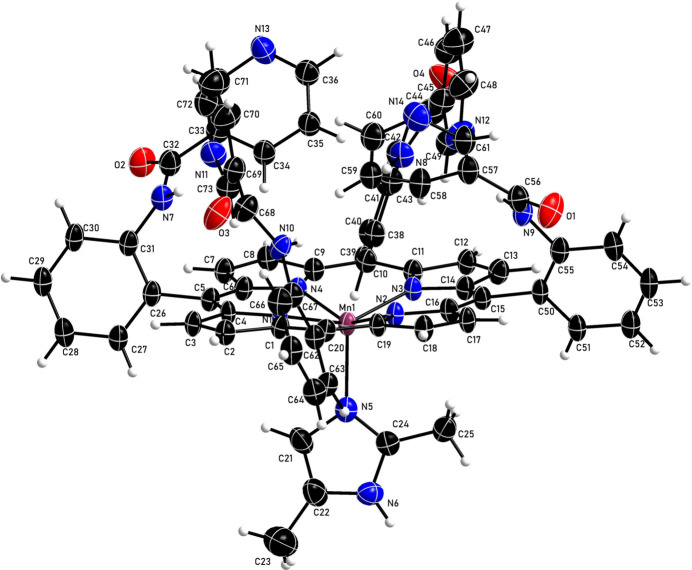
In the title compound, the central MnII ion is coordinated by four pyrrole N atoms of the porphyrin core in the basal sites and one N atom of the 2,5-dimethylimidazole ligand in the apical site. Two chlorobenzene solvent molecules are also present in the asymmetric unit.
Keywords: crystal structure, porphyrin derivative, hydrogen bonds
Abstract
In the title compound, [Mn(C68H44N12O4)(C5H8N2)]·2C6H5Cl, the central MnII ion is coordinated by four pyrrole N atoms of the porphyrin core in the basal sites and one N atom of the 2,5-dimethylimidazole ligand in the apical site. Two chlorobenzene solvent molecules are also present in the asymmetric unit. Due to the apical imidazole ligand, the Mn atom is displaced out of the 24-atom porphyrin mean plane by 0.66 Å. The average Mn—Np (p = porphyrin) bond length is 2.143 (8) Å, and the axial Mn—NIm (Im = 2,5-dimethylimidazole) bond length is 2.171 (8) Å. The structure displays intermolecular and intramolecular N—H⋯O, N—H⋯N, C—H⋯O and C—H⋯N hydrogen bonding. The crystal studied was refined as a two-component inversion twin.
Structure description
Metalloporphyrins combined with imidazole(ate) ligands have long been utilized to replicate metalloenzymes, specifically five-coordinate heme complexes (Liang et al., 2023 ▸; Yu et al., 2015 ▸; Yao et al., 2019 ▸; Krishna Deepak & Sankararamakrishnan, 2016 ▸). Imidazole and imidazolates have been extensively employed as axial ligands to imitate histidine residues, which also possess a five-membered ring and play significant roles in the properties and functions of hemoproteins (Nappa et al., 1977 ▸). The first imidazole manganese porphyrin adduct, [Mn(TPP)(1-MeIm)], (TPP = 5,10,15,20-tetraphenylporphyrin, 1-MeIm = 1-methylimidazole) was documented by Scheidt and colleagues in 1977 (Kirner et al., 1977 ▸). Subsequently, in 1980, Reed and coworkers reported the first imidazolate manganese porphyrin adduct (Landrum et al., 1980 ▸). In this study, the synthesis and crystal structure of the title manganese(II) porphyrin solvated complex, [Mn(C68H44N12O4)(C5H8N2)]·2C6H5Cl, is presented.
The asymmetric unit of the title compound contains one (2,5-dimethylimidazole){N,N′,N′′,N′′′-[porphyrin-5,10,15,20-tetrayltetra(2,1-phenylene)]tetrakis(pyridine-3-carboxamide)}manganese(II) molecule and two chlorobenzene solvate molecules. As illustrated in Fig. 1 ▸, the metal atom exhibits a five-coordinate structure (Table 1 ▸) with a significant metal out-of-plane displacement of 0.66 Å, indicative of the high-spin state of MnII. Additional quantitative information on the structure is provided in supplementary Fig. 1 ▸, presenting the displacements of each porphyrin core atom from the 24-atom mean plane. Averaged values of the chemically unique bond lengths (Å) and angles (°) are also displayed. The hindered 2,5-dimethylimidazole ligand may also contribute to the large out-of-plane displacement for the metal atom. The dihedral angle formed by the 2,5-dimethylimidazole axial ligand plane and the closest Mn—Np vector is 37.3°. The average Np—Mn—Np angle is 86.0 (7)° and the axial Mn—NIm bond length is 2.171 (8) Å. The average Mn—Np distance of 2.143 (8) Å is a typical value for high-spin manganese porphyrin derivatives.
Figure 1.
The molecular structure of the title compound with displacement ellipsoids drawn at the 50% probability level. The solvent molecules have been omitted for clarity.
Table 1. Selected geometric parameters (Å, °).
| Mn1—N1 | 2.138 (6) | Mn1—N4 | 2.154 (6) |
| Mn1—N2 | 2.141 (6) | Mn1—N5 | 2.171 (8) |
| Mn1—N3 | 2.141 (6) | ||
| N1—Mn1—N2 | 86.9 (2) | N2—Mn1—N5 | 101.4 (3) |
| N1—Mn1—N3 | 150.0 (3) | N3—Mn1—N2 | 85.3 (2) |
| N1—Mn1—N4 | 85.5 (2) | N3—Mn1—N4 | 86.4 (2) |
| N1—Mn1—N5 | 98.1 (3) | N3—Mn1—N5 | 111.8 (3) |
| N2—Mn1—N4 | 148.9 (3) | N4—Mn1—N5 | 109.5 (3) |
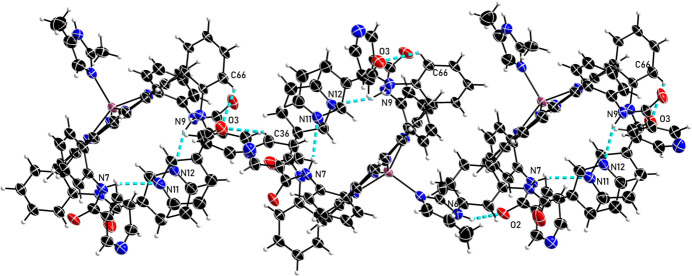
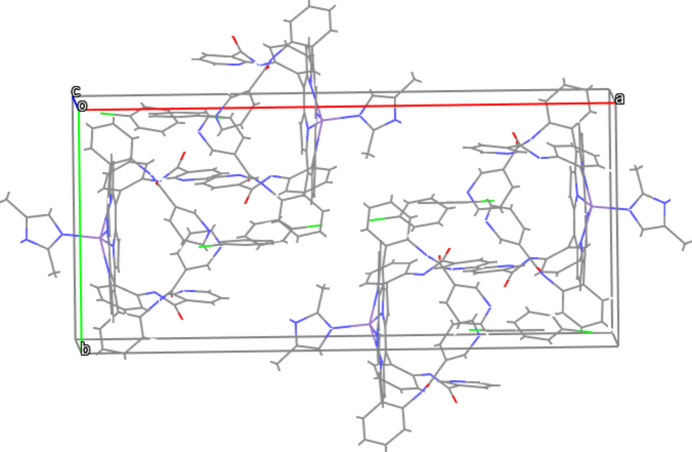
Several intra- and inter-molecular interactions are identified in the title compound (Table 2 ▸, Fig. 2 ▸): the distances between N7 and N11, N9 and N12, C42 and O4 are 3.098 (12), 3.011 (11) and 2.880 (13) Å, respectively. The distance between N6 and O2, as well as the N6—H6⋯O2 angle, are found to be 2.856 (12) Å and 153°, respectively, consistent with the N—H⋯O interaction criteria of 2.7 < N⋯O < 3.05 Å and N—H⋯O > 130° (Landrum et al., 1980 ▸). The molecular packing is shown in Fig. 3 ▸.
Table 2. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| N6—H6⋯O2i | 0.88 | 2.05 | 2.858 (11) | 153 |
| N7—H7A⋯N11 | 0.88 | 2.24 | 3.094 (11) | 164 |
| N9—H9⋯N12 | 0.88 | 2.17 | 3.009 (10) | 159 |
| C5S—H5S⋯N14ii | 0.95 | 2.57 | 3.421 (16) | 150 |
| C36—H36⋯O3ii | 0.95 | 2.35 | 2.99 (2) | 124 |
| C60—H60⋯O4iii | 0.95 | 2.40 | 3.062 (18) | 126 |
Symmetry codes: (i) 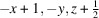 ; (ii)
; (ii) 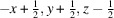 ; (iii)
; (iii) 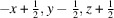 .
.
Figure 2.
Intra- and inter-molecular interactions in the crystal structure of the title compound.
Figure 3.
A view of the packing of the title compound. H atoms have been omitted for clarity.
Synthesis and crystallization
All experimental manipulations in this work were conducted under an argon atmosphere using a double-manifold vacuum line, Schlenkware and cannula techniques. With the exception of the solvent used in column chromatography, all solvents utilized in the experimental procedures were subjected to anhydrous and anaerobic conditions. Chlorobenzene, benzene and n-hexane were distilled over P2O5 and potassium–sodium alloy, respectively. All solvents employed in the anhydrous and anaerobic operations (Schlenk system) underwent the freeze–pump–thaw method three times before use. The precursors H2(TPyPP), [Mn(TPyPP)]Cl, and [Mn(TPyPP)]OH were prepared following literature methods (Gunter et al., 1984 ▸), with slight modifications.
[Mn(TPyPP)]OH (10 mg) was dried under vacuum for 30 minutes and dissolved in 5 ml of benzene. After adding 1 ml of ethanethiol, the solution was stirred for 1 day and then evacuated under vacuum to yield a purple powder. The resulting purple solid of [Mn(TPyPP)] (10 mg) was dried for 60 minutes, and excess 2,5-dimethylimidazole in PhCl (5 ml) was added using a cannula. The mixture was stirred for 1 h and transferred into glass tubes, which were layered with n-hexane as a non-polar solvent. Several weeks later, X-ray quality crystals of the title compound in the form of black blocks were collected.
Refinement
Crystal data, data collection and structure refinement details are summarized in Table 3 ▸. The crystal studied was refined as a 2-component inversion twin.
Table 3. Experimental details.
| Crystal data | |
| Chemical formula | [Mn(C68H44N12O4)(C5H8N2)]·2C6H5Cl |
| M r | 1469.32 |
| Crystal system, space group | Orthorhombic, Pna21 |
| Temperature (K) | 101 |
| a, b, c (Å) | 30.247 (4), 13.713 (2), 17.205 (2) |
| V (Å3) | 7136.2 (16) |
| Z | 4 |
| Radiation type | Mo Kα |
| μ (mm−1) | 0.33 |
| Crystal size (mm) | 0.61 × 0.55 × 0.35 |
| Data collection | |
| Diffractometer | Bruker APEXII CCD |
| Absorption correction | Multi-scan (SADABS; Krause et al., 2015 ▸) |
| Tmin, Tmax | 0.568, 0.745 |
| No. of measured, independent and observed [I > 2σ(I)] reflections | 142482, 14653, 12171 |
| R int | 0.071 |
| (sin θ/λ)max (Å−1) | 0.629 |
| Refinement | |
| R[F2 > 2σ(F2)], wR(F2), S | 0.080, 0.240, 1.06 |
| No. of reflections | 14653 |
| No. of parameters | 946 |
| No. of restraints | 1 |
| H-atom treatment | H-atom parameters constrained |
| Δρmax, Δρmin (e Å−3) | 0.98, −1.03 |
| Absolute structure | Refined as an inversion twin. |
| Absolute structure parameter | 0.41 (4) |
Supplementary Material
Crystal structure: contains datablock(s) I. DOI: 10.1107/S2414314624004978/hb4469sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2414314624004978/hb4469Isup2.hkl
Porphyrin ring displacement data. DOI: 10.1107/S2414314624004978/hb4469sup3.docx
CCDC reference: 2358337
Additional supporting information: crystallographic information; 3D view; checkCIF report
full crystallographic data
(2,5-Dimethylimidazole){N,N',N'',N'''-[porphyrin-5,10,15,20-tetrayltetra(2,1-phenylene)]tetrakis(pyridine-3-carboxamide)}manganese(II) chlorobenzene disolvate . Crystal data
| [Mn(C68H44N12O4)(C5H8N2)]·2C6H5Cl | Dx = 1.368 Mg m−3 |
| Mr = 1469.32 | Mo Kα radiation, λ = 0.71073 Å |
| Orthorhombic, Pna21 | Cell parameters from 9672 reflections |
| a = 30.247 (4) Å | θ = 2.5–26.3° |
| b = 13.713 (2) Å | µ = 0.33 mm−1 |
| c = 17.205 (2) Å | T = 101 K |
| V = 7136.2 (16) Å3 | Block, black |
| Z = 4 | 0.61 × 0.55 × 0.35 mm |
| F(000) = 3044 |
(2,5-Dimethylimidazole){N,N',N'',N'''-[porphyrin-5,10,15,20-tetrayltetra(2,1-phenylene)]tetrakis(pyridine-3-carboxamide)}manganese(II) chlorobenzene disolvate . Data collection
| Bruker APEXII CCD diffractometer | 12171 reflections with I > 2σ(I) |
| φ and ω scans | Rint = 0.071 |
| Absorption correction: multi-scan (SADABS; Krause et al., 2015) | θmax = 26.6°, θmin = 2.0° |
| Tmin = 0.568, Tmax = 0.745 | h = −37→37 |
| 142482 measured reflections | k = −17→17 |
| 14653 independent reflections | l = −21→21 |
(2,5-Dimethylimidazole){N,N',N'',N'''-[porphyrin-5,10,15,20-tetrayltetra(2,1-phenylene)]tetrakis(pyridine-3-carboxamide)}manganese(II) chlorobenzene disolvate . Refinement
| Refinement on F2 | Hydrogen site location: inferred from neighbouring sites |
| Least-squares matrix: full | H-atom parameters constrained |
| R[F2 > 2σ(F2)] = 0.080 | w = 1/[σ2(Fo2) + (0.1079P)2 + 24.1645P] where P = (Fo2 + 2Fc2)/3 |
| wR(F2) = 0.240 | (Δ/σ)max < 0.001 |
| S = 1.06 | Δρmax = 0.98 e Å−3 |
| 14653 reflections | Δρmin = −1.03 e Å−3 |
| 946 parameters | Absolute structure: Refined as an inversion twin. |
| 1 restraint | Absolute structure parameter: 0.41 (4) |
| Primary atom site location: dual |
(2,5-Dimethylimidazole){N,N',N'',N'''-[porphyrin-5,10,15,20-tetrayltetra(2,1-phenylene)]tetrakis(pyridine-3-carboxamide)}manganese(II) chlorobenzene disolvate . Special details
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
| Refinement. Refined as a 2-component inversion twin |
(2,5-Dimethylimidazole){N,N',N'',N'''-[porphyrin-5,10,15,20-tetrayltetra(2,1-phenylene)]tetrakis(pyridine-3-carboxamide)}manganese(II) chlorobenzene disolvate . Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| Mn1 | 0.45525 (4) | 0.06614 (8) | 0.17877 (7) | 0.0281 (3) | |
| N1 | 0.4348 (2) | −0.0793 (4) | 0.2054 (4) | 0.0262 (13) | |
| N2 | 0.4298 (2) | 0.1066 (5) | 0.2903 (4) | 0.0279 (13) | |
| N3 | 0.4393 (2) | 0.2123 (5) | 0.1452 (4) | 0.0289 (13) | |
| N4 | 0.4429 (2) | 0.0271 (5) | 0.0595 (4) | 0.0271 (13) | |
| C4 | 0.4360 (3) | −0.1573 (5) | 0.1553 (4) | 0.0251 (15) | |
| C1 | 0.4293 (3) | −0.1147 (5) | 0.2795 (4) | 0.0270 (15) | |
| C19 | 0.4243 (2) | 0.0426 (5) | 0.3515 (4) | 0.0255 (14) | |
| C16 | 0.4268 (3) | 0.1998 (6) | 0.3193 (5) | 0.0300 (16) | |
| C14 | 0.4347 (3) | 0.2913 (5) | 0.1942 (4) | 0.0299 (16) | |
| C11 | 0.4395 (3) | 0.2489 (6) | 0.0699 (5) | 0.0287 (15) | |
| C9 | 0.4409 (3) | 0.0900 (6) | −0.0030 (4) | 0.0279 (15) | |
| C6 | 0.4424 (3) | −0.0655 (6) | 0.0300 (4) | 0.0292 (16) | |
| C3 | 0.4323 (3) | −0.2477 (5) | 0.2004 (4) | 0.0295 (16) | |
| H3 | 0.432566 | −0.312405 | 0.180631 | 0.035* | |
| C2 | 0.4283 (3) | −0.2206 (6) | 0.2763 (5) | 0.0318 (17) | |
| H2 | 0.425412 | −0.263366 | 0.319432 | 0.038* | |
| C18 | 0.4181 (3) | 0.0991 (6) | 0.4221 (4) | 0.0339 (18) | |
| H18 | 0.413455 | 0.073443 | 0.472722 | 0.041* | |
| C17 | 0.4200 (3) | 0.1945 (6) | 0.4031 (4) | 0.0321 (17) | |
| H17 | 0.417493 | 0.248084 | 0.437833 | 0.038* | |
| C13 | 0.4340 (3) | 0.3799 (6) | 0.1479 (5) | 0.0344 (17) | |
| H13 | 0.431639 | 0.444585 | 0.167184 | 0.041* | |
| C12 | 0.4372 (3) | 0.3535 (6) | 0.0724 (5) | 0.0335 (17) | |
| H12 | 0.437852 | 0.396232 | 0.028974 | 0.040* | |
| C8 | 0.4404 (3) | 0.0337 (6) | −0.0748 (5) | 0.0343 (18) | |
| H8 | 0.439129 | 0.059094 | −0.126106 | 0.041* | |
| C7 | 0.4419 (3) | −0.0621 (6) | −0.0543 (5) | 0.0337 (17) | |
| H7 | 0.442578 | −0.116402 | −0.088618 | 0.040* | |
| C5 | 0.4391 (3) | −0.1523 (6) | 0.0742 (4) | 0.0286 (16) | |
| C20 | 0.4238 (3) | −0.0582 (6) | 0.3470 (4) | 0.0279 (15) | |
| C15 | 0.4287 (3) | 0.2852 (6) | 0.2751 (4) | 0.0287 (16) | |
| C10 | 0.4390 (3) | 0.1915 (5) | 0.0011 (5) | 0.0285 (15) | |
| C38 | 0.4317 (3) | 0.2450 (6) | −0.0742 (4) | 0.0328 (17) | |
| C43 | 0.3902 (3) | 0.2874 (6) | −0.0896 (5) | 0.0346 (17) | |
| C42 | 0.3823 (3) | 0.3348 (7) | −0.1599 (5) | 0.041 (2) | |
| H42 | 0.354400 | 0.363857 | −0.170375 | 0.049* | |
| C41 | 0.4162 (4) | 0.3385 (7) | −0.2143 (5) | 0.043 (2) | |
| H41 | 0.411037 | 0.370699 | −0.262340 | 0.052* | |
| C40 | 0.4568 (3) | 0.2973 (7) | −0.2011 (5) | 0.041 (2) | |
| H40 | 0.479286 | 0.299728 | −0.239652 | 0.050* | |
| C39 | 0.4645 (3) | 0.2518 (6) | −0.1300 (5) | 0.0370 (18) | |
| H39 | 0.492859 | 0.224862 | −0.119639 | 0.044* | |
| C26 | 0.4362 (3) | −0.2455 (6) | 0.0290 (4) | 0.0299 (16) | |
| C31 | 0.3979 (3) | −0.2690 (6) | −0.0133 (5) | 0.0333 (17) | |
| C30 | 0.3956 (3) | −0.3541 (6) | −0.0573 (5) | 0.0387 (19) | |
| H30 | 0.369625 | −0.368807 | −0.085973 | 0.046* | |
| C29 | 0.4311 (3) | −0.4166 (6) | −0.0592 (5) | 0.041 (2) | |
| H29 | 0.429615 | −0.474211 | −0.089785 | 0.049* | |
| C28 | 0.4693 (4) | −0.3962 (6) | −0.0166 (5) | 0.043 (2) | |
| H28 | 0.493690 | −0.439754 | −0.018096 | 0.052* | |
| C27 | 0.4714 (3) | −0.3119 (6) | 0.0279 (5) | 0.0342 (17) | |
| H27 | 0.497053 | −0.299127 | 0.058142 | 0.041* | |
| C62 | 0.4133 (3) | −0.1143 (5) | 0.4194 (4) | 0.0289 (16) | |
| C67 | 0.3725 (3) | −0.1613 (6) | 0.4269 (4) | 0.0318 (17) | |
| C66 | 0.3630 (3) | −0.2179 (7) | 0.4948 (5) | 0.041 (2) | |
| H66 | 0.335455 | −0.250347 | 0.500374 | 0.049* | |
| C65 | 0.3950 (3) | −0.2241 (6) | 0.5522 (5) | 0.040 (2) | |
| H65 | 0.389080 | −0.261811 | 0.597376 | 0.048* | |
| C64 | 0.4355 (3) | −0.1768 (7) | 0.5457 (5) | 0.039 (2) | |
| H64 | 0.457141 | −0.182661 | 0.585507 | 0.047* | |
| C63 | 0.4439 (3) | −0.1204 (6) | 0.4797 (5) | 0.0334 (17) | |
| H63 | 0.470918 | −0.085468 | 0.475856 | 0.040* | |
| C50 | 0.4216 (3) | 0.3793 (5) | 0.3174 (5) | 0.0304 (16) | |
| C55 | 0.3806 (3) | 0.4020 (6) | 0.3517 (5) | 0.0332 (17) | |
| C54 | 0.3749 (3) | 0.4881 (6) | 0.3924 (5) | 0.0395 (19) | |
| H54 | 0.347039 | 0.502786 | 0.414984 | 0.047* | |
| C53 | 0.4101 (4) | 0.5537 (6) | 0.4006 (5) | 0.043 (2) | |
| H53 | 0.406239 | 0.612243 | 0.429356 | 0.052* | |
| C52 | 0.4507 (3) | 0.5327 (6) | 0.3662 (5) | 0.041 (2) | |
| H52 | 0.474763 | 0.576813 | 0.371583 | 0.049* | |
| C51 | 0.4560 (3) | 0.4474 (6) | 0.3244 (5) | 0.0343 (18) | |
| H51 | 0.483525 | 0.434614 | 0.299801 | 0.041* | |
| C21 | 0.5500 (3) | −0.0324 (7) | 0.1896 (7) | 0.050 (2) | |
| H21 | 0.539801 | −0.088773 | 0.163089 | 0.059* | |
| C22 | 0.5911 (4) | −0.0208 (8) | 0.2188 (6) | 0.055 (3) | |
| C23 | 0.6282 (5) | −0.0939 (14) | 0.2290 (11) | 0.099 (5) | |
| H23A | 0.654723 | −0.070274 | 0.202121 | 0.148* | |
| H23B | 0.619323 | −0.156791 | 0.206977 | 0.148* | |
| H23C | 0.634676 | −0.101800 | 0.284452 | 0.148* | |
| C24 | 0.5516 (3) | 0.1122 (6) | 0.2425 (5) | 0.0347 (17) | |
| C25 | 0.5403 (3) | 0.2105 (7) | 0.2705 (6) | 0.044 (2) | |
| H25A | 0.565198 | 0.236989 | 0.300271 | 0.066* | |
| H25B | 0.514134 | 0.206798 | 0.303958 | 0.066* | |
| H25C | 0.534110 | 0.253002 | 0.226096 | 0.066* | |
| C44 | 0.3205 (3) | 0.3335 (7) | −0.0220 (5) | 0.042 (2) | |
| C45 | 0.2967 (3) | 0.3183 (7) | 0.0538 (5) | 0.0381 (19) | |
| C49 | 0.3200 (3) | 0.2978 (7) | 0.1214 (5) | 0.042 (2) | |
| H49 | 0.351180 | 0.291514 | 0.118252 | 0.051* | |
| C46 | 0.2518 (4) | 0.3300 (9) | 0.0590 (6) | 0.054 (3) | |
| H46 | 0.235080 | 0.347755 | 0.014461 | 0.064* | |
| C47 | 0.2312 (4) | 0.3157 (12) | 0.1291 (7) | 0.069 (4) | |
| H47 | 0.199932 | 0.321103 | 0.133299 | 0.082* | |
| C48 | 0.2562 (4) | 0.2938 (10) | 0.1924 (6) | 0.060 (3) | |
| H48 | 0.241461 | 0.283059 | 0.240442 | 0.072* | |
| C32 | 0.3453 (3) | −0.1570 (7) | −0.0738 (5) | 0.040 (2) | |
| C33 | 0.3165 (4) | −0.0722 (7) | −0.0565 (6) | 0.046 (2) | |
| C34 | 0.3268 (4) | −0.0108 (7) | 0.0064 (6) | 0.055 (3) | |
| H34 | 0.351483 | −0.025289 | 0.038401 | 0.065* | |
| C37 | 0.2820 (5) | −0.0483 (8) | −0.1046 (7) | 0.068 (4) | |
| H37 | 0.276962 | −0.085239 | −0.150433 | 0.081* | |
| C35 | 0.3018 (5) | 0.0688 (8) | 0.0218 (7) | 0.077 (5) | |
| H35 | 0.308722 | 0.110647 | 0.064047 | 0.093* | |
| C36 | 0.2662 (8) | 0.0871 (11) | −0.0256 (9) | 0.117 (8) | |
| H36 | 0.248724 | 0.143239 | −0.015163 | 0.141* | |
| C68 | 0.3074 (3) | −0.2106 (7) | 0.3468 (5) | 0.042 (2) | |
| C69 | 0.2886 (3) | −0.1948 (7) | 0.2677 (5) | 0.0401 (19) | |
| C73 | 0.3156 (3) | −0.1769 (6) | 0.2041 (5) | 0.0387 (19) | |
| H73 | 0.346403 | −0.169205 | 0.212802 | 0.046* | |
| C70 | 0.2435 (4) | −0.2042 (11) | 0.2552 (7) | 0.066 (3) | |
| H70 | 0.223972 | −0.218568 | 0.296836 | 0.079* | |
| C71 | 0.2279 (4) | −0.1921 (12) | 0.1808 (8) | 0.078 (4) | |
| H71 | 0.196986 | −0.194992 | 0.171200 | 0.094* | |
| C72 | 0.2571 (4) | −0.1758 (9) | 0.1193 (7) | 0.059 (3) | |
| H72 | 0.245625 | −0.168762 | 0.068217 | 0.071* | |
| C56 | 0.3202 (3) | 0.2986 (7) | 0.4006 (5) | 0.0395 (19) | |
| C57 | 0.2937 (3) | 0.2114 (6) | 0.3789 (5) | 0.0370 (18) | |
| C58 | 0.3101 (4) | 0.1405 (7) | 0.3272 (6) | 0.050 (2) | |
| H58 | 0.337908 | 0.149512 | 0.302639 | 0.060* | |
| C61 | 0.2534 (4) | 0.1957 (8) | 0.4125 (7) | 0.058 (3) | |
| H61 | 0.243320 | 0.241724 | 0.449769 | 0.070* | |
| C59 | 0.2858 (6) | 0.0588 (9) | 0.3128 (7) | 0.080 (5) | |
| H59 | 0.296744 | 0.009203 | 0.279320 | 0.096* | |
| C60 | 0.2464 (6) | 0.0501 (10) | 0.3467 (8) | 0.094 (6) | |
| H60 | 0.230164 | −0.007726 | 0.336369 | 0.113* | |
| C1S | 0.3496 (2) | 0.5649 (7) | 0.1213 (5) | 0.065 (3) | |
| H1S | 0.377136 | 0.566780 | 0.094747 | 0.078* | |
| C5S | 0.3103 (3) | 0.5676 (7) | 0.0795 (5) | 0.101 (6) | |
| H5S | 0.310965 | 0.571380 | 0.024379 | 0.121* | |
| C4S | 0.2700 (2) | 0.5649 (7) | 0.1184 (7) | 0.122 (9) | |
| C2S | 0.2691 (3) | 0.5594 (8) | 0.1990 (7) | 0.088 (5) | |
| H2S | 0.241542 | 0.557478 | 0.225562 | 0.106* | |
| C6S | 0.3084 (3) | 0.5566 (8) | 0.2408 (5) | 0.100 (6) | |
| H6S | 0.307712 | 0.552877 | 0.295932 | 0.120* | |
| C3S | 0.3486 (3) | 0.5594 (7) | 0.2020 (5) | 0.084 (5) | |
| H3S | 0.375509 | 0.557528 | 0.230526 | 0.101* | |
| C7S | 0.0972 (4) | 0.0367 (8) | 0.1522 (6) | 0.052 (2) | |
| C8S | 0.1144 (4) | 0.0883 (8) | 0.2146 (6) | 0.058 (3) | |
| H8S | 0.095431 | 0.111217 | 0.254540 | 0.069* | |
| C9S | 0.1574 (5) | 0.1054 (10) | 0.2181 (8) | 0.073 (4) | |
| H9S | 0.168861 | 0.144867 | 0.258808 | 0.088* | |
| C10S | 0.1236 (4) | −0.0035 (9) | 0.0967 (6) | 0.057 (3) | |
| H10S | 0.111343 | −0.041601 | 0.055922 | 0.068* | |
| C11S | 0.1681 (5) | 0.0125 (12) | 0.1011 (8) | 0.076 (4) | |
| H11S | 0.187073 | −0.012897 | 0.062139 | 0.091* | |
| C12S | 0.1858 (5) | 0.0665 (14) | 0.1633 (9) | 0.093 (5) | |
| H12S | 0.216837 | 0.076154 | 0.167499 | 0.111* | |
| Cl1S | 0.22099 (16) | 0.5730 (4) | 0.0715 (3) | 0.1114 (16) | |
| Cl2S | 0.04023 (12) | 0.0223 (4) | 0.1439 (2) | 0.0998 (15) | |
| N5 | 0.5252 (2) | 0.0496 (5) | 0.2041 (4) | 0.0356 (15) | |
| N6 | 0.5922 (3) | 0.0716 (6) | 0.2505 (5) | 0.0437 (18) | |
| H6 | 0.615329 | 0.099384 | 0.272268 | 0.052* | |
| N7 | 0.3617 (2) | −0.2033 (5) | −0.0110 (4) | 0.0355 (15) | |
| H7A | 0.349239 | −0.192075 | 0.034294 | 0.043* | |
| N8 | 0.3568 (3) | 0.2791 (5) | −0.0313 (4) | 0.0365 (15) | |
| H8A | 0.360605 | 0.232130 | 0.002861 | 0.044* | |
| N9 | 0.3452 (2) | 0.3352 (5) | 0.3425 (4) | 0.0347 (15) | |
| H9 | 0.339016 | 0.315991 | 0.294934 | 0.042* | |
| N10 | 0.3418 (2) | −0.1535 (5) | 0.3659 (4) | 0.0355 (15) | |
| H10 | 0.345720 | −0.102892 | 0.335286 | 0.043* | |
| N11 | 0.3005 (3) | −0.1697 (6) | 0.1306 (5) | 0.0476 (19) | |
| N12 | 0.3006 (3) | 0.2865 (7) | 0.1911 (4) | 0.0485 (19) | |
| N13 | 0.2545 (6) | 0.0296 (9) | −0.0866 (7) | 0.101 (5) | |
| N14 | 0.2268 (4) | 0.1181 (9) | 0.3960 (8) | 0.085 (4) | |
| O1 | 0.3205 (3) | 0.3298 (6) | 0.4678 (4) | 0.0564 (19) | |
| O2 | 0.3549 (2) | −0.1787 (5) | −0.1415 (4) | 0.0496 (17) | |
| O3 | 0.2929 (4) | −0.2738 (8) | 0.3903 (5) | 0.091 (4) | |
| O4 | 0.3066 (3) | 0.3919 (8) | −0.0691 (5) | 0.082 (3) |
(2,5-Dimethylimidazole){N,N',N'',N'''-[porphyrin-5,10,15,20-tetrayltetra(2,1-phenylene)]tetrakis(pyridine-3-carboxamide)}manganese(II) chlorobenzene disolvate . Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Mn1 | 0.0395 (6) | 0.0223 (5) | 0.0227 (5) | −0.0016 (4) | 0.0000 (5) | 0.0008 (5) |
| N1 | 0.041 (4) | 0.017 (3) | 0.021 (3) | −0.003 (2) | −0.002 (2) | −0.002 (2) |
| N2 | 0.043 (4) | 0.021 (3) | 0.020 (3) | −0.003 (3) | 0.003 (3) | 0.001 (2) |
| N3 | 0.044 (4) | 0.022 (3) | 0.021 (3) | 0.001 (3) | −0.003 (3) | 0.001 (3) |
| N4 | 0.039 (3) | 0.022 (3) | 0.020 (3) | 0.000 (3) | −0.001 (3) | 0.001 (2) |
| C4 | 0.041 (4) | 0.011 (3) | 0.023 (3) | 0.000 (3) | −0.001 (3) | 0.000 (3) |
| C1 | 0.035 (4) | 0.021 (4) | 0.025 (4) | −0.004 (3) | 0.001 (3) | 0.002 (3) |
| C19 | 0.034 (4) | 0.022 (3) | 0.020 (3) | 0.001 (3) | −0.001 (3) | −0.002 (3) |
| C16 | 0.045 (4) | 0.022 (4) | 0.023 (4) | 0.004 (3) | 0.001 (3) | 0.006 (3) |
| C14 | 0.042 (4) | 0.023 (3) | 0.025 (4) | −0.001 (3) | −0.002 (3) | −0.002 (3) |
| C11 | 0.039 (4) | 0.022 (4) | 0.026 (4) | 0.000 (3) | −0.001 (3) | 0.005 (3) |
| C9 | 0.034 (4) | 0.028 (4) | 0.021 (3) | 0.000 (3) | 0.001 (3) | 0.002 (3) |
| C6 | 0.041 (4) | 0.028 (4) | 0.019 (3) | −0.003 (3) | −0.003 (3) | −0.003 (3) |
| C3 | 0.048 (4) | 0.016 (3) | 0.025 (4) | −0.005 (3) | 0.002 (3) | 0.004 (3) |
| C2 | 0.045 (4) | 0.024 (4) | 0.026 (4) | −0.002 (3) | 0.002 (3) | 0.004 (3) |
| C18 | 0.057 (5) | 0.026 (4) | 0.019 (4) | 0.000 (3) | 0.003 (3) | 0.001 (3) |
| C17 | 0.055 (5) | 0.021 (4) | 0.019 (4) | 0.003 (3) | −0.002 (3) | −0.005 (3) |
| C13 | 0.044 (4) | 0.028 (4) | 0.031 (4) | −0.005 (3) | −0.004 (3) | 0.000 (3) |
| C12 | 0.049 (5) | 0.018 (4) | 0.034 (4) | −0.003 (3) | −0.003 (4) | 0.002 (3) |
| C8 | 0.050 (5) | 0.031 (4) | 0.022 (4) | 0.003 (4) | 0.000 (3) | 0.004 (3) |
| C7 | 0.048 (5) | 0.030 (4) | 0.024 (4) | −0.001 (3) | 0.001 (3) | −0.004 (3) |
| C5 | 0.036 (4) | 0.027 (4) | 0.023 (4) | −0.004 (3) | −0.001 (3) | 0.000 (3) |
| C20 | 0.034 (4) | 0.031 (4) | 0.019 (3) | −0.002 (3) | −0.001 (3) | 0.002 (3) |
| C15 | 0.039 (4) | 0.023 (4) | 0.024 (4) | 0.004 (3) | −0.003 (3) | 0.001 (3) |
| C10 | 0.038 (4) | 0.024 (4) | 0.024 (4) | −0.001 (3) | −0.002 (3) | 0.008 (3) |
| C38 | 0.053 (5) | 0.024 (4) | 0.021 (4) | −0.003 (3) | 0.000 (3) | 0.006 (3) |
| C43 | 0.048 (5) | 0.027 (4) | 0.028 (4) | −0.001 (3) | 0.001 (4) | 0.003 (3) |
| C42 | 0.053 (5) | 0.037 (5) | 0.032 (4) | 0.002 (4) | −0.002 (4) | 0.012 (4) |
| C41 | 0.068 (6) | 0.037 (5) | 0.025 (4) | −0.003 (4) | 0.000 (4) | 0.004 (3) |
| C40 | 0.056 (5) | 0.036 (4) | 0.032 (4) | −0.001 (4) | 0.006 (4) | 0.006 (4) |
| C39 | 0.045 (4) | 0.029 (4) | 0.038 (4) | 0.000 (3) | 0.005 (4) | 0.004 (3) |
| C26 | 0.042 (4) | 0.028 (4) | 0.020 (3) | −0.002 (3) | 0.002 (3) | −0.003 (3) |
| C31 | 0.050 (5) | 0.025 (4) | 0.025 (4) | −0.003 (3) | −0.005 (3) | −0.002 (3) |
| C30 | 0.059 (5) | 0.030 (4) | 0.027 (4) | −0.003 (4) | −0.004 (4) | −0.009 (3) |
| C29 | 0.065 (6) | 0.027 (4) | 0.032 (4) | −0.001 (4) | 0.002 (4) | −0.005 (3) |
| C28 | 0.070 (6) | 0.026 (4) | 0.033 (4) | 0.005 (4) | 0.005 (4) | −0.003 (3) |
| C27 | 0.047 (5) | 0.026 (4) | 0.030 (4) | −0.001 (3) | −0.001 (3) | 0.000 (3) |
| C62 | 0.044 (4) | 0.024 (4) | 0.019 (3) | 0.002 (3) | 0.000 (3) | 0.001 (3) |
| C67 | 0.042 (4) | 0.031 (4) | 0.022 (4) | −0.001 (3) | 0.003 (3) | 0.008 (3) |
| C66 | 0.053 (5) | 0.037 (4) | 0.032 (4) | −0.003 (4) | 0.006 (4) | 0.011 (4) |
| C65 | 0.068 (6) | 0.032 (4) | 0.021 (4) | −0.001 (4) | 0.003 (4) | 0.004 (3) |
| C64 | 0.061 (6) | 0.036 (4) | 0.021 (4) | 0.008 (4) | 0.000 (4) | 0.003 (3) |
| C63 | 0.042 (4) | 0.034 (4) | 0.024 (4) | −0.004 (3) | −0.003 (3) | 0.001 (3) |
| C50 | 0.048 (5) | 0.015 (3) | 0.028 (4) | 0.000 (3) | −0.001 (3) | 0.000 (3) |
| C55 | 0.046 (4) | 0.021 (3) | 0.032 (4) | −0.003 (3) | 0.001 (3) | 0.000 (3) |
| C54 | 0.059 (5) | 0.022 (4) | 0.037 (5) | 0.004 (4) | 0.010 (4) | −0.003 (3) |
| C53 | 0.070 (6) | 0.021 (4) | 0.038 (5) | −0.005 (4) | 0.008 (4) | −0.008 (3) |
| C52 | 0.061 (6) | 0.025 (4) | 0.036 (5) | −0.002 (4) | −0.002 (4) | 0.000 (3) |
| C51 | 0.050 (5) | 0.019 (4) | 0.034 (4) | −0.001 (3) | 0.001 (3) | 0.000 (3) |
| C21 | 0.060 (6) | 0.039 (4) | 0.050 (6) | 0.017 (4) | −0.009 (5) | −0.005 (4) |
| C22 | 0.065 (7) | 0.055 (6) | 0.043 (6) | 0.018 (5) | −0.007 (5) | −0.015 (5) |
| C23 | 0.064 (9) | 0.117 (14) | 0.115 (13) | 0.024 (9) | −0.013 (9) | −0.025 (11) |
| C24 | 0.046 (5) | 0.033 (4) | 0.025 (4) | 0.000 (3) | 0.001 (3) | 0.006 (3) |
| C25 | 0.054 (5) | 0.033 (4) | 0.044 (5) | −0.004 (4) | −0.015 (4) | 0.007 (4) |
| C44 | 0.048 (5) | 0.046 (5) | 0.032 (5) | 0.006 (4) | −0.003 (4) | 0.008 (4) |
| C45 | 0.045 (5) | 0.039 (5) | 0.031 (4) | 0.000 (4) | −0.003 (4) | 0.003 (4) |
| C49 | 0.050 (5) | 0.042 (5) | 0.034 (4) | −0.004 (4) | 0.000 (4) | 0.005 (4) |
| C46 | 0.053 (6) | 0.074 (7) | 0.034 (5) | 0.004 (5) | −0.002 (4) | −0.005 (5) |
| C47 | 0.041 (5) | 0.117 (11) | 0.048 (6) | −0.003 (6) | 0.001 (5) | −0.012 (7) |
| C48 | 0.057 (6) | 0.087 (8) | 0.036 (6) | −0.017 (6) | −0.001 (4) | −0.006 (5) |
| C32 | 0.058 (5) | 0.033 (4) | 0.030 (4) | 0.003 (4) | −0.007 (4) | −0.001 (3) |
| C33 | 0.067 (6) | 0.030 (4) | 0.039 (5) | 0.005 (4) | −0.017 (5) | 0.004 (4) |
| C34 | 0.085 (8) | 0.031 (5) | 0.048 (6) | 0.015 (5) | −0.029 (5) | −0.008 (4) |
| C37 | 0.104 (10) | 0.043 (6) | 0.056 (7) | 0.020 (6) | −0.040 (7) | −0.007 (5) |
| C35 | 0.141 (13) | 0.031 (5) | 0.060 (7) | 0.032 (6) | −0.055 (8) | −0.017 (5) |
| C36 | 0.21 (2) | 0.067 (9) | 0.075 (10) | 0.075 (12) | −0.072 (12) | −0.023 (8) |
| C68 | 0.047 (5) | 0.042 (5) | 0.036 (5) | −0.015 (4) | 0.005 (4) | 0.005 (4) |
| C69 | 0.048 (5) | 0.038 (5) | 0.035 (5) | −0.002 (4) | −0.001 (4) | −0.010 (4) |
| C73 | 0.051 (5) | 0.033 (4) | 0.032 (4) | 0.001 (4) | −0.003 (4) | 0.005 (3) |
| C70 | 0.047 (6) | 0.106 (10) | 0.045 (6) | −0.013 (6) | 0.002 (5) | −0.022 (6) |
| C71 | 0.041 (5) | 0.132 (12) | 0.062 (7) | 0.006 (6) | −0.008 (6) | −0.038 (9) |
| C72 | 0.060 (6) | 0.068 (7) | 0.051 (6) | 0.017 (6) | −0.008 (5) | −0.024 (5) |
| C56 | 0.046 (5) | 0.043 (5) | 0.030 (4) | 0.002 (4) | 0.004 (4) | −0.003 (4) |
| C57 | 0.058 (5) | 0.024 (4) | 0.029 (4) | −0.001 (3) | 0.005 (4) | 0.005 (3) |
| C58 | 0.076 (7) | 0.032 (5) | 0.041 (5) | −0.010 (5) | 0.010 (5) | −0.005 (4) |
| C61 | 0.068 (7) | 0.047 (6) | 0.059 (7) | −0.016 (5) | 0.018 (6) | −0.013 (5) |
| C59 | 0.144 (13) | 0.040 (6) | 0.056 (7) | −0.037 (7) | 0.040 (8) | −0.020 (5) |
| C60 | 0.171 (17) | 0.051 (7) | 0.060 (8) | −0.060 (9) | 0.029 (9) | −0.008 (6) |
| C1S | 0.057 (7) | 0.060 (7) | 0.077 (8) | 0.003 (5) | −0.007 (6) | 0.019 (6) |
| C5S | 0.094 (12) | 0.062 (9) | 0.146 (17) | 0.009 (8) | −0.003 (11) | −0.005 (10) |
| C4S | 0.076 (10) | 0.041 (7) | 0.25 (3) | −0.005 (6) | 0.030 (13) | 0.032 (11) |
| C2S | 0.065 (8) | 0.127 (14) | 0.072 (10) | −0.005 (8) | 0.006 (7) | 0.031 (10) |
| C6S | 0.097 (12) | 0.102 (13) | 0.101 (13) | 0.011 (10) | 0.028 (10) | 0.038 (11) |
| C3S | 0.082 (9) | 0.055 (7) | 0.114 (14) | −0.009 (6) | −0.005 (8) | 0.021 (8) |
| C7S | 0.067 (6) | 0.051 (6) | 0.039 (5) | 0.010 (5) | 0.004 (5) | 0.016 (4) |
| C8S | 0.082 (8) | 0.050 (6) | 0.040 (6) | 0.019 (6) | 0.007 (5) | 0.010 (5) |
| C9S | 0.102 (11) | 0.065 (8) | 0.052 (7) | −0.004 (7) | −0.005 (7) | −0.019 (6) |
| C10S | 0.076 (8) | 0.058 (6) | 0.037 (5) | 0.004 (6) | 0.001 (5) | −0.001 (5) |
| C11S | 0.077 (9) | 0.103 (11) | 0.048 (7) | 0.019 (8) | 0.004 (6) | −0.012 (7) |
| C12S | 0.075 (9) | 0.128 (14) | 0.075 (10) | −0.009 (9) | 0.003 (7) | −0.026 (9) |
| Cl1S | 0.085 (3) | 0.131 (4) | 0.118 (4) | −0.001 (3) | −0.002 (3) | 0.021 (3) |
| Cl2S | 0.0616 (18) | 0.170 (5) | 0.068 (2) | 0.012 (2) | −0.0020 (16) | 0.030 (3) |
| N5 | 0.045 (4) | 0.034 (4) | 0.029 (3) | −0.002 (3) | 0.001 (3) | 0.001 (3) |
| N6 | 0.039 (4) | 0.055 (5) | 0.038 (4) | 0.003 (3) | −0.004 (3) | −0.003 (4) |
| N7 | 0.051 (4) | 0.031 (3) | 0.024 (3) | 0.002 (3) | −0.004 (3) | 0.001 (3) |
| N8 | 0.049 (4) | 0.035 (4) | 0.026 (3) | 0.001 (3) | −0.001 (3) | 0.007 (3) |
| N9 | 0.047 (4) | 0.030 (3) | 0.027 (3) | −0.004 (3) | 0.005 (3) | −0.003 (3) |
| N10 | 0.043 (4) | 0.030 (4) | 0.034 (4) | −0.010 (3) | −0.003 (3) | 0.004 (3) |
| N11 | 0.056 (5) | 0.051 (5) | 0.036 (4) | 0.008 (4) | −0.006 (4) | 0.001 (4) |
| N12 | 0.055 (5) | 0.063 (5) | 0.028 (4) | −0.011 (4) | 0.003 (3) | 0.002 (4) |
| N13 | 0.163 (13) | 0.070 (8) | 0.071 (7) | 0.059 (8) | −0.047 (8) | −0.023 (6) |
| N14 | 0.109 (9) | 0.066 (7) | 0.080 (8) | −0.043 (7) | 0.026 (7) | −0.012 (6) |
| O1 | 0.077 (5) | 0.059 (4) | 0.033 (4) | −0.019 (4) | 0.016 (3) | −0.015 (3) |
| O2 | 0.069 (5) | 0.056 (4) | 0.023 (3) | 0.002 (3) | −0.007 (3) | −0.002 (3) |
| O3 | 0.136 (9) | 0.099 (7) | 0.038 (4) | −0.084 (7) | −0.013 (5) | 0.015 (4) |
| O4 | 0.098 (7) | 0.102 (7) | 0.045 (4) | 0.056 (6) | 0.017 (4) | 0.031 (5) |
(2,5-Dimethylimidazole){N,N',N'',N'''-[porphyrin-5,10,15,20-tetrayltetra(2,1-phenylene)]tetrakis(pyridine-3-carboxamide)}manganese(II) chlorobenzene disolvate . Geometric parameters (Å, º)
| Mn1—N1 | 2.138 (6) | C22—C23 | 1.515 (17) |
| Mn1—N2 | 2.141 (6) | C22—N6 | 1.380 (13) |
| Mn1—N3 | 2.141 (6) | C23—H23A | 0.9800 |
| Mn1—N4 | 2.154 (6) | C23—H23B | 0.9800 |
| Mn1—N5 | 2.171 (8) | C23—H23C | 0.9800 |
| N1—C4 | 1.373 (9) | C24—C25 | 1.471 (13) |
| N1—C1 | 1.374 (10) | C24—N5 | 1.347 (11) |
| N2—C19 | 1.379 (10) | C24—N6 | 1.356 (12) |
| N2—C16 | 1.374 (10) | C25—H25A | 0.9800 |
| N3—C14 | 1.380 (10) | C25—H25B | 0.9800 |
| N3—C11 | 1.389 (10) | C25—H25C | 0.9800 |
| N4—C9 | 1.380 (10) | C44—C45 | 1.504 (13) |
| N4—C6 | 1.367 (10) | C44—N8 | 1.336 (12) |
| C4—C3 | 1.467 (9) | C44—O4 | 1.214 (12) |
| C4—C5 | 1.402 (10) | C45—C49 | 1.388 (13) |
| C1—C2 | 1.454 (11) | C45—C46 | 1.371 (14) |
| C1—C20 | 1.406 (11) | C49—H49 | 0.9500 |
| C19—C18 | 1.453 (10) | C49—N12 | 1.344 (12) |
| C19—C20 | 1.385 (11) | C46—H46 | 0.9500 |
| C16—C17 | 1.459 (10) | C46—C47 | 1.371 (16) |
| C16—C15 | 1.398 (11) | C47—H47 | 0.9500 |
| C14—C13 | 1.452 (11) | C47—C48 | 1.361 (16) |
| C14—C15 | 1.406 (11) | C48—H48 | 0.9500 |
| C11—C12 | 1.436 (11) | C48—N12 | 1.347 (14) |
| C11—C10 | 1.421 (11) | C32—C33 | 1.482 (13) |
| C9—C8 | 1.455 (11) | C32—N7 | 1.349 (11) |
| C9—C10 | 1.395 (11) | C32—O2 | 1.237 (11) |
| C6—C7 | 1.452 (11) | C33—C34 | 1.406 (13) |
| C6—C5 | 1.416 (11) | C33—C37 | 1.371 (14) |
| C3—H3 | 0.9500 | C34—H34 | 0.9500 |
| C3—C2 | 1.362 (11) | C34—C35 | 1.352 (15) |
| C2—H2 | 0.9500 | C37—H37 | 0.9500 |
| C18—H18 | 0.9500 | C37—N13 | 1.391 (16) |
| C18—C17 | 1.349 (11) | C35—H35 | 0.9500 |
| C17—H17 | 0.9500 | C35—C36 | 1.376 (19) |
| C13—H13 | 0.9500 | C36—H36 | 0.9500 |
| C13—C12 | 1.352 (12) | C36—N13 | 1.360 (18) |
| C12—H12 | 0.9500 | C68—C69 | 1.491 (13) |
| C8—H8 | 0.9500 | C68—N10 | 1.341 (11) |
| C8—C7 | 1.362 (12) | C68—O3 | 1.226 (12) |
| C7—H7 | 0.9500 | C69—C73 | 1.388 (13) |
| C5—C26 | 1.498 (11) | C69—C70 | 1.386 (14) |
| C20—C62 | 1.499 (10) | C73—H73 | 0.9500 |
| C15—C50 | 1.497 (11) | C73—N11 | 1.349 (12) |
| C10—C38 | 1.505 (10) | C70—H70 | 0.9500 |
| C38—C43 | 1.409 (12) | C70—C71 | 1.373 (18) |
| C38—C39 | 1.386 (12) | C71—H71 | 0.9500 |
| C43—C42 | 1.394 (12) | C71—C72 | 1.396 (18) |
| C43—N8 | 1.427 (11) | C72—H72 | 0.9500 |
| C42—H42 | 0.9500 | C72—N11 | 1.329 (14) |
| C42—C41 | 1.389 (14) | C56—C57 | 1.488 (12) |
| C41—H41 | 0.9500 | C56—N9 | 1.350 (11) |
| C41—C40 | 1.373 (14) | C56—O1 | 1.232 (11) |
| C40—H40 | 0.9500 | C57—C58 | 1.408 (13) |
| C40—C39 | 1.392 (12) | C57—C61 | 1.365 (14) |
| C39—H39 | 0.9500 | C58—H58 | 0.9500 |
| C26—C31 | 1.406 (11) | C58—C59 | 1.362 (15) |
| C26—C27 | 1.400 (12) | C61—H61 | 0.9500 |
| C31—C30 | 1.393 (11) | C61—N14 | 1.364 (15) |
| C31—N7 | 1.417 (11) | C59—H59 | 0.9500 |
| C30—H30 | 0.9500 | C59—C60 | 1.33 (2) |
| C30—C29 | 1.373 (13) | C60—H60 | 0.9500 |
| C29—H29 | 0.9500 | C60—N14 | 1.39 (2) |
| C29—C28 | 1.397 (14) | C1S—H1S | 0.9500 |
| C28—H28 | 0.9500 | C1S—C5S | 1.3900 |
| C28—C27 | 1.388 (12) | C1S—C3S | 1.3900 |
| C27—H27 | 0.9500 | C5S—H5S | 0.9500 |
| C62—C67 | 1.399 (11) | C5S—C4S | 1.3900 |
| C62—C63 | 1.391 (11) | C4S—C2S | 1.3900 |
| C67—C66 | 1.432 (11) | C4S—Cl1S | 1.692 (8) |
| C67—N10 | 1.405 (11) | C2S—H2S | 0.9500 |
| C66—H66 | 0.9500 | C2S—C6S | 1.3900 |
| C66—C65 | 1.385 (13) | C6S—H6S | 0.9500 |
| C65—H65 | 0.9500 | C6S—C3S | 1.3900 |
| C65—C64 | 1.392 (14) | C3S—H3S | 0.9500 |
| C64—H64 | 0.9500 | C7S—C8S | 1.387 (16) |
| C64—C63 | 1.398 (12) | C7S—C10S | 1.360 (15) |
| C63—H63 | 0.9500 | C7S—Cl2S | 1.740 (12) |
| C50—C55 | 1.409 (12) | C8S—H8S | 0.9500 |
| C50—C51 | 1.404 (12) | C8S—C9S | 1.322 (19) |
| C55—C54 | 1.384 (11) | C9S—H9S | 0.9500 |
| C55—N9 | 1.417 (11) | C9S—C12S | 1.38 (2) |
| C54—H54 | 0.9500 | C10S—H10S | 0.9500 |
| C54—C53 | 1.399 (14) | C10S—C11S | 1.366 (19) |
| C53—H53 | 0.9500 | C11S—H11S | 0.9500 |
| C53—C52 | 1.395 (14) | C11S—C12S | 1.41 (2) |
| C52—H52 | 0.9500 | C12S—H12S | 0.9500 |
| C52—C51 | 1.382 (12) | N6—H6 | 0.8800 |
| C51—H51 | 0.9500 | N7—N11 | 3.098 (12) |
| C21—H21 | 0.9500 | N8—H8A | 0.8800 |
| C21—C22 | 1.349 (15) | N9—H9 | 0.8800 |
| C21—N5 | 1.374 (11) | N10—H10 | 0.8800 |
| N1—Mn1—N2 | 86.9 (2) | C52—C51—H51 | 119.3 |
| N1—Mn1—N3 | 150.0 (3) | C22—C21—H21 | 125.0 |
| N1—Mn1—N4 | 85.5 (2) | C22—C21—N5 | 109.9 (9) |
| N1—Mn1—N5 | 98.1 (3) | N5—C21—H21 | 125.0 |
| N2—Mn1—N4 | 148.9 (3) | C21—C22—C23 | 130.4 (11) |
| N2—Mn1—N5 | 101.4 (3) | C21—C22—N6 | 106.1 (9) |
| N3—Mn1—N2 | 85.3 (2) | N6—C22—C23 | 122.9 (11) |
| N3—Mn1—N4 | 86.4 (2) | C22—C23—H23A | 109.5 |
| N3—Mn1—N5 | 111.8 (3) | C22—C23—H23B | 109.5 |
| N4—Mn1—N5 | 109.5 (3) | C22—C23—H23C | 109.5 |
| C4—N1—Mn1 | 125.8 (5) | H23A—C23—H23B | 109.5 |
| C4—N1—C1 | 108.0 (6) | H23A—C23—H23C | 109.5 |
| C1—N1—Mn1 | 124.3 (5) | H23B—C23—H23C | 109.5 |
| C19—N2—Mn1 | 124.2 (5) | N5—C24—C25 | 127.4 (8) |
| C16—N2—Mn1 | 126.1 (5) | N5—C24—N6 | 109.0 (8) |
| C16—N2—C19 | 107.9 (6) | N6—C24—C25 | 123.6 (8) |
| C14—N3—Mn1 | 126.3 (5) | C24—C25—H25A | 109.5 |
| C14—N3—C11 | 106.7 (6) | C24—C25—H25B | 109.5 |
| C11—N3—Mn1 | 126.1 (5) | C24—C25—H25C | 109.5 |
| C9—N4—Mn1 | 126.5 (5) | H25A—C25—H25B | 109.5 |
| C6—N4—Mn1 | 125.9 (5) | H25A—C25—H25C | 109.5 |
| C6—N4—C9 | 106.9 (6) | H25B—C25—H25C | 109.5 |
| N1—C4—C3 | 109.0 (6) | N8—C44—C45 | 114.9 (8) |
| N1—C4—C5 | 126.0 (7) | O4—C44—C45 | 120.3 (9) |
| C5—C4—C3 | 125.0 (7) | O4—C44—N8 | 124.9 (9) |
| N1—C1—C2 | 108.7 (7) | C49—C45—C44 | 120.8 (8) |
| N1—C1—C20 | 125.9 (7) | C46—C45—C44 | 121.0 (8) |
| C20—C1—C2 | 125.4 (7) | C46—C45—C49 | 118.1 (9) |
| N2—C19—C18 | 108.3 (6) | C45—C49—H49 | 118.3 |
| N2—C19—C20 | 126.4 (7) | N12—C49—C45 | 123.4 (9) |
| C20—C19—C18 | 125.3 (7) | N12—C49—H49 | 118.3 |
| N2—C16—C17 | 108.7 (6) | C45—C46—H46 | 120.3 |
| N2—C16—C15 | 125.4 (7) | C47—C46—C45 | 119.5 (10) |
| C15—C16—C17 | 125.9 (7) | C47—C46—H46 | 120.3 |
| N3—C14—C13 | 108.9 (6) | C46—C47—H47 | 120.6 |
| N3—C14—C15 | 124.8 (7) | C48—C47—C46 | 118.8 (10) |
| C15—C14—C13 | 126.2 (7) | C48—C47—H47 | 120.6 |
| N3—C11—C12 | 109.4 (7) | C47—C48—H48 | 118.0 |
| N3—C11—C10 | 125.2 (7) | N12—C48—C47 | 123.9 (10) |
| C10—C11—C12 | 125.3 (7) | N12—C48—H48 | 118.0 |
| N4—C9—C8 | 109.3 (6) | N7—C32—C33 | 115.2 (8) |
| N4—C9—C10 | 125.8 (7) | O2—C32—C33 | 121.0 (8) |
| C10—C9—C8 | 124.9 (7) | O2—C32—N7 | 123.7 (9) |
| N4—C6—C7 | 110.0 (7) | C34—C33—C32 | 119.7 (8) |
| N4—C6—C5 | 125.7 (7) | C37—C33—C32 | 120.8 (9) |
| C5—C6—C7 | 124.2 (7) | C37—C33—C34 | 119.3 (9) |
| C4—C3—H3 | 126.8 | C33—C34—H34 | 119.6 |
| C2—C3—C4 | 106.4 (7) | C35—C34—C33 | 120.8 (10) |
| C2—C3—H3 | 126.8 | C35—C34—H34 | 119.6 |
| C1—C2—H2 | 126.1 | C33—C37—H37 | 119.8 |
| C3—C2—C1 | 107.9 (7) | C33—C37—N13 | 120.4 (11) |
| C3—C2—H2 | 126.1 | N13—C37—H37 | 119.8 |
| C19—C18—H18 | 126.0 | C34—C35—H35 | 121.0 |
| C17—C18—C19 | 108.0 (7) | C34—C35—C36 | 118.0 (11) |
| C17—C18—H18 | 126.0 | C36—C35—H35 | 121.0 |
| C16—C17—H17 | 126.5 | C35—C36—H36 | 118.1 |
| C18—C17—C16 | 107.1 (7) | N13—C36—C35 | 123.7 (13) |
| C18—C17—H17 | 126.5 | N13—C36—H36 | 118.1 |
| C14—C13—H13 | 126.2 | N10—C68—C69 | 115.8 (8) |
| C12—C13—C14 | 107.5 (7) | O3—C68—C69 | 121.5 (9) |
| C12—C13—H13 | 126.2 | O3—C68—N10 | 122.6 (9) |
| C11—C12—H12 | 126.3 | C73—C69—C68 | 121.4 (8) |
| C13—C12—C11 | 107.5 (7) | C70—C69—C68 | 120.3 (9) |
| C13—C12—H12 | 126.3 | C70—C69—C73 | 118.2 (9) |
| C9—C8—H8 | 126.5 | C69—C73—H73 | 118.2 |
| C7—C8—C9 | 107.0 (7) | N11—C73—C69 | 123.6 (9) |
| C7—C8—H8 | 126.5 | N11—C73—H73 | 118.2 |
| C6—C7—H7 | 126.6 | C69—C70—H70 | 120.9 |
| C8—C7—C6 | 106.8 (7) | C71—C70—C69 | 118.2 (11) |
| C8—C7—H7 | 126.6 | C71—C70—H70 | 120.9 |
| C4—C5—C6 | 125.4 (7) | C70—C71—H71 | 119.8 |
| C4—C5—C26 | 118.2 (7) | C70—C71—C72 | 120.5 (11) |
| C6—C5—C26 | 116.3 (7) | C72—C71—H71 | 119.8 |
| C1—C20—C62 | 115.4 (7) | C71—C72—H72 | 119.2 |
| C19—C20—C1 | 126.5 (7) | N11—C72—C71 | 121.6 (11) |
| C19—C20—C62 | 118.0 (7) | N11—C72—H72 | 119.2 |
| C16—C15—C14 | 126.4 (7) | N9—C56—C57 | 114.5 (8) |
| C16—C15—C50 | 116.8 (7) | O1—C56—C57 | 121.2 (8) |
| C14—C15—C50 | 116.7 (7) | O1—C56—N9 | 124.2 (9) |
| C11—C10—C38 | 116.6 (7) | C58—C57—C56 | 121.6 (8) |
| C9—C10—C11 | 126.5 (7) | C61—C57—C56 | 120.1 (8) |
| C9—C10—C38 | 116.7 (7) | C61—C57—C58 | 118.2 (9) |
| C43—C38—C10 | 119.6 (7) | C57—C58—H58 | 120.2 |
| C39—C38—C10 | 121.6 (8) | C59—C58—C57 | 119.6 (11) |
| C39—C38—C43 | 118.7 (7) | C59—C58—H58 | 120.2 |
| C38—C43—N8 | 117.7 (7) | C57—C61—H61 | 117.9 |
| C42—C43—C38 | 120.5 (8) | N14—C61—C57 | 124.1 (11) |
| C42—C43—N8 | 121.8 (8) | N14—C61—H61 | 117.9 |
| C43—C42—H42 | 120.8 | C58—C59—H59 | 120.8 |
| C41—C42—C43 | 118.4 (9) | C60—C59—C58 | 118.4 (12) |
| C41—C42—H42 | 120.8 | C60—C59—H59 | 120.8 |
| C42—C41—H41 | 118.8 | C59—C60—H60 | 117.0 |
| C40—C41—C42 | 122.3 (8) | C59—C60—N14 | 126.0 (11) |
| C40—C41—H41 | 118.8 | N14—C60—H60 | 117.0 |
| C41—C40—H40 | 120.7 | C5S—C1S—H1S | 120.0 |
| C41—C40—C39 | 118.7 (9) | C5S—C1S—C3S | 120.0 |
| C39—C40—H40 | 120.7 | C3S—C1S—H1S | 120.0 |
| C38—C39—C40 | 121.3 (8) | C1S—C5S—H5S | 120.0 |
| C38—C39—H39 | 119.4 | C1S—C5S—C4S | 120.0 |
| C40—C39—H39 | 119.4 | C4S—C5S—H5S | 120.0 |
| C31—C26—C5 | 120.8 (7) | C5S—C4S—Cl1S | 122.5 (7) |
| C27—C26—C5 | 121.1 (7) | C2S—C4S—C5S | 120.0 |
| C27—C26—C31 | 118.1 (7) | C2S—C4S—Cl1S | 117.4 (7) |
| C26—C31—N7 | 118.5 (7) | C4S—C2S—H2S | 120.0 |
| C30—C31—C26 | 120.9 (8) | C4S—C2S—C6S | 120.0 |
| C30—C31—N7 | 120.6 (7) | C6S—C2S—H2S | 120.0 |
| C31—C30—H30 | 120.1 | C2S—C6S—H6S | 120.0 |
| C29—C30—C31 | 119.8 (8) | C3S—C6S—C2S | 120.0 |
| C29—C30—H30 | 120.1 | C3S—C6S—H6S | 120.0 |
| C30—C29—H29 | 119.7 | C1S—C3S—H3S | 120.0 |
| C30—C29—C28 | 120.5 (8) | C6S—C3S—C1S | 120.0 |
| C28—C29—H29 | 119.7 | C6S—C3S—H3S | 120.0 |
| C29—C28—H28 | 120.2 | C8S—C7S—Cl2S | 119.6 (9) |
| C27—C28—C29 | 119.7 (9) | C10S—C7S—C8S | 121.9 (11) |
| C27—C28—H28 | 120.2 | C10S—C7S—Cl2S | 118.5 (10) |
| C26—C27—H27 | 119.6 | C7S—C8S—H8S | 120.1 |
| C28—C27—C26 | 120.9 (8) | C9S—C8S—C7S | 119.8 (11) |
| C28—C27—H27 | 119.6 | C9S—C8S—H8S | 120.1 |
| C67—C62—C20 | 120.0 (7) | C8S—C9S—H9S | 119.7 |
| C63—C62—C20 | 120.6 (7) | C8S—C9S—C12S | 120.7 (13) |
| C63—C62—C67 | 119.4 (7) | C12S—C9S—H9S | 119.7 |
| C62—C67—C66 | 120.1 (8) | C7S—C10S—H10S | 120.9 |
| C62—C67—N10 | 118.7 (7) | C7S—C10S—C11S | 118.3 (11) |
| N10—C67—C66 | 121.2 (8) | C11S—C10S—H10S | 120.9 |
| C67—C66—H66 | 120.8 | C10S—C11S—H11S | 119.9 |
| C65—C66—C67 | 118.4 (8) | C10S—C11S—C12S | 120.2 (12) |
| C65—C66—H66 | 120.8 | C12S—C11S—H11S | 119.9 |
| C66—C65—H65 | 119.0 | C9S—C12S—C11S | 118.9 (14) |
| C66—C65—C64 | 121.9 (8) | C9S—C12S—H12S | 120.5 |
| C64—C65—H65 | 119.0 | C11S—C12S—H12S | 120.5 |
| C65—C64—H64 | 120.6 | C21—N5—Mn1 | 125.6 (6) |
| C65—C64—C63 | 118.9 (8) | C24—N5—Mn1 | 127.6 (6) |
| C63—C64—H64 | 120.6 | C24—N5—C21 | 106.6 (8) |
| C62—C63—C64 | 121.2 (8) | C22—N6—H6 | 125.8 |
| C62—C63—H63 | 119.4 | C24—N6—C22 | 108.3 (8) |
| C64—C63—H63 | 119.4 | C24—N6—H6 | 125.8 |
| C55—C50—C15 | 121.4 (7) | C31—N7—H7A | 117.9 |
| C51—C50—C15 | 120.6 (8) | C32—N7—C31 | 124.2 (7) |
| C51—C50—C55 | 118.0 (7) | C32—N7—H7A | 117.9 |
| C50—C55—N9 | 118.3 (7) | C43—N8—H8A | 115.8 |
| C54—C55—C50 | 120.7 (8) | C44—N8—C43 | 128.4 (7) |
| C54—C55—N9 | 121.0 (8) | C44—N8—H8A | 115.8 |
| C55—C54—H54 | 119.8 | C55—N9—H9 | 117.3 |
| C55—C54—C53 | 120.4 (9) | C56—N9—C55 | 125.4 (8) |
| C53—C54—H54 | 119.8 | C56—N9—H9 | 117.3 |
| C54—C53—H53 | 120.2 | C67—N10—H10 | 114.7 |
| C52—C53—C54 | 119.6 (8) | C68—N10—C67 | 130.6 (7) |
| C52—C53—H53 | 120.2 | C68—N10—H10 | 114.7 |
| C53—C52—H52 | 120.1 | C72—N11—C73 | 117.8 (9) |
| C51—C52—C53 | 119.8 (9) | C49—N12—C48 | 116.2 (9) |
| C51—C52—H52 | 120.1 | C36—N13—C37 | 117.4 (12) |
| C50—C51—H51 | 119.3 | C61—N14—C60 | 113.5 (12) |
| C52—C51—C50 | 121.5 (8) | ||
| Mn1—N1—C4—C3 | 163.1 (5) | C41—C40—C39—C38 | 2.0 (13) |
| Mn1—N1—C4—C5 | −18.4 (11) | C39—C38—C43—C42 | 0.3 (12) |
| Mn1—N1—C1—C2 | −163.2 (5) | C39—C38—C43—N8 | 179.7 (7) |
| Mn1—N1—C1—C20 | 18.9 (11) | C26—C31—C30—C29 | 0.5 (13) |
| Mn1—N2—C19—C18 | 164.8 (5) | C26—C31—N7—C32 | 118.2 (9) |
| Mn1—N2—C19—C20 | −16.6 (11) | C31—C26—C27—C28 | 3.1 (12) |
| Mn1—N2—C16—C17 | −163.8 (5) | C31—C30—C29—C28 | 0.8 (14) |
| Mn1—N2—C16—C15 | 17.8 (12) | C30—C31—N7—C32 | −61.0 (12) |
| Mn1—N3—C14—C13 | 167.0 (5) | C30—C29—C28—C27 | −0.1 (14) |
| Mn1—N3—C14—C15 | −17.1 (12) | C29—C28—C27—C26 | −1.9 (13) |
| Mn1—N3—C11—C12 | −166.6 (6) | C27—C26—C31—C30 | −2.5 (12) |
| Mn1—N3—C11—C10 | 17.3 (12) | C27—C26—C31—N7 | 178.4 (7) |
| Mn1—N4—C9—C8 | 169.2 (5) | C62—C67—C66—C65 | 0.3 (13) |
| Mn1—N4—C9—C10 | −11.9 (11) | C62—C67—N10—C68 | 158.0 (9) |
| Mn1—N4—C6—C7 | −168.4 (5) | C67—C62—C63—C64 | 3.5 (12) |
| Mn1—N4—C6—C5 | 16.3 (12) | C67—C66—C65—C64 | 0.4 (14) |
| N1—C4—C3—C2 | 0.8 (9) | C66—C67—N10—C68 | −20.7 (14) |
| N1—C4—C5—C6 | 1.2 (14) | C66—C65—C64—C63 | 0.8 (13) |
| N1—C4—C5—C26 | −175.0 (7) | C65—C64—C63—C62 | −2.8 (13) |
| N1—C1—C2—C3 | −1.3 (9) | C63—C62—C67—C66 | −2.2 (12) |
| N1—C1—C20—C19 | −2.0 (13) | C63—C62—C67—N10 | 179.2 (7) |
| N1—C1—C20—C62 | 173.4 (7) | C50—C55—C54—C53 | −0.5 (13) |
| N2—C19—C18—C17 | −0.3 (10) | C50—C55—N9—C56 | 128.5 (9) |
| N2—C19—C20—C1 | 0.8 (13) | C55—C50—C51—C52 | 2.6 (13) |
| N2—C19—C20—C62 | −174.5 (7) | C55—C54—C53—C52 | 1.1 (14) |
| N2—C16—C17—C18 | −1.5 (10) | C54—C55—N9—C56 | −52.5 (13) |
| N2—C16—C15—C14 | −1.2 (14) | C54—C53—C52—C51 | 0.1 (14) |
| N2—C16—C15—C50 | 175.9 (8) | C53—C52—C51—C50 | −2.0 (13) |
| N3—C14—C13—C12 | 1.1 (10) | C51—C50—C55—C54 | −1.3 (12) |
| N3—C14—C15—C16 | 0.7 (14) | C51—C50—C55—N9 | 177.7 (7) |
| N3—C14—C15—C50 | −176.3 (7) | C21—C22—N6—C24 | 2.2 (12) |
| N3—C11—C12—C13 | −2.2 (10) | C22—C21—N5—Mn1 | −174.5 (7) |
| N3—C11—C10—C9 | −4.3 (14) | C22—C21—N5—C24 | 0.2 (12) |
| N3—C11—C10—C38 | 170.1 (8) | C23—C22—N6—C24 | −169.7 (13) |
| N4—C9—C8—C7 | 0.1 (9) | C25—C24—N5—Mn1 | −5.7 (12) |
| N4—C9—C10—C11 | 1.3 (14) | C25—C24—N5—C21 | 179.7 (9) |
| N4—C9—C10—C38 | −173.1 (7) | C25—C24—N6—C22 | 179.3 (9) |
| N4—C6—C7—C8 | −2.4 (10) | C44—C45—C49—N12 | 177.9 (9) |
| N4—C6—C5—C4 | −0.1 (14) | C44—C45—C46—C47 | −179.8 (11) |
| N4—C6—C5—C26 | 176.2 (8) | C45—C44—N8—C43 | −168.8 (8) |
| C4—N1—C1—C2 | 1.8 (9) | C45—C49—N12—C48 | 1.5 (15) |
| C4—N1—C1—C20 | −176.1 (8) | C45—C46—C47—C48 | 2 (2) |
| C4—C3—C2—C1 | 0.3 (9) | C49—C45—C46—C47 | −3.7 (17) |
| C4—C5—C26—C31 | 105.8 (9) | C46—C45—C49—N12 | 1.7 (15) |
| C4—C5—C26—C27 | −73.7 (10) | C46—C47—C48—N12 | 1 (2) |
| C1—N1—C4—C3 | −1.6 (9) | C47—C48—N12—C49 | −2.9 (18) |
| C1—N1—C4—C5 | 176.8 (8) | C32—C33—C34—C35 | 177.9 (12) |
| C1—C20—C62—C67 | −68.3 (10) | C32—C33—C37—N13 | 178.1 (13) |
| C1—C20—C62—C63 | 111.7 (9) | C33—C32—N7—C31 | −163.5 (8) |
| C19—N2—C16—C17 | 1.3 (9) | C33—C34—C35—C36 | 0 (2) |
| C19—N2—C16—C15 | −177.2 (8) | C33—C37—N13—C36 | 8 (3) |
| C19—C18—C17—C16 | 1.0 (10) | C34—C33—C37—N13 | −7 (2) |
| C19—C20—C62—C67 | 107.5 (9) | C34—C35—C36—N13 | 0 (3) |
| C19—C20—C62—C63 | −72.5 (10) | C37—C33—C34—C35 | 3 (2) |
| C16—N2—C19—C18 | −0.6 (9) | C35—C36—N13—C37 | −4 (3) |
| C16—N2—C19—C20 | 178.1 (8) | C68—C69—C73—N11 | 174.6 (9) |
| C16—C15—C50—C55 | −65.6 (10) | C68—C69—C70—C71 | −177.9 (12) |
| C16—C15—C50—C51 | 114.0 (9) | C69—C68—N10—C67 | −165.4 (9) |
| C14—N3—C11—C12 | 2.9 (9) | C69—C73—N11—C72 | 3.2 (15) |
| C14—N3—C11—C10 | −173.1 (8) | C69—C70—C71—C72 | 3 (2) |
| C14—C13—C12—C11 | 0.7 (10) | C73—C69—C70—C71 | −2.0 (18) |
| C14—C15—C50—C55 | 111.7 (9) | C70—C69—C73—N11 | −1.2 (15) |
| C14—C15—C50—C51 | −68.7 (10) | C70—C71—C72—N11 | −1 (2) |
| C11—N3—C14—C13 | −2.5 (9) | C71—C72—N11—C73 | −2.0 (17) |
| C11—N3—C14—C15 | 173.4 (8) | C56—C57—C58—C59 | 175.9 (11) |
| C11—C10—C38—C43 | −68.7 (10) | C56—C57—C61—N14 | 179.8 (12) |
| C11—C10—C38—C39 | 113.0 (9) | C57—C56—N9—C55 | −164.5 (8) |
| C9—N4—C6—C7 | 2.4 (9) | C57—C58—C59—C60 | 2 (2) |
| C9—N4—C6—C5 | −172.8 (8) | C57—C61—N14—C60 | 6 (2) |
| C9—C8—C7—C6 | 1.3 (10) | C58—C57—C61—N14 | −3.9 (18) |
| C9—C10—C38—C43 | 106.3 (9) | C58—C59—C60—N14 | 1 (3) |
| C9—C10—C38—C39 | −72.0 (10) | C61—C57—C58—C59 | −0.4 (17) |
| C6—N4—C9—C8 | −1.6 (9) | C59—C60—N14—C61 | −5 (3) |
| C6—N4—C9—C10 | 177.4 (8) | C1S—C5S—C4S—C2S | 0.0 |
| C6—C5—C26—C31 | −70.7 (10) | C1S—C5S—C4S—Cl1S | 177.2 (8) |
| C6—C5—C26—C27 | 109.7 (9) | C5S—C1S—C3S—C6S | 0.0 |
| C3—C4—C5—C6 | 179.4 (8) | C5S—C4S—C2S—C6S | 0.0 |
| C3—C4—C5—C26 | 3.2 (12) | C4S—C2S—C6S—C3S | 0.0 |
| C2—C1—C20—C19 | −179.6 (8) | C2S—C6S—C3S—C1S | 0.0 |
| C2—C1—C20—C62 | −4.2 (12) | C3S—C1S—C5S—C4S | 0.0 |
| C18—C19—C20—C1 | 179.2 (8) | C7S—C8S—C9S—C12S | −5 (2) |
| C18—C19—C20—C62 | 3.9 (12) | C7S—C10S—C11S—C12S | 2 (2) |
| C17—C16—C15—C14 | −179.3 (8) | C8S—C7S—C10S—C11S | −3.5 (18) |
| C17—C16—C15—C50 | −2.3 (13) | C8S—C9S—C12S—C11S | 3 (3) |
| C13—C14—C15—C16 | 175.9 (8) | C10S—C7S—C8S—C9S | 4.8 (17) |
| C13—C14—C15—C50 | −1.1 (12) | C10S—C11S—C12S—C9S | −2 (3) |
| C12—C11—C10—C9 | −179.7 (8) | Cl1S—C4S—C2S—C6S | −177.3 (7) |
| C12—C11—C10—C38 | −5.3 (12) | Cl2S—C7S—C8S—C9S | −175.1 (10) |
| C8—C9—C10—C11 | −179.9 (8) | Cl2S—C7S—C10S—C11S | 176.5 (10) |
| C8—C9—C10—C38 | 5.7 (12) | N5—C21—C22—C23 | 169.6 (14) |
| C7—C6—C5—C4 | −174.7 (8) | N5—C21—C22—N6 | −1.5 (13) |
| C7—C6—C5—C26 | 1.6 (12) | N5—C24—N6—C22 | −2.1 (10) |
| C5—C4—C3—C2 | −177.7 (8) | N6—C24—N5—Mn1 | 175.8 (5) |
| C5—C6—C7—C8 | 172.9 (8) | N6—C24—N5—C21 | 1.2 (10) |
| C5—C26—C31—C30 | 178.0 (8) | N7—C31—C30—C29 | 179.6 (8) |
| C5—C26—C31—N7 | −1.1 (11) | N7—C32—C33—C34 | 37.1 (14) |
| C5—C26—C27—C28 | −177.3 (8) | N7—C32—C33—C37 | −148.0 (11) |
| C20—C1—C2—C3 | 176.6 (8) | N8—C43—C42—C41 | −179.0 (8) |
| C20—C19—C18—C17 | −179.0 (8) | N8—C44—C45—C49 | 34.5 (13) |
| C20—C62—C67—C66 | 177.8 (8) | N8—C44—C45—C46 | −149.5 (10) |
| C20—C62—C67—N10 | −0.9 (11) | N9—C55—C54—C53 | −179.5 (8) |
| C20—C62—C63—C64 | −176.5 (8) | N9—C56—C57—C58 | 36.3 (13) |
| C15—C16—C17—C18 | 177.0 (8) | N9—C56—C57—C61 | −147.5 (10) |
| C15—C14—C13—C12 | −174.7 (8) | N10—C67—C66—C65 | 178.9 (8) |
| C15—C50—C55—C54 | 178.3 (8) | N10—C68—C69—C73 | 39.6 (13) |
| C15—C50—C55—N9 | −2.7 (12) | N10—C68—C69—C70 | −144.7 (11) |
| C15—C50—C51—C52 | −177.0 (8) | O1—C56—C57—C58 | −140.0 (10) |
| C10—C11—C12—C13 | 173.8 (8) | O1—C56—C57—C61 | 36.2 (14) |
| C10—C9—C8—C7 | −178.8 (8) | O1—C56—N9—C55 | 11.7 (15) |
| C10—C38—C43—C42 | −178.1 (8) | O2—C32—C33—C34 | −139.0 (11) |
| C10—C38—C43—N8 | 1.3 (11) | O2—C32—C33—C37 | 35.9 (16) |
| C10—C38—C39—C40 | 176.8 (8) | O2—C32—N7—C31 | 12.6 (15) |
| C38—C43—C42—C41 | 0.4 (13) | O3—C68—C69—C73 | −138.5 (12) |
| C38—C43—N8—C44 | 159.6 (9) | O3—C68—C69—C70 | 37.2 (17) |
| C43—C38—C39—C40 | −1.6 (13) | O3—C68—N10—C67 | 12.7 (18) |
| C43—C42—C41—C40 | 0.0 (14) | O4—C44—C45—C49 | −145.3 (11) |
| C42—C43—N8—C44 | −21.0 (14) | O4—C44—C45—C46 | 30.7 (16) |
| C42—C41—C40—C39 | −1.2 (14) | O4—C44—N8—C43 | 11.0 (17) |
(2,5-Dimethylimidazole){N,N',N'',N'''-[porphyrin-5,10,15,20-tetrayltetra(2,1-phenylene)]tetrakis(pyridine-3-carboxamide)}manganese(II) chlorobenzene disolvate . Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| N6—H6···O2i | 0.88 | 2.05 | 2.858 (11) | 153 |
| N7—H7A···N11 | 0.88 | 2.24 | 3.094 (11) | 164 |
| N9—H9···N12 | 0.88 | 2.17 | 3.009 (10) | 159 |
| C5S—H5S···N14ii | 0.95 | 2.57 | 3.421 (16) | 150 |
| C36—H36···O3ii | 0.95 | 2.35 | 2.99 (2) | 124 |
| C60—H60···O4iii | 0.95 | 2.40 | 3.062 (18) | 126 |
Symmetry codes: (i) −x+1, −y, z+1/2; (ii) −x+1/2, y+1/2, z−1/2; (iii) −x+1/2, y−1/2, z+1/2.
References
- Bruker (2014). APEX2 and SAINT. Bruker AXS Inc., Madison, Wisconsin, USA.
- Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K. & Puschmann, H. (2009). J. Appl. Cryst.42, 339–341.
- Gunter, M. J., McLaughlin, G. M., Berry, K. J., Murray, K. S., Irving, M. & Clark, P. E. (1984). Inorg. Chem.23, 283–300.
- Kirner, J. F., Reed, C. A. & Scheidt, W. R. (1977). J. Am. Chem. Soc.99, 2557–2563. [DOI] [PubMed]
- Krause, L., Herbst-Irmer, R., Sheldrick, G. M. & Stalke, D. (2015). J. Appl. Cryst.48, 3–10. [DOI] [PMC free article] [PubMed]
- Krishna Deepak, R. N. V. & Sankararamakrishnan, R. (2016). Biochemistry, 55, 3774–3783. [DOI] [PubMed]
- Landrum, J. T., Hatano, K., Scheidt, W. R. & Reed, C. A. (1980). J. Am. Chem. Soc.102, 6729–6735.
- Liang, X., Zhao, J., Ren, W., Yuan, Y., Guo, W. & Li, J. (2023). Dyes Pigments, 211, 111068.
- Nappa, M., Valentine, J. S. & Snyder, P. A. (1977). J. Am. Chem. Soc.99, 5799–5800. [DOI] [PubMed]
- Sheldrick, G. M. (2015a). Acta Cryst. A71, 3–8.
- Sheldrick, G. M. (2015b). Acta Cryst. C71, 3–8.
- Yao, Z., Li, H., Liang, X., Xu, X. & Li, J. (2019). Dyes Pigments, 162, 75–79.
- Yu, Q., Liu, Y., Liu, D. & Li, J. (2015). Dalton Trans.44, 9382–9390. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) I. DOI: 10.1107/S2414314624004978/hb4469sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2414314624004978/hb4469Isup2.hkl
Porphyrin ring displacement data. DOI: 10.1107/S2414314624004978/hb4469sup3.docx
CCDC reference: 2358337
Additional supporting information: crystallographic information; 3D view; checkCIF report