NA; Biddinika MK; Widiastuti I; Hijriawan M; Wibowo KM Immersion Cooling as the Next Technology for Data Center Cooling: A Review. J. Phys.: Conf. Ser 2019, 1402, 044057. [Google Scholar]
- (849).Baccour E; Foufou S; Hamila R; Erbad A Green Data Center Networks: A Holistic Survey and Design Guidelines. Proceedings from the 2019 15th International Wireless Communications & Mobile Computing Conference (IWCMC), June 24–28, 2019; IEEE, 2019; pp 1108–1114. [Google Scholar]
- (850).Hwang B; Lee J-S Recent Advances in Memory Devices with Hybrid Materials. Adv. Electron. Mater 2019, 5, 1800519. [Google Scholar]
- (851).Forte AE; Hanakata PZ; Jin L; Zari E; Zareei A; Fernandes MC; Sumner L; Alvarez J; Bertoldi K Inverse Design of Inflatable Soft Membranes through Machine Learning. Adv. Funct. Mater 2022, 32, 2111610. [Google Scholar]
- (852).Afanasenkau D; Kalinina D; Lyakhovetskii V; Tondera C; Gorsky O; Moosavi S; Pavlova N; Merkulyeva N; Kalueff AV; Minev IR; et al. Rapid Prototyping of Soft Bioelectronic Implants for Use as Neuromuscular Interfaces. Nat. Biomed. Eng 2020, 4, 1010–1022. [DOI] [PubMed] [Google Scholar]
- (853).Driscoll N; Erickson B; Murphy Brendan B; Richardson Andrew G; Robbins G; Apollo Nicholas V; Mentzelopoulos G; Mathis T; Hantanasirisakul K; Bagga P; et al. Mxene-Infused Bioelectronic Interfaces for Multiscale Electrophysiology and Stimulation. Sci. Transl. Med 2021, 13, No. eabf8629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (854).Jeong H; Wang L; Ha T; Mitbander R; Yang X; Dai Z; Qiao S; Shen L; Sun N; Lu N Modular and Reconfigurable Wireless E-Tattoos for Personalized Sensing. Adv. Mater. Technol 2019, 4, 1900117. [Google Scholar]
- (855).Bariya M; Shahpar Z; Park H; Sun J; Jung Y; Gao W; Nyein HYY; Liaw TS; Tai LC; Ngo QP; et al. Roll-to-Roll Gravure Printed Electrochemical Sensors for Wearable and Medical Devices. ACS Nano 2018, 12, 6978–6987. [DOI] [PubMed] [Google Scholar]
- (856).Shen PC; Su C; Lin Y; Chou AS; Cheng CC; Park JH; Chiu MH; Lu AY; Tang HL; Tavakoli MM; et al. Ultralow Contact Resistance between Semimetal and Monolayer Semiconductors. Nature 2021, 593, 211–217. [DOI] [PubMed] [Google Scholar]
- (857).Fernandez SV; Sadat D; Tasnim F; Acosta D; Schwendeman L; Shahsavari S; Dagdeviren C Ubiquitous Conformable Systems for Imperceptible Computing. Foresight 2022, 24, 75–98. [Google Scholar]
- (858).Meng K; Xiao X; Wei W; Chen G; Nashalian A; Shen S; Xiao X; Chen J Wearable Pressure Sensors for Pulse Wave Monitoring. Adv. Mater 2022, 34, 2109357. [DOI] [PubMed] [Google Scholar]
- (859).Fu Y; Zhao S; Wang L; Zhu R A Wearable Sensor Using Structured Silver-Particle Reinforced PDMS for Radial Arterial Pulse Wave Monitoring. Adv. Healthc. Mater 2019, 8, 1900633. [DOI] [PubMed] [Google Scholar]
- (860).Fu Y; Zhao S; Zhu R A Wearable Multifunctional Pulse Monitor Using Thermosensation-Based Flexible Sensors. IEEE Trans. Biomed. Eng 2019, 66, 1412–1421. [DOI] [PubMed] [Google Scholar]
- (861).Kotov NA Sustainability of the Academic Enterprise in the United States. ACS Nano 2015, 9, 1–2. [DOI] [PubMed] [Google Scholar]
- (862).Luchs MG; Swan KS; Griffin A Design Thinking; John Wiley & Sons, Inc, 2015. [Google Scholar]
- (863).Lu S; Liu A Innovative Design Thinking for Breakthrough Product Development. Procedia CIRP 2016, 53, 50–55. [Google Scholar]
- (864).Liu A; Lu S Functional Design Framework for Innovative Design Thinking in Product Development. CIRP J. Manuf. Sci. Tec 2020, 30, 105–117. [Google Scholar]
- (865).Oh YS; Kim JH; Xie Z; Cho S; Han H; Jeon SW; Park M; Namkoong M; Avila R; Song Z; et al. Battery-Free, Wireless Soft Sensors for Continuous Multi-Site Measurements of Pressure and Temperature from Patients at Risk for Pressure Injuries. Nat. Commun 2021, 12, 5008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (866).Kim JJ; Wang Y; Wang H; Lee S; Yokota T; Someya T Skin Electronics: Next-Generation Device Platform for Virtual and Augmented Reality. Adv. Funct. Mater 2021, 31, 2009602. [Google Scholar]
- (867).Chung HU; Rwei AY; Hourlier-Fargette A; Xu S; Lee K; Dunne EC; Xie Z; Liu C; Carlini A; Kim DH; et al. Skin-Interfaced Biosensors for Advanced Wireless Physiological Monitoring in Neonatal and Pediatric Intensive-Care Units. Nat. Med 2020, 26, 418–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (868).Yousefi H; Ali MM; Su HM; Filipe CDM; Didar TF Sentinel Wraps: Real-Time Monitoring of Food Contamination by Printing Dnazyme Probes on Food Packaging. ACS Nano 2018, 12, 3287–3294. [DOI] [PubMed] [Google Scholar]
- (869).Basu B; Ghosh S Assessment of Technology and Manufacturing Readiness Levels. In Biomaterials for Musculoskeletal Regeneration: Applications, Indian Institute of Metals Series, Springer; Singapore, 2017; pp 235–246. [Google Scholar]
- (870).Imec R&D, Nano Electronics and Digital Technologies. https://www.imec-int.com/en (accessed 2022-11-30).
- (871).Innovationlab: Your Expert for Flexible Printed Sensors. https://www.innovationlab.de/en/printed-electronics/ (accessed 2022-11-30).
- (872).Frith JT; Lacey MJ; Ulissi U A Non-Academic Perspective on the Future of Lithium-Based Batteries. Nat. Commun 2023, 14, 420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (873).Lee Y; Chung Jong W; Lee Gae H; Kang H; Kim J-Y; Bae C; Yoo H; Jeong S; Cho H; Kang S-G; et al. Standalone Real-Time Health Monitoring Patch Based on a Stretchable Organic Optoelectronic System. Sci. Adv 2021, 7, No. eabg9180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (874).Yu Y; Li J; Solomon SA; Min J; Tu J; Guo W; Xu C; Song Y; Gao W All-Printed Soft Human-Machine Interface for Robotic Physicochemical Sensing. Sci. Robot 2022, 7, No. eabn0495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (875).Song J Mechanics of Stretchable Electronics. Curr Opin. Solid State Mater. Sci 2015, 19, 160–170. [Google Scholar]
- (876).Root SE; Savagatrup S; Pais CJ; Arya G; Lipomi DJ Predicting the Mechanical Properties of Organic Semiconductors Using Coarse-Grained Molecular Dynamics Simulations. Macromolecules 2016, 49, 2886–2894. [Google Scholar]
- (877).Rodriquez D; Kim JH; Root SE; Fei Z; Boufflet P; Heeney M; Kim TS; Lipomi DJ Comparison of Methods for Determining the Mechanical Properties of Semiconducting Polymer Films for Stretchable Electronics. ACS Appl. Mater. Interfaces 2017, 9, 8855–8862. [DOI] [PubMed] [Google Scholar]
- (878).Li Y. Twist-Enhanced Stretchability of Graphene Nanoribbons: A Molecular Dynamics Study. J. Phys. D: Appl. Phys 2010, 43, 495405. [Google Scholar]
- (879).Hanakata PZ; Cubuk ED; Campbell DK; Park HS Accelerated Search and Design of Stretchable Graphene Kirigami Using Machine Learning. Phys. Rev. Lett 2018, 121, 255304. [DOI] [PubMed] [Google Scholar]
- (880).Ding W-L; Lu Y; Peng X-L; Dong H; Chi W-J; Yuan X; Sun Z-Z; He H Accelerating Evaluation of the Mobility of Ionic Liquid-Modulated Pedot Flexible Electronics Using Machine Learning. J. Mater. Chem. A 2021, 9, 25547–25557. [Google Scholar]
- (881).Abbasi Shirsavar M; Taghavimehr M; Ouedraogo LJ; Javaheripi M; Hashemi NN; Koushanfar F; Montazami R Machine Learning-Assisted E-Jet Printing for Manufacturing of Organic Flexible Electronics. Biosens. Bioelectron 2022, 212, 114418. [DOI] [PubMed] [Google Scholar]
- (882).Wu L; Liu L; Wang Y; Zhai Z; Zhuang H; Krishnaraju D; Wang Q; Jiang H A Machine Learning-Based Method to Design Modular Metamaterials. Extreme Mech. Lett 2020, 36, 100657. [Google Scholar]
- (883).Chortos A. Extrusion 3D Printing of Conjugated Polymers. J. Polym. Sci 2022, 60, 486–503. [Google Scholar]
- (884).Deo KA; Jaiswal MK; Abasi S; Lokhande G; Bhunia S; Nguyen TU; Namkoong M; Darvesh K; Guiseppi-Elie A; Tian L; et al. Nanoengineered Ink for Designing 3D Printable Flexible Bioelectronics. ACS Nano 2022, 16, 8798–8811. [DOI] [PubMed] [Google Scholar]
- (885).Valentine AD; Busbee TA; Boley JW; Raney JR; Chortos A; Kotikian A; Berrigan JD; Durstock MF; Lewis JA Hybrid 3D Printing of Soft Electronics. Adv. Mater 2017, 29, 1703817. [DOI] [PubMed] [Google Scholar]
- (886).Lee K; Shang Y; Bobrin VA; Kuchel R; Kundu D; Corrigan N; Boyer C xs3D Printing Nanostructured Solid Polymer Electrolytes with High Modulus and Conductivity. Adv. Mater 2022, 34, 2204816. [DOI] [PubMed] [Google Scholar]
- (887).Saleh MS; Ritchie SM; Nicholas MA; Gordon HL; Hu C; Jahan S; Yuan B; Bezbaruah R; Reddy JW; Ahmed Z; et al. CMU Array: A 3D Nanoprinted, Fully Customizable High-Density Microelectrode Array Platform. Sci. Adv 2022, 8, No. eabj4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (888).Kim F; Yang SE; Ju H; Choo S; Lee J; Kim G; Jung S.-h.; Kim S; Cha C; Kim KT; et al. Direct Ink Writing of Three-Dimensional Thermoelectric Microarchitectures. Nat. Electron 2021, 4, 579–587. [Google Scholar]
- (889).Ouyang X; Su R; Ng DWH; Han G; Pearson DR; McAlpine MC 3D Printed Skin-Interfaced UV-Visible Hybrid Photodetectors. Adv. Sci 2022, 9, 2201275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (890).Xu R; He P; Lan G; Behrouzi K; Peng Y; Wang D; Jiang T; Lee A; Long Y; Lin L Facile Fabrication of Multilayer Stretchable Electronics via a Two-Mode Mechanical Cutting Process. ACS Nano 2022, 16, 1533–1546. [DOI] [PubMed] [Google Scholar]
- (891).Yang S; Chen YC; Nicolini L; Pasupathy P; Sacks J; Su B; Yang R; Sanchez D; Chang YF; Wang P; et al. ″Cut-and-Paste″ Manufacture of Multiparametric Epidermal Sensor Systems. Adv. Mater 2015, 27, 6423–6430. [DOI] [PubMed] [Google Scholar]
- (892).Zhao G; Ling Y; Su Y; Chen Z; Mathai Cherian J; Emeje O; Brown A; Alla Dinesh R; Huang J; Kim C; et al. Laser-Scribed Conductive, Photoactive Transition Metal Oxide on Soft Elastomers for Janus On-Skin Electronics and Soft Actuators. Sci. Adv 2022, 8, No. eabp9734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (893).Zhang S; Fei W; Jiang Q; Jiang J; Shi K; Xue L; Wu Z Facile Fabrication of Sensitivity-Tunable Strain Sensors Based on Laser-Patterned Micro-Nano Structures. J. Micromech. Microeng 2021, 31, 085003. [Google Scholar]
- (894).Choi C; Kim H; Kang J-H; Song M-K; Yeon H; Chang CS; Suh JM; Shin J; Lu K; Park B-I; et al. Reconfigurable Heterogeneous Integration Using Stackable Chips with Embedded Artificial Intelligence. Nat. Electron 2022, 5, 386–393. [Google Scholar]
- (895).Jung YH; Yoo J-Y; Vázquez-Guardado A; Kim J-H; Kim J-T; Luan H; Park M; Lim J; Shin H-S; Su C-J; et al. A Wireless Haptic Interface for Programmable Patterns of Touch across Large Areas of the Skin. Nat. Electron 2022, 5, 374–385. [Google Scholar]
- (896).Li T; Hou J; Yan J; Liu R; Yang H; Sun Z Chiplet Heterogeneous Integration Technology-Status and Challenges. Electronics 2020, 9, 670. [Google Scholar]
- (897).Huang Y; Tang W; Feng L; Chen S; Zhao J; Liu Z; Han L; Ouyang B; Guo X Printable Low Power Organic Transistor Technology for Customizable Hybrid Integration Towards Internet of Everything. IEEE J. Electron. Devices Soc 2020, 8, 1219–1226. [Google Scholar]
- (898).Ouyang B; Song Y; Cai W; Tang Y; Si Y; Yin X; Chen S; Tang W; Zhou H; Huang B, et al. RF Powered Flexible Printed Ion-Sensitive Organic Field Effect Transistor Chip with Design-to-Manufacturing Automation for Mobile Bio-Sensing. Proceedings from the 2021 IEEE International Electron Devices Meeting (IEDM), December 11–16, 2021, San Francisco, CA; IEEE, 2021. [Google Scholar]
- (899).Zhao Y; Wang T; Zhao Z; Wang Q Track-Etch Membranes as Tools for Template Synthesis of Highly Sensitive Pressure Sensors. ACS Appl. Mater. Interfaces 2022, 14, 1791–1799. [DOI] [PubMed] [Google Scholar]
- (900).Huang Q; Zhu Y Printing Conductive Nanomaterials for Flexible and Stretchable Electronics: A Review of Materials, Processes, and Applications. Adv. Mater. Technol 2019, 4, 1800546. [Google Scholar]
- (901).Ogilvie SP; Large MJ; O’Mara MA; Sehnal AC; Amorim Graf A; Lynch PJ; Cass AJ; Salvage JP; Alfonso M; Poulin P; et al. Nanosheet-Stabilized Emulsions: Near-Minimum Loading and Surface Energy Design of Conductive Networks. ACS Nano 2022, 16, 1963–1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (902).Kamyshny A; Magdassi S Conductive Nanomaterials for 2D and 3D Printed Flexible Electronics. Chem. Soc. Rev 2019, 48, 1712–1740. [DOI] [PubMed] [Google Scholar]
- (903).Su R; Park SH; Ouyang X; Ahn SI; McAlpine MC 3D-Printed Flexible Organic Light-Emitting Diode Displays. Sci. Adv 2022, 8, No. eabl8798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (904).Hong SY; Jee SM; Ko Y; Cho J; Lee KH; Yeom B; Kim H; Son JG Intrinsically Stretchable and Printable Lithium-Ion Battery for Free-Form Configuration. ACS Nano 2022, 16, 2271–2281. [DOI] [PubMed] [Google Scholar]
- (905).Li Y; Sivan M; Niu JX; Veluri H; Zamburg E; Leong J; Chand U; Samanta S; Wang X; Feng X, et al. Aerosol Jet Printed WSe2 Based RRAM on Kapton Suitable for Flexible Monolithic Memory Integration. In 2019 IEEE International Conference on Flexible and Printable Sensors and Systems (FLEPS), July 8–10, 2019, Glasgow, UK; IEEE, 2019; pp 1–3. [Google Scholar]
- (906).Lin Y; Chen J; Tavakoli MM; Gao Y; Zhu Y; Zhang D; Kam M; He Z; Fan Z Printable Fabrication of a Fully Integrated and Self-Powered Sensor System on Plastic Substrates. Adv. Mater 2019, 31, 1804285. [DOI] [PubMed] [Google Scholar]
- (907).Li P; Zhang Y; Zheng Z Polymer-Assisted Metal Deposition (PAMD) for Flexible and Wearable Electronics: Principle, Materials, Printing, and Devices. Adv. Mater 2019, 31, 1902987. [DOI] [PubMed] [Google Scholar]
- (908).Go GT; Lee Y; Seo DG; Lee TW Organic Neuroelectronics: From Neural Interfaces to Neuroprosthetics. Adv. Mater 2022, 34, No. e2201864. [DOI] [PubMed] [Google Scholar]
- (909).Tao X; Liao S; Wang Y Polymer-Assisted Fully Recyclable Flexible Sensors. EcoMat 2021, 3, No. e12083. [Google Scholar]
- (910).Liu J; Zhang X; Liu Y; Rodrigo M; Loftus PD; Aparicio-Valenzuela J; Zheng J; Pong T; Cyr KJ; Babakhanian M; et al. Intrinsically Stretchable Electrode Array Enabled in Vivo Electrophysiological Mapping of Atrial Fibrillation at Cellular Resolution. Proc. Natl. Acad. Sci. U. S. A 2020, 117, 14769–14778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (911).Ju J; Hu N; Cairns DM; Liu H; Timko BP Photo-Cross-Linkable, Insulating Silk Fibroin for Bioelectronics with Enhanced Cell Affinity. Proc. Natl. Acad. Sci. U. S. A 2020, 117, 15482–15489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (912).Yao S; Ren P; Song R; Liu Y; Huang Q; Dong J; O’Connor BT; Zhu Y Nanomaterial-Enabled Flexible and Stretchable Sensing Systems: Processing, Integration, and Applications. Adv. Mater 2020, 32, 1902343. [DOI] [PubMed] [Google Scholar]
- (913).Guo Y; Shi E; Zhu J; Shen P-C; Wang J; Lin Y; Mao Y; Deng S; Li B; Park J-H; et al. Soft-Lock Drawing of Super-Aligned Carbon Nanotube Bundles for Nanometre Electrical Contacts. Nat. Nanotechnol 2022, 17, 278–284. [DOI] [PubMed] [Google Scholar]
- (914).Li M-Y; Su S-K; Wong H-SP; Li L-J How 2D Semiconductors Could Extend Moore’s Law. Nature 2019, 567, 169–170. [DOI] [PubMed] [Google Scholar]
- (915).Andrews AM; Liao WS; Weiss PS Double-Sided Opportunities Using Chemical Lift-Off Lithography. Acc. Chem. Res 2016, 49, 1449–1457. [DOI] [PubMed] [Google Scholar]
- (916).Rim YS; Bae S-H; Chen H; Yang JL; Kim J; Andrews AM; Weiss PS; Yang Y; Tseng H-R Printable Ultrathin Metal Oxide Semiconductor-Based Conformal Biosensors. ACS Nano 2015, 9, 12174–12181. [DOI] [PubMed] [Google Scholar]
- (917).Yang H; Valenzuela SO; Chshiev M; Couet S; Dieny B; Dlubak B; Fert A; Garello K; Jamet M; Jeong DE; et al. Two-Dimensional Materials Prospects for Non-Volatile Spintronic Memories. Nature 2022, 606, 663–673. [DOI] [PubMed] [Google Scholar]
- (918).Zheng Y; Gao J; Han C; Chen W Ohmic Contact Engineering for Two-Dimensional Materials. Cell Rep. Phys. Sci 2021, 2, 100298. [Google Scholar]
- (919).Cho D; Li R; Jeong H; Li S; Wu C; Tzavelis A; Yoo S; Kwak SS; Huang Y; Rogers JA Bitter Flavored, Soft Composites for Wearables Designed to Reduce Risks of Choking in Infants. Adv. Mater 2021, 33, 2103857. [DOI] [PubMed] [Google Scholar]
- (920).Okutani C; Yokota T; Someya T Ultrathin Fiber-Mesh Polymer Thermistors. Adv. Sci 2022, 9, 2202312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (921).Godwin H; Nameth C; Avery D; Bergeson LL; Bernard D; Beryt E; Boyes W; Brown S; Clippinger AJ; Cohen Y; et al. Nanomaterial Categorization for Assessing Risk Potential to Facilitate Regulatory Decision-Making. ACS Nano 2015, 9, 3409–3417. [DOI] [PubMed] [Google Scholar]
- (922).Lee I; Probst D; Klonoff D; Sode K Continuous Glucose Monitoring Systems-Current Status and Future Perspectives of the Flagship Technologies in Biosensor Research. Biosens. Bioelectron 2021, 181, 113054. [DOI] [PubMed] [Google Scholar]
- (923).Sun H; Saeedi P; Karuranga S; Pinkepank M; Ogurtsova K; Duncan BB; Stein C; Basit A; Chan JCN; Mbanya JC; et al. IDF Diabetes Atlas: Global, Regional and Country-Level Diabetes Prevalence Estimates for 2021 and Projections for 2045. Diabetes Res. Clin. Pract 2022, 183, 109119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (924).Williams R; Karuranga S; Malanda B; Saeedi P; Basit A; Besancon S; Bommer C; Esteghamati A; Ogurtsova K; Zhang P; et al. Global and Regional Estimates and Projections of Diabetes-Related Health Expenditure: Results from the International Diabetes Federation Diabetes Atlas, 9th Edition. Diabetes Res. Clin. Pract 2020, 162, 108072. [DOI] [PubMed] [Google Scholar]
- (925).Beh B. Continuous Glucose Monitors: A New Era for Diabetes Management; IDTechEx, 2022. https://www.idtechex.com/en/webinar/continuous-glucose-monitors-a-new-era-for-diabetes-management/421 (accessed 2022-11-10). [Google Scholar]
- (926).Klonoff DC; Nguyen KT; Xu NY; Gutierrez A; Espinoza JC; Vidmar AP Use of Continuous Glucose Monitors by People without Diabetes: An Idea Whose Time Has Come? J. Diabetes Sci. Technol 2022, 19322968221110830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (927).Liang W; Elrod S; McFarland DA; Zou J Systematic Analysis of 50 Years of Stanford University Technology Transfer and Commercialization. Patterns 2022, 3, 100584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (928).Koga H; Nagashima K; Suematsu K; Takahashi T; Zhu L; Fukushima D; Huang Y; Nakagawa R; Liu J; Uetani K; et al. Nanocellulose Paper Semiconductor with a 3D Network Structure and Its Nano-Micro-Macro Trans-Scale Design. ACS Nano 2022, 16, 8630–8640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (929).Holst Centre to Host Launch of Ecotron Project. https://holstcentre.com/insights/news/holst-centre-to-host-launc-of-ecotron-project/ (accessed 2022-11-30).
- (930).Sustainable Semiconductor Technologies and Systems ∣ Imec. https://www.imec-int.com/en/expertise/cmos-advanced/sustainable-semiconductor-technologies-and-systems-ssts (accessed 2022-12-13).
- (931).Deng B; Wang X; Luong DX; Carter RA; Wang Z; Tomson MB; Tour JM Rare Earth Elements from Waste. Sci. Adv 2022, 8, No. eabm3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (932).Li F; Zhu J; Sun P; Zhang M; Li Z; Xu D; Gong X; Zou X; Geim AK; Su Y; et al. Highly Efficient and Selective Extraction of Gold by Reduced Graphene Oxide. Nat. Commun 2022, 13, 4472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (933).Das P; Gabriel JP; Tay CY; Lee JM Value-Added Products from Thermochemical Treatments of Contaminated E-Waste Plastics. Chemosphere 2021, 269, 129409. [DOI] [PubMed] [Google Scholar]
- (934).Teng L; Ye S; Handschuh-Wang S; Zhou X; Gan T; Zhou X Liquid Metal-Based Transient Circuits for Flexible and Recyclable Electronics. Adv. Funct. Mater 2019, 29, 1808739. [Google Scholar]
- (935).Shi C; Zou Z; Lei Z; Zhu P; Zhang W; Xiao J Heterogeneous Integration of Rigid, Soft, and Liquid Materials for Self-Healable, Recyclable, and Reconfigurable Wearable Electronics. Sci. Adv 2020, 6, No. eabd0202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (936).Brown MS; Somma L; Mendoza M; Noh Y; Mahler GJ; Koh A Upcycling Compact Discs for Flexible and Stretchable Bioelectronic Applications. Nat. Commun 2022, 13, 3727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (937).Zou Z; Zhu C; Li Y; Lei X; Zhang W; Xiao J Rehealable, Fully Recyclable, and Malleable Electronic Skin Enabled by Dynamic Covalent Thermoset Nanocomposite. Sci. Adv 2018, 4, No. eaaq0508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (938).Liu Y; Wang H; Zhu Y Recycling of Nanowire Percolation Network for Sustainable Soft Electronics. Adv. Electron. Mater 2021, 7, 2100588. [Google Scholar]
- (939).Li J; Zeng X; Stevels A Ecodesign in Consumer Electronics: Past, Present, and Future. Crit. Rev. Environ. Sci. Technol 2015, 45, 840–860. [Google Scholar]
- (940).Cao J; Sim Y; Tan XY; Zheng J; Chien SW; Jia N; Chen K; Tay YB; Dong JF; Yang L; et al. Upcycling Silicon Photovoltaic Waste into Thermoelectrics. Adv. Mater 2022, 34, 2110518. [DOI] [PubMed] [Google Scholar]
- (941).Baumgartner M; Hartmann F; Drack M; Preninger D; Wirthl D; Gerstmayr R; Lehner L; Mao G; Pruckner R; Demchyshyn S; et al. Resilient yet Entirely Degradable Gelatin-Based Biogels for Soft Robots and Electronics. Nat. Mater 2020, 19, 1102–1109. [DOI] [PubMed] [Google Scholar]
- (942).Guo Y; Zhong M; Fang Z; Wan P; Yu G A Wearable Transient Pressure Sensor Made with Mxene Nanosheets for Sensitive Broad-Range Human-Machine Interfacing. Nano Lett. 2019, 19, 1143–1150. [DOI] [PubMed] [Google Scholar]
- (943).Guidetti G; d’Amone L; Kim T; Matzeu G; Mogas-Soldevila L; Napier B; Ostrovsky-Snider N; Roshko J; Ruggeri E; Omenetto FG Silk Materials at the Convergence of Science, Sustainability, Healthcare, and Technology. Appl. Phys. Rev 2022, 9, 011302. [Google Scholar]
- (944).Cui Y; Zhang F; Chen G; Yao L; Zhang N; Liu Z; Li Q; Zhang F; Cui Z; Zhang K; et al. A Stretchable and Transparent Electrode Based on Pegylated Silk Fibroin for in Vivo Dual-Modal Neural-Vascular Activity Probing. Adv. Mater 2021, 33, 2100221. [DOI] [PubMed] [Google Scholar]
- (945).Zhao Z; Hwang Y; Yang Y; Fan T; Song J; Suresh S; Cho NJ Actuation and Locomotion Driven by Moisture in Paper Made with Natural Pollen. Proc. Natl. Acad. Sci. U. S. A 2020, 117, 8711–8718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (946).Hwang Y; Sadhu A; Shin S; Leow SW; Zhao Z; Deng J; Jackman JA; Kim M; Wong LH; Cho NJ An Intrinsically Micro-/Nanostructured Pollen Substrate with Tunable Optical Properties for Optoelectronic Applications. Adv. Mater 2021, 33, 2100566. [DOI] [PubMed] [Google Scholar]
- (947).Kadumudi FB; Trifol J; Jahanshahi M; Zsurzsan TG; Mehrali M; Zeqiraj E; Shaki H; Alehosseini M; Gundlach C; Li Q; et al. Flexible and Green Electronics Manufactured by Origami Folding of Nanosilicate-Reinforced Cellulose Paper. ACS Appl. Mater. Interfaces 2020, 12, 48027–48039. [DOI] [PubMed] [Google Scholar]
- (948).Chen Y; Xie R; Zou B; Liu Y; Zhang K; Li S; Zheng B; Zhang W; Wu J; Huo F CNT@Leather-Based Electronic Bidirectional Pressure Sensor. Sci. China Technol. Sc 2020, 63, 2137–2146. [Google Scholar]
- (949).Zhang K; Kang N; Zhang B; Xie R; Zhu J; Zou B; Liu Y; Chen Y; Shi W; Zhang W; et al. Skin Conformal and Antibacterial PPy-Leather Electrode for ECG Monitoring. Adv. Electron. Mater 2020, 6, 2000259. [Google Scholar]
- (950).Zou B; Chen Y; Liu Y; Xie R; Du Q; Zhang T; Shen Y; Zheng B; Li S; Wu J; et al. Repurposed Leather with Sensing Capabilities for Multifunctional Electronic Skin. Adv. Sci 2019, 6, 1801283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (951).Guan Q-F; Ling Z-C; Han Z-M; Yang H-B; Yu S-H Ultra-Strong, Ultra-Tough, Transparent, and Sustainable Nano-composite Films for Plastic Substitute. Matter 2020, 3, 1308–1317. [Google Scholar]
- (952).Lan L; Ping J; Xiong J; Ying Y Sustainable Natural Bio-Origin Materials for Future Flexible Devices. Adv. Sci 2022, 9, 2200560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (953).Yan N. Recycling Plastic Using a Hybrid Process. Science 2022, 378, 132–133. [DOI] [PubMed] [Google Scholar]
- (954).Kobashi K; Ata S; Yamada T; Futaba DN; Okazaki T; Hata K Classification of Commercialized Carbon Nanotubes into Three General Categories as a Guide for Applications. ACS Appl. Nano Mater 2019, 2, 4043–4047. [Google Scholar]
- (955).Moon J-H; Baek DH; Choi YY; Lee KH; Kim HC; Lee S-H Wearable Polyimide-PDMS Electrodes for Intrabody Communication. J. Micromech. Microeng 2010, 20, 025032. [Google Scholar]
- (956).Ghosal K; Freeman BD Gas Separation Using Polymer Membranes: An Overview. Polym. Adv. Technol 1994, 5, 673–697. [Google Scholar]
- (957).Cheng Z; Pang C-S; Wang P; Le ST; Wu Y; Shahrjerdi D; Radu I; Lemme MC; Peng L-M; Duan X; et al. How to Report and Benchmark Emerging Field-Effect Transistors. Nat. Electron 2022, 5, 416–423. [Google Scholar]
- (958).Khan Y; Mauriello ML; Nowruzi P; Motani A; Hon G; Vitale N; Li J; Kim J; Foudeh A; Duvio D; et al. Design Considerations of a Wearable Electronic-Skin for Mental Health and Wellness: Balancing Biosignals and Human Factors. bioRxiv, 2021.01.20.427496, ver. 1. https://www.biorxiv.org/content/10.1101/2021.01.20.427496v1 (accessed 2022-09-15). [Google Scholar]
- (959).Ledger D; McCaffrey D Inside Wearables Part 1: How Behavior Change Unlocks Long-Term Engagement. https://medium.com/@endeavourprtnrs/inside-wearable-how-the-science-of-human-behavior-change-offers-the-secret-to-long-term-engagement-a15b3c7d4cf3 (accessed 2022-04-22). [Google Scholar]
- (960).Hermsen S; Moons J; Kerkhof P; Wiekens C; De Groot M Determinants for Sustained Use of an Activity Tracker: Observational Study. JMIR mhealth and uhealth 2017, 5, No. e164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (961).Piwek L; Ellis DA; Andrews S; Joinson A The Rise of Consumer Health Wearables: Promises and Barriers. PLoS Med. 2016, 13, No. e1001953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (962).Kao Y-S; Nawata K; Huang C-Y An Exploration and Confirmation of the Factors Influencing Adoption of IoT-Based Wearable Fitness Trackers. Int. J. Environ. Res. Public. Health 2019, 16, 3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (963).Kononova A; Li L; Kamp K; Bowen M; Rikard RV; Cotten S; Peng W The Use of Wearable Activity Trackers among Older Adults: Focus Group Study of Tracker Perceptions, Motivators, and Barriers in the Maintenance Stage of Behavior Change. JMIR mhealth uhealth 2019, 7, No. e9832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (964).Nan K; Babaee S; Chan WW; Kuosmanen JLP; Feig VR; Luo Y; Srinivasan SS; Patterson CM; Jebran AM; Traverso G Low-Cost Gastrointestinal Manometry via Silicone-Liquid-Metal Pressure Transducers Resembling a Quipu. Nat. Biomed. Eng 2022, 6, 1092–1104. [DOI] [PubMed] [Google Scholar]
- (965).Ni X; Ouyang W; Jeong H; Kim JT; Tzaveils A; Mirzazadeh A; Wu C; Lee JY; Keller M; Mummidisetty CK; et al. Automated, Multiparametric Monitoring of Respiratory Biomarkers and Vital Signs in Clinical and Home Settings for COVID-19 Patients. Proc. Natl. Acad. Sci. U.S.A 2021, 118, No. e2026610118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (966).Teymourian H; Tehrani F; Longardner K; Mahato K; Podhajny T; Moon JM; Kotagiri YG; Sempionatto JR; Litvan I; Wang J Closing the Loop for Patients with Parkinson Disease: Where Are We? Nat. Rev. Neurol 2022, 18, 497–507. [DOI] [PubMed] [Google Scholar]
- (967).Yin L; Cao M; Kim KN; Lin M; Moon J-M; Sempionatto JR; Yu J; Liu R; Wicker C; Trifonov A; et al. A Stretchable Epidermal Sweat Sensing Platform with an Integrated Printed Battery and Electrochromic Display. Nat. Electron 2022, 5, 694–705. [Google Scholar]
- (968).Xu K; Lu Y; Takei K Flexible Hybrid Sensor Systems with Feedback Functions. Adv. Funct. Mater 2021, 31, 2007436. [Google Scholar]
- (969).Wang M; Yan Z; Wang T; Cai P; Gao S; Zeng Y; Wan C; Wang H; Pan L; Yu J; et al. Gesture Recognition Using a Bioinspired Learning Architecture That Integrates Visual Data with Somatosensory Data from Stretchable Sensors. Nat. Electron 2020, 3, 563–570. [Google Scholar]
- (970).Rusk N. Deep Learning. Nat. Meth 2016, 13, 35. [Google Scholar]
- (971).Lee GH; Park JK; Byun J; Yang JC; Kwon SY; Kim C; Jang C; Sim JY; Yook JG; Park S Parallel Signal Processing of a Wireless Pressure-Sensing Platform Combined with Machine-Learning-Based Cognition, Inspired by the Human Somatosensory System. Adv. Mater 2020, 32, 1906269. [DOI] [PubMed] [Google Scholar]
- (972).Wang M; Wang T; Luo Y; He K; Pan L; Li Z; Cui Z; Liu Z; Tu J; Chen X Fusing Stretchable Sensing Technology with Machine Learning for Human-Machine Interfaces. Adv. Funct. Mater 2021, 31, 2008807. [Google Scholar]
- (973).Zhang K; Wang J; Liu T; Luo Y; Loh XJ; Chen X Machine Learning-Reinforced Noninvasive Biosensors for Healthcare. Adv. Healthc. Mater 2021, 10, 2100734. [DOI] [PubMed] [Google Scholar]
- (974).Zhou Z; Chen K; Li X; Zhang S; Wu Y; Zhou Y; Meng K; Sun C; He Q; Fan W; et al. Sign-to-Speech Translation Using Machine-Learning-Assisted Stretchable Sensor Arrays. Nat. Electron 2020, 3, 571–578. [Google Scholar]
- (975).Pan J; Li Y; Luo Y; Zhang X; Wang X; Wong DLT; Heng CH; Tham CK; Thean AV Hybrid-Flexible Bimodal Sensing Wearable Glove System for Complex Hand Gesture Recognition. ACS Sens. 2021, 6, 4156–4166. [DOI] [PubMed] [Google Scholar]
- (976).Park H; Lee H; Park K; Mo S; Kim J Deep Neural Network Approach in Electrical Impedance Tomography-Based Real-Time Soft Tactile Sensor. Proceedings from the 2019 IEEE/RSJ International Conference on Intelligent Robots and Systems (IROS), November 4–8, 2019, Macau, China; IEEE, 2019; pp 7447–7452. [Google Scholar]
- (977).Husain Z; Madjid NA; Liatsis P Tactile Sensing Using Machine Learning-Driven Electrical Impedance Tomography. IEEE Sens. J 2021, 21, 11628–11642. [Google Scholar]
- (978).Li F; Wang R; Song C; Zhao M; Ren H; Wang S; Liang K; Li D; Ma X; Zhu B; et al. A Skin-Inspired Artificial Mechanoreceptor for Tactile Enhancement and Integration. ACS Nano 2021, 15, 16422–16431. [DOI] [PubMed] [Google Scholar]
- (979).Bian L; Wang Z; White DL; Star A Machine Learning-Assisted Calibration of Hg2+ Sensors Based on Carbon Nanotube Field-Effect Transistors. Biosens. Bioelectron 2021, 180, 113085. [DOI] [PubMed] [Google Scholar]
- (980).Gibney E. How to Shrink AI’s Ballooning Carbon Footprint. Nature 2022, 607, 648. [DOI] [PubMed] [Google Scholar]
- (981).Willcox KE; Ghattas O; Heimbach P The Imperative of Physics-Based Modeling and Inverse Theory in Computational Science. Nat. Comput. Sci 2021, 1, 166–168. [DOI] [PubMed] [Google Scholar]
- (982).Alber M; Buganza Tepole A; Cannon WR; De S; Dura-Bernal S; Garikipati K; Karniadakis G; Lytton WW; Perdikaris P; Petzold L; et al. Integrating Machine Learning and Multiscale Modeling-Perspectives, Challenges, and Opportunities in the Biological, Biomedical, and Behavioral Sciences. npj Digit. Med 2019, 2, 115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (983).Jia X; Zwart J; Sadler J; Appling A; Oliver S; Markstrom S; Willard J; Xu S; Steinbach M; Read J, et al. Physics-Guided Recurrent Graph Model for Predicting Flow and Temperature in River Networks. Proceedings of the 2021 SIAM International Conference on Data Mining (SDM), April 29–ay 1, 2021virtual; Society for Industrial and Applied Mathematics, 2021; pp 612–620. [Google Scholar]
- (984).Faghmous JH; Kumar V A Big Data Guide to Understanding Climate Change: The Case for Theory-Guided Data Science. Big Data 2014, 2, 155–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (985).Karpatne A; Atluri G; Faghmous JH; Steinbach M; Banerjee A; Ganguly A; Shekhar S; Samatova N; Kumar V Theory-Guided Data Science: A New Paradigm for Scientific Discovery from Data. IEEE T. Knowl. Data En 2017, 29, 2318–2331. [Google Scholar]
- (986).Rai R; Sahu CK Driven by Data or Derived through Physics? A Review of Hybrid Physics Guided Machine Learning Techniques with Cyber-Physical System (CPS) Focus. IEEE Access 2020, 8, 71050–71073. [Google Scholar]
- (987).Karniadakis GE; Kevrekidis IG; Lu L; Perdikaris P; Wang S; Yang L Physics-Informed Machine Learning. Nat. Rev. Phys 2021, 3, 422–440. [Google Scholar]
- (988).Khan WZ; Ahmed E; Hakak S; Yaqoob I; Ahmed A Edge Computing: A Survey. Future Gener. Comput. Syst 2019, 97, 219–235. [Google Scholar]
- (989).Satyanarayanan M. How We Created Edge Computing. Nat. Electron 2019, 2, 42–42. [Google Scholar]
- (990).Pan J; Luo Y; Li Y; Tham C-K; Heng C-H; Thean AV-Y A Wireless Multi-Channel Capacitive Sensor System for Efficient Glove-Based Gesture Recognition with AI at the Edge. IEEE T. Circuits-II 2020, 67, 1624–1628. [Google Scholar]
- (991).Zhou F; Chai Y Near-Sensor and in-Sensor Computing. Nat. Electron 2020, 3, 664–671. [Google Scholar]
- (992).Jiang J; Parto K; Cao W; Banerjee K Ultimate Monolithic-3D Integration with 2D Materials: Rationale, Prospects, and Challenges. IEEE J. Electron. Devices Soc. 2019, 7, 878–887. [Google Scholar]
- (993).Cheng Y; Guo X; Pavlidis VF Emerging Monolithic 3D Integration: Opportunities and Challenges from the Computer System Perspective. Integration 2022, 85, 97–107. [Google Scholar]
- (994).Dhananjay K; Shukla P; Pavlidis VF; Coskun A; Salman E Monolithic 3D Integrated Circuits: Recent Trends and Future Prospects. IEEE T. Circuits-II 2021, 68, 837–843. [Google Scholar]
- (995).Biggs J; Myers J; Kufel J; Ozer E; Craske S; Sou A; Ramsdale C; Williamson K; Price R; White S A Natively Flexible 32-Bit Arm Microprocessor. Nature 2021, 595, 532–536. [DOI] [PubMed] [Google Scholar]
- (996).Ozer E; Kufel J; Myers J; Biggs J; Brown G; Rana A; Sou A; Ramsdale C; White S A Hardwired Machine Learning Processing Engine Fabricated with Submicron Metal-Oxide Thin-Film Transistors on a Flexible Substrate. Nat. Electron 2020, 3, 419–425. [Google Scholar]
- (997).Zhang H; Xiang L; Yang Y; Xiao M; Han J; Ding L; Zhang Z; Hu Y; Peng LM High-Performance Carbon Nanotube Complementary Electronics and Integrated Sensor Systems on Ultrathin Plastic Foil. ACS Nano 2018, 12, 2773–2779. [DOI] [PubMed] [Google Scholar]
- (998).Shen H; He Z; Jin W; Xiang L; Zhao W; Di CA; Zhu D Mimicking Sensory Adaptation with Dielectric Engineered Organic Transistors. Adv. Mater 2019, 31, 1905018. [DOI] [PubMed] [Google Scholar]
- (999).Tang H; Liang Y; Liu C; Hu Z; Deng Y; Guo H; Yu Z; Song A; Zhao H; Zhao D; et al. A Solution-Processed n-Type Conducting Polymer with Ultrahigh Conductivity. Nature 2022, 611, 271–277. [DOI] [PubMed] [Google Scholar]
- (1000).Krauhausen I; Koutsouras DA; Melianas A; Keene ST; Lieberth K; Ledanseur H; Sheelamanthula R; Giovannitti A; Torricelli F; McCulloch I; et al. Organic Neuromorphic Electronics for Sensorimotor Integration and Learning in Robotics. Sci. Adv 2021, 7, No. eabl5068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (1001).Wang M; Tu J; Huang Z; Wang T; Liu Z; Zhang F; Li W; He K; Pan L; Zhang X; et al. Tactile Near-Sensor Analogue Computing for Ultrafast Responsive Artificial Skin. Adv. Mater 2022, 34, 2201962. [DOI] [PubMed] [Google Scholar]
- (1002).Benatti S; Casamassima F; Milosevic B; Farella E; Schonle P; Fateh S; Burger T; Huang Q; Benini L A Versatile Embedded Platform for EMG Acquisition and Gesture Recognition. IEEE T. Biomed. Circ. S 2015, 9, 620–630. [DOI] [PubMed] [Google Scholar]
- (1003).Liu X; Sacks J; Zhang M; Richardson AG; Lucas TH; Van der Spiegel J The Virtual Trackpad: An Electromyography-Based, Wireless, Real-Time, Low-Power, Embedded Hand-Gesture-Recognition System Using an Event-Driven Artificial Neural Network. IEEE T. Circuits-II 2017, 64, 1257–1261. [Google Scholar]
- (1004).Milosevic B; Farella E; Benatti S Exploring Arm Posture and Temporal Variability in Myoelectric Hand Gesture Recognition. Proceedings from the 2018 7th IEEE International Conference on Biomedical Robotics and Biomechatronics (Biorob), August 26–29, 2018, Enschede, The Netherlands; IEEE, 2018; pp 1032–1037. [Google Scholar]
- (1005).Moin A; Zhou A; Rahimi A; Menon A; Benatti S; Alexandrov G; Tamakloe S; Ting J; Yamamoto N; Khan Y; et al. A Wearable Biosensing System with in-Sensor Adaptive Machine Learning for Hand Gesture Recognition. Nat. Electron 2021, 4, 54–63. [Google Scholar]
- (1006).He Z; Ye D; Liu L; Di CA; Zhu D Advances in Materials and Devices for Mimicking Sensory Adaptation. Mater. Horiz 2022, 9, 147–163. [DOI] [PubMed] [Google Scholar]
- (1007).He Z; Shen H; Ye D; Xiang L; Zhao W; Ding J; Zhang F; Di C.-a.; Zhu D An Organic Transistor with Light Intensity-Dependent Active Photoadaptation. Nat. Electron 2021, 4, 522–529. [Google Scholar]
- (1008).Liao F; Zhou Z; Kim BJ; Chen J; Wang J; Wan T; Zhou Y; Hoang AT; Wang C; Kang J; et al. Bioinspired in-Sensor Visual Adaptation for Accurate Perception. Nat. Electron 2022, 5, 84–91. [Google Scholar]
- (1009).Mennel L; Symonowicz J; Wachter S; Polyushkin DK; Molina-Mendoza AJ; Mueller T Ultrafast Machine Vision with 2D Material Neural Network Image Sensors. Nature 2020, 579, 62–66. [DOI] [PubMed] [Google Scholar]
- (1010).Kaspar C; Ravoo BJ; van der Wiel WG; Wegner SV; Pernice WHP The Rise of Intelligent Matter. Nature 2021, 594, 345–355. [DOI] [PubMed] [Google Scholar]
- (1011).Yasuda H; Buskohl PR; Gillman A; Murphey TD; Stepney S; Vaia RA; Raney JR Mechanical Computing. Nature 2021, 598, 39–48. [DOI] [PubMed] [Google Scholar]
- (1012).Yu C; Guo H; Cui K; Li X; Ye YN; Kurokawa T; Gong JP Hydrogels as Dynamic Memory with Forgetting Ability. Proc. Natl. Acad. Sci. U. S. A 2020, 117, 18962–18968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (1013).Xia X; Spadaccini CM; Greer JR Responsive Materials Architected in Space and Time. Nat. Rev. Mater 2022, 7, 683–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (1014).Wehner M; Truby RL; Fitzgerald DJ; Mosadegh B; Whitesides GM; Lewis JA; Wood RJ An Integrated Design and Fabrication Strategy for Entirely Soft, Autonomous Robots. Nature 2016, 536, 451–455. [DOI] [PubMed] [Google Scholar]
- (1015).Jin Y; Lin Y; Kiani A; Joshipura ID; Ge M; Dickey MD Materials Tactile Logic via Innervated Soft Thermochromic Elastomers. Nat. Commun 2019, 10, 4187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (1016).Chae S; Choi WJ; Fotev I; Bittrich E; Uhlmann P; Schubert M; Makarov D; Wagner J; Pashkin A; Fery A Stretchable Thin Film Mechanical-Strain-Gated Switches and Logic Gate Functions Based on a Soft Tunneling Barrier. Adv. Mater 2021, 33, 2104769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (1017).El Helou C; Grossmann B; Tabor CE; Buskohl PR; Harne RL Mechanical Integrated Circuit Materials. Nature 2022, 608, 699–703. [DOI] [PubMed] [Google Scholar]
- (1018).Chen J; Zhu C; Cao G; Liu H; Bian R; Wang J; Li C; Chen J; Fu Q; Liu Q; et al. Mimicking Neuroplasticity via Ion Migration in van der Waals Layered Copper Indium Thiophosphate. Adv. Mater 2022, 34, 2104676. [DOI] [PubMed] [Google Scholar]
- (1019).Park HL; Lee Y; Kim N; Seo DG; Go GT; Lee TW Flexible Neuromorphic Electronics for Computing, Soft Robotics, and Neuroprosthetics. Adv. Mater 2020, 32, 1903558. [DOI] [PubMed] [Google Scholar]
- (1020).Ashtiani F; Geers AJ; Aflatouni F An On-Chip Photonic Deep Neural Network for Image Classification. Nature 2022, 606, 501–506. [DOI] [PubMed] [Google Scholar]
- (1021).Wang Z; Wu H; Burr GW; Hwang CS; Wang KL; Xia Q; Yang JJ Resistive Switching Materials for Information Processing. Nat. Rev. Mater 2020, 5, 173–195. [Google Scholar]
- (1022).Liu X; Cao J; Qiu J; Zhang X; Wang M; Liu Q Flexible and Stretchable Memristive Arrays for in-Memory Computing. Front. Nanotechnol 2022, 3, 821687. [Google Scholar]
- (1023).Torrejon J; Riou M; Araujo FA; Tsunegi S; Khalsa G; Querlioz D; Bortolotti P; Cros V; Yakushiji K; Fukushima A; et al. Neuromorphic Computing with Nanoscale Spintronic Oscillators. Nature 2017, 547, 428–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (1024).He Y; Jiang S; Chen C; Wan C; Shi Y; Wan Q Electrolyte-Gated Neuromorphic Transistors for Brain-Like Dynamic Computing. J. Appl. Phys 2021, 130, 190904. [Google Scholar]
- (1025).Seo D-G; Go G-T; Park H-L; Lee T-W Organic Synaptic Transistors for Flexible and Stretchable Artificial Sensory Nerves. MRS Bull. 2021, 46, 321–329. [Google Scholar]
- (1026).Liu L; Xu W; Ni Y; Xu Z; Cui B; Liu J; Wei H; Xu W Stretchable Neuromorphic Transistor That Combines Multisensing and Information Processing for Epidermal Gesture Recognition. ACS Nano 2022, 16, 2282–2291. [DOI] [PubMed] [Google Scholar]
- (1027).Liang K; Ren H; Wang Y; Li D; Tang Y; Song C; Chen Y; Li F; Wang H; Zhu B Tunable Plasticity in Printed Optoelectronic Synaptic Transistors by Contact Engineering. IEEE Electron Device Lett. 2022, 43, 882–885. [Google Scholar]
- (1028).Kireev D; Liu S; Jin H; Patrick Xiao T; Bennett CH; Akinwande D; Incorvia JAC Metaplastic and Energy-Efficient Biocompatible Graphene Artificial Synaptic Transistors for Enhanced Accuracy Neuromorphic Computing. Nat. Commun 2022, 13, 4386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (1029).Dai S; Dai Y; Zhao Z; Xia F; Li Y; Liu Y; Cheng P; Strzalka J; Li S; Li N; et al. Intrinsically Stretchable Neuromorphic Devices for On-Body Processing of Health Data with Artificial Intelligence. Matter 2022, 5, 3375–3390. [Google Scholar]
- (1030).Sarwat SG; Kersting B; Moraitis T; Jonnalagadda VP; Sebastian A Phase-Change Memtransistive Synapses for Mixed-Plasticity Neural Computations. Nat. Nanotechnol 2022, 17, 507–513. [DOI] [PubMed] [Google Scholar]
- (1031).Tsai S-H; Fang Z; Wang X; Chand U; Chen C-K; Hooda S; Sivan M; Pan J; Zamburg E; Thean AV-Y Stress-Memorized HZO for High-Performance Ferroelectric Field-Effect Memtransistor. ACS Appl. Electron. Mater 2022, 4, 1642–1650. [Google Scholar]
- (1032).Li C; Hu M; Li Y; Jiang H; Ge N; Montgomery E; Zhang J; Song W; Dávila N; Graves CE; et al. Analogue Signal and Image Processing with Large Memristor Crossbars. Nat. Electron 2018, 1, 52–59. [Google Scholar]
- (1033).Wang R; Shi T; Zhang X; Wei J; Lu J; Zhu J; Wu Z; Liu Q; Liu M Implementing in-Situ Self-Organizing Maps with Memristor Crossbar Arrays for Data Mining and Optimization. Nat. Commun 2022, 13, 2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (1034).Oh S; Cho J-I; Lee BH; Seo S; Lee J-H; Choo H; Heo K; Lee SY; Park J-H Flexible Artificial Si-in-Zn-O/Ion Gel Synapse and Its Application to Sensory-Neuromorphic System for Sign Language Translation. Sci. Adv 2021, 7, No. eabg9450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (1035).Fuller EJ; Keene ST; Melianas A; Wang Z; Agarwal S; Li Y; Tuchman Y; James CD; Marinella MJ; et al. Parallel Programming of an Ionic Floating-Gate Memory Array for Scalable Neuromorphic Computing. Science 2019, 364, 570–574. [DOI] [PubMed] [Google Scholar]
- (1036).Abnavi A; Ahmadi R; Hasani A; Fawzy M; Mohammadzadeh MR; De Silva T; Yu N; Adachi MM Free-Standing Multilayer Molybdenum Disulfide Memristor for Brain-Inspired Neuromorphic Applications. ACS Appl. Mater. Interfaces 2021, 13, 45843–45853. [DOI] [PubMed] [Google Scholar]
- (1037).Li G; Xie D; Zhong H; Zhang Z; Fu X; Zhou Q; Li Q; Ni H; Wang J; Guo EJ; et al. Photo-Induced Non-Volatile VO2 Phase Transition for Neuromorphic Ultraviolet Sensors. Nat. Commun 2022, 13, 1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (1038).Kim D; Lee JS Neurotransmitter-Induced Excitatory and Inhibitory Functions in Artificial Synapses. Adv. Funct. Mater 2022, 32, 2200497. [Google Scholar]
- (1039).Wang T; Wang M; Wang J; Yang L; Ren X; Song G; Chen S; Yuan Y; Liu R; Pan L; et al. A Chemically Mediated Artificial Neuron. Nat. Electron 2022, 5, 586–595. [Google Scholar]
- (1040).Keene ST; Lubrano C; Kazemzadeh S; Melianas A; Tuchman Y; Polino G; Scognamiglio P; Cina L; Salleo A; van de Burgt Y; et al. A Biohybrid Synapse with Neurotransmitter-Mediated Plasticity. Nat. Mater 2020, 19, 969–973. [DOI] [PubMed] [Google Scholar]
- (1041).Qiu J; Cao J; Liu X; Chen P; Feng G; Zhang X; Wang M; Liu Q A Flexible Organic Electrochemical Synaptic Transistor with Dopamine-Mediated Plasticity. IEEE Electron Device Lett. 2023, 44, 176–179. [Google Scholar]
- (1042).Zhu Y; He Y; Chen C; Zhu L; Wan C; Wan Q IGZO-Based Neuromorphic Transistors with Temperature-Dependent Synaptic Plasticity and Spiking Logics. Sci. China Inf. Sci 2022, 65, 1–8. [Google Scholar]
- (1043).Zhou F; Zhou Z; Chen J; Choy TH; Wang J; Zhang N; Lin Z; Yu S; Kang J; Wong HP; et al. Optoelectronic Resistive Random Access Memory for Neuromorphic Vision Sensors. Nat. Nanotechnol 2019, 14, 776–782. [DOI] [PubMed] [Google Scholar]
- (1044).Seo D-G; Lee Y; Go G-T; Pei M; Jung S; Jeong YH; Lee W; Park H-L; Kim S-W; Yang H; et al. Versatile Neuromorphic Electronics by Modulating Synaptic Decay of Single Organic Synaptic Transistor: From Artificial Neural Networks to Neuro-Prosthetics. Nano Energy 2019, 65, 104035. [Google Scholar]
- (1045).Harikesh PC; Yang CY; Tu D; Gerasimov JY; Dar AM; Armada-Moreira A; Massetti M; Kroon R; Bliman D; Olsson R; et al. Organic Electrochemical Neurons and Synapses with Ion Mediated Spiking. Nat. Commun 2022, 13, 901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (1046).Beck ME; Shylendra A; Sangwan VK; Guo S; Gaviria Rojas WA; Yoo H; Bergeron H; Su K; Trivedi AR; Hersam MC Spiking Neurons from Tunable Gaussian Heterojunction Transistors. Nat. Commun 2020, 11, 1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (1047).Subbulakshmi Radhakrishnan S; Sebastian A; Oberoi A; Das S; Das S A Biomimetic Neural Encoder for Spiking Neural Network. Nat. Commun 2021, 12, 2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (1048).Chen C; He Y; Mao H; Zhu L; Wang X; Zhu Y; Zhu Y; Shi Y; Wan C; Wan Q A Photoelectric Spiking Neuron for Visual Depth Perception. Adv. Mater 2022, 34, 2201895. [DOI] [PubMed] [Google Scholar]
- (1049).Han JK; Kang M; Jeong J; Cho I; Yu JM; Yoon KJ; Park I; Choi YK Artificial Olfactory Neuron for an in-Sensor Neuromorphic Nose. Adv. Sci 2022, 9, 2106017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (1050).Xie Z; Zhu X; Wang W; Guo Z; Zhang Y; Liu H; Sun C; Tang M; Gao S; Li RW Temporal Pattern Coding in Ionic Memristor-Based Spiking Neurons for Adaptive Tactile Perception. Adv. Electron. Mater 2022, 8, 2200334. [Google Scholar]
- (1051).Sarkar T; Lieberth K; Pavlou A; Frank T; Mailaender V; McCulloch I; Blom PWM; Torricelli F; Gkoupidenis P An Organic Artificial Spiking Neuron for in Situ Neuromorphic Sensing and Biointerfacing. Nat. Electron 2022, 5, 774–783. [Google Scholar]
- (1052).Zhang H-T; Park Tae J; Islam ANMN; Tran Dat SJ; Manna S; Wang Q; Mondal S; Yu H; Banik S; Cheng S; et al. Reconfigurable Perovskite Nickelate Electronics for Artificial Intelligence. Science 2022, 375, 533–539. [DOI] [PubMed] [Google Scholar]
- (1053).Wan C; Cai P; Wang M; Qian Y; Huang W; Chen X Artificial Sensory Memory. Adv. Mater 2020, 32, 1902434. [DOI] [PubMed] [Google Scholar]
- (1054).Yu H; Zhu Y; Zhu L; Lin X; Wan Q Recent Advances in Transistor-Based Bionic Perceptual Devices for Artificial Sensory Systems. Front. Nanotechnol 2022, 4, 954165. [Google Scholar]
- (1055).Kim Y; Chortos A; Xu W; Liu Y; Oh Jin Y; Son D; Kang J; Foudeh Amir M; Zhu C; Lee Y; et al. A Bioinspired Flexible Organic Artificial Afferent Nerve. Science 2018, 360, 998–1003. [DOI] [PubMed] [Google Scholar]
- (1056).Wan C; Chen G; Fu Y; Wang M; Matsuhisa N; Pan S; Pan L; Yang H; Wan Q; Zhu L; et al. An Artificial Sensory Neuron with Tactile Perceptual Learning. Adv. Mater 2018, 30, 1801291. [DOI] [PubMed] [Google Scholar]
- (1057).Kim SH; Baek GW; Yoon J; Seo S; Park J; Hahm D; Chang JH; Seong D; Seo H; Oh S; et al. A Bioinspired Stretchable Sensory-Neuromorphic System. Adv. Mater 2021, 33, 2104690. [DOI] [PubMed] [Google Scholar]
- (1058).Liu F; Deswal S; Christou A; Sandamirskaya Y; Kaboli M; Dahiya R Neuro-Inspired Electronic Skin for Robots. Sci. Robot 2022, 7, No. eabl7344. [DOI] [PubMed] [Google Scholar]
- (1059).Lee TJ; Yun KR; Kim SK; Kim JH; Jin J; Sim KB; Lee DH; Hwang GW; Seong TY Realization of an Artificial Visual Nervous System Using an Integrated Optoelectronic Device Array. Adv. Mater 2021, 33, 2105485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (1060).Shim H; Jang S; Thukral A; Jeong S; Jo H; Kan B; Patel S; Wei G; Lan W; Kim HJ; et al. Artificial Neuromorphic Cognitive Skins Based on Distributed Biaxially Stretchable Elastomeric Synaptic Transistors. Proc. Natl. Acad. Sci. U. S. A 2022, 119, No. e2204852119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (1061).Wan C; Cai P; Guo X; Wang M; Matsuhisa N; Yang L; Lv Z; Luo Y; Loh XJ; Chen X An Artificial Sensory Neuron with Visual-Haptic Fusion. Nat. Commun 2020, 11, 4602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (1062).Wan H; Zhao J; Lo LW; Cao Y; Sepulveda N; Wang C Multimodal Artificial Neurological Sensory-Memory System Based on Flexible Carbon Nanotube Synaptic Transistor. ACS Nano 2021, 15, 14587–14597. [DOI] [PubMed] [Google Scholar]
- (1063).Han JK; Park SC; Yu JM; Ahn JH; Choi YK A Bioinspired Artificial Gustatory Neuron for a Neuromorphic Based Electronic Tongue. Nano Lett. 2022, 22, 5244–5251. [DOI] [PubMed] [Google Scholar]
- (1064).He K; Liu Y; Wang M; Chen G; Jiang Y; Yu J; Wan C; Qi D; Xiao M; Leow WR; et al. An Artificial Somatic Reflex Arc. Adv. Mater 2020, 32, 1905399. [DOI] [PubMed] [Google Scholar]
- (1065).Sun L; Du Y; Yu H; Wei H; Xu W; Xu W An Artificial Reflex Arc That Perceives Afferent Visual and Tactile Information and Controls Efferent Muscular Actions. Research 2022, 2022, 9851843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (1066).Lee Y; Liu Y; Seo DG; Oh JY; Kim Y; Li J; Kang J; Kim J; Mun J; Foudeh AM; et al. A Low-Power Stretchable Neuromorphic Nerve with Proprioceptive Feedback. Nat. Biomed. Eng 2022, 6, 1085. [DOI] [PubMed] [Google Scholar]
- (1067).Jo SH; Chang T; Ebong I; Bhadviya BB; Mazumder P; Lu W Nanoscale Memristor Device as Synapse in Neuromorphic Systems. Nano Lett. 2010, 10, 1297–1301. [DOI] [PubMed] [Google Scholar]
- (1068).Zhang M; Tang Z; Liu X; Van der Spiegel J Electronic Neural Interfaces. Nat. Electron 2020, 3, 191–200. [Google Scholar]
- (1069).Lee Y; Oh JY; Xu W; Kim O; Kim TR; Kang J; Kim Y; Son D; Tok JBH; Park MJ; et al. Stretchable Organic Optoelectronic Sensorimotor Synapse. Sci. Adv 2018, 4, No. eaat7387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (1070).Rus D; Tolley MT Design, Fabrication and Control of Soft Robots. Nature 2015, 521, 467–475. [DOI] [PubMed] [Google Scholar]
- (1071).Chun S; Kim J-S; Yoo Y; Choi Y; Jung SJ; Jang D; Lee G; Song K-I; Nam KS; Youn I; et al. An Artificial Neural Tactile Sensing System. Nat. Electron 2021, 4, 429–438. [Google Scholar]
- (1072).Wang J; Wang C; Cai P; Luo Y; Cui Z; Loh XJ; Chen X Artificial Sense Technology: Emulating and Extending Biological Senses. ACS Nano 2021, 15, 18671–18678. [DOI] [PubMed] [Google Scholar]
- (1073).Zhu S; Li Y; Yelemulati H; Deng X; Li Y; Wang J; Li X; Li G; Gkoupidenis P; Tai Y An Artificial Remote Tactile Device with 3D Depth-of-Field Sensation. Sci. Adv 2022, 8, No. eabo5314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (1074).Xie L; Zhang Z; Wu Q; Gao Z; Mi G; Wang R; Sun HB; Zhao Y; Du Y Intelligent Wearable Devices Based on Nanomaterials and Nanostructures for Healthcare. Nanoscale 2023, 15, 405–433. [DOI] [PubMed] [Google Scholar]
- (1075).Piroozmand F; Mohammadipanah F; Faridbod F Emerging Biosensors in Detection of Natural Products. Synth. Syst. Biotechnol 2020, 5, 293–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (1076).Alizadeh N; Salimi A Ultrasensitive Bioaffinity Electrochemical Sensors: Advances and New Perspectives. Electroanal. 2018, 30, 2803–2840. [Google Scholar]
- (1077).Bhalla N; Jolly P; Formisano N; Estrela P Introduction to Biosensors. Essays Biochem. 2016, 60, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (1078).Barreiros dos Santos M; Rodriguez-Lorenzo L; Queirós R; Espiña B Fundamentals of Biosensors and Detection Methods. In Microfluidics and Biosensors in Cancer Research: Applications in Cancer Modeling and Theranostics, Caballero D; Kundu SC; Reis RL Eds.; Advances in Experimental Medicine and Biology; Springer International Publishing, 2022; pp 3–29, Vol. 1379. [DOI] [PubMed] [Google Scholar]
- (1079).Liu H; Ge J; Ma E; Yang L Advanced Biomaterials for Biosensor and Theranostics. In Biomaterials in Translational Medicine, Yang L, Bhaduri SB, Webster TJ Eds.; Academic Press, 2019; pp 213–255. [Google Scholar]
- (1080).Lin W; Wang B; Peng G; Shan Y; Hu H; Yang Z Skin-Inspired Piezoelectric Tactile Sensor Array with Crosstalk-Free Row+Column Electrodes for Spatiotemporally Distinguishing Diverse Stimuli. Adv. Sci 2021, 8, 2002817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (1081).Dowling NE Mechanical Behavior of Materials: Engineering Methods for Deformation, Fracture, and Fatigue; Pearson Education, 2013. [Google Scholar]
- (1082).Heterogeneous Integration Roadmap. IEEE, 2017. https://eps.ieee.org/technology/heterogeneous-integration-roadmap.html (accessed 2022-11-15). [Google Scholar]
- (1083).Zadpoor AA Mechanical Meta-Materials. Mater. Horiz 2016, 3, 371–381. [Google Scholar]
- (1084).Bertoldi K; Vitelli V; Christensen J; Van Hecke M Flexible Mechanical Metamaterials. Nat. Rev. Mater 2017, 2, 1–11. [Google Scholar]
- (1085).Li L; Pan L; Ma Z; Yan K; Cheng W; Shi Y; Yu G All Inkjet-Printed Amperometric Multiplexed Biosensors Based on Nano-structured Conductive Hydrogel Electrodes. Nano Lett. 2018, 18, 3322–3327. [DOI] [PubMed] [Google Scholar]
- (1086).Hu W-L; Akash K; Jain N; Reid T Real-Time Sensing of Trust in Human-Machine Interactions. IFAC-PapersOnLine 2016, 49, 48–53. [Google Scholar]
- (1087).Héder M. From NASA to EU: The Evolution of the TRL Scale in Public Sector Innovation. Innov. J 2017, 22, 1–23. [Google Scholar]