Abstract
Here, we aimed to isolate an acetic acid bacterium that is suitable for the production of unripe Citrus unshiu vinegar from traditional fermented vinegars. We compared the halo sizes of isolates to select a strain with superior acetic acid production capabilities and selected Komagataeibacter kakiaceti P6 (P6) as the final strain. Using Acetobacter pasteurianus CY (CY) and A. pasteurianus KACC 17058 (KACC 17058) as controls, we analyzed the total phenolic compounds, total flavonoid content, antioxidant activities, and organic acids of the selected strain to verify its suitability for acetic acid fermentation. On the 30th day of the fermentation period, P6 showed a total acidity of 4.86%, which was higher than that of control groups (CY, 4.16%; KACC 17058, 4.01%). The total phenolic compounds, total flavonoid content, 1,1-diphenyl-2-picrylhydrazyl scavenging activity, and ferric ion reducing antioxidant power values significantly increased during fermentation with P6 compared with the initial C. unshiu wine, and no significant differences were observed from the vinegars produced by CY and KACC 17058. Moreover, organic acid analysis revealed that the unripe C. unshiu vinegar produced with P6 had an acetic acid content of 26.15 mg/mL, which was significantly higher than those produced with CY and KACC 17058, indicating that the P6 strain effectively produces acetic acid without adversely affecting other quality aspects during fermentation. In conclusion, the novel P6 strain is expected to be used as a starter for fermenting unripe C. unshiu vinegar, and its excellent acetic acid production capabilities suggest potential applications for other vinegars.
Keywords: acetic acid bacteria, Citrus unshiu, fermentation, Komagataeibacter kakiaceti, vinegar
INTRODUCTION
Fermented vinegar has traditionally been used as a seasoning to enhance the taste of various foods (Lee et al., 2019; De Leonardis et al., 2022). It is primarily produced using fruits and vegetables rich in nutritional components (e.g., amino acids, organic acids, phenols, vitamins, and minerals), and numerous studies have reported that these components can help with digestion, fatigue recovery, and diabetes and possess antiobesity and anticancer properties (Ousaaid et al., 2020; Özdemir et al., 2022). Recently, the incidence of various diseases has been continuously increasing with the increase in the elderly population; consequently, foods for wellbeing, including health functional foods and health protectants aimed at preventing chronic diseases such as cancer, diabetes, and cardiovascular diseases, are gaining increasing attention (Park et al., 2020).
In the Republic of Korea, the majority of Citrus unshiu, commonly known as mandarins, are produced on Jeju Island, with an annual production of about 560,000 Mg (Jang et al., 2004). C. unshiu contains about 60 types of bioactive compounds, including flavonoids, carotenoids, phenylpropanoids, and limonoids (Lee et al., 2022b), along with various nutrients (e.g., free sugars, organic acids, dietary fibers, vitamins, and minerals) (Ahn et al., 2007). Consequently, numerous preclinical and clinical trials have reported on the various physiological activities induced by antioxidants in C. unshiu peels and pulp (Ahn et al., 2007; Park et al., 2020; Lee et al., 2022a). In the past, unripe C. unshiu fruits, known as green tangerines, were largely considered to have no industrial value and were often discarded in large quantities (Yi et al., 2022). However, their nutritional value has been recognized recently, leading to increased sales through the internet and direct transactions, and their distribution is now allowed in Jeju Special Self-Governing Province, Republic of Korea (Lee and Joo, 2021).
Unripe C. unshiu is rich in functional substances, including flavonoids, polyphenols, limonoids, and vitamin C (Shin and Lee, 2021), and various products (e.g., green tangerine marmalade and vinegar) that contain these substances have been marketed. Nevertheless, because of the antimicrobial activities of essential oils (e.g., limonene) and flavonoids (e.g., hesperidin and naringin), acetic acid fermentation using unripe C. unshiu is challenging compared with other fruits (Yi et al., 2014). The total acidity, which is a quality indicator of fermented vinegar, depends on the type of acetic acid bacteria used during the manufacturing process of vinegar; thus, the screening and utilization of excellent acetic acid bacteria are important (Park et al., 2005; Şengün et al., 2022).
This study aims to isolate and select an excellent acetic acid bacterial strain that is suitable for unripe C. unshiu vinegar production, analyze the fermentation characteristics during the fermentation process of vinegar, and investigate the strain’s potential for developing high-quality unripe C. unshiu vinegar by analyzing the bioactive substance content, antioxidant activity, and organic acid composition of its vinegar.
MATERIALS AND METHODS
Strains and materials
Unripe C. unshiu was purchased in July 2020 from Ommapum Agricultural Co., Ltd., which is located in Jeju Special Self-Governing Province, and used to produce vinegar. The pulp and peel of unripe C. unshiu were separated by hand, and the juice obtained from squeezing was used for the experiments. Four types of commercially available traditional fermented vinegars were purchased from Goljaknara Research Institute Agricultural Co., Ltd.; Yeongdong Dried Persimmon Farming Association Co.; Yeongyang Green Food Co., Ltd.; and Hanhongsoon Agricultural and Fisheries and used as sources for isolation to select an appropriate acetic acid bacterium for unripe C. unshiu vinegar. The commercial Saccharomyces cerevisiae Fermivin (DMS Food Specialties) was used as yeast in the production of unripe C. unshiu wine. Acetobacter pasteurianus CY (KACC 92333p), which was previously isolated and preserved in the Food Microbiology and Biotechnology Lab at Kyungpook National University, and A. pasteurianus KACC 17058, which was obtained from the Rural Development Administration, were used as controls for the selected isolated strains.
Vinegar production
Fifteen kilograms of unripe C. unshiu was washed with baking soda (100% sodium bicarbonate, Church & Dwight Co., Inc.), peeled, and extracted to obtain the juice. Then, 10 L of unripe C. unshiu juice was placed in a 20-L sterilized fermentation container made of PET (height, 45 cm; diameter, 28 cm), equipped with an airlock, adjusted to 17.5°Brix sugar content, treated with 100 mg/L of K2S2O5 (potassium metabisulfite), and left to stabilize for 2 h. Afterward, the prepared juice was inoculated with 0.02% (w/v) S. cerevisiae Fermivin. Subsequently, the mixture was fermented at 20°C for 5 days to produce unripe C. unshiu wine. To prepare the starter cultures for acetic acid fermentation, each control and isolated acetic acid bacterial strain were inoculated into 5% (v/v) YPM liquid medium (0.5% yeast extract, 0.3% peptone, and 2.5% D-mannitol) and cultured at 30°C for 48 h at 150 rpm. Then, they were added to sterilized unripe C. unshiu wine and incubated at 30°C for 10 days at 150 rpm. The starter culture and unripe C. unshiu wine were mixed at a 2:8 (v/v) ratio and subjected to acetic acid fermentation in a 5-L fermentation container at 30°C for 30 days to produce unripe C. unshiu vinegar.
Screening for acetic acid-producing bacteria
A 5% (v/v) inoculation was conducted in YPM liquid medium followed by cultivation at 30°C for 48 h at 150 rpm to isolate strains with excellent acetic acid production capabilities from four commercially available traditional fermented vinegars. Subsequently, streak plating was performed using a platinum loop, and 64 single colonies showing the characteristics of acetic acid bacteria were primarily screened. These colonies were then inoculated on GYC solid medium [3% glucose, 0.5% yeast extract, 1.0% CaCO3, 3% (v/v) ethanol, and 1.5% agar] to observe the formation of clear zones. Based on the presence of clear zones, six colonies were selected for further analysis. To compare the acetic acid production capabilities of the six isolates, relative halo sizes were determined by comparing their clear zones and colony sizes on GYC solid medium (Guo et al., 2008), and the final acetic acid-producing strain was selected through comparison with control strains (A. pasteurianus CY and KACC 17058).
The 16S rRNA gene sequences of the final selected strain were analyzed and then compared with sequences recorded in the gene bank using the National Center for Biotechnology Information’s Basic Local Alignment Search Tool. Multiple sequence alignment was performed using ClustalW in the BioEdit program (v7.2.5) (Thompson et al., 1997). Meanwhile, phylogenetic tree analysis was conducted using the neighbor-joining method in the MEGA (v6.06) program, and the reliability of branches within the molecular phylogeny was assessed using the bootstrap method with 1,000 replications (Felsenstein, 1981; Tamura et al., 2013).
Analysis of fermentation characteristics
The pH levels of fermentation cultures were measured using a pH meter (MP225K, Mettler-Toledo CH). The supernatant of unripe C. unshiu vinegar was obtained by centrifugation at 4°C and 4,973 g for 15 min. To determine the total acidity, 10 mL of the supernatant was titrated with 0.1 N NaOH solution to pH 8.3 after adding 2∼3 drops of 1% phenolphthalein, and the amount of 0.1 N NaOH consumed was converted into an organic acid coefficient equivalent to acetic acid (Sim et al., 2018). To measure the viable cell count, 1 mL of the vinegar sample was added with 9 mL of sterile distilled water. Next, the sample was diluted to the appropriate concentration using the serial dilution method and spread on YPM solid medium. Thereafter, it was incubated at 30°C for 48 h, and the growing colonies were counted as colony forming units (CFU/mL). The alcohol content was analyzed in accordance with the National Tax Service Liquor License Support Center (2017) regulations. After centrifugation, 100 mL of the supernatant was distilled to obtain 70 mL of distillate. Next, 30 mL of distilled water was added to this distillate, and after adjusting to 15°C, the alcohol content was measured using an alcohol meter and converted to alcohol by volume using the Gay-Lussac alcohol conversion table.
Analysis of the physicochemical properties of vinegar
The organic acid content was analyzed through high-performance liquid chromatography (Model Prominence, Shimadzu Co.) using a PL Hi-Plex H column (7.7×300 mm, Agilent Technologies) with a flow rate of 0.6 mL/min and temperature of 65°C. The mobile phase was 0.005 M sulfuric acid. The supernatant of unripe C. unshiu vinegar was filtered through a Sep-Pak C18 Plus Cartridge (Waters Co., Ltd.) and a 0.45-μm nylon syringe filter (SN02545, Lubitech Technologies Co., Ltd.) before analysis.
The total phenolic compound content was quantified colorimetrically in accordance with the Folin-Denis method (Amerine and Ough, 1980). One milliliter of 50% Folin-Ciocalteu phenol reagent was added to 1 mL of the supernatant of unripe C. unshiu vinegar, and the solution was left at room temperature for 3 min. Then, 1 mL of Na2CO3 solution was added, and the mixture was allowed to react in the dark at room temperature for 1 h before measuring the absorbance at 700 nm using a spectrophotometer. The total phenolic compound content was calculated using a standard curve prepared with gallic acid.
To determine the total flavonoid content, 430 μL of 50% ethanol and 50 μL of 5% sodium nitrite were added to 70 μL of the supernatant of unripe C. unshiu vinegar. The mixture was left at room temperature for 30 min. Then, 50 μL of 10% aluminum nitrate nonahydrate was added, and the reaction was allowed to proceed at room temperature for 6 min. After adding 500 μL of 1 N sodium hydroxide, the absorbance was measured at 510 nm using a spectrophotometer, and the total flavonoid content was calculated using a standard curve prepared with catechin (Zhishen et al., 1999).
Antioxidant activity
The 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging activity of vinegar was measured in accordance with Blois’ (1958) method. Fifty microliters of the supernatant of unripe C. unshiu vinegar was mixed with 150 μL of 0.1 mM DPPH solution in a 96-well plate and incubated in the dark at room temperature for 15 min. Distilled water and 100 μg/mL of ascorbic acid were used as negative and positive controls, respectively. The absorbance was measured spectrophotometrically at 517 nm (Benzie and Strain, 1996).
The ferric ion reducing antioxidant power (FRAP) assay was performed to assess the antioxidant capacity of vinegar. First, 25 μL of the supernatant of unripe C. unshiu vinegar was added to a 96-well plate. Then, a 10:1:1 (v/v/v) solution of 300 mM acetate buffer (pH 3.6), 10 mM 2,4,6-tripyridyl-s-triazine, and 20 mM ferric chloride was prewarmed at 37°C for 15 min. Thereafter, 175 μL was added to each well of the plate. Then, the mixture was incubated in the dark at room temperature for 30 min, and the absorbance was measured at 590 nm. The results were converted to μg TE (Trolox equivalents)/g using a standard curve.
Statistical analysis
All experiments were conducted with at least three replicates, and the results are presented as mean±standard deviation. Analyses of variance and Duncan’s multiple range tests were performed using SAS (version 9.4, SAS Institute Inc.) to determine the significance of the findings (P<0.05).
RESULTS AND DISCUSSION
Screening and identification of acetic acid-producing bacteria
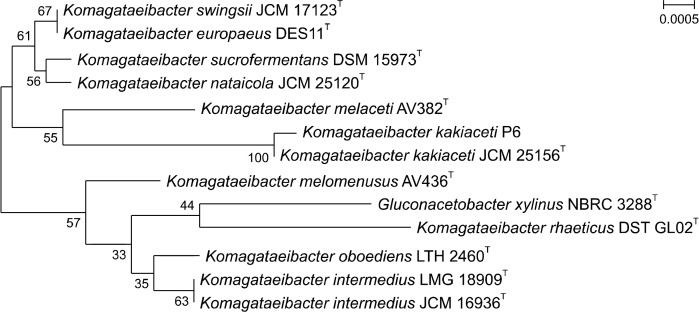
Sixty-four potential isolates from four types of traditional fermented vinegar were primarily screened for the identification of excellent acetic acid-producing bacteria (data not shown). Utilizing the fact that acetic acid bacteria can dissolve CaCO3 in GYC solid medium, forming a clear zone (Bang et al., 2022), six isolates forming clear zones were selected for secondary screening. A. pasteurianus CY and KACC 17058 were used as controls to compare the acetic acid production capability of these isolates, and the colony and clear zone sizes of eight strains, including the controls, were examined, comparing their acetic acid production ability through their relative halo sizes (Table 1). The relative halo sizes revealed that the P6 strain exhibited the highest relative halo size at 2.57, indicating that it had the highest acetic acid production capability. Ultimately, this strain was selected as the optimal fermentation strain for unripe C. unshiu vinegar. The analysis of the 16S rRNA gene sequence of the P6 strain showed 100% homology with the type strain Komagataeibacter kakiaceti JCM 25156 (Fig. 1).
Table 1.
Colony size, clear zone, and relative halo size of each isolate
| Strain | Colony size (cm) | Clear zone (cm) | Relative halo size1) |
|---|---|---|---|
| S1 | 0.41±0.10b | 0.72±0.00c | 1.75±0.07c |
| S2 | 0.42±0.10b | 0.00±0.00d | 0.00±0.00e |
| BR3 | 0.74±0.00a | 1.41±0.10b | 2.00±0.05b |
| P5 | 0.64±0.00a | 1.31±0.10b | 2.17±0.04b |
| P6 | 0.73±0.20a | 1.83±0.00a | 2.57±0.05a |
| R7 | 0.72±0.10a | 1.44±0.00b | 2.00±0.05b |
| Acetobacter pasteurianus KACC 17058 | 0.70±0.00a | 1.31±0.10b | 1.86±0.03c |
| A. pasteurianus CY | 0.44±0.00b | 0.64±0.10c | 1.50±0.05d |
Values are presented as mean±SD (n=3).
Different letters within the same column (a-e) indicate significantly different means (P<0.05).
1)Relative halo size=clear zone/colony size.
Fig. 1.
Phylogenetic tree based on the 16S rRNA gene sequences of the P6 strain (the selected isolate) and related sequences. The related sequences were obtained using a Basic Local Alignment Search Tool search on the National Center for Biotechnology Information website (www.ncbi.nlm.nih.gov). All sequences were aligned using the Clustal X software. The tree was constructed using the neighbor-joining method and the Kimura two-parameter calculation model in MEGA 6.
Fermentation characteristics of unripe C. unshiu vinegar
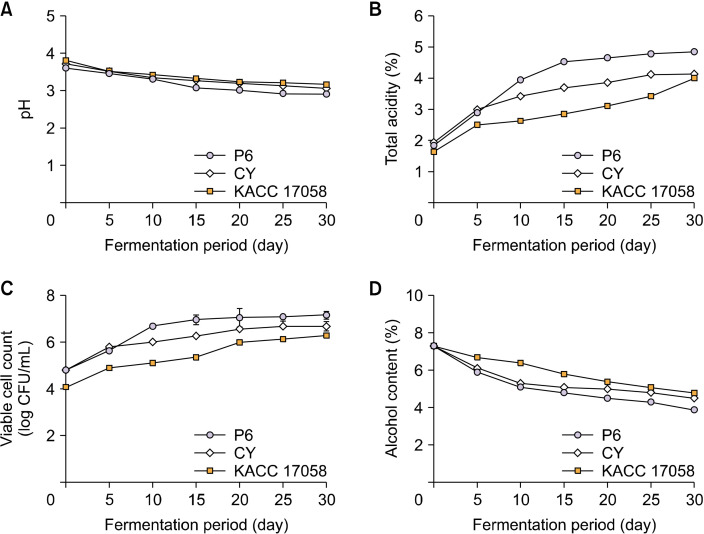
The newly isolated K. kakiaceti P6 strain was inoculated into unripe C. unshiu wine with an initial alcohol concentration of 7.3% to verify its potential as a starter culture bacterium for vinegar production. For comparison, the control strains A. pasteurianus CY and KACC 17058, which were previously isolated from traditional fermented vinegar, were also inoculated into wine with the same initial alcohol concentrations. The fermentation characteristics of unripe C. unshiu vinegar during fermentation are shown in Fig. 2. As fermentation progressed, the pH generally decreased across all experimental groups, with the most significant reduction seen in P6 vinegar (Fig. 2A). This decrease in pH is attributed to acetic acid bacteria utilizing alcohol as an energy source and fermentation substrate to produce acetic acid (Bang et al., 2020). The total acidity serves as a vinegar quality indicator as acetic acid bacteria can break down alcohol to produce acetic acid and CO2 (Kwon et al., 2014; Park et al., 2021). The total acidity contents of vinegars are shown in Fig. 2B. As the fermentation period increased, the total acidity increased in all experimental groups, with the highest value (4.86%) recorded on the 30th day of fermentation in the P6 group. According to Kim et al. (2001) who used kelp extract to produce vinegar, while pH decreases, the total acidity increases as ethanol, sugar, and other solution components are converted to acetic acid. Kim et al. (2020) reported that the total acidity increased to 7.3% when A. pasteurianus YJ17, which was isolated from brown rice, was used to ferment mulberry fruits. The total acidity of vinegar varies depending on the type of acetic acid bacteria. In the present study, the highest total acidity content was observed when the P6 strain was used, indicating that it is the most suitable strain for producing unripe C. unshiu vinegar.
Fig. 2.
Changes in pH (A), total acidity (B), viable cell count (C), and alcohol content (D) during the fermentation of unripe Citrus unshiu vinegar using three bacterial strains: Komagataeibacter kakiaceti P6 (P6), Acetobacter pasteurianus CY (CY), and A. pasteurianus KACC 17058 (KACC 17058).
The viable cell count results of unripe C. unshiu vinegars are shown in Fig. 2C. As fermentation progressed, the viable cell counts increased in all experimental groups, with the P6 group showing a higher rate of increase, surpassing the other strains by day 10 and reaching the highest viable cell count (7.18 log CFU/mL) at the end of fermentation.
The alcohol contents of unripe C. unshiu vinegars are shown in Fig. 2D. As the fermentation period increased, the alcohol content decreased in all experimental groups likely because of the conversion of alcohol to organic acids during the acetic acid fermentation process (Lee et al., 2012). Compared with other vinegars, the P6 vinegar exhibited the lowest alcohol content at the end of fermentation. As the P6 group showed the highest rate of reduction in alcohol and increase in total acidity during the acetic acid fermentation process, it was considered as the optimal fermentation strain for unripe C. unshiu vinegar.
Total polyphenol and flavonoid contents
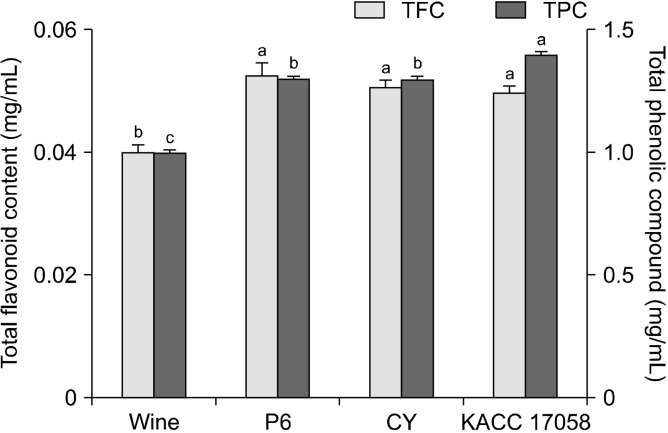
Phenolic compounds, which are widely distributed in plants, are mostly secondary metabolites possessing various biological activities, including antioxidant, anticancer, and anti-inflammatory properties, because of their hydroxyl groups (Lee et al., 2005; Kim et al., 2009). Belonging to the polyphenol group, flavonoids are categorized into flavonols, flavanones, catechins, and isoflavones based on their chemical structure, which influences their biochemical activity (Kim et al., 2010). The unripe C. unshiu wine was analyzed as a control to observe the effect of each strain on the phenolic and flavonoid compounds during acetic acid fermentation. The total phenolic and flavonoid contents in unripe C. unshiu vinegar are shown in Fig. 3. Unripe C. unshiu wine contained the lowest total phenolic compound content (0.98 mg/mL), and all vinegar samples exhibited a significant increase in phenolic content compared with the control, showing approximate increases of 30%∼40%. In addition, all vinegar samples contained higher total flavonoid content than wine, with the P6 group showing the highest value at 0.053 mg/mL, approximately 32.5% higher than that of the control. Similarly, Gao et al. (2022) observed changes in phenolic compounds and flavonoid contents during the acetic acid fermentation of black wolfberry. They found that the total phenolic compounds and flavonoid contents increased as fermentation progressed.
Fig. 3.
Total phenolic compounds (TPC) and total flavonoid contents (TFC) of unripe Citrus unshiu vinegars and unripe C. unshiu wine that was used to make them. Three bacterial strains were used for fermentation: Komagataeibacter kakiaceti P6 (P6), Acetobacter pasteurianus CY (CY), and A. pasteurianus KACC 17058 (KACC 17058). Different letters above the bar (a-c) mean scores that are significantly different (P<0.05) by Duncan’s multiple range test.
Several studies have examined bioactive compounds in vinegars created by fermentation using the same or similar fruits. In Yi et al.’s (2014) study, which analyzed the total phenolic and flavonoid contents in vinegar made from immature and ripe citrus fruits, immature citrus vinegar showed an approximately 6.7-fold increase in total phenolic compounds and a greater than 5.7-fold increase in flavonoid content compared with vinegar made from ripe fruits. According to Park et al. (2020), premature mandarin vinegar had a higher flavonoid content (3 μg CE/mL) than vinegars made from premature mandarin mixed with 10% dried or roasted Citri Unshius Pericarpium Immaturus. Lee et al. (2014) studied the production of rice vinegar with the addition of Akebia quinata fruit during fermentation. They found that the total polyphenol and flavonoid contents in vinegar increased with increasing amount of A. quinata fruit and the progression of acetic acid fermentation. These changes in bioactive substances are because of the conversion of bound polyphenolic compounds in the fermentation substrate to free forms by microbe-derived acids, sugars, or lipolytic enzymes during the fermentation process (Cho et al., 2017).
Antioxidant activity
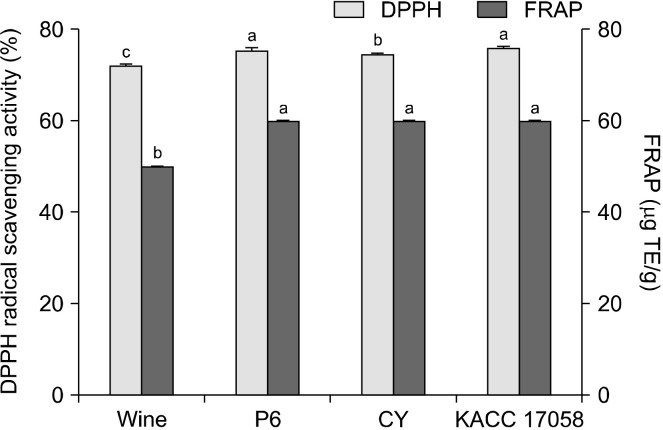
The antioxidant activities of unripe C. unshiu vinegars are shown in Fig. 4. With regard to DPPH radical scavenging activity, unripe C. unshiu wine showed an activity of 72.08%, and all vinegar samples exhibited an increase because of acetic acid fermentation, with the KACC 17058 group showing the highest activity (76.08%). The FRAP assay produced similar results, wherein all vinegar samples showed significant increases in antioxidant activity compared with the control (50 μg TE/g). Yi et al. (2014) analyzed the DPPH radical scavenging activity of vinegar made from immature and ripe C. unshiu. They reported that immature C. unshiu vinegar showed greater DPPH radical scavenging activity and higher total phenolic and flavonoid contents than ripe C. unshiu vinegar. Chen et al. (2017) analyzed the changes in DPPH radical scavenging activity in citrus vinegar, which underwent primary alcohol fermentation with S. cerevisiae (dry yeast) and Lactobacillus plantarum AS1.555 and secondary acetic acid fermentation with A. pasteurianus AS1.41. They found that, along with an increase in polyphenol content, the DPPH radical scavenging activity increased from 25.5% to 37.4% with the progression of acetic acid fermentation. In another study, the DPPH radical scavenging activity increased in kombuchas brewed with various fruit peels as fermentation progressed, which was attributed to the increase in polyphenol and flavonoid contents (Lee and Yi, 2023). In their study comparing the antioxidant activities of 10 commercially available fermented vinegar products in Korea, Pyo et al. (2021) found that citrus vinegar had high polyphenol and flavonoid contents and FRAP antioxidant activity. The high FRAP values were attributed to the high content of quercetin derivatives and polyphenols. In another study that analyzed the FRAP activity of commercially available fruit juices, Lee et al. (2008) reported high FRAP values in orange and grapefruit juices (117.62 and 115.72 μM TE, respectively). In addition, these juices contained significantly higher DPPH radical scavenging activity and FRAP antioxidant activity compared with others, noting that the two values were significantly correlated.
Fig. 4.
1,1-Diphenyl-2-picrylhydrazyl (DPPH) radical scavenging activity and ferric ion reducing antioxidant power (FRAP) of unripe Citrus unshiu vinegars and unripe C. unshiu wine that was used to make them. Three bacterial strains were used for fermentation: Komagataeibacter kakiaceti P6 (P6), Acetobacter pasteurianus CY (CY), and A. pasteurianus KACC 17058 (KACC 17058). Different letters above the bar (a-c) mean scores that are significantly different (P<0.05) by Duncan’s multiple range test.
Organic acid contents
The organic acid contents of unripe C. unshiu vinegars are shown in Table 2. Five types of organic acids were detected, with acetic acid and citric acid having the highest concentrations. Citric acid, which is primarily found in citrus juices (Song et al., 1998), had high concentrations even after acetic acid fermentation. The acetic acid contents in unripe C. unshiu vinegars produced with K. kakiaceti P6 and A. pasteurianus CY were 26.15 and 20.52 mg/mL, respectively, representing the highest organic acid content in these vinegars. Similarly, Park et al. (2020) found that when acetic acid fermentation was conducted using premature mandarins harvested in July, the organic acids in the resulting vinegar contained (in descending order) acetic (13,288 mg/L), citric, succinic, and tartaric acids. In their study comparing organic acids found in vinegars produced with 30%, 35%, and 40% immature C. unshiu juice in 5% fermented alcohol, 5% seed vinegar, and distilled water, Yi et al. (2014) found that lactic acid and acetic acid were the most abundant organic acids, with the latter having the highest concentration (3,990 mg%) in the group with 40% immature C. unshiu juice. Additionally, Yi et al. (2017) reported that six types of organic acids were detected in lemongrass vinegar, with acetic acid being the predominant organic acid (3,658.58 mg%). The present study found that unripe C. unshiu vinegars fermented with K. kakiaceti P6 and A. pasteurianus CY also showed significant amounts of acetic acid, indicating that normal acetic acid fermentation occurred.
Table 2.
Organic acid contents of unripe Citrus unshiu vinegars produced by different bacterial strains
| Strain | Organic acid (mg/mL) | ||||
|---|---|---|---|---|---|
|
| |||||
| Citric acid | Malic acid | Succinic acid | Lactic acid | Acetic acid | |
| Komagataeibacter kakiaceti P6 | 12.00±0.02b | 2.72±0.02c | 1.55±0.03b | 0.11±0.02a | 26.15±0.02a |
| Acetobacter pasteurianus KACC 17058 | 14.58±0.01a | 3.80±0.03a | 1.81±0.02a | 0.08±0.01b | 11.63±0.04c |
| A. pasteurianus CY | 11.84±0.03c | 3.41±0.03b | 1.52±0.00c | 0.11±0.01a | 20.52±0.02b |
Values are presented as mean±SD (n=3).
Different letters within the same column (a-c) indicate significantly different means (P<0.05).
In this study, while all unripe C. unshiu vinegars exhibited similar increases in antioxidant activity, different organic acid contents, total acid production, and polyphenol contents were observed when using three types of acetic acid bacteria, including a novel isolate (K. kakiaceti P6). This variation can be attributed to differences in metabolic and enzyme activities, leading to different substrate degradation characteristics when different acetic acid bacteria were used (Chen et al., 2022). In conclusion, the novel strain K. kakiaceti P6 demonstrated higher acid production and physiological activity characteristics during vinegar fermentation than either A. pasteurianus strain, indicating its high potential as a starter culture bacterium for unripe C. unshiu vinegar.
ACKNOWLEDGEMENTS
This research was supported by biological materials Specialized Graduate Program through the Korea Environmental Industry & Technology Institute (KEITI) funded by the Ministry of Environment (MOE).
Footnotes
FUNDING
This research was supported by the National Research Foundation of Korea, Korea, grant number NRF-2022R1I1A3072406.
AUTHOR DISCLOSURE STATEMENT
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
Concept and design: SHW, HDP, SBL. Analysis and interpretation: SHW, YJK, KTC, JSC, SBL. Data collection: SHW, YJK, SBL. Writing the article: SHW, YJK, SBL. Critical revision of the article: HDP, SBL. Final approval of the article: all authors. Statistical analysis: KTC, JSC, SBL. Obtained funding: SBL. Overall responsibility: SHW, HDP, SBL.
References
- Ahn MS, Kim HJ, Seo MS. A study on the antioxidative and antimicrobial activities of the Citrus unshju peel extracts. J Korean Soc Food Cult. 2007;22:454–461. [Google Scholar]
- Amerine MA, Ough CS. Methods for analysis of musts and wines. John Wiley & Sons; 1980. pp. 176–180. [Google Scholar]
- Bang KH, Kim CW, Kim CH. Isolation of an acetic acid bacterium Acetobacter pasteurianus CK-1 and its fermentation characteristics. J Life Sci. 2022;32:119–124. [Google Scholar]
- Bang SI, Gwon GH, Cho EJ, Lee AY, Seo WT. Characteristics of fermented vinegar using mulberry and its antioxidant activity. Korean J Food Preserv. 2020;27:651–662. doi: 10.11002/kjfp.2020.27.5.651. [DOI] [Google Scholar]
- Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of "antioxidant power": the FRAP assay. Anal Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Blois MS. Antioxidant determinations by the use of a stable free radical. Nature. 1958;181:1199–1200. doi: 10.1038/1811199a0. [DOI] [Google Scholar]
- Chen C, Wu S, Li Y, Huang Y, Yang X. Effects of different acetic acid bacteria strains on the bioactive compounds, volatile compounds and antioxidant activity of black tea vinegar. LWT. 2022;171:114131. doi: 10.1016/j.lwt.2022.114131. https://doi.org/10.1016/j.lwt.2022.114131. [DOI] [Google Scholar]
- Chen Y, Huang Y, Bai Y, Fu C, Zhou M, Gao B, et al. Effects of mixed cultures of Saccharomyces cerevisiae and Lactobacillus plantarum in alcoholic fermentation on the physicochemical and sensory properties of citrus vinegar. LWT. 2017;84:753–763. doi: 10.1016/j.lwt.2017.06.032. [DOI] [Google Scholar]
- Cho KM, Hwang CE, Joo OS. Change of physicochemical properties, phytochemical contents and biological activities during the vinegar fermentation of Elaeagnus multiflora fruit. Korean J Food Preserv. 2017;24:125–133. doi: 10.11002/kjfp.2017.24.1.125. [DOI] [Google Scholar]
- De Leonardis A, Macciola V, Iftikhar A, Lopez F. Antioxidant effect of traditional and new vinegars on functional oil/vinegar dressing-based formulations. Eur Res Technol. 2022;248:1573–1582. doi: 10.1007/s00217-022-03986-0. [DOI] [Google Scholar]
- Felsenstein J. Evolutionary trees from DNA sequences: a maximum likelihood approach. J Mol Evol. 1981;17:368–376. doi: 10.1007/BF01734359. [DOI] [PubMed] [Google Scholar]
- Gao Q, Song Y, Liang Y, Li Y, Chang Y, Ma R, et al. Dynamics of physicochemical properties, functional compounds and antioxidant capacity during spontaneous fermentation of Lycium ruthenicum Murr. (Qinghai-Tibet plateau) natural vinegar. Foods. 2022;11:1344. doi: 10.3390/foods11091344. https://doi.org/10.3390/foods11091344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo P, Seo S, Zhang L, Kim H, Oh Y, Jahng D. Isolation of lysozyme producing bacteria capable of solubilizing microbial cells. Korean J Biotechnol Bioeng. 2008;23:187–192. [Google Scholar]
- Jang SY, Choi HK, Ha NY, Kim OM, Jeong YJ. Study on the antimicrobial effects of citrus peel by different extract methods. Korean J Food Preserv. 2004;11:319–324. [Google Scholar]
- Kim DW, Kim DH, Kim JK, Yeo SH, Choi HS, Kim YH, et al. Comparison of volatile compounds in Maclura tricuspidata fruit vinegar and commercial vinegars. Korean J Food Preserv. 2020;27:85–97. doi: 10.11002/kjfp.2020.27.1.85. [DOI] [Google Scholar]
- Kim HS, Hong MJ, Kang IY, Jung JY, Kim HK, Shin YS, et al. Radical scavenging activities and antioxidant constituents of oriental melon extract. J Bio Environ Control. 2009;18:442–447. [Google Scholar]
- Kim KE, Choi OS, Lee YJ, Kim HS, Bae TJ. Processing of vinegar using the sea tangle (Laminaria japonica) extract. Korean J Life Sci. 2001;11:211–217. [Google Scholar]
- Kim NY, Chae HS, Lee IS, Kim DS, Seo KT, Park SJ. Analysis of chemical composition and antioxidant activity of Codonopsis lanceolata skin. J Korean Soc Food Sci Nutr. 2010;39:1627–1633. doi: 10.3746/jkfn.2010.39.11.1627. [DOI] [Google Scholar]
- Kwon WY, Lee EK, Yoon JA, Chung KH, Lee KJ, Song BC, et al. Quality characteristics and biological activities of vinegars added with young leaves of Akebia quinata. J Korean Soc Food Sci Nutr. 2014;43:989–998. doi: 10.3746/jkfn.2014.43.7.989. [DOI] [Google Scholar]
- Lee EK, Kwon WY, Lee JW, Yoon JA, Chung KH, Song BC, et al. Quality characteristics and antioxidant activity of vinegar supplemented added with Akebia quinata fruit during fermentation. J Korean Soc Food Sci Nutr. 2014;43:1217–1227. doi: 10.3746/jkfn.2014.43.8.1217. [DOI] [Google Scholar]
- Lee HR, Jung BR, Park JY, Hwang IW, Kim SK, Choi JU, et al. Antioxidant activity and total phenolic contents of grape juice products in the Korean market. Korean J Food Preserv. 2008;15:445–449. [Google Scholar]
- Lee JC, Han WC, Lee JH, Jang KH. Quality evaluation of vinegar manufactured using rice and Rosa rugosa Thunb. Korean J Food Sci Technol. 2012;44:202–206. doi: 10.9721/KJFST.2012.44.2.202. [DOI] [Google Scholar]
- Lee JE, Ahn JH, Kim DS, Kim SS, Park SM, Yun SH, et al. LC/MS-based metabolomic analysis of peels from citrus varieties. J Korean Soc Food Sci Nutr. 2022a;51:150–161. doi: 10.3746/jkfn.2022.51.2.150. [DOI] [Google Scholar]
- Lee SJ, Kim SH, Kim SY, Yeo SH. Quality characteristics of Kujippong (Cudrania tricuspidata) vinegar fermented by various acetic acid bacteria. Korean J Food Preserv. 2019;26:766–776. doi: 10.11002/kjfp.2019.26.7.766. [DOI] [Google Scholar]
- Lee SL, Joo SY. Effects of premature mandarin powder on the quality characteristics and antioxidant activities of scone. Korean J Food Preserv. 2021;28:231–239. doi: 10.11002/kjfp.2021.28.2.231. [DOI] [Google Scholar]
- Lee SO, Lee HJ, Yu MH, Im G, Lee IS. Total polyphenol contents and antioxidant activities of methanol extracts from vegetables produced in Ullung island. Korean J Food Sci Technol. 2005;37:233–240. [Google Scholar]
- Lee TY, Yi YH. Physicochemical properties of kombucha with fruit peels during fermentation. Korean J Food Preserv. 2023;30:321–333. doi: 10.11002/kjfp.2023.30.2.321. [DOI] [Google Scholar]
- Lee YH, Yeo MH, Yoon SA, Hyun HB, Ham YM, Jung YH, et al. Extracts of citrus juice processing wastes induce weight gain and decrease serum glucose in Sprague-Dawley rats. Prev Nutr Food Sci. 2022b;27:70–77. doi: 10.3746/pnf.2022.27.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Tax Service Liquor License Support Center, author. Analysis Regulations of Alcoholic Beverages. 2017. [cited 2020 Oct 31]. Available from: https://www.law.go.kr/LSW/admRulLsInfoP.do?admRulSeq=2100000100372 .
- Ousaaid D, Laaroussi H, Bakour M, ElGhouizi A, Aboulghazi A, Lyoussi B, et al. Beneficial effects of apple vinegar on hyperglycemia and hyperlipidemia in hypercaloric-fed rats. J Diabetes Res. 2020;2020:9284987. doi: 10.1155/2020/9284987. https://doi.org/10.1155/2020/9284987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Özdemir N, Pashazadeh H, Zannou O, Koca I. Phytochemical content, and antioxidant activity, and volatile compounds associated with the aromatic property, of the vinegar produced from rosehip fruit (Rosa canina L.) LWT. 2022;154:112716. doi: 10.1016/j.lwt.2021.112716. https://doi.org/10.1016/j.lwt.2021.112716. [DOI] [Google Scholar]
- Park B, Choi JW, Kim SH, Yun YR, Lee Y, Lee Y, et al. Antioxidant activity of premature mandarin vinegar according to harvest period and raw material conditions. Korean J Food Sci Technol. 2020;52:333–341. [Google Scholar]
- Park HJ, Jeon SH, Kim SY, Yeo SH, Gwon HM. Improvement in the manufacturing process and quality of jujube vinegar in the ancient literature 『Sangayorok』. Korean J Food Preserv. 2021;28:107–116. doi: 10.11002/kjfp.2021.28.1.107. [DOI] [Google Scholar]
- Park MH, Lee JO, Lee JY, Yu SJ, Ko YJ, Kim YH, et al. Isolation and characteristics of acetic acid bacteria for persimmon vinegar fermentation. J Korean Soc Food Sci Nutr. 2005;34:1251–1257. doi: 10.3746/jkfn.2005.34.8.1251. [DOI] [Google Scholar]
- Pyo YH, Noh YH, Lee DB, Lee YW, Yoon SM, Lee AR, et al. Profile chemical compounds and antioxidant activity of Korean commercial vinegars produced by traditional fermentation. Chem Pap. 2021;75:2537–2547. doi: 10.1007/s11696-020-01437-2. [DOI] [Google Scholar]
- Şengün İY, Kilic G, Charoenyingcharoen P, Yukphan P, Yamada Y. Investigation of the microbiota associated with traditionally produced fruit vinegars with focus on acetic acid bacteria and lactic acid bacteria. Food Biosci. 2022;47:101636. doi: 10.1016/j.fbio.2022.101636. https://doi.org/10.1016/j.fbio.2022.101636. [DOI] [Google Scholar]
- Shin KS, Lee JH. Optimization of enzymatic hydrolysis of immature citrus (Citrus unshiu Marcov.) for flavonoid content and antioxidant activity using a response surface methodology. Food Sci Biotechnol. 2021;30:663–673. doi: 10.1007/s10068-021-00897-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim SY, Ahn HY, Seo KI, Cho YS. Physicochemical properties and biological activities of Protaetia brevitarsis seulensis larvae fermented by several kinds of micro-organisms. J Life Sci. 2018;28:827–834. [Google Scholar]
- Song EY, Choi YH, Kang KH, Koh JS. Free sugar, organic acid, hesperidin, naringin and inorganic elements changes of Cheju citrus fruits according to harvest date. Korean J Food Sci Technol. 1998;30:306–312. [Google Scholar]
- Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: molecular evolutionary genetics analysis version 6. Mol Biol Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997;25:4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi HY, Park JW, Yu SK, Kim DS, Chun JY. Optimization of spray drying conditions for immature Citrus unshiu concentrates using response surface methodology. J Korean Soc Food Sci Nutr. 2022;51:845–854. doi: 10.3746/jkfn.2022.51.8.845. [DOI] [Google Scholar]
- Yi MR, Hwang JH, Oh YS, Oh HJ, Lim SB. Quality characteristics and antioxidant activity of immature Citrus unshiu vinegar. J Korean Soc Food Sci Nutr. 2014;43:250–257. doi: 10.3746/jkfn.2014.43.2.250. [DOI] [Google Scholar]
- Yi MR, Kang CH, Bu HJ. Acetic acid fermentation properties and antioxidant activity of lemongrass vinegar. Korean J Food Preserv. 2017;24:680–687. doi: 10.11002/kjfp.2017.24.5.680. [DOI] [Google Scholar]
- Zhishen J, Mengcheng T, Jianming W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999;64:555–559. doi: 10.1016/S0308-8146(98)00102-2. [DOI] [Google Scholar]