Abstract
This study aimed to investigate the potential in vitro antihyperglycemic activity of honey sourced from three different species of stingless bees (Heterotrigona itama, Geniotrigona thoracica, and Kelulut matahari) by assessing their α-glucosidase enzyme inhibition, antioxidant activity, and total phenolic and flavonoid contents in comparison with honey from Apis cerana, obtained from West Sumatra, Indonesia. The honey samples were obtained from stingless bee farms at the Faculty of Animal Science, Universitas Andalas. Variations were observed in α-glucosidase enzyme inhibition, antioxidant activity (half maximal inhibitory concentration, IC50), and total phenolic and flavonoid contents among the honey samples from H. itama, G. thoracica, K. matahari, and A. cerana. In terms of α-glucosidase inhibition, honey from the stinging bee A. cerana demonstrated higher inhibition than that from the other three stingless bees species. Honey derived from K. matahari exhibited the lowest IC50 value, indicating its superior antioxidant activity, followed by honey from A. cerana, H. itama, and G. thoracica. The highest total phenolic and flavonoid contents were found in honey from A. cerana, followed by honey from K. matahari, H. itama, and G. thoracica. Analysis using Fourier-transform infrared spectroscopy revealed that the predominant absorptions in all four honey samples were observed at 767∼1,643 cm−1, indicating that absorptions are primarily ascribed to monosaccharides and disaccharides. Additionally, some peaks implied the presence of phenolic and flavonoid compounds. Overall, honey from stingless bees shows promise as an antihyperglycemic food, as evidenced by its α-glucosidase enzyme inhibition activity, antioxidant activity, and relatively high total phenolic content.
Keywords: antihyperglycemic, functional food, honey, stingless bee
INTRODUCTION
Hyperglycemia, often called as diabetes mellitus, is a metabolic disorder characterized by elevated blood glucose levels and carbohydrate, lipid, and protein metabolism disruptions. This condition arises from insulin deficiency or resistance (Surya et al., 2014). Hyperglycemia is caused by lipid peroxidation and oxidative stress, which result from an increase in free radical compounds, leading to decreased pancreatic β-cell secretion, inflammation, and endothelial damage (Vijay and Vimukta, 2014).
Antioxidant and phenolic compounds play a substantial role in various biological activities, including acting as antidiabetic agents. Furthermore, the consumption of polyphenol-based antioxidant-rich foods helps in mitigating the formation of reactive free radicals and oxidative stress within the body. Consequently, this can protect pancreatic β-cells and promote their proliferation, thereby activating insulin signaling and secretion (Fatima et al., 2022). Additionally, polyphenolic compounds contribute to inhibiting the α-glucosidase enzyme, which represents another mechanism of antihyperglycemic action. This inhibition leads to reduced blood glucose levels postcarbohydrate consumption in individuals with diabetics as the enzyme aids in the breakdown of complex carbohydrates (Sansenya et al., 2023).
Stingless bee honey, locally known as Galo-galo honey in West Sumatra, is sourced from stingless bees (Apidae; Meliponini). In addition to being a beneficial nutritional source, honey is consumed for its health-promoting properties. Its primary constituents are carbohydrates, specifically fructose and sucrose (Bogdanov et al., 2008). Moreover, honey contains high levels of antioxidants and polyphenolic compounds. Honey from stingless bees exhibits higher antioxidant activity and total polyphenol content compared to honey produced by stinging bees of the genus Apis (Rodríguez-Malaver, 2009). Several studies have reported the various benefits of stingless bee honey, including its antimicrobial (Tuksitha et al., 2018), anti-inflammatory (Chong et al., 2021), anticancer (Al-hatamleh et al., 2020), and antihyperglycemic properties (Sahlan et al., 2020).
Traditionally, high-carbohydrate foods are believed to increase blood glucose levels and are not recommended for consumption by individuals with diabetes. However, recent research suggests that honey can act as an antidiabetic agent. The antihyperglycemic mechanism of honey involves the role of fructose and oligosaccharides in glycemic control (Erejuwa et al., 2012), contribution of flavonoid compounds and its antioxidant activity, which mitigate oxidative stress (Tadera et al., 2006; Garba et al., 2012).
The nutritional profile of honey, including antioxidants and flavonoids, plays a crucial role in its potential as an antihyperglycemic food. However, the composition of honey is influenced by factors such as its geographic origin, botanical nectar source, environmental conditions, climate, and processing techniques. Bee preferences for foraging, behavior, and body size can affect the honey composition, causing variations in the total and types of flavonoids present (Ismail et al., 2016). Despite these factors, the potential antihyperglycemic properties of stingless bee honey from West Sumatra, Indonesia, remain largely unexplored. Therefore, there is a pressing need for studies to investigate the potential of stingless bee honey as an antihyperglycemic food source.
MATERIALS AND METHODS
Materials
Raw honey samples were sourced from bee farms at the Faculty of Animal Science, Universitas Andalas. These farms encompass three varieties of stingless bees: Heterotrigona itama, Geniotrigona thoracica, and Kelulut matahari and one species of stinging bee, Apis cerana. Samples were collected in September 2023, and honey was extracted from the honeycombs using a syringe needle and transferred into dark glass bottles. All samples were stored under cool conditions until the analysis was performed.
Experimental design
The study was designed to assess the potential of honey sourced from three varieties of stingless bees as antihyperglycemic food, juxtaposed with honey from the stinging bee, A. cerana. This comparison was based on their biological properties, including α-glucosidase enzyme inhibition activity−a crucial parameter of the antihyperglycemic mechanism. By inhibiting α-glucosidase, which is involved in the breakdown of complex carbohydrates, thereby reducing blood sugar levels. Additionally, assessments of half maximal inhibitory concentration (IC50) antioxidant capability, total phenolic and flavonoid contents, and the chemical surface properties via Fourier-transform infrared (FTIR) analysis were conducted to bolster the potential of stingless bee honey as an antihyperglycemic food source. Notably, all measurements were performed in triplicate to ensure the robustness and reliability of the findings.
Determination of α-glucosidase enzyme inhibition activity
The α-glucosidase inhibition activity was assessed following the method outlined by Sancheti et al. (2009). The reaction mixture comprised 50 μL of 0.1 M phosphate buffer (pH 7.0), 25 μL of 4-nitrophenyl a-D-glucopyranoside (dissolved in 0.1 M phosphate buffer, pH 7.0), 10 μL of the sample (10 mg dissolved in 1 mL of dimethyl sulfoxide and aquabides solvent), and 25 μL of α-glucosidase enzyme solution (0.04 units mL−1 in 0.1 M phosphate buffer, pH 7.0). The mixture was then incubated at 37°C for 30 min. Following incubation, the reaction was terminated by adding 100 μL of 0.2 M sodium carbonate solution. The extent of enzymatic hydrolysis of the substrate was determined by measuring the amount of p-nitrophenol released during the reaction. This measurement was conducted using an microplate reader at a wavelength of 410 nm.
Determination of antioxidant activity (IC50)
The antioxidant activity was assessed following the method proposed by Salazar-Aranda et al. (2011). Initially, samples (100 g) underwent extraction using the maceration method with methanol (3×600 mL) for 2 h at 29°C. Subsequently, the obtained extracts were filtered and concentrated. For the assay, standard solution, blank, and samples (concentrations of 0.625, 1.25, 2.5, 5, 10, and 20 mg/mL) were added to microplate wells in aliquots of 100 μL. Following this, ethanol (100 μL) and a 1,1-diphenyl-2-picrylhydrazyl (DPPH) solution were added up to 300 μL. The mixture was then incubated for 30 min at 29°C. After incubation, the absorbance was measured using a microplate reader at a wavelength of 517 nm. The free radical scavenging capacity was calculated as the percentage inhibition of DPPH, representing the percentage of the scavenging effect.
Determination of total phenolic content
The analysis of total phenolic content followed the method proposed by Hodzic et al. (2009). Initially, a sample weighing 100 mg was added to 5 mL of 95% ethanol in a covered reaction tube, followed by vortexing. The tube containing the mixture was centrifuged at 1,409 g for 15 min. Supernatants from the sample and standard solutions were withdrawn in 0.5 mL aliquots and transferred to clean reaction tubes. Subsequently, 0.5 mL of 95% ethanol, 2.5 mL of aquades, and 2.5 mL of the Folin-Ciocalteu reagent (50%) were added to each reaction tube. After allowing the mixture to stand for 5 min, 0.5 mL of 5% sodium carbonate solution was added to it and vortexed. The reaction tubes were wrapped in aluminum foil and stored in the dark for 1 h. Following incubation, the absorbance was measured using a ultraviolet-visible (UV-Vis) spectrophotometer at a wavelength of 725 nm. The quantitative analysis of the total phenolic content was conducted by constructing a standard curve of gallic acid concentrations (0, 50, 100, 150, 200, and 250 ppm). The total phenolic content of the sample was then determined using the prepared gallic acid standard curve.
Determination of total flavonoid content
The total flavonoid content was determined following the method described by Pontis et al. (2014), with slight modifications. Initially, 2.5 g sample was dissolved in 5 mL of ethanol, followed by sonication for 15 min and filtration. Subsequently, 1.5 mL of the sample solution was mixed with 0.1 mL of 10% AlCl3 and 0.1 mL of 1 M Na-acetate in 3.3 mL of ethanol. The resulting mixture was incubated for 30 min, after which the absorbance was measured using a UV-Vis spectrophotometer at a wavelength of 425 nm. Quantitative analysis was performed by constructing a standard curve of quercetin concentrations (6, 8, 10, 12, and 14 ppm). Subsequently, the total flavonoid content of the sample was determined using the quercetin standard curve.
FTIR analysis
Honey spectra were recorded following a method Gok et al. (2015) outlined, with slight modifications. For the measurement of honey spectra, 0.1 g of each sample was analyzed using an FTIR spectrometer (PerkinElmer Inc.) equipped with a zinc selenide crystal-fitted diamond single reflection attenuated total reflectance (ATR) accessory. Spectral acquisition was conducted using PerkinElmer Frontier C90704 Spectrum IR Version 10.6.1 software. Infrared spectra were collected within the 4,000∼600 cm−1 range with a 4 cm−1 spectral resolution.
Data analysis
Data analysis was performed utilizing the one-way ANOVA procedure in IBM SPSS version 22 (IBM Corp.). Upon detection of differences indicated by ANOVA, Duncan’s multiple range test was applied at a significance level of 5%.
RESULTS AND DISCUSSION
α-Glucosidase enzyme inhibition activity
The enzyme α-glucosidase plays a pivotal role in elevating blood glucose, and its inhibition can yield beneficial antidiabetic effects by reducing blood glucose levels. Table 1 presents the inhibitory activities of α-glucosidase enzyme in honey sourced from H. itama, G. thoracica, K. matahari, and A. cerana.
Table 1.
α-Glucosidase inhibition activity of stingless bee honey from West Sumatra
| Sample | % Inhibition |
|---|---|
| Heterotrigona itama | 6.79±0.37b |
| Geniotrigona thoracica | 2.81±0.27a |
| Kelulut matahari | 6.61±0.28b |
| Apis cerana | 9.56±0.31c |
Values are presented as mean±SD of three replication.
Different letters in a column (a-c) show a significant difference (P<0.05).
The results indicated that the inhibitory activities against α-glucosidase enzymes varied among the different types of honey, ranging from 2.81% to 9.56%. Among the honey samples from stingless bees, no substantial difference (P>0.05) was observed between honey sourced from H. itama and K. matahari species. However, a significant difference (P<0.05) was noted in honey from both H. itama and K. matahari compared with honey from the G. thoracica species, which exhibited the lowest α-glucosidase enzyme inhibition activity. Furthermore, all three honey samples procured from stingless bees demonstrated lower α-glucosidase enzyme inhibition activities than that from the stinging bee species, A. cerana.
The findings of this study aligned with those reported by Peláez-Acero et al. (2022), who observed that α-glucosidase enzyme inhibition activities of honey from Mexico ranged from 5.28% to 14.50%. Honey from stingless bees Tetragonula laeviceps and Tetragonula biroi exhibited higher α-glucosidase enzyme inhibition activities than those under investigation in this study, ranging from 15.56% to 40.10% (Rahmawati et al., 2019). Additionally, Krishnasree and Ukkuru (2017) noted that honey from stingless bees exhibited higher α-glucosidase enzyme inhibition activities than that from stinging bees. However, contrary to their findings, the results obtained in this study indicated that the inhibition activities of honey from the stinging bee against the α-glucosidase enzyme A. cerana were higher than those from stingless bees. The inhibition of the α-glucosidase enzyme is closely associated with the presence of flavonoid compounds in honey, as flavonoids are known to possess high inhibitory activity against the α-glucosidase enzyme (Kim et al., 2000).
Antioxidant activity (IC50)
The antioxidant activity in this study was assessed using IC50 values, which indicate the sample concentration required to reduce DPPH activity by 50%. Table 2 presents honey’s antioxidant activity values (IC50) from H. itama, G. thoracica, K. matahari, and A. cerana.
Table 2.
Antioxidant activity (IC50) of stingless bee honey from West Sumatra
| Sample | IC50 (mg/mL) |
|---|---|
| Heterotrigona itama | 52.08±0.87c |
| Geniotrigona thoracica | 64.19±0.82d |
| Kelulut matahari | 8.82±0.06a |
| Apis cerana | 23.56±0.04b |
Values are presented as mean±SD of three replication.
Different letters in a column (a-d) show a significant difference (P<0.05).
IC50, half maximal inhibitory concentration.
The results revealed considerable differences (P<0.05) in the IC50 values for antioxidant activity among all honey samples, ranging from 8.82 to 64.19 mg/mL. Lower IC50 values indicate the higher antioxidant activity of a substance (Molyneux, 2004). Herein, honey sourced from K. matahari exhibited the highest antioxidant activity among all samples. Bastos et al. (2009) corroborated that honey from stingless bees generally exhibits superior antioxidant activity than that from the stinging bee Apis mellifera, aligning with the findings of this study. Honey from K. matahari demonstrated higher antioxidant activity with a lower IC50 than that from A. cerana, whereas honey from H. itama and G. thoracica exhibited lower antioxidant activity than the other samples.
Regarding honey from H. itama and G. thoracica, previous studies have reported that H. itama honey possesses a lower IC50 value than that from G. thoracica, indicating higher antioxidant activity (Tuksitha et al., 2018; Shamsudin et al., 2019). These variations in antioxidant activity might be arising from differences in floral sources and the ecosystems where the bees reside. However, in this study, because all four honey samples came from the same farming area with identical floral sources, disparities in antioxidant activity could be linked to variations in the bee species themselves.
Antioxidant compounds play a crucial role in antihyperglycemic activity, with one mechanism involving the reduction of free radicals by these compounds present in honey. This process effectively diminishes oxidative stress and shields organs from oxidative damage (Erejuwa et al., 2009). Sahhugi et al. (2014) also demonstrated that honey sourced from the stingless bee Gelam could mitigate oxidative damage by reducing malondialdehyde levels in rats.
Total phenolic and flavonoid contents
The total phenolic and flavonoid contents in honey sourced from H. itama, G. thoracica, K. matahari, and A. cerana are presented in Table 3. The results indicated significant differences (P<0.05) in the total phenolic content among all samples, ranging from 39.98 to 81.02 mg gallic acid equivalent (GAE)/g. Honey from A. cerana exhibited the highest total phenolic content, followed by honey from K. matahari, H. itama, and G. thoracica. The total phenolic content observed in this study falls within the range da Silva et al. (2013) reported for honey obtained from Melipona (Apidae; Meliponini), namely 17.0∼66.0 mg GAE/g. However, it was significantly higher than that reported by Shamsudin et al. (2019) for honey from stingless bees H. itama and G. thoracica, which ranged from only 0.27 to 0.55 mg GAE/g.
Table 3.
Total phenolic and flavonoid contents of stingless bee honey from West Sumatra
| Sample | Total phenolic content (mg GAE/g) | Total flavonoid content (mg EQ/100 g) |
|---|---|---|
| Heterotrigona itama | 59.98±0.72b | 1.51±0.01b |
| Geniotrigona thoracica | 39.98±0.28a | 1.23±0.01a |
| Kelulut matahari | 75.80±1.20c | 1.96±0.02c |
| Apis cerana | 81.02±0.54d | 2.18±0.01d |
Values are presented as mean±SD of three replication.
Different letters in a column (a-d) show a significant difference (P<0.05).
GAE, gallic acid equivalent; EQ, quercetin equivalent.
The total flavonoid content mirrored the pattern observed in the total phenolic content across all four honey samples, indicating significant differences (P<0.05) among them. The highest value was recorded in honey sourced from A. cerana [2.18 mg quercetin equivalent (QE)/100 g], while the lowest was observed in honey from G. thoracica (1.23 mg QE/100 g). Consequently, in this study, honey from A. cerana exhibited elevated levels of total phenolic and flavonoid contents compared to honey from other stingless bee species. However, the total flavonoid content in this study was comparatively lower than that reported by Shamsudin et al. (2019) and da Silva et al. (2013), ranging from 2.80 to 9.31 mg QE/100 g and 2.6 to 31.0 mg QE/100 g, respectively. Discrepancies in total phenolic and flavonoid contents compared with other studies may be attributed to variations in nectar sources, climate conditions, and harvest seasons. The diversity in total phenolic and flavonoid contents among the four study samples might be attributable to differences in bee species.
The total phenolic and flavonoid contents positively correlate with antioxidant activity, wherein higher levels of polyphenolic compounds are typically associated with higher antioxidant activity (Islam et al., 2017). However, in this study, although honey from A. cerana exhibited the highest total phenolic and flavonoid contents, the highest antioxidant activity (IC50) was observed in honey from K. matahari, despite both these honeys having higher total phenolic and flavonoid contents and antioxidant activity than honeys from other species. This may be because the antioxidant activity originates from factors other than the phenolic and flavonoid contents alone. Tuksitha et al. (2018) highlighted that the overall antioxidant activity of honey is influenced by various antioxidant components and the complexity of its composition. Moreover, polyphenolic compounds, Maillard reaction products, organic acids, and various peptides found in honey collectively influence its antioxidant activity (Gheldof and Engeseth, 2002).
Phenolic and flavonoid compounds have been reported to inhibit α-glucosidase. The findings of this study elucidated a correlation between the total phenolic and flavonoid contents and α-glucosidase enzyme inhibition activity. Specifically, honey sourced from A. cerana exhibited the highest α-glucosidase enzyme inhibition activity and total phenolic and flavonoid contents. Conversely, honey from G. thoracica demonstrated the lowest α-glucosidase enzyme inhibition activity and total phenolic and flavonoid contents. Phenolic compounds regulate the metabolic pathways associated with diabetes by enhancing glucose transporter type 4 cascade signaling, promoting glycogen production, modulating inflammatory mediators, and regulating oxidative responses (Sharma et al., 2020). Furthermore, the increase in α-glucosidase inhibition activity is linked to an increased number of hydroxyl groups on the B-ring structure of flavonoid compounds (Tadera et al., 2006).
FTIR spectra interpretations
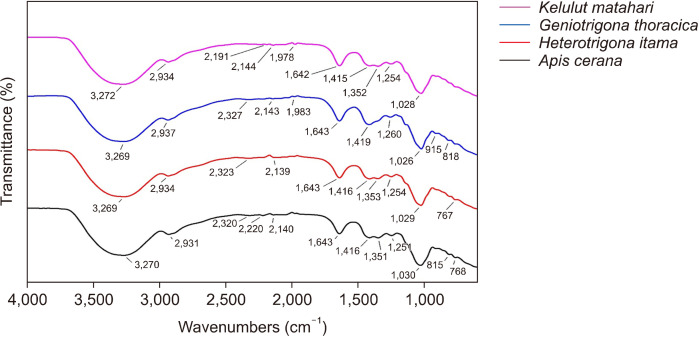
The results of the FTIR analysis for the four honey samples in the wavenumbers 4,000∼650 cm−1 are depicted in Fig. 1. According to Huang et al. (2020), the region 1,500∼750 cm−1 corresponds to the primary absorption of fructose, glucose, and sucrose, while the peaks at 1,642 and 3,297 cm−1 are indicative of water absorption in honey. Overall, the FTIR spectra of all honey samples exhibited similar patterns. The sample from A. cerana displayed 12 peaks, the sample from G. thoracica showed 11 peaks, and the samples from H. itama and K. matahari displayed 10 peaks (Fig. 1 and Table 4).
Fig. 1.
Fourier-transform infrared-attenuated total reflectance spectra of honey from stingless bee procured from West Sumatra.
Table 4.
Prediction of Fourier-transform infrared spectrum peaks of stingless bee honey from West Sumatra
| Apis cerana | Heterotrigona itama | Geniotrigona thoracica | Kelulut matahari | Functional group | Reference |
|---|---|---|---|---|---|
| 768 | 767 | − | − | Anomeric region of carbohydrate | Gallardo-Velázquez et al. (2009) |
| 815 | − | 818,915 | − | C-H bending (carbohydrate) | Gallardo-Velázquez et al. (2009) |
| 1,030 | 1,029 | 1,026 | 1,028 | C-O stretching | Subari et al. (2012) |
| 1,251 | 1,254 | 1,260 | 1,254 | C-O stretching (C-OH group) in carbohydrate structure | Tewari and Irudayaraj (2004); Gallardo-Velázquez et al. (2009) |
| 1,351 | 1,353 | − | 1,352 | O-H bending on the C-OH group | Gallardo-Velázquez et al. (2009) |
| 1,416 | 1,416 | 1,419 | 1,415 | Combination of O-H bending from the C-OH group and C-H bending of alkenes | Gallardo-Velázquez et al. (2009) |
| 1,643 | 1,643 | 1,643 | 1,642 | H-O-H bending vibration | Nayik et al. (2019) |
| 2,931 | 2,934 | 2,937 | 2,934 | C-H stretching (carboxylic acids) and NH3 stretching band (free amino acids) | Gallardo-Velázquez et al. (2009) |
| 3,270 | 3,269 | 3,269 | 3,272 | C-H stretching (carboxylic acids) and NH3 stretching band (free amino acids) |
Sucrose exhibits bands in the range of 928∼1,427 cm−1 with notable peaks at 994 and 1,049 cm−1, fructose demonstrates in the range of 923∼1,418 cm−1 with a prominent peak at 1,053 cm−1, glucose in the range of 902∼1,431 cm−1 with a key peak at 1,032 cm−1, and maltose in the range of 920∼1,353 cm−1 with a key peak at 1,032 cm−1 (Wang et al., 2010). In this study, dominant peaks were observed at 767∼1,643 cm−1 across all four honey samples, indicating a sugar profile consisting of sucrose, fructose, glucose, and maltose. Fructose and oligosaccharides present in honey, such as palatinose, turanose, raffinose, and isomaltose, have been identified as potential antidiabetic agents (Erejuwa et al., 2012). Fructose in honey can enhance hepatic glucose absorption, glycogen synthesis, and storage, thereby improving glycemic control in diabetes mellitus (Eteraf-Oskouei and Najafi, 2013).
Oligosaccharides found in honey have been associated with several roles as antihyperglycemic agents through various mechanisms, such as their involvement in the gut and gut microbiota as prebiotics, enhancement in glucose-stimulated insulin secretion, and reduction in the rate of glucose absorption (Erejuwa et al., 2011). Additionally, isomaltose, a compound detected in honey, exhibits inhibitory effects on the human incretin-degrading enzyme dipeptidyl peptidase IV (DPP-IV), which is responsible for deactivating glucose-regulating hormones such as glucagon-like peptide-1. Therefore, the inhibitory action of DPP-IV can alleviate diabetic conditions (Bharti et al., 2015; Belay et al., 2017).
Regarding phenolic compounds, peaks in the vibration region spanning 1,175∼940 cm−1 represent C-OH groups and stretching of C-C and C-O in carbohydrate moieties as well as C-O in phenol (Masek et al., 2014); in this study, these peaks are observed at 1,026, 1,028, 1,029, and 1,030 cm−1 in all four honey samples. The region at 1,540∼1,175 cm−1 represents deformations of O-H, C-O, C-H, and C=C corresponding to phenol and flavanol (Masek et al., 2014); in this study these peaks are observed at 1,419∼1,251 cm−1 in all four honey samples.
Thummajitsakul et al. (2023) reported that the abundance of peaks generated by FTIR indicates the high total phenolic content present in ethanol extracts of Glycine max L. and Phaseolus vulgaris, establishing a positive and significant correlation between the total phenolic content and FTIR spectra. Peaks observed at 2,934∼2,931, 1,419∼1,415, and 1,353∼1,351 cm−1 in this study are consistent with those reported by Wongsa et al. (2022). These peaks are attributed to the presence of phenolic components exhibiting antihyperglycemic properties, particularly the compound p-coumaric acid. p-Coumaric acid has a positive correlation with α-glucosidase inhibition and is a dominant phenolic compound in honey (Halagarda et al., 2020).
Differences in bee species contribute to differences in honey composition, affecting α-glucosidase enzyme inhibition, antioxidant activity (IC50), and total phenolic and flavonoid contents. A. cerana honey exhibited the highest inhibition activity and total phenolic and flavonoid contents, whereas honey from G. thoracica showed the lowest, demonstrating a correlation between the α-glucosidase enzyme inhibition activity and total phenolic and flavonoid contents. Regarding antioxidant activity (IC50), K. matahari honey displayed the superior antioxidant activity (lowest IC50 value), followed by A. cerana, H. itama, and G. thoracica.
The FTIR results revealed that all four honey samples mostly exhibited absorption in the region of 767∼1,643 cm−1, indicating dominant absorption by monosaccharides and disaccharides. These findings underscore the potential of stingless bee honey as an antihyperglycemic food, as evidenced by its inhibitory activity against the α-glucosidase enzyme, antioxidant activity, and comparatively high total phenolic content.
Footnotes
FUNDING
The funding for this study is provided by Faculty of Animal Science, Universitas Andalas (contract no. 001.e/UN. 16.06.D/PT.01/SPP.RDP/FATERNA/2023).
AUTHOR DISCLOSURE STATEMENT
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
Concept and design: RDS, SM, IJ. Analysis and interpretation: RDS, SM, IJ, R. Data collection: RDS, R. Writing the article: RDS. Critical revision of the article: RDS. Final approval of the article: all authors. Statistical analysis: RDS. Obtained funding: RDS, SM. Overall responsibility: RDS.
References
- Al-Hatamleh MAI, Boer JC, Wilson KL, Plebanski M, Mohamud R, Mustafa MZ. Antioxidant-based medicinal properties of stingless bee products: recent progress and future directions. Biomolecules. 2020;10:923. doi: 10.3390/biom10060923. https://doi.org/10.3390/biom10060923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastos DHM, dos Santos MCM, Mendonça S, Torres EAFS. Antioxidant capacity and phenolic content of stingless bee honey from Amazon in comparison to Apis bee honey. Acta Hortic. 2009;841:483–485. doi: 10.17660/ActaHortic.2009.841.64. [DOI] [Google Scholar]
- Belay A, Haki GD, Birringer M, Borck H, Lee YC, Cho CW, et al. Sugar profile and physicochemical properties of Ethiopian monofloral honey. Int J Food Prop. 2017;20:2855–2866. doi: 10.1080/10942912.2016.1255898. [DOI] [Google Scholar]
- Bharti SK, Krishnan S, Kumar A, Gupta AK, Ghosh AK, Kumar A. Mechanism-based antidiabetic activity of Fructo- and isomalto-oligosaccharides: Validation by in vivo, in silico and in vitro interaction potential. Process Biochem. 2015;50:317–327. doi: 10.1016/j.procbio.2014.10.014. [DOI] [Google Scholar]
- Bogdanov S, Jurendic T, Sieber R, Gallmann P. Honey for nutrition and health: a review. J Am Coll Nutr. 2008;27:677–689. doi: 10.1080/07315724.2008.10719745. [DOI] [PubMed] [Google Scholar]
- Chong KY, Chin NL, Yusof YA, Fakurazi S. Anti-inflammatory activity of raw and processed stingless bee honey. Food Res. 2021;5:201–208. doi: 10.26656/fr.2017.5(S1).007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva IA, da Silva TM, Camara CA, Queiroz N, Magnani M, de Novais JS, et al. Phenolic profile, antioxidant activity and palynological analysis of stingless bee honey from Amazonas, Northern Brazil. Food Chem. 2013;141:3552–3558. doi: 10.1016/j.foodchem.2013.06.072. [DOI] [PubMed] [Google Scholar]
- Erejuwa OO, Sulaiman SA, Ab Wahab MS. Honey: a novel antioxidant. Molecules. 2012;17:4400–4423. doi: 10.3390/molecules17044400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erejuwa OO, Sulaiman SA, Ab Wahab MS. Oligosaccharides might contribute to the antidiabetic effect of honey: a review of the literature. Molecules. 2011;17:248–266. doi: 10.3390/molecules17010248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erejuwa OO, Sulaiman SA, Ab Wahab MS, Sirajudeen KNS, Salzihan MS. Effects of Malaysian Tualang honey supplementation on glycemia, free radical scavenging enzymes and markers of oxidative stress in kidneys of normal and streptozotocin-induced diabetic rats. Int J Cardiol. 2009;137:S45. doi: 10.1016/j.ijcard.2009.09.148. https://doi.org/10.1016/j.ijcard.2009.09.148. [DOI] [Google Scholar]
- Eteraf-Oskouei T, Najafi M. Traditional and modern uses of natural honey in human diseases: a review. Iran J Basic Med Sci. 2013;16:731–742. [PMC free article] [PubMed] [Google Scholar]
- Fatima MT, Bhat AA, Nisar S, Fakhro KA, Al-Shabeeb Akil AS. The role of dietary antioxidants in type 2 diabetes and neurodegenerative disorders: An assessment of the benefit profile. Heliyon. 2022;9:e12698. doi: 10.1016/j.heliyon.2022.e12698. https://doi.org/10.1016/j.heliyon.2022.e12698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallardo-Velázquez T, Osorio-Revilla G, Zuñiga-de Loa M, Rivera-Espinoza Y. Application of FTIR-HATR spectroscopy and multivariate analysis to the quantification of adulterants in Mexican honeys. Food Res Int. 2009;42:313–318. doi: 10.1016/j.foodres.2008.11.010. [DOI] [Google Scholar]
- Garba AM, Mohammed B, Garba SH, Numan AI, Dalori BM. The effects of honey and Aloe vera extract on ibuprofen induced liver damage in rats. IOSR J Pharm Biol Sci. 2012;3:6–10. doi: 10.9790/3008-03206106. [DOI] [Google Scholar]
- Gheldof N, Engeseth NJ. Antioxidant capacity of honeys from various floral sources based on the determination of oxygen radical absorbance capacity and inhibition of in vitro lipoprotein oxidation in human serum samples. J Agric Food Chem. 2002;50:3050–3055. doi: 10.1021/jf0114637. [DOI] [PubMed] [Google Scholar]
- Gok S, Severcan M, Goormaghtigh E, Kandemir I, Severcan F. Differentiation of Anatolian honey samples from different botanical origins by ATR-FTIR spectroscopy using multivariate analysis. Food Chem. 2015;170:234–240. doi: 10.1016/j.foodchem.2014.08.040. [DOI] [PubMed] [Google Scholar]
- Halagarda M, Groth S, Popek S, Rohn S, Pedan V. Antioxidant activity and phenolic profile of selected organic and conventional honeys from Poland. Antioxidants. 2020;9:44. doi: 10.3390/antiox9010044. https://doi.org/10.3390/antiox9010044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodzic Z, Pasalic H, Memisevic A, Srabovic M, Saletovic M, Poljakovic M. The influence of total phenols content on antioxidant capacity in the whole grain extracts. Eur J Sci Res. 2009;28:471–477. [Google Scholar]
- Huang F, Song H, Guo L, Guang P, Yang X, Li L, et al. Detection of adulteration in Chinese honey using NIR and ATR-FTIR spectral data fusion. Spectrochim Acta A Mol Biomol Spectrosc. 2020;235:118297. doi: 10.1016/j.saa.2020.118297. https://doi.org/10.1016/j.saa.2020.118297. [DOI] [PubMed] [Google Scholar]
- Islam MR, Pervin T, Hossain H, Saha B, Hossain SJ. Physicochemical and antioxidant properties of honeys from the Sundarbans mangrove forest of Bangladesh. Prev Nutr Food Sci. 2017;22:335–344. doi: 10.3746/pnf.2017.22.4.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail NI, Abdul Kadir MR, Mahmood NH, Singh OP, Iqbal N, Zulkifli RM. Apini and Meliponini foraging activities influence the phenolic content of different types of Malaysian honey. J Apic Res. 2016;55:137–150. doi: 10.1080/00218839.2016.1207388. [DOI] [Google Scholar]
- Kim JS, Kwon CS, Son KH. Inhibition of alpha-glucosidase and amylase by luteolin, a flavonoid. Biosci Biotechnol Biochem. 2000;64:2458–2461. doi: 10.1271/bbb.64.2458. [DOI] [PubMed] [Google Scholar]
- Krishnasree V, Ukkuru PM. In vitro antidiabetic activity and glycemic index of bee honeys. Indian J Tradit Knowl. 2017;16:134–140. [Google Scholar]
- Masek A, Chrzescijanska E, Kosmalska A, Zaborski M. Characteristics of compounds in hops using cyclic voltammetry, UV-VIS, FTIR and GC-MS analysis. Food Chem. 2014;156:353–361. doi: 10.1016/j.foodchem.2014.02.005. [DOI] [PubMed] [Google Scholar]
- Molyneux P. The use of the stable free radical diphenylpicrylhydrazyl (DPPH) for estimating antioxidant activity. Songklanakarin J Sci Technol. 2004;26:211–219. [Google Scholar]
- Nayik GA, Dar BN, Nanda V. Physico-chemical, rheological and sugar profile of different unifloral honeys from Kashmir valley of India. Arab J Chem. 2019;12:3151–3162. doi: 10.1016/j.arabjc.2015.08.017. [DOI] [Google Scholar]
- Peláez-Acero A, Garrido-Islas DB, Campos-Montiel RG, González-Montiel L, Medina-Pérez G, Luna-Rodríguez L, et al. The application of ultrasound in honey: antioxidant activity, inhibitory effect on α-amylase and α-glucosidase, and in vitro digestibility assessment. Molecules. 2022;27:5825. doi: 10.3390/molecules27185825. https://doi.org/10.3390/molecules27185825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontis JA, da Costa LAMA, da Silva SJR, Flach A. Color, phenolic and flavonoid content, and antioxidant activity of honey from Roraima, Brazil. Food Sci. 2014. Technol;34:69–73. doi: 10.1590/S0101-20612014005000015. [DOI] [Google Scholar]
- Rahmawati O, Pratami DK, Raffiudin R, Sahlan M. Alpha-glucosidase inhibitory activity of stingless bee honey from Tetragonula biroi and Tetragonula laeviceps. AIP Conf Proc. 2019;2092:030001. doi: 10.1063/1.5096705. https://doi.org/10.1063/1.5096705. [DOI] [Google Scholar]
- Rodríguez-Malaver AJ, Rasmussen C, Gutiérrez MG, Gil F, Nieves B, Vit P. Properties of honey from ten species of Peruvian stingless bees. Nat Prod Commun. 2009;4:1221–1226. doi: 10.1177/1934578X0900400913. [DOI] [PubMed] [Google Scholar]
- Sahhugi Z, Hasenan SM, Jubri Z. Protective effects of gelam honey against oxidative damage in young and aged rats. Oxid Med Cell Longev. 2014;2014:673628. doi: 10.1155/2014/673628. https://doi.org/10.1155/2014/673628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahlan M, Rahmawati O, Pratami DK, Raffiudin R, Mukti RR, Hermasyah H. The effects of stingless bee (Tetragonula biroi) honey on streptozotocin-induced diabetes mellitus in rats. Saudi J Biol Sci. 2020;27:2025–2030. doi: 10.1016/j.sjbs.2019.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar-Aranda R, Pérez-López LA, López-Arroyo J, Alanís-Garza BA, Waksman de Torres N. Antimicrobial and antioxidant activities of plants from northeast of Mexico. Evid Based Complement Alternat Med. 2011;2011:536139. doi: 10.1093/ecam/nep127. https://doi.org/10.1093/ecam/nep127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancheti S, Sancheti S, Seo SY. Chaenomeles sinensis: a potent α- and β-glucosidase inhibitor. Am J Pharmacol Toxicol. 2009;4:8–11. doi: 10.3844/ajptsp.2009.8.11. [DOI] [Google Scholar]
- Sansenya S, Payaka A, Mansalai P. Inhibitory efficacy of cycloartenyl ferulate against α-glucosidase and α-amylase and its increased concentration in gamma-irradiated rice (germinated rice) Prev Nutr Food Sci. 2023;28:170–177. doi: 10.3746/pnf.2023.28.2.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamsudin S, Selamat J, Sanny M, Abd Razak SB, Jambari NN, Mian Z, et al. Influence of origins and bee species on physicochemical, antioxidant properties and botanical discrimination of stingless bee honey. Int J Food Prop. 2019;22:239–264. doi: 10.1080/10942912.2019.1576730. [DOI] [Google Scholar]
- Sharma R, Martins N, Chaudhary A, Garg N, Sharma V, Kuca K, et al. Adjunct use of honey in diabetes mellitus: A consensus or conundrum? Trends Food Sci Technol. 2020;106:254–274. doi: 10.1016/j.tifs.2020.10.020. [DOI] [Google Scholar]
- Subari N, Mohamad Saleh J, Md Shakaff AY, Zakaria A. A hybrid sensing approach for pure and adulterated honey classification. Sensors. 2012;12:14022–14040. doi: 10.3390/s121014022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surya S, Salam AD, Tomy DV, Carla B, Kumar RA, Sunil C. Diabetes mellitus and medicinal plants-a review. Asian Pac J Trop Dis. 2014;4:337–347. doi: 10.1016/S2222-1808(14)60585-5. [DOI] [Google Scholar]
- Tadera K, Minami Y, Takamatsu K, Matsuoka T. Inhibition of alpha-glucosidase and alpha-amylase by flavonoids. J Nutr Sci Vitaminol. 2006;52:149–153. doi: 10.3177/jnsv.52.149. [DOI] [PubMed] [Google Scholar]
- Tewari J, Irudayaraj J. Quantification of saccharides in multiple floral honeys using fourier transform infrared microattenuated total reflectance spectroscopy. J Agric Food Chem. 2004;52:3237–3243. doi: 10.1021/jf035176+. [DOI] [PubMed] [Google Scholar]
- Thummajitsakul S, Paensanit P, Saeieo T, Sirirat J, Silprasit K. FTIR and multivariate analysis of total phenolic content, antioxidant and anti-amylase activities of extracts and milk of Glycine max L. and Phaseolus vulgaris L. Electron J Biotechnol. 2023;64:69–75. doi: 10.1016/j.ejbt.2023.04.001. [DOI] [Google Scholar]
- Tuksitha L, Chen YLS, Chen YL, Wong KY, Peng CC. Antioxidant and antibacterial capacity of stingless bee honey from Borneo (Sarawak) J Asia Pac Entomol. 2018;21:563–570. doi: 10.1016/j.aspen.2018.03.007. [DOI] [Google Scholar]
- Vijay PVP, Vimukta SVS. The role of natural antioxidants in oxidative stress induced diabetes mellitus. Res J Pharm Sci. 2014;3:1–6. [Google Scholar]
- Wang J, Kliks MM, Jun S, Jackson M, Li QX. Rapid analysis of glucose, fructose, sucrose, and maltose in honeys from different geographic regions using fourier transform infrared spectroscopy and multivariate analysis. J Food Sci. 2010;75:C208–C214. doi: 10.1111/j.1750-3841.2009.01504.x. [DOI] [PubMed] [Google Scholar]
- Wongsa P, Phatikulrungsun P, Prathumthong S. FT-IR characteristics, phenolic profiles and inhibitory potential against digestive enzymes of 25 herbal infusions. Sci Rep. 2022;12:6631. doi: 10.1038/s41598-022-10669-z. https://doi.org/10.1038/s41598-022-10669-z. [DOI] [PMC free article] [PubMed] [Google Scholar]