Abstract
In this study, we investigated the effect of roasting conditions and time on the physicochemical properties of pomegranate seed oil. We analyzed the fatty acid, total phenolic, flavonoid, tocopherol, and phytosterol contents of pomegranate seed oil extracted under four conditions: raw, heated at 160°C for 15 min, heated at 160°C for 20 min, and heated at 180°C for 10 min, which included three that were well-established to enhance nutritional and flavor properties. Furthermore, the oxidative stability was evaluated based on the acid value, peroxide value, and induction period. Roasting significantly decreased the contents of punicic acid, polyunsaturated fatty acids, tocopherol, and phytosterol and the 2,2-diphenyl-1-picrylhydrazyl radical scavenging capacity (P<0.05) of the oil. Conversely, saturated fatty acids, monounsaturated fatty acids, acid value, peroxide value, total phenolic and flavonoid contents, and induction period were significantly increased (P<0.05). Our results suggest that the roasting conditions were nutritionally and oxidatively stable, thereby enhancing the roasting process and providing a database for essential roasting treatments for pomegranate seed oil.
Keywords: antioxidants, fatty acid, food analysis, pomegranate
INTRODUCTION
Pomegranate (Punica granatum L.) is a tree species native to India that is widely cultivated in China, India, Iran, Mediterranean countries, and the United States (Khoddami et al., 2014). It is a functional product that provides nutritional and health benefits and it has been cultivated for nutritional and pharmacological uses since ancient times (Kaseke et al., 2020b). All parts of the pomegranate plant, including the skin, flesh, and seeds, contain healthy compounds. The skin and flesh are rich in punicalagin, tannins, anthocyanins, and ellagic acid, and are frequently consumed as fresh fruit or in various products, including juice, syrup, jam, and wine (Drinić et al., 2020; Kaseke et al., 2021). Pomegranate seeds, a by-product of pomegranate processing (Kaseke et al., 2021), are rich in bioactive compounds, such as polyphenols, tocopherols, phytosterols, and punicic acid (Kaseke et al., 2021), and contain approximately 12%∼20% oil (Musa Özcan and Uslu, 2023).
Pomegranate seed oil is a valuable industrial and health alternative to pomegranate seeds, which are waste products (Musa Özcan and Uslu, 2023). Similar to pomegranate, it has attracted much scientific interest because of its uniqueness in terms of fatty acid composition and bioactivity (Iriti et al., 2023). Pomegranate seed oil comprises palmitic acid, stearic acid, oleic acid, linoleic acid, and four isomers of linolenic acid (Khoddami et al., 2014). Punicic acid, an ω-5 isomer of linolenic acid, is the primarycomponent and has antioxidant and anticancer properties (Drinić et al., 2020). Furthermore, major biomarkers include palmitic acid (saturated fatty acids), oleic acid (monounsaturated fatty acids), β-sitosterol (phytosterol), and γ-tocopherol (tocopherol) (Iriti et al., 2023), and it is rich in phenolic compounds (Musa Özcan and Uslu, 2023). Therefore, pomegranate seed oil reduces total cholesterol, wound inflammation, type 2 diabetes risk, insulin resistance, and glucose intake, provides antioxidant and anticancer protection, and prevents obesity (Khoddami et al., 2014; Iriti et al., 2023), making pomegranate seed oil a uniquely valuable product (Iriti et al., 2023).
Thermal treatment of foods affects their bioactive and physicochemical properties. Thermal processing is a traditional processing method that improves the nutritional value and organoleptic properties of foods; however, proper heating is essential because it affects the antioxidant capacity, composition, and concentration of volatile compounds (Musa Özcan and Uslu, 2023). Kaseke et al. (2020b) evaluated the quality and antioxidant capacity of pomegranate seed oil following microwave treatment, and Kaseke et al. (2020a) investigated the effects of blanching on the physicochemical and bioactive substances and antioxidant properties of pomegranate seed oil. This study aims to investigate the effects of roasting temperature and time on the physicochemical and phytochemicals of pomegranate seed oil by comparing its composition under different roasting conditions.
MATERIALS AND METHODS
Experimental materials
Frozen pomegranate (P. granatum L.) seeds were purchased online from Turkey. The pulp was removed and dehydrated using a dry oven (WOF-W155, DAIHAN Scientific Co., Ltd.), and then frozen at −18°C until further use.
Roasting and oil extraction of pomegranate seed oil
Four roasting conditions were applied, including three roasting conditions (160°C_15 min, 160°C_20 min, 180°C_10 min) and raw, which were selected based on antioxidant properties and flavor analysis results from previous studies. One hundred grams of pomegranate seeds were roasted at 160°C for 15 min, 160°C for 20 min, or 180°C for 10 min using the oven function of a multi-light oven (EON-C200F, Guangdong Midea Kitchen Appliances Manufacturing Co., Ltd.). Subsequently, they were finely ground using a grinder (CSM-309, SY Electronics Co.) and placed in a glass vial. Then, hexane (500 mL) was added, and the powder was stored at 4°C for 24 h. Next, the hexane-containing pomegranate seeds were filtered and excess moisture was removed using anhydrous sodium sulfate. A liquid layer was obtained and the pomegranate seeds were removed. A rotary evaporator (Rotavapor R-3, Buchi Korea) was used to remove hexane from the liquid layer, which was then used for further analysis.
Fatty acid composition analysis
We added 0.5 mL of heptadecanoic acid (C17:0, 1 mg/mL hexane), which is the internal standard for fatty acid components, to approximately 100 mg of the sample and dissolved it in 2 mL of 0.5 N NaOH/methanol solution. After heating it at 100°C for 10 min, it was cooled, and 4 mL of BF3 was added. Then, the sample was heated at 100°C for 40 min to proceed with derivatization. After cooling, the upper layer was extracted by adding 5 mL of hexane and saturated NaCl, filtered, and analyzed. Gas chromatography (GC; Agilent 7890A, Agilent Technologies) was used to analyze fatty acids. The column used for the analysis was an SP-2560 capillary column (100 m×0.25 mm i.d., 0.25 μm film thickness, Agilent Technologies), and the injector and detector temperatures were set at 250°C. The oven was heated to 130°C for 5 min and then increased by 4°C per min to 240°C, then maintained for 15 min. Each isolated fatty acid was identified by comparing its retention time (RT) with that of a fatty acid standard (Lim et al., 2016).
Total phenolic content (TPC) analysis
Each sample (1 mL) was mixed with ethanol (1 mL) and distilled water (5 mL); 0.5 mL of Folin-Ciocalteu’s phenol (Sigma-Aldrich) was added to the solution, and the mixture was stirred for 5 min. One milliliter of 10% sodium carbonate (Na2CO3) solution was added to the solution, and the solution was centrifuged at 1,764 g for 10 min. Then, 200 μL of the sample was added to a 96-well plate, and the absorbance was measured at a wavelength of 750 nm using a UV Spectrophotometer (Multiskan Go, Thermo Fisher Scientific). The TPC was expressed as milligrams of gallic acid equivalents per mL of oil (mg GAE/mL) using an equation obtained from the standard gallic acid calibration curve (0.01∼0.08 mg/mL) (Hong et al., 2020).
Total flavonoid content (TFC) analysis
We mixed 0.5 mL of the sample with 1.5 mL of 95% ethanol, 0.1 mL of 10% aluminum nitrate and 1 M potassium acetate. The mixture was vortexed for 5 min, then 2.8 mL of distilled water was added, and the sample was left to react at 23°C for 30 min. Subsequently, the TFC was computed using a calibration curve obtained by adding 200 μL of the mixture to a 96-well plate and measuring the absorbance at a wavelength of 415 nm. The TFC was expressed as milligrams of quercetin equivalents per mL of oil (mg QE/mL) using an equation obtained from the standard gallic acid calibration curve (0.01∼0.08 mg/mL) (Hong et al., 2020).
Tocopherol content analysis
Approximately 50 mg of sample was dissolved with 50 mL of hexane, filtered through a 0.45 μm hydrophobic polytetrafluoroethylene syringe filter, and used for analysis. High performance liquid chromatography (HPLC; Agilent 1260N, Agilent Technologies) using the LiChrosorb Si-60 (4×250 mm, 5 μm particle size, HibarⓇ Fertigsäle RT, Merck Millipore) was used to analyze the mixture. The HPLC detector was operated at an excitation and emission wavelength of 290 and 330 nm, respectively, using a fluorescence detector. The analyzed peaks were identified by comparing their RT with those of tocopherol standards (Shin, 2013).
Phytosterol content analysis
To approximately 1 g of sample, we added 500 μL of internal standard 5α-cholestane (1 mg/mL hexane), 8 mL of 3% (w/v) pyrogallol/ethanol, and 1 mL of prepared saturated KOH solution. After mixing, the samples were incubated at 80°C for 1 h and then cooled. Subsequently, 7 mL of distilled water and hexane were added, then the hexane layer was removed under a nitrogen stream. Subsequently, we added 0.5 mL of a N,O-Bis(trimethylsilyl)trifluoroacetamide solution containing 1% trimethylsilyl and 1 mL of a pyridine solution to redissolve it by mixing, followed by 1 hour of derivatization at 80°C. Phytosterols were analyzed using GC-FID (Agilent 7890A), and the column was an HP-5 (30 m×0.32 mm i.d., 0.25 μm film thickness, Agilent Technologies). The oven temperature was 260°C, with a 3°C per min increase until it reached 300°C, where it was maintained for 15 min. The injector and detector temperatures were set at 300°C and 320°C, respectively (Shin et al., 2010).
2,2-Diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity analysis
DPPH was prepared by mixing 3.9 mg of DPPH with 10 mL of isooctane, making a 1 mM DDPH solution. We then mixed 5 mL of this solution with 45 mL of isooctane, preparing a 0.1 mM DPPH solution. Approximately 10 μL of the sample was mixed with 1 mL of 0.01 mM DPPH solution (Sigma-Aldrich) and allowed to stand at room temperature in the dark for 20 min. The samples were then centrifuged at 125.44 g for 10 min, and the absorbance was measured using a spectrophotometer at 517 nm using a 96-well plate. The measured absorbance values were substituted into the following equation to calculate the DPPH radical scavenging capacity (Hong et al., 2020).
DPPH radical scavenging activity (%)=(1−sample absorbance/blank absorbance)×100
Acid value (AV) and peroxide value (PV) analysis
For AV analysis, approximately 4 g of sample was dissolved in a 1:1 (v/v) mixture of ether:ethanol, then approximately 200 μL of 1% phenolphthalein solution was added. Titration was performed with the prepared 0.1 N KOH/ethanol solution until the mixture became pale red. The AV was calculated in comparison to the blank test.
For the PV assay, approximately 1 g of the sample was mixed with 10 mL of chloroform, 15 mL of acetic acid, and 1 mL of a prepared KI saturation solution. The mixture was allowed to stand for 10 min in a dark room. Distilled water (75 mL) and 1 mL of 1% starch solution (indicator) were added, followed by titration with the prepared 0.01 N Na2S2O3 solution until clear. The PV was calculated in comparison to the blank test. The AV and POV were determined using the AOCS Ca 5a-40, Cd 8-53 official methods, respectively (AOCS, 2003, 2017).
Induction period (IP) analysis
The oxygen IP was measured using Rancimat (Metrohm CH series 892, Metrohm AG). Approximately 3 g of the sample was placed in the reaction vessel and forced oxidation was performed by injecting air at 20 L/h and 100°C. Upon heating and oxidation, polar substances, such as ketones, aldehydes, and carboxylic acids, were produced, and the oxygen IP was determined based on the value obtained from an electrolyte sensor mounted in deionized water (Kim et al., 2019).
Statistical methods
The results were expressed as the mean±standard deviation of at least three replicates, and the significance of the results was tested by Tukey’s multiple range test (P<0.05) using SAS version 9.0 (SAS Institute Inc.).
RESULTS AND DISCUSSION
Fatty acid composition
The fatty acids contained in the pomegranate seed oil were analyzed, and the findings are shown in Table 1. The highest percentage of punicic acid (C18:3n5), the most representative fatty acid in pomegranate seed oil, was observed in the raw oil (86.80%; P<0.05). This was followed by linoleic acid (C18:2n6c), oleic acid (C18:1n9c), palmitic acid (C16:0), and stearic acid (C18:0) at proportions of 4.49%, 4.55%, 2.43%, and 1.73%, respectively. After roasting, the percentage of punicic acid varied from 82.96%∼85.87%, demonstrating a decreasing pattern of approximately 1.07%∼4.42% (P<0.05). Oleic, linoleic, palmitic, and stearic acids demonstrated increasing patterns, varying between 4.83%∼5.78%, 4.91%∼5.92%, 2.59%∼3.15%, and 1.80%∼2.20%, respectively (P<0.05). Therefore, the percentage of saturated and monounsaturated fatty acids in pomegranate seed oil increased from 4.16%∼5.34% and 4.55%∼5.78% (P<0.05), respectively. Conversely, the percentage of polyunsaturated fatty acids decreased to 88.88%∼91.29% (P<0.05).
Table 1.
Changes in fatty acid composition of raw and roasted pomegranate seed oils
| Fatty acid | Fatty acid profile (%) | |||
|---|---|---|---|---|
|
| ||||
| Raw | 160°C_15 min | 160°C_20 min | 180°C_10 min | |
| Palmitic acid (C16:0) | 2.43±0.01d | 2.59±0.01c | 2.68±0.01b | 3.15±0.01a |
| Stearic acid (C18:0) | 1.73±0.01d | 1.80±0.01c | 1.85±0.01b | 2.20±0.01a |
| Oleic acid (C18:1n9c) | 4.55±0.01d | 4.83±0.02c | 4.92±0.01b | 5.78±0.01a |
| Linoleic acid (C18:2n6c) | 4.49±0.01d | 4.91±0.02c | 5.02±0.01b | 5.92±0.01a |
| Punicic acid (C18:3n5) | 86.80±0.01a | 85.87±0.06b | 85.54±0.01c | 82.96±0.01d |
| SFA | 4.16±0.01d | 4.39±0.02c | 4.53±0.01b | 5.34±0.01a |
| MUFA | 4.55±0.01d | 4.83±0.02c | 4.92±0.01b | 5.78±0.01a |
| PUFA | 91.29±0.01a | 90.78±0.04b | 90.55±0.01c | 88.88±0.01d |
Values are presented as mean±standard deviation in triplicate.
Means with different letters (a-d) indicate significant differences by Tukey’s multiple range test (P<0.05).
SFA, saturated fatty acid; MUFA, monounsaturated fatty acid; PUFA, polyunsaturated fatty acid.
The fatty acid composition is a most important quality attribute of seed oils because the type of fatty acid has a significant impact on the suitability for eating, functional foods, and other uses (Kaseke et al., 2021). Among the unsaturated fatty acids, polyunsaturated fatty acids are the most sensitive to heat treatment and are the first to be affected (Kim et al., 2019; Kaseke et al., 2021). Heat treatment affects the fatty acid composition by breaking the polymerization and double bonds of unsaturated fatty acids, turning them into smaller molecules (Kim et al., 2019; Kaseke et al., 2021). Wroniak et al. (2016) reported that heating decreases the amount of polyunsaturated fatty acids and increases the amount of saturated and monounsaturated fatty acids in vegetable oils. In this study, we observed a trend toward a decrease in the proportion of polyunsaturated fatty acids and a slight increase in the proportion of saturated and monounsaturated fatty acids. The proportion of punicic acid, the most common polyunsaturated fatty acid in pomegranate seed oil, decreased after roasting. Furthermore, Kaseke et al. (2021) reported a decrease in punicic acid content by approximately 7% in microwave-treated pomegranate seed oil. In pomegranate seed oil, a reduction in punicic acid is undesirable because punicic acid is highly associated with the oil’s bioactive properties. However, the reduction in punicic acid was <5% in this study; this reduction was, unlikely to have a significant effect on the biochemical properties of pomegranate seed oil.
TPC and TFC
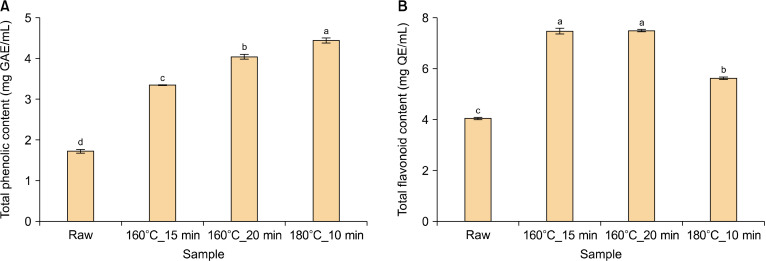
The TPC and TFC of raw and roasted pomegranate seed oils were analyzed, and the results are shown in Fig. 1. The TPC (Fig. 1A) was found to be 1.72 mg GAE/mL in raw oil, the lowest among the four conditions (P<0.05). Postroasting, the TPC demonstrated a continuous and significant increase, with the highest content being 4.45 mg GAE/mL after heating at 180°C for 10 min (P<0.05). The TFC (Fig. 1B) was noted to be the lowest in the raw oil, at 4.05 mg QE/mL, and increased significantly postroasting (P<0.05). For TFC, the highest contents were observed in the oil heated at 160°C for 15 min and 160°C for 20 min, with 7.48 and 7.50 mg QE/mL, respectively, whereas treatment at 180°C for 10 min (5.64 mg QE/mL) demonstrated a decreasing pattern (P<0.05).
Fig. 1.
Total phenolic (A) and flavonoid (B) contents of pomegranate seed oil under four conditions. The means, indicated by different letters (a-d), are significantly different between the four conditions by Tukey’s multiple range test (P<0.05). GAE, gallic acid equivalent; QE, quercetin equivalent.
Phenolic and flavonoid compounds are polyphenols, secondary plant metabolites, and widely distributed in seed oils (Zhang et al., 2021). Polyphenols are the most abundant antioxidants in plants and have multiple functions, including antioxidant, antimicrobial, and anti-inflammatory activities, which may aid in reducing the risk of chronic diseases (Kaseke et al., 2020a; Zhang et al., 2021). Specifically, pomegranate seeds oil is a good source of phenolic compounds (Kaseke et al., 2020b). Generally, phenolic and flavonoid compounds are bound with other compounds, such as polysaccharides, proteins, and pectin (Kaseke et al., 2020b). Thermal processing, such as roasting, increases the content of phenolic and flavonoid compounds by breaking them down or degrading them into their free forms, which releases them as small molecules (Mohamed Ahmed et al., 2021). However, excessive roasting can cause heat-induced degradation; therefore, appropriate roasting conditions are essential (Ghafoor et al., 2020).
The TPC of pomegranate seed oil was observed to increase with increasing blanching time in a previous study (Kaseke et al., 2020b), and the TPC of three pomegranate seed oils increased following microwave treatment (Kaseke et al., 2020a). Furthermore, in a previous study by Yang et al. (2018), the TPC and TFC of camellia seed oil continued to increase up to a suitable roasting temperature. With treatment of 180°C for 10 min, the TFC tended to decrease because of pyrolysis; thus, 160°C for 15 min or 20 min were considered positive conditions.
Tocopherol content
The tocopherol content in pomegranate seed oil was analyzed for α- and γ-tocopherol, the two main tocopherols found in vegetable oils (Kim et al., 2019). Our findings are displayed in Table 2. The most abundant tocopherol in pomegranate seed oil was identified as γ-tocopherol. α-Tocopherol was found at a concentration of 1.28 mg/100 g in the raw oil, the highest content among the four conditions. After roasting, the content showed a decreasing tendency (P<0.05) and varied between 1.02 and 1.24 mg/100 g. However, in the case of the oil heated at 160°C for 15 min, there was a slight decrease compared to the raw sample; however, the difference was not significant (P>0.05). The oil treated at 160°C for 20 min and 180°C for 10 min showed a significant decrease (P<0.05) compared to the raw sample, with contents of 1.08 and 1.02 mg/100 g, respectively. Moreover, γ-tocopherol showed the highest content (105.79 mg/100 g) in the raw sample, with a decreasing tendency postroasting (P<0.05). Treatment at 160°C for 20 min and 180°C for 10 min showed a significant decrease (P<0.05) compared to the raw sample, with contents of 97.79 and 101.78 mg/100 g, respectively. γ-Tocopherol showed the highest content (105.79 mg/100 g) in the raw sample, with a noted decreasing tendency postroasting (P<0.05). However, the 160°C_15 min treatment showed a slight decrease compared to the raw sample, but the difference was not significant (P>0.05). After heating at 160°C for 20 min and 180°C for 10 min, the contents were 97.79 and 101.78 mg/100 g, respectively.
Table 2.
Changes in tocopherol and phytosterol contents of raw and roasted pomegranate seed oils
| Phytochemical | Contents | ||||
|---|---|---|---|---|---|
| Raw | 160°C_15 min | 160°C_20 min | 180°C_10 min | ||
| Tocopherol (mg/100 g) | α-Tocopherol | 1.28±0.03a | 1.24±0.05a | 1.08±0.04b | 1.02±0.04b |
| γ-Tocopherol | 105.79±0.31a | 105.54±0.43a | 97.79±0.34c | 101.78±0.77b | |
| Phytosterol (mg/100 g) | Stigmasterol | 63.93±0.24a | 57.31±0.59b | 57.25±0.20b | 45.79±0.17c |
| β-Sitosterol | 1,298.61±6.39a | 1,137.54±10.74b | 1,134.80±7.43b | 932.39±3.56c | |
| Campesterol | 85.20±5.54a | 65.13±0.51b | 60.90±0.73bc | 54.38±0.93c | |
Values are presented as mean±standard deviation of three independent experiments.
Means with different letters (a-c) indicate significant differences by Tukey’s multiple range test (P<0.05).
Tocopherols are lipophilic and powerful natural antioxidants (Chirinos et al., 2016; Zhang et al., 2021). Some tocopherols are bound to proteins or phospholipids, and several studies have shown that heat treatment breaks these bonds and releases them, increasing their content (Zhang et al., 2021). However, as the intensity of thermal processing increases, pyrolysis occurs, reducing the tocopherol content (Zhang et al., 2021). In this study, the tocopherol content was slightly decreased by roasting. Zou et al. (2018) noted a slight decrease in the tocopherol content of wheat germ oil after roasting, and Demnati et al. (2018) found a continuous decrease in tocopherol content with roasting temperature and time, with 3%∼20% for α-tocopherol and 0%∼7% for γ-tocopherol. The relatively greater reduction of α-tocopherol is attributed to the fact that α-tocopherol is less stable at high temperatures than other tocopherol isomers (Oracz et al., 2014). In this study, the tocopherol content showed a general decreasing trend. However, after treatment at 160°C for 15 min, it decreased by 0%∼3% and did not show a significant difference from the raw oil, which is considered the most positive of the three roasting conditions.
Phytosterol content
The phytosterol content of pomegranate seed oil was analyzed, focusing on campesterol, stigmasterol, and β-sitosterol, which are reported to be commonly present in vegetable oils (Table 2) (Kim et al., 2019). The most abundant phytosterol in pomegranate seed oil was identified as β-sitosterol, followed by campesterol and stigmasterol. Campesterol, stigmasterol, and β-sitosterol had the highest contents in the raw oil at concentration of 85.20, 63.93, and 1,298.61 mg/100 g, respectively. After roasting, the levels of all three phytosterols significantly decreased (P<0.05). Stigmasterol showed a decrease of around 10%∼28% compared to raw oil and varied in the range of 45.79∼57.31 mg/100 g. β-Sitosterol decreased by approximately 12%∼28% after roasting, with changes ranging from 932.39∼1,137.54 mg/100 g. Campesterol showed the greatest percentage reduction (23%∼36%), ranging from 54.38∼65.13 mg/100 g.
Phytosterols and tocopherols are natural antioxidants that prevent oil rancidity (Zhou et al., 2016). Structurally similar to cholesterol, they can lower cholesterol levels by reducing the concentration of low density lipoprotein-cholesterol in the body, thereby reducing the risk of coronary heart disease. They also exhibit antioxidant activity (Kim et al., 2019; Kaseke et al., 2020b). Similar to tocopherols, proper roasting increases the phytosterol content, but excessive roasting leads to their oxidation and degradation (Zhang et al., 2021). In the present study, the phytosterol content tended to decrease after roasting. Zhou et al. (2016) reported that the phytosterol content of walnut oil decreased with increasing microwave heat treatment time, and Arab et al. (2022) confirmed that the phytosterol content decreased with increasing roasting temperature. In this study, the phytosterol content of pomegranate seed oil decreased with roasting time and temperature.
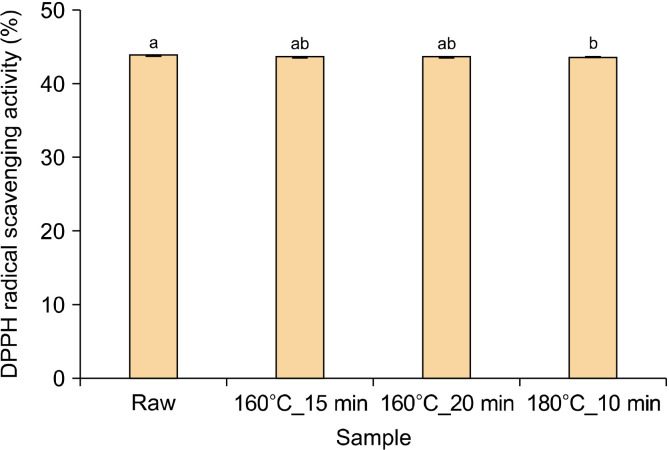
DPPH radical scavenging activity
The DPPH radical scavenging capacities of the four conditions of pomegranate seed oil were analyzed and are shown in Fig. 2. The highest DPPH radical scavenging capacity was observed in the raw samples (43.95%). After roasting at 160°C for 15 min or 20 min, the DPPH radical scavenging capacity revealed a slight decrease; however, no significant difference was found (P>0.05). The 180°C_10 min treatment demonstrated the lowest antioxidant capacity (43.65%).
Fig. 2.
2,2-Diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity of pomegranate seed oil under four conditions. The means, indicated by different letters (a,b), are significantly different between the four conditions by Tukey’s multiple range test (P<0.05).
The DPPH radical scavenging capacity test is a commonly used method for evaluating the antioxidant capacity because it measures the ability to scavenge free radicals or hydrogen donors (Oracz et al., 2014). Thermal processing, such as roasting, increases the antioxidant capacity owing to the release of bioactive compounds, such as phenols and flavonoids (Mohamed Ahmed et al., 2021). However, in this study, the antioxidant capacity measurements showed a slight decrease. This was determined by a decrease in tocopherols and phytosterols.
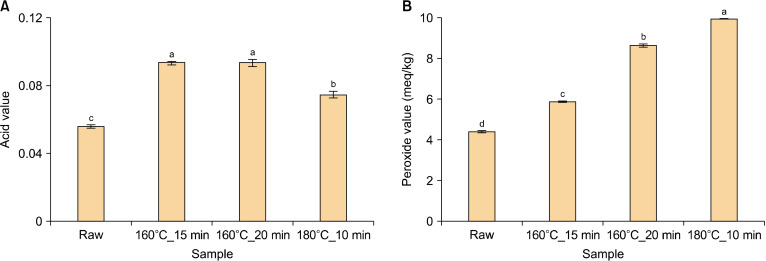
AV and PV
Thermal processing, such as roasting, affects the fatty acid composition of the oil as well as the AV/PV ratio, which is an indicator of oxidative stability (Kim et al., 2019). The results of the AV analysis of pomegranate seed oil is shown in Fig. 3A. In the raw state, the AV of pomegranate seed oil was found to be 0.06. After roasting, the AV showed an increasing pattern of 0.09, 0.09, and 0.07 at 160°C_15 min, 160°C_20 min, and 180°C_10 min, respectively. The AV measures the amount of free fatty acids that have separated from the glyceride form and is an indicator of the degree of fatty acid hydrolysis (Kim et al., 2019; Drinić et al., 2020). Chirinos et al. (2016) reported that the AV of sacha inchi oil increased with increasing temperature and roasting time (Chirinos et al., 2016), while Kim et al. (2019) determined that the AV of various vegetable oils continues to increase with increasing thermal processing (Kim et al., 2019).
Fig. 3.
Acid value (A) and peroxide value (B) of pomegranate seed oil under four conditions. The means, indicated by different letters (a-d), are significantly different between the four conditions by Tukey’s multiple range test (P<0.05).
The results of the PV analysis of pomegranate seed oil are shown in Fig. 3B. The PV of the raw pomegranate seed oil was 4.39 meq/kg. After roasting, the PV increased by about 1.34∼2.27 times to 5.88, 8.64, and 9.96 meq/kg at 160°C_15 min, 160°C_20 min, and 180°C_10 min, respectively. The PV is a measure of the primary oxidation products generated during the auto-oxidation of oil and an indicator of the degree of initial rancidity of the oil (Kim et al., 2019). The PV guideline for refined oils set by FAO and WHO (2001) is 10 meq/kg, with a value of >10 meq/kg indicating oil oxidation (Chirinos et al., 2016). Chirinos et al. (2016) identified a consistent increase in the PV of sacha inchi oil with increasing roasting degrees. This increase in PV was associated with the degradation of fatty acids, particularly polyunsaturated fatty acids (Chirinos et al., 2016).
The AV and PV of pomegranate seed oil under the four conditions were analyzed, and both oxidation indicators were found to be increased after roasting. This suggests that roasting affects the oxidative stability of pomegranate seed oil.
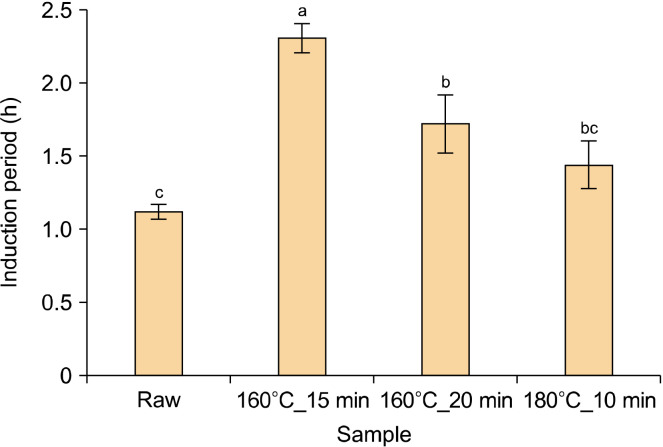
IP
The IPs of the four conditions of pomegranate seed oil were measured and are shown in Fig. 4. The IP of the raw sample was 1.12 h. The IPs of the roasted pomegranate seed oil were all increased compared to the raw oil. The longest IP was measured at 160°C_15 min, which was significantly increased by more than two times compared to the raw sample (P<0.05). The oils that received treatments of 160°C_20 min and 180°C_10 min were measured at 1.72 and 1.44 h, respectively.
Fig. 4.
Induction period of pomegranate seed oil under four conditions. The means, indicated by different letters (a-c), are significantly different between the four conditions by Tukey’s multiple range test (P<0.05).
IP, which is used as an indicator of oxidative stability, is an important factor in the shelf-life and quality control of edible oils (Kim et al., 2019). In addition, the IP is the period when the amount of oxygen absorption increases rapidly owing to external factors such as heat or oxygen, while oil freshness is maintained. Generally, the IP shows a pattern of shorter duration for oils with a higher unsaturated content (Kim et al., 2019). In a study by Habibnia et al. (2012), the IP of five pomegranate seed oils was measured at 110°C and ranged between 0.73 and 1.02 h, similar to the IP of the raw samples in this study. Thermal processing, such as roasting, causes oxidation of lipids but generally improves oxidative stability (Zhang et al., 2021). This is due to an increase in antioxidants such as phenols, flavonoids, and tocopherols, as well as compounds produced through the Maillard reaction, which have antioxidant activities, such as melanoidin (Rabadán et al., 2018). Rabadán et al. (2018) observed that the IP of pistachio oil increased continuously with the roasting degree, and Arab et al. (2022) confirmed that the IP of sesame oil increased continuously with increasing roasting temperature.
In this study, the IP of pomegranate seed oils was measured in four different conditions, and all of them increased compared to the raw sample, indicating the positive effect of the roasting treatment. Particularly, 160°C_15 min increased by about two times, which is considered to be the most optimal roasting condition.
This study analyzed the fatty acids, antioxidant properties, and oxidative stability of pomegranate seed oil extracted under raw, 160°C_15 min, 160°C_20 min, and 180°C_10 min conditions. Fatty acid analysis confirmed that the major fatty acid in pomegranate seeds was the C18:5 isomer (>90%), along with four other fatty acids. As the roasting degree increased, the proportions of punicic acid and polyunsaturated fatty acids decreased, whereas the proportions of saturated and monounsaturated fatty acids increased. Regarding antioxidant properties, TPC and TFC were significantly increased compared to the raw sample, whereas tocopherols and phytosterols showed no significant difference when compared to the raw sample. The AV and PV increased after roasting but were within the acceptable range. However, the IP increased due to the positive effects of roasting. Our findings provided basic data on the physicochemical properties of pomegranate seed oil and are expected to be used as a reference for setting conditions for extracting pomegranate seed oil via oven-roasting.
Footnotes
FUNDING
This research was supported by Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education (NRF-2022 R1I1A3066192).
AUTHOR DISCLOSURE STATEMENT
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
Concept and design: SY, HJ, ECS. Analysis and interpretation: SY, HJ, SJH, SMJ. Data collection: SY, HJ, HP, YB. Writing the article: SY, HJ. Critical revision of the article: MYY, ECS. Final approval of the article: all authors. Statistical analysis: SY, HP, YB. Obtained funding: ECS. Overall responsibility: ECS.
References
- AOCS, author. Official methods and recommended practices of the AOCS. AOCS official method Ca 5a-40. 7th ed. American Oil Chemists' Society; 2017. [Google Scholar]
- AOCS, author. Official methods and recommended practices of the AOCS. AOCS surplus method Cd 8-53. 7th ed. American Oil Chemists' Society; 2003. [Google Scholar]
- Arab R, Casal S, Pinho T, Cruz R, Freidja ML, Lorenzo JM, et al. Effects of seed roasting temperature on sesame oil fatty acid composition, lignan, sterol and tocopherol contents, oxidative stability and antioxidant potential for food applications. Molecules. 2022;27:4508. doi: 10.3390/molecules27144508. https://doi.org/10.3390/molecules27144508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirinos R, Zorrilla D, Aguilar-Galvez A, Pedreschi R, Campos D. Impact of roasting on fatty acids, tocopherols, phytosterols, and phenolic compounds present in Plukenetia huayllabambana seed. J Chem. 2016;2016:6570935. doi: 10.1155/2016/6570935. https://doi.org/10.1155/2016/6570935. [DOI] [Google Scholar]
- Demnati D, Pacheco R, Martínez L, Sánchez S. Effect of roasting temperature and time on the chemical composition and oxidative stability of argan (Argania spinosa L.) oils. Eur J Lipid Sci Technol. 2018;120:1700136. doi: 10.1002/ejlt.201700136. https://doi.org/10.1002/ejlt.201700136. [DOI] [Google Scholar]
- Drinić Z, Mudrić J, Zdunić G, Bigović D, Menković N, Šavikin K. Effect of pomegranate peel extract on the oxidative stability of pomegranate seed oil. Food Chem. 2020;333:127501. doi: 10.1016/j.foodchem.2020.127501. https://doi.org/10.1016/j.foodchem.2020.127501. [DOI] [PubMed] [Google Scholar]
- FAO, author; WHO, author. Codex alimentarius: Vol. 8. Fats, oils and related products. 2nd ed. Food and Agriculture Organization of the United Nations, 2001. World Health Organization; pp. 9–38. [Google Scholar]
- Ghafoor K, Ahmed IAM, Özcan MM, Al-Juhaimi FY, Babiker EE, Azmi IU. An evaluation of bioactive compounds, fatty acid composition and oil quality of chia (Salvia hispanica L.) seed roasted at different temperatures. Food Chem. 2020;333:127531. doi: 10.1016/j.foodchem.2020.127531. https://doi.org/10.1016/j.foodchem.2020.127531. [DOI] [PubMed] [Google Scholar]
- Habibnia M, Ghavami M, Ansaripour M, Vosough S. Chemical evaluation of oils extracted from five different varieties of Iranian pomegranate seeds. J Food Biosci Technol. 2012;2:35–40. [Google Scholar]
- Hong SJ, Cho JJ, Boo CG, Youn MY, Lee SM, Shin EC. Comparison of physicochemical and sensory properties of bean sprout and peanut sprout extracts, subsequent to roasting. J Korean Soc Food Sci Nutr. 2020;49:356–369. doi: 10.3746/jkfn.2020.49.4.356. [DOI] [Google Scholar]
- Iriti G, Bonacci S, Lopreiato V, Frisina M, Oliverio M, Procopio A. Functional compounds of cold-pressed pomegranate seed oil: fatty acids and phytosterols profile as quality biomarkers for origin discrimination. Foods. 2023;12:2599. doi: 10.3390/foods12132599. https://doi.org/10.3390/foods12132599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaseke T, Opara UL, Fawole OA. Effect of blanching pomegranate seeds on physicochemical attributes, bioactive compounds and antioxidant activity of extracted oil. Molecules. 2020a;25:2554. doi: 10.3390/molecules25112554. https://doi.org/10.3390/molecules25112554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaseke T, Opara UL, Fawole OA. Effect of microwave pretreatment of seeds on the quality and antioxidant capacity of pomegranate seed oil. Foods. 2020b;9:1287. doi: 10.3390/foods9091287. https://doi.org/10.3390/foods9091287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaseke T, Opara UL, Fawole OA. Quality and antioxidant properties of cold-pressed oil from blanched and microwave-pretreated pomegranate seed. Foods. 2021;10:712. doi: 10.3390/foods10040712. https://doi.org/10.3390/foods10040712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoddami A, Man YBC, Roberts TH. Physico-chemical properties and fatty acid profile of seed oils from pomegranate (Punica granatum L.) extracted by cold pressing. Eur J Lipid Sci Technol. 2014;116:553–562. doi: 10.1002/ejlt.201300416. https://doi.org/10.1002/ejlt.201300416. [DOI] [Google Scholar]
- Kim MS, Kim DS, Cho JJ, Hong SJ, Boo CG, Shin EC. Oxidative stability, physicochemical, and sensory characteristics of vegetable oils at their induction periods. J Korean Soc Food Sci Nutr. 2019;48:649–660. doi: 10.3746/jkfn.2019.48.6.649. [DOI] [Google Scholar]
- Lim HJ, Kim MS, Yoo HS, Kim JK, Shin EC. Analysis of nutritional components and sensory attributes of grilled and fast-chilled mackerels. J Korean Soc Food Sci Nutr. 2016;45:452–459. doi: 10.3746/jkfn.2016.45.3.452. [DOI] [Google Scholar]
- Mohamed Ahmed IA, Uslu N, Musa Özcan M, Al Juhaimi F, Ghafoor K, Babiker EE, et al. Effect of conventional oven roasting treatment on the physicochemical quality attributes of sesame seeds obtained from different locations. Food Chem. 2021;338:128109. doi: 10.1016/j.foodchem.2020.128109. https://doi.org/10.1016/j.foodchem.2020.128109. [DOI] [PubMed] [Google Scholar]
- Musa Özcan M, Uslu N. Influence of microwave heating on bioactive properties, phenolic compounds and fatty acid profiles of pomegranate seed oil. Food Chem. 2023;422:136207. doi: 10.1016/j.foodchem.2023.136207. https://doi.org/10.1016/j.foodchem.2023.136207. [DOI] [PubMed] [Google Scholar]
- Oracz J, Nebesny E, Żyżelewicz D. Effect of roasting conditions on the fat, tocopherol, and phytosterol content and antioxidant capacity of the lipid fraction from cocoa beans of different Theobroma cacao L. cultivars. Eur J Lipid Sci Technol. 2014;116:1002–1014. doi: 10.1002/ejlt.201300474. https://doi.org/10.1002/ejlt.201300474. [DOI] [Google Scholar]
- Rabadán A, Gallardo-Guerrero L, Gandul-Rojas B, Álvarez-Ortí M, Pardo JE. Effect of roasting conditions on pigment composition and some quality parameters of pistachio oil. Food Chem. 2018;264:49–57. doi: 10.1016/j.foodchem.2018.05.030. [DOI] [PubMed] [Google Scholar]
- Shin EC. Relationships between fatty acids and tocopherols of conventional and genetically modified peanut cultivars grown in the United States. J Korean Soc Food Sci Nutr. 2013;42:1618–1628. doi: 10.3746/jkfn.2013.42.10.1618. [DOI] [Google Scholar]
- Shin EC, Pegg RB, Phillips RD, Eitenmiller RR. Commercial peanut (Arachis hypogaea L.) cultivars in the United States: phytosterol composition. J Agric Food Chem. 2010;58:9137–9146. doi: 10.1021/jf102150n. [DOI] [PubMed] [Google Scholar]
- Wroniak M, Rękas A, Siger A, Janowicz M. Microwave pretreatment effects on the changes in seeds microstructure, chemical composition and oxidative stability of rapeseed oil. LWT. 2016;68:634–341. doi: 10.1016/j.lwt.2016.01.013. [DOI] [Google Scholar]
- Yang KM, Hsu FL, Chen CW, Hsu CL, Cheng MC. Quality characterization and oxidative stability of camellia seed oils produced with different roasting temperatures. J Oleo Sci. 2018;67:389–396. doi: 10.5650/jos.ess17190. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Li X, Lu X, Sun H, Wang F. Effect of oilseed roasting on the quality, flavor and safety of oil: A comprehensive review. Food Res Int. 2021;150:110791. doi: 10.1016/j.foodres.2021.110791. https://doi.org/10.1016/j.foodres.2021.110791. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Fan W, Chu F, Pei D. Improvement of the flavor and oxidative stability of walnut oil by microwave pretreatment. J Am Oil Chem Soc. 2016;93:1563–1572. doi: 10.1007/s11746-016-2891-9. https://doi.org/10.1007/s11746-016-2891-9. [DOI] [Google Scholar]
- Zou Y, Gao Y, He H, Yang T. Effect of roasting on physico-chemical properties, antioxidant capacity, and oxidative stability of wheat germ oil. LWT. 2018;90:246–253. doi: 10.1016/j.lwt.2017.12.038. [DOI] [Google Scholar]