Abstract
Aims
Although low-intensity pulsed ultrasound (LIPUS) combined with disinfectants has been shown to effectively eliminate portions of biofilm in vitro, its efficacy in vivo remains uncertain. Our objective was to assess the antibiofilm potential and safety of LIPUS combined with 0.35% povidone-iodine (PI) in a rat debridement, antibiotics, and implant retention (DAIR) model of periprosthetic joint infection (PJI).
Methods
A total of 56 male Sprague-Dawley rats were established in acute PJI models by intra-articular injection of bacteria. The rats were divided into four groups: a Control group, a 0.35% PI group, a LIPUS and saline group, and a LIPUS and 0.35% PI group. All rats underwent DAIR, except for Control, which underwent a sham procedure. General status, serum biochemical markers, weightbearing analysis, radiographs, micro-CT analysis, scanning electron microscopy of the prostheses, microbiological analysis, macroscope, and histopathology evaluation were performed 14 days after DAIR.
Results
The group with LIPUS and 0.35% PI exhibited decreased levels of serum biochemical markers, improved weightbearing scores, reduced reactive bone changes, absence of viable bacteria, and decreased inflammation compared to the Control group. Despite the greater antibiofilm activity observed in the PI group compared to the LIPUS and saline group, none of the monotherapies were successful in preventing reactive bone changes or eliminating the infection.
Conclusion
In the rat model of PJI treated with DAIR, LIPUS combined with 0.35% PI demonstrated stronger antibiofilm potential than monotherapy, without impairing any local soft-tissue.
Cite this article: Bone Joint Res 2024;13(7):332–341.
Keywords: Low-intensity pulsed ultrasound; Periprosthetic joint infection; Rat model; povidone-iodine; low-intensity pulsed ultrasound (LIPUS); rat model; periprosthetic joint infection (PJI); saline; biofilms; debridement, antibiotics, and implant retention; bacteria; soft-tissues; micro-CT scans
Article focus
Does low-intensity pulsed ultrasound (LIPUS) or 0.35% povidone-iodine (PI) treat acute periprosthetic joint infection (PJI) in rats?
Does LIPUS augment the efficacy of 0.35% PI to eliminate bacterial biofilm in vivo?
Does LIPUS combined with 0.35% PI impair the soft-tissue?
Key messages
LIPUS combined with 0.35% PI alleviated acute PJI in rats more effectively than monotherapy, and did not impair the soft-tissue.
Strengths and limitations
This study was the first to combine LIPUS with 0.35% PI in a rat debridement, antibiotics, and implant retention model of acute PJI, and to observe its safety in soft-tissue.
A limitation is the absence of adjuvant oral antibiotics to completely mimic clinical conditions.
Introduction
Periprosthetic joint infection (PJI) is a challenging complication affecting approximately 1% to 2% of patients undergoing primary arthroplasty.1 By 2030, it is projected that the numbers of annual procedures for total knee arthroplasty and total hip arthroplasty in the USA will reach 935,000 and 635,000, respectively.2 The economic burden associated with PJI is substantial, with estimated annual costs of approximately $1.1 billion and $750 million for hip and knee infections, respectively.3
Methicillin-resistant Staphylococcus aureus (MRSA) infections have accounted for up to 19.2% of all PJIs, presenting substantial challenges for both surgeons and patients.4,5 When treating acute MRSA PJIs, orthopaedic surgeons prefer debridement, antibiotics, and implant retention (DAIR) over one-stage or two-stage approaches due to its less disruptive nature and ability to preserve the prosthesis.6-9 However, despite the use of rifampin-based combination therapy, which was used for antibiofilm and has improved the success rate, the failure rate of DAIR remains relatively high, ranging from 42% to 45%.10,11 These failures are often attributed to the presence of residual biofilm during the DAIR procedure.12 To mitigate this risk, numerous antiseptics and antibiotics solutions have been used as adjuncts to eradicate biofilm,13-20 of which povidone-iodine (PI) is a common antiseptic solution for the treatment of PJI intraoperatively. Nevertheless, the success rate of DAIR for acute PJI using PI remains unsatisfactory, ranging from 69% to 74%.21-23 However, the specific concentration of PI used in these studies was not mentioned, although 0.35% PI is frequently employed during arthroplasty procedures to prevent PJI.24 Therefore, it is crucial to explore methods that can enhance the efficacy of 0.35% PI in biofilm eradication.
The use of ultrasound to augment the antibacterial activity of antibiotics was initially introduced in 1994 by Pitt et al,25 who demonstrated that low-intensity pulsed ultrasound (LIPUS) enhances antibiofilm activity of antibiotics in vivo.26,27 Furthermore, ultrasound has been found to augment the antibiofilm activity of disinfectants such as ozone in vitro.28,29
The biological impact of ultrasound on microorganisms is attributed to its acoustical cavitation,26 which effectively eliminates extracellular polysaccharides and proteins present in microbial biofilms. Additionally, ultrasound can modify the structure of proteins that constitute the extracellular polymeric substances (EPS), which initially act as barriers against chemical disinfectants.30 Based on previous studies,26-29 we believe that the combination of LIPUS and 0.35% PI can effectively eliminate biofilms. However, no studies have been reported on this specific topic. Therefore, the aim of this study was to investigate the antibiofilm potential and safety of LIPUS combined with 0.35% PI in a rat DAIR model of PJI. Various parameters, including general status, serum biochemical markers, weightbearing analysis, radiographs, micro-CT analysis, scanning electron microscopy (SEM) of the prostheses, microbiological analysis, macroscope evaluation, and histopathology assessment, were examined. We hypothesized that LIPUS can enhance the efficacy of 0.35% PI in biofilm eradication without impairing local soft-tissue in these models.
Methods
Animals and ethical approval
A total of 56 Sprague-Dawley rats of specific pathogen-free (SPF) grade (male, aged 11 weeks, mean weight 259 g (standard deviation (SD) 6)) were procured from the Animal Center of Xinjiang Medical University (Ürümqi, China). The rats were individually tagged and housed in ventilated cages, with five animals per cage, under controlled conditions of 22°C ± 2°C temperature (humidity: 55% ± 5%) and a 12-hour dark/light cycle. The rats were provided with ad libitum access to a standard rodent diet and water. The study protocol received approval from the Animal Center of Xinjiang Medical University, and was conducted in accordance with the guidelines of the Institutional Animal Care and Use Committee (IACUC). An ARRIVE checklist is included in the Supplementary Material to show that the ARRIVE guidelines were adhered to in this study.
Bacteria
Individual strains (MRSA; ATCC BAA-1556) were grown in tryptic soy broth (TSB; Solarbio, China). The log-phase bacterial suspension was centrifuged (the supernatant was discarded), and was then resuspended with PBS to achieve a concentration of approximately 1.5 × 107 colony-forming units (CFUs)/ml.
In vivo rat model of acute PJI
As occurred in our previous experiments, all the rats survived.31 A total of 56 rats were randomly divided into four groups: 1) Control group (sham, n = 14); 2) PI group (0.35% PI, n = 14); 3) LIPUS and saline group (LIPUS combined with saline, n = 14); and 4) LIPUS and PI group (LIPUS combined with 0.35% PI, n = 14). LIPUS with a frequency of 25 KHz and intensity of 300 mW/cm2 (duty cycle of 70%) was applied.32 Based on a study investigating DAIR for acute knee PJI in mice,33 general anaesthesia was induced by intraperitoneal administration of ketamine (60 mg/kg) and xylazine (6 mg/kg). The surgeon (TW, CY) was blinded to group allocations. Subsequently, the left legs of all the rats were shaved and disinfected. A medial parapatellar approach was employed, followed by patellar dislocation to expose the distal part of the femur. In four groups, the femur medullary canal was drilled using a 1.2 mm Kirschner wire, and a medical-grade screw (1.5 mm diameter, 14 mm length; Baishe, China) made of titanium alloy (Ti-6AI-4V) was implanted into the canal. Following suturing of the capsule, 30 μl of 1.5 × 107 CFUs/ml suspension of ATCC BAA-1556 was injected into the articular cavity (Supplementary Figure a). Post-surgery pain was managed using buprenorphine at a dosage of 0.1 mg/kg for three days.
Surgical procedure for DAIR
On day 5, all rats were anaesthetized by intraperitoneal administration of ketamine (60 mg/kg) and xylazine (6 mg/kg). Blinding was not possible as the PI could not be concealed from the surgeon. The left knee was shaved and disinfected. After sterile draping of the surgical site, a 2 cm skin incision was made along the old incision. After the articular cavity was exposed, infected and inflammatory synovium and soft-tissues were removed. Then, according to their dedicated groups, the knee was immersed in 80 ml PI or saline loaded into a sterile funnel (diameter 90 mm) for three minutes, or combined with a LIPUS probe located in the middle of the funnel for three minutes (Supplementary Figure b). Thereafter, the joint was washed with saline prior to the capsule closure. Subsequently, they received intraperitoneal injections of vancomycin (88 mg/kg, 1 ml, every 12 hours, equivalent to 1 g intravenous (IV) every 12 hours in a patient weighing 70 kg) for two weeks, but the Control group received just 1 ml of saline.34 On day 19, all rats were euthanized with overdose of ketamine and xylazine. Then, the tissue was harvested in compliance with the approved protocol of the IACUC (Figure 1).
Fig. 1.
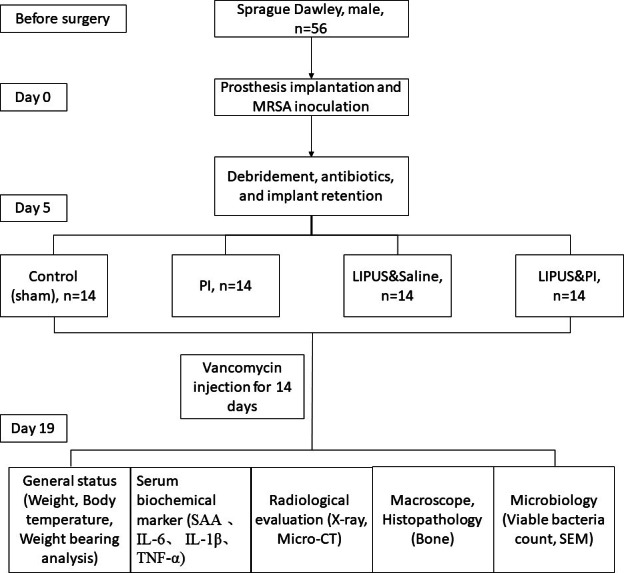
Flowchart illustrating the treatment scheme for the animals in this experiment. After all the rats developed acute periprosthetic joint infection (PJI), the four treatment groups subjected to debridement, antibiotics, and implant retention (DAIR) were as follows: Control group (sham); PI group (0.35% povidone-iodine); LIPUS and saline group (low-intensity pulsed ultrasound combined with saline); and LIPUS and PI group (low-intensity pulsed ultrasound combined with 0.35% povidone-iodine). IL, interleukin; MRSA, methicillin-resistant Staphylococcus aureus; SAA, serum amyloid A; SEM, scanning electron microscopy; TNF-α, tumour necrosis factor-alpha.
General status and serum biochemical markers
The body temperature and weight of the rats (n = 6 per group) were measured before surgery and on days 1, 3, 5, 9, 13, and 19. The serum amyloid A (SAA), interleukin (IL)-6, tumour necrosis factor-alpha (TNF-α), and IL-1β were used as serum biochemical markers of acute infection in rats (n = 6 per group), as previously described,31,35 and were measured with an enzyme-linked immunosorbent assay kit (Cusabio, China).
Weightbearing analysis
The weightbearing analysis of the rats (n = 6 per group) was assessed using ink blot analysis, and was graded for each rat as full (3 points), partial (2 points), toe-touch (1 point), or no weightbearing (0 points).36 The front paws of the rats were covered with yellow ink, and the hind paws were covered with green ink.
Radiograph evaluation and micro-CT analysis of the knee
Anteroposterior and lateral radiograph images were obtained for the left limbs of the animals (n = 6 per group) on day 19 (Kangpai Medical Technology, China). A high-resolution micro-CT of the femur (n = 5 per group) was conducted using a SkyScan 1172 scanner (Bruker, Germany). The distal 5 mm of the stem and 3 mm from the implant surface were selected as the regions of interest. 3D structural parameters, including bone mineral density (BMD) (g cm-3), bone volume fraction (BV/TV) (%), and trabecular thickness (Tb.Th) (mm), were analyzed to evaluate reactive bone changes. The results of radiographs and micro-CT were assessed by a single, experienced observer (YW) blinded to the treatment group.
Microbiological analysis
After euthanasia, tissues, bone, and prostheses (n = 6 per group) were collected with sterile instruments. The tissues and bone were cut into small pieces, weighed, placed in sterile saline solution (ratio of tissues or bone to saline solution was 1:4, w/v), and homogenized using a sterile tissue grinder; the retrieved prosthesis was placed in 2 ml sterile saline solution, sonicated for five minutes, and vortexed for 30 seconds to dislodge any attached bacteria.37 The suspensions from the homogenized tissues, bone, and sonicated prostheses were serially diluted, and 100 µl of each dilution was plated on TSB agar plates, which were incubated overnight at 37°C.
Scanning electron microscopy
The prostheses (n = 3 per group) were fixed in 2.5% glutaraldehyde at 4°C for 24 hours and osmium acid for two hours, dehydrated in alcohol gradient, dried with a critical point dryer, coated with a conductive coating using a sputter coater, and observed using a JSM-6390 LV scanning electron microscope (JEOL, Japan).
Macroscope and histopathology evaluation
Local soft-tissue and cartilage damage was evaluated on the basis of the criteria of the modified Rissing scale score (n = 14 per group).38 Bone histopathology staining (n = 6 per group) with haematoxylin and eosin (H&E) was used to evaluate the tissue morphology of any inflammation. The histological score of the tissues referred to the modified Petty’s scale.39-41 Histopathology was assessed and scored by a single, experienced observer (YW) blinded to the treatment group.
Statistical analysis
According to prior rat PJI studies,42,43 an appropriate sample size (n = 14 per group) was chosen. Data were analyzed using GraphPad Prism 9 (GraphPad Software, USA), and are presented as means and standard errors of the means. Normal distribution of data was tested with Shapiro-Wilk test. The independent-samples t-test or Mann-Whitney U test was used for comparing two groups. One-way analysis of variance (ANOVA) with Tukey’s multiple comparison test, or Kruskal-Wallis test with Dunn’s multiple comparison test, was used for more than two groups, but Mann-Whitney U test was used for the comparison of colony counts between different groups. Statistical significance was set at p < 0.05.
Results
General status, serum biochemical markers, and weightbearing analysis
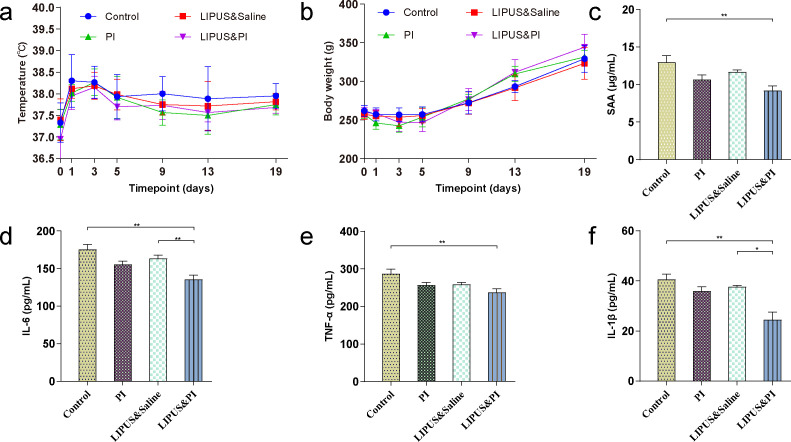
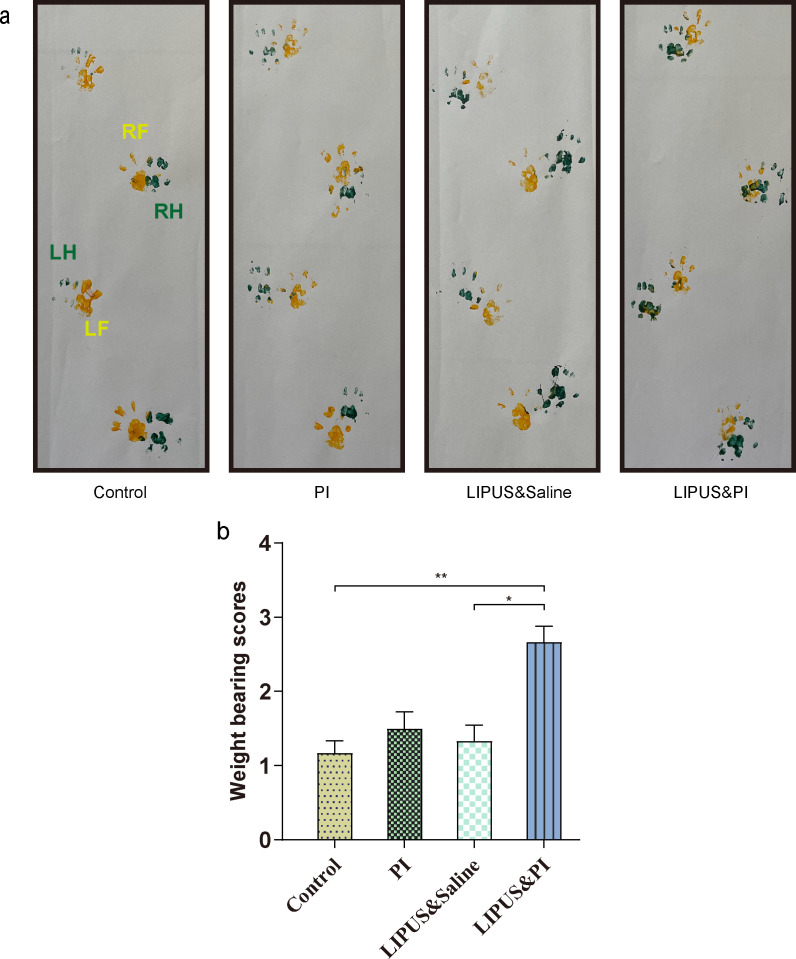
No significant differences were observed in body temperature or weight among the four groups (Figures 2a and 2b). The Control group demonstrated higher serum levels of SAA, IL-6, IL-1β, and TNF-α compared to the LIPUS and PI group (Figures 2c to 2f), however LIPUS and PI exhibited a significant reduction in the levels of IL-6 and IL-1β compared with the LIPUS and saline group (Figures 2d and 2f; p = 0.008, one-way ANOVA; p = 0.015, Kruskal-Wallis test, respectively). The weightbearing scores in the LIPUS and PI group were higher than those of the Control group and LIPUS and saline group (Figures 3a and 3b).
Fig. 2.
General status (temperature and body weight) and serum inflammatory markers. a) Changes in body temperature before surgery (day 0) and on days 1, 3, 5, 9, 13, and 19 (n = 6 per group). b) Changes in body weight before surgery (day 0) and on days 1, 3, 5, 9, 13, and 19 (n = 6 per group). c) to f) The level of: c) serum amyloid A (SAA); d) interleukin (IL)-6; e) tumour necrosis factor-alpha (TNF-α); and f) IL-1β (n = 6 per group). The four treatment groups were as follows: Control group (sham); PI group (0.35% povidone-iodine); LIPUS and saline group (low-intensity pulsed ultrasound combined with saline); and LIPUS and PI group (low-intensity pulsed ultrasound combined with 0.35% povidone-iodine). Data are presented as means and standard errors of the means. *p < 0.05, **p < 0.01; one-way analysis of variance with Tukey’s multiple comparison test or Kruskal-Wallis test with Dunn’s multiple comparison test.
Fig. 3.
Evaluation of weightbearing analysis. a) Representative images of ink blotting trails of different groups. RH, right hind (green); LH, left hind (green); RF, right front (yellow); LF, left front (yellow). b) Grade of weightbearing score among the different groups (n = 6 per group). The four treatment groups were as follows: Control group (sham); PI group (0.35% povidone-iodine); LIPUS and saline group (low-intensity pulsed ultrasound combined with saline); and LIPUS and PI group (low-intensity pulsed ultrasound combined with 0.35% povidone-iodine). Data are presented as means and standard errors of the means. *p < 0.05, **p < 0.01; one-way analysis of variance.
Radiological evaluation
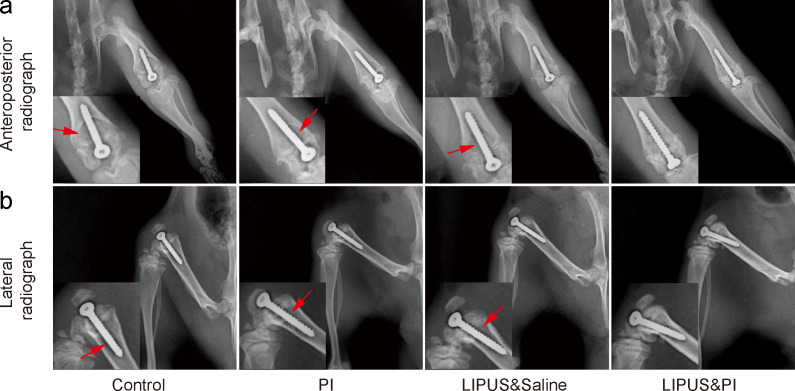
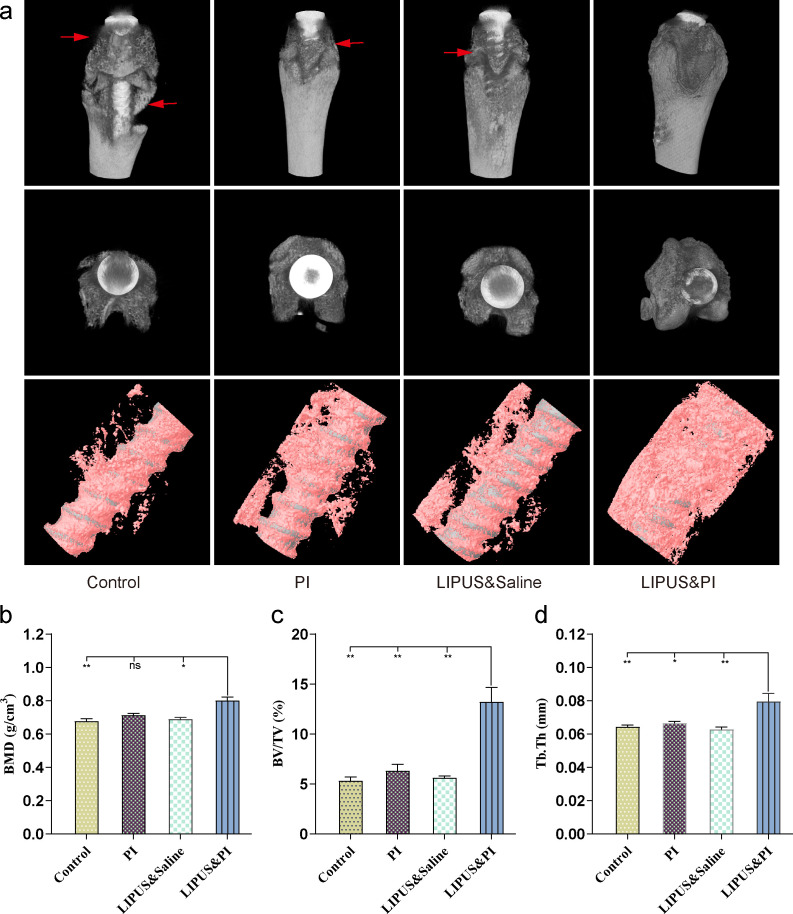
Radiographs showed that the prostheses were still in position within the distal femoral metaphysis. All except the LIPUS and PI groups were accompanied by signs of osteolysis around the prosthesis, of which the Control group was the most serious, while milder osteolysis was observed in rats from the PI group, and LIPUS and saline groups. No osteolysis was observed in the LIPUS and PI group (Figures 4a and 4b). Additionally, 3D micro-CT scans indicated severe bone destruction in the Control group, whereas only mild bone destruction was seen in the PI and LIPUS and saline groups. There was no bone destruction observed in the LIPUS and PI group (Figure 5a). Moreover, the LIPUS and PI group exhibited higher values in BMD, BV/TV, and Tb.Th compared to the Control, PI, or LIPUS and saline groups (Figures 5b to 5d).
Fig. 4.
Radiograph evaluation of the knee joint and prosthesis on day 19. a) Anteroposterior radiograph and b) lateral radiograph. Red arrows indicate osteolysis (n = 6 per group). The four treatment groups were as follows: Control group (sham); PI group (0.35% povidone-iodine); LIPUS and saline group (low-intensity pulsed ultrasound combined with saline); and LIPUS and PI group (low-intensity pulsed ultrasound combined with 0.35% povidone-iodine).
Fig. 5.
Micro-CT evaluation of the knee joint in different groups on day 19. a) Micro-CT scan of 3D and bone reconstruction of the femur with retained implants. Red arrows indicate osteolysis. b) to d) Quantitative micro-CT analysis of distal femur of: b) bone mineral density (BMD); c) bone volume fraction (BV/TV); and d) trabecular thickness (Tb.Th) (n = 5 per group). The four treatment groups were as follows: Control group (sham); PI group (0.35% povidone-iodine); LIPUS and saline group (low-intensity pulsed ultrasound combined with saline); and LIPUS and PI group (low-intensity pulsed ultrasound combined with 0.35% povidone-iodine). Data are presented as means and standard errors of the means. *p < 0.05, **p < 0.01; one-way analysis of variance with Tukey’s multiple comparison test or Kruskal-Wallis test with Dunn’s multiple comparison test. ns, non-significant.
Evaluation of microbial counts
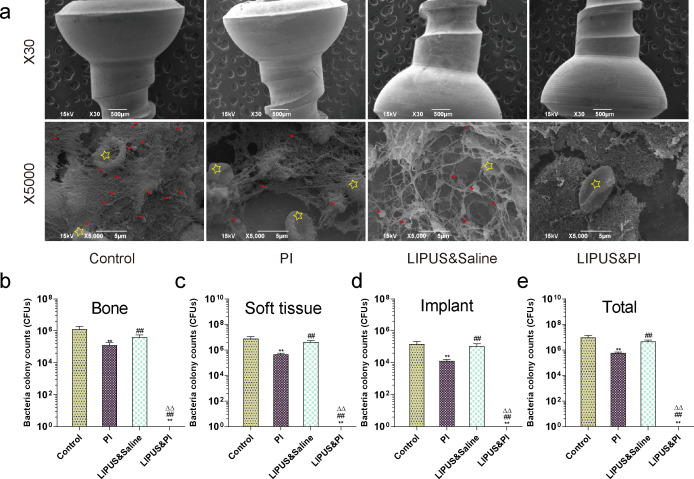
A substantial presence of MRSA bacteria was observed on the implants in the Control, PI, and LIPUS and saline groups by SEM in stark contrast to the LIPUS and PI group; these bacteria were found to be surrounded by erythrocytes. Conversely, no bacterial colonies were detected in the LIPUS and PI group (Figure 6a). The CFU counts of each sample in the LIPUS and PI group were lower than those of the Control, PI, and LIPUS and saline groups (Figures 6b to 6e).
Fig. 6.
Microbiological evaluation in each treatment group on day 19. a) The bacteria on the surface of the implant in different groups were observed by scanning electron microscopy (SEM), with high magnification (30× and 5,000×). The red arrows indicate bacteria and yellow stars indicate erythrocytes (n = 3 per group). b) to e) Analysis of bacteria culture counts from: b) knee joint bone; c) soft-tissues; d) implants; and e) total knee (n = 6 per group). The four treatment groups were as follows: Control group (sham); PI group (0.35% povidone-iodine); LIPUS and saline group (low-intensity pulsed ultrasound combined with saline); and LIPUS and PI group (low-intensity pulsed ultrasound combined with 0.35% povidone-iodine). Data are presented as means and standard errors of the means. **p < 0.01 (compared with Control group), ##p < 0.01 (compared with PI group), ΔΔp < 0.01 (compared with LIPUS and saline group); Mann-Whitney U test.
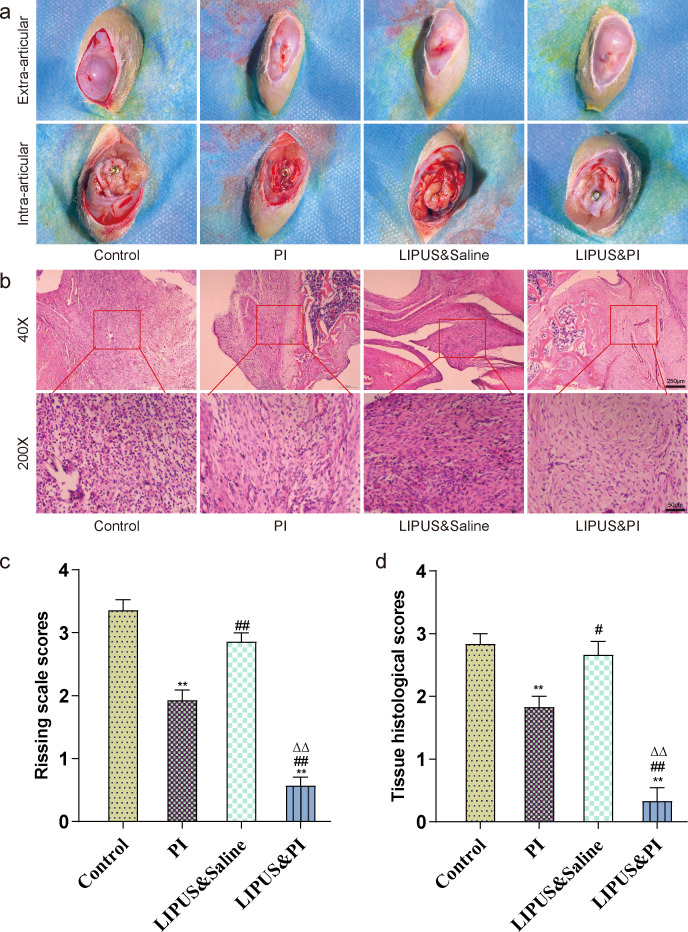
Evaluation of knee macroscope and inflammation
Macroscopic examination of the surgical knee revealed joint swelling, purulent formation, and cartilage destruction in the Control group and LIPUS and saline group. In contrast, the PI group exhibited mild changes, while the LIPUS and PI group showed complete healing without any signs of joint swelling, purulent formation, or cartilage destruction (Figure 7a). Additionally, on day 19, the Rissing scale scores in the LIPUS and PI group were lower compared to the Control, PI, and LIPUS and saline groups (Figure 7c). The bone tissues of the Control, PI, and LIPUS and saline groups exhibited purulent inflammation. However, this inflammation was significantly reduced in the LIPUS and PI group, which showed minimal inflammatory cell infiltration (Figure 7b). The LIPUS and PI group had lower Petty’s scale scores compared to the Control, PI, and LIPUS and saline groups (Figure 7d).
Fig. 7.
Macroscopic examination and histopathological assessment of the bone surrounding the prosthesis. a) Macroscopic examination of the extra-articular capsule and intra-articular bone and the prosthesis on day 19. b) Histopathological haematoxylin and eosin (H&E) staining of the femur bone in each group on day 19. c) Rissing scale scores used for assessment of soft-tissue and bone damage on day 19 (n = 14 per group). d) Mean femur bone histopathological scores based on the criteria of modified Petty’s scale on day 19 in each treatment group (n = 6 per group). The four treatment groups were as follows: Control group (sham); PI group (0.35% povidone-iodine); LIPUS and saline group (low-intensity pulsed ultrasound combined with saline); and LIPUS and PI group (low-intensity pulsed ultrasound combined with 0.35% povidone-iodine). Data are presented as means and standard errors of the means. **p < 0.01 (compared with Control group), #p < 0.05, ##p < 0.01 (compared with PI group), ΔΔp < 0.01 (compared with LIPUS and saline group); one-way analysis of variance.
Discussion
Although the efficacy of combining LIPUS with disinfectants to eliminate biofilm has been demonstrated in vitro,28,29 its effectiveness in vivo remains unclear. In this study, we investigated the use of LIPUS combined with 0.35% PI and demonstrated its ability to alleviate acute PJI compared to LIPUS or 0.35% PI alone. Furthermore, its combination did not impair local soft-tissue. This study is the first to assess the antibiofilm efficacy of LIPUS combined with 0.35% PI therapy using a rat DAIR model of PJI. Based on our results, further investigation is warranted to determine the clinical applicability of this method for effectively eliminating biofilm.
Chronic PJIs are known to cause osteolysis due to bone metabolism disorders. Radiological analysis revealed substantial radiolucency and reactive bone changes around infected implants.43 Similarly, we observed similar phenomena and substantial reactive bone changes in the control group using radiographs and micro-CT. In line with this result, previous studies have also demonstrated substantial bone destruction around infected implants caused by bacteria.34,44,45 Interestingly, the LIPUS and 0.35% PI combination group exhibited no radiolucency and minimal reactive bone changes, surpassing the efficacy of monotherapy. This finding suggests that the combination therapy may effectively eliminate bacteria and prevent osteolysis.
Biofilm is a major contributor to infection recurrence, as it provides protection to enclosed organisms against conventional antimicrobial agents and the host immune system.46 Therefore, managing biofilm is crucial in controlling PJI. PI has been recommended for controlling PJI in the context of the DAIR procedure, as stated by the International Consensus Meeting on PJI.6 In a previous in vitro study, a one-minute exposure to 0.35% PI reduced MRSA biofilm by 88.24% after 72 hours of incubation.47 Furthermore, Premkumar et al48 found that 10% PI showed the highest efficacy against biofilm-based bacteria in vitro, surpassing 0.3% PI and other commercial antiseptics after a three-minute exposure, regardless of the toxicity of high concentrations of PI. Conversely, a study in vivo showed that 0.35% PI as prophylaxis for exposure for three minutes did not change the rate of infection.49 Similarly, PI monotherapy in our study did not effectively control PJI from the result of SEM and bacterial count, possibly due to the complex and compact structure of biofilm, limiting the penetration of PI to internal bacteria.50 However, although LIPUS can eliminate EPS of biofilms by acoustic cavitation as we described previously, the LIPUS and saline group was less effective in antibiofilm than the PI group. Pitt and Ross51 confirmed that the 2 W/cm2 ultrasound simultaneously increases the rate of transport of oxygen and nutrients to bacteria, and increases the rate of transport of waste products away from bacteria, thereby enhancing biofilm growth while preserving microbial viability. Pitt and Ross51 also showed in vivo that 100 mW/cm2 LIPUS alone did not reduce bacterial viability in Escherichia coli biofilm, whereas 300 mW/cm2 LIPUS combined with gentamicin substantially reduced bacterial viability.27,32 This is in line with our results. Of note, when LIPUS was combined with 0.35% PI, biofilm elimination was observed on the implants, likely because LIPUS disrupted the biofilm, facilitating the penetration of PI to kill internal bacteria. These results align with recent studies, indicating that ultrasound can enhance the efficacy of disinfectants against biofilm in vitro.29,52 Previous studies have also found a synergistic effect of LIPUS and antibiotics on bacterial biofilm in vivo due to LIPUS, increasing penetration of the antibiotics.53,54 Notably, the antibiofilm efficacy observed through SEM and bacterial count correlated with the absence of radiolucency and minimal reactive bone changes in radiological evaluation.
While the efficacy of biofilm elimination is important, the safety of LIPUS and PI should also be considered. Rediske et al32 reported that continuous ultrasound with an intensity of 300 mW/cm2 damages soft tissue in vivo, but LIPUS with the same intensity does not cause any detrimental impact on the surrounding tissue. Additionally, LIPUS with an intensity of 300 mW/cm2 does not affect cell viability in vitro.55 However, for PI, the potential toxicity of high concentrations should not be ignored, as evidenced by the study by Newton Ede et al,56 wherein they demonstrated that osteoblasts exposed to 0.35% PI for three minutes experienced reduced bone nodule mineralization. Further, a large clinical series has demonstrated the safety and cost-effectiveness of 0.35% PI in reducing PJI for primary arthroplasty.57 However, we built a PJI model to observe local tissue damage due to inflammation, and observed that the incision of the LIPUS and PI group completely healed without sinuses or abscesses. Therefore, we believe that LIPUS combined with 0.35% PI is safe to use in procedures, without impairing local soft-tissue.
This study has several limitations. First, we used a single-strain in vivo model, which inherently limits its clinical applicability. Second, while previous in vivo studies have demonstrated the presence of immature biofilm on implants as early as day 3 using SEM,58,59 we believe that allowing the infection to progress for five days in this DAIR model results in an excessive infection that exceeds the severity observed clinically within five days. Third, our study solely employed systemic vancomycin without the inclusion of other adjunctive oral antibiotics, such as rifampicin, which could yield a more favourable outcome in the current experiment. Fourth, only male rats were included in our experiments, as the male sex is one of the risk factors associated with the development of PJI.60,61 Lastly, this was a preliminary animal study, and the period of debridement in rats was shorter than at clinical stage, and rats may not fully recapitulate human PJI. The results need to be verified through a large number of clinical studies to popularize and apply this clinically.
In conclusion, our study has demonstrated the robust antibiofilm potential of combining LIPUS with 0.35% PI in treating acute PJI in a rat model. Furthermore, we observed no adverse effects of LIPUS and 0.35% PI on local soft-tissues. This method holds promise for assisting in the future treatment of acute PJI.
Author contributions
T. Wang: Methodology, Investigation, Formal analysis, Writing – original draft.
C. Yang: Methodology, Investigation, Formal analysis.
G. Li: Methodology, Formal analysis, Writing – review & editing.
Y. Wang: Methodology, Formal analysis, Writing – review & editing.
B. Ji: Methodology, Formal analysis, Writing – review & editing.
Y. Chen: Methodology, Formal analysis, Writing – review & editing.
H. Zhou: Methodology, Formal analysis, Writing – review & editing.
L. Cao: Methodology, Formal analysis, Writing – review & editing.
Funding statement
The authors disclose receipt of the following financial or material support for the research, authorship, and/or publication of this article: this work was supported by grants from the Major Project of Science and Technology Program of Xinjiang Uygur Autonomous Region (Grant No.2022A03011), and the Science and Technology Innovation Team Project of Xinjiang Uygur Autonomous Region Science and Technology Department (Grant No.2023TSYCTD0014).
ICMJE COI statement
All authors report grants from the Major Project of Science and Technology Program of Xinjiang Uygur Autonomous Region (Grant No.2022A03011) and the Science and Technology Innovation Team Project of Xinjiang Uygur Autonomous Region Science and Technology Department (Grant No.2023TSYCTD0014), related to this study.
Data sharing
The datasets generated and analyzed in the current study are not publicly available due to data protection regulations. Access to data is limited to the researchers who have obtained permission for data processing. Further inquiries can be made to the corresponding author.
Acknowledgements
We would like to thank the Charlesworth Author Services for providing linguistic assistance during the preparation of this manuscript.
Ethical review statement
All rat studies have been performed in accordance with the ethical standards in the 1964 Declaration of Helsinki. The protocol of all animal experiments was approved by the Animal Center of Xinjiang Medical University (Permission number: IACUC-202300523-11). All animal experimental procedures were performed following the Guidelines for the Care and Use of Laboratory Animals of the Chinese Animal Welfare Committee.
Open access funding
The authors report that they received open access funding for their manuscript from the Major Project of Science and Technology Program of Xinjiang Uygur Autonomous Region (Grant No.2022A03011) and the Science and Technology Innovation Team Project of Xinjiang Uygur Autonomous Region Science and Technology Department (Grant No.2023TSYCTD0014).
Supplementary material
Figures illustrating the establishment of the periprosthetic joint infection rat model and the debridement, antibiotics, and implant retention procedure process of the low-intensity pulsed ultrasound device combined with 0.35% povidone-iodine. An ARRIVE checklist is also included to show that the ARRIVE guidelines were adhered to in this study.
© 2024 Wang et al. This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial No Derivatives (CC BY-NC-ND 4.0) licence, which permits the copying and redistribution of the work only, and provided the original author and source are credited. See https://creativecommons.org/licenses/by-nc-nd/4.0/
Contributor Information
Tianxing Wang, Email: 286268302@qq.com, 286268302@qq.com.
Chenchen Yang, Email: 2596243576@qq.com, 2596243576@qq.com.
Yang Wang, Email: 41481718@qq.com, 41481718@qq.com.
Baochao Ji, Email: 254422281@qq.com.
Yongjie Chen, Email: 342992871@qq.com, 342992871@qq.com.
Haikang Zhou, Email: zhkvip@sina.cn, 834551670@qq.com.
Li Cao, Email: xjbone@sina.com.
Data Availability
The datasets generated and analyzed in the current study are not publicly available due to data protection regulations. Access to data is limited to the researchers who have obtained permission for data processing. Further inquiries can be made to the corresponding author.
References
- 1. Widmer AF. New developments in diagnosis and treatment of infection in orthopedic implants. Clin Infect Dis. 2001;33 Suppl 2:S94–S106. doi: 10.1086/321863. [DOI] [PubMed] [Google Scholar]
- 2. Sloan M, Premkumar A, Sheth NP. Projected volume of primary total joint arthroplasty in the U.S., 2014 to 2030. J Bone Joint Surg Am. 2018;100-A(17):1455–1460. doi: 10.2106/JBJS.17.01617. [DOI] [PubMed] [Google Scholar]
- 3. Premkumar A, Kolin DA, Farley KX, et al. Projected economic burden of periprosthetic joint infection of the hip and knee in the United States. J Arthroplasty. 2021;36(5):1484–1489. doi: 10.1016/j.arth.2020.12.005. [DOI] [PubMed] [Google Scholar]
- 4. Hays MR, Kildow BJ, Hartman CW, et al. Increased incidence of methicillin-resistant Staphylococcus aureus in knee and hip prosthetic joint infection. J Arthroplasty. 2023;38(6S):S326–S330. doi: 10.1016/j.arth.2023.02.025. [DOI] [PubMed] [Google Scholar]
- 5. Parvizi J, Azzam K, Ghanem E, Austin MS, Rothman RH. Periprosthetic infection due to resistant staphylococci: serious problems on the horizon. Clin Orthop Relat Res. 2009;467(7):1732–1739. doi: 10.1007/s11999-009-0857-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Argenson JN, Arndt M, Babis G, et al. Hip and Knee Section, treatment, debridement and retention of implant: proceedings of International Consensus on Orthopedic Infections. J Arthroplasty. 2019;34(2S):S399–S419. doi: 10.1016/j.arth.2018.09.025. [DOI] [PubMed] [Google Scholar]
- 7. Sendi P, Lötscher PO, Kessler B, Graber P, Zimmerli W, Clauss M. Debridement and implant retention in the management of hip periprosthetic joint infection: outcomes following guided and rapid treatment at a single centre. Bone Joint J. 2017;99-B(3):330–336. doi: 10.1302/0301-620X.99B3.BJJ-2016-0609.R1. [DOI] [PubMed] [Google Scholar]
- 8. Kildow BJ, Patel SP, Otero JE, et al. Results of debridement, antibiotics, and implant retention for periprosthetic knee joint infection supplemented with the use of intraosseous antibiotics. Bone Joint J. 2021;103-B(6 Supple A):185–190. doi: 10.1302/0301-620X.103B6.BJJ-2020-2278.R1. [DOI] [PubMed] [Google Scholar]
- 9. Moore AJ, Wylde V, Whitehouse MR, et al. Development of evidence-based guidelines for the treatment and management of periprosthetic hip infection. Bone Jt Open. 2023;4(4):226–233. doi: 10.1302/2633-1462.44.BJO-2022-0155.R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lora-Tamayo J, Murillo O, Iribarren JA, et al. A large multicenter study of methicillin-susceptible and methicillin-resistant Staphylococcus aureus prosthetic joint infections managed with implant retention. Clin Infect Dis. 2013;56(2):182–194. doi: 10.1093/cid/cis746. [DOI] [PubMed] [Google Scholar]
- 11. Scheper H, Gerritsen LM, Pijls BG, Van Asten SA, Visser LG, De Boer MGJ. Outcome of debridement, antibiotics, and implant retention for staphylococcal hip and knee prosthetic joint infections, focused on rifampicin use: a systematic review and meta-analysis. Open Forum Infect Dis. 2021;8(7):ofab298. doi: 10.1093/ofid/ofab298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Indelli PF, Ghirardelli S, Valpiana P, Bini L, Festini M, Iannotti F. Debridement, antibiotic pearls, and retention of the implant (DAPRI) in the treatment of early periprosthetic joint infections: a consecutive series. Pathogens. 2023;12(4):605. doi: 10.3390/pathogens12040605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Farhan-Alanie OM, Kennedy JW, Meek RMD, Haddad FS. Is it time to reconsider the use of hydrogen peroxide in hip and knee arthroplasty? Bone Joint J. 2023;105-B(2):97–98. doi: 10.1302/0301-620X.105B2.BJJ-2022-1090.R1. [DOI] [PubMed] [Google Scholar]
- 14. Li J, Cheung W-H, Chow SK, Ip M, Leung SYS, Wong RMY. Current therapeutic interventions combating biofilm-related infections in orthopaedics: a systematic review of in vivo animal studies. Bone Joint Res. 2022;11(10):700–714. doi: 10.1302/2046-3758.1110.BJR-2021-0495.R3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Deng Z, Liu F, Li C. Therapeutic effect of ethylenediaminetetraacetic acid irrigation solution against wound infection with drug-resistant bacteria in a rat model: an animal study. Bone Joint Res. 2019;8(5):189–198. doi: 10.1302/2046-3758.85.BJR-2018-0280.R3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lin J, Suo J, Bao B, et al. Efficacy of EDTA-NS irrigation in eradicating Staphylococcus aureus biofilm-associated infection. Bone Joint Res. 2024;13(1):40–51. doi: 10.1302/2046-3758.131.BJR-2023-0141.R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pijls BG, Sanders IMJG, Kuijper EJ, Nelissen RGHH. Effectiveness of mechanical cleaning, antibiotics, and induction heating on eradication of Staphylococcus aureus in mature biofilms. Bone Joint Res. 2022;11(9):629–638. doi: 10.1302/2046-3758.119.BJR-2022-0010.R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wiesli MG, Livio F, Achermann Y, Gautier E, Wahl P. Wound fluid ceftriaxone concentrations after local application with calcium sulphate as carrier material in the treatment of orthopaedic device-associated hip infections. Bone Joint Res. 2022;11(11):835–842. doi: 10.1302/2046-3758.1111.BJR-2022-0180.R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ji B, Li G, Zhang X, et al. Effective single-stage revision using intra-articular antibiotic infusion after multiple failed surgery for periprosthetic joint infection: a mean seven years’ follow-up. Bone Joint J. 2022;104-B(7):867–874. doi: 10.1302/0301-620X.104B7.BJJ-2021-1704.R1. [DOI] [PubMed] [Google Scholar]
- 20. Rajput V, Meek RMD, Haddad FS. Periprosthetic joint infection: what next? Bone Joint J. 2022;104-B(11):1193–1195. doi: 10.1302/0301-620X.104B11.BJJ-2022-0944. [DOI] [PubMed] [Google Scholar]
- 21. Ernest EP, Machi AS, Karolcik BA, LaSala PR, Dietz MJ. Topical adjuvants incompletely remove adherent Staphylococcus aureus from implant materials. J Orthop Res. 2018;36(6):1599–1604. doi: 10.1002/jor.23804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Duque AF, Post ZD, Lutz RW, Orozco FR, Pulido SH, Ong AC. Is there still a role for irrigation and debridement with liner exchange in acute periprosthetic total knee infection? J Arthroplasty. 2017;32(4):1280–1284. doi: 10.1016/j.arth.2016.10.029. [DOI] [PubMed] [Google Scholar]
- 23. Royo A, Bertrand ML, Ramos L, Fernandez-Gordillo F, Guerado E. Is there still a place for continuous closed irrigation in the management of periprosthetic total knee infection? Open Orthop J. 2013;7:205–210. doi: 10.2174/1874325001307010205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Calkins TE, Culvern C, Nam D, et al. Dilute betadine lavage reduces the risk of acute postoperative periprosthetic joint infection in aseptic revision total knee and hip arthroplasty: a randomized controlled trial. J Arthroplasty. 2020;35(2):538–543. doi: 10.1016/j.arth.2019.09.011. [DOI] [PubMed] [Google Scholar]
- 25. Pitt WG, McBride MO, Lunceford JK, Roper RJ, Sagers RD. Ultrasonic enhancement of antibiotic action on gram-negative bacteria. Antimicrob Agents Chemother. 1994;38(11):2577–2582. doi: 10.1128/AAC.38.11.2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Carmen JC, Roeder BL, Nelson JL, et al. Ultrasonically enhanced vancomycin activity against Staphylococcus epidermidis biofilms in vivo. J Biomater Appl. 2004;18(4):237–245. doi: 10.1177/0885328204040540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rediske AM, Roeder BL, Brown MK, et al. Ultrasonic enhancement of antibiotic action on Escherichia coli biofilms: an in vivo model. Antimicrob Agents Chemother. 1999;43(5):1211–1214. doi: 10.1128/AAC.43.5.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chen X, Tang R, Wang Y, et al. Effect of ultrasonic and ozone pretreatment on the fate of enteric indicator bacteria and antibiotic resistance genes, and anaerobic digestion of dairy wastewater. Bioresour Technol. 2021;320(Pt A):124356. doi: 10.1016/j.biortech.2020.124356. [DOI] [PubMed] [Google Scholar]
- 29. Case PD, Bird PS, Kahler WA, George R, Walsh LJ. Treatment of root canal biofilms of Enterococcus faecalis with ozone gas and passive ultrasound activation. J Endod. 2012;38(4):523–526. doi: 10.1016/j.joen.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 30. Yang G, Lin J, Zeng EY, Zhuang L. Extraction and characterization of stratified extracellular polymeric substances in Geobacter biofilms. Bioresour Technol. 2019;276:119–126. doi: 10.1016/j.biortech.2018.12.100. [DOI] [PubMed] [Google Scholar]
- 31. Li Y, Wuermanbieke S, Zhang X, et al. Effects of intra-articular D-amino acids combined with systemic vancomycin on an experimental Staphylococcus aureus-induced periprosthetic joint infection. J Microbiol Immunol Infect. 2022;55(4):716–727. doi: 10.1016/j.jmii.2022.01.005. [DOI] [PubMed] [Google Scholar]
- 32. Rediske AM, Roeder BL, Nelson JL, et al. Pulsed ultrasound enhances the killing of Escherichia coli biofilms by aminoglycoside antibiotics in vivo. Antimicrob Agents Chemother. 2000;44(3):771–772. doi: 10.1128/AAC.44.3.771-772.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sosa BR, Niu Y, Turajane K, et al. 2020 John Charnley Award: The antimicrobial potential of bacteriophage-derived lysin in a murine debridement, antibiotics, and implant retention model of prosthetic joint infection. Bone Joint J. 2020;102-B(7_Supple_B):3–10. doi: 10.1302/0301-620X.102B7.BJJ-2019-1590.R1. [DOI] [PubMed] [Google Scholar]
- 34. Wei J, Tong K, Wang H, Wen Y, Chen L. Intra-articular versus systemic vancomycin for the treatment of periprosthetic joint infection after debridement and spacer implantation in a rat model. Bone Joint Res. 2022;11(6):371–385. doi: 10.1302/2046-3758.116.BJR-2021-0319.R3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Carli AV, Ross FP, Bhimani SJ, Nodzo SR, Bostrom MPG. Developing a clinically representative model of periprosthetic joint infection. J Bone Joint Surg Am. 2016;98-A(19):1666–1676. doi: 10.2106/JBJS.15.01432. [DOI] [PubMed] [Google Scholar]
- 36. Carli AV, Bhimani S, Yang X, et al. Quantification of peri-implant bacterial load and in vivo biofilm formation in an innovative, clinically representative mouse model of periprosthetic joint infection. J Bone Joint Surg Am. 2017;99-A(6):e25. doi: 10.2106/JBJS.16.00815. [DOI] [PubMed] [Google Scholar]
- 37. Karau MJ, Schmidt-Malan SM, Albano M, et al. Novel use of rifabutin and rifapentine to treat methicillin-resistant Staphylococcus aureus in a rat model of foreign body osteomyelitis. J Infect Dis. 2020;222(9):1498–1504. doi: 10.1093/infdis/jiaa401. [DOI] [PubMed] [Google Scholar]
- 38. Rissing JP, Buxton TB, Weinstein RS, Shockley RK. Model of experimental chronic osteomyelitis in rats. Infect Immun. 1985;47(3):581–586. doi: 10.1128/iai.47.3.581-586.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lovati AB, Drago L, Monti L, et al. Diabetic mouse model of orthopaedic implant-related Staphylococcus aureus infection. PLoS One. 2013;8(6):e67628. doi: 10.1371/journal.pone.0067628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lovati AB, Romanò CL, Bottagisio M, et al. Modeling Staphylococcus epidermidis-induced non-unions: subclinical and clinical evidence in rats. PLoS One. 2016;11(1):e0147447. doi: 10.1371/journal.pone.0147447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lovati AB, Bottagisio M, Maraldi S, et al. Vitamin E phosphate coating stimulates bone deposition in implant-related infections in a rat model. Clin Orthop Relat Res. 2018;476(6):1324–1338. doi: 10.1097/01.blo.0000534692.41467.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wei J, Tong K, Wang H, Wen Y, Chen L. Dosage, efficacy, and safety of intra-articular vancomycin for prophylaxis of periprosthetic joint infection caused by Methicillin-resistant Staphylococcus aureus after total knee arthroplasty in a rat model. Antimicrob Agents Chemother. 2022;66(2):e0164121. doi: 10.1128/AAC.01641-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yagi H, Kihara S, Mittwede PN, et al. Development of a large animal rabbit model for chronic periprosthetic joint infection. Bone Joint Res. 2021;10(3):156–165. doi: 10.1302/2046-3758.103.BJR-2019-0193.R3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ibrahim MM, Liu Y, Ure K, Hall CW, Mah TF, Abdelbary H. Establishment of a novel rat model of gram-negative periprosthetic joint infection using cementless hip hemiarthroplasty. J Bone Joint Surg Am. 2023;105-A(1):42–52. doi: 10.2106/JBJS.22.00094. [DOI] [PubMed] [Google Scholar]
- 45. Hinz N, Butscheidt S, Jandl NM, et al. Increased local bone turnover in patients with chronic periprosthetic joint infection. Bone Joint Res. 2023;12(10):644–653. doi: 10.1302/2046-3758.1210.BJR-2023-0071.R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. del Pozo JL, Patel R. The challenge of treating biofilm-associated bacterial infections. Clin Pharmacol Ther. 2007;82(2):204–209. doi: 10.1038/sj.clpt.6100247. [DOI] [PubMed] [Google Scholar]
- 47. O’Donnell JA, Wu M, Cochrane NH, et al. Efficacy of common antiseptic solutions against clinically relevant microorganisms in biofilm. Bone Joint J. 2021;103-B(5):908–915. doi: 10.1302/0301-620X.103B5.BJJ-2020-1245.R2. [DOI] [PubMed] [Google Scholar]
- 48. Premkumar A, Nishtala SN, Nguyen JT, Bostrom MPG, Carli AV. The AAHKS Best Podium Presentation Research Award: Comparing the efficacy of irrigation solutions on staphylococcal biofilm formed on arthroplasty surfaces. J Arthroplasty. 2021;36(7S):S26–S32. doi: 10.1016/j.arth.2021.02.033. [DOI] [PubMed] [Google Scholar]
- 49. Sweet FA, Forsthoefel CW, Sweet AR, Dahlberg RK. Local versus systemic antibiotics for surgical infection prophylaxis in a rat model. J Bone Joint Surg Am. 2018;100-A(18):e120. doi: 10.2106/JBJS.18.00105. [DOI] [PubMed] [Google Scholar]
- 50. Bridier A, Briandet R, Thomas V, Dubois-Brissonnet F. Resistance of bacterial biofilms to disinfectants: a review. Biofouling. 2011;27(9):1017–1032. doi: 10.1080/08927014.2011.626899. [DOI] [PubMed] [Google Scholar]
- 51. Pitt WG, Ross SA. Ultrasound increases the rate of bacterial cell growth. Biotechnol Prog. 2003;19(3):1038–1044. doi: 10.1021/bp0340685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Baumann AR, Martin SE, Feng H. Removal of Listeria monocytogenes biofilms from stainless steel by use of ultrasound and ozone. J Food Prot. 2009;72(6):1306–1309. doi: 10.4315/0362-028x-72.6.1306. [DOI] [PubMed] [Google Scholar]
- 53. Ensing GT, Roeder BL, Nelson JL, et al. Effect of pulsed ultrasound in combination with gentamicin on bacterial viability in biofilms on bone cements in vivo. J Appl Microbiol. 2005;99(3):443–448. doi: 10.1111/j.1365-2672.2005.02643.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Carmen JC, Roeder BL, Nelson JL, et al. Treatment of biofilm infections on implants with low-frequency ultrasound and antibiotics. Am J Infect Control. 2005;33(2):78–82. doi: 10.1016/j.ajic.2004.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Li X, Zhong Y, Zhou W, et al. Low-intensity pulsed ultrasound (LIPUS) enhances the anti-inflammatory effects of bone marrow mesenchymal stem cells (BMSCs)-derived extracellular vesicles. Cell Mol Biol Lett. 2023;28(1):9. doi: 10.1186/s11658-023-00422-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Newton Ede MP, Philp AM, Philp A, Richardson SM, Mohammad S, Jones SW. Povidone-iodine has a profound effect on in vitro osteoblast proliferation and metabolic function and inhibits their ability to mineralize and form bone. Spine (Phila Pa 1976) 2016;41(9):729–734. doi: 10.1097/BRS.0000000000001332. [DOI] [PubMed] [Google Scholar]
- 57. Shohat N, Goh GS, Harrer SL, Brown S. Dilute povidone-iodine irrigation reduces the rate of periprosthetic joint infection following hip and knee arthroplasty: an analysis of 31,331 cases. J Arthroplasty. 2022;37(2):226–231. doi: 10.1016/j.arth.2021.10.026. [DOI] [PubMed] [Google Scholar]
- 58. Tomizawa T, Nishitani K, Ito H, et al. The limitations of mono- and combination antibiotic therapies on immature biofilms in a murine model of implant-associated osteomyelitis. J Orthop Res. 2021;39(2):449–457. doi: 10.1002/jor.24956. [DOI] [PubMed] [Google Scholar]
- 59. Nishitani K, Sutipornpalangkul W, de Mesy Bentley KL, et al. Quantifying the natural history of biofilm formation in vivo during the establishment of chronic implant-associated Staphylococcus aureus osteomyelitis in mice to identify critical pathogen and host factors. J Orthop Res. 2015;33(9):1311–1319. doi: 10.1002/jor.22907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. McMaster Arthroplasty Collaborative (MAC) Risk factors for periprosthetic joint infection following primary total hip arthroplasty: a 15-year, population-based cohort study. J Bone Joint Surg Am. 2020;102-A(6):503–509. doi: 10.2106/JBJS.19.00537. [DOI] [PubMed] [Google Scholar]
- 61. Baier C, Adelmund S, Schwab F, et al. Incidence and risk factors of surgical site infection after total knee arthroplasty: results of a retrospective cohort study. Am J Infect Control. 2019;47(10):1270–1272. doi: 10.1016/j.ajic.2019.04.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and analyzed in the current study are not publicly available due to data protection regulations. Access to data is limited to the researchers who have obtained permission for data processing. Further inquiries can be made to the corresponding author.