Abstract
In Asia, Rosa spp. has been used in traditional medicine for the treatment of osteoarthritis, rheumatoid arthritis, and edema. In this study, we investigated the effect of rose petal extract (RPE) on high fat diet (HFD)-induced obesity in mice. C57BL/6J mice were fed with either an AIN-93G diet (normal control), a 60% HFD, or a HFD plus supplementation with RPE at 100 or 200 mg/kg body weight (HFD+R100, HFD+R200) for 14 weeks. The HFD increased the body weight gain, liver and fat weight, lipid profiles (total cholesterol, triglyceride, high density lipoprotein cholesterol, and low density lipoprotein cholesterol), and the serum aspartate aminotransferase and alanine aminotransferase levels of mice, while RPE supplementation significantly decreased these parameters compared with the HFD group. Furthermore, the HFD increased the protein expressions of adipogenesis- and lipogenesis-related factors and decreased the protein expression of lipolysis- and energy metabolism-related factors. Conversely, RPE supplementation significantly decreased the protein expression of adipogenesis- and lipogenesis-related factors and increased the protein expression of lipolysis- and energy metabolism-related factors compared to the HFD group. Taken together, the results provide preliminary evidence for the potential protective effects of the RPE against obesity.
Keywords: high fat diet, obesity, Rosa spp., rose petal extract
INTRODUCTION
Obesity is a high interest disease owing to its contribution to cardiovascular disease, hypertension, diabetes, and cancer. Obesity prevalence is increasing worldwide, including in Korea, where its prevalence increased from 26.0% in 1998 to 38.3% in 2020 (Rasouli and Kern, 2008; Korea Disease Control and Prevention Agency, 2022; Huang et al., 2024). Obesity is described as excessive fat accumulation resulting from an imbalance between energy intake and energy expenditure, an increased number of preadipocytes, and increased differentiation into mature adipocytes (de Ferranti and Mozaffarian, 2008; Patterson et al., 2016). Treatments for obesity include exercise, antiobesity drug supplementation, and surgery such as gastrotomy. Antiobesity drugs and surgery have side effects such as headache, stomachache, nausea, insomnia, and diarrhea; therefore, several studies have been conducted into the mechanisms of adipogenesis, lipogenesis, and lipolysis for the development of a functional food. Natural materials frequently contain a diverse array of bioactive compounds that can target obesity through multiple mechanisms, such as by reducing fat absorption, increasing energy expenditure, and modulating metabolic pathways. However, it is challenging to replicate the multifunctionality with single-target synthetic drugs. There is growing consumer demand for “natural” and plant-based solutions for health issues like obesity, driven by perceptions of safety and sustainability. Therefore, the use of natural materials can improve product acceptance and marketability (Cercato et al., 2009; Karri et al., 2019; Chan et al., 2021; Seo et al., 2021; Lee et al., 2023; Cho et al., 2024; Lin et al., 2024; Zhao et al., 2024).
In this study, we investigated the effects of rose petal (Rosa spp.) extract on high fat diet (HFD)-induced obesity. Rosa spp., known as many-flowered rose, baby rose, seven-sisters rose, or multiflora rose, is popular in Asia, Europe, and the United States. This rose is used as rootstock for ornamental roses in Europe and the United States, whereas all parts of it have been used as conventional medicine for rheumatoid arthritis, osteoarthritis, and edema in Asia. Several studies have reported that the extracts of its fruits and roots have anti-inflammatory and antioxidant activities (Guo et al., 2011; Park et al., 2011; Wu et al., 2014; Fougère-Danezan et al., 2015). Sudeep et al. (2021) indicated that the supplementation of 100 or 200 mg/kg/d of Rosa multiflora var. platyphylla extract for 14 weeks decreased body weight (BW) gain, fat mass, and serum lipid levels, while increasing insulin sensitivity. In this study, we examined the antiobesity effects of rose petal extract (RPE) on HFD-induced mice by investigating adipogenesis, lipogenesis, and lipolysis mechanisms.
MATERIALS AND METHODS
RPE preparation and standardization
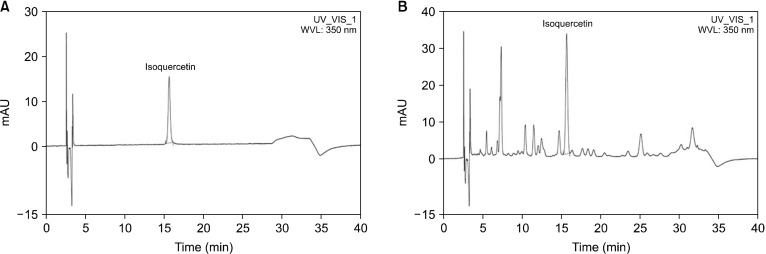
The RPE was a standardized flower petal extract of Rosa spp., which was provided by Daehan Chemtech Co., Ltd. The dried flower petal of Rosa spp. was extracted using 70% ethyl alcohol. Then, the extracted solution was filtered, concentrated with an evaporator under vacuum, and vacuum dried. High-performance liquid chromatography (HPLC) was used to determine the phytochemical profile of the RPE using isoquercetin as the standard compound. The analyses were performed using an HPLC UltiMate 3000 system (Dionex Co.). Chromatography was conducted on a Capcell Pak C18 column (250×4.6 mm, 5 μm) at 30°C. The elution solvents were 15.0% acetonitrile and 85.0% deionized water containing 0.3% phosphoric acid. The elution was performed in isocratic mode at a flow rate of 1 mL/min. Finally, the RPE was standardized with 2.4% to 3.6% isoquercetin (Fig. 1).
Fig. 1.
High-performance liquid chromatography chromatograms of isoquercetin in rose petal extract (RPE) at 350 nm. (A) Isoquercetin standard and (B) RPE chromatogram.
Animals
The experiments were licensed by the Institutional Animal Care and Use Committee of Kyung Hee University (protocol: KHGASP-22-399). Thirty-two male C57BL/6J mice (5-week-old) were acquired from Saeronbio Inc. and transported in cages under administered conditions with 50%±10% relative humidity and a semidiurnal light/dark cycle at 22°C±2°C during the experimental term. Mice were adapted to the conditions for 1 week and then separated into four groups of eight animals each: normal control group (NC group, AIN-93G diet), HFD (60% HFD control), HFD+R100 (60% HFD+Rosa spp. extract 100 mg/kg BW), and HFD+R200 (60% HFD+Rosa spp. extract 200 mg/kg BW). At the end of 12th week, the mice were fasted for 16 h. Blood was collected via heart puncture and centrifuged at 200 g for 20 min at 4°C. The white adipose tissue (WAT), brown adipose tissue (BAT), and liver were excised, rinsed with phosphate-buffered saline, weighed, and stored at −80°C until further analysis.
Biochemical analysis
The levels of aspartate aminotransferase (AST), alanine aminotransferase (ALT), total cholesterol (TC), triglycerides (TG), high density lipoprotein cholesterol (HDL-chol), low density lipoprotein cholesterol (LDL-chol), and cyclic adenosine monophosphate (cAMP) were measured in the serum, feces, and WAT using the AST Activity Assay kits (K552-100, BioVision Inc.), ALT Activity Assay kits (K752-100, BioVision Inc.), Total Cholesterol and Cholesteryl Ester Colorimetric Assay kits (K603-100, BioVision Inc.), Triglycerides Quantification Colorimetric Assay kits (K622-100, BioVision Inc.), HDL&LDL/VLDL Cholesterol Quantification Colorimetric Assay kits (K613-100, BioVision Inc.), and cAMP enzyme-linked immunosorbent assay (ELISA) kits (ADI-900-163, Enzo Life Sciences), respectively. All procedures strictly adhered to the protocols outlined in the respective manufacturer’s manuals, ensuring precision and reproducibility in the experimental procedures.
Protein extraction and western blot analysis
The WAT (100 mg) was lysed using CelLyticTM MT Cell Lysis Reagent (Sigma-Aldrich) with HaltTM Protease & Phosphatase Inhibitor Cocktail (Thermo Fisher Scientific), then centrifuged at 19,320 g at 4°C for 20 min. Protein samples (50 μg each) were loaded into 10% Mini-PROTEANⓇ TGXTM Precast Gels (Bio-Rad) and transferred to membranes using the Trans-BlotⓇ TurboTM transfer system (Bio-Rad). The membranes were blocked with buffer (5% skim milk in Tris-buffered saline with 1% TweenⓇ 20) for 1 h at room temperature. After washing, the membranes were exposed to primary antibodies overnight at 4°C, then the washed membranes were incubated with secondary antibodies (anti-rabbit/mouse/goat IgG HRP conjugated, 1:5,000) for 1 h at room temperature. The primary and secondary antibodies are explained in Table 1.
Table 1.
Antibodies used for western blot analysis
| Biomarker | Host animal | Dilution for western blot | Distributor |
|---|---|---|---|
| MAPK | Rabbit | 1:1,000 | CST |
| SREBP-1c | Rabbit | 1:1,000 | Abcam |
| PPAR-γ | Rabbit | 1:1,000 | CST |
| p-CREB | Rabbit | 1:1,000 | CST |
| CREB | Rabbit | 1:1,000 | CST |
| C/EBPα | Rabbit | 1:1,000 | CST |
| Leptin | Rabbit | 1:1,000 | Abcam |
| Adiponectin | Rabbit | 1:1,000 | Abcam |
| G6PDH | Rabbit | 1:1,000 | CST |
| Citrate synthase | Rabbit | 1:1,000 | CST |
| p-ACL | Rabbit | 1:1,000 | CST |
| ACL | Rabbit | 1:1,000 | CST |
| p-ACC | Rabbit | 1:1,000 | CST |
| ACC | Rabbit | 1:1,000 | CST |
| FAS | Rabbit | 1:1,000 | CST |
| LPL | Rabbit | 1:1,000 | CST |
| PKA | Rabbit | 1:1,000 | CST |
| PDE3B | Rabbit | 1:1,000 | Abcam |
| p-HSL | Rabbit | 1:1,000 | CST |
| HSL | Rabbit | 1:1,000 | CST |
| Perilipin | Rabbit | 1:1,000 | CST |
| ATGL | Rabbit | 1:1,000 | CST |
| p-AMPK | Rabbit | 1:1,000 | CST |
| AMPK | Rabbit | 1:1,000 | CST |
| UCP1 | Rabbit | 1:1,000 | Abcam |
| CPT1A | Rabbit | 1:1,000 | LSBio |
| FABP4 | Rabbit | 1:1,000 | Abcam |
| b-Actin | Rabbit | 1:3,000 | LSBio |
MAPK, mitogen-activated protein kinase; SREBP-1c, sterol regulatory element-binding protein-1c; PPAR-γ, peroxisome proliferator-activated receptor-γ; CREB, cAMP response element-binding protein; C/EBPα, CCAAT/enhancer-binding protein α; G6PDH, glucose-6-phosphate dehydrogenase; ACL, ATP-citrate lyase; ACC, acetyl-CoA carboxylase; FAS, fatty acid synthase; LPL, lipoprotein lipase; PKA, protein kinase A; PDE3B, phosphodiesterase 3B; HSL, hormone-sensitive lipase; ATGL, adipose triglyceride lipase; AMPK, AMP-activated protein kinase; UCP1, uncoupling protein 1; CPT1A, carnitine palmitoyltransferase-1A; FABP4, fatty acid binding protein 4; CST, Cell Signaling Technology.
Statistical analysis
All results were expressed as the mean±standard deviation. The data were statistically estimated using Duncan’s multiple range tests after one-way ANOVA using SPSS software (IBM SPSS Statistics v.23.0, IBM Corp.). Differences were considered statistically significant at a P-value of <0.05.
RESULTS
Effects of RPE on body and organ weight change in HFD-induced obese mice
To determine the effects of RPE on obesity, mice were fed a HFD diet supplemented with RPE (100 or 200 mg/kg BW) for 14 weeks. A significant increase in BW gain was observed in the HFD group compared to the NC group (P<0.05). Mice supplemented with RPE (HFD+R100, HFD+R200) exhibited a significant reduction in BW gain compared to the HFD group (P<0.05; Table 2). The food efficiency ratio (FER) was significantly increased in the HFD group compared to the NC group (P<0.05), whereas the mice supplemented with RPE (HFD+R100, HFD+R200) demonstrated no significant differences in FER compared with the HFD group (P<0.05; Table 2). Liver and fat tissue (subcutaneous, visceral, epididymal, and brown) weight revealed a significant increase in the HFD group compared to the NC group (P<0.05), while mice supplemented with RPE (HFD+R100, HFD+R200) showed a significant reduction in liver and fat weight compared to HFD group (P<0.05). The ratio of BAT to total adipose tissue showed a significant decrease in the HFD group compared to the NC group, while mice supplemented with RPE showed no significant differences compared with the HFD group (Table 2).
Table 2.
Effects of rose petal extract (RPE) on the body and organ weights in high fat diet (HFD)-induced obese mice
| NC | HFD | HFD+R100 | HFD+R200 | |
|---|---|---|---|---|
| Initial BW (g) | 20.99±1.42ns | 20.35±1.34 | 20.43±1.48 | 20.07±1.44 |
| Final BW (g) | 31.54±1.15c | 50.24±1.09a | 47.34±0.81b | 46.27±1.85b |
| Weight gain (g) | 10.55±1.50c | 29.89±1.58a | 26.91±1.95b | 26.20±1.93b |
| FER | 4.83±0.69b | 16.47±0.87a | 15.64±1.13a | 15.66±1.15a |
| Tissue weights | ||||
| Liver | 1.38±0.11c | 2.05±0.14a | 1.68±0.10b | 1.58±0.10b |
| Subcutaneous fat | 0.91±0.26d | 2.84±0.31a | 2.18±0.23b | 1.88±0.22c |
| Visceral fat | 0.32±0.13d | 1.84±0.31a | 0.93±0.14b | 0.77±0.11c |
| Epididymis fat | 0.83±0.22d | 2.21±0.32a | 1.73±0.25b | 1.43±0.18c |
| Brown fat | 0.13±0.02d | 0.24±0.03a | 0.20±0.02b | 0.17±0.02c |
| White adipose tissue/total adipose tissue (%) | 93.77±0.74b | 96.29±0.24a | 96.09±0.32a | 96.06±0.34a |
| Brown adipose tissue/total adipose tissue (%) | 6.23±0.74a | 3.71±0.24b | 3.91±0.32b | 3.94±0.34b |
Values are presented as mean±SD.
Different letters (a-d) represent significant differences at P<0.05, as determined by Duncan’s multiple range test.
BW, body weight; NC (normal control), AIN-93G diet; HFD, 60% HFD; HFD+R100, 60% HFD+RPE 100 mg/kg BW; HFD+R200, 60% HFD+RPE 200 mg/kg BW; ns, not significant; FER, food efficiency ratio=[weight gain (g)/total food consumption (g)]×100.
Effects of RPE on serum ALT and AST and lipid profiles of serum and feces in HFD-induced obese mice
We analyzed the serum of mice fed a HFD diet using ELISA to determine the effects of RPE on serum ALT and AST levels. The levels of ALT and AST were observed to be significantly increased in the HFD group compared to the NC group (P<0.05). The mice supplemented with RPE (HFD+R100, HFD+R200) showed a reduction in serum ALT level compared to the HFD group. The serum ALT levels of the HFD+R100 group were not significantly different compared with HFD group, while the HFD+R200 showed significantly decreased serum ALT levels compared to HFD group (P<0.05). The mice supplementwith RPE (HFD+R100, HFD+R200) exhibited a significant reduction in serum AST level compared to the HFD group (P<0.05; Table 3).
Table 3.
Effects of rose petal extract (RPE) on the biochemical levels of serum, feces, and white adipose tissue in high fat diet (HFD)-induced obese mice
| NC | HFD | HFD+R100 | HFD+R200 | |
|---|---|---|---|---|
| ALT in serum (mU/mL) | 0.14±0.09c | 0.59±0.06a | 0.55±0.09ab | 0.49±0.07b |
| AST in serum (mU/mL) | 0.45±0.19c | 2.09±0.20a | 1.74±0.29b | 1.74±0.26b |
| Total cholesterol in serum (μg/μL) | 58.00±5.88d | 119.34±6.70a | 93.12±6.40b | 74.76±6.50c |
| Triglyceride in serum (μM) | 26.70±4.03d | 60.87±4.32a | 45.37±4.88b | 39.20±4.39c |
| HDL-chol in serum (μg/μL) | 8.93±1.36d | 15.95±1.18a | 13.84±1.36b | 13.07±1.17c |
| LDL-chol in serum (μg/μL) | 31.22±6.10d | 73.87±5.64a | 59.55±6.56b | 49.20±4.97c |
| HDL-chol/LDL-chol ratio | 0.29±0.04a | 0.22±0.03c | 0.23±0.03bc | 0.27±0.04ab |
| Total cholesterol in feces (μg/μL) | 24.64±2.33d | 32.62±2.09c | 37.83±2.38b | 41.41±2.02a |
| Triglyceride in feces (μg/μL) | 22.93±2.45d | 33.06±2.52c | 43.16±2.13b | 50.09±2.40a |
Values are presented as mean±SD.
Different letters (a-d) represent significant differences at P<0.05, as determined by Duncan’s multiple range test.
NC (normal control), AIN-93G diet; HFD, 60% HFD; HFD+R100, 60% HFD+RPE 100 mg/kg body weight (BW); HFD+R200, 60% HFD+RPE 200 mg/kg BW; ALT, alanine aminotransferase; AST, aspartate aminotransferase; HDL-chol, high density lipoprotein cholesterol; LDL-chol, low density lipoprotein cholesterol.
To determine the effects of RPE on the lipid profiles of serum and feces, we analyzed the serum and feces of mice fed a HFD diet using ELISA. The levels of TC, TG, HDL-chol, and LDL-chol in the serum and feces were significantly increased in the HFD group compared to the NC group (P<0.05). The mice supplemented with RPE (HFD+R100, HFD+R200) showed significantly reduced serum lipid profiles and significantly increased feces lipid profiles compared to the HFD group (P<0.05).
Effects of RPE on factors related to adipogenesis and lipogenesis in HFD-induced obese mice
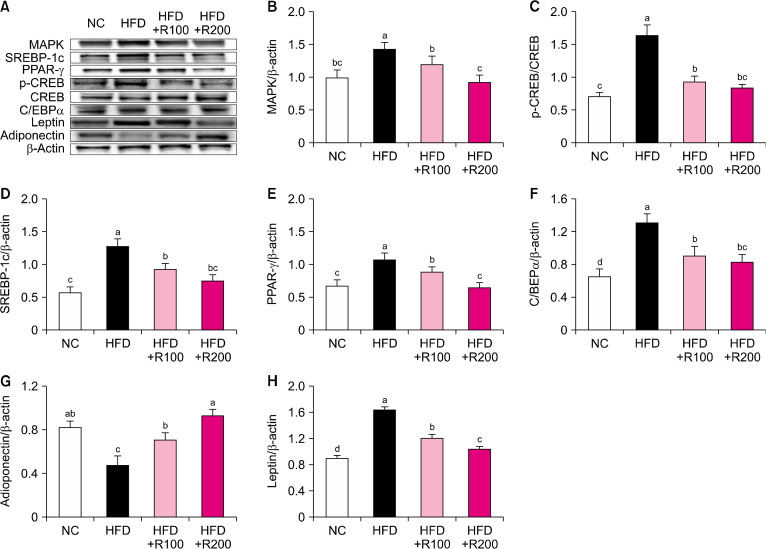
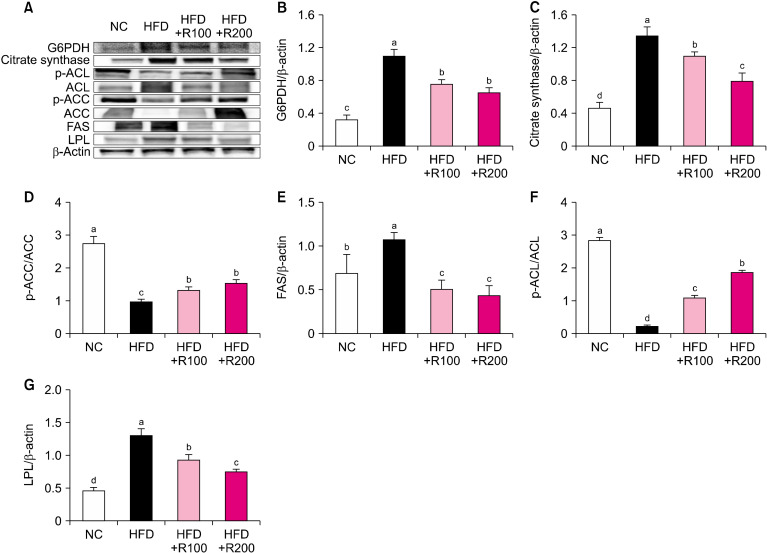
To investigate the adipogenesis and lipogenesis-related factors of RPE, we analyzed the WAT of HFD-induced obese mice using western blotting. The protein expressions of mitogen-activated protein kinase (MAPK), p-cAMP response element-binding protein (CREB)/CREB, sterol regulatory element-binding protein (SREBP)-1c, peroxisome proliferator-activated receptor (PPAR)-γ, CCAAT/enhancer-binding protein (C/EBP), and leptin were significantly increased in the HFD group compared to the NC group, however, the RPE-supplemented mice (HFD+R100, HFD+R200) showed a significant decrease in the levels of these proteins compared with the HFD group (P<0.05; Fig. 2A∼2F and 2H). The protein expression of adiponectin was significantly decreased in the HFD group compared to the NC group, while the RPE-supplemented mice (HFD+R100, HFD+R200) showed a significant increase in the expression of adiponectin compared to the HFD group (P<0.05; Fig. 2G). The protein expression of glucose-6-phosphate dehydrogenase (G6PDH), citrate synthase, fatty acid synthase (FAS), and lipoprotein lipase (LPL) was significantly increased in the HFD group compared to the NC group; however, RPE-supplemented mice (HFD+R100, HFD+R200) showed a significant decrease in the expression of these proteins compared with the HFD group (P<0.05; Fig. 3A∼C, 3E, and 3G). The protein expression of p-acetyl-CoA carboxylase (ACC)/ACC and p-ATP-citrate lyase (ACL)/ACL was significantly decreased in the HFD group compared to the NC group, while RPE-supplemented mice (HFD+R100, HFD+R200) showed a significant increase in the expression of these proteins compared with the HFD group (P<0.05; Fig. 3D and 3F). These results suggest that RPE supplementation may block adipogenesis and lipogenesis in HFD-induced obese mice.
Fig. 2.
Effects of rose petal extract (RPE) on protein expression-related adipogenesis in high fat diet (HFD)-induced obese mice. (A) Protein band, (B) mitogen-activated protein kinase (MAPK), (C) p-cAMP response element-binding protein (CREB)/CREB, (D) sterol regulatory element-binding protein (SREBP)-1c, (E) peroxisome proliferator-activated receptor (PPAR)-γ, (F) CCAAT/enhancer-binding protein (C/EBP)α, (G) adiponectin, (H) leptin. Normal control (NC), AIN-93G diet; HFD, 60% HFD; HFD+R100, 60% HFD+RPE 100 mg/kg body weight (BW); HFD+R200, 60% HFD+RPE 200 mg/kg BW. Values are presented as mean±SD. Different letters (a-d) represent significant differences at P<0.05, as determined by Duncan’s multiple range test. Antibodies used for western blot analysis are listed in Table 1.
Fig. 3.
Effects of rose petal extract (RPE) on protein expression-related lipogenesis in high fat diet (HFD)-induced obese mice. (A) Protein band, (B) G6PDH, (C) citrate synthase, (D) p-acetyl-CoA carboxylase (ACC)/ACC, (E) fatty acid synthase (FAS), (F) p-ATP-citrate lyase (ACL)/ACL, (G) LPL. Normal control (NC), AIN-93G diet; HFD, 60% HFD; HFD+R100, 60% HFD+RPE 100 mg/kg body weight (BW); HFD+R200, 60% HFD+RPE 200 mg/kg BW. Values are presented as mean±SD. Different letters (a-d) represent significant differences at P<0.05, as determined by Duncan’s multiple range test. Antibodies used for western blot analysis are listed in Table 1.
Effects of RPEs on factors related to lipolysis in HFD-induced obese mice
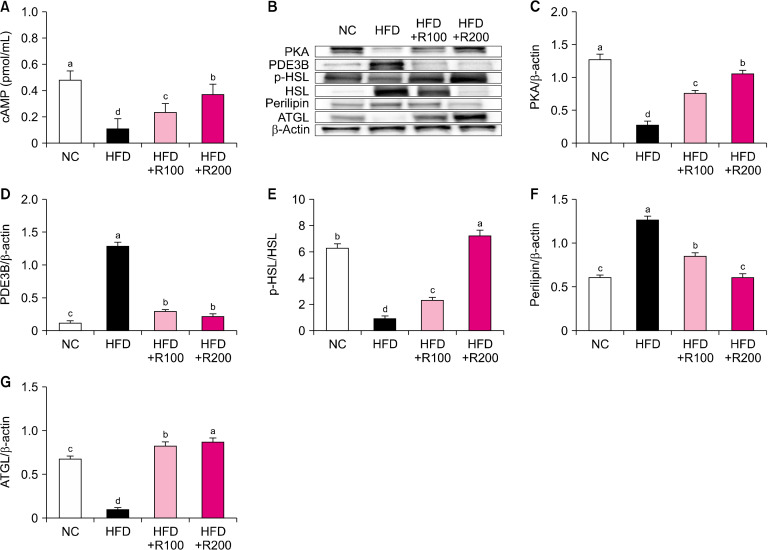
To investigate the lipolysis-related factors of RPE, we analyzed the WAT of HFD-induced obese mice using ELISA and western blot. The levels of cAMP were significantly decreased in HFD group compared NC group; however, RPE treatment to HFD-induced mice groups (HFD+R100, HFD+R200) induced a significant dose-dependent increase in cAMP compared with the HFD group (P<0.05; Fig. 4A). The protein expressions of protein kinase A (PKA), p-hormone-sensitive lipase (HSL)/HSL, and adipose triglyceride lipase (ATGL) were significantly decreased in HFD group compared NC group; however, RPE treatment to HFD-induced mice groups (HFD+R100, HFD+R200) induced a significant dose-dependent increase in these factors compared with the HFD group (P<0.05; Fig. 4B, 4C, 4E, and 4G). The protein expressions of phosphodiesterase 3B (PDE3B) and perilipin were significantly increased in the HFD group compared to the NC group; however, RPE treatment to HFD-induced mice (HFD+R100, HFD+R200) induced a significant decrease in these proteins compared with the HFD group (P<0.05; Fig. 4D and 4F). These results suggest that RPE supplementation can stimulate lipolysis in HFD-induced obese mice.
Fig. 4.
Effects of rose petal extract (RPE) on protein expression-related lipolysis in high fat diet (HFD)-induced obese mice. (A) Cyclic adenosine monophosphate (cAMP) level, (B) protein band, (C) protein kinase A (PKA), (D) phosphodiesterase 3B (PDE3B), (E) p-hormone-sensitive lipase (HSL)/HSL, (F) perilipin, (G) adipose triglyceride lipase (ATGL). Normal control (NC), AIN-93G diet; HFD, 60% HFD; HFD+R100, 60% HFD+RPE 100 mg/kg body weight (BW); HFD+R200, 60% HFD+RPE 200 mg/kg BW. Values are presented as mean±SD. Different letters (a-d) represent significant differences at P<0.05, as determined by Duncan’s multiple range test. Antibodies used for western blot analysis are listed in Table 1.
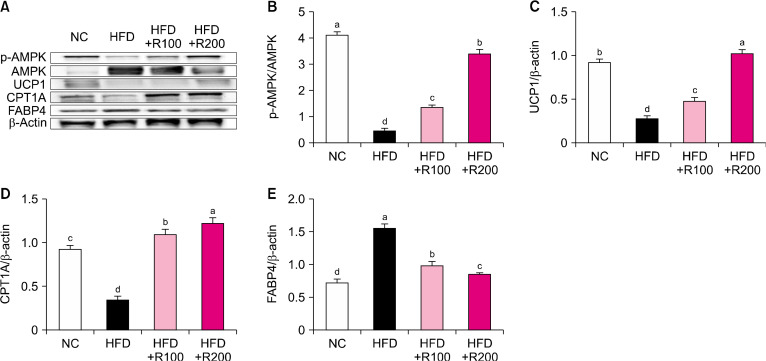
Effects of RPEs on factors related to energy metabolism in HFD-induced obese mice
To investigate the energy metabolism-related factors of RPE, we analyzed the BAT of HFD-induced obese mice using western blotting. The protein expressions of p-AMP-activated protein kinase (AMPK)/AMPK, uncoupling protein 1 (UCP1), and carnitine palmitoyltransferase-1A (CPT1A) were significantly decreased in the HFD group compared to the NC group; however RPE treatment to HFD-induced mice groups (HFD+R100, HFD+R200) induced a significant dose-dependent increase in these factors compared with the HFD group (P<0.05; Fig. 5A∼5D). The protein expression level of fatty acid binding protein 4 (FABP4) was significantly increased in the HFD group compared to the NC group; however, RPE treatment to HFD-induced mice groups (HFD+R100, HFD+R200) induced a significant dose-dependent decrease in FABP4 compared with the HFD group (P<0.05; Fig. 5E). These results suggest that RPE supplementation can stimulate energy metabolism in HFD-induced obese mice.
Fig. 5.
Effects of rose petal extract (RPE) on protein expression-related energy metabolism in high fat diet (HFD)-induced obese mice. (A) Protein band, (B) p-(AMP-activated protein kinase) AMPK/AMPK, (C) uncoupling protein 1 (UCP1), (D) carnitine palmitoyltransferase-1A (CPT1A), (E) fatty acid binding protein 4 (FABP4). Normal control (NC), AIN-93G diet; HFD, 60% HFD; HFD+R100, 60% HFD+RPE 100 mg/kg body weight (BW); HFD+R200, 60% HFD+RPE 200 mg/kg BW. Values are presented as mean±SD. Different letters (a-d) represent significantly differences at P<0.05, as determined by Duncan’s multiple range test. Antibodies used for western blot analysis are listed in Table 1.
DISCUSSION
The prevalence of obesity has been constantly increasing globally over the past few decades. Obesity is a complex chronic condition; it is characterized by excess body fat accumulation, which can lead to negative health outcomes such as cardiovascular disease, diabetes, and certain types of cancer (Vinodhini and Rajeswari, 2019; Karadogan et al., 2022). Depending on the severity of the condition, the treatment for obesity involves a multi-faceted approach that includes lifestyle modifications, bariatric surgery, and pharmacotherapy. Lifestyle modifications are the first-line treatment for obesity, and they include dietary changes, increased physical activity, and behavioral therapy. Medications may include appetite suppressants or medications that reduce the absorption of dietary fat. However, these medications are not without side effects, which include gastrointestinal symptoms such as nausea and diarrhea, and cardiovascular complications such as high blood pressure or palpitations (Bray et al., 2003; Jackson et al., 2015; Broughton and Moley, 2017). Therefore, several researchers have studied natural resources with minimal side effects for improving obesity using cell and animal models (Anhê et al., 2019; Lee et al., 2019; Zang et al., 2021).
The present study investigated whether RPE could alleviate HFD-induced obesity through effects on adipogenesis, lipogenesis, lipolysis, and energy metabolism. The HFD group exhibited an increased BW gain, liver weight, and WAT; the RPE treatment to HFD-induced mice groups (HFD+R100, HFD+R200) significantly reduced these abovementioned parameters. The reason for the lack of difference in the ratio of WAT to BAT is that the total fat weight decreased. Moreover, RPE supplementation decreased the levels of AST, ALT, TG, TC, and LDL-chol in the serum and the TG and TC in the feces of obese mice. In human clinical practice, it is typical for HDL-chol levels to decline with a HFD. Conversely, in mice fed a HFD, all blood lipids, including HDL-chol, rise. Thus, monitoring HDL-chol levels and HDL-LDL-chol ratio becomes crucial for assessing lipid metabolism (Liang et al., 2021; Rašković A et al., 2023).
Adipogenesis and lipogenesis are separate pathways involved in the production and storage of fat in the body. Both processes result in the formation of fatty acids and TG; however, they differ in their mechanisms and physiological functions. Adipogenesis is the process by which preadipocytes mature into adipocytes, which are specialized cells responsible for storing excess energy in the form of TG. Adipogenesis factors include MAPK, PPAR-γ, C/EBPα, and SREBP, among others. Adipogenesis is primarily involved in the expansion of adipose tissue mass in response to excess energy intake, as well as in the maintenance of metabolic homeostasis by regulating energy storage and release (Mota de Sá et al., 2017; Lee et al., 2019). Lipogenesis, in contrast, is a process of de novo synthesis of fatty acids and their conversion into TG for storage. Lipogenesis factors include ACL, ACC, and FAS, among others. Lipogenesis is primarily responsible for producing TG for energy storage and export, as well as regulating lipid metabolism (Imi et al., 2018; Song et al., 2018). In this study, RPE decreased adipogenesis-related protein expression such as that of MAPK, p-CREB/CREB, SREBP-1c, C/EBPα, PPAR-γ, and leptin, and increased adiponectin protein expression in the WAT of HFD-induced obese mice. Furthermore, RPE decreased lipogenesis-related protein expression such as that of G6PDH, citrate synthase, FAS, and LPL, and increased p-ACC/ACC and p-ACL/ACL protein expression in the WAT of HFD-induced obese mice. These results suggest that RPE suppresses adipogenesis and lipogenesis in HFD-induced obese mice. However, the specific mechanisms by which RPE modulates the key adipogenesis and lipogenesis pathways were not completely elucidated.
Lipolysis is the breakdown of TG into free fatty acids and glycerol, which can be used as an energy source. In WAT, this process is regulated by HSL and ATGL, which are activated by signals such as glucagon and epinephrine. These signals activate adenylate cyclase, leading to an increase in cAMP levels, which in turn activate PKA. PKA then phosphorylates HSL and ATGL, activating them and leading to the breakdown of TG into free fatty acids and glycerol (Duncan et al., 2007; Yang and Mottillo, 2020). In BAT, energy metabolism is regulated by a different pathway. BAT contains a protein called UCP1, which uncouples oxidative phosphorylation from ATP synthesis, leading to the production of heat instead of ATP. The activation of UCP1 is regulated by a variety of signals, including norepinephrine and thyroid hormones. These signals activate the cAMP-PKA pathway, leading to the phosphorylation and activation of UCP1. Furthermore, the activation of AMPK can activate UCP1 by increasing the expression of genes involved in thermogenesis (Fenzl and Kiefer, 2014; Marlatt and Ravussin, 2017; Bódis and Roden, 2018). In this study, RPE decreased lipolysis-related protein expression such as that of PDE3B and perilipin, and increased the expression of PKA, p-HSL/HSL, and ATGL in the WAT of HFD-induced obese mice. Moreover, RPE increased energy metabolism-related protein expression such as that of p-AMPK/AMPK, UCP1, and CPT1A, and decreased FABP4 protein expression in the BAT of HFD-induced obese mice. These findings suggest that RPE activates lipolysis and energy metabolism in HFD-induced obese mice. Although the study focused on the effects of RPE on the expression of key proteins involved in lipolysis and energy metabolism, it did not provide a comprehensive understanding of the underlying molecular mechanisms. Moreover, the study did not evaluate the impact of RPE on energy expenditure, thermogenesis, or other physiological processes that contribute to weight management. Therefore, further research would provide a more comprehensive understanding of the therapeutic potential of RPE for obesity management.
We discovered that RPE led to improvements in obesity by inhibiting adipogenesis- and lipogenesis-related factors, while activating lipolysis- and energy metabolism-related factors. These results provide preliminary evidence for the potential protective effects of RPE against obesity. The authors suggest that further research is needed to fully elucidate the mechanisms and potential therapeutic applications of RPE for obesity management.
Footnotes
FUNDING
None.
AUTHOR DISCLOSURE STATEMENT
JK and SE are the current employees of a commercial company which holds a patent for the RPE. JK and SE are listed as inventors.
AUTHOR CONTRIBUTIONS
Concept and design: ML, SE, JL. Analysis and interpretation: JJ, ML, SHP, WC, JK. Data collection: ML, JJ. Writing the article: ML, JL. Critical revision of the article: ML, JK, JL. Final approval of the article: all authors. Statistical analysis: JJ. Overall responsibility: JL.
References
- Anhê FF, Nachbar RT, Varin TV, Trottier J, Dudonné S, Le Barz M, et al. Treatment with camu camu (Myrciaria dubia) prevents obesity by altering the gut microbiota and increasing energy expenditure in diet-induced obese mice. Gut. 2019;68:453–464. doi: 10.1136/gutjnl-2017-315565. [DOI] [PubMed] [Google Scholar]
- Bódis K, Roden M. Energy metabolism of white adipose tissue and insulin resistance in humans. Eur J Clin Invest. 2018;48:e13017. doi: 10.1111/eci.13017. https://doi.org/10.1111/eci.13017. [DOI] [PubMed] [Google Scholar]
- Bray GA, Hollander P, Klein S, Kushner R, Levy B, Fitchet M, et al. A 6-month randomized, placebo-controlled, dose-ranging trial of topiramate for weight loss in obesity. Obes Res. 2003;11:722–733. doi: 10.1038/oby.2003.102. [DOI] [PubMed] [Google Scholar]
- Broughton DE, Moley KH. Obesity and female infertility: potential mediators of obesity's impact. Fertil Steril. 2017;107:840–847. doi: 10.1016/j.fertnstert.2017.01.017. [DOI] [PubMed] [Google Scholar]
- Cercato C, Roizenblatt VA, Leança CC, Segal A, Lopes Filho AP, Mancini MC, et al. A randomized double-blind placebo-controlled study of the long-term efficacy and safety of diethylpropion in the treatment of obese subjects. Int J Obes. 2009;33:857–865. doi: 10.1038/ijo.2009.124. [DOI] [PubMed] [Google Scholar]
- Chan Y, Ng SW, Tan JZX, Gupta G, Negi P, Thangavelu L, et al. Natural products in the management of obesity: Fundamental mechanisms and pharmacotherapy. S Afr J Bot. 2021;143:176–197. doi: 10.1016/j.sajb.2021.07.026. [DOI] [Google Scholar]
- Cho SY, Choi JS, Jung UJ. Effects of Ecklonia stolonifera extract on metabolic dysregulation in high-fat diet-induced obese mice. J Med Food. 2024;27:242–249. doi: 10.1089/jmf.2023.K.0252. [DOI] [PubMed] [Google Scholar]
- de Ferranti S, Mozaffarian D. The perfect storm: obesity, adipocyte dysfunction, and metabolic consequences. Clin Chem. 2008;54:945–955. doi: 10.1373/clinchem.2007.100156. [DOI] [PubMed] [Google Scholar]
- Duncan RE, Ahmadian M, Jaworski K, Sarkadi-Nagy E, Sul HS. Regulation of lipolysis in adipocytes. Annu Rev Nutr. 2007;27:79–101. doi: 10.1146/annurev.nutr.27.061406.093734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenzl A, Kiefer FW. Brown adipose tissue and thermogenesis. Horm Mol Biol Clin Investig. 2014;19:25–37. doi: 10.1515/hmbci-2014-0022. [DOI] [PubMed] [Google Scholar]
- Fougère-Danezan M, Joly S, Bruneau A, Gao XF, Zhang LB. Phylogeny and biogeography of wild roses with specific attention to polyploids. Ann Bot. 2015;115:275–291. doi: 10.1093/aob/mcu245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo D, Xu L, Cao X, Guo Y, Ye Y, Chan CO, et al. Anti-inflammatory activities and mechanisms of action of the petroleum ether fraction of Rosa multiflora Thunb. hips. J Ethnopharmacol. 2011;138:717–722. doi: 10.1016/j.jep.2011.10.010. [DOI] [PubMed] [Google Scholar]
- Huang Q, Liu Z, Wei M, Feng J, Huang Q, Liu Y, et al. Metabolically healthy obesity, transition from metabolic healthy to unhealthy status, and carotid atherosclerosis. Diabetes Metab Res Rev. 2024;40:e3766. doi: 10.1002/dmrr.3766. https://doi.org/10.1002/dmrr.3766. [DOI] [PubMed] [Google Scholar]
- Imi Y, Yabiki N, Abuduli M, Masuda M, Yamanaka-Okumura H, Taketani Y. High phosphate diet suppresses lipogenesis in white adipose tissue. J Clin Biochem Nutr. 2018;63:181–191. doi: 10.3164/jcbn.17-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson VM, Breen DM, Fortin JP, Liou A, Kuzmiski JB, Loomis AK, et al. Latest approaches for the treatment of obesity. Expert Opin Drug Discov. 2015;10:825–839. doi: 10.1517/17460441.2015.1044966. [DOI] [PubMed] [Google Scholar]
- Karadogan SR, Canbolat E, Cakıroglu FP. The effect of obesity on metabolic parameters: a cross sectional study in adult women. Afr Health Sci. 2022;22:241–251. doi: 10.4314/ahs.v22i4.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karri S, Sharma S, Hatware K, Patil K. Natural anti-obesity agents and their therapeutic role in management of obesity: A future trend perspective. Biomed Pharmacother. 2019;110:224–238. doi: 10.1016/j.biopha.2018.11.076. [DOI] [PubMed] [Google Scholar]
- Korea Disease Control and Prevention Agency, author. Prevalence of obesity (1998-2022) 2022. [cited 2023 Mar 4]. Available from: https://www.index.go.kr/unify/idx-info.do?idxCd=8021 .
- Lee HS, Lim SM, Jung JI, Kim SM, Lee JK, Kim YH, et al. Gynostemma pentaphyllum extract ameliorates high-fat diet-induced obesity in C57BL/6N mice by upregulating SIRT1. Nutrients. 2019;11:2475. doi: 10.3390/nu11102475. https://doi.org/10.3390/nu11102475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YR, Lee HB, Oh MJ, Kim Y, Park HY. Thyme extract alleviates high-fat diet-induced obesity and gut dysfunction. Nutrients. 2023;15:5007. doi: 10.3390/nu15235007. https://doi.org/10.3390/nu15235007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang H, Jiang F, Cheng R, Luo Y, Wang J, Luo Z, et al. A high-fat diet and high-fat and high-cholesterol diet may affect glucose and lipid metabolism differentially through gut microbiota in mice. Exp Anim. 2021;70:73–83. doi: 10.1538/expanim.20-0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SX, Yang C, Jiang RS, Wu C, Lang DQ, Wang YL, et al. Flavonoid extracts of Citrus aurantium L. var. amara Engl. promote browning of white adipose tissue in high-fat diet-induced mice. J Ethnopharmacol. 2024;324:117749. doi: 10.1016/j.jep.2024.117749. https://doi.org/10.1016/j.jep.2024.117749. [DOI] [PubMed] [Google Scholar]
- Marlatt KL, Ravussin E. Brown adipose tissue: an update on recent findings. Curr Obes Rep. 2017;6:389–396. doi: 10.1007/s13679-017-0283-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mota de Sá P, Richard AJ, Hang H, Stephens JM. Transcriptional regulation of adipogenesis. Compr Physiol. 2017;7:635–674. doi: 10.1002/cphy.c160022. [DOI] [PubMed] [Google Scholar]
- Park GH, Lee JY, Kim DH, Cho YJ, An BJ. Anti-oxidant and antiinflammatory effects of Rosa multiflora root. J Life Sci. 2011;21:1120–1126. doi: 10.5352/JLS.2011.21.8.1120. [DOI] [Google Scholar]
- Patterson E, Ryan PM, Cryan JF, Dinan TG, Ross RP, Fitzgerald GF, et al. Gut microbiota, obesity and diabetes. Postgrad Med J. 2016;92:286–300. doi: 10.1136/postgradmedj-2015-133285. [DOI] [PubMed] [Google Scholar]
- Rašković A, Martić N, Tomas A, Andrejić-Višnjić B, Bosanac M, Atanasković M, et al. Carob extract (Ceratonia siliqua L.): effects on dyslipidemia and obesity in a high-fat diet-fed rat model. Pharmaceutics. 2023;15:2611. doi: 10.3390/pharmaceutics15112611. https://doi.org/10.3390/pharmaceutics15112611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasouli N, Kern PA. Adipocytokines and the metabolic complications of obesity. J Clin Endocrinol Metab. 2008;93:S64–S73. doi: 10.1210/jc.2008-1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo SH, Fang F, Kang I. Ginger (Zingiber officinale) attenuates obesity and adipose tissue remodeling in high-fat diet-fed C57BL/6 mice. Int J Environ Res Public Health. 2021;18:631. doi: 10.3390/ijerph18020631. https://doi.org/10.3390/ijerph18020631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Z, Xiaoli AM, Yang F. Regulation and metabolic significance of de novo lipogenesis in adipose tissues. Nutrients. 2018;10:1383. doi: 10.3390/nu10101383. https://doi.org/10.3390/nu10101383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudeep HV, Gouthamchandra K, Ramanaiah I, Raj A, Shyamprasad K. An edible bioactive fraction from Rosa multiflora regulates adipogenesis in 3T3-L1 adipocytes and high-fat diet-induced C57Bl/6 mice models of obesity. Pharmacogn Mag. 2021;17:84–92. doi: 10.4103/pm.pm_175_20. [DOI] [Google Scholar]
- Vinodhini S, Rajeswari VD. Exploring the antidiabetic and anti-obesity properties of Samanea saman through in vitro and in vivo approaches. J Cell Biochem. 2019;120:1539–1549. doi: 10.1002/jcb.27385. [DOI] [PubMed] [Google Scholar]
- Wu J, Liu X, Chan CO, Mok DK, Chan SW, Yu Z, et al. Petroleum ether extractive of the hips of Rosa multiflora ameliorates collagen-induced arthritis in rats. J Ethnopharmacol. 2014;157:45–54. doi: 10.1016/j.jep.2014.09.026. [DOI] [PubMed] [Google Scholar]
- Yang A, Mottillo EP. Adipocyte lipolysis: from molecular mechanisms of regulation to disease and therapeutics. Biochem J. 2020;477:985–1008. doi: 10.1042/BCJ20190468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zang L, Shimada Y, Nakayama H, Katsuzaki H, Kim Y, Chu DC, et al. Preventive effects of green tea extract against obesity development in zebrafish. Molecules. 2021;26:2627. doi: 10.3390/molecules26092627. https://doi.org/10.3390/molecules26092627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao T, Chen Q, Chen Z, He T, Zhang L, Huang Q, et al. Anti-obesity effects of mulberry leaf extracts on female high-fat diet-induced obesity: Modulation of white adipose tissue, gut microbiota, and metabolic markers. Food Res Int. 2024;177:113875. doi: 10.1016/j.foodres.2023.113875. https://doi.org/10.1016/j.foodres.2023.113875. [DOI] [PubMed] [Google Scholar]