Abstract
To identify the domains of Vpr that are involved nuclear localization, we transfected HeLa cells with a panel of expression vectors that encode mutant Vpr protein with deletions or substitutions within putative domains. Immunofluorescence staining of transfected cells revealed that wild-type Vpr was localized predominantly in the nucleus and the nuclear envelope and certainly in the cytoplasm. Introduction of substitutions or deletions within αH1 or αH2 resulted, by contrast, in diffuse expression over the entire cell. In addition, double mutations within both of these α-helical domains led to the complete absence of Vpr from nuclei. Next, we prepared HeLa cells that express chimeric proteins which consist of the αH1 and αH2 domains fused individually with green fluorescent protein (GFP) and a Flag tag and extracted them with digitonin and Triton X-100 prior to fixation. Flag-αH1-GFP was detected in the nucleus but not in the cytoplasm, while Flag-αH2-GFP was retained predominantly in the nucleus and in a small amount in the cytoplasm. The immunostaining patterns were almost eliminated by substitutions in each chimeric protein. Thus, it appeared that the two α-helical domains might be involved in nuclear import by binding to certain cellular factors. Taken together, our data suggest that the two putative α-helical domains mediate the nuclear localization of Vpr by at least two mechanisms.
The mechanisms of nuclear import of the preintegration complex (PIC) are necessary for infection by human immunodeficiency virus (HIV) of nondividing cells, such as quiescent T lymphocytes, terminally differentiated dendritic macrophages, and microglial cells (5, 38). At least three HIV type 1 (HIV-1) proteins, namely, the matrix protein (MA) (4), integrase (IN) (13), and viral protein R (Vpr) (29, 36), have been identified as possible mediators of the nuclear import of the PIC. MA and IN contain classical, simian virus (SV40)-like, nuclear localization signal (NLS) sequences (5, 14), and their roles appear to be mediated by importin α/β heterodimers (1). By contrast to MA and IN, Vpr contains two discrete nuclear targeting signals that use two different import pathways, both of which are distinct from the NLS- and M9-dependent pathways (17). Vpr interacts with importin α (29, 36), but this interaction is not blocked by a peptide that corresponds to the NLS of the SV40 large T antigen, a result that suggests that the site at which Vpr binds to importin α is not the NLS cargo site (14, 29). Likewise, Vpr interacts with several nuclear pore proteins that contain FXFG repeats, such as Nsp1p, Nup1p, and Pom121 (9, 28, 36). However, the NLS of Vpr has not been fully characterized and the nuclear import pathway that involves Vpr has also not been defined.
In addition to its role in nuclear transport, Vpr has a number of other functions, which include the induction of cell cycle arrest at the G2/M phase (16, 18, 30) or at the G1 phase (25b, 28) both positive and negative regulation of apoptosis (2, 7, 12, 33; Nishizawa, M., M. Kamata, T. Myojin, Y. Nakai, and Y. Aida, submitted for publication), weak activation of several viral promoters that include those of HIV-1 (6), and induction of the terminal differentiation of certain types of cell (20). The various biological activities of Vpr have been correlated with specific structural features of the protein (8, 21–25, 37, 39, 40). Computer-assisted secondary-structural analysis of Vpr predicts the presence of two α-helical segments between residues 17 and 34 and between residues 46 and 74 (22, 24, 34). Mutational analysis has suggested the importance of the first of these two α-helical domains in nuclear localization and also in the expression, stability, and incorporation into virions of Vpr (8, 22, 24, 39). A peptide derived from the first α-helical domain has been reported to mediate nuclear transport of peptide-conjugated bovine serum albumin in permeabilized cells (19). Moreover, the second of these domains appears to contain a determinant that is involved in translocation to the nucleus of the PIC in nondividing cells (25). By contrast, recent work has indicated that a 20-amino-acid (aa) arginine-rich region in the carboxy-terminal tail of the protein (residues 77 to 96) might be necessary for its nuclear localization (17, 39, 41). Thus, the domain(s) of Vpr that might be important for nuclear localization remains controversial.
To identify in further detail the functional domains of Vpr that are involved in nuclear localization, we used immunofluorescent staining and the powerful technique of confocal microscopy, which allows three-dimensional imaging of the localization of protein in cells. In another study, we have identified the localization of Vpr during the 24-h period after transfection, namely, during the time when Vpr is expressed at a detectable level but is minimally effective in inducing G2 arrest (M. Nishizawa, M. Kamata, T. Myojin, Y. Nakai, and Y. Aida, submitted). Using a panel of expression vectors that encode mutated forms of Vpr, we first demonstrated that both α-helical domains, but not the carboxy-terminal arginine-rich tail, contribute to the subcellular localization of Vpr. We then constructed expression plasmids that encode chimeric proteins that consist of the putative α-helical domains of Vpr fused to green fluorescent protein (GFP) and a Flag tag. The results we obtained with these chimeric proteins indicate that the two α-helical domains both mediate nuclear transport of Vpr and that they operate by at least two mechanisms.
Identification of domains that are involved in the nuclear localization function of Vpr.
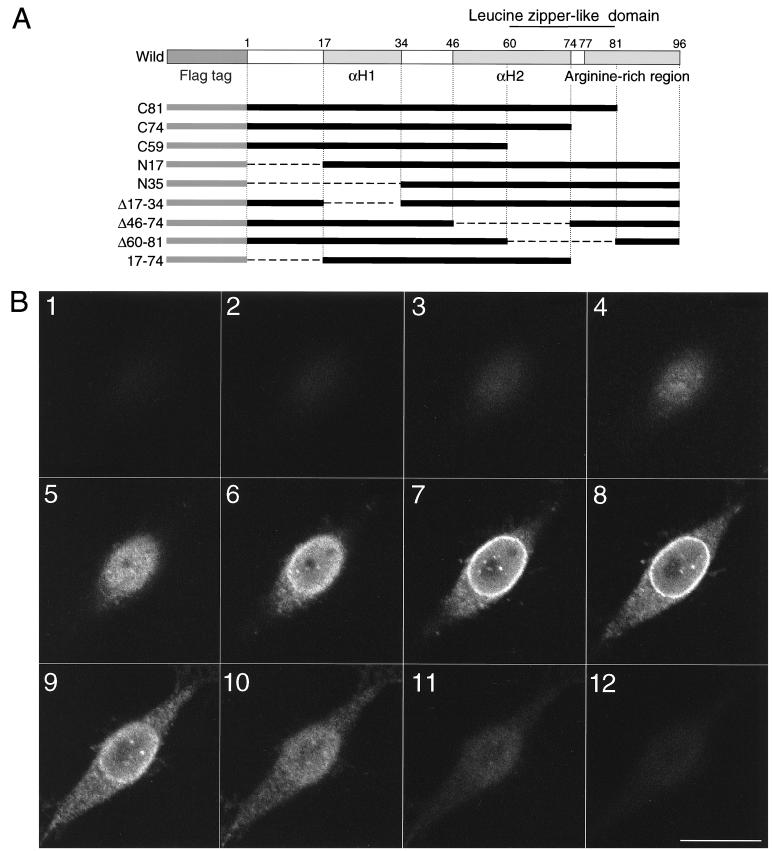
To identify the domain(s) involved in nuclear localization of Vpr, we used a series of plasmids that encode variants of Vpr with deletions in different regions of the protein (26), as shown schematically in Fig. 1A. In designing these mutants, we targeted five putative structural regions on the basis of the amino acid sequence (8, 22–24, 37, 39): (i) the amino-terminal domain from aa 1 to aa 16; (ii) the first amphipathic α-helical region, extending from aa 17 to aa 34 (αH1); (iii) the second amphipathic α-helical region, extending from aa 46 to aa 74 (αH2); (iv) the leucine zipper-like domain from aa 60 to aa 81; and (v) the arginine-rich carboxy-terminal domain from aa 77 to aa 96.
FIG. 1.
Subcellular localization of wild-type and mutant forms of Vpr with specific deletions. (A) Plasmids that encode mutant forms of Vpr and were generated by PCR from HIV-1NL43. The predicted first α-helical domain (αH1) is located near the amino terminus, and a second α-helical domain (αH2) is located near the carboxyl terminus. A leucine zipper-like domain and an arginine-rich region are also indicated. The Flag tag is in gray, and the Vpr portions are in black. (B) Laser sectioning analysis of the localization of immunostained wild-type Vpr. HeLa cells were transfected with pME18Neo, which encodes Flag-tagged wild-type Vpr. At 24 h after transfection, cells were subjected to immunofluorescence staining with Flag-specific MAb M2 and Cy3-conjugated antibodies against mouse IgG. Serial laser sections through a representative cell that expressed wild-type Vpr protein were obtained by confocal laser scanning microscopy at 0.6-μm intervals. Photomicrographs are numbered from 1, which corresponds to the nuclear periphery, through 12, which corresponds to the region through which the cell was adsorbed to the slide. The staining of the cytoplasm becomes more obvious toward the center of the cell in sections 8 through 10. (C) Photomicrographs of HeLa cells expressing wild-type and mutant forms of Vpr. HeLa cells were transfected with pME18Neo, which encodes Flag-tagged wild-type Vpr (a and l); C81 (b); C74 (c); C59 (d); N17 (e); N35 (f); Δ17-34 (g); Δ46-74 (h); Δ60-81 (i); 17-74 (j); or control vector pME18Neo-Flag (k). At 24 h after transfection, cells were subjected to immunofluorescence staining with either Flag-specific MAb M2 (a to k) or normal mouse IgG (l) and Cy3-conjugated antibodies against mouse IgG. Cells were then analyzed by confocal laser scanning microscopy on a focal plane near the center of each nucleus. Bar, 20 μm.
We transfected HeLa cells with expression vectors that encode wild-type or mutant Vpr with an amino-terminal Flag tag by electroporation as described previously (25a). Twenty-four hours after transfection, cells growing on coverslips were fixed for 15 min at 4°C in phosphate-buffered saline (PBS) that contained 1% formaldehyde and then permeabilized for 5 min in PBS that contained 0.2% Triton X-100. The coverslips were then incubated either with monoclonal antibody (MAb) M2, which recognizes the Flag tag (Sigma), or with normal mouse immunoglobulin G (IgG) and then with Cy3-conjugated goat antibodies against mouse IgG (Jackson ImmunoResearch). Cells were then mounted on glass slides in PBS that contained 50% glycerol, 0.1% p-phenylene diamine, and 0.02% sodium azide. Cells were examined with a confocal laser scanning microscope (LSM 10 or LSM 510; Carl Zeiss).
Serial sections through positively immunostained cells showed that wild-type Vpr was localized predominantly in the nucleus and nuclear envelope and a certain amount was present in the cytoplasm of transfected cells (Fig. 1B). In addition, a small number of discrete dots that represented the products of immunostaining were present in the nuclei of most cells. The C74 and C81 proteins with deletion of 22 and 15 aa at the carboxyl terminus gave patterns of immunofluorescence that were not significantly different from that observed with wild-type Vpr (Fig. 1C). The N17 mutant, which lacked 16 aa at the amino terminus of Vpr, showed a slightly increased ability to reach the interior of nuclei (Fig. 1C). Similarly, the 17-74 mutant protein gave exclusively strong nuclear staining that was distinct from the nuclear staining pattern obtained with wild-type Vpr, which was more diffuse. These results indicated that 16 aa in the amino-terminal region and 22 aa in the carboxy-terminal region of Vpr are not essential for the nuclear localization of Vpr. By contrast, Vpr with a deletion of the αH1 domain (Δ17-34) and the amino-terminally truncated Vpr that lacks the 34 aa that include the αH1 domain (N35) had completely lost the specific capacity of wild-type Vpr for perinuclear localization. The aberrant localization was associated with punctate cytoplasmic staining, indicating that the first α-helical domain was a major determinant of the perinuclear localization of Vpr (Fig. 1C). Vpr with a deletion of the αH2 domain or of the leucine zipper-like domain (Δ46-74 or Δ60-81, respectively) and a carboxyl-terminally truncated Vpr that lacks 37 aa that include part of the αH2 domain (C59) were barely detectable after immunofluorescence staining (Fig. 1C). These mutant proteins were diffusely distributed throughout cells, including their nuclei. By contrast, C74, which lacks part of the leucine zipper-like domain, was associated with a nuclear localization pattern similar to that of wild-type Vpr. Disruption of the second α-helical domain, but not that of the leucine zipper-like domain, had a dramatic effect on subcellular localization and on the level of expression or stability of Vpr. The specificity of the immunostaining was demonstrated by the absence of signals in cells transfected with the control vector (Fig. 1C) and in mock-transfected cells (data not shown) and by the absence of staining in cells transfected with the plasmid that encodes wild-type Vpr that were immunostained with normal mouse IgG (Fig. 1C). Collectively, our results indicate that the putative α-helical domains that extend from aa 17 to aa 34 and from aa 46 to aa 74 influence the localization of Vpr.
Effects on the localization of Vpr of mutations within the two α-helical domains.
To confirm the roles of the two α-helical domains in the nuclear localization of Vpr, we constructed plasmids that encode Vpr with site-directed mutations (Fig. 2A). To disrupt helical structures, four bulky nonpolar leucine residues at positions 20, 22, 23, and 26 within αH1 of wild-type Vpr were replaced with smaller alanine residues (21, 22) by ExSite PCR-based site-directed mutagenesis (Stratagene). As primer and template DNAs, we used 5′-CCGAGGAAGCCAAGAGTGAAGCTGTTAGA-3′, 5′-CCGCCTCGGCTGTCCATTCATTGTATGGCTCC-3′, and pSK-Fvpr (26), which encoded wild-type Vpr and a Flag tag with the amino acid sequence NH2-Met-Asp-Tyr-Lys-Asp-Asp-Asp-Asp-Lys. An XhoI-NotI fragment which includes the site-mutated vpr gene and the Flag sequence of pSK-FαLA was then excised and subcloned into the expression vector pME18Neo (35). The resultant plasmid encodes a protein designated αLA. Next, isoleucine at position 60 and leucine at position 67 within αH2 of wild-type Vpr were changed to proline residues to yield I60P and L67P, respectively, as described previously (26). Both of these residues are located on the hydrophobic side of the helix with an orientation favorable for dimerization through leucine zipper interactions (31, 32). In addition, both amino acids are strongly conserved in various isolates of HIV-1 (26), an observation that suggests that they play important roles in the activities of Vpr. Moreover, to generate double mutants with mutations in αH1 and αH2 (αLA/I60P and αLA/L67P), we performed ExSite PCR-based site-directed mutagenesis using pSK-FI60P or pSK-FL67P (26) as templates and the primers described above. The XhoI-NotI fragments were then subcloned into pME18Neo. Twenty-four hours after transfection with each expression plasmid, we examined the localization of Vpr by immunofluorescence staining with Flag-specific MAb M2 and Cy3-conjugated antibodies against mouse IgG (Fig. 2B).
FIG. 2.
Subcellular localization of mutant forms of Vpr with specific substitutions. (A) Replacement of isoleucine and leucine residues within predicted α-helical domains αH1 and αH2 (represented by shaded boxes) of Vpr. The positions of the substituted amino acids are numbered, and amino acids are shown in the single-letter code. Hatched boxes represent the Flag tag. (B) Transfection of HeLa cells with pME18Neo, which encodes Flag-tagged wild-type or mutant Vpr, or with control vector pME18Neo-Flag. At 24 h after transfection, cells were subjected to immunofluorescence staining as described in the legend to FIG. 1 and analyzed by confocal laser scanning microscopy with the focal plane near the center of each nucleus. Bar, 20 μm.
The isoleucine and leucine substitutions in αH2 (I60P and L67P) resulted in complete loss of the highly specific wild-type pattern of perinuclear staining, with diffuse staining over the entire cell. Expression of αLA resulted in cytoplasmic staining that was much more widespread and diffuse than the perinuclear foci of wild-type Vpr. However, single substitutions in αH1 and αH2 still yielded intense signals in the nucleus, suggesting that both of the α-helical domains act together to determine the subcellular localization of Vpr. To confirm this possibility, we examined the intracellular localization of double-mutant proteins with mutations in both the αH1 and αH2 domains (αLA/I60P and αLA/L67P). As expected, we observed exclusively diffuse cytosolic staining that was distinct from the cytosolic staining of wild-type Vpr, which was much less intense, and we detected almost no nuclear staining (Fig. 2B). A similar pattern was observed when HeLa cells were transfected with another double mutant, Δ17-34/L67P, with the αH1 domain deleted and replacement of the leucine at position 67 with proline (data not shown). Moreover, when we examined the participation of isoleucine at positions 74 and 81 in the leucine zipper-like domain in the subcellular localization of Vpr (26), we found almost the same localization pattern as with wild-type Vpr (data not shown). These results indicate that both α-helical domains were necessary for the nuclear distribution of Vpr.
Both α-helical regions of Vpr mediate nuclear uptake of chimeric proteins that include the Flag tag and GFP.
We examined whether the α-helical regions of Vpr is sufficient for the nuclear localization of proteins in transfected cells. We constructed plasmids that express chimeric proteins that consist of the αH1 or αH2 domain, or of these domains with site-specific mutations, fused individually at the carboxyl terminus to GFP to monitor the effects of each α-helical domain and fused at the amino terminus to the Flag tag to facilitate immunofluorescence staining (Fig. 3A). The expression vectors included ATG initiation codons for the Flag tag and for GFP, and the chimeric proteins were detected by indirect immunofluorescence after reaction with the Flag-specific MAb M2 and Cy3-conjugated antibodies against mouse IgG, to avoid confusion by green fluorescence derived from nonchimeric GFP, which might have been translated from the initiation codon of GFP. First, to generate a plasmid that encodes a Flag-Vpr-GFP fusion protein, the fragment containing vpr and Flag sequences was amplified by PCR with the primers 5′-TAATCTCGAGATGGACTACAAGGACGAC-3′ and 5′-TACCTCGAGATAGGATCTACTGGCTCC-3′ with pSK-Fvpr (26) as the template. The fragment obtained by PCR was subcloned in the GFP expression vector pEGFP-N1 (Clontech Laboratories) to yield pGFP-Fvpr. Next, plasmids pGFP-FαH1 and pGFP-FαH2 and plasmids with site-specific mutations, such as pGFP-FαLA/αH1, pGFP-FI60P/αH2, and pGFP-FL67P/αH2, were generated by PCR with the following primers and templates: αH1, 5′-GCGGATATCCGAATGGACACTAGAG-3′ and 5′-ATCCCCGCGGAAAATGTCTAACAGC-3′ with pSK-Fvpr as the template; αLA/αH1, 5′-GCGGATATCCGAATGGACAGCCGA-3′ and 5′-ATCCCCGCGGAAAATGTCTAACAGC-3′ with pSK-FαLA as the template; αH2, I60P/αH2, and L67P/αH2, 5′-GGCGGATATCCATCTATGAAAC-3′ and 5′-CGCGGATCCCCAATTCTGAAA-3′ with pSK-Fvpr, pSK-FI60P (26), and pSK-FL67P (26) as the templates, respectively. The αH1 and αLA/αH1 fragments were prepared by digestion with EcoRV and SacII, and the αH2, I60P/αH2, and L67P/αH2 fragments were prepared by digestion with EcoRV and BamHI. Each fragment was subcloned into pGFP-Fvpr that had been digested with same respective enzymes to introduce the Flag tag at the amino terminus of each GFP fusion protein. We also constructed expression vectors that encode a chimeric Flag-GFP fusion protein as a negative control and a chimeric Flag-SV40 NLS-GFP fusion protein as a positive control. The expression of each chimeric protein was examined by radioimmunoprecipitation assay with Flag-specific MAb M2. We observed bands of proteins with apparent molecular masses consistent with the predicted sequences in our analysis of HeLa cells that had been transfected with each of our constructs (Fig. 3C). The molecular masses of Flag, GFP, and Vpr are 1, 27, and 15 kDa, respectively. Thus, the sizes of the various fusion proteins were substantially below the limit of 40 to 60 kDa for passive diffusion of proteins through the nuclear pore complex (15). Therefore, we examined whether the fusion proteins were retained in the nuclear compartment by binding to cellular factors after their entry into the nucleus. To eliminate nonanchored chimeric proteins after transfection of HeLa cells, we treated cells with digitonin at 40 μg/ml in PBS for 3 min on ice and washed them once with cold PBS and then once with 0.025% Triton X-100 for 5 min on ice before fixation in 1% formaldehyde and permeabilization in 0.2% Triton X-100. The cells on coverslips were then examined by immunofluorescence staining. To confirm the validity of this assay, we used expression vectors that encode chimeric Flag-GFP and Flag-SV40 NLS-GFP fusion proteins (Fig. 3D, a to d). The NLS in the large T antigen of SV40, as previously reported (1), is imported into the nucleus via the importin α/β pathway. As we had expected, in transiently transfected HeLa cells, the chimeric Flag-GFP fusion protein was distributed throughout the cells in the absence of prior treatment with digitonin and Triton X-100 (Fig. 3D, a). Treatment with digitonin and Triton X-100 completely eliminated the fluorescence of Flag-GFP (Fig. 3D, b). By contrast, the chimeric Flag-SV40 NLS-GFP fusion protein was localized in the nucleus in both untreated and digitonin- and Triton X-100-treated cells (Fig. 3D, c and d). Thus, it appears that treatment with digitonin and Triton X-100 prior to fixation did not affect the signal-mediated nuclear transport process.
FIG. 3.
Nuclear import by predicted α-helical domains αH1 and αH2. (A) Construction of plasmids derived from pEGFP-N1 that encode wild-type Vpr, αH1, αH2, or a substitution mutant form of Vpr fused at the carboxyl terminus to GFP and at the amino terminus to the Flag tag. The helical regions of Vpr encoded by each plasmid are in gray; the positions of the substituted amino acids are numbered, and amino acids are indicated in the single-letter code. (B) Amino acid sequences of αH1, αH2, and the NLS of the large T antigen of SV40. (C) Analysis by radiolabeling and immunoprecipitation of transfected HeLa cells that express the indicated fusion proteins. One day after transfection with the pEGFP-N1 plasmids indicated above the lanes, HeLa cells were incubated with Redivue Pro-Mix (a mixture of [35S]methionine and [35S]cysteine [1,000 Ci/mmol]; Amersham Pharmacia Biotech) at a concentration of 200 μCi/ml for 2 h. The cells were lysed at 4°C in lysis buffer {0.15 M NaCl, 0.05 M Tris-HCl [pH 8.5], 5 mM 3-[(3-cholamidopropyl) dimethylammonio]-1-propanesulfonic acid [CHAPS; Sigma]} with a cocktail of protease inhibitors (Boehringer Mannheim Biochemicals) and centrifuged for 20 min at 17,000 × g and 4°C to remove cell debris. Supernatants were subjected to immunoprecipitation with Flag-specific MAb M2 and protein A-Sepharose 4FF (50% [vol/vol]; Amersham Pharmacia Biotech.). The immunoprecipitates were dissolved in 0.05 M Tris-HCl (pH 6.8)–0.1 M dithiothreitol–2% sodium dodecyl sulfate–10% glycerol–0.001% bromphenol blue, heated at 100°C for 5 min, and analyzed by sodium dodecyl sulfate–5 to 20% polyacrylamide gel electrophoresis with subsequent fluorography. Arrowheads indicate the positions of chimeric proteins. (D) Photomicrographs of transfected HeLa cells that express the indicated fusion constructs. HeLa cells were transfected with derivatives of pEGFP-N1 that encode Flag-Vpr-GFP (e and f), Flag-αH1-GFP (g and h), Flag-αLA/αH1-GFP (i and j), Flag-αH2-GFP (k and l), Flag-I60P/αH2-GFP (m and n), Flag-L67P/αH2-GFP (o and p), Flag-SV40 NLS-GFP (c and d), or the control protein Flag-GFP (a and b). At 24 h after transfection, cells were treated (+) with digitonin at 40 μg/ml and then with 0.025% Triton X-100 (b, d, f, h, j, l, n, and p) or were not treated (−) with these reagents (a, c, e, g, i, k, m, and o) and then they were fixed in 1% formalin. The fusion proteins were detected by indirect immunofluorescence staining with Flag-specific MAb M2 and Cy3-conjugated antibodies against mouse IgG. Then they were examined by confocal laser scanning microscopy at a focal plane near the center of each nucleus. Bar, 20 μm.
In the absence of initial treatment with digitonin and Triton X-100, the pattern of immunofluorescence staining of the Flag-Vpr-GFP fusion protein was not significantly different from that of wild-type Vpr, as shown in Fig. 1B (Fig. 3D, e). The subcellular localization was almost completely retained even after the initial treatment with digitonin and Triton X-100 (Fig. 3D, f). The chimeric protein with the first α-helical domain of Vpr, Flag-αH1-GFP, was targeted to the nucleus, with weak and diffuse cytoplasmic staining, in cells that had not been treated with digitonin and Triton X-100 (Fig. 3D, g). Treatment with digitonin and Triton X-100 eliminated much of the diffuse cytoplasmic staining but not the nuclear staining of Flag-αH1-GFP (Fig. 3D, h). Thus, it appeared that the αH1 domain might be anchored in the nuclear compartment by binding to cellular factors. After replacement of the leucine residues at positions 20, 22, 23, and 26 within the first α-helical domain with alanine residues, the capacity for nuclear localization of αH1 was attenuated (Fig. 3D, i) and treatment with digitonin and Triton X-100 almost eliminated the fluorescence of Flag-αLA/αH1-GFP (Fig. 3D, j). Likewise, the nuclear localization of the chimeric protein with the αH2 domain, Flag-αH2-GFP, was more clearly evident than that of Flag-αH1-GFP in the absence of treatment with digitonin and Triton X-100 (Fig. 3D, k). After treatment with digitonin and Triton X-100, Flag-αH2-GFP was observed in the cytoplasm, as well as in the nucleus, as in the case of Flag-Vpr-GFP (Fig. 3D, l), suggesting that the αH2 domain remains in both the nuclear and cytosolic compartments as a result of binding to cellular factors. In the cases of Flag-I60P/αH2-GFP and Flag-L67P/αH2-GFP, the capacity for nuclear localization was almost totally absent (Fig. 3D, m and o) and the immunostaining patterns were partially resistant to the initial treatment with digitonin and Triton X-100 (Fig. 3D, n and p). In addition, detection of Flag-αH1-GFP was more sensitive to treatment with Triton X-100 than that of Flag-αH2-GFP: Flag-αH1-GFP, but not Flag-αH2-GFP, was almost completely undetectable in cells that had been treated with digitonin at 40 μg/ml and 0.05% Triton X-100 (data not shown). These observations suggest that maintenance of the two α-helical structures is important for binding to cellular factors and, in addition, that the nuclear targeting mediated by the αH1 and αH2 domains involves at least two mechanisms.
Our results suggest that the karyophilic properties of HIV-1 Vpr can be attributed to the two putative α-helical domains located between residues 17 and 34 and between residues 46 and 74. (i) Introduction of mutations into the αH1 and αH2 domains of Vpr partially impaired the capacity for nuclear localization. (ii) Double mutations within the αH1 and αH2 domains resulted in complete loss of the nuclear and nuclear-membrane localization of Vpr. By contrast, the carboxyl terminus of Vpr, which most closely resembles a classical NLS, is greatly involved in its nuclear localization (17, 39, 41). However, contrary results have also been reported: this region can be deleted with no impairment of nuclear localization (8). Moreover, a peptide derived from this region did not function as an active NLS (19). Our results clearly indicate that the carboxy-terminal region of Vpr is not absolutely required for nuclear localization. The C81 mutant, namely, Vpr with a deletion of 15 aa at its carboxyl terminus, gave an immunofluorescence staining pattern similar to that of wild-type Vpr. Furthermore, substitutions within the αH1 or αH2 domain of Vpr with an intact carboxy-terminal region resulted in diffuse expression throughout cells. We also showed that the αH1 and αH2 domains independently targeted chimeric proteins that included GFP and a Flag tag to the nuclei of HeLa cells, and in addition, each fusion protein was apparently retained in the nucleus by binding to cellular factors after entry into the nucleus. These results support previous suggestions that nuclear localization of Vpr is a signal-mediated process (9, 17) and that Vpr interacts with importin α and with several nuclear pore proteins that contain FXFG repeats, such as Nsp1p, Nup1p, and Pom121 (9, 28, 36). Thus, the two α-helical domains might be involved in nuclear import via binding to various cellular factors.
How does Vpr mediate nuclear targeting? It has been reported that Vpr uses a signal-mediated import pathway that does not involve NLS or M9 signals (17, 29). Jenkins and colleagues (17) showed that the nuclear import of Vpr in vitro proceeds independently of protein factors provided by a rabbit reticulocyte lysate and also independently of exogenously added nucleoside triphosphates. These characteristics of Vpr do not coincide with those of NLS- or M9-binding proteins (1, 27), suggesting that as yet unidentified cellular factors are involved in the import process. We did confirm that maintenance of α-helical structure is important for retention in specific cellular compartments via binding to cellular factors, and we showed that the αH1 or αH2 domain might mediate nuclear localization of Vpr by at least two mechanisms. Further studies of the nuclear import of Vpr should yield new insights into the mechanism(s) by which proteins move from one side of the nuclear pore complex to the other. It is now essential to identify the receptor that is involved in the nuclear localization of Vpr.
For entry of the PIC into the nucleus of a host cell, HIV has evolved specific mechanisms that are independent of the breakdown of the nuclear membrane. These mechanisms involve mainly Vpr and, to some extent, MA (10, 11). Such functional redundancy underscores the importance of infection by HIV of nondividing cells, such as macrophages and dendritic cells, which are presumed to be among the first cells infected (3). Therefore, further study is required to define clearly the function of the αH1 or αH2 domain in the infection of nondividing cells by HIV. Moreover, suppression of the nuclear import of the PIC is likely to have a profound effect on the ability of HIV to infect host cells. Thus, the αH1 and αH2 domains of Vpr might be attractive targets for the development of novel anti-HIV therapies.
Acknowledgments
This study was supported in part by a grant from the Japan Health Science Foundation, by grants for AIDS research from the Ministry and Education, Science and Culture of Japan, and by a special grant for the promotion of research from RIKEN.
REFERENCES
- 1.Adam S A, Gerace L. Cytosolic proteins that specifically bind nuclear location signals are receptors for nuclear import. Cell. 1991;66:837–847. doi: 10.1016/0092-8674(91)90431-w. [DOI] [PubMed] [Google Scholar]
- 2.Ayyavoo V, Mahboubi A, Mahalingam S, Ramalingam R, Kudchodkar S, Williams W V, Green D R, Weiner D B. HIV-1 Vpr suppresses immune activation and apoptosis through regulation of nuclear factor κB. Nat Med. 1997;3:1117–1123. doi: 10.1038/nm1097-1117. [DOI] [PubMed] [Google Scholar]
- 3.Berger E A, Murphy P M, Farber J M. Chemokine receptors as HIV-1 coreceptors: roles in viral entry, tropism, and disease. Annu Rev Immunol. 1999;17:657–700. doi: 10.1146/annurev.immunol.17.1.657. [DOI] [PubMed] [Google Scholar]
- 4.Bukrinsky M I, Haggerty S, Dempsey M P, Sharova N, Adzhubel A, Spitz L, Lewis P, Goldfarb D, Emerman M, Stevenson M. A nuclear localization signal within HIV-1 matrix protein that governs infection of non-dividing cells. Nature. 1993;365:666–669. doi: 10.1038/365666a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bukrinsky M I, Sharova N, Dempsey M P, Stanwick T L, Bukrinskaya A G, Haggerty S, Stevenson M. Active nuclear import of human immunodeficiency virus type 1 preintegration complexes. Proc Natl Acad Sci USA. 1992;89:6580–6584. doi: 10.1073/pnas.89.14.6580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen E A, Terwilliger E F, Jalinoos Y, Proulx J, Sodroski J G, Haseltine W A. Identification of HIV-1 vpr product and function. J Acquired Immune Defic Syndr. 1990;3:11–18. [PubMed] [Google Scholar]
- 7.Conti L, Rainaldi G, Matarrese P, Varano B, Rivabene R, Columba S, Sato A, Belardelli F, Malorni W, Gessani S. The HIV-1 vpr protein acts as a negative regulator of apoptosis in a human lymphoblastoid T cell line: possible implications for the pathogenesis of AIDS. J Exp Med. 1998;187:403–413. doi: 10.1084/jem.187.3.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Di Marzio P, Choe S, Ebright M, Knoblauch R, Landau N R. Mutational analysis of cell cycle arrest, nuclear localization, and virion packaging of human immunodeficiency virus type 1 Vpr. J Virol. 1995;69:7909–7916. doi: 10.1128/jvi.69.12.7909-7916.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fouchier R A M, Meyer B E, Simon J H, Fischer U, Albright A V, Gonzalez-Scarano F, Malim M H. Interaction of the human immunodeficiency virus type 1 Vpr protein with the nuclear pore complex. J Virol. 1998;72:6004–6013. doi: 10.1128/jvi.72.7.6004-6013.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fouchier R A M, Meyer B E, Simon J H M, Fischer U, Malim M H. HIV-1 infection of non-dividing cells: evidence that the amino-terminal basic region of the viral matrix protein is important for Gag processing but not for post-entry nuclear import. EMBO J. 1997;16:4531–4539. doi: 10.1093/emboj/16.15.4531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Freed E O, Martin M A. HIV-1 infection of non-dividing cells. Nature. 1994;369:107–108. doi: 10.1038/369107b0. [DOI] [PubMed] [Google Scholar]
- 12.Fukumori T, Akari H, Iida S, Hata S, Kagawa S, Aida Y, Koyama A H, Adachi A. The HIV-1 Vpr displays strong anti-apoptotic activity. FEBS Lett. 1998;432:17–20. doi: 10.1016/s0014-5793(98)00824-2. [DOI] [PubMed] [Google Scholar]
- 13.Gallay P, Hope T, Chin D, Trono D. HIV-1 infection of nondividing cells through the recognition of integrase by the importin/karyopherin pathway. Proc Natl Acad Sci USA. 1997;94:9825–9830. doi: 10.1073/pnas.94.18.9825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gallay P, Stitt V, Mundy C, Oettinger M, Trono D. Role of the karyopherin pathway in human immunodeficiency virus type 1 nuclear import. J Virol. 1996;70:1027–1032. doi: 10.1128/jvi.70.2.1027-1032.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Görlich D. Transport into and out of the cell nucleus. EMBO J. 1998;17:2721–2727. doi: 10.1093/emboj/17.10.2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.He J, Choe S, Walker R, Di Marzio P, Morgan D O, Landau N R. Human immunodeficiency virus type 1 viral protein R (Vpr) arrests cells in the G2 phase of the cell cycle by inhibiting p34cdc2 activity. J Virol. 1995;69:6705–6711. doi: 10.1128/jvi.69.11.6705-6711.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jenkins Y, McEntee M, Weis K, Greene W C. Characterization of HIV-1 vpr nuclear import: analysis of signals and pathways. J Cell Biol. 1998;143:875–885. doi: 10.1083/jcb.143.4.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jowett J B M, Planelles V, Poon B, Shah N P, Chen M-L, Chen I S Y. The human immunodeficiency virus type 1 vpr gene arrests infected T cells in the G2 + M phase of the cell cycle. J Virol. 1995;69:6304–6313. doi: 10.1128/jvi.69.10.6304-6313.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karni O, Friedler A, Zakai N, Gilon C, Loyter A. A peptide derived from the N-terminal region of HIV-1 Vpr promotes nuclear import in permeabilized cells: elucidation of the NLS region of the Vpr. FEBS Lett. 1998;429:421–425. doi: 10.1016/s0014-5793(98)00645-0. [DOI] [PubMed] [Google Scholar]
- 20.Levy D N, Fernandes L S, Williams W V, Weiner D B. Induction of cell differentiation by human immunodeficiency virus 1 vpr. Cell. 1993;72:541–550. doi: 10.1016/0092-8674(93)90073-y. [DOI] [PubMed] [Google Scholar]
- 21.Mahalingam S, Ayyavoo V, Patel M, Kieber-Emmons T, Weiner D B. Nuclear import, virion incorporation, and cell cycle arrest/differentiation are mediated by distinct functional domains of human immunodeficiency virus type 1 Vpr. J Virol. 1997;71:6339–6347. doi: 10.1128/jvi.71.9.6339-6347.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mahalingam S, Collman R G, Patel M, Monken C E, Srinivasan A. Functional analysis of HIV-1 Vpr: identification of determinants essential for subcellular localization. Virology. 1995;212:331–339. doi: 10.1006/viro.1995.1490. [DOI] [PubMed] [Google Scholar]
- 23.Mahalingam S, Khan S A, Jabbar M A, Monken C E, Collman R G, Srinivasan A. Identification of residues in the N-terminal acidic domain of HIV-1 Vpr essential for virion incorporation. Virology. 1995;207:297–302. doi: 10.1006/viro.1995.1081. [DOI] [PubMed] [Google Scholar]
- 24.Mahalingam S, Khan S A, Murali R, Jabbar M A, Monken C E, Collman R G, Srinivasan A. Mutagenesis of the putative alpha-helical domain of the Vpr protein of human immunodeficiency virus type 1: effect on stability and virion incorporation. Proc Natl Acad Sci USA. 1995;92:3794–3798. doi: 10.1073/pnas.92.9.3794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nie Z, Bergeron D, Subbramanian R A, Yao X J, Checroune F, Rougeau N, Cohen E A. The putative alpha helix 2 of human immunodeficiency virus type 1 Vpr contains a determinant which is responsible for the nuclear translocation of proviral DNA in growth-arrested cells. J Virol. 1998;72:4104–4115. doi: 10.1128/jvi.72.5.4104-4115.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25a.Nishino Y, Myojin T, Kamata M, Aida Y. Human immunodeficiency virus type 1 vpr gene product prevents cell proliferation on mouse NIH3T3 cells without the G2 arrest of the cell cycle. Biochem Biophys Res Commun. 1997;232:550–554. doi: 10.1006/bbrc.1997.6186. [DOI] [PubMed] [Google Scholar]
- 25b.Nishizawa M, Kamata M, Katsumata R, Aida Y. A carboxy-terminally truncated form of the human immunodeficiency virus type 1 Vpr protein induces apoptosis via G1 cell cycle arrest. J Virol. 2000;74:6058–6067. doi: 10.1128/jvi.74.13.6058-6067.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nishizawa M, Myojin T, Nishino Y, Nakai Y, Kamata M, Aida Y. A carboxy-terminally truncated form of the Vpr protein of human immunodeficiency virus type 1 retards cell proliferation independently of G2 arrest of the cell cycle. Virology. 1999;263:313–322. doi: 10.1006/viro.1999.9905. [DOI] [PubMed] [Google Scholar]
- 27.Pollard V W, Michael W M, Nakielny S, Siomi M C, Wang F, Dreyfuss G. A novel receptor-mediated nuclear protein import pathway. Cell. 1996;86:985–994. doi: 10.1016/s0092-8674(00)80173-7. [DOI] [PubMed] [Google Scholar]
- 28.Popov S, Rexach M, Ratner L, Blobel G, Bukrinsky M. Viral protein R regulates docking of the HIV-1 preintegration complex to the nuclear pore complex. J Biol Chem. 1998;273:13347–13352. doi: 10.1074/jbc.273.21.13347. [DOI] [PubMed] [Google Scholar]
- 29.Popov S, Rexach M, Zybarth G, Reiling N, Lee M A, Ratner L, Lane C M, Moore M S, Blobel G, Bukrinsky M. Viral protein R regulates nuclear import of the HIV-1 pre-integration complex. EMBO J. 1998;17:909–917. doi: 10.1093/emboj/17.4.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Re F, Braaten D, Franke E K, Luban J. Human immunodeficiency virus type 1 Vpr arrests the cell cycle in G2 by inhibiting the activation of p34cdc2-cyclin B. J Virol. 1995;69:6859–6864. doi: 10.1128/jvi.69.11.6859-6864.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roques B P, Morellet N, de Rocquigny H, Déméné H, Schueler W, Jullian N. Structure, biological functions and inhibition of the HIV-1 proteins Vpr and NCp7. Biochimie. 1997;79:673–680. doi: 10.1016/s0300-9084(97)83501-8. [DOI] [PubMed] [Google Scholar]
- 32.Schüler W, Wecker K, de Rocquigny H, Baudat Y, Sire J, Roques B P. NMR structure of the (52-96) C-terminal domain of the HIV-1 regulatory protein Vpr: molecular insights into its biological functions. J Mol Biol. 1999;285:2105–2117. doi: 10.1006/jmbi.1998.2381. [DOI] [PubMed] [Google Scholar]
- 33.Stewart S A, Poon B, Jowett J B, Chen I S Y. Human immunodeficiency virus type 1 Vpr induces apoptosis following cell cycle arrest. J Virol. 1997;71:5579–5592. doi: 10.1128/jvi.71.7.5579-5592.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Subbramanian R A, Yao X J, Dilhuydy H, Rougeau N, Bergeron D, Robitaille Y, Cohen E A. Human immunodeficiency virus type 1 Vpr localization: nuclear transport of a viral protein modulated by a putative amphipathic helical structure and its relevance to biological activity. J Mol Biol. 1998;278:13–30. doi: 10.1006/jmbi.1998.1685. [DOI] [PubMed] [Google Scholar]
- 35.Tajima S, Zhuang W Z, Kato M V, Okada K, Ikawa Y, Aida Y. Function and conformation of wild-type p53 protein are influenced by mutations in bovine leukemia virus-induced B-cell lymphosarcoma. Virology. 1998;243:235–246. [PubMed] [Google Scholar]
- 36.Vodicka M A, Koepp D M, Silver P A, Emerman M. HIV-1 Vpr interacts with the nuclear transport pathway to promote macrophage infection. Genes Dev. 1998;12:175–185. doi: 10.1101/gad.12.2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang L, Mukherjee S, Jia F, Narayan O, Zhao L J. Interaction of virion protein Vpr of human immunodeficiency virus type 1 with cellular transcription factor Sp1 and trans-activation of viral long terminal repeat. J Biol Chem. 1995;270:25564–25569. doi: 10.1074/jbc.270.43.25564. [DOI] [PubMed] [Google Scholar]
- 38.Weinberg J B, Matthews T J, Cullen B R, Malim M H. Productive human immunodeficiency virus type 1 (HIV-1) infection of nonproliferating human monocytes. J Exp Med. 1991;174:1477–1482. doi: 10.1084/jem.174.6.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yao X J, Subbramanian R A, Rougeau N, Boisvert F, Bergeron D, Cohen E A. Mutagenic analysis of human immunodeficiency virus type 1 Vpr: role of a predicted N-terminal alpha-helical structure in Vpr nuclear localization and virion incorporation. J Virol. 1995;69:7032–7044. doi: 10.1128/jvi.69.11.7032-7044.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhao L J, Mukherjee S, Narayan O. Biochemical mechanism of HIV-I Vpr function. Specific interaction with a cellular protein. J Biol Chem. 1994;269:15577–15582. [PubMed] [Google Scholar]
- 41.Zhou Y, Lu Y, Ratner L. Arginine residues in the C-terminus of HIV-1 Vpr are important for nuclear localization and cell cycle arrest. Virology. 1998;242:414–424. doi: 10.1006/viro.1998.9028. [DOI] [PubMed] [Google Scholar]